Note: Descriptions are shown in the official language in which they were submitted.
CA 02602044 2014-01-09
PACKAGING SYSTEM AND
METHOD OF ALERTING A PRACTITIONER
Background
[00011 This patent is directed to a packaging system and methods of use,
and, in
particular, to a packaging system with informational features and methods of
use.
100021 One common way of administering pharmaceutical products is by
injecting the product in liquid form. Often, the liquid product will be
packaged in a
vial in a condition ready for administration. The vial will have a stopper at
one end,
through which a needle of a syringe may be passed so as to draw the product
out of
the vial.
[0003] A label is affixed to the outside of the vial so that a medical
professional
can determine the contents of the vial. A textual description of the product
will be
oriented about the periphery of the vial, or aligned with the axis of the
vial.
Sometimes, a portion or region of the label will be color-coded to
differentiate
different products and/or dosages for the professional that will be
administering the
product.
[0004] It is important that the label accurately convey the
identification of the
product and dosage to the administering professional. Certain pharmaceuticals
(e.g.,
morphine) are so powerful that administration of the pharmaceutical except
where
indicated is to be avoided whenever possible. Other pharmaceutical products
may
contain the same active agent, but at different concentrations and, therefore,
are
intended for completely different purposes (compare Heparin and Hep-Lock
products). In either instance, administration of a product where not indicated
(or
contraindicated) may have severe consequences, and may even lead to the death
of the
patient.
Summary of the Invention
[0005] In one aspect, there is provided a packaging system comprising: a
container having a closed end, an open end, a longitudinal axis running
through the
closed end and the open end, and an outer surface; a closure assembly fitted
to the open
end of the container and having an access port; a detachable cap disposed over
the
access port; and a label disposed about the container, the label having a
first region
angled relative to the longitudinal axis, the first region consisting
essentially of a name
CA 02602044 2014-01-09
of an injectable drug product and a second region angled relative to the
longitudinal
axis, the second region consisting essentially of a concentration of the
injectable drug
product.
[0006] In another aspect, a method of alerting a practitioner to contents
of a
container is provided. The method includes providing a container having a
closed
end, an open end, a longitudinal axis running through the closed end and the
open
end, and an outer surface, and disposing a label about the container, the
label having a
first region angled relative to the longitudinal axis, the first region
consisting
essentially of a name of a product.
[0007] Additional aspects of the disclosure are defined by the claims of
this
patent.
Brief Description of the Drawings
[0008] Fig. 1 is a perspective view of the packaging system according to
the
present disclosure with a two-part label, the label being intact;
[0009] Fig. 2 is a perspective view of the packaging system of Fig. 1
with the
sections of the label separated and the cap removed;
[0010] Fig. 3 is a flowchart illustrating the method of use of the
packaging
system of Fig. 1;
[0011] Fig. 4 is a cross-sectional view of the packaging system with the
label
intact as in Fig. 1;
[0012] Fig. 5 is a perspective view of the packaging system illustrating
the
angle, taken relative to the longitudinal axis, of the first and second
regions of the first
section of the label;
[0013] Fig. 6 is a perspective view of an alternative embodiment of the
packaging system according to the present disclosure with a one-part label;
and
[0014] Fig. 7 is a plan view of an alternative embodiment of a cap for
use with
the packaging system of Fig. 1.
Detailed Description of Various Embodiments
[0015] Although the following text sets forth a detailed description of
different
embodiments of the invention, it should be understood that the legal scope of
the
invention is defined by the words of the claims set forth at the end of this
patent. The
2
CA 02602044 2014-01-09
detailed description is to be construed as exemplary only and does not
describe every
possible embodiment of the invention since describing every possible
embodiment
would be impractical, if not impossible. The scope of the claims should not be
limited
by the preferred embodiments set forth herein, but should be given the
broadest
interpretation consistent with the description as a whole.
[0016] It should also be understood that, unless a term is expressly
defined in
this patent using the sentence "As used herein, the term' 'is hereby
defined to
mean..." or a similar sentence, there is no intent to limit the meaning of
that term,
either expressly or by implication, beyond its plain or ordinary meaning, and
such
term should not be interpreted to be limited in scope based on any statement
made in
any section of this patent (other than the language of the claims). To the
extent that
any term recited in the claims at the end of this patent is referred to in
this patent in a
manner consistent with a single meaning, that is done for sake of clarity only
so as to
not confuse the reader, and it is not intended that such claim term be
limited, by
implication or otherwise, to that single meaning.
[0017] Figs. 1-5 illustrate an embodiment of packaging system 50
according to
the present disclosure and its use. Packaging system 50 may be used for a
pharmaceutical product, such as heparin or morphine. Alternatively, packaging
system 50 may be used with other products. Examples of injectable drugs
employed
in system 50 may include, but are not limited to: abarelix, abciximab,
acetazolamide,
acetonide, acetylcysteine, acyclovir, adalimumab, adenosine, adipiodone,
agalsidase
beta, albumin, aldesleukin, aldesleukin, alefacept, alemtuzumab, alfentanil,
alglucosidase, allopurinol, alpha 1-proteinase inhibitor, alphacon-1,
alprostadil,
alteplase, amifostine, amikacin, aminocaproic acid, aminophylline, amiodarone,
amobarbital, amphotericin b, ampicillin, amrinone, anakinra, antithrombin
antivenom serum, apomorphine, aprotinin, aredia, argatroban, arginine,
aripiprazple,
asparaginase, atenolol, atracurium, atropine, aurothioglucose, axetil,
azacitidine,
azathioprine, azithromycin, aztreonam, bacillus calmette-guerin, bacitracin,
basiliximab, benzoic acid, benztropine, betamethasone, bevacizumab,
bivalirudin,
bleomycin, bortezomib, botulinum a toxin, bretylium, bumetanide, bupivacaine,
buprenorphine, busulfan, butorphanol, caffeine, calcitonin (salmon),
calcitriol,
3
CA 02602044 2007-09-24
capreomycin, carboplatin, carboprost, carmine, carmustine, camitine,
caspofungin,
cefazolin, cefepime, cefotaxime, cefotetan, cefoxitin, ceftazidime,
ceftizoxime,
ceftriaxone, cefuroxime, cefuroxime, cetuximab, chloramphenicol,
chloroprocaine,
chlorothiazide, chlorpromazine, chondroitin, choriogonadotropin alfa,
cidofovir,
cilastatin, cimetidine, cinacalcet, ciprofloxacin, cisatracurium, cisplatin,
cladribine,
clavulanic acid, clindamycin, clofarabine, clonidine, codeine, colchicine,
colistin,
conivaptan, corticorelin, corticotrophin, cosyntropin, cyanocobalarnin,
cyclophosphamide, cyclosporine, cysteine, cytarabine, dacarbazine, daclizumab,
dactinomycin, dalfopristin, dalteparin, dantrolene, daptomycin, darbepoetin
alfa,
daunorubicin, ddavp, decitabine, deferoxamine, denileukin diftitox,
desmopressin,
dexamethasone, dexmedetomidine, dexpanthenol, dexrazoxane, dextasone,
diatrizoic
acid, diazepam, diazoxide, dicyclomine, digibind, digoxin, dihydroergotamine,
diltiazem, dimenhydrinate, diphenhydramine, dipyridamole, dobutamine,
docetaxel,
dolasetron, dopamine, dornase alfa, doxacurium, doxapram, doxercalciferol,
doxorubicin, doxycycline, droperidol, drotrecogin alfa, dyphylline,
eculizumab, edetic
acid, edrophonium, efalizumab, enalaprilat, enoxaparin, ephedrine,
epinephrine,
epirubicin, epoetin alpha, epoprostenol, eptacog alfa, eptifibatide,
ergocalciferol,
ergocalciferol, ertapenem, erythromycin, erythropoietin alpha, esmolol,
esomeprazole,
estradiol, estrogen, etanercept, ethacrynic acid, ethanolamine, ethiodized
oil, etidronic
acid, etomidate, etoposide, exenatide, factor ii, factor ix, factor vii,
factor viii, factor
x, famotidine, fenoldopam, fentanyl, filgrastim, floxuridine, fluconazole,
fludarabine,
fltunazenil, fluorescein, fluphenazine, follicle-stimulating hormone,
follitropin,
fomepizole, fondaparinux, foscamet, fosphenytoin, fulvestrant, furosemide,
gadobenic
acid, gadodiamide, gadopentetate, gadoteridol, gadoversetamide, gallium,
galsulfase,
ganciclovir, ganirelex, gatifloxacin, gemcitabine, gemtuzumab, gentamicin,
glatiramer, glucagon, glycopyrrolate, gm-csf, gold sodium thiomalate,
gonadorelin,
gonadotropin, goserelin, granisetron, haemophilus b polysaccaride,
haloperidol,
hemin, heparin, hetastarch, hexacetonide, histamine, hyaluronic acid,
hyaluronidase,
hydralazine, hydrocortisone, hydromorphone, hydroxocobalamin, hydroxyzine,
hylan,
hyoscyamine, hypromellose, ibuprofen, ibutilide, idarubicin, idursulfase,
ifosfamide,
imatinib mesylate, imiglucerase, imipenem, immune globulin, indigo,
indomethacin,
infliximab, insulin, interferons, iodine, iodixanol, iohexol, iopamidol,
iopromide,
iothalamic acid, ioversol, ioxaglic acid, ioxilan, irinotecan, iron dextran,
isoniazid,
4
CA 02602044 2007-09-24
isophane, isoproterenol, kanamycin, ketamine, ketorolac, labetalol,
lansoprazole,
laronidase, lepirudin, leucovorin, leuprolide, levetiracetam, levobupivacaine,
levofloxacin, levothyroxine, lidocaine, lincomycin, linezolid, liothyronine,
lispro,
lorazepam, luteinising hormone, mechlorethamine, medroxyprogesterone,
melphalan,
meperidine, meperidine, mepivacaine, meropenem, mesna, metaraminol, methadone,
methocarbamol, methohexital, methotrexate, methoxamine, methyldopate,
methylene
blue, methylergonovine, methylprednisolone, metoclopramide, metoprolol,
metronidazole, micafungin, midazolam, milrinone, minocycline, mitomycin,
mitoxantrone, mivacurium, mofetil, molybdenum, morphine, morrhuic acid,
moxifloxacin, muromonab-cd3, mycophenolate, nafcillin, nalbuphine, nalmefene,
naloxone, nandrolone, natalizumab, nelarabine, neostigmine, nesiritide,
nicardipine,
nitroglycerin, nitroprusside, norepinephiine, octreotide, olanzapine,
omalizumab,
ondansetron, oprelvekin, orphenadrine, ovine, oxacillin, oxaliplatin,
oxymorphone,
oxytetracycline, oxytocin, ozogamicin, paclitaxel, palifermin, palivizumab,
palonosetron, pamidronic acid, pancuronium, panitumumab, pantoprazole,
papaverine, paricalcitol, pegalated interferon alfa-2b, pegaptanib,
pegaspargase,
pegfilgrastim, pegvisomant, pegylated liposomal doxorubicin, pemetrexed,
penicillin
g, pentamidine, pentazocine, pentobarbital, pentostatin, phenobarbital,
phentolamine,
phenylacetic acid, phenylephrine, phenytoin, physostigrnine, phytonadione,
piperacillin, polymyxin b, porfimer, pralidoxime, pramlintide, prilocaine,
procainamide, procaine, prochlorperazine, progesterone, promethazine,
propofol,
propranolol, protamine, pyridostigmine hydroxide, pyridoxine, quinidine,
quinupristin, ranitidine, rasburicase, remifentanil, rho d immune globulin,
rifampin,
rituximab, rocuroniurn, ropivacaine, scopolamine, secretin, sermorelin,
sincalide,
somatrem, somatropin, spectinomycin, streptokinase, streptomycin,
streptozocin,
succinylcholine, sufentanil, sulbactam, sulfamethoxazole, sulphan blue,
sumatriptan,
tacrolimus, tazobactam, teniposide, terbutaline, teriparatide, testosterone,
tetracaine,
tetradecyl sulfate, theophylline, thiamine, thiopental, thiotepa, thyroid
stimulating
hormone, thyrotropin, ticarcillin, tigecycline, tinzaparin, tirofiban,
tobramycin,
topotecan, torsemide, tranexarnic acid, trastuzumab, treprostinil,
triamcinolone,
triflutate, trimethobenzamide, trimethoprim, trimetrexate, triptorelin,
tromethamine,
tuberculin, typhoid vaccine, urofollitropin, urokinase, valproic acid,
vancomycin,
varicella, vasopressin, vecuronium, verapamil, verteporfin, vinblastine,
vincristine,
CA 02602044 2007-09-24
vinorelbine, voriconazole, warfarin, ziconotide, zidovudine, ziprasidone, and
zoledronic acid. System 50, in its various embodiments, may also be useful for
the
provision of vaccines, vitamins and other nutritional agents.
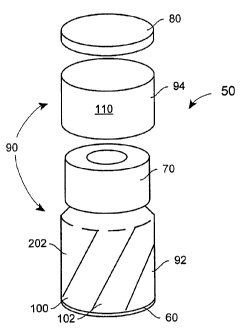
[0018] Referring to Figs. 1 and 2, packaging system 50 includes container
60,
closure assembly 70, cap 80, and label 90. Closure assembly 70 is fitted to an
open
end of container 60, and includes an access port. Closure assembly 70 may be
resealable. Cap 80 is fitted over the access port, and label 90 is disposed
about
container 60.
[0019] Label 90 has first and second sections 92, 94, separated by
perforated
boundary 96. First section 92 of label 90 is attached at least in part to an
outer surface
of container 60. Label 90 may partially or fully cover the perimeter of
container 60.
First section 92 of label 90 covers container 60 up to perforated boundary 96.
Second
section 94 extends at least between perforated boundary 96 and cap 80.
[0020] First section 92 of label 90 has first region 100 and, optionally,
second
region 102. First and second regions 100, 102 extend from perforated boundary
96 to
the opposing edge of first section 92. First and second regions 100, 102 are
angled
relative to longitudinal axis 104 of system 50 (see Fig. 5). First region 100
consists
essentially of the name of a product to be packaged in container 60. Optional
second
region 102 consists essentially of a concentration of the product, or other
description
of the product. Second section 94 of label 90 may include informational or
warning
message 110.
[0021] A method of use of packaging system 50 is illustrated in Fig. 3.
First, a
user grasps container 60 (block 112). The user then breaks label 90 along
perforated
boundary 96, thereby separating first section 92 of label 90 from second
section 94
(block 114). Boundary 96 may be broken by grasping an edge of second section
94,
preferably normal to the axis 104, and tearing second section 94 loose from
first
section 92 and cap 80. This motion also may result in removal of the section
94
(block 116). Finally, cap 80 may be removed from container 60 (118).
Optionally,
though not preferred, it is possible to remove cap 80 first, followed by the
breaking of
boundary 96. Also, the removal of section 92 and cap 80 may be achieved in a
single
step.
[0022] Packaging system 50 in general, and label 90 in particular, includes
a
number of informational features that assist the user administering the
product
6
CA 02602044 2007-09-24
packaged in container 60. For instance, label 90 has been designed to
highlight the
name and, optionally, the concentration of the product, or other description
of the
product. One way in which the name and concentration have been highlighted is
through the unconventional orientation of regions 100, 102 of label 90, which
regions
100, 102 are neither parallel nor orthogonal to axis 104. Further, by
segregating
essentially only the name and concentration to regions 100, 102, label 90
reduces the
informational "clutter" that may accompany conventional presentations. System
50
also requires active manipulation of label 90 during the use of system 50,
namely the
separation of label 90 along perforated boundary 96. It is believed that
active
manipulation of label 90 will improve the user's appreciation of the
informational
content of label 90. Each of these features may contribute to the improvements
provided by system 50.
[0023] The embodiment of system 50 illustrated in general terms in Figs. 1
and 2
is now described in greater detail with regard to Fig. 4.
[0024] According to the present embodiment of system 50, container 60 may
be
in the form of a vial. Vial 60 may be cylindrical in shape, and may be made of
a
materials such as glass or plastic, for example. The vial may have wall 120
that
defines first, closed end 122 and second, open end 124. Wall 120 may have
outer
surface 126 and inner surface 128, which in turn defines receptacle 130. Wall
120
may also define flange-like rim 132 that is disposed about open end 124. Given
the
cylindrical shaped of vial 60, rim 132 may have an annular shape, with
outermost
edge 134 and opposing surfaces 136, 138.
[0025] As mentioned previously, system 50 has longitudinal axis 104. As
illustrated best in Figs. 1 and 5, closed end 122 and open end 124 of vial 60
may have
a substantially circular shape, with centerpoints, one of which (142) is
shown.
According to at least the illustrated embodiment of vial 60, longitudinal axis
104 may
pass through centerpoints 142 of closed and open ends 122, 124.
[0026] Fitted to open end 124 of vial 60 is closure assembly 70. According
to
the illustrated embodiment, closure assembly 70 may include valve 150 and
crimp
ring 152. Valve 150 controls access to open end 124 of vial 60, while crimp
ring 152
maintains the position of valve 150 at open end 124.
[0027] Valve 150 may be defined by a layer of Teflon-coated rubber, the
layer
having opposing first and second surfaces 162, 164 and outer edge 166. Given
the
7
= CA 02602044 2007-09-24
cylindrical geometry of vial 60, outer edge 166 of valve 150 may be circular
in shape.
Given the material used, the layer may be punctured repeatedly by a needle,
for
example, but reseal thereafter so as to limit movement of product disposed in
receptacle 130 through open end 124.
[0028] Crimp ring 152 may be defined by cylindrical metal
sleeve 170 having a
cylindrical shape. Sleeve 170 has first end 172 with annular metal band 174
that
defines circular opening 176. Sleeve 170 also has intermediate, tube-like
portion 178
and second end 180.
[0029] As assembled, valve 150 is disposed with second surface
164 disposed
over open end 124 of vial 60 abutting surface 136. Edge 166 of valve 150 may,
but
need not necessarily, extend to outermost edge 134 of rim 132. Crimp ring 152
may
be disposed over the assembly of valve 150 and vial 60, such that first end
172 abuts
first surface 162 of valve 150. Opening 176 thereby defines access port 182
for
closure assembly 70. With first end 172 abutting first surface 162, second end
180 is
disposed over and about rim 132 such that second end 180 abuts surface 138.
[0030] Disposed over access port 182 is cap 80. Cap 80 may be
made of a plastic
or metal material. Cap 80 may have opposing surfaces 190, 192 and downturned
edge
194. Cap 80 is disposed over crimp ring 152 such that surface 192 abuts first
end 172
of ring 152. Furthermore, cap 80 may be attached to closure assembly 70, and
in
particular the portion of closure assembly 70 that defines access port 182,
with a
releasable adhesive or by friction fitting, for example.
[0031] Returning to label 90, label 90 may be made of paper or
other material
suitable for printing that has been backed, at least in part, with an
adhesive. The
adhesive used with label 90 may require label 90 to be tom to remove label 90
from
the surface to which it is affixed. First section 92 of label 90 may be
attached at least
in part to outer surface 126 of vial 60. Second section 94 of label 90 may be
attached
at least in part to cap 80, and in particular edge 194.
[0032] First section 92 may extend from first edge 200 generally
aligned with
closed end 122 of vial 60 to perforated boundary 96. First region 100 of first
section
92 may thus extend substantially from closed end 122 of vial 60 to perforated
boundary 96. Similarly, second region 102 of first section 92 may also
substantially
extend from closed end 122 to perforated boundary 96.
8
CA 02602044 2007-09-24
[0033] First and second regions 100, 102 may, as noted above, be angled
relative
to longitudinal axis 104. The nature of this relationship is shown
particularly in Fig.
5. As will be noted, to be angled relative to the longitudinal axis means that
regions
100, 102 are not aligned with axis 104 (parallel to axis 104), nor do regions
100, 102
lie in a plane that is orthogonal or substantially orthogonal to longitudinal
axis 104.
Rather, regions 100, 102 lie in planes that make non-zero, non-right angle 0
with
longitudinal axis 104. By contrast, the text in remainder 202 of first section
92 may
be aligned either along axis 104 or orthogonal or substantially orthogonal to
axis 104.
[0034] According to certain embodiments, the angle of regions 100, 102
relative
to axis 104 may lie in the range of between twenty and eighty degrees, and
more
preferably in the range of between thirty degrees and sixty degrees. As
illustrated, the
angle may be forty-five degrees. Shallower and steeper angles may be possible
in
certain embodiments.
[0035] However, in selecting the angle of regions 100, 102, it is presently
believed that the angle should not be selected so shallow as to extend region
100, 102
more than half-way about the periphery of container 60. That is, if region
100, 102
extends through more than about 180 degrees about the periphery of container
60, the
user may not be able to visualize all of the information contained in region
100, 102 at
a single time. To maximize the possibility that all of the information in a
given region
100, 102 may be read by the user at one time, the angle of inclination of
region 100,
102 may thus be limited.
[0036] First and second regions 100, 102 may have a contrasting background
color in regard to remainder 202 of first section 92 of label 90. That is, if
remainder
202 of label 90 has a tan background color, for example, regions 100, 102 may
have a
neutral color or white for the background color. A contrasting background
color may
further differentiate regions 100, 102 in combination with the angled nature
of regions
100, 102.
[0037] A still further differentiation of regions 100, 102, and in
particular the text
used in regions 100, 102, may be provided through the use of a contrasting
font type
or font size. For example, while the text in remainder 202 of first section 92
of the
label may have a six point font size, the text in regions 100, 102 may have a
ten point
font size. In fact, it may be recognized that by angling regions 100, 102
relative to
axis 104, regions 100, 102 may include more area than a region oriented
parallel to or
9
= CA 02602044 2007-09-24
orthogonal to axis 104, thus permitting use of a larger font size. Similar to
the
contrasting background color, the contrasting background font may further
differentiate regions 100, 102. Any font size may be printed on regions 100,
102 and
sections 92, 94, so long as such sizes identify the product, other
descriptions and
warnings to the user of the product.
100381 It will be recognized that while the illustrated
embodiment uses angled
regions 100, 102 with contrasting color and font size, it is not a requirement
of the
present disclosure that all three features be used in combination. For
example, angled
regions 100, 102 may be used in combination with neither, either or both of
the
contrasting color and the contrasting font size.
[0039] Second section 94 may extend from perforated boundary 96
to second
edge 210. Second edge 210 may be disposed above edge 194 of cap 80. According
to
certain embodiments, such as the embodiment illustrated, second edge 210 may
be
generally aligned with first surface 190 of cap 80.
[0040] Second section 94 may have a contrasting background
color relative to
first section 92 of label 90. In particular, while first section 92 may
feature
background colors of tan and white, for example, second ection 94 may feature
a
background color such as red. Preferably, colors such as red, orange and
fluorescent
yellow may be used for the background color of second section 94. Any color
combination may be employed in sections 92, 94, but preferably such section
colors
contrast to aid the practitioner in using system 50. As noted above, it is not
necessary
for all embodiments of present system 50 to use such colors, although it may
aid in
differentiating warning message 110 displayed in second section 94 from other
portions of label 90.
100411 Warning message 110 may be textual, in the form of
alphanumeric
characters, for example. However, warning message 110 is not limited to
alphanumeric characters. For example, icons or symbols may be used in
combination
with or in substitution for alphanumeric message 110. For that matter, message
110
may be conveyed without any icons, symbols, or characters at all, but by the
color of
section 94 alone. If a textual message is incorporated into warning message
110, then
the font size of the text may be varied relative to that used in one or more
regions 100,
102, 202 of first section 92 of label 90 to create differentiation.
CA 02602044 2007-09-24
[0042] It will be further recognized that a number of variants are
possible, not
only relative to the structures already discussed, but relative to additional
features that
may be combined with or substituted for those already described.
[0043] For example, second section 92 of label 90 may be optional, and may
not
be included according to all embodiments of system 50 according to the present
disclosure. An illustration of such an embodiment is illustrated in Fig. 6,
with similar
parts numbered similarly. Label 220 is disposed about vial 60. Label 220 has a
single
section with first region 222 and, optionally, second region 224. The
remainder of
label 220 is indicated as 226. Regions 222, 224 lie in planes that make a non-
zero,
non-right angle with a longitudinal axis of the vial 60, similar to regions
100, 102. In
general, other than the fact that the label 220 has but a single section, the
comments
made relative regions 100, 102 and remainder 202 may apply with equal force to
regions 222, 224 and reminder 226. In fact, such a label with angled regions
may be
used with containers other than a vial, as shown; the label may be used with
ampuls,
syringes, and other devices. It will also be recognized that the perforated
boundary
may be used in a label without the angled regions in the first section of the
label.
[0044] As another example of an alternative embodiment, crimp ring 152 may
have a color that contrasts with one or more of regions 100, 102, 202 of first
section
92 of label 90. In this regard, color of crimp ring 152 may preferably be red,
orange
or fluorescent yellow, similar to the color of second section 94 of label 90.
[0045] Further, crimp ring 152 may have a warning message displayed on a
portion or area of ring 152. For example, ring 152 may have a warning message
defined or displayed on intermediate region 178 between first and second ends
172,
180. Alternatively, the warning message may be defined or displayed on band
174
disposed about opening 176 that defines, in part, access port 182. Similar
comments
to those made above relative to warning message 110 may be made in regard to
the
warning message displayed on crimp ring 152.
[0046] Additionally or in the alternative, warning message 240 may be
displayed
or defined on a portion or area of cap 80. For example, the message may be
displayed
or defined on surface 190 of cap 80. In particular, as illustrated in Fig. 7,
message
240 may be disposed about the periphery of surface 190 of cap 80.
11