Note: Descriptions are shown in the official language in which they were submitted.
CA 2602222 2017-04-12
CONTAINER CLOSURE WITH OVERLYING NEEDLE PENETRABLE AND
SEALABLE PORTION AND UNDERLYING PORTION COMPATIBLE WITH FAT
CONTAINING LIQUID PRODUCT, AND RELATED APPARATUS AND METHOD
Field Of The Invention
[0001] The present invention relates to containers having container bodies
and stoppers
for sealing openings in the container bodies, such as containers having
polymeric stoppers
that are needle penetrable for filling the closed bodies with liquids, such as
fat containing
liquid nutrition products, and that are laser resealable for laser resealing
the needle
penetrated region of the stopper.
Background Of The Invention
[0002] Prior art needle penetrable and laser resealable containers include
thermoplastic
elastomer ("TPE") stoppers or portions of stoppers that are needle penetrable
to needle fill
the containers with a product, and are thermally resealable at the resulting
needle holes by
applying laser radiation thereto to hermetically seal the product within the
containers. An
exemplary such container and stopper is disclosed in commonly assigned U.S.
Patent No.
6,604,561. One of the drawbacks of such TPE stoppers is that they can be
difficult to use
with fat containing liquid products, such as infant or baby formulas, or other
milk-based or
low acid products. For example, many such TPE materials contain leachables
that can
leach into the fat containing product, or otherwise can undesirably alter a
taste profile of
the product.
1
CA 02602222 2007-07-25
[0004] Conventional containers and systems for aseptically filling
containers with fat
containing liquid products, such as infant or baby formulas, or other milk-
based or low acid
products, employ a container having an open mouth and a screw cap or other
type of cap
that is secured to the open mouth after aseptically filling the container with
the product. In
many such systems, the open containers are pre-sterilized by flushing the
interior and
exterior surfaces of the open containers with a fluid sterilant, such as
peroxide vapor or
vaporized hydrogen peroxide, to sterilize the food contacting surfaces. Then,
the
containers are flushed with heated sterile air in order to re-vaporize any
fluid sterilant that
condenses on the container surfaces and to flush away the sterilant. After
flushing with
heated sterile air, the open containers are filled through the open mouths of
the containers
= with the desired product, and after filling, the containers are capped to
seal the product
within the containers. Typically, the sterilizing, flushing, filling and
capping processes are
all performed within the same sterile zone of the filling system.
(00051 One of the drawbacks of this type of filling system is that
it can be difficult to
remove all of the fluid sterilant from the interior surfaces of the
containers, thus leaving
sterilant residue, such as hydrogen peroxide, within the containers and
thereby
contaminating the product filled into the containers. If the level of residue
is sufficiently
high, the product must be discarded. Alternatively, the sterilant residue can
negatively
affect the taste or taste profile of the product.
NON Another drawback of such prior art systems is that because
the sterilizing,
flushing, filling and capping processes are all performed within the same
sterile zone, the
apparatus forming the sterile zone tends to be relatively large and complex.
Moreover,
because the product is open filled (i.e., poured into the open mouths of the
containers), the
product is not as well contained within the sterile zone as otherwise desired,
thus creating
hygiene problems within the sterile zone. Such apparatus can require cleaning
more
frequently than desired due, for example, to the collection of sterilant
and/or product
residue within the sterile zone. Cleaning such large. and complex apparatus
can result in
substantial down time and expense. As a result, such prior art systems can
have
undesirably short run times between cleaning and sterilization of the sterile
zone. Yet
another drawback of such systems is that because they sterilize the packaging,
fill and seal
2
CA 02602222 2007-07-25
apparatus all within the same enclosure and sterile zone, if any part of the
system goes
down, the entire system must be subjected to clean in place ("CP") and
sterilize in place
("SIP") procedures prior to re-starting, which can further contribute to
substantial down
time and expense.
[0007] Yet another drawback of such prior art systems is that the
containers are filled
immediately prior to capping resulting in poor closure seals due to the
presence of wet
product at the sealing surfaces or interfaces.
100081 Another drawback of such prior art systems is that in many cases
product must be
sterilized after filling by employing a retort process that can undesirably
alter the taste of ,
the product.
100091 Accordingly, it is an object of the present invention to overcome
one or more of
the above-described drawbacks and disadvantages of the prior art.
Summary Of The Invention
100010] In accordance with a first aspect, the present invention is
directed to a container
for storing a fat containing liquid product. The container is penetrable by a
needle for
aseptically -filling a storage chamber of the container through the needle
with the fat
containing liquid product, and the resulting needle hole is thermally
resealable to seal the
fat containing liquid product within the container. The container comprises a
body
defining a storage chamber therein for receiving the fat containing liquid
product and a first
aperture in fluid communication with the storage chamber. The body does not
leach more
than a predetermined amount of leachables into the fat containing liquid
product and does
not undesirably alter a taste profile of the fat containing liquid product. A
container closure
assembly (lithe container includes a stopper receivable within the first
aperture for
hermetically sealing the storage chamber. The stopper includes a .first
material portion
defining an internal surface in fluid communication with the storage chamber
forming at
least most of the surface area of the container closure that can contact any
fat containing
liquid product within the storage chamber. The first material portion does not
leach more
than a predetermined amount of leachables into the fat containing liquid
product or
CA 02602222 2007-07-25
undesirably alter a taste profile of the fat containing liquid product. The
predetermined
amount of leachables is less than about 100 parts per million ("PPM"), is
preferably less
than or equal to about 50 PPM, and most preferably is less than or equal to
about 10 PPM.
A second material portion of the stopper either (i) overlies the first
material portion and
cannot contact any fat containing liquid product within the storage chamber,
or (ii) forms a
substantially lesser surface area of the container closure that can contact
any fat containing
liquid product within the storage chamber in comparison to the first material
portion. The
second material portion is needle penetrable for aseptically filling the
storage chamber with
the fat containing liquid product, and a resulting needle aperture formed in
the second -
material portion is thermally resealable to seal the fat containing liquid
product within the
storage chamber. A sealing portion of the container closure assembly is
engageable with
the body prior to aseptically filling the storage chamber with the fat
containing liquid
product to thereby form a substantially dry hermetic seal between the
container closure and
body. A securing member or cap is connectable between the stopper and body for
securing
the stopper to the body.
[000111 In one embodiment of the present invention, the first material
portion is selected
from the group including (i) a low mineral oil or mineral oil free
thermoplastic; (ii) a low
mineral oil or mineral oil free thermoplastic defining a predetermined
durometer; (iii) a
liquid injection moldable silicone; and (iv) a silicone. The predetermined
durometer is
within the range of about 20 Shore A to about 50 Shore A, and preferably is
within the
range of about 25 Shore A to about 35 Shore A.
[00012] In one embodiment of the present invention, the second material
portion is a
thermoplastic elastomer that is heat resealable to hermetically seal the
needle aperture by
applying laser radiation at a predetermined wavelength and power thereto. The
second
material portion defines (i) a predetermined valI thickness, (ii) a
predetermined color and
opacity that substantially absorbs the laser radiation at the predetermined
wavelength and
substantially prevents the passage of the radiation through the predetermined
wall thickness
thereof, and (iii) a predetermined color and opacity that causes the laser
radiation at the
predetermined wavelength and power to hermetically seal the needle aperture
formed in the
4
CA 02602222 2007-07-25
needle penetration region thereof in a predetermined time period of less than
or equal to
about 5 seconds and substantially without burning the needle penetration
region.
100013] In one embodiment of the invention, the second material portion is
a
thermoplastic elastomer that is heat resealable to hermetically seal the
needle aperture by
applying laser radiation at a predetermined wavelength and power thereto. The
second
material portion includes (i) a styrene block copolymer; (ii) an olefin; (iii)
a predetermined
amount of pigment that allows the second material portion to substantially
absorb laser
radiation at the predetermined wavelength and substantially prevent the
passage of
radiation through the predetermined wall thickness thereof, and hermetically
seal the
needle aperture formed in the needle penetration region thereof in a
predetermined time
period of less than or equal to about 5 seconds; and (iv) a predetermined
amount of
lubricant that reduces friction forces at an interface of the needle and
second material
portion during needle penetration thereof
1000141 In one embodiment of the invention, the second material portion is
a
thermoplastic elastomer that is heat resealable to hermetically seal the
needle aperture by
applying laser radiation at a predetermined wavelength and power thereto. The
second
material portion includes (i) a first polymeric material in an amount within
the range of
about 80% to about 973"c by weight and defining a first elongation; (ii) a
second polymeric
material in an amount within the range of about 3% to about 20% by weight and
defining a
second elongation that is less than the first elongation of the first
polymeric material; (iii) a
pigment in an mount that allows the second material portion to substantially
absorb laser
radiation at the predetermined wavelength and substantially prevent the
passage of
radiation through the predetermined wall thickness thereof, and hermetically
seal a needle
aperture formed in the needle penetration region thereof in a predetermined
time period of
less than or equal to about 5 seconds; and (iv) a lubricant in an amount that
reduces friction
forces at an interface of the needle and second material portion during needle
penetration
thereof.
1000151 In one embodiment of the invention, the first material portion
defines a second
aperture, the second material portion overlies the second aperture, and the
second aperture
CA 02602222 2007-07-25
constitutes less than about 15% of the surface area of the first material
portion exposed to
the storage chamber. In one such embodiment, the second aperture constitutes
less than
about 10% of the surface area of the first material portion exposed to the
storage chamber.
In another embodiment of the present invention, the first material portion is
interposed
entirely between the second material portion and any fat containing liquid
product stored
within the storage chamber to thereby prevent contact between the second
material portion
and fat containing liquid product during storage thereof in the container. In
one
embodiment of the invention, the first material portion is co-molded with the
second
material portion. In one such ethbodiment, either the first material portion
or the second
material portion is over-molded to the other. In one embodiment of the
invention, the
second material portion defines a relatively raised portion, and at least one
of the first and
second material portions defines a relatively recessed portion spaced
laterally relative to the
relatively raised portion. The relatively raised configuration inherently
laterally
compresses the needle penetration region to facilitate resealing thereof. In
one such
embodiment, the relatively raised portion is substantially dome shaped.
[00016] In one embodiment of the invention, the securing member is a cap
movable
between a first position engaging the body and securing the stopper to the
body, and a
second position spaced away from the body and engaged with the stopper for
removing the
container closure from the body. Also in a currently preferred embodiment, the
first
material portion defines a peripheral flange that is releasably connectable to
the body. In
one such embodiment, the peripheral flange includes a plurality of peripheral
flange
portions angularly spaced relative to each other. Preferably, either the
peripheral flange or
the body defines a raised securing surface, and the other defines a
corresponding recessed =
securing surface engageable with the raised surface for securing the
peripheral flange and
the body to each other. In one embodiment of the invention, the stopper is
snap fit to the
body, and the securing member or cap is threadedly enugeable with the body.
[90017] In accordance with another aspect, the present invention is
directed to a method
for aseptically needle filling and laser resealing a container with a fat
containing liquid
product. The method comprises the following steps:
CA 02602222 2007-07-25
(i) providing a container including a body defining a sterile storage chamber
therein for receiving the fat containing liquid product and a first aperture
in fluid
communication with the storage,chamber, wherein the body does not leach more
than a
predetermined amount of leachables into the fat containing liquid product and
does not
undesirably alter a taste profile of the fat containing liquid product; and a
container closure
assembly including a stopper receivable within the first aperture for
hermetically sealing the
storage chamber, wherein the stopper includes a first material portion
defining an internal
surface in fluid communication with the storage chamber forming at least most
of the surface
area of the container closure that can contact any fat containing liquid
product within the
storage chamber and that does not leach more than a predetermined amount of
leachables
into the fat containing liquid product or undesirably alter a taste profile of
the fat containing
liquid product, and a second material portion that either (a) overlies the
first material portion
and cannot contact any fat containing liquid product within the storage
chamber, or (b) forms
a substantially lesser surface area of the container closure that can contact
any fat containing
liquid product within the storage chamber in comparison to the first material
portion. The
predetermined amount of leachables is less than about 100 PPM, is preferably
less than or
equal to about 50 PPM, and most preferably is less than or equal to about 10
PPM. The
second material portion is needle penetrable for aseptically filling the
storage chamber with
the fat containing liquid product, and a resulting needle aperture formed in
the second
material portion is thermally resealable to seal the fat containing liquid
product within the
storage chamber;
(ii) mounting the sealed, empty container defining a sterile storage chamber
on a
conveyor, and moving the conveyor through a sterile zone;
(iii) transmitting within the sterile zone a fluid sterilant onto at least an
exposed
portion of the stopper of the container and, in turn, sterilizing with the
fluid sterilant at least
the exposed portion of the stopper of the container;
(iv) transmitting within the sterile zone a heated gas onto the portion of the
container exposed to the fluid sterilant, flushing away with the heated gas
the fluid sterilant
from at least the exposed portion of the stopper of the container and, in
turn, forming a
needle penetration region of the stopper substantially free of fluid
sterilant;
7
CA 02602222 2007-07-25
(v) penetrating the needle penetration region of the stopper with a filling
needle
coupled in fluid communication with a source of the fat containing liquid
product, and
introducing fat containing liquid product through the needle and into the
storage chamber;
(vi) withdrawing the filling needle from the stopper; and
(vii) applying laser radiation to a resulting needle hole in the stopper to
thermally
reseal the second material portion and, in turn, hermetically seal the fat
containing liquid
product within the storage chamber.
[00018] In one embodiment of the present invention, the method further
comprises
moving the filled container outside of the sterile zone, and applying outside
of the sterile zone a
cap to the container that overlies at least an exposed portion of the stopper
of the container. The
method also preferably further comprises directing an over pressure of sterile
gas within the
sterile zone, and directing at least a portion of the sterile gas in a flow
direction generally from
an outlet end toward an inlet end of the sterile zone to, in turn, prevent
fluid sterilant from
contacting a container during needle filling thereof.
[00019] One advantage of the present invention is that the needle
penetrable and laser
resealable portion of the stopper defined by the second material portion is
isolated, or
substantially isolated from the fat containing liquid product by the first
material portion that
- does not leach into (or leaches less than a predetermined amount), or
undesirably affect the
taste profile of the product. As a result, the containers of the present
invention can be needle
filled and laser resealed without the above-described problems encountered
using prior art
needle penetrable and laser resealable stoppers formed in whole or in part
with TIT, or other
materials that contain leachables when used in connection with fat containing
liquid
products.
[00020] Yet another advantage of the present invention is that the stopper
is sealed to the
container body prior to filling the container, thereby forming a dry seal
between the stopper
and body and avoiding the seal integrity problems encountered with "wet" seals
in the prior
art.
[00021] Another advantage of the present invention is that because the fat
containing
liquid product is needle filled through a stopper into a sealed, empty,
sterile container, there
8
CA 02602222 2007-07-25
is significantly better product containment within the sterile zone in
comparison to the above-
described liquid food filling systems, thus requiring less frequent cleaning
of the sterile zone
and enabling longer run times between cleaning and sterilization of the
sterile zone than
encountered in such prior art.
[000221 Yet another advantage of the present invention is that container
sterilization is de-
linked from container filling since the interior of the sealed, empty
container is sterilized
prior to introducing the container into the sterile zone for filling. As a
result, the closed
containers do not require the post-filling assembly required with prior art
liquid food
containers and systems, thus enabling the filling apparatus to be
significantly smaller, less
complex, and more efficient. In addition, the sealed containers can be
manufactured off-site
from the filling apparatus to thereby avoid problems associated with space
constraints in
manufacturing and filling facilities.
1000231 Another advantage of the present invention is that the product can
be aseptically
filled into sealed, empty sterile containers, thus avoiding the need to
sterilize the product by
retort after filling and the negative effects of retort on the filled product.
1000241 Other advantages of the present invention and/or of the currently
preferred
embodiments thereof will become more readily apparent in view of the following
detailed
description of the currently preferred embodiments and accompanying drawings.
Brief Description Of The Drawings
[000251 FIGS. IA, 1B, and IC are a series of side devotional views of a
container
embodying the present invention illustrating respectively (i) the container
body itself, (ii) the
container body with the stopper snap-fit thereto, and (iii) the container body
with the stopper
and securing member threadedly engaged to the body.
[000261 FIG. 2 is a partial, cross-sectional view of the assembled
container of FIGS. I A,
113 and IC.
[00027] FIG. 3A is a side elevational view of an apparatus embodying the
present
invention for needle filling and laser resealing the containers of FIGS. IA,
1B, IC and 2.
9
CA 02602222 2007-07-25
[00028] FIG. 3B is a perspective view of the apparatus of FIG. 3A.
[00029] FIG, 4 is a partial, perspective cross-sectional view of another
embodiment of a
container of the present invention wherein the stopper is thrcadedly engaged
with the body,
and the cap is snap lit to the stopper.
[00030] FIG. 5 is a partial, perspective cross-sectional view of another
embodiment of a
container of the present invention wherein the securing member is in the form
of a disk
overlying the stopper and fixedly secured thereto.
Detailed Description Of The Currently Preferred Embodiments
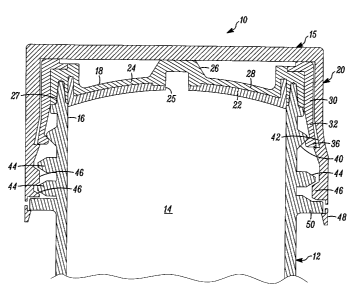
[00031] In FIGS. 1A, 1B, 1C and 2, a container embodying the present
invention is
indicated generally by the reference numeral 10. The container 10 comprises a
body 12
defining a storage chamber 14 therein for receiving a substance, such as a fat
containing
liquid product, and a first aperture 16 in fluid communication with the
storage chamber 14.
A container closure 15 includes a stopper 18 receivable within the first
aperture 16 for
hermetically sealing the storage chamber 14 with respect to the ambient
atmosphere, and a
securing member or cap 20 for securing the stopper to the body. As described
further below,
the stopper 18 includes a first material portion 22 and a second material
portion 24. The first
material portion 22 is connectable between the stopper 18 and body 12 for
securing the
stopper to the body, and in the illustrated embodiment, defines a second
aperture 25 for
exposing a predetermined portion of the second material portion 24
therethrough, As can be
seen, the first material portion 22 defines an internal surface in fluid
communication with the
storage chamber 14 forming at least most of the surface area of the container
closure 15 that
can contact any fat containing liquid product within the storage chamber and
that does not
leach more than a predetermined amount of leachables into the fat containing
liquid product
or undesirably alter a taste profile of the fat containing liquid product. The
fat containing
liquid product may be any of numerous different products that are currently
known, or that
later become known, including without limitation infant or baby formulas,
growing-up milks,
milks, creams, half-and-halfs, yogurts, ice creams, juices, syrups,
condiments, milk-based or
milk-containing products, liquid nutrition products, liquid health care
products, and
pharmaceutical products. The term "leachable" is used herein to mean any
chemical
CA 02602222 2010-03-08
. =
compound (volatile or non-volatile) that leaches into the product within the
container from a
component of the container during the period of storage through expiry of the
product. An
exemplary leachable to be avoided in connection with fat containing liquid
nutrition
products, such as infant or baby formulas, is mineral oil. Accordingly, as
indicated below, in
the exemplary embodiments of the present invention, the first material portion
22 does not
contain mineral oil, or contains sufficiently low amounts of mineral oil such
that it does not
leach mineral oil into the fat containing liquid nutrition product, or
substantially does not
leach mineral oil into the fat containing liquid nutrition product (i.e., if
any mineral oil is
leached into the product, any such amount is below the maximum amount
permitted under
applicable regulatory guidelines for the respective product, such as FDA or
LFCA
guidelines). In accordance with the present invention, the second material
portion 22 and the
body 12 each do not leach more than a predetermined amount of leachables into
the product.
The predetermined amount of leachables is less than about 100 PPM, is
preferably less than
or equal to about 50 PPM, and most preferably is less than or equal to about
10 PPM.
[00031] The second material portion 24 either (i) overlies at least a
portion of the first
material portion 22, or (ii) forms a substantially lesser surface area, if
any, of the container
closure 15 that can contact any fat containing liquid product within the
storage chamber 14 in
comparison to the first material portion 22. In addition, the second material
portion 24 is
needle penetrable for aseptically filling the storage chamber 14 with the fat
containing liquid
product, and a resulting needle hole formed in the second material portion 24
after
withdrawing the needle is thermally resealable to seal the fat containing
liquid product within
the storage chamber. As shown typically in FIG. 2, the second material portion
22 of the
stopper defines an annular groove 27 formed in a peripheral flange portion
thereof, and the
end portion of the container body 12 is received therein to form a
substantially hermetic seal
between the stopper and body.
[00032] One advantage of the present invention is that the stopper 18 is
sealed to the body
12 prior to filling the storage chamber 14 with the product, and therefore a
dry seal is formed
between the stopper and body. As a result, the containers of the present
invention can
provide significantly higher seal integrity in comparison to prior art
containers in which the
cap is sealed after filling the container thus giving rise to a significantly
higher likelihood of
11
CA 02602222 2007-07-25
forming a less reliable "wet" seal. Yet another advantage of the illustrated
embodiment of
the invention is that the stopper 18 is assembled and sealed to the body 12 by
inserting or
pressing the stopper into the mouth or opening 16 of the body. Accordingly,
the rotational or
screwing motions encountered in prior art containers are avoided within the
sterile zone, thus
simplifying the assembly process within the sterile zone, and thereby enabling
an increased
level of sterility assurance and reduced complexity within the sterile zone in
comparison to
prior art containers wherein the seals are created by screwing a cap onto a
container body. If
desired, however, the stoppers can be threadedly or rotatably attached and/or
the caps can be
applied to the containers within the sterile zone if for some reason this is
desired or otherwise
required.
[00034] The securing member or cap 20 is movable between a first position
engaging the
body 12 and securing the stopper 18 to the body, and a second position spaced
away from the
body 12 for exposing the second aperture 16 and allowing access to the
substance within the
storage chamber 14. In the first position, the cap 20 is engaged with the
stopper 18 for
removing the assembled container closure from the body. In the embodiment of
the present
invention wherein the product stored within the container is a fat containing
liquid nutrition
product, such as a baby or infant formula, a nipple (not shown) of a type
known to those of
ordinary skill in the pertinent art may be threadedly attached to the threads
44 or otherwise
attached to the body 12 to allow a baby or child to drink the product within
the storage
chamber through the nipple.
1000351 As shown typically in FIG. 2, the second material portion 24 is
superimposed
over the first material portion 22. In the illustrated embodiment, the first
material portion 22
and second material portion 24 are co-molded, such as by over-molding the
second material
portion to the first material portion, or vice-versa. However, as may be
recognized by those
of ordinary skill in the pertinent art based on the teachings herein, the
first and second
material portions may be thermally fused or otherwise assembled in any of
numerous
different ways that are current known, or that later become known. Although in
the
illustrated embodiment a small portion of the second material portion 24 is
exposed to the
storage chamber 14, if desired, the first material portion 22 may completely
underlie the
CA 02602222 2007-07-25
second material portion 24 and/or otherwise fully isolate the second material
portion from the
storage chamber 14 and product stored therein.
1000361 As also shown typically in FIG. 2, the second material portion 24
defines a
relatively raised portion 26 overlying the second aperture 25 of the first
material portion 22,
and a relatively recessed portion 28 spaced laterally relative to, and
surrounding the relatively
raised portion. The raised portion 26 defines the needle penetration and
thermally resealable
region of the second material portion 24. In the illustrated embodiment, the
relatively raised
portion is substantially dome shaped. One advantage of forming the needle
penetrable and
thermally resealable portion 26 in a relatively raised configuration, such as
a dome shape, is
that the septum material (i.e., the needle penetrable and thermally resealable
portion) is
maintained in compression, and thus is substantially self-resealing.
Accordingly, when the
filling needle (not shown) is removed, the septum compresses itself about the
resulting
needle hole, thus closing or substantially closing the needle hole. As a
result, when thermally
resealed, such as by the application of laser or light energy thereto, a high
integrity seal may
be obtained. If, on the other hand, the septum material is in tension, such as
may occur if the
septum material is attached about its periphery to the first material portion,
it may prevent
thermal resealing of the resulting needle hole and/or may prevent the
formation of a high
integrity seal. If desired, a device (not shown) can be employed to place the
needle
penetration region of the stopper in compression during needle filling
thereof. As may be
recognized by those of ordinary skill in the pertinent art based on the
teachings herein,
although there can be significant advantages derived from the illustrated
septum
configuration, or otherwise from placing the needle penetration region of the
septum into
compression to facilitate resealing thereof, these and other aspects of the
stopper may take
any of numerous different shapes and/or configurations that are currently
known, or that later
become known.
[000371 The first material portion 22 defines a peripheral flange 30 that
is releasably
connectable to the body 12. In the illustrated embodiment, and as shown
typically in FIG. 1,
the peripheral flange 30 includes a plurality of peripheral flange portions 32
angularly spaced
relative to each other with angularly-extending gaps 34 formed therebetween.
As a result,
the peripheral flange portions 32 are radially flexible to facilitate forming
a snap-fit
13
CA 02602222 2007-07-25
connection between the peripheral flange and the body. As shown typically in
FIG. 6, each
peripheral flange portion 32 defines an angularly-extending raised securing
surface 36, and
the body 12 defines a corresponding angularly-extending recessed securing
surface 40 that is
engageable with the raised surface 36 for securing the peripheral flange and
body to each
other. In the illustrated embodiment, the peripheral flange 30 is snap fit to
the body 12.
However, as may be recognized by those of ordinary skill in the pertinent art
based on the
teachings herein, other connecting mechanisms or structures that are currently
known, or that
later become known, equally may be used. As also shown typically in FIG. 6,
the securing
member or cap 20 defines an annular recess 42 for receiving therein the
exterior edges of the
peripheral flange portions 32 to thereby interlock the first material portion
22 and cap 20 to
each other when the cap is moved into the second or closed position. The body
12 defines
first threads 44 and the securing member or cap 20 defines second threads 46
that threadedly
engage each other to secure the cap to the body.
[000381 As can be seen, the second material portion 24 overlies the first
material portion
22, and the first material portion substantially isolates the second material
portion relative to
the storage chamber 14 and thus relative to the product contained within the
storage chamber.
Preferably, substantially the only portion of the second material portion 24,
if any, exposed to
the storage chamber 14 (or the product contained therein) is the portion 26
overlying the
second aperture 25. In the illustrated embodiment, the second aperture 25
preferably
constitutes less than about 15% of the surface area of the first material
portion 22 exposed to
the storage chamber 14 or product contained therein, and most preferably
constitutes less
than about 10% of the surface area of the first material portion 22 exposed to
the storage
chamber or product contained therein. As indicated above, if desired, the
first material
portion 22 may completely underlie the second material portion 24 to thereby
eliminate the
second aperture 25 and/or otherwise fully isolate the second material portion
from the storage
chamber 14 and/or product stored therein.
[000391 As can be seen, the securing member or cap 20 includes a frangible
portion 48
that is snap-fit and thereby interlocked with a peripheral flange 50 ilomied
on the body 12,
and that frangibly connects the cap to the body to thereby provide a tamper-
evident or
tamper-proof closure.
14
CA 02602222 2010-03-08
[00039] As indicated above, the second material portion 24 is preferably co-
molded with
the first material portion 22, such as by over-molding the second material
portion to the first
material portion. In addition, the stopper 18 may be molded in the same mold
as the
container body 12, and at least one of the stopper and the body may be
assembled within or
adjacent to the mold in accordance with the teachings of commonly-assigned
U.S. Published
Patent Application Serial Nos. 2005/0217211 and 2005/0223677 and U.S.
Provisional Patent
Application serial no. 60/727,899 filed October 17, 2005, entitled "Sterile De-
Molding
Apparatus And Method".
[00040] In addition, the sterile, empty stopper and body assemblies are
needle filled and
thermally resealed in accordance with the teachings of any of the following
patent
applications and patents: U.S. Patent 7,032,631 issued April 25, 2006 entitled
"Medicament
Vial Having A Heat-Sealable Cap, And Apparatus and Method For Filling The
Vial", which
is a continuation-in-part of a similarly titled U.S. Patent Application which
is now U.S.
Patent 6,805, 170 issued October 19, 2004, which is a continuation of a
similarly titled U.S.
Patent Application which is now U.S. Patent 6,684,916 dated February 3, 2004,
which is a
divisional of similarly titled U.S. Patent Application Serial No. 09/781,846,
filed February
12, 2001, now U.S. Patent No. 6,604,561, issued August 12, 2003, which, in
turn, claims the
benefit of similarly titled U.S. Provisional Application Serial No.
60/182,139, filed February
11, 2000; similarly titled U.S. Provisional Patent Application No. 60/443,526,
filed January
28, 2003; similarly titled U.S. Provisional Patent Application No. 60/484,204,
filed June 30,
2003; U.S. Patent Application No. 10/655,455, filed September 3, 2003,
entitled "Sealed
Containers And Methods Of Making And Filling Same" (now U.S. Patent 7,100,646
dated
September 5, 2006); U.S. Published Patent Application No. 2005/0145295 dated
July 7,
2005, entitled "Adjustable Needle Filling and Laser Sealing Apparatus and
Method; U.S.
Published Patent Application No. 2005/0194059 dated September 8, 2005,
entitled
"Apparatus and Method for Needle Filling and Laser Resealing"; U.S. Published
Patent
Application No. 2005/0223677 dated October 13, 2005, entitled "Apparatus for
Molding and
Assembling Containers with Stoppers and Filling Same; and U.S. Published
Patent
Application No. 2005/0217211 dated October 6, 2005, entitled "Method for
Molding and
Assembling Containers with Stoppers and Filling Same".
= -
CA 02602222 2010-03-08
[00041] In FIGS. 3A and 3B, an exemplary needle filling and laser resealing
apparatus for
use in filling and resealing the containers of the present invention is
indicated generally by
the reference numeral 58. The apparatus 58 includes a closed loop or endless
conveyor 60
for indexing and thereby conveying the containers 10 through the apparatus.
The containers
that are fed by the conveyor 60 into the apparatus 58 include the stoppers 18
sealed to the
openings 16 of the bodies 12, but do not include the caps 20 (FIG. 2). The
interior chamber
14 of each container is sterile, such as by assembling the stoppers and
containers in the mold
and/or within a sterile zone within or adjacent to the mold as described in
any of the co-
pending patent applications and patents mentioned above, by transmitting
radiation, such as
gamma or ebeam radiation, onto the sealed, empty stopper and body assembly, or
by
employing a fluid sterilant, such as vaporized hydrogen peroxide. The
apparatus 58 includes
an elongated housing 62 defining within it a sterile zone 64 and through which
the conveyor
60 with the containers 10 located thereon passes. The term "sterile zone" is
used herein
within the meaning of the applicable regulatory guidelines as promulgated, for
example, by
the FDA (the United States Food and Drug Administration) or other national or
applicable
regulatory agency, and including applicable Low Acid Canned Food ("LACF")
regulations,
and is preferably defined by a commercially sterile area that is maintained
sterile by means of
an over pressure of sterile air in a manner known to those of ordinary skill
in the pertinent
art. In the illustrated embodiment, the housing 62 includes side walls formed
by see-through
panels in order to allow an operator to view the interior of the apparatus. If
desired, however,
the side walls could be opaque, or could include an arrangement of opaque and
see-through
portions different than that shown. As shown, one or more of the side panels
may be
mounted to the housing frame by hinges 61 in order to pivot the respective
side panel
outwardly to access the interior of the housing to, for example, perform
maintenance and/or
repairs. Otherwise, the side and top walls of the housing 62 are sealed with
respect to the
ambient atmosphere to maintain the sterility of the sterile zone 64.
[00042] The apparatus 58 includes on its inlet end an inlet transfer
station 66 through
which the conveyor 60 passes for transferring the containers 10 mounted on the
conveyor 60
into the sterile zone 64. A sterilizing station 68 is located within the
housing 62 immediately
downstream of the inlet transfer station 66 in the direction of conveyor
movement (clockwise
in FIGS. 3A and 3B) and includes one or more sterilizing heads 70 coupled to a
source of
16
CA 02602222 2007-07-25
fluid sterilant (not shown) such as a hydrogen peroxide, vaporized hydrogen
peroxide
sterilant ("VFW") or other fluid sterilant that is currently or later known,
for transmitting the
fluid sterilant onto the exterior surfaces of the containers to sterilize the
exterior surfaces.
The apparatus 58 further includes within the housing 62 a first sterilant
removing station 72
located downstream of the sterilizing station 68 in the direction of conveyor
movement, and a
second sterilant removing station 74 located downstream of the first sterilant
removing
station 72. Each sterilant removing station 72, 74 includes one or more
respective sterilant
flushing heads 76 for transmitting heated sterile air or other gas over the
exterior surfaces of
the containers at a sufficient temperature, flow rate and/or volume, and for a
sufficient time
period to substantially entirely remove the fluid sterilant therefrom. The
vaporized peroxide
may condense at least in part on the surfaces of the containers and/or
conveyor, and therefore
it is desirable to flush such surfaces with a heated, sterile air or other gas
to re-vaporize any
condensed hydrogen peroxide and flush it out of the sterile zone. In the
currently preferred
embodiment, the temperature of the sterile air is at least about 60 C;
however, as may be
recognized by those of ordinary skill in the pertinent art based on the
teachings herein, the
. temperature may be set as desired or otherwise required by a particular
application. A needle
filling station 78 is located within the housing 62 downstream of the second
sterilant
removing station 74 for needle filling each container 10 with product from a
product fill tank
80, and -first and second laser resealing stations 82 and 84, respectively,
are located
downstream of the needle filling station 78 for laser resealing the resulting
needle holes
formed in the stoppers of the containers after filling the containers and
withdrawing the
needles. An exit transfer station 86 is located downstream of the laser
resealing stations 82,
84 for transferring the filled containers 10 on the conveyor 60 out of the
sterile zone 64.
After exiting the sterile zone 64, the containers 10 are capped with the caps
or securing
members 20 and ready for shipment.
[000441 The over pressure of sterile air or other gas is provided by a
sterile gas source 88
including one or more suitable filters, such as URA filters, for sterilizing
the air or other gas
prior to introducing same into the sterile zone 64. A fluid conduit 90 is
coupled in fluid
communication between the sterile air source 88 and the sterile zone 64 for
directing the
sterile air into the sterile zone. The apparatus 58 includes one or more
vacuum pumps or
other vacuum sources (not shown) mounted within a base support 87 of the
apparatus and of
17
CA 02602222 2007-07-25
a type known to those of ordinary skill in the pertinent art The vacuum
source(s) are
coupled in fluid communication with an exhaust manifold at the inlet transfer
station 66 and
an exhaust manifold at the exit transfer station 86 for drawing the air and
fluid sterilant out of
the sterile zone 64 and exhausting same through a catalytic converter 92 and
exhaust conduit
94. The catalytic converter 92 is of a type known to those of ordinary skill
in the pertinent
art to break down the exhausted hydrogen peroxide into water and oxygen. In
the illustrated
embodiment, the exhaust manifolds are mounted at the base of the inlet and
outlet stations
and extend into the base support 87. As can be seen, the exhaust manifolds at
the inlet and
outlet stations 66 and 86, respectively, draw into the exhaust passageways
located within the
base support 87 (not shown) both sterile air and fluid sterilant from the
sterile zone 64, and
non-sterile ambient air located either within the inlet station or outlet
station. As a result, any
ambient non-sterile air (including any other ambient gases or contaminants) in
the inlet and
outlet stations are drawn into the exhaust manifolds, and thereby prevented
from entering the
sterile zone 64 to maintain the sterility of the sterile zone. Similarly, any
sterile air or
sterilant is substantially prevented from being re-circulated within the
sterile zone, and
instead, is drawn into the exhaust manifolds after passage over the containers
and/or
conveyor portion located within the sterile zone. If desired, one or more
exhaust manifolds
may be located at the base of the sterile zone (i.e., beneath the conveyor 60
or between the
overlying and underlying portions of the conveyor 60) for fully exhausting the
air and fluid
sterilant and otherwise for avoiding the creation of any "dead" zones where
air and/or fluid
sterilant may undesirably collect. In one embodiment of the present invention,
the flow of
sterile air within the sterile zone 64 is controlled to cause the air to flow
generally in the
direction from right to left in FIG. 3A (i.e., in the direction from the
needle filling station 78
toward the sterilizing station 68) to thereby prevent any fluid sterilant from
flowing into the
needle filling and laser resealing stations 78, 82 and 84. This flow pattern
may be effected
by creating a higher vacuum at the inlet station 66 in comparison to the
outlet station 86.
However, as may be recognized by those of ordinary skill in the pertinent art
based on the
teachings herein, this flow pattern or other desired flow patterns may be
created within the
sterile zone in any of numerous different ways that are currently known, or
that later become
known.
18
CA 02602222 2007-07-25
[000451 In the illustrated embodiment, the conveyor 60 includes a plurality
of flights or
like holding mechanisms 96 that clamp each container 10 at or below its neck
finish (i.e., at
the peripheral region immediately below the mouth or opening 16 of the body
12) or other
desired container region. The flights 96 are pivotally mounted on a belt 98
defining a closed
loop and rotatably mounted on rollers 100 located on opposite sides of the
apparatus relative
to each other. One or more drive motors and controls (not shown) may be
mounted within
the base support 87 and are coupled to one or both rollers 100 for rotatably
driving the
conveyor 60 and, in turn, controlling movement of the containers 10 through
the apparatus in
a manner known to those of ordinary skill in the pertinent art. Each flight 96
of the conveyor
60 includes a plurality of container-engaging recesses 102 laterally spaced
relative to each
other and configured for engaging the respective necks or other desired
portions of the
containers 10 to support the containers on the conveyor. Although the
container-engaging
recesses 102 are illustrated as being semi-circular in order to engage the
containers 10, they
equally may be formed in any of numerous different shapes that are currently
known, or that
later become known, in order to accommodate any desired container shape, or
otherwise as
desired. The flights 96 further define a plurality of vent apertures 104 that
are laterally
spaced relative to each other, and are formed between and adjacent to the
container-engaging
recesses 102. The vent apertures 104 are provided to allow the sterile air and
fluid sterilant
to flow over the portions of the containers 10 located above the flights 96 of
the conveyor
and, in turn, through the conveyor prior to being exhausted through the
exhaust manifolds.
In the illustrated embodiment, the vent apertures 104 are provided in the form
of elongated
slots; however, as may be recognized by those of' ordinary skill in the
pertinent art based on
the teachings herein, the vent apertures may take any of numerous different
configurations
that are currently known, or that later become known. Preferably, the flights
96 laterally
engage the neck portions of the containers 10, and effectively isolate the
sterile portions of
- the containers above the flights from the portions of the containers
located below the flights
that may not be sterile, or that may include surface portions that are not
sterile.
[000461 The conveyor 60 defines an inlet end 106 for receiving the
containers 10 to be fed
into the apparatus, and an outlet end 108 for removing the filled and laser
resealed containers
from the apparatus. As can be seen, the adjacent flights 96 located at the
inlet and outlet ends
106 and 108, respectively, are pivoted relative to each other upon passage
over the rollers
19
CA 02602222 2007-07-25
100 to thereby define a loading gap 110 at the inlet end of the conveyor and
an unloading gap
112 at the outlet end of the conveyor. Accordingly, at the inlet end, the
containers 10 may be
fed on their sides into the loading gap 110 and received within the container-
engaging
recesses 102 of the respective -flight 96. Then, as the conveyor 60 is rotated
in the clockwise
direction in FIGS. 3A and 3B, the opposing -flights 96 are pivoted toward each
other to
thereby engage the containers 10 between the opposing recesses 102 of adjacent
flights.
Similarly, at the outlet end 108, the formation of the unloading gap 112
between the
respective flights 96 allows the containers loaded thereon to be removed from
the conveyor.
Any of numerous different devices for automatically, semi-automatically, or
manually
loading and/or unloading the containers onto the conveyor that are currently
known, or that
later become known, may be employed. In addition, any of numerous different
apparatus
that are currently known, or that later become known, may be employed to cap
the filled
containers after exiting the sterile zone. As may be recognized by those of
ordinary skill in
the pertinent art based on the teachings herein, the conveyor, the devices for
holding the
containers onto the conveyor, and/or the apparatus for driving and/or
controlling the
conveyor may take any of numerous different configurations that are currently
known, or that
later become known.
[000471 In the
illustrated embodiment, each flight 96 of the conveyor is configured to hold
four containers 10 spaced laterally relative to each other. Accordingly, in
the illustrated
embodiment, each sterilizing head 70 located within the sterilizing station 70
includes two
sterilant manifolds 114, and four sterilizing nozzles 116 mounted on each
sterilant manifold.
Each sterilizing nozzle 116 is located over a respective container position on
the conveyor to
direct fluid sterilant onto the respective container. Similarly, each
sterilant flushing head 76
located within the sterilant removing stations 72 and 74 includes two flushing
manifolds 118,
and each flushing manifold 118 includes four flushing nozzles 120. Each
flushing nozzle
120 is located over a respective container position on the conveyor to direct
heated sterile air
or other gas onto the respective container to re-vaporize if necessary and
flush away the fluid
sterilant. In the illustrated embodiment, the conveyor 60 is indexed by two
rows of
containers (or flights) at a time, such that at any one time, two rows of
containers are each
being sterilized, needle filled, and laser resealed within the respective
stations, and four rows
of containers are being flushed within the two sterilant removing stations
(i.e., the first
CA 02602222 2007-07-25
sterilant removing station 72 applies a first flush, and the second sterilant
removing station
74 applies a second flush to the same containers). When each such cycle is
completed, the
conveyor is indexed forward (or clockwise in FIGS. 3A and 3B) a distance
corresponding to
two rows of containers, and the cycle is repeated. As may be recognized by
those of
ordinary skill in the pertinent art based on the teachings herein, the
apparatus may define any
desired number of stations, any desired number of container positions within
each station,
and if desired, any desired number of apparatus may be employed to achieve the
desired
throughput of containers.
[00048] The needle filling station 78 comprises a needle manifold 122
including a
plurality of needles 124 spaced relative to each other and movable relative to
the flights 96
on the conveyor 60 for penetrating a plurality of containers 10 mounted on the
portion of the
conveyor within the filling station, filling the containers through the
needles, and
withdrawing the needles from the filled containers. Each of the laser
resealing stations 82
and 84 comprises a plurality of laser optic assemblies 126, and each laser
optic assembly is
located over a respective container position of the conveyor flights located
within the
respective laser resealing station. Each laser optic assembly is connectable
to a source of
laser radiation (not shown), and is focused substantially on a penetration
spot on the second
material portion 24 of the stopper 18 of the respective container 10 for
applying laser
radiation thereto and resealing the respective needle aperture. Also in the
illustrated
embodiment, each laser resealing station 82 and 84 further comprises a
plurality of optical
sensors (not shown). Each optical sensor is mounted adjacent to a respective
laser optic
assembly 126 and is focused substantially on the laser resealed region of a
stopper 18 of the
respective laser optic assembly, and generates signals indicative of the
temperature of the
laser resealed region to thereby test the integrity of the thermal seal.
[00049] In one embodiment of the present invention, a non-coring filling
needle 124
defines dual channels (i.e., a double lumen needle), wherein one channel
introduces the
substance into the storage chamber 14 and the other channel withdraws the
displaced air
and/or other gas(es) from the storage chamber. In another embodiment, a first
non-coring
needle introduces the substance into the chamber and a second Don-coring
needle (preferably
mounted on the same needle manifold for simultaneously piercing the stopper)
is laterally
21
CA 02602222 2007-07-25
spaced relative to the first needle and withdraws the displaced air and/or
other gas(es) from
the chamber. In another embodiment, grooves are formed in the outer surface of
the needle
to vent the displaced gas from the storage chamber. In one such embodiment, a
cylindrical
sleeve surrounds the grooves to prevent the septum material from filling or
blocking the
grooves (partially or otherwise) and thereby preventing the air and/or other
gases within the
container from venting therethrough. In each case, the channels or passageways
may be
coupled to a double head (or channel) peristaltic pump such that one
passageway injects the
product into the storage chamber, while the other passageway simultaneously
withdraws the
displaced air and/or Other gases from the storage chamber. In some embodiments
of the
present invention, there is preferably a substantially zero pressure gradient
between the
interior of the filled storage chamber 14 and the ambient atmosphere. Also in
some
embodiments of the present invention, the substance substantially entirely
fills the storage
chamber (or is filled to a level spaced closely to, or substantially in
contact with the interior
surface of the first material portion 22, but not in contact with the exposed
portion 26 of the
second material portion 24).
100050] As shown typically in FIGS. 1A-1C, in one embodiment of the
invention, the
body 12 defines a base 52, a mid-portion 54, and an upper portion 56 axially
spaced from the
base on an opposite side of the mid-portion relative to the base, and each of
the base and
upper portion define a laterally-extending dimension greater than a maximum
laterally-
extending dimension of the mid-portion. As a result, as also shown typically
in FIGS. 1A-
1C, in the illustrated embodiment, the assembled container defines a
substantially diabolo or
spool shape. During needle filling and resealing, the container engaging
recesses 102 of the
flights 96 engage the mid-portion 54 of the body 12 immediately below the
upper portion 56.
Accordingly, the.upper portion 56 of the body is engageable with the upper
surface of the
respective flight or other container support for substantially preventing
axial movement of
the body relative thereto during at least one of needle penetration and
withdrawal with
respect to the stopper, and the base 52 of the body 12 is engageable with the
lower surface of
the respective flight or other container support for substantially preventing
axial movement
of the body relative thereto during at least one of needle penetration and
withdrawal with
respect to the stopper.
22
CA 02602222 2007-07-25
[00051] In the illustrated embodiment of the present invention, the second
material portion
24 is preferably made of a thermoplastic/elastomer blend, and may be the same
material as
those described in the co-pending patent applications and/or patents
incorporated by
reference above. Accordingly, in one such embodiment, the second material
portion 24 is a
thermoplastic elastomer that is heat resealable to hermetically seal the
needle aperture by
applying laser radiation at a predetermined wavelength and power thereto, and
defines (i) a
predetermined wall thickness, (ii) a predetermined color and opacity that
substantially
absorbs the laser radiation at the predetermined wavelength and substantially
prevents the
passage of the radiation through the predetermined wall thickness thereof, and
(iii) a
predetermined color and opacity that causes the laser radiation at the
predetermined
wavelength and power to hermetically seal the needle aperture formed in the
needle
penetration region thereof in a predetermined time period of less than or
equal to about 5
seconds and substantially without burning the needle penetration region.
[00052] In one embodiment, the second material portion 24 is a
thermoplastic elastomer
that is heat resealable to hermetically seal the needle aperture by applying
laser radiation at a
predetermined wavelength and power thereto, and includes (i) a styrene block
copolymer; (ii)
an olefin; (iii) a predetermined amount of pigment that allows the second
material portion to
substantially absorb laser radiation at the predetermined wavelength and
substantially prevent
=the passage of radiation through the predetermined wall thickness thereof',
and hermetically
seal the needle aperture formed in the needle penetration region thereof in a
predetermined
time period of less than or equal to about 5 seconds; and (iv) a predetermined
amount of
lubricant that reduces friction forces at an interface of the needle and
second material portion
during needle penetration thereof In one such embodiment, the second material
portion
includes less than or equal to about 40% by weight styrene block copolymer,
less than or
equal to about 15% by weight olefin, less than or equal to about 60% by weight
mineral oil,
and less than or equal to about 3% by weight pigment and any processing
additives of a type
known to those of ordinary skill in the pertinent art.
[00053] in one embodiment, the second material portion 24 is a
thermoplastic elastomer
that is heat resealable to hermetically seal the needle aperture by applying
laser radiation at a
predetermined wavelength and power thereto, and includes (i) a -first
polymeric material in an
23
CA 02602222 2007-07-25
amount within the range of about 80% to about 97% by weight and defining a
first
elongation; (ii) a second polymeric material in an amount within the range of
about 3% to
about 20% by weight and defining a second elongation that is less than the
first elongation of
the first polymeric material; (iii) a pigment in an mount that allows the
second material
portion to substantially absorb laser radiation at the predetermined
wavelength and
substantially prevent the passage of radiation through the predetermined wall
thickness
thereof, and hermetically seal a needle aperture formed in the needle
penetration region
thereof in a predetermined time period of less than or equal to about 5
Seconds; and (iv) a
lubricant in an amount that reduces friction forces at an interface of the
needle and second
material portion during needle penetration thereof.
[00054] In one embodiment of the invention, the pigment is sold under the
brand name
LumogenThl IR 788 by BASF Aktiengesellschaft of Ludwigshafen, Germany. The
Lumogen
IR products are highly transparent selective near infrared absorbers designed
for absorption
of radiation from semi-conductor lasers with wavelengths near about 800 nm. In
this
embodiment, the Lumogen pigment is added to the elastomeric blend in an amount
sufficient
to convert the radiation to heat, and melt the stopper material, preferably to
a depth equal to
at least about 1/3 to about V2 of the depth of the needle hole, within a time
period of less than
or equal to about 5 seconds, preferably less than about 3 seconds, and most
preferably less
than about 1-1/2 seconds. The Lumogen IR 788 pigment is highly absorbent at
about 788
mn, and therefore in connection with this embodiment, the laser preferably
transmits
radiation at about 788 urn (or about 800 nm). One advantage of the Lumogen IR
788
pigment is that very small amounts of this pigment can be added to the
elastomeric blend to
achieve laser resealing within the time periods and at the resealing depths
required or
otherwise desired, and therefore, if desired, the needle penetrable and laser
resealable stopper
may be transparent or substantially transparent. This may be a significant
aesthetic
advantage. In one embodiment of the invention, the Lumogen IR 788 pigment is
added to
the elastomeric blend in a concentration of less than about 150 ppm, is
preferably within the
range of about 10 ppm to about 100 ppm, and most preferably is within the
range of about 20
ppm to about 80 ppm. In this embodiment, the power level of the 800 rim laser
is preferably
less than about 30 Watts, or within the range of about 8 Watts to about 18
Watts.
24
CA 02602222 2007-07-25
[000551 In one embodiment of the present invention, the substance or
product contained
within the storage chamber is a fat containing liquid product, such as infant
or baby formula,
and the first material portion 22, the second material portion 24, and the
body 12 each are
selected from materials (i) that are regulatory approved for use in connection
with nutritional
foods, and preferably are regulatory approved at least for indirect contact,
and preferably for
direct contact with nutritional foods, (ii) that do not leach an undesirable
level of
contaminants or non-regulatory approved leachables into the fat containing
product, such
mineral oil, and (iii) that do not undesirably alter the taste profile
(including no undesirable
aroma impact) of the fat containing liquid product to be stored in the
container. In certain
embodiments of the invention, the needle penetrable and thermally resealable
second
material portion 24 provides lesser or reduced barrier properties in
comparison to the first
material portion, and therefore the first material portion 22 and/or over cap
20 are selected to
provide the requisite barrier properties of the container closure 15 for
purposes of storing the
product to be contained therein.
1000561 In the embodiment of the present invention wherein the product is a
fat containing
liquid nutrition product, such as an infant or baby formula, exemplary
materials for the
second material portion 24 are selected from the group including (11_,S 254-
071, C-Flex R70-
001, Evoprene TS 2525 4213, Evoprene SG 948 4213 and Cawiton 7193,
modifications of
any of the foregoing, or similar thermoplastic elastomers. In one such
embodiment, the body
12 is an injection molded multi-layer of PP/EVOH. In another such embodiment,
the body
12 is blow molded, such as by extrusion blow molding, and is an HDPE/EVOH
multi layer.
In some such embodiments, the first material portion 22 is selected from the
group including
(i) a low mineral oil or mineral oil free thermoplastic; (ii) a low mineral
oil or mineral oil free
thermoplastic defining a predetermined durometer; (iii) a liquid injection
moldable silicone;
and (iv) a silicone. The predetermined duromcter is within the range of about
20 Shore A to
about 50 Shore A, and preferably is within the range of about 25 Shore A to
about 35 Shore
A. In some such embodiments, the first material portion is formed of
polyethylene, an
LIDPETrpE blend or multi layer, or a PP/TPE blend or multi layer. Also in some
such
embodiments, the securing member or cap 20 is made of a plastic sold under the
trademark
Ccicon"TM, a PP/EV014 multi layer, an IMPE/EVOM multi layer or blend, or a
HDPE/EV01-1
multi layer or blend. As may be recognized by those or ordinary skill in the
pertinent art
CA 02602222 2007-07-25
based on the teachings herein, these materials are only exemplary, and
numerous other
materials that are currently known, or that later become known, equally may be
used.
1000571 In FIG. 4, another container embodying the present invention is
indicated
generally by the reference number 110. The container 110 is substantially
similar to the
container 10 described above, and therefore like numbers preceded by the
number "1" are
used to indicate like elements. The primary difference of the container 110 in
comparison to
the container 10 is that the first material portion 122 of the stopper 118
includes a peripheral
flange 132 defining internal female threads 146 that threadedly engage male
threads 144 on
the body 112 to threadedly secure the stopper to the body. In this embodiment,
the seal
between the stopper and body can be formed in any of numerous different ways
that are
currently known, or that later become known, including, for example, by a
"plug" seal, a
"valve" seal, or a "direct" seal between the top edge of the body and a gasket
formed on the
stopper. In the latter case, the gasket can be formed by the second material
portion 124 at the
time of co-molding the first and second material portions 122 and 124,
respectively, or at the
time of over-molding the second material portion 124 to the first material
portion 122. In
this embodiment, the cap 120 does not secure the closure 115 to the body 112,
but rather is
snap fit at 133 to the depending flange 132 of the first material portion 122
and provides the
requisite barrier properties for the container closure (i.e., an oxygen and
moisture-vapor
transmission ("MVT") barrier). In the illustrated embodiment, as can be seen,
the snap fit
connection 133 is formed by an annular protuberance on the cover 120 received
within a
corresponding annular groove on the flange 132. However, as may be recognized
by those of
ordinary skill in the pertinent art based on the teachings herein, the cap 120
may be fixedly
secured to the stopper 118 in any of numerous different ways that are
currently known, or
that later become known. Also in this embodiment, a frangible tamper evident
ring 148 is
formed at the base of the depending flange 132 of the -first material portion
122 of the stopper
118 and slides over a tamper evident ridge 150 of the body 112 to releasably
engage the
tamper evident ring and cap to the body.
[00058] In FIG. 5, another container embodying the present invention is
indicated
generally by the reference number 210. The container 210 is substantially
similar to the
container 110 described above, and therefore like reference numerals preceded
by the
26
CA 02602222 2007-07-25
numeral "2" instead of the numeral "1" are used to indicate the same or
similar elements.
The primary difference of the container 210 in comparison to the container 110
described
above is that the container 210 does not include a conventional cap, but
rather includes a
barrier disk 220 that is received within a recess 221 formed in the upper
surface of the first
material portion 222 of the stopper 218. As can be seen, the barrier disk 220
overlies the
container closure 215 and forms a seal between the first material portion 224
and the ambient
atmosphere to thereby provide the requisite barrier properties between the
storage chamber
214 and ambient atmosphere. In the illustrated embodiment, the barrier disk
220 is fixedly
secured to the first material portion 222 of the stopper 218 such as by
ultrasonic or induction
welding or sealing. However, as may be recognized by those of ordinary skill
in the
pertinent art based on the teachings herein, the barrier disk can be fixedly
secured to the
stopper in any of numerous different ways that are currently known, or that
later become
known, As with the caps of the embodiments described above, the barrier disk
220 is
assembled to the stopper 218 after needle filling and laser resealing the
stopper, and
preferably outside of the sterile filling zone.
[00059] As may be recognized by those skilled in the pertinent art based on
the teachings
herein, numerous changes and modifications may be made to the above-described
and other
embodiments of the present invention without departing from its scope as
defined in the
appended claims. For example, the first and second material portions, body and
cap may be
made of any of numerous different materials that are currently known, or that
later become
known for performing their functions and/or depending on the container
application(s),
including the product to be stored within the container. In addition, the body
and container
closure may take any of numerous different shapes and/or configurations, and
may be
adapted to receive and store within the storage chamber any of numerous
different substances
or products that are currently known or that later become known, including
without
limitation, any of numerous different food and beverage products, including
low acid or fat
. containing liquid products, such as milk-based products, including
without limitation milk,
evaporated milk, infant formula, growing-up milks, condensed milk, cream, half-
and-half,
yogurt, and ice cream (including dairy and non-diary, such as soy-based ice
cream), other
liquid nutrition products, liquid healthcare products, juice, syrup, coffee,
condiments, such as
ketchup, mustard, and mayonnaise, and soup, and pharmaceutical products.
Accordingly,
27
CA 02602222 2007-07-25
this detailed description of preferred embodiments is to be taken in an
illustrative, as opposed
to a limiting sense.
28