Note: Descriptions are shown in the official language in which they were submitted.
CA 02602282 2007-10-04
62406-168D
- 1 -
AQUEOUS COATING COMPOSITION
This application is a divisional of Canadian patent application No. 2,219,878
filed June 4, 1996.
BACKGROUND AND SUMMARY OF THE INVENTION
It is to be understood that the expression "the present invention" or the like
used in this specification encompasses the subject-matter of both this
divisional application
and the parent application.
This invention relates to coating compositions and
systems, and more particularly to aqueous or water-base coating
compositions, and methods of making and using the same.
Coating untreated polyolefin-containing substrates, such
as polypropylene substrates, is generally difficult because of poor
adhesion of coatings to this type of substrate.
?n an effort to solve this problem, polyolefinic and other
2.5 similar plastic or synthetic substrates have been typically coated
with coating systems employing an organic solvent base. While the use
of organic solvents appears to assist, to some degree, the organic
contents of the coatings often render the coating composition or
system undesirable from a number of aspects. For example, most
2D organic solvents are derived from petroleum products, and thus are
generally e}:pensive and in short supply. Further, unless suitable
costly recovery or disposal procedures are used, organic solvents,
such as the commonly used aromatic organic solvents, may create a
potential pollution problem.
25 other popularly employed coatings include chromium-
containing coatings. And like petroleum-based systems, unless
properl.y controlled costly recovery or treatment methods are also
employed, chromium-containing coatings are also potentially
undesirable from an environmental standpoint.
3D Aqueous-based primer coatings, additionally employing
substantial levelsof aromatic organic solvents, have been suggested
in the art. For example, such compositions have been disclosed by
manufacturers or distributors such as Eastman Chemical Corp. Likewise
Emco Chemical Corporation has disclosed a primer for polypropylene
35 that includes, as initial ingredients 25% by weight of a chlorinated
polyolefin having a chlorine content of 300 (25t in xylene), 24.925%
toluene, 0.025% sulfonated castor oil, 0.050o sodium lauryl sulfate,
and 50.000% deionized water (as a final ingredi.ent).
U.S. Patent No. 9,959,573 discloses a chlorinated
40 polyolefin composition for use as a primer or coating or
t-arious types of substrates, such as polyolefins.
CA 02602282 2007-10-04
62406-168D
- 2 -
The use of chlorinated polyolefin materials as a
primer for polyolefin substrates has also been proposed.
For instance, U.S. Patent No. 4,303,697 discloses priming a
polyolefinic substrate with a chlorinated polymeric
material, such as chlorinated polypropylene, containing
carboxylic acid anhydride groups, and exposing the primed
substrate to ultraviolet radiation.
U.S. Patent No. 4,070,421 discloses chlorinated
polyolefinic compositions useful as primers for adhesion
improvement of decorative or protective coatings to
polyolefins.
U.S. Patent No. 3,579,485 discloses chlorinated
carboxyl group-containing poly-a-olefins, which form primer
coatings for use on untreated poly-a-olefin substrates, and
have aromatic solvents as a preferred solvent.
The following United States patents relate more
generally to coating compositions for substrates, including
coating compositions for polyolefin substrates: 4,710,408;
4,567,106; 4,417,022; 4,343,925; 4,317,894; 4,314,918;
4,263,411; 4,246,319; 4,214,039; 4,144,363; 4,046,587;
4,028,329; 3,849,148; 3,317,330; 3,218,189; and 2,998,324.
In one aspect of the present invention there is
provided an aqueous-based coating composition, with a
reduced level of, or substantially free of, volatile organic
components ("VOC's") or solvents (such as aromatic organic
solvents) for coating substrates such as polyolefin-
containing substrates.
The compositions and methods of the present
invention preferably employ: (a) a resin; (b) an ethylene
glycol; (c) a surfactant; (d) an aliphatic amine; and
CA 02602282 2007-10-04
62406-168D
- 2a -
(e) water. In another embodiment the compositions and
method further employ a thickening agent, a filler, or both.
In one exemplary embodiment of the invention of
the parent application, there is provided a method for
preparing a conductive, aqueous, carbon black coating
composition comprising: (a) milling a carbon black material
in two or fewer milling passes to a particle size which does
not sacrifice the conductivity of the carbon black;
(b) preparing a carbon black dispersion comprising a first
adhesion promoter composition and the milled carbon black
material, wherein the first adhesion promoter composition
comprises by weight about 3 to about 25 parts surfactant,
about 3 to about 9 parts amine, about 35 to about 150 parts
resin and about 300 to about 500 parts water; and
(c) admixing the carbon black dispersion with a second
adhesion promoter composition comprising by weight about 3
to about 25 parts surfactant, about 3 to about 9 parts
amine, about 35 to about 150 parts resin and about 300 to
about 500 parts water.
In another exemplary embodiment of the invention
of the parent application, there is provided a method for
preparing a conductive, carbon black coating composition
comprising: (a) milling a carbon black material in two or
fewer milling passes to a particle size which does not
sacrifice the conductivity of the carbon black;
(b) preparing a first carbon black concentrate composition
comprising by weight about 40 to about 75 parts of a first
adhesion promoter and about 12 to about 25 parts of the
milled carbon black; and (c) admixing the carbon black
concentrate composition with a second adhesion promoter,
wherein the first and second adhesion promoters comprise by
weight of the first and second adhesion promoters about 3 to
about 25 parts surfactant, about 3 to about 9 parts amine,
CA 02602282 2007-10-04
62406-168D
- 2b -
about 35 to about 150 parts resin and about 300 to about 500
parts water.
In an exemplary embodiment of the invention of
this divisional application, there is provided an aqueous
coating composition, comprising: (a) up to about 40 percent
of a polyol; (b) about 0.5 percent to about 40 percent of a
resin, which is a halogenated polyolefinic resin material, a
halogenated vinyl resin, an epihydrin resin, or a
halogenated epoxy resin, or a mixture thereof; (c) about
0.05 to about 15 percent of a surfactant; (d) an amine;
(e) a mixture comprising an acrylic/polyurethane dispersion;
and (f) about 30 to about 95 percent water, wherein the
total volatile content in said composition is less than
about 5 percent by weight, and wherein said composition is
substantially free of aromatic organic solvents.
BRIEF DESCRIPTION OF THE DRAWINGS
Other objects and advantages of the invention will
become apparent upon reading the following detailed
description and upon reference to the drawings, in which:
Figures la and lb illustrate how samples prepared
according to the methods of the present invention are
tested.
Detailed Description Of The Invention
The present invention comprises aqueous or water-
based coating compositions or systems useful in coating
polyolefinic-containing substrates. The compositions are
also useful in coating other troublesome metallic and
synthetic substrates, i.e., substrates that are difficult to
coat with good adhesion. The compositions or systems
preferably comprise:
CA 02602282 2007-10-04
62406-168D
- 2c -
(i) a polyol;
(ii) a surfactant, such as a non-ionic surfactant;
(iii) a halogenated polyolefinic resin material or
other similar halogenated resin, such as a PVC resin;
(iv) an aliphatic amine, such as an amino-
substituted alkanol; and
CA 02602282 2007-10-04
V- 140819 PCTIUS96/09466
- 3 -
(v) water.
In another preferred embodiment, the compositions or
systems further comprise, in addition to the above, a thickening
agent, a filler, or both.
In another preferred embodiment, the compositions or
systems comprise a carbon black dispersion.
In another preferred embodiment, the compositions or
systems comprise a mixture comprising an acrylic and a polyurethane.
The compositions can be used to efficaciously coat
polyolefinic-like substrates while employing substantially reduced
levels of volatile organic solvents or volatile organic components
(VOC's). In a preferred embodiment, the compositions and systems of
the present invention contain very low levels or is substantially free
of volatile organic solvents, especially aromatic solvents, such as
benzene, xylene; toluene, or similar materials or components. In a
highly preferred embodiment, the compositions and systems of the
present invention are substantially-free of volatile organic
components or materials, especially aromatic organic solvents such as
benzene, toluene, xylene, and the like.
The coatings provided by the compositions and methods of
the present invention may be decorative, protective, or act as a base
or pre-treatment for another coating or treatment step to come; they
may also be employed to perform two or more of these functions.
They may be applied or deposited by any convenient method,
such as spraying, dipping, roller-coating, electrostatic deposition,
or the like.
While it may have other functions as well, (and without
intending to be bound by theory) the polyols employed in the
compositions and methods of the present invention are used primarily,
without limitation, as coalescing agents. The polyols employed in the
methods and compositions of the present invention are preferably
distillable polyols. Thus, several different classes of polyols are
suitable for use in the compositions and methods of the present
invention.
The polyols may be any suitable polyol having any suitable
cbain length or -OH functionality. The preferred polyols for use in
the present invention are glycol-type polyols, and still more
preferably are alkylene polyols (e.g. ethylene polyols). In
particular, preferred glycol-type polyols include alkylene glycols,
such as ethylene glycol, diethylene glycol, triethylene glycol,
tetraethylene glycol, propylene glycol, dipropylene glycol,
tripropylene glycol, and hexylene glycol as well as other glycols such
as 1,3-butylene glycol, and ethoxytriglycol. Mixtures of two or more
of these materials may also be employed. The most preferred glycols
.45 are alkylene glycols. Ethylene glycol is one of the most preferred.
CA 02602282 2007-10-04
Wt. 10819 PCT/US96/09466
- 4 -
Preferably the polyol is generally volatile and has a
hydroxyl functiona'yity of at least 2, and preferably about 2 - 6
hydroxy groups, per mole of polyol. Further, the polyol preferably
has an average molecular weight of about 50 to about 500, more
preferably about 62 to about 500, even more preferably about 62 to
about 425, and still more preferably about 62 to about 250.
The final coating compositions or systems and methods of
the present invention preferably employ the selected polyol at a level
of between about 3o to about 40%, by weight of the final composition.
In a more preferred embodiment, the polyol constitutes about 3o to
about 20%, and still more preferably about 3% to about 10%. In one
highly preferred embodiment the polyol comprises about 4.9% of the
total weight of the final coating composition or system.
The selection of the resin for use in the compositions and
methods of the present invention is extremely important. While the
skilled artisan will appreciate that its selection will depend upon
many factors, such as the nature of the ultimate surface to be coated
(or related utility), processing conditions, the other components
selected (i.e., the glycol, amine, surfactant, etc.), environmental
concerns, costs, and the like, the following discussion relates to
presently preferred materials.
The resins preferred for use in the composition and
methods of the present invention include halogenated polyolefinic
resin materials, as well as other art-disclosed halogenated materials
and resins, such as PVC and related materials, and homo-, co- or
terpolymers of such materials. It should be noted, however, that
under certain conditions, non-halogen-containing resins may be
employed, alone (as homopolymers) or as co- or terpolymers, along with
halogenated materials. Rubbers may also be employed.
As noted, one class of preferred resins is halogenated
polyolefinic materials. The halogenated polyolefinic materials
preferred for use in the compositions and methods of the present
invention are chlorinated polyolefinic materials. The chlorinated
polyolefinic resin materials selected for use in the present coating
compositions and methods should preferably have a chlorine content of
from about 10 to about 40 weight percent, more preferably about 10 to
about 30 weight percent, even more preferably about 18 to about 22
weight percent, and still more preferably about 19 to about 21 weight
percent. In a present highly preferred embodiment, the chlorine
content of a resin should comprise about 19.9 weight percent of the
material.
The chlorinated resin, e.g., chlorinated polyolefin, may
be supplied for use in the compositions or systems in any suitable
form, with powder or pelletized forms being the preferred forms, and
the powder form being the most preferred form.
~
CA 02602282 2007-10-04
62 6-168
- 5 -
The chlorinated polyolefin may be prepared according to
known methods, such as those described in U.S. Patent Nos. 4,070,421
and 4,954,573 . For use in the preserit compositions
ard methods, the chlorinated polyolefin materials
can he selected from comniercially available
materials such as those supplied by Eastman Chemical Company,
Kingsport, Tennessee under the trade name CPO-343-1 (100%) . Other
commercially available materials that may be employed under certain
conditions include PM 12075-00, and 12075-OF, also supplied by Eastman
Chemical Company, Kingsport, Tennessee, and CP-26P, CP-30P and CP-32P,
products of Toyo Kasei Kogyo Co. Ltd., Osaka, Japan.
As noted in U.S. Patent No. 4,070,421, the resins of the
present invention can be admixed with other hydrocarbon-type resins;
see Col. 2, 1. 15-3B of said '421 patent.
Preferably, the chlorinated polyolefins and other
preferred chlorinated resins of the present compositions and methods
have a melting point in the range of about 150 F to about 350 F, more
preferably about 150 F to about 250 F, and still more preferably in
the range of about 180 F to about 210 F. Moreover, the preferred
chlorinated polyolefins or other preferred chlorinated resins for use
in the compositions and methods of the present invention have average
molecular weights in the range of about 10,000 to about 40,000, more
preferably about 10,000 to about 30,000, even more preferably about
15,000 to about 30,000, and still more preferably about 22,000 to
about 29,000. The chlorinated polyolefins and other preferred
chlorinated resins employed in the coating compositions and methods of
the present invention preferably constitute about 0.596 to about 300
(and perhaps as high as about 40%), more preferably about 2% to about
10%, still more preferably about 2% to about 5% by weight of the total
3D composition. In one highly preferred embodiment, it is employed at a
level of about 4.3%; in yet another it is about 10%.
Another group of useful resins, as noted above, include
various polyvinyl resins, preferably chlorinated polyvinyl resins or
PVC's; such materials also include PVC-like materials. Exemplary
materials include polyvinyl alcohol, polyvinyl chloride,
polyvinylidene chloride, and polyvinyl chloride-vinyl acetate or
-maleic anhydride resins. Homo-, co- and tei-polymeric materials made
from such materials may be employed; mixtures of such materials may
also be used.
Other similar useful materials include resins prepared
from polyepichlorohydrins, brominated epoxies, all rubbers, and the
like. These materials may be employed as homopolymers, copolymers and
terpolymers; again, mixtures of such materials may also be employed.
As with the polyolefin materials described above, it is
preferred that the final resin material employed in the compositions
CA 02602282 2007-10-04
624,, 168
- 6 -
and methods of the present invention be chlorinated and have general
physical propertiE:s similar to the olefinic materials. For example,
an average molecular weight in the range of about 10,000 to about
30,000 is preferred. Likewise, it is highly preferred that at least
a part of the final resin material be chlorinated and have a chlorine
content of about 10 to about 30 percent. They are employed in the
methods in similar fashions and in the compositions at similar levels.
The preferred compositions and methods of the present
invention further include surface active agents, such as surfactants.
Without intending to be bound by theory, the surface active agent
serves primarily as an emulsifying agent in the present compositions.
However, the surface active agent may also serve, without limitation,
as an agent for wetting the surface of a material coated with the
present coating compositions.
The surfactants preferred for use in the compositions and
methods of the present invention include both high molecular weight
(average molecular weight of 500 or more) and low molecular weight
(average molecular weight of less than 500) nonionic, amphoteric,
cationic and anionic materials. Polymeric surfactants may also be
employed.
Exemplary nonionic materials include, for example, organic
materials which contain groups of varying polarity whereby one part of
the molecule is hydrophilic and the other is hydrophobic. Examples of
such materials include polyethylene polyols, polyvinyl alcohol,
polyethers, polyesters and polyhalides. Preferably, the non-ionic
surface active agent or other surface active agent selected is a
surfactant and is present in an amount of about 0.05% to about 15%,
more preferably about 0.05o to about 10%, still more preferably about
0_1% to about 10%, and still more preferably at a level of about 0.5%
to about 7.0%, by weight of the total weight of the composition.
A highly preferred class of nonionic surface active agents
or surfactants includes those that belong to the series of
nonylphenoxy polyethoxy ethanol surfactants. In particular, these
highly preferred surfactants preferably contain about 5 to about 25,
and more preferably about 7 to about 15, and still more preferably
about 7 to about l0 ethylene oxide groups per molecule of surfactant.
Thus, commercial surfactants suitable for the present invention
include, without limitation, Triton N-101"'' manufactured by Rohm and
Haas; as well as other commercially available materials such as Triton*
Y.-100, GAF CO-630 and Makon* 10 manufactured by Stepan Chemical,
Voeppe, France.
Alternative preferredsurfactants includefluorosurfactan*_
materials such as FC- 129, 430 manufactured by Minnesota Mining and
Manufacturing.
*Trade-mark
CA 02602282 2007-10-04
62 6-168
- 7 -
Ex.emplary anionic surface - active agents include
materials such as those containing a carboxylate group attached
directly to a hydrophobic group, or, in the alternative, where there
is an intermediate functionality such as an ester, amide, sulfonamide,
or the like. Other useful materials include anionic agents derived
from sulfuric or sulfonic acids in which the hydrophobic groups are
selected from aliphatic or aromatic groups of varying polarity, such
as halides, hydroxyls, ether and/or ester groups. A preferred anionic
material is a material such as sodium lauryl sulfate.
lp Exemplary cationic surface - active agents are those
derived from amino groups (including primary; secondary; and/or
tertiary amine salts) wherein the hydrophilic character is obtained by
groups of varying polarity. Also exemplary are materials such as
auaternary ammonium compounds, guanidine, and thiuronium salts.
Exemplary polymeric surfactants would include those
manufactured by ICI Americas under the Tradename designations of
Hypermer P52 and Hypermer P53.
As will be appreciated by the skilled artisan, there are
a wide variety of such surface-active agents available. They are
conveniently listed, by class, in "McCutcheon's Emulsifier's &
Detergents", North American Edition, 1982, pp. 322-327.
They are employed in a similar fashion and at similar
levels to the preferred non-ionic materials discussed above.
The preferred amines for use in the compositions and
methods of the present invention include primary, secondary and
tertiary aliphatic amines. In a highly preferred embodiment, the
aliphatic amine has an amine functionality of between 1 and 3, and
optionally contains other oxygen-containing functional groups as well.
Preferred amines include primary, secondary and tertiary alkylamines,
alkyldiamines, alkynolamines, dialkynolamines, and
poly(oxyalkylene)diamines. A highly preferred group of amines further
contains amines having one or more hydroxy or alkoxy (ether) groups
and an average molecular weight in the range of about 5o to about
7,000.
A highly preferred group of amines are primary, secondary
and tertiary aliphatic amines having a functionality of 1 to 3 and can
be generally represented by the general formulae:
RZ
Rl ~T P,3 ; or
*Trade-mar}:
CA 02602282 2007-10-04
62 S-168
- 8 -
Iz 4
Rl ~; R, N R5 ; or
lU I
2 4 I6
Rl N R3 N RS 17 R,
wherein R, - R, are independently selected from H or straight or
branched chain alkyl, hydroxyalkyl, or alkoxylalkyl groups of about 1
to about 20 carbon atoms; R, - R, can additionally include a
substituted alkyl group, i.e., where one or more of the carbons in the
radical is replaced with or has substituted thereon another
functionality, e.g., an amine, ether, hydroxy or -mercapto moiety,
e.g., tris-(3-aminopropyl) amine.
Another group of highly preferred amines within the above
classes are those primary, secondary or tertiary aliphatic amines of
the above Formulae in which R1 - R. is specifically substituted with
or contains one or more hydroxyl (-OH) functionalities.
Another group of preferred amines can be represented by
the formulae:
R9 R9
I I
RB--(NH2)n or P.e-rNH)õ or Re---EP,lo-N-Rll)
wherein n is 1 or 2 and Re, R9, Rlo and Rll are independently selected
from straight or branched chain alkyl, hydroxyal}:yl or alkoxyalkyl
groups of about 1 to about 20 carbon atoms. These chains may also be
substituted with another functionality as described above.
Yet another group (or subgroup of the above) which
comprise amines preferred in the practice of the present invention are
primary, secondary and tertiary aliphatic amines with an amine
functionality of about 1-3 which also contain one or more ether or
alkoxy linkages. Such materials are sometimes referred to as
poly(oxyalkylene)diamines. Ethoxylated or propoxylated materials are
particularly preferred. For example, a useful variety of such
materials are those manufactured by Texaco Co. and marketed under the
Trade Name or designation of "Jeffamines". Such useful materials are
typically poly(oxyethylene) or poly(oxypropylene) amine or diamine
materials having molecular weights of about 400 to about 2000. These
materials typically carry the Jeffamine* mark, or designation and
"Trade-inark
CA 02602282 2007-10-04
\. 0/40819 PCTIUS96/09466
- 9 -
include a "series" designation of A"; M"; "D"; "ED"; and DU . Many
such materials have been found to be useful.
Exemplary amines preferred for use in the present
invention include:
2-amino-l-butanol;
4-amino-l-butanol;
2-aminoethanethiol;
2-aminoheptane;
2-amino-l-hexanol;
6-amino-l-hexanol;
allylamine;
2-amino-3-methyl-l-butanol;
2-amino-2-methyl-1,3-propanediol;
2-amino-2-methyl-l-propanol;
2-amino-l-pentanol;
5-amino-l-pentanol;
3-amino-l-propanol;
amylamine;
butylamine;
N,N'-bis(2-aminoethyl)-1,3-propanediamine;
N,NI-bis(3-aminopropyl)-1,3-propanediamine;
1,3-bis(dimethylamino)-2-propanol;
1- [N,N-bis (2-hydroxyethyl) amino] -2-propanol;
N,N'-bis (2 -hydroxyethyl) ethylenediamine;
decylamine;
1,4-diaminobutane;
1,10-diaminodecane;
1,12-diaminododecane;
1,7-diaminoheptane;
1,3-diamino-2-hydroxypropane;
3,3'-diamino-N-methyldipropylamine;
1,2-diamino-2-methylpropane;
1,9-diaminononane;
1,8-diaminooctane;
1,5-diaminopentane;
1,2-diaminopropane;
1,3-diaminopropane;
dibutylamine;
3-(dibutylamino)propylamine;
y 40 diethanolamine;
diethylamine;
5-diethylamino-2-pentanol;
3-(diethylamino)-1,2-propanediol;
CA 02602282 2007-10-04
N. , 6140819 PCT/LIS96/09466
- 10 -
1-diethylamino-2-propanol;
3-diethylamino-l-propanol;
3-diethylaminopropylamine;
diethylenetriamine;
N,N-diethylethanolamine;
N,N-diethylethylenediamine;
N,N-diethylmethylamine;
N,N'-diethyl-1,3-propanediamine;
diisobutylamine;
diisopropanolamine;
diisopropylamine;
2-(diisopropylamino)ethanol;
3-diisopropylamino-l,2-propanediol;
N,N-diisopropylethylamine;
1-dimethylamino-2-propanol;
3-dimethylamino-l-propanol;
3-dimethylaminopropylamine;
1,3-dimethylbutylamine;
3,3-dimethylbutylamine;
N,N-dimethylethanolamine;
N,N-dimethylethylamine;
N,N-dimethylethylenediamine;
N,N-dimethyl-N'-ethylethylenediamine;
N,N'-dimethyl-1,6-hexanediamine;
2,5-dimethyl-2,5-hexanediamine;
1,5-dimethylhexylamine;
2,2-dimethyl-1,3-propanediamine;
( )-1,2-dimethylpropylamine;
dipropylamine;
dodecylamine;
ethanolamine;
3-ethoxypropylamine;
ethylamine;
2-(ethylamino)ethanol;
N-ethylbutylamine;
2-ethylbutylamine;
N-ethyldiethanolamine;
ethylenediamine;
hexamethylenediamine;
1,6-hexanediamine;
hexylamine;
isoamylamine;
CA 02602282 2007-10-04
6z-406-168
- 11 -
isopropylamine;
N-isopropylethylenediamine;
N'-isopropyl-2-methyl-1,2-propanediamine;
/(!N,N;N'-tetramethyl-1,4-butanediamine;
3 NN,N;N'-tetramethyldiaminometl-,ane;
/ll,N,N;N'-tetramethylethylenediamine;
N,N,N;N'- tetramethyl -1, 6 -hexanediamine;
N,NN;N'-tetramethyl-1,3-propane-diamine;
N,N,2,2-tetramethyl-l,3-propanediamine;
tributylamine;
tridecylamine;
triethanolamine;
triethylamine;
triisooctylamine;
triisopropanolamine;
trimethylamine;
methylamine;
2-(methylamino)ethanol;
N-Methylbutylamine;
1-methylbutylamine;
2-methylbutylamine;
JU-methyldiethanolamine;
N-methylethylenediamine;
N-methyl-1,3-propanediamine;
nonylamine;
octylamine;
tert-octylamine ;
propylamine;
2-(propylamino)ethanol;
1-tetradecylamine; and
tris(3-aminopropyl)amine. Mixtures of such materials may also be
employed.
Without intending to be bound by theory, the amine,
(especially an amino substituted alkanol) is present to further
facilitate emulsification of the coating composition.
In a highly preferred embodiment, the amine is an amino-
substituted alkanol, and more preferably is 2-amino - 2-methyl - 1-
~
propanol, e.g., AMP-95. Alternatively, the amino-substituted alkanol
may be substituted with a constituent selected from the group
consisting of triethylamine, triethanolamine, diethanolamine,
*Trade-mark
CA 02602282 2007-10-04
,62406-168
- 12 -
dimethylethanoiamine; dimethyl-amino-ethanol, or a 2-dimethylamino-2-
methyl-l-propanol, and mixtures thereof.
Commercial amino-substituted alkanols suitable for the
present invention would include, without limitation ANIP-95'", and D.MF-M
P-BO', both manufactured by Angus Chemical.
Highly preferred materials include:
2-amino-2-methyl-l-propanol;
ethanolamine;
dibutylamine;
dimethylethanolamine;
N-ethyldiethanolamine; and
tris-(3-aminopropyl)amine.
Highly preferred poly(or_yal}:ylene)diamines include
Jeffamine M600; D230; D400; D200; ED600; ED900; ED2001; ED4000;
ED6000; DU700; T3000, and the like.
Preferably, the amine, such as an amino-substituted
alkanol, is present in an amount of about 0.01% to about 10% percent,
more preferably about 0.05% to about 50, and still more preferably
about 0.05o to about 3% of the total weight of the final coating
composition or system.
The coating compositions and systems of the present
invention further include water. Water is preferably present in an
amount equal to about 30o to about 95%, more preferably about 50o to
95%, still more preferably about 75% to about 95 s, and still more
preferably about 90% to about 95%, by weight of the total composition.
However, it will be appreciated that the amount of water may be varied
to meet the coating properties required for a particular application.
For instance, where a more viscous coating is desired, the amount of
water may be reduced. The level of water may also be dramatically
reduced in order to prepare a concentrate where dilution is intended
later.
Preferably, the pH of the final coating composition or
system of the preferred embodiments herein is in the range of 7-10.5,
and is more preferably about 7.5 to about 9Ø
The compositions of the present invention are preferably
substantially free of organic solvents, and especially organic
solvents, and still more especially aromatic organic solvents, e.g.,
benzene, or substituted benzene materials such as xylene, toluene, and
the like. By "substantially free of aromatic organic solvents," as
used herein, it is meant that the solvent is present at less than
about 5% by weight, more preferably less than about 2.5%, and still
more preferably less than about 1%, by weight of the final coating
composition. it will be appreciated that this term does not apply to
the required components of the invention, such as the halogenated
polyolefin, glycol, etc, disclosed herein.
*Trade-mark
CA 02602282 2007-10-04
Yr .. .6/40819 PCTIUS96/09466
- 13 -
it will be appreciated that all of the above-identified
materials and ranges are for preferred compositions. The ultimate
level of any component may vary according to many factors such as the
type, purpose or function of the desired coating, the coating weight,
the substrates to be coated, the shape of the article to be coated,
the preparation of surface to be coated, and many other factors
recognized and understood by the skilled artisan. For example, it may
be desirable to prepare a concentrate that would be later diluted with
water after shipment or just prior to use. Accordingly, it will be
appreciated that the ranges given above will be for the final use
composition or system and a concentrate would have correspondingly
higher level of the basic components prior to dilution.
While it will again be appreciated that any suitable
sequence of processing steps may be employed to prepare the
compositions of the present invention, or a concentrate thereof, the
following is a preferred method. However, the sequence of steps may
be varied under some circumstances. As used herein, the phrase
"starting material ratio" refers to the ratio of constituent amounts
as measured before any admixing steps have occurred i.e. while all
materials are still in an initial starting material state.
A presently preferred composition may be prepared by
admixing, using conventional means, a predetermined amount of the
polyol, which is preferably a glycol and more preferably ethylene
glycol, with a predetermined amount of the surf actant. Preferably the
starting material ratio of glycol to surfactant is up to 8:1, and more
preferably about 1:1, 1:2 or lower, by weight. The glycol-surfactant
admixture is then heated, while under agitation, to a first
predetermined'temperature, preferably in the range of about 150 F to
about 275 F, and more preferably in the range of 210 F to about 250 F.
In a preferred embodiment, this temperature is slightly greater than
the melting point of the chlorinated polyolefin employed in the
coating composition.
When the above admixture reaches the predetermined desired
temperature, the temperature is then maintained relatively constant
and the resin, which is preferably chlorinated polyolefin, is added
thereto, optionally under a nitrogen blanket. Preferably the starting
materials ratio of chlorinated polyolefin to ethylene glycol is about
11:1.5 to about 1:3, and more preferably about 1:1.63.
To the resulting admixture, a predetermined amount of the
amine, which is preferably amino-substituted alkanol, is added.
Preferably the starting materials ratio of chlorinated polyolefin to
amino-substituted alkanol is about 25:1 to about 40:1.
The components are admixed for about 3 to about 5 minutes
in a sealed mixing tank, or alternatively in a reflux condenser.
CA 02602282 2007-10-04
- 14 -
The chlorinated polyolefin is admixed with the
glycol surfactant admixture for an amount of time sufficient
to permit the chlorinated polyolefin to melt. The temperature
of the admixture is preferably maintained for at least about
minutes at a temperature of about 35 F above the melting
point of the chlorinated polyolefin. Thus, the temperature
should preferably be maintained at about 205 F to about 250 F,
and more preferably at about 210 F to about 240 F, again,
depending upon the melting point of the chlorinated poly-
10 olefin. The admixture then forms a molten mass. At this
point, preheated water is added to form an emulsion.
Water is added to the composition in three separate
steps. The first step preferably includes adding hot water
i.e. water at a temperature of 125 F to 160 F, in a starting
material ratio of chlorinated polyolefin to water of about
2.5:1 to about 4.5:1 and more preferably about 3.4:1. The
composition is agitated. As the hot water becomes absorbed
into the molten mass, an additional amount of hot water is
then added under continued agitation. Preferably the second
addition of hot water is added in a starting material ratio of
chlorinated polyolefin to water of about 2.5:1.0 to about
4.5:1.0, and more preferably about 3.4:1. At this point an
inversion preferably takes place.
Throughout the water additions, the temperature of
the materials admixture is preferably maintained at a tempera-
ture of about 20 F above the melting point of the chlorinated
polyolefin. Thus, it is maintained at about 165 F to about
210 F, and more preferably in the range of about 195 F to
about 210 F, depending upon the melting point of the chlori-
nated polyolefin. To the resulting admixture, and after the
second amount of water has been absorbed by the molten mass, a
third amount of water is added under increased agitation.
Preferably the amount of the third addition of water is suffi-
cient for the addition to have a chlorinated polyolefin to
water starting material ratio of about 1:33 to about 1:40, and
62406-168
CA 02602282 2007-10-04
- 14a -
more preferably about 1:30 to about 1:35. The amount of water
preferably yields a final viscosity at room temperature in the
range of about 25 to about 50 CPS. The viscosity can be
adjusted by changing the amount of water added, or by adding a
suitable amount of a thickener to the resulting material. A
suitable amount of a filler may also be added to the resulting
material. Further, the resulting admixture has a volatile
organic content of less than about 5%, and preferably about
3.5%. It is also preferably substantially free of volatile
organic solvents or components. In a highly preferred embodi-
ment, the organic volatile content is less than about 0.5%.
The methods of the present invention may also
comprise contacting a prepared (e.g., cleaned and dried)
olefinic-based
62406-168
CA 02602282 2007-10-04
V. ~6/40819 PCTIUS96/09466
- 15 -
surface, such as the surface of a thermoplastic polyolefinic substrate
(e.g., polypropylene), with a composition as described above.
The above-described compositions and methods are useful
for producing a water-based coating composition suitable for coating
a variety of substrates. Without limitation, the compositions are
suitable for coating plastics, wood, ceramic, metal, wallboard and the
like. Particularly useful applications include coating the present
coating compositions onto a plastic substrate such as a polyolefinic
substrate, including polypropylene substrates and thermoplastic olef in
substrates. The present water-based compositions are especially
useful as primer compositions because of their ability to adhere well
to heretofore difficult substrates, such as polyolefinic substrates,
including polypropylene.
After suitable preparation of a substrate surface, the
compositions may be applied to a substrate surface in any suitable
manner including, without limitation, spraying, dipping, brushing,
rolling, and flow-coating methods.
Moreover, it is contemplated that one or more conventional
additives may be included in the present compositions. For instance
it is possible that pigment for coloration purposes may be added.
Moreover, as indicated above, thickeners such as a functional
polyacrylate, available commercially as Alcoqum L-31' , manufactured by
Alco Chemical, can be added in suitable proportions to control
viscosity and flow of the material. A filler may be employed in
suitable proportions in the composition of the present invention, and
can be any suitable conventional filler material for plastic materials
including, but not limited to calcium carbonate, silicates or the
like.
Also, it is possible to add materials, such as carbon
black, and conductive pigments, to render the resulting composition
conductive for purposes of electrostatic coating application
techniques. When an additive that includes carbon black is added to
the above-described coating compositions or systems, it is preferred
that an additive mixture of carbon black, glycol, surfactant and water
is mixed at high speed, dispersed on an apparatus such as a sandmill,
and then added to the above-described coating compositions.
More preferably the additive mixture includes about. 20%- to
about 25% by weight carbon black, such as commercially available Cabot
XC-72R'", about 15sk to about 2551. by weight ethylene glycol, about i$ to
about 3% by weight of a nonylphenoxy polyethoxy ethanol surfactant,
and about 50t to about 65t by weight water. After mixing and
grinding, the additive is added to the coating composition in a ratio
of approximately 9.5 parts additive to 100 parts emulsion.
ALTERNATIVE PREFERRED ENffiODIMEbiT
CA 02602282 2007-10-04
V., 96/40819 PCT/US96/09466
- 16 -
WITH CARBON BLACK DISPERSION
In a preferred embodiment, the coating compositions and
systems of the present invention further include a carbon black
dispersion. The carbon black dispersion incorporates (1) a first
carbon black concentrate composition, which includes a first adhesion
promoter composition and carbon black, admixed with (2) a second
adhesion promoter composition, which includes an aqueous admixture of
a surfactant, an amine and a resin, preferably a chlorinated
polyolefin resin.
The carbon black dispersion of the present preferred
embodiment employs the following as the first adhesion promoter
composition, expressed as parts by weight (on a dry basis for solids-
containing materials):
Preferred More
Component Amount Amount Preferred
Amount
Surfactant about 3 to about 5 to about 5
about 25 parts about 12.5 parts
parts
Amine about 3 to about 4 to about 6.22
about 9 parts about 7 parts parts
Resin about 35 to about 60 to about 75
(preferably a about 150 parts about 90 parts parts
chlorinated
polyolefin
resin}
Water about 300 to about 350 to about 431
about 500 parts about 450 parts parts
To obtain the desired first carbon black concentrate
composition, approximately 12 to about 25 parts, preferably about 15
to about 22 parts, and more preferably about 19.25 parts of carbon
black is added into approximately 40 to about 75 parts, preferably
about 55 to about 65 parts, and more preferably about 61.5 parts, of
the first adhesion promoter composition. Adjustments are made, such
as by additions of suitable amounts of resin, surfactant or both, in
order to yield the first carbon black concentrate composition.
Preferably, after the adjustments, the first carbon black concentrate
composition includes about 40 to about 75 parts, preferably about 55
to about 65 parts, and more preferably about 61.5 parts, of the first
adhesion promoter composition; about 12 to about 25 parts, preferably
about 15 to about 22 parts, and more preferably about 19.25 parts, of
carbon black; and about 15 to about 25 parts, preferably about 15 to
about 22 parts, and more preferably about 19.25 parts, of a
surfactant-water blend (about 20 percent surfactant in water); and has
CA 02602282 2007-10-04
' Wli o6/40819 PCTIUS96/09466
- 17 -
a total solids content of about 12 to about 45 parts, preferably about
20 to about 40 parts, and more preferably about 37.74 parts, by
weight. The solids include about 12 to about 25 parts, preferably
about 15 to about 22 parts, and more preferably about 19.25 parts, of
carbon black; about 10 to about 25 parts, and more preferably about
14.64 parts, of the resin; and about 1 to about 6 parts, and more
preferably about 3.85 parts, of the surfactant. Additionally, a
suitable amount (e.g., about 0.1 parts by weight) of a suitable
antifoam agent, such as isopropyl alcohol, is incorporated into the
first carbon black concentrate composition.
The first carbon black concentrate composition is admixed
with a second adhesion promoter composition, which includes the first
adhesion promoter composition (or one substantially identical thereto)
preferably further diluted with water in the below-indicated amount,
and any additional additives employed to yield a final carbon black
dispersion. In one preferred aspect, the final carbon black
dispersion includes, expressed as parts by weight (on a dry basis for
solids-containing materials):
CA 02602282 2007-10-04
W v 96/40819 PCT/US96/09466
- 18 -
Preferred More
Component Amount Amount Preferred
Amount
First adhesion about 325 to about 350 to about 450
promoter about 500 parts about 475 parts parts
composition
First carbon about 20 to about 30 to about 42
black about 60 parts about 45 parts parts
concentrate
composition
Water about 325 to about 350 to about 430
about 500 parts about 475 parts parts
In a further preferred embodiment, the final carbon black
dispersion also includes a thickener and ammonium hydroxide in the
following relative amounts, expressed as parts by weight (on a dry
basis for solids-containing materials):
Preferred More
Component Amount Amount Preferred
Amount
First adhesion about 325 to about 350 to about 450
promoter about 500 parts about 475 parts parts
composition
First carbon about 20 to about 30 to about 42
black about 60 parts about 45 parts parts
concentrate
composition
Thickener about 15 to about 25 to about 30
(e.g., Alcoqum about 40 parts about 35 parts parts
L-31)
Ammonium about 1 to about 2 to about 4
hydroxide (26 ) about 6 parts about 5 parts parts
Water about 325 to about 350 to about 430
about 500 parts about 475 parts parts
Examples of suitable surfactants, amines, resins and other
additives employed in the first adhesion promoter composition and,
thus, employed also in the first carbon black concentrate composition
and in the second adhesion promoter composition, have been discussed
in detail elsewhere herein. In a present highly preferred embodiment
the surfactant is Triton N-101'". Alternatively, it may be a
suxfactant available from Texaco under the designation N-85 or N-35.1.
A preferred amine is dimethylaminoethanol or dimethylethanolamine
(DMEA). A preferred resin is a chlorinated polyolef in resin, such as
CPO-343-1, supplied by Eastman Chemical Company, Kingsport, Tennessee.
A highly preferred carbon black is available commercially from Cabot
CA 02602282 2007-10-04
62406-168
- 19 -
Corporation, Special Blacks Division, under the designation vulcan+"XC72R. Of
course, other suitable tyoes or sources of material may be
employed in accordance with the teachings herein.
In a highly preferred embodiment, which includes a carbon
black dispersion, the carbon black dispersion is made by admixing the
first adhesion promoter composition and carbon black, shot milling the
first adhesion promoter composition and carbon black to result in the
first carbon black concentrate composition, and admixing the milled
material of the first carbon black concentrate composition with the
second adhesion promoter composition. Preferably, milling of the
first carbon black concentrate composition begins at a temperature of
about 20 to about 30 C, and more preferably about 29 C, and will rise
during milling to a temperature of about 35 to about 50 C, and more
preferably about 40 C or higher. The admixture is shot milled, in a
suitable mill (e.g., a horizontal shot mill, such as that commercially
available from Chicago Boiler Co. (CB Mills Division), under the
product name Dyno-Mill) for an amount of time sufficient to achieve a
grinding of the carbon black so that about 950 of it could pass
through a 10 micron (absolute) filter.
2D It is observed that a relatively rapid temperature
increase in the mill, potentially to as high as about 60 C, takes
place during this milling step. The mill optionally is cooled by
running kerosene through a jacket enveloping at least a portion of
the mill.
Preferably the carbon black is milled for a sufficient
time that the desired particle size can be obtained without
sacrificing any of the efficacious properties (e.g., its conductivity)
of the material. In one embodiment, no more than two passes are
employed for milling.
Throughout the present steps, viscosity of the first
carbon black concentrate composition is monitored and adjusted through
suitable amounts of water additions (to render less viscous) or
thickener additions (to render more viscous), in order to maintain a
viscosity level of about 13 sec (#3 Zahn). The pH of the admixture is
also monitored and controlled to maintain it at about pH 7 to about
10, and more preferably about pH 9Ø The pH can be controlled
through additions of sufficient quantities of amine (or even ammonium
hydroxide or the like) to render the admixture more basic, or carbon
black to render the admi-xture more acidic.
.4 D Optionally, as indicated, suitable amounts of an anti-
foaming agent may be added to control foaming. Isopropyl alcohol is
an example of one such type of agent. It is added in an amount of
about 0.1 parts by weight of the first carbon black concentrate
comDosition.
FTrade-mark
CA 02602282 2007-10-04
1+- 16/40819 ' PCT/US96/09466
- 20 -
After the first carbon black concentrate composition has
been milled for its desired time (for example, for a sample having a
total weight of about 150 pounds, a milling time of about 8 minutes is
used), it is admixed with the second adhesion promoter composition of
the present preferred embodiment. This latter admixing step
preferably takes place in a suitable mixing vessel (e.g., a suitable
stainless steel or polypropylene vessel), at any suitable temperature,
and for a sufficient amount of time to assure a substantially
homogeneous admixture. For example, for an admixture of about 3500
1D pounds, a starting temperature of about 25 C, a time of about 60
minutes is employed. The time of mixing, of course, will vary with
such factors as the temperature, the volumes of materials, and the
like. Also, during this latter admixing step, water is added in
sufficient quantities to obtain the ultimate desired concentration of 15
materials in the resulting adhesion promoter composition.
The resulting preferred carbon black dispersion of the
present preferred embodiment can be admixed with the other components
of the present compositions and systems by adding the carbon black to
the composition (either with or without the polyurethane/acrylic
20 dispersion) and slowly mixing for approximately 10 to 15 minutes, at
an ambient temperature. In a preferred embodiment, ammonium hydroxide
is then added to control the pH of the composition and a thickener
such as L-31 supplied by Alco Chemical is also added.
The preferred primer composition of the present invention,
25 which includes carbon black, can be applied to a substrate and dried
using conventional applying and drying techniques, such as, without
limitation, those described herein. In a highly preferred embodiment
the final primer composition is coated onto a surface of a
thermoplastic polyolefin (TPO) article. For coating a typical TPO
30 substrate (which may be a virgin material or even a regrind) a primer
thickness of about 0.2 to about 0.4 mil is employed. Smaller or
greater thicknesses may be employed. The resulting coated TPO
substrate is thereafter further coated.
in a particularly preferred aspect of the present
35 embodiment, the surface of the TPO article coated with the resulting
primer composition is further coated with a second layer of material
(e.g., a top coat), and then dried at a suitable temperature, for a
suitable amount of time (e.g., about 160 F to about 185 F for about 30
minutes, for a two-component polyurethane top coat thickness of about
40 1.5 mils). The second layer of material preferably includes a
polyurethane, such as, without limitation, a two-component
polyurethane paint system. Resulting coated substrates exhibit
excellent paint surface and adhesion characteristics, including
surface smoothness, gloss, adhesion, weatherability (e.g., humidity)
45 and thermal cycling.
CA 02602282 2007-10-04
= 162406-168
- 21 -
without intending to be bound by theory, it is believed
that upon molding of articles of typical TPO materials, the surFace of
the molded article comprises a boundary layer having a substantially
high-crystallinity content by comparison with the relatively
substantially amorphous nature of the material toward the central
portions of the article. The relatively high crystallinity is
believed to inhibit adhesion of a coating applied to a surface of the
molded article, absent a step of eliminating the boundary layer by
high temperature heating (e.g., about 200 F to about 250 F, for at
least two minutes) . The present preferred primer composition is
believed to interact with and modify the surface characteristics of
the aforenoted crystalline boundary layer without the need for a
separate high temperature heating step, and thus permits obtention of
a high quality surface finish and excellent adhesion of the primer
composition to the molded article even using conventional drying steps
(e.g., without limitation, not exceeding a temperature of about
185 F) -
ALTERNATIVE PREFERRED EMSODIMENT WITH
POLYIIRETHANE/ACRYLIC DISPERSION
In one preferred embodiment, the coating compositions and
systems of the present invention further include a mixture comprising
an acrylic and a polyurethane, more preferably, a polyurethane/acrylic
dispersion. The polyurethane/acrylic dispersion preferably uses a low
co-solvent level and is externally crosslinked by post addition. The
polyurethane/acrylic dispersion is preferably grafted. An example of
a preferred polyurethane/acrylic dispersion of the present invention
is UPACO WL-305XL supplied by Worthen industries, Inc., Nashua, NH.
UPACO WL-305XL contains about 50% solids, has a VOC of
3D about 3%, an aliphatic polyurethane/acrylic co-solvent,
and a pH of about 7.5 to about 8Ø
The polyurethane/acrylic dispersion of the present
invention is preferably present in an amount of about 10 to about 45
percent, more preferably about 12 to about 40 percent, still more
preferably about 14 to about 20 percent, and still more preferably
about 14.7 percent of the total weight of the final composition or
system.
The polyurethane/acrylic dispersion is admixed with the
other components of the present compositions and systems by adding the
polyurethane/acrylic dispersion to the composition and mixing at
ambient temperature for approximately 10 to 15 minutes. In a
preferred embodiment, one or more suitable additives such as ammonium
hydroxide is added to control the pH of the composition. A thickener
such as a urethane-based or acrylic-based thickener, and in
CA 02602282 2007-10-04
WO 96/40819 PCT/US96/09466
- 22 -
particular, Alcoqum L-31' , supplied by Alco Chemical, Chattanooga,
Tennessee, may also be added.
APPLICATIONS
Because of the unexpected adhesion characteristics
resulting from the composition of the present invention, the
composition is particularly attractive in many adhesive applications.
By way of illustration, the composition of the present invention may
be employed as an adhesive promoter to enhance the adherence of
numerous types of materials to other similar or dissimilar materials
to form multi-layer articles. One preferred combination of materials
includes adhering to a substrate using the composition of the present
invention a material selected from acrylics, rubbers, urethanes,
epoxies, vinyls or mixtures thereof. A particularly attractive
combination of materials for making multi-layer articles includes
thermoplastic polyolefin (TPO) adhered to another layer of TPO or to
a material such as that containing an acrylic, styrene-butadiene
rubber, polyurethane, epoxy, nitrile butadiene rubber, polyvinyl
butyral, and mixtures thereof. Further, the composition is useful to
bond materials that contain pigment.
in general, for making the above articles, the composition
of the present invention is coated or applied to a desired thickness
on either of the materials to-be-joined. The materials are then
coupled. The resulting articles are baked at a suitable temperature
preferably in the range of about 70 C to about 800C for a time
sufficient to dry the composition of the present invention (e.g. for
about 1 hour or longer).
In a particularly preferred embodiment, to prepare multi-
layered articles a latex (or emulsion) is prepared, using conventional
methods, having as its base material a material containing an acrylic,
styrene-butadiene rubber, polyurethane, epoxy, nitrile butadiene
rubber, natural rubber, polyvinyl butyral, or mixtures thereof. A
substrate is coated with the composition of the present invention.
The latex is then applied over the coated substrate, and the entire
article is treated as described previously.
In another embodiment the materials described above; that
is, the latex and the composition of the present invention, are
admixed together in suitable proportions prior to coating the
substrate. The admixture then is applied to the substrate and can be
processed according to the above-outlined steps.
In another particularly preferred embodiment two or more
thermoplastic polyolefin substrates are bonded together by using the
composition of the present invention as an adhesive, either alone or
in combination with other conventional adhesives (e.g. a polyurethane
adhesive).
CA 02602282 2007-10-04
' 62906-168
- 23 -
The composition of the present invention also finds
utility as a primer for several adhesive systems. That is, when used
in combination with known adhesives the resulting materials have
improved adhesion characteristics as compared with using such adhesive
by itself. In general, for this aspect of the invention, articles to
whs.ch adhesive is to be applied are first coated with a"primer" layer
of the composition of the present invention. The adhesive is then
applied to the primer layer and can be bonded to other articles. The
adhesive is cured and the primer layer is dried. It may also be
possible to admix the adhesive with the composition of the present
invention and then apply the resulting admixture to an article to be
joined. Preferred adhesive systems for use with the composition of
the present invention include those selected from the group consisting
of acrylics, epoxies, polyurethanes, silicones, and mixtures thereof.
2.5 The compositions of the present invention including a
carbon black dispersion, are particularly suitable as primers for
thermoplastic polyolefin surfaces. In such embodiment, the carbon
black dispersion is particularly useful to obtain superior adhesion
results between a primed surface of an article and a top coat or paint
coating (more preferably a two-component polyurethane paint system
coating) disposed on the primed surface. Particularly preferred two-
component polyurethane paint systems are available commercially from
any of a variety of suppliers including but not limited to PPG
Industries Automotive Products (DMT-DBU is preferred); Morton
International Inc. Bee Chemical (2YB-2KC is preferred); and Akzo
Coatings Inc.
The coatings and systems of the present invention
including the polyurethane/acrylic dispersion are particularly
suitable as a weatherable primer.
3D In another application, the composition of the present
invention is useful, by itself, as an adhesive for bonding a substrate
to a foam, such as a polyurethane foam (rigid, flexible, or both).
Preferably the composition of the present invention is applied to a
substrate and before the composition is dry, a polyurethane is foamed
in place, using knovm methods, over the composition of the present
invention.
Substrates useful in the above applications include not
only substrates of a substantially homogeneous material, but may also
include blends or admixtures of materials, reinforced material (i.e.,
composites) or the like. An example of a particularly attractive
reinforced material is a thermoplastic polyolefin substrate reinforced
with fiberglass. For instance, without limitation, "glass filled
polypropylene" like that supplied, typically in sheet form, by Er.3:on
Corporation under the trade designation TAFFEIQ*, or AZDEL, supplied by
4S 7-LZDEL, INC. is useful.
*Trade-marr
CA 02602282 2007-10-04
62406-168
- 24 -
It should also be noted that the compositions of the
present invention can be incorporated into coating formulations such
as paints and inks to provide a material suitable for simultaneously
priming and coating a substrate surface.
The following examples are illustrative of the described
invention.
EXAMPLE I
Ethylene glycol in the amount of 110 grams is admixed with
17 grams of surfactant, such as Triton N-101'M. The admixture is
heated to 210 F under agitation. When the product reaches 210 F, 67.5
grams of a chlorinated polyolefinic resin, such as CPO-343-1 (100
percent) , is mixed in until the chlorinated polyolefin melts and
disperses. The temperature is maintained at about 210 F for about 10
minutes. At that time, 2.1 grams of an amine, such as a 2-amino-2-
methyl-l-propanol (AMP 95'") is added. The mixture is mixed for three
to five minutes to increase temperature to 240 - 250 F. A hot water
supply is heated to about 140 F and is maintained at about 140 F
throughout the several water additions. Twenty grams of the hot water
is added to the admixture at a slow rate. As the hot water becomes
absorbed into the molten mass, agitation is increased. Upon
absorption of the hot water, 20 additional grams of the hot water from
the hot water supply is slowly added to the molten mass. Temperature
is maintained at about 200 - 210 F. Agitation is increased, and 1970
grams of hot water, from the hot water supply, is added to the
admixture.
The coatings are then tested for adhesion by spraying on
a polypropylene panel with the above emulsion and drying the coating
for a time of about 10 to about 15 minutes at a temperature of about
160 F. to about 165 F. and even as high as about 175 F. The coated
3D surface of the substrate can be coated with a suitable top coat and
cured for about 15 minutes at 160 F. and cooled. Substantially
similar results using longer times and higher temperatures also are
contemplated (e.g. about one hour at about 25D F.). The coated
surface is then cross-hatched with a sharp blade and tested with
pressure sensitive adhesive tape (3M 610 or equivalent) by firmly
pressing the tape over the crosshatched region and then quickly
removing the tape. Upon removal of the tape, adhesion to the
substrate of about 95o to 1000 of the coating is observed.
Satisfactory test results are also demonstrated using alternative
testing methods of the type including test method GM 9502P, described
in a July, 1988 publication by General Motors entitled Engineering
Materials and Processes, Procedures Manual; and test method ASTM D3359 B.
Substantially similar results may be obtained by variation
of the above according to the teachings of the present disclosure.
CA 02602282 2007-10-04
WO 96/40819 PCTIUS96/09466
- 25 -
For example, the AMP 95'" may be replaced, in whole or in part, with
another form or brand of 2-amino-2-methyl-l-propanol; ethanolamine;
dibutylamine; N-ethyldiethanolamine; tris-(3-aminopropyl)amine;
dimethylethanolamine triethylamine; diethanolamine;
dimethylaminoethanol;2-dimethylamino-2-methyl-l-propanol;DMAM P-80'";
Jeffamine' M600, D230, D400, D2000, ED600, ED900, ED2001, ED4000,
ED6000, DV7000 or T3000; mixtures may also be employed. Thickeners,
filler, or both, likewise may be added to the composition in suitable
proportions to achieve substantially similar results.
Further, the levels of the various components may be
varied. For example, the level of surfactant and/or glycol can be
reduced by 50t.
The following are two preferred compositions of the
present invention which may be made according to the above directions:
25 lbs. ethylene glycol, 25 lbs. Triton N-101 ", 100 lbs. CPO-343-1,
6.22 lbs. AMP 95TM and 350 lbs. water; and, 12.5 lbs. ethylene glycol,
lbs. Triton N-101", 100 lbs. CPO-343-1, 6.22 AMP 95'" and 370 lbs.
water.
EXAMPLE II
20 A polyol such as ethylene glycol, a surfactant, an amine
and a resin, are mixed for example, in a reactor with two shafts or in
a conventional high speed mixer., and heated to about 20 F above the
melting point of the resin, e.g., about 245 F to about 2500F. When
the mixture reaches about 240 F, the mixing is stopped and the
25 admixture is examined to assure a homogenous melt, e.g., clarity and
elongation to a fine molten thread, should be observed. A hot water
supply is heated to preferably about 165 F to about 195 F and is
maintained at this temperature throughout the several water additions.
The hot water is added to the admixture at a slow rate. As the hot
water becomes absorbed into the molten mass, agitation is increased.
When the admixture becomes thin, the process is complete.
It will be noted that in one preferred embodiment, the
resin is tested prior to use for its ability to be dispersed and
emulsified. Those skilled in the art will appreciate that this test
may be performed on a small scale, e.g., in a laboratory in a glass
vessel.
EXAMPLE III
Multilayer articles are formed that comprise a layer of
latex and a thermoplastic polyolefin (TPO) layer or substrate. The
latex is coated onto a TPO substrate. Several samples are prepared
using different base materials for the latex. Some of the TPO
substrates, however, are coated to a thickness of up to about 1 mil,
with an intermediate layer between the latex and substrate (i.e.,
"primer" layer) having the coating composition of Example I, and
further containing a thickening agent. Some samples are not coated
CA 02602282 2007-10-04
,62406-168
- 26 -
with the "primer" layer. The articles are baked at about 80 C for
about 1 hour and then aged at about room tem'oerature for about 24
hours. The articles are then tested for adhesion of the latex.
To test the articles, two test methods (X-scribe tape
adhesion test and 180 peel strength test -- ASTM D903-49) are used.
For the X-scribe tape adhesion test, two diagonally intersecting lines
of about 5 cm in length are cut, using a sharp razor blade, in the
layer of the latex. Permacel * brand 703 masking tape is firmly
attached to the cut surface. The tape (or its equivalent) is then
pulled off rapidly, by pulling it back upon itself at any angle of
close to about 180 . The "X-cut" area is visually inspected and the
adhesion of the latex layer is rated according to the following
standards:
Desiqnation Observation
SA No peeling or removal of latex
4A Trace peeling or removal of latex along
incisions
3A Jagged removal of latex along incisions up to
1.6 mm on either side
2A Jagged removal of latex along incisions up to
3.2 mm on either side
lA Removal of latex from most of the area of the
"X" under the tape
OA Removal of latex beyond the area of the "X"
For tests conducted according to the 180 peel strength
test, prior to testing, testing samples are baked overnight (e.g. at
least about 12 hours) at about 70 C, rather than baking at about 80 C
for about 1 hour and aging at about room temperature for about 24
hours.
rTrade-mark
CA 02602282 2007-10-04
W.. >6/40819 PCT/[JS96/09466
- 27 -
The results are summarized in Table I.
TABLE I'
Latex 1800 Peel Strength (lb/in) X-Scribe tape adhesion
Coatings With primer without primer With primer Without primer
Acrylics
RES 2301 1.1 (A) 0.8 (A) OA OA
RES 1019 14.5 (C) 2.4 (A) 5A OA
A 1052 8.1 (C) 2.1 (A) 5A OA
Styrene
Butadiene
Rubber
(SBR) 15.1 (C) 1.9 (A) 5A 1A
Polyurethane
(PU) 12.3 (C) 1.3 (A) 5A 1A
Epoxy 7.6 (C) 5.4 (C) - OA
Natural
Rubber (NR) 3.2 (C) 4.3 (A) OA OA
Nitrile
Butadiene
Rubber
(NBR) 2.1 (C) 0.5 (A) 5A OA
Polyvinyl
Butyral
(PVB) 5.6 (C) 1.3 (A) OA OA
Acrylics RES 2301 and RES 1019 are supplied by Unico
Chemical Division. A 1052 is supplied by ICI Chemical Corp.
EXAMPLE IV
An emulsion (referred to in this example as an "adhesion
promoter") having the coating composition of Example III is prepared
and is admixed with the latex materials of Example III prior to
applying to a substrate. The emulsion is added into the latex at
about 209. by weight (based on 10096 solids of both the latex and
emulsion). A thermoplastic polyolefin substrate is coated with the
resulting admixture to a thickness of up to about 1 mil. Dried and
cured samples are tested and the results are summarized in Table II.
'The letters in parentheses indicates that (A)dhesive damage, or
(C)ohesive bonding is observed. By adhesive damage it is meant that
the tape removes both the adhesive and latex from the substrate. By
cohesive bonding it is meant that latex and primer remain on the
substrate after tape removal.
CA 02602282 2007-10-04
,62406-168
- 28 -
TF.BLE 2
1800 Peel StrenGth (lb/in) X-Scribe *atDe adhesion
With adhesion Without adhesion With adhesion Without adhesion
Latex promoter promoter promoter promoter
S
Acrylics
RES 2301 3.2 (C) 0.8 (A) 3A OA
RES 1019 4.1 (C) 2.4 (A) SA OA
A 1052 5.6 (C) 2.1 (A) 5A OA
SBR 3.5 (C) 1.9 (A) SA lA
PU 3.0 (C) 1.3 (P_) SA lA
Epoxy - 5.4 (C) - OA
NR 3.5 (A) 4.3 (A) SA OA
NBR 3.5 (C) 0.5 (A) 5A OA
PVB 5.9 (C) 1.3 (A) OA OA
EhAMPLE V
Two articles are prepared for each adhesive system of this
example. The articles have a layer of adhesive and a thermoplastic
polyolefin substrate. For one of the articles for each adhesive
system, a"primer" layer having a thickness of up to about 1 mil and
the coating composition of Example III lies between the adhesive and
3D the substrate. For the other article the adhesive lies directly on
the substrate, without the "primer" layer. Four adhesive systems are
employed and are listed as follows:
(1) acrylic based adhesive (such as that supplied by
Boston S.P.A. under the trade designation of Gemini Adhesive);
(2) epoxy-based adhesive (formulated by admixing at about
a 1/1 equivalent ratio, D.E.R. 332 bisphenol-type epoxy supplied by
Dow Chemical Co., and Ancamine, an aliphatic amine curing agent,
supplied by Pacific Anchor Co.);
(3) polyurethane-based adhesive (formulated from a
4D reaction using an aliphatic polyisocyanate (e.g. Desmodur* N-100,
supplied by Mobay Chemical) as a starting material, which is admixed
with an aromatic diamine (e.g. Ethacure 300, supplied by Ethyl Corp.)
at an equivalent ratio of about 1/1);
(4) silicone-based adhesive, such as one component room
temperature curable RTV silicone rubber adhesive sealant (supplied by
GE).
The adhesives are cured, and the test samples are aged at
about room temperature for about 24 hours. The samples are then
tested for lap shear strength, according to test method ASTM D 1002.
The results are summarized in Table 3.
*Trade-mark_
CA 02602282 2007-10-04
62906-168
- 29 -
TP?LE 3
Lap Shear Strength, psi
P.dhesive
S System With primer Without primer
Acrylic 29S 93
Epoxy 368 172
Polyurethane >S74* 131
Silicone 115 76
*The thermoplastic polyolefin panel is observed to be broken, but the
adhesive remains in tact.
EXAMPLE VI
The composition of Example III is used as an adhesive for
adhering polyurethane foams (both rigid and flexible foams) to a
reinforced thermoplastic polyolefin substrate, such as a thermoplastic
polyolefin substrate reinforced with fiberglass.
A flexible foam (e.g. having a density of about 4.0 lb/ft')
is made by reacting suitable amounts of Isocyanate tt80 (supplied by
~
BP.SF), Pluracol 380 (from BASF), water, a catalyst such as that
supplied by Union Carbide under the trade designation Niar.*C-174, a
catalyst such as that supplied by Air Products under the trade
designation Dabco* 33LV, diethanolamine, and a silicon surf actant like
DC-190 (supplied by Air Products).
A rigid foam (e.g. having a density lower than the flexible
foam), is made by reacting suitable amounts of an aromatic
* *
polyisocyanate such as PAPI-27 (supplied by Dow Chemical), Pluracol
824 (supplied by BASF), water, dibutyltin dilaurate, such as T-12
(supplied by Air Products), Dabco 33LV and a silicon surfactant such
as Dow Corning;-193 (supplied by Air Products).
Both the flexible and rigid foams are f.oamed-in-place
(using conventional methods) on the reinforced thermoplastic
polyolefin substrates. The resulting layers of foam have a thickness
of about 1/2 inch. However, one sample of each foam-type has a
"primer" layer, having a thickness of up to about 1 mil and the
coating composition of Example III, lying between the foam and the
thermoplastic polyolefin substrate.
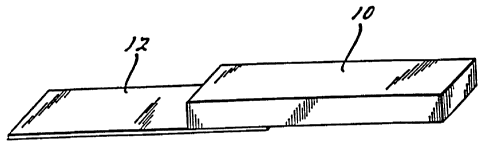
Articles comprising the rigid foam are tested for lap shear
strength by a method like test method ASTM D1002 as shown in
F'ig. la. Articles comprising the flexible foam are tested for 180
peel strength by a method like test method ASTM D903-49, as shown in
4' Fig. lb. In Figs. la and lb the foam is designated generally by
reference numeral 10, ar;d the substrate by reference numeral 12.
Fig. lb illustrates how the samples are tested by showing results of
force that is applied to test the samp'_es.
*Trade-mark
CA 02602282 2007-10-04
W,, Y6140819 PCT/US96/09466
- 30 -
TABLE 4
With primer Without primer
Rigid foam
Lap shear strength 25.9 13.0
(psi)
Flexible foam
180 peel strength 6.6 (adhesive strength
(psi) too weak to test)
EXAMPLE VII
Two types of polypropylene sheets (one with pigment, and
one without) each having dimensions of about 1 inch by about 6 inches
by about 0.04 inches thickness are coated with a primer layer having
a thickness of up to about 1 mil and the coating composition of
Example III, and are then baked at about 75 C for about 30 minutes.
The polypropylene sheets are then bonded together using the
polyurethane adhesive of Example V. The adhesive is cured by baking
at about 70 C for about 30 minutes. The samples are then aged at
about room temperature for about 24 hours and are tested for lap shear
strength by a method like test method ASTM D1002. The results are
summarized in Table S.
TABLE 5
Sample Type of Sheets Lap Shear Imperfections
Strength, psi Observed
1 Polypropylene Sheets 14.7 Adhesive not
without primer intact
2 Polypropylene Sheets >72 Sheet deformed
.with primer only; adhesive
intact
3 Polypropylene Sheets (2% Adhesive not
pigment) without primer 14.1 intact
4 Polypropylene Sheets (2% Sheet deformed
pigment) with primer >54 only; adhesive
intact
EXAMPLE VIII
Surfactant, such as Triton N- 101", in the amount of 17
grams is heated to 210 F under agitation. When the product reaches
210 F, 67.5 grams of a chlorinated polyolefinic resin, such as CPO-
343-1 (100 percent), is mixed in until the chlorinated polyolefin '
melts and disperses. The temperature is maintained at about 210 F for
about 10 minutes. At that time, 2.1 grams of an amine, such as a 2-
amino-2-methyl-l-propanol (AMP 95 1) is added. The mixture is mixed
CA 02602282 2007-10-04
Vti.. A/40819 PCT/US96/09466
- 31 -
for three to five minutes to increase temperature to 240 - 2500F. A
hot water supply is heated to about 140 F and is maintained at about
140 F throughout the several water additions. Twenty grams of the hot
water is added to the admixture at a slow rate. As the hot water
becomes absorbed into the molten mass, agitation is increased. Upon
absorption of the hot water, 20 additional grams of the hot water from
the hot water supply is slowly added to the molten mass. Temperature
is maintained at about 200 - 210 F. Agitation is increased, and 1970
grams of hot water, from the hot water supply, is added to the
admixture.
The following is a preferred composition of the present
invention which may be made according to the above directions: 25
lbs. Triton N-101'", 100 lbs. CPO-343-1, 6.22 lbs. AMP 95'" and 874.5
lbs. water.
In a preferred embodiment, a polyurethane/acrylic
dispersion described above, such as UPACO WL-305XL, is added to the
above-described admixture (after the admixture is cooled) and mixed
for about 10 to 15 minutes. Ammonium hydroxide is then added to
control pH and a thickener such as L-31 is added. The mixture is then
mixed for about 20 minutes.
In another preferred embodiment, for example where a highly
conductive coating is desired, the carbon black dispersion, described
above, is added before the addition of the ammonium hydroxide and
thickener. The mixture is slowly mixed for about 10 to 15 minutes at
ambient temperature. Ammonium hydroxide and thickener are then added
as described above.
In a highly preferred embodiment, containing a carbon black
dispersion, the weight of the above components based on total wet
weight of the composition is as follows:
Admixture (described above): 67.12%
Polyurethane/acrylic dispersion: 16.550
Carbon black dispersion (described above): 14.48%
Ammonium hydroxide: .120
Thickener: 1.73g
In a highly preferred embodiment that does not contain a
carbon black dispersion, the weight percent of the above components
based on total wet weight of the composition is as follows:
Admixture (described above): 78,48%-
= Polyurethane/acrylic dispersion (described above): 19.35%
Ammonium hydroxide: .14e
Thickener: 2.03%
CA 02602282 2007-10-04
~. ., 96/40819 PCT/US96/09466
- 32 -
Substantially similar results may be obtained by variation
of the above according to the teachings of the present disclosure.
For example, as described in Example I, a suitable polyol such as
ethylene glycol may be added in accordance with the steps of Example
I. Further, the AMP 95' may be replaced, in whole or in part, with
another form or brand of 2-amino-2-methyl-l-propanol; ethanolamine;
dibutylamine; N-ethyldiethanolamine; tris-(3-aminopropyl)amine;
dimethylethanolamine triethylamine; diethanolamine;
dimethylaminoethanol;2-dimethylamino-2-methyl-l-propanol;DMAM P-80'0;
Jeffamine' M600, D230, D400, D2000, ED600, ED900, ED2001, ED4000,
ED6000, DV7000 or T3000; mixtures may also be employed. Thickeners,
filler, or both, likewise may be added to the composition in suitable
proportions to achieve substantially similar results.
Further, the levels of the various components may be
varied. For example, the level of surfactant can be reduced by 50%.
Although the invention has been described with particular
reference to certain preferred embodiments, variations and
modifications can be effected without deviating from the spirit and
scope of the invention as defined in the following claims.