Note: Descriptions are shown in the official language in which they were submitted.
CA 02602435 2007-10-09
WO 98/32412 PCT/US98/01310
"< <
1
BISTABLE SPRING CONSTRUCTYON FOR A STE_rIT AND OTHEP. ?1TEDYCAL
APPARATUS
Rack2mund Ofthhe Invention
There are several kinds of stents on the market with either balloon
expandable or self expanding function. Balloon expandable stents are generally
made
from a material that can easily be plastically deformed into two dircctions.
Before
insertion, the sten[ is placed around the balloon scction at the distal end of
a catheter and
pressed together to reduce the outer dimensions.
As soon as the stent is brought into the body in ttie proper axial position it
can be cxpanded and thereby plastically deformed by ptunping up the balloon.
In this
final position, the stent is at its largest diameter and should function to
support the
surrounding tissue, preventing an undrsired shape change into a much smaller
diameter,
at least locally.
Therefore, the stent needs to have sufficient rigidity in the radial
direction,
but also some flexibility in the axial direction when it is in the final
position_ Further, the
amount of material s}iould be as small as possible and in the inner surface of
the stent
should not obstruct the flow through the channel (e.g., for blood) or cause
too much
turbulence.
Problems that generally occur with these stents are as follows: After
compressing the stent to its smttllest diameter around the ba[loon, the stent
will always
have some elastic spring back to a slightly targer diameter, which can cause
problems
when the catheter is brought into the patient's body. Inaddition, the axial
friction
between balloon and stent can become so small that the stent slips off the
catheter.
Further, a larger size stent is typically a disadvantage.
A further piobIem is the so called recoil of these stents. This means that
after expansion by the balloon pressure, the outcr diameter will always become
slightly
stnaller as soon as the balloon is clef7ated. This dcgec of reeoil ean be as
niueh as 10%,
which can cause-migration of the stent.
A different type of stent is'made of a more or less elastically expanding
RECTIFIED SHEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
2
structure, which has to be held on the catheter by some external means. An
example
of this type is a stent that is held in its constrained state by a delivery
sheath, that is
removed at the moment that the stent should deploy to its natural form.
Some of these stents are made of shape memory material with either
superelastic behaviour or temperature sensitive triggering of the expansion
function.
A disadvantage of these self-expanding stents is the need for the delivery
sheath, causing a larger insertion diameter. The removal of the sheath also
requires a
sheath retraction mechanism, which has to be activated at the proximal end.
Most stents of both types further have the disadvantage of relatively large
length change during expansion and a poor hydrodynamic behaviour because of
the
shape of the metal wires or struts.
Another disadvantage of some stents is the positive spring rate, which means
that further expansion can only be achieved by higher balloon pressure.
The construction of prior stents is typically made in such a way that the
external forces, working on the stent in the radial direction, merely cause
bending
forces on the struts or wires of the structure.
For example, a unit cell of a Palmaz-Schatz-stent, as produced by Johnson &
Johnson Interventional Systems or the ACT One Coronary stent, produced by
Progressive Angioplasty Systems, Inc. has in its collapsed condition a flat,
rectangular
shape and in its expanded condition a more or less diamond-shaped form with
almost
straight struts (Palmaz-Schatz) or more curved struts (ACT-One).
The shape of the unit cell of such stents is typically symmetrical with four
struts each having the same cross section. In addition, the loading of the
cell in the
axial direction will typically cause an elastic or plastic deformation of all
of the struts,
resulting in an elongation of the unit cell in the axial direction. These unit
cells have a
positive spring rate. In stents based upon these unit cells the stability
against radial
pressure is merely dependent on the banding strength of the struts and their
connections.
Summary of the Invention
In one aspect, the invention provides an expandable tubular device comprising
one or more bistable cells, each cell comprising a rigid segment coupled to a
relatively flexible segment, each cell being capable of transitioning between
only a
CA 02602435 2007-10-09
2a
. .,
first stable state and a second stable state, wherein the device is expanded
by applying
a force thereto, and during expansion, the force required to move between the
first
stable state and the second stable state decreases when the cell expands
beyond an
intermediate transition point.
A new type of stent is described hereafter with a unit cell, having a negative
spring rate and a bistable function. Such a unit cell can also be used in
CA 02602435 2007-10-09
WO 98/32412 PCTIUS98/01310
3
other medical applications. This means that it has two configurations in which
it is stable
without the need for an extemal force to hold it in that shape. The unit cell
is formed
using at least two different sections. One section is less pliable than the
other one and
acts a relatively rigid support that hinders the shape change of the more
pliable section in
one direction. In the other direction the pliable section can be deformed, but
because of
the opposing force from the rigid section, the stability of the pliable or
flexible section is
strongly increased.
External forces in a direction perpendicular to the most pliable section are
distributed to the rigid section and the cross section of the pliable section
is merely
loaded in compression mode. This makes the construction much stronger than
prior
stents. In prior stents, all struts have generally the same cross section and
mechanical
properties and are merely used in the bending mode.
The construction of a stent, based upon this unit cell results in an
apparatus, that can easily be elastically compressed around the balloon by
finger pressure.
Below a certain critical diameter, the present stent snaps further to a
stable, smallest diameter, thus holding the deflated balloon firmly on to the
surface of the
catheter, with an insertion diameter that is as small as possible. An
additional sheath is
not required, but may be used for extra safety.
After the stent has been brought into the patient's body at the proper axial
position, the balloon can be inflated until the stent reaches its critical
elastic equilibrium
diameter. Slightly above this diameter the stent automatically expands further
to its final
largest diameter, where it reaches its maximum stability against radial
pressure. The
design enables a constant length large expansiori ratio, a reliable
expandability and/or a
small surface ratio.
A further embodiment of this invention is the possibility of a kind of
stepwise expanding stent with a range of stable diameters.
Another part of the invention is a stent with several external diameters
along its length, to adapt as good as possible to the shape of the body cavity
where it is
placed.
Another part of the invention is the possibility to modify the stress and
strain pattern in the unit cell by means of a heat treatment in such a way,
that the force
CA 02602435 2007-10-09
- ~ ... "~
-WO 98/32412 PCT/US98/01310
4
displ_acement characteristic of this unit cell becomes asymar:etrical or even
exhibits a
rnonostable instead of a bictable function, either yArnth the expanded
diarTleter or the
collapsed diameter being the most stable condition. =
Another embodiment of the invention is the modification of the geometry
of the cross section of some struts to achieve the symmetric or asymmetric
bistable or monostable force against displacement characteristics of a unit
cell. =
Another part of the invention is the use of one or more unit cells in other
medical applications such as, for example, an expander or a clip, either to
spread a body
cavity open or to clamp or hold a body part or some body tissue.
The invention is also directed to the use of the inventive stents in
conjunction with inventive expander rings to join together two vessels.
The invention is also directed to a bistable valve having a snap-action
bipositiorial unit cell and uses for the same, in particular, to prevent
incontinence.
The invention is also directed to multistable cells and their use in medical
devices.
Description of the Construction.
The construction of the present stent includes a series of elements with an
arrangement of unit cells that enable the stability in a special way. Each
unit cell exists
out of at least two distinct, mechanically connected sections with different
mechanical
behaviors. One section acts as a relatively rigid support for the more
flexible
counteracting section. The more flexible section is responsible for most, if
not all, of the
expansion of the stent. There are several ways -to manufacture a stent based
upon this
principle and it can be made from several materials, like polymers,
composites,
conventional metals or shape memory alloys with superelastic behavior or with
temperature sensitive behavior.
It can be made from an arrangement of wire or strip, welded together at
specific places. Another possibility is metal deposition in the desired
pattern onto a
substrate or the use of sintering of prealloyed powder.
A further method is making the stent from a tubular shaped starting
material, with a pattern of slits or slots made in the wall by means of
etching, grinding,
CA 02602435 2007-10-09
~-.
WO 98/32412 PCT/[JS98/01310
cutting (e.g., with a laser, water, etc.), spark erosion or any other suitable
method. The
pattern can also be made in a flat plate and then welded, brazed or crimped to
a more or
less cylindrical shape or a cylindrical mid section with two conical ends with
larger
diameters.
5
Brief Description of the Drawings
Fig. I shows the principle of a bistable mechanism;
Fig. 2 shows the force-displacement characteristic of the mechanism of
Fig. 1;
Fig. 3 shows another bistable mechanism with an asymmetric bistability;
Fig. 4 shows the force-displacement characteristic of the mechanism of
Fig. 3;
Fig. 5a shows an inventive tubular stent in the stable, fully collapsed
configuration;
Fig. 5b shows an inventive tubular stent in the stable fully expanded
configuration;
Fig. 6 shows a part of a stent with one bistable unit cell, drawn in the
stable expanded shape;
Fig. 7 shows the part of the stent of Fig. 6 near its elastic bistable
equilibrium position;
Fig. 8 shows the part of the stent of Figs. 6 and 7 in its stable collapsed
shape; and
Fig. 9 shows a larger section of the stent of Figs. 6 and 8, showing some
unit cells in the collapsed shape and some unit cells in the expanded shape.
Fig. 10 shows an inventive stent formed of a plurality of smaller inventive
stents joined together with flexible connectors.
Fig. 11 shows a partially expanded inventive stent having more than one
type of bistable unit cell;
Fig. 12 shows an inventive stent having a range of diameters along its
length;
Fig. 13 shows an inventive expansion ring in expanded state;
CA 02602435 2007-10-09
= ~ ~ ~
WO 98/32412 PGT/1JS98/01310
6
Fig. 14 shows the expansion ring of Fig. 13 in contracted state;
Fig. 15 shows an inventive stent joining two vessels together and further
securcd with inventive expansion rings, the stent exterior to the vessels;
Fig. 16 shows a cross-sectional view of Fig. 15 along section line 16-16;
Fig. 17 shows an inventive stent joining two vessels together, the stent
interior to the vessels; =
Fig. 18 shows two vesselsjoined together with an itiventive cxpansion
ring and a clamp
Fig. 19 shows a bistablc valve in the closed position;
Fig. 20 shows the bistable valve of Fig. 19 in the open position;
Fig. 21 a shows a multistable cell in the fully contracted state;
Fig. 21b shows the multistable cell of Fig. 21a in the fully expandcd stare;
Fig. 22a shows another multistable cell in the fully contracted state;
Fig. 22b shows the rnultistable cell of Fig. 22a in the fully expanded state;
Fig. 23 shows several unit cells as shown in Figs. 21a,b joined together
and in the fully expanded state;
Fig. 24a shows several unit cells as shown in Figs. 22a,b joined together
and in the contracted state;
Fig. 24b shows the interconnected cells of Fig. 24a in fully cxpanded
state;
Fig. 24c shows the interconnected units cells of Fig. 24a in the process of
expanding; and
Fig. 24d shows several strips of interconnected cells as in Figs. 24a,b
joined together and in the process of expanding.
1etail ]2 escription of the Drawings
Fig. I shows the principle on whicl-s the stent is basod, Fig. la stiows a rod
I with a length L, which is compressed in its axial direction unit; it reaches
its buelrling
stress. Then the central part of the rod will bend out in a sidewards
direction, either to
position 2 or-3 (dashed lines in Fig. 1b). When the axia1 displacement L of
the ends of
the rod is held stable by extcanal clamps 4, it is possible to move the
central section of
RECTIFIED SHEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
WO 98/32412 PCT/US98/01310
7
the rod -betwe.en the two stable positions 2 and 3. This movement is in a
direction X,
perpendicular to the original length axis A-A ofthe rod. All positions between
the stable
positions 2 and 3 are unstable. In Fig. lb the eentral part of the rod has to
rotate over an
angle (3 before the rod can be moved in direction X. Fig. 1C shows a second
order
curvature in rod 1, whiah occurs when the mtation over angle P is opposed by
clamping
the central part of rod I and maintaining this part parallel to the axis A-A.
Fig. 2 shows the force F as a function of displaccment X, with X
displayed in the hnrizonal direetion. The rod is iuoved from the upper 2 to
the lower 3
stable position of Fig. I. The force incrcascs rapidiv from zero to Fmax. At
that moment
the onset of cither the first or second order ctmature of Fig.'lb and lc is
reached. Further
displacemcrzt in direction X costs lcss force, because this spring svstem has
a negative
spring ratc. The force even becomes zero in the mid position and further
movement
occurs automatically. It can be seen in Fig. 2 that the system is completely
symmetrical
and thc forcc needed to move back from the lower to the iiPrer positioII has
the same
characteristic.
Fig. 3 shows rod 5, which will have an asymmetrical force displacement
characteristic, because it already has a preset curvatue, even in the
unload.ed position,
where the length is already L-eL. This can be nchievcd bv prior plastic
deformatian, heat
treatment or the use of an asymmctrical geomctry of the cross section of the
rod (not
shown). The rod 5 in Fig. 3 can be mounted between two clamps on a length.L -
dL, and
if it is elastically deforined in the same way as the rod in Figs lb and lc,
it Nill have a
different stress distribution in the cross section in end position 2 and 3,
compared to the
rod of Fig. 1. This means that the rod has becomc a prefcrcnt unloaded stable
position,
shown in Fig. 3.
Fig, 4 shows the asynzmetrical force-displacement character.stic of the
precurved rod of Fig. 3. The initial displacement from the stable upper
position needs a
starting forrz Fl and if the rod is in its stable lower oosition the starting
force in the
opposite direction is only F2, being smaller than FL Force F2 can be made as
small as
dcsired,. even zero or negative, but needs to. have a positive value if
stability of the lower
position is required.
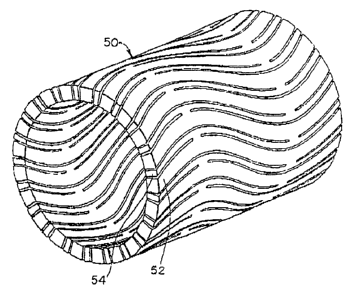
Figs. 5d and 5b show the general appearance of an inventive tubular stent
RECTIFIED SHEET (RULE 91)
ISA./EP
CA 02602435 2007-10-09
- ~ --~ ,
t ;
~
WO 98/32412 PGT/US98/01310
8
in fully contracted and fully expanded configuration respectively. The stent,
in its fully
contracted .CittKiv Jhcns s,m generally at S/V and in itJ 1411Y Lx-GLllded
state sh~, W2~ ge~~erd.liy at
60, is comprised of a plurality of interconnected bistable unit cells (shown
in the =
expanded state at 64 in Fig. 5b). The bistable unit cells are formed from a
first relatively
rigid segment 52 (66 in Fig. 5b) and a second relatively flexible segment 54
(68 in Fig.
5b), joined together at ends 70 and 72. Second relatively flexible segments 68
are
interconnected with adjacent relatively rigid members 66. Adjacent cells in
the
longitudinal sense (the longitudinal axis is denoted by reference numeral 75)
are joined at
ends 70 and 72. By applying a uniform radially outward or inward force, .the
stent may
be switched directly from a fully contracted to a fully expanded configuration
or vice
versa.
Fig. 6 (corresponding to inset 6 in Figure 5b) shows a small part of a stent
such as that shown in Figs .5 which uses the bistable function of a unit cell,
according to
the present invention. The drawing shows a horizontal line A-A, which is
parallel to the
central axis of the stent. There are two series of sinusoidal segments with
distinct size
(see also Fig. 9 for an overview). The segments 7 and 9 have a relatively
large cross
section. Only segment 9 is shown entirely. The segments 9 and 10 have a
relatively
smatler cross section, and here only segment 8 is entirely shown. The segments
are
interconnected for example welded, at joints 11 and 12.
Because of the difference between the cross section of segment 8 and 9.
the deformation force of segment 8 is much lower than for segment 9.
Therefore,
segment 9 can be considered as a relatively rigid clamp, like the clamps 4 in
Fig. lb
opposing relative displacement between the joints 12 in the axial direction,
parallel to
axis A-A. In contrast, segment 8 acts as a flexible rod, like rod 1, described
in Fig. 1 or
rod 5, described in Fig. 3. This combination of segments 7 and 8 or 9 and 10
defines a
unit cell, acting as a bistable spring system with a force-displacement curve
F-X like the
described curves of Fig 2 and 4, depending on the unloaded condition and
geometry of
the segments. Alternatively, instead of using segments or struts of different
diameter, the
segments can have the same diameters (i.e., cross sectional area) and exhibit
different
strengths or rigidity and still accomplish the same effect. One way to obtain
such
differences in strength or rigidity would be to use different materials for
the segments.
CA 02602435 2007-10-09
WO 98/32412 PGT/US98/01310
9
Another way would be to use the same material, like a metal, for all the
segments but
selectivelv strengthen (e.g., by heat treating) thuse segiixents that need to
be rigid. It
should be noted that heat treatment will not strengthen all materials.
Nitinol, for example
becomes mare pliable as a result of heat treatment. This propeny of Nitinol
can be
exploited, however, to render one section of Nitinol more pliable relative to
a second,
non-heat-treated section of Nitinol.
Fig. 7 shows the sar.:e part of the stent (as depicted in Fig. 6) near the
elastic equilibrium position. Segment 8 has been deformed into the dircction
X, causcd
by force F, but segment 9 has alinost its original shapc, because of its
larger rigidity.
Fig. 8 shows the same unit cell of the stent of Figs. 6-7 after it has been
pressed through the elastic equilibrit!rn. Fosition. It automatir.ally snaps
into its stable
position of Fig. 8. This snapping force can be strong enough to hold a
deflated balloon
tight on the catheter shaft (not shown), depending on the mechanical
charaeteristics (e.g.,
the strength) of the material(s) used to make the segntents. With the geometry
shown in
these figures, the segments 8 and 9 fit close together. taking up a minimum
amount of
spacc whcn the stent is in its smallest stable diameter.
Fig. 9 shows a section of the st.ent of Figs. 5, flattened for illustrative
purposes, showing several flexible segments in the collapsed stable shape
(segments 14,
18 and 20) and one segment element 16 in the expanded stable shape. Segments
13, 15,
17,-and 19 are relatively rigid segments and substantiaily maintain tlieir
original shapc:.
The distance between two relatively rigid seginents is shown as (h) in che
collapscd
stable shape and (H) in the expanded stable shape. The value of the
displacement (H-h)
in the direction X depends on the heigYtt of an cxpanded unit cell or
amplitude of thc
segments and the size of the connecting joints. '['he dcscribed part of the
stent is showzl
as a flat surface, but it may be clear that a cylindrical stent such as that
shown in Pigs. 5
is shaped if segments 13 and 20 are directly connected to reach other with
joints 21. In
other words, the stent is shown separated along the joints 21 and in a
1lattened condition.
The rangc of stable diameters of the stent changes v&ith the value (K-h)/-;:,
each ti-me that a flexiblc segment snaps from the collaPscd stable position to
the
expanded stable position. The resvlt is a stent with an extremely rigid -
stuface at all
-diameters being able to withstattd the external forces better thwt with
conventional stents.
RECTIFIED SAEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
~-- ~"~ . . =
i.. I~
WO 98/32412 PCTIUS98ro1310
In the length direction, the flexibility of the stent can be increased by
disconnecting
several unit cells from thrõir nr ighbor ~~nit ce!ls, for example, by c~~tting
the center of one
or more joints while maintaining the several joint pieces as joints.
Another method to increase flexibility is to change the geometry of
5 several sections of the unit cells in the length direction from the relative
flexible to the
relative rigid shape several times along the total length of the stent. In
other words, =
referring to Fig. 9 one or more or each of the segments 13 - 20 could be
constructed with
larger and smaller diameter (or otherwise flexible and rigid) sections which
alternate
after each joint 21.
10 Another possibility, as shown in Figure 10 is the use of a series of short
multistable stents 100 aligned lengthwise end to end and connected with
flexibility joints
104 having the same or a different geometry or configuration as the joints
forming
individual unit cells.
The scope of the invention should include all types of material. One of
the most interesting materials is superelastic Nitinol, because of its large
elastic strain,
well defined stress values, caused by their plateau stresses and the
possibility to define
the desired curvature into the metal by means of a heat treatment. A stent of
Nitinol can
be made by forming slits or slots in a tube, while in its collapsed or smaller
stable
diameter. The slotted tube is then expanded by a separate shaping tool and
heat treated
on this tool to define the expanded stable diameter as the unstrained shape.
In a more general sense, the present invention is directed to a device
having a plurality of stable configurations. The device is comprised of a
plurality of
interconnected multistable cells. The cells incliide one or more relatively
rigid sections
and one or more relatively flexible sections interconnected so as to define a
cell structure
in the form of a multistable spring system having a plurality of stable
configurations. In
a preferred embodiment, the cells comprise a first arcuate member having first
and
second ends and a second arcuate member having first and second ends, the
first end of
the first member in communication with the first end of the second member, and
the
second end of the first member in communication with the second end of the
second
member. It should be noted, however that members need not be rigorously
arcuate.
Other shaped members, including relatively straight members are
contemplated'as well.
CA 02602435 2007-10-09
WO 98/32412 PCT/US98/01310
ll
The invention, in particular, contemplates bistable cells, that is cells
having two stable configurations. In one such cell, the distance between
corresponding
points on the f rst and second secd ons is larger in the first stable state of
the cell than in
the seeond.stable state of the cell. The cells themselves are constructed and
arranged so
that the device itself is at least bistable and possibly multistable. One such
device, a
cylindrical stent having two or mort configurations with an initial diameter
size and a
final larger diamcter size has been described above. However, multistable
stents are also
contemplated. Thus; for example, a stent may be constructed in which the cells
are
designed and arranged to provide a range of diameters in step-wise fashion.
One such
way this may be accomplished would be to employ several different types of
cells in the
stcnt, each type of cell having a different spring constant so that depending
on the
amount of force used, the stent would assume a different diameter. Such a
stent in a
partially expanded state is shown schematically in Fig. 11. A partially
expanded stent is
shown generally at 120. The stent is comprised- of relatively rigid segments
123, 127,
131 and 135 which substantially maintain their original shape, and relativety
flexible
segxnents-125,129, and 133. The segments are interconnected, with joints 122.
As
depicted, -first flexible elcments 125, and 133 are in an expanded
configuration while
sccond flexible clcment 129 is in a contracted configuration. By applying a
radially
outward or tangential force, flexible element 129 may be flipped to its -
fiilly expanded
configuration resulting in a stent (not shown) with a larger diameter. As
shown in Fig.
11, ctlls 138 are larger than cells 136 even in the contracted state. First
flexible elements
125 and 133 are characterized by a different degrcc of flexibility than second
flexible
element 129.
Another form of stcnt, as shown generally at 150 in schematic Fig. 12, has
an first diameter at a first end 152, a second diameter at a second end 154
and one (or
more) intermediate diameters in a reeien 156 between rrtit. crid 152 and
second end 154,
the intermediate diameter differing froin the first alid second diamctcrs. The
interconnected cells in such a stent, as showtt gcnerally at 150 in Fig. 12
may all have the
same force constant and hence be openable all at once with the application of
the
necessary force or there may be several different types of cells, each with
their own force
constant In order to achieve the multiplicity of diameters, cells of differing
sizes may be
RECTIFIED SHEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
WO 98132412 PCT/US98l01310
12
used. In one embodiment of this type of stent, the first and second diameters
are the
c~.,~P , Pr}~;1A ;n ~.~nt},o~ Pmhn~;mPnt tha first and sPrnnri riiamatPrc
riiffer
.,c.ua.v auav ua viaav. . - , + _ -
The present invention is also directed to a method of implanting an
expandable stent having a plurality of stable configurations. The method
comprises the
steps of applying the stent to an expanding means on a catheter, delivering
the stent to a
desired bodily location, expanding the expanding means so as to expand the
stent from a,
first stable configuration to a desired second stable configuration, the
second stable
configuration having a larger diameter than the first stable configuration,
and deploying
the expanded stent at the desired bodily location. The expanding means may be
a
balloon, a mechanical device on or in the catheter, a heat source where the
cells can be
induced to change states by heating or any other suitable expanding means. The
stent
may be applied to the balloon in the first stable configuration or may be
applied in the
second stable (expanded) configuration during the applying step. In the latter
case
radially inward pressure may be applied to the stent so as to urge the stent
into the first
stable configuration to snap it onto the catheter. Where the stent has
additional stable
states, the stent may be applied to the balloon in an intermediate stable
state in which the
diameter of the stent is intermediate between the diameter in the first state
and the
diameter in the second state. Again, the stent may be locked on the expanding
means by
further applying a radially inward pressure.
A further object of the invention is the use of a single bistable unit cell as
an expander (expansion ring), that can be brought into a narrow place and then
triggered
to snap back into its expanded stable shape. As shown in Fig. 13 an expansion
ring
shown generally in its expanded state at 250 coinsists of a first rigid member
254 having
first 258 and second 262 ends and a second more flexible member 266 having
first 270
and second 274 ends. First end 258 of first member 254 is connected to first
end 270 of
second member 266 and second end 262 of first member 254 is connected to
second end
274 of second member 266. Fig. 14 depicts the expansion ring of Fig. 13 in its
contracted state. Second member 266 is seen to be in a second stable position.
Another object of the invention is the use of a single bistable loop (unit
cell) as a clip, that can be used to clamp on an artery, fallopian tube or any
other body
part, to close or hold it for some time. For such a clip it may be desirable
to define the
CA 02602435 2007-10-09
WO 98/32412 PCTlUS98/01310
13
collapsed stab[e shape as the unstrained shape, because the collapsed stable
shape has to
be the most stable one. In the collapsed state, ihe clip would resemble the
collapsed
expansion ring of Fig.14: A triggering means would be used in conjunction with
the
clamp to switch the bistable loop from one state to another. The triggering
means may be
pneumatic, hydraulic, mechanicaI, therrnul or elcctromechanical means.
Examples -of
such triggering means include a human hand applying force to the bistable
loop, and the application of heat to the loop. dkher triggering means include
pulling on the device,
pushing on the device, bending the rigid section of the device or releasing a
restraint
holding the flexible member in place.
1 ~ Another part of the present invention involves constructions between one
or more rinb-shaped elements acearding to the present invenuon, combined with
a
tubular sleeve that is reinforced or held open with such elements. An exnmple
is a so-
called graft stent made of a po137ner with one or more expansion rings. The
expaiision
rings may consist of the above-described bi-stable cells. The surface of'the
stent
comprises a skin mounted on the expansion rings. In mounting the skin, the
skin may
surround, be in or between the expansion rings. The skin may be human or
animal slin,
zi polyzncric rnatcrial or any other suitable bio-compatible matcria.l. Such a
stcnt may
comprise one or more expansion rings, such as a first expansion ring at a
first end of the
stent and a second expansion ring at a second end of the stent. The stent may
be of
constant diameter along its length or may have a first diameter at the first
end and a
second diameter at the second end.
The present invention is also directcd to a stcnt having an unCxpandcd
configuration and an expanded configuration, and comprising a plurality of
generally
longitudinal, wave-like first members characterized by a first wavelength, and
having
peaks and troughs and a plurality of generally longitudinal wave-like second
members
chaxacterized by a second wavelength, and having peaks and troughs. The
wavelengths
of the tlrst and second longitudinal members are substantially equaL '1'he
second
members are capable of stably assuming two positions, a first position
corresponding to
the unexpanded configuration in which the first and second members are in
phase and a
30. second position correspond"uig to the expanded configuration, in which the
first and
second members azt;180 out of phase. The first members are tnorc rigid than
the
RECTIFIED SHEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
..+-=~ - . '
.-- ~'
WO 98/32412 PCT/US98101310
14
second members. The first and second longitudinal members are disposed on the
surface
of the stent such that the longitudinal fust and second members alternate. In
the
unexpanded state, each peak of each first member is connected to one adjacent
peak of a
second member in a region of attachment and each trough of each first member
is
attached to one adjacent tmugh of a second member in a region of attachment,
as can be
seen from rig. 9. The regions of attachment are separated along the
longitudinal
direction by one wavelength. The so described stent em be snapped from the
unexpandcd configuration to the expanded configuration by applying a radially
outward
force and similarly can be snapped from the expanded to;he unexpanded
configuration
by applying a mdiatly inward force. While such stents may be used intemal to a
bodily
vessel, it may also be used extemal to vessels to join two vessels together.
The invention also contemplates a inethod of joining together two vessels
cotnpr:sing the steps of delivering an inventive stent in an unexpanded
configuration in a
'first stable state to a bodily site, expanding the stent to a second smble
state, the diameter
ofthe stent in the second stable state exceeding that of the vessels to be
joined and
placing the stent over the vessels to be joined. The stent may then be
contracted to a
third stable state, the stent in the third stable state having a diameter-
inter.nediate
between the diameters of the stent in the unexpanded state and in the second
stable state.
The stent may further be secured to the vessel with the aid of one or more of
the above-
described expw.sion rings (a bistable loop). One or more expansion rings, such
as that
depicted in Figs. 13 and 14 or small clamping stents (such as that formed from
the strip
= shown in Fig. 23) may be delivered to each side of the stent in a contracted
state and
deployed so as to clamp the vessels between the ring(s). Multiple rings may be
use-d for
additional clamping. As shown generally at 300 in Fig. 15, a first vessel=304
and a
second vesse1308 are joined together with inventive stent 312. Vessel 304
overlaps stent
312 in a first overlap region 316 while vessel 308 overlaps stent 312 in a
second overlap
region 320. Vessel 304 is clamped between expansion ring 324 (shown in the
expanded
state) and stent 312 whilc vcssel=308 is clamped between expansion ring 328
(shown in
the unexpande:d state for illutrative purposes only) and stent 312. the dotted
lines
assooiated with expansion ring 328 illustrate expansion ring 328 in its
expanded statc. It
should be additionally noted that Fig. 15 provides a cut-away view of vessels
'showinb
RECTIFIED SHEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
WO 98132412 PCT/US98101310
the rings contained therein. Fig. 16 shows a cross-sectional view of Fig. 15
along section
line 16-16. Vesse1304 is shown sandwiched between stent 312 and expansion ring
324.
In another embodiment, as shown in Fig. 17, a first vesse1404 and a
second vessel 408 are joined together by a'stent 412. First end 416 of stent
412 rests in
5 vesse1404 while second end 420 of stent 412 rests within vesse1408. Optional
clamps
(such as a small portion of a collapsible inventive stent shown later in strip
form in Fig. 23) 424 and 428 residing on the outside of vessels 404 and 408
clamp the stent to the
vessel. Additional clamps may be used as needed.
In another embodiment, a combination of the embodiments of Figs 15 and
10 17, the first end of the stent may protrude from one of the vessels and the
second end of
the stent may extend over the second vessel. Again, clamps and expansion rings
may be
used to further secure the stent to the vessels.
I In another embodiment, as shown in Fig. 18, vessel 454 and vessel 458
are held together by an expansion ring 462 internal to the vessel and a clamp
466,
15 consisting of, for example, a small section of collapsible stent, the stent
chosen so that
the diameter of the stent in a collapsed state affords a snug fit with vessels
454 and 458
and expansion ring 462. Either the expansion ring or the clamp, but not both,
may be
replaced by a suitable support such as a rigid collar.
The invention also contemplates a method ofjoining together two vessels
comprising the steps of delivering an inventive stent in an unexpanded
configuration in a
first stable state to a bodily site, placing two bodily vessels over the stent
and expanding
the stent to a second stable state, the diameter of the stent in the second
stable state
exceeding that of the vessels to be joined. The diameter of the stent in the
second stable
state is preferably chosen so that the vessels fit snugly over the stent. The
delivery of the
stent may be accomplished by delivering the stent in an unexpanded
configuration
through a bodily vessel and subsequently expanding the stent to rest snugly in
the vessels
to be joined (where a portion of the stent resides in a vessel), or by
expanding the stent
to its most expanded state, placing the stent over the vessel and then
contracting the stent
to an intermediate state over the vessel. The collars and expansion rings
mentioned
above may similarly be delivered. Alternatively, the stent, collars and
expansion rings
may be delivered by surgically exposing the vessel in question.
CA 02602435 2007-10-09
~ ~ i %'~'; ~'= '' =
-WO 98/32412 J PGT/US98/01310
16
The present invention is also directed to a bistable valve. The valve, as
shown generally at 600 in Fig. 19 includes a snap-actior bipositional unit
ccII shown
generally at 604 located within a conduit 606. Snap-action bipositional unit
ceII 604
consists of a(substantially arcuate) tlexible member 608 having a first end
612 and a
second end 616. First end 612 is in communication witt: a trigocring means 620
which is
supported, in turti by a support means 624 enierging from the inner surface of
conduit
606. Second end 616 oftlexible member 608 is anchored to siop surface 628
which
extends across conduit 606. Suppon means 624 and stop surface 628 must be
sufficiently rigid to hold flexible member 608 in place and must be more rigid
than
flexible naember 608. Stop surface 628 extends substantially obliquely across
conduit
606 in oblique regions 630 and has a opening 632 within in longitudinal region
634 to
allow the flow therel,hrough of a fluid. Although openiiig 632 is oriented
along the
longitudinal axis 636 of conduit 606, those of ordinary skill in the art will
recognize
other possible orientations of the opening and stop surface. Valve closure
member 640,
actuated between open and closed positions by flexible member 608, is
constructed and
arranged so as to block the flow of fluid through opening 632 when flexible
member 608
is in the closed position. When flexible member 608 is in the open position,
as depicted
in Fig. 20 valve closure member 640 no longer obstructs opening 632, thereby
allowing
the flow of fluid therethrough.
While triggering means 620 may be any suitable inechanical, hydraulic,
pneumatic, or thermal based trigger known in the art at present or in the
future, in a
preferred embodiment, triggering means 620 is a piezoeicctric elemcnt: In
operation; if
the piezoclem.ent shown in Fig. 19 at 620 is not ac;ivated, valve closure
member 640 is
closed. Activation of piezoelement 620, as shovkm. in Fio. 20 causes a small
shorteni..-ig in
the longitudinal length (denoted by Y in Fig. 15) of piezoelement 620 which in
turn
releases flexible member 608 from its first position. With member 608
relesised, valve
closure member 640 'is frec to open under the pressure uansmitted from the
fluid.
Member 608 a,ssumes a second, inverted, position, as depicted in Fig. 20.
Dv'liile the fluid
pressure maintains member 608 in its second position, even in the a.hcence of
any fluid.
member 608 remains in its second position, as depicted in Fig. 20 if
the.triggering is
tumed off and piezoelemcnt 620 assumes'its original length. Valve closure
membcr.640
RECTIFIED SHEET (RULE 91)
LSA/EP
CA 02602435 2007-10-09
WO 98/32412 PCT/US98/01310
17
may be closed again, in the absence of fluid, by a subsequent triggering of
piezoelement
620 allowing member 608 to transition to its second (closed) position which is
the
preferred position of member 608. Member 608 has been treated to receive a
preferred
position as shown in Fig. 3.
The valve depicted in Figs. 19 and 20 may be applied to medical and non-
medical devices. It is, in particular, an aim of the present invention to
apply the inventive
bistable valve to the control of urinary incontinence. In a patient with
incontinence, the
above described valve may be implanted in the urethra using any suitable means
including the use of the above-described expansion rings to clamp the valve to
the
urethra. Although the valve in the default state is closed, the valve may be
triggered
when the bladder is full, to void the bladder. Upon voiding the bladder, the
valve may be
triggered again to close it. Another such application is to employ the
inventive valve in
conjunction with a shunt. The shunt may be activated by triggering the device
and
similarly may be closed by triggering the device.
Of course the valve may be used in other medical and non-medical
applications as well.
In addition to the bistable unit cells disclosed above, bistable unit cells
and more generally, multistable unit cells of other shapes are also
contemplated by the
present invention. Figs. 21 a and 21 b are schematic representations of
another
embodiment of an inventive hinged multistable cell in its contracted and
expanded states,
respectively. The contracted cell, shown generally at 700, and the expanded
cell, shown
generally at 705, consist of four interconnected relatively rigid members. Two
side
members 709 are connected to opposite ends of top member 713 via hinges 715.
Side
members 709 are connected at their opposite ends to opposite ends of bottom
member
717 via hinges 719. Preferably, the hinges are elastic or plastically
deformable. The
hinges may be fixedly attached to the side, top and bottom members or may be
integral
with these members. In the latter case, the hinges may be formed bv removing
material
from the cell in the region of the hinges so that the hinges are thinner or
have a different
geometry from the side, top and bottom members. In the process of
transitioning from
the expanded to the collapsed state, bottom member 717 opens slightly. The
cell of Figs.
21 a,b also has two additional intermediate states in which one or the other
(but riot both)
CA 02602435 2007-10-09
WO 98/32412 PCT/US98/01310
18
of side members 709 and top member 713 are collapsed downward.
A hexagonai hinged ~ii~.;ltistabie unit ceii is shown schematically in Fig.
22a in the collapsed state and in Fig. 22b in the expanded state. The cell,
shown
generally at 750, consists of top member 754 and bottom member 758, and upper
side
members 762. Two upper side members 762 are connected to opposite ends of top
member 754 via hinges 756. Upper side members 762 are connected to bottom
member '
758 via hinges 768. Bottom member 758 is shaped like a'U' with the two
uprights of the
'U' modified to lie at oblique angles with respect to the bottom part of
the'U'. As with
the previously discussed inventive cells, hinges 756 and 768 may be elastic or
plastically
deformable and may be fixedly attached to the members or integral with the
members.
The hexagonal unit cell exhibits multiple stable states. In addition to the
fully expanded
and fully contracted states shown in Figs. 22a and 22b, the hexagonal cell can
also
achieve two intermediate stable configurations in which only one of the two
upper side
members 762 is collapsed inward along with top member 754.
The above described hinged multistable cells may be used in any of the
above discussed applications e.g. to form stents, clamps, clips, expander
rings, bistable
valves.
In one such application a ring or stent is formed of the hinged cells of
Figs. 2 I a and 21 b. As shown in Fig. 23, a series of unit cells of the type
depicted in Figs.
21 are joined together so that the top member of a cell forms a portion of the
bottom
member of an adjoining cell. As depicted, top member 814 of cell 810 fonns a
portion of
bottom element 818 of cell 820. Similarly, top member 824 of cell 828 forms a
portion
of bottom element 832 of cell 836. Although the ring or stent in Fig. 23 has
been cut for
illustrative purposes, the two ends 840 and 844 are normally joined together
with a
portion of lower member 848 of cell 852 serving as an upper member for cell
856. The
ring so formed has a range of stable stable states including a fully expanded
state and a
fully contracted state. Where the individual cells are made identically, only
the fully
expanded states may be accessed by the application of a uniform radially
outward force
to the stent in the fully contracted state. It may serve as a clamp or collar,
an expansion
ring or a stent. Larger stents may be formed by interconnecting a plurality of
such rings.
Similar products may also be formed from other multistable units cells.
CA 02602435 2007-10-09
WO 98/32412 PCTIUS98/01310
19
Figs. 24a and 24b illustrate one such possibility schematically in which
hexagonal unit
cells such as those shown in Figs. 22a, b may be joined together to form. a
rino: The top
member 884 of each cel1880 is joined with a the bottom portion 886 or
modified'U'
shaped bottom member 890. Although shown in strip form in Figs. 24a and 24b,
end 894
can be joined to end 898 to ibrm a ring. The strip of Fig. 24a is shown in
fully expanded
state in Fig. 24b. Adjacent cells 880 are seen in their expanded state. For
the sake of completeness, the hinges are designated 902. Fig. 24c shows or.e
cell 920 in the process
of expanding and one atlready expandcd cei1924. The cells 920 and 924 are
joined at
bottom member 92$ and top member 932. 1-Tinaes are shown at 936. Multiple
strips may
also be joined togcther so as to form a stent whose length is a multiple of
the length of
the unit cell. In such a casc, upper side members of adjacent cells would be
joined
togcther. This is illustrated in Fig. 24d which, like Fig. 24c shows cells 940
in the
expanded state and eells 944 in the process of expanding. Upper side members
948 are
shown by dashed lines_ Adjacent strips of interconnected eclls 952 are joined
together by
tipper side members 948 as well as by oblique regions 956 of bottom members
960.
It should be noted that the inventive devices of the present application
may be used on a temportuy basis or on a permauent basis in the body. i hus,
for
example, permanent stents atid clamps are contempiated, as are removable
stents and
clamps.
It should further be noted that in expanding some of the inventive
multistable cells, there rnay be components of expansion/contraction in u
direction
perpendictilar to the direction of ttie fo= applitd to expand thc eells.
Finally, for the purposes of this application, the terrn 'multistable' is
intended to include 'bistable'.
In the described drawings and text only some examples of different
embodiments have been given_ While the stents of the present invention can
appear
similar to prior stents, the mechruiical results are eotnpletely different due
to the special
combination of a rigid section and a.tnore flexible section in the same unit
cell. Of
course there are, beside the illustrated sinusoidal shape many other possible
basic shapes
for the unit cells, with similar characteristic behavior.
From the above disclosure of the general principles of the present
RECTIFIED SHEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
-WO 98/32412 19a PCT/US98/01310
invention and the preceding detailed description, those skilled in this art
will readily
RECTHUD SHEET (RULE 91)
ISA/EP
CA 02602435 2007-10-09
WO 9W2412 PCTlUS98101310
comprehend the various modifications to which the present invention is
susceptible. It is
intended for the coverage of the present application to include different
geometries,
different constructions and different combinations of one or more materials to
obtain the
same basic mechanical behavior as exhibited by the above described examples.
5