Note: Descriptions are shown in the official language in which they were submitted.
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
Microprojections With Capillary Control
Features and Method
FIELD OF THE PRESENT INVENTION
[0001] The present invention relates to devices and methods for delivering a
biologically
active agent transdermally using a coated microprojection array. More
particularly, the
invention relates to devices and methods for reducing the variability in the
amount of
active agent coated on the microprojections, thus improving the consistency of
delivered
amount.
BACKGROUND OF THE INVENTION
[0002] Active agents (or drugs) are most conventionally administered either
orally or by
injection. Unfortunately, many active agents are completely ineffective or
have radically
reduced efficacy when orally administered, since they eitlier are not absorbed
or are
adversely affected before entering the bloodstream and thus do not possess the
desired
activity. On the other hand, the direct injection of the agent into the
bloodstream, while
assuring no modification of the agent during administration, is a difficult,
inconvenient,
painful and uncomfortable procedure which sometimes results in poor patient
compliance.
[0003] As an alternative, transdermal delivery provides for a method of
administering
biologically active agents that would otherwise need to be delivered via
hypodermic
injection, intravenous infusion or orally. Transdermal delivery, when compared
to oral
delivery avoids the harsh environment of the digestive tract, bypasses
gastrointestinal
drug metabolism, reduces first-pass effects, and avoids the possible
deactivation by
digestive and liver enzymes.
[0004] As is well known in the art, the word "transdermal" is used that is
used to refer
to delivery of an active agent (e.g., a nucleic acid or other therapeutic
agent such as a
drug) through the skin to the local tissue or systemic circulatory system
without
substantial cutting or piercing of the skin, such as cutting with a surgical
knife or
piercing the skin with a hypodermic needle.
[0005] Transdermal agent delivery includes delivery via passive diffusion as
well as by
external energy sources, including electricity (e.g., iontophoresis) and
ultrasound (e.g.,
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
phonophoresis). While most agents will diffuse across both the stratum corneum
and the
epidermis, the rate of diffusion through the stratum corneu.m is often the
limiting step.
Many compounds, in order to achieve a therapeutic dose, require higher
delivery rates
than can be achieved by simple passive transdermal diffusion.
[0006] One common method of increasing the passive transdertn.al diffusional
agent flux
involves pre-treating the skin with, or co-delivering with the agent, a skin
permeation
enhancer. A permeation enhancer, when applied to a body surface through which
the
agent is delivered, enhances the flux of the agent therethrough. However, the
efficacy of
these methods in enhancing transdermal agent flux has been limited,
particularly for larger
molecules.
[0007] There also have been many techniques and systems developed to
mechanically
penetrate or disrupt the outermost skin layers thereby creating pathways into
the skin in
order to enhance the amount of agent being transdermally delivered.
Illustrative are skin
scarification devices, or scarifiers, which typically provide a plurality of
tines or needles
that are applied to the skin to scratch or make small cuts in the area of
application. The
agent, such as a vaccine, is applied either topically on the skin, such as
disclosed in U.S.
Patent No. 5,487,726, or as a wetted liquid applied to the scarifier tines,
such as disclosed
in U.S. Patent Nos. 4,453,926, 4,109,655, and 3,136,314.
[0008] Other devices that use tiny skin piercing elements or microprojections
to
enhance transdernial agent delivery are disclosed in European Patent EP
0407063A1,
U.S. Patent Nos. 5,879,326 issued to Godshall, et al., 3,814,097 issued to
Ganderton, et
al., 5,279,544 issued to Gross, et al., 5,250,023 issued to Lee, et al.,
3,964,482 issued to
Gerstel, et al., Reissue 25,637 issued to Kravitz, et al., and PCT Publication
Nos. WO
96/37155, WO 96/37256, WO 96/17648, WO 97/03718, WO 98/11937, WO 98/00193,
WO 97/48440, WO 97/48441, WO 97/48442, WO 98/00193, WO 99/64580, WO
98/28037, WO 98/29298, and WO 98/29365; all incorporated by reference in their
entirety.
[0009] The piercing elements disclosed in the noted references generally
extend
perpendicularly from a thin, flat member, such as a pad or sheet. The piercing
elements
are typically extremely small, some having dimensions (i.e., a microblade
length and
width) of only about 25 - 400 gm and a microblade thickness of only about 5 -
50 m.
2
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
[0010] The disclosed systems generally include a reservoir for holding the
active agent
and a delivery system to transfer the active agent from the reservoir through
the stratum
corneum, such as by hollow tines or needles.
[0011] Alternatively, a formulation containing the active agent can be coated
on the
microprojections. Illustrative are the systems disclosed in U.S. Patent Pub.
Nos.
2002/0132054, 2002/0193729, 2002/0177839, 2002/ 0128599, and Application No.
10/045,842, which are fully incorporated by reference herein. Coated
microprojection
systems eliminate the necessity of a separate physical reservoir and the
development of
an agent formulation or composition specifically for the reservoir.
[0012] However, one challenge associated with this method of delivery lies in
achieving a reproducible dose of the coated agent. Specifically, conventional
means of
coating can result in a significant variation in the amount of active agent
loaded onto the
delivery device.
[0013] For example, dip-coating is a method of applying an active agent to the
microprojections of a delivery device that generally involves placing the tips
of the
microprojections in a reservoir of fluid. Capillary action causes the fluid to
wick up the
sides of the microprojections to variable heights, creating inconsistency in
the amount of
agent coated and the location of the agent on the microprojection array.
[0014] As will be appreciated by one having ordinary skill in the art, the
distance the
fluid rises up the microprojection is a function the depth the tip is dipped
into the fluid,
the viscosity of the fluid, the contact angle of the fluid with the
microprojection material
and the duration the tip is dipped into the fluid. Furthermore, the proximity
of the
microprojections in the array to each other creates an environment in which
the fluid
wicks higher in the center of the array than on the perimeter of the array.
[0015) Due to the noted effects, there can be substantial variability in the
amount of
active agent loaded on the microprojection delivery device.
[0016] Accordingly, it is an object of this invention to provide methods and
compositions for enhancing transdermal delivery of biologically active agents
using
microprojection devices.
3
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
[0017] It is a further object of the invention to provide a device and method
that
reduces the variability in the amount of active agent coated on the
microprojections.
[0018] It is another object of the invention to a device for and method of
delivering a
more consistent amount of a biologically active agent using a coated
microprojection
device.
[0019] It is yet another objection of the invention to provide a device and
method that
limits the capillary action when applying an active agent formulation to a
microprojection delivery device.
[0020] Another object of the invention is to provide a device and method for
more
precisely controlling the coating depth on a microprojection.
SUMMARY OF THE INVENTION
[0021] In accordance with the above objects and those that will be mentioned
and will
become apparent below, one aspect of the invention comprises a transdermal
delivery
device comprising a microprojection member having at least one stratum corneum-
piercing microprojection with a capillary control feature, wherein the
microprojection
has a length running from a distal tip to a proximal end and a thickness,
wherein the
capillary control feature is located between the distal tip and the proximal
end, and
wherein the microprojection has a first width at the capillary control feature
location.
Preferably, the capillary control feature is located in the range of
approximately 25 m
to 200 m from the distal tip of the microprojection.
[0022] In one embodiment of the invention, the capillary control feature
comprises a
scribe line running perpendicular to the microprojection length. Preferably,
the scribe
line extends at least 50% of the first width on each side of the
microprojection. The
scribe line can be configured as a ridge or a trough. Also preferably, the
scribe line has a
thickness in the range of approximately 5 m and 25% of the thickness of the
microproj ection.
4
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
[0023] In another embodiment of the invention, the capillary control feature
comprises
a void. Preferably, the void has a horizontal dimension up to approximately
half the
width of the microprojection at the location of the capillary control feature.
[0024] In yet another embodiment of the invention, the capillary control
feature
comprises a transition from a maximum width to a minimum width at the
capillary
control feature location. Preferably, the microproj ection has a minimum width
in the
range of approximately 25% to 100% of the maximum width, and more preferably,
in
the range of approximately 35% to 70% of the maximum width. Even more
preferably,
the minimum width is approximately 50% of the maximum width. Alternately, the
microprojection has a minimum width that is in the range of approximately 10
.m to
120 m less than said maximum width.
[0025] In yet another embodiment of the invention, the capillary control
feature
comprises a hydrophobic coating. Presently preferred hydrophobic coatings are
selected
from the group consisting of polytetrafluoroethylene, parylene and silicon.
[0026] Preferably, the delivery devices of the invention further comprise a
coating of a
biologically active agent applied to the microprojection from the distal tip
to the
capillary control feature.
[0027] In another aspect of the invention, the coating is applied to the
microprojection
with a static contact angle greater than 20 degrees, and more preferably,
between 30 and
60 degrees.
[0028] In one embodiment of the invention, the coating comprises a formulation
having a biologically active agent selected from the group consisting of
growth hormone
release hormone (GHRH), growth hormone release factor (GHRF), insulin,
insultropin,
calcitonin, octreotide, endorphin, TRN, NT-36 (chemical name: N-[[(s)-4-oxo-2-
azetidinyl] carbonyl]-L-histidyl-L-prolinamide), liprecin, pituitary hormones
(e.g., HGH,
HMG, desmopressin acetate, etc), follicle luteoids, aANF, growth factors such
as growth
factor releasing factor (GFRF), bMSH, GH, somatostatin, bradykinin,
somatotropin,
platelet-derived growth factor releasing factor, asparaginase, bleomycin
sulfate,
chymopapain, cholecystokinin, chorionic gonadotropin, erythropoietin,
epoprostenol
(platelet aggregation inhibitor), gluagon, HCG, hirulog, hyaluronidase,
interferon alpha,
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
interferon beta, interferon gaznma, interleukins, interleukin- 10 (IL- 10),
erythropoietin
(EPO), granulocyte macrophage colony stimulating factor (GM-CSF), granulocyte
colony stimulating factor (G-CSF), glucagon, leutinizing hormone releasing
hormone
(LHRH), LHRH analogs (such as goserelin, leuprolide, buserelin, triptorelin,
gonadorelin, and napfarelin, menotropins (urofollitropin (FSH) and LH)),
oxytocin,
streptokinase, tissue plasminogen activator, urokinase, vasopressin, deamino
[Va14, D-
Arg8] arginine vasopressin, desmopressin, corticotropin (ACTH), ACTH analogs
such
as ACTH (1-24), ANP, ANP clearance inhibitors, angiotensin II antagonists,
antidiuretic
hormone agonists, bradykinn antagonists, ceredase, CSI's, calcitonin gene
related peptide
(CGRP), enkephalins, FAB fragments, IgE peptide suppressors, IGF-1,
neurotrophic
factors, colony stimulating factors, parathyroid hormone and agonists,
parathyroid
hormone antagonists, parathyroid hormone (PTH), PTH analogs such as PTH (1-
34),
prostaglandin antagonists, pentigetide, protein C, protein S, renin
inhibitors, thymosin
alpha-1, thrombolytics, TNF, vasopressin antagonists analogs, alpha-1
antitrypsin
(recombinant), and TGF-beta.
[0029] In another embodiment of the invention, the biologically active agent
comprises
an immunologically active agent selected from the group consisting of
proteins,
polysaccharide conjugates, oligosaccharides, lipoproteins, subunit vaccines,
Bordetella
pertussis (recombinant PT accince - acellular), Clostridium tetani (purified,
recombinant), Corynebacterium diphtheriae (purified, recombinant),
Cytomegalovirus
(glycoprotein subunit), Group A streptococcus (glycoprotein subunit,
glycoconjugate
Group A polysaccharide with tetanus toxoid, M protein/peptides linked to
toxing subunit
carriers, M protein, multivalent type-specific epitopes, cysteine protease,
C5a peptidase),
Hepatitis B virus (recombinant Pre Sl, Pre-S2, S, recombinant core protein),
Hepatitis C
virus (recombinant - expressed surface proteins and epitopes), Human
papillomavirus
(Capsid protein, TA-GN recombinant protein L2 and E7 [from HPV-6], MEDI-501
recombinant VLP L1 from HPV-11, Quadrivalent recombinant BLP LI [from HPV-6],
HPV-11, HPV-16, and HPV-18, LAMP-E7 [from HPV-16]), Legionella pneumophila
(purified bacterial survace protein), Neisseria meningitides (glycoconjugate
with tetanus
toxoid), Pseudomonas aeruginosa (synthetic peptides), Rubella virus (synthetic
peptide),
Streptococcus pneumoniae (glycoconjugate [1, 4, 5, 6B, 9N, 14, 18C, 19V, 23F]
conjugated to meningococcal B OMP, glycoconjugate [4, 6B, 9V, 14, 18C, 19F,
23F]
6
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
conjugated to CRM197, glycoconjugate [1, 4, 5, 6B, 9V, 14, 18C, 19F, 23F]
conjugated
to CRM1 970, Treponema pallidum (surface lipoproteins), Varicella zoster virus
(subunit, glycoproteins), Vibrio cholerae (conjugate lipopolysaccharide),
whole virus,
bacteria, weakened or killed viruses, cytomegalo virus, hepatitis B virus,
hepatitis C
virus, human papillomavirus, rubella virus, varicella zoster, weakened or
killed bacteria,
bordetella pertussis, clostridium tetani, corynebacterium diphtheriae, group A
streptococcus, legionella pneumophila, neisseria meningitidis, pseudomonas
aeruginosa,
streptococcus pneumoniae, treponema pallidum, vibrio cholerae, flu vaccines,
Lyme
disease vaccine, rabies vaccine, measles vaccine, mumps vaccine, chicken pox
vaccine,
small pox vaccine, hepatitis vaccine, pertussis vaccine, diphtheria vaccine,
nucleic acids,
single-stranded and double-stranded nucleic acids, supercoiled plasmid DNA,
linear
plasmid DNA, cosmids, bacterial artificial chromosomes (BACs), yeast
artificial
chromosomes (YACs), mammalian artificial chromosomes, and RNA molecules.
[0030] The invention also comprises methods of applying a coating of a
biologically
active agent to a transdermal delivery device, generally including the steps
of providing
a microprojection member having at least one stratum corneum-piercing
microprojection
with a capillary control feature, wherein the microprojection has a length
running from a
distal tip to a proximal end, a thickness, wherein the capillary control
feature is located
between the distal tip and the proximal end, and wherein the microprojection
has a first
width at the capillary control feature location; applying a formulation of the
biologically
active agent to a location proximal the distal tip of the microprojection so
that the
formulation migrates to the capillary control feature; and drying the
formulation to form
a coating. Preferably, the step of applying the formulation comprises dip
coating.
BRIEF DESCRIPTION OF THE DRAWINGS
[0031] Further features and advantages will become apparent from the following
and
more particular description of the preferred embodiments of the invention, as
illustrated in
the accompanying drawings, and in which like referenced characters generally
refer to the
same parts or elements throughout the views, and in which:
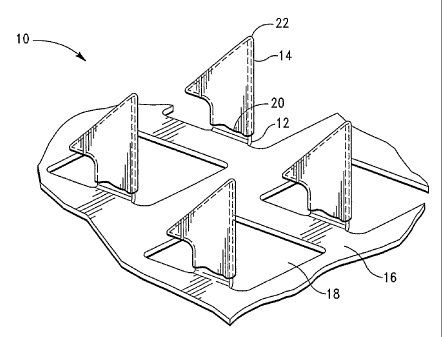
[0032] FIGURE 1 is a perspective view of a microprojection member having a
coating
deposited on the microprojections, according to the invention;
7
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
[0033] FIGURE 2 is a detail view of an embodiment of a microprojection having
a
scribed capillary control feature, according to the invention;
[0034] FIGURE 3 is a detail view of an alternate embodiment of a
microprojection
having a capillary control feature comprising a void, according to the
invention;
[0035] FIGURE 4 is a detail view of another embodiment of a microprojection
having a
capillary control feature comprising a reduced width configuration, according
to the
invention;
[0036] FIGURE 5 is a detail view of yet another embodiment of a
microprojection
having a capillary control feature comprising a hydrophobic coating, according
to the
invention;
[0037] FIGURES 6 and 7 are graphical illustrations comparing capillary rise
heights for
microprojections having features of the invention to prior art
microprojections; and
[0038] FIGURES 8 and 9 are graphical illustrations comparing meniscus volumes
for
microprojections having features of the invention to prior art
microprojections.
DETAILED DESCRIPTION OF THE INVENTION
[0039] Before describing the present invention in detail, it is to be
understood that this
invention is not limited to particularly exemplified materials, methods or
structures as such
may, of course, vary. Thus, although a number of materials and methods similar
or
equivalent to those described herein can be used in the practice of the
present invention,
the preferred materials and methods are described herein.
[0040] It is also to be understood that the terminology used herein is for the
purpose of
describing particular embodiments of the invention only and is not intended to
be limiting.
[0041] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one having ordinary slcill in the art
to which
the invention pertains.
[0042] Further, all publications, patents and patent applications cited
herein, whether
supra or infra, are hereby incorporated by reference in their entirety.
8
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
[0043] Finally, as used in this specification and the appended claims, the
singular forms
"a, "an" and "the" include plural referents unless the content clearly
dictates otherwise.
Thus, for example, reference to "an active agent" includes two or more such
agents;
reference to "a microprojection" includes two or more such microprojections
and the like.
Definitions
[0044] The term '"transdermal", as used herein, means the delivery of an agent
into
and/or through the skin for local or systemic therapy.
[0045] The term "biologically active agent", as used herein, refers to a
composition of
matter or mixture containing an active agent or drug, which is
pharmacologically effective
when administered in a therapeutically effective amount.
[0046] It is to be understood that more than one biologically active agent can
be
incorporated into the agent source and/or coatings of this invention, and that
the use of the
term "active agent" in no way excludes the use of two or more such active
agents or drugs.
[0047] As used herein, the term "microprojection array," "microprojection
member," and
the like, all refer to a device for delivering an active agent into or through
the skin that
comprises a plurality of microprojections on which the active agent can be
coated. The
term "microprojections" refers to piercing elements that are adapted to pierce
or cut
through the stratum comeum into the underlying epidermis layer, or epidermis
and dermis
layers, of the skin of a living animal, particularly a human.
[0048] Typically the microprojections have a blade length of less than 1000
m, and
preferably less than 500 m. In one embodiment, the microprojections have a
length in
the range of 50 - 145 m. The microprojections typically have a width in the
range of
about 75 - 500 m and a thickness in the range of about 5 - 50 m.
[0049] The microprojections can be formed in different shapes, for example by
etching
or punching a plurality of microprojections from a thin sheet and folding or
bending the
microprojections out of the plane of the sheet to form a configuration, such
as that shown
in Fig. 1. The microprojection member can also be formed in other known
manners, such
as by forming one or more strips having microprojections along an edge of each
of the
strip(s).
9
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
[0050] Exemplary methods of forming metal microprojection are disclosed in
Trautman
et al., U.S. Patent No. 6,083,196; Zuck, U.S. Patent No. 6,050,988; and
Daddona et al.,
U.S. Patent No. 6,091,975; the disclosures of which are incorporated by
reference herein
in their entirety.
[0051] Other microprojection members that can be used with the present
invention are
formed by etching silicon using silicon chip etching techniques or by molding
plastic
using etched micro-molds. Silicon and plastic microprojection members are
disclosed in
Godshall et al., U.S. Patent No. 5,879,326; the disclosure of which is
incorporated by
reference herein.
[0052] As used herein, the terms "deliver," "delivering," and all variations
thereof, refer
to and include any means by which an active agent can be administered into or
through the
skin.
[0053]' As used herein, the term "thickness," as it relates to coatings,
refers to the average
thickness of a coating as measured over substantially all of the portion of a
substrate that is
covered with the coating.
[0054] Referring now to Fig. 1, there is shown one embodiment of a stratum
comeum-
piercing microprojection member 10 for use with the present invention. As
illustrated in
Fig. 1, the member 10 includes a plurality of microprojections 12 having a
coating 14
disposed thereon. Coating 14 comprises a dried formulation having one or more
biologically active agents. In the illustrated embodiment, the
microprojections 12 extend
at substantially a 90 angle from a substrate, such as sheet 16, having
openings 18.
[0055] The microprojections 12 are preferably formed by etching or punching a
plurality
of microprojections 12 from a thin metal sheet 16 and bending the
microprojections 12 out
of a plane of the sheet. Metals such as stainless steel, titanium and nickel
titanium alloys
are preferred.
[0056] According to the invention, the coating 14 preferably covers the
microprojection
12 from a capillary control feature 20 to the distal tip 22. According to the
invention, the
coating 14 can be formed upon the microprojections 12 by a variety of known
methods.
Generally, a liquid formulation is applied to microprojection 12 and then
dried to form
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
coating 14. Preferably, capillary control feature 20 is positioned within the
nominal rise
height of the coating formulation at a location selected to result in a
desired coating depth.
Thus, the applied fluid formulation wicks along the microprojection 12 until
capillary
control feature 20 restricts the migration.
[0057] A presently preferred means of applying a formulation to the
microprojections of
the invention means is dip-coating. This method generally involves immersing
microprojections 12 into a coating formulation. Depending upon the properties
of the
coating formulation and the desired loading amount, the microprojections can
be lowered
into the formulation to any depth up to the capillary control feature 20. In
some
embodiments, it may be desirable to dip only a distal portion of the
microprojection tip
into the formulation.
[0058] The capillary control features of the invention are applicable to other
means of
applying coatings, so long as the applied formulation is fluid or otherwise
susceptible to
migration. As will be appreciated by one having ordinary skill in the art, the
use of the
capillary control features of the invention minimizes such migration and
restricts the
coating depth.
[0059] One alternative coating method is roller coating, which employs a
roller coating
mechanism that similarly limits the coating 14 to the tips of the
microprojections 12. The
roller coating method is disclosed in U.S. Application No. 10/099,604 (Pub.
No.
2002/0132054), which is incorporated by reference herein in its entirety. As
discussed in
detail in the noted application, the disclosed roller coating method provides
a smooth
coating that is not easily dislodged from the microprojections 12 during skin
piercing.
[0060] A further coating method that can be employed within the scope of the
present
invention comprises spray coating. According to the invention, spray coating
can
encompass formation of an aerosol suspension of the coating composition. In
one
embodiment, an aerosol suspension having a droplet size of about 10 to 200
picoliters is
sprayed onto the microprojections 10 and then dried.
[0061] Pattern coating can also be employed to coat the microprojections 12.
The
pattern coating can be applied using a dispensing system for positioning the
deposited
liquid onto the microprojection surface. The quantity of the deposited liquid
is preferably
-11
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
in the range of 0.1 to 20 nl/microprojection. Examples of suitable precision-
metered
liquid dispensers are disclosed in U.S. Patent Nos. 5,916,524; 5,743,960;
5,741,554; and
5,738,728; which are fully incorporated by reference herein.
[0062] Microprojection coating formulations or solutions can also be applied
using ink
jet technology using known solenoid valve dispensers, optional fluid motive
means and
positioning means which is generally controlled by use of an electric field.
Other liquid
dispensing technology from the printing industry or similar liquid dispensing
technology
known in the art can be used for applying the pattern coating of this
invention.
[0063] The invention is directed to microprojection designs and methods having
reduced
coating variability. To achieve minimal coating variability, the
microprojection has a
capillary control feature, located so that capillary action is disrupted or
minimized at the
desired coating depth.
[0064] In a first embodiment, shown in Fig. 2, the invention includes a
microprojection
30 having a capillary control feature comprising a scribe line 32. A scribe
line 32
generally is a trough or ridge that runs substantially perpendicular to the
length of the
microprojection. Preferably, scribe line 32 runs continuously from edge to
edge on both
sides of the microprojection. Alternatively, scribe line 32 can run
intermittently across at
least half the distance. The thickness of scribe line 32 refers to depth of
the trough or
height of the ridge, and is measured as the differential from the plane of the
microprojection. Preferably, the thickness of scribe line 32 is approximately
equal to its
width. More preferably, the thickness is in the range of approximately 5 m
and 25% of
the thickness of the microprojection.
[0065] To maximize effectiveness in controlling capillary action, the edges of
the scribe
line preferably have a sharp configuration. Scribe line 32 is located the
distance from the
tip 34 of the microprojection that the fluid is intended to coat. Preferably,
scribe line 32 is
located in the range of approximately 25 m to 200 m from the distal tip 34
of the
microprojection 30.
[0066] In an alternate embodiment of the invention, shown in Fig. 3,
microprojection 40
has a capillary control feature comprising at least one void 42. In
embodiments with a
single hole, the width of the microprojection on each side of void 42 is
preferably in the
12
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
range of approximately 25 m and half the width of the microprojection. Also
preferably,
void 42 is located so that the distal portion of the void (that closest to the
tip of the
microprojection) corresponds to the desired coating depth. For example, the
distal portion
of void 42 is preferably located in the range of approximately 25 m to 200 m
from the
tip 44 of microprojection 40.
[0067] In another embodiment of the invention, the microprojection is
configured to
minimize the effect of capillary action to wick fluid beyond a desired region.
As shown in
Fig. 4, microprojection 50 has a width that increases from distal tip 52 to
location 54 of
maximum width. The width of microprojection 50 then decreases to location 56
of
minimum width. The capillary control feature is the reduction of width at
location 56.
Preferably, location 56 corresponds to the desired coating depth. As shown in
Fig. 4,
coating 58 wicking action causes migration only in the minimum width
561ocation.
Microprojection 50 still presents adequate surface area below minimum width 56
to allow
a desired amount of coating. As one having skill in the art can appreciate,
decreasing the
width of the microprojection reduces variability in coating height but must be
balanced
against the need to retain sufficient structural integrity.
[0068] Preferably, the maximum width at location 54 is in the range of
approximately
m to 120 m wider than the minimum width at location 56. Alternatively, the
minimum width at location 56 of the microprojection is preferably in the range
of
approximately 25% to 100%, and more preferably, in the range of approximately
35% to
70%, of the maximum width at location 54. In one presently preferred
embodiment, the
minimum width at location 56 is approximately 50% of the maximum width at
location
54. The reduction to minimum width is located at the desired coating depth,
such as in
the range of approximately 25 m to 200 m from the distal tip of the
microprojection.
[0069] In another embodiment of the invention, the capillary control feature
comprises a
hydrophobic coating. As shown in Fig. 5, microprojection 60 has a hydrophobic
coating
62 located at the proximal boundary of the location 64 corresponding to the
desired
coating depth. Preferably, the hydrophobic coating is located in the range of
approximately 25 m to 200 m from the distal tip 66 of the microprojection.
Also
preferably, the hydrophobic coating is selected from the group consisting of
polytetrafluoroethylene, parylene and silicon.
13
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
[0070] Presently preferred characteristics of the microprojection members of
the
invention include a microproj ection density in the range of approximately 10
to
2000 per cm2, a microprojection length in the range of approximately 50 to 500
m, a
microprojection maximum width in the range of approximately 20 to 300 m, and
a
microprojection thickness in the range of approximately 10 to 50 m.
[0071] The capillary control features of the invention minimize variations in
coating
depth as compared to prior art microprojection designs as demonstrated by the
graphical
illustrations shown in Figs. 6 and 7. These graphs show the capillary rise
measured for
five tip microprojection members, each having the same boundary conditions and
tip
configurations, with Fig. 6 showing prior art designs and Fig. 7 showing
microprojections
having capillary control features.
[0072] As can be seen in Fig. 6, the rise heights show a significant amount of
variation,
15 m or more. The graph also shows that neighboring microprojections affect
capillary
rise heights, leading to different loading amounts in different positions of
the
microprojection array.
[0073] In contrast, Fig. 7 shows that microprojections having capillary
control features
offer consistent capillary rise heights and exhibit minimal variability. Also,
the position of
the microprojection within the array does not have a significant effect on
coating depth for
designs incorporating capillary control features.
[0074] The capillary control features of the invention also significantly
increase the
potential loading amount. Figs. 8 and 9 are graphical illustrations that
compare the
meniscus volume for a conventional microprojection tip with a microprojection
tip having
capillary control features, respectively, when dipped to a depth of 400 m.
The fluid
loading on the tip shown in Fig. 8 is calculated to be 6.3 x 10"12 m3 as
compared to the
36.2 x 10-12 m3 for the capillary controlled microprojection of Fig. 9.
Accordingly, the use
of capillary control features can result in approximately a six-fold increase
in loading.
[0075] Further, without a capillary control feature, the contact angle of the
meniscus
limits the volume of coating on the microprojection. Microprojections formed
from
titanium, for example, exhibit a contact angle of approximately 65 as shown
in Fig. 8, and
microprojections formed from stainless steel have an even lower contact angle.
In
14
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
contrast, the use of a capillary control feature allows the contact angle to
approach 90 ,
effectively removing contact angle as a limiting factor. As shown in Fig. 9,
the contact
angle with a microprojection having a capillary control feature is
approximately 88 .
Accordingly, the use of capillary control features allows coatings to be
applied to the
microprojection at contact angles greater than would be possible without such
features.
[0076] For example, using the capillary control features of the invention, the
coating
formulation can be applied with a contact angle greater than approximately 25
degrees.
More preferably, the coating formulation can be applied with a contact angle
between
approximately 30 and 60 degrees.
[0077] In one aspect of the invention, the biologically active agent comprises
a
therapeutic agent in all the major therapeutic areas including, but not
limited to, anti-
infectives, such as antibiotics and antiviral agents; analgesics, including
buprenorphine
and analgesic combinations; anesthetics; anorexics; antiarthritics;
antiasthinatic agents,
such as terbutaline; anticonvulsants; antidepressants; antidiabetic agents;
antidiarrheals;
antihistamines; anti-inflammatory agents; antimigraine preparations;
antimotion sickness
preparations, such as scopolamine and ondansetron; antinauseants;
antineoplastics ;
antiparkinsonism drugs; antipruritics; antipsychotics; antipyretics;
antispasmodics,
including gastrointestinal and urinary; anticholinergics; sympathomimetrics;
xanthine
derivatives; cardiovascular preparations, including calcium channel blockers
such as
nifedipine; beta blockers; beta-agonists, such as dobutamine and ritodrine;
antiarrythmics;
antihypertensives, such as atenolol; ACE inhibitors, such as ranitidine;
diuretics;
vasodilators, including general, coronary, peripheral, and cerebral; central
nervous system
stimulants; cough and cold preparations; decongestants; diagnostics; hormones,
such as
parathyroid hormone; hypnotics; immunosuppressants; muscle relaxants;
parasympatholytics; parasympathomimetrics; prostaglandins; proteins; peptides;
psychostimulants; sedatives; and tranquilizers. Other suitable agents include
vasoconstrictors, anti-healing agents and pathway patency modulators. One or
more
biologically active agents can also be combined as desired.
[0078] In a preferred embodiment, the biologically active agent is selected
from the
group consisting of growth hormone release hormone (GHRH), growth hormone
release
factor (GHRF), insulin, insultropin, calcitonin, octreotide, endorphin, TRN,
NT-36
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
(chemical name: N-[[(s)-4-oxo-2-azetidinyl] carbonyl]-L-histidyl-L-
prolinamide), liprecin,
pituitary hormones (e.g., HGH, HMG, desmopressin acetate, etc), follicle
luteoids, aANF,
growth factors such as growth factor releasing factor (GFRF), bMSH, GH,
somatostatin,
bradykinin, somatotropin, platelet-derived growth factor releasing factor,
asparaginase,
bleomycin sulfate, chymopapain, cholecystokinin, chorionic gonadotropin,
erythropoietin,
epoprostenol (platelet aggregation inhibitor), gluagon, HCG, hirulog,
hyaluronidase,
interferon alpha, interferon beta, interferon gamma, interleukins, interleukin-
10 (IL- 10),
erythropoietin (EPO), granulocyte macrophage colony stimulating factor (GM-
CSF),
granulocyte colony stimulating factor (G-CSF), glucagon, leutinizing hormone
releasing
hormone (LHRH), LHRH analogs (such as goserelin, leuprolide, buserelin,
triptorelin,
gonadorelin, and napfarelin, menotropins (urofollitropin (FSH) and LH)),
oxytocin,
streptokinase, tissue plasminogen activator, urokinase, vasopressin, deamino
[Val4, D-
Arg8] arginine vasopressin, desmopressin, corticotropin (ACTH), ACTH analogs
such as
ACTH (1-24), ANP, ANP clearance inhibitors, angiotensin II antagonists,
antidiuretic
hormone agonists, bradykinn antagonists, ceredase, CSI's, calcitonin gene
related peptide
(CGRP), enkephalins, FAB fragments, IgE peptide suppressors, IGF-1,
neurotrophic
factors, colony stimulating factors, parathyroid hormone and agonists,
parathyroid
hornione antagonists, parathyroid hormone (PTH), PTH analogs such as PTH (1-
34),
prostaglandin antagonists, pentigetide, protein C, protein S, renin
inhibitors, thymosin
alpha-1, thrombolytics, TNF, vasopressin antagonists analogs, alpha-1
antitrypsin
(recombinant), and TGF-beta..
[0079] Other suitable biologically active agents comprise immunologically
active agents,
such as vaccines and antigens in the form of proteins, polysaccharide
conjugates,
oligosaccharides, and lipoproteins. Specific subunit vaccines in include,
without
limitation, Bordetella pertussis (recombinant PT accince - acellular),
Clostridium tetani
(purified, recombinant), Corynebacterium diphtheriae (purified, recombinant),
Cytomegalovirus (glycoprotein subunit), Group A streptococcus (glycoprotein
subunit,
glycoconjugate Group A polysaccharide with tetanus toxoid, M protein/peptides
linked to
toxing subunit carriers, M protein, multivalent type-specific epitopes,
cysteine protease,
C5a peptidase), Hepatitis B virus (recombinant Pre S1, Pre-S2, S, recombinant
core
protein), Hepatitis C virus (recombinant - expressed surface proteins and
epitopes),
Human papillomavirus (Capsid protein, TA-GN recombinant protein L2 and E7
[from
16
CA 02602814 2007-09-28
WO 2006/105233 PCT/US2006/011530
HPV-6], MEDI-501 recombinant VLP L1 from HPV-11, Quadrivalent recombinant BLP
Ll [from HPV-6], HPV-11, HPV-16, and HPV-18, LAMP-E7 [from HPV-16]),
Legionella pneumophila (purified bacterial survace protein), Neisseria
meningitides
(glycoconjugate with tetanus toxoid), Pseudomonas aeruginosa (synthetic
peptides),
Rubella virus (synthetic peptide), Streptococcus pneumoniae (glycoconjugate
[1, 4, 5, 6B,
9N, 14, 18C, 19V, 23F] conjugated to meningococcal B OMP, glycoconjugate [4,
6B, 9V,
14, 18C, 19F, 23F] conjugated to CRM197, glycoconjugate [1, 4, 5, 6B, 9V, 14,
18C,
19F, 23F] conjugated to CRM1970, Treponema pallidum (surface lipoproteins),
Varicella
zoster virus (subunit, glycoproteins), and Vibrio cholerae (conjugate
lipopolysaccharide).
[0080] Suitable immunologically active agents also include nucleic acids, such
as single-
stranded and double-stranded nucleic acids, supercoiled plasnlid DNA, linear
plasmid
DNA, cosmids, bacterial artificial chromosomes (BACs), yeast artificial
chromosomes
(YACs), mammalian artificial chromosomes, and RNA molecules.
[0081] For storage and application (in accordance with one embodiment of the
invention), the microproj ection member 10 is preferably suspended in a
retainer ring by
adhesive tabs, as described in detail in Co-Pending U.S. Application No.
09/976,762 (Pub.
No. 2002/0091357), which is incorporated by reference herein in its entirety.
[0082] After placement of the microprojection member 10 in the retainer ring,
the
microprojection member 10 is applied to the patient's skin. Preferably, the
microprojection member 10 is applied to the skin using an impact applicator,
such as
disclosed in Co-Pending U.S. Application No. 09/976,798, which is incorporated
by
reference herein in its entirety.
[0083] From the foregoing description, one of ordinary skill in the art can
easily
ascertain that the present invention, among other things, provides an
effective and efficient
means for enhancing the transdermal flux of a biologically active agent into
and through
the stratum corneum of a patient.
[0084] Without departing from the spirit and scope of this invention, one of
ordinary
skill can make various changes and modifications to the invention to adapt it
to various
usages and conditions. As such, these changes and modifications are properly,
equitably,
and intended to be, within the full range of equivalence of the following
claims.
17