Note: Descriptions are shown in the official language in which they were submitted.
CA 02603314 2007-10-02
WO 2006/113572 PCT/US2006/014320
-1-
METHODS OF INCREASING NATURAL KILLER CELL ACTIVITY FOR
THERAPY
RELATED APPLICATION
This application claims the benefit of U.S. Provisional Application No.
60/671,910, filed on April 15, 2005. The entire teachings of the above
application
are incorporated herein by reference.
BACKGROUND OF THE INVENTION
Natural killer (NK) cells, a type of white blood cell, are known to be an
important component of the body's inmzune system. Because the defming function
of NK cells is spontaneous cytotoxicity without prior immunization, NK cells
can be
the first line of defense in the immune system, and are believed to play a
role in
attacking cancer cells and infectious diseases. Many conditions, such as
immunodeficiency diseases, aging, toxin exposure, endometriosis, and the like
can
leave subjects with lowered NK cell activity or dysfunctional NK cells.
For example, subjects can have decreased or deficient NK cell activity, in
conditions such as chronic fatigue syndrome (chronic fatigue immune
dysfunction
syndrome) or Epstein-Barr virus, post viral fatigue syndrome, post-
transplantation
syndrome or host-graft disease, exposure to drugs such as anticancer agents or
nitric
oxide synthase inhibitors, natural aging, and various immunodeficiency
conditions
such as severe combined immunodeficiency, variable immunodeficiency syndrome,
and the like. (Caligiuri M, Murray C, Buchwald D, Levine H, Cheney P, Peterson
D,
Komaroff AL, Ritz J. Phenotypic and functional deficiency of natural killer
cells in
patients with chronic fatigue syndrome. Jouinal of Immunology 1987; 139: 3306-
13;
Morrison LJA, Behan WHM, Behan PO. Changes in natural killer cell phenotype in
patients with post-viral fatigue syndrome. Clinical and Experimental
Immunology
1991; 83: 441-6; Klingemann, HG Relevance and Potential of Natural Killer
Cells in
Stem Cell Transplantation Biology of Blood and Marrow Transplantation
CA 02603314 2007-10-02
-2-
WO 2006/113572 PCT/US2006/014320
2000;6:90-99; Ruggeri L, Capanni M, Mancusi A, Aversa F, Martelli MF, Velardi
A. Natural killer cells as a therapeutic tool in mismatched transplantation.
Best Pract
Res Clin Haematol. 2004 Sep;17(3):427-38; Cifone MG, Ulisse S, Santoni A.
Natural killer cells and nitric oxide. Int Immunopharmacol. 2001 Aug;1(8):1513-
24;
Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells.
J
Leukoc Biol. 2004 Aug;76(2):291-9. Epub 2004 Mar 23; Alpdogan 0, van den
Brink MR. IL-7 and IL- 15: therapeutic cytokines for immunodeficiency. Trends
Immunol. 2005 Jan;26(1):56-64; Heusel JW, Ballas ZK. Natural killer cells:
emerging concepts in immunity to infection and implications for assessment of
immunodeficiency. Curr Opin Pediatr. 2003 Dec;15(6):586-93; Hacein-Bey-Abina
S, Fischer A, Cavazzana-Calvo M. Gene therapy of X-linked severe combined
immunodeficiency. Int J Hematol. 2002 Nov;76(4):295-8; Baumert E, Schlesier M,
Wolff-Vorbeck G, Peter HH.Alterations in lymphocyte subsets in variable
irnmunodeficiency syndrome Immun Infekt. 1992 Ju1;20(3):73-5.)
NK cells are known to have activity against a wide range of infectious
pathogens such as bacteria, viruses, fungi, protozoan parasites, combined
infections,
e.g., combined bacterial/viral infections, and the like. NK cells are believed
to be
particularly important in combating intracellular infections where the
pathogens
replicate in the subjects cells, e.g., a substantial fraction of viruses and
many other
pathogens that can form intracellular infections.
For example, a wide range of fungal infections are reported to be targeted by
NK cells such as Cryptococcus neoformans, dermatophytes, e.g., Trichophyton
rubrum, Candida albicans, Coccidioides immitis, Paracoccidioides brasiliensis,
or
the like (Hidore MR, Mislan TW, Murphy JW. Responses of murine natural killer
cells to binding of the fungal target Cryptococcus neoformans Infect Immun.
1991
Apr;59(4):1489-99; Akiba H, Motoki Y, Satoh M, Iwatsuki K, Kaneko F;
Recalcitrant trichophytic granuloma associated with NK-cell deficiency in a
SLE
patient treated with corticosteroid. Eur J Dermatol. 2001 Jan-Feb;l l(1):58-
62;
Mathews HL, Witek-Janusek L. Antifungal activity of interleukin-2-activated
natural killer (NK1.1+) lymphocytes against Candida albicans. J Med Microbiol.
1998 Nov;47(11):1007-14; Ampel NM, Bejarano GC, Galgiani JN. Killing of
Coccidioides immitis by human peripheral blood mononuclear cells. Infect
Immun.
1992 Oct;60(10):4200-4; Jimenez BE, Murphy JW. In vitro effects of natural
killer
CA 02603314 2007-10-02
-3-
WO 2006/113572 PCT/US2006/014320
cells against Paracoccidioides brasiliensis yeast phase. Infect Immun. 1984
Nov;46(2):552-8.)
Also targeted by NK cells are bacteria, especially intracellular bacteria,
e.g.,
Mycobacteriuna tuberculosis, Mycobacterium avium, Listeria monocytogenes, many
different viruses, such as human immunodeficiency virus, herpesviruses,
hepatitis,
and the like, and viral/bacterial co-infection (Esin S, Batoni G, Kallenius G,
Gaines
H, Campa M, Svenson SB, Andersson R, Wigzell H. Proliferation of distinct
human
T cell subsets in response to live, leilled or soluble extracts of
Mycobacterium
tuberculosis and Myco. avium. Clin Exp Immunol. 1996 Jun;104(3):419-25;
Kaufinann SH. Immunity to intracellular bacteria. Annu Rev Immunol.
1993;11:129-63; See DM, Khemka P, Sahl L, Bui T, Tilles JG. The role of
natural
killer cells in viral infections. Scand J hrununol. 1997 Sep;46(3):217-24;
Brenner
BG, Dascal A, Margolese RG, Wainberg MA. Natural killer cell function in
patients
with acquired immunodeficiency syndrome and related diseases. J Leukoc Biol.
1989 Ju1;46(1):75-83; Kottilil S. Natural ltiller cells in HIV-1 infection:
role of NK
cell-mediated non-cytolytic mechanisms in pathogenesis of HIV-1 infection.
Indian
J Exp Biol. 2003 Nov;41(11):1219-25; Herman RB, Koziel MJ. Natural killer
cells
and hepatitis C: is losing inhibition the key to clearance? Clin Gastroenterol
Hepatol.
2004 Dec;2(12):1061-3; Beadling C, Sliflca MK. How do viral infections
predispose
patients to bacterial infections? Curr Opin Infect Dis. 2004 Jun;17(3):185-91)
In addition, NK cells combat protozoal infections including toxoplasmosis,
trypanosomiasis, leishmaniasis and malaria, especially intracellular
infections
(Korbel DS, Finney OC, Riley EM. Natural killer cells and innate immunity to
protozoan pathogens. Int J Parasitol. 2004 Dec;34(13-14):1517-28; Ahmed JS,
Mehlhorn H. Review: the cellular basis of the immunity to and
immunopathogenesis
of tropical theileriosis. Parasitol Res. 1999 Jul;85(7):539-49; Osman M,
Lausten SB,
El-Sefi T, Boghdadi I, Rashed MY, Jensen SL. Biliary parasites. Dig Surg.
1998; 15(4):287-96; Gazzinelli RT, Denkers EY, Sher A. Host resistance to
Toxoplasma gondii: model for studying the selective induction of cell-mediated
immunity by intracellular parasites. Infect Agents Dis. 1993 Jun;2(3):139-49;
Askonas BA, Bancroft GJ. Interaction of African trypanosomes with the immune
system. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):41-9;
Allison
AC, Eugui EM. The role of cell-mediated immune responses in resistance to
CA 02603314 2007-10-02
-4-
WO 2006/113572 PCT/US2006/014320
malaria, with special reference to oxidant stress. Annu Rev Immunol.
1983;1:361-92.)
Therefore, NK cells are known to be such an important component of the
immune system. There is a continuing need in the art for effective treaiments
for
increasing NK cell activity.
SUMMARY OF THE INVENTION
It is now found that certain bis(thio-hydrazide) amides are surprisingly
effective at maintaining or increasing NK cell activity. The methods disclosed
herein demonstrate surprising biological activity by raising NK cell activity
in
humans (see Examples 3-6). Moreover, these surprising results were obtained in
the
presence of paclitaxel, which is lcnown in the art to reduce NK cell activity.
Disclosed are methods employing bis(thio-hydrazide amides) to increase NK
cell activity in a subject in need thereof, provided the disorder is not
cancer, a
proliferative cell disorder, a non-infective heat shoclc protein 70 (Hsp70)
responsive
disorder, or a proteasome-inhibitor responsive disorder.
Typically, a subject, e.g., a human, can be in need of increased NK cell
activity has an immunodeficiency or is treated for an infection (e.g., a
bacterial,
viral, fungal, or parasite infection, or a combination thereof).
The method includes administering to the subject an effective amount of a
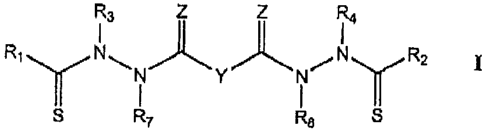
compound represented by Structural Formula I:
R3 Z Z R4
R,yN\ )t" Y ) 1 1 " /N R2 I
I I y
S R7 R$ S
Y is a covalent bond or an optionally substituted straight chained
hydrocarbyl group, or, Y, taken together with both >C=Z groups to which it is
bonded, is an optionally substituted aromatic group.
Rl-R4 are independently -H, an optionally substituted aliphatic group, an
optionally substituted aryl group, or R, and R3 taken together with the carbon
and
nitrogen atoms to which they are bonded, and/or R2 and R4 taken together with
the
carbon and nitrogen atoms to which they are bonded, form a non-aromatic
heterocyclic ring optionally fused to an aromatic ring.
CA 02603314 2007-10-02
-5-
WO 2006/113572 PCT/US2006/014320
R7-R8 are independently -H, an optionally substituted aliphatic group, or an
optionally substituted aryl group.
ZisOorS.
As used herein, the term "bis(thio-hydrazide amide)" also includes
pharmaceutically acceptable salts and solvates of the compounds represented by
Structural Formula I.
The methods described herein for increasing NK cell activity are believed to
be effective for restoring or augmenting immune function, for example in
subjects
with immunodeficiency disorders, and to treating subjects (therapeutically or
prophylactically) for infection, e.g., infections due to bacteria, fungi,
viruses,
parasites, or combinations thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
FIGs 1A, 1 B, and 1 C are bar graphs showing the percent increase in Hsp70
plasma
levels associated with administration of the Compound (1)/paclitaxel
combination therapy at 1 hour (FIG 1A), 5 hours (FIG 1B), and 8 hours (FIG
1C) after administration.
FIG 2 is a Kaplan-Meier graph of time-to-progression (resumption of cancer
growth)
in studies of various combinations of platinum anticancer drugs and taxanes.
Also shown is the disclosed combination of a bisthiohydrazide (Compound
(1)), a taxane (paclitaxel) and also a platinum anticancer drug, carboplatin.
The preliminary data shows that the disclosed method is superior to prior
platin/taxane combinations alone.
DETAILED DESCRIPTION OF THE INVENTION
A description of preferred embodiments of the invention follows.
The bis(thio-hydrazide amides) employed in the disclosed invention are
represented by Structural Formula I and pharmaceutically acceptable salts and
solvates of the compounds represented by Structural Formula I.
In one embodiment, Y in Structural Formula I is a covalent bond, -C(R5R6)-,
-(CH2CH2)-, trans-(CH=CH)-, cis-(CH=CH)- or -(C ~Q- group, preferably
-C(R5R6)-. Rl-R4 are as described above for Structural Fonnula I. R5 and R6
are
each independently -H, an aliphatic or substituted aliphatic group, or R5 is -
H and R6
CA 02603314 2007-10-02
-6-
WO 2006/113572 PCT/US2006/014320
is an optionally substituted aryl group, or, R5 and R6, talcen together, are
an
optionally substituted C2-C6 alkylene group. The pharmaceutically acceptable
cation is as described in detail below.
In specific embodiments, Y talcen together with both >C=Z groups to which
it is bonded, is an optionally substituted aromatic group. In this instance,
certain
bis(thio-hydrazide amides) are represented by Structural Formula II:
i3 ~/ i R4
R N\ R2 II
N Y
y IN I
S R7 R$ S
wherein Ring A is substituted or unsubstituted and V is -CH- or -N-. The other
variables in Structural Formula II are as described herein for Structural
Formula I or
III.
In particular embodiments, the bis(thio-hydrazide amides) are represented by
Structural Formula III:
R3 Z Z R4
R N~11 N N/NyR2 III
y I I S R7 R5 R6 R$ S
Rl-R$ and the pharmaceutically acceptable cation are as described above for
Structural Formula I.
In Structural Fonnulas I-III, R, and R2 are the same or different and/or R3
and R4 are the same or different; preferably, RI and K, are the same and R3
and R4
are the same. In Structural Formulas I and III, Z is preferably O. Typically
in
Structural Formulas I and III, Z is 0; Rj and R2 are the same; and R3 and R4
are the
same. More preferably, Z is 0; Rl and R2 are the saine; R3 and R4 are the
same, and
R7 and R8 are the same.
In other embodiments, the bis(thio-hydrazide amides) are represented by
Structural Formula III: R, and R2 are each an optionally substituted aryl
group,
preferably an optionally substituted phenyl group; R3 and R4 are each an
optionally
substituted aliphatic group, preferably an allcyl group, more preferably,
methyl or
ethyl; and R5 and R6 are as described above, but R5 is preferably -H and R6 is
preferably -H, an aliphatic or substituted aliphatic group.
CA 02603314 2007-10-02
-7-
WO 2006/113572 PCT/US2006/014320
Alternatively, Rl and R2 are each an optionally substituted aryl group; R3 and
R4 are each an optionally substituted aliphatic group; R5 is -H; and R6 is -H,
an
aliphatic or substituted aliphatic group. Preferably, R, and R2 are each an
optionally
substituted aryl group; R3 and R4 are each an alkyl group; and R5 is -H and R6
is -H
or methyl. Even more preferably, R, and R2 are each an optionally substituted
phenyl group; R3 and R4 are each methyl or ethyl; and R5 is -H and R6 is -H or
methyl. Suitable substituents for an aryl group represented by Rl and R2 and
an
aliphatic group represented by R3, R4 and R6 are as described below for aryl
and
aliphatic groups.
In another embodiment, the bis(thio-hydrazide amides) are represented by
Structural Formula III: Rl and R2 are each an optionally substituted aliphatic
group,
preferably a C3-C8 cycloallcyl group optionally substituted with at least one
alkyl
group, more preferably cyclopropyl or 1-inethylcyclopropyl; R3 and R4 are as
described above for Structural Formula I, preferably both an optionally
substituted
alkyl group; and R5 and R6 are as described above, but R5 is preferably -H and
R6 is
preferably -H, an aliphatic or substituted aliphatic group, more preferably -H
or
methyl.
Alternatively, the bis(thio-hydrazide amides) are represented by Structural
Formula III: RI and R2 are each an optionally substituted aliphatic group; R3
and R4
are as described above for Structural Formula I, preferably both an optionally
substituted alkyl group; and R5 is -H and R6 is -H or an optionally
substituted
aliphatic group. Preferably, R, and R2 are both a C3-C8 cycloalkyl group
optionally
substituted with at least one alkyl group; R3 and R4 are both as described
above for
Structural Formula I, preferably an alkyl group; and R5 is -H and R6 is -H or
an
aliphatic or substituted aliphatic group. More preferably, Rl and R2 are both
a
C3-C8 cycloalkyl group optionally substituted with at least one alkyl group;
R3 and
R4 are both an alkyl group; and R5 is -H and R6 is -H or methyl. Even more
preferably, Rl and R2 are both cyclopropyl or 1-methylcyclopropyl; R3 and R4
are
both an alkyl group, preferably methyl or ethyl; and R5 is -H and R6 is -H or
methyl.
In specific embodiments, the bis(thio-hydrazide amides) are represented by
Structural Formula IV:
CA 02603314 2007-10-02
-8-
WO 2006/113572 PCT/US2006/014320
IR3 O O I4
R, N~N N R2 IV
Y H H Y
S R5 R6 S
wherein: R, and R2 are both phenyl, R3 and R4 are both methyl, and R5 and
R6 are both -H; R, and R2 are both phenyl, R3 and R4 are both ethyl, and R5
and R6
are both -H; Rl and R2 are both 4-cyanophenyl, R3 and R4 are both methyl, RS
is
methyl, and R6 is -H; R, and R2 are both 4-methoxyphenyl, R3 and R4 are both
methyl, and R5 and R6 are both -H; R, and R2 are both phenyl, R3 and R4 are
both
methyl, R5 is methyl, and R6 is -H; Rl and R2 are both phenyl, R3 and R4 are
both
ethyl, R5 is methyl, and R6 is -H; R, and R2 are both 4-cyanophenyl, R3 and R4
are
both methyl, and R5 and R6 are both -H; RI and R2 are both 2,5-
dimethoxyphenyl,
R3 and R4 are both methyl, and R5 and R6 are both -H; R, and R2 are both
2,5-dimethoxyphenyl, R3 and R4 are both methyl, R5 is methyl, and R6 is -H; Rl
and
R2 are both 3-cyanophenyl, R3 and R4 are both methyl, and R5 and R6 are both -
H; Rl
and R2 are both 3-fluorophenyl, R3 and R4 are both methyl, and R5 and R6 are
both
-H; Rl and R2 are both 4-chlorophenyl, R3 and R4 are both methyl, R5 is
methyl, and
R6 is -H; Rl and R2 are both 2-dimethoxyphenyl, R3 and R4 are both methyl, and
R5
and R6 are both -H; Rl and R2 are both 3-methoxyphenyl, R3 and R4 are both
methyl,
and R5 and R6 are both -H; R, and R2 are both 2,3-dimethoxyphenyl, R3 and R4
are
both methyl, and RS and R6 are both -H; Rl and R2 are both 2,3-
dimethoxyphenyl,
R3 and R4 are both methyl, R5 is methyl, and R6 is -H; RI and R2 are both
2,5-difluorophenyl, R3 and R4 are both methyl, and R5 and R6 are both -H; Rl
and R2
are both 2,5-difluorophenyl, R3 and R4 are both methyl, R5 is methyl, and R6
is -H;
RI and R2 are both 2,5-dichlorophenyl, R3 and R4 are both methyl, and R5 and
R6 are
both -H; Rl and R2 are botli 2,5-dimethylphenyl, R3 and R4 are both methyl,
and R5
and R6 are both -H; R, and R2 are both 2,5-dimethoxyphenyl, R3 and R4 are both
methyl, and R5 and R6 are both -H; Rl and R2 are both phenyl, R3 and R4 are
both
methyl, and R5 and R6 are both -H; Rj and R2 are both 2,5-dimethoxyphenyl, R3
and
R4 are both methyl, R5 is methyl, and R6 is -H; R, and R2 are both
cyclopropyl, R3
and R4 are both methyl, and R5 and R6 are both -H; Ri and R2 are both
cyclopropyl,
R3 and R4 are both ethyl, and R5 and R6 are both -H; Rl and R2 are both
cyclopropyl,
R3 and R4 are both methyl, R5 is methyl, and R6 is -H; Rl and R2 are both
1-methylcyclopropyl, R3 and R4 are both methyl, and R5 and R6 are both -H; Rl
and
CA 02603314 2007-10-02
-9-
WO 2006/113572 PCT/US2006/014320
R2 are both 1-methylcyclopropyl, R3 and R4 are both methyl, R5 is methyl and
R6 is
-H; Rl and R2 are both 1-methylcyclopropyl, R3 and R4 are both methyl, R5 is
ethyl,
and R6 is -H; R1 and R2 are both 1-methylcyclopropyl, R3 and R4 are both
methyl, R5
is n-propyl, and R6 is -H; Rl and R2 are both 1-methylcyclopropyl, R3 and R4
are
both methyl, and R5 and R6 are both methyl; Rl and R2 are both
1-methylcyclopropyl, R3 and R4 are both ethyl, and R5 and R6 are both -H; Rl
and R2
are both 1-methylcyclopropyl, R3 is methyl, R4 is ethyl, and RS and R6 are
both -H;
Rl and R2 are both 2-methylcyclopropyl, R3 and R4 are both methyl, and RS and
R6
are both -H; Rt and R2 are both 2-phenylcyclopropyl, R3 and R4 are both
methyl, and
R5 and R6 are both -H; R, and R2 are both 1-phenylcyclopropyl, R3 and R4 are
both
methyl, and R5 and R6 are both -H; Rl and R2 are both cyclobutyl, R3 and R4
are
both methyl, and R5 and R6 are both -H; Rl and R2 are both cyclopentyl, R3 and
R4
are both methyl, and R5 and R6 are both -H; Rl and R2 are both cyclohexyl, R3
and
R4 are both methyl, and R5 and R6 are both -H; Rl and R2 are both cyclohexyl,
R3
and R4 are both phenyl, and R5 and R6 are both -H; Rl and R2 are both methyl,
R3
and R4 are both methyl, and R5 and R6 are both -H; R, and R2 are both methyl,
R3
and R4 are both t-butyl, and R5 and R6 are both -H; R, and R2 are both methyl,
R3
and R4 are both phenyl, and R5 and R6 are both -H; Rl and R2 are both t-butyl,
R3
and R4 are both methyl, and R5 and Rg are both -H; R, and R2 are ethyl, R3 and
R4
are both methyl, and R5 and R6 are both -H; or Rt and R2 are both n-propyl, R3
and
R4 are both methyl, and R5 and R6 are both -H.
In specific embodiments, the bis(thio-hydrazide amides) are represented by
Structural Formula V:
R3 O O R4
( I
R, N\ N R
y 2 V
H H Ys S
wherein: Rl and R2 are both phenyl, and R3 and R4 are both o-CH3-phenyl; Rl
and
R2 are both o-CH3C(O)O-phenyl, and R3 and R4 are phenyl; Rl and R2 are both
phenyl, and R3 and R4 are both methyl; R, and R2 are both phenyl, and R3 and
R4 are
both ethyl; Rl and R2 are both phenyl, and R3 and R4 are both n-propyl; Rl and
R2
are bothp-cyanophenyl, and R3 and R4 are both methyl; Rl and R2 are bothp-
nitro
phenyl, and R3 and R4 are both methyl; Rl and R2 are both 2,5-dimethoxyphenyl,
and R3 and R4 are both methyl; Rl and R2 are both phenyl, and R3 and R4 are
both
CA 02603314 2007-10-02
-10-
WO 2006/113572 PCT/US2006/014320
n-butyl; R, and R2 are both p-chlorophenyl, and R3 and R4 are both methyl; Rl
and
R2 are both 3-nitrophenyl, and R3 and R4 are both methyl; R, and R2 are both
3-cyanoplienyl, and R3 and R4 are both methyl; Rl and RZ are both 3-
fluorophenyl,
and R3 and R4 are both methyl; R, and R2 are both 2-furanyl, and R3 and R4 are
both
phenyl; R, and R2 are both 2-methoxyphenyl, and R3 and R4 are both methyl; R,
and
R2 are both 3-methoxyphenyl, and R3 and R4 are both methyl; RI and R2 are both
2,3-dimethoxyphenyl, and R3 and R4 are both methyl; Ri and R2 are both
2-methoxy-5-chlorophenyl, and R3 and R4 are both ethyl; Rl and R2 are both
2,5-difluorophenyl, and R3 and R4 are both methyl; Rl and R2 are both
2,5-dichlorophenyl, and R3 and R4 are both methyl; RI and R2 are both
2,5-dimethylphenyl, and R3 and R4 are both methyl; R, and R2 are both
2-methoxy-5-chlorophenyl, and R3 and R4 are both methyl; R, and R2 are both
3,6-dimethoxyphenyl, and R3 and R4 are both methyl; Ri and Rz are both phenyl,
and R3 and R4 are both 2-ethylphenyl; Rl and R2 are both 2-methyl-5-pyridyl,
and
R3 and R4 are both methyl; or Rl is phenyl; R2 is 2,5-dimethoxyphenyl, and R3
and
R4 are both methyl; Rl and R2 are both methyl, and R3 and R4 are both
p-CF3-phenyl; R, and R2 are both methyl, and R3 and R4 are both o-CH3-phenyl;
Rl
and R2 are both -(CH2)3COOH; and R3 and R4 are both phenyl; RI and R2 are both
aY
represented by the following structural formula: 0 and R3
and R4 are both phenyl; R, and Rz are both n-butyl, and R3 and R4 are both
phenyl;
RI and RZ are both n-pentyl, R3 and R4 are both phenyl; Rl and RZ are both
methyl,
and R3 and R4 are both 2-pyridyl; R, and RZ are both cyclohexyl, and R3 and R4
are
both phenyl; Rl and RZ are both methyl, and R3 and R4 are both 2-ethylphenyl;
R,
and R2 are both methyl, and R3 and R4 are both 2,6-dichlorophenyl; Rl-R4 are
all
methyl; Rl and R2 are both methyl, and R3 and R4 are both t-butyl; Rl and R,,
are
both ethyl, and R3 and R4 are both methyl; RI and R2 are both t-butyl, and R3
and R4
are both methyl; RI and R? are both cyclopropyl, and R3 and R4 are both
methyl; RI
and R2 are both cyclopropyl, and R3 and R4 are both ethyl; Rl and R2 are both
1-methylcyclopropyl, and R3 and R4 are both methyl; Rl and R2 are both
2-methylcyclopropyl, and R3 and R4 are both methyl; Rl and R2 are both
1-phenylcyclopropyl, and R3 and R4 are both methyl; Rl and RZ are both
CA 02603314 2007-10-02
-11-
WO 2006/113572 PCT/US2006/014320
2-phenylcyclopropyl, and R3 and R4 are both methyl; Rl and R2 are both
cyclobutyl,
and R3 and R4 are both methyl=, R, and R2 are both cyclopentyl, and R3 and R4
are
both methyl; R, is cyclopropyl, R2 is phenyl, and R3 and R4 are both methyl.
Preferred examples of bis(thio-hydrazide amides) include Compounds
(1)-(18) and pharmaceutically acceptable salts and solvates thereof:
OL1rOyO
S S
Compound (1)
\ \
O O
,Y NN
S N N S
Compound (2)
\ \
0 0
N~N N~N
S H H S
Compound (3)
0 0
NNIN "N
S H H
Compound (4)
(
OY 0 0 /
\
N --))A N \
H H
S S
Compound (5)
CA 02603314 2007-10-02
-12-
WO 2006/113572 PCT/US2006/014320
H3CO OCH3
/ O O /
N"~N \
S
H H S
Compound (6)
NC CN
/ O O Ya
S \ N~~N'N H H S
Compound (7)
OCH3 OCH3
/ O O
N, NA~"~N,N \
CH30 s H H S OCH3
Compound (8)
OCH3 OCH3
~
yNNNNy
--,)A
C
H30 s H H S OCH3
Compound (9)
F F
o o
N, N N_-N
S H H Yi:
R s
Compound (10)
N 0 0
3C0 ~N"~N"N OCH3
yl;)~ H
CH3O S H H S OCH3
Compound (11)
CA 02603314 2007-10-02
- 13-
WO 2006/113572 PCT/US2006/014320
O O I / I
H3CO N~N OCH3
CH30 S H H S OCH3
Compound (12)
OCH3 OCH3
/ I I O O I / I
NA~N~N
H H Yc
Compound (13)
CI CI
/ I I O O ( / I
N"~AN'N
Y-
ci S H H S CI
Compound (14)
OCH3
IDY O O I / I
N N~N \
H H Y
S S OCH3
Compound (15)
ID O O ~ /
N~ iN I
N N
H H
S
Compound (16)
CA 02603314 2007-10-02
-14-
WO 2006/113572 PCT/US2006/014320
O O I
Y Nl~ N)L"~N"IN
H H
S S
Compound (17) ; and
O o I
N" N'JL~"AN~N
H H
S Compound (18) S
Particular examples of bis(thio-hydrazide amides) include Compounds (1),
(17), and (18) and pharmaceutically acceptable salts and solvates thereof.
A "straight chained hydrocarbyl group" is an alkylene group, i.e., -(CH2)y-,
with one, or more (preferably one) internal methylene groups optionally
replaced
with a linkage group. y is a positive integer (e.g., between 1 and 10),
preferably
between 1 and 6 and more preferably 1 or 2. A "linkage group" refers to a
functional group which replaces a metliylene in a straight chained
hydrocarbyl.
Examples of suitable linkage groups include a ketone (-C(O)-), alkene, alkyne,
phenylene, ether (-0-), thioether (-S-), or amine (-N(Ra)-), wherein Ra is
defmed
below. A preferred linkage group is -C(R5R6)-, wherein R5 and R6 are defined
above. Suitable substituents for an allcylene group and a hydrocarbyl group
are those
which do not substantially interfere with the activity of the bis(thio-
hydrazide)
amides. R5 and R6 are preferred substituents for an alkylene or hydrocarbyl
group
represented by Y.
An aliphatic group is a straight chained, branched or cyclic non-aromatic
hydrocarbon which is completely saturated or which contains one or more units
of
unsaturation. Typically, a straight chained or branched aliphatic group has
from 1 to
about 20 carbon atoms, preferably from 1 to about 10, and a cyclic aliphatic
group
has from 3 to about 10 carbon atoms, preferably from 3 to about 8. An
aliphatic
group is preferably a straight chained or branched alkyl group, e.g., methyl,
ethyl,
n-propyl, iso-propyl, n-butyl, sec-butyl, tert-butyl, pentyl, hexyl, pentyl or
octyl, or a
cycloalkyl group with 3 to about 8 carbon atoms. A Cl-C20 straight chained or
branched alkyl group or a C3-C8 cyclic alkyl group is also referred to as a
"lower
alkyl" group.
CA 02603314 2007-10-02
-15-
WO 2006/113572 PCT/US2006/014320
The term "aromatic group" may be used interchangeably with "aryl," "aryl
ring," "aromatic ring," "aryl group" and "aromatic group." Aromatic groups
include
carbocyclic aromatic groups such as phenyl, naphthyl, and anthracyl, and
heteroaryl
groups such as imidazolyl, thienyl, furanyl, pyridyl, pyrimidyl, pyranyl,
pyrazolyl,
pyrroyl, pyrazinyl, thiazole, oxazolyl, and tetrazole. The term "heteroaryl
group"
may be used interchangeably with "heteroaryl," "heteroaryl ring,"
"heteroaromatic
ring" and "heteroaroinatic group." The term "heteroaryl," as used herein,
means a
mono-or multi-cyclic aromatic heterocycle which comprise at least one
heteroatom
such as nitrogen, sulfur and oxygen, but may include 1, 2, 3 or 4 heteroatoms
per
ring. Aromatic groups also include fused polycyclic aromatic ring systems in
which
a carbocyclic aromatic ring or heteroaryl ring is fused to one or more other
heteroaryl rings. Examples include benzothienyl, benzofuranyl, indolyl,
quinolinyl,
benzothiazole, benzooxazole, benzimidazole, quinolinyl, isoquinolinyl and
isoindolyl.
The term "arylene" refers to an aryl group which is connected to the
remainder of the molecule by two other bonds. By way of example, the structure
of a
1,4-phenylene group is shown below:
~ 0 ~
Substituents for an arylene group are as described below for an aryl group.
Non-aromatic heterocyclic rings are non-aromatic rings which include one or
more heteroatoms such as nitrogen, oxygen or sulfur in the ring. The ring can
be
five, six, seven or eight-membered. Examples include tetrahydrofuranyl,
tetrahydrothiophenyl, morpholino, thiomorpholino, pyrrolidinyl, piperazinyl,
piperidinyl, and thiazolidinyl.
Suitable substituents on an aliphatic group (including an alkylene group),
non-aromatic heterocyclic group, benzylic or aryl group (carbocyclic and
heteroaryl)
are those which do not substantially interfere with the activity of the
bis(thio-hydrazide) amides. A substituent substantially interferes with
activity when
the activity is reduced by more than about 50% in a compound with the
substituent
compared with a compound without the substituent. Examples of suitable
substituents include -R, -OH, -Br, -Cl, -I, -F, -ORa, -O-CORa, -CORa, -CN, -
NO2,
-COOH, -SO3H, -NH2, -NHRa, -N(RaRb), -COORa, -CHO, -CONH2, -CONHRa,
-CON(RaRb), -NHCORa4-NR CORa, -NHCONH2, -NHCONRaH, -NHCON(RaRb),
CA 02603314 2007-10-02
-16-
WO 2006/113572 PCT/US2006/014320
-NR CONH2, -NR CONRaH, -NR CON(RaR), -C(=NH)-NH2, -C(=NH)-NHRa,
-C(=NH)-N(RaR'), -C(=NR )-NHza -C(=NR )-NHRa, -C(=NR )-N(RaR),
-NH-C(=NH)-NH2, -NH-C(=NH)-NHRa, -NH-C(=NH)-N(RaRb),
-NH-C(=NR')-NHz, -NH-C(=NR )-NHRa, -NH-C(=NR )-N(RaRb),
-NRdH-C(=NH)-NHz, -NR-C(=NH)-NHRa, -NRd-C(=NH)-N(RaRb),
-NRa-C(=NRc)-NH2, -NRa-C(=NW)-NHRa, -NRd-C(=NRc)-N(RaRb), -NHNH2,
-NHNHRa, -NHRaRb, -SO2NH2a -SO2NHW, -SO2NRaRb, -CH=CHRa, -CH=CRaRb,
-CW=CRaRb,-CR =CHRa, -CR =CRaRb, -CCRa, -SH, -SRa, -S(O)Ra, -S(O)2Ra.
Ra-Rd are each independently an alkyl group, aromatic group, non-aromatic
heterocyclic group or -N(RaRb), taken together, form an optionally substituted
non-aromatic heterocyclic group. The alkyl, aromatic and non-aromatic
heterocyclic
group represented by Ra-Rd and the non-aromatic heterocyclic group represented
by
-N(RaR) are each optionally and independently substituted with one or more
groups
represented by R#.
R# is W, -OR+, -O(haloallcyl), -SR+, -NOZ, -CN, -NCS, -N(R+)2, -NHCO2R+,
-NHC(O)R+, -NHNHC(O)R+, -NHC(O)N(R+)2, -NHNHC(O)N(R+)2,
-NHNHCOzR+, -C(O)C(O)R+, -C(O)CH2C(O)R+, -CO2W, -C(O)R+, -C(O)N(R+)2,
-OC(O)R+, -OC(O)N(R+)2, -S(O)2R+, -SO2N(R+)2, -S(O)R+, -NHSO2N(R+)2,
-NHSO2R+, -C(=S)N(R+)Z, or -C(=NH)-N(R+)2.
R+ is -H, a C 1-C4 allcyl group, a monocyclic heteroaryl group, a
non-aromatic heterocyclic group or a phenyl group optionally substituted with
alkyl,
haloalkyl, alkoxy, haloalkoxy, halo, -CN, -NOZ, amine, alkylamine or
dialkylamine.
Optionally, the group N(W)2 is a non-aromatic heterocyclic group, provided
that
non-aromatic heterocyclic groups represented by R+ and N(W)2 that comprise a
secondary ring amine are optionally acylated or alkylated.
Preferred substituents for a phenyl group, including phenyl groups
represented by Rl-R4, include C1-C4 alkyl, C1-C4 alkoxy, C1-C4 haloalkyl, C1-
C4
haloalkoxy, phenyl, benzyl, pyridyl, -OH, -NHZ, -F, -Cl, -Br, -I, -NOZ or -CN.
Preferred substituents for an aliphatic group, including aliphatic groups
represented by R, -R4, include C1-C4 allcyl, C1-C4 alkoxy, C1-C4 haloalkyl, C1-
C4
haloalkoxy, phenyl, benzyl, pyridyl, -OH, -NH2, -F, -Cl, -Br, -I, -NO2 or -CN.
Preferred substituents for a cycloallcyl group, including cycloalkyl groups
represented by Rl and R2, are alkyl groups, such as a methyl or ethyl groups.
CA 02603314 2007-10-02
-17-
WO 2006/113572 PCT/US2006/014320
Also included in the present invention are phannaceutically acceptable salts
of the bis(thio-hydrazide) amides employed herein. These compounds can have
one
or more sufficiently acidic protons that can react with a suitable organic or
inorganic
base to form a base addition salt., Base addition salts include those derived
from
inorganic bases, such as aminonium or alkali or alkaline earth metal
hydroxides,
carbonates, bicarbonates, and the lilce, and organic bases such as alkoxides,
alkyl
amides, allcyl and aryl amines, and the like. Such bases useful in preparing
the salts
of this invention thus include sodium hydroxide, potassium hydroxide, ammonium
hydroxide, potassium carbonate, and the lilce.
For example, phannaceutically acceptable salts of bis(thio-hydrazide) amides
employed herein (e.g., those represented by Structural Formulas I-VI,
Compounds
1-18,) are those formed by the reaction of the compound with one equivalent of
a
suitable base to form a monovalent salt (i.e., the compound has single
negative
charge that is balanced by a pharmaceutically acceptable counter cation, e.g.,
a
monovalent cation) or with two equivalents of a suitable base to form a
divalent salt
(e.g., the compound has a two-electron negative charge that is balanced by two
pharmaceutically acceptable counter cations, e.g., two pharmaceutically
acceptable
monovalent cations or a single pharmaceutically acceptable divalent cation).
Divalent salts of the bis(thio-hydrazide amides) are preferred.
"Pharmaceutically
acceptable" means that the cation is suitable for administration to a subject.
Examples include Li+, Na~, K+, MgZ+, Ca'+ and NR4+, wherein each R is
independently hydrogen, an optionally substituted aliphatic group (e.g., a
hydroxyalkyl group, aminoalkyl group or ammoniumalkyl group) or optionally
substituted aryl group, or two R groups, taken together, form an optionally
substituted non-aromatic heterocyclic ring optionally fased to an aromatic
ring.
Generally, the phannaceutically acceptable cation is Li+, Na+, K+,
NH3(C2H5OH)+ or
N(CH3)3(C2H5OH)+, and more typically, the salt is a disodium or dipotassium
salt,
preferably the disodium salt.
Bis(thio-hydrazide) amides employed herein having a sufficiently basic
group, such as an amine can react with an organic or inorganic acid to form an
acid
addition salt. Acids commonly employed to form acid addition salts from
compounds with basic groups are inorganic acids such as hydrochloric acid,
hydrobromic acid, hydroiodic acid, sulfuric acid, phosphoric acid, and the
like, and
organic acids such as p-toluenesulfonic acid, methanesulfonic acid, oxalic
acid,
CA 02603314 2007-10-02
-18-
WO 2006/113572 PCT/US2006/014320
p-bromophenyl-sulfonic acid, carbonic acid, succinic acid, citric acid,
benzoic acid,
acetic acid, and the lilce. Examples of such salts include the sulfate,
pyrosulfate,
bisulfate, sulfite, bisulfite, phosphate, monohydrogenphosphate,
dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide,
acetate, propionate, decanoate, caprylate, acrylate, formate, isobutyrate,
caproate,
heptanoate, propiolate, oxalate, malonate, succinate, suberate, sebacate,
fumarate,
maleate, butyne-1,4-dioate, hexyne-1,6-dioate, benzoate, chlorobenzoate,
methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate,
sulfonate, xylenesulfonate, phenylacetate, phenylpropionate, phenylbutyrate,
citrate,
lactate, gainma-hydroxybutyrate, glycolate, tartrate, methanesulfonate,
propanesulfonate, naphthalene-1-sulfonate, naphthalene-2-sulfonate, mandelate,
and
the like.
Particular salts of the bis(thio-hydrazide amide) compounds described herein
can be prepared according to methods described in copending, co-owned Patent
Application Serial No. 60/582,596, filed June 23, 2004.
The neutral bis(thio-hydrazide) amides can be prepared according to methods
described in U.S. Patent Nos. 6,800,660, and 6,762,204, both entitled
"Synthesis of
Taxol Enhancers" and also according to methods described in the co-pending and
co-owned U.S. Pat. Appl. Ser. Nos. 10/345,885 filed January 15, 2003, and
10/758,589, January 15, 2004. The entire teachings of each document referred
to in
this application is expressly incorporated herein by reference.
It will also be understood that certain compounds employed in the invention
maybe obtained as different stereoisomers (e.g., diastereomers and
enantiomers)
and that the invention includes all isomeric forms and racemic mixtures of the
disclosed compounds and methods of treating a subject with both pure isomers
and
mixtures thereof, including racemic mixtures. Stereoisomers can be separated
and
isolated using any suitable method, such as chromatography.
A "subject" includes mammals, e.g., humans, companion animals (e.g., dogs,
cats, birds, aquarium fish, reptiles, and the like), famz animals (e.g., cows,
sheep,
pigs, horses, fowl, farm-raised fish and the like) and laboratory animals
(e.g., rats,
mice, guinea pigs, birds, aquarium fish, reptiles, and the like).
Alternatively, the
subject is a warm-blooded animal. More preferably, the subject is a mammal.
Most
preferably, the subject is human.
CA 02603314 2007-10-02
-19-
WO 2006/113572 PCT/US2006/014320
A subject in need of treatment is in need of immune system augmentation
because of infection or the possibility thereof. In some embodiments, such a
subject
can have an infection (or has been exposed to an infectious environment where
pathogens are present, e.g., in a hospital) the symptoms of which may be
alleviated
by the methods disclosed herein. For example, a subject in need of treatment
can
have an infection (bacterial, viral, fungal, or parasitical (protozoal) for
which the
disclosed methods of activating NK cells can be a treatment.
In some embodiments, a subject in need of treatment is in need of immune
system augmentation because the subject has an immunodeficiency. Such a
subject
is in need of or can benefit from prophylactic therapy, for example, a subject
that has
incomplete, damaged or otherwise compromised defenses against infection, or is
subject to an infective environment, or the like. For example, a subject can
be in an
infectious environment where pathogens are present, e.g., in a hospital; can
have an
open wound or bum injury; can have an inherited or acquired immune deficiency
(e.g., severe combined immunodeficiency or "bubble boy" syndrome, variable
immunodeficiency syndrome acquired immune deficiency syndrome (AIDS), or the
like); can have a depressed immune system due to physical condition, age,
toxin
exposure, drug effect (immunosuppressants, e.g., in a transplant recipient) or
side
effect (e.g., due to an anticancer agent); or the like.
In some embodiments, NK activity can be increased in subjects that have
decreased or deficient NK cell activity, in conditions such as chronic fatigue
syndrome (chronic fatigue immune dysfunction syndrome) or Epstein-Barr virus
infection, post viral fatigue syndrome, post-transplantation syndrome
(especially
allogeneic transplants) or host-graft disease, exposure to drugs such as
anticancer
agents or nitric oxide synthase inhibitors, natural aging, and various
immunodeficient conditions such as severe combined immunodeficiency, variable
immunodeficiency syndrome, and the like.
In some embodiments, the subject is in need of treatment for bacteremia.
Bacteremia is the condition of bacterial infection in the bloodstream. Septic
shock
includes serious localized or bacteremic infection accompanied by systemic
inflammation, in other words sepsis with hypoperfusion and hypotension
refractory
to fluid therapy. Sepsis, or systemic inflammatory response syndrome, includes
various severe conditions such as infections, pancreatitis, bums, trauma) that
can
cause acute inflammation. Septic shock is typically related to infections by
CA 02603314 2007-10-02
-20-
WO 2006/113572 PCT/US2006/014320
gram-negative organisms, staphylococci, or meningococci. Septic shock can be
characterized by acute circulatory failure, typically with hypotension, and
multiorgan failure.
In some embodiments, the methods do not include sepsis.
Transient bacteremia can be caused by surgical or trauma wounds.
Gram-negative bacteremia can be intermittent and opportunistic; although it
may
have no effect on a healthy person, it may be seriously important in
immunocompromised patients with debilitating underlying diseases, after
chemotherapy, and in settings of malnutrition. The infection can typically be
in the
lungs, in the GU or GI tract, or in soft tissues, e.g., skin in patients with
decubitus
ulcer, oral ulcers in patients at risk, and patients with valvular heart
disease,
prosthetic heart valves, or other implanted prostheses.
Typically, gram-negative bacteremia can manifest in chronically ill and
immunocompromised patients. Also in such patients, bloodstream infections can
be
caused by aerobic bacilli, anaerobes, and fungi. Bacteroides can lead to
abdominal
and pelvic infective complications, especially in females. Transient or
sustained
bacteremia can typically result in metastatic infection of the meninges or
serous
cavities, such as the pericardium or larger joints. Enterococcus,
staphylococcus, or
fungus can lead to endocarditis, but is less common with gram-negative
bacteremia.
Staphylococcal bacteremia can be typical of IV drug users, and can be a
typical
cause of gram-positive bacterial endocarditis.
The incidence of systemic fungal infections has undergone a significant
increase, particularly in hunians, due in part to increases in the number of
subjects
with compromised immune systems, for example, the elderly, AIDS patients,
patients undergoing chemotherapy, bum patients, patients with diabetic
ketoacidosis,
and transplant patients on immunosuppressive drugs. A study found that about
40%
of deaths from infections acquired during hospitalization were due to mycoses;
see
Stemberg et. al, Science, Vol. 266, (1994), pp.1632-1634, the entire teachings
of
which are incorporated herein by reference.
In various embodiments, the subject can be treated for a fungal infection
from a pathogenic dermatophyte, a pathogenic filamentous fungus, and/or a
pathogenic non- filamentous fungus, e.g., a yeast, or the like. Pathogenic
dermatophytes can include, e.g., species of the genera Trichophyton, Tinea,
Microsporum, Epidermophyton, or the lilce. Pathogenic filamentous fungus can
CA 02603314 2007-10-02
-21-
WO 2006/113572 PCT/US2006/014320
include, e.g., species of genera such as Aspergillus, Histoplasma,
Cryptococcus,
Microsporum, or the like. Pathogenic non- filamentous fungus, e.g., yeasts,
can
include, for example, species of the genera Candida, Malassezia, Trichosporon,
Rhodotorula, Torulopsis, Blastomyces, Paracoccidioides, Coccidioides, or the
like.
In various embodiments, the subject can be treated for a fungal infection from
a
species of the genera Aspergillus or Trichophyton. Species of Trichophyton can
include, for example, Trichophyton mentagroplzytes, Trichophyton rubrum,
Trichophyton schoenleinii, Trichoplzyton toiasurans, Ti ichoplzyton
verrucosum, and
Trichoplayton violaceuin. Species of Aspergillus can include, for example,
Aspergillusfumigatus, Aspergillus flavus, Aspergillus niger, Aspergillus
amstelodwni, Aspergillus candidus, Aspergillus carneus, Aspergillus nidulans,
A
oryzae, Aspergillus restrictus, Aspergillus sydowi, Aspergillus terreus,
Aspergillus
ustus, Aspergillus versicolor, Aspergillus caesiellus, Aspergillus clavatus,
Aspergillus avenaceus, and Aspergillus deflectits. In some embodiments, the
subject
can be treated for a ftmgal infection from a pathogenic dermatophyte, e.g.,
Trichophyton (e.g., Trichophyton rubrum), Tinea, Microsporum, or
Epidermophyton; or Cryptococcus (e.g., Cryptococcus neoformans) Candida (e.g.,
Candida albicans), Paracoccidioides (e.g., Paracoccidioides brasiliensis), or
Coccidioides (e.g., Coccidioides imrnitis). In particular embodiments, the
subject
can be treated for a fungal infection from Trichophyton rubrum, Cryptococcus
neoformans, Candida albicans, Paracoccidioides brasiliensis, or Coccidioides
immitis.
Thus, in various embodiments, a subject can have an infection caused by a
fungus selected from the genera Trichophyton, Tinea, Microsporum,
Epidermophyton, Aspergillus, Histoplasma, Cryptococcus, Microsporum, Candida,
Malassezia, Trichosporon, Rhodotorula, Torulopsis, Blastomyces,
Paracoccidioides,
and Coccidioides. In some embodiments, the subject can have an infection
caused
by a fungus selected from the genera Trichophyton, Tinea, Microsporum,
Epidermophyton; Cryptococcus, Candida, Paracoccidioides, and Coccidioides. In
certain embodiments, the subject can have an infection caused by a fungus
selected
from Trichophyton rubruin, Cryptococcus neoformans, Candida albicans,
Paracoccidioides brasiliensis, and Coccidioides irnmitis.
In various embodiunents, the subject can be treated for a bacterial infection
caused by a bacteria of a genus selected from Allochromatium, Acinetobacter,
CA 02603314 2007-10-02
-22-
WO 2006/113572 PCT/US2006/014320
Bacillus, Campylobacter, Chlamydia, Chlamydophila, Clostridium, Citrobacter,
Escherichia, Enterobacter, Enterococcus, Francisella, Haemophilus,
Helicobacter,
Klebsiella, Listeria, Moraxella, Mycobacterium, Micrococcus, Neisseria,
Proteus,
Pseudomonas, Salmonella, Serratia, Shigella, Stenotrophomonas, Staphyloccocus,
Streptococcus, Synechococcus, Vibrio, and Yersina; or anerobic bacterial
genera
such as Peptostreptococci, Porphyroinonas, Actinomyces, Clostridium,
Bacteroides,
Prevotella, Anaerobiospirillum, Fusobacterium, and Bilophila. In some
embodiments, the subject can be treated for a bacterial infection from
Allochromatium vinosum, Acinetobacter baumanii, Bacillus antlaracis,
Campylobacter jejuni, Chlamydia trachomatis, Chlarnydia pneutnoniae,
Clostridium
spp., Citrobacter spp., Escherichia coli, Enterobacter spp.,
Enterococcusfaecalis.,
Enterococcus faeciuzn, Francisella tularensis, Haemophilus influenzae,
Helicobacter pylori, Klebsiella spp., Listeria monocytogenes, Moraxella
catarrhalis,
Mycobacterium tuberculosis, Neisseria meningitidis, Neisseria gonorrhoeae,
Proteus mirabilis, Proteus vulgaris, Pseudonaonas aeruginosa, Salmonella spp.,
Serratia spp., Slzigella spp., Stenotrophomonas maltophilia, Staphyloccocus
aureus,
Staphyloccocus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes,
Streptococcus agalactiae, Yersina pestis, and Yersina enterocolitica, or the
like; or
Peptostreptococci asaccharolyticus, Peptostreptococci magnus,
Peptostreptococci
micros, Peptostreptococci prevotii, Porphyromonas asaccharolytica,
Porphyromonas canoris, Porplayrornonas gingivalis, Porphyromonas macaccae,
Actinonzyces israelii, Actinomyces odontolyticus, Clostridium innocuum,
Clostridium clostridiofornie, Clostridium diff cile, Bacteroides tectum,
Bacteroides
ureolyticus, Bacteroides gracilis (Cainpylobacter gracilis), Prevotella
intermedia,
Prevotella heparinolytica, Prevotella oris-buccae, Prevotella bivia,
Prevotella
melaninogenica, Fusobacteriurn naviforme, Fusobacterium n.ecrophorum,
Fusobacterium varium, Fusobacterium ulcerans, Fusobacterium russii, Bilophila
wadsworthia, Haemophilus ducreyi; Calymnaatobacterium granulomatis,or the
like.
It is believed that the method can be particularly useful for treating a
subject
with an intracellular infection. It is generally believed in the art that NK
cells are
particularly effective against intracellular infections. Intracellular
infections are
those wherein a portion of the infecting pathogen resides within cells of the
subject.
For example, intracellular infections can be caused by one or more bacteria
selected from: Ehrlichia (e.g., obligate, intracellular bacteria that can
appear as small
CA 02603314 2007-10-02
- 23 -
WO 2006/113572 PCT/US2006/014320
cytoplasmic inclusions in lymphocytes aiid neutrophils such as Ehrliclzia
sennetsu,
Ehrlichia canis, Elarlichia chaffeensis, Ehrlichia phagocytophilia, or the
like);
Listeria (e.g., Listeria monocytogenes); Legionella (e.g., Legionella
pneumophila);
Rickettsiae (e.g., Riclcettsiae prowazelcii, Ricleettsiae typhi (Rickettsiae
mooseri),
Rickettsiae rickettsii, Rickettsiae tsutsugamushi, Rickettsiae sibirica;
Rickettsiae
australis; Rickettsiae conorii; Riclcettsiae alcari; Riclcettsiae burnetii);
Chlamydia
(e.g., Chlamydia psittaci; Cltlanzydia pneunzoniae; Chlamydia trachornatis, or
the
like); Mycobacterium (Mycobacterium tuberculosis; Mycobacterium marinum;
Mycobacterium Avium Complex; Mycobacterium bovis; Mycobacterium
scrofulaceum; Mycobacterium ulcerans; MycobacteriunZ leprae (Leprosy, Hansen's
Bacillus)); Brucella (e.g., Brucella melitensis; Brucella abortus; Brucella
suis;
Brucella canis); genus Coxiella (e.g., Coxiella burnetii); or the like. Thus,
in some
embodiments, the subject can have an intracellular bacterial infection caused
by a
bacterium selected from the genera Ehrlichia; Listeria; Legionella;
Rickettsiae;
Chlamydia; Mycobacterium; Brucella; and Coxiella.
In various embodiments, the subject can be treated for a bacterial infection
from one or more upper respiratory tract bacteria. Examples of upper
respiratory
tract bacteria include those belonging genera such as Legionella, Pseudomonas,
and
the like. In some embodiments, the bacteria can be Pseudomonas aeruginosa. In
particular embodiments, the bacteria can be Legionella pneurnophila (e.g.,
including
serogroups 1, 2, 3, 4, 5, 6, 7, 8, and the like), Legionella dumoffli,
Legionella
longbeacheae, Legionella micdadei, Legionella oakridgensis, Legionellafeelei,
Legionella anisa, Legionella sainthelensi, Legionella bozemanii, Legionella
gormanii, Legionella wadswortlaii., Legionellajordanis, or Legionella
gormanii.
In some embodiments, the subject can be treated for a bacterial infection
from one that causes acute bacterial exacerbation of chronic bronchitis
(ABECB)in
the subject. Typically, ABECB can be caused by Streptococcus pneumoniae,
Haemophilus injhcenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
In some embodiments, the subject can be treated for a bacterial infection
from one that causes acute community acquired pneumonia (CAP) in the subject.
Typically, CAP can be caused by Streptococcus pneumoniae, Haemophilus
influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, Chlamydia
pneumoniae, or Klebsiella pneumoniae. In a particular embodiment, the CAP can
be
CA 02603314 2007-10-02
-24-
WO 2006/113572 PCT/US2006/014320
caused by drug resistant bacteria, e.g., a multi-drug resistant strain of
Streptococcus
pneurnoniae.
In various embodiments, the subject can be treated for a bacterial infection
from Streptococcus pneunaoniae, Haeinophilus influenzae, Haemophilus
parainfuenzae, Moraxella catarrizalis, Mycoplasina pneumoniae, Clalainydia
pneumoniae, Klebsiella pneumoniae, Stapliylococcus aureus, Streptococcus
pyogenes, Acinetobacter lwoffi, Klebsiella oxytoca, Legionella pneumophila, or
Proteus vulgaris.
In various embodiments, the subject can be treated for a bacterial infection
from maxillary sinus pathogenic bacteria. As used herein, maxillary sinus
pathogenic bacteria is a bacterial strain isolated from acute or chronic
maxillary
sinusitis, or, for example, a maxillary sinus isolate of Staphylococcus
aureus,
Streptococcus pneumoniae, Haemophilus spp., Moraxella catarrhalis, an
anaerobic
strain of non-fermentative Gram negative bacilli, Neisseria nieningitides or 0-
haemolytic Streptococcus. In various embodiments, maxillary sinus pathogenic
bacteria can include a bacterial strain isolated from acute or chronic
maxillary
sinusitis; a maxillary sinus isolate of Staplaylococcus aureus, Streptococcus
pneumoniae, Haemophilus spp., Moraxella catarrizalis, an anaerobic strain of
non-fermentative Gram negative bacilli, Neisseria meningitidis, 0 -haemolytic
Streptococcus, Haemophilus influenzae, an Enterobacteriaceae, a non-
fermentative
Gram negative bacilli, Streptococcus pneumoniae, Streptococcus pyogenes, a
methicillin-resistant Staphylococcus spp., Legionella pneumophila, Mycoplasma
spp. and Chlamydia spp., Haemophilus influenzae, Haemophilus parainfluenzae, ~
Peptostreptococcus, Bacteroides spp., and Bacteroides urealyti.cus.
In various embodiments, the subject can be treated for a bacterial infection
that causes a urinary tract infection (UTI) in the subject. Examples of UTIs
include
urethritis, cystitis, prostatitis, pyelonephritis (acute, chronic, and
xanthogranulomatous), and hematogenous UTI (e.g., from bacteremia with
virulent
bacilli such as Salmonella, Staphylococcus aureus, and the like). Typically,
UTIs
can be caused by gram-negative aerobic bacteria, e.g., Escherichia (e.g.,
Escherichia
coli), Klebsiella, Proteus, Enterobacter, Pseudomonas, and Serratia; gram-
negative
anaerobic bacteria; gram-positive bacteria, e.g., Enterococci (e.g.,
Enterococcus
faecalis) and Staphylococcus sp (e.g., Staphylococcus saprophyticus,
Staplzylococcus aureus, and the like); Mycobacterium tuberculosis; and
sexually
CA 02603314 2007-10-02
WO 2006/113572 - 25 PCT/US2006/014320
transmitted bacterial infections (e.g., Chlanzydia trachoinatis, Neisseria
gonorrhoeae, and the like).
In certain embodiments, it is believed the methods can be effective in
treating infections from microorganisms that cause sexually transmitted
diseases, for
example, Treponema pallidum; Trichomonas vaginalis; Candidia (Candida
albicans); Neisseria gonorrlaoeae; Chlarnydia trachomatis; Mycoplasrna
genitalium,
Ureaplasma urealyticum; Haemophilus ducreyi; Calymnzatobacterium granulonzatis
(formerly Donovania granulomatis); herpes simplex viruses (HSV-1 or HSV-2);
human papillomavirus [HPV];human immunodeficiency virus (HIV);various
bacterial (Shigella, Campylobacter, or Salmonella), viral (hepatitis A), or
parasitic
(Giardia or amoeba, e.g., Entanzoeba dispar (previously Entamoeba
histolytica); or
the like.
Thus, in various einbodiments, the subject can have an infection resulting in
upper respiratory tract bacterial infection, acute bacterial exacerbation of
chronic
bronchitis; acute community acquired pneumonia, maxillary sinus pathogenic
bacteria; a urinary tract infection; or a sexually transmitted infection.
It is believed that the methods can be particularly effective for treating a
subject with a viral infection. Thus, in various embodiments, a subject can be
treated
for infection from viruses such as Picornaviruses (e.g., Polio Virus,
rhinoviruses and
certain echoviruses and coxsackieviruses); Parvoviridae (Human Parvovirus
B19);
Hepatitis, e..g, Hepadnavirus (Hepatitis B); Papovavirus (JC Virus);
Adenovirus
(Human Adenovirus); Herpesvirus (e.g., Cytomegalovirus, Epstein Barr Virus
(Mononucleosis), Mononucleosis-Like Syndrome, Roseola Infantum, Varicella
Zoster Virus(Chicken Pox), Herpes Zoster (Shingles), Herpes Simplex Virus
(Oral
Herpes, Genital Herpes)), Poxvirus (Smallpox); Calicivirus (Norwalk Virus),
Arbovirus (e.g., Togavirus (Rubella virus, Dengue virus), Flavivirus (Yellow
Fever
virus), Bunyavirus (California Encephalitis Virus), Reovirus (Rotavirus));
Coronavirus (Coronavirus); Retrovirus (Human Immunodeficiency Virus 1, Human
Immunodeficiency Virus 2); Rhabdovirus (Rabies Virus), Filovirus (Marburg
Virus,
Ebola virus, other hemorrhagic viral diseases); Paramyxovirus (Measles Virus,
Mumps Virus); Orthomyxovirus (Influenza Virus);Arenavirus (Lassa Fever); human
T-cell Lymphotrophic virus type I and II (HTLV-I, HTLV II); human
papillomavirus [HPV]; or the like. Thus, in various embodiments, the subject
can
have an infection caused by a virus selected from Picornavirus; Parvoviridae;
CA 02603314 2007-10-02
-26-
WO 2006/113572 PCT/US2006/014320
Hepatitis virus; Papovavirus; Adenoviius; Herpesvirus, Poxvirus; Calicivirus;
Arbovirus; Coronavirus; a Retrovirus; Rhabdovirus; Paramyxovirus;
Orthomyxovirus; Arenavirus; human T-cell Lymphotrophic virus; human
papillomavirus; and human immunodeficiency virus.
In some embodiments, a subject can be treated for infection from viruses or
infections thereof such as human immunodeficiency virus-1, human
immunodeficiency virus-2, Cytomegalovirus, Epstein Barr Virus,
Mononucleosis-Like Syndrome, Roseola Infantum, Varicella Zoster Virus, Herpes
Zoster, Herpes Simplex Virus, or hepatitis.
It is believed that the methods can be particularly effective for treating a
subject with a parasitic infection. Thus, in various embodiments, a subject
can be
treated for infection from Plasmodia (e.g., Plasnaodia falciparum, Plasmodia
vivax,
Plasmodia ovale, and Plasmodia malariae, typically transmitted by anopheline
mosquitoes); Leishmania (transmitted by sandflies and caused by obligate
intracellular protozoa, e.g., Leishmania donovani, Leishmania infantum,
Leishmania
chagasi, Leishmania mexicana, Leishmania amazonensis, Leishmania
venezuelensis,
Leishmania tropica; Leishmania major; Leishinania aethiopica; and the subgenus
Viannia, Leishmania Viannia braziliensis, Leishmania Viarinia guyanensis,
Leishmania Viannia panamensis, and Leishmania Viannia peruviana); Trypanosoma
(e.g., sleeping sickness caused by Tyypanosoma bf-ucei gambiense, and
Trypanosoma brucei rhodesiense); amoebas of the genera Naegleria or
Acanthamoeba; pathogens such as genus Entamoeba (Entamoeba histolytica and
Entainoeba dispar); Giardia lamblia; Cryptosporidium; Isospora; Cyclospora;
Microsporidia; Ascaris lumbricoides; infection with blood flukes of the genus
Schistosoma (e.g.; S. haematobium; S. mansoni; S. japonicum; S. mekongi; S.
intercalatum); Toxoplasmosis (e.g., Toxoplasma gondii); TNeponema pallidum;
Trichomonas vaginalis; or the like.
In some embodiments, the subject can have an infection caused by a
protozoa selected from Toxoplasnaa gondii, Trypanosoma brucei gambiense,
Trypanosoma brucei rhodesiense, Leishmania donovani, Leishmania infantum,
Leishmania chagasi, Leishrnania mexicana, Leishmania amazonensis, Leishmania
venezuelensis, Leishmania tropica; Leishmania major; Leishmania aethiopica;
and
the subgenus Viannia, Leishrnania Viannia braziliensis, Leishmania Viannia
CA 02603314 2007-10-02
-27-
WO 2006/113572 PCT/US2006/014320
guyanensis, Leishmania Viannia panamensis, Leishmania Viannia peruviana,
Plasmodiafalciparum, Plasmodia vivax, Plasmodia ovale, and Plasmodia
rnalariae.
In the last century, antibiotics were developed that led to significant
reductions in mortality. Unfortunately, widespread use has led to the rise of
antibiotic resistant bacteria, e.g., methicillin resistant Staplzyloccocus
aureus
(MRSA), vancomycin resistant eizterococci (VRE), and penicillin-resistant
Streptococcus pneumoniae (PRSP). Some bacteria are resistant to a range of
antibiotics, e.g., strains of Mycobacterium tuberculosis resist isoniazid,
rifampin,
ethambutol, streptomycin, ethionamide, kanamycin, and rifabutin. In addition
to
resistance, global travel has spread relatively unknown bacteria from isolated
areas
to new populations. Furthennore, there is the threat of bacteria as biological
weapons. These bacteria may not be easily treated with existing antibiotics.
It is believed that the methods can be particularly effective for treating a
subject for drug-resistant pathogens, for example, drug resistant bacteria, or
pathogens for which no drugs are available, e.g., many viruses. Without
wishing to
be bound by theory, it is believed that because the methods can act by
increasing NK
cell activity, and thus the NK cells can kill infective microorganisms or
infected
cells separately from any direct action of the compounds on the pathogen or
infected
cells. Thus, it is believed that the methods can have at least one mode of
action that
is separate from typical anti-infective drugs such as antibiotics which can
typically
act directly on the bacteria themselves.
Drug resistant pathogens can be resistant to at least one and typically
multiple agents, for example, drug resistant bacteria can be resistant to one
antibiotic, or typically at least two antibiotics such as penicillin,
Methicillin, second
generation cephalosporins (e.g., cefuroxime, and the like), macrolides,
tetracyclines,
trimethoprim/methoxazole, vancomycin, or the like. For example, in some
embodiments, a subject can be treated for bacteria selected from a strain of
multiple
drug resistant Streptococcus pneumoniae (MDRSP, previously known as penicillin
resistant Streptococcus pneumoniae, PRSP), vancomycin resistant Enterococcus,
methicillin resistant Staplaylococcus Aureus, penicillin resistant
Pneumococcus,
antibiotic resistant Salmonella, resistant and multi-resistant Neisseria
Gonorrhea
(e.g., resistant to one, two or more of tetracycline, penicillin,
fluoroquinolones,
cephalosporins, ceftriaxone (Rocephin), Cefixime (Suprax), Azithromycin, or
the
like), and resistant and multi-resistant Tuberculosis (e.g., resistant to one,
two or
CA 02603314 2007-10-02
-28-
WO 2006/113572 PCT/US2006/014320
more of Isoniazid, Rifampin, Ethambutol, Pyrazinamide, Aminoglycoside,
Capreomycin, Ciprofloxacin, Ofloxacin, gemifloxacin, Cycloserine, Ethionamide,
para-aminosalicylic acid or the like).
In some embodiments, NIC activity can be increased in subjects that have an
immunodeficiency. In various embodiments, this can be due to decreased or
deficient NK cell activity. In some embodiments, the immunodeficiency can be
any
known immunodeficiency, even those that do not directly impact NK cells.
Without
wishing to be bound by theory, it is believed that boosting NK cell activity
can
augment immune function in many immunodeficiency conditions to "make-up" at
least in part, for aspects of immunodeficiency separate from those aspects
directly
concerned with NK cell activity.
In various embodiments, immunodeficiency disorders can include disorders
with increased susceptibility to infection, for example, one or more disorders
selected from: circulatory and systemic disorders (sickle cell disease,
diabetes
mellitus, nephrosis, varicose veins, congenital cardiac defects); obstructive
disorders
(ureteral or urethral stenosis, bronchial asthma, bronchiectasis, allergic
rhinitis,
blocked Eustachian tubes); integumentary defects (eczema, bums, skull
fractures,
midline sinus tracts, ciliary abnormalities); primary immunodeficiencies (X-
linked
agammaglobulinemia, DiGeorge anomaly, chronic granulomatous disease, C3
deficiency); secondary immunodeficiencies (malnutrition, prematurity,
lymphoma,
splenectomy, uremia, inununosuppressive therapy, protein-losing enteropathy,
chronic viral diseases);unusual microbiologic factors (antibiotic overgrowth,
chronic
infections with resistant organism, continuous reinfection (contaminated water
supply, infectious contact, contaminated inhalation therapy equipment));
foreign
bodies, trauma (ventricular shunts, central venous catheter, artificial heart
valves,
urinary catheter, aspirated foreign bodies) allogeneic transplant, graft-
versus-host
disease, uterine dysfunction (e.g., endometriosis),or the like.
In various embodiments, immunodeficiency disorders can include for
example, transient hypogammaglobulinemia of infancy, selective IgA deficiency,
X-
linked agammaglobulinemian (Bruton's Agammaglobulinemia; Congenital
Agammaglobulinemia), common variable immunodeficiency (Acquired
Agammaglobulinemia), hyper-IgM immunodeficiency, IgG subclass deficiency,
chronic mucocutaneous Candidiasis, combined immunodeficiency, Wiskott-Aldrich
syndrome, ataxia-telangiectasia, X-linked lymphoproliferative syndrome, hyper-
IgE
CA 02603314 2007-10-02
-29-
WO 2006/113572 PCT/US2006/014320
syndrome (Job-Buckley Syndrome), chronic granulotomatous disease, leukocyte
adhesion deficiency (MAC-1/LFA-l/CR3 deficiency), or the like
In various embodiments, immunodeficiency disorders can include primary
immunodeficiency disorders for example: B-cell (antibody) deficiencies (X-
linked
agammaglobulinemia; Ig deficiency with hyper-IgM (XL); IgA deficiency); IgG
subclass deficiencies, Antibody deficiency with normal or elevated Igs,
Immunodeficiency with theymoma, Common variable immunodeficiency, Transient
hypogammaglobulinemia of infancy); T-cell (cellular) deficiencies (Predominant
T-
cell deficiency: DiGeorge anomaly, Chronic mucocutaneous candidiasis, Combined
immunodeficiency with Igs (Nezelof syndrome), Nucleoside phosphorylase
deficiency (AR), Natural killer cell deficiency, Idiopathic CD4
lymphocytopenia,
Combined T- and B-cell deficiencies: Severe combined immunodeficiency (AR or
XL), Adenosine deaminase deficiency (AR), Reticular dysgenesis, Bare
lymphocyte
syndrome, Ataxia-telangiectasia (AR), Wislcott-Aldrich syndrome (XL), Short-
limbed dwarfism, XL lymphoproliferative syndrome); Phagocytic disorders
(Defects
of cell movement: Hyperimmunoglobulinemia E syndrome, Leukocyte adhesion
defect type 1 (AR), Defects of microbicidal activity: Chronic granulomatous
disease
(XL or AR), Neutrophil G6PD deficiency, Myeloperoxidase deficiency (AR),
Chediak-Higashi syndrome (AR)); Compleinent disorders (Defects of complement
components: Clq deficiency, Defects of control proteins: Cl inhibitor
deficiency
(Dl), Factor I (C3b inactivator) deficiency (ACD), Factor H deficiency (ACD),
Factor D deficiency (ACD), Properdin deficiency (XL)); or the like
In various embodiments, immunodeficiency disorders can include secondary
immunodeficiency disorders, for example, one or more conditions selected from:
Premature and newborn infants (Physiologic immunodeficiency due to immaturity
of immune system); Hereditary and metabolic diseases (Chromosome abnormalities
(e.g., Down syndrome), Uremia, Diabetes (i.e., complications from diabetes
such as
gangrene associated with peripheral circulatory and nerve dysfunction),
Malnutrition, Vitamin and mineral deficiencies, Protein-losing enteropathies,
Nephrotic syndrome, Myotonic dystrophy, Sickle cell disease);
Immunosuppressive
agents (Radiation, Immunosuppressive drugs, Corticosteroids, Anti-lymphocyte
or
anti-thymocyte globulin, Anti-T-cell monoclonal antibodies); Infectious
diseases
(Congenital rubella, Viral exanthems (e.g., measles, varicella), HIV
infection,
Cytomegalovirus infection, Infectious mononucleosis, Acute bacterial disease,
CA 02603314 2007-10-02
-30-
WO 2006/113572 PCT/US2006/014320
Severe mycobacterial or fangal disease); Infiltrative and hematologic diseases
(Histiocytosis, Sarcoidosis, Hodgltin's disease and lymphoma, Leukemia,
Myeloma,
Agranulocytosis and aplastic anemia); Surgery and trauma (Bums, Splenectomy,
Anesthesia, wounds); and Miscellaneous (SLE, Chronic active hepatitis,
Alcoholic
cirrhosis, Aging, Anticonvulsive drugs, Graft-vs.-host disease); or the like.
In certain embodiments, the subject can be treated for burns or wounds.
Typically, such a wound or bum is a severe injury that places a significant
burden on
the subject's immune defenses. For example, in some embodiments, the subject
is
treated for a second or third degree bum covering at least about 5%, 10%, 15%,
20%, 25%, 30%, 40%, 50%, 75%, or more of the surface area of the subject's
body.
Also, in some embodiments, the subject is treated for a wound or wounds, e.g.,
an
open wound of at least about 1 cm2, 2 cmZ, 5 cm2, 10 cm2, 20 cm2, 50 cm2 or
larger,
or 1%, 2%, 3%, 4%, 5%, 10%, 15%, or more of the surface area of the subject's
body; or one or more incisions penetrating the skin totaling at least 1 cm, 2
cm, 3
cm, 4 cm, 5 cm, 7 cm, 10 cm, 20 cm, 25 cm, 50 cm in length; an amputation; and
the
like.
In various embodiments, the subject can have an infection caused by
antibiotic resistant bacteria. In some embodiments, the subject can have an
infection
caused by a bacterium selected from multiple drug resistant Streptococcus
pneumoniae, vancomycin resistant Enterococcus, methicillin resistant
Staphylococcus Aureus, penicillin resistant Pneumococcus, antibiotic resistant
Salmonella, resistant/multi-resistant Neisseria Gonorrhea, and
resistant/multi-resistant Tuberculosis. In some embodiments, the subject can
have a
bacterial infection resistant to at least one antibiotic selected from
penicillin,
Methicillin, second generation cephalosporins, macrolides, tetracyclines,
trimethoprim/methoxazole, vancomycin, tetracycline, fluoroquinolones,
ceftriaxone,
Cefixime, Azithromycin, Isoniazid, Rifampin, Ethambutol, Pyrazinamide,
Aminoglycoside, Capreomycin, Ciprofloxacin, Ofloxacin, gemifloxacin,
Cycloserine, Ethionamide, and para-aminosalicylic acid.
Thus, various embodiments, the subject can have an immunodeficiency
disorder. In some embodiments, the subject can have a primary immunodeficiency
disorder. In some embodiments, the subject can have a secondary
immunodeficiency disorder.
CA 02603314 2007-10-02
-31-
WO 2006/113572 PCT/US2006/014320
In some embodiments, immunodeficiency disorders can include uremia,
diabetes (infective complications thereof, malnutrition, vitamin and mineral
deficiencies, protein-losing enteropathies, nephrotic syndrome, myotonic
dystrophy,
sickle cell disease; or the like.
In some embodiments, immunodeficiency disorders can include
immunosuppressive agents, e.g., radiation, immunosuppressive drugs,
corticosteroids, anti-lymphocyte or anti-thymocyte globulin, anti-T-cell
monoclonal
antibodies; or the like.
In some embodiments, immunodeficiency disorders can include surgery and
trauma, e.g., bums, splenectomy, anesthesia, wounds, implanted medical
devices; or
the like.
In some embodiments, immunodeficiency disorders can include chronic
fatigue syndrome (chronic fatigue immune dysfunction syndrome); Epstein-Barr
virus infection, post viral fatigue syndrome, post-transplantation syndrome
(host-graft disease), exposure to nitric oxide synthase inhibitors, aging,
severe
combined immunodeficiency, variable immunodeficiency syndrome, and the like.
As used herein, a"pharmaceutical composition" can be a formulation
containing the disclosed compounds, in a form suitable for administration to a
subject. The pharmaceutical composition can be in bulk or in unit dosage form.
The
unit dosage form can be in any of a variety of forms, including, for example,
a
capsule, an IV bag, a tablet, a single pump on an aerosol inhaler, or a vial.
The
quantity of active ingredient (i.e., a formulation of the disclosed compound
or salts
thereof) in a unit dose of composition can be an effective amount and can be
varied
according to the particular treatment involved. It may be appreciated that it
can be
necessary to make routine variations to the dosage depending on the age and
condition of the patient. The dosage can also depend on the route of
administration.
A variety of routes are contemplated, including topical, oral, pulmonary,
rectal,
vaginal, parentemal, including transdermal, subcutaneous, intravenous,
intramuscular, intraperitoneal and intranasal.
The compounds described herein, and the pharmaceutically acceptable salts
thereof can be used in pharmaceutical preparations in combination with a
pharmaceutically acceptable carrier or diluent. Suitable pharmaceutically
acceptable
carriers include inert solid fillers or diluents and sterile aqueous or
organic solutions.
The compounds can be present in such pharmaceutical compositions in amounts
CA 02603314 2007-10-02
-32-
WO 2006/113572 PCT/US2006/014320
sufficient to provide the desired dosage amount in the range described herein.
Techniques for formulation and administration of the disclosed compounds of
the
invention can be found in Refnington: tlae Science and Practice of Phat=macy,
19cn
edition, Mack Publishing Co., Easton, PA (1995).
For oral administration, the disclosed compounds or salts thereof can be
combined with a suitable solid or liquid carrier or diluent to fonn capsules,
tablets,
pills, powders, syrups, solutions, suspensions, or the like.
The tablets, pills, capsules, and the like can contain from about 1 to about
99
weight percent of the active ingredient and a binder such as gum tragacanth,
acacias,
corn starch or gelatin; excipients such as dicalciuin phosphate; a
disintegrating agent
such as corn starch, potato starch or alginic acid; a lubricant such as
magnesium
stearate; and/or a sweetening agent such as sucrose, lactose or saccharin.
When a
dosage unit form is a capsule, it may contain, in addition to materials of the
above
type, a liquid carrier such as a fatty oil.
Various other materials can be present as coatings or to modify the physical
form of the dosage unit. For instance, tablets may be coated with shellac,
sugar or
both. A syrup or elixir may contain, in addition to the active ingredient,
sucrose as a
sweetening agent, methyl and propylparabens as preservatives, a dye and a
flavoring
such as cherry or orange flavor, and the like.
For parental administration, the bis(thio-hydrazide) amides can be combined
with sterile aqueous or organic media to form injectable solutions or
suspensions.
For example, solutions in sesame or peanut oil, aqueous propylene glycol and
the
like can be used, as well as aqueous solutions of water-soluble
pharmaceutically-acceptable salts of the compounds. Dispersions can also be
prepared in glycerol, liquid polyethylene glycols and mixtures thereof in
oils. Under
ordinary conditions of storage and use, these preparations contain a
preservative to
prevent the growth of microorganisms.
In addition to the formulations previously described, the compounds may
also be formulated as a depot preparation. Suitable formulations of this type
include
biocompatible and biodegradable polymeric hydrogel formulations using
crosslinked
or water insoluble polysaccharide formulations, polymerizable polyethylene
oxide
formulations, impregnated membranes, and the like. Such long acting
formulations
may be administered by implantation or transcutaneous delivery (for example
subcutaneously or intramuscularly), intramuscular injection or a transdennal
patch.
CA 02603314 2007-10-02
-33-
WO 2006/113572 PCT/US2006/014320
Typically, they can be implanted in, or applied to, the microenvironment of an
affected organ or tissue, for example, a membrane impregnated with the
disclosed
compound can be applied to an open wound or burn injury. Thus, for example,
the
compounds may be formulated with suitable polymeric or hydrophobic materials,
for example, as an emulsion in an acceptable oil, or ion exchange resins, or
as
sparingly soluble derivatives, for example, as a sparingly soluble salt.
For topical administration, suitable formulations may include biocompatible
oil, wax, gel, powder, polymer, or other liquid or solid carriers. Such
formulations
may be administered by applying directly to affected tissues, for example, a
liquid
fonnulation to treat infection of conjunctival tissue can be administered
dropwise to
the subject's eye, a cream formulation can be administer to a wound site, or a
bandage may be impregnated with a formulation, and the like.
For rectal administration, suitable pharmaceutical compositions are, for
example, topical preparations, suppositories or enemas.
For vaginal administration, suitable pharmaceutical compositions are, for
example, topical preparations, pessaries, tampons, creams, gels, pastes, foams
or
sprays.
In addition, the compounds may also be formulated to deliver the active
agent by pulmonary administration, e.g., administration of an aerosol
formulation
containing the active agent from, for example, a manual pump spray, nebulizer
or
pressurized metered-dose inhaler. Suitable formulations of this type can also
include
other agents, such as antistatic agents, to maintain the disclosed compounds
as
effective aerosols.
The term "pulmonary" as used herein refers to any part, tissue or organ
whose primary function is gas exchange with the external environment, i.e.,
02/C02
exchange, within a patient. "Pulmonary" typically refers to the tissues of the
respiratory tract. Thus, the phrase "pulmonary administration" refers to
administering the formulations described herein to any part, tissue or organ
whose
primary function is gas exchange with the external environment (e.g., mouth,
nose,
pharynx, oropharynx, laryngopharynx, larynx, trachea, carina, bronchi,
bronchioles,
alveoli). For purposes of the present invention, "pulmonary" is also meant to
include a tissue or cavity that is contingent to the respiratory tract, in
particular, the
sinuses.
CA 02603314 2007-10-02
-34-
WO 2006/113572 PCT/US2006/014320
A drug delivery device for delivering aerosols can comprise a suitable
aerosol canister with a metering valve containing a pharmaceutical aerosol
formulation as described and an actuator housing adapted to hold the canister
and
allow for drug delivery. The canister in the drug delivery device has a head
space
representing greater than about 15% of the total volume of the canister.
Often, the
polymer intended for pulmonary administration is dissolved, suspended or
emulsified in a mixture of a solvent, surfactant and propellant. The mixture
is
maintained under pressure in a canister that has been sealed with a metering
valve.
For nasal administration, either a solid or a liquid carrier can be used. The
solid carrier includes a coarse powder having particle size in the range of,
for
example, from about 20 to about 500 microns and such formulation is
administered
by rapid inhalation through the nasal passages. Where the liquid carrier is
used, the
formulation may be administered as a nasal spray or drops and may include oil
or
aqueous solutions of the active ingredients.
In addition to the formulations described above, a formulation can optionally
include, or be co-administered with one or more additional drugs, e.g., other
antifungals, anti-inflammatories, anti-biotics, antivirals, immunomodulators,
antiprotozoals, steroids, decongestants, bronchodialators, antihistamines,
anticancer
agents, and the like. For example, the disclosed compound can be co-
administered
with drugs such as such as ibuprofen, prednisone (corticosteroid)
pentoxifylline,
Amphotericin B, Fluconazole, Ketoconazol, Itraconazol, penicillin, ampicillin,
amoxicillin, and the like. The formulation may also contain preserving agents,
solubilizing agents, chemical buffers, surfactants, emulsifiers, colorants,
odorants
and sweeteners.
Hsp70-responsive disorders excluded by proviso from various embodiments
include any such disorder identified in Barsoum, U.S. Provisional Application
No.:
60/629,595 (Attorney's Docket No. 3211.1017-000); filed November 19, 2004, the
entire teachings of which are incorporated by reference. As used herein, a
non-infective heat shock protein 70 (Hsp70) responsive disorder, e.g., the
Hsp70
disorders excluded by proviso from various embodiments, can be a medical
condition wherein stressed cells can be treated by increased Hsp70 expression.
Such
disorders can be caused by a wide variety of cellular stressors, including,
but not
limited to Alzheimers' disease; Huntington's disease; Parkinson's disease;
spinal/bulbar muscular atrophy (e.g., Kennedy's disease), spinocerebellar
ataxic
CA 02603314 2007-10-02
-35-
WO 2006/113572 PCT/US2006/014320
disorders, and other neuromuscular atrophies; familial amyotrophic lateral
sclerosis;
ischemia; seizure; hypothermia; hyperthermia; burn trauma; atherosclerosis;
radiation exposure; glaucoma; toxin exposure; mechanical injury; inflammation;
and
the like.
As used herein, "Hsp70" includes each member of the family of heat shock
proteins having a mass of about 70-kiloDaltons, including forms such as
constituitive, cognate, cell-specific, glucose-regulated, inducible, etc.
Examples of
specific Hsp70 proteins include hsp70, hsp70hom; hsc70; Grp78/BiP;
mt-hsp70/Grp75, and the like). Typically, the disclosed methods increase
expression of inducible Hsp70. Functionally, the 70-1cDa HSP (HSP70) family is
a
group of chaperones that assist in the folding, transport, and assembly of
proteins in
the cytoplasm, mitochondria, and endoplasmic reticulum. In humans, the Hsp70
family encompasses at least 11 genes encoding a group of highly related
proteins.
See, for example, Tavaria, et al., Cell Stress Chaperones, 1996;1(1):23-28;
Todryk,
et al., Immunology. 2003, 110(1): 1-9; and Georgopoulos and Welch, Annu Rev
Cell Biol. 1993;9:601-634; the entire teachings of these documents are
incorporated
herein by reference.
An example of Hsp70 disorders excluded by proviso from various
embodiments can include a neurodegenerative disorder. As used herein, a
neurodegenerative disorder involves degradation of neurons such as cereberal,
spinal, and peripheral neurons (e.g., at neuromuscular junctions), more
typically
degradation of cerebral and spinal neurons. Neurodegenerative disorders can
include Alzheimers' disease; Huntington's disease; Parlcinson's disease;
spinal/bulbar muscular atrophy and other neuromuscular atrophies; and familial
amyotrophic lateral sclerosis or other diseases associated with superoxide
dismutase
(SOD) mutations. Neurodegenerative disorders can also include degradation of
neurons caused by ischemia, seizure, thermal stress, radiation, toxin
exposure,
infection, injury, and the like.
Other examples of Hsp70 disorders excluded by proviso from various
embodiments can include a disorder of protein aggregation/misfolding, such as
Alzheimers' disease; Huntington's disease; Parlcinson's disease; and the like.
Additional examples of Hsp70 disorders excluded by proviso from various
embodiments can include ischemia. Ischemia can damage tissue through multiple
routes, including oxygen depletion, glucose depletion, oxidative stress upon
CA 02603314 2007-10-02
-36-
WO 2006/113572 PCT/US2006/014320
reperfusion, and/or glutamate toxicity, and the lilce. Ischemia can result
from an
endogenous condition (e.g., stroke, heart attack, and the lilce), from
accidental
mechanical injury, from surgical injury (e.g., reperfusion stress on
transplanted
organs), and the like. Alternatively, tissues that can be damaged by ischemia
include neurons, cardiac muscle, liver tissue, skeletal muscle, kidney tissue,
puhnonary tissue, pancreatic tissue, and the like.
Also, examples of Hsp70 disorders excluded by proviso from various
embodiments can include seizure, e.g., eplileptic seizure, injury-induced
seizure,
chemically-induced seizure, and the like.
More examples of Hsp70 disorders excluded by proviso from various
embodiments can include disorders due to thermal stress. Thermal stress
includes
hyperthermia (e.g., from fever, heat stroke, burns, and the like) and
hypothermia.
Further examples of Hsp70 disorders excluded by proviso from various
embodiments can include radiation damage, e.g., due to visible light,
ultraviolet
light, microwaves, cosmic rays, alpha radiation, beta radiation, gamma
radiation,
X-rays, and the like. For example, the damage could be radiation damage to
non-cancerous tissue in a subject treated for cancer by radiation therapy.
Certain examples of Hsp70 disorders excluded by proviso from various
embodiments can include mechanical injury, e.g., trauma from surgery,
accidents,
certain disease conditions (e.g., pressure damage in glaucoma) and the like.
Particular examples of Hsp70 disorders excluded by proviso from various
embodiments can include exposure to a toxin. e.g., exposure to a neurotoxin
selected
from methamphetamine; antiretroviral HIV therapeutics (e.g., nucleoside
reverse
transcriptase inhibitors; heavy metals (e.g., mercury, lead, arsenic, cadmium,
compounds thereof, and the lilce), amino acid analogs, chemical oxidants,
ethanol,
glutamate, metabolic inhibitors, antibiotics, and the like.
Cancer is excluded from the present invention. Examples include those
identified in Koya, et al, U.S. Patent Nos.: 6,800,660, issued October 5;
6,762,204,
issued, July 13, 2004; and Koya, et al U.S. Application No. 10/758,589; Filed:
January 15, 2004; the entire teachings of which are incorporated by reference.
For
example, such cancers can be human sarcomas and carcinomas, e.g.,
fibrosarcoma,
myxosarcoma, liposarcoma, chondrosarcoma, osteogenic sarcoma, chordoma,
angiosarcoma, endotheliosarcoma, lymphangiosarcoma,
lymphangioendotheliosarcoma, synovioma, mesothelioma, Ewing's tumor,
CA 02603314 2007-10-02
-37-
WO 2006/113572 PCT/US2006/014320
leiomyosarcoma, rhabdomyosarcoma, colon carcinoma, pancreatic cancer, breast
cancer, ovarian cancer, prostate cancer, squamous cell carcinoma, basal cell
carcinoma, adenocarcinoma, sweat gland carcinoma, sebaceous gland carcinoma,
papillary carcinoma, papillary adenocarcinomas, cystadenocarcinoma, medullary
carcinoma, bronchogenic carcinoma, renal cell carcinoma, hepatoma, bile duct
carcinoma, choriocarcinoma, seminoma, embryonal carcinoma, Wilms' tumor,
cervical cancer, testicular tumor, lung carcinoma, small cell lung carcinoma,
bladder
carcinoma, epithelial carcinoma, glioma, astrocytoma, medulloblastoma,
craniopharyngioma, ependymoma, pinealoma, hemangioblastoma, acoustic
neuroma, oligodendroglioma, meningioma, melanoma, neuroblastoma,
retinoblastoma; leukemias, e.g., acute lymphocytic leukemia and acute
myelocytic
leukemia (myeloblastic, promyelocytic, myelomonocytic, monocytic and
erythroleukemia); chronic leukemia (chronic myelocytic (granulocytic) leukemia
and chronic lymphocytic leulcemia); and polycythemia vera, lymphoma (Hodgkin's
disease and non-Hodglcin's disease), multiple myeloma, Waldenstrobm's
macroglobulinemia, and heavy chain disease.
Other examples of cancer excluded by proviso from various embodiments
include leukemias include acute and/or chronic leukemias, e.g., lymphocytic
leukemia (e.g., as exemplified by the p388 (murine) cell line), large granular
lymphocytic leukemia, and lymphoblastic leukemia; T-cell leukemias, e.g., T-
cell
leukemia (e.g., as exemplified by the CEM, Jurlcat, and HSB-2 (acute),
YAC-1(murine) cell lines), T-lymphocytic leukemia, and T-lymphoblastic
leukemia;
B cell leukemia (e.g., as exemplified by the SB (acute) cell line), and B-
lymphocytic
leukemia; mixed cell leukemias, e.g., B and T cell leukemia and B and T
lymphocytic leukemia; myeloid leukemias, e.g., granulocytic leukemia,
myelocytic
leukemia (e.g., as exemplified by the HL-60 (promyelocyte) cell line), and
myelogenous leukemia (e.g., as exemplified by the K562(chronic)cell line);
neutrophilic leulcemia; eosinopliilic leukemia; monocytic leulcemia (e.g., as
exemplified by the THP- 1 (acute) cell line); myelomonocytic leulcemia;
Naegeli-type
myeloid leukemia; and nonlymphocytic leukemia. Other examples of leukemias are
described in Chapter 60 of The Claemotherapy Sourcebook, Michael C. Perry Ed.,
Williams & Williams (1992) and Section 36 of Holland Frie Cancer Medicine 5th
Ed., Bast et al. Eds., B.C. Decker Inc. (2000). The entire teachings of the
preceding
references are incorporated herein by reference.
CA 02603314 2007-10-02
-38-
WO 2006/113572 PCT/US2006/014320
Other examples of cancer excluded by proviso from various embodiments
include non-solid tumors such as multiple myeloma, T-leukemia (e.g., as
exemplified by Jurlcat and CEM cell lines); B-leukemia (e.g., as exemplified
by the
SB cell line); promyelocytes (e.g., as exeinplified by the HL-60 cell line);
uterine
sarcoma (e.g., as exemplified by the MES-SA cell line); monocytic leukemia
(e.g.,
as exemplified by the THP-1(acute) cell line); and lymphoma (e.g., as
exemplified
by the U937 cell line).
Other examples of cancer excluded by proviso from various embodiments
include colon cancer, pancreatic cancer, melanoma, renal cancer, sarcoma,
breast
cancer, ovarian cancer, lung cancer. stomach cancer, bladder cancer and
cervical
cancer.
Other examples of cancer excluded by proviso from various embodiments
include cancer has become "multi-drug resistant". A cancer which initially
responded to an anti-cancer drug becomes resistant to the anti-cancer drug
when the
anti-cancer drug is no longer effective in treating the subject with the
cancer. For
example, many tumors will initially respond to treatnient with an anti-cancer
drug by
decreasing in size or even going into remission, only to develop resistance to
the
drug. Drug resistant tumors are characterized by a resumption of their growth
and/or
reappearance after having seemingly gone into remission, despite the
administration
of increased dosages of the anti-cancer chug. Cancers that have developed
resistance
to two or more anti-cancer drugs are said to be "multi-drug resistant". For
example,
it is common for cancers to become resistant to three or more anti-cancer
agents,
often five or more anti-cancer agents and at times ten or more anti-cancer
agents.
Proliferative cell disorders are excluded from the present invention.
Examples include those disorders identified in Sherman et al, U.S. Provisional
Application Ser. No 60/610,270; filed September 16, 2004 (Attorney's docket
No.
3211.1015-000), the entire teachings of which are incorporated by reference.
For
example, non-cancerous proliferative disorders excluded by proviso from
various
embodiments include smooth muscle cell proliferation, systemic sclerosis,
cirrhosis
of the liver, adult respiratory distress syndrome, idiopathic cardiomyopathy,
lupus
erythematosus, retinopathy, e.g., diabetic retinopathy or other retinopathies,
cardiac
hyperplasia, reproductive system associated disorders such as benign prostatic
hyperplasia and ovarian cysts, pulmonary fibrosis, endometriosis,
fibromatosis,
harmatomas, lymphangiomatosis, sarcoidosis, desmoid tumors and the like.
CA 02603314 2007-10-02
-39-
WO 2006/113572 PCT/US2006/014320
Non-cancerous proliferative disorders excluded by proviso from various
embodiments also include smooth muscle cell proliferation, e.g., proliferative
vascular disorders, for example, intimal smooth muscle cell hyperplasia,
restenosis
and vascular occlusion, particularly stenosis following biologically- or
mechanically-mediated vascular injury, e.g., vascular injury associated with
balloon
angioplasty or vascular stenosis. Moreover, intimal smooth muscle cell
hyperplasia
can include hyperplasia in smooth muscle other than the vasculature, e.g.,
hyperplasia in bile duct blockage, in bronchial airways of the lung in asthma
patients, in the kidneys of patients with renal interstitial fibrosis, and the
like.
Non-cancerous proliferative disorders excluded by proviso from various
embodiments also include hyperproliferation of cells in the skin such as
psoriasis
and its varied clinical forms, Reiter's syndrome, pityriasis rubra pilaris,
and
hyperproliferative variants of disorders of keratinization (e.g., actinic
keratosis,
senile keratosis), scleroderma, and the lilce.
Proteasome inhibitor responsive disorders excluded from the present
invention. Examples include those disorders identified in Mei Zhang, et al,
U.S.
Provisional Application Ser. No 60/629,858; filed: November 19, 2004,
(Attorney's
Docket No. 3211.1018-000), the entire teachings of which are incorporated by
reference. Such conditions include for example, the above cancer and non-
cancerous
proliferative conditions, conditions marlced by excessive or accelerated
protein
degradation, and Hsp70-responsive disorders. Additional examples of proteasome
inhibitor responsive disorders excluded by proviso from various embodiments
include muscle-wasting diseases (e.g., fever, muscle disuse (atrophy) and
denervation, nerve injury, fasting, renal failure associated with acidosis,
hepatic
failure, uremia, diabetes, and sepsis), skeletal system disorders resulting
from bone
loss or low bone density (e.g., closed fractures, open fractures, non-union
fractures,
age-related osteoporosis, post-menopausal osteoporosis, glucocorticoid-induced
osteoporosis, disuse osteoporosis, arthritis), growth deficiencies (e.g.,
periodontal
disease and defects, cartilage defects or disorders), disorders of hair growth
(e.g.,
male pattern baldness, alopecia caused by chemotherapy, hair thinning
resulting
from aging, genetic disorders resulting in deficiency of hair coverage), dry-
eye
disorders (e.g., excessive inflammation in relevant ocular tissues, such as
the
lacrimal and meibomian glands, dry eye associated with refractive surgery
(e.g.,
LASIK surgery)) and cystic fibrosis.
CA 02603314 2007-10-02
-40-
WO 2006/113572 PCT/US2006/014320
EXEMPLIFICATION
Example 1: Measurement of Heat Shock Protein 70 (Hsp70)
Plasma Hsp70 was measured by a sandwich ELISA kit (Stressgen
Bioreagents Victoria, British Columbia, CANADA) according to a modified
protocol in house. In brief, Hsp70 in plasma specimens and serial
concentrations of
Hsp70 standard were captured onto 96-well plate on which anti-Hsp70 antibody
was
coated. Then captured Hsp70 was detected with a biotinylated anti-Hsp70
antibody
followed by incubation with europium-conjugated streptavidin. After each
incubation unbound materials were removed by washing. Finally, antibody-Hsp70
complex was measured by time resolved fluorometry of europium. Concentration
of
Hsp70 was calculated from a standard curve.
Example 2: Measurement of Natural 1Uller Cell Cytotoxic Activity
The following procedure can be employed to assay NK cell activity in a
subject. The procedure is adapted from Kantakamalalcul W, Jaroenpool J,
Pattanapanyasat K. A novel enhanced green fluorescent protein (EGFP)-K562 flow
cytometric method for measuring natural killer (NK) cell cytotoxic activity. J
Immunol Methods. 2003 Jan 15; 272:189-197, the entire teachings of which are
incorporated herein by reference.
Materials and methods: Human erythroleukaemic cell line, K562, was
obtained from American Type Culture Collection (CCL-243, American Type
Culture Collection, Manassas, VA), and cultured in RPMI-1640 medium
(Cat#11875-093Gibco Invitrogen Corp, Carlsbad, CA) supplemented with 10% heat
inactivated fetal calf serum (Gibco), 2mM L-glutamin, 100 g/mi streptomycin
and
100 IU/ml penicillin at 37 C with 5% CO2. K562 cells were transduced with
retroviral vector which encode green fluorescent protein (eGFP). Stable cell
line
was selected with antibiotic, G418. About 99.6% G418 resistant cells were eGFP
positive after section.
The subject's peripheral blood mononuclear cells (PBMCs) were prepared by
clinical study sites and received in BD Vacutainer Cell Preparation Tube with
sodium heparin (Product Number: 362753, Becton Diclcinson, Franklin Lakes,
NJ).
Two-fold serial dilution of 800 l effector cells (patient's PBMC) starting at
concentration of 1X106 cells/mL were put into four individual polystyrene
12X75-
mm tubes. Log phase growing target cells (K562/eGFP) were adjusted with growth
CA 02603314 2007-10-02
-41-
WO 2006/113572 PCT/US2006/014320
medium (RPMI-1640) to a concentration of 1X105 cells/mL and 100 L targets
then
added into the tubes to provide effector/target (E/T) ratios of 80:1, 40:1,
20:1, 10:1.
Effector cells alone and target cells alone were used as controls. All tubes
were
incubated at 37 C with 5% COZ for about 3.5 hr. Ten microliters of propidium
iodide (PI) at a concentration of 1 mg/mL was added t each tube including
effector
and target control tubes and then incubated at room temperature for 15 min.
Cytotoxic activity was analyzed with a FACSCalibur flow cytometer
(Becton Dickinson). Linear amplification of the forward and side scatter
(FSC/SSC)
signals, as well as logarithmic amplification of eGFP and PI emission in green
and
red fluorescence were obtained. Ten thousand events per sample tube with no
gating for acquisition were collected for analysis. Data analysis for two-
parameter
dot plots for eGFP versus PI was performed using CELLQuest (Becton Dickinson
Biosciences) software to enumerate live and dead target cells. Debris and dead
cells
were excluded by setting a threshold of forward light scatter.
Example 3: The Disclosed Combination Therapy Induces Hsp70
A Phase I trial was conducted for combined administration of a
bis(thio-hydrazide) amide (Compound (1)) and a taxane (paclitaxel) to human
subjects with various advanced solid tumors. Compound (1) and paclitaxel were
co-administered intravenously over 3 hours every 3 weeks. Starting doses were
44
milligrams/meterz (mg/m2, or 110 micromoles/meterz ( mol/m2)) Compound (1)
and 135 mg/m2 (158 mol/m2) paclitaxel . Paclitaxel was then increased to 175
mg/m2 (205 mol/m2), followed by escalation of Compound (1) to establish the
maximum tolerated dose based on first cycle toxicity in 3 to 6 patients at
each dose
level. Pharmacokinetic (PK) studies were performed during cycle 1 using liquid
chromatography/mass spectrometry (LC/MS) to measure both compounds in
plasma. Heat shock protein 70 (Hsp70) was measured in plasma before and after
treatment. 35 patients were evaluated at 8 dose levels, including paclitaxel
at 135
mg/m2 (158 mol/m2) and Compound (1) at 44 mg/m2, and paclitaxel at 175
mg/m2 (205 mol/m2) and Compound (1) at a doses ranging among 44-525 mg/m2
(110-1311 mol /m2). Table 1 shows the eight different doses #1-#8 in mg/m2
and
mol/mz.
CA 02603314 2007-10-02
WO 2006/113572 - 42 PCT/US2006/014320
Table 1 #1 #2 #3 #4 #5 #6 #7 #8
Compound (1), m/mz 44 44 88 175 263 350 438 525
Com ound 1, mol/mz 110 110 220 437 657 874 1094 1311
Paclitaxel, mg/m2 135 175 175 175 175 175 175 175
Paclitaxel, moUmz 158 205 205 205 205 205 205 205
No serious effects specifically attributable to Compound (1) were observed.
Paclitaxel dose limiting toxicities occurred in a single patient in each of
the top three
dose levels (neutropenia, arthralgia, and febrile neutropeiiia with mucositis)
resulting
in cohort expansion. Compound (1) exhibited linear PK that was unaffected by
paclitaxel dose, and was rapidly eliminated from plasma with terminal-phase
half
life of 0.94 0.23 hours (h) and total body clearance of 28 8
Liters/hour/meter2
(L/h/m2). Its apparent volume of distribution was comparable to total body
water
(Vss 23 + 16 L/mz). Paclitaxel PK appeared to be moderately dependent on the
Compound (1) dose, as indicated by a significant trend toward decreasing
clearance,
and increase in peak plasma concentration and Vss, but without affecting the
terminal phase half-life. These observations are consistent with competitive
inhibition of paclitaxel hepatic metabolism. Increased toxicity at higher dose
levels
was consistent with a moderate increase in systemic exposure to paclitaxel.
Induction of Hsp70 protein in plasma was dose dependent, peaking between about
8
hours to about 24 hours after dosing.
FIGs 1A, 1B, and 1C are bar graphs showing the percent increase in Hsp70
plasma levels associated with administration of the Compound (1)/paclitaxel
combination therapy at 1 hour (FIG 1A), 5 hours (FIG 1B), and 8 hours (FIG 1C)
after administration. Significant rises in Hsp701evels occurred for at least
one
patient at the 88 mg/m2 (220 mol /m2) Compound (1) dose, where Hsp70 levels
nearly doubled in a percent increase of about 90%. At the 175 mg/m2 (437
mol/m2) Compound (1) dose, Hsp70 concentrations more than doubled in two
patients; at the 263 mg/m2 (657 mol/m2) Compound (1) dose, Hsp70
concentrations roughly doubled in two patients and increased by more than 250%
in
a third patient; at the 350 mg/m2 (874 mol/m2) Compound (1) dose, Hsp70
concentrations increased more than 200% in all patients and increased by as
much as
500% in two patients; at the 438 mg/m2 (1094 mol/m2) Compound (1) dose,
CA 02603314 2007-10-02
- 43 -
WO 2006/113572 PCT/US2006/014320
Hsp70 concentrations roughly doubled in two patients, increased by over 2005
in
one patient, and increased by as much as 500% in another patient.
Time to progression will be measured as the time from patient randomization
to the time the patient is first recorded as having tumor progression
according to the
RECIST (Response Evaluation Criteria in Solid Tumors Group) criteria; see
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et
al.
New guidelines to evaluate the response to treatment in solid tumors. J Natl
Cancer
Inst 2000;92:205-16, the entire teachings of which are incorporated by
reference.
Death from any cause will be considered as progressed.
Time to progression can be performed on the randomized sample as well as
the efficacy sample. Treatment groups can be compared using the log-rank test
and
Kaplan-Meier curves of time to progression can be presented.
FIG 2 is a Kaplan-Meier graph of time-to-progression (resumption of cancer
growth) in studies of various combinations of platinum anticancer drugs and
taxanes. Also shown is the disclosed combination of a bisthiohydrazide
(Compound
(1)), a taxane (paclitaxel) and also a platinum anticancer drug, carboplatin.
The
preliminary data in show that the disclosed method is superior to the
platin/taxane
combination alone.
Thus, the conibination of a bi(thio-hydrazide) amide and taxane dramatically
increased plasma Hsp70 levels in patients, giving significant increases for
patients at
a combined paclitaxel dose of 175 mg/m2 (205 mol/m2) and Compound (1) doses
ranging from 88 through 438 mg/m2 (220-1094 mol/m2). Moreover, the
combination was well-tolerated, with adverse events consistent with those
expected
for paclitaxel alone.
Example 4: A Phase 2 Study Shows the Disclosed Combination Therapy with
Carboplatin is Effective for Treating Non-Small Cell Lung Carcinoma
The following study of Compound (1) and paclitaxel in patients with
non-small cell lung carcinoma was initiated based on the biological activity
shown
by the results of the above Phase I study, where the combined administration
Compound (1) and paclitaxel led to dose-related Hsp70 induction.
Phase 1(safety/PK/MTD (maximum tolerated dose) was followed by a
Phase 2 randomized two arm portion. Two dose levels were evaluated in Phase 1.
CA 02603314 2007-10-02
-44-
WO 2006/113572 PCT/US2006/014320
Cohort 1 was dosed with carboplatin AUC (area under the curve) 6,
paclitaxel 175 mg/m2 and Compound (1) 233 mg/m2. If the maximum tolerated
dose was not observed, Cohort 2 was enrolled with carboplatin AUC 6,
paclitaxel
200 mg/m2 and Compound (1) 266 mg/m2.
Dosing was IV q 3 weeks for up to 6 cycles in the absence of dose-limiting
toxicity or progression. In the phase 2 portion, 86 patients are planned to be
randomized 1:1 to carboplatin AUC 6 + paclitaxe1200 mg/m2 IV q 3 weeks or
carboplatin AUC 6, paclitaxe1200 mg/m2 and Coinpound (1) 266 mg/m2. The
phase 2 primary endpoint is time to progression, with secondary endpoints of
response rate, survival, and quality of life. Study pharmacodynamic parameters
include NK cell activity and Hsp701eve1.
Sixteen patients were treated in Phase 1, 7 in Cohort 1, and 9 in Cohort 2.
No first cycle dose-limiting toxicities were seen in either cohort. Phase
adverse
effects (AEs) included (usually Grade 1-2) arthralgia and myalgia, peripheral
neuropathy, rash, nausea, and vomiting, fatigue, alopecia, edema, dehydration,
constipation, and decreased blood counts. Eleven patients completed 6 cycles
of
therapy. Eight patients (50%) achieved a partial response (PR). Seven of the 8
patients with evaluable samples showed increased NK cell activity when assayed
7
days after the second dose.
The carboplatin:paclitaxel:Compound (1) combination is well tolerated at the
dose levels studied, and the overall safety profile appears similar to that of
carboplatin:paclitaxel alone. Encouraging clinical activity was observed, as
well as
correlative NK activity that supports a conclusion that Compound (1) is
biologically
active in vivo.
The RECIST criteria used to determine objective tumor response for target
lesions, taking into account the measurement of the longest diameter for all
target
lesions. RECIST criteria include:
Complete Response (CR): Disappearance of all target lesions
Partial Response (PR): At least a 30% decrease in the sum of the
longest diameter (LD) of target lesions, taking as reference the baseline sum
LD
Progressive Disease (PD): At least a 20% increase in the sum of the LD
of target lesions, taking as reference the smallest sum LD recorded since the
treatment started or the appearance of one or more new lesions
CA 02603314 2007-10-02
-45-
WO 2006/113572 PCT/US2006/014320
Stable Disease (SD): Neither sufficient shrinlcage to qualify for PR nor
sufficient increase to qualify for PD, talcing as reference the smallest sum
LD since
the treatment started
Table 2 shows the substantial anticancer efficacy and NK cell activity results
for different subjects. The Effector/Target data shows the ratio of the
subjects
PBMC cells to the NK assay target cells. The pre and post dose column values
show the percent of tumor cells lysed before dosing with Paclitaxel and
Compound
(1). Best Response indicates an evaluation of the patient's tumor: PR = at
least a
30% decrease in the sunl of the longest diameters as compared to baseline,
while SD
indicates less than 20% of an increase and less than 30% of a decrease in the
sum of
the longest diameters as compared to baseline. Target Lesions indicates the
percent
change in targeted melanoma lesions in the subjects. NK Activity indicates the
change in NK activity before and after dosing.
Table 2 shows that for patients completing the study (#1-#8) there was a
substantial decrease in target lesion size for each patient. Also, 5 of the 8
patients
completing the study had the best response evaluation category, at least a 30%
decrease in the sum of the longest diameters compared to baseline. For NK cell
activity, 8 of the 11 original patients showed an increase between pre- and
post-dose
treatment, though in this example the difference was not significant according
to
paired t-test (p=0.06).
Table 2 % tumor cell
lysis dosing information
Sub'ect Effector/ pre- post- Paclitaxel, Cmpnd (1) Best Target NK
~ Target dose dose mg/M- mg/M' Response Lesions activity
1 80:1 9.55 16.14 175 233 SD -5.9% increase
2 80:1 3,12 8.76 175 233 SD -30% inerease
3 80:1 7.84 10.05 175 233 PR -67% increase
4 80:1 8.4 5.5 200 266 PR -38% decrease
5 80:1 7,79 30.8 175 233 PR -34% increase
6 80:1 3.59 7.81 200 266 PR -44% inerease
7 80:1 0.92 7.75 175 233 SD -24% no change
8 80:1 10.7 14.61 175 233 PR -62% increase
9 80:1 7.21 10.11 NA NA increase
10 80:1 8 3.8 NA NA decrease
11 80:1 36.19 45.98 NA NA increase
CA 02603314 2007-10-02
-46-
WO 2006/113572 PCT/US2006/014320
Given the safety profile of Cohort 2, this dose level (carboplatin AUC 6,
paclitaxe1200 mg/m2 and Compound (1) 266 mg/m2) was used in Phase 2.
Example 5: A 2 Stage Phase 2 Study Shows the Disclosed Combination Therapy
is Effective for Treating Advanced Metastatic Melanoma
The following study of Compound (1) and paclitaxel in patients with
advanced metastatic melanoma was initiated based on the biological activity
shown
by the results of the above Phase I study, where the combined administration
Compound (1) and paclitaxel led to dose-related Hsp70 induction.
The study included a Stage 1 initial safety assessment of the weekly dose
schedule, where Compound (1) 106 mg/m2 (265 mol/m2) and paclitaxel at 80
mg/m2 (94 mol/m2) were administered weeldy for 3 weeks out a 4 week period.
The dose of Compound (1) was then escalated to 213 mg/m2 (532 mol/m2) in
combination with the paclitaxel at 80 mg/m2 (94 inol/m2). The higher
tolerated
dose level was expanded to a total of 20 patients (Stage 1).
A total of 7 patients were treated in the initial safety assessment, 3 at the
lower dose level and 4 at the higher. In the absence of dose-limiting
toxicities in
either group, the higher dose level was chosen as the dose of interest and
additional
patients were enrolled to complete stage 1. Adverse events seen were as
expected
for paclitaxel chemotherapy administration. Of 20 evaluable patients, 11 were
stable
at 3 months for 55% NPR.
The study will continue to Stage 2 if 7 or more patients have a response of
stable disease or better, or at least 2 patients have a partial response or
better. A
safety assessment was performed with the first 6 patients enrolled a s the
weekly
dose schedule had not previously been studied in humans. The primary endpoint
is
non-progression rate (NPR) at 3 months and response rate. Pharmacodynamic
parameters include pre and post-dose NK cell activity in blood and when
possible,
tumor biopsies.
Table 3 shows the significant preliminary results of anticancer efficacy and
NK cell activity results when assayed 7 days after the second dose for
different
subjects. The Effector/Target data shows the ratio of the subjects PBMC cells
to the
NK assay target cells. The pre and post dose column values show the percent of
tumor cells lysed before dosing with Paclitaxel and Compound (1). Best
Response
indicates an evaluation of the patient's tumor: SD indicates less than 20% of
an
CA 02603314 2007-10-02
-47-
WO 2006/113572 PCT/US2006/014320
increase and less than 30% of a decrease in the sum of the longest diameters
as
compared to baseline; and PD = at least a 20% increase in the sum of the
longest
diameters as compared to baseline. NK Activity indicates the change in NK
activity
before and after dosing.
Table 3 shows that for patients completing the study (#12-#20, #22), three
patients had less than 20% of an increase and less than 30% of a decrease in
the sum
of the longest diameters as compared to baseline, while seven patients had at
least a
20% increase in the sum of the longest diameters as compared to baseline. For
NK
cell activity, four of the original patients showed a statistically
significant increase
between pre- and post-dose treatment.
Table 3 % tumor cell lysis dosing information Best Response
Subject Effector/ pre- post- Paclitaxel, 1, Cmpnd Z1) cycle 2 NK
Target dose dose mg/M mg/M week 4 activity
12 80:1 2.32 7.74 80 106 SD increase
13 80:1 6.13 2.43 80 106 PD decrease
14 80:1 3.83 10.77 80 213 SD increase
(40:1) 3.5 10.01 80 213 PD (increase)
16 80:1 19.71 19.78 80 213 SD no change
17 80:1 41.61 26.52 80 213 PD decrease
18 80:1 8.6 8.64 80 213 PD no change
19 80:1 24.76 18.77 80 213 PD decrease
80:1 16.49 5.2 80 213 PD decrease
21 80:1 15.4 26.31 80 213 NA increase
22 80:1 10.81 7.2 80 213 PD decrease
The combination therapy was well-tolerated on the weekly schedule.
Enrollment in the randomized portion will assess the activity of Compound (1)
in
15 combination with paclitaxel versus paclitaxel alone.
Stage 2 is planned to be a randomized 2-arm study comparing the drug
combination to paclitaxel alone. The criterion for continuation to Stage 2 is
>= 50%
non-progression rate (NPR) at two months. A total of 78 patients are to be
randomized 2:1 (combination:control). The primary endpoint is time to
progression;
20 secondary endpoints are response rate, survival, and quality of life.
Pharmacodynamic parameters will include pre- and post-dose measurements of NK
cell activity in blood and, when possible, tumor biopsies.
CA 02603314 2007-10-02
-48-
WO 2006/113572 PCT/US2006/014320
Example 6: A Phase 2 Study Shows the Disclosed Combination Therapy is
Effective for Treating Soft Tissue Sarcomas
The following study of Compound (1) and paclitaxel in patients with soft
tissue sarcomas was initiated based on the biological activity shown by the
results of
the above Phase I study, where the combined administration Compound (1) and
paclitaxel led to dose-related Hsp70 induction.
The study is a 2 stage design, enrolling 30 patients in the first stage and
adding 50 patients to tota180 if certain continuation criteria are met. Major
inclusion criteria are refractory or recurrent soft tissue sarcomas other than
gastrointestinal stromal tumor (GIST), with evidence of recent progression.
Patients
are treated weekly, 3 weelcs out of every 4 week cycle with 213 mg/m2 Compound
(1) and 80 mg/m2 paclitaxel. For example, the compounds were administered
together 3 weeks out of 4 on Days 1, 8, and 15 of a 28 day cycle as a 1 hour
IV
infusion. 30 Patients have been enrolled to completed accrual of Stage 1.
As used herein, "soft-tissue sarcomas" (STS)are cancers that begin in the soft
tissues that support, connect, and surround various parts of the body for
example,
soft tissues such as muscles, fat, tendons, nerves, and blood vessels, lymph
nodes, or
the like. Such STSs can occur anywhere in the body, though typically about one
half occur in the limbs. In various embodiments, STSs can include one or more
cancers selected from liposarcoma, fibrosarcoma, malignant fibrous
histiocytoma
leiomyosarcoma, neurofibrosarcoma, rhabdomyosarcoma, synovial sarcoma, or the
like.
Table 4 shows the significant preliminary results of anticancer efficacy and
NK cell activity results when assayed 7 days after the second dose for
different
subjects. The Effector/Target data shows the ratio of the subjects PBMC cells
to the
NK assay target cells. The pre and post dose column values show the percent of
tumor cells lysed before dosing with Paclitaxel and Compound (1). Best
Response
indicates an evaluation of the patient's tumor: PR = at least a 30% decrease
in the
sum of the longest diameters as compared to baseline; SD indicates less than
20% of
an increase and less than 30% of a decrease in the sum of the longest
diameters as
compared to baseline; and PD = at least a 20% increase in the sum of the
longest
diameters as compared to baseline. NK Activity indicates the change in NK
activity
before and after dosing.
CA 02603314 2007-10-02
WO 2006/113572 - 49 - PCT/US2006/014320
Table 4 shows that for patients completing the study (#23-#29, #31-33), five
patients had less than 20% of an increase and less than 30% of a decrease in
the sum
of the longest diameters as compared to baseline, while five patients had at
least a
20% increase in the sum of the longest diameters as compared to baseline. For
NK
cell activity, seven of the original patients showed a statistically
significant increase
or no change between pre- and post-dose treatment, wliile only four of the
original
patients showed a decrease statistically significant increase between pre- and
post-
dose treatment.
Table 4 % tumor cell dosing information Best Response
lysis
Subject Effector/ pre- post- Paclitaxel, Cmpnd (1) cycle 2 NK
Target dose dose ing/M mg/M' activity
23 80:1 4.28 30.48 80 213 PD increase
24 80:1 20.74 20.04 80 213 SD no change
25 80:1 34.28 11.86 80 213 PD decrease
26 80:1 22.33 14.74 80 213 SD decrease
27 80:1 10.6 22.9 80 213 SD increase
28 80:1 17.93 28.13 80 213 SD increase
29 80:1 6.58 17.18 80 213 PD increase
30 (40:1) 9.88 9.91 80 213 NA no change
31 80:1 2.62 5.46 80 213 SD increase
32 80:1 13.03 7.41 80 213 PD decrease
33 80:1 15.77 7.84 80 213 PD decrease
Patients are currently being evaluated through 3 months. Adverse events
seen were typical for paclitaxel administration on a similar schedule.
Assessment of
NK activity is ongoing. The addition of Compound (1) to the weekly paclitaxel
schedule was well-tolerated. Stage 1 accrual has completed, and patients are
currently being evaluated for the study continuation decision.
While this invention has been particularly shown and described with
references to preferred embodiments thereof, it will be understood by those
skilled
in the art that various changes in form and details may be made therein
without
departing from the scope of the invention encompassed by the appended claims.