Note: Descriptions are shown in the official language in which they were submitted.
CA 02604700 2007-09-28
LOW DENSITY SPREADING METHODS FOR SOMATIC EMBRYOGENESIS
FIELD OF THE INVENTION
The present invention relates to methods for producing plant embryos in vitro,
and
optionally producing plants from plant embryos.
BACKGROUND OF THE INVENTION
The demand for coniferous trees, such as pines and firs, to make wood products
continues to increase. One proposed solution to the problem of providing an
adequate supply of
coniferous trees is to identify individual coniferous trees that possess
desirable characteristics,
such as a rapid rate of growth, and to produce numerous, genetically
identical, clones of the
superior trees by somatic cloning.
Somatic cloning is the process of creating genetically identical trees from
tree somatic
tissue. Tree somatic tissue is tree tissue other than the male and female
gametes. In one
approach to somatic cloning, tree somatic tissue is cultured in an initiation
medium which
includes hormones, such as auxins and/or cytokinins, that initiate formation
of embryogenic
cells that are capable of developing into somatic embryos. The embryogenic
cells are then
further cultured in a maintenance medium that promotes multiplication of the
embryogenic cells
to form pre-cotyledonary embryos (i.e., embryos that do not possess
cotyledons). The
multiplied embryogenic cells are then cultured in a development medium that
promotes
development and maturation of cotyledonary somatic embryos which can, for
example, be
placed within artificial seeds and sown in the soil where they germinate to
yield conifer
seedlings. The seedlings can be transplanted to a growth site for subsequent
growth and
eventual harvesting to yield lumber, or wood-derived products. Alternatively,
the cotyledonary
somatic embryos can also be germinated in a germination medium, and thereafter
transferred to
soil for further growth.
A continuing problem with somatic cloning of conifer embryos is stimulating
efficient
and cost effective formation of somatic embryos that are capable of
germinating to yield plants.
Preferably conifer somatic embryos, formed in vitro, are physically and
physiologically similar,
-1-
CA 02604700 2007-09-28
or identical, to conifer zygotic embryos formed in vivo in conifer seeds.
There is, therefore, a
continuing need for methods for producing viable conifer somatic embryos from
conifer
embryogenic cells.
SUMMARY OF THE INVENTION
In one aspect, the present invention provides methods of producing conifer
cotyledonary
somatic embryos from pre-cotyledonary embryos. The methods of this aspect of
the invention
include the step of (a) dispensing a plurality of pre-cotyledonary embryos
onto a porous material
horizontally disposed over a non-porous surface in a volume of sterile
dilution medium
sufficient to submerge at least the surface of the porous material, thereby
uniformly dispersing
the pre-cotyledonary embryos; (b) removing the sterile dilution medium from
the porous
material, thereby trapping the uniformly dispersed pre-cotyledonary embryos on
the porous
material; and (c) contacting the pre-cotyledonary embryos trapped on the
porous material with
development medium for a period of time sufficient to produce conifer
cotyledonary somatic
embryos.
In another aspect, the present invention provides methods of producing conifer
cotyledonary somatic embryos from conifer somatic cells. The methods of this
aspect of the
invention include the steps of (a) culturing conifer somatic cells in an
induction medium to yield
embryogenic cells; (b) culturing the embryogenic cells prepared in step (a) in
a liquid
maintenance medium to form pre-cotyledonary conifer somatic embryos; (c)
dispensing a
plurality of pre-cotyledonary embryos prepared in step (b) onto a porous
material horizontally
disposed over a non-porous surface in a volume of sterile dilution medium
sufficient to
submerge at least the surface of the porous material, thereby uniformly
dispersing the pre-
cotyledonary embryos; (d) removing the sterile dilution medium from the porous
material,
thereby trapping the uniformly dispersed pre-cotyledonary embryos on the
porous material; and
(e) contacting the pre-cotyledonary embryos trapped on the porous material
with development
medium for a period of time sufficient to produce conifer cotyledonary somatic
embryos.
-2-
CA 02604700 2007-09-28
The methods of the invention produce a higher yield of conifer somatic embryos
than an
equivalent method in which the pre-cotyledonary embryos are not uniformly
dispersed over
development medium. In some embodiments, the plurality of the pre-cotyledonary
embryos are
dispensed in sterile dilution medium at a density of less than 0.1 gram wet
cell weight per square
inch of porous material, such as from 0.005 to 0.1 gram wet cell weight per
square inch of
porous material. In some embodiments, the plurality of the pre-cotyledonary
embryos are
dispensed in sterile dilution medium at a density of less than 0.05 gram wet
cell weight per
square inch of porous material, such as from 0.001 to 0.05 gram wet cell
weight per square inch
of porous material.
The methods of the invention are useful, for example, for preparing conifer
somatic
embryos that can be used for later maturation steps and/or that can be
germinated to yield
conifer plants that can be grown into mature conifer trees, if so desired.
Thus, for example, the
methods of the invention can be used to produce clones of individual conifer
trees that possess
one or more desirable characteristics, such as a rapid growth rate or improved
wood quality. For
example, a population of conifer somatic embryos produced using the methods of
the invention
can be used to produce a stand, or forest, of conifer trees possessing one or
more desirable
characteristics, such as a rapid growth rate or improved wood quality. The
trees, in turn, can be
utilized to produce wood products.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing aspects and many of the attendant advantages of this invention
will
become more readily appreciated as the same become better understood by
reference to the
following detailed description, when taken in conjunction with the
accompanying drawings,
wherein:
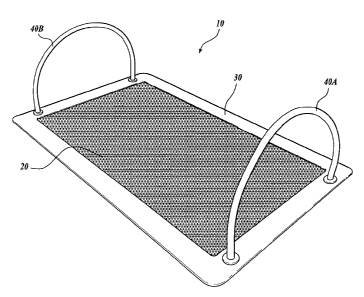
FIGURE 1 illustrates an exemplary plating frame comprising porous material
disposed
on a support frame, for use in accordance with an embodiment of the method of
the invention;
and
-3-
CA 02604700 2007-09-28
FIGURES 2A-H provides photographic images demonstrating that the growth of
cotyledonary embryos plated according to the methods of the invention is
improved in
comparison to a standard plating method, as described in Example 3. FIGURES 2A-
D show the
drop plating method controls for genotypes A, E, F, and B, respectively.
FIGURES 2E-H show
the results of the liquid dispersion confluent spread plating method for
genotypes A, E, F, and B,
respectively.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
Unless specifically defined herein, all terms used herein have the same
meaning as they
would to one skilled in the art of the present invention.
As used herein, the term "embryogenic cells" refers to any cells, including
cells that are
organized to form a tissue or an organ, derived from a plant of the order
Coniferales, that are
capable of producing one or more conifer somatic embryos when treated in
accordance with the
methods of the invention. Thus, the term "embryogenic cells" includes, for
example, conifer
embryonal suspensor masses.
As used herein, the term "pre-cotyledonary embryo" refers to an embryo that
does not
yet possess any cotyledons.
As used herein, the term "cotyledonary embryo" refers to an embryo that
possesses at
least one cotyledon.
The present inventors have discovered that methods of the invention produce a
higher
yield of conifer somatic embryos than an equivalent method in which the pre-
cotyledonary
embryos are not uniformly dispersed over development medium. The inventors
have further
observed that plating pre-cotyledonary embryos according to the methods of the
invention at a
plating density of less than 0.1 gram wet cell weight per square inch of
porous material, such as
from 0.001 to 0.1 gram wet cell weight per square inch of porous material,
produces an
increased yield of cotyledonary embryos per unit area as compared to pre-
cotyledonary embryos
plated at a higher density and/or plated using a traditional pipette drop
method, as further
described in Examples 2 and 3.
-4-
CA 02604700 2007-09-28
In accordance with the foregoing, in one aspect, the present invention
provides methods
of producing conifer cotyledonary somatic embryos from pre-cotyledonary
embryos. The
methods of this aspect of the invention include the step of (a) dispensing a
plurality of pre-
cotyledonary embryos onto a porous material disposed horizontally over a non-
porous surface in
a volume of sterile dilution medium sufficient to submerge at least the
surface of the porous
material, thereby uniformly dispersing the pre-cotyledonary embryos; (b)
removing the sterile
dilution medium from the porous material, thereby trapping the uniformly
dispersed pre-
cotyledonary embryos on the porous material; and (c) contacting the pre-
cotyledonary embryos
trapped on the porous material with development medium for a period of time
sufficient to
produce conifer cotyledonary somatic embryos.
The methods of the invention can be used to produce cotyledonary somatic
embryos
from any conifer, such as members of the genus Pinus, such as Loblolly Pine
(Pinus taeda) and
Radiata pine. Again, by way of example, Douglas-fir cotyledonary somatic
embryos can be
produced by the methods of the invention.
In accordance with the methods of the invention, a plurality of pre-
cotyledonary conifer
somatic embryos to be plated are suspended into a volume of sterile dilution
medium sufficient
to submerge at least the surface of the porous material disposed on the non-
porous substrate.
The plurality of pre-cotyledonary embryos may be generated using the methods
described
herein. For example, suspension cultures of immature somatic embryos (pre-
cotyledonary
embryos) may be cultured in a liquid maintenance medium and the cells allowed
to settle. The
settled cell volume (SCV) is then measured, using any suitable method, such as
the method
described in Example 2. The desired amount of SCV is then diluted with the
sterile dilution
media for plating at a desired density. In some embodiments, the amount of
dilution medium
used to dilute the SCV is chosen based on the surface area of the plating
frame, in order to be
sufficient to submerge at least the surface of the porous material attached to
the frame. For
example, the SCV may be diluted with an amount of sterile dilution media at
least about 3 to 4
times or more of the SCV volume.
-5-
CA 02604700 2007-09-28
The sterile dilution medium may be any suitable liquid medium that maintains
the ability
of the embryos to survive and maintain their developmental status, such as,
for example,
maintenance media or the exemplary dilution media shown below in TABLE 2.
In some embodiments of the method, the pre-cotyledonary embryos are plated at
a low
density, such as less than about 0.1 gram wet cell weight per square inch of
porous material
plating area, assuming an average wet weight of about 0.lg/ml SCV. The average
wet weight of
SCV may be determined as described in Example 2. For example, the embryos may
be plated at
less than 0.05 gram, or less than 0.025 grams wet cell weight per square inch
of porous material
plating area. In some embodiments, the pre-cotyledonary embryos are plated at
a low density in
a range from about .001 gram to about 0.1 gram wet cell weight per square inch
of porous
material plating area (e.g., from 1 ml SCV to 0.01 ml SCV per square inch of
porous material
plating area). In one embodiment, the pre-cotyledonary embryos are plated at a
density in a
range from about 0.01 grams to about .08 grams wet cell weight per square inch
of porous
material plating area, such as from about 0.02 grams to about 0.05 grams wet
cell weight per
square inch of porous material plating area.
Porous materials that are useful in the practice of the present invention have
a pore
diameter in the range of from about 5 microns to about 1200 microns, such as
from about
50 microns to about 500 microns, such as from about 70 to about 150 microns,
such as about
100 microns. The porous material is typically planar and may be any desired
shape and
dimension. The shape and dimension of the porous material are chosen for ease
of manipulation
and for placement onto a growth substrate such as development media. Suitable
shapes include
square, rectangular or circular shapes. Exemplary dimension are from a surface
area of about 14
square inches to 28 square inches or greater, such as 50 square inches, 100
square inches up to
500 square inches or greater. Preferred porous materials are sterilizable and
sufficiently strong
to resist tearing when the materials are lifted in order to transfer somatic
embryos after plating to
subsequent stages of the somatic embryo production process. Examples of useful
porous
materials include membranes, nylon fiber, woven mesh (e.g., nylon, stainless
steel or plastic)
-6-
CA 02604700 2007-09-28
and polymeric fibers. In some embodiments, the porous material is non-
absorbent. In some
embodiments, the porous material is a woven mesh, such as a stainless steel or
nylon mesh.
In accordance with an embodiment of the method of the invention, a porous
material,
such as for example, a woven mesh, is used to plate and support plant tissue
during the
development phase of plant somatic embryo production. The pre-cotyledonary
somatic embryos
are initially dispensed onto a planar porous material which is disposed
horizontally over a non-
porous surface. The non-porous surface may be any suitable sterile surface,
such as, for
example, the surface of a solid or semi-solid growth medium, such as a petri
dish containing
development medium, or any other sterile or sterilizable surface capable of
retaining liquid, such
as a plastic, rubber, or glass surface. In some embodiments, the non-porous
surface is semi-solid
development medium contained in a box, such as a cambro box. In some
embodiments, the non-
porous surface is contained within a bioreactor vessel, wherein the bioreactor
vessel is drainable.
In one embodiment of the method, the porous material is attached to a plating
frame. A
representative example of a plating frame 10 is shown in FIGURE 1. As shown in
FIGURE 1,
the plating frame 10 comprises a planar porous material 20 attached to a
support frame 30 that
surrounds the porous material 20. Optional handles 40A, 40B are provided that
are attached to
the support frame 30. The plating frame is preferably made of materials that
are sterilizable.
For example, the support frame 30 may be made of a metal or plastic material.
The handles
40A, 40B may be made of any suitable sterilizable material, such as, for
example, autoclavable
tubing. An exemplary method for constructing the plating frame 10 is described
in Example 2.
In one embodiment, the support frame 30 is metal and the porous material is a
nylon mesh
which is attached to the frame prior to autoclaving and moderately shrinks
after autoclaving to
produce a taut attachment to the frame and a substantially level plating
surface.
In accordance with the methods of the invention, a plurality of pre-
cotyledonary embryos
are dispensed onto a porous material disposed over a non-porous surface in a
volume of sterile
dilution medium sufficient to submerge at least the surface of the porous
material. The pre-
cotyledonary embryos are thereby uniformly dispersed across the submerged
surface of the
porous material. In some embodiments, the dispensed embryos are gently mixed
or agitated to
-7-
CA 02604700 2007-09-28
facilitate dispersion of the embryos in the sterile dilution medium. Gentle
agitation may be
achieved by any suitable means, such as, for example, via the use of an
instrument contacting
the embryos, or via vibration of the porous material and/or the non-porous
substrate.
Once the dispensed pre-cotyledonary embryos are substantially uniformly
dispersed over
the porous material, the sterile dilution medium is removed from the porous
material. In one
embodiment, the sterile dilution medium is removed from the porous material by
vertically
lifting the porous material off the non-porous substrate, thereby trapping the
uniformly dispersed
pre-cotyledonary embryos on the surface of the porous material. For example,
the porous
material attached to a plating frame 10 comprising handles 40A, 40B, may be
vertically lifted by
the handles using any suitable means, such as manually or through robotic
means.
In an alternative embodiment, once the dispensed pre-cotyledonary embryos are
substantially uniformly dispersed over the porous material, the sterile
dilution medium is
removed by reducing the volume of the sterile dilution medium to a level below
the surface of
the porous material, thereby trapping the uniformly dispersed pre-cotyledonary
embryos on the
surface of the porous material. The volume of sterile dilution medium may be
reduced using
any method that avoids disturbing the distribution of plated cells, such as,
for example,
suctioning, draining, tipping, or blotting off the sterile dilution medium.
The uniformly dispersed pre-cotyledonary embryos trapped on the surface of the
porous
material are then contacted with development medium for a period of time
sufficient to produce
conifer cotyledonary somatic embryos.
In one embodiment, the porous material is either continuously or
intermittently contacted
with liquid development medium. For example, the porous material may be placed
on an
absorbent pad which is soaked in development medium so that the development
medium passes
through the porous material and contacts the embryos. The porous material,
such as a nylon
mesh bearing embryonic cells, is typically enclosed within a sealed space
which contains a
humid atmosphere that ensures that the embryos remain moist. In another
embodiment, the
porous material is disposed on a growth substrate comprising solid or semi-
solid development
media.
-8-
CA 02604700 2007-09-28
The development medium for use in the methods of the invention contains
nutrients that
sustain the somatic embryos. Maltose and glucose may be included in the
development medium
as the principal or sole source of sugar for the somatic embryos. Useful
maltose and glucose
concentrations are within the range of from about 1% to about 2.5%. Suitable
development
media typically do not include growth-promoting hormones, such as auxins and
cytokinins, but
may include the hormone abscisic acid. When abscisic acid is utilized in the
development
medium, it is typically utilized at a concentration in the range of from about
1 mg/L to about
200 mg/L. The development medium may contain gellan gum, typically present at
a
concentration of up to about 0.40%. The osmolality of the development medium
can be adjusted
to a value that falls within a desired range, using osmoticants such as PEG
8000 molecular
weight, such as from about 250 mM/Kg to about 450 mM/Kg. Typically, an
osmolality of 300-
350 mM or higher is advantageous. An example of suitable liquid or solid
development medium
is provided in Example 1 and Example 2.
By way of example, pre-cotyledonary conifer somatic embryos may be cultured on
a
porous material, such as a nylon mesh or membrane that is at least
intermittently contacted with
development medium, for a period of from 4 weeks to 14 weeks, such as from 8
weeks to
12 weeks, or such as about 12 weeks, at a temperature of from 10 C to 30 C,
such as from 15 C
to 25 C, or such as from 20 C to 23 C.
In one embodiment, the pre-cotyledonary conifer somatic embryos are cultured
on a
porous material contacted with liquid development media that is applied to an
absorbent
substrate, such as a substrate made from cellulose (e.g., cellulose fibers),
such as one or more
filter papers, or some other absorbent material. The substrate absorbs the
liquid development
medium which passes through the porous material disposed on the substrate and
contacts conifer
precotyledonary somatic embryos disposed on the porous material. The
development medium
promotes the development of the conifer precotyledonary somatic embryos to
form cotyledonary
somatic embryos.
In another embodiment, the pre-cotyledonary conifer somatic embryos are
cultured on a
porous material contacted with liquid development medium using an atomiser
which sprays the
-9-
CA 02604700 2007-09-28
porous material with development medium. The somatic embryos are disposed on
an upper
surface of the porous material and the opposite, lower surface of the porous
material is sprayed
with liquid development medium. By way of further example, the porous material
bearing
somatic embryos can be disposed over liquid development medium that includes a
rotating stir
bar which rotates sufficiently fast to spray liquid development medium up onto
the lower surface
of the porous material.
In another aspect, the present invention provides methods of producing conifer
cotyledonary somatic embryos from conifer somatic cells. The methods of this
aspect of the
invention include the step of (a) culturing conifer somatic cells in an
induction medium to yield
embryogenic cells; (b) culturing the embryogenic cells prepared in step (a) in
a liquid
maintenance medium to form pre-cotyledonary conifer somatic embryos; (c)
dispensing a
plurality of pre-cotyledonary embryos prepared in step (b) onto a porous
material disposed over
a non-porous surface in a volume of sterile dilution medium sufficient to
submerge at least the
surface of the porous material, thereby uniformly dispersing the pre-
cotyledonary embryos;
(d) removing the sterile dilution medium from the porous material, thereby
trapping the
uniformly dispersed pre-cotyledonary embryos on the porous material; and (e)
contacting the
pre-cotyledonary embryos trapped on the porous material with development
medium for a
period of time sufficient to produce conifer cotyledonary somatic embryos.
Thus, in some embodiments, conifer somatic cells are cultured in, or on, an
induction
medium to yield embryogenic cells. Embryogenic cells are capable of producing
one or more
cotyledonary conifer somatic embryos. Examples of embryogenic cells are
embryonal
suspensor masses (ESMs).
The induction medium typically includes inorganic salts and organic nutrient
materials.
The osmolality of the induction medium is typically about 160 mM/kg or even
lower, but it may
be as high as 170 mM/kg. The induction medium typically includes growth
hormones. Examples
of hormones that can be included in the induction medium are auxins (e.g.,
2,4-dichlorophenoxyacetic acid (2,4-D)) and cytokinins (e.g., 6-
benzylaminopurine (BAP)).
-10-
CA 02604700 2007-09-28
Auxins can be utilized, for example, at a concentration of from 1 mg/L to 200
mg/L. Cytokinins
can be utilized, for example, at a concentration of from 0.1 mg/L to 10 mg/L.
The induction medium may contain an adsorbent composition, especially when
very high
levels of growth hormones are used. The adsorbent composition can be any
composition that is
not toxic to the embryogenic cells at the concentrations utilized in the
practice of the present
methods, and that is capable of adsorbing growth-promoting hormones, and toxic
compounds
produced by the plant cells during embryo development, that are present in the
medium. Non-
limiting examples of useful adsorbent compositions include activated charcoal,
soluble
poly(vinyl pyrrolidone), insoluble poly(vinyl pyrrolidone), activated alumina,
and silica gel.
The adsorbent composition may be present in an amount, for example, of from
about 0.1 g/L to
about 5 g/L. The induction medium is typically solid, and may be solidified by
inclusion of a
gelling agent. An example of an induction medium useful in the practice of the
present
invention is set forth in Example 1.
Conifer somatic cells are typically cultured in, or on, an induction medium
for a period
of from 3 weeks to 12 weeks, such as from 8 weeks to 10 weeks, or such as
about 8 weeks, at a
temperature of from 10 C to 30 C, such as from 15 C to 25 C, or such as from
20 C to 23 C.
The maintenance medium may be a solid medium, or it may be a liquid medium
which
can be agitated to promote growth and multiplication of the embryogenic
tissue. The osmolality
of the maintenance medium is typically higher than the osmolality of the
induction medium,
typically in the range of 180-400 mM/kg. The maintenance medium may contain
nutrients that
sustain the embryogenic tissue, and may include hormones, such as one or more
auxins and/or
cytokinins, that promote cell division and growth of the embryogenic tissue.
Typically, the
concentrations of hormones in the maintenance medium is lower than their
concentration in the
induction medium.
It is generally desirable, though not essential, to include maltose as the
sole, or principal,
metabolizable sugar source in the maintenance medium. Examples of useful
maltose
concentrations are within the range of from about 1% to about 2.5%. An example
of a suitable
maintenance medium is set forth in Example 1 herein.
-11-
CA 02604700 2007-09-28
Conifer embryogenic cells are typically cultured in, or on, a maintenance
medium for a
period of up to 6 months by weekly subculture, at a temperature of from 10 C
to 30 C, such as
from 15 C to 25 C, or such as from 20 C to 23 C.
Conifer embryogenic cells are typically transferred to fresh maintenance
medium once
per week or as growth exhausts media components.
Useful development media are described supra. After being cultured in
continuous, or
periodic, contact with a development medium, the cotyledonary somatic embryos
can optionally
be transferred to a maturation medium, and then to a stratification medium,
for a further period
of culture.
The methods of the invention can be used, for example, to produce clones of
individual
conifer trees that possess one or more desirable characteristics, such as a
rapid growth rate.
Thus, in one aspect, the present invention provides methods for producing a
population of
genetically-identical, conifer, cotyledonary, somatic embryos. The methods of
this aspect of the
invention each include the step of culturing genetically-identical, conifer,
precotyledonary
somatic embryos on a porous material (e.g., porous nylon mesh) that is in
continuous, or
periodic, contact with a development medium, for a period of time sufficient
to produce
genetically-identical, conifer, cotyledonary, somatic embryos from the
precotyledonary somatic
embryos, wherein the development medium passes through the porous material and
contacts the
somatic embryos.
The conifer cotyledonary somatic embryos produced using the methods of the
invention
can optionally be germinated to form conifer plants which can be grown into
coniferous trees, if
desired. The cotyledonary embryos may also be disposed within artificial seeds
for subsequent
germination. The conifer cotyledonary somatic embryos can be germinated, for
example, on a
solid germination medium, such as the germination medium described in Example
2 herein. The
germinated plants can be transferred to soil for further growth. For example,
the germinated
plants can be planted in soil in a greenhouse and allowed to grow before being
transplanted to an
outdoor site. Typically, the conifer cotyledonary somatic embryos are
illuminated to stimulate
-12-
CA 02604700 2007-09-28
germination. Typically, all the steps of the methods of the invention, except
germination, are
conducted in the dark.
The methods of the invention produce a higher yield of conifer somatic embryos
per
surface area plated than an equivalent method in which the embryogenic cells
are plated at a
higher density and/or in the presence of excess liquid, as further described
in Examples 2 and 3
supra.
The methods of the invention can be used, for example, to produce clones of
individual
conifer trees that possess one or more desirable characteristics, such as a
rapid growth rate. The
methods described herein can be used to produce populations of genetically-
identical, mature
somatic conifer embryos.
The following examples merely illustrate the best mode now contemplated for
practicing
the invention, but should not be construed to limit the invention.
EXAMPLE 1
This Example shows a representative method of the invention for producing
somatic pine
embryos from Loblolly Pine.
Female gametophytes containing zygotic embryos are removed from seeds four to
five
weeks after fertilization. The seed coats are removed but the embryos are not
further dissected
out of the surrounding gametophyte other than to excise the nucellar end. The
cones were stored
at 4 C until used. Immediately before removal of the immature embryos, the
seeds are sterilized
utilizing an initial washing and detergent treatment followed by a ten minute
sterilization in 15%
H202. The explants were thoroughly washed with sterile distilled water after
each treatment.
Tables 1 and 2 set forth exemplary compositions of media useful for producing
pine
somatic embryos.
-13-
CA 02604700 2010-08-03
TABLE 1
Pinus Taeda Basal Medium (BM)
Constituent Concentration (m L)
NH4NO3 150.0
KNO3 909.9
KH2PO4 136.1
Ca(N03)2.4H20 236.2
CaC12.4H2O 50.0
MgS04.7H20 246.5
Mg(N03)2.6H20 256.5
MgC12.6H20 50.0
KI 4.15
H3BO3 15.5
MnSO4.H2O 10.5
ZnS04.7H20 14.4
NaMoO4.2H2O 0.125
CuSO4.5H20 0.125
CoC12.6H20 0.125
FeSO4.7H2O 27.86
Na2EDTA 37.36
Maltose 30,000
myo-Inositol 200
Casamino acids 500
L-Glutamine 1000
Thiamine-HCl 1.00
Pyridoxine-HCI 0.50
Nicotinic acid 0.50
Glycine 2.00
GelriteTM+ 1600
pH adjusted to 5.7
+Used if a solid medium is desired.
14
CA 02604700 2007-09-28
TABLE 2
Composition of Media for Different Stage Treatments
BM1-Induction Medium BM+2,4-D (15 M)+Kinetin (2 M)+BAP (2 M).
BM2-Maintenance Medium BM+2,4-D (5 M)+Kinetin (0.5 M)+BAP (0.5 M).
GELRITE (1600 mg/L) is added when a solid medium is
desired.
Dilution Medium BM + I Omg/mL abscisic acid + 100- 1 000mg/mL additional
myo-inositol, + 2.5% Maltose. The following amino acid
mixture is added: L-proline (100mg/L), L-asparagine
(100mg/L), L-arginine (50mg/L), L-alanine (20mg/L), and
L-serine (20mg/L). Preferably no maintenance hormones
are present.
BM3-Development Medium BM+25 mg/L abscisic acid +12% PEG-8000 +800 mg/L
additional myo-inositol +0.1 % activated charcoal +1 %
glucose, +2.5% Maltose. The following amino acid mixture
is added: L-proline (100 mg/L), L-asparagine (100 mg/L),
L-arginine (50 mg/L), L-alanine (20 mg/L), and L-serine
(20 mg/L). GELRITE (2500 mg/L) is added when a solid
medium is desired.
BM5-Stratification Medium BM3 modified by omitting abscisic acid, and PEG-
8000.
GELRITE (2500 mg/L) is added when a solid medium is
desired.
BM6-Germination Medium BM modified by replacing maltose with 2% sucrose.
Myo-inositol is reduced to 100.0 mg/L, glutamine and
casamino acids are reduced to 0.0 mg/L. FeSO4.7H2O is
reduced to 13.9 mg/L and Na2EDTA reduced to 18.6 mg/L.
Agar at 0.8% and activated charcoal at 0.25% are added.
Induction: Sterile gametophytes with intact embryos are placed on a solid BM1
culture
medium and held in an environment at 22 -25 C with a 24 hour dark photoperiod
for a time of
3-5 weeks. The length of time depends on the particular genotype being
cultured. At the end of
this time, a white mucilaginous mass forms in association with the original
explants.
Microscopic examination typically reveals numerous early stage embryos
associated with the
mass. These are generally characterized as having a long thin-walled suspensor
associated with
a small head with dense cytoplasm and large nuclei.
Osmolality of the induction medium may in some instances be as high as 150
mM/kg.
Normally it is about 120 mM/kg or even lower (such as 110 mM/kg).
Maintenance and Multiplication of Pre-cotyledonary Embryos: Early stage
embryos
removed from the masses generated in the induction stage are first placed on a
BM2 gelled
-15-
CA 02604700 2007-09-28
maintenance and multiplication medium. This differs from the induction medium
in that the
growth hormones (both auxins and cytokinins) are reduced by at least a full
order of magnitude.
Osmolality of this medium is at 130 mM/kg or higher (typically within the
range of about 120-
150 mM/kg for Pinus taeda). The temperature is again 22 -25 C in the dark.
Embryos are
cultured 12-14 days on the BM2 solid medium before transferring to a liquid
medium for further
subculturing. This liquid medium has the same composition as BM2, but lacks
the gellant. The
embryos at the end of the solid maintenance stage are typically similar in
appearance to those
from the induction stage. After 5 to 6 weekly subcultures on the liquid
maintenance medium,
advanced early stage embryos have formed. These are characterized by smooth
embryonal
heads, estimated to typically have over 100 individual cells, with multiple
suspensors.
Embryo Development: Embryo development is conducted as described below in
Examples 2 and 3.
The osmotic potential of this development medium may be raised substantially
over that
of the maintenance medium. It has been found advantageous to have an
osmolality as high as
300 mM/kg or even higher. Development is preferably carried out in complete
darkness at a
temperature of 22 -25 C until cotyledonary embryos have developed. Development
time is
typically several weeks, such as 7 to 12 weeks.
Stratification: Cotyledonary embryos are singulated and transferred to
stratification
medium BM5. This medium is similar to development medium but lacks abscisic
acid,
PEG-8000, and gellan gum. Embryos are cultivated on stratification medium at
between about
1 C and about 10 C in the dark for between three to six weeks.
Conditioning over water: The mature embryos still on the porous material are
lifted
from the growth substrate and placed in a closed container over H2O at a
relative humidity of
97%, for a period of about three weeks.
Germination: The conditioned mature embryos were placed on solid BM6 medium
for
germination. This is a basal medium lacking growth hormones which has been
modified by
reducing sucrose, myo-inositol and organic nitrogen. The embryos are incubated
on BM6
medium for sufficient time under environmental conditions of 23 -25 C, until
the resulting
-16-
CA 02604700 2007-09-28
plantlets have a well developed radicle and hypocotyl and green cotyledonary
structure and
epicotyl.
Because of the reduced carbohydrate concentration, the osmotic potential of
the
germination medium is further reduced below that of the development medium. It
is normally
below about 150 mM/kg (such as about 100 mM/kg).
EXAMPLE 2
This example describes the construction an exemplary plating frame and use
according to
an embodiment of the method of the invention.
Construction of a Plating Frame: A metal plating frame was constructed to
which a
100 micron nylon weave mesh was attached by silicone. The plating frame (10)
is shown in
FIGURE 1. In the embodiment of the plating frame (10) shown in FIGURE 1, the
nylon weave
mesh (20) is rectangular in shape, with a length of 7 inches and a width of 4
inches, having an
exposed surface area of 28 square inches. Tubing handles (40A, 40B) were
attached to the metal
frame (30) to facilitate movement of the plating frame (10). A silicon bead
was added along the
edge of the frame and across the middle of the frame to create 2 separated
plating areas of equal
size (not shown). The plating frame (10) was then autoclaved, resulting in a
taut plating surface
due to shrinkage of the nylon weave mesh (20) onto the metal frame (30) during
autoclaving.
Plating cells on the Plating Frame:
Preparation of the SCV for plating: Conifer somatic embryo cells of genotype A
that
were grown in proliferation medium (made as described in TABLE 4) in 1 liter
Ehrlemeyer
flasks were allowed to settle. The settled cell volume (SCV) was measured by
drawing a line on
the flask, and supernatant above the settled cells was withdrawn via a fritted
glass wand under
aspiration. The settled cells were then resuspended in a 3X SCV supplemental
amount of sterile
dilution medium, made as described in Example 1.
Determination of the wet weight of SCV: In order to determine wet weight of
SCV, SCV
was measured as described above. The supernatant was then removed using a
Buchnar funnel
(13 inches Hg) and 1 ml of the SCV sample was placed onto a pre-weighed, pre-
moistened
-17-
CA 02604700 2007-09-28
VWR grade 417 filter paper. Weight measurements were taken at a fixed time (30
seconds) on a
4-point balance. Wet weight measurements of four representative genotypes per
ml of SCV are
shown below in TABLE 3.
TABLE 3
Genotype wet weight mg/ml SCV
A 92 mg/ml
B 102 mg/ml
C 102 in ml
D 118 mg/ml
From the results shown in TABLE 3, the average wet weight across 4 genotypes
tested:
103.5 mg/ml SCV. Therefore, the average wet weight of 1 ml of SCV from the
four genotypes
tested is about 0.1 g/mL SCV.
A plating frame (10), made as described above, was provided and placed onto
the surface
of semi-solid development media (made as described in TABLE 5). The volume of
the rinsed,
settled cells was then measured, and plated onto the first half of the plating
frame at a density of
3 mis SCV (0.3 g/14 square inches = 0.02 g/square inch), and onto the second
half of the plating
frame at a density of 12 mis SCV (1.2 g/14 square inches = 0.08 g/square
inch), each in a total
volume of 9 mls and 36 mls, respectively.
Plating of the cells was done over the plating frame placed onto the surface
of a semi-
solid development media contained in a box. This allowed cells and media to
disperse evenly
across the nylon mesh in order to minimize variation in cell density and
initial cell depth. Once
the plated cells were evenly dispersed over the submerged nylon mesh, the
plating frame (10)
was lifted vertically off the semi-solid development media, thereby capturing
the evenly
dispersed embryos and allowing the excess media to disperse while remaining in
the first box.
The plating frame containing the evenly dispersed embryos was then placed onto
the surface of a
semi-solid development media of the same formulation as the first in a new
box. The plated
cells on the plating frame were then allowed to grow for 12 weeks and
monitored for total
biomass, embryo suspensor mass, and embryo structure formation, as shown below
in
TABLE 6.
-18-
CA 02604700 2010-08-03
TABLE 4
Proliferation/Maintenance Medium
(Loblolly Pine)
Constituent Concentration (mg/1L)
NH4NO3 150.0
KNO3 909.9
KH2PO4 136.0
Ca(N03)2.4H20 236.15
CaC12.2H2O 50.0
MgSO4.7H2O 246.5
Mg(N03)2.6H20 256.5
MgC12.6H20 50.0
KI 4.15
H3BO3 15.5
MnSO4.H2O 10.5
ZnSO4.7H2O 14.4
Na2MoO4.2H2O 0.125
CuSO4.5H20 0.125
CoC12.6H2O 0.125
FeSO4.7H2O 27.86
Na2EDTA.2H2O 37.36
Maltose 30,000
Myo-Inositol 200
Casamino acids 500
L-Glutamine 1000
Thiamine-HO 1.00
Pyridoxine-HC1 0.50
Nicotinic acid 0.50
Glycine 2.00
*GelriteTM+ 1600*
2,4 D (10 mg/mL) 1.1 mg/L
6-BAP (10 m /mL) 0.1 m L
KinetinTM (10 mg/mL) 0.1 mg/L
*ABA (2 mg/mL) 1.0 mg/L*
pH adjusted to 5.7 (*=optional)
19
CA 02604700 2007-09-28
TABLE 5
Development Medium (Loblolly Pine)
Constituent Concentration
(m /L)
NH4NO3 150.0
KNO3 909.9
KH2PO4 136.0
Ca(N03)2.4H20 236.15
CaC12.2H20 50.0
MgSO4.7H2O 246.5
Mg(N03)2.6H20 256.5
MgC12.6H20 50.0
KI 4.15
H3BO3 15.5
MnSO4.H2O 10.5
ZnSO4.7H2O 14.4
Na2MoO4.2H2O 0.125
CuSO4.5H2O 0.125
CoC12.6H2O 0.125
FeSO4.7H2O 27.86
Na2EDTA.2H20 37.36
Maltose 25,000.
Glucose 10,000
Myo-Inositol 100-1000
Casamino acids 500
L-Glutamine 1000
Thiamine.HC1 1.00
Pyridoxine.HC1 0.50
Nicotinic acid 0.50
Glycine 2.00
Proline 100
L-Arginine 50
L-As ara ine 100
L-Alanine 20
L-Serine 20
PEG 100000
Charcoal 1000
Gelrite+ 2500
ABA (2 mg/mL) 25.0 mg/L
pH adjusted to 5.7
-20-
CA 02604700 2007-09-28
Results:
The cultures plated at different densities within the plating frame were
examined after 12
weeks in culture for total biomass, embryo suspensor mass (ESM), and embryo
structure
formation. Select embryos refers to the presence of at least 4 cotyledons and
no large scale
deformities. The results are shown below in TABLE 6.
TABLE 6
Number of
Plating Final Wet Total Dry Dry ESM Dry Embryo Select
Density Biomass Biomass (mg) (mg) (mg) Embryos
Low 17.0 g total 1277.6 mg 235 mg total 291.8 mg 720 total
Density total total
(half frame) (5.67 g/ml (425.87 mg/ml (78.33 mg/ml (240 per ml
3 ml SCV SCV plated) SCV plated) SCV plated) (97.27 mg/ml SCV plated)
plated SCV plated)
High 14.0 g total 1302.4 mg 138.7 mg 312.0 mg 771 total
Density (1.17 g/ml total total total (64.25 per
(half frame) SCV plated) ml SCV
12 ml SCV (108.53 mg/ml (11.56 mg/ml (26.0 mg/ml plated)
plated SCV plated) SCV plated) SCV plated)
As shown above in TABLE 6, when the embryos were plated at a lower density
(e.g.,
3 ml SCV/14 square inches (approx 0.3 g/14 square inches = 0.02 g/square
inch)), the
subsequent proliferation of the embryo suspensor masses yielded as much total
biomass as the
cells plated at a higher density of 12 mls SCV/14 square inches (approx. 1.2
g/14 square inches
= 0.08 g/square inch). As further shown in TABLE 6, the total amount of
embryos produced on
a per area basis was nearly equal between the low density and high density.
This was made
possible by the greatly enhanced embryo suspensor mass (ESM) growth as shown
by the
difference in dry weight, e.g., 235 mg dry ESM from the low density, versus
only 138.7 mg dry
ESM from the high density plated frame. Therefore, the select embryo formation
(the end
product of interest) per ml of SCV plated was clearly better in the low
density plating
(240 embryos/ml SCV) as compared to the high density plating (64.25 embryos/ml
SCV).
-21-
CA 02604700 2010-08-03
EXAMPLE 3
This Example describes a comparison between embryo yields using a standard
pipette
drop plating method and the liquid dispersion confluent spread plating method
according to an
embodiment of the present invention using four different genotypes of Loblolly
Pine.
Methods: An experiment was carried out to directly compare methods of plating
cells
onto plating frames (10) using the same amount of biomass of four different
genotypes of
Loblolly Pine, A, B, E, and F, while varying the plating density of the plated
SCV. The yield of
embryos and germination outcomes were measured.
Embryonic somatic (ESM) cells from Genotypes A, B, E and F were grown in
proliferation medium (made as described in Example 2) in 1 liter Erlenmeyer
flasks. The ESM
cells were allowed to settle and the settled cell volume (SCV) was measured as
described in
Example 2.
For each genotype, as a control representing the standard drop plating method,
6 ml of
SCV was directly plated as 12 drops (0.5 ml SCV each) onto an entire plating
frame (7" x 4"
total area = 28 square inches) placed on semi-solid development media in a
shallow plating box.
In this Example, a cambro box (CambroTM Manufacturing Co., Huntington Beach,
CA) that
holds two plating frames (10) was utilized. To test the spread plating method,
a second aliquot
of 6 ml SCV from each genotype was rinsed with 3X (18 ml) of development
media. The 24 ml
of SCV plus rinse media was then plated onto an entire plating frame (28
square inches) disposed
over a first semi-solid development media in a cambro box, such that the ESM
cells floated in
the media and dispersed uniformly over the submerged surface of the plating
frame. The
uniform dispersal was aided by gentle agitation of the plating frame. The
plating frames were
then lifted vertically off the first semi-solid media in the cambro box using
the attached handles
while maintaining the plating surface in a horizontal orientation, thereby
trapping the uniformly
dispersed ESM cells while allowing the media to flow through the porous mesh.
The plating frames containing plated cells were then moved to a fresh cambro
box
containing semi-solid development media (described in Example 2) and allowed
to develop for
12 weeks. At the end of 12 weeks, the embryos were put through late
development treatments to
22
CA 02604700 2007-09-28
induce germination, counted, and a sample was germinated. A normal germinant
was scored as
having the presence of a 1 mm white root, the presence of approximately 5
epicotyl leaves
approximately 5 mm long, no large scale hypocotyl ruptures, and a hypocotyl
not having a bend
greater than 90 degrees.
The total yield of developed embryos was counted after the 12 week development
period. The results are shown below in TABLE 7. Photographs were taken at the
end of the
development period, as shown in FIGURE 2. FIGURES 2A-D show the controls for
genotypes A, E, F, and B, respectively. FIGURES 2E-H show the results of the
liquid
dispersion confluent spread plating method for genotypes A, E, F, and B,
respectively.
At the end of 12 weeks, the embryos were stratified using BM5 stratification
media
(described in Example 1) for 4 weeks. After 4 weeks of stratification, the
embryos were spray
separated and conditioned over water for 10 days.
For each genotype, 25 embryos were selected for germination assessment. The
embryos
were selected for germination based on the presence of at least 4 cotyledons
and the absence of
gross deformities or split hypocotyls.
Results:
TABLE 7
Genotype Drop Method Spread Method Fold Increase in
Embryo Yield per Embryo Yield per Embryo Yield using
box box Spread Method
(Germination %) (Germination
A 2241 (52%) 5416 (49%) 2.4
E 2267 (15%) 3187 (27%) 1.4
F 1803 (8%) 2545 (3%) 1.4
B 2356(10%) 3188(19%) 1.3
As shown above in TABLE 7, all four genotypes tested showed at least a 1.3
fold or
greater increase in embryo yield using the low density confluent spreading
method as compared
to the traditional pipette drop plating method. The results of the germination
studies showed no
-23-
CA 02604700 2007-09-28
statistically significant difference between successful germination of samples
plated using the
drop method versus the spreading method.
When the results of the four genotypes shown in TABLE 7 are combined, the
overall
mean control value of embryos produced is 2166.7 using the drop method versus
the overall
mean value of 3692 embryos produced using the liquid dispersion confluent
spreading method,
which is a 1.7 fold higher embryo yield, with a p value of 0.0708. This is a
very significant
improvement in embryo yields which allows a genotype to be plated in a single
cambro unit to
achieve the mean yield target for germination yields, and decreases the number
of units required
to handle a single clone down to only one in contrast to the at least 30
separate units (petri
plates) required using the traditional pipette drop plating method.
The combined results recorded across the four genotypes tested are provided
below in
TABLE 8.
TABLE 8
Parameter Control Drop Plating Spread Plating p-value
(6 ml SCV in.5 ml (6 ml SCV diluted
drops x 12) in 18 ml)
Total embryo yield per box 2167 3692 0.0708
Mean embryo yield per ml 180.6 307.6
SCV
Category 1 Germination 17.2 20.9 0.4261
Germinants per ml SCV 40 88.9 0.1768
Mean root length (mm) 32.1 23.7 0.1481
The data shown above in TABLE 8 shows a statistically significant (0.10p value
cutoff)
70% increase in embryo yield with no apparent loss in germination percent,
although there is
some indication of difference in root length. While not wishing to be bound by
theory, the
difference in root size observed may be due to nutritional issues associated
with the greater
amount of growth. The nutritional issues can be addressed by either moving the
plated embryos
-24-
CA 02604700 2007-09-28
to a fresh growth substrate after a period of time, or by adjusting media
composition to a greater
concentration, and/or modifying the plating density for particular cell lines
based upon their
growth rate.
As shown in TABLE 8, the spread plating method of the invention resulted in
120%
yield of germinants as compared to the drop plating method, suggesting a very
large
improvement in culture productivity as measured by both embryo generation and
germination,
on a per box unit and surface area basis. This observed improvement in embryo
yield allows for
scale up with no limitation upon the size of the plated surface, in contrast
to the previous drop
method grown in petri dishes. Such scale up increases the amount of embryos
formed per plated
unit and decreases per unit costs by reducing the amount of manipulation
required.
Although this Example describes the use of Cambro boxes as plating containers,
the
plating containers for the development step following plating can be any
suitable plating surface,
such as any box type containers, such as commercially available food
preparation containers that
are heat stable and have a lid that can be used.
The liquid dispersion confluent spreading method has also been successfully
carried out
using a plating frame disposed over a variety of non-porous sterile surfaces
besides semi-solid
media, such as for example, a sterile plastic lid, or a silicon sheet or a
rubber mat placed into a
vessel capable of retaining liquid, followed by contacting the plated embryos
with development
medium.
The liquid dispersion confluent spreading method has also been successfully
carried out
by first plating an amount of sterile dilution media sufficient to submerge a
plating surface
comprising a porous material disposed over a solid (non-porous) substrate. The
desired volume
of SCV is then added to the dilution media and gently mixed with a pipette.
The floating cells
are then dispersed with the optional aid of a pipette and/or gentle agitation
of the first plating
surface. The plating frame is then picked up and placed over a second growth
substrate
comprising development media and incubated as described supra.
-25-
CA 02604700 2007-09-28
Finally, the confluent spreading method has also been successfully used to
generate
embryos from various Loblolly Pine genotypes using aliquots of from 1 ml up to
12 ml SCV
plated onto an entire plating frame (7" x 4") placed on semi-solid development
media.
While the preferred embodiment of the invention has been illustrated and
described, it
will be appreciated that various changes can be made therein without departing
from the spirit
and scope of the invention.
-26-