Note: Descriptions are shown in the official language in which they were submitted.
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
METHOD FOR ADDING BORON TO METAL ALLOYS
BACKGROUND OF THE INVENTION
Field of the Invention:
[0001] This invention relates to a process to manufacture boron containing
precious metal
alloys and master alloys. More particularly a solid compound that is either a
boron
containing metal hydride, and preferably a solid tetrahydroborate, or a boron
containing
metal fluoride is dispersed throughout a molten precious metal alloy or master
alloy.
Description of the Related Art:
[0002] Precious metal jewelry alloys are frequently worked into complex
ornamental
shapes. To withstand extensive working without fracture, the precious metal
alloys
require high ductility and high strength. High ductility and high strength are
facilitated by
an alloy having a low oxygen content and a fine grain structure.
[0003] Boron is known to both deoxidize and refine the grain of precious metal
alloys.
When boron scavenges oxygen from a melt and other oxides in the melt, it
cleanses
surfaces of the metal. U.S. Patent No. 5,384,089 to Diamond discloses the use
of boron as
a deoxidizer for gold-base alloys. This patent discloses that boron causes
hard spots. U.S.
Patent No. 6,168,071 to Johns discloses a diffusion bondable silver-copper-
germanium
alloy that may contain up to 20 parts per million of boron as a grain refiner.
The boron is
disclosed as added as a component of a copper-2%, by weight, boron master
alloy.
Throughout this patent application, all percentages are weight percent, unless
otherwise
specified.
[0004] A conventional method of introducing boron into a precious metal alloy
or master
alloy is through the use of the 98% copper-2% boron master alloy. However, the
use of
such a master alloy frequently introduces hard spots into the products. These
hard spots
are believed to be non-equilibrium phase CuB22 particles that form in copper
saturated
with boron when cooled from the liquid phase to the solid phase. Hard spots
can also
form with other metal-boride compounds such as iron borides (for example Fe5B2
and
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
FeB2). The hard spots are frequently not detected until after the precious
metal jewelry
alloy is polished and inspected resulting in needless expense for the
processing of
ultimately unsatisfactory product.
[0005] Copper - 2% boron master alloys are frequently contaminated with
silicon. The
silicon contamination may lead to brittleness, as a result of the formation of
brittle
intermetallic compounds, oxides and low melting eutectics. A low silicon
content is
required for fine, white gold and fine silver.
1o [0006] A further disadvantage with the use of a copper - 2% boron master
alloy is that the
high mass percent of copper may not be desired for the alloy product. Excess
copper may
cause a silver-base alloy to be subject to tarnish and / or firestain.
[0007] There remains, therefore, a need for a more effective way to introduce
boron as a
grain refiner and oxygen/oxide scavenger into a precious metal melt.
BRIEF SUMMARY OF THE INVENTION
[0008] In accordance with the invention, there is provided a method to produce
a precious
metal alloy or master alloy. This method includes the steps of (a) forming a
molten
precursor alloy of the precious metal alloy or master alloy, (b) disbursing a
boron
containing compound throughout the molten precursor alloy, and (c) solidifying
the boron
containing precursor alloy.
[0009] It is feature of the invention that the boron containing compound is
either a boron
containing metal hydride or a boron containing metal fluoride. When a boron
containing
metal hydride, the metal may be sodium, lithium, potassium, calcium, zinc and
mixtures
thereof. When a boron containing metal fluoride, the metal is sodium. Most
preferred as a
boron containing compound is sodium boro-hydride.
-2-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
[0010] It is another feature of the invention that more than 20 ppm of boron
can be
incorporated into a silver-base, or other precious metal, alloy without the
development of
hard spots.
[0011] The details of one or more embodiments of the invention are set forth
in the
accompanying drawings and the description below. Other features, objects and
advantages of the invention will be apparent from the description and
drawings, and from
the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
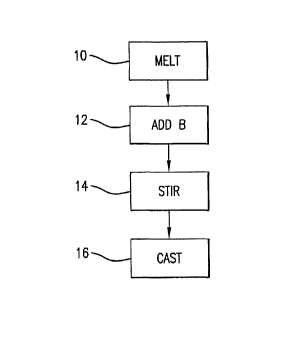
[0012] Figure 1 illustrates in flow chart representation an initial processing
sequence for
the alloys of the invention.
[0013] Figure 2 illustrates in flow chart representation subsequent processing
of the alloys
of the invention in accordance with a first embodiment of the invention.
[0014] Figure 3 illustrates in flow chart representation subsequent processing
of the alloys
of the invention in accordance with a second embodiment of the invention.
[0015] Figure 4 graphically illustrates the rate of boron loss in a batch
process of the
invention.
[0016] Like reference numbers and designations in the various drawings
indicate like
elements.
DETAILED DESCRIPTION
[0017] The following definitions are used throughout this patent application:
[0018] Master Alloy - Constituents of a precious metal alloy omitting the
predominant
precious metal. For example, a yellow 10, 14 or 18 karat alloy may contain
both silver
and gold, only the gold would be omitted in the master alloy. Silver would be
present.
For a sterling silver alloy, the silver would be omitted and there would be no
precious
-3-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
metal constituent present. Master alloys are usually sent to an end user who
adds the
required amount of precious metal.
[0019] Precious Metal Alloy - An alloy having a desired composition for
jewelry
applications. The alloy includes required amounts of gold, silver, palladium
and/or
platinum.
[0020] Precursor Alloy - A composition slightly off specification for a
desired master
alloy or precious metal alloy. The addition of a metal foil containing boron
compound
places the composition on specification. If the boron compound is not wrapped
in metal
foil, for example wrapped in paper or not wrapped, the precursor alloy
composition is on
specification for the desired master alloy or precious metal alloy.
[0021] The process of the invention is useful to add boron to precious metal
alloys and to
master alloys with a minimal formation of hard spots. Exemplary of the
precious metal
alloys are sterling silver alloys and silver alloys containing in excess of
75% silver with
the balance being alloying elements, including, but not limited to, copper and
zinc, and
inevitable impurities. Silver alloys and sterling silver alloys having between
80% and
97% silver are most benefited by the process of the invention.
[0022] The process is also useful for gold jewelry alloys having at least 33%,
by weight,
of gold (8 karat) with the balance being alloying elements including, but not
limited to
silver, nickel, copper and zinc as well as inevitable impurities. Most
benefited by the
process of the invention are those gold alloys having between 37.5% and 77%
gold.
[0023] Figure 1 illustrates in flow chart representation an initial processing
sequence of
the alloys of the invention. A precursor melt of the precious metal alloy or
master alloy is
formed by melting 10 appropriate amounts of the precious metal and alloying
elements in
a suitable crucible. As described below, a boron containing compound may be
wrapped in
a metallic foil formed from either the precious metal or one of the alloying
elements and
added to the precursor melt. Accordingly, the additional metal content of the
foil is taken
into consideration and the composition of the precursor melt is typically
slightly different
than the composition of the desired end product.
-4-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
[0024] The alloy is melted 10 in a suitable crucible. For silver alloys, one
suitable
crucible is formed from clay-graphite and for gold alloys one suitable
crucible is ceramic.
Other suitable crucibles for silver-base and gold-base alloys include clay-
graphite, fused
silica, silicon carbide, graphite and zirconia. The metals are heated to a
temperature
effective to fully liquify and flow the mixture, typically in the range of
from 1066 C
(1950 F) to 1260 C (2300 F), with a nominal temperature on the order of 1177 C
(2150 F). The melting temperature influences the kinetics of boron
evaporation which
determines the fmal boron concentration in the cast precious metal alloy or
master alloy.
The selected temperature should be sufficiently above the liquidous
temperature of the
alloy to prevent freezing in a die during continuous casting or freezing in a
grain box
during grain making. While the alloys are readily cast at atmospheric
pressures, higher or
lower pressures should not affect the benefits of the invention, but will
affect the kinetics
of boron evaporation.
[0025] To reduce the formation of an oxide slag, the molten precursor alloy
should be
covered to isolate the metal surface from oxygen. Suitable gas covers include,
but are not
limited to, a carbon monoxide flame, forming gas flame, argon, nitrogen,
hydrogen flame
and natural gas flame. Suitable powdered solid covers include, but are not
limited to,
borax, boric acid, graphite and charcoal.
[0026] Once the precursor melt is at the desired molten temperature, a boron
containing
compound is added 12 to the precursor melt. Boron is incorporated into
precious metal
alloys as an oxygen scavenger and, for silver alloys, additionally or
alternatively as a grain
refiner. It may be added to molten silver by bubbling a gaseous borane, e.g.
diborane into
the alloy in admixture with a non-reactive gas such as argon, by introducing
into the alloy
a borane which is solid at ambient temperature, e.g. decaborane B10H14
(melting point =
100 C, boiling point = 213 C), or by adding an alkylated borane, e.g.
triethylborane or tri-
n-butyl borane, although the latter reagents are spontaneously combustible and
require
care in handling.
[0027] When added to the precious metal in the gas phase, the boron compound
is
advantageously an admixture with a carrier gas that assists in creating a
stirring action in
the molten alloy and dispersing the boron content of the gas mixture into the
alloy.
Suitable carrier gases include, hydrogen, nitrogen and argon. The gaseous
boron
-5-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
compound and the carrier gas may be introduced into a vessel containing molten
silver or
silver-base alloy by using a metallurgical lance that may be an elongated
tubular body of
refractory material, e.g. graphite, or it may be a metal tube clad in
refractory material. The
lance is preferably of sufficient length to permit injection of the gaseous
boron compound
and carrier gas deep within the molten alloy.
[0028] Alternatively, the boron-containing gas may be introduced into the
molten alloy
from a side or bottom of the alloy-containing vessel through a gas transport
member,
such as a gas permeable bubbling plug or a submerged injection nozzle.
Rautomead
International of Dundee, Scotland, manufactures horizontal continuous casting
machines
in the RMK series for the continuous casting of semi-finished silver-base and
gold-base
products. The alloy to be heated is placed in a solid graphite crucible,
protected by an
inert gas atmosphere which may, for example, be oxygen-free nitrogen
containing <5
ppm oxygen and <2 ppm moisture and is heated by electrical resistance heating
using
graphite blocks. Such furnaces have a built-in facility for bubbling inert gas
through the
melt. Addition of small quantities of thermally decomposable boron-containing
gas to
the inert gas being bubbled through the melt readily provides a desired few
ppm or few
tens of ppm boron content. The introduction of the boron compound into the
alloy as a
dilute gas stream over a period of time, the carrier gas of the gas stream
serving to stir the
molten metal or alloy, rather than in one or more relatively large quantities
is believed to
be favorable from the standpoint of avoiding development in the metal or alloy
of boron
hard spots.
[0029] Compounds which may be introduced into molten silver or gold or alloys
thereof
as a gas include boron trifluoride, diborane or trimethylboron which are
available in
pressurized cylinders diluted with hydrogen, argon, nitrogen or helium,
diborane being
preferred because apart from the boron, the only other element is introduced
into the alloy
is hydrogen. A yet further possibility is to bubble carrier gas through the
molten silver to
effect stirring thereof and to add a solid boron compound e.g. NaBH4 or NaBF4
into the
fluidized gas stream as a finely divided powder which forms an aerosol.
[0030] The boron compound may also be introduced into the molten silver or
gold alloy
in the liquid phase, either as such or in an inert organic solvent. Compounds
which may
-6-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
be introduced in this way include alkylboranes or alkoxy-alkyl boranes such as
triethylborane, tripropylborane, tri-n-butylborane and methoxydiethylborane
which for
safe handling may be dissolved in hexane or tetrahydrofuran (THF). The liquid
boron
compound may be filled and sealed into containers of silver-base or of copper-
base foil
resembling a capsule or sachet using known liquid/capsule or liquid/sachet
filling
machinery and using a protective atmosphere to give filled capsules sachets or
other small
containers typically of capacity 0.5-5 ml, more typically about 1-1.5 ml. As
an alternative,
especially for gold casting, the capsules or sachets may be of a polymer film,
e.g.
polyethylene or polypropylene. The filled capsules or sachets in appropriate
number may
then be plunged individually or as one or more groups into the molten silver
or gold or
alloy thereof. A yet further possibility is to atomize the liquid boron-
containing
compound into a stream of carrier gas which is used to stir the molten silver
as described
above. The droplets may take the form of an aerosol in the carrier gas stream,
or they may
become vaporized therein.
[0031] Preferably, the boron is added as a metal borohydride, e.g. a
borohydride of an
alkali metal, a pseudo-alkali metal or an alkaline earth metal, e.g. lithium
borohydride.
Sodium borohydride is especially preferred because it is widely commercially
available
and can be obtained in the form of relatively large pellets that are
convenient to handle
during precious metal melting operations.
[0032] The boron is advantageously solid, e.g. a metal borohydride or a higher
borane,
such as decaborane, and is in the form of pellets or granules which are
advantageously
wrapped in a layer of foil of precious metal and plunged as a group into the
molten metal.
Boron can be added to the other molten components both on first melting and at
intervals
during casting to make up for boron loss if the alloy is held in the molten
state for a period
of time, such as in a continuous casting process for grain. Addition of boron
to a molten
copper-base master alloy is not recommended because adding boron changes the
copper
content and hence the overall proportions of the various constituents in the
alloy.
100331 The boron is added in the form of either a boron containing metal
hydride, and
preferably as a solid tetrahydroborate, or a boron containing metal fluoride.
When a boron
containing metal hydride, suitable metals include sodium, lithium, potassium,
calcium,
-7-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
zinc and mixtures thereof. When a boron containing metal fluoride, sodium is
the
preferred metal. Most preferred is sodium borohydride, NaBH4, also referred to
as sodium
tetrahydroborate. Sodium borohydride has a molecular weight of 37.85 and
contains
28.75% boron.
[0034] Sufficient boron is added so that an effective amount remains in the
cast precious
metal alloy or master alloy for effective grain refinement and deoxidation.
Between 1
ppm and 1600 ppm boron remaining is effective. Preferably, the boron content
is between
100 ppm and 1600 ppm for a master alloy and between 1 ppm and 1000 ppm for a
silver-
or gold-base precious metal alloy. A nominal boron content in the cast
precious metal
lo alloy or master alloy of about 250 ppm is most preferred. Typically, from
0.001% to
0.16% of boron added to the precursor alloy melt is effective.
[0035] Boron reacts to form a gas that evaporates at elevated temperatures and
it may be
necessary to make sequential additions of boron as described hereinbelow to
maintain an
adequate concentration for grain refining. To enable better mixing into the
precursor
alloy, the boron compound may be wrapped in a thin metallic foil. The foil may
be any
constituent of the master melt or an inert material, such as paper, and is
preferably a
ductile metal that may be formed into a relatively thin foil. Preferred metals
for the foil
include silver, copper and gold. The foil has a thickness of from about 0.01
millimeter to
about 0.3 millimeter to enable the foil wrapped boron compound to be well
submerged in
the master melt before the foil melts through releasing the boron compound.
Once
released, the constituents of the boron compound combine with oxygen in the
precursor
melt to effectively deoxidize the melt and the boron reacts with some of the
elements in
the melt to form discrete insoluble particles dispersed througliout the base
material which
act as nucleation sites promoting the formation of fine grains that are
uniform in size and
resist growth.
[0036] When sodium borohydride is first added to the master melt, the initial
reaction is
believed to be decomposition of the boron containing grain refiner.
(1) NaBH4(s)--,-Na(g)+B(S)+2H2(g)
When diborane is first added to the molten metal, the decomposition reaction
is believed
to be:
-8-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
(2) B2H6 --+ 3B(s) + 3H2(g)
The hydrogen is effective to deoxidize the melt.
[0037] After decomposition, the sodium, hydrogen and boron are all effective
to
deoxidize the melt as follows:
(3) Na(g)+0.5O2(g) --+Na2O(s)
(4) H2(g)+0.502(g) ->HZO(g)
(5) B(s)+0.502(g)+0.5H2(g) -+HBO(g)
[0038] To achieve a uniform casting, the boron is dispersed throughout the
precursor melt
by stirring 14. Preferably, the boron is stirred 14 for in excess of 1 minute
and typically
for from 1-5 minutes. Stirring may be by any means which does not contaminate
the
precursor melt such as with a graphite stirring rod.
[0039] The molten precious metal alloy or master alloy is then cast 16 by a
method
suitable for forming a desired end product.
[0040] One such useful end product is casting grains. Casting grains are
roughly
spherical particles which are sold to jewelry manufacturers who then
investment cast to
form a desired article of jewelry. Subsequent to stirring 14, molten precious
metal alloy is
poured into a grain box 18, Figure 2. A grain box is a container with openings
in the
bottom, through which the liquid metal flows to make the desired shape and
size of grains.
The grain box is made from materials similar to the crucible, such as, but not
limited to,
graphite, clay/graphite, ceramic and silicon carbide. The molten precious
metal alloy is
formed into discrete droplets in the grain box as it flows through the
openings and is then
solidified into roughly spherical particles in grain tank 20. A grain tank 20
contains water
into which the droplets fall and solidify.
[0041] The particles are then removed from the grain tank 20 and dried 22 by
centrifugal
force and hot air. The roughly spherical grains have a typical diameter of
from about 0.1
millimeter to about 5 mm.
-9-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
[0042] In accordance with a second embodiment of the invention, continuous
casting may
be used to form wrought mill products such as sheet, tubing and wire that is
later made
into finished products such as jewelry. The stirred boron containing molten
metal alloy is
transferred to a die 24, Figure 3, and partially solidified in the die such
that a cohesive
structure may be withdrawn from the die and subjected to secondary cooling to
26 such as
by impact with water spray or passing through a chilled coil. The continuous
cast
structure is then finished 28 such as by passing through rolling mills and
shears to achieve
a desired cross-sectional shape and surface finish and then coiled 30 for
shipment to
jewelry manufacturers. Continuous cast sheet has a more consistent boron
content than
achieved by prior art processes which enables more consistent welding into
tubing.
[0043] For a well stirred batch process, as illustrated in the combination of
Figures 1 and
2, the concentration of boron decreases over time in accordance with the
equation
(5) CB=CB,oexp(-kpt/m)
Where CB = the present boron content in ppm.
CB,o = the initial boron content in ppm.
k= a rate constant dependent on alloy composition, temperature, gas cover and
melt cover
expressed in units of inch3/minute.
p = the density of the alloy in troy ounces / inch3.
t = time in minutes.
m = melt weight in troy ounces.
[0044] Equation (5) predicts that the rate of boron evaporation is faster for
smaller melt
sizes and this was observed in practice. Figure 4 illustrates in graphical
representation the
kinetics of boron loss in the batch melting process in the exemplary condition
of a CO gas
cover and a graphite powder solid cover.
[0045] For a continuous casting process as illustrated in the combination of
Figures 1 and
3, the material balance must take into account the change in mass in the
casting crucible
with time. The amount of boron present may be calculated by the equation:
(6) Cs=CB,o(mo/(mo-Fot))exp-(kp/Fo)
-10-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
Where CB, CB,o, t, k and p were previously defined.
mo = the initial mass (in troy ounces) of alloy in a crucible at time = 0.
Fo = the casting rate in troy ounces / minute.
[0046] The time, t, is reset to zero and the initial alloy mass mo, is
recalculated after each
incremental addition of boron.
[0047] The above-described process may be used to make alloys of any precious
metal,
especially fine silver, fine gold and alloys thereof that are required to
incorporate boron.
In particular, it may be used to make silver/germanium alloys having an Ag
content of at
least 77%, by weight, a Ge content of between 0.5 and 3%, by weight, an amount
of boron
1o effective to enhance ductility and strength and the balance copper along
with incidental
ingredients and impurities. If desired, the germanium content may be
substituted, in part,
by one or more incidental ingredient elements selected from Al, Ba, Be, Cd,
Co, Cr, Er,
Ga, In, Mg, Mn, Ni, Pb, Pd, Pt, Si, Sn, Ti, V, Y, Yb and Zr, provided that the
effect of
germanium in providing firestain and tarnish resistance is not unduly
affected. The weight
ratio of germanium to incidental ingredient elements may range from 100:0 to
60:40, and
preferably ranges from 100:0 to 80:0. The term "incidental ingredient" permits
the
ingredient to have ancillary fiuictionality within the alloy, e.g. to improve
color or as-
molded appearance and includes the metals or metalloids Si, Zn, Sn and In in
amounts
appropriate for deoxidation. The invention is also applicable for the
manufacture of mater
2o alloys, such as Cu/Ge/B and CuB.
[0048] The alloys that may be made according to the process of the invention
include
coinage grade, 800-grade (80% by weight silver) (including 830 and 850 grades
(83% and
85%, by weight, silver, respectively) and the like) and standard Sterling
silver and an alloy
of silver containing an amount of germanium effective to reduce firestain and
/ or
tarnishing. The ternary Ag-Cu-Ge alloys and quaternary Ag-Cu-Zn-Ge alloys that
can
suitably be made are those having a minimum silver content of 80%, by weight,
and more
preferably, a minimum silver content, by weight, of 92.5%. The maximum silver
content
is 98%, by weight, and preferably, the maximum silver content is 97%, by
weight. The
germanium content is at least 0.1%, and preferably at least 0.5%, more
preferably at least
1.1%, and most preferably at least 1.5%, by weight. The maximum germanium
content is
preferably 6.5% and more preferably 4.0%, by weight.
-11-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
[0049] Silicon may be added to silver alloys in an amount of up to 5%, by
weight,
preferably from 0.5 to 3 weight percent and most preferably in an amount of
from 0.1 to
0.2 weight percent. When incorporated into casting grain of an Ag-Cu-Ge
ternary alloy, it
can provide bright investment casting immediately on removal from the mold. It
may be
added to casting grain, e.g. before investment casting, or it may be
incorporated into the
silver at the time of first melting to form an alloy.
[0050] The process of the invention may be used to manufacture gold jewelry
alloys
having at least 33%, by weiglit, of gold (8 karat) with the balance being
alloying elements
including, but not limited to silver, nickel, copper and zinc, as well as
inevitable
1o impurities. Particular gold alloys that may be processed in accord with the
invention
include:
[0051] 24K gold - a minimum of 99.7%, by weight, of gold with the balance
being grain
refiners, hardening additives and impurities.
[0052] 22K gold - exemplary is, by weight, 91.67% Au, 5% Ag, 2% Cu and 1.33%
Zn.
[0053] 18K gold - exeinplary are, by weight, 75% Au, 20% Ag and 5% Cu; 75% Au,
15% Ag and 10% Cu; 75% Au, 13% Ag and 12% Cu; 75% Au, 5 % Ag and 20% Cu; 75%
Au, 2.75% Ag and 22.25% Cu; 75% Au and 25% Cu; 80% Au and 20% Al; 75% Au, 25%
Pt, Pd or Ag; 75% Au, 10% Pd, 10% Ni and 5% Zn; 75%Au, 17% Fe and 8% Cu and
75%
Au, 23% Cu and 2% Cd.
[0054] 14K gold - exemplary are, by weight, 58.33% Au, 24,78% Cu and 0.14% Zn;
58.33% Au, 4.00% Ag, 31.24% Cu, 6.43% Zn, 0.10% Ni, 0.05% Fe; and 0.01% Si and
58.33% Au, 2.08% Ag and 39.59% Cu.
[0055] 10K gold - exemplary are, by weight, 41.70% Au, 11.66% Ag, 40.81% Cu,
5.83%
Zn, 0.03% Si and 0.02% B; 41.70% Au, 5.50% Ag, 43.80% Cu and 9.00% Zn; and
41.70% Ag, 2.82% Ag and 55.48% Cu.
[0056] The above-described invention is better understood by the examples
which follow:
-12-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
Examples
Example 1 (Prophetic) - Afz-Cu-Ge-Si Alloy
[0057] A silver alloy is made by melting together 93.2 weight percent fine
silver casting
grains, 1.3 weight percent germanium in the form of small broken pieces, 0.2
weight
percent silicon (added as a Cu/Si master alloy containing 10 weight percent
silicon) and
the balance being copper granules. Melting is by means of a gas-fired furnace
heated to a
pour temperature of about 1093 C (2000 F). The melt is covered with graphite
to protect
against atmospheric oxidation. In addition, a hydrogen gas protective flame is
provided.
Stirring is by hand using a graphite stirring rod.
[0058] When the alloy constituents liquefy, 20.2 grams (0.65 troy ounce) of
sodium
borohydride per 46.7 kilogram (1500 oz.) melt are wrapped in a pure silver
foil, about 0.15
mm thick. The foil wrapper holds the sodium borohydride to prevent it from
floating to
the surface of the melt. The wrapped sodium borohydride is placed into a
hollow cup-
shaped end of a graphite stirring rod and plunged beneath the surface of the
melt. The
melt is covered with a ceramic fiber blanket to quench a flame resulting from
decomposition of the borohydride. The hydrogen and sodium burns off with a
bright
yellow flame over a period of 1- 2 minutes during which time the melt is
continuously
stirred. When the evolution of hydrogen ceases, the boron is substantially
incorporated
into the melt together with at least some sodium.
[0059] After the boron is added, the crucible is pivoted to permit pouring the
molten alloy
into a tundish having a bottom formed with very fine holes. The molten silver
flows into
the tundish and through the holes in fine streams that break into fine pellets
and fall into a
stirred water bath becoming solidified and cooled. The cast pellets are then
removed from
the bath and dried.
[0060] The pellets are tested by investment casting using a calcium sulphate
bonded
investment. The resulting casting has a matte silvery finish when removed from
the mold,
a fine grain structure, and can be easily polished. It is free from boron hard
spots and was
ductile as exhibited by a capability to make a ring stretch 4- 6 sizes whereas
a similar
material made using copper/boron can only be stretched about two sizes.
-13-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
Example 2 (Working) - Manufacture of Sterling Silver Casting Grain
[0061] 6.11 kilograms (two hundred troy ounces) of a sterling silver precursor
melt were
melted in a clay-graphite crucible. The precursor melt had a nominal
composition, by
weight, of 93% silver, 5.7% copper and 1.3% germanium. The precursor melt
constituents
were mixed together and heated under a carbon monoxide flame and covered with
a 2.54
cm (one inch) thick layer of borax salt. When the precursor melt temperature
reached the
flow temperature, 0.0125% boron was added as NaBH4. The boron compound was
wrapped in 0.15 mm silver foil for introduction to the master melt. Sufficient
power was
provided to maintain the temperature of the molten precious metal alloy at the
flow
temperature. The molten precious metal alloy was then stirred with a graphite
stirring rod
for 3.7 minutes and poured into a grain box. The molten precious metal alloy
was
protected by a reducing atmosphere during pouring at the flow temperature.
After about
0.25 minutes, the entire molten precious metal alloy was converted into
casting grains.
[0062] The casting grains were assayed and found to have 13.8 ppm boron. The
grains
were mounted, polished and etched for examination of grain structure and hard
spots. The
resulting grain structure was fine and contained no boron hard spots. The
material was not
brittle when reduced 75% by thickness in a rolling mill. Investment cast rings
formed
from the casting grains contained no fire scale or hard spots. The rings were
stretched
2o 3.25 sizes without annealing before failure.
Example 3 (Working) - Manufacture of SterlingLSilver Grains by Batch Process
[0063] Table 1 illustrates that the process of the invention is effective to
add boron to a
sterling silver precursor alloy and that the melt cover appears to have more
of an effect on
the boron content in the precious metal alloy than does the pour gas. In no
instance were
hard spots detected on the cast grains.
-14-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
Table 1
Melt Melt Size Amount Pour Melt Pour Boron Time Boron
Number (troy NaBH4 Gas Cover Temp. Content Between Content
Kg oz) Added C ( F) in Boron in Cast
(grams) Precursor Addition Grains
Alloy and Pour (ppm)
(ppm) (minutes)
0281 6.22 200 2.78 N2/142 Borax 1177 2150 125 3.7 13.8
0322 46.7 1500 3.33 N2/112 Borax 1177 2150 20 4.0 9.5
0326 6.22 200 3.04 CO Borax 1177 2150 137 4.25 23.5
0327 6.22 200 3.04 CO Borax 1177 2150 137 6.5 6.1
0338/3 12.4 400 2.02 CO Borax 1177 2150 45.5 5.0 23.9
0353 46.7 1500 5.05 N2/HZ Borax 1177 2150 30.3 5.25 12.2
0339 2.24 72 8.09 CO Borax 1177 2150 1011 3.0 9.0
081006 9.33 300 3.03 CO Borax 1177 2150 91 4.0 11.4
B01 6.22 200 17.77 CO Graphite 1093 2000 800 5.0 0.6
B02 6.22 200 8.89 CO Graphite 1093 2000 400 5.0 0.6
B03 6.22 200 5.55 CO Graphite 1093 2000 250 5.0 1.3
BS4 6.22 200 35.53 CO Graphite 1204 2200 1600 6.4 2.4
BS05 6.22 200 8.89 CO Graphite 1204 2200 400 5.0 0.5
0605 31.1 1000 20.2 N2/H2 Borax 1177 2150 182 5 42.9
0610 31.1 1000 20.2 N2/H2 Borax 1177 2150 182 5 59.9
0611 31.1 1000 20.2 N2/H2 Borax 1177 2150 182 5 85.7
Example 4 (Working) - Manufacture of Sterling Silver Continuous Cast Products
[0064] 140 kilograms (4500 troy ounces) of a sterling silver precursor melt
were melted
in a clay-graphite crucible. The master melt had a nominal composition, by
weight, of
93% silver, 5.7% copper and 1.3% germanium. The precursor melt constituents
were
mixed together and heated under a natural gas flame and covered with a layer
of charcoal.
When the precursor melt temperature reached the flow temperature, 0.0020%
boron was
added as NaBH4. The boron compound was wrapped in 0.15 mm silver foil for
introduction to the precursor melt. Sufficient power was provided to maintain
the
temperature of the molten precious metal alloy at the flow temperature. The
NaBH4 was
added incrementally to maintain a good boron concentration by taking into
account the
change in melt weight and the evaporation of boron with time. A timer was used
to add
-15-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
the boron as scheduled below. Each boron addition was placed inside a graphite
plunger
and mixed into the molten precious metal alloy at the times indicated in Table
2.
Table 2
Time From Boron Added as NaBH4, Time From Boron Added as NaBH4,
Transfer (minutes) (dry weight grams) Transfer (minutes) (dry weight grams)
0 6.50 30 3.25
6 5.85 36 2.60
12 5.20 42 1.95
18 4.55 48 1.30
24 3.9 54 0.65
[0065] At transfer, to the continuous casting die, the molten precious metal
alloy was
heated to the transfer temperature and cast into a continuous two inch
diameter cylindrical
bar at the casting temperature under a natural gas flame and charcoal cover.
Samples of
the cast precious metal alloy were assayed for boron level. Table 3 summarizes
the assay
results.
Table 3
Time (min.) Boron Concentration (ppm)
8.5 2.9
17 1.8
25.48 2.5
34 1.0
42.5 1.4
Example 5 (Working) - Manufacture of 18 Karat White Gold Casting Grain
[0066] 3.89 kilograms (125 troy ounces) of a white gold precious metal alloy
were melted
in a ceramic crucible. The precious metal alloy melt had a nominal composition
of 75%
gold and the balance 6% nickel, 14% copper and 5% zinc. The precious metal
alloy
constituents were inelted together and heated under a carbon monoxide flame to
the flow
temperature at which time 0.08% boron as NaBH4 was added. The boron compound
was
wrapped in a paper envelope for introduction to the melt. Sufficient power was
provided
to maintain the temperature of the molten precious metal alloy at the flow
temperature.
The molten precious metal alloy was stirred for 1.75 minutes subsequent to the
boron
addition and then poured into a grain box. The gas cover during pour into the
grain box
-16-
CA 02605502 2007-10-18
WO 2006/113847 PCT/US2006/014835
was a reducing atmosphere. After about 0.25 minutes, the entire molten
precious metal
alloy was converted into casting grain. Analysis of the casting grain showed a
clean
surface, no hard spots and a fine grain size.
[0067] One or more embodiments of the present invention have been described.
Nevertheless, it will be understood that various modifications may be made
without
departing from the spirit and scope of the invention. Accordingly, other
embodiments are
within the scope of the following claims.
-17-