Note: Descriptions are shown in the official language in which they were submitted.
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 VERIFICATION METHOD & SYSTEM FOR MEDICAL TREATMENT
2
3 CROSS-REFERENCE TO RELATED APPLICATIONS
4 [0001] This application claims the benefit of priority to U.S. Provisional
Application Ser.
No. 60/682,969, filed May 19, 2005, U.S. Provisional Application Ser. No.
60/683,280 filed May
6 19, 2005, and U.S. Provisional Application Ser. No. 60/683,333, filed May
19, 2005.
7
8 BACKGROUND OF THE INVENTION
9 FIELD OF THE INVENTION
[0002] The present invention relates to the management of medical treatments.
More
11 specifically it relates to a permission-based fluid dispensing device.
12
13 DESCRIPTION OF THE PRIOR ART
14 [0003] Despite remarkable advances in health care technology and delivery,
a large number
of patients die or are disabled as a result of medical errors. These errors
occur in health care
16 settings, such as hospitals, clinics, nursing homes, urgent care centers,
physicians' offices,
17 pharmacies, and the care delivered in the home, and they usually result
from systems problems
18 rather than one single action or decision.
19 [0004] For many years, bar code labelling has been the technology of choice
in ensuring
patient safety. Recently, the Food and Drug Administration (FDA) issued a new
rule which
21 requires certain human drug and biological product labels to have bar
codes. As such, the bar
22 code for human drug products and biological products (other than blood,
blood components, and
23 devices regulated by the Center for Biologics Evaluation and Research) must
contain the
24 National Drug Code (NDC) number in a linear barcode. The rule is geared
toward reducing the
number of medication errors in hospitals and other health care settings by
allowing health care
26 professionals to use bar code scanning equipment to verify that the right
drug (in the right dose
27 and right route of administration) is being given to the right patient at
the right time. The rule
28 also requires the use of machine-readable information on blood and blood
component container
29 labels to help reduce medication errors.
-1-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 [0005] However, bar codes require line of sight with a reader in order to be
read and they
2 cannot store additional information apart from simple identification data,
such as a serial no. or
3 SKU. For example, a bar-coded wristband on a patient is not easy to read if
the patient gets it wet
4 or is sleeping on top of the arm bearing the wristband, or when the patient
is on an emergency
room gurney or operating table; these are instances where mistakes in
medication or blood
6 transfizsion are most prevalent.
7
8 [0006] It is an object of the present invention to mitigate or obviate at
least one of the above-
9 mentioned disadvantages.
11 SUMMARY OF THE INVENTION
12 In one of its aspects, the present invention provides a system for the
collection, treatment and
13 delivery of a blood sample, the system comprising:
14 an article for association with a patient having a patient identifier;
a first syringe having:
16 a first syringe inlet for drawing an untreated blood sample from the
patient,
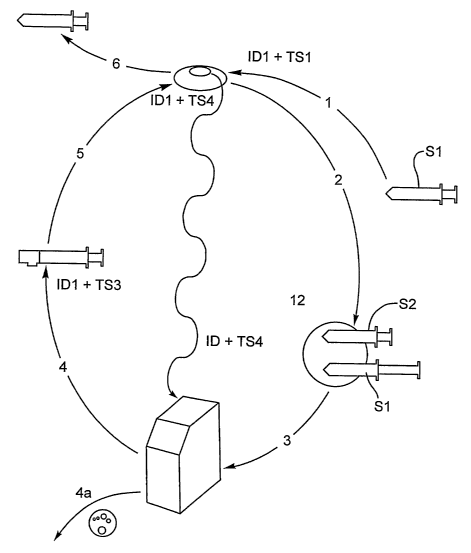
17 a first fluid chamber for receiving the untreated blood,
18 a first syringe outlet for dispensing the untreated blood sample from the
first
19 chamber,
a first incremental counter for recording temporal data corresponding to
untreated
21 blood events related to the collection of blood,
22 the first syringe being associated with a first unique identifier
correlatable to the
23 patient identifier;
24 a vessel for processing the blood sample, the vessel having:
a blood sample processing chamber, the vessel having a chamber inlet; the
first
26 syringe outlet being operable to establish a dedicated first fluid coupling
with the
27 chamber inlet to dispense the untreated blood sample to the blood sample
processing
28 chamber; the vessel having a chamber outlet for dispensing a treated blood
sample
29 following treatment to a second syringe,
the second syringe having:
-2-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 a second syringe inlet operable to form a dedicated second fluid coupling
with the
2 chamber outlet to receive the blood sample from the blood sample treatment
chamber;
3 a second chamber for receiving the treated blood;
4 a second syringe outlet;
a passage in communication with the second chamber and the second syringe
6 outlet;
7 a second incremental counter for recording temporal data corresponding to
blood
8 treatment events, treated blood events and delivery events; the second
incremental
9 counter being operable independently of the first incremental counter and
being non-
synchronized with the first incremental counter;
11 the second syringe being associated with a second unique identifier, the
second
12 unique identifier operatively associated with the first syringe and
correlatable to the first
13 unique identifier;
14 a releasable lock formed within the passage for operating the second
syringe
outlet between a plurality of states; -
16 a processor having:
17 a comparator for comparing the patient identifier to the first unique
identifier to
18 determine the correlation between same; and comparing the second unique
identifier to
19 the patient identifier to determine the correlation between same, the
comparator issuing
an output signal;
21 logic for receiving the output signal and the temporal data to determine
time
22 delays between the events and for determining whether the time delays are
within
23 predefined ranges;
24 a release signal generator coupled to the logic for issuing a release
signal to the releasable
lock;
26 whereby the release signal is issued upon confirmation of the correlation
between the patient
27 identifier and the first unique identifier, and the correlation between the
patient identifier and the
28 second unique identifier, and provided that the time delays are within
predetermined ranges.
29 [0007] In another of its aspects, the present invention provides
identification means for
identifying an originating patient of the untreated blood sample, verification
means for verifying
-3-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 a match between the originating patient and the treated blood sample, and
release signal
2 generating means for generating a release signal in response to a positive
verification by the
3 verification means. The identification means and/or the release signal
generating means may be
4 located on the second syringe body, or on an external article. The external
article may worn,
carried, attached or ingested by the patient, such as a pinned or self
adhesive label, or a coated
6 object, and the like. Preferably, the external article contains a removable
portion containing
7 audit data relating to the patient and/or the treated blood sample. For
example, the external
8 article may be conveniently provided as a wristband to be worn by the
originating patient.
9 [0008] In yet another of its aspects, the second syringe body may also
include a filtered vent
outlet in the passage for expelling one or more gas constituents in the
treated blood sample.
11 [0009]1! As a further aspect, the present invention provides a method of
monitoring a material
12 sample from a patient, comprising the steps of:
13 (a) collecting the sample from the patient with a first collection device;
14 (b) associating the patient with a first signal carrying data
representative of the sample;
(c) associating the first collection device with a second signal carrying data
representative
16 of the ,sample;
17 (d) delivering the sample to a sample treatment chamber;
18 (e) processing the sample to form a processed sample;
19 (f) collecting the sample in a second collection device;
(g) associating the second collection device with a third signal carrying data
21 representative of the processed sample;
22 (h) comparing the data in the first and third signals to link the processed
sample with the
23 patient; and thereafter;
24 (i) associating at least one of the steps (a) to (h) with temporal data;
(j) determining at least one time delay using the temporal data to determine
whether the
26 at least one of the steps (a) to (h) occurs within acceptable time limits;
27 (k) delivering the processed sample to the patient upon a positive outcome
from step (h)
28 and step (j); and
29 (1) assembling an audit record having temporal data collected from step
(i), the outcome
from step (h) and step (j), and data associated with the sample.
-4-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 [0010] The events related to the collection of untreated blood are tracked
by the first
2 increniental counter, while the treatment and post treatment events are
tracked by the second
3 increniental counter, such that time delays may be determined from the
temporal data
4 Advaritageously, these two counters operate independently of one another and
do not require to
be syrichronized with each other, unlike real-time clocks. The counters only
operate during the
6 steps (a) to (j) described above, and thus do not require substantial
battery power. As such, the
7 battery is sufficient to maintain substantial accuracy of the clock within
the time period from
8 steps (a) to (f), and thus the possibility of losing time or decreasing
clock accuracy as the
9 battery's power runs down is substantially eliminated.
[0011] In yet another of its aspects, the system includes a releasable lock
means operable by
11 a solerioid configured to receive the release signal.
12 [0012] In yet another of its aspects, the system includes a releasable lock
means operable by
13 a motorized means configured to receive the release signal.
14 [0013] The term "treatment device" used herein below is intended to mean a
device used
directly or indirectly in the course of a treatment. It may include devices
which actually perform
16 a treatment on the patient or a patient-derived sample, or alternatively be
an article for
17 perforrning functions associated with treatments, such as carrying or
otherwise transferring the
18 sample to or from a treatment.
19
BRIEF DESCRIPTION OF THE DRAWINGS
21 [0014] These and other features of the preferred embodiments of the
invention will become
22 more apparent in the following detailed description in which reference is
made to the appended
23 drawings wherein:
24 [0015] Figure 1 is a perspective view of a blood treatment system;
[0016] Figure 2 is a sectional view of a first syringe shown in Figure 1,
taken along line 1-1';
26 [0017] Figure 3 is a perspective view of the first syringe of Figure 1
coupled to a sodium
27 citrate lbag;
28 [0018] Figure 4 is a perspective view of a blood treatment chamber of
Figure 1;
29 [0019] Figure 5 is a perspective view of a second syringe of Figure 1;
[0020] Figure 6 is a sectional view of the second syringe of Figure 5 taken
along line 5-5';
-5-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 [0021] Figure 7 is another perspective view of the blood treatment chamber
carrying the first
2 syringe and the second syringe;
3 [0022] Figure 8 is a sectional view of the blood treatment chamber of Figure
7 taken along
.4 line 7=-7';
[0023] Figure 9 is a sectional view of the blood treatment chamber of Figure 7
taken along
6 line 9--9';
7 [0024] Figure 10 is an exploded view of an outlet port of the second syringe
of Figure 5;
8 [0025] Figure 11 is a perspective view of a outlet valve;
9 [0026] Figure 12 is a sectional view of the outlet valve element of Figure
10 taken along line
11-11";
11 [0027] Figure 13(a) is a perspective view of the a portion of locking
mechanism in a locked
12 state;
13 [0028] Figure 13(b) is a perspective view of the a portion of locking
mechanism in an open
14 state;
[0029] Figure 13(c) is a perspective view of the portion of locking mechanism
in a
16 permanently locked state;
17 [0030] Figure 13(d) is a perspective view of the portion of locking
mechanism adjacent to
18 the outlet port of Figure 10, in a permanently locked state;
19 [0031] Figure 14 is a perspective view of the portion of locking mechanism
in a cooperating
arrangement with the outlet port;
21 [0032] Figure 15 is a flowchart outlining a verification protocol of the
system of Figure 1;
22 [0033] Figure 16 is a flowchart outlining a verification portion protocol
of Figure 15;
23 [0034] Figure 17 is a detailed perspective view of the blood treatment
system;
24 [0035] Figure 18 is a schematic view of a verification protocol;
[0036] Figure 19 is a perspective view of a wristband as shown in Figure 1,
prior to
26 operation;
27 [0037] Figure 20 is a perspective view of a wristband as shown in Figure 1,
in operation;
28 and;
29 [0038] Figure 21 is a perspective view of a wristband as shown in Figure 1,
prior to
operation.
-6-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1
2 DESC;RIPTION OF THE PREFERRED EMBODIMENTS
3 [0039] As shown Figure 1, there is provided a system 10 for the collection,
treatment and
4 delivery of an autologous blood sample. The system 10 includes a plurality
of entities which are
used at different stages during the handling of the blood sample, such as, a
first syringe 11 (Sl),
6 a sample management unit 12 (SMU), a blood treatment unit 14 (BTU), a second
syringe 15
7 (S2), and a wristband 16 (WB). The first syringe 11 is used to collect an
untreated blood sample
8 from an originating patient 17. Following collection of the untreated blood
sample, the blood
9 collection syringe 11 is coupled to the sample management unit with the
blood delivery syringe
15 already mounted thereon, and the sample management unit is introduced into
the blood
11 treatment unit, in which the untreated blood sample is subjected to one or
more stressors, such as
12 ozone or ozone/gas mixture, ultra-violet (UV) light and infra-red (IR)
energy.
13
14 [0040] Following treatment, the treated blood sample is delivered to a
second syringe 15,
from which the treated blood sample is administered to the originating patient
17. At one or
16 more critical stages, the system 10 provides for a verification check,
aimed at reducing the
17 possibility of error, and thus ensure that the correct blood sample is
returned to the correct
18 originating patient 17. The verification check includes the steps of
matching the blood sample,
19 either in its treated or untreated form or both, with the originating
patient 17. Typically, the
wristband 16, the first syringe 11, the sample management unit 12, the second
syringe 15,
21 include identification data associated with the originating patient, the
data may include indicia,
22 or may be machine-readable via optical or electro/magnetic means.
23 [0041] As shown in Figure 2, the first syringe 11 has a first body portion
18 which provides a
24 cylindi-ical cavity 19 which in cooperation with a syringe plunger 20 forms
a sample receiving
chamber 21. The first syringe 11 includes a first channel portion 22 with a
channel 23 in
26 communication with the first sample receiving chamber 21, and a first
syringe inlet port 24 for
27 ingress of the untreated blood sample from the patient 17. The first
channel portion 22 also
28 includes a first syringe outlet port 26 for dispensing the untreated blood
sample therefrom to the
29 sample management unit 12. The first syringe outlet port 26 includes a
channel 27 in
commiulication with the first sample receiving chamber 21 and channe124.
-7-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 100421 The first syringe inlet port 24 is provided with a first syringe
inlet valve means 28 in
2 channel 24 for controlling the flow of blood through the first syringe inlet
24. In this case, the
3 first irilet valve means 28 includes a housing 29 containing a valve 30
arranged to be opened by a
4 complementary valve member 31, located on an external device 32, as shown in
Figure 3. The
external device 32 may be a blood collection unit, such as a "butterfly"
needle or a sodium citrate
6 bag, and so forth. Extending outwardly from the first syringe outlet port 26
is a pair of bayonet
7 pins 72 for coupling the first syringe 11 to the blood treatment chamber 12.
Included within the
8 channel 27 of the first syringe 11 is a valve element 74 biased to a closed
position against a valve
9 seat 76 on an end cap 78 which forms the outer end of the first syringe
outlet port 26.
[00431 Within the first channel portion 22, is a printed circuit board (PCB)
34 having
11 circuitry for transmitting and receiving data related to the syringe and/or
its contents, or a patient
12 17, such as identification data, SKU, serial no., manufacturing date,
expiry date, fluid data,
13 health facility data, health practitioner data, medication data, and so
forth. The circuitry includes,
14 but is not limited to, a transmitter, a receiver, logic means or processor,
a computer readable
memory for data storage, a timing circuit, an antenna and a power source. In
the preferred
16 embodiment, the circuitry also includes an RFID reader/writer for reading
RFID tags associated
17 with other entities within the treatment system. The RFID reader/writer is
also coupled to other
18 elemerits of the circuitry to perform at least one verification check, and
other functions. Also
19 coupled to the PCB 34 are input/output devices such as a display, an LED
36, a speaker, and a
switch, such as pullout tab 38. The first channel portion 22 also includes a
cover 40 with a bore
21 42 contiguous with an opening 44 of the first outlet port 26. The first
syringe 11 also includes a
22 compartment 46 for housing a power supply unit 48 to provide electrical
power to the PCB 34
23 and the input/output devices. The power supply unit 48 typically comprises
one or more batteries
24 which may be removed following the single use of the first syringe 11, in
order to enable use in
anotheir device or allow for proper recycling in compliance with current
environmental
26 regulations. In order to facilitate easy battery installation or removal,
the batteries 48 may be
27 placed on a tray which is slidably received by the compartment 46.
28 [0044] As shown in Figure 4, the sample management unit 12 is a vessel 49
having an open
29 top portion 51, a closed bottom portion 56 and a rigid walled portion 58
therebetween, and a
cover portion 54 to define a cylindrical treatment cavity 52, or treatment
chamber. The cover
-8-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 portion 54 has a chamber inlet 50 to form a dedicated first fluid coupling
with the first syringe
2 outlet port 26, such that the untreated blood sample may be dispensed into
the treatment cavity
3 52 of the blood sample treatment chamber 12. The cover portion 54 also has a
gas inlet port 60
4 for delivery of ozone to treat a blood sample, a gas outlet port 62 for the
discharge of the ozone,
and other gases. The bottom portion 56 has a bowl 66 to receive the blood
sample during
6 treatment.
7 [0045] In the course of the treatment of the blood sample, the treatment
cavity 52 is
8 subjected to stressors, such as, UV A, B and C radiation, infrared radiation
and ozone is bubbled
9 through the blood sample. As such, the walled portion 58 and the bowl 66 are
made from
appropriate materials capable of transmitting such radiation, such as low
density polyethylene
11 (LDPE) containing a small amount (about 5%) of ethylene vinyl acetate.
12 [0046] The chamber inlet 50 has a female collar portion 68 with a pair of
helically oriented
13 passages or grooves 70 extending through its wall, or in its wall, to
receive the pair of
14 corresponding bayonet pins 72 of the first syringe outlet port 26. In
operation, the first syringe 11
is rotated to urge the bayonet pins 72 along the helical passages 70 and
downwardly into the
16 female collar portion 68 until the valve element 74 abuts the valve-
actuating element 80.
17 Subsequently, the valve element 74 is displaced by the valve-actuating
element 80 from its
18 closed position against the valve seat 76 to open the fluid coupling. Once
fully engaged within
19 the chamber inlet 50, the first syringe 11 is supported in place by a
saddle member 79, which
minimizes motion of the first syringe about the chamber inlet 50.
21 [0047] The cover portion 54 has a chamber outlet 81 to form a dedicated
second fluid
22 coupling with the second syringe 15, as shown in Figure 8. The second
syringe 15, shown in
23 more cletail in Figure 5 and Figure 6, has a second body portion 82 having
a barrel 83 with a
24 proximal end 84, at which is disposed a second inlet port 85, a second
outlet port 86; and a distal
end 87 with a cylindrical wall 88 therebetween to define a second sample
receiving chamber 89.
26 The second inlet port 85 is disposed at an angle to the second outlet port
86, and intermediate the
27 second sample receiving chamber 89 and the second outlet port 86. A plunger
90 is slidably
28 disposed at the distal end 87 and is in tight fluid engagement with the
cylindrical wall 88. The
29 plungei- 90 serves to draw fluid into the second sample receiving chamber
89 and urge the fluid
therefrom. The second syringe 15 also includes a second channel portion 92
with a channel 94 in
-9-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 communication with the second sample receiving chamber 89 and the second
outlet port 86, and
2 a chatmel 96 in communication with the second inlet port 85 and the second
sample receiving
3 chamber 89 via a portion of the channel 94. In order to prevent large
particulate from entering
4 the second outlet port 86, a second end cap 97 is removably attached
thereto, while the second
inlet port 85 includes a slidable cap 98 to prevent contamination prior to use
with the blood
6 treatment unit 14. The treated blood sample is dispensed from the second
syringe 15 to the
7 originating patient 17 via the second syringe outlet port 86 operable
between an open position
8 and a closed position by a releasable lock means 100, as will be described
below.
9 [0048] Similar to the first syringe 11, within the second channel portion 92
is a printed
circuit board (PCB) 102 having circuitry for transmitting, receiving and
storing data related to
11 the syringe and/or its contents or the originating patient 17, such as
identification data, SKU,
12 serial no., manufacturing date, expiry date, fluid data, health facility
data, health practitioner
13 data, rnedication data, and so forth. The circuitry includes RFID
reader/writer functionality for
14 reading RFID tags associated with other entities within the treatment
system. The RFID
reader/writer is also coupled to other elements of the circuitry to perform at
least one verification
16 check, and other functions As such, the circuitry includes, but is not
limited to, a transmitter, a
17 receiver, logic means or processor, a computer readable medium for data
storage, a timing
18 circuit., an antenna and a power source. Also coupled to the PCB 102 are
input/output devices
19 such as a display, LED 103, a speaker or a button. In addition, the PCB 102
also includes
circuiti-y for controlling the operation of the releasable lock means 100. A
compartment 104
21 houses a power supply unit 106 comprising one or more batteries, and a
power circuit resident on
22 the PCB 102 for regulating the power therein and input/output devices. The
batteries 106 may
23 be removed after the single use of the second syringe 15, in order to
enable use in another device
24 or allow for proper recycling in compliance with current environmental
regulations. In order to
facilitate easy battery installation or removal, the batteries 106 may be
placed on a tray which is
26 slidably received by the battery compartment 104.
27 [0049] The syringe 10 is typically maintained in a low power state, when
not in use, to
28 conserve battery energy. However, when the sample management unit 12 is
introduced into the
29 blood treatment unit, the syringe 15 is placed into an operating state from
the lower power state.
Such a transition may be effected via a mechanical switch which is closed
before insertion of the
- 10-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 sample management unit into the blood treatment unit, or the switch is
closed by the blood
2 treatment unit following insertion of the sample management unit into the
blood treatment unit.
3 Other ways include an electronic switch actuable by an RF signal or a DC
signal from the blood
4 treatment unit, or a DC magnetic reed relay enabled by a magnet in the blood
treatment unit.
[0050;1 As shown in Figures 4 and 7 to 9, the chamber outlet 81 has a female
collar portion
6 108 with a pair of helically oriented passages or grooves 110 extending
through or in its wall to
7 engag., a corresponding one or more pins 112 extending outwardly from the
second syringe inlet
8 port 85. Similarly, a valve element 114 is located in the channel 96 and
biased to a closed
9 position against a valve seat 116 on an end cap 118 forming the outer end of
the second syringe
outlet 96. The valve element 114 is also aligned for abutment with a valve
actuating element 120
11 which is positioned in the chamber outlet 81. The valve actuating element
120 is thus operable
12 to displace the valve element 114 from its closed position against the
valve seat 116 to open the
13 seconci fluid coupling. The cover portion 54 is also provided with a saddle
member 122 for
14 supporting the second syringe 15 when it is in a fully engaged position
with chamber outlet 81.
[0051] The cover portion 54 has a top cap 124 and a cap lock 126 bonded,
welded or
16 otherwise fixed thereto. The cap lock 1261atches on an upper periphery of
the bottom portion 56.
17 The chamber inlet 50 and the chamber outlet 81 are each in fluid
communication with the inner
18 treatment cavity 52 by way of conduits 128, 130 extending below the valve
actuating elements
19 80, 120 respectively.
[0052] As shown in Figures 6, 10, 11 and 12, the second syringe body portion
84 has a
21 cylindi-ical cavity which in cooperation with the plunger 90 provides a
second sample receiving
22 chamber 89. The passage 94 of the blood sample transfer portion 92 has a
second access
23 location 132 for fluid communication with the second syringe outlet port
86.
24 [0053] The second syringe outlet port 86 and the blood transfer portion 92
are further
provided with the releasable lock means shown generally at 100 for forming a
locked third fluid
26 coupling between the second access location 132 and the second syringe
outlet port 86. As will
27 be described, the releasable lock means 100 is operable in response to a
release signal to release
28 the third fluid coupling, as shown in Figures 13(a) to 13(d). With the
releasable lock means
29 unlocked, the second syringe outlet port 86 is operable to form a fourth
fluid coupling with a
-11-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 fluid fitting on a common blood sample delivery unit with a complementary
LUER 31 or similar
2 fitting, such as the needle 32.
3 [00541 As best shown in Figure 10, the second syringe outlet port 86
includes a male Luer
4 insert 134, an outlet valve means generally shown at 136 for opening and
closing the access to
the fluid channel 92 to control the flow of the blood sample therethrough. The
male Luer insert
6 134 iricludes an opening 138 and a thread for the LUER fitting for coupling
with female Luer 31
7 of a nieedle 32. The outlet valve means 136 includes a valve element portion
140 and a valve
8 seat portion 142 and first actuating means generally shown at 144 for
actuating the valve element
9 portion 140 relative to the valve seat portion 142.' A pair of resilient
members 148, such as a
spring;, biases the outlet valve means 136 in a closed position. As will be
described, the first
11 actuating means 144 is operable to displace the valve element portion 140
in different directions
12 when the second syringe body portion 84 is either engaged or disengaged
with a female Luer 31.
13 [0055] The first actuating means 144 takes the form of a plurality of first
actuating elements
14 150 which extend outwardly from a central web 152, and also second
actuating means such as a
tab 154 extending therefrom. The central web 152 is fixed to a block 156
positioned in a channel
16 94 in the body portion 92 of the second syringe 15. The block 156 has a
central bore 158
17 carrying a tubular valve stem 160 having one end carrying the valve element
portion 140 and an
18 opposilte end carrying a valve stem head 162, which has a peripheral edge
region with a sealing
19 element such as an 0-ring or the like. The valve stem 160 has a pair of
fluid transfer holes as
shown at 164 immediately beside the valve element portion 140, thereby forming
an inner valve
21 passag;e which is in fluid communication with the second sample receiving
chamber 89, as
22 shown in Figures 11 and 12. The female Luer 31 includes complementary first
actuating
23 elements which displace the first actuating elements 150, when the female
Luer 31 member is
24 introduced into the male Luer insert 134. Consequently, the valve stem 160
and the valve
element portion 140 are caused to open the central bore 158 within the valve
stem 160 to the
26 channe:l 96 to allow fluid flow through the outlet port 86.
27 [00561 The outlet port 86 of the second syringe 15 is operable between
three states, a locked
28 state, an open state and permanently locked state, by a releasable lock
means, such as locking
29 mechanism 100, as shown in Figures 13(a) to 13(d). The locking mechanism
100 includes a pawl
168 coupled to the outlet valve means 136 to control the coupling of the
female Luer 31 to the
-12-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 male Luer insert 134 of the second syringe 15. The pawl 168 has one end 170
with an opening
2 172 fDr receiving a pivoting pin 174, protruding from a board 176, to allow
pivoting thereabout.
3 The pawl 168 is positioned between a first spring plate 178 and a second
spring plate 180 which
4 control its swinging motion. Typically, the first spring plate 178 is made
from fuse material
which temporarily changes consistency under the presence of the predetermined
electric current
6 signal, such as nickel titanium naval ordinance laboratory intermetallic
material (NITINOL).
7 Nitinol exhibits superelasticity and shape memory, such that nitinol is
caused to heat up due to
8 the predetermined electric current signal, as such it is mechanically
deformed under stress above
9 a specific temperature, and returns to the pre-stressed position when the
stress is removed.
[0057) On the other end 182 of the pawl 168 is a first finger 184 and a second
finger 186
11 defining a recess 188 with an opening 189. Adjacent to the recess 188 is a
punched out slot 190
12 which includes a plurality of interconnected slots 192, 194, 196. These
interconnected slots 192,
13 194, 196 correspond to the above-mentioned locked state, the open state and
the permanently
14 locked state, respectively. The slots 192 and 196 are opposite each other
and separated by a pawl
tooth 198 on one side of slot 190 and linked to one another by slot 194 on the
other side of slot
16 190. The slot 192 is L-shaped and includes one arm 200 and another arm 202
which links to slot
17 194.
18 [0058] The first spring plate 178 is secured to the board 176 at one end
and includes an
19 arcuate portion 204 positioned above the pawl 168. The arcuate portion 204
is bent at
approximately 90 degrees at point 208, and adjacent to the point 208 is an
abutment flange 210
21 which engages the arm 200 of slot 192, in the locked position, as shown in
Figure 13(a). The
22 subsequent positioning of the abutment flange 210 determines the operating
state of the syringe
23 15.
24 [0059] The motion of the pawl 168 through the three different positions
will now be
described. Starting in the rest position, the abutment flange 210 is
positioned in the arm 200 of
26 slot 1912. Upon receipt of the release signal following the verification
process, a predetermined
27 electric signal is caused to flow through the first spring plate 178, and
the electric signal is
28 sufficient to cause the first spring plate 178 to relax. The first spring
plate 178 is sufficiently
29 relaxecl such that the second spring plate 180 forces the abutment flange
210 out of the arm 200
into arm 202, and finally into slot 194 corresponding to the open position, as
shown in Figure
-13-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 13(b). A female Luer 31 of a needle 32 can now be attached to the second
syringe 15 and the
2 treated blood is expressed from the second sample receiving chamber 89 via
the open outlet
3 valve into the patient 17, as shown in Figure 14.
4 [00601 After a predetermined time, such as 20 minutes, the predetermined
electric signal is
once again caused to flow through the first spring plate 178, and causes the
first spring plate 178
6 to relax. The second spring plate 180 forces the abutment flange 210 out of
the slot 194 into slot
7 196 corresponding to the permanently locked position, as shown in Figure
13(c) and 13(d). If
8 the female Luer 31 is still attached when the release signal is issued, then
the abutment flange
9 210 is prevented from sliding into the permanently locked position until the
female Luer 31 is
removed. By permanently locking the second syringe 15, subsequent use of the
second syringe
11 15 is precluded, thus substantially eliminating contamination risks.
12 [0061] The operation of the outlet valve means 136 in conjunction with the
locking
13 mechanism 100 will now be described with particular reference to Figures 10-
14. In the locked
14 position of the second syringe 15, the tab 154 rests on the finger 184 and
thus restricts the central
web 152 from longitudinal displacement away from the opening 138. Any attempt
to couple a
16 female Luer 31 fails, since the complementary first actuating elements
cannot displace the first
17 actuating elements 150 and therefore the female Luer 31 and male Luer
insert 134 cannot mate.
18 Correspondingly, the outlet valve means 136 is biased closed by the pair of
resilient members
19 148 acting on the central web 152, and thus the central bore 158 within the
valve stem 160 is
closed.
21 [00621 Upon energising the first spring plate 178, the pawl 168 is caused
to rotate in a
22 clockwise direction and the abutment flange 210 is forced out of the arm
200 into arm 202, and
23 slides into slot 194 corresponding to the unlocked or open position.
Concurrently, the finger 184
24 of the pawl 168 moves away from the tab 154 such that the tab 154 is now
aligned with the
recess 188. The female Luer 31 is now be introduced into the male Luer insert
134, and the
26 complementary first actuating elements abut the first actuating elements
150. The force applied
27 to mate the female Luer 31 to the male Luer insert 134 displaces the first
actuating elements 150
28 away from the opening 138, the central web 152 moves in sympathy. The tab
154 enters the
29 recess 188 via the opening 189 and travels the length of the recess 188.
The force applied to
-14-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
I couple the Luers 31 and 134 is sufficient to compress the resilient members
148 and thus open
2 the central bore 158 within the valve stem 160.
3 [0063] As the treated blood often includes bubbles of gases used during
treatment, the
4 second syringe 15 includes a de-bubbling system or bubble removal mechanism
to expel gas
from syringe, before the treated blood sample is administered to the
originating patient 17.
6 Alterrratively, a separate vent cap is attached to the proximal end 84 to
interface with the Luer
7 134. "The vent cap includes a hydrophobic gas permeable membrane to prevent
blood from
8 escaping. Generally, more air can be introduced into the second sample
receiving chamber 89 to
9 coalesce the existing bubbles, thus facilitating removal of otherwise small
bubbles. Thus, the
barrel 83 is transparent such that a user can inspect the treated blood sample
to verify that gas
11 bubbles have been removed.
12 [0064] After the treated blood has been administered to the patient 17, the
female Luer 31 is
13 uncoupled from the male Luer insert 134, as the needle 32 is removed. With
the complementary
14 first actuating elements removed from the male Luer insert 134, the
resilient members 148
expand to push the central web 152 towards the opening 138 and the tab 154
travels out of the
16 recess 188 and faces the recess opening 189. At the predetermined time, a
predetermined electric
17 signal is caused to flow through the first spring plate 178, and the
abutment flange 210 is forced
18 out of the slot 194 into slot 196. The tab 154 now abuts the finger 186,
and thus any longitudinal
19 displacement of the central web 152 from away from the opening 138 is
precluded. With the
abutment flange 210 unable to be forced to return to slot 194, the second
syringe 15 is now
21 permanently locked, and so a female Luer 31 can not be subsequently coupled
to the male Luer
22 insert134, as shown in Figure 13(d).
23 [0065] As will be described, the system 10 provides a verification protocol
which involves
24 number of verification checks to be sure that the proper treated blood
sample is delivered to the
proper originating patient 17, and that certain events in the collection,
treatment and delivery of
26 the blood sample to the patient 17 occurs within prescribed time periods.
To that end, and as
27 shown in Figure 15, the system has identification means 211 for identifying
an originating
28 patient 17, and the untreated blood sample in the first syringe 11,
verification means 212 for
29 verifying a match between the originating patient 17 and the treated blood
sample in second
syringe 15, and release signal generating means 214 for generating a release
signal in response
-15-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 to a positive outcome by the verification means. The release signal is
transmitted to the
2 releasable lock means 100 to deliver the predetermined current to the first
spring plate 178,
3 thereby to render the second syringe 15 operable to deliver the treated
blood sample to the
4 originiating patient 17.
[0066] As will be described, the identification means 211 and the release
signal generating
6 means 214 are located on the second syringe 15, but may be located in the
aforementioned
7 entities. The releasable lock means 100 has a signal receiving means 216 for
receiving the
8 release signal.
9 [00671 As shown in Figure 16, the verification means 212 includes comparison
means 218
for comparing patient identity data with treated blood sample identity data,
both stored in
11 memory means 220, and signal receiving means 216 to receive one or more
signals associated
12 with the originating patient identity data and/or the blood sample identity
data (either untreated,
13 treated or both). In this case, the one or more signals contain the
originating patient identity data
14 and/or the blood sample identity data. However, as an alternative, the one
or more signals may
contain data which is associated with or related to the patient 17 or blood
sample identity data.
16 For example, the data in the signals may include one or more codes which
allow the patient
17 identity data or the blood sample identify data to be obtained from a data
structure in the memory
18 means 220 or some other location, for example in the form of a look up
table, for instance
19 [0068] The verification means 212 also includes a counter means 221 which
provides
temporal data related to a predetermined event including and/or between an
untreated blood
21 sample collection event and a treated blood sample delivery event. The
temporal data may also
22 include at least one elapsed time value between two predetermined events
including or between
23 the untreated blood sample collection event and the treated blood sample
delivery event. The
24 counter means 221 may be implemented as a first incremental counter 222 on
first syringe 11
and a second incremental counter 224 on the second syringe 15 are used to
track time delay. The
26 first incremental counter 222 tracks the events related to the collection
of untreated blood, while
27 the treatment and post treatment events are tracked by the second
incremental counter 224. These
28 two incremental counters 222 and 224 operate independently of one another
and do not require
29 to be synchronized with each other. The battery power is sufficient to
maintain substantial
accuracy of their internal clock within the time period from collection of the
untreated blood
-16-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 sample to the delivery of the treated blood sample to the patient 17.
Therefore, the possibility of
2 losing time or decreasing clock accuracy as the battery's power runs down is
substantially
3 eliminated.
4 [0069] In this case, the verification means 212 may be operable to prevent
release of the
locked third fluid coupling when the elapsed time value has exceeded a
predetermined value.
6 Before treatment of the untreated blood sample, the verification means 212
is also operable to
7 prevent treatment of the blood sample when the elapsed time value has
exceeded a
8 predetermined value. Similarly, following treatment, the verification means
212 is operable to
9 verify a match between the untreated blood sample in the first syringe 11
and the originating
patient 17.
11 [0070] The verification protocol may be implemented in a number of forms,
although the
12 most preferred at present is by the use of one or more radio frequency
signal transmitters and
13 receivers and RFID tags. As shown in Figure 17, the wristband 16 is
provided with a passive
14 RFID tag, such as WB RFID tag 226, while the first syringe 11 and the
second syringe 15
include the aforementioned printed circuit board (PCB) 102 having circuitry
for transmitting,
16 receiving and storing data related to the syringe and/or its contents or
the originating patient 17,
17 including a S 1 RFID reader/writer 228 and a S2 RFID reader/writer 230,
respectively. The
18 passive WB RFID tag 226 comprises an antenna coil and a silicon chip that
includes modulation
19 circuitry and non-volatile memory. The passive WB RFID tag 226 is energized
by an external
time-varying electromagnetic radio frequency (RF) wave that is transmitted by
a RFID
21 reader/writer, such as the S 1 RFID reader/writer 228 or the S2 RFID
reader/writer 230.
22 Therefore, S 1 RFID reader/writer 228 or the S2 RFID reader/writer 230 is
capable of writing
23 data onto the WB RFID tag 226, and reading data back from WB RFID tag 226
by detecting the
24 backscatter modulation.
[0071] The blood treatment unit 14 is also equipped with a BTU RFID
reader/writer 232 to
26 receive: a pre-treatment identity data from the S 1 RFID reader/writer 228
and to receive post
27 treatment data from the S2 RFID reader/writer 230. Similarly, the blood
treatment chamber 12 is
28 equipped with a passive SMU RFID tag 234 to provide an identification code.
The BTU RFID
29 reader/writer 232 issues query signals to the SMU RFID tag 234 to determine
whether the
-17-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
I blood treatment chamber 12 is valid for use in the treatment process, that
is, whether the blood
2 treatnient chamber 12 is an authentic product or whether it has been
previously used.
3 [0072] As shown in Figures 19 to 21, the wristband 16 (WB) contains a
removable portion
4 236 containing the WB RFID tag 226 and audit data written onto it relating
to the patient 17
and/or the treated blood sample. The wristband 16 may also include a buckle
assembly 238
6 having a base portion 240 and cover portion 241. The base portion 240 is
integrally formed with
7 a banci 242 of resilient material which a number of perforations forming
passages 244 to receive
8 the buckle assembly 238. The base portion 240 has pins 246, 247, 248 that
are dimensioned to
9 fit through the passages 244. The cover portion 242 is hinged to the base
portion 240 by way of a
hinge shown at 250. The cover portion 242 also has a pair of cavities 252,
each for receiving one
11 of the pins 246 or 248. The pin 247 may press against a switch (not shown)
in the base portion
12 240 to activate portions of the circuitry of the wristband 16, upon
securement of the band 242
13 around the patient's 17 arm.
14 [0073] The method of monitoring a material sample will now be described
with reference to
the Figures 1 to 21. The verification protocol makes use of a number of
identification codes,
16 such as a first syringe identity code representative of the untreated blood
sample therein, and the
17 a wristband identity code representative of the originating patient 17. To
simplify the data
18 transfer, the first syringe identity code and the wristband identity code
may include common
19 identity data, though the data between them may be different or related as
the case may be. The
first syringe identity code may, if desired, include a first time value
representative of the time of
21 untreated sample collection from the originating patient 17 (or a
designated event either before or
22 after the sample collection step) and/or verification thereof.
23 [0074] Thus, the S 1 RFID reader/writer 228 functions as a first signal
emitter for emitting a
24 first signal carrying the first syringe identification code data, and/or
common identity data, while
the W13 RFID tag 226 on the wristband 16 functions as a first signal receiver
to receive the first
26 signal. The second syringe 15 is assigned a second syringe identity code,
which is representative
27 of the treated blood sample therein. The second syringe identity code
includes a second time
28 value representative of the time of the treated sample delivery thereto
from the treatment cavity
29 52 (or a designated event either before or after the treated sample
delivery step) and/or
verification thereof.
-18-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 [0075] Thus, the S2 RFID reader/writer 230 functions as a second signal
emitter for emitting
2 a second signal carrying the treated blood sample identity data and the WB
RFID tag 226
3 functions as a second signal receiver means to receive the second signal,
wherein the verification
4 means 212 is operable to compare the first signal data with data
representative of the treated
blood sample.
6 [0076] Referring to Figure 18, the verification protocol will now be
discussed along with a
7 typical blood treatment procedure. As shown in Figure 1, a kit for a blood
treatment procedure is
8 asserr.ibled including, among other things, the wristband 16, the first
syringe 11, the second
9 syringe 15, the sample management unit 12 and a number of prepared labels
258 with patient
identification printed thereon. The procedure starts with the activation of
the first syringe 11 via
11 an actuating means such as the pullout tab button 38. Once activated, the
circuitry on PCB 34 is
12 powered on by the batteries 48 and conducts a power-on-self-test (POST)
procedure and
13 subsequently the first syringe I1 is ready for use, barring any detected
faults during the POST
14 procedure. The S 1 RFID reader/writer 228 is then activated and starts
transmitting query signals
and waits for an acknowledgement response from the passive WB RFID tag 226.
The first
16 increniental counter 222 is also initiated and outputs temporal data, and
keeps track of the
17 untreated blood events and log time stamps associated with predefined
untreated blood events, in
18 association with the logic means. To that end, a timestamp TSO indicative
of the event of power-
19 on is recorded by the second incremental counter 224 and stored in memory.
The S1 RFID
reader/writer 228 and the WB RFID tag 226 each contain common patient identity
data or
21 sample treatment data, coded as IDI.
22 [0077] Before the first syringe 11 is used to draw blood from the patient
17, a blood anti-
23 coagulant, such as sodium citrate solution, is also drawn into the first
sample receiving chamber
24 21 to prevent clotting of the blood, as shown in Figure 3. A sample of
blood is then withdrawn
from the patient 17, and once primed, the first syringe 11 is brought to
within RF range of the
26 wristband 16.The S 1 RFID reader/writer 228 queries the WB RFID tag 226 to
verify that the
27 data read from or emitted by the WB RFID tag 226 corresponds to the common
patient identity
28 data II)1 on S 1 RFID reader/writer 228. The process is terminated if there
is no correlation
29 between the data on the wristband 16 and the first syringe 11. However, if
a positive correlation
has been made, the S I RFID reader/writer 228 records a "time data stamp" TS 1
stamp on the S 1
-19-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 RFID reader/writer 228, and writes the same time-stamp to the WB RFID tag
226, Therefore the
2 S 1 RFID reader/writer 228 and the WB RFID tag 226 now carry TSO, TSI and ID
1. As an
3 example, the data now on the S 1 RFID reader/writer 228 and the WB RFID tag
226, the may be
4 represented as: S 1 ID1 TSO TS 1 meaning that the untreated blood sample
drawn into the first
syringe 11 is from a patient with the identification ID 1, the first syringe
11 was powered on at
6 time ']CSO, and the common patient identity data ID1 on the first syringe 11
and the wristband 16
7 was matched at time TS 1.
8 [00781 The first syringe 11 logic means receives temporal data from the
first incremental
9 counter 222 and determines the elapsed time from the start of the procedure
(TSO) and the instant
that the common patient identity data ID1 on the first syringe 11 and the
wristband 16 is
11 matched. The process advances as long as the time unit difference between
TSO and TS 1 is
12 within an acceptable predefined range.
13 [0079] In the next step, the first syringe 11 is installed on the blood
treatment chamber 12
14 (with the second syringe 15 already positioned thereon), which is then
delivered to the blood
treatment unit 14. As such, the S1 RFID reader/writer 228 emits the data TSO,
TS1, ID1 to the
16 BTU RFID reader/writer 232. The data also include a time value TS2 denoting
a treatment start
17 time. The blood treatment unit 14 then calculates the time delay between
TSl and TS2 of the
18 first syringe 11. In addition, the blood treatment unit 14 issues a query
signal to the SMU RFID
19 tag 234 on the sample management unit 12 and, in response thereto, the SMU
RFID tag 234
issues a signal containing its identification code to the blood treatment unit
14. A determination
21 as to whether the SMU RFID tag 234 is valid, and also whether the delay is
acceptable. If the
22 SMU RFID tag 234 is invalid, and/or the delay is unacceptable then the
process ends, otherwise
23 the process continues. This identification code, in this case, includes an
"enable" code indicating
24 that the blood treatment chamber 12 has not been previously used for a
blood treatment, thus
reducir-g the risk of contamination the current untreated blood sample SI.
Alternatively, the
26 SMU RFID tag 234 need not issue an enable code, but rather merely emit a
signal containing
27 identity data such as a SKU or the like.
28 [0080] If the time delay between TS 1 and TS2 is acceptable, the blood
treatment unit 14 the
29 procedure continues with the untreated blood sample in the first syringe 11
being delivered to the
treatment cavity 52, via the chamber inlet 50 and conduit 128. The S 1 RFID
reader/writer 228 is
-20-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 subsequently disabled to prevent further use by including a disable code
thereon. In addition, a
2 SMU RFID tag 234 on the blood treatment chamber 12 receives a disable code
from the BTU 14
3 after the blood sample is delivered to it, thereby preventing the reuse of
the blood treatment
4 chamber 12. Alternatively, the SMU RFID tag 234 may be disabled in other
ways without
writirig a disable code thereon. For example, the SMU RFID tag 234 may be
rendered
6 inoperable by issuing the SMU RFID tag 234 a signal causing a fuse to be
blown therein.
7 [0081] In the course of the treatment, the second syringe 15 is powered on
and starts
8 querying the BTU RFID reader/writer 232 for data. A new time stamp
signifying the end of the
9 blood sample treatment "TS3" is written to the BTU RFID reader/writer 232,
and subsequently
TS3 is read by the S2 RFID reader/writer 230, and stored thereon. The treated
blood is then
11 delivered from the treatment cavity 52 via the conduit 130 and to the
second syringe 15, and. If
12 desired, the blood treatment unit 14 may also include the TS 1 stamp,
meaning that the data
13 written to the S2 RFID reader/writer 230 would include ID1, TSO, TSI, TS2,
and TS3. In this
14 case, the second syringe 15 includes the treatment start time TS2 and the
treatment end time TS3.
Alternatively, or in addition, TS2 or TS3 may include a treatment duration
time, or some other
16 code indicating that all previous verification steps have been successfully
carried out.
17 [0082] For example, the blood treatment unit 14 may record the following
data:
18 Sl ID1 TSO TS1
19 PATIENT ID
TREATMENT START TS2
21 TREATMENT END TS3
22 S1 ID1 TSO TS1 TS3
23 [0083] In this case, the PATIENT ID code may include other patient-related
data that is
24 manually or automatically entered into the blood treatment unit 14.
Alternatively, the patient-
related data is transferred to the blood treatment unit 14 from a central data
storage centre, a
26 server computer, a memory bank or the like.
27 [0084] The second syringe 15 is then transported back to the originating
patient 17 wearing
28 the wristband 16 and the S2 RFID reader/writer 230 continually polls the WB
RFID tag 226 until
29 the latter is within range of the query signals. In response to the query
signals, the WB RFID tag
226 then emits ID1 data, at time "TS4". The S2 RFID reader/writer 230 then
calculates the time
-21-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 delay between TS3 data and the time of arrival, TS4, of the second syringe
15 back to the
2 wristband 16. If the expected time delay is exceeded, then the second
syringe 15 remains locked
3 by the locking mechanism 100, otherwise the process continues.
4 [0085] The second syringe 15 records ID1, and the time stamp "TS4". In
addition, the
second syringe 15 may include the PATIENT ID data as well as the ID1, TSI,
TS2, TS3. This
6 data is subsequently written onto the WB RFID 226. At this stage, the S2
RFID reader/writer 230
7 issues a release signal to the locking mechanism 100 to unlock the second
syringe 15, by issuing
8 a predetermined current to the spring plate 178 to force the abutment flange
into slot 194,
9 thereby rendering the second syringe 15 operable for injection.
[0086] As an example, the WB RFID tag 226 therefore records:
11 S1IDITSOTSI
12 S2 15 IDl TSO TSl TS2
13 SAMPLE MATCH TS3
14 S2 UNLOCK TS4
[0087] The verification protocol is then completed when the TS4 is recorded in
the WB
16 RFID tag 226 after it performs a sample match between the IDl data on the
S2 RFID
17 reader/writer 230 and the WB RFID tag 226. As shown in Figure 21, the
removable portion 236
18 of the wristband 16 is then separated therefrom and matched with the
originating patient's record
19 and the patient record is returned to the blood treatment unit 14 for a
data exchange between the
WB RFID tag 226 and the blood treatment unit 14, to complete the audit trail.
21 [0088] Alternatively, an RF reading audit record capture station may be
provided which is
22 local to the patient 17 or to a patient record area in a medical facility,
thereby eliminating the
23 need for the patient record to be returned to the blood treatment unit 14.
In this case, the audit
24 record capture station may be capable of downloading the patient record to
complete the audit
trail. The RF reading audit record capture station may be part of the internal
network of the
26 medical facility, either through a wired or wireless data port, or may be
part of a network
27 localized to one or more blood treatment unit systems in the medical
facility. It may collect data
28 and allow for later batch recording to a computer readable medium, such as
an optical disc, hard
29 drive or other storage device. It may be attached to or integrally formed
with a computing
device, personal digital assistant, a mobile phone or the like. It may also be
embodied as
-22-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 software configured to run on a computing device, together with an RFID
reading attachment
2 thereon.
3 [00891 The data ID1 and TS4 is delivered to blood treatment unit 14 or other
system to
4 complete the audit trail. The time stamp may also include an "event" code,
which may comprise
five ma}or events:
6 [0090]
7 1) S 1 start time
8 2) WB acknowledges with S 1
9 3) Start of Treatment
4) End of Treatment
11 5) Match between the Treated Sample and the Originating Patient.
12
13 The tiine stamp may also include any one or more of a number of Error
events
14 1) No match
2) S 1 does not match with WB at before/after collection
16 3) S2 does not match with WB on return after Treatment.
17 4) Time Delay- exceed time to collect of blood
18 5) Time Delay- exceed time to deliver sample to BTU
19 6) Time Delay- exceeds time to return to patient.
The TS3 time stamp may also include a "match" code as follows:
21 01 Match
22 02 No match
23 [0091] In another embodiment, the wristband includes electronic circuitry
coupled to the
24 passive WB RFID tag 226, and a battery for providing power to the
electronic circuitry. As
shown in Figure 20, the wristband 16 includes outputs means, such as LEDs 260,
262, 264, or a
26 speakeir (not shown), which are operated in different combinations of one
or more thereof. For
27 example, the LEDs 260, 262 may be operable to illuminate in accordance to a
predetermined
28 cycle indicative of the communication associated with verification process
with the first syringe
29 11 and the second syringe 15. The third LED 264 may be provided for alarm
situations.
-23-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 [0092] In another embodiment, the wristband includes electronics circuitry
coupled to the
2 passive WB RFID tag 226, and a battery for providing power to the electronic
circuitry. As
3 shown in Figure 20, the wristband 16 includes outputs means, such as LEDs
260, 262, 264, or a
4 speaker (not shown), which are operated in different combinations of one or
more thereof. For
example, the LEDs 260, 262 may be operable to illuminate in accordance to a
predetermined
6 cycle indicative of the communication associated with verification process
with the first syringe
7 11 and the second syringe 15. The third LED 264 may be provided for alarm
situations.
8 [0093]
9 [0094] The wristband 16 may be replaced by some other article to be wom,
carried, attached
or ingested by the patient 17, such as a pinned or self adhesive label 258 and
the like.
11 [0095] The second syringe 15 may also include a second sample receiving
chamber 89
12 volurne detector to determine whether the received treated blood from the
treatment cavity 52 is
13 withir.t a predefined range suitable for injection into the patient 17 to
provide the desired medical
14 treatment.
[0096;1 In another embodiment, the system 10 includes a blood sample treatment
chamber,
16 similar to the sample blood treatment chamber 12 of Figure 4, with an
expandable treatment
17 cavity 52 formed by a cover portion 54, a bottom portion 56 and a flexible
walled portion 58
18 therebetween.
19 [0097] In yet another embodiment, the system includes a locking mechanism
100 operable
by a solenoid or motorized means configured to receive the release signal.
21 [0098] In another embodiment, the system includes a wristband 16 with
electronic circuitry
22 for transmitting, receiving and storing data related the originating
patient 17, such as
23 identification data or an identifier, SKU, serial no., manufacturing date,
expiry date, health
24 facility data, health practitioner data, medication data, and so forth. The
circuitry includes, but is
not limited to, a transmitter, a receiver, logic means or processor, a
computer readable memory
26 for data storage, a timing' circuit, an antenna and a power source. The
circuitry also includes an
27 RFID reader/writer for reading RFID tags associated with other entities
within the treatment
28 system, such as the first syringe 11, the second syringe 15, or the sample
management unit 12. A
29 wristband tag. This wristband 16 acts as the archive data storage for the
entire treatment and
therefore provides the audit trail once the treatment has been completed. The
data may be stored
-24-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 in the computer readable medium, such as RAM, ROM, flash memory, and so
forth. or.the
2 wristband may include an RFID tag to which the data is written.
3 [0099] The first syringe 11 and the second syringe 15 include a printed
circuit board (PCB)
4 having circuitry for transmitting, receiving and storing data related to the
syringe and/or its
contetits or the originating patient 17, such as identification data or
identifiers, SKU, serial no.,
6 manufacturing date, expiry date, fluid data, health facility data, health
practitioner data,
7 medication data, and so forth. The circuitry is implemented as an active
RFID tag having a
8 transmitter, a receiver, logic means or processor, a computer readable
medium for data storage, a
9 timing circuit, an antenna and a power source such as a batteries. Also
coupled to the PCB are
input/output devices such as a display, LED, a speaker or a button. The second
syringe 15 also
11 includes circuitry for controlling the operation of a releasable lock means
or electromechanical
12 interlock to prevent re-injection of treated blood in the event that the
wristband 16 identifier and
13 second syringe identifiers do not match.
14 [00100] Similar to the preferred embodiment, the system includes a BTU
reader/writer which
can communicate (read and write) to RFID tags of the first syringe 11 and the
second syringe 15
16 and to a tag on the sample management unit 12. The first syringe 11 RFID
tag stores and record
17 data relating to the patient for example, the time blood was removed for
treatment. It will also
18 ensure that the syringe 11 cannot be re-used. The first syringe 11 RFID tag
will also include an
19 elapsed time counter and a matching identifier to that contained in the
wristband written at the
time of manufacture or packaging. The second syringe 15 RFID tag includes
similar functions
21 and includes logic and circuitry to drive an electromechanical interlock.
22 [00101.] The flow of treatment events are similar to the one described
above. Prior to removal
23 of blood, a check is performed to verify that the unique treatment set ID
numbers contained in
24 the wristband 16 and in the first syringe 11 match, by having the syringe
11 active tag emit the
data to the wristband 16 reader/writer. If there is a match, this event is
recorded by the wristband
26 and blood is withdrawn from the patient. At the same time the elapsed time
counters in the
27 syringe 11 tag and wristband 16 will start.
28 [00102] The first syringe 11 is then be fitted onto the sample management
unit (SMU) 12,
29 which is already fitted with a single-use second syringe 15. The SMU 12
with both syringes
11,15 is then taken to the blood treatment unit (BTU) 14 with a BTU
reader/writer. The patient
-25-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 details are entered into the BTU reader at this stage. The blood treatment
unit 12 reader/writer
2 will read the first syringe 11 tag and write the details (including the
unique ID) to the second
3 syringe 11 tag. The BTU reader/writer will also write a message to the SMU
tag to indicate it has
4 been used. The BTU reader/writer will read the elapsed time from the first
syringe 11 tag and
calculate treatment time details. These are then written to the second syringe
11 tag along with
6 patierit ID.
7 [00103] Following the blood treatment, the BTU reader/writer writes the
completed treatment
8 time to the second syringe 15 tag. The SMU 12 is removed from BTU. S2
syringe is removed
9 from the SMU 12, and the SMU 12 and S i syringe are discarded. The second
syringe 15 is then
preserited to the wristband 12 on the patient and, provided the unique IDs
match and elapsed
11 time is within set parameters, the second syringe 15 locking mechanism is
released and the
12 second syringe 15 can be used to inject the treated blood into the patient.
The wristband 12
13 reader/writer writes the patient data and procedure details to the
wristband 12 tag or computer
14 readable medium, for subsequent removal for storage with patient records.
The wristband 12
reader/writer is then deactivated and its strap is cut to allow removal from
the patient and
16 disposal. A network RFID reader is used to read the encrypted data in the
wristband tag memory
17 unit or computer readable medium for transfer to the health facility
database on a computer or
18 network.
19 [00104] In another embodiment, the BTU reader/writer or an external
reader/writer provides
all the verification checks
21 [00105] Even though the description above is in large part focused on the
use of system 10 in
22 the treatment of autologous blood samples, it will be understood that the
system 10, its
23 components and alternatives thereof, may be used for samples other than
blood samples, such as
24 bone rnarrow or, lymphatic fluids, semen, ova- fluid mixtures, other bodily
fluids or other
medical fluids which may or may not be "autologous", for example fluid
mixtures perhaps
26 contairiing a patient's desired solid sample such as from organs, body
cells and cell tissue, skin
27 cells and skin samples, spinal cords. The system 10 may also be used for
medical testing where
28 it is irriportant to ensure that test results of a particular test can be
delivered to the originating
29 patient 17.
-26-
CA 02605675 2007-10-23
WO 2006/122400 PCT/CA2006/000781
1 [00106] While the system 10 makes use of syringes 11 and 15, it will be
understood that other
2 devices may be used such as, alone or in combination, one or more syringes,
IV bottles, powder
3 and/or atomized fluids and/or gas inhalant dispensers, implant delivery
dispensers, ventilators,
4 syringe pumps, intubation tubes, gastrointestinal feeding tubes, or a
plurality and/or a
combination thereof. One of the treatment devices may also comprise a blood
treatment device
6 such as that disclosed in International Publication No. WO0119318A1 entitled
"APPARATUS
7 AND PROCESS FOR CONDITIONING MAMMALIAN BLOOD" (the entire contents of
8 which are incorporated herein by reference). Alternatively, one treatment
device may be
9 equipped to perform a range of invasive and non-invasive treatments such as
surgeries,
treatments for diseases such as cancer, as well as exploratory or diagnostic
investigations such as
11 X-rays, CAT Scans, MRI's and the like.
12 [00107] Although the invention has been described with reference to certain
specific
13 embocliments, various modifications thereof will be apparent to those
skilled in the art without
14 departing from the spirit and scope of the invention as outlined in the
claims appended hereto.
-27-