Note: Descriptions are shown in the official language in which they were submitted.
= CA 02606480 2007-11-06
Direct Flow Injection Analysis Nebulization Electrospray and APCI Mass
Spectrometry
Field of the Invention
The present invention relates to apparatus and methods in the field of mass
spectrometry.
Background of the Invention
Flow injection analysis (FIA) performed with an atmospheric pressure ion (API)
source
interfaced to a mass spectrometer (MS) is a common method for introducing
sample into an API
MS apparatus. Typically, API MS FIA is performed by connecting a sample
injector valve in-
line between a solvent or solution delivery system and an inlet probe to an
API source. When the
injector valve is switched to the load position, sample solution is loaded
into the injector valve
through an injector needle while the separate solvent delivery system provides
liquid flow to the
API source probe through a separate channel in the injector valve. The
injector valve is then
switched so that the loaded sample solution, usually contained in a tubing
loop or channel, is
connected to the solvent delivery channel and the sample solution flows into
the API source
through the API probe at a flow rate set by the solvent delivery system. The
injector valve is
generally connected to the API source probe with a transfer line or tube. The
sample is
introduced into the injector valve through an injector needle that is
connected to a fluid reservoir
or transfer line. The injector needle may be connected to a syringe for manual
injection, a syringe
configured in an autoinjector or transfer tube or line that is connected to a
remote fluid delivery
means configured in an autoinjector. In some commercially available
autoinjectors, the injector
needle is attached directly to a syringe. The syringe and injector needle are
loaded, emptied and
positioned by the autoinjector apparatus. The autoinjector moves the injector
needle into a vial or
container holding sample solution and loads a programmed volume of sample
solution into the
injector needle and attached syringe. The autoinjector apparatus then moves
the syringe and
injector needle position to the injector valve and loads the sample solution
into the injector valve.
To avoid sample carryover from one sample solution injection to the next, the
injection needle
inner bore and outer surface and the syringe inner volume may be washed or
flushed between
sample injections.
1
µ . CA 02606480 2007-11-06
' .
Other commercially available autoinjectors do not attach the injector needle
directly to a syringe
but instead connect the injector needle to a fluid transfer line or tube that
is in turn connected to a
syringe or fluid pump which may or may not translate with the injector needle
position. Sample
solutions are drawn into the injector needle and connected tubing and injected
into the injector
valve by activating the remote syringe or fluid delivery pump when the
injector needle is
appropriately positioned in a sample vial or the injector valve respectively.
A number of
apparatus and methods have been employed in commercial autoinjectors to flush
or wash the
outer and inner bore of injection needles and the connected tubing between
sample injections.
Typically, autoinjector needles are metal tubes with sufficient rigidity to
push through the seal of
an injector valve or sample vial top. The flow rate of sample solution pulled
into or delivered
from the injection needle is programmably controlled by the autoinjector
syringe or positive
displacement fluid flow pump. Autoinjectors can be programmed to inject sample
solutions
drawn from multiple sample vials or containers in an unattended sequence. Each
sample loaded
into the injector valve is subsequently injected or delivered to the API MS
where a portion of the
sample is ionized and mass to charge analysis. Alternatively, some or all
sample solution
transfer, injection and injector needle cleaning steps performed by an
autoinjector can be
performed manually as well with a handheld syringe or a syringe mounted on an
syringe pump
that is connected through a transfer line and an injection needle to the
injector valve.
API MS performance can be reduced using conventional FIA configured with
injector valves and
transfer lines. MS signal resulting from ES and APCI source ionization is
essentially sample
concentration dependent. Dilution of the sample can occur in injector valves
and transfer lines
due to diffusion of sample solution into the mobile solvent, mixing connection
points and in dead
volumes and adsorption to the walls. Such dilution can result in reduced MS
signal or tailing of
injection peaks. Sample that has adsorbed to surfaces in the injector valve or
liquid transfer lines
can bleed off during subsequent sample injections. Such sample carry over can
appear as added
peaks or chemical noise in subsequent injections and may cause errors in trace
component or
quantitative MS analysis. The effects of the dead volumes from injection
valves, connections and
fluid deliver or transfer lines become increasingly pronounced as the liquid
flow rate or sample
concentration decreases. When the liquid flow rate is decreased, the sample
transit time in the
injector valve and transfer tubing increases for a given dead volume. Longer
sample transit times
2
CA 02606480 2012-04-03
=60412-4221D
allow increased sample diffusion into the solvent, diluting the sample. Higher
liquid
flow rates may require more total sample to be injected to accommodate slower
MS
data acquisition rates encountered with scanning mass spectrometers such as
quadrupoles.
The Electrospray needle in some commercial ES sources is operated at kilovolt
potentials during spraying. For such ES sources, a longer dielectric liquid
transfer
line of several inches is typically configured between the ground potential
injector
valve and the ES needle to allow a gradual drop in kilovolt potential through
the
sample solution. A high electric field gradient in the transfer tube is
avoided to
minimize sample heating, electrophoretic and electrolysis effects during FIA.
Liquid
transfer lines can be reduced in length when an ES source in configured with a
grounded needle, however, even with grounded ES needles, the dead volume due
to
the transfer lines cannot be entirely eliminated. For API MS FIA applications
where
small amounts of sample are available for injection, sample dilution or losses
due to
injector valve, connector and transfer line dead volumes and surfaces may
compromise the limit of detection. Sample handling techniques employed in
conventional FIA apparatus and methods may be the primary limitation in
achieving
lower limits of detection in API MS FIA analysis.
Embodiments of the invention may reduce or eliminate those elements configured
and used in conventional FIA apparatus and methods that reduce API MS FIA
performance. In one preferred embodiment of the invention, the injector needle
and
an ES source has been configured such that the sample solution can be sprayed
directly from the injector needle tip. The injector needle tip is introduced
into the ES
source chamber through a probe that serves as a needle guide, seal, electrical
connection and pneumatic nebulization second needle layer. The injector needle
can
be introduced into an APCI source through a similar probe apparatus serving as
a
needle guide, seal and pneumatic nebulization sprayer second tube layer.
Multiple
injection needles can be configured to spray in a multiplexed manner through
one or
more API probes to increase FIA sample throughput. The injector needle can be
configured as a reusable or disposable tip. The liquid spray flow rate is set
by the
3
CA 02606480 2012-07-09
60412-4221D
auto or manual injector sample injection flow rate. This flow rate can be set
to
optimize MS analysis and sample throughput. Embodiments of the invention may
reduce instrument cost by eliminating the need for an injector valve and
controls,
transfer lines and a separate solvent flow pump in API TOF FIA. Embodiments of
the
invention may also minimize solvent consumption and waste.
Embodiments of the invention may allow increased sample throughput in API MS
FIA
applications by eliminating steps and the time associated with liquid transfer
per
injector needle. In one embodiment of the invention, multiple injector needles
can be
sequentially introduced into one API source probe or multiple injector needles
can be
introduced into an API source through multiple API source probes. T. Wang et.
al.,
Proceedings of the 46th ASMS Conference on Mass Spectrometry and Allied
Topics,
1034, 1998 have reported the configuration and use of multiple injector
needles and
valves to shorten analysis run time and increase sample throughput.
Commercially
available autoinjectors, such as the Gilson Multiprobe 215 liquid handler,
have been
configured with up to eight autoinjector needles dispensing to eight
autoinjector
valves which transfer sample through an additional selector valve to an API
source.
Fluid flow through such a system is provided by a separate liquid flow pump.
The
transfer lines have increased length from multiple injector valves when
compared to
the single injector valve configuration. The increased transfer and dead
volumes
from each injector valve through the transfer lines and the switching valve to
the API
source must be thoroughly flushed between injections. The speed of injections
even
with such a multiple injector valve configuration is still limited to some
extent by the
washing and flushing of the eight injector valves, transfer lines and
switching valve.
In one embodiment of the invention, multiple injector needles can be
configured for
introduction into one or more API probes without the need to add multiple
injector
valves, transfer lines, switching valves or an additional fluid flow pump.
Increases in
sample throughput can be achieved with the invention at a lower cost, when
compared with commercially available systems, without a reduction in
performance
that is unavoidable in API MS FIA apparatus with higher dead volumes. In an
embodiment of the invention internal flushing or cleaning is limited to the
injector
4
CA 02606480 2012-04-03
= 60412-4221D
needle and the attached reservoir and external flushing is limited to the
injector
needle only to avoid cross talk or contamination sample carry over from one
injection
to another. Flushing or cleaning of valves or transfer lines may be eliminated
in FIA
according to some embodiments of the invention.
Summary of the Invention
According to an aspect of the invention, there is provided an apparatus for
producing
ions from chemical species comprising: an ion source operated substantially at
atmospheric pressure, comprising a probe to produce ions from sample bearing
solutions; a needle configured both to withdraw a sample bearing solution from
a vial
and to spray said solution from said probe after insertion therein; and a
means for
delivering said ions into a vacuum region, wherein said needle is a syringe or
said
needle is an injection needle and storage reservoir from an autoinjector.
In another aspect, the invention comprises a reusable or disposable injector
needle
configured in an autoinjector or a manual injector which serves as the means
to
remove a sample solution from a container and transport such solution to an
API
source wherein the injector needle, when introduced into the API source,
serves as
the spray needle to deliver sample directly into the API source chamber. Such
fixed
or disposable injector needle, when introduced into an API source, becomes the
liquid introduction channel or tube in the nebulizer probe of an APCI source,
the
nebulizer apparatus of a pneumatically assisted Electrospray probe or an
Electrospray tip in an unassisted ES ion source probe. Ions produced from
samples
introduced through such sprayers into an API source are subsequently directed
into
vacuum where they are mass to charge analyzed. Ions transported into vacuum
from
such API source apparatus may also be subject to mass to charge selection
and/or
fragmentation in MS/MS or MS/MSn analysis. An API source may be configured
with
multiple direct injection needles and/or probes for introducing samples at an
increased rate into an API source. Autoinjectors may be configured with
multiple
injector needles configured for direct delivery of sample into an API source
through
one or more probes. Such multiple needle autoinjectors may deliver samples in
a
5
CA 02606480 2012-04-03
60412-4221D
sequential or multiplexed manner to such single or multiple direct injection
API source
ports or probes to maximize sample throughput. In one embodiment of the
invention,
a reusable or disposable sampling and spray injection needle may be packed
with
material, such as C18 coated beads, to aid in desalting, sample cleanup or the
separation of sample compounds in solution during the sample pickup, delivery
and
spray steps. Different solvent composition layers can be pulled sequentially
into such
packed sampling and spray needles with attached reservoirs prior to sample
pickup.
The sample can then be sprayed into an API source from such a loaded injection
needle using solvent gradients to aid in sample desalting, additional cleanup
or
sample compound separation during spraying.
Washing or flushing of a packed or open disposable injection needle, according
to an
embodiment of the invention, is not required between injections allowing an
increase
in sample throughput. In one embodiment of the invention, sample solution may
be
drawn up into a packed or open disposable injection needle. The injection
needle is
subsequently introduced into the API source and sample solution is sprayed
from the
injection needle tip with or without a solvent gradient to elute sample from
any packed
material. Alternately, a sample solution can be loaded into a non-disposable
or
reusable needle and the needle is then inserted into and forms a seal with a
packed
disposable injection needle. The packed injection needle is then introduced
into an
API source and the sample solution and any solvent gradient flows from the non-
disposable needle through the packed disposable needle. The resulting solvent
and
sample solution is sprayed from the disposable needle tip into an API source.
The
packing material in the disposable tip serves to desalt or further clean the
sample
solution as well as to provide some sample component separation due to solvent
gradient flow, if desired. Depending on the requirements of a specific
analytical
application, packing material may be replaced by filter media according to the
invention to aid in sample cleanup with a minimum of dead volume.
Some embodiments of the invention may eliminate the need for sample injector
valves or transfer lines into an API source, reducing sample dilution, loss
and
contamination due to sample handing and transfer. When a reusable needle is6
CA 02606480 2012-04-03
60412-4221D
configured in the invention, the needle inner bore and outer surface can be
washed in
between each sample delivery and spraying step to reduce or eliminate,
chemical
noise, cross talk or carry over from one sample to the next. The use of
disposable
needles, configured according to an embodiment of the invention, eliminates
sample
to sample cross talk or contamination without a wash step between sample
injections
into the API source. Faster cycle times or more rapid sample injection
throughput
can be achieved by eliminating wash steps. Alternatively, a wash step can be
run for
one or more reusable injection needles while sample delivery and spraying is
occurring with another injection needle or needles. Some embodiments of the
invention may reduce apparatus costs, sample losses, sample contamination, and
sample handling and minimizes solvent consumption and waste while increasing
sample throughput in flow injection analysis with Atmospheric pressure ion
sources.
A direct injection needle apparatus may be configured with other API inlets in
the
same API source chamber as a means to increase analytical flexibility within
one API
source apparatus. Ions produced from the API source may be analyzed by
apparatus other than MS including but not limited to ion mobility analyzers.
Brief Description of the Figures
Figure us a diagram of a sampling and spray injection needle that is
introduced into
an ES source through an ES probe assembly whose axis is configured at an angle
orthogonal to the axis of the vacuum entrance orifice.
6a
CA 02606480 2007-11-06
. .
Figure 2 is a diagram of the progression of sample loading and spraying with a
reusable injection
needle attached to a syringe reservoir in FIA according to the invention.
Figure 3 includes two diagrams of reusable injector needles inserted into API
probe assemblies
with two different gas seals. The reusable injector needles are configured
with syringes.
Figure 4 is a diagram of a sampling and spray injection needle that is
introduced into an ES
source through an ES probe assembly whose axis is configured at an angle
substantially the same
as the axis to the vacuum entrance orifice.
Figure 5 is a diagram of a sampling and spray injection needle that is
introduced into an APCI
source through an APCI pneumatic nebulizer probe whose axis is configured at
an angle
orthogonal to the axis of the vacuum entrance orifice.
Figure 6 is a diagram of a sampling and spray needle that is introduced into
an APCI source
through an APCI pneumatic nebulizer probe assembly whose axis is configured
substantially the
aligned with the axis of the orifice into vacuum.
Figure 7 is a diagram of a reusable injector needle configured with a syringe
wherein the injector
needle is filled with material to remove contaminants and separate sample
components during
FIA. Operation with solvent gradients in the syringe is illustrated.
Figure 8 is a diagram of removable injector needles configured with and
without conductive
elements attached and configured with and without filled tips.
Figure 9 is a diagram of the progression of sample loading and spraying with
removable injector
needles into an API source through an API source probe assembly according to
the invention.
7
CA 02606480 2007-11-06
Figure 10 is a diagram syringe and pipette devices attached to different
configurations of
removable injector needles.
Figure 11 includes a Total Ion Chromatogram and five representative mass
spectra acquired in
accordance with the invention by repeated injections of different samples
using an autoinjector
configured with a sampling and spray injector needle that is introduced into
an ES source
interfaced to a mass spectrometer.
Detailed Description of the Invention and the Preferred Embodiments
The apparatus and methods used for flow injection analysis typically include
an injector valve,
transfer lines, fluid line connections, an addition fluid delivery pump, a
sprayer probe with
internal volume for ES and APCI sources and a switching valve when multiple
injector valves
are configured. Each of these elements adds to the dead volume or mixing
volume encountered
when delivering a sample solution into an API source in flow injection
analysis. Added dead or
mixing volumes can cause sample dilution due to diffusion or mixing of the
sample with solvent
during sample solution flow into an API source. Sample can adsorb to the walls
of the valve,
transfer line and probe transfer tube. Dilution of sample and loss of sample
to the inner surfaces
of the flow pathway results in reduced ion signal and analytical sensitivity.
As liquid flow rates
are reduced the sample solution spends more time in the transfer dead volumes.
Increased
transfer time results in increased sample dilution and loss to transfer
surfaces. Adsorbed sample
can bleed off valve, transfer line, connector and probe surfaces in subsequent
injections,
contributing chemical noise and interference peaks to acquired mass spectrum.
Chemical noise
or interference peaks due to contamination from prior injected sample can
reduce the accuracy of
quantitative measurements and compromise the limits of detection. Increased
valve, connector,
transfer line and probe surfaces require increased solvent flushing or
cleaning time in between
sample injections to minimize subsequent sample carry over or bleed. This
required flushing
increases solvent consumption and increases the time between injections.
Increased cleaning
time between injections decreases the number of samples that can be injected
in a given time
period, reducing sample throughput.
8
CA 02606480 2007-11-06
The invention allows rapid flow injection analysis over a wide range of liquid
flow rates while
minimizing solvent consumption and waste and eliminating all injector valves,
fluid line
connectors, transfer lines, probe liquid transfer tubes and additional liquid
flow delivery system
apparatus. Sample dilution or adsorption losses and solvent consumption are
minimized with the
invention and apparatus costs are reduced by elimination of components. Sample
carry over or
cross talk can be minimized with washing of reusable injection needles or
eliminated with
disposable or removable injection needles configured according to the
invention. The invention
comprises the configuration and use of an injector needle to draw up sample
solution from a
sample vial or container into the injector needle and attached solvent
reservoir, transfer of the
sample solution to an API source probe, passing of the injector needle through
the API source
probe channel and spraying of the sample solution from the tip of the injector
needle into an API
source. Ions are produced from the sprayed solution in the API source and are
directed into
vacuum where they are mass to charge analyzed. Alternatively, the ion
population produced in
the API source can be mass selected and fragmented in MS/MS or MS/MS"
analysis. API
sources may include but are not limited to ES, APCI or Inductively Coupled
Plasma (ICP) ion
sources. Mass to charge analysis can be conducted by any type of mass
spectrometer including
but not limited to quadrupoles, triple quadrupoles, Time-Of-Flight, three
dimensional ion traps,
Fourier Transform Mass Spectrometers (FT'MS) or magnetic sector mass
spectrometers.
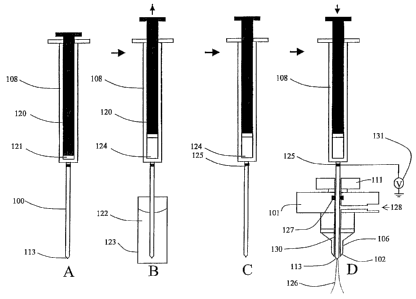
One embodiment of the invention is diagrammed in Figures 1 and 2 in which
reusable injection
needle 100 connected to syringe solvent reservoir 108 is configured to serve
as an Electrospray
needle in ES source 105. Figure 2 is a diagram of the uptake of a sample
solution from a sample
vial or container, transfer of said sample solution to an ES source probe and
the spraying of such
sample solution into the ES source chamber using the same injector needle with
attached solvent
reservoir. Referring to Figure 2, reusable injector needle 100 and attached
syringe 108 are used
to draw up sample solution 122 from sample vial or container 123 in diagrams A
and B. In
diagram A, syringe plunger 120 with plunger seal 121 is located in the full
forward position in
syringe 108. The inside bore of syringe 108 and injector needle 100 have been
flushed with
solvent to remove any previously injected sample. The outside surface of
injector needle 100 has
also been rinsed with solvent to remove any previously injected sample. With
plunger 120
moved to the full forward position, cleaned injector needle 100 with tip 113
is inserted into
9
= CA 02606480 2007-11-06
sample solution 122 in sample vial or container 123. Plunger 120 is retracted
or moved in the
reverse direction to pull sample solution from sample vial 123 through the
bore of injector needle
100 and into syringe volume 124 as diagrammed in Figure 2B. Syringe 108 loaded
with sample
solution is moved from the sample vial position as shown in diagram C to a
position where
injection needle 100 is in-line with bore 130 of ES probe assembly 101 as
shown in Figure 1.
Injector needle 100 is then moved through bore 130 of ES probe assembly 101
passing through
entrance port 111, seal 127, tube 106 and tip 102. Electrical connection with
electrically
conductive injector needle 100 and ground or voltage supply 131 is made
through contact 125
attached to injector needle 100.
Kilovolt electrical potentials are applied to cylindrical lens 115, nosepiece
112 and the capillary
entrance lens 114 in ES source 105 when the injection needle and ES probe
assembly 101 are
operated at ground or zero electrical potential. Nebulization gas 128 is
introduced through gas
connection 107 and flows through the annulus described by the inner bore of
tube 106 and the
outer surface of injector needle 100 exiting at tip 102. Plunger 120 is moved
forward causing
sample solution to exit the syringe at injector needle tip 113. The sample
solution is
Electrosprayed with pneumatic nebulization assist from tip 113 due to the
local electrical field
gradient at tip 113 and the exiting nebulizing gas 128. Spray 126 comprises
charged liquid
droplets that evaporate as they move through ES chamber 103. A portion of the
evaporating
charged liquid droplets are directed by the electric field to move against
counter current drying
gas flow 110 toward capillary entrance orifice 109. Ions are released from
such evaporating
charged droplets and are directed into vacuum through capillary 104. Ions
entering vacuum are
subsequently mass to charge analyzed with a mass spectrometer. Details of the
counter current
drying gas 110 and capillary 104 configuration and function in API sources in
described in U. S.
Patent Numbers 4,531,056 and 4,542,293 respectively. The liquid flow rate of
spray 126 is
controlled by the forward movement rate of syringe plunger 120. The filling,
emptying and
positioning of syringe 108 with injector needle 100 may be manually controlled
or mechanically
controlled as part of a programmable autoinjector or automated sample handling
system to
achieve API MS FIA.
10
= CA 02606480 2007-11-06
Commercially available autoinjectors such as the Leap HTS PAL system are
configured with
syringes for the uptake, movement and injection of samples into injector
valves. The syringes
and attached injector needles are typically mounted to a programmable x-y-z
position translator
arm. Under pre-programmed control, sample solution is removed from a selected
sample vial or
vials, the loaded injector needle is moved to a position directly in-line with
the bore of an ES
probe assembly and the injector needle is introduced through the bore of the
ES probe assembly
in an ES source as diagrammed in Figures 1 and 2. Some commercially available
autoinjectors
are configured with multiple syringes. FIA sample throughput can be increased
according to the
invention when such multiple syringe autoinjectors are used. Such a multiple
syringe
autoinjector configuration can be operated whereby one syringe is spraying
sample solution into
ES source 105 while a second syringe is being flushed and cleaned prior to
loading the next
sample solution to be sprayed into the second injector needle and syringe. The
syringes can be
partially or completely filled with sample solution for each FIA run. The fill
and spray liquid
flow rates are determined by the syringe size used and the plunger movement
rate as
programmed in the autoinjector. Commercially available autoinjectors are
configured to flush the
internal bore of the syringe and injection needle and wash the injection
needle external surface.
In an alternative embodiment to the invention, injector needle 100 is
connected to a fluid transfer
tube that may also serve as a sample solution reservoir instead of syringe
108. A fluid pump is
connected to the opposite end of the fluid transfer tube controlling the
sample solution flow into
and out of injector needle 100. In such an embodiment, the fluid pump may or
may not translate
with the injector needle. Commercially available autoinjectors like the
Hewlett Packard 1100 are
configured with an injector needle connected to a tube made of peek, stainless
steel or other
material and the sample solution is loaded in the reverse flow direction
through the injector
needle and into such tube prior to injection of the sample solution in the
forward flow direction
through the injector needle tip. The sample solution volume removed from the
sample vials and
flow rate of the sample solution loading and spraying can be programmably
controlled in
commercially available autoinjectors configured with transfer tubing connected
to the injector
needle. Other commercially available autoinjectors are configured with a
variations of injector
needle and attached solution reservoir designs. Autoinjectors can be custom
designed or
modified to accommodate the translation and orientation of loaded injector
needles into ES probe
11
, = CA 02606480 2007-11-06
assembly bore 130. Such autoinjectors require an injector needle with
sufficient rigidity to
penetrate the septa of a sample vial and to push past seal 127 in ES probe
assembly 101. Sample
solution 122 is removed from sample vial 123 with reverse flow through
injector needle 100 and
held in solution reservoir 124. Loaded injector needle 100 is moved from
sample vial 123,
translated to ES source probe 101 and slid through bore 130 with tip 113
location programmably
positioned inside, even with or slightly past tip 102 depending on the
application and spraying
conditions desired. Sample solution is then sprayed from tip 113 with or
without pneumatic
nebulization assist into ES chamber 103. A portion of the ions that are
produced in ES source
105 are directed into vacuum where they are mass to charge analyzed.
Variations of autoinjector
designs may be configured which include the apparatus and methods of the
invention. In the
embodiment of the invention diagrammed in Figures 1 and 2, injector needle 100
and syringe
108 are configured as the only sample solution transport and delivery means.
In this embodiment
of the invention, sample cross contamination from one FIA sample to the next
is restricted to
injector needle 100 and syringe or storage reservoir 108. Consequently,
internal flushing of
injector needle 100 and syringe or storage reservoir 108 and external flushing
of injector needle
100 minimizes or eliminates cross sample contamination in FIA. Injection
needle and solution
reservoir cleaning or flushing apparatus and programmable methods are
available on most
commercial autoinjectors.
As shown in the embodiment of the invention diagrammed in Figure 1, the
centerline of ES
probe assembly 101 is oriented orthogonal to the bore of capillary 104. The
position of probe tip
102 is located a distance ri from the capillary centerline and a distance Z1
from the face of
nosepiece 112. Injector needle 100 and ES probe assembly 101 can be operated
at ground
potential during Electrospraying when an ES source is configured with
dielectric capillary tube
104. The potential of an ion being swept through the bore of dielectric
capillary tube 104 into
vacuum is described in U. S. Patent Number 4,542,293. In alternative
embodiments of the
invention, the dielectric capillary can be replaced with a nozzle or
conductive (metal) capillary
and ES sources can be configured with or without heated counter current drying
gas 110. When
conductive capillaries or orifices are configured in ES source 105, injector
needle 100 and ES
probe tip 102 will be operated at high electrical potential relative to
counterelectrodes 115,112
and 114. Power supply 131 can be connected to injector needle 100 at contact
125 and to ES
12
. ' . = CA 02606480 2007-11-06
probe assembly 101 to apply the required high electrical potential, typically
3,000 to 6,000 volts
during Electrospray operation. The Electrospray chamber 103 may be configured
shorter in
length and smaller in diameter due to the reduced flow rate range and total
solution volume
sprayed in FIA applications.
ES probe assembly 101 may be integrated into the walls of Electrospray chamber
103 or may be
configured to penetrate through the wall of ES chamber 103. ES probe assembly
101 may be
configured with alternative seals from that shown as seal 127 in Figure 2D.
Seal 127 in Figure 2
may be replaced by ferrule 132 and seal 133 of ES probe assembly 135 or by
septum seal 134 in
ES probe assembly 136 as diagrammed in Figure 3. Seals 127, 132 and 136
prevent nebulizer gas
flow from exiting through entrance port 111 during Electrospraying with
pneumatic nebulization
assist. 0-ring seal 127 may not close when injector needle 100 is removed from
ES probe
assembly 101. If nebulizing gas flow remains on when injector needle 100 has
been removed,
gas can flow out port 111 as well as through tip 102. Flushing nebulizer gas
flow through ES
probe assembly 101 during the time period between each sample solution
spraying may be
desirable to evaporate any solvent deposited in bore 130 when injector needle
100 is removed.
Alternatively, nebulizing gas flow and even ES electrical potentials may be
turned off when
injector needle 100 is removed from ES probe assembly 101. Nebulizing gas and
ES electrical
potentials can be turned on when injector needle 100 is inserted into ES probe
assembly 101.
Commercial autoinjectors can be programmed to delay the dispensing of liquid
from injector
needle 100 for a period of time after injector needle 100 is inserted into ES
probe assembly 101.
This programmed delay allows the MS data acquisition system time to start, the
nebulizing gas
flow to stabilize, and the ES lens voltages to stabilize before the sample
solution is
Electrosprayed. Autoinjectors can also be programmed to dispense the sample at
variable flow
rates to optimize FIA performance. Ferrule 132 with seal 133 captured in ES
probe assembly 135
of Figure 3 may be configured to provide adequate gas sealing while imposing
minimum
resistance when inserting or removing injector needle 100. Septum seal 134
mounted in ES
probe assembly 136 reseals when injector needle 100 is removed. Additional
force may be
required to reinsert injector needle 100 into bore 138 of ES probe assembly
136 when compared
with the force required to insert injector needle 100 through seals 127 and
133. With alternative
seals 133 or 136, nebulizer gas and ES electrical potentials can remain on at
all times during FIA
13
= ' CA 02606480 2007-11-06
injection or they may be turned on only when injection needle 100 is inserted
into an ES probe
assembly. Turning off nebulizing gas flow 128 reduces gas consumption between
each sample
FIA.
Bore 130 of ES probe assembly 101 as diagrammed in Figure 1 is positioned
orthogonal to the
bore of capillary 104. Commercially available ES sources include off axis and
on axis ES probes
with the angles of ES probe assembly centerlines ranging from zero to ninety
degrees. U. S.
Patent Number 5,495,108 even describes ES probe positions with angles greater
than 90 degrees.
Figure 4 shows a diagram of an embodiment of the invention in which the bore
of ES probe
assembly 301 is positioned on the same axis as the bore of capillary 304. The
distance from ES
probe tip 312 to nosepiece 309 is set at Z2 in Figure 4. The value for Z2
ranges typically from 0.5
to 2 cm for ES flow rates ranging from 0.2 to 200 ul/min. ES source chamber
305 is configured
with heated drying gas flow 310. ES probe assembly 301 is configured with
inlet port 311,
nebulizer gas port 307 and exit end 302 with tip 312. Injector needle 300 with
attached reservoir
308 is inserted into ES probe assembly 305 as described in Figures 1 and 2 for
the orthogonal ES
probe embodiment. Higher sensitivity can be achieved for lower solution flow
rates using on-
axis ES probes compared with off axis probes. ES probe assemblies 101 and/or
301 can be
oriented at any angle relative to the capillary bore axis. Multiple ES probe
assemblies
configured according to the invention can be mounted in the same ES chamber.
ES probes
configured according to the invention can be mounted with standard ES probes
in the same ES
source. Multiple ES and APCI probe assemblies configured in an API source are
described in U.
S. Patent No. 6,207,954. Autoinjectors can be configured to position injector
needles in the
orientation required for insertion into ES probe assemblies configured
according to the invention
that are oriented at various angles in an ES chamber.
An alternative embodiment to the invention is diagrammed in Figure 5 wherein
injector needle
200 attached to reservoir 208 is configured to deliver sample solution through
APCI nebulizer
probe assembly 201. APCI source assembly 218 comprises two APCI inlet probe
assemblies 201
and 213, droplet separator ball 212 in droplet transfer assembly 203,
vaporizer heater 214,
corona discharge needle 215 nosepiece 216, capillary 204, heated counter
current gas 210 and
cylindrical lens 217 in APCI chamber 205. Droplet separator ball 212 may or
may not be
14
= CA 02606480 2007-11-06
included depending on the liquid flow rates being sprayed from API probes 201
and/or 213. Bore
220 of APCI nebulizer probe assembly 201 is oriented orthogonal to the APCI
source centerline
defined by the extension of the capillary orifice 209 centerline. The
centerline of a second APCI
nebulizer probe 213 which connects to a liquid transfer line, is positioned
along the APCI source
centerline. Sample solutions delivered through injector needle 200 and APCI
probe assembly
213 are sprayed into chamber 222 before being swept through vaporizer 214.
Sample solutions
may be sprayed simultaneously or sequentially from APCI nebulizer probe
assemblies 201 and
213. Sprayed liquid droplets from APCI probe assembly 201 or 213 evaporate as
they pass
through vaporizer heater 214. Kilovolt electrical potentials are applied to
corona discharge
needle 215 to form a corona discharge near the exit end of vaporizer heater
214. Sample bearing
vapor is ionized as it passed through the corona discharge region near the tip
of needle 215. A
portion of the ions formed in the corona discharge region are directed against
counter current gas
flow 210 toward capillary orifice 209 by the electric fields formed from the
electrical potentials
applied to cylindrical lens 217 and needle 215, nosepiece 216 and capillary
entrance lens 223.
Ions are swept into vacuum through the bore of capillary 204 where they are
mass to charge
analyzed.
In an alternative embodiment of the invention, Figure 6 shows a diagram of
APCI nebulizer
probe 401 configured similar to APCI probe 201. Injector needle 400 attached
to solution
reservoir 408 can be slid through adjustable needle port 411(211 in Fig.5),
seal 413 and bore
412 of APCI nebulizer probe assembly 401. Nebulizer gas flow enters port 407
(207 in Fig. 5)
and flows between the inner diameter of bore 412 and the outer diameter of
inserted injector
needle 400. The tip of the inserted injector needle 400 is positioned relative
to exit orifice 202
and 402 of APCI probe assemblies 201 or 401 respectively to optimize the
nebulization
efficiency of the liquid spray. The tip of injector needle 400 may be
positioned slightly inside,
even with or extended beyond exit orifice 202 or 402 depending on liquid and
nebulizer gas flow
rates. Sample solution sprayed from injector needle 400 passes through the
droplet transfer
region 403, through vaporizer heater 414 and into APCI chamber 405. Ions
formed from the
vaporized sample solution in the corona discharge region are directed against
counter current gas
flow 410 into orifice 409 of capillary 404 by the electric fields in APCI
source chamber 405.
15
= CA 02606480 2007-11-06
Ions entering capillary orifice 409 are swept into vacuum through the bore to
capillary 404 where
they are mass to charge analyzed with a mass spectrometer.
The sequence of sample solution pick up and transfer to an API probe shown in
Figure 2 can be
applied to injector needles 200 and 400 and APCI nebulizer probe assemblies
201 and 401. As in
the Electrospray ion source embodiment, an APCI source can be operated in flow
injection
analysis mode using apparatus and methods according to the invention. Injector
valves, transfer
tubing, fluid line connections, probe liquid transfer tubing and additional
fluid flow pumps can
be eliminated when an injector needle and sample solution reservoir is
configured and operated
according to the invention in APCI MS FIA. Alternative seals may be configured
in APCI probe
assemblies 201 or 401 as diagrammed in Figure 3 for ES probe assemblies 135
and 136. Bore
220 of APCI nebulizer probe assembly 201 may be oriented at any angle relative
to the APCI
source centerline. Multiple APCI nebulizer probes configured according to the
invention may be
mounted to droplet transfer assembly 203. As in the ES probe assembly
embodiment of the
invention, removable injector needle 200 or 400 may be attached to a syringe,
a solution
reservoir or a liquid transfer tube. Such a liquid transfer tube may be
connected to a fluid flow
pump to deliver sample solution through injector needle 200. Sample solution
may be introduced
in this manner to deliver a calibration solution into the ES or APCI sources
sequentially or
simultaneously with a second sample solution spray as is described in U. S.
Patent No.
6,207,954. The movement and positioning of injector needle 200 and attached
solution reservoir
208 may be controlled manually or using a programmable mechanical apparatus
such as an
autoinjector. Autoinjectors can be configured to insert injector needle 208
into bore 220 of APCI
probe assembly 201 at any angle required by the APCI source probe geometry.
The total sample
solution volume loaded and the liquid flow rates passing through
injector needle 200 can be programmably controlled by an autoinjector
apparatus. Multiple
solution samples can be run sequentially in an ES and/or APCI source delivered
from an
autoinjector containing multiple sample vials from which sample solution can
be loaded into an
injector needle configured according to the invention. It will be apparent to
one skilled in the art
that variations of embodiments of the invention may include but are not
limited to combinations
of:
1. The ES or APCI probe assembly centerline to vacuum orifice centerline
angles (t) may range from 0 to 180';
16
= CA 02606480 2007-11-06
2. ES or APCI probe assembly tip locations(rlizi,) wherein ri may equal any
distance, and ri may equal any distance within an ES chamber;
3. An API source capillary or orifice mounted in a position with its
centerline
ranging from horizontal to vertical;
4. The ES or APCI probe assembly can be combined with other ES probe types
or APCI probes in the same API source as described in U. S. Patent No
6,207,954;
5. The ES probe assembly may include pneumatic assisted Electrospray
nebulization, ultrasonic assisted Electrospray nebulization or other nebulizer
type or may be configured for unassisted Electrospray operation;
6. Electrospray probe may include two, three or more layer construction for
use with or without additional liquid layer flow;
7. Multiple injector needles directed to one ES or APCI probe assembly or
multiple injector needles directed to multiple ES or APCI probe assemblies;
and
8. Multiple ES or APCI probe assemblies configured according to the invention
and mounted in one API source apparatus.
An alternative embodiment of the reusable injector needle is shown in Figure
7, wherein the
internal bore of injector needle 420 is filled with packing material 421.
Packing material 421
may comprise but is not limited to coated beads similar to those used in
liquid chromatography
columns or filter material. Frits 422 and 423 are configured at each end of
the bore of injector
needle 420 to retain packing material 421 during loading and delivery of
sample solution through
injector needle 420. Figure 7A shows an external view of injector needle 420
attached to syringe
424 shown in cross section with plunger 425 and plunger seal 426 located in
the full forward or
empty position. Figure 7B is a diagram of the cross section of packed injector
needle 420 and
syringe 424 prior to loading solvent and sample solution. The inclusion of
packing material in
injector needle 420 allows desalting and other sample cleanup as well as
separation of
components in the sample solution during API MS FIA. As sample solution is
loaded from the
17
. , .' CA 02606480 2007-11-06
sample vial, it passes through the packing material in injector needle 420. As
an example, when
FIA of peptides is performed, the packing material may be selected to be C18
coated beads. This
C18 packing material is similar to the media packed in high pressure liquid
chromatography
columns that are used to conduct gradient separations of peptides. A partial
sample component
gradient separation can be achieved in FIA when the sample solution is
delivered to an API
source in through the packed injector needle 420 connected to a syringe loaded
with a solvent
gradient as shown in Figure 7C.
Referring to Figure 7C, syringe 424 with injector needle 420 is loaded with a
series of solutions
of different compositions 428 through 433 from sequentially from different
containers to form a
solvent gradient along the length of syringe reservoir 436. For example the
composition of
solution volume 428 may be 10% water and 90% acetonitrile with 0.05% TFA,
solution volume
429, 30% water and 70% acetonitrile with 0.05 % TFA to solution volume 433
which may be
90% water and 10% acetonitrile with 0.05% TFA. A solvent gradient is formed in
syringe
reservoir 436 from loading partial syringe volumes consecutively from a series
of vials
containing solution compositions ranging, in this example from 10% to 90%
acetonitrile with
0.05% TFA. To divide the solvent gradient from the sample solution, air bubble
volume 434 can
be drawn into syringe 424 before loading sample solution volume 435. The
sample solution
containing a high percentage of water passes through the C18 media packed in
the bore of
injector needle 420 during loading. Salts and other contaminants pass through
injector needle
420 into internal volume 436 of syringe 424 during loading of the sample
solution while the
peptide sample components of interest adsorb or stick to available sites on
the C18 media in
injector needle 420. When syringe 424 is loaded, it is moved and aligned with
the bore of an API
probe assembly and the injector needle is introduced through the API probe
bore according to the
invention as diagrammed in Figures 1 and 2 above. As syringe plunger 425 is
moved forward,
sample solution solvents, salts and contaminants contained in volume 435 are
sprayed into the
API source chamber while the peptide sample components remain adsorbed to
packing material
421. Air bubble 434 and solvent gradient volume 433 through 428 pass through
injector needle
420 as syringe 424 is emptied during ES or APCI MS FIA. As solvent volumes 433
through 428
pass through injector needle media 421, the adsorbed peptides will release
from the C18 packing
material with increasing organic content of the solvent solution. Some peptide
component
18
CA 02606480 2007-11-06
separation may occur as syringe 424 is emptied because different peptides may
release from the
C18 packing material at different organic solvent concentrations. Peptides
spraying from packed
injector needle 420 into an API source may be separated from salts or other
contaminants
contained in the original sample solution using this method. Some degree of
chromatographic
separation may also be achieved using this method during API MS flow injection
analysis,
improving the quality of acquired MS data. Flushing or cleaning between sample
runs is limited
to flushing packed injector needle 420 and the inner bore of syringe 424 and
washing the outside
surface of injector needle 420.
In alternative embodiments of the invention, a fluid transfer line or other
solution reservoir may
replace syringe 424 in Figure 7. The loading, emptying and positioning of
packed injector needle
420 with syringe 424 may be controlled manually or mechanically with
programmable control.
Syringe 420 configured with packed injector needle 420 may be mounted in an
autoinjector
which also contains several sample vials required to conduct the method
described above.
Packing material may extend into a solution reservoir in alternative
embodiments of the
invention. U. S. Patent Number 5,572,023 describes the filling of fixed
position Electrospray
needles with chromatography packing material. In the apparatus described in U.
S. Patent
Number 5,572,023, the packed Electrospray needle is connected to sample
injector valves,
transfer lines and multiple solvent pumps for conducting sample desalting of
gradient separations
in a single liquid flow direction. Sample cleanup and chromatographic
separation in FIA can be
achieved with the apparatus configured according to the invention without
injector valves, liquid
transfer lines or additional liquid flow pumps. According to the invention,
solution flow moves
through the injector needle in the reverse direction during loading and in the
forward direction
during spraying. The loading of solvents and/or sample solution through the
injector needle
packing material may be aided by pressurizing the solution vials or
containers.
The sample solution can alternatively be loaded without including bubble 434
if it is desirable to
have a continuous liquid column when loading or spraying. A continuous liquid
column provides
increased tensile strength when pulling sample solution from a vial through
packing material.
When desalting is desired without chromatographic separation, a two part
solvent front may be
preloaded in syringe 424 instead of a number of different organic
concentrations. In a stepwise
19
= = CA 02606480 2007-11-06
manner, the first high percentage aqueous solvent volume will flush out salts
and other
contaminants through injector needle 420 while spraying and the second high
percentage organic
solvent volume passing through packing material 421 will release the adsorbed
peptide
components simultaneously during API source spraying. Injector needle 420 may
be packed with
filter media instead of chromatography packing material to remove contaminants
from
the sample solution prior to sample injection into the API source.
In an alternative embodiment of the invention, the reusable injection needle
is replaced by a
removable or disposable injection needle. The removable injection needle may
be comprised of a
dielectric material such as molded plastic, a combination of dielectric and
conductive material or
entirely of conductive material. Figure 8 is a diagram of four embodiments of
removable or
disposable injector needles configured according to the invention. Removable
injector needle
444 is fabricated from dielectric material such as molded polyethylene.
Removable injector
needle 444 comprises the narrower needle portion 445 and the larger diameter
upper portion 446.
A portion of the removable injector needle inner volume may be filled with
media such as filter
or chromatography packing material. Such packing material can be used to
remove contaminants
from the sample solution or separate sample components as described for the
reusable needle
embodiment. Removable injector needle 440 in Figure 8 is shown with packing
material 442
filling the narrower needle portion of removable injector needle 440. In the
embodiment shown,
packing material is prevented from moving out of the bore of removable
injector needle 440 by
frits 441 an 443. Dielectric removable injector needles 440 and 444 may be
used to spray
samples into APCI sources where the formation of charged liquid droplets is
not required. A
conductive tip is preferred for Electrospraying from a removable injector
needle. Two alternative
embodiments of removable injector needles 448 and 454 with conductive elements
configured in
each tip are diagrammed in Figure 8. In one embodiment, conductive strip 452
beginning at tip
450 and extending to the opposite opening end 451 is molded into or attached
to the inside
surface of removable injector needle 448. Conductive strip 452 provides
electrical contact with
the sample solution at tip 450 and a contact point at end 451 during
Electrospraying. An
alternative means to provide an electrical contact with the Electrospraying
solution is shown with
removable injection needle 454. Conductive material 455 is attached to the
external surface of
removable injection needle 454 surrounding tip 456. Conductive material 455
serves to provide
20
= CA 02606480 2007-11-06
electrical contact between the solution spraying from tip 456 with an
electrical conductor
external to removable injector needle 454 during Electrospray operation.
Figure 9 diagrams embodiments of the invention wherein removable injection
needles are used
to load sample solution and conduct API MS flow injection analysis. Referring
to Figure 9,
pipette device 470 is moved into position and inserted into removable injector
needle 474 in set
473. Contact between the pipette device taper end 497 and removable injector
needle 474 at
point 475 provides a seal and sufficient friction to allow temporary
attachment of removable
injector needle 474 to taper end 497 of pipette device 470. Plunger 471 of
pipette device 470 is
located in its forward position in Figure 9A when picking up removable
injector needle 474.
Channel 472 in pipette device 470 connects the plunger chamber volume with the
internal
volume of the attached injector needle 474. Removable injector needle 474,
configured with
electrical conductor 476 surrounding tip 498, is moved from set 473 to vial
480 containing
sample solution 479 as shown in Figure 9B. Plunger 471 is moved in the reverse
direction to load
sample solution 478 into removable injection needle 474 through the opening at
tip 498. Volume
477 created by retracting plunger 471 determines the volume of sample solution
loaded into
removable injector needle 474. The desired sample solution volume 481 is
loaded into removable
injector tip 474, and if necessary for greater volume, into plunger volume
477. Pipette device 470
with loaded attached injector needle 474 are moved from container 480 to a
position where tip
498 can be inserted into to the bore of API probe assembly 482 as shown in
Figure 9C and D.
When removable injector needle 474 is fully inserted into API probe assembly
482, the outer
diameter of removable injector needle 474 forms a gas seal with probe seal
483. Electrical
contact to ground or power supply 494 is made to conductive material 476
through contact 484
and connection 488. Nebulizer gas flow 487 enters API probe assembly 482
through port 468
and exits at nebulizer gas flow 485 at probe tip 499 flowing through the
annulus between
conductive material 476 and the bore of API probe 482. The forward movement of
plunger 471
pushes sample solution 481 out through tip 498 creating ES or APCI spray 486.
An alternative embodiment of API probe assembly 489 is shown in Figure 9E. A
gas seal is
formed by contact point 491 between the API probe assembly body and removable
injector
needle 474. Electrical contact is made directly between conductive material
476 and the
21
CA 02606480 2007-11-06
conductive body of API probe assembly 489 due to the taper of tip 489 mating
with API probe
assembly inner bore 469. API probe assembly 489 may be grounded or connected
to voltage
supply 493 for ES sources where it is required that the ES probe be operated
at kilovolt
potentials. Nebulizer gas 490 enters API probe assembly 489 through port 466
and exits at API
probe tip 465 where nebulizer gas flow 495 aids in forming spray 496 from
sample solution in
ES and APCI source operation. Typically, tip 498 of removable injection needle
474 has a small
diameter and consequently is not sufficiently rigid to push through a seal or
a septum. Entrance
end 467 of API probe assembly 489 is tapered to guide the thin filament tube
end 498
through inner bore 469. A manual injector or autoinjector may be configured
with a separate
rigid tube that is use to pierce a septum sealing a sample solution vial. The
removable injection
needle can then be passed through the rigid tube passing through the septum to
load sample
solution from the sample vial. After flow injection analysis is completed,
pipette device 470 with
attached injector needle 474 is withdrawn from API probe assembly 489 or 482.
Removable
injector needle 474 is detached from pipette device 470, a new removable
injector needle is
attached to pipette device 470 and the FIA cycle as diagrammed in Figure 9 is
repeated. No wash
cycle is required when removable injector needles are used in flow injection
analysis resulting in
decreased cycle time per sample. Total sample throughput can be increased when
using
removable injector needles in FIA without any sample carryover or
contamination from sample
to sample.
Filling, emptying and positioning of pipette device 470 can be controlled
manually or using
programmable mechanical devices such as autoinjectors or automatic pipette
controllers.
Alternative embodiments of the invention can be configured to control the
filling, emptying and
positioning of removable injection needles as is diagrammed in Figure 10.
Referring to Figure
10A, syringe 540 with attached needle 553 when inserted into removable
injector needle 544
forms a seal a point 547 and electrical contact with inner conducting strip
551. Removable
injector needle 544 is shown with tip end 555 filled with packing material 548
contained by frits
549 and 550. Electrical contact between ground or voltage supply 557 and tip
556 is made
through conducting strip 545, needle 553 and electrical contact 552 when tip
556 is operated as
an Electrospray tip. Reverse and forward movement of plunger 541 fills or
empties reservoir
542 with sample solvent or sample solution respectively through removable
injector needle 544
22
= == CA 02606480 2007-11-06
during API MS FIA. An alternative embodiment of the invention is shown in
Figure 10B.
Removable injector needle 560 is configured with conductive material 565
coating the outer
surface of packed tip 562. Electrical connection with tip 567 to ground or
voltage supply 563 is
made through contact between conductive material 565 and an API probe assembly
as was
diagrammed in Figure 9. No direct electrical connection is made between tip
567 and syringe
needle 568 or syringe 561 in the embodiment diagrammed in Figure 10B
eliminating any
electrical connections between syringe 561 and a voltage supply or ground
potential. An
alternative embodiment of the invention is diagrammed in Figure 10C wherein
removable
injector needle 570 with electrically conductive strip 571 makes an electrical
connection to
ground or voltage supply 575 through electrical contact with the conductive
tapered portion 573
of pipette device 574. Variations and/or combinations of embodiments shown may
be configured
and used according to the invention to perform flow injection analysis with
atmospheric pressure
ion sources interfaced to mass spectrometers, ion mobility analyzers or other
analytical devices.
Figure 11 shows the results acquired from ES MS flow injection analysis using
the embodiment
of the invention diagrammed in Figures 1 and 2. Figure 11 includes a total ion
current (TIC)
trace in curve 500 and five mass spectra 501-505 acquired by spraying sample
solution into an
Electrospray ion source configured as diagrammed in Figure 1. A 100 ul syringe
mounted in a
Leap HTS PAL autoinjector was used to load and deliver sample solution to the
ES probe
assembly. The autoinjector flow rate when spraying the sample solution from
the injector needle
attached to the syringe was set at 200 ul/min for the data acquired in Figure
11. Three injections
each were made of five different samples as are shown in TIC trace 500 of
Figure 11. The first
three TIC peaks 506 are of Tri-Tyrosine injected at a concentration of 50
pmole/pL. Mass
spectrum 501 was acquired under one of the TIC peaks of 506. Mass spectrum 501
shows the
singly charged protonated molecular ion peak 511 of ,Tri-Tyrosine peak which
has a measured
mass to charge value of 508. Injections of 10 pmole/ul sample solutions of
protein cytochrome-C
form the second three TIC peaks 507 corresponding to injections four through
six. Mass
spectrum 502 of cytochrome-C was acquired under one of the TIC peaks in 507.
Mass spectrum
502 shows peaks 512, 513, 514, 515, and 516 of multiply charged protonated
molecular ions of
cytochrome-C corresponding to mass to charge values 688, 728, 773, 825, and
884 respectfully.
The third set of TIC peaks 508 were acquired by injecting sample solution
containing 1 pmole/ul
23
. ' = CA 02606480 2007-11-06
of gramicidin-S. Mass spectrum 503 acquired under one of these injection peaks
503, shows the
doubly charged protonated molecular ion peak 517 of gramicidin-S. The fourth
set of three TIC
peaks 509 were acquired by injecting a sample solution containing 11 pmole/ul
of Lincomycin.
Mass spectrum 504 acquired under one of the TIC peaks 509, shows the singly
charged
protonated molecular ion peak 518 of Lincomycin with a measured mass to charge
of 407. The
last three TIC peaks 510 were acquired by sequentially injecting sample
solutions containing 82
pmole/ul of reserpine. Mass spectrum 505 was acquired under one of the TIC
peaks 510 and
shows the singly charged protonated molecular ion peak 519 of reserpine which
has a measured
mass to charge value of 609. The data shown in Figure 11 is an example of ES
MS FIA acquired
according to the invention with no injector valve, transfer lines, probe
transfer volumes, tubing
connectors or additional fluid flow pumps. No cross talk or sample carryover
is observed in TIC
trace 500 or acquired mass spectra 501-505.
Having described this invention with regard to specific embodiments, it is to
be understood that
the description is not meant as a limitation since further modifications and
variations may be
apparent or may suggest themselves. It is intended that the present
application cover all such
modifications and variations, including those as fall within the scope of the
appended claims.
References Cited:
The following references are referred to above:
U. S. Patent Documents:
5,495,108 Feb. 27,1996, Apffel, James; Werlich, Mark; Bertach,
James.
6,207,954 Sept. 12,1997, Adrien Jr., Bruce A, Sansone, Michael A,
Whitehouse, Craig M.
4,542,293 Sept. 17,1985, Fenn,John B., Yamashita, Masamichi,
Whitehouse, Craig.
24
. ' . CA 02606480 2007-11-06
5,572,023 Nov. 5,1996, Caprioli, Richard.
4,531,056 July 23,1985 Labowski, Michael, Fenn John, B.,
Yamashita, Masamichi
Publications:
T. Wang, L. Zeng, T. Strader, L. Burton, and Daniel B. Kassel, Proceedings of
the 4661
ASMS Conference on Mass Spectrometry, 1034, 1998.
25