Note: Descriptions are shown in the official language in which they were submitted.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
1
Method for the purification of antibodies
Field of the Invention
The current invention relates to the field of polypeptide purification. A
novel
method for the purification of immunoglobulins with an ion exchange resin is
de-
scribed herein. At the same time a method for the fast determination of
purification
conditions is given.
Background of the Invention
Proteins and especially immunoglobulins play an important role in today's
medical
portfolio. For human application every pharmaceutical substance has to meet
dis-
tinct criteria. To ensure the safety of biopharmaceutical agents to humans
nucleic
acids, viruses, and host cell proteins, which would cause severe harm, have to
be
removed especially. To meet the regulatory specification one or more
purification
steps have to follow the manufacturing process. Among other, purity,
throughput,
and yield play an important role in determining an appropriate purification
pro-
cess.
Different methods are well established and widespread used for protein
purifica-
tion, such as affinity chromatography with microbial proteins (e.g. protein A
or
protein G affinity chromatography), ion exchange chromatography (e.g. cation
exchange (carboxymethyl resins), anion exchange (amino ethyl resins) and mixed-
mode exchange), thiophilic adsorption (e.g. with beta-mercaptoethanol and
other
SH ligands), hydrophobic interaction or aromatic adsorption chromatography
(e.g.
with phenyl-sepharose, aza-arenophilic resins, or m-aminophenylboronic acid),
metal chelate affinity chromatography (e.g. with Ni(II)- and Cu(II)-affinity
mate-
rial), size exclusion chromatography, and electrophoretical methods (such as
gel
electrophoresis, capillary electrophoresis) (Vijayalakshmi, M.A., Appl.
Biochem.
Biotech. 75 (1998) 93-102).
Necina, R. et al., Biotechnol. Bioeng. 60 (1998) 689-698, reported the capture
of
human monoclonal antibodies directly from cell culture supernatants by ion ex-
change media exhibiting high charge density. In WO 89/05157 a method is
reported
for the purification of product immunoglobulins by directly subjecting the
cell cul-
ture medium to a cation exchange treatment. A one-step purification of mono-
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 2 -
clonal IgG antibodies from mouse ascites is described by Danielsson, A., et
al., J.
Immun. Meth. 115 (1988), 79-88.
A combination of methods, i.e. a caprylic acid precipitation and ion exchange
chromatography, was used by Raweerith, R. et al. (J. Immun. Meth. 282 (2003)
63-
72), as means to fractionate pepsin-digested horse antivenom F(ab')2 antibody.
In
WO 2003/102132 the combination of a non-affinity purification step and a high-
performance tangential-flow filtration is reported for the purification of
proteins.
The combination of two affinity chromatography steps is reported in
WO 92/19973.
Follman, D.K., and Fahrner, R.L., reported a factorial screening of antibody
purifi-
cation processes using three chromatography steps without protein A (J. Chrom.
A
1024 (2004) 79-85). Mhatre, R. et al. (J. Chrom. A 707 (1995) 225-231),
explored
the purification of antibody Fab fragments by cation exchange chromatography
and
pH gradient elution.
WO 94/00561 reports human monoclonal anti-rhesus antibodies and cell lines pro-
ducing the same. A method for purifying a polypeptide by ion exchange chroma-
tography is reported in WO 2004/024866 in which a gradient wash is used to re-
solve a polypeptide of interest from one or more contaminants. Schwarz, A. et
al.
(Laborpraxis 21(1997) 62-66), report the purification of monoclonal antibodies
with a CM-HyperD-column.
WO 2004/076485 reports a process for antibody purification by protein A and
ion
exchange chromatography. In EP 0 530 447 a process for purifying IgG
monoclonal
antibodies by a combination of three chromatographic steps is reported. The re-
moval of protein A from antibody preparations is reported in US 4,983,722.
WO 95/16037 reports the purification of anti-EGF-R/anti-CD3 bispecific mono-
clonal antibodies from hybrid hybridoma performed by protein A cation exchange
chromatography. The separation of antibody monomers from its multimers by use
of ion exchange chromatography is reported in EP 1 084 136. US 5,429,746
relates
to the application of hydrophobic interaction chromatography combination chro-
matography to the purification of antibody molecule proteins.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 3 -
Summary of the Invention
Thus it is the objective of the current invention to provide another method
for the
purification of recombinantly produced immunoglobulins and for the separation
of
monomeric and multimeric immunoglobulin species.
The current invention provides a method for purifying an immunoglobulin,
wherein the method comprises the following steps
a) providing a solution comprising an immunoglobulin, a buffer substance,
and optionally a salt;
b) bringing the solution and a weak ion exchange material in contact under
conditions whereby the immunoglobulin binds to the weak ion exchange
material;
c) recovering the immunoglobulin from the weak ion exchange material in a
single step by using a solution comprising a buffer substance and a salt.
The invention further provides a method for determining the concentration of a
salt for eluting a polypeptide from an ion exchange chromatography material in
a
single step purification process, comprising the following two steps
a) step one comprising the following sub-steps
al) providing a solution comprising a polypeptide, a buffer sub-
stance, and optionally a salt;
a2) bringing a first aliquot of the solution containing the polypeptide
and an ion exchange material in contact under conditions whereby
the polypeptide binds to the ion exchange material;
a3) recovering the polypeptide from the ion exchange material by
using a solution comprising a buffer substance and a salt whereby
the concentration of the salt is increased linearly;
a4) determining the starting concentration of the salt where the first
fraction of the polypeptide starts to elute from the ion exchange col-
umn;
b) step two comprising the following sub-steps
bl) bringing a second aliquot of the solution containing the poly-
peptide and an ion exchange material in contact under conditions
whereby the polypeptide binds to the ion exchange material;
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 4 -
b2) recovering the polypeptide from the ion exchange material by
using a three step elution method, whereby
i) the salt concentration of the first elution step is calculated
as the sum of
the product of the starting concentration of the salt as
determined in step a4) and the total number of
monovalent cations different from hydrogen denoted in
the molecular formula of the salt
and
the product of the concentration of the buffer salt and the
total number of monovalent cations different from
hydrogen denoted in the molecular formula of the buffer
salt;
ii) the salt concentration of the second elution step is the
product of the salt concentration of the first elution step and
a factor between 1.25 and 1.35;
iii) the salt concentration of the third elution step is the pro-
duct of the salt concentration of the first elution step and a
factor between 1.50 and 1.70;
whereby the factors of step ii) and iii) are determined as follows: at a
starting concentration between 10 mM and 40 mM the factors are
1.35 and 1.70 respectively, at a starting concentration between
40 mM and 70 mM the factors are 1.30 and 1.60 respectively, and at
a starting concentration of more than 70 mM the factors are 1.25
and 1.50 respectively.
b3) determining at which sub-step of the three step elution method
of step b2) the polypeptide is eluted from the ion exchange column
thereby determining the concentration of a salt for eluting a poly-
peptide from an ion exchange chromatography material in a single
step purification process.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 5 -
Detailed Description of the Invention
These terms are used within this application in accordance with the following
defi-
nition:
The term "ion exchange resin" or "ion exchange material" as used within this
appli-
cation denotes an immobile high molecular weight matrix that carries
covalently
bound charged substituents. For overall charge neutrality not covalently bound
counter ions are bound thereto. The "ion exchange material" has the ability to
ex-
change its not covalently bound counter ions for similarly charged ions of the
sur-
rounding solution. Depending on the charge of its exchangeable counter ions
the
"ion exchange resin" is referred to as cation exchange resin or as anion
exchange
resin. Depending on the nature of the charged group (substituent) the "ion ex-
change resin" is referred to as, e.g. in the case of cation exchange resins,
sulfonic
acid resin (S), or carboxymethyl resin (CM). Depending on the chemical nature
of
the charged group/substituent the "ion exchange resin" can additionally be
classi-
fled as strong or weak ion exchange resin, depending on the strength of the
cova-
lently bound charged substituent. For example, strong cation exchange resins
have
a sulfonic acid group as charged substituent, weak cation exchange resins have
a
carboxylic group, preferably a carboxymethyl group, as charged substituent,
and
weak anion exchange resins have a diethylaminoethyl group as charged
substituent.
Cation exchange resins are available under different names from a multitude of
companies such as e.g. Bio-Rex, Macro-Prep CM (available from Biorad Laborato-
ries, Hercules, CA, USA), weak cation exchanger WCX 2 (available from Cipher-
gen, Fremont, CA, USA), Dowex MAC-3 (available from Dow chemical company
¨ liquid separations, Midland, MI, USA), Mustang C (available from Pall
Corpora-
tion, East Hills, NY, USA), Cellulose CM-23, CM-32, CM-52, hyper-D, and par-
tisphere (available from Whatman plc, Brentford, UK), Amberlite IRC 76,
IRC 747, IRC 748, GT 73 (available from Tosoh Bioscience GmbH, Stuttgart, Ger-
many), CM 1500, CM 3000 (available from BioChrom Labs, Terre Haute, IN,
USA), and CM-SepharoseTm Fast Flow (available from GE Healthcare ¨ Amersham
Biosciences Europe GmbH, Freiburg, Germany).
Preferably, the charged substituents of the weak ion exchange material are at
least
about 90 % carboxylic acid groups, more than 90% carboxylic acid groups, or
more
than 95 % carboxylic acid groups.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 6 -
The terms "single step elution" and "single step gradient elution", which are
used
interchangeably within this application, denote a method wherein e.g. the
concen-
tration of a substance causing elution, i.e. the dissolution of a bound
compound
from a material, is raised or lowered at once, i.e. directly from a starting
value/level
to a final value/level, i.e. in a single step.
A "polypeptide" is a polymer of amino acid residues joined by peptide bonds,
whether produced naturally or synthetically. Polypeptides of less than about
20
amino acid residues are referred to as "peptides."
A "protein" is a macromolecule comprising one or more polypeptide chains or a
polypeptide chain of more than 100 amino acid residues. A protein may also com-
prise non-peptidic components, such as carbohydrate groups. Carbohydrates and
other non-peptidic substituents may be added to a protein by the cell in which
the
protein is produced, and will vary with the type of cell. Proteins are defined
herein
in terms of their amino acid backbone structures; substituents such as
carbohydrate
groups are generally not specified, but may be present nonetheless.
The terms "antibody" and "immunoglobulin" which can be used interchangeably
within this application comprise at least two light polypeptide chains and two
heavy
polypeptide chains. Each of the heavy and light polypeptide chains contains a
vari-
able region (generally the amino terminal portion of the polypeptide chain)
which
contains a binding domain for interaction with the antigen. Each of the heavy
and
light polypeptide chains also comprises a constant region (generally the
carboxyl
terminal portion) which may mediate the binding of the antibody to host
tissues or
factors including various cells of the immune system, some phagocytic cells
and a
first component (Clq) of the classical complement system. Typically, the light
and
heavy polypeptide chains are complete chains, each consisting essentially of a
vari-
able region, i.e. VL or VH, and a complete constant region, i.e. of CL in case
of a light
polypeptide chain or of CH1, CH2, CH3, and optionally CH4 in case of a heavy
poly-
peptide chain. The variable regions of the antibody according to the invention
can
be grafted to constant regions of other isotypes. For example, a
polynucleotide en-
coding the variable region of a heavy chain of the 1-isotype can be grafted to
polynucleotide encoding the constant region of another heavy chain class (or
sub-
class).
As used herein, the term "antibody" or "immunoglobulin" refers to a protein
con-
sisting of one or more polypeptides substantially encoded by antibody genes.
The
recognized antibody genes include the different constant region genes as well
as the
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 7 -
myriad antibody variable region genes. Antibodies may exist in a variety of
forms,
including, for example, Fv, Fab, and F(ab)2 as well as single chains (e.g.
Huston,
J.S., et al., PNAS USA 85 (1988) 5879-5883; Bird et al., Science 242 (1988)
423-426;
and, in general, Hood et al., Immunology, Benjamin N.Y., 2nd edition (1984)
and
Hunkapiller and Hood, Nature 323 (1986) 15-16). In one embodiment antibodies
according to the invention comprise monoclonal antibodies and fragments
thereof,
for example isolated heavy or light chains, or heavy or light chains only
consisting
of constant regions as well as fragments thereof.
General chromatographic methods and their use are known to a person skilled in
the art. See for example, Chromatography, 5th edition, Part A: Fundamentals
and
Techniques, Heftmann, E. (ed), Elsevier Science Publishing Company, New York,
(1992); Advanced Chromatographic and Electromigration Methods in Biosciences,
Deyl, Z. (ed.), Elsevier Science By, Amsterdam, The Netherlands, (1998);
Chroma-
tography Today, Poole, C. F., and Poole, S. K., Elsevier Science Publishing
Corn-
pany, New York, (1991); Scopes, Protein Purification: Principles and Practice
(1982); Sambrook, J., et al. (ed), Molecular Cloning: A Laboratory Manual ,
Second
Edition, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1989;
or
Current Protocols in Molecular Biology, Ausubel, F. M., et al. (eds), John
Wiley
& Sons, Inc., New York.
The current invention provides a method for purifying an immunoglobulin,
wherein the method comprises the following steps
a) providing a solution comprising an immunoglobulin, a buffer substance,
and optionally a salt;
b) bringing the solution and a weak ion exchange material in contact under
conditions whereby the immunoglobulin binds to the weak ion exchange
material;
c) recovering the immunoglobulin from the weak ion exchange material in a
single step by using a solution comprising a buffer substance and a salt.
The purification process of immunoglobulins in general comprises a multistep
chromatographic part. In the first step non-immunoglobulin polypeptides and
pro-
teins are separated from the immunoglobulin fraction by an affinity
chromatogra-
phy, e.g. with protein A. Afterwards an ion exchange chromatography can be per-
formed to disunite the individual immunoglobulin classes and to remove traces
of
protein A, which has been coeluted from the first column. Finally a third
chroma-
tographic step is necessary to separate immunoglobulin monomers from multimers
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 8 -
and fragments of the same class. Sometimes the amount of aggregates is high (5
%
or more) and it is not possible to separate them efficiently in the third
purification
step necessitating further purification steps.
With the recombinant production of specific immunoglobulins the separation
step
for the separation of different immunoglobulin classes is dispensable. Thus
the
overall purification process of recombinantly produced immunoglobulins may be
reduced to two chromatographic steps.
The conditioned protein A eluate is in general chromatographically processed
on a
cation exchange material at pH values below the isoelectric point of the
respective
immunoglobulin protein.
The anti IL-1R antibody (WO 2005/023872) and Herceptin , an anti-HER2 anti-
body (WO 99/57134), were available in sufficient quantities in our
laboratories at
the time of the invention and therefore the current invention is exemplified
with
these two immunoglobulins. Likewise the invention is in general practicable
with
immunoglobulins. This exemplified description is done only by way of example
and
not by way of limitation of the invention. These examples are provided to aid
the
understanding of the present invention, the true scope of which is set forth
in the
appended claims.
The current invention describes a purification method for the separation of
immu-
noglobulin monomers from aggregates and fragments as well as the depletion of
other polypeptide impurities. As can be seen from the experiments this
purification
is achieved by the use of weak ion exchange resins, preferably by the use of
weak
cation exchange resins. As exemplified by the comparison of strong and weak
ion
exchange materials the weak ion exchange material provides a separation of
monomeric and aggregated forms of the immunoglobulins (see examples 1 and 2
and examples 9 and 12).
In one embodiment of the invention the pH value of the buffer material (sub-
stance) is of from pH 3.0 to pH 10.0, preferably of from pH 3.0 to pH 7.0,
more
preferred of from pH 4.0 to pH 6.0 and most preferred of from pH 4.5 to pH
5.5.
In one embodiment the pH value is kept constant in the single step, i.e. it is
main-
tained at the same value in the single step.
Another preferred item of the current invention is that the method of the
current
invention is applicable to immunoglobulins that have an isoelectric point (pI)
of
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
-9-
6.0 or more (pI > 6.0) and therefore have a net positive charge in the pH
range
starting from pH 6.0 to pH 14.
A preferred embodiment of the invention is the purification of an
immunoglobulin
of the IgG or IgE class.
A weak cation exchange material is used in a preferred embodiment of the
current
invention.
The buffer material is preferably employed in a concentration range between 5
mM
and 100 mM as exemplified.
For the recovering of the bound immunoglobulins from the weak ion exchange
material the conductivity of the buffer/solution is increased. This can be
accom-
plished either by an increased buffer salt concentration or by the addition of
other
salts, so called elution salts, to the buffer solution. Preferred elution
salts are sodium
citrate, sodium chloride, sodium sulphate, sodium phosphate, potassium
chloride,
potassium sulfate, potassium phosphate, as well as other salts of citric and
phospho-
ric acid, and any mixture of these components. Especially preferred are sodium
citrate, sodium chloride, potassium chloride and mixtures thereof.
The concentration of the salt, causing the elution, is preferably in the range
of be-
tween 5 mM and 500 mM, more preferred between 5 mM and 400 mM, and espe-
cially preferred between 5 mM and 250 mM.
Another preferred embodiment of the invention is the use of the salt, causing
the
elution, at the same time as buffer substance, especially with citric acid and
salts
thereof or phosphoric acid and salts thereof.
The method of the current invention is preferably a chromatographic or batch
method, especially preferred is a method comprising a batch elution.
Another preferred embodiment of the current invention is that the purification
is a
single step purification process.
A "single step" denotes a process wherein one or more conditions, for example
the
pH, the ionic strength, concentration of a salt, and/or the flow of a
chromatogra-
phy, is/are changed all at once from a starting value to a final value, i.e.
the condi-
tions are changed incrementally, i.e. stepwise, in contrast to a linear
change.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 10 -
Still a preferred embodiment of the current invention is that the method
comprises
the additional step of a purification of the immunoglobulin by a protein A
affinity
chromatography before step a) of the method.
The current invention further provides a method for determining the
concentration
of a salt for eluting a polypeptide from an ion exchange chromatography
material
in a single step purification process, comprising the following two steps
a) step one comprising the following sub-steps
al) providing a solution comprising a polypeptide, a buffer sub-
stance, and optionally a salt;
a2) bringing a first aliquot of the solution containing the polypeptide
and an ion exchange material in contact under conditions whereby
the polypeptide binds to the ion exchange material;
a3) recovering the polypeptide from the ion exchange material by
using a solution comprising a buffer substance and a salt whereby
the concentration of the salt is increased linearly;
a4) determining the starting concentration of the salt where the first
fraction of the polypeptide starts to elute from the ion exchange col-
umn;
b) step two comprising the following sub-steps
b 1 ) bringing a second aliquot of the solution containing the poly-
peptide and an ion exchange material in contact under conditions
whereby the polypeptide binds to the ion exchange material;
b2) recovering the polypeptide from the ion exchange material by
using a three step elution method, whereby
i) the salt concentration of the first elution step is calculated
as the sum of
the product of the starting concentration of the salt as
determined in step a4) and the total number of
monovalent cations different from hydrogen denoted in
the molecular formula of the salt
and
the product of the concentration of the buffer salt and the
total number of monovalent cations different from
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 11 -
hydrogen denoted in the molecular formula of the buffer
salt;
ii) the salt concentration of the second elution step is the
product of the salt concentration of the first elution step and
a factor between 1.25 and 1.35;
iii) the salt concentration of the third elution step is the
product of the salt concentration of the first elution step and
a factor between 1.50 and 1.70;
whereby the factors of step ii) and iii) are determined as follows: at a
starting concentration between 10 mM and 40 mM the factors are
1.35 and 1.70 respectively, at a starting concentration between
40 mM and 70 mM the factors are 1.30 and 1.60 respectively, and at
a staring concentrations of more than 70 mM the factors are 1.25
and 1.50 respectively.
b3) determining at which sub-step of the three step elution method
of step b2) the polypeptide is eluted from the ion exchange column
thereby determining the concentration of a salt for eluting a poly-
peptide from an ion exchange chromatography material in a single
step purification process.
The factors of step b2) define a range that has been determined
experimentally.
These values are no absolute values but merely a target value. A deviation of
10% is
maintainable.
The current invention describes a method for determining the concentration of
a
salt for eluting a polypeptide from an ion exchange chromatography material in
a
single step purification process for the purification of polypeptides from
other pro-
teinaceous material.
The concentration, at which the elution of the polypeptide, preferably of an
immu-
noglobulin, from the ion exchange resin starts, provides the basis for the
second
optimization step b), a three step elution method. The approximate buffer/salt
con-
centrations for the step elution are calculated as follows:
- the salt concentration of the first elution step is equal to the sum of
as first summand the product of the concentration of the salt, at which the
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 12 -
elution from the ion exchange column starts as determined with the linear
increasing salt gradient, and the total number of monovalent cations
different from hydrogen denoted in the molecular formula of the salt causing
the elution
and
as second summand the product of the concentration of the buffer salt and
the total number of monovalent cations different from hydrogen denoted in
the molecular formula of the buffer salt;
- the salt concentration of the second elution step is equal to the product
of the
salt concentration of the first elution step and a factor of between 1.25 and
1.35;
- the salt concentration of the third elution step is equal to the product
of the salt
concentration of the first elution step and a factor between 1.50 and 1.70.
The factor included in the calculation of the concentration steps accounts for
the
interval between the concentration levels and is adjusted depending on the
starting
concentration. At small starting concentrations, i.e. between 10 mM and 40 mM,
the factors are 1.35 and 1.70 respectively, at medium starting concentrations
be-
tween 40 mM and 70 mM the factors are 1.30 and 1.60 respectively, and at high
starting concentrations of more than 70 mM the factors are 1.25 and 1.50
respec-
lively. These factors define a range that has been determined experimentally.
These
values are no absolute values but merely a target value. A deviation of 10% is
main-
tainable.
The buffer salt has to be accounted for in the calculation because it is
possible, as
outlined in example 3, that the elution of a protein from an ion exchange
resin can
be effected by a change of the buffer salt concentration during the
chromatography.
If the buffer salt concentration is kept constant during the chromatography or
is
small compared to the stating concentration of the salt causing the elution it
may be
neglected during the calculation to reduce complexity.
In one embodiment the salt causing the elution is not the buffer salt and the
salt
concentration of step b2i) is the product of the concentration of the salt, at
which
the elution from the ion exchange column starts as determined with the linear
in-
creasing salt gradient in step a4), and the total number of monovalent cations
dif-
ferent from hydrogen denoted in the molecular formula of the salt causing the
elu-
tion.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 13 -
The calculation will be exemplified based on example 4 with the anti IL-1R
anti-
body. With a starting concentration of 15 mM sodium citrate, as determined in
example 3, consisting of a 10 mM buffer concentration and a 5 mM contribution
from the linear gradient, the three steps are calculated as follows:
- the target concentration for step one is calculated to be 30 mM (5 mM*2 +
mM*2) sodium citrate
in detail: 5 mM (starting concentration) multiplied with two (cit-
ric acid is a trivalent acid, employed as di-sodium salt;
therefore two monovalent cations different from hydro-
10 gen are
present in the molecular formula) plus 10 mM
(buffer salt concentration) multiplied with two (citric
acid is a trivalent acid, employed as di-sodium salt;
therefore two monovalent cations different from hydro-
gen are present in the molecular formula)
- the target concentration for step two is calculated to be 40.5 mM (30 mM *
1.35) sodium citrate
in detail: 30 mM sodium citrate is the concentration of step one
multiplied by 1.35 (the starting concentration is 15 mM,
therefore as factor 1.35 is selected)
- the target concentration for step three is calculated to be 51 mM (30 mM *
1.70) sodium citrate
in detail: 30 mM sodium citrate is the concentration of step one
multiplied by 1.70 (the starting concentration is 15 mM,
therefore as factor 1.70 is selected)
As can be seen from the experiments this purification is achieved in a
preferred em-
bodiment by the use of a weak ion exchange material, especially preferred of a
weak
cation exchange material.
A preferred embodiment of the invention is that the polypeptide is an immu-
noglobulin, especially preferred an immunoglobulin of the IgG or IgE class.
In one embodiment of the invention the pH value of the buffer
material/substance
is of from pH 3.0 to pH 10.0, preferably of from pH 3.0 to pH 7.0, more
preferred
of from pH 4.0 to pH 6.0 and most preferred of from pH 4.5 to pH 5.5.
CA 02607375 2013-01-08
- 14 -
Another preferred item of the current invention is that the method of the
current
invention is applicable to immunoglobulins that have an isoelectric point (pI)
of
6.0 or more (p1? 6.0) and therefore have a net positive charge in the pH range
starting from pH 6.0 to pH 14.
The buffer material/substance is preferably employed in a concentration range
be-
tween 5 mM and 100 mM as exemplified.
For the recovering of the bound immunoglobulins from the ion exchange material
the conductivity of the buffer/solution is increased. This can be accomplished
either
by an increased buffer salt concentration or by the addition of other salts,
so called
elution salts, to the buffer solution. Preferred elution salts are sodium
citrate, so-
dium chloride, sodium sulphate, sodium phosphate, potassium chloride,
potassium
sulfate, potassium phosphate, as well as other salts of citric and phosphoric
acid,
and any mixture of these components. Especially preferred are sodium citrate,
so-
dium chloride, potassium chloride and any mixture thereof.
The concentration of the salt, causing the elution, is preferably in the range
of be-
tween 5 mM and 500 mM, more preferred between 5 mM and 400 mM, and espe-
cially preferred between 5 mM and 250 mM.
Another preferred embodiment of the invention is the use of the salt, causing
the
elution, at the same time as buffer substance, especially with citric acid and
salts
thereof or phosphoric acid and salts thereof.
The method of the current invention is preferably a chromatographic or batch
method, especially preferred is a method comprising a batch elution.
Another preferred embodiment of the current invention is that the purification
is a
single step purification process.
Still a preferred embodiment of the current invention is that the method
comprises
the additional step of a purification of the immunoglobulin by a protein A
affinity
chromatography before step a) of the method.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 15 -
Description of the Figures
Figure 1 Single step elution of anti IL-1R antibody from strong
cation ex-
change resin SP-Sepharose; monomeric and aggregated forms of
the receptor antibody are not separated and elute as one peak.
Figure 2 Single step elution of anti IL-1R antibody from weak cation ex-
change resin CM-Toyopearl; monomeric and aggregated forms of
the receptor antibody are partially separated and elute as one
main peak comprising the monomeric form of the immu-
noglobulin and as a second peak, comprising monomeric and ag-
gregated forms of the immunoglobulin as well as protein A.
Figure 3 Linear gradient elution of anti IL-1R antibody from weak
cation
exchange resin CM-Toyopearl with sodium citrate at pH 5.0;
monomeric and aggregated forms of the receptor antibody are
partially separated and elute at a sodium citrate starting concen-
tration of 15 mM as a main peak comprising the monomeric form
of the immunoglobulin and as a second peak, comprising a mix-
ture of monomeric form, aggregated forms of the immunoglobu-
lin, and protein A. The SEC analysis of the two fractions is in-
serted into the ion exchange chromatogram. In the main peak ag-
gregates are absent. In contrast in the second peak aggregated
forms of the immunoglobulin are present.
Figure 4 Three step gradient elution of anti IL-1R antibody from weak
cation exchange resin CM-Toyopearl with sodium citrate at pH
5.0; monomeric and aggregated forms of the receptor antibody
are partially separated and elute as a main peak comprising the
monomeric form of the immunoglobulin at a sodium citrate con-
centration of 34 mM and as a second peak, comprising mono-
meric and aggregated forms of the immunoglobulin as well as
protein A at a sodium citrate concentration of 44 mM.
Figure 5 Single step gradient elution of anti IL-1R antibody from weak
cation exchange resin CM-Sepharose with 150 mM sodium chlo-
ride at pH 5.5; monomeric and aggregated forms of the receptor
antibody are separated and elute as a main peak comprising the
monomeric form of the immunoglobulin and as a second peak,
comprising monomeric and aggregated forms of the immu-
noglobulin as well as protein A.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 16 -
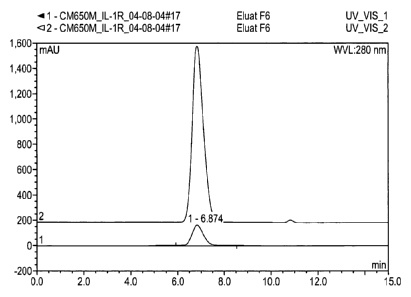
Figure 6a Elution profile on a CM-Sepharose fast flow; two peaks can
be
identified: a main peak, corresponding to the monomeric anti
IL-1R antibody, and a smaller second peak, that contains mainly
aggregates and other impurities.
Figure 6b Elution profile of anti IL-1R antibody on a SP-Sepharose fast
flow; one peak can be identified which contains monomeric im-
munoglobulin, aggregates and other impurities which have not
been separated on this column.
Figure 7a Single step gradient elution of anti IL-1R antibody from
weak
cation exchange resin CM-Sepharose with 100 mM sodium chlo-
ride at pH 5.5.
Figure 7b Single step gradient elution of anti IL-1R antibody from
weak
cation exchange resin CM-Sepharose with 150 mM sodium chlo-
ride at pH 5.5.
Figure 7c Single step gradient elution of anti IL-1R antibody from weak
cation exchange resin CM-Sepharose with 200 mM sodium chlo-
ride at pH 5.5.
Figure 8a Single step gradient elution of anti IL-1R antibody from
weak
cation exchange resin CM-Sepharose with 150 mM sodium chlo-
ride at pH 4Ø
Figure 8b Single step gradient elution of anti IL-1R antibody from
weak
cation exchange resin CM-Sepharose with 150 mM sodium chlo-
ride at pH 6Ø
Figure 9a Single step elution of anti IL-1R antibody from weak cation
ex-
change resin CM-Sepharose; size exclusion chromatography
(SEC) of the starting material showing both monomeric and ag-
gregated forms of the immunoglobulin.
Figure 9b Single step elution of anti IL-1R antibody from weak cation
ex-
change resin CM-Sepharose; monomeric and aggregated forms of
the receptor antibody are partially separated and elute as one
main peak comprising the monomeric form of the immu-
noglobulin and as a second peak, comprising monomeric and ag-
gregated forms of the immunoglobulin as well as other protein. In
this figure the size exclusion chromatography of the first (main)
peak is shown. Only one peak is eluted from the SEC-column,
which is the monomeric immunoglobulin.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 17 -
Figure 9c Single step elution of anti IL-1R antibody from weak cation
ex-
change resin CM-Sepharose; monomeric and aggregated forms of
the receptor antibody are partially separated and elute as one
main peak comprising the monomeric form of the immu-
noglobulin and as a second peak, comprising monomeric and ag-
gregated forms of the immunoglobulin as well as other protein. In
this figure the size exclusion chromatography of the second peak
is shown. In the chromatogram at least three peaks can be seen,
equivalent to monomeric and aggregated forms of the immu-
noglobulin and other protein.
Figure 10 Single step elution of anti-HER-2 antibody from strong
cation
exchange resin SP-Sepharose; monomeric and aggregated forms
of the antibody are not separated and elute as one peak.
Figure 11 Linear gradient elution of anti-HER-2 antibody from weak
cation
exchange resin CM-Sepharose with sodium chloride in sodium
citrate buffer at pH 5.5; monomeric and aggregated forms of the
antibody are partially separated and elute with a starting concen-
tration of 80 mM sodium chloride as one main peak comprising
the monomeric form of the immunoglobulin; the monomeric and
aggregated forms elute as mixture in the tailing of the main peak
together with protein A.
Figure 12 Three step gradient elution of anti-HER-2 antibody from weak
cation exchange resin CM-Sepharose with sodium chloride in so-
dium citrate buffer at pH 5.5; monomeric and aggregated forms
of the antibody are separated and elute as one main peak com-
prising the monomeric form of the immunoglobulin at a sodium
chloride concentration of 80 mM and as a second peak compris-
ing monomeric and aggregated forms of the immunoglobulin as
well as protein A at a sodium chloride concentration of 100 mM.
Figure 13 Single step gradient elution of anti-HER-2 antibody from weak
cation exchange resin CM-Sepharose with 80 mM sodium chlo-
ride at pH 5.5; the monomeric form is eluted free of aggregated
forms; the aggregated forms elute after a second sodium chloride
step to 120 mM as a second defined peak.
Figure 14 Single step gradient elution of anti-HER-2 antibody from strong
cation exchange resin SP-Sepharose with 80 mM sodium chloride
at pH 5.5; two peaks are obtained: peak 1 contains only mono-
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 18 -
meric Herceptin , peak 2, which is eluted after a second sodium
chloride step to 120 mM, contains a high amount of monomeric
Herceptin and aggregates; the yield of Herceptin in its mono-
meric form is less than 60%.
Experimental Part
Material:
An IgG4 immunoglobulin anti IL-1R antibody (hereinafter referred to as immu-
noglobulin, WO 2005/023872) was purified in a first step with a protein A
affinity
chromatography. Elution from the protein A column was carried out under acidic
conditions (10 mM sodium citrate buffer, pH value 3.0 0.5). Before the
filtration
step the pH value of the fraction containing the immunoglobulin was adjusted
with
a concentrated, e.g. 1 M, buffer solution of pH 9.0 (e.g. tris-hydroxymethyl-
amino-
methane (TRIS) or phosphate buffer) to pH 5Ø The protein A eluate is a
solution
with a protein concentration between 5 mg/ml and 15 mg/ml and is buffered with
sodium citrate. This material is referred to in the following as conditioned
pro-
tein A eluate, which is prepared for loading onto a cationic exchange resin.
Example 1
In this comparative example an ion exchange chromatography with a strong
cation
exchange resin and single step elution is described.
Chromatographic conditions:
Resin: SP-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.0
Loading: max. 20 g protein/L gel matrix
Elution: 25 mM sodium citrate buffer with 100 mM sodium chloride, ad-
justed to pH 5.0
The conditioned protein A eluate was applied to a chromatography column con-
taming a strong cation exchange resin (SP-Sepharose). After the loading step
at a
flow rate of 160 cm/h the column was washed with equilibration buffer (10
column
volumes). The bound immunoglobulins were eluted with a single step elution
method, whereby the pH value was kept constant and the conductivity was varied
(increased) by the addition of sodium chloride.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 19 -
In figure 1 the elution chromatogram of the cation exchange chromatography of
the anti IL-1R antibody on the strong cation exchange resin SP-Sepharose is
pre-
sented. The elution is a single step replacement elution with sodium chloride
with-
out altering the pH value of the chromatographic system. The monomeric and ag-
gregated immunoglobulin molecules are not separated and thus with this method
no purification by reduction of the aggregate content in the eluate compared
to the
loaded material can be obtained.
Example 2
In this example an ion exchange chromatography with a weak cation exchange
resin
and single step elution is described.
To achieve a separation of monomeric and aggregated forms of an immunoglobu-
lin, a weak cation exchange resin was employed. By using this kind of resin an
in-
crease of the conductivity by a step elution is accompanied by a particular pH
shift
on the resin (even when the pH of the eluting buffer remains constant). This
effect
facilitates the discrimination e.g. between monomeric immunoglobulins and
aggre-
gated forms. Furthermore, other impurities, like traces of host cell proteins
or pro-
tein A, can efficiently be separated from the monomeric mean fraction, without
significant loss in yield.
Chromatographic conditions:
Resin: CM-Toyopearl
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.0
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.0
Elution: 25 mM sodium citrate buffer with 100 mM sodium chloride, ad-
justed to pH 5.0
The conditioned protein A eluate was applied to a chromatography column con-
taining a weak cation exchange resin (CM-Toyopearl). After the loading step at
a
flow rate of 160 cm/h the column was washed with equilibration buffer (10
column
volumes). The bound immunoglobulins were eluted with a single step elution
method, whereby the pH value in the mobile phase was kept constant and the con-
ductivity was varied by the addition of sodium chloride.
In figure 2 the elution profile with the same chromatographic conditions as in
ex-
ample 1 but this time with a weak cation exchange resin, CM-Toyopearl, is pre-
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 20 -
sented. Herein a second peak as shoulder of the main peak appears. This
separation
behavior is different to that with a strong cation exchange resin, such as SP-
Sepharose. An analysis of fractions corresponding to the main peak and to the
sec-
ond shoulder peak showed a significant amount of aggregates to be present in
the
shoulder peak fraction. No aggregates were detectable in the main peak
fractions
(see also figures 9a to c).
Example 3
Optimization of the chromatographic method ¨ first step: linear concentration
gradient.
To optimize the cation exchange chromatography on a weak cation exchange mate-
rial an optimization procedure, which is consisting of three steps, was put
into
practice:
The first step is a chromatography using a linear concentration gradient of
the
buffer salt, sodium citrate. Just as well is it possible to keep the
concentration of the
buffer salt constant and to admix a linearly increasing concentration of a
salt caus-
ing the elution of the immunoglobulin. In both cases is the conductivity of
the solu-
tion increased without an alteration of the pH value of the mobile phase.
Salts suit-
able for the elution are e.g. sodium chloride, sodium sulphate, sodium
phosphate,
potassium chloride, potassium sulfate, potassium phosphate, citric acid and
salts
thereof as well as mixtures of these components. The concentrations of from
10 mM to 500 mM, which are applied, are adjusted accordingly to set
conductivity
in the range of from about 1 milli S/cm to about 50 milli S/cm.
Chromatographic conditions:
Resin CM-Toyopearl
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.0
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.0
Elution: linear gradient; from 10 mM sodium citrate buffer, adjusted to
pH 5.0, to 100 mM sodium citrate buffer, adjusted to pH 5.0
The conditioned protein A eluate was applied to a chromatography column con-
taining a weak cation exchange resin (CM-Toyopearl). After the loading step at
a
flow rate of 160 cm/h the column was washed with equilibration buffer (10
column
volumes). The bound immunoglobulins were eluted with a linear gradient elution
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 21 -
method, whereby the pH value in the mobile phase was kept constant. The concen-
tration of the buffer salt, sodium citrate, was raised linearly from 10 mM to
100 mM over 40 column volumes. After the final sodium citrate concentration
was
reached the elution was continued for an additional 40 column volumes.
In figure 3 the chromatogram of the linear buffer gradient elution of the anti
IL-1R
antibody is presented. The monomeric and the aggregated form of the immu-
noglobulin elute in a semi-detached peak starting at a concentration of 15 mM
so-
dium citrate and ending at a concentration of 55 mM sodium citrate.
The recovery of the bound immunoglobulin from the cation exchange resin is de-
pending on the conductivity of the applied solution. Therefore the cations of
the
buffer salt present in the eluting solution during the recovery step have to
be con-
sidered as effecting the elution of the immunoglobulin from the cation
exchange
resin. The conductivity as well as the ionic strength of the mobile phase are
effected
by the total number of ions in the solution. Thus the number of monovalent
cations different from hydrogen in one molecular formula of the employed
buffer
salt and the employed salt causing the elution have to be considered hereby.
Example 4
Optimization of the chromatographic method ¨ second step: three step concentra-
tion gradient elution.
The concentration, at which the elution of the immunoglobulin from the ion ex-
change resin starts, as determined in example 3, provides the basis for the
second
optimization step, a three step elution method. The approximate buffer/salt
con-
centrations for the step elution are calculated as follows:
- the salt concentration of the first elution step is equal to the sum of
as first summand the product of the concentration of the salt, at
which the elution from the ion exchange column starts as deter-
mined with the linear increasing salt gradient, and the total number
of monovalent cations different from hydrogen denoted in the mo-
lecular formula of the salt causing the elution
and
as second summand the product of the concentration of the buffer
salt and the total number of monovalent cations different from hy-
drogen denoted in the molecular formula of the buffer salt;
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 22 -
- the salt concentration of the second elution step is equal to the product
of
the salt concentration of the first elution step and a factor of between
1.25 and 1.35;
- the salt concentration of the third elution step is equal to the product
of
the salt concentration of the first elution step and a factor between
1.50 and 1.70.
The factor included in the calculation of the concentration steps accounts for
the
interval between the concentration levels and is adjusted depending on the
starting
concentration. At small starting concentrations, i.e. between 10 mM and 40 mM,
the factors are 1.35 and 1.70 respectively, at medium starting concentrations
be-
tween 40 mM and 70 mM the factors are 1.30 and 1.60 respectively, and at high
starting concentrations of more than 70 mM the factors are 1.25 and 1.50
respec-
tively.
The factors define a range that has been determined experimentally. These
values
are no absolute values but merely a target value. A deviation of 10% is
maintain-
able.
The buffer salt has to be accounted for in the calculation because it is
possible as
outlined in example 3 that the elution of a protein from an ion exchange resin
can
be effected by a change of the buffer salt concentration during the
chromatography.
If the buffer salt concentration is kept constant during the chromatography or
is
small compared to the stating concentration (< 15% of the salt concentration)
it
may be neglected during the calculation to reduce complexity.
With a starting concentration of 15 mM sodium citrate, as determined in exam-
ple 3, consisting of a 10 mM buffer concentration and a 5 mM contribution from
the linear gradient, the three steps can be calculated as follows:
- the target concentration for step 1 is calculated to be 30 mM (5 mM*2 +
10 mM*2) sodium citrate
in detail: 5 mM (starting concentration) multiplied with two (cit-
ric acid is a trivalent acid, employed as di-sodium salt;
therefore two monovalent cations different from hydro-
gen are present in the molecular formula) plus 10 mM
(buffer salt concentration) multiplied with two (citric
acid is a trivalent acid, employed as di-sodium salt;
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 23 -
therefore two monovalent cations different from hydro-
gen are present in the molecular formula)
- the target concentration for step 2 is calculated to be 40.5 mM (30 mM *
1.35) sodium citrate
in detail: 30 mM sodium citrate is the concentration of step 1 mul-
tiplied by 1.35 (the starting concentration is 15 mM,
therefore as factor 1.35 is selected)
- the target concentration for step 3 is calculated to be 51 mM (30 mM *
1.70) sodium citrate
in detail: 30 mM sodium citrate is the concentration of step 1 mul-
tiplied by 1.70 (the starting concentration is 15 mM,
therefore as factor 1.70 is selected)
Chromatographic conditions:
Resin: CM-Toyopearl
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.0
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.0
Elution: step 1: 34 mM sodium citrate buffer, adjusted to pH 5.0
step 2: 44 mM sodium citrate buffer, adjusted to pH 5.0
step 3: 54 mM sodium citrate buffer, adjusted to pH 5.0
The conditioned protein A eluate was applied to a chromatography column con-
taining a weak cation exchange resin (CM-Toyopearl). After the loading step at
a
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 24 -
In figure 4 the elution profile of the three step gradient elution of anti IL-
1R anti-
body is presented. The monomeric immunoglobulin elutes in the first step
fraction
and the aggregates elute in the second step fraction.
Example 5
Optimization of the chromatographic method ¨ third step: single step elution
with
sodium chloride.
The final step of the optimization procedure is the adaptation to a single
step elu-
tion method (= a method wherein the concentration of the elution salt is
changed
at once from a starting value to a final value). For this purpose the pH of
the chro-
matography is raised from 5.0 to 5.5. This pH shift improves the separation
from
protein A, due that protein A has an isoelectric point below 5.5. Additionally
the
elution salt is changed from sodium citrate, which is further on used as
buffer salt,
to sodium chloride. Additional analyses have been carried out (DNA, host cell
pro-
tein, protein A content, and glycosylation pattern with LC-MS) with the
fractions
after this chromatographic run.
Chromatographic conditions:
Resin: CM-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate, adjusted to pH 5.5
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate, adjusted to pH 5.5
Elution: 10 mM sodium citrate with 150 mM sodium chloride, adjusted to
pH 5.5
The conditioned protein A eluate was applied to a chromatography column con-
taining a weak cation exchange resin (CM-Sepharose). After the loading step at
a
flow rate of 160 cm/h the column was washed with equilibration buffer (10
column
volumes). The bound immunoglobulins were eluted with a single step gradient
elu-
tion method (= a method wherein the concentration of the elution salt is
changed
at once from a starting value to a final value), whereby the pH value in the
mobile
phase was kept constant. The concentration of the buffer salt, sodium citrate,
was
kept constant and 150 mM sodium chloride was admixed. After the increase of
the
salt concentration fifteen column volumes of the elution buffer were passed
through the column to elute the bound immunoglobulin.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 25 -
The elution chromatogram of the single step elution with sodium chloride is
pre-
sented in figure 5. The single step gradient chromatography effects resolution
of the
main monomeric fraction and the aggregate/protein A fraction. The yield of
monomeric immunoglobulin is more than 80 %. Even more than 95% yield is pos-
sible.
Example 6
Comparison between the separation with a strong cation exchange resin (SP-
Sepharose fast flow) and a weak cation exchange resin (CM-Sepharose fast
flow).
A comparison between the strong SP-Sepharose if exchanger and CM-Sepharose if
was done. Experiments were performed according to example 5 in duplicates
(only
one from each column is shown in figure 6a and b) and additional analyses have
been carried out (DNA, host cell protein, protein A content, and glycosylation
pat-
tern with LC-MS).
Analytical Methods:
Size Exclusion Chromatography: resin: TSK 3000 (Tosohaas)
column: 300 x 7.8 mm
flow rate: 0.5 ml/min
buffer: 200 mM potassium phosphate
containing
250 mM potassium chloride,
adjusted to pH 7.0
DNA-threshold-system: see e.g. Merrick, H., and Hawlitschek, G., Biotech
Forum Europe 9 (1992) 398-403
Protein A ELISA: The wells of a micro titer plate are coated with a poly-
clonal protein A-IgG derived from chicken. After
binding non-reacted antibody is removed by washing
with sample buffer. For protein A binding a defined
sample volume is added to the wells. The protein A
present in the sample is bound by the chicken anti-
body and retained in the wells of the plate. After the
incubation the sample solution is removed and the
wells are washed. For detection are added subse-
quently a chicken derived polyclonal anti-protein A-
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 26 -
IgG-biotin conjugate and a streptavidin peroxidase
conjugate. After a further washing step substrate solu-
tion is added resulting in the formation of a colored
reaction product. The intensity of the color is propor-
tional to the protein A content of the sample. After a
defined time the reaction is stopped and the absorb-
ance is measured.
Host cell protein (HCP) ELISA:
The walls of the wells of a micro titer plate are coated
with a mixture of serum albumin and streptavidin. A
goat derived polyclonal antibody against HCP is
bound to the walls of the wells of the micro titer plate.
After a washing step different wells of the micro titer
plate are incubated with a HCP calibration sequence
of different concentrations and sample solution. After
the incubation not bound sample material is removed
by washing with buffer solution. For the detection the
wells are incubated with an antibody peroxidase con-
jugate to detect bound host cell protein. The fixed
peroxidase activity is detected by incubation with
ABTS and detection at 405 nm.
Chromatographic conditions:
Resin: CM-Sepharose; SP-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.5
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.5
Elution: 10 mM sodium citrate buffer with 150 mM sodium chloride, ad-
justed to pH 5.5
In figures 6a and 6b a comparison between the elution chromatogram of a weak
and a strong cation exchange resin is presented. Using a weak cation exchange
resin
(figure 6a) a separation of the monomeric anti IL-1R antibody from other
impuri-
ties is achieved. With the strong cation exchange resin (figure 6b) no
separation is
possible under the same conditions. The fractions corresponding to the peaks
have
been collected and analyzed. The analysis results, which are listed in table
1, show
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 27 -
that with the weak cation exchange resin aggregates and other impurities can
effec-
tively be depleted from the immunoglobulin preparation.
The data presented in table 1 show that it is possible to separate with a weak
cation
exchange resin monomeric anti IL-1R antibody from aggregated forms of the anti-
body. Furthermore DNA- and protein A-impurities can be depleted.
Table 1: Analysis of the eluates: comparison between SP-Sepharose and CM-
Sepharose, results of two different separations are presented.
analyte conditioned SP-Sepharose eluate CM-Sepharose eluate
protein A single peak Peak 1 Peak 2
eluate
amount of between 20 31 26 7.5 11 1638 550
protein A and 50 ng/mg ng/mg ng/mg ng/mg ng/mg ng/mg
ng/mg
HCP between 20 3.88 3.98 3.13 3.27 946 1424
and 120 ng/mg ng/mg ng/mg ng/mg ng/mg ng/mg
ng/mg
DNA between 36 16 157 131 1918 1222
2800 and pg/mg pg/mg pg/mg pg/mg pg/mg pg/mg
3500 pg/mg
aggregates present present present not not present present
present present in high in
high
amount amount
mass no differences were found between SP- and CM-Sepharose
analysis
Example 7
Comparative example ¨ elution at different conductivities.
Chromatographic conditions:
Resin: CM-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.5
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.5
Elution: 10 mM sodium citrate buffer with 100 mM, 150 mM or 200 mM
sodium chloride, adjusted to pH 5.5
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 28 -
The conditioned protein A eluate was applied to a chromatography column con-
taining a weak cation exchange resin (CM-Sepharose). After the loading step at
a
flow rate of 160 cm/h the column was washed with equilibration buffer (10
column
volumes). The bound immunoglobulins were eluted with a single step gradient
elu-
tion method, whereby the pH value in the mobile phase was kept constant. The
concentration of the buffer salt, sodium citrate, was kept constant and in
three dif-
ferent runs 100 mM, 150 mM, and 200 mM sodium chloride respectively were ad-
mixed. After the increase of the salt concentration fifteen column volumes of
the
elution buffer were passed through the column to elute the bound immunoglobu-
lin. The elution chromatograms are displayed in figures 7a to c.
Good separations have been obtained using 150 mM sodium chloride and 200 mM
sodium chloride as elution salt concentration.
Example 8
Comparative example ¨ elution at different pH values.
Chromatographic conditions:
Resin: CM-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.5
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.5
Elution: 10 mM sodium citrate buffer with 150 mM sodium chloride, ad-
justed to pH 4.0, or 6.0
The conditioned protein A eluate was applied to a chromatography column con-
taming a weak cation exchange resin (CM-Sepharose). After the loading step at
a
flow rate of 160 cm/h the column was washed with equilibration buffer (10
column
volumes). The bound immunoglobulins were eluted with a single step gradient
elu-
tion method, whereby the pH value in the mobile phase was kept constant at pH
4.0
or 6.0 respectively. The concentration of the buffer salt, sodium citrate, was
kept
constant, and 150 mM sodium chloride was admixed. After the increase of the
salt
concentration fifteen column volumes of the elution buffer were passed through
the
column to elute the bound immunoglobulin. The elution chromatograms are dis-
played in figures 8a and b.
At pH 4.0 is the tendency to form aggregates of this immunoglobulin increased.
But
the CM-Sepharose is able to separate this higher amount of aggregates in two
peaks.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 29 -
Example 9
Chromatographic separation of a monoclonal anti-HER-2 antibody
(WO 99/57134) with a strong cation exchange resin (SP-Sepharose).
The current invention is further exemplified in the following with Herceptin ,
a
monoclonal anti-HER-2 antibody.
The purification of Herceptin with a cation exchange chromatography on
SP-Sepharose, a strong cation exchange resin, was carried out. Under standard
con-
ditions of the current invention, i.e. step elution with e.g. sodium chloride,
a sepa-
ration of monomeric and aggregated forms of the antibody is not effected
(figure
10).
Chromatographic conditions:
Resin: SP-Sepharose
Flow rate: 160 cm/h
Equilibration: 25 mM 2-morpholinoethanesulfonic acid, 50 mM sodium chloride,
adjusted to pH 5.6
Loading: max. 20 g protein/L gel matrix
Wash: 25 mM 2-morpholinoethanesulfonic acid, 50 mM sodium chloride,
adjusted to pH 5.6
Elution: 25 mM 2-morpholinoethanesulfonic acid, 95 mM sodium chloride,
adjusted to pH 5.6
The monoclonal anti-HER-2 antibody (hereinafter referred to as Herceptin') was
purified in a first step with a protein A affinity chromatography. Elution
from the
protein A column is carried out under acidic conditions (10 mM sodium citrate
buffer, pH value of 3.0 0.5). Before the filtration step the pH value of the
fraction
containing the antibody is adjusted with a concentrated tris-hydroxymethyl-
amino-
methane (TRIS) buffer to pH 5.6. The protein A eluate is a solution with a
protein
concentration between 5 mg/ml and 15 mg/ml and is buffered with sodium
citrate.
The conditioned protein A eluate was applied to a chromatography column con-
taming a strong cation exchange resin (SP-Sepharose). After the loading step
at a
flow rate of 160 cm/h the column was washed with equilibration buffer (10
column
volumes). The bound immunoglobulins were eluted with a single step elution
method, whereby the pH value was kept constant and the conductivity was varied
by the (stepwise) increase of the sodium chloride concentration. The elution
chro-
matogram is displayed in figure 10.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 30 -
No separation of monomeric and aggregated forms of the antibody was achieved.
Example 10
Optimization of the chromatographic method ¨ first step: linear concentration
gradient.
To improve the separation of the two fractions the separation conditions have
been
optimized in accordance with the procedure as outlined with the anti IL-1R
anti-
body.
In contrast to the anti IL-1R antibody optimization process a linear gradient
of a
(elution) salt, i.e. of sodium chloride, was used instead of a gradient of the
buffer
substance. The chromatogram of the linear sodium chloride gradient elution,
which
corresponds to the first step of the optimization procedure, is presented in
figure
11. Analysis confirmed that the tail of the main peak is enriched with
aggregated
forms of the antibody.
Chromatographic conditions:
Resin CM-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.5
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.5
Elution: linear gradient; from 10 mM sodium citrate buffer, adjusted to pH
5.5, to 10 mM sodium citrate buffer containing 400 mM sodium
chloride, adjusted to pH 5.5
The conditioned protein A eluate as described in example 9 was applied to a
chro-
matography column containing a weak cation exchange resin (CM-Sepharose).
After the loading step at a flow rate of 160 cm/h the column was washed with
equilibration buffer (10 column volumes). The bound immunoglobulins were
eluted with a linear gradient elution method, whereby the pH value in the
mobile
phase and the concentration of the buffer salt was kept constant. The
concentration
of the elution salt, sodium chloride, was raised linearly from 0 mM to 400 mM
over
column volumes. The elution chromatogram is displayed in figure 11.
The replacement of the strong cation exchange resin by a weak cation exchange
resin caused the detachment of a second peak as shoulder of the first main
peak.
This observation is similar to the observation in case of the anti IL-1R
antibody.
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 31 -
The immunoglobulins start to elute from the column at a sodium chloride concen-
tration of 80 mM.
Example 11
Optimization of the chromatographic method ¨ second step: three step concentra-
tion gradient elution.
The starting concentration, at which the immunoglobulin starts to elute, as
deter-
mined in example 10 and as derived from the chromatogram presented in figure
11,
is 80 mM sodium chloride. For the calculation of the three concentration steps
for
the second optimization step the buffer concentration can be neglected as it
is low
and kept constant during the chromatography.
The starting concentration of the sodium chloride is 80 mM and sodium chloride
has one cation different from hydrogen in its molecular formula. Accordingly
the
concentrations for the three step elution are calculated to be 80 mM, 100 mM
(= 80
mM multiplied with 1.25), and 120 mM (= 80 mM multiplied with 1.50) sodium
chloride respectively.
Chromatographic conditions:
Resin: CM-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate buffer, adjusted to pH 5.5
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate buffer, adjusted to pH 5.5
Elution: step 1: 10 mM sodium citrate buffer with 80 mM sodium
chloride,
adjusted to pH 5.5
step 2: 10 mM sodium citrate buffer with 100 mM sodium chloride,
adjusted to pH 5.5
step 3: 10 mM sodium citrate buffer with 120 mM sodium chloride,
adjusted to pH 5.5
The conditioned protein A eluate as described in example 9 was applied to a
chro-
matography column containing a weak cation exchange resin (CM-Sepharose).
After the loading step at a flow rate of 160 cm/h the column was washed with
equilibration buffer (10 column volumes). The bound immunoglobulins were
eluted with a step gradient elution method, whereby the pH value in the mobile
phase and the concentration of the buffer salt, sodium citrate, was kept
constant.
The concentration of the elution salt, sodium chloride, was raised from 0 mM
as
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 32 -
starting condition to 80 mM in the first step, to 100 mM in the second step,
and to
120 mM in the final step. After each increase of the salt concentration ten
column
volumes of the elution buffer with the specified sodium chloride
concentrations
were passed through the column prior to the next concentration step. After the
final
sodium citrate concentration was reached the elution was continued for an addi-
tional 10 column volumes. The elution chromatogram is displayed in figure 12.
In the three step elution method the monomeric antibody is eluted at the step
with
a sodium chloride concentration of 80 mM. Size exclusion analysis confirmed
that
only monomeric antibody is eluted. After the sodium chloride concentration was
increased to 100 mM in the second step, the aggregated forms eluted (figure
12).
Example 12
Optimization of the chromatographic method ¨ third step: single step elution
with
sodium chloride.
Chromatographic conditions:
Resin: CM-Sepharose; SP-Sepharose
Flow rate: 160 cm/h
Equilibration: 10 mM sodium citrate, adjusted to pH 5.5
Loading: max. 20 g protein/L gel matrix
Wash: 10 mM sodium citrate, adjusted to pH 5.5
Elution: 10 mM sodium citrate with 80 mM sodium chloride, adjusted to
pH 5.5
The conditioned protein A eluate as described in example 9 was applied to a
chro-
matography column containing a weak cation exchange resin (CM-Sepharose).
After the loading step at a flow rate of 160 cm/h the column was washed with
equilibration buffer (10 column volumes). The bound immunoglobulins were
eluted with a single step gradient elution method, whereby the pH value in the
mo-
bile phase and the concentration of the buffer salt was kept constant. The
concen-
tration of the buffer salt, sodium citrate, was kept constant and 80 mM sodium
chloride was admixed. After the increase of the salt concentration fifteen
column
volumes of the elution buffer with sodium chloride were passed through the col-
umn to elute the bound anti-HER-2 antibody in monomeric form. To affirm the
separation of monomeric and aggregated forms of the antibody a second step,
which is not necessary for the preparation of monomeric antibodies, to a
sodium
chloride concentration of 120 mM was performed. After this second increase the
CA 02607375 2007-11-07
WO 2006/125599
PCT/EP2006/004863
- 33 -
aggregated forms of the antibody eluted from the column. The elution chroma-
togram is displayed in figure 13.
If the same method is performed with a strong cation-exchange resin a
significant
loss of monomeric antibody is observed (yield of approximately 60 % only corn-
pared to 95% and more on a weak cation exchange column), although the separa-
tion of monomeric and aggregated form of the antibody can be seen (figure 14).
Applying the conditions suitable for the separation on a weak cation exchange
ma-
terial to a strong cation exchange resin, SP-Sepharose, is not beneficial.
Albeit the
two fractions can be separated the yield of the monomeric antibody is reduced
to
60 % or even less.
Table 2: Analysis of the eluates: comparison between SP-Sepharose and CM-
Sepharose, results of two different separations are presented.
analyte conditioned SP-Seph arose CM-Sepharose
protein A
eluate
Peak 1 Peak 2 Peak 1 Peak 2
amount of 17 ng/mg <3.9 5.7 <3.9 <3.9 32.2 39.7
protein A ng/mg ng/mg ng/mg ng/mg ng/mg ng/mg
HCP 3243 ng/mg 49.0 183.4 107.5 126.2 1247 988
ng/mg ng/mg ng/mg ng/mg ng/mg ng/mg
DNA 1615 pg/mg 830 2635 319 682 10554 <9300
pg/mg pg/mg pg/mg pg/mg pg/mg pg/mg
aggregates 0.97 % 0 % 32 % 0 % 0 % 15.2 % 23.0 %
(SEC)