Note: Descriptions are shown in the official language in which they were submitted.
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
IN THE UNITED STATES PATENT AND T1tADEMARK OFFICE
APPLICATION FOR UNITED STATES PATENT
INVENTOR(S): ANANDA CHAKRABARTY
TAPAS DAS GUPTA
ANITA CHAUDHARI
ARSENIO FIALHO
CHANG SOO HONG
TOHRU YAMADA
TITLE: COMPOSITIONS AND METHODS FOR
TREATING MALARIA WITH
CUPREDOXIN AND CYTOCHROME
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
COMPOSITIONS AND METHODS FOR TREATING MALARIA
WITH CUPREDOXIN AND CYTOCHROME
RELATED APPLICATIONS
This application claims priority to co-filed U.S. Provisional Patent
Application Serial
No. , entitled "Compositions and Methods for Treating HIV Infection with
Cupredoxin and Cytochrome c", filed , U.S. Provisional Patent Application
Serial
No. 60/780,868, filed March 10, 2006, U.S. Provisional Patent Application
Serial No.
60/682,813, filed May 20, 2005, and U.S. Patent Application No. 11/244,105,
filed October
6, 2005, which claims priority to U.S. Provisional Patent Application Serial
No. 60/616,782,
filed October 7, 2004, and U.S. Provisional Patent Application Serial No.
60/680,500, filed
May 13, 2005, and is a continuation-in-part of U.S. Patent Application Serial
Number
10/720,603, filed November 11, 2003, which claims priority to U.S. Provisional
Patent
Application Serial No. 60/414,550, filed August 15, 2003, and which is a
continuation-in-part
of U.S. Patent Application Serial Number 10/047,710, filed January 15, 2002,
which claims
priority to U.S. Provisional Patent Application Serial Number 60/269,133,
filed February 15,
2001. The entire content of these prior applications is fully incorporated
herein by reference.
STATEMENT OF GOVERNMENTAL INTEREST
The subject matter of this application has been supported by research grants
from the
National Institutes of Health (NIH), Bethesda, Maryland, U.S.A., (Grant
Numbers Al 16790-
21, ES 04050-16, Al 45541, CA09432 and N01-CM97567). The government may have
certain rights in this invention.
FIELD OF THE INVENTION
The present invention relates to cupredoxin and cytochrome and their use,
separately
or in combination, in inhibiting parasitemia of the malaria parasite, and in
particular
inhibiting parasitemia of Plasrnodium falciparufn in mammalian red blood
cells. The
invention also relates to variants and derivatives of cupredoxin and
cytochrome that retain the
ability to inhibit parasitemia by the malaria parasite. Finally, the invention
provides methods
to inhibit the spread of malaria infection in insect vectors.
2
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
BACKGROUND
About one quarter of the world's population is exposed.to the risk of malaria
and more
than a million people die of malaria each year. Of the four species of
malarial parasites that
infect humans, the two major species are Plasmodium falciparum and P. vivax.
The P. falciparuna blood stage merozoites bind to and parasitize the
erythrocytes using
a variety of surface proteins (Cowman et al., FEBS Lett. 476:84-88 (2000);
Baum et al., J.
Biol. Chem. 281:5197-5208 (2006)), a major antigenic member of which is called
Merozoite
Surface Protein 1(MSP1), a 195 kDa protein. MSPI is present in all the
erythrocyte-invasive
species of Plasm.odiurn, anchored to the merozoite surface by a glycosyl-
phosphatidylinositol
linkage. During early stages of the erythrocyte invasion process, soon after
release from
infected erythrocytes, the merdzoite MSP1 protein undergoes proteolytic
cleavage,
producing a C-terminal cleavage product MSP1-42, which subsequently undergoes
a second
cleavage, producing an 11 kDa peptide MSP1-19, which remains attached to the
parasite
surface as it enters the erythrocyte. The formation of the cleavage product
MSP1-19 is very
important for successful invasion by the parasite since inhibition of its
proteolytic formation
or its neutralization by monoclonal antibodies prevents entry of the parasite
to the
erythrocytes (Blackman et al., J. Exptl., Med. 180:389-393 (1994)).
The MSPI-19 peptide is one of the most important malaria vaccine candidates
available. MSP1-19-specific antibodies from malaria-resistant human sera react
with the
antigen and include a major erythrocyte-invasion inhibitory component (Holder
& Riley,
Parasitol. Today, 12: 173-174 (1996); O'Donnell et al., J. Expt. Med. 193:
1403-1412
(2001)). Serum from donors in malaria-endemic regions usually demonstrates
strong
antibody reactivity towards Pf MSPI-19. (Nwuba et al., Infect. Immun. 70: 5328-
5331
(2002))
The monoclonal antibody (mAb) G17.12 was raised against recombinant Pf MSP1-19
and recognizes its epitope on the parasite surface, demonstrating that this
region of the
antigen is accessible on the native MSP1 polypeptide complex (Pizarro et al.,
J. Mol. Biol.
328:1091-1103 (2003)). Interestingly, erythrocyte invasion experiments in
vitro showed that
infection is not inhibited in the presence of G 17.12, even at 200 g/ml
concentration and
G17.12 does not inhibit in vitro secondary processing of MSP1. Id. The
presence of
antibodies that block the binding of invasion - inhibitory antibodies, thereby
facilitating
parasite survival, has also been demonstrated (Guevara Patino et aL, J. Expt.
Med. 186: 1689-
3
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
1699(1997)), and may be responsible for the failure of G17.12 mAb to inhibit
erythrocyte
invasion by M. falciparuna.
Cerebral malaria, a rare but fatal infection restricted to P. falciparuzia
invasion of brain
capillaries because of the sequestration of parasitized erythrocytes, is often
untreatable
because most drugs cannot cross the blood-brain barrier to reach the brain
capillaries.
Adhesion of P. falciparum - infected erythrocytes to brain capillaries is
mediated by the
interaction of parasite ligands Pf Emp-1 family of proteins expressed on the
surface of
infected erythrocytes with ICAM-1 and CD36 expressed on the surface of
capillary
endothelium cells in cerebral vessels. (Smith et al., Proc. Natl. Acad. Sci.
USA 97:1766-
1771 (2000); Franke-Fayard et al., Proc. Natl. Acad. Sci. USA 102, 11468-11473
(2005))
Although a few drugs, such as chloroquine that targets the heme detoxification
pathway, are used to treat malaria, there are increasing incidence of parasite
resistance to
drugs and mosquito vector resistance to insecticides. Chloroquine antagonizes
heme
polymerization mediated by parasite-induced HRPs (histidine-rich proteins), as
heme
monomers are highly toxic for malaria parasites. The polymerization of heme
allows
detoxification, which is reversed by chloroquine. Another drug, artemisinin,
is effective
against chloroquine-resistant P. falciparum in cerebral malaria. Artemisinin
forms adducts
with globin-bound heme in hemoglobin, which binds FIRPs to prevent heme
polymerization.
There is an urgent need to find new drugs for this dreaded disease that is
particularly
prevalent in Africa and Asia. Present attempts at drug development are
directed towards
deciphering the complete parasite genome sequence, molecular modeling of the
malaria
parasite proteins and a search for novel drug targets.
SUMMARY OF THE INVENTION
The present invention relates to cupredoxin and cytochrome and their use,
separately
or together, to inhibit the spread of parasitemia in mammalian red blood cells
and other
tissues infected by the malaria parasite, and in particular the parasitemia of
human red blood
cells by P. falciparurn.
One aspect of the invention is an isolated peptide that is a variant,
derivative or
structural equivalent of a cupredoxin or cytochrome; and that can inhibit
intracellular
replication of a malarial parasite in malaria-infected human red blood cells.
4
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Another aspect of the invention is an isolated peptide that is a variant,
derivative or
structural equivalent of a cupredoxin; and that can bind a protein selected
from the group
consisting of PfMSPl-19 and PfMSP1-42.
Another aspect of the invention is an isolated peptide that is a variant,
derivative or
structural equivalent of a cupredoxin or cytochrome, and that can inhibit
parasitemia by
malaria in malaria-infected red blood cells. Specifically, the isolated
peptide can inhibit
parasitemia by malaria in P. falciparum-infected human red blood cells. In
some
embodiments, the cupredoxin is an azurin, pseudoazurin, plastocyanin,
rusticyanin, Laz or
auracyanin. Specifically, the cupredoxin may be rusticyanin, azurin or Laz. In
some
embodiments, the cupredoxin is from Pseudomonas aeruginosa,
Alcaligenesfaecalis,
Achromobacter xylosoxidan, Bordetella bronchiseptica, Methylon2onas sp.,
Neisseria
meningitidis, Neisseria gonorrhea, Pseudomonasfluorescens, Pseudomonas
chlororaphis,
Xylellafastidiosa or Vibrio parahaemolyticus. Specifically, the cupredoxin may
be from
Thiobacillusferrooxidans, Pseudomonas aeruginosa, Neisseria gonorrhea or
Neisseria
meningitidis.
In other embodiments of this aspect, the cytochrome is cytochrome c or
cytochrome f.
In particular, the cytochrome c may be from human or Pseudomonas aeruginosa.
The
cytochrome f may be from a cyanobacteria.
In other embodiments of this aspect, the isolated peptide is a truncation of a
peptide
selected from the group consisting of SEQ ID NOS: 1-20 and 22. In some
embodiments,
SEQ ID NOS: 1-20 or 22 has at least 90% amino acid sequence identity to the
sequence of
the isolated peptide.
In some embodiments of this aspect, the isolated peptide is a truncation of
cupredoxin
or cytochrome. In some embodiments, the isolated peptide is more than about 10
residues
and not more than about 100 residues. The isolated peptide may comprise azurin
residues 36-
89. Alternatively, the isolated peptide may consist of azurin residues 36-89.
Alternatively,
the isolated peptide may comprise equivalent residues of a cupredoxin as
azurin 36-89.
In other embodiments of this aspect, the isolated peptide is fused to a H.8
region of
Laz. In another embodiment, the isolated peptide is a structural equivalent of
monoclonal
antibody G17.12.
Another aspect of the invention is a composition comprising at least one
cupredoxin,
cytochrome, or isolated peptide that is a variant, derivative or structural
equivalent of a
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
cupredoxin or cytochrome that can inhibit parasitemia by malaria in malaria-
infected red
blood cells, in a pharmaceutical composition. Specifically, the pharmaceutical
composition
may be formulated for intravenous administration. The composition may comprise
another
anti-malarial drug or an anti-HIV drug.
In some embodiments of the composition, the cupredoxin is from Pseudomonas
aeruginosa, Alcaligenes faecalis, Achromobacter xylosoxidan, Bordetella
bronchiseptica,
Methylomonas sp., Neisseria meningitidis, Neisseria gonorrhea, Pseudomonas
fluorescens,
Pseudomonas chlororaphis, Xylella fastidiosa or Vibrib parahaemolyticus.
Specifically, the
cupredoxin may be from Thiobacillus ferrooxidans, Pseudomonas aeruginosa,
Neisseria
gonorrhea or Neisseria meningitidis.
In some embodiments of the composition, the cytochrome is cytochrome c or
cytochrome f. Specifically, the cytochrome c may be from human or Pseudomonas
aeruginosa. The cytochrome f may be from a cyanobacteria. In some embodiments,
the
cupredoxin or cytochrome c is SEQ ID NOS: 1-20 or 22.
Another aspect of the invention is a method to treat a patient suffering from
an
infection by a malaria parasite by administering to the patient an effective
amount of the
composition of the invention. In specific embodiments, the peptide inhibits
parasitemia by
malaria in the patient's malaria-infected human red blood cells. In some
embodiments, the
malaria parasite is Plasmodium vivax or Plasmodium falciparum. In some
embodiments, the
patient is additionally suffering from HIV infection. In some embodiments, the
composition
is administered with a second composition that may contain an anti-malarial
drug and/or an
anti-HIV drug. In some embodiments, the composition of the invention is
administered
within 0 minutes to 12 hours of the administration of second composition. In
some
embodiments, the composition of the invention is administered to the patient
orally, by
inhalation, intravenously, intramuscularly or subcutaneously; and,
specifically, the
composition may be administered to the patient intravenously.
Another aspect of the invention is a method to treat a patient suspected of
having
contact with a malaria parasite, comprising administering to the patient an
effective amount
of the composition of the invention.
Another aspect of the invention is a method to prevent malaria in mammals,
comprising administering to an insect vector in a population of insect vectors
harboring a
6
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
malaria parasite an amount of the composition of the invention. In some
embodiments of this
method, the composition is administered to the insect vector orally.
These and other aspects, advantages, and features of the invention will become
apparent from the following figures and detailed description of the specific
embodiments.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1. Figure 1 depicts surface plasmon resonance binding titrations
depicting the
interactions of Azurin, H.8-azurin (H.8-Az), Laz, and GST-azurin (GST-Azu)
constructs with
MSP1-19,and MSPl-42. (A) Binding curves demonstrating the interactions of
azurin and its
analogues with MSP1-19 immobilized on carboxymethyldextran coated gold sensor
chips
(MSP1-19-CM5). Concentration dependent binding of the azurin proteins to MSP1-
19 was
determined via injection of various concentrations (0.05-300 nM) over the
sensor surface and
the extent of binding was evaluated as a function of the equilibrium resonance
response value
measured in resonance units (RU). While H.8-Az and Laz bound somewhat more
strongly
than azurin, no binding was seen with GST or H.8-GST. (B) In vitro binding
titrations for
immobilized MSP1-42 with azurin and its analogues was followed in a similar
manner to that
for MSPl-19 as shown in (A). Relative binding affinities were determined via
fitting the data
to Req = Rmax/(1+(Kd/C)) with the curve fits connecting the data points in the
graphs. The
MSP1-19 binding Kd values are: 32.2 2.4 nM (azurin), 26.2 :L 2.4 nM (Laz),
11.8 0.3 nM
(H.8-Az), and those for MSP1-42 binding are: 54.3 7.6 nM (azurin), 45.6
2.4 nM (Laz)
and 14.3 .+ 1.7 nM (H.8-Az). (C) Binding titrations for the interactions of
GST-Azu fusion
proteins over the MSP1-19-CM5 sensors surface demonstrate the recognition of
GST-Azu
36-128 and GST-Azu 36-89 with MSP1-19. No binding was seen with GST or GST-Azu
88-
113.
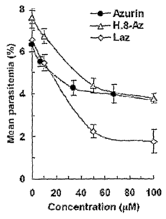
Figure 2. Figure 2 depicts inhibition of P. falciparum parasitemia (parasite
growth
within the RBC) by different concentrations, as shown, of Azurin, H.8-azurin
(H.8-Az) and
Laz. In these experiments, normal red blood cells were infected with schizonts
in absence or
in presence of the proteins at different concentrations, incubated overnight
and the number of
intracellular parasites was scored by thin blood smear and Giemsa staining.
Figure 3. Figure 3 depicts surface plasmon resonance binding curves for the
binding
of ICAMs (ICAM-1, ICAM-2, ICAM-3 and NCAM, inset) with immobilized azurin. Due
to
large nonspecific binding to the bare Au-CM5 chip, CM5 was added as an eluent
to the
7
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
running buffer (1 mg/ml CM5 to HBS-EP buffer). The selective recognition of
azurin with
ICAM-3, but not with ICAM-1 or ICAM-2, is notable and the binding strength was
19.5 ~
5.4 nM. The Kd for NCAM binding with azurin, as shown in the inset, was 20 =L
5.0 nM.
BRIEF DESCRIPTION OF THE SEQUENCES
SEQ ID NO: 1 is the amino acid sequence of azurin from Pseudonaonas
aeruginosa.
SEQ ID NO: 2 is the amino acid sequence of cytochrome c551 from Pseudomonas
aeruginosa.
SEQ ID NO: 3 is the amino acid sequence of Laz from Neisseria meningitidis
MC58.
SEQ ID NO: 4 is the amino acid sequence of plastocyanin from Phormidiuni
laminosum.
SEQ ID NO: 5 is the amino acid sequence of rusticyanin from Thiobacillus
ferrooxidans (Acidithiobacillus ferrooxidans).
SEQ ID NO: 6 is the amino acid sequence of pseudoazurin from Achromobacter
cycloclastes.
SEQ ID NO: 7 is the amino acid sequence of azurin from Alcaligenesfaecalis.
SEQ ID NO: 8 is the amino acid sequence of azurin from Achromobacter
xylosoxidans ssp. denitrificans I.
SEQ ID NO: 9 is the amino acid sequence of azurin from Bordetella
bronchiseptica.
SEQ ID NO: 10 is the amino acid sequence of azurin from Methylomonas sp. J.
SEQ ID NO: I 1 is the amino acid sequence of azurin from Neisseria
meningitidis
Z2491.
SEQ ID NO: 12 is the amino acid sequence of azurin from Pseudomonas f
uorescens.
SEQ ID NO: 13 is the amino acid sequence of azurin from Pseudomonas
chlororaphis.
SEQ ID NO: 14 is the amino acid sequence of azurin from Xylella fastidiosa
9a5c.
SEQ ID NO: 15 is the amino acid sequence of stellacyanin from Cucumis sativus
SEQ ID NO: 16 is the amino acid sequence of auracyanin A from Chloroflexus
aurantiacus
SEQ ID NO: 17 is the amino acid sequence of auracyanin B from Chloroflexus
aur'antiacus
8
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
SEQ ID NO: 18 is the amino acid sequence of cucumber basic protein from
Cucumis
sativus
SEQ ID NO: 19 is the amino acid sequence of cytochrome c from Homo sapiens.
SEQ ID NO: 20 is the amino acid sequence of cytochrome f from cyanobacteria
PhoNmidium laminosum.
SEQ ID NO: 21 is the amino acid sequence of the H.8 region of Laz from
Neisseria
gonorrhoeae F62.
SEQ ID NO: 22 is the amino acid sequence, of Laz from Neisseria gonorrhoeae
F62.
SEQ ID NO: 23 is the forward primer to PCR amplify the Laz-encoding gene (laz)
of
Neisseria gonorrhoeae.
SEQ ID NO: 24 is the reverse primer to PCR amplify the Laz-encoding gene (laz)
of
Neisseria gonorrhoeae.
SEQ ID NO: 25 is the forward primer to PCR amplify a 3.1 kb fragment of pUC 18-
laz .
SEQ ID NO: 26 is the reverse primer to PCR amplify a 3.1 kb fragment of pucl8-
laz.
SEQ ID NO: 27 is the forward primer to PCR amplify a 0.4 kb fragment of pUC 19-
paz.
SEQ ID NO: 28 is the reverse primer to PCR amplify a 0.4 kb fragment of pUC 19-
paz.
SEQ ID NO: 29 is the forward primer for pGST-azu 36-128.
SEQ ID NO: 30 is the reverse primer for pGST-azu 36-128.
SEQ ID NO: 31 is the forward primer for pGST-azu 36-89.
SEQ ID NO: 32 is the reverse primer for pGST-azu 36-89.
SEQ ID NO: 33 is the forward primer for pGST-azu 88-113.
SEQ ID NO: 34 is the reverse primer for pGST-azu 88-113.
SEQ ID NO: 35 is an oligonucleotide for site directed mutagenesis for the
preparation
of pGST-azu 88-113.
SEQ ID NO: 36 is an oligonucleotide for site directed mutagenesis for the
preparation
of pGST-azu 88-113.
9
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
DETAILED DESCRIPTION OF THE INVENTION
Definitions
As used herein, the term "cell" includes both the singular or the plural of
the term,
unless specifically described as a "single cell."
As used herein, the terms "polypeptide," "peptide," and "protein" are used
interchangeably to refer to a polymer of amino acid residues. The terms apply
to amino acid
polymers in which one or more amino acid residue is an artificial chemical
analogue of a
corresponding naturally occurring amino acid. The terms also apply to
naturally occurring
amino acid polymers. The terms "polypeptide," "peptide," and "protein" are
also inclusive of
modifications including, but not limited to, glycosylation, lipid attachment,
sulfation, gamma-
carboxylation of glutamic acid residues, hydroxylation and ADP-ribosylation.
It will be
appreciated that polypeptides are not always entirely linear. For instance,
polypeptides may
be branched as a result of ubiquitination and they may be circular (with or
without
branching), generally as a result of post-translation events, including
natural processing event
and events brought about by human manipulation which do not occur naturally.
Circular,
branched and branched circular polypeptides may be synthesized by non-
translation natural
process and by entirely synthetic methods as well.
As used herein, the term "pathological condition" includes anatomic and
physiological deviations from the normal that constitute an impairment of the
normal state of
the living animal or one of its parts, that interrupts or modifies the
performance of the bodily
functions, and is a response to various factors (as malnutrition, industrial
hazards, or climate),
to specific infective agents (as worms, parasitic protozoa, bacteria, or
viruses), to inherent
defects of the organism (as genetic anomalies), or to combinations of these
factors.
As used herein, the term "condition" includes anatomic and physiological
deviations
from the normal that constitute an impairment of the norrnal state of the
living animal or one
of its parts, that interrupts or modifies the performance of the bodily
functions.
As used herein, the term "suffering from" includes presently exhibiting the
symptoms
of a pathological condition, having a pathological condition even without
observable
symptoms, in recovery from a pathological condition, or recovered from a
pathological
condition.
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
As used herein, the term "parasitemia" includes a condition in which parasites
are
present in the blood and other tissues, and in particular to indicate the
presence of parasites
with or without clinical symptoms.
As used herein, the term "inhibition of parasitemia" refers to a decrease or a
lessening
of the rate of increase of the presence of the parasite in the blood of a
mammal. Inhibition is
any decrease or lessening of the rate of increase that is statistically
significant as compared to
control treatments.
A used herein, the term "treatment" includes preventing, lowering, stopping,
or
reversing the progression or severity of the condition or symptoms associated
with a
condition being treated. As such, the term "treatment" includes medical,
therapeutic, and/or
prophylactic administration, as appropriate.
As used herein, "anti-malarial activity" includes any activity that decreases
the
infectivity, the reproduction, or inhibits the progress of the lifecycle of a
malaria parasite.
"Anti-malarial activity" includes inhibition of the growth of malaria
infection by all of the
means of observed with current anti-malarial drugs.
As used herein, the term "anti-malarial drug" refers to drugs with anti-
malarial
activity that may be used to decrease the infectivity, the reproduction, or
inhibit the progress
of the lifecycle of a malaria parasite.
As used herein, the term "anti-HIV drug" refers to drugs with anti-HIV
activity HIV
by which HIV infection in mammals is decreased, or prevented from increasing
in the human
body, by any means including, but are not limited to, inhibition of
replication of the HIV
genome, inhibition of synthesis and/or assembly of the HIV coat proteins, and
inhibition of
HIV entry into uninfected cells.
The term "substantially pure", when used to modify the term a polypeptide or
other
compound, as used herein, refers to a polypeptide or compound, for example, a
polypeptide
isolated from the growth medium, in a form substantially free of, or
unadulterated by, active
inhibitory agents. The term "substantially pure" refers to a compound in an
amount of at
least about 75%, by dry weight, of isolated fraction, or "75% substantially
pure." More
specifically, the term "substantially pure" refers to a compound of at least
about 85%, by dry
weight, active compound, or "85% substantially pure." Most specifically, the
term
"substantially pure" refers to a compound of at least about 95%, by dry
weight, active
compound, or "95% substantially pure." The substantially pure cupredoxin or
cytochrome
11
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
c551 or a variant or derivative thereof can be used in combination with one or
more other
substantially pure compounds, or another isolated cupredoxin or cytochrome.
The phrases "isolated," "purified" or "biologically pure" refer to material
which is
substantially or essentially free from components which normally accompany the
material as
it is found in its native state. Thus, isolated peptides in accordance with
the invention
preferably do not contain materials normally associated with the peptides in
their in situ
environment. An "isolated" region refers to a region that does not include the
whole
sequence of the polypeptide from which the region was derived. An "isolated"
nucleic acid,
protein, or respective fragment thereof has been substantially removed from
its in vivo
environment so that it may be manipulated by the skilled artisan, such as but
not limited to
nucleotide sequencing, restriction digestion, site-directed mutagenesis, and
subcloning into
expression vectors for a nucleic acid fragment as well as obtaining the
protein or protein
fragment in substantially pure quantities.
The term "variant" as used herein with respect to a peptide, refers to amino
acid
sequence variants which may have amino acids replaced, deleted, or inserted as
compared to
the wild-type polypeptide. Variants may be truncations of the wild-type
peptide. Thus, a
variant peptide may be made by manipulation of genes encoding the polypeptide.
A variant
may be made by altering the basic composition or characteristics of the
polypeptide, but not
at least some of its fundamental activities. For example, a "variant" of
azurin can be a
mutated azurin that retains its ability to inhibit parasitemia in malaria-
infected human red
blood cells. In some cases, a variant peptide is synthesized with non-natural
amino acids,
such as s-(3,5-dinitrobenzoyl)-Lys residues. (Ghadiri &Fernholz, J. Am. Chem.
Soc.,
112:9633-9635 (1990)) In some embodiments, the variant has not more than 20
amino acids
replaced, deleted or inserted compared to wild-type peptide. In some
embodiments, the
variant has not more than 15, 14, 13, 12 or 11 amino acids replaced, deleted
or inserted
compared to wild-type peptide. In some embodiments, the variant has not more
than 10, 9, 8
or 7 amino acids replaced, deleted or inserted compared to wild-type peptide.
In some
embodiments, the variant has not more than 6 amino acids replaced, deleted or
inserted
compared to wild-type peptide. In some embodiments, the variant has not more
than 5 or 4
amino acids replaced, deleted or inserted compared to wild-type peptide. In
some
embodiments, the variant has not more than 3, 2 or 1 amino acids replaced,
deleted or
inserted compared to wild-type peptide.
12
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
The term "amino acid," as used herein, means an amino acid moiety that
comprises
any naturally-occurring or non-naturally occurring or synthetic amino acid
residue, i.e., any
moiety comprising at least one carboxyl and at least one amino residue
directly linked by one,
two three or more carbon atoms, typically one (a) carbon atom.
The term "derivative" as used herein with respect to a peptide refers to a
peptide that
is derived from the subject peptide. A derivation includes chemical
modifications of the
peptide such that the peptide still retains some of its fundamental
activities. For example, a
"derivative" of azurin can be a chemically modified azurin that retains its
ability to inhibit
parasitemia in malaria-infected red blood cells. Chemical modifications of
interest include,
but are not limited to, amidation, acetylation, sulfation, polyethylene glycol
(PEG)
modification, phosphorylation or glycosylation of the peptide. In addition, a
derivative
peptide maybe a fusion of a polypeptide or fragment thereof to a chemical
compound, such as
but not limited to, another peptide, drug molecule or other therapeutic or
pharmaceutical
agent or a detectable probe.
The term "percent (%) amino acid sequence identity" is defined as the
percentage of
amino acid residues in a polypeptide that are identical with amino acid
residues in a candidate
sequence when the two sequences are aligned. To determine % amino acid
identity,
sequences are aligned and if necessary, gaps are introduced to achieve the
maximum %
sequence identity; conservative substitutions are not considered as part of
the sequence
identity. Amino acid sequence alignment procedures to determine percent
identity are well
known to those of skill in the art. Often publicly available computer software
such as
BLAST, BLAST2, ALIGN2 or Megalign (DNASTAR) software is used to align peptide
sequences. In a specific embodiment, Blastp (available from the National
Center for
Biotechnology Information, Bethesda MD) is used using the default parameters
of long
complexity filter, expect 10, word size 3, existence 11 and extension 1.
When amino acid sequences are aligned, the % amino acid sequence identity of a
given amino acid sequence A to, with, or against a given amino acid sequence B
(which can
alternatively be phrased as a given amino acid sequence A that has or
comprises a certain %
amino acid sequence identity to, with, or against a given amino acid sequence
B) can be
calculated as:
% amino acid sequence identity = X/Y* 100
where
13
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
X is the number of amino acid residues scored as identical matches by the
sequence alignment program's or algorithm's alignment of A and B and
Y is the total number of amino acid residues in B.
If the length of amino acid sequence A is not equal to the length of amino
acid
sequence B, the % amino acid sequence identity of A to B will not equal the %
amino acid
sequence identity of B to A. When comparing longer sequences to shorter
sequences, the
shorter sequence will be the "B" sequence, unless stated otherwise. For
example, when
comparing truncated peptides to the corresponding wild-type polypeptide, the
truncated
peptide will be the "B" sequence.
A "therapeutically effective amount" is an amount effective to prevent or slow
the
development of, or to partially or totally alleviate the existing symptoms in
a particular
condition, pathological or otherwise, for which the subject being treated.
Determination of a
therapeutically effective amount is well within the capability of those
skilled in the art.
General
The present invention provides compositions and methods that use cupredoxin
and/or
cytochrome to inhibit parasitemia of malaria-infected mammalian red blood
cells and bodily
tissues, such as brain tissue and bone tissue.
Previously it was known that several bacterial redox proteins belonging to a
family of
the blue copper-containing proteins called cupredoxins, or the iron (haem) -
containing
proteins called cytochromes, enter mammalian cells, including cancer cells,
and either induce
apoptotic cell death or cause growth inhibition through G1 arrest of the cell
cycle. (Yamada
et al., Cell Cycle 3:752-755 (2004); Yamada et aL, Cell Cycle 3:1182-1187
(2004)) Single
bacterial proteins such as the cupredoxin azurin or the cytochrome c551, both
elaborated by
Pseudomonas aeruginosa, can demonstrate either activity based on their
hydrophobicity.
Thus wild type (wt) azurin induces apoptosis in the murine J774 cells (Yamada
et al.,
Infection and Immunity 70:7054-7062 (2002)) while a mutant M44KM64E azurin
causes cell
cycle inhibition at the G1 phase in J774 cells. (Yamada et al., PNAS 101:4770-
4775 (2004))
In contrast, wt cytochrome c551 causes cell cycle inhibition at the G1 phase
in J774 cells
while a mutant V23DI59E cytochrome c551 induces apoptosis. (Hiraoka et al.,
PNAS
101:6427-6432 (2004))
14
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
In accordance with the present invention, it is surprisingly now known that
cupredoxins and cytochromes will inhibit in vitro parasitemia in human red
blood cells by
the malaria parasite Plasmodium falciparum. In particular, the cupredoxins
azurin and Laz
inhibit parasitemia in P. falciparum by about 50% and about 75% respectively.
See, Example
6. Further, rusticyanin and cytochromes c and f inhibited parasitemia by 20-30
%. See,
Example 1. Further, it is now known that azurin has a discernable structural
homology to the
Fab fragment of G17.12 mouse monoclonal antibody when complexed to the Pf MSPl-
19
fragment of the MSP 1 surface protein of P. falciparum. See, Example 2. While
not limiting
the mode of inhibition to any one means, it is thought that azurin may inhibit
parasitemia of
P. falciparum by interaction with the MSP1 protein on the parasite's surface.
Surprisingly, it is now known that azurin and Laz bind both the PfMSP1-19 and
PfMSP1-42 P. falciparuni surface proteins in vitro. Further, it is now lcnown
that azurin
amino acid residues 36-89 are required for binding to PfMSP1-19 and PfMSPI-42.
Further,
it is now known that the H.8 domain of Laz from N. gonorrhea increases both
the binding of
a fused azurin to PfMSPl-19 as well as inhibition of parasitemia by P.
falcipaNum. See,
Examples 5 and 6.
Because of the high structural homology between the cupredoxins, it is
contemplated
that other cupredoxins will have the same anti-malarial activity as the
azurin, rusticyanin, or
Laz. In some embodiments, the cupredoxin is, but is not limited to, azurin,
pseudoazurin,
plastocyanin, auracyanin, Laz or rusticyanin. In specific embodiments, the
cupredoxin is
Laz, azurin or rusticyanin. In other embodiments, the cupredoxin is from a
pathogenic
bacteria. In a more specific embodiment, the cupredoxin is azurin. In
particularly specific
embodiments, the azurin is derived from Pseudornonas aeruginosa, Alcaligenes
faecalis,
Achrornobacter xylosoxidans ssp.denitrificans I, Bordetella bronchiseptica,
Metlaylomonas
sp., Neisseria meningitidis Z2491, Pseudomonasfluorescens, Pseudomonas
chlororaphis,
Xylellafastidiosa 9a5 or Vibnio parahaemolyticus. In a most specific
embodiment, the azurin
is from P. aeruginosa. In other specific embodiments, the cupredoxin comprises
an amino
acid sequence that is SEQ ID NO:1, 2-18 or 21.
In accordance with the present invention, it has been learned that P.
aeruginosa
cytochrome c551, human cytochrome c and Phorrnidiutn laminosum cytochrome f
will inhibit
parasitemia in malaria-infected human red blood cells. In a specific
embodiment, the
cytochrome is cytochrome e551 from P. aeruginosa, human cytochrome c or
cytochrome f. In
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
other specific embodiments, the cytochrome comprises an amino acid sequence
that is SEQ
ID NO: 2, 19 or 20.
Because of the structural homology between the cytochrome c's, it is
contemplated
that other cytochromes will have the same anti-malarial activity.as P.
aeruginosa cytochrome
cssl and human cytochrome c. In some embodiments, the cytochrome is from a
pathogenic
bacterium. In another specific embodiment, the cytochrome inhibits parasitism
in malaria-
infected red blood cells, and more specifically, human red blood cells. In
another specific
embodiment, the cytochrome inhibits cell cycle progression in a mammalian
cancer cell, and
more specifically in a J774 cell.
Compositions of the Invention
The invention provides for peptides that are variants, derivatives or
structural
equivalents of cupredoxin or cytochrome. In some embodiments, the peptide is
substantially
pure. In other embodiments, the peptide is isolated. In some embodiments, the
peptide is
less that a full length cupredoxin or cytochrome, and retains some of the
functional
characteristics of the cupredoxin or cytochrome. In some embodiments, the
peptide retains
the ability to inhibit parasitemia in malaria-infected red blood cells, and
more specifically the
ability to inhibit P. falciparum infection in human red blood cells. In
specific embodiments,
the cytochrome is P. aeruginosa cytochrome cssi, human cytochrome c, or
cyanobacterial
cytochrome f, and specifically SEQ ID NOS: 2, 19, and 20.. In another specific
embodiment,
the peptide does not raise an immune response in a mammal, and more
specifically a human.
The invention also provides compositions comprising at least one peptide that
is a
cupredoxin, cytochrome, or variant, derivative or structural equivalent of a
cupredoxin or
cytochrome. The invention also provides compositions comprising at least one
peptide that is
a cupredoxin or variant, derivative or structural equivalent of a cupredoxin.
The invention
also provides compositions comprising at least one peptide that is a
cytochrome, or variant,
derivative or structural equivalent of a cytochrome. In other embodiments, the
composition
consists essentially of the peptide. The invention also provides compositions
comprising at
least one peptide that is a cupredoxin, cytochrome, or variant, derivative or
structural
equivalent of a cupredoxin or cytochrome in a pharmaceutical composition.
Because of the high structural homology between the cupredoxins, it is
contemplated
that other cupredoxins will have the same anti-malarial activity as
Pseudonzonas aeruginosa
16
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
azurin with regards to inhibition of parasitemia in malaria-infected red blood
cells. In some
embodiments, the cupredoxin is, but is not limited to, azurin, pseudoazurin,
plastocyanin,
rusticyanin, Laz or auracyanin. In particularly specific embodiments, the
cupredoxin is
derived from Pseudomonas aeruginosa, Alcaligenesfaecalis, Achromobacter
xylosoxidans
ssp.denitrificans I, Bordetella bronchiseptica, Methylomonas sp., Neisseria
meningitidis
Z2491, Neisseria gonorrhea, Pseudornonas fluorescens, Pseudomonas
chlororaphis, Xylella
fastidiosa 9a5 or Vibrio parahaemolyticus. In a very specific embodiment, the
cupredoxin is
azurin from Pseudomonas aeruginosa. In other specific embodiments, the
cupredoxin
comprises an amino acid sequence that is SEQ ID NO: 1, 3-18, or 22. In other
specific
embodiments, the cupredoxin is the Laz protein from Neisseria meningitidis or
Neisseria
gonorrhea.
The invention provides for amino acid sequence variants of a cupredoxin or
cytochrome which have amino acids replaced, deleted, or inserted as compared
to the wild-
type polypeptide. Variants of the invention may be truncations of the wild-
type polypeptide.
In some embodiments, the composition comprises a peptide that consists of a
region of a
cupredoxin or cytochrome that is less that the full length wild-type
polypeptide. In some
embodiments, the composition comprises a peptide that consists of more than
about 10
residues, more than about 15 residues or more than about 20 residues of a
truncated
cupredoxin or cytochrome. In some embodiments, the composition comprises a
peptide that
consists of not more than about 100 residues, not more than about 50 residues,
not more than
about 40 residues or not more than about 30 residues of a truncated cupredoxin
or
cytochrome. In some embodiments, composition comprises a peptide to which a
cupredoxin
or cytochrome, and more specifically to SEQ ID NOS: 1-20 or 22 has at least
about 90%
amino acid sequence identity, at least about 95% amino acid sequence identity
or at least
about 99% amino acid sequence identity.
In specific embodiments, the variant of cupredoxin comprises P. aeruginosa
azurin
residues 36-89. In other embodiments, the variant of cupredoxin consists of P.
aeruginosa
azurin residues 36-89. In other specific embodiments, the variant consists of
the equivalent
residues of a cupredoxin other that azurin.
It is contemplated that other cupredoxin variants can be designed that have a
similar
activity to azurin residues 36-89. To do this, the subject cupredoxin amino
acid sequence
will be aligned to the Pseudornonas aeruginosa azurin sequence using BLAST,
BLAST2,
17
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
ALIGN2 or Megalign (DNASTAR), the relevant residues located on the P.
aeruginosa azurin
amino acid sequence, and the equivalent residues found on the subject
cupredoxin sequence,
and the equivalent residues of the cupredoxin thus identified.
The variants also include peptides made with synthetic amino acids not
naturally
occurring. For example, non-naturally occurring amino acids may be integrated
into the
variant peptide to extend or optimize the half-life of the composition in the
bloodstream.
Such variants include, but are not limited to, D,L-peptides (diastereomer),
(Futaki et al., J.
Biol. Chem. 276(8):5836-40 (2001); Papo et al., Cancer Res. 64(16):5779-86
(2004); Miller
et al, Biochem. Pharmacol. 36(1):169-76, (1987)); peptides containing unusual
amino acids
(Lee et al., J. Pept. Res. 63(2):69-84 (2004)), and incorporation of olefin-
containing non-
natural amino acid followed by hydrocarbon stapling (Schafineister et al., J.
Am. Chem. Soc.
122:5891-5892 (2000); Walenski et al., Science 305:1466-1470 (2004)) and
peptides
comprising s-(3,5-dinitrobenzoyl)-Lys residues.
In other embodiments, the peptide of the invention is a derivative of a
cupredoxin or
cytochrome. The derivatives of cupredoxin or cytochrome are chemical
modifications of the
peptide such that the peptide still retains some of its fundamental
activities. For example, a
"derivative" of azurin can be a chemically modified azurin that retains its
ability to inhibit the
malaria parasitemia in mammalian cells. Chemical modifications of interest
include, but are
not limited to, amidation, acetylation, sulfation, polyethylene glycol (PEG)
modification,
phosphorylation and glycosylation of the peptide. In addition, a derivative
peptide maybe a
fusion of a cupredoxin or cytochrome, or variant, derivative or structural
equivalent thereof to
a chemical compound, such as but not limited to, another peptide, drug
molecule or other
therapeutic or pharmaceutical agent or a detectable probe. Derivatives of
interest include
chemical modifications by which the half-life in the bloodstream of the
peptides and
compositions of the invention can be extended or optimized, such as by several
methods well
known to those in the art, including but not limited to, circularized peptides
(Monk et al.,
BioDrugs 19(4):261-78, (2005); DeFreest et al., J. Pept. Res. 63(5):409-19
(2004)), N- and
C- terminal modifications (Labrie et al., Clin. Invest. Med. 13(5):275-8,
(1990)), and
incorporation of olefin-containing non-natural amino acid followed by
hydrocarbon stapling
(Schafmeister et al., J. Am. Chem. Soc. 122:5891-5892 (2000); Walenslci et
al., Science
305:1466-1470 (2004)).
18
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
In one embodiment, the cupredoxin or cytochrome, or variant, derivative or
structural
equivalent thereof, is fused to a H.8 region of Laz from Neisseria
meningitidis or Neisseria
gonorrhea. One example of such a peptide is the H.8-Paz fusion protein
described in
Example 4. In a specific embodiment, the H.8 is fused to the C-terminus of the
cupredoxin or
cytochrome, or variant, derivative or structural equivalent thereof. In
another specific
embodiment, the H.8 region is SEQ ID NO: 21, or a variant, derivative or
structural
equivalent thereof.
It is contemplated that the peptide of the composition of invention may be
more than
one of a variant, derivative and structural equivalent of a cupredoxin or
cytochrome. For
example, the peptide may be a truncation of azurin that has been PEGylated,
thus making it
both a variant and a derivative. In one embodiment, the peptides of the
invention are
synthesized with a,a-disubstituted non-natural amino acids containing olefin-
bearing tethers,
followed by an all-hydrocarbon "staple" by ruthenium catalyzed olefin
metathesis.
(Scharmeister et al., J. Am. Chem. Soc. 122:5891-5892 (2000); Walensky et al.,
Science
305:1466-1470 (2004)) Additionally, peptides that are structural equivalents
of azurin may
be fused to other peptides, thus making a peptide that is both a structural
equivalent and a
derivative. These examples are merely to illustrate and not to limit the
invention. Variants,
derivatives or structural equivalents of cupredoxin or cytochrome may or may
not bind
copper.
In another embodiment, the peptide may be a structural equivalent of a
cupredoxin or
cytochrome. Examples of studies that determine significant structural homology
between
cupredoxins and cytochromes and other proteins include Toth et al.
(Developmental Cell
1:82-92 (2001)). Specifically, significant structural homology between a
cupredoxin or
cytochrome and its structural equivalents are determined by using the VAST
algorithm
(Gibrat et al., Curr Opin Struct Biol 6:377-385 (1996); Madej et al., Proteins
23:356-3690
(1995)). In specific embodiments, the VAST p value from a structural
comparison of a
cupredoxin or cytochrome to the structural equivalent is less than about 10-3,
less than about
10"5, or less than about 10-7. In other embodiments, significant structural
homology between
a cupredoxin or cytochrome and its structural equivalents are determined by
using the DALI
algorithm (Holm & Sander, J. Mol. Biol. 233:123-138 (1993)). In specific
embodiments, the
DALI Z score for a pairwise structural comparison is at least about 3.5, at
least about 7.0, or
at least about 10Ø
19
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
In another embodiment, the variant or derivative of cupredoxin has a
significant
structural homology to the Fab fragment of G17.12 mouse monoclonal antibody.
An
example of how this structural similarity can be determined can be foutid in
Example 3.
Specifically, significant structural homology between a cupredoxin and the Fab
fragment of
G 17.12 mouse monoclonal antibody can be determined by using the VAST
algorithm (Gibrat
et al., id.; Madej et al., id.). In specific embodiments, the VAST p-value
from a structural
comparison of a cupredoxin to the Fab fragment of G17.12 mouse monoclonal
antibody can
be less than about 10"4, less than about 10"5, less than about 10"6, or less
than about 10"7. In
other specific embodiments, the VAST score from a structural comparison of a
cupredoxin to
the Fab fragment of G17.12 mouse monoclonal antibody can be greater than about
9, greater
than about 10, greater than about 11 or greater than about 12.
In some embodiments, the variant, derivative or structural equivalent thereof
has
some of the functional characteristics of the P. aeruginosa azurin, P.
aeruginosa cytochrome
cssi, human cytochrome c or cyanobacterial cytochrome f. In a specific
embodiment, the
peptide of the invention inhibits parasitemia by malaria in malaria-infected
red blood cells,
and more specifically parasitemia by P. falciparum in P. falciparum-infected
human red
blood cells. The invention also provides for the variants, derivatives and
structural
equivalents of cupredoxin and cytochrome c551 that retain the ability to
inhibit parasitemia in
malaria-infected red blood cells, and more specifically parasitemia by P.
falciparum in P.
falciparum-infected human red blood cells. The inhibition of parasitemia by P.
falciparum in
P. falciparurn-infected human red blood cells may be determined by the method
described in
Example 6.
Because it is now known that cupredoxins and cytochrome can inhibit
parasitemia in
malaria-infected red blood cells, it is now possible to design variants,
derivatives and
structural equivalents of cupredoxins and cytochrome that retain this anti-
malarial activity.
Such variants and derivatives can be made by, for example, creating a
"library" of various
variants and derivatives of cupredoxins and cytochromes, and then testing each
for anti-
malarial activity using one of many methods known in the art, such the
exemplary method in
Example 6. It is contemplated that the resulting variants, derivatives and
structural
equivalents of cupredoxins and cytochromes with anti-malarial activity can be
used in the
methods of the invention, in place of or in addition to the cupredoxins and
cytochromes
mentioned herein. This method of selecting variants and derivatives may be
adapted for any
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
of the activities of P. aeruginosa azurin, P. aeruginosa cytochrome cssl,
human cytochrome c
or cyanobacterial cytochrome f disclosed herein.
In other embodiments, the peptide of the invention inhibits intracellular
replication of
the malaria parasite in human red blood cells. Methods to determine the
intracellular
replication of the malaria parasite are well known in the art, and one such
method is
described in Example 2.
In some embodiments, the peptide of the invention binds to the PfMSP1-19
and/or
PfMSP1-42 P. falciparum surface proteins with a relative binding affinity that
is statistically
greater a non-binding control protein. A peptide can be tested for this
activity by using
surface plasmon resonance analysis as described in Example 5. Other methods to
determine
whether one protein binds to another are well known in the art and may be used
as well.
In another embodiment, the peptide of the invention binds to ICAM-3 or NCAM
with
a relative binding affinity that is statistically greater a non-binding
control protein. A peptide
can be tested for this activity by using surface plasmon resonance analysis as
described in
Examples 7 and 5. Other methods to determine whether one protein binds to
another are
well known in the art and may be used as well.
In some specific embodiments, the peptides of the invention induce apoptosis
in a
mammalian cancer cell, more specifically a J774 cell. The ability of a peptide
to induce
apoptosis may be observed by mitosensor ApoAlertTM confocal microscopy using a
MITOSENSORTM APOLERTTM Mitochondrial Membrane Sensor kit (Clontech
Laboratories, Inc., Palo Alto, California, U.S.A.), by measuring caspase-8,
caspase-9 and
caspase-3 activity using the method described in Zou et al. (J. Biol. Chem.
274: 11549-11556
(1999)), and by detecting apoptosis-induced nuclear DNA fragmentation using,
for example,
the APOLERTTM DNA fragmentation kit (Clontech Laboratories, Inc., Palo Alto,
California,
U.S.A.).
In another specific embodiment, the peptide of the invention induces cellular
growth
arrest in a mammalian cancer cell, more specifically a J774 cell. Cellular
growth arrest can
be determined by measuring the extent of inhibition of cell cycle progression,
such as by the
method found in Yamada et al. (PNAS 101:4770-4775 (2004)). In another specific
embodiment, the peptide of the invention inhibits cell cycle progression in a
mammalian
cancer cell, more specifically a J774 cell.
21
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Cupredoxins
These small blue copper proteins (cupredoxins) are electron transfer proteins
(10-20
kDa) that participate in bacterial electron transfer chains or are of unknown
function. The
copper ion is solely bound by the protein matrix. A special distorted trigonal
planar ,
arrangement to two histidine and one cysteine ligands around the copper gives
rise to very
peculiar electronic properties of the metal site and an intense blue color. A
number of
cupredoxins have been crystallographically characterized at medium to high
resolution.
The cupredoxins in general have a low sequence homology but high structural
homology. (Gough & Clothia, Structure 12:917-925 (2004); De Rienzo et al.,
Protein
Science 9:1439-1454 (2000).) For example, the amino acid sequence of azurin is
31%
identical to that of auracyanin B, 16.3% to that of rusticyanin, 20.3 % to
that of plastocyanin,
and 17.3% to that of pseudoazurin. See Table 1. However, the structural
similarity of these
proteins is more pronounced. The VAST p value for the comparison of the
structure of
azurin to auracyanin B is 10"7.4, azurin to rusticyanin is 10-5, azurin to
plastocyanin is 10"5.6,
and azurin to psuedoazurin is 10-4'1.
All of the cupredoxins possess an eight-stranded Greek key beta-barrel or beta-
sandwich fold and have a highly conserved site architecture. (De Rienzo et
al., Protein
Science 9:1439-1454 (2000).) A prominent hydrophobic patch, due to the
presence of many
long chain aliphatic residues such as methionines and leucines, is present
around the copper
site in azurins, amicyanins, cyanobacterial plastocyanins, cucumber basic
protein and to a
lesser extent, pseudoazurin and eukaryotic plastocyanins. Id. Hydrophobic
patches are also
found to a lesser extent in stellacyanin and rusticyanin copper sites, but
have different
features. Id.
22
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Table 1. Sequence and structure alignment of azurin (1JZG) from P. aeruginosa
to
other proteins using VAST algorithm.
PDB Alignment % aa P-value 2 SSeore3
lengthl identity RMSD4 Description
1AOZ A 2 82 18.3 10 e-7 12.2 1.9 Ascorbate oxidase
1QHQ A 113 31 10e-7.4 12.1
1.9 AuracyaninB
1V54 B 1 79 20.3 lOe-6.0 11.2 2.1 Cytocrome c oxidase
1GY2 A 92 16.3 lOe-5.0 11.1
1.8 Rusticyanin
3MSP A 74 8.1 lOe-6.7 10.9 2.5 Motile Major Sperm
Protein5
1IUZ 74 20.3 l Oe-5 .6 10.3
2.3 Plastocyanin
1KGY E 90 5.6 10e-4.6 10.1
3.4 Ephrinb2
1 PMY 75 17.3 lOe-4.1 9.8
2.3 Pseudoazurin
'Aligned Length: The number of equivalent pairs of C-alpha atoms superimposed
between the two structures, i.e. how many residues have been used to calculate
the 3D
superposition.
ZP-VAL: The VAST p value is a measure of the significance of the comparison,
expressed as a probability. For example, if the p value is 0.001, then the
odds are 1000 to 1
against seeing a match of this quality by pure chance. The p value from VAST
is adjusted for
the effects of multiple comparisons using the assumption that there are 500
independent and
unrelated types of domains in the MMDB database. The p value shown thus
corresponds to
the p value for the pairwise comparison of each domain pair, divided by 500.
3Score: The VAST structure-similarity score. This number is related to the
number of
secondary structure elements superimposed and the quality of that
superposition. Higher
VAST scores correlate with higher similarity.
4RMSD: The root mean square superposition residual in Angstroms. This number
is
calculated after optimal superposition of two structures, as the square root
of the mean square
distances between equivalent C-alpha atoms. Note that the RMSD value scales
with the
extent of the structural alignments and that this size must be taken into
consideration when
using RMSD as a descriptor of overall structural similarity.
23
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
C. elegans major sperm protein proved to be an ephrin antagonist in oocyte
maturation (Kuwabara, 2003 "The multifaceted C. elegans major sperm protein:
an ephrin
signalling antagonist in oocyte maturation" Genes and Development, 17:155-161.
Azurin
The azurins are copper containing proteins of 128 amino acid residues which
belong
to the family of cupredoxins involved in electron transfer in plants and
certain bacteria. The
azurins include those from P. aeruginosa (PA) (SEQ ID NO: 1), A. xylosoxidans,
andA.
denitnificans (SEQ ID NO: 8). (Murphy et al., J. Mol. Biol. 315:859-871
(2002)) The amino
acid sequence identity between the azurins varies between 60-90%, these
proteins showed a
strong structural homology. All azurins have a characteristic (3-sandwich with
Greek key
motif and the single copper atom is always placed at the same region of the
protein. In
addition, azurins possess an essentially neutral hydrophobic patch surrounding
the copper
site. Id.
Plastocyanins
The plastocyanins are soluble proteins of cyanobacteria, algae and plants that
contain
one molecule of copper per molecule and are blue in their oxidized form. They
occur in the
chloroplast, where they function as electron carriers. Since the determination
of the structure
of poplar plastocyanin in 1978, the structure of algal (Scenedesmus,
Enteromorpha,
Chlamydomonas) and plant (French bean) plastocyanins has been determined
either by
crystallographic or NMR methods, and the poplar structure has been refined to
1.33 A
resolution. SEQ ID NO: 4 shows the amino acid sequence of plastocyanin from
Phorinidium
laminosum, a thermophilic cyanobacterium.
Despite the sequence divergence among plastocyanins of algae and vascular
plants
(e.g., 62% sequence identity between the Chlamydoinonas and poplar proteins),
the three-
dimensional structures are conserved (e.g., 0.76 A rms deviation in the C
alpha positions
between the Chlanzydonaonas and Poplar proteins). Structural features include
a distorted
tetrahedral copper binding site at one end of an eight-stranded antiparallel
beta-barrel, a
pronounced negative patch, and a flat hydrophobic surface. The copper site is
optimized for
its electron transfer function, and the negative and hydrophobic patches are
proposed to be
involved in recognition of physiological reaction partners. Chemical
modification, cross-
24
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
linking, and site-directed mutagenesis experiments have confirmed the
importance of the
negative and hydrophobic patches in binding interactions with cytochrome f,
and validated
the model of two functionally significant electron transfer paths involving
plastocyanin. One
putative electron transfer path is relatively short (approximately 4 A) and
involves the
solvent-exposed copper ligand His-87 in the hydrophobic patch, while the other
is more
lengthy (approximately 12-15 A) and involves the nearly conserved residue Tyr-
83 in the
negative patch, Redinbo et al., J. Bioenerg. Biomembr. 26:49-66 (1994).
Rusticyanins
Rusticyanins are blue-copper containing single-chain polypeptides obtained
from a
Thiobacillus (now called Acidithiobacillus). The X-ray crystal structure of
the oxidized form
of the extremely stable and highly oxidizing cupredoxin rusticyanin from
Thiobacillus
ferrooxidans (SEQ ID NO: 5) has been determined by multiwavelength anomalous
diffraction and refined to 1.9A resolution. The rusticyanins are composed of a
core beta-
sandwich fold composed of a six- and a seven-stranded b-sheet. Like other
cupredoxins, the
copper ion is coordinated by a cluster of four conserved residues (His 85,
Cys138, His143,
Met148) arranged in a distorted tetrahedron. Walter, R.L. et al., J. Mol.
Biol., vol. 263, pp-
730-51 (1996).
Pseudoazurins
The pseudoazurins are a family of blue-copper containing single-chain
polypeptide.
The amino acid sequence of pseudoazurin obtained from Achromobacter
cycloclastes is
shown in SEQ ID NO: 6. The X-ray structure analysis of pseudoazurin shows that
it has a
similar structure to the azurins although there is low sequence homology
between these
proteins. Two main differences exist between the overall structure of the
pseudoazurins and
azurins. There is a carboxy terminus extension in the pseudoazurins, relative
to the azurins,
consisting of two alpha-helices. In the mid-peptide region azurins contain an
extended loop,
shortened in the pseudoazurins, which forms a flap containing a short a-helix.
The only
major differences at the copper atom site are the conformation of the MET side-
chain and the
Met-S copper bond length, which is significantly shorter in pseudoazurin than
in azurin.
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Phytocyanins
The proteins identifiable as phytocyanins include, but are not limited to,
cucumber
basic protein, stellacyanin, mavicyanin, umecyanin, a cucumber peeling
cupredoxin, a
putative blue copper protein in pea pods, and a blue copper protein from
Arabidopsis
thaliana. In all except cucumber basic protein and the pea-pod protein, the
axial methionine
ligand normally found at blue copper sites is replaced by glutamine.
Auracyanin
Three small blue copper proteins designated auracyanin A, auracyanin B-l, and
auracyanin B-2 have been isolated from the thermophilic green gliding
photosynthetic
bacterium Chloroflexus aurantiacus. The two B forms are glycoproteins and have
almost
identical properties to each other, but are distinct from the A form. The
sodium dodecyl
sulfate-polyacrylamide gel electrophoresis demonstrates apparent monomer
molecular
masses as 14 (A), 18 (B-2), and 22 (B-1) kDa.
The amino acid sequence of auracyanin A has been determined and showed
auracyanin A to be a polypeptide of 139 residues. (Van Dreissche et al.,
Protein Science
8:947-957 (1999).) His58, Cys123, Hisl28, and Met132 are spaced in a way to be
expected
if they are the evolutionary conserved metal ligands as in the lcnown small
copper proteins
plastocyanin and azurin. Secondary structure prediction also indicates that
auracyanin has a
general beta-barrel structure similar to that of azurin from Pseudomonas
aeruginosa and
plastocyanin from poplar leaves. However, auracyanin appears to have sequence
characteristics of both small copper protein sequence classes. The overall
similarity with a
consensus sequence of azurin is roughly the same as that with a consensus
sequence of
plastocyanin, namely 30.5%. The N-terminal sequence region 1-18 of auracyanin
is
remarkably rich in glycine and hydroxy amino acids. Id. See exemplary amino
acid
sequence SEQ ID NO: 16 for chain A of auracyanin from Chloroflexus aurantiacus
(NCBI
Protein Data Bank Accession No. AAM12874).
The auracyanin B molecule has a standard cupredoxin fold. The crystal
structure of
auracyanin B from Chloroflexus aurantiacus has been studied. (Bond et al., J.
Mol. Biol.
306:47-67 (2001).) With the exception of an additional N-teririinal strand,
the molecule is
very similar to that of the bacterial cupredoxin, azurin. As in other
cupredoxins, one of the
Cu ligands lies on strand 4 of the polypeptide, and the other three lie along
a large loop
26
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
between strands 7 and 8. The Cu site geometry is discussed with reference to
the amino acid
spacing between the latter three ligands. The crystallographically
characterized Cu-binding
domain of auracyanin B is probably tethered to the periplasmic side of the
cytoplasmic
membrane by an N-terminal tail that exhibits significant sequence identity
with known tethers
in several other membrane-associated electron-transfer proteins. The amino
acid sequences
of the B forms are presented in McManus et al. (J. Biol Chem. 267:6531-6540
(1992).). See
exemplary amino acid sequence SEQ ID NO: 17 for chain B of auracyanin from
Chloroflexus
aurantiacus (NCBI Protein Data Bank Accession No. 1QHQA).
Stellacyanin
Stellacyanins are a subclass of phytocyanins, a ubiquitous family of plant
cupredoxins. An exemplary sequence of a stellacyanin is included herein as SEQ
ID NO: 15.
The crystal structure of umecyanin, a stellacyanin from horseradish root (Koch
et al., J. Am.
Chem. Soc. 127:158-166 (2005)) and cucumber stellacyanin (Hart el al., Protein
Science
5:2175-2183 (1996).). The protein has an overall fold similar to the other
phytocyanins. The
ephrin B2 protein ectodomain tertiary structure bears a significant similarity
to stellacyanin.
(Toth et al., Developmental Cell 1:83-92 (2001).) An exemplary amino acid
sequence of a
stellacyanin is found in the National Center for Biotechnology Information
Protein Data Bank
as Accession No. 1JER, SEQ ID NO: 15.
Cucumber basic protein
An exemplary amino acid sequence from a cucumber basic protein is included
herein
as SEQ ID NO: 18. The crystal structure of the cucumber basic protein (CBP), a
type 1 blue
copper protein, has been refined at 1.8 A resolution. The molecule resembles
other blue
copper proteins in having a Greek lcey beta-barrel structure, except that the
barrel is open on
one side and is better described as a "beta-sandwich" or "beta-taco". (Guss et
al., J. Mol.
Biol. 262:686-705 (1996).) The ephrinB2 protein ectodomian tertiary structure
bears a high
similarity (rms deviation 1.5A for the 50 a carbons) to the cucumber basic
protein. (Toth et
al., Developmental Cell 1:83-92 (2001).)
The Cu atom has the normal blue copper NNSS' co-ordination with bond lengths
Cu-
N(His39) = 1.93 A, Cu-S(Cys79) = 2.16 A, Cu-N(His84) = 1.95 A, Cu-S(Met89) =
2.61 A.
A disulphide link, (Cys52)-S-S-(Cys85), appears to play an important role in
stabilizing the
27
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
molecular structure. The polypeptide fold is typical of a sub-family of blue
copper proteins
(phytocyanins) as well as a non-metalloprotein, ragweed allergen Ra3, with
which CBP has a
high degree of sequence identity. The proteins currently identifiable as
phytocyanins are
CBP, stellacyanin, mavicyanin, umecyanin, a cucumber peeling cupredoxin, a
putative blue
copper protein in pea pods, and a blue copper protein from Arabidopsis
thaliana. In all except
CBP and the pea-pod protein, the axial methionine ligand normally found at
blue copper sites
is replaced by glutamine. An exemplary sequence for cucumber basic protein is
found in
NCBI Protein Data Bank Accession No. 2CBP, SEQ ID NO: 18.
Cytochromes
Cytochrome C551
Cytochrome Cssl from P. aeruginosa (Pa-C55 1) is a monomeric redox protein of
82
amino-acid residues (SEQ ID NO: 2), involved in dissimilative denitrification
as the
physiological electron donor of nitrite reductase. The functional properties
of Pa-C551 have
been extensively investigated. The reactions with non-physiological small
inorganic redox
reactants and with other macromolecules, like blue copper proteins, eukaryotic
cytochrome c
and the physiological partner nitrite reductase have provided a test for
protein-protein
electron transfer.
The three-dimensional structure of Pa-C55 1, which is a member of bacterial
class I
cytochromes, shows a single low-spin heme with His-Met ligation and the
typical
polypeptide fold which however leaves the edges of pyrrole rings II and III of
the heme
exposed (Cutruzzola et al., J. Inorgan. Chem. 88:353-61 (2002)). The lack of a
20-residue
omega loop, present in the mammalian class I cytochromes, causes further
exposure of the
heme edge at the level of propionate 13. The distribution of charged residues
on the surface
of Pa-C551 is very anisotropic: one side is richer in acidic residues whereas
the other displays
a ring of positive side chains, mainly lysines, located at the border of a
hydrophobic patch
which surrounds the heme crevice. This patch comprises residues Glyll, Va113,
Alal4,
Met22, Va123, Pro58, Ile59, Pro60, Pro62, Pro63 and A1a65. The anisotropic
charge
distribution leads to a large dipolar moment which is important for electron
transfer complex
formation.
The charge distribution described above for Pa-C551 has been reported for
other
electron transfer proteins and their electron acceptors. Moreover,
modification by site-
28
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
directed mutagenesis of residues within the hydrophobic or charged patch has
shown for
different proteins the importance of surface complementarity for binding and
electron
transfer. As an example, evidence for the relevance of the hydrophobic patch
for the electron
transfer properties of azurin from P. aeruginosa came from the studies carried
out on mutants
of residues Met44 and Met64 changed to positively and negatively charged amino
acids. Id.
The cytochrome c-type domain has a fold consisting of a series of alpha
helices and
reverse turns that serve to envelop the covalently bound haem within a
hydrophobic pocket.
This domain can be found in monodomain cytochrome c proteins, such as
cytochrome c6,
cytochrome c552, cytochrome c459 and mitochondrial cytochrome c. The
cytochrome c-type
domain occurs in a number of other proteins, such as in cytochrome cdl-nitrite
reductase as
the N-terminal haem c domain, in quinoprotein alcohol dehydrogenase as the C-
terminal
domain, in Quinohemoprotein amine dehydrogenase A chain as domains 1 and 2,
and in the
cytochrome bc1 complex as the cytochrome bcl domain. Structural analysis with
VAST
algorithm (cytochrome cssl from Pseudornonas aeNuginosa as a query) showed
significant
structural neighbors (P values between 10-10-3 to 10"4'5) only for
cytochromes.
Methods of Use
The invention provides methods to treat patients with a malarial infection or
at danger
of acquiring one, or inhibit the spread of the malaria parasite. These methods
comprise
administering to a patient or an insect vector a cupredoxin or cytochrome, or
variant,
derivative or structural equivalent thereof which inhibits parasitemia of
malaria-infected
mammalian cells. The inhibition of parasitemia can be determined by many
methods well
known in the art. One method is described in Example 6, and determines the
inhibition of
parasitemia in malaria-infected human red blood cells. In other embodiments,
the cupredoxin
or cytochrome, or variant, derivative or structural equivalent thereof
inhibits intracellular
replication of the malaria parasite in human red blood cells and is
administered to the patient
or insect vector. Methods to determine the intracellular replication of the
malaria parasite are
well known in the art, and one such method is described in Example 2. The mode
of the
invention is not limited to any particular mechanism, and inhibition of
parasitemia may result
from many factors, including but not limited to, inhibition of replication of
the parasite in
infected blood cells, inhibition of parasite infecting uninfected blood cells,
inhibition in the
growth cycle of the parasite and inhibition of parasite entry into the
mammalian cell.
29
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
The invention provides methods to treat patients suffering from infection by a
malaria
parasite by administering an effective amount of at least one protein that is
a cupredoxin or
cytochrome, or variant, derivative or structural equivalent thereof. The
patients that may be
treated by this method are any mammal that can be infected by a malaria
parasite, and
specifically are human patients. Malaria parasites known to infect mammals
include, but are
not limited to, Plasrnodium falciparum, P. vivax, P. berghei (rodent-
specific), P. yoelli
(murine-specific), P. cynonaolgi and P. knowlesi (monkey-specific).
It has also been learned that cupredoxins and cytochrome cssl are also
effective
against HIV-1 infections, as disclosed in a co-filed application.
"Compositions And Methods
For Treating HIV Infection Witli Cupredoxin And Cytochrome c," U.S Provisional
Patent
Application Serial No. , whose disclosure is expressly incorporated herein
by reference. Further, co-infections with HIV and malaria are very common in
many areas of
the world, and in particular sub-Saharan Africa. In some embodiments, the
patient suffering
from infection by a malaria parasite is also suffering from infection by HIV.
In some
embodiments, the method of treatment of the invention also comprises
administering anti-
HIV drugs. In some embodiments, the anti-HIV drugs are co-administered.
The invention also provides methods to treat a patient suspected of having
contact
with a malaria parasite by administering an effective amount of at least one
peptide that is a
cupredoxin and/or a cytochrome, or a variant, derivative or structural
equivalent of a
cupredoxin or a cytochrome. A patient can be suspected of having contact with
a malaria
parasite, for example, if that patient lives or has traveled in a region of
the world where
malaria infection of others of the patient's species is common. Treatment by
this method
may be commenced when the patient is about to, or has already, come into
contact with the
malaria parasite. Contact with malaria parasites most often occurs by contact
with an insect
vector such as mosquitoes, so that areas abundant in these insects and the
malaria parasite are
considered to be among the areas where a patient would have a high probability
of coming in
contact with a malaria parasite. Such areas of the world include, but are not
limited to, parts
of Africa, Asia and Latin America. Further, a patient can be suspected of
having contact with
the malaria parasite if they have come into contact with blood infected with a
malaria
parasite, are intentionally exposed to the malaria parasite, or accidentally
injected with blood
or drugs contaminated with the parasite.
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
The cupredoxin or cytochrome, or variant, derivative or structural equivalent
of
cupredoxin or cytochrome can be administered to the patient by many routes and
in many
regimens that will be well known to those in the art. In specific embodiments,
the
cupredoxin or cytochrome, or variant, derivative or structural equivalent of
cupredoxin or
cytochrome is administered orally, topically, by inhalation, by injection,
more specifically,
intravenously, intramuscularly or subcutaneously.
In one embodiment, the methods may comprise co-administering to a patient one
unit
dose of compositions comprising a cupredoxin or cytochrome, or a variant,
derivative or
structural equivalent thereof, and one unit dose of compositions comprising an
anti-malarial
drug and/or an anti-HIV drug, in either order. These compositions may be
administered at
about the same time, or within about a given time following the administration
of the other,
for example, about one minute to about 6o minutes, or about 1 hour to about 12
hours of the
other.
The invention also provides methods to inhibit the spread of the malaria
parasite in an
insect vector population harboring a malaria parasite by administering to an
insect vector in
the population at least one of a cupredoxin or cytochrome, or variant,
derivative or structural
equivalent of cupredoxin or cytochrome, at an amount that is effective to
reduce the
infectivity of the parasite in a co-existant mammalian population. In specific
embodiments,
the insect vector is a mosquito, and more specifically a mosquito from the
species Anopheles
gambiae. In this method, the administration of the cupredoxin or cytochrome,
or variant,
derivative or structural equivalent of cupredoxin or cytochrome can be
accomplished by
placing the peptides in compositions that will be consumed by the insect
vector, however any
manner that brings the peptide into contact with the malaria parasite in the
insect vector's gut
is contemplated. Many methods to administer chemicals to insect populations
which produce
such consumption are known in the art.
In another embodiment, a transmissible genetic element that passes from one
mosquito to another will be operably connected to the cupredoxin coding
sequence operably
connected to a constitutive promoter, the cupredoxin or cytochrome, or
variant, derivative or
structural equivalent of cupredoxin or cytochrome will be produced inside the
Anopheles
ganabiae infected with P. falciparuna and will interfere with its
replication/survival in the
mosquito.
31
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Other manners of administration of the peptides to the insect vector include,
but are
not limited to, fusing the cupredoxin or cytochrome, or variant, derivative or
structural
equivalent of cupredoxin or cytochrome genes to genes from other proteins
normally
consumed by the insects. The amount of peptides administered to the insect
vector should be
an amount effective to reduce the infectivity of the malaria parasite in a
mammal when the
insect vector comes into contact with a mammal. In specific embodiments, the
amount
administered should be effective to reduce the infectivity of the malaria
parasite when the
insect vector comes into contact with a human.
Mosquito larvae are suitable for use in the present invention and preferably,
the
promoter used is a strong promoter. Two alternative categories of promoter are
available for
use: inducible and constitutive promoters. Inducible promoters include, for
example, heat
shock promoters. Preferably, the heat shock promoter is an insect heat shock
promoter, for
example the Drosophila melanogaster hsp70 promoter, which is capable of
driving the
expression of genes in heterologous organisms, including medfly. The invention
also
encompasses the use of the medfly hsp70 promoter (Papadimitriou et al., Insect
Mol Biol
7:279-90(1998) ). Alternative systems may be based on induction with the
antibiotic
tetracycline. (Heinrich and Scott, PNAS 97:8229-8232, (2000))
Heat shock promoters are inducible by raising the temperature of the
conditions under
which the medfly are being cultured. For example, at 23-25 C., the hsp70
promoter is active
at low levels or not at all. This allows the insect larva to develop without
stress induced by
the production of a heterologous protein. At higher temperatures, however,
such as 37-42
C., the hsp70 promoter is induced and expresses the heterologous protein at a
high level.
Inducible promoters may be constructed based on known inducible gene control
elements. For example, inducible promoters may be constructed by combining an
element
responsive to a drug or hormone which may be administered in the diet. In a
preferred
embodiment, a human oestrogen responsive element (ERE) may be used to regulate
expression of the protein of interest, as long as the insect is transformed
with a second coding
sequence which expresses the human oestrogen receptor.
Constitutive promoters may also be used to express the protein and/or other
proteins
required in the insect larva. For example, the constitutive promoter may be a
cytoplasmic
actin promoter. The D. melanogaster cytoplasmic actin promoter has been cloned
(Act5C)
and is highly active in mosquitoes (Huynh and Zieler, J. Mol. Biol. 288:13-
20(1999) ).
32
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Cytoplasmic actin genes and their promoters may also be isolated from other
insects,
including medfly. Other examples include the cytoplasmic tubulin promoter, for
instance the
medfly cytoplasmic tubulin promoter.
Promoters which control secreted polypeptides may be used, optionally together
with
appropriate signal sequences, to direct secretion of the protein to the
haemolymph. For
example, the larval serum protein promoter may be employed (Benes et al., Mol.
Gen. Genet
249(5):545-556 (1995)).
Mass rearing technology for mosquitoes is highly developed. For protein
production,
larval cultures that have been initiated at different times can be
synchronized by appropriate
temperature shift regimes. This is possible because growth rates depend on the
temperature of
the environment; at 18 C larval growth rates decrease by approximately 50%.
Pharmaceutical Compositions Comprising Cupredoxin and/or Cytochrome and
Variants and Derivatives Thereof
Pharmaceutical compositions comprising cupredoxin or cytochrome, or variant,
derivative or structural equivalent thereof can be manufactured in any
conventional manner,
e.g., by conventional mixing, dissolving, granulating, dragee-making,
emulsifying,
encapsulating, entrapping, or lyophilizing processes. The substantially pure
cupredoxin
and/or cytochrome, and variants, derivatives and structural equivalents
thereof can be readily
combined with a pharmaceutically acceptable carrier well-known in the art.
Such carriers
enable the preparation to be formulated as a tablet, pill, dragee, capsule,
liquid, gel, syrup,
slurry, suspension, and the like. Suitable carriers or excipients can also
include, for example,
fillers and cellulose preparations. Other excipients can include, for example,
flavoring
agents, coloring agents, detackifiers, thiclceners, and other acceptable
additives, adjuvants, or
binders. In some embodiments, the pharmaceutical preparation is substantially
free of
preservatives. In other embodiments, the pharmaceutical preparation may
contain at least one
preservative. General methodology on pharmaceutical dosage forms is found in
Ansel et al.,
Pharmaceutical Dosage Forms and Drug Delivery Systenas (Lippencott Williams &
Wilkins,
Baltimore MD (1999)).
The composition comprising a cupredoxin or cytochrome, or variant, derivative
or
structural equivalent thereof used in the invention may be administered in a
variety of ways,
including by injection (e.g., intradermal, subcutaneous, intramuscular,
intraperitoneal and the
33
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
like), by inhalation, by topical administration, by suppository, by using a
transdermal patch or
by mouth. General information on drug delivery systems can be found in Ansel
et al., Id.. In
some embodiments, the composition comprising a cupredoxin or cytochrome, or
variant,
derivative or structural equivalent thereof can be formulated and used
directly as injectibles,
for subcutaneous and intravenous injection, among others. The injectable
formulation, in
particular, can advantageously be used to treat patients that are at risk of
an malaria-infection,
likely to have an malaria-infection or have an malaria-infection. The
composition
comprising a cupredoxin or cytochrome, or variant, derivative or structural
equivalent thereof
can also be taken orally after mixing with protective agents such as
polypropylene glycols or
similar coating agents.
When administration is by injection, the cupredoxin or cytochrome, or variant,
derivative or structural equivalent thereof may be formulated in aqueous
solutions,
specifically in physiologically compatible buffers such as Hanks solution,
Ringer's solution,
or physiological saline buffer. The solution may contain formulatory agents
such as
suspending, stabilizing and/or dispersing agents. Alternatively, the
cupredoxin or
cytochrome, or variant, derivative or structural equivalent thereof may be in
powder form for
constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before
use. In some
embodiments, the pharmaceutical composition does not comprise an adjuvant or
any other
substance added to enhance the immune response stimulated by the peptide. In
some
embodiments, the pharmaceutical composition comprises a substance that
inhibits an immune
response to the peptide.
When administration is by intravenous fluids, the intravenous fluids for use
administering the cupredoxin or cytochrome, or variant, derivative or
structural equivalent
thereof may be composed of crystalloids or colloids. Crystalloids as used
herein are aqueous
solutions of mineral salts or other water-soluble molecules. Colloids as used
herein contain
larger insoluble molecules, such as gelatin. Intravenous fluids may be
sterile.
Crystalloid fluids that may be used for intravenous administration include but
are not
limited to, normal saline (a solution of sodium chloride at 0.9%
concentration), Ringer's
lactate or Ringer's solution, and a solution of 5% dextrose in water sometimes
called D5W, as
described in Table 2.
34
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Table 2. Composition of Common Crystalloid Solutions
Solution Other Name [Na+] [Cl"] [Glucose]
D5W 5% Dextrose 0 0 252
2/3 & 1/3 3.3% Dextrose 51 51 168
/ 0.3% saline
Half-normal 0.45% NaCI 77 77 0
saline
Normal saline 0.9% NaC1 154 154 0
Ringer's Ringer's 130 109 0
lactate* solution
*Ringer's lactate also has 28 mmol/L lactate, 4 mmol/L K+ and 3 mmol/L Ca2+.
When administration is by inhalation, the cupredoxin or cytochrome, or
variant,
derivative or structural equivalent thereof may be delivered in the form of an
aerosol spray
from pressurized packs or a nebulizer with the use of a suitable propellant,
e.g.,
dichlorodifluoromethane, trichlorofluoromethane, carbon dioxide or other
suitable gas. In the
case of a pressurized aerosol, the dosage unit may be determined by providing
a valve to
deliver a metered amount. Capsules and cartridges of, e.g., gelatin, for use
in an inhaler or
insufflator may be formulated containing a powder mix of the proteins and a
suitable powder
base such as lactose or starch.
When administration is by topical administration, the cupredoxin or
cytochrome, or
variant, derivative or structural equivalent thereof may be formulated as
solutions, gels,
ointments, creams, suspensions, and the like, as are well known in the art. In
some
embodiments, administration is by means of a transdermal patch. When
administration is by
suppository (e.g., rectal or vaginal), cupredoxin and/or cytochrome c and
variants and
derivatives thereof compositions may also be formulated in compositions
containing
conventional suppository bases.
When administration is oral, a cupredoxin or cytochrome, or variant,
derivative or
structural equivalent thereof can be readily formulated by combining the
cupredoxin or
cytochrome, or variant, derivative or structural equivalent thereof with
pharmaceutically
acceptable carriers well known in the art. A solid carrier, such as mannitol,
lactose,
magnesium stearate, and the like may be employed; such carriers enable the
cupredoxin
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
and/or cytochrome and variants and derivatives thereof to be formulated as
tablets, pills,
dragees, capsules, liquids, gels, syrups, slurries, suspensions and the like,
for oral ingestion
by a subject to be treated. For oral solid formulations such as, for example,
powders,
capsules and tablets, suitable excipients include fillers such as sugars,
cellulose preparation,
granulating agents, and binding agents.
Other convenient carriers, as well-known in the art, also include multivalent
carriers,
such as bacterial capsular polysaccharide, a dextran or a genetically
engineered vector. In
addition, sustained-release formulations that include a cupredoxin or
cytochrome, or variant,
derivative or structural equivalent thereof allow for the release of
cupredoxin or cytochrome,
or variant, derivative or structural equivalent thereof over extended periods
of time, such that
without the sustained release formulation, the cupredoxin or cytochrome, or
variant,
derivative or structural equivalent thereof would be cleared from a subject's
system, and/or
degraded by, for example, proteases and simple hydrolysis before eliciting or
enhancing a
therapeutic effect.
The half-life in the bloodstream of the compositions of the invention can be
extended
or optimized by several methods well known to those in the art, including but
not limited to,
circularized peptides (Monk et al., BioDrugs 19(4):261-78, (2005); DeFreest et
al., J. Pept.
Res. 63(5):409-19 (2004)), D,L-peptides (diastereomer), (Futaki et al., J.
Biol. Chem. Feb
23;276(8):5836-40 (2001); Papo et al., Cancer Res. 64(16):5779-86 (2004);
Miller et al.,
Biochem. Pharmacol. 36(1):169-76, (1987)); peptides containing unusual amino
acids (Lee et
al., J. Pept. Res. 63(2):69-84 (2004)), N- and C- terminal modifications
(Labrie et al., Clin.
Invest. Med. 13(5):275-8, (1990)), and hydrocarbon stapling (Schafineister et
al., J. Am.
Chem. Soc. 122:5891-5892 (2000); Walenski et al., Science 305:1466-1470
(2004)). Of
particular interest are d-isomerization (substitution) and modification of
peptide stability via
D-substitution or L- amino acid substitution and hydrocarbon stapling.
In various embodiments, the pharmaceutical composition includes carriers and
excipients (including but not limited to buffers, carbohydrates, mannitol,
proteins,
polypeptides or amino acids such as glycine, antioxidants, bacteriostats,
chelating agents,
suspending agents, thickening agents and/or preservatives), water, oils,
saline solutions,
aqueous dextrose and glycerol solutions, other pharmaceutically acceptable
auxiliary
substances as required to approximate physiological conditions, such as
buffering agents,
tonicity adjusting agents, wetting agents and the like. It will be recognized
that, while any
36
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
suitable carrier known to those of ordinary skill in the art may be employed
to administer the
compositions of this invention, the type of carrier will vary depending on the
mode of
administration. Compounds may also be encapsulated within liposomes using well-
known
technology. Biodegradable microspheres may also be employed as carriers for
the
pharmaceutical compositions of this invention. Suitable biodegradable
microspheres are
disclosed, for example, in U.S. Patent Nos. 4,897,268; 5,075,109; 5,928,647;
5,811,128;
5,820,883; 5,853,763; 5,814,344 and 5,942,252.
The pharmaceutical compositions may be sterilized by conventional, well-known
sterilization techniques, or may be sterile filtered. The resulting aqueous
solutions may be
packaged for use as is, or lyophilized, the lyophilized preparation being
combined with a
sterile solution prior to administration.
Administration Of Cupredoxin And/Or Cytochrome And Variants And Derivatives
Thereof
The cupredoxin or cytochrome, or variant, derivative or structural equivalent
thereof
can be administered formulated as pharmaceutical compositions and administered
by any
suitable route, for example, by oral, buccal, inhalation, sublingual, rectal,
vaginal,
transurethral, nasal, topical, percutaneous, i.e., transdermal or parenteral
(including
intravenous, intramuscular, subcutaneous and intracoronary) administration.
The
pharmaceutical formulations thereof can be administered in any amount
effective to achieve
its intended purpose. More specifically, the composition is administered in a
therapeutically
effective amount. In specific embodiments, the therapeutically effective
amount is generally
from about 0.01-20 mg/day/kg of body weight.
The compounds comprising cupredoxin or cytochrome, or variant, derivative or
structural equivalent thereof are useful for the treatment and/or prophylaxis
of malaria
infection, alone or in combination with other active agents. The appropriate
dosage will, of
course, vary depending upon, for example, the compound of cupredoxin or
cytochrome, or
variant, derivative or structural equivalent thereof employed, the host, the
mode of
administration and the nature and severity of the conditions being treated.
However, in
general, satisfactory results in humans are indicated to be obtained at daily
dosages from
about 0.01-20 mg/kg of body weight. An indicated daily dosage in humans is in
the range
from about 0.7 mg to about 1400 mg of a compound of cupredoxin or cytochrome
c551, or
37
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
variant, derivative or structural equivalent thereof conveniently
administered, for example, in
daily doses, weekly doses, monthly doses, and/or continuous dosing. Daily
doses can be in
discrete dosages from 1 to 12 times per day. Alternatively, doses can be
administered every
other day, every third day, every fourth day, every fifth day, every sixth
day, every week, and
similarly in day increments up to 31 days. Alternatively, dosing can be
continuous using
patches, i.v. administration and the like.
The method of introducing cupredoxin or cytochrome, or variant, derivative or
structural equivalent thereof to patients is, in some embodiments, through the
co-
administration of cupredoxin or cytochrome, or variant, derivative or
structural equivalent
thereof with other drugs used for malaria therapy. Such methods are well-known
in the art.
In a specific embodiment, the cupredoxin and/or cytochrome c are part of an
cocktail or co-
dosing containing or with other malaria therapeutics. Malaria therapeutics of
interest include,
but are not limited to, proguanil, chlorproguanil, trimethoprim, chloroquine,
mefloquine,
lumefantrine, atovaquone, pyrimethamine=sulfadoxine, pyrimethamine-dapsone,
halofantrine,
quinine, quinidine, amodiaquine, amopyroquine, sulphonamides, artemisinin,
arteflene,
artemether, artesunate, primaquine, pyronaridine, proguanil, chloroquine,
mefloquine,
pyrimethamine-sulfadoxine, pyrimethamine-dapsone, halofantrine, quinine,
proguanil,
chloroquine, mefloquine, 1,16-hexadecamethylenebis(N-
methylpyrrolidinium)dibromide, and
combinations thereof.
The method of introducing cupredoxin or cytochrome c551, or variant,
derivative or
structural equivalent thereof to patients is, in some embodiments, the same as
currently used
to introduce anti-HIV drugs, such as the protease-inhibitor-containing
cocktails. Such
methods are well-known in the art. In a specific embodiment, the cupredoxin or
cytochrome
c551, or variant, derivative or structural equivalent thereof are part of an
cocktail or co-dosing
with anti-HIV therapeutics. Anti-HIV drugs include, but are not limited to,
reverse
transcriptase inhibitors: AZT (zidovudine [Retrovir]), ddC (zalcitabine
[Hivid],
dideoxyinosine), d4T (stavudine [Zerit]), and 3TC (lamivudine [Epivir]),
nonnucleoside
reverse transcriptase inhibitors (NNRTIS): delavirdine (Rescriptor) and
nevirapine
(Viramune), protease inhibitors: ritonavir (Norvir), a lopinavir and ritonavir
combination
(Kaletra), saquinavir (Invirase), indinavir sulphate (Crixivan), amprenavir
(Agenerase), and
nelfinavir (Viracept). In some embodiments, a combination of several drugs
called highly
active antiretroviral therapy (HAART) is used to treat the patients.
38
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
The exact formulation, route of administration, and dosage is determined by
the
attending physician in view of the patient's condition. Dosage amount and
interval can be
adjusted individually to provide plasma levels of the active cupredoxin or
cytochrome, or
variant, derivative or structural equivalent thereof which are sufficient to
maintain therapeutic
effect. Generally, the desired cupredoxin or cytochrome, or variant,
derivative or structural
equivalent thereof is administered in an admixture with a pharmaceutical
carrier selected with
regard to the intended route of administration and standard pharmaceutical
practice.
In one aspect, the cupredoxin or cytochrome, or variant, derivative or
structural
equivalent thereof is delivered as DNA such that the polypeptide is generated
in situ. In one
embodiment, the DNA is "naked," as described, for example, in Ulmer et al.,
(Science
259:1745-1749 (1993)) and reviewed by Cohen (Science 259:1691-1692 (1993)).
The uptake
of naked DNA may be increased by coating the DNA onto a carrier, e.g.,
biodegradable
beads, which are then efficiently transported into the cells. In such methods,
the DNA may
be present within any of a variety of delivery systems known to those of
ordinary skill in the
art, including nucleic acid expression systems, bacterial and viral expression
systems.
Techniques for incorporating DNA into such expression systems are well known
to those of
ordinary skill in the art. See, e.g., W090/11092, W093/24640, WO 93/17706, and
U.S. Pat.
No. 5,736,524.
Vectors, used to shuttle genetic material from organism to organism, can be
divided
into two general classes: cloning vectors are replicating plasmid or phage
with regions that
are essential for propagation in an appropriate host cell and into which
foreign DNA can be
inserted; the foreign DNA is replicated and propagated as if it were a
component of the
vector. An expression vector (such as a plasmid, yeast, or animal virus
genome) is used to
introduce foreign genetic material into a host cell or tissue in order to
transcribe and translate
the foreign DNA, such as the DNA of a cupredoxin and/or a cytochrome. In
expression
vectors, the introduced DNA is operably-linked to elements such as promoters
that signal to
the host cell to highly transcribe the inserted DNA. Some promoters are
exceptionally useful,
such as inducible promoters that control gene transcription in response to
specific factors.
Operably-linking a cupredoxin or cytochrome and variants and derivatives
thereof
polynucleotide to an inducible promoter can control the expression of the
cupredoxin or
cytochrome and variants and derivatives thereof in response to specific
factors. Examples of
classic inducible promoters include those that are responsive to a-interferon,
heat shock,
39
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
heavy metal ions, and steroids such as glucocorticoids (Kaufman, Methods
Enzyrnol.
185:487-511 (1990)) and tetracycline. Other desirable inducible promoters
include those that
are not endogenous to the cells in which the construct is being introduced,
but, however, are
responsive in those cells when the induction agent is exogenously supplied. In
general,
useful expression vectors are often plasmids. However, other forms of
expression vectors,
such as viral vectors (e.g., replication defective retroviruses, adenoviruses
and
adeno-associated viruses) are contemplated.
Vector choice is dictated by the organism or cells being used and the desired
fate of
the vector. In general, vectors comprise signal sequences, origins of
replication, marker
genes, polylinker sites, enhancer elements, promoters, and transcription
termination
sequences. As an example, one may clone a cupredoxin or a cytochrome gene into
a vector
transmissible in the malaria parasite-harboring mosquitoes to prevent the
parasite from
replicating inside the mosquitoes. The transmissibility of the vector will
allow the spread of
the cupredoxin/cytochrome to neighboring mosquitoes that are infected with the
malaria
parasites as well.
The exact formulation, route of administration, and dosage is determined by
the
attending physician in view of the patient's condition. Dosage amount and
interval can be
adjusted individually to provide plasma levels of the active cupredoxin and/or
cytochrome
and variants and derivatives thereof which are sufficient to treat the patient
and/or maintain
therapeutic effect. Generally, the desired cupredoxin and/or cytochrome and
variants and
derivatives thereof can be administered in an admixture with a pharmaceutical
carrier
selected with regard to the intended route of administration and standard
pharmaceutical
practice. Pharmaceutical compositions used in accordance with the present
invention can be
formulated in a conventional manner using one or more physiologically
acceptable carriers
comprising excipients and auxiliaries that facilitate processing of the
cupredoxin and/or
cytochrome and variants and derivatives thereof, active agents, for inhibiting
or stimulating
the secretion of cupredoxin and/or cytochrome and variants and derivatives
thereof, or a
mixture thereof into preparations which can be used therapeutically.
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Kits Comprising Cupredoxin And/Or Cytochrome C And Variants And Derivatives
Thereof
In one aspect, the invention provides kits containing one or more of the
following in a
package or container: (1) a biologically active composition comprising a
cupredoxin or
cytochrome, or variant, derivative or structural equivalent thereof; (2) a
pharmaceutically
acceptable adjuvant or excipient; (3) a vehicle for administration, such as a
syringe; (4)
instructions for administration. Embodiments in which two or more of
components (1) - (4)
are found in the same container are also contemplated.
In another aspect, the invention provides kits containing one or more of the
following
in a package or container: (1) a biologically active composition comprising a
cupredoxin or
cytochrome, or variant, derivative or structural equivalent thereof; (2) an
malaria therapeutic,
including, but not limited to, proguanil, chlorproguanil, trimethoprim,
chloroquine,
mefloquine, lumefantrine, atovaquone, pyrimethamine-sulfadoxine, pyrimethamine-
dapsone,
halofantrine, quinine, quinidine, amodiaquine, amopyroquine, sulphonamides,
artemisinin,
arteflene, artemether, artesunate, primaquine, pyronaridine, proguanil,
chloroquine,
mefloquine, pyrimethamine-sulfadoxine, pyrimethamine-dapsone, halofantrine,
quinine,
proguanil, chloroquine, mefloquine, 1,16-hexadecamethylenebis(N-
methylpyrrolidinium)dibromide; (3) a pharmaceutically acceptable adjuvant or
excipient; (4)
a vehicle for administration, such as a syringe; (5) instructions for
administration.
Embodiments in which two or more of components (1) - (5) are found in the same
packaging
or container are also contemplated.
In some embodiments, the kit also comprises an anti-HIV therapeutic in a
package or
container. Anti-HIV therapeutics of interest include, but are not limited to,
reverse
transcriptase inhibitors: AZT (zidovudine [Retrovir]), ddC (zalcitabine
[Hivid],
dideoxyinosine), d4T (stavudine [Zerit]), and 3TC (lamivudine [Epivir]),
nonnucleoside
reverse transcriptase inhibitors (NNRTIS): delavirdine (Rescriptor) and
nevirapine
(Viramune), protease inhibitors: ritonavir (Norvir), a lopinavir and ritonavir
combination
(Kaletra), saquinavir (Invirase), indinavir sulphate (Crixivan), amprenavir
(Agenerase), and
nelfinavir (Viracept). In some embodiment, a combination of several drugs
called highly
active antiretroviral therapy (HAART) is included in the kit.
When a kit is supplied, the different components of the composition may be
packaged
in separate containers and admixed immediately before use. Such packaging of
the
41
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
components separately may permit long-term storage without losing the active
components'
functions.
The reagents included in the kits can be supplied in containers of any sort
such that
the life of the different components are preserved and are not adsorbed or
altered by the
materials of the container. For example, sealed glass ampules may contain the
lyophilized
polypeptide or polynucleotide of cupredoxin and/or cytochrome c and variants
and
derivatives thereof, or buffers that have been packaged under a neutral, non-
reacting gas,
such as nitrogen. Ampules may consist of any suitable material, such as glass,
organic
polymers, such as polycarbonate, polystyrene, etc., ceramic, metal or any
other material
typically employed to hold similar reagents. Other examples of suitable
containers include
simple bottles that may be fabricated from similar substances as ampules, and
envelopes, that
may comprise foil-lined interiors, such as aluminum or an alloy. Other
containers include
test tubes, vials, flasks, bottles, syringes, or the like. Containers may have
a sterile access
port, such as a bottle having a stopper that can be pierced by a hypodermic
injection needle.
Other containers may have two compartments that are separated by a readily
removable
membrane that upon removal permits the components to be mixed. Removable
membranes
may be glass, plastic, rubber, etc.
Kits may also be supplied with instructional materials. Instructions may be
printed on
paper or other substrate, and/or may be supplied as an electronic-readable
medium, such as a
floppy disc, CD-ROM, DVD-ROM, Zip disc, videotape, audiotape, flash memory
device,
etc.. Detailed instructions may not be physically associated with the kit;
instead, a user may
be directed to an internet web site specified by the manufacturer or
distributor of the kit, or
supplied as electronic mail.
Modification Of Cupredoxin And/Or Cytochrome
Cupredoxin or cytochrome may be chemically modified or genetically altered to
produce variants and derivatives as explained above. Such variants and
derivatives may be
synthesized by standard techniques.
In addition to naturally-occurring allelic variants of cupredoxin and
cytochrome,
changes can be introduced by mutation into cupredoxin or cytochrome coding
sequence that
incur alterations in the amino acid sequences of the encoded cupredoxin or
cytochrome that
do not significantly alter the ability of cupredoxin or cytochrome to inhibit
parasitemia in
42
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
malaria-infected red blood cells. A"non-essentiaP' amino acid residue is a
residue that can
be altered from the wild-type sequences of the cupredoxin without altering
biological
activity, whereas an "essential" amino acid residue is required for such
biological activity.
For example, amino acid residues that are conserved among the cupredoxins are
predicted to
be particularly non-amenable to alteration, and thus "essential."
Amino acids for which conservative substitutions that do not change the
activity of
the polypeptide can be made are well known in the art. Useful conservative
substitutions are
shown in Table 3, "Preferred substitutions." Conservative substitutions
whereby an amino
acid of one class is replaced with another amino acid of the same type fall
within the scope of
the invention so long as the substitution does not materially alter the
biological activity of the
compound.
Table 3. Preferred substitutions
Original residue Exemplary substitutions Preferred
substitutions
Ala (A) Val, Leu, Ile Val
Arg (R) Lys, Gln, Asn Lys
Asn (N) Gln, His, Lys, Arg Gln
Asp (D) Glu Glu
Cys (C) Ser Ser
Gln (Q) Asn Asn
Glu (E) Asp Asp
Gly (G) Pro, Ala Ala
His (H) Asn, Gln, Lys, Arg Arg
Ile (I) Leu, Val, Met, Ala, Phe, Leu
Norleucine
Leu (L) Norleucine, Ile, Val, Met, Ala, Ile
Phe
Lys (K) Arg, Gln, Asn Arg
Met (M) Leu, Phe, Ile Leu
Phe (F) Leu, Val, Ile, Ala, Tyr Leu
Pro (P) Ala Ala
Ser(S) Thr Thr
Thr (T) Ser Ser
Trp (W) Tyr, Phe Tyr
Tyr (Y) Trp, Phe, Thr, Ser Phe
Val (V) Ile, Leu, Met, Phe, Ala, Leu
Norleucine
43
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Non-conservative substitutions that affect (1) the structure of the
polypeptide
backbone, such as a(3-sheet or a-helical conformation, (2) the charge, (3)
hydrophobicity, or
(4) the bulk of the side chain of the target site can modify the cytotoxic
factor function.
Residues are divided into groups based on common side-chain properties as
denoted in Table
4. Non-conservative substitutions entail exchanging a member of one of these
classes for
another class. Substitutions may be introduced into conservative substitution
sites or more
specifically into non-conserved sites.
Table 4. Amino'acid classes
Class Amino acids
hydrophobic Norleucine, Met, Ala, Val, Leu, Ile
neutral hydrophilic Cys, Ser, Thr
acidic Asp, Glu
basic Asn, Gln, His, Lys, Arg
disrupt chain conformation Gly, Pro
aromatic Trp, Tyr, Phe
The variant polypeptides can be made using methods known in the art such as
oligonucleotide-mediated (site-directed) mutagenesis, alanine scanning, and
PCR
mutagenesis. Site-directed mutagenesis (Carter, Biochem J. 237:1-7 (1986);
Zoller and
Smith, Methods Enzymol. 154:329-350 (1987)), cassette mutagenesis, restriction
selection
mutagenesis (Wells et al., Gene 34:315-323 (1985)) or other known techniques
can be
performed on the cloned DNA to produce the cupredoxin or cytochrome c551
variant DNA.
Known mutations of cupredoxins and cytochrome c551 can also be used to create
variant cupredoxin and cytochrome c551 to be used in the methods of the
invention. For
example, the C 112D and M44KM64E mutants of azurin are known to have cytotoxic
and
growth arresting activity that is different frorift the native azurin, and
such altered activity can
be useful in the treatment methods of the present invention. One embodiment of
the methods
of the invention utilize cupredoxin and/or cytochrome and variants and
derivatives thereof
retaining the ability inhibit the growth of malaria infection in mammalian
cells. In another
embodiment, the methods of the present invention utilize cupredoxin variants
such as the
M44KM64E mutant, having the ability to cause cellular growth arrest.
44
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
A more complete understanding of the present invention can be obtained by
reference
to the following specific Examples. The Examples are described solely for
purposes of
illustration and are not intended to limit the scope of the invention. Changes
in form and
substitution of equivalents are contemplated as circumstances may suggest or
render
expedient. Although specific terms have been employed herein, such terms are
intended in a
descriptive sense and not for purposes of limitations. Modifications and
variations of the
invention as hereinbefore set forth can be made without departing from the
spirit and scope
thereof, and, therefore, only such limitations should be imposed as are
indicated by the
appended embodiments.
EXAMPLES.
Example 1: In Vitro Inhibition of P. falciparunz Parasitemia by Cupredoxin and
Cytochrome
The cupredoxins bacterial wt azurin, M44KM64E azurin, rusticyanin and
cyanobacterial plastocyanin, as well as the cytochromes Pseudomonas aeruginosa
cytochrome c551, human cytochrome c and Phormidiuna laminosum cytochrome f
were tested
in a normal red blood cell (RBC) assay at 200 g/ml concentrations at 30 hours
post
inoculation. In these experiments, the normal RBCs were washed twice in serum
free media
and resuspended to 10% hematocrit in complete RPMI. 200 l of 10% Hct RBCs
were added
to each of 24 wells (final 2% Hct at 1rn1) in addition to 30 l complete RPMI
containing
recombinant cupredoxin or cytochrome proteins at 666 M for a final
concentration of 200
M. Schizont-stage parasites were prepared by centrifuging a late-stage culture
through a
Percoll cushion at 3200 rpm for 10 minutes. For infection, 4 x 106
parasites/well in 500 l
volume were added at t=0 hr.. The plate was incubated for 30 hours and scored
by thin blood
smear and Giemsa stain at that time.
The control showed 9.5% parasitemia (standard error 1.3%), wt azurin 6.9%
(s.e.
1.4%), M44KM64E azurin 9.1% (s.e. 1.0%), rusticyanin 7.2% (s.e. 0.7%),
cytochrome c551
7.5% (s.e. 1.5%), human cytochrome c 8.4% (s.e. 0.4%), plastocyanin 8.1% (s.c.
1.3%) and
cytochrome f 6.6% (s.e. 1.0%), suggesting that cupredoxins such as wt azurin
and rusticyanin
and cytochromes such as cytochrome f or cytochrome c551 demonstrated 20 to 30%
inhibition
of parasitemia.
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
When the cupredoxins were tested for their effects at various stages of the
parasite life
cycle (0 - 24 hours, ring formation; 24-36 hours, trophozoite; 36-48 hours,
schizont), the
control showed 0.1 % average ring formation and 9.4% trophozoite formation
while wt azurin
showed no ring formation but 6.9% trophozoite formation; cytochrome f showed
0.2% ring
formation but had significantly low (6.3%) trophozoite formation. Remarkably,
rusticyanin
exhibited very high (2.0%) ring formation and significantly reduced (5.2%)
trophozoite
formation. The others had no significant effect. The parasites in rusticyanin -
treated
samples looked sick and dying as compared to the rest of the samples, showing
a significant
inhibitory and toxic effect of rusticyanin on parasite development.
Example 2: Inhibition In Vitro of P. falciparum Intracellular Replication by
Rusticyanin
To determine if the bacterial redox proteins can inhibit intracellular
replication of the
malarial parasites, red blood cells were loaded to an intracellular
recombinant protein
concentration of 200 g/ml using a hypotonic ghost preparation. Cells where
then washed,
resuspended and infected with schizont-stage parasites (P. falciparum) as
described in
Example 1. The red blood cell ghosts were incubated for 19 hours and 40 hours
and giemsa
smears were made.
Compared to the infections of nornial red blood cells in Example 1, only
rusticyanin
decreased total parasitemia in loaded cell ghost cultures. At 19 hours, there
was no
significant difference in invasion and ring formation, with empty ghosts at
5.0 0.4% and
rusticyanin-loaded ghosts at 4.5 1.0%. However, at 40 hours, rusticyanin-
loaded ghosts
had a lower level of infection. No major effects were seen at 19 hour with any
of the
bacterial proteins. However, at 40 hours, control untreated ghosts showed 4.6
0.3%
parasitemia while rusticyanin-treated ghosts had 2.7 :L 0.8% parasitemia, an
almost 50%
reduction. See, Table 5. Wt azurin, M44KM64E mutant azurin, plastocyanin,
cytochrome
cssi, human cytochrome c and cynobacterial cytochrome f proteins showed
parasitemia
varying from 4.2 to 5.4%.
46
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Table 5. Cupredoxin and cytochrome inhibition of P. falciparuni infection of
red blood cell
ghosts.
Treatment Mean Parasitemia Std. Error
at 40 hr
Empty 4.6% 0.3%
Wild Type Azurin 5.4% 1.0%
M44KM64E Azurin 4.7% 0.5%
Rusticyanin 2.7% 0.8%
Cytochrome c551 4.2% 0.4%
Human Cytochrome c 4.6% 0.8%
Plastocyanin 4.3% 0.3%
Cytochrome f 4.5% 0.9%
Example 3: Structural Homology between Azurin and Fab Fragment of G17.12
Monoclonal Antibody Complexed with Pf MSPl-19
Previous studies have shown that cupredoxins show structural similarity to the
variable domains of the immunoglobulin superfamily members. (Gough & Chothia,
Structure 12:917-925 (2004); Stevens et al., J. Mol. Recognit. 18:150-157
(2005)) The DALI
algorithm (Holm & Park, Bioinformatics 16:566-567 (2000)) was used to search
the 3D
databases for structural homologs of azurin (1JZG) from P. aeruginosa. Azurin
exhibits
structural similarity to the Fab fragment of G17.12 monoclonal antibody in
complexation
with Pf MSPl-19 fragment of the MSP1 merozoite surface protein of P.
falciparufn. (Pizarro
et al., J. Mol. Biol. 328:1091-1103 (2003).) (Table 6) Azurin also exhibits a
structural
similarity to ICAM-1 (Table 6), which is involved in cerebral malaria and
implicated as a
receptor on the endothelial cells in the microvasculture of the brain and
other tissues for
sequestering P. falciparum-infected erythrocytes. (Smith et al., Proc. Nati.
Acad. Sci. USA
97:1766-1771 (2000); Franke-Fayard et al., Proc. Natl. Acad. Sci. USA
102:11468-11473
(2005))
This example shows that cupredoxins including azurin demonstrate structural
similarities in having two anti-parallel 0 sheets packed face to face and
linked by a disulfide
bridge to the variable domains of the immunoglobulin superfamily members as
well as
extracellular domains of the intercellular adhesion molecules (ICAM) and their
ligands.
47
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Table 6. Structural similarity of P. aeruginosa azurin with various
pathogenesis-related
proteins
Azurin (ljzg)
PDB Annotation Reference DALI z score(l)
1VCA Human Vascular Cell Adhesion Molecle-1, 17 3.5
B1 VCAM-1
1 ZXQ 1 The Crystal Structure of ICAM-2 19 3.3
1IAM1 Structure of The Two Amino-Terminal 20 3.0
Domains of, ICAM-1
1 OB 1 Crystal Structure of a Fab complex with 21 2.9
Al Plasmodium falciparum MSP1-19
1TOP B The complex Structure of Binding Domains 22 2.5
of ICAM-3 and Alphabeta2
2NCM Neural Cell Adhesion Molecule, NCAM 23 2.4
(1) - Structural aligment to azurin were made using DALI (16). Structure pairs
with DALI z
scores <2 are considered dissimilar.
Example 4. Cloning And Expression of the Laz and H.8-Azurin Fusion Genes
The laz gene from Neisseria gonorrhoeae was cloned based on its known sequence
(SEQ ID NO: 22). The P. aeruginosa azurin gene (SEQ ID NO: 1), termed paz ,
and the
sequence of the H.8 epitope of laz from N. gonnerrhoeae (SEQ ID NO: 21), were
used to
clone in frame the H.8 epitope gene in the 5'-end of paz to produce H.8-paz or
in the 3'-end
of paz to generate paz-H.8.
Table 7. Cancer cells, bacterial strains and genetic constructs
Cells/strains/ Relevant characteristics* Reference
plasmids
P. aeruginosa Prototroph, FP- (sex factor minus) Holloway, et al.,
PAOI Microbiol. Rev. 43:73-102
(1979)
E. coli JM109 Cloning and azurin expression strain Yanisch-Perron, et al.,
Gene 33:103-119 (1985)
E. coli BL21 GST expression strain Novagen
(DE3)
48
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
N. Prototroph used for DNA isolation American Type Culture
gonorrhoeae Collection
F62
pU 18 General cloning vector, Ap' Yanisch-Perron, et al., id.
pUC 19 General cloning vector, Apr Yanisch-Perron, et al., id.
pUC 18-1az A 1 kb PCR fragment from genomic Herein
DNA of N. gonorrhoeae F62 cloned
into pUC 18
pUC19 paz A 0.55 kb PCR fragment from P. Yamada, et al., Proc. Natl.
aeruginosa PAO1 cloned into Hindlll Acad. Sci. USA 99:14098-
and Pstl digested pUC 19, Apr 14103 (2002); Yamada, et
al., Proc. Natl. Acad. Sci.
USA 101:4770-4775
(2004)
pUC18-H.8- Fusion plasmid encoding H.8 from N. Herein
paz gonorrhoeae and azurin from P.
aeruginosa PAO1, Ap'
pGEX-5X-3 GST gene fusion vectors, Ap' Amersham
pET29a E. coli expression vector, Km' Novagen
pET29a-gst pET29a derivative containing the gst Herein
gene, Kmr
pGEX-5X-3- pGEX-5X-3 derivative containing H.8- Herein
H.8 encoding region, Apr
pET29a-gst- pET29a derivative containing gst-H.8 Herein
H.8 gene, Kmr
*Ap, ampicillin; Km, kanamycin; GST, Glutathione S-transferase.
Cloning and Expression of the paz and laz Genes. The cloning and
hyperexpression of the azurin gene has been described. (Yamada, et al., Proc.
Natl. Acad.
Sci. USA 99:14098-14103 (2002); Punj, et al., Oncogene 23:2367-2378 (2004))
The Laz-
encoding gene (laz) of Neisseria gonorrhoeae was amplified by PCR with genomic
DNA of
N. gonorflhoeae strain F62 as template DNA. The forward and reverse primers
used were
5'-CCGGAATTCCGGCAGGGATGTTGTAAATATCCG-3' (SEQ ID NO: 23) and
5'-GGGGTACCGCCGTGGCAGGCATACAGCATTTCAATCGG-3' (SEQ ID NO: 24)
49
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
where the additionally introduced restriction sites of EcoRI and Kpnl sites
are underlined
respectively. The amplified DNA fragment of 1.0 kb, digested with EcoRI and
Kpnl, was
inserted into the corresponding sites of pUC 18 vector (Yanisch-Perron, et
al., Gene 33:103-
119 (1985)) so that the laz gene was placed downstream of the lac promoter to
yield an
expression plasmid pUC18-laz (Table 7).
The plasmids expressing fusion H.8 of N. gonorrizoeae Laz and azurin of P.
aeruginosa (Paz) were constructed by PCR with pUC 19 paz and pUC 18-laz as
templates.
For H.8-Paz fusion, a 3.1 kb fragment was amplified with pUC 18-laz as a
template and
primers, 5'-(phosphorylated)GGCAGCAGGGGCTTCGGCAGCATCTGC-3' (SEQ ID NO:
25) and 5'-CTGCAGGTCGACTCTAGAGGATCCCG-3' (SEQ ID NO: 26) where a SaII site
is underlined. A PCR amplified a 0.4 kb fragment was obtained from pUC 19 paz
as a
template and primers, 5'-(phosphorylated)GCCGAGTGCTCGGTGGACATCCAGG-3' (SEQ
ID NO: 27) and 5'-TACTCGAGTCACTTCAGGGTCAGGGTG-3' (SEQ ID NO: 28) where
a XhoI site is underlined. A SaII digested PCR fragment from pUC 1 8-laz and
Xhol digested
PCR fragment from pUC 19paz were cloned to yield an expression plasmid pUC18-
H.8 paz
(Table 7).
E. coli JM109 was used as a host strain for expression of azurin and its
derivative
genes. Recombinant E. coli strains were cultivated in 2 X YT medium containing
100 g/ml
ampicillin, 0.1 mM IPTG and 0.5 mM CuSO4 for 16 h at 37 C to produce the
azurin proteins.
When E. coli strains harboring these plasmids were grown in presence of IPTG,
cells
lysed and the proteins purified as described for azurin (Yamada, et al., Proc.
Natl. Acad. Sci.
USA 99:14098-14103 (2002); Punj, et al., Oncogene 23:2367-2378 (2004);Yamada,
et al.,
Cell. Microbiol. 7:1418-1431 (2005)), the various azurin derivatives migrated
on SDS-PAGE
as single components, although the H.8 containing proteins (about 17 kDa)
showed
anomalous migrations, as noted before (Cannon, Clin. Microbiol. Rev. 2:S1-S4
(1989);
Fisette, et al., J. Biol. Chem. 278:46252-46260 (2003)).
Plasmid Construction for Fusion GST Proteins. Plasmids expressing fusion
glutathione S-transferase (GST)-truncated wt-azurin (azu) derivatives were
constructed by a
polymerase chain reaction using proofreading DNA polymerase. For pGST-azu 36-
128, an
amplified PCR fragment was introduced into the BamHl and EcoRl sites of the
commercial
GST expression vector pGEX-5X (Amersham Biosciences, Piscataway, NJ). The
fragment
was amplified with pUC19-azu as a template and primers, 5'-CGGGATCC CCG GCA
ACC
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
TGC CGA AGA ACG TCA TGG GC-3'(SEQ ID NO: 29) and 5'-CGGAATTC GCA TCA
CTT CAG GGT CAG GG-3' (SEQ ID NO: 30), where the additionally introduced BamHl
and EcoRl sites are underlined respectively. Carboxyl-terminus truncation of
azu gene was
cumulatively performed by introducing a stop codon using QuickChange site-
direct
mutagenesis kit (Stratagene, La Jolla, CA).
For pGST-azu 36-89, a stop codon were introduced into G1y90. The plasmid
carrying
pGST-azu 36-128 was used as template DNA. Three sets of oligonuclotides for
site-direct
mutagenesis are shown as follows. For pGST-azu 36-89: 5'-CCA AGC TGA TCG GCT
CGT GAG AGAAGG ACT CGG TGA CC-3' (SEQ ID NO: 31), and 5'-GGT CAC CGA
GTC CTT CTC TCA CGA GCC GAT CAG CTT GG-3 (SEQ ID NO: 32).
For pGST-azu 88-113, carboxyl terminus truncation of azu gene was cumulatively
performed by introducing stop codon using QuiclcChange site directed
mutagenesis kit
(Stratagene, La Jolla, CA). For pGST-azu 88-113, a stop codon was introduced
into Phe114.
The plasmid carrying pGST-azu 88-128 was used as the template. For pGST-azu 88-
128 an
amplified PCR fragment was introduced into the BairrHl and EcoRl sites of the
commercial
GST expression vector pGEX-5X (Amersham Biosciences). The fragment was
amplified
with pUC19-azu as the template and primers, 5'-CGGGGATCC CCG GCT CGG GCG AGA
AGG AC-3' (SEQ ID NO: 33) and 5'-CGGGAATTC TCC ACT TCA GGG TCA GGG TG-
3' (SEQ ID NO: 34) where the additionally introduced BanaHl and EcoRl sites
are
underlined respectively.
One set of oligonucleotides for site directed mutagenesis are shown as follows
for the
preparation of pGST-azu 88-113: 5'-GTT CTT CTG CAC CTA GCC GGG CCA CTC CG-
3' (SEQ ID NO: 35) and 5'-CGG AGT GGC CCG GCT AGG TGC AGA AGA AC-3' (SEQ
ID NO: 36). pGST-azu 88-113 was used to transform E. colf XL-1 Blue strains.
Plasmid
extraction was performed using a commercial kit (Qiagen, Venlo, The
Netherlands) and PCR
sequencing were performed to assess plasmid insertion and transfection.
E. coli BL21 (DE3) was used as a host strain for expression of the gst and its
fusions
derivatives. E. colf strain XL1-Blue transformed with pGST-azu plasmids was
grown in LB
media with ampicillin for three hours at 37 C upon which IPTG induction (0.4
mM) was
performed an subsequent incubation for 2-4 h at 37 C to maximize the
expression levels.
Cells were isolated by centrifugation, resuspended in 25 mL of IX PBS buffer.
Subsequent
cell lysis involved two sequential treatments of the cell suspension via
sonication (20 min on
51
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
ice) and heat-cold shock in acetone-dry ice bath (using the appropriate
protease inhibitors).
Supematants of the cell lysis mixture were isolated and passed through a
freshly packed and
PBS equilibrated 1 mL glutathione-sepharose 4B (Amersham Biosciences) column.
After
column washing and subsequent elution of GST-azu product using 10 mM
glutathione in 20
mM Tris-HCl pH 8. GST-Azu 88-113 purity was tested via electrophoresis using a
10%
SDS-PAGE Tris-Gly gel stained with Coomassie Brilliant Blue R reagent. Protein
concentration was determined using the Bradford Method.
Example 5. Azurin Binds to the C-Terminal Fragments MSP1-19 and MSP1-42 of the
P. falciparum Merozoite Surface Protein MSPl.
Given the structural similarity (Table 6) between azurin and the fab fragment
of the
monoclonal antibody G17.12 in complexation with Pf MSP1-19 (Pizarro et al.,
id), the ability
of azurin to form a complex with Pf MSPl-42 or Pf MSP1-19 was determined. Two
derivatives of azurin, Laz, an azurin-like protein from gonnococci and
meningococci such as
Neisseria meningitidis with an additional 39 amino acid epitope called an H.8
epitope
(Gotschlich & Seiff, FEMS Microbiol. Lett. 43:253-255 (1987); Kawula et al.,
Mol.
Microbiol. 1:179-185 (1987)) and H.8-azurin, where the H.8 epitope of Laz has
been fused in
the N-terminal part of P. aeruginosa azurin in frame (as described in Example
4) were tested.
In vitro protein-protein interactions were evaluated using a Biacore X
spectrometer
from Biacore AB International. All experiments were conducted at 25 C in HBS-
EP running
buffer (0.01 M HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v Surfactant
P20)
using Au-CM5 sensor chips (Biacore). Protein immobilizations on CM5 chips were
conducted according to the amine coupling procedure. Proteins were immobilized
after
NHS/EDC preactivation of the CM5 surface: 50 l injections of azurin (510
IVI).
Subsequent treatment of CM5 surface with ethanolamine (1M, pH 8.8) removed
uncrosslinked proteins. Binding studies were performed by injecting protein
eluents (50 l)
over the protein-CM5 surface at flow rates of 30 l/min with a 120 sec time
delay at the end
of the injections. Protein eluents included GST-azurin fusion proteins (GST,
GST-Azu 36-
128, GST-Azu 36-89, and GST-Azu 88-113, as described in Example 4). Sensor
chip
surfaces were regenerated between protein injections using 100 mM NaOH (10 l
injection
pulse). All binding studies were run in parallel against a negative flow
channel with bare Au-
CM5 sensor surface to correct for nonspecific binding to the chips. To
generate binding
52
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
constant data, titration experiments were designed via injection of increasing
concentrations
of protein eluents (0.05-2000 nM). The SPR data were fit to a Langmuir (1:1)
equilibrium
binding model [Req = Rmax/(1 + Kd/C] as specified in the Biacore software from
which
binding constants (Kd) were extrapolated.
Specific interactions of the Pf MSPl-19 and Pf MSP1-42 proteins with azurin,
H.8-
azurin and Laz were determined by surface plasmon resonance (SPR) analysis and
the data
are presented in Figure 1. SPR sensorgrams for binding of immobilized Pf MSP1-
19 and Pf
MSP1-42 with azurin and its derivatives indicated selective recognition among
these proteins.
While nanomolar concentrations of azurin allowed significant binding with the
immobilized
MSP1-19 (Fig. 1A) or MSP1-42 (Fig. 1B), both H.8-azurin and Laz demonstrated a
higher
affinity of binding with the merozoite surface protein MSP1 cleavage products,
with
characteristic Kd values of 32.2 nM between azurin and MSP1-19 and 54.3 nM
between
azurin and MSP1-42. The Kd values between H.8-azurin and MSPI-19 and MSPI-42
were
11.8 nM and 14.3 nM while such values between Laz and MSP1-19 and MSP1-42
ranged
from 26.2 nM and 45.6 nM respectively.
To examine if the H.8 epitope might facilitate binding of the H.8-azurin or
Laz to the
PfMSPI-19 or PfMSP1-42 moieties, the ability of glutathione S-transferase
(GST) and a
fusion derivative H.8-GST where the H.8 epitope was fused in the N-terminal of
GST (see
Example 4), to bind MSP1-19 or MSP1-42 was tested. Neither the GST nor the H.8-
GST
bound PfMSP1-19 (Fig. 1A) or MSP1-42 (Fig. 1B), although H.8-GST showed a weak
binding with MSP1-42.
Glutathione S transferase (GST) and some of the fusion proteins where parts of
azurin
were fused to GST (Yamada et al., Cell. Microbiol. 7:1418-1431 (2005), and
Example 4)
were tested for their ability to bind to MSP1-19. GST alone, or GST-Azu 88-
113, where the
azurin amino acid sequence 88 to 113 out of 128 amino acids of azurin was
fused to GST in
frame, did not show any binding (Fig. 1C) while GST-Azu 36-89 with amino acid
sequence
36 to 89 and GST-Azu 36-128 with ainino acid sequence 36 to 128 showed
significant
binding with MSPI-19 with Kd values of 20.9 nM and 24.5 nM respectively.
53
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Example 6. Inhibition of Plasrtzodium falciparunz Parasitemia by Azurin, H.8-
Azurin
and Laz.
The extent of parasitemia was determined using schizont stage parasites and
normal
red blood cells (RBC). Normal red blood cells (RBCs) were washed twice in
serum-free
medium and resuspended to 10% hematocrit in complete RPMI. 200 l of 10%
hematocrit
RBCs were added to each of 24 wells in addition to 300 l complete RPMI
without or with
azurin, H.8-azurin or Laz at various concentrations. Schizont stage P.
falciparum parasites
were prepared by centrifuging a late-stage culture through a Percoll cushion
at 3200 rpm for
min. For infection, 4x106 parasites per well in 500 l volume were added at
time zero.
The plate was incubated overnight (about 16 h) and then scored by thin blood
smear and
Giemsa stain at that time.
Azurin, H.8-azurin or Laz all demonstrated significant inhibition of
parasitemia in a
dose-dependent manner (Fig. 2), although at relatively high concentrations
(about 50 M).
Such high concentrations presumably reflect the multiple ways the malarial
parasites invade
the erythrocytes (Cowman et al., FEBS Lett. 476:84-88 (2000); Baum et al., J.
Biol. Chem.
281:5197-5208 (2006)) and a high concentration of azurin or Laz is necessary
to interfere in
the entry process. As indicated by their enhanced binding affinities to MSPI-
19, both H.8-
azurin and Neisserial Laz protein showed a higher level of inhibition of P.
falciparuna
parasitemia as compared to azurin (Fig. 2).
When azurin was labeled with the red fluorescent dye Alexa fluor 568 and used
during the invasion assay, very little red fluorescence was detectable inside
the RBC,
suggesting that azurin seems not to enter the RBC as part of bound MSPl-19, or
more likely,
that the RBCs that showed the presence of the schizonts were the ones where
azurin failed to
bind with the MSPl-19. These data fully agree with our previous observation
(Yamada et al.,
Cell. Microbiol. 7:1418-1431 (2005)) that azurin does not enter normal cells
such as
macrophages, mast cells, etc, and the effect of azurin, H.8-azurin or Laz is
at the entry level
rather than the intracellular replication of the parasite. Taken together, the
data in Fig. 2
demonstrate the potential antimalarial action of azurin, H.8-azurin and Laz
through
interference in the invasion of the RBC by the parasites.
54
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
Example 7. Azurin binds ICAMs
An interesting structural similarity between azurin and ICAMs (Table 6) that
are
known to be involved as receptors for P. falciparum-infected erythrocytes
(Wassmer et al.,
P1oS Med. 2:885-890 (2005); Dormeyer et al., Antimicrob. Agents Chemotherap.
50:724-730
(2006)) prompted test analysis of protein-protein interactions as measured by
SPR between
azurin and ICAMs such as ICAM-1, ICAM-2, ICAM-3 and NCAM. With immobilized
azurin on the CM5 chip, ICAM-3 (Fig. 3, Kd = 19.5 + 5.4 nM) and NCAM (Fig. 3,
inset), but
interestingly not ICAM-1 and ICAM-2, showed strong binding. While not limiting
the
manner in which the invention operates, part of effect of azurin on inhibition
of P. falciparum
parasitemia might also be mediated through its interaction with ICAM-3 or
NCAM.
Example 8. Treatment Of Patients Likely Exposed to or Exposed to Malaria
Clinical use for the prevention malaria, a pharmaceutical comprising one or
more
cupredoxin and/or a cytochrome is administered to a patient.
Fifteen healthy male volunteers, aged 22-50, who have a no history of
preexisting
antibodies to blood-stage P. falciparum parasites, as determined by
immunofluorescent assay,
but reside in an area where malaria is endemic, will be injected with a
pharmaceutical
preparation of purified cupredoxin and purified cytochrome. Two such men will
serve as
non-treated controls.
The sterile pharmaceutical preparation is in the form of 0.5 ml single-dose
ampules of
sterile Pseudomonas aerugninosa azurin in a pharmaceutical preparation
designed for
intraveneous administration, as will be well known to those in the art.. The
pharmaceutical
preparation is stored at 4 C. and protected from light before administration.
In one clinical
trial, azurin is prepared at five different concentrations: 10 g, 30 g, 100
g, 300 g and 800
g azurin per 0.5 ml dose.
The pharmaceutical preparation is given intraveneously to thirteen volunteers
for each
doses. Volunteers receive primary treatment at day 0 and subsequent doses
identical
doses at every other day for three weeks. Volunteers are observed for
immediate toxic effects
for twenty minutes after injection. Twenty-four and forty-eight hours later,
they are
examined for evidence of fever, local tenderness, erythema, warmth, induration
and
lymphadenopathy, and are asked about complaints of headache, fever, chills,
malaise, local
pain, nausea and joint pain. Before each dose, blood and urine samples are
taken for full
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
laboratory examination. Complete blood count and serum chemistry profiles are
recheclced
two days after each dose. The presence of the malaria parasite are determined
by light
microscopic examination (ME) of the stained blood smears, or the ICT Malaria
P.f.IP.v. test
kits ( Binax, Inc., Portland, ME) . The results demonstrate the effectiveness
of the therapy.
Example 9: Control of Malaria Infection of Insects
A transmissible genetic element that passes from one mosquito to another will
be
operably connected to the cupredoxin coding sequence operably connected to a
constitutive
promoter. The P. aeruginosa azurin will therefore be produced inside the
Anopheles
gambiae infected with P. falciparum and will interfere with its
replication/survival in the
mosquito. This mosquito will then be introduced to an endemic area so that the
azurin-
harboring genetic element will spread to other P. falciparum-infected A.
gambiae mosquitoes,
inhibiting P. falciparum growth or survival.
Example 10. Treatment of patients infected by Malaria
Clinical use of a malaria therapy, comprising one or more cupredoxin and/or a
cytochrome, for treatment of malaria infection in humans.
Fifteen healthy male volunteers, aged 22-50, who exhibit a history of
preexisting antibodies
to blood-stage P. falciparum parasites, as determined by immunofluorescent
assay are
injected with a pharmaceutical preparation of purified P. aeruginosa azurin.
Two such men
serve as treated controls.
The sterile pharmaceutical preparation is in the form of 0.5 ml single-dose
ampules of
sterile P. aeruginosa azurin in a pharmaceutical preparation designed for
intraveneous
administration, as will be well known to those in the art.. The pharmaceutical
preparation is
stored at 4 C. and protected from light before administration. In one
clinical trial, P.
aeruginosa azurin is prepared at five different concentrations: 10 g, 30 g,
100 g, 300 g
and 800 g azurin/cytochrome C551 (1:1 on molecule basis) per 0.5 ml dose.
The pharmaceutical preparation is given intraveneously to thirteen volunteers
for each
doses. Volunteers receive primary treatment at day 0 and subsequent doses
identical
doses at every other day for three weeks. Volunteers are observed for
immediate toxic effects
for twenty minutes after injection. Twenty-four and forty-eight hours later,
they are
examined for evidence of fever, local tenderness, erythema, warmth, induration
and
56
CA 02607739 2007-11-07
WO 2006/127477 PCT/US2006/019492
lymphadenopathy, and are asked about complaints of headache, fever, chills,
malaise, local
pain, nausea and joint pain. Before each dose, blood and urine samples are
taken for full
laboratory examination. Complete blood count and serum chemistry profiles are
rechecked
two days after each dose. The presence of the malaria parasite are determined
by light
microscopic examination (ME) of the stained blood smears, or the ICT Malaria
P.f./P.v. test
kits ( Binax, Inc., Portland, ME) . The results demonstrate the effectiveness
of the therapy.
57