Note: Descriptions are shown in the official language in which they were submitted.
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
PYRIDINE [3,4-b] PYRAZINONES
Cross Reference to Related Applications
This application claims priority from U. S. Provisional Application Serial
Number 60/684,139
filed May 24, 2005, the disclosure of which is incorporated herein by
reference in its entirety.
FIELD OF THE INVENTION
The present invention comprises a class Pyridine [3,4-b] Pyrazinone compounds
having the
structure of Formula I and pharmaceutical compositions comprising a compound
of Formula I.
The present invention also comprises methods of treating a subject by
administering a
therapeutically effective amount of a compound of Formula I to the subject. In
general, these
compounds inhibit, in whole or in part, the enzyme: cyclic guanylate
monophosphate-specific
phosphodiesterase type 5 (PDE-5).
BACKGROUND OF THE INVENTION
The prevalence of hypertension in developed countries is about 20% of the
adult population,
rising to about 60-70% of those aged 60 or more. Hypertension is associated
with an increased
risk of stroke, myocardial infarction, atrial fibrillation, heart failure,
peripheral vascular disease
and renal impairment. Despite the large number of anti-hypertensive drugs
available in various
pharmacological categories, additional agents useful for the treatment of
hypertension are stili
needed.
Vascular endothelial cells secrete nitric oxide (NO). This acts on vascular
smooth muscle
cells and leads to the activation of guanylate cyclase and the accumulation of
cyclic guanosine
monophosphate (cGMP). The accumulation of cGMP causes the muscles to relax and
the
blood vessels to dilate, leading to a reduction in blood pressure. The cGMP is
inactivated by
hydrolysis to guanosine 5'-monophosphate (GMP) by a cGMP-specific
phosphodiesterase. One
important cGMP-phosphodiesterase has been identified as Phosphodiesterase type
5 (PDE5).
Inhibitors of PDE5 decrease the rate of hydrolysis of cGMP and so potentiate
the actions of nitric
oxide.
Improved drug therapies for the treatment of subjects suffering from or
susceptible to a
cardiovascular condition are desirable. In particular, there still is a need
for a new class of PDE-
5 inhibitors for treating cGMP-mediated conditions and corresponding drug
therapies.
SUMMARY OF THE INVENTION
In one embodiment, the invention comprises compounds having the structure of
Formula I:
1
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
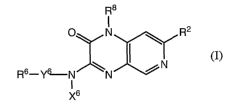
R8
O R2
N
~ I N
R6-Y6N N
I
X6
tI)
wherein R2, X6, Y6, R6, and R8 are as defined in the detailed description of
the invention.
In another embodiment, the invention comprises a pharmaceutical composition
comprising a
compound having the structure of Formula I.
In another embodiment, the invention comprises methods of treating a condition
in a subject
by administering a therapeutically effective amount of a compound having the
Formula I to the
subject. The conditions that can be treated in accordance with the present
invention include
cardiovascular diseases, metabolic diseases, central nervous system diseases,
pulmonary
diseases, sexual dysfunction, and renal dysfunction.
In another embodiment, the invention comprises a method for inhibiting PDE-5,
and
particularly methods for treating a condition (typically a pathological
condition) mediated by PDE-
5 activity by administering a compound having a structure of Formula I to the
subject.
In another embodiment, the invention comprises intermediates useful in the
synthesis of
compounds having the structure of Formula I.
DETAILED DESCRIPTION OF THE INVENTION
This detailed description of embodiments is intended only to acquaint others
skilled in the art
with Applicants' inventions, its principles, and its practical application so
that others skilled in the
art may adapt and apply the inventions in their numerous forms, as they may be
best suited to
the requirements of a particular use. These inventions, therefore, are not
limited to the
embodiments described in this specification, and may be variously modified.
A. Abbreviations and Definitions
As used in reference to'H NMR, the symbol "5"refers to a'H NMR chemical shift.
As used in reference to'H NMR, the abbreviation "br" refers to a broad'H NMR
signal.
As used in reference to'H NMR, the abbreviation "d" refers to a doublet'H NMR
peak.
As used in reference to'H NMR, the abbreviation "dd" refers to a doublet of
doublets'H
NMR peak.
The abbreviation "HRMS" refers to High Resolution Mass Spectrocopy
(electrospray
ionisation positive scan).
The abbreviation "m/z" refers to a Mass spectrum peak.
As used in reference to iH NMR, the abbreviation "m" refers to a multiplet 1H
NMR peak.
As used in reference to'H NMR, the abbreviation "q" refers to a quartet'H NMR
peak.
As used in reference to'H NMR, the abbreviation "s" refers to a singlet'H NMR
peak.
2
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
As used in reference to'H NMR, the abbreviation 'Y' refers to a triplet iH NMR
peak.
The abbreviation 'TFA" refers to trifluoroacetic acid.
The term "alkyl" (alone or in combination with other term(s)) refers to a
linear or branched-
chain saturated hydrocarbyl substitutent (i.e., a substitutent containing only
carbon and
hydrogen) typically containing from about one to about twenty carbon atoms or;
in another
embodiment from about one to about twelve carbon atoms; in another embodiment,
from about
one to about ten carbon atoms; in another embodiment, from about one to about
six carbon
atoms; and in another embodiment, from about one to about four carbon atoms.
Examples of
such substituents include methyl, ethyl, propyl (including n-propyl and
isopropyl), butyl (including
n-butyl, isobutyl, sec-butyl and tert-butyl), pentyl, iso-amyl, hexyl and the
like.
The term "alkenyl" (alone or in combination with other term(s)) refers to a
linear or branched-
chain hydrocarbyl substituent containing one or more double bonds and from
about two to about
twenty carbon atoms; in another embodiment, from about two to about twelve
carbon atoms;in
another embodiment, from about two to about six carbon atoms; and in another
embodiment,
from about two to about four carbon atoms. Examples of alkenyl radicals
include ethenyl, allyi,
propenyl, butenyl and 3-methylbutenyl.
The terms "alkenyl'", and "lower alkenyl", embrace radicals having "cis" and
"trans"
- - ---or-ientations,-or alternatively,-"Z"and"EO' orientations-
The term "alkynyl" (alone or in combination with other term(s)) refers to
linear or branched-
chain heterocarbyl substituents containing one or more triple bonds and from
about two to about
twenty carbon atoms; in another embodiment, from about two to about twelve
carbon atoms; in
another embodiment, from about two to about six carbon atoms; and in another
embodiment,
from about two to about four carbon atoms. Examples of alkynyl radicals
include 1-propynyl, 2-
propynyl, 1-butyne, 2-butynyl and 1-pentynyl.
The term "amino", alone or in combination with another term(s), refers to -NH2
when it is at a
terminal position or to -NH-when it is used in combination with another
term(s) and is not at a
terminal position.
The term "aryl", alone or in combination with another term(s), refers to a
carbocyclic aromatic
system containing one, two or three rings wherein such rings may be attached
together in a
pendent manner or may be fused. Examples of aryl moieties include phenyl,
naphthyl,
tetrahydronaphthyl, indanyl and biphenyl.
The term "carboxy", alone or in combination with another term(s),. refers to a
radical of the
formula -C(O)OH.
The term "cyano", alone or in combination with another term(s), means -CN,
which also may
- -C N
be depicted:
The term "cycloalkyl", alone or in combination with another term(s), refers to
saturated
carbocyclic radicals having three to about twelve carbon atoms. In another
embodiment,
3
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
cycloalkyl radicals are "lower cycloalkyl" radicals having three to about
eight carbon atoms.
Examples of such radicals include cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
The term "cycloalkylalkyl", alone or in combination with another term(s),
refers to alkyl
substituted with cycloalkyl. Examples of such substituents include
cyclopropylmethyl,
cyclobutylmethyl, cyclopentylmethyl, and cyclohexylmethyl.
The term "cycloalkenyl", alone or in combination with another term(s), refers
to a partially
unsaturated carbocyclyl substituent. Examples of such substituents include
cyclobutenyl,
cyclopentenyl, and cyclohexenyl.
The term "halogen" or "halo", alone or in combination with another term(s),
refers to means a
fluorine radical (which may be depicted as -F), chlorine radical (which may be
depicted as -CI),
bromine radical (which may be depicted as -Br), or iodine radical (which may
be depicted as -I).
In another embodiment, the halogen is a fluorine or chlorine radical. In
another embodiment, the
halogen is a fluorine radical.
When used in combination with another term(s), the prefix "halo" indicates
that the
substituent to which the prefix is attached is substituted with one or more
independently selected
halogen radicals. For example, haloalkyl refers to an alkyl substituent
wherein at least one
hydrogen radical is replaced with a halogen radical. Where there are more than
one hydrogens
- ------r-eplaced-w-ith-halogens;-the-halogens-may be-the-same-or-different.-
Exampies of-haloalkyls
include chloromethyl, dichloromethyl, difluorochloromethyl,
dichlorofluoromethyl, trichloromethyl,
1-bromoethyl, fluoromethyl, difluoromethyl, trifluoromethyl, 2,2,2-
trifluoroethyl, difluoroethyl,
pentafluoroethyl, difluoropropyl, dichloropropyl, and heptafluoropropyl.
Illustrating further,
"haloalkoxy' means an alkoxy substituent wherein at least one hydrogen radical
is replaced by a
halogen radical. Examples of haloalkoxy substituents include chloromethoxy, 1 -
bromoethoxy,
fluoromethoxy, difluoromethoxy, trifluoromethoxy (also known as
"perfluoromethyloxy"), and
2,2,2,-trifluoroethoxy. If a substituent is substituted by more than one
halogen radical, those
halogen radicals may be identical or different (unless otherwise stated).
The term "heterocyclyl" refers to a saturated, partially saturated, or
completely unsaturated
ring structure containing a total of 3 to 14 ring atoms. At least one of the
ring atoms is a
heteroatom (i.e., oxygen, nitrogen, or sulfur), with the remaining ring atoms
being independently
selected from the group consisting of carbon, oxygen, nitrogen, and sulfur.
A heterocyclyl may be a single ring, which typically contains from 3 to 10
ring atoms, more
typically from 3 to 7 ring atoms, and even more typically 5 to 6 ring atoms.
Examples of
single-ring heterocyclyls include furanyl, dihydrofurnayl, tetradydrofurnayl,
thiophenyl (also
known as "thiofuranyl"), dihydrothiophenyl, tetrahydrothiophenyl, pyrrolyl,
isopyrrolyl, pyrrolinyl,
pyrrolidinyl, imidazolyl, isoimidazolyl, imidazolinyl, imidazolidinyl,
pyrazolyl, pyrazolinyl,
pyrazolidinyl, triazolyl, tetrazolyl, dithiolyl, oxathiolyl, oxazolyl,
isoxazolyl, thiazolyl, isothiazolyl,
thiazolinyl, isothiazolinyl, thiazolidinyl, isothiazolidinyl, thiodiazolyl,
oxathiazolyl, oxadiazolyl
(including oxadiazolyl, 1,2,4-oxadiazolyl (also known as "azoximyl"), 1,2,5-
oxadiazolyl (also
known as "furazanyl"), or 1,3,4-oxadiazolyl), oxatriazolyl (including 1,2,3,4-
oxatriazolyl or
1,2,3,5-oxatriazolyl), dioxazolyl (including 1,2,3-dioxazoiyl, 1,2,4-
dioxazolyl, 1,3,2-dioxazolyl, or
4
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
1,3,4-dioxazolyl), oxathiazolyl, oxathiolyl, oxathiolanyl, pyranyl (including
1,2-pyranyl or
1,4-pyranyl), dihydropyranyl, pyridinyl (also known as "azinyl"), piperidinyl,
diazinyl (including
pyridazinyl (also known as "1,2-diazinyP'), pyrimidinyl (also known as "1,3-
diazinyP" or "pyrimidyl"),
or pyrazinyl (also known as "1,4-diazinyP')), piperazinyl, triazinyl
(including s-triazinyl (also known
as "1,3,5-triazinyP'), as-triazinyl (also known 1,2,4-triazinyl), and v-
triazinyl (also known as
"1,2,3-triazinyP')), oxazinyl (including 1,2,3-oxazinyl, 1,3,2-oxazinyl, 1,3,6-
oxazinyl (also known as
"pentoxazolyP'), 1,2,6-oxazinyl, or 1,4-oxazinyl), isoxazinyl (including o-
isoxazinyl or p-isoxazinyl),
oxazolidinyl, isoxazolidinyl, oxathiazinyl (including 1,2,5-oxathiazinyl or
1,2,6-oxathiazinyl),
oxadiazinyl (including 1,4,2-oxadiazinyl or 1,3,5,2-oxadiazinyl), morpholinyl,
azepinyl, oxepinyl,
thiepinyl, and diazepinyl. A heterocyclyl alternatively may comprise 2 or 3
rings fused together,
wherein at least one such ring contains a heteroatom as a ring atom (e.g.,
nitrogen, oxygen, or
sulfur). Examples of 2-fused-ring heterocyclyls include, indolizinyl,
pyrindinyl, pyranopyrrolyl,
4H-quinolizinyl, purinyl, naphthyridinyl, pyridopyridinyl (including
pyrido[3,4-b]-pyridinyl,
pyrido[3,2-b]-pyridinyl, or pyrido[4,3-b]-pyridinyl), and pteridinyl, indolyl,
isoindolyl, indoleninyl,
isoindazolyl, benzazinyl, phthalazinyl, quinoxalinyl, quinazolinyl,
benzodiazinyl, benzopyranyl,
benzothiopyranyl, benzoxazolyl, indoxazinyl, anthranilyl, benzodioxolyl,
benzodioxanyl,
benzoxadiazolyl, benzofuranyl, isobenzofuranyl, benzothienyl, isobenzothienyl,
benzothiazolyl,
------benzothiadiazolyl,-benzimidazolyl,--benzotriazolyl, benzoxazinyl,
benzisoxazinyl, and
tetrahydroisoquinolinyl. Other examples of fused-ring heterocyclyls include
benzo-fused
hetes-ccyclyls, such as indolyl, isoindolyl (also known as "isobenzazolyl" or
"pseudoisoindolyl"),
indoleninyl (also known as "pseudoindolyl"), isoindazolyl (also known as
"benzpyrazolyl"),
benzazinyl (including quinolinyl (also known as "1-benzazinyl") or
isoquinolinyl (also known as
"2-benzazinyl")), phthalazinyl, quinoxalinyl, quinazolinyl, benzodiazinyl
(including cinnolinyl (also
known as "1,2-benzodiazinyl") or quinazolinyl (also known as "1,3-
benzodiazinyP')), benzopyranyl
(including "chromanyl" or "isochromanyl"), benzothiopyranyl (also known as
"thiochromanyl"),
benzoxazolyl, indoxazinyl (also known as "benzisoxazolyl"), anthranilyl,
benzodioxolyl,
benzodioxanyl, benzoxadiazolyl, benzofuranyl (also known as "coumaronyP'),
isobenzofuranyl,
benzothienyl (also known as "benzothiophenyl," "thionaphthenyl," or
"benzothiofuranyl"),
isobenzothienyl (also known as "isobenzothiophenyl," "isothionaphthenyl," or
"isobenzothiofuranyl"), benzothiazolyl, benzothiadiazolyl, benzimidazolyl,
benzotriazolyl,
benzoxazinyl (including 1,3,2-benzoxazinyl , 1,4,2-benzoxazinyl , 2,3,1-
benzoxazinyl , or
3,1,4-benzoxazinyl ), benzisoxazinyl (including 1,2-benzisoxazinyl or 1,4-
benzisoxazinyl),
tetrahydroisoquinolinyl , carbazolyl, xanthenyl, and acridinyl.
The term "heteroaryl", alone or in combination with another term(s), refers to
a completely
unsaturated (i.e., aromatic) heterocyclyl containing from 5 to 14 ring atoms.
A heteroaryl may
comprise a single ring or 2 or 3 fused rings. In one embodiment, heteroaryl
radicals are 5- or 6-
membered heteroaryl, containing one or two heteroatoms selected from sulphur,
nitrogen and
oxygen, selected from thienyl, furanyl, thiazolyl, imidazolyl, pyrazolyl,
isoxazolyl, isothiazolyl,
pyridyl and pyrazinyl. Examples of heteroaryl substituents include 6-membered
ring substituents
such as pyridyl, pyrazyl, pyrimidinyl, and pyridazinyl; 5-membered ring
substituents such as
5
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
1,3,5-, 1,2,4- or 1,2,3-triazinyl, imidazyl, furanyl, thiophenyl, pyrazolyl,
oxazolyl, isoxazolyl, and
thiazolyl; 1,2,3-, 1,2,4-, 1,2,5-, or 1,3,4-oxadiazolyl and isothiazolyl; 6/5-
membered fused ring
substituents such as benzothiofuranyl, isobenzothiofuranyl, benzisoxazolyl,
benzoxazolyl,
purinyl, and anthranilyl; and 6/6-membered fused rings such as 1,2-, 1,4-, 2,3-
and 2,
1-benzopyronyl, quinolinyl, isoquinolinyl, cinnolinyl, quinazolinyl, and 1,4-
benzoxazinyl. Other
heteroaryls include unsaturated 5 to 6 membered heteromonocyclyl groups
containing 1 to 4
nitrogen atoms, for example, pyrrolyl, imidazolyl, pyrazolyl, 2-pyridyl, 3-
pyridyl, 4-pyridyl,
pyrimidyl, pyrazinyl, pyridazinyl, triazolyl [e.g., 4H-1,2,4-triazolyl, 1H-
1,2,3-triazolyl, 2H-1,2,3-
triazolyl]; unsaturated condensed heterocyclic groups containing 1 to 5
nitrogen atoms, for
example, indolyl, isoindolyl, indolizinyl, benzimidazolyl, quinolyl,
isoquinolyl, indazolyl,
benzotriazolyl, tetrazolopyridazinyl [e.g., tetrazolo [1,5-b]pyridazinyl];
unsaturated 3 to 6-
membered heteromonocyclic groups containing an oxygen atom, for example,
pyranyl, 2-furyl, 3-
furyl, etc.; unsaturated 5 to 6-membered heteromonocyclic groups containing a
sulfur atom, for
example, 2-thienyl, 3-thienyl, etc.; unsaturated 5- to 6-membered
heteromonocyclic groups
containing 1 to 2 oxygen atoms and 1 to 3 nitrogen atoms, for example,
isoxazolyi, oxadiazolyl
[e.g., 1,2,4-oxadiazolyl, 1,3,4-oxadiazolyl, 1,2,5-oxadiazolyl]; unsaturated
condensed heterocyclic
groups containing 1 to 2 oxygen atoms and 1 to 3 nitrogen atoms [e.g.
benzoxazolyl,
---------benzoxadiazoiyl]; unsaturated-5-to-6=membered-heteromonocyclic-groups
containing- 1-to 2 sulfur
atoms and 1 to 3 nitrogen atoms, for example, thiazolyl, thiadiazolyl [e.g.,
1,2,4- thiadiazolyl,
1,3,4-thiadiazolyl, 1,2,5-thiadiazolyl]; unsaturated condensed heterocyclic
groups containing 1 to
2 sulfur atoms and 1 to 3 nitrogen atoms [e.g., benzothiazolyl,
benzothiadiazolyl] and the like.
The term also embraces radicals where heterocyclic radicals are fused with
aryl radicals.
Examples of such fused bicyclic radicals include benzofuran, benzothiophene,
and the like.
The term "heterocyclylalkyl", alone or in combination with another term(s),
refers to alkyl
substituted with a heterocyclyl.
The term "hydroxy", alone or in combination with another term(s), refers to -
OH.
The term "mercapto" or "thiol" refers to a sulfhydryl substituent, which also
may depicted as -
SH.
The term "nitro", alone or in combination with another term(s), refers to -
NOZ.
The term "sulfonyl", alone or in combination with another term(s), refers to -
S(O)2-, which
also may be depicted as:
OO
'2Z s ~.
Thus, for example, "alkyl-sulfonyl-alkyl" refers to alkyl-S(O)2-alkyl.
Examples of typically
preferred alkylsulfonyl substituents include methylsulfonyl, ethylsulfonyl,
and propylsulfonyl.
The term "sulfoxyl" , alone or in combination with another term(s), refers to -
S(O) -, which
also may be depicted as:
6
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
0
S
The term "thio" or "thia", alone or in combination with another term(s),
refers to a thiaether
substituent, i.e., an ether substituent wherein a divalent sulfur atom is in
the place of the ether
oxygen atom. Such a substituent may be depicted as -S-. This, for example,
"alkyl-thio-alkyl"
means alkyl-S-alkyl.
If a substituent is described as being "optionally substituted", the
substituent may be either
(1) not substituted, or (2) substituted. If a carbon of a substituent is
described as being optionally
substituted with one or more of a list of substituents, one or more of the
hydrogens on the carbon
(to the extent there are any) may separately and%or together be replaced with
an independently
selected optional substituent. This specification uses the terms "substituent"
and "radical"
interchangeably.
The term "cGMP-mediated condition" refers to any condition mediated by cGMP,
whether
through direct regulation by cGMP, or through indirect regulation by cGMP as a
component of a
signaling pathway.
The term "composition" refers to an article of manufacture which results from
the mixing or
combining of more than one element or ingredient.
The term "hypertensive subject" refers to a subject having hypertension,
suffering from the
effects of hypertension or susceptible to a hypertensive condition if not
treated to prevent or
control such hypertension.
The term "pharmaceutically acceptable carrier" refers to a carrier that is
compatible with the
other ingredients of the composition and is not deleterious to the subject.
Such carriers may be
pharmaceutically acceptable material, composition or vehicle, such as a liquid
or solid filler,
diluent, excipient, solvent or encapsulating material, involved in carrying or
transporting a
chemical agent. The preferred composition depends on the method of
administration.
The terms "prevent," "prevention" or "preventing" refer to either preventing
the onset of a
preclinically evident condition altogether or preventing the onset of a
preclinical evident stage of
a condition in a subject. Prevention includes, but is not limited to,
prophylactic treatment of a
subject at risk of developing a condition.
The term "therapeutically effective amount" refers to that amount of drug or
pharmaceutical
agent that will elicit the biological or medical response of a tissue, system
or animal that is being
sought by a researcher or clinician.
The term "treatment" (and corresponding terms "treat" and "treating") includes
palliative,
restorative, and preventative treatment of a subject. The term "palliative
treatment" refers to
treatment that eases or reduces the effect or intensity of a condition in a
subject without curing
the condition. The term "preventative treatment" (and the corresponding term
"prophylactic
treatment") refers to treatment that prevents the occurrence of a condition in
a subject. The term
7
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
"restorative treatment" refers to treatment that halts the progression of,
reduces the pathologic
manifestations of, or entirely eliminates a condition in a subject.
B. Compounds
The present invention comprises, in part, a novel class of pyridine [3,4-b]
pyrazinone
compounds. These compounds are useful as inhibitors of PDE5.
Compounds of Formula (I)
As used herein, compounds of the present invention include tautomers of the
compounds
and pharmaceutically acceptable salts of the compounds and tautomers.
The present invention is directed, in part, to a class of compounds having the
structure of
Formula I:
R8
O N ~ R2
s_ ( ~ N
R YQN N
I
- -- X6
-(I) --------- ----- ---
wherein
R2 is selected from the group consistirig of aryl and 3 to 10 membered ring
heterocycyl
wherein R2 may be optionally substituted with one or more substituents
independently selected
from the group consisting of halogen, cyano, nitro, oxo, alkyl, alkenyl,
alkynyl, cycloalkyl, -OR100,
-C(O)Ri o -OC(O)R100, -C(O)OR'00, -NRi R101 -N(R1 )C(O)R1 1-C(O)NRi R'01, -
C(O NR10 C O R101, -SR10 -S O R10 and -S O R10 ) ( ) ( ) ( )2 , aziridinyl,
azetidinyl, pyrrolidinyl,
piperidinyl, morpholinyl, thiomorpholinyl, and piperazinyl wherein (a) the
alkyl, alkenyl, alkylnyl
and cycloalkyl substituents may be optionally substituted with one or more
substituents
independently selected from the group consisting of halogen, oxo, -OR102, and -
C(O)OR102; and
(b) the aziridinyl, azetidinyl, pyrrolidinyl, piperidinyl, morpholinyl,
thiomorpholinyl, and piperazinyl
substituents may be optionally substituted with one or more substituents
selected from the group
consisting of alkyl, hydroxy and alkoxy;
R10 , R101 and R102 are independently selected from the group consisting of
hydrogen, alkyl,
alkenyl and alkynyl, wherein the alkyl may be optionally substituted with one
or more substituents
independently selected from the group consisting of halogen, hydroxy, alkoxy, -
C(O)OH and -
C(O)NH2;
X6 is selected from the group consisting of hydrogen and alkyl wherein the X6
alkyl
substituent may be optionally substituted with one or more substituents
selected from the group
consisting of chloro, fluoro, hydroxy and alkoxy;
8
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Y6 represents a bond or is selected from the group consisting of alkyl,
alkenyl and alkynyl,
wherein Y6 may be optionally substituted with one ormore substituents
independently selected
from the group consisting of halogen, cyano, oxo, cycloalkyl, -OR103, -
C(O)R103, -C(O)OR103, -
OC O R'03' -NR1 03R104' -N R103 C O R104 1 3 1 4
( ) ( ) ( ) , and -C(O)NR R ;
R1 3 and R'04 are independently selected from the group consisting of hydrogen
and alkyl,
wherein the alkyl may be optionally substituted with one or more substituents
independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkynyl,
haloalkynyl, hydroxyalkynyl, carboxyalkynyl, alkoxy, haloalkoxy,
hydroxyalkoxy, and
carboxyalkoxy;
R6 is selected from the group consisting of aryl, aryl-C(O)-, heterocyclyl,
aryl-C(O)-NR105-
heterocyclyl-C(O)-, and heterocyclyl-C(O)-NR105- wherein R 6 may be optionally
substituted with
one or more substituents independently selected from the group consisting of
halogen, cyano,
oxo, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heterocyclyl, -OR106, -
C(O)R106, -C(O)OR'oe, _
OC(O)R106, -NR106Rim -N(R1 06)C(O)R107, -C(O)NR10sRio7, -C(O)NR'06 C(O)R1 07 -
SR106
-
S(O)R106, -S(O)2R106, -N(R106)S(O)2R107, and -S(O)2NR106R107; wherein the
alkyl, alkenyl, alkynyl,
cycloalkyl, aryl and heterocyclyl susbstituents may be optionally substituted
with one or more
substituents independently selected from the group consisting of halogen,
hydroxy, carboxy,
cyano, oxo, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy;
R105 is independently selected from the group consisting of hydrogen and
alkyl;
R106 and R107 are independently selected from the group consisting of
hydrogen, alkyl,
alkenyl, and alkynyl, wherein (a) the R106 and R107 alkyl and alkenyl
substituents may be
optionally substituted with one or more substituents independently selected
from the group
consisting of halogen, hydroxy, carboxy, cyano, oxo, alkynyl, haloalkynyl,
hydroxyalkynyl,
carboxyalkynyl, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy, and (b)
the R106 and R107
alkynyl substituents may be optionally substituted with one or more
substituents independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkoxy, haloalkoxy,
hydroxyalkoxy, and carboxyalkoxy;
RB is alkyl; wherein RB may be optionally substituted with one or more
substituents
independently selected from the group consisting of halogen, hydroxy, carboxy,
cyano, alkynyl, -
OR108, -C(O)R108, -C(O)OR108, -OC(O)Ri08, -NR108 R109, -N(R108)C(O)R109, -
C(O)NR10eR1e9
, -
SR108, -S(O)R108, -S(O)2R108, and -C(O)NR108C(O)R109, wherein the alkynyl
substituents may be
optionally substituted with one or more substituents independently selected
from the group
consisting of halogen, hydroxy, carboxy, cyano, oxo, alkyl, and alkoxy; and
9
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
R108 and R109 are independently selected from the group consisting of
hydrogen, alkyl,
alkenyl and alkynyl, wherein (a) when the alkyl is methyl, the methyl may be
optionally
substituted with 1, 2, or 3 fluoro substituents, (b) when the alkyl comprises
at least two carbon
atoms, the alkyl may be optionally substituted with one or more substituents
independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkynyl,
haloalkynyl, hydroxyalkynyl, carboxyalkynyi, alkoxy, haloalkoxy,
hydroxyalkoxy, and
carboxyalkoxy, and (c) the R10Band R109 alkenyl and alkynyl substituents may
be optionally
substituted with one or more substituents independently selected from the
group consisting of
halogen, hydroxy, carboxy, cyano, oxo, alkoxy, haloalkoxy, hydroxyalkoxy, and
carboxyalkoxy.
Selected subclasses of compounds of interest that fall within the scope of the
compounds of
Formula I are shown in Table A, wherein R 2, X6, Ys, Rs, and R8 can be as
defined for compounds
of Formula I and as defined in the various embodiments described thoughout
this specification.
Illustrative embodiments of these subclasses of compounds are described later
in the
specification.
TABLE A
R10 R1o
R8 R9 R11 R8 R9 Rii
O N \\ N O N N
~
Rs_YsN ,N i N Ris R6_Y6 N ,N N R13
Xs (I-A) H (I-B)
R8 R11 R11
O N R8
N 0 N N
Rs-YO-N ~N N R13 s_ s 1 , N
Xs (I C) R Y XN s N (I D)
R8 / O
O
O N \\ N O N N
\
Rs-YsN ~N ~ N ~ N
R YG-N N
Xs (I-E) ~6 (I-F)
Rio Rio
Re R9 N RS R9 / Rii
O N
I R12 0 N \ R12
s_ 6~ N R13 ~ N R13
R Y N N Rs-Y~N N
X s (I-G) Xs (I-H)
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
R10 R10
Rs R9 / R11 Rg R11
O N . \ R12 0 N
6_ 6 ~ I,N R13 6_ ~~ N
R Y H N R Y N N
I
(I I) X6 (I-J)
R8 / R11 R$ R9 N' R11
O N \ \ O N Nr
6_ fi ~ N 6_ 6 ~ N R13
R Y N N R Y N N
X6 (I-K) X6 (I-L)
11 Rs cAh1
R8 R9 N R
O N / ~ \ II
O N
\ \ N ~
s_ ~~ i N R13 R6_Y6 N ~N N R13
R Y H N (I-M) X6 (I-N)
R11 R11
_ .. ------ ------- -- --..._ ---R9--- -- -- -- _.- -- - ------- - R9
8 ,
O N N p N8 \ p
6_ fi- /\ I N R13 ~ I ~ R13
R Y N N R6 -Y6 N N
j~s (1-0) j~s (I-P)
R1o R1o
Rs R9 O R8 R9 O
O N p /\N \ \ ~
s _ /' / N R13 I N R13
R Y6 N N R6-Y6 N N
j(s (I-Q) j~s (I-R)
Ra R6
O I R2 0 I
R2
/N
R6-Y N \N N R6-YG-N \N
H ~
(I-S) (I-T)
8 R8
O\N I R2 O N R2
Rs-Y N N R6_N \N
(I-U) x6 (I-V)
11
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
R8
~ R8
2
O N R
I 0 N ~ fl2
R6N N N
R6N \N
Xs (I-W) x6 (I-X)
\O~ O--)
O N R2 0 N
R2
R6-Y~N \N N Rs-YsN \N
x6 (I-Y) X6 (I-Z)
~
O//>
O N R2 O R2 R6-Y6N ~N N R6-Y6 N ~N
j~6 (I-AA) X6 (I-BB)
~~o~ ~'/~O
O N R 2 O N I R 2
Rs-YcH \N Rs-YsN \N N
(I-CC) H (I-DD)
R11
2 O N N
O N R
R6-Y6 N N N
R6-Y6N H (I-FF)
H (I-EE)
2
N
R 0 N R2
0
R6-N \N /
H (I-GG) H N
(I-HH)
12
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Rii
O N Rz O N N
s"\ I N
Rs/\N \N N R N N
(I H (I JJ)
H
-II)
Q~ R11 Ri i
Q--)
N \ \ N O N N
IN
R
s\~~H ~N Rs_ ~N ~ N
0
(I-KK) H (I-LL)
Rii
F F O N R2 O N N
Rs-YaN \N I Rs-Ys-N N N
H (I-MM) H (INN)
2 ~
O N =~ R O N ~ Rz
I \IY
Rs-N N R\~\ \ I~ N
H (I-00) H N
(I-PP)
v \Q ~/ 'Q R11
O Rz O N \
H N
s~\ I N
s/\ N R N N
R N H (I RR)
(I-QQ)
O R11 ~~\Q R11
--)
N I I N O N
R \~H CN N R6_N N / N
(I SS) H
(I-TT)
13
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
O N \ N
~
R6-YGN \N ~ N
H (I-UU)
Embodiments of Substituent
In one embodiment of Formula I, R 2 is selected from the group consisting of
aryl and 3 to 10
membered ring heterocycyl wherein R2 may be optionally substituted with one or
more
substituents independently selected from the group consisting of halogen,
cyano, nitro, oxo,
alkyl, alkenyl, alkynyl, cycloalkyl, -OR100, -C(O)Ri00, -OC(O)Rioo, -C(O)OR10
, -NR10 R101, -
N(R10 )C(O)R101, -C(O)NR100R101, -C(O)NR10 C(O)R101, and -S(O)mR10 wherein
the alkyl,
alkenyl, alkynyl and cycloalkyl substituents may be optionally substituted
with one or more
substituents independently selected from the group consisting of halogen, oxo,
-OR102, and -
C(0)OR102; wherein R10 , R101 and R102 are independently selected from the
group consisting of
hydrogen, alkyl, alkenyl and alkynyl, wherein the alkyl may be optionally
substituted with one or
more substituents independently selected from the group consisting of halogen,
hydroxy, alkoxy,
-C(O)OH and -C(O)NH2; and m is 0, 1, or 2.
In one embodiment of Formula I, R2 is selected from the group consisting of
phenyl and a 3
to 10 membered ring heteroaryl, optionally substituted as described in Formula
I. In another
embodiment of Formula I, R2 is selected 'from the group consisting of phenyl
and a 5 to 7
membered ring heterocyclyl, optionally substituted as described in Formula I.
In another
embodiment of Formula I, R2 is selected from the group consisting of phenyl
and a 5 to 7
membered ring heteroaryl, optionally substituted as described in Formula I. In
another
embodiment of Formula I, R2 is selected from the group consisting of phenyl
and a 5-6
membered ring heteroaryl, optionally substituted as described in Formula I. In
another
embodiment of Formula I, R2 is a 5 to 6 membered ring heteroaryl that
comprises 1, 2, or 3 ring
heteroatoms selected from the group consisting of oxygen and nitrogen.
In one embodiment of Formula I, R2 is selected from the group consisting of
phenyl, thienyl,
furanyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, pyrazinyl
pyridinyl, triazinyl,
imidazyl, thiophenyl, pyrazolyl, oxazolyl, oxadiazolyl, pyridyl, pyrazyl,
pyrimidinyl, pyridazinyl,
benzofuran, and benzodioxolyl. In another embodiment of Formula I, R2 is
selected from the
group consisting of phenyl, pyridinyl, pyrimidinyl, isoxazolyl, pyrazolyl,
benzofuran, and
benzodioxolyl, optionally substituted as described in Formula I. In another
embodiment of
Formula I, R2 is selected from the group consisting of phenyl,
N\NH \O ) \ N N \
N N
'L N I I I N
_Z and
14
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
each optionally substituted as provided in Formula I. In another embodiment of
Formula I, R2 is
selected from the group consisting of phenyl and pyridinyl, optionally
substituted as described in
Formula I.
In one embodiment of Formula I, R2 is optionally substituted with one or more
substituents
selected from the group consisting of halogen, oxo, alkyl, -OR10 , -C(O)R10 , -
OC(O)R'oo, -
C(O)OR10 , -NR10 R101 and -C(O)NR10 R101, wherein the alkyl substitutent may
be optionally
substituted with one or more substituents independently selected from the
group consisting of
halogen, oxo, -OR102, and -C(O)OR102; wherein R'oo R'o' and R102 are
independently selected
from the group consisting of hydrogen and C, to C4 alkyl.
In another embodiment of Formula I, R 2 is optionally substituted with one or
more
substituents independently selected from the group consisting of halogen,
cyano, oxo, C, to C4
alkyl, -OR100, -C(O)OR10 , and -C(O)NR10 R'01, wherein (a) when the alkyl is
methyl, the methyl
may be optionally substituted with 1, 2, or 3 halogen substituents, (b) when
the alkyl comprises at
least two carbon atoms, the alkyl may be optionally substituted with one or
more substituents
selected from the group consisting of halogen, C, to C4 alkoxy and hydroxy;
and wherein R'oo
and R101 are independently selected from the group consisting of hydrogen and
Ci to C2 alkyl.
In another embodiment of Formula I, R2 is optionally substituted with one or
more
substituents selected from the group consisting of halogen, C, to C4 alkyl, -
OR100, and -
NR100R101; wherein (a) when the alkyl is methyl, the methyl may be optionally
substituted with 1,
2, or 3 halogen substituents, (b) when the alkyl comprises at least two carbon
atoms, the alkyl
may be optionally substituted with one or more substituents selected from the
group consisting of
halogen, oxo, C1 to C2 alkoxy and hydroxy; and wherein R10 and R101 are
independently
selected from the group consisting of hydrogen and Ci to C2 alkyl.
In one embodiment of Formula I, R2 is optionally substituted with one or more
substituents
selected from the group consisting of chloro, fluoro, methyl, ethyl, propyl,
butyl, pentyl, hexyl,
trifluoromethyl, hydroxy, methoxy, ethoxy, propoxy, butoxy, amino,
methylamino, dimethylamino,
ethylamino, and diethylamino. In another embodiment of Formula I, R2 is
optionally substituted
with one or more substituents selected from the group consisting of fluoro,
methyl,
trifluoromethyl, methoxy, trifluoromethoxy, amino, methylamino, and
dimethylamino.
In one embodiment of Formula I, R2 is substituted with one or more fluoro
substituents. In
another embodiment of Formula I, R2 is substituted=with one fluoro
substituent. In another
embodiment of Formula I, R2 is substituted with two fluoro substituents.
In one embodiment of Formula I, R2 is substituted with methoxy.
In one embodiment of Formula I, R2 is substituted at the para position with a
substituent
selected from the group consisting of fluoro, methyl, trifluoromethyl,
methoxy, trifluoromethoxy,
amino, methylamino, and dimethylamino. In another embodiment of Formula I, R2
is substituted
at the para position with a substituent selected from the group consisting of
fluoro, methyl,
trifluoromethyl, methoxy, and trifluoromethoxy.
In one embodiment of Formula I, R2 is selected from the group in Table A
consisting of
Formula I-A, Formula I-C, Formula I-G, Formula I-H, Formula I-I, Formula I-J,
Formula I-K,
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Formula I-L, Formula I-M, Formula I-N, Formula 1-0, Formula I-P, Formula I-Q
and Formula I-R,
wherein R9, R10, R", R'2 and R'3 are independently selected from the group
consisting of
hydrogen, halogen, oxo, alkyl, -OR10 , -C(O)R100, -OC(O)R10 , -C(O)OR10 , -
NR10 R101 and -
C(O)NR100R101, wherein the alkyl substitutent may be optionally substituted
with one or more
substituents independentiy selected from the group consisting of halogen, oxo,
-OR102, and -
C(O)OR102; wherein R10 , R101, and R102 are independently selected from the
group consisting of
hydrogen and Ci toC4 alkyl. In another embodiment of Formula I, R2 is selected
from the group
in Table A consisting of Formula I-H, Formula I-A and Formula I-L, wherein R9,
R'o, R", R'Z and
R13 are independently selected from the group consisting of hydrogen, halogen,
oxo, alkyl, -
OR100, -C(O)R100, -OC(0)R100, -C(O)OR10 , -NR10 R101 and -C(O)NR'ooR10'
wherein the alkyl
substitutent may be optionally substituted with one or more substituents
independently selected
from the group consisting of halogen, oxo, -OR102, and -C(O)OR102; wherein
R'oo R101, and R102
are independently selected from the group consisting of hydrogen and Ci to C4
alkyl. In another
embodiment of Formula I, R 2 is selected from the group in Table A consisting
of Formula I-A,
Formula I-D, Formula I-H and Formula I-L, wherein R9, R'o, R", R'2 and R13 are
independently
selected from the group consisting of hydrogen, halogen, alkyl, haloalkyl,
oxy, alkoxy, hydroxy,
and carboxy. In another embodiment of Formula I, R 2 is selected from the
group in Table A
consistingof_Formula, -A,_ Formula I-C, Formula I-D and Formula I-H, wherein
R9, R10, R", R'z
and R13 are independently selected from the group consisting of hydrogen,
fluoro, methyl,
trifluoromethyl, and methoxy. In another embodiment of Formula I, 'r~2 is
selected from the group
in Table A consisting of Formula I-D and Formula I-H, wherein R9, R'O. R", R12
and R13 are
independently selected from the group consisting of hydrogen, fluoro, methyl,
trifluoromethyl, and
methoxy.
In one embodiment of Formula I, R2 is as described by the structures in Table
A consisting
of Formula I-D and Formula I-H wherein R9, R'o, R", R12and R13 are
independently selected from
the group consisting of hydrogen, fluoro, methyl, trifluoromethyl, and
methoxy. In another
embodiment of Formula I, R2 is as described in Formula I-D in Table A, wherein
R" is selected
from the group consisting of hydrogen, fluoro, methyl, trifluoromethyl, and
methoxy. In another
embodiment of Formula I, the R2 substituent is as described in Formula I-E in
Table A.
Embodiments of X6 Substituent
In one embodiment of Formula I, X6 is selected from the group consisting of
hydrogen and Ci
to C4 alkyl wherein the X6 Cy to C4 alkyl substituent may be optionally
substituted with one or
more substituents selected from the group consisting of chloro, fluoro,
hydroxy and alkoxy.
In one embodiment of Formula I, X6 is alkyl, optionally substituted with 1 to
3 substituents
independently selected from the group consisting of fluoro and chloro. In
another embodiment of
Formula I, X6 alkyl is optionally substituted with 1 to 3 fluoro substituents.
In one embodiment of Formula I, X6 is selected from the group consisting of
hydrogen and Ci
to C6 alkyl. In another embodiment of Formula I, X6 is selected from the group
consisting of
hydrogen, m,ethyl, and ethyl. In another embodiment, X6 is hydrogen.
16
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Embodiments of Ys Substituent
In one embodiment of Formula I, Y6 represents a bond or is selected from the
group
consisting of alkyl, alkenyl and alkynyl, wherein the Y6 alkyl, alkenyl and
alkynyl substituents may
be optionally substituted with one or more substituents independently selected
from the group
consisting of halogen, cyano, oxo, cycloalkyl, -OR103, -C(O)R103, -C(O)OR103,
_OC(O)R'03_
NR103R104, -N R103 C O R104, and -C O NR'03R10a 103 and R10a
( )() () ; and wherein R are independently
selected from the group consisting of hydrogen and alkyl, wherein the alkyl
may be optionally
substituted with one or more substituents independently selected from the
group consisting of
halogen, hydroxy, carboxy, cyano, oxo, alkynyl, haloalkynyl, hydroxyalkynyl,
carboxyalkynyl,
alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy.
In one embodiment of Formula I, Ys represents a bond or is alkyl, optionally
substituted as
described in Formula I. In another embodiment of Formula I, Y6 represents a
bond or is Ci to C6
alkyl, optionally substituted as described in Formula I. In another embodiment
of Formula I, Y6
represents a bond or is selected from the group consisting of Ci to C4 alkyl
and hydroxyCi to C6.
alkyi. In another embodiment of Formula I, Y6 represents a bond or is selected
from the group
consisting of methyl, ethyl, propyl, butyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl,
_dihydroxyeth~rl,.and dihy_droxybutyl,_ In another embodiment of Formula I, Y6
represents a bond
or is selected from the group consisting of methyl, ethyl, propyl, butyl,
hydroxyethyl,
hydroxypropyl, hydroxybutyl, dihydroxyethyl,
HO HO HO T HO,,__HO~ HO--,,,_ HO
~ (R6)
(R6) (Rs) (Rs) (R6) (R6) \,(R6), and HO
In one embodiment of Formula I, Ys represents a bond or is selected from the
group
consisting of methyl, ethyl, propyl and butyl. In another embodiment of
Formula I, Y6 represents
a bond.
In one embodiment of Formula I, Y6 is alkyl optionally substituted with one or
more
substituents independently selected from the group consisting of halogen,
cyano, oxo, cycloalkyl,
-OR103, -C(O)R103, _C(O)OR103, _OC(O)R103, _NR103R104, -N(R103)C(O)R104 , and -
C(O)NR103 104
R ;
wherein R103 and R104 are independently selected from the group consisting of
hydrogen and Ci
to C4 alkyl. In another embodiment of Formula I, Y6 is alkyl substituted with
one or more
substituents independently selected from the group consisting of fluoro,
chloro, oxo, cycloalkyl,
hydroxy, and carboxy. In another embodiment of Formula I, Y6 is alkyl
substituted with one to
three substituents independently selected from the group consisting of hydroxy
and cyclohexyl.
In another embodiment of Formula I, Y6 is unsubstituted alkyl. In another
embodiment of
Formula I, Ys is selected from the group consisting of methyl, ethyl and
propyl. In another
embodiment, Y6 is selected from the group consisting of -CH2-, -CH2CH2-, -
CH2CH2CH2-, -
CH2(CH3)-, -CH2CH2(CH3)-, -CH2(CH3)CH2-, -CH2C(CH3) 2CH-, -C(CH3) 2CH-, -
CH2C(CH3)-, and
-CH2CH2CH2CH2-. In another embodiment of Formula I, Y6 is methyl.
17
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Embodiments of 146 Substituent
In one embodiment of Formula I, R 6 is selected from the group consisting of
phenyl, phenyl-
C(O)-, 3 to 10 membered ring heterocyclyl, phenyl-C(O)-NR105-, 3 to 10
membered ring
heterocyclyl-C(O)-, and 3 to 10 membered ring heterocyclyl-C(O)-NR105-,
wherein R6 is optionally
substituted as described in Formula I, wherein R105 is selected from the group
consisting of
hydrogen and Ci to C6 alkyl. In another embodiment of Formula I, R6 is
selected from the group
consisting of phenyl, phenyl-C(O)-, 5 to 7 membered ring heterocyclyl, phenyl-
C(O)-NR105- 5 to
7 membered ring heterocyclyl-C(O)-, and 5 to 7 membered ring heterocyclyl-C(O)-
NR1o5-
wherein R6 is optionally substituted as described in Formula I, wherein R105
is selected from the
group consisting of hydrogen and Ci to C4 alkyl. In another embodiment of
Formula I, R6 is
selected from the group consisting of phenyl, phenyl-C(O)-, 5 to 6 membered
ring heterocyclyl,
phenyl-C(O)-NR105-, 5 to 6 membered ring heterocyclyl-C(O)-, and 5 to 6
membered ring
heterocyclyl-C(O)-NR105-, wherein R6 is optionally substituted as described in
Formula I wherein
R105 is hydrogen. In another embodiment, R6 is selected from the group
consisting of phenyl,
pyridinyl, piperidinyl, piperizinyl, morpholino, pyrazinyl, tetrahydropyran,
tetrahydrofuran,
isoxazole, imidazole, pyrrolidine, wherein R6 is optionally substituted as
described in Formula I.
In another embodiment, R 6 is selected from the group consisting of
morpholinyl,
tetrahydropyranyl, tetrahydrofuranyl, piperidinyl, and pyrrolidinyl, wherein
R6 is optionally
substituted as provided iR? Formula I.
In one embodiment of Formula I, R6 is selected from the group consisting of
phenyl, phenyl
C(O)NH-, and phenyl C(O)-.
In one embodiment of Formula I, R6 is selected from the group consisting of 5
to 7
membered ring heteroaryl, 5 to 7 membered ring heteroaryl-C(O)-, and 5 to 7
membered ring
heteroaryl-C(O)-NR105-, wherein R6 is optionally substituted as described in
Formula I, wherein
R105 is selected from the group consisting of hydrogen and Ci to C4 alkyl. In
another
embodiment of Formula I, R6 is a 3 to 10 membered ring heteroaryl, optionally
substituted as
described in Formula I. In another embodiment of Formula I, R6 is a 5 to 7
membered ring
heteroaryl, optionally substituted as described in Formula I. In another
embodiment of Formula I,
R6 is a 5 to 6 membered ring heteroaryl, optionally substituted as described
in Formula I. In
another embodiment of Formula I, R6 is a 5 to 6 membered ring heteroaryl
containing one to
three heteroatoms selected from the group consisting of 0 and N, optionally
substituted as
described in Formula I. In another embodiment of Formula I, R6 is selected
from the group
consisting of imidazole, isoxazole, pyridinyl and pyrazynyl, optionally
substituted as described in
Formula I. In another embodiment of Formula I, R6 is selected from the group
consisting of
imidazole, isoxazole, pyridinyl and pyrazynyl, optionally substituted with one
or more substituents
independently selected from the group consisting of alkyl, -OR105, and -
C(O)R105, wherein R105 is
18
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
selected from the group consisting of hydrogen, methyl, and ethyl. In another
embodimeht, R6 is
~ffi.
N N ~ n(Zr"?.
N~ O-N NJ
selected from the group consisting of , , , and
In one embodiment of Formula I, R6 is selected from the group consisting of 3
to 10
membered ring fully or partially saturated heterocylyl, 3 to 10 membered ring
fully or partially
saturated heterocyclyl-C(O)-, 3 to 10 membered ring fully or partially
saturated heterocyclyl-
C(O)NH-, optionally substituted as described in Formula I. In another
embodiment of Formula I,
R6 is a 5 to 7 membered ring fully or partially saturated heterocylyl, 5 to 7
membered ring fully or
partially saturated heterocyclyl-C(O)-, 5 to 7 membered ring fully or
partially saturated
heterocyclyl-C(O)NH-, wherein R6 is optionally substituted as described in
Formula I. In another
embodiment of Formula I, R6 is a 5 to 6 membered ring fully saturated
heterocylyl, 5 to 6
membered ring fully saturated heterocyclyl-C(O)-, 5 to 6 membered ring fully
saturated
heterocyclyl-C(O)NH-, wherein R6 is optionally substituted as described in
Formula I. In another
embodiment of Formula I, R6 is a 5 to 6 membered ring fully saturated
heterocylyl, wherein R6 is
optionally substituted as described in Formula I. In another embodiment of
Formula I, Rs is
selected from the group consisting of tetrahydrofuran, tetrahydropyran,
pyrrolidinyl, piperidinyl,
-- --- piper-azinyl;-and-morpholino; wher-ein-R6 is-optionallysubstituted as-
described-in Formula I. In
another embodiment of Formula I, R6 is selected from the group consisting of
tetrahydrofuran,
tetrahydropyran, pyrrolidinyl, piperidinyl, piperazinyl, and morpholino,
wherein R6 is optionally
substituted with one or more substituents independently selected from the
group consisting of
alkyl, -OR105, -C(O)R'05 and -C(O)OR105 wherein R1 5 is selected from the
group consisting of
hydrogen, methyl, and ethyl. In another embodiment, R6 is selected from the
group consisting
O
~-Z-
n1'~ N
of > > O > > ,
O
~. N
~~
HN
N
> > , ,
19
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
0
O N
olf r J~
HNJ NJ
> > a
O
N ~ rN
\/NJ NJ
O o
O
_JN~' JN
~ rN
/ \O N O~/ O~/ O
N JL, H
OJ
and
In another embodiment of Formula I, R6 is optionally substituted with one or
more
substituents independently selected from the group consisting of -OR'06, -
C(O)R106, -C(O)OR106,
-NR106Ri07 -N(Ry06)C(O)Ri07, -N(R1 06)C(O)ORi07, -C(O)NR106R107, -NHC(O)NR106
Ri07, -
N(R106)S(O)2R107, wherein R106 and R107 are independently selected from the
group consisting of
hydrogen, methyl, ethyl, methylethyl, tert-butyl, cyclopentyl, cyclohexyl,
optionally substituted
with one or more substituent selected from the group consisting of halogen,
oxo, hydroxy, and
methyl. In another embodiment of Formula I, R6 is optionally substituted with
one or more
substituents independently selected from the group consisting of -OH, -OCH3, -
OCH2CH3, -
OCH2(CH3)CH3, -C(O)CH2CH2OH, -C(O)OH, -C(O)OCH2(CH3)(CH3)(CH3), -NH2, -
NH(CH2CH3), -
N(CH3)(CH3), -N(CH2CH3)CH2CH3, -N(H)CH2(CH3)CH3, -N(H)CH2CH2CH2OH, -
N(H)CH2C(O)CH3, -NH(CH2CH2OH), -N(H)C(O)OCH3, -N(H)C(O)CH2(CH3)(CH3)(CH3), -
NHC(O)OCH2(CH3)(CH3)(CH3), -NHC(O)COCH3, -NHC(O)NHCOCH3, -NCH3C(O)CH3, -
NCH3(O)C(O)CH3i N(CH(O)CH3)C(O)CH3, -C(O)NH2, -C(O)NH(CH3), -C(O)NCH3(CH3), -
C(O)NHCH2(CH3)(CH3)(CH3), -C(O)NHR106, -N(H)S(O)2CH3i -N(H)S(O)2CHF3, wherein
R106 is
independently selected from the group consisting of cyclopentyl and
cyclohexyl, and wherein the
R106 cyclohexyl substituent is optionally substituted with hydroxy; and
wherein (b) the cyclohexyl
R6 substituent is optionally substituted with hydroxy. In another embodiment
of Formula I, R6 is
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
optionally substituted with one or more substituents independ-ently selected
from the group
consisting of alkyl, --C(O)R'osand -C(O)OR106, wherein R106 is selected from
the group consisting
of hydrogen, methyl, and ethyl. In another embodiment of Formula I, R6 is
optionally substituted
with one or more substituents independently selected from the group consisting
of -OH, -CH3, -
CH2CH3i -C(O)CH3, -C(O)CH2CH3, -C(O)OCH2CH3.
Embodiments of A8 Substituent
In one embodiment of Formula I, RB is alkyl; wherein the R8 substituent may be
optionally
substituted with one or more substituents independently selected from the
group consisting of
halogen, hydroxy, carboxy, cyano, alkynyl, -OR10S, -SR108, -C(O)R108, -
C(O)OR108, -OC(O)R108, -
NR'08R10s, -N(R108)C(O)R109, -C(O)NR108R109, and -C(O)NR108C(O)R109, wherein
the alkynyl
substituents may be optionally substituted with one or more substituents
independently selected
from the group consisting of halogen, hydroxy, carboxy, cyano, oxo, alkyl, and
alkoxy; and
wherein R10S and R109 are independently selected from the group consisting of
hydrogen, alkyl,
and alkynyl, wherein (a) when the alkyl is methyl, the methyl may be
optionally substituted with 1,
2, or 3 fluoro substituents, (b) when the alkyl comprises at least two carbon
atoms, the alkyl may
be optionally substituted with one or more substituents independently selected
from the group
_- -consisting_of-halogen,h_y_dx_oxy, carh.oxy,_.cyano,_oxo,-
alkyny_i,h.aloalkynayl,hydr.oxyalkynyl,
carboxyalkynyl, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy, and (c)
the R108and R109
alkynyl substituents may be optionally substituted with one or more
substituents independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkoxy, haloalkoxy,
hydroxyalkoxy, and carboxyalkoxy.
In one embodiment of Formula I, R8 is Ci to C10 alkyl, optionally substituted
as described in
Formula I. In another embodiment of Formula I, R8 is C. to C$ alkyl,
optionally substituted as
described in Formula I. In another embodiment of Formula I, R8 is Ci to C6
alkyl, optionally
substituted as described in Formula I. In another embodiment of Formula I, R8
is Ci to C4 alkyl,
optionally substituted as described in Formula I. In another embodiment of
Formula I, R8 is ethyl,
optionally substituted as described in Formula I.
In one embodiment of Formula I, R8 is optionally substituted with one or more
substituents
independently selected from the group consisting of halogen, hydroxy, carboxy,
cyano, -OR'08-
C(O)R108, -C(O)OR108, -OC(O)R108, -NR108R109-N(R108)C(O)R709 -C(O)NR108R109,
and -
C(O)NR108C(O)R109, wherein R108 and R109 are independently selected from the
group consisting
of hydrogen and Ci to C6 alkyl, wherein (a) when the C, to C6 alkyl is methyl,
the methyl may be
optionally substituted with 1, 2, or 3 fluoro substituents, (b) when the Ci to
C6 alkyl comprises at
least two carbon atoms, the alkyl may be optionally substituted with one or
more substituents
independently selected from the group consisting of halogen, hydroxy, carboxy,
cyano, oxo,
alkynyl, haloalkynyl, hydroxyalkynyl, carboxyalkynyl, alkoxy, haloalkoxy,
hydroxyalkoxy, and
carboxyalkoxy. In another embodiment of Formula I, R8 is optionally
substituted with one or
more substituents independently selected from the group consisting of halogen,
hydroxy,
carboxy, cyano, alkoxy, haloalkyl, hydroxyalkyl, carboxyalkyl, haloalkoxy,
hydroxyalkoxy, and
21
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
carboxyalkoxy. In another embodiment of Formula I, Ra is optionally
substituted with one or
more substituents independently selected from the group consisting of halogen,
haloalkoxy,
hydroxy, carboxy, cyano, oxo, and alkoxy. In another embodiment of Formula I,
R8 is optionally
substituted with one or more substituents independently selected from the
group consisting of
halogen, haloalkoxy, hydroxy, oxo, and alkoxy. In another embodiment of
Formula I, R8 is
optionally substituted with one or more substituents independently selected
from the group
consisting of haloalkoxy and alkoxy. In another embodiment of Formula I, R 8
is ethyl optionally
substituted with one or more substituents independently selected from the
group consisting of
halogen, haloalkoxy, hydroxy, carboxy, cyano, oxo, and alkoxy. In another
embodiment of
Formula I, R8 is ethyl optionally substituted with one or more substituents
independently selected
from the group consisting of haloalkoxy and alkoxy.
In another embodiment of Formula I, R8 is alkoxyalkyl, optionally substituted
as described in
Formula I. In another embodiment of Formula I, R8 is a(Cl to C4)alkoxy(C, to
C4)alkyl, optionally
substituted as described in Formula I. In another embodiment of Formula I, R 8
is methoxyethyl,
as described in Formula 1-Y in Table A. In another embodiment of Formula I, R8
is ethoxyethyl,
as described in Formula I-Z in Table A. In another embodiment of Formula I, R$
is propoxyethyl,
as described in Formulae I-AA and I-BB in Table A. In another embodiment of
Formula I, R8 is
trifluoroethoxyethyl. In another embodiment of Formula I, R8 is selected from
the group
consisting of propoxyethyl and ethoxyethyl as described in Formulae I-Z, I-AA
and I-BB in Table
A, respectively.
Embodiments of Multiple Substituents
The following are additional embodiments of the compounds of Formula I. Unless
otherwise specified, substituents are as described in Formula I. Further
embodiments of
Formula I described when R2, X6, Y6, R6 and R8 are selected from the various
embodiments
described above.
Embodiments where X6 is Hydrogen, R2 is phenyl or 3-10 membered heterocyclyl
In one embodiment of Formula I, X6 is hydrogen and R2 is selected from the
group
consisting of phenyl, thienyl, furanyl, thiazolyl, imidazolyl, pyrazolyl,
isoxazolyl, isothiazolyl,
pyrazinyl pyridinyl, triazinyl, imidazyl, thiophenyl, pyrazolyl, oxazolyl,
oxadiazolyl, pyridyl, pyrazyl,
pyrimidinyl, pyridazinyl, benzofuran, and benzodioxolyl. In another embodiment
of Formula I, X6
is hydrogen and RZ is selected from the group consisting of phenyl, pyridinyl,
pyrimidinyl,
isoxazolyl, pyrazolyl, benzofuran, and benzodioxolyl. In another embodiment of
Formula I, X6 is
hydrogen and R2 is selected from the group consisting of phenyl, pyridinyl and
pyrimidinyl, as
described in Formulae I-I, I-B and I-M of Table A. In another embodiment of
Formula I, X6 is
hydrogen and R2 is selected from the group consisting of phenyl and pyridinyl,
as described in
Formulae I-I and I-B of Table A. In another embodiment of Formula I, X6 is
hydrogen and R2 is
phenyl, as described in Formulae I-B of Table A, wherein R9, R1o R11, R12and
R13are
independently selected from the group consisting of hydrogen, hydroxy, and
fluoro. In another
22
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
embodiment of Formula I, X6 is hydrogen and R 2 is pyridinyl, as described in
Formulae I-B of
Table A, wherein R9, R10 and R13 are each hydrogen, and R" is methoxy.
Embodiments where X6 is Hydrogen, R8 is alkoxyalkyl
In one embodiment of Formula I, X6 is hydrogen and R8 is alkoxyalkyl,
optionally substituted
as described in Formula I. In another embodiment of Formula I, X6 is hydrogen
and Ra is a(C1
to C4)alkoxy(C1 to C4)alkyl, optionally substituted as described in Formula I.
In another
embodiment of Formula I, X6 is hydrogen and R8 is selected from the group
consisting of
methoxyethyl, ethoxyethyl, and propoxyethyl, optionally substituted as
described in Formula I.
In another embodiment, X6 is hydrogen and R8 is selected from the group
consisting of
methoxyethyl, ethoxyethyl, propoxyethyl, and trifluoroethylethoxy. In another
embodiment of
Formula I, X6 is hydrogen and RB is selected from the group consisting of
propoxyethyl and
ethoxyethyl, as described in Formulae I-Z, I-AA and I-BB of Table A,
respectively.
Embodiments where X6 is Hydrogen, R8 is alkoxyalkyl, and Ys represents a bond
or is
alkyl
In one embodiment of Formula I, X6 is hydrogen, R8 is alkoxyalkyl, optionally
substituted as
--- - described-in-Formula-I-and Y6 r-epresents-a-bond-or-is-alkyl,-optionally-
substituted as described in
Formula I. In another embodiment of Formula I, X6 is hydrogen and R8 is a(Ci
to C4)alkoxy(Ci
to C4)alkyl, optionally substituted as described in Formula I, and Y6
represents a bond or is C, to
C6 alkyl, optionally substituted as described in Formula I. In another
embodiment of Formula I,
X6 is hydrogen, R8 is selected from the group consisting of methoxyethyl,
ethoxyethyl, and
propoxyethyl, wherein R8 is optionally substituted as described in Formula I,
and Y6 represents a
bond or is selected from the group consisting of methyl, ethyl, propyl, butyl,
methylethyl, tert-
butyl, hydroxyethyl, hydroxypropyl, hydroxybutyl, dihydroxyethyl,
HO HO HO HO~HO~ HO~_ HO
s
(R6 )
(Rs) (R6) (R6) (R6) (R6), and R ( ) HO
In another embodiment of Formula I, X6 is hydrogen, Ra is selected from the
group
consisting of methoxyethyl, ethoxyethyl, propoxyethyl, and
trifluoroethoxyethyl, and Ys
represents a bond or is selected from the group consisting of inethyl, ethyl,
propyl and butyl. In
another embodiment of Formula I, X6 is hydrogen, R8 is selected from the group
consisting of
propoxyethyl and ethoxyethyl, and Y6 represents a bond or is selected from the
group consisting
of methyl and ethyl. In another embodiment of Formula I, X6 is hydrogen, R8 is
ethoxyethyl, and
Y6 represents a bond or is selected from the group consisting of methyl and
ethyl, as described
in Formulae I-GG, I-HH and I-II in Table A, respectively. In another
embodiment of Formula I, X6
is hydrogen, RB is propoxyethyl, and Y6 represents a bond or is selected from
the group
consisting of methyl and ethyl, as described in Formulae 1-00, I-QQ and I-PP
in Table A,
respectively.
23
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Embodiments where Ra is alkoxyalkyl, and R2 is phenyl or 3-10 membered
heterocyclyl
In one embodiment of Formula I, R8 is alkoxyalkyl, optionally substituted as
described in
Formula I and R2 is selected from the group consisting of phenyl, thienyl,
furanyl, thiazolyl,
imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, pyrazinyl pyridinyl,
triazinyl, imidazyl, thiophenyl,
pyrazolyl, oxazolyl, oxadiazolyl, pyridyl, pyrazyl, pyrimidinyl, pyridazinyl,
benzofuran, and
benzodioxolyl, wherein R2 is optionally substituted as described in Formula I.
In another
embodiment of Formula I, R8 is a(C, to C4)alkoxy(Cy to C4)alkyl, optionally
substituted as
described in Formula I, and R2 is selected from the group consisting of
phenyl, pyridinyl,
pyrimidinyl, isoxazolyl, pyrazolyl, benzofuran, and benzodioxolyl, wherein R2
is optionally
substituted as described in Formula I. In another embodiment of Formula I, R8
is selected from
the group consisting of trifluroethoxyethyl, ethoxyethyl, and propoxyethyl,
and R2 is selected from
the group consisting of phenyl, pyridinyl and pyrimidinyl, wherein R2 is
optionally substituted as
described in Formula I. In another embodiment of Formula I, R8 is selected
from the group
consisting of ethoxyethyl and propoxyethyl, and R2 is selected from the group
consisting of
phenyl and pyridinyl, wherein R2 is optionally substituted as described in
Formula I. In another
embodiment of Formula I, R8 is ethoxyethyl, and R2 is pyridinyl, optionally
substituted as
described in Formula I. In another embodiment of Formula I, R8 is ethoxyethyl
and R2 is a
--- methoxy-pyr-idinyl,-as-descr-ibed-in-For-mula-I-F-of Table A. In-another-
embodiment of Formula I,
R8 is propoxyethyl and R2 is a methoxy pyridinyl.
Embodiments where X6 is hydrogen, R8 is alkoxyalkyl, and R2 is phenyl or 3-10
membered
heterocyclyl
In one embodiment of Formula I, X6 is hydrogen, R8 is alkoxyalkyl, optionally
substituted as
described in Formula I and R2 is selected from the group consisting of phenyl,
thienyl, furanyl,
thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, pyrazinyl
pyridinyl, triazinyl, imidazyl,
thiophenyl, pyrazolyl, oxazolyl, oxadiazolyl, pyridyl, pyrazyl, pyrimidinyl,
pyridazinyl, benzofuran,
and benzodioxolyl, wherein R2 is optionally substituted as described in
Formula I. In another
embodiment of Formula I, X6 is hydrogen, R$ is a(Cl to C4)alkoxy(Ci to
C4)alkyl, optionally
substituted as described in Formula I, and R2 is selected from the group
consisting of phenyl,
pyridinyl, pyrimidinyl, isoxazolyl, pyrazolyl, benzofuran, and benzodioxolyl,
wherein R2 is
optionally substituted as described in Formula I. In another embodiment of
Formula I, X6 is
hydrogen, R8 is selected from the group consisting of trifluoroethoxyethyl,
ethoxyethyl, and
propoxyethyl, wherein R8 is optionally substituted as described in Formula I
and R2 is selected
from the group consisting of phenyl, pyridinyl and pyrimidinyl, wherein R2 is
optionally substituted
as described in Formula I. In another embodiment of Formula I, X6 is hydrogen,
R 8 is selected
from the group consisting of ethoxyethyl and propoxyethyl, and R2 is selected
from the group
consisting of phenyl and pyridinyl wherein R2 is optionally substituted as
described in Formula I.
In another embodiment of Formula I, X6 is hydrogen, R8 is ethoxyethyl, and R2
is pyridinyl,
optionally substituted as described in Formula I. In another embodiment of
Formula I, X6 is
hydrogen, R$ is ethoxyethyl and R2 is a para-substitued pyridinyl, as
described in Formula I-FF of
24
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Table A. In another embodiment of Formula I, X6 is hydrogen, R8 is
propoxyethyl and R2 is a
para-substitued pyridinyl, as described in Formula I-NN of Table A. In another
embodiment of
Formula I, X6 is hydrogen, R8 is propoxyethyl and R2 is a pyridinyl,
substituted at the para
position with a methoxy as described in Formula I-UU of Table A.
Embodiments where X6 is hydrogen, R8 is alkoxyalkyl, Y6 represents a bond or
is alkyl and
R2 is phenyl or 3-10 membered heterocyclyl
In one embodiment of Formula I, X6 is hydrogen, Y6 is a bond or is alkyl,
optionally
substituted as described in Formula I, R8 is alkoxyalkyl, optionally
substituted as described in
Formula I, and R2 is selected from the group consisting of phenyl, thienyl,
furanyl, thiazolyl,
imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, pyrazinyl pyridinyl,
triazinyl, imidazyl, thiophenyl,
pyrazolyi, oxazolyl, oxadiazolyl, pyridyl, pyrazyl, pyrimidinyl, pyridazinyl,
benzofuran, and
benzodioxolyl, wherein R2 is optionally substituted as described in Formula I.
In another
embodiment of Formula I, X6 is hydrogen, Y6 represents a bond or is Ci to C6
alkyl, optionally
substituted as described in Formula I, R8 is a(C1 to C4)alkoxy(Ci to C4)alkyl,
optionally
substituted as described in Formula I, and R2 is selected from the group
consisting of phenyl,
pyridinyl, pyrimidinyl, isoxazolyl, pyrazolyl, benzofuran, and benzodioxolyl
wherein R2 is
optionally substituted as described in Formula I. In another embodiment of
Formula I, X6 is
- -_- hydrogen,_R$_is.selected from_the_group_consisting
of_triflur.oethoxyethyl,_ethoxyethyl,_and
propoxyethyl, optionally substituted as described in Formula I and R 2 is
selected from the group
consisting of phenyl, pyridinyl and pyrimidinyl wherein R2 is optionally
substituted as described in
Formula I. In another embodiment of Formula I, X6 is hydrogen, Y6 represents a
bond or is
selected from the group consisting of methyl, ethyl, propyl, butyl,
hydroxyethyl, hydroxypropyl,
hydroxybutyl, dihydroxyethyl, and dihydroxybutyl, R8 is selected from the
group consisting of
ethoxyethyl and propoxyethyl, and R2 is selected from the group consisting of
phenyl and
pyridinyl wherein R 2 is optionally substituted as described in Formula I. In
another embodiment
of Formula I, X6 is hydrogen, Y6 represents a bond or is selected from the
group consisting of
methyl, ethyl, methylethyl, propyl and butyl, R8 is selected from the group
consisting of
ethoxyethyl and propoxyethyl, and R2 is pyridinyl, optionally substituted as
described in Formula
1. In another embodiment of Formula I, X6 is hydrogen, Ys represents a bond, R
8 is ethoxyethyl
and R2 is a para-substitued pyridinyl, as described in Formula I-LL of Table
A. In another
embodiment of Formula I, X6 is hydrogen, Y6 is methyl, R8 is ethoxyethyl and
R2 is a para-
substitued pyridinyl, as described in Formula I-JJ of Table A. In another
embodiment of Formula
I, X6 is hydrogen, Y6 is ethyl, R 8 is ethoxyethyl and R2 is a para-substitued
pyridinyl, as described
in Formula I-KK of Table A. In another embodiment of Formula I, X6 is
hydrogen, Y6 represents a
bond, R8 is propoxyethyl and R2 is a para-substitued pyridinyl, as described
in Formula I-TT of
Table A. In another embodiment of Formula I, X6 is hydrogen, Y6 is methyl, Ra
is propoxyethyl
and R2 is a para-substitued pyridinyl, as described in Formula I-RR of Table
A. In another
embodiment of Formula I, X6 is hydrogen, Y6 is ethyl, Re is propoxyethyl and
R2 is a para-
substitued pyridinyl, as described in Formula I-SS of Table A.
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Embodiments where X6 is hydrogen, R8 is alkoxyalkyl, Y6 represents a bond or
is alkyl and
R2 is phenyl or 3-10 membered heterocyclyl, and R6 is selected from various
embodiments
In one embodiment of Formula I, X6 is hydrogen, Y6 represents a bond, R8 is
selected from
the group consisting of propoxyethyl and ethoxyethyl and R2 is a para-
substitued pyridinyl, R6 is
selected from the group consisting of selected from the group consisting of
phenyl, phenyl
C(O)NH-, phenyl C(O)-, 3 to 10 membered ring heterocyclyl, phenyl-C(O)-NH-, 3
to 10
membered ring heterocyclyl-C(O)-, and 3 to 10 membered ring heterocyclyl-C(O)-
NH, optionally
substituted with one or more substituents independently selected from the
group consisting of -
OR105, -C(O)R105, -C(O)OR105, -NR. 105R106, -N(R105)C(O)R106, -
N(R105)C(O)OR106, -C(O)NR105 R106
,
-NHC(O)NR'05R106, -N(R105)S(O)2R106, wherein R105 and R106 are independently
selected from
the group consisting of hydrogen, methyl, ethyl, methylethyl, tert-butyl,
cyclopentyl, cyclohexyl,
optionally substituted with one or more substituent selected from the group
consisting of halogen,
oxo, hydroxy, and methyl. In another embodiment of Formula I, X6 is hydrogen,
Y6 is methyl, R8
is selected from the group consisting of propoxyethyl and ethoxyethyl, and R2
is a para-
substitued pyridinyl, optionally substituted as described in Formula I, and R6
is selected from the
group consisting of phenyl, phenyl C(O)NH-, phenyl C(O)-, 5 to 7 membered ring
heterocyclyl,
phenyl-C(O)-NH-, 5 to 7 membered ring heterocyclyl-C(O)-, and 5 to 7 membered
ring
----heterocyel-yl-C(O)-NH,-optionally-s-ubstituted-with-one-or-more-
substituents-independently
selected from the group consisting of of -OR106, -C(O)R106, -C(O)OR'06 -
NR106R107, -
N(R106)C(O)R107 -N(R106)C(O)OR107, -C(O)NR106R107 -NHC(O)NR106R107, -
N(R106)S(O)2R107,
wherein R106 and R107 are independently selected from the group consisting of
hydrogen, methyl,
ethyl, methylethyl, tert-butyl, cyclopentyl, cyclohexyl, optionally
substituted with one or more
substituent selected from the group consisting of halogen, oxo, hydroxy, and
methyl. In another
embodiment of Formula I, X6 is hydrogen, Y6 is ethyl, R8 is selected from the
group consisting of
propoxyethyl and ethoxyethyl, R2 is a para-substitued pyridinyl, optionally
substituted as
described in Formula I, and R6 is selected from the group consisting of 5 to 6
membered ring
heteroaryl, 5 to 6 membered ring heteroaryl-C(O)-, and 5 to 6 membered ring
heteroaryl-C(O)-
NH, optionally substituted with one or more substituents independently
selected from the group
consisting of alkyl, -OR106, and -C(O)R106, wherein R106 is selected from the
group consisting of
hydrogen, methyl, and ethyl. In another embodiment of Formula I, X6 is
hydrogen, Y6 is ethyl, R8
is selected from the group consisting of propoxyethyl and ethoxyethyl, R2 is a
para-substitued
pyridinyl optionally substituted as described in Formula I, and R6 is selected
from the group
consisting of 5 to 6 membered ring fully saturated heterocyclyl, 5 to 6
membered ring fully
saturated heterocyclyl-C(O)-, and 5 to 6 membered ring fully saturated
heterocyclyl-C(O)-NH,
optionally substituted with one or more substituents independently selected
from the group
consisting of alkyl, -OR106, and -C(O)R106, wherein R106 is selected from the
group consisting of
hydrogen, methyl, and ethyl.
Compounds of Formula (I-B)
26
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
The present invention is directed, in part, to a class of compounds having the
structure of
Formula I-B:
Rio
R$ R9 Rii
O N
\ \ N
(
R6-Yrl'N ~N ~ N R13
H (I-B)
wherein
R9, R10, R" and R13 are independently selected from the group consisting of
hydrogen,
halogen, oxo, alkyl, -OR100, -C(O)R100, -OC(O)R100, -C(O)OR'o -NR100R101 and -
C(O)NR100R101,
wherein the alkyl substitutent may be optionally substituted with one or more
substituents
independently selected from the group consisting of halogen, oxo, -OR102, and -
C(O)OR102;
wherein R10 , R101, and R102 are independently selected from the group
consisting of hydrogen
and Ci to C4 alkyl;
y6 represents a bond or is selected from the group consisting of alkyl,
alkenyl and alkynyl,
wherein (a) the Y6 alkyl, alkenyl and alkynyl substituents may be optionally
substituted with one
or more substituents independently selected from the group consisting of
halogen, cyano, oxo,
cycloaiky,, -OR103, -C(O)R103, -C(O)OR103, -OC(O)R103, -NR103R104, -
N(R103)C(O)R104, and -
C(O)NR103R104;
R103 and R104 are independently selected from the group consisting of hydrogen
and alkyl,
wherein the alkyl may be optionally substituted with one or more substituents
independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkynyl,
haloalkynyl, hydroxyalkynyl, carboxyalkynyl, alkoxy, haloalkoxy,
hydroxyalkoxy, and
carboxyalkoxy;
R6 is selected from the group consisting of aryl, aryl-C(O)-, heterocyclyl,
aryl-C(O)-NR105-,
heterocyclyl-C(O)-, and heterocyclyl-C(O)-NR105- wherein R6 may be optionally
substituted with
one or more substituents independently selected from the group consisting of
halogen, cyano,
oxo, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heterocyclyi, -OR106, -
C(O)R106, -C(O)OR106, -
OC(O)R 106, -NR106 R107, -N(R106)C(O)R107, -C(O)NR10sR107, -C(O)NR10sC(O)R107,
-SR106
, -
S(O)R106, -S(O)2R106, -N(R106)S(O)2R107, and -S(O)2NR106R107; wherein the
alkyl, alkenyl, alkynyl,
cycloalkyl, aryl and heterocyclyl susbstituents may be optionally substituted
with one or more
substituents independently selected from the group consisting of halogen,
hydroxy, carboxy,
cyano, oxo, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy;
R105 is independently selected from the group consisting of hydrogen and
alkyl;
27
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
R106 and R107 are independently selected from the group consisting of
hydrogen, alkyl,
alkenyl, and alkynyl, wherein (a) the R106 and R107 alkyl and alkenyl
substituents may be
optionally substituted with one or more substituents independently selected
from the group
consisting of halogen, hydroxy, carboxy, cyano, oxo, alkynyl, haloalkynyl,
hydroxyalkynyl,
carboxyalkynyl, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy, and (b)
the R106 and R107
alkynyl substituents may be optionally substituted with one or more
substituents independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkoxy, haloalkoxy,
hydroxyalkoxy, and carboxyalkoxy;
RB is alkoxyalkyl, optionally substituted with halogen.
Embodiments of R9. RYO, R1 i and R13
In one embodiment of the compounds of Formula I-B, R9, R10, R" and R'3are
selected from
the group consisting of hydrogen, chloro, fluoro, methyl, ethyl, propyl,
butyl, pentyl, hexyl,
trif luorom ethyl, hydroxy, methoxy, ethoxy, propoxy, butoxy, amino,
methylamino, dimethylamino,
ethylamino, and diethylamino. In another embodiment of Formula I-B, R9, R10,
R" and R'3are
- ---selected-fr-om-the gr-oup-consisting-of-hydr-ogen,-fluor-o,-rnethyl,-
trifluor-omethyl; methoxy,
trifluoromethoxy, amino, methylamino, and dimethylamino. In another embodiment
of Formula I-
B, R" and R13 are hydrogen, and R9 and R1 are selected from the group
consisting ot hydrogen,
chloro, fluoro, methyl, ethyl, propyl, butyl, pentyl, hexyl, trifluoromethyl,
hydroxy, methoxy,
ethoxy, propoxy, butoxy, amino, methylamino, dimethylamino, ethylamino, and
diethylamino. In
another embodiment of Formula I-B, R9 and R10 are hydrogen, and R" and R'3are
selected from
the group consisting of hydrogen, methoxy, and fluoro. In another embodiment
of Formula I-B,
R9 R10 and R13 are hydrogen, and R" is methoxy.
Embodiments of 1 6
In one embodiment of the compounds of Formula I-B, Ys represents a bond or is
selected
from the group consisting of alkyl, alkenyl and alkynyl, wherein the Y6 alkyl,
alkenyl and alkynyl
substituents may be optionally substituted with one or more substituents
independently selected
from the group consisting of halogen, cyano, oxo, cycloalkyl, -OR103, -
C(O)R103, -C(O)OR103, -
OC(O)R'03, -NR103R104, -N(R103)C(O)R'04 , and -C(O)NR 103 R104 ; and wherein
R103 and R104
are
independently selected from the group consisting of hydrogen and alkyl,
wherein the alkyl may
be optionally substituted with one or more substituents independently selected
from the group
consisting of halogen, hydroxy, carboxy, cyano, oxo, alkynyl, haloalkynyl,
hydroxyalkynyl,
carboxyalkynyl, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy.
In one embodiment of Formula I-B, Y6 represents a bond or is alkyl, optionally
substituted as
described in Formula I-B. In another embodiment of Formula I-B, Y6represents a
bond or is Ci
to C6 alkyl, optionally substituted as described in Formula I-B. In another
embodiment of
Formula I-B, Y6 represents a bond or is selected from the group consisting of
Ci to C4 alkyl and
hydroxyCi to C4-alkyl. In another embodiment of Formula I-B, Y6 represents a
bond or is
28
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
selected from the group consisting of methyl, ethyl, propyl, butyl,
hydroxyethyl, hydroxypropyl,
hydroxybutyl, dihydroxyethyl, and dihydroxybutyl. In one embodiment of Formula
I-B, Y6
represents a bond or is selected from the group consisting of methyl, ethyl,
propyl and butyl.
In one embodiment of Formula I-B, Y6 is alkyl optionally substituted with one
or more
substituents independently selected from the group consisting of halogen,
cyano, oxo, cycloalkyl,
-OR'03, -C(O)R103, -C(O)OR103, -OC(O)R 103, -NR 103 R104, -N(R103)C(O)R 104,
and -C(O)NR103 R104
;
wherein R103 and R104 are independently selected from the group consisting of
hydrogen and Ci
to C4 alkyl. In another embodiment of Formula I-B, Y6 is alkyl substituted
with one or more
substituents independently selected from the group consisting of fluoro,
chloro, oxo, cycloalkyl,
hydroxy, and carboxy. In another embodiment of Formula I-B, Y6 is alkyl
substituted with one to
three substituents independently selected from the group consisting of hydroxy
and cyclohexyl.
In another embodiment of Formula I-B, Y6 is unsubstituted alkyl.
Embodiments of R6 Substituent
In one embodiment of Formula I-B, R6 is selected from the group consisting of
phenyl,
phenyl-C(O)-, 3 to 10 membered ring heterocyclyl, phenyl-C(O)-NR105-, 3 to 10
membered ring
heterocyclyl-C(O)-, and 3 to 10 membered ring heterocyclyl-C(O)-NR105-,
wherein R6 is optionally
substituted as described in Formula I-B, wherein R105 is selected from the
group consisting of
_..by_dr_o.geriansLC1-to_C6-aIkylJn_an_olher-embodiment_oiEormu.la1,J36.is
~elecfed_fr_om_the_group
consisting of phenyl, phenyl-C(O)-, 5 to 7 membered ring heterocyclyl, phenyl-
C(O)-NR105-, 5 to
7 membered ring heterocyclyl-C(O)-, and 5t07 membered ring I,Eterocyclyl-C(O)-
NR105-,
wherein R6 is optionally substituted as described in Formula I-B, wherein R105
is selected from
the group consisting of hydrogen and C7 to C4 alkyl. In another embodiment of
Formula I-B, R6 is
selected from the group consisting of phenyl, phenyl-C(O)-, 5 to 6 membered
ring heterocyclyl,
phenyl-C(O)-NR105-, 5 to 6 membered ring heterocyclyl-C(O)-, and 5 to 6
membered ring
heterocyclyl-C(O)-NR105-, wherein R6 is optionally substituted as described in
Formula I-B
wherein R105 is hydrogen. In another embodiment, R6 is selected from the group
consisting of
phenyl, pyridinyl, piperidinyl, piperizinyl, morpholino, pyrazinyl,
tetrahydropyran, tetrahydrofuran,
isoxazole, imidazole, pyrrolidine, wherein R 6 is optionally substituted as
described in Formula I.
In another embodiment, R6 is selected from the group consisting of
morpholinyl,
tetrahydropyranyl, tetrahydrofuranyl, piperidinyl, and pyrrolidinyl, wherein
R6 is optionally
substituted as provided in Formula I.
In one embodiment of Formula I, R6 is selected from the group consisting of
phenyl, phenyl
C(O)NH-, and phenyl C(O)-.
In one embodiment of Formula I, R6 is selected from the group consisting of 5
to 7
membered ring heteroaryl, 5 to 7 membered ring heteroaryl-C(O)-, and 5 to 7
membered ring
heteroaryl-C(O)-NR105-, wherein R 6 is optionally substituted as described in
Formula I, wherein
R105 is selected from the group consisting of hydrogen and Ci to C4 alkyl. In
another
embodiment of Formula I, R6 is a 3 to 10 membered ring heteroaryl, optionally
substituted as
described in Formula I. In another embodiment of Formula I, R6 is a 5 to 7
membered ring
heteroaryl, optionally substituted as described in Formula I. In another
embodiment of Formula I,
29
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
R6 is a 5 to 6 membered ring heteroaryl, optionally substituted as described
in Formula 1. In
another embodiment of Formula I, R 6 is a 5 to 6 membered ring heteroaryl
containing one to
three heteroatoms selected from the group consisting of 0 and N, wherein R6 is
optionally
substituted as described in Formula I. In another embodiment of Formula I, R6
is selected from
the group consisting of imidazole, isoxazole, pyridinyl and pyrazynyl, wherein
R6 is optionally
substituted as described in Formula I. In another embodiment of Formula I, R6
is selected from
the group consisting of imidazole, isoxazole, pyridinyl and pyrazynyl, wherein
R 6 is optionally
substituted with one or more substituents independently selected from the
group consisting of
alkyl, -OR105, and -C(O)R105, wherein R105 is selected from the group
consisting of hydrogen,
N\
~
methyl, and ethyl. In another embodiment, R6 is selected from the group
consisting of
N tq~
N O-N NJ'
and
In one embodiment of Formula I, R 6 is selected from the group consisting of 3
to 10
membered ring fully or partially saturated heterocylyl, 3 to 10 membered ring
fully or partially
- -saturated-heterocYclYI-C()- O-,-to-1-0-membered-rin g-fullY-orpartiallY
saturated heterocYclYI-
C(O)NH-, wherein R6 is optionally substituted as described in Formula I. In
another embodiment
of Formula I, R6 is a 5 to 7 membered ring fully or partially saturated
heterocylyl, 5 to 7
membered ring fully or partially saturated heterocyclyl-C(O)-, 5 to 7 membered
ring fully or
partially saturated heterocyclyi-C(0)NH-, wherein R 6 is optionally
substituted as described in
Formula 1. In another embodiment of Formula I, R6 is a 5 to 6 membered ring
fully saturated
heterocylyl, 5 to 6 membered ring fully saturated heterocyclyl-C(O)-, 5 to 6
membered ring fully
saturated heterocyclyl-C(O)NH-, wherein R6 is optionally substituted as
described in Formula I.
In another embodiment of Formula I, R6 is a 5 to 6 membered ring fully
saturated heterocylyl,
optionally substituted as described in Formula I. In another embodiment of
Formula I, R6 is
selected from the group consisting of tetrahydrofuran, tetrahydropyran,
pyrrolidinyl, piperidinyl,
piperazinyl, and morpholino, wherein R 6 is optionally substituted as
described in Formula I. In
another embodiment of Formula I, R6 is selected from the group consisting of
tetrahydrofuran,
tetrahydropyran, pyrrolidinyl, piperidinyl, piperazinyl, and morpholino,
wherein R6 is optionally
substituted with one or more substituents independently selected from the
group consisting of
alkyl, -OR105, -C(O)R105 and -C(O)OR105 wherein R105 is selected from the
group consisting of
hydrogen, methyl, and ethyl. In another embodiment, R6 is selected from the
group consisting
O
JL
N
of
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
0
~ O
~
HN
N
O
O N "N' N J~*-'
HNJ N
~O N
O
N N'''
N J~-
~ ~
J N
O O
O
N-Zi' rN
rN OJ Oj
O N
j
and
O
rN'jL'N-~-
HOJ
.
In another embodiment of Formula I, Rs is optionally substituted with one or
more
substituents independently selected from the group consisting of -OR105, -
C(O)R'05 -C(O)OR'05
-NR'0eRi06s -N(Ri05)C(O)R106-N(R10e)C(O)ORi06, -C(O)NR105Ri06, -
NHC(O)NRioa.RiOS' -
N(R105)S(O)2R106, wherein R105 and R'06 are independently selected from the
group consisting of
hydrogen, methyl, ethyl, methylethyl, tert-butyl, cyclopentyl, cyclohexyl,
optionally substituted
with one or more substituent selected from the group consisting of halogen,
oxo, hydroxy, and
methyl.. In another embodiment of Formula I, R6 is optionally substituted with
one or more
substituents independently selected from the group consisting of alkyl, --
C(O)R105 and -
C(O)OR105, wherein R105 is selected from the group consisting of hydrogen,
methyl, and ethyl. In
another embodiment of Formula I, R6 is optionally substituted with one or more
substituents
31
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
independently selected from the group consisting of -OH, -CH3, -CH2CH3, -
C(O)CH3, -
C(O)CH2CH3, -C(O)OCH2CH3.
Embodiments of R8 Substituent
In one embodiment of Formula I-B, R8 is a(C, to C4)alkoxy(Cl to C4)alkyl,
optionally
substituted with one to three substituents selected from fluoro and chloro. In
another
embodiment of Formula I, R8 is methoxyethyl. In another embodiment of Formula
I, R8 is
ethoxyethyl. In another embodiment of Formula I, R8 is propoxyethyl. In
another embodiment of
Formula I, R8 is trifluoroethoxyethyl. In another embodiment of Formula I, R8
is selected from the
group consisting of propoxyethyl and ethoxyethyl.
Embodiments of Multiple Substituents
The following are additional embodiments of the compounds of Formula I-B.
Unless
otherwise specified, substituents are as described in Formula I-B. Further
embodiments of
Formula I-B described when R9, R10, R", R'3, Y6, R6 and R8 are selected from
the various
embodiments described above.
Embodiments where R9, R10 are hydrogen, Ys represents a bond or is alkyl
In one embodiment of Formula I-B, Y6 is a bond or is alkyl, optionally
substituted as
-- --descr-ibed-in-For-r-nula-I-B, and-R9 and-R'- are-hydr-ogen-In-another-
embodiment-of Formula I-B,
y6 is a bond or is alkyl, optionally substituted as described in Formula I-B,
R9 and R10 are
hydrogen and R" and R13 are selected from the group consisting of hydrogen,
methoxy, and
fiuoro. In another embodiment of Formula I-B, Y6 is a bond or is alkyl,
optionally substituted as
described in Formula I-B, R9 R10 and R'3 are hydrogen, and R" is methoxy.
Embodiments where R9, R10, R13 are hydrogen, Y6 represents a bond or isaikyl
In one embodiment of Formula I-B, Y6 is a bond or is alkyl, optionally
substituted as
described in Formula I-B, and R9, R10 and R13 are hydrogen. In another
embodiment of Formula
I-B, Y6 is a bond or is alkyl, optionally substituted as described in Formula
I-B, R9, R10 and R'3
are hydrogen and R" is selected from the group consisting of hydrogen,
methoxy, and fluoro. In
another embodiment of Formula I-B, Y6 is a bond or is alkyl, optionally
substituted as described
in Formula I-B, R9 R10 and R'3 are hydrogen, and R" is methoxy.
Embodiments where R9, R10, R'3 are hydrogen and R8 is ethoxyethyl or
propoxyethyl
In one embodiment of Formula I-B, R9, R10 and R'3 are hydrogen, and R8 is
selected from
the group consisting of ethoxyethyl and propoxyethyl. In another embodiment of
Formula I-B,
R9, R10 and R'3 are hydrogen and R" is selected from the group consisting of
hydrogen,
methoxy, and fluoro, and R8 is selected from the group consisting of
ethoxyethyl and
propoxyethyl. In another embodiment of Formula I-B, R9 R10 and R'3 are
hydrogen, R" is
methoxy, and R8 is ethoxyethyl, as described in Formula I-FF in Table A. In
another
embodiment of Formula I-B, R9 R10 and R'3 are hydrogen, R" is methoxy, and R8
is
propoxyethyl, as described in Formula 1-UU in Table A.
Embodiments of Formula I-FF and 1-UU
32
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
In one embodiment of Formulae I-FF and I-UU, Y6 represents a bond or is alkyl
optionally
substituted as described in Formula I-B, and R6 is selected from the group
consisting of
hydrogen, halogen, oxo, hydroxy, amino, aminoalkyl, alkyl, alkynyl, alkoxy,
alkylamino, and
cycloalkyl, optionally substituted as described in Formula I-B. In another
embodiment of
Formulae I-FF and I-UU, Y6 represents a bond or is selected from the group
consisting of
unsubstituted Cl to C4 alkyl and unsubstituted hydroxyC, to C4-alkyl, and R6
is selected from
the group consisting of phenyl, phenyl-C(O)-, 5 to 6 membered ring
heterocyclyl, phenyl-C(O)-
NR105-, 5 to 6 membered ring heterocyclyl-C(O)-, and 5 to 6 membered ring
heterocyclyl-C(O)-
NR105-, wherein R6 is optionally substituted as described in Formula I-B
wherein R105 is
hydrogen. In another embodiment of Formulae I-FF and I-UU, Y6 represents a
bond or is
selected from the group consisting of methyl, ethyl, propyl, butyl,
hydroxyethyl, hydroxypropyl,
hydroxybutyl, dihydroxyethyl, and dihydroxybutyl, and R 6 is selected from the
group consisting
of phenyl, phenyl-C(O)-, 5 to 6 membered ring heterocyclyi, phenyl-C(O)-NH-, 5
to 6
membered ring heterocyclyl-C(O)-, and 5 to 6 membered ring heterocyclyl-C(O)-
NH-, wherein
R6 is optionally substituted with one or more substituents independently
selected from the
group consisting of -OH, -CH3, -CH2CH3, -C(O)CH3, -C(O)CH2CH3, -C(O)OCH2CH3.
Embodiments where R9, R10, R'3 are hydrogen, R8 is ethoxyethyl or propoxyethyl
and R6 is
selected from various embodiments
In one embodiment of Formula I-B, R9, R10 and R'3 are hydrogen, R8 is selected
from the
group consisting of ethoxyethyl and propoxyethyl, and R 6 is selected from the
group consisting of
phenyl, phenyl C(O)NH-, phenyl C(O)-, 5 to 7 membered ring heterocyclyl,
pher,yl-C(O)-NH-, 5
to 7 membered ring heterocyclyl-C(O)-, and 5 to 7 membered ring heterocyclyl-
C(O)-NH,
optionally substituted with one or more substituents independently selected
from the group
consisting of of -OR105, -C(O)R105, -C(O)OR'05-NR105R106, -N(R105)C(O)R1 6, -
N(R105)C(O)OR106, -C(O)NR1 5R'06, -NHC(O)NR'0eRt06-N(R108)S(O)2R,1)6,0s 106
, wherein R and R
are independently selected from the group consisting of hydrogen, methyl,
ethyl, methylethyl,
tert-butyl, cyclopentyl, cyclohexyl, optionally substituted with one or more
substituent selected
from the group consisting of halogen, oxo, hydroxy, and methyl. In another
embodiment of
Formula I-B, R9, R10 and R'3 are hydrogen, R8 is selected from the group
consisting of
ethoxyethyl and propoxyethyl, and R6 is selected from the group consisting of
5 to 6 membered
ring heteroaryl, 5 to 6 membered ring heteroaryl-C(O)-, and 5 to 6 membered
ring heteroaryl-
C(O)-NH, optionally substituted with one or more substituents independently
selected from the
group consisting of alkyl, -OR105, and -C(O)R105, wherein R105 is selected
from the group
consisting of hydrogen, methyl, and ethyl. In another embodiment of Formula I-
B, R9, R10 and
R13 are hydrogen, R8 is selected from the group consisting of ethoxyethyl and
propoxyethyl, and
R6 is selected from the group consisting of 5 to 6 membered ring fully
saturated heterocyclyl, 5 to
6 membered ring fully saturated heterocyclyl-C(O)-, and 5 to 6 membered ring
fully saturated
heterocyclyl-C(O)-NH, optionally substituted with one or more substituents
independently
selected from the group consisting of alkyl, -OR105, and -C(O)R105, wherein
R105 is selected from
the group consisting of hydrogen, methyl, and ethyl.
33
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Compounds of Formula (I-I)
The present invention is directed, in part, to a class of compounds having the
structure of
Formula I-I:
R1o
8 R9 / R"
R
O ~ N \ ~ I R12
R6-Y6N N N R13
H
(I-I)
wherein
Ro, R10, R", R'2 and R'3 are independently selected from the group consisting
of hydrogen,
halogen, oxo, alkyl, -OR100, -C(O)R100, -OC(O)R100, -C(O)OR100, NR100R101 and -
C(O)NR100R101
- ,
wherein the alkyl substitutent may be optionally substituted with one or more
substituents
independently selected from the group consisting of halogen, oxo, -OR102, and -
C(O)OR102;
wherein R10 , R101, and R102 are independently selected from the group
consisting of hydrogen
- - - and C1 to C4 alkyl; - - - - ---- --- - - -- - - -
y6 represents a bond or is selected from the group consisting of alkyl,
alkenyl and alkynyl,
wherein (a) the Y6 alkyl, alkenyl and alkynyl substituents may be optionally
substituted with one
or more substituents independently selected from the group consisting of
halogen, cyano, oxo,
cycloalkyl, -OR'03, -C(O)R103, -C(O)OR103, -OC(O)R103, -NR103R104, -
N(R103)C(O)R104' and -
C(O)NR103R104,
,
R103 and R104 are independently selected from the group consisting of hydrogen
and alkyl,
wherein the alkyl may be optionally substituted with one or more substituents
independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkynyl,
haloalkynyl, hydroxyalkynyl, carboxyalkynyl, alkoxy, haloalkoxy,
hydroxyalkoxy, and
carboxyalkoxy;
R6 is selected from the group consisting of aryl, aryl-C(O)-, heterocyclyl,
aryl-C(O)-NR105-,
and heterocyclyl-C(O)-NR105- wherein R6 may be optionally substituted with
one or more substituents independently selected from the group consisting of
halogen, cyano,
~ -
oxo, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heterocyclyl, -OR106, -
C(O)R106, -C(O)OR'06
OC(O)R106, -NR106R107, -N(R106)C(O)R107, -C(O)NR106 R107, -C(O)NR10sC(O)R107, -
SR106-
S(O)R106, -S(O)2R106, -N(R106)S(O)2R'07, and -S(O)2NR106R107; wherein the
alkyl, alkenyl, alkynyl,
cycloalkyl, aryl and heterocyclyl susbstituents may be optionally substituted
with one or more
34
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
substituents independently selected from the group consisting of halogen,
hydroxy, carboxy,
cyano, oxo, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy;
R105 is independently selected from the group consisting of hydrogen and
alkyl;
R106 and R107 are independently selected from.the group consisting of
hydrogen, alkyl,
alkenyl, and alkynyl, wherein (a) the R106 and R107 alkyl and alkenyl
substituents may be
optionally substituted with one or more substituents independently selected
from the group
consisting of halogen, hydroxy, carboxy, cyano, oxo, alkynyl, haloalkynyl,
hydroxyalkynyl,
carboxyalkynyl, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy, and (b)
the R106and R107
alkynyl substituents may be optionally substituted with one or more
substituents independently
selected from the group consisting of halogen, hydroxy, carboxy, cyano, oxo,
alkoxy, haloalkoxy,
hydroxyalkoxy, and carboxyalkoxy;
R8 is alkoxyalkyl, optionally substituted with halogen.
Embodiments of R9, R10. Ri1, R'2 and R13
In one embodiment of the compounds of Formula I-I, R9, R10, R" R12, and R13
are selected
from the group consisting of hydrogen, chloro, fluoro, methyl, ethyl, propyl,
butyl, pentyl, hexyl,
___ tr-if-luoromethylr.-hydr-oxy,-methoxy,-ethoxy, propoxy, butoxy, amino,
methylamino, dimethylamino,
ethylamino, and diethylamino. In another embodiment of Formula I-I, R9, R10,
R" R'2, and R13
are selected from the group consisting of hydrogen, fluoro, methyl,
trifluoromethyl, methoxy,
trifluoromethoxy, amino, methylamino, and dimethylamino. In another embodiment
of Formula I-
I, R9, R12 and R13 are hydrogen, and R10 and R'1 are selected from the group
consisting of
hydrogen, chloro, fluoro, methyl, ethyl, propyl, butyl, pentyl, hexyl,
trifluoromethyl, hydroxy,
methoxy, ethoxy, propoxy, butoxy, amino, methylamino, dimethylamino,
ethylamino, and
diethylamino. In another embodiment of Formula I-I, R9, R12 and R13 are
hydrogen, and R" and
R'3are selected from the group consisting of hydrogen, methoxy,
trifluoromethyl, and fluoro. In
another embodiment of Formula I-I, R9, R10, R'2 and R'3 are hydrogen, and R"
is fluoro.
Embodiments of Y6
In one embodiment of the compounds of Formula I-I, Ys represents a bond or is
selected
from the group consisting of alkyl, alkenyl and alkynyl, wherein the Y6 alkyl,
alkenyl and alkynyl
substituents may be optionally substituted with one or more substituents
independently selected
from the group consisting of halogen, cyano, oxo, cycloalkyl, -OR103, -
C(O)R103, -C(O)OR103, -
OC(O)R103, -NR'03R104, -N(R103)C(O)R104, and -C(O)NR1 3R'04; and wherein R103
and R104 are
independently selected from the group consisting of hydrogen and alkyl,
wherein the alkyl may
be optionally substituted with one or more substituents independently selected
from the group
consisting of halogen, hydroxy, carboxy, cyano, oxo, alkynyl, haloalkynyl,
hydroxyalkynyl,
carboxyalkynyl, alkoxy, haloalkoxy, hydroxyalkoxy, and carboxyalkoxy.
In one embodiment of Formula I-I, Y6 represents a bond or is alkyl, optionally
substituted as
described in Formula I-I. In another embodiment of Formula I-I, Y6 represents
a bond or is C1 to
C6 alkyl, optionally substituted as described in Formula I-I. In another
embodiment of Formula I-
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
I, Y6 represents a bond or is selected from the group consisting of C, to C4
alkyl and hydr6xyC1 to
C4_alkyl. In another embodiment of Formula I-I, Y6 represents a bond or is
selected from the
group consisting of methyl, ethyl, propyl, butyl, hydroxyethyl, hydroxypropyl,
hydroxybutyl,
dihydroxyethyl, and dihydroxybutyl. In one embodiment of Formula I-I, Y6
represents a bond or is
selected from the group consisting of methyl, ethyl, propyl and butyl.
In one embodiment of Formula I-I, Ys is alkyl optionally substituted with one
or more
substituents independently selected from the group consisting of halogen,
cyano, oxo, cycloalkyl,
-OR103, -C(O)R1 031 -C(O)ORi03, -OC(O)Rioa, -NR703R' oa, -N(R10a)C(O)Rioa, and
-C(O)NR,oaR,oa;
wherein R103 and R104 are independently selected from the group consisting of
hydrogen and C,
to C4 alkyl. In another embodiment of Formula I-I, Y6 is alkyl substituted
with one or more
substituents independently selected from the group consisting of fluoro,
chloro, oxo, cycloalkyl,
hydroxy, and carboxy. In another embodiment of Formula I-I, Y6 is alkyl
substituted with one to
three substituents independently selected from the group consisting of hydroxy
and cyclohexyl.
In another embodiment of Formula I-I, Y6 is unsubstituted alkyl.
Embodiments of R6 Substituent
In one embodiment of Formula I-I, R6 is selected from the group consisting of
phenyl, phenyl-
C(O)-, 3 to 10 membered ring heterocyclyi, phenyl-C(O)-NR105-, 3 to 10
membered ring
heterocyclyl-C(O)-, and 3 to 10 membered ring heterocyclyl-C(O)-NR105- wherein
R6 is optionally
substituted as described in Formula I-I, wherein R105 is selected from the
group consisting of
hydrogen and C1 to C6 alkyl. In another embodiment of Formula I-I, R6 is
selected from the group
consisting of phenyl, phenyl-C(O)-, 5 to 7 membered ring heterocyclyi, phenyl-
C(O)-NR105-, 5 to
7 membered ring heterocyclyl-C(O)-, and 5 to 7 membered ring heterocyclyl-C(O)-
NR105-,
wherein R6 is optionally substituted as described in Formula I-I, wherein R105
is selected from the
group consisting of hydrogen and C1 to C4 alkyl. In another embodiment of
Formula I-I, R6 is
selected from the group consisting of phenyl, phenyl-C(O)-, 5 to 6 membered
ring heterocyclyl,
phenyl-C(O)-NR105-, 5 to 6 membered ring heterocyclyl-C(O)-, and 5 to 6
membered ring
heterocyclyl-C(O)-NR105-, wherein R 6 is optionally substituted as described
in Formula I-I wherein
R105 is hydrogen. In another embodiment, R6 is selected from the group
consisting of phenyl,
pyridinyl, piperidinyl, piperizinyl, morpholino, pyrazinyl, tetrahydropyran,
tetrahydrofuran,
isoxazole, imidazole, pyrrolidine, wherein R6 is optionally substituted as
described in Formula I-I.
In another embodiment, R6 is selected from the group consisting of
morpholinyl,
tetrahydropyranyl, tetrahydrofuranyl, piperidinyl, and pyrrolidinyl, wherein
R6 is optionally
substituted as provided in Formula I-I.
In one embodiment of Formula I-I, R6 is selected from the group consisting of
phenyl,
phenyl C(O)NH-, and phenyl C(O)-.
In one embodiment of Formula I-I, R6 is selected from the group consisting of
5 to 7
membered ring heteroaryl, 5 to 7 membered ring heteroaryl-C(O)-, and 5 to 7
membered ring
heteroaryl-C(O)-NR105- wherein R6 is optionally substituted as described in
Formula I-I, wherein
R105 is selected from the group consisting of hydrogen and C, to C4 alkyl. In
another
embodiment of Formula I-I, R6 is a 3 to 10 membered ring heteroaryl,
optionally substituted as
36
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
described in Formula I-I. In another embodiment of Formula I-I, R 6 is a 5 to
7 membered ring
heteroaryl, optionally substituted as described in Formula I-I. In another
embodiment of Formula
I-I, R 6 is a 5 to 6 membered ring heteroaryl, optionally substituted as
described in Formula I-I. In
another embodiment of Formula I-I, R6 is a 5 to 6 membered ring heteroaryl
containing one to '
three heteroatoms selected from the group consisting of 0 and N, optionally
substituted as
described in Formula I-I. In another embodiment of Formula I-I, R6 is selected
from the group
consisting of imidazole, isoxazole, pyridinyl and pyrazynyl, wherein R6 is
optionally substituted as
described in Formula I-I. In another embodiment of Formula I-I, R6 is selected
from the group
consisting of imidazole, isoxazole, pyridinyl and pyrazynyl, wherein R6 is
optionally substituted
with one or more substituents independently selected from the group consisting
of alkyl, -OR'05and -C(O)R105, wherein R105 is selected from the group
consisting of hydrogen, methyl, and ethyl.
N~'2. N
~ NJ
In another embodiment, R6 is selected from the group consisting of
~ ~~ ~p.
O-N NJ
,and
- - ---- --
-In one enibodirnenf of Formula I-I,-R is selec~d from~he group consisting of-
3 to 10-
membered ring fully or partially saturated heterocylyi, 3 to 10 membered ring
fully or partially
saturated heterocyclyl-C(O)-, 3 to 10 membered ring fully or partially
saturated heterocyclyl-
C(O)NH-, wherein R6 is optionally substituted as described in Formula I-I. In
another
embodiment of Formula I-I-I, R6 is a 5 to 7 membered ring fully or partially
saturated heterocylyl,
5 to 7 membered ring fully or partially saturated heterocyclyl-C(O)-, 5 to 7
membered ring fully or
partially saturated heterocyclyl-C(O)NH-, wherein R6 is optionally substituted
as described in
Formula I-I. In another embodiment of Formula I-I, R6 is a 5 to 6 membered
ring fully saturated
heterocylyl, 5 to 6 membered ring fully saturated heterocyclyl-C(O)-, 5 to 6
membered ring fully
saturated heterocyclyl-C(O)NH-, wherein R 6 is optionally substituted as
described in Formula I-I.
In another embodiment of Formula I-I, R 6 is a 5 to 6 membered ring fully
saturated heterocylyl,
optionally substituted as described in Formula I-I. In another embodiment of
Formula I-I, R6 is
selected from the group consisting of tetrahydrofuran, tetrahydropyran,
pyrrolidinyl, piperidinyl,
piperazinyl, and morpholino, wherein R 6 is optionally substituted as
described in Formula I-I. In
another embodiment of Formula I-I, R 6 is selected from the group consisting
of tetrahydrofuran,
tetrahydropyran, pyrrolidinyl, piperidinyl, piperazinyl, and morpholino,
wherein R6 is optionally
substituted with one or more substituents independently selected from the
group consisting of
alkyl, -OR105, -C(O)R105 and -C(O)OR'05 wherein R105 is selected from the
group consisting of
hydrogen, methyl, and ethyl. In another embodiment, R6 is selected from the
group consisting
37
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
O
of , , O , , N. ,
O
O
~.
HN
N
O
O N N
r~
HNJ N
~O N
O
rN rN
N " NJ
O O
O
~ N rNNI rN
N J OJ OJ
and
O
N H
OJ
In another embodiment of Formula I-I, R6 is optionally substituted with one or
more
substituents independently selected from the group consisting of -OR105, -
C(O)R105, -C(O)OR105,
-NR105R106, -N(R105)~=i(O)R106, -N(R105)C(0)OR106, -C(O)NR105 R106, -
NHC(O)NR105R 106
,-
N(R1 5)S(O)2R106, wherein R105 and R106 are independently selected from the
group consisting of
hydrogen, methyl, ethyl, methylethyl, tert-butyl, cyclopentyl, cyclohexyl,
optionally substituted
with one or more substituent selected from the group consisting of halogen,
oxo, hydroxy, and
38
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
methyl.. In another embodiment of Formula I-I, R6 is optionally substituted
with one or more
substituents independently selected from the group consisting of alkyi, --
C(O)R105 and -
C(O)OR105, wherein R105 is selected from the group consisting of hydrogen,
methyl, and ethyl. In
another embodiment of Formula I-I, R6 is optionally substituted with one or
more substituents
independently selected from the group consisting of -OH, -CH3, -CH2CH3, -
C(O)CH3, -
C(O)CH2CH3, -C(O)OCH2CH3.
Embodiments of R( Substituent
In one embodiment of Formula I-I, R8 is a(C1 to C4)alkoxy(C1 to C4)alkyl,
optionally
substituted with one to three substituents selected from fluoro and chloro. In
another
embodiment of Formula I-I, R 8 is trifluroethoxyethyl. In another embodiment
of Formula I-I, R8 is
ethoxyethyl. In another embodiment of Formula I, R 8 is propoxyethyl. In
another embodiment of
Formula I-I, R8 is trifluoroethoxyethyl. In another embodiment of Formula I-I,
R8 is selected from
the group consisting of propoxyethyl and ethoxyethyl.
Embodiments of Multiple Substituents
The following are additional embodiments of the compounds of Formula I-I.
Unless
otherwise specified, substituents are as described in Formula I-I. Further
embodiments of
----For-mula-l--I -descr-ibed when-R9-, R10; Rii-R-'3;-Y6, Rs and-R8 are
selected from the various
embodiments described above.
Embodiments where R9, R12 and R13 are hydrogen, Ys represents a bond or is
alkyl
In one embodiment of Formula I-I, Y6 is a bond or is alkyl, optionally
substituted as
described in Formula I-I, and R9, R12 and R13 are hydrogen. In another
embodiment of Formula I-
(, y6 is a bond or is alkyl, optionally substituted as described in Formula I-
I, R9, R'Z and R13 are
hydrogen and R10 and R" are selected from the group consisting of hydrogen,
methyl, methoxy,
trifluoromethyl, and fluoro. In another embodiment of Formula I-I, Y6 is a
bond or is alkyl,
optionally substituted as described in Formula I-I, R9, R10, R'2 and R'3 are
hydrogen, and R" is
fluoro.
Embodiments where R9, R10, R13 are hydrogen and Re is ethoxyethyl or
propoxyethyl
In one embodiment of Formula I-I, R9, R10 and R'3 are hydrogen, and R8 is
selected from
the group consisting of ethoxyethyl and propoxyethyl. In another embodiment of
Formula I-I, R9,
R10 and R'3 are hydrogen and R" and R'2 are independently selected from the
group consisting
of hydrogen, methyl, methoxy, fluoro and trifluoromethyl, and R8 is selected
from the group
consisting of ethoxyethyl and propoxyethyl. In another embodiment of Formula I-
I, R9 R1 and
R13 are hydrogen, R" and R12 are independently selected from the group
consisting of hydrogen
and fluoro, and R8 is selected from the group consisting of ethoxyethyl and
propoxyethyl.
Embodiments where R9, R70, R13 are hydrogen, R8 is ethoxyethyl or propoxyethyl
and R 6 is
selected from various embodiments
In one embodiment of Formula I-I, R9, R10 and R'3 are hydrogen, R8 is selected
from the
group consisting of ethoxyethyl and propoxyethyl, and R6 is selected from the
group consisting of
phenyl, phenyl C(O)NH-, phenyl C(O)-, 5 to 7 membered ring heterocyclyl,
phenyl-C(O)-NH-, 5
39
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
to 7 membered ring heterocyclyl-C(O)-, and 5 to 7 membered ring heterocyclyi-
C(O)-NH, wherein
R 6 is optionally substituted with one or more substituents independently
selected from the group
consisting of of -OR105, -C(O)R105, -C(O)OR105,-NR'05R'0s, -N(R105)C(O)R'0s, -
N(R105)C(O)OR106, -C(O)NR105R106, -NHC(O)NR105Ri06, -N(R1e5)S(O)2R106, wherein
R105 and R106
are independently selected from the group consisting of hydrogen, methyl,
ethyl, methylethyl,
tert-butyl, cyclopentyl, cyclohexyl, optionally substituted with one or more
substituent selected
from the group consisting of halogen, oxo, hydroxy, and methyl. In another
embodiment of
Formula I-I, R9, R10 and R'3 are hydrogen, R8 is selected from the group
consisting of ethoxyethyl
and propoxyethyl, and R 6 is selected from the group consisting of 5 to 6
membered ring
heteroaryl, 5 to 6 membered ring heteroaryl-C(O)-, and 5 to 6 membered ring
heteroaryl-C(O)-
NH, wherein R 6 is optionally substituted with one or more substituents
independently selected
from the group consisting of alkyl, -OR105, and -C(O)R105, wherein R105 is
selected from the group
consisting of hydrogen, methyl, and ethyl. In another embodiment of Formula I-
I, R9, R10 and R13
are hydrogen, R8 is selected from the group consisting of ethoxyethyl and
propoxyethyl, and R 6 is
selected from the group consisting of 5 to 6 membered ring fully saturated
heterocyclyl, 5 to 6
membered ring fully saturated heterocyclyl-C(O)-, and 5 to 6 membered ring
fully saturated
heterocyclyl-C(O)-NH, wherein R 6 is optionally substituted with one or more
substituents
independently selected from the group consisting of alkyl, -OR105, and -
C(O)R105, wherein R105 is
selected from the group consisting of hydrogen, methyl, and ethyl.
C. Isomers
When an asymmetric center is present in a compound of Formulae (I) through (I-
UU) the
compound will exist in the form of enantiomers. In one embodiment, the present
invention
comprises optical isomers and mixtures, including racemic mixtures of the
compounds of
Formulae (I) through (I-UU). In another embodiment, the present invention
comprises
diastereomeric forms (individual diastereomers and mixtures thereof) of
compounds of Formulae
(I) through (I-UU). When a compound of Formulae (I) through (1-UU) contains an
alkenyl group or
moiety, geometric isomers may arise.
D. Tautomeric Forms
The present invention comprises the tautomeric forms of compounds of Formulae
(I) through
(1-UU). For instance, a tautomeric form of the following compound:
OH
O N
0 N ~
ON, /-N \N N
H
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
may be represented by:
0 N
NH
O
N
ON ---HN I i
The various ratios of the tautomers in solid and liquid form is dependent on
the various
substituents on the molecule as well as the particular crystallization
technique used to isolate a
compound.
E. Salts
The compounds of this invention may be used in the form of salts derived from
inorganic or
organic acids. Depending on the particular compound, a sait of the compound
may be
advantageous due to one or more of the salt's physical properties, such as
enhanced
pharmaceutical stability in differing temperatures and humidities, or a
desirable solubility in water
or oil. In some instances, a salt of a compound also may be used as an aid in
the isolation,
purification, and/or resolution of the compound.
Wher-e-a-salt is-intended to be administer-ed-to-a-patient (as opposed-to, for-
example, being
used in an in vitro context), the salt preferably is pharmaceutically
acceptable. The term
"pharmaceutically acceptable salt" refers to a salt prepared by combining a
compound of
Formuiae (I) - (I-CC) with an acid whose anion, or a base whose cation, is
generally considered
suitable for human consumption. Pharmaceutically acceptable salts are
particularly useful as
products of the methods of the present invention because of their greater
aqueous solubility
relative to the parent compound. For use in medicine, the salts of the
compounds of this
invention are non-toxic "pharmaceutically acceptable salts." Salts encompassed
within the term
"pharmaceutically acceptable salts" refer to non-toxic salts of the compounds
of this invention
which are generally prepared by reacting the free base with a suitable organic
or inorganic acid.
Suitable pharmaceutically acceptable acid addition salts of the compounds of
the present
invention when possible include those derived from inorganic acids, such as
hydrochloric,
hydrobromic, hydrofluoric, boric, fluoroboric, phosphoric, metaphosphoric,
nitric, carbonic,
sulfonic, and sulfuric acids, and organic acids such as acetic,
benzenesulfonic, benzoic, citric,
ethanesulfonic, fumaric, gluconic, glycolic, isothionic, lactic, lactobionic,
maleic, malic,
methanesulfonic, trifluoromethanesulfonic, succinic, toluenesulfonic,
tartaric, and trifluoroacetic
acids. Suitable organic acids generally include, for example, aliphatic,
cycloaliphatic, aromatic,
araliphatic, heterocyclyl, carboxyic, and sulfonic classes of organic acids.
Specific examples of suitable organic acids include acetate, trifluoroacetate,
formate,
propionate, succinate, glycolate, gluconate, digluconate, lactate, malate,
tartaric acid, citrate,
ascorbate, glucuronate, maleate, fumarate, pyruvate, aspartate, glutamate,
benzoate, anthranilic
acid, mesylate, stearate, salicylate, p-hydroxybenzoate, phenylacetate,
mandelate, embonate
(pamoate), methanesulfonate, ethanesulfonate, benzenesulfonate, pantothenate,
41
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
toluenesulfonate, 2-hydroxyethanesulfonate, sufanilate,
cyclohexylaminosulfonate, algenic acid,
f3-hydroxybutyric acid, galactarate, galacturonate, adipate, alginate,
butyrate, camphorate,
camphorsulfonate, cyclopentanepropionate, dodecylsulfate, glycoheptanoate,
glycerophosphate,
heptanoate, hexanoate, nicotinate, 2-naphthalesulfonate, oxalate, palmoate,
pectinate,
3-phenylpropionate, picrate, pivalate, thiocyanate, and undecanoate.
In another embodiment, examples of suitable addition salts formed include the
acetate,
aspartate, benzoate, besylate, bicarbonate/carbonate, bisulphate/sulphate,
borate, camsyate,
citrate, edisylate, esylate, formate, fumarate, gluceptate, gluconate,
glucuronate,
hexafluorophosphate, hibenzate, hydrochloride/chloride, hydrobromide/bromide,
hydroiodide/iodide, isethionate, lactate, malate, maleate, nitrate, orotate,
oxalate, palmitate,
pamoate, phosphate/hydrogen phosphate/dihidrogen phosphate, saccharate,
stearate,
succinate, tartrate, tosylate and trifluoroacetate salts.
In another embodiment, representative salts include benzenesulfonate,
hydrobromide and
hydrochloride.
Furthermore, where the compounds of the invention carry an acidic moiety,
suitable
pharmaceutically acceptable salts thereof may include alkali metal salts,
e.g., sodium or
potassium salts; alkaline earth metal salts, e.g., calcium or magnesium salts;
and salts formed
____with suitable organic ligands, e.g.., quaternary amrnonium_salts.._._.
In another embodiment, base salts are formed from bases which form non-toxic
salts,
including aluminum, arginine, benzathine, choline, diethylamine, diolamine,
glycine, lysine,
meglumine, olamine, tromethamine and zinc salts.
Organic salts may be made from secondary, tertiary or quaternary amine salts,
such as
tromethamine, diethylamine, N,N'-dibenzylethylenediamine, chloroprocaine,
choline,
diethanolamine, ethylenediamine, meglumine (N-methylglucamine), and procaine.
Basic
nitrogen-containing groups may be quaternized with agents such as lower alkyl
(Ci to C6) halides
(e.g., methyl, ethyl, propyl, and butyl chlorides, bromides, and iodides),
dialkyl sulfates (e.g.,
dimethyl, diethyl, dibuytl, and diamyl sulfates), long chain halides (e.g.,
decyl, lauryl, myristyl, and
stearyl chlorides, bromides, and iodides), arylalkyl halides (e.g., benzyl and
phenethyl bromides),
and others.
In one embodiment, salts of the compounds of this invention include
hydrochloric acid (HCI)
salts, trifluoroacetate (CF3COOH or 'TFA") salts, mesylate salts, and tosylate
salts.
Pharmaceutically acceptable salts of compounds of Formulae (I) to (I-UU) may
be prepared
by one or more of three methods:
(i) by reacting the compound of any one of Formulae (I)- (I-UU) with the
desired acid or
base;
(ii) by removing an acid- or base-labile protecting group from a suitable
precursor of the
compound of any one of Formulae (I)- (I-UU) or by ring-opening a suitable
cyclic
precursor, for example, a lactone or lactam, using the desired acid or base;
and
42
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
(iii) by converting one salt of the a compound of Formulae (I) through (I-UU)
to another
by reaction with an appropriate acid or base or by means of a suitable ion
exchange
column.
All three reactions are typically carried out in solution. The resulting salt
may precipitate out
and be collected by filtration or may be recovered by evaporation of the
solvent. The degree of
ionization in the resulting salt may vary from completely ionized to almost
non-lonised.
F. Prodrups
Also within the scope of the present invention are so-called "prodrugs" of the
compounds of
Formulae (I) through (1-UU). Thus, certain derivatives of compounds of any of
Formulae (I)
through (1-UU) which may have little or no pharmacological activity themselves
can, when
administered into or onto the body, be converted into compounds of any of
Formulae (I) through
(1-UU) having the desired activity, for example, by hydrolytic cleavage. Such
derivatives are
referred to as "prodrugs". Further information on the use of prodrugs may be
found in "Pro-drugs
as Novel Delivery Systems, Vol. 14, ACS Symposium Series (T Higuchi and W
Stella) and
"Bioreversible Carriers in Drug Design", Pergamon Press, 1987 (ed. E B Roche,
American
-Pharm_aceutical Association)._ P_r_odrugs .in.acoordance with_the ir_wention
can, for_example,._be
produced by replacing appropriate functionalities present in the compounds of
any of Formulae
(I) through (1-UU) with certain moieties known to those skilled iri the art as
"pro-moieties" as
described, for example, in "Design of Prodrugs" by H Bundgaard New York, NY
(Elseview, 1985).
G. Methods of Treatment
The present invention further comprises methods for treating a condition in a
subject having
or susceptible to having such a condition, by administering to the subject a
therapeutically-
effective amount of one or more compounds of Formulae (I) through (1-UU) as
described above.
In one embodiment, the treatment is preventative treatment.
In another embodiment, the treatment is palliative treatment.
In another embodiment, the treatment is restorative treatment.
The conditions that can be treated in accordance with the present invention
include, but are
not limited to, cardiovascular diseases, metabolic diseases, central nervous
system diseases,
pulmonary diseases, sexual dysfunction, and renal dysfunction.
Conditions
The present invention further comprises methods for treating a condition in a
subject having
or susceptible to having such a condition, by administering to the subject a
therapeutically-
effective amount of one or more compounds of Formula (I) through (I-UU). In
another
embodiment, the condition is a cGMP-mediated condition.
The conditions that can be treated in accordance with the present invention
are PDE-5
mediated conditions. Such conditions include cardiovascular diseases,
metabolic diseases,
43
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
central nervous system diseases, pulmonary diseases, sexual dysfunction, and
renal
dysfunction.
In one embodiment, the condition is a cardiovascular disease, particularly a
cardiovascular
disease selected from the group consisting of hypertension (such as essential
hypertension,
pulmonary hypertension, secondary hypertension, isolated systolic
hypertension, hypertension
associated with diabetes, hypertension associated with atherosclerosis, and
renovascular
hypertension) ; complications associated with hypertension (such as vascular
organ damage,
congestive heart failure, angina, stroke, glaucoma and impaired renal
function); valvular
insufficiency; stable, unstable and variant (Prinzmetal) angina; peripheral
vascular disease;
myocardial infarct; stroke; thromboembolic disease; restenosis;
arteriosclerosis; atherosclerosis;
pulmonary arterial hypertension; angiostenosis after bypass; angioplasty (such
as percutaneous
transluminal angioplasty, or percutaneous transluminal coronary angioplasty);
hyperlipidemia;
hypoxic vasoconstriction; vasculitis, such as Kawasaki's syndrome; heart
failure (such as
congestive, decompensated, systolic, diastolic, left ventricular heart
failure, right ventricular heart
failure, left ventricular hypertrophy); Raynaud's disease; preeclampsia;
pregnancy-induced high
blood pressure; cardiomyopathy; and arterial occlusive disorders.
In another embodiment, the condition is hypertension. In another embodiment,
the condition
_______is_pulm.onary_aderial_h_yp.er_tension-IrLarLother-embodiment, the
condition is heart failure. In
another embodiment, the condition is diastolic heart failure. In another
embodiment, the
condition is systolic heart failure. In another embodiment, the condition is
angina. In another
embodiment, the condition is thrombosis. In another embodiment, the condition
is stroke.
In another embodiment, the condition is a metabolic disease, particularly a
metabolic disease
selected from the group consisting of Syndrome X; insulin resistance or
impaired glucose
tolerance; diabetes (such as type I and type II diabetes); syndromes of
insulin resistance (such
as insulin receptor disorders, Rabson-Mendenhall syndrome, leprechaunism,
Kobberling-
Dunnigan syndrome, Seip syndrome, Lawrence syndrome, Cushing syndrome,
acromegaly,
pheochomocytoma, glucagonoma, pri'mary aldosteronism, somatostatinoma,
Lipoatrophic
diabetes, R-cell toxin induced diabetes, Grave's disease, Hashimoto's
thyroiditis and idiopathic
Addison's disease); diabetic complications (such as diabetic gangrene,
diabetic arthropathy,
diabetic nephropathy, diabetic glomerulosclerosis, diabetic deramatopathy,
diabetic neuropathy,
peripheral diabetic neuropathy, diabetic cataract, and diabetic retinopathy);
hyperglycemia; and
obesity.
In another embodiment, the condition is insulin resistance. In another
embodiment, the
condition is nephropathy.
In another embodiment, the condition is a disease of the central nervous
system, particularly
a disease of the central nervous system selected from the group consisting of
vascular dementia;
craniocerebral trauma; cerebral infarcts; dementia; concentration disorders;
Alzheimer's disease;
Parkinson's disease; amyolateral sclerosis (ALS); Huntington's disease;
multiple sclerosis;
Creutzfeld-Jacob; anxiety; depression; sleep disorders; and migraine. In one
embodiment, the
condition is Alzheimer's disease. In another embodiment, the condition is
Parkinson's disease.
44
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
In one embodiment, the condition is ALS. In another embodiment, the condition
is a
concentration disorder.
In one embodiment, the condition is a pulmonary disease, particularly a
pulmonary disease,
selected from the group consiting of asthma; acute respiratory distress;
cystic fibrosis; chronic
obstructive pulmonary disease (COPD); bronchitis; and chronic reversible
pulmonary obstruction.
In one embodiment, the condition is sexual dysfunction, particularly sexual
dysfunction
selected from the group consiting of impotence (organic or psychic); male
erectile dysfunction;
clitoral dysfunction; sexual dysfunction after spinal cord injury; female
sexual arousal disorder;
female sexual orgasmic dysfunction; female sexual pain disorder; and female
hypoactive sexual
desire disorder. In another embodiment, the condition is erectile dysfunction.
In another embodiment, the condition is renal dysfunction, particularly a
renal dysfunction
selected from the group consisting of acute or chronic renal failure;
nephropathy (such as
diabetic nephropathy); glomerulopathy; and nephritis.
In another embodiment, the condition is pain. In another embodiment, the
condition is acute
pain. Examples of acute pain include acute pain associated with injury or
surgery. In another
embodiment, the condition is chronic pain. Examples of chronic pain include
neuropathic pain
(including postherpetic neuralgia and pain associated with peripheral, cancer
or diabetic
-neur-opathy),-carpal't-unnel-syndrorne,-back-pain-(including-pain-associated-
with-her.niated or
ruptured intervertabral discs or abnormalities of the lumber facet joints,
sacroiliac joints,
paraspinal muscles or the posterior longitudinal ligament), headache, cancer
pain (including
tumour related pain such as bone pain, headache, facial pain or visceral pain)
or pain associated
with cancer therapy (including postchemotherapy syndrome, chronic postsurgical
pain syndrome,
post radiation syndrome, pain associated with immunotherapy, or pain
associated with hormonal
therapy), arthritic pain (including osteoarthritis and rheumatoid arthritis
pain), chronic post-
surgical pain, post herpetic neuralgia, trigeminal neuralgia, HIV neuropathy,
phantom limb pain,
central post-stroke pain and pain associated with chronic alcoholism,
hypothyroidism, uremia,
multiple sclerosis, spinal cord injury, Parkinson's disease, epilepsy and
vitamin deficiency. In
another embodiment, the condition is nociceptive pain (including pain from
central nervous
system trauma, strains/sprains, burns, myocardial infarction and acute
pancreatitis, post-
operative pain (pain following any type of surgical procedure), posttraumatic
pain, renal colic,
cancer pain and back pain). In another embodiment, the condition is pain
associated with
inflammation (including arthritic pain (such as osteoarthritis and rheumatoid
disease pain),
ankylosing spondylitis, visceral pain (including inflammatory bowel disease,
functional bowel
disorder, gastro-esophageal reflux, dyspepsia, irritable bowel syndrome,
functional abdominal
pain syndrome, Crohn's disease, ileitis, ulcerative colitis, dysmenorrheal,
cystitis, pancreatitis
and pelvic pain). In another embodiment, the condition is pain resulting from
musculo-skeletal
disorders (including myalgia, fibromyalgia, spondylitis, sero-negative (non-
rheumatoid)
arthropathies, non-articular rheumatism, dystrophinopathy, glycogenolysis,
polymyositis and
pyomyositis). In another embodiment, the condition is selected from the group
consisting of
heart and vascular pain (including pain caused by angina, myocardical
infarction, mitral stenosis,
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
pericarditis, Raynaud's phenomenon, scieredoma and skeletal muscle ischemia).
In another
embodiment, the condition is selected from the group consisting of head pain
(including migraine
such as migraine with aura and migraine without aura), cluster headache,
tension-type headache
mixed headache and headache associated with vascular disorders; orofacial
pain, including
dental pain, otic pain, burning mouth syndrome and temporomandibular
myofascial pain).
In another embodiment, the condition is a urologic condition selected from the
group
consisting of bladder outlet obstruction; incontinence and benign prostatic
hyperplasia.
In another embodiment, the condition is an ophthalmic condition selected from
retinal
disease; macular degeneration and glaucoma.
In another embodiment, the condition is selected from the group consisting of
tubulointerstitial disorders; anal fissure; baldness; cancerous cachexia;
cerebral apoplexy;
disorders of gut motility; enteromotility disorders; dysmenorrhoea (primary
and secondary);
glaucoma; macular degeneration; antiplatelet; haemorrhoids; incontinence;
irritable bowel
syndrome (IBS); tumor metastasis; multiple sclerosis; neoplasia; nitrate
intolerance; nutcracker
oesophagus; osteoporosis; infertility; premature labor; psoriasis; retinal
disease; skin necrosis;
and urticaria. In another embodiment, the condition is osteoporosis.
In another embodiment, the condition is associated with endothelial
dysfunction, particularly
----- c-onditions-selec#ed from-the-group-consisting-of--atherosclerotic-
lesions, myocardial ischaemia,
peripheral ischaemia, valvular insufficiency, pulmonary arterial hypertension,
angina, vascular
complications after vascular bypass, vascular dilation, vascular
repermeabilisation, and heart
transplantation.
The methods and compositions of the present invention are suitable for use
with, for
example, mammalian subjects such as humans, other primates (e.g., monkeys,
chimpanzees),
companion animals (e.g., dogs, cats, horses), farm animals (e.g., goats,
sheep, pigs, cattle),
laboratory animals (e.g., mice, rats), and wild and zoo animals (e.g., wolves,
bears, deer). In
another embodiment, the subject is a human.
Hypothesized Mechanism
Without being held to a particular theory, it is hypothesized that compounds
of Formulae (I)
through (I-UU) inhibit PDE-5 and increase intracellular cGMP levels. This
increase in
intracellular cGMP reduces intracellular calcium signaling, resulting in
vascular smooth muscle
relaxation, and a reduction in hypertension.
Selected embodiments of the invention, therefore, comprise methods for
treating a cGMP-
mediated condition. via PDE-5 inhibition. A condition in which, for instance,
insufficient cGMP is
a major component, and whose production or action is modulated in response to
PDE-5, would
therefore be considered a disorder mediated by cGMP. Thus, compounds of
Formulae (I)
through (I-UU) would be therapeutically useful in methods for treating
hypertension by
administering to a hypertensive subject a therapeutically-effective amount of
a compound of
Forumulae (I) through (I-UU). Other examples of circulatory-related disorders
which can be
46
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
treated by compounds of the invention include congestive heart failure, renal
failure, angina, and
glaucoma.
Co-administration
One or more compounds of the present invention can be used, alone or in
combination with
other therapeutic agents, in the treatment of various conditions or disease
states. The
compound(s) of the present invention and other therapeutic agent(s) may be may
be
administered simultaneously (either in the same dosage form or in separate
dosage forms) or
sequentially.
For instance, in one embodiment, one or more compounds of Formulae (I) through
(I-UU)
may be administered with aspirin.
In one embodiment, one or more compounds of Formulae (I) through (I-UU) may be
co-
administered with one or more angiotensin converting enzyme (ACE) inhibitors.
Examples of
the one or more ACE inhibitors for use with the one or morecompound of
Formulae (I) -(I-UU)
include quinapril (such as ACCUPRILTM), perindopril (such as ACEONTM),
captopril (such as
CAPOTENTM), enalapril (such as VASOTECTM), ENALAPRILATTM, ramipril (such as
ALTACETM),
cilazapril, delapril, fosenopril (such as MONOPRILTM), zofenopril, indolapril,
benazepril (such as
L-O-T-E-NSINT); lisinopr-il-(such-as-PRINIVILTM or-ZESTRILT-"'); spirapril;
trandolapr-il-(such as
MAVIKTM), perindep, pentopril, moexipril (such as UNIVASCTM) or pivopril.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more alpha blockers such as dozazosin (such as
CA;DURAT"~),
phenoxybenzamine (such as DIBENZYLINETM), or terazosin (such as HYTRINTM),
CDRI-93/478
and CR-2991.
In another embodiment, one or more compounds of Formulae (I) through (1-UU)
may be co-
administered with one or more alpha-beta blockers such as Iabetalol (such as
NORMODYNETM
or TRANDATETM).
In another embodiment, one or more compounds of Formulae (I) through (1-UU)
may be co-
administered with one or more angiotensin II receptor blockers such as
candesartan (such as
ATACANDTM), eprosartan (such as TEVETENTM), irbesartan (such as AVEPROTM),
losartan
(such as COZAARTM), olmesartan, olmesartan medoxomil (such as BENICARTM),
tasosartan,
telmisartan (such as MICARDISTM), valsartan (such as DIOVANTM) or zolasartan,
FI-6828K,
RNH-6270, UR-7198, Way-126227, KRH-594, TAK-536, BRA-657, and TA-606.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more alpha-2-delta ligands such as gabapentin,
pregabalin (such as
LYRICATM), [(1R,5R,6S)-6-(aminomethyl)bicyclo[3.2.0]hept-6-yI]acetic acid, 3-
(1-aminomethyl-
cyclohexyimethyl)-4H-[1,2,4]oxadiazol-5-one, C-[1-(1 H-tetrazol-5-ylmethyl)-
cycloheptyl]-
methylamine, (3S,4S)-(1-aminomethyl-3,4-dimethyl-cyclopentyl)-acetic acid,
(1a,3a,5(x)-(3-
amino-methyl-bicyclo[3.2.0]hept-3-yl)-acetic acid, (3S,5R)-3-aminomethyl-5-
methyl-octanoic acid,
(3S,5R)-3-amino-5-methyl-heptanoic acid, (3S,5R)-3-amino-5-methyl-nonanoic
acid and
47
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
(3S,5R)-3-amino-5-methyl-octanoic acid), (2S,4S)-4-(3-Chlorophenoxy)praline,
or (2S,4S)-4-(3-
Fluorobenzyl)praline.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be
co-administered with one or more beta blockers such as timolol (such as
BLOCARDENTM),
carteolol (such as CARTROLTM), carvedilol (such as COREGTM), nadolol (such as
CORGARDTM), propranolol (such as INNOPRAN XLTM), betaxolol (such as
KERLONETM),
penbutolol (such as LEVATOLTM), metoprolol (such as LOPRESSORTM or TOPROL-
XLTM),
atenolol (such as TENORMINTM), or pindolol (such as VISKENTM), and bisoprolol.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more calcium channel blockers such as nifedipine
(such as ADALATTM,
ADALAT CCTM or PROCARDIATM), verapamil (such as CALANTM, COVERA-HST"', ISOPTIN
SRTM or VERELANTM), diltiazem (such as CARDIZEMTM CARDIZEM CDTM, CARDIZEM
LATM,
CARDIZEM SRTM, DILACORTM, TIAMATETM or TIAZACTM), isradipine (such as
DYNACIRCTM or
DYNACIRC CRTM), amlodipine (such as NORVASCT'"), felodipine (such as
PLENDILTM),
nisoldipine (such as SULARTM), or bepridil (such as VASCORTM), vatanidipine,
clevidipine,
lercanidipine, dilitiazem, and NNC-55-0396.
In another embodiment, one or more compounds Formulae (I) through (1-UU) may
be co-
ister-ed with_one_or-mor_ecentral_antiadr.energics-such_as_methyldopa-(such-as-
_- .-.
ALDOMETTM), clonidine (such as CATAPRESTM or CATAPRES-TTSTM), guanfacine (such
as
TENEXTM), or guanabenz (such as WYTENSINTM).
In another embodiment, one or more compounds oF Formulae (I) through (1-UU)
may be co-
administered with one or more diruretics such as hydroclorothiazide (such as
MICROZIDETM or
ORETICTM), hydroflumethiazide (such as SALURONTM), bemetanide (such as
BUMEXTM),
torsemide (such as DEMADEXTM), metolazone (such as ZAROXOLYNTM),
chlorothiazide (such
as DIURILTM, ESIDRIXTM or HYDRODIURILTM), triamterene (such as DYRENIUMTM),
ethacrynic
acid (such as EDECRINTM), chlorthalidone (such as HYGROTONTM), furosemide
(such as
LASIXTM), indapamide (such as LOZOLTM), or amiloride (such as MIDAMORTM or
MODURETICTM).
In another embodiment, one or more compounds of Formulae (I) through (1-UU)
may be co-
administered with one or more glycosides / inotropic agents such as digoxin
(such as
LANOXINTM).
In another embodiment, one or more compounds of Formulae (I) through (1-UU)
may be co-
administered with one or more organic nitrates or an NO donors. "Nitric oxide
donor" or "NO
donor" refers to a compound that donates, releases and/or directly or
indirectly transfers a
nitrogen monoxide species, and/or stimulate the endogenous production of
nitric oxide or
endothelium-derived relaxing factor (EDRF) in vivo and/or elevate endogenous
levels of nitric
oxide or EDRF in vivo. "NO donor" also includes compounds that are substrates
for nitric oxide
synthase. Examples of the one or more NO donors for use with one or more
compounds of
Formulae (I) through (I-UU) include S-nitrosothiols, nitrites, nitrates, N-oxo-
N-nitrosamines, SPM
3672, SPM 5185, SPM 5186 and analogues thereof, sodium nitroprusside,
nitroglycerin,
48
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
isosorbide dinitrate, isosorbide mononitrate, molsidomine, SIN-1 or substrates
of the various
isozymes of nitric oxide synthase.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more human B-type natriuretic peptides (hBNP) such as
nesiritide (such
as NATRECORTM).
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more renin inhibitors such as Aliskiren (SPP 100),
SPP-500/600 and
YS-004-39.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be
co-administered with one or more soluble guanylate cyclase activator ("sGCa").
An example of a
suitable soluble guanylate cyclase activator is BAY-41-8543.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more neutral endopeptidase (NEP) inhibitors, such as,
for example,
( \
O
NH N
SH C02H
omapatrilat, fasidotril, mixanpril, sampatrilat, Z13752A , BMS-189921 , or
H
N
0
H3
CYS'-AN O O OH
O = H
~ MDL-100240
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more aldosterone receptor antagonists such as
eplerenone (such as
INSPRATM) or spironolactone (such as ALDACTONETM).
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more bradykinin agonists.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more endothelian antagonists. Examples of suitable
endothelin
antagonists include ambrisentan, darusentan, J-1 04132, SPP-301, TBC-371 1, YM-
62899, YM-
91746, and BMS-193884.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with niacin or one or more nicotinic acid derivatives, such as
NIACORTM,
NIASPANTM, NICOLARTM, or SLO-NIACINr"'.
49
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more fibric acid derivatives, such as clofibrate
(such as ATROMID-STM),
gemfibrozil (such as LOPIDTM), or fenofibrate (such as TRICORTM).
In another embodiment, one or more compounds of Formulae (1) through (I-UU)
may be co-
administered with one or more cholesteryl ester transport protein inhibitors
(CETPi), such as
torcetrapib.
In another embodiment, one or more compounds of Formulae (I) through (1-UU)
may be co-
administered with one or more bile acid sequestants, such as colestipol (such
as COLESTIDTM),
cholestyramine (such as LOCHOLESTTM, PREVALITETM, QUESTRANTM, or QUESTRAN
LIGHTTM), colesevelam (such as WELCHOLTM).
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with an apical sodium-dependent bile acid cotransporter
inhibitors, such as SD-
5613, AZD7806 or 264W94.
In another embodiment, one or more compounds of Formulae (I) through (1-UU)
may be co-
administered with one or more cholesterol absorbtion inhibitors, such as
ezetimibe (such as
ZETIATM).
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
_ -administexedLwith one_or_rnoce_3=b-ydr_oxy--3--meth_yJglutar_yJ
coQnzyme_A_(HMG-CoA) reductase
inhibitors (statins) such as fluvastatin (such as LESCOLTM), atorvastatin
(such as LIPITORTM),
lovastatin (such as ALTOCORTM or MEVACORTM), pravastatin (such as
PRAVACHOLTM),
rosuvastatn (such as CRESTORTM), or simvastatin (such as ZOCORTM).
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more alpha glucosidase inhibitors, such as miglitol
(such as
GLYSETT"'), or acarbose (such as PRECOSETM).
In another embodiment, one or more compounds of Formulae (I) through (1-UU)
may be co-
administered with one or more biguanides, such as roseiglitazone (such as
AVANDAMETT"'), or
metformin (such as GLUCOPHAGETM or GLUCOPHAGE XRTM).
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more insulins, such as HUMALOGTM, HUMALOG 50/50T"',
HUMALOG
75/25TM, HUMULIN 50/50T"', HUMALIN 75/25TM, HUMALIN LTM, HUMALIN NTM, HUMALIN
RrM,
HUMALIN R U-500T"', HUMALIN UTM, ILETIN II LENTETM, ILETIN II NPHTM, ILETIN II
REGULART"~, LANTUSTM, NOVOLIN 70/30T~~, NOVILIN NTM, NOVILIN RT~", NOVOLOGTM,
or
VELOSULIN BRTM, and EXUBERATM.
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more meglitnides, such as repaglinide (such as
PRANDINTM) or
nateglinide (such as STARLIXTM).
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more sulfonylureas, such as glimepiride (such as
AMARYLTM),
glyburide (such as DIABETAT"~, GLYNASE PRESTABTM or MICRONASET"'), or
glipizide (such
as GLUCOTROLTM, or GLUCOTROL XLTM).
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
In another embodiment, one or more compounds of Formulae (I) through (I-UU)
may be co-
administered with one or more thiazolidinediones, such as pioglitazone (such
as ACTOSTM) or
rosiglitazone (such as AVANDIATM).
Administration and Dosing
Typically, a compound described in this specification is administered in an
amount effective
to inhibit PDE-5. The compounds of the present invention are administered by
any suitable route
in the form of a pharmaceutical composition adapted to such a route, and in a
dose effective for
the treatment intended. Therapeutically effective doses of the compounds
required to prevent or
arrest the progress of or to treat the medical condition are readily
ascertained by one of ordinary
skill in the art using preclinical and clinical approaches familiar to the
medicinal arts.
The dosage regimen for the compounds and/or compositions containing the
compounds is
based on a variety of factors, including the type, age, weight, sex and
medical condition of the
patient; the severity of the condition; the route of administration; and the
activity of the particular
compound employed. Thus the dosage regimen may vary widely. Dosage levels of
the order
from about 0.01 mg to about 100 mg per kilogram of body weight per day are
useful in the
treatment of the above-indicated conditions. In one embodiment, the total
daily dose of a
_ compound of Formulae_(I)_throu-gh (I_U_lJ)_(administered in singleor_divided
doses) is typically
from about 0. 01 to about 100 mg/kg. In another embodiment, total daily dose
of the compound
of Formulae (I) through (IX) is from about 0.1 to about 50 mg/kg, aizcf in
another embodiment,
from about 0.5 to about 30 mg/kg (i.e., mg compound of Formulae (I).through (1-
UU) per kg body
weight). In one embodiment, dosing is from 0.01 to 10 mg/kg/day. In another
embodiment,
dosing is from 0.1 to 1.0 mg/kg/day. Dosage unit compositions may contain such
amounts or
submultiples thereof to make up the daily dose. In many instances, the
administration of the
compound will be repeated a plurality of times in a day (typically no greater
than 4 times).
Multiple doses per day typically may be used to increase the total daily dose,
if desired.
For oral adminisiration, the compositions may be provided in the form of
tablets containing
0.01, 0.05, 0.1, 0.5, 1.0, 2.5, 5.0, 10.0, 15.0, 25.0, 50.0, 75.0, 100, 125,
150, 175, 200, 250 and
500 milligrams of the active ingredient for the symptomatic adjustment of the
dosage to the
patient to be treated. A medicament typically contains from about 0.01 mg to
about 500 mg of
the active ingredient, or in another embodiment, from about 1 mg to about 100
mg of active
ingredient. Intravenously, doses may range from about 0.1 to about 10
mg/kg/minute during a
constant rate infusion.
H. Use in the Preparation of a Medicament
In one embodiment, the present invention comprises methods for the preparation
of a
pharmaceutical composition (or "medicament) comprising the compounds of
Formulae (I)
through (I-UU) in combination with one or more pharmaceutically-acceptable
carriers and/or
other active ingredients for use in treating a cGMP-mediated condition.
51
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
In another embodiment, the invention comprises the use of one or more
compounds of
Formulae (I) through (1-UU) in the preparation of a medicament for the
treatment of hypertension.
In another embodiment, the invention comprises the use of one or more
compounds of
Formulae (I) through (I-UU) in the preparation of a medicament for the
treatment of angina.
In another embodiment, the invention comprises the use of one or more
compounds of
Formulae (I) through (1-UU) in the preparation of a medicament for the
treatment of congestive
heart failure.
In another embodiment, the invention comprises the use of one or more
compounds of
Formulae (I) through (1-UU) in the preparation of a medicament for the
treatment of thrombosis.
In another embodiment, the invention comprises the use of one or more
compounds of
Formulae (I) through (I-UU) in the preparation of a medicament for the
treatment of erectile
dysfunction.
1. Pharmaceutical Compositions
For the treatment of the conditions referred to above, the compounds of
Formulae (I) through
(I-UU) can be administered as compound per se. Alternatively, pharmaceutically
acceptable
salts are suitable for medical applications because of their greater aqueous
solubility relative to
__ the parent compound.___
In another embodiment, the present invention comprises pharmaceutical
compositions.
Such pharmaceutical compositions comprise compounds of Formulae (I) through (I-
UU)
presented with a pharmaceutically-acceptable carrier. The carrier can be a
solid, a liquid, or
both, and may be formulated with the compound as a unit-dose composition, for
example, a
tablet, which can contain from 0.05% to 95% by weight of the active compounds.
Compounds of
Formulae (I) through (I-UU) may be coupled with suitable polymers as
targetable drug carriers.
Other pharmacologically active substances can also be present.
The active compounds of the present invention may be administered by any
suitable route,
preferably in the form of a pharmaceutical composition adapted to such a
route, and in a dose
effective for the treatment intended. The active compounds and compositions,
for example, may
be administered orally, rectally, parenterally, or topically.
Oral administration of a solid dose form may be, for example, presented in
discrete units,
such as hard or soft capsules, pills, cachets, lozenges, or tablets, each
containing a
predetermined amount of at least one compound of the present invention. In
another
embodiment, the oral administration may be in a powder or granule form. In
another
embodiment, the oral dose form is sub-lingual, such as, for example, a
lozenge. In such solid
dosage forms, the compounds of Formulae (I) through (I-UU) are ordinarily
combined with one or
more adjuvants. In the case of capsules, tablets, and pills, the dosage forms
also may comprise
buffering agentsor may be prepared with enteric coatings.
In another embodiment, oral administration may be in a liquid dose form.
Liquid dosage
forms for oral administration include, for example, pharmaceutically
acceptable emulsions,
solutions, suspensions, syrups, and elixirs containing inert diluents commonly
used in the art
52
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
(e.g., water). Such compositions also may comprise adjuvants, such as wetting,
emulsifying,
suspending, flavoring (e.g., sweetening), and/or perfuming agents.
In another embodiment, the present invention comprises a parenteral dose form.
"Parenteral
administration" includes, for example, subcutaneous injections, intravenous
injections,
intraperitoneally, intramuscular injections, intrasternal injections, and
infusion. Injectable
preparations (e.g., sterile injectable aqueous or oleaginous suspensions) may
be formulated
according to the known art using suitable dispersing, wetting agents, and/or
suspending agents.
In another embodiment, the present invention comprises a topical dose form.
"Topical
administration" includes, for example, transdermal administration, such as via
transdermal
patches or iontophoresis devices, intraocular administration, or intranasal or
inhalation
administration. Compositions for topical administration also include, for
example, topical gels,
sprays, ointments, and creams. A topical formulation may include a compound
which enhances
absorption or penetration of the active ingredient through the skin or other
affected areas. When
the compounds of this invention are administered by a transdermal device,
administration will be
accomplished using a patch either of the reservoir and porous membrane type or
of a solid
matrix variety. Formulations suitable for topical administration to the eye
include, for example,
eye drops wherein the compound of this invention is dissolved or suspended in
suitable carrier.
For intranasal administration or administration by inhalation, the active
compounds of the
invention are conveniently delivered in the form of a solution or suspension
from a pump spray
container that is squeezed or pumped by the patient or as an aerosol spray
presentation from a
pressurized container or a nebulizer, with the use of a suitable propellant.
In another embodiment, the present invention comprises a rectal dose form.
Such rectal
dose form may be in the form of, for example, a suppository.
Other carrier materials and modes of administration known in the
pharmaceutical art may
also be used. Pharmaceutical compositions of the invention may be prepared by
any of the well-
known techniques of pharmacy, such as effective formulation and administration
procedures.
The above considerations in regard to effective formulations and
administration procedures are
well known in the art and are described in standard textbooks. Formulation of
drugs is discussed
in, for example, Hoover, John E., Remington's Pharmaceutical Sciences, Mack
Publishing Co.,
Easton, Pennsylvania, 1975; Liberman, et al., Eds., Pharmaceutical Dosage
Forms, Marcel
Decker, New York, N.Y., 1980; and Kibbe, et al., Eds., Handbook of
Pharmaceutical Excipients
(3rd Ed.), American Pharmaceutical Association, Washington, 1999.
J. Kits
The present invention further comprises kits that are suitable for use in
performing the
methods of treatment or prevention described above. In one embodiment, the kit
contains a first
dosage form comprising one or more of the compounds of the present invention
and a container
for the dosage, in quantities sufficient to carry out the methods of the
present invention.
n another embodiment, the kit of the present invention comprises one or more
compounds of
Formulae (I) through (I-UU) and an ACE inhibitor.
53
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
In another embodiment, the kit of the present invention comprises one or more
compounds
of Formulae (I) through (I-UU) and an angiotensin II receptor antagonist.
In another embodiment, the kit of the present invention comprises one or more
compounds
of Formulae (I) through (I-UU) and an aidosterone receptor antagonist.
In another embodiment, the kit of the present invention comprises one or more
compounds
of Formulae (I) through (I-UU) and a NO donor.
L. Compound Preparations
Schemes
The starting materials used herein are commercially available or may prepared
by routine
methods well known to those of ordinary skill in the art (such as those
methods disclosed in
standard reference books such as the COMPENDIUM OF ORGANIC SYNTHETIC METHODS,
Vol. I-VI (published by Wiley-Interscience)).
The compounds of the present invention may be prepared using the methods
illustrated in
the general synthetic schemes and experimental procedures detailed below. The'
general
synthetic schemes are presented for purposes of illustration and are not
intended to be limiting.
Scheme 1
54
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
CI Boc20 CI nBuLi F CI
I\ I\ then F+ I\
H2N ~ N BocHN ~ N. BocHN N
II III IV
Ra Ra
i i
NH2R8 HN \ CI Acid HN CI
I~N I~
BocHN H2N
V V VI
CIC(O)CO2Et R$ POCI3 R8
NR3, heat O N Cl nPrCN, reflux O N CI
OR ' I OR I
Oxalic acid, O H (COCI)2, DMF, CI N
H3O + VII CH2CI2, rt VIII
R8 R$
NHXs(Ys-Rs) s O N ~ CI ArB(OH)2 O N Ar
R.Ys o R2.
N N ~ Pd N N
, N i
Xs IX R2 I
Scheme 1 outlines a general procedure for the preparation of 7-aryl, 1, 3-
disubstituted
pyrido[3,4-b]pyrazin-2(1 H)-one of formula I. The starting material is the
commercially available 6-
chloropyridin-3-amine II. 6-chloropyridin-3-amine II is converted to tert-
butyl 6-chloropyridin-3-
ylcarbamate III by treatment with reagents such as di-tert-butyl dicarbonate,
(2E)-{[(tert-
butoxycarbonyl)oxy]imino}(phenyl)acetonitrile and tert-butyl phenyl carbonate.
This reaction is
carried out in solvents such as dioxane, tetrahydrofuran, water, ethyl acetate
or dichloromethane,
in the presence or absence of inorganic bases such as potassium carbonate or
sodium
bicarbonate or organic bases such as triethylamine, 4-methylmorpholine,
pyridine or N,N-
diisopropylethylamine at temperatures ranging from room temperature to 110
C. tert-butyl 6-
chloropyridin-3-ylcarbamate III is converted to tert-butyl 6-chloro-4-
fluoropyridin-3-ylcarbamate
IV by metallation followed by quenching with an electrophilic fluorine source.
Lithation could be
achieved be treating tert-butyl 6-chloropyridin-3-ylcarbamate III with an
organolithium such as n-
butyl lithium or t-butyl lithium in the presence or absence of additives such
as N,N,N;N'-
tetramethylethylenediamine in solvents such as diethyl ether or
tetrahydrofuran at temperatures
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
ranging from -80 C to 0 C. Suitable electrophilic fluorine sources include
N-
flurobenzenesulfonimide. Addition of primary and benzylic amines to tert-butyl
6-chloro-4-
fluoropyridin-3-ylcarbamate IV afforded amines of the formula V. This
conversion could be
achieved by treatment of IV with amines in solvents such as ethyl alcohol,
isopropyl alcohol,
dimethylformamide, dimethylactemide, toluene, dioxane and dichloroethane in
the presence or
absence of inorganic bases such as potassium carbonate or sodium bicarbonate
or organic
bases such as triethylamine, 4-methylmorpholine, pyridine or N,N-
diisopropylethylamine at
temperatures ranging from room temperature to 110 C. Amines of the formula V
could be
converted to diamines of the formula VI by removing the carbamate protecting
group under
standard conditions, as described in Green, T., Wuts, P. Protecting Groups in
Organic Synthesis,
John Wiley & Sons, INC, Second edition, 1991, pp 309-405. The diamines of
formula VI could
be converted to the diones of formula VII using various reaction procedures.
In one procedure,
this conversion is achieved by refluxing an aqueous solution of VI in the
presence of oxalic acid
and a catalytic amount of a mineral acid such as HCI. Alternatively, this
conversion to structures
of formula VII could be achieved by addition of either methyl chlorooxoacetate
or oxalyl chloride
to a solution of VI in the presence of an organic base such as triethylamine,
4-methylmorpholine,
or N,N-diisopropylethylamine, at 0 C, followed by warming to either room temp
or the reflux
temperature of the solvent. Suitable solvents include toluene,
dichloromethane, dicholroethane,
dioxane, or tetrahydrofuran. The chloroimidate of formula VIII could be
prepared by a number of
methods. In one procedure, a dione of formula VII could be heated to reflux in
the presence of
phosphorous oxychloride and a phase transfer catalyst such as
tetraethylammonium chloride.
Suitable solvents for this reaction include propionitrile or acetonitrile. In
an alternate procedure,
the formation of chloroimidate VII is achieved by dissolving VII in a suitable
solvent such as
dichloromethane, tetrahydrofuran, or dioxane and treating it with oxalyl
chloride in the presence
of a catalytic amount of dimethylformamide between 0 C and rt. The 6-
aminopyrazinones of
formula IX could be prepared by the addition of various primary and secondary
amines to
chloroimidate VIII in the presence of an organic base such as triethylamine, 4-
methylmorpholine,
or N,N-diisopropylethylamine at temperatures ranging from 0 C to rt. Suitable
solvents include
dich lorom ethane, tetrahydrofuran, and dioxane. Formation of the desired
pteridinone of formula I
could be prepared through a standard palladium catalyzed Suzuki coupling
between chloride IX
and suitable boronic acids, as described in Miyaura, N., Suzuki, A; Chem Rev.
1995, 95, 2457-
2483. A solution of the chloride, IX, in a suitable solvent such as
tetrahydrofuran or dioxane is
heated to reflux in the presence of the desired boronic, an inorganic base
such as sodium
carbonate or cesium carbonate, and a palladium(0) source such palladium(II)
acetate or
tetrakis(triphenylphosphine)palladium to give compounds of formula I.
56
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Scheme 2
CI nBuLi, I+ ' CI NH2Rg RBHN z CI
BocHN N BocHN ~N BocHN I ~N
III x V
Scheme 2 outlines an alternate conversion of tert-butyl 6-chloropyridin-3-
ylcarbamate III to
amines of formula V. tert-butyl 6-chloropyridin-3-ylcarbamate III is converted
to tert-butyl 6-
chloro-4-iodopyridin-3-ylcarbamate X by metallation followed by quenching with
an electrophilic
iodine source. Lithation could be achieved be treating tert-butyl 6-
chloropyridin-3-ylcarbamate III
with an organolithium such as n-butyl lithium or t-butyl lithium in the
presence or absence of
additives such as N,N,N;N'tetramethylethylenediamine in solvents such as
diethyl ether or
tetrahydrofuran at temperatures ranging from -80 C to 0 C. Suitable
electrophilic iodine
sources include molecular iodine and 1-iodopyrrolidine-2,5-dione. Addition of
primary and
benzylic amines to tert-butyl 6-chloro-4-iodopyridin-3-ylcarbam ate IV
afforded amines of the
formula V. Amines of the formula X could be converted to diamines of the
formula V by standard
coupling techniques as described in Ley, S., Thomas, A.; Angew. Chem. Int. Ed.
2003, 42, 5400-
5449. A solution of iodide, X, in a suitable solvent such as tetrahydrofuran,
dioxane, toluene,
benzene, N,N dimethylformamide, isopropanol, ethanol or propionitrile is
stirred at temperatures
ranging from room temperature to reflux in the presence of the desired amine,
a base such as
sodium carbonate, cesium carbonate, potassium phosphate, or sodium tert-
butoxide and a
palladium with ligand and/or a copper source. Suitable sources of palladium
include palladium(II)
acetate, tetrakis(triphenylphosphine)palladium, dichloro[1,1'-
bis(diphenylphosphino)ferrocene]palladium (II) dichloromethane adduct and
tris(dibenzylideneacetone)dipalladium(0). Suitable ligands include
triphenylphosphine, tri-2-
furylphosphine, 4,5-bis(diphenylphosphine)-9-9-dimethylxathene,
tricyclohexylphospine, tert-
butylphospine and 2,2'-bis(diphenylphosphino)-1,1'-binapthyl. Suitable sources
of copper
include copper(II) acetate, copper(l) iodide and copper(l) chloride.
Scheme 3 outlines a one-pot procedure for the conversion of diaminopyridine of
formula VI
to amino substituted pyrazinone of formula IX. The pyridine VI is dissolved in
a solvent such as
dichloromethane, tetrahydrofuran, or dioxane and cooled to 0 C. The mixture is
treated with
oxalyl chloride and allowed to slowly warm to room temperature. The reaction
is typically mixed
for 4-24 hours. The reaction mixture is then recooled to 0 C, treated with an
organic base such
as triethylamine, 4-methylmorpholine, or N,IV diisopropylethylamine, followed
by addition of the
requisite primary or secondary amine leading to isolation of the desired amine
of formula IX.
57
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Scheme 3
1) (COCI)2 R
R1 CH2CI2, 0 C_ rt O Ni
I ~ CI
HN CI 2) Cool to 0 C R21
N~ ~ N
add organic base i N
H2N H Rs
~N, VI Rs R2 IX
The preparation of substituted amine analogs in the C(6) position similar to
compounds of
formula XIII or XIV are shown in Scheme 5. The starting amines XI can be
prepared by utilizing
mono-protected diamines in the final step of Scheme 1. The protecting groups
used are typically
carbamates which are removed using standard conditions to afford free amines
of formula XII.2
Derivatives of formula XIII can be prepared by adding the desired aldehyde to
a solution of XII in
the presence of a catalytic amount of acids such as hydrochloric or acetic
acid. A reducing agent
such as sodium borohydride, sodium cyanoborohydride, or sodium
triacetoxyborohydride is then
added to the mixture leading to amines of formula XIII. Suitable solvents
include tetrahydrofuran,
dioxane, or dichloromethane. Amide derivatives of formula XIV could be
prepared by treatment
of the amine XII with activated esters such a acid chlorides, or acid
derivatives prepared from
acids utilizing_peptide coupling reagents such as 1-[3_(Dimethylaminopropy]-3-
ethylcarbodiimide
methiodide , 1,3-Dicyclohexylcarbodiimide, or 0-(7-Azabenzotriazol-1-yl)-
1,1,3,3-
tetramethyluromium hexafluorophosphate in the presence of an organic base such
as
triethylamine, N, N-diisopropylethylamine, 4-methyimorpholine. Suitable
solvents include
dichloromethane, tetrahydrofuran, or dioxane.
Scheme 5
58
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
R8
O N "z Ar R$
Acid O N Ar
N
~N~H TIN H2N ~N
I ~ N
~pp H
XI XII
R8
p 0 N Ar.
RAH \I-H N
N
n H
H+, Reducing agent
s
O N Ar XIII
N
H2N N N
1n H O
XII R6-I-CI
R$
Base, 0 C I
- O N Ar
- --- -- ~ . ~ p - -------
OR R ' NH~~,- ~N_ ~ N
O 11n H
RsA-pH xiv
Coupling agent,
base, rt
Compound Examples
The following illustrate the synthesis of various compounds of the Formulae
(I)-(I-EE). Other
compounds of this invention may be prepared using the methods illustrated in
these Examples,
either alone or in combination with techniques generally known in the art.
Example 1
0f
~~ N
O O )CCra
N N
H
7-(6-methoxypyridin-3-yl)-3-[(2-morpholin-4-ylethyl)amino]-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one.
Step 1: Preparation of tert-butyl 6-chloropyridin-3-yicarbamate.
A solution of 5-amino-2-chloropyridine (30.94 g, 236 mmol) and di-tert-
butyldicarbonate
(65.36 g, 299 mmol) in 1,4-dioxane (300 mL) was stirred at reflux for 20
hours. Additional di-tert-
59
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
butyidicarbonate (8.30 g, 38 mmol) was added and the reaction was stirred at
reflux for 7 hours.
The reaction was cooled to room temperature and poured into water. The layers
were separated
and the aqueous layer was extracted with ethyl acetate. The combined organic
layers were
washed with brine, dried over magnesium sulfate, and solvent was removed at
reduced pressure
to give a brown oil. The oil was triturated with diethyl ether and filtered to
give tert-butyl 6-
chloropyridin-3-ylcarbamate as a tan solid. (49.84 g, 92% yield). 'H NMR
(CDCI3) b 8.24 (m,
1 H), 7.96 (1 H), 7.27 (1 H), 6.65 (1 H), 1.51 (9H).
Step 2: Preparation of tert-butyl 6-chloro-4-fluoropyridin-3-ylcarbamate.
F
H
OyN ~
~ 0 ~ ~
N CI
To a-63 C solution of tert-butyl 6-chloropyridin-3-ylcarbamate (24.99 g, 109.3
mmol) and
TMEDA (39 mL, 260.0 mmol) in diethyl ether (700 mL) was added a 1.6M n-butyl
lithium solution
in hexane (193 mL, 308.8 mmol) over a period of 30 minutes while maintaining
the temperature
of the reaction at -60 to -50 C. The reaction was stirred at -60 C for an
additional 10 minutes
after the addition was complete then warmed to -10 C and stirred at -25 to -
10 C for 2.0 hours.
The reaction was cooled to -60 C and a solution of N-fluorobenzenesulfonimide
(53.49 g, 169.6
15-mmel)-in tetrahydrofu"rari (155-mQ-Was ad"ded'while keeping-the temperature
below -50 C. It
precipitated on addition and stirring became difficult. The reaction was then
allowed to slowly
warm to 0 C over 1 hour. The reaction was quenched with saturated ammonium
chloride solution
(400 mL). The layers were separated and the aqueous layer was extracted with
ethyl acetate (2 x
250 mL). The combined organic layers were washed with brine, dried over
magnesium sulfate,
and solvent was removed at reduced pressure to give an oily brown solid. The
material was
passed through a column of silica gel with 20% ethyl acetate/ hexane. The 6-
chloro-4-
fluoropyridin-3-ylcarbamate was obtained as a yellow solid. (15.88 g, 59%
yield). 'H NMR
(CDCI3) b 9.09 (1 H), 7.12 (1 H), 6.55 (1 H), 1.54 (s, 9H).
Step 3: Preparation of tert-butyl 6-chloro-4-f(2-propoxyethyl)aminolpyridin-3-
ylcarbamate.
O
INH
H
>rOYN
0 (
N CI
A solution of tert-butyl 6-chloro-4-fluoropyridin-3-ylcarbamate (11.96 g, 48.5
mmol) and 2-n-
propoxyethylamine (11.8 mL, 97.2 mmol) in ethanol (120 mL) was stirred at
reflux for 22 hours.
The reaction was cooled to room temperature and solvent was removed at reduced
pressure to
give a yellow solid which was triturated with diethyl ether and filtered to
give 6-chloro-4-[(2-
propoxyethyl)amino]pyridin-3-ylcarbamate as a white solid. (13.08 g, 82%
yield). 'H NMR
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
(CDCI3) S 7.92 (1 H), 6.54 (1 H), 5.77 (1 H), 5.11 (1 H), 3.65 (2H), 3.44
(2H), 3.34-3.29 (2H), 1.65-
1.56 (2H), 1.49 (9H), 0.94 (3H).
Step 4: Preparation of 6-ch!oro-N4-(2-propoxyethyl)pyridine-3,4-diamine.
O
HN ~ CI
~ /N
H2N
A solution of tert-butyl 6-ch!oro-4-[(2-propoxyethyl)amino]pyridin-3-
y!carbamate (7.08 g, 21.4
mmol) in 1,4-dioxane (20 mL) was treated with 4N HCI in 1,4-dioxane (100 mL)
and stirred at
room temperature for one hour. The reaction was partitioned between ethyl
acetate and
saturated sodium bicarbonate solution. The layers were separated and the
aqueous layer was
extracted with ethyl acetate. The combined organic layers were washed with
brine, dried over
magnesium sulfate, and solvent was removed at reduced pressure to give 6-
ch!oro-N4-(2-
propoxyethyl)pyridine-3,4-diamine as a brown oil. (4.93 g, 100% yield). 1H NMR
(CDCI3) 8 7.63
(1 H), 6.45 (1 H), 4.67 (1 H), 3.67 (2H), 3.43 (2H), 3.32-3.27 (2H), 2.92
(2H), 1.64-1.55 (2H), 0.93
(3H).
Step 5: Preparation of 3,7-dich!oro-1-(2-propoxyethy!)pyr!do[3,4-blpyrazin-
2(1H)-one.
0J"'
~
O N CI
CI~N N
A 0 C solution of 6-ch!oro-N4-(2-propoxyethyl)pyridine-3,4-diamine (2.80 g,
12.2 mmol) and
diisopropylethylamine (4.6 mL, 25.7 mmol) in dichloromethane (100 mL) was
treated with methyl
ch!orooxoacetate(1.1 mL, 11.7 mmol), allowed to warm to room temperature and
stirred for four
hours. The reaction was diluted with dichloromethane and washed with saturated
sodium
bicarbonate solution, dried over magnesium sulfate, and solvent was removed at
reduced
pressure. The residue was dissolved in toluene (30 mL) and heated at 105 C for
four hours. The
solvent was removed at reduced pressure and the resulting solid taken up in
dichloromethane
(100 mL) and treated with oxalyl chloride (2.1 mL, 24.1 mmol) and DMF (3
drops). The reaction
was stirred at room temperature for 6 hours. The solvent was removed at
reduced pressure to
give a brown solid. This was passed through a column of silica gel with 70%
ethyl acetate/
hexane to give 3,7-dich!oro-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-one
as a white solid.
(2.44 g, 66% yield). 1 H NMR (CDCI3) b 8.78 (1 H), 7.59 (1 H), 4.40 (2H), 3.80
(2H), 3.35 (2H),
1.52-1.46 (2H), 0.82 (3H).
61
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Step 6: Preparation of 7-chloro-3-f(2-morpholin-4-ylethyl)aminol-l-(2-
propoxyethyl)pyridof3,4-
blpyrazin-2(1 H)-one.
)-10
~
O N CI
N,,_~ N
N N
H
A solution of 3,7-dichloro-1 -(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-one
(193 mg, 0.64
mmol), 4-(2-aminoethyl)morpholine (118 mg, 0.91 mmol) and triethylamine (0.15
mL, 1.07 mmol)
in THF (3 mL) was stirred at room temperature for one hour. The reaction was
partitioned
between ethyl acetate and water. The layers were separated and the aqueous
layer was
extracted with ethyl acetate. The combined organic layers were washed with
brine, dried over
magnesium sulfate, and solvent was removed at reduced pressure to give a brown
oil. This was
passed through a column of silica gel with 80-100% ethyl acetate/ hexane to
give 7-chloro-3-[(2-
morpholin-4-ylethyl)amino]-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one as
a pink oil. (183
mg, 72% yield). 1H NMR (CDCI3) b 8.49 (1 H), 7.36 (1 H), 6.93 (1 H), 4.36
(2H), 3.77-3.72 (6H),
- - - ----3:64 3.-58-(2H), 3.36 (2H),--2:66-(2H), 2.-53-2.50 (-H)-, 1:54-1.47
(2H); 0.83 (3H).
Step 7: Preparation of 7-(6-methoxypyridin-3-yl)-3-f(2-morpholin-4-
ylethyl)aminol-l-(2-
propoxyethyl)pyridof3,4-blpyrazin-2(1 H)-one.
0
~ oll,
cx
N N
H
A solution of 7-chloro-3-[(2-morpholin-4-ylethyl)amino]-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one (72 mg, 0.18 mmol) in 1,4-dioxane (2.5 mL) was treated
with"
tetrakis(triphenylphosphine) palladium(0) (19 mg, 0.016 mmol) and stirred at
room temperature
for five minutes. A warm solution of 2-methoxy-5-pyridineboronic acid (41 mg,
0.27 mmol) in
ethanol (0.5 mL) and 2.0 M aqueous sodium carbonate 1.5 mL) were added. The
mixture was
refluxed for 2.0 hours, filtered hot through celite and the filtrate was
concentrated under reduced
pressure. The residue was partitioned between ethyl acetate and water, and the
Cayers were
separated. The aqueous layer was extracted with ethyl acetate. The combined
organic layers
were washed with brine, dried over magnesium sulfate, concentrated under
reduced pressure,
and passed through a column of silica gel with 2% methanol/ dichloromethane.
Fractions were
concentrated at reduced pressure and triturated with diethyl ether. The 7-(6-
methoxypyridin-3-yl)-
3-[(2-morpholin-4-ylethyl)amino]-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-
one was obtained
as a pink powder. (47 mg, 47% yield).
62
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
'H NMR (CDCI3) b 8.78 (1 H), 8.74 (1 H), 8.23 (1 H), 7.66 (1 H), 6.92 (1 H),
6.86 (1 H), 4.46 (2H),
4.00 (3H), 3.80 (2H), 3.76-3.73 (4H), 3.69-3.63 (2H), 3.37 (2H), 2.68 (2H),
2.55-2.52 (4H), 1.53-
1.46 (2H), 0.77 (3H); HRMS m/z469.2559 (calcd for M+H, 469.2558).
Example 2
0
0~1
O N N
N~N \ N
N
~H
/
7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-[(pyridin-2-
ylmethyl)amino]pyrido[3,4-b]pyrazin-
2(1 H)-one.
Prepared as described in example 1 using 2-(aminomethyl)pyridine in step 6.
' H NMR (CDCI3) b 8.83 (1 H), 8.76 (1 H), 8.62 (1 H), 8.25 (1 H), 7.73-7.67
(2H), 7.61-7.59 (1 H),
7.40 (1 H), 7.24-7.22 (1 H), 6.87 (1 H), 4.88 (2H), 4.49 (2H), 4.01 (3H), 3.82
(2H), 3.37 (2H), 1.53-
1.46 (2H), 0.77 (3H); HRMS m/z447.2129 (calcd for M+H, 447.2134).
- -- ---Example 3- _ . _ .__. - ---- -_,_ .-- - -
oJI"
I - oNI
xx
~
7-(6-methoxypyridin-3-y1)-1-(2-propoxyethyl)-3-[(tetrahydrofuran-2-
ylmethyl)amino]pyrido[3,4-
b]pyrazin-2(1 H)-one.
Prepared as described in example 1 using tetrahydrofurfurylamine in step 6.
'H NMR (CDCI3) b 8.77 (1H), 8.74 (1H), 8.23 (1H), 7.66 (1H), 6.85 (1H), 6.76-
6.72 (1H), 4.45
(2H), 4.22-4.14 (1 H), 4.00 (3H), 3.97-3.90 (1 H), 3.84-3.76 (4H), 3.59-3.50
(1 H), 3.36 (2H), 2.10-
2.02 (1 H), 1.99-1.89 (2H), 1.73-1.63 (1 H), 1.55-1.43 (2H), 0.77 (3H); HRMS
m/z 440.2272 (calcd
for M+H, 440.2292).
Example 4
63
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
O1~,
~;c / i O\O N \ N
~N\ NN N
II H
ri
N
1-(2-isopropoxyethyl)-7-(6-methoxypyridin-3-yl)-3-{[(5-methy/pyrazin-2-
yl)methyl]amino)pyrido[3,4-b]pyrazin-2(1 H)-one.
Prepared as described in example 1 using 2-isopropoxyethylamine in step 3 and
(5-
m ethyl pyrazin-2-yl)m ethylam ine in step 6.
'H NMR (CDCI3) 8 8.80 (1 H), 8.75 (1 H), 8.60 (1 H), 8.44 (1 H), 8.25 (1 H),
7.71 (1 H), 7.33-7.29
(1 H), 6.86 (1 H), 4.88 (2H), 4.44 (2H), 4.00 (3H), 3.79 (2H), 3.56-3.50 (1
H), 2.57 (3H), 1.77 (3H);
HRMS m/z462.2253 (calcd for M+H, 462.2248).
Example 5
O~
0~1
O N N ---
NN N
H
N
7-(6-methoxypyridin-3-yl)-3-{[(5-methylpyrazin-2-yl)methyl]amino)-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one.
Prepared as described in example 1 using (5-methylpyrazin-2-yl)methylamine in
step 6.
'H NMR (CDCI3) b 8.79 (1 H), 8.74 (1 H), 8.60 (1 H), 8.43 (1 H), 8.22 (1 H),
7.67 (1 H), 7.33-7.29
(1 H), 6.84 (1 H), 4.88 (2H), 4.46 (2H), 3.99 (3H), 3.80 (2H), 3.36 (2H), 2.56
(3H), 1.52-1.46 (2H),
0.76 (3H); HRMS m/z462.2218 (calcd for M+H, 462.2248).
Example 6
O' \ I
I u
O N N
N N \ N
OH
7 -(2-isopropoxyethyl)-7-(6-methoxypyridin-3-yl)-3-[(tetrahydro-2H-pyran-4-
ylmethyl)amino]pyrido[3,4-b]pyrazin-2(1 H)-one.
Prepared as described in example 1 using 2-isopropoxyethylamine in step 3 and
tetrahydro-
2H-pyran-4-ylmethylamine in step 6.
64
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
' H NMR (CDCI3) S 8.80 (1 H), 8.76 (1 H), 8.26 (1 H), 7.70 (1 H), 6.87 (1 H),
6.53-6.49 (1 H), 4.44
(2H), 4.04-3.99 (5H), 3.80 (2H), 3.60-3.46 (3H), 3.42-3.38 (2H), 2.01-1.95
(1H), 1.76-1.72 (2H),
1.52-1.38 (2H), 1.09 (6H); HRMS m/z454.2451 (calcd for M+H, 454.2449).
Example 7
), O
~ O"
O N ,' .N
HN1-N ~N
r:~
Oly N
tert-butyl 4-({[7-(6-meth oxypyridin-3-yl)-2-oxo-1-(2-propoxyethyl)-1,2-
dihydropyrido[3, 4-b]pyrazin-
3-yl]amino]methyl)piperidine-1-carboxylate.
Prepared as described in example 1 using tert-butyl 4-(aminomethyl)piperidine-
l-carboxylate
in step 6.
' H NMR (CDCI3) b 8.80 (1 H), 8.75 (1 H), 8.24 (1 H), 7.68 (1 H), 6.87 (1 H),
6.53-6.49 (1 H); 4.47
(2H), 4.16-4.12 (2H), 4.01 (3H), 3.81 (2H), 3.50 (2H), 3.38 (2H), 2.74-2.69
(2H), 1.90-1.78 (3H),
1.54-1.49 (2H), 1.47 (9H), 1.29-1.24 (2H), 1.09 (6H); HRMS m/z 553.3145 (calcd
for M+H,
553.3133).
Example 8
o~
O\
I
ON N
y
r N \N
HNI~H
7-(6-methoxypyridin-3-yl)-3-[(piperidin-4-ylmethyl)amino]-1-(2-
propoxyethyl)pyrido(3,4-b]pyrazin-
2(1 H)-one.
A solution of tert-butyl 4-({[7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-
propoxyethyl)-1,2-
dihydropyrido[3,4-b]pyrazin-3-yl]amino}methyl)piperidine-1-carboxylate from
example 10 (60 mg,
0.11 mmol) in 4N HCI in 1,4-dioxane (10 mL) was stirred at room temperature
for two hours. The
solvent was removed at reduced pressure and the residue was recrystallized
from methanol/ethyl
acetate to give 7-(6-methoxypyridin-3-yl)-3-[(piperidin-4-ylmethyl)amino]-1-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-one as a white solid. (18 mg, 34%
yield). 'H NMR
(CD3OD) 5 8.73 (1 H), 8.72 (1 H), 8.24 (1 H), 8.22 (1 H), 7.13 (1 H), 4.68
(2H), 4.06 (3H), 3.85 (2H),
3.54 (2H), 3.46-3.36 (4H), 3.05-2.96 (2H), 2.18 (1H), 2.08-2.03 (2H),1.61-
1.53~2H), 1.49-1.40
(2H), 0.72 (3H); HRMS m/z453.2638 (calcd for M+H, 453.2609).
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Example 9
o 'f~ I
I
I ~ o
O N N
N" N N
\ ~l H
p-N
7-(6-methoxypyridin-3-yl)-3-{((5-methylisoxazol-3-yl)methyl]amino]-1-(2-
propoxyethyl)pyridol3,4-
6]pyrazin-2(1 H)-one.
Prepared as described in example 1 using (5-methylisoxazol-3-yl)methylamine in
step 6.
'H NMR (CDCI3) b 8.83 (1 H), 8.76 (1 H), 8.25 (1 H), 7.70 (1 H), 6.87-6.84 (1
H), 6.07 (1 H), 4.82
(2H), 4.46 (2H), 4.01 (3H), 3.81 (2H), 3.37 (2H), 2.42 (3H), 1.53-1.46 (2H),
0.78 (3H); HRMS m/z
451.2057 (calcd for M+H, 451.2088).
Example 10
0
I
o
O N N
N~\H N N
NJ
3-{(3-(1 H-imidazol-1-yl)propyl]amin o]-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido(3, 4-
b]pyrazin-2(1 H)-one.
Prepared as described in example 1 using 3-(1H-imidazol-1-yl)propylamine in
step 6.
'H NMR (CDCI3) 8 8.80 (1 H), 8.75 (1 H), 8.25 (1 H), 7.68 (s, 1 H), 7.58 (1
H), 7.11(1 H), 7.01 (1 H),
6.87 (1 H), 6.50-6.46 (1 H), 4.47 (2H), 4.11(2H), 4.01 (3H), 3.81 (2H), 3.65-
3.58 (2H), 3.38 (2H),
2.25-2.21 (2H), 1.53-1.46 (2H), 0.78 (3H); HRMS m/z464.2384 (calcd for M+H,
464.2405).
Example 11
o'r
N'
O~1 O Nr / I \ 1N
N
H
3-[(2-morpholin-4-ylethyl)amino]-1-(2-propoxyethyl)-7-pyrimidin-5-ylpyrido[3,4-
b]pyrazin-2(1 H)-
one.
Prepared as described in example 1 using pyrimidine-5-boronic acid in step 7.
' H NMR (CDCI3) b 9.29 (2H), 9.18 (1 H), 8.78 (1 H), 7.78 (1 H), 7.02-7.00 (1
H), 4.44 (2H), 3.79
(2H), 3.72-3.69 (4H), 3.66-3.60 (2H), 3.32 (2H), 2.64 (2H), 2.51-2.48 (4H),
1.47-1.40 (2H), 0.71
(3H); HRMS m/z440.2427 (calcd for M+H, 440.2405).
66
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Example 12
of~
I r I
\ N
ON Xll~N /
r N N
OI~H
7-(6-methoxypyridin-3-y1)-1-(2-propoxyethyl)-3-((tetrahydro-2H-pyran-4-
ylmethyl)amino]pyrido(3,4-b]pyrazin-2(1 H)-one.
Prepared as described in example 1 using tetrahydro-2H-pyran-4-ylmethylamine
in step 6.
' H NMR (CDCI3) b 8.80 (1 H), 8.74 (1 H), 8.24 (1 H), 7.67 (1 H), 6.86 (1 H),
6.53-6.49 (1 H), 4.46
(2H), 4.04-4.00 (5H), 3.80 (2H), 3.52-3.45 (2H), 3.42-3.35 (4H), 2.00-1.94 (1
H), 1.76-1.72 (2H),
1.53-1.39 (4H), 0.78 (3H); HRMS m/z454.2401 (calcd for M+H, 454.2449).
Example 13
of~
I
f i I O~
O~ O IN I N
~1N N " _N N
Y"'-H
O
3-(2-morpholino-2-oxoethylamino)-7-(6-m ethoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-morpholin-4-yl-2-oxoethylamine in
step 6.
'H NMR (CDCI3) b 8.77 (1 H), 8.75 (1 H), 8.22 (1 H), 7.68 (1 H), 7.38 (1 H),
6.85 (1 H), 4.46 (2H),
4.33 (2H), 4.00 (3H), 3.81-3.71 (8H), 3.58-3.56 (2H), 3.35 (2H), 1.50-1.45
(2H), 0.78 (3H);
HRMS m/z483.2348 (calcd for M+H, 483.2350).
Example 14
o-1-
o
O N I N
HN" 'N IN
O
7-(6-mefhoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(((2S)-tetrahydrofuran-2-
ylmethyl]amino)pyrido[3,4-b]pyrazin-2(1 H)-one
Prepared as described in example 1 using (S)-(+)-tetrahydrofurfurylamine in
step 6.
' H NMR (CDCI3) b 8.75 (1 H), 8.72 (1 H), 8.20 (1 H), 7.64 (1 H), 6.83 (1 H),
6.75-6.71 (1 H), 4.44
(2H), 4.18-4.15 (1 H), 3.98 (3H), 3.96-3.89 (1 H), 3.83-3.75 (4H), 3.58-3.50
(1 H), 3.35 (2H), 2.07-
67
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
2.01 (1 H), 1.95-1.91 (2H), 1.69-1.62 (1 H), 1.51-1.44 (2H), 0.76 (3H); HRMS
m/z440.2299 (calcd
for M+H, 440.2292).
Example 15
0
I "" 0~1
O N / \ N
O N~ N \ N
~H
7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-{((2R)-tetrahydrofuran-2-
ylmethyl]amino)pyrido[3, 4-b]pyrazin-2(1 H)-one.
Prepared as described in example 1 using (R)-(-)-tetrahydrofurfurylamine in
step 6.
' H NMR (CDCI3) b 8.78 (1 H), 8.74 (1 H), 8.24 (1 H), 7.67 (1 H), 6.86 (1 H),
6.76-6.72 (1 H), 4.46
(2H), 4.20-4.16 (1 H), 4.00 (3H), 3.98-3.90 (1 H), 3.87-3.77 (4H), 3.60-3.51
(1 H), 3.36 (2H), 2.11-
2.03 (1 H), 2.00-1.90 (2H), 1.73-1.62 (1 H), 1.55-1.43 (2H), 0.77 (3H); HRMS
m/z 440.2306 (calcd
for M+H, 440.2292).
Example 16
of'*'
0 ? ~ i O~1
N O N N
~N" 'N N
H
ethyl 4-{[7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyethyl)-1,2-
dihydropyrido[3,4-b]pyrazin-3-
yl]amino}piperidine-l-carboxylate.
Prepared as described in example 1 using ethyl 4-am inopiperidine-1 -
carboxylate in step 6.
'H NMR (CDCI3) b 8.79 (1 H), 8.74 (1 H), 8.24 (1 H), 7.68 (1 H), 6.87 (1 H),
6.35 (1 H), 4.46 2H),
4.20-4.12 (5H), 4.01 (3H), 3.80 (2H), 3.37 (2H), 3.11-3.07 (2H), 2.15-2.11
(2H), 1.53-1.46 (4H),
1.28 (3H), 0.78 (3H); HRMS m/z511.2686 (calcd for M+H, 511.2663).
Example 17
68
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
O~
O~1
I I
HNO N N
N" 'N \ N
H
7-(6-methoxypyridin-3-yl)-3-(piperidin-4-ylamino)-1-(2-propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-
one.
Prepared as described in example 1 using 4-aminopiperidine in step 6.
iH NMR (CDCI3) b 8.74 (2H), 8.22 (1H), 7.60 (1H), 6.84 (1H), 4.84-4.80 (2H),
4.40 (2H), 3.98
(3H), 3.77 (2H), 3.35 (2H), 3.13-3.04 (2H), 2.99 (1 H), 1.98-1.94 (2H), 1.54-
1.45 (4H), 0.77 (3H);
HRMS m/z439.2455 (calcd for M+H, 439.2452).
Example 18
0
0 ~
~N O N N
~N" 'N \ N
H
3-((1-acetylpiperidin-4-yl)amino]-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3, 4-
b]pyrazin-2(1 H)-one.
A solution of 7-(6-methoxypyridin-3-yl)-3-(piperidin-4-ylamino)-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one from example 28 (72 mg, 0.16 mmol), acetyl chloride (27
mg, 0.34 mmol)
and triethylamine (0.05 mL, 0.36 mmol) in dichloromethane (2 mL) was stirred
at room
temperature for 15 hours. The reaction was partitioned between ethyl acetate
and water. The
layers were separated and the aqueous layer was extracted with ethyl acetate.
The combined
organic layers were washed with brine, dried over magnesium sulfate, solvent
was removed at
reduced pressure to give and passed through a column of silica gel with 2.5%
methanol/
dichloromethane to give 3-[(1-acetylpiperidin-4-yl)amino]-7-(6-methoxypyridin-
3-yl)-1-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one as a yellow solid. (15 mg, 18%
yield).
'H NMR (CDCI3) b 8.77-8.76 (2H), 8.29 (1 H), 7.65 (1 H), 6.87 (1 H), 5.38-5.35
(1 H), 4.87-4.82
(2H), 4.43 (2H), 4.11-4.07 (1 H), 4.01 (3H), 3.79 (2H), 3.37 (2H), 3.18-3.10
(2H), 2.12-2.09 (2H),
2.04 (3H), 1.53-1.44 (4H), 0.78 (3H); HRMS m/z481.2538 (calcd for M+H,
481.2558).
Example 19
69
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
~
O
O\
O N N
NXN N
H
7-(6-methoxypyridin-3-yl)-3-[(2-piperidin-1-ylethyl)amino]-1-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-
2(1 H)-one.
Prepared as described in example 1 using 2-piperidin-1-ylethylamine in step 6.
iH NMR (CDCI3) b 8.77 (1 H), 8.73 (1 H), 8.22 (1 H), 7.64 (1 H), 7.00-6.97 (1
H), 6.84 (1 H), 4.44
(2H), 3.99 (3H), 3.79 (2H), 3.64-3.59 (2H), 3.36 (2H), 2.61 (2H), 2.44 (4H),
1.62-1.56 (4H), 1.52-
1.42 (4H), 0.76 (3H); HRMS m/z467.2762 (calcd for M+H, 467.2765).
Example 20
0f
\ O\
I N
O N /N
NHN \N
7-(6-methoxypyridin-3-y1)-1-(2-prc; yoxyethyl)-3-[(2-pyridin-2-
ylethyl)amino]pyrido[3,4-b]pyrazin-
2(1H)-one.
Prepared as described in example 1 using 2-(2-aminoethyl)pyridine in step 6.
'H NMR (CDCI3) b 8.80 (s, 1 H), 8.74 (1 H), 8.62-8.60 (1 H), 8.26 (1 H), 7.69-
7.63 (2H), 7.24-7.15
(3H), 6.86 (1 H), 4.45 (2H), 4.04-3.98 (5H), 3.79 (2H), 3.36 (2H), 3.22 (2H),
1.52-1.45 (2H), 0.77
(3H); HRMS m/z461.2346 (calcd for M+H, 461.2296).
Example 21
o~~
OMe
N~ O N N
\~lN' N1 N N
I0 I H
3-(2-(4-ethylpiperazin-1-yl)-2-oxoethylamino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-amino-l-(4-ethylpiperazin-1-
yl)ethanone in step
6. The Suzuki reaction of step 7 in example 1 was performed using the
following modified
procedure. A solution of 3-(2-(4-ethylpiperazin-1-yl)-2-oxoethylamino)-7-
chloro-l-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one (306 mg, 0.70 mmol) in 1,4-dioxane
(5.0 mL) was
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
treated with dichloro[1,1'-bis(diphenylphosphino)ferrocene]palladium (II)
dichloromethane adduct
(54 mg, 0.066 mmol), 6-methoxypyridin-3-yl-3-boronic acid (250 mg, 1.6 mmol)
and 2.5 mL of
water containing Na2CO3 (264 mg, 2.5 mmol). The mixture was refluxed for 4.0
hours. The
mixture was partitioned between ethyl acetate and water, and the layers were
separated. The
aqueous layer was extracted with ethyl acetate. The combined organic layer
were dried over
sodium sulfate, concentrated under reduced pressure, and purified by column
chromatography.
3-(2-(4-ethylpiperazin-1 -yl)-2-oxoethylamino)-7-(6-methoxypyridin-3-yl)-1 -(2-
propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-one was obtained. (177 mg, 48%
yield).Prepared as
described in example 1 using in step 6 and the Suzuki conditions of example 47
step 7.
1H NMR (CDCI3) 58.79 (1 H), 8.75 (1 H), 8.24 (1 H), 7.68 (1 H), 7.42 (1 H),
6.84 (1 H), 4.47 (t, 2H, J
= 5.3 Hz), 4.33 (2H), 4.00 (3H), 3.80 (2H), 3.75 (2H), 3.58 (2H), 3.36 (2H),
2.54 - 2.43 (6H), 1.52
-1.45 (2H), 1.47 (3H), 0.77 (3H); HRMS m/z 510.2837 (calcd for M+H, 510.2823).
Example 22
O OMe
N~ O N N 11
~IN N11N N
~H
ethyl4-(2-(1,2-dihydro-7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-3-
y/amino)acetyl)piperazine-1-carboxylate
Prepared as described in example 1 using ethyl 4-(2-aminoacetyl)piperazine-l-
carboxylate in
step 6 and the Suzuki conditions of example 21 step 7.
yH NMR (CDCI3) 08.78 (1 H), 8.75 (1 H), 8.24 (1 H), 7.68 (1 H), 7.37 (1 H),
6.84 (1 H), 4.47 (2H),
4.35 (2H), 4.22 - 4.15 (2H), 4.00 (3H), 3.80 (2H), 3.72 - 3.68 (2H), 3.58 -
3.51 (7H), 3.36 (2H),
1.52 - 1.45 (2H), 1.31 - 1.25 (5H), 0.77 (3H); HRMS m/z 554.2738 (calcd for
M+H, 554.2722).
Example 23
OMe
r I
O N N
ON N~N N
~H
3-(2-oxo-2-(pyrrolidin-1-yl)ethylamino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-amino-l-(pyrrolidin-1-yl)ethanone
in step 6 and
the Suzuki conditions of example 21 step 7.
iH NMR (CDCI3) 08.78 (1 H), 8.75 (1 H), 8.24 (1 H), 7.68 (1 H), 7.37 (1 H),
6.84 (1 H), 4.46 (2H),
4.25 (2H), 4.00 (3H), 3.80 (2H), 3.60 - 3.53 (4H), 3.36 (2H), 2.08 - 2.01
(2H), 1.97 - 1.90 (2H),
1.52 -1.45 (2H), 0.77 (3H); HRMS m/z467.2394 (calcd for M+H, 467.2401).
Example 24
71
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
o~~
O
O~ O N ~ I o N
'~ NJ=N N
101 H
3-(2-morpholino-2-oxoethylamino)-7-(3,5-dimethylisoxazol-4-yl)-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1H)-one
Prepared as described in example 1 using 2-amino-l-morpholinoethanone in step
6 and and
the Suzuki conditions of example 21 step 7 with 3,5-dimethylisoxazol-4-yl-4-
boronic acid.
'H NMR (CDCI3) 08.78 (1 H), 7.41 (1 H), 7.34 (1 H), 4.41 (2H), 4.33 (2H), 3.99
- 3.97 (1 H), 3.81 -
3.73 (10H), 3.59 - 3.56 (2H), 3.43 (1 H), 3.34 (2H), 2.59 (3H), 2.44 (3H),
0.75 (3H); HRMS m/z
471.2374 (calcd for M+H, 471.2350).
Example 25
J / OMe
( I
O N N
HN N N
~/ ~H N
0
3-(2-oxo-2-(piperazin-1-yl)ethylamino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-amino-l-(piperazin-1-yl)ethanone in
step 6,and
the Suzuki conditions of example 21 step 7.
' H NMR (CDCI3) 08.78 - 8.77 (2H), 8.28 - 8.26 (1 H), 7.67 (1 H), 6.85 (1 H),
4.44 (2H), 4.01 - 3.99
(6H), 3.81 - 3.78 (4H), 3.54 (2H), 3.37 (2H), 1.70 (4H), 1.53 - 1.46 (2H),
1.27 - 1.25 (2H), 0.76
(3H); HRMS m/z 482.2541 (calcd for M+H, 482.2510).
Example 26
OMe
r I
O N ~ ~ N
Me ~ ~N
-~'H N
O
3-(2-(4-methylpiperazin-1-yl)-2-oxoethylamino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-amino-l-(4-methylpiperazin-1-
yl)ethanone in
step 6 and the Suzuki conditions of example 21 step 7.
'H NMR (CDCI3) 08.78 (1 H), 8.75 (1 H), 8.26 - 8.22 (1 H), 7.68 (1 H), 7.43 -
7.40 (1 H), 6.85 (1 H),
4.47 (2H), 4.33 (2H), 4.00 (3H), 3.80 (2H), 3.73 - 3.71 (2H), 3.58 (2H), 3.36
(2H), 2.51 - 2.43
(4H), 2.34 (3H), 1.52 - 1.45 (2H), 0.77 (3H); HRMS m/z496.2632 (calcd for M+H,
496.2667).
Example 27
72
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
O~/
OMe
( I
OI N N
HO~
N ~H NN
O
3-(2-(4-hydroxypiperidin-1-yl)-2-oxoethylamino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3,4-b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-amino-l-(4-hydroxypiperidin-1-
yl)ethanone in
step 6 and the Suzuki conditions of example 21 step 7.
iH NMR (CDCI3) b8.79 (1 H), 8.75 (1 H), 8.26 - 8.22 (1 H), 7.69 (1 H), 7.44 (1
H), 6.84 (1 H), 4.47
(2H), 4.33 (2H), 4.09 - 4.05 (1 H), 4.00 (3H), 3.82 - 3.78 (2H), 3.43 - 3.31
(3H), 3.17 -1.96 (1 H),
1.67 (6H), 1.55 - 1.42 (2H), 0.77 (3H); HRMS m/z 497.2543 (calcd for M+H,
497.2507).
Example 28
o~~
OMe
r I
ON N
C N' ~H ~N 1 N
o
3-(2-oxo-2-(piperidin-1-yl)ethylamino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-amino-l-(piperidin-1-yi)P.thanone
in step 6 and
the Suzuki conditions of example 21 step 7.
' H NMR (CDCI3) 08.79 (1 H), 8.75 (1 H), 8.26 - 8.22 (1 H), 7.68 (1 H), 7.48
(1 H), 6.85 (1 H), 4.46
(2H), 4.31 (2H), 4.00 (3H), 3.80 (2H), 3.66 - 3.63 (2H), 3.50 - 3.46 (2H),
3.38 - 3.34 (2H), 1.68 -
1.43 (8H), 0.77 (3H); HRMS m/z481.2574 (calcd for M+H, 481.2558).
Example 29
o/\
N O~
O Nf \ \ ~
~, N'-/-'N1 N I N
H
3-(2-morpholinoethylamino)-1-(2-isopropoxyethyl)-7-(6-methoxypyridin-3-
yl)pyrido[3,4-b]pyrazin-
2(1 H)-one
Prepared as described in example 1 using 2-isopropoxyethanamine in step 3.
iH NMR (CDCI3) b8.78 (1 H), 8.75 (1 H), 8.27 - 8.24 (1 H), 7.69 (1 H), 6.93 (1
H), 6.84 (1 H), 4.43
(2H), 3.99 (3H), 3.80 - 3.67 (8H), 3.57 - 3.51 (1 H), 2.70 (2H), 2.62 (4H),
1.06 (6H); HRMS m/z
469.2524 (calcd for M+H, 469.2558).
Example 30
73
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
O-t--I
N O~
O Nr \ \ I
N N (NN
l- H
3-((pyridin-2-y1)methylamino)-1-(2-isopropoxyethyl)-7-(6-methoxypyridin-3-
yl)pyrido[3,4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-isopropoxyethanamine in step 3 and
(pyridin-2-
yl)methanamine in step 6.
iH NMR (CDCI3) b8.82 (1 H), 8.76 (1 H), 8.62 - 8.61 (1 H), 8.30 - 8.27 (1 H),
7.72 - 7.67 (2H), 7.60
- 7.58 (1 H), 7.38 (1 H), 7.26 - 7.22 (1 H), 6.85 (1 H), 4.87 (2H), 4.45 (2H),
4.00 (3H), 3.80 (2H),
3.57 - 3.51 (1 H), 1.05 (6H); HRMS m/z 447.2120 (calcd for M+H, 447.2139).
Example 31
o' \
N O~
O IN I
0~,. O.
N N
H
3-(((S)-tetrahydrofuran-2-yl)methylamino)-1-(2-isopropoxyethyl)-7-(6-
methoxypyridin-3-
yl)pyrido[3,4-b]p_~, razin-2(1 H)-one
Prepared as described in example 1 using 2-isopropoxyethanamine in step 3 and
((S)-
tetrahydrofuran-2-yl)methanamine in step 6 and the Suzuki conditions of
example 21 step 7.
iH NMR (CDCI3) b8.84 (1 H), 8.76 (1 H), 8.26 - 8.22 (1 H), 7.69 (1 H), 6.84 (1
H), 6.74 (1 H), 4.42
(2H), 4.28 - 4.16 (1 H), 4.00 (3H), 3.97 - 3.90 (1 H), 3.85 - 3.72 (4H), 3.59 -
3.50 (2H), 2.10 -
1.94 (3H), 1.72 - 1.63 (1 H), 1.06 (6H); HRMS m/z 440.2270 (calcd for M+H,
440.2292).
Example 32
r
0
O~
~ U
0 Nf N N (NN
H
1-(3-ethoxypropyl)-7-(6-methoxypyridin-3-yl)-3-[(pyridin-2-
ylmethyl)amino]pyrido[3, 4-b]pyrazin-
2(1 H)-one
Prepared as described in example 1 using 3-ethoxypropan-l-amine in step 3 and
(pyridin-2-
yl)methanamine in step 6 and the Suzuki conditions of example 21 step 7.
' H NMR (CDCI3) 08.82 (1 H), 8.79 (1 H), 8.62 - 8.61 (1 H), 8.27 - 8.25 (1 H),
7.70 - 7.56 (3 H),
7.37 (1 H), 7.24 - 7.21 (1 H), 6.84 - 6.82 (1 H), 4.87 (2H), 4.40 (2H), 3.99
(3H), 3:52 - 3.45 (4H),
2.09 - 2.04 (2H), 1.24 - 1.19 (3H); HRMS m/z 447.2139 (calcd for M+H,
447.2139).
74
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Example 33
r
O
N O~
O N \ \ I
N N11 N N
JI NH
1-(3-ethoxypropyl)-7-(6-methoxypyridin-3-yl)-3-{[(5-methylpyrazin-2-
yl)methyl]amino)pyrido[3,4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 3-ethoxypropan-l-amine in step 3 and
(5-
methylpyrazin-2-yl)methanamine in step 6 and the Suzuki conditions of example
21 step 7.
' H NMR (CDCI3) b8.81 (1 H), 8.78 (1 H), 8.59 (1 H), 8.43 (1 H), 8.26 - 8.23
(1 H), 7.63 (1 H), 7.30
(1 H), 6.82 (1 H), 4.86 (2H), 4.39 (2H), 3.99 (3H), 3.52 - 3.45 (4H), 2.56
(3H), 2.16 - 2.02 (2H),
1.24 - 1.19 (3H); HRMS m/z 462.2270 (calcd for M+H, 462.2248).
Example 34
r
0
N o~
O N \ \ I
0,. N N I N
H
1-(3-ethoxypropyl)-7-(6-methoxypyridin-3-yl)-3-{[(2S)-tetrahydrofuran-2-
ylmethyl]amino)pyrido[3, 4-b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 3-ethoxypropan-l -am ine in step 3
and ((S)-
tetrahydrofuran-2-yl)methanamine in step 6 and the Suzuki conditions of
example 21 step 7.
'H NMR (CDCI3) 08.77 - 8.76 (2H), 8.25 - 8.22 (1 H), 7.61 (1 H), 6.82 (1 H),
6.76 - 6.74 (1 H), 4.37
(2H), 4.18 - 4.15 (1 H), 3.98 (3H), 3.95 - 3.89 (1 H), 3.84 - 3.76 (2H), 3.56 -
3.44 (4H), 2.08 -
2.02 (3H), 1.97 -1.91 (2H), 1.68 -1.65 (1 H), 1.23 -1.19 (4H); HRMS m/z
440.2282 (calcd for
M+H, 440.2292).
Example 35
I
O
OMe
O-') O IN N
~,N NX N ~N
~H
3-(2-morpholino-2-oxoethylamino)-1-(3-ethoxypropyl)-7-(6-methoxypyridin-3-
yl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-amino-l-morpholinoethanone in step
3 and 3-
methoxypropan-l-amine in step 6 and the Suzuki conditions of example 21 step
7.
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
iH NMR (CDCI3) 08.78 - 8.77 (2H), 8.23 (1 H), 7.62 (1 H), 7.39 (1 H), 6.82 (1
H), 4.38 (2H), 4.31
(2H), 3.98 (3H), 3.84 - 3.71 (6H), 3.57 - 3.55 (2H), 3.51 - 3.44 (4H), 2.16 -
2.01 (2H), 1.21 (3H);
HRMS m/z483.2376 (calcd for M+H, 483.2350).
Example 36
Oi
N
O
O ON
N~~N ~N I ~ N
H
7-(3,5-dimethylisoxazol-4-y1)-1-(2-ethoxyethyl)-3-[(2-morpholin-4-
ylethyl)amino]pyrido[3,4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and and
the Suzuki
conditions of example 21 step 7 with 3,5-dimethylisoxazol-4-yl-4-boronic acid.
' H NMR (CDCI3) 08.79 (1 H), 7.31 (1 H), 6.96 (1 H), 4.40 (2H), 3.81 - 3.68
(8H), 3.50 - 3.43 (3H),
2.64 (2H), 2.59 - 2.57 (6H), 2.44 (3H), 1.09 (3H); HRMS m/z443.2418 (calcd for
M+H,
443.2401).
Example 37
OJ
N O1~
O N(
0. NN N
H
3-(((S)-tetrahydrofuran-2-yl)methylamino)- 1-(2-ethoxyethyl)-7-(6-
methoxypyridin-3-yl)pyrido[3,4-
b]pyrazin-2(1H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and ((S)-
tetrahydrofuran-2-yi)methanamine in step 6 and the Suzuki conditions of
example 21 step 7.
iH NMR (CDCI3) b8.77 (1 H), 8.73 (1 H), 8.26 - 8.22 (1 H), 7.66 (1 H), 7.15 (1
H), 6.84 (1 H), 6.75
(1 H), 4.45 (2H), 4.21 - 4.14 (1 H), 3.99 (3H), 3.97 - 3.90 (1 H), 3.86 - 3.74
(4H), 3.59 - 3.39 (2H),
2.11 -1.89 (3H), 1.72 -1.64 (1 H), 1.14 (3H); HRMS m/z 426.2121 (calcd for
M+H, 426.2136).
Example 38
OJ
N O~
O Nr ~
N'~-NXN N
H
3-(2-(pyrrolidin-1-yl)ethylamino)-1-(2-ethoxyethyl)-7-(6-methoxypyridin-3-
yl)pyrido[3,4-b]pyrazin-
2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and 2-
(pyrrolidin-l-
yl)ethanamine in step 6 and the Suzuki conditions of example 21 step 7.
76
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
' H NMR (CDCI3) 08.78 (1 H), 8.74 (1 H), 8.25 (1 H), 7.66 (1 H), 7.00 (1 H),
6.84 (1 H), 4.45 (2H),
4.00 (3H), 3.82 - 3.76 (4H), 3.51 - 3.44 (2H), 2.93 (2H), 2.76 (4H), 1.89
(4H), 1.08 (3H); HRMS
m/z 439.2424 (calcd for M+H, 439.2452).
Example 39
OJ
N O1-1
0 Nf
ON"--- N11 N N
H
3-(2-(piperidin- y-yl)ethylamino)-1-(2-ethoxyethyl)-7-(6-methoxypyridin-3-
yl)pyrido[3,4-b]pyrazin-
2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and 2-
(piperidin-l-
yl)ethanamine in step 6 and the Suzuki conditions of example 21 step 7.
'H NMR (CDCI3) 08.78 (1 H), 8.75 (1 H), 8.25 (1 H), 7.66 (1 H), 7.26 (1 H),
6.85 (1 H), 4.45 (2H),
4.00 (3H), 3.80 (2H), 3.66 (2H), 3.48 (2H), 2.68 (2H), 2.51 (4H), 1.65 (4H),
1.48 (2H), 1.12 (3H);
HRMS m/z453.2642 (calcd for M+H, 453.2609).
Example 40
O~
-i
O~1 O N
~N'/~N~N N
H
3-(2-morpholinoethylamino)-1-(2-ethoxyethyl)-7-(pyridin-3-yl)pyrido[3,4-
b]pyrazin-2(1H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with pyridin-3-yl-3-boronic acid.
'H NMR (CDCI3) 09.20 - 9.19 (1 H), 8.83 (1 H), 8.64 - 8.62 (1 H), 8.37 - 8.33
(1 H), 7.79 (1 H), 7.43
- 7.38 (1 H), 7.00 (1 H), 4.49 (2H), 3.84 - 3.66 (8H), 3.48 (2H), 2.71 (2H),
2.56 (4H), 1.12 (3H);
HRMS m/z425.2303 (calcd for M+H, 425.2296).
Example 41
OJ
OMe
r I
O~\ O N N
N N~N iN
~H
3-(2-morpholino-2-oxoethylamino)- i -(2-ethoxyethyl)-7-(6-methoxypyridin-3-
yl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and 2-
amino-l-
morpholinoethanone in step 6 and the Suzuki conditions of example 21 step 7.
77
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
' H NMR (CDCI3) 08.78 - 8.74 (2H), 8.28 - 8.25 (1 H), 7.69 (1 H), 7.40 (1 H),
6.85 (1 H), 4.46 (2H),
4.32 (2H), 4.00 (3H), 3.82 - 3.72 (8H), 3.58 (2H), 3.47 (2H), 1.11 (3H); HRMS
m/z469.2183
(calcd for M+H, 469.2194).
Example 42
oJ
OMe
f I
O N N
N~ N~N N
J NH
3-((5-methylpyrazin-2-yl)methylamino)- 1-(2-ethoxyethyl)-7-(6-methoxypyridin-3-
yl)pyrido[3,4-
b]pyrazin-2(1H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and (5-
methylpyrazin-2-yl)methanamine in step 6 and the Suzuki conditions of example
21 step 7.
'H NMR (CDCI3) 08.82 (1 H), 8.75 - 8.74 (1 H), 8.60 (1 H), 8.44 (1 H), 8.30 -
8.27 (1 H), 7.70 (1 H),
7.32 (1 H), 6.86 (1 H), 4.88 (2H), 4.47 (2H), 4.00 (3H), 3.81 (2H), 3.48 (2H),
2.58 (3H), 1.11 (3H);
HRMS m/z448.2050 (calcd for M+H, 448.2092).
Example 43
oJ
OMe
f
0 N N
I ,N
N'~-, H N
/
3-((pyridin-2-yl)methylamino)-1-(2-ethoxyethyl)-7-(6-methoxypyridin-3-
yl)pyrido[3,4-b]pyrazin-
2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and
(pyridin-2-
yl)methanamine in step 6 and the Suzuki conditions of example 21 step 7.
'H NMR (CDCI3) 08.83 (1 H), 8.75 (1 H), 8.61 (1 H), 8.29 (1 H), 7.74 - 7.68
{2H), 7.61 (1 H), 7.39
(1 H), 7.25 - 7.22 (1 H), 6.86 (1 H), 4.88 (2H), 4.48 (2H), 4.00 (3H), 3.82
(2H), 3.48 (2H), 1.12
(3H); HRMS m/z433.2018 (calcd for M+H, 433.1983).
Example 44
OJ
OMe
f
O O N N
N~'N~N I N
H
3-(2-morpholinoethylamino)-1-(2-ethoxyethyl)-7-(6-m ethoxypyridin-3-
yl)pyrido[3, 4-b]pyrazin-
2(1H)-one
78
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7.
' H NMR (CDCI3) 08.78 (1 H), 8.74 (1 H), 8.26 (1 H), 7.67 (1 H), 6.97 (1 H),
6.85 (1 H), 4.46 (2H),
4.00 (3H), 3.82 - 3.72 (8H), 3.48 (2H), 2.75 (2H), 2.60 (4H), 1.12 (3H); HRMS
m/z455.2378
(calcd for M+H, 455.2401).
Example 45
JI",
0
~ / F
o:xxr
3-(2-morpholinoethylamino)-7-(4-fluorophenyl)- 1-(2-propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 and the Suzuki conditions of example 21
step 7 with 4-
fluorophenylboronic acid.
1 H NMR (CDCI3) b 8.78 (1 H), 7.99-7.94 (2H), 7.68 (1 H), 7.17-7.11 (2H), 6.93-
6.90 (1 H), 4.46
(2H), 3.80 (2H), 3.76-3.72 (4H), 3.68-3.62 (2H), 3.37 (2H), 2.67 (2H), 2.54-
2.51 (4H), 1.524.45
(2H), 0.78 (3H); HRMS m/z456.2407 (calcd for M+H, 456.2405).
Example 46
Jr
0
o
0 N O >
o I
N'-~NN \ N
H
7-(1, 3-benzodioxol-5-yl)-3-[(2-morpholin-4-ylethyl)amino]-1-(2-
propoxyethyl)pyrido[3, 4-b]pyrazin-
2(1 H)-one.
Prepared as described in example 1 and the Suzuki conditions of example 21
step 7using
3,4-(methylenedioxy)phenylboronic acid.
'H NMR (CDCI3) b 8.75 (1H), 7.62 (1H), 7.51-7.47 (2H), 6.90-6.87 (2H), 6.01
(2H), 4.46 (2H),
3.80 (2H), 3.76-3.72 (4H), 3.68-3.62 (2H), 3.37 (2H), 2.67 (2H), 2.54-2.51
(4H), 1.54-1.47 (2H),
0.79 (3H); HRMS m/z482.2354 (calcd for M+H, 482.2398).
Example 47
oJ
ON,
oXcc
H
79
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
3-(2-morpholinoethylamino)-1-(2-ethoxyethyl)-7-(4-methoxyphenyl)pyrido[3,4-
b]pyrazin-2(1 H)-
one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with 4-methoxyphenylboronic acid.
'H NMR (CDCI3) b8.78 (1 H), 7.95 (2H), 7.66 (1 H), 7.01 (2H), 6.91 (1 H), 4.47
(2H), 3.87 (3H),
3.83 - 3.67 (8H), 3.49 (2H), 2.71 (2H), 2.57 (4H), 1.13 (3H); HRMS m/z
454.2425 (calcd for
M+H, 454.2449).
Example 48
OJ
?;C~ O
O , NN1 N N
H
7-(2,3-dihydro-1-benzofuran-5-yl)-1-(2-ethoxyethyl)-3-[(2-morpholin-4-
ylethyl)amino]pyrido(3,4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with 2,3-dihydrobenzofuran-5-yl-5-boronic
acid.
'H NMR (CDCI3) b 8.77 (1 H), 7.91 (1 H), 7.75 - 7.71 (1 H), 7.63 (1 H), 6.89 -
6.85 (2H), 4.64 (2H),
4.47 (2H), 3.83 - 3.70 (6 H), 3.68 - 3.64 (2H), 3.51 (2H), 3.28 (2H), 2.72 -
2.70 (2H), 2.57 - 2.53
(4H), 1.14 (3H); HRMS m/z466.2420 (calcd for M+H, 466.2449).
Example 49
oJ
? CF3
O~1 O N ' I
NNIN I N
H
1-(2-ethoxyethyl)-3-[(2-morpholin-4-ylethyl)amino]-7-[4-
(trifluoromethyl)phenyl]pyrido[3, 4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with 4-(trifluoromethyl)phenylboronic acid.
1 H NMR (CDCI3) 08.83 (1 H), 8.13 (2H), 7.82 (1 H), 7.73 (2H), 7.01 (1 H),
4.49 (2H), 3.85 - 3.70
(8H), 3.46 (2H), 2.73 (2H), 2.58 (4H), 1.12 (3H); HRMS m/z492.2181 (calcd for
M+H, 492.2217).
Example 50
OJ CF3
O~I O N 11
, N---~-NX, N . ~ N
H
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
1-(2-ethoxyethyl)-3-[(2-morpholin-4-ylethyl)amino]-7-(3-
(trifluoromethyl)phenyl]pyrido[3, 4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with 3-(trifluoromethyl)phenylboronic acid.
' H NMR (CDCI3) 08.82 (s), 8.28 (s), 8.21 (1 H), 7.82 (1 H), 7.66 - 7.59 (2H),
7.0 (1 H), 4.49 (2H),
3.85 - 3.69 (8H), 3.51 (2H), 2.72 (2H), 2.57 (4H), 1.13 (3H); HRMS m/z
492.2194 (calcd for
M+H, 492.2217).
Example 51
oJ
ON O N
~~ N~N N
H
3-(2-morpholinoethylamino)-1-(2-ethoxyethyl)-7-phenylpyrido[3,4-b]pyrazin-
2(1H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with phenylboronic acid.
' H NMR (CDCI3) 08.82 (1 H), 8.00 (2H), 7.74 (1 H), 7.50 - 7.37 (3H), 6.94 (1
H), 4.48 (2H), 3.83 -
3.68 (8H), 3.49 (2H), 2.71 (2H), 2.56 (4H), 1.14 (3H); HRMS m/z 424.2306
(calcd for M+H,
424.2343).
Example 52
oJ
J / F
f I
co
H
3-(2-morpholinoethylamino)-1-(2-ethoxyethyl)-7-(4-fluorophenyl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with 4-fluorophenylboronic acid.
1H NMR (CDCI3) b8.79 (1 H), 8.01 - 7.96 (2H), 7.69 (1 H), 7.15 (2H), 6.95 (1
H), 4.47 (2H), 3.83 -
3.68 (8H), 3.48 (2H), 2.27 (2H), 2.57 (4H), 1.13 (3H); HRMS m/z442.2222 (calcd
for M+H,
442.2249).
Example 53
oJ
O N F
0 ~'N~N N
H
3-(2-morpholinoethylamino)-1-(2-ethoxyethyl)-7-(3-fluorophenyl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
81
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with 3-fluorophenylboronic acid.
'H NMR (CDCI3) 08.80 (1 H), 7.79 - 7.71 (3H), 7.47 - 7.39 (1 H), 7.08 - 7.05
(1 H), 6.97 (1 H), 4.48
(2H), 3.84 - 3.65 (8H), 3.49 (2H), 2.72 (2H), 2.56 (4H), 1.13 (3H); HRMS m/z
442.2262 (calcd for
M+H, 442.2249).
Example 54
oJ
F
O O Nf I F
NNX N N
H
3-(2-morpholinoethylamino)-1-(2-ethoxyethyl)-7-(3,4-difluorophenyl)pyrido[3,4-
b]pyrazin-2(1 H)-
one
Prepared as described in example 1 using 2-ethoxyethanamine in step 3 and the
Suzuki
conditions of example 21 step 7 with 3,4-difluorophenylboronic acid.
' H NMR (CDCI3) ~8.78 (1 H), 7.90 - 7.84 (1 H), 7.75 - 7.71 (2H), 7.23 - 7.20
(1 H), 7.00 (1 H), 4.48
(2H), 3.84 - 3.79 (8H), 3.48 (2H), 2.74 (2H), 2.60 (4H), 1.13 (3H); HRMS m/z
460.2122 (calcd for
_--M+H; 460:2155).-----
Example 55
o.,/
OMe
o;I::Jcc*N
3-(2-morpholinoethylam ino)-1-(3-ethoxypropyl)-7-(6-methoxypyridin-3-
yl)pyrido(3, 4-b]pyrazin-
2(1 H)-one
Example 56
OMe
O Nf N
0
X, N N
H
O
1-(3-(1,2-dihydro-7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyethyl)pyrido(3,4-
b]pyrazin-3-
ylamino)propyl)pyrrolidine-2, 5-dione
Example 57
82
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
HO OMe
O N N
tN--"~,-N X N N
H
3-(2-(3-hydroxypyrrolidin-1-yl)ethylam6io)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
Example 58
OMe
HO O fN \ \ N
N,_~ N11 N I ~ N
H
3-(2-(4-hydroxypiperidin-1-yl)ethy/amino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one
Example 59
- -- --- ---- - -- - -- - - -- _ _-- -- ~/- - -- -------
O OMe
H2N I O Nf N
N~~N I N N
H
1-(2-(1,2-dihydro-7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyethyl)pyrido(3,
4-bJpyrazin-3-
ylamino)ethyl)piperidine-4-carboxamide
Example 60
o--~
O NH2 ? OMe
I
O N
N-,---N cx:xr
H
1-
(2-(1,2-dihydro-7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyethyl)pyrido[3,4-
b]pyrazin-3-
ylamino)ethyl)piperidine-3-carboxamide
Example 61
o~~
J / OMe
ON O Nf N
I N
83
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
3-(N-methyl-N-(2-morpholinoethyl)amino)-7-(6-methoxypyridin-3-yl)-1-(2-
propoxyethyl)pyrido[3, 4-
b]pyrazin-2(1 H)-one
Example 62
o~\J
OMe
r I
O N \ \ N
NNX, N I ~ N
~
3-(N-methyl-N-(2-(piperidin-1-y1)ethyl)amino)-7-(6-methoxypyridin-3-y1)-1-(2-
propoxyethyl)pyrido(3,4-b]pyrazin-2(yH)-one
Example 63
OMe
f I
o ON N
NJk H~H ~N N
o1-,)
N-(3-(1,2-dihydro-7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyethyl)pyrido[3,4-
b]pyrazin-3-
ylamino)propyl)morpholine-4-carboxamide
Example 64
J / OMe
r I
O O N N
N-/~N~N N H
0 "
N-(2-(1,2-dihydro-7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyethyl)pyrido(3,
4-b]pyrazin-3-
ylamino)ethyl)morpholine-4-carboxamide
Example 65
o~~
OMe
r I
O N N
X, a_N'--"N ~N 20 H N
3-(3-(pyridin-2-ylamino)propylamino)-7-(6-methoxypyridin-3 yl)-1-(2-
propoxyethyl)pyrido[3,4-
b]pyrazin-2(1 H)-one
Additional compounds of Formula I that can be prepared in accordance with the
synthetic
methods of the present invention include those compounds described in Table B:
84
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Table B
O'CF3 OCF3
N OMe N OMe
O N \ \ ~ ~N~ O N \ \ ~
ON N~N I N ~N ~N~N N
~H H O H
O~CF3 OCF3
O N OMe
N ~ ~N ~ N
oXO HO N I
H 'O' H
o~ O~CF3 O~CF3
O O
O \ I > O N >
N O HN O
~ H IN
I N N I N
/ N
' N
N N ' O H
O a O
O N > ~ O N >
HN O N \ O
N~N N ~N NX N I N
~H ~H
OCF3
0 OMe
O N \ I ~ HO O N \ N
/-N
N N1N N NN ~N ~N
H
~H
M. In Vitro Assays
Method 1: Human Platelet PDE5 Enzyme Inhibition Scintillation Proximity Assay
The IC50 of test compounds can be measured using an in vitro assay using PDE5
enzyme isolated from human platelets. The IC50 is the concentration of test
compound required
to inhibit the hydrolysis of cGMP to GMP by the PDE5 enzyme by 50% relative to
the activity of
uninhibited controls. The PDE5 enzyme for use in the assay can be obtained
from human
platelets by appropriate modification of the method of Thompson, WJ et al.;
Biochemistry 18(23),
5228-5237, 1979, as described by Ballard SA et al.; J. Urology 159(6), 2164-
2171, 1998. The
PDE5 enzyme so obtained can be used to catalyze the hydrolysis of [3H]cGMP
(Amersham
Biosciences) to 5' nucleotide [3H]GMP. The [3H]GMP binds to yttrium silicate
SPA beads
(Amersham Biosciences) and is detected by scintillation counting. More
specifically, the effect of
the test compound at different concentrations can be evaluated in the assay by
contacting the
compound with a fixed amount of PDE5 enzyme in the presence of substrate (cGMP
or cAMP in
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
a 3:1 ratio unlabelled to [3H]-labeled). Scintillation counting can be used as
described above to
determine relative PDE5 enzyme activity. The inhibition of PDE5 enzyme
activity is then
calculated relative to.total PDE5 enzyme activity of uninhibited controls.
PDE5 IC50 Assay: 96-well microtiter plate format
Reagents
Buffer A: 20 mM Tris-HCI, 5 mM MgCI2, pH 7.4
Buffer B: 2 mg/mi BSA in Buffer A (enzyme buffer)
cGMP substrate: Final concentration of 500 nM in assay
The amount of 3H-labeled substrate added depends upon the specific activity of
[3H]cGMP, and
the cGMP substrate is diluted with a 10 mM stock of cold cGMP in Buffer A for
a final substrate
concentration of 500 nM in the assay.
PDE enzyme: Prepared in Buffer B. The dilution factor is determined by enzyme
activity.
SPA beads: 20 mg/mi suspension prepared in dH2O.
Positive Control Negative Control Standard/Test compound
2 pl 100% DMSO 2 pl 100% DMSO 2 pl Standard/Test compound
pl Buffer A 25 pi Buffer A 25 pl Buffer A
25 NI Enzyme 25 pl Buffer B 25 pl Enzyme
20 50 pl Substrate 50 pl Substrate 50 pl Substrate
50 pl SPA to stop 50 pl SPA to stop 50 pl SPA to stop
Stocks of standard and test compounds are prepared at 5 mM in 100% DMSO. The
compound is serially diluted in a dilution plate using a 10-point 1/2 log
dilution format. 2 pl of the
25 compound dilution is added in duplicate to the wells of the assay plate. 2
pl of 100% DMSO are
added to designated control wells. 25 NI of Buffer A are added to all wells.
25 pl of Buffer B are
added to the negative control wells. 25 pl of enzyme are added to the
remaining wells. 50 pl of
substrate are added to each well. Plates are sealed and incubated for 60
minutes on a plate
shaker at 30 C. 50 pl of SPA beads are added to stop the reaction. The plates
are again sealed
and shaken for 15 minutes to allow the beads to bind the GMP product. The
beads are allowed
to settle for 30 minutes and then read on a NXT TopCount scintillation
counter. Data are
analyzed with a curve fitting application for plate-based screening. Percent
inhibition in this assay
is calculated as follows:
Inhibition (%) = [(mean maximum - compound value/ (mean maximum - mean
minimum)] x 100.
The IC50value is determined from sigmoid dose-response curves of enzyme
activity
versus compound concentration.
Method 2: Alternative Human Platelet PDE5 Enzyme Inhibition Scintillation
Proximity Ass~
86
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
The IC50 of test compounds also can be measured in an alternative in vitro
assay that
varies from Method 1 as described below:
PDE5 IC50 Assay: 96-well microtiter plate format
Reagents
Buffer A: 20 mM Tris-HCI, 5 mM MgCI2, pH 7.4
Buffer B: 2 mg/mI BSA in Buffer A (enzyme buffer)
cGMP substrate: Final concentration of 50 nM in assay
The amount of 3H-labeled substrate added depends upon the specific activity of
[3H]cGMP, and it
is diluted in Buffer A.
PDE enzyme: Prepared in Buffer B. The dilution factor is determined by enzyme
activity.
SPA beads: 4 mg/mi suspension prepared in dH2O.
Positive Control Negative Control Standard/Test compound
3 pl 100% DMSO 3 pl 100% DMSO 3 pl Standard/Test compound
27 pl Buffer A 27 pl Buffer A 27 pl Buffer A
30 pl Enzyme 30 pl Buffer B 30 pl Enzyme
30 pl Substrate 30 pl Substrate 30 pl Substrate
30 pl SPA to stop 30 pi SPA to stop 30 pl SPA to stop
Stocks of standard and test compound are prepared at 2 mM in 100% DMSO. The
test
compound is serially diluted in a dilution plate using an 8-point 1/5 log
dilution format such that
the starting concentration in the assay is 2 pM for an initial IC50 screen. 27
ial of.Buffer A are
added to the wells of the assay plates. From the dilution plate, 3 pl of
diluted compound is
delivered in duplicate or 3 pl of 100 % DMSO (for positive and negative
controls) are added. 30
pl of enzyme are added. For the negative control wells, Buffer B is
substituted in place of the
enzyme. 30 pl of labeled substrate are added to all wells.
After incubating for 60 minutes at room temperature, the reaction is stopped
with the
addition of 30 pl of the yttrium silicate beads. These beads are dense and
require constant
agitation while being added to the plate. The plates are sealed and shaken on
a plate shaker for
fifteen minutes to allow the beads to bind the GMP product.
After allowing the beads to settle for 30 minutes, plates are read on a NXT
TopCount
scintillation counter and the data are analyzed as follows. Percent inhibition
values are calculated
using the means of the 0% and 100% controls on each plate. The estimates of
the 4-parameters
of the logistic, sigmoid dose-response model are then calculated using the
well-level percent
inhibition value for the compound. The formula for the four-parameter logistic
model may be
expressed as Y=((a - d) / (1 + ( X / c) A b) ) + d, where Y is the response, X
is the
concentration, a is the lower asymptote (minimum response), d is the upper
asymptote
(maximum response), c is the model IC50 (in the same units as X),.and b is the
slope (as
87
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
described in De Lean, A., P. J. Munson, and D. Rodbard ("Simultaneous analysis
of families of
sigmoidal curves: application to bioassay, radioligand assay, and
physiological dose-response
curves." Am. J. Physiol. 235(2): E97-E102, 1978). These estimates are used to
calculate the
concentration that corresponds to 50% inhibition.
IC50 values are shown in Table C for compounds tested in accordance with
Method 2 above.
88
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Table C Example IC50 Enzyme (nM)
Example IC50 Enzyme (nM) #
# (Method B)
(Method B) 33 1.38
1 0.0710 34 1.35
2 0.0440 35 4.25
3 0.0450 36 1.93
4 0.121 37 0.116
0.0600 38 6.22
6 0.0890 39 1.66
7 0.038 40 36.9
8 0.0660 41 0.812
9 0.169 42 0.168
20.0 43 0.141
11 0.0550 44 0.273
12 0.150 45 0.356
13 0.0450 46 0.0220
14 0.0400 47 3.61
2.96 48 14.5
16 0.284 49 132
17 0.466 50 3.48
18 0.0740 51 4.97
19 1.01 52 2.00
0.127 53 1.80
21 0.0860 54 0.925
22 -- 0.0820 55 2.21
23 2.42 56 0.093
24 3.07 57 0.337
1.65 58 0.353
26 0.0720 59 0.139
27 0.134 60 0.115
28 0.0690 61 0.506
29 0.333 62 1.51
0.249 63 0.117
31 0.261 64 0.047
32 1.02 65 0.034
N. Biological Protocols--/n Vivo Assays
Method 5: CulexTM Assay
The effect of the test compound on systemic arterial blood pressure can be
evaluated in a
5 conscious pre-cannulated spontaneously hypertensive rat ("SHR") model. This
assay is
conducted using an automated blood sampler ("ABS") system. The CulexT~" ABS
system
(Bioanalytical System, Inc., West Lafayette, IN) comprises a laptop computer,
four control units
and metabolic cages. This ABS system allows for the collection of multiple
blood samples from a
single rat without causing undue stress to the animal. In addition, the ABS
system allows for the
10 collection of urine samples that can be potentially used for biomarker
identifications. Through
this approach, efficacy and standard pharmacokinetic studies are conducted in
the conscious
unrestrained SHR rats simultaneously to define the relationship between plasma
free drug
concentration or potential biomarker(s) and pharmacological effect (reduction
of mean arterial
blood pressure).
89
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
SHR rats at 12 to 16 weeks of age, weighing about 300g, undergo surgerical
cannulation of
both jugular veins and the right carotid artery. After surgical recovery,
animals are placed in the
CulexTM cages and tethered to a movement-responsive arm with a sensor that
controls cage
movement when the animal moves to prevent the catheters from being twisted.
Connections are
made between the right jugular catheter and the CulexTM sterile tubing set for
blood sampling,
and the left jugular catheter for compound administration, and the catheter in
the right carotid
artery is connected to a pressure transducer for monitoring blood pressure. To
keep the patency
of the catheters, the right jugular cannula is maintained by the "tend"
function of the CulexTM that
flushes the catheter with 20 pL heparin saline (10 units/mL) every 12 minutes
or between
sampling events, and the left jugular cannula is filled with heparin saline
(20 units/mL). The
patency of the right carotid cannula is maintained by slow infusion of heparin
saline either directly
into the extend tubing when blood pressure is not recorded or through the
pressure transducer
during the blood pressure monitoring. Animals are allowed to acclimate for at
least two hours
before compound evaluation. The test compound may be administered
intravenously or by oral
gavage. Blood sampling protocols (sampling time and volume) are programmed
using the
CulexTM software. The total amount of blood withdrawn from each animal will
not exceed 750
pU24 hrs and 10 mUkg within two weeks. Heart rate, blood pressure, and drug
concentration
are monitored. Systemic arterial blood pressure and heart rate are recorded by
PONEMAH
(Gould Instrument System, Valley View, OH), a pressure transducer through a
data acquisition
system for recording blood pressure and heart rate, for 6 to 24 hours based on
experimental
protocol. Mean arterial blood pressure (primary endpoint) is analyzed for
assessing the efficacy
of the compound.
Blood samples are analyzed for measuring plasma drug concentration, using the
LC/MS/MS
method described below, and for evaluating potential biomarkers.
LC/MS/MS Method
Sample Preparation: Plasma samples (50 pL unknown, control or blank) are mixed
with 10
pL acetonitrile:water or a standard solution of the test compound and 150 pL
of internal standard
solution (100 ng/mL of the test compound in acetonitrile). The mixture is
centrifuged at 3000 rpm
for 5 min, and 125 pL of the supernatant transferred to a 96 well plate. The
solvent is
evaporated under a stream of nitrogen and the residue is reconstituted with 80
pL
acetonitrile/0.1% aqueous formic acid (20:80 v/v).
A 20 pL volume of each prepared sample is injected onto a Phenomenex Synergi 4
pm
MAX-RP 2.0 x 75 mm column and eluted at 0.4 mUmin using gradient elution from
0.1%
aqueous formic acid (mobile phase A) to acetonitrile (mobile phase B). The
gradient program
consists of initial application of 90% mobile phase A, followed by a linear
gradient to 75% mobile
phase B from 0.2 to 1.15 min after injection and held at 75% mobile phase B
until 2.0 min. The
mobile phase was linearly changed back to 90% mobile phase A from 2.00 to 2.10
minutes, and
the next injection took place at 3.00 min. Detection was performed by mass
spectrometry using
positive ion electrospray (ESI) with multiple reaction monitoring of the
transitions m/z 454.00
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
(MH+ the Carboxypiperidine Compound) - m/z 408.00, m/z 466.24 (MH+ the
Carboxypiperidine
Compound) -> 409.33 . The ion spray voltagea is set at 5000. A calibration
curve is constructed
by using peak area ratios of the analyte relative to the internal standard.
Subject concentrations
are determined by inverse prediction from their peak area ratios against the
calibration curve.
Method 6: Implantation of Radio Transmitters and Subsequent Blood Pressure
Screening by
Telemetry in Spontaneously Hypertensive Rats
SHR Rats are anesthetized with isoflurane gas via an isoflurane anesthesia
machine that is
calibrated to deliver isoflurane over a range of percentages as oxygen passes
through the
machine's inner chambers. The animals are placed in an induction chamber and
administered
isoflurane at 4-5% to reach a surgical plane of anesthesia. They are then
maintained at 1-2%
during the surgical procedure via a nose cone, with isoflurane delivered via a
smaller isoflurane
anesthesia device on the surgical table.
Following administration of anesthesia, the rats are implanted with
transmitters using
aseptic procedures with commercially available sterile radio-telemetry units
(Data Sciences,
International, Roseville, MN 551 1 3-1 1 36). Prior to surgery the surgical
field is shaved, scrubbed
with DialT"' brand antimicrobial solution (containing 4% chlorhexidine
gluconate and 4% isopropyl
alcohol) followed by an application of iodine (10%) spray solution. A 2.5 to
3.0 cm laparotomy is
preformed and the radio-telemetry units implanted into the abdomen, with the
catheter tip
inserted into the abdominal aorta. Baby Weitlaner retractors are used to
retain soft tissue. A 1
cm section of the abdominal aorta is partially dissected and. that section
cross-clamped briefly,
punctured with a 21-gauge needle and the transmitter catheter tip introduced
into the vessel and
secured by a single 4.0 silk suture anchored to the adjacent psoas muscle. The
transmitter body
is then inserted into the abdominal cavity and simultaneously secured to the
abdominal muscle
wall while closing with running 4.0 silk suture. The skin layer is closed with
subdermal continuous
4.0 absorbable suture. A subcutaneous (s.c.) administration of marcaine
followed by a topical
application of iodine is administered into and around the suture line,
respectively, upon closing.
All rats receive a postoperative injection of buprenorphine C 0.05mg/kg, s.c.
before regaining
consciousness. A typical dose volume for a 0.300kg rat will be 0.050m1. The
rats must be fully
recovered from their operative anesthesia before the administration of
buprenorphine. They then
receive the same dose once daily for 2 consecutive days, unless the animal
demonstrates that it
is in compromising postoperative pain.
Following surgery, the rats are returned to their cages and housed
individually on solid
bottom caging with paper bedding. A period of no less than 7 days is allowed
for recovery before
experimental procedures are initiated. It has been observed that the rats are
typically
hypertensive for several days following surgery and return to "normotensive"
levels by
approximately the 7th day post-surgery. They are fed standard rat chow and
water ad libitum
throughout the experimental time line.
91
CA 02608672 2007-11-16
WO 2006/126082 PCT/IB2006/001387
Test compounds are administered intragastrically (i.g.) via gavage, using of a
stainless
steel, 21h inch, 18 gauge gavage needle with a balled end. For single daily
dosing, the target
volume is 3.33 ml/kg, i.g. The dose volume for the test compound is
approximately 1 ml/ rat.
The vehicles in which the test compound is administered is methylcellulose
(0.5%) + Tween 80
(0.1%) in 50mM citrate buffer pH=5Ø
Blood pressure data will be obtained using Data Sciences International's data
acquisition
program (Version 3.0). Blood pressure samples are recorded at 1.5-3 minute
intervals for a 5
second duration 24 hours per day for the entire study. This data is processed
by Data Science's
data analysis software into averages of a desired time inervals. All other
data reduction is
performed in Microsoft ExcelTM spreadsheets.
As various changes could be made in the above invention(s) without departing
from the
scope of the invention(s), it is intended that all matter contained in the
above description be
interpreted as illustrative and not in a limiting sense. All documents
mentioned in this application
are expressly incorporated by reference as if fully set forth at length, with
the definitions of the
present application controlling. When introducing elements of the present
invention or the
various embodiment(s) thereof, the articles "a", "an", "the" and "said" are
intended to mean that
-- --there are one or-more of-the-elements: -The terms "comprising",
"including" and "having" are
intended to be inclusive and mean that there may be additional elements other
than the listed
elements.
92