Note: Descriptions are shown in the official language in which they were submitted.
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
1
DESCRIPTION
ASPIRATION CATHETER
TECHNICAL FIELD
[0001] The
present invention relates to a catheter for blood vessel and
specifically a catheter to aspirate and remove wastes such as thrombi in the
blood vessel
or to inject drug solution into the blood vessel.
BACKGROUND ART
[0002]
Aspiration catheters conventionally have been used for removing thrombi
and the like in the blood vessel. An operator inserts a catheter into a blood
vessel and
moves it along the blood vessel and locates the catheter at a target position
to aspirate and
remove the thrombi and the like. For example, the below patent document 1
discloses an
aspiration catheter with dilators, having a double tubular structure
comprising a central
catheter and an outer catheter with almost the same diameters and same
lengths, which
aspirates and removes thrombi usually through the central catheter and when
the central
catheter is plugged up by the clots, the operator easily pulls out the central
catheter from
the outer catheter to exchange it for a substitute central catheter. And the
outer catheter
itself also functions as a catheter and can aspirate clots and the like.
However the central
catheter of the device of Patent Document 1 has a dilator, such a dilator
decreases the
effective diameter for aspiration of the catheter, also there is a flexible
conical chip at the
distal end of the dilator, which forms the narrowest part of the catheter and
decreases the
aspiration amount.
[Patent Document 1] Japanese Laid-Open Patent No. 24058/95
[0003]
Patent Document 2 discloses a catheter device comprising a guide
catheter for aspirating and removing thrombi and an evacuation sheath formed
of an
aspiration inlet of thrombi located inside the distal end thereof. The
catheter aspirates
thrombi while blocking the blood flow in the blood vessel by a balloon located
outside the
sheath to create a retrograde flow to prevent dispersion of debris of thrombi
and to prevent
blocking of the other blood vessels by the debris of thrombi. However in the
combination
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
2
of the guide catheter and the evacuation sheath in Patent Document 2, the
space between
the guide catheter and the evacuation sheath is sealed by a balloon, which
makes inner
diameter of the evacuation sheath to aspirate thrombi considerably narrower
than the outer
diameter of the guide catheter. Consequently the concept of the present
invention to
make the effective diameter for aspiration of the evacuation sheath as large
as possible is
not disclosed in the Patent Document 2.
And as the catheter of the Patent Document 2 aspirates thrombi while the
balloon
is dilated, it cannot move back and forth in the blood vessel during
aspiration. Thereby
when removing thrombi dissipated in the wide area of the blood vessel,
frequent and
troublesome procedures are unavoidable to repeat aspiration and movement of
the
catheter in blood vessels.
[Patent Document 2] W002/087677
DISCLOSURE OF THE INVENTION
[Problem to be solved by the invention]
[0004] As
aspiration catheters in blood vessels are surgical instruments which
are used by inserting into blood vessels and guided to affected parts in the
blood vessels
by a guide wire and the like to treat inner walls of the blood vessels or
inside the blood
vessels, it is necessary to make the catheters very thin, for example, usually
the outer
diameters thereof are less than 1 mm. Furthermore in the case of aspirating
and
removing high-viscosity thrombi accumulated on the inner wall of the blood
vessel using a
thin catheter, there raise problems such that as the aspiration pressure
increases it
becomes difficult or impossible to aspirate thrombi or it takes a long time
even if aspiration
is possible, and an excessive burden is loaded on the patient. And in the case
of
conventional aspiration catheters, it is inevitable that the aspiration
resistance of the whole
length of the catheter is loaded on the aspiration device as it is mounted
along the
extension line of the proximal side of the longitudinal axis of the catheter.
10005] However as the catheters are used while inserting into
blood vessels,
there is a limitation of a thickness of the catheter, and it is substantially
impossible to make
the outer diameter thicker than conventional catheters. Consequently it is a
long-felt
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
3
desire that a catheter which can aspirate and remove thrombi promptly while
maintaining
the aspiration pressure as low as possible. Further in the case of the
catheter of above
Patent Document 2 which uses a balloon and by the balloon the catheter is
fixed to an
inner wall of a blood vessel for aspiration, it is difficult to clean up clots
promptly in a wide
area in the blood vessel as it is inevitable to repeat aspiration and movement
of the
catheter in the blood vessel. As viscous clots adhere to a wide area of walls
of blood
vessels, it takes a long time for the catheter of Patent Document 2 to clean
up such viscous
clots in a wide area, consequently frequent and busy procedures are necessary,
and it is a
long-felt desire to realize a catheter which can easily and promptly remove
clots in a wide
area and can lighten the patient's burden.
[Means for solving the problem]
[0006]
The present invention relates to an aspiration catheter wherein a tubular
member is located at the distal side of a catheter shaft and the catheter
shaft itself is used
for an aspiration tube at the proximal side of the tubular part of the
catheter shaft in order to
secure the effective diameter for aspiration as close as the outer diameter of
the catheter,
setting a Y connector at the proximal end of the tubular part of the catheter
shaft and
aspirating from the branch conduit of the Y connector by means of the
aspiration device to
increase the aspiration rate by shortening the length of the aspiration
conduit in which an
aspiration resistance is created because of thin aspiration conduit comparing
with the
conventional catheter which aspirating with the aspiration device located at
the extension
of the proximal end of the catheter, the catheter with no balloon can smoothly
aspirate
thrombi of wide area in the blood vessel because the catheter can aspirate
while moving
the catheter in the blood vessel.
[0007]
The present invention relates to a catheter member of an aspiration
catheter for aspirating and removing useless wastes in blood vessel such as
thrombi
and/or injecting medical agent into blood vessel by inserting the catheter
into blood vessel
wherein the catheter device has a catheter member and a catheter shaft, the
catheter
member has a flexible shaft having a distal end and a proximal end and the
tubular
member connected to the distal end of the flexible shaft, and the proximal
inner cavity of
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
4
the tubular member located in the catheter shaft is connected to the cavity of
the tubular "
part of the catheter shaft.
[0008]
The catheter member of the present invention constructing a part of the
catheter system comprises a flexible shaft having a distal end and a proximal
end, a
tubular member connected to the distal end of the flexible shaft and a hub
connected to the
proximal end thereof. The inner space of the proximal end of the tubular
member is
connected to the tubular part of the catheter shaft. The flexible shaft is a
shaft that
connects with the tubular member and the operating hub. There is no limitation
on its
sectional shape in particular, and the shape is preferably a round bar, a
circular arc
sectional shape extending a part of the tubular wall of the tubular member,
more preferably
a round bar having no orientational flexibility differences. A solid-core bar
and a tubular
bar can be used. In the case of the tubular bar, another surgical instrument
can be
inserted into the tubular member and further a blood vessel through the
tubular cavities of
the operational hub and the flexible shaft.
[0009] The
flexible shaft is connected to the proximal end of the tubular member
and used to move the location of the tubular member in the tubular part of the
catheter
shaft. It is important that the lumen of the tubular member which aspirates
thrombi
connects to the inner space of the catheter shaft at the position of the
catheter shaft into
which the flexible shaft is inserted. There are a lot of methods for the
connection. In the
case of a flexible shaft having a very small outer diameter comparing with the
inner space
of the catheter shaft, the flexible shaft with no substantial space inside is
advantageous for
back-and-forth movement of the tubular member, although one can make a space
therein.
In this case, the inner space of the tubular member connects with mainly a
space between
the outside of the flexible shaft and an inside the catheter shaft. Also when
the outer
diameter of the flexible shaft is similar to the inner diameter of the
catheter shaft, it is
necessary to make a space for aspiration or injection inside the flexible
shaft. In this case,
it is necessary to connect the space inside the flexible shaft with the inner
space of the
catheter shaft by means of a slit, a cut, a hole, mesh and the like. And the
inner space of
the tubular member connects with the space between the outside the flexible
shaft and
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
inside the catheter shaft through the inner space of the flexible shaft. The
length of the
flexible shaft is normally 20 cm to 200 cm, and preferably 30 cm to 130 cm.
The material
of the flexible shaft can be flexible plastics or metals, and for example,
polyethylene,
polyurethane, nylon, nylon elastomer, ethylene-vinyl acetate copolymers,
polyisoprene,
5 polycarbonate, polystyrene, polyoxymethylene, acrylonitrile-styrene resins,
m-polyphenylether, polyvinylchloride, polymethyl methacrylate, polyethylene
terephthalate,
polybutylene terephthalate and the like, and combination thereof can be used.
Metal
materials such as stainless steels and super-elastic metal (Ni-Ti alloy) can
be preferably
used. Also composite materials formed by using a metal as a core material and
coating
the outside of the core material with a polymer can be used.
[0010] The hub can be any shape as far as the operator can
conveniently
manipulate the flexible shaft, however too large or too heavy hub is
inappropriate because
the catheter member may get out of the clean area. When a tubular cavity is
formed in
the flexible shaft, the hole which connects with the cavity may be formed in
the hub. And
to connect with the other instruments, a medical lure taper or a medical
double-start thread
mechanism may be formed. As the material, polyethylene, polyurethane, nylon,
nylon
elastomer, ethylene-vinyl acetate copolymer, polyisoprene, polycarbonate,
polystyrene,
polyoxymethylene, acrylonitrile-styrene resin, m-polyphenyl ether,
polyvinylchloride,
polymethyl methacrylate, polyethylene terephthalate, polybutylene
terephthalate and the
like and combination thereof can be used.
[0011] The length of the tubular member of the catheter member can
be usually
3 cm to 50 cm, and preferably 5 cm to 15 cm. The outer diameter of the tubular
member
is specified by the inner diameter of the catheter shaft. As the tubular
member is used by
inserting into the catheter shaft, it is necessary that the outer diameter of
the tubular
member is close enough to the inner diameter of the catheter shaft in order
that blood does
not leak in from the end of the aspiration catheter member or the drug
solution does not
leak out of the gap between the tubular member and the catheter shaft, on the
other hand it
is important to have a slight clearance between the tubular member and the
catheter shaft
so that the movement of the tubular member in the catheter shaft is not
disturbed so that
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
6
the operator can often move the catheter member in the back and forth
directions during
aspiration or injection operation. In practice, the clearance is preferably
from 0.02 % to
25 %, more preferably from 0.1% to 15 % of the outer diameter of the catheter
shaft used.
The radial thickness of the tubular member can be from 0.02 mm to 2 mm.
[0012] It is
preferred that the distal end and the proximal end of the tubular
member are obliquely cut so as to move smoothly during insertion of the
tubular member
into the connection part of the catheter shaft or blood vessel or extraction
thereof. The
oblique angle can be 5 ¨70 0 preferably 20 ¨50 to the lengthwise
direction. The
shape of cutting can be a single linear line, a composite line composed of
several linear
lines or a fluent arc line. The tubular member can be made by a single
material or a
composite material. As the single material, polyethylene, nylon, nylon
elastomer,
polyurethane, fluorocarbon resin, ethylene-vinyl acetate copolymer, silicone
resin and the
like can be used. As the composite material, two layer structure material can
be used.
In this case, as the inner layer, fluorocarbon resin, polyamide, polyamide
elastomer,
polyethylene and the like can be used, and as the outer layer, polyethylene,
polyurethane,
nylon, nylon elastomer, ethylene-vinyl acetate copolymer, silicone resin and
the like can be
used. It is possible to insert a blade (a reinforcing member in the form of a
net or a spiral
fiber) of metal or resin.
[0013]
An X-ray-opaque marker may be made at the aspiration end of the
tubular member, near the cutting parts of both the aspiration end and the
opposite end to
the aspiration end of the tubular member so as to prevent dropout of the
tubular member
from the catheter shaft by eye-observation. The marker can be formed at only
one end of
the two. The marker can be formed at the surface, inside or in the radial
thickness of the
tubular member in the shape of tube, coil, paint, powder and the like. Also it
is possible to
admix an X-ray-opaque material in the whole tubular member material to
visualize the
whole tubular member under X-ray. The materials of the marker can be any
materials that
is opaque to X-rays, and stainless steel, platinum, tungsten or alloy thereof
and further
contrast agents such as bismuth subcarbonate, bismuth oxide and barium sulfate
may be
used.
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
7
[0014]
Surface treatment may be made to attain lubricating property at the outer
surface of the tubular member to reduce resistance during insertion into the
catheter shaft
and movement in the blood vessel. The surface treatment agent is optional and
may be
hydrophobic materials such as fluorocarbon resin and silicone resin or
hydrophilic
materials such as polyethylene glycol (PEG), polyethylene oxide (PEO),
polyvinyl alcohol,
polyacrylamide, and homopolymers and copolymers of methacrylic acid
derivatives.
Further to enforce lubricating effect, surface treatment may be made at the
inner surface of
the catheter shaft as well as the surface of the tubular member.
[0015]
The thrombi in the blood vessel is aspirated from the distal end of the
tubular member, transferred from the proximal end of the tubular member to
inner cavity of
the catheter shaft, and removed through the branch conduit of Y connector by
means of
the aspiration device.
[0016]
Although an inner lumen of the tubular member may be used as a guide
wire channel, it is preferred to make an independent path for guide wire. In
this case, the
position of the guide wire channel can be any part against the tubular member,
however
whole length of the tubular member in the lengthwise direction is preferred
and it is
possible to form the channel only near to the aspiration end. Although the
guide wire
channel may be made outside the outer diameter of the tubular member, it is
preferred to
make the guide wire channel in the radial thickness or inside the outer
diameter of the
tubular member to keep the sectional shape of the tubular member round in
order to
prevent flow-in of the blood and flow-out of the drug solution between the
catheter shaft
and the tubular member. The guide wire channel may be made by the same
material as
the tubular member and it is possible to be integrally made with the tubular
member or
made independently, and also it is possible to make a part or whole part of
the guide wire
channel in the space in the tubular member. The inner diameter of the guide
wire channel
is appropriately selected according to the guide wire diameter to be used, and
may be 0.1
mm to 3 mm. The guide wire channel may be made inside the flexible shaft or
outside the
flexible shaft independently, or it is possible not to make the guide wire
channel in
particular after getting out the proximal end of the tubular member.
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
8
[0017]
The guide wire channel can protrude out of the aspiration end of the
tubular member, and the appropriate protrusion length may be 0.3 mm to 10 mm,
and
preferably 0.5 mm to 4 mm. The length of the guide wire channel including the
protrusion
length may be 5 mm to 500 mm, and preferably 10 mm to 300 mm.
[0018] The
sectional shape of the guide wire channel is preferably round and its
inner diameter is from 0.1 mm to 3 mm and from 0.3 mm to 1 mm is particularly
preferred
when used for coronary arteries. The radial thickness of the guide wire
channel when
formed as an independent member from the tubular member may be 0.02 mm to 2
mm,
preferably 0.05 mm to 0.25 mm when used for coronary arteries. The material in
such
case may be a single or composite material. As the single material,
polyethylene, nylon,
nylon elastomer, polyurethane, fluorocarbon resin, ethylene-vinyl acetate
copolymer,
silicone resin and the like can be used. As the composite material, two layer
structure
material can be used. In this case, for the inner layer of the composite
material,
fluorocarbon resin, polyamide, polyamide elastomer, polyethylene and the like
can be used,
and for the outer layer of the composite material, polyethylene, polyurethane,
nylon, nylon
elastomer, ethylene-vinyl acetate copolymer, silicone resin and the like can
be used.
[0019]
In the case of forming the end tip of the guide wire channel independent
from the tubular member, a surface treatment may be made to provide
lubricating property
outside the end tip to reduce resistance during insertion into the catheter
shaft or its
movement in the blood vessel. The surface treatment agent is optional and may
be
hydrophobic materials such as fluorocarbon resin and silicone resin or
hydrophilic
materials such as polyethylene glycol (PEG), polyethylene oxide (PEO),
polyvinyl alcohol,
polyacrylamide, and homopolymers and copolymers of methacrylic acid
derivatives.
[0020]
It is preferred in view of safety to detect the position of the flexible shaft
by X-ray imaging. It is possible to mix a contrast agent such as tungsten,
bismuth
subcarbonate, bismuth oxide and barium sulfate to the material especially in
the case of
resinous material. An X-ray opaque marker may be made at the tip of the
protrusion part
of the guide wire channel from the aspiration end in order to indicate the
protrusion part.
The marker can be formed on the surface, inner surface or in the radial
thickness of the
CA 02609966 2009-12-10
9
flexible shaft in the shape of tube, coil, paint, powder and the like. The
materials of
the marker can be any materials that is opaque to X-rays as set forth above.
[0021] Commercial products may be used as the guide wire and the thickness may
be
adopted according to the thickness of the blood vessel in which the catheter
is
inserted. The guide wires for blood vessels having thicknesses of
0.012"\0.014"\
0.018"\ 0.025", 0.035" and the like are available on the market. 0.014"
thickness
guide wire is often used for coronary arteries of the heart. Various lengths
from 30 cm
to 300 cm are available on the market. The lengths from 175 cm to 190 cm or
300 cm
are often used for the coronary arteries. Catheters having various structures
are
available and they are designed to be soft in order not to injure the blood
vessels by
the tip end of the guide wire. The guide wires such as those formed on the
whole
length of a metal coil such as stainless steel, a metal wire with a 30cm coil
at the
distal end which is initially inserted into blood vessel, those manufactured
by
composite materials such as stainless steel and super-elastic alloy (Ni-Ti
alloy) and
those covered with polyurethane or nylon polymers are available. The guide
wire may
be manipulated to guide the catheter in the blood vessel.
[0022] The catheter shaft of the present invention is a tubular
shape and has an
approximate uniform sectional shape and an approximate uniform sectional area.
The
tubular member is set in the catheter shaft as said above when the catheter
shaft is
used for the blood vessel catheter. The tubular member can protrude out of the
distal
end of the catheter shaft, however it is necessary that the proximal end of
the tubular
member must be always kept in the catheter shaft. Although the catheter for
exclusive
use may be used as the catheter shaft, commercial guiding catheters, catheters
for
injection of contrast agent for diagnosis and the like may be used. There are
catheters
of 3 Fr. to 10 Fr. in diameters and 90 cm to 120 cm in effective lengths, and
there are
various kinds of the distal end shape so that the catheters are stable in the
blood
vessels. There are products having curvatures, for example, JudkinsTM left,
Judkins
right, AmplatzTM right, Amplatz left, Border, Multipurpose, Kimney, Champ, and
the
like.
[0023] In a structure of a catheter shaft for exclusive use, a tubular part
which
becomes gradually soft against bending is formed at a distal side of the
catheter shaft
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
having a distal end and a proximal end, and a connector for connecting with a
connection
part at the proximal end. The outer diameter of the tubular member is from 0.3
mm to 12
mm and for coronary arteries 1 mm to 3.5 mm may be used. The radial thickness
can be
0.03 mm to 2 mm and for coronary arteries 0.05 mm to 0.8 mm is preferred. The
tubular
5
member can be made by a single material or a composite material. As the single
material,
polyethylene, nylon, nylon elastomer, polyurethane, fluorocarbon resin (FEP,
PTFE, PFA,
PVDF and the like), stainless steel tube, super-elastic alloy (Ni-Ti alloy)
and the like and as
the composite material, two layer structure material can be used. In this
case, as the
inner layer of the composite material, fluorocarbon resin, polyamide,
polyamide elastomer,
10
polyethylene and the like can be used, and as the outer layer of the composite
material,
polyethylene, polyurethane, nylon, nylon elastomer and the like can be used.
It is
preferred to insert metal blades (a reinforcing member in the form of a net or
a spiral fiber)
or resin blade between the inner layer and the outer layer to improve anti-
kink property or
shape-retentive property of the tube.
[0024] The
tubular part of the catheter shaft has an inner diameter that can
contain the tubular member and also similar to an outer diameter of the
tubular member.
The hardness of the tubular part can be uniform on the whole or can change
gradually
without boundaries. It is also possible to change stepwise by more than two
steps. An
example of the hardness change is such that: forming a tubular part of the
proximal side
having a uniform hardness, and forming a tubular part of the distal side which
gradually
softens from the proximal side thereof toward the distal end. The length of
the tubular part
having changing hardness is normally no more than 35 % of the effective length
of the
catheter shaft which is the total lengths of the tubular part and the tubular
part. Effective
length of the catheter shaft is usually 70 cm to 130 cm.
The tubular part can be made of a single material or a composite material. For
the single material, polyethylene, nylon, nylon elastomer, polyurethane,
fluorocarbon resin,
ethylene- vinyl acetate copolymer, silicone resin and the like can be used.
For the
composite material, two layer structure material can be used. In this case,
for the inner
layer material, fluorocarbon resin, polyamide, polyamide elastomer,
polyethylene and the
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
11
like can be used, and for the outer layer material, polyethylene,
polyurethane, nylon, nylon
elastomer, ethylene-vinyl acetate copolymer, silicone resin and the like can
be used. It is
possible to insert a blade (a reinforcing member in the form of a net or a
spiral fiber) of
metal or resin between the inner layer and the outer layer.
[0025] A
connector is used for manipulating the catheter shaft itself and for
connecting to a connection part, and has a space inside thereof so as to
retain a diameter
larger than the outer diameter of the tubular member. The outer shape can be
optional
while considering easiness of handling and connectability to the connection
part. As the
material of the connector, polycarbonate, polystyrene, polyoxymethylene,
acrylonitrile-styrene copolymer, m-polyphenyl ether, polyvinylchloride,
polynnethyl
methacrylate, polyethylene terephthalate, polybutylene terephthalate and the
like can be
adopted however a lighter-weight material is preferred so as to prevent
dropout of the
catheter shaft from a patient.
[0026]
A surface treatment may be made to provide lubricating property on the
outer surface of the catheter shaft to reduce resistance during insertion of
the catheter
shaft into a blood vessel. Methods of the surface treatment is optional and
may be to coat
the surface with hydrophobic materials such as fluorocarbon resin and silicone
resin or
hydrophilic materials such as polyethylene glycol (PEG), polyethylene oxide
(PEO),
polyvinyl alcohol, polyacrylamide, and homopolymers and copolymers of
methacrylic acid
derivatives.
[0027]
Y connector as a connection part for connecting to the catheter shaft is
used for separating the flexible shaft and the guide wire from the aspiration
device and for
connecting them to outside. The flexible shaft and the guide wire usually pass
through
the main conduit and the connection to the aspiration device is through the
branch conduit
although the reverse connection may be possible.
[0028]
The aspiration device in the present invention is optional and not
restricted in particular, and aspiration devices such as injection type and
aspiration-pump
type may be adopted.
EXAMPLES
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
12
[0029]
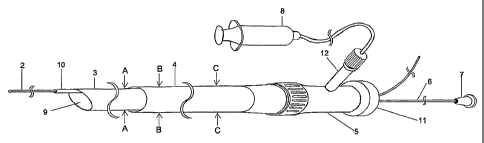
Fig. 1 shows an example of the present invention for catheter system 1
which is used by inserting into a blood vessel for aspirating thrombi and/or
injecting drug
solution into blood vessel. The figure shows whole the catheter system 1 and
the figure
depicts the state that the catheter member 13 is inserted into the catheter
shaft 4. The
catheter member 13 (not shown) comprises a tubular member 3, a flexible shaft
6, a hub 7
and a guide wire channel 10 shown in Fig. 3. The connection part between the
tubular
member 3 and the flexible shaft 6 is in the tubular part of the catheter shaft
4 and not
shown. The connection part 5 is a Y connector in Fig. 1 and the flexible shaft
6 passes
through the main conduit 11. A round bar type is used for the flexible shaft
6. A guide wire
2 also can passes through the main conduit 11 and in Fig. 1 passes through the
main
conduit 11 of Y connector. An aspiration device 8 is connected to the branch
conduit 12.
[0030]
A distal end of the guide wire 2 projects out of the tubular member 3 and
the guide wire channel 10, and a proximal end of the guide wire 2 protrude
from Y
connector. An aspiration opening 9 is at the distal end of the tubular member
3.
[0031] An example
of practical use of a catheter for aspirating thrombi of the
present invention is hereafter described. First at access position to the
body, a path to an
artery is specified by so-called a sheath introducer by making a small hole
through the skin,
for example, at the wrist or the inguinal region near the root of the femoral
region; the
tubular part of the catheter shaft 4 containing a guide wire 2 commercially
available are
proceeded to near the target area through the styptic valve of the sheath
introducer.
[0032]
Near the target area, only the guide wire 2 is preceded to secure the
route for medical treatment. After the position of the guide wire 2 and the
catheter shaft 4
are roughly fixed, the guide wire 2 is passed through the guide wire channel
10 of the
catheter member 13 and proceeds the catheter member 13 to approximate the area
where
there is targets such as thrombi between the distal end of the guide wire 2
and the distal
end of the tubular part of the catheter shaft 4. The guide wire 2 precedes the
catheter
member 13 to prevent dropout of the guide wire 2. And a built-in styptic valve
in the main
conduit of the connection part 5 is slightly closed to minimize the leakage of
blood.
Further necessary parts such as an extension tube and a stop cock are
connected to the
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
13
branch conduit 12 of the connection part 5 and the aspiration device 8 is
connected to the
proximal end. After arrangement of the catheter system 1 has been completed, a
reduced pressure is applied using the aspiration device 8 to the system 1 to
aspirate
thrombi, or an elevated pressure is applied to inject a drug solution after
filling the
necessary drug solution into the aspiration device. The catheter member 13 is
moved in
the blood vessels to approximate target area to aspirate thrombi or to inject
the drug
solution for therapy while maintaining the reduced pressure or elevated
pressure.
[0033]
The catheter member 13 has the flexible shaft 6 at the proximal side of
the tubular member 3 having a continuous lumen between the distal end and the
proximal
end as shown in Fig. 1, Fig.2 and Fig. 6, and further the hub 7 at the
proximal end of the
flexible shaft 6. The guide wire channel 10 for the guide wire to pass through
is located at
the tubular member 3 and an X-ray opaque marker is located at the distal end
of the guide
wire channel 10.
[0034]
Although the shape of the flexible shaft 6 is optional, it is possible to make
the shaft a simple solid cylindrical shape as shown in Fig. 5 or hollow
cylindrical shape as
shown in Fig. 7. Further it is possible that a part of the flexible shaft
which is more
proximal than the proximal end of the tubular member 3 inside the catheter
shaft 4 may
have holes or slits on the whole or a part thereof while making the outer
diameter of the
flexible shaft close to the inner diameter of the catheter shaft as shown in
Fig. 8 to connect
between the inner space of the catheter shaft 4 and that of the tubular member
3 on
condition the inner space of the tubular part 4 of the catheter shaft is
connected to the inner
space of the tubular member 3 of the catheter member.
[0035]
The connection means are optional and there are a number of forms, for
example, more than one slits are formed at whole or a part of the flexible
shaft 6 as in Fig.
11, and more than one holes having various shapes instead of slits as in Fig.
13. The
holes can be uniform sizes or various sizes or such holes can be mixed. Fig.
12 shows a
drawing of the flexible shaft 6 where a part or whole length of the shaft is
cut in the
longitudinal direction. The flexible shaft 6 is not restricted to one, for
example, a plurality
of flexible shafts 6 are possible as shown in Fig. 9. In the case of a
plurality of the flexible
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
14
shafts, the shafts may be integrated at an optional position as shown in Fig.
9 or Fig. 10.
In such case, it is necessary to design while considering the position of the
styptic valve at
the main conduit 11 of the connection part 5 so as not to leak blood
therefrom. It is
preferred in view of safety to detect the position of the flexible shaft by X-
ray imaging.
When resinous materials are used for the flexible shaft, it is preferred to
mix a contrast
agent such as tungsten, bismuth subcarbonate, bismuth oxide and barium sulfate
considering imaging.
BRIEF DESCRIPTION OF DRAWINGS
[Fig. 1] The aspiration catheter of the present invention.
[Fig. 2] The catheter member of the aspiration catheter in Fig. 1.
[Fig. 31 The sectional drawing of A-A section of Fig. 1. A section of the
tubular member
protruding out of the catheter shaft, the guide wire channel 10 is formed in
the tubular wall
and the guide wire is passing through therein.
[Fig. 4]
B-B sectional shape of Fig. 1. The tubular member is inserted into the
catheter shaft. The part of the tubular member is the same as Fig. 3 and the
guide wire
channel 10 is formed in the tubular wall and the guide wire is passing through
therein.
[Fig. 51 C-C sectional shape of Fig. 1. There is no guide wire channel and
guide wire
is in the inner space.
[Fig. 6]
The drawing of longitudinal section of the aspiration catheter system of the
present invention constructing one flexible shaft.
[Fig. 71
The drawing of longitudinal section of the aspiration catheter system of the
present invention constructing one flexible shaft.
[Fig. 8]
The drawing of longitudinal section of the aspiration catheter system of the
present invention constructing one flexible shaft.
[Fig. 9] The
drawing of longitudinal section of the aspiration catheter system of the
present invention constructing three flexible shafts at the proximal end of
the flexible shaft.
[Fig. 10]
The drawing of longitudinal section of the aspiration catheter system of the
present invention constructing three flexible shafts at the proximal end of
the flexible shaft.
[Fig. 11]
The drawing of longitudinal section of the aspiration catheter system of the
CA 02609966 2007-11-27
WO 2006/132434 PCT/JP2006/311973
present invention constructing one flexible shaft.
[Fig. 12] The drawing of longitudinal section of the aspiration catheter
system of the
present invention constructing one flexible shaft.
[Fig. 13] The drawing of longitudinal section of the aspiration catheter
system of the
5 present invention constructing one flexible shaft.
DESCRIPTION OF SYMBOLS
1 = = = catheter system, 2 = = = guide wire, 3 = = = tubular member, 4 = = =
tubular part of
the catheter shaft, 5 = = = connection part, 6 = = = flexible shaft, 7 = = =
hub, 8 = = = aspiration
device, 9 = = = aspiration end, 10 = = = guide wire channel, 11 = = = main
conduit, 12 = = =
10 branch conduit, 13 = = = catheter member.