Note: Descriptions are shown in the official language in which they were submitted.
CA 02610818 2009-03-30
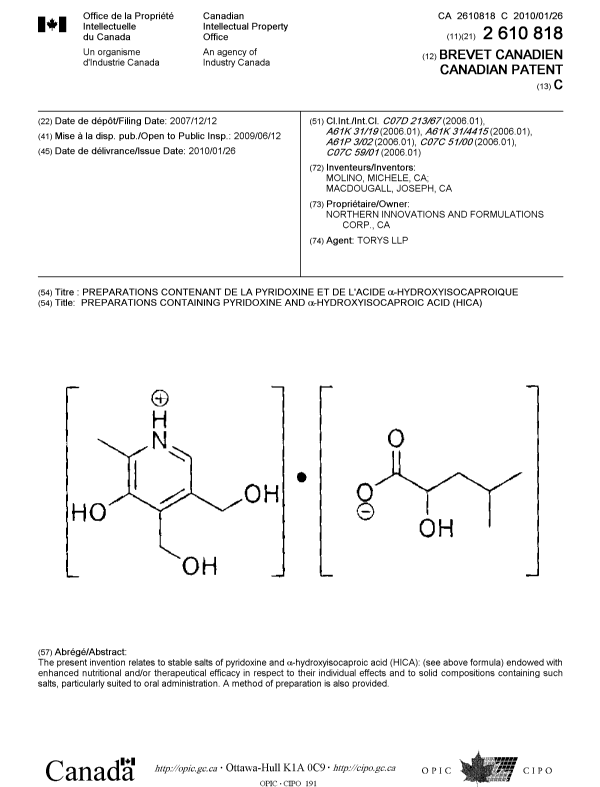
Preparations containing Pyridoxine and a- Hydroxyisocaproic acid (HICA)
Field of the Invention
The present invention relates to a structure and method for producing stable
salts of pyridoxine and a-hydroxyisocaproic acid (HICA). More specifically,
formed
salts of the present invention are particularly well suited for oral
administration
thereby providing enhanced nutritional and/or therapeutical efficacy in
relation to the
individual components alone.
Background of the Invention
Pyridoxine is often referred to as vitamin B6, however, it is actually only
one of
three components which constitute vitamin B6; the others being pyridoxal and
pyridoxamine. The active form of pyridoxine in the body is pyridoxal 5-
phosphate,
which is a coenzyme for all transamination and some decarboxylation and
deamination reactions. Furthermore, pyridoxal 5-phosphate is required as a
coenzyme for all transamination reactions which occur in the body (Peterson
DL,
Martinez-Carrion M. The mechanism of transamination. Function of the histidyl
residue at the active site of supernatant aspartate transaminase. J Biol Chem.
1970
Feb 25;245(4):806-13).
CA 02610818 2007-12-12
a-hydroxyisocaproic acid (HICA), is an end product of the metabolism of
the branched chain amino acids, and is a nitrogen-free pre-cursor from which
25 amino acids can be synthesized. Since branched chain amino acid analogs may
be reaminated back to their correspondent amino acid (e.g. HICA can be
converted to ketoisocaproic acid (KICA), which can subsequently be converted
back to Leucine), they can act to provide the dietary requirements for BCAA
without increasing level of ingested nitrogen (Boebek KP, Baker DH.
30 Comparative utilization of the a-keto and D- and L-a-hydroxy analogs of
Leucine,
Isoleucine and Valine by chicks and rats. J Nutr. 1982 Oct;112(10):1929-39).
Transamination is the transfer of the amino group from an amino acid to an a-
keto acid, e.g. a-ketoisocaproic acid can be converted to Leucine in this
manner.
As the product of transamination reactions depend on the availability of a-
keto
35 acids, providing exogenous HICA would make the formation of Leucine more
favorable. Thus oral administration of analogues of branched-chain amino acids
will increase the cellular content of the corresponding branched-chain amino
acid, while substantially simultaneously reducing plasma and cellular ammonia.
There is now an extensive and ever growing body of literature, particularly
40 patents, disclosing the formation of various salts having physiological
functions in
mammals.
UK Patent No. 1,248,324 ('324') discloses the formation of pyridoxine and
a-ketoglutarate salts. a-Ketoglutarate is the deaminated form of glutamate,
and
is an intermediate in the citric acid cycle. Transamination of branch chain
amino
2
CA 02610818 2007-12-12
45 acids occurs primarily with a-ketoglutarate to form glutamate; however the
reverse reaction of Glutamate to branch chain amino acids does not occur.
It would be desirable for the development of new salts of amino acid
metabolites capable of being reaminated and transaminated to branch chain
amino acids for use in the body of a mammal using the nitrogen found in the
50 body of said mammal without the requirement of adding additional nitrogen
to the
system. It would therefore also be desirable to produce a compound having the
aforementioned qualities while additionally providing required co-factors for
transamination reactions to occur.
Summary of the Invention
55 In the present invention, a compound and methods for its production are
disclosed. Specifically, the compound is a salt comprising a molecule of
pyridoxine and a molecule of a-hydroxyisocaproic acid (HICA), and having a
structure of Formula 1:
Formula 1
H
0
HO OH ~ $
OH
--Y
60 OH
Detailed Description of the Invention
In the following description, for the purposes of explanations, numerous
specific details are set forth in order to provide a thorough understanding of
the
present invention. It will be apparent, however, to one of ordinary skill in
the art
65 that the present invention may be practiced without these specific details.
3
CA 02610818 2009-03-30
The present invention is directed towards the structure and synthesis of salts
of pyridoxine and a-hydroxyisocaproic acid (HICA).
90 The present invention provides for the production of a stable salt which
may
afford a synergistic combination of pyridoxine and HICA, free of
physiologically
unsafe additives to an individual upon administration to a mammal.
Furthermore, the
present invention is particularly well suited for use in tablets, capsules,
powders,
granules, powdered beverage mixes and other forms known in the art of dietary
95 supplements.
As used herein, the term `pyridoxine a-hydroxyisocaproate' is to be
understood as the salt of pyridoxine with HICA reacted in an equimolar ratio.
Pyridoxine a-hydroxyisocaproate is a non-hygroscopic crystalline powder,
which is stable in storage and can be processed without special precautions.
Due to
100 the non-hygroscopic nature of the pyridoxine a-hydroxyisocaproate it would
be
understood by one of skill in the art, that the salt is easy to process and is
particularly
suitable for processing with rapidly running machines, since it does not tend
to stick
together or become lumpy.
As used herein, `pyridoxine' refers to the chemical 2-methyl-3-hydroxy-4,5-
105 dihydroxymethylpyridine, (CAS Registry No. 65-23-6), also known as 3-
hydroxy-4,5-
bis(hydroxymethyl)-2-methylpyridine, 3-hydroxy-4,5-dimethyl-a-picoline, 5-
hydroxy-6-
methyl-3,4-pyridinedimethanol, or Vitamin B6. The invention also includes
derivatives of pyridoxine such as esters, and amides, and salts, as well as
other
derivatives, including derivatives having substantially similar
pharmacoproperties to
110 pyridoxine upon metabolism to an active form.
4
CA 02610818 2009-03-30
As used herein, `a-hydroxyisocaproic acid' refers to the chemical 2-hydroxy-4-
methylvaleric acid, (CAS Registry No. 498-36-2), also known as HICA, or leucic
acid.
The invention also includes `a-hydroxyisocaproic acid' also includes
derivatives of a-
hydroxyisocaproic acid such as esters, and amides, and salts, as well as other
115 derivatives, including derivatives having substantially similar
pharmacoproperties to
a-hydroxyisocaproic acid upon metabolism to an active form.
As used herein, `lower alcohol' refers to aliphatic alcohols having about 1 to
about 4 carbon atoms as is known in the art, such as, without limitation,
methanol,
ethanol, propanol, and isopropanol. These lower alcohols may be used singly or
in
120 admixture containing two or more alcohols.
As used herein, 'pharmaceutically acceptable excipients' refers to substances
added to produce quality tablets, chewable tablets, capsules, granulates or
powders,
but which do not provide nutritive value. A non-exhaustive list of examples of
excipients includes monoglycerides, magnesium stearate, modified food starch,
125 gelatin, microcrystalline cellulose, glycerin, stearic acid, silica,
yellow beeswax,
lecithin, hydroxypropylcellulose, croscarmellose sodium, and crosprovidone.
According to the present invention, the compounds disclosed herein comprise
an a-hydroxyisocaproic acid molecule combined with a pyridoxine molecule to
form a
salt having a structure according to Formula 1. The
CA 02610818 2007-12-12
aforementioned compound being prepared according to the reaction as set forth
for the purposes of the description in Scheme 1:
Scheme 1
N 1) Heat
H
O 2) Cool (RT to 4 C) N O
HO OH + HO =
OH Y Lower Alcohol [HOoH] g~
OH OH
OH
2 3
115 With reference to Scheme 1, in the first step of the reaction the
pyridoxine
(1) is dissolved in an excess of hot lower alcohol. The lower alcohol is
considered to be hot when it is heated to a temperature below the boiling
point of
the corresponding lower alcohol.
In various embodiments of the present invention, the lower alcohol is
120 selected from the group consisting of methanol, ethanol, propanol, and
isopropanol. These lower alcohols may be used singly or in admixture
containing
two or more alcohols.
Concurrently, in the second step of the reaction the a-hydroxyisocaproic
acid (2) is dissolved into an excess of hot lower alcohol. The lower alcohol
is
125 considered to be hot when it is heated to a temperature below the boiling
point of
the corresponding lower alcohol.
Both solutions above are then mixed together and heated to about the
boiling point of the corresponding lower alcohol. If there are solids still
present
the solution is filtered at this temperature to remove unreacted starting
materials.
130 The solution is then allowed to cool to room temperature and then covered
and
placed in a refrigerator until crystallization occurs, preferably for between
about
6
CA 02610818 2007-12-12
24 to about 48 hours. The resultant crystals are filtered under vacuum and
washed with ice cold lower alcohol, yielding a crystalline powder, the
pyridoxine
a-hydroxyisocaproate (3).
135 In larger scale preparations of the present invention diethyl ether can be
added until the cloud point, as would be known to one of skill in the art, is
reached after the mixture is cooled to room temperature. This will facilitate
greater precipitation of the product thus yielding more of the pyridoxine a-
hydroxyisocaproate (3), which would be desired in industrial settings.
140 Pyridoxine a-hydroxyisocaproate is used advantageously alone or with
additional active ingredients, such as, trace elements, vitamins, mineral
substances, or other amino acids as well as, optionally, excipients usually
used
for the preparation of the respective forms of administration. The forms of
administration include, particularly, all varieties of tablets, both those
that are
145 swallowed without being chewed, and tablets to be chewed or dissolved in
the
mouth of an individual, as well as those that are dissolved in a liquid before
being
ingested by an individual. The tablet forms include uncoated tablets, one-
layer or
multilayer or encased forms or effervescent tablets. Further preferred forms
of
administration are capsules of hard and soft gelatin, the latter having
particularly
150 suitable to include a liquid core. Additionally, pyridoxine a-
hydroxyisocaproate
can be used advantageously for the preparation of solutions and suspensions
and as a powder, either effervescent or granulated.
While not wishing to be bound by theory, it is understood by the inventors
that pyridoxine a-hydroxyisocaproate and its derivatives corresponding to
7
CA 02610818 2007-12-12
155 Formula 1 above, are useful compounds, since they combine within a single
molecule both the pyridoxine and the a-hydroxyisocaproate, thus resulting in
the
increase of the useful activities of these two compounds. Particularly, it is
herein
understood by the inventors that pyridoxine a-hydroxyisocaproate will have
enhanced pH stability in water within a substantially broad range of
160 concentrations.
The examples given below explain the execution of the invention with
respect to the production of pyridoxine a-hydroxyisocaproate. Provided below
is
the a basic method of producing pyridoxine a-hydroxyisocaproate, however those
of skill in the art will appreciate certain changes may be made in the process
of
165 "scaling-up" the reaction to manufacture larger batches of pyridoxine a-
hydroxyisocaproate which may be required for commercial uses and supply
requirements. Other methods of synthesis may also be apparent to those of
skill
in the art.
Examples
N 1) Heat (60 to 70 C) H
0 2) Cool (RT to 4 C) N 0
OH + ~ ^ /
Ho HO" ~ Y EtOH [HOTx I g
OH OH I OH
OH
170 1 2 3
Example I (Laboratory Scale)
132.16 g (1 mol) of a-hydroxyisocaproic acid (2) is dissolved into 200mL of
hot ethanol, solution 1. Concurrently, 169.18 g (1 mol) of pyridoxine (1) is
dissolved in 300mL of hot ethanol, solution 2. Solution 2 is added to solution
1
175 with stirring and the resultant solution is heated to the boiling point.
If there are
8
CA 02610818 2007-12-12
solids still present the solution is filtered at this temperature to remove
unreacted
starting materials. The solution is then allowed to cool to room temperature
and
then covered and placed in a refrigerator until crystallization occurs; about
24
hours. The resultant crystals are filtered under vacuum and washed with ice
cold
180 ethanol, yielding a crystalline powder, the pyridoxine a-
hydroxyisocaproate (3).
Example 2 (Industrial Scale)
781.179 kg (5910.86 mol) of a-hydroxyisocaproic acid (2) is dissolved into
800 L of hot ethanol, solution 3. Concurrently, 1000 kg (5910.86 mol) of
pyridoxine (1) is dissolved in 1000 L of hot ethanol, solution 4. Solution 3
is
185 added to solution 4 with stirring and the resultant solution is heated to
the boiling
point. If there are solids still present the solution is filtered at this
temperature to
remove unreacted starting materials. The solution is then allowed to cool to
room temperature, diethyl ether is added until the cloud point is reached, and
the
mixture is then cooled for 48 hours to induce crystallization. The resultant
190 crystals are then vacuum-filtered and washed with ice cold ethanol,
yielding a
crystalline powder, the pyridoxine a-hydroxyisocaproate (3).
Extensions and Alternatives
In the foregoing specification, the invention has been described with
specific embodiments thereof; however, it will be evident that various
195 modifications and changes may be made thereto without departing from the
broader spirit and scope of the invention.
9