Note: Descriptions are shown in the official language in which they were submitted.
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
NIACIN RECEPTOR AGONISTS, COMPOSITIONS CONTAINING SUCH COMPOUNDS AND
METHODS OF TREATMENT
BACKGROUND OF THE INVENTION
The present invention relates to cycloalkene compounds, their derivatives,
compositions
containing such compounds and methods of treatment or prevention in a mammal
relating to
dyslipidemias. Dyslipidemia is a condition wherein serum lipids are abnormal.
Elevated cholesterol and
low levels of high density lipoprotein (HDL) are independent risk factors for
atherosclerosis associated
with a greater-than-normal risk of atherosclerosis and cardiovascular disease.
Factors known to affect
serum cholesterol include genetic predisposition, diet, body weight, degree of
physical activity, age and
gender. While cholesterol in normal amounts is a vital building block for cell
membranes and essential
organic molecules such as steroids and bile acids, cholesterol in excess is
known to contribute to
cardiovascular disease. For example, cholesterol, through its relationship
with foam cells, is a primary
component of plaque which collects in coronary arteries, resulting in the
cardiovascular disease termed
atherosclerosis.
Traditional therapies for reducing cholesterol include medications such as
statins (which
reduce production of cholesterol by the body). More recently, the value of
nutrition and nutritional
supplements in reducing blood cholesterol has received significant attention.
For example, dietary
compounds such as soluble fiber, vitamin E, soy, garlic, omega-3 fatty acids,
and niacin have all received
significant attention and research funding.
Niacin or nicotinic acid (pyridine-3-carboxylic acid) is a drug that reduces
coronary
events in clinical trials. It is commonly known for its effect in elevating
serum levels of high density
lipoproteins (HDL). Importantly, niacin also has a beneficial effect on other
lipid profiles. Specifically,
it reduces low density lipoproteins (LDL), very low density lipoproteins
(VLDL), and triglycerides (TG).
However, the clinical use of nicotinic acid is limited by a number of adverse
side-effects including
cutaneous vasodilation, sometimes called flushing.
Despite the attention focused on traditional and alternative means for
controlling serum
cholesterol, serum triglycerides, and the like, a significant portion of the
population has total cholesterol
levels greater than about 200 mg/dL, and are thus candidates for dyslipidemia
therapy. There thus
remains a need in the art for compounds, compositions and alternative methods
of reducing total
cholesterol, serum triglycerides, and the like, and raising HDL.
The present invention relates to compounds that have been discovered to have
effects in
modifying serum lipid levels.
The invention thus provides compositions for effecting reduction in total
cholesterol and
triglyceride concentrations and raising HDL, in accordance with the methods
described.
- l -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Consequently one object of the present invention is to provide a nicotinic
acid receptor
agonist that can be used to treat dyslipidemias, atherosclerosis, diabetes,
metabolic syndrome and related
conditions while minimizing the adverse effects that are associated with
niacin treatment.
Yet another object is to provide a pharmaceutical composition for oral use.
These and other objects will be apparent from the description provided herein.
SUMMARY OF THE INVENTION
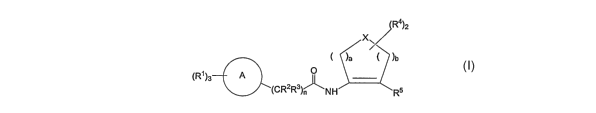
A compound represented by formula I:
R4)2
X
)a )b
0
(R')3 ~
CRZR3)n \NH R5
I
or a pharmaceutically acceptable salt or solvate thereof is disclosed wherein:
X represents CH2, 0, S, S(O), SO2 or NH, such that when X represents NH, the
nitrogen
atom may be optionally substituted with R6, C(O)R6, or SO2R6, wherein:
R6 represents C1_3alkyl optionally substituted with 1-3 groups, 0-3 of which
are halo, and
0-1 of which are selected from the group consisting of: OC,_3alkyl, OH, NH2,
NHCI_3alkyl, N(C1_3alkyl)2,
CN, Hetcy, Aryl and HA.R,
said Aryl and HAR being further optionally substituted with 1-3 groups, 1-3 of
which are
halo, and 0-1 of which are selected from the group consisting of: OH, NH2,
C1_3alkyl, C1_3alkoxy, haloCl_
3alkyl and haloCl_3alkoxy groups;
a and b are each integers 1, 2 or 3, such that the sum of a and b is 2, 3 or
4;
ring A represents a 6-10 membered aryl, a 5-13 membered heteroaryl or a
partially
aromatic heterocyclic group, said heteroaryl and partially aromatic
heterocyclic group containing at least
one heteroatom selected from 0, S, S(O), S(O)a and N, and optionally
containing 1 other heteroatom
selected from 0 and S, and optionally containing 1-3 additional N atoms, with
up to 5 heteroatoms being
present;
each RZ and R3 is independently H, C1_3alkyl, haloC1_3alkyl, OCl_3alkyl,
haloC1_3alkoxy,
OH or F;
n represents an integer of from 1 to 5;
each R4 is H or is independently selected from halo and R6;
-2-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
H
<\ H
N\N
RS represents -CO2H, N~N or -C(O)NHSO2W wherein W
represents Cz_4alkyl or phenyl, said C1_4a1ky1 and phenyl each being
optionally substituted with 1-3
groups, 1-3 of which are selected from halo and CI_3allcyl, and 1-2 of which
are selected from the group
consisting of: OCl_3alkyl, haloC1.3alkyl, haloCl_3alkoxy, OH, NH2 and
NHC1_3allcyl;
and each R' is H or is independently selected from the group consisting of:
a) halo, OH, CO2H, CN, NH2, S(O)0_2Re, C(O)Re, OC(O)Re and CO2Re , wherein Re
is as
previously defined;
b) Cl_6 alkyl and OC,_6alkyl, said Cl_6alkyl and alkyl portion of OCI_6alkyl
being
optionally substituted with 1-3 groups, 1-3 of which are halo and 1-2 of which
are selected from: OH,
CO2H, CO2C1_4alkyl, COzCl4haloalkyl, OCO2C14alkyl, NH2, NHC,-4alkyl, N(C,-
4a1ky1) Z, Hetcy and CN;
c) NHC1_4alkyl and N(C,-4alkyl) 2, the alkyl portions of which are optionally
substituted
as set forth in (b) above;
d) C(O)NH2, C(O)NHC14alkyl, C(O)N(Cl4alkyl) 2, C(O)Hetcy, C(O)NHOC,_4alkyl and
C(O)N(C,.4alkyl)(OCI-4alkyl), the alkyl portions of which are optionally
substituted as set forth in (b)
above;
e) NR'C(O)R", NR'SO2R", NR'CO2R" and NR'C(O)NR"R"' wherein:
R' represents H, C,_3alkyl or haloC1_3alkyl,
R" represents (a) C1_8alkyl optionally substituted with 1-4 groups, 0-4 of
which are
halo, and 0-1 of which are selected from the group consisting of: OC,_6alkyl,
OH, CO2H, CO2C,.4alkyl,
COzC,-4haloalkyl, NH2, NHCI-4alkyl, N(Cl_aalkyl) 2, CN, Hetcy, Aryl and HAR,
said Hetcy, Aryl and HAR being further optionally substituted with 1-3 halo,
Cl_
aalkyl, C14alkoxy, haloCl_4alkyl or haloC,-4alkoxy groups;
(b) Hetcy, Aryl or HAR, each being optionally substituted with 1-3
members selected from the group consisting of: halo, Cl4alkyl, Cl-4alkoxy,
haloCl-4alkyl and haloCl_
aalkoxy groups;
and R"' representing H or R";
f) phenyl or a 5-6 membered heteroaryl or a Hetcy group attached at any
available ring
atom and each being optionally substituted with 1-3 groups, 1-3 of which are
selected from halo, Cl_
3alkyl and haloCl_3alkyl groups, and 1-2 of which are selected from OC,_3alkyl
and haloOC1_3alkyl
groups, and 0-1 of which is selected from the group consisting of:
i) OH; CO2H; CN; NH2 and S(O)o_ZRe wherein Re is as described above;
-3-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
ii) NHC1.4a11cyl and N(Cl.4allcyl) a, the allcyl portions of which are
optionally
substituted with 1-3 groups, 1-3 of which are halo and 1-2 of which are
selected from: OH, CO2H,
CO2C1_4alkyl, COZC1.4haloallcyl, NH2, NHCi-4allcyl, N(Cl.~alkyl) 2 and CN;
iii) C(O)NH2, C(O)NHCI_4alkyl, C(O)N(Ci4alkyl) Z, C(O)NHOC1_4alkyl and
C(O)N(Cl_4alkyl)(OC1.4alkyl), the alkyl portions of which are optionally
substituted as set forth in b)
above; and
iv) NR'C(O)R", NR'SO2R", NR'CO2R" and NR'C(O)NR"R"' wherein R', R"
and R"' are as described above.
DETAILED DESCRIPTION OF THE INVENTION
The invention is described herein in detail using the terms defined below
unless
otherwise specified.
"Alkyl", as well as other groups having the prefix "alk", such as alkoxy,
alkanoyl and the
like, means carbon chains which may be linear, branched, or cyclic, or
combinations thereof, containing
the indicated number of carbon atoms. If no number is specified, 1-6 carbon
atoms are intended for
linear and 3-7 carbon atoms for branched alkyl groups. Examples of alkyl
groups include methyl, ethyl,
propyl, isopropyl, butyl, sec- and tert-butyl, pentyl, hexyl, heptyl, octyl,
nonyl and the like. Cycloalkyl is
a subset of alkyl; if no number of atoms is specified, 3-7 carbon atoms are
intended, forming 1-3
carbocyclic rings that are fused. "Cycloalkyl" also includes monocyclic rings
fused to an aryl group in
which the point of attachment is on the non-aromatic portion. Examples of
cycloalkyl include
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
tetrahydronaphthyl, decahydronaphthyl,
indanyl and the like. Haloalkoxy and halo0alkyl are used interchangeably and
refer to halo substituted
alkyl groups linked through the oxygen atom. Haloalkyl and haloalkoxy include
mono- substituted as
well as multiple substituted alkyl and alkoxy groups, up to perhalo
substituted alkyl and alkoxy. For
example, trifluoromethyl and trifluoromethoxy are included.
"Alkenyl" means carbon chains which contain at least one carbon-carbon double
bond,
and which may be linear or branched or combinations thereof. Examples of
alkenyl include vinyl, allyl,
isopropenyl, pentenyl, hexenyl, heptenyl, 1-propenyl, 2-butenyl, 2-methyl-2-
butenyl, and the like.
"Alkynyl" means carbon chains which contain at least one carbon-carbon triple
bond,
and which may be linear or branched or combinations thereof. Examples of
alkynyl include ethynyl,
propargyl, 3-methyl-l-pentynyl, 2-heptynyl and the like.
"Aryl" (Ar) means mono- and bicyclic aromatic rings containing 6-10 carbon
atoms.
Examples of aryl include phenyl, naphthyl, indenyl and the like.
"Heteroaryl" (HAR) unless otherwise specified, means mono-, bicyclic and
tricyclic
aromatic ring systems containing at least one heteroatom selected from 0, S,
S(O), SO2 and N, with each
ring containing 5 to 6 atoms. HAR groups may contain from 5-14, preferably 5-
13 atoms. Examples
include, but are not limited to, pyrrolyl, isoxazolyl, isothiazolyl,
pyrazolyl, pyridyl, oxazolyl, oxadiazolyl,
-4-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
thiadiazolyl, thiazolyl, imidazolyl, triazolyl, tetrazolyl, furanyl,
triazinyl, thienyl, pyrimidyl, pyridazinyl,
pyrazinyl, benzoxazolyl, benzothiazolyl, benzimidazolyl, benzofuranyl,
benzothiophenyl,
benzopyrazolyl, benzotriazolyl, furo(2,3-b)pyridyl, benzoxazinyl,
tetrahydrohydroquinolinyl,
tetrahydroisoquinolinyl., quinolyl, isoquinolyl, indolyl, dihydroindolyl,
quinoxalinyl, quinazolinyl,
naphthyridinyl, pteridinyl, 2,3-dihydrofuro(2,3-b)pyridyl and the like.
Heteroaryl also includes aromatic
carbocyclic or heterocyclic groups fused to heterocycles that are non-aromatic
or partially aromatic, and
optionally containing a carbonyl. Examples of additional heteroaryl groups
include indolinyl,
dihydrobenzofuranyl, dihydrobenzothiophenyl, dihydrobenzoxazolyl, and aromatic
heterocyclic groups
fused to cycloalkyl rings. Examples also include the following:
N-O O-N N-NH
X~ X~ ;X'/ \ I / NV"' O
XN\ I X' N\ ~
N- ~~ I N=N
N=N
N-NH N-O
I> ~ // I N\\ N ~\ S~
/
N S H N
N- R
NH N O
~s~'
I / S N OO N
N-NH N-NH
DNH X\ ~ 7 / X\
X N ~ I /
== is a single or double bond
X'=CHorN
R = H or CH3
Heteroaryl also includes such groups in charged form, e.g., pyridinium.
"Heterocyclyl" (Hetcy) unless otherwise specified, means mono- and bicyclic
saturated
and partially saturated rings and ring systems containing at least one
heteroatom selected from N, S and
0, each of said ring having from 3 to 10 atoms in which the point of
attachment may be carbon or
nitrogen. Examples of "heterocyclyl" include, but are not limited to,
azetidinyl, pyrrolidinyl, piperidinyl,
piperazinyl, imidazolidinyl, tetrahydrofuranyl, 1,4-dioxanyl, morpholinyl,
thiomorpholinyl,
-5-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
tetrahydrothienyl and the like. Heterocycles can also exist in tautomeric
forms, e.g., 2- and 4-pyridones.
Heterocycles moreover includes such moieties in charged form, e.g.,
piperidinium.
"Halogen" (Halo) includes fluorine, chlorine, bromine and iodine.
The phrase "in the absence of substantial flushing" refers to the side effect
that is often
seen when nicotinic acid is administered in therapeutic amounts. The flushing
effect of nicotinic acid
usually becomes less frequent and less severe as the patient develops
tolerance to the drug at therapeutic
doses, but the flushing effect still occurs to some extent and can be
transient. Thus, "in the absence of
substantial flushing" refers to the reduced severity of flushing when it
occurs, or fewer flushing events
than would otherwise occur. Preferably, the incidence of flushing (relative to
niacin) is reduced by at
least about a third, more preferably the incidence is reduced by half, and
most preferably, the flushing
incidence is reduced by about two thirds or more. Likewise, the severity
(relative to niacin) is preferably
reduced by at least about a third, more preferably by at least half, and most
preferably by at least about
two thirds. Clearly a one hundred percent reduction in flushing incidence and
severity is most preferable,
but is not required.
One aspect of the invention relates to a compound represented by formula I:
R4)2
XQ-I (RI)3 O A ~
(CRR3); \NH Ra
I
or a pharmaceutically acceptable salt or solvate thereof is disclosed wherein:
X represents CH2, 0, S, S(O), SO2 or NH, such that when X represents NH, the
nitrogen
atom may be optionally substituted with R6, C(O)R6, or SO2R6, wherein:
R6 represents Cl_3alkyl optionally substituted with 1-3 groups, 0-3 of which
are halo, and
0-1 of which are selected from the group consisting of: OCl_3alkyl, OH, NH2,
NHC,_3alkyl, N(C,_3alkyl)2,
CN, Hetcy, Aryl and HAR,
said Aryl and HAR being further optionally substituted with 1-3 groups, 1-3 of
which are
halo, and 0-1 of which are selected from the group consisting of: OH, NH2,
C1_3alkyl, C1_3alkoxy, haloCl_
3alkyl and haloC1_3alkoxy groups;
a and b are each integers 1, 2 or 3, such that the sum of a and b is 2, 3 or
4;
ring A represents a 6-10 membered aryl, a 5-13 membered heteroaryl or a
partially
aromatic heterocyclic group, said heteroaryl and partially aromatic
heterocyclic group containing at least
one heteroatom selected from 0, S, S(O), S(O)2 and N, and optionally
containing 1 other heteroatom
-6-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
selected from 0 and S, and optionally containing 1-3 additional N atoms, with
up to 5 heteroatoms being
present;
each Ra and R3 is independently H, Cl.3alkyl, haloC1.3alkyl, OCI.3alkyl,
haloQ_3alkoxy,
OH or F;
n represents an integer of from 1 to 5;
each R4 is H or is independently selected from halo and R6;
H
~ ~~
N\N
RS represents -CO2H, N-N or -C(O)NHSO2Re wherein Re
represents C1.4a1ky1 or phenyl, said Cl-4alkyl and phenyl each being
optionally substituted with 1-3
groups, 1-3 of which are selected from halo and Cl_3alkyl, and 1-2 of which
are selected from the group
consisting of: OC1_3alkyl, haloC1.3alkyl, haloCl_3alkoxy, OH, NH2 and
NHC1.3alkyl;
and each R' is H or is independently selected from the group consisting of:
a) halo, OH, CO2H, CN, NH2, S(O)o.aRe, C(O)Re, OC(O)Re and CO2Re , wherein Re
is as
previously defined;
b) C1_6 alkyl and OC1_6alkyl, said C1_6alkyl and alkyl portion of OC,_6alkyl
being
optionally substituted with 1-3 groups, 1-3 of which are halo and 1-2 of which
are selected from: OH,
COzH, CO2C,-4alkyl, CO2C,-4haloalkyl, OCO2CI-4alkyl, NH2, NHC,-,alkyl,
N(C1.4alkyl) Z, Hetcy and CN;
c) NHCx4alkyl and N(Cl4alkyl) 2, the alkyl portions of which are optionally
substituted
as set forth in (b) above;
d) C(O)NH2, C(O)NHCl-4alkyl, C(O)N(C,-4alkyl) 2, C(O)Hetcy, C(O)NHOCI-4alkyl
and
C(O)N(C,4alkyl)(OC1.4a1ky1), the alkyl portions of which are optionally
substituted as set forth in (b)
above;
e) NR'C(O)R", NR'SOZR", NR'CO2R" and NR'C(O)NR"R"' wherein:
R' represents H, C1.3alkyl or haloC1.3alkyl,
R" represents (a) Cl_$alkyl optionally substituted with 1-4 groups, 0-4 of
which are
halo, and 0-1 of which are selected from the group consisting of: OC1.6alkyl,
OH, COzH, CO2Ci4alkyl,
CO2C,-4haloalkyl, NH2, NHCl4alkyl, N(Cl-4alkyl) Z, CN, Hetcy, Aryl and HAR,
said Hetcy, Aryl and HAR being further optionally substituted with 1-3 halo,
Cl.
4alkyl, Cl_4alkoxy, haloC1_4alkyl or haloC,-4alkoxy groups; or
(b) Hetcy, Aryl or HAR, each being optionally substituted with 1-3
members selected from the group consisting of: halo, Cl.4alkyl, Cl-4alkoxy,
haloCl-4alkyl and haloCi_
4alkoxy groups;
and R"' representing H or R";
-7-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
f) phenyl or a 5-6 membered heteroaryl or a Hetcy group attached at any
available ring
atom and each being optionally substituted with 1-3 groups, 1-3 of which are
selected from halo, Cl.
3alkyl and haloC1.3alkyl groups, and 1-2 of which are selected from OCI.3alkyl
and haloOC1.3al1ry1
groups, and 0-1 of which is selected from the group consisting of:
i) OH; CO2H; CN; NH2 and S(O)o-2Re wherein W is as described above;
ii) NHC14alkyl and N(Cl 4allryl) 2, the allcyl portions of which are
optionally
substituted with 1-3 groups, 1-3 of which are halo and 1-2 of wliich are
selected from: OH, CO2H,
CO2C14a1ky1, CO2C,.4haloalkyl, NH2, NHC14alkyl, N(C,.4alkyl) Z and CN;
iii) C(O)NH2, C(O)NHC1.4alkyl, C(O)N(Cl4alkyl) a, C(O)NHOCI-4alkyl and
C(O)N(C,.4alkyl)(OC14alkyl), the alkyl portions of which are optionally
substituted as set forth in b)
above; and
iv) NR'C(O)R", NR'SO2R", NR'CO2R" and NR'C(O)NR"R"' wherein R', R"
and R"' are as described above.
One aspect of the invention that is of interest relates to a compound of
formula I wherein
up to 4 RZ and R3 moieties are selected from the group consisting of:
C1_3alkyl, haloC1-3alkyl, OC1.3alkyl,
haloC1.3alkoxy, OH and F, and any remaining R2 and R3 moieties represent H.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein ring A is a phenyl or naphthyl group, a 5-6 membered monocyclic
heteroaryl group or a 9-13
membered bicyclic or tricyclic heteroaryl group. Within this subset of
compounds, all other variables are
as defined with respect to formula I.
More particularly, a subset of compounds that is of interest relates to
compounds of
forrnula I wherein ring A is selected from the group consisting of: phenyl;
naphthyl;
HAR which is a member selected from the group consisting of: pyrrolyl,
isoxazolyl,
isothiazolyl, pyrazolyl, pyridyl, oxazolyl, oxadiazolyl, thiadiazolyl,
thiazolyl, imidazolyl, triazolyl,
tetrazolyl, furanyl, triazinyl, thienyl, pyrimidyl, pyridazinyl, pyrazinyl,
benzoxazolyl, benzothiazolyl,
benzimidazolyl, benzofuranyl, benzothiophenyl, benzopyrazolyl, benzotriazolyl,
furo(2,3-b)pyridyl,
benzoxazinyl, tetrahydrohydroquinolinyl, tetrahydroisoquinolinyl., quinolyl,
isoquinolyl, indolyl,
dihydroindolyl, quinoxalinyl, quinazolinyl, naphthyridinyl, pteridinyl, 2,3-
dihydrofuro(2,3-b)pyridyl
indolinyl, dihydrobenzofuranyl, dihydrobenzothiophenyl, dihydrobenzoxazolyl,
or a member selected
from the group consisting of:
-~-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
N-O O-N N-NH
\, X.. X~
c)c\ p ~ c-
N-N
N
% N-NH N-O
N / S/>
N S H
N, R
~/~ ~ i%H N
N N~
O
N-NH N-NH
X\ X'
/
NH / N~
X'-N N
is a single or double bond
X'=CHorN
R = H or CH3
Even more particularly, an aspect of the invention that is of interest relates
to a
compound of formula I wherein ring A is selected from the group consisting of:
phenyl; naphthyl;
HAR which is a member selected from the group consisting of: isoxazolyl,
pyrazolyl,
oxazolyl, oxadiazolyl, thiazolyl, triazolyl, thienyl, benzothiazolyl, or a
member selected from the group
consisting of:
-9-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
N-O O-N N-NH
X. ~ ,' X. X~
~ / /
N\ o c
N~ ~ =NN-NH N.O
~ s
~ ,~ I N~ /\ S N N
H
R
/k,L N_NH N cx
I / S p O \.
N-NH N-NH
X\ 7 X
\
/
NH ~ / N~ I
x--N N
is a single or double bond
X'=CHorN
R=HorCH3
Within this subset of compounds, all other variables are as defined with
respect to formula I.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein each R' is H or is selected from the group consisting of:
a) halo, OH, CN, NHZ and S(O)o_zRe wherein Re is methyl or phenyl optionally
substituted with 1-3 halo groups;
b) C1_3 alkyl and OC1_3alkyl, each being optionally substituted with 1-3
groups, 1-3 of
which are halo and 1-2 of which are selected from: OH, NH2, NHC1_4alkyl and
CN;
c) NR'SO2R" and NR'C(O)NR"R"' wherein:
R' represents H, Cl_3alkyl or haloC1_3alkyl,
R" represents (a) C,_$alkyl optionally substituted with 1-4 groups, 0-4 of
which are
halo, and 0-1 of which are selected from the group consisting of: OC1_6alkyl,
OH, COaH, CO2C1_4alkyl,
CO2C,_4haloalkyl, OCOzC,-4alkyl, NH2, NHCI.4alkyl, N(C,4alkyl) a, CN, Hetcy,
Aryl and HAR,
said Hetcy, Aryl and HAR being further optionally substituted with 1-3 groups
selected from: halo, C14alkyl, Cl-4alkoxy, haloCl-4alkyl and haloC,-4alkoxy;
(b) Hetcy, Aryl or HAR, said Aryl and HAR being optionally substituted
with 1-3 groups selected from: halo, Cl-4alkyl, C,-4alkoxy, ha1oC,-4alkyl and
ha1oC14alkoxy;
and R"' representing H or R"; and
-10-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
d) phenyl or a 5-6 membered heteroaryl or a heterocyclic group attached at any
available
point and being optionally substituted with 1-3 groups, 1-3 of which are halo,
C1.3alkyl or haloCl_3alkyl
groups, 1-2 of which are OCI_3allcyl or haloOCI_3allcyl groups, and 1 of which
is selected from the group
consisting of:
i) OH; CO2H; CN; NH2 and S(O)o_2W wherein Re is as described above;
ii) NHCI.4alkyl, the alkyl portion of which is optionally substituted with 1-3
groups, 1-3 of which are halo and 1 of which is selected from: OH, CO2H,
CO2C1_4alkyl, C02C1_
4haloalkyl, NH2, NHCI_4alkyl, N(Cl_dalkyl) 2 and CN;
iii) C(O)NH2a C(O)NHC1_4alkyl and C(O)N(Cl 4alkyl) 2, the alkyl portions of
which are optionally substituted as set forth in (b) above; and
iv) NR'C(O)R" and NR' SOzR" wherein R' and R" are as described above.
Within this subset of compounds, all other variables are as defined with
respect to formula I.
In particular, another subset of compounds that is of interest relates to
compounds of
formula I wherein each R' is H or is selected from the group consisting of:
a) halo, OH, CN and NH2 ;
b) C1_3a11cy1 and OCI_3alkyl, each being optionally substituted with 1-3
groups, 1-3 of
which are halo and 1-2 of which are selected from: OH, NH2, NHCl-4alkyl and
CN;
c) phenyl or a 5-6 membered heteroaryl or a heterocyclic group attached at any
available
point and being optionally substituted with 1-3 groups, 1-3 of which are halo,
Cl_3alkyl or haloCl_3alkyl
groups, 1-2 of which are OC,_3alkyl or haloOCj_3alkyl groups, and 1 of which
is selected from the group
consisting of:
i) OH, CN and NHa . Within this subset of compounds, all other variables are
as
defined with respect to formula I.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein a and b are 1 or 2 such that the sum of a and b is 2 or 3. Within this
subset of compounds, all
other variables are as defined with respect to formula I.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein X represents 0, S, N or CH2. Within this subset of compounds, all
other variables are as defined
with respect to formula I.
More particularly, another subset of compounds that is of interest relates to
compounds
of formula I wherein X represents 0 or CH2. Within this subset of compounds,
all other variables are as
defined with respect to formula I.
Another subset of compounds that is of interest relates to compounds of
fonnula I
wherein R~ and R3 are independently H, Cl_3alkyl, OH or haloC1_3alkyl. Within
this subset of
compounds, all other variables are as defined with respect to formula I.
-11-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
More particularly, another subset of compounds that is of interest relates to
compounds
of formula I wherein R2 and R3 are independently H, CI-3alkyl or
haloC1_3alkyl. Within this subset of
compounds, all other variables are as defined with respect to formula I.
More particularly, a subset of compounds that is of interest relates to
compounds of
formula I wherein R2 and R3 are independently H or methyl. Within this subset
of compounds, all other
variables are as defined with respect to formula I.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein n represents an integer of from 2 to 4. Within this subset of
compounds, all other variables are
as defined with respect to formula I.
More particularly, a subset of compounds that is of interest relates to
compounds of
formula I wherein n is 2. Within this subset of compounds, all other variables
are as defined with
respect to formula I.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein each R~ is H or is independently selected from the group consisting
of: halo, CI-3alkyl optionally
substituted with 1-3 halo groups and 0-1 OC,_3alkyl groups. Within this subset
of compounds, all other
variables are as defined with respect to formula I.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein each R4 is H or is independently selected from halo or CI-3alkyl
optionally substituted with 1-3
halo groups. Within this subset of compounds, all other variables are as
defined with respect to formula
I.
Another subset of compounds that is of interest relates to compounds of
formula I
wherein RS represents -COaH. Within this subset of compounds, all other
variables are as defined with
respect to formula I.
A particular subset of compounds that is of interest relates to compounds of
forrnula I or
a pharmaceutically acceptable salt or solvate thereof wherein:
ring A is a phenyl or naphthyl group, a 5-6 membered monocyclic heteroaryl
group or a
9-13 membered bicyclic or tricyclic heteroaryl group;
each R' is H or is selected from the group consisting of:
a) halo, OH, CN, NH2 and S(O)o_2Re wherein Re is methyl or phenyl optionally
substituted with 1-3 halo groups;
b) Cl_3 alkyl and OCl_3alkyl, each being optionally. substituted with 1-3
groups, 1-3 of
which are halo and 1-2 of which are selected from: OH, NH2, NHC14alkyl and CN;
c) NR'SO2R" and NR'C(O)NR"R"' wherein:
R' represents H, C,_3alkyl or haloCl_sallcyl,
R" represents (a) C,_$alkyl optionally substituted with 1-4 groups, 0-4 of
which are
halo, and 0-1 of which are selected from the group consisting of: OC1_6alkyl,
OH, CO2H, CO2C1_4alkyl,
CO2C1_4haloalkyl, OC02C1-4alkyl, NH2, NHC14alkyl, N(Cl4alkyl) a, CN, Hetcy,
Aryl and HAR,
-12-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
said Hetcy, Aryl and HAR being further optionally substituted with 1-3 halo,
Cl.
4alkyl, Cl.4alkoxy, haloCl.4alkyl and haloC1.4alkoxy groups;
(b) Hetcy, Aryl or HAR, said Aryl and HAR being further optionally
substituted with 1-3 halo, CI_4alkyl, Cl-4allcoxy, haloC1.4alkyl and
haloCl_4alkoxy groups;
and R"' representing H or R"; and
d) phenyl or a 5-6 membered heteroaryl or a heterocyclic group attached at any
available
point and being optionally substituted with 1-3 groups, 1-3 of which are halo,
Cl_3alkyl or haloCI.3alkyl
groups, 1-2 of which are OCI.3alkyl or haloOC1.3alkyl groups, and I of which
is selected from the group
consisting of:
i) OH; COzH; CN; NH2; S(O)0.2Re wherein Re is as described above;
ii) NHC1.4a1ky1 the alkyl portion of which is optionally substituted with 1-3
groups, 1-3 of which are halo and 1 of which is selected from: OH, COZH,
CO2C14alkyl, COzCI.
ahaloalkyl, NH2, NHC14alkyl, N(C,.4alkyl) 2 and CN;
iii) C(O)NH2, C(O)NHC14alkyl, C(O)N(CI.4alkyl) 2, the alkyl portions of which
are optionally substituted as set forth in (b) above; and
iv) NR'C(O)R" and NR'SO2R" wherein R' and R" are as described above;
a and b are 1 or 2 such that the sum of a and b is 2 or 3;
X represents 0 or CH2;
Rz and R3 are independently H, OH, C,.3alkyl or haloCl_3alkyl;
n represents 2;
R4 is H or is independently selected from the group consisting of: halo,
Cl_3alkyl
optionally substituted with 1-3 halo groups or 0-1 OC1.3alkyl groups; and
R5 represents -CO2H.
A more particular subset of compounds that is of interest relates to compounds
of
formula I or a pharmaceutically acceptable salt of solvate thereof wherein:
ring A is selected from the group consisting of:
\
j
HN N
O-N N I / S
/S~ N0 I NPi and HNN~
_N \-~ N
\N \
each R' is independently H, CH3, phenyl, 4-hydroxy-phenyl, OH, 2-hydroxy-
phenyl, 3-
hydroxy-phenyl, 3-amino-phenyl, 2,3-dihydro-benzofuran-6-yl, 2-chloro-4-
hydroxy-phenyl, 1H-pyrazol-
4-yl, 5-hydroxy-pyridin-2-yl, 4-hydroxy-pyrazol-1-yl, 1H-[1,2,3]triazol-4-yl,
or 5-fluoro-pyridin-2-yl;
-13-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
a and b are 1 or 2 such that the sum of a and b is 2 or 3;
X represents CH2;
each Ra and R3 is independently H, OH or CH3;
n represents 2;
R4 is H, CH3, CH 2CH3, CF3 or CH2OCH3a and
RS represents -CO2H.
Representative examples of species that are of interest are shown below in
Table I.
Within this subset of compounds, all other variables are as originally defined
with respect to formula I.
TABLE 1
Compound 1 Compound 2 Compound 3
~ 0 0
N \ \ C / N \ \ / H \ \
HOOG H HO O H HO 0 OH
Compound 4 Compound 5 Compound 6
OH O
/ N O
H \ \ I ~ H O I~ ~ I S NH N'O
HO O HO 0
HO :CO
OH
Compound 7 Compound 8 Compound 9
o /~
\
H ~ / C N 0 ~. \ /
HO O HO O H H I/ \
HO O
Compound 10 Compound 11 Compound 12
HO / OH
O
/ \\ ~ o H NHZ
H QNc~ HO 0
~
HO O HO O H
Compound 13 Compound 14 Compound 15
o 0
~
O
C
QNLCOO H HOOC HO O I \ 0 / HO
OH
-14-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Compound 16 Compound 17 Compound 18
I o oII O
HO e \ ,J~N ~' HO ~
NN~J I H COZH N,J I H COzH ~ N~~{
~ / HOZC
HO S
Compound 19 Compound 20 Compound 21
o
N CO2H
N~ SN /
N H
\
I H
~ ~ >%N HO2C N_NH Ci O
HO ~ S
Compound 22 Compound 23 Compound 24
N 1 H C02H HO ~\ (N-O H O OH O~
HN N / ~=N N~N / HO ~ N N ~~~
N\ , S O N-J H CO2H
Compound 25 Compound 26 Compound 27
O
N (~1-0 COOH
~N ~ O~ HO ' N /
HO N
HO \ YN N N
N,O H COzH N. ~O H COzH p
Compound 28 Compound 29 Compound 30
o
~H COZH ~ ~ HN
/ NO N / HO /\ NN H COOH N=N H ~
HO--~N
HO
O
Compound 31 Compound 32 Compound 33
O OH O
HO Nj~ N HO NN P
N 0 N N v ~ N
~ NN
O ~O H CO2H HO - N ~O
H COZH
Compound 34 Compound 35 Compound 36
COOH
N O
s
~ ~
N Ny~/ ~ 0 N OH F3C NH
HO /
~ I N O OHH N ~~ N N
N-0 H COzH O' O_ OH
N
Compound 37 Compound 38 Compound 39
a COOH IO
0
OH NH O
HO O H O-N N O~N / N-
F N~F
O--N O_N N
O
HO
-15-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Compound 40 Compound 41
OH Nzz: OH
o o
NH NH
ccOOH I COOH
Pharmaceutically acceptable salts and solvates thereof are included as well.
Many of the compounds of formula I contain asymmetric centers and can thus
occur as
racemates and racemic mixtures, single enantiomers, diastereomeric mixtures
and individual
diastereomers. All such isomeric forms are included.
Moreover, chiral compounds possessing one stereocenter of general formula I,
may be
resolved into their enantiomers in the presence of a chiral environment using
methods known to those
skilled in the art. Chiral compounds possessing more than one stereocenter may
be separated into their
diastereomers in an achiral environment on the basis of their physical
properties using methods known to
those skilled in the art. Single diastereomers that are obtained in racemic
form may be resolved into their
enantiomers as described above.
If desired, racemic mixtures of compounds may be separated so that individual
enantiomers are isolated. The separation can be carried out by methods well
known in the art, such as
the coupling of a racemic mixture of compounds of Formula I to an
enantiomerically pure compound to
form a diastereomeric mixture, which is then separated into individual
diastereomers by standard
methods, such as fractional crystallization or chromatography. The coupling
reaction is often the
formation of salts using an enantiomerically pure acid or base. The
diasteromeric derivatives may then be
converted to substantially pure enantiomers by cleaving the added chiral
residue from the diastereomeric
compound.
The racemic mixture of the compounds of Formula I can also be separated
directly by
chromatographic methods utilizing chiral stationary phases, which methods are
well known in the art.
Alternatively, enantiomers of compounds of the general Formula I may be
obtained by
stereoselective synthesis using optically pure starting materials or reagents.
Some of the compounds described herein exist as tautomers, which have
different points
of attachment for hydrogen accompanied by one or more double bond shifts. For
example, a ketone and
its enol form are keto-enol tautomers. Or for example, a 2-hydroxyquinoline
can reside in the tautomeric
2-quinolone form. The individual tautomers as well as mixtures thereof are
included.
DosingInformation
The dosages of compounds of formula I or a pharmaceutically acceptable salt or
solvate
thereof vary within wide limits. The specific dosage regimen and levels for
any particular patient will
depend upon a variety of factors including the age, body weight, general
health, sex, diet, time of
administration, route of administration, rate of excretion, drug combination
and the severity of the
-16-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
patient's condition. Consideration of these factors is well within the purview
of the ordinarily slcilled
clinician for the purpose of determining the therapeutically effective
or_prophylactically effective dosage
amount needed to prevent, counter, or arrest the progress of the condition.
Generally, the compounds
will be administered in amounts ranging from as low as about 0.01 mg/day to as
high as about 2000
mg/day, in single or divided doses. A representative dosage is about 0.1
mg/day to about 1 g/day. Lower
dosages can be used initially, and dosages increased to further minimize any
untoward effects. It is
expected that the compounds described herein will be administered on a daily
basis for a length of time
appropriate to treat or prevent the medical condition relevant to the patient,
including a course of therapy
lasting months, years or the life of the patient.
Combination Therapy
One or more additional active agents may be administered with the compounds
described
herein. The additional active agent or agents can be lipid modifying compounds
or agents having other
pharmaceutical activities, or agents that have both lipid-modifying effects
and other pharmaceutical
activities. Examples of additional active agents which may be employed include
but are not limited to
HMG-CoA reductase inhibitors, which include statins in their lactonized or
dihydroxy open acid forms
and pharmaceutically acceptable salts and esters thereof, including but not
limited to lovastatin (see US
Patent No. 4,342,767), simvastatin (see US Patent No. 4,444,784), dihydroxy
open-acid simvastatin,
particularly the ammonium or calcium salts thereof, pravastatin, particularly
the sodium salt thereof (see
US Patent No. 4,346,227), fluvastatin particularly the sodium salt thereof
(see US Patent No. 5,354,772),
atorvastatin, particularly the calcium salt thereof (see US Patent No.
5,273,995), pitavastatin also referred
to as NK-104 (see PCT international publication number WO 97/23200) and
rosuvastatin, also known as
CRESTOR ; see US Patent No. 5,260,440); HMG-CoA synthase inhibitors; squalene
epoxidase
inhibitors; squalene synthetase inhibitors (also known as squalene synthase
inhibitors), acyl-coenzyme A:
cholesterol acyltransferase (ACAT) inhibitors including selective inhibitors
of ACAT-1 or ACAT-2 as
well as dual inhibitors of ACAT-1 and -2; microsomal triglyceride transfer
protein (MTP) inhibitors;
endothelial lipase inhibitors; bile acid sequestrants; LDL receptor inducers;
platelet aggregation
inhibitors, for example glycoprotein IIb/IIIa fibrinogen receptor antagonists
and aspirin; human
peroxisome proliferator activated receptor gamma (PPAR-gamma) agonists
including the compounds
commonly referred to as glitazones for example pioglitazone and rosiglitazone
and, including those
compounds included within the structural class known as thiazolidine diones as
well as those PPAR-
gamma agonists outside the thiazolidine dione structural class; PPAR-alpha
agonists such as clofibrate,
fenofibrate including micronized fenofibrate, and gemfibrozil; PPAR dual
alpha/ganuna agonists;
vitamin B6 (also known as pyridoxine) and the pharmaceutically acceptable
salts thereof such as the HCl
salt; vitamin B 12 (also known as cyanocobalamin); folic acid or a
pharmaceutically acceptable salt or
ester thereof such as the sodium salt and the methylglucamine salt; anti-
oxidant vitamins such as vitamin
C and E and beta carotene; beta-blockers; angiotensin II antagonists such as
losartan; angiotensin
-17-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
converting enzyme inhibitors such as enalapril and captopril; renin
inhibitors, calcium channel blockers
such as nifedipine and diltiazem; endothelin antagonists; agents that enhance
ABCAl gene expression;
cholesteryl ester transfer protein (CETP) inhibiting compounds, 5-lipoxygenase
activating protein
(FLAP) inhibiting compounds, 5-lipoxygenase (5-LO) inhibiting compounds,
farnesoid X receptor
(FXR) ligands including both antagonists and agonists; Liver X Receptor (LXR)-
alpha ligands, LXR-
beta ligands, bisphosphonate compounds such as alendronate sodium;
cyclooxygenase-2 inhibitors such
as rofecoxib and celecoxib; and compounds that attenuate vascular
inflanunation.
Cholesterol absorption inhibitors can also be used in the present invention.
Such
compounds block the movement of cholesterol from the intestinal lumen into
enterocytes of the small
intestinal wall, thus reducing serum cholesterol levels. Examples of
cholesterol absorption inhibitors are
described in U.S. PatentNos. 5,846,966, 5,631,365, 5,767,115, 6,133,001,
5,886,171, 5,856,473,
5,756,470, 5,739,321, 5,919,672, and in PCT application Nos. WO 00/63703, WO
00/60107, WO
00/38725, WO 00/34240, WO 00/20623, WO 97/45406, WO 97/16424, WO 97/16455, and
WO
95/08532. The most notable cholesterol absorption inhibitor is ezetimibe, also
known as 1-(4-
fluorophenyl)-3(R)-[3(S)-(4-fluorophenyl)-3-hydroxypropyl)]-4(S)-(4-
hydroxyphenyl)-2-azetidinone,
described in U.S. Patent Nos. 5,767,115 and 5,846,966.
Therapeutically effective amounts of cholesterol absorption inhibitors include
dosages of
from about 0.01 mg/kg to about 30 mg/kg of body weight per day, preferably
about 0.1 mg/kg to about 15
mg/kg.
For diabetic patients, the compounds used in the present invention can be
administered
with conventional diabetic medications. For example, a diabetic patient
receiving treatment as described
herein may also be taking insulin or an oral antidiabetic medication. One
example of an oral antidiabetic
medication useful herein is metformin.
In the event that these niacin receptor agonists induce some degree of
vasodilation, it is
understood that the compounds of formula I may be co-dosed with a vasodilation
suppressing agent.
Consequently, one aspect of the methods described herein relates to the use of
a compound of formula I
or a pharmaceutically acceptable salt or solvate thereof in combination with a
compound that reduces
flushing. Conventional compounds such as aspirin, ibuprofen, naproxen,
indomethacin, other NSAIDs,
COX-2 selective inhibitors and the like are useful in this regard, at
conventional doses. Alternatively,
DP antagonists are useful as well. Doses of the DP receptor antagonist and
selectivity are such that the
DP antagonist selectively modulates the DP receptor without substantially
modulating the CRTH2
receptor. In particular, the DP receptor antagonist ideally has an affinity at
the DP receptor (i.e., K;) that
is at least about 10 times higher (a numerically lower K; value) than the
affinity at the CRTH2 receptor.
Any compound that selectively interacts with DP according to these guidelines
is deemed "DP selective".
This is in accordance with US Published Application No. 2004/0229844A1
published on November 18,
2004, incorporated herein by reference.
-18-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Dosages for DP antagonists as described herein, that are useful for reducing
or
preventing the flushing effect in mammalian patients, particularly humans,
include dosages ranging from
as low as about 0.01 mg/day to as high as about 100 mg/day, administered in
single or divided daily
doses. Preferably the dosages are from about 0.1 mg/day to as high as about
1.0 g/day, in single or
divided daily doses.
Examples of compounds that are particularly useful for selectively
antagonizing DP
receptors and suppressing the flushing effect include the following:
Compound A Compound B Compound C
MeOZS
qCc_CO2H CO H 2 N
p1~s vCH3 O~S~CH3 0 \/ CI
ci a
Compound D Compound E Compound F
MeO2S \ F i CO H N
.''s,COaH N N\ CO2H
o=S=O s
\
HO /CI CH3 ci CI
CI
Compound G Compound H Compound I
SO2-Me SMe ci
S \
/ CI SOzMe I I
SaCI CCO2H
N N COaH
Compound J Compound K Compound L
O SOZMe ci
SO2Me CI \ S 0 Br SO Me
ci
\ ~ ~ Z
N N COZH N~ I S \
N N CO2H N
C
OZH
Compound M Compound N Compound 0
ci SOZMe CI
N S \ ~ CF3 SOZMe -
SO2Me _
S \/ CI S ~~ F
\ N N CO2H
/ N CO2H N N CO2H
Compound P Compound Q Compound R
SO2Me Cl CI SO2Me
S \ ~ \ SO2Me ~ S \ ~ CH3
CCOH I~ ~ N N CO~H
N N COZH
-19-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Compound S Compound T Compound U
SOZMe ci S02Me
S SOZMe S f ci
~ /
ci
~~ ~ ~ I N 1
N N CO2H N N coZH N CO2H
Compound V Compound W Compound X
SO2Me H3cOZS F
b~N S~~ Ci N CozH \ I~ Co2H
I O1,S ~
~ N
CO H ci 0'~CH H3C I
2 3 ~ ci
Compound Y Compound Z Compound AA
O cl
F\ N COZH F/ I \ OH S ci
N~ ~ o
O; ~ N
p'S ci O=S=O ci I
CHg I N 0
CH3
Compound AB Compound AC Compound AD
HO2C o CO2H
O
~/ C\ CH3 ~/ N OH3 l\
N ~ NH H
O \
O CH3 O
O
CH3
Compound AE Compound AF Compound AG
cozH
F =.
.,
,,,///CO2H
I ~ VN
" 'eICO2H NH \ \ ~
\ ~ CI
O \ ~ 0 O ci
/
~
NI /
CH3
Compound AH Compound Al Compound AJ
F N
C02H 11 "" ,oIC02H F CO2H
N
S CF3
0 1~ CI ~~/CI CH3O2S H C
~
as well as the pharmaceutically acceptable salts and solvates thereof.
The compound of formula I or a pharmaceutically acceptable salt or solvate
thereof and
the DP antagonist can be administered together or sequentially in single or
multiple daily doses, e.g., bid,
- 20 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
tid or qid, without departing from the invention. If sustained release is
desired, such as a sustained
release product showing a release profile that extends beyond 24 hours,
dosages may be administered
every other day. However, single daily doses are preferred. Likewise, morning
or evening dosages can
be utilized.
Salts and Solvates
Salts and solvates of the compounds of formula I are also included in the
present
invention, and numerous pharmaceutically acceptable salts and solvates of
nicotinic acid are useful in
this regard. Alkali metal salts, in particular, sodium and potassium, form
salts that are useful as
described herein. Likewise alkaline earth metals, in particular, calcium and
magnesium, form salts that
are useful as described herein. Various salts of amines, such as ammonium and
substituted ammonium
compounds also form salts that are useful as described herein. Similarly,
solvated forms of the
compounds of formula I are useful within the present invention. Examples
include the hemihydrate,
mono-, di-, tri- and sesquihydrate.
The compounds of the invention also include esters that are pharmaceutically
acceptable,
as well as those that are metabolically labile. Metabolically labile esters
include Cl-4 alkyl esters ,
preferably the ethyl ester. Many prodrug strategies are known to those skilled
in the art. One such
strategy involves engineered amino acid anhydrides possessing pendant
nucleophiles, such as lysine,
which can cyclize upon themselves, liberating the free acid. Similarly,
acetone-ketal diesters, which can
break down to acetone, an acid and the active acid, can be used.
The compounds used in the present invention can be administered via any
conventional
route of administration. The preferred route of administration is oral.
Pharmaceutical Compositions
The pharmaceutical compositions described herein are generally comprised of a
compound of formula I or a pharmaceutically acceptable salt or solvate
thereof, in combination with a
pharmaceutically acceptable carrier.
Examples of suitable oral compositions include tablets, capsules, troches,
lozenges,
suspensions, dispersible powders or granules, emulsions, syrups and elixirs.
Examples of carrier
ingredients include diluents, binders, disintegrants, lubricants, sweeteners,
flavors, colorants,
preservatives, and the like. Examples of diluents include, for example,
calcium carbonate, sodium
carbonate, lactose, calcium phosphate and sodium phosphate. Examples of
granulating and disintegrants
include corn starch and alginic acid. Examples of binding agents include
starch, gelatin and acacia.
Examples of lubricants include magnesium stearate, calcium stearate, stearic
acid and talc. The tablets
may be uncoated or coated by known techniques. Such coatings may delay
disintegration and thus,
absorption in the gastrointestinal tract and thereby provide a sustained
action over a longer period.
-21-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
One embodiment of the invention that is of interest is a tablet or capsule
that is
comprised of a compound of formula I or a pharmaceutically acceptable salt or
solvate thereof in an
amount ranging from about 0.1mg to about 1000mg, in combination with a
pharmaceutically acceptable
carrier.,
In another embodiment of the invention, a compound of formula I or a
pharmaceutically
acceptable salt or solvate thereof is combined with another therapeutic agent
and the carrier to form a
fixed combination product. This fixed combination product may be a tablet or
capsule for oral use.
More particularly, in anotlier embodiment of the invention, a compound of
formula I or a
pharmaceutically acceptable salt or solvate thereof (about 0.1 to about 1000
mg) and the second
therapeutic agent (about 0.1 to about 500 mg) are combined with the
pharmaceutically acceptable carrier,
providing a tablet or capsule for oral use.
Sustained release over a longer period of time may be particularly important
in the
formulation. A time delay material such as glyceryl monostearate or glyceryl
distearate may be
employed. The dosage form may also be coated by the techniques described in
the U.S. Patent Nos.
4,256,108; 4,166,452 and 4,265,874 to form osmotic therapeutic tablets for
controlled release.
Other controlled release technologies are also available and are included
herein. Typical
ingredients that are useful to slow the release of nicotinic acid in sustained
release tablets include various
cellulosic compounds, such as methylcellulose, ethylcellulose,
propylcellulose, hydroxypropylcellulose,
hydroxyethylcellulose, hydroxypropylmethylcellulose, microcrystalline
cellulose, starch and the like.
Various natural and synthetic materials are also of use in sustained release
formulations. Examples
include alginic acid and various alginates, polyvinyl pyrrolidone, tragacanth,
locust bean gum, guar gum,
gelatin, various long chain alcohols, such as cetyl alcohol and beeswax.
Optionally and of even more interest is a tablet as described above, comprised
of a
compound of formula I or a pharmaceutically acceptable salt or solvate
thereof, and further containing an
HMG Co-A reductase inhibitor, such as simvastatin or atorvastatin. This
particular embodiment
optionally contains the DP antagonist as well.
Typical release time frames for sustained release tablets in accordance with
the present
invention range from about 1 to as long as about 48 hours, preferably about 4
to about 24 hours, and
more preferably about 8 to about 16 hours.
Hard gelatin capsules constitute another solid dosage form for oral use. Such
capsules
similarly include the active ingredients mixed with carrier materials as
described above. Soft gelatin
capsules include the active ingredients mixed with water-miscible solvents
such as propylene glycol,
PEG and ethanol, or an oil such as peanut oil, liquid paraffin or olive oil.
Aqueous suspensions are also contemplated as containing the active material in
admixture with excipients suitable for the manufacture of aqueous suspensions.
Such excipients include
suspending agents, for example sodium carboxymethylcellulose, methylcellulose,
hydroxypropylmethylcellulose, sodium alginate, polyvinylpyrrolidone,
tragacanth and acacia; dispersing
-22-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
or wetting agents,e.g., lecithin; preservatives, e.g., ethyl, or n-propyl para-
hydroxybenzoate, colorants,
flavors, sweeteners and the like.
Dispersible powders and granules suitable for preparation of an aqueous
suspension by
the addition of water provide the active ingredients in admixture with a
dispersing or wetting agent,
suspending agent and one or more preservatives. Suitable dispersing or wetting
agents and suspending
agents are exemplified by those already mentioned above.
Syrups and elixirs may also be formulated.
More particularly, a pharmaceutical composition that is of interest is a
sustained release
tablet that is comprised of a compound of formula I or a pharmaceutically
acceptable salt or solvate
thereof, and a DP receptor antagonist that is selected from the group
consisting of compounds A through
AJ in combination with a pharmaceutically acceptable carrier.
Yet another pharmaceutical composition that is of more interest is comprised
of a
compound of formula I or a pharmaceutically acceptable salt or solvate thereof
and a DP antagonist
compound selected from the group consisting of compounds A, B, D, E, X, AA,
AF, AG, AH, Al and AJ,
in combination with a pharmaceutically acceptable carrier.
Yet another pharmaceutical composition that is of more particular interest
relates to a
sustained release tablet that is comprised of a compound of formula I or a
pharmaceutically acceptable
salt or solvate thereof, a DP receptor antagonist selected from the group
consisting of compounds A, B,
D, E, X, AA, AF, AG, AH, AI and AJ, and simvastatin or atorvastatin in
combination with a
pharmaceutically acceptable carrier.
The term "composition", in addition to encompassing the pharmaceutical
compositions
described above, also encompasses any product which results, directly or
indirectly, from the
combination, complexation or aggregation of any two or more of the
ingredients, active or excipient, or
from dissociation of one or more of the ingredients, or from other types of
reactions or interactions of
one or more of the ingredients. Accordingly, the pharmaceutical composition of
the present invention
encompasses any composition made by admixing or otherwise combining the
compounds, any additional
active ingredient(s), and the pharmaceutically acceptable excipients.
Another aspect of the invention relates to the use of a compound of formula I
or a
pharmaceutically acceptable salt or solvate thereof and a DP antagonist in the
manufacture of a
medicament. This medicament has the uses described herein.
More particularly, another aspect of the invention relates to the use of a
compound of
formula I or a pharmaceutically acceptable salt or solvate thereof, a DP
antagonist and an HMG Co-A
reductase inhibitor, such as simvastatin, in the manufacture of a medicament.
This medicament has the
uses described herein.
Compounds of the present invention have anti-hyperlipidemic activity, causing
reductions in LDL-C, triglycerides, lipoprotein (a), free fatty acids and
total cholesterol, and increases in
HDL-C. Consequently, the compounds of the present invention are useful in
treating dyslipidemias. The
-23-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
present invention thus relates to the treatment, prevention or reversal of
atherosclerosis and the other
diseases and conditions described herein, by administering a compound of
formula I or a
pharmaceutically acceptable salt or solvate in an amount that is effective for
treating, preventing or
reversing said condition. This is achieved in humans by administering a
compound of formula I or a
pharmaceutically acceptable salt or solvate thereof in an amount that is
effective to treat or prevent said
condition, while preventing, reducing or minimizing flushing effects in terms
of frequency and/or
severity.
One aspect of the invention that is of interest relates to a compound in
accordance
with formula I or a pharmaceutically acceptable salt or solvate thereof for
use in a method of
treatment of the human or animal body by therapy.
Another aspect of the invention that is of interest relates to a compound in
accordance
with formula I or a pharmaceutically acceptable salt or solvate thereof for
use in a method for the
treatment of atherosclerosis, dyslipidemia, diabetes, metabolic syndrome or a
related condition in the
human or animal body by therapy.
More particularly, an aspect of the invention that is of interest is a method
of treating
atherosclerosis in a human patient in need of such treatment comprising
administering to the patient a
compound of formula I or a pharmaceutically acceptable salt or solvate thereof
in an amount that is
effective for treating atherosclerosis in the absence of substantial flushing.
Another aspect of the invention that is of interest relates to a method of
raising serum
HDL levels in a human patient in need of such treatment, comprising
administering to the patient a
compound of formula I or a pharmaceutically acceptable salt or solvate thereof
in an amount that is
effective for raising serum HDL levels.
Another aspect of the invention that is of interest relates to a method of
treating
dyslipidemia in a human patient in need of such treatment comprising
adniinistering to the patient a
compound of formula I or a pharmaceutically acceptable salt or solvate thereof
in an amount that is
effective for treating dyslipidemia.
Another aspect of the invention that is of interest relates to a method of
reducing serum
VLDL or LDL levels in a human patient in need of such treatment, comprising
administering to the
patient a compound of formula I or a pharmaceutically acceptable salt or
solvate thereof in an amount
that is effective for reducing serum VLDL or LDL levels in the patient in the
absence of substantial
flushing.
Another aspect of the invention that is of interest relates to a method of
reducing serum
triglyceride levels in a human patient in need of such treatment, comprising
administering to the patient a
compound of formula I or a pharmaceutically acceptable salt or solvate thereof
in an amount that is
effective for reducing serum triglyceride levels.
Another aspect of the invention that is of interest relates to a method of
reducing serum
Lp(a) levels in a human patient in need of such treatment, comprising
administering to the patient a
- 24 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
compound of formula I or a pharmaceutically acceptable salt or solvate thereof
in an amount that is
effective for reducing serum Lp(a) levels. As used herein Lp(a) refers to
lipoprotein (a).
Another aspect of the invention that is of interest relates to a method of
treating diabetes,
and in particular, type 2 diabetes, in a human patient in need of such
treatment comprising administering
to the patient a compound of formula I or a pharmaceutically acceptable salt
or solvate thereof in an
amount that is effective for treating diabetes.
Another aspect of the invention that is of interest relates to a method of
treating
metabolic syndrome in a human patient in need of such treatment comprising
administering to the patient
a compound of formula I or a pharmaceutically acceptable salt or solvate
thereof in an amount that is
effective for treating metabolic syndrome.
Another aspect of the invention that is of particular interest relates to a
method of
treating atherosclerosis, dyslipidemias, diabetes, metabolic syndrome or a
related condition in a human
patient in need of such treatment, comprising administering to the patient a
compound of formula I or a
pharmaceutically acceptable salt or solvate thereof and a DP receptor
antagonist, said combination being
administered in an amount that is effective to treat atherosclerosis,
dyslipidemia, diabetes or a related
condition in the absence of substantial flushing.
Another aspect of the invention that is of particular interest relates to the
methods
described above wherein the DP receptor antagonist is selected from the group
consisting of compounds
A through AJ and the pharmaceutically acceptable salts and solvates thereof.
METHODS OF SYNTHESIS FOR COMPOUNDS OF FORMULA I
Compounds of Formula I have been prepared by the following representative
reaction
schemes. It is understood that similar reagents, conditions or other synthetic
approaches to these
structure classes are conceivable to one slcilled in the art of organic
synthesis. Therefore these reaction
schemes should not be construed as limiting the scope of the invention. All
substituents are as defined
above unless indicated otherwise.
Scheme I R R
0 NH2 O~NH O--J\ NH
NH4OAc ~COOMe RCOOH,MsCI
(R~)2 COOMe [-\~COOMe - ~ COOH
MeOH a ~ ~
(R)2 DMAP, DCM ~
1 2 (R4)2 3 (R4)~ 4
Compounds of Formula I, where X represents CH2, a and b equal 1 and RCOOH
represents:
(Rl)3 A (CR2R3)n-C02H
- 25 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
can be prepared as illustrated in Scheme 1 by treatment of commercially
available methyl 2-
oxocyclopentane-l-carboxylate 1 with ammonium acetate in a polar solvent such
a methanol or ethanol
to give the methyl 2-amino cyclopent-l-ene-l-carboxylate 2. The amine 2 can be
coupled with the
appropriate acid in the presence of methanesulfonyl chloride (MsCI) and DMAP
to give the desired
amide 3. Finally, the ester can be saponified by one slcilled in the art using
such methods as NaOH or
LiOH -dioxane to give compounds with the structure 4.
Scheme 2
(R42 (R4)2~
R4)2 / II
NH2 MsCI, DMAP NxR -=- ~ NR
RCOOH H H
R'O 0 R'O O HO O
R=Me, 5
R= Et, 6
7 8
Compounds of Formula I, where X represents CH2, a represents 2, and b
represents 1
such that the sum of a and b is 3, can be prepared as illustrated in Scheme 2
by coupling commercially
available methyl or ethyl 2-amino-cyclo-hex-l-ene-l-carboxylate 5 or 6 with
the appropriate acid in the
presence of methanesulfonyl chloride and DMAP to give the desired amide 7. The
ester can be
saponified to the desired compound 8 by methods known to those skilled in the
art.
Scheme 3
O O
OH H2 OH
(R~)s ~ / / ~
Pd/C
9 (R')$ 10
Shown in Scheme 3 is the preparation of acid of the structure 10 from
commercially
available material 9 by methods known to one skilled in the art, such as
hydrogenation in a polar
solvent, such as methanol or ethanol, using Pd/C as a catalyst.
-26-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 4
0
CHO (Ph3)sP II O~ 0 H2
O
Me0 Toluene Me0 Pd/C
11 12
O O O
BBr3 NaOH
I ~ O -= I ~ ~ OMe ( OH
MeO ~ ~ ~ HO ~ ~ HO 15
13 14
O O
TBSOTf, TEA ~ ~ OTBS AcOH ~ ~ OH MsCI, DMAP
TBSOf/ / THF/H20 TBSOJ// NHZ
16 17 COOMe
O O
~ ~ NaOH H I ~ ~
Me0 O H OTBS THF-MeOH HO O 19 ~ ~ OH
18 f~ ~
Compounds with the structure 19 can be prepared by the chemistry outlined in
Scheme 4.
Thus, 6-methoxy-2-naphthaldehyde 11 can be treated with a suitable ylide such
as (tert-Butoxycarbonyl-
methylene)triphenyl-phospharane in a non-polar solvent such as toluene or
xylenes under refluxing
conditions to give the desired olefin 12. Hydrogenation of the double bond can
be accomplished using
standard conditions such as H2(g), Pd/C in a suitable polar solvent like
methanol or ethanol to give 13.
Removal of the methyl group in the methoxynaphthyl moiety can be accomplished
with boron tribromide
at low temperature, followed by a careful quenching of the reaction with
methanol to give the trans-
esterified product 14. Saponification of the ester was accomplished using
conditions described earlier.
The naphthol can be protected as the TBS ether using TBSOTf or TBS-Cl in the
presence of a suitable
base such as triethylamine or imidazole in dichloromethane. The TBS ester can
be hydrolyzed with a
mild acid such as acetic acid in THF-H20 to give the desired acid 17. This
acid can be coupled to the
methyl or ethyl2-aminocyclo-hex-1-ene-l-carboxylate in the presence of
methanesulfonyl chloride and
DMAP to give the desired amide 18. Finally, TBS ether removal and methyl ester
saponification can be
achieved using NaOH/THF-Ha0 providing compounds of the structure 19.
-27-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 5
~ 1) LDA ~ OMe LIH OH
Me I 2) CIC CCHOC a HZ OZMe Me O Me I O
20 2'I 22
O-N O O-N O TBSCI, Imid
~ ~ ~ ~ DMAP
~ BBr3 -~. I ~
NH20H HCI, Et3N OH OH
Me H
23 24
O
O-N O N
1) DMF, (COCIh I~ ~ H
TBS ~ NaOH, TBAF
I ~ OTBS ~ --
2);)0 NTBS OMe
25 26
OMe
DMAP
N O O-N O
H I% HIJ LiOH H HN-~1
~OMe 0OH
27 28
Compounds with the structure 28 can be prepared by the chemistry outlined in
Scheme 5.
Thus, treating a suitable tetralone such as 20 with LDA at low temperature
followed by the addition of a
suitable acylating agent such as 4-chloro-4-oxobutyrate provides the desired
diketo-ester 21. The ester
can be saponified using standard conditions known to one skilled in the art to
give the acid 22. The di-
ketone 22 can be converted to the fused isoxazole of the structure 23 by
refluxing with hydroxylamine
hydrochloride in the presence of a base such as triethylamine in an alcoholic
solvent such as methanol or
ethanol. De-protection of the methyl ether can be done with boron tribromide
in a suitable solvent such
as dichloromethane to give the desired alcoho124. Treatment of the
intermediate 24 with a silylating
agent such as TBS-Cl in the presence of a base such as imidazole or
triethylamine in a chlorinated
solvent like DCM gives the bis-silyl protected ester 25. The silyl ester 25
can be treated with oxalyl
chloride in a solvent such as DCM under anhydrous conditions followed by
coupling with methyl2-
aminocyclo-pent-l-ene-l-carboxylate to give the desired amide 26. The TBS
group can be removed
using aqueous TBAF. Finally, the methyl ester can be saponified using standard
conditions to give
compounds of the structure 28.
-28-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 6
Br COOH O \ B(OH)2 O OH
N~ Z9 N \ Br Ho I~ N \ \ ~
MsCI, DMAP, DCM ti I/ B H
Me0 O Me0 O (' uPhFerrPdCla Me0 0
30 1:1 THF:1M K2CO3 31
NaOH O / I OH
H I \ \
HO O
32
Scheme 6 outlines the strategy used to synthesize compounds of the structure
32.
Coupling conunercially available methyl or ethyl 2-aminocyclo-hex- 1 -ene- 1 -
carboxylate 5 or 6 with 3-(3-
5 bromophenyl) propionic acid 29 in the presence of methanesulfonyl chloride
and DMAP gives the
desired amide 30. The bromide 30 can be converted to 31 via a Suzulci reaction
with a suitable boronic
acid such as 4-hydroxy phenyl boronic acid in the presence of a catalyst such
as Bis-tert-butyl-ferrocene
palladium dichloride. The ester can be saponified by methods known to those
slcilled in the art providing
compounds of the structure 32.
Scheme 7
0
O Ph3Pyk0Et O
Me Me ~~ N\ \ OEt Pd/C, H2 Me ~~ OEt
1301C, o/n N-
MeOH ~
34
33 1
O O
Me0
~N ~ OEt + Me /\ N \OEt
N' j HCI/HOAc
O O O
~ BBr3 Me0 ~ \
TBSG, Imid H / OH OH
.i~0 OTBS
DMAP Ntt- _ tJ~
J N'
36 35
(COG)2, DMF ~S ~ ~ LiOH H ~ ~
DMAP N C~Me--" ~-N ~l' ~~{N"' 111C~H
~COZMe
~ 37
NH2
Scheme 7 outlines the strategy used to synthesize compounds of the structure
37.
Homologating aldehyde 33, followed by reduction provides 34 which may be
resolved into its
enantiomers via chiral HPLC . One enantiomer is shown in Scheme 7 for
illustrative purposes.
Hydrolysis of the ethyl esters provides the acid 35, followed by demethylation
and silylation to give 36.
This intermediate can be acylated with the cyclopentene fragment, and
saponified to provide biaryl
products such as 37.
- 29 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 8
~OEt
BrCH2C(O)CO2Et N p EtOH \ D~BALH
~}-NHZ . 1 ~NHZ*Br ~ ~ , CH2CIa
S DME ~O S reflux S -78C
38 reflux 39
p p 0
H n-BuLVTHF ' Ol' tosylhydrazide /--~OMe
-a I ~ N
I~~N -- I~ YN M OH
S (MeO)2POCH2CO2Me S
p p OII
LiOH ~ BBr3 /'~ OH TBSCI, Et3N /~p v OTBS
~ OH N ~ IN
THF/MeOH \ ~/ S N CHaGZ HO I/ S N TBSO I/ g
O
41 42
O
(COCI)2, DMF N LiOH
DMAP ~HO~ I%~{~N HO~C
CpzMe TBSO" S HO SJ
~ 43
NH2
Scheme 8 outlines the strategy used to synthesize compounds of the structure
43. The
aminobenzothiazole 38 may be N-alkylated and cyclized to form intermediate 39.
The ester can be
reduced to the aldehyde, and homologated to the enoate 40. This intermediate
can then be reduced and
5 saponified to provide the acid 41. Demethylation and silylation affords
intermediate 42, which can be
acylated and saponified once again as in Scheme 7 above, to provide products
such as 43.
Scheme 9
~ COOH O
NC~~CN
-78 LDA ~ NH2 Br I~ 46 NH ~~ Br
C to -20 C
44 MsCI, DMAP CN
47
B(OH)2 O O
4 ~ NaN3, ZnBrz
(tBuP)zFerrPdClz '// NH 2:1 dioxane-H20 N~
1:1 THF:1M K2C03 ~CN
49 Ni NH 50
N%N
10 Scheme 9 outlines the strategy used to synthesize compounds of the
structure 50.
Adiponitrile can be converted to the amino-nitrile 45 via a Thorpe-Ziegler
reaction using a suitable base
such as LDA in a solvent such as THF. Coupling the amino nitrile 45 to 3-(4-
bromophenyl) propionic
acid 46 in the presence of methanesulfonyl chloride and DMAP gives the desired
amide 47. The bromide
47 can be converted to 49 via a Suzuki reaction with a suitable boronic acid
such as phenyl boronic acid
15 in the presence of a catalyst such as 1,1 bis(di-tert-butylphosphino)-
ferrocene palladium dichloride.
Finally, treatment of the nitrile 49 with NaN3 in the presence of a Lewis acid
such as zinc bromide, in a
suitable solvent mixture such as dioxane-water gives tetrazoles such as 50.
-30-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 10
/N~ NaBH4 /N, PPh3, CBr4 /N~ NaH/THF ~, i COZEt
Br~\S CHO Br~\SJ~CHzOH --~ Br~S CH ~ Br -JG
51 MeOH ZBr MeCH(CO2Et)2 S CO2Et
Me0 B(OH)z
LIOH Br _,~ COzH DMF Br~\ ~ - ~I - Me0 r N
S COZH S COzH S COZH
52 Pd(PPh3)4, NaHCO3
CI
BBr3 N 1. TBS-CI, TEA/DMAP N, I CO H
HO /S~COzH 2.(COCI)2/DMF HO Si~N z
HZN II
CI 53 3. ~ CI O
EtO2C 54
4. LIOH
Scheme 10 outlines the strategy used to synthesize compounds of the structure
54. The
heterocyclic bromo aldehyde 51 can be homologated to the intermediate 52 via
several transformations
including the displacement of an activated bromide with a malonate anion. The
bromide 52 can be
arylated and demethylated to provide acid 53, which in turn may be acylated
and deprotected to provide
compounds such as 54.
Scheme 11
HZN~
//N~ I (COCI)2/DMF EtOzCJTI~ N H COZEt HNO
Br~\SJOH --= --. BrN / N-
52 IOI 0 4~-P(tBu
I )2
Fe PdCl2
N H CO2Et LiOH N I H CO2H
HN \ /SN / -y HN \ /S~ J~ N / ~P(tBu)z
- -
0 O THF/K2CO3
15
Scheme 11 displays a method for generating compounds of the structure 55. The
acid
intermediate 52 from Scheme 10 above may be acylated, the bromide coupled with
a heterocyclic
boronate ester, and this intermediate deprotected to provide compounds such as
55.
-31-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 12
0
NaH/PMBOH NHaOH.HCI ~ N-OH CI 11 O~
Br~CN PMBO~CN PMBO
N DMF N NaOH/EtOH N NHZ
56 Pyrfdine, 120-130 C
N (COCI)2/DMF/CH2CI2
PMBO ~~ r~ LIOH PMBO C N~
N N p~ THF/MeOWH20 N N OH HZN
57 0 58 p I
MeOaC
PMBO ~~ N_H p p~ TFA HO ~~ N~p O O~ LiOH
N N N ) Pr3SiH/CHZCIZ N NN
p THF/MeOH/HZO
O
HO ~ ){N~0 0 OH
'=N N~N
59 0
Scheme 12 demonstrates a synthetic strategy to access compounds of the
structure 59.
Starting from a pyridyl bromo nitrile such as 56, the bromide may be
displaced, the nitrile transformed to
an N-hydroxy amidine, this intermediate acylated and then cyclized to provide
the intermediate 57.
Saponification of 57 can give acid 58, which may be acylated, and after
deprotection provide compounds
such as 59.
Scheme 13
~CO2Me
1. KH, diglyme N N
HN~ 2 OZN /\ N~ NBS,hv OZN /~ N~Br NOHMe
60 OaN / \ Br
r _COaMe DMF, heat pZN / N N/~\
OZN / N N~COZMe LiOH pZN / N N~ HO~C
COZMe
JNMeOZC' ~ N~J I61
ZNHOAc H N / N \ H2SO4, NaNO2 HO / N \ 1) TBSCI, Et3N
a -NCOZMe N-COZH
N 90 C, 2 h 2) DMF, (COCI)Z
62 3) ~COZMe
I NHZ
O LiOH N
TBSO / N ~ Hp /~ N
1COZMe H COaH
63
- 32 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 13 outlines the strategy used to synthesize compounds of the structure
63. The
pyrazole 60 may be N-arylated, this intermediate homologated to nitro 61,
which in turn may be
transformed to hydroxy acid 62. Upon acylation and deprotection, compounds
such as 63 may be
obtained.
Scheme 14
0
G v 'OH
N DIBALH N NH?OH N ~NOH
PMBO ~ ~ CN PMBO ~ ~ CHO PMBOC~
NaOCI, DMF
64
O O
PMBO ~ N \~ OH TF~? ? HO ~ N \~ OH Et3N, TBSCI
N-O N-
O 65
N 1) (COCI)2 N O
TBSO OTBS TBSO ~ \ N P
N- 2) CO2Me ~ N- H CO2Me
aNH2
LiOH N O
HO N p
N_O H CO2H
66
Scheme 14 displays a method to access compounds of the structure 66. The
intermediate
64 generated in Scheme 12, may be elaborated into 65 via an intermediate oxime
cycloaddition with an
alkyne. The hydroxy acid 65 can be protected, acylated and deprotected to
provide compounds such as
66.
Scheme 15
O HZSO4 I O 1) OsOg, NMO O
PrOH \w~OPr 2) NaIOq ~OPr
3) NaC102 OH 67
1) SOCIZ O O
PMBO N~OPr LiOH PMBO ~ N \NOH
N NOH
2) PMBO C~ N N-
NHZ
O 0 1) (COCI)2 PMBO N N TFA . HO ~ N N~ Nq
2) ~COzMe - N~O H COZMe iPr3SfH N-O CO2Me
INHZ
O
LiOH N
HO ~ N_ N p
N-O H CO~H
68
-33-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 15 shows a synthetic route used to generate compounds of the structure
68. The
intermediate 67 may be accessed from an oxidative cleavage of the requisite
olefin. This alpha-methyl
acid 67 may then be condensed with an N-hydroxy amidine, and this intermediate
elaborated into
compounds such as 68 using methods illustrated in the Schemes above.
Scheme 16
O 1) OsOg, NMO ~ 1)SOCIZ B NO
~OEt 2) NaiOq ~O OH PM ~N~O~
3) NaCIOZ O 2) C~ N-OH O
PMBO N NH2
(COCI)Z DMF
(~ N,O LiOH PMBO N~ ~ IOH PMBO c NO 1 H COOPMB
PMB N NOEt N O N N N
COOPMB
0 HaN~) 69 O
TFA, iPr3SiH HO /\\ N O H COOH
NN~
70 0
Scheme 16 outlines the strategy used to synthesize compounds of the structure
70. This
methodology follows closely to that illustrated in Scheme 15 above, where
intermediate 69 can now be
doubly deprotected in one step to provide compounds such as 70, containing a
methyl group alpha to the
amide moiety.
-34-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 17
/1~ amyl nitrite, CuCiZ ' ~1~ I ~ N H ~~--CO2Me
HZN~S C02Me CIS CO2Me -> I~N
NaH N
71
1) iPrMgCI, B(OMe)3 N~ j-CO2Me C
O2Me TBSCI, Et3N S DIBALH
-a
2) H202 HO ~ N~S TBSO CN
N
72
/N II~--CH2OH DMP JI-CHO nBuLi, MeOCOCH2P(O)(OMe)2
TBSOJ\ TBSO-CN
N ~ CO2Me
pTsNHNH2 N- 11 ~COZMe LiOH
TBSO N
~NJ\ TBSO-~\NJ~
73
1. (COCI)2, DMF H CO2H
N~ CO2H jj~rN /
HZN CO2Me HON ~S O
HONS)--' 2.
CN / ~ 74
3. LiOH
Scheme 17 displays methodology to synthesize compounds of the structure 74. An
amino heterocycle such as 71 can be converted to its halide and displaced with
a nitrogen anionic
heterocycle, followed by hydroxyl introduction to provide intermediate 72.
This ester 72 may be
homologated to 73, and upon acylation and protecting group manipulation,
converted to compounds such
as 74.
- 35 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 18
0
0--/ \-NHz NaNO2 O / %Nz+ CNCHZCOZMe O / %NN_-~Oi LIBHq
10%HCI NaOAc, MeOH \--N
0 0
O / \N~~OH Dess-Martin's reagent\ O/~ N~(Me0)zP(O)CHZCOZMe
\
NVN H n-BuLi ONN' v O
0 0
Hz, Pd/C LiOH BBr3
N~N THF/MeOH/H20 \O N~~OH
76
O O HzN
O
HO /~ N~OH (COC i)z HO NNiCI MeOZC HO /~ NN COZMe
O
LIOH / ~ N~N ~
HO -N~N H COOH
77
Scheme 18 displays a method for generating compounds of the structure 77. The
intermediate 75 can be accessed from 4-methoxyaniline via dipolar
cycloaddition, and then homologated
5 into 76. Following some of the methods illustrated in the Schemes above, 76
can be converted into
compounds such as 77.
Scheme 19
Sf~ ~N\
~ N N
\\ ~0 (M O)iPOCHyCOOMe \\ - - _
BuLVTHF \ / \ Cul/OMHMaOH ~ -N \ / \ O LIOH ry N \ / \ O
H
78 /P O H OH
79
NHz
I O\ NN
O I N N O LIOH ' N N ~ ~ O
LIOHIMoOH N-N H H '- \
H OH H H ~
p 80 HO
10 O O
Scheme 19 displays a method to access compounds of the structure 80. Alkyne 78
may
be homologated and undergo a cycloaddition reaction to generate intermediate
79. The enoate 79 may
then be converted into compounds such as 80 using methods illustrated in the
Schemes above.
-36-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 20
0 OH H3CO" 'OCH3 0 OA- 1. CDI N'O O
HOA~~ OH HO~ O PMBO ~
IOI p-TSOH 'O' 2= ~~ N-OH N N~
81 PMBO
N NHZ O
1)TFA H-O OH TBSCI NO OTBS
TBSO (~ ~ OTBS
2) 5 aKOH -N N~ TEA/DMAP -N
82 0 0
1.Oxalyl-CI O\ 0 OH
DMF/CH2CI2 NO OTBA O LiOH ~~ N~ OH H
2. HONN o HON~N
H3CO 0 83 O
H2N I
Scheme 20 illustrates a strategy used to synthesize compounds of the structure
83. Malic
acid 81 can be orthogonally protected, condensed with an N-hydroxy amidine,
and deprotected to
generate 82. The bis-hydroxyacid 82 may be globally silylated, and then
acylated and deprotected to
provide alpha-hydroxy compounds such as 83.
Scheme 21
0
0
OH HZSO4, EtOH 0
OEt OsO4, NMO /~ O~ NalO4 H-~OEt
HC/1\I ~\ OEt
OH O
NaCIO2 O 1) (COCI)2, DMF N 0 LiOH
HO-~oEt PMBO ~ \ NOEt
O 2) N NOH N,p
~ PMBO ~
NH2
TfO
~ l OH 1) N-OH -OH ry NH~
PMBO ~ 1 N~ EDCI PMBO_~~'\~N, COzMe
Pd2(dba)3, Xantphos,
N-O N_O
2) NH3, H20 85 Cs2CO3, dioxane
O N~ 1) TFA, iPr3SiH O
N ~ N (~\/1
PMBO ~ N 2) UGH
HO \ N,
N
N-O H CO2Me - N~p H COzH
86
Scheme 21 displays a strategy used to generate compounds of the structure 86.
Orthogonally protected acid ester intermediate 84 can be obtained via
oxidative degradation of the
requisite olefinic starting material. The acid 84 may then be condensed with
an N-hydroxy amidine, and
manipulated to provide a primary carboxamide 85. A primary carboxamide
intermediate such as 85 may
-37-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
undergo a coupling reaction with an enol triflate, and upon further
deprotection reactions, provide
geminal dimethyl compounds such as 86.
Scheme 22
' NHZ O
O O LIHMDS, NCCOZMe NH4OAc, MeOH
1) HCI, H20 50Me ~OMe
87
(S)-pulegone
N ~' ~ J~ HCI, HOAc HO N\' ~0 1) (COCI)2 N
PMBO O
' HO ~ ~ \ L'r~~' xN ~
~N ' Y 'OEt (' ~)_ sN r ~' OH Z)
N-O I N-p COZMe N- p H COZMe
IaNH2
LiOH O 87
HO ~/ N NN ~
N_O H COZH
88
Scheme 22 outlines a methodology to access compounds of the structure 88.
Commercially available (S)-pulegone can be converted into intermediate 87 via
reverse aldol, acylation
with Mander's reagent, and enamine formation. Using a similar alpha-methyl
ester intermediate
illustrated in Scheme 16, chiral amine 87 may be elaborated into compounds
such as 88.
Scheme 23
0 0 0
UHMDS T OMe NaH
NGCOgMe Ct Tf0 9
~
I COaMe O
N NTfZ OH
O OH O'N N
89
pMBO (~/ N N~ NH3,MeOH N O
OMe pMBO ~ N ~~NHZ
N-O N-O
Scheme 23 displays a strategy to access compounds of the structure 89.
Conunercially
available symmetrical ketones, such as 4-ethylcyclohexanone, can be acylated
with Mander's reagent,
followed by enol triflate formation with Comins' reagent. Using similar metal
catalyzed coupling
methodology illustrated in Scheme 21, different regioisomerically substituted
cyclohexene compounds
such as 89 may be accessed.
- 38 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 24
OH 0 1) LIHMDS NH2
F3Cb 1)Rh ,Hz ~ NCCO2Me COZMe
CF3
2) D=M 2) NH40Ac
FaC F3C ~ O
N O HN N OH
PMBO 1 N~~OH 0 OH
& 90
N-O
Scheme 24 illustrates a methodology to access compounds of the structure 90.
Commercially available 3-(trifluoromethyl)phenol can be reduced to a hydroxy
cyclohexane, oxidized
with Dess-Martin reagent to the ketone, acylated with Mander's reagent, and
followed by enamine
formation. Using similar methodologies illustrated above, compounds such as 90
may be obtained that
possess a trifluoromethyl substituted cyclohexene.
Scheme 25
0 0 1) LiHMDS NHy
Cul, MeLi ~ NCCOZMe ttt
COZMe
2) NHyOAc
Of
OH
N O H N N
PMBO ~ 1 NOI..I O OH
~ 91
N-O
Scheme 25 displays a strategy to access compounds of the structure 91.
Commercially
available 3-methyl-2-cyclohexen-1-one can be substituted to generate a 3-
geminal-dialkyl
cyclohexanone. Upon acylation with Mander's reagent, enamine formation, and
following similar
methodologies illustrated above, compounds such as 91 may be obtained that
possess a geminal dialkyl
substituted cyclohexene.
-39-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 26
0
ciA~ ~
HZN /\ CN NaNOa FCN NHaOH FN-OH O }
N HF-pyridine N N NFiZ
Pyridlne, 120-130 C
92
/ \ N-O NaOH F ( \ N,O (COCI)2/DMF/CH2CI2
F
N N-O-' N NOH HzN
93 O O
MeOzC
H / N'O OH
/\ N-O LiOH F~
F---~~N ~N
~N N O 94 O
Scheme 26 shows a method to access compounds of the structure 94. Commercially
available pyridine 92 can be fluorinated and incorporated into a fluoro biaryl
intermediate such as 93.
Subsequent hydrolysis, acylation, and saponification following similar
methodologies illustrated above,
may provide fluoropyridyl compounds such as 94.
Scheme 27
OH 1) TBSCI OTBS 1) NaH, Mel OH 1) Swem NHZ C Me
Oz
(/ CHO 2) NaBH4 6 OH 2) TBAF ~O,, 2) NaH, Me2CO3 ~O \
3) Rh, H2 3) NH4OAc
95 96
NH2 I
~COZMe O
0 0
N / ~~N
F/ 1 N~OH -. H _0. ~ N j F
N-O OH N
97
Scheme 27 illustrates a method to generate compounds of the structure 97.
Commercially available 3-hydroxybenzaldehyde can be converted into the ether
substituted cyclohexane
intermediate 95 via the key reduction of the phenyl ring. Upon ketone
formation and similar
methodologies described above, the ether substituted cyclohexene aminoester 96
can be obtained. This
enamine intermediate may be acylated and deprotected as described above, to
generate compounds such
as 97 that possess an ether substituted cyclohexene.
- 40 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Scheme 28
LDA
OO NCCO2Me OOOMe Ci NaH O
~ O~COOMe
I
N NTf2 98
O 1) Pd/C, H2
~ CHO Toluene O~ O 2) BBr3
Me0 ~ (Ph3)3P' Me0
O 3) NHdOH
~ /OTf
O (~
O O
I NHZ O COOMe H
~ ~
98
HO ~
1) Pd2(dba)3, Xantphos HO O 100 OH
99 2) NaOH
Scheme 28 displays methodology to access compounds of the structure 100.
Commercially available tetrahydro-4-H-pyran-4-one can be converted into the
dihydropyran triflate
intermediate 98 via similar methodologies described above. In parallel,
commercially available 6-
methoxy-2-naphthaldehyde can be converted into the primary carboxamide
intermediate 99, also via
similar methodologies described above. Intermediates 98 and 99 may be coupled
under similar metal
catalyzed methodology illustrated in Scheme 21, to generate compounds such as
100 that possess a
dihydropyran carboxylic acid moiety.
Scheme 29
0 1) LiHMDS OTf 0
O O ppph3 O O 1) Pd/C NCC02Me
KHMDS_ MeOH OMe
toluene 2) HCIlTHF/ 2) NaH
MeOH CI
O I Et Pr ~~ Pr
102 103 N NTf2 104
101
HO Nzz
NHZ Ok-I
99 O
NH
1. Pd2(dba)3, XANTPHOS, CsZCO3 p
COOH
2. THF/MeOH, LiOH 105
Scheme 29 illustrates a methodology to access compounds of the structure 105.
The
ketone 101 (for preparation see Danishefsky, et al J. Am. Claena. Soc. 2004,
126, 14358) can be converted
to the olefin 102, followed by reduction of the double bond using standard
hydrogenation conditions, and
acid catalyzed removal of the ketal protecting group to provide the ketone
103. This material can be
acylated using Mander's reagent to give the desired ketoester that is
converted to the enol trifate 104 with
Comins' reagent. Intermediates 104 and 99 may be coupled using similar metal
catalyzed methodology
-41-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
illustrated in Scheme 21. Saponification of the methyl ester using standard
conditions can generate
vicinal disubstituted cyclohexene compounds such as 105.
The various organic group transformations and protecting groups utilized
herein can be
performed by a number of procedures other than those described above.
References for other synthetic
procedures that can be utililized for the preparation of intermediates or
compounds disclosed herein can
be found in, for example, M.B. Smith, J. March Advanced Organic Chemistry, 5"'
Edition, Wiley-
Interscience (2001); R.C. Larock Comprehensive Organic Transformations, A
Guide to Functional Group
Preparations, 2"d Edition, VCH Publishers, Inc. (1999); T.L. Gilchrist
Heterocyclic Chemistry, 3d
Edition, Addison Wesley Longman Ltd. (1997); J.A. Joule, K. Mills, G.F. Smith
Heterocyclic Chemistry,
3d Edition, Stanley Thornes Ltd. (1998); G.R. Newkome, W.W. Paudler Contempory
Heterocyclic
Chemistry, John Wiley and Sons (1982);or Wuts, P. G. M.; Greene, T. W.;
Protective Groups in Organic
Synthesis, 3'd Edition, John Wiley and Sons, (1999), all six incorporated
herein by reference in their
entirety.
REPRESENTATIVE EXAMPLES
The following examples are provided to more fully illustrate the present
invention, and
shall not be construed as limiting the scope in any manner. Unless stated
otherwise:
(i) all operations were carried out at room or ambient temperature (RT), that
is, at a
temperature in the range 18-25 C;
(ii) evaporation of solvent was carried out using a rotary evaporator under
reduced
pressure (4.5-30 imnHg) with a bath temperature of up to 50 C;
(iii) the course of reactions was followed by thin layer chromatography (TLC)
and/or
tandem high performance liquid chromatography (HPLC) followed by mass
spectroscopy (MS), herein
termed LCMS, and any reaction times are given for illustration only;
(iv) yields, if given, are for illustration only;
(v) the structure of all final compounds was assured by at least one of the
following
techniques: MS or proton nuclear magnetic resonance (1H NMR) spectrometry, and
the purity was
assured by at least one of the following techniques: TLC or HPLC;
(vi) 1H NMR spectra were recorded on either a Varian Unity or a Varian Inova
instrument at 500 or 600 MHz using the indicated solvent; when line-listed,
NMR data is in the form of
delta values for major diagnostic protons, given in parts per million (ppm)
relative to residual solvent
peaks (multiplicity and number of hydrogens); conventional abbreviations used
for signal shape are: s.
singlet; d. doublet (apparent); t. triplet (apparent); m. multiplet; br.
broad; etc.;
(vii) MS data were recorded on a Waters Micromass unit, interfaced with a
Hewlett-
Packard (Agilent 1100) HPLC instrument, and operating on MassLynx/OpenLynx
software; electrospray
ionization was used with positive (ES+) or negative ion (ES-) detection; the
method for LCMS ES+ was
1-2 mL/min, 10-95% B linear gradient over 5.5 min (B = 0.05% TFA-acetonitrile,
A= 0.05% TFA-
- 42 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
water), and the method for LCMS ES- was 1-2 mL/min, 10-95% B linear gradient
over 5.5 min (B =
0.1 lo formic acid - acetonitrile, A = 0.1% formic acid - water), Waters
XTerra C 18 - 3.5 um - 50 x 3.0
mmID and diode array detection;
(viii) automated purification of compounds by preparative reverse phase HPLC
was
performed on a Gilson system using a YMC-Pack Pro C18 colunm (150 x 20 mm
i.d.) eluting at 20
mL/min with 0- 50% acetonitrile in water (0.1% TFA);
(ix) the manual purification of compounds by preparative reverse phase HPLC
(RPHPLC) was conducted on either a Waters Symmetry Prep C18 - 5 um - 30 x 100
mmID, or a Waters
Atlantis Prep dCl8 - 5 um - 20 x 100 mmID; 20 mL/min, 10-100% B linear
gradient over 15 min (B =
0.05% TFA-acetonitrile, A = 0.05% TFA-water), and diode array detection;
(x) the purification of compounds by preparative thin layer chromatography
(PTLC)
was conducted on 20 x 20 cm glass prep plates coated with silica gel,
commercially available from
Analtech;
(xi) flash column chromatography was carried out on a glass silica gel column
using
Kieselgel 60, 0.063-0.200 mm (Si02), or a Biotage SiO2 cartridge system
including the Biotage Horizon
and Biotage SP-1 systems;
(xii) chemical symbols have their usual meanings, and the following
abbreviations
have also been used: h (hours), min (minutes), v (volume), w (weight), b.p.
(boiling point), m.p. (melting
point), L (litre(s)), mI. (millilitres), g (gram(s)), mg (milligrams(s)), mol
(moles), mmol (millimoles), eq
or equiv (equivalent(s)), IC50 (molar concentration which results in 50% of
maximum possible
inhibition), EC50 (molar concentration which results in 50% of maximum
possible efficacy), uM
(micromolar), nM (nanomolar);
(xiii) definitions of acronyms are as follows:
BBr3 is boron tribromide B(OMe)3 is trimethyl borate
Comins' reagent is 2-[N,N- CDI is 1,1'-carbonyl diimidazole
Bis(trifluromethylsulfonyl)amino]-5-
chloropyridine
DCM is dichloromethane (methylene chloride) DIBALH is diisobutyl aluminum
hydride
DMF is dimeth lformamide DMAP is 4-dimethyl amino pyridine
DMSO is dimethyl sulfoxide iPrMgCl is iso ro l magenisium chloride
KHMDS is potassium bis trimethylsil 1 amide LDA is lithium diiso ro yl amide
LiHMDS is lithium bis(trimeth lsil l) amide Mander's reagent is methyl
cyanoformate
NBS is N-bromo-succinimide
NaOCI is sodium h ochlorite NMO is 4-meth lmo holine N-oxide
OTf is triflate Pd(PPh3)4 is tetrakis tri henyl hos hine palladium (0)
Pd2(dba)3 is Tris(dibenzylideneacetone) TBAF is tetrabutylammonium fluoride;
di alladium (0);
TBS Chloride is t-bulyl dimethyl silyl chloride TBSOTF is t-butyl dimethyl
silyl trifluoromethane
sulfonate
TFA is trifluoroacetic acid THF is tetrahydrofuran
XANTPHOS is 9,9-Dimethyl-4,5-bis(diphenyl-
hos hino xanthene
- 43 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
EXAMPLE 1
O
? \ \
HOOC H ~ / /
To a solution of inethyl-2-oxocyclopentane-l-carboxylate (1.5g, 10.55 mmol) in
methanol was added ammonium acetate (4.07 g, 52.76 mmol). After stirring the
reaction at room
temperature for 18 h, it was concentrated in vacuo. The residue was dissolved
in DCM, washed with
water, brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuo.
This cyclopentene
aminoester intermediate was used without any further purification.
To a solution of 3-(2-naphthyl) acrylic acid (1.5 g, 7.56 mmol) in 1:1 ethanol-
ethyl
acetate (50 mL) was added Pd/C and the resulting mixture stirred under a Ha
balloon for 18 h. The
reaction mixture was filtered through celite, and concentrated in vacuo to
give the desired saturated
naphthyl acid as a white solid.
To a solution of this saturated naphthyl acid intermediate (150 mg, 0.75 mmol)
in DCM
(6 mL) cooled to 0 C was added DMAP (201 mg, 1.65 mmol) followed by
methanesulfonyl chloride
(0.059 mL, 0.75 mmol). After 5 min, the cyclopentene aminoester intermediate
(95 mg, 0.67 mmol) was
added as a solid. The mixture was stirred at RT for 18 h, and quenched with
saturated NH4Cl solution.
The resulting mixture was extracted with DCM. The organic layer was dried over
anhydrous Na2SO4
filtered and concentrated in vacuo. The residue was purified by flash
chromatography using 10% ethyl
acetate-hexanes as the eluant to give the desired amide product as a methyl
ester.
To a solution of this ester intermediate (15 mg, 0.046 mmol) in THF (2 mL),
was added
methanol (1 mL) followed by 1 N NaOH (1 mL). The resulting reaction mixture
was stirred at 23 C for
6 h. It was neutralized to pH=7 by the addition of IN HCI and extracted with
ethyl acetate. The organic
layer was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The
residue was purified by
reverse phase HPLC (Gilson) to provide Example 1. 'H NMR (500 MHz, CD3OD) 8
1.85 (m, 211), 2.49
(m, 2H), 2.8 (t, 2H), 3.1 (m, 4H), 7.4 (m, 3H), 7.7 (bs, 111), 7.8 (m, 311);
LCMS m/z 308 (M-1).
PREPARATION OF CYCLOHEXENE AMINOESTER AND ENOL TRIFLATE ESTER
INTERMEDIATES
NH 40Ac NaH TfO 9
HZN O CI
CO2Me COzMe CO2Me
N NTf2
-44-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
To a slurry of NaH (5.6 g, 60%) in 200 mL of THF was slowly added methyl
cyclohexanone 2-carboxylate (19.9 g, 90%) at 0 C. After 30 min, the mixture
was warmed to 23 C and
stirred for 15 min. The resulting mixture was cooled to 0 C, and to it was
added Commin's reagent (50
g) in portions. The resulting mixture was warmed to RT and stirred for 2.5 h.
The solution was then
concentrated, and the residue was partitioned between ethyl acetate and water.
The organic layer was
dried with sodium sulfate and concentrated. The residue was purified by
Biotage (5-10% ethyl acetate in
hexane) to give the cyclohexene enol triflate ester.
To a solution of methyl cyclohexanone 2-carboxylate (10.3 g, 90%) in 100 mL of
methanol was added ammonium acetate (8.5 g). The resulting mixture was stirred
at room temperature
overnight. The mixture was then concentrated, and the residue was dissolved in
ethyl acetate. The solid
was filtered, and the filtrate was washed with water, brine and dried over
sodium sulfate. The resulting
solution was concentrated to give the cyclohexene aminoester as an oil, which
crystallized from hexane
as a white solid.
EXAMPLE 2
O
~ \ \
N
Ho O
H
To a solution of the saturated naphthyl acid intermediate from Example 1 (194
mg, 0.97
nunol) in DCM (6 mL), was added DMAP (236 mg, 1.93 mmol) followed by
methanesulfonyl chloride
(0.05 mL, 0.64 mmol). After 5 min, a solution of methyl 2-aminocyclohex-l-ene-
l-carboxylate (100 mg,
0.64 mmol) in DCM (1 mL) was added. The reaction mixture was stirred at RT for
18 h, and quenched
with saturated NH4C1 solution. The resulting mixture was extracted with DCM
dried over anhydrous
Na2SO4, filtered and concentrated in vacuo. The residue was purified by flash
chromatography using 7%
ethyl acetate-hexanes to provide the amide as a methyl ester.
To a solution of this ester intermediate in THF (2 mL) was added MeOH (1 mL)
and 1N
NaOH (1 mL). The resulting reaction mixture was stirred at room temperature
for 18 h, then neutralized
to pH=7 by the addition of 1N HCI, and extracted with ethyl acetate. The
organic layer was dried over
anhydrous Na2SO4, filtered, concentrated in vacuo and purified by reverse
phase HPLC (Gilson) to
provide Example 2. 1H NMR (500 MHz, CD3OD) 6 1.55 (m, 4H), 2.3 (m, 2H), 2.7
(t, 2H), 2.85 (m, 2H),
3.1 (t, 2H), 7.45 (m, 3H), 7.66 (s, 1H), 7.77 (m, 3H); LCMS m/z 324 (M+1).
- 45 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
EXAMPLE 3
O
H I \ \
HO O ~ ~ OH
To a solution of 6-methoxy-2-naphthaldehyde (3.6 g, 19.38 mmol) in toluene
(100 mL)
placed in a pressure vessel, was added (tert-butoxycarbonyl-
methylene)triphenyl-phospharane (8.76 g,
23.25 mmol). The resulting mixture was refluxed at 120 C for 18 h. The
reaction mixture was
concentrated in vacuo and purified using a Biotage flash 40M column with 15%
ethyl acetate-hexanes as
the eluant to give the enoate intermediate.
To a solution of this tert-butyl-3-(6-methoxy-2-naphthyl) acrylate (4.88 g,
17.16 mmol)
in ethanol (100 mL) was added Pd/C. The resulting mixture was stirred under a
H2 balloon for 18 h. The
reaction mixture was filtered through celite and concentrated in vacuo to give
the saturated ester as a
white solid.
To a solution of this ether ester intermediate (150 mg, 0.52 nunol) in DCM
cooled to 0
C, was added BBr3 (5.23 mL, 1.OM in DCM). After 30 min, the reaction mixture
was quenched by the
addition of methanol (2 mL). The reaction mixture was concentrated in vacuo,
and the residue was
dissolved in ethyl acetate and washed with water. The organic layer was dried
over anhydrous Na2SO4,
filtered and concentrated in vacuo. The residue was purified by flash
chromatography using 20% ethyl
acetate-hexanes as eluant. This transesterified methyl ester (97 mg, 0.42
mmol) was dissolved in THF (3
mL) and MeOH was added (2 mL) followed by 1N NaOH (2 mL). After stirring for 6
h, the mixture was
neutralized to pH=7 by the addition of iN HCI. The resulting solution was
extracted with ethyl acetate,
dried over anhydrous NaZSO4, filtered and concentrated in vacuo. This
naphtholic acid was used in the
next step without any further purification.
To a solution of this naphtholic acid (106 mg, 0.49 nurnol) in DCM (5 mL)
cooled to 0
C, was added TBSOTf (0.17 mL, 0.73 mmol) followed by triethylamine (0.14 mL,
0.98 nunol). After
warming the mixture to 23 C and stirring for 2 h, it was quenched by the
addition of water. The
resulting mixture was extracted with DCM, dried over anhydrous NaZSO4i
filtered and concentrated in
vacuo. This bis-silylated material was dissolved in 1:1 THF/H20 (2 mL) and
AcOH (3 niL) was added.
After stirring the mixture at RT for 1 h, it was diluted with water and
extracted with ethyl acetate. The
organic layer was dried over anhydrous Na2SO4, filtered and concentrated in
vacuo to give the desired
acid intermediate.
To a solution of this acid (51 mg, 0.154 mmol) in DCM (2 mL), was added DMAP
(48
mg, 0.39 mmol) followed by methanesulfonyl chloride (0.012 mL, 0.154 mmol).
After 5 niin, methyl2-
aminocyclohex-l-ene-l-carboxylate (20 mg, 0.128 mmol) was added as a solid.
The reaction mixture
was heated to 50 C for 18 h, and then cooled to RT and quenched by the
addition of saturated
-46-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
ammonium chloride. The resulting mixture was extracted with DCM, dried over
anhydrous NaZSO4
filtered and concentrated in vacuo. The residue was purified by flash
chromatography using 10% ethyl
acetate-hexanes as the eluant to provide the amide product.
To a solution of this intermediate ester (109 mg, 0.23 mmol) in THF (3 mL),
was added
1N NaOH (1 mL) followed by MeOH (1.5 mL). After the reaction was complete, it
was neutralized to
pH=7 by the addition of 1N HCI. The resulting mixture was extracted with ethyl
acetate, dried over
anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was purified
by reverse phase HPLC
(Gilson) to provide Example 3. 'H NMR (500 MHz, DMSO-d6) 8 1.5 (m, 4H), 2.2
(bt, 2H), 2.63 (t, 211),
2.8 (bt, 2H), 2.92 (t, 2H), 7.1 (m, 2H), 7.27 (d, 1H), 7.52 (m, 2H), 7.65 (d,
1H), 9.6 (bs, 1H), 11.6 (bs,
1H), 12.5 (bs, 1H); LCMS m/z 338 (M-1).
EXAMPLE 4
O ~I
H
HO O
To a solution of inethyl3-(3-bromophenyl)propionate (100 mg, 0.411 nnnol) in
toluene
(2 mL) was added phenyl boronic acid (100 mg, 0.82 mmol), 1M Na2CO3 solution
(1 mL) followed by
Pd(PPh3)4. The resulting reaction mixture was refluxed in a pressure tube.
After 2 h the mixture was
cooled to 23 C, diluted with ethyl acetate, washed with water, brine, dried
over anhydrous Na2SO4,
filtered and concentrated in vacuo. The residue was purified by flash
chromatography using 10 % ethyl
acetate-hexanes to give the biaryl product.
To a solution of this ester intermediate (85 mg, 0.35 mmol) in THF (1 mL) was
added
MeOH (1 mL) and 5N NaOH (1 mL). After stirring for 1 h, the reaction mixture
was neutralized to
pH=7 by the addition of 1N HCI. The resulting mixture was extracted with ethyl
acetate, and the organic
phase was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The
acid was used in the
next step without any further purification. This acid intermediate was coupled
to methyl-2-amino-
cyclohexene using the similar procedures as described in the Examples above.
Example 4 was prepared
by saponification of the penultimate ester using similar procedures as
described in the Examples above.
'H NMR (500 MHz, CD3OD) 6 1.6 (m, 4H), 2.3 (m, 2H), 2.68 (t, 2H), 2.85 (m,
2H), 3.01 (t, 2H), 7.2 (d,
111), 7.35 (m, 2H), 7.45 (m, 4H), 7.58 (d, 211); LCMS m/z 348 (M-1).
EXAMPLE 5
O OH
N
H
HO O
-47-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
The coupling of 3-(3-bromophenyl) propionic acid with methyl 2-aminocyclohex-l-
ene-
1-carboxylate followed the similar procedures as described in the Examples
above. To a solution of this
aryl bromide intermediate (50 mg, 0.136 mmol) and 4-hydoxy-phenyl boronic acid
(28 mg, 0.2 mmol) in
THF (0.5 mL), was added K2C03 (0.5 mL, 1.OM solution), followed by 1, 1 (bis-
tert-butyl-phosphino)
ferrocene palladium dichloride ligand. The reaction vessel was flushed with N2
and heated to 85 C.
After 30 min, the reaction mixture was cooled to RT and diluted with ethyl
acetate. The resulting
mixture was washed with water, brine, dried over anhydrous Na2SO4, filtered
and concentrated in vacuo.
The residue was purified by flash chromatography using 15% ethyl acetate-
hexanes as the eluant to
obtain the hydroxy biaryl methyl ester.
This intermediate ester was saponified following similar procedures described
in the
Examples above. 'H NMR (500 MHz, DMSO-d6) S 1.55 (m, 4H), 2.24 (m, 2H), 2.63
(t, 2H), 2.80 (bt,
2H), 2.9 (t, 2H), 6.83 (d, 214), 7.13 (d, 111), 7.33 (t, 1H), 7.38 (d, 1H),
7.42 (s, 1H), 7.45 (d, 211), 9.52 (s,
1H); LCMS m/z 364 (M-1).
EXAMPLE 6
O
N-O
t NH
HO O
OH
To a solution of diisopropylamine (5.3 g, 52 mmol) in 200 mL of THF was added
n-
butyllithium (22.4 mL, 56 mmol, 2.5 M in hexane) at -78 C. The resulting
solution was stirred at -78 C
for 30 min, and then at RT for an additional 30 niin. The solution was re-
cooled to -78 C, and to this
solution, was added dropwise a solution of tetralone 20 (7.03 g, 39.9 mmol) in
80 mL of THF. After 1 h
at -78 C, to the above solution was added 4-chloro-4-oxobutyrate (8.43 g,
6.84 mL, 56 mmol) in one
portion. The resulting solution was warmed to 23 C over 2 h. The solvent was
then evaporated, and the
residue was diluted with 200 mL of THF/MeOH/water (v:v:v=3:1:1). To this
mixture was added 100 niL
of lithium hydroxide (1 M in water), and the resulting solution was stirred
overnight. After removing
some solvent in vacuo, the remaining aqueous layer was extracted with ethyl
acetate. The aqueous phase
was acidified with HCl until pH=3. The mixture was extracted with ethyl
acetate, and the combined
organic fractions were dried with sodium sulfate and concentrated in vacuo to
give the ketoacid as a grey
solid.
To a solution of this ketoacid intermediate (0.72 g, 2.6 mmol) in 15 mL of
ethanol were
added hydroxylamine hydrochloride (0.22 g, 3.1 mmoL) and triethylamine (320
mg, 0.44 mL, 3.1 nimol).
The resulting mixture was heated at reflux for 5 h. After removing ethanol in
vacuo, the residue was
diluted with ethyl acetate (100 mL) and 1N HCl (20 mL). The aqueous layer was
further extracted with
- 48 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
30% of isopropanol in chloroform (2 x 30 mL). The organic fractions were
combined, dried with sodium
sulfate and concentrated in vacuo to give the tricycle as a pale yellow solid.
This intermediate was
dissolved in dichloromethane (20 mL) and borontribromide (10 mL, 1 M in
dichloromethane) was added
at 0 C. The resulting dark solution was stirred at room temperature for 4 h
before it was quenched with
100 mL of water at 0 C. The mixture was extracted with 30% isopropanol in
chloroform. The aqueous
layer contained a lot of product as a yellow solid, which was collected by
filtration. The aqueous layer
was further extracted with 30% isopropanol in chloroform. The organic phase
was dried with sodium
sulfate and concentrated in vacuo to give the hydroxy product as a yellow
solid after reverse phase-HPLC
purification.
To a solution of this hydroxy acid intermediate (110 mg, 0.42 mmol) in 15 mL
of
dichloromethane were added imidazole (87 mg, 1.3 mmol) and tert-
butyldimethylsilyl chloride (192 mg,
1.3 mmoL) at RT. The resulting mixture was stirred for 4 h. The mixture was
then purified by Biotage
to give the product as a colorless oil.
To a solution of this bis-silyl intermediate (50 mg, 0.10 mmol) in 3 mL of
dichloromethane were added 1 drop of DMF and oxalyl chloride (0.13 mL, 0.25
mmol, 2 M in
dichloromethane) at 0 C. After 2 h at 0 C, the mixture was warmed to room
temperature and stirred for
30 min. The volatiles were removed in vacuo, and to the residue was added 2 mL
of dichloromethane
followed by methyl2-aminocyclopent-l-ene-l-carboxylate (35 mg, 0.25 mmol). The
mixture was stirred
overnight and then DMAP (10 mg) was added. The resulting mixture was stirred
for an additional 2 h.
The crude mixture was directly purified by Biotage (2-10% ethyl
acetate/hexane) to give the amide
product as a colorless oil.
To a solution of this silyl ether methyl ester intermediate (14 mg, 0.023
mmol, 23%) in 5
mL of THF/MeOH/water (v:v:v= 3:1:1), was added 1 N sodium hydroxide (1 mL) and
3 drops of TBAF
(1 M in THF). After 5 min at 23 C, the mixture was concentrated in vacuo and
the residue was
dissolved in DMSO, which was purified by reverse phase HPLC (Gilson) to give a
colorless oil. This
material was then dissolved in 3 mL of THF/MeOH/water (v:v:v= 3:1:1). To this
solution was added
lithium hydroxide (2 mL, 1 M in water). The resulting mixture was stirred at
room temperature for 3 h.
The mixture was concentrated and dissolved in DMSO. The mixture was purified
by reverse phase
HPLC (Gilson) to provide Example 6 as a light brown solid. 'H NMR (acetone-d6,
500 MHz) S 10.4
(1H, s), 7.44 (1H, d), 6.85 (s, 1H), 6.80 (1H, dd), 3.13 (2H, t), 2.98 (4H,
q), 2.83 (2H, t), 2.73 (2H, t),
2.49 (2H, t), 1.87 (2H, t); LCMS m/z 369 (M+1).
EXAMPLES 7-15
The following compounds were prepared under conditions similar to those
described in
Examples 1-6 above and illustrated in Schemes 1-6. Example 15 utilized
triethylamine as base instead of
imidazole/DMAP described for the TBSCl silylation step in Example 6.
- 49 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
EXAMPLE LCMS
7 N 322 (M-1)
H
HO O
8 0 336 (M-1)
X H
O
9 cIIN 348 (M-1)
Ho O
O HO
364 (M-1)
H
HO O
OH
11 0 364 (M-1)
H
HO O
O
12 N NH2 363 (M-1)
H
HO 0
O
13 N O 390 (M-1)
H
HO O
O
14 ?--N 334 (M-1)
HOOC H
/
-50-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
O
15 NH N'O 383 (M+1)
HO O
OH
NMR data for selected Examples:
EXAMPLE 7
'H NMR (500 MHz, DMSO-d6) S 1.5 (m, 4H), 2.2 (bt, 2H), 2.65 (t, 2H), 2.8 (bt,
2H), 3.34 (t, 2H), 7.4
(m, 2H), 7.54 (m, 2H), 7.78 (d, 1H), 7.92 (d, 1H), 8.08 (d, 1H).
EXAMPLE 8
'H NMR (500 MHz,CD3OD) 8 0.9 (d, 3H), 1.3 (m, 1H), 1.57 (m, 1H), 1.7 (m, 1H),
2.24 (m, 1H), 2.35
(m, 1H), 2.45 (m, 1H), 2.7 (t, 2H), 3.05 (dd, 1H), 3.1 (t, 2H), 7.34-7.43 (m,
3H), 7.65 (s, 1H), 7.72 (m,
3H).
EXAMPLE 9
'H NMR (500 MHz, DMSO-d6) S 1.55 (m, 4H), 2.2 (bt, 2H), 2.6 (t, 2H), 2.8 (bt,
2H), 2.9 (t, 2H), 7.3 (m,
3H), 7.44 (t, 2H), 7.56 (d, 211), 7.62 (d, 2H), 11.6 (bs, 1H).
EXAMPLE 10
'H NMR (500 MHz, DMSO-d6) S 1.55 (m, 4H), 2.4 (t, 2H), 2.62 (t, 2H), 2.84 (bt,
2H), 2.9 (t, 2H), 6.85
(t, 1H), 6.95 (d, 1H), 7.15 (m, 2H), 7.22 (d, 1H), 7.3 (t, 1H), 7.38 (m, 2H),
9.4 (s, 1H), 11.6 (s, 1H), 12.55
(bs, 1H).
EXAMPLE 11
'H NMR (500 MHz, DMSO-d6) 6 1.55 (m, 4H), 2.24 (bt, 2H), 2.66 (t, 2H), 2.82
(bt, 2H), 2.94 (t, 2H),
6.75 (d, 1H), 6.9 (m, 1H), 7.05 (d, 1H), 7.25 (m, 2H), 7.35 (t, 1H), 7.4 (d,
1H), 7.45 (s, 1H), 9.49 (s, 1H).
EXAMPLE 12
'H NMR (500 MHz, DMSO-d6) 8 1.55 (m, 4H), 2.22 (bt, 2H), 2.62 (t, 2H), 2.8
(bt, 2H), 2.9 (t, 2H), 6.9
(d, 1H), 7.2 (m, 3H), 7.3-7.4 (m, 3H), 7.44 (s; 1H), 11.6 (s, 1H).
EXAMPLE 13
'H NMR (500 MHz, DMSO-d6) 8 1.55 (m, 4H), 2.2 (m, 2H), 2.6 (t, 2H), 2.8 (m,
2H), 2.9 (t, 2H), 3.2 (t,
2H), 4.55 (t, 2H), 6.82 (d, 1H), 7.14 (d, 1H), 7.3 (t, 1H), 7.39 (m, 2H), 7.42
(s, 1H), 7.49 (s, 1H), 11.62
(s, 1H), 12.5 (bs, 1H).
-51-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
EXAMPLE 14
'H NMR (500 MHz, DMSO-d6) 8 1.75 (m, 2H), 2.35 (bt, 2H), 2.7 (t, 2H), 2.85 (t,
2H), 3.02 (t, 2H), 7.35
(m, 3H), 7.44 (t, 2H), 7.56 (d, 211), 7.62 (d, 2H).
EXAMPLE 15
'H NMR (acetone-d6, 500 MHz) 8 11.8 (1H, s), 7.43 (1H, d), 6.81 (1H, d), 6.80
(1H, dd), 2.96 (8H, m),
2.72 (4H, q), 1.61 (4H, m).
EXAMPLE 16
O
HO~
-\ N H NJ:
CO2H
% A solution of the aldehyde intermediate (1.45 g, 6.7 mmol), ethyl
triphenylphosphonium
methyl acetate (3.1 g, 8.1 nunol) in 15 mL of toluene was heated at 130 C for
16 h. The mixture was
directly purified by Biotage (5-20% ethyl acetate in hexane) to give the
enoate as a light yellow solid.
This enoate intermediate (1.74 g, 5.8 nunol) and Pd/C (10%, 170 mg) in 200 niL
of
methanol was stirred under 1 atm of hydrogen gas (balloon) for 12 h. The
slurry was filtered and
concentrated in vacuo. The residue was dissolved in ethanol/methanol (1:1) and
purified by chiral OJ-H
(9 mL/min, 28% isopropanol/heptane, isocratic, 40 niin/run) to give the
enantiomers as white solids.
Elution times of these enantiomeric intermediates were 18 min and 22 min using
analytical Chiralcel-OJ,
(25% isopropanol in heptane, isocratic).
The ethyl ester enantiomer (400 mg, 1.32 mmoL) was combined with concentrated
HCI
(2 mL) and 4 mL of acetic acid, and was heated at 80 C for 3 h. The mixture
was concentrated in vacuo,
and to it was added 15 niL of water. The mixture was extracted with 30%
isopropanol/chloroform. The
organic layer was dried with sodium sulfate and concentrated in vacuo to give
the acid product as a white
solid.
To a solution of the methyl ether (410 mg, 1.50 mmol) in 20 niL of
dichloromethane was
added borontribromide (7.5 mL, 1 M in dichloromethane) at 0 C. The mixture was
warmed to RT and
stirred for 18 h. The mixture was quenched with water at 0 C and concentrated
in vacuo without further
purification.
To a solution of the phenol in 60 mL of dichloromethane were added TBSCI (0.57
g, 3.8
mmol), imidazole (0.26 g, 3.8 mmol) and DMAP (37 mg, 0.3 mmol). The mixture
was stirred at 23 C
for 5 h. The mixture was concentrated and purified by RP-HPLC to give
monosilyl ether (0.37 g), which
was resubmitted to a solution of TBSCI (225 mg), triethylamine (0.21 mL) and
DMAP (20 mg) in 15 mL
of dichloromethane. The reaction mixture was stirred for 3 h and washed with
brine. The mixture was
-52-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
then dried with sodium sulfate and concentrated in vacuo to give the bis-
silylated intermediate as a crude
brown oil. Following the previously described amide formation and hydrolysis
procedures using oxalyl
chloride and lithium hydroxide respectively, the enantiomers of Example 16
were obtained. 'H NMR
(methanol-d4, 500 MHz) 8 7.41 (1H, s), 7.16 (2H, d), 6.88 (2H, d), 3.07 (2H,
m), 2.73 (3H, m), 2.46 (2H,
m), 2.15 (3H, s), 1.87 (2H, m), 1.23 (3H, d); LCMS m/z 370 (M+1).
EXAMPLE 17
O
HO_/ ~
-~\ H CO2H
The enantiomers of Example 17 were prepared under similar conditions as
described in
the Examples above. 'H NMR (methanol-d4, 500 MHz) 8 7.43 (1H, s), 7.18 (2H,
dd), 6.89 (2H, dd), 2.86
(2H, m), 2.76 (1H, dd), 2.63 (1H, dd), 2.58 (1H, m), 2.30 (2H, m), 2.16 (3H,
s), 1.60 (4H, m), 1.23 (3H,
d); LCMS m/z 384 (M+1).
EXAMPLE 18
O
N ~AH \
H02C
J:: ~ ~N
HO ~ S
A mixture of the methoxy aminobenzothiazole (8.5 g, 47 mmoL) and ethyl
a-bromopyruvate (12.9 g, 59 mmol) was heated in 120 niL of DME under reflux
for 2 h. After cooling
to RT, the precipitate was collected by filtration to afford the product as a
yellow solid, which was then
heated in a solution of ethanol (200 mL) under reflux for 4 h. The
partitioning of the resulting residue
after concentration using ethyl acetate and saturated aqueous sodium carbonate
solution gave an organic
fraction, which was dried with sodium sulfate. The concentration in vacuo led
to the tricyclic
intermediate as a solid.
To a solution of this ester (2.67 g, 9.65 mmol) in 100 mL of dichloromethane
was added
DIBALH (14.5 mL, 1 M in hexane, 14.5 mmol) at -78 C. After 1 h at -78 C, the
mixture was quenched
with water and slowly warmed to 23 C. A saturated aqueous Rochelle's salt
solution was added, and the
mixture turned clear overnight. The organic phase was washed with water and
concentrated. The
resulting residue was filtered to give the aldehyde as a yellow solid.
To a solution of trimethyl phosphonoacetate (0.71 mL, 4.33 mmol) in 40 mL of
THF was
added nBuLi (2.9 mL, 4.6 mmoL, 1.6 M in hexane) at 0 C. After 30 min, to the
solution was added the
aldehyde (0.67 g, 2.88 mmol). After 10 min, the mixture was quenched with
water and diluted with ethyl
acetate. The organic phase was concentrated and purified by Biotage (20-30%
ethyl acetate/hexane) to
give the enoate as a white solid.
-53-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
To a solution of this enoate intermediate (0.43 g, 1.49 mmol) in 200 mL of
methanol was
added tosylhydrazide (2.77 g, 14.9 mmol). The mixture was heated under reflux
for overnight. The
resulting clear solution was concentrated and purified by Gilson to give the
product as a white solid.
To a solution of this ester (230 mg) in 50 mL of THF/MeOH/water (v:v:v= 3:1:1)
was
added 10 mL of 1 N aqueous LiOH solution. After 1.5 h, the mixture was
acidified using HCl to pH=4.
The mixture was extracted with 30% of isopropanol in chloroform. The organic
phase was concentrated
to dryness to give the acid as a white solid.
To a solution of the methyl ether (220 mg, 0.80 mmol) in 40 mL of
dichloromethane was
added borontribromide (6.4 mL, 1 M in dichloromethane) at 0 C. The mixture was
warmed to RT and
stirred for 12 h. The mixture was quenched with water at 0 C and washed with
30% of isopropanol in
chloroform. After concentration of the organic solvent, the product was
obtained as a solid.
To a solution of this phenol in 60 mL of dichloromethane were added TBSCI (380
mg)
and triethylamine (3 mL). The reaction mixture was stirred for 3 h and washed
with water. After
concentration of the organic fraction, the residue was purified by RP-HPLC to
give the mono-TBS
product (0.26 g), which was resubmitted to a solution of TBSCI (156 mg),
triethylaniine (0.24 mL) and
DMAP (13 mg) in 60 mL of dichloromethane. The reaction mixture was stirred at
0 C for 1 hr, and to
the mixture was added additional 1 equivalent of triethylamine and TBSCI. The
solution was stirred
overnight at RT before it washed with water. The organic phase was then dried
with sodium sulfate and
concentrated in vacuo to give the bis-silylated product as a crude brown oil.
Following the previously
described amide formation and hydrolysis procedures using oxalyl chloride and
lithium hydroxide
respectively, Example 18 was obtained.'H NMR (methanol-d4, 500 MHz) S 8.13
(1H, s), 7.88 (1H, d),
7.41 (1H, d), 7.11 (1H, dd), 3.16 (4H, m), 2.89 (2H, t), 2.50 (2H, t), 1.91
(2H, m); LCMS m/z 372 (M+1).
EXAMPLE 19
O
H
>%N H02C
HO S
Example 19 was prepared under similar conditions as described in the Examples
above.
'H NMR (methanol-d4i 500 MHz) S 8.04 (1H, s), 7.83 (1H, d), 7.37 (1H, d), 7.08
(1H, dd), 3.12 (2H, t),
2.93 (2H, m), 2.81 (2H, t), 2.34 (2H, m), 1.65 (4H, m); LCMS m/z 386 (M+1).
-54-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
EXAMPLE 20
O
i
N~ H I / \
\_INH
N~N
To a solution of adiponitrile (0.569 mL, 5.0 mmol) in anhydrous THF cooled to -
78 C
under a nitrogen atmosphere, was added LDA (2.62 mL, 5.25 nnnol, 2.0 M
solution in THF). The
reaction was warmed to -20 C over 10 min, and then quenched with saturated
NH4CI solution. The
resulting mixture was extracted with ethyl acetate. The organic layer was
washed with brine, dried over
anhydrous Na2SO4, filtered and concentrated in vacuo. This material was
purified by flash
chromatography using 15 % ethyl acetate-hexanes as the eluant to give the
cyclopentene aminonitrile as
an off white solid. This intermediate cyclopentene aminonitrile was coupled
with 3-(4-bromophenyl)
propionic acid, using similar procedures as described in the Examples above,
to provide the amide
bromide.
To a solution of this arylbromide intermediate (88 mg, 0.28 mmol) in THF (0.5
mL) was
added phenyl boronic acid (48 mg, 0.41 mmol) followed by 1M K2C03 (0.5 mL) and
1, lbis(di-tert-
butylphosphino)ferrocene palladium dichloride ligand (18 mg, 0.03 mmol). After
stirring the reaction in
a sealed tube at 85 C for 18 h, it was diluted with ethyl acetate, washed
with H20 and saturated NaCI.
The organic layer was dried over anhydrous Na2SO4, filtered and concentrated
in vacuo. The residue was
purifed by flash chromatography using 10% ethyl acetate-hexanes to give the
biaryl intermediate as a
colorless oil.
To a solution of this intermediate nitrile (34 mg, 0.11 nunol) in a 2:1
mixture of dioxane-
H20 (0.9 mL) was added sodium azide (21 mg, 0.32 mmol) and zinc bromide (29
mg, 0.13 nnnol). The
reaction mixture was stirred at 120 C in a sealed tube for 18 h. The mixture
was then cooled to room
temperature and 1N HCl was added until the the pH=7. The reaction mixture was
concentrated in vacuo
and purified by reverse phase HPLC (Gilson) to provide Example 20. 1H NMR (500
MHz, CD3OD) 6
2.02 (m, 2H), 2.46 (m, 2H), 2.85 (m, 211), 3.15 (m, 4H), 7.18 (d, 2H), 7.31
(t, 1H), 7.41 (t, 211), 7.52 (d,
2H), 7.56 (t, 211); LCMS m/z 360 (M+1).
EXAMPLE 21
HO N N C02H
~
CI O
-55-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
To the commercially available thiazole bromo aldehyde (4.97 g) shown in Scheme
10, in
200 mL of methanol was added NaBH4 (0.98 g) in portions at 0 C. The mixture
was stirred for 2 h and
concentrated. The residue was suspended in saturated NH4CI solution (200 mL)
to adjust the pH to 6.
The mixture was then basified by NaOH (aq) to pH 11 before the extraction with
ethyl acetate (4 x 200
mL). The combined organic fractions were dried with sodium sulfate and
concentrated to give the
alcohol.
To a solution of this hydroxy intermediate (4.91 g) in 120 mL of
dichloromethane at 0 C
was added triphenylphosphine (9.96 g). To this solution was then added
dropwise, carbon tetrabromide
(12.6 g) in 30 mL of dichloromethane. After 2.5 h at 23 C, the mixture was
stirred at -20 C overnight.
The solvent was then removed, and the residue was purified by Biotage (5-10%
ethyl acetate in hexane)
to give the bromide as a white solid.
To a solution of diethyl methylmalonate (5.19 g) in 100 mL of THF was added
NaH (1.2
g, 60%) at 0 C in portions. The mixture was stirred at 0 C for 10 min, and to
this solution was then
added the bromide intermediate (3.84 g) in one portion. The mixture was warmed
to 23 C and stirred
for 1 h before the addition of water. The resulting mixture was extracted with
ethyl acetate, concentrated
and purified by Biotage (5-10% ethyl acetate in hexane) to give the diester
containing some diethyl
methyl malonate contaminant as a crude oil.
To this diester (5 g) was added THF/MeOH/water (-100 mL, 3:1:1), LiOH (-50 mL,
1
N) at 23 C. The resulting solution was stirred overnight. After the removal
of the organic solvent, to
the residue was added concentrated HCl until pH=4. The mixture was extracted
with ethyl acetate (5 x
100 mL). The combined organic layers were dried with sodium sulfate and
concentrated to give the
diacid as a white solid containing some a-methyl malonate acid contaminant.
The solution of this diacid intermediate (4.9 g) in 20 mL of DMF was heated at
150 C
for 7 min and then cooled to 0 C. The solution was diluted with ethyl acetate,
washed with brine, dried
with sodium sulfate and concentrated to give the monoacid containing some
propanoic acid. The mixture
was further purified by RP-HPLC to give pure alpha-methylacid as a colorless
oil.
The mixture of this bromoacid intermediate (0.71 g), the aryl boronic acid
(0.67 g),
Pd(PPh3)4 (323 mg), NaHCO3 solution (11.2 mL, 1 N) and dioxane (40 mL) was
heated at 100 C under
nitrogen overnight in a sealed tube. The mixture was then partitioned between
ethyl acetate and 1 N
NaOH solution. The organic layer was washed with 1 N NaOH solution. The
combined aqueous layers
were acidified with concentrated HCI until pH=4-5. The resulting mixture was
extracted with ethyl
acetate three times. The combined organic layers were washed with brine and
dried with sodium sulfate.
The removal of solvent afforded the biaryl product.
To a solution of the methoxyaryl intermediate (0.88 g) in 60 n-iL of
dichloromethane was
added BBr3 (22.7 mL, 1 M in dichloromethane) at 0 C. The mixture was warmed to
room temperature
and stirred overnight. To the mixture was then added water at 0 C, and the
mixture was extracted with
30% isopropanol in chloroform. After concentrating the organic layer, the
residue was hydrolyzed with
-56-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
LiOH (1 N) in 3:1:1 THF/MeOH/water for 2 h. After the removal of the organic
solvent, the residue was
washed with ethyl acetate. The alkaline aqueous phase was acidified with HCI
until pH=4-5, and the
mixture extracted with ethyl acetate (2 x). The combined organic layers were
washed with brine, dried
with sodium sulfate and concentrated to give the phenol as a brown solid.
To a mixture of this phenolic acid intermediate (0.51 g) in 10 mL of
dichloromethane
was added triethylamine (0.84 mL). To this solution at 0 C was added tert-
butyldimethylsilyl chloride
(0.91 g) and DMAP (42 mg). After 6 h at 23 C, the mixture was washed with
water, brine and dried
with sodium sulfate. The resulting organic fraction was concentrated in vacuo.
To the resulting residue
in dichloromethane (40 mL) was added one drop of DMF, and then a solution of
oxalyl chloride (4 mL, 2
N in dichloromethane). The mixture was warmed to 23 C and stirred for
additional 4 h. The resulting
mixture was concentrated in vacuo and then dissolved in dichloromethane (28
mL). To the resulting
solution was then added the enamine fragment (717 mg) as in the examples
above. The resulting mixture
was stirred for overnight. The crude material was purified by RP-HPLC to give
430 mg of amide. To the
resulting amide was added 6 mL of THF:methanol:water (3:1:1) and a solution of
lithium hydroxide (10
mL, iN). After 5 h, most of the low boiling solvent was removed in vacuo. To
the residue was added
concentrated HCI until pH=3. The mixture was extracted with 30% isopropanol in
chloroform. The
organic layer was concentrated and purified by RP-HPLC to give the desired
Example 21. 1H NMR
(DMSO-d6, 500 MHz) S 12.6 (1H, s), 11.6 (1H, s), 10.4 (1H, s), 7.95 (1H, d),
7.62 (1H, s), 6.94 (1H, d),
6.85 (1H, dd), 3.10 (1H, dd), 2.89 (IH, dd), 2.79 (2H, m), 2.62 (1H, m), 2.20
(2H, m), 1.52 (4H, m), 1.15
(3H, d); LCMS m/z 421 (M+1).
EXAMPLE 22
N H C02H
0(
HN2r-ls3 - "~ ~~N
To the intermediate alpha-methylacid bromothiazole above (197 mg, Compound 52
in
Scheme 11) in dichloromethane (10 mL) was added one drop of DMF, and then a
solution of oxalyl
chloride (1.6 mL, 2 N in dichloromethane). The mixture was warmed to room
temperature and stirred for
I h. The resulting mixture was concentrated in vacuo and then dissolved in
dichloromethane (10 niL).
To the resulting solution was then added the common 2-aminocyclohex-1-ene-l-
carboxylate ester (400
mg). The resulting mixture was stirred overnight. The crude material was
purified by Biotage (5-10%
ethyl acetate in hexane) to give the amide as a colorless oil.
The niixture of this bromo intermediate (55 mg), phosphine ligand (14 mg),
K2CO3 (440
mg, in 3.2 mL of water) in THF (4 mL) was degassed with argon followed by the
addition of boronate
ester (35 mg). The mixture was heated at 55 C for 1 h and then 65 C
overnight. The resulting mixture
was partitioned between ethyl acetate and brine. The organic layer was dried
with sodium sulfate and
-57-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
purified by RP-HPLC to give the biaryl product. The similar hydrolysis
procedure as described in the
Examples above gave Example 22 as a white solid. 'H NMR (DMSO-d6, 500 MHz) S
11.6 (1H, s), 8.05
(1H, s), 7.43 (1H, d), 3.04 (1H, dd), 2.90 (1H, dd), 2.76 (2H, m), 2.58 (1H,
m), 2.18 (2H, m), 1.50 (4H,
m), 1.13 (3H, d); LCMS rn/z 361 (M+1).
EXAMPLE 23
~ ~ N~O O OH
HO
N N~N z
O
To NaH (7.2 g, 60%) was added DMF (100 mL) followed by 4-methoxybenzyl alcohol
(18.7 mL) at 0 C. After 25 min at 0 C, the mixture was warmed to 23 C and
stirred for additiona130
min. To the resulting solution was added the pyridyl cyanobromide (22.9 g) in
one portion. The reaction
was exothermic and stirred for 10 min before it was cooled to room
temperature. The mixture was
diluted with 500 mL of ethyl acetate, washed with water (500 mL x 3). The
first two aqueous phases
were extracted with dichloromethane (500 mL x 2). The combined dichloromethane
phase was washed
with water (500 mL x 3). The combined organic phases were dried over sodium
sulfate and concentrated
to give the PMB ether as a white solid.
To the suspension of this intermediate (24.6 g) and hydroxylamine
hydrochloride (8.55
g) in ethanol (500 mL) was added NaOH (4.92 g in 50 mL of water) dropwise. The
mixture was stirred
at RT overnight. The solid was collected by filtration to give the N-hydroxy
amidine as a white solid.
To this amidine intermediate (15.4 g) was added pyridine (40 mL) and the acid
chloride
shown in Scheme 12 (8.3 mL). The mixture was heated at 120 C for 2 h and then
130 C for 1 h. After
removing most pyridine, the residue was partitioned between water and
dichloromethane. The organic
phase was washed with water four times and then dried with sodium sulfate.
After removing the solvent,
to the residue was added some methanol. The resulting slurry was filtered. The
solid collected by the
filtration was washed with methanol and dried in vacuo to give the methyl
ester intermediate as a pale
pink solid.
To this ester (30 g) suspended in 3:1:1 THF/MeOH/water (700 mL) was added LiOH
(300 mL, 1 N). The mixture was stirred at RT for 1 h. After removing most of
the solvent, the aqueous
layer was acidified to pH=3. Filtration of the resulting slurry gave a white
solid, which was washed with
water, diethyl ether and azeotroped with toluene to give the acid as a white
solid.
To a mixture of this acid (26.9 g) in 300 niL of dichloromethane were added
0.1 mL of
DMF, and then a solution of oxalyl chloride (76 mL, 2 N in dichloromethane) at
0 C. After 0.5 h, the
mixture was warmed to RT and stirred for additiona10.5 h. The resulting
mixture was concentrated in
vacuo and then dissolved in dichloromethane (250 mL). To the resulting
solution was then added the
methyl2-aminocyclohex-l-ene-l-carboxylate (29 g). The resulting mixture was
stirred for overnight.
- 58 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
The solution was then washed with water (200 mL), saturated sodium bicarbonate
solution (200 mL) and
dried with sodium sulfate to give the amide as a crude material.
To a solution of the PMB ether intermediate (10.2 g) in 50 mL of
dichloromethane was
added triisopropylsilane (12.3 mL) and trifluoroacetic acid (20 mL) dropwise.
The mixture was stirred at
RT for 10 min, and the solvent was removed in vacuo. To the residue containing
this hydroxy product
was added 300 mL of THF:methanol:water (3:1:1) followed by a solution of
lithium hydroxide (200 mL,
1N). After 12 h, most of the low-boiling solvent was removed in vacuo. To the
residue was added ethyl
acetate (200 mL x 2), then the aqueous layer was neutralized to pH=5. The
precipitate was collected by
filtration to give the desired Example 23 as a light brown solid. 'H NMR (DMSO-
d6, 500 MHz) 8 12.6
(1H, s), 11.7 (1H, s), 10.6 (1H, s), 8.25 (1H, d), 7.88 (1H, d), 7.29 (1H,
dd), 3.18 (2H, t), 2.89 (2H, t),
2.48 (2H, m), 2.21 (2H, m), 1.51 (4H, m); LCMS m/z 359 (M+1).
EXAMPLE 24
O
HO C N Nsi~N
~ H CO2H
To a flask containing 20 mL diglyme and KH (1.33 g, 30%) at room temperature
was
added methylpyrazole (820 mg) in one portion. After 2 h, to this mixture was
added the pyridyl
nitrobromide (1.83 g). The mixture was then heated at 130 C overnight. To the
resulting mixture was
added 100 mL of water and 100 niL of ethyl acetate. The aqueous layer was
extracted with 100 niL of
dichloromethane. The combined organic layers were dried with sodium sulfate
and concentrated in
vacuo to give a green slurry. To the slurry was added hexane to remove the
mineral oil. The mixture
was then filtered to give the pyridylpyrazole as a green solid.
The mixture of this methylated intermediate (204 mg), NBS (330 mg) and 5 mL of
CC14
under light was refluxed for 3.5 h. The mixture was filtered and the filtrate
was washed with saturated
aqueous sodium sulfi'te (100 mL). The mixture was extracted with 30% of
isopropanol in chloroform.
The organic layer was washed with water, dried with sodium sulfate and
concentrated. The residue was
purified by Biotage eluting with 5-30% ethyl acetate in hexane/dichloromethane
to give the bromide as a
light yellow solid.
To NaH (350 mg, 60%) in 20 niL of THF was added dimethyl malonate (1.14 g) at
0 C.
After 20 min, to the clear solution was added the bromomethylene intermediate
(490 mg) in 10 mL of
THF dropwise. The mixture was warmed to RT and stirred for additional 1 h. To
the mixture was then
added water (50 mL). The mixture was extracted with ethyl acetate (200 mL).
The combined organic
layers were concentrated, and the residue was submitted to 10 mL of LiOH (1N)
and 50 mL of
THF/MeOH/water (3:1:1). After 3 h, the mixture was acidified to pH=4 using
concentrated HCI. The
mixture was concentrated to remove organic solvents, and the residue was
extracted with 30%
- 59 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
isopropanol in chloroform. The organic layer was concentrated and purified by
RP-HPLC to give the
acidester. The mixture of this alpha-carboxyacid (1 g) in 10 mL of DMF was
heated at 150 C for 10
min. The mixture was then purified by RP-HPLC to give the monoester (880 mg)
as a yellow solid. To
this nitro intermediate (100 mg) in 6 mL of acetic acid was added Zn (234 mg).
The slurry was heated at
60 C for 30 min and filtered through celite. The filtrated was purified by RP-
HPLC to give the amino
methyl ester as a reddish oil.
To the mixture of this aminopyridine (580 mg), sodium nitrite (200 mg) was
added in 2.5
mL of 10% sulfuric acid. The mixture was heated at 80 C for 1 h. The mixture
was purified by RP-
HPLC to give the hydroxypyridine. To this hydroxyacid (86 mg) was added 5 mL
of dichloromethane,
0.18 niL of triethyl amine and 139 mg of TBSCI. After 3 h, to the mixture was
added water. The
mixture was extracted with dichloromethane and 30% isopropanol in chloroform.
The combined organic
fractions were dried with sodium sulfate and concentrated in vacuo. The
resulting residue was dissolved
in 5 mL of dichloromethane. To the solution was added 1 drop of DMF, and 1 mL
of oxalyl chloride (2
M in dichloromethane) at 0 C. The resulting mixture was warmed to 23 C and
stirred for 30 min before
the mixture was concentrated in vacuo. The residue was diluted into 5 mL of
dichloromethane, and to
the solution was added 100 mg of inethyl2-aminocyclohex-l-ene-l-carboxylate.
The mixture was stirred
overnight. The reaction mixture was concentrated, and to the residue was added
20 mL of
THF/MeOH/water (3:1:1) and 8 mL of LiOH (1 N). The mixture was stirred at RT
for 8 h and
concentrated to a smaller volume. To the residue was added concentrated HCl
dropwise until pH<3.
The mixture was extracted with 30% isopropanol in chloroform. The organic
fraction was concentrated
and the residue was purified by RP-HPLC to give Example 24. 'H NNMR (Acetone-
d6, 500 MHz) S 11.7
(1H, s), 8.32 (1H, s), 8.01 (1H, s), 7.78 (1H, d), 7.56 (1H, s), 7.40 (1H, d),
2.93 (2H, m), 2.87 (2H, t),
2.63 (2H, t), 2.32 (2H, m), 1.60 (4H, m); LCMS m/z 357 (M+1).
EXAMPLE 25
O
HO ~ N
N-0 H COZH
The cyanopyridine prepared above, was reduced with DIBAL-H under standard
conditions, and the aldehyde (173 mg), in 5 mL of THF and 2 mL of water was
combined with
hydroxylamine hydrochloride (99 mg). The mixture was stirred for 5 h and
concentrated in vacuo. The
residue was purified by Biotage eluting with 5%-20% of ethyl acetate in 1:1
mixture of dichloromethane
and hexane to give the oxime.
To this oxime (50 mg) and 4-pentynoic acid (76 mg) in 10 mL of dichloromethane
at 0
C was added 0.4 mL of NaOCl (>=4% in water). After 12 h, the solvent was
removed, and to the
-60-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
residue was added 6 mL of DMF and 3 mL of NaOCI (>=4% in water). The mixture
was stirred at RT
for 2 days. The mixture was filtered, and the filtrate was purified with RP-
HPLC to give the isoxazole.
To this PMB ether intermediate (42 mg), was added 1 mL of dichloromethane and
1 mL
of TFA. After 30 min, the mixture was concentrated, and to the residue was
added 10 mL of
dichloromethane, 73 uL of triethyl amine and 48 mg of TBSCI. After 3 h, to the
mixture was added
water. The mixture was then extracted with dichloromethane and 30% isopropanol
in chloroform. The
combined organic fractions were dried with sodium sulfate and concentrated in
vacuo. Following similar
procedures as described in the Examples above, acylation and deprotection
provided Example 25. 'H
NMR (Acetone-d6, 500 MHz) S 11.8 (1H, s), 8.31 (1H, s), 7.94 (1H, s), 7.39
(1H, d), 6.73 (1H, s), 2.93
(2H, t), 2.82 (2H, t), 2.68 (2H, m), 2.33 (2H, m), 1.60 (4H, m); LCMS m/z 358
(M+1).
EXAMPLE 26
O
HO ~ ~ N
N
~ N-O H CO2H
To the commercially available olefin (5 g) in 20 mL of propanol was added 0.5
mL of
concentrated sulfuric acid. The mixture was heated at reflux for 2 days. The
reaction mixture was
purified by Biotage (5% ethyl acetate in hexane) to give the propyl ester as a
colorless oil.
To a solution of this ester (4.8 g) and NMO (9.9 g) in 30 mL of
dichloromethane was
added OsO4 (4.2 mL, 4% in water). The mixture was stirred for 12 h at RT. To
the resulting solution
was added water (150 mL) and dichloromethane (300 mL). The organic layer was
concentrated. To the
residue was added acetone (300 mL) and sodium periodate (14.4 g) in water (80
mL). A white slurry
was formed. After 30 min, the slurry was filtered, and the filtrate was
concentrated in vacuo. The
residue was purified by Biotage (5% ethyl acetate in hexane) to give the
corresponding aldehyde as a
colorless oil. To this aldehyde was added t-butanol (25 mL), 2-methyl-2-butene
(15 mL), a mixture of
sodium chlorite (14.5 g, 80%) and sodium dihydrophosphate (18 g) in water (75
mL) at 0 C. The
resulting brown solution was slowly warmed to 23 C and stirred for 1.5 h. To
this mixture was added
NaOH (1 N) until pH=8. The organic layer was removed. To the aqueous layer was
added concentrated
HCl until pH=3. The mixture was then extracted with ethyl acetate (200 niL x
3). The combined organic
layers were dried to give the monoacid as a colorless oil.
To this acid intermediate (1 g) in 10 mL of toluene was added thionyl chloride
(2 mL) at
room temperature. The mixture was heated at 80 C for 1 h, and the volatiles
were removed and
azetroped with toluene. The residue was then dissolved in pyridine (10 mL),
and to the mixture was
added the N-hydroxy amidine (1.0 g) described in the above Examples. The
resulting mixture was
heated at 120 C for 2 h and then purified by Biotage (5-40% ethyl acetate in
hexane) to give the
oxadiazole as a brown oil. Following similar procedures described in the
Examples above, acylation and
-61-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
deprotection gave the desired Example 26 as a white solid. 'H NMR (DMSO-d6,
500 MHz) S 11.6 (1H,
s), 10.7 (1H, bs), 8.27 (1H, d), 7.90 (1H, d), 7.31 (1H, dd), 2.86 (1H, dd),
2.80 (1H, dd), 2.74 (3H, m),
2.22 (2H, m), 1.52 (4H, m), 1.37 (3H, d); LCMS m/z 373 (M+1).
EXAMPLE 27
HO ~~ N O H COOH
N_'~ N 6
O
The similar procedures as described for the preparation of Example 26 above,
gave the
racemic oxadiazole ester intermediate shown below.
N-O
PMBO ~ ~ O\/
N' v II
O
This oxadiazole intermediate (5 g) was purified by chrial AD-H to give two
enantiomers.
To each enantiomer (1.5 g) in 100 mL of THF/MeOH/water was added LiOH (15 mL,
1 N) at 0 C. After
30 min at 0 C, the mixture was acidified with HCl to pH=2-3. After the removal
of the organic solvent
in vacuo, the residue was extracted with 30% isopropanol in chloroform. The
organic layer was dried
with sodium sulfate. The removal of solvent in vacuo gave the acid containing
some inorganic salt.
This material was submitted to amide formation following the same procedures
as
described in the Examples above, which was subsequently treated with
dichloromethane (20 mL),
triisopropylsilane (2 mL) and treated dropwise with TFA (10 mL). The resulting
mixture was stirred at 0
C for 25 min, and the mixture was concentrated in vacuo. The residue was
dissolved in DMSO and
purified by RP-HPLC to give an enriched single enantiomer of Example 27 (83%
ee determined by chiral
OJ-R). The same procedure starting with the opposite enantiomer gave
enantiomerically enriched
Example 27 (71% ee determined by chrial OJ-R).
These enantiomers of Example 27 were subsequently repurified and resolved by
preparative SFC chiral chromatography (ChiralPak AD, 35% methanol(TFA) - CO2)
to obtain each
enantiomer at 98-99% ee. Enantiomer A: 'H NMR (DMSO-d6, 500 MHz) S 11.7 (1H,
s), 10.7 (1H, bs),
8.27 (1H, d), 7.88 (1H, d), 7.31 (1H, dd), 3.24 (1H, dd), 3.07 (1H, dd), 3.02
(1H, m), 2.75 (2H, m), 2.21
(2H, m), 1.51 (4H, m), 1.27 (3H, d); LCMS m/z 373 (M+1). Enantiomer B: 'H NMR
(CD3OD-d6, 500
MHz) S 8.25 (1H, d), 8.05 (1H, d), 7.42 (1H, dd), 3.34 (1H, dd), 3.12 (2H, m),
2.87 (2H, m), 2.34 (2H,
m), 1.62 (4H, m), 1.37 (3H, d); LCMS m/z 373 (M+1).
-62-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
EXAMPLE 28
H CO2H
N~,
hS~ 0
HO CN
To a preheated (50 C) slurry of copper (II) chloride (932 mg), 10 mL of
acetonitrile was
added, along with the thiazole aminoester (1 g) and amyl nitrite (737 mg). The
mixture was heated at
50 C for 2 h. The resulting mixture was concentrated and purified by Biotage
(5-10% ethyl acetate in
hexane) to give the chloride as a brown solid.
To 4-iodopyrazole (715 mg) in 15 mL of THF was added NaH (161 mg, 60%) at 0 C.
After 30 min, to this mixture was added the chloride intermediate (595 mg).
After 30 min at 0 C, the
mixture was warmed to RT and stirred for 8 h. The mixture was quenched with
water and extracted with
ethyl acetate. The organic layer contained some white solid which is pure
biaryl product, and was
collected by filtration. The filtrate was concentrated and further purified by
Biotage (20-100% ethyl
acetate in hexane) to give additional biaryl product as a white solid.
To this iodo intermediate (750 mg) in 30 mL of THF was dropwise added iPrMgCl
(1.4
mL, 2 M in diethyl ether) at -78 C under nitrogen to give a light brown
solution. After 1 h at -78 C, to
the resulting solution was added B(OMe)3 (0.29 mL). The mixture was slowly
wanned to 23 C and
stirred for 12 h. The mixture was partitioned between ethyl acetate and water.
The organic layer was
concentrated and treated with 10 mL of 30% hydrogen peroxide and 50 mL of THF.
The mixture was
heated at 50 C for overnight. The mixture was then concentrated and purified
by RP-HPLC to give the
hydroxypyrazole.
To this alcohol intermediate (200 mg) was added 15 mL of dichloromethane, 0.15
niL of
triethylamine and 148 mg of TBSCI. The crude mixture was concentrated and
purified by Biotage (5-
10% ethyl acetate in hexane) to give the silyl ether as an off-white solid.
To this methyl ester (265 mg) in 20 mL of dichloromethane was added DIBAL-H (5
mL,
1 M in hexane) at -78 C. The mixture was warmed to RT and stirred for 5 h
before it was quenched with
a saturated solution of Rochelle's salt. The slurry was stirred vigorously,
and the aqueous layer was
extracted with dichloromethane. The combined organic layers were dried with
sodium sulfate and
concentrated to give the hydroxymethylene product as a crude oil, which was
directly used for the next
step.
To this crude alcohol (300 mg) in 10 mL of dichloromethane was added sodium
bicarbonate (121 mg) and Dess-Martin periodinane (490 mg). After 2 h, the
crude mixture was purified
by Biotage (10% ethyl acetate in hexane) to give the aldehyde.
To a solution of trimethyl phosphonate acetate (182 mg) in 20 mL of THF was
added n-
butyllithium (0.75 mL, 1.6 M in hexane) at 0 C. The resulting solution was
stirred at this temperature
for 30 min. To this solution was added a THF solution (5 mL) of the aldehyde
intermediate (270 mg).
- 63 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
The mixture was slowly warmed to RT and stirred for 2 hours. After quenching
the mixture with water,
the mixture was extracted with ethyl acetate, concentrated and purified by
Biotage to give the methyl
enoate as a white solid.
A mixture of methyl enoate (159 mg) and p-toluenesulfonyl hydrazide (2 g) in
30 mI. of
methanol was heated at 65 C for 2.5 days. The solvent was removed, and the
residue was purified by
RP-HPLC to give the saturated methyl ester as a white solid.
To this methyl ester (40 mg) in 5 mL of THF:MeOH:water (3:1:1) was added LiOH
(1.5
mL, 1 M). The mixture was stirred for 2 hours. After being acidified with
concentrated HCl until pH =
3, the slurry was extracted with 30% isopropanol in chloroform, dried with
sodium sulfate and
concentrated in vacuo to give the acid as an oily solid.
Following the similar amide formation and hydrolysis procedures described in
the
Examples above, Example 28 was obtained as a white solid. 'H NMR (Acetone-d6,
500 MHz) S 11.8
(1H, s), 7.84 (1H, s), 7.42 (1H, s), 7.27 (1H, s), 3.13 (2H, t), 2.95 (2H, t),
2.71 (2H, t), 2.34 (2H, m), 1.62
(4H, m); LCMS m/z 363 (M+1).
EXAMPLE 29
O
/ ~ N N ~
HN H COOH
To a solution of 4-methoxyaniline (1.57 g) in 10% HCl (18 mL) was added sodium
nitrite (0.87 g) in 4 mL of water at 0 C. After being stirred at 0 C for 30
min, to this mixture was added
dropwise, a solution of methyl isocyanoacetate (1.05 g) and sodium acetate
(6.63 g) in methanol (40 mL)
and water (12 mL) at 0 C. The mixture was stirred at 0 C for 1.5 h. The
solvent was removed in vacuo
and the residue was extracted with ethyl acetate, washed with 5% HCI,
saturated sodium bicarbonate
solution and brine. The solution was then dried with sodium sulfate and
purified by Biotage (40-80%
ethyl acetate in hexane) to give the triazole intermediate.
To a solution of this triazole ester (0.7 g) in THF (40 mL) was added LiBH4
(79 mg).
The mixture was heated under reflux for 1 h and cooled to 23 C. The mixture
was then quenched with 1
N HCI. After removing the solvent, the residue was dissolved in ethyl acetate,
washed with saturated
sodium bicarbonate solution, brine and dried with sodium sulfate. The
concentration of this mixture
gave the hydroxymethylene intermediate.
To this alcohol (0.46 g) was added 50 mL of dichloromethane and Dess-Martin
reagent
(227 mg) at 0 C. The mixture was warmed to RT and stirred for an additional 3
h. The mixture was
purified by Biotage (40-80% ethyl acetate in hexane) to give the aldehyde.
To a solution of trimethyl phosphosphonoacetate (301 mg) in 20 mL of THF was
added
n-BuLi (0.73 mL, 2.5 M in hexane) at 0 C. After 30 min, to this solution was
added the aldehyde
-64-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
intermediate (0.30 g). The resulting solution was stirred at RT for 1 h. To
the solution was then added
dichloromethane/water. The mixture was extracted with 30% isopropanol in
chloroform. The organic
layer was concentrated and purified by Biotage (40-80% ethyl acetate in
hexane) to give the methyl
enoate.
A mixture of the methyl enoate intermediate (186 mg), ca. 60 mg Pd/C (10%) and
200
mL of inethanol/dichloromethane (1:1) was subjected to hydrogenation under a
hydrogen balloon. After
20 min, the reaction mixture was filtered and the filtrate was concentrated to
give the saturated methyl
ester as a white solid.
Following the similar amide formation and hydrolysis procedures described in
the
Examples above, Example 29 was obtained as a white solid. 'H NMR (DMSO-d6a 500
MHz) S 12.5 (1H,
bs), 11.7 (1H, s), 9.77 (1H, s), 7.57 (2H, d), 6.88 (2H, dd), 2.95 (2H, t),
2.82 (2H, m), 2.72 (2H, t), 2.23
(2H, m), 1.53 (4H, m); LCMS m/z 357 (M+1).
EXAMPLE 30
HN_N ~ f O
H ~
HO
O
BuLi (10 mmol, 1.3 eq, 2.5M/THF, 4 mL) was added to a THF (8 mL) solution of
trimethyl phosphonoacetate (9.23 mmol, 1.2 eq, 1.68 g) at -78 C and stirred
for 30 min. The solution
was warmed to 0 C for 10 min and re-cooled to -78 C. Then a THF ( 5 mL)
solution of 4-
ethynylbenzaldehyle (7.69 mmol, 1 eq, 1.OOg) was added dropwise and stirred
for 2 h at RT. The
reaction was partitioned between AcOEt and H20. The organic layer was dried,
and the residue was
recrystalized with CH2C12/MeOH to obtain a light yellow solid product.
A mixture of this methyl enoate acetylide (460 mg, 1 eq, 2.47 mmol), Cul (0.1
eq. 24
mg) and azidotrimethylsilane (427 mg, 1.5 eq, 3.71 mmol) were mixed in
DMF/MeOH (5 mL, 9/1) in a
sealed tube and heated to 100 C for 15 h. The reaction solution was cooled to
RT and diluted with
AcOEt (10 mL). The solution was filtered through celite and dried under
reduced pressure. The residue
was recrystalized with CH2ClZ/MeOH to obtain a light yellow solid product
triazole.
Then LiOH (0.5 M, 8 mL) was added to this methyl ester (450 mg, 1.97 mmol) in
MeOH/THF (10 mL, 1/9) and stirred until all solids were dissolved (about 2 h).
Then 20 mL of MeOH
was added to this solution, followed by Pd/C (10 mg), and the mixture was
subjected to hydrogenation
under balloon pressure for 15 h. The reaction solution was filtered and
acidified to pH = 7. The product
white solid was obtained by filtration of the precipitate.
- 65 -
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
Following the similar amide formation and hydrolysis procedures described in
the
Examples above, Example 30 was obtained. 'H NMR (CD3OD, 500 MHz) S 8.12 (s,
1.H), 7.75(d, 2H),
7.33 (d, 2H), 3.00 (t, 2H), 2.89 (t, 2H), 2.65 (t, 2H), 2.32 (t, 2H), 1.62 (m,
4H); LCMS m/z 339 (M-1).
EXAMPLE 31
O OH
HO N O OH H
N
N
O
To racemic malic acid (1.03 g) was added 2,2-dimethoxypropane (25 mL) and p-
TsOH
hydrate (30 mg). The mixture was stirred overnight before the addition of
sodium acetate. The mixture
was stirred for additional 3 h and filtered. The filtrate was concentrated,
and the mono-protected acid
was crystallized from chloroform/hexane as a white solid.
To a solution of this acid intermediate (281 mg) in 10 mL of dichloromethane
was added
CDI (524 mg). The resulting mixture was stirred for 1 h, and to this mixture
was added the N-hydroxy
amidine (1.32 g) and dichloromethane (10 mL). The mixture was stirred over 2
days and then filtered.
The filtrate was concentrated, and the residue was suspended in toluene (40
mL) and heated to 120 C for
6 h and 130 C for 2 h. After removing the solvent, the residue was purified
by Biotage (20-40% ethyl
acetate in hexane) to give the oxadiazole intermediate, which was dissolved in
chloroform (5 mL) and
treated with TFA (2.5 mL) for 20 min. The mixture was concentrated, and to the
residue was added 5%
KOH in ethanol (50 ml). The resulting mixture was stirred for 6 h and
acidified with HCI until pH=4.
The solution was extracted with 30% isopropanol in chloroform. The extracts
were concentrated and
purified by RP-HPLC to give the hydroxypyridyl alpha-hydroxy acid
intermediate. Following similar
procedures as described for the Examples above, Example 31 was obtained after
silylation, amide
formation and hydrolysis. 'H NMR (CD3OD, 500 MHz) 6 8.25 (1H, s), 8.06 (1H,
dd), 7.42 (1H, dd),
4.63 (1H, dd), 3.62 (1H, m), 3.52 (1H, m), 2.92 (1H, m), 2.35 (1H, m), 1.63
(6H, m); LCMS m/z 375
(M+1).
EXAMPLE 32
0
HO '/ N N-r-~N P
N-O H CO2H
A solution of the commercially available olefinic acid shown in Scheme 21 (20
g) in
ethanol (150 mL) in the presence of 0.5 niL of concentrated sulfuric acid, was
heated under reflux for 1
day. A pad of 3A molecular sieves above the reaction flask was used to absorb
the water generated from
-66-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
the reaction. The mixture containing the volatile ester was cooled to 0 C, and
to the mixture was added
NMO (21.9 g) and 4% of Os04 (1 mL). The solution was stirred at 0 C for 1 h
and then warmed to 23
C and stirred overnight. Most of the solvent in this mixture was removed in
vacuo, the residue was
partitioned between water and ethyl acetate. The organic layer was
concentrated to give the diol
intermediate as a brown oil.
To a solution of this diol in 300 mL of acetone at 0 C was added a slurry of
sodium
periodate (87 g) in 400 mL of water. The resulting white slurry was slowly
warmed to RT and stirred
for 1.5 h. The slurry was filtered and washed with acetone, and the filtrate
was extracted with
dichloromethane. The combined organic layers were carefully concentrated to
provide the volatile
aldehyde as a brown oil. At 0 C, to the solution of this aldehyde and tert-
butanol (150 mL) was added 2-
methyl-2-butene (20 mL) and a solution of sodium dihydrophosphate (15 g) and
sodium chlorite (41 g,
-80%). The resulting brown mixture was slowly warmed to RT, and the mixture
was stirred for 5 h. To
the mixture was added 10% sodium hydroxide until pH>11. The mixture was then
washed with ethyl
acetate, and the aqueous layer was acidified with concentrated HCl until pH=4.
The resulting aqueous
fraction was extracted with ethyl acetate. The combined organic fractions were
dried with sodium
sulfate and concentrated in vacuo to give the mono-acid mono-ester as a
colorless oil.
To a solution of this acid intermediate (13.5 g) in 120 mL of dichloromethane
were
added 50 L of DMF and 97 mL of oxalyl chloride (2 M in dichloromethane) at 0
C. The mixture was
stirred at 0 C for 30 min, warmed to 23 C, and the resulting solution was
stirred for an additional 2 h.
After removing the volatiles, to the residue was added the N-hydroxy amidine
(21.2 g) and 100 mL of
pyridine. The mixture was then heated at 130 C for overnight. The pyridine
was removed in vacuo, and
the residue was partitioned between water and dichloromethane. The organic
phase was concentrated
and purified by Biotage (eluting with 10-40% ethyl acetate in hexane) to give
the bi-heterocyclic
intermediate as a white solid.
To this ester (15.1 g) in 400 mL of THF/MeOH/water (3:1:1), was added LiOH
(200 mL,
1 N) dropwise. The mixture was stirred at room temperature for 2 h. After
removing most organic
solvent in vacuo, the aqueous layer was acidified with 1 N HCl to pH=3. The
precipitate was extracted
with dichloromethane thrice. The combined organic phase was dried with sodium
sulfate and
concentrated to give the acid as a white solid.
To a solution of this acid intermediate (14.3 g) in 300 niL of
dichloromethane, were
added N-hydroxysuccinimide (4.52 g) and EDCI (7.53 g). The mixture was stirred
for 2.5 h and then
diluted to 1 L of dichloromethane. The resulting solution was washed with
brine and dried with sodium
sulfate. The solution was concentrated, and the resulting residue was
dissolved in 700 niL of dioxane.
To this solution was added ammonia in water (60 mL, 28-30%) at 0 C. The
resulting mixture was
stirred at RT for 15 min. The volatiles were removed in vacuo, and the residue
was partitioned between
water and dichloromethane. The organic phase was washed with water, saturated
sodium bicarbonate,
-67-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
and then dried with sodium sulfate. The resulting solution was concentrated to
give the primary
carboxamide as a white solid.
A mixture of this carboxamide (6.83 g), the enol triflate (12.9 g), Pd2(dba)3
(1.31 g),
Cs2CO3 (9.9 g, anhydrous), Xantphos (2.48 g) and 200 mL of dioxane was heated
under argon at 80 C
for overnight. The mixture was cooled and filtered through celite,
concentrated, and purified by Biotage
(20-40% ethyl acetate in hexane) to give the cyclohexenylamide ester as an
oil.
To this ester (8.6 g) in 84 mL of dichloromethane was added triisopropylsilane
(8.4 mL)
and TFA (40 mL) at 0 C. The mixture was stirred at room temperature for 15
min, the solvents
removed, the residue dissolved in 200 mL of THF/MeOH/water (3:1:1), and the
mixture treated dropwise
with excess of LiOH (1 N). The mixture was stirred at RT overnight, and after
removing most organic
solvents in vacuo, the aqueous layer was washed with ethyl acetate. The
aqueous layer was then
acidified with 1N HCl to pH=5. The precipitate was collected by filtration,
washed with water and
diethyl ether to give the product as a crude material. This material was
dissolved in 30% isopropanol in
chloroform and filtered. The filtrate was concentrated to a small volume and
the homogeneous mixture
was kept at 0 C overnight. The precipitate was collected, washed with
methanol, then diethyl ether and
dried under vacuum to provide Example 32 as a white solid. 'H NMR (Acetone-d6,
500 MHz) 6 12.1
(1H, s), 8.36 (1H, s), 7.98 (1H, d), 7.41 (1H, d), 3.27 (2H, s), 2.95 (2H, m),
2.37 (2H, m), 1.63 (4H, m),
1.46 (6H, s); LCMS m/z 387 (M+1).
EXAMPLE 33
O
HO ~ N N~N
N-0 CO2H
To (S)-pulegone (4 g) was added concentrated HCl (3.8 mL) and 12 mL of water.
The
mixture was heated at reflux for 20 h under vigorous stirring. The mixture was
distilled to remove
acetone and the heating oil was heated at 180 C to distill off both organic
layer and HCI (aq). The
organic layer was separated from the aqueous layer and then was diluted with
diethyl ether and washed
with sodium bicarbonate solution. The organic layer was then dried with sodium
sulfate and
concentrated carefully to remove most of the diethyl ether to give the (S)-3-
methylcyclohexanone.
To this (S)-3-methylcyclohexanone (2 g) in 50 mL of THF, was added LiHMDS (20
mL,
1 M in THF) at -78 C. The mixture was slowly warmed to 0 C and stirred for 30
min before the
solution was re-cooled to -78 C. To this mixture was added methyl
cyanoformate (1.55 mL), and the
mixture was stirred at -20 C for 2 h before the addition of 1 N HCl solution.
The aqueous layer was
extracted with diethyl ether and purified by Biotage (10-20% diethyl ether in
hexane) to give the
ketoester as a colorless oil.
-68-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
To this ketoester intermediate (1 g) in 20 mL of methanol, was added ammonium
acetate
(4.6 g), and the mixture was stirred overnight. After the removal of the
solvent, the residue was diluted
with ethyl acetate. The solid was filtered off, and the filtrate was washed
with water, brine and dried
with sodium sulfate. The liquid was then concentrated to give the (S)-
cyclohexene aminoester as an oily
solid.
The enantiomeric ester intermediates from Example 27 above (200 mg) were each
heated
at 70 C in 1 mL of concentrated HCl/HOAc (v:v=1:2) for 30 min. The resulting
mixture was
concentrated under high vacuum overnight. The enantiomeric acids were then
submitted directly for the
next amidation step with the (S)-cyclohexene aminoester as described in the
Examples above.
Following the similar hydrolysis procedures as described in the Examples
above,
provided two of the four possible diasteromers of Example 33 containing minor
epimerization. (S)-
Diastereomer A: 1H NMR (DMSO-d6, 500 MHz) S 12.6 (1H, s), 11.7 (1H, s), 10.6
(1H, s), 8.26 (1H, s),
7.88 (1H, d), 7.30 (1H, d), 3.02 (4H, m), 2.33 (2H, m), 2.16 (1H, m), 1.60
(2H, m), 1.27 (3H, s), 1.09
(1H, m), 0.91 (3H, d); LCMS m/z 387 (M+1). (S)-Diastereomer B:'H NMR (DMSO-d6,
500 MHz) S
12.6 (1H, s), 11.69 (1H, s), 10.6 (1H, s), 8.26 (1H, s), 7.88 (IH, d), 7.30
(1H, d), 3.02 (4H, m), 2.33 (2H,
m), 2.16 (1H, m), 1.60 (2H, m), 1.27 (3H, s), 1.09 (1H, m), 0.91 (3H, d); LCMS
m/z 387 (M+1).
Utilizing the commercially available (R)-3-methylcyclohexanone, provided
access to the
additional other two diastereomers of Example 33. Thus to a suspension of
sodium hydride (3.57 g,
89.15 mmol, 60% dispersion in oil) in anhydrous dioxane (25 mL) was added
dimethyl carbonate (30
mL, 356.6 mmol). The resulting mixture was heated to 85 C, and a solution of
(R)-3-
methylcyclohexanone (5.0 g, 44.64 mmol) in dioxane (50 mL) was added dropwise
via an addition
funnel. After stirring at 80 C for 2 hours, the reaction mixture was cooled
to room temperature and
quenched with 1N HCI. The resulting mixture was concentrated, the residue was
extracted with ether,
and the organic layer was washed with brine, dried over magnesium sulfate,
filtered and concentrated.
The residue was purified by Biotage using a gradient of 0-5% ethyl acetate-
hexanes to give the desired
product as a white crystalline solid.
To a solution of this methyl ketoester intermediate (2.5 g, 14.7 mmol) in
methanol (50
mL) was added ammonium acetate (5.66 g, 73.52 mmol). The reaction mixture was
left stirring at RT for
16 h. It was then concentrated, and the residue was diluted with ethyl acetate
and washed with water.
The organic layer was washed with brine, dried over anhydrous sodium sulfate,
filtered and concentrated.
A white solid was obtained for this methyl (R)-cyclohexene aminoester
intermediate.
As before, the enantiomeric ester intermediates from Example 27 above were
each
converted to their respective acids. One enantiomeric acid was then submitted
directly for the next
amidation step with the methyl (R)-cyclohexene aminoester as described in the
Examples above.
Following the similar hydrolysis procedures as described in the Examples
above, and subsequent
purification by reverse phase HPLC, provided the third diastereomer of Example
33 containing minor
-69-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
epimerization. (R)-Diastereomer A: 'H NMR (500 MHz, DMSO-d6) 8 11.73 (s, 1H),
8.26 (s, 1H), 7.9 (d,
IH), 7.37 (dd, 1H), 3.26 (m, 1H), 3.0 (m, 3H), 2.4 (m, 2H), 2.1 (m, 1H), 1.6
(rn, 2H), 1.28 (d, 3H), 1.08
(m, 1H), 0.93 (d, 3H); LCMS m/z 387 (M+1).
To obtain the fourth diastereomer of Example 33, a solution of the (R)-3-
methyl
cyclohexanone (2.18 g, 19.46 mmol) in anhydrous THF (50 mL), cooled to -78 C,
was treated with
LiHMDS (23.35 mL, 1.0 M in THF). After 15 minutes, benzyl cyanoformate was
added. The reaction
mixture was slowly wanned to 0 C over an hour, and quenched by the addition of
1N HCI. The
resulting mixture was extracted with ethyl acetate. The organic layer was
washed with brine, dried over
anhydrous sodium sulfate, filtered and concentrated. The residue was purified
by Biotage SP-I using a
gradient of 0-15% ethyl acetate-hexanes to give the desired product as a
colorless oil.
To a solution of this benzyl ketoester intermediate (1.0 g, 4.06 mmol) in
methanol (20
mL) was added ammonium acetate (1.57 g, 20.32 mmol). After stirring the
reaction at 23 C for 16 hours
it was concentrated. The residue was diluted with ethyl acetate and washed
with water. The organic
layer was washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated. A white
solid was obtained for this benzyl (R)-cyclohexene aminoester intermediate.
As before, the enantiomeric ester intermediates from Example 27 above were
each
converted to their respective acids. One enantiomeric acid was then submitted
directly for the next
amidation step with the benzyl (R)-cyclohexene aminoester as described in the
Examples above.
Following the similar PMB-ether deprotection procedures as described in the
Examples above, provided
the benzyl ester penultimate intermediate.
To a solution of this benzyl ester intermediate (27 mg, 0.056 rnmol) in
methanol (2 mL)
was added Pd/C (10 mg). The resulting solution was stirred under a hydrogen
balloon for 15 minutes.
The reaction mixture was filtered through celite. The filtrate was
concentrated and purified by reverse
phase HPLC to provide the fourth diastereomer of Example 33 containing minor
epimerization. (R)-
Diastereomer B: 1H NNIR (500 MHz, DMSO-d6) S 11.69 (s, 1H), 8.26 (s, 1H), 7.88
(d, 1H), 7.3 (dd, 1H),
3.24 (m, 1H), 3.0 (m, 3H), 2.4 (m, 2H), 2.1 (m, 1H), 1.6 (m, 2H), 1.26 (d,
3H), 1.1 (m, 1H), 0.92 (d, 3H);
LCMS m/z 387 (M+1).
EXAMPLE 34
O
HO
N-0 H CO2H
The enantiomers of Example 34 were generated from the respective methyl (R)-
cyclohexene aminoester and methyl (S)-cyclohexene aminoester intermediates
prepared in Example 33
above. These enantiomeric methyl cyclohexene aminoesters were acylated with
the requisite carboxylic
acid intermediate from Example 23, and the amides converted to the desired
products following the
-70-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
procedures described in the Examples above. (S)-Enantiomer: 'H NMR (CD3OD, 500
MHz) S 8.23 (1H,
s), 8.01 (1H, d), 7.34 (1H, d), 3.29 (2H, m), 3.08 (1H, bd), 2.99 (2H, bs),
2.50 (1H, bd), 2.41 (1H, m),
2.28 (1H, m), 1.70 (1H, m), 1.66 (1H, m), 1.18 (1H, m), 1.01 (3H, d); LCMS m/z
373 (M+1). (R)-
Enantiomer: 'H NMR (500 MHz, DMSO-d6) 8 11.66 (s, 1H), 8.26 (s, 1H), 7.91 (d,
1H), 7.32 (dd, 1H),
3.2 (t, 2H), 3.0 (dd, 1H), 2.9 (m, 2H), 2.4 (m, 2H), 2.1 (m, 1H), 1.6 (m, 2H),
1.15 (m, 1H), 0.95 (d, 3H);
LCMS m/z 373 (M+1).
EXAMPLE 35
O
H~~N OH
O OH O-N N
Commercially available 4-ethylcyclohexanone was converted to its methyl
ketoester via
Mander's reagent under the conditions described in the Examples above. This
material as an orange oil
was used in the next step without any further purification.
To a solution of this methyl ketoester intermediate (475mg, 2.6 mmol) in
anhydrous THF
(25 mL) cooled to 0 C, was added NaH (113 mg, 2.84 mmol, 60%). After 30 min, 2-
[]V,N-
bis(trifluromethylsulfonyl)amino]-5-chloropyridine (1.13 g, 2.84 mmol) was
added, and the resulting
reaction stirred at room temperature for 2 h. The reaction mixture was
quenched with 1N HCI, then
extracted with EtOAc. The organic layers were washed with brine, dried over
NazSO4i filtered and
concentrated to give a brown oil. This material was purified by Biotage using
50% EtOAc/hexanes as
eluant to give the desired enol triflate intermediate.
As shown in Scheme 23, the methyl ester intermediate from Example 23 can be
directly
converted to its primary carboxamide. Thus to a solution of this methyl ester
in dioxane in a pressure
tube was added 7N ammonia in methanol. The resulting solution was heated at 70
C for 16 hours. The
reaction mixture was cooled to room temperature and concentrated to give the
desired primary
carboxamide intermediate as a white solid.
To a solution of the enol triflate intermediate (150 mg, 0.37 9 nunol) in
anhydrous
dioxane (3 mL) was added the primary carboxamide intermediate (112 mg, 0.316
mmol), XANTPHOS
(37 mg, 0.06 mmol), cesium carbonate (46 mg, 0.36 mmol) and Pd2(dba)3 (20 mg,
0.019 mmol). The
resulting mixture was de-gassed for 2 minutes by bubbling N2. The reaction was
heated at 60 C under a
N2 atmosphere for 18 h. The reaction mixture was cooled to RT, and filtered
through celite. The filtrate
was concentrated, and the residue was purified by Prep-TLC (30% ethyl acetate-
hexanes) to give the
desired amide product.
-71-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
To a solution of this intermediate (89 mg, 0.171 mmol) in DCM (2 mL) was added
triisopropyl silane (0.3 mL) followed by TFA (1 mL). After stirring the
mixture for 20 min, it was
cooled to 0 C and carefully quenched by the addition of saturated sodium
bicarbonate solution. The
resulting mixture was extracted with 30% isopropanol/chloroform. The organic
layer was dried over
anhydrous sodium sulfate, filtered and concentrated. This material was
dissolved in THF (5 mL), 1N
NaOH (2 mL) was added followed by enough MeOH to obtain a homogenous solution.
After stirring the
reaction at room temperature for 18 h, it was neutralized with 1N HCI. The
resulting mixture was
extracted with 20% isopropanol/chloroform. The organic layer was washed with
brine and dried over
Na2SO4, filtered and concentrated. The residue was purified by reverse phase
HPLC (10-100%
acetonitrile/HZO (1%TFA)) to provide Example 35. 1H NMR (CD3OD, 500 MHz), S
8.25 (d, 1H), 7.90-
7.88 (d, 1H) 7.31-7.29 (dd, 1H), 3.18 (t, 2H), 2.99-2.88 (m, 3H), 2.69-2.63
(m, 1H), 2.44-2.40 (m, 1H),
1.78-1.73 (m, 2H), 1.30-1.23 (m, 3H), 1.10-1.06 (m, 1H), 0.866 (t, 3H); LCMS
m/z 387 (M+1).
EXAMPLE 36
COOH
~
F3C NH
p N N-"
/ ~ ~ OH
oN
To a solution of 3-(trifluromethyl)-phenol (3.0g, 18.51 mmol) in methanol (20
mL) was
added Rh/Ala03 (100 mg). The resulting mixture was stirred under hydrogen
atmosphere (50 psi) for 16
h. The reaction was filtered through celite and concentrated to give the
cyclohexanol as a colorless oil.
To a solution of this cyclohexanol intermediate (3.0g, 17.85 mmol) in
dichloromethane
(100 mL) was added Dess-Martin reagent (9.0g, 21.42 mmol). After stirring at
room temperature for 4 h,
the mixture was quenched with saturated sodium bicarbonate solution. The
resulting mixture was
extracted with dichloromethane. The organic layer was dried over anhydrous
sodium sulfate, filtered and
concentrated to give the ketone as a colorless oil.
To a solution of this cyclohexanone intermediate (2.0 g, 12.04 nunol) in
anhydrous THF
cooled to -78 C was added LiHMDS (14.5 mL, 14.5 mmol, 1.0 M in THF). The
resulting mixture was
warmed to 0 C and stirred for 20 min. The reaction mixture was cooled back to -
78 C and methyl
cyanoforniate (1.15 mL, 14.46 nunol) was added. The reaction mixture was
warmed to -20 C and
quenched with 1N HCI. The resulting mixture was extracted with ethyl acetate.
The organic layer was
washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated. The residue was
purified by flash chromatography using 10% ethyl acetate-hexanes to give the
ketoester as a colorless oil.
-72-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
To this ketoester intermediate (860 mg, 3.83 mmol) in methanol was added
ammonium
acetate (1.48 g, 19.2 mmol). After stirring the mixture at room temperature
for 16 h, it was concentrated.
The residue was diluted with ethyl acetate and washed with water. The organic
layer was washed with
brine, dried over anhydrous sodium sulfate, filtered and concentrated. A white
solid of the
trifluorocyclollexene aminoester was obtained.
As shown in Scheme 24, the carboxylic acid intermediate from Example 23 can be
used
to acylate this trifluorocyclohexene aminoester. Thus to a solution of the
carboxylic acid (100 mg, 0.281
mmol) in anhydrous dichloromethane (5 mL) cooled to 0 C under nitrogen
atmosphere, was added DMF
(10 L) followed by oxalyl chloride (0.562 mL, 2.0 M solution in DCM). The
reaction mixture was
warmed to 23 C and stirred for 30 min. The reaction mixture was concentrated,
and the residue was
dissolved in anhydrous dichloromethane. A solution of the trifluorocyclohexene
aminoester (140 mg,
0.627 mmol) in dichloromethane (2 mL) was added. After stirring the mixture at
RT for 16 h, it was
quenched by the addition of saturated sodium bicarbonate solution. The
resulting mixture was extracted
with dichloromethane. The organic layer was dried over anhydrous sodium
sulfate, filtered and
concentrated. The residue was purified by flash chromatography using 40% ethyl
acetate-hexanes to
give the desired amide methyl ester as a white solid.
To a solution of this ester intermediate (44 mg) in THF (2 mL) was added 0.5 N
NaOH
(2 mL), followed by MeOH (1 mL). After stirring the mixture for 1 h, it was
quenched by neutralizing
with 1N HCl (1 mL). The resulting mixture was concentrated, and the residue
was diluted with water (5
mL) and extracted with ethyl acetate. The organic layer was dried over
anhydrous sodium sulfate,
filtered and concentrated. The residue was purified by reverse phase HPLC to
provide Example 36. 'H
NMR (500 MHz, DMSO-d6) S 11.64 (s, 1H), 10.62 (bs, 1H), 8.26 (bs, 1H), 7.9 (d,
1H), 7.32 (dd, 1H), 3.2
(m, 3H), 2.9 (m, 211), 2.75 (m, 1H), 2.6 (m, 2H), 2.2 (m, 1H), 1.9 (m, 1H),
1.4 (m, 1H); LCMS m/z 427
(M+1).
EXAMPLE 37
O
~ NJ~N OH
HO O H O-N N
To a suspension of copper(I) iodide (3.8 g, 20 mmol) in anhydrous ether (20
mL) cooled
to 0 C under a nitrogen atmosphere was added dropwise a solution of methyl
lithium (25 mL, 40 mmol,
1.6 M in diethylether). After stirring the mixture at 0 C for 20 min, it was
cooled to -78 C and 3-methyl
2-cyclohexen-l-one was added. The reaction mixture was slowly warmed to -20 C
over I h and
-73-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
quenched by the addition of conc. ammonium hydroxide solution (10 mL). The
resulting biphasic
solution was stirred for 20 min. The aqueous layer was extracted with ether,
and the organic layer was
washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated. The residue was
purified by flash chromatography using 10% ethyl acetate-hexanes to give the
desired geminal dimethyl
cyclohexanone as a yellow oil.
To a solution of this cyclohexanone intermediate (740 mg, 5.86 mmol) in
anhydrous
THF (20 mL) cooled to -78 C under a nitrogen atmosphere, was added LiHMDS (7
mL, 7 mmol, 1.0 M
solution). After 15 min, methyl cyanoformate (0.558mL, 7.03 mmol) was added.
After stirring the
mixture at -78 C for 20 min, it was quenched with 1N HCI. The biphasic
mixture was extracted with
ethyl acetate, washed with brine and dried over anhydrous sodium sulfate. The
organic layer was
filtered, concentrated and purified by flash chromatography using 10% ethyl
acetate hexanes to give the
ketoester as a colorless oil.
To a solution of this ketoester intermediate (500 mg, 2.72 mmol) in methanol
(30 mL)
was added ammonium acetate (1.05 g, 13.6mmo1). After stirring the reaction
mixture at room
temperature for 48 h, it was concentrated, and the residue was diluted with
ethyl acetate and washed with
water. The organic layer was washed with brine, dried over anhydrous sodium
sulfate, filtered and
concentrated. A white solid was obtained for the geminal dimethyl cyclohexene
aminoester intermediate.
As shown in Scheme 25, the carboxylic acid intermediate from Example 23 can be
used
to acylate this cyclohexene aminoester. Thus to a solution of the carboxylic
acid (100 mg, 0.281 nnnol)
in methylene chloride (4 mL) and DMF (20 uL), was added oxalyl chloride (0.281
mL, 0.563 mmol) at 0
C. The solution was stirred at RT for 30 min, then concentrated. To the
resulting residue was added
methylene chloride (3 mL) followed by a solution of the cyclohexene aminoester
intermediate (129 mg,
0.703 mmol) in methylene chloride (2 mL). The reaction was stirred at 23 C
under N2 for 3 h, then
quenched with saturated NaHC03 solution and extracted with methylene chloride.
The combined
organic layers were dried over anhydrous sodium sulfate, filtered and
concentrated. The residue was
purified on silica, eluting with a gradient of 20% - 60% EtOAc/hexanes over 10
column volumes, then
60% - 100% EtOAc/hexanes over 4 column volumes, affording the desired amide as
a white solid.
To a solution of this PMB ether intermediate (84 mg, 0.161 mmol) in methylene
chloride
(4 mL) was added triisopropylsilane (500 uL, 2.441 mmol) then TFA (2 ml, 26.0
mmol). The reaction
was stirred for 5 min, then slowly quenched at 0 C with saturated aqueous
NaHCO3 in an ice bath. This
mixture was extracted with methylene chloride then 30% IPA/CHC13. The combined
organic layers were
dried over anhydrous sodium sulfate, filtered and concentrated. The solid
residue was used in the next
step without further purification.
To this ester intermediate was added THF (2 mL), NaOH (2 mL, 1.0 mmol) and
methanol (1 mL). The mixture was stirred overnight, then quenched with 1N HCl
(1 mL) and
concentrated. The mixture was diluted with water and extracted with EtOAc. The
combined organic
layers were dried over anhydrous sodium sulfate, filtered and concentrated to
an oily residue. The
-74-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
residue was purified by reverse phase HPLC to provide Example 37. 'H NMR (500
MHz, DMSO-d6)
S 11.67 (s, 1H), 8.26 (d, 1H), 7.89 (d, 1H), 7.30 (dd, 1H), 3.18 (t, 2H), 2.90
(t, 2H), 2.59 (s, 2H), 2.25 (t,
2H), 1.29 (t, 2H), 0.87 (s, 6H); LCMS m/z 369 (M-17).
EXAMPLE 38
COOH
I NH
N
F
oN
To a suspension of 5-amino-2-cyano pyridine (20.0 g, 0.168 mol) in. HF-
pyridine (100 g)
in an Erlenmeyer flask cooled to 0 C was added sodium nitrite (17.4 g, 0.251
mol) in four portions.
After 45 min at 0 C the reaction mixture was stirred at room temperature for
30 min and then heated to
80 C for 90 min. The reaction mixture was quenched by pouring into ice/water
mixture. The resulting
mixture was extracted with DCM. The organic layer was dried over anhydrous
sodium sulfate, filtered
and concentrated to give the fluoropyridine as an orange solid.
To a suspension of this fluoropyridine nitrile intermediate (16.0 g, 0.131
mol) in
methanol (200 mL) was added hydroxylamine (9.63 niL, 0.157 mmol, 50% by wt).
After stirring the
reaction at room temperature for 48 h, it was filtered through a fritted
funnel. The precipitate was
washed with ether and dried under vacuum to give the N-hydroxy amidine as a
yellow solid.
To a suspension of this amidine intermediate (5.32 g, 34.32 mmol) in anhydrous
pyridine
(10 mL) was added 4-chloro-4-oxo-methyl butyrate (5 mL, 41.18 mmol). The
resulting reaction mixture
was heated at 120 C for 2 h. The niixture was cooled to RT and concentrated.
The residue was
dissolved in ethyl acetate and washed with 1N HCI, water and brine. The
organic layer was dried over
anhydrous sodium sulfate, filtered and concentrated to give a dark brown
solid. This material was
purified by Biotage using 25%-60% ethyl acetate-hexanes gradient to give the
heterobiaryl intermediate
as a light yellow solid.
To a solution of this ester intermediate (900 mg, 3.58 mmol) in THF (4 mL) was
added
methanol (2 mL) followed by 5N NaOH (1 mL). After 30 min, the reaction mixture
was neutralized by
the addition of 1N HCl (5 mL). The reaction mixture was concentrated. The
residue was extracted with
ethyl acetate, and the organic layer was washed with brine, dried over
anhydrous sodium sulfate, filtered
and concentrated to give a light yellow solid of the carboxylic acid.
To a solution of this acid intermediate (50 mg, 0.21 mmol) in anhydrous
dichloromethane (4 mL) cooled to 0 C under nitrogen atmosphere was added DMF
(10 L) followed by
oxalyl chloride (0.21mL, 2.0 M solution in DCM). The reaction was warmed to 23
C and stirred for 30
min. The reaction mixture was concentrated, and the residue was dissolved in
anhydrous
-75-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
dichloromethane (2 mL) and cooled to 0 C. A dichloromethane (2 mL) solution of
the methyl (R)-
cyclohexene aminoester described in the Examples above (90 mg, 0.525 mmol) was
then added. The ice-
bath was renioved, and the resulting solution was stirred at RT for 16 h. The
reaction mixture was
quenched by the addition of saturated sodium bicarbonate solution. The
resulting mixture was extracted
with dichloromethane. The organic layer was dried over anhydrous sodium
sulfate, filtered and
concentrated. The residue was purified by flash chromatography using 25% then
35% ethyl acetate-
hexanes to give the amide as a white solid.
To a solution of this amide ester intermediate (41 mg) in THF was added 1N
LiOH (1
mL). The resulting mixture was stirred for 16 h. The reaction mixture was
neutralized by the addition of
IN HCI (1 mL). The resulting biphasic mixture was extracted with ethyl
acetate. The organic layer was
dried over anhydrous sodium sulfate, filtered and concentrated. The residue
was purified by reverse
phase HPLC to provide Example 38 as the (R)-enantiomer. 'H NMR (500 MHz, DMSO-
d6) 8 12.6 (bs,
111), 11.64 (s, 1H), 8.76 (d, 1H), 8.14 (dd, 1H), 7.93 (dt, 1H), 3.2 (t, 2H),
2.9 (m, 3H), 2.3 (m, 2H), 2.15
(m, 1H), 1.6 (m, 2H), 1.1 (m, 1H), 0.92 (d, 3H); LCMS m/z 397 (M+Na).
EXAMPLE 39
0
F
HO O O--N N
To a solution of 3-hydroxy benzaldehyde (1.2g, 10 mmol) in anhydrous DCM (50
mL)
was added imidazole (1.02 g, 15 mmol) followed by TBSCI (1.65 g, 11 mmol).
After stirring the
reaction at room temperature for 1 h, it was quenched by pouring into
saturated sodium bicarbonate
solution. The resulting mixture was extracted with DCM, and the organic layer
was dried over
anhydrous sodium sulfate, filtered and concentrated to give the silyl ether
aldehyde.
To a solution of this aldehyde intermediate (2.24 g, 9.5 mmol) in ethanol (50
mL) was
added sodium borohydride (0.5g, 14.5 mmol). After stirring the mixture at RT
for 1 h, it was
concentrated, and the residue was dissolved in ethyl acetate and washed with
water and brine. The
organic layer was dried over anhydrous sodium sulfate, filtered and
concentrated to give the hydroxy
methylene intermediate.
To a solution of this alcohol (2.25 g, 9.5 mmol) in anhydrous THF (50 mL)
cooled to 0
C under a nitrogen atmosphere was added sodium hydride (0.57 g, 14.25 mmol).
After 15 min, methyl
iodide (0.89 mL, 14.25 mmol) was added. After stirring the mixture at room
temperature for 1 h, it was
quenched with saturated ammonium chloride solution. The resulting mixture was
extracted with ethyl
acetate, washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated. The crude
-76-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
material was dissolved in THF (20 mL) and TBAF (2 mL) was added. After 1 h,
the reaction mixture
was concentrated and the residue purified by flash chromatography using 30%
ethyl acetate hexanes to
give the methyl ether as a colorless oil.
To a solution of this phenol intermediate (0.9 g, 6.52 mmol) in methanol (20
mL) was
added Rh/A1203 (50 mg). The resulting mixture was stirred under a hydrogen
balloon for 18 h. The
mixture was filtered through celite and concentrated to give the desired
cyclohexanol as a colorless oil.
To a solution of this cyclohexanol (870 mg, 6 mmol) in DCM cooled to -78 C
was
added DMSO (0.85 ml, 12 mmol) followed by oxalyl chloride (4.5 mL, 2M in DCM).
After 10 min,
triethylamine (1.67 mL, 12 mmol) was added and the reaction mixture slowly
warmed to 0 C over 1 h.
The mixture was quenched by pouring into saturated sodium bicarbonate
solution. The resulting mixture
was extracted with DCM. The organic layer was dried over anhydrous sodium
sulfate, filtered and
concentrated. The residue was purified by flash chromatography using 10% ethyl
acetate-hexanes to
give the cyclohexanone intermediate.
To a suspension of sodium hydride (0.225 g, 5.62 mmol, 60% dispersion in oil)
in
anhydrous dioxane (5 mL) was added dimethyl carbonate (1 mL, 11.87 mmol). The
resulting mixture
was heated to 85 C, and a solution the cyclohexanone intermediate (400 mg,
2.81 mmol) in dioxane (5
mL) was added dropwise via an addition fiuuiel. After stirring at 80 C for 2
h, the reaction mixture was
cooled to room temperature and quenched with 1N HCI. The resulting mixture was
concentrated. The
residue was extracted with ether. The organic layer was washed with brine,
dried over magnesium
sulfate, filtered and concentrated. The residue was purified by flash
chromatography 30% ethyl acetate-
hexanes to give the ketoester.
To a solution of this ketoester (199 mg, 0.994 mmol) in methanol (12 mL), was
added
ammonium acetate (383 mg, 4.97 mmol). The reaction was left stirring at room
temperature, and
concentrated. The residue was dissolved in EtOAc and washed with water and
brine. The organic phase
was dried over anhydrous sodium sulfate, filtered and concentrated to give the
methoxymethyl ether
substituted cyclohexene aminoester.
Following similar procedures as described for the Examples above, Example 39
was
obtained after amide formation and hydrolysis. The residue was purified by
reverse phase HPLC to
provide Example 39. 1H NMR (500 MHz, DMSO-d6) S 11.66 (s, 1H), 8.76 (d, 1H),
8.14 (dd, 1H), 7.94
(m, 1), 3.24 - 3.20 (m, 7H), 3.01 - 2.89 (m, 3H), 2.43 (t, 1H), 2.37 (d, 1H),
2.16 (m, 1H), 1.75 (m, 1H),
1.68 (br d, IH), 1.14 (m, 1H); LCMS m/z 403 (M-1).
EXAMPLE 40
-77-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
OH
NH
OCI(COOH
To a solution of tetrahydro-4-H-pyran-4-one (1 mL, 10.82 mmol) in anhydrous
THF (50
mL) cooled to -78 C under a nitrogen atmosphere, was added lithium
diisopropylamide (6.5 mL, 13.02
rnmol, 2.0 M solution). After 20 min, methyl cyanoformate (1.03 mL, 13.03
mmol) was added. The
resulting mixture was slowly warmed to -20 C, and quenched with saturated
ammonium chloride
solution. The biphasic mixture was extracted with ethyl acetate, washed with
brine and dried over
anhydrous sodium sulfate. The organic layer was filtered, concentrated and
purified by flash
chromatography using 30% ethyl acetate hexanes to give the ketoester as a
colorless oil.
To a solution of this ketoester intermediate (0.450 g, 2.85 mmol) in anhydrous
THF (20
mL) cooled to 0 C, was added sodium hydride (0.171 g, 4.27 mmol, 60% by
weight). After 30 min, 2-
[N,N-Bis(trifluromethylsulfonyl)amino]-5-chloropyridine (1.34 g, 3.42 nunol)
was added. After stirring
the reaction mixture at room temperature for 2 h, it was quenched with
saturated ammonium chloride
solution. The resulting mixture was extracted with ethyl acetate, and the
combined organic layers were
washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated. The residue was
purified by flash chromatography using 30% ethyl acetate-hexanes to give the
enol triflate as colorless
oil.
To a solution of 6-methoxy-2-naphthaldehyde (3.72g, 20.0 mmol) in toluene (40
mL)
placed in a pressure vessel, was added
methyl(triphenylphosphoranylidene)acetate (6.7 g, 20 nunol). The
resulting mixture was refluxed at 120 C for 18 h. The reaction mixture was
concentrated and purified
using a Biotage flash 40M column with 15% ethyl acetate-hexanes as the eluant,
to provide the enoate.
To a solution of this enoate (4.64 g, 19.14 mmol) in 1:1 dichloromethane-
methanol (100
mL) was added Pd/C. The resulting mixture was stirred under a H2 balloon for
18 h. The reaction
mixture was filtered through celite and concentrated to give the methoxy ester
as a white solid.
To a solution of this methyl ether intermediate (3.0 g, 12.3 mmol) in DCM (80
mL)
cooled to 0 C, was added BBr3 (61.5 mL, 1.OM in DCM). After 30 min, the
mixture was quenched with
methanol (50 mL) followed by cold water. The resulting mixture was
concentrated, and the residue
diluted with water and extracted with dichloromethane. The organic layer was
dried over anhydrous
Na2SO4i filtered and concentrated. This naphtholic ester was used in the next
step without any further
purification.
To a solution of this ester intermediate (3.0 g, 12.3 mmol) in 1,4-dioxane (50
mL) placed
in a pressure tube, was added concentrated NH4OH solution. The resulting
mixture was stirred at RT for
18 h. The reaction mixture was concentrated and the residue was suspended in
ethyl acetate, washed
-78-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
with water, dried over anhydrous sodium sulfate filtered and concentrated. The
residue was purified by
flash chromatography using 50% ethyl acetate-hexanes then 100% ethyl acetate
as the eluant to give the
naphtholic primary carboxamide as an off-white solid.
To a solution of the enol triflate intermediate (100 mg, 0.344 mmol) in
anhydrous
dioxane (3 mL) was added the primary carboxamide intermediate (61 mg, 0.287
mmol), XANTPHOS (40
mg, 0.068 mmol), cesium carbonate (157 mg, 0.481 mmol) and Pd2(dba)3 (19 mg,
0.02 mmol). The
resulting mixture was degassed for 2 min by bubbling N2 gas. The reaction
mixture was heated at 50 C
under a N2 atmosphere for 2 h. The reaction mixture was cooled to room
temperature, and filtered
through celite. The filtrate was concentrated and purified by flash
chromatography using 40% ethyl
acetate-hexanes to give the amide product.
To a solution of this ester penultimate intermediate (44 mg) in THF (2 mL) was
added
1N NaOH (1 mL) followed by MeOH (1 mL). The resulting mixture was stirred at
23 C for 5 h. The
reaction mixture was quenched by the addition of 1N HCI (1 mL). The resulting
mixture was
concentrated, and the residue was extracted with ethyl acetate. The combined
organic layers were
washed with brine, dried over anhydrous sodium sulfate, filtered and
concentrated. The residue was
purified by reverse phase HPLC (Gilson) to provide Example 40. 'H NMR (500
MHz, DMSO-d6) S
11.38 (s, 1H), 9.59 (bs, 1H), 7.65 (d, 1H), 7.56 (m, 2H), 7.28 (d, 1H), 7.05
(m, 2H), 4.16 (s, 2H), 3.65 (t,
2H), 2.9 (t, 2H), 2.86 (bt, 2H), 2.65 (t, 2H); LCMS m/z 342 (M+1).
EXAMPLE 41
OH
NH
~
COOH
Potassium hexamethyldisilazide (35 niL of a 0.5 M solution in TIIF, 17.5
nmmol) was
added to propyl triphenylphosphonium bromide (7.1g, 18.5 mmol) in toluene (75
mL) at 0 C. The
solution was stirred for 15 min, and the ketone (2.1 g, 12.3 mmol) in toluene
(50 mL) was added. The
solution was stirred at 0 C for 1 h and then heated at 100 C overnight.
Solvent was removed, and the
product was purified by flash chromatography (Biotage, Horizon) 0 to 10% ethyl
acetate/hexanes. The
product was dissolved in methanol (150 rnL) and stirred over palladium on
carbon (5%, lg) under an
atmosphere of hydrogen overnight. The solution was filtered through celite and
solvent was removed.
The product was dissolved in THF/MeOH/3N HCl (50mL/20mL/10mL) for 36 h. The
mixture was
neutralized with saturated sodium bicarbonate, and the solvent was removed.
The solution was washed
with ethyl acetate, and the resulting organic layer was washed with brine and
dried over Na2SO4. The
product was purified by flash chromatography (Biotage, Horizon) 0 to 20% ethyl
acetate/hexanes.
-79-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
To a solution of the ketone (796 m g, 5.2 mmol) in anhydrous THF (25 mL)
cooled to -
78 C under a N2 atmosphere, was added LiHMDS (6.2 mL, 6.2 mmol, 1.0 M in
THF). After 30 min,
methyl cyanoformate (0.538 mL, 6.7 mmol) was added, and the reaction mixture
was allowed to warm to
0 C over several hours. The mixture was quenched with 1N HCl and extracted
with EtOAc (2X). The
organic layer was washed with brine and dried over Na2SO4a filtered and
concentrated in vacuo. This
material was used in the next step without any further purification.
To a solution of the ketoester (1095 mg, 5.2 mmol) in anhydrous THF (50 mL)
was
added NaH (309 mg, 7.7 mmol, 60%). After 15 min, 2-[N,N-
Bis(trifluromethylsulfonyl)amino]-5-
chloropyridine (2.02 g, 5.2 mmol) was added. The reaction mixture was stirred
at room temperature for
18 h and then quenched with water. The resulting mixture was extracted with
EtOAc (2X). The organic
layer was washed with brine, dried over Na2SO4, filtered, and concentrated in
vacuo. The crude material
was purified by flash chromatography (Biotage, Horizon) (0% EtOAc/Hexane to
20% EtOAc/Hexane) to
give the desired product.
To a solution of the vinyl triflate (200 mg, 0.58 mmol) in anhydrous dioxane
(11 mL)
was added the amide (15 mg, 0.07 mmol), XANTPHOS (32 mg, 0.05 mmol), cesium
carbonate (22 mg,
0.17 mmol) and Pd2(dba)3 (20 mg, 0.02 mmol). The resulting mixture was de-
gassed for 2 min by
bubbling gaseous N2. The mixture was heated at 60 C under a N2 atmosphere for
18 h. The reaction
mixture was cooled to room temperature, and filtered through celite. The
filtrate was concentrated in
vacuo, and the residue was purified by reverse phase HPLC (Gilson) to give the
desired product.
To a solution of the methyl ester in dioxane (3 mL) was added MeOH (1 mL) and
1N
LiOH (1 mL). The resulting mixture was stirred at room temperature for 18 h,
and then neutralized to
pH=7 by the addition of 1N HCI, and purified by reverse phase HPLC (Gilson) to
provide Example 41.
'H NMR (500 MHz, DMSO-d6) S 7.65 (d, 1H), 7.58 (d, 1H), 7.56 (s, 1H), 7.28 (d,
1H), 7.06-7.02 (m,
2H), 2.97-2.88 (m, 3H), 2.65-2.63 (m, 3H), 2.45-2.33 (m, 2H), 1.84-1.71 (m,
1H), 1.65-1.62(m, 1H),
1.51-1.15(m, 4H),0.901 (d, 3H), 0.86 (t, 3H); LCMS m/z 396 (M+1).
BIOLOGICAL ASSAYS
The activity of the compounds of the present invention regarding niacin
receptor affinity
and function can be evaluated using the following assays:
3H-Niacin bindingassay:
1. Membrane: Membrane preps are stored in liquid nitrogen in:
20 mM HEPES, pH 7.4
0.1 mM EDTA
Thaw receptor membranes quickly and place on ice. Resuspend by pipetting up
and down
vigorously, pool all tubes, and mix well. Use clean human at 15 g/well, clean
mouse at lOug/well, dirty
preps at 30ug/well.
-80-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
la. (human): Dilute in Binding Buffer.
lb. (human+ 4% serum): Add 5.7% of 100% human serum stock (stored at -20 C)
for a final
concentration of 4%. Dilute in Binding Buffer.
1 c. (mouse): Dilute in Binding Buffer.
2. Wash buffer and dilution buffer: Make 101iters of ice-cold Binding Buffer:
20 mM HEPES, pH 7.4
1 mM MgC12
0.01% CHAPS (w/v)
use molecular grade or ddHZO water
3. [5, 6 3H] - nicotinic acid: American Radiolabeled Chemicals, Inc. (cat #
ART-689). Stock is -50
Ci/mrnol, 1 mCi/ml, 1 ml total in ethanol4 20 M
Make an intermediate 3H-niacin working solution containing 7.5% EtOH and 0.25
M tracer.
40 L of this will be diluted into 200 gL total in each well-> 1.5% EtOH, 50 nM
tracer final.
4. Unlabeled nicotinic acid:
Make 100mM, 10mM, and 80 M stocks; store at -20 C. Dilute in DMSO.
5. Preparing Plates:
1) Aliquot manually into plates. All compounds are tested in duplicate. 10mM
unlabeled nicotinic
acid must be included as a sample compound in each experiment.
2) Dilute the 10mM compounds across the plate in 1:5 dilutions (8 1:40 1).
3) Add 195 L binding buffer to all wells of Intermediate Plates to create
working solutions (250 M
4 0). There will be one Intermediate Plate for each Drug Plate.
4) Transfer 54 from Drug Plate to the Intermediate Plate. Mix 4-5 times.
6. Procedure:
1) Add 140 L of appropriate diluted 19CD membrane to every well. There will
be three plates for
each drug plate: one human, one human+serum, one mouse.
2) Add 20 L of compound from the appropriate intermediate plate
3) Add 40 L of 0.25 M 3H-nicotinic acid to all wells.
4) Seal plates, cover with aluminum foil, and shake at RT for 3-4 hours, speed
2, titer plate shaker.
5) Filter and wash with 8 X 200 L ice-cold binding buffer. Be sure to rinse
the apparatus with > 1
liter of water after last plate.
6) Air dry overnight in hood (prop plate up so that air can flow through).
-81-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
7) Seal the back of the plate
8) Add 40 L Microscint-20 to each well.
9) Seal tops with sealer.
10) Count in Packard Topcount scintillation counter.
11) Upload data to calculation program, and also plot raw counts in Prism,
determining that the
graphs generated, and the IC50 values agree.
The compounds of the invention generally have an IC50 in the 3H-nicotinic acid
competition binding assay within the range of 1 nM to about 25 M.
35S-GTP7S binding assay:
Membranes prepared from Chinese Hamster Ovary (CHO)-Kl cells stably expressing
the
niacin receptor or vector control (7 g/assay) were diluted in assay buffer
(100 mM HEPES, 100 mM
NaCl and 10 mM MgCIZ, pH 7.4) in Wallac Scintistrip plates and pre-incubated
with test compounds
diluted in assay buffer containing 40 M GDP (final [GDP] was 10 M) for - 10
minutes before addition
of 35S-GTPyS to 0.3 nM. To avoid potential compound precipitation, all
compounds were first prepared
in 100% DMSO and then diluted with assay buffer resulting in a final
concentration of 3% DMSO in the
assay. Binding was allowed to proceed for one hour before centrifuging the
plates at 4000 rpm for 15
minutes at room temperature and subsequent counting in a TopCount
scintillation counter. Non-linear
regression analysis of the binding curves was performed in GraphPad Prism.
Membrane Preparation
Materials:
CHO-K1 cell culture medium: F-12 Kaighn's Modified Cell Culture Medium with
10% FBS, 2 mM L-
Glutamine, 1 mM Sodium Pyruvate and 400 g/ml G418
Membrane Scrape Buffer: 20 mM HEPES
10 mM EDTA, pH 7.4
Membrane Wash Buffer: 20 mM HEPES
0.1 mM EDTA, pH 7.4
Protease Inhibitor Cocktail: P-8340, (Sigma, St. Louis, MO)
Procedure:
(Keep everything on ice throughout prep; buffers and plates of cells)
-82-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
= Aspirate cell culture media off the 15 cm2 plates, rinse with 5 mL cold PBS
and aspirate.
= Add 5 ml Membrane Scrape Buffer and scrape cells. Transfer scrape into 50 mL
centrifuge tube.
Add 50uL Protease Inhibitor Cocktail.
= Spin at 20,000 rpm for 17 minutes at 4 C.
= Aspirate off the supernatant and resuspend pellet in 30 mL Membrane Wash
Buffer. Add 50 L
Protease Inhibitor Cocktail.
= Spin at 20,000 rpm for 17 minutes at 4 C.
= Aspirate the supematant off the membrane pellet. The pellet may be frozen at
-80 C for later use
or it can be used immediately.
Assay
Materials:
Guanosine 5'-diphosphate sodium salt (GDP, Sigma-Aldrich Catalog #87127)
Guanosine 5'-[y35S] thiotriphosphate, triethylammonium salt ([35S]GTPyS,
Amersham Biosciences
Catalog #SJ1320, -1000Ci/mmol)
96 well Scintiplates (Perkin-Elmer #1450-501)
Binding Buffer: 20 mM HEPES, pH 7.4
100 mM NaCI
10 mM MgC12
GDP Buffer: binding buffer plus GDP, ranging from 0.4 to 40 M, make fresh
before assay
Procedure:
(total assay volume =100 well)
25g.L GDP buffer with or without compounds (final GDP lO M - so use 40 M
stock)
50 L membrane in binding buffer (0.4mg protein/mL)
25gL [35S]GTPyS in binding buffer. This is made by adding 5 l [35S]GTPyS
stock into lOmL
binding buffer (This buffer has no GDP)
= Thaw compound plates to be screened (daughter plates with 5 L compound @
2mM in 100%
DMSO)
= Dilute the 2 mM compounds 1:50 with 245 L GDP buffer to 40 p,M in 2% DMSO.
(Note: the
concentration of GDP in the GDP buffer depends on the receptor and should be
optimized to
obtain maximal signal to noise; 40 pM).
-83-
CA 02611552 2007-12-07
WO 2007/002557 PCT/US2006/024740
= Thaw frozen membrane pellet on ice. (Note: they are really membranes at this
point, the cells
were broken in the hypotonic buffer without any salt during the membrane prep
step, and most
cellular proteins were washed away)
= Homogenize membranes briefly (few seconds - don't allow the membranes to
warm up, so keep
on ice between bursts of homogenization) until in suspension using a POLYTRON
PT3 100
(probe PT-DA 3007/2 at setting of 7000 rpm). Determine the membrane protein
concentration
by Bradford assay. Dilute membrane to a protein concentrations of 0.40 mg/ml
in Binding
Buffer. (Note: the final assay concentration is 20 g/well).
= Add 25 L compounds in GDP buffer per well to Scintiplate.
= Add 50 L of membranes per well to Scintiplate.
= Pre-incubate for 5-10 minutes at room temperature. (cover plates with foil
since compounds may
be light sensitive)
= Add 25 L of diluted [35S]GTPyS. Incubate on shaker (Lab-Line model #1314,
shake at setting
of 4) for 60 minutes at room temperature. Cover the plates with foil since
some compounds
might be light sensitive.
= Assay is stopped by spinning plates sealed with plate covers at 2500 rpm for
20 minutes at 22 C
= Read on TopCount NXT scintillation counter - 35S protocol.
The compounds of the invention generally have an EC50 in the functional in
vitro GTPyS
binding assay within the range of about less than 1 M to as high as about 100
M.
.~~
Flushing via Laser Doppler
Male C57B16 niice (-25g) are anesthetized using lOmg/ml/kg Nembutal sodium.
When
antagonists are to be administered they are co-injected with the Nembutal
anesthesia. After ten minutes
the animal is placed under the laser and the ear is folded back to expose the
ventral side. The laser is
positioned in the center of the ear and focused to an intensity of 8.4-9.0 V
(with is generally -4.5cm
above the ear). Data acquisition is initiated with a 15 by 15 image format,
auto interval, 60 images and a
20sec time delay with a medium resolution. Test compounds are administered
following the 10th image
via injection into the peritoneal space. Images 1-10 are considered the
animal's baseline and data is
normalized to an average of the baseline mean intensities.
Materials and Methods - Laser Doppler Pirimed PirnII; Niacin (Sigma); Nembutal
(Abbott labs).
All patents, patent applications and publications that are cited herein are
hereby
incorporated by reference in their entirety. While certain preferred
embodiments have been described
herein in detail, numerous alternative embodiments are seen as falling within
the scope of the invention.
-84-