Note: Descriptions are shown in the official language in which they were submitted.
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
METHODS AND RELATED COMPOSITIONS FOR TREATING OR
PRE'VENTING OBESITY, IlVSULIN RESISTANCE DISORDERS, AND
MITOCHONDRIAL-ASSOCIATED DISORDERS
Background
Obesity is a clironic condition that is characterized by a body mass index
(BMI) over 25. Both congenital and environmental factors, such as exercise and
eating habits, contribute to the disease. For instance, the hormone leptin has
been
shown to be involved in fat accumulation and regulating eating behavior.
Several
animal models of obesity result from mutations in the leptin and/or leptin
receptor
gene. In addition to affecting the lifestyle of an individual, obesity can
lead to a
number of complications and diseases, including insulin resistance, Type II
diabetes,
gallbladder disease, hypertension, cardiovascular disease, hyperlipidemia,
sleep
apnea, coronary artery disease, knee osteoarthritis, gout, infertility, breast
cancer,
endometrial cancer, colon cancer and lower back pain.
Diabetes is a disease that shows an acute symptom due to a remarkably high
blood sugar or ketoacidosis, or as well as chronic, general metabolic
abnormalities
arising from a prolonged high blood sugar status or a decrease in glucose
tolerance.
Both congenital and environmental factors, such as exercise and eating habits,
contribute to the disease. The pathogenic causes of diabetes are insulin
productive
disorders, secretion disorders or reductions in activities and sensitivities
of the
secreted insulin. Diabetes is largely grouped into the following two types:
insulin-
dependent diabetes mellitus (also known as Type I diabetes) and non-insulin-
dependent diabetes mellitus (also known as Type II diabetes). The incidence of
Type II diabetes is remarkably increased in obese patients.
Treatments for obesity are generally directed to suppressing the appetite of
the subject. Whereas a number of appetite suppressants are available
(diethylpropion tenuate, mazindol, orlistat, pllendimetrazine, phentermine,
sibutramine), these compounds may not be effective in all subjects or may be
of
limited efficacy. Accordingly, new treatments for obesity are needed.
A number of treatinents for diabetes are well known and include oral
hypoglycemic agents such as sulfonylureas that increase insulin secretion (for
CA 02613141 2007-12-20
WO 2007/008548 atPCT/US2006/026272:)-014
example, tolbutamide, chlorpropamide and glibenclamide), biguanides (for
example,
metformin and buformin) that increase glucose uptake and utilization and a-
glucosidase inhibitors (for example, acarbose and voglibose). In addition,
tliiazolidinediones, such as troglitazone, rosiglitazone and pioglitazone, are
used to
ameliorate insulin-resistance. However, thiazolidinedione intake is usually
associated with a weight gain. Thus, there is a still a need for more
effective
therapies for diabetes.
Currently 8% and 15% of adults in the United States are diabetic or obese,
respectively. With the number of individuals affected with diabetes,
particularly
with type II diabetes, and obesity on the increase, there is a dire need for
medications that prevent and treat these conditions.
Summary
In one aspect, the invention provides methods for treating and/or preventing
metabolic disorders, such as diabetes and obesity, by administering to a
subject a
high dose a sirtuin activator. The sirtuin activator may be administered alone
or in
combination with another lipid-lowering, anti-obesity and/or anti-diabetes
agent.
When administering a sirtuin activator as a combination with another
therapeutic
agent, it may be possible to administer a lower dose of the therapeutic agent
than is
typically required. By using a lower dose of the therapeutic agent, it is
possible to
reduce or eliminate undesirable side effects, such as, hypertension, elevated
heart
rate, etc. that may be associated with such agents. In certain embodiments, co-
administration of a sirtuin activating agent with an anti-diabetic or anti-
obesity drug
may reduce or eliminate side effects because the activity of the sirtuin
activator
counteracts or prevents the side effects associated with the therapeutic
agent.
In other aspects, the invention provides pharmaceutical compositions
comprising a high dose of a sirtuin activator in a single dosage form. Such
pharmaceutical compositions may be formulated for sustained release over at
least
about 6 to 48 hours or more. Also provided are neutraceuticals, such as food
or
beverages, that are supplemented with a sirtuin activator.
In another aspect, the invention provides methods for treating or preventing a
variety diseases or disorders by administering to a subject a high dose of a
sirtuin
2
CA 02613141 2007-12-20
WO 2007/008548 ActoiPCT/US2006/026272 014
activating compound. Exemplary diseases and disorders that may be treated with
a
high dose of a sirtuin activating compound include, for example, diseases or
disorders related to aging or stress, diabetes, obesity, neurodegenerative
diseases,
diseases or disorders associated with mitochondrial dysfunction,
chetnotherapeutic
induced neuropathy, neuropathy associated with an ischemic event, ocular
diseases
and/or disorders, cardiovascular disease, blood clotting disorders,
inflatnmation,
and/or flushing, etc. As described further below, the methods comprise
administering to a subject in need thereof a high dose of a sirtuin activating
compound.
In certain aspects, a liigh dose of a sirtuin activating compound may be
administered alone or in combination with other compounds, including other
sirtuin-
modulating compounds, or other therapeutic agents.
Brief Description of the Drawings
Figure 1 shows examples of plant polyphenol sirtuin 1(SIRTI) activators.
Figure 2 shows examples of stilbene and chalcone SIRT1 activators.
Figure 3 shows examples of flavone SIRT1 activators.
Figure 4 shows exatnples of flavone SIRT1 modulators
Figure 5 shows examples of isoflavone, flavanone and anthocyanidin SIRT1
modulators.
Figure 6 shows examples of catechin (Flavan-3-ol) SIRT1 modulators.
Figure 7 shows examples of free radical protective SIRT1 modulators.
Figure 8 shows examples of SIRT1 modulators.
Figure 9 shows examples of SIRTI modulators.
Figure 10 shows examples of resveratrol analog SIRTI activators.
Figure 11 shows further examples of resveratrol analog SIRT1 activators.
Figure 12 shows further examples of resveratrol analog SIRT1 activators.
Figure 13 shows examples of resveratrol analog SIRT1 modulators.
Figure 14 shows further examples of resveratrol analog SIRTI modulators.
Figures 15A-G shows examples of sirtuin activators.
Figure 16 shows examples of sirtuin inhibitors.
3
CA 02613141 2007-12-20
WO 2007/008548 AttopCT/US2006/026272-014
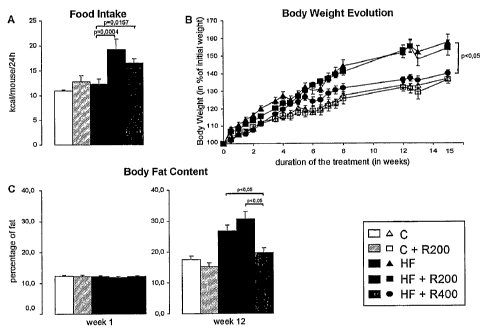
Figures 17 A-C are graphs showing that the Sirt-1 activator resveratrol (400
mg/kg/day), co-administered with a high fat diet, prevents diet-induced
obesity in
male C57BL/6J mice. (A) Food intake of mice expressed as kcal per 24 h. (B)
Body
weight evolution over time. (C) Comparison of body fat content, as analyzed by
dexa scanning, at week 1 and week 12 of treatment. Values are represented as
the
mean f SEM (n=10). Significant differences are irftiicated (p value).
Figures 18 A-C are graphs showing that the Sirt-1 activator resveratrol (400
mg/kg/day) increases energy expenditure in male C57BL/6J mice when co-
administrated with a high fat diet. (A) Average oxygen consumption (V02) in 8
male
mice over a period of 13 h where time 0 is 7:00 pm. The mean area under the
curve
is represented in the adjacent histogram. (B) Respiratory quotient (R.Q.) i.e.
VCO2 /
V02 (n=8). (C) Body temperature as measured at room temperature (n=10). Values
are represented as the mean SEM. Significant differences are indicated (p
value).
Figures 19 A-C are graphs showing that the Sirt-1 activator resveratrol (400
mg/kg/day) significantly diminishes the circadian locomotor activity in male
C57BL/6J mice. Resting heart rate (A) and blood pressure (B) in control and
resveratrol treated mice on a high fat diet. (C) Circadian activity including
total
ambulatory locomotor activity (top graph) and number of rears (bottom graph).
The
adjacent histograms represent the circadian activity measurement as area under
the
curve. Values are represented as the mean SEM (n=8). Significant differences
are
indicated (p value).
Figures 20 A-B are graphs showing that the Sirt-1 activator resveratrol (400
mg/kg/day) increases glucose tolerance in high fat diet fed C57BL/6J mice. (A)
Blood glucose levels during an intraperitoneal glucose tolerance test (2g
glucose/kg)
and (B) during an oral glucose tolerance test (2g glucose/kg). The adjacent
histograms represent the mean area under the curve and body weight of the
experimental groups. Values are represented as the mean SEM (n=5).
Significant
differences are indicated (p value).
Figure 21 shows the results of an intraperitoneal glucose tolerance test in
mice.
Figure 22 shows the Sirtl activator resveratrol (400 mg/kg/day, R400)
coadministered with a high fat diet (HF) in male C57BL/6 mice enhances
adaptive
4
CA 02613141 2007-12-20
WO 2007/008548 Att01PCT/US2006/026272 014
thermogenesis. Curves represent the body temperature of mice as measured
hourly
during a 6 h cold test and are presented as the mean +/- SEM, with p<0.05.
Figure 23 shows the results of an oral glucose tolerance test in Zucker
diabetic fatty rats treated with resveratrol for 42 days.
Figure 24 shows that the Sirt-1 activator resveratrol (400 mg/kg/day), co-
administered with a high fat diet, prevents diet-induced obesity in male
C57BL/6J
mice. The top left panel shows a graph of body weight evolution for mice in
the four
dietary groups over a nine week period. The top right panel shows a graph of
food
intake of mice in the four dietary groups expressed as kcal per 24 h. The
bottom
panels show comparisons of body fat content, as analyzed by dexa scanning, at
week
9 of treatment for mice in the four dietary groups. Values are represented as
the
mean SEM (n=10). BAT is brown adipose tissue (bottom right panel); Inguinal
WAT is inguinal white adipose tissue (bottom left panel); and Retroperitoneal
WAT
is retroperitoneal white adipose tissue (bottom middle panel). Significant
differences are indicated (p value). Animals were maintained on a control diet
(C),
control diet plus 400 mg/kg/day resveratrol (C + R400), high fat diet (HF) or
high
fat diet plus 400 mg/kg/day resveratrol (HF + R400) diets for the indicated
period.
Figure 25 shows the results of serum biochemical analysis of animals
following 16 weeks on control (C), high fat (HF) or high fat plus 400
mg/kg/day
resveratrol (HF + R400) diets (values are average of 10 animals from each
group).
Figure 26 shows hematoxylin and eosin staining of liver and epididymal
adipose tissue sections of animals following 16 weeks on control (C), high fat
(HF)
or high fat plus 400 mg/kg/day resveratrol (HF + R400) diets.
Figure 27 shows hematoxylin and eosin staining of brown adipose tissue and
gastrocnemius muscle sections of animals following 16 weeks on control (C),
high
fat (HF) or high fat plus 400 mg/kg/day resveratrol (HF + R400) diets.
Figure 28 shows succinate dehydrogenase staining of brown adipose tissue
and gastrocnemius and soleus muscle of animals following 16 weeks on high fat
(HF) or high fat plus 400 mg/kg/day resveratrol (HF + R400) diets.
Figure 29 shows transmission electron microscopy of gastrocnemius muscle
(non-oxidative fibers) of animals following 16 weeks on control (C), high fat
(HF)
5
CA 02613141 2007-12-20
WO 2007/008548 Aa'PCT/US2006/026272'014
or high fat supplemented with 400 mg/kg/day resveratrol (HF + R400) diets at
10,000 and 20,000 magnification. Inset shows schematic of muscle fiber
anatomy.
Figure 30 shows transmission electron microscopy of brown adipose tissue
of animals following 16 weeks on control (C), high fat (HF) or high fat plus
400
mg/kg/day resveratrol (HF + R400) diets at 4,000 and 20,000 magnifications.
Figure 31 shows Sirtl mRNA level measured in the brown adipose tissue,
liver and muscle of animals treated with control (C), high fat (HF) or high
fat plus
400 mg/lcg/day resveratrol (HF + R400) diets (values are average of 6 animals
from
each group). Values are expressed relative to the housekeeping gene 18s and
then
expressed relative to chow diet (arbitrarily equal to 1).
Figure 32 shows relative gene expression of PEPCK, glucose-6-phosphatase,
Foxo 1, PGCI-alpha and Sirtl in liver, brown adipose tissue and muscle on
either
control (unshaded), high fat (light shading) or high fat plus 400 mg/kg
resveratrol
(dark shading) diets (n = pool of 6 animals for each condition).
Figure 33 shows the results of an immunoblot indicating that resveratrol
increases PGCI alpha deacetylation. IP is immunoprecipitation; IB is
immunoblot;
HF is high fat diet; and HF + R400 is high fat diet plus 400 mg/kg
resveratrol.
Figure 34 shows an analysis of the fecal lipid content for mice fed diets of
chow (C), high fat (HF), or high fat plus 400 mg/kg resveratrol (HF + R400).
The
left panel shows the total fecal weight per mouse. The right panel shows the
amount
of cholesterol and triglycerides excreted by the animals in the different diet
groups.
Figure 35 are graphs showing that the Sirt-l activator resveratrol (400
mg/kg/day), co-administered with a high fat diet, prevents diet-induced
obesity in
male C57BL/6J mice. Left Paenl: Body weight evolution over time (graphs from
top
to bottom are: HF, HF + R400, C, and C + R400). Right Panel: Area under the
curve
for the graphs shown in the panel to the left.
Figure 36 is a diag'ram illustrating the treadmill endurance protocol for mice
/fed chow (top line) and high fat (bottom line) diets.
Figure 37 is a graph showing the results of the endurance test for mice fed
chow or high fat diets. Each line on the graph shows an individual animal
tested
using the endurance protocol illustrated in Figure 33,
6
CA 02613141 2007-12-20
AI 0-014
WO 2007/008548 PCT/US2006/026272
Figure 38 shows the effect of resveratrol on insulin sensitivity as measured
by hyperinsulinemic (18mU/kg/min) euglycemic (5.5mmol/l) clamp. The left hand
panel shows glucose infusion rates (GIR) for groups of animals following 14
weeks
on either a control diet (C), control diet plus 400 mg/kg resveratrol
(C+R400), high
fat diet (HF) or high fat diet plus 400 mg/kg resvertrol (HF+R400). The right
hand
panel shows average GIR at steady state clamp.
Detailed Description
Defataitions
As used herein, the following terms and phrases shall have the meanings set
forth below. Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as commonly understood to one of ordinary skill in the
art.
The singular forms "a," "an," and "the" include plural reference unless the
context clearly dictates otherwise.
The term "agent" is used herein to denote a chemical compound, a mixture
of chemical compounds, a biological macromolecule (such as a nucleic acid, an
antibody, a protein or portion thereof, e.g., a peptide), or an extract made
from
biological materials such as bacteria, plants, fungi, or animal (particularly
mammalian) cells or tissues. The activity of such agents may render it
suitable as a
"therapeutic agent" which is a biologically, physiologically, or
pharmacologically
active substance (or substances) that acts locally or systemically in a
subject.
A "form that is naturally occurring", when referring to a compound, means a
compound that is in a form, e.g., a composition, in which it can be found
naturally.
For example, since resveratrol can be found in red wine, it is present in red
wine in a
form that is naturally occurring. A compound is not in a form that is
naturally
occurring if, e.g., the compound has been purified and separated from at least
some
of the other molecules that are found with the compound in nature. A
"naturally
occurring compound" refers to a compound that can be found in nature, i.e., a
compound that has not been designed by man. A naturally occurring compound
may have been made by man or by nature.
"Sirtuin modulator" refers to a compound that up regulates (e.g., activate or
stimulate), down regulates (e.g., inhibit or suppress) or otherwise changes a
7
CA 02613141 2007-12-20
A.. ,....m .....0-014
WO 2007/008548 PCT/US2006/026272
functional property or biological activity of a sirtuin protein. Sirtuin
modulators
may act to modulate a sirtuin protein either directly or indirectly. In
certain
embodiments, a sirtuin modulator may be a sirtuin activator or a sirtuin
inhibitor.
The terins "sirtuin activator" or "sirtuin activating compound" refer to a
compound that increases the level of a sirtuin protein and/or increases at
least one
activity of a sirtuin protein. In an exemplary embodiment, a sirtuin activator
may
increase at least one biological activity of a sirtuin protein by at least
about 10%,
25%, 50%, 75%, 100%, or more. Exemplary biological activities of sirtuin
proteins
include deacetylation, e.g., of histones and p53; extending lifespan;
increasing
genomic stability; silencing transcription; and controlling the segregation of
oxidized proteins between mother and daughter cells. Exemplary sirtuin
activating
compounds include, for example, compounds having a formula selected from the
group of formulas 1-25, 30, 32-65, and 69-88.
A "high dose of a sirtuin activating compound" refers to a quantity of a
sirtuin activator having a sirtuin activating effect equal to or greater than
the sirtuin
activating effect of 18 mg/kg resveratrol (e.g., in humans). In certain
embodiments,
a high dose of a sirtuin activating compound refers to a quantity of a sirtuin
activator having a sirtuin activating effect equal to or greater than the
sirtuin
activating effect of 18 mg/kg of resveratrol which is administered (i) orally,
(ii)
released from a sustained release form over 6 to 48 hours, and/or (iii) for an
equivalent amount of time. In certain embodiments, a high dose of a sirtuin
activating compound refers to a quantity of a sirtuin activator having a
sirtuin
activating effet equal to or greater than the sirtuin activating effect of at
least about
20, 25, 30, 35, 40, 50, 60, 75, 100, 150 mg/kg, or more, or resveratrol.
"Sirtuin activating effect" refers to the level or extent of one or more
therapeutic effects obtained upon administration of a high dose of a sirtuin
activating compound. Therapeutic effects include, for example, (i) preventing
or
inhibiting weight gain upon consuming a diet having an increased fat and/or
calorie
content without an increase in activity, heart rate, and/or blood pressure;
and/or (ii)
improved blood glucose levels. Such therapeutic effects include, for example,
the
therapeutic effects illustrated in the Examples.
8
CA 02613141 2007-12-20
A.. ~ . . 1O-014
WO 2007/008548 PCT/US2006/026272
"Sirtuin inhibitor" refers to a compound that decreases the level of a sirtuin
protein and/or decreases at least one activity of a sirtuin protein. In an
exemplary
embodiment, a sirtuin inhibitor may decrease at least one biological activity
of a
sirtuin protein by at least about 10%, 25%, 50%, 75%, 100%, or more. Exemplary
biological activities of sirtuin proteins include deacetylation, e.g., of
histones and
p53; extending lifespan; increasing genomic stability; silencing
transcription; and
controlling the segregation of oxidized proteins between mother and daughter
cells.
"Sirtuin protein" refers to a member of the sirtuin deacetylase protein family
or preferably to the Sir2 family, which include yeast Sir2 (GenBank Accession
No.
P53685), C. elegans Sir-2.1 (GenBank Accession No. NP_501912), and human
SIRT1 (GenBank Accession No. NM 012238 and NP036370 (or AF083106)) and
SIRT2 (GenBank Accession No. NM 030593 and AF083107) proteins. Other
family members include the four additional yeast Sir2-like genes termed "HST
genes" (homologues of Sir two) HSTl, HST2, HST3 and HST4, and the five other
human homologues hSIRT3, hSIRT4, hSIRT5, hSIRT6 and hSIRT7 (Brachmann et
al. (1995) Genes Dev. 9:2888 and Frye et al. (1999) BBRC 260:273). Preferred
sirtuins are those that share more similarities with SIRT1, i.e., hSIRTI,
and/or Sir2
than with SIRT2, such as those members having at least part of the N-terminal
sequence present in SIRTl and absent in SIRT2 such as SIRT3 has.
"SIRTl protein" refers to a member of the sir2 family of sirtuin
deacetylases. In one embodiment, a SIRTI protein includes yeast Sir2 (GenBank
Accession No. P53685), C. elegans Sir-2.1 (GenBank Accession No. NP_501912),
human SIRTI (GenBank Accession No. NM 012238 and NP 036370 (or
AF083106)), human SIRT2 (GenBank Accession No. NM 012237, NM 030593,
NP036369, NP 085096, and AF083107) proteins, and equivalents and fragments
thereof. In another embodiment, a SIRTI protein includes a polypeptide
comprising a sequence consisting of, or consisting essentially of, the amino
acid
sequence set forth in GenBank Accession Nos. NP_036370, NP 501912,
NP_085096, NP 036369, and P53685. SIRT1 proteins include polypeptides
comprising all or a portion of the amino acid sequence set forth in GenBank
Accession Nos. NP 036370, NP_501912, NP 085096, NP 036369, and P53685;
the amino acid sequence set forth in GenBank Accession Nos. NP_036370,
9
CA 02613141 2007-12-20
WO 2007/008548 Attorne 4
PCT/US2006/026272
NP 501912, NP_085096, NP 036369, and P53685 with 1 to about 2, 3, 5, 7, 10,
15, 20, 30, 50, 75 or more conservative amino acid substitutions; an amino
acid
sequence that is at least 60%, 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99%
identical to GenBank Accession Nos. NP_036370, NP 501912, NP_085096,
NP_036369, and P53685 and functional fragments thereof. Polypeptides of the
invention also include homologs (e.g., orthologs and paralogs), variants, or
fragments, of GenBank Accession Nos. NP_036370, NP 501912, NP 085096,
NP 036369, and P53685.
"Biologically active portion of a sirtuin" refers to a portion of a sirtuin
protein having a biological activity, such as the ability to deacetylate.
Biologically
active portions of sirtuins may comprise the core domain of sirtuins. For
example,
amino acids 62-293 of the SIRTI protein sequence, which are encoded by
nucleotides 237 to 932 of the SIRT1 nucleic acid sequence, encompass the NAD+
binding domain and the substrate binding domain. Therefore, this region is
sometimes referred to as the core domain. Other biologically active portions
of
SIRT1, also sometimes referred to as core domains, include about amino acids
261
to 447 of the SIRT1 protein sequence, which are encoded by nucleotides 834 to
1394 of the SIRT1 nucleic acid sequence; about amino acids 242 to 493 of the
SIRT1 protein sequence, which are encoded by nucleotides 777 to 1532 of the
SIRTI nucleic acid sequence; or about amino acids 254 to 495 of the SIRT1
protein
sequence, which are encoded by nucleotides 813 to 1538 of the SIRT1 nucleic
acid
sequence.
A "direct activator" of a sirtuin is a molecule that activates a sirtuin by
binding to it. A "direct inhibitor" of a sirtuin is a molecule that inhibits a
sirtuin by
binding to it.
The terms "comprise" and "comprising" are used in the inclusive, open
sense, meaning that additional elements may be included.
The term "including" is used to mean "including but not limited to".
"Including" and "including but not limited to" are used interchangeably.
The term "percent identical" refers to sequence identity between two amino
acid sequences or between two nucleotide sequences. Identity can each be
determined by comparing a position in each sequence which may be aligned for
CA 02613141 2007-12-20
WO 2007/008548 Ait PCT/US2006/026272'014
purposes of comparison. When an equivalent position in the compared sequences
is
occupied by the same base or amino acid, then the molecules are identical at
that
position; when the equivalent site occupied by the same or a similar amino
acid
residue (e.g., similar in steric and/or electronic nature), then the molecules
can be
referred to as homologous (similar) at that position. Expression as a
percentage of
homology, similarity, or identity refers to a function of the number of
identical or
similar amino acids at positions shared by the compared sequences. Expression
as a
percentage of homology, similarity, or identity refers to a function of the
number of
identical or similar amino acids at positions shared by the compared
sequences.
Various alignment algorithms and/or programs may be used, including FASTA,
BLAST, or ENTREZ. FASTA and BLAST are available as a part of the GCG
sequence analysis package (University of Wisconsin, Madison, Wis.), and can be
used with, e.g., default settings. ENTREZ is available through the National
Center
for Biotechnology Information, National Library of Medicine, National
Institutes of
Health, Bethesda, MD. In one embodiment, the percent identity of two sequences
can be determined by the GCG program with a gap weight of 1, e.g., each amino
acid gap is weighted as if it were a single amino acid or nucleotide mismatch
between the two sequences.
Other techniques for alignment are described in Methods in Enzymology,
vol. 266: Computer Methods for Macromolecular Sequence Analysis (1996), ed.
Doolittle, Academic Press, Inc., a division of Harcourt Brace & Co., San
Diego,
California, USA. Preferably, an alignment program that permits gaps in the
sequence is utilized to align the sequences. The Smith-Waterman is one type of
algorithm that permits gaps in sequence alignments. See Meth. Mol. Biol. 70:
173-
187 (1997). Also, the GAP program using the Needleman and Wunsch alignment
method can be utilized to align sequences. An alternative search strategy uses
MPSRCH software, which runs on a MASPAR computer. MPSRCH uses a Smith-
Waterman algorithm to score sequences on a massively parallel computer. This
approach improves ability to pick up distantly related matches, and is
especially
tolerant of small gaps and nucleotide sequence errors. Nucleic acid-encoded
amino
acid sequences can be used to search both protein and DNA databases.
11
CA 02613141 2007-12-20
WO 2007/008548 AttompCT/US2006/026272 ~
The terms "polynucleotide" and "nucleic acid" are used interchangeably.
They refer to a polymeric form of nucleotides of any length, either
deoxyribonucleotides or ribonucleotides, or analogs thereof. Polynucleotides
may
have any three-dimensional structure, and may perform any function, known or
unknown. The following are non-limiting examples of polynucleotides: coding or
non-coding regions of a gene or gene fragment, loci (locus) defined from
linkage
analysis, exons, introns, messenger RNA (mRNA), transfer RNA, ribosomal RNA,
ribozymes, cDNA, recombinant polynucleotides, branched polynucleotides,
plasmids, vectors, isolated DNA of any sequence, isolated RNA of any sequence,
nucleic acid probes, and primers. A polynucleotide may comprise modified
nucleotides, such as methylated nucleotides and nucleotide analogs. If
present,
modifications to the nucleotide structure may be imparted before or after
assembly
of the polymer. The sequence of nucleotides may be interrupted by non-
nucleotide
components. A polynucleotide may be further modified, such as by conjugation
with a labeling component. The term "recombinant" polynucleotide means a
polynucleotide of genomic, cDNA, semisynthetic, or synthetic origin which
either
does not occur in nature or is linked to another polynucleotide in a
nonnatural
arrangement.
A "patient", "subject" or "host" refers to either a human or a non-human
animal. Non-human animals include farm animals (e.g., cows, horses, pigs,
sheep,
goats) and companion animals (e.g., dogs, cats).
The term "substantially homologous" when used in connection with amino
acid sequences, refers to sequences which are substantially identical to or
similar in
sequence with each other, giving rise to a homology of conformation and thus
to
retention, to a useful degree, of one or more biological (including
immunological)
activities. The term is not intended to imply a common evolution of the
sequences.
The term "modulation" is art-recognized and refers to up regulation (i.e.,
activation or stimulation), down regulation (i.e., inhibition or suppression)
of a
response, or the two in combination or apart.
The term "prophylactic" or "therapeutic" treatment is art-recognized and
refers to administration of a drug to a host. If it is administered prior to
clinical
manifestation of the unwanted condition (e.g., disease or other unwanted state
of the
12
CA 02613141 2007-12-20
WO 2007/008548 AttoPCT/US2006/026272'ot4
host animal) then the treatment is prophylactic, i.e., it protects the host
against
developing the unwanted condition, whereas if administered after manifestation
of
the unwanted condition, the treatment is therapeutic (i.e., it is intended to
diminish,
ameliorate or maintain the existing unwanted condition or side effects
therefrom).
The term "mammal" is known in the art, and exemplary mammals include
humans, primates, bovines, porcines, canines, felines, and rodents (e.g., mice
and
rats).
The term "bioavailable" when referring to a compound is art-recognized and
refers to a form of a compound that allows for it, or a portion of the amount
of
compound administered, to be absorbed by, incorporated to, or otherwise
physiologically available to a subject or patient to whom it is administered.
The term "pharmaceutical" refers to any compound having a
pharmacological effect. For example, the term pharmaceutical encompasses
natural
compounds as well as nonnatural compounds that have a pharmacological effect.
The term "pharmaceutically-acceptable salts" is art-recognized and refers to
the relatively non-toxic, inorganic and organic acid addition salts of
compounds, as
well as solvates, co-crystals, polymorphs and the like of the salts,
including, for
example, those contained in compositions described herein.
The term "pharmaceutically acceptable carrier" is art-recognized and refers
to a pharmaceutically-acceptable material, composition or vehicle, such as a
liquid
or solid filler, diluent, excipient, solvent or encapsulating material. Each
carrier
must be "acceptable" in the sense of being compatible with the subject
composition
and its components and not injurious to the patient. Some examples of
materials
which may serve as pharmaceutically acceptable carriers include: (1) sugars,
such as
lactose, glucose and sucrose; (2) starches, such as corn starch and potato
starch; (3)
cellulose, and its derivatives, such as sodium carboxymethyl cellulose, ethyl
cellulose and cellulose acetate; (4) powdered tragacanth; (5) malt; (6)
gelatin; (7)
talc; (8) excipients, such as cocoa butter and suppository waxes; (9) oils,
such as
peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and
soybean
oil; (10) glycols, such as propylene glycol; (11) polyols, such as glycerin,
sorbitol,
mannitol and polyethylene glycol; (12) esters, such as ethyl oleate and ethyl
laurate;
(13) agar; (14) buffering agents, such as magnesium hydroxide and aluminum
13
CA 02613141 2007-12-20 Attor - ~__,,,, *r., . etn r_n1xrn 014
WO 2007/008548 PCT/US2006/026272
hydroxide; (15) alginic acid; (16) pyrogen-free water; (17) isotonic saline;
(18)
Ringer's solution; (19) ethyl alcohol; (20) phosphate buffer solutions; and
(21) other
non-toxic compatible substances employed in pharmaceutical formulations.
The terms "systemic administration," "administered systemically,"
"peripheral administration" and "administered periplierally" are art-
recognized and
refer to the administration of a subject composition, therapeutic or other
material
other than directly into the central nervous system, such that it enters the
patient's
system and, thus, is subject to metabolism and other like processes.
The terms "parenteral administration" and "administered parenterally" are
art-recognized and refer to modes of administration other than enteral and
topical
administration, usually by injection, and includes, without limitation,
intravenous,
intramuscular, intraarterial, intrathecal, intracapsular, intraorbital,
intracardiac,
intradermal, intraperitoneal, transtracheal, subcutaneous, subcuticular, intra-
articulare, subcapsular, subarachnoid, intraspinal, and intrasternal injection
and
infusion.
"Transcriptional regulatory sequence" is a generic term used throughout the
specification to refer to DNA sequences, such as initiation signals,
enhancers, and
promoters, which induce or control transcription of protein coding sequences
with
which they are operable linked. In preferred embodiments, transcription of one
of
the recombinant genes is under the control of a promoter sequence (or other
transcriptional regulatory sequence) which controls the expression of the
recombinant gene in a cell-type which expression is intended. It will also be
understood that the recombinant gene can be under the control of
transcriptional
regulatory sequences which are the same or which are different from those
sequences which control transcription of the naturally-occurring forms of
genes as
described herein.
A "vector" is a self-replicating nucleic acid molecule that transfers an
inserted nucleic acid molecule into and/or between host cells. The term
includes
vectors that function primarily for insertion of a nucleic acid molecule into
a cell,
replication of vectors that function primarily for the replication of nucleic
acid, and
expression vectors that function for transcription and/or translation of the
DNA or
RNA. Also included are vectors that provide more than one of the above
functions.
14
CA 02613141 2007-12-20
WO 2007/008548 AttornePCT/US2006/026272
As used herein, "expression vectors" are defined as polynucleotides which,
when
introduced into an appropriate host cell, can be transcribed and translated
into a
polypeptide(s). An "expression system" usually connotes a suitable host cell
comprised of an expression vector that can function to yield a desired
expression
product.
An "indicator of mitochondrial function" is any parameter that is indicative
of mitochondrial function that can be measured by one skilled in the art. In
certain
embodiments, the indicator of mitochondrial function is a mitochondrial
electron
transport chain enzyme, a Krebs cycle enzyme, a mitochondrial matrix
component, a
mitochondrial membrane component or an ATP biosynthesis factor. In other
embodiments, the indicator of mitochondrial function is mitochondrial number
per
cell or mitochondrial mass -per cell. In other embodiments, the indicator of
mitochondrial function is an ATP biosynthesis factor. In other embodiments,
the
indicator of mitochondrial function is the amount of ATP per mitochondrion,
the
amount of ATP per unit mitochondrial mass, the amount of ATP per unit protein
or
the amount of ATP per unit mitochondrial protein. In other embodiments, the
indicator of mitochondrial function comprises free radical production. In
other
embodiments, the indicator of mitochondrial function comprises a cellular
response
to elevated intracellular calcium. In other embodiments, the indicator of
mitochondrial function is the activity of a mitochondrial enzyme such as, by
way of
non-limiting example, citrate synthase, hexokinase II, cytochrome c oxidase,
phosphofructokinase, glyceraldehyde phosphate dehydrogenase, glycogen
phosphorylase, creatine kinase, NADH dellydrogenase, glycerol 3-phosphate
dehydrogenase, triose phosphate dehydrogenase or malate dehydrogenase. In
other
embodiments, the indicator of mitochondrial function is the realtive or
absolute
amount of mitochondrial DNA per cell in the patient.
"Improving mitochondrial function" or "altering mitochondrial function"
may refer to (a) substantially (e.g., in a statistically significant manner,
and
preferably in a manner that promotes a statistically significant improvement
of a
clinical parameter such as prognosis, clinical score or outcome) restoring to
a normal
level at least one indicator of glucose responsiveness in cells having reduced
glucose
responsiveness and reduced mitochondrial mass and/or impaired mitochondrial
CA 02613141 2007-12-20
Ar. "." " "...0 014
WO 2007/008548 PCT/US2006/026272
function; or (b) substantially (e.g., in a statistically significant manner,
and
preferably in a manner that promotes a statistically significant improvement
of a
clinical parameter such as prognosis, clinical score or outcome) restoring to
a normal
level, or increasing to a level above and beyond normal levels, at least one
indicator
of mitochondrial function in cells having impaired mitochondrial function, or
in
cells having normal mitochondrial function, respectively. Improved or altered
mitochondrial function may result from changes in extramitochondrial
structures or
events, as well as from mitochondrial structures or events, in direct
interactions
between mitochondrial and extramitochondrial genes and/or their gene products,
or
in structural or functional changes that occur as the result of interactions
between
intermediates that may be formed as the result of such interactions, including
metabolites, catabolites, substrates, precursors, cofactors and the like.
"Impaired mitochondrial function" may include a full or partial decrease,
inhibition, diminution, loss or other impairment in the level and/or rate of
any
respiratory, metabolic or other biochemical or biophysical activity in some or
all
cells of a biological source. As non-limiting examples, markedly impaired
electron
t'ransport chain (ETC) activity may be related to impaired mitochondrial
function, as
may be generation of increased reactive oxygen species (ROS) or defective
oxidative phosphorylation. As further examples, altered mitochondrial membrane
potential, induction of apoptotic pathways and formation of atypical chemical
and
biochemical crosslinked species within a cell, whether by enzymatic or non-
enzymatic mechanisms, may all be regarded as indicative of mitochondrial
function.
These and other non-limiting examples of impaired mitochondrial function are
described in greater detail below.
"Treating" a condition or disease refers to curing as well as ameliorating at
least one symptom of the condition or disease.
The term "cis" is art-recognized and refers to the arrangement of two atoms
or groups around a double bond such that the atoms or groups are on the same
side
of the double bond. Cis configurations are often labeled as (Z)
configurations.
The term "trans" is art-recognized and refers to the arrangement of two
atoms or groups around a double bond such that the atoms or groups are on the
16
CA 02613141 2007-12-20
WO 2007/008548 AttonPCT/US2006/026272~14
opposite sides of a double bond. Trans configurations are often labeled as (E)
configurations.
The term "covalent bond" is art-recognized and refers to a bond between two
atoms where electrons are attracted electrostatically to both nuclei of the
two atoms,
and the net effect of increased electron density between the nuclei
counterbalances
the internuclear repulsion. The term covalent bond includes coordinate bonds
when
the bond is with a metal ion.
The term "therapeutic agent" is art-recognized and refers to any compound
that is a biologically, physiologically, or pharmacologically active substance
that
acts locally or systemically in a subject. The term also means any substance
intended for use in the diagnosis, cure, mitigation, treatment or prevention
of disease
or in the enhancement of desirable physical or mental development and/or
conditions in an animal or human.
The term "therapeutic effect" is art-recognized and refers to a local or
systemic effect in animals, particularly mammals, and more particularly humans
caused by a pharmacologically active substance. The phrase "therapeutically-
effective amount" means that amount of such a substance that produces some
desired local or systemic effect at a reasonable benefit/risk ratio applicable
to any
treatment. The therapeutically effective amount of such substance will vary
depending upon the subject and disease condition being treated, the weight and
age
of the subject, the severity of the disease condition, the manner of
administration and
the like, which can readily be determined by one of ordinary skill in the art.
For
example, certain compositions described herein may be administered in a
sufficient
amount to produce a desired effect on metabolic disorders or diabetes or
complications thereof, at a reasonable benefit/risk ratio applicable to such
treatment.
The term "synthetic" is art-recognized and refers to production by in vitro
chemical or enzymatic synthesis.
The term "meso compound" is art-recognized and refers to a chemical
compound which has at least two chiral centers but is achiral due to a plane
or point
of symmetry.
The term "chiral" is art-recognized and refers to molecules which have the
property of non-superimposability of the mirror image partner, while the term
17
CA 02613141 2007-12-20
At 0-014
WO 2007/008548 PCT/US2006/026272
"achiral" refers to molecules which are superimposable on their mirror image
partner. A "prochiral molecule" is a molecule which has the potential to be
converted to a chiral molecule in a particular process.
The term "stereoisomers" is art-recognized and refers to compounds which
have identical chemical constitution, but differ with regard to the
arrangement of the
atoms or groups in space. In particular, "enantiomers" refer to two
stereoisomers of
a compound which are non-superimposable mirror images of one another.
"Diastereomers", on the other hand, refers to stereoisomers with two or more
centers
of dissymmetry and whose molecules are not mirror images of one another.
Furthermore, a "stereoselective process" is one which produces a particular
stereoisomer of a reaction product in preference to other possible
stereoisomers of
that product. An "enantioselective process" is one which favors production of
one
of the two possible enantiomers of a reaction product.
The term "regioisomers" is art-recognized and refers to compounds which
have the same molecular formula but differ in the connectivity of the atoms.
Accordingly, a "regioselective process" is one which favors the production of
a
particular regioisomer over others, e.g., the reaction produces a
statistically
significant increase in the yield of a certain regioisomer.
The term "epimers" is art-recognized and refers to molecules with identical
chemical constitution and containing more than one stereocenter, but which
differ in
configuration at only one of these stereocenters.
The term "ED50" is art-recognized. In certain embodiments, ED50 means the
dose of a drug which produces 50% of its maximum response or effect, or
alternatively, the dose which produces a pre-determined response in 50% of
test
subjects or preparations. The term "LD50" is art-recognized. In certain
embodiments,
LD50 means the dose of a drug which is lethal in 50% of test subjects. The
term
"tlierapeutic index" is an art-recognized term which refers to the therapeutic
index of
a drug, defined as LD50/ED50.
The term "structure-activity relationship" or "SAR" is art-recognized and
refers to the way in which altering the molecular structure of a drug or other
compound alters its biological activity, e.g., its interaction with a
receptor, enzyme,
nucleic acid or other target and the like.
18
CA 02613141 2007-12-20
Att' - - - - 7-014
WO 2007/008548 PCT/US2006/026272
The term "aliphatic" is art-recognized and refers to a linear, branched,
cyclic
alkane, alkene, or alkyne. In certain embodiments, aliphatic groups in the
present
compounds are linear or branched and have from I to about 20 carbon atoms.
The term "alkyl" is art-recognized, and includes saturated aliphatic groups,
including straight-chain alkyl groups, branched-chain allcyl groups,
cycloallcyl
(alicyclic) groups, allcyl substituted cycloalkyl groups, and cycloalkyl
substituted
alkyl groups. In certain embodiments, a straight chain or branched chain alkyl
has
about 30 or fewer carbon atoms in its backbone (e.g., CI-C30 for straight
chain, C3-
C30 for branched chain), and alternatively, about 20 or fewer. Likewise,
cycloalkyls
have from about 3 to about 10 carbon atoms in their ring structure, and
alternatively
about 5, 6 or 7 carbons in the ring structure.
The term "aralkyl" is art-recognized and refers to an alkyl group substituted
with an aryl group (e.g., an aromatic or heteroaromatic group).
The terms "alkenyl" and "alkynyl" are art-recognized and refer to
unsaturated aliphatic groups analogous in length and possible substitution to
the
alkyls described above, but that contain at least one double or triple bond
respectively.
Unless the number of carbons is otherwise specified, "lower alkyl" refers to
an alkyl group, as defined above, but having from one to about ten carbons,
alternatively from one to about six carbon atoms in its backbone structure.
Likewise, "lower alkenyl" and "lower alkynyl" have similar chain lengths.
The term "heteroatom" is art-recognized and refers to an atom of any
element other than carbon'or hydrogen. Illustrative heteroatoms include boron,
nitrogen, oxygen, phosphorus, sulfur and selenium.
The term "aryl" is art-recognized and refers to 5-, 6- and 7-membered single-
ring aromatic groups that may include from zero to four heteroatoms, for
example,
benzene, naphtalene, anthracene, pyrene, pyrrole, furan, thiophene, imidazole,
oxazole, thiazole, triazole, pyrazole, pyridine, pyrazine, pyridazine and
pyrimidine,
and the like. Those aryl groups having heteroatoms in the ring structure may
also be
referred to as "aryl heterocycles" or "heteroaromatics." The aromatic ring may
be
substituted at one or more ring positions with such substituents as described
above,
for example, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl,
hydroxyl,
19
CA 02613141 2007-12-20
Attc -014
WO 2007/008548 PCT/US2006/026272
alkoxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate,
carbonyl,
carboxyl, silyl, ether, alkylthio, sulfonyl, sulfonamido, ketone, aldehyde,
ester,
heterocyclyl, aromatic or heteroaromatic moieties, -CF3, -CN, or the like. The
term
"aryl" also includes polycyclic ring systems having two or more cyclic rings
in
which two or more carbons are common to two adjoining rings (the rings are
"fused
rings") wherein at least one of the rings is aromatic, e.g., the other cyclic
rings may
be cycloalkyls, cycloalkenyls, cycloalkynyls, aryls and/or heterocyclyls. In
compounds 77-88, "aryl" is intended to refer to both carbocyclic and
heterocyclic
aromatic groups.
The terms ortlzo, rraeta and para are art-recognized and refer to 1,2-, 1,3-
and
1,4-disubstituted benzenes, respectively. For example, the names 1,2-
dimethylbenzene and ortho-dilnethylbenzene are synonymous.
The terms "heterocyclyl" or "heterocyclic group" are art-recognized and
refer to 3- to about 10-membered ring structures, alternatively 3- to about 7-
membered rings, whose ring structures include one to four heteroatoms.
Heterocycles may also be polycycles. Heterocyclyl groups include, for example,
thiophene, thianthrene, furan, pyran, isobenzofuran, chromene, xanthene,
phenoxanthene, pyrrole, imidazole, pyrazole, isothiazole, isoxazole, pyridine,
pyrazine, pyrimidine, pyridazine, indolizine, isoindole, indole, indazole,
purine,
quinolizine, isoquinoline, quinoline, phthalazine, naphthyridine, quinoxaline,
quinazoline, cinnoline, pteridine, carbazole, carboline, phenanthridine,
acridine,
pyrimidine, phenanthroline, phenazine, phenarsazine, phenothiazine, furazan,
phenoxazine, pyrrolidine, oxolane, thiolane, oxazole, piperidine, piperazine,
morpholine, lactones, lacta.ms such as azetidinones and pyrrolidinones,
sultams,
sultones, and the like. The heterocyclic ring may be substituted at one or
more
positions with such substituents as described above, as for example, halogen,
alkyl,
aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulthydryl,
imino,
amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio,
sulfonyl, ketone, aldehyde, ester, a heterocyclyl, an aromatic or
heteroaromatic
moiety, -CF3, -CN, or the like.
The terms "polycyclyl" or "polycyclic group" are art-recognized and refer to
two or more rings (e.g., cycloalkyls, cycloalkenyls, cycloalkynyls, aryls
and/or
CA 02613141 2007-12-20
WO 2007/008548 AttornPCT/US2006/02627214
lleterocyclyls) in which two or more carbons are common to two adjoining
rings,
e.g., the rings are "fused rings". Rings that are joined through non-adjacent
atoms
are termed "bridged" rings. Each of the rings of the polycycle may be
substituted
with such substituents as described above, as for example, halogen, alkyl,
aralkyl,
alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sultlhydryl, imino,
amido,
phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio,
sulfonyl,
ketone, aldehyde, ester, a heterocyclyl, an aromatic or heteroaromatic moiety,
-CF3,
-CN, or the like.
The term "carbocycle" is art-recognized and refers to an aromatic or non-
aromatic ring in which each atom of the ring is carbon.
The terin "nitro" is art-recognized and refers to -NO2; the term "halogen" is
art-recognized and refers to -F, -Cl, -Br or -I; the term "sulfllydryl" is art-
recognized
and refers to -SH; the term "hydroxyl" means -OH; and the term "sulfonyl" is
art-
recognized and refers to -S02 ."Halide" designates the corresponding anion of
the
halogens, and "pseudohalide" has the definition set forth on 560 of "Advanced
Inorganic Chemistry" by Cotton and Wilkinson.
The terms "amine" and "amino" are art-recognized and refer to both
unsubstituted and substituted amines, e.g., a moiety that may be represented
by the
general formulas:
R50
SR50 I
+
N N R53
R51 R52
wherein R50, R51 and R52 each independently represent a hydrogen, an alkyl, an
alkenyl, -(CH2).-R61, or R5 0 and R5 1, taken together with the N atom to
which
they are attached complete a heterocycle having from 4 to 8 atoms in the ring
structure; R61 represents an aryl, a cycloalkyl, a cycloalkenyl, a heterocycle
or a
polycycle; and m is zero or an integer in the range of 1 to 8. In certain
embodiments, only one of R50 or R51 may be a carbonyl, e.g., R50, R51 and the
nitrogen together do not form an imide. In other embodiments, R50 and R51 (and
optionally R52) each independently represent a hydrogen, an alkyl, an alkenyl,
or -
(CH2)R,-R61. Thus, the term "alkylamine" includes an amine group, as defined
21
CA 02613141 2007-12-20
WO 2007/008548 Att PCT/US2006/026272D-01~
above, having a substituted or unsubstituted alkyl attached thereto, i.e., at
least one
of R50 and R51 is an alkyl group.
The term "acylamino" is art-recognized and refers to a moiety that may be
represented by the general formula:
O
N---'~-R54
I
R50
wherein R50 is as defined above, and R54 represents a hydrogen, an alkyl, an
alkenyl or -(CH2),,,-R61, where m and R61 are as defined above.
The term "amido" is art recognized as an amino-substituted carbonyl and
includes a moiety that may be represented by the general formula:
O
R51
N
(
R50
wherein R50 and R51 are as defined above. Certain embodiments of amides may
not include imides which may be unstable.
The term "alkylthio" refers to an alkyl group, as defined above, having a
sulfur radical attached thereto. In certain embodiments, the "alkylthio"
moiety is
represented by one of -S-alkyl, -S-alkenyl, -S-alkynyl, and -S-(CH2)m-R61,
wherein
m and R61 are defined above. Representative alkylthio groups include
methylthio,
ethyl thio, and the like.
The term "carbonyl" is art recognized and includes such moieties as may be
represented by the general formulas:
O O
R55
",KX50 X50 R56
wherein X50 is a bond or represents an oxygen or a sulfur, and R55 and R56
represents a hydrogen, an alkyl, an alkenyl, -(CH2)õ-R61or a pharmaceutically
acceptable salt, R56 represents a hydrogen, an alkyl, an alkenyl or -(CHz)m
R61,
where m and R61 are defined above. Where X50 is an oxygen and R55 or R56 is
22
CA 02613141 2007-12-20
WO 2007/008548 Att~PCT/US2006/026272)-014
not hydrogen, the formula represents an "ester". Where X50 is an oxygen, and
R55
is as defined above, the moiety is referred to herein as a carboxyl group, and
particularly when R55 is a hydrogen, the formula represents a"carboxylic
acid".
Where X50 is an oxygen, and R56 is hydrogen, the formula represents
a"formate".
In general, where the oxygen atom of the above formula is replaced by sulfur,
the
formula represents a "thiolcarbonyl" group. Where X50 is a sulfur and R55 or
R56
is not hydrogen, the formula represents a "thiolester." Where X50 is a sulfur
and
R55 is hydrogen, the formula represents a "thiolcarboxylic acid." Where X50 is
a
sulfur and R56 is hydrogen, the formula represents a "thiolformate." On the
other
hand, where X50 is a bond, and R55 is not hydrogen, the above formula
represents a
"ketone" group. Where X50 is a bond, and R55 is hydrogen, the above formula
represents an "aldeliyde" group.
The terms "alkoxyl" or "alkoxy" are art-recognized and refer to an alkyl
group, as defined above, having an oxygen radical attached thereto.
Representative
alkoxyl groups include methoxy, ethoxy, propyloxy, tert-butoxy and the like.
An
"ether" is two hydrocarbons covalently linked by an oxygen. Accordingly, the
substituent of an alkyl that renders that alkyl an ether is or resembles an
alkoxyl,
such as may be represented by one of -O-alkyl, -0-alkenyl, -0-alkynyl, -0--
(CH2)m-
R61, where m and R61 are described above.
The term "sulfonate" is art recognized and refers to a moiety that may be
represented by the general formula:
0
(I
S OR57
I I
0
in which R57 is an electron pair, hydrogen, alkyl, cycloalkyl, or aryl.
The term "sulfate" is art recognized and includes a moiety that may be
represented by the general formula:
0
11
O S OR57
I I
O
in which R57 is as defined above.
23
CA 02613141 2007-12-20
WO 2007/008548 AtPCT/US2006/0262720-014
The term "sulfonamido" is art recognized and includes a moiety that may be
represented by the general formula:
0
11
N S OR56
I II
R50 O
in which R50 and R56 are as defined above.
The term "sulfamoyl" is art-recognized and refers to a moiety that may be
represented by the general formula:
O
II /R50
. ~~ N\
R51
O
in which R50 and R51 are as defined above.
The term "sulfonyl" is art-recognized and refers to a moiety that may be
represented by the general formula:
0
11
S R58
I I
O
in which R58 is one of the following: hydrogen, alkyl, alkenyl, alkynyl,
cycloalkyl,
heterocyclyl, aryl or heteroaryl.
The term "sulfoxido" is art-recognized and refers to a moiety that may be
represented by the general formula:
O
R58
in which R58 is defined above.
The term "phosphoryl" is art-recognized and may in general be represented
by the formula:
24
CA 02613141 2007-12-20
WO 2007/008548 Att'PCT/US2006/026272'-014
Q50
11
P
I
OR59
wherein Q50 represents S or 0, and R59 represents hydrogen, a lower alkyl or
an
aryl. When used to substitute, e.g., an alkyl, the phosphoryl group of the
phosphorylalkyl may be represented by the general formulas:
Q50 Q50
-Q51-II O -Q51-II-OR59
I
OR59 OR59
wherein Q50 and R59, each independently, are defined above, and Q51 represents
0, S or N. When Q50 is S, the phosphoryl moiety is a "phosphorothioate".
The term "phosphoramidite" is art-recognized and may be represented in the
general formulas:
0 0
-Q51-I O -Q51-II-OR59
I
fN\ SN\
R50 R51 R50 R51
wherein Q51, R50, R5 1 and R59 are as defined above.
The term "phosphonamidite" is art-recognized and may be represented in the
general formulas:
R60 0 R60
0
-Q51-p O -Q51-p-OR59
I
R50 R51 R50 R51
wherein Q51, R50, R51 and R59 are as defined above, and R60 represents a lower
alkyl or an aryl.
Analogous substitutions may be made to alkenyl and alkynyl groups to
produce, for example, aminoalkenyls, aminoalkynyls, amidoalkenyls,
CA 02613141 2007-12-20
A '0-014
WO 2007/008548 PCT/US2006/026272
amidoalkynyls, iminoalkenyls, iminoallcynyls, thioalkenyls, thioalkynyls,
carbonyl-
substituted allcenyls or alkynyls.
The definition of each expression, e.g. alkyl, m, n, and the like, when it
occurs more
than once in any structure, is intended to be independent of its definition
elsewhere
in the same structure.
The term "selenoalkyl" is art-recognized and refers to an alkyl group having
a substituted seleno group attached thereto. Exemplary "selenoethers" which
may
be substituted on the alkyl are selected from one of -Se-alkyl, -Se-alkenyl, -
Se-
alkynyl, and -Se-(CHz),,,-R61, m and R61 being defined above.
The terins triflyl, tosyl, mesyl, and nonaflyl are art-recognized and refer to
trifluoromethanesulfonyl, p-toluenesulfonyl, methanesulfonyl, and
nonafluorobutanesulfonyl groups, respectively. The terms triflate, tosylate,
mesylate, and nonaflate are art-recognized and refer to
trifluorometlianesulfonate
ester, p-toluenesulfonate ester, methanesulfonate ester, and
nonafluorobutanesulfonate ester functional groups and molecules that contain
said
groups, respectively.
The abbreviations Me, Et, Ph, Tf, Nf, Ts, and Ms represent methyl, ethyl,
phenyl, trifluoromethanesulfonyl, nonafluorobutanesulfonyl, p-toluenesulfonyl
and
methanesulfonyl, respectively. A more comprehensive list of the abbreviations
utilized by organic chemists of ordinary skill in the art appears in the first
issue of
each volume of the Journal of Organic Chemistry; this list is typically
presented in a,
table entitled Standard List ofAbbreviations.
Certain compounds contained in compositions described herein may exist in
particular geometric or stereoisomeric forms. In addition, compounds may also
be
optically active. Contemplated herein are all such compounds, including cis-
and
trans-isomers, R- and S-enantiomers, diastereomers, (D)-isomers, (L)-isomers,
the
racemic mixtures thereof, and other mixtures thereof. Additional asymmetric
carbon
atoms may be present in a substituent such as an alkyl group. All such
isomers, as
well as mixtures thereof, are encompassed herein.
If, for instance, a particular enantiomer of a compound is desired, it may be
prepared by asymmetric synthesis, or by derivation with a chiral auxiliary,
where the
resulting diastereomeric mixture is separated and the auxiliary group cleaved
to
26
CA 02613141 2007-12-20
WO 2007/008548 AttornPCT/US2006/02627214
provide the pure desired enantiomers. Alternatively, where the molecule
contains a
basic functional group, such as amino, or an acidic functional group, such as
carboxyl, diastereomeric salts are formed with an appropriate optically-active
acid or
base, followed by resolution of the diastereomers thus formed by fractional
crystallization or chromatographic means well known in the art, and subsequent
recovery of the pure enantiomers.
It is understood that compounds disclosed herein are intended to represent
the compound itself, along with solvates, co-crystals, polymorphs and the like
of the
compound.
It will be understood that "substitution" or "substituted with" includes the
implicit proviso that such substitution is in accordance with permitted
valence of the
substituted atom and the substituent, and that the substitution results in a
stable
compound, e.g., which does not spontaneously undergo transformation such as by
rearrangement, cyclization, elimination, or other reaction.
The term "substituted" is also contemplated to include all permissible
substituents of organic compounds. In a broad aspect, the permissible
substituents
include acyclic and cyclic, branched and unbranched, carbocyclic and
heterocyclic,
aromatic and nonaromatic substituents of organic compounds. Illustrative
substituents include, for example, those described herein above. The
permissible
substituents may be one or more and the same or different for appropriate
organic
compounds. Heteroatoms such as nitrogen may have hydrogen substituents and/or
any permissible substituents of organic compounds described herein which
satisfy
the valences of the heteroatoms. Compounds are not intended to be limited in
any
manner by the permissible substituents of organic compounds.
The chemical elements are identified in accordance with the Periodic Table
of the Elements, CAS version, Handbook of Chenzistry and Physics, 67th Ed.,
1986-
87, inside cover.
The term "protecting group" is art-recognized and refers to temporary
substituents that protect a potentially reactive functional group from
undesired
chemical transformations. Examples of such protecting groups include esters of
carboxylic acids, silyl ethers of alcohols, and acetals and ketals of
aldehydes and
ketones, respectively. The field of protecting group chemistry has been
reviewed by
27
CA 02613141 2007-12-20
WO 2007/008548 AttopCT/US2006/026272.014
Greene and Wuts in Protective Groups in Organic Synthesis (2"d ed., Wiley: New
York, 1991).
The term "hydroxyl-protecting group" is art-recognized and refers to those
groups intended to protect a hydrozyl group against undesirable reactions
during
synthetic procedures and includes, for example, benzyl or other suitable
esters or
ethers groups known in the art.
The term "carboxyl-protecting group" is art-recognized and refers to those
groups intended to protect a carboxylic acid group, such as the C-terminus of
an
amino acid or peptide or an acidic or hydroxyl azepine ring substituent,
against
undesirable reactions during synthetic procedures and includes. Examples for
protecting groups for carboxyl groups involve, for example, benzyl ester,
cyclohexyl
ester, 4-nitrobenzyl ester, t-butyl ester, 4-pyridylmethyl ester, and the
like.
The term "amino-blocking group" is art-recognized and refers to a group
which will prevent an amino group from participating in a reaction carried out
on
some other functional group, but which can be removed from the amine when
desired. Such groups are discussed by in Ch. 7 of Greene and Wuts, cited
above,
and by Barton, Protective Groups in Organic Chemistry ch. 2 (McOmie, ed.,
Plenum Press, New York, 1973). Examples of suitable groups include acyl
protecting groups such as, to illustrate, formyl, dansyl, acetyl, benzoyl,
trifluoroacetyl, succinyl, methoxysuccinyl, benzyl and substituted benzyl such
as
3,4-dimethoxybenzyl, o-nitrobenzyl, and triphenylmethyl; those of the formula -
COOR where R includes such groups as methyl, ethyl, propyl, isopropyl, 2,2,2-
trichloroethyl, 1-methyl-l-phenylethyl, isobutyl, t-butyl, t-amyl, vinyl,
allyl, phenyl,
benzyl, p-nitrobenzyl, o-nitrobenzyl, and 2,4-dichlorobenzyl; acyl groups and
substituted acyl such as formyl, acetyl, chloroacetyl, dichloroacetyl,
trichloroacetyl,
trifluoroacetyl, benzoyl, and p-methoxybenzoyl; and other groups such as
methanesulfonyl, p-toluenesulfonyl, p-bromobenzenesulfonyl, p-
nitrophenylethyl,
and p-toluenesulfonyl-aminocarbonyl. Preferred amino-blocking groups are
benzyl
(-CH2C6H5), acyl [C(O)R1] or SiR13 where Rl is C1-C4 alkyl, halomethyl, or 2-
halo-substituted-(C2-C4 alkoxy), aromatic urethane protecting groups as, for
example, carbonylbenzyloxy (Cbz); and aliphatic urethane protecting groups
such as
t-butyloxycarbonyl (Boc) or 9-fluorenylmethoxycarbonyl (FMOC).
28
CA 02613141 2007-12-20
WO 2007/008548 AkT/US2006/026272D-014
The definition of each expression, e.g. lower alkyl, m, n, p and the like,
when
it occurs more than once in any structure, is intended to be independent of
its
definition elsewhere in the same structure.
The term "electron-withdrawing group" is art-recognized, and refers to the
tendency of a substituent to attract valence electrons from neighboring atoms,
i.e.,
the substituent is electronegative with respect to neighboring atoms. A
quantification of the level of electron-withdrawing capability is given by the
Hammett sigma (6) constant. This well known constant is described in many
references, for instance, March, Advanced Organic Chenaistiy 251-59 (McGraw
Hill
Book Company: New York, 1977). The Hammett constant values are generally
negative for electron donating groups (a(P) = - 0.66 for NH2) and positive for
electron withdrawing groups (a(P) = 0.78 for a nitro group), 6(P) indicating
para
substitution. Exemplary electron-withdrawing groups include nitro, acyl,
forinyl,
sulfonyl, trifluoromethyl, cyano, chloride, and the like. Exemplary electron-
donating groups include amino, methoxy, and the like.
1. Exemplary methods and compositions for increasing the activity or
protein level of sirtuin proteins
In one embodiment, exemplary sirtuin-activating compounds are those
described in Howitz et al. (2003) Nature 425: 191 and include, for example,
resveratrol (3,5,4'-Trihydroxy-trans-stilbene), butein (3,4,2',4'-
Tetrahydroxychalcone), piceatannol (3,5,3',4'-Tetrahydroxy-trans-stilbene),
isoliquiritigenin (4,2',4'-Trihydroxychalcone), fisetin (3,7,3',4'-
Tetrahyddroxyflavone), quercetin (3,5,7,3',4'-Pentahydroxyflavone),
Deoxyrhapontin (3,5-Dihydroxy-4'-methoxystilbene 3-O-B-D-glucoside); trans-
Stilbene; Rhapontin (3,3',5-Trihydroxy-4'-methoxystilbene 3-0-13-D-glucoside);
cis-Stilbene; Butein (3,4,2',4'-Tetrahydroxychalcone); 3,4,2'4'6'-
Pentahydroxychalcone; Chalcone; 7,8,3',4'-Tetrahydroxyflavone; 3,6,2',3'-
Tetrahydroxyflavone; 4'-Hydroxyflavone; 5,4'-Dihydroxyflavone; 5,7-
Dihydroxyflavone; Morin (3,5,7,2',4'- Pentahydroxyflavone); Flavone; 5-
Hydroxyflavone; (-)-Epicatechin (Hydroxy Sites: 3,5,7,3',4'); (-)-Catechin
(Hydroxy Sites: 3,5,7,3',4'); (-)-Gallocatechin (Hydroxy Sites:
3,5,7,3',4',5') (+)-
29
CA 02613141 2007-12-20
WO 2007/008548 AtPCT/US2006/0262720-014
Catechin (Hydroxy Sites: 3,5,7,3',4'); 5,7,3',4',5'-pentahydroxyflavone;
Luteolin
(5,7,3',4'-Tetrahydroxyflavone); 3,6,3',4'-Tetrahydroxyflavone; 7,3',4',5'-
Tetrahydroxyflavone; Kaempferol (3,5,7,4'-Tetrahydroxyflavone); 6-
Hydroxyapigenin (5,6,7,4'-Tetrahydoxyflavone); Scutellarein); Apigenin (5,7,4'-
Trihydroxyflavone); 3,6,2',4'-Tetrahydroxyflavone; 7,4'-Dihydroxyflavone;
Daidzein (7,4'-Dihydroxyisoflavone); Genistein (5,7,4'-Trihydroxyflavanone);
Naringenin (5,7,4'-Trihydroxyflavanone); 3,5,7,3',4'-Pentahydroxyflavanone;
Flavanone; Pelargonidin chloride (3,5,7,4'-Tetrahydroxyflavylium chloride);
Hinokitiol (b-Thujaplicin; 2-hydroxy-4-isopropyl-2,4,6-cycloheptatrien-l-one);
L-
(+)-Ergothioneine ((S)-a-Carboxy-2,3-dihydro-N,N,N-trimethyl-2-thioxo-1 H-
imidazole-4-ethanaminium inner salt); Caffeic Acid Phenyl Ester; MCI-186 (3-
Methyl-l-phenyl-2-pyrazolin-5-one); HBED (N,N'-Di-(2-hydroxybenzyl)
ethylenediamine-N,N'-diacetic acid=H2O); Ambroxol (trans-4-(2-Amino-3,5-
dibromobenzylamino) cyclohexane=HCI; and U-83836E ((-)-2-((4-(2,6-di-1-
Pyrrolidinyl-4-pyrimidinyl)-1-piperzainyl)methyl)-3,4-dihydro-2,5,7,8-
tetrarnethyl-
2H-1-benzopyran-6-ol=2HC1). Analogs and derivatives thereof can also be used.
Other sirtuin-activating compounds may have any of formulas 1-25, 30, 32-
65, and 69-88 below. In one embodiment, a sirtuin-activating compound is a
stilbene or chalcone compound of formula 1:
R'2
R R'l Rfa
z
R3 I~ R1 B R'4
R15
Rq
R5 M
n
wherein, independently for each occurrence,
RI, R2, R3, R4, R5, R' i, R'2, R'3, R'4, and R'5 represent H, alkyl, aryl,
heteroaryl, aralkyl, alkaryl, heteroaralkyl, halide, NO2, SR, OR, N(R)2, or
carboxyl;
R represents H, alkyl, aryl, heteroaryl, or aralkyl;
M represents 0, NR, or S;
CA 02613141 2007-12-20
WO 2007/008548 Att'PCT/US2006/026272'"014
A-B represents a bivalent alkyl, alkenyl, alkynyl, amido, sulfonamido, diazo,
ether, alkylamino, alkylsulfide, hydroxylamine, or hydrazine group; and
nis0or1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 1 and the attendant definitions, wherein n is 0. In a further
embodiment, a
sirtuin-activating compound is a compound of formula 1 and the attendant
definitions, wherein n is 1. In a further embodiment, a sirtuin-activating
compound
is a compound of formula 1 and the attendant definitions, wherein A-B is
ethenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 1
and the attendant definitions, wherein A-B is -CH2CH(Me)CH(Me)CH2-. In a
further embodiment, a sirtuin-activating compound is a compound of formula 1
and
the attendant definitions, wherein M is O. In a further embodiment, the
methods
comprises a compound of formula 1 and the attendant definitions, wherein Ri,
R2,
R3, R4, R5, R' I, R'2, R'3, R'4, and R'5 are H. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 1 and the attendant definitions,
wherein R2, R4, and R'3 are OH. In a further embodiment, a sirtuin-activating
compound is a compound of formula 1 and the attendant definitions, wherein R2,
R4,
R'2 and R'3 are OH. In a further embodiment, a sirtuin-activating compound is
a
compound of formula 1 and the attendant definitions, wherein R3, R5, R'2 and
R'3
are OH. In a further embodiment, a sirtuin-activating compound is a compound
of
formula 1 and the attendant definitions, wherein Rl, R3, R5, R'2 and R'3 are
OH. In
a further embodiment, a sirtuin-activating compound is a compound of formula 1
and the attendant definitions, wherein R2 and R'2 are OH; R4 is O-(3-D-
glucoside;
and R'3 is OCH3. In a further embodiment, a sirtuin-activating compound is a
compound of formula 1 and the attendant definitions, wherein R2 is OH; R4 is O-
(3-
D-glucoside; and R'3 is OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 1 and the attendant definitions, wherein n is 0; A-B is ethenyl; and
RI, R2,
R3, R4, R5, R'1, R'2, R'3, R'4, and R'5 are H (trans stilbene). In a further
embodiment, a sirtuin-activating compound is a compound of formula 1 and the
attendant definitions, wherein n is 1; A-B is ethenyl; M is 0; and Rl, R2, R3,
R4, R5,
R' 1, R'2, R'3, R'4, and R'5 are H (chalcone). In a further embodiment, a
sirtuin-
31
CA 02613141 2007-12-20
WO 2007/008548 Att PCT/US2006/026272)"014
activating compound is a compound of formula 1 and the attendant definitions,
wherein n is 0; A-B is ethenyl; R2, R4, and R'3 are OH; and Rl, R3, R5, R'l,
R'2, R'4,
and R'5 are H(resveratrol). In a further embodiment, a sirtuin-activating
compound is a compound of formula 1 and the attendant definitions, wherein n
is 0;
A-B is ethenyl; R2, R4, R'2 and R'3 are OH; and Rl, R3, R5, R'l, R'4 and R'5
are H
(piceatannol). In a further embodiment, a sii-tuin-activating compound is a
compound of formula 1 and the attendant definitions, wherein n is 1; A-B is
ethenyl;
M is 0; R3, R5, R'2 and R'3 are OH; and Rl, R2, R4, R'1, R'4, and R'5 are H
(butein).
In a further embodiment, a sirtuin-activating compound is a compound of
formula 1
and the attendant definitions, wherein n is 1; A-B is ethenyl; M is 0; Rl, R3,
R5, R'2
and R'3 are OH; and R2, R4, R'l, R'4, and R'5 are H(3,4,2',4',6'-
pentahydroxychalcone). In a further embodiment, a sirtuin-activating compound
is
a compound of formula 1 and the attendant definitions, wherein n is 0; A-B is
ethenyl; R2 and R'2 are OH, R4 is O-(3-D-glucoside, R'3 is OCH3; and Rl, R3,
R5,
R'l, R'4, and R'5 are H (rhapontin). In a further embodiment, a sirtuin-
activating
compound is a compound of formula 1 and the attendant definitions, wherein n
is 0;
A-B is ethenyl; R2 is OH, R4 is 0-p-D-glucoside, R'3 is OCH3; and RI, R3, R5,
R'l,
R'2, R'4, and R'5 are H (deoxyrhapontin). In a further embodiment, a sirtuin-
activating compound is a compound of formula 1 and the attendant definitions,
wherein n is 0; A-B is -CH2CH(Me)CH(Me)CH2-; R2, R3, R'2, and R'3 are OH; and
RI, R4, R5, R'l, R'4, and R'5 are H (NDGA).
In another embodiment, a sirtuin-activating compound is a flavanone
compound of formula 2:
R'2
R1 R1 Rs
RZ I ~ Z~Y R4
R3 / X, Rõ R'5
R4 M
2
wherein, independently for each occurrence,
RI, R2, R3, R4, R' l, R'2, R'3, R'4, R'5, and R" represent H, alkyl, aryl,
heteroaryl, aralkyl, alkaryl, heteroaralkyl, halide, NOa, SR, OR, N(R)2, or
carboxyl;
32
CA 02613141 2007-12-20
WO 2007/008548 '4tto~PCT/US2006/026272114
R represents H, alkyl, aryl, heteroaryl, or aralkyl;
M represents H2, 0, NR, or S;
Z represents CR, 0, NR, or S;
X represents CR or N; and
Y represents CR or N.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 2 and the attendant definitions, wherein X and Y are both CH. In a
further
embodiment, a sirtuin-activating compound is a compound of formula 2 and the
attendant definitions, wherein M is O. In a further embodiment, a sirtuin-
activating
compound is a compound of formula 2 and the attendant definitions, wherein M
is
H2. In a further embodiment, a sirtuin-activating compound is a compound of
formula 2 and the attendant definitions, wherein Z is O. In a further
embodiment, a
sirtuin-activating compound is a compound of formula 2 and the attendant
definitions, wherein R" is H. In a further embodiment, a sirtuin-activating
compound is a compound of formula 2 and the attendant definitions, wherein R"
is
OH. In a further embodiment, a sirtuin-activating compound is a compound of
formula 2 and the attendant definitions, wherein R" is an alkoxycarbonyl. In a
further embodiment, a sirtuin-activating compound is a compound of formula 2
and
OH
OH
the attendant definitions, wherein Rl is OH . In a further embodiment, a
sirtuin-activating compound is a compound of formula 2 and the attendant
definitions, wherein Rl, R2, R3, R4, R'1, R'2, R'3, R'4, R'5 and R" are H. In
a further
embodiment, a sirtuin-activating compound is a compound of formula 2 and the
attendant defmitions, wherein R2, R4, and R'3 are OH. In a further embodiment,
a
sirtuin-activating compound is a compound of forniula 2 and the attendant
definitions, wherein R4, R'2, R'3, and R" are OH. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 2 and the attendant definitions,
wherein R2, R4, R'2, R'3, and R" are OH. In a further embodiment, a sirtuin-
activating compound is a compound of formula 2 and the attendant definitions,
wherein R2, R4, R'2, R'3, R'4, and R" are OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 2 and the attendant definitions, wherein X and Y are CH; M is 0; Z and
0;
33
CA 02613141 2007-12-20
At D-014
WO 2007/008548 PCT/US2006/026272
R" is H; and RI, R2, R3, R4, R'i, R'2, R'3, R'4, R'5 and R" are H (flavanone).
In a
further embodiment, a sirtuin-activating compound is a compound of formula 2
and
the attendant definitions, wherein X and Y are CH; M is 0; Z and 0; R" is H;
R2,
R4, and R'3 are OH; and Ri, R3, R'1, R'2, R'4, and R'5 are H (naringenin). In
a
further embodiment, a sirtuin-activating compound is a compound of formula 2
and
the attendant definitions, wherein X and Y are CH; M is 0; Z and 0; R" is OH;
R2,
R4, R'2, and R'3 are OH; and Rl, R3, R'I, R'4, and R'5 are H(3,5,7,3',4'-
pentahydroxyflavanone). In a further embodiment, a sirtuin-activating compound
is a compound of formula 2 and the attendant definitions, wherein X and Y are
CH;
M is H2; Z and 0; R" is OH; R2, R4, R'2, and R'3, are OH; and Rl, R3, R'1, R'4
and
R'5 are H (epicatechin). In a furthe'r embodiment, a sirtuin-activating
compound is
a compound of formula 2 and the attendant definitions, wherein X and Y are CH;
M
is H2; Z and 0; R" is OH; Rz, R4, R'2, R'3, and R'4 are OH; and Rl, R3, R'1,
and R'5
are H (gallocatechin). In a further embodiment, a sirtuin-activating compound
is a
compound of formula 2 and the attendant definitions, wherein X and Y are CH; M
is
OH
~ -O
H2; Z and 0; ~ R" is \/OH, H= R2, R" are OH; = and R1R
, e R'2, R'3, R'4, ~ 3e
R'I, and R'5 are H (epigallocatechin gallate).
In another embodiment, a sirtuin-activating compound is an isoflavanone
compound of formula 3:
Rl
RZ \ Z\Y~R~~, R
I '
R / X ~ R~2
3
R4 M R1 I/ R15 3
R14
3
wherein, independently for each occurrence,
Rt, R2, R3, R4, R'l, R'2, R'3, R'4, R'5, and R"I represent H, alkyl, aryl,
heteroaryl, aralkyl, alkaryl, heteroaralkyl, halide, NOZ, SR, OR, N(R)2, or
carboxyl;
R represents H, alkyl, aryl, heteroaryl, or aralkyl;
M represents H2, 0, NR, or S;
34
CA 02613141 2007-12-20
WO 2007/008548 AttPCT/US2006/026272)-014
Z represents C(R)2, 0, NR, or S;
X represents CR or N; and
Y represents CR or N.
In another embodiment, a sirtuin-activating compound is a flavone
compound of formula 4:
R12
R~ / R Rs
R2 R1 Z ~ I
~ I 4
R3 X'RõR5
R4 M
4
wherein, independently for each occurrence,
Rl, R2, R3, R4, R' I, R'2, R'3, R'4, and R'5, represent H, alkyl, aryl,
heteroaryl,
aralkyl, alkaryl, heteroaralkyl, halide, NO2, SR, OR, N(R)2, or carboxyl;
R represents H, alkyl, aryl, heteroaryl, or aralkyl;
M represents H2, 0, NR, or S;
Z represents CR, 0, NR, or S; and
X represents CR" or N, wherein
R" is H, alkyl, aryl, heteroaryl, alkaryl, heteroaralkyl, halide, NO2, SR, OR,
N(R)2, or carboxyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 4 and the attendant definitions, wherein X is C. In a further
embodiment, a
sirtuin-activating compound is a compound of formula 4 and the attendant
definitions, wherein X is CR. In a further embodiment, a sirtuin-activating
compound is a compound of formula 4 and the attendant definitions, wherein Z
is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 4
and the attendant definitions, wherein M is O. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 4 and the attendant definitions,
wherein R" is H. In a further embodiment, a sirtuin-activating compound is a
compound of formula 4 and the attendant definitions, wherein R" is OH. In a
further
embodiment, a sirtuin-activating compound is a compound of formula 4 and the
attendant definitions, wherein Rl, R2, R3, R4, R'1, R'2, R'3, R'4, and R'5 are
H. In a
CA 02613141 2007-12-20 Aff,,,,,,,, n-L- AL. = o1- nn10-014
WO 2007/008548 PCT/US2006/026272
further embodiment, a sirtuin-activating compound is a compound of formula 4
and
the attendant definitions, wherein R2, R'2, and R'3 are OH. In a further
embodiment,
a sirtuin-activating compound is a compound of formula 4 and the attendant
definitions, wherein R2, R4, R'2, R'3, and R'4 are OH. In a further
embodiment, a
sirtuin-activating compound is a compound of forinula 4 and the attendant
definitions, wherein R2, R4, R'2, and R'3 are OH. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 4 and the attendant definitions,
wherein R3, R'2, and R'3 are OH. In a further embodiment, a sirtuin-activating
compound is a compound of formula 4 and the attendant definitions, wherein R2,
R4,
R'2, and R'3 are OH. In a further embodiment, a sirtuin-activating compound is
a
compound of formula 4 and the attendant definitions, wherein R2, R'2, R'3, and
R'4
are OH. In a further embodiment, a sirtuin-activating compound is a compound
of
formula 4 and the attendant definitions, wherein R2, R4, and R'3 are OH. In a
further
embodiment, a sirtuin-activating compound is a compound of formula 4 and the
attendant definitions, whe'rein R2, R3, R4, and R'3 are OH. In a further
embodiment,
a sirtuin-activating compound is a compound of formula 4 and the attendant
definitions, wherein R2, R4, and R'3 are OH. In a further embodiment, a
sirtuin- .
activating compound is a compound of formula 4 and the attendant definitions,
wherein R3, R' I, and R'3 are OH. In a further embodiment, a sirtuin-
activating
compound is a compound of formula 4 and the attendant definitions, wherein R2
and
R'3 are OH. In a further embodiment, a sirtuin-activating compound is a
compound
of formula 4 and the attendant definitions, wherein Rl, R2, R'2, and R'3 are
OH. In a
further embodiment, a sirtuin-activating compound is a compound of formula 4
and
the attendant definitions, wherein R3, R' 1, and R'z are OH. In a further
embodiment,
a sirtuin-activating compound is a compound of formula 4 and the attendant
definitions, wherein R'3 is' OH. In a furtlier embodiment, a sirtuin-
activating
compound is a compound of formula 4 and the attendant definitions, wherein R4
and
R'3 are OH. In a further embodiment, a sirtuin-activating compound is a
compound
of formula 4 and the attendant definitions, wherein Ra and R4 are OH. In a
further
embodiment, a sirtuin-activating compound is a compound of formula 4 and the
attendant definitions, wherein R2, R4, R'1, and R'3 are OH. In a further
embodiment,
a sirtuin-activating compound is a compound of formula 4 and the attendant
36
CA 02613141 2007-12-20
WO 2007/008548 '"PCT/US2006/026272D-014
definitions, wherein R4 is OH. In a further embodiment, a sirtuin-activating
compound is a compound of formula 4 and the attendant definitions, wherein R2,
R4,
R'2, R'3, and R'4 are OH. In a further embodiment, a sirtuin-activating
compound is
a compound of formula 4 and the attendant definitions, wherein R2, R'2, R'3,
and R'4
are OH. In a further embodiment, a sirtuin-activating compound is a compound
of
formula 4 and the attendant definitions, wherein RI, R2, R4, R'2, and R'3 are
OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 4 and the attendant definitions, wherein X is CH; Z is 0; M is 0; and
Ri,
R2, R3, R4, R'l, R'2, R'3, R'4, and R'5 are H (flavone). In a further
embodiment, a
sirtuin-activating compound is a compound of formula 4 and the attendant
definitions, wherein X is COH; Z is 0; M is 0; R2, R'2, and R'3 are OH; and
RI, R3,
R4, R'1, R'4, and R'5 are H (fisetin). In a further embodiment, a sirtuin-
activating
compound is a compound of formula 4 and the attendant definitions, wherein X
is
CH; Z is 0; M is 0; R2, R4, R'2, R'3, and R'4 are OH; and Rl, R3, R'l, and R'5
are H
(5,7,3',4',5'-pentahydroxyflavone). In a further embodiment, a sirtuin-
activating
compound is a compound of formula 4 and the attendant definitions, wherein X
is
CH; Z is 0; M is 0; R2, R4, R'2, and R'3 are OH; and Ri, R3, R' 1, R'4, and
R'5 are H
(luteolin). In a further embodiment, a sirtuin-activating compound is a
compound
of formula 4 and the attendant definitions, wherein X is COH; Z is 0; M is 0;
R3,
R'2, and R'3 are OH; and Rl, R2, R4, R'1 R'4, and R'S are H (3,6,3',4'-
tetrahydroxyflavone). In a further embodiment, a sirtuin-activating compound
is a
compound of formula 4 and the attendant definitions, wherein X is COH; Z is 0;
M
is 0; R2, R4, R'2, and R'3 are OH; and RI, R3, R'1, R'4, and R'5 are H
(quercetin).
In a further embodiment, a sirtuin-activating compound is a compound of
formula 4
and the attendant definitions, wherein X is CH; Z is 0; M is 0; R2, R'2, R'3,
and R'4
are OH; and RI, R3, R4, R'I, and R'5 are H. In a further embodiment, a sirtuin-
activating compound is a compound of formula 4 and the attendant definitions,
wherein X is COH; Z is 0; M is 0; R2, R4, and R'3 are OH; and Ri, R3, R'r,
R'2, R'4,
and R'5 are H. In a further embodiment, a sirtuin-activating compound is a
compound of formula 4 and the attendant definitions, wherein X is CH; Z is 0;
M is
0; R2, R3, R4, and R'3 are OH; and Rl, R'1, R'2, R'4, and R'5 are H. In a
further
embodiment, a sirtuin-activating compound is a compound of formula 4 and the
37
CA 02613141 2007-12-20 AttorneyDocketNo.:SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
attendant definitions, wherein X is CH; Z is 0; M is 0; R2, R4, and R'3 are
OH; and
RI, R3, R'l, R'2, R'4, and R'S are H. In a further embodiment, a sirtuin-
activating
compound is a compound of formula 4 and the attendant definitions, wherein X
is
COH;ZisO;MisO;R3,R'I,andR'3areOH;andRl,Ra,R4,R'a,R'd,andR'5are
H. In a further embodiment, a sirtuin-activating compound is a compound of
formula 4 and the attendant definitions, wherein X is CH; Z is 0; M is 0; R2
and R'3
are OH; and Rt, R3, R4, R' I, R'z, R'4, and R'5 are H. In a further
embodiment, a
sirtuin-activating compound is a compound of formula 4 and the attendant
definitions, wherein X is COH; Z is 0; M is 0; Ri, R2, R'2, and R'3 are OH;
and Rt,
R2, R4, R'3, R'4, and R'5 are H. In a further embodiment, a sirtuin-activating
compound is a compound of formula 4 and the attendant definitions, wherein X
is
COH; Z is 0; M is 0; R3, R' I, and R'2 are OH; and RI, R2, R4; R'3, R'4, and
R'5 are
H. In a further embodiment, a sirtuin-activating compound is a compound of
formula 4 and the attendant definitions, wherein X is CH; Z is 0; M is 0; R'3
is OH;
and RI, R2, R3, R4, R'l, R'2, R'4, and R'5 are H. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 4 and the attendant definitions,
wherein X is CH; Z is 0; M is 0; R4 and R'3 are OH; and RI, R2, R3, R'I, R'2,
R'4,
and R'5 are H. In a further embodiment, a sirtuin-activating compound is a
compound of formula 4 and the attendant definitions, wherein X is CH; Z is 0;
M is
0; R2 and R4 are OH; and RI, R3, R'l, R'2, R'3, R'4, and R'5 are H. In a
further
embodiment, a sirtuin-activating compound is a compound of formula 4 and the
attendant definitions, wherein X is COH; Z is 0; M is 0; R2, R4, R' I, and R'3
are
OH; and RI, R3, R'2, R'4, and R'5 are H. In a further embodiment, a sirtuin-
activating compound is a compound of formula 4 and the attendant definitions,
wherein X is CH; Z is 0; M is 0; R4 is OH; and RI, R2, R3, R' I, R'2, R'3,
R'4, and
R'5 are H. In a further embodiment, a sirtuin-activating compound is a
compound of
formula 4 and the attendant definitions, wherein X is COH; Z is 0; M is 0; R2,
R4,
R'2, R'3, and R'4 are OH; and RI, R3, R'l, and R'5 are H. In a further
embodiment, a
sirtuin-activating compound is a compound of formula 4 and the attendant
definitions, wherein X is COH; Z is 0; M is 0; R2, R'2, R'3, and R'4 are OH;
and RI,
R3, R4, R'l, and R'5 are H. In a further embodiment, a sirtuin-activating
compound
38
CA 02613141 2007-12-20
WO 2007/008548 AttorPCT/US2006/026272014
is a compound of formula 4 and the attendant definitions, wherein X is COH; Z
is 0;
M is 0; Ri, R2, R4, R'2, and R'3 are OH; and R3, R' i, R'4, and R'5 are H.
In another embodiment, a sirtuin-activating compound is an isoflavone
compound of formula 5:
R1
~~
::1~ix::
I
R4 M
R'5 R3
R'a
5
wherein, independently for each occurrence,
Rl, R2, R3, R4, R'i, R'2, R'3, R'4, and R'5, represent H, alkyl, aryl,
heteroaryl,
aralkyl, alkaryl, heteroaralkyl, halide, N02, SR, OR, N(R)2, or carboxyl;
R represents H, alkyl, aryl, heteroaryl, or aralkyl;
M represents H2, 0, NR, or S;
Z represents C(R)2, 0, NR, or S; and
Y represents CR" or N, wherein
R" represents H, alkyl, aryl, heteroaryl, alkaryl, heteroaralkyl, halide, NOz,
SR, OR, N(R)2, or carboxyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 5 and the attendant definitions, wherein Y is CR". In a further
embodiment,
a sirtuin-activating compound is a compound of formula 5 and the attendant
definitions, wherein Y is CH. In a further embodiment, a sirtuin-activating
compound is a compound of formula 5 and the attendant definitions, wherein Z
is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 5
and the attendant definitions, wherein M is 0. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 5 and the attendant definitions,
wherein R2 and R'3 are OH. In a further embodiment, a sirtuin-activating
compound
is a compound of formula 5 and the attendant definitions, wherein R2, R4, and
R'3
are OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 5 and the attendant definitions, wherein Y is CH; Z is 0; M is 0; R2
and R'3
39
CA 02613141 2007-12-20
Attoi - -- - 014
WO 2007/008548 PCT/US2006/026272
are OH; and Ri, R3, R4, R'1, R'2, R'4, and R'5 are H. In a further embodiment,
a
sirtuin-activating compound is a compound of formula 5 and the attendant
definitions, wherein Y is CH; Z is 0; M is 0; R2, R4, and R'3 are OH; and Rl,
R3,
R' 1, R'2, R'4, and R'5 are H.
In another embodiment, a sirtuin-activating compound is an anthocyanidin
compound of formula 6:
R3
R'2 R14
R$A'+
R7 I ~ ~ 0 \ R15
R16
R6 R3
R5 R4
6
wherein, independently for each occurrence,
R3, R4, R5, R6, R7, R8, R'2, R'3, R'4, R'5, and R'6 represent H, alkyl, aryl,
heteroaryl, aralkyl, alkaryl, heteroaralkyl, halide, NO2, SR, OR, N(R)2, or
carboxyl;
R represents H, alkyl, aryl, heteroaryl, or aralkyl; and
A" represents an anion selected from the following: C1", Br", or F.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 6 and the attendant definitions, wherein A- is Cl". In a further
embodiment,
a sirtuin-activating compound is a compound of formula 6 and the attendant
definitions, wherein R3, R5, R7, and R'4 are OH. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 6 and the attendant definitions,
wherein R3, R5, R7, R'3, and R'4 are OH. In a further embodiment, a sirtuin-,
activating compound is a compound of formula 6 and the attendant definitions,
wherein R3, R5, R7, R'3, R'4, and R'5 are OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 6 and the attendant definitions, wherein A- is Cl"; R3, R5, R7, and
R'4 are
OH; and R4, R6, R8, R'2, R'3, R'5, and R'6 are H. In a further embodiment, a
sirtuin-
activating compound is a compound of formula 6 and the attendant definitions,
wherein A- is Cl"; R3, R5, R7, R'3, and R'4 are OH; and R4, R6, R8, R'2, R'5,
and R'6
are H. In a further embodiment, a sirtuin-activating compound is a compound of
CA 02613141 2007-12-20
WO 2007/008548 AiPCT/US2006/0262720-014
formula 6 and the attendant definitions, wherein A- is Cl"; R3, R5, R7, R'3,
R'4, and
R'5 are OH; and R4, R6, R8, R'2, and R'6 are H.
In a further embodiment, a sirtuin-activating compound is a stilbene,
chalcone, or flavone compound represented by formula 7:
R'2
R11 R13
R1
Ra
RM
R
5 R4 0 n
7
wherein, independently for each occurrence,
M is absent or 0;
Ri, R2, R3, R4, R5, R' l, R'2, R'3, R'4, and R'5 represent H, alkyl, aryl,
heteroaryl, aralkyl, alkaryl, heteroaralkyl, halide, NO2, SR, OR, N(R)2, or
carboxyl;
Ra represents H or the two instances of Ra form a bond;
R represents H, alkyl, aryl, heteroaryl, aralkyl; and
nis0or1.
In a further embodiment, a sirtuin-activating compound is an activating
compound represented by formula 7 and the attendant definitions, wherein n is
0. In
a further embodiment, a sirtuin-activating compound is an activating compound
represented by formula 7 and the attendant definitions, wherein n is 1. In a
further
embodiment, a sirtuin-activating compound is an activating compound
represented
by formula 7 and the attendant definitions, wherein M is absent. In a further
embodiment, a sirtuin-activating compound is an activating compound
represented
by formula 7 and the attendant definitions, wherein M is O. In a further
embodiment, a sirtuin-activating compound is an activating compound
represented
by formula 7 and the attendant definitions, wherein Ra is H. In a further
embodiment, a sirtuin-activating compound is an activating compound
represented
by formula 7 and the attendant definitions, wherein M is 0 and the two Ra form
a
bond.
41
CA 02613141 2007-12-20
At - 0-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is an activating
compound represented by formula 7 and the attendant definitions, wherein R5 is
H.
In a further embodiment, a sirtuin-activating compound is an activating
compound
represented by formula 7 and the attendant definitions, wherein R5 is OH. In a
further embodiment, a sirtuin-activating compound is an activating compound
represented by formula 7 and the attendant definitions, wherein Rl, R3, and
R'3 are
OH. In a further embodiment, a sirtuin-activating compound is an activating
compound represented by formula 7 and the attendant definitions, wherein R2,
R4,
R'2, and R'3 are OH. In a further embodiment, a sirtuin-activating compound is
an
activating compound represented by formula 7 and the attendant definitions,
wherein
R2, R'2, and R'3 are OH. In a further embodiment, a sirtuin-activating
compound is
an activating compound represented by formula 7 and the attendant definitions,
wherein R2 and R4 are OH.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 7 and the attendant definitions, wherein n is 0; M is
absent;
Ra is H; R5 is H; Rl, R3, and R'3 are OH; and R2, R4, R'l, R'2, R'4, and R'5
are H. In
a further embodiment, a sirtuin-activating compound is an activating compound
represented by formula 7 and the attendant definitions, wherein n is 1; M is
absent;
Ra is H; R5 is H; R2, R4, R'2, and R'3 are OH; and Rl, R3, R'l, R'4, and R'5
are H. In
a further embodiment, a sirtuin-activating compound is an activating compound
represented by formula 7 and the attendant definitions, wherein n is 1; M is
0; the
two Ra form a bond; R5 is OH; R2, R'2, and R'3 are OH; and Rl, R3, R4, R'l,
R'4, and
R'5 are H.
Other sirtuin-activating compounds include compounds having a formula
selected from the group consisting of formulas 8-25 and 30 set forth below.
42
CA 02613141 2007-12-20
At ~ ,._ . . .....0-014
WO 2007/008548 PCT/US2006/026272
,/OH
/ OH
Ri (~J n RI l~
\ ~N\ õ/OH I''~
~ C~Tn H OA \ N OH
HO~N ~ D I n
~) n R R3 B
HO Z
OH R2
8 9
Rl, R2 = H, aryl, heterocycle, small alkyl Rl, R2 = H, aryl, heterocycle,
small alkyl
A,B,C,D = CR,,N R3 = H, small alkyl
n = 0,1,2,3 A,B = CRI,N
n = 0,1,2,3
R'2
R'
R R'l R13 2
~ R R1 R'3
I
4B" R14 HO AR'
HO~N D R5 4
H ~) n R R3 B R'5
0 ~
OH R2
11
Rl, R2 = H, aryl, heterocycle, small alkyl Rl, R2 = H, aryl, heterocycle,
small alkyl
R'j-R'5 = H, OH R3 = H, small alkyl
A,B,C,D = CRj,N R'l-R'5 = H, OH
n=0,1,2,3 A,B=CRj,N
n = 0,1,2,3
Rl R2
CH3
H3C-,,N +
H3C' n CO2-
OH n R
0
12 13
RI,R2 = H, alkyl, alkenyl R Heterocycle, aryl
n=0-10
43
CA 02613141 2007-12-20 AttornPv nnr.kPtNn -STRT_PWn-OI4
WO 2007/008548 PCT/US2006/026272
R2 R2
R'l R1 R3 R 2 Rl R3
:::R4 5 R5 R1I A ~ R5
4
R1a R' O
14 5 15
R2 R'l
Rl R1 / R3 R'2 Z~Y~R111 R
I '
R 2 Z~ i \ Ra X R2
X R'3
~ 1- X~ ~, R5 R'4 O
R3 R l R5 R3
R'a O 17 Ra
16
R2
R~1 Rl R3
R2 Z I Ra
R'13 / R"1 R5
R1a 18
R, = H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R2 =H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R3 =H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R4 =H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R5 =H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R', =H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R'2 =H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R'3 = H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R'4 = H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R'5 =H, halogen,NO2,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
R", = H, halogen,N02,SR(R=H,alkyl,aryl),OR(R = H, alkyl, aryl),
NRR'(R,R'=alkyl,aryl), alkyl, aryl, carboxy
A-B = ethene,ethyne,amide,sulfonamide,diazo,alkyi ether,alkyl amine,alkyl
sulfide, hydroxyamine,hydrazine
X=CR,N
Y = CR,N
Z = O,S,C(R)2,NR
R = H, alkyl, aryl, aralkyl
44
CA 02613141 2007-12-20
WO 2007/008548 AttornPCT/US2006/02627214
0
R' OR
R'
R' R'
R'
19
wherein, independently for each occurrence,
R = H, alkyl, aryl, heterocyclyl, heteroaryl, or aralkyl; and
R' = H, halogen, NOz, SR, OR, NR2, alkyl, aryl, or carboxy.
R
R
NR
O N
R
wherein, independently for each occurrence,
R = H, alkyl, aryl, heterocyclyl, heteroaryl, or aralkyl.
HO2C, N/-\NIICO2H
R' R'
R' R'
R' R'
R' R'
10 R' R'
21
wherein, independently for each occurrence,
R' = H, halogen, NO2, SR, OR, NR2, alkyl, aryl, aralkyl, or carboxy; and
R = H, alkyl, aryl, heterocyclyl, heteroaryl, or aralkyl.
CA 02613141 2007-12-20
AY - ' " ---- ----0-014
WO 2007/008548 PCT/US2006/026272
R'
R' R'
R' R'
L L
R' L R R'
R'
L
R
R'
R' R'
R'
22
wherein, independently for each occurrence,
L represents CR2, 0, NR, or S;
R represents H, alkyl, aryl, aralkyl, or heteroaralkyl; and
R' represents H, halogen, NO2, SR, OR, NR2, alkyl, aryl, arallcyl, or carboxy.
L R' R'
Ar/ W
W L
W- L
R'
R' R, R, R,
23
wherein, independently for each occurrence,
L represents CR2, 0, NR, or S;
W represents CR or N;
R represents H, alkyl, aryl, aralkyl, or heteroaralkyl;
Ar represents a fused aryl or heteroaryl ring; and
R' represents H, halogen, NO2, SR, OR, NR2, alkyl, aryl, aralkyl, or carboxy.
R'
R' R'
R'
\ L _
R'
R'
R L L R
24
wherein, independently for each occurrence,
46
CA 02613141 2007-12-20
WO 2007/008548 Attom PCT/US2006/026272 14
L represents CR2, 0, NR, or S;
R represents H, alkyl, aryl, aralkyl, or heteroaralkyl; and
R' represents H, halogen, NO2, SR, OR, NR2, alkyl, aryl, aralkyl, or carboxy.
R' R'
R' R'
~ \
R' ~ L L
R'
25
wherein, independently for each occurrence,
L represents CR2, 0, NR, or S;
R represents H, alkyl, aryl, aralkyl, or heteroaralkyl; and
R' represents H, halogen, NO2, SR, OR, NR2, alkyl, aryl, aralkyl, or carboxy.
In a further embodiment, a sirtuin-activating compound is a stilbene,
chalcone, or flavone compound represented by formula 30:
R'2
R'l R'3
R~ D
RZ AB R,
R3 R5 R'5
R4
wherein, independently for each occurrence,
15 D is a phenyl or cyclohexyl group;
Rl, R2, R3, R4, R5, R' 1, R'2, R'3, R'4, and R'5 represent H, alkyl, aryl,
heteroaryl, alkaryl, heteroaralkyl, halide, NO2, SR, OR, N(R)2, carboxyl,
azide,
ether; or any two adjacent R or R' groups taken together form a fused benzene
or
cyclohexyl group;
20 R represents H, alkyl, aryl, or aralkyl; and
A-B represents an ethylene, ethenylene, or imine group;
provided that when A-B is ethenylene, D is phenyl, and R'3 is H: R3 is not
OH when Rl, R2, R4, and R5 are H; and R2 and R4 are not OMe when RI, R3, and
R5
are H; and R3 is not OMe when RI, R2, R4, and R5 are H.
47
CA 02613141 2007-12-20
WO 2007/008548 AttokT/US2006/026272 014
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein D is a phenyl
group.
In a further embodiment, a sirtuin-activating compound is a coinpound
represented by formula 30 and the attendant definitions, wherein A-B is an
ethenylene or imine group.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is an
ethenylene group.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein R2 is OH.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein R4 is OH
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein R2 and R4 are
OH.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein D is a phenyl
group; and A-B is an ethenylene group.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein D is a phenyl
group; A-B is an ethenylene group; and R2 and R4 are OH.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is Cl.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is OH.
In a further embodiment, a sirtuin-activating coinpound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is H.
48
CA 02613141 2007-12-20
WO 2007/008548 AttPCT/US2006/026272)-014
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is CH2CH3.
In a furtlier embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is F.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is Me.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is an azide.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is SMe.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; Rz and R4 are OH; and R'3 is NO2.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is CH(CH3)2.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2and R4 are OH; and R'3 is OMe.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; R'2 is OH; and R'3 is OMe.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 is OH; R4 is carboxyl; and R'3 is OH.
49
CA 02613141 2007-12-20
WO 2007/008548 AnPCT/US2006/026272D-014
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is carboxyl.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 and R'4 taken together form a
fused
benzene ring.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; and R4 is OH.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OCH2OCH3i and R'3 is SMe.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is carboxyl.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a cyclohexyl ring; and Rz and R~ are OH.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; and R3 and R4 are OMe.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 30 and the attendant definitions, wherein A-B is
ethenylene;
D is a phenyl ring; R2 and R4 are OH; and R'3 is OH.
In anotlier embodiment, a sirtuin-activating compound is a compound of
formula 32:
S R1
(R)2N)~ N,N Rz
R
32
wherein, independently for each occurrence:
CA 02613141 2007-12-20
WO 2007/008548 ACPCT/US2006/0262720-014
R is H, or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
Rl and R2 are a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 32 and the attendant definitions wherein R is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 32 and the attendant definitions wherein Ri is 3-hydroxyphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 32 and the attendant definitions wherein R2 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 32 and the attendant definitions wherein R is H and Rl is 3-
hydroxyphenyl.
In a further embodiment, a sirtuin-activating coinpound is a compound of
formula 32 and the attendant definitions wherein R is H, Rl is 3-
hydroxyphenyl, and
R2 is methyl.
In another embodiment, a sirtuin-activating compound is a compound of
formula 33:
0 R
,
RL I ~N
R2
33
wherein, independently for each occurrence:
R is H, or a substituted or uhsubstituted alkyl, alkenyl, or alkynyl;
RI and R2 are a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
L is O, S, or NR.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 33 and the attendant definitions wherein R is alkynyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 33 and the attendant definitions wherein R, is 2,6-dichlorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 33 and the attendant definitions wherein R2 is methyl.
51
CA 02613141 2007-12-20
Ai - 0-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 33 and the attendant definitions wherein L is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 33 and the attendant definitions wherein R is alkynyl and R, is 2,6-
dichlorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 33 and the attendant definitions wherein R is alkynyl, R, is 2,6-
dichlorophenyl, and R2 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 33 and the attendant definitions wherein R is alkynyl, Rl is 2,6-
dichlorophenyl, R2 is methyl, and L is O.
In another embodiment, a sirtuin-activating compound is a compound of
formula 34:
R~ O
R1f\N N,R2
~
34
wherein, independently for each occurrence:
R, Rl, and RZ are H, or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
n is an integer from 0 to 5 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 34 and the attendant definitions wherein R is 3,5-dichloro-2-
hydroxyphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 34 and the attendant definitions wherein R, is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 34 and the attendant definitions wherein R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 34 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 34 and the attendant definitions wherein R is 3,5-dichloro-2-
hydroxyphenyl
and Rt is H.
52
CA 02613141 2007-12-20
A 0-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 34 and the attendant definitions wherein R is 3,5-dicliloro-2-
hydroxyphenyl,
R, is H, and R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 34 and the attendant definitions wherein R is 3,5-dichloro-2-
hydroxyphenyl,
RI isH,RaisH,andnis 1.
In another embodiment, a sirtuin-activating compound is a compound of
formula 35:
(R2)m
X \
R-L O
NR
(R2)o O n 1
35
wherein, independently for each occurrence:
R is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Rl is a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R2 is hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, heteroaralkyl;
LisO,NR,orS;
m is an integer from 0 to 3 inclusive;
n is an integer from 0 to 5 inclusive; and
o is an integer from 0 to 2 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein R is phenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein RI is pyridine.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein L is S.
53
CA 02613141 2007-12-20
WO 2007/008548 AttorPCT/US2006/026272014
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein m is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating coinpound is a compound of
formula 35 and the attendant definitions wherein o is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein R is phenyl and Rl is
pyridine.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein R is phenyl, R, is pyridine,
and L
is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein R is phenyl, Rl is pyridine,
L is S,
and m is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein R is phenyl, Rl is pyridine,
L is S,
mis0,andnisl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 35 and the attendant definitions wherein R is phenyl, RI is pyridine,
L is S,
mis0,nis 1,andois0.
In another embodiment, a sirtuin-activating compound is a compound of
formula 36:
R
R4~L: LZ-Rl
R3 :(~
RZ L3
36
wherein, independently for each occurrence:
R, R3, and R4 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester,
amido, ketone, carboxylic acid, nitro, or a substituted or unsubstituted
alkyl, aryl,
aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroaralkyl;
Rl and R2 are H or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, heteroaralkyl;
54
CA 02613141 2007-12-20 AttoTnev nnckPtNn = cTTiT_P\xln,014
WO 2007/008548 PCT/US2006/026272
Li is 0, NRI, S, C(R)2, or SO2; and
L2 and L3 are 0, NRi, S, or C(R)2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein RI is 4-chlorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R2 is 4-chlorophenyl.
In a further embodiment, a sir-tuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R3 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R4 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein Lj is SOz.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein L2 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein L3 is O.
In a further embodiment, a sirtuin-activating compound is a coinpound of
formula 36 and the attendant definitions wherein R is H and Rl is 4-
chlorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R is H, Rl is 4-chlorophenyl,
and
R2 is 4-chlorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R is H, Rl is 4-chlorophenyl,
R2 is
4-chlorophenyl, and R3 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R is H, Rl is 4-chlorophenyl,
R2 is
4-chlorophenyl, R3 is H, and R4 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R is H, Rl is 4-chlorophenyl,
R2 is
4-chlorophenyl, R3 is H, R4 is H, and LI is SO2.
CA 02613141 2007-12-20
Atf - " " '...0-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R is H, R, is 4-chlorophenyl,
R2 is
4-chlorophenyl, R3 is H, R4 is H, Li is SO2, and L2 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 36 and the attendant definitions wherein R is H, R, is 4-chlorophenyl,
R2 is
4-chlorophenyl, R3 is H, R4 is H, L, is SO2, L2 is NH, and L3 is O.
In another embodiment, a sirtuin-activating compound is a compound of
formula 37:
N,L~Rl
i
~R)n N I R2
~ ~N R3
37
wherein, independently for each occurrence:
R is hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, heteroaralkyl;
RI is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, heteroaralkyl;
R2 and R3 are H or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, heteroaralkyl;
L is 0, NRI, or S; and
n is an integer from 0 to 4 inclusive:
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein R is methyl.
In a further embodiment, a sirtuin-activating corripound is a compound of
formula 37 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein RI is 3-fluorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein R3 is 4-chlorophenyl.
56
CA 02613141 2007-12-20
Att - . . _. _m .....-)-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein L is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein R is methyl and n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein R is methyl, n is 1, and Rl
is 3-
fluorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein R is methyl, n is 1, Rt is 3-
fluorophenyl, and R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 37 and the attendant definitions wherein R is methyl, n is 1, Rl is 3-
fluorophenyl, R2 is H, and R3 is 4-chlorophenyl.
In another embodiment, a sirtuin-activating compound is a compound of
formula 38:
O
R11 ~L2,
L~ R1
38
wherein, independently for each occurrence:
R and Rt are H or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
L, and L2 are 0, NR, or S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 38 and the attendant definitions wherein R is 3-methoxyphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 38 and the attendant definitions wherein Rl is 4-t-butylphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 38 and the attendant definitions wherein L, is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 38 and the attendant definitions wherein L2 is O.
57
CA 02613141 2007-12-20
Atto -" ~ ~ 014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 38 and the attendant definitions wherein R is 3-methoxyphenyl and Rl
is 4-
t-butylphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 38 and the attendant definitions wherein R is 3-methoxyphenyl, Ri is 4-
t-
butylphenyl, and L1 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 38 and the attendant definitions wherein R is 3-methoxyphenyl, R1 is 4-
t-
butylphenyl, LI is NH, and L2 is O.
In another embodiment, a sirtuin-activating compound is a compound of
formula 39:
0
L~ Ri
L
39
wherein, independently for each occurrence:
R is H, hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
RI is H or a substituted or unsubstituted alkyl, aryl, alkaryl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Li and L2 are 0, NR, or S; and
n is an integer from 0 to 4 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein R is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein Rl is 3,4,5-trimethoxyphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein L1 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein L2 is NH.
58
CA 02613141 2007-12-20 Attor- Ilneket Nnt SfRT-PWn-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein R is methyl and n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein R is methyl, n is 1, and R,
is 3,4,5-
trimethoxyphenyl.
In a further embodiment, a sii-tuin-activating compound is a compound of
formula 39 and the attendant definitions wherein R is methyl, n is 1, RI is
3,4,5-
trimethoxyphenyl, and L, is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 39 and the attendant definitions wherein R is methyl, n is 1, R1 is
3,4,5-
trimethoxyphenyl, L, is S, and L2 is NH.
In another embodiment, a sirtuin-activating compound is a compound of
formula 40:
0 R3
R.N ~iR41"
R, R2 L
J2
L~
40
wherein, independently for each occurrence:
R, Ri, R2, R3 are H or a substituted or unsubstituted alkyl, aryl, alkaryl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R4 is hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
L, and L2 are 0, NR, or S; and
n is an integer from 0 to 3 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein Rl is perfluorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R2 is H.
59
CA 02613141 2007-12-20
Ad
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R3 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein L, is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein n is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R is H and Rl is
perfluorophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R is H, Rl is
perfluorophenyl, and
R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions R is H, RI is perfluorophenyl, R2 is
H, and
R3 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R is H, Rl is
perfluorophenyl, R2 is
H, R3 is H, and L, is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R is H, Rl is
perfluorophenyl, R2 is
H,R3isH,Lt isO,andL2is0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 40 and the attendant definitions wherein R is H, Rt is
perfluorophenyl, R2 is
H,R3isH,L, isO,L2isO,andnis0.
In another embodiment, a sirtuin-activating compound is a compound of
formula 41:
R, O
LlL
~R~n N I2
R2
N
(R3)m ' J
L3
CA 02613141 2007-12-20 Ay õT LutAT.. = QTAT iri11O-o14
WO 2007/008548 PCT/US2006/026272
41
wherein, independently for each occurrence:
R, Ri, and R3 are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R2 is H or a substituted or unsubstituted alkyl, aryl, arallcyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Ll, L2, and L3 are 0, NR2, or S; and
m and n are integers from 0 to 8 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein n is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein R, is cyano.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein R2 is ethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein m is 0.
In a further embodiment, a sirtuin-activating oompound is a compound of
formula 41 and the attendant definitions wherein Ll is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein L3 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein n is 0 and RI is cyano.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein n is 0, -R, is cyano, and R2
is ethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein n is 0, Rl is cyano, R2 is
ethyl, and
mis0.
61
CA 02613141 2007-12-20 Attorr- n-4 r Un = CiRT_PW(LQ14
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein n is 0, Rt is cyano, R2 is
ethyl, m is
0, and Ll is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein n is 0, Rl is cyano, Ra is
ethyl, m is
0,L1 isS,andL2isO.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 41 and the attendant definitions wherein n is 0, Rl is cyano, R2 is
ethyl, m is
0,L1 isS,LzisO,andL3 isO.
In another embodiment, a sirtuin-activating compound is a compound of
formula 42:
(R) n~ O
L.2'R,
L3
O I ~N
t N
4
Ra \~Rz)n,
42
wherein, independently for each occurrence:
R and R2 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Rl and R3 are H or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
LI, L2, L3, and L4 are 0, NRI, or S;
m is an integer from 0 to 6 inclusive; and
n is an integer from 0 to 8 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein Rl is methyl.
62
CA 02613141 2007-12-20
WO 2007/008548 Att~PCT/US2006/026272)"014
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein R2 is CF3 and m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
forinula 42 and the attendant definitions wherein R3 is 4-methylphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein L, is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein L3 is NRI.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein L4 is NRI.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0 and Rl is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0, R, is methyl, R2 is
CF3, and
mis1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0, Rl is methyl, Rz is
CF3, m is
1; and R3 is 4-methylphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0, RI is methyl, R2 is
CF3, m is
1; R3 is 4-methylphenyl; and L, is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0, Rl is methyl, R2 is
CF3, m is
1; R3 is 4-methylphenyl; Ll is S, and L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0, R, is methyl, R2 is
CF3, m is
1; R3 is 4-methylphenyl; Li is S, L2 is 0; and L3 is NRI.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 42 and the attendant definitions wherein n is 0, Rl is methyl, R2 is
CF3, m is
1; R3 is 4-methylphenyl; L, is S, Lz is 0; L3 is NRI, and L4 is NRI.
63
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In another embodiment, a sirtuin-activating compound is a compound of
formula 43:
R1
R L
1
R3 IL2
N
R2
43
wherein, independently for each occurrence:
R and R, are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R2 and R3 are H or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
Ll and L2 are 0, NR2, or S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R is cyano.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R, is NH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R2 is 4-bromophenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R3 is 3-hydroxy-4-
methoxyphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein L1 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein L2 is NR2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R is cyano and RI is NH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R is cyano, Rl is NH2, and R2
is 4-
bromophenyl.
64
CA 0 2 613141 2 0 0 7-12 - 2 0 Attornev Docket No.: 51RT-PWO-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R is cyano, R, is NH2, Rz is
4-
bromoplienyl, and R3 is 3-hydroxy-4-methoxyphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R is cyano, Rl is NH2, R2 is
4-
bromophenyl, R3 is 3-hydroxy-4-methoxyphenyl, and Li is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 43 and the attendant definitions wherein R is cyano, R, is NH2, R2 is
4-
bromophenyl, R3 is 3-hydroxy-4-methoxyphenyl, Ll is 0, and L2 is NR2.
In another embodiment, a sirtuin-activating compound is a compound of
formula 44:
/ ~(R1)
\ I
L3
r-1-- O
Li N L2
N~ I R
O
44
wherein, independently for each occurrence:
R is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Ri is hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
LI, L2, and L3 are 0, NR, or S; and
n is an integer from 0 to 5 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein R is 3-
trifluoroinethylphenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein R, is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein LI is NR.
CA 02613141 2007-12-20
Attt - 014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein L3 is NR.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein n is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein R is 3-trifluoromethylphenyl
and
Rt is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein R is 3-trifluoromethyiphenyl,
R, is
C(O)OCH3, and Lt is NR.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein R is 3-trifluoromethylphenyl,
R, is
C(O)OCH3, Lt is NR, and L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein R is 3-trifluoromethylphenyl,
RI is
C(O)OCH3, LI is NR, Lz is S, and L3 is NR.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 44 and the attendant definitions wherein R is 3-trifluoromethylphenyl,
R, is
C(O)OCH3i Ll is NR, L2 is S, L3 is NR, and n is 2.
In another embodiment, a sirtuin-activating compound is a compound of
formula 45:
0
RI
(R) ~ ~ N.
n
N Ll
L2-Rz
O
45
wherein, independently for each occurrence:
R is hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
66
CA 02613141 2007-12-20
WO 2007/008548 Att PCT/US2006/0262720-014
Rt and R2 are H or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
L, and L2 are 0, NRI, or S; and
n is an integer from 0 to 4 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein n is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein Rl is 2-
tetrahydrofuranylmethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein R2 is -CH2CH2C6H4SOZNH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein Ll is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein L2 is NRI.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein n is 0 and R, is 2-
tetrahydrofuranylmethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein n is 0, Rl is 2-
tetrahydrofuranylmethyl, and R2 is -CH2CH2C6H4SO2NH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein n is 0, R, is 2-
tetrahydrofuranylmethyl, R2 is -CH2CH2C6H4SO2NH2, and L, is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 45 and the attendant definitions wherein n is 0, Rl is 2-
tetrahydrofuranylmethyl, R2 is -CH2CH2C6H4SO2NH2, Ll is S, and L2 is NRl.
In another embodiment, a sirtuin-activating compound is a compound of
formula 46:
67
CA 02613141 2007-12-20
WO 2007/008548 Atto PCT/US2006/026272 -014
(R3)p
11{~
L2
(R1)m 0-~ 0
(R2)o
Ll
(R) n
46
wherein, independently for each occurrence:
R, RI, R2, and R3 are hydroxy, amino, cyano, halide, alkoxy, ether, ester,
amido, ketone, carboxylic acid, nitro, or a substituted or unsubstituted
alkyl, aryl,
aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Ll and L2 are 0, NR4, or S;
R4 is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
n is an integer from 0 to 4 inclusive;
m is an integer from 0 to 3 inclusive;
o is an integer from 0 to 4 inclusive; and
p is an integer from 0 to 5 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein n is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein Rl is Cl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein o is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein R2 is Cl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein p is 3.
68
CA 02613141 2007-12-20 pttamnv Tlnrrknt Nn = STRT-PWO-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein R3 is OH or I.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein n is 0 and m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein n is 0, m is 1, and o is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein n is 0, m is 1, o is 1, and
Rl is Cl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 46 and the attendant definitions wherein n is 0, m is 1, o is 1, Rl is
Cl, and p
is 3.
In a further embodiment, a sirtuin-activating compound is a compound of
forlnula 46 and the attendant definitions wherein n is 0, m is 1, o is 1, Rl
is Cl, p is
3,andR2isOHor1.
In another embodiment, a sirtuin-activating compound is a compound of
formula 47:
(R1)m
L1
1
P=0
/ L2
~
47
wherein, independently for each occurrence:
R and Rl are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Ll and L2 are 0, NR4, or S;
R4 is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
m and n are integers from 0 to 4 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein n is 2.
69
CA 02613141 2007-12-20
AttOflN" --- T.r.. = QiRT_AVUtIl1l4
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein R is methyl or t-butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein m is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
forinula 47 and the attendant definitions wherein Ri is methyl or t-butyl.
In a further embodiinent, a sirtuin-activating compound is a compound of
forinula 47 and the attendant definitions wherein L, is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein n is 2 and R is methyl or t-
butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein n is 2, R is methyl or t-
butyl, and m
is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein n is 2, R is methyl or t-
butyl, m is
2, and RI is metliyl or t-butyl.
In a further embodiment, a sirtuin-activating conlpound is a compound of
formula 47 and the attendant definitions wherein n is 2, R is methyl or t-
butyl, m is
2, R, is methyl or t-butyl, and L, is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 47 and the attendant definitions wherein n is 2, R is methyl or t-
butyl, m is
2, Rl is methyl or t-butyl, L, is 0, and L2 is O.
In anotlier embodiment, a sirtuin-activating compound is a compound of
formula 48:
R2
Lz Rs
R~ \ \ R4
Ll
L3
, \ \
(R) n N R5
R R6
7
48
CA 02613141 2007-12-20
Attorne "'- = TnT-n~x,n n,4
WO 2007/008548 PCT/US2006/026272
wherein, independently for each occurrence:
R, Rl, R2, R3, R4, R5, and R6 are hydroxy, amino, cyano, halide, alkoxy,
ether, ester, amido, ketone, carboxylic acid, nitro, or a substituted or
unsubstituted
alkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or
heteroaralkyl;
R7 is H or a substituted or unsubstituted alkyl, acyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Li, L2, and L3 are 0, NR7, or S and
n is an integer from 0 to 4 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein R is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein R, is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein R2 is C(O)OCH3.
a
In a further embodirnent, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein R3 is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein R4 is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein RS is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein R6 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein R7 is C(O)CF3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein L, is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein L3 is S.
71
CA 02613141 2007-12-20
Attorne 4
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1 and R is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, and Ri
is
C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, R, is
C(O)OCH3, and R2 is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, R} is
C(O)OCH3, R2 is C(O)OCH3, and R3 is C(O)OCH3.
In a further embodiment, a sirtuin-activatirig compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R3 is C(O)OCH3, and R4 is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3,R3 is C(O)OCH3, R4 is C(O)OCH3, and R5 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R3 is C(O)OCH3, R4 is C(O)OCH3, R5 is methyl, and
R6 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, R, is
C(O)OCH3, R2 is C(O)OCH3, R3 is C(O)OCH3, R4 is C(O)OCH3, R5 is metllyl, R6 is
methyl, and R7 is C(O)CF3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R3 is C(O)OCH3, R4 is C(O)OCH3, R5 is methyl, R6 is
methyl, R7 is C(O)CF3, and Li is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, Ri is
72
CA 02613141 2007-12-20 pttnrnPv llnrkPt Nn = CiRT-PWO-014
WO 2007/008548 PCT/US2006/026272
C(O)OCH3, R2 is C(O)OCH3, R3 is C(O)OCH3, R4 is C(O)OCH3, R5 is methyl, R6 is
methyl, R7 is C(O)CF3, L, is S, and L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 48 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R31s C(O)OCH3, R4 lS C(O)OC,H3a R5 is methyl, R6 is
methyl, R7 is C(O)CF3, Li is S, L2 is S, and L3 is S.
In another embodiment, a sirtuin-activating compound is a compound of
formula 49:
Ri R
L2
I L3
~R)n i R3
N R
~ 4
0 R5
49
wherein, independently for each occurrence:
R, Rl, R2, R3, R4, and R5 are hydroxy, amino, cyano, halide, alkoxy, ether,
ester, amido, ketone, carboxylic acid, nitro, or a substituted or
unsubstituted alkyl,
aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Ll, L2, and L3 are 0, NR6, or S;
R6 is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
n is an integer from 0 to 4 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein R is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein RI is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein R2 is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein R3 is methyl.
73
CA 02613141 2007-12-20
WO 2007/008548 AttornkT/US2006/026272 4
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein R4 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein R5 is CH2CH(CH3)2.
In a further embodiment, a sirtuin-activating compound is a compound of
forinula 49 and the attendant definitions wherein LI is S.
In a further einbodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein L3 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1 and R is methyl.
In a further embodiment, a sirtuin-activating,compound is a compound of
formula 49 and the attendant definitions wherein n is 1', R is methyl, and R,
is
C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is methyl, RI is
C(O)OCH3, and R2 is C(O)OCH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is methyl, Rt is
C(O)OCH3, R2 is C(O)OCH3, and R3 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is methyl, RI is
C(O)OCH3, R2 is C(O)OCH3, R3 is methyl, and R4 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R3 is methyl, R4 is methyl, and R5 is CH2CH(CH3)2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R3 is methyl, R4 is methyl, R5 is CH2CH(CH3)2, and
LI is S.
74
CA 02613141 2007-12-20
AltornF" ' ern m own n14
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wllerein n is 1, R is methyl, Ri is
C(O)OCH3, R2 is C(O)OCH3, R3 is methyl, R4 is methyl, R5 is CH2CH(CH3)2, and
L, is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R3 is methyl, R4 is methyl, RS is CH2CH(CH3)2, L, is
S, and L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is metliyl, Ri is
C(O)OCH3, R2 is C(O)OCH3, R3 is methyl, R4 is methyl, R5 is CH2CH(CH3)2, Ll is
S, and L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 49 and the attendant definitions wherein n is 1, R is methyl, Rl is
C(O)OCH3, R2 is C(O)OCH3, R3 is methyl, Rd is methyl, R5 is CH2CH(CH3)2, L, is
S,L2isS,andL3isS.
In another embodiment, a sirtuin-activating compound is a compound of
formula 50:
N i (R1)m
~L1
S
1 N
N N-L~RZ
(R)õ-C
\ /~
50
wherein, independently for each occurrence:
R and R, are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R2 is H, hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
LI and L2 are 0, NR3, or S;
CA 02613141 2007-12-20
WO 2007/008548 AttorPCT/US2006/026272314
R3 is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
n is an integer from 0 to 5 inclusive; and
m is an integer from 0 to 4 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein R is CO2Et.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein m is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein R2 is cyano.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein Ll is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein L2 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein n is 1 and R is CO2Et.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein n is 1, R is CO2Et, and m is
0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein n is 1, R is COzEt, m is 0,
and R2 is
cyano.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein n is 1, R is CO2Et, m is 0,
R2 is
cyano, and Ll is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 50 and the attendant definitions wherein n is 1, R is CO2Et, m is 0,
R2 is
cyano, Ll is S, and L2 is S.
In another embodiinent, a sirtuin-activating compound is a compound of
formula 51:
76
CA 02613141 2007-12-20 Attornev Docket No.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
~ N
(R)~ / (R1)m
N
51
wherein, independently for each occurrence:
R and Rl are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
n is an integer from 0 to 4 inclusive; and
m is an integer from 0 to 2 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein R is CI or trifluoromethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein m is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein Rl is phenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is 2 and R is Cl or
trifluoromethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is 2, R is Cl or
trifluoromethyl,
and m is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is 2, R is Cl or
trifluoromethyl, m
is 2, and R, is phenyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein R is F.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein R, is 4-methylphenyl.
77
CA 02613141 2007-12-20
WO 2007/008548 AttorPCT/US2006/026272D14
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is I and R is F.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is 1, R is F, and m is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 51 and the attendant definitions wherein n is 1, R is F, m is 2, and
RI is 4-
inethylphenyl.
In another embodiment, a sirtuin-activating compound is a compound of
formula 52:
R3
RZ R4
L2 R5
(R1)n
3
R, Ll O (R6)p o
52
wherein, independently for each occurrence:
R is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Rt and R6 are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R2 is alkylene, alkenylene, or alkynylene;
R3, R4, and R5 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester,
amido, ketone, carboxylic acid, nitro, or a substituted or unsubstituted
alkyl, aryl,
aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
LI, L2, and L3 are 0, NR, or S;
n and p are integers from 0 to 3 inclusive; and
m and o are integers from 0 to 2 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein n is 1.
78
CA 02613141 2007-12-20
WO 2007/008548 AttPCT/US2006/026272)'014
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein Ri is I.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R2 is alkynylene.
In a further embodiment, a sirtuin-activating compound is a compound of
forinula 52 and the attendant definitions wherein m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R3 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R4 is C(O)OEt.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein o is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein p is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein L1 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein L3 is O.
In a furtlier embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH and n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CHaCH2OH, n is 1, and RI
is
I.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH, n is 1, RI is
I,
and Ra is alkynylene.
79
CA 02613141 2007-12-20
Attome -- 4
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CHaCHaOH, n is 1, R, is
1, R2
is alkynylene, and m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CHaOH, n is 1, R, is
1, R2
is alkynylene, m is 1, and R3 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CHaOH, n is 1, Ri is
I, R2
is alkynylene, m is 1, R3 is OH, and R4 is C(O)OEt.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH, n is 1, Rl is
I, R2
is alkynylene, m is 1, R3 is OH, R4 is C(O)OEt, and o is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH, n is 1, R, is
I, R2
is alkynylene, m is 1, R3 is OH, R4 is C(O)OEt, o is 1, and R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compouiid of
formula 52 and the attendant definitions wherein R is CHzCHZOH, n is 1, Rl is
I, R2
is alkynylene, m is 1, R3 is OH, R4 is C(O)OEt, o is 1, R5 is OH, and p is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH, n is 1, Rl is
I, R2
is alkynylene, m is 1, R3 is OH, R4 is C(O)OEt, o is 1, R5 is OH, p is 0, and
Lz is
NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH, n is 1, Rl is
I, R2
is alkynylene, m is 1, R3 is OH, R4 is C(O)OEt, o is 1, RS is OH, p is 0, L1
is NH,
and L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 52 and the attendant definitions wherein R is CH2CH2OH, n is 1, Ri is
I, R2
is alkynylene, m is 1, R3 is OH, R4 is C(O)OEt, o is 1, R5 is OH, p is 0, Ll
is NH, L2
is O, and L3 is O.
In another embodiment, a sirtuin-activating compound is a compound of
formula 53:
CA 02613141 2007-12-20
WO 2007/008548 AttornPCT/US2006/02627214
R2y O
R3
L4I
L3 N
O ~nR4
L L2
R R~-1'N R5
53
wherein, independently for each occurrence:
R, Rl, R2, R3, R4, and R5 are H, hydroxy, amino, cyano, halide, alkoxy, ether,
ester, amido, ketone, carboxylic acid, nitro, or a substituted or
unsubstituted alkyl,
aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
La, L2, L3, and L4 are 0, NR6, or S;
R6 is and H, or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
n is an integer from 0 to 5 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is 0-t-butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein Rl is t-butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R2 is O-t-butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R3 is t-butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R4 is C(O)OMe.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R5 is C(O)OMe.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein L1 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein L2 is O.
81
CA 02613141 2007-12-20 Attomey DocketNo.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein L3 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein L4 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein n is 1.
In a filrther embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is 0-t-butyl and Rl is t-
butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is O-t-butyl, RI is t-
butyl, and R2
is 0-t-butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is 0-t-butyl, RI is t-
butyl, R2 is
0-t-butyl, and R3 is t-butyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is 0-t-butyl, RI is t-
butyl, R2 is
0-t-butyl, R3 is t-butyl, and R4 is C(O)OMe.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is 0-t-butyl, RI is t-
butyl, R2 is
0-t-butyl, R3 is t-butyl, R4 is C(O)OMe, and R5 is C(O)OMe.
In a further embodiment, a sirtuin-activating compound is a compound of
formula53 and the attendant definitions wherein R is O-t-butyl, Rl is t-butyl,
R2 is
0-t-butyl, R3 is t-butyl, R4 is C(O)OMe, R5 is C(O)OMe, and LI is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is 0-t-butyl, RI is t-
butyl, R2 is
0-t-butyl, R3 is t-butyl, R4 is C(O)OMe, R5 is C(O)OMe, Li is NH, and L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is 0-t-butyl, RI is t-
butyl, R2 is
O-t-butyl, R3 is t-butyl, R4 is C(O)OMe, R5 is C(O)OMe, LI is NH, L2 is 0, and
L3
is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 53 and the attendant definitions wherein R is O-t-butyl, RI is t-
butyl, R2 is
82
CA 02613141 2007-12-20
Attoi
WO 2007/008548 PCT/US2006/026272
O-t-butyl, R3 is t-butyl, R.4 is C(O)OMe, R5 is C(O)OMe, L1 is NH, L2 is 0, L3
is 0,
and Ld is NH.
In a furtlier embodiment, a sirtuin-activating compound is a colnpound of
formula 53 and the attendant definitions wherein R is O-t-butyl, Rl is t-
butyl, R2 is
O-t-butyl, R3 is t-butyl, R4 is C(O)OMe, R5 is C(O)OMe, L, is NH, L2 is 0, L3
is 0,
L~isNH,andnisl.
In another embodiment, a sirtuin-activating compound is a compound of
formula 54:
(R2)m
Rl N
" ~
/N R R3 (R4)0
R 7 R6~
R
5
54
wherein, independently for each occurrence:
R and Rl are H or a substituted or unsubstituted alkyl, aryl, aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R2, R4, and R5 are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R3, R6, and R7 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester,
amido, ketone, carboxylic acid, nitro, or a substituted or unsubstituted
alkyl, aryl,
aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
L is O, NR, or S;
n and o are integers from 0 to 4 inclusive; and
m is an integer from 0 to 3 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein Rl is ethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein m is 0.
83
CA 02613141 2007-12-20
WO 2007/008548 AttokT/US2006/026272 014
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R3 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein o is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R5 is Cl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R6 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R7 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein L is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl and RI is ethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, RI is ethyl, and
m is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, Rl is ethyl, m is
0, and
R3 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, Rl is ethyl, m is
0, R3 is
H, and o is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, RI is ethyl, m is
0, R3 is
H,ois0,andR5isC1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, Rl is ethyl, m is
0, R3 is
H, o is 0, R5 is Cl, and R6 is H.
84
CA 02613141 2007-12-20
WO 2007/008548 Attorn~PCT/US2006/026272 4
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, R, is etliyl, m
is 0, R3 is
H, o is 0, RS is Cl, R6 is H, and R7 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, Ri is ethyl, m is
0, R3 is
H, o is 0, R5 is Cl, R6 is H, R7 is methyl, and L is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 54 and the attendant definitions wherein R is ethyl, Rl is ethyl, m is
0, R3 is
H, o is 0, R5 is Cl, R6 is H, R7 is methyl, L is NH, and n is 1.
In another embodiment, a sirtuin-activating compound is a compound of
formula 55:
Li
Ri
i i2 R2
N
R R3
R4 N
N~L3
R5
L4
wherein, independently for each occurrence:
15 R, Rl, R4, and R5 are H or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
R2 and R3 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
20 LI, L2, L3, and L4 are 0, NR, or S.
In a further einbodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein RI is H.
25 In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R2 is OEt.
CA 02613141 2007-12-20
Attornc
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R3 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein Ra is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R5 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein Ll is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein Lz is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein L3 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein L4 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H and RI is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H, R, is H, and R2 is
OEt.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H, RI is H, R2 is OEt,
and R3
is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wlierein R is H, RI is H, R2 is OEt,
R3 is
methyl, and R4 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H, Rl is H, Rz is OEt,
R3 is
methyl, R4 is H, and R5 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H, Rl is H, R2 is OEt,
R3 is
methyl, R4 is H, R5 is H, and Li is S.
86
CA 02613141 2007-12-20 Attomey DocketNo.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H, R, is H, R2 is OEt,
R3 is
methyl, R4 is H, R5 is H, L, is S, and L2 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H, RI is H, R2 is OEt,
R3 is
methyl, R4 is H, R5 is H, Li is S, L2 is NH, and L3 is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 55 and the attendant definitions wherein R is H, R, is H, R2 is OEt,
R3 is
methyl, R4 is H, R5 is H, Li is S, L2 is NH, L3 is NH, and L4 is S.
In another embodiment, a sirtuin-activating compound is a compound of
formula 56:
Li
N L2 ~ _ ~R1)m
y
L3
56
wherein, independently for each occurrence:
R and RI are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Ll, L2, and L3 are 0, NR2, or S;
R2 is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
n is an integer from 0 to 4 inclusive; and
m is an integer from 0 to 5 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein n is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein m is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein LI is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein L2 is S.
87
CA 02613141 2007-12-20
WO 2007/008548 AttornPCT/US2006/02627214
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein L3 is S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein m is 0 and n is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein m is 0, n is 0, and L, is NH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein m is 0, n is 0, Lt is NH, and
L2 is
S.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 56 and the attendant definitions wherein m is 0, n is 0, Ll is NH, L2
is S,
and L3 is S.
In another embodiment, a sirtuin-activating compound is a compound of
formula 57:
(R1)ml (R2)0
(R) A
n (R3)p
57
wherein, independently for each occurrence:
R, Rl, R2, and R3 are hydroxy, amino, cyano, halide, alkoxy, ether, ester,
amido, ketone, carboxylic acid, nitro, or a substituted or unsubstituted
alkyl, aryl,
aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
A is alkylene, alkenylene, or alkynylene;
n is an integer from 0 to 8 inclusive;
m is an integer from 0 to 3 inclusive;
o is an integer from 0 to 6 inclusive; and
p is an integer from 0 to 4 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein R is OH or methyl.
88
CA 02613141 2007-12-20
WO 2007/008548 AttOrnPCT/US2006/02627214
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein R, is methyl.
In a further embodimeht, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein o is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein R2 is C(O)CH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein p is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein R3 is COaH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein A is alkenylene.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2 and R is OH or methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2, R is OH or methyl,
and m is
1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2, R is OH or methyl, m
is 1,
and Rl is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2, R is OH or methyl, m
is 1,
RI is methyl, and o is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2, R is OH or methyl, m
is 1,
Rl is methyl, o is 1, and R2 is C(O)CH3.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2, R is OH or methyl, m
is 1,
RI is methyl, o is 1, R2 is C(O)CH3, and p is 2.
89
CA 02613141 2007-12-20
Attomi 14
WO 2007/008548 PCT/US2006/026272
IIn a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2, R is OH or methyl, m
is 1,
RI is methyl, o is 1, R2 is C(O)CH3, p is 2, and R3 is CO2H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 57 and the attendant definitions wherein n is 2, R is OH or methyl, m
is 1,
R, is methyl, o is 1, R2 is C(O)CH3, p is 2, R3 is COaH, and A is alkenylene.
In another embodiment, a sirtuin-activating compound is a compound of
formula 58:
O
LZ
RZ R3
R R
1
R4
Ll R
5
R9 ~L3
R6
R$
R7
58
wherein, independently for each occurrence:
R, Ri, R2, R3, R4, R5, R6, R7, R8, and R9 are liydroxy, amino, cyano, halide,
alkoxy, ether, ester, amido, ketone, carboxylic acid, nitro, or a substituted
or
unsubstituted alkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl,
heteroaryl, or
heteroaralkyl;
Ll, L2, and L3 are 0, NRIO, or S; and
Rio is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein Rl is CH2OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R2 is OH.
CA 02613141 2007-12-20 Attorne~ '"' *Y... amm nii - ,,,~
WO 2007/008548 PCT/US2006/026272
tr= q.nõ n , ,,.e ,.,,.. õ.a. ..rv . n.... ,o,n ,.... .,.....
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R3 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R4 is OH.
In a fiirther embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R6 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R7 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R8 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R9 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein L, is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein L3 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH and Rl is CH2OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CH2OH, and R2
is
OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, R, is CH2OH, R2 is
OH,
and R3 is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CHzOH, R2 is
OH,
R3 is methyl, and R4 is OH.
91
CA 02613141 2007-12-20
Att
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, R1 is CHaOH, R2 is
OH,
R3 is methyl, R4 is OH, and R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Ri is CHaOH, RZ is
OH,
R3 is methyl, R4 is OH, RS is OH, and R6 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CH2OH, R2 is
OH,
R3 is methyl, R4 is OH, R5 is OH, R6 is OH, and R7 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CH2OH, R2 is
OH,
R3 is methyl, R4 is OH, R5 is OH, R6 is OH, R7 is OH, and R$ is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CH2OH, R2 is
OH,
R3 is methyl, R4 is OH, R5 is OH, R6 is OH, R7 is OH, R8 is OH, and R9 is
methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CH2OH, R2 is
OH,
R3 is methyl, R4 is OH, R5 is OH, R6 is OH, R7 is OH, R8 is OH, R9 is methyl,
and
Ll is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CH2OH, R2 is
OH,
R3 is methyl, R4 is OH, R5 is OH, R6 is OH, R7 is OH, R8 is OH, R9 is methyl,
L1 is
0, and L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 58 and the attendant definitions wherein R is OH, Rl is CH2OH, R2 is
OH,
R3 is methyl, R4 is OH, R5 is OH, R6 is OH, R7 is OH, R8 is OH, R9 is methyl,
Ll is
0, L2 is 0, and L3 is O.
In another embodiment, a sirtuin-activating compound is a compound of
formula 59:
R,N,~ /L' ~NR2
\'n~ '/m i
R~ Ra
59
92
CA 02613141 2007-12-20
WO 2007/008548 AttcpCT/US2006/0262721-014
wherein, independently for each occurrence:
R, RI, R2, and R3 are H or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
L is 0, NR, S, or Se; and
n and m are integers from 0 to 5 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein RI is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R3 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein L is Se.
In a furtlier embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R is H and Ri is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R is H, Rl is H, and R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 aiid the attendant definitions wherein R is H, Rl is H, R2 is H,
and R3 is
H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R is H, R, is H, R2 is H, R3
is H,
and L 'zs Se.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R is H, Rl is H, R2 is H, R3
is H, L
isSe,andnis 1.
93
CA 02613141 2007-12-20
Attorn 14
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 59 and the attendant definitions wherein R is H, R, is H, R2is H, R3
is H, L
is Se, n is l, and m is 1.
In another embodiment, a sirtuin-activating compound is a compound of
formula 60:
~ L
(R) n ~
Rl
( m R
2
wherein, independently for each occurrence:
R is hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido, ketone,
10 carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
Rl and R2 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
15 L is 0, NR3, S, or SOz;
R3 is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
n is an integer from 0 to 4 inclusive; and
m is an integer from 1 to 5 inclusive.
20 In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein n is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein R is Cl.
In a further embodiment, a sirtuin-activating compound is a compound of
25 formula 60 and the attendant definitions wherein Rl is NH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein R2 is COZH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein L is SOa.
94
CA 02613141 2007-12-20
Attorni
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
forinula 60 and the attendant definitions wherein m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein n is 1 and R is Cl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein n is 1, R is Cl, and R, is
NHz.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein n is 1, R is Cl, R, is NH2,
and R2 is
CO2H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein n is 1, R is Cl, Rl is NHz,
R2 is
COzH, and L is SO2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 60 and the attendant definitions wherein n is 1, R is Cl, Ri is NH2,
R2 is
CO2H, L is SO2, and m is 1.
In another embodiment, a sirtuin-activating compound is a compound of
formula 61:
R, / (R3)
\ ~
(R)
"' / R2
61
wherein, independently for each occurrence:
R, Rl, R2, and R3 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester,
amido, ketone, carboxylic acid, nitro, or a substituted or unsubstituted
alkyl, aryl,
aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl;
n and m are integers from 0 to 5 inclusive.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein n is 2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein R is 3-hydroxy and 5-hydroxy.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein Rt is H.
CA 02613141 2007-12-20
WO 2007/008548 AttorrPCT/US2006/02627214
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein R2 is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein m is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein in is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein R3 is 4-hydroxy.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein R3 is 4-methoxy.
In a further embodiment, a sirtuin-activatihg compound is a compound of
formula 61 and the attendant definitions wherein n is 2 and R is 3-hydroxy and
5-
hydroxy.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein n is 2, R is 3-hydroxy and 5-
hydroxy, and Rl is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein n is 2, R is 3-hydroxy and 5-
hydroxy, Rl is H, and Rz is H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein n is 2, R is 3-hydroxy and 5-
hydroxy, RI is H, R2 is H, and m is 0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein n is 2, R is 3-hydroxy and 5-
hydroxy, R, is H, R2 is H, and m is 1.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein n is 2, R is 3-hydroxy and 5-
hydroxy, Rl is H, R2 is H, m is 1, and R3 is 4-hydroxy.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 61 and the attendant definitions wherein n is 2, R is 3-hydroxy and 5-
hydroxy, Ri is H, R2 is H, m is 1, and R3 is 4-methoxy.
96
CA 02613141 2007-12-20 Attom- n,,,.te '" . crWr_ourn 014
WO 2007/008548 PCT/US2006/026272
In another embodiment, a sirtuin-activating compound is a compound of
formula 62:
R~
R 0
~ ~
I ~ I ' R2
R6 L
R5
R4 R3
62
wherein, independently for each occurrence:
R, Rl, R2, R3, R4, R5, and R6 are H, hydroxy, amino, oyano, halide, alkoxy,
ether, ester, amido, ketone, carboxylic acid, nitro, or a substituted or
unsubstituted
alkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or
heteroaralkyl;
L is 0, NR7, or S; and
R7 is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
for>.nula 62 and the attendant definitions wherein RI is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R2 is CH2OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R3 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R4 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R6 is CH2OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein L is O.
97
CA 02613141 2007-12-20
WO 2007/008548 AttomPCT/US2006/02627214
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH and Ri is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH, R, is OH, and R2 is
CHzOH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH, R1 is OH, R2 is
CH2OH,
and R3 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH, Rl is OH, R2 is
CH2OH,
R3 is OH, and R4 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH, RI is OH, R2 is
CH2OH,
R3 is OH, R4 is OH, and R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH, Rl is OH, R2 is
CH2OH,
R3 is OH, R4 is OH, R5 is OH, and R6 is CH2OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 62 and the attendant definitions wherein R is OH, R, is OH, Rz is
CH2OH,
R3 is OH, R4 is OH, R5 is OH, R6 is CH2OH, and L is O.
In another embodiment, a sirtuin-activating compound is a compound of
formula 63:
R
O ~
~ N'R
I ,
NN
R2
63
wherein, independently for each occurrence:
R, RI, and R2 are H, hydroxy, amino, cyano, halide, alkoxy, ether, ester,
amido, ketone, carboxylic acid, nitro, or a substituted or unsubstituted
alkyl, aryl,
aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl.
98
CA 02613141 2007-12-20 Attomey DocketNo.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
In a further elnbodiment, a sirtuin-activating compound is a compound of
formula 63 and the attendant definitions wherein R is COZH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 63 and the attendant definitions wherein Rl is ethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 63 and the attendant definitions wherein R2 is N-1-pyrrolidine.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 63 and the attendant definitions wherein R is CO2H and Rl is ethyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 63 and the attendant definitions wherein R is CO2H and R2 is N-1-
pyrrolidine.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 63 and the attendant definitions wherein R, is ethyl and R2 is N-1-
pyrrolidine.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 63 and the attendant definitions wherein R is CO2H, Rl is ethyl, and
R2 is
N-1-pyrrolidine.
In another embodiment, a sirtuin-activating compound is a compound of
formula 64:
R L1. R, R2
R3
cc4
R7 L3 R6 5 L2 64
wherein, independently for each occurrence:
R, Ri, R2, R3, R4, R5, R6, and R7 are H, hydroxy, amino, cyano, halide,
alkoxy, ether, ester, amido, ketone, carboxylic acid, nitro, or a substituted
or
unsubstituted alkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl,
heteroaryl, or
heteroaralkyl;
Ll, L2, and L3 are CH2, 0, NR8, or S; and
R$ is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl.
99
CA 02613141 2007-12-20
WO 2007/008548 AttornPCT/US2006/02627214
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wlierein R is Cl.
In a furtlier embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H.
In a fiirther embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R, is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R2 is N(Me)2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R3 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R4 is C(O)NH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R6 is OH.
In a further embodiment a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R7 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein L, is CHa.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein L3 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl and Rz is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, R, is OH, and R2 is
N(Me)2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Rl is OH, R2 is
N(Me)2,
and R3 is OH.
100
CA 02613141 2007-12-20 Attorney DocketNo.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Ri is OH, R2 is
N(Me)2, R3
is OH, and R4 is C(O)NH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Rl is OH, Ra is
N(Me)2, R3
is OH, R4 is C(O)NHz, and RS is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Rl is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, and Rb is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Rl is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, Rc, is OH, and R7 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Ri is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, R6 is OH, R7 is OH, and Ll is CH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Rl is OH, Rz is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, R6 is OH, R7 is OH, LI is CH2, and L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is Cl, Rl is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, R6 is OH, R7 is OH, Ll is CH2, L2 is 0, and L3
is
0.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H and Rl is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, Rl is OH, and R2 is
N(Me)2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, Rl is OH, Ra is
N(Me)2,
and R3 is OH.
101
CA 02613141 2007-12-20 Attomey DocketNo.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, Rl is OH, R2 is
N(Me)2, R3
is OH, and R4 is C(O)NH2.
In a further embodiment, a sirtuin-activating compound is a compourid of
formula 64 and the attendant definitions wherein R is H, Rl is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, and R5 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, RI is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, and R6 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, RI is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, R6 is OH, and R7 is OH.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, R, is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, R6 is OH, R7 is OH, and LI is CH2.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, RI is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, R6 is OH, R7 is OH, LI is CH2, and L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 64 and the attendant definitions wherein R is H, RI is OH, R2 is
N(Me)2, R3
is OH, R4 is C(O)NH2, R5 is OH, R6 is OH, R7 is OH, L, is CH2, L2 is 0, and L3
is
0.
In another embodim.ent, a sirtuin-activating compound is a compound of
formula 65:
R.N~ ~ ~R1
1
N N
IR3 R2
~2
wherein, independently for each occurrence:
R is H or a substituted or unsubstituted alkyl, aryl, aralkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, or heteroaralkyl;
102
CA 02613141 2007-12-20
Attorn.... ,,..,.,....,.,,. . o.nT n.a,r% n14
WO 2007/008548 PCT/US2006/026272
Rl, R2, and R3 are hydroxy, amino, cyano, halide, alkoxy, ether, ester, amido,
ketone, carboxylic acid, nitro, or a substituted or unsubstituted alkyl, aryl,
aralkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroaralkyl; and
L1 and L2 are 0, NR, or S.
In a further einbodiment, a sirtuin-activating compound is a compound of
forlnula 65 and the attendant definitions wherein R is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein Rl is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein R2 is CO2H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein R3 is F.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein Ll is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein L2 is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein R is methyl and Rl is methyl.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein R is methyl, Rl is methyl,
and R2 is
CO2H.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein R is methyl, Rl is methyl, R2
is
CO2H, and R3 is F.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein R is methyl, RI is methyl, R2
is
CO2H, R3 is F, and Ll is O.
In a further embodiment, a sirtuin-activating compound is a compound of
formula 65 and the attendant definitions wherein R is methyl, Rl is methyl, R2
is
CO2H, R3 is F, L1 is 0, and L2 is O.
Exemplary activating compounds are those listed in the appended Tables
having a ratio to control rate of more than one. A preferred compound of
formula 8
103
CA 02613141 2007-12-20
Attorn - - l4
WO 2007/008548 PCT/US2006/026272
is Dipyridamole; a preferred compound of formula 12 is Hinokitiol; a preferred
compound of formula 13 is L-(+)-Ergothioneine; a preferred compound of formula
19 is Caffeic Acid Phenol Ester; a preferred compound of formula 20 is MCI-186
and a preferred compound of formula 21 is HBED (Supplementary Table 6).
Activating compounds may also be oxidized forins of the compounds of Table 21.
Also included are pharmaceutically acceptable addition salts and complexes
of the compounds of formulas 1-25, 30, 32-65, and 69-88. In cases wherein the
compounds may have one or more chiral centers, unless specified, the compounds
contemplated herein may be a single stereoisomer or racemic mixtures of
stereoisomers.
In one embodiment, a sirtuin-activating compound is a stilbene, chalcone, or
flavone compound represented by formula 7:
R'2
R Rf1 R13
,
Ra
RZ M Ra R 4
( / ( R'5
R3 R5
R4 O n
7
wherein, independently for each occurrence,
M is absent or 0;
RI, R2, R3, R4, R5, R' I, R'2, R'3, R'4, and R'5 represent H, alkyl, aryl,
heteroaryl, aralkyl, alkaryl, heteroaralkyl, halide, NO2, SR, OR, N(R)2, or
carboxyl;
Ra represents H or the two instances of Ra form a bond;
R represents H, alkyl, or aryl; and
nis0orl.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 7 and the attendant definitions, wherein n is 0. In a
further
embodiment, a sirtuin-activating compound is a compound represented by formula
7
and the attendant definitions, wherein n is 1. In a further embodiment, a
sirtuin-
activating compound is a compound represented by formula 7 and the attendant
104
CA 02613141 2007-12-20
Attorne" ~~~' ~"'~ ' emT_nwn_n14
WO 2007/008548 PCT/US2006/026272
definitions, wherein M is absent. In a further embodiment, a sirtuin-
activating
compound is a compound represented by formula 7 and the attendant definitions,
wherein M is O. In a further embodiment, a sirtuin-activating compound is a
compound represented by formula 7 and the attendant definitions, wherein Ra is
H.
In a further embodiment, a sirtuin-activating compound is a compound
represented
by formula 7 and the attendant definitions, wherein M is 0 and the two R,,
form a
bond. In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 7 and the attendant definitions, wherein R5 is H. In a
further
embodiment, a sirtuin-activating compound is a compound represented by formula
7
and the attendant definitions, wherein R5 is OH. In a further embodiment, a
sirtuin-
activating compound is a compound represented by formula 7 and the attendant
definitions, wherein Rl, R3, and R'3 are OH. In a further embodiment, a
sirtuin-
activating compound is a compound represented by formula 7 and the attendant
definitions, wherein R2, R4, R'2, and R'3 are OH. In a further embodiment, a
sirtuin-
activating compound is a compound represented by formula 7 and the attendant
definitions, wherein R2, R'2, and R'3 are OH.
In a further embodiment, a sirtuin-activating compound is a compound
represented by formula 7 and the attendant definitions, wherein n is 0; M is
absent;
Ra is H; R5 is H; Rl, R3, and R'3 are OH; and R2, R4, R'l, R'2, R'4, and R'5
are H. In
a further embodiment, a sirtuin-activating compound is a compound represented
by
formula 7 and the attendant definitions, wherein n is 1; M is absent; Ra is H;
R5 is H;
R2, R4, R'2, and R'3 are OH; and Rl, R3, R'1, R'4, and R'5 are H. In a further
embodiment, a sirtuin-activating compound is a compound represented by formula
7
and the attendant definitions, wherein n is 1; M is 0; the two Ra form a bond;
R5 is
OH; RZ, R'2, and R'3 are OH; and Ri, R3, R4, R'1, R'4, and R'5 are H.
In another embodiment, exemplary sirtuin-activating compounds are
isonicotinamide analogs, such as, for example, the isonicotinamide analogs
described in U.S. Patent Nos. 5,985,848; 6,066,722; 6,228,847; 6,492,347;
6,803,455; and U.S. Patent Publication Nos. 2001/0019823; 2002/0061898;
2002/0132783; 2003/0149261; 2003/0229033; 2003/0096830; 2004/0053944;
2004/0110772; and 2004/0181063, the disclosures of which are hereby
incorporated
by reference in their entirety. In an exemplary emobidment, sirtuin-activating
105
CA 02613141 2007-12-20
Atton
WO 2007/008548 PCT/US2006/026272
compounds may be an isonicotinamide analog having any of formulas 69-72 below.
In one embodiment, a sirtuin-activating compound is an isonicotinamide analog
compound of formula 69:
H H
A
O
D H
H B
OH C
69
Wherein A is a nitrogen-, oxygen-, or sulfur-linked aryl, alkyl, cyclic, or
heterocyclic group. The A moieties thus described, optionally have leaving
group
characteristics. In embodiments encompassed herein, A is further substituted
with an
electron contributing moiety. B and C are both hydrogen, or one of B or C is a
halogen, amino, or thiol group and the other of B or C is hydrogen; and D is a
primary alcohol, a hydrogen, or an oxygen, nitrogen, carbon, or sulfur linked
to
phosphate, a phosphoryl group, a pyrophosphoryl group, or adenosine
monophosphate through a phosphodiester or carbon-, nitrogen-, or sulfur-
substituted
phosphodiester bridge, or to adenosine diphosphate through a phosphodiester or
carbon-, nitrogen-, or sulfur-substituted pyrophosphodiester bridge.
In one example, A is a substituted N-linked aryl or heterocyclic group, an 0-
linked aryl or heterocyclic group having the formula -0-Y, or an S-linked aryl
or
heterocyclic group having the formula -0-Y; both B and C are hydrogen, or one
of
B or C is a halogen, amino, or thiol group and the other of B or C is
hydrogen; and
D is a primary alcohol or hydrogen. Nonlimiting preferred examples of A are
set
forth below, where each R is H or an electron-contributing moiety and Z is an
alkyl,
aryl, hydroxyl, OZ' where Z' is an alkyl or aryl, amino, NHZ' where Z' is an
alkyl or
aryl, or NHZ'Z" where Z' and Z" are independently an alkyl or aryl.
Examples of A include i-xiv below:
106
CA 02613141 2007-12-20
Attomi ~ ~ - 14
WO 2007/008548 PCT/US2006/026272
R ZHN R 0 R ZHN
R R R R
HN
O N O
~vinr winr iv Inr ,MIII
I I i
iii iv
NH2 ' NH2 R R
R
O 0 R R N~
HN N-N
ri,nn niõr ~'~ innr
v vi vii viii
H2N R
NHZ X
HO N /
R / ~\O N
N I
\
N
\ N-N
~r N ,nnJI
~~ ~ nrir~n
ix x xi xii
S/Y
.rvtiv' rvvtr
I
xiii xiv
where Y = a group consistent with leaving group function.
Examples of Y include, but are not limited to, xv-xxvii below:
107
CA 02613141 2007-12-20 Attornev nocketNo.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
NH2 NH2
NOZ
ZZ-' NO2
nnrv.iwv~ "L' _ \~
xv xvi xvii xviii
H2N H2N
02N X
HO / 5~~' 1
~ \ N/ I O N ]Jo
NO2 ~ \ nnnn
'V%AP
xix xx xxi xxii
H2N 0
N'~~N yaqxoHwq
~ ,iwAP n I n~ 'n~I'~ N
Irv i v nnN
I I
xxiii xxiv xxv xxvi xxvii
Wherein, for i-xxvii, X is halogen, thiol, or substituted thiol, amino or
substituted amino, oxygen or substituted oxygen, or aryl or alkyl groups or
heterocycles.
In certain embodiments, A is a substituted nicotinamide group (i above,
where Z is H), a substituted pyrazolo group (vii above), or a substituted 3-
carboxamid-imidazolo group (x above, where Z is H). Additionally, both B and C
may be hydrogen, or one of B or C is a halogen, amino, or thiol group and the
other
of B or C is hydrogen; and D is a primary alcohol or hydrogen.
In other embodiments, one of B or C may be halogen, amino, or thiol group
when the other of B or C is a hydrogen. Furthermore, D may be a hydrogen or an
108
CA 02613141 2007-12-20 Attorn- n~~4n! Un = CTT?T_bl717I1 n14
WO 2007/008548 PCT/US2006/026272
oxygen, nitrogen, carbon, or sulfur linked to phosphate, a phosphoryl group, a
pyrophosphoryl group, or adenosine monophosphate through a phosphodiester or
carbon-, nitrogen-, or sulfur-substituted phosphodiester bridge, or to
adenosine
diphosphate through a phosphodiester or carbon-, nitrogen-, or sulfur-
substituted
pyrophosphodiester bridge. Analogues of adenosine monophosphate or adenosine
diphosphate also can replace the adenosine monophosphate or adenosine
diphosphate groups.
In some embodiments, A has two or more electron contributing moieties.
In other embodiments, a sirtuin-activating compound is an isonicotinamide
analog compound of formulas 70, 71, or 72 below.
F
E NHZ
\
O
/
N
O
HO
OH
wherein Z is an alkyl, aryl, hydroxyl, OZ' where Z' is an alkyl or aryl,
amino, NHZ'
where Z' is an alkyl or aryl, or NHZ'Z" where Z' and Z" are independently an
alkyl
15 or aryl; E and F are independently H, CH3, OCH3, CH2CH3, NH2, OH, NHCOH,
NHCOCH3, N(CH3)2, C(CH3)2, an aryl or a C3-C10 alkyl, preferably provided
that,
when one of of E or F is H, the other of E or F is not H;
K
O / I
~N
N
0
HO
OH
20 71
109
CA 02613141 2007-12-20
Attome
WO 2007/008548 PCT/US2006/026272
wherein G, J or K is CONHZ, Z is an alkyl, aryl, hydroxyl, OZ' wllere Z' is an
alkyl
or aryl, amino, NHZ' where Z' is an alkyl or aryl, or NHZ'Z" where Z' and Z"
are
independently an alkyl or aryl, and the other two of G, J and K is
independently
CH3, OCH3, CH2CH3, NH2, OH, NHCOH, NHCOCH3;
ZHN
N O
0
HO
OH
72
wherein Z is an alkyl, aryl, hydroxyl, OZ' where Z' is an alkyl or aryl,
amino, NHZ'
where Z' is an alkyl or aryl, or NHZZ" where Z' and Z" are independently an
alkyl
or aryl; and L is CH3, OCH3, CH2CH3, NH2, OH, NHCOH, NHCOCH3.
In an exemplary embodiment, the compound is formula 70 above, wherein E
and F are independently H, CH3, OCH3, or OH, preferably provided that, when
one
of E or F is H, the other of E or F is not H.
In another exemplary embodiment, the compound is (3-1'-5-methyl-
nicotinamide-2'-deoxyribose, (3-D- l'-5-methyl-nico-tinamide-2'-
deoxyribofuranoside, (3-l'-4,5-dimethyl-nicotinamide-2'-de-oxyribose or P-D-1'-
4,5-
dimethyl-nicotinamide-2'-deoxyribofuranoside.
In yet another embodiment, the compound is (3-1'-5-methyl-nicotinamide-2'-
deoxyribose.
Without being bound to any particular mechanism, it is believed that the
electron-contributing moiety on A stabilizes the compounds of the invention
such
that they are less susceptible to hydrolysis from the rest of the compound.
This
improved chemical stability improves the value of the compound, since it is
available for action for longer periods of time in biological systems due to
resistance
to hydrolytic breakdown. The skilled artisan could envision many electron-
contributing moieties that would be expected to serve this stabilizing
function. Non-
110
CA 02613141 2007-12-20
WO 2007/008548 Attorn~PCT/US2006/02627214
limiting examples of suitable electron contributing moieties are methyl,
ethyl, 0-
methyl, amino, NMe2, hydroxyl, CMe3, aryl and alkyl groups. Preferably, the
electron-contributing moiety is a inethyl, ethyl, 0-methyl, amino group. In
the most
preferred embodiments, the electron-contributing moiety is a methyl group.
The compounds of formulas 69-72 are useful both in free form and in the
form of salts. The term "pharmaceutically acceptable salts" is intended to
apply to
non-toxic salts derived from inorganic or organic acids and includes, for
example,
salts derived from the following acids: hydrochloric, sulfuric, phosphoric,
acetic,
lactic, fumaric, succinic, tartaric, gluconic, citric, methanesulfonic, and p-
toluenesulfonic acids.
Also provided are compounds of formulas 69-72 that are the tautomers,
pharmaceutically-acceptable salts, esters, and pro-drugs of the inhibitor
compounds
disclosed herein.
The biological availability of the compounds of formulas 69-72 can be
enhanced by conversion into a pro-drug form. Such a pro-drug can have improved
lipophilicity relative to the unconverted compound, and this can result in
enhanced
membrane permeability. One particularly useful form of pro-drug is an ester
derivative. Its utility relies upon the action of one or more of the
ubiquitous
intracellular lipases to catalyse the hydrolysis of ester groups, to release
the active
compound at or near its site of action. In one form of pro-drug, one or more
hydroxy
groups in the compound can be 0-acylated, to make an acylate derivative.
Pro-drug forms of a 5-phosphate ester derivative of compounds of formulas
69-72 can also be made. These may be particularly useful, since the anionic
nature
of the 5-phosphate may limit its ability to cross cellular membranes.
Conveniently,
such a 5-phosphate derivative can be converted to an uncharged
bis(acyloxymethyl)
ester derivative. The utility of such a pro-drug relies upon the action of one
or more
of the ubiquitous intracellular lipases to catalyse the hydrolysis of ester
groups,
releasing a molecule of formaldehyde and a compound of the present invention
at or
near its site of action. Specific examples of the utility of, and general
methods for
making, such acyloxymethyl ester pro-drug forms of phosphorylated carbohydrate
derivatives have been described (Kang et al., 1998; Jiang et al., 1998; Li et
al., 1997;
Kruppa et al., 1997).
111
CA 02613141 2007-12-20 AttornevDocketNo.: SIRT-PWO-014
WO 2007/008548 PCT/US2006/026272
In another embodiment, exemplary sirtuin-activating compounds are 0-
acetyl-ADP-ribose analogs, including 2'-O-acetyl-ADP-ribose and 3'-O-acetyl-
ADP-
ribose, and analogs thereof. Exemplary O-acetyl-ADP-ribose analogs are
described,
for example, in U.S. Patent Publication Nos. 2004/0053944; 2002/0061898; and
2003/0149261, the disclosures of which are hereby incorporated by reference in
their entirety. In an exemplary emobidment, sirtuin-activating compounds may
be
an O-acetyl-ADP-ribose analog having any of formulas 73-76 below. In one
embodiment, a sirtuin-activating compound is an O-acetyl-ADP-ribose analog
compound of formula 73:
B
H
N
Z A\ N
CHz N
Y
w x
73
wherein:
A is selected from N, CH and CR, where R is selected from halogen,
optionally substituted alkyl, aralkyl and aryl, OH, NHzi NHR1, NR1R2 and SR3,
where Rl, RZ and R3 are each optionally substituted alkyl, aralkyl or aryl
groups;
B is selected from OH, NH2, NHR4, H and halogen, where R4 is an
optionally substituted alkyl, aralkyl or aryl group;
D is selected from OH, NH2, NHRS, H, halogen and SCH3, where RS is an
optionally substituted alkyl, aralkyl or aryl group;
X and Y are independently selected from H, OH and halogen, with the
proviso that when one of X and Y is hydroxy or halogen, the other is hydrogen;
Z is OH, or, when X is hydroxy, Z is selected from hydrogen, halogen,
hydroxy, SQ and OQ, where Q is an optionally substituted alkyl, aralkyl or
aryl
group; and
W is OH or H, with the proviso that when W is OH, then A is CR where R is
as defined above;
112
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
or a tautomer thereof; or a pharmaceutically acceptable salt thereof; or an
ester thereof; or a prodrug thereof.
In certain embodiments, when B is NHR4 and/or D is NHR5, then R4 and/or
R5 are C 1-C4 alkyl.
In other embodiments, when one or more halogens are present they are
chosen from chlorine and fluorine.
In another embodiment, when Z is SQ or OQ, Q is C1-C5 alkyl or phenyl.
In an exemplary embodiment, D is H, or when D is other than H, B is OH.
In another embodiment, B is OH, D is H, OH or NHz, X is OH or H, Y is H,
most preferably with Z as OH, H, or methylthio, especially OH.
In certain embodiments W is OH, Y is H, X is OH, and A is CR where R is
methyl or halogen, preferably fluorine.
In other embodiments, W is H, Y is H, X is OH and A is CH.
In other embodiments, a sirtuin-activating compound is an O-acetyl-ADP-
ribose analog compound of formula 74:
H
N E
z
1--1OHz H
G
Y
OH X
74
wherein A, X, Y, Z and R are defined for compounds of formula (73) where
first shown above; E is chosen from CO2H or a corresponding salt form, COaR,
CN,
CONH2, CONHR or CONR2; and G is chosen from NH2, NHCOR, NHCONHR or
NHCSNHR; or a tautomer thereof, or a pharmaceutically acceptable salt thereof,
or
an ester thereof, or a prodrug thereof.
In certain embodiments, E is CONH2 and G is NH2.
In other embodiments, E is CONH2, G is NH2, X is OH or H, is H, most
preferable with Z as OH, H or methylthio, especially OH.
Exemplary sirtuin-activating compounds include the following:
113
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
(1 S)-1,4-d ideoxy-l-C-(4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-
D-ribitol
(1 S)-1-C-(2-am ino-4-hydroxypyrrolo [3, 2-d] pyrimidin-7-yl)-1,4-dideoxy-1, 4-
imino-D-ribitol
(1R)-1-C-(4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-1,2,4-trideoxy-
D-erythro-pentitol
(1 S)-1-C-(4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-1,4,5-trideoxy-
D-ribitol
(1 S)-1,4-dideoxy-l-C-(4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-5-
methylthio-D-ribitol
(1 S)-1,4-dideoxy-l-C-(2,4-dihydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-
imino-D-ribitol
(1 R)-1-C-(2,4-dihydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-1,2,4-
trideoxy-D-erthro-pentitol
(1S)-1-C-(2,4-dihydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-1,4,5-
trideoxy-D-ribitol
(1 S)-1,4-dideoxy-l-C-(2,4-dihydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-
imino-5 -ethylthio-D-rib ito l
(1 R)-1-C-(2-arnino-4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-1,2,4-
trideoxy-D-erythro-pentitol
(1 S)-1-C-(2-amino-4-hydroxypyrro lo [3,2-d] pyrimidin-7-yl)-1,4-imino-1,4, 5 -
trideoxy-D-ribitol
(1 S)-1-C-(2-amino-4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-dideoxy-1,4-
imino-5 -methylthio-D-rib ito 1
(1S)-1,4-dideoxy-l-C-(7-hydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-imino-
D-ribitol
(1 R)-1-C-(7-hydroxypyrazolo [4, 3 -d] pyrimidin-3 -yl)- 1,4-imino- 1,2,4-
trideoxy-D-erythro-pentitol
(1 S)-1-C-(7-hydroxypyrazolo [4, 3 -d]pyrimidin-3 -yl)-1,4-imino-1,4, 5-
trideoxy-D-ribitol
(1 S)-1,4-dideoxy-l-C-(7-hydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-imino-
5 -ethylth io-D-rib ito l
114
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
(1 S)-1,4-dideoxy-l-C-(5, 7-dihydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-
imino-D-ribitol
(1 R)-1-C-(5,7-dihydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-irnino-1,2,4-
trideoxy-D-erythro-pentitol
(1S)-1-C-(5,7-dihydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-imino-1,4,5-
trideoxy-D-ribitol
(1 S)-1,4-dideoxy-l-C-(5, 7-dihydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-
imino-5 -methylthio-D-ribitol
(1 S)-1-C-(5-amino-7-hydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-dideoxy-
1,4-imino-D-ribitol
(1 R)-1-C-(S-amino-7-hydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-imino-
1,2,4-trideoxy-D-erythro-pentito 1
(1 S)-1-C-(5-amino-7-hydroxypyrazolo [4,3-d]pyrimidin-3 -yl)-1,4-imino-
1,4, 5-trideoxy-D-ribitol
(1 S)-1-C-(5-amino-7-hydroxypyrazolo[4,3-d]pyrimidin-3-yl)-1,4-dideoxy-
1,4-imino-5 -methylthio-D-ribitol
(1 S)-1-C-(3-amino-2-carboxamido-4-pyrroly)-1,4-dideoxy-1,4-imino-D-
ribitol.
(1 S)-1,4-dideoxy-l-C-(4-hydroxypyrrolo [3,2-d]pyrimidin-7-yl)-1,4-imino-
D-ribitol 5-phosphate
(1 S)-1-C-(2-amino-4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-D-
ribitol 5-phosphate
(1 S)-1-C-(3-amino-2-carboxamido-4-pyrrolyl)-1,4-dideoxy-1,4-imino-D-
ribitol
In yet other embodiments, sirtuin-activating compounds are O-acetyl-ADP-
ribose analog compounds of formula 75 and 76, their tautomers and
pharmaceutically acceptable salts.
115
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
OH
H
N
HO~ \ I \ N
CHa H
N N/
OH OH
OH
H
N
H0~ \ ' \ N
CH2 H
N N
NH2
OH OH
76
5 The biological availability of a compound of formula (73) or formula (74)
can be enhanced by conversion into a pro-drug form. Such a pro-drug can have
improved lipophilicity relative to the compound of formula (73) or formula
(74), and
this can result in enhanced membrane permeability. One particularly useful
form of
a pro-drug is an ester derivative. Its utility relies upon the action of one
or more of
10 the ubiquitous intracellular lipases to catalyse the hydrolysis of these
ester group(s),
to release the compound of formula (73) and formula (74) at or near its site
of
action.
In one form of a prodrug, one or more of the hydroxy groups in a compound
of formula (73) or formula (74) can be 0-acylated, to make, for example a 5-0-
15 butyrate or a 2,3-di-O-butyrate derivative.
Prodrug forms of 5-phosphate ester derivative of a compounds of formula
(73) or formula (74) can also be made and may be particularly useful, since
the
anionic nature of the 5-phosphate may limit its ability to cross cellular
membranes.
Conveniently, such a 5-phosphate derivative can be converted to an uncharged
20 bis(acyloxymethyl) ester derivative. The utility of such a pro-drug relies
upon the
action of one or more of the ubiquitous intracellular lipases to catalyse the
116
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
hydrolysis of these ester group(s), releasing a molecule of formaldehyde and
the
compound of formula (73) or formula (74) at or near its site of action.
In an exemplary embodiment, analogs of 2'-AADPR or 3'-AADPR that are
designed to have increased stability from esterase action through the use of
well-
known substitutes for ester oxygen atoms that are subject to esterase attack.
The
esterase-labile oxygen atoms in 2'-AADPR and 3'-AADPR would be understood to
be the ester oxygen linking the acetate group with the ribose, and the ester
oxygen
between the two phosphorus atoms. As is known in the art, substitution of
either or
both of these ester oxygen atoms with a CF2, a NH, or a S would be expected to
provide a 2'-AADPR or 3'-AADPR analog that is substantially more stable due to
increased resistance to esterase action.
Thus, in some embodiments, the invention is directed to analogs 2'-O-acetyl-
ADP-ribose or 3'-O-acetyl-ADP-ribose exhibiting increased stability in cells.
The
preferred analogs comprise a CF2, a NH, or a S instead of the acetyl ester
oxygen or
the oxygen between two phosphorus atoms. The most preferred substitute is CFa.
Replacement of the acetyl ester oxygen is particularly preferred. In other
preferred
embodiments, both the ester oxygen and the oxygen between the two phosphorus
atoms are independently substituted with a CF2, a NH, or a S.
Also included are pharmaceutically acceptable addition salts and complexes
of the sirtuin-activity compounds described herein. In cases wherein the
compounds
may have one or more chiral centers, unless specified, the compounds
contemplated
herein may be a single stereoisomer or racemic mixtures of stereoisomers.
In one embodiment, sirtuin modulators for use in the invention are
represented by Formula 77 or 78:
117
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
0
0 R304
R304 I
R3o5
R
305 \~I~
/ NR3o1R302
// NR301R302 / \I
l\~ ~ R306 N-- R303
R3o N= R303
R311 R311
OR307 X OR307
X
R312 R312
R314 R314
R309 R313 OR308 R309 R313 OR308
OR310 77 or OR310
78
or a pharmaceutically acceptable salt thereof, where:
R301 and R302 are independently -H, a substituted or unsubstituted alkyl
group, a substituted or unsubstituted alkenyl group, a substituted or
unsubstituted
alkynyl group, a substituted or unsubstituted non-aromatic heterocyclic group
or a
substituted or unsubstituted aryl group, or R3oi and R302 taken together form
a
substituted or unsubstituted non-aromatic heterocyclic group;
R303, R304, R305 and R306 are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
group, halogen, -OR, -CN, -CO2R, -OCOR, -OCO2R, -C(O)NRR', -OC(O)NRR',
-C(O)R, -COR, -SR, -OSO3H, -S(O)nR, -S(O)nOR, -S(O)nNRR', -NRR',
-NRC(O)OR', -NO2 and -NRC(O)R';
R307, R308 and R31o are independently selected from the group consisting of
-H, a substituted or unsubstituted alkyl group, a substituted or unsubstituted
aryl
group, -C(O)R, -C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR and -C(O)SR;
R309 is selected from the group consisting of -H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, a
substituted or
unsubstituted non-aromatic heterocyclic group, halogen, -OR, -CN, -CO2R, -
OCOR, -
OCOZR, -C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -SR, -OSO3H, -S(O)õR,
-S(O)õOR, -S(O)õNRR', -NRR', -NRC(O)OR' and -NRC(O)R';
118
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
R31 i, R312, R313 and R314 are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
group, halogen, -CN, -CO2R, -OCOR, -OCOaR, -C(O)NRR', -OC(O)NRR', -C(O)R,
-COR, -OSO3H, -S(O)õR, -S(O)õOR, -S(O)nNRR', -NRR', -NRC(O)OR', -NOa and
-NRC(O)R';
R and R' are independently -H, a substituted or unsubstituted alkyl group, a
substituted or unsubstituted aryl group or a substituted or unsubstituted non-
aromatic
heterocyclic group;
X is O or S; and
nislor2.
A group of suitable compounds encompassed by Formulas 77 and 78 is
represented by Structural Formulas 79 and 80:
R204 0 R204 0
R2a5 R2os
NR201R202 NR20IR202
I / I I
R206 N R203 R206 N R203
R217 R211
x OR207 X OR207
R212 R212
Rz~a R214
R209 R213 OR2oa R209 R213 OR20e
OR210 79 or OR210
S0
or a pharmaceutically acceptable salt thereof, where:
RZoj and R202 are independently -H, a substituted or unsubstituted alkyl
group, a substituted or unsubstituted alkenyl group, a substituted or
unsubstituted
alkynyl group, a substituted or unsubstituted non-aromatic heterocyclic group
or a
substituted or unsubstituted aryl group, or Rzol and R202 taken together form
a
substituted or unsubstituted non-aromatic heterocyclic group;
R203, R2o4,1Zzos and R206 are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
119
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
group, halogen, -OR, -CN, -CO2R, -OCOR, -OCO2R, -C(O)NRR', -OC(O)NRR',
-C(O)R, -COR, -SR, -OSO3H, -S(O)õR, -S(O)nOR, -S(O)õNRR', -NRR',
-NRC(O)OR', -NOz and -NRC(O)R';
R207, R208 and R21 are independently selected from the group consisting of
-H, a substituted or unsubstituted alkyl group, a substituted or unsubstituted
aryl
group, -C(O)R, -C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR and -C(O)SR;
R209 is selected from the group consisting of -H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, a
substituted or
unsubstituted non-aromatic heterocyclic group, halogen, -OR, -CN, -CO2R, -
OCOR,
-OCO2R, -C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -SR, -OSO3H, -S(O)õR,
-S(O)õOR, -S(O)õNRR', -NRR', -NRC(O)OR' and -NRC(O)R';
R21 t, R212, R213 and R214 are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
group, halogen, -CN, -CO2R, -OCOR, -OCO2R, -C(O)NRR', -OC(O)NRR',
-C(O)R, -COR, -OSO3H, -S(O)nR, -S(O)õOR, -S(O)nNRR', -NRR', -NRC(O)OR',
-NO2 and -NRC(O)R';
R and R' are independently -H, a substituted or unsubstituted alkyl group, a
substituted or unsubstituted aryl group or a substituted or unsubstituted non-
aromatic
heterocyclic group;
X is 0 or S, preferably 0; and
nislor2.
In a particular group of compounds represented by Formula 79 or 80, at least
one of R207, R208 and R210 is a substituted or unsubstituted alkyl group, a
substituted
or unsubstituted aryl group, -C(O)R, -C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR or -
C(O)SR. Typically, at least one of R207, R208 and R210 is -C(O)R or -C(O)OR.
More typically, at least one of R207, R208 and R210 is -C(O)R. In such
compounds, R
is preferably a substituted or unsubstituted alkyl, particularly an
unsubstituted alkyl
group such as methyl or ethyl.
In another particular group of compounds represented by Formula 79 or 80,
R204 is a halogen (e.g., fluorine, bromine, chlorine) or hydrogen (including a
deuterium and/or tritium isotope). Suitable compounds include those where at
least
120
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
one of R207, R20$ and R21o is a substituted or unsubstituted alkyl group, a
substituted
or unsubstituted aryl group, -C(O)R, -C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR or -
C(O)SR and R204 is a halogen or hydrogen.
Typically, for compounds represented by Formulas 79 and 80, R203-R206 are
-H. In addition, R209 and Ra1 1-R214 are typically -H. Particular compounds
represented by Formulas 79 and 80 are selected such that R203-R206, R2og and
R21 I -
R214 are all -H. For these compounds, R204, R207, R208 and R210 have the
values
described above.
R2ol and R202 are typically -H or a substituted or unsubstituted alkyl group,
more typically -H. In compounds having these values of R201 and R202, R203-
R206,
R209 and R211-R214 typically have the values described above.
In certain methods of the invention, at least one of R201-R214 is not -H when
X isO.
In certain methods of the invention, R206 is not -H or -NH2 when R2oi-R2o5
and R207-R214 are each -H.
In one embodiment, a sirtuin modulator is represented by Formula 81 or 82:
R4 O R4 Rs :IIIIIIII3NRIR2
I 1R2 R6 3 R11 R11
x OR7 x OR7
R12 R12
R14 R14
R9 R13 OR8 Rg R~3 OR8
OR10 81 or OR1a 82,
or a pharmaceutically acceptable salt thereof, wherein:
Rl and R2 are independently -H, a substituted or unsubstituted alkyl group, a
substituted or unsubstituted alkenyl group, a substituted or unsubstituted
alkynyl
group, a substituted or unsubstituted non-aromatic heterocyclic group or a
substituted or unsubstituted aryl group, or Ri and R2 taken together form a
121
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
substituted or unsubstituted non-aromatic heterocyclic group, provided that
when
one of Ri and R2 is -H, the other is not an alkyl group substituted by
-C(O)OCH2CH3;
R3, R4 and R5 are independently selected from the group consisting of-H, a
substituted or unsubstituted alkyl group, a substituted or unsubstituted aryl
group, a
substituted or unsubstituted non-aromatic heterocyclic group, halogen, -OR, -
CN,
-COaR, -OCOR, -OCOzR, -C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -SR,
-OSO3H, -S(O)nR, -S(O)nOR, -S(O)nNRR', -NRR', -NRC(O)OR', -NO2 and
-NRC(O)R';
R6 is selected from the group consisting of -H, a substituted or unsubstituted
alkyl group, a substituted or unsubstituted aryl group, a substituted or
unsubstituted
non-aromatic heterocyclic group, halogen, -OR, -CN, -CO2R, -OCOR, -OCOzR, -
C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -SR, -OSO3H, -S(O)nR, -S(O)nOR,
-S(O)nNRR', -NRC(O)OR', -NOz and -NRC(O)R';
R7, R8 and RIo are independently selected from the group consisting of -H, a
substituted or unsubstituted alkyl group, a substituted or unsubstituted aryl
group,
-C(O)R, -C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR and -C(O)SR;
R9 selected from the group consisting of -H, a substituted or unsubstituted
alkyl group, a substituted or unsubstituted aryl group, a substituted or
unsubstituted
non-aromatic heterocyclic group, halogen, -OR, -CN, -CO2R, -OCOR, -OCO2R, -
C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -SR, -OSO3H, -S(O)õR, -S(O)õOR,
-S(O)õNRR', -NRR', -NRC(O)OR' and -NRC(O)R';
R11, R12, R13 and R14 are independently selected from the group consisting of
-H, a substituted or unsubstituted alkyl group, a substituted or unsubstituted
aryl
group, a substituted or unsubstituted non-aromatic heterocyclic group,
halogen, -CN,
-CO2R, -OCOR, -OCO2R, -C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -OSO3H,
-S(O)nR, -S(O)nOR, -S(O)nNRR', -NRR', -NRC(O)OR', -NO2 and -NRC(O)R';
R and R' are independently H, a substituted or unsubstituted alkyl group, a
substituted or unsubstituted aryl group or a substituted or unsubstituted non-
aromatic
heterocyclic group;
X is 0 or S, preferably 0; and
n is 1 or 2,
122
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
provided that Ri-R14 are not each -H and that RI-R9 and Rl 1-R14 are not each
-H when Rio is -C(O)C6H5.
In certain embodiments, Rl is -H.
In certain embodiments, R7, R8 and RIo are independently -H, -C(O)R or
-C(O)OR, typically -H or -C(O)R such as -H or -C(O)CH3. In particular
embodiments, R, is -H and R7, R8 and Rio are independently -H, -C(O)R or
-C(O)OR.
In certain embodiments, R9 is -H. In particular embodiments, R9 is -H when
Rl is -H and/or R7, R8 and RIo are independently -H, -C(O)R or -C(O)OR.
. In certain embodiments, R2 is -H. In particular embodiments, R2 is -H when
Ry is -H, Rl is -H and/or R7, R8 and Rio are independently -H, -C(O)R or -
C(O)OR.
Typically, R2 is -H when R9 is -H, RI is -H and R7, Rg and RIo are
independently -
H, -C(O)R or -C(O)OR.
In certain embodiments, R4 is -H or a halogen, such as deuterium or fluorine.
In one embodiment, a sirtuin modulator is represented by Formula 83 or 84:
0
0 R104
i;04 R105
R105 \j
/~ NR1olRioz ~'NR,oRjo2
~ R1os N- R1o3
R1os N- N1o3
R111 R111
X OR107 x OR107
R112 R112
R114 R114
R1os R113 QR1o6 R1as R113 OR108
OR11o 83 or OR11o 84
or a pharmaceutically acceptable salt thereof, wherein:
RIo, and R102 are independently -H, a substituted or unsubstituted alkyl
group, a substituted or unsubstituted alkenyl group, a substituted or
unsubstituted
alkynyl group, a substituted or unsubstituted non-aromatic heterocyclic group
or a
substituted or unsubstituted aryl group, or Rlot and Rla2 taken together form
a
substituted or unsubstituted non-aromatic heterocyclic group;
123
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Rios, R104, R105 and R106 are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
group, halogen, -OR, -CN, -CO2R, -OCOR, -OCOaR, -C(O)NRR', -OC(O)NRR',
-C(O)R, -COR, -SR, -OSO3H, -S(O)nR, -S(O)nOR, -S(O)nNRR', -NRR',
-NRC(O)OR', -NO2 and -NRC(O)R';
R107 and Rlo$ are selected from the group consisting of -H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, -C(O)R,
-C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR and -C(O)SR, wherein at least one of R107
and R108 is a substituted or unsubstituted alkyl group, a substituted or
unsubstituted
aryl group, -C(O)R, -C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR or -C(O)SR;
Rlog is selected from the group consisting of-H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, a
substituted or
unsubstituted non-aromatic heterocyclic group, halogen, -OR, -CN, -CO2R, -
OCOR,
-OCO2R, -C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -SR, -OSO3H, -S(O)nR,
-S(O)nOR, -S(O)nNRR', -NRR', -NRC(O)OR' and -NRC(O)R';
R110 is selected from the group consisting of -H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, -C(O)R,
-C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR and -C(O)SR, provided that Rlto is not
-C(O)C6H5;
RI 11, Rl 12, R113 and R, 1~ are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
group, halogen, -CN, -CO2R, -OCOR, -OCO2R, -C(O)NRR', -OC(O)NRR',
-C(O)R, -COR, -OSO3H, -S(O)õR, -S(O)õOR, -S(O)õNRR', -NRR', -NRC(O)OR',
-NO2 and -NRC(O)R'; ,
R and R' are independently -H, a substituted or unsubstituted alkyl group, a
substituted or unsubstituted aryl group or a substituted or unsubstituted non-
aromatic
heterocyclic group;
X is O or S; and
nislor2.
124
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In another embodiment, a sirtuin modulator is represented by Formula 85 or
86:
R104 0 R104 0
R
R1o8 NR1o1R102 105
I NR1o1R1o2
I ~
R1os N R1o3 R1os N R1o3
IR111 R111
X OR1o7 X ORio7
R112 R112
R91A R114
R109 R113 OR108 R109 R113 oR108
OR110 85 or oR,10 86,
or a pharmaceutically acceptable salt thereof, where:
Rlol and R102 are independently -H, a substituted or unsubstituted alkyl
group, a substituted or unsubstituted alkenyl group, a substituted or
unsubstituted
alkynyl group, a substituted or unsubstituted non-aromatic heterocyclic group
or a
substituted or unsubstituted aryl group, or Rlol and R102 taken together form
a
substituted or unsubstituted non-aromatic heterocyclic group;
RI03, R104, Rio5 and R106 are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
group, halogen, -OR, -CN, -CO2R, -OCOR, -OCOaR, -C(O)NRR', -OC(O)NRR',
-C(O)R, -COR, -SR, -OSO3H, -S(O)õR, -S(O)õOR, -S(O)õNRR', -NRR',
-NRC(O)OR', -NO2 and -NRC(O)R';
R107and Rlo$ are selected from the group consisting of -H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, -C(O)R,
-C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR and -C(O)SR, wherein at least one of R107
and R108 is a substituted or unsubstituted alkyl group, a substituted or
unsubstituted
aryl group, -C(O)R, -C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR or -C(O)SR;
R109 is selected from the group consisting of-H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, a
substituted or
unsubstituted non-aromatic heterocyclic group, halogen, -OR, -CN, -CO2R, -
OCOR,
125
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
-OCO2R, -C(O)NRR', -OC(O)NRR', -C(O)R, -COR, -SR, -OSO3H, -S(O)õR,
-S(O)õOR, -S(O)õNRR', -NRR', -NRC(O)OR' and -NRC(O)R';
Rilo is selected from the group consisting of -H, a substituted or
unsubstituted alkyl group, a substituted or unsubstituted aryl group, -C(O)R,
-C(O)OR, -C(O)NHR, -C(S)R, -C(S)OR and -C(O)SR, provided that Rllo is not
-C(O)C6H5;
Rl 11, Rl 12, Ri 13 and Rt 14 are independently selected from the group
consisting of -H, a substituted or unsubstituted alkyl group, a substituted or
unsubstituted aryl group, a substituted or unsubstituted non-aromatic
heterocyclic
group, halogen, -CN, -COZR, -OCOR, -OCOzR, -C(O)NRR', -OC(O)NRR',
-C(O)R, -COR, -OSO3H, -S(O)nR, -S(O)nOR, -S(O)nNRR', -NRR', -NRC(O)OR',
-NOZ and -NRC(O)R';
R and R' are independently -H, a substituted or unsubstituted alkyl group, a
substituted or unsubstituted aryl group or a substituted or unsubstituted non-
aromatic
heterocyclic group;
X is 0 or S; and
nis1or2.
For compounds represented by Formulas 83-86, typically at least one of R107
and R10$ is -C(O)R, such as -C(O)CH3. In particular embodiments, R107, Rios
and
R, Io are independently -H or -C(O)R (e.g., -C(O)CH3).
In certain embodiments, such as when Rio7, Rios and RIIO have the values
described above, Rtol and RI02 are each -H.
In certain embodiments, Rlo9 is -H.
In certain embodiments, R103-R106 are each -H.
In certain embodiments, Rl l 1-R114 are each -H.
In particular embodiments, Rlo7, R108 and RI Io have the values described
above and Rio,-Rto6, Rlo9 and R111-R11a are each H.
In certain embodiments, R104 is -H or a halogen, typically deuterium or
fluorine. The remaining values are as described above.
For sirtuin modulators represented by Formula 87 or 88:
126
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Ra O Ra O
R5 R5
NRIR2 NR1R2
I I I
R6 N R3 R6 N R3
R11 R11
X OR7 x OR7
R12 5RI2
R1a R1a
R9 ORB Ry ORe
R13 R13
OR10 87 or OR10 88,
R4 in certain embodiments is -H (e.g., deuterium, tritium) or a halogen (e.g.,
fluorine, bromine, chlorine).
In embodiments of the invention where Rl-R6 can each be -H, they typically
are each -H. In embodiments of the invention where one of RI-Rb is not -H,
typically the remaining values are each -H and the non-H value is a
substituted or
unsubstituted alkyl group or a halogen (RI and R2 are typically a substituted
or
unsubstituted alkyl group).
In certain embodiments, R11-R14 are each -H. When R> >-R14 are each -H,
Ri-R6 typically have the values described above.
In certain embodiments, R9 is -H. When R9 is -H, typically R11-R14 are each
-H and RI-Rb have the values described above.
Specific examples of sirtuin modulators (e.g., sirtuin activators and sirtuin
inliibitors) are described in U.S. Patent Publication Nos. 2005/0136537 and
2005/0096256 and include, for example, the compounds shown in Figures 1-16.
Also included are pharmaceutically acceptable addition salts and complexes
of the sirtuin modulators described herein. In cases wherein the compounds may
have one or more chiral centers, unless specified, the compounds contemplated
herein may be a single stereoisomer or racemic mixtures of stereoisomers.
The compounds and salts thereof described herein also include their
corresponding hydrates (e.g., hemihydrate, monohydrate, dihydrate, trihydrate,
127
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
tetrahydrate) and solvates. Suitable solvents for preparation of solvates and
hydrates
can generally be selected by a skilled artisan.
The compounds and salts thereof can be present in amorphous or crystalline
(including co-crystalline and polymorph) forms.
Sirtuin modulating compounds also include the related secondary
metabolites, such as phosphate, sulfate, acyl (e.g., acetyl, fatty acid acyl)
and sugar
(e.g., glucurondate, glucose) derivatives (e.g., of hydroxyl groups),
particularly the
sulfate, acyl and sugar derivatives. In other words, substituent groups -OH
also
include -OS03' M+, where M+ is a suitable cation (preferably H+, NH4+ or an
alkali
metal ion such as Na+ or K) and sugars such as
OH
HO2C O 0~ O O~
~ ~--
HO\\\\\~ ,/i/OH He\', iOH
OH and OH
These groups are generally cleavable to -OH by hydrolysis or by metabolic
(e.g.,
enzymatic) cleavage.
In cases in which the sirtuin-activating compounds have unsaturated carbon-
carbon double bonds, both the cis (Z) and trans (E) isomers are contemplated
herein.
In cases wherein the compounds may exist in tautomeric forms, such as keto-
enol
0 OR'
tautomers, such as -"I and -1-L-1- , each tautomeric form is contemplated as
being included within the methods presented herein, whether existing in
equilibrium
or locked in one form by appropriate substitution with R. The meaning of any
substituent at any one occurrence is independent of its meaning, or any other
substituent's meaning, at any other occurrence.
Also included in the methods presented herein are prodrugs of the sirtuin-
activating compounds described herein. Prodrugs are considered to be any
covalently bonded carriers that release the active parent drug in vivo.
Analogs and derivatives of the sirtuin-activating compounds described herein
can also be used for activating a member of the sirtuin protein family. For
example,
128
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
derivatives or analogs may make the compounds more stable or improve their
ability
to traverse cell membranes or being phagocytosed or pinocytosed. Exemplary
derivatives include glycosylated derivatives, as described, e.g., in U.S.
Patent
6,361,815 for resveratrol. Other derivatives of resveratrol include cis- and
trans-
resveratrol and conjugates thereof with a saccharide, such as to forin a
glucoside
(see, e.g., U.S. Patent 6,414,037). Glucoside polydatin, referred to as piceid
or
resveratrol 3-O-beta-D-glucopyranoside, can also be used. Saccharides to which
compounds may be conjugated include glucose, galactose, maltose, lactose and
sucrose. Glycosylated stilbenes are further described in Regev-Shoshani et al.
Biochemical J. (published on 4/16/03 as BJ20030141). Other derivatives of
compounds described herein are esters, amides and prodrugs. Esters of
resveratrol
are described, e.g., in U.S. patent 6,572,882. Resveratrol and derivatives
thereof can
be prepared as described in the art, e.g., in U.S. patents 6,414,037;
6,361,815;
6,270,780; 6,572,882; and Brandolini et al. (2002) J. Agric. Food.
Chem.50:7407.
Derivatives of hydroxyflavones are described, e.g., in U.S. patent 4,591,600.
Resveratrol and other activating compounds can also be obtained commercially,
e.g.,
from Sigma.
In certain embodiments, if a sirtuin-activating compound occurs naturally, it
may be at least partially isolated from its natural environment prior to use.
For
example, a plant polyphenol may be isolated from a plant and partially or
significantly purified prior to use in the methods described herein. An
activating
compound may also be prepared synthetically, in which case it would be free of
other compounds with which it is naturally associated. In an illustrative
embodiment, an activating composition comprises, or an activating compound is
associated with, less than about 50%, 10%, 1 lo, 0.1 %, 10"2 10 or 10'30/a of
a
compound with which it is naturally associated.
In certain embodiments, a certain biological function (e.g., modulating
metabolic activity) is modulated by a sirtuin-activating compound with the
proviso
that the term sirtuin-activating compound does not include one or more
specific
compounds. For example, in certain embodiments, a sirtuin-activating compound
may be any compound that is capable of increasing the level of expression
and/or
activity of a sirtuin protein with the proviso that the compound is not
resveratrol,
129
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
flavone, any other compound specifically cited herein. In an exemplary
embodiment, a sirtuin-activating compound may be a compound of any one of
formulas 1-25, 30, 32-65, and 69-88 with the proviso that the compound is not
resveratrol, flavone, or any other compound specifically cited herein. In an
exemplary embodiment, a sirtuin-activating compound does not include any of
the
compounds cited in U.S. Patent Nos. 6,410,596 or 6,552,085, the disclosures of
which are hereby incorporated by reference in their entirety.
In certain embodiments, the subject sirtuin activators, such as SIRT1
activators, do not have any substantial ability to inhibit P13-kinase, inhibit
aldoreductase and/or inhibit tyrosine protein kinases at concentrations (e.g.,
in vivo)
effective for activating the deacetylase activity of the sirtuin, e.g., SIRT1.
For
instance, in preferred embodiments the sirtuin activator is chosen to have an
EC50
for activating sirtuin deacetylase activity that is at least 5 fold less than
the EC50 for
inhibition of one or more of aldoreductase and/or tyrosine protein kinases,
and even
more preferably at least 10 fold, 100 fold or even 1000 fold less. Methods for
assaying PI3-Kinase activity, aldose reductase activity, and tyrosine kinase
activity
are well known in the art and kits to perform such assays may be purchased
commercially. See e.g., U.S. Patent Publication No. 2003/0158212 for P13-
kinase
assays; U.S. Patent Publication No. 2002/20143017 for aldose reductase assays;
tyrosine kinase assay kits may be purchased commercially, for example, from
Promega (Madison, WI; world wide web at promega.com), Invitrogen (Carlsbad,
CA; world wide web at invitrogen.com) or Molecular Devices (Sunnyvale, CA;
world wide web at moleculardevices.com).
In certain embodiments, the subject sirtuin activators do not have any
substantial ability to transactivate EGFR tyrosine kinase activity at
concentrations
(e.g., in vivo) effective for activating the deacetylase activity of the
sirtuin. For
instance, in preferred embodiments the sirtuin activator is chosen to have an
EC50
for activating sirtuin deacetylase activity that is at least 5 fold less than
the EC50 for
transactivating EGFR tyrosine kinase activity, and even more preferably at
least 10
fold, 100 fold or even 1000 fold less. Methods for assaying transactivation of
EGFR tyrosine kinase activity are well known in the art, see e.g., Pai et al.
Nat.
Med. 8: 289-93 (2002) and Vacca et al. Cancer Research 60: 5310-5317 (2000).
130
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In certain embodiments, the subject sirtuin activators do not have any
substantial ability to cause coronary dilation at concentrations (e.g., in
vivo)
effective for activating the deacetylase activity of the sirtuin. For
instance, in
preferred embodiments the sirtuin activator is chosen to have an EC50 for
activating
sirtuin deacetylase activity that is at least 5 fold less than the EC5o for
coronary
dilation, and even more preferably at least 10 fold, 100 fold or even 1000
fold less.
Methods for assaying vasodilation are well known in the art, see e.g., U.S.
Patent
Publication No. 2004/0236153.
In certain embodiments, the subject sirtuin activators do not have any
substantial spasmolytic activity at concentrations (e.g., in vivo) effective
for
activating the deacetylase activity of the sirtuin. For instance, in preferred
embodiments the sirtuin activator is chosen to have an EC50 for activating
sirtuin
deacetylase activity that is at least 5 fold less than the EC50 for
spasmolytic effects
(such as on gastrointestinal muscle), and even more preferably at least 10
fold, 100
fold or even 1000 fold less. Methods for assaying spasmolytic activity are
well
known in the art, see e.g., U.S. Patent Publication No. 2004/0248987.
In certain embodiments, the subject sirtuin activators do not have any
substantial ability to inhibit hepatic cytochrome P450 IB1 (CYP) at
concentrations
(e.g., in vivo) effective for activating the deacetylase activity of the
sirtuin. For
instance, in preferred embodiments the sirtuin activator is chosen to have an
EC50
for activating sirtuin deacetylase activity that is at least 5 fold less than
the EC5o for
inhibition of P450 1B 1, and even more preferably at least 10 fold, 100 fold
or even
1000 fold less. Methods for assaying cytochrome P450 activity are well known
in
the art and kits to perform such assays may be purchased commercially. See
e.g.,
U.S. Patent Nos. 6,420,131 and 6,335,428 and Promega (Madison, WI; world wide
web at promega.com).
In certain embodiments, the subject sirtuin activators do not have any
substantial ability to inhibit nuclear factor-kappaB (NF-xB) at concentrations
(e.g.,
in vivo) effective for activating the deacetylase activity of the sirtuin. For
instance,
in preferred embodiments the sirtuin activator is chosen to have an EC50 for
activating sirtuin deacetylase activity that is at least 5 fold less than the
EC50 for
inhibition of NF-xB, and even more preferably at least 10 fold, 100 fold or
even
131
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
1000 fold less. Methods for assaying NF-xB activity are well known in the art
and
kits to perform such assays may be purchased commercially (e.g., from Oxford
Biomedical Research (Ann Arbor, MI; world wide web at oxfordbiomed.com)).
In certain embodiments, the subject sirtuin activators do not have any
substantial ability to inhibit a histone deacetylase (HDACs) class I, a HDAC
class
II, or HDACs I and II, at concentrations (e.g., in vivo) effective for
activating the
deacetylase activity of the sirtuin. For instance, in preferred embodiments
the
sirtuin activator is chosen to have an EC50 for activating sirtuin deacetylase
activity
that is at least 5 fold less than the EC50 for inhibition of an HDAC I and/or
HDAC
II, and even more preferably at least 10 fold, 100 fold or even 1000 fold
less.
Methods for assaying HDAC I and/or HDAC II activity are well known in the art
and kits to perform such assays may be purchased commercially. See e.g.,
BioVision, Inc. (Mountain View, CA; world wide web at biovision.com) and
Thomas Scientific (Swedesboro, NJ; world wide web at tomassci.com).
In certain embodiments, the subject SIRT1 activators do not have any
substantial ability to activate SIRT1 orthologs in lower eukaryotes,
particularly
yeast or human pathogens, at concentrations (e.g., in vivo) effective for
activating
the deacetylase activity of human SIRT1. For instance, in preferred
embodiments
the SIRT1 activator is chosen to have an EC50 for activating human SIRTI
deacetylase activity that is at least 5 fold less than the EC50 for activating
yeast Sir2
(such as Candida, S. cerevisiae,etc), and even more preferably at least
10'fold, 100
fold or even 1000 fold less.
In certain embodiments, the sirtuin activating compounds may have the
ability to activate one or more sirtuin protein homologs, such as, for
example, one
or more of human SIRTl, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, or SIRT7. In
other embodiments, a SIRTI activator does not have any substantial ability to
activate other sirtuin protein homologs, such as, for example, one or more of
human
SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, or SIRT7, at concentrations (e.g., in vivo)
effective for activating the deacetylase activity of human SIRT1. For
instance, the
SIRTI activator may be chosen to have an EC50 for activating human SIRT1
deacetylase activity that is at least 5 fold less than the EC50 for activating
one or
132
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
more of human SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, or SIRT7, and even more
preferably at least 10 fold, 100 fold or even 1000 fold less.
In certain embodiments, SIRT3 and SIRT4 modulators may be used to
modulate fat mobilization. For example, SIRT3 and/or SIRT4 activators may be
used to induce fat mobilization and may be used to treat, e.g., obesity and
insulin
resistance disorders.
In other embodiments, the subject sirtuin activators do not have any
substantial ability to inhibit protein kinases; to phosphorylate mitogen
activated
protein (MAP) kinases; to inhibit the catalytic or transcriptional activity of
cyclo-
oxygenases, such as COX-2; to inhibit nitric oxide synthase (iNOS); or to
inhibit
platelet adhesion to type I collagen at concentrations (e.g., in vivo)
effective for
activating the deacetylase activity of the sirtuin. For instance, in preferred
embodiments, the sirtuin activator is chosen to have an EC50 for activating
sirtuin
deacetylase activity that is at least 5 fold less than the EC50 for performing
any of
these activities, and even more preferably at least 10 fold, 100 fold or even
1000
fold less. Methods for assaying protein kinase activity, cyclo-oxygenase
activity,
nitric oxide synthase activity, and platelet adhesion activity are well known
in the
art and kits to perform such assays may be purchased commercially. See e.g.,
Promega (Madison, WI; world wide web at promega.com), Invitrogen (Carlsbad,
CA; world wide web at invitrogen.com); Molecular Devices (Sunnyvale, CA;
world wide web at moleculardevices.com) or Assay Designs (Ann Arbor, MI;
world wide web at assaydesigns.com) for protein kinase assay kits; Amersham
Biosciences (Piscataway, NJ; world wide web at amershambiosciences.com) for
cyclo-oxygenase assay kits; Amersham Biosciences (Piscataway, NJ; world wide
web at amershambiosciences.com) and R&D Systems (Minneapolis, MN; world
wide web at rndsystems.com) for nitric oxide synthase assay kits; and U.S.
Patent
Nos. 5,321,010; 6,849,290; and 6,774,107 for platelet adhesion assays.
In certain embodiments, a compound described herein, e.g., a sirtuin
activator or inhibitor, does not have significant or detectable anti-oxidant
activities,
as determined by any of the standard assays known in the art. For example, a
compound does not significantly scavenge free-radicals, such as 02 radicals. A
133
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
compound may have less than about 2, 3, 5, 10, 30 or 100 fold anti-oxidant
activity
relative to another compound, e.g., resveratrol.
In certain embodiments, a sirtuin activating compound may have a binding
affinity for a sirtuin of about 10"9 M, 10"1 M, 10-11 M, 10"12 M or less. A
sirtuin
activating compound may reduce the K,n of a sirtuin for its substrate or NAD+
by a
factor of at least about 2, 3, 4, 5, 10, 20, 30, 50 or 100. A sirtuin
activating
compound may increase the V,n,,, of a sirtuin by a factor of at least about 2,
3, 4, 5,
10, 20, 30, 50 or 100. Exemplary compounds that may increase the Vmax of a
sirtuin include, for example, analogs of isonicotinamide, such as, for
example,
compounds of formulas 69-72, and/or analogs of O-acetyl-ADP-ribose, such as,
for
example, compounds of formulas 73-76. A compound may have an EC50 for
activating the deacetylase activity of a sirtuin of less than about 1 nM, less
than
about 10 nM, less than about 100 nM, less than about 1 .M, less than about 10
M,
less than about 100 M, or from about 1-10 nM, from about 10-100 nM, from
about
0.1-1 M, from about 1-10 M or from about 10-100 M. A compound may
activate the deacetylase activity of a sirtuin by a factor of at least about
5, 10, 20,
30, 50, or 100, as measured in an acellular assay or in a cell based assay as
described in the Examples. A compound may cause at least a 10%, 30%, 50%,
80%, 2 fold, 5 fold, 10 fold, 50 fold or 100 fold greater induction of the
deacetylase
activity of SIRT1 relative to the same concentration of resveratrol or other
compound described herein. A compound may also have an EC50 for activating
SIRT5 that is at least about 10 fold, 20 fold, 30 fold, 50 fold greater than
that for
activating SIRT1.
In an exemplary embodiment, the methods and compositions described
herein may include a combination therapy comprising (i) at least one sirtuin-
activating compound that reduce the K,,, of a sirtuin for its substrate or
NAD+ by a
factor of at least about 2, 3, 4, 5, 10, 20, 30, 50 or 100, and (ii) at least
one sirtuin-
activating compound that increases the Vm,., of a sirtuin by a factor of at
least about
2, 3, 4, 5, 10, 20, 30, 50 or 100. In one embodiment, a combination therapy
may
comprise at least two of the following: (i) at least one sirtuin-activating
compound
of formula 1-25, 30, and 32-65, (ii) at least one sirtuin-activating compound
of
formula 69-76, and (iii) at least one sirtuin-activating compound of formula
77-88.
134
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
A compound may traverse the cytoplasmic membrane of a cell. For
example, a compound may have a cell-permeability of at least about 20%, 50%,
75%, 80%, 90% or 95%.
Compounds described herein may also have one or more of the following
characteristics: the compound may be essentially non-toxic to a cell or
subject; the
compound may be an organic molecule or a small molecule of 2000 amu or less,
1000 amu or less; a compound may have a half-life under normal atmospheric
conditions of at least about 30 days, 60 days, 120 days, 6 months or 1 year;
the
compound may have a half-life in solution of at least about 30 days, 60 days,
120
days, 6 months or 1 year; a compound may be more stable in solution than
resveratrol by at least a factor of about 50%, 2 fold, 5 fold, 10 fold, 30
fold, 50 fold
or 100 fold; a compound may promote deacetylation of the DNA repair factor
Ku70;
a compound may promote deacetylation of ReIA./p65; a compound may increase
general turnover rates and enhance the sensitivity of cells to TNF-induced
apoptosis.
II. Exemplary therapeutic applications of the sirtuin-activating
compounds
In certain embodiments, the invention provides methods for treating and/or
preventing a wide variety of diseases and disorders by administering to a
subject a
high dose of a sirtuin activator. In an exemplary embodiments, a quantity of a
sirtuin activator having a sirtuin activating effect equal to or greater than
the sirtuin
activating effect of 18 mg/kg resveratrol may be administered to a subject. A
high
dose may be administered to a subject once, or multiple times (e.g., daily)
until a
desired therapeutic effect is achieved. For example, a high dose may be
administered daily for 1 day, 1 week, 2 weeks, 1 month, 2 months, 3 montlis, 6
months, 1 year, or more depending on the disease or disorder being treated. A
high
dose of a sirtuin activator may be administered daily in a single dosage or
may be
divided into multiple dosages, e.g., that are taken twice or three times per
day. In an
exemplary embodiment, a high dose of a sirtuin activator may be administered
in a
sustained release formulation. Exemplary diseases or disorders that may be
teated
135
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
using a high dose of a sirtuin activator include, for example, diseases or
disorders
related to aging or stress, diabetes, obesity, neurodegenerative diseases,
diseases or
disorders associated with mitochondrial dysfunction, cardiovascular disease,
blood
clotting disorders, inflainmation, cancer, and/or flushing, etc. The methods
comprise administering to a subject in need thereof a high dose of a sirtuin
activating compound.
In certain einbodiments, a high dose of a sirtuin activating compound may be
taken alone or in combination with other compounds. In one embodiment, a
mixture
of a high dose of two or more sirtuin activating compounds may be administered
to a
subject in need thereof. In another embodiment, a high dose of a sirtuin
activating
compound may be administered with one or more of the following compounds:
resveratrol, butein, fisetin, piceatannol, or quercetin. In an exemplary
embodiment,
a high dose of a sirtuin activating compound may be administered in
combination
with nicotinic acid. In yet another embodiment, a high dose of one or more
sirtuin
activating compound may be administered with one or more therapeutic agents
for
the treatment or prevention of various diseases, including, for example,
cancer,
diabetes, neurodegenerative diseases, diseases or disorders associated with
mitochondrial dysfunction, cardiovascular disease, blood clotting,
inflammation,
flushing, obesity, ageing, stress, etc. In various embodiments, combination
therapies
comprising a high dose of a sirtuin activating compound may refer to (1)
pharmaceutical compositions that comprise a high dose of one or more sirtuin
activating compounds in combination with one or more therapeutic agents; and
(2)
co-administration of a high dose of one or more sirtuin activating compounds
with
one or more therapeutic agents wherein the sirtuin activating compound and
therapeutic agent have not been formulated in the same compositions. When
using
separate formulations, the high dose of the sirtuin activating compound may be
administered at the same, intermittent, staggered, prior to, subsequent to, or
combinations thereof, with the administration of another therapeutic agent.
Metabolic Dis rclers/Diabetes/Weiglat Control
Described herein are methods for treating or preventing obesity or generally
weight gain, in a subject, such as to reduce the weight of the subject or
reduce
weight gain. A method may comprise administering to a subject, such as a
subject
136
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
in need thereof, a high dose of an agent that increases the activity or
protein level of
a sirtuin, such as SIRTI or Sir2, e.g., a sirtuin activator. A subject in need
of such a
treatment may be a subject who is obese, or likely to become obese, or who
has, or
is, likely to gain excess weight, as predicted, e.g., from family history.
Exemplary
agents are those described herein. A combination of agents may also be
administered (e.g., a combination of a high dose of a sirtuin activator with
an anti-
obesity agent). A method may further comprise monitoring the weight of the
subject
and/or the level of activation of sirtuins, for example, in adipose tissue.
Also described herein are methods for treating or preventing a metabolic
disorder, such as insulin-resistance or other precursor symptom of type II
diabetes or
complications thereof. Methods may increase insulin sensitivity or decrease
insulin
levels in a subject. A method may comprise administering to a subject, such as
a
subject in need thereof, a high dose of an agent that increases the activity
or protein
level of a sirtuin, such as SIRTI or Sir2. A subject in need of such a
treatment may
be a subject who has insulin resistance or other precusor symptom of type II
diabetes, who has type II diabetes, or who is likely to develop any of these
conditions. For example, the subject may be a subject having insulin
resistance, e.g.,
having high circulating levels of insulin and/or associated conditions, such
as
hyperlipidemia, dyslipogenesis, hypercholesterolemia, impaired glucose
tolerance,
high blood glucose sugar level, other manifestations of syndrome X,
hypertension,
atherosclerosis and lipodystrophy. Exemplary agents are those described
herein.
In certain embodiments, a high dose of a sirtuin activating compound may be
used to decrease the amount of fat absorption in the gastrointestinal tract of
an
individual thereby promoting weight loss and/or preventing gain. In certain
embodiments, a sirtuin modulating compound may be administered in combination
with another agent that inhibits fat absorption, such as, Orlistat (also known
as
tetrahydrolipstatin and sold under the brand name XENICALTM). Orlistat is a
potent
inhibitor of gastrointestinal lipases, i.e. lipases that are responsible for
breaking
down ingested fat (gastric lipase, carboxylester lipase, pancreatic lipase).
As a
consequence of lipase inhibition, the undigested fats cannot be absored and
are
excreted in the feces. In certain embodiments, sirtuin modulating compound may
permit a lower dose of a fat absorption inhibitor to be administered and still
achieve
137
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
therapeutically desirable results. Such combination therapy may permit
avoidance
of undesirable side affects associated with the fat absorption inhibitor.
A combination of agents may also be administered (e.g., a combination of a
high dose of a sirtuin activating compound with an anti-diabetic agent). A
method
may further comprise monitoring in the subject the state of any of these
conditions
and/or the level of activation of sirtuins, for example, in adipose tissue.
The sirtuin-activitating compounds described herein may be taken alone or in
combination with other compounds. The other compounds may be other sirtuin
and/or AMPK activators. For example, LongevinexTM, which is a red wine
extract,
and contains, in addition to resveratrol, other sirtuin activators, such as
quercetin, is
a particularly potent agent for mobilizing fat. LongevinexTM can be obtained
on the
world wide web at longevinex.com.
In an exemplary embodiment, a high dose of a sirtuin-activating compound
may be administered as a combination therapy with a lipid lowering, an anti-
obesity
and/or an anti-diabetic agent. Examples of lipid lowering, anti-obesity or
anti-
diabetic agents suitable for administration in combination with a high dose of
a
sirtuin activator include chromium, fat binding polymers, carbohydrate binding
polymers, lipase inhibitors, thermogenic agents, catecholamine reuptake
inhibitors,
and thyroid hormone. For example, for reducing weight, preventing weight gain,
or
treatment or prevention of obesity, a high dose of one or more sirtuin-
activating
compounds may be used in combination with one or more anti-obesity agents such
as the following: phenylpropanolamine, ephedrine, pseudoephedrine,
phentermine, a
cholecystokinin-A agonist, a monoamine reuptake inhibitor (such as
sibutramine), a
sympathomimetic agent, a serotonergic agent (such as dexfenfluramine or
fenfluramine), a dopamine agonist (such as bromocriptine), a melanocyte-
stimulating hormone receptor agonist or mimetic, a melanocyte-stimulating
hormone
analog, a cannabinoid receptor antagonist, a melanin concentrating hormone
antagonist, the OB protein (leptin), a leptin analog, a leptin receptor
agonist, a
cannabinoid receptor modulator (such as ramonibant), a galanin antagonist or a
GI
lipase inhibitor or decreaser (such as orlistat). Other anorectic agents
include
bombesin agonists, dehydroepiandrosterone or analogs thereof, glucocorticoid
receptor agonists and antagonists, orexin receptor antagonists, urocortin
binding
138
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
protein antagonists, agonists of the glucagon-like peptide-1 receptor such as
Exendin, anticonvulsants and ciliary neurotrophic factors such as Axokine.
In other embodiments, a high dose of one or more sirtuin activating
compounds may be used in combination with one or more anti-diabetic agents
such
as the following: an aldose reductase inhibitor, a glycogen phosphorylase
inhibitor, a
sorbitol dehydrogenase inhibitor, a protein tyrosine phosphatase 1B inhibitor,
a
dipeptidyl protease inhibitor, insulin (including orally bioavailable insulin
preparations), an insulin mimetic, metformin, acarbose, a peroxisome
proliferator-
activated receptor-y (PPAR-7) ligand such as troglitazone, rosaglitazone,
pioglitazone or GW-1929, a sulfonylurea, glipazide, glyburide, or
chlorpropamide
wherein the amounts of the first and second compounds result in a therapeutic
effect.
Other anti-diabetic agents include a glucosidase inhibitor, a glucagon-like
peptide-1
(GLP-1), insulin, a PPAR a/y dual agonist, a meglitimide and an aP2 inhibitor.
In
an exemplary embodiment, an anti-diabetic agent may be a dipeptidyl peptidase
IV
(DP-IV or DPP-IV) inhibitor, such as, for example LAF237 from Novartis (NVP
DPP728; 1-[[[2-[(5-cyanopyridin-2-yl)amino] ethyl]amino]acetyl]-2- cyano-(S)-
pyrrolidine) or MK-04301 from Merck (see e.g., Hughes et al., Biochemistry 38:
11597-603 (1999)).
In other embodiments, a high dose of one or more sirtuin activating
compounds may be used in combination with one or more lipid lowering agents
such as the following: statins such as simvastatin (Zocor), pravastatin
(Pravachol),
lovostatin (Mevacor), fleuvastatin (Lescol), cerivastatin (Baycol),
rosuvastatin
(Crestcor) and atorvastatin (Lipitor) as well as niacin.
In certain embodiments, administration of a high dose of a sirtuin activator
in
combination with a lipid lowering, anti-obesity and/or anti-diabetic agent may
reduce, alleviate or eliminate undesirable side effects associated with the
anti-
obesity and/or anti-diabetic agents. For example, administration of a lipid
lowering,
anti-obesity and/or anti-diabetic agent in combination with a high dose of a
sirtuin
activator may permit a therapeutically beneficial result upon administration
of a
lower dose of the lipid lowering, anti-obesity and/or anti-diabetic agent than
would
be necessary in the absence of the combination with the sirtuin activator. For
example, a high dose of a sirtuin activator may be administered in combination
with
139
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
a low dose (e.g., an amount that reduces or prevents undesirable side effects
such as
increased heart rate and/or blood pressure) of a lipid lowering, anti-obesity
and/or
anti-diabetic drug. In certain embodiments, a high dose of a sirtuin activator
may be
administered in combination with thyroid hormone. In other embodiments, a high
dose of a sirtuin activator may be administered in combination with an
absorption
blocker, such as an a-glucosidase inhibitor. In yet other embodiments, a high
dose
of a sirtuin activator may be administered in combination with inetformin. In
certain
embodiments, a high dose of a sirtuin activator may be administered in
combination
with a catecholamine reuptake inhibitor, such as sibutramine. Additional
examples
of lipid lowering, anti-obesity or anti-diabetic agents are described herein.
In other
embodiments, the dose of the lipid lowering, anti-obesity and/or anti-diabetic
agent
may not be reduced as compared to a normal dose and administration of the
sirtuin
activator reduces, alleviates or abolishes a side effect of the lipid
lowering, anti-
obesity and/or anti-diabetic agent.
In certain embodiments, the use of sirtuin activating compounds can be used
to reduce the amount of a lipid lowering, anti-obesity or anti-diabetic agent
that is
taken. This may be desirable in instances where a lipid lowering, anti-obesity
and/or
anti-diabetic agent dosing regimen produces unwanted side effects in the
patient in
need thereof. For example, administration of thyroid hormone to a subject
typically
causes an increase in heart rate and/or blood pressure. In certain
embodiments, the
invention provides methods to adjust (e.g., reduce) the amount of thyroid
hormone
administered to the patient to an amount that does not have an undesirable
effect on
heart rate and/or blood pressure by administering the thyroid hormone in
combination with a high dose of a sirtuin activator. In other embodiments, the
invention provides methods to adjust (e.g., reduce) the amount of inetformin
taken
by a subject in need thereof by administering metformin in combination with a
high
dose of a sirtuin activator. In yet other embodiments, a high dose of a
sirtuin
activator is used to reduce the amount of sibutramine that is taken by a
subject in
need thereof.
The methods described herein may comprise administering daily, or every
other day, or once a week, a high dose of a sirtuin activating compound, e.g.,
in the
form of a pill, to a subject. In embodiments where the high dose of a sirtuin
140
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
activating compound is administered daily to the subject, the sirtuin
activating
compound may be administered once a day. In other embodiments, it is
administered twice or three times a day.
In some embodiments, the high dose of a sirtuin activating compound is
administered in a sustained release formulation, e.g., by embedding or
encapsulating
the sirtuin activator into nanoparticles for delivery over a period of at
least 12 hours,
to a subject. In embodiments where the sirtuin activator is administered to a
subject
in a sustained release formulation, a high dose of the sirtuin activator may
be
administered for sustained delivery over a period of for example, at least
about 12,
15, 18, 24, or 36 hours, or longer. In other embodiments, it is administered
for a
sustained delivery over a period of one or more days. In yet other
embodiments, it is
administered for a sustained delivery over a period of one or more weeks.
In certain embodiments, the sirtuin activating compound is administered in a
nutraceutical formulation. A "nutraceutical" is any functional food (including
beverages) that provides an additional benefit other than its nutritional
benefit. In a
preferred embodiment, a nutraceutical is provided and contains from about 0.1
% to
about 99%, or from about 0.1% to about 10% of a sirtuin activator by weight.
In
preferred embodiments, a high dose as described herein of a sirtuin activator
is
administered in a single serving of a food or beverage. In a preferred
formulation, a
single dosage form is provided (e.g., an 8 fluid ounce serving of a beverage
such as
water, flavored water, or fruit juice) that contains a quantity of total
sirtuin activator
that has a sirtuin activating effect equal to or greater than the sirtuin
activating effect
of 25 mg resveratol. In other embodiments, a single dosage form is provided
that
contains a quantity of total sirtuin activator that has a sirtuin activating
effect equal
to or greater than the sirtuin activating effect of about 10, 15, 20, 25, 50,
60, 75, 80,
100, 150, 200, or more, mg resveratrol per 8 fluid ounces. In other preferred
embodiments, a single dosage form is provided (e.g., a serving of food such as
a
nutrition bar) that contains a total quantity of sirtuin activator that has a
sirtuin
activating effect equal to or greater than the sirtuin activating effect of
100 mg
resveratol. In some embodiments, the food supplies 100 to 500 kcal per
serving. In
other embodiments, a single dosage form is provided that contains a total
quantity of
sirtuin activator that has a sirtuin activating effect equal to or greater
than the sirtuin
141
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
activating effect of 25, 50, 60, 75, 80, 100, 150, 200, 250, or more, mg
resveratrol
per 100 to 500 kcal. The phrase "total quantity of sirtuin activator" refers
to the total
amount of sirtuin activator(s) present in the single dosage form.
In various embodiments, a nutraceutical comprising a sirtuin activator may
be any variety of food or drink. For example, nutraceuticals may include
drinks
such as nutritional drinks, diet drinks (e.g., SlimfastTM, BoostTM and the
lilce) as well
as sports, herbal and other fortified beverages. Additionally, nutraceuticals
may
include foods intended for lluman or animal consumption such as baked goods,
for
example, bread, wafers, cookies, crackers, pretzels, pizza, and rolls, ready-
to-eat
breakfast cereals, hot cereals, pasta products, snacks such as fruit snacks,
salty
snacks, grain snacks, nutrition bars, and microwave popcorn, dairy products
such as
yogurt, cheese, and ice cream, sweet goods such as hard candy, soft candy, and
chocolate, beverages, animal feed, pet foods such as dog food and cat food,
aqua-
culture foods such as fish food and shrimp feed, and special purpose foods
such as
baby food, infant formulas, hospital food, medical food, sports food,
performance
food or nutritional bars, or fortified foods, food preblends or mixes for home
or food
service use, such as preblends for soups or gravy, dessert mixes, dinner
mixes,
baking mixes such as bread mixes, and cake mixes, and baking flour. In certain
embodiments, the food or beverage does not include one or more of grapes,
mulberries, blueberries, raspberries, peanuts, milk, yeast, or extracts
thereof. The
present invention provides nutraceutical compositions that may be used to
promote
weight loss in a subject in need thereof. For example, in certain aspects, the
present
invention provides nutraceutical compositions that are useful for treating or
preventing obesity and/or diabetes.
In addition to the sirtuin activator, the nutraceutical also may contain a
variety of other beneficial 'components including but not limited to essential
fatty
acids, vitamins and minerals. Additional disclosure describing the contents
and
production of nutritional supplements may be found in e.g., U.S. Pat. No.
5,902,797;
U.S. Pat. No. 5,834,048; U.S. Pat. No. 5,817,350; U.S. Pat. No. 5,792,461;
U.S. Pat.
No. 5,707,657 and U.S. Pat. No. 5,656,312 (each incorporated herein by
reference).
When ingested in a solid form, a nutraceutical composition of the invention
may additionally contain a solid carrier such as a gelatin or an adjuvant.
When
142
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
administered in liquid form, a liquid carrier such as water, petroleum, oils
of animal
or plant origin such as peanut oil, mineral oil, soybean oil, or sesame oil,
or synthetic
oils may be added. The nutraceutical composition of the present invention may
also
contain stabilizers, preservatives, buffers, antioxidants, or other additives
known to
those of slcill in the art.
In other embodiments, a food or beverage comprises a supplement of one or
more sirtuin activating compounds. In certain embodiments, the supplement
comprises a quantity of a sirtuin activating compound that has a sirtuin
activating
effect equal to or greater than the sirtuin activating effect of 11 mg/g
resveratol. In
other embodiments, the supplement comprises a quantity of a sirtuin activating
compound that has a sirtuin activating effect equal to or greater than the
sirtuin
activating effect of 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 20, 25, 50, or
more, mg/g
resveratrol.
Other methods include administering to a subject a combination of a high
dose of a sirtuin activator and an agent that increases the activity or
protein level of
an AMPK, e.g., other than an agent that activates a sirtuin. Activators of
AMPK
include AICAR or Metformin. Alternatively, the protein level of AMPIC may be
increased by introducing into the cell a nucleic acid encoding AMPK. The
nucleotide sequence of the catalytic domain (al) of human AMPK has the
nucleotide sequence set forth in GenBank Accession No. NM 206907 and encodes a
protein having the aniino acid sequence set forth in GenBank Accession No.
NP 996790. The nucleotide sequence of the non-catalytic domain ((il) of human
AMPK has the nucleotide sequence set forth in GenBank Accession No.
NM 006253 and encodes a protein having the amino acid sequence set forth in
GenBank Accession No. NP006244. The nucleotide sequence of the non-catalytic
domain (y l) of human AMPK has the nucleotide sequence set forth in GenBank
Accession No. NM 212461 and encodes a protein having the amino acid sequence
sets forth in GenBank Accession No. NP_997626. To increase the protein level
of
human AMPK in a cell, it may be necessary to introduce nucleic acids encoding
each of the subunits of the protein. Nucleic acid sequences encoding the
different
subunits may be contained on the same or separate nucleic acid molecules.
143
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Other diseases that may be treated by administering a high dose of a sirtuin
activator include certain renal diseases including glomerulonephritis,
gloinerulosclerosis, nephrotic syndrome, hypertensive nephrosclerosis.
In other embodiments, a high dose of a sirtuin activator may be used to treat
a disease or condition that will benefit from weight loss such as, for
example: high
blood pressure, llypertension, high blood cholesterol, dyslipidemia, type 2
diabetes,
insulin resistance, glucose intolerance, hyperinsulinemia, coronary heart
disease,
angina pectoris, congestive heart failure, stroke, gallstones, cholescystitis
and
cholelithiasis, gout, osteoarthritis, obstructive sleep apnea and respiratory
problems,
some types of cancer (such as endometrial, breast, prostate, and colon),
complications of pregnancy, poor female reproductive health (such as menstrual
irregularities, infertility, irregular ovulation), bladder control problems
(such as
stress incontinence); uric acid nephrolithiasis; psychological disorders (such
as
depression, eating disorders, distorted body image, and low self esteem).
Stunkard
AJ, Wadden TA. (Editors) Obesity: theory and therapy, Second Edition. New
York:
Raven Press, 1993. Finally, patients with AIDS can develop lipodystrophy or
insulin resistance in response to combination therapies for AIDS. Accordingly,
any
of these conditions can be treated or prevented by the methods described
herein for
reducing or preventing weight gain.
Other diseases and conditions that can be treated by the methods described
herein include chlomicronemia syndrome, polycistic ovarian syndrome,
hypothermia, fat pad syndrome in the knee, alcoholic fatty liver, and non-
alcoholic
fatty liver.
In another embodiment, a high dose of a sirtuin-activating compound may be
administered to reduce drug-induced weight gain. For example, a high dose of a
sirtuin-activating compound may be administered as a combination therapy with
medications that may stimulate appetite or cause weight gain, in particular,
weight
gain due to factors other than water retention. Examples of medications that
may
cause weight gain, include for example, diabetes treatments, including, for
example,
sulfonylureas (such as glipizide and glyburide), thiazolidinediones (such as
pioglitazone and rosiglitazone), meglitinides, nateglinide, repaglinide,
sulphonylurea
medicines, and insulin; anti-depressants, including, for example, tricyclic
144
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
antidepressants (such as amitriptyline and imipramine), irreversible monoamine
oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs),
bupropion, paroxetine, and mirtazapine; steroids, such as, for example,
prednisone;
hormone therapy; lithium carbonate; valproic acid; carbamazepine;
chiorpromazine;
thiothixene; beta blockers (such as propranolo); alpha blockers (such as
clonidine,
prazosin and terazosin); and contraceptives including oral contraceptives
(birth
control pills) or other contraceptives containing estrogen and/or progesterone
(Depo-
Provera, Norplant, Ortho), testosterone or Megestrol. In another exemplary
embodiment, a high dose of a sirtuin-activating compound may be administered
as
part of a smoking cessation program to prevent weight gain or reduce weight
already
gained.
The methods described herein can also be used in veterinary applications,
such as to treat metabolic disorders in pets (e.g., obesity or diabetes in
dogs, cats,
etc.) or farm animals (e.g., fat cow syndrome in cows).
In certain embodiments, a high dose of a sirtuin activator may be used to
reduce weight, prevent weight gain, or reduce the rate of weight gain in a
subject
that consumes a high fat diet. For example, the invention provides methods and
compositions to promote weight loss in a subject that consumes a high fat diet
where
lipids represent at least 30% of the average daily calorie consumption of the
subject.
In other embodiments, lipids represent at least 35%, at least 40%, at least
45%, at
least 50%, at least 55%, at least 60% of the average daily calorie comsumption
of
the subject. In certain embodiments, a high fat diet includes at least about
10%,
20%, 30%, 40%, 50%, 60%, or more, of the daily calorie consumption from
carbohydrates.
The methods and compositions of the present invention may also be used to
reduce weight gain in a subject that is refractory to diet and exercise. In
exemplary
embodiments, a high dose of a sirtuin activating compound may promote weight
loss in a subject that does not reduce calorie consumption, increase activity,
or a
combination thereof, to an extent sufficient to cause weight loss in the
absence of a
sirtuin activating compound.
In certain embodiments, a high dose of a sirtuin-activating compound may be
directed specifically to a certain tissue (e.g., liver) rather than the whole
body.
145
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Tissue specific treatments may be used to treat, e.g., obesity and insulin
resistance
disorder.
In certain embodiments the methods are useful for preventing fat
accumulation in cells with lipogenic capacity, e.g. liver, pancreas and muscle
cells.
In certain embodiments, the invention provides methods for increasing the
life span or preventing cell death of pancreatic (3-cells. The methods involve
contacting pancreatic (3-cells with a sirtuin activating compound. In other
embodiments, the methods involve administering to a subject in need thereof
(e.g., a
subject having type 1 diabetes, type 2 diabetes, impaired glucose tolerance,
etc.) a
therapeutically effective amount of a sirtuin activating compound.
Susceptibility to
type 2 diabetes requires both genetic and acquired factors. Its continuing
pathogenesis involves an interplay of progressive cellular insulin resistance
and
pancreatic P-cell failure. Free radical generation and induced nitric oxide
synthase
(iNOS) production secondary to the hyperglycemia of type 2 diabetes can lead
to
pancreatic P-cell destruction, and the production of diagnostic enzymatic
indicators
characteristic of type 1 diabetes. In this scenario, 0-cells are not only
"exhausted"
by the progression of pathology from insulin resistance to type 2 diabetes but
may
also undergo destruction induced by chronic hyperglycemia. Pancreatic P-cell
apoptosis is responsible for irreversible progression toward insulin
dependence in
type 2 diabetes. The compounds described herein can be used to inhibit or
prevent
progression to type 2 diabetes in a subject in need thereof. For example, in
certain
embodiments, the compounds of the subject invention inhibit or prevent
pancreatic
P-cell death. As described below, prevention of pancreatic P-cell death or
dysfunction may be through increased mitochondrial activity or number.
In certain embodiments, the invention provides methods for treating a
metabolic disorder comprising administration of a sirtuin activating compound
in
combination with a sirtuin inhibitor. In an exemplary embodiment, the method
involves administering a sirtuin activating compound to the fat cells of a
patient in
combination with administering a sirtuin inhibitor to the liver of a subject
in need
thereof.
Mitochondrial-Associated Diseases and Disorders
146
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In certain embodiments, the invention provides methods for treating diseases
or disorders that would benefit from increased mitochondrial activity. The
methods
involve administering to a subject in need thereof a therapeutically effective
amount
of a sirtuin activating compound. Increased mitochondrial activity refers to
increasing activity of the mitochondria while maintaining the overall numbers
of
mitochondria (e.g., mitochondrial mass), increasing the numbers of
mitochondria
thereby increasing mitochondrial activity (e.g., by stimulating mitochondrial
biogenesis), or combinations thereof. In an exemplary embodiment, the methods
involve administering a high dose of a sirtuin activating compound. In certain
embodiments, diseases and disorders that would benefit from increased
mitochondrial activity include diseases or disorders associated with
mitochondrial
dysfunction.
In certain embodiments, methods for treating diseases or disorders that
would benefit from increased mitochondrial activity may comprise identifying a
subject suffering from a mitochondrial dysfunction. Methods for diagnosing a
mitochondrial dysfunction may involve molecular genetic, pathologic and/or
biochemical analysis are summarized in Cohen and Gold, Cleveland Clinic
Journal
of Medicine, 68: 625-642 (2001). One method for diagnosing a mitochondrial
dysfunction is the Thor-Byrne-ier scale (see e.g., Cohen and Gold, supra;
Collin S.
et al., Eur Neurol. 36: 260-267 (1996)).
Mitochondria are critical for the survival and proper function of almost all
types of eukaryotic cells. Mitochondria in virtually any cell type can have
congenital
or acquired defects that affect their function. Thus, the clinically
significant signs
and symptoms of mitochondrial defects affecting respiratory chain function are
heterogeneous and variable depending on the distribution of defective
mitochondria
among cells and the severity of their deficits, and upon physiological demands
upon
the affected cells. Nondividing tissues with high energy requirements, e.g.
nervous
tissue, skeletal muscle and cardiac muscle are particularly susceptible to
mitochondrial respiratory chain dysfunction, but any organ system can be
affected.
Diseases and disorders associated with mitochondrial dysfunction include
diseases and disorders in which deficits in mitochondrial respiratory chain
activity
contribute to the development of pathophysiology of such diseases or disorders
in a
147
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
mammal. This includes 1) congenital genetic deficiencies in activity of one or
more
components of the mitochondrial respiratory chain; and 2) acquired
deficiencies in
the activity of one or more components of the mitochondrial respiratory chain,
wherein such deficiencies are caused by a) oxidative damage during aging; b)
elevated intracellular calcium; c) exposure of affected cells to nitric oxide;
d)
hypoxia or ischemia; e) microtubule-associated deficits in axonal transport of
mitochondria, or f) expression of mitochondrial uncoupling proteins.
Diseases or disorders that would benefit from increased mitochondrial
activity generally include for example, diseases in which free radical
mediated
oxidative injury leads to tissue degeneration, diseases in which cells
inappropriately
undergo apoptosis, and diseases in which cells fail to undergo apoptosis.
Exemplary
diseases or disorders that would benefit from increased mitochondrial activity
include, for example, AD (Alzheimer's Disease), ADPD (Alzheimer's Disease and
Parkinsons's Disease), AMDF (Ataxia, Myoclonus and Deafness), auto-immune
disease, cancer, CIPO (Chronic Intestinal Pseudoobstruction with myopathy and
Ophthalmoplegia), congenital muscular dystrophy, CPEO (Chronic Progressive
External Ophthalmoplegia), DEAF (Maternally inherited DEAFness or
aminoglycoside-induced DEAFness), DEMCHO (Dementia and Chorea), diabetes
mellitus (Type I or Type II), DIDMOAD (Diabetes Insipidus, Diabetes Mellitus,
Optic Atrophy, Deafness), DMDF (Diabetes Mellitus and Deafness), dystonia,
Exercise Intolerance, ESOC (Epilepsy, Strokes, Optic atrophy, and Cognitive
decline), FBSN (Familial Bilateral Striatal Necrosis), FICP (Fatal Infantile
Cardiomyopathy Plus, a MELAS-associated cardiomyopathy), GER
(Gastrointestinal Reflux), HD (Huntington's Disease), KSS (Kearns Sayre
Syndrome), "later-onset" myopathy, LDYT (Leber's hereditary optic neuropathy
and
DYsTonia), Leigh's Syndrome, LHON (Leber Hereditary Optic Neuropathy), LIMM
(Lethal Infantile Mitochondrial Myopathy), MDM (Myopathy and Diabetes
Mellitus), MELAS (Mitochondrial Encephalomyopathy, Lactic Acidosis, and
Stroke-like episodes), MEPR (Myoclonic Epilepsy and Psychomotor Regression),
MERME (MERRF/MELAS overlap disease), MERRF (Myoclonic Epilepsy and
Ragged Red Muscle Fibers), MHCM (Maternally Inherited Hypertrophic
CardioMyopathy), MICM (Maternally Inherited Cardiomyopathy), MILS
148
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
(Maternally Inherited Leigh Syndrome), Mitochondrial Encephalocardiomyopathy,
Mitochondrial Encephalomyopathy, MM (Mitochondrial Myopathy), MMC
(Maternal Myopathy and Cardiomyopathy), MNGIE (Myopathy and external
ophthalmoplegia, Neuropathy, Gastro-Intestinal, Encephalopathy), Multisystem
Mitochondrial Disorder (myopathy, encephalopathy, blindness, hearing loss,
peripheral neuropathy), NARP (Neurogenic muscle weakness, Ataxia, and
Retinitis
Pigmentosa; alternate phenotype at this locus is reported as Leigh Disease),
PD
(Parkinson's Disease), Pearson's Syndrome, PEM (Progressive Encephalopathy),
PEO (Progressive External Ophthalhnoplegia), PME (Progressive Myoclonus
Epilepsy), PMPS (Pearson Marrow-Pancreas Syndrome), psoriasis, RTT (Rett
Syndrome), schizophrenia, SIDS (Sudden Infant Death Syndrome), SNHL
(Sensorineural Hearing Loss), Varied Familial Presentation (clinical
manifestations
range from spastic paraparesis to multisystem progressive disorder & fatal
cardiomyopathy to truncal ataxia, dysarthria, severe hearing loss, mental
regression,
ptosis, ophthalmoparesis, distal cyclones, and diabetes mellitus), or Wolfram
syndrome.
Other diseases and disorders that would benefit from increased mitochondrial
activity include, for example, Friedreich's ataxia and other ataxias,
amyotrophic
lateral sclerosis (ALS) and other motor neuron diseases, macular degeneration,
epilepsy, Alpers syndrome, Multiple mitochondrial DNA deletion syndrome,
MtDNA depletion syndrome, Complex I deficiency, Complex II (SDH) deficiency,
Complex III deficiency, Cytochrome c oxidase (COX, Complex IV) deficiency,
Complex V deficiency, Adenine Nucleotide Translocator (ANT) deficiency,
Pyruvate dehydrogenase (PDH) deficiency, Ethylmalonic aciduria with lactic
acidemia, 3-Methyl glutaconic aciduria with lactic acidemia, Refractory
epilepsy
with declines during infection, Asperger syndrome with declines during
infection,
Autism with declines during infection, Attention deficit hyperactivity
disorder
(ADHD), Cerebral palsy with declines during infection, Dyslexia with declines
during infection, materially inherited thrombocytopenia and leukemia syndrome,
MARIAHS syndrome (Mitrochondrial ataxia, recurrent infections, aphasia,
hypouricemia/hypomyelination, seizures, and dicarboxylic aciduria), ND6
dystonia,
Cyclic vomiting syndrome with declines during infection, 3-Hydroxy isobutryic
149
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
aciduria with lactic acidemia, Diabetes mellitus with lactic acidemia, Uridine
responsive neurologic syndrome (URNS), Dilated cardiomyopathy, Splenic
Lymphoma, and Renal Tubular Acidosis/Diabetes/Ataxis syndrome.
In other embodiments, the invention provides methods for treating a subject
suffering from mitochondrial disorders arising from, but not limited to, Post-
traumatic head injury and cerebral edema, Stroke (invention methods useful for
preventing or preventing reperfusion injury), Lewy body dementia, Hepatorenal
syndrome, Acute liver failure, NASH (non-alcoholic steatohepatitis), Anti-
metastasis/prodifferentiation therapy of cancer, Idiopathic congestive heart
failure,
Atrial fibrilation (non-valvular), Wolff-Parkinson-White Syndrome, Idiopathic
heart
block, Prevention of reperfusion injury in acute myocardial infarctions,
Familial
migraines, Irritable bowel syndrome, Secondary prevention of non-Q wave
myocardial infarctions, Premenstrual syndrome, Prevention of renal failure in
hepatorenal syndrome, Anti-phospholipid antibody syndrome, Eclampsia/pre-
eclampsia, Oopause infertility, Ischemic heart disease/Angina, and Shy-Drager
and
unclassified dysautonomia syndromes.
In still another embodiment, there are provided methods for the treatment of
mitochondrial disorders associated with pharmacological drug-related side
effects.
Types of pharmaceutical agents that are associated with mitochondrial
disorders
include reverse transcriptase inhibitors, protease inhibitors, inhibitors of
DHOD, and
the like. Examples of reverse transcriptase inhibitors include, for example,
Azidothymidine (AZT), Stavudine (D4T), Zalcitabine (ddC), Didanosine (DDI),
Fluoroiodoarauracil (FIAU), Lamivudine (3TC), Abacavir and the like. Examples
of
protease inhibitors include, for example, Ritonavir, Indinavir, Saquinavir,
Nelfinavir
and the like. Examples of inhibitors of dihydroorotate dehydrogenase (DHOD)
include, for example, Leflunomide, Brequinar, and the like.
Reverse transcriptase inhibitors not only inhibit reverse transcriptase but
also
polymerase gamma which is required for mitochondrial function. Inhibition of
polymerase gamma activity (e.g., with a reverse transcriptase inhibitor)
therefore
leads to mitochondrial dysfunction and/or a reduced mitochondrial mass which
manifests itself in patients as hyperlactatemia. This type of condition may
benefit
150
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
from an increase in the number of mitochondria and/or an improvement in
mitochondrial function, e.g., by administration of a sirtuin activating
compound.
Common symptoms of mitochondrial diseases include cardiomyopathy,
muscle weakness and atrophy, developmental delays (involving motor, language,
cognitive or executive function), ataxia, epilepsy, renal tubular acidosis,
peripheral
neuropathy, optic neuropathy, autonomic neuropathy, neurogenic bowel
dysfunction, sensorineural deafness, neurogenic bladder dysfunction, dilating
cardiomyopathy, migraine, hepatic failure, lactic acidemia, and diabetes
mellitus.
In certain embodiments, the invention provides methods for treating a
disease or disorder that would benefit from increased mitochondrial activity
that
involves administering to a subject in need thereof one or more sirtuin
activating
compounds in combination with another therapeutic agent such as, for example,
an
agent useful for treating mitochondrial dysfunction (such as antioxidants,
vitamins,
or respiratory chain cofactors), an agent useful for reducing a symptom
associated
with a disease or disorder involving mitochondrial dysfunction (such as, an
anti-
seizure agent, an agent useful for alleviating neuropathic pain, an agent
for'treating
cardiac dysfunction), a cardiovascular agent (as described further below), a
chemotherapeutic agent (as described further below), or an anti-
neurodegeneration
agent (as described further below). In an exemplary embodiment, the invention
provides methods for treating a disease or disorder that would benefit from
increased
mitochondrial activity that involves administering to a subject in need
thereof one or
more sirtuin activating compounds in combination with one or more of the
following: coenzyme Q10, L-camitine, thiamine, riboflavin, niacinamide,
folate,
vitamin E, selenium, lipoic acid, or prednisone. Compositions comprising such
combinations are also provided herein.
In exemplary embodiments, the invention provides methods for treating
diseases or disorders that would benefit from increased mitochondrial acitivty
by
administering to a subject a therapeutically effective amount of a sirtuin
activating
compound. Exemplary diseases or disorders include, for example, neuromuscular
disorders (e.g., Friedreich's Ataxia, muscular dystrophy, multiple sclerosis,
etc.),
disorders of neuronal instability (e.g., seizure disorders, migrane, etc.),
developmental delay, neurodegenerative disorders (e.g., Alzheimer's Disease,
151
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Parkinson's Disease, amyotrophic lateral sclerosis, etc.), ischemia, renal
tubular
acidosis, age-related neurodegeneration and cognitive decline, chemotherapy
fatigue, age-related or chemotherapy-induced menopause or irregularities of
menstrual cycling or ovulation, mitochondrial myopathies, mitochondrial damage
(e.g., calcium accumulation, excitotoxicity, nitric oxide exposure, hypoxia,
etc.), and
mitochondrial deregulation.
A gene defect underlying Friedreich's Ataxia (FA), the most common
hereditary ataxia, was recently identified and is designated "frataxin". In
FA, after a
period of normal--development, deficits in coordination develop which progress
to
paralysis and death, typically between the ages of 30 and 40. The tissues
affected
most severely are the spinal cord, peripheral nerves, myocardium, and
pancreas.
Patients typically lose motor control and are confined to wheel chairs, and
are
commonly afflicted with heart failure and diabetes. The genetic basis for FA
involves GAA trinucleotide repeats in an intron region of the gene encoding
frataxin. The presence of these repeats results in reduced transcription and
expression of the gene. Frataxin is involved in regulation of mitochondrial
iron
content. When cellular frataxin content is subnormal, excess iron accumulates
in
mitochondria, promoting oxidative damage and consequent mitochondrial
degeneration and dysfunction. When intermediate numbers of GAA repeats are
present in the frataxin gene intron, the severe clinical phenotype of ataxia
may not
develop. However, these intermediate-length trinucleotide extensions are found
in
to 30% of patients with non-insulin dependent diabetes mellitus, compared to
about 5% of the nondiabetic population. In certain embodiments, sirtuin
activating
compounds may be used for treating patients with disorders related to
deficiencies or
25 defects in frataxin, including Friedreich's Ataxia, myocardial dysfunction,
diabetes
mellitus and complications of diabetes like peripheral neuropathy.
Muscular dystrophy refers to a family of diseases involving deterioration of
neuromuscular structure and function, often resulting in atrophy of skeletal
muscle
and myocardial dysfunction. In the case of Duchenne muscular dystrophy,
mutations
or deficits in a specific protein, dystrophin, are implicated in its etiology.
Mice with
their dystrophin genes inactivated display some characteristics of muscular
dystrophy, and have an approximately 50% deficit in mitochondrial respiratory
152
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
chain activity. A final common pathway for neuromuscular degeneration in most
cases is calcium-mediated impairment of mitochondrial function. In certain
embodiments, sirtuin activating compounds may be used for reducing the rate of
decline in muscular functional capacities and for improving muscular
functional
status in patients with muscular dystrophy.
Multiple sclerosis (MS) is a neuromuscular disease characterized by focal
inflammatory and autoimmune degeneration of cerebral white matter. Periodic
exacerbations or attacks are significantly correlated with upper respiratory
tract and
other infections, both bacterial and viral, indicating that mitochondrial
dysfunction
plays a role in MS. Depression of neuronal mitochondrial respiratory chain
activity
caused by Nitric Oxide (produced by astrocytes and other cells involved in
inflammation) is implicated as a molecular mechanism contributing to MS. In
certain embodiments, sirtuin activating compounds may be used for treatment of
patients with multiple sclerosis, both prophylactically and during episodes of
disease
exacerbation.
Epilepsy is often present in patients with mitochondrial cytopathies,
involving a range of seizure severity and frequency, e.g. absence, tonic,
atonic,
myoclonic, and status epilepticus, occurring in isolated episodes or many
times
daily. In certain embodiments, sirtuin activating compounds may be used for
treating
patients with seizures secondary to mitochondrial dysfunction, including
reducing
frequency and severity of seizure activity.
Metabolic studies on patients with recurrent migraine headaches indicate that
deficits in mitochondrial activity are commonly associated with this disorder,
manifesting as impaired-oxidative phosphorylation and excess lactate
production.
Such deficits are not necessarily due to genetic defects in mitochondrial DNA.
Migraineurs are hypersensitive to nitric oxide, an endogenous inhibitor of
Cytochrome c Oxidase. In addition, patients with mitochondrial cytopathies,
e.g.
MELAS, often have recurrent migraines. In certain embodiments, sirtuin
activating
compounds may be used for treating patients with recurrent migraine headaches,
including headaches refractory to ergot compounds or serotonin receptor
antagonists.
153
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Delays in neurological or neuropsychological development are often found in
children with mitochondrial diseases. Development and remodeling of neural
connections requires intensive biosynthetic activity, particularly involving
synthesis
of neuronal membranes and myelin, both of which require pyrimidine nucleotides
as
cofactors. Uridine nucleotides are involved inactivation and transfer of
sugars to
glycolipids and glycoproteins. Cytidine nucleotides are derived from uridine
nucleotides, and are crucial for synthesis of major membrane phospholipid
constituents like phosphatidylcholine, which receives its choline moiety from
cytidine diphosphocholine. In the case of mitochondrial dysfunction (due to
either
mitochondrial DNA defects or any of the acquired or conditional deficits like
exicitoxic or nitric oxide-mediated mitochondrial dysfunction) or other
conditions
resulting in impaired pyrimidine synthesis, cell proliferation and axonal
extension is
impaired at crucial stages in development of neuronal interconnections and
circuits,
resulting in delayed or arrested development of neuropsychological functions
like
language, motor, social, executive function, and cognitive skills. In autism
for
example, magnetic resonance spectroscopy measurements of cerebral phosphate
compounds indicates that there is global undersynthesis of membranes and
membrane precursors indicated by reduced levels of uridine diphospho-sugars,
and
cytidine nucleotide derivatives involved in membrane synthesis. Disorders
characterized by developmental delay include Rett's Syndrome, pervasive
developmental delay (or PDD-NOS "pervasive developmental delay not otherwise
specified" to distinguish it from specific subcategories like autism), autism,
Asperger's Syndrome, and Attention Deficit/Hyperactivity Disorder (ADHD),
which
is becoming recognized as a delay or lag in development of neural circuitry
underlying executive functions. In certain embodiments, sirtuin activating
compounds may be useful for treating treating patients with neurodevelopmental
delays (e.g., involving motor, language, executive function, and cognitive
skills), or
other delays or arrests of neurological and neuropsychological development in
the
nervous system and somatic development in non-neural tissues like muscle and
endocrine glands.
The two most significant severe neurodegenerative diseases associated with
aging, Alzheimer's Disease (AD) and Parkinson's Disease (PD), both involve
154
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
mitochondrial dysfunction in their pathogenesis. Complex I deficiencies in
particular
are frequently found not only in the nigrostriatal neurons that degenerate in
Parkinson's disease, but also in peripheral tissues and cells like muscle and
platelets
of Parkinson's Disease patients. In Alzheimer's Disease, mitochondrial
respiratory
chain activity is often depressed, especially Complex IV (Cytochrome c
Oxidase).
Moreover, mitochondrial respiratory function altogether is depressed as a
consequence of aging, further amplifying the deleterious sequelae of
additional
molecular lesions affecting respiratory chain function. Other factors in
addition to
primary mitochondrial dysfunction underlie neurodegeneration in AD, PD, and
related disorders. Excitotoxic stimulation and nitric oxide are implicated in
both
diseases, factors which both exacerbate mitochondrial respiratory chain
deficits and
whose deleterious actions are exaggerated on a background of respiratory chain
dysfunction. Huntington's Disease also involves mitochondrial dysfunction in
affected brain regions, with cooperative interactions of excitotoxic
stimulation and
mitochondrial dysfunction contributing to neuronal degeneration. In certain
embodimeiits, sirtuin activating compounds may be useful for treating and
attenuating progression of age-related neurodegenerative disease including AD
and
PD.
One of the major genetic defects in patients with Amyotrophic Lateral
Sclerosis (ALS or Lou Gehrig's Disease) is mutation or deficiency in Copper-
Zinc
Superoxide Dismutase (SOD 1), an antioxidant enzyme. Mitochondria both produce
and are primary targets for reactive oxygen species. Inefficient transfer of
electrons
to oxygen in mitochondria is the most significant physiological source of free
radicals in mammalian systems. Deficiencies in antioxidants or antioxidant
enzymes
can result in or exacerbate mitochondrial degeneration. Mice transgenic for
mutated
SODI develop symptoms and pathology similar to those in human ALS. The
development of the disease in these animals has been shown to involve
oxidative
destruction of mitochondria followed by functional decline of motor neurons
and
onset of clinical symptoms. Skeletal muscle from ALS patients has low
mitochondrial Complex I activity. In certain embodiments, sirtuin activating
compounds may be useful for treating ALS, for reversing or slowing the
progression
of clinical symptoms.
155
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Oxygen deficiency results in both direct inhibition of mitochondrial
respiratory chain activity by depriving cells of a terminal electron acceptor
for
Cytochrome c reoxidation at Complex IV, and indirectly, especially in the
nervous
system, via secondary post-anoxic excitotoxicity and nitric oxide formation.
In
conditions like cerebral anoxia, angina or sickle cell anemia crises, tissues
are
relatively hypoxic. In such cases, compounds that increase mitochondrial
activity
provide protection of affected tissues fi-om deleterious effects of hypoxia,
attenuate
secondary delayed cell death, and accelerate recovery from hypoxic tissue
stress and
injury. In certain embodiments, sirtuin activating compounds may be useful for
preventing delayed cell death (apoptosis in regions like the hippocampus or
cortex
occurring about 2 to 5 days after an episode of cerebral ischemia) after
ischemic or
hypoxic insult to the brain.
Acidosis due to renal dysfunction is often observed in patients with
mitochondrial disease, whether the underlying respiratory chain dysfunction is
congenital or induced by ischemia or cytotoxic agents like cisplatin. Renal
tubular
acidosis often requires administration of exogenous sodium bicarbonate to
maintain
blood and tissue pH. In certain embodiments, sirtuin activating compounds may
be
useful for treating renal tubular acidosis and other forms of renal
dysfunction caused
by mitochondrial respiratory chain deficits.
During normal aging, there is a progressive decline in mitochondrial
respiratory chain function. Beginning about age 40, there is an exponential
rise in
accumulation of mitochondrial DNA defects in humans, and a concurrent decline
in
nuclear-regulated elements of mitochondrial respiratory activity. Many
mitochondrial DNA lesions have a selection advantage during mitochondrial
turnover, especially in postmitotic cells. The proposed mechanism is that
mitochondria with a defective respiratory chain produce less oxidative damage
to
themselves than do mitochondria with intact functional respiratory chains
(mitochondrial respiration is the primary source of free radicals in the
body).
Therefore, normally-functioning mitochondria accumulate oxidative damage to
membrane lipids more rapidly than do defective mitochondria, and are therefore
"tagged" for degradation by lysosomes. Since mitochondria within cells have a
half
life of about 10 days, a selection advantage can result in rapid replacement
of
156
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
functional mitochondria with those with diminished respiratory activity,
especially
in slowly dividing cells. The net result is that once a mutation in a gene for
a
mitochondrial protein that reduces oxidative damage to mitochondria occurs,
such
defective mitochondria will rapidly populate the cell, diminishing or
eliminating its
respiratory capabilities. The accumulation of such cells results in aging or
degenerative disease at the organismal level. This is consistent with the
progressive
mosaic appearance of cells with defective electron transport activity in
muscle, with
cells almost devoid of Cytochrome c Oxidase (COX) activity interspersed
randomly
amidst cells with normal activity, and a higher incidence of COX-negative
cells in
biopsies from older subjects. The organism, during aging, or in a variety of
mitochondrial diseases, is thus faced with a situation in which irreplaceable
postmitotic cells (e.g. neurons, skeletal and cardiac muscle) must be
preserved and
their function maintained to a significant degree, in the face of an
inexorable
progressive decline in mitochondrial respiratory chain function. Neurons with
dysfunctional mitochondria become progressively more sensitive to insults like
excitotoxic injury. Mitochondrial failure contributes to most degenerative
diseases
(especially neurodegeneration) that accompany aging. Congenital mitochondrial
diseases often involve early-onset neurodegeneration similar in fundamental
mechanism to disorders that occur during aging of people born with normal
mitochondria. In certain embodiments, sirtuin activating compounds may be
useful
for treating or attenuating cognitive decline and other degenerative
consequences of
aging.
Mitochondrial DNA damage is more extensive and persists longer than
nuclear DNA damage in cells subjected to oxidative stress or cancer
chemotherapy
agents like cisplatin due to both greater vulnerability and less efficient
repair of
mitochondrial DNA. Although mitochondrial DNA may be more sensitive to
damage than nuclear DNA, it is relatively resistant, in some situations, to
mutagenesis by chemical carcinogens. This is because mitochondria respond to
some types of mitochondrial DNA damage by destroying their defective genomes
rather than attempting to repair them. This results in global mitochondrial
dysfunction for a period after cytotoxic chemotherapy. Clinical use of
chemotherapy
agents like cisplatin, mitomycin, and cytoxan is often accompanied by
debilitating
157
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
"chemotherapy fatigue", prolonged periods of weakness and exercise intolerance
which may persist even after recovery from hematologic and gastrointestinal
toxicities of such agents. In certain embodiments, sirtuin activating
compounds may
be useful for treatment and prevention of side effects of cancer chemotherapy
related
to mitochondrial dysfunction.
A crucial function of the ovary is to maintain integrity of the mitochondrial
genome in oocytes, since mitochondria passed onto a fetus are all derived from
those
present in oocytes at the time of conception. Deletions in mitochondrial DNA
become detectable around the age of menopause, and are also associated with
abnormal menstrual cycles. Since cells cannot directly detect and respond to
defects
in mitochondrial DNA, but can only detect secondary effects that affect the
cytoplasm, like impaired respiration, redox status, or deficits in pyrimidine
synthesis, such products of mitochondrial function participate as a signal for
oocyte
selection and follicular atresia, ultimately triggering menopause when
maintenance
of mitochondriai genomic fidelity and functional activity can no longer be
guaranteed. This is analogous to apoptosis in cells with DNA damage, which
undergo an active process of cellular suicide when genomic fidelity can no
longer be
achieved by repair processes. Women with mitochondrial cytopathies affecting
the
gonads often undergo premature menopause or display primary cycling
abnormalities. Cytotoxic cancer chemotherapy often induces premature
menopause,
with a consequent increased risk of osteoporosis. Chemotherapy-induced
amenorrhea is generally due to primary ovarian failure. The incidence of
chemotherapy-induced amenorrhea increases as a function of age in
premenopausal
women receiving chemotherapy, pointing toward mitochondrial involvement.
Inhibitors of mitochondrial respiration or protein synthesis inhibit hormone-
induced
ovulation, and furthermore inhibit production of ovarian steroid hormones in
response to pituitary gonadotropins. Women with Downs syndrome typically
undergo menopause prematurely, and also are subject to early onset of
Alzheimer-
like dementia. Low activity of cytochrome oxidase is consistently found in
tissues of
Downs patients and in late-onset Alzheimer's Disease. Appropriate support of
mitochondrial function or compensation for mitochondrial dysfunction therefore
is
useful for protecting against age-related or chemotherapy-induced menopause or
158
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
irregularities of menstrual cycling or ovulation. In certain embodiments,
sirtuin
activating compounds may be useful for treating and preventing amenorrhea,
irregular ovulation, menopause, or secondary consequences of menopause.
In certain embodiments, sirtuin modulating compounds may be useful for
treatment mitochondrial myopathies. Mitochondrial myopathies range from mild,
slowly progressive wealcness of the extraocular muscles to severe, fatal
infantile
myopathies and multisystem encephalomyopathies. Some syndromes have been
defined, with some overlap between them. Established syndromes affecting
muscle
include progressive external ophthalmoplegia, the Kearns-Sayre syndrome (with
ophthalmoplegia, pigmentary retinopathy, cardiac conduction defects,
cerebellar
ataxia, and sensorineural deafness), the MELAS syndrome (mitochondrial
encephalomyopathy, lactic acidosis, and stroke-like episodes), the MERFF
syndrome (myoclonic epilepsy and ragged red fibers), limb-girdle distribution
weakness, and infantile myopathy (benign or severe and fatal). Muscle biopsy
specimens stained with modified Gomori's trichrome stain show ragged red
fibers
due to excessive accumulation of mitochondria. Biochemical defects in
substrate
transport and utilization, the Krebs cycle, oxidative phosphorylation, or the
respiratory chain are detectable. Numerous mitochondrial DNA point mutations
and
deletions have been described, transmitted in a maternal, noninendelian
inheritance
pattern. Mutations in nuclear-encoded mitochondrial enzymes occur.
In certain embodiments, sirtuin activating compounds may be useful for
treating patients suffering from toxic damage to mitochondria, such as, toxic
damage
due to calcium accumulation, excitotoxicity, nitric oxide exposure, drug
induced
toxic damage, or hypoxia.
A fundamental mechanism of cell injury, especially in excitable tissues,
involves excessive calcium entry into cells, as a result of either leakage
through the
plasma membrane or defects in intracellular calcium handling mechanisms.
Mitochondria are major sites of calcium sequestration, and preferentially
utilize
energy from the respiratory chain for taking up calcium rather than for ATP
synthesis, which results in a downward spiral of mitochondrial failure, since
calcium
uptake into mitochondria results in diminished capabilities for energy
transduction.
159
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Excessive stimulation of neurons with excitatory amino acids is a common
mechanism of cell death or injury in the central nervous system. Activation of
glutamate receptors, especially of the subtype designated NMDA receptors,
results
in mitochondrial dysfunction, in part through elevation of intracellular
calcium
during excitotoxic stimulation. Conversely, deficits in mitochondrial
respiration and
oxidative phosphorylation sensitizes cells to excitotoxic stimuli, resulting
in cell
death or injury during exposure to levels of excitotoxic neurotransmitters or
toxins
that would be innocuous to normal cells.
Nitric oxide (about 1 micromolar) inhibits cytochrome oxidase (Complex IV)
and thereby inhibits mitochondrial respiration; moreover, prolonged exposure
to
nitric oxide (NO) irreversibly reduces Complex I activity. Physiological or
pathophysiological concentrations of NO thereby inhibit pyrimidine
biosynthesis.
Nitric oxide is implicated in a variety of neurodegenerative disorders
including
inflammatory and autoimmune diseases of the central nervous system, and is
involved in mediation of excitotoxic and post-hypoxic damage to neurons.
Oxygen is the terminal electron acceptor in the respiratory chain. Oxygen
deficiency impairs electron transport chain activity, resulting in diminished
pyrimidine synthesis as well as diminished ATP synthesis via oxidative
phosphorylation. Human cells proliferate and retain viability under virtually
anaerobic conditions if provided with uridine and pyruvate (or a similarly
effective
agent for oxidizing NADH to optimize glycolytic ATP production).
In certain embodiments, sirtuin activating compounds may be useful for
treating diseases or disorders associated with mitochondrial deregulation.
Transcription of mitochondrial DNA encoding respiratory chain components
requires nuclear factors. In neuronal axons, mitochondria must shuttle back
and forth
to the nucleus in order to maintain respiratory chain activity. If axonal
transport is
impaired by hypoxia or by drugs like taxol which affect microtubule stability,
mitochondria distant from the nucleus undergo loss of cytochrome oxidase
activity.
Accordingly, treatment with a sirtuin activating compound may be useful for
promoting nuclear-mitochondrial interactions.
Mitochondria are the primary source of free radicals and reactive oxygen
species, due to spillover from the mitochondrial respiratory chain, especially
when
160
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
defects in one or more respiratory chain components impairs orderly transfer
of
electrons from metabolic intermediates to molecular oxygen. To reduce
oxidative
damage, cells can compensate by expressing mitochondrial uncoupling proteins
(UCP), of which several have been identified. UCP-2 is transcribed in response
to
oxidative damage, inflammatory cytokines, or excess lipid loads, e.g. fatty
liver and
steatohepatitis. UCPs reduce spillover of reactive oxygen species from
mitochondria
by discharging proton gradients across the mitochondrial inner membrane, in
effect
wasting energy produced by metabolism and rendering cells vulnerable to energy
stress as a trade-off for reduced oxidative injury.
Muscle Perfornzance
In other embodiments, the invention provides methods for enhancing muscle
performance by administering a therapeutically effective amount of a sirtuin
activating compound. For example, sirtuin activating compounds may be useful
for
improving physical endurance (e.g., ability to perform a physical task such as
exercise, physical labor, sports activities, etc.), inhibiting or retarding
physical
fatigues, enhancing blood oxygen levels, enhancing energy in healthy
individuals,
enhance working capacity and endurance, reducing muscle fatigue, reducing
stress,
enhancing cardiac and cardiovascular function, improving sexual ability,
increasing
muscle ATP levels, and/or reducing lactic acid in blood. In certain
embodiments,
the methods involve administering an amount of a sirtuin activating compound
that
increase mitochondrial activity, increase mitochondrial biogenesis, increase
mitochondrial mass, or a high dose of a sirtuin activating compound.
Sports performance refers to the ability of the athlete's muscles to perform
when participating in sports activities. Enhanced sports performance,
strength, speed
and endurance are measured by an increase in muscular contraction strength,
increase in amplitude of muscle contraction, shortening of muscle reaction
time
between stimulation and contraction. Athlete refers to an individual who
participates in sports at any level and who seeks to achieve an improved level
of
strength, speed and endurance in their performance, such as, for example, body
builders, bicyclists, long distance runners, short distance runners, etc. An
athlete
may be hard training, that is, performs sports activities intensely more than
three
days a week or for competition. An athlete may also be a fitness enthusiast
who
161
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
seeks to improve general health and well-being, improve energy levels, who
works
out for about 1-2 hours about 3 times a week. Enhanced sports performance in
manifested by the ability to overcome muscle fatigue, ability to maintain
activity for
longer periods of time, and have a more effective workout.
In the arena of athlete muscle performance, it is desirable to create
conditions
that permit competition or training at higher levels of resistance for a
prolonged
period of time. However, acute and intense anaerobic use of skeletal muscles
often
results in impaired athletic performance, with losses in force and work
output, and
increased onset of muscle fatigue, soreness, and dysfunction. It is now
recognized
that even a single exhaustive exercise session, or for that matter any acute
trauma to
the body such as muscle injury, resistance or exhaustive muscle exercise, or
elective
surgery, is characterized by perturbed metabolism that affects muscle
performance
in both short and long term phases. Both muscle metabolic/enzymatic activity
and
gene expression are affected. For example, disruption of skeletal muscle
nitrogen
metabolism as well as depletion of sources of metabolic energy occur during
extensive muscle activity. Amino acids, including branclied-chain amino acids,
are
released from muscles followed by their deamination to elevate serum ammonia
and
local oxidation as muscle fuel sources, which augments metabolic acidosis. In
addition, there is a decline in catalytic efficiency of muscle contraction
events, as
well as an alteration of enzymatic activities of nitrogen and energy
metabolism.
Further, protein catabolism is initiated where rate of protein synthesis is
decreased
coupled with an increase in the degradation of non-contractible protein. These
metabolic processes are also accompanied by free radical generation which
further
damages muscle cells.
Recovery from fatigue during acute and extended exercise requires reversal
of metabolic and non-metabolic fatiguing factors. Known factors that
participate in
human muscle fatigue, such as lactate, ammonia, hydrogen ion, etc., provide an
incomplete and unsatisfactory explanation of the fatigue/recovery process, and
it is
likely that additional unknown agents participate (Baker et al., J. Appl.
Physiol.
74:2294-2300, 1993; Bazzarre et al., J Am. Coll. Nutr. 11:505-511, 1992; Dohm
et
al., Fed. Proc. 44:348-352, 1985; Edwards In: Biochemistry of Exercise,
Proceedings of the Fifth International Symposium on the Biochemistry of
Exercise
162
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
(Kutrgen, Vogel, Poormans, eds.), 1983; MacDougall et al., Acta Physiol.
Scand.
146:403-404, 1992; Walser et al., Kidney Int. 32:123-128, 1987). Several
studies
have also analyzed the effects of nutritional supplements and herbal
supplements in
enhancing muscle performance.
Aside from muscle performance during endurance exercise, free radicals and
oxidative stress parameters are affected in pathophysiological states. A
substantial
body of data now suggests that oxidative stress contributes to muscle wasting
or
atrophy in pathophysiological states (reviewed in Clarkson, P. M. Antioxidants
and
physical performance. Crit. Rev. Food Sci. Nutr. 35: 31-41; 1995; Powers, S.
K.;
Lennon, S. L. Analysis of cellular responses to free radicals: Focus on
exercise and
skeletal muscle. Proc. Nutr. Soc. 58: 1025-1033; 1999). For example, with
respect to
muscular disorders where both muscle endurance and function are compensated,
the
role of nitric oxide (NO), has been implicated. In muscular dystrophies,
especially
those due to defects in proteins that make up the dystrophin-glycoprotein
complex
(DGC), the enzyme that synthesizes NO, nitric oxide synthase (NOS), has been
associated. Recent studies of dystrophies related to DGC defects suggest that
one
mechanism of cellular injury is functional ischemia related to alterations in
cellular
NOS and disruption of a normal protective action of NO. This protective action
is
the prevention of local ischemia during contraction-induced increases in
sympathetic
vasoconstriction. Rando (Microsc Res Tech 55(4):223-35, 2001), has shown that
oxidative injury precedes pathologic changes and that muscle cells with
defects in
the DGC have an increased susceptibility to oxidant challenges. Excessive
lipid
peroxidation due to free radicals has also been shown to be a factor in
myopathic
diseases such as McArdle's disease (Russo et al., Med Hypotheses. 39(2):147-
51,
1992). Furthermore, mitochondrial dysfunction is a well-known correlate of age-
related muscle wasting (sarcopenia) and free radical damage has been
suggested,
though poorly investigated, as a contributing factor (reviewed in Navarro, A.;
Lopez-Cepero, J. M.; Sanchez del Pino, M. L. Front. Biosci. 6: D26-44; 2001).
Other indications include acute sarcopenia, for example muscle atrophy and/or
cachexia associated with burns, bed rest, limb immobilization, or major
thoracic,
abdominal, andlor orthopedic surgery. It is contemplated that the methods of
the
163
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
present invention will also be effective in the treatment of muscle related
pathological conditions.
In certain embodiments, the invention provides novel dietary compositions
comprising sirtuin modulators, a method for their preparation, and a method of
using
the compositions for improvement of sports performance. Accordingly, provided
are
therapeutic compositions, foods and beverages that have actions of improving
physical endurance and/or inhibiting physical fatigues for those people
involved in
broadly-defined exercises including sports requiring endurance and labors
requiring
repeated muscle exertions. Such dietary compositions may additional comprise
electrolytes, caffeine, vitamins, carbohydrates, etc.
Aging/Stress
In certain embodiments, the invention provides methods for increasing
cellular lifespan or preventing apoptosis comprising administering a high dose
of a
sirtuin activating compound to a subject that would benefit from increased
cell
lifespan or decreased apoptosis. For example, skin can be protected from aging
(e.g., developing wrinkles, loss of elasticity, etc.) by treating skin or
epithelial cells
with a high dose of a sirtuin activating compound. In an exemplary embodiment,
skin is contacted with a pharmaceutical or cosmetic composition comprising a
high
dose of a sirtuin activating compound. Exemplary skin afflictions or skin
conditions that may be treated in accordance with the methods described herein
include disorders or diseases associated with or caused by inflammation, sun
damage or natural aging. For example, the compositions find utility in the
prevention or treatment of contact dermatitis (including irritant contact
dermatitis
and allergic contact dermatitis), atopic dermatitis (also known as allergic
eczema),
actinic keratosis, keratinization disorders (including eczema), epidermolysis
bullosa
diseases (including penfigus), exfoliative dermatitis, seborrheic dermatitis,
erythemas (including erythema multiforme and erythema nodosum), damage
caused by the sun or other light sources, discoid lupus erythematosus,
dermatomyositis, psoriasis, skin cancer and the effects of natural aging. In
another
embodiment, a high dose of a sirtuin activating compound may be used for the
treatment of wounds and/or burns to promote healing, including, for example,
first-,
second- or third-degree burns and/or a thermal, chemical or electrical burns.
The
164
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
formulations may be administered topically, to the skin or mucosal tissue, as
an
ointment, lotion, cream, microemulsion, gel, solution or the like, as further
described herein, within the context of a dosing regimen effective to bring
about the
desired result.
Topical formulations comprising a high dose of one or more sirtuin
activating compounds may also be used as preventive, e.g., chemopreventive,
compositions. When used in a chemopreventive method, susceptible skin is
treated
prior to any visible condition in a particular individual.
Sirtuin activating compounds may be delivered locally or systemically to a
subject. In one embodiment, a high dose of a sirtuin activating compound is
delivered locally to a tissue or organ of a subject by injection, topical
formulation,
etc.
In another embodiment, a high dose of a sirtuin activating compound may
be used for treating or preventing a disease or condition induced or
exacerbated by
cellular senescence in a subject; methods for decreasing the rate of
senescence of a
subject, e.g., after onset of senescence; methods for extending the lifespan
of a
subject; methods for treatihg or preventing a disease or condition relating to
lifespan; methods for treating or preventing a disease or condition relating
to the
proliferative capacity of cells; and methods for treating or preventing a
disease or
condition resulting from cell damage or death. In certain embodiments, the
method
does not act by decreasing the rate of occurrence of diseases that shorten the
lifespan of a subject. In certain embodiments, a method does not act by
reducing
the lethality caused by a disease, such as cancer.
In yet another embodiment, a high dose of a sirtuin activating compound
may be administered to a subject in order to generally increase the lifespan
of its
cells and to protect its cells against stress and/or against apoptosis. It is
believed
that treating a subject with a compound described herein is similar to
subjecting the
subject to hormesis, i.e., mild stress that is beneficial to organisms and may
extend
their lifespan.
A high dose of a sirtuin activating compound may be administered to a
subject to prevent aging and aging-related consequences or diseases, such as
stroke,
heart disease, heart failure, arthritis, high blood pressure, and Alzheimer's
disease.
165
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Other conditions that can be treated include ocular disorders, e.g.,
associated with
the aging of the eye, such as cataracts, glaucoma, and macular degeneration. A
high dose of a sirtuin activating compound can also be administered to
subjects for
treatment of diseases, e.g., chronic diseases, associated with cell death, in
order to
protect the cells from cell death. Exemplary diseases include those associated
with
neural cell death, neuronal dysfunction, or muscular cell death or
dysfunction, such
as Parkinson's disease, Alzheimer's disease, multiple sclerosis, amniotropic
lateral
sclerosis, and muscular dystrophy; AIDS; fulminant hepatitis; diseases linked
to
degeneration of the brain, such as Creutzfeld-Jakob disease, retinitis
pigmentosa
and cerebellar degeneration; myelodysplasis such as aplastic anemia; ischemic
diseases such as myocardial infarction and stroke; hepatic diseases such as
alcoholic hepatitis, hepatitis B and hepatitis C; joint-diseases such as
osteoarthritis;
atherosclerosis; alopecia; damage to the skin due to UV light; lichen planus;
atrophy of the skin; cataract; and graft rejections. Cell death can also be
caused by
surgery, drug therapy, chemical exposure or radiation exposure.
A high dose of a sirtuin activating compound can also be administered to a
subject suffering from an acute disease, e.g., damage to an organ or tissue,
e.g., a
subject suffering from stroke or myocardial infarction or a subject suffering
from a
spinal cord injury. A high dose of a sirtuin activating compound may also be
used
to repair an alcoholic's liver.
Cardiovascular Disease
In another embodiment, the invention provides a method for treating and/or
preventing a cardiovascular disease by administering to a subject in need
thereof a
high dose of a sirtuin activating compound.
Cardiovascular diseases that can be treated or prevented using a high dose
of a sirtuin activating compound include cardiomyopathy or myocarditis; such
as
idiopathic cardiomyopathy, metabolic cardiomyopathy, alcoholic cardiomyopathy,
drug-induced cardiomyopathy, ischemic cardiomyopathy, and hypertensive
cardiomyopathy. Also treatable or preventable using compounds and methods
described herein are atheromatous disorders of the major blood vessels
(macrovascular disease) such as the aorta, the coronary arteries, the carotid
arteries,
the cerebrovascular arteries, the renal arteries, the iliac arteries, the
femoral arteries,
'166
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
and the popliteal arteries. Other vascular diseases that can be treated or
prevented
include those related to platelet aggregation, the retinal arterioles, the
glomerular
arterioles, the vasa nervorum, cardiac arterioles, and associated capillary
beds of
the eye, the kidney, the heart, and the central and peripheral nervous
systems. A
high dose of a sirtuin activating compound may also be used for increasing HDL
levels in plasma of an individual.
Yet other disorders that may be treated with a high dose of a sirtuin
activating compound include restenosis, e.g., following coronary intervention,
and
disorders relating to an abnormal level of high density and low density
cholesterol.
In one embodiment, a high dose of a sirtuin activating compound may be
administered as part of a combination therapeutic with another cardiovascular
agent
including, for example, an anti-arrhythmic agent, an antihypertensive agent, a
calcium channel blocker, a cardioplegic solution, a cardiotonic agent, a
fibrinolytic
agent, a sclerosing solution, a vasoconstrictor agent, a vasodilator agent, a
nitric
oxide donor, a potassium channel blocker, a sodium channel blocker, statins,
or a
naturiuretic agent.
In one embodiment, a high dose of a sirtuin activating compound may be
administered as part of a combination therapeutic with an anti-arrhythmia
agent.
Anti-arrhythmia agents are often organized into four main groups according to
their
mechanism of action: type I, sodium channel blockade; type II, beta-adrenergic
blockade; type III, repolarization prolongation; and type IV, calcium channel
blockade. Type I anti-arrhythmic agents include lidocaine, moricizine,
mexiletine,
tocainide, procainamide, encainide, flecanide, tocainide, phenytoin,
propafenone,
quinidine, disopyramide, and flecainide. Type II anti-arrhythmic agents
include
propranolol and esmolol. Type III includes agents that act by prolonging the
duration of the action potential, such as amiodarone, artilide, bretylium,
clofilium,
isobutilide, sotalol, azimilide, dofetilide, dronedarone, ersentilide,
ibutilide,
tedisamil, and trecetilide. Type IV anti-arrhythmic agents include verapamil,
diltaizem, digitalis, adenosine, nickel chloride, and magnesium ions.
In another embodiment, a high dose of a sirtuin activating compound may be
administered as part of a combination therapeutic with another cardiovascular
agent.
Examples of cardiovascular agents include vasodilators, for example,
hydralazine;
167
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
angiotensin converting enzyme inhibitors, for example, captopril; anti-anginal
agents, for example, isosorbide nitrate, glyceryl trinitrate and
pentaerythritol
tetranitrate; anti-arrhythmic agents, for example, quinidine, procainaltide
and
lignocaine; cardioglycosides, for example, digoxin and digitoxin; calcium
antagonists, for example, verapamil and nifedipine; diuretics, such as
thiazides and
related compounds, for example, bendrofluazide, chlorothiazide,
chlorothalidone,
hydrochlorothiazide and other diuretics, for example, fursemide and
triamterene, and
sedatives, for example, nitrazepam, flurazepam and diazepam.
Other exemplary cardiovascular agents include, for example, a
cyclooxygenase inhibitor such as aspirin or indomethacin, a platelet
aggregation
inhibitor such as clopidogrel, ticlopidene or aspirin, fibrinogen antagonists
or a
diuretic such as chlorothiazide, hydrochlorothiazide, flumethiazide,
hydroflumethiazide, bendroflumethiazide, methylchlorthiazide,
trichloromethiazide,
polythiazide or benzthiazide as well as ethacrynic acid tricrynafen,
chlorthalidone,
furosemide, musolimine, bumetanide, triamterene, amiloride and spironolactone
and
salts of such compounds, angiotensin converting enzyme inhibitors such as
captopril, zofenopril, fosinopril, enalapril, ceranopril, cilazopril,
delapril, pentopril,
quinapril, ramipril, lisinopril, and salts of such compounds, angiotensin II
antagonists such as losartan, irbesartan or valsartan, thrombolytic agents
such as
tissue plasminogen activator (tPA), recombinant tPA, streptokinase, urokinase,
prourokinase, and anisoylated plasminogen streptokinase activator complex
(APSAC, Eminase, Beecham Laboratories), or animal salivary gland plasminogen
activators, calcium channel blocking agents such as verapamil, nifedipine or
diltiazem, thromboxane receptor antagonists such as ifetroban, prostacyclin
mimetics, or phosphodiesterase inhibitors. Such combination products if
formulated
as a fixed dose employ the compounds of this invention within the dose range
described above and the other pharmaceutically active agent within its
approved
dose range.
Yet other exemplary cardiovascular agents include, for example,
vasodilators, e.g., bencyclane, cinnarizine, citicoline, cyclandelate,
cyclonicate,
ebumamonine, phenoxezyl, flunarizine, ibudilast, ifenprodil, lomerizine,
naphlole,
nikamate, nosergoline, nimodipine, papaverine, pentifylline, nofedoline,
vincamin,
168
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
vinpocetine, vichizyl, pentoxifylline, prostacyclin derivatives (such as
prostaglandin
E 1 and prostaglandin 12), an endothelin receptor blocking drug (such as
bosentan),
diltiazem, nicorandil, and nitroglycerin. Examples of the cerebral protecting
drug
include radical scavengers (such as edaravone, vitamin E, and vitamin C),
glutamate
antagonists, AMPA antagonists, kainate antagonists, NMDA antagonists, GABA
agonists, growth factors, opioid antagonists, phosphatidylcholine precursors,
serotonin agonists, Na"/Ca2+chamiel inhibitory drugs, and K+ channel opening
drugs. Examples of the brain metabolic stimulants include amantadine,
tiapride, and
y-aminobutyric acid. Examples of the anticoagulant include heparins (such as
heparin sodium, heparin potassium, dalteparin sodium, dalteparin calcium,
heparin
calcium, parnaparin sodium, reviparin sodium, and danaparoid sodium),
warfarin,
enoxaparin, argatroban, batroxobin, and sodium citrate. Examples of the
antiplatelet
drug include ticlopidine hydrochloride, dipyridamole, cilostazol, ethyl
icosapentate,
sarpogrelate hydrochloride, dilazep hydrochloride, trapidil, a nonsteroidal
antiinflammatory agent (such as aspirin), beraprostsodium, iloprost, and
indobufene.
Examples of the thrombolytic drug include urokinase, tissue-type plasminogen
activators (such as alteplase, tisokinase, nateplase, pamiteplase, monteplase,
and
rateplase), and nasaruplase. Examples of the antihypertensive drug include
angiotensin converting enzyme inhibitors (such as captopril, alacepril,
lisinopril,
imidapril, quinapril, temocapril, delapril, benazepril, cilazapril,
trandolapril,
enalapril, ceronapril, fosinopril, imadapril, mobertpril, perindopril,
ramipril,
spirapril, and randolapril), angiotensin II antagonists (such as losartan,
candesartan,
valsartan, eprosartan, and irbesartan), calcium channel blocking drugs (such
as
aranidipine, efonidipine, nicardipine, bamidipine, benidipine, manidipine,
cilnidipine, nisoldipine, nitrendipine, nifedipine, nilvadipine, felodipine,
amlodipine,
diltiazem, bepridil, clentiazem, phendilin, galopamil, mibefradil,
prenylamine,
semotiadil, terodiline, verapamil, cilnidipine, elgodipine, isradipine,
lacidipine,
lercanidipine, nimodipine, cinnarizine, flunarizine, lidoflazine, lomerizine,
bencyclane, etafenone, and perhexiline), (3-adrenaline receptor blocking drugs
(propranolol, pindolol, indenolol, carteolol, bunitrolol, atenolol,
acebutolol,
metoprolol, timolol, nipradilol, penbutolol, nadolol, tilisolol, carvedilol,
bisoprolol,
betaxolol, celiprolol, bopindolol, bevantolol, labetalol, alprenolol,
amosulalol,
169
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
arotinolol, befunolol, bucumolol, bufetolol, buferalol, buprandolol,
butylidine,
butofilolol, carazolol, cetamolol, cloranolol, dilevalol, epanolol,
levobunolol,
mepindolol, metipranolol, moprolol, nadoxolol, nevibolol, oxprenolol, practol,
pronetalol, sotalol, sufinalol, talindolol, tertalol, toliprolol, xybenolol,
and esmolol),
a-receptor blocking drugs (such as amosulalol, prazosin, terazosin, doxazosin,
bunazosin, urapidil, phentolamine, arotinolol, dapiprazole, fenspiride,
indoramin,
labetalol, naftopidil, nicergoline, tamsulosin, tolazoline, trimazosin, and
yohimbine),
sympathetic nerve inhibitors (such as clonidine, guanfacine, guanabenz,
methyldopa,
and reserpine), hydralazine, todralazine, budralazine, and cadralazine.
Examples of
the antianginal drug include nitrate drugs (such as amyl nitrite,
nitroglycerin, and
isosorbide), (3-adrenaline receptor blocking drugs (such as propranolol,
pindolol,
indenolol, carteolol, bunitrolol, atenolol, acebutolol, metoprolol, timolol,
nipradilol,
penbutolol, nadolol, tilisolol, carvedilol, bisoprolol, betaxolol, celiprolol,
bopindolol, bevantolol, labetalol, alprenolol, amosulalol, arotinolol,
befunolol,
bucumolol, bufetolol, buferalol, buprandolol, butylidine, butofilolol,
carazolol,
cetainolol, cloranolol, dilevalol, epanolol, levobunolol, mepindolol,
metipranolol,
moprolol, nadoxolol, nevibolol, oxprenolol, practol, pronetalol, sotalol,
sufinalol,
talindolol, tertalol, toliprolol, andxybenolol), calcium channel blocking
drugs (such
as aranidipine, efonidipine, nicardipine, bamidipine, benidipine, manidipine,
cilnidipine, nisoldipine, nitrendipine, nifedipine, nilvadipine, felodipine,
amlodipine,
diltiazem, bepridil, clentiazem, phendiline, galopamil, mibefradil,
prenylamine,
semotiadil, terodiline, verapamil, cilnidipine, elgodipine, isradipine,
lacidipine,
lercanidipine, nimodipine, cinnarizine, flunarizine, lidoflazine, lomerizine,
bencyclane, etafenone, and perhexiline) trimetazidine, dipyridamole,
etafenone,
dilazep, trapidil, nicorandil, enoxaparin, and aspirin. Examples of the
diuretic
include thiazide diuretics (such as hydrochlorothiazide, methyclothiazide,
trichlormethiazide, benzylhydrochlorothiazide, and penflutizide), loop
diuretics
(such as furosemide, etacrynic acid, bumetanide, piretanide, azosemide, and
torasemide), K+ sparing diuretics (spironolactone, triamterene,
andpotassiumcanrenoate), osmotic diuretics (such as isosorbide, D-mannitol,
and
glycerin), nonthiazide diuretics (such as meticrane, tripamide,
chlorthalidone, and
mefruside), and acetazolamide. Examples of the cardiotonic include digitalis
170
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
formulations (such as digitoxin, digoxin, methyldigoxin, deslanoside,
vesnarinone,
lanatoside C, and proscillaridin), xanthine formulations (such as
aminophylline,
choline theophylline, diprophylline, and proxyphylline), catecholamine
forinulations
(such as dopamine, dobutamine, and docarpamine), PDE III inhibitors (such as
amrinone, olprinone, and milrinone), denopamine, ubidecarenone, pimobendan,
levosimendan, aminoethylsulfonic acid, vesnarinone, carperitide, and colforsin
daropate. Examples of the antiarrhythmic drug include ajmaline, pirmenol,
procainamide, cibenzoline, disopyramide, quinidine, aprindine, mexiletine,
lidocaine, phenyloin, pilsicainide, propafenone, flecainide, atenolol,
acebutolol,
sotalol, propranolol, metoprolol, pindolol, amiodarone, nifekalant, diltiazem,
bepridil, and verapamil. Examples of the antihyperlipidemic drug include
atorvastatin, simvastatin, pravastatin sodium, fluvastatin sodium,
clinofibrate,
clofibrate, simfibrate, fenofibrate, bezafibrate, colestimide, and
colestyramine.
Examples of the immunosuppressant include azathioprine, mizoribine,
cyclosporine,
tacrolimus, gusperimus, and methotrexate.
Cell Death/Cancer
A high dose of a sirtuin activating compound may be administered to
subjects who have recently received or are likely to receive a dose of
radiation or
toxin. In one embodiment, the dose of radiation or toxin is received as part
of a
work-related or medical procedure, e.g., working in a nuclear power plant,
flying an
airplane, an X-ray, CAT scan, or the administration of a radioactive dye for
medical
imaging; in such an embodiment, the high dose of the sirtuin activating
compound
is administered as a prophylactic measure. In another embodiment, the
radiation or
toxin exposure is received unintentionally, e.g., as a result of an industrial
accident,
habitation in a location of natural radiation, terrorist act, or act of war
involving
radioactive or toxic material. In such a case, the high dose of the sirtuin
activating
compound is preferably administered as soon as possible after the exposure to
inhibit apoptosis and the subsequent development of acute radiation syndrome.
Neuronal Diseases/Disorders
In certain aspects, a high dose of a sirtuin activating compound can be used
to treat patients suffering from neurodegenerative diseases, and traumatic or
mechanical injury to the central nervous system (CNS), spinal cord or
peripheral
171
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
nervous system (PNS). Neurodegenerative disease typically involves reductions
in
the mass and volume of the human brain, which may be due to the atrophy and/or
death of brain cells, which are far more profound than those in a healthy
person that
are attributable to aging. Neurodegenerative diseases can evolve gradually,
after a
long period of normal brain function, due to progressive degeneration (e.g.,
nerve
cell dysfunction and death) of specific brain regions. Alternatively,
neurodegenerative diseases can have a quick onset, such as those associated
with
trauma or toxins. The actual onset of brain degeneration may precede clinical
expression by many years. Examples of neurodegenerative diseases include, but
are not limited to, Alzheimer's disease (AD), Parkinson's disease (PD),
Huntington's disease (HD), amyotrophic lateral sclerosis (ALS; Lou Gehrig's
disease), diffuse Lewy body disease, chorea-acanthocytosis, primary lateral
sclerosis, ocular diseases (ocular neuritis), chemotherapy-induced
neuropathies
(e.g., from vincristine, paclitaxel, bortezomib), diabetes-induced
neuropathies and
Friedreich's ataxia. Sirtuin-modulating compounds that increase the level
and/or
activity of a sirtuin protein can be used to treat these disorders and others
as
described below.
AD is a chronic, incurable, and unstoppable CNS disorder that occurs
gradually, resulting in memory loss, unusual behavior, personality changes,
and a
decline in thinking abilities. These losses are related to the death of
specific types of
brain cells and the breakdown of connections and their supporting network
(e.g. glial
cells) between them. AD has been described as childhood development in
reverse.
In most people with AD, symptoms appear after the age 60. The earliest
symptoms
include loss of recent memory, faulty judgment, and changes in personality.
Later in
the disease, those with AD may forget how to do simple tasks like washing
their
hands. Eventually people with AD lose all reasoning abilities and become
dependent on other people for their everyday care. Finally, the disease
becomes so
debilitating that patients are bedridden and typically develop coexisting
illnesses.
PD is a chronic, incurable, and unstoppable CNS disorder that occurs
gradually and results in uncontrolled body movements, rigidity, tremor, and
dyskinesia. These motor system problems are related to the death of brain
cells in
an area of the brain that produces dopamine, a chemical that helps control
muscle
172
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
activity. In most people with PD, symptoms appear after age 50. The initial
symptoms of PD are a pronounced tremor affecting the extremities, notably in
the
hands or lips. Subsequent characteristic symptoms of PD are stiffness or
slowness
of movement, a shuffling walk, stooped posture, and impaired balance. There
are
wide ranging secondary symptoms such as memory loss, dementia, depression,
emotional changes, swallowing difficulties, abnormal speech, sexual
dysfunction,
and bladder and bowel problems. These symptoms will begin to interfere with
routine activities, such as holding a fork or reading a newspaper. Finally,
people
with PD become so profoundly disabled that they are bedridden.
ALS (motor neuron disease) is a chronic, incurable, and unstoppable CNS
disorder that attacks the motor neurons, components of the" CNS that connect
the
brain to the skeletal muscles. In ALS, the motor neurons deteriorate and
eventually
die, and though a person's brain normally remains fully functioning and alert,
the
command to move never reaches the muscles. Most people who get ALS are
between 40 and 70 years old. The first motor neurons that weaken are those
controlling the arms or legs. Those with ALS may have trouble walking, they
may
drop things, fall, slur their speech, and laugh or cry uncontrollably.
Eventually the
muscles in the limbs begin to atrophy from disuse. This muscle weakness will
become debilitating and a person will need a wheel chair or become unable to
function out of bed.
The causes of these neurological diseases have remained largely unknown.
They are conventionally defined as distinct diseases, yet clearly show
extraordinary
similarities in basic processes and commonly demonstrate overlappiiig symptoms
far
greater than would be expected by chance alone. Current disease definitions
fail to
properly deal with the issue of overlap and a new classification of the
neurodegenerative disorders has been called for.
HD is another neurodegenerative disease resulting from genetically
programmed degeneration of neurons in certain areas of the brain. This
degeneration causes uncontrolled movements, loss of intellectual faculties,
and
emotional disturbance. HD is a familial disease, passed from parent to child
through
a dominant mutation in the wild-type gene. Some early symptoms of HD are mood
swings, depression, irritability or trouble driving, learning new things,
remembering
173
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
a fact, or making a decision. As the disease progresses, concentration on
intellectual
tasks becomes increasingly difficult and the patient may have difficulty
feeding
himself or herself and swallowing.
Tay-Sachs disease and Sandhoff disease are glycolipid storage diseases
caused by the lack of lysosomal (i-hexosaminidase (Gravel et al., in The
Metabolic
Basis of Inherited Disease, eds. Scriver et al., McGraw-Hill, New York, pp.
2839-
2879, 1995). In both disorders, GM2 ganglioside and related
glycolipidssubstrates
for (3-hexosaminidase accumulate in the nervous system and trigger acute
neurodegeneration. In the most severe forms, the onset of symptoms begins in
early
infancy. A precipitous neurodegenerative course then ensues, with affected
infants
exhibiting motor dysfunction, seizure, visual loss, and deafness. Death
usually
occurs by 2-5 years of age. Neuronal loss through an apoptotic mechanism has
been
demonstrated (Huang et al., Hum. Mol. Genet. 6: 1879-1885, 1997).
It is well-known that apoptosis plays a role in AIDS pathogenesis in the
immune system. However, HIV-1 also induces neurological disease. Shi et al.
(J.
Clin. Invest. 98: 1979-1990, 1996) examined apoptosis induced by HIV-1
infection
of the CNS in an in vitro model and in brain tissue from AIDS patients, and
found
that HIV-1 infection of primary brain cultures induced apoptosis in neurons
and
astrocytes in vitro. Apoptosis of neurons and astrocytes was also detected in
brain
tissue from 10/11 AIDS patients, including 5/5 patients with HIV-1 dementia
and
4/5 nondemented patients.
There are four main peripheral neuropathies associated with HIV, namely
sensory neuropathy, AIDP/CIPD, drug-induced neuropathy and CMV-related.
The most common type of neuropathy associated with AIDS is distal
symmetrical polyneuropathy (DSPN). This syndrome is a result of nerve
degeneration and is characterized by numbness and a sensation of pins and
needles.
DSPN causes few serious abnormalities and mostly results in numbness or
tingling
of the feet and slowed reflexes at the ankles. It generally occurs with more
severe
immunosuppression and is steadily progressive. Treatment with tricyclic
antidepressants relieves symptoms but does not affect the underlying nerve
damage.
A less frequent, but more severe type of neuropathy is known as acute or
chronic inflammatory demyelinating polyneuropathy (AIDP/CIDP). In AIDP/CIDP
174
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
there is damage to the fatty membrane covering the nerve impulses. This kind
of
neuropathy involves inflammation and resembles the muscle deterioration often
identified with long-term use of AZT. It can be the first manifestation of HIV
infection, where the patient may not complain of pain, but fails to respond to
standard reflex tests. This kind of neuropathy may be associated with
seroconversion, in which case it can sometimes resolve spontaneously. It can
serve
as a sign of HIV infection and indicate that it might be time to consider
antiviral
therapy. AIDP/CIDP may be auto-immune in origin.
Drug-induced, or toxic, neuropathies can be very painful. Antiviral drugs
commonly cause peripheral neuropathy, as do other drugs e.g. vincristine,
dilantin
(an anti-seizure medication), high-dose vitamins, isoniazid, and folic acid
antagonists. Peripheral neuropathy is often used in clinical trials for
antivirals as a
dose-limiting side effect, which means that more drugs should not be
administered.
Additionally, the use of such drugs can exacerbate otherwise minor
neuropathies.
Usually, these drug-induced neuropathies are reversible with the
discontinuation of
the drug.
CMV causes several neurological syndromes in AIDS, including
encephalitis, myelitis, and polyradiculopathy.
Neuronal loss is also a salient feature of prion diseases, such as Creutzfeldt-
Jakob disease in human, BSE in cattle (mad cow disease), Scrapie Disease in
sheep
and goats, and feline spongiform encephalopathy (FSE) in cats. A high dose of
a
sirtuin activating compound may be useful for treating or preventing neuronal
loss
due to these prior diseases.
In another embodiment, a high dose of a sirtuin activating compound may be
used to treat or prevent any disease or disorder involving axonopathy. Distal
axonopathy is a type of peripheral neuropathy that results from some metabolic
or
toxic derangement of peripheral nervous system (PNS) neurons. It is the most
common response of nerves to metabolic or toxic disturbances, and as such may
be
caused by metabolic diseases such as diabetes, renal failure, deficiency
syndromes
such as malnutrition and alcoholism, or the effects of toxins or drugs. The
most
common cause of distal axonopathy is diabetes, and the most common distal
axonopathy is diabetic neuropathy. The most distal portions of axons are
usually the
175
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
first to degenerate, and axonal atrophy advances slowly towards the nerve's
cell
body. If the noxious stimulus is removed, regeneration is possible, though
prognosis
decreases depending on the duration and severity of the stimulus. Those with
distal
axonopathies usually present with symmetrical glove-stocking sensori-motor
disturbances. Deep tendon reflexes and autonomic nervous system (ANS)
functions
are also lost or diminished in affected areas.
Diabetic neuropathies are neuropathic disorders that are associated with
diabetes mellitus. These conditions usually result from diabetic microvascular
injury
involving small blood vessels that supply nerves (vasa nervorum). Relatively
common conditions which may be associated with diabetic neuropathy include
third
nerve palsy; mononeuropathy; mononeuritis multiplex; diabetic amyotrophy; a
painful polyneuropathy; autonomic neuropathy; and thoracoabdominal neuropathy.
Clinical manifestations of diabetic neuropathy include, for example,
sensorimotor
polyneuropathy such as numbness, sensory loss, dysesthesia and nighttime pain;
autonomic neuropathy such as delayed gastric emptying or gastroparesis; and
cranial
neuropathy such as oculomotor (3rd) neuropathies or Mononeuropathies of the
thoracic or lumbar spinal nerves.
Peripheral neuropathy is the medical term for damage to nerves of the
peripheral nervous system, which may be caused either by diseases of the nerve
or
from the side-effects of systemic illness. Peripheral neuropathies vary in
their
presentation and origin, and may affect the nerve or the neuromuscular
junction.
Major causes of peripheral neuropathy include seizures, nutritional
deficiencies, and
HIV, though diabetes is the most likely cause. Mechanical pressure from
staying in
one position for too long, a tumor, intraneural hemorrhage, exposing the body
to
extreme conditions such as radiation, cold temperatures, or toxic substances
can also
cause peripheral neuropathy.
In an exemplary embodiment, a high dose of a sirtuin activating compound
may be used to treat or prevent multiple sclerosis (MS), including relapsing
MS and
monosymptomatic MS, and other demyelinating conditions, such as, for example,
chromic inflammatory demyelinating polyneuropathy (CIDP), or symptoms
associated therewith.
176
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
MS is a chronic, often disabling disease of the central nervous system.
Various and converging lines of evidence point to the possibility that the
disease is
caused by a disturbance in the immune function, although the cause of this
disturbance has not been established. This disturbance permits cells of the
immune
system to "attack" myelin, the fat containing insulating sheath that surrounds
the
nerve axons located in the central nervous system ("CNS"). When myelin is
damaged, electrical pulses cannot travel quickly or normally along nerve fiber
pathways in the brain and spinal cord. This results in disruption of normal
electrical
conductivity within the axons, fatigue and disturbances of vision, strength,
coordination, balance, sensation, and bladder and bowel function.
As such, MS is now a common and well-known neurological disorder that is
characterized by episodic patches of inflammation and demyelination which can
occur anywhere in the CNS. However, almost always without any involvement of
the peripheral nerves associated therewith. Demyelination produces a situation
analogous to that resulting from cracks or tears in an insulator surrounding
an
electrical cord. That is, when the insulating sheath is disrupted, the circuit
is "short
circuited" and the electrical apparatus associated therewith will function
intermittently or nor at all. Such loss of myelin surrounding nerve fibers
results in
short circuits in nerves traversing the brain and the spinal cord that thereby
result in
symptoms of MS. It is further found that such demyelination occurs in patches,
as
opposed to along the entire CNS. In addition, such demyelination may be
intermittent. Therefore, such plaques are disseminated in both time and space.
Tt is believed that the pathogenesis involves a local disruption of the blood
brain barrier which causes a localized immune and inflammatory response, with
consequent damage to myelin and hence to neurons.
Clinically, MS exists in both sexes and can occur at any age. However, its
most common presentation is in the relatively young adult, often with a single
focal
lesion such as a damage of the optic nerve, an area of anesthesia (loss of
sensation),
or paraesthesia (localize loss of feeling), or muscular weakness. In addition,
vertigo,
double vision, localized pain, incontinence, and pain in the arms and legs may
occur
upon flexing of the neck, as well as a large variety of less common symptoms.
177
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
An initial attack of MS is often transient, and it may be weeks, months, or
years before a further attack occurs. Some individuals may enjoy a stable,
relatively
event free condition for a great number of years, while other less fortunate
ones may
experience a continual downhill course ending in complete paralysis. There is,
most
commonly, a series of remission and relapses, in which each relapse leaves a
patient
somewhat worse than before. Relapses may be triggered by stressful events,
viral
infections or toxins. Therein, elevated body temperature, i.e., a fever, will
make the
condition worse, or as a reduction of temperature by, for example, a cold
bath, may
make the condition better.
In yet another embodiment, a high dose of a sirtuin activating compound
may be used to treat trauma to the nerves, including, trauma due to disease,
injury
(including surgical intervention), or environmental trauma (e.g., neurotoxins,
alcoholism, etc.).
A high dose of a sirtuin activating compound may also be useful to prevent,
treat, and alleviate symptoms of various PNS disorders, such as the ones
described
below. The PNS is composed of the nerves that lead to or branch off from the
spinal
cord and CNS. The peripheral nerves handle a diverse array of functions in the
body, including sensory, motor, and autonomic functions. When an individual
has a
peripheral neuropathy, nerves of the PNS have been damaged. Nerve damage can
arise from a number of causes, such as disease, physical injury, poisoning, or
malnutrition. These agents may affect either afferent or efferent nerves.
Depending
on the cause of damage, the nerve cell axon, its protective myelin sheath, or
both
may be injured or destroyed.
The term "peripheral neuropathy" encompasses a wide range of disorders in
which the nerves outside of the brain and spinal cord-peripheral nerves-have
been damaged. Peripheral neuropathy may also be referred to as peripheral
neuritis,
or if many nerves are involved, the terms polyneuropathy or polyneuritis may
be
used.
Peripheral neuropathy is a widespread disorder, and there are many
underlying causes. Some of these causes are common, such as diabetes, and
others
are extremely rare, such as acrylamide poisoning and certain inherited
disorders.
The most common worldwide cause of peripheral neuropathy is leprosy. Leprosy
is
178
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
caused by the bacterium Mycobacterium leprae, which attacks the peripheral
nerves
of affected people.
Leprosy is extremely rare in the United States, where diabetes is the most
commonly known cause of peripheral neuropathy. It has been estimated that more
than 17 -nillion people in the United States and Europe have diabetes-related
polyneuropathy. Many neuropathies are idiopatllic; no known cause can be
found.
The most common of the inherited peripheral neuropathies in the United States
is
Charcot-Marie-Tooth disease, which affects approximately 125,000 persons.
Another of the better known peripheral neuropathies is Guillain-Barre
syndrome, which arises from complications associated with viral illnesses,
such as
cytomegalovirus, Epstein-Barr virus, and human immunodeficiency virus (HIV),
or
bacterial infection, including Campylobacter jejuni and Lyme disease. The
worldwide incidence rate is approximately 1.7 cases per 100,000 people
annually.
Other well-known causes of peripheral neuropathies include chronic alcoholism,
infection of the varicella-zoster virus, botulism, and poliomyelitis.
Peripheral
neuropathy may develop as a primary symptom, or it may be due to another
disease.
For example, peripheral neuropathy is only one symptom of diseases such as
amyloid neuropathy, certain cancers, or inherited neurologic disorders. Such
diseases may affect the PNS and the CNS, as well as other body tissues.
Other PNS diseases treatable with sirtuin-modulating compounds that
increase the level and/or activity of a sirtuin protein include: Brachial
Plexus
Neuropathies (diseases of the cervical and first thoracic roots, nerve trunks,
cords,
and peripheral nerve components of the brachial plexus. Clinical
manifestations
include regional pain, paresthesia; muscle weakness, and decreased sensation
in the
upper extremity. These disorders may be associated with trauma, including
birth
injuries; thoracic outlet syndrome; neoplasms, neuritis, radiotherapy; and
other
conditions. See Adams et al., Principles of Neurology, 6th ed, pp l351-2);
Diabetic
Neuropathies (peripheral, autonomic, and cranial nerve disorders that are
associated
with diabetes mellitus). These conditions usually result from diabetic
microvascular
injury involving small blood vessels that supply nerves (vasa nervorum).
Relatively
common conditions which may be associated with diabetic neuropathy include
third
nerve palsy; mononeuropathy; mononeuritis multiplex; diabetic amyotrophy; a
179
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
painful polyneuropathy; autonomic neuropathy; and thoracoabdominal neuropathy
(see Adams et al., Principles of Neurology, 6th ed, p 1325); mononeuropathies
(disease or trauma involving a single peripheral nerve in isolation, or out of
proportion to evidence of diffuse peripheral nerve dysfunction). Mononeuritis
multiplex refers to a condition characterized by multiple isolated nerve
injuries.
Mononeuropathies may result from a wide variety of causes, including ischemia;
traumatic injury; compression; connective tissue diseases; cumulative trauma
disorders; and other conditions; Neuralgia (intense or aching pain that occurs
along
the course or distribution of a peripheral or cranial nerve); Peripheral
Nervous
System Neoplasms (neoplasms which arise from peripheral nerve tissue). This
includes neurofibromas; Schwannomas; granular cell tumors; and malignant
peripheral nerve sheath tumors. See DeVita Jr et al., Cancer: Principles and
Practice
of Oncology, 5th ed, pp1750-1); and Nerve Compression Syndromes (mechanical
compression of nerves or nerve roots from internal or external causes. These
may
result in a conduction block to nerve impulses, due to, for example, myelin
sheath
dysfunction, or axonal loss. The nerve and nerve sheath injuries may be caused
by
ischemia; inflammation; or a direct mechanical effect; Neuritis (a general
term
indicating inflammation of a peripheral or cranial nerve). Clinical
manifestation
may include pain; paresthesias; paresis; or hyperesthesia; Polyneuropathies
(diseases
of multiple peripheral nerves). The various forms are categorized by the type
of
nerve affected (e.g., sensory, motor, or autonomic), by the distribution of
nerve
injury (e.g., distal vs. proximal), by nerve component primarily affected
(e.g.,
demyelinating vs. axonal), by etiology, or by pattern of inheritance.
In another embodiment, a high dose of a sirtuin activating compound may be
used to treat or prevent chemotherapeutic induced neuropathy. The high dose of
the
sirtuin activating compound may be administered prior to administration of the
chemotherapeutic agent, concurrently with administration of the
chemotherapeutic
drug, -and/or after initiation of administration of the chemotherapeutic drug.
If the
high dose of the sirtuin activating compound is administered after the
initiation of
administration of the chemotherapeutic drug, it is desirable that the high
dose of the
sirtuin activating compound be administered prior to, or at the first signs,
of
chemotherapeutic induced neuropathy,
180
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Chemotherapy drugs can damage any part of the nervous system.
Encephalopathy and myelopathy are fortunately very rare. Damage to peripheral
nerves is much more common and can be a side effect of treatment experienced
by
people with cancers, such as lymphoma. Most of the neuropathy affects sensory
rather than motor nerves. Thus, the common symptoms are tingling, numbness or
a
loss of balance. The longest nerves in the body seem to be most sensitive
hence the
fact that most patients will report numbness or pins and needles in their
hands and
feet.
The chemotherapy drugs which are most commonly associated with
neuropathy, are the Vinca alkaloids (anti-cancer drugs originally derived from
a
member of the periwinkle - the Vinca plant genus) and a platinum-containing
drug
called Cisplatin. The Vinca alkaloids include the drugs vinblastine,
vincristine and
vindesine. Many combination chemotherapy treatments for lymphoma for example
CHOP and CVP contain vincristine, which is the drug known to cause this
problem
most frequently. Indeed, it is the risk of neuropathy that limits the dose of
vincristine that can be administered.
Studies that have been performed have shown that most patients will lose
some reflexes in their legs as a result of treatment with vincristine and many
will
experience some degree of tingling (paresthesia) in their fingers and toes.
The
neuropathy does not usually manifest itself right at the start of the
treatment but
generally comes on over a period of a few weeks. It is not essential to stop
the drug
at the first onset of symptoms, but if the neuropathy progresses this may be
necessary. It is very important that patients should report such symptoms to
their
doctors, as the nerve damage is largely reversible if the drug is
discontinued. Most
doctors will often reduce the dose of vincristine or switch to another form of
Vinca
alkaloid such as vinblastine or vindesine if the symptoms are mild.
Occasionally, the
nerves supplying the bowel are affected causing abdominal pain and
constipation.
In another embodiment, a high dose of a sirtuin activating compound may be
used to treat or prevent a polyglutainine disease. Huntington's Disease (HD)
and
Spinocerebellar ataxia type 1(SCAI) are just two examples of a class of
genetic
diseases caused by dynamic mutations involving the expansion of triplet
sequence
repeats. In reference to this common mechanism, these disorders are called
181
CA 02613141 2007-12-20
WO 2007/008548 PCTIUS2006/026272
trinucleotide repeat diseases. At least 14 such diseases are known to affect
human
beings. Nine of them, including SCAI and Huntington's disease, have CAG as the
repeated sequence (see Table A below). Since CAG codes for an amino acid
called
glutamine, these nine trinucleotide repeat disorders are collectively known as
polyglutamine diseases.
Altliough the genes involved in different polyglutamine diseases have little
in common, the disorders they cause follow a strikingly similar course. Each
disease
is characterized by a progressive degeneration of a distinct group of nerve
cells. The
major symptoms of these diseases are similar, although not identical, and
usually
affect people in midlife. Given the similarities in symptoms, the
polyglutamine
diseases are hypothesized to progress via common cellular mechanisms. In
recent -
years, scientists have made great strides in unraveling what the mechanisms
are.
Above a certain threshold, the greater the number of glutamine repeats in a
protein, the earlier the onset of disease and the more severe the symptoms.
This
suggests that abnormally long glutamine tracts render their host protein toxic
to
nerve cells.
To test this hypothesis, scientists have generated genetically engineered mice
expressing proteins with long polyglutamine tracts. Regardless of whether the
mice
express full-length proteins or only those portions of the proteins containing
the
polyglutamine tracts, they develop symptoms of polyglutamine diseases. This
suggests that a long polyglutamine tract by itself is damaging to cells and
does not
have to be part of a functional protein to cause its damage.
For example, it is thought that the symptoms of SCA1 are not directly caused
by the loss of normal ataxin-1 function but rather by the interaction between
ataxin- 1
and another protein called LANP. LANP is needed for nerve cells to communicate
with one another and thus for their survival. When the mutant ataxin-1 protein
accumulates inside nerve cells, it "traps" the LANP protein, interfering with
its
normal function. After a while, the absence of LANP function appears to cause
nerve cells to malfunction.
Table A. Summary of Polyglutamine Diseases.
Disease Gene Chromosomal Pattern of Protein Normal Disease
name location inheritance repeat repeat
len th lezz th
Spinobulbar AR Xq13-21 X-linked androgen 9-36 38-62
182
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Disease Gene Chromosomal Pattern of Protein Normal Disease
name location inheritance repeat repeat
len th Zen th
muscular recessive receptor
atrophy (AR)
(Kennedy
disease)
Huntington's HD 4p16.3 autosomal huntingtin 6-35 36-121
disease dominant
Dentatorubral- DRPLA 12p13.31 autosomal atrophin- 6-35 49-88
pallidoluysian dominant 1
atrophy (Haw
River
syndrome)
Spinocerebellar SCAI 6p23 autosomal ataxin-1 6-44 39-82
ataxia type 1 dominant
Spinocerebellar SCA2 12q24.1 autosomal ataxin-2 15-31 36-63
ataxia type 2 dominant
Spinocerebellar SCA3 14q32.1 autosomal ataxin-3 12-40 55-84
ataxia type 3 dominant
(Machado-
Joseph disease)
Spinocerebellar SCA6 19p13 autosomal a1A- 4-18 21-33
ataxia type 6 dominant voltage-
dependent
calcium
channel
subunit
Spinocerebellar SCA7 3p12-13 autosomal ataxin-7 4-35 37-306
ataxia type 7 dominant
Spinocerebellar SCA17 6q27 autosomal TATA 25-42 45-63
ataxia type 17 dominant binding
protein
Many transcription factors have also been found in neuronal inclusions in
different diseases. It is possible that these transcription factors interact
with the
polyglutamine-containing proteins and then become trapped in the neuronal
inclusions. This in turn might keep the transcription factors from turning
genes on
and off as needed by the cell. Another observation is hypoacetylation of
histones in
affected cells. This has led to the hypothesis that Class I/II Histone
Deacetylase
(HDAC I/II) inhibitors, which are known to increase histone acetylation, may
be a
novel therapy for polyglutamine diseases (US Patent application 10/476,627;
183
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
"Method of treating neurodegenerative, psychiatric, and other disorders with
deacetylase inhibitors").
In yet another embodiment, the invention provides a method for treating or
preventing neuropathy related to ischemic injuries or diseases, such as, for
example,
coronary heart disease (including congestive heart failure and myocardial
infarctions), stroke, emphysema, hemorrhagic shock, peripheral vascular
disease
(upper and lower extremities) and transplant related injuries.
In certain embodiments, the invention provides a method to treat a central
nervous system cell to prevent damage in response to a decrease in blood flow
to the
cell. Typically the severity of damage that may be prevented will depend in
large
part on the degree of reduction in blood flow to the cell and the duration of
the
reduction. By way of example, the normal amount of perfusion to brain gray
matter
in humans is about 60 to 70 mL/100 g of brain tissue/min. Death of central
nervous
system cells typically occurs when the flow of blood falls below approximately
8-10
mL/100 g of brain tissue/min, while at slightly higher levels (i.e. 20-35
mL/100 g of
brain tissue/min) the tissue remains alive but not able to function. In one
embodiment, apoptotic or necrotic cell death may be prevented. In still a
further
embodiment, ischemic-mediated damage, such as cytoxic edema or central nervous
system tissue anoxemia, may be prevented. In each embodiment, the central
nervous
system cell may be a spinal cell or a brain cell.
Another aspect encompasses administrating a high dose of a sirtuin
activating compound to a subject to treat a central nervous system ischemic
condition. A number of central nervous system ischemic conditions may be
treated
by the sirtuin activating compounds described herein. In one embodiment, the
ischemic condition is a stroke that results in any type of ischemic central
nervous
system damage, such as apoptotic or necrotic cell death, cytoxic edema or
central
nervous system tissue anoxia. The stroke may impact any area of the brain or
be
caused by any etiology commonly known to result in the occurrence of a stroke.
In
one alternative of this embodiment, the stroke is a brain stem stroke.
Generally
speaking, brain stem strokes strike the brain stem, which control involuntary
life-
support functions such as breathing, blood pressure, and heartbeat. In another
alternative of this embodiment, the stroke is a cerebellar stroke. Typically,
cerebellar
184
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
strokes impact the cerebellum area of the brain, which controls balance and
coordination. In still another embodiment, the stroke is an embolic stroke. In
general
terms, embolic strokes may impact any region of the brain and typically result
from
the blockage of an artery by a vaso-occlusion. In yet another alternative, the
stroke
may be a hemorrhagic stroke. Like ischemic strokes, hemorrhagic stroke may
impact
any region of the brain, and typically result from a ruptured blood vessel
characterized by a hemorrhage (bleeding) within or surrounding the brain. In a
further embodiment, the stroke is a thrombotic stroke. Typically, thrombotic
strokes
result from the blockage of a blood vessel by accumulated deposits,.
In another embodiment, the ischemic condition may result from a disorder
that occurs in a part of the subject's body outside of the central nervous
system, but
yet still causes a reduction in blood flow to the central nervous system.
These
disorders may include, but are not limited to a peripheral vascular disorder,
a venous
thrombosis, a pulmonary embolus, arrhythmia (e.g. atrial fibrillation), a
myocardial
infarction, a transient ischemic attack, unstable angina, or sickle cell
anemia.
Moreover, the central nervous system ischemic condition may occur as result of
the
subject undergoing a surgical procedure. By way of example, the subject may be
undergoing heart surgery, lung surgery, spinal surgery, brain surgery,
vascular
surgery, abdominal surgery, or organ transplantation surgery. The organ
transplantation surgery may include heart, lung, pancreas, kidney or liver
transplantation surgery. Moreover, the central nervous system ischemic
condition
may occur as a result of a trauma or injury to a part of the subject's body
outside the
central nervous system. By way of example, the trauma or injury may cause a
degree
of bleeding that significantly reduces the total volume of blood in the
subject's body.
Because of this reduced total volume, the amount of blood flow to the central
nervous system is concomitantly reduced. By way of further example, the trauma
or
injury may also result in the formation of a vaso-occlusion that restricts
blood flow
to the central nervous system.
Of course it is contemplated that the high dose of the sirtuin activating
compound may be employed to treat the central nervous system ischemic
condition
irrespective of the cause of the condition. In one embodiment, the ischemic
condition results from a vaso-occlusion. The vaso-occlusion may be any type of
185
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
occlusion, but is typically a cerebral thrombosis or an embolism. In a further
embodiment, the ischemic condition may result from a hemorrhage. The
hemorrhage
may be any type of hemorrhage, but is generally a cerebral hemorrhage or a
subararachnoid hemorrhage. In still another embodiment, the ischemic condition
may result from the narrowing of a vessel. Generally speaking, the vessel may
narrow as a result of a vasoconstriction such as occurs during vasospasms, or
due to
arteriosclerosis. In yet another embodiment, the ischemic condition results
from an
injury to the brain or spinal cord.
In yet another aspect, a high dose of a sirtuin activating compound may be
administered to reduce infarct size of the ischemic core following a central
nervous
system ischemic condition. Moreover, a high dose of a sirtuin activating
compound
may also be beneficially administered to reduce the size of the ischemic
penumbra
or transitional zone following a central nervous system ischemic condition.
In one embodiment, a combination drug regimen may include drugs or
compounds for the treatment or prevention of neurodegenerative disorders or
secondary conditions associated with these conditions. Thus, a combination
drug
regimen may include a high dose of one or more sirtuin activators and one or
more
anti-neurodegeneration agents. For example, one or more sirtuin-activating
compounds can be combined with an effective amount of one or more of: L-DOPA;
a dopamine agonist; an adenosine A2A receptor antagonist; a COMT inhibitor; a
MAO inhibitor; an N-NOS inhibitor; a sodium channel antagonist; a selective N-
methyl D-aspartate (NMDA) receptor antagonist; an AMPA/kainate receptor
antagonist; a calcium channel antagonist; a GABA-A receptor agonist; an acetyl-
choline esterase inhibitor; a matrix metalloprotease inhibitor; a PARP
inhibitor; an
inhibitor of p38 MAP kinase or c-jun-N-terminal kinases; TPA; NDA antagonists;
beta-interferons; growth factors; glutamate inhibitors; and/or as part of a
cell
therapy.
Exemplary N-NOS inhibitors include 4-(6-amino-pyridin-2-yl)-3-
methoxypheno 16-[4-(2-dimethylamino-ethoxy)-2-methoxy-phenyl]-pyridin-2-yl-
amine, 6-[4-(2-dimethylamino-ethoxy)-2,3-dimet-hyl-phenyl]-pyridin-2-yl-amine,
6-
[4-(2-pyrrolidinyl-ethoxy)-2,3-dimethyl-p-henyl]-pyridin-2-yl-amine, 6-[4-(4-
(n-
methyl)piperidinyloxy)-2,3-dimethyl-p-henyl]-pyridin-2-yl-amine, 6-[4-(2-
186
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
dimethylamino-ethoxy)-3-methoxy-phenyl]-pyridin-2-yl-amine, 6-[4-(2-
pyrrolidinyl-ethoxy)-3-methoxy-phenyl]-pyridin-2-yl-amine, 6-{4-[2-(6,7-
dimethoxy-3,4-dihydro-1 h-isoquinolin-2-yl)-ethoxy]-3-methoxy-phenyl} -pyridin-
2-
yl-amine, 6-{3-methoxy-4-[2-(4-phenethyl-piper-azin-1-yl)-ethoxy]-phenyl}-
pyridin-2-yl-amine, 6-{3-methoxy-4-[2-(4-methyl-piperazin-1-yl)-ethoxy]-
phenyl}-
pyridin-2-yl-amine, 6-{4-[2-(4-dimethylamin-o-piperidin-1-yl)-ethoxy]-3-
methoxy-
phenyl}-pyridin-2-yi-amine, 6-[4-(2-dimethylamino-ethoxy)-3-ethoxy-phenyl]-
pyridin-2-yl-amine, 6-[4-(2-pyrrolidinyl-ethoxy)-3-ethoxy-phenyl]-pyridin-2-yl-
amine, 6-[4-(2-dimethylamino-ethoxy)-2-isopropyl-phenyl]-pyridin-2-yl-amine, 4-
(6-amino-pyridin-yl)-3 -cyclopropyl-phenol6-[2-cyclopropyl-4-(2-dimethy-lamino-
ethoxy)-phenyl]-pyridin-2-yl-amine, 6-[2-cyclopropyl-4-(2-pyrrolidin-1-yl-
ethoxy)-
phenyl]-pyridin-2-yl-amine, 3-[3-(6-amino-pyridin-2y1)-4-cycl-opropyl-phenoxy]-
pyrrolidine-1-carboxylic acid tert-butyl ester 6-[2-cyclopropyl-4-(1-methyl-
pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 4-(6-amino-pyridin-2-yl)-3-
cyclobutyl-phenol6-[2-cyclobutyl-4-(2-dime-thylamino-ethoxy)-phenyl]-pyridin-2-
yl-amine, 6-[2-cyclobutyl-4-(2-pyrrolid-in-1-yl-ethoxy)-phenyl]-pyridin-2-yl-
amine,
6-[2-cyclobutyl-4-(1-methyl-pyr-rolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine,
4-(6-
amino-pyridin-2-yl)-3 -cy-c lopentyl-phenol6-[2-cyclopentyl-4-(2-dimethylamino-
ethoxy)-phenyl]-pyrid-in-2-yl-amine, 6-[2-cyclopentyl-4-(2-pyrrolidin-lyl-
ethoxy)-
phenyl]-pyridin-2-yl-amine, 3-[4-(6-amino-pyridin-2y1)-3-methoxy-phenoxy]-
pyrrolidine-l-ca-rboxylic acid tert butyl ester 6-[4-(1-methyl-pyrrolidin-3-yl-
oxy)-2-
metho-xy-phenyl]-pyridin-2-yl-amine, 4-[4-(6-amino-pyridin-2yl)-3-methoxy-
phenoxy-]-piperidine-l-carboxylic acid tert butyl ester 6-[2-methoxy-4-(1-
methyl-p-
iperidin-4-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[4-(allyloxy)-2-methoxy-ph-
enyl]-
pyridin-2-yl-amine, 4-(6-amino-pyridin-2-yl)-3-methoxy-6-allyl-phenol 12 and 4-
(6-
amino-pyridin-2-yl)-3-methoxy-2-allyl-phenol 13 4-(6-amino-pyridin-2-yl)-3-
methoxy-6-propyl-phenol 6-[4-(2-dimethylamino-ethoxy)-2-methoxy-5-propyl-
phenyl]-pyridin-yl-amine, 6-[2-isopropyl-4-(pyrrolidin-3-yl-oxy)-phenyl]-
pyridin-2-
yl-amine, 6-[2-isopropyl-4-(piperidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-
[2-
isopropyl-4-(1-methyl-azetidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-
isopropyl-4-(1-methyl-piperidin-4-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-
isopropyl-4-(1-methyl-pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amin-e 6-[2-
187
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
isopropyl-4-(1-methyl-pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-
isopropyl-4-(2-methyl-2-aza-bicyclo[2.2.1 ]hept-5-yl-oxy)-phenyl]-p-yridin-2-
yl-
amine, 6-[4-(2-dimethylamino-ethoxy)-2-methoxy-phenyl]-pyridin-2-yl-amine, 6-
{4-[2-(benzyl-methyl-amino)-ethoxy]-2-methoxy-phenyl}-pyridin-2-yl-amine, 6-[2-
methoxy-4-(2-pyrrolidin-l-yl-ethoxy)-phenyl]-pyridin-2-yl-amine, 2-(6-amino-
pyridin-2-yl)-5 -(2-d imethyl am ino-ethoxy)-pheno l 2-[4-(6-am ino-pyridin-2-
yl)-3 -
methoxy-phenoxy]-acetamide 6-[4-(2-amino-ethoxy)-2-methoxy-phenyl]-pyridin-2-
yl-amine, 6-{4-[2-(3,4-dihydro-lh-isoquinolin-2-yl)-ethoxy]-2-methoxy-phenyl}-
pyrid-in-2-yl-amine, 2-[4-(6-amino-pyridin-2-yl)-3 -methoxy-phenoxy] -ethanol
6-
{2-methoxy-4-[2-(2,2,6,6-tetramethyl-piperidin-1-yl)-ethoxy]-phenyl}-py-ridin-
2-
yl-amine, 6-{4-[2-(2,5-dimethyl-pyrrolidin-1-yl)-ethoxy]-2-methoxy-phenyl}-
pyridin-2-yl-amine, 6-{4-[2-(2,5-dimethyl-pyrrolidin-1-yl)-ethoxy]-2-methoxy-
phenyl}-pyridin-2-yl-amine, 2-[4-(6-amino-pyridin-2-yl)-3-methoxy-phenoxy]-1-
(2,2,6,6-tetramethyl-piperidin-l-yl)-ethanone 6-[2-methoxy-4-(1-methyl-
pyrrolidin-
2-yl-methoxy)-phenyl]-pyridin-2-yl-amine, 6-[4-(2-dimethylamino-ethoxy)-2-
propoxy-phenyl]-pyridin-2-yl-amine, 6-{4-[2-(benzyl-methyl-amino)-ethoxy]-2-
propoxy-phenyl}-pyridin-2-yl-amin-e 6-[4-(2-ethoxy-ethoxy)-2-methoxy-phenyl]-
pyridin-2-yl-amine, 6-[4-(2-dimethylamino-ethoxy)-2-isopropoxy-phenyl]-pyridin-
2-yl-amine, 6-[4-(2-ethoxy-ethoxy)-2-isopropoxy-phenyl]-pyridin-2-yl-amine, 6-
[2-
methoxy-4-(3-methyl-butoxy)-phenyl]-pyridin-2- yl-amine, 6-[4-(2-dimethylamino-
ethoxy)-2-ethoxy-phenyl]-pyridin-2-yl-amine, 6-{4-[2-(benzyl-methyl-amino)-
ethoxy]-2-ethoxy-phenyl}-pyridin-2-yl-amine, 6-[2-ethoxy-4-(3-methyl-butoxy)-
phenyl]-pyridin-2-yl-amine, 1-(6-amino-3-aza-bicyclo[3.1.0]hex-3-yl)-2-[4-(6-
amino-pyridin-2-yl)-3-et-hoxy-phenoxy]-ethanone 6-[2-ethoxy-4-(2-pyrrolidin-1-
yl-
ethoxy)-phenyl]-py-ridin-2-yl-amine, 3-{2-[4-(6-amino-pyridin-2-yl)-3-ethoxy-
phenoxy]-ethyl}-3-aza-bicyclo[3.1.0]hex-6-yl-amine, 1-(6-amino-3-aza-
b icyc lo [3 .1.0] hex-3 -yl)-2-[4-(6-amino-pyridin-2-yl)-3 -methoxy-phenoxy]-
ethanone
3-{2-[4-(6-amino-pyridin-2-yl)-3-methoxy-phenoxy]-ethyl} -3 -aza-bicyclo [3.-
1.0]hex-6-yl-amine, 6-[2-isopropoxy-4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-py-
ridin-
2-yl-amine, 6-{4-[2-(benzyl-methyl-amino)-ethoxy]-2-isopropoxy-phenyl-}-
pyridin-
2-yl-amine, 6-[4-(2-dimethylamino-ethoxy)-2-methoxy-5-propyl-phen-yl]-pyridin-
2-yl-amine, 6-[5-allyl-4-(2-dimethylamino-ethoxy)-2-methoxy-phe-nyl]-pyridin-2-
188
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
yl-amine, 6-[5-allyl-2-methoxy-4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-pyridin-2-
yl-
amine, 6-[3-allyl-4-(2-dimethylamino-ethoxy)-2-methoxy-phenyl]-pyridin-2-yl-
amine, 6-[2-methoxy-4-(pyrrolidin-3-yl-oxy)-phenyl]-p-yridin-2-yl-amine, 6-[2-
methoxy-4-(1-methyl-pyrrolidin-3-yl-oxy)-phenyl]-py-ridin-2-yl-amine, 6-[2-
ethoxy-4-(pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-isopropoxy-4-
(pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-methoxy-4-(piperidin-4-
yl-
oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-methoxy-4-(2,2,6,6-tetramethyl-piperidin-
4-
yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-isopropoxy-4-(pyrrolidin-3-yl-oxy)-
phenyl]-pyridin-2-yl-amine, 3-[4-(6-amino-pyridin-2-yl)-3-methoxy-phenoxy]-
azetidine-l-carboxylic acid tert-butyl ester 6-[4-(azetidin-3-yl-oxy)-2-
methoxy-
phenyl]-pyridin-2-yl-amine, 6-[2-methoxy-4-(1-methyl-azetidin-3-yl-oxy)-
phenyl]-
pyridin-2-y-l-amine, 6-[2-isopropoxy-4-(pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-
yl-
amine, 6-[2-isopropoxy-4-(pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-
[2-
methoxy-4-(pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-methoxy-4-(1-
methyl-pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-methoxy-4-(1-
methyl-
pyrrolidin-3-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[2-methoxy-4-(2-methyl-2-
aza-
bicyclo[2.2.1]hept-5-yl-oxy)-phenyl]-pyrid-in-2-yl-amine, 6-[2-methoxy-4-(1-
methyl-piperidin-4-yl-oxy)-phenyl]-pyridin-2-yl-amine, 6-[4-(1-ethyl-piperidin-
4-
yl-oxy)-2-methoxy-phenyl]-pyridin-2-yl-amine, 6-[5-allyl-2-methoxy-4-(1-methyl-
pyrrolidin-3-yl-oxy)-phenyl]-pyr-idin-2-yl-amine, 6-[4-(2-dimethylamino-
ethoxy)-
2,6-dimethyl-phenyl]-pyridin-2-yl-amine, 6-[2,6-dimethyl-4-(3-piperidin-l-yl-
propoxy)-phenyl]-pyridin-2-yl-amine, 6-[2,6-dimethyl-4-(2-pyrrolidin-1-yl-
ethoxy)-
phenyl]-pyridin-2-y-l-amine, 6-{2,6-dimethyl-4-[3-(4-methyl-piperazin-l-yl)-
propoxy]-phenyl}-py-ridin-2-yl-amine, 6-[2,6-dimethyl-4-(2-morpholin-4-yl-
ethoxy)-phenyl]-pyrid-in-2-yl-amine, 6-{4-[2-(benzyl-methyl-amino)-ethoxy]-2,6-
dimethyl-phenyl}-p-yridin-2-yl-amine, 2-[4-(6-amino-pyridin-2-yl)-3,5-dimethyl-
phenoxy]-acetam-ide 6-[4-(2-amino-ethoxy)-2,6-dimethyl-phenyl]-pyridin-2-yl-
amine, 6-[2-isopropyl-4-(2-pyrrolidin-l-yl-ethoxy)-phenyl]-pyridin-2-yl-amine,
2-
(2,5-dimethyl-pyrrolidin-l-yl)-6-[2-isopropyl-4-(2-pyrrolidin-l-yl-etho-xy)-
phenyl]-
pyridine 6-{4-[2-(3,5-dimethyl-piperidin-1-yl)-ethoxy]-2-isopr-opyl-phenyl}-
pyridin-2-yl-amine, 6-[4-(2-dimethylamino-ethoxy)-2-isopropyl-phenyl]-pyridin-
2-
yl-amine, 6-[2-tert-butyl-4-(2-dimethylamino-ethoxy)-phen-yl]-pyridin-2-yl-
amine,
189
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
6-[2-tert-butyl-4-(2-pyrrolidin-1-yl-ethoxy)-phenyl-]-pyridin-2-yl-amine, 6-[4-
(2-
pyrro lid inyl-ethoxy)-2,5 -dimethyl-phenyl]-pyr-idin-2-yl-am ine, 6-[4-(2-
dimethylamino-ethoxy)-2,5-dimethyl-phenyl]-pyridin-2-yl-amine, 6-[4-(2-(4-
phenethylpiperazin-1-yl)-ethoxy)-2,5-dimethyl-pheny-1]-pyridin-2-yl-amine, 6-
[2-
cyclopropyl-4-(2-dimethylamino-l-methyl-ethoxy)-phenyl]-pyridin-2-yl-amine, 6-
[cyclobutyl-4-(2-dimethylamino-l-methyl-etho-xy)-phenyl]-pyridin-2-yl-amine, 6-
[4-(allyloxy)-2-cyclobutyl-phenyl]-pyridi-n-2ylamine, 2-allyl-4-(6-amino-
pyridin-2-
yl)-3-cyclobutyl-phenol and 2-allyl-4-(6-amino-pyridin-2-yl)-5-cyclobutyl-
phenol4-
(6-amino-pyridin-2y1)-5 -cyclobutyl-2-propyl-phenol4-(6-amino-pyridin-2y1)-3 -
cyclobutyl-2-propyl-phenol6-[2-cyclobutyl-4-(2-dimethylamino-l-methyl-ethoxy)-
5-propyl-phenyl]-pyri-din-2-yl-amine, 6-[2-cyclobutyl-4-(2-dimethylamino-l-
methyl-ethoxy)-3-propy-l-phenyl]-pyridin-2-yl-amine, 6-[2-cyclobutyl-4-(2-
dimethylamino-ethoxy)-5-propyl-phenyl]-pyridin-2-yl-amine, 6-[2-cyclobutyl-4-
(2-
dimethylamino-ethox-y)-3-propyl-phenyl]-pyridin-2-yl-amine, 6-[2-cyclobutyl-4-
(1-
methyl-pyrroli-din-3-yl-oxy)-5-propyl-phenyl]-pyridin-2-yl-amine, 6-
[cyclobutyl-4-
(1-methy-l-pyrrolidin-3-yl-oxy)-3-propyl-phenyl]-pyridin-2-yl-amine, 2-(4-
benzyloxy-5-hydroxy-2-methoxy-phenyl)-6-(2,5-dimethyl-pyrrol=l-yl)-p-yridine 6-
[4-(2-dimethylamino-ethoxy)-5-ethoxy-2-methoxy-phenyl]-pyridin-2-yl-amine, 6-
[5-ethyl-2-methoxy-4-(1-methyl-piperidin-4-yl-oxy)-phenyl]-pyr-idin-2-yl-
amine, 6-
[5-ethyl-2-methoxy-4-(piperidin-4-yl-oxy)-phenyl]-pyridi-n-2-yl-amine, 6-[2,5-
dimethoxy-4-(1-methyl-pyrrolidin-3-yl-oxy)-phenyl]-pyr-idin-2-yl-amine, 6-[4-
(2-
dimethylamino-ethoxy)-5-ethyl-2-methoxy-phenyl]-py-ridin-2-yl-amine.
Exemplary NMDA receptor antagonist include (+)-(1S, 2S)-1-(4-hydroxy-
phenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-pro-panol, (1 S, 2S)-1-(4-hydroxy-3-
methoxyphenyl)-2-(4-hydroxy-4-phenylpiperi-dino)-1-propanol, (3R, 4S)-3-(4-(4-
fluorophenyl)-4-hydroxypiperidin-1-yl-)-chroman-4,7-diol, (1R*, 2R*)-1-(4-
hydroxy-3 -methylphenyl)-2-(4-(4-fluoro-phenyl)-4-hydroxypiperidin-1-yl)-
propan-
1-ol-mesylate or a pharmaceutically acceptable acid addition salt thereof.
Exemplary dopamine agonist include ropininole; L-dopa decarboxylase
inhibitors such as carbidopa or benserazide, bromocriptine,
dihydroergocryptine,
etisulergine, AF-14, alaptide, pergolide, piribedil; dopamine D1 receptor
agonists
such as A-68939, A-77636, dihydrexine, and SKF-38393; dopamine D2 receptor
190
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
agonists such as carbergoline, lisuride, N-0434, naxagolide, PD-118440,
pramipexole, quinpirole and ropinirole; dopamine/(3-adrenegeric receptor
agonists
such as DPDMS and dopexamine; dopamine/5-HT uptake inhibitor/5-HT-lA
agonists such as roxindole; dopamine/opiate receptor agonists such as NIH-
10494;
a2-adrenergic antagonist/dopamine agonists such as terguride; a2-adrenergic
antagonist/dopamine D2 agonists such as ergolines and talipexole; dopainine
uptalce
inhibitors such as GBR-12909, GBR-13069, GYKI-52895, and NS-2141;
monoamine oxidase-B inhibitors such as selegiline, N-(2-butyl)-N-
methylpropargylamine, N-methyl-N-(2-pentyl)propargylamine, AGN-1 133, ergot
derivatives, lazabemide, LU-53439, MD-280040 and mofegiline; and COMT
inhibitors such as CGP-28014.
Exemplary acetyl cholinesterase inhibitors include donepizil, 1-(2-methyl-
1H-benzimida-zol-5-yl)-3-[1-(phenylmethyl)-4-piperidinyl]-1-propanone; 1-(2-
phenyl-lH-benzimidazol-5-yl)-3-[1-(phenylmethyl)-4-piperidinyl]-1-pr-opanone;
1-
(1-ethyl-2-methyl-lH-benzimidazol-5-yl)-3-[1-(phenylmethyl)-4-p-iperidinyl]-1-
propanone; 1-(2-methyl-6-benzothiazolyl)-3-[1-(phenylmethyl)-4-piperidinyl]-1-
propanone; 1-(2-methyl-6-benzothiazolyl)-3-[1-[(2-methyl-4-thiazolyl)methyl]-4-
piperidinyl]-l-propanone; 1-(5-methyl-benzo[b]thie-n-2-yl)-3-[1-
(phenylmethyl)4-
piperidinyl]-1-propanone; 1-(6-methyl-benzo[b]thien-2-yl)-3-[1-(phenylmethyl)-
4-
piperidinyl]-1-prop-anone; 1-(3,5-dimethyl-benzo[b]thien-2-yl)-3-[1-
(phenylmethyl)-4-piperidin-yl]-1-propanone; 1-(benzo[b]thien-2-yl)-3-[1-
(phenylmethyl)-4-piperidinyl]-1-propanone; 1-(benzofuran-2-yl)-3-[i-
(phenylmethyl)-4-piperidinyl]-1-pro-panone; 1-(1-phenylsulfonyl-6-methyl-indol-
2-
yl)-3-[l-(phenylmethyl)-4-pip-eridinyl]-1-propanone; 1-(6-methyl-indol-2-yl)-3-
[1-
(phenylmethyl)-4-piper-idinyl]-1-propanone; 1-(1-phenylsulfonyl-5-amino-indol-
2-
yl)-3-[1-(phenylm-ethyl)-4-piperidinyl]-1-propanone; 1-(5-amino-indol-2-yl)-3-
[1-
(phenylmet-hyl)-4-piperidinyl]-1-propanone; and 1-(5-acetylamino-indol-2-yl)-3-
[1-
(ph-enylmethyl)-4-piperidinyl]-1-propanone. 1-(6-quinolyl)-3-[ 1-
(phenyhnethyl)-4-
piperidinyl]-1-propanone; 1-(5-indolyl)-3-[1-(phenylmethyl)-4-piperidiny-1]-1-
propanone; 1-(5-benzthienyl)-3-[1-(phenylmethyl)-4-piperidinyl]-1-pro-panone;
1-
(6-quinazolyl)-3-[1-(phenylmethyl)-4-piperidinyl]-1-propanone; 1-(6-
benzoxazolyl)-
3-[1-(phenylmethyl)-4-piperidinyl]-1-propanone; 1-(5-benzofuranyl)-3-[1-
191
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
(phenylmethyl)-4-piperidinyl]-1-propanone; 1-(5-methyl-benzimidazol-2-yl)-3-[1-
(phenylmethyl)-4-piperidinyl]-1-propa-none; 1-(6-methyl-benzimidazol-2-yl)-3-
[1-
(phenylmethyl)-4-piperidinyl]-1-propanone; 1-(5-chloro-benzo[b]thien-2-yl)-3-
[1-
(phenylmethyl)-4-piperidin-yl]-1-propanone; 1-(5-azaindol-2-yl)-3-[1-
(phenyhnethyl)4-piperidinyl]-1-p-ropanone; 1-(6-azabenzo[b]thien-2-yl)-3-[1-
(phenylmethyl)-4-piperidinyl]-1-propanone; 1-(1H-2-oxo-
pyrrolo[2',3',5,6]benzo[b]thieno-2-yl)-3-[ 1-(phenylmethyl)-4-piperidinyl]-1-
propanone; 1-(6-methyl-benzothiazol-2-yl)-3-[1-(phenylmethyl)-4-piperidinyl]-1-
propanone; 1-(6-methoxy-indol-2-yl)-3-[ 1-(phenylmethyl)-4-piperidinyl]-1-
propanone; 1-(6-methoxy-benzo[b]thien-2-yl)-3-[1-(phenylmethyl)-4-piperidinyl]-
1-
pro-panone; 1-(6-acetylamino-benzo[b]thien-2-yl)-3-[1-(phenylmethyl)-4-piperid-
inyl]-1-propanone; 1-(5-acetylamino-benzo[b]thien-2-yl)-3-[1-(phenylmethyl-)-4-
piperidinyl]-1-propanone; 6-hydroxy-3-[2-[1-(phenylmethyl)-4-piperidin-
yl]ethyl]-
1,2-benzisoxazole; 5-methyl-3-[2-[1-(phenylmethyl)-4-piperidinyl-]ethyl]-1,2-
benzisoxazole; 6-methoxy-3[2-[1(phenylmethyl)-4-piperidinyl]et-hyl]-1,2-
benzisoxazole; 6-acetamide-3-[2-[1-(phenylmethyl)-4-piperidinyl]-ethyl]-1,2-
benzisoxazole; 6-amino-3-[2-[ 1-(phenymethyl)-4-piperidinyl]ethy-1]-1,2-
benzisoxazole; 6-(4-morpholinyl)-3 - [2-[1 -(phenylmethyl)-4-piperidin-yl]
ethyl] - 1,2-
benzisoxazole; 5,7-dihydro-3-[2-[ 1-(phenylmethyl)-4-piperidi-nyl]ethyl]-6H-
pyrrolo[4,5-f]-1,2-benzisoxazol-6-one; 3-[2-[1-(phenylmethyl)-4-
piperidinyl]ethyl]-
1,2-benzisothiazole; 3-[2-[1-(phenylmethyl)-4-piperidinyl]ethenyl]-1,2-
benzisoxazole; 6-phenylamino-3-[2-[1-(phenylmethyl)-4-piperidinyl]ethyl]-1,2,-
benzisoxaz-ole; 6-(2-thiazoly)-3-[2-[1-(phenylmethyl)-4-piperidinyl]ethyl]-1,2-
benzis-oxazole; 6-(2-oxazolyl)-3-[2-[1-(phenylmethyl)-4-piperidinyl]ethyl]-1,2-
be-
nzisoxazole; 6-pyrrolidinyl-3-[2-[1-(phenylmethyl)-4-piperidinyl]ethyl]-1,-2-
benzisoxazole; 5,7-dihydro-5,5-dimethyl-3-[2-[1-(phenylmethyl)-4-piperid-
inyl]ethyl]-6H-pyrrolo[4,5-f]-1,2-benzisoxazole-6-one; 6,8-dihydro-3-[2-[1-
(phenylmethyl)-4-piperidinyl]ethyl]-7H-pyrrolo[5,4-g]-1,2-benzisoxazole-7-one;
3-
{2-[ 1-(phenylmethyl)-4-piperidinyl] ethyl] -5,6,-8-trihydro-7H-isoxazolo[4,5 -
g]-
quinolin-7-one; 1-benzyl-4-((5,6-dimethoxy-l-indanon)-2-yl)methylpiperidine, 1-
benzyl-4-((5,6-dimethoxy-l-indanon)-2-ylidenyl)methylpiperidine, 1-benzyl-4-
((5-
methoxy-l-indanon)-2-yl)methylp-iperidine, 1-benzyl-4-((5,6-diethoxy-l-
indanon)-
192
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
2-yl)methylpiperidine, 1-benzyl-4-((5,6-methnylenedioxy-l-indanon)-2-
yl)methylpiperidine, 1-(m-nitrobenzyl)-4-((5,6-dimethoxy-l-indanon)-2-
yl)methylpiperidine, 1-cyclohexymethyl-4-((5,6-dimethoxy-l-indanon)-2-
yl)methylpiperidine, 1-(m-florobenzyl)-4-((5,6-dimethoxy-l-indanon)-2-
yl)methylpiperidine, 1-benzyl-4-((5,6-dimethoxy-l-indanon)-2-
yl)propylpiperidine,
and 1-benzyl-4-((5-isopropoxy-6-methoxy-l-indanon)-2-yl)methylpiperidine.
Exemplary calcium channel antagonists include diltiazem, omega-conotoxin
GVIA, methoxyverapamil, amlodipine, felodipine, lacidipine, and mibefradil.
Exemplary GABA-A receptor modulators include clomethiazole; IDDB;
gaboxadol (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol); ganaxolone (3a-
hydroxy-3(3-methyl-5a-pregnan-20-one); fengabine (2-[(butylimino)-(2-
chlorophenyl)methyl]-4-chlorophenol); 2-(4-methoxyphenyl)-2,5,6,7,8,9-
hexahydro-pyrazolo[4,3-c]cinnolin-3-one; 7-cyclobutyl-6-(2-methyl-2H-1,2,4-
triazol-3-ylmethoxy)-3-phenyl-1,2,4-triazolo[4,3-b]pyridazine; (3-fluoro-4-
methylphenyl)-N-({-1-[(2-methylphenyl)methyl]-benzimidazol-2-yl}methyl)-N-
pentylcarboxamide; and 3-(aminomethyl)-5-methylhexanoic acid.
Exemplary potassium channel openers include diazoxide, flupirtine,
pinacidil, levcromakalim, rilmakalim, chromakalim, PCO-400 and SKP-450 (2-
[2"(1 ", 3"-dioxolone)-2-methyl]-4-(2'-oxo-1'-pyrrolidinyl)-6-nitro-2H-1-
benzopyra-
n).
Exemplary AMPA/kainate receptor antagonists include 6-cyano-7-
nitroquinoxalin-2,3-di-one (CNQX); 6-nitro-7-sulphamoylbenzo[f]quinoxaline-2,3-
dione (NBQX); 6,7-dinitroquinoxaline-2,3-dione (DNQX); 1-(4-aminophenyl)-4-
methyl-7,8-m-ethylenedioxy-5H-2,3-benzodiazepine hydrochloride; and 2,3-
dihydroxy-6-nitro-7-sulfamoylbenzo-[flquinoxaline.
Exemplary sodium channel antagonists include ajmaline, procainamide,
flecainide and riluzole.
Exemplary matrix-metalloprotease inhibitors include 4-[4-(4-
fluorophenoxy)benzenesulfon-ylamino]tetrahydropyran-4-carboxylic acid
hydroxyamide; 5-Methyl-5-(4-(4'-fluorophenoxy)-phenoxy)-pyrimidine-2,4,6-
trione; 5-n-Butyl-5-(4-(4'-fluorophenoxy)-phenoxy)-pyrimidine-2,4,6-trione and
prinomistat.
193
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Poly(ADP ribose) polymerase (PARP) is an abundant nuclear enzyme which
is activated by DNA strand single breaks to synthesize poly (ADP ribose) from
NAD. Under normal conditions, PARP is involved in base excision repair caused
by oxidative stress via the activation and recruitment of DNA repair enzymes
in the
nucleus. Thus, PARP plays a role in cell necrosis and DNA repair. PARP also
participates in regulating cytokine expression that mediates inflammation.
Under
conditions where DNA damage is excessive (such as by acute excessive exposure
to
a pathological insult), PARP is over-activated, resulting in cell-based
energetic
failure characterized by NAD depletion and leading to ATP consumption,
cellular
necrosis, tissue injury, and organ damage/failure. PARP is thought to
contribute to
neurodegeneration by depleting nicotinamide adenine dinucleotide (NAD+) which
then reduces adenosine triphosphate (ATP; Cosi and Marien, Ann. N.Y. Acad.
Sci.,
890:227, 1999) contributing to cell death which can be prevented by PARP
inhibitors. Exemplory PARP inhibitors can be found in Southan and Szabo,
Current
Medicinal Chemistry, 10:321, 2003.
Exemplary inhibitors of p38 MAP kinase and c jun-N-terminal kinases
include pyridyl imidazoles, such as PD 169316, isomeric PD 169316, SB 203580,
SB 202190, SB 220026, and RWJ 67657. Others are described in US Patent
6,288,089, and incorporated by reference herein.
In an exemplary embodiment, a combination therapy for treating or
preventing MS comprises a high dose of one or more sirtuin activating
compounds
and one or more of Avonex (interferon beta-la), Tysabri (natalizumab), or
Fumaderm (BG-12/Oral Fumarate).
In another embodiment, a combination therapy for treating or preventing
diabetic neuropathy or conditions associated therewith comprises a high dose
of one
or more sirtuin activating compounds and one or more of tricyclic
antidepressants
(TCAs) (including, for example, imipramine, ainytriptyline, desipramine and
nortriptyline), serotonin reuptake inhibitors (SSRIs) (including, for example,
fluoxetine, paroxetine, sertralene, and citalopram) and antiepileptic drugs
(AEDs)
(including, for example, gabapentin, carbamazepine, and topimirate).
In another embodiment, the invention provides a metliod for treating or
preventing a polyglutamine disease using a combination comprising a high dose
of
194
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
one or more sirtuin activating compounds and at least one HDAC I/II inhibitor.
Examples of HDAC I/Il inhibitors include hydroxamic acids, cyclic peptides,
benzamides, short-chain fatty acids, and depudecin.
Examples of hydroxamic acids and hydroxamic acid derivatives, but are not
limited to, trichostatin A (TSA), suberoylanilide hydroxamic acid (SAIHA),
oxamflatin, suberic bishydroxamic acid (SBHA), m-carboxy-cinnamic acid
bishydroxamic acid (CBHA), valproic acid and pyroxamide. TSA was isolated as
an
antifungi antibiotic (Tsuji et al (1976) J. Antibiot (Tokyo) 29:1-6) and found
to be a
potent inhibitor of mammalian HDAC (Yoshida et al. (1990) J. Biol. Chem.
265:17174-17179). The finding that TSA-resistant cell lines have an altered
HDAC
evidences that this enzyme is an important target for TSA. Other hydroxamic
acid-
based HDAC inhibitors, SAHA, SBHA, and CBHA are synthetic compounds that
are able to inhibit HDAC at micromolar concentration or lower in vitro or in
vivo.
Glick et al. (1999) Cancer Res. 59:4392-43 99. These hydroxamic acid-based
HDAC
inhibitors all possess an essential structural feature: a polar hydroxamic
terminal
linked through a hydrophobic methylene spacer (e.g. 6 carbon at length) to
another
polar site which is attached to a terminal hydrophobic moiety (e.g., benzene
ring).
Compounds developed having such essential features also fall within the scope
of
the hydroxamic acids that may be used as HDAC inhibitors.
Cyclic peptides used as HDAC inhibitors are mainly cyclic tetrapeptides.
Examples of cyclic peptides include, but are not limited to, trapoxin A,
apicidin and
depsipeptide. Trapoxin A is a cyclic tetrapeptide that contains a 2-amino-8-
oxo-
9,10-epoxy-decanoyl (AOE) moiety. Kijima et al. (1993) J. Biol. Chem.
268:22429-
22435. Apicidin is a fungal metabolite that exhibits potent, broad-spectrum
antiprotozoal activitity and inhibits HDAC activity at nanomolar
concentrations.
Darkin-Rattray et al. (1996) Proc. Natl. Acad. Sci. USA. 93;13143-13147.
Depsipeptide is isolated from Chromobacterium violaceum, and has been shown to
inhibit HDAC activity at micromolar concentrations.
Examples of benzamides include but are not limited to MS-27-275. Saito et
al. (1990) Proc. Natl. Acad. Sci. USA. 96:4592-4597. Examples of short-chain
fatty
acids include but are not limited to butyrates (e.g., butyric acid, arginine
butyrate
and phenylbutyrate (PB)). Newmark et al. (1994) Cancer Lett. 78:1-5; and
Carducci
195
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
et al. (1997) Anticancer Res. 17:3972-3973. In addition, depudecin which has
been
shown to inhibit HDAC at micromolar concentrations (Kwon et al. (1998) Proc.
Natl. Acad. Sci. USA. 95:3356-3361) also falls within the scope of histone
deacetylase inhibitor as described herein.
Blood Coagulation Disorders
In other aspects, a high dose of a sirtuin activating compound can be used to
treat or prevent blood coagulation disorders (or hemostatic disorders). As
used
interchangeably herein, the terms "hemostasis", "blood coagulation," and
"blood
clotting" refer to the control of bleeding, including the physiological
properties of
vasoconstriction and coagulation. Blood coagulation assists in maintaining the
integrity of mammalian circulation after injury, inflammation, disease,
congenital
defect, dysfunction or other disruption. After initiation of clotting, blood
coagulation proceeds through the sequential activation of certain plasma
proenzymes
to their enzyme forms (see, for example, Coleman, R. W. et al. (eds.)
Hemostasis
and Tlirombosis, Second Edition, (1987)). These plasma glycoproteins,
including
Factor XII, Factor XI, Factor IX, Factor X, Factor VII, and prothrombin, are
zymogens of serine proteases. Most of these blood clotting enzymes are
effective on
a physiological scale only when assembled in complexes on membrane surfaces
with
protein cofactors such as Factor VIII and Factor V. Other blood factors
modulate
and localize clot formation, or dissolve blood clots. Activated protein C is a
specific
enzyme that inactivates 'procoagulant components. Calcium ions are involved in
many of the component reactions. Blood coagulation follows either the
intrinsic
pathway, where all of the protein components are present in blood, or the
extrinsic
pathway, where the cell-membrane protein tissue factor plays a critical role.
Clot
formation occurs when fibrinogen is cleaved by thrombin to form fibrin. Blood
clots are composed of activated platelets and fibrin.
Further, the formation of blood clots does not only limit bleeding in case of
an injury (hemostasis), but may lead to serious organ damage and death in the
context of atherosclerotic diseases by occlusion of an important artery or
vein.
Thrombosis is thus blood clot formation at the wrong time and place. It
involves a
cascade of complicated and regulated biochemical reactions between circulating
196
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
blood proteins (coagulation factors), blood cells (in particular platelets),
and
elements of an injured vessel wall.
Accordingly, the present invention provides anticoagulation and
antithrombotic treatments aiming at inhibiting the formation of blood clots in
order
to prevent or treat blood coagulation disorders, such as myocardial
infarction, stroke,
loss of a limb by peripheral artery disease or pulmonary embolism.
As used interchangeably herein, "modulating or modulation of hemostasis"
and "regulating or regulation of hemostasis" includes the induction (e.g.,
stimulation
or increase) of hemostasis, as well as the inhibition (e.g., reduction or
decrease) of
hemostasis.
In one aspect, the invention provides a method for reducing or inliibiting
hemostasis in a subject by administering a high dose of a sirtuin activating
compound. The compositions and methods disclosed herein are useful for the
treatment or prevention of thrombotic disorders. As used herein, the term
"thrombotic disorder" includes any disorder or condition characterized by
excessive
or unwanted coagulation or hemostatic activity, or a hypercoagulable state.
Thrombotic disorders include diseases or disorders involving platelet adhesion
and
thrombus formation, and may manifest as an increased propensity to form
thromboses, e.g., an increased number of thromboses, thrombosis at an early
age, a
familial tendency towards thrombosis, and thrombosis at unusual sites.
Examples of
thrombotic disorders include, but are not limited to, thromboembolism, deep
vein
thrombosis, pulmonary embolism, stroke, myocardial infarction, miscarriage,
thrombophilia associated with anti-thrombin III deficiency, protein C
deficiency,
protein S deficiency, resistance to activated protein C, dysfibrinogenemia,
fibrinolytic disorders, homocystinuria, pregnancy, inflammatory disorders,
myeloproliferative disorders, arteriosclerosis, angina, e.g., unstable angina,
disseminated intravascular coagulation, thrombotic thrombocytopenic purpura,
cancer metastasis, sickle cell disease, glomerular nephritis, and drug induced
thrombocytopenia (including, for example, heparin induced thrombocytopenia).
In
addition, a high dose of a sirtuin activating compound may be administered to
prevent thrombotic events or to prevent re-occlusion during or after
therapeutic clot
lysis or procedures such as angioplasty or surgery.
197
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In another embodiment, a combination drug regimen may include drugs or
compounds for the treatment or prevention of blood coagulation disorders or
secondary conditions associated with these conditions. Thus, a combination
drug
regimen may include a high dose of one or more sirtuin activating compounds
and
one or more anti-coagulation or anti-thrombosis agents. For example, a high
dose of
one or more sirtuin activating compounds can be combined with an effective
amount
of one or more of: aspirin, heparin, and oral Warfarin that inhibits Vit K-
dependent
factors, low molecular weight heparins that inhibit factors X and II, thrombin
inhibitors, inhibitors of platelet GP IIbIIIa receptors, inhibitors of tissue
factor (TF),
inhibitors of human von Willebrand factor, inhibitors of one or more factors
involved in hemostasis (in particular in the coagulation cascade). In
addition, a high
dose of one or more sirtuin activating compounds can be combined with
thrombolytic agents, such as t-PA, streptokinase, reptilase, TNK-t-PA, and
staphylokinase.
Inf lanzmatory Diseases
In other aspects, a high dose of one or more sirtuin activating compounds
can be used to treat or prevent a disease or disorder associated with
inflammation.
A high dose of one or more sirtuin activating compounds may be administered
prior
to the onset of, at, or after the initiation of inflammation. When used
prophylactically, the compounds are preferably provided in advance of any
inflammatory response or symptom. Administration of the high dose of the
sirtuin
activating compound may prevent or attenuate inflammatory responses or
symptoms.
Exemplary inflammatory conditions include, for example, multiple
sclerosis, rheumatoid arthritis, psoriatic arthritis, degenerative joint
disease,
spondouloarthropathies, gouty arthritis, systemic lupus erythematosus,
juvenile
arthritis, rheumatoid arthritis, osteoarthritis, osteoporosis, diabetes (e.g.,
insulin
dependent diabetes mellitus or juvenile onset diabetes), menstrual cramps,
cystic
fibrosis, inflammatory bowel disease, irritable bowel syndrome, Crohn's
disease,
mucous colitis, ulcerative colitis, gastritis, esophagitis, pancreatitis,
peritonitis,
Alzheimer's disease, shock, ankylosing spondylitis, gastritis, conjunctivitis,
pancreatis (acute or chronic), multiple organ injury syndrome (e.g., secondary
to
198
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
septicemia or trauma), myocardial infarction, atherosclerosis, stroke,
reperfusion
injury (e.g., due to cardiopulmonary bypass or kidney dialysis), acute
glomerulonephritis, vasculitis, thermal injury (i.e., sunburn), necrotizing
enterocolitis, granulocyte transfusion associated syndrome, and/or Sjogren's
syndrome. Exemplary inflammatory conditions of the skin include, for example,
eczema, atopic dermatitis, contact dermatitis, urticaria, schleroderma,
psoriasis, and
dermatosis with acute inflammatory components.
In another embodiment, a high dose of one or more sirtuin activating
compounds may be used to treat or prevent allergies and respiratory
conditions,
including asthma, bronchitis, pulmonary fibrosis, allergic rhinitis, oxygen
toxicity,
emphysema, chronic bronchitis, acute respiratory distress syndrome, and any
chronic obstructive pulmonary disease (COPD). The high dose of one or more
sirtuin activating compounds may be used to treat chronic hepatitis infection,
including hepatitis B and hepatitis C.
Additionally, a high dose of one or more sirtuin activating compounds may
be used to treat autoimmune diseases and/or inflammation associated with
autoimmune diseases such as organ-tissue autoimmune diseases (e.g., Raynaud's
syndrome), scleroderma, myastlienia gravis, transplant rejection, endotoxin
shock,
sepsis, psoriasis, eczema, dermatitis, multiple sclerosis, autoimmune
thyroiditis,
uveitis, systemic lupus erythematosis, Addison's disease, autoimmune
polyglandular disease (also known as autoimmune polyglandular syndrome), and
Grave's disease.
In certain embodiments, a high dose of one or more sirtuin activating
compounds may be taken alone or in combination with other compounds useful for
treating or preventing inflammation. Exemplary anti-inflammatory agents
include,
for example, steroids (e.g., cortisol, cortisone, fludrocortisone, prednisone,
6a-
methylprednisone, triamcinolone, betamethasone or dexamethasone) and
nonsteroidal antiinflammatory drugs (NSAIDS) (e.g., aspirin, acetaminophen,
tolmetin, ibuprofen, mefenamic acid, piroxicam, nabumetone, rofecoxib,
celecoxib,
etodolac or nimesulide). In another embodiment, the other therapeutic agent is
an
antibiotic (e.g., vancomycin, penicillin, amoxicillin, ampicillin, cefotaxime,
ceftriaxone, cefixime, rifampinmetronidazole, doxycycline or streptomycin). In
199
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
another embodiment, the other therapeutic agent is a PDE4 inhibitor (e.g.,
roflumilast or rolipram). In another embodiment, the other therapeutic agent
is an
antihistamine (e.g., cyclizine, hydroxyzine, promethazine or diphenhydramine).
In
another embodiment, the other therapeutic agent is an anti-malarial (e.g.,
artemisinin, artemether, artsunate, chloroquine phosphate, mefloquine
hydrochloride, doxycycline hyclate, proguanil hydrochloride, atovaquone or
halofantrine). In one embodiment, the other therapeutic agent is drotrecogin
alfa.
Further examples of anti-inflammatory agents include, for example,
aceclofenac, acemetacin, e-acetamidocaproic acid, acetaininophen,
acetaminosalol,
acetanilide, acetylsalicylic acid, S-adenosylmethionine, alclofenac,
alclometasone,
alfentanil, algestone, allylprodine, alminoprofen, aloxiprin, alphaprodine,
aluminum bis(acetylsalicylate), amcinonide, amfenac, aminochlorthenoxazin, 3-
amino-4-hydroxybutyric acid, 2-amino-4-picoline, aminopropylon, aminopyrine,
amixetrine, ammonium salicylate, ampiroxicam, amtolmetin guacil, anileridine,
antipyrine, antrafenine, apazone, beclomethasone, bendazac, benorylate,
benoxaprofen, benzpiperylon, benzydamine, benzylmorphine, bermoprofen,
betamethasone, betamethasone-17-valerate, bezitramide, a-bisabolol, bromfenac,
p-bromoacetanilide, 5-bromosalicylic acid acetate, bromosaligenin, bucetin,
bucloxic acid, bucolome, budesonide, bufexamac, bumadizon, buprenorphine,
butacetin, butibufen, butorphanol, carbamazepine, carbiphene, carprofen,
carsalam,
chlorobutanol, chloroprednisone, chlorthenoxazin, choline salicylate,
cinchophen,
cinmetacin, ciramadol, clidanac, clobetasol, clocortolone, clometacin,
clonitazene,
clonixin, clopirac, cloprednol, clove, codeine, codeine methyl bromide,
codeine
phosphate, codeine sulfate, cortisone, cortivazol, cropropamide, crotethamide,
cyclazocine, deflazacort, dehydrotestosterone, desomorphine, desonide,
desoximetasone, dexamethasone, dexamethasone-21-isonicotinate, dexoxadrol,
dextromoramide, dextropropoxyphene, deoxycorticosterone, dezocine,
diampromide, diamorphone, diclofenac, difenamizole, difenpiramide,
diflorasone,
diflucortolone, diflunisal, difluprednate, dihydrocodeine, dihydrocodeinone
enol
acetate, dihydromorphine, dihydroxyaluminum acetylsalicylate, dimenoxadol,
dimepheptanol, dimethylthiambutene, dioxaphetyl butyrate, dipipanone,
diprocetyl,
dipyrone, ditazol, droxicam, emorfazone, enfenamic acid, enoxolone, epirizole,
200
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
eptazocine, etersalate, ethenzamide, ethoheptazine, ethoxazene,
ethylmethyltliiambutene, ethylmorphine, etodolac, etofenamate, etonitazene,
eugenol, felbinac, fenbufen, fenclozic acid, fendosal, fenoprofen, fentanyl,
fentiazac, fepradinol, feprazone, floctafenine, fluazacort, flucloronide,
flufenamic
acid, flumethasone, flunisolide, flunixin, flunoxaprofen, fluocinolone
acetonide,
fluocinonide, fluocinolone acetonide, fluocortin butyl, fluocortolone,
fluoresone,
fluorometholone, fluperolone, flupirtine, fluprednidene, fluprednisolone,
fluproquazone, flurandrenolide, flurbiprofen, fluticasone, formocortal,
fosfosal,
gentisic acid, glafenine, glucametacin, glycol salicylate, guaiazulene,
halcinonide,
halobetasol, halometasone, haloprednone, heroin, hydrocodone, hydrocortamate,
hydrocortisone, hydrocortisone acetate, hydrocortisone succinate,
hydrocortisone
hemisuccinate, hydrocortisone 21 -lysinate, hydrocortisone cypionate,
hydromorphone, hydroxypethidine, ibufenac, ibuprofen, ibuproxam, imidazole
salicylate, indomethacin, indoprofen, isofezolac, isoflupredone, isoflupredone
acetate, isoladol, isomethadone, isonixin, isoxepac, isoxicam, ketobemidone,
ketoprofen, ketorolac, p-lactophenetide, lefetamine, levallorphan,
levorphanol,
levophenacyl-morphan, lofentanil, lonazolac, lornoxicam, loxoprofen, lysine
acetylsalicylate, mazipredone, meclofenamic acid, medrysone, mefenamic acid,
meloxicam, meperidine, meprednisone, meptazinol, mesalamine, metazocine,
methadone, methotrimeprazine, methylprednisolone, methylprednisolone acetate,
methylprednisolone sodium succinate, methylprednisolone suleptnate, metiazinic
acid, metofoline, metopon, mofebutazone, mofezolac, mometasone, morazone,
morphine, morphine hydrochloride, morphine sulfate, morpholine salicylate,
myrophine, nabumetone, nalbuphine, nalorphine, 1-naphthyl salicylate,
naproxen,
narceine, nefopam, nicomorphine, nifenazone, niflumic acid, nimesulide, 5'-
nitro-
2'-propoxyacetanilide, norlevorphanol, normethadone, normorphine, norpipanone,
olsalazine, opium, oxaceprol, oxametacine, oxaprozin, oxycodone, oxymorphone,
oxyphenbutazone, papaveretum, paramethasone, paranyline, parsalmide,
pentazocine, perisoxal, phenacetin, phenadoxone, phenazocine, phenazopyridine
hydrochloride, phenocoll, phenoperidine, phenopyrazone, phenomorphan, phenyl
acetylsalicylate, phenylbutazone, phenyl salicylate, phenyramidol,
piketoprofen,
piminodine, pipebuzone, piperylone, pirazolac, piritramide, piroxicam,
pirprofen,
201
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
pranoprofen, prednicarbate, prednisolone, prednisone, prednival, prednylidene,
proglumetacin, proheptazine, promedol, propacetamol, properidine, propiram,
propoxyphene, propyphenazone, proquazone, protizinic acid, proxazole,
ramifenazone, remifentanil, rimazolium metilsulfate, salacetamide, salicin,
salicylamide, salicylamide o-acetic acid, salicylic acid, salicylsulfuric
acid,
salsalate, salverine, simetride, sufentanil, sulfasalazine, sulindac,
superoxide
dismutase, suprofen, suxibuzone, talniflumate, tenidap, tenoxicam,
terofenamate,
tetrandrine, thiazolinobutazone, tiaprofenic acid, tiaramide, tilidine,
tinoridine,
tixocortol, tolfenamic acid, tolmetin, tramadol, triamcinolone, triamcinolone
acetonide, tropesin, viminol, xenbucin, ximoprofen, zaltoprofen and zomepirac.
In an exemplary embodiment, a high dose of one or more sirtuin activating
compounds may be administered with a selective COX-2 inhibitor for treating or
preventing inflammation. Exemplary selective COX-2 inhibitors include, for
example, deracoxib, parecoxib, celecoxib, valdecoxib, rofecoxib, etoricoxib,
lumiracoxib, 2-(3,5-difluorophenyl)-3-[4-(methylsulfonyl)phenyl]-2-cyclopenten-
l-
one, (S)-6,8-dichloro-2-(triflu- oromethyl)-2H-1-benzopyran-3-carboxylic acid,
2-
(3,4-difluorophenyl)-4-(3-- hydroxy-3-methyl-l-butoxy)-5-[4-
, (methylsulfonyl)phenyl]-3-(2H)-pyridazinone, 4-[5-(4-fluorophenyl)-3-
(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide, tert-butyl 1 benzyl-4-
[(4-
oxopiperidin-1-yl)sulfonyl]piperidine-4-carboxylate, 4-[5-(phenyl)-3-
(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide, salts and prodrugs
thereof.
Flushiug
In another aspect, a high dose of one or more sirtuin activating compounds
may be used for reducing the incidence or severity of flushing and/or hot
flashes
which are symptoms of a disorder. For instance, the subject method includes
the use
of a high dose of one or more sirtuin activating compounds, alone or in
combination
with other agents, for reducing incidence or severity of flushing and/or hot
flashes in
cancer patients. In.other embodiments, the method provides for the use of a
high
dose of one or more sirtuin activating compounds to reduce the incidence or
severity
of flushing and/or hot flashes in menopausal and post-menopausal woman.
In another aspect, a high dose of one or more sirtuin activating compounds
may be used as a therapy for reducing the incidence or severity of flushing
and/or
202
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
hot flashes which are side-effects of another drug therapy, e.g., drug-induced
flushing. In certain embodiments, a method for treating and/or preventing drug-
induced flushing comprises administering to a patient in need thereof a
formulation
comprising at least one flushing inducing compound and a high dose of at least
one
sirtuin activating compound. In other embodiments, a method for treating drug
induced flushing comprises separately administering one or more compounds that
induce flushing and a high dose of one or more sirtuin activating compounds,
e.g.,
wherein the sirtuin-modulating compound and flushing inducing agent have not
been formulated in the same compositions. When using separate formulations,
the
sirtuin-modulating compound may be administered (1) at the same as
administration
of the flushing inducing agent, (2) intermittently with the flushing inducing
agent,
(3) staggered relative to administration of the flushing inducing agent, (4)
prior to
administration of the flushing inducing agent, (5) subsequent to
administration of the
flushing inducing agent, and (6) various combination thereof. Exemplary
flushing
inducing agents include, for example, niacin, faloxifene, antidepressants,
anti-
psychotics, chemotherapeutics, calcium channel blockers, and antibiotics.
In one embodiment, a high dose of one or more sirtuin activating compounds
may be used to reduce flushing side effects of a vasodilator or an antilipemic
agent
(including anticholesteremic agents and lipotropic agents). In an exemplary
embodiment, a high dose of one or more sirtuin activating compounds may be
used
to reduce flushing associated with the administration of niacin.
, Nicotinic acid, 3-pyridinecarboxylic acid or niacin, is an antilipidemic
agent
that is marketed under, for example, the trade names Nicolaro, SloNiacin ,
Nicobid
and Time Release Niacin . Nicotinic acid has been used for many years in the
treatment of lipidemic disorders such as hyperlipidemia, hypercholesterolemia
and
atherosclerosis. This compound has long been known to exhibit the beneficial
effects of reducing total cholesterol, low density lipoproteins or "LDL
cholesterol,"
triglycerides and apolipoprotein a (Lp(a)) in the human body, while increasing
desirable high density lipoproteins or "HDL cholesterol".
1 Typical doses range from about 1 gram to about 3 grams daily. Nicotinic
acid is normally administered two to four times per day after meals, depending
upon
the dosage form selected. Nicotinic acid is currently commercially available
in two
203
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
dosage forms. One dosage form is an immediate or rapid release tablet which
should
be administered three or four times per day. Immediate release ("IR")
nicotinic acid
formulations generally release nearly all of their nicotinic acid within about
30 to 60
minutes following ingestion. The other dosage form is a sustained release form
which is suitable for administration two to four times per day. In contrast to
IR
formulations, sustained release ("SR") nicotinic acid formulations are
designed to
release significant quantities of drug for absorption into the blood stream
over
specific timed intervals in order to maintain therapeutic levels of nicotinic
acid over
an extended period such as 12 or 24 hours after ingestion.
As used herein, the term "nicotinic acid" is meant to encompass nicotinic
acid or a compound other than nicotinic acid itself which the body metabolizes
into
nicotinic acid, thus producing essentially the same effect as nicotinic acid.
Exemplary compounds that produce an effect similar to that of nicotinic acid
include, for example, nicotinyl alcohol tartrate, d-glucitol hexanicotinate,
aluminum
nicotinate, niceritrol and d,1-alpha-tocopheryl nicotinate. Each such compound
will
be collectively referred to herein as "nicotinic acid."
In another embodiment, the invention provides a method for treating and/or
preventing hyperlipidemia with reduced flushing side effects. The method
comprises
the steps of administering to a subject in need thereof a therapeutically
effective
amount of nicotinic acid and a high dose of one or more sirtuin activating
compounds. In an exemplary embodiment, the nicotinic acid and/or sirtuin-
modulating compound may be administered nocturnally.
In another representative embodiment, the method involves the use of a high
dose of one or more sirtuin activating compounds to reduce flushing side
effects of
raloxifene. Raloxifene acts like estrogen in certain places in the body, but
is not a
hormone. It helps prevent osteoporosis in women who have reached menopause.
Osteoporosis causes bones to gradually grow thin, fragile, and more likely to
break.
Evista slows down the loss of bone mass that occurs with menopause, lowering
the
risk of spine fractures due to osteoporosis. A common side effect of
raloxifene is hot
flashes (sweating and flushing). This can be uncomfortable for women who
already
have hot flashes due to menopause.
204
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In another representative embodiment, the method involves the use of a high
dose of one or more sirtuin activating compounds to reduce flushing side
effects of
antidepressants or anti-psychotic agent. For instance, a high dose of one or
more
sirtuin activating compounds can be used in conjunction (adininistered
separately or
together) with a serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an
anticonvulsant, a norepinephrine reuptake inhibitor, an a-adrenoreceptor
antagonist,
an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an
Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A
receptor
antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase
inhibitor, or a sedative-hypnotic drug.
In certain embodiments, a high dose of one or more sirtuin activating
compounds may be used as part of a treatment with a serotonin reuptake
inhibitor
(SRI) to reduce flushing. In certain preferred embodiments, the SRI is a
selective
serotonin reuptake inhibitor (SSRI), such as a fluoxetinoid (fluoxetine,
norfluoxetine) or a nefazodonoid (nefazodone, hydroxynefazodone,
oxonefazodone).
Other exemplary SSRI's include duloxetine, venlafaxine, milnacipran,
citalopram,
fluvoxamine, paroxetine and sertraline. A high dose of one or more sirtuin
activating compounds can also be used as part of a treatment with sedative-
hypnotic
drug, such as selected from the group consisting of a benzodiazepine (such as
alprazolam, chlordiazepoxide, clonazepam, chlorazepate, clobazam, diazepam,
halazepam, lorazepam, oxazepam and prazepam), zolpidem, and barbiturates. In
still other embodiments, a high dose of one or more sirtuin activating
compounds
may be used as part of a treatment with a 5-HT1A receptor partial agonist,
such as
selected from the group consisting of buspirone, flesinoxan, gepirone and
ipsapirone. A high dose of one or more sirtuin activating compounds can also
used
as part of a treatment with a norepinephrine reuptake inhibitor, such as
selected from
tertiary amine tricyclics and secondary amine tricyclics. Exemplary tertiary
amine
tricyclic include amitriptyline, clomipramine, doxepin, imipramine and
trimipramine. Exemplary secondary amine tricyclic include amoxapine,
desipramine, maprotiline, nortriptyline and protriptyline. In certain
embodiments, a
high dose of one or more sirtuin activating compounds may be used as part of a
treatment with a monoamine oxidase inhibitor, such as selected from the group
205
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
consisting of isocarboxazid, pheneizine, tranylcypromine, selegiline and
moclobemide.
In still another representative embodiment, a high dose of one or more sirtuin
activating compounds may be used to reduce flushing side effects of
chemotherapeutic agents, such as cyclophosphamide, tamoxifen.
In another embodiment, a high dose of one or more sirtuin activating
compounds may be used to reduce flushing side effects of calcium channel
blockers,
such as amlodipine.
In another embodiment, a high dose of one or more sirtuin activating
compounds may be used to reduce flushing side effects of antibiotics. For
example,
a high dose of one or more sirtuin activating compounds can be used in
combination
with levofloxacin. Levofloxacin is used to treat infections of the sinuses,
skin,
lungs, ears, airways, bones, and joints caused by susceptible bacteria.
Levofloxacin
also is frequently used to treat urinary infections,. including those
resistant to other
antibiotics, as well as prostatitis. Levofloxacin is effective in treating
infectious
diarrheas caused by E. coli, campylobacterjejuni, and shigella bacteria.
Levofloxacin also can be used to treat various obstetric infections, including
mastitis.
Ocular Disorders
One aspect of the present invention is a method for inhibiting, reducing or
otherwise treating vision impairment by administering to a patient a high dose
of one
or more sirtuin activating compounds.
In certain aspects of the invention, the vision impairment is caused by
damage to the optic nerve or central nervous system. In particular
embodiments,
optic nerve damage is caused by high intraocular pressure, such as that
created by
glaucoma. In other particular embodiments, optic nerve damage is caused by
swelling of the nerve, which is often associated with an infection or an
immune
(e.g., autoimmune) response such as in optic neuritis.
Glaucoma describes a group of disorders which are associated with a visual
field defect, cupping of the optic disc, and optic nerve damage. These are
commonly
referred to as glaucomatous optic neuropathies. Most glaucomas are usually,
but not
always, associated with a rise in intraocular pressure. Exemplary forms of
glaucoma
206
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
include Glaucoma and Penetrating Keratoplasty, Acute Angle Closure, Chronic
Angle Closure, Chronic Open Angle, Angle Recession, Aphakic and Pseudophakic,
Drug-Induced, Hyphema, Intraocular Tumors, Juvenile, Lens-Particle, Low
Tension,
Malignant, Neovascular, Phacolytic, Phacomorphic, Pigmentary, Plateau Iris,
Primary Congenital, Primary Open Angle, Pseudoexfoliation, Secondary
Congenital, Adult Suspect, Unilateral, Uveitic, Ocular Hypertension, Ocular
Hypotony, Posner-Schlossman Syndrome and Scleral Expansion Procedure in
Ocular Hypertension & Primary Open-angle Glaucoma.
Intraocular pressure can also be increased by various surgical procedures,
such as phacoemulsification (i.e., cataract surgery) and implanation of
structures
such as an artificial lens. In addition, spinal surgeries in particular, or
any surgery in
which the patient is prone for an extended period of time can lead to
increased
interoccular pressure.
Optic neuritis (ON) is inflammation of the optic nerve and causes acute loss
of vision. It is highly associated with multiple sclerosis (MS) as 15-25% of
MS
patients initially present with ON, and 50-75% of ON patients are diagnosed
with
MS. ON is also associated with infection (e.g., viral infection, meningitis,
syphilis),
inflammation (e.g., from a vaccine), infiltration and ischemia.
Another condition leading to optic nerve damage is anterior ischemic optic
neuropathy (AION). There are two types of AION. Arteritic AION is due to giant
cell arteritis (vasculitis) and leads to acute vision loss. Non-arteritic AION
encompasses all cases of ischemic optic neuropathy other than those due to
giant cell
arteritis. The patliophysiology of AION is unclear although it appears to
incorporate
both inflammatory and ischemic mechanisms.
Other damage to the optic nerve is typically associated with demyleination,
inflammation, ischemia, toxins, or trauma to the optic nerve. Exemplary
conditions
where the optic nerve is damaged include Demyelinating Optic Neuropathy (Optic
Neuritis, Retrobulbar Optic Neuritis), Optic Nerve Sheath Meningioma, Adult
Optic
Neuritis, Childhood Optic Neuritis, Anterior Ischemic Optic Neuropathy,
Posterior
Ischemic Optic Neuropathy, Compressive Optic Neuropathy, Papilledema,
Pseudopapilledema and Toxic/Nutritional Optic Neuropathy.
207
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Other neurological conditions associated with vision loss, albeit not directly
associated with damage to the optic nerve, include Amblyopia, Bells Palsy,
Chronic
Progressive External Ophthalmoplegia, Multiple Sclerosis, Pseudotumor Cerebri
and Trigeminal Neuralgia.
In certain aspects of the invention, the vision impairment is caused by
retinal
damage. In particular embodiments, retinal damage is caused by disturbances in
blood flow to the eye (e.g., arteriosclerosis, vasculitis). In particular
embodiments,
retinal damage is caused by disrupton of the macula (e.g., exudative or non-
exudative macular degeneration).
Exemplary retinal diseases include Exudative Age Related Macular
Degeneration, Nonexudative Age Related Macular Degeneration, Retinal
Electronic
Prosthesis and RPE Transplantation Age Related Macular Degeneration, Acute
Multifocal Placoid Piginent Epitheliopathy, Acute Retinal Necrosis, Best
Disease,
Branch Retinal Artery Occlusion, Branch Retinal Vein Occlusion, Cancer
Associated and Related Autoimmune Retinopathies, Central Retinal Artery
Occlusion, Central Retinal Vein Occlusion, Central Serous Chorioretinopathy,
Eales
Disease, Epimacular Membrane, Lattice Degeneration, Macroaneurysm, Diabetic
Macular Edema, Irvine-Gass Macular Edema, Macular Hole, Subretinal Neovascular
Membranes, Diffuse Unilateral Subacute Neuroretinitis, Nonpseudophakic Cystoid
Macular Edema, Presumed Ocular Histoplasmosis Syndrome, Exudative Retinal
Detachment, Postoperative Retinal Detachment, Proliferative Retinal
Detachment,
Rhegmatogenous Retinal Detachment, Tractional Retinal Detachment, Retinitis
Pigmentosa, CMV Retinitis, Retinoblastoma, Retinopathy of Prematurity,
Birdshot
Retinopathy, Background Diabetic Retinopathy, Proliferative Diabetic
Retinopathy,
Hemoglobinopathies Retinopathy, Purtscher Retinopathy, Valsalva Retinopathy,
Juvenile Retinoschisis, Senile Retinoschisis, Terson Syndrome and White Dot
Syndromes.
Other exemplary diseases include ocular bacterial infections (e.g.
conjunctivitis, keratitis, tuberculosis, syphilis, gonorrhea), viral
infections (e.g.
Ocular Herpes Simplex Virus, Varicella Zoster Virus, Cytomegalovirus
retinitis,
Human Immunodeficiency Virus (HIV)) as well as progressive outer retinal
necrosis
secondary to HIV or other HIV-associated and other immunodeficiency-associated
208
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
ocular diseases. In addition, ocular diseases include fungal infections (e.g.
Candida
choroiditis, histoplasmosis), protozoal infections (e.g. toxoplasmosis) and
others
such as ocular toxocariasis and sarcoidosis.
One aspect of the invention is a method for inhibiting, reducing or treating
vision impairment in a subject undergoing treatment with a chemotherapeutic
drug
(e.g., a neurotoxic drug, a drug that raises intraocular pressure such as a
steroid), by
administering to the subject in need of such treatment a high dose of one or
more
sirtuin activating compounds.
Another aspect of the invention is a method for inhibiting, reducing or
treating vision impairment in a subject undergoing surgery, including ocular
or other
surgeries performed in the prone position such as spinal cord surgery, by
administering to the subject in need of such treatment a high dose of one or
more
sirtuin activating compounds. Ocular surgeries include cataract, iridotomy and
lens
replacements.
Another aspect of the invention is the treatment, including inhibition and
prophylactic treatment, of age related ocular diseases include cataracts, dry
eye,
retinal damage and the like, by administering to the subject in need of such
treatment
a high dose of one or more sirtuin activating compounds.
The forination of cataracts is associated with several biochemical changes in
the lens of the eye, such as decreased levels of antioxidants ascorbic acid
and
glutathione, increased lipid, amino acid and protein oxidation, increased
sodium and
calcium, loss of amino acids and decreased lens metabolism. The lens, which
lacks
blood vessels, is suspended in extracellular fluids in the anterior part of
the eye.
Nutrients, such as ascorbic acid, glutathione, vitamin E, selenium,
bioflavonoids and
carotenoids are required to maintain the transparency of the lens. Low levels
of
selenium results in an increase of free radical-inducing hydrogen peroxide,
which is
neutralized by the selenium-dependent antioxidant enzyme glutathione
peroxidase.
Lens-protective glutathione peroxidase is also dependent on the amino acids
methionine, cysteine, glycine and glutamic acid.
Cataracts can also develop due to an inability to properly metabolize
galactose found in dairy products that contain lactose, a disaccharide
composed of
209
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
the monosaccharide galactose and glucose. Cataracts can be prevented, delayed,
slowed and possibly even reversed if detected early and metabolically
corrected.
Retinal damage is attributed, inter alia, to free radical initiated reactions
in
glaucoma, diabetic retinopathy and age-related macular degeneration (AMD). The
eye is a part of the central nervous system and has limited regenerative
capability.
The retina is composed of numerous nerve cells which contain the highest
concentration of polyunsaturated fatty acids (PFA) and subject to oxidation.
Free
radicals are generated by UV light entering the eye and mitochondria in the
rods and
cones, which generate the energy necessary to transform light into visual
impulses.
Free radicals cause peroxidation of the PFA by hydroxyl or superoxide radicals
which in turn propagate additional free radicals. The free radicals cause
temporary
or permanent damage to retinal tissue.
Glaucoma is usually viewed as a disorder that causes an elevated intraocular
pressure (IOP) that results in permanent damage to the retinal nerve fibers,
but a
sixth of all glaucoma cases do not develop an elevated IOP. This disorder is
now
perceived as one of reduced vascular perfusion and an increase in neurotoxic
factors.
Recent studies have implicated elevated levels of glutamate, nitric oxide and
peroxynitirite in the eye as the causes of the death of retinal ganglion
cells.
Neuroprotective agents may be the future of glaucoma care. For example, nitric
oxide synthase inhibitors block the formation of peroxynitrite from nitric
oxide and
superoxide. In a recent study, animals treated with aminoguanidine, a nitric
oxide
synthase inhibitor, had a reduction in the loss of retinal ganglion cells. It
was
concluded that nitric oxide in the eye caused cytotoxicity in many tissues and
neurotoxicity in the central nervous system.
Diabetic retinopathy occurs when the underlying blood vessels develop
microvascular abnormalities consisting primarily of microaneurysms and
intraretinal
hemorrhages. Oxidative metabolites are directly involved with the pathogenesis
of
diabetic retinopathy and free radicals augment the generation of growth
factors that
lead to enhanced proliferative activity. Nitric oxide produced by endothelial
cells of
the vessels may also cause smooth muscle cells to relax and result in
vasodilation of
segments of the vessel. Ischemia and hypoxia of the retina occur after
thickening of
the arterial basement membrane, endothelial proliferation and loss of
pericytes. The
210
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
inadequate oxygenation causes capillary obliteration or nonperfusion,
arteriolar-
venular shunts, sluggish blood flow and an impaired ability of RBCs to release
oxygen. Lipid peroxidation of the retinal tissues also occurs as a result of
free radical
damage.
The macula is responsible for our acute central vision and coinposed of light-
sensing cells (cones) while the underlying retinal pigment epithelium (RPE)
and
choroid nourish and help remove waste materials. The RPE nourishes the cones
with
the vitamin A substrate for the photosensitive pigments and digests the cones
shed
outer tips. RPE is exposed to high levels of UV radiation, and secretes
factors that
inhibit angiogenesis. The choroid contains a dense vascular network that
provides
nutrients and removes the waste materials.
In AMD, the shed cone tips become indigestible by the RPE, where the cells
swell and die after collecting too much undigested material. Collections of
undigested waste material, called drusen, form under the RPE. Photoxic damage
also
causes the accumulation of lipofuscin in RPE cells. The intracellular
lipofuscin and
accumulation of drusen in Bruch's membrane interferes with the transport of
oxygen
and nutrients to the retinal tissues, and ultimately leads to RPE and
photoreceptor
dysfunction. In exudative AMD, blood vessels grow from the choriocapillaris
through defects in Bruch's membrane and may grow under the RPE, detaching it
from the choroid, and leaking fluid or bleeding.
Macular pigment, one of the protective factors that prevent sunlight from
damaging the retina, is formed by the accumulation of nutritionally derived
carotenoids, such as lutein, the fatty yellow pigment that serves as a
delivery vehicle
for other important nutrients and zeaxanthin. Antioxidants such as vitamins C
and E,
beta-carotene and lutein, as well as zinc, selenium and copper, are all found
in the
healthy macula. In addition to providing nourishment, these antioxidants
protect
against free radical damage that initiates macular degeneration.
Another aspect of the invention is the prevention or treatment of damage to
the eye caused by stress, chemical insult or radiation, by administering to
the subject
in need of such treatment a high dose of one or more sirtuin activating
compounds.
Radiation or electromagnetic damage to the eye can include that caused by
CRT's or
exposure to sunlight or UV.
211
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In one einbodiinent, a combination drug regimen may include drugs or
compounds for the treatment or prevention of ocular disorders or secondary
conditions associated with these conditions. Thus, a combination drug regimen
may
include a high dose of one or more sirtuin activating compounds and one or
more
therapeutic agents for the treatment of an ocular disorder. For example, a
high dose
of one or more sii-tuin activating compounds can be combined with an effective
amount of one or more of: an agent that reduces intraocular pressure, an agent
for
treating glaucoma, an agent for treating optic neuritis, an agent for treating
CMV
Retinopathy, an agent for treating multiple sclerosis, and/or an antibiotic,
etc.
In one embodiment, a high dose of one or more sirtuin activating compounds
can be administered in conjunction with a therapy for reducing intraocular
pressure.
One group, of therapies involves blocking aqueous production. For example,
topical
beta-adrenergic antagonists (timolol and betaxolol) decrease aqueous
production.
Topical timolol causes IOP to fall in 30 minutes with peak effects in 1-2
hours. A
reasonable regimen is Timoptic 0.5%, one drop every 30 minutes for 2 doses.
The
carbonic anhydrase inhibitor, acetazolamide, also decreases aqueous production
and
should be given in conjunction with topical beta-antagonists. An initial dose
of 500
mg is administered followed by 250 mg every 6 hours. This medication may be
given orally, intramuscularly, or intravenously. In addition, alpha 2-agonists
(e.g.,
Apraclonidine) act by decreasing aqueous production. Their effects are
additive to
topically administered beta-blockers. They have been approved for use in
controlling
an acute rise in pressure following anterior chamber laser procedures, but has
been
reported effective in treating acute closed-angle glaucoma. A reasonable
regimen is
1 drop every 30 minutes for 2 doses.
A second group of therapies for reducing intraocular pressure involve
reducing vitreous volume. Hyperosmotic agents can be used to treat an acute
attack.
These agents draw water out of the globe by making the blood hyperosmolar.
Oral
glycerol in a dose of 1 mL/kg in a cold 50% solution (mixed with lemon juice
to
make it more palatable) often is used. Glycerol is converted to glucose in the
liver;
persons with diabetes may need additional insulin if they become hyperglycemic
after receiving glycerol. Oral isosorbide is a metabolically inert alcohol
that also can
be used as an osmotic agent for patients with acute angle-closure glaucoma.
Usual
212
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
dose is 100 g taken p.o. (220 cc of a 45% solution). This inert alcohol should
not be
confused with isosorbide dinitrate, a nitrate-based cardiac medication used
for
angina and for congestive heart failure. Intravenous mannitol in a dose of 1.0-
1.5
mg/kg also is effective and is well tolerated in patients with nausea and
vomiting.
These hyperosmotic agents should be used with caution in any patient with a
history
of congestive heart failure.
A third group of therapies involve facilitating aqueous outflow from the eye.
Miotic agents pull the iris from the iridocorneal angle and may help to
relieve the
obstruction of the trabecular meshwork by the peripheral iris. Pilocarpine 2%
(blue
eyes)-4% (brown eyes) can be administered every 15 minutes for the first 1-2
hours.
More frequent administration or higher doses may precipitate a systemic
cholinergic
crisis. NSAIDS are sometimes used to reduce inflammation.
Exemplary therapeutic agents for reducing intraocular pressure include
ALPHAGAN P (Allergan) (brimonidine tartrate ophthalmic solution), AZOPT
(Alcon) (brinzolamide ophthalmic suspension), BETAGAN (Allergan)
(levobunolol hydrochloride ophthalmic solution, USP), BETIMOL (Vistakon)
(timolol ophthalmic solution), BETOPTIC S (Alcon) (betaxolol HCl),
BRIMONIDINE TARTRATE (Bausch & Lomb), CARTEOLOL
HYDROCHLORIDE (Bausch & Lomb), COSOPT (Merck) (dorzolamide
hydrochloride-timolol maleate ophthalmic solution), LUMIGAN (Allergan)
(bimatoprost ophthalmic solution), OPTIPRANOLOL (Bausch & Lomb)
(metipranolol ophthalmic solution), TIMOLOL GFS (Falcon) (timolol maleate
ophthalmic gel forming solution), TIMOPTIC (Merck) (timolol maleate
ophthalmic solution), TRAVATAN (Alcon) (travoprost ophthalmic solution),
TRUSOPT (Merck) (dorzolamide hydrochloride ophthalmic solution) and
XALATAN (Pharmacia & Upjohn) (latanoprost ophthalmic solution).
In one embodimen't, a high dose of one or more sirtuin activating compounds
can be administered in conjunction with a therapy for treating and/or
preventing
glaucoma. An example of a glaucoma drug is DARANIDE Tablets (Merck)
(Dichlorphenamide).
In one embodiment, a high dose of one or more sirtuin activating compounds
can be administered in conjunction with a therapy for treating and/or
preventing
213
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
optic neuritis. Examples of drugs for optic neuritis include DECADRON
Phosphate Injection (Merck) (Dexamethasone Sodium Phosphate), DEPO-
MEDROL (Pharmacia & Upjohn)(methylprednisolone acetate),
HYDROCORTONE Tablets (Merck) (Hydrocortisone), ORAPRED (Biomarin)
(prednisolone sodium phosphate oral solution) and PEDIAPRED (Celltech)
(prednisolone sodium phosphate, USP).
In one embodiinent, a high dose of one or more sirtuin activating compounds
can be administered in conjunction with a therapy for treating and/or
preventing
CMV Retinopathy. Treatments for CMV retinopathy include CYTOVENE
(ganciclovir capsules) and VALCYTE (Roche Laboratories) (valganciclovir
hydrochloride tablets).
In one embodiment, a high dose of one or more sirtuin activating compounds
can be administered in conjunction with a therapy for treating and/or
preventing
multiple sclerosis. Examples of such drugs include DANTRIUM (Procter &
Gamble Pharmaceuticals) (dantrolene sodium), NOVANTRONE (Serono)
(mitoxantrone), AVONEX (Biogen Idec) (Interferon beta-la), BETASERON
(Berlex) (Interferon beta-lb), COPAXONE (Teva Neuroscience) (glatiramer
acetate injection) and REBIF (Pfizer) (interferon beta-la).
In addition, macrolide and/or mycophenolic acid, which has multiple
activities, can be co-administered with a high dose of one or more sirtuin
activating
compounds. Macrolide antibiotics include tacrolimus, cyclosporine, sirolimus,
everolimus, ascomycin, erythromycin, azithromycin, clarithromycin,
clindamycin,
lincomycin, dirithromycin, josamycin, spiramycin, diacetyl-midecamycin,
tylosin,
roxithromycin, ABT-773, telithromycin, leucomycins, and lincosamide.
III. Exemplary Assays
In certain aspects, the present invention provides screening methods for
identifying compounds (agents) for treating or preventing metabolic disorders.
Candidate compounds identified by the subject screening methods can be
administered to a subject, such as a subject in need thereof. A subject in
need of
such a treatment may be a subject who suffers from obesity or diabetes, or who
has,
214
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
or is, likely to have these disorders, as predicted, e.g., from family
history.
Exemplary agents are those described herein.
The effect of a compound on the activity of a sirtuin, such as SIRT1, may be
determined as described, e.g., in Howitz et al., supra or as follows. For
instance,
sirtuin proteins may be contacted with a compound in vitro, e.g., in a
solution or in a
cell. In one embodiment, a sirtuin protein is contacted with a compound in a
solution and an activity of the sirtuin, e.g., its ability to deacetylate a
protein, such as
a histone, p53, or portions thereof, is determined. Generally, a sirtuin is
activated or
inhibited by a compound when at least one of its biological activities, e.g.,
deacetylation activity, is higher or lower, respectively, in the presence of
the
compound than in its absence. Activation or inhibition may be by a factor of
at least
about 10%, 30%, 50%, 100% (i.e., a factor of two), 3, 10, 30, or 100.
Whether a sirtuin is activated or inhibited can be determined, e.g., by
contacting the sirtuin or a cell or cell extract containing the sirtuin with a
deacetylation target, such as a histone, p53 protein, or portions thereof, and
determining the level of acetylation of the deacetylation target. A higher
level of
acetylation of the target incubated with the sirtuin that is being tested
relative to the
level of acetylation of a control sirtuin indicates that the sirtuin that is
being tested is
activated. Conversely, a lower level of acetylation of the target incubated
with the
sirtuin that is being tested relative to the level of acetylation of a control
sirtuin
indicates that the sirtuin that is being tested is inhibited. The control
sirtuin may be
a recombinantly produced sirtuin that has not been contacted with a sirtuin-
activating or -inhibiting compound.
Assays for determining the likelihood that a subject has or will develop
weight gain, obesity, insulin resistance, diabetes or precursor symptoms or
conditions resulting therefrom, are also provided. Such assays may comprise
determining the level activity or expression (e.g., mRNA, pre-mRNA or protein)
of
a sirtuin, such as SIRTI, or AMPK in a subject. A low level of sirtuin
activity or
expression in a subject is likely to indicate that the subject has or
is.likely to develop
weight gain, obesity, insulin resistance, diabetes, precursor symptoms thereof
or
secondary conditions thereof. Alternatively, a higher level of sirtuin
activity or
expression in a subject is likely to indicate that the subject has or is
likely to develop
215
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
weight loss and be protected from developing high weight associated diseases,
such
as insulin resistance and diabetes. Other assays include determining the
activity or
level of expression of a sirtuin and AMPK.
Also provided herein are methods for identifying compounds that modulate
weight gain and/or treat or prevent insulin resistance (or sensitivity) or
diabetes. A
method may comprise identifying an agent that modulates the activity or
protein
level of a sirtuin and testing whether the test agent modulates weight gain
and/or can
be used for treating or preventing insulin resistance or diabetes. The first
step of the
method may comprise contacting a sirtuin with a test agent and determining the
effect of the test agent on the activity of the sirtuin, e.g., SIRT1, as
described, e.g., in
Howitz et al., supra. The first step of the method may also comprise
contacting a
cell comprising a sirtuin with a test agent and determining the effect of the
test agent
on the activity of or expression level of the sirtuin. Expression levels of a
sirtuin
may be determined by measuring the mRNA, pre-mRNA or protein level of the
sirtuin. Other steps may comprise testing the agent in an animal model for
obesity,
insulin resistance and/or diabetes. Such animal models are well known in the
art.
Screening methods may further comprise a step to determine the toxicity or
adverse
effects of the agents.
Other screening assays comprise identifying agents that modulate AMPK
activity or protein levels. There is a need for compounds that activate AMPK
but do
not have the toxicities or adverse effects of known AMPK activators, such as
metformin/phenformin.
In other embodiments, the invention provides methods for determining
and/or monitoring a subject's intake of a sirtuin modulating compound. Such
methods may be useful for monitoring progress during therapeutic
administration of
a sirtuin modulator. Such assays may also be used to identify individuals that
have
been dosed with a sirtuin modulator. For example, such assays may be used to
identify individuals, such as student athletes, professional athletes, Olympic
athletes,
etc., who have taken sirtuin modulators to enhance their athletic performance
and/or
endurance. Such methods may involve measuring the amount of a sirtuin
modulator, or metabolite thereof, in the blood and/urine of an individual.
Exemplary
metabolites of resveratrol include, for example, resveratrol glucuronides and
216
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
resveratrol sulfates, such as resveratrol monoglucuronide, dihydroresveratrol
monosulfate, resveratrol monosulfate, dihydroresveratrol, trans-resveratrol-3-
O-
glucuronide, cis-resveratrol-3-O-glucuronide, cis-resveratrol-3-O-glucoside,
trans-
resveratrol-4' -sulfate, trans-resveratrol-3,5-disulfate, trans-resveratrol-
3,4'-disulfate,
trans-resveratrol-3,4',5-trisulfate, and trans-resveratrol-3-O-beta-D-
glucuronide, as
well as resveratrol aglycone and free trans-resveratrol. The methods may
involve
obtaining a biological sainple, such as urine, blood, saliva, tissue, feces,
hair, skin,
etc., from an individual and analyzing the sample to' identify the presence or
a sirtuin
modulating compound or metabolite thereof, the amount of a sirtuin modulating
compound or inetabolite thereof, and/or the type of sirtuin modulating
compound or
metabolite thereof. Identification, quantitation and characterization of
sirtuin
acmodulating compounds or metabolites thereof from a biological sample may be
achieved by a variety of methods known to one of skill in the art, such as,
for
example, an immunoassay, chromatography, mass spectroscopy (MS), including
liquid chromatography (LC)- -MS/MS, LC-ESI-MS/MS (electrospray ionization,
ESI), high performance liquid chromatography-diode array detection (HPLC-DAD),
or on-line ultraviolet-photodiode array detection and mass spectrometric
detection
(LC-DAD-MS and LC-UV-MS-MS) (see e.g., Wang et al., J. Chromatrogr B analyt
Technol Biomed Life Sci 829: 97-106 (2005); Urpi-sarda et al., Anal Chem 77:
3149-55 (2005); Wenzel et al., Mol Nutr Food Res 49: 472-81 (2005); Wenzel et
al.,
Mol Nutr Food Res 49: 482-94 (2005); Wang et al., J Pharm Sci 93: 2448-57
(2004); Meng et al., J Agric Food Chem 52: 935-42 (2004); and Yu et al., Pharm
Res 19: 1907-14 (2002)). In certain embodiments, the methods may involve
extracting, purifying or partially purifying the sirtuin modulators or
metabolites
thereof from the biological sample before analysis. In other embodiments, it
may be
desirable to compare the results to one or more known standards of sirtuin
modulating compounds or metabolites thereof.
In yet other embodiments, provided are methods (e.g., assays such as
screening assays or high throughput screens) for identifying agents, such as
sirtuin
modulating compounds, that are useful for modulating mitochondrial mass and/or
mitochondrial function in cells of an animal or human subject. In certain
embodiments, candidate agents are screened for their ability to increase
217
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
mitochondrial mass and/or improve mitochondrial function. In an exemplary
embodiment, the inethods described herein may be used to identify an agent
that
increases mitochondrial mass and/or improves mitochondrial function in cells;
such
as, for example, a sirtuin-activating compound.
In one embodiment, a method for identifying an agent that modulates
mitochondrial mass and/or function comprises contacting a candidate agent with
a
sample comprising a cell containing a mitochondrion, and determining a level
of at
least one indicator of mitochondrial function, wherein the candidate agent
that alters
the level of the indicator of mitochondrial function relative to the level of
said
indicator in the absence of the agent is indicative of an agent that alters
mitochondrial function.
In another embodiment, a method for identifying an agent that modulates
mitochondrial mass and/or function comprises identifying a regulator of
mitochondrial biogenesis. The method may comprise contacting a stimulus with a
cell comprising a mitochondrion under conditions and for a time sufficient to
induce
mitochondrial biogenesis; and detecting an altered level of a candidate
signaling
molecule, wherein an altered level of the candidate signaling molecule in a
cell that
has been contacted with the stimulus that induces mitochondrial biogenesis
relative
to the level of the candidate signaling molecule in a cell that has not been
contacted
with the stimulus indicates that the candidate signaling molecule is a
regulator of
mitochondrial biogenesis. In a further embodiment the stimulus is selected
cold
stress, an electrical stimulus or an adrenergic stimulus. In certain other
embodiments
mitochondrial biogenesis is detected by determining an indicator of
mitochondrial
function that is oxygen consumption, amount of mitochondrial DNA,
mitochondrial
mass or an ATP biosynthesis factor. In certain other embodiments the candidate
signaling molecule regulates activity of a gene that is a PGC gene or a NRF
gene. In
certain other embodiments the candidate signaling molecule is regulated by a
gene
that is a PGC gene or a NRF gene. In certain other embodiments the altered
level of
the candidate signaling molecule is a level of a nucleic acid, a level of a
polypeptide
and a level of phosphorylation of a protein.
In certain embodiments, the indicator of mitochondrial function may be a
mitochondrial electron transport chain enzyme. The methods may involve
measuring
218
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
electron transport chain enzyme catalytic activity, determining enzyme
activity per
initochondrion in the sample, determining enzyme activity per unit of protein
in the
sample, measuring electron transport chain enzyme quantity, determining enzyme
quantity per mitochondrion in the sample, and/or determining enzyme quantity
per
unit of protein in the sample. In certain embodiments the mitochondrial
electron
transport chain enzyme comprises at least one subunit of mitochondrial complex
1,
mitochondrial complex II, mitochondrial complex III, mitochondrial complex IV,
and/or mitochondrial complex V. The mitochondrial complex IV subunit may be
COX1, COX2 or COX4 and the mitochondrial complex V subunit may be an ATP
synthase subunit 8 or ATP synthase subunit 6.
In other embodiments, the indicator of mitochondrial function may be a
mitochondrial matrix component. a mitochondrial membrane component, and/or a
mitochondrial inner membrane component. The mitochondrial membrane
component may be an adenine nucleotide translocator (ANT), voltage dependent
anion channel (VDAC), malate-aspartate shuttle, calcium uniporter, UCP-1, UCP-
2,
UCP-3 (e.g., Boss et al., 2000 Diabetes 49:143; Klingenberg 1999 J.
Bioenergetics
Biomembranes 31:419), a hexokinase, a peripheral benzodiazepine receptor, a
mitochondrial intermembrane creatine kinase, cyclophilin D, a Bcl-2 gene
family
encoded polypeptide, tricarboxylate carrier or dicarboxylate carrier.
In certain embodiments the indicator of mitochondrial function is a Krebs
cycle enzyme. The methods may involve measuring Krebs cycle enzyme catalytic
activity, determining enzyme activity per mitochondrion in the sample,
determining
enzyme activity per unit of protein in the sample, measuring Krebs cycle
enzyme
quantity, determining enzyme quantity per mitochondrion in the sample, and/or
determining enzyme quantity per unit of protein in the sample. The Krebs cycle
enzyme may be citrate synthase, aconitase, isocitrate dehydrogenase, alpha-
ketoglutarate dehydrogenase, succinyl-coenzyme A synthetase, succinate
dehydrogenase, fumarase or malate dehydrogenase.
In other embodiments, the indicator of mitochondrial function may be
mitochondrial mass per cell in the sample. Mitochondrial mass may be
determined
using a mitochondria selective agent (such as nonylacridine orange) or by
morphometric analysis. In certain embodiments, the indicator of mitochondrial
219
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
function may be the number of mitochondria per cell in the sample which may be
determined using a mitochondrion selective reagent, such as a fluorescent
reagent.
In other embodiments, the indicator of mitochondrial function may be the
amount of mitochondrial DNA ("mtDNA") per cell in the sample. The amount of
mitochondrial DNA per cell may be measured and/or expressed in absolute (e.g.,
mass of mtDNA per cell) or relative (e.g., proportion of mtDNA relative to
nuclear
DNA) terms. In certain embodiments, mitochondrial DNA is measured by
contacting a biological sample containing tnitochondrial DNA with an
oligonucleotide primer having a nucleotide sequence that is complementary to a
sequence present in the mitochondrial DNA, under conditions and for a time
sufficient to allow hybridization of the primer to the mitochondrial DNA; and
detecting hybridization of the primer to the mitochondrial DNA, and therefrom
quantifying the mitochondrial DNA. In certain embodiments the step of
detecting
comprises a technique that may be polymerase chain reaction, oligonucleotide
primer extension assay, ligase chain reaction, or restriction fragment length
polymorphism analysis. In certain embodiments, mitochondrial DNA is measured
by contacting a sample containing amplified mitochondrial DNA with an
oligonucleotide primer having a nucleotide sequence that is complementary to a
sequence present in the amplified mitochondrial DNA, under conditions and for
a
time sufficient to allow hybridization of the primer to the mitochondrial DNA;
and
detecting hybridization of the primer to the mitochondrial DNA, and therefrom
quantifying the mitochondrial DNA. In certain embodiments the step of
detecting
comprises a technique that may be polymerase chain reaction, oligonucleotide
primer extension assay, ligase chain reaction, or restriction fragment length
polymorphism analysis. In certain embodiments the mitochondrial DNA is
amplified
using a technique that may be polymerase chain reaction, transcriptional
amplification systems or self-sustained sequence replication. In certain
embodiments, mitochondrial DNA is measured by contacting a biological sample
containing mitochondrial DNA with an oligonucleotide primer having a
nucleotide
sequence that is complementary to a sequence present in the mitochondrial DNA,
under conditions and for a time sufficient to allow hybridization of the
primer to the
mitochondrial DNA; and detecting hybridization and extension of the primer to
the
220
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
mitochondrial DNA to produce a product, and therefrom quantifying the
mitochondrial DNA. In certain embodiinents the step of comparing comprises
measuring mitochondrial DNA by contacting a sample containing amplified
mitochondrial DNA with an oligonucleotide primer having a nucleotide sequence
that is coinplementary to a sequence present in the amplified mitochondrial
DNA,
under conditions and for a time sufficient to allow hybridization of the
primer to the
mitochondrial DNA; and detecting hybridization and extension of the primer to
the
mitochondrial DNA to produce a product, and therefrom quantifying the
mitochondrial DNA. In certain embodiments the mitochondrial DNA is amplified
using a technique that may be the polymerase chain reaction (PCR), including
quantitaive and competitive PCR (Ahmed et al., BioTechniques 26:290-300,
1999),
transcriptional amplification systems or self-sustained sequence replication.
In
certain embodiments, the amount of mitochondrial DNA in the sample is
determined
using an oligonucleotide primer extension assay. In other embodiments, the
amount
of mitochondrial DNA is determined by subjecting a sample to a cesium chloride
gradient to separate it from nuclear DNA (see, e.g., Welter et al., Mol. Biol.
Rep.
13:17-120, 1988) in the presence of a detectably labeled compound that binds
to
double-stranded nucleic acids (e.g., ethidium bromide) and comparing the
relative
and/or absolute signals corresponding to the mitochondrial and nuclear DNAs.
In other embodiments, the indicator of mitochondrial function is the amount
of ATP per cell in the sample. The methods may comprise measuring the amount
of
ATP per mitochondrion in the sample, measuring the amount of ATP per unit
protein in the sample, measuring the amount of ATP per unit mitochondrial mass
in
the sample, measuring the amount of ATP per unit mitochondial protein in the
sample. In certain embodiments, the indicator of mitochondrial function is the
rate
of ATP synthesis in the sample or an ATP biosynthesis factor. The methods may
comprise measuring ATP biosynthesis factor catalytic activity, determining ATP
biosynthesis factor activity per mitochondrion in the sample, determining ATP
biosynthesis factor activity per unit mitochondrial mass in the sample,
determining
ATP biosynthesis factor activity per unit of protein in the sample, measuring
ATP
biosynthesis factor quantity, determining ATP biosynthesis factor quantity per
221
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
mitochondrion in the sample, and/or determining ATP biosynthesis factor
quantity
per unit of protein in the sample.
In other embodiments, the indicator of mitochondrial function may be one or
more of the following: free radical production, reactive oxygen species,
protein
nitrosylation, protein carbonyl modification, DNA oxidation, mtDNA oxidation,
protein oxidation, protein carbonyl modification, malondialdehyde adducts of
proteins, a glycoxidation product, a lipoxidation product, 8'-OH-guanosine
adducts,
BARS, cellular response to elevated intracellular calcium, and/or cellular
response
to at least one apoptogen. In certain embodiments the indicator of
mitochondrial
function is oxygen consumption, which may be determined according to any of a
variety of known methodologies (e.g., Wu et al., 1999 Cell 98:115; Li et al.
1999 J.
Biol. Chem. 274:17534).
Functional mitochondria contain gene products encoded by mitochondrial
genes situated in mitochondrial DNA (mtDNA) and by extramitochondrial genes
(e.g., nuclear genes) not situated in the circular mitochondrial genome. The
16.5 kb
mtDNA encodes 22 tRNAs, two ribosomal RNAs (rRNA) and 13 enzymes of the
electron transport chain (ETC), the elaborate multi-complex mitochondrial
assembly
where, for example, respiratory oxidative phosphorylation takes place. The
overwhelming majority of mitochondrial structural and functional proteins are
encoded by extramitochondrial, and in most cases presumably nuclear, genes.
Accordingly, mitochondrial and extramitochondrial genes may interact directly,
or
indirectly via gene products and their downstream intermediates, including
metabolites, catabolites, substrates, precursors, cofactors and the like.
Alterations in
mitochondrial function, for example impaired electron transport activity,
defective
oxidative phosphorylation or increased free radical production, may therefore
arise
as the result of defective mtDNA, defective extramitochondrial DNA, defective
mitochondrial or extramitochondrial gene products, defective downstream
intermediates or a combination of these and other factors.
In certain embodiments, an enzyme is the indicator of mitochondrial function
as provided herein. The enzyme may be a mitochondrial enzyme, which may
further
be an ETC enzyme or a Krebs cycle enzyme. The enzyme may also be an ATP
biosynthesis factor, which may include an ETC enzyme and/or a Krebs cycle
222
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
enzyme, or other enzymes or cellular components related to ATP production as
provided herein. A "non-enzyme" refers to an indicator of mitochondrial
function
that is not an enzyme (i.e., that is not a mitochondrial enzyme or an ATP
biosynthesis factor as provided herein). In certain other embodiments, an
enzyme is
a co-indicator of mitochondrial function. The following enzymes may not be
indicators of mitochondrial function according to the present invention, but
may be
co-indicators of mitochondrial function as provided herein: citrate synthase
(EC
4.1.3.7), hexokinase II (EC 2.7.1.1), cytochrome c oxidase (EC 1.9.3.1),
phosphofructokinase (EC 2.7.1.11), glyceraldehyde phosphate dehydrogenase (EC
1.2.1.12), glycogen phosphorylase (EC 2.4.1.1) creatine kinase (EC 2.7.3.2),
NADH
dehydrogenase (EC 1.6.5.3), glycerol 3-phosphate dehydrogenase (EC 1.1.1.8),
triose phosphate dehydrogenase (EC 1.2.1.12) and malate dehydrogenase (EC
1.1.1.37).
In other embodiments, the indicator of mitochondrial function is any ATP
biosynthesis factor, ATP production, mitochondrial mass or mitochondrial
number,
free radical production, a cellular response to elevated intracellular calcium
and/or a
cellular response to an apoptogen. In certain embodiments, mitochondrial DNA
content may not be an indicator of mitochondrial finction but may be a co-
predictor
of initochondrial function or a co-indicator of mitochondrial function, as
provided
herein.
i. Indicators of mitoclzondrial function that are enzymes
In certain embodiments, methods for identifying agents that modulate
mitochondrial mass and/or function include the detection and/or absolute or
relative
measurement of at least one indicator of mitochondrial function in biological
test
samples, wherein the indicator of mitochondrial function is an enzyme. As
provided
herein, such an enzyme may be a mitochondrial enzyme or an ATP biosynthesis
factor that is an enzyme, for example an ETC enzyme or a Krebs cycle enzyme.
Reference to "enzyme quantity", "enzyme catalytic activity" or "enzyme
expression level" in the context of the methods for identifying agents that
modulate
mitochondrial mass and/or function, is meant to include a reference to any of
a
mitochondrial enzyme quantity, activity or expression level or an ATP
biosynthesis
factor quantity, activity or expression level; either of which may further
include, for
223
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
example, an ETC enzyme quantity, activity or expression level or a Krebs cycle
enzyme quantity, activity or expression level. In the most preferred
embodiments of
the invention, an enzyme is a natural or recombinant protein or polypeptide
that has
enzyme catalytic activity as provided herein. Such an enzyme may be, by way of
non-limiting examples, an enzyme, a holoenzyme, an enzyme complex, an enzyme
subunit, an enzyme fragment, derivative or analog or the like, including a
truncated,
processed or cleaved enzyme.
A mitochondrial enzyme that may be an indicator of mitochondrial function
as provided herein refers to a initochondrial molecular component that has
enzyme
catalytic activity and/or functions as an enzyme cofactor capable of
influencing
enzyme catalytic activity. As used herein, mitochondria are comprised of
"mitochondrial molecular components", which may be a protein, polypeptide,
peptide, amino acid, or derivative thereof; a lipid, fatty acid or the like,
or derivative
thereof; a carbohydrate, saccharide or the like or derivative thereof, a
nucleic acid,
nucleotide, nucleoside, purine, pyrimidine or related molecule, or derivative
thereof,
or the like; or any covalently or non-covalently complexed combination of
these
components, or any other biological molecule that is a stable or transient
constituent
of a mitochondrion.
A mitochondrial enzyme that may be an indicator of mitochondrial function
or a co-indicator of mitochondrial function as provided herein, or an ATP
biosynthesis factor that may be an indicator of mitochondrial function as
provided
herein, may comprise an ETC enzyme, which refers to any mitochondrial
molecular
component that is a mitochondrial enzyme component of the mitochondrial
electron
transport chain (ETC) complex associated with the inner mitochondrial membrane
and mitochondrial matrix. An ETC enzyme may include any of the multiple ETC
subunit polypeptides encoded by mitochondrial and nuclear genes. The ETC is
typically described as comprising complex I (NADH:ubiquinone reductase),
complex II (succinate dehydrogenase), complex III (ubiquinone: cytochrome c
oxidoreductase), complex IV (cytochrome c oxidase) and complex V
(mitochondrial
ATP synthetase), where each complex includes multiple polypeptides and
cofactors
(for review see, e.g., Walker et al., 1995 Meths. Enzymol. 260:14; Emster et
al.,
1981 J. Cell Biol. 91:227s-255s, and references cited therein).
224
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
A mitochondrial enzyme that may be an indicator of mitochondrial function
as provided herein, or an ATP biosynthesis factor that may be an indicator of
mitochondrial function as provided herein, may also comprise a Krebs cycle
enzyme, wllich includes mitochondrial molecular components that mediate the
series
of biochemical/bioenergetic reactions also known as the citric acid cycle or
the
tricarboxylic acid cycle (see, e.g., Lehninger, Biochemistry, 1975 Worth
Publishers,
New York; Voet and Voet, Biochemistry, 1990 John Wiley & Sons, New York;
Mathews and van Holde, Biochemistry, 1990 Benjamin Cummings, Menlo Park,
Calif.). Krebs cycle enzymes include subunits and cofactors of citrate
synthase,
aconitase, isocitrate dehydrogenase, the a-ketoglutarate dehydrogenase
complex,
succinyl CoA synthetase, succinate dehydrogenase, fumarase and malate
dehydrogenase. Krebs cycle enzymes further include enzymes and cofactors that
are
functionally linked to the reactions of the Krebs cycle, such as, for example,
nicotinamide adenine dinucleotide, coenzyme A, thiamine pyrophosphate,
lipoamide, guanosine diphosphate, flavin adenine dinucloetide and nucleoside
diphosphokinase.
The methods described herein also pertain in part to the correlation of type 2
diabetes with an indicator of mitochondrial function that may be an ATP
biosynthesis factor, an altered amount of ATP or an altered amount of ATP
production. For example, decreased mitochondrial ATP biosynthesis may be an
indicator of mitochondrial finction from which a risk for type 2 diabetes rnay
be
identified.
An "ATP biosynthesis factor" refers to any naturally occurring cellular
component that contributes to the efficiency of ATP production in
mitochondria.
Such a cellular component may be a protein, polypeptide, peptide, amino acid,
or
derivative thereof, a lipid, fatty acid or the like, or derivative thereof; a
carbohydrate,
saccharide or the like or derivative thereof, a nucleic acid, nucleotide,
nucleoside,
purine, pyrimidine or related molecule, or derivative thereof, or the like. An
ATP
biosynthesis factor includes at least the components of the ETC and of the
Krebs
cycle (see, e.g., Lehninger, Biochemistry, 1975 Worth Publishers, New York;
Voet
and Voet, Biochemistry, 1990 John Wiley & Sons, New York; Mathews and van
Holde, Biochemistry, 1990 Benjamin Cummings, Menlo Park, Calif.) and any
225
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
protein, enzyme or other cellular component that participates in ATP
synthesis,
regardless of whether such ATP biosynthesis factor is the product of a nuclear
gene
or of an extranuclear gene (e.g., a mitochondrial gene). Participation in ATP
synthesis may include, but need not be limited to, catalysis of any reaction
related to
ATP synthesis, transmembrane import and/or export of ATP or of an enzyme
cofactor, transcription of a gene encoding a mitochondrial enzyme and/or
translation
of such a gene transcript.
Compositions and methods for determining whether a cellular component is
an ATP biosynthesis factor are well known in the art, and include methods for
determining ATP production (including' determination of the rate of ATP
production
in a sample) and methods for quantifying ATP itself. The contribution of an
ATP
biosynthesis factor to ATP production can be determined, for example, using an
isolated ATP biosynthesis factor that is added to cells or to a cell-free
system. The
ATP biosynthesis factor may directly or indirectly mediate a step or steps in
a
biosynthetic pathway that influences ATP production. For example, an ATP
biosynthesis factor may be an enzyme that catalyzes a particular chemical
reaction
leading to ATP production. As another example, an ATP biosynthesis factor may
be
a cofactor that enhances the efficiency of such an enzyme. As another example,
an
ATP biosynthesis factor may be an exogenous genetic element introduced into a
cell
or a cell-free-system that directly or indirectly affects an ATP biosynthetic
pathway.
Those having ordinary skill in the art are readily able to compare ATP
production by
an ATP biosynthetic pathway in the presence and absence of a candidate ATP
biosynthesis factor. Routine determination of ATP production may be
accomplished
using any known method for quantitative ATP detection, for example by way of
illustration and not limitation, by differential extraction from a sample
optionally
including chromatographic isolation; by spectrophotometry; by quantification
of
labeled ATP recovered from a sample contacted with a suitable form of a
detectably
labeled ATP precursor molecule such as, for example, 32P; by quantification of
an
enzyme activity associated with ATP synthesis or degradation; or by other
techniques that are known in the art. Accordingly, in certain embodiments of
the
present invention, the amount of ATP in a biological sample or the production
of
ATP (including the rate of ATP production) in a biological sample may be an
226
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
indicator of mitochondrial function. In one embodiment, for instance, ATP may
be
quantified by measuring luminescence of luciferase catalyzed oxidation of D-
luciferin, an ATP dependent process.
"Enzyme catalytic activity" refers to any function performed by a particular
enzyme or category of enzymes that is directed to one or more particular
cellular
function(s). For example, "ATP biosynthesis factor catalytic activity" refers
to any
function performed by an ATP biosynthesis factor as provided herein that
contributes to the production of ATP. Typically, enzyme catalytic activity is
manifested as facilitation of a chemical reaction by a particular enzyme, for
instance
an enzyme that is an ATP biosynthesis factor, wherein at least one enzyme
substrate
or reactant is covalently modified to form a product. For example, enzyme
catalytic
activity may result in a substrate or reactant being modified by formation or
cleavage of a covalent chem-ical bond, but the invention need not be so
limited.
Various methods of measuring enzyme catalytic activity are known to those
having
ordinary skill in the art and depend on the particular activity to be
determined.
For many enzymes, including mitochondrial enzymes or enzymes that are
ATP biosynthesis factors as provided herein, quantitative criteria for enzyme
catalytic activity are well established. These criteria include, for example,
activity
that may be defined by international units (IU), by enzyme turnover number, by
catalytic rate constant (K,,at), by Michaelis-Menten constant (Km), by
specific
activity or by any other enzymological method known in the art for measuring a
level of at least one enzyme catalytic activity. Specific activity of a
mitochondrial
enzyme, such as an ATP biosynthesis factor, may be expressed as units of
substrate
detectably converted to product per unit time and, optionally, further per
unit sample
mass (e.g., per unit protein or per unit mitochondrial mass).
In certain embodiments, enzyme catalytic activity may be expressed as units
of substrate detectably converted by an enzyme to a product per unit time per
unit
total protein in a sample, as units of substrate detectably converted by an
enzyme to
product per unit time per unit mitochondrial mass in a sample, or as units of
substrate detectably converted by an enzyme to product per unit time per unit
mitochondrial protein mass in a sample. Products of enzyme catalytic activity
may
be detected by suitable methods that will depend on the quantity and
227
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
physicochemical properties of the particular product. Thus, detection may be,
for
example by way of illustration and not limitation, by radiometric,
colorimetric,
spectrophotometric, fluorimetric, immunometric or mass spectrometric
procedures,
or by other suitable means that will be readily apparent to a person having
ordinary
skill in the art.
In certain embodiments, detection of a product of enzyme catalytic activity
may be accomplished directly, and in certain other embodiments detection of a
product may be accomplished by introduction of a detectable reporter moiety or
label into a substrate or reactant such as a marker enzyme, dye, radionuclide,
luminescent group, fluorescent group or biotin, or the like. The amount of
such a
label that is present as unreacted substrate and/or as reaction product,
following a
reaction to assay enzyme catalytic activity, is then determined using a method
appropriate for the specific detectable reporter moiety or label. For
radioactive
groups, radionuclide decay monitoring, scintillation counting, scintillation
proximity
assays (SPA) or autoradiographic methods are generally appropriate. For
immunometric measurements, suitably labeled antibodies may be prepared
including, for example, those labeled with radionuclides, with fluorophores,
with
affinity tags, with biotin or biotin mimetic sequences or those prepared as
antibody-
enzyme conjugates (see, e.g., Weir, D. M., Handbook of Experimental
Immunology,
1986, Blackwell Scientific, Boston; Scouten, W. H., Methods in Enzymology
135:30-65, 1987; Harlow and Lane, Antibodies: A Laboratory Manual, Cold Spring
Harbor Laboratory, 1988; Haugland, 1996 Handbook of Fluorescent Probes and
Researcli Chemicals--Sixth Ed., Molecular Probes, Eugene, Oreg.; Scopes, R.
K.,
Protein Purification: Principles and Practice, 1987, Springer-Verlag, New
York;
Hermanson, G. T. et al., Immobilized Affinity Ligand Techniques, 1992,
Academic
Press, Inc., New York; Luo et al., 1998 J. Biotechnol. 65:225 and references
cited
therein). Spectroscopic methods may be used to detect dyes (including, for
example,
colorimetric products of enzyme reactions), luminescent groups and fluorescent
groups. Biotin may be detected using avidin or streptavidin, coupled to a
different
reporter group (commonly a radioactive or fluorescent group or an enzyme).
Enzyme reporter groups may generally be detected by the addition of substrate
(generally for a specific period of time), followed by spectroscopic,
228
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
spectrophotometric or other analysis of the reaction products. Standards and
standard additions may be used to determine the level of enzyme catalytic
activity in
a sample, using well known techniques.
As noted above, enzyme catalytic activity of an ATP biosynthesis factor may
further include other functional activities that lead to ATP production,
beyond those
involving covalent alteration of a substrate or reactant. For example by way
of
illustration and not limitation, an ATP biosynthesis factor that is an enzyme
may
refer to a transmembrane transporter molecule that, through its enzyme
catalytic
activity, facilitates the movement of metabolites between cellular
compartments.
Such metabolites may be ATP or other cellular components involved in ATP
synthesis, such as gene products and their downstream intermediates, including
metabolites, catabolites, substrates, precursors, cofactors and the like. As
another
non-limiting example, an ATP biosynthesis factor that is an enzyme may,
through its
enzyme catalytic activity, transiently bind to a cellular component involved
in ATP
synthesis in a manner that promotes ATP synthesis. Such a binding event may,
for
instance, deliver the cellular component to another enzyme involved in ATP
synthesis and/or may alter the conformation of the cellular component in a
manner
that promotes ATP synthesis. Further to this example, such conformational
alteration may be part of a signal transduction pathway, an allosteric
activation
pathway, a transcriptional activation pathway or the like, where an
interaction
between cellular components leads to ATP production.
Thus, an ATP biosynthesis factor may include, for example, a mitochondrial
membrane protein. Suitable mitochondrial membrane proteins include such
mitochondrial components as the adenine nucleotide transporter (ANT; e.g.,
Fiore et
al., 1998 Biochimie 80:137; Klingenberg 1985 Ann. New York Acad. Sci.
456:279),
the voltage dependent anion channel (VDAC, also referred to as porin; e.g.,
Manella,
1997 J. Bioenergetics Biomembr. 29:525), the malate-aspartate shuttle, the
mitochondrial calcium uniporter (e.g., Litsky et al., 1997 Biochem. 36:7071),
uncoupling proteins (UCP-1, -2, -3; see e.g., Jezek et al., 1998 Int. J.
Biochem. Cell
Biol. 30:1163), a hexokinase, a peripheral benzodiazepine receptor, a
mitochondrial
intermembrane creatine kinase, cyclophilin D, a Bcl-2 gene family encoded
polypeptide, the tricarboxylate carrier (e.g., Iocobazzi et al., 1996 Biochim.
Biophys.
229
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Acta 1284:9; Bisaccia et al., 1990 Biochim. Biophys. Acta 1019:250) and the
dicarboxylate carrier (e.g., Fiermonte et al., 1998 J. Biol. Chem. 273:24754;
Indiveri
et al., 1993 Biochim. Biophys. Acta 1143:310; for a general review of
mitochondrial
membrane transporters, see, e.g., Zonatti et al., 1994 J. Bioenergetics
Biomembr.
26:543 and references cited therein).
Enzyme quantity as used herein with reference to the methods for identifying
modulators of mitochondrial mass and/or function refers to an amount of an
enzyme
including mitochondrial enzymes or enzymes that are ATP biosynthesis factors
as
provided herein, or of another ATP biosynthesis factor, that is present, i.e.,
the
physical presence of an enzyme or ATP biosynthesis factor selected as an
indicator
of mitochondrial function, irrespective of enzyme catalytic activity.
Depending on
the physicochemical properties of a particular enzyme or ATP biosynthesis
factor,
the preferred method for determining the enzyme quantity will vary. In the
most
highly preferred embodiments of the invention, determination of enzyme
quantity
will involve quantitative determination of the level of a protein or
polypeptide using
routine methods in protein chemistry with which those having skill in the art
will be
readily familiar, for example by way of illustration and not limitation, those
described in greater detail below.
Accordingly, determination of enzyme quantity may be by any suitable
method known in the art for quantifying a particular cellular component that
is an
enzyme or an ATP biosynthesis factor as provided herein, and that in preferred
embodiments is a protein or polypeptide. Depending on the nature and
physicochemical properties of the enzyme or ATP biosynthesis factor,
determination
of enzyme quantity may be by densitometric, mass spectrometric,
spectrophotometric, fluorimetric, immunometric, chromatographic,
electrochemical
or any other means of quantitatively detecting a particular cellular
component.
Methods for determining enzyme quantity also include methods described above
that are useful for detecting products of enzyme catalytic activity, including
those
measuring enzyme quantity directly and those measuring a detectable label or
reporter moiety. In certain preferred embodiments of the invention, enzyme
quantity
is determined by immunometric measurement of an isolated enzyme or ATP
biosynthesis factor. In certain preferred embodiments of the invention, these
and
230
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
other immunological and immunochemical techniques for quantitative
determination
of biomolecules such as an enzyme or ATP biosynthesis factor may be employed
using a variety of assay formats known to those of ordinary skill in the art,
including
but not limited to enzyme linked immunosorbent assay (ELISA), radioimmunoassay
(RIA), immunofluorimetry, immunoprecipitation, equilibrium dialysis,
immunodiffusion and other techniques. (See, e.g., Harlow and Lane, Antibodies:
A
Laboratory Manual, Cold Spring Harbor Laboratory, 1988; Weir, D. M., Handbook
of Experimental Immunology, 1986, Blackwell Scientific, Boston.) For example,
the
assay may be performed in a Western blot format, wherein a preparation
comprising
proteins from a biological sample is submitted to gel electrophoresis,
transferred to a
suitable membrane and allowed to react with an antibody specific for an enzyme
or
an ATP biosynthesis factor that is a protein or polypeptide. The presence of
the
antibody on the membrane inay then be detected using a suitable detection
reagent,
as is well known in the art and described above.
In certain embodiments, an indicator (or co-indicator) of mitochondrial
function including, for example, an enzyme as provided herein, may be present
in an
isolated form, e.g., removed from its original environment (e.g., the natural
environment if it is naturally occurring). For example, a naturally occurring
polypeptide present in a living animal is not isolated, but the same
polypeptide,
separated from some or all of the co-existing materials in the natural system,
is
isolated. Such polypeptides could be part of a composition, and still be
isolated in
that such composition is not part of its natural environment.
Affinity techniques are useful in the context of isolating an enzyme or an
ATP biosynthesis factor protein or polypeptide for use according to the
methods of
the present invention, and may include any method that exploits a specific
binding
interaction involving an enzyme or an ATP biosynthesis factor to effect a
separation.
For example, because an enzyme or an ATP biosynthesis factor protein or
polypeptide may contain covalently attached oligosaccharide moieties, an
affinity
technique such as binding of the enzyme (or ATP biosynthesis factor) to a
suitable
immobilized lectin under conditions that permit carbohydrate binding by the
lectin
may be a particularly useful affinity technique.
231
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Other useful affinity techniques include immunological techniques for
isolating and/or detecting a specific protein or polypeptide antigen (e.g., an
enzyme
or ATP biosynthesis factor), which techniques rely on specific binding
interaction
between antibody combining sites for antigen and antigenic determinants
present on
the factor. Binding of an antibody or other affinity reagent to an antigen is
"specific"
where the binding interaction involves a Ka of greater than or equal to about
104 M"1,
preferably of greater than or equal to about 105 M-1, more preferably of
greater than
or equal to about 106 M"1 and still more preferably of greater than or equal
to about
107 M"1. Affinities of binding partners or antibodies can be readily
determined using
conventional techniques, for example those described by Scatchard et al., Ann.
New
York Acad. Sci. 51:660 (1949).
Immunological techniques include, but need not be limited to,
immunoaffinity chromatography, immunoprecipitation, solid phase
immunoadsorption or other immunoaffinity methods. For these and other useful
affinity techniques, see, for example, Scopes, R. K., Protein Purification:
Principles
and Practice, 1987, Springer-Verlag, New York; Weir, D. M., Handbook of
Experimental Immunology, 1986, Blackwell Scientific, Boston; and Hermanson, G.
T. et al., Immobilized Affinity Ligand Techniques, 1992, Academic Press, Inc.,
California; which are hereby incorporated by reference in their entireties,
for details
regarding techniques for isolating and characterizing complexes, including
affinity
techniques.
As noted above, an indicator of mitochondrial function can be a protein or
polypeptide, for example an enzyme or an ATP biosynthesis factor. The protein
or
polypeptide may be an unmodified polypeptide or may be a polypeptide that has
been posttranslationally modified, for example by glycosylation,
phosphorylation,
fatty acylation including glycosylphosphatidylinositol anchor modification or
the
like, phospholipase cleavage such as phosphatidylinositol-specific
phospholipase c
mediated hydrolysis or the like, protease cleavage, dephosphorylation or any
other
type of protein posttranslational modification such as a modification
involving
formation or cleavage of a covalent chemical bond.
ii. Indicators of 'nzitochondrial function that are nzitochondrial mass,
niitoclzondrial volunze or mitochondrial itumber
232
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In certain embodiments, methods for identifying agents that modulate
mitochondrial mass and/or function include the detection and/or measurement of
at
least one indicator of mitochondrial function in biological test samples,
wherein the
indicator of mitochondrial function is absolute or relative mitochondrial
mass,
mitochondrial volume or mitochondrial number.
Methods for quantifying mitochondrial mass, volume and/or mitochondrial
number are known in the art, and may include, for example, quantitative
staining of
a representative biological sample. Typically, quantitative staining of
mitochondrial
may be performed using organelle-selective probes or dyes, including but not
limited to mitochondrion selective reagents such as fluorescent dyes that bind
to
mitochondrial molecular components (e.g., nonylacridine orange, MitoTrackers)
or
potentiometric dyes that accumulate in mitochondria as a function of
mitochondrial
inner membrane electrochemical potential (see, e.g., Haugland, 1996 Handbook
of
Fluorescent Probes and Research Chemicals, Sixth Ed., Molecular Probes,
Eugene,
Oreg.). As another example, mitochondrial mass, volume and/or number may be
quantified by morphometric analysis (e.g., Cruz-Orive et al., 1990 Am. J.
Physiol.
258:L148; Schwerzmann et al., 1986 J. Cell Biol. 102:97). These or any other
means
known in the art for quantifying mitochondrial mass, volume and/or
mitochondrial
number in a sample are within the contemplated scope of the invention. For
example, the use of such quantitative determinations for purposes of
calculating
mitochondrial density is contemplated and is not intended to be limiting. In
certain
embodiments, mitochondrial protein mass in a sample is determined using well
known procedures. For example, a person having ordinary skill in the art can
readily
prepare an isolated mitochondrial fraction from a biological sample using
established cell fractionation techniques, and therefrom determine protein
content
using any of a number of protein quantification methodologies well known in
the
art.
W. I-zdicators of niitochondrial function that include mitochondrial DNA
coutent
In other embodiments, methods for identifying modulators of mitochondrial
mass and/or function include the detection and/or measurement of at least one
indicator of mitochondrial function in biological test samples, wherein the
indicator
233
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
of mitochondrial function is the absolute or relative amount of mitochondrial
DNA.
Quantification of mitochondrial DNA (mtDNA) content may be accomplished by
any of a variety of established techniques that are useful for this purpose,
including
but not limited to oligonucleotide probe hybridization or polymerase chain
reaction
(PCR) using oligonucleotide primers specific for mitocllondrial DNA sequences
(see, e.g., Miller et al., 1996 J. Neurochem. 67:1897; Fahy et al., 1997 Nucl.
Ac.
Res. 25:3102; U.S. patent application Ser. No. 09/098,079; Lee et al., 1998
Diabetes
Res. Clin. Practice 42:161; Lee et al., 1997 Diabetes 46(suppl. 1):175A). A
particularly useful method is the primer extension assay disclosed by Fahy et
al.
(Nucl. Acids Res. 25:3102, 1997) and by Ghosh et al. (Am. J. Hum. Genet.
58:325,
1996). Suitable hybridization conditions may be found in the cited references
or may
be varied according to the particular nucleic acid target and oligonucleotide
probe
selected, using methodologies well known to those having ordinary skill in the
art
(see, e.g., Ausubel et al., Current Protocols in Molecular Biology, Greene
Publishing, 1987; Sambrook et al., Molecular Cloning: A Laboratory Manual,
Cold
Spring Harbor Press, 1989).
Examples of other useful techniques for determining the amount of specific
nucleic acid target sequences (e.g., mtDNA) present in a sample based on
specific
hybridization of a primer to the target sequence include specific
amplification of
target nucleic acid sequences and quantification of amplification products,
including
but not limited to polymerase chain reaction (PCR, Gibbs et al., Nucl. Ac.
Res.
17:2437, 1989), transcriptional amplification systems (e.g., Kwoh et al., 1989
Proc.
Nat. Acad. Sci. 86:1173); strand displacement amplification (e.g., Walker et
al.,
Nucl. Ac. Res. 20:1691, 1992; Walker et al., Proc. Nat. Acad. Sci. 89:392,
1992) and
self-sustained sequence replication (3SR, see, e.g., Ghosh et al, in Molecular
Methods for Virus Detection, 1995 Academic Press, New York, pp. 287-314;
Guatelli et al., Proc. Nat. Acad. Sci. 87:1874, 1990), the cited references
for which
are incorporated herein by reference in their entireties. Other useful
amplification
techniques include, for example, ligase chain reaction (e.g., Barany, Proc.
Nat.
Acad. Sci. 88:189, 1991), Q-beta replicase assay (Cahill et al., Clin. Chem.
37:1482,
1991; Lizardi et al., Biotechnol. 6:1197, 1988; Fox et al., J. Clin. Lab.
Analysis
3:378, 1989) and cycled probe technology (e.g., Cloney et al., Clin. Chem.
40:656,
234
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
1994), as well as other suitable methods that will be known to those familiar
with
the art.
Sequence length or molecular mass of primer extension assay products may
be determined using any known method for characterizing the size of nucleic
acid
sequences with which those skilled in the art are familiar. In one embodiment,
primer extension products are characterized by gel electrophoresis. In another
embodiment, primer extension products are characterized by mass spectrometry
(MS), which may further include matrix assisted laser desorption
ionization/time of
flight (MALDI-TOF) analysis or other MS techniques known to those skilled in
the
art. See, for example, U.S. Pat. Nos. 5,622,824, 5,605,798 and 5,547,835. In
another
embodiment, primer extension products are characterized by liquid or gas
chromatography, which may further include high performance liquid
chromatography (HPLC), gas chromatograpliy-mass spectrometry (GC-MS) or other
well known chromatographic methodologies.
iv. Indicators of mitochondrial futzction that are cellular responses to
elevated intracellular calcium
Certain aspects of the present invention, as it relates detecting and/or
measuring an indicator of mitochondrial function, involve monitoring
intracellular
calcium homeostasis and/or cellular responses to perturbations of this
homeostasis,
including physiological and pathophysiological calcium regulation. The range
of
cellular responses to elevated intracellular calcium is broad, as is the range
of
methods and reagents for the detection of such responses. Many specific
cellular
responses are known to those having ordinary skill in the art; these responses
will
depend on the particular cell types present in a selected biological sample.
As non-
limiting examples, cellular responses to elevated intracellular calcium
include
secretion of specific secretory products, exocytosis of particular . pre-
formed
components, increased glycogen metabolism and cell proliferation (see, e.g.,
Clapham, 1995 Cell 80:259; Cooper, The Cell--A Molecular Approach, 1997 ASM
Press, Washington, D.C.; Alberts, B., Bray, D., et al., Molecular Biology of
the Cell,
1995 Garland Publishing, New York).
As a brief background, normal alterations of intramitochondrial calcium are
associated with normal metabolic regulation (Dykens, 1998 in Mitochondria &
Free
235
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Radicals in Neurodegenerative Diseases, Beal, Howell and Bodis-Wollner, Eds.,
Wiley-Liss, New York, pp. 29-55; Radi et al., 1998 in Mitochondria & Free
Radicals in Neurodegenerative Diseases, Beal, Howell and Bodis-Wollner, Eds.,
Wiley-Liss, New York, pp. 57-89; Gunter and Pfeiffer, 1991, Am. J. Physio. 27:
C755; Gunter et al., 1994, Am. J. Physiol. 267:313). For example, fluctuating
levels
of mitochondrial free Calcium may be responsible for regulating oxidative
metabolism in response to increased ATP utilization, via allosteric regulation
of
enzymes (reviewed by Crompton et al., 1993 Basic Res. Cardiol. 88: 513-523;)
and
the glycerophosphate shuttle (Gunter et al., 1994 J. Bioenerg. Biomembr. 26:
471).
Normal mitochondrial function includes regulation of cytosolic free calcium
levels by sequestration of excess calcium within the mitochondrial matrix.
Depending on cell type, cytosolic calcium concentration is typically 50-100
nM. In
normally functioning cells, when calcium levels reach 200-300 nM, mitochondria
begin to accumulate calcium as a function of the equilibrium between influx
via a
calcium uniporter in the inner mitochondrial membrane and calcium efflux via
both
sodium dependent and sodium independent calcium carriers. In certain
instances,
such perturbation of intracellular calcium homeostasis is a feature of
diseases (such
as type 2 diabetes) associated with mitochondrial function, regardless of
whether the
calcium regulatory dysfunction is causative of, or a consequence of,
mitochondrial
function.
Elevated mitochondrial calcium levels thus may accumulate in response to
an initial elevation in cytosolic free calcium, as described above. Such
elevated
mitochondrial calcium concentrations in combination with reduced ATP or other
conditions associated with mitochondrial pathology, can lead to collapse of
mitochondrial inner membrane potential (see Gunter et al., 1998 Biochim.
Biophys.
Acta 1366:5; Rottenberg and Marbach, 1990, Biochim. Biophys. Acta 1016:87).
The extramitochondrial (cytosolic) level of calcium in a biological sample
that is
greater than that present within mitocllondria may be used as a risk factor
for type 2
diabetes in an individual. In the case of type 2 diabetes, mitochondrial or
cytosolic
calcium levels may vary from the above ranges and may range from, e.g., about
1
nM to about 500 mM, more typically from about 10 nM to about 100 mM and
usually from about 20 nM to about 1 mM, where "about" indicates +/-10 /o. A
236
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
variety of calcium indicators are known in the art, including but not limited
to, for
example, fura-2 (McCormack et al., 1989 Biochim. Biophys. Acta 973:420); mag-
fura-2; BTC (U.S. Pat. No. 5,501,980); fluo-3, fluo-4 and fluo-5N (U.S. Pat.
No.
5,049,673); rhod-2; benzothiaza-1; and benzothiaza-2 (all of which are
available
from Molecular Probes, Eugene, Oreg.). These or any other means for monitoring
intracellular calcium are contemplated according to the subject invention
method for
identifying a risk for type 2 diabetes.
For monitoring an indicator of mitochondrial function that is a cellular
response to elevated intracellular calcium, compounds that induce increased
cytoplasmic and mitochondrial concentrations of calcium, including calcium
ionophores, are well known to those of ordinary skill in the art, as are
methods for
measuring intracellular calcium and intramitochondrial calcium (see, e.g.,
Gunter
and Gunter, 1994 J. Bioenerg. Biomeinbr. 26: 471; Gunter et al., 1998 Biochim.
Biophys. Acta 1366:5; McCormack et al., 1989 Biochim. Biophys. Acta 973:420;
Orrenius and Nicotera, 1994 J. Neural. Transm. Suppl. 43:1; Leist and
Nicotera,
1998 Rev. Physiol. Biochem. Pharmacol. 132:79; and Haugland, 1996 Handbook of
Fluorescent Probes and Research Chemicals, Sixth Ed., Molecular Probes,
Eugene,
Oreg.). Accordingly, a person skilled in the art may readily select a suitable
ionophore (or another compound that results in increased cytoplasmic and/or
mitochondrial concentrations of calcium ions) and an appropriate means for
detecting intracellular and/or intramitochondrial calcium for use in the
present
invention, according to the instant disclosure and to well known methods.
Calcium ion influx into mitochondria appears to be largely dependent, and
may be completely dependent, upon the negative transmembrane electrochemical
potential (DY) established at the inner mitochondrial membrane by electron
transfer,
and such influx fails to occur in the absence of DY even when an eight-fold
Calcium
concentration gradient is imposed (Kapus et al., 1991 FEBS Lett. 282:61).
Accordingly, mitochondria may release Calcium when the membrane potential is
dissipated, as occurs with uncouplers like 2,4-dinitrophenol and carbonyl
cyanide p-
trifluoro-methoxyphenylhydrazone (FCCP). Thus, according to certain
embodiments
of the present invention, collapse of DY may be potentiated by influxes of
cytosolic
free calcium into the mitochondria, as may occur under certain physiological
237
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
conditions including those encountered by cells of a subject having type 2 DM.
Detection of such collapse may be accomplished by a variety of means as
provided
herein.
Typically, mitochondrial inembrane potential may be determined according
to methods with which those skilled in the art will be readily familiar,
including but
not limited to detection and/or measurement of detectable compounds such as
fluorescent indicators, optical probes and/or sensitive pH and ion-selective
electrodes (See, e.g., Ernster et al., 1981 J. Cell Biol. 91:227s and
references cited;
see also Haugland, 1996 Handbook of Fluorescent Probes and Research Chemicals,
Sixth Ed., Molecular Probes, Eugene, Oreg., pp. 266-274 and 589-594.). For
example, by way of illustration and not limitation, the fluorescent probes 2-
,4-
dimethylaminostyryl-N-methyl pyridinium (DASPMI) and tetramethylrhodamine
esters (e.g., tetramethylrhodamine methyl ester, TMRM; tetramethylrhodamine
ethyl
ester, TMRE) or related compounds (see, e.g., Haugland, 1996, supra) may be
quantified following accumulation in mitochondria, a process that is dependent
on,
and proportional to, mitochondrial membrane potential (see, e.g., Murphy et
al.,
1998 in Mitochondria & Free Radicals in Neurodegenerative Diseases, Beal,
Howell
and Bodis-Wollner, Eds., Wiley-Liss, New York, pp. 159-186 and references
cited
therein; and Molecular Probes On-line Handbook of Fluorescent Probes and
Research Chemicals, on the world wide web at probes.com/handbook/toc.html).
Other fluorescent detectable compounds that may be used include but are not
limited
to rhodamine 123, rhodamine B hexyl ester, DiOC6(3), JC-1 [5,5',6,6'-
Tetrachloro-
1,1',3,3'-Tetraethylbez- imidazolcarbocyanine Iodide] (see Cossarizza, et al.,
1993
Biochem. Biophys. Res. Comm. 197:40; Reers et al., 1995 Meth. Enzymol.
260:406), rhod-2 (see U.S. Pat. No. 5,049,673; all of the preceding compounds
are
available from Molecular Probes, Eugene, Oreg.) and rhodamine 800 (Lambda
Physik, GmbH, Gottingen, Germany; see Sakanoue et al., 1997 J. Biochem.
121:29).
Methods for monitoring mitochondrial membrane potential are also disclosed in
U.S. patent application Ser. No. 09/161,172.
Mitochondrial membrane potential can also be measured by non-fluorescent
means, for example by using TTP (tetraphenylphosphonium ion) and a TTP-
sensitive electrode (Kamo et al., 1979 J. Membrane Biol. 49:105; Porter and
Brand,
238
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272;
1995 Am. J. Physiol. 269:RI213). Those skilled in the art will be able to
select
appropriate detectable compounds or other appropriate means for measuring DYm.
By way of example and not limitation, TMRM is somewhat preferable to TMRE
because, following efflux from mitochondria, TMRE yields slightly inore
residual
signal in the endoplasmic reticulicum and cytoplasm than TMRM.
As another non-limiting example, membrane potential may be additionally or
alternatively calculated from indirect measurements of mitochondrial
permeability
to detectable charged solutes, using matrix volume and/or pyridine nucleotide
redox
determination combined with spectrophotometric or fluorimetric quantification.
Measurement of membrane potential dependent substrate exchange-diffusion
across
the inner mitochondrial membrane may also provide an indirect measurement of
membrane potential. (See, e.g., Quinn, 1976, The Molecular Biology of Cell
Membranes, University Park Press, Baltimore, Md., pp. 200-217 and references
cited therein.)
Exquisite sensitivity to extraordinary mitochondrial accumulations of
calcium that result from elevation of intracellular calcium, as described
above, may
also characterize type 2 diabetes. Such mitochondrial sensitivity may provide
an
indicator of mitochondrial finction according to the present invention.
Additionally,
a variety of physiologically pertinent agents, including hydroperoxide and
free
radicals, may synergize with calcium to induce collapse of DY (Novgorodov et
al.,
1991 Biochem. Biophys. Acta 1058: 242; Takeyaina et al., 1993 Biochem. J. 294:
719; Guidox et al., 1993 Arch. Biochem. Biophys. 306:139).
v. Indicators of initochondrial funetion that include responses to
apoptogenic stinzuli
In another embodiment, methods for identifying a modulator of
mitochondrial mass and/or function may include the detection and/or
measurement
of an indicator of mitochondrial function, wherein the mitochondrial function
involves programmed cell death or apoptosis. The range of responses to various
known apoptogenic stimuli is broad, as is the range of methods and reagents
for the
detection of such responses.
Mitochondrial dysfunction is thought to be critical in the cascade of events
leading to apoptosis in various cell types (Kroemer et al., FASEB J 9:1277-87,
239
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
1995). Mitochondrial physiology may be among the earliest events in programmed
cell death (Zamzami et al., J. Exp. Med. 182:367-77, 1995; Zamzami et al., J.
Exp.
Med. 181:1661-72, 1995) and elevated reactive oxygen species (ROS) levels that
result from such mitochondrial function may initiate the apoptotic cascade
(Ausserer
et al., Mol Cell Biol 14:5032-42, 1994). In several cell types, reduction in
the
mitochondrial membrane potential (DYm) precedes the nuclear DNA degradation
that accompanies apoptosis. In cell-free systems, mitochondrial, but not
nuclear,
enriched fractions are capable of inducing nuclear apoptosis (Newmeyer et al.,
Cell
70:353-64, 1994). Perturbation of mitochondrial respiratory activity leading
to
altered cellular metabolic states, such as elevated intracellular ROS, may
occur in
type 2 diabetes and may further induce pathogenetic events via apoptotic
mechanisms.
Oxidatively stressed mitochondria may release a pre-formed soluble factor
that can induce chromosomal condensation, an event preceding apoptosis
(Marchetti
et al., Cancer Res. 56:2033-38, 1996). In addition, members of the Bcl-2
family of
anti-apoptosis gene products are located within the outer mitochondrial
membrane
(Monaghan et al., J. Histochem. Cytochem. 40:1819-25, 1992) and these proteins
appear to protect membranes from oxidative stress (Korsmeyer et al., Biochim.
Biophys. Act. 1271:63, 1995). Localization of Bcl-2 to this membrane appears
to be
indispensable for modulation of apoptosis (Nguyen et al., J. Biol. Chem.
269:16521-
24, 1994). Thus, changes in mitochondrial physiology may be important
mediators
of apoptosis.
Impaired mitochondrial function may therefore be reflected in a lower
threshold for induction of apoptosis by one or more apoptogens. A variety of
apoptogens are known to those familiar with the art (see, e.g., Green et al.,
1998
Science 281:1309 and references cited therein) and may include by way of
illustration and not limitation: tumor necrosis factor-alpha (TNF-a); Fas
ligand;
glutamate; N-methyl-D-aspartate (NMDA); interleukin-3 (IL-3); herbimycin A
(Mancinitet al., 1997 J. Cell. Biol. 138:449-469); paraquat (Costantini et
al., 1995
Toxicology 99:1-2); ethylene glycols; protein kinase inhibitors, e.g.,
staurosporine,
calphostin C, caffeic acid phenethyl ester, chelerythrine chloride, genistein;
1-(5-
isoquinolinesulfonyl)-2-methylpiperazine; KN-93; N-[2-((p-bromocinnamyl)amino)
240
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
ethyl)-5-5-isoquinolinesulfonamide; d-erythrosphingosine derivatives; UV
irradiation; ionophores, e.g., ionomycin and valinomycin; MAP kinase inducers,
e.g., anisomycin, anandamine; cell cycle blockers, e.g., aphidicolin,
colcemid, 5-
fluorouracil, homoharringtonine; acetylcholinesterase inhibitors, e.g.
berberine; anti-
estrogens, e.g., tamoxifen; pro-oxidants, e.g.,: tert-butyl peroxide, hydrogen
peroxide; free radicals, e.g., nitric oxide; inorganic metal ions, e.g.,
cadmium; DNA
synthesis inhibitors, e.g., actinomycin D; DNA intercalators, e.g.,
doxorubicin,
bleomycin sulfate, hydroxyurea, methotrexate, mitoinycin C, camptothecin,
daunorubicin; protein synthesis inhibitors, e.g., cycloheximide, puromycin,
rapamycin; agents that affect microtubulin formation or stability, e.g.,
vinblastine,
vincristine, colchicine, 4-hydroxyphenylretinamide, paclitaxel; Bad protein,
Bid
protein and Bax protein (see, e.g., Jurgenmeier et al., 1998 Proc. Nat. Acad.
Sci.
USA 95:4997-5002 and references cited therein); calcium and inorganic
phosphate
(Kroemer et al., 1998 Ann. Rev. Physiol 60:619).
In one embodiment, wherein the indicator of mitochondrial function is a
cellular response to an apoptogen, cells in a biological sample that are
suspected of
undergoing apoptosis may be examined for morphological, permeability or other
changes that are indicative of an apoptotic state. For example by way of
illustration
and not limitation, apoptosis in many cell types may cause altered
morphological
appearance such as plasma membrane blebbing, cell shape change, loss of
substrate
adhesion properties or other morphological changes that can be readily
detected by a
person having ordinary skill in the art, for example by using light
microscopy. As
another example, cells undergoing apoptosis may exhibit fragmentation and
disintegration of chromosomes, which may be apparent by microscopy and/or
through the use of DNA-specific or chromatin-specific dyes that are known in
the
art, including fluorescent dyes. Such cells may also exhibit altered plasma
membrane permeability properties as may be readily detected through the use of
vital dyes (e.g., propidium iodide, trypan blue) or by the detection of
lactate
dehydrogenase leakage into the extracellular milieu. These and other means for
detecting apoptotic cells by morphologic criteria, altered plasma membrane
permeability and related changes will be apparent to those familiar with the
art.
241
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In another einbodiment, wherein the indicator of mitochondrial function is a
cellular response to an apoptogen, cells in a biological sample may be assayed
for
translocation of cell membrane phosphatidylserine (PS) from the inner to the
outer
leaflet of the plasma membrane, which may be detected, for example, by
measuring
outer leaflet binding by the PS-specific protein annexin. (Martin et al., J.
Exp. Med.
182:1545, 1995; Fadok et al., J. Immunol. 148:2207, 1992.) In still another
embodiment, a cellular/biochemical response to an apoptogen is determined by
an
assay for induction of specific protease activity in any member of a family of
apoptosis-activated proteases known as the caspases (see, e.g., Green et al.,
1998
Science 281:1309). Those having ordinary skill in the art will be readily
familiar
with methods for determining caspase activity, for example by determination of
caspase-mediated cleavage of specifically recognized protein substrates. These
substrates may include, for example, poly-(ADP-ribose) polymerase (PARP) or
other naturally occurring or synthetic peptides and proteins cleaved by
caspases that
are known in the art (see, e.g., Ellerby et al., 1997 J. Neurosci. 17:6165).
Synthetic
peptide substrates have been defined (Kluck et al., 1997 Science 275:1132;
Nicholson et al., 1995 Nature 376:37). Other non-limiting examples of
substrates
include nuclear proteins such as U1-70 kDa and DNA-PKcs (Rosen and Casciola-
Rosen, 1997 J. Cell. Biochem. 64:50; Cohen, 1997 Biochem. J. 326:1).
As described above, the mitochondrial inner membrane may exhibit highly
selective and regulated permeability for many small solutes, but is
impermeable to
large (less than around 10 kDa) molecules. (See, e.g., Quinn, 1976 The
Molecular
Biology of Cell Membranes, University Park Press, Baltimore, Md.). In cells
undergoing apoptosis, however, collapse of mitochondrial membrane potential
may
be accompanied by increased permeability permitting macromolecule diffusion
across the mitochondrial membrane. Thus, in another embodiment of the subject
invention method wherein the indicator of mitochondrial function is a cellular
response to an apoptogen, detection of a mitochondrial protein, for example
cytochrome c that has escaped from mitochondria in apoptotic cells, may
provide
evidence of a response to an apoptogen that can be readily determined. (Liu et
al.,
Cell 86:147, 1996) Such detection of cytochrome c may be performed
242
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
spectrophotometrically, immunochemically or by other well established methods
for
determining the presence of a specific protein.
For instance, release of cytochrome c from cells challenged with apoptotic
stimuli (e.g., ionomycin, a well known calcium ionophore) can be followed by a
variety of immunological methods. Matrix-assisted laser desorption ionization
time-
of-flight (MALDI-TOF) mass spectrometry coupled with affinity capture is
particularly suitable for such analysis since apo-cytochrome c and holo-
cytochrome
c can be distinguished on the basis of their unique molecular weights. For
example,
the Surface-Enhanced Laser Desorption/Ionization (SELDI) system (Ciphergen,
Palo Alto, Calif.) may be utilized to detect cytochrome c release from
mitochondria
in apoptogen treated cells. In this approach, a cytochrome c specific antibody
immobilized on a solid support is used to capture released cytochrome c
present in a
soluble cell extract. The captured protein is then encased in a matrix of an
energy
absorption molecule, (EAM) and is desorbed from the solid support surface
using
pulsed laser excitation. The molecular mass of the protein is determined by
its time
of flight to the detector of the SELDI mass spectrometer.
A person having ordinary skill in the art will readily appreciate that there
may be other suitable techniques for quantifying apoptosis, and such
techniques for
purposes of determining an indicator of mitochondrial function that is a
cellular
response to an apoptogenic stimulus are within the scope of the methods
provided by
the present invention.
vi. Free radical production as an iudicator of mitoclaondrial fuuctiou
In certain embodiments methods for identifying modulators of mitochondrial
mass and/or function involve detecting free radical production in a biological
sample
as an indicator of mitochondrial function. Although mitochondria are a primary
source of free radicals in biological systems (see, e.g., Murphy et al., 1998
in
Mitochondria and Free Radicals in Neurodegenerative Diseases, Beal, Howell and
Bodis-Wollner, Eds., Wiley-Liss, New York, pp. 159-186 and references cited
therein), the methods described herein should not be so limited and free
radical
production can be an indicator of mitochondrial function regardless of the
particular
subcellular source site. For example, numerous intracellular biochemical
pathways
that lead to the formation of radicals through production of metabolites such
as
243
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
hydrogen peroxide, nitric oxide or superoxide radical via reactions catalyzed
by
enzymes such as flavin-linked oxidases, superoxide dismutase or nitric oxide
synthetase, are known in the art, as are methods for detecting such radicals
(see, e.g.,
Kelver, 1993 Crit. Rev. Toxicol. 23:21; Halliwell B. and J. M. C. Gutteridge,
Free
Radicals in Biology aild Medicine, 1989 Clarendon Press, Oxford, UK; Davies,
K. J.
A. and F. Ursini, The Oxygen Paradox, Cleup Univ. Press, Padova, IT).
Mitochondrial function, such as failure at any step of the ETC, may also lead
to the
generation of highly reactive free radicals. As noted above, radicals
resulting from
mitochondrial function include reactive oxygen species (ROS), for example,
superoxide, peroxynitrite and hydroxyl radicals, and potentially other
reactive
species that may be toxic to cells. Accordingly, in certain embodiments, an
indicator
of mitochondrial function may be a detectable free radical species present in
a
biological sample. In certain embodiments, the detectable free radical will be
a ROS.
Methods for detecting a free radical that may be useful as an indicator of
mitochondrial finction are known in the art and will depend on the particular
radical.
Typically, a level of free radical production in a biological sample may be
determined according to methods with which those skilled in the art will be
readily
familiar, including but not limited to detection and/or measurement of:
glycoxidation
products including pentosidine, carboxymethylysine and pyrroline; lipoxidation
products including glyoxal, malondialdehyde and 4-hydroxynonenal;
thiobarbituric
acid reactive substances (TBARS; see, e.g., Steinbrecher et al., 1984 Proc.
Nat.
Acad. Sci. USA 81:3883; Wolff, 1993 Br. Med. Bull. 49:642) and/or other
chemical
detection means such as salicylate trapping of hydroxyl radicals (e.g.,
Ghiselli et al.,
1998 Meths. Mol. Biol. 108:89; Halliwell et al., 1997 Free Radic. Res. 27:239)
or
specific adduct formation (see, e.g., Mecocci et al. 1993 Ann. Neurol. 34:609;
Giulivi et al., 1994 Meths. Enzymol. 233:363) including malondialdehyde
formation, protein nitrosylation, DNA oxidation including mitochondrial DNA
oxidation, 8-OH-guanosine adducts (e.g., Beckman et al., 1999 Mutat. Res.
424:51),
protein oxidation, protein carbonyl modification (e.g., Baynes et al., 1991
Diabetes
40:405; Baynes et al., 1999 Diabetes 48:1); electron spin resonance (ESR)
probes;
cyclic voltametry; fluorescent and/or chemiluminescent indicators (see also
e.g.,
Greenwald, R. A. (ed.), Handbook of Methods for Oxygen Radical Research, 1985
244
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
CRC Press, Boca Raton, Fla.; Acworth and Bailey, (eds.), Handbook of Oxidative
Metabolism, 1995 ESA, Inc., Chelmsford, Mass.; Yla-Herttuala et al., 1989 J.
Clin.
Invest. 84:1086; Velazques et al., 1991 Diabetic Medicine 8:752; Belch et al.,
1995
Int. Angiol. 14:385; Sato et al., 1979 Biochem. Med. 21:104; Traverso et al.,
1998
Diabetologia 41:265; Haugland, 1996 Handbook of Fluorescent Probes and
Research Chemicals--Sixth Ed., Molecular Probes, Eugene, Oreg., pp. 483-502,
and
references cited therein). For example, by way of illustration and not
limitation,
oxidation of the fluorescent probes dichlorodihydrofluorescein diacetate and
its
carboxylated derivative carboxydichlorodihydrofluorescein diacetate (see,
e.g.,
Haugland, 1996, supra) may be quantified following accumulation in cells, a
process
that is dependent on, and proportional to, the presence of reactive oxygen
species
(see also, e.g., Molecular Probes On-line Handbook of Fluorescent Probes and
Research Chemicals, world wide web at probes.com/handbook/toc.html). Other
fluorescent detectable compounds that may be used in the invention for
detection of
free radical production include but are not limited to dihydrorhodamine and
dihydrorosamine derivatives, cis-parinaric acid, resorufin derivatives,
lucigenin and
any other suitable compound that may be known to those familiar with the art.
Thus, as also described above, free radical mediated damage may inactivate
one or more of the myriad proteins of the ETC and in doing so, may uncouple
the
mitochondrial chemiosmotic mechanism responsible for oxidative phosphorylation
and ATP production. Indicators of mitochondrial function that are ATP
biosynthesis
factors, including determination of ATP production, are described in greater
detail
herein. Free radical mediated damage to mitochondrial functional integrity is
also
just one example of multiple mechanisms associated with mitochondrial fuction
that
may result in collapse of the electrochemical potential maintained by the
inner
mitochondrial membrane.
In other embodiments, provided are methods for treating an individual that
may benefit from increased mitochondrial mass and/or function. The methods may
involve first identifying a patient suffering from a mitochondrial
dysfunction. The
methods described above for identifying an agent that modulates mitochondrial
mass
and/or function may also be used for identifying an individual that would
benefit
from increased mitochondrial mass and/or activity. For example, the methods
245
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272 - -
described above may be used to measure mitochondrial mass and/or function in a
biological sample from one individual as compared to an individual (e.g., an
individual having normal mitochondrial mass and/or function), a control
population,
or standard predetermined values of mitochondrial mass and/or function.
IV. Pliarmaceutical formulations and administration modes
Pharmaceutical compositions for use in accordance with the present methods
may be formulated in any conventional manner using one or more physiologically
acceptable carriers or excipients. Thus, sirtuin-activating or -inhibiting
compounds
and their physiologically acceptable salts and solvates may be formulated for
administration by, for example, injection (e.g. subcutaneous, intramuscular,
intraperitoneal), inhalation or insufflation (either through the mouth or the
nose) or
oral, buccal, parenteral, sublingual or rectal administration. In one
embodiment, the
compound is administered locally, at the site where of target cells, e.g.,
adipose
cells.
Compounds can be formulated for a variety of loads of administration,
including systemic and topical or localized administration. Techniques and
formulations generally may be found in Remmington's Pharmaceutical Sciences,
Meade Publishing Co., Easton, PA. For systemic administration, injection is
preferred, including intramuscular, intravenous, intraperitoneal, and
subcutaneous.
For injection, the compounds can be formulated in liquid solutions, preferably
in
physiologically compatible buffers such as Hank's solution or Ringer's
solution. In
addition, the compounds may be formulated in solid form and redissolved or
suspended immediately prior to use. Lyophilized forms are also included.
For oral administration, the phannaceutical compositions may take the form
of, for example, tablets, lozanges, or capsules prepared by conventional means
with
pharmaceutically acceptable excipients such as binding agents (e.g.,
pregelatinised
maize starch, polyvinylpyrrolidone or hydroxypropyl methylcellulose); fillers
(e.g.,
lactose, microcrystalline cellulose or calcium hydrogen phosphate); lubricants
(e.g.,
magnesium stearate, talc or silica); disintegrants (e.g., potato starch or
sodium starch
glycolate); or wetting agents (e.g., sodium lauryl sulphate). The tablets may
be
coated by methods well known in the art. Liquid preparations for oral
246
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
administration may take the form of, for example, solutions, syrups or
suspensions,
or they may be presented as a dry product for constitution with water or other
suitable vehicle before use. Such liquid preparations may be prepared by
conventional means with pharinaceutically acceptable additives such as
suspending
agents (e.g., sorbitol syrup, cellulose derivatives or hydrogenated edible
fats);
emulsifying agents (e.g., lecithin or acacia); non-aqueous vehicles (e.g.,
ationd oil,
oily esters, ethyl alcohol or fractionated vegetable oils); and preservatives
(e.g.,
methyl or propyl-p-hydroxybenzoates or sorbic acid). The preparations may also
contain buffer salts, flavoring, coloring and sweetening agents as
appropriate.
Preparations for oral administration may be suitably formulated to give
controlled
release of the active compound.
Polyphenols such as resveratrol can oxidize and lose sirtuin-stimulatory
activity, especially in a liquid or semi-solid form. To prevent oxidation and
preserve
the sirtuin-stimulatory activity of polyphenol-containing compounds, the
compounds
may be stored in a nitrogen atmosphere or sealed in a type of capsule and/or
foil
package that excludes oxygen (e.g., CapsugelTM)
For administration by inhalation, the compounds may be conveniently
delivered in the form of an aerosol spray presentation from pressurized packs
or a
nebuliser, with the use of a suitable propellant, e.g.,
dichlorodifluoromethane,
trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other
suitable
gas. In the case of a pressurized aerosol the dosage unit may be determined by
providing a valve to deliver a metered amount. Capsules and cartridges of
e.g.,
gelatin, for use in an inhaler or insufflator may be formulated containing a
powder
mix of the compound and a suitable powder base such as lactose or starch.
The compounds may be formulated for parenteral administration by
injection, e.g., by bolus injection or continuous infusion. Formulations for
injection
may be presented in unit dosage form, e.g., in ampoules or in multi-dose
containers,
with an added preservative. The compositions may take such forms as
suspensions,
solutions or emulsions in oily or aqueous vehicles, and may contain
formulatory
agents such as suspending, stabilizing and/or dispersing agents.
Alternatively, the
active ingredient may be in powder form for constitution with a suitable
vehicle,
e.g., sterile pyrogen-free water, before use.
247
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
The compounds may also be formulated in rectal compositions such as
suppositories or retention enemas, e.g., containing conventional suppository
bases
such as cocoa butter or other glycerides.
In addition to the formulations described previously, the compounds may
also be formulated as a depot preparation. Such long acting formulations may
be
administered by implantation (for example subcutaneously or intramuscularly)
or by
intramuscular injection. Thus, for example, the compounds may be formulated
with
suitable polymeric or hydrophobic materials (for example as an emulsion in an
acceptable oil) or ion exchange resins, or as sparingly soluble derivatives,
for
example, as a sparingly soluble salt. Controlled release formula also includes
patches.
In certain embodiments, pharmaceutical compositions can be administered
with medical devices known in the art. For example, a pharmaceutical
composition
described herein can be administered with a needle-less hypodermic injection
device, such as the devices disclosed in U.S. Pat. Nos. 5,399,163, 5,383,851,
5,312,335, 5,064,413, 4,941,880, 4,790,824, or 4,596,556. Examples of well-
known
implants and modules useful in the invention include: U.S. Pat. No. 4,487,603,
which discloses an implantable micro-infusion pump for dispensing medication
at a
controlled rate; U.S. Pat. No. 4.,486,194, which discloses a therapeutic
device for
administering medicants through the skin; U.S. Pat. No. 4,447,233, which
discloses
a medication infusion pump for delivering medication at a precise infusion
rate; U.S.
Pat. No. 4,447,224, which discloses a variable flow implantable infusion
apparatus
for continuous drug delivery; U.S. Pat. No. 4,439,196, which discloses an
osmotic
drug delivery system having multi-chamber compartments; and U.S. Pat. No.
4,475,196, which discloses an osmotic drug delivery system. Of course, many
otlier
such implants, delivery systems, and modules also are known.
In certain embodiments, the compounds described herein can be formulated
for delivery to the central nervous system (CNS) (reviewed in Begley,
Pharmacology & Therapeutics 104: 29-45 (2004)). Conventional approaches for
drug delivery to the CNS include: neurosurgical strategies (e.g.,
intracerebral
injection or intracerebroventricular infusion); molecular manipulation of the
agent
(e.g., production of a chimeric fusion protein that comprises a transport
peptide that
248
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
has an affinity for an endothelial cell surface molecule in combination with
an agent
that is itself incapable of crossing the blood-brain barrier (BBB)) in an
attempt to
exploit one of the endogenous transport pathways of the BBB; pharmacological
strategies designed to increase the lipid solubility of an agent (e.g.,
conjugation of
water-soluble agents to lipid or cholesterol carriers); and the transitory
disruption of
the integrity of the BBB by hyperosmotic disruption (resulting from the
infusion of a
mannitol solution into the carotid artery or the use of a biologically active
agent such
as an angiotensin peptide).
One possibility to achieve sustained release kinetics is embedding or
encapsulating the active compound into nanoparticles. Nanoparticles can be
administrated as powder, as a powder mixture witli added excipients or as
suspensions. Colloidal suspensions of nanoparticles can easily be
administrated
through a cannula with small diameter.
Nanoparticles are particles with a diameter from about 5 nm to up to about
1000 nm. The term "nanoparticles" as it is used hereinafter refers to
particles formed
by a polymeric matrix in which the active compound is dispersed, also known as
"nanospheres", and also refers to nanoparticles which are composed of a core
containing the active compound which is surrounded by a polymeric membrane,
also
known as "nanocapsules". In certain embodiments, nanoparticles are preferred
having a diameter from about 50 nm to about 500 nm, in particular from about
100
nm to about 200 nm.
Nanoparticles can be prepared by in situ polymerization of dispersed
monomers or by using preformed polymers. Since polymers prepared in situ are
often not biodegradable and/or contain toxicological serious byproducts,
nanoparticles from preformed polymers are preferred. Nanoparticles from
preformed
polymers can be prepared by different techniques, e.g., by emulsion
evaporation,
solvent displacement, salting-out, mechanical grinding, microprecipitation,
and by
emulsification diffusion.
With the methods described above, nanoparticles can be formed with various
types of polymers. For use in the method of the present invention,
nanoparticles
made from biocompatible polymers are preferred. The term "biocompatible"
refers
to material that after introduction into a biological environment has no
serious
249
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
effects to the biological environment. From biocompatible polymers those
polymers
are especially preferred which are also biodegradable. The term
"biodegradable"
refers to material that after introduction into a biological environment is
enzymatically or chemically degraded into smaller molecules, which can be
eliminated subsequently. Examples are polyesters from hydroxycarboxylic acids
such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), polycaprolactone
(PCL),
copolymers of lactic acid and glycolic acid (PLGA), copolymers of lactic acid
and
caprolactone, polyepsilon caprolactone, polyhyroxy butyric acid and
poly(ortho)esters, polyurethanes, polyanhydrides, polyacetals,
polydihydropyrans,
polycyanoacrylates, natural polymers such as alginate and other
polysaccharides
including dextran and cellulose, collagen and albumin.
Suitable surface modifiers can preferably be selected from known organic
and inorganic pharmaceutical excipients. Such excipients include various
polymers,
low molecular weiglit oligomers, natural products and surfactants. Preferred
surface
modifiers include nonionic and ionic surfactants. Representative examples of
surface
modifiers include gelatin, casein, lecithin (phosphatides), gum acacia,
cholesterol,
tragacanth, stearic acid, benzalkonium chloride, calcium stearate, glycerol
monostearate, cetostearyl alcohol, cetomacrogol emulsifying wax, sorbitan
esters,
polyoxyethylene alkyl ethers, e.g., macrogol ethers such as cetomacrogol 1000,
polyoxyethylene castor oil derivatives, polyoxyethylene sorbitan fatty acid
esters,
e.g., the commercially available TweensTM, polyethylene glycols,
polyoxyethylene
stearates, colloidal silicon dioxide, phosphates, sodium dodecylsulfate,
carboxymethylcellulose calcium, carboxymethylcellulose sodium,
methylcellulose,
hydroxyethylcellulose, hydroxy propylcellulose, hydroxypropylmethylcellulose
phthalate, noncrystalline cellulose, magnesium aluminum silicate,
triethanolamine,
polyvinyl alcohol, and polyvinylpyrrolidone (PVP). Most of these surface
modifiers
are known pharmaceutical excipients and are described in detail in the
Handbook of
Pharmaceutical Excipients, published jointly by the American Pharmaceutical
Association and The Pharmaceutical Society of Great Britain, the
Pharmaceutical
Press, 1986.
250
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Further description on preparing nanoparticles can be found, for example, in
US Patent No. 6,264,922, the contents of which are incorporated herein by
reference.
Liposomes are a further drug delivery system which is easily injectable.
Accordingly, in the method of invention the active compounds can also be
administered in the form of a liposome delivery system. Liposomes are well-
known
by a person skilled in the art. Liposomes can be formed from a variety of
phospholipids, such as cholesterol, stearylamine of phosphatidylcholines.
Liposomes
being usable for the method of invention encompass all types of liposomes
including, but not limited to, small unilamellar vesicles, large unilamellar
vesicles
and multilamellar vesicles.
Liposomes are used for a variety of therapeutic purposes, and in particular,
for carrying therapeutic agents to target cells. Advantageously, liposome-drug
formulations offer the potential of improved drug-delivery properties, which
include,
for example, controlled drug release. An extended circulation time is often
needed
for liposomes to reach a target region, cell or site. In particular, this is
necessary
where the target region, cell or site is not located near the site of
administration. For
example, when liposomes are administered systemically, it is desirable to coat
the
liposomes with a hydrophilic agent, for example, a coating of hydrophilic
polymer
chains such as polyethylene glycol (PEG) to extend the blood circulation
lifetime of
the liposomes. Such surface-modified liposomes are commonly referred to as
"long
circulating" or "sterically stabilized" liposomes.
One surface modification to a liposome is the attachment of PEG chains,
typically having a molecular weight from about 1000 daltons (Da) to about 5000
Da,
and to about 5 mole percent (%) of the lipids making up the liposomes (see,
for
example, Stealth Liposomes, CRC Press, Lasic, D. and Martin, F., eds., Boca
Raton,
Fla., (1995)), and the cited references therein. The pharmacokinetics
exhibited by
such liposomes are characterized by a dose-independent reduction in uptake of
liposomes by the liver and spleen via the mononuclear phagocyte system (MPS),
and
significantly prolonged blood circulation time, as compared to non-surface-
modified
liposomes, which tend to be rapidly removed from the blood and accumulated in
the
liver and spleen.
251
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
In certain embodiments, the complex is shielded to increase the circulatory
half-life of the complex or shielded to increase the resistance of nucleic
acid to
degradation, for example degradation by nucleases.
As used herein, the term "shielding", and its cognates such as "shielded",
refers to the ability of "shielding moieties" to reduce the non-specific
interaction of
the complexes described herein with serum complement or with other species
present in serum in vitro or in vivo. Shielding moieties may decrease the
complex
interaction with or binding to these species through one or more mechanisms,
including, for example, non-specific steric or non-specific electronic
interactions.
Examples of such interactions include non-specific electrostatic interactions,
charge
interactions, Van der Waals interactions, steric-hindrance and the like. For a
moiety
to act as a shielding moiety, the mechanism or mechanisms by which it may
reduce
interaction with, association with or binding to the serum complement or other
species does not have to be identified. One can determine whether a moiety can
act
as a shielding moiety by determining whether or to what extent a complex binds
serum species.
It should be noted that "shielding moieties" can be multifunctional. For
example, a shielding moiety may also function as, for example, a targeting
factor. A
shielding moiety may also be referred to as multifunctional with respect to
the
mechanism(s) by which it shields the complex. While not wishing to be limited
by
proposed mechanism or theory, examples of such a multifunctional shielding
moiety
are pH sensitive endosomal membrane-disruptive synthetic polymers, such as
PPAA
or PEAA. Certain poly(alkylacrylic acids) have been shown to disrupt endosomal
membranes while leaving the-outer cell surface membrane intact (Stayton et al.
(2000) J. Controll. Release 65:203-220; Murthy et al. (1999) J. Controll.
Release
61:137-143; WO 99/3483 1), thereby increasing cellular bioavailability and
functioning as a targeting factor. However, PPAA reduces binding of serum
complement to complexes in which it is incorporated, thus functioning as a
shielding
moiety.
Another way to produce a formulation, particularly a solution, of a sirtuin
modulator such as resveratrol or a derivative thereof, is through the use of
cyclodextrin. By cyclodextrin is meant a-, (3-, or y-cyclodextrin.
Cyclodextrins are
252
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
described in detail in Pitha et al., U.S. Pat. No. 4,727,064, which is
incorporated
herein by reference. Cyclodextrins are cyclic oligomers of glucose; these
compounds
form inclusion complexes with any drug whose molecule can fit into the
lipophile-
seeking cavities of the cyclodextrin inolecule.
The cyclodextrin of the compositions according to the invention may be a-,
(3-, or y-cyclodextrin. a-cyclodextrin contains six glucopyranose units; (3-
cyclodextrin contains seven glucopyranose units; and y-cyclodextrin contains
eight
glucopyranose units. The molecule is believed to form a truncated cone having
a
core opening of 4.7-5.3 angstroms, 6.0-6.5 angstroms, and 7.5-8.3 angstroms in
a-,
(3-, or y-cyclodextrin respectively. The composition according to the
invention may
comprise a mixture of two or more of the a-, (3-, or 7-cyclodextrins.
Typically,
however, the composition according to the invention will comprise only one of
the
a-, (3-, or y-cyclodextrins.
Most preferred cyclodextrins in the compositions according to the invention
are amorphous cyclodextrin compounds. By amorphous cyclodextrin is meant non-
crystalline mixtures of cyclodextrins wherein the mixture is prepared from a-,
(3-, or
y-cyclodextrin. In general, the amorphous cyclodextrin is prepared by non-
selective
alkylation of the desired cyclodextrin species. Suitable alkylation agents for
this
purpose include but are not limited to propylene oxide, glycidol,
iodoacetarnide,
chloroacetate, and 2-diethylaminoethlychloride. Reactions are carried out to
yield
mixtures containing a plurality of components thereby preventing
crystallization of
the cyclodextrin. Various alkylated cyclodextrins can be made and of course
will
vary, depending upon the starting species of cyclodextrin and the alkylating
agent
used. Among the amorphous cyclodextrins suitable for compositions according to
the invention are hydroxypropyl, hydroxyethyl, glucosyl, maltosyl and
maltotriosyl
derivatives of (3-cyclodextrin, carboxyamidomethyl-(3-cyclodextrin,
carboxymethyl-
(3-cyclodextrin, hydroxypropyl-(3-cyclodextrin and diethylamino-(3-
cyclodextrin.
One example of resveratrol dissolved in the presence of a cyclodextrin is
provided in Marier et al., J. Phaf=macol. Exp. Therap. 302:369-373 (2002), the
contents of which are incorporated herein by reference, where a 6 mg/mL
solution of
resveratrol was prepared using 0.9% saline containing 20% hydroxylpropyl-p-
cyclodextrin.
253
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
As mentioned above, the compositions of matter of the invention comprise
an aqueous preparation of preferably substituted amorphous cyclodextrin and
one or
more sirtuin modulators. The relative amounts of sirtuin modulators and
cyclodextrin will vary depending upon the relative amount of each of the
sirtuin
modulators and the effect of the cyclodextrin on the compound. In general, the
ratio
of the weight of compound of the sirtuin modulators to the weight of
cyclodextrin
compound will be in a range between 1:1 and 1:100. A weight to weight ratio in
a
range of 1:5 to 1:50 and more preferably in a range of 1:10 to 1:20 of the
compound
selected from sirtuin modulators to cyclodextrin are believed to be the most
effective
for increased circulating availability of the sirtuin modulator.
Importantly, if the aqueous solution comprising the sirtuin modulators and a
cyclodextrin is to be administered parenterally, especially via the
intravenous route,
a cyclodextrin will be substantially free of pyrogenic contaminants. Various
forms
of cyclodextrin, such as forms of amorphous cyclodextrin, may be purchased
from a
number of vendors including Sigma-Aldrich, Inc. (St. Louis, Mo., USA). A
method
for the production of hydroxypropyl-(3-cyclodextrin is disclosed in Pitha et
al., U.S.
Pat. No. 4,727,064 which is incorporated herein by reference.
Additional description of the use of cyclodextrin for solubilizing compounds
can be found in US 2005/0026849, the contents of which are incorporated herein
by
reference.
Rapidly disintegrating or dissolving dosage forms are useful for the rapid
absorption, particularly buccal and sublingual absorption, of pharmaceutically
active
agents. Fast melt dosage forms are beneficial to patients, such as aged and
pediatric
patients, who have difficulty in swallowing typical solid dosage forms, such
as
caplets and tablets. Additionally, fast melt dosage forms circumvent drawbacks
associated with, for example, chewable dosage forms, wherein the length of
time an
active agent remains in a patient's mouth plays. an important role in
determining the
amount of taste masking and the extent to which a patient may object to throat
grittiness of the active agent.
To overcome such problems manufacturers have developed a number of fast
melt solid dose oral formulations. These are available from manufacturers
including
Cima Labs, Fuisz Technologies Ltd., Prographarm, R. P. Scherer, Yamanouchi-
254
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Shaklee, and McNeil-PPC, Inc. All of these manufacturers market different
types of
rapidly dissolving solid oral dosage forms. See e.g., patents and publications
by
Cima Labs such as U.S. Pat. No. 5,607,697, 5,503,846, 5,223,264, 5,401,513,
5,219,574, and 5,178,878, WO 98/46215, WO 98/14179; patents to Fuisz
Technologies, now part of BioVail, such as U.S. Pat. No. 5,871,781, 5,869,098,
5,866,163, 5,851,553, 5,622,719, 5,567,439, and 5,587,172; U.S. Pat. No.
5,464,632
to Prographarm; patents to R. P. Scherer such as U.S. Pat. No. 4,642,903,
5,188,825,
5,631,023 and 5,827,541; patents to Yamanouchi-Shaklee such as U.S. Pat. No.
5,576,014 and 5,446,464; patents to Janssen such as U.S. Pat. No. 5,807,576,
5,635,210, 5,595,761, 5,587,180 and 5,776,491; U.S. Pat. Nos. 5,639,475 and
5,709,886 to Eurand America, Inc.; U.S. Pat. Nos. 5,807,578 and 5,807,577 to
L.A.B. Pharmaceutical Research; patents to Schering Corporation such as U.S.
Pat.
Nos. 5,112,616 and 5,073,374; U.S. Pat. No. 4,616,047 to Laboratoire L. LaFon;
U.S. Pat. No. 5,501,861 to Takeda Chemicals Inc., Ltd.; and U.S. Pat. No.
6,316,029 to Elan.
In one example of fast melt tablet preparation, granules for fast melt tablets
made by either the spray drying or pre-compacting processes are mixed with
excipients and compressed into tablets using conventional tablet making
machinery.
The granules can be combfned with a variety of carriers including low density,
high
moldability saccharides, low moldability saccharides, polyol combinations, and
then
directly compressed into a tablet that exhibits an improved dissolution and
disintegration profile.
The tablets according to the present invention typically have a hardness of
about 2 to about 6 Strong-Cobb units (scu). Tablets within this hardness range
disintegrate or dissolve rapidly when chewed. Additionally, the tablets
rapidly
disentegrate in water. On average, a typical 1.1 to 1.5 gram tablet
disintegrates in 1-
3 minutes without stirring. This rapid disintegration facilitates delivery of
the active
material.
The granules used to make the tablets can be, for example, mixtures of low
density alkali earth metal salts or carbohydrates. For example, a mixture of
alkali
earth metal salts includes a combination of calcium carbonate and magnesium
hydroxide. Similarly, a fast melt tablet can be prepared according to the
methods of
255
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
the present invention that incorporates the use of A) spray dried extra light
calcium
carbonate/maltodextrin, B) magnesium hydroxide and C) a eutectic polyol
combination including Sorbitol Instant, xylitol and mannitol. These materials
have
been combined to produce a low density tablet that dissolves very readily and
promotes the fast disintegration of the active ingredient. Additionally, the
pre-
compacted and spray dried granules can be combined in the same tablet.
For fast melt tablet preparation, a sirtuin modulator useful in the present
invention can be in a form such as solid, particulate, granular, crystalline,
oily or
solution. The sirtuin modulator for use in the present invention may be a
spray dried
product or an adsorbate that has been pre-compacted to a harder granular form
that
reduces the medicament taste. A pharmaceutical active ingredient for use in
the
present invention may be spray dried with a carrier that prevents the active
ingredient from being easily extracted from the tablet when chewed.
In addition to being directly added to the tablets of the present invention,
the
medicament drug itself can be processed by the pre-compaction process to
achieve
an increased density prior to being incorporated into the formulation.
The pre-compaction process used in the present invention can be used to
deliver poorly soluble pharmaceutical materials so as to improve the release
of such
pharmaceutical materials over traditional dosage forms. This could allow for
the use
of lower dosage levels to deliver equivalent bioavailable levels of drug and
thereby
lower toxicity levels of both currently marketed drug and new chemical
entities.
Poorly soluble pharmaceutical materials can be used in the form of
nanoparticles,
which are nanometer-sized particles.
In addition to the active ingredient and the granules prepared from low
density alkali earth metal salts and/or water soluble carbohydrates, the fast
melt
tablets can be formulated using conventional carriers or excipients and well
established pharmaceutical techniques. Conventional carriers or excipients
include,
but are not limited to, diluents, binders, adhesives (i.e., cellulose
derivatives and
acrylic derivatives), lubricants (i.e., magnesium or calcium stearate,
vegetable oils,
polyethylene glycols, talc, sodium lauryl sulphate, polyoxy ethylene
monostearate),
disintegrants, colorants, flavorings, preservatives, sweeteners and
miscellaneous
materials such as buffers and adsorbents.
256
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
Additional description of the preparation of fast melt tablets can be found,
for example, in U.S. Pat. No. 5,939,091, the contents of which are
incorporated
herein by reference.
Pharmaceutical compositions (including cosmetic preparations) may
comprise from about 0.00001 to 100% such as from 0.001 to 10% or from 0.1 % to
5% by weight of one or more compounds described herein.
In one embodiment, a compound described herein, is incorporated into a
topical formulation containing a topical carrier that is generally suited to
topical
drug administration and comprising any such material known in the art. The
topical
carrier may be selected so as to provide the composition in the.desired form,
e.g., as
an ointment, lotion, cream, microemulsion, gel, oil, solution, or the like,
and may be
comprised of a material of either naturally occurring or synthetic origin. It
is
preferable that the selected carrier not adversely affect the active agent or
other
components of the topical formulation. Examples of suitable topical carriers
for use
herein include water, alcohols and other nontoxic organic solvents, glycerin,
mineral
oil, silicone, petroleum jelly, lanolin, fatty acids, vegetable oils,
parabens, waxes,
and the like.
Formulations may be colorless, odorless ointments, lotions, creams,
microemulsions and gels.
Compounds may be incorporated into ointments, which generally are
semisolid preparations which are typically based on petrolatum or other
petroleum
derivatives. The specific ointment base to be used, as will be appreciated by
those
skilled in the art, is one that will provide for optimum drug delivery, and,
preferably,
will provide for other desired characteristics as well, e.g., emolliency or
the like. As
with other carriers or vehicles, an ointment base should be inert, stable,
nonirritating
and nonsensitizing. As explained in Remington's, cited in the preceding
section,
ointment bases may be grouped in four classes: oleaginous bases; emulsifiable
bases; emulsion bases; and water-soluble bases. Oleaginous ointment bases
include,
for example, vegetable oils, fats obtained from animals, and semisolid
hydrocarbons
obtained from petroleum. Emulsifiable ointment bases, also known as absorbent
ointment bases, contain little or no water and include, for example,
hydroxystearin
sulfate, aiihydrous lanolin and hydrophilic petrolatum. Emulsion ointment
bases are
257
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
either water-in-oil (W/0) emulsions or oil-in-water (O/W) emulsions, and
include,
for example, cetyl alcohol, glyceryl monostearate, lanolin and stearic acid.
Exemplary water-soluble ointment bases are prepared from polyethylene glycols
(PEGs) of varying molecular weight; again, reference may be had to
Remington's,
supra, for further information.
Compounds may be incorporated into lotions, which generally are
preparations to be applied to the skin surface without friction, and are
typically
liquid or semiliquid preparations in which solid particles, including the
active agent,
are present in a water or alcohol base. Lotions are usually suspensions of
solids, and
may comprise a liquid oily emulsion of the oil-in-water type. Lotions are
preferred
formulations for treating large body areas, because of the ease of applying a
more
fluid composition. It is generally necessary that the insoluble matter in a
lotion be
finely divided. Lotions will typically contain suspending agents to produce
better
dispersions as well as compounds useful for localizing and holding the active
agent
in contact with the skin, e.g., methylcellulose, sodium
carboxymethylcellulose, or
the like. An exemplary lotion formulation for use in conjunction with the
present
method contains propylene glycol mixed with a hydrophilic petrolatum such as
that
which may be obtained under the trademark AquaphorRT" from Beiersdorf, Inc.
(Norwalk, Conn.).
Compounds may be incorporated into creams, which generally are viscous
liquid or semisolid emulsions, either oil-in-water or water-in-oil. Cream
bases are
water-washable, and contain an oil phase, an emulsifier and an aqueous phase.
The
oil phase is generally comprised of petrolatum and a fatty alcohol such as
cetyl or
stearyl alcohol; the aqueous phase usually, although not necessarily, exceeds
the oil
phase in volume, and generally contains a humectant. The emulsifier in a cream
formulation, as explained in Remington's, supra, is generally a nonionic,
anionic,
cationic or amphoteric surfactant.
Compounds may be incorporated into microemulsions, which generally are
thermodynamically stable, isotropically clear dispersions of two immiscible
liquids,
such as oil and water, stabilized by an interfacial film of surfactant
molecules
(Encyclopedia of Pharmaceutical Technology (New York: Marcel Dekker, 1992),
volume 9). For the preparation of microemulsions, surfactant (emulsifier), co-
258
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
surfactant (co-emulsifier), an oil phase and a water phase are necessary.
Suitable
surfactants include any surfactants that are useful in the preparation of
emulsions,
e.g., emulsifiers that are typically used in the preparation of creams. The co-
surfactant (or "co-emulsifer") is generally selected from the group of
polyglycerol
derivatives, glycerol derivatives and fatty alcohols. Preferred emulsifier/co-
emulsifier combinations are generally although not necessarily selected from
the
group consisting of: glyceryl monostearate and polyoxyethylene stearate;
polyethylene glycol and ethylene glycol palmitostearate; and caprilic and
capric
triglycerides and oleoyl macrogolglycerides. The water phase includes not only
water but also, typically, buffers, glucose, propylene glycol, polyethylene
glycols,
preferably lower molecular weight polyethylene glycols (e.g., PEG 300 and PEG
400), and/or glycerol, and the like, while the oil phase will generally
comprise, for
example, fatty acid esters, modified vegetable oils, silicone oils, mixtures
of mono-
di- and triglycerides, mono- and di-esters of PEG (e.g., oleoyl macrogol
glycerides),
etc.
Compounds may be incorporated into gel formulations, which generally are
semisolid systems consisting of either suspensions made up of small inorganic
particles (two-phase systems) or large organic molecules distributed
substantially
uniformly throughout a carrier liquid (single phase gels). Single phase gels
can be
made, for example, by combining the active agent, a carrier liquid and a
suitable
gelling agent such as tragacanth (at 2 to 5%), sodium alginate (at 2-10%),
gelatin (at
2-15%), methylcellulose (at 3-5%), sodium carboxymethylcellulose (at 2-5%),
carbomer (at 0.3-5%) or polyvinyl alcohol (at 10-20%) together and mixing
until a
characteristic semisolid product is produced. Other suitable gelling agents
include
methylhydroxycellulose, polyoxyethylene-polyoxypropylene,
hydroxyethylcellulose
and gelatin. Although gels commonly employ aqueous carrier liquid, alcohols
and
oils can be used as the carrier liquid as well.
Various additives, known to those skilled in the art, may be included in
formulations, e.g., topical formulations. Examples of additives include, but
are not
limited to, solubilizers, skin permeation enhancers, opacifiers, preservatives
(e.g.,
anti-oxidants), gelling agents, buffering agents, surfactants (particularly
nonionic
and amphoteric surfactants), emulsifiers, emollients, thickening agents,
stabilizers,
259
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
humectants, colorants, fragrance, and the like. Inclusion of solubilizers
and/or skin
permeation enhancers is particularly preferred, along with emulsifiers,
emollients
and preservatives. An optimum topical formulation comprises approximately: 2
wt.
% to 60 wt. %, preferably 2 wt. % to 50 wt. %, solubilizer and/or skin
permeation
enhancer; 2 wt. % to 50 wt. %, preferably 2 wt. % to 20 wt. %, emulsifiers; 2
wt. %
to 20 wt. % emollient; and 0.01 to 0.2 wt. % preservative, with the active
agent and
carrier (e.g., water) making of the remainder of the formulation.
A skin permeation enhancer serves to facilitate passage of therapeutic levels
of active agent to pass through a reasonably sized area of unbroken skin.
Suitable
enhancers are well known in the art and include, for example: lower alkanols
such as
methanol ethanol and 2-propanol; alkyl methyl sulfoxides such as
dimethylsulfoxide
(DMSO), decylmethylsulfoxide (Clo MSO) and tetradecylmethyl sulfboxide;
pyrrolidones such as 2-pyrrolidone, N-methyl-2-pyrrolidone and N-(-
hydroxyethyl)pyrrolidone; urea; N,N-diethyl-m-toluamide; C2-C6 alkanediols;
miscellaneous solvents such as dimethyl formamide (DMF), N,N-dimethylacetamide
(DMA) and tetrahydrofurfuryl alcohol; and the 1-substituted azacycloheptan-2-
ones,
particularly 1-n-dodecylcyclazacycloheptan-2-one (laurocapram; available under
the
trademark AzoneRTM from Whitby Research Incorporated, Richmond, Va.).
Examples of solubilizers include, but are not limited to, the following:
hydrophilic ethers such as diethylene glycol monoethyl ether (ethoxydiglycol,
available commercially as TranscutolRTM) and diethylene glycol monoethyl ether
oleate (available commercially as SoftcutolRTM); polyethylene castor oil
derivatives
such as polyoxy 35 castor oil, polyoxy 40 hydrogenated castor oil, etc.;
polyethylene
glycol,'particularly lower molecular weight polyethylene glycols such as PEG
300
and PEG 400, and polyethylene glycol derivatives such as PEG-8 caprylic/capric
glycerides (available commercially as LabrasolRTM); alkyl methyl sulfoxides
such as
DMSO; pyrrolidones such as 2-pyrrolidone and N-methyl-2-pyrrolidone; and DMA.
Many solubilizers can also act as absorption enhancers. A single solubilizer
may be
incorporated into the formulation, or a mixture of solubilizers may be
iricorporated
therein.
Suitable emulsifiers and co-emulsifiers include, without limitation, those
emulsifiers and co-emulsifiers described with respect to microemulsion
260
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
formulations. Emollients include, for exainple, propylene glycol, glycerol,
isopropyl
myristate, polypropylene glycol-2 (PPG-2) myristyl ether propionate, and the
like.
Other active agents may also be included in formulations, e.g., anti-
inflammatory agents, analgesics, antimicrobial agents, antifungal agents,
antibiotics,
vitamins, antioxidants, and sunblock agents commonly found in sunscreen
formulations including, but not limited to, anthranilates, benzophenones
(particularly
benzophenone-3), camphor derivatives, cinnamates (e.g., octyl
methoxycinnamate),
dibenzoyl methanes (e.g., butyl methoxydibenzoyl methane), p-aminobenzoic acid
(PABA) and derivatives thereof, and salicylates (e.g., octyl salicylate).
In certain topical formulations, the active agent is present in an amount in
the
range of approximately 0.25 wt. % to 75 wt. % of the forinulation, preferably
in the
range of approximately 0.25 wt. % to 30 wt. % of the formulation, more
preferably
in the range of approximately 0.5 wt. % to 15 wt. % of the formulation, and
most
preferably in the range of approximately 1.0 wt. % to 10 wt. % of the
formulation.
Topical skin treatment compositions can be packaged in a suitable container
to suit its viscosity and intended use by the consumer. For example, a lotion
or
cream can be packaged in a bottle or a roll-ball applicator, or a propellant-
driven
aerosol device or a container fitted with a pump suitable for finger
operation. When
the composition is a cream, it can simply be stored in a non-deformable bottle
or
squeeze container, such as a tube or a lidded jar. The composition may also be
included in capsules such as those described in U.S. Pat. No. 5,063,507.
Accordingly, also provided are closed containers containing a cosmetically
acceptable composition as herein defined.
In an alternative embodiment, a pharmaceutical formulation is provided for
oral or parenteral administration, in which case the formulation may comprise
an
activating compound-containing microemulsion as described above, and may
contain alternative pharinaceutically acceptable carriers, vehicles,
additives, etc.
particularly suited to oral or parenteral drug administration. Alternatively,
an
activating compound-contairiing microemulsion may be administered orally or
parenterally substantially as described above, without modification.
Administration of a sirtuin activator or inhibitor may be followed by
measuring a factor in the subject, such as measuring the activity of the
sirtuin. In an
261
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
illustrative embodiment, a cell is obtained from a subject following
administration of
an activating or inhibiting compound to the subject, such as by obtaining a
biopsy,
and the activity of the sirtuin or sirtuin expression level is determined in
the biopsy.
Alternatively, biomarkers, such as plasma biomarkers may be followed. The cell
may be any cell of the subject, but in cases in which an activating compound
is
administered locally, the cell is preferably a cell that is located in the
vicinity of the
site of administration. For example, the cell may be an adipose cell.
V. Exemplary kits
Also provided herein are kits, e.g., kits for therapeutic purposes, including
kits for treating or preventing metabolic disorders, such as obesity or
diabetes, or
secondary conditions thereof. A kit may comprise one or more high dosage
formulations of a sirtuin activator, such as those described herein, and
optionally
devices for contacting cells with the agents. Devices include syringes, stents
and
other devices for introducing a compound into a subject or applying it to the
skin of
a subject.
Further, a kit may also contain components for measuring a factor, e.g.,
described above, such as the activity of sirtuin proteins, e.g., in tissue
samples.
Other kits include kits for diagnosing the likelihood of having or developing
a metabolic disorder, such as obesity or diabetes, or secondary conditions
thereof. A
kit may comprise an agent for measuring the activity and or expression level
of a
sirtuin.
Kits for screening assays are also provided. Exemplary kits comprise one or
more agents for conducting a screening assay, such as a sirtuin or a
biologically
active portion thereof, or a cell or cell extract comprising such. Any of the
kits may
also comprise instructions for use.
The present description is further illustrated by the following examples,
which should not be construed as limiting in any way. The contents of all
cited
references (including literature references, issued patents, published patent
applications and GenBank Accession numbers as cited throughout this
application)
are hereby expressly incorporated by reference.
262
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
The practice of the present methods will employ, unless otherwise indicated,
conventional techniques of cell biology, cell culture, molecular biology,
transgenic
biology, microbiology, recombinant DNA, and immunology, which are witliin the
skill of the art. Such techniques are explained fully in the literature. See,
for
example, Molecular Cloning A Laboratory Manual, 2 d Ed., ed. by Sambrook,
Fritsch and Maniatis (Cold Spring Harbor Laboratory Press: 1989); DNA Cloning,
Volumes I and II (D. N. Glover ed., 1985); Oligonucleotide Syntliesis (M. J.
Gait
ed., 1984); Mullis et al. U.S. Patent No: 4,683,195; Nucleic Acid
Hybridization (B.
D. Hames & S. J. Higgins eds. 1984); Transcription And Translation (B. D.
Hames
& S. J. Higgins eds. 1984); Culture Of Animal Cells (R. I. Freshney, Alan R.
Liss,
Inc., 1987); Immobilized Cells And Enzymes (IRL Press, 1986); B. Perbal, A
Practical Guide To Molecular Cloning (1984); the treatise, Methods In
Enzymology
(Academic Press, Inc., N.Y.); Gene Transfer Vectors For Mammalian Cells (J. H.
Miller and M. P. Calos eds., 1987, Cold Spring Harbor Laboratory); Methods In
Enzymology, Vols. 154 and 155 (Wu et al. eds.), Immunochemical Methods In Cell
And Molecular Biology (Mayer and Walker, eds., Academic Press, London, 1987);
Handbook Of Experimental Immunology, Volumes I-IV (D. M. Weir and C. C.
Blackwell, eds., 1986); Manipulating the Mouse Embryo, (Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, N.Y., 1986).
EXEMPLIFICATION
The invention now being generally described, it will be more readily
understood by reference to the following examples which are included merely
for
purposes of illustration of certain aspects and embodiments of the present
invention,
and are not intended to limit the invention in any way.
EXAMPLE 1: Metabolic Activities of Sirtuirz Activators in a Diet Induced
Obesity
(DIO) Mouse Model
In order to define whether SIRT-1 activators protect against the development
of obesity and associated insulin-resistance, resveratrol is chronically
administered
(via food admix) to male C57BL6J mice that are subjected during 16 weeks to a
high fat diet. The mice undergo an extensive phenotypic and molecular analysis
to
263
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
define the regulatory pathways affected by Sirt-1 activation. See, for
example, the
results presented in Figures 17-21.
Resveratrol, as a food additive, has been shown to be well tolerated and does
not cause food aversion. In this long-term study, 50 male C57BL6J mice (5
weeks
of age) are analyzed during a period of 18 weeks. Five groups of 10 animals
are
assigned as follows:
1: chow diet
2: chow diet + resveratrol (200 nig/kg/day)
3: high fat diet
4: high fat diet + resveratrol (200 mg/kg/day)
5: high fat diet + resveratrol (400 mg/kg/day)
During the entire study, body weight and food intake are monitored twice
weekly.
During week 1, body composition is analyzed, for all groups, by dual energy
X-ray absorptiometry (dexascan).
During week 2, serum levels of glucose, triglycerides, cholesterol, HDL-C,
LDL-C and insulin are measured in all groups after a fasting period of 12 h
and mice
are then placed on the diets as indicated (Day 0).
During week 10, glucose tolerance is determined by subjecting all the
aniinals to an intraperitoneal glucose tolerance test (IPGTT). Animals are
fasted for
12 h prior to this test.
Nocturnal energy expenditure of groups 1, 3 and 5 (chow diet, high fat diet
and high fat diet 400 mg) is measured by indirect calorimetry.
During week 12, body weight composition is again analysed by dexascan for
all groups.
During week 13, circadian activity of groups 3, 4 and 5 (high fat diet fed
mice) is studied during a period of 30 h.
During week 14, measurement of blood pressure and heart rate is performed
on groups 3, 4 and 5.
During week 15, rectal temperature of all animals is measured at room
temperature at 10:00 am.
A circadian activity measurement is performed on groups 1, 2 and 3.
264
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
During week 16, glucose tolerance is analysed by performing an oral glucose
tolerance test (OGTT) on a subset of animals (n=5) of groups 3, 4 and 5, and
an
intraperitoneal insulin sensitivity test (IPIST) on another subset of animals
(n=5).
During these experiments, blood is also collected to analyze insulin levels.
Animals
are fasted 12 h prior these tests.
Feces are collected in all groups over a 24 h time period and fecal lipids
content are measured.
During week 17, serum levels of resveratrol are measured on a subset of
mice (n=5) at 7:00 ain which corresponds to the beginning of the light cycle
and on
another subset of mice (n=5) three hours later (10:00 am). Moreover, thyroid
hormone T3 levels are measured in the blood collected at 7:00 am and plasma
lipoproteins levels are measured in the blood collected at 10:00 am.
During week 18, a cold test is performed on all animals by measuring body
temperature of animals exposed to 4 C.
Three days later, animals are sacrified.
At sacrifice, blood is collected and analyzed for: plasma lipids (TC, TG,
HDL-C, FFAs); liver functions (ALAT, ASAT, alkaline Pase, y-GT); and glucose
and insulin lipoprotein profiles of selected groups of plasma (size-exclusion
chomatography).
Liver, small intestine, adipose tissues (WAT and BAT), pancreas, heart and
muscle are collected and weighed. These can be analyzed by standard histology
(HE staining, succinate dehydrogenase staining, oil-red-O staining and cell
morphology); for tissue lipid content; and by electron microscopy on BAT and
muscle to analyze mitochondria. RNA isolation can be conducted for expression
studies of selected genes involved in metabolism and energy homeostasis by
quantitative RT-PCR. Microarray experiments can also be performed on selected
tissues. In addition, protein extraction can be performed for the study of
changes in
protein level and post-translational modifications such as acetylation of
proteins of
interest (e.g. PGC-la).
Methods
Aninaal housing and handling. Mice are group housed (5 animals / cage) in
specific pathogen-free conditions with a 12 h: 12 h (on at 7:00) light-dark
cycle, in a
265
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
temperature (20-22 C) and humidity controlled vivarium, according to the
European
Community specifications. Animals are'allowed free access to water and food.
Drinking water. Chemical composition of the tap water is regularly analyzed
to verify the absence of potential toxic substances at the Institut
d'Hydrologie, ULP,
Strasbourg. Drinking water is treated with HCI and HC1O4 to maintain pH
between 5
and 5.5 and chlorin concentration between 5 and 6 ppm.
Diet. The standard rodent chow diet is obtained from UAR and the high fat
diet is obtained from Research Diet. Mice are fed, either with chow diet (16%
protein, 3% fat, 5% fiber, 5% ash) or with high fat diet (26,2% protein, 26,3%
carbohydrate, 34,9% fat). Resveratrol is mixed with either powdered chow diet
or
powdered high fat diet and pellets are reconstituted. Control groups receive
pellets
as provided by the company. Due to the consistency of the high fat diet, it is
not
necessary to add water to mix it with the resveratrol. In case of the chow,
which is
harder to reconstitute, a minimal amount of water is added to the powder to
reconstitute pellets, which are then air-dried. New batches of food are
prepared
weekly.
Blood collection. Blood is collected either from the retro-orbital sinus or
from the tail vein.
Anesthesia. For the dexa scanning experiment, animals are anesthesized with
a mixture of ketamine (200 mg/kg) / Xylasine (10 mg/kg) administred by intra-
peritoneal injection.
Bioc/zeinistry
Tests are performed with an Olympus AU-400 automated laboratory work
station using commercial reagents (Olympus).
Analysis of lipids and lipoproteins. Serum triglycerides, total and HDL
cholesterol are determined by enzymatic assays. Serum HDL cholesterol content
is
determined after precipitation of apo B-containing lipoproteins with
phosphotungstic
acid/Mg (Roche Diagnostics, Mannheim, Germany). Free fatty acids level is
determined with a kit from Wako (Neuss, Germany) as specified by the provider.
Metabolic and endocrine exploration. Blood glucose concentration is
measured by a Precision Q.I.D analyzer (Medisense system), using Medisense
Precis
electrodes (Abbot Laboratories, Medisense products, Bedford, USA). This method
266
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
has been validated, by comparing Precision Q.I.D analyzer values with
classical
glucose measurements. The Precision Q.I.D method was chosen since it requires
a
minimal amount of blood and can hence be employed for multiple measurements
such as during an IPGTT. Plasma insulin (Crystal Chem, Chicago, IL) is
determined by ELISA according to the manufacturer's specifications. Plasma
level
of T3 is determined by standard radio-immunoassays (RIA) according to the
protocol specified by the providers.
Metabolic Testittg
Lipoprotein profiles. Lipoprotein profiles are obtained by fast protein liquid
chromatography, allowing separation of the three major lipoprotein classes
VLDL,
LDL, and HDL.
Intraperitoneal glucose tolerance test - Oral glucose tolerance test. IPGTT
and OGTT are performed in mice which are fasted overnight (12 h). Mice are
either
injected intraperitoneally (IPGTT) or orally gavaged (OGTT) with a solution of
20
% glucose in sterile saline (0.9% NaCI) at a dose of 2g glucose/kg body
weight.
Blood is collected from the tail vein, for glucose and insulin monitoring,
prior to and
at 15, 30, 45, 75, 90, 120, 150, 180 min after administration of the glucose
solution.
The incremental area of the glucose curve is calculated as a measure of
insulin
sensitivity, whereas the corresponding insulin levels indicate insulin
secretory
reserves.
Intraperitoneal insulin sensitivity test. Fasted animals are submitted to an
IP
injection of regular porcine insulin (0.5-1.0 IU/kg; Lilly, Indianapolis, IN).
Blood is
collected at 0, 15, 30, 45, 60, and 90 min after injection and glucose
analyzed as
described above. Insulin sensitivity is measured as the slope of the fall in
glucose
over time after injection of insulin.
Energy expenditure. Energy expenditure is evaluated through indirect
calorimetry by measuring oxygen consumption with the Oxymax apparatus
(Columbus Instruments, Columbus, OH) during 12 h. This system consists of an
open circuit with air coming in and out of plastic cages (one mouse per cage).
Animals are allowed free access to food and water. A very precise COa and 02
sensor measures the difference in OZ and CO2 concentrations in both air
volumes,
which gives the amount of oxygen consumed in a period of time given that the
air
267
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
flow of air coming in the cage is constant. The data coming out of the
apparatus are
processed in a connected computer, analyzed, and shown in an exportable Excel
file.
The values are expressed as ml'kg'1'h-1, which is commonly known as the V02.
Determination of body fat content by Dexa scanning. The Dexa analyses are
performed by the ultra high resolution PIXIMUS Series Densitometer (0.18 x
0.18
mm pixels, GE Medical Systems, Madison, WI, USA). Bone mineral density (BMD
in g/cm2) and body composition are determined by using the PIXIMUS software
(version 1.4x, GE Medical Systems).
Non-invasive Blood Pressure and heart Rate measurenzents
The Visitech BP-2000 Blood Pressure Analysis System is a computer-
automated tail cuff system that is used for taking multiple measurements on 4
awake
mice simultaneously without operator intervention. The mice are contained in
individual dark chambers on a heated platform with their tails threaded
through a tail
cuff The system measures blood pressure by determining the cuff pressure at
which
the blood flow to the tail is eliminated. A photoelectric sensor detects the
specimen's
pulse. The system generates results that Applicants have shown correspond
closely
with the mean intra-arterial pressure measured simultaneously in the carotid
artery.
This allows obtaining reproducible values of systolic blood pressure and heart
beat
rate. This requires training of the animals for one week in the system.
Circadian Activity
Spontaneous locomotor activity is measured using individual boxes, each
composed with a sliding floor, a detachable cage, and equipped with infra-red
captors allowing measurement of ambulatory locomotor activity and rears. Boxes
are linked to a computer using an electronic interface (Imetronic, Pessac,
France).
Mice are tested for 32 h in order to measure habituation to the apparatus as
well as
nocturnal and diurnal activities. The quantity of water consumed is measured
during
the test period using an automated lickometer.
EXAMPLE 2: Metabolic Activities of Sirtuin Activators in a Zucker Diabetic Rat
Model
Resveratrol (200 mg/kg), metformin (200 mg/kg), the combination (200
mg/kg each), or vehicle (2% Tween 80, 10 ml/kg) were administered orally twice
a
day (total dose 400 mg/kg/day) for 42 days to Zucker diabetic fatty rats
268
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
(ZOF/Gmicrl falfa). Groups of 8 rats were used for each group (6 weeks old,
190
grams). Animals were fasted for 24 hours prior to Oral Glucose Tolerance Test
on day 43 (2 g/kg glucose load, PO). Blood samples were collected from the
retro-
orbital sinus 35 minutes before glucose load (Fasting glucose) and 90 minutes
post
5 oral glucose load. Serum glucose levels were determined by means of a
Hitachi
Model 750 Automatic Analyzer. The results of this experiment are shown in
Figure
23. Daily body weights and food intake over the same 43 days demonstrated no
statistical difference between the four groups. In addition, no difference in
fasting
serum glucose levels (day 8, 15, 22, 29, 36 and 43) was seen between the four
10 groups.
EXAMPLE 3: Biochemical and Histological Analysis in a Diet Induced Obesity
(DIO) Mouse Model
Diet induced obesity was established in mice as described above in Example
1. Biochemical and histological analyses were conducted on mice fed with a
control
diet (C), high fat diet (HF), or high fat diet plus 400 mg/kg/day resveratrol
(HF +
R400) (see Example 1).
Figure 24 shows the results of body weight evolution experiments, food
intake experiments and body fat content experiment which were conduced as
described above in Example 1. Animals were niaintained on a control diet (C),
control diet plus 400 mg/kg/day resveratrol (C + R400), high fat diet (HF) or
high
fat diet plus 400 mg/kg/day resveratrol (HF + R400) diets for a 9 week period.
The
top left panel shows a graph of body weight evolution for mice in the four
dietary
groups over a nine week period. The top right panel shows a graph of food
intake of
mice in the four dietary groups expressed as kcal per 24 h. The bottom panels
show
comparisons of body fat content, as analyzed by dexa scanning, at week 9 of
treatment for mice in the four dietary groups. Values are represented as the
mean ~
SEM (n=10). BAT is brown adipose tissue (bottom right panel); Inguinal WAT is
inguinal white adipose tissue (bottom left panel); and Retroperitoneal WAT is
retroperitoneal white adipose tissue (bottom middle panel). Significant
differences
are indicated (p value).
Figure 25 shows the results of serum biochemical analysis of animals
following 16 weeks on a control (C), high fat (HF) or high fat plus 400
mg/kg/day
269
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
reservatrol (HF + R400). The values shown are based on an average of
measurements from 10 animals for each dietary group. Levels of total
cholesterol,
HDL-cholestrol, LDL-cholesterol, triglycerides, free fatty acids, aspartate
aminotransferase (ALAT), alanine aminotransferase (ALAT), and alkaline
phosphate (ALP) were determined using standard procedures. ASAT, ALP and
ALAT were measured by kinetic UV and colour tests using methods based on the
recommendations of the 'International Federation for Clinical Chemistry'
(IFCC) on
an Olympus AU-400 automated laboratory work station. AST was quantified using
the OSR6109 reagent system which is based on the activity of AST that
catalyzes
the transamination of aspartate and 2-oxoglutarate to L-glutamate and
oxalacetate.
The subsequent reduction of oxalacetate to L-malate by malate dehydrogenase
results in the conversion of NADH to NAD. The decrease in absorbance due to
the
consumption of NADH is measured at 340 nM and is proportional to the AST
activity in the sample. ALT was quantified using the OSR6107 reagent system
which is based on the activity of ALT that transfers the amino group from
alanine to
2-oxoglutarate to form pyruvate and glutamate. The pyruvate is then reacted
upon by
lactate dehydrogenase which results in the conversion of NADH to NAD. As with
the AST measurement, consumption of NADH is measured at 340 nM and is
proportional to the amount of ALT activity in the sample. ALP is measured by
determining the rate of conversion of p-nitro-phenyl phosphate to p-
nitrophenol
(pNP). The rate of change in absorbance due to the formation of pNP is
measured
bichromatically at 410/480 nM and is directly proportional to the amount of
ALP
activity in the sample. Values of all serum biochemical markers fell within
the
normal range for each dietary group.
Figures 26 and 27 shows hematoxylin and eosin staining of liver, epididymal
white adipose tissue (WAT), brown adipose tissue (BAT), and gastrocnemius
muscle sections of animals following 16 weeks on control (C), high fat (HF),
or high
fat plus 400 mg/kg/day resveratrol (HF + R400) diets. After collection,
tissues were
fixed in 4% paraformaldehyde, processed and embedded in paraffin prior to
sectioning (10 microns) and staining. Tissue processing, paraffin embedding,
tissue
sectioning and hematoxylin and eosin staining of histological sections were
carried
out using standard procedures and commercially available materials (see e.g.,
270
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
McManus J.F.A. and Mowry, R.W., Staining Methods. Histologic and
Histochenicial, Harper and Row, New York 1960; Luna L.G., Hitopathological
Methods and Color Atlas of Special Stains and Tissue Artifacts, Johnson
Printers,
Downers Grove, IL 1992; Gabe M., Techniques histologiques. Masson, Paris 1968;
and world wide web at statlab.com). As shown in the figures, few histological
changes were observed in any of the tissues between the various dietary
groups.
Figure 28 shows succinate dehydrogenase staining of brown adipose tissue
and muscle tissue (soleus and gastrocnemius) from mice following 16 weeks of
high
fat (HF) or high fat plus 400 mg/kg/day resveratrol (HF + R400) diets.
Succinate
dehydrogenase is a marker of mitochondrial activity and produces a dark stain
in the
photos. Tissues were collected and immediately frozen in methylbutane, then
kept
at -80 C prior to sectioning and staining. Succinate Dehydrogenase staining
was
carried out using standard procedures and commercially available reagents (see
e.g.,
Reichmann H and Wildenauer D, Histochemistry, 96: 251-3 (1991)). As shown in
Figure 28, succinate dehydrogenase staining was significantly higher for the
mice
receiving the HF + R400 diet in the brown adipose tissue and gastrocnemius
tissues
indicating the mitochondrial activity was higher in these tissues following
administration of resveratrol. In contrast, little change was observed in the
succinate
dehydrogenase staining in the soleus tissue for mice on the HF and HF + R400
diets.
Figure 29 shows transmission electron microscopy of gastrocnemius muscle
(non-oxidative fibers) of mice following 16 weeks on control (C), high fat
(HF) or
high fat plus 400 mg/kg/day resveratrol (HF + R400) diets at 10,000 and 20,000
fold
magnification. Transmission electron microscopy was carried out using standard
techniques as described below. The mitochondria can be seen as the darker
oblong
Z lines crossing the I lines. As shown in the figure, the mice fed with the HF
+
R400 diet display more mitochondria than the mice fed the control or high fat
diets.
Figure 30 shows transmission electron microscopy of brown adipose tissue
of mice following 16 weeks on control (C), high fat (HF), or high fat plus 400
mg/kd/day resveratrol (HF + R400) at 4,000 and 20,000 fold magnification.
Transmission electron microscopy was carried out using standard techniques as
described below. Fat droplets may be observed at either magnification as white
or
light gray droplets and mitochondria can be observed at the higher
magnification as
271
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
roundish striated structures. Animals fed the high fat diet plus resveratrol
had
smaller fat droplets (top panel) and more mitochondria (bottom panel).
Transrraission Electronic Micr=oscopy/Preparation of Samples: The biopsies
of gastrocnemius muscle and brown adipose tissue were cut in pieces of 1 mm
and
fixed immediately after collection in Karnovsky fixative (glutaraldehyde in
cacodylate buffer) and kept at 4 C without time limitation. The second step is
the
post-fixation with 1% osmium tetraoxide in 0.1M cacodylate buffer for lh at 4
C.
Tissues were then dehydrated through successive baths of graded alcohol
followed
by a propylene oxide bath, and then a treatment with a propylene oxide and
resine
mix before to be embedded in a pu're epoxy resine (araldite, Epon 812) which
becomes solid after 48h at 60 C. Semithin sections were cut at 2 m and
stained
with toluidine blue, and histologically analysed by light microscopy.
Ultrathin
sections were cut at 70 nm and contrasted with uranyl acetate and lead
citrate, and
examined with a Philips 208 electron microscope.
Figure 31 shows Sirtl mRNA levels measured in brown adipose tissue, liver
and muscle of mice treated with control (C), high fat (HF), or high fat plus
400
mg/kg/day resveratrol (HF + R400) diets. The values shown are based on the
average values from 6 animals for each dietary group. Values are expressed
relative
to housekeeping gene 18s and then expressed relative to chow diet (arbitrarily
equal
to 1). Relative gene expression was performed by real-time quantitative PCR
using
Sybrgreen incorporation (Lightcycler , Roche Applied Science, Indianapolis,
IN).
Levels of protein expression and protein activity may also be determined.
Protein
expression level is determined by separating liver nuclear extracts by SDS-
polyacrylamide gel electrophoresis and then immunoblotting is performed using
a
primary antibody specific for Sirtl (rabbit IgG anti-Sir2, Upstate
Biotechnology,
Lake Placid, NY). Determination of Sirtl activity is performed in liver
nuclear
extracts that are immunoprecipitated with an anti-PGCIa antibody (PGC1 H300:sc-
13063, Santa Cruz Biotecnology, Inc., Santa Cruz, CA) followed by separation
by
SDS-PAGE and immunoblotting with an anti-acetylated lysine antibody (Cell
Signaling Technology, Inc., Beverly, MA).
Figure 32 shows relative gene expression of phosphenolpyruvate
carboxykinase (PEPCK), glucose-6-phosphate (G6Pase), Foxo 1, PGC 1-alpha
272
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
(peroxisome proliferative activated receptor, gamma, coactivator 1, alpha),
and Sirtl
from liver, uncoupling protein 1(UCP1), acyl-CoA oxidase (ACO), Foxol, PGC1-
alpha, and Sirtl from brown adipose tissue (BAT), and uncoupling protein 3
(UCP3), muscle-type carnitine palmitoyltransferase (mCPT), Foxol, PGC1-alpha,
and Sirtl from muscle. Relative gene expression was performed by real-time
quantitative PCR using Sybrgreen incorporation (Lightcycler , Roche Applied
Science, Indianapolis, IN).
Figure 33 shows the results of an immunoblot which demonstrates that
resveratrol increases PGC1 alpha deacetylation. Nuclear extracts were prepared
from gastrocnemius muscle from individual mice fed either a high fat diet (HF)
or
high fat diet with 400 mg/kg resveratrol (HF+R400) for 15 weeks. Following
immunoprecipitation with a PGClalpha antibody (Santa Cruz Biotechnology, cat
#SC-13067), secondary western blots were probed with either a acetylated
lysine
specific monoclonal (Cell Signaling Technology, Cat #9441; top left panel) or
a
PGClalpha antibody (Santa Cruz Biotechnology, cat #SC-13067; lower left
panel).
Exposures were scanned and the acetylation status of PGC lalpha compared to
total
PGC 1 alpha of animals on either the HF or HF+R400 is shown in the right hand
panel.
EXAMPLE 4: Analysis o, f'Fat Absorption in a Diet Induced Obesity (Dio) Mouse
Model
The fecal lipid content of mice fed diets of chow (C), high fat (HF) or high
fat plus 400 mg/kg resveratrol (HF + R400) as described above was determined
to
investigate fat abosorption in the mice on the different dietary protocols. To
conduct
the analysis of fecal lipid content, mice are placed in metabolic cages
consisting of a
metal floor grid in place of mouse bedding. Food intake during a 24 h period
is
monitored and feces are collected in parallel to determine fat balance. Feces
are
dried in a vacuum oven at 70 C and then carefully cleaned free of
contaminating
mouse bedding and/or food bits. Lipids are extracted from 100 mg aliquots
using
chloroform/methanol (2:1, v/v) for 30 min at 60 C under constant agitation.
Samples
are cooled and then filtered through a Whatman No. 1 filter into a glass tube.
An
additional volume of chloroform/methanol is added and the sample is back-
extracted
by adding water and mixed well by vortexing. Phase separation is induced by
low
273
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
speed centrifugation and then the lower chloroform phase is transferred to a
new
tube. The sample is then evaporated to dryness and initially resuspended in
chloroform/triton and then finally water. Fat extracts are partitioned
according to
total cholesterol (Biomerieux, enzymatic colour test CHOD-PAP) and
triglyceride
(Biomerieux, enzymatic colour test, GPO-PAP) content using enzymatic kit
assays
and manufacture provided protocols. Data is expressed as the amount of lipid
per
total amount of fecal weight. The results of the fecal lipid content analysis
are
presented in Figure 34.
EXAMPLE 5: Analysis of Endurance and Fat Absorption in a Diet Induced
Obesity (Dio) Mouse Model
A second group of animals were subjected to a diet induced obesity study
involving 16 weeks of a high fat diet as described above in Example 1. The
animals
in this study were divided into the following four dietary groups:
1: chow diet
2: chow diet + resveratrol (400 mg/kg/day)
3: high fat diet
4: high fat diet + resveratrol (400 mg/kg/day).
Body weight analyses for mice in all four dietary categories over a 16 week
period are presented in Figure 35.
After 14-15 weeks on the indicated diet, the mice were subjected to an
endurance study. A standard method for assessing exercise performance uses the
treadmill, a system composed of a variable speed belt, enclosed in a
plexiglass
chamber, with a stimulus device consisting of a metal shock grid attached to
the rear
of the belt. Initially animals are acclimatized to the treadmill by using a
habituation
protocol on the day preceding the running test. With this procedure mice are
placed
in the chamber and run at 27 cm/s for 10 minutes with a 5 incline. For the
actual
running test, two incremental exercise protocols are used, one for high fat
fed
animals and one for chow. For chow animals, the experiment starts at 25cm/s
and a
5 incline and then increases in speed and incline are adjusted according to
the
outline in Figure 36. For the high fat fed animals which generally weigh more
and
perform less easily, the beginning speed is 18 cm/s with a 0 incline and then
increased according to Figure 36. The distance run and the number of shocks
274
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
obtained over 5 minute intervals are recorded. A mouse is considered exhausted
and
removed from the experiment when it receives approximately 100 shocks (at 2 mA
each shock) in a period of five minutes. The duration of running and the total
distance covered evaluates the performance of the mice (Figure 37). All mice
were
fasted for 2 hours prior to running; the habituation protocol is performed in
the
afternoon and the running experiment the following morning.
EXAMPLE 6: Effect of'Resveratrol on Insulin Resistaace
The current gold standard method for measuring insulin resistance is the
euglycemic clamp. In this method glucose is "clamped" at a predetermined value
(5
mmol/L for euglycaemia) by titrating a variable-rate of glucose (glucose
infusion
rate: GIR) against a fixed-infusion rate of insulin. Two to three days in
advance of
the study, a catheter is established in the femoral vein, under anesthesia
(ketamine
and xylazine), with the catheter fed underneath the mouse's skin and affixed
behind
their head. After surgery, mice are housed individually and allowed to recover
for
at least 48 hours, preferably enough time for them to regain their body
weight. The
clamps are performed in awake, unrestrained, unstressed and light-cycle
inverted
mice following a 5 hour fast. Mice are acclimatized (1 hour) to the tops of
cages
while their catheter is attached to a syringe-infusion pump. The catheter from
the
mouse is bifurcated to allow for simultaneous constant and variable injection
of
insulin and glucose, respectively. Base-line glucose values are measured by
tail
vein sampling prior to the injection of insulin. Catheter placement is
assessed with a
short priming dose (6 l/min, 1 min) of insulin prior to the constant infusion
of
insulin at a flow rate of 2 l/min equivalent to 18mU of insulin/kg/min. Blood
glucose values are monitored every 5 minutes throughout the test and within 15
minutes blood glucose is lowered and glucose infusion (20% solution in saline)
can
be started. The glucose infusion rate (GIR) is varied until euglycemia (4:15%)
has
been reached and maintained. At this point the animal is "clamped" and the
degree
of insulin resistance is inversely related to the amount of glucose necessary
to
maintain the required blood glucose concentrations. The GIR (mg glucose /kg
animal *min) is then calculated as an average during the last 60 minutes of
the
clamp. When the average GIR of one animal is greater than another, it
indicates
275
CA 02613141 2007-12-20
WO 2007/008548 PCT/US2006/026272
better insulin sensitivity or that the clearance of glucose from the plasma is
much
faster.
Figure 38 shows the effect of resveratrol on insulin sensitivity as measured
by hyperinsulinemic (18mU/kg/min) euglycemic (5.5mmol/1) clamp. The left hand
panel shows glucose infusion rates (GIR) for groups of animals following 14
weeks
on either a control diet (C), control diet plus 400 mg/kg resveratrol
(C+R400), high
fat diet (HF) or high fat diet plus 400 mg/kg resvertrol (HF+R400). The right
hand
panel shows average GIR at steady state clamp.
EQUIVALENTS
The present invention provides among other things sirtuin-activating
compounds and methods of use thereof. While specific embodiments of the
subject
invention have been discussed, the above specification is illustrative and not
restrictive. Many variations of the invention will become apparent to those
skilled
in the art upon review of this specification. The full scope of the invention
should
be determined by reference to the claims, along with their full scope of
equivalents,
and the specification, along with such variations.
INCORPORATION BY REFERENCE
All publications and patents mentioned herein, including those items listed
below, are hereby incorporated by reference in their entirety as if each
individual
publication or patent was specifically and individually indicated to be
incorporated
by reference. In case of conflict, the present application, including any
definitions
herein, will control.
Also incorporated by reference in their entirety are any polynucleotide and
polypeptide sequences which reference an accession number correlating to an
entry
in a public database, such as those maintained by The Institute for Genomic
Research (TIGR) (www.tigr.org) and/or the National Center for Biotechnology
Information (NCBI) (www.ncbi.nlm.nih.gov).
Also incorporated by reference are the following: PCT Publications WO
2005/002672; 2005/002555; and 2004/016726.
276