Note: Descriptions are shown in the official language in which they were submitted.
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
SYSTEMS AND METHODS FOR ORGANIC MATERIAL CONVERSION AND USE
CROSS REFERENCES TO RELATED APPLICATIONS
[0001] The present application is a continuation-in-part of U.S. Patent
Application Serial No.
11/425,347 filed June 20, 2006 (which claims the benefit under 35 U.S.C. 119
of U.S.
Provisional Patent Application Serial No. 60/692,099 filed June 20, 2005)
which is a
continuation-in-part of U.S. Patent Application Serial No. 11/379,404, filed
April 20, 2006 (which
claims the benefit, under 35 U.S.C. 119 of 60/675,511, filed April 27,
2005). The present
application also claims the benefit under 35 U.S.C. 119 of U.S. Provisional
Patent Application
Serial No. 60/695,608, filed June 30, 2005. The contents of all these
applications are
incorporated herein in their entirety by reference.
FIELD OF THE INVENTION
[0002] The present invention relates to the thermal conversion of sludge and
other
organic/carbonaceous materials into energy and other products.
BACKGROUND OF THE INVENTION
[0003] Industrial and municipal wastewater treatment plants produce
significant amounts of
sludge, a material comprised of water, organic material (such as proteins,
lipids and
carbohydrates), and inorganic materials (such as clay and grit) that have not
been eliminated
during the treatment process. While most facilities have some form of onsite
sludge treatment in
order to reduce the volume and volatility of sludge, the final sludge product
must ultimately be
removed from the treatment plant for disposal.
[0004] In some cases, sludge is dewatered and dried to reduce the size and
weight required
for transport and disposal. In other cases, sludge is removed from the
treatment plant in liquid
form. In rare cases, facilities may utilize onsite incineration for final
sludge disposal.
[00051 Because disposal at sea was banned several years ago, today's most
common
methods of final disposal for non-incinerated sludge have been land
application and landfill. In
land applications, sludge is sprayed or spread as a fertilizer on nonfood-crop
agricultural fields.
In landfill applications, sludge is simply buried, often alongside traditional
municipal solid wastes.
[0006] All of the above sludge disposal scenarios contain significant
environmental risks. For
example, despite containing valuable plant nutrients such as phosphorus and
nitrogen, sludge
can also contain high levels of heavy metals and pathogens. The presence of
these hazardous
materials/substances and their potential concentration in agriculture fields
over time, have made
land application less desirable in recent years. Similarly these same
contaminants can escape
1
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
into groundwater near landfills and into the air via incinerator emissions.
Given these issues, it
is clear that there have historically been few environmentally safe methods
for sludge disposal.
[0007] In recent years, new thermal processing technologies such as
gasification and starved
air incineration have emerged as viable sludge disposal options. These
processes not only
meet the primary goal of eliminating sludge, but they also do so in a way that
converts much of
the energy found in sludge into methane rich gasses. These gasses, in turn,
can be used to
create steam or heat for the generation of electrical power. Unfortunately,
the gasses produced
using these technologies are generally not condensable and have a relatively
low energy
content. They therefore cannot easily be stored and must be consumed as soon
as they are
created. This poses challenges when used for electrical generation because
electricity demand
falls at different points during a typical 24 hour period. During these low
demand times, the
gases cannot be used to provide additional electricity to the grid and must be
flared to the
atmosphere creating airborne pollution and generally wasting a valuable source
of energy.
[0008] A more efficient form of sludge conversion involves the oxygen free
thermal process
known as pyrolysis. In pyrolysis, sludge material can be heated under high
pressure or ambient
pressure to form a gas that contains vaporized oils. Liquid oil can then be
condensed from the
gas in a process that is energy self-sufficient. In fact, the condensed oil is
excess energy in a
form that can be stored and transported for use at a later date. This process
therefore provides
at least two beneficial outcomes - economical sludge disposal and net energy
generation in a
form (e.g., liquid oil) that can be stored and transported as desired.
[0009] U.S. Patent Nos. 4,618,735 and 4,781,796 describe a pyrolysis process
and apparatus
for the conversion of organic sludge into materials that may be useful as
industrial fuels,
including liquid oils. This process involves heating the sludge in an oxygen
free environment to
induce volatilization of the organic material contained therein, resulting in
an energy rich
gaseous byproduct and sludge residue. In another phase of the process, the
gasses are further
contacted with the residue at even higher temperatures to create oil producing
reactions and
gaseous products containing the oil products. The oil products are then
condensed from the
gasses in a separate phase of the process and may be stored and used as an
industrial fuel. As
described in these patents, char, the final solid form of sludge residue, is
also removed from the
process as a more easily disposed of material. The process described in these
applications is
known as a "single reactor" system.
[0010] In U.S. Patent Nos. 5,847,248 and 5,865,956 a new process and apparatus
that are
based upon U.S. Patent Nos. 4,618,735 and 4,781,796 are described. This
updated process
and apparatus incorporate a second reactor designed to improve the quality of
the final oil
through reductive, heterogenic, catalytic gas/solid phase reactions. This
process and apparatus
also include the addition of a new screw conveyor to remove char and solids
from the second
2
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
reactor, convey it through a cooling device, and ultimately discharge it from
the process. The
overall process described in these two patents is commonly referred to as a
"dual reactor"
system.
[0011] International Patent Application PCT/AUOO/00206 ("the'206 application")
describes a
simplified version of the process and apparatus described in U.S. Patent Nos.
5,847,248 and
5,865,956 that could allow for more cost-effective operation. The updated
design incorporated a
catalytic converter to receive gasses from the first reactor. These gasses
were subsequently
condensed to produce reaction water and an oil product. Detailed descriptions
of the catalytic
converter temperatures and catalysts, and their effect on the formation or
destruction of several
gaseous compounds are outlined in the '206 application. This process and
apparatus are
commonly known as "catalytic converter" systems.
[0012] Finally, International Patent Application PCT/AU2003/001099 ("the '099
application")
describes a process and apparatus based upon the prior art described above. In
this process
and apparatus, features were incorporated to closely control the Solids
Retention Time (SRT)
and thus the resulting Weight Hour Space Velocity (WHSV) - a parameter
directly related to the
viscosity and overall quality of the final oil product.
[0013] In versions of the processes and apparatuses prior to the '099
application, sludge was
positively conveyed through reaction zone(s) using screw conveyors. The speed
of material
conveyance, and thus the overall retention time of the solids in the reaction
zone, was
dependent upon the speed and pitch of these conveyors. However, for the best
overall reaction
producing the highest quantity and quality of oil, the sludge/char had to
remain in the reaction
zone for a relatively long period of time. This forced operators to operate
the conveyors at very
slow speeds. At such slow speeds, the heat and mass transfer within the
reactor was
compromised due to the lack of a mixing action from the slow moving screws.
This design
hindered the overall reaction, causing less than optimal oil viscosity.
[0014] In an attempt to address this problem, the '099 application described a
process to
allow for a more precise control of the inventory of char in the reactor and
the WHSV. The
application further provided data demonstrating the oil viscosity is closely
tied to the WHSV
regardless of sludge type or reactor configuration (i.e., single or dual
reactor).
[0015] The first feature described in the '099 application involved the
replacement of screw
conveyors with a series of pitched paddles affixed to a central rotating shaft
in order to convey
material through the reactor. By altering the number of paddles, the angle at
which they
address the sludge/char bed, and the speed at which they rotate, it was
expected that operators
could more easily control the amount of time material was held in the reaction
zone. The
paddles were also intended to provide proper mixing of char and vapor as well
as enhanced
3
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
heat transfer. With these factors under greater control, operators were
expected to have much
greater control over the WHSV.
[0016] A great deal of detail is provided in the '099 application regarding
the position of
paddles on the shaft, paddle shape, paddle angle, shaft rotational speed
(RPM), paddle tip
speed, and other parameters. These elements of the paddle conveyance system
must all be
calculated and designed prior to building the reactor, and many are not
adjustable once the
reactor is put into service. This is a major limitation of the '099
application. It is very difficult to
predict precisely which combination of those factors will result in the best
overall process priorto
testing the apparatus. In fact, the prior approaches acknowledged the
difficulty in keeping
sludge from accumulating in certain areas of the reactor causing a torque
overload on the
rotating shaft and paddles.
[0017] Further, the '099 application described the overall reaction as
occurring in two
separate functional zones within the same reactor vessel in a single reactor
system - a heating
"zone" and a reaction "zone." The heating zone provided a heating rate of 5-30
C/minute to
induce volatilization and production of initial vapor and solid residue/char.
The reaction zone
was heated to a temperature of 400-450 C to promote vapor-phase catalytic
reactions through
further mixing and increased collision of the vapors and solid residues. This
is a limitation in that
it is very difficult to create and distinguish a heating zone and a reaction
zone in an open single
reactor chamber.
[0018] Additionally, the '099 application described the use of an adjustable
weir (or a fixed
weir if the desired WHSV is known prior to manufacture) mechanism to control
the inventory of
char within the reactor. The adjustable weir was described as being rotated
off center by
approximately 30 degrees to conform to the position of the char bed caused by
the paddle
rotation, and was located immediately before the char outlet. No description
was provided
regarding the maximum or minimum height of the weir or its specific design.
However, iterations
of the adjustable weir in use at the time of the '099 application did not
allow the reactor vessel to
be filled to a level greater than a 30% coefficient of fill - thus limiting
the overall inventory of
solid material in the process.
[0019] Another problem in prior designs that remains to be addressed is the
creation and
disposal of reaction water during the gas condensation phase of the process.
In known
processes, vapors from the reactor are condensed using common water and oil-
based direct
spray condensers. Direct spray condensation chamber temperatures would
routinely fall below
100 C (for example, without limitation, to about 35 C - 45 C), causing not
only the oil in the
vapor to condense but also any latent water vapor to condense into liquid
water. A separate
oil/water separation phase would then be required to separate clean oil from
the reaction water.
The reaction water would then return to the head works of the wastewater
treatment plant where
4
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
it could be combined with fresh influent and recycled through the entire
wastewater treatment
process.
[0020] A major limitation of this design is the quality of the water being
returned to the
treatment plant. Reaction water can be extremely high in nitrogen. Most
treatment facilities can
remove the relatively low levels of nitrogen found in typical municipal and
industrial influent
streams. When reaction water is added to the influent at the facility head
works, however, the
artificially high concentration of nitrogen can create substantial upsets in
the overall treatment
process leading to the discharge of sub-standard effluent water to local
rivers and streams.
Furthermore, if reaction water is not or cannot be returned to the head works,
it must be stored
onsite prior to other means of disposal. Storing the reaction water requires
the capacity of a
large wastewater treatment facility, which may not be obtainable or desirable
for smaller
operations. Further, because it is an extremely pungent material, the reaction
water also
requires storage in expensive leak-proof containers. Disposal of such water
can also be costly
and can release harmful gases into the air.
[0021] Another limitation of prior designs related to reaction water includes
the requirement
for a three-phase centrifugal separator to clean and separate the three
constituents in the final
condensed liquids (oil, particulate matter, and reaction water). If reaction
water is eliminated
from the process altogether, a much simpler two-phase centrifugal separator
could be used.
This advance would produce a key benefit because most centrifugal separators
rely upon
differences in material densities for proper separation. Many of the bio-oils
produced in the prior
processes, however, have very similar densities to the reaction water making
separation difficult
and time consuming.
[0022] A further limitation of prior approaches is related to the discharge of
char from the
reactor. In previous versions of pyrolysis processes and apparatuses, char was
viewed as a
waste product of the process and was passively transported from the end of the
reactor (via
gravity) through a vertical solid material outlet or "chute." This outlet was
sealed with a rotary
valve mechanism designed to keep vapor from the reactor from escaping along
with the char.
Over time, however, it has become clear that there are two major drawbacks to
a vertical chute
and rotary valve design. First, the char material has a tendency to get stuck
in the chute when
there is no active process to push it through. A plugged outlet chute requires
that the entire
reactor be shut down and manually cleaned. Second, the rotary valves currently
used have
proven inherently leaky, allowing vapors from the reaction chamber to escape
through the chute,
out of the valve and into the surrounding atmosphere. These vapors, like the
reaction water,
have an extremely pungent odor, making operation of the unit uncomfortable for
operators.
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
[0023] Based on the foregoing, there is room for improvement in pyrolysis
systems and
methods. The present invention provides numerous improvements addressing a
number of
described drawbacks inherent in prior approaches.
SUMMARY OF THE INVENTION
[0024] The present invention provides improved pyrolysis systems and methods
that address
a number of described drawbacks associated with the prior art. The present
invention also
forms char with a variety of beneficial uses such that char need no longer be
viewed as a waste
product of pyrolysis processes.
[0025] As stated, one major drawback of the prior art is the production of
reaction water due
to the presently used condensation methods and resulting need for three-phase
centrifugal
separation of oil, particulate matter and reaction water. The present
invention provides methods
to avoid the production of reaction water, thus requiring only a two-phase
centrifugal separation
of oil and particulate matter and avoiding inefficiencies and environmental
issues associated
with reaction water. The present invention avoids the production of reaction
water by
condensing oils at a temperature above that at which water will condense. In
one embodiment,
this benefit is achieved by condensing oil with other oil cooled enough to
condense additional oil
but not cooled enough to condense water. This advance removes the numerous
drawbacks
associated with the production of reaction water that currently exist in
presently used pyrolysis
methods.
[0026] An additional drawback of the prior art is that conveyance of sludge
through a reaction
chamber occurred so slowly that the material was not mixed sufficiently during
the process to
allow sufficient contact between the sludge and vapors in the reaction
chamber. International
Patent Application PCT/AU2003/001099 ("the'099 application") addressed this
issue by moving
sludge through a reaction chamber with paddles that mixed the sludge with its
surrounding
environment while simultaneously transporting it through the chamber. While
this approach
addressed the previous issue of insufficient mixing, it produced drawbacks of
its own including
the inability to independently control speed and amount of mixing with time of
sludge in the
reaction chamber and the need to calculate paddle parameters before the
reactor was put into
service (with an inability to readily adjust these parameters thereafter).
Further with this
approach, it was difficult to keep sludge from accumulating in certain areas
of the reactor
causing a torque overload on the rotating shaft and paddles. Thus, this
process required that
the entire system be shut down while manually actuated valves, screws, and
other moving parts
were adjusted and/or cleaned.
[0027] The present invention addresses these particular drawbacks by adopting
mixing
elements that can increase contact between sludge and vapors as the sludge
moves through a
6
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
reaction chamber (and becomes char) but do not convey the sludge/char material
through the
reaction chamber. By separating the function of conveying material through the
reaction
chamber and mixing the materials, various advantages are obtained including
the advantage
that operators can independently control time in the reaction chamber versus
amount of mixing
while in the chamber. This allows adjustment during sludge processing so that
batch processing
can be avoided. This approach also allows greater fill coefficients of the
reaction chamber
because, irregardless of the amount of mixing that occurs, the sludge and/or
char can remain in
the reactor chamberfor any desired period of time. Thus, separating time spent
in the chamber
from the amount of mixing can increase contact between the shell and contents
of the chamber
to facilitate efficient heat transfer and production of quality bio-oil and/or
chars. In one
embodiment, adjustments can also occur through automated controls while the
pyrolysis
process remains on-going. The present invention also provides numerous other
benefits over
prior art approaches that will become clear through the entirety of the
present disclosure.
[0028] The present invention also recognizes the numerous beneficial uses of
char produced
by pyrolysis processes. Accordingly, embodiments according to the present
invention can
include methods comprising (i) producing char using a pyrolysis process
wherein the char is
utilized for an industrial purpose or (ii) utilizing char produced through a
pyrolysis process for an
industrial purpose, wherein the pyrolysis process for both (i) and (ii)
utilizes a system comprising
a reactor module comprising a reaction chamber and a separation chamber;
wherein in the
reaction chamber sludge can be heated in an oxygen free state after which the
sludge becomes
vapor and char and wherein the separation chamber conveys the vapor and char
out of the
reactor module and wherein the system further comprises mixing elements within
the reactor
module that mix the sludge without substantially conveying the sludge through
the reactor
module.
[0029] In certain embodiments according to the present invention, the system
further
comprises an active sludge transport mechanism in the reactor module wherein
the mixing
elements and the active sludge transport mechanism can be independently
controlled.
[0030] In certain embodiments according to the present invention, the char
comprises a
Brunauer, Emmett and Teller (BET) surface area of between about 400 m2/g and
about 600
m2/g. In certain embodiments, the pyrolysis process can utilize waste heat. In
another
embodiment, during the pyrolysis process the char can be exposed to a
temperature of at least
about 550 C.
[0031] Industrial purposes according to the present invention can include,
without limitation,
the char being used as a pore generator in brick manufacturing, as a carbon
black substitute, or
both. In certain embodiments, before being utilized for an industrial purpose,
the char can be
converted to activated carbon. Activated carbon can be used for, without
limitation, the
7
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
absorption of metals; air purification; liquid purification, catalyst support;
decolorization of
beverages, sugar refining, deoderization, emergency poison treatment, solvent
recovery and
whiskey manufacturing.
[0032] Another embodiment according to the present invention includes a method
of
manufacturing bricks wherein the method comprises at least in part using char
produced as the
result of a pyrolysis process. In another embodiment, this method can comprise
adding the char
to raw materials used in brick manufacturing to form a mixture; drying the
mixture to reduce
moisture; and firing the mixture in a high temperature device wherein during
the firing the char
releases vapors creating stable micropores in the bricks thus improving the
insulation properties
of the manufactured bricks. In another embodiment, the drying step and/or the
firing step can
utilize waste heat. In another embodiment, the manufactured bricks comprise a
heat transfer
coefficient of less than about 0.27 W/m2/K.
BRIEF DESCRIPTION OF THE DRAWINGS
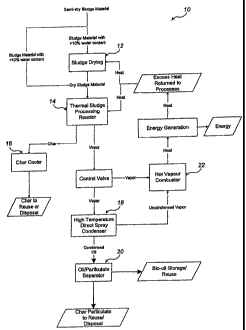
[0033] Figure 1 is a flow chart illustrating a process of conversion according
to the present
invention;
[0034] Figure 2 is a cross-sectional illustration of a converter system formed
according to the
present invention;
[0035] Figures 3A-3C are enlarged views of an overflow weir, a control valve,
and a char plug
screw, respectively, in a reactor module according to the present invention;
[0036] Figure 4 is an illustration of a condenser module according to the
present invention;
and
[0037] Figures 5A-5B are illustrations of a hot vapor combustion module
according to the
present invention.
DEFINITION OF TERMS
[0038] To aid in understanding the following detailed description of the
present invention, the
terms and phrases used herein shall have the following, non-limiting,
definitions.
[0039] As used herein, the term "sludge" includes any organic material that
can be converted
into an energy source at least in part or can be treated for disposal through
the use of heat. In
one embodiment, sludge includes sewage material from treatment plants, however
the present
invention is not so limited and can be used to treat any sort of organic
material that can benefit
in its conversion to energy, an energy source, an energy product, or a value-
added product such
as, without limitation, char.
8
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
[0040] As used herein, the term "oxygen free" means an atmosphere with an
oxygen
concentration that is too low to allow combustion or gasification of sludge.
[0041] As used herein, the term "purified" does not require absolute purity,
rather, it is used as
a relative term. Thus, a substance that is purified contains less contaminants
after going
through a process than it did before going through the process.
[0042] As used herein, the term "facility" includes any place, industrial or
otherwise, that
produces excess heat in a sufficient amount to contribute to the drying of
sludge. Facilities
include but are not limited to plants, factories and mills.
[0043] As used herein, the term "waste heat" includes heat generated from a
process wherein
the heat can be captured and directed. Thus, another appropriate term for the
presently
described waste heat could be "available heat."
[0044] As used herein, the term "industrial purpose" includes a use to which
char generated
through a pyrolysis process can be put. While purpose is preceded by the
modifier "industrial"
the purpose is not so limited and can include any use to which the char can be
put prior to its
disposal (note that some uses can remove the necessity of disposing of the
char altogether).
Non-limiting examples of industrial purposes in accordance with the present
invention include
converting char into activated carbon for use in, without limitation, the
absorption of metals; air
purification; liquid purification, catalyst support; decolorization of
beverages, sugar refining,
deoderization, emergency poison treatment, solvent recovery and whiskey
manufacturing; as
well as for use as a pore generator (in one example in brick manufacturing)
and as a carbon
black substitute.
DETAILED DESCRIPTION OF THE INVENTION
[0045] Industrial and municipal wastewater treatment plants produce
significant amounts of
sludge that must be properly treated for disposal. Thermal conversion
processes such as
pyrolysis can be used to convert sludge into bio-oil and char that can have a
wide variety of
commercial and industrial applications. However, prior approaches to these
processes have
suffered from many drawbacks. Some of these drawbacks include the creation of
reaction water
and the inability to independently control the mixing of sludge material
within a reaction chamber
and the time the sludge spends within the reaction chamber. This particular
drawback requires
that operation be conducted in batches whereby a batch of sludge material is
fully processed
before the quality of the resulting oil can be tested. This batch process is
time consuming and,
when adjustments are needed, requires that the entire pyrolysis process be
shut down while
manually actuated valves, screws, and other moving parts are adjusted.
Previous approaches
also encountered difficulties in obtaining quality char for industrial and/or
commercial
applications as well as in removing char from the reactor after processing.
Based on these
9
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
difficulties, char was viewed as a waste product of the pyrolysis process
requiring disposal. The
present invention addresses these and other drawbacks related to prior
pyrolysis systems and
methods and recognizes the commercial potentials of char produced by pyrolysis
methods.
[0046] One aspect of the present invention provides pyrolysis systems and
methods that do
not produce reaction water. This advance is significant because the production
of reaction
water causes various inefficiencies and environmental problems as is
understood by those of
ordinary skill in the art. The present invention can prevent the production of
reaction water by
condensing bio-oils at a temperature above that at which water vapor
condenses.
[0047] Other aspects according to the present invention allow operators to
more precisely
control the pyrolysis process, eliminating the need for batch processing, by
making the speed
through which sludge travels through a reactor module and the amount of mixing
that occurs
while therein independently controlled. The present invention allows for this
independent control
by separating the functions of moving sludge and mixing sludge to different
system components.
The present invention also allows more heat to be applied to material as it
goes through the
pyrolysis process. Additional heat (and longer exposure to the additional
heat) creates a char
that is more suitable for use as a precursor to activated carbon than chars
created using
previous pyrolysis methods. This benefit of the present invention is created
by providing
systems and methods that allow for a higher fill coefficient in the reactor
chambers according to
the present invention. A higher fill coefficient increases the available
surface area for conductive
heat transfer, thus allowing more heat to be applied and absorbed by the
system. Other
important features and advantages of the present invention will become
apparent through the
following detailed description.
1. Overview of Systems and Methods
[0048] Figure 1 depicts a flow chart of one method according to the present
invention. In this
depicted embodiment, sludge arrives at a system according to the present
invention. If the
arriving sludge has a water content of greater than about 20%, greater than
about 10% or
greater than about 5%, the sludge can enter a sludge drying module 12. If the
sludge is below a
pre-determined water content, the sludge can bypass sludge drying module 12.
Once sludge
has an acceptable water content, the sludge can enter a thermal reactor module
14. The
reactor module 14 has a reaction chamber (also called a conversion zone
herein) and a
separation chamber. Within the reactor module 14, sludge is heated and
processed to become
char and vapors. Within the separation chamber, the vapors and char are
separated. After
leaving the separation chamber, char can enter a char cooler module 16 and can
subsequently
be safely disposed of or put to a number of beneficial commercial uses. After
leaving the
separation chamber, vapors are funneled through control valves directly to one
or more of a
condenser module 18 or a combuster module 22. If funneled to the condenser
module 18,
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
vapors are condensed to form oils. These oils can be purified in an
oil/particulate separator
module 20 following condensation after which removed particulate can be safely
disposed of or
put to other beneficial commercial uses. The oils collected following
condensation and
separation can be stored for use at a later time. Uncondensed vapors from the
condenser
module 18 as well as vapors directly from the control valve can also be
funneled to a combuster
module 22. This combuster module 22 combusts the vapors to generate energy.
Generated
energy can be diverted for uses such as the generation of electrical power or
can be returned to
the pyrolysis process as heat used in a drying module 12 or reaction module
14. The following
description provides a more detailed explanation of embodiments according to
the present
invention.
II. Optional Drying
[0049] Figure 1 depicts one beneficial embodiment according to the present
invention. In this
Figure 1, the process 10 includes drying sludge in a dryer module (12 in
Figure 1; 32 in Figure
2) before the sludge's entrance into the reactor module 14. Generally, when
sludge does not
arrive de-watered, it can be beneficial to dry it before processing. Drying
sludge generally is
beneficial because a higher water content means that more energy must be
applied to a
reaction chamber within the reactor module 14 to heat and volatilize the
incoming material. As
s.uch, there can be great benefit in an integrated system that dries sludge to
a low water content
before it enters the thermal conversion process. In embodiments according to
the present
invention incorporating drying, sludge can be dried to a water content of less
than about 20%,
less than about 10%, or less than about 5% before entering the thermal
conversion process.
Dryers 12 (32) used in accordance with the present invention can be any
appropriate form of
commercial dryer including, without limitation, direct and indirect heated
drum dryers as well as
surface drum dryers. Drying can occur through, without limitation,
centrifugation or heating
provided by, for example, a source of existing waste heat or the combustion
modules presently
described. Drying mechanisms used in accordance with the present invention can
also entail
those described in co-pending U.S. Patent Application Serial No. 11/379,404,
filed April 20,
2006, of co-pending U.S. Patent Application Serial No. 11/425,347 filed June
20, 2006 and of
U.S. Provisional Patent Application Serial Number 60/695,608, filed June 30,
2005, the contents
all of which are incorporated by reference in their entirety herein.
III. Reaction Chamber Comprising One Conversion Zone
[0050] Referring to Figure 2, in accordance with the present invention, sludge
40 is heated in
an oxygen free reaction chamber 36 of a reaction module to produce vapors and
char. In one
embodiment, the reaction chamber 36 can have a single heating/reaction zone
for both heating
the incoming material and thermally converting the material. When the reaction
chambers of the
present invention comprise a combined and continuous heating and reaction
zone, this zone
11
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
can be collectively referred to as a conversion zone 38. Sludge 40 can enter
the reaction
chamber 36 through a sealed material inlet 42 and can be immediately heated to
a desired
reaction temperature. In one embodiment, a rotating horizontal shaft 44 can
extend the length
of the reactor module (or a subset of this length) and can contain one or more
mixing elements
46. The mixing elements 46 can rotate through the material bed 48 causing the
material to be
mixed and lifted into an upper portion 50 of the reaction chamber
36/conversion zone 38. Other
methods of mixing and forms of mixing elements can also be adopted so long as
the approach
increases vapor and solid contact above that which would otherwise occur
without such mixing.
Mixing and contact can promote vapor-phase catalytic reactions and
heterogenetic solid phase
vapor phase catalytic reactions which, along with temperatures used in
accordance with the
present invention can help to ensure that carbohydrates are nearly completely
converted to
graphite with a high active surface area, classifying the char as an
especially appropriate
precursor for activated carbon manufacturing. Importantly, when mixing of the
sludge occurs in
accordance with the present invention, the rate of mixing and the rate of
sludge movement
through the reaction chamber 36/conversion zone 38 can be independently
controlled. Thus,
the material can be mixed in the reaction chamber 36/conversion zone 38 to
promote vapor and
char contact, but the mixing mechanism has little to no effect on material
inventory and will not
actively convey material through the reactor. This aspect of the present
invention provides an
important advance over previous pyrolysis methods allowing further control and
adjustment of
the pyrolysis process.
[0051] In one embodiment according to the present invention sludge is moved
through the
reactor module using gravity as a passive means to convey materials within the
reactor module.
In another embodiment sludge can be actively transported through the reactor
module through a
number of different mechanisms including, without limitation, a conveyor belt.
Regardless of the
transport mechanism used, these embodiments can further comprise an adjustable
overflow
weir 52 at the end of the reaction chamber 36/conversion zone 38 to control
both the volume of
material within the reaction chamber 36/conversion zone 38 and the rate of
conveyance out of
the reaction chamber 36/conversion zone 38. These adjustable overflow weirs 52
can include,
without limitation, one or more gates. By controlling the volume of material
in the reaction
chamber 36/conversion zone 38 and its passage rate out of the reaction chamber
36/conversion
zone 38, the weir 52 can allow variability in filling coefficients.
Embodiments adopting active
transport mechanisms allow for even more control than those adopting passive
gravity control.
In one specific embodiment, the filling coefficient is at least about 50%,
which can allow for an
efficient heat transfer from the shell of the reactor to the solids inside the
reactor. Fill
coefficients of at least about 50% can allow materials within the chamber to
be heated to a
higher temperature (for example, in one embodiment, to at least about 550 C)
than lower fill
12
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
coefficients allow. These higher temperatures can allow for a more efficient
and complete
thermal conversion of sludge inside the reactor module and can produce higher
quality chars for
commercial and/or industrial applications. For instance, char exposed to these
higher
temperatures are especially suitable as precursors for activated carbon uses.
Additionally, by
controlling the rate of conveyance of material in a reactor module, the
reactor module can
maintain an appropriate WHSV for optimal vapors/bio-oil production.
[0052] Following heating, and in one embodiment mixing, in the reaction
chamber
36/conversion zone 38 the produced vapors and char must be removed. Following
removal,
vapors can either be condensed to produce bio-oil, combusted to generate heat
or to generate
energy via one of many secondary heat-to-energy generation processes or both.
Char can also
be used in a variety of commercial endeavors. In one non-limiting example, the
char is activated
for filtering processes including, in one embodiment, mercury chelation. The
removal and
treatment of vapors is addressed first.
IV. Removal and Use of Vapors
[0053] Still referring to Figure 2, vapors 58 are produced as sludge material
passes through
the reaction chamber 36/conversion zone 38. One limitation of prior approaches
concerns the
amount of particulate matter contained in the hot vapor as it exits the
reaction chamber
36/conversion zone 38. In previous designs, the vapor outlet was unprotected
and positioned in
such a way as to allow vapor to be drawn directly from the main reactor
chamber. This allowed
char particles, disturbed by the mixing of mixing elements, to become airborne
and exit the
reaction chamber along with the vapor. This particulate matter created several
problems in the
rest of the process. First, the particulate matter had a tendency to clog
valves in the oil
condensation phase of the process requiring extensive filtering. Second, the
filters routinely
filled with particulate sludge and had to be cleaned, creating more disposal
and odor issues.
[0054] The present invention addresses these issues by having the vapors 58
move through
the conversion zone 38 toward a converter gas outlet 60. Prior to reaching the
converter gas
outlet 60, the vapors 58 can pass through a series of baffles 24 that can
separate particulate
matter from the vapors prior to their exit from the reaction chamber 36. These
baffles 24,
representing an improvement over prior approaches, can significantly reduce
the amount of
particulate matter such as char or dust near the gas outlet, which can reduce
the amount of
impurities in the resulting bio-oil.
[0055] In another embodiment, after passing through the converter gas outlet
60, the vapors
58 can pass through one or more control valves (64 in Figure 3B). These
control valves 64 can
be either automatically or manually actuated and can direct the flow of vapors
to a direct spray
condenser module, a hot vapor combustion module or both, depending upon the
desired final
product. If bio-oil is desired, the vapors 58 are directed to the direct spray
condenser module. If
13
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
immediate heat and/or energy are desired, the vapors 58 are directed to the
hot vapor
combustion module. When both are desired, vapors are directed to both a
condenser module
and a combustion module.
A. Condensor Module
[0056] When directed to a condenser module, the vapors are condensed at
temperatures
sufficient to avoid the condensation of free water found in the vapor thus
preventing the
production of reaction water. This aspect of the present invention represents
one significant
benefit of the systems and methods of the present invention. Free water can
remain in vapor
form and can be discharged from the condenser module along with other non-
condensed
vapors.
[0057] If directed to a direct spray condenser module, the vapors 58 can enter
the Direct
Spray Condenser module 68 as depicted in Figure 4. The vapors 58 can be piped
through an
inlet opening 70 in the condensation chamber 72 where they can be immediately
met with a
direct spray of cooled bio-oil 74. The cooled bio-oil in turn can cool the
vapors to a level that
allows condensation of bio-oils out of the vapors. In one specific embodiment,
the temperature
in the condensation chamber 72 can remain at or above about 110 C, preventing
water vapor
from condensing into liquid water. In another specific embodiment, the
temperature in the
condensation chamber 72 can remain at about 100 C. In another specific
embodiment, water
vapor and uncondensed vapors 78 can exit the condensation chamber 72 via the
outlet valve
and piping 80 leading to a hot vapor combustion module.
[0058] In another embodiment, the bio-oil can be transferred via a pump 90 to
a heat
exchanger 92 designed to cool the bio-oil prior to re-introduction into the
condensation chamber
72. The bio-oil can enter the heat exchanger 92 where it can be indirectly
cooled by a source of
incoming cooling water 94. The cooling water 94, which can be effluent from
the wastewater
process, can then be discharged from the heat exchanger 92 via a cooling water
outlet 96.
Because there can be no direct contact between the water and the bio-oil,
further treatment of
the water can be avoided. In another embodiment, the purified bio-oil can be
pumped via pump
90 into storage barrels/tanks 98, where bio-oil can be stored for future use.
[0059] In another embodiment, condensed bio-oil (as well as a portion of the
now re-heated
bio-oil originally sprayed into the condensation chamber 72) can gather at the
bottom 84 of the
condensation chamber 72 where a U-Tube overflow device 86 can allow excess bio-
oil to exit
the condensation chamber 72. The excess bio-oil can then be directed to a
centrifuge 88 for
separation of particulate matter. In one specific embodiment, the centrifuge
88 can be a two-
phase centrifugal separator that is configured to separate bio-oil and
particulate. This is an
advance over prior approaches requiring a three-phase centrifugal separator
configured to
separate bio-oil, particulate and water. In one specific embodiment, the
purified bio-oil can be
14
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
converted into oil-derived products, including without limitation diesel fuel,
gasoline or heating
oil.
B. Combustion Module
[0060] In one embodiment, the vapors can be directed to a hot vapor combustion
(HVC)
module 82 as shown in Figures 5A and 5B. The vapors can enter the module 82
through an
inlet valve 100, which can precisely control the rate of process gas
introduction into the HVC
module 82. Water within the vapor can be combusted along with other non-
condensed vapors
in the HVC module portion of the process. Because such HVC devices are readily
commercially
available, the HVC module 82 will not be described in detail herein. Briefly,
a burner 102 can
provide heat to the combustion chamber 104, and a flue gas exit 106 can
provide an outlet.
[0061] The HVC module 82 can be designed to meet regulatory requirements.
Forexample,
in Europe the only requirement the HVC module 82 has to meet is a minimal
temperature of
about 850 C with a minimal gas residence time of two seconds. The reasoning
behind this is
that sewage sludge is classified waste within the European Union environmental
jurisdiction and
as a consequence of this any product from sludge is also classified as waste
and subsequently
has to meet waste incineration regulation. Past experience has shown that the
minimal
combustion chamber has to be about 650 C to avoid the generation of soot. In
the United
States the combustion temperature and the gas residence time at the combustion
temperature
may be regulated completely differently, and as a consequence the dimensions
of the HVC
module 82 can vary. After the HVC module 82 an air pollution control device
(APCD) (not
shown) can be used to clean the emissions from the HVC to meet all applicable
regulatory
requirements.
[0062] One limitation of prior pyrolysis approaches concerns the lack of
automated controls to
monitor and control the process and apparatus for optimal oil production.
Thus, previous
designs relied upon "batch" operation whereby a batch of sludge material was
fully processed
before the quality of the resulting oil could be tested. The process was then
manually adjusted
when necessary to produce a higher quality end product. This iterative batch
process was time
consuming and required that the process be completely shut down while manually
actuated
valves, screws, and other moving parts are adjusted.
[0063] The present invention addresses this drawback of prior processes by
providing for the
control of the process through the use of advanced instrumentation and
automated control
systems. In one specific embodiment, the process can include an automatic
control system to
control the valving of vapors, which can enable precise control of the
temperature inside the
reactor. This can in turn control bio-oil production because bio-oil
production can be more
efficient and more manageable when run at lower temperatures such as 400-500
C. The
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
control system can measure temperature in real time and manage the supply of
vapors to the
HVC, which can provide heat to the reactor module of the present invention.
V. Removal and Use of Char
[0064] Removal and use of char from the reaction chamber is also an important
aspect of the
present invention. Referring back to Figure 2, in one embodiment, following
the processing of
sludge, char accumulates in the reaction chamber 36/conversion zone 38 until
the material bed
level rises above the level of the weir plate gates 52. Referring to Figure
3A, when the material
bed level rises above the level of the wier plate gates 52, gravity can convey
the material
through weir gate openings 54 and, in one embodiment, onto a char plug screw
device (56 in
Figure 2) device. The adjustable weir plate gates 52 can control the depth of
the material bed
48 and thus the overall volume of material in the conversion zone 38.
[0065] In one embodiment, the char can exit the reaction chamber in a manner
designed to
eliminate contact with outside air or accidental leaking of vapors. In one
specific embodiment,
the char at the end of the process can be removed by actively conveying the
char from the
downstream side of the weir to a char cooler. This can be accomplished by,
without limitation,
the use of a char plug screw. A char plug screw is an active device that can
be used to convey
char material from the reaction chamber 36/conversion zone 38 after it has
passed through the
adjustable weir gates. The char plug screw can provide an air tight seal to
prevent hot vapors
from leaking from the reaction chamber 36/conversion zone 38. In one specific
embodiment as
shown in Figure 3C, the char plug screw 56 can actively convey the char
material out of the
bottom portion of reactor and onto a char cooling conveyor 66. The char plug
screw 56 can
convey char at different speeds, which can help eliminate clogging issues that
are common in
non-active char conveyor designs. This approach represents a significant
advance over
previously used methods that removed char from reaction chambers using gravity
and chutes
alone.
[0066] The disclosed embodiments according to the present invention also
provide a char
obtained from the thermal conversion of sludge. As stated, previous pyrolysis
methods treated
generated char as a waste product requiring disposal. Aspects according to the
present
invention recognize various beneficial uses of chars products by pyrolysis
processes, including
the chars produced by the systems and methods described herein. For example,
chars can be
processed to generate activated carbon. Activation can be carried out by, for
example and
without limitation, contact of the char with carbon dioxide or steam, as
described in U.S. Patent
No. 6,537,947, which is incorporated by reference herein. There are many uses
for this
activated carbon including, without limitation, in the absorption of metals,
such as mercury, in
purification and/or chemical recovery operations as well as in environmental
remediation. Other
particular non-limiting examples of uses for activated carbon produced using
chars generated
16
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
from pyrolysis processes include in the application of air purification,
catalyst support,
decolorization in beverages and sugar refining, deoderization, metal
recovery/removal, liquid
purification, emergency poison treatment, solvent recovery, and/or whiskey
manufacturing.
[0067] Chars made in accordance with the systems and methods of the present
invention can
be particularly useful in a variety of contexts due to the ability to achieve
higher reaction
temperatures due to higher filling coefficients, improved mixing and improved
sealing of the
reaction chamber among otherfeatures. These features of the present invention
can alter the
physical and/or chemical characteristics of the char, including, without
limitation, its density,
structure (geometric composition of carbon plates, etc.), Brunauer, Emmett and
Teller (BET)
surface area, number of active sites, and chemical compositions. By way of
example, and not
as a limitation, the BET surface areas of the char produced by previous
pyrolysis methods
ranged from about 100-200 m2/g. The BET surface areas of the chars according
to the present
invention, in contrast, can range from about 400 to about 600 m2/g. This
increase in BET
surface area can make chars formed in accordance with the systems and methods
described
herein highly appropriate activated carbon precursors.
[0068] Chars can also be useful in brick manufacturing. In one embodiment, the
char can
have a relatively fine particle size and can be added to the raw materials
used in brick
manufacturing (for example and without limitation, natural clay minerals) to
form a
homogeneous mixture. Small amounts of manganese, barium, and other additives
can also be
added to the mixture to produce different shades and/or to improve the brick's
chemical
resistance to the elements. The mixture can be dried to remove excess moisture
and then can
be fired in high temperature fumaces or kilns according to methods known to
those of ordinary
skill in the art. During firing, the char can release vapors at high
temperatures (in one
embodiment at a temperature of about 550 C or above) creating stable
micropores in the bricks.
These micropores can help reduce the thermal conductivity of the bricks
improving their
insulation properties. This use of chars can be especially useful in countries
that have set
standards to meet the C02-reduction goals set forth in the Kyoto protocol. For
example,
countries in Europe have introduced tighter standards with regard to heat
transfer coefficients of
construction materials. In Germany, new solid structure buildings must utilize
building materials
with a heat transfer coefficient of <0,27 W/m2/K. Chars can help achieve these
goals by, without
limitation, reducing the heat transfer coefficient of building materials
either by generating
micropores in building materials, being used as an insulation material, being
used as a fuel
reducing primary energy source or combinations thereof.
[0069] Chars can also be used as a carbon black substitute in a variety of
manufacturing
processes to reduce cost fluctuation. Carbon black is derived from the
incomplete combustion
of natural gas or petroleum oil and, as such, the cost of carbon black rises
or falls with increases
17
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
or decreases in oil and/or natural gas prices. The price of char on the other
hand, can be
independent from the prices of oil and/or natural gas, and can remain stable
over a longer
period time. These provided non-limiting examples help to illustrate the wide
range of beneficial
uses chars made during pyrolysis processes can have.
VI. Use of Waste Heat to Power Processes
[0070] Certain embodiments according to the present invention utilize waste
heat. Waste
heat can be produced by a number of different facilities, including, without
limitation, power
generation (coal-fired, natural gas fired, nuclear, etc.), wood product
processing (pulp & lumber
mills) and various other heat-producing manufacturing processes. The methods
according to
the present invention can include constructing one or more such facilities or
processes in order
to create a readily available source of waste heat for the downstream sludge
drying, processing,
and/or power generation processes, can use one or more already-existing
sources of waste
heat or both.
[0071] When waste heat is used, the systems and methods according to the
present invention
can include an apparatus to collect heat from the waste heat source in the
form of, without
limitation, heated air, steam, liquid, or another useable form. This apparatus
can consist of heat
exchangers installed in the exhaust stream from the heat source, where heat
can be captured
prior to other forms of disposal. As will be understood by one of skill in the
art, the apparatus
can include all necessary valves, ducts, fans, pumps, and piping to redirect
the heated material.
[0072] The necessary valves, ducts, fans, pumps, and piping can control the
delivery of waste
heat to the downstream sludge drying and/or thermal processing stages using,
in one
embodiment, an automated control system. Using sensors located throughout one
or more
modules and processes, instantaneous heat requirements can be measured and the
necessary
valves, ducts, piping, fans and pumps can be affected to deliver the required
heat from the
waste heat source. Systems and methods to utilize waste heat in accordance
with the systems
and methods of the present invention are described more fully in U.S. Patent
Application No.
11/379,404 which is fully incorporated by reference herein.
[0073] Unless otherwise indicated, all numbers expressing quantities of
ingredients, reaction
conditions, and so forth used in the specification and claims are to be
understood as being
modified in all instances by the term "about." Accordingly, unless indicated
to the contrary, the
numerical parameters set forth herein are approximations that may vary
depending upon the
desired properties sought to be obtained by the present invention. At the very
least, and not as
an attempt to limit the application of the docti-ine of equivalents to the
scope of the claims, each
numerical parameter should at least be construed in light of the number of
reported significant
digits and by applying ordinary rounding techniques. Notwithstanding that the
numerical ranges
and parameters setting forth the broad scope of the invention are
approximations, the numerical
18
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
values set forth in the specific examples are reported as precisely as
possible. Any numerical
value, however, inherently contains certain errors necessarily resulting from
the standard
deviation found in their respective testing measurements.
[0074] The terms "a" and "an" and "the" and similar referents used in the
context of describing
the invention (especially in the context of the following claims) are to be
construed to cover both
the singular and the plural, unless otherwise indicated herein or clearly
contradicted by context.
Recitation of ranges of values herein is merely intended to serve as a
shorthand method of
referring individually to each separate value falling within the range. Unless
otherwise indicated
herein, each individual value is incorporated into the specification as if it
were individually recited
herein. All methods described herein can be performed in any suitable order
unless otherwise
indicated herein or otherwise clearly contradicted by context. The use of any
and all examples,
or exemplary language (e.g., "such as") provided herein is intended merely to
better illuminate
the invention and does not pose a limitation on the scope of the invention
otherwise claimed.
No language in the specification should be construed as indicating any non-
claimed element
essential to the practice of the invention.
[0075] Groupings of alternative elements or embodiments of the invention
disclosed herein
are not to be construed as limitations. Each group member may be referred to
and claimed
individually or in any combination with other members of the group or other
elements found
herein. It is anticipated that one or more members of a group may be included
in, or deleted
from, a group for reasons of convenience and/or patentability. When any such
inclusion or
deletion occurs, the specification is herein deemed to contain the group as
modified thus
fulfilling the written description of all Markush groups used in the appended
claims.
[0076] Certain embodiments of this invention are described herein, including
the best mode
known to the inventors for carrying out the invention. Of course, variations
of these
embodiments will become apparent to those of ordinary skill in the art upon
reading the
foregoing description. The inventors expect skilled artisans to employ such
variations as
appropriate, and the inventors intend for the invention to be practiced
otherwise than specifically
described herein. Accordingly, this invention includes all modifications and
equivalents of the
subject matter recited in the claims appended hereto as permitted by
applicable law. Moreover,
any combination of the above-described elements in all possible variations
thereof is
encompassed by the invention unless otherwise indicated herein or otherwise
clearly
contradicted by context.
[0077] Furthermore, references have been made to patents and/or printed
publications
throughout this specification. Each of the above cited references and printed
publications are
herein individually incorporated by reference in their entirety.
19
CA 02613599 2007-12-27
WO 2007/005771 PCT/US2006/025878
[0078] In closing, it is to be understood that the embodiments of the
invention disclosed
herein are illustrative of the principles according to the present invention.
Other modifications
that may be employed are within the scope of the invention. Thus, by way of
example, but not
of limitation, alternative configurations according to the present invention
may be utilized in
accordance with the teachings herein. Accordingly, the present invention is
not limited to that
precisely as shown and described.