Note: Descriptions are shown in the official language in which they were submitted.
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
1
PROCESS FOR THE MANUFACTURE OF DIESEL RANGE HYDRO-
CARBONS
Field of the invention
The invention relates to an improved process for the manufacture of diesel
range
hydrocarbons from bio oils and fats with reduced hydrogen consumption. Particu-
larly the invention relates to an improved process for the manufacture of
diesel
range hydrocarbons with high selectivity and which process yields a product
with
improved cold flow properties concurrently without decreasing diesel yield
during
isomerisation.
Background of the invention
Environmental interests and an increasing demand for diesel fuel, especially
in
Europe, encourage fuel producers to employ more intensively renewable sources
available. In the manufacture of diesel fuel based on biological raw materials
the
main interest has concentrated on vegetable oils and animal fats comprising
triglycerides of fatty acids. Long, straight and mostly saturated hydrocarbon
chains of fatty acids correspond chemically to the hydrocarbons present in
diesel
fuels. However, neat vegetable oils display inferior properties, particularly
ex-
treme viscosity and poor stability and therefore their use in transportation
fuels is
limited.
Conventional approaches for converting vegetable oils or other fatty acid
deriva-
tives into liquid fuels comprise processes such as transesterification,
catalytic hy-
drotreatment, hydrocracking, catalytic cracking without hydrogen and thermal
cracking. Typically triglycerides, forming the main component in vegetable
oils,
are converted into the corresponding esters by the transesterification
reaction with
an alcohol in the presence of catalysts. The obtained product is a fatty acid
alkyl
ester, most commonly fatty acid methyl ester (FAME). Poor low-temperature
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
2
properties of FAME however limit its wider use in regions with colder climatic
conditions. Poor cold flow properties are a result of the straight chain
nature of the
FAME molecule and thus double bonds are needed in order to create even bear-
able cold flow properties. Carbon-carbon double bonds and ester groups however
decrease the stability of fatty acid esters, which is a major disadvantage of
trans-
esterification technology. Further, Schmidt, K., Gerpen J.V.: SAE paper 961086
teaches that the presence of oxygen in esters results in undesired and higher
emis-
sions of NOX in comparison to conventional diesel fuels.
Undesired oxygen may be removed from fatty acids or esters by deoxygenation
reactions. The deoxygenation of bio oils and fats, which mean oils and fats
based
on biological material, to hydrocarbons suitable as diesel fuel products, may
be
carried out in the presence of a catalyst under controlled hydroprocessing
condi-
tions, known as hydrotreating or hydrocracking processes.
During hydrodeoxygenation oxogroups are reacted with hydrogen and removed
through formation of water. The hydrodeoxygenation reaction requires
relatively
high amounts of hydrogen. Due to the highly exothermic reactions the control
of
reaction heat is extremely important. Unnecessary high reaction temperature,
in-
sufficient control of reaction temperature or unnecessary low hydrogen
availabil-
ity in the feed stream cause increased formation of unwanted side reaction
prod-
ucts and coking of catalyst. Unwanted side reactions, such as cracking,
polymeri-
sation, ketonisation, cyclisation and aromatisation decrease the yield and the
prop-
erties of diesel fraction. Unsaturated feeds and free fatty acids in
triglyceridic bio
oils may also promote the formation of heavy molecular weight compounds.
Patents US 4,992,605 and US 5,705,722 describe processes for the production of
diesel fuel additives by conversion of bio oils into saturated hydrocarbons
under
hydroprocessing conditions with NiMo and CoMo catalysts. The hydrotreatment
operates at high temperatures of 350-450 C and produces n-paraffins and other
hydrocarbons. The product has high cetane number but poor cold properties,
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
3
which limit the amount of product that can be blended in conventional diesel
fuel
in summer time and prevent its use during winter time. The formation of heavy
compounds with a boiling point above 343 C was observed, especially when a
fatty acid fraction was used as a feed. A lower limit of 350 C for reaction
tem-
perature was concluded as a requirement for trouble-free operation.
A two-step process is disclosed in Fl 100248 for producing middle distillates
from
vegetable oils by hydrogenating fatty acids or triglycerides of vegetable oil
origin
using commercial sulphur removal catalysts, such as NiMo and CoMo, to give n-
paraffms, followed by isomerizing said n-paraffins using metal containing
molecule sieves or zeolites to obtain branched-chain paraffins. The
hydrotreating
was carried out at rather high reaction temperatures of 330-450 C, preferably
390 C. Hydrogenating fatty acids at those high temperatures leads to shortened
catalyst life resulting from coking and formation of side products.
EP 1 396 531 describes a process containing at least two steps, the first one
being
a hydrodeoxygenation step and the second one being a hydroisomerisation step
utilizing counter-current flow principle, and biological raw material
containing
fatty acids and/or fatty acid esters serving as the feedstock. The process
comprises
an optional stripping step.
Cracking is significant side reaction in isomerisation of n-paraffins.
Cracking in-
creases with higher isomerisation conversion (more severe reaction conditions)
and decrease the yield of diesel. The severity of isomerisation conditions
(isomer-
isation conversion) controls also the amount of methyl branches formed and
their
distance from each other and therefore cold properties of bio diesel fraction
pro-
duced.
FR 2,607,803 describes a process for hydrocracking of vegetable oils or their
fatty
acid derivatives under elevated pressure to give hydrocarbons and to some
extent
acid. The catalyst contains metal dispersed on a support. A high reaction
tempera-
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
4
ture of 370 C did not result in complete conversion and high selectivity of n-
paraffins. The product formed contained also some intermediate fatty acid com-
pounds.
Water formation during hydrotreatment mainly results from deoxygenation of
triglyceride oxygen by the means of hydrogen (hydrodeoxygenation). Deoxygena-
tion using hydrodeoxygenation conditions is to some extent accompanied by de-
carboxylation reaction path, described below as reaction A, and
decarbonylation
reaction path (reaction BI and B2). Deoxygenation of fatty acid derivatives by
decarboxylation and/or decarbonylation reactions forms carbon oxides (CO2 and
CO) and aliphatic hydrocarbon chains with one carbon atom less than in the
origi-
nal fatty acid molecule. Thereafter water-gas-shift reaction may balance the
con-
centrations of CO and CO2 (reaction E). Methanation reaction uses hydrogen and
forms H2O and methane if it is active during hydrotreatment conditions
(reaction
D). Hydrogenation of fatty acids gives aliphatic hydrocarbons and water
(reaction
Q. Reaction schemes A - E are described below.
Decarboxylation: C17H35COOH -> C17H36 + CO2 (A)
Decarbonylation: C17H35000H + H2 -> C17H36 + CO + H2O (B1)
C17H35000H -> C17H34 + CO + H2O (B2)
Hydrogenation: C17H35000H + 3H2 -> C18H38 + 2 H2O (C)
Methanation: CO + 3H2 -> CH4 + H2O (D)
Water-Gas-shift: CO + H2O -> H2 + CO2 (E)
The feasibility of decarboxylation varies greatly with the type of carboxylic
acid
or derivative thereof used as the starting material. Alpha-hydroxy, alpha-
carbonyl
and dicarboxylic acids are activated forms and they are more easily
deoxygenated
by decarb-reactions, which mean here decarboxylation and/or decarbonylation.
Linear aliphatic acids are not activated this way and generally they are
difficult to
deoxygenate through the decarb-reaction path and they need much more severe
reaction conditions.
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
5 Decarboxylation of carboxylic acids to hydrocarbons by contacting carboxylic
acids with heterogeneous catalysts was suggested by Maier, W. F. et al:
Chemische Berichte (1982), 115(2), 808-12. Maier et al tested Ni/A1203 and
Pd/Si02 catalysts for decarboxylation of several carboxylic acids. During the
reac-
tion the vapors of the reactant were passed through a catalytic bed together
with
hydrogen. Hexane represented the main product of the decarboxylation of the
tested compound heptanoic acid.
Patent US 4,554,397 discloses a process for the manufacture of linear olefins
from
saturated fatty acids or esters, suggesting a catalytic system consisting of
nickel
and at least one metal selected from the group consisting of lead, tin and
germa-
nium. With other catalysts, such as Pd/C, low catalytic activity and cracking
to
saturated hydrocarbons, or formation of ketones when Raney-Ni was used, were
observed.
Decarboxylation, accompanied with hydrogenation of oxo-compound, is de-
scribed in Laurent, E., Delmon, B.: Applied Catalysis, A: General (1994),
109(1),
77-96 and 97-115, wherein hydrodeoxygenation of biomass derived pyrolysis oils
over sulphided CoMo/y-A1203 and NiMo/y-A1203 catalysts was studied. Di-
ethyldecanedioate was used among others as a model compound and it was ob-
served that the rates of formation of the decarboxylation product, nonane and
the
hydrogenation product, decane were comparable under hydrotreating conditions
(260-300 C, 7 MPa, in hydrogen). The presence of hydrogen sulphide (H2S) in
feed promoted the decarboxylation selectivity compared with zero sulphur in
feed.
Different sulphur levels studied however had no effect on the decarboxylation
selectivity of diethyldecanedioate.
Biological raw materials often contain several impurities, such as metal com-
pounds, organic nitrogen, sulphur and phosphorus compounds, which are known
catalyst inhibitors and poisons inevitably reducing the service life of
catalysts and
necessitating more frequent catalyst regeneration or change. Metals in bio
oils/fats
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
6
inevitable build up on catalyst surface and change the activity of catalyst.
Metals
can promote some side reactions and blocking of active sites of catalysts
typically
decreases the activity.
Fatty acid composition, size and saturation degree of the fatty acid may vary
con-
siderably in feedstock of different origin. Melting point of bio oil or fat is
mainly
consequence of saturation degree. Fats are more saturated than liquid oils and
in
this respect need less hydrogen for hydrogenation of double bonds. Double
bonds
in fatty acid chains contribute also to different kinds of side reactions,
such as
oligomerisation/polymerization, cyclisation/aromatisation and cracking
reactions,
which deactivate catalyst, increase hydrogen consumption and reduce diesel
yield.
Hydrolysis of triglycerides produces also diglycerides and monoglycerides,
which
are partially hydrolyzed products. Diglycerides and monoglycerides are surface-
active compounds, which can form emulsions and make liquid/liquid separations
of water and oil more difficult. Bio oils and fats can also contain other
glyceride-
like surface-active impurities like phospholipids, suck as lecithin, which
have
phosphorus in their structures. Phospholipids are gum like materials, which
can be
harmful for catalysts. Natural oils and fats also contain non-glyceride
components.
These are among others waxes, sterols, tocopherols and carotenoids, some
metals
and organic sulphur compounds as well as organic nitrogen compounds. These
compounds can be harmful for catalysts or pose other problems in processing.
Plant oils/fats and animal oils/fat may contain free fatty acids, which are
formed
during processing of oils and fats through hydrolysis of triglycerides. Free
fatty
acids are a class of problematic components in bio oils and fats, their
typical con-
tent being between 0 and 30% by weight. Free fatty acids are corrosive in
their
nature, they can attack the materials of the process unit or catalyst and they
can
promote side reactions like formation of metal carboxylates in the presence of
metal impurities. Due to the free fatty acids contained in bio oils and fats,
the
formation of heavy molecular weight compounds is significantly increased when
7
compared to triglyceridic bio-feedstock having only low amounts of free fatty
acids,
typically below I% by weight.
Deoxygenation of plant oils/fats and animal oils/fats with hydrogen requires
rather much
hydrogen and at the same time releases significant amount of heat. Heat is
produced
from the deoxygenation reactions and from double bond hydrogenation. Different
feedstocks produce significantly different amounts of reaction heat. The
variation in
reaction heat produced is mainly dependent of double bond hydrogenation. The
average
amount of double bonds per triglyceride molecule can vary from about 1.5 to
over 5
depending on the source of bio oil or fat.
Object of the invention
An object of the invention is an improved process for the manufacture of
diesel range
hydrocarbons from bio oils and fats with reduced hydrogen consumption.
A further object of the invention is an improved process for the manufacture
of diesel
range hydrocarbons from bio oils and fats with high selectivity and which
process yields
a product with improved cold flow properties concurrently without decreasing
diesel
yield during isomerisation.
A further object of the invention is an improved process for the manufacture
of high
quality diesel range hydrocarbons from bio oils and fats with decreased
hydrogen
consumption and high diesel yield.
The present invention provides a process for the manufacture of diesel range
hydrocarbons wherein a feed is hydrotreated in a hydrotreating step and
isomerised in an
isomerisation step, characterized in that the feed comprises fresh feed
containing at least
20 % by weight of triglyceride C12-C16 fatty acids or C12-C16 fatty acid
esters or C12-C16
fatty acids or combinations of thereof and the total feed contains 50 - 20000
w-ppm
sulphur calculated as elemental sulphur.
7a
The fresh feed can contain at least 30 % by weight and preferably at least 40
% by weight
of triglyceride C12-C16 fatty acids or other fatty acid esters or combinations
of thereof.
The fresh feed can contain more than 5 % by weight of free fatty acids. The
feed can
contain less than 10 w-ppm alkaline and alkaline earth metals, calculated as
elemental
alkaline and alkaline earth metals, less than 10 w-ppm other metals,
calculated as
elemental metals and less than 30 w-ppm phosphorus, calculated as elemental
phosphorus.
The feed can comprise less than 20 wt-% of fresh feed and additionally at
least one
diluting agent. The diluting agent can be selected from hydrocarbons and
recycled
products of the process or mixtures thereof and the diluting agent / fresh
feed-ratio is
5-30:1, preferably 10-30:1 and most preferably 12-25:1. The feed can contain
1000-8000
w-ppm and preferably 2000-5000 w-ppm of sulphur calculated as elemental
sulphur. At
least one inorganic or organic sulphur compound or a refinery gas and/or
liquid stream
containing sulphur compounds can be added to the feed. The fresh feed is of
biological
origin selected from plant oils/fats, animal fats/oils, fish fats/oils, fats
contained in plants
bred by means of gene manipulation, recycled fats of the food industry and
mixtures
thereof.
The fresh feed can be selected from rapeseed oil, colza oil, canola oil, tall
oil, sunflower
oil, soybean oil, hempseed oil, olive oil, linseed oil, mustard oil, palm oil,
peanut oil,
castor oil, coconut oil, lard, tallow, train oil or fats contained in milk or
mixtures thereof.
The fresh feed can comprise feed of biological origin and a
hydrocarbon/hydrocarbons.
In the hydrotreatment step a catalyst bed system can be used comprising one or
more
catalyst beds. In the hydrotreating step, the pressure varies in the range of
2-15 MPa,
preferably in the range of 3-10 MPa, the temperature varying between 200 and
400 C,
preferably between 250 and 350 C, and most preferably between 280 and 345 C.
In the isomerisation step, the pressure can vary in the range of 2-15 MPa,
preferably in
the range of 3-10 MPa, the temperature varying between 200 and 500 C,
preferably
between 280 and 400 C. The hydrotreatment can be carried out in the presence
of a
hydrogenation catalyst, said hydrogenation catalyst containing a metal from
the Group
7b
VIII and/or VIB of the Periodic System. The hydrotreating catalyst can be a
supported
Pd, Pt, Ni, NiMo or a CoMo catalyst, the support being alumina and/or silica.
An isomerisation catalyst containing molecular sieve can be used in the
isomerisation
step. The isomerisation catalyst can comprise a metal from the Element Group
VIII. The
isomerisation catalyst can contain A 1203 or Si02. The isomerisation catalyst
can contain
SAPO-11 or SAPO-41 or ZSM-22 or ZSM-23 or ferrierite and Pt or Pd or Ni and
A1203
or Si02.
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
8
Definitions
Here hydroprocessing is understood as catalytic processing of organic material
by
all means of molecular hydrogen.
Here hydrotreatment is understood as a catalytic process, which removes oxygen
from organic oxygen compounds as water (hydrodeoxygenation, HDO), sulphur
from organic sulphur compounds as dihydrogen sulphide (H2S) (hydrodesulphuri-
sation, HDS), nitrogen from organic nitrogen compounds as ammonia (NH3) (hy-
drodenitrogenation, HDN) and halogens, such as chloride from organic chloride
compounds as hydrochloric acid (HC1) (hydrodechlorination, HDCI), typically
under the influence of a sulphided NiMo or sulphided CoMo catalysts.
Here deoxygenation is understood to mean removal of oxygen from organic
molecules, such as fatty acid derivatives, alcohols, ketones, aldehydes or
ethers by
any means previously described.
Here hydrodeoxygenation (HDO) of triglycerides or other fatty acid derivatives
or
fatty acids is understood to mean the removal of carboxyl oxygen as water by
the
means of molecular hydrogen under the influence of a catalyst.
Here decarboxylation and/or decarbonylation of triglycerides or other fatty
acid
derivatives or fatty acids is understood to mean removal of carboxyl oxygen as
CO2 (decarboxylation) or as CO (decarbonylation) with or without the influence
of molecular hydrogen. Decarboxylation and/or decarbonylation reactions are
referred to as decarb-reactions.
Here hydrocracking is understood as catalytic decomposition of organic hydro-
carbon materials using molecular hydrogen at high pressures.
CA 02614014 2010-11-22
9
Here hydrogenation means saturation of carbon-carbon double bonds by the
means of molecular hydrogen under the influence of a catalyst.
Here n-paraffins mean normal alkanes or linear alkanes that do not contain
side
chains.
Here isoparaffins means alkanes having one or mere C1- C9, typically C1- C2
alkyl side chains, typically mono-, di-, to - or tetramethylalkanes.
The feed ( total feed) to the hydrotreatment step is to be understood
comprising
fresh feed and optionally at least one dilution agent.
Summary of the invention
The present invention relates to an improved process comprising a
hydrotreatment
step and anisomerisation step, for the manufacture of diesel range
hydrocarbons
from renewable sources like bio oils and fats, such as plant oils/fats and
animal
and fish oils/fats, particularly C12-C16 fatty acids and/or derivatives
thereof in the
presence of sulphur. The invention relates to hydrotreating of the feed
comprising
triglycerides, fatty acids and derivatives of fatty acids and particularly C12-
C16
fatty acids and/or derivatives thereof or combinations of thereof, into n-
paraffins
with reduced hydrogen consumption during hydrotreating, in the presence of sul-
phur, followed by converting the n-paraffins into diesel range branched
alkanes
using isomerisation with high diesel yield. The hydrocarbon oil product formed
via this method is a high quality diesel component. In the hydrotreating step
the
feed is contacted with a sulphided hydrotreatment catalyst in the presence of
sul-
phur, followed by-the isomerisation step with an isomerisation catalyst.
According to one aspect of the invention there is provided process for the
manufacture of diesel range hydrocarbons, wherein total feed comprising
fresh feed and 50-20000 w-ppm sulphur calculated as elemental sulphur is
hydrotreated in a hydrotreating step to form a hydrotreated product, and the
hydrotreated product is isomerised in an isomerisation step to form diesel
range hydrocarbons;
wherein the fresh feed comprises at least 20 % by weight of
triglyceride C12-C16 fatty acids, C12-C16 fatty acid esters, C12-C16 fatty
acids,
or any combination thereof;
CA 02614014 2010-11-22
9a
wherein at least one inorganic or organic sulphur compound or a
refinery gas or a liquid stream containing a sulphur compound is added to
the total feed or the fresh feed;
wherein during the hydrotreating step, the pressure varies in the
range of 2-15 MPa, and the temperature varies between 200 and 400 C;
and
wherein during the isomerisation step, the pressure varies in the
range of 2-15 MPa, and the temperature varies between 200 and 500 C.
Detailed description of the invention
It was surprisingly found that hydrogen consumption in the hydrotreatment
step,
deoxygenation of fatty acids and/or fatty acid derivatives, and cracking
during
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
5 isomerisation of n-paraffins can be significantly reduced by adding one or
more
sulphur compounds to the feed to achieve sulphur content of 50-20000 w-ppm,
preferably 1000-8000 w-ppm, most preferably 2000-5000 w-ppm in the feed, cal-
culated as elemental sulphur, particularly when bio oils and fats comprising
C12-
C16 fatty acids and/or derivatives thereof are used as the fresh feed for the
hy-
10 drotreatment step.
Feedstock
The bio oil and/or fat used as the fresh feed in the process of the present
invention
originates from renewable sources, such as fats and oils from plants and/or
ani-
mals and/or fish and compounds derived from them. The basic structural unit of
a
typical plant or vegetable or animal oil/fat useful as the feedstock is a
triglyceride,
which is a triester of glycerol with three fatty acid molecules, having the
structure
presented in the following formula I:
i 0 ez
Formula 1. Structure of triglyceride
In formula I R1, R2 and R3 are alkyl chains. Fatty acids found in natural
triglyc-
erides are almost solely fatty acids of even carbon number. Therefore R1, R2,
and
R3 typically are C5 - C23 alkyl groups, mainly C11-C19 alkyl groups and most
typi-
cally C15 or C17 alkyl groups. R1, R2, and R3 may contain carbon-carbon double
bonds. These alkyl chains can be saturated, unsaturated or polyunsaturated.
Suit-
able bio oils are plant and vegetable oils and fats, animal fats, fish oils,
and mix-
tures thereof containing fatty acids and/or fatty acid esters. Examples of
said ma-
terials are wood-based and other plant-based and vegetable-based fats and oils
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
11
such as rapeseed oil, colza oil, canola oil, tall oil, sunflower oil, soybean
oil,
hempseed oil, olive oil, linseed oil, mustard oil, palm oil, peanut oil,
castor oil,
coconut oil, as well as fats contained in plants bred by means of gene
manipula-
tion, animal-based fats such as lard, tallow, train oil, and fats contained in
milk, as
well as recycled fats of the food industry and mixtures of the above.
Typically a bio oil or fat, suitable as feedstock, comprises C12 - C24 fatty
acids,
derivatives thereof such as anhydrides or esters of fatty acids as well as
triglyc-
erides of fatty acids or combinations of thereof. The fatty acids or fatty
acid de-
rivatives, such as esters may be produced via hydrolysis of bio oils or by
their
fractionalization or esterification reactions of triglycerides.
In the process according to the invention the fresh feed contains at least 20
%,
preferably at least 30 % and most preferably at least 40 % by weight of
triglyc-
eride C12-C16 fatty acids or C12-C16 fatty acid esters or C12-C16 fatty acids
or com-
binations of thereof. Examples of this kind of feed are palm oils and animal
fats
containing lower carbon numbers fatty acids, which are typically more
saturated
than C18 fatty acids and their decarboxylation tendency is lower than that of
higher carbon number fatty acids during hydrodeoxygenation. The fresh feed may
also comprise feedstock of biological origin and a hydrocarbon or
hydrocarbons.
C12-C16 fatty acids can be bound to glycerol as triglycerides or other esters.
Ani-
mal fats and palm oil triglycerides contain significant amounts of C16 fatty
acids,
typically 15-45 wt-% and especially palmitic acid. Other vegetable
triglycerides
contain only 1-13 wt% C16 fatty acids, for example rapeseed oil only 1-5 wt-%.
In order to avoid catalyst deactivation and undesired side reactions the feed
shall
comply with the following requirements: The amount of alkaline and alkaline
earth metals, calculated as elemental alkaline and alkaline earth metals, in
the feed
is below 10, preferably below 5 and most preferably below 1 w-ppm. The amount
of other metals, calculated as elemental metals, in the feed is below 10,
preferably
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
12
below 5 and most preferably below 1 w-ppm. The amount of phosphorus, calcu-
lated as elemental phosphorus is below 30, preferably below 15 and most
prefera-
bly below 5 w-ppm.
In many cases the feedstock, such as crude plant oil or animal fat, is not
suitable
as such for processing because of high impurity content and thus the feedstock
is
preferably purified using suitable one or more conventional purification proce-
dures, before introducing it to the hydrotreating step of the process.
Examples of
some conventional procedures are provided in the following.
Degumming of plant oils/fats and animal oils/fats means removal of phosphorus
compounds, such as phospholipids. Solvent extracted vegetable oils contain
often
significant amounts of gums, typically 0.5-3% by weight, which are mostly phos-
phatides (phospholipids) and therefore a degumming stage is needed for crude
plant oils and animal fats in order to remove phospholipids and metals present
in
crude oils and fats. Iron and also other metals may be present in the form of
metal-phosphatide complexes. Even a trace amount of iron is capable of catalys-
ing oxidation of the oil or fat.
Degumming is performed by washing the feed at 90-105 C, 300-500 kPa(a), with
H3PO4, NaOH and soft water and separating the formed gums. A major amount of
metal components, which are harmful for the hydrotreatment catalyst, are also
removed from the feedstock during the degumming stage. The moisture content
of the degummed oil is reduced in dryer at 90-105 C, 5-50 kPa(a).
The amount of free fatty acids present in vegetable oils is typically 1-5 wt %
and
in animal fat 10-25 wt-%. High amounts of free fatty acids in a feedstock may
be
reduced using a deacidification stage, which may be performed for example by
steam stripping. A feedstock, which is optionally degummed, is typically first
degassed under 5-10 kPa(a)a pressure at a temperature of approx. 90 C.
Thereaf-
ter the obtained oil is heated to approx. 250-280 C, 5-10 kPa(a) and directed
to a
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
13
stripping column, where life steam strips at 230-260 C the free fatty acids
and
deodorizes the oil under vacuum. The fatty acid fraction is withdrawn from the
column overhead.
A feedstock, which is optionally degummed or refined in another conventional
way, may be bleached. In bleaching the degummed or refined feedstock is heated
and mixed with natural or acid-activated bleaching clay. Bleaching removes
vari-
ous impurity traces left from other pretreatment steps like degumming, such as
chlorophyll, carotenoids, phosphoipids, metals, soaps and oxidation products.
Bleaching is typically carried out under vacuum to minimize possible
oxidation.
Bleaching is used to reduce the color pigments in order to produce an oil of
ac-
ceptable color and to reduce the oxidation tendency of oil.
In the following the process according to the invention comprising a
hydrotreating
step and an isomerisation step is described in more detail.
Hydrotreating step
The feed to the hydrotreating unit comprises fresh feed and optionally at
least one
diluting agent. The diluting agent can be a hydrocarbon of biological origin
and/or
non biological origin. In the case the feed comprises additionally at least
one di-
luting agent it is preferable that the feed contains less than 20 wt-% of
fresh feed.
The diluting agent can also be recycled product from the process (product
recycle)
and then the diluting agent / fresh feed-ratio is 5-30:1, preferably 10-30:1
and
most preferably 12-25:1.
The total feed comprising fresh feed containing at least 20 %, preferably at
least
30 % and most preferably at least 40 % by weight of triglyceride C12-C16 fatty
acids or C12-C16 fatty acid esters or C12-C16 fatty acids or combinations of
thereof,
is hydrotreated in the presence of hydrogen with a catalyst at hydrotreating
condi-
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
14
tions in the presence of 50-20000 w-ppm, preferably 1000-8000 w-ppm, most
preferably 2000-5000 w-ppm of sulphur in the total feed, calculated as
elemental
sulphur.
In the hydrotreating step of the process fatty acids, triglycerides and fatty
acid
derivatives are deoxygenated, denitrogenated, desulphurisated and
dechlorinated.
In the hydrotreating step, known hydrogenation catalysts containing metals
from
Group VIII and/or VIB of the Periodic System may be used. Preferably, the hy-
drogenation catalysts are supported Pd, Pt, Ni, NiMo or a CoMo catalysts, the
support being alumina and/or silica, as described for instance in FI 100248.
Typi-
cally, NiMo/A1203 and CoMo/A1203 catalysts are used.
In the hydrotreating step, the pressure range can be varied between 2 and 15
MPa,
preferably between 3 and 10 MPa and most preferably between 4 and 8 MPa, and
the temperature between 200 and 400 C, preferably between 250 and 350 C and
most preferably 280-345 C.
It was found that the deoxygenation of starting materials originating from
renew-
able sources can be controlled between two partially alternative reaction
routes:
hydrodeoxygenation and decarboxylation and/or decarbonylation (decarb-
reactions). The selectivity of decarb-reactions and the deoxygenation through
de-
carb-reactions can be promoted during hydrotreating over the hydroteatment
cata-
lyst, by using sulphur content of 50 - 20000 w-ppm in the total feed. The
specific
sulphur content in the feed is able to double the extent of n-paraffins formed
by
removal of COx. Complete deoxygenation of triglycerides by decarb-reactions
can theoretically lower the consumption of hydrogen about 60% (max) compared
with pure deoxygenation by hydrogen as can be seen in Table 3.
At least one organic or inorganic sulphur compound may be fed along with hy-
drogen or with the feed to achieve the desired sulphur content. The inorganic
sul-
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
5 phur compound can be for example H2S or elemental sulphur or the sulphur com-
pound may be an easily decomposable organic sulphur compound such as di-
methyl disulphide, carbon disulphide and butyl thiol or a mixture of easily de-
composable organic sulphur compounds. It is also possible to use refinery gas
or
liquid streams containing decomposable sulphur compounds.
It was surprisingly observed from the examples that with added sulphur com-
pounds in the feed, resulting in sulphur contents of 100 - 10000 w-ppm in the
feed the decarboxylation of short chain fatty acids and derivatives, such as
C16
fatty acids increases significantly more than that of C18 fatty acids.
When C16 containing fatty acids and derivatives thereof are hydrodeoxygenated,
n-C15 and n-C16 paraffins are formed, with melting points of 9.9 C and 18.2 C
respectively. The conversion of other vegetable oils like rapeseed oil and
soybean
oil produces almost totally n-C17 and n-C18 paraffms with significantly higher
melting points of 22.0 and 28.2 C.
Hydrodeoxygenation of triglycerides facilitates controlled decomposition of
the
triglyceride molecule contrary to uncontrolled cracking. Double bonds are also
hydrogenated during the controlled hydrotreatment. Light hydrocarbons and
gases
formed, mainly propane, water, C02, CO, H2S and NH3, are removed from hy-
drotreated product.
In the case the fresh feed comprises more than 5 wt-% free fatty acids, it is
prefer-
able to use diluting agent or product recycle in the process as described in
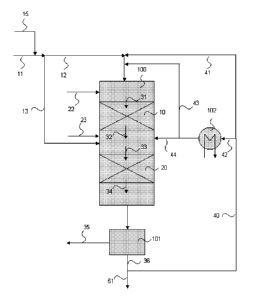
Figure
1, wherein an improved reactor configuration is presented, particularly for
the
control of the increase of temperature over catalyst bed and side reaction
forma-
tion. In Figure 1 a hydrotreatment process configuration is provided,
comprising
one or more catalyst beds in series, hydrotreated product recycle introduction
on
the top of the first catalyst bed and fresh feed, quench liquid and hydrogen
intro-
duction on top of each catalyst beds. This results in improved control of the
reac-
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
16
tion temperature in the catalyst beds and hence diminishes undesired side reac-
tions.
In Figure 1 the hydrotreatment reactor 100 comprises two catalyst beds 10 and
20.
Fresh feed 11 is introduced as streams 12 and 13 on the catalyst beds 10 and
20,
respectively, and hydrogen as stream 22 and 23 on the catalyst beds 10 and 20,
respectively. The fresh feed stream 12 is first mixed with the hydrotreated
product
recycle stream 41 and quench liquid stream 43 and the resulting mixture 31, di-
luted in the fresh feed concentration, is then introduced on the catalyst bed
10. In
order to obtain a required sulphur concentration in the feed stream 31,
required
amount of sulphur make up is added to the fresh feed stream 11 via stream 15.
As
mixture 31 passes through the catalyst bed 10 with the hydrogen stream 22,
fatty
acids and fatty acid derivatives of the fresh feed stream 12 are converted to
the
corresponding reaction products. A two-phase stream 32 is withdrawn from the
bottom of the catalyst bed 10 and is mixed with the fresh feed stream 13,
quench
liquid stream 44 and the hydrogen stream 23. The formed vapor-liquid mixture
33, diluted in the fresh feed concentration, is then introduced on the
catalyst bed
20 at reduced temperature due to cooling effect of the hydrogen, quench liquid
and fresh feed, passed through the catalyst bed 20 and finally withdrawn from
the
catalyst bed as a product stream 34. The stream 34 is separated in to a vapor
stream 35 and liquid stream 36 in the high temperature separator 101. Vapor
stream 35 is rich in hydrogen and is directed to further treatment. Part of
the liquid
stream 36 is returned to the reactor 100 as recycle stream 40, which is
further di-
vided to dilution stream 41 and total quench liquid stream 42. The quench
liquid
stream 42 is cooled in the heat exchanger 102 to provide adequate cooling
effect
on the top of the catalyst beds 10 and 20. Hydrotreated product stream 51 is
di-
rected from the hydrotreatment step to further processing.
The catalyst beds 10 and 20 may be located in the same pressure vessel or in
sepa-
rate pressure vessels. In the embodiment where the catalyst beds are in the
same
pressure vessels the hydrogen streams 22 and 23 may alternatively be
introduced
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
17
on the catalyst bed 10 and then be passed through the catalyst beds 10 and 20.
In
the embodiment where the catalyst beds are in separate pressure vessels, the
cata-
lyst beds may operate in parallel mode with separate dilution streams,
hydrogen
streams and quench liquid streams. The number of catalyst beds may be one or
two or more than two.
The sulphur make up to the hydrotreatment step may be introduced with the
fresh
feed stream 11. Alternatively, required amount of sulphur may be fed with the
hydrogen streams 22 and 23 as gaseous sulphur component such as hydrogen sul-
phide.
Hydrogen is fed to the hydrotreating reactor in excess of the theoretical
hydrogen
consumption. During the hydrotreating step, triglyceride oils, fatty acids and
de-
rivatives thereof are almost theoretically converted to n-paraffins without or
al-
most without side reactions. Additionally, propane is formed from the glycerol
part of the triglycerides, water and CO and/or CO2 from carboxylic oxygen, H2S
from organic sulphur compounds and NH3 from organic nitrogen compounds.
Using the above described procedures in the hydrotreating step, the
temperature
needed for reactions to start up is achieved in the beginning of each catalyst
bed,
the temperature increase in the catalyst beds is limited, harmful and
partially con-
verted product intermediates can be avoided and the catalyst life is extended
con-
siderably. The temperature at the end of the catalyst bed is controlled by net
heat
of reactions and to the extent of diluting agent used. Diluting agent may be
any
hydrocarbon available, bio-origin or non bio-origin. It can also be product
recycle.
If diluting agent is used, fresh feed content from total feed is less than 20
wt-%. If
the product recycle is used, product recycle/fresh feed ratio is 5-30:1,
preferably
10-30:1, most preferably 12-25:1. After the hydrotreatment step, the product
is
subjected to an isomerisation step.
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
18
Isomerisation of n-paraffins formed during hydrotreatment
In order to improve the cold properties of the products, isomerisation of n-
paraffms are needed. During the isomerisation branched isoparaffms are formed.
Isoparaffms may typically have mono-, di-, tri- or tetramethyl branches.
The product obtained from the hydrotreatment step is isomerised with a
catalyst
under isomerisation conditions. The feed into the isomerisation reactor is a
mix-
ture of pure n-paraffins and the composition of the feed can be predicted from
the
fatty acid distribution of each individual bio oil used as feed to the
hydrotreat-
ment.
The isomerisation step may comprise an optional purification step, wherein the
reaction product from the hydrotreatment step may be purified using a suitable
method such as stripping with water vapour or a suitable gas such as light
hydro-
carbon, nitrogen or hydrogen. Preferably acid gases and water impurities are
re-
moved as completely as possible before the hydrocarbons are contacted with the
isomerization catalyst.
In the isomerisation step, the pressure varies in the range of 2-15 MPa,
preferably
in the range of 3-10 MPa and the temperature varies between 200 and 500 C,
preferably between 280 and 400 C.
In the isomerisation step, isomerisation catalysts known in the art may be
used.
Suitable isomerisation catalysts contain a molecular sieve and/or a metal
selected
from Group VIII of the Periodic Table and/or a carrier. Preferably, the
isomerisa-
tion catalyst contains SAPO-11 or SAPO-41 or ZSM-22 or ZSM-23 or ferrierite
and Pt, Pd or Ni and A1203 or Si02. Typical isomerisation catalysts are, for
exam-
ple, Pt/SAPO-11/A1203, Pt/ZSM-22/A1203, Pt/ZSM-23/A1203 and Pt/SAPO-
11/Si02. Most of these catalysts require the presence of hydrogen to reduce
the
catalyst deactivation.
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
19
The isomerised diesel product consists mainly of branched hydrocarbons and
also
linear hydrocarbons and it has a boiling range of 180 - 350 C. Additionally
some
gasoline and gas may be obtained.
Advantages of the invention
The process according to the invention provides a way to reduce the formation
of
higher molecular weight compounds during the hydrotreatment of the fresh feed,
which may contain fatty acids and derivatives thereof. The process according
to
the invention provides selective manufacture of diesel range hydrocarbons from
bio oils and fats with high diesel yield and without significant side-
reactions.
Branched hydrocarbons can be manufactured from plant and vegetable oils and
fats as well as animal and fish oils and fats using promoted assistance of
decarb-
reactions during hydrodeoxygenation and therefore the consumption of hydrogen
is decreased by 20-60 %, typically 20-40 %.
During the deoxygenation of the feed through decarboxylation and/or decarbon-
ylation, oxygen is removed in the form of CO and CO2. The decarb-reactions de-
crease hydrogen consumption, theoretically in complete deoxygenation about 60-
70 % compared to complete hydrodeoxygenation route, but depends on the
triglyceride source. C12-C16 fatty acids and their derivatives have typically
lower
amount of double bonds and their decarboxylation tendency is lower than higher
carbon number fatty acids and their derivatives during hydrodeoxygenation. How-
ever, it was surprisingly found that when 50-20 000 w-ppm of sulphur,
calculated
as elemental sulphur, was present in the feed comprising fresh feed containing
at
least 20 % by weight of C12-C16 fatty acids and/or their derivatives, the
decar-
boxylation of C16 fatty acids and derivatives thereof increases significantly
more
than that of C18 fatty acids and its derivatives. This results in still lower
consump-
tion of hydrogen. Added sulphur compounds in hydrodeoxygenation feed facili-
tate the control of catalyst stability and reduce hydrogen consumption.
Feedstock
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
5 like palm oil or animal fat, containing more saturated fatty acid
derivatives, pro-
duces less heat.
It was also found that feeds having a high content of C12-C16 fatty acids
and/or
their derivatives decreases hydrogen consumption in the isomerisation step and
10 also improve cold properties of diesel fuel. The yield of diesel range
hydrocarbons
is especially increased during isomerisation of n-paraffins due to the lower
crack-
ing of n-paraffins formed from the fatty acid derivative feed to
hydrotreatment.
The C11-C16 n-paraffins formed during hydrotreatment need lower conversion and
lower reaction temperature during isomerisation in order to maintain same cold
15 properties of diesel and thus significantly lower the extent of cracking
and coke
formation compared to heavier n-paraffms. Alternatively improved cold proper-
ties can be achieved at the same reaction temperature without yield loss. The
sta-
bility of the catalysts during hydrotreating and isomerisation is increased.
20 The invention is illustrated in the following with examples presenting some
pref-
erable embodiments of the invention. However, it is evident to a man skilled
in
the art that the scope of the invention is not meant to be limited to these
examples.
Examples
Example 1. Effect of sulphur content of total feed
Palm oil containing 0.3 area-% of free fatty acids was used as the fresh feed,
along with product recycle 5:1 in the presence of hydrogen. The content of
triglyceride C12-C16 fatty acids in the fresh feed was 58.3 wt-%. The total
feed
contained alkaline and alkaline earth metals, calculated as elemental alkaline
and
alkaline earth metals in a amount of below 10 w-ppm. The amount of other met-
als, calculated as elemental metals, in the feed was below 10 w-ppm. The
amount
of phosphorus, calculated as elemental phosphorus was below 30 w-ppm.
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
21
During the test runs various amounts of dimethyl disulfide in the total feed
were
used. The reaction temperature was 305 C, reactor pressure was 5 MPa and
space
velocity was 0.5 g/g for fresh feed. Higher content of sulphur in feed
significantly
increased the total deoxygenation reactions through CO and CO2 (decarb-
reactions, production of one carbon less n-paraffins than original fatty acid)
in-
stead of deoxygenation by hydrogen (HDO, production of same carbon number n-
paraffins than original fatty acid). However the decarb-reactions of C16-fatty
ac-
ids increased significantly more than decarb-reactions of higher C18 or C20
fatty
acids. High content of sulphur in the feed decreased the double bond hydrogena-
tion activity of catalyst and also decreased decarb-reactions as can be seen
from
table 1, wherein the effect of sulphur content of total feed calculated as
elemental
sulphur, on decarb-% of different carbon number fatty acids observed in
product
oil (decarb-% calculated from fresh feed) is presented. Table 2 discloses
relative
increase of decarb-reactions compared to the feed with 100 w-ppm of sulphur
and
table 3 presents theoretical decrease of hydrogen consumption due to decarb-
reactions.
Table 1. Effect of sulphur content of total feed calculated as elemental
sulphur
Sulphur Sulphur Sulphur Sulphur Sulphur Sulphur
100 570 w- 1000 w- 3000 w- 5000 w- 10000
w-ppm ppm ppm ppm ppm w-ppm
C15/(C15+C16) 29.1 % 45.6 % 52.6 % 55.1 % 56.2 % 47.5 %
C17/(C17+C18) 30.2% 37.5% 40.1% 42.5% 43.3% 39.7%
C19/(C19+C20) 36.6 % 43.4 % 46.0 % 48.1 % 49.2 % 46.5 %
Total decarb-% 32.0 % 42.2 % 46.2 % 48.6 % 49.5 % 44.6 %
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
22
Table 2. Relative increase of decarb-reactions
Sulphur Sulphur Sulphur Sulphur Sulphur
570 ppm vs 1 000 ppm vs 3 000 ppm vs 5 000 ppm vs 10 000 ppm
100 ppm 100 ppm 100 ppm 100 ppm vs 100 ppm
C16 56.8 % 80.9 % 89.5 % 93.2 % 63.3 %
C18 24.1 % 32.7% 40.7% 43.2% 31.4%
C20 18.7 % 25.7 % 31.5 % 34.4 % 27.1 %
Total 31.9 % 44.6 % 52.0 % 55.0 % 39.5 %
Table 3. Theoretical hydrogen consumption with and without decarb-reactions
Rapeseed Palm stearin Palm oil Animal fat
oil
Hydrogen consumption (H2 molecules per triglyceride), 100% deoxygenation by hy-
drodeox enation
Water 6 6 6 6
Subst. Hy-
drogen 6 6 6 6
Double
bonds 4 1.16 1.8 2
Ttotal 16 13.16 13.8 14
Hydrogen consumption (H2 molecules per triglyceride),100% deoxygenation by de-
carb-reactions
Water 0 0 0 0
Subst. Hy-
drogen 3 3 3 3
Double bonds 4 1.16 1.8 2
Total 7 4.16 4.8 5
H2 reduction
(max) 56% 68% 65% 64%
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
23
Example 2. Effect of C16 fatty acids on cracking during isomerisation and
diesel yield at same pour point level with palm oil feed
Palm oil containing 44.8 wt-% of triglyceride C12-C16 fatty acids was used in
the
fresh feed. Dimethyl disulphide was added to palm oil to obtain sulphur
content of
about 600 w-ppm in the feed, calculated as elemental sulphur. The feed purity
was
same as in example 1, but the amount of free fatty acids was 0.2 area-%. No
dilut-
ing agent was used. The feed was hydrotreated at 305 C in the presence of hy-
drogen, reactor pressure was 5 MPa and space velocity was 2 g/g for fresh
feed.
The products contained mainly n-paraffins. The n-paraffin feeds were
isomerised
at 317 C, 4 MPa and WHSV was 3 1/h in presence of hydrogen. The catalyst (A)
contained Pt, SAPO-11 and an alumina support. The amount of hydrocarbons
>C 10 was 97 wt-% in the product. The cloud point of the liquid product was -
22
C. Results of analysis of the product are provided in table 4.
A comparative test was carried out with rapeseed oil feed. Rapeseed oil
contained
of 4.5 wt-%. of triglyceride C12-C16 fatty acids. Rapeseed oil was
hydrotreated and
isomerised at the same reaction conditions as described above. The amount of
hydrocarbons >C 10 was 96 wt-% in the product. The cloud point of the liquid
product was -15 C. Results of analysis of the product are provided in table
4.
Example 3. Effect of C16 fatty acids on pour point of isomerised diesel oil at
same diesel yield with palm oil feed
The hydrotreated palm oil obtained in Example 2 was isomerised at 312 C, 4
MPa and WHSV was 3 1/h in the presence of hydrogen with catalyst A. This
yielded a liquid product with a cloud point of -14 C. The amount of hydrocar-
bons >C10 was now 98 wt-% in the product. A small amount of lighter hydrocar-
bons can be concluded from the flash point and in the distillation curve of
the
products as can be seen from table 4, which presents analysis results of hy-
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
24
drotreated and isomerised products from rapeseed oil and palm oil, and
HRO=hydrotreated rapeseed oil, HPO=hydrotreated palm oil.
Table 4. Analysis results of hydrotreated and isomerised products from
rapeseed
oil and palm oil.
Feed Method Unit HRO HPO HPO
Isomerisation T C 317 317 312
EN ISO
Density 15 C 12185 kg/m3 782.7 779.2 779.3
ASTM
Cloud point D5773 C -15 -22 -14
ASTM
Pour point D5949 C -24 -33 -24
CFPP EN 116 C -15 -22 -15
Flash point EN 22719 C 52 53 65
Distillation TA C 117 123 185
EN ISO 3405 5 vol-% C 274 264 268
vol-% C 282 270 274
30 vol-% C 290 279 280
50 vol-% C 292 283 283
70 vol-% C 294 287 287
90 vol-% C 299 294 294
95 vol-% C 306 298 299
TL C 327 311 308
Example 4. Effect of C16 fatty acids on cracking during isomerisation and
diesel yield at same pour point level with animal fat feed
Animal fat containing 30 wt-% of triglyceride C12-C16 fatty acids was used as
fresh feed. The feed contained alkaline and alkaline earth metals, calculated
as
CA 02614014 2008-01-02
WO 2007/003708 PCT/F12006/050300
5 elemental alkaline and alkaline earth metals in the amount of below 10 w-
ppm.
The amount of other metals, calculated as elemental metals, in the feed was
below
10 w-ppm. The amount of phosphorus, calculated as elemental phosphorus was
below 30 w-ppm. Dimethyl disulphide was added to animal fat to obtain sulphur
content of about 100 w-ppm in the feed. Fresh feed contained free fatty acids
0.6
10 area-%. The feed was hydrotreated at 300 C in the presence of hydrogen,
reactor
pressure was 5 MPa and space velocity was 2 g/g for fresh feed without any
dilut-
ing agent. The products contained mainly n-paraffins. The n-paraffin feeds
were
isomerised at 316 C, 4 MPa and WHSV was 1.5 1/h in the presence of hydrogen.
The catalyst (B) contained Pt, SAPO-11 and an alumina support. The amount of
15 hydrocarbons >C10 was 95 wt-% in the product. The cloud point of the liquid
product was -20 C.
As a comparative example, rapeseed oil was hydrotreated and isomerisated at
the
same reaction conditions as described above. Rapeseed oil contained 4.5 wt-%
of
20 triglyceride C12-C16 fatty acids. In the isomerised product, the amount of
hydro-
carbons >C10 was 95 wt-%. The cloud point of the liquid product was -14 C.
Example 5. Effect of C16 fatty acids on pour point of isomerised diesel oil at
same diesel yield with animal fat feed
The hydrotreated animal fat obtained in Example 4 was isomerised at 312 C, 4
MPa and WHSV was 1.5 1/h in the presence of hydrogen with catalyst B. This
yielded a liquid product with a cloud point of -13 C. The amount of hydrocar-
bons >C10 was now 98 wt-%.