Note: Descriptions are shown in the official language in which they were submitted.
CA 02614171 2007-12-10
WO 03/029276 PCT/11 SO 2,13
COPOLYMERS FOR SUPPRESSION OF AUTOIMMUNE
DISEASES, AND METHODS OF USE
Technical Field
The invention relates to design of copolymers having particular amino acids in
specific molar ratios, synthesized into polypeptides of predetermined length
and capable of
suppression of symptoms and frequency of recurrent episodes of an autoirnmune
disease.
Background
Multiple sclerosis (MS) is an inflammatory disease of the central nervous
system
affecting 0.1 % of the population, and is associated in northern European
Caucasoid MS
patients with the HLA-DR-2 (DRB I * 1501) haplotype (Olerup, O. et al. 1991.
Tissue
Antigens 38:1-15). An animal model of MS, experimental autoimmune
encephalomyelitis
(ERAE), is a T cell-mediated autoimmune disease. EAE can be induced by
subcutaneous
injection of peptides derived from myelin components such as myelin basic
protein (MBP;
Madsen, L.S. et al. 1999. Nat. Genet. 23:343-347), proteolipid protein (PLP;
Greer, J.M. et al.
1992. J. Inununol. 149:783-788) or myelin oligodendrocyte glycoprotein (MOG;
Mendel, 1.
et al. 1995. Eur. J. hnmunol. 25:1951-1959).
In the course of EAE, autoreactive CD4} T cells recognize self-antigens
presented by
murine class II MHC molecules (e.g. H-2A), ultimately leading to pathological
changes that
can be monitored as clinical signs of disease. EAE provides a well studied
system for testing
the efficacy of potential therapeutic compounds to suppress the disease. These
compounds
have included cytokines (Leonard, J.P. et al. 1996. Ann. N. Y. Acad Sci.
795:216-226),
peptide antigens that induce anergy (Gaur, A. et al. 1992. Science 258:1491-
1494) or that
induce oral tolerance (Kennedy, K.J. et al. 1997. J. Imnninol. 159:1036-1044;
Weiner, H.L.
1997. hnmunol. Today 18:335-343), or altered peptide ligands (Pfeiffer, C. et
al. 1995-1
Exp. Med. 181:1569-1574; Nicholson, L.B. et al. 1997. Proc. Natl. Acad. Sci.
USA
94:9279-9284).
Copolymer 1 (Copt; Copaxone YEAK) is a random amino acid copolymer of
alanine (A), lysine (K), glutamic acid (E) and tyrosine (Y) in a molar ratio
of approximately
5:3:1.5:1. Copt is synthesized in solution using N-carboxyamino acid
anhydrides
(Teitelbaum D. et al. 1971. Eur. J. Innnsnol. 1:242-248). Initially, this and
other related
copolymers were used to define the genetic basis of immune responsiveness, now
known as
class II M-IC genes (McDevitt, HØ, and M. Sela. 1965. J. Exp. Med. 122:517-
532;
McDevitt, H.O., and M. Sela. 1967. J. Exp. Med. 126:969-978). Cop 1, also
known as poly
(Y,E,A,K) or YEAK was found to be effective both in suppression of
experimental allergic
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/313;,
encephalomyelitis (Teitelbaum D. et al. 1971. Eur. J. hmmunol. 1:242-248;
Teitelbaum D. et
al_ 1973. Eur. J. hnmunol. 3:273--279; Teitelbaum D, et al. 1974; Clin.
Immunol.
Imnnmopathol. 3:256-262; Aharoni R. et al. 1993. Eur. J. Immunol. 23:17-25)
and in the
treatment of relapsing forms of multiple sclerosis (MS; Bornstein, M.B. et al.
1987. N. Engl.
J Med. 317:408-414; Johnson, K.'. et .1. 1995. Neurology 45:1268-1276;
Johnson, K.P. et
al. 1998. Neurology 50:701-708).
CopI has been approved as a therapy for MS and currently is in wide use.
However,
while Cop] reduces the MS relapse rate, it does not eliminate relapse, and is
not curative for
the disease. It is important to develop improved compositions and methods of
use for
treatment of MS, and for other autoimmune diseases.
Summary
A feature of the invention is a linear random amino acid copolymer YFAK
comprising tyrosine (Y), phenylalanine (F), alanine (A) and lysine (K) in a
molar ratio of
(Y+F):A:K of about 1:5:3. The expression "(Y+F)" means the sum of the molar
ratios of Y
and F, compared to the molar ratios of each of A and K.
The amino acids are polymerized by a solid phase reaction; in an alternative
embodiment, the amino acids are polymerized by solution chemistry. In a
related
embodiment, the molar ratio of F to Y is about 1, for example, the molar ratio
of F to Y is at
least about 2, or Y is about 4.
In an alternative embodiment, the molar ratio of Y is greater than F, for
example, the
molar ratio of Y to F is at least about 2, or the molar ratio of Y to F is at
least about 4. In
general, the copolymer is at least about 25 amino acid residues in length, for
example, the
copolymer is at least about 35amino acid residues, at least about 50 amino
acid residues, or at
least about 70 amino acid residues in length.
In one embodiment, the invention provides a linear random amino acid copolymer
comprising Y:F:A:K in a molar ratio of about 0.2:0.8:5:3. In a related
embodiment, the
invention provides a linear random amino acid copolymer comprising Y:F:A:K in
a molar
ratio of about 0.5:0.5:5:3. In another related embodiment, the invention
provides a linear
random amino acid copolymer comprising Y:F:A:K in a molar ratio of about
0.8:0.2:5:3. In
general, the copolymer amino acids are polymerized using a solid phase
reaction;
alternatively, the copolymer amino acids are polymerized by solution phase
chemistry.
In another aspect, the invention provides a linear random amino acid copolymer
VFAK comprising valine (V), phenylalanine (F), alanine (A) and lysine (K). In
another
aspect, the invention provides a linear random amino acid copolymer VWAK
comprising
2
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/3139>
valine (V), tryptophan (W), alanine (A) and lysine (K). In another aspect, the
invention
provides a linear random amino acid copolymer VYAK comprising valine (V),
tyrosine (Y),
alanine (A) and lysine (K). In another aspect, the invention provides a linear
random amino
acid copolymer FAK comprising phenylalanine (F), alanine (A) and lysine (K),
in a molar
ratio F:A:K of about 1:5:3. In another aspect, the invention provides a linear
random amino
acid copolymer VAK comprising valine (V), alanine (A) and lysine (K) in a
molar ratio
V:A:K of about 1:5:3. In another aspect, the invention provides a linear
random amino acid
copolymer WAK comprising tryptophan (W), alanine (A) and lysine (K) in a molar
ratio
W:A:K of about 1:5:3. In another aspect, the invention provides a linear
random amino acid
copolymer V WAK comprising valine (V), tryptophan (W), alanine (A) and lysine
(K), in a
molar ratio (V+W):A:K of about 1:5:3. The expression "(V+W)" means the sum of
the molar
ratios of V and W, compared to the molar ratios of each of A and K.
In another aspect, the invention provides a linear random amino acid copolymer
VWAK comprising valine (V), tryptophan (W), alanine (A) and lysine (K), in a
molar ratio
V:W:A:K of about 0.5:0.5:5:3. In another aspect, the invention provides a
linear random
amino acid copolymer VEAK comprising valine (V), glutamic acid (E), alanine
(A) and
lysine (K), in a molar ratio V:E:A:K of about 1: I.5:5:3. In another aspect,
the invention
provides a linear random amino acid copolymer FEAK comprising phenylalanine
(F),
glutamic acid (E), alanine (A) and lysine (K), comprising F:E:A:K in a molar
ratio of about
1:1.5:5:3. In another aspect, the invention provides a linear random amino
acid copolymer
VYAK comprising valine (V), tyrosine (Y), alanine (A) and lysine (K), in a
molar ratio
(V+Y):A:K of about 1:5:3. The expression "(V+Y)" means the sum of the molar
ratios of V
and Y, compared to the molar ratios of each of A and K. In another aspect, the
invention
provides a linear random amino acid copolymer VYAK comprising valine (V),
tyrosine (Y),
alanine (A) and lysine (K), in a molar ratio V:Y:A:K of about 0.5:0.5:5:3.
Further, any of the
compositions provided here may be provided in a pharmaceutically acceptable
buffer, and/or
in a unit dosage.
The featured copolymers herein are comprised of amino acids as described, and
are
further considered to be equivalent to copolymers sharing the amino acid
compositions as
described and also containing one or more additional substituents, for
example, have one or
more additional amino acids, such that the resulting copolymer has about the
same function.
For example, a copolymer FEAK, FAK, VWAK, VYAK, YFAK, or any of the copolymer
compositions as provided herein, which is comprised substantially of this
composition, i.e, is
at least about 60%, or at least about 70%, or at least about 80%, or at least
about 90%, or at
CA 02614171 2007-12-10
51621-3 (S)
least about 95% or about 99% the composition provided herein, and has about
the same
functional properties as a copolymer provided herein, is considered equivalent
to the
composition as provided herein. The function is considered to be about the
same if a dosage
of a composition herein that is effective for treating an autoimmune disease
is about the same
as a dosage of a copolymer comprising substantially the same subsitutents as a
composition
herein, for treating the autoimmune disease.
The featured copolymer compositions herein can be combined with at least one
additional therapeutic agent. In related embodiments, the additional therapeut
ic agent is an
antibody, an enzyme inhibitor, an antibacterial, an antiviral, a steroid, a
nonsteroidal
anti-inflammatory, an antimetabolite, a cytokine, a cytokine blocking agent,
an adhesion
molecule blocking agent or a soluble cytokine receptor. For example, the
cytokine is selected
from the group consisting of a-interferon, interleukin-4 and interleukin-10.
An embodiment of the invention is a kit comprising at least one unit dosage of
a
copolymer described above, and a pharmaceutically acceptable carrier.
A feature of the invention is a method of manufacture of a composition for use
in
treating a subject having an autoimmune disease, wherein the composition
comprises any of
random linear amino acid copolymers FAK, YFAK, VYAK, VWAK, VEAK and FEAK. In
general, the copolymer has a length of at least about 50 residues, for
example, at least about
70 residues. Further, in such a use, the composition further comprises a
pharmaceutically
acceptable carrier. Further, the use can involve administering the composition
in an effective
dose. An "effective dose" is an amount of the composition that remediates
either or both of
clinical symptoms and frequencey of recurrence of an autoimmune disease. Prior
to
administering, the copolymer is selected for inhibiting binding of an
autoantigenic peptide to
an MHC class II protein associated with the autoinunune disease. For example,
the
copolymer that inhibits a class II-specific T cell response to an MHC class II
protein-peptide
complex is selected. The autoimmune disease is selected from the group
consisting of
Hashimoto's thyroiditis; idiopathic myxedema, a severe hypothyroidism;
multiple sclerosis, a
demyelinating disease; myasthenia gravis; Guillain-Barre syndrome; systemic
lupus
erythematosis; uveitis; autoimmune oophoritis; chronic immune thrombocytopenic
purpura;
colitis; diabetes; Grave's disease; psoriasis; pemphigus vulgaris; and
rheumatoid arthritis, and
others. In a preferred embodiment, the autoimmune disease is multiple
sclerosis; the
autoimmune disease is rheumatoid arthritis; or the autoimmune disease is
diabetes. An
additional therapeutic agent, can be co-administered, for example, the
additional therapeutic
agent is an antibody, an enzyme inhibitor, an antibacterial agent, an
antiviral agent, a steroid,
4
CA 02614171 2010-03-25
51621-3D
a nonsteroidal anti-inflammatory agent, an antimetabolite, a
cytokine, a cytokine blocking agent, an adhesion molecule
blocking agent, or a soluble cytokine receptor. The
cytokine is: interferon-a, interleukin-4, or
interleukin-10. The enzyme inhibitor is a protease
inhibitor or a cyclooxygenase inhibitor.
In another aspect of the present invention, there
is provided a linear random amino acid copolymer YFAK
comprising tyrosine (Y), phenylalanine (F), alanine (A) and
lysine (K) in a molar ratio of (Y+F):A:K other than about
1:5:3.
In another aspect of the present invention, there
is provided a linear random amino acid copolymer comprising
substantially tyrosine (Y), phenylalanine (F), alanine (A)
and lysine (K) in a molar ratio of (Y+F):A:K other than
about 1:5:3, further comprising at least one amino acid
modification at a residue location and in an amount
sufficient to inhibit proteolytic degradation of the
copolymer in a subject, compared to a copolymer which is
otherwise identical and lacking the amino acid modification.
In another aspect of the present invention, there
is provided a kit comprising at least one unit dosage of the
copolymer as described above and a pharmaceutically
acceptable carrier.
In another aspect of the present invention, there
is provided use in the preparation of a medicament for
treating a subject having an autoimmune disease of a random
linear amino acid copolymer as described above or a
combination thereof.
In another aspect of the present invention, there
is provided use for treating a subject having an autoimmune
disease of a random linear amino acid copolymer as described
above or a combination thereof.
5
CA 02614171 2007-12-10
51621-3D
In another aspect of the present invention, there
is provided a random linear amino acid copolymer as defined
above for use for treating a subject having an autoimmune
disease.
In another aspect of the present invention, there
is provided a kit comprising the copolymer as described
above, or a combination thereof and instructions for use for
treating a subject having an autoimmune disease.
In another aspect of the present invention, there
its provided a pharmaceutical composition comprising a
copolymer as described above, or a combination thereof,
combined with a pharmaceutically acceptable carrier or
diluent.
5a
CA 02614171 2007-12-10
51621-3D
Brief Description of the Drawings
Figure I is a set of panels of graphs showing inhibition of 1-ILA-DR-2-
restricted MBP
84-1 02-specific T cell lines 2E12 (panel A), 8073 (panel B) and Hy1B (panel
C), in the
presence of the random copolymers. Irradiated L466 (A) or MGAR (B, C) cells
were
co-incubated in duplicate with MBP 85-99 (SEQ ID NO: 2) at a final
concentration of 4 pM
(A) or 12.5 pM (B, C) and different concentrations of each of the random
copolymers as
indicated for 2 hr at 37 C, then T cells were added and incubated for 24 hr at
37 C.
Supernatants (30 pl) were incubated with IL-2-dependent c)tolytic T-cell
lymphocytes
(CTLL), followed by labeling with 3H-thymidine (1 pCi/well) for 12 hr.
Figure 2 is a set of line graphs (A) showing inhibition of HLA-DR-2-restricted
PLP
40-60-specific human T cells 1 06 A, and a set of bar graphs (B and C) showing
inhibition of
H-2'-restricted PLP 139-151-specific mouse T cell hybridomas (hPLP/I and
hPLP/c4,
respectively), in the presence of random copolymers. Irradiated L466 (Figure
3A) or
splenocytes from SJLJJ (B and C) mice were co-incubated with the proteolipid
protein
peptide PLP 40-60 (SEQ ID NO: 3) at a final concentration of 60 pM (A) and the
concentrations of different copolymers as indicated on the abscissa, or with
PLP 139-1-51
peptide (SEQ ID NO: 4; in B and C) at the final concentration of 24 pM, and
the different
copolymers (28 pM) for 2 hr at 37 C, then T cells were added and incubated for
24 hr at
37 C. Supernatants (30 pl) were incubated with IL-2-dependent CTLL, followed
by labeling
with 3H-thymidine (1 pCiiwell) for 12 hr. * indicates 0% inhibition.
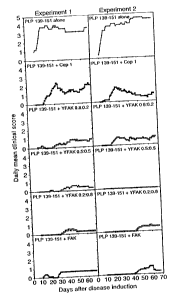
Figure 3 is a set of graphs showing suppression by different random
=copolymers
VEAK., FEAK, and Copaxone , of EAE induced with PLP 139-151 (SEQ ID NO: 4)
.peptide. SJL/J mice were co-injected subcutaneously with 50 Vg of PLP 139-151
(SEQ ID
NO: 4) peptide and 500 pg of the indicated random copolymers, or with PLP 139-
151 (SEQ
ID NO: 4) alone. Progression of the disease was monitored for the appearance
of clinical
symptoms, scored on the ordinate, for the days shown on the abscissa Results
shown on the
ordinate represent the mean daily score of clinical symptoms.
Figure 4 is a graph showing inhibition of binding of biotinylated MBP 86-
100(SEQ
ID NO: 1) to HLA-DR-2 molecules by random copolymers FAK, YFAK (0.8:0.2), YFAK
5b
CA 02614171 2007-12-10
51621-3(S)
(0.2:0.8), YFAK (0.5:0.5), and Cop 1. Recombinant water-soluble HLA-DR-2
molecules
were incubated with biotinylated MBP 86-100 (SEQ ID NO: 1; 0.13 pM) and with
the
unlabeled random copolymers or the synthetic unlabeled peptide control MBP 85-
99 (SEQ
ID NO: 2), at concentrations shown on the abscissa. Incubations were carried
out in
duplicate at pH 7.0 for 40 hr at 37 C. Results shown as inhibition of binding
on the ordinate
represent one out of two independent experiments. Specific binding is
expressed as
percentage of inhibition using the formula: percentage of inhibition = 100% -
[(absorbance at
410 nm with competitor - background)/absorbance without competitor -
background) x 100].
The signals at 410 nm without competitor were 0.8-0.9 and the background was
0.1.
Figure 5 is a set of graphs showing inhibition of HLA-DR-2-restricted MBP
84-102-specific T cells for each of cell lines 2E12, 8073 and Hy1B, in the
presence of
random copolymers FAK, YFAK (0.8:0.2), YFAK (0.2:0.8), YFAK (0.5:0.5), and Cop
L,
Irradiated MGAR cells were co-incubated in duplicates with MBP 85-99 (SEQ ID
NO: 2) at
the final concentration of 12.5 pM and different concentrations of the random
copolymers for
2 hr at 37 C, then T cells were added and incubated for 24 hr at 37 C.
Supernatants (30 pl)
were incubated with each of the IL-2-dependent CTLL cell lines as indicated,
and were
labeled with 3H-thymidine (1 pCi/well) for 12 hr.
Figure 6 is a set of graphs showing suppression by different random copolymers
FAX,
YFAK 0.2:0.8, YFAK 0.8:0.2, YFAK 0.5:0.5, or Copaxone of EAE induced with PLP
139-151 (SEQ ID NO: 4) peptide. SJ IJ mice were co-injected subcutaneously
with 50 pg of
PLP 139-151 (SEQ ID NO: 4) peptide and 500 pg of the indicated random
copolymers, or
immunized with PLP 139-151 (SEQ ID NO: 4) alone. Progression of the disease
was
monitored for the appearance of clinical symptoms for the days after disease
induction shown
on the abscissa. Figure 6A shows the results of the mean daily score of
clinical symptoms as
shown on the ordinate for each group of five to nine mice per group in each of
two
experiments. Figure 6B shows data for each individual mouse, with the
copolymer treatment
of the group listed at the top of each column, and the maximal clinical score
observed for the
mouse indicated in the upper right hand corner of each box, for a
representative experiment
Figure 7 is-a set of line graphs showing suppression, by different random
copolymers
YFAK, VWAK, VWAK, or Cop 1, of EAE induced with MBP 85-89 (SEQ ID NO: 2)
peptide, and control mice not treated with copolymer. Humanized mice (Madsen,
L.S. et al.
1999 Nat. Genet. 23(3): 343 -34 7 )
carry transgenes HLA DR-2 (DRA* 0101 and DRBI * 1501) and TCR from MS patient
Ob,
which is a V(D)J rearrangement of TCRa and TCR3 amplified from clone Ob.1A12.
6
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/313;.
Co-immunized mice were co-injected on day 0 with 500 g of the copolymer or
control
material as indicated, and 50 g of the EAE inducing peptide MBP 85-89 (SEQ ID
NO: 2).
Pre-immunized mice were preinjected with the copolymer two days prior to EAE
induction.
The copolymers VYAK and VWAK respectively, have molar ratios of 0.5:0.5:5:3 of
V:Y:A:K and of V:W:A:K, respectively. The data points indicate progression of
the disease
by scoring of clinical symptoms, on the ordinate, on each of days 3, 5, 7, 9,
11, 14, 16, 18, 22,
25, 28, 32, 37, 40, 43 and 50, on the abscissa.
Figure 8 is a set of line graphs, replotted together from data for three of
the groups of
animals from Figure 7: diamonds are control EAE-induced mice not further
receiving
copolymer treatment; squares are EAE-induced mice treated with YFAK 0.5:0.5;
and
triangles are EAE-induced mice treated with Copl. Each treatment in this
figure was
administered two days prior to EAE induction, i.e., vaccination against
disease.
Description of Specific Embodiments
Unless the context otherwise requires, as used in this description and in the
following
claims, the terms below shall have the meanings as set forth:
The term "autoimmune condition" or "autoimmune disease" means a disease state
caused by an inappropriate immune response that is directed to a self-encoded
entity which is
known as an autoantigen. The copolymer compounds provided herein can be used
to treat
symptoms of an autoimmune disease, a class of disorder which include
Hashimoto's
thyroiditis; idiopathic myxedema, a severe hypothyroidism; multiple sclerosis,
a
demyelinating disease marked by patches or hardened tissue in the brain or the
spinal cord;
myasthenia gravis which is a disease having progressive weakness of muscles
caused by
autoimmune attack on acetylcholine receptors at neuromuscular junctions;
Guillain-Barre
syndrome, a polyneuritis; systemic lupus erythematosis; uveitis; autoimmune
oophoritis;
chronic immune thrombocytopenic purpura; colitis; diabetes; Grave's disease,
which is a
form of hypothyroidism; psoriasis; pemphigus vulgaris; and rheumatoid
arthritis (RA).
The term "demyelinating condition" includes a disease state in which a portion
of the
myelin sheath, consisting of plasma membrane wrapped around the elongated
portion of the
nerve cell, is removed by degradation. A demyelinating condition can arise
post-vaccination,
post-anti TNF treatment, post-viral infection, and in MS.
The term "derivative" of an amino acid means a chemically related form of that
amino
acid having an additional substituent, for example, N-carboxyanhydride group,
a y-benzyl
group, an s,N-trifluoroacetyl group, or a halide group attached to an atom of
the amino acid.
7
CA 02614171 2007-12-10
WO 03/029276 PCTIUS02/31399
The term "analog" means a chemically related form of that amino acid having a
different configuration, for example, an isomer, or a D-configuration rather
than an
L-configuration, or an organic molecule with the approximate size, charge, and
shape of the
amino acid, or an amino acid with modification to the atoms that are involved
in the peptide
bond, so that the copolymer having the analog residue is more protease
resistant than an
otherwise similar copolymer lacking such analog, whether the analog is
interior or is located
at a terminus of the copolymer, compared to the copolymer without the analog.
The phrases "amino acid" and "amino acid copolymer" can include one or more
components which are amino acid derivatives and/or amino acid analogs as
defined herein,
the derivative or analog comprising part or the entirety of the residues for
any one or more of
the 20 naturally occurring amino acids indicated by that composition. For
example, in an
amino acid copolymer composition having one or more tyrosine residues, a
portion of one or
more of those residues can be substituted with homotyrosine. Further, an amino
acid
copolymer having one or more non-peptide or peptidomimetic bonds between two
adjacent
residues, is included within this definition.
The term "hydrophobic" amino acid means aliphatic amino acids alanine (A, or
ala),
glycine (G, or gly), isoleucine (I, or ile), leucine (L, or leu), methionine
(M, or met), proline
(P, or pro), and valine (V, or val), the terms in parentheses being the one
letter and three letter
standard code abbreviations for each amino acid, and aromatic amino acids
tryptophan (W, or
trp), phenylalanine (F, or phe), and tyrosine (Y, or tyr). These amino acids
confer
hydrophobicity as a function of the length of aliphatic and size of aromatic
side chains, when
found as residues within a copolymer or other polypeptide.
The term "charged" amino acid means amino acids aspartic acid (D or asp),
glutamic
acid (E or glu), arginine (R or arg) and lysine (K or lys), which confer a
positive (lys, and
arg) or negative (asp, glu) charge at physiological values of pH on an aqueous
solution of a
copolymer or other amino acid composition containing one or more residues of
these amino
acids. Histidine (H or his) is hydrophobic at pH 7, and charged at pH 6.
The term "anergy" means unresponsiveness of the immune system of a subject to
an
antigen.
The term "subject" as used herein indicates a mammal, including a human.
The term "heterologous cell" means a cell for production of an MHC protein
which is
unrelated to a cell of a subject, e.g., the heterologous cell is not a cell of
a mammal. The
heterologous cell for example can be from a cold blooded animal, for example,
from an
8
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31..
invertebrate; the heterologous cell is an insect cell, or a cell of a
microorganism such as a
yeast cell.
The term "surfaces of Class H IV[HC HLA-DR-2 protein" includes the portions of
the
protein molecule in its three-dimensional configuration which are in contact
with its external
environment, including those features of the protein that interact with
aqueous solvent and are
capable of binding to other cell components such as nucleic acids, other
proteins, and
peptides.
The terms "P 1 pocket" and "P4 pocket" include three dimensional polymorphic
regions on the peptide binding surface of the Class II MHC protein molecule
that
accommodate amino acid residue side chains from a peptide that is bound to the
Class II
MHC protein (Fridkis-Hareli, M. et al. 1998. J. Immunol. 160:4386-4397;
Fridkis-Hareli, M.
et. al. 2000. Human Immunol 61:640; Fridkis-Hareli, M. et al. 2001.Human
Immunol.
62:753-763), including a bound naturally occurring antigen or epitope, and a
bound synthetic
peptide or copolymer.
The terms "P-I position" and "P5 position" refer to amino acid residues on the
Class II MHC protein molecule peptide complex which directly contact the T-
cell receptor
(FridkistHareli, M. et. al. 2000. Human Imniunol 61:640; Fridkis-Hareli, M. et
al.
2001.Human bnmunol. 62:753-763). The P-1 position refers to the amino acid
which
precedes the amino acid residue of the peptide that occupies the PI pocket.
The P5 position
refers to the amino acid residue that follows the amino acid residue that
occupies the P4
pocket.
The term "antigen binding groove" refers to a three dimensional antigen
interactive
site on the surface of the Class II MHC protein molecule (Stern, L.J. et. al.,
Nature 368:215
(1994)) that is formed by surfaces of both the a and 0 subunits of the Class
II MHC protein
molecule.
The term "pharmaceutically acceptable carrier" includes any and all solvents,
dispersion media, coatings, antimicrobials such as antibacterial and
antifungal agents,
isotonic and absorption delaying agents and the like that are physiologically
compatible.
Preferably, the carrier is suitable for intravenous, intramuscular, oral,
intraperitoneal,
transdermal, or subcutaneous administration, and the active compound can be
coated in a
material to protect it from inactivation by the action of acids or other
adverse natural
conditions.
An autoimmune disease results when a host's immune response fails to
distinguish
foreign antigens from self molecules (autoantigens) thereby eliciting an
aberrant immune
9
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
response. The immune response towards self molecules in an autoimmune disease
results in a
deviation from the normal state of self-tolerance, which involves the
destruction of T cells
and B cells capable of reacting against autoantigens, which has been prevented
by events that
occur in the development of the immune system early in life. The cell surface
proteins that
play a central role in regulation of immune responses through their ability to
bind and present
processed peptides to T cells are the major histocompatibility complex (MHC)
molecules
(Rothbard, J.B. et al., Annu. Rev. Immunol. 9:527 (1991)).
In addition to MS, other demyelinating conditions have been found to occur,
for
example, post-viral infection, post-vaccination, post-encephalomyelitis
(Wucherpfennig
K.W. et al. 1991. Immunol. Today 12:277-282) and following administration of
certain
anti-TNF agents (FDA Talk Paper, Food and Drug Administration Public Health
Service,
Rockville, MD, http://www.fda.gov/bbs/topics/ANSWERS/ANSDO954.html).
Copolymers of amino acids as therapeutic agents for autoimmune diseases
Methods of the invention include use of a class of agents that can bind to
Class II
MHC proteins encoded by particular alleles. Such an agent can bind to a
particular Class II
MHC protein, and thus inhibit and/or prevent the binding of an autoantigen
involved in an
autoimmune disease, or upon binding can induce anergy, so that there is no
response of the
immune system to the autoantigen.
A number of therapeutic agents have been developed to treat autoimmune
diseases.
For example, agents have been developed that can, by inhibiting a
cyclooxygenase, prevent
formation of low molecular weight inflammatory compounds. Also, agents are
available that
can function by inhibiting a protein mediator of inflammation, by sequestering
the
inflammatory protein tumor necrosis factor (TNF) with an anti-TNF specific
monoclonal
antibody fragment, or with a soluble form of the TNF receptor. Finally, agents
are available
that target and inhibit the function of a protein on the surface of a T cell
(the CD4 receptor or
the cell adhesion receptor ICAM-1) thereby preventing a productive interaction
with an
antigen presenting cell (APC).However, compositions which are natural folded
proteins as
therapeutic agents can incur problems in production, formulation, storage, and
delivery.
Further, natural proteins can be contaminated with pathogenic agents such as
viruses and
priors.
An additional target for inhibition of an autoimmune response is the set of
lymphocyte surface proteins represented by the MHC molecules. Specifically,
these proteins
are encoded by the Class II MI-IC genes designated as HLA (human leukocyte
antigen) -DR,
-DQ and -DP. Each of the MHC genes is found in a large number of alternative
or allelic
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/313>
forms within a mammalian population. The genomes of subjects affected with
certain
autoimmune diseases, for example, MS and rheumatoid arthritis (RA), are more
likely to
carry one or more characteristic Class II MHC alleles, to which that disease
is linked.
A potential source of agents for treatment of MS and other demyelinating
conditions
is to identify peptides that bind selectively in vitro to a purified Class II
MHC allele protein
molecule, particularly to a protein which is a product of an Class II MHC
allele associated
with demyelinating conditions. In addition, the agent should bind to that
protein as it occurs
on the surfaces of antigen presenting cells in vivo, and thereby block,
anergize, or inactivate
the class of T cells that are responsible for the demyelinating condition,
such as MS.
The Class II MHC protein consists of two approximately equal-sized subunits, a
and
0, which are transmembrane proteins. A peptide-binding cleft, which is formed
by protein
features of both a and (i subunits, is the site of presentation of the antigen
to T cells. There
are at least three types of Class II MHC molecules: HLA-DR, -DQ, and -DP, and
there are
numerous alleles of each type. The Class II MHC molecules are expressed
predominantly on
the surfaces of B lymphocytes and antigen presenting cells such as macrophages
and
dendritic cells (Mengle-Gaw, L., The Major Histocompatibility Complex (MHC),
in the
Encyclopedia of Molecular Biology, Oxford: Blackwell Science Ltd., 1994, pp.
602-606).
An embodiment of the invention includes a novel method for treating autoimmune
diseases, by targeting Class Ii MHC molecules with a class of compounds
identified as
copolymers that include three or more different amino acids.
A copolymer of the invention can be synthesized using Fmoc or t-boc initiating
amino
acid analogs, or the like, which are immobilized on a resin in an automated
peptide synthesis
apparatus for further polymerization (solid state synthesis). The amino acids
are polymerized
in molar ratios that can be adjusted to provide a copolymer with optimal
binding
characteristics.
Synthesis procedures can include providing a solution which is a mixture of
the
chosen amino acids in an activated form, for example, activated as an N-
carboxy anhydride,
in the appropriate molar ratios of each of the appropriately derivatized amino
acid precursors
(derivatized to protect certain functional groups, such as the c amino group
of L-lysine, for
example the precursor c,N-trifiuoroacetyl-L-lysine). Alternatively, the
synthesis procedure
can involve online mixing during the synthetic procedure of derivatized
precursors of the
selected amino acids in the preferred molar ratios. Heteropolymer synthesis
services can be
obtained commercially, for example, at Chiron Technologies, Clayton,
Australia, the Harvard
11
CA 02614171 2007-12-10
51621-3(S)
Medical School Biopolymer Laboratory, Boston, MA, and at Advanced ChemTech,
Inc.,
Louisville, KY.
Examples of such resin supports for peptide synthesis include a Merrifield
resin,
chloromethylated polystyrene with I% DVB cross-links; an Fmoc amino acid Wang
resin,
4-benzyloxybenzyl alcohol, the resins being pre-loaded with an amino acid (for
example,
Fmoc-D-trp(boc)-Wang resin). Resins are available in different mesh-sizes, for
example
100-200 mesh, and high loading or low loading densities of functionalization
of the initiating
amino acid.
A solution of the different derivatized amino acids to be polymerized into the
composition of the invention, preferably protected as conventional in peptide
synthesis, is
added to sample of beads e.g., Fmoc. Reagents for synthesis, for deblocking,
and for
cleavage of the complete copolymer molecules for removal from the resin are
available from
manufacturers of the apparatus (Applied Biosystems Peptide' Synthesizer,
Foster City, CA, or
Advanced ChemTech, Louisville, KY); see e.g., M. Bodansky, Principles of
Peptide
Synthesis, 2nd Ed., Springer-Verlag, 1991.
Additional amino acids or analogs or derivatives of amino acids, can be added
to
the at least three amino acids selected to comprise the copolymers, to
substitute for a small
proportion of those amino acids, to provide, for example, a copolymer having
increased
protease resistance and therefore having enhanced pharmacological properties
such as longer
in vivo lifetime. Examples of analogs are bomotyrosine, or other substituted
tyrosine
derivatives, and aminobutyric acid, each available as an Fmoc derivative from
Advanced
ChemTech.
Therapeutic Compositions in the Methods of the Invention
A pharmaceutically acceptable carrier includes any and all solvents,
dispersion media,
coatings, antimicrobials such as antibacterial and antifungal agents, isotonic
and absorption
delaying agents and the like that are physiologically compatible. Preferably,
the carrier is
suitable for intravenous, intramuscular, oral, intraperitoneal, transdermal,
or subcutaneous
administration, and the active compound can be coated in a material to protect
it from
inactivation by the action of acids or other adverse natural conditions.
The methods of the invention include incorporation of a copolymer into a
pharmaceutical composition suitable for administration to a subject. A
composition of the
present invention can be administered by a variety of methods known in the art
as will be
appreciated by the skilled artisan. The active compound can be prepared with
carriers that
will protect it against rapid release, such as a controlled release formulati
on, including
12
CA 02614171 2007-12-10
WO 03/029276 PCTIUS02131399
implants, transdermal patches, and microencapsulated delivery systems. Many
methods for
the preparation of such formulations are patented and are generally known to
those skilled in
the art. See, e.g., Sustained and Controlled Release Drug Delivery Systems,
J.R. Robinson,
Ed., Marcel Dekker, Inc., NY, 1978. Therapeutic compositions for delivery in a
pharmaceutically acceptable carrier are sterile, and are preferably stable
under the conditions
of manufacture and storage. The composition can be formulated as a solution,
microemulsion, liposome, or other ordered structure suitable to high drug
concentration.
Dosage regimens can be adjusted to provide the optimum desired response (e.g.,
a
therapeutic response). For example, a single bolus can be administered,
several divided doses
can be administered over time, or the dose can be proportionally reduced or
increased as
indicated by the exigencies of the disease situation.
In general, an embodiment of the invention is to administer a suitable daily
dose of a
therapeutic copolymer composition that will be the lowest effective dose to
produce a
therapeutic effect, for example, mitigation of symptoms. The therapeutic
heteropolymer
compounds of the invention are preferably administered at a dose per subject
per day of at
least about 2 mg, at least about 5 mg, at least about 10 mg or at least about
20 mg as
appropriate minimal starting dosages. In general, the compound of the
effective dose of the
composition of the invention can be administered in the range of about 50 to
about 400
micrograms of the compound per kilogram of the subject per day.
A physician or veterinarian having ordinary skill in the art can readily
determine and
prescribe the effective dose of the pharmaceutical composition required. For
example, the
physician or veterinarian could start doses of the compound of the invention
employed in the
pharmaceutical composition at a level lower than that required in order to
achieve the desired
therapeutic effect, and increase the dosage with time until the desired effect
is achieved.
5 In another embodiment, the pharmaceutical composition includes also an
additional
therapeutic agent. Thus in a method of the invention the pharmaceutical
copolymer
composition can be administered as part of a combination therapy, i.e. in
combination with an
additional agent or agents. Examples of materials that can be used as
combination
therapeutics with the copolymers for treatment of autoimmune disease and
arthritic
0 conditions as additional therapeutic agents include: an antibody or an
antibody fragment that
can bind specifically to an inflammatory molecule or an unwanted cytokine such
as
interleukin-6, interleukin-8, granulocyte macrophage colony stimulating
factor, aid tumor
necrosis factor-a; an enzyme inhibitor which can be a protein, such as a,-
antitrypsin, or
aprotinin; an enzyme inhibitor which can be a cyclooxygenase inhibitor; an
engineered
13
{
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/313i;,9
binding protein, for example, an engineered protein that is a protease
inhibitor such an
engineered inhibitor of a kallikrein; an antibacterial agent, which can be an
antibiotic such as
amoxicillin, rifampicin, erythromycin; an antiviral agent, which can be a low
molecular
weight chemical, such as acyclovir; a steroid, for example a corticosteroid,
or a sex steroid
such as progesterone; a non-steroidal anti-inflammatory agent such as aspirin,
ibuprofen, or
acetaminophen; an anti-cancer agent such as methotrexate or adriamycin; a
cytokine blocking
agent; an adhesion molecule blocking agent; or a cytokine.
An additional therapeutic agent can be a cytokine, which as used herein
includes
without limitation agents which are naturally occurring proteins or variants
and which
function as growth factors, lymphokines, interferons particularly interferon-
P, tumor necrosis
factors, angiogenic or antiangiogenic factors, erythropoietins,
thrombopoietins, interleukins,
maturation factors, chemotactic proteins, or the like. An additional agent to
be added to a
copolymer of amino acids which are embodiments of the invention herein can be
a different
copolymer, for example, Copaxone which is a YEAK or Cop 1, or a copolymer
comprising
a subset of these or other amino acids (Aharoni et al. WO 00/05250,
PCT/US99/16747), or an
oligopeptide or peptide derivative (Strominger et al. WO 00/05249,
PCT/US99/16617; WO
02/59143, PCT/US02/02071). Preferred therapeutic agents to be used in
combination with a
composition of the invention and which are cytokines include interferon-0,
interleukin-4 and
interleukin-10.
A therapeutic agent to be used with the composition of the invention can be an
engineered binding protein, known to one of skill in the art of remodeling a
protein that is
covalently attached to a virion coat protein by virtue of genetic fusion
(Ladner, R. et al., U.S.
Patent 5,233,409; Ladner, R. et al., U.S. Patent 5,403,484), and can be made
according to
methods known in the art. A protein that binds any of a variety of other
targets can be
engineered and used in the present invention as a therapeutic agent in
combination with a
heteropolymer of the invention.
An improvement in the symptoms as a result of such administration is noted by
a
decrease in frequency of recurrences of episodes of MS, by decrease in
severity of symptoms,
and by elimination of recurrent episodes for a period of time after the start
of administration.
A therapeutically effective dosage preferably reduces symptoms and frequency
of recurrences
by at least about 20%, for example, by at least about 40%, by at least about
60%, and by at
least about 80%, or by about 100% elimination of one or more symptoms, or
elimination of
recurrences of the autoimmune disease, relative to untreated subjects. The
period of time can
be at least about one month, at least about six months, or at least about one
year.
14
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
Methods of use of random synthetic copolymers can be the basis of treating
other
autoimmune diseases which are associated with HLA-DR gene products, by
competing with
candidate autoantigens for binding to these protein receptor molecules, or by
inducing T cell
anergy or even T cell apoptosis, or by suppression of T cells, such that
subsequent T cell
response to an autoantigen is inhibited in vivo. Further, synthetic copolymers
having one or
more additional components, such as amino acid analogs or derivatives added in
varying
quantities into the polymerization reaction, can be effective inhibitors of a
variety of
autoimmune T cell responses.
The activity of Copl appears to involve, as a first step, binding to the
surface of
antigen-presenting cells (APC), for example to class II MHC proteins (Fridkis-
Hareli M. et
al. 1994. Proc. Natl. Acad. Sc!. USA 91:4872-4876), following which its
effectiveness may be
due either to competition with myelin antigens (for example, MBP, PLP, MOG)
for
activation of specific effector T cells recognizing peptide epitopes derived
from these
proteins (Ben-Nun, A. et al. 1996. J. Neurol. 243:514-22; Teitelbaum, D. et
al. 1996. J.
Neuroimmunol. 64:209-217), and/or induction of antigen-specific regulatory T
cells (Aharoni
R_ et al_ 1993. Eur. J. Immunol. 23:17-25).
Examination of additional copolymers and investigation of the mechanisms
involved
in their activities could potentially result in information that could lead to
improved
therapeutic reagents. Recent studies have shown that virtually all of the
large variety of
copolymers found in the random mixture of YEAK bound to purified molecules of
each of
human HLA-DRI, -DR-2 and -DR4 molecules, showing that YEAK generally binds to
purified class II MHC proteins (Fridkis-Hareli, M., and J.L. Strominger. 1998.
J. Immunol.
160:4386-4397). CopI further competes for binding of MBP 85-99 to HLA-DR-2
(DRBI * 1501) and inhibits responses of DR-2-restricted T cells to MBP 85-99.
Study of the
binding to class II MHC molecules of random copolymers containing only 3 of
the 4 amino
acids of Copl, for example, YAK, revealed that YAK is the most effective
(Fridkis-Hareli,
M. et al. 1999. Int. Immunol. 11:635-641).
The binding motif of Copt to the MS- associated molecule HLA DR-2 (DRB I *
1501)
shows E at P-2, K at P-i and Y at P1, with no preferences observed at other
positions
(Fridkis-Hareli. M. et al. 1999. J. Immmol. 162:4697-4704). Further, A is
overrepresented at
P1. As P1 is the anchor position, binding of Y at this position was not
anticipated. The PI
pocket in proteins encoded by the DR-2 allele is small (due to the presence of
1386Va1 rather
than 086Gly), and overrepresentation of A at this position may result from
this fact. The
effect of K at P-I appears to be due to stabilization of binding by the
interaction of K with
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/313,
residues in the top of the al helix, similarly to residue K at P-1 of HA 306-
318 complexed
with HLA-DRI which can interact with the side chains of al helix residues at
Sa53 or Ea55
(Stem, L.J. et al. 1994. Nature 368:215-221).
Copolymers designed according to the binding motif of MBP 85-99
(Wucherpfennig,
K.W. et al. 1994. J. Exp. Med. 179:279-290) might be better therapeutic agents
than Copl.
As provided herein, several random three- and four-amino acid copolymers, each
synthesized
as 14-, 35- and 50-mers in length, were made by the solid phase method. Design
of these
copolymers was made primarily by choice of amino acids with reference to the
anchor
residues of MBP 85-99 bound to HLA-DR-2 (DRBI * 1501) (Wucherpfennig, K.W. et
al.
1994. J. Exp. Med. 179:279-290; Smith, K.J. et a1. 1998. J. Exp. Med. 19:1511-
1520),
particularly the P1 anchor, to improve the effectiveness of the copolymers.
Effects of these
copolymers on autoantigen-specific T cell responses in MS, and on disease
progression of
EAE, an animal model of MS, are shown in the Examples below.
A major goal in the treatment of autoinunune diseases has been development of
antigen-specific immunomodulating therapies that interfere with the
trimolecular interaction
of the autoreactive T cell receptor (TCR) with the autoantigenic peptides
presented by self
MHC receptors at the surface of antigen-presenting cells. These
immunotherapies of T
cell-mediated autoirnmune diseases have been successful in animal models with
known target
antigens (see, for example, Weiner, H.L. 1997. Immunol. Today 18:335-343;
Nicholson, L.B.
et al. 1997. Proc. Natl. Acad. Sci. USA 94:9279-9284). The use of altered
peptide ligands
(APL) has been used both to treat EAE (Nicholson, L.B. et al. 1997. Proc.
Natl. Acad. Sci.
USA 94:9279-9284; Brocke, S. et al. 1996. Nature 379:343-346) and recently to
treat MS
(Bielekova, B. et al. 2000. Nat. Med. 10:1167-1175; Kappos, L. et al. 2000.
Nat. Med.
10:1176-1182), with contradictory findings.
CopI (Copaxone , an approved therapy for relapsing-remitting MS, was proposed
to
act as a promiscuous binder to class II MHC molecules (Fridkis-Hareli, M., and
J.L.
Strominger. 1998. J Immunol. 160:4386-4397), as an antagonist of the TCR
(Aharoni, R. et
al., 1999. Proc. Natl. Acad. Sci. USA 96: 634-639), and/or as an inducer of
suppressor cells
(Aharoni R. et al. 1993. Eur. J. Immunol. 23:17-25). Copaxonee is currently in
wide use, has
shown little or no toxicity, and has sustained efficacy in MS patients over a
period of 6 years
(Johnson, K.P. et al. 2000. Mult. Scler. 6:255-266). However, this agent was
found to reduce
frequency of relapse by at 30%, but did not eliminate relapse. Development of
novel
compounds may provide improved therapeutic agents for MS and possibly for
other
autoimmune disorders.
16
CA 02614171 2007-12-10
51621-3 (S)
In Examples 1-6, an optimal size of copolymers described herein was determined
using copolymers which are 14-, 35- or 50-mers in length. Since the 50-mers
are shown
herein to be most efficient in binding HLA-DR-2 and in inhibiting MBP-specific
T cell
responses, the additional copolymers used in Examples 7-11 were all
synthesized as 50-mers.
A size of 50 amino acids or longer, found here to provide efficient inhibition
of antigen
presentation and suppression of EAE, suggests that the random copolymers
herein act by
binding to and then clustering class Il MHC molecules in one portion of the
cell membrane,
similarly to Copaxone (Fridkis-Hareli, M. et al. 1997 Int. Immunol. 9: 925-
34) or
oligomerized T cell epitopes (Rotzschke, O. et al. 1997 Proc. Natl. Acad. Sci.
USA 94:
14642-14647).
The residues in the random copolymers in Examples 7-11 herein were designed
mainly on the basis of the anchor residues of the immunodominant T cell
epitope MBP 85-99
peptide (SEQ ID NO: 2). The Y in Copolymer I was found in the presumed PI
pocket of the
HLA DR -2 (DRBI * 1501) molecule (Fridis-Hareli, M. et al. 1999 J. Immunol.
162:
4697-4704), although Y may be too large for this pocket which has a good fit
with F, and
accommodates V89 in MBP85-99. Moreover, the F92 in MBP 85-99 (SEQ ID NO: 2) is
in
the P4 pocket (Smith, KJ. et al. 1998 J. Exp. Med.19: 1511-1520), but Y or W
may be a
tighter fit for this pocket. The interrelationship between these two residues
in the Y- and
F-containing copolymers provided herein is examined using copolymers
synthesized at
different ratios of Y:F. Further, V- and W-containing copolymers and V- and Y-
containing
copolymers, selected for synthesis on the basis of the need for differently
sized aromatic
groups to accommodate the differing sizes of the P1 and P4 pockets, are shown
in Example
I I to be particularly effective in treating EAE symptoms. With present
knowledge of the
size, shape and charge distributions of each of The PI and P4 pockets, and the
data on V- and
W- containing polymers as therapeutic agents for EAE, it is possible to design
amino acids
with novel organic side chains that could substitute for V and W,
respectively, in synthesis of
a copolymer, to provide an agent having an equivalent or even tighter fit of
the side chain into
these sites than V and W. A copolymer containing such a compound might be an
even more
useful therapeutic agent for an autoimmune disease such as EAE or MS.
The invention having now been fully described, additional embodiments of the
invention can be found in the Examples and in the claims below, which-
embodiments are not
to be construed as further limiting.
17
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/3135,
EXAMPLES
Materials and Methods
Copolymers, peptides and antibodies. Poly (Y,E,A,K), referred to as YEAK,
poly(V,E,A,K) or VEAK; and poly(F,E,A,K) or FEAK, in molar ratios
approximating those
found in Copl (wherein the V or F are present in the same molar ratio as the Y
in Copl),
were synthesized by the solid phase method as 14-, 35- and 50-mers (Chiron
Technologies,
Clayton, Australia), by using Fmoc amino acids mixed in the desired ratios at
each cycle.
Cop] batch 52596, in the molar ratio of I Y: 1.5 E: 4.3 A: 3.3 K (indicated
herein as
Y:E:A:K having a molar ratio of 1:1.5:4.4:3.3, with an average molecular
weight (MW) of
8,150, (Teitelbaum D. et al. 1971. Eur. J. bnmunol. 1:242-248), was obtained
from Teva
Pharmaceutical Industries (Petach Tikva, Israel). Glatiramer acetate (Cop 1,
Copaxone ) was
obtained from Teva Marion Partners, Kansas City, MO. Biotinylation of Copl was
performed with excess N-hydroxysuccinimide biotin (Sigma) in DMSO as described
(Fridkis-Hareli M. et al. 1994. Proc. Natl. Acad Sci. USA 91:4872-4876).
Unreacted biotin
was removed by dialysis (Spectra/Por membrane MWCO 500; Spectrum Medical
Industries, Laguna Hills, CA).
Peptides were synthesized using solid phase techniques (Barany, G., and R.
Merrifield. 1979. Academic Press, New York, NY) on an Applied Biosystems
Peptide
Synthesizer and purified by reversed-phase HPLC(RP-HPLC). Peptide sequences
were MBP
(human basic myelin protein) 86-100, NPVVHFFKNIVTPRT (SEQ ID NO: 1); MBP 85-
99,
ENPVVHFFKNIVTPR (SEQ ID NO: 2), MW 1795; PLP (human proteolipid protein) 40-
60,
TGTEKLIETYFSKNYQDYEYL (SEQ ID NO: 3), MW 2603; and PLP 139-151,
HSLGKWLGHPDKF (SEQ ID NO: 4), MW 1520, either unlabeled or labeled with biotin
linked to the N-terminus by the spacer SGSG and free acid at the C-terminus.
FAK (molar ratio 1:5:3), YFAK (molar ratio 0.2:0.8:5:3), YFAK (molar ratio
0.8:0.2:5:3) and YFAK (molar ratio 0.5:0.5:5:3) were synthesized by solid
phase chemistry as
50-mers (Chiron Technologies, Clayton, Australia). A variance of about 10%
from the input
molar ratios and observed the amino acid compositions of the resulting
polymers was found
consistent with previously reported data from use of this procedure.
Protein expression and pur/cation. Soluble HLA-DR-2 molecules were expressed
in
Drosophila S2 cells and purified as described (Kalandadze, A. et al. 1996. J.
Biol. Chem.
271:20156-20162). Cells were grown at 26 C in roller bottles in ExCell 401
medium (JRH
Biosciences, Lenexa, KS) supplemented with 0-5% fetal bovine serum (Sigma
Chemicals, St.
18
CA 02614171 2007-12-10
51621-3 (S)
Louis, MO). Cells were harvested 4-5 days after induction by I mM CuSO4.
Supernatant
from harvested cells was sequentially passed through Protein A, Protein G and
Protein
A-LB3.1 columns, followed by elution of the bound HLA-DR with 50 mM
3-[cyclohexylamino]-1-propanesulfonic acid (CAPS), pH 11.5, and neutralized
with 200 mM
phosphate (pH 6.0). Proteins were concentrated on a Centriprep 10 membrane
(Amicon,
Beverly, MA).
HPLC separation and microsequencing. Different copolymers were separated and
pool
sequenced as previously described (Fridlds-Hareli, M. et al. 1999. J. Immunol.
162:4697-4704). Briefly, the fractionation was by microbore HPLC using a
Zorbax C181.0
mm reverse-phase column on a Hewlett-Packard 1090 HPLC with 1040 diode array
detector.
Copolymers were eluted at a flow rate of 54 plhnin with a gradient of 0.055%
trifluoroacetic.
acid (TFA) in acetonitrile (0% at 0 to 10 min, 33% at 73 min and 60% at 105
min). Strategies
for peak selection, reverse phase separation and Edman microsequencing have
been
previously described (Chic; R..M. et al. 1993. J. Exp Med.178: 27-47). Pooled
fractions were
submitted to automated Edman degradation on a Hewlett-Packard G1005A (Palo
Alto, CA)
protein sequencer using the manufacturer's Routine 3.5.
Assays for peptide binding to class 11 MHC proteins
(A). Solutions. The solutions used in this assay are the following: binding
buffer is 20 mM
2-[N-morpholino]ethanesulfonic acid (MES), 140 mM NaCl, 0.05% NaN3, pH 5.0,
unless
otherwise specified; PBS is 150 mM sodium chloride, 7.5 mM sodium phosphate,
dibasic, 2.5
mM sodium phosphate, monobasic, pH 7.2; TBS is 137 mM sodium chloride, 25 mM
Tris
pH 8.0, 2.7 mM potassium chloride; TTBS is TBS plus 0.05% Tween-20.
(B). Microtter assay plate preparation. Immunoassay plates (96-well
microliter,
PRO-B1NDT"', Falcon, Lincoln Park, NJ) were coated with I pg/well affinity-
purified LB3.1
monoclonal antibodies in PBS (100 p1 total) for 18 hrs at 4 C. The wells were
then blocked
with TBS/3% BSA for l hr at 37 C and washed three times with TTBS. Before
sample
addition, 50 pl of TBS/1 % BSA was added to each well.
(C). Inhibition reactions. Biotinylated peptide MBP 86-100 (SEQ ID NO: 1),
final
concentration 0.13 pM in 50 pl of the binding buffer, was co-incubated with
unlabeled
inhibitors (random copolymers or MBP 85-99, SEQ ID NO: 2), and IRA-DR-2
molecules
for 40 hr at 37 C.
(D). Detection of class II MHC protein/peptide complexes. Bound peptide-biotin
was
detected using streptavidin-conjugated alkaline phosphatase, as follows.
Plates were washed
*Trade-mark
19
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/333ji
three times with TTBS and incubated with 100 l of streptavidin-conjugated
alkaline
phosphatase (1:3000, BioRad, Richmond, CA) for 1 hr at 37 C, followed by
addition of
p-nitrophenyl phosphate in triethanolamine buffer (BioRad). Absorbance at 410
nm was
monitored by a microplate reader (model MR4000; Dynatech, Chantilly, VA).
Antigen presentation assays. HLA-DR-2-restricted T cells were MBP
84-102-specific transfectants carrying the genes for TCR obtained from
patients with
relapsing-remitting MS carrying DR-2 (8073, patient Ob (DRB 1 * 1501) and Hy l
B, patient
Hy (DRB 1* 1602)), into BW 58 TCR a-/P- cells (Fridkis-Hareli, M. et al. 2001
Human
Immunol. 62: 753-763); and MBP 84-102-specific (2E12) and PLP 40-60-specific
(106A)
hybridomas from HLA-DR-2-transgenic mice (Madsen, L.S. et al. 1999. Nat.
Genet.
23:343-347). Mouse T cell hybridomas were PLP 139-151-specific H-25-restricted
(hPLP/1
and hPLP/c4, Santambrogio, L. et al. 1993. J. Immunol. 151: 1116). Antigen
presenting cells
(APC) were L466 (L cells transfected with HLA-DR-2b (DRB 1* 1501)), L416 (L
cells
transfected with HLA-DR-2a (RB5*0101)), MGAR (EBV-transformed B cells
homozygous for DRB1 * 1501), and splenocytes from SJL/J (H-2) mice. T cell
stimulation
experiments were performed in a total volume of 200 l in 96-well microtiter
plates.
Irradiated (3000 rad) APC (2.5 x 104/well) were co-incubated with MBP 85-99
(SEQ ID
NO: 2), PLP 40-60 (SEQ ID NO: 3) or PLP 139-151 (SEQ ID NO: 4) and the random
copolymers, at concentrations indicated, for 2 hr at 37 C. Then T cells (5 x
104/well) were
added, and plates were incubated for 24 hr at 37 C. Supernatants (30 l) were
taken and
were incubated with IL-2-dependent CTLL (5 x 104/well) for 12 hr, followed by
labeling
with 3H-thymidine (1 llCi/well) for 12 hr. Plates were harvested, and the
radioactivity was
monitored using a 1450 microbeta Plus liquid scintillation counter (Wallac,
Gaithersburg,
MD).
Mouse strains. SJL/J (H-2) mice (8-12 weeks of age) were purchased from
Jackson
Laboratories (Bar Harbor, ME) and were maintained in the animal facility at
Harvard
University according to the Guidelines of the Committee on Animals of Harvard
University
and the Committee on Care and Use of Laboratory Animal Resources, National
Research
Counsel (Department of Health and Human Services Publication 85-23, revised
1987).
Humanized mice (Madsen, L.S. et al. 1999 Nat. Genet 23(3): 343-347; and D.
Altman, D.
Hafler, and V. Kuchroo, unpublished) carry transgenes HLA DR-2 (DRA* 0101 and
DRBI
1501) and TCR from MS patient Ob, which is a V(D)J rearrangement of TCRa and
TCR(3
amplified from clone Ob.1A12.
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
Induction and suppression of EAE. Mice were injected subcutaneously both in
the
base of the tail and the nape of the neck with either whole spinal cord
homogenate (WSCH,
500 g/mouse, prepared as previously described (Santambrogio, L. et al. 1993.
J. Immunol.
151:1116-1127), or with PLP 139-151 peptide (50 pg/mouse) together with 400 pg
Mycobacterium tuberculosis H37Ra (BD Difco Laboratories, Sparks, MD) in an
emulsion
containing equal parts of PBS and complete Freund's adjuvant (CFA; Sigma
Chemical Co.,
St. Louis, MO). Pertussis toxin (List Biological Laboratories, Campbell, CA,
200 ng) was
injected intravenously into the tail one day after immunization. Mice were
scored daily for
clinical signs of EAE on a scale 1-5, according to the severity of disease
symptoms as
previously described (Santambrogio, L. et al. 1993. J. Iminunol. 151: 1116).
For
determination of suppression of EAE, each copolymer (500 pg/mouse) was mixed
and
injected with the encephalitogenic emulsion as described above.
Neuropathology. For assessment of inflammation and demyelination, mice were
perfused under anesthesia through the ascending aorta with 40 ml of Trump's
fixative (4%
paraformaldehyde, I% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4). Slices
of the brain
and spinal cord were postfixed in cold I% osmium tetroxide for 1 hr, were
dehydrated
through a graded series of solvents having increasing ethanol, and were
embedded in epoxy
resin. Sections of one m were obtained, and were stained with toluidine blue
and examined
by light microscopy.
Example 1. Synthesis and microchemical analysis of novel copolymers.
Random four-amino acid copolymers YEAK, VEAK, and FEAK, each of 14-, 35- and
50-mer in length, were synthesized by the solid phase method. V or F were
chosen to be
substituted for Y because of the following structural information: the P1
pocket of
DRB I * 1501 includes 086V resulting in a small pocket that can accommodate V
or F but for
which Y is too large to be accommodated (except at high peptide concentration;
Krieger, J.I.
et al. 1991. J. Imniunol. 146:2331-2340); the residue occurring at P1 in the
binding of MBP
85-99 (SEQ ID NO: 2) is V, and F might provide a tighter fit; and the residue
occurring at P4
in MBP 85-99 is F.
To determine whether the solid phase synthesis procedure yielded copolymers
similar
in amino acid composition, distribution, hydrophobicity and size, as compared
to copolymer
that had previously been generated only by solution chemistry, the novel
compounds were
subjected to amino acid analysis, RP-HPLC separation and microsequencing.
Amino acid analysis revealed molar ratios of Y, V, F, E and K in different
copolymers
to be similar to the predicted ratios, except for A, the molar ration of which
was increased in
21
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/313y9
all the copolymers, and particularly in the 35- and 50-mers. For example, in
the 50-mer of
VEAK, the molar ratios observed were 1.0 V: 2.1 E: 10.7 A: 2.9 K, as compared
with the
expected values of 1.0 V. 1.5 E: 5.0 A: 3.0 K. Separation of the copolymers by
HPLC using
an acetonitrile gradient showed a broad peak with several smaller peaks, which
spread
between about 40 and about 120 min elution time, similar to that of untreated
Copl
(Fridkis-Hareli, M., and J.L. Strominger 2001 Hum. Immunol. 62: 753-763).
Edman
sequencing of the first 10 amino acids showed constant ratios at each cycle,
similar to those
found by amino acid analysis, and indicating that the sequences of amino acids
in the
copolymers were random.
Example 2. Binding of the novel random copolymers to HLA-DR-2 molecules.
Copi and certain three amino acid random copolymers synthesized in solution
using
N-carboxyamino acid anhydrides (Teitelbaum D. et al. 1971. Eur. J. Immunol.
1:242-248),
viz., those containing three of the amino acids Y, E, A and K have been shown
to bind to
purified HLA-DR-2 and to compete for binding with MBP 85-99 (Fridkis-Hareli,
M., and
J.L. Strominger. 1998. J. Immunol. 160:4386-4397; Fridkis-Hareli, M. et al.
1999. Int.
Immunol. 11:635-641).
To determine whether copolymers synthesized by the solid phase method also
competed with this autoantigenic epitope for binding to HLA-DR-2, competitive
binding
assays were carried out with biotinylated MBP 86-100 (SEQ ID NO: 1) and the
unlabeled
peptides and random copolymers. Binding of biotinylated MBP 86-100 (SEQ ID NO:
1) to
HLA-DR-2 molecules was inhibited most efficiently by the 50-mers of YEAK, the
unlabeled
MBP 85-99 (SEQ ID NO: 2) peptide or by Copl. All other random copolymers
tested here,
i.e., those of 14 and 35 amino acid residues in length, were less effective in
this assay.
Example 3. Proliferative responses of MBP-specific T cells in the presence of
the random
copolymers.
A series of proliferation assays was performed to determine biological
activity of each
of the random copolymers with several MBP 84-102-specific T cell clones (see
Materials and
Methods).
Three types of APC, each expressing HLA-DR-2 molecules, were tested to
determine
which one presented the MBP 85-99 (SEQ ID NO: 2) peptide most efficiently.
Higher levels
of proliferation were observed when this peptide was presented by the human B
cell line
MGAR [DR-2b (DRB 1 * 1501)-expressing] than by L466 (DR-2b-expressing L cell
transfectant) cells. When L416 [DR-2a (DRB5*0101)-expressing L cells] were
used, no
response was detected, confirming that all the T cell clones were restricted
to the DR-2b
22
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
(DRB 1* 1501) allele. Therefore, MGAR cells, or sometimes L466 cells, were
subsequently
used in the antigen presentation assays described below.
The inhibition of proliferation in the presence of different copolymers of
three different
T cell clones by the MBP 85-99 peptide was examined. Generally, 14-mers were
not
inhibitory, regardless of the T cell clone tested, whereas 35- and 50-mers
showed higher
levels of inhibition. For all clones, YEAK 50-mer was approximately equivalent
to Copl
(which on average is a 70-mer). Inhibition fell off markedly with the YEAK 35-
mer, and was
very low with the YEAK 14-mer.
Inhibition of proliferation of the 2E12 T cell clone was efficient in the
presence of the
35- and 50-mers of FEAK, and in the presence of Copl (Figure 1A, lower left
panel). VEAK
did not inhibit the 2E12 clone (Figure IA, lower right panel). The 50-mer of
FEAK was
somewhat less inhibitory than Copt. In the case of the HyIB clone, CopI was
the best
inhibitor, and lower levels of inhibition by the 50-mers of FEAK and VEAK were
observed
(Figure 1C).
The combination of V, E, A and K resulted in a low affinity binding to HLA-DR-
2
molecules and low levels of inhibition of HLA-DR-2-restricted MBP 85-99-
specific T cells.
This is in despite the observation that in the MBP 85-99/HLA-DR-2 complex, V
is the anchor
residue at position 89 of the peptide (SEQ ID NO: 2), interacting with P86VaI
in the P1
pocket of the HLA-DR-2 protein (Smith, K.J. et al. 1998. J. Exp. Med. 19:1511-
1520). The F
side chain also fits in the P4 pocket, thus making the FEAK a better binding
agent. Residue A
may interact with the PI pocket and Y with the P4 pocket (Smith, K.J. et al.
1998. J. Exp.
Med. 19:1511-1520). MBP 85-99 (SEQ ID NO: 2) may be a relatively low affinity
peptide
because of V89.
Residue K in FEAK is most likely important for the interaction with the TCR,
similarly
to K at position 93 of MBP 85-99 (SEQ ID NO: 2; Wucherpfennig, K.W. et al.
1994. J. Exp.
Med. 179:279-290; Smith, K.J. et al. 1998. J. Exp. Med. 19:1511-1520). On the
other hand, K
located near the N-terminus of the copolymer in the binding site may
contribute to stable
interactions with the HLA-DR molecules and the TCR, similarly to residue K at
P-1 of HA
306-318 (SEQ ID NO: 5) bound to HLA-DR1 which can interact with the side
chains of al
helix residues at Sa53 or Ea55 (Stem, L.J. et al. 1994. Nature 368:215-221).
Example 4. Proliferation ofPLP-specific T cell clones.
To determine whether the random copolymers were able to inhibit the
presentation of
another potential autoantigen in MS,namely PLP, two different PLP ewere
epitopes
employed: human PLP 40-60 (SEQ ID NO: 3) that binds to DRB 1* 1501
(Krogsgaard, M. et
23
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/313y,
al. 2000. J. Exp. Med. 191:1395-1412), and mouse PLP 139-151 (SEQ ID NO: 4)
peptide that
binds to H-2S and is encephalitogenic in SJL/J mice (Tuohy, V.K. et al. 1989 J
bnmunol.
142:1523-1527). The T cells used in this assay were 106A (PLP 40-60-specific
hybridomas
from HLA-DR-2-transgenic mice; Madsen, L.S. et al. 1999. Nat. Genet. 23:343-
347), and
hPLP/ I and hPLP/c4 (PLP 139-151-specific H-2'-restricted hybridomas from
SJL/J mice;
Santambrogio, L. et al. 1993. J Immunol. 151:1116-1127). Proliferation of the
T cell
hybridomas was induced by the corresponding peptides in a dose-dependent
manner. Each of
the different copolymers was then added to the antigen presentation assay.
Presentation of the PLP 40-60 (SEQ ID NO: 3) epitope by the L466 APC to the
106A T
cells was inhibited most efficiently by the 35- and 50-mers of FEAK (Figure
2A, bottom
panel). The levels of inhibition were somewhat higher than in the presence of
Cop I. As in the
case of the MBP-specific T cells, the PEAK 50-mer approximated Copl (Figure
2A, top
panel), while VEAK inhibited PLP 40-60-specific T cells only at the highest
concentrations
(Figure 2A, middle panel).
Proliferation of mouse H-2S-restricted PLP 139-151-specific T cell hybridoma
hPLP/1
was best inhibited by Copl. FEAK or VEAK were somewhat less effective (Figure
2B). The
hPLP/c4 hybridoma was best inhibited by the 50-mers of FEAK and Copt (Figure
2C).
Without being limited by any particular theory, several mechanisms have been
postulated by which the copolymers may suppress a self-reactive T cell
response: MHC/TCR
blockage, competition, anergy induction, apoptosis, and bystander suppression.
The first two
mechanisms imply an effect of the copolymers on the early phase of the
induction phase,
when autoreactive T cells start expanding in number. Bystander suppression may
act both on
the induction and the effector phases, to promote development of regulatory T
cells, or
expansion of cross-reactive T cells and thereby suppress self-reactive
encephalitogenic T
cells. During ex-vivo proliferation, T cells of mice immunized with PLP 139-
151 (SEQ ID
NO: 4) developed a response only to the immunizing peptide, without any cross-
reactivity to
the tested copolymers. However when PLP 139-151 T cells were challenged in
vitro in the
presence both of the self-peptides and the copolymer, the response of the T
cells to PLP
139-151 (SEQ ID NO: 4) was strongly abolished. Also, in several co-immunized
mice, T
cells proliferated in response to PLP139-151 (SEQ ID NO: 4) as well as to the
copolymers.
The copolymers which when administered in vivo show greatest suppression of
EAE in
Examples below are also the best in suppressing T cell proliferative response
to PLP 139-151
(SEQ ID NO: 4) in vitro. In such light, it appears that the more likely
mechanism of action of
the copolymers is blockage of MHC, and competition for antigen presentation.
24
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/3139
Example 5. In vivo effect of VEAK and FEAK random copolymers on EAE induced by
WSCH.
To find out whether VEAK and FEAK random copolymers affected the clinical
course
of EAE in SJL/J mice, a number of in vivo experiments were performed. The
protocol for
disease induction was subcutaneous injection to co-immunize with both WSCH
(500 g) and
each copolymer (500 g), similar to the protocol of previous studies of
suppression of EAE
by Cop 1 (Teitelbaum, D. et al. 1996. J. Neuroifnmunol. 64:209-217). Following
disease
induction, mice were observed daily for 40 days for appearance of typical
clinical signs of
EAE (Table 1).
The data show that mice injected with WSCH developed EAE at around day 14-15
(Table 1, line 1) and had a maximal clinical score of about 2.2 (incidence:
18/32, mortality:
3%). Co-immunization with 35- or 50-mer VEAK (Table 1, lines 6 and 4,
respectively) did
not significantly affect the course of EAE, and resulted in an incidence and
maximal score
similar to the group injected with only WSCH, although in these co-immunized
mice the
onset of the disease may have been slightly delayed.
In contrast, mice treated with either 35-mer or 50-mer of FEAK (Table 1, lines
7 and 5
respectively) did not even develop symptoms of EAE. Treatment with Copaxone
(Table 1,
line 2) suppressed EAE. One out of fourteen of the mice treated with Copaxone
developed
the disease on day 20, with a maximal score of 3Ø Similarly, two out of
sixteen mice
injected with the 50-mer of YEAK (Table 1, line 3) developed mild EAE on day
14, with a
maximal score of 1Ø
To determine the extent of inflammation and demyelination in mice injected
with each
of the different copolymers, central nervous system immunohistochemistry was
performed on
spinal cord samples. Samples from the lumbar cord of diseased mice injected
with WSCH
only, or with WSCH and VEAK 50-mer, showed extensive submeningeal,
perivascular and
parenchymal infiltration, as well as demyelination. In contrast, no symptoms
of infiltration or
demyelination were detected in samples from those mice that had not developed
any signs of
disease after treatment with the other copolymers.
Among different random copolymers synthesized and characterized in examples
herein,
FEAK was most efficient in suppression of EAE induced by WSCH.
Example 6. Treatment with VEAK or FEAK random copolymers of EAE induced by PLP
139-151 peptide (SEQ ID NO. 4).
To find out whether random copolymers provided herein might affect development
of
chronic-relapsing EAE, mice were injected subcutaneously with 50 pg of PLP 139-
151 (SEQ
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
ID NO: 4; the encephalitogenic epitope in the SJL/J strain) alone, or with 50
pg of PLP
139-151 (SEQ ID NO: 4) and 500 pg of the copolymer. Mice were examined on a
daily basis
for 90 days after the induction of the disease.
Immunization with the PLP 139-151 (SEQ ID NO: 4) epitope alone in CFA resulted
in EAE with more severe clinical signs (Figure 3A) compared to EAE induced by
WSCH
(Table 1). For example, five of eight mice developed severe symptoms of EAE
with a
mortality of 33%. The first attack occurred at about day 14 after
immunization, with a
maximal clinical score of 4.0, followed by subsequent fluctuation in disease
attacks peaking
approximately at days 30, 50, 70 and 85.
Co-injection with the various copolymers differentially reduced the clinical
signs of
EAE. In the VEAK 50-mer-treated group (Figure 3B), four out of eight mice
showed clinical
signs of EAE (mortality: 12%). The first attack developed on day 13 and peaked
at about day
(mean maximal score: 1.6).
Co-injection with the FEAK 50-mer (Figure 3C) resulted in three sick mice out
of
15 eight (mortality: 0%). The first attack was delayed and was less
symptomatically severe
(days 23-25, mean maximal score of 1.1) compared to the control receiving the
peptide alone,
or to the VEAK-treated group. Clinical symptoms were almost entirely
remediated by about
day 40.
Treatment with Copaxone (Figure 3D) led to delay of the first attack
(starting on day
20 26, peak at day 34, maximal mean score: 1.25), similarly to results
obtained with FEAK. In
the Copaxone group, two out of eight mice developed EAE with a mortality of
12%.
The data in Table I and Figure 3 indicate that EAE induced by either WSCH or
by
PLP 139-151 (SEQ ID NO: 4) peptide was efficiently suppressed by the FEAK 50-
mers.
Further, these data demonstrate that 50-mers of FEAK suppressed EAE induced by
either
WSCH or the PLP 139-151 (SEQ ID NO: 4) peptide more efficiently than Copl.
This
observation was evident when both the encephalitogenic material and the
copolymer were
injected simultaneously into SJL/J mice. Copl inhibits EAE induced by either
WSCH or the
synthetic PLP peptides, and interferes with PLP-specific T cell responses only
when mice
were co-immunized with both antigens (Teitelbaum, D. et al. 1996. J.
Neurointmunol.
64:209-217), suggesting that they compete for binding to class II MHC
molecules.
Without being limited by any particular theory, the mechanism of activity of
the
50-mer random copolymers provided herein might be similar to that of Copl,
leading to
inhibition of binding of potential autoantigenic peptides to class II MHC
proteins, and
subsequent T cell suppression.
26
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31395
Example 7. Synthesis and microchemical analysis of Y- and F- containing
copolymers.
It is shown supra that 50-mers compared to 14- or 35-mers of random copolymers
composed of the amino acids Y, E, A and K are potent inhibitors of the binding
of human
immunodominant epitopes MBP 85-99 (SEQ ID NO: 2) to MS-associated HLA-DR-2
(DRB 1 * 1501). Some of these copolymers inhibited the response of HLA DR-2-
restricted
MBP 84-102-specific T cells, and also suppressed EAE in the susceptible SJL/J
strain
induced by the encephalitogenic epitope PLP 139-151 (SEQ ID NO: 4).
Here, analysis of each of amino acid composition and amino acid ratios within
the
copolymers, is shown for random three- copolymer FAK 50-mer, and for the four-
amino
acid copolymer YFAK, at different ratios of Y:F (the "Y- and F-containing"
polymers), each
50-mer copolymer synthesized as a 50-mer by the solid phase method. Amino
acids that
comprise these copolymers were chosen according to the anchor residues of the
MBP 85-99
(SEQ ID NO: 2) epitope bound to HLA-DR-2 (DRB1 * 1501) molecules.
Copolymers having different ratios of Y and F were designed according to the
following structural criteria: the PI pocket of DRBI * 1501 includes 086V
resulting in a small
pocket that can accommodate F but for which Y is too large to be accommodated;
thus F
would provide a tighter fit for PI although the residue occurring at PI in the
binding of MBP
85-99 is V; and the residue occurring at P4 in MBP 85-99 is F, but this pocket
is large enough
to accommodate Y, which may be a better fit than F. To determine whether the
synthesis
procedure yielded substances similar in amino acid composition, distribution,
hydrophobicity
and size, as compared to those generated by previous techniques, the novel
compounds were
subjected to amino acid analysis, RP-HPLC separation and microsequencing.
Amino acid analysis revealed that the molar ratios of Y, F and K in each of
the
different
copolymers were similar to the expected input molar ratios, except for A, the
molar ratio of
which was increased in all the copolymers. HPLC separation of the copolymers,
using an
acetonitrile gradient as previously described for Copt (Fridkis-Hareli, M. et
al. (1999) J.
Immunol. 162, 4697-4704), showed a broad peak with several smaller peaks,
which eluted
between about 40 and 80 min, similar to elution of untreated Cop 1.
Pool sequencing of the first several amino acids of each copolymer synthesized
here
showed random patterns, with significantly higher levels of A over the levels
of each of Y, F,
or K, which corresponded to the initially higher molar ratio of A found by
analyzing the
composition of these random copolymers. No sequence specificity or
preferential positioning
27
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31.kv)
of any amino acid in the copolymers was observed, indicating that the polymers
were of
random sequence.
Example 8. Binding of the Y- and F-containing random copolymers to HLA-DR-2
molecules.
To determine whether the Y- and F-containing copolymers synthesized herein by
the
solid phase method can compete with autoantigenic MS-associated epitope MBP 85-
99 (SEQ
ID NO: 2) for binding to HLA-DR-2 molecules, competitive binding assays were
carried out
with biotinylated MBP 86-100 (SEQ ID NO: 1) and each of the unlabeled random
copolymers.
Binding of biotinylated MBP 86-100 to HLA-DR-2 molecules was efficiently
inhibited by FAK 50-mer and the YFAK 50-mer copolymer (having the molar ratio
Y0.8:F0.2; Figure 4). Thus, the Y- and F-containing 50-mer random copolymers
herein
compete with the MS-related epitope (SEQ ID NO: 2) for binding to MS-
associated
HLA-DR-2 molecules.
Example 9. Proliferative responses of MBP-specific T cells in the presence of
the random
50-mer copolymers.
Effects of the presence of each of 50-mer copolymers FAK, YFAK (0.2:0.8), YFAK
(0.5:0.5), and YFAK (0.8:0.2) on proliferation of three different T cell
clones, in response to
the MBP 85-99 (SEQ ID NO, 2) peptide, were examined, and results from two
independent
experiments are shown in Figure 5.
The data show that for each of three MBP-specific HLA-DR-2-restricted clones,
the
three Y- and F-containing YFAK copolymers and the FAK copolymer were efficient
inhibitors. Among these copolymers, YFAK 0.2:0.8, YFAK 0.5:0.5, and FAK were
better
inhibitors than YFAK 0.8:0.2, and were superior to Cop 1.
The superior inhibitor activities of the three YFAK copolymers having
different Y:F
ratios and of the FAK copolymer were observed at lower concentrations (e.g.,
at about 20
M) of each of these better inhibitors for clone 2E 12, and at several low
copolymer
concentrations with the other T cell clones. At higher concentrations, e.g.,
greater than about
100 M, the observed levels of inhibition were similar for all of the
copolymers tested in this
example (Figure 5).
Example 10. Treatment of EAR; induced by PLP 139-151 (SEQ ID NO. 4) with Y-
and
F-containing copolymers.
In vivo experiments were carried out to determine whether the Y- and F-
containing
50-mer random copolymers would affect the clinical course of EAE in SJL/J
mice. As in
Examples above, the protocol for co-immunization was subcutaneous injection of
SJL/J mice
28
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/3139
with, in this example, the encephalitogenic epitope PLP 139-151 (SEQ ID NO: 4;
50 g) and
a copolymer preparation (500 kg). Following disease induction, mice were
observed daily for
appearance of typical signs of EAE, during a period of 70 days.
Immunization with PLP 139-151 (SEQ ID NO: 4) epitope alone in CFA resulted in
chronic-relapsing EAE (Figure 6; Table 2). All 13 mice receiving this
treatment developed
severe EAE, with a mortality of 77%. The first signs appeared around day 11,
followed by
subsequent fluctuation in disease attacks, with a mean maximal score of 4.6
(Figure 6).
Co-injection of random copolymers herein differentially reduced the clinical
signs of
EAE. In the YFAK 0.2:0.8-treated group, only two out of 16 mice showed
clinical signs of
EAE (mortality: 6%), and these clinical signs occurred with a delay in the
first attack which
occurred about day 37 (mean maximal score: 0.6; Figure 6, Table 2) rather than
day II as in
the untreated group.
Similarly, in the YFAK 0.5:0.5-treated group, one sick mouse of 16 was
observed
(mortality: 0%), with the first attack developing on day 33. Further, of mice
treated with
YFAK 0.8:0.2, eight of 17 developed EAE, with no mortality. In this group, the
observed
mean maximal clinical score of 1.5 and the time of onset (day 27) were each
indicative of a
less therapeutic benefit than these data obtained for mice treated with the
YFAK preparations
having the lower ratios of Y to F shown above.
Co-injection with FAK resulted in three sick mice of 17, with 12% mortality,
mean
maximal score of 0.9 and mean onset of day 25 (Table 2, line 5). Copaxone co-
injected with
PLP 139-151 (SEQ ID NO: 4), resulted in 12 of 16 mice developing EAE, with
mean onset at
day 22, and a mean clinical score of 2.6 (Table 2, line 6).
Observation of clinical symptoms in individual mice in another experiment
(Figure
6B) shows that YFAK 0.5:0.5 treatment eliminated all symptoms in the entire
group of mice
treated with this copolymer. From these data on individual mice, it is clear
that F-containing
copolymers are more effective in remediation of PLP-induced EAE than Cop 1,
and that a
greater molar ratio of F to Y is associated with superior remediation of EAE.
In summary, EAE induced by PLP 139-151 (SEQ ID NO: 4) was efficiently
suppressed by the three different YFAK copolymers and by FAK, with the order
of efficacy
being YFAK 0.5:0.5>YFAK 0.2:0.8>FAK>YFAK 0.8:0.2. The F-containing copolymers
remediated PLP-induced EAE more effectively than Cop 1.
The Y- and F- containing random amino acid copolymers synthesized and analyzed
herein are more potent in binding to HLA-DR-2 molecules, inhibition of
autoantigen-specific
29
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
T cells, and suppression of EAE, than Cop I (Copaxone ). These copolymers were
designed
and synthesized mainly based on those residues of immunodominant T cell
epitope MBP
85-99 (SEQ ID NO:2) interacting with the MS-associated HLA-DR-2 (DRB 1 * 1501)
molecules. The length of the copolymer preparations is shown herein to be
important for
activity, with the 50-mers being most efficient. Longer polypeptides may be
able to link
adjacent class H molecules.
The 50-mer random copolymer FAK and the YFAK 50-mer copolymers of different
molar ratios of Y to F herein are more potent than control Copaxone in the
following
functional activities: binding to HLA-DR-2 molecules, inhibition of MBP-
specific DR-
2-restricted T cells, and suppression of EAE. Random copolymer VEAK showed low
affinity binding to HLA-DR-2 molecules, low levels of inhibition of HLA-DR-2-
restricted
MBP 85-99-specific T cells and no effect on progression of EAE, in spite of
having an amino
acid residue V at a position that is equivalent to the P 1 of the MBP 85-99
auto antigen (SEQ
ID NO: 2). Data herein show that substitution of V by F resulted in a better
inhibitory
compound, probably due to a tighter fit of F into the PI pocket, and Y into
the P4 pocket.
Most significant is the effect of the copolymers herein on progression of EAE
induced
by encephalitogenic epitope PLP 139-151 (SEQ ID NO: 4). Clinical signs of EAE
were
significantly reduced by treatment with the YFAK copolymers or with FAK, when
the
encephalitogenic material and the copolymer were injected simultaneously into
SJL/J mice.
Without being limited by any particular theory, these data support a mechanism
of
activity of the random copolymers involving the copolymers as efficient
blockers of antigen
presentation by class II MHC molecules, leadomg to inhibition of binding of
the potential
autoantigenic peptides and subsequent autoimmune T cell suppression.
The YFAK 50-mer and FAK 50-mer copolymers are candidates for use in treatment
of MS, a disease in which 60% of the patients are of HLA-DR-2 (DRB I * 1501)
haplotype.
Given the promiscuous binding abilities of random copolymers (Fridkis-Hareli,
M., et al.
1998 J. Immunol.160: 4386-4397; Fridkis-Hareli, M. et al. 1999 Int.
Immunol.11: 635-641),
the copolymers herein may be beneficial also in MS patients having other HLA-
DR
specificities, and might provide new therapeutic compounds for use in other
autoimmune
conditions.
Example 11. Co/Pre-immunization treatment with valine (I'7- and tyrosine (I)-
or valine (I/)-
and tryptophan- (T9- containing copolymers suppresses MBP 85-99 (SEQ ID NO: 2)
induced
EAE in humanized mice.
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
The peptide-binding pockets of HLA-DR-2 DRB I * 1501 have a 1386 Val residue
at
P1, and is of a size that can accommodate a residue which is a V or F, but not
of sufficient
size to accommodate a Y or W. In contrast, the large hydrophobic pocket P4
contains a $371
Ala, therefore it can accommodate a residue of large size such as Y or W; and
the P9 pocket
is promiscuous. Based on these structural considerations, copolymers
containing valine (V)
and tyrosine (Y), or valine (V) and tryptophan (W), along with A and K, were
synthesized
and tested for effect on progression and symptoms of EAE induced by MBP 85-99
(SEQ ID
NO: 2).
Experimental animals were humanized mice carrying transgenes HLA DR-2
(DRA* 0101 and DRB I * 1501)and TCR from MS patient Ob, which is a V(D)J
rearrangement of TCRa and TCR1, amplified from clone Ob.1A12. Mice in each
group were
injected with MBP 85-99 (SEQ ID NO: 2) subcutaneously to induce EAE. As shown
in
Figure 7, groups of mice were pre-immunized with a single injection two days
prior to EAE
induction, either with Copt, YFAK 0.5:0.5, or control MBP 85-89, or were
simultaneously
co-immunized with Copl, YFAK 0.5:0.5, YFAK 0.2:0.8, VYAK 0.5:0.5, or with VWAK
0.5:0.5, and with the EAE-inducing MBP 85-99 (SEQ ID NO: 2). Clinical symptoms
were
monitored over a course of 50 days on days indicated.
Mice in the control group that were induced with MBP 85-99 (SEQ ID NO: 2) and
otherwise untreated showed a severity of symptoms that exceeded a clinical
score of 4 at
about day 25. Clinical symptoms in this group generally rose to a high level
of 3-4 for eight
time points (days 11 to 32), prior to stabilizing at a level of severity
between 2 and 3.
Duration of symptoms was observed over a total of 14 time points
(corresponding to day 7 to
the end of the observation period, day 50), with symptoms stabilizing at
between 2 and 3 in
severity.
In contrast, mice induced with MBP 85-99 (SEQ ID NO: 2) and co-immunized with
VWAK showed minimal EAE clinical symptoms (Figure 7). During the 50 day course
of the
experiment, the mice exhibited a return to a normal clinical appearance by day
37. The
symptoms recorded for VWAK-treated mice that appeared at about day 9 were
observed at a
greatest clinical score of about or less than about 1. Other copolymers shown
in Figure 7,
while providing some symptom remediation compared to the MBP 85-99 (SEQ ID NO:
2)
control, did not so substantially reduce the severity of symptoms, which
ranged from I to
slightly above 2 (for the group co-immunized with YFAK 0.2:0.8), 1 to 2 (for
the group
pre-immunized with YFAK 0.5:0.5), and slightly greater than 1 (for the group
co immunized
with YFAK 0.5:0.5).
31
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31399
The greatest remediation of symptoms was found in the group that was co-
immunized
with VWAK, and the shortest duration of symptoms was found in the group that
was
pre-immunized with YFAK 0.5:0.5. In the latter group, symptoms were observed
for a total
of only five time points, followed by disappearance of clinical symptoms. The
YFAK 0.5:0.5
pre-treatment data are co-plotted in Figure 8 (square symbols) to show the
contrasts in
severity and duration of symptoms of the YFAK 0.5:0.5-treated group with the
control group
of MBP 85-99 (SEQ ID NO: 2; diamond-shaped symbols) induced and otherwise
untreated,
and the CopI-treated group (Figure 8, triangular symbols). The pre-
immunization protocol
used here is equivalent to vaccination against the autoimmune disease EAE.
In contrast to pre-immunization with YFAK 0.5:0.5, Copl pre-immunization or
co-immunization in the same assay, while remediating symptoms, provided relief
of
symptoms to a level of a clinical score of about 2 to 3 (Cop 1 co-
immunization), or slightly
greater than 3 (Cop I pre-immunization). Further, symptoms were observed for
nine time
points, taken from days 7 through 37 (Copl co-immunization) prior to mice
becoming
asymptomatic, or over a period of 14 time points from days 7 through 50 (Copl
pre-immunization), with mice achieving a stable level of symptoms of greater
than about I in
severity, rather than elimination of symptoms as in the YFAK-treated group.
These data
show that YFAK 0.5:0.5 is most effective in pre-immunization of animals
against
development of the EAE disease condition.
These data indicate that the presence of W or F in a random copolymer with
amino
acids V, A, and K may increase tightness of fit of the copolymer into a
position of the class II
MHC major groove, for example, into both the P1 and the P4 position. The data
show that
YFAK and VWAK are promising potential therapeutic agents for MS, for
demyelinating
conditions, and possibly for other autoimmune diseases.
32
CA 02614171 2007-12-10
PCT/U502/31349
WO 03/029276
W
0
o
a) O N
C~l
cd
0 4-
4- M
0 C~ M C~ o
O
I O _ ~~ t3 N
TS O
kn N
o6 ON
a - ; w
0
v a) O v
a) Ln
cd O cd
0 - O p p p V_
0
N N ,_ , a En 0 a) 0
cd +-
0 -0
cd
cd *-
Cl)
0
o o 0 0 0
~ o 0 0 0
Q
0 0 o o
0 as U cy N
r. cd
~ 5-
Cl) O
a) ~,O U 30
La
CA O O p o ~+ - U O
co 4-4 4-4 0 0 O =
~ L1. ~ ~, 4~ O O o
14 a O
3UOW~
. ~ r
tea) 0 S)
_ 00~-+ N o0 O M 3-.
W Cy.~ a) a) a) a)
to i
"ca t4_4 4-+ N Cl) V ue
N ~ N 't3
0
U t. O pt - s+ ~. + s + r.
{ ~+ V7 Vl to M M Cl) N 4 O\
03 ca
-s' c'" o W W W W ri'J >>
0
U > w > w
33
CA 02614171 2007-12-10
WO 03/029276 PCT/US02/31 0 0
N t7 O
3 O co N O
o
4-1
Q) r,
t
N ~, N o .-~ ...
-H -H -H .}f -H -H U
cti o v~ v~ ~D O t~ j p .~
I
O O "-+ M M N N N c+ U U
U p O
'Zo
-O Ti
t3 O 00 v-) O tN 00 ~O ti n, 'C7
H I H -H 41 -H j -
acs ry 4n rn \0
Q> U
~U O O+ cd cct N U
0~0 CA
0 N N U 0 0
0 aa)
O M N 00 to N Q ~' 9
Q' 't3 7 -~ N 0 2
ul --
c 1 am., 0
P-4 ca
1 O
~1 U O cd
a> M '.0 p., O
-0 M ZZ,
N 5 ca
R3 "C3
U ,V 4-+
W "d o
U~+ bAcn~
v wv'3~~t`
kr~ 00 cv o . rn
O
V
V N
W) 00 P, 4, co
=Zi
N ~, O O c0 0 a O ''
P.
0
0 (D 0;
bl)
En =L
34
CA 02614171 2007-12-10
SEQUENCE LISTING
<110> Strominger, Jack L.
Fridkis-Hareli, Masha
<120> COPOLYMERS FOR SUPPRESSION OF AUTOIMMUNE DISEASES, AND METHODS OF USE
<130> 24655-015-061
<140> PCT/US02/31399
<141> 2002-10-03
<150> 60/326,705
<151> 2001-10-03
<160> 5
<170> Patentln Ver. 2.1
<210> 1
<211> 15
<212> PRT
<213> Human
<220>
<221> DOMAIN
<222> (86) ... (100)
<223> myelin basic protein
<400> 1
Asn Pro Val Val His Phe Phe Lys Asn Ile Val Thr Pro Arg Thr
1 5 10 15
<210> 2
<211> 15
<212> PRT
<213> Human
<220>
<221> DOMAIN
<222> (85)...(99)
<223> myelin basic protein
<400> 2
Glu Asn Pro Val Val His Phe Phe Lys Asn Ile Val Thr Pro Arg
1 5 10 15
<210> 3
<211> 21
<212> PRT
<213> Human
<220>
<221> DOMAIN
<222> (40)...(60)
<223> proteolipid protein
<400> 3
Thr Gly Thr Glu Lys Leu Ile Glu Thr Tyr Phe Ser Lys Asn Tyr Gln
1 5 10 15
i
CA 02614171 2007-12-10
Asp Tyr Glu Tyr Leu
<210> 4
<211> 13
<212> PRT
<213> Mouse
<220>
<221> DOMAIN
<222> (139) ... (151)
<223> proteolipid protein
<400> 4
His Ser Leu Gly Leu Trp Leu Gly His Pro Asp Lys Phe
1 5 10
<210> 5
<211> 13
<212> PRT
<213> Influenza virus
<220>
<221> DOMAIN
<222> (30G) ... (318)
<223> hemagglutinin protein
<400> 5
Pro Lys Tyr Val Lys Gln Asn Thr Leu Lys Leu Ala Thr
1 5 10
2