Note: Descriptions are shown in the official language in which they were submitted.
CA 02614886 2007-11-28
WO 98/24377 PCT/US97/22197
1
DESCRIPTION
CEREBRAL PROTECTION DURING CAROTID ENDARTERECTOMY
AND DOWNSTREAM VASCULAR PROTECTION
DURING OTHER SURGERIES
Field of the Invention
This invention relates to carotid endarterectomy surgery. More
particularly, it relates to methods and apparatus for improving endarterectomy
procedures by using blood filtration to protect the patient from embolization
during these vascular surgeries.
Background of the Invention
Endarterectomy is a surgical procedure which generally includes the
removal of the lining of an artery. Typically the artery is dissected
longitudinally
to expose an affected region from which plaque and other materials may be
removed. Endarterectomy can be performed on almost any major artery that is
diseased or blocked, and is most commonly used for the carotid, femoral, and
popliteal arteries.
In a typical procedure, the surgeon makes a standard vertical
iricision in the neck of a patient, or a transverse incision corresponding to
a skin
line of the neck. The incision is deepened through and around subcutaneous
adipose tissue, platysma muscle, the branches of the external jugular vein,
and the
border of the sternocleidomastoid muscle in order to expose the carotid
sheath.
Careful dissection is used to expose the common carotid artery and its
external and
internal branches. Vascular clamps are applied to the internal carotid artery,
external carotid artery, and common carotid artery, and a vertical arteriotomy
is
made in the common carotid artery, typically below the bifurcation. The
incision
may be advanced into the internal carotid artery to a point beyond the area
which
contains plaque material.
. An indwelling shunt may then be installed in order to bypass the
CA 02614886 2007-11-28
WO 98/Z4377 PCT/US97122197
2
clamped region of the artery so that brain perfusion is not disrupted. The
artery is
then clamped proximal and distal about the shunt in order to isolate a
bloodless
region for endarterectomy. Atheromatous material is then removed, first from
the
common carotid artery, then from the external carotid artery, and generally
last
from the internal carotid artery. After the endarterectomy procedure has been
performed, the surgeon cleans the region of plaque fragments before removal of
the shunt and closure of the vascular incision.
The above-described procedure, however, suffers from a deficiency
which relates to the escape of embolic material which may lead to devastating
neurologic complications, particularly when emboli escape through the internal
carotid artery. Emboli may be produced through any step of the procedure where
mechanical forces are applied to the artery, and these manipulations include
clamping, unclamping, applying a tourniquet, dissecting the vessel, inserting
and
removing a bypass shunt, removing atheromatous material, cleaning the affected
site, and suturing the vessel. Therefore, a need exists for an improved
endarterectomy procedure and apparatus which will enable the surgeon to
minunize the production of embolic material and to prevent the escape of
embolic
material during carotid endarterectomy, arteriotomy, and other vascular
surgeries.
$ummary of the Invention
A dramatic improvement in the neurologic outcome of patients
undergoing carotid endarterectomy, and arteriotomy procedures generally, can
be
achieved by using a blood filter device to capture and remove dislodged
embolic
material during the surgical procedure in accordance with our invention. Thus,
the invention provides novel methods and apparatus for protecting a patient
from
embolization during arteriotomy procedures. In one embodiment, the invention
provides a bypass tubing or indwelling shunt, having a main lumen for blood
bypass and a second, branching lumen adapted to receive an elongated blood
filtration instrument, or other surgical device (e.g., an angioplasty
catheter, stent
catheter, atherectomy catheter) and to allow passage of same into an artery
distal
to the endarterectomy region. The branching secondary lumen can either merge
CA 02614886 2007-11-28
WO 98na371 rcrtv897n2197
3
and communicate with the main lumen of the shunt, or may extend to a distal
= opening separate from the blood bypass lumen of the device.
In another embodiment, a standard single-lumen indwelling shunt is
used in accordance with the disclosure of Loftus, Carotid Endarterectomy
Principles and Techniques; Quality Medical Publishing, Inc.: St. Louis,
Missouri,
1995 (this and all other references cited herein are expressly incorporated by
reference as if fully set forth in their entirety herein), and an introducer
sheath and
filtration catheter are provided for deployment distal to the site of standard
carotid
endarterectomy. The introducer sheath includes a hemostatic valve adapted to
receive a filtration catheter. The filtration catheter typically includes a
catheter
sheath, an elongated control member, a control mechanism at a proximal end of
the control member, and a filtration assembly which includes an expansion
frame
and filter mesh at a distal region of the control member, the expansion frame
being operable to enlarge from a contracted condition to an expanded condition
which covers all of, or a substantial portion of the cross-sectional area of a
vessel.
In alternative embodiments, a filter is disposed on a guidewire or tubing for
use in
carotid artery bypass to capture clots and atherosclerotic material released
during
endarterectomy.
According to the methods of the present invention, an affected
region of an artery is isolated, clamped, and dissected as disclosed in
Loftus,
Carotid Endarterectomy Principles and Techniques; Quality Medical Publishing,
Inc.: St. Louis, Missouri, 1995, and Smith, The Surgical Treatment of
Peripheral
Vascular Disease, Chapter 142, in "The Heart, Arteries, and Veins," Vol. 2,
Ed.
J. Willis Hurst; McGraw-Hill Information Services Corp., 1990. An indwelling
shunt as described herein is then inserted so that the distal region
penetrates into
the distal artery and is secured by a distal artery clamp, while the proximal
region
penetrates into the proximal artery and is secured by a clamp proximal to the
region of arteriotomy. A blood filter device is deployed through the second
lumen
of the indwelling shunt as disclosed herein, is advanced within the blood
vessel,
and then expanded to cover a substantial cross-sectional area of the artery
distal to
the arteriotomy region. Endarterectomy is performed in accordance with
standard
CA 02614886 2007-11-28
WO 98/24377 PGT/US97122197
4
procedures to remove atherosclerotic material from the affected region of the
artery.
According to an alternative method, a non-indwelling shunt or
plastic tubing as disclosed herein is used to bypass an affected region of the
artery.
After the carotid artery is exposed, an incision is made proximal to the site
where
the conunon carotid artery cross-clamp will be placed. Plastic tubing having
an
appropriate size is placed in this incision and then extended distally, past
the site
where the internal carotid artery cross-clamp will be placed, and distat to
the
atherosclerotic plaque, where the plastic tubing reenters the carotid artery
through
a second incision. A filter device is deployed in the internal carotid artery
through
a side-port on the shunt, or the filter may be deployed by an expansion
mechanism intrinsic to the tubing itself. The conunon and internal carotid
arteries
are then clamped. The carotid artery is incised, plaque removed, the operative
site rinsed with sterile saline or water, and the carotid artery, with or
without a
graft, is closed. The proximal and distal cross-clamps are removed, and
circulation through the repaired carotid artery is restored as discussed
herein. The
proximal end of the plastic tubing is removed from the common carotid artery
and
the proximal incision is closed. The filter, including captured embolic
material, is
retracted after several minutes, typically at least 5 minutes, more preferably
at
least 10 minutes, and the distal end of the shunt is removed. Finally, the
distal
incision is closed.
Brief Description of the Drawings
Fig. 1 depicts a common or merging lumen shunt in accordance
with one embodiment of the present invention;
Fig. 2 depicts a non-communicating lumen shunt in accordance
with another aspect of the present invention;
Fig. 3 depicts an indwelling common lumen shunt and filter
deployed within an artery during an endarterectomy procedure;
Fig. 3A is a cross-sectional view taken through section line 3A-3A
of the shunt and vessel depicted in Fig. 3;
CA 02614886 2007-11-28
WO 98n4377 Pcr/US97n2197
Fig. 4 depicts a standard indwelling shunt during endarterectomy
and a filtration catheter deployed through an introducer sheath;
Fig. 5 depicts a non-indwelling shunt bypassing a region of the
common and internal carotid arteries during endarterectomy; and
5 Fig. 6 depicts an indwelling common lumen shunt and filter
deployed within an artery during an endarterectomy procedure.
Detailed Descrintion of e Invention
The devices and methods disclosed herein function to prevent
embolic material from migrating downstream (into the brain, organs,
extremities
of the limbs, etc.) during vascular surgery. The devices and methods herein
are
useful during any procedure where vessels are cut open for the purpose of
removing occlusions or performing other types of repair that may require the
use
of shunting to maintain distal blood flow.
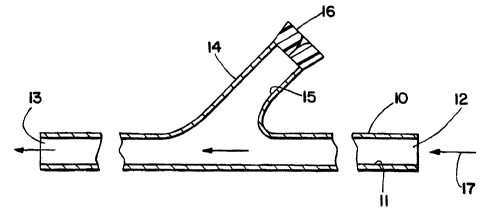
According to one embodiment, the shunt is as depicted in Fig. 1.
The shunt includes elongated tubular member 10 having lumen 11 which extends
from proximal opening 12 to distal opening 13. Within an intermediate section
of
tubular member 10, the shunt includes a "Y" arm, or second tubular member 14
having lumen 15 which branches away from main lumen 11. Thus, at one end,
lumen 15 merges and communicates with lumen 11, while at the other end, lumen
15 terminates at hemostatic valve 16 which permits device introduction. The
direction of blood flow through the shunt during use is depicted by arrow 17.
The
"communicating lumen" shunt as depicted in Fig. 1 will typically be
constructed
from a very soft, atraumatic material, e.g., silicon, latex, urethane. The
length of
tubular member 10 from its proximal opening to its distal opening will
typically be
greater than 5 cm, more typically greater than 8 cm, more typically greater
than
10 cm, more typically greater than 12 cm, more typically 15 cm or more in
length. Meanwhile, the outer diameter of tubular member 10 will generally be 3
mtn or greater, more generally 4 mm or greater, more generally 5 mm or
greater.
The foregoing ranges are set forth solely for the purpose of illustrating
typical
device dimensions. The actual dimensions of a device constructed according to
CA 02614886 2007-11-28
WO 98l24377 PCTIUS97/22197
6
the principles of the present disclosure may obviously vary outside of the
listed
ranges without departing from those basic principles disclosed herein.
In a second embodiment, the shunt includes elongated tubular
member 10 with lumen 11 as depicted in Fig. 2. A second tubular member 14 is
provided having lumen 15 extending from hemostatic valve 16 at a proximal end
to opening 18 at a distal end thereof. Lumen 15 therefore includes a first
segment
which runs substantially parallel to lumen 11 of main tubular member 10, and a
second segment which branches away from lumen 11 and terminates proximally at
hemostatic valve 16. This "non-communicating lumen" shunt allows blood to
flow in the direction of arrow 17 when the shunt is in use, and this shunt is
constructed from the materials and according to the device parameters given
above.
In use, it will be understood that secondary lumen 15 defines a
passageway for introduction of a medical instrument, e.g., a blood filter
device,
within an artery during an arteriotomy or endarterectomy procedure. With
reference to Fig. 3, the use of a shunt as disclosed herein will be described
in the
context of an endarterectomy procedure. A typical site of atherosclerotic
plaque
build-up is in the common carotid artery near the segment which branches to
the
internal carotid artery and external carotid artery. Segment 62 of artery 61
having
plaque build-up is located and exposed through an incision made in the neck of
a
patient. Tourniquet 31 (Rummel tourniquet) is placed loosely around the common
carotid artery. A Bulldog clamp (not shown) is then secured on the internal
carotid artery. Next, a DeBakey clamp (not shown) is placed on the common
carotid artery proximal (upstream) of the tourniquet. The external carotid
artery
(not shown) is secured with a Bulldog clamp. This order of vessel clamping is
significant because the clamp on the internal carotid artery is effective to
catch any
embolic debris dislodged by the DeBakey clamp placed on the conunon carotid
artery.
With the clamps in place, the surgeon makes a longitudinal incision
in the artery using scissors to expose the region of the artery containing
plaque
material. A- shunt as depicted in Fig. 1 or Fig. 2 is gripped with forceps in
the
CA 02614886 2007-11-28
WO 98/24377 P(.TIUS97122197
7
distal region. A second forceps is secured to the proximal region of the shunt
to
prevent blood escape. The Bulldog clamp which secures the internal carotid
artery
is loosened to allow back-bleeding while the distal opening 13 of shunt 10 is
advanced distally into the internal carotid artery. When the shunt has been
successfully placed in the internal carotid artery, it is secured by Javid
clamp 32 to
prevent further back-bleeding. It should be noted that during advancement of
the
distal opening of the shunt into the internal carotid artery, care must be
taken to
avoid scraping and thereby dislodging debris from the walls of the vessel. For
this reason, the clamp on the internal carotid artery is loosened and allowed
to
back-bleed during the process so that antegrade blood flow blows the vessel
walls
apart so that tubular member 10 can be advanced through the center.
The second forceps secured to the proximal region of the shunt is
released in order to vent air from the interior lumen of the shunt. A blood
filter
device is deployed through the hemostatic valve and advanced through lumen 15
into common lumen 11 and through distal opening 13 into artery 61. The blood
filter device will typically include an elongated member 41 (guidewire,
sheath,
etc.) having a proximal end with control mechanism 43 for activating the
filter,
and filtration mesh 42 suspended on an expansion frame disposed about the
distal
end of elongated member 41. The construction and use of expansion frame,
associated filter mesh 42, and control mechanism 43 have been thoroughly
discussed in earlier applications including Barbut et al., U.S. Application
Serial
No. 08/553,137, filed November 7, 1995, Barbut et al., U.S. Application Serial
No. 08/580,223, filed December 28, 1995, Barbut et al., U.S. Application
Serial
No. 08/584,759, filed January 9, 1996, Barbut et al., U.S. Application Serial
No.
08/640,015, filed Apri130, 1996, Barbut et al., U.S. Application Serial No.
08/645,762, filed May 14, 1996, and Barbut et al., U.S. Application Serial No.
08/683,503, filed July 17, 1996, and the contents of each of these prior
applications are incorporated herein by reference in their entirety. It will
be
understood that the design and use of a filter mesh, associated expansion
frame,
and control mechanism as discussed in these applications is fully applicable
to the
use of such filter and expansion frame on a guidewire or arterial catheter
system
CA 02614886 2007-11-28
WO 98/24377 PCT/1Js97122197
8
as disclosed herein.
The filter is maintained in a contracted state during entry through
lumen 15, and lumen 11. Once the filter has been advanced beyond distal
opening
13 of shunt 10, filter 42 is expanded to an enlarged diameter which covers a
substantial portion of the cross-sectional area of vesse161. Filter 42 is
maintained
in place during the remaining surgery in order to capture embolic material
dislodged during the procedure.
Next, the proximal opening of the shunt is advanced proximally into
the conunon carotid artery until it abuts against the DeBakey clamp.
Tourniquet
31 is tightened and the DeBakey clamp released to allow the surgeon to slide
the
shunt further proximal. Once the shunt and filter are in place and operational
as
depicted in Fig. 3, it is generally desirable to evaluate shunt function using
a
Doppler probe. An audible flow signal will typically confirm patency. Fig. 3A
shows a cross-sectional view of shunt 10 and elongate member 41 within vessel
segment 62, taken through section line 3A-3A. The endarterectomy procedure is
then performed within the dissected region of the artery. The plaque or
atheroma
material typically has the consistency of a thick shell. This material is
dissected
and peeled out of the vessel, preferably in one or a small number of large
pieces.
Such a monolithic removal is preferred to breaking of the plaque into small
pieces
as the latter may be lost in the circulation and result in emboli.
The dissected vessel is then closed by suturing both ends of the slit
toward the center until a small hole remains in the common carotid artery, as
d'escribed in Loftus, Carotid Endarterectonry Principles and Techniques;
Quality
Medical Publishing, Inc.: St. Louis, Missouri, 1995. The shunt is then gripped
by
two clamps spaced by a short distance. Filter mesh 42 is contracted to a small
diameter, holding captured embolic material trapped within the mesh. The
filter is
then withdrawn from vessel 61 into lumen 11, and then into lumen 15 and
removed from hemostatic valve 16. The shunt is cut by scissors between the
clamps. Both resulting pieces of the shunt are removed from the common carotid
artery.
The clamp on the internal carotid artery is briefly loosened and
CA 02614886 2007-11-28
WO 98/24377 PCT/US97/22197
9
allowed to back-bleed in order to purge air from the dissected region 62 of
vessel
61. The clamp on the external carotid artery is similarly loosened briefly to
back-bleed and purge air from the affected segment of the external carotid
artery.
The surgeon checks for thrombi enclosed within the affected segment 62 of
vessel
61, and for inadvertent closure from the suture line having caught an
unintended
portion of the back of the vessel. Heparinized saline is injected into the
small
opening which remains. The last suture is tied to completely close the
incision in
the dissected region of vessel 61. The clamp on the external carotid artery is
removed, and the clamp on the common carotid artery is removed. After a delay
of 10 seconds, the clamp on the internal carotid artery is removed. This
sequence
ensures that any inadvertent debris or air is flushed to the external carotid
artery
rather than the internal carotid artery and the patient thereby avoids
neurologic
harm.
In another embodiment, the shunt is secured to the vessel walls
using one or more balloon occluders as depicted in Fig. 6. The use of balloon
occlusion eliminates the need to apply compressive clamps (numerals 31 and 32
in
Fig. 3) to secure the shunt within the vessel, and thereby reduces the risk of
debris
dislodgement during shunt installation. With reference to Fig. 6, shunt 10
includes one or more balloon occluder 35 at its proximal and/or distal ends,
the
balloon occiuder being disposed circumferentially around the tubing of the
shunt.
Occluder 35 is in fluid communication with inflation lumen 37, inflation port
38,
and optionally tubing 39 for saline injection. Thus, in use, the proximal or
distal
end of the shunt is positioned as described above, while occluder 35 is in a
deflated state. Saline, or other biotolerable fluid, is injected through port
38 until
occluder 35 enlarges into contact with the inner diameter of vessel 61,
thereby
sealing the vessel from blood flow. A cuff or C-clamp 36 may be fitted about
the
vessel to prevent hyperexpansion, minimize internal slippage of the balloon
occluder, and provide a tight seal within the vessel. After the endarterectomy
procedure, saline is withdrawn to deflate occluder 35 before the shunt is
removed
from the vessel.
In another embodiment, the shunt and filtration assembly are
CA 02614886 2007-11-28
WO 9&W77 PCT/US97/22197
separated from another as depicted in Fig. 4. Deployment of the filtration
assembly makes use of introducer sheath 51 having hemostatic valve 52 at one
end
thereof. Introducer sheath 51 is inserted through an incision in the wall of
artery
61 downstream or distal to the site of arteriotomy 62. Introducer 51 is shaped
to
5 receive catheter sheath 43 which receives elongate member 44 having filter
mesh
42 operably disposed at a distal region thereof. Expansion and contraction of
filter 42 is controlled by mechanism 45 which operates at the proximal region
of
elongate member 44. Thus, in use, after the artery is selected and isolated,
introducer 51 is inserted through an incision created in the wall of artery
61.
10 Filter catheter 43 is inserted through hemostatic valve 52 and into the
lumen of
vessel 61 with filtration assembly 42 being in a contracted condition. Once in
place, control mechanism 45 is operated to expand filter 42 so that it covers
most,
if not all, of the cross-sectional area of vessel 61. With the filter in
place, an
endarterectomy procedure, which includes steps of clamping (using Bulldog
clamp
32 and optionally vascular occlusion clamp 33), installation of a normal
shunt,
arteriotomy, shunt removal, and unclamping, is conducted in accordance with
the
description given above. Thereafter, filter 42 is contracted and removed from
artery 61 through introducer 51. In a final step, introducer 51 is removed
from
the artery and the opening in artery 61 is sutured.
It will be understood that the ordering of steps can be modified so
that introduction of filter 42 may occur at any point in the procedure.
However,
in a preferred embodiment, the filter is deployed before arteriotomy begins
and the
filter is removed after arteriotomy has been completed. In this manner, filter
42 is
available to capture all embolic material which results from the manipulative
steps
of the arteriotomy procedure, e.g., clamping, unclamping, installation of
tourniquet, installation and movement of shunt 10, cutting of vesse161,
suturing
the vessel, and shunt removal. Thus, the method depicted in Fig. 4 constitutes
a
preferred embodiment insofar as it allows the surgeon to maintain filter 42
deployed within the vessel throughout the arteriotomy procedure.
In another embodiment, a non-indwelling shunt is used to bypass an
endarterectomy region as depicted in Fig. 5. This figure shows common carotid
CA 02614886 2007-11-28
WO 98/24377 PCTlUS97/22197
11
artery 65 which branches into external carotid artery 66 and internal carotid
artery
67, and which includes an affected region 62 having atherosclerotic plaque 63
disposed on the lumen thereof. Distal opening 13 of shunt 10 is inserted into
vesse161 through an incision. Back-bleeding through the shunt occurs from the
distal opening 13 of lumen 11 in order to purge air from within the shunt.
After
the shunt is purged, proximal opening 12 is secured by a clamp (not shown).
Filter catheter 43 having elongate member 44, filter mesh 42, and control
mechanism 45 disposed thereon, is inserted into the lumen of shunt 10 through
hemostatic valve 16 and thereafter advanced into the internal carotid artery
67
with filter 42 in a collapsed state. Using control mechanism 45, filter 42 is
enlarged to cover substantially all of the cross-sectional area of the
internal carotid
artery lumen. Proximal opening 12 of shunt 10 is then inserted into conunon
carotid artery 65 through an incision.
With the filter in place, an endarterectomy procedure is conducted
on affected region 62 in order to remove deposits 63 as depicted in Fig. 5.
This
region of the carotid artery is isolated using clamps 31, 33, and 34 as
described
above, and incision 64 is created to expose plaque deposits 63 within region
62.
The plaque is removed, the area is cleaned, the incision is closed, and the
clamps
are removed in order to purge any remaining gas. The final sutures are then
installed to complete the closure of incision 64, and blood flow is
reestablished
while filter mesh 42 remains in place. This sequence ensures that any
remaining
debris is captured by filter 42 and is not allowed to enter the brain as
emboli.
Filter 42 is then collapsed and removed from the internal carotid artery 67
into
shunt 10, and thereafter through hemostatic valve 16. Finally, the distal end
of
the shunt is removed from the internal carotid artery, the incision is
sutured, the
proximal end of the shunt is removed from the common carotid artery, and the
incision in the common carotid artery is sutured. In this manner, the patient
is
protected from embolization to the brain throughout the arteriotomy procedure.
Referring again to Fig. 4, the introducer sheath 51 will typically
have an external diameter of 5-12 French, more preferably 6-8 French. With
reference to the filter device, the diameter at the distal end will typically
be 1-3
CA 02614886 2007-11-28
WO 98/?d377 PCT/US97/22197
12
mm, more preferably 1.5-2.5 mm. The filter is generally activated from the
proximal end and is deployed from within a small sheath or on the outside of a
guidewire or small tube. The length of the filter device is generally 20-40 cm
and
the deployed diameter of filter mesh 42 will typically be 2 mm or larger, more
preferably 4 mm or larger, more preferably 6 mm or larger, more preferably 8
mm or larger, more preferably 10 mm or larger, and generally will be 2-10 mm.
The foregoing ranges are set forth solely for the purpose of illustrating
typical
device dimensions. The actual dimensions of a device constructed according to
the principles of the present disclosure may obviously vary outside of the
listed
ranges without departing from the basic principles disclosed herein.
It will be understood that filtration is an important aspect of the
endarterectomy shunt and methods disclosed herein. To filter blood
effectively,
i.e., to capture embolic material, without unduly disrupting blood flow, the
mesh
must have the appropriate physical characteristics, including area (AM),
thread
diameter (DT), and pore size (Sp). In the carotid arteries, the mesh 42 must
permit
flow rates as high as 0.15 L/minute or more, more preferably 0.2 L/minute or
more, more preferably 0.25 L/minute or more, more preferably 0.3 L/minute or
more, more preferably 0.35 L/minute or more, more preferably 0.4 L/minute or
more, more preferably 0.45 L/minute or more, and most preferably 0.5 L/minute
or more at pre-filter maxiunum systolic pressures (proximal to the mesh) of
around
200 mm Hg or less.
In order to capture as much of the dislodged material as possible,
mesh with the appropriate pore size must be chosen. With reference to embolic
material dislodged from the aorta, individual particle diameter ranges from
0.05
mm to 2.88 mm, with a mean diameter of 0.85 mm, and individual particle
volume ranges from 6.5x10'1 mm3 to 12.45 mm3, with a mean particle volume of
0.32 mm 3. Approximately 27 percent of the particles have been found to
measure
0.6 mm or less in diameter. During cardiac bypass surgery in particular, the
total
aortic embolic load has been found to range from 570 mm3 to 11200 mm3, with a
mean of 3700 mm3, and an estimated cerebral embolic load has been found to
range from 60 mm3 to 510 mm3, with a mean of 276 mm'. During carotid
CA 02614886 2007-11-28
WO 98/Z4377 PCT/US97122197
13
endarterectomy, materials dislodged as emboli have similar characteristics to
those
of aortic materials.
It should also be understood that the embolic material against which
the present devices and method protect may include gaseous bubbles
inadvertently
introduced during the surgical procedure. Air emboli are a common and
dangerous occurrence during all types of surgeries. They are potentially most
dangerous if allowed to enter the cerebral circulation and cause ischemic
events,
which may lead to stroke. The type of surgery where this is most likely to
occur
is surgery on the heart and ascending aorta, but may also occur during
endarterectomy. Currently, surgeons make great efforts to de-air and vent the
heart and vasculature after a procedure to eliminate air prior to closing the
incision
and/or taking a patient off of cardiopulmonary bypass. Nevertheless, a small
amount of air always remains and is potentially dangerous.
Thus, the filter assembly disclosed herein acts to retain large air
bubbles, and under sufficient pressure, causes them to be broken into much
smaller bubbles which are much less potentially harmful. A typical pore size
for
the aortic filter is about 100 m. When a bubble greater than 100 m diameter
encounters the filter, there must be sufficient pressure on the proximal side
of the
filter to force the bubble through the pore. The surface tension of the blood
generally prevents the bubble from deforming and extruding through the pore,
but
rather the bubble breaks apart into a plurality of bubbles small enough to
pass
freely through the pore. The filter thereby acts as a bubble sieve.
The benefit of reducing the size of the interactive bubbles is
twofold. First, the potential of a bubble to cause ischemia is directly
related to its
diameter. The larger the bubble, the more likely it is to block blood flow to
a
larger area of the brain. Smaller bubbles may block smaller arteries, but will
have
less overall ischemic effect. Second, smaller bubbles will be absorbed into
tissue
and cells more quickly than large bubbles, because of their greater surface
area to
volume ratio. The net effect is smaller bubbles which may make their way into
the brain, and bubbles which will be more quickly metabolized further reducing
risk of embolic ischemia.
CA 02614886 2007-11-28
WO 98/24377 PCr/US97/22197
14
Another method by which large bubbles can be rendered into
smaller bubbles is due to velocity and momentum effects. During moments of
peak systolic cardiac output, the blood velocity from the heart is at its
maximum
(100-150 cm/s). If a bubble is trapped against the intraaortic filter and is
subject
to instantaneous high velocity blood flow, the momentum of the blood on the
bubble will cause the bubble to shatter into smaller bubbles. The smaller
bubbles
will then "escape" through the pores in the filter if they have been rendered
small
enough.
The area of the mesh required for the device herein, having all of
the desirable properties disclosed herein, for use in the carotid arteries is
calculated from Bernoulli's equation as described in Barbut et al., U.S.
Application Serial No., U.S. Application Serial No. 08/553,137, filed November
7, 1995, Barbut et al., U.S. Application Serial No. 08/580,223, filed December
28, 1995, Barbut et al., U.S. Application Serial No. 08/584,759, filed January
9,
1996, Barbut et al., U.S. Application Serial No. 08/640,015, filed April 30,
1996,
and Barbut et al., and U.S. Application Serial No. 08/645,762, filed May 14,
1996. Thus, in one embodiment, a filter for use in the carotid arteries is
provided
with a mesh having dimensions within the following ranges: mesh area is 10-200
mm2, more preferably 20-150 mmz, more preferably 35-100 mm2, more
preferably 50-75 mm2; mesh thickness is 60-280 m, more preferably 70-270
,um, more preferably 80-260 /zm, more preferably 90-250 m, more preferably
100-250 m, more preferably 120-230 jcm, more preferably 140-210 /cm; thread
diameter is 30-145 m, more preferably 40-135 Am, more preferably 50-125
m, more preferably 60-115 ,um, more preferably 70-105 Am; and pore size is
500 m or less, more preferably 50-180 E.cm, more preferably 50-170 icm, more
preferably 50-160 gm, more preferably 60-150 ,um, more preferably 60-140 Am,
more preferably 60-130 gm, more preferably 60-120 gm, more preferably
60-110 m, more preferably 60-100 m, more preferably 60-90 gm, more
preferably 60-80 Am, and usually larger than at least a red blood cell. In a
preferred embodiment of the invention, mesh area is 50-75 mm2, mesh thickness
is
100-150 m, thread diameter is 30-100 m, and pore size is 50-150 m.
CA 02614886 2007-11-28
wo 981Z4377 PCT/US97n2197
Once appropriate physical characteristics are determined, suitable
mesh can be found among standard meshes known in the art. For example,
polyester meshes may be used, such as meshes made by Saati Corporation and
Tetko Inc. These are available in sheet form and can be easily cut and formed
5 into a desired shape. In a preferred embodiment, the mesh is sonic welded
into a
cone shape. Other meshes known in the art, which have the desired physical
characteristics, are also suitable. Anticoagulants, such as heparin and
heparinoids,
may be applied to the mesh to reduce the chances of blood clotting on the
mesh.
Anticoagulants other than heparinoids may also be used, e.g., monoclonal
10 antibodies such as ReoPro (Centocor). The anticoagulant may be painted or
sprayed onto the mesh. A chemical dip comprising the anticoagulant also may be
used. Other methods known in the art for applying chemicals to mesh may be
used.
In an embodiment of the devices suited for placement in the carotid
15 arteries, the expansion frame comprises an inflation seal with inflation
system as
discussed in U.S. Application Serial Nos. 08/580,223, 08/584,759, 08/640,015,
08/645,762, and 08/683,503. The expansion means, when fully inflated, has a
thickness of 0.5-1 mm. The dimensions of the expansion means may be adjusted
in alternative embodiments adapted for use in vessels other than the carotid
arteries. Alternatively, an expandable frame other than a balloon inflation
seal
may be used with the devices and methods disclosed herein. Expandable frames
include umbrella frames with a plurality of arms as described in U.S.
Application
Serial Nos. 08/533,137, 08/580,223, and 08/584,759.
All components of this device should be composed of materials
suitable for insertion into the body. Additionally, sizes of all components
are
determined by dimensional parameters of the vessels in which the devices are
intended to be used. These parameters are known by those skilled in the art.
Filtration of blood in the carotid arteries will usually be conducted
while the heart is functioning normally, i.e., without the use of
cardiopulmonary
bypass. Thus, blood pressure will be typically 50-200 mm Hg, blood flow will
be
approximately between 0.15-0.5 L/minute, and the pressure gradient will have
no
CA 02614886 2007-11-28
WO 98/24377 PCT/US97/22197
16
more than a 40 mm Hg drop across the filter when open (i.e., the filter may
not be
used in some embodiments). Modification of the operational characteristics set
forth above for use in vessels other than the carotid arteries are readily
ascertainable by those skilled in the art in view of the present disclosure.
An
advantage of all embodiments including a filter disclosed herein is that both
the
shunt and filter enter the vessel through a single incision created for the
shunt, and
therefore the devices and methods herein economize on incisions made in the
arteries.
It will also be understood that the filter device may be deployed by
insertion through the "Y" arm on the shunt during or after installation in an
artery, and for each disclosed method, the shunt and "Y" arm lumens may be
common (merging) or separate lumens as depicted in Fig. 1 and Fig. 2,
respectively. Moreover, insertion may be made next to the shunt, before,
during,
or after the shunt is installed. Insertion of the filter device may occur
distal to the
arteriotomy site, the shunt, and the occlusion clamp through an introducer,
either
intraoperatively or percutaneously. Where insertion of the filter device
occurs
percutaneously, distal to the region, the filter device may be inserted and
deployed
prior to interventional therapy such as arteriotomy, angioplasty, or stent
deployment.
Although the foregoing invention has been described in some detail
by way of illustration and example for purposes of clarity of understanding,
it will
be obvious that certain changes and modifications may be practiced which will
still
fall within the scope of the appended claims. In particular, it should be
understood that, although certain features (such as balloon occlusion) are
shown
by reference to only a single embodiment, those features are applicable to all
other
embodiments disclosed herein.