Note: Descriptions are shown in the official language in which they were submitted.
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
Express Mail No.:EV487233205US-
Attorney Docket No.: 1318_003 PCT
ORTHOPEDIC DEVICES WITH COMPRESSIVE ELASTOMER FORMED
DIRECTLY ONTO A BASE MATERIAL
FIELD OF THE INVENTION
[0001] The present invention relates to orthopedic devices, and, in
particular, to
orthopedic support devices that comprise an elastomeric material formed
directly onto,
atop, or around a base material in order to provide not only support but also
a desirable
combination of compression, protection and suspension to an injured body part.
BACKGROUND OF THE INVENTION
[0002] Many people develop injuries in an area of their body (e.g., knee,
ankle,
elbow, wrist) that is utilized on a daily basis such that the injured area
cannot be
immobilized while the injury heals. Thus, the goal becomes to stabilize and
protect the
injured body part to an extent whereby some usage of the body part can occur
while still
allowing for there to be simultaneous healing. To that end, orthopedic support
devices
have been developed consisting of a layer of flexible, resilient material
(e.g., neoprene)
which, when stretched over a body part, provides support thereto.
[0003] Various problems have been observed with regard to these traditional
orthopedic devices. For example, resilient materials neither effectively
dissipate heat nor
absorb/wick perspiration away from the skin. Thus, those who wear devices
formed of
such materials in warm climates and/or while engaged in strenuous physical
activity may
develop skin irritation, abrasions, heat rashes and/or dermatitis due to
perspiration,
particularly at points of bending such as the back, the knee, the elbow or the
wrist.
[0004] Moreover, conventional resilient material orthopedic supports tend to
migrate from their desired area of coverage, again owing to perspiration.
Migration
leaves the injured area entirely or partially unsupported, which, in turn, can
result in
slowed healing or even aggravation of the underlying injury. In a similar
vein, resilient
orthopedic supports have been known to sag, lose its shape or "bunch up,"
e.g., when the
supported body part is flexed. Bunching also can leave injured areas
unprotected or only
partially protected, and can either expedite the onset of skin problems
already associated
with such devices or create still other skin problems such as chafing or
bruising.
1
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
[0005] Those in the art have made various attempts to solve these problems,
including by providing support devices with multiple layers. However, these
multilayer
supports tend to be comparatively bulkier and/or more expensive than single
layer
supports. Plus, the presence of the extra layer(s) tend to cause discomfort to
the wearer
of the device, and/or may exacerbate skin problems caused by single layer
devices.
[0006] One particular manufacturing approach that seeks to curb the problem of
"bunching" is to utilize compression molding to form the support device.
However, this
compression molding technique tends to produce a support that, although
bunching-
resistant, lacks the flexibility and elasticity to be worn comfortably and to
permit an
acceptable range of movement for the injured area.
[0007] To combat the problem of support devices sagging or losing their shape,
some have produced devices that include a buttress made of a comparatively
stiffer
material. However, the added firmness of the buttress can irritate the injured
area if the
buttress is either too firm and/or placed too near the injured area. Moreover,
the process
for attaching the buttress (e.g., sewing, gluing) is labor-intensive and the
end product may
not be the ideal shape and size for people of different anatomies.
Customization is an
option; however, that renders the manufacturing process slower and more
expensive for
only mildly improved results.
[0008] There have been other attempts to stiffen orthopedic supports without
the
addition of a buttress, such as through the use of an injection molding
manufacturing
process. However, support devices produced by injection molding suffer from
similar
drawbacks as those that include buttresses, e.g., limited range of motion and
comparative
lack of compression. Moreover, injection molding equipment is particularly
expensive to
implement and operate.
[0009] Two other approaches have likewise proven unsuccessful, namely the
insertion of webbing within cuts in the support material and utilization of
standalone gel
components that are attached by straps or other devices.
[0010] Therefore, a need exists for orthopedic supports that avoid the litany
of
problems of conventional devices, yet that still can promote healing and
enable freedom
of movement while being worn.
2
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
SUMMARY OF THE INVENTION
[0011] These and other needs are met by the present invention, which,
according
to an exemplary aspect of the invention, provides orthopedic support devices
that include
a support body wherein a first layer formed of first material (e.g., an
elastomeric
material) is attached directly atop or around a second layer formed of a
different material
(e.g., a spacer material), wherein the first material layer provides support
and/or
compression, and the second material layer provides breathability and wicking.
[0012] The present invention advantageously provides flexibility with regard
to
both design and treatment options for orthopedic support devices, since
certain
characteristics (e.g., hardness, shape, thickness, location, pattern, modulus
of elasticity)
of the first material layer can be varied precisely in order to controllably
vary the location
and/or the amount/level of compression provided by the support device. That,
in turn,
enables orthopedic support devices in accordance with the present invention to
provide
targeted compression in order to offer an optimal combination of healing,
comfort and
freedom of movement while the devices are being worn, yet without resulting in
devices
that are prohibitively expensive to produce.
[0013] By way of non-limiting example, the support body for the orthopedic
support device can be a sleeve wherein the first material layer is formed to
have a
constant pattern (e.g., a web-like pattern with openings defined therein) and
a
substantially constant thickness to provides a substantially constant
level/amount of
compressive force to the injured body part (e.g., a knee) around which the
sleeve is worn.
To provide varied compression to the injured body part, the thickness of the
first layer
can be modified (e.g., increased by providing a buttress) in certain
predetermined areas of
the sleeve and/or the constant pattern can be made non-uniform.
[0014] According to another exemplary aspect of the present invention, the
support body for an orthopedic sleeve includes elastomeric material in the
form of one or
more buttresses that are sited on the sleeve such that when the sleeve is worn
the
buttress(es) correspond to the location of an injured area and thus provide
highly targeted
compression thereto. By way of non-limiting example, an orthopedic sleeve
meant to be
worn over a knee can include an elastomeric buttress that, when the sleeve is
properly
worn, will be positioned over the kneecap and/or a knee ligament.
3
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
[0015] In accordance with yet another exemplary aspect of the present
invention,
the support body for the orthopedic support device can be in the form of a
support strap,
e.g., a patella tendon bearing strap, whereby certain portions of the strap
can be formed
either entirely or partially of the elastomeric material in order to provide
targeted
compression to certain injured areas, e.g., the patella tendon.
[0016] Still other aspects, embodiments and advantages of the present
invention
are discussed in detail below.
BRIEF DESCRIPTION OF THE DRAWINGS
100171 For a fuller understanding of the nature and desired objects of the
present
invention, reference is made to the following detailed description taken in
conjunction
with the accompanying figures, wherein like reference characters denote
corresponding
parts throughout the views, and in which:
100181 FIG. 1 is front, perspective view of an orthopedic support sleeve of
the
present invention being worn on a knee;
[0019] FIG. 2 is a top view of the orthopedic support sleeve of FIG. 1 in an
unworn condition;
[0020] FIG. 3 is a front, perspective view of an alternative embodiment of the
orthopedic support sleeve of FIG. 1 being worn on a knee;
[0021] FIG. 4 is top view of a front panel of an orthopedic support sleeve of
the
present invention that includes a shaped buttress;
[0022] FIG. 5 is a side, sectional view of the front panel of FIG. 4 along the
line
5-5; -
[0023] FIG. 6 is an exploded view of the front panel of FIG. 4;
[0024] FIG. 7 is a top view of orthopedic patellar tendon bearing support
device
of the present invention;
[0025] FIG. 8 is a top view of the support buttress of the orthopedic device
of
FIG. 7
[0026] FIG. 9 is a bottom view of the support buttress of the orthopedic
device of
FIG. 7; and
[0027] FIG. 10 is a front view of the orthopedic support device of FIG. 7 as
worn.
4
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
DETAILED DESCRIPTION
[0028] The present invention provides embodiments of orthopedic support
devices in which a first material layer is attached directly onto, atop, or
around a second
material layer, wherein the first material layer provides support and/or
compression, and
the second material layer provides breathability and/or wicking. As will be
described in
detail below, and in accordance with such embodiments, certain characteristics
(e.g.,
hardness, modulus of elasticity, shape, thickness, and/or location) of the
first material
layer can be varied to vary the location and/or the amount/level of
compression provided
by the support device. That, in turn, enables cost effective formation of an
support device
that can provide targeted compression to an injured body part with an optimal
combination of healing and freedom of movement while the device is being worn.
[0029] In such embodiments, it is the first material layer that generally
provides
compression when the orthopedic device is worn. In an exemplary embodiment of
the
present invention, the first material layer is formed of one or more
elastomeric or rubber
materials. Suitable such materials include, but are not limited to materials
having a
hardness in the range of Shore 00-30 to Shore A-50 and/or a modulus of
elasticity in the
range of about 20 psi to about 150 psi, such as a thermoplastic rubber
material (e.g., a
thermoplastic elastomer), a silicone material (e.g., a silicone elastomer), a
polyurethane
material, a polyvinylchloride material, a styrene material (e.g., styrene-
butadiene rubber,
styrene-butadiene rubber and natural rubber blend), an acrylic rubber (e.g.,
polyacrylate,
ethylene acrylic rubber), a polyester-urethane (e.g., ADIPRENE ), a butadiene-
based
material (e.g., polybutadiene, butyl rubber, hi-nitrile butadiene rubber), a
choloroprene
material (e.g., neoprene), a chlorosulfonated elastomer, an ethylene
copolymer, an
ethylene vinyl acetate material, a fluoro-rubber, a natural rubber, a nitrile
elastomer, a hi-
nitrile phenolic rubber, an epicholorohydrin material, and a vinyl plasticol
material.
[0030] Generally, the material that forms the first material layer will have a
higher modulus of elasticity than the material that forms the second material
layer, so as
to provide the orthopedic support with a desirable overall combination of
support,
compression and breathability, while still allowing for freedom of movement of
the area
on or over which the support is worn. However, it is understood that the
material that
forms the first material layer can have a modulus of elasticity less than or
equal to the
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
modulus of elasticity of the material that forms the second material layer
without
departing from the scope of the present invention.
[0031] The second material layer generally provides a buffer layer between the
first material layer and the wearer's skin, but can have other functions and
placements as
well. According to an exemplary embodiment of the present invention, the
second
material layer is made of a material that is substantially breathable, has
good wicking
characteristics, and/or is resistant to bunching and migration when the
orthopedic device
is worn. Suitable such materials include, but are not limited to elastic or
inelastic spacer
materials such as nylon or polyester.
[0032] In accordance with various exemplary embodiments of the present
invention, the first material layer can have a substantially constant or
varied thickness,
design and/or shape over some or all of its overall length. For example, and
as will be
discussed in greater detail below, the first material layer can have a uniform
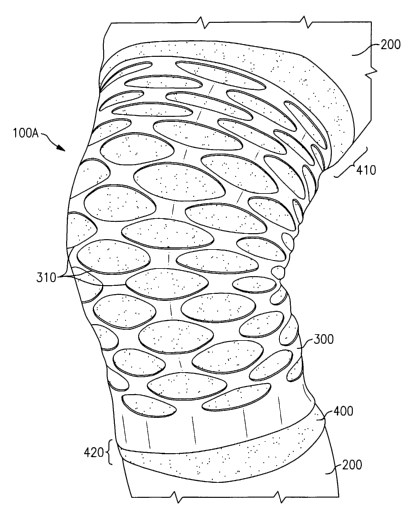
patterned
shape of substantially constant thickness (see, e.g., the sleeve 100A
illustrated in FIG. 1)
or the first material layer can have a non-uniform patterned shape of non-
constant
thickness (see, e.g., the sleeve 100B illustrated in FIG. 3), and/or the first
material layer
can entirely comprise a shaped area of added thickness (see, e.g., the sleeve
100C
depicted in FIGS. 4-6).
[0033] Referring initially to FIGS. 1 and 2, an exemplary embodiment of an
orthopedic support device 100A according to the present invention is shown. In
the
depicted embodiment, the support device 100A is a sleeve, which, as shown in
FIG. 1,
can be worn over a portion of a wearer's leg 200 such that the first material
layer 300
provides a compressive force on and around the wearer's knee and such that the
second
material layer 400 is in direct contact with the wearer's skin, thus acting as
a barrier layer
between the first material layer and the wearer.
[0034] The overall length of the second material layer 400 of the sleeve 100A
can
be less than, substantially equal to or greater than the overall length of the
first material
layer 300 of the sleeve 100A. As depicted in FIG. 1, and in accordance with an
exemplary embodiment of the present invention, the length of the second
material layer
400 of the sleeve 100A is greater than the length of the first material layer
300 such that
one or more portions 410, 420 of the second material layer 400 extend above
and/or
6
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
below the first material layer 300, thus enabling the sleeve 100A to serve as
a lower
modulus control top in order to minimize displacement of soft tissue.
[0035] According to an alternate embodiment of the present invention, the
extended portion(s) 410 and/or 420 can be formed of a third material different
from the
material that comprises either the first or second material layers 300, 400.
By way of
non-limiting example, and in accordance with such an alternate embodinient,
the first
material layer 300 can be formed of an elastomeric layer, the second material
layer 400
can be formed of a spacer material, and the third material that forms the
extended
portion(s) 410 and/or 420 can be formed of a resilient material (e.g.,
neoprene).
[0036] As best illustrated in FIG. 2, the first and second material layers
300, 400
are present around the entire circumference of the sleeve 100A in accordance
with this
exemplary embodiment of the present invention. FIG. 2 also illustrates that
the first
material layer 300 is formed directly atop/around and not encapsulated within
the second
material layer 400; however, it is understood that can be at least partially
encapsulated
within the second material layer 400 if instead desired. Also, although FIGS.
1 and 2
depict the first material layer 300 being present around the entire sleeve
100A (i.e.,
having 360 of coverage), it should be noted that the amount of coverage of
the first
material layer 300 can be about less than 360 in accordance with the present
invention,
e.g., such that when the sleeve device 100A is worn the first material layer
can
correspond to the front, back or side of a knee.
[0037] FIGS. 1 and 2 depict an exemplary embodiment of a sleeve 100A wherein
the first material layer 300 has a substantially repeating, patterned shape,
which, in this
exemplary embodiment, resembles a web, but which can take on other shapes as
well. To
form the FIG. 1 pattern, the first material layer 300 of the sleeve 100A has a
plurality of
linked circular or elliptical openings 310 defined therein. The presence of
these openings
310 is beneficial, since they better enable dissipation of heat and/or
moisture (i.e., heat
and/or moisture that has accumulated between the'wearer's skin and the second
material
400) through the breathable second material layer 400, thus reducing the
occurrence, or at
least the severity of skin problems associated with heat and/or moisture
retention between
the sleeve 100A and the wearer's skin. Moreover, the web-like pattern of
openings 310
within the first material layer 300 provides an improved fit as well as
increased comfort
7
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
and an increased range of motion to the wearer, while still providing
necessary targeted
compression along substantially the entire sleeve I OOA.
[0038] In an exemplary embodiment of the present invention wherein the sleeve
I OOA is designed for placement over a knee, the diameter of each opening 310
is
generally in the range of about 0.25 inch to about 1.25 inch, and the total
number of
openings is generally in the range of about 100 about 200, and the distance
between each
opening is generally in the range of about 0.15 inch to about 0.80 inch. It is
understood
that the number, and/or the diameter, and /or the distance between one, some
or all of the
openings 310 within the first material layer of sleeve I OOA can be modified
within or
outside these ranges for various reasons, e.g., to modify the level of
compression sought
to be provided by the sleeve I OOA, to provide non-uniform compression at one
or more
areas, to adapt to an atypical anatomy of a wearer, to relieve compression at
one or more
pressure points, and/or to vary the range of motion provided to a wearer,
and/or to
improve the overall fit of the device. It is further understood that one or
more portions of
the first material layer 300 between and/or around the openings 310 can have
various
complex and/or compound radii and/or can have filleted areas and/or edges,
thus
allowing the modulus of elasticity of such portion(s) to be precisely
tailored.
[0039] In accordance with an exemplary embodiment of the present invention in
which the first material layer 300 has a plurality of openings 310 defined
therein, at least
a portion of the length of the first material layer does not include any
openings in order to
enhance the structural integrity of the device. By way of non-limiting
example, such a
portion can be located at the bottom and/or top end(s) of the first material
layer 300 in
order to prevent migration and bunching and to increase the durability of the
sleeve
100A. Also by way of non-limiting example, and as shown in FIG. 1, such a
portion can
be located at the bottom end 320 of the first material layer 300 and generally
will have a
length in the range of about 0.10 inch to about 2.50 inch, wherein this length
can be
modified within or outside of this range for various reasons, e.g., to improve
the overall
fit of the device.
[0040] Because of the presence of the repeating pattern of openings 310 within
its
first material layer 300, the sleeve 100A of FIG. 1 provides substantially
uniform,
targeted compression and pressure when worn over an injured body part. It may
be
desired, however, to provide varied (i.e., non-uniform) compression through
some
8
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
portions of a sleeve 100A. For example, it may be desired to design the sleeve
100A to
provide increased compression at the portion of the sleeve that will be worn
directly over
an injured area, e.g., a kneecap or a knee ligament. Such varied compression
can be
accomplished, e.g., by varying one or more of the shape of the openings 310
and/or by
varying the thickness of the first material layer 300, and/or by varying the
modulus of
elasticity of the first material layer.
[0041] Referring now to FIG. 3, an alternate embodiment of the FIG. 1 sleeve
100A is shown that differs from the FIG. 1 sleeve in that its sleeve 100B
provides a non-
uniform level of compression by including a varied pattern of openings 310 and
a varied
thickness of the first material layer 300 in certain predetermined areas of
the sleeve.
Whereas the pattern of the first material layer 300 in the FIG. 1 sleeve 100A
was
substantially repeating, the pattern of the first material layer in the FIG. 3
sleeve 100B is
substantially non-uniform (i.e., non-repeating). By way of non-limiting
example, and as
shown in the FIG. 3 exemplary embodiment, some openings 310A in the first
material
layer 300 of the sleeve 100B can have a pentagonal shape, while other openings
310B
can have a triangular shape, and still other openings 310C can have a rhomboid
shape,
and yet still other openings 310D can have a trapezoidal shape. Each of these
shapes
represents not only a differently shaped opening, but also a somewhat
differently sized
opening; thus, in turn, each differently shaped opening provides a somewhat
different
level of compression beneath the opening. In general, the number of total
openings
310A, 310B, 310C, 310D and the distance between each opening provided in the
FIG. 3
sleeve 100B will be less than the total number of openings 310 and the
distance between
each opening in the FIG. 1 sleeve, whereas the size of the openings 310A,
310B, 310C,
310D in the FIG. 3 sleeve 100B generally will be greater than the size of the
openings
310 in the FIG. 1 sleeve 100A.
[0042] Further, whereas the thickness of the first material layer 300 was
substantially constant in the FIG. 1 sleeve 100A, one or more portions of the
first
material layer in the FIG. 3 sleeve 100B generally will have an increased
thickness in
order to provide one or more areas of increased, targeted compression. Also,
the added
thickness will prevent or at least further deter bunching and migration of the
sleeve 100B.
The number, location and/or shape of these increased thickness portions can
vary;
however, according to an exemplary embodiment of the present invention, the
FIG. 3
9
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
sleeve includes at least two added thickness portions: a first added thickness
portion 330
(i.e., a buttress) surrounding one of the circular openings 310 of the first
material layer
300 that provides increased compression at a kneecap, and a second added
thickness
portion 340 that spans between the top and bottom ends of the first material
layer to
provide increased compression to the knee ligaments and to specifically deter
migration
and bunching of the sleeve 100B.
[0043] In the FIG. 1 embodiment 100A of the present invention, the thickness
of
the first material layer 300 is substantially constant and is generally in the
range of about
0.05 inch to about 0.20 inch, and the thickness of the second material layer
400 is
substantially constant and is generally in the range of about 0.05 inch to
about 0.25 inch.
In the FIG. 3 embodiment 100B, the thickness of the second material layer 400
is
generally constant and within the same range as its thickness in the FIG. 1
embodiment
and the thickness of the first material layer 300 is generally constant and
within the same
range as its thickness in the FIG. 1 embodiment, except at the increased
thickness
portions 330, 340 where the thickness of the first material layer is generally
up to about
50% greater than that of the remainder of the first material layer. By way of
non-limiting
example, in a FIG. 3 embodiment wherein the thickness of the first material
layer 300 is
about 0.125 inch, the thickness of each of the increased thickness portions
330, 340
generally will be up to about 0.1875 inch. The hardness of the first material
300
generally will be about 20 Shore A in both the FIG. 1 embodiment 100A and the
FIG. 2
embodiment 100B.
[0044] As noted above, the FIG. 3 sleeve 100B includes a buttress 330, which
comprises a portion of the first material layer 300 and which has
comparatively increased
thickness to provide increased targeted compression. FIGS. 4-10 depict other
exemplary
embodiments of orthopedic devices 100C (see FIG. 4-6), 100D (see FIG. 7-10) in
accordance with the present invention that also include one or more buttress
portions
formed at least partially of an elastomeric material.
[0045] FIGS. 4-6 depict a top panel of an orthopedic device 100C. The device
100C can be a sleeve that is worn over a knee, in which case the top panel can
be
connected to a back panel (not shown) or can be fashioned into a sleeve, e.g.,
by
extending the front panel to form a sleeve that would fit over a leg as do the
sleeves
100A, 100B shown in FIGS. 1-3. The device 100C includes a panel/sleeve body
520
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
having an opening 525 (see FIG. 6), wherein a first buttress 500 is formed on
the inside
of the panel body and a second buttress 510 is formed on the outside of the
panel body.
According to an exemplary embodiment of the present invention, the first and
second
buttresses 500, 510 are located at the same position on the device 100C,
wherein that
position will correspond to a kneecap when the device is worn over a leg, and
wherein
the first, inner buttress 500 would be in direct contact with the kneecap.
Each buttress
500, 510 generally includes an opening 530, wherein at least a portion of each
buttress
opening will overlap at least a portion of (optionally, substantially the
entirety of) the
opening 525.
[0046] Although two buttresses 500, 510 are illustrated in FIGS. 4-6, it
should be
noted that the FIG. 4 device 100C can include only one buttress or greater
than two
buttresses. In an embodiment wherein the device 100C includes only one
buttress, the
buttress generally would be located on the inside of the panel body 520, e.g.,
in a position
that will correspond to a kneecap when the device is worn over a leg. In an
embodiment
wherein more than two buttresses are included, the additional buttress(es) can
be located,
e.g., inside and/or outside the sleeve 100C so as to correspond to positions
at either or
both sides of the knee in order to protect knee ligaments.
[0047] The first and second buttresses 500, 510 can have the same or different
shapes and the same or different thicknesses. According to the exemplary
embodiment of
the FIGS. 4-6 sleeve 100C, the first and second buttresses 500, 510 have
identical
"shieldlike" shapes (i.e., resembling a rounded triangle) and each buttress
has an elliptical
opening 530 defined therein. Without wishing to be bound by theory, such a
shape is
believed to be well suited for providing targeted support and compression to a
kneecap
when the sleeve 100C is worn over a knee; however, other shapes for the
buttresses 500,
510 and/or the openings 530 are within the scope of the present invention. The
size of
the openings 530 can vary, as can the size of the buttresses 500, 510 in which
the opening
is defined; however, according to an exemplary embodiment of the present
invention,
each opening covers about 35% to about 80% of the overall surface area of each
buttress.
[0048] According to an exemplary embodiment of the present invention, and as
best shown in FIG. 6, a fabric layer 600 can be provided between the inner
buttress 500
and the outer buttress 510. The fabric layer 600 is selected to be highly
breathable to
allow air and/or moisture generated within the openings 530 to be breathed
and/or wicked
11
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
away from the skin. According to an exemplary embodiment of the present
invention,
the buttresses 500, 510 are made of an elastomeric material, the panel body
520 is made
of a resilient material (e.g., neoprene) and the fabric layer 600 is made of
mesh material.
[0049] Optionally, the device IOOC can include first and second control top
portions 610, 620, which are provided at a top portion and a bottom portion of
the
sleeve/panel body 520 as described above with regard to the FIG. 1 sleeve
100A.
According to an exemplary embodiment of the present invention, the control top
portions
610, 620 of the device 100C are made of a spacer material and again are
present in order
to minimize displacement of soft tissue.
[0050] The thickness and/or the hardness of each buttress 500, 510 can be
identical or can vary. According to an exemplary embodiment of the present
invention,
the thickness of the first, inner buttress 500 is greater than the thickness
of the second,
outer buttress 510 and the hardness of the first, inner buttress is less than
the hardness of
the second, outer buttress. This enables the first, inner buttress 500 to
provide cushioned
support and the second, outer buttress 510 provides firmer, structural
support.
[0051] The first, inner buttress 500 is generally about three to five times
(e.g.,
four times) as thick as the second, outer buttress 510, wherein the thickness
of the first,
inner buttress 500 generally is in the range of about 0.15 inch to about 0.35
inch (e.g.,
about 0.25 inch) and the thickness of the second, outer buttress 510 generally
is in the
range of about 0.0425 inch to about 0.0825 inch (e.g., about 0.0625 inch). The
hardness
of the first, inner buttress 500 generally is about 40 Shore 00, and the
hardness of the
second, outer buttress 510 generally is about 40 Shore A.
[0052] Another exemplary orthopedic support device 100D in accordance with
the present invention is shown in FIGS. 7-10. This device 100D is patella
tendon bearing
device and includes a support buttress 610 having an attached strap 620,
wherein the
strap has a first strap portion 630 and a second strap portion 640, and
wherein the second
strap portion culminates in a D-ring or buckle 660 that is configured to
accept the first
strap portion 650. By way of non-limiting example, the first strap portion 630
can be
made of hook material (e.g., velcro) and the second strap portion 640 can be
made of
loop material (e.g., velcro) to help maintain the device 100D in place when
worn as
shown in FIG. 10.
12
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
[0053] As best shown in FIG. 8, the top side 670 of the support buttress 610
includes first and second raised portions 680A, 680B (e.g., rails) shaped to
function as a
strap guide, wherein a strap guide area 685 is defined between the first and
second rails
on which the strap portion 620 will rest when the device 100D is being worn
(see FIG.
10). In accordance with an exemplary embodiment of the present invention, the
first and
second raised potions 680A, 680B of the support buttress 610 are made solely
of an
elastomeric material, and the strap guide area 685 between the first and
second raised
portions are made of a combination of an elastomeric material and a spacer
material.
Generally, the same elastomeric material is utilized to form both raised
portions 680A,
680B of the buttress 610, wherein according to an exemplary embodiment of the
present
invention the hardness of the elastomeric material is about 40 Shore A. The
elastomeric
material within the combination of materials utilized to form the strap guide
area 685 can
have the same or different hardness as the elastomeric material that comprises
the raised
portions 680A, 680B.
[0054] Support buttress 610 can have a variety of shapes; however, as shown in
FIGS. 8 and 9, and according to an exemplary embodiment of the present
invention, the
support buttress has a concave shape resembling a bowtie or hourglass. When
the device
100D is worn as shown in FIG. 10, the underside 690 (see FIG. 9) of support
buttress 610
is in direct contact with the wearer's leg 200 and the strap 620 is wrapped
around the
support buttress such that the strap is in direction communication with the
strap guide
area 685 between the raised portions 680A, 680B of the support buttress.
[0055] In accordance with an exemplary embodiment of the present invention,
and as best shown in FIG. 9, the inner segment 692 of the underside 690 of
support
buttress 610 protrudes from the underside by a predetermined height and is
formed of an
elastomeric material, whereas the outer segment 694 that surrounds the inner
segment
692 is formed of a combination of elastomeric material and spacer material.
Generally,
the elastomeric material that forms the inner segment 692 of the underside 690
of the
support buttress 610 has a lower hardness (e.g., 40 Shore 00) than the
hardness (e.g., 40
Shore A) of the elastomeric material that forms the outer segment 694.
[0056] The orthopedic support devices of the present invention can be formed
by
one or more techniques known in the art, including, but not limited to, a
transfer molding
process. Transfer molding is an advantageous manufacturing process in
accordance with
13
CA 02615870 2008-01-18
WO 2007/018484 PCT/US2005/025627
the present invention because it inexpensively allows a manufacturer to vary
one or more
of the size, shape, pattern, thickness and location of some or all of the
materials that form
the end product (i.e., the support device) in order to vary the level/amount
and location of
compression provided by the support device. Moreover, in further accordance
with the
present invention, a transfer molding process enables a first material layer
to be deposited
onto a second material layer or substrate such that some, none or all of the
first material
layer flows through or encapsulates the second material layer/substrate.
[0057] It is understood that although the exemplary embodiments depicted in
the
figures and described herein pertain either to knee support devices or patella
support
devices, such devices in accordance with the present invention can be modified
without
departing froni the scope of the present invention so as to be wearable to
provide support
to other injured body parts, e.g., an elbow, an arm, a leg, an ankle, the
back, a shoulder, a
wrist, the neck, that can become injured to an extent that the body part
requires support or
would benefit from being supported while the injured body part heals.
100581 Although the present invention has been described herein with reference
to
details of currently preferred embodiments, it is not intended that such
details be regarded
as limiting the scope of the invention, except as and to the extent that they
are included in
the following claims - that is, the foregoing description of the present
invention is merely
illustrative, and it should be understood that variations and modifications
can be effected
without departing from the scope or spirit of the invention as set forth in
the following
claims. Moreover, any document(s) mentioned herein are incorporated by
reference in
their entirety, as are any other documents that are referenced within the
document(s)
mentioned herein.
14