Note: Descriptions are shown in the official language in which they were submitted.
CA 02616559 2008-01-18
PCT/CA2006/001218
Devices and Methods for the Selection of Agents with Efficacy against Blofilm
Infections
This application is claims priority from U.S. Provisional Application No.
60/701,858 filed on July 22, 2005.
1. Field of the Invention
This invention relates to methods and devices for the analysis of biofilms,
and
to determining microbial sensitivity to anti-microbial or anti-biofilm
reagents,
preferably combinations of anti-biofilm reagents, such as antibiotics or
biocides. In a
preferred embodiment of the invention, methods and devices include selecting
appropriate individual and combinations of anti-biofilm agents with enhanced
efficacy
for the treatment of biofilm disease, including but not limited to Pseudomonas
aeruginosa, specifically lung infections in cystic fibrosis (CF) patients.
This invention provides a method and device for the selection of appropriate
anti-biofilm agents with enhanced efficacy for the treatment of biofilm
disease.
II. Background of the Invention
The characterization of microorganisms has traditionally employed methods of
batch culture studies, where the organisms exist in a dispersed or planktonic
state.
Over the past 25 years, it has been recognized that the major component of the
bacterial biomass in many environments are sessile bacteria. Recent
technological
advances in microbial ecology have allowed for careful study of microbes as
they
actually exist in nature and disease. These studies have indicated that most
microorganisms are capable of growth in biofilms, and that the growth of
organisms
in biofilms is physically and physiologically different than growth of the
same
organisms in batch culture. These differences contribute to observed
alterations in
both the pathogenesis of these organisms and their susceptibilities to
antimicrobial
agents. The antibiotic resistance is generally attributed to the production of
a
protective exopolysaccharide matrix and alterations in microbial physiology.
P. aeruginosa which is a gram-negative rod, is one of many organisms found
in a wide variety of industrial, commercial and processing operations such as
sewer
discharges, re-circulating water systems (cooling tower, air conditioning
systems
etc.), water condensate collections, paper pulping operations and, in general,
any
--1-
CA 02616559 2008-01-18
PCT/CA2006/001218
water bearing, handling, processing, collection etc. systems. Just as biofiims
are
ubiquitous in water handling systems, it is not surprising that P. aeruginosa
is also
found in association with these biofilms. In many cases, P. aeruginosa is the
major
microbial component.
In addition to its importance in industrial processes, P. aeruginosa and its
associated biofiim structure has far-reaching medical implications and is the
basis of
many pathological conditions. P. aeruginosa is an opportunistic bacterium that
is
associated with a wide variety of infections, e.g., chronically colonizes the
lung of
patients with cystic fibrosis. Pseudomonas aeruginosa growing as biofilms are
highly resistant to antibiotics and are resistant to phagocytes.
The inventors have developed assays with a specific purpose of identifying
anti-biofilm agents and anti-biofilm agent combinations that are effective in
eliminating and controlling biofilms. A device and method have been developed
specifically for Pseudomonas aeruginosa biofilms. Such a product should
improve
the selection of antimicrobial drug therapy for patients with cystic fibrosis
lung
infections and other Pseudomonas infections.
The organisms present on these surfaces include a number of pathogenic and
nonpathogenic bacteria and fungi. Staphylococcus spp. infections are
frequently
associated with implanted medical devices composed of stainless steel,
silicone,
polyurethane). Implanted medical devices first become coated with glycoprotein
such as fibronectin which allows the Staphylococcus organism to adhere to the
surface and eventuaily form a microbial biofilm. It is well recognized that
Staph does
not respond to antibiotic treatment when associated with a medical device.
Evaluation of antimicrobial activity to sessile bacteria should better predict
clinical
efficacy for Staph infections.
Pathogenic fungi such as Candida and Aspergillus fumigatus are now
recognized as important infections and are responsible for significant
morbidity and
mortality. Often they associated with implanted devices or in
immunocompromised
patients. It is only recentiy that it has been recognized that treatment
failures are
associated with the formation of biofllms. Although resistance genes to
antifungal
agents have been described, physiological resistance based on the biofilm mode
of
growth may be equally or more significant with respect to treatment failures.
It is now widely known that bacteria in the form of biofilms are more
resistant
to antibacterial reagents than planktonic bacteria. Yet testing for the
presence of
-2-
CA 02616559 2008-01-18
PCT/CA2006/001218
bacteria and the testing the efficacy of antibiotics against bacteria has
traditionally
involved testing for planktonic bacteria. Studies have shown a greater than
hundred-
fold resistance to antibiotics of biofilms when compared to the same bacteria
in a
planktonic (free floating) state. This resistance is multi-factorial due to
many
phenotypic adaptations as part of the biofilm mode of growth, including but
not
limited to the mucopolysaccharide coating that is developed, and a
physiological
alteration in the microorganism.
Selecting antibiotics and combinations of antibiotics for treating biofilm
infections continues to rely on minimal inhibitory concentration (MIC) assays
despite
the recognized lack of efficacy of these tests. Some have suggested the use of
biofilm inhibitory concentrations (BIC) (Moskowitz, et al.; J. Clin.
Microbiology,
42:1915-1922 (May 2004)), but the evidence suggests that both 131C and MIC
address planktonic bacteria, not sessile bacteria. For example, Moskowitz et
al. use
centrifugation in their process, and do not remove the vast majority of cells
derived
from the challenged biofilm, therefore resulting in an assay for the
planktonic
bacteria alone, or an assay of only a portion of the biofilm. The assay
therefore may
miss viable cells left on the pegs, therefore leading to a potentially
inaccurate
conclusion. Further, the prior art typically grows the biofilm in a static
(non-flowing)
environment, which sometimes affects the results.
In contrast, the present invention uses sonication or re-growing biofilm on a
separate recovery plate in its processing so that the complete, intact biofilm
can be
obtained and assayed. Also, the processes of the present invention include
growing
the biofilm under dynamic or flowing conditions, and neutralizing the anti-
microbials,
both of which individually and collectively fortify any assay results.
Therefore a need exists for improved processing and assaying devices and
methods for selecting effective compositions against biofilm, including anti-
biofilm
compositions that are effective against biofilm mediated diseases of man and
animals, including but not limited to CF lung infections.
lil. Summary of the Invention
The invention comprises improved methods and devices for the selection of
one or more active agents, either alone or in combination, effective against
biofilm.
In preferred embodiments of the invention, the devices and methods may be used
in
the treatment of a biofilm infection. The biofilm may be any biofiim, e.g.,
those
-3-
CA 02616559 2008-01-18
PCT/CA2006/001218
formed from bacteria, fungi, or algae, viruses, and parasites; or a
microorganism that
is incorporated within a biofilm as it is formed; or mixed biofiims, e.g.,
containing
more than one bacterial, viral, fungal, parasitic, or algal biofilm.
The devices and methods of the present invention also include developing a
treatment protocol. In preferred embodiments, the treatment protocol can be
tailored to a specific patient and or may form the basis of developing a
personalized
medical treatment or approach.
The devices and methods of the present invention are also effective in
treating a wide variety of microorganisms, inciuding but not limited to
Pseudomonas
aeruginosa, Staphylococcus ssp., Candida ssp., and Aspergillus fumigatus.
The devices and methods of the present invention are also effective in
treating a wide variety of diseases and conditions mediated by one or more
biofilms,
the diseases including but not limited to cystic fibrosis (CF), including
disease and
conditions caused or mediated by one or more bacteria, viruses, fungi,
parasites,
algae, or combinations thereof.
The invention also provides a clinically significant assay tailored to growing
a
particular biofilm or biofilms, and to determining the appropriate active
agent or
agents effective against that biofilm. In preferred embodiments of the
invention, the
assay provides the minimum biofilm eradication concentration (MBEC), the
minimum
inhibitory concentration (MIC), or the minimum biocidal concentration (MBC),
or
combinations thereof.
The present invention provides a panel of individual and/or combined active
agents for selecting a composition containing one or more active agents with
efficacy
against a biofilm. These agents or combination of agents may be useful in
treating
patient-specific infectious organisms. The present invention provides a method
and
apparatus for the selection of combinatorial antibiotic treatment of biofilm
associated
infectious diseases.
The devices and methods of the present invention may also be useful in
determining and developing a pharmaceutical composition specific for an
individual
patient.
The devices and methods of the present invention also provide an alternative
to existing treatments that contribute to well-publicized antibiotic
resistance.
--4--
CA 02616559 2008-01-18
PCT/CA2006/001218
The devices and methods of the present invention may also be used to
identify genetic shift, antibiotic resistance, and genetic variations in the
process of
developing the appropriate treatment protocol tailored for the particular
patient.
The invention also provides an in vitro assay tailored to the presence of a
biofilm, namely an assay based on determining the minimum biofilm eradication
concentration (MBEC). In preferred embodiments of the invention, the devices
and
methods provide any combination of MBEC, minimum inhibitory concentration
(MIC),
and minimum biocidal concentration (MBC) values.
The devices and methods of the present invention are improved over prior art
devices in one or more of the following: the device and process involve
testing intact
biofilm; using sonication to remove the intact biofllm; the devices and
process apply
to a wider range of biofilms, e.g., fungal, etc.; the anti-biofilm agent
covers a wider
range of agents, including biocides, etc.; the devices and methods are high-
throughput and therefore more efficient and cost effective; and growing the
biofilm is
improved, involving increased understanding and application of process
conditions to
enhance biofilm growth.
Microbial biofilms exist in a number of medical, veterinary, agricultural and
industrial systems, processes, processing equipment, and surfaces. The
organisms
present on these surfaces include a number of pathogenic and nonpathogenic
biofilm.
The methods and devices of the present invention may be used to degrade
biofiims wherever they occur, e.g., in industrial processes where fouling
occurs, e.g.,
de-fouling pulp and paper mill equipment, treating of a gas/oil pipe line, and
decontaminating food processing equipment, or implanted medical devices,
including
catheters, hip implants, and cannulae. It is within the scope of this
invention that the
principles outlined here also apply to all biofilms in all circumstances in
which they
occur.
The invention also includes the use of an integrated device or assembly,
multiple or plural assemblies, multiple or plural sub-assemblies, or
combinations
thereof.
These and other aspects of the invention will be made apparent in the figures,
description, and claims that follow.
_5_
CA 02616559 2008-01-18
PCT/CA2006/001218
lll. Brief Description of the Drawings
Figure 1 is a bottom view of plural biofilm adherent sites on a lid of a
vessel.
Figure 2 is a top view of a vessel for receiving the plural biofilm adherent
sites of
FIG. 1.
Figure 3 is a side view, partly broken away, of the lid and vessel of FIGS. I
and 2.
Figure 4 is a flow diagram of the process steps is an exemplary embodiment of
the
invention.
Figure 5 shows an example of a biofilm growth and formation process of the
present
invention.
Figure 6 shows an example of a biofilm susceptibility assay of the present
invention.
Figure 7 shows an example of a process for recovering intact biofilm in
accordance
with the present invention.
Figure 8 shows an example of a process for establishing MBEC and MIC
determinations in accordance with the present invention.
Figure 9 is a chart of the results of the experiment described in Example 6.
Figure 10 shows the configuration of a challenge plate used in Example 7.
Figure 11 shows the configuration of a challenge plate used in Example 10.
Figure 12 is a chart of the MIC, MFC, and MBEC values determined biofilms.
IV. Detailed Description of the Invention
The invention comprises improved methods and devices for the selection of
one or more active agents, either alone or in combination, effective against
biofilm.
In preferred embodiments of the invention, the devices and methods may be used
in
the treatment of a biofilm infection. In the most preferred embodiments of the
invention, the devices and methods may be used as a diagnostic tool to
determine
various compositions, including the optimum composition, for treating one or
more
biofilms and/or one or more disease or conditions mediated by the biofilm.
The invention comprises improved methods and devices for selecting
appropriate combinations of anti-biofilm agents for the treatment of biofilm.
In
preferred embodiments of the invention, the methods and devices provide
diagnostic
susceptibility testing and in the most preferred embodiments, provide MBEC,
MBC,
and MIC values in a single experiment. An embodiment of the invention may
include
a method and device for selecting combinations of active agents against
specific
biofilms or groups of biofilms.
-6-
CA 02616559 2008-01-18
PCT/CA2006/001218
The methods and devices of the present invention may be used to degrade
biofilms wherever they occur, e.g., in industrial processes where fouling
occurs;
implanted medical devices, including catheters, hip implants, and cannulae; or
for a
wide variety of infections such as: ophthalmic applications (infections,
implants,
contact lenses, surgical manipulations etc.), respiratory infections,
including
pneumonia and cystic fibrosis, ear infections, recurrent joint related
infections,
urinary tract infections, skin and soft tissue infections, infections that
occur in bum
victims, endocarditis, vaginal infections, and gastrointestinal tract
infections. It is
within the scope of this invention that the principles outlined here also
apply to all
biofilms in all circumstances in which they occur.
The present invention also includes methods and devices for treating a patient
or subject having a disease or condition mediated or caused by a biofilm. In
these
embodiments of the invention, a biological sample from a patient or subject is
processed with a biofilm formation device; the biofllm is then processed with
a biofilm
susceptibility device to provide one or more agents active against the
biofilm.
An embodiment of the invention includes an assembly comprising one or
more plates pre-loaded with one or more pre-selected anti-biofiim agents
against a
specific biofilm or biofilms, said plates may be used to identify efficacious
individual
or combined active agents for treating biofilm-mediated diseases or
conditions.
In some embodiments of the invention, the method may also include one or
more of the following: growing multiple or plural biofilms under conditions
that
promote the production of substantially uniform biofilms; screening the
biological
sample against a large group of active agents; selecting a subgroup of active
agents;
loading an assay device with muftiple or plural active agents in the subgroup;
growing biofilm from a specific patient's or subject's sample; screening the
biofilm
from the specific patient or subject against the subgroup of active agents;
reading
the results; determining the appropriate active agent or combination of active
agents
suitabte for the particular biofilm; conducting a turbidity assay if the
microorganism
produces visible turbidity when growing (e.g. Pseudomonas); and conducting a
plating assay if the microorganism does not grow with visible turbidity.
An embodiment of the invention includes methods for selecting specific
combinations of antibiotics that have efficacy against isolates of a
particular
pathogen as a biofilm by screening a broad range of clinical isolates of a
species
--7--
CA 02616559 2008-01-18
PCT/CA2006/001218
against an extensive panel of antibiotics alone or in combination to identify
combinations with efficacy against biofilm grown organisms.
An embodiment of the invention includes forming biofiims of patient isolates
where biofilms are grown using a biofilm assay device as described in one or
more
of U.S. Patents Nos. 6051423, 6326190, 6410256, 6596505, 6599696 and 6599714
for the testing of biofilm antibiotic susceptibility.
An embodiment of the invention includes determining the antibiotic(s) of
choice for the treatment of a biofilm infection by challenging the biofilm of
the
patient's specific isolate against the diagnostic plate specific for the
species that
forms the biofilm.
An embodiment of the invention includes rehydrating a species specific plate
of preloaded antibiotics as the challenge plate to identify antibiotics with
efficacy
against the specific pathogen. Plates may be frozen (no rehydration required),
or
lyophilized, freeze dried or vacuum dried.
An embodiment of the invention includes a well plate containing frozen or
lyophilized antibiotic combinations that can be re-hydrated to be used in
antibiotic
susceptibility assay.
An embodiment of the invention includes growing biofilm obtained from an
isolated pathogen of a patient, and using the biofilm in a susceptibility
assay.
An embodiment of the invention includes challenging a biofilm against
selected combinations of an anti-microbial or an anti-biofilm agent, thereby
selecting
the most appropriate combination.
An embodiment of the invention includes providing MBEC values in the
diagnosis and treatment of any microorganism capable of biofilm formation, and
using those values to treat or develop a treatment protocol for any
microorganism-
mediated disease, infection, or condition. The invention may further include
providing MIC and/or MBC values.
In a further aspect of the invention, after growing the biofilm on adherent
sites
on a lid or plate, dislodging the biofilm from the biofilm adherent sites and
further
incubating the biofilm. Dislodging the biofilm from the biofilm adherent sites
may
include dislodging the biofilm from each biofilm adherent site into a separate
well of a
microtiter plate or base. In preferred embodiments of the invention, the
biofilm is
dislodged using any process that results in intact biofllm being removed from
the
adherent sites. The inventors have found that using centrifugation removes
only a
-8--
CA 02616559 2008-01-18
PCT/CA2006/001218
portion of the microorganism, and therefore any assay may be incomplete or
inaccurate.
Preferably, the plural biofilm adherent sites are formed in plural rows, with
plural sites in each row; and the container includes plural channels, with one
channel
for each row of plural biofilm adherent sites. Devices or assemblies so
configured
permit high throughput analysis of the biofilm.
In its entirety, the present invention comprises a biofilm growth assembly 1,
a
biofilm challenge assembly 2, a rinsing assembly 3, and a biofilm dislodging
and re-
growth assembly 4. Used in concert, the assemblies provide MIC, MBC, and MBEC
values in a single experiment.
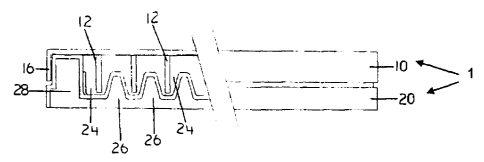
In accordance with the present invention, the biofilm growth assembly 1 may
include a base or plate 20 configured to receive a lid 10. Lid 10 may be
configured
to include one or more projections 12 that extend into a space defined by base
20.
In most preferred embodiments of the invention, the biofilm growth assembly 1
is
rocked, moved, or the like so that the growth fluid in the assembly flows or
moves
across projections 12. In preferred embodiments of the invention, base 20 is
an
incubation base and is configured to provide each projection with
substantially
equivaient exposure to the source of microorganisms and its nutrient/growth
broth.
As noted elsewhere in this specification, the typical base includes one of
more
channels 26. An exemplary configuration is shown in Figure 3.
In accordance with the present invention, the biofilm challenge assembly 2
comprises a second base or plate 21 configured to receive a{id 60 having
projections 61 typically covered by biofilm. Projections 61 extend into one or
more
wells configured in plate 21. A typical second base 21 is a standard 96 well
microtiter plate, although one skilled in the art will readily recognize that
other
configurations may be used. Second base 21 includes one or more anti-biofiim
agents in the wells. In accordance with the present invention, second plate 21
may
be removed and used for determining the MIC value of the non-biofiim (e.g.,
planktonic) microorganism (see Figure 8).
In accordance with the present invention, the biofilm rinsing assembly 3
comprises a third base or plate 40 configured to receive a lid 60 having
projections
61 typically covered by biofilm. Projections 61 extend into one or more wells
configured in plate 40. A typical third plate 40 is a standard 96 well
microtiter plate,
although one skilled in the art will readily recognize that other
configurations may be
-9-
CA 02616559 2008-01-18
PCT/CA2006/001218
used. Third plate 40 includes one or more rinsing and/or neutralizing agents
in the
wells.
After rinsing, lid 60 may then be joined with a fourth base 50, also referred
to
as a recovery plate. Lid 60 and fourth base 50 form the biofilm disruption
assembly
4. The recovery plate contains recovery media, and, in accordance with the
present
invention, assembly 4 may be subjected to sonication and biofilm re-growth
(confirming that the biofilm has not been removed). In preferred embodiments
of the
invention, the recovery medium includes one or more neutralizing agents. As
shown
in the examples, assaying the projections on lid 60 after it has been exposed
to
recovery media provides an MBEC value of the microorganism, and plating from
the
recovery plate provides an MBC value.
An exemplary embodiment of the invention is described below. As shown
most particularly in FIGS. 1, 2 and 3, an exemplary biofilm growth assembly of
the
present invention includes a lid 10 comprising projections 12, and a base 20
adapted
to receive lid 10 and projections 12 and comprising at least one channel 24 or
well.
As illustrated in the Figures, the device includes biofilm lid 10 composed of
tissue
grade plastic or other suitable material (e.g. stainless steel, titanium) with
projections
12 extending downwardly from the lid 10. The projections 12 may be biofilm
adherent sites to which a biofilm may adhere, and may be configured into any
pattern or shape suitable for use in conjunction with a channel or well-
containing
bottom, such as base 20. The pattem of projections 12 preferably mirror the
pattern
of channels and/or wells in convention plates, e.g. a 96 microtiter or well
plate
commonly used in assay procedures. In most preferred embodiments of the
invention, the projections 12 are preferably formed in at least eight rows 14
of at
least twelve projections each. Other numbers of rows or numbers of projections
in a
row may be used, but this is a convenient number since it matches the 96 well
plates
commonly used in biomedical devices. Additional or some of the projections as
shown may be used to determine the initial biofiim concentration after
incubation.
The exemplary projections 12 shown are about 1.5 cm long and 2 mm wide, but
may
be any size and/or shape.
The biofilm growth assembly I also includes an incubation base 20 configured
and adapted to receive lid 10 with projections 12. The lid 10 forms a support
for the
projections 12 for supporting the biofilm adherent sites within the channels
24. The
--10-
CA 02616559 2008-01-18
PCT/CA2006/001218
lid 10 has a surrounding lip 16 that fits tightly over a surrounding wall 28
of the
vessel 20 to avoid contamination of the inside of the vessel during
incubation.
Base 20 serves two important functions for biofilrn development, The first is
a
reservoir for liquid growth medium containing the bacterial population which
will form
a biofilm on projections 12. The second function is having a configuration
suitable
for generating shear force across the projections. While not intending to be
limited to
any particular theory of operation, the inventors believe that shear force
formed by
fluid passing across the projections promotes optimal biofilm production and
formation on the projections.
Shear force on the projections 12 may be generated by rocking the vessel 20
with lid 10 on a tilt table 30. The inventors have found that using a rocking
table that
tilts to between about 7 and about 11 is suitable for most applications. In
preferred
embodiments of the invention, the rocking table should be set on about 9 . It
is
intended that the invention should not be limited by the use of an actual
degree of tilt,
but that any tilt used for any particular machine be appropriate for growing
biofilm in
accordance with the present invention.
The projections 12 may be suspended in the channels 24 so that the tips of
the projections 12 may be immersed in liquid growth medium flowing in the
channels
24. The ridges 26 channel the liquid growth medium along the channels 24 past
and
across the projections 12, and thus generate a shear force across the
projections.
Rocking the vessel 10 causes a repeated change in direction of flow, in this
case a
repeated reversal of flow of liquid growth medium, across the projections 10,
which
helps to ensure a biofilm of equal proportion on each of the projections 12 of
the lid
10. Rocking the vessel so that liquid flows backward and forward along the
channels
provides not only an excellent biofilm growth environment, but also simulates
naturally occurring conditions.
Each projection 12 and each channel 24 preferably has substantially the
same shape (within manufacturing tolerances) to ensure uniformity of shear
flow
across the projections during biofilm formation. In preferred embodiments of
the
invention, channels 24 should all be configured or connected so that they
share the
same liquid nutrient and bacterial mixture filling the basin 22. The inventors
have
found that substantially uniform channel configuration and access to the same
source of microorganisms promotes the production of an equivalent biofilm on
each
projection, equivalent at least to the extent required for testing anti-
biofilm agents.
--11--
CA 02616559 2008-01-18
PCT/CA2006/001218
Biofilms thus produced are considered to be uniform. Results have been
obtained
within P<0.05 for random projections on the plate.
Sensitivity of a biofilm may be measured by treating the biofilm adherent
sites
with one or more anti-biofilm agents, i.e., challenging the biofilm, and then
assaying
the biofilm. This may be accomplished by placing the lid 60 (having a biofilm
formed
on the projections) into a second base 21 adapted to receive lid 10 and
projections
12. In preferred embodiments of the invention, lid 60 engages second base 21
in a
manner sufficient to prevent contamination of the assembly. As used herein, a
manner sufficient to prevent contamination refers to the configuration and
assembly
of mating structures so that the contents of the closed assembly are free of
outside
contamination.
In accordance with the present invention, one skilled in the art may use any
arrangement or scheme for challenging a group of biofilms. For example, all of
the
wells of the challenge plate may include the same anti-biofilm agent; plural
or
multiple wells may include different doses of the same anti-biofilm agent;
plural or
multiple wells in a single row may include the same dose or different doses of
anti-
biofilm agent; plural or multiple rows may include the same dose or different
doses of
anti-biofilm agent; plural or multiple wells or plural or multiple rows may
include more
than one anti-biofilm agent; or plural or multiple wells or plural or multiple
rows may
include more than one anti-biofilm agent, varying the dose by well, by row,
and/or by
anti-biofilm agent. It is intended that the configuration and arrangement of
wells,
type and number of anti-biofilm agents, and dose in each well should be
variable as
desired by one skilled in the art to achieve a specific purpose, e.g., testing
one or
more biofilms with one or more anti-biofilm agents using as many variables as
reasonable to the intended purpose.
For example, projections 12 that have been incubated in the same channel 24
of the vessel 20 may be treated with a different anti-bacterial reagent. In
this manner,
consistent results may be obtained since the growth conditions in any one
channel
will be very similar along the entire channel and thus for each projection 12
suspended in that channel. This helps improves the reliability of treatment of
different
projections 12 with different anti-bacterial reagents. The examples show
different
arrangements suitable for use with the assemblies of the present invention.
Several different conventional methods may be used to count the bacteria. It
may be done by incubating the sonicated bacteria, taking serial dilutions and
visually
--12--
CA 02616559 2008-01-18
PCT/CA2006/001218
counting the colony forming units, or automated methods may be used, as for
example using an optical reader to determine optical density. It has been
found
however that the optical reader of turbidity is too imprecise for practical
application,
and it is preferred that vital dye technology be applied to automate the
measurement
of viability, by treating the biofilm with a vital dye, and measuring the
intensity of light
given off by the dyed biofilm. In the case of using vital dye technology, the
biofllm
need not be further incubated. One skilled in the art will recognize that
other dyes
for cell mass may be used; these dyes may be later extracted and read for OD
(a
measure of remaining cell biomass). In a further embodiment, the assay may be
carried out by sonicating the cells until they lyse and release ATP and then
adding
luciferase to produce a mechanically readable light output. In a still further
embodiment, the assay may be carried out directly on the biofilm on the
projections
using a confocal microscope, although it should be considered that this is
difficult to
automate. In the examples that follow, the results are obtained from a manual
count
following serial dilution.
The concentration (MBEC) of anti-bacterial reagent at which the survival of
bacteria falls to zero may be assessed readily from the assay. Likewise, the
MIC
may also be determined from the assay.
The inventors have found that in some instances a biofilm will not form
without
the inclusion of host components in the biofilm. Host components may therefore
be
added to the growth medium in the vessel during incubation of the bacteria to
form
the biofilm. Host components that may be added include serum protein and cells
from a host organism. For the testing of the effect of different host cells
and
components, the ends 25 of the channels 24 may be sealed by walls to prevent
growth medium in one channel from flowing into another, thus isolating the
bacteria
growth in each channel from other channels. he device thus described may also
be
used to test coatings used to inhibit biofilm growth and to test coatings
which may
enhance biofilm formation. In an initial step, the projections 12 may be
coated with a
coating to be tested, and then the biofilm grown on the projections. The
biofilm may
then be assayed, or treated with anti-bacterial reagent and then assayed. The
assay
may be in situ or after dislodging of the biofilm. Different coatings may be
tested on
different rows of pegs. Enhanced biofilm formation may be used to create large
viable biofilms for biofermentation.
--13--
CA 02616559 2008-01-18
PCT/CA2006/001218
As used herein, assembly refers to an integrated collection of elements or
components designed or configured to work in concert. A typical assembly of
the
present invention includes a lid and its corresponding base. In some
embodiments
of the invention, an element of one assembly may function or work with a
separate
assembly. For example, the lid of assembly I may be used as the lid in
assembly 2,
i.e., with a different base. In preferred embodiments of the invention, a lid
may
engage a base in a removable, sealingly fashion. In other embodiments of the
invention, a lid may engage a base in a closed, sealingly fashion; in these
embodiments, it may be desirable to adapt other elements of the assembly so
that
they are removable, e.g., one or more removable projections.
As used herein, challenge plate refers to any base having one, multiple, or
plural configurations of wells or the like, said plate being used to expose
one or more
biofilms to one or more anti-biofilm agents. A typical challenge may be used
to
determine biofilm growth in an environment that includes one or more anti-
biofilm
agents. In a later step of a process of the present invention, the challenge
plate may
be used to determine the MIC value of any planktonic microorganism. An
exemplary
challenge plate is shown in Figures 6 and 8.
As used herein, recovery plate refers to any base one, multiple, or plural
configurations of wells or the like, said plate being used to rinse biofilm
after it has
been exposed to an anti-biofilm agent, neutralize any anti-biofilm agent, to
collect
any disrupted biofilm after the assembly has been sonicated, or combinations
thereof. In a later step of a process of the present invention, the recovery
plate may
be used to determine the MBEC value of any biofilm formed in the process. An
exemplary recovery plate is shown in Figure 7 and 8.
As used herein, neutralizing agent refers top any composition suitable for
reducing or counteracting any toxicity caused by an anti-biofilm agent. A
neutralizing
agent is appropriate if it is effective for the anti-biofilm agent(s) being
used and for a
particular biofilm. The choice of neutralizing agent is within the skill of
the art.
Several neutralizing agents and compositions are shown in the Examples. As
shown
in Figure 7 and described in the Examples, recovery medium is a composition
that
includes one or more neutralizing agents.
As used herein, active agent or anti-biofilm agent refers to one or more
agents
that are effective in reducing, degrading, or eliminating a biofilm or biofilm-
like
structures. The present invention includes but is not limited to active agents
that are
--14--
CA 02616559 2008-01-18
PCT/CA2006/001218
already well known, e.g., antibiotics, anti-microbials, and biocides. One or
more
active agents may act independently; one or more active agents may act in
combination or synergistically; one or more active agents may be used
sequentially
or serially.
As used herein, a panel or library of active agents refers to a collection of
multiple or plural active agents grouped according to a pre-determined
strategy. For
example, a first library may include one or more active agents that show some
degree of potential in being effective against a particular biofilm. A second
library
may begin with a subset of the first library, and is designed to narrow the
choices
effective active agents, or to provide more information about a particular
subset of
active agents. A panel or library may also include a proprietary or non-
proprietary
group of active agents grouped according to a pre-determined strategy, e.g.,
variable
doses.
As used herein a composition containing an active agent includes one or
more active agents, and may further include one or more additional agents,
including
but not limited to bacteriocins or other anti-bacterial peptides or
polypeptides, one or
more disinfectants or the like, one or more surfactants or the like, one or
more
carriers, physiological saline or the like, one or more diluents or the like,
and one or
more preservatives or the like.
As used herein, sample refers to a biological or fluid sample taken from a
patient, animal, or environment; sample expressly includes any source or
potential
source of microorganism. A patient's isolate is derived by standard laboratory
methods and prepared for assay again by standard laboratory practice (CLSI).
Inoculum for the challenge plate includes biofilms formed to standard density
using
existing technology, U.S. Patents Nos. 6051423, 6326190, 6410256, 6596505,
6599696 and 6599714.
As used herein, biofilm challenge involves the placement of the biofilm
culture
grown on the pegs MBEC device into the wells of the prepackaged challenge tray
such that the patient's isolate is exposed to a range of concentrations of a
spectra of
antibiotics selected for their synergy against the target organism. Incubation
time and
conditions and medium used will vary with isolate.
As used herein, efficacy is based on the ability of the combined antibiotics
to
have activity of the biofilm and is defined on the basis of MIC (minimal
inhibitory
concentration), MBC (minimal biocidal concentration), and MBEC (minimal
biofilm
--15--
CA 02616559 2008-01-18
PCT/CA2006/001218
eradication concentration). The standard assay for testing the antibiotic
susceptibility of bacteria is the minimum inhibitory concentration (MIC),
which tests
the sensitivity of the bacteria in their planktonic phase. The MIC is of
limited value in
determining the true antibiotic suscepti:bility of the bacteria in its biofilm
phase. The
MBEC assay, on the other hand, allows direct determination of the bacteria in
the
biofilm phase, and involves forming a biofilm in a biofilm growth device or
plate,
exposing the biofilm to one or more test antibiotics or acfive agents for a
defined
period, transferring the biofilm to a second plate having fresh bacteriologic
medium,
and incubating the biofilm overnight. The MBEC value is the lowest active
agent
dilution that prevents re-growth of bacteria from the treated biofilm. As used
herein,
treatment protocol refers to dose of active agent, the composition of the
active agent,
and how often it should be administered. With the devices and methods of the
present invention, the treatment protocol can be tailored to a specific human
or
animal, a specific biofilm or biofitms, and/or a specific disease or
condition. For
some diseases and conditions, e.g., CF, it may be desirable to perform
separate
assays at different times to optimize the course of treatment. For example, it
is
believed that a CF patient's condition changes over time as both the patient
and the
infection change; it would be a beneficial result to monitor those changes and
alter
any treatment as required.
As used herein beneficial result refers to any degree of efficacy against a
microorganism or biofilm. Examples of benefits include but are not limited to
reduction, elimination, eradication, or decrease in a biofilm or a
microorganism that
forms a biofilm; and the capability of treating a microorganism hidden or
protected by
a biofilm. Exemplary examples of an improvement in the manner in which a
patient
is treated includes but is not limited to the ability or capability of
treating a specific
patient, of the ability to tailor a treatment protocol for a particular
patient at a
particular time; and of the increased ability of being able to choose a
particular active
agent or agents.
As used herein susceptibility testing or similar phrases refers to determining
if
and by how much an active agent affects the growth or condition of a
microorganism
in a biofilm. In the devices and methods of the present invention,
susceptibility
testing is distinguished from prior art methods by using high through-put
devices, by
forming a biofilm in a non-static environment, by generating biofilms through
a flow
system.
--16-
CA 02616559 2008-01-18
PCT/CA2006/001218
As used herein, high throughput refers to the capability of growing and/or
assaying a high number of biofilms and/or a high number of anti-biofilm agents
at the
same time or in the same procedure. Typically, high throughput translates into
structural elements in one or more of the assemblies in order to increase
speed or
quantities of materials being grown or tested, e.g., a 96 well assay plate, a
top
adapted to and configured to engage the 96 well plate, a top with pegs
corresponding to the wells, and a biofilm growth plate with channels so that
you can
process a large number of individual biofilms at the same time.
EXAMPLES:
The following is used for examples 1-4:
Antibiotic and other antimicrobial stock solutions should be prepared in
advance at 5 x the highest concentration to be used in the challenge plate.
For
example, de-ionized water or an appropriate solvent is used to prepare stock
solutions of antibiotics at 5120 pg ml"l of active agent. Consult Clinical
Laboratory
Standards Institute (CLSI) document M100-S8 for details of which solvents and
diluents to use. Stock solutions of most antibiotics are stable for a minimum
of 6
months at -70 C.
For research applications it is appropriate to employ a neutralizing agent for
determination of minimum bactericidal and fungicidal concentrations. These
agents
reduce toxicity from the carry-over of biologically active compounds from
cFiallenge
to recovery media. As examples, it is possible to use ~-Iactamase to
neutralize
penicillin, or L-cysteine to neutralize Hg2+and some other heavy metal
cations. The
following experiments use a universal neutralizer in biocide susceptibility
assays that
is required for regulatory aspects of product development. This example is
presented
below:
1.0 g L-Histidine
1.0 g L-Cysteine
2.0 g Reduced glutathione
Make up to 20 ml in double distilled water. Pass through a syringe with a 0.20
pm filter to sterilize. This solution may be stored at -20 C. Make up 1 liter
of the
-17-
CA 02616559 2008-01-18
PCT/CA2006/001218
appropriate growth medium (cation adjusted MHB). Supplement this medium with
20.0 g per liter of saponin and 10.0 g per liter of Tween-80. Adjust with
dilute NaOH
to the correct pH (7.0 0.2 at 200 C). Add 500 NI of the universal
neutralizer to each
20 ml of the surfactant supplemented growth medium used for recovery plates.
An overview of this experimental protocol is provided in Figure 4. The number
of days required to complete this protocol is dependent on the growth rate of
the
microorganism being examined. The protocol has been divided into 6 sequential
steps, each of which is detailed in the sections below.
This protocol has been developed for use with Nunc Brand, flat bottom, 96-
well microtiter plates. These microplates have a maximum volume of 300 NI per
well.
The medium and buffer volumes listed here may need to be adjusted for
different
brands of microtiter plates.
Example 1. Step 1- growing sub-cultures of the desired microorganism
1. If using a cryogenic stock (at -70 C), streak out a first sub-culture of
the
desired bacterial or fungal strain on an appropriate agar plate. Incubate at
the
optimum growth temperature of the microorganism for an appropriate period of
time.
For most bacterial strains, the first sub-culture may be wrapped with
ParafilmTM' and
stored at 4 C for up to 14 days.
2. Check the first sub-culture for purity (ie. only a single colony morphology
should be present on the plate).
3. From the first sub-cuiture or from a clinical isolate, streak out a second
sub-
culture on an appropriate agar plate. Incubate at the optimum growth
temperature of
the microorganism for an appropriate period of time. The second sub-culture
should
be used within 24 h starting from the time it was first removed from
incubation.
4. Verify the purity of the second sub-culture.
It is not recommended to grow subcultures on media containing selective
agents. Antibiotics and other antimicrobials may trigger an adaptive stress
response
in bacteria andJor may increase the accumulation of mutants in the population.
This
may result in an aberrant susceptibility determination.
Step 2 - inoculate the assembly
This step, inoculating the assembly, is illustrated in Figure 5. In summary, a
fresh second sub-culture is used to create an inoculum that matches a 1.0
--18-
CA 02616559 2008-01-18
PCT/CA2006/001218
McFarland Standard. This solution is diluted 1 in 30 with growth medium. 22 ml
of
the 1 in 30 dilution is added to the trough of the base in an assembly of the
present
invention. The device is placed on a rocking table to assist the formation of
biofilms
on the polystyrene pegs.
It is recommended that the following steps be carried out in a biological
safety
cabinet (if available). However, it is possible to use aseptic technique on a
bench
top:
1. Open a sterile 96-well microtiter plate. For each high throughput assay,
fill 4
'columns' of the microtiter plate from 'rows' A to F with 180 NI of a
physiological
saline solution.
2. Put 1.5 ml (plus 1.0 ml for each additional device being inoculated at the
same time) of the desired broth growth medium into a sterile glass test tube.
3. Using a sterile cotton swab, collect the bacterial colonies on the surface
of
the second agar sub-culture. Cover the tip of the cotton swab with a thin
layer of
bacteria.
4. Dip the cotton swab into the broth to suspend the bacteria. The goal is to
a -1
create a suspension that matches a 1.0 McFarland standard (ie. 3.0 x 10 cfu ml
).
Be careful not to get clumps of bacteria in the solution.
5. Repeat step 2, parts 3 and 4 as many times as required to match the
optical standard.
6. Put 29 ml of the appropriate broth growth medium (e.g. TSB) into a sterile
50 ml polypropylene or glass tube. To this, add 1.0 ml of the 1.0 McFarland
standard
bacterial suspension. This 30 fold dilution of the 1.0 McFarland standard (ie.
1.0 x
7 -1
10 cfu ml ) serves as the inoculum for the device.
7. Open the sterile package of the device. Pour the inoculum into a reagent
reservoir. Using a sterile pipette, add 22 ml of the inoculum to the trough
packaged
with the device. Place the peg lid onto the trough.
The volume of inoculum used in this step has been calibrated such that the
biofilm covers a surface area that is immersed, entirely, by the volume of
antimicrobials used in the challenge plate set up in Step 3 (below). Using a
larger
volume of inoculum may lead to biofilm formation high on the peg that
physically
escapes exposure in this challenge step.
--19-
CA 02616559 2008-01-18
PCT/CA2006/001218
8. Place the device on the rocking table in a humidified incubator at the
appropriate temperature. The table should be set to between 3 and 5 rocks per
minute. It is critical that the angle of the rocking table is set to between
90 and 16 of
inclination. This motion must be symmetrical.
9. Serially dilute (ten-fold) a sample of the inoculum (do 3 or 4 replicates).
These are controls used to verify the starting cell number in the inoculum.
10. Spot plate the serial 10 fold dilutions of the inoculum from 10-6 to 10 ,
on
an appropriately labeled series of agar plates. Incubate the spot plates for
an
appropriate period of time and score for growth.
Example 2. Step 3 -- Set up the antimicrobial challenae plate
The following section describes how to set up a serial two-fold dilution
gradient of a single antimicrobial in the challenge plate. This is only one
example.
The antimicrobial challenge plate may be set up in any manner desired with any
combination of antimicrobials. It is important that the final volume in each
well of the
challenge plate is 200 NI. This is to ensure complete submersion of the
biofilm in the
antimicrobial.
1. Get a brand new, sterile 96-well microtiter plate and open in it in the
laminar
flow hood.
2. Setup a working solution of the desired antimicrobial in the appropriate
growth medium. Do not dilute the antimicrobial by more than 20% (i.e., no more
than
1 part stock antimicrobial solution per 4 parts of growth medium). The working
solution of the antimicrobial should be made at a concentration equal to the
highest
concentration to be tested in the challenge plate.
3. Add 200 NI of growth medium to 'column' 1 and 'column' 12 of the
challenge plate. These will serve as sterility and growth controls,
respectively.
4. Add 100 NI of growth medium to 'columns' 3 to 11 of the microtiter plate.
5. Add 200 NI of the working solution to 'column' 2 of the microtiter plate.
6. Add 100 NI of the working solution to 'column' 3 and 'column' 4 of the
microtiter plate.
7. Using the multi-channel micropipette, mix the contents of 'column' 4 by
pipetting up and down. After mixing, transfer 100 NI from the wells in
'column' 4 to
the corresponding wells in 'column' 5.
--20-
CA 02616559 2008-01-18
PCT/CA2006/001218
8. Mix and transfer 100 pl from 'column' 5 to 'column' 6. Serially repeat this
mix and transfer process down the length of the microtiter plate until
reaching
'column' 11.
9. Mix the contents of column 11 up and down. Extract 100 pl from each well
in 'column' 11 and discard.
10. Add 100 NI of growth media to the wells in 'columns' 4 through 11.
11. Replace the lid on the challenge plate. Gently tap the plate to facilitate
mixing of
biocide/antibiotic and media.
Step 4 - Expose the biofilms
This step, exposing the biofilm to one or more anti-microbials, is illustrated
in
Figure 6. In summary, the assembly prepared above is removed from the
gyrorotary
shaker and the biofilms are rinsed in a physiological saline solution. The
rinsed
biofilms are then immersed in the antimicrobials of the challenge plate and
incubated
for the desired exposure time.
1. Setup a sterile microtiter plate with 200 pl of physiological saline
solution in
every well. This plate will be used to rinse the pegs to remove loosely
adherent
planktonic cells from the biofilm (this is termed a 'rinse plate').
2. This step will be used to determine biofilm growth on four sample pegs and
from four wells of the planktonic cultures. Setup a sterile microtiter plate
with 200 NI
of physiological saline solution in 4'columns' of row A for each device
inoculated
(i.e., 1 microtiter plate is required for every 3 devices). Fill rows B to F
with 180 pl of
physiological saline solution. In a second microtiter plate, fill 4'columns'
from rows A
to H with 180 pl of physiological saline solution for each device inoculated.
The first
microtiter plate will be used to do serial dilutions of biofilm cultures, the
second will
be used to check the growth of planktonic cells in the wells of the microtiter
plate that
contained the inoculum.
3. Following the desired period of incubation, remove the high throughput
assembly from the rocking table and into the laminar flow hood. Remove the peg
lid
from the trough and submerse the pegs in the wells of the rinse plate. Let the
rinse
plate sit for 1 to 2 minutes while performing step 4 below.
4. Use a micropipette to transfer 20 Ni of the planktonic culture (in the
corrugated trough of the device) into the 180 NI of saline in row 'A' of the
latter plate
-21-
CA 02616559 2008-01-18
PCT/CA2006/001218
set up in step 2 (immediately above). Repeat this three more times for a total
of 4 x
20 pl aliquots.
5. Discard the planktonic culture into the appropriate biohazard waste.
6. In the laminar flow hood, dip a pair of pliers into 95% ethanol. Flame the
pliers using the ethanol lamp in the flow hood. Be cautious when using the
ethanol
lamp. Do not light the ethanol lamp and do not flame the pliers before your
hands
have dried following disinfection using 70% ethanol.
7. Using the flamed pliers, break off pegs Al, Cl, El and Gi from the lid of
the assembly and immerse them in the 200 NI of saline in row A (and each in a
different 'column') of the first plate setup in step 2.
8. Using the flamed pliers, break off pegs BI, Dl, Fl and H1 and discard.
9. Insert the peg lid of the assembly into the challenge plate. Place the
challenge plate in the appropriate incubator for the desired exposure time.
Incubations may be carried out at altemative temperatures, taking into
consideration
extended times for MICdeterminations.
10. Place the microtiter plate containing the sample pegs in the tray of the
ultrasonic cleaner (the sonicator). Sonicate on the setting 'high' for 5 to 30
minutes
(the time required depends on the microorganism being assayed). The vibrations
created in the water by the sonicator transfer first through the water, then
through the
steel insert tray, and finally to the device to use vibrations to disrupt
biofilms from the
surface of the 96 pegs into the saline.
11. Serially dilute 20 NI aliquots of the planktonic cultures (from step 4) in
the
wells of the corresponding microtiter plate. Once sonication is complete,
repeat this
serial dilution process with the biofilm cultures.
12. Spot plate the serial 10 fold dilutions of the planktonic and biofilm
cultures
from 10$ to 103 and 10s to 10 on an appropriately labeled series of agar
plates.
Incubate the spot plates for an appropriate period of time and score for
growth.
Step 5 - neutralize and recover
This step, neutralizing the anti-microbials and recovering surviving biofilm
bacteria, is illustrated in Figure 7. In summary, after exposure, biofilms are
rinsed
twice in physiological saline. The biofilms are then transferred to a
microtiter plate
--22--
CA 02616559 2008-01-18
PCT/CA2006/001218
containing a neutralizing agent and recovery medium. The biofilms are
disrupted into
this by sonication on a water table sonicator.
1. Add 200 NI of the appropriate recovery medium (containing a neutralizing
agent, see example in section 3.2) to each well of a brand new 96-well
microtiter
plate. This plate is termed the 'recovery plate'.
2. Prepare 2 rinse plates for every assembly used.
3. Remove the challenge plate from the incubator and place in the laminar
flow hood (or use careful aseptic technique). Remove the peg lid and immerse
the
pegs in the physiological saline of a rinse plate. Cover the challenge plate
with the
sterile lid of the rinse plate. After approximately 1 min, transfer the peg
lid from the
first rinse plate into the second rinse plate. Cover the challenge plate and
retain for
an MIC determination if appropriate.
4. Transfer the peg lid from the second rinse plate into the recovery plate
setup above. Transfer the recovery plate (containing the pegs of the device)
onto the
tray of the sonicator. Sonicate on high for 5 to 30 min. (depending on the
thickness
of the biofilm). The vibrations will disrupt biofilms from the surface of the
96 pegs into
the recovery plate.
5. After sonication, remove the peg lid from the recovery plate and replace
the
original lid of the microtiter plate. The lid of the device may now be
discarded into
autoclave garbage.
6. Place the recovery plate in the incubator and incubate a minimum of 24 to
72 h, depending on the organism being examined.
Viable cell counting
For viable cell counts of biofilms after treatment with an antimicrobial,
transfer
100 NI of the recovery media (containing the sonicated biofilms) from the
recovery
plate to row A of a serial dilution plate. This plate additionally set up to
contain 180 NI
of physiological saline solution in each well of rows B to F. Serially dilute
20 NI from
row A using the multi-channel pipette. Ensure that the tips on the multi-
channel
pipette are changed between transfers to each row in the microtiter plate.
Spot plate
biofilm cultures (which have been serially diluted ten-fold) on appropriately
labeled
agar plates. Incubate for a minimum of 48 hours to ensure maximum recovery of
the
surviving microorganisms.
--23-
CA 02616559 2008-01-18
PCT/CA2006/001218
Following incubation, enumerate bacteria recovered on plates. Use the
formulas in the following section to determine killing of the biofilm
population.
To calculate death and survival (log-kill), use the following formula:
log-kill = Iog10(initial cfu/ml) - Iog10(remaining cfu/ml after exposure)
Alternatively,
log-kill = log1o[1/(1 - % kill (as a decimal))]
To calculate percent kill, use the following formula:
% kill = [1 - (remaining cfu/ml) / (initial cfu/ml)] x 100
To calculate percent survival, use the following formula:
% survival = [(remaining cfu/ml after exposure) /(initial cfu/ml)] x 100
To calculate log percent survival, use the following formula:
log % survival = loglo(% survival)
Microscopy
For many microscopy techniques, it may be desirable to fix the biofilms to the
surface of the pegs of the assembly. The following protocols may be used to
prepare biofilms for scanning electron microscopy (SEM) and confocal laser
scanning microscopy (CLSM). In the standard experimental procedure above, each
challenge plate has eight growth controls (before exposure). Four of these are
used
for growth controls. The remaining four may be used for microscopy instead of
being
discarded.
Fixing Biofilms for Scannina Electron Microscopy (SEM)
Preparing Working Solutions
--24-
CA 02616559 2008-01-18
pCT/CA2006/001218
Wear protective gloves in the following steps and handle these highly toxic
chemicals in a fume hood.
Cacodylate buffer 0.1 M: dissolve 16 g of cacodylic acid in 1 liter of double
distilled H20; adjust to pH 7.2.
Glutaraldehyde 2.5% in cacodylate buffer: dissolve 2 mi of 70%
glutaraldehyde in 52 ml of cacodylate buffer (yields a 2.5% solution). It is
also
possible to use a 5% solution (2 ml of glutaraldehyde into 26 ml of cacodylate
buffer).
Standard protocol
This fixing technique is destructive to biofilms. However, this allows for an
examination of the cell structure of the underlying bacteria.
1. Break pegs from the MBECT"'-HTP device using a pair of flamed pliers.
2. Rinse pegs in 0.9% saline for 1 min. This disrupts loosely-adherent
pianktonic bacteria.
3. Fix the pegs in 2.5% glutaraldehyde in 0.1 M cacodylic acid (pH 7.2). Pegs
are placed in this solution at 4 C for 16 h.
4. Following this fixing step, wash the pegs once in 0.1 M cacodylic acid for
approximately 10 min.
5. Wash the pegs once in double distilled water for approximately 10 min.
6. Dehydrate the pegs in 70% ethanol for 15 to 20 minutes.
7. Air dry for a minimum of 24 h.
8. Mount specimens and examine by SEM.
Alternative protocol
This fixing technique is less destructive. It is possible to observe the
extracellular polymeric matrix and some (albeit dehydrated) biofilm structure.
1. Break pegs from the MBECT"-HTP device using a pair of flamed pliers.
2. Rinse pegs in 0.9% saline for 2 min. This disrupts loosely-adherent
planktonic bacteria.
3. Fix the pegs in 2.5% glutaraidehyde in 0.1 M cacodylic acid (pH 7.2). Pegs
are placed in this solution at 20 C for 2 to 3 h.
4. Air dry for at least 120 h.
-25-
CA 02616559 2008-01-18
PCT/CA2006/001218
5. Mount specimens and examine by SEM.
Fixing Biofilms for Confocal Scanning Laser Microscopy (CLSM)
Glutaraldehyde 5% in phosphate buffered saline: dissolve 2 ml of 70%
glutaraldehyde in 26 ml of phosphate buffered saline (yields a 5% solution).
Standard protocol
1. Break pegs from the lid using a pair of flamed pliers.
2. Rinse pegs in 0.9% saline for I min. This disrupts loosely-adherent
planktonic bacteria.
3. Fix the pegs in 5% glutaraldehyde in phosphate buffered saline (pH 7.2).
Pegs are placed in this solution at 30 C for 0.5 to 1 h.
4. Rinse pegs in 0.9% saline for 1 min.
5. Stain pegs with the appropriate fluorphores and examine using the confocal
laser scanning microscope.
Surface Coating the projections of the assembly
The surface of the pegs or projections may be coated with a number of
materials to facilitate the growth of fastidious microorganisms. For example,
biofilm
formation by certain Candida spp. is enhanced by coating the pegs with a
solution of
1.0 lo L-lysine. The peg lid may also be coated with hydroxyapetite, collagen,
or
platinum.
Example 3 Determine MBEC values
To determine the minimum biofilm eradication concentration (MBEC) values,
check for turbidity (visually) in the wells of the recovery plate.
Altematively, use a
microtiter plate reader to obtain optical density measurements at 650 nm
(OD650).
Clear wells (OD650 < 0.1) are evidence of biofilm eradication.
Example 4 Determine MIC values
To determine the minimum inhibitory concentration (MIC) values, check for
turbidity (visually) in the wells of the challenge plate. Alternatively, use a
microtiter
plate reader to obtain optical density measurements at 650 nm (OD650). The MIC
is
--26-
CA 02616559 2008-01-18
PCT/CA2006/001218
defined as the minimum concentration of antibiotic that inhibits growth of the
organism. Clear wells (OD850 < 0.1) are evidence of inhibition following a
suitable
period of incubation.
Example 5
Background: Pseudomonas aeruginosa (Ps) and Staphylococcus aureus
(Staph) form biofilms on tissue and implanted surfaces resulting in persistent
infections that are frequently unresponsive to antimicrobial therapy due to
biofilm-
specific resistance mechanisms. The use of MIC to select antimicrobial
therapeutics
for biofilm infections is usually not suitable. The MBEC assay was used for
evaluation of antimicrobial susceptibility of biofilm and planktonic bacteria
to single
and combinations of agents.
Methods: Biofilms of Ps (12 isolates from Cystic Fibrosis patients) and Staph
(12 isolates from device associated infections) were formed on the pins of an
MBEC
assay lid. Biofilm and Planktonic bacteria were then exposed to various
antibiotic
and antibiotic combinations for 24 hours (Table I and 2). The assay provides
qualitative sensitivity of each isolate as a biofilm and planktonic organism
to
antimicrobial agents alone or in combination.
Results:
Table 1. Staph resistance to individual antibiotics and antibiotic
combinations
Antibiotic Planktonic Biofilm Antibiotic Planktonlc Biofilm
GM/CLOX 1 12 VAN/GM 1 12
GM/AMP 3 12 CLIN/AMP 6 12
GM/CFZ 3 12 VAN/RIF 7 11
GM/CLIN 10 12 CIPRO/RIF 12 10
GM/RIF 12 12 CIPRO/CFZ 4 12
GM/CIPRO 9 12 CLOX/RIF 8 12
CLIN/RIF 12 12 GM 6 10
CLIN/CLOX 4 12 AMP 12 12
CLINNAN 4 12 CFZ 2 12
CLIN/CFZ 4 12 CIPRO 10 12
CLIN/CIPRO 10 12 VAN 6 12
VAN/CFZ 1 12 CLIN 9 12
VAN/CLOX 3 12 CLOX 3 12
VAN/CIPRO 1 12
-27--
CA 02616559 2008-01-18
PCT/CA2006/001218
Table 2. Ps resistance to individual antibiotics and antibiotic combinations
Antibiotic Planktonic Biofrlm Antibiotic Planktonic Bioi'tlm
GM/AZTR 1 12 CLO/TMS 0 3
GM/CFTZ 3 12 CFTZ/AZTR 11 12
TB/AZTR 1 12 CIPRO/AK 5 12
TB/CFTZ 3 12 CIPRO/AZTR 0 3
P+T/TB 1 12 P+T 4 12
P+T/GM 1 12 CLO 2 12
AK/AZTR 2 12 AZTR 0 9
AK/P+T 2 12 CIPRO 4 12
TB/CIPRO 3 12 GM 8 12
TB/IMP 1 12 AK 8 11
GMlIMP 8 12 TB 3 12
CLO/RIF 8 12 TMS 1 6
AK/CFTZ 2 12 CFTZ 3 12
AK/IMP 4 11 IMP 12 12
Conclusions: The resistance pattems were unique to each strain. Ps and
Staph strains were sensitive to multiple antibiotics as planktonic forms but
significantly more resistant as a biofilm. Certain antibiotics were more
effective as
combinations than as individual agents. The MBEC assay may be useful in the
selection of antibiotics for treatment of biofilm associated infections.
Example 6
bioFILM PA Antimicrobial Susceptibility System
bioFILM PA panels are designed for use in determining antimicrobial agent
susceptibility of both planktonic and biofilm Pseudomonas aeruginosa.
Summary and Principles
This broth dilution antimicrobial susceptibility test has various
antimicrobial
agents alone and in combination which are diluted in cation adjusted Mueller-
Hinton
broth (CAMHB) at categorical breakpoint concentrations defined by Clinical and
Laboratory Standard InstituteTM (CLSI). Panel wells are inoculated with
planktonic and
biofilm Pseudomonas aeruginosa using a 95 peg inoculation lid. Panels and
pegged
lids are then incubated at 35 C for a minimum of 16 hours. Planktonic
susceptibility and
resistance is determined by measuring inhibition and growth in the presence of
antimicrobial agents after 16-24 hours incubation at 35 C. The pegged lid
containing the
biofilm bacteria that have been exposed to the antimicrobial agents is placed
in a
-28--
CA 02616559 2008-01-18
PCT/CA2006/001218
recovery media containing only CAMHB in 96 well plate. Biofllm susceptibility
and
resistance is determined by measuring inhibition and growth after incubation
for
additional 16-24 hours at 35 C.
Procedure Materials
bioFILM PA Breakpoint Panel
Sterile recovery panel with lid
Sterile MBECTM 95 pegged inoculation lid
Sterile MBECTM tray (for growth of inoculum)
Sterile rinse panel
0.5 McFarland Barium Sulfate Turbidity Standard
Inoculum water
Inoculum Broth (Tryptic Soy Broth)
Test and recovery broth, Cation Adjusted Mueller Hinton Broth (CAMHB)
100 N1 pipette with disposable tips
Multichannel micropipettes (50-300 N1 with 12 channels recommended)
Rocking Platform (9 tilt angle)
Incubator
25 ml pipette
Sterile 50 ml tube with screw top
Quality control organism (Pseudomonas aeruginosa, ATCC 27853)
Quality control report forms
96 well microtiter plates (1 for peg washing and 1 for recovery plate)
Vortex
Procedure Outline
A. Inoculum Preparation
CLSI recommends periodically checking inoculum densities by doing colony
counts. The expected results for Pseudomonas aeruginosa ATCC 27853
should closely approximate 5x105 CFU/m12,3.
1. Primary Inoculation Method
-29-
CA 02616559 2008-01-18
PCT/CA2006/001218
The turbidity standard technique is recommended for direct inoculation
of Pseudomonas aeruginosa.
a. Using a sterile wooden applicator stick or bacteriological loop, touch the
surface of 4-5 large or 5-10 small morphologically similar, well-isolated
colonies from an 18-24 hour noninhibitory agar plate.
b. Emulsify in 3 ml of Inoculum Water (autoclaved distilled water) and
compare with 0.5 McFarland standard.
c. Vortex the suspension for 2-3 seconds.
d. Pipette 88 g1 of the standardized suspension into sterile 22 ml of
Tryptic Soy Broth (TSB). Cap tightly. Invert 8-10 times or vortex sample
for few seconds to mix
2. Preparation of bioFILM PA Inoculation Peg Lid
a. Remove MBECTM tray and 95 pegged lid from the package (Do not use if
integrity of the packaging is compromised).
b. Remove pegged lid from tray.
c. Pipette 22 ml of Pseudomonas aeruginosa suspension (see Id) in TSB to
slotted tray.
d. Place 95 pegged inoculation lid on the tray (check that the pegged lid is
properly aligned to fit securely over the tray)
e. Place the assembled pegged lid and tray on rocking platform with
approximately 90 tilt. Align the troughs parallel to the direction of rocking.
Incubate at 35 C with 3-4 rocks per minute. Some rockers are not suitable for
the bioFILM PA assay as the tilt angle is too great or the platform rocking is
asymmetrical. The operator should check to see that the rocker meets the
necessary requirements for this assay.
f. Incubate for 4-6 hours. This is sufficient to generate a biofilm of
approximately
105 cfu/peg.
B. Panel Preparation
1. Remove the panels to be used from frozen storage. Cut open the
pouch and remove the panel and allow to equilibrate to room
temperature for 30 to 60 minutes.
-30-
CA 02616559 2008-01-18
PCT/CA2006/001218
C. Planktonic Antimicrobial Sensitivity Testing
1. Place prepared MBECTM 95 pegged lid on thawed bioFILM PA panel.
2. A final well concentration of planktonic Pseudomonas aeruginosa of 3-
7x105 CFU/ml should be achieved 2.
3. A purity plate should be prepared by streaking the inoculum on blood
agar plate and incubate for 16-20 hours. If more than one colony
morphology is present on the purity plate, re-isolation of test colonies
and retesting of the panel is warranted.
D. Antimicrobial Panel Incubation
1. Stack the panels in groups of 3-5 to ensure even thermal distribution
during incubation.
2. Incubate the panels for 16-24 hours at 35 C in a non-CO2 incubator.
3. After incubation for 16-24 hours the Planktonic panel is ready to be
read.
4. Remove pegged lid (see/follow section E 1.)
5. Read planktonic antimicrobial sensitivity results (see below F).
E. Biofilm Antimicrobial Sensitivity Testing
1. Place pegged lid in 96 well plate containing 200 g1 10 g1 per well of
Inoculum Water for approximately 30 seconds. This is performed to
remove any residual antibiotics from pegs.
2. Place pegged lid in 96 well recovery plate containing 200 g1 of CAMHB
in each well.
3. To ensure even thermal distribution during incubation, stack the panels
in groups of no more than 5.
4. Incubate the panels for 16-24 hours at 35 C in a non-CO2 incubator.
5. After incubation for 16-24 hours the Biofilm recovery panel is ready to
be read.
6. Remove pegged lid and discard.
7. Read Biofilm antimicrobial sensitivity results (see below F).
F Reading the Panels for Planktonic and Biofilm Susceptibility
--31--
CA 02616559 2008-01-18
PCT/CA2006/001218
1. Remove the panels after 16-20 hours incubation.
2. Wipe off the bottom of the panel with a lint-free tissue to remove any
condensation or debris that may be present.
3. Growth in the antimicrobial wells appears as turbidity, which may take
the form of a white haze throughout the well, a white button in the
center of the well, or a fine granular growth throughout the well.
Inadequate or no growth is defined as a slight whiteness in the well or
the broth.
4. Precautions for reading the panels:
a. Only read the panels if the growth well is turbid.
b. Do not read the antimicrobial sensitivity wells if the Sterility
Control well (SC) is turbid, or if there is no growth in the growth well or
Growth Control (GC).
G. Reading Planktonic Antimicrobial Susceptibilities:
Description Conclusion Code
Following 16-20 hours incubation, no growth in the
antimicrobial higher and lower concentration of Susceptible S
antimicrobial agent
rowth in the lower concentration of the antimicrobial
gent, but not in the higher concentration. Intermediate I
Growth in both concentrations of the antimicrobial agent. Resistant R
Interpretation of Results
Susceptibility is determined by comparing the breakpoint susceptibility of an
organism with either the attainable blood or urine level of the antimicrobial
agent.
The following table lists the interpretive criteria as indicated in the CLSI
document
M100-S9
J. Interpretive Breakpoints* Susceptible Intermediate Resistant
Antimicrobial Agent
Amikacin < 16 32 > 64
Aztreonam < 8 46 > 32
Cefepime <8 16 >_ 32
Ceftazidime < 8 16 > 32
-32-
CA 02616559 2008-01-18
PCT/CA2006/001218
Chloramphenicol < 8 16 > 32
Ciprofloxacin < 1 2 > 4
Colistin < 2 - > 4
<4 8 > 16
Gentamicin
Meropenem < 4 8 >_ 16
Piperacillin/tazobactam < 16/4 32/4-64/4 > 128/4
Trimethoprimisulfamethoxazole < 2/38 - > 4/76
Tobramycin < 4 8 > 16
Amikacin/aztreonam < 16/8 32116 > 64/32
Amikacin/cefepime < 16/8 32/16 > 64/32
Amikacin/ceftazidime < 16/8 32/16 > 64/32
Amikacin/ciprofloxacin < 16/1 32/2 > 64/4
Amikacin/colistin < 16/2 - > 64/4
Amikacin/meropenem < 16/4 32/8 > 64/16
Amikacin/piperacillin/tazobactam < 16116/4 32/3214-32164/4 > 64/128/4
Amikacin/trimethoprimisulfamethoxazole < 16/2/38 - > 64/4/76
Chloramphenicol/ceftazidime < 8/8 16/16 > 32/32
ChloramphenicoUmeropenem < 814 16/8 > 32/16
Ciprofloxacin/aztreonam < 118 2/16 > 4/32
Ciprofloxacin/colistin < 1/2 - > 4/4
Ciprofloxacin/meropenem < 1/4 2/8 > 4/16
Ciprofloxacin/piperacillin/tazobactam < 1/16/4 2/32/4-2/64/4 > 4/128/4
Ciprofloxacin/trimethoprim/sulfamethoxazofe < 1/2/38 - > 4/4176
Gentamicin/aztreonam < 4/8 8/16 > 16/32
Gentamicin/cefepime < 4/8 8/16 > 16/32
Gentamicin/ceftazidime < 4/8 8/16 > 16/32
Gentamicin/ciprofloxacin < 4/1 8/2 > 16/4
Gentamicin/colistin < 4/2 - > 16/4
Gentamicin/meropenem < 4/4 8/8 > 16116
Gentamicin/piperacillin/tazobactam < 4/16/4 8/32/4-8/64/4 > 16/128/4
Gentamicin/trimethoprim/sulfamethoxazole < 4/2/38 - > 16/4/76
Tobramycin/aztreonam < 4/16 8/32 > 16/64
Tobramycin/cefepime < 4/8 8/16 > 16/32
Tobramycin/ceftazidime < 4/8 8/16 > 16/32
Tobramycin/ciprofloxacin < 4/1 8/2 > 16/4
Tobramycin/cotistin < 4/2 - > 16/4
Tobramycin/meropenem < 4/4 8/8 > 16/16
Tobramycin/piperacillin/tazobactam < 4/16/4 8/32/4-8/64/4 > 16/128/4
Tobramycin/trimethoprim/sulfamethoxazole < 4/2/38 - > 16/4/76
Trimethoprimisulfamethoxazole/aztreonam < 2/38/16 - > 4/76/64
Trimethoprim/sulfamethoxazole/ceftazidime < 2/38/8 - > 4/76/32
Trimethoprim/sulfamethoxazole/meropenem -< 2/38/4 - >_ 4/76/16
Trimethoprim/sulfamethoxazole/piperacillin/ < 2/38/16/4 - > 4/76/128/4
Based on Interpretive Breakpoints as indicated in CLSI Document M100-S16
-33--
CA 02616559 2008-01-18
PCT/CA2006/001218
References
1. Murray, PR., E.J. Baron, M.A. Pfaller, F.C. Tenover and R.H. Yolken (eds)
2003.
Manual of Clinical Microbiology, 8th Ed. American Society of Microbiology
Washington, D.C.
2. Clinical Standard Laboratory Institute TM (2006). Methods for dilution
antimicrobial susceptibility tests for bacteria that grow aerobically; 6th ed.
Approved
standard M7-A7.
3. Clinical Standard Laboratory InstituteTM (2006). Performance Standards For
Antimicrobial Susceptibility Testing; 16th informational supplement, Wayne,
Pennsylvania CLSI document M100-S16, Clinical and Laboratory Standards
Institute, 940 West Road Suite 1400, Wayne, Pennsylvania.
Example 7
The experiment described in Example 6 was repeated using a challenge plate
configuration and breakpoints shown in Figure 10.
Example 8
In this study, a Pseudomonas aeruginosa biofilm assay kit was used to test the
effect of 10 antibiotics and combinations of these antibiotics at different
concentrations, and to compare the effects of antibiotics on two strains of P.
aeruginosa, CF 6649 and CF 6106.
Antibiotic and antibiotic combinations were selected based on the results of
preliminary studies that demonstrated effectiveness among antibiotic
combinations
to microbial biofilms.
Forminci the biofilm: A suspension of the organism such that the turbidity
matches a
McFarland standard of 1.0 (approx. 3.0 X 108 cfu/mL) in TSB was prepared. A 30
mL inoculum was prepared by diluting the suspension 1/30 for an initial
inoculum of
7
10 cfu/mL. 22 mL of the inoculum was placed into a trough of an assembly of
the
present invention, and the peg lid was replaced. The device was placed on a
rocking platform at 350C, approx. 90 tilt, and 3-4 rocks per minute, with the
troughs
--34--
CA 02616559 2008-01-18
PCT/CA2006/001218
parallel to the direction of rocking. The target was to generate a biofilm of
> 10
cfu/peg; this was achieved in less than 24 hour incubation.
A 96-well tissue culture plate was used to prepare the challenge plate.
20NL of each test antibiotic was placed in the 96-well tissue culture plate
and 180 NL
5 of Cation Adjusted Mueller Hinton Broth (CAMHB) to was added to each well of
the
microtiter plate to achieve a 1:10 dilution of test drug. Two wells (G12 &
H12) were
empty or included 200 pL of Sterile Normal Saline. G12 and H12 served as
Sterility
Control. Similarly, A12 & B12 served as Growth Control.
The lid with the pegs were placed on the challenge plate and incubated at 35 C
for 24 hours.
A rinse plate(s) of saline (200 NL per well) in a sterile 96 well microtiter
plate was prepared. A recovery plate(s) of CAMHB (200 pL per well) in another
96
well microtiter plate was also prepared. Pegs were placed in saiine. Pegs were
transferred to recovery media, and then sonicated on high for 5 minutes to
dislodge
surviving biofilm. The pegs were then incubated at 35 C for 20 to 24 hours to
allow
surviving bacteria to grow to turbidity.
Planktonic MIC was determined by visually checking turbidity in the wells of
the
challenge plate and on a plate reader at 650 nm. The MIC (minimum inhibitory
concentration) for each antibiotic for the planktonic bacteria shed from the
biofilm
during the challenge incubation was determined. The MIC is defined as the
minimum
concentration of antibiotic that inhibits growth of the organism. Clear wells
(AsSO <
0.1) are evidence of inhibition.
Biofilm MBEC (minimum biofilm elimination concentration) was determined for
each antibiotic by reading the turbidity of the recovery plate. The MBEC is
defined as
the minimum concentration of antibiotic that inhibits re-growth of the biofilm
bacteria
in the recovery media. Clear wells (A650 < 0.1) are evidence of inhibition.
Pseudomonas aeruainosa MBEC Test plate: GM = gentamicin, AK = amikacin,
CFTZ = ceftazidime, TMS = trimethoprim/sulfamethoxazole, P+T =
piperacillin/tazobactam (concentration given as the piperacillin), AZTR =
aztreonam,
IMP = imipenem, TB = tobramycin, CIPRO = ciprofloxacin, GC = growth control,
SC
= sterility control.
--35-
CA 02616559 2008-01-18
PCT/CA2006/001218
The sensitivity of planktonic and biofilm forms of P. aeruginosa to individual
and combination antimicrobial agents can be determined rapidly (48 hours) and
reproducibly (Table 1). The resistance patterns were unique for each isolate.
P.
aeruginosa was sensitive to multiple antibiotics as planktonic forms but
significantly
more resistant as a biofilm.
Table 1: Number of P. aeruginosa isolates resistant to individual antibiotics
and
antibiotic combinations
Antibiotic Planktonic Biofilm Antibiotic Planktonic Biofllm
GEN/ATM 1 12 CT/SXT 0 3
GEN/CAZ 3 12 CAZ/ATM 11 12
TOB/ATM 1 12 CIP/AK 5 12
TOB/CAZ 3 12 CIP/ATM 0 3
TZP/TOB 1 12 TZP 4 12
TZP/GEN 1 12 CT 2 12
AK/ATM 2 12 ATM 0 9
AKlTZP 2 12 CIP 4 12
TOB/CtP 3 12 GEN 8 12
TOB/IPM 1 12 AK 8 11
GEN/IPM 8 12 TOB 3 12
CT/RA 8 12 SXT 1 6
AK/CAZ 2 12 CAZ 3 12
AK/IPM 4 11 IPM 12 12
Certain antibiotics were more effective as combinations than as individual
agents. The assay offers the clinician 10 single and 82 combinations of
antibiotics at
breakpoint concentrations. This assay may be useful for clinicians in the
selection of
antibiotics for treatment of biofilm associated infections that are common in
cystic
fibrosis patients.
This experiment demonstrates that each strain has unique biofilm sensitivity.
This is surprising because the planktonic data is very similar among isolates.
These
results clearly indicate that organisms have different sensitivity toward
individual
antibiotic or combinations of antibiotics, depending on their growth condition
(planktonic or biofilm).
Example 9 Staph Test Plate Assembly
A prototype Staphylococcus test plate was developed to evaluate antibiotics
and antibiotic combinations that can be used to treat Staphylococcus
infections. The
antibiotic and antibiotic combinations selected are based on the results of
preliminary
-36-
CA 02616559 2008-01-18
PCT/CA2006/001218
studies that demonstrated effectiveness among antibiotic combinations to
microbial
biofilms. The prototypes 96 well plate is described below:
Staahvlococcus Test glate: GM = gentamicin; CLIN = clindamycin; CFZ =
cefazolin;
CLOX = cloxacillin; RIF = rifampin; VAN = vancomycin; LIZD = Linezolid; AMP =
ampicillin sublactamj; Cipro = Ciprofloxacin; GC = growth control; SC =
sterility
control
The sensitivity of planktonic and biofilm forms of Staphylococcus aureus to
individual and combination antimicrobial agents can be determined rapidly
(within
about 48 hours) and reproducibly (Table 2). The resistance patterns were
unique for
each isolate. Staphylococcus aureus was sensitive to multiple antibiotics and
antibiotic combinations as planktonic forms, but significantly more resistant
as a
biofilm.
Table 2: Number of Staphylococcus aureus isolates resistant to individual
antibiotics
and antibiotic combinations
Antibiotic Pianktonic Biofilm Antibiotic Planktonic Biofiim
GEN2/CLO 2 10 CLIN1/CIP 9 12
GEN4/CLO 0 10 CLIN21CIP 10 12
GEN2/AMP 0 09 VAN2/CFZ 0 12
GEN4/AMP 1 12 VAN4/CFZ 1 12
GEN2/CFZ 3 09 VAN2/CLO 0 12
GEN4/CFZ 1 10 VAN4/CLO 0 12
GEN2/CLN 5 12 VAN2/CIP 1 12
GEN4/CLN 7 12 VAN4/CIP 0 12
GEN2/RIF 11 09 VAN2/GM 0 09
GEN4/RIF 11 10 VAN4/GM 0 08
GEN2/CIP 4 11 CLIIIAM 4 12
GEN4/CIP 2 11 CLI2/AM 2 12
CLIN1/RIF 10 12 VAN4/RIF 7 10
CLIN2/RIF 10 12 VAN8/RIF 7 11
CLIN1/CLO 2 12 CIP2/CFZ 3 09
CLIN2/CLO 2 12 CIP4/CFZ 7 10
CLININAN 1 12 CIP2/RIF 4 12
CLIN2NAN 0 12 CIP4/RIF 3 11
CLIN1/CFZ 4 12 CLO2/RIF 5 12
CLIN2/CFZ 2 12 CLO4/RIF 4 12
GM2 4 11 CIP2 7 12
GM4 3 10 VAN2 5 12
AMP4/2 10 12 VAN4 1 12
AMP8/4 10 12 CLI1 8 12
CFZ4 4 12 CL14 5 12
CFZ8 6 12 CLO2 2 12
CIP1 7 12 CLO4 2 12
--37--
CA 02616559 2008-01-18
PCT/CA2006/001218
Certain antibiotics were more effective as combinations than as individual
agents. The assay offers the clinician 10 single and 82 combinations of
antibiotics at
breakpoint concentrations.
This experiment demonstrates that each strain has unique biofilm sensitivity.
This is surprising because the planktonic data is very similar among isolates.
These
results clearly indicate that organisms have different sensitivity towards
individual
antibiotic or combinations of antibiotics depending on their growth condition
(planktonic or biofilm).
Example 10: Comparative Susceptibility of Planktonic and Biofilm Forms of
Candida sap and Aspergillus fumipatus to Antifungal Agents
Three clinical isolates of Candida were used in this study, including one
albicans
and two non-albicans species. C. albicans ATCC 14053 was obtained from the
University Of Calgary, Department Of Biological Sciences. C. tropicalis 99916
and
C. glabrata 14326 were obtained from the dialysate of patients undergoing
continued
ambulatory peritoneal dialysis (CAPD). Aspergillus fumigatus was also tested.
Biofilm formation and measurement of antimicrobial sensitivity of Candida and
Aspergillus biofilms were performed using an assembly of the present
invention. '
The device features a microtiter plate lid with 96 pegs or projections
distributed on
the lid. Each peg provided the surface for microorganism to adhere, colonize
and
form a uniform biofilm. The pegs fit precisely into the wells of a standard 96-
well
microtiter plate. The lid was used in conjunction with a base having special
troughs
for growing, washing, and incubating fungi. Colonies of Candida sp. were
picked
from 24 hour cultures on Sabouraud Dextrose agar (SDA) (BBL Microbiological
Systems, Cockeysville, Md) and placed into Mueller-Hinton Broth (MHB) (Difco
Laboratories, Detroit Mich.) such that the suspension matched a McFarland
standard
of 1Ø This suspension was then diluted 1:30 in MHB, and 25 ml were pipetted
into
the trough chamber of the base. The closed assembly (lid with pegs positioned
on
the base) was placed on a Hoeffer Red Rocker (VWR, Scientific) set to rock the
plates 3.5 cycles per minute in a 37 C incubator. C. albicans and C.
tropicalis were
incubated aerobically, while C. glabrata was incubated in 10% CO2. It was
-38--
CA 02616559 2008-01-18
PCT/CA2006/001218
determined in preliminary experiments that C. glabrata required 10% CO2
atmosphere for biofilms to form.
The growth curves were obtained for each isolate by randomly removing 3
pegs from the lid of the device at 1, 2, 3, 4, 5, 6, 7, 22, 23, 24 and 26
hours post-
inoculation. The removed pegs were placed in microfuge tubes containing -200
NI of
saline, and sonicated (Aquasonic sonicator, VWR Scientific, ) for 5 minutes.
Serial
dilutions were performed and plate counts of viable Candida spp. cells were
performed on SDA. Additional pegs containing 22 hour Candida spp. biofilms
were
fixed with 2.5% glutaraldehyde in phosphate buffered saline solution (PBSS),
air-
dried overnight, and prepared for scanning electron microscopy.
Optimal conditions for the formation of A. fumigatus biofilms were determined
in preliminary studies. The pegs were first soaked ovemight in 1 % L-lysine
(Sigma
Chemical Co, St. Louis, Mo) and then air-dried inside a laminar flow hood. A
50 ul
volume of A. fumigatus spore suspension was added to 250 ml of Tryptic Soy
Broth
(TSB) (Difco, Detroit, Mich.) in a 500 ml Erlynmeyer flask. The flask was
shaken at
150 rpm for 20 hours at 37 C. The adherent mycelial cells growing on the glass
at
the apex of the liquid broth were removed with sterile cotton swab. This was
transferred to a blender (Waring) with 250 ml of fresh TSB equilibrated to 4
C. The
mycelium was blended at medium speed over ice for two minutes. This was
repeated three times. The A. fumigatus suspension (25 ml) was then transferred
to
the trough of the biofllm growth device. The lid containing the lysine-treated
pegs
was placed in the suspension and the device was transferred to a Red Rocker as
described above. The plates were incubated for 25 hours at 37 C, establishing
a
visible biofilm on each peg. Scanning electron microscopy examination of pegs
containing 24 hour Aspergillus biofilms was performed.
Minimum Biofilm Eradication Concentration Assay
Biofilm susceptibility testing uses the pegged lid of the assembly, now
containing biofilms formed after rocking in the tray for 24 hour. Each peg on
the lid
was gently washed once in 200uI of phosphate buffered saline solution (PBSS)
in a
96-well microtiter plate (Falcon). The pegged lid was then transferred to
another 96
well microtiter plate containing 2 fold dilutions antifungal agent in 200NI of
RPMI
1640 (Sigma, St. Louis, Mo) or RPMI 1640 containing 1 k DMSO (see test drug
section). After the pegs were exposed to the drugs for 24 hours, the pegged
lid was
-39-
CA 02616559 2008-01-18
PCT/CA2006/001218
removed and gently rinsed twice in saline. The pegged lid was then placed on a
96
well plate containing RPMI 1640 recovery medium. The pegs were sonicated for 5
minutes (Candida spp.) or 7 minutes (Aspergillus) in an uFtrasonicator to
dislodge
adherent cells into the recovery medium. Aliquots of 20 pi of the recovery
medium
were spot plated on SDA (Candida spp) or Rose Bengal Agar (A. fumigatus) to
obtain the MBEC. The assembly was also used to determine the minimum
inhibitory concentration (MIC) and minimum fungicidal concentration (MFC). The
turbidity of the wells that contained the antibiotic and planktonic cells
which were
shed from the biofilm was measured at 650 rlm to obtain the MIC. A 20 pi
sample
from each well was also spot plated onto Sabouraud Dextrose agar (Candida spp)
or
Rose Bengal Agar (A. fumigatus) to obtain the MFC.
Planktonic sensitivity of Candida sap. and A. fumiaatus:
Minimum Inhibitory Concentrations (MIC) and Minimum Fungicidal
Concentrations (MFC) were determined according to the guidelines of the
National
Committee for Clinical laboratory Standards (NCCLS). Briefly, Candida sp. were
maintained on polystyrene spheres at -70 C until plating. These organisms were
streaked onto Sabouraud Dextrose agar (Microbiologie) plates 24 hours before
test
initiation. The 24 hour colonies were picked from the agar plates and placed
in
Muellar-Hinton Broth (MHB, Microbiologie) such that the turbidity matched a
McFarland standard of 0.5. This was then diluted 1:10 in MHB to obtain a
suspension of approximately 1x105 to 5 x 105 CFU/mi. A 5 NI volume of this
suspension was added to wells containing 200 NI of antifungal agent serially
diluted
in RPMI 1640 media (Sigma) in a 96 well microtiter plate (Nunclon). A.
fumigatus
was maintained as a spore suspension at 4 C until testing. The spore
suspension
(50 NI) was diluted into 250ml of TSB, and 5 ul of this suspension was added
to each
test well containing dilution of antifungal agent as above.
The test wells were then incubated at 37 C for 24 hours with the antifungal
drugs. MICs for Candida were obtained after incubation by reading the
turbidity at
650 nm on a microtiter plate reader (Softmax, VWR). A 20 NI aliquot of each
well
was also plated (SDA) and MFCs obtained from them after 24 hours incubation at
37 C. MFCs were determined for Aspergillus by plating 100 pi from each well
onto
--40--
CA 02616559 2008-01-18
PCT/CA2006/001218
Rose Bengal Agar, followed by spreading with a sterile glass spreader. The
plated
organisms were maintained at 25 C for three days before colony enumeration.
Drugs tested. Itraconazole, Fluconazole, and Ketoconazole were obtained
from Janssen Pharmaceuticals in Brussels, Belgium. Nystatin 5-Fluorocytosine,
Griseofulvin, Amphotericin B and Polymyxin B were obtained from Sigma Chemical
Co, St. Louis, Mo. 5-Fluorocytosine, polymyxin B sulphate and nystatin were
dissolved in double-distilled water to a concentration of 10.24 mg/mI and
diluted in
the test wells through a range of 1024 pg/ml to 0.125 pg/mI in RPMI.
Amphotericin B
was dissolved in neat dimethyisulfoxone (DMSO) to a concentration maximum of
51.2 mg/mI. This was serially diluted to achieve 512 pg/mI to 0.016 Ng/mI of
Amphotericin B in RPMI 1640 (Sigma, St. Louis Mo) and 1% DMSO in the test
wells.
Fluconazole, itraconazole, ketoconazole and griseofulvin were dissolved in
neat
DMSO to a concentration of 102.4 mg/mI and further diluted to achieve a range
range of 1024 pg/mI to 0.032 pg/mI drug in RPMI 1640 and 1% DMSO in the test
wells.
To determine the effect of 1 /a DMSO on the fungal celis, a control well
containing 1% DMSO in RPMI 1640 with no drug was run in parallel to all test
wells
containing DMSO. In addition, all testing involved sterility control wells
which were
not inoculated, as well as growth control wells containing no antifungal
agent.
Biofilm Growth on the device surface: Each Candida species formed biofilms on
each peg of the device to achieve 105 to 106 CFU/peg after 22 hours after
inoculation. C. glabrata was the most fastidious, requiring 10% COZ to
establish
bioffms. After 20 hours, C. glabrata grew only to about 5 x103 CFU/peg when
incubated aerobicaily, while it grew to an average of 4.1 x104 CFU/peg in 10%
COZ .
Aspergillus biofilms seemed to adhere more tightly to the pegs. Aspergillus
grew to
s
10 CFU/peg after 24 hour incubation (data not shown).
The biofilm that formed on the pegs of the MBEC device was similar for all
species of Candida. Candida cells uniformly coated the entire peg and were
encased is an extensive exopolysaccharide matrix. The Candida cells grew as
raised clusters of elongated cells in certain regions. Aspergillus biofilms
were
composed of organized conidiophores which swarmed over the entire peg after 24
--41-
CA 02616559 2008-01-18
PCT/CA2006/001218
hours. Exopolysaccharide was attached to the peg surface and surrounded each
Aspergillus conidiophore.
Anti-fungal Susceptibilitv: The concentration of antibiotic required to
inhibit
planktonic cells (MIC), kill planktonic cells (MFC) and kill biofilm fungi
(MBEC) are
summarized in Table 3. The MIC and MFC values obtained from the NCCLS
protocol and planktonic cells released from the biofilms which formed on the
device
pegs were similar or identical. The MIC and MFC obtained from the device were
highly reproducible. Fungal biofilms were universally more difficult to
eliminate than
planktonic cells (Table 3).
Similar results were obtained for all species of Candida tested. In general
the
MBEC exceeded the MIC or MFC by several orders of magnitude. Although
Amphoteracin B, nystatin, 5-flurocytosine, fluconazole, itraconazole and
ketoconazole were all effective in killing planktonic Candida cells only
nystatin was
effective against the same Candida growing as a biofilm. For example,
amphotericin
B, a polyene antifungal drug, was found to have an average MIC of 0.09 Ng/ml
and
an average MFC of 0.02 Ug/ml when used against planktonic cultures of C.
albicans.
Conversely, an average of 12 Ng/mI of the same drug was required to kill
biofilm cells
of C. albicans. Griseofulvin and Polymyxin B were ineffective against
planktonic and
sessile Candida.
The MIC of Aspergillus fumigatus, could not be obtained due to the clumping
of Aspergillus cells in the 96 well microtiter plate, which renders analysis
by the plate
reader inaccurate. The MFC values (gathered by spot plating 100 pI of the well
contents onto Rose Bengal Agar) demonstrated sensitivity of planktonic
Aspergillus
to amphotericin B, itraconazole, ketoconazole, and nystatin (Table 1). In
contrast,
none of the antifungal agents were effective against A. fumigatus biofilms
even at the
highest concentrations tested.
Azole drugs inhibited, but did not kill biofilm cells even at extremely high
concentrations. Survival of viable cells is not a favorable result following
drug
therapy, and may contribute to the rise in azole-resistant strains of Candida
(8). One
may speculate that the failure of these drugs to eliminate biofilm cells is
that they
must be actively taken up by the cell. The decreased rate of drug uptake or
inhibition of the exopolysaccharide by these biofilm organisms may prevent the
drug
from reaching its target enzyme. A fluconazole MIC less than or equal to 8
pg/mI
--42-
CA 02616559 2008-01-18
PCT/CA2006/001218
against Candida species indicates that the species is susceptible to the drug
(16).
The C. albicans and C. tropicalis strains tested would be classified as
susceptible to
fluconazole according to these criteria. However, the MBEC values for
fluconazole
and itraconazole (>1024 Ng/mI) was well above the breakpoint of 64 Ng/mI for
both of
these isolates, indicating that the biofilm cells are resistant. Although
fluconazole and
itraconazole are the only drugs for which these tentative breakpoints have
been
established, the same trend was seen for ketoconazole and 5-fluorocytosine.
This
may explain why azoles are frequently ineffective in treating some cases of
chronic
candidiasis and Candida associated with implanted devices (4,5,12).
The polyenes, nystatin and amphotericin B, were the most effective agents in
elimination of Candida biofilms. However, the MBEC of 16 pg/mI for
amphotericin B
may not be achievable under clinical situations -- peak permissible human
serum
concentrations are 2 Ng/mI. The ability of the polyenes to work on the plasma
membrane of fungi, without requiring uptake into the cell, may explain their
relative
effectiveness among the drugs tested against biofilm cells.
Once a protein surface such as L-lysine was provided, the Aspergillus readily
formed an organized biofilm on the surface of the peg. The morphological
features
of the AspergiUus biofilm are not unlike that which occurs within tissue and
on
medical devices. Although the biofilm rapidly formed, it was still resistant
to all
agents tested. As with the Candida biofilms, it appears that growth rate does
not
influence resistance. An extensive exopolysaccharide was observed in the
Aspergillus biofilms, which may be important in resistance. The crude
mortality rate
of patients treated with amphoteracin B for invasive pulmonary, sinus and
cerebral
aspergillosis has been reported to be 86%, 66% and 99% respectively. Only 54%
of
cases show any response to 14 days of treatment. Although polyenes show in
vitro
efficacy to Aspergillus they are largely infective for the treatment of
invasive
aspergillosis. The results of the MBEC assay would predict this treatment
failure.
Although to date there are no alternative chemotherapeutic agents for
elimination of
A. fumigatus, the assembly may be used for screening agents which could be
potentially developed for future therapies.
It is established that success of a drug in vitro cannot be extrapolated to
success in treatment therapy, but that failure of a drug in vitro should
predict
therapeutic failure. From this study, the azoles, 5-fluorocytosine,
griseofulvin, and
--43-
CA 02616559 2008-01-18
PCT/CA2006/001218
polymyxin B would be predicated to be unsuccessful in treating biofilm Candida
infections, while the polyenes may or may not be effective.
What is clear from the research is that Candida and Aspergillus species
adhere to plastics, and that the formation of a biofilm tends to allow these
organisms
to withstand exposure to antimicrobial agents in concentrations many times
greater
than the same species grown in batch culture. It is no longer satisfactory to
characterize antifungal agents against organisms in batch cultures when they
are
capable of growth in biofilms. In order to accurately assess the ability of a
particular
agent to clear an infection of a biofilm organism, the susceptibility testing
should be
carried out on the cells as they would be found to exist in the host or in
nature, i.e.,
displaying a profoundly altered physiology and encased in a protective
exopolysaccharide matrix.
Example 11
As noted above, a device of the present invention may be loaded with one or
more anti-biofiim agents. An incomplete and exemplary list of possible anti-
biofilm
agents include, but are not limited to: Antibiotics. Including, but not
limited to the
following classes and members within a class: Aminoglycosides, such as
Gentamicin, Tobramycin, Netilmicin, Amikacin, Kanamycin, Streptomycin,
Neomycin,
Quinolones/Fluoroquinolones, Nalidixic Acid, Cinoxacin, Norfloxacin,
Ciprofloxacin,
Perfloxacin, Ofloxacin, Enoxacin, Fleroxacin, and Levofloxacin ;
Antipseudomonal,
such as Carbenicillin, Carbenicillin Indanyl, Ticarcillin, Azlocillin,
Mezlocillin,
Piperacillin Cephalosporins, Cephalothin, Cephaprin, Cephalexin, Cephradine,
Cefadroxil, Cefazolin Cefamandole, Cefoxitin, Cefaclor, Cefuroxime, Cefotetan,
Ceforanide, Cefuroxine Axetil, Cefonicid Cefotaxime, Moxalactam, Ceftizoxime,
Ceftriaxone, Cefoperazone, Cftazidime, Other Cephalosporins, such as
Cephaloridine, and Cefsulodin; other beta.-Lactam; Antibiotics, such as
Imipenem,
Aztreonam beta.-Lactamase Inhibitors Clavulanic Acid, Augmentin, Sulbactam;
Sulfonamides, such as Sulfanilamide, Sulfamethoxazole, Sulfacetamide,
Sulfadiazine, Sulfisoxazole, Sulfacytine, Sulfadoxine, Mafenide, p-
Aminobenzoic
Acid, Trimethoprim-Sulfamethoxazole; Urinary Tract Antiseptics, such as
Methenamine, Nitrofurantoin, Phenazopyridine and other napthpyridines;
Penicillins,
such as Penicillin G and Penicillin V, Penicillinase Resistant Methicillin,
Nafcillin,
Oxaciliin, Cloxacillin, Dicloxacillin Penicillins for Gram-Negative/Amino
Penicillins
--44--
CA 02616559 2008-01-18
PCT/CA2006/001218
Ampicillin (Polymycin), Amoxicillin, Cyclacillin, Bacampicillin;
Tetracyclines, such as
Tetracycline, Chlortetracycline, Demeclocycline, Methacycline, Doxycycline,
Minocycline; other Antibiotics, such as Chloramphenicol (Chlormycetin),
Erythromycin, Lincomycin, Clindamycin, Spectinomycin, Polymyxin B (Colistin),
Vancomycin, Bacitracin; Tuberculosis Drugs, such as Isoniazid, Rifampin,
Ethambutol, Pyrazinamide, Ethinoamide, Aminosalicylic Acid, Cycloserine; Anti-
Fungal Agents, such as Amphotericin B, Cyclosporine, Flucytosine lmidazoles
and
Triazoles Ketoconazole, Miconazaole, Itraconazole, Fluconazole, Griseofulvin;
Topical Anti Fungal Agents, such as Clotrimazole, Econazole, Miconazole,
Terconazole, Butoconazole, Oxiconazole, Sulconazole, Ciclopirox Olamine,
Haloprogin, Tolnaftate, Naftifine, Polyene, Amphotericin B, Natamycin
EXAMPLE 12
Assay for High-throughput Screening (HTP) Using a 96-Peg Lid and Trough
The procedure provided is an example of how to evaluate antimicrobial
activity of compounds against biofilm and planktonic bacteria using the
devices and
methods of the present invention.
Materials List:
Sterile lid and trough (1)
Sterile 96 well tissue culture plate (3)
Platform Rocker (tilt angle approx 90)
Ultrasonicator
Needle nose pliers (optional)
Description of Procedure
Forming the biofilm:
1. Prepare a suspension of the organism such that the turbidity matches a
McFarland standard of 1.0 (approx. 3.0 X 10 cfu/mL) in TSB or other suitable
media
using single colonies from a fresh overnight streak plate. Prepare 30 mL
inoculum by
diluting the suspension 1/30 for an initial inoculum of 107 cfu/mL. Fastidious
organisms may require supplemented media for growth in broth.
2. Open a sterile growth assembly. Add 22 mL of the inoculum to the trough
and replace the peg lid. Place the device on a rocking platform at 35 C,
approx. 9
--45--
CA 02616559 2008-01-18
PCT/CA2006/001218
tilt, and 3-4 rocks per minute, with the troughs parallel to the direction of
rocking.
The target is to generate a biofilm of > 105 cfu/peg, usually 24 hour
incubation is
sufficient.
Note: Some rockers are not suitable for the assay because as the tilt angle is
too
great or the platform rocking is asymmetrical. The operator should check to
see that
the rocker meets the necessary requirements for this assay.
3. Dilute and spot plate a sample of the inoculum to check inoculum numbers
(should contain approx. lx 107 cfu/mL) and to check for contaminants in the
culture.
4. Sterility Controls (optional). Using alcohol flamed pliers, break off pegs
Al,
B1, Cl and Dl such that there will no longer be protrusions to which bacteria
could
adhere. These positions will serve as sterility controls for the assay.
Day 2: Antibiotic Stock Solutions:
Antibiotic stock solutions should be prepared in advance and stored at -70 C.
De-ionized water or appropriate solvent is used to prepare stock solutions at
5120
pg/mL of active agent. Consult NCCLS document M100-S8 for details of which
solvents and diluents to use. Store stock solutions in 250 pL aliquots (enough
for 2
challenge plates). Stock solutions of most antibiotics are stable for a
minimum of 6
months at -70 C.
Day 2: Preparation of Antibiotic Challenge Plate:
1. Use a 96-well tissue culture plate to prepare the challenge plate.
Designate
the antibiotics to be tested in the assay and assign them to rows A through H.
This
plate set-up will allow 8 different antibiotics at 10 concentrations to be
tested.
Note: the MBEC plate fits into 96 well plates (eg. Nunc) but not all 96 well
plates are
compatible.
2.. Prepare working solutions at 1024 Ng/mL by adding 200 pL of 5120
pg/mL stock to 800 pL of Cation Adjusted Mueller Hinton Broth (CAMHB) which
provides sufficient working solution for two rows. Working solutions must be
used the
same day that they are prepared.
3. An example of how the Challenge plate can be prepared is as follows:
--46--
CA 02616559 2008-01-18
PCT/CA2006/001218
Add 200 NL CAMHB into wells Al, B1, Cl and D1. These will be the sterility
controls.
Add 200 NL CAMHB into wells A-H of column #2. These will be the growth
controls.
Add 100 pL CAMHB into the all wells of columns 4-12. These will be used to
dilute the working concentration of antibiotic.
Add 200 NL of the working solution of the designated antibiotics to the wells
of
column 3.
Add 100 pL of the working solution of antibiotics to the wells of columns 4
and
5.
Use an 8-channel pipette to prepare the dilutions, changing tips between
columns: Well #5: mix and transfer 100 pL to well #6 ; Well #6: mix and
transfer 100
NL to well #7, continue through to well #12; Well #12: mix and discard 100 NL
Add 100 NL CAMHB to the wells of columns 5-12 to bring the volume of all
wells to 200 pL. This dilution scheme will result in declining antibiotic
concentrations
of 1024 Ng/mL in column 3 to 2 Ng/mL in column 12. These concentrations can be
adjusted accordingly to suit the needs of the study.
Antibiotic Challenge of Biofilm:
1. Prepare rinse plate(s) of 0.9% saline (200NL per well). Rinse planktonic
bacteria from pegs by placing the pegs into the rinse plate for approx. 1
minute.
2. Biofilm inoculum check (optional): using flamed pliers remove pegs El, Fl,
G1 and H1, placing each in 200pL saline in a dilution plate. Sonicate the
sample
pegs E1-H1 for 5 minutes on high to dislodge the biofilm bacteria then
serially dilute
to 10-7 and spot plate on TSA (or appropriate media) and incubate overnight to
determine cfu/peg.
3. Transfer the lid with the remaining pegs to the challenge plate and
incubate
at 35 C for 24 hours.
Recovering Surviving Biofilm
1. Prepare rinse plate(s) of saline (200 pL per well) in a sterile 96 well
microtiter plate.
-47--
CA 02616559 2008-01-18
pCT/CA2006/001218
2. Prepare recovery plate(s) of CAMHB (200 pL per well) in another 96 well
microtiter plate.
3. Rinse pegs in saline for approx. 1 minute. Do not discard the challenge
plate. Transfer pegs to recovery media then sonicate on high for 5 minutes to
dislodge surviving biofilm. Discard the peg lid and cover the recovery plate.
Incubate
at 35 C for 20 to 24 hours to allow surviving bacteria to grow to turbidity.
If surviving
cfu/peg data for each well is required, 50 NL can be removed from each well of
the
recovery plate immediately after the sonication step and transferred to
dilution plates
and serially diluted in saline and spot plated (100 through 10T) on
appropriate media.
Determination of Planktonic MIC
1. Check for turbidity (visually) in the wells of the challenge plate or on a
plate
reader at 650 nm.
2. Determine the MIC for each antibiotic for the planktonic bacteria shed from
the biofilm during the challenge incubation. The MIC is defined as the minimum
concentration of antibiotic that inhibits growth of the organism. Clear wells
(A650 <
0.1) are evidence of inhibition.
3. Record MIC values for each antibiotic.
Determination of Biofilm MBEC
1. Determine the MBEC for each antibiotic by reading the turbidity of the
recovery plate. The MBEC is defined as the minimum concentration of antibiotic
that
inhibits regrowth of the biofilm bacteria in the recovery media. Clear wells
(A650 <
0.1) are evidence of inhibition.
2. Record MBEC values for each antibiotic.
EXAMPLE 13
Assay for Physiology & Genetics (P&G) Using a 96-Well Microtiter Plate
Using a 96 well microtiter plate, multiple biofilms of different organisms or
equivalent biofilms of the same organism may be formed. This procedure can be
used for studying variability in biofilm formation or antimicrobial testing.
It should be
noted that this assay can be used to screen for genetic mutants in a biofilm
format,
to compare MBEC values of different isolates or species of bacteria, to
compare
--48-
CA 02616559 2008-01-18
PCT/CA2006/001218
gene expression in different isolates grown as biofilms, or in many other
formats
where biofilms of different isolates are needed.
The procedure described below describes an assay for testing multiple
organisms grown as a biofilm against a single antimicrobial agent.
Materials List:
Sterile lid and Tray
Sterile 96 well tissue culture plate (3)
Gyrotary shaker
Ultrasonicator
Needle nose pliers (optional)
Description of Procedure
Day 1: Forming the biofilm:
1. Prepare a suspension for each organism (max. 12 per plate) such that the
turbidity matches a McFarland standard of 1.0 (approx. 3.0 X 108 cfu/mL) in
TSB or
other suitable media using single colonies from a fresh ovemight streak plate.
2. Dilute 1/30 to obtain an inoculum of 10' cfu/mL. Place 150 pL per test well
of the starting inoculum for each organism in the designated column. Columns
can
be set up to test a maximum of 8 isolates, with 1 well for growth control and
11
antibiotic concentrations or 12 isolates, with 1 well for growth control and 7
antibiotic
concentrations (one column can be designated for sterility control if
desired).
3. Open a sterile assembly. Place the lid on the 96 well microtitre plate
containing the bacterial inoculum. Place the device on a gyrating platform at
35 C at
approximately 150 rpm. Some species may require a lower incubation temperature
or elevated CO2. The target is to generate a biofilm of > 105 cfu/peg; usually
24 hour
incubation is sufficient.
4. Dilute and spot plate a sample of the inoculum to check inoculum numbers
(should contain approx. 1 x 10' cfu/mL) and to check for contaminants in the
culture.
Day 2: Antibiotic Stock Solution:
Antibiotic stock solutions should be prepared in advance and stored at -70 C.
De-ionized water or appropriate solvent is used to prepare stock solutions at
5120
g/mL of active agent. Consult NCCLS document M100-S8 for details of which
-49-
CA 02616559 2008-01-18
PCT/CA2006/001218
solvents and diluents to use. Stock solutions of most antibiotics are stable
for a
minimum of 6 months at -70 C.
Day 2: Preparation of Antibiotic Challenge Plate:
1. Using a 96-well tissue culture plate prepare the challenge plate.
Note: the plate fits into 96 well plates (eg. Nunc) but not all 96 well plates
are
compatible.
2. Each test antibiotic concentration, made up in Cation Adjusted Mueller
Hinton Broth (CAMHB), is placed in one lane of the microtitre plate (200NL
total
volume per well) at 2 fold dilutions of antibiotic in the range necessary.
Day 2: Antibiotic Challenge of Biofilm:
1. Prepare rinse plate(s) of 0.9% saline (200NL per well). Rinse planktonic
bacteria from pegs by placing the pegs into the rinse plate for approx. 1
minute.
2. Transfer the peg lid to the challenge plate and incubate at 35 C for 24
hours.
Day 3: Recovery of Surviving Biofilm
1. Prepare rinse plate(s) of saline (200 pL per well) in a sterile 96 well
microtitre plate.
2. Prepare recovery plate(s) of CAMHB (200 pL per well) in another 96 well
microtitre plate.
3. Rinse pegs in saline for approx. 1 minute. Do not discard the challenge
plate. Transfer pegs to recovery media then sonicate on high for 5 minutes to
dislodge surviving biofilm. Discard the peg lid and cover the recovery plate.
Incubate
at 35 C for 20 to 24 hours to allow surviving bacteria to grow to turbidity.
Note: If surviving cfu/peg data for each well is required, 50 NL can be
removed from
each well of the recovery plate immediately after the sonication step and
transferred
to dilution plates and serially diluted in saline and spot plated (100 through
107) on
appropriate media.
-50--
CA 02616559 2008-01-18
PCT/CA2006/001218
Day 3: Determination of Planktonic MIC
1. Check for turbidity (visually) in the wells of the challenge plate or on a
plate
reader at 650 nm.
2. Determine the MIC (minimum inhibitory concentration) for each antibiotic
for the planktonic bacteria shed from the biofilm during the challenge
incubation. The
MIC is defined as the minimum concentration of antibiotic that inhibits growth
of the
organism. Clear wells (A650 < 0.1) are evidence of inhibition.
3. Record MIC values for each antibiotic.
Day 4: Determination of Biofilm MBEC
1. Determine the MBEC (minimum biofilm elimination concentration) for each
antibiotic by reading the turbidity of the recovery plate. The MBEC is defined
as the
minimum concentration of antibiotic that inhibits regrowth of biofilm bacteria
in the
recovery media. Clear wells (A650 < 0.1) are evidence of inhibition.
2. Record MBEC values for each antibiotic.
Although a few preferred embodiments have been descrrbed, it will be
appreciated by those skilled in the art that various changes and modifications
might
be made without departing from the scope of the invention. The terms and
expressions in the preceding specification have been used therein as terms of
description and not of limitation, and there is no intention in the use of
such terms
and expressions of excluding equivalents of the features shown and described
or
portions thereof, it being recognized that the scope of the invention is
defined and
limited only by the claims that follow.
-=51-.