Note: Descriptions are shown in the official language in which they were submitted.
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-1-
STENT DELIVERY SYSTEM AND METHOD OF USE
TECHNICAL FIELD
In one of its aspects, the present invention relates to a balloon dilation
catheter. In another of its aspects, the present invention relates to a
catheterization method.
BACKGROUND ART
Balloon dilation catheters have been known for many years. Originally,
such catheters were used in interventional techniques such as angioplasty.
In recent years, balloon dilation catheters have also been used to facilitate
delivery of endovascular prosthesis' such as stents. Stents are generally
known.
Indeed, the term "stent" has been used interchangeably with terms such as
"intraluminal vascular graft" and "expansible prosthesis". As used throughout
this specification, the term "stent" is intended to have a broad meaning and
encompasses any expandable prosthetic device for implantation in a body
passageway (e.g., a lumen or artery).
In the past dozen years, the use of stents has attracted an increasing
amount of attention due to the potential of these devices to be used, in
certain
cases, as an alternative to surgery. Generally, a stent is used to obtain and
maintain the patency of the body passageway while maintaining the integrity of
the passageway. As used in this specification, the term "body passageway" is
intended to have a broad meaning and encompasses any duct (e.g., natural or
iatrogenic) within the human body and can include a member selected from the
group comprising: blood vessels, respiratory ducts, gastrointestinal ducts and
the
like.
Stent development has evolved to the point where the vast majority of
currently available stents rely on controlled plastic deformation of the
entire
structure of the stent at the target body passageway so that only sufficient
force
to maintain the patency of the body passageway is applied during expansion of
the stent.
Generally, in many of these systems, a stent, in association with a balloon,
is delivered to the target area of the body passageway by a catheter system.
Once
CA 02616856 2008-02-08
WO 01/58383 PCT/CAOI/00154
-2-
the stent has been properly located (for example, for intravascular
implantation
the target area of the vessel can be filled with a contrast medium to
facilitate
visualization during fluoroscopy), the balloon is expanded thereby plastically
deforming the entire structure of the stent so that the latter is urged in
place
against the body passageway. As indicated above, the amount of force applied
is at least that necessary to expand the stent (i.e., the applied force
exceeds the
minimum force above which the stent material will undergo plastic deformation)
while maintaining the patency of the body passageway. At this point, the
balloon
is deflated and withdrawn within the catheter, and is subsequently removed.
Ideally, the stcnt will remain in place and maintain the target area of the
body
passageway substantially free of blockage (or narrowing).
See, for example, any of the following patents:
United States patent 4,323,071 (Simpson et al.),
United States patent 4,411,055 (Simpson et al.),
United States patent 4,616,648 (Simpson),
United States patent 4,661,094 (Simpson),
United States patent 4,733,665 (Palmaz),
United States patent 4,739,762 (Palmaz),
United States patent 4,800,882 (Gianturco),
United States patent 4,907,336 (Gianturco),
United States patent 5,035,706 (Gianturco et al.),
United States patent 5,037,392 (Hillstead),
United States patent 5,041,126 (Gianturco),
United States patent 5,092,873 (Simpson et al.),
United States patent 5,102,417 (Pahnaz),
United States patent 5,147,385 (Beck et al.),
United States patent 5,269,793 (Simpson),
United States patent 5,282,824 (Gianturco),
United States patent 5,316,023 (Palmaz et al.),
United States patent 5,415,634 (Glynn et al.),
United States patent 5,462,529 (Simpson et al.),
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-3-
United States patent 5,755,771 (Penn et al.),
United States patent 5,980,570 (Simpson),
International patent application PCT/CA97/00151 (Penn et al.), and
Intemational patent application PCT/CA97/00152 (Penn et al.),
for a discussion on previous stent designs and deployment systems.
Given the development of stent design, the prior art has also focussed on
delivery systems for stent deployment.
One particular delivery system is taught by United States patent 4,748,982
[Horzewski et al. (Horzewski)]. Horzewski teaches a reinforced balloon
dilation
catheter with a slitted exchange sleeve. Essentially, the catheter comprises a
tubular member having a first lumen and a second lumen. A dilation balloon is
mounted on the distal end of the tubular member and is in communication with
the first lumen. An opening (or notch) is disposed in the tubular member
intermediate its proximal and distal ends for receiving a guidewire which
travels
through the second lumen and emanates out of the distal end of the tubular
member. A slit is disposed on the longitudinal portion of the tubular member
between the opening and an area 0.5-1 cm proximal the dilation balloon. Thus,
as illustrated in Figure 1 of Horzewski, the guidewire travels partly within a
lumen in the catheter (approximately 10-15 cm) and partly along the outside of
the catheter (approximately 80-90 cm). This approach is also known as a
"monorail" delivery system. The principal advantage of this approach is that
it
permits so-called "rapid exchange" of the balloon catheter with another
balloon
catheter. In design, the exchange is facilitated by the provision of the above-
mentioned slit so that the actual exchange is done over the balloon portion
only
(approximately 3 cm). The principal disadvantages of this approach include:
less
than optimum steerability of the guidewire, difficulties in moving the
guidewire
with respect to the catheter, less than optimum torque control and inability
to
exchange the guidewire while leaving the catheter in place. The catheter
illustrated by Horzewski has not gained widespread commercial popularity.
Another approach for catheterization is the so-called "over the wire"
approach - this approach is discussed in many of the above-mentioned United
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-4-
States patents naming John P. Simpson as an inventor. In this approach, the
catheter comprises a tubular member having a first lumen and a second lumen.
A dilation balloon is mounted on the distal end of the tubular member and is
in
communication with the first lumen. The second lumen runs through the length
of the tubular member. An opening is disposed in the tubular member at its
proximal end for receiving a guidewire which travels through second lumen and
emanates out of the distal end of the tubular member. Thus, in the "over the
wire" approach, the guidewire is encompassed by the second lumen along the
entire length of the tubular member (approximately 90-105 cm). The principal
advantages of the this approach include: optimum steerability, smoother
movement of the guidewire with respect to the catheter (due to the coaxial
relationship thereof), optimum torque control and the ability to exchange the
guidewire while leaving the catheter in place. The principal is disadvantage
of
this approach is that exchange with another balloon catheter is relatively
cumbersome (i.e., compared to the "monorail" approach discussed above.
A purported improvement over the "monorail" delivery system is
described in United States patent 5,195,978 [Schiffer]. Schiffer teaches a
"rapid
exchange over-the-wire catheter". The purported point of novelty in Schiffer
is
the provision of one or more breakaway elements for progressively exposing the
guidewire from the proximal end toward the distal end of the catheter. In the
illustrated embodiment the breakaway element is a pull tab or tear strip. The
tab
strip form from a plurality of longitudinally extending generally parallel
grooves
form in the tubular shaft of the catheter. The Schiffer catheter is
disadvantageous
since it requires the physician to execute two distinct and sequential steps
to
achieve "rapid exchange". First, the physician must take one hand off the
guidewire or the catheter and thereafter grasp and remove the pull tab or tear
strip
to expose the guidewire. Second, the physician must remove the catheter while
the guidewire is held in position. The requirement for these two distinct and
sequential steps renders the Schiffer catheter cumbersome, time consuming and
impractical to use.
Accordingly, it would be desirable to have a balloon dilation catheter
which combined the advantages of the above-mentioned "monorail" approach
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-5-
and "over the wire" approach while obviating or mitigating the disadvantages
of
these approaches. It would be further advantageous if the balloon dilation
catheter were readily adaptable to be used in various interventional
techniques
such as endovascular prosthesis delivery, angioplasty and the like.
DISCLOSURE OF THE INVENTION
It is an object of the present invention to provide a novel balloon dilation
catheter.
It is another object of the present invention to provide a novel
catheterization method.
Accordingly, in one of its aspects, the present invention provides a
balloon dilation catheter comprising: a tubular member having a proximal end
and a distal end;
an inflatable balloon disposed at the distal end of the tubular member;
a first lumen disposed in the tubular member and in communication with
an interior of the inflatable balloon;
a second lumen disposed in the tubular member for receiving a guidewire
along at least a portion its length (preferably the entire length), the second
lumen
having a first opening in the proximal region of the tubular member and a
second
opening at the distal region of the tubular member; and
a first slit disposed longitudinally in the tubular member and extending
along at least a portion of the tubular member, the first slit comprising a
first pair
of longitudinal edges in a side by side relationship, the tubular member being
constructed of a resilient material such that, as the guidewire is separated
from the
second lumen, the longitudinal edges are biassed open from a first position to
a
second position having a gap greater than or equal a diameter of the
guidewire.
In another of its aspects, the present invention provides a balloon dilation
catheter comprising:
a tubular member having a proximal end and a distal end;
an inflatable balloon disposed at the distal end of the tubular member;
a first lumen disposed in the tubular member and in communication with
an interior of the inflatable balloon;
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-6-
a second lumen disposed in the tubular member for receiving a guidewire
along a portion of its length (preferably the entire length), the second lumen
having a first opening in the proximal region of the tubular member and a
second
opening at the distal region of the tubular member;
a first slit disposed longitudinally in the tubular member and extending
along at least a portion of the tubular member, the slit permitting withdrawal
of
the guidewire from the second lumen; and
an adapter attached to the proximal region of the tubular member, the
adaptor comprising a valve comprising a second slit and third lumen for
receiving
the guidewire, the second lumen and the third lumen in communication with one
anothcr, the second slit comprising a pair of longitudinal edges in a side by
side
relationship, the valve being constructed of a resilient material such that,
as the
guidewire is separated from the third lumen, the longitudinal edges are
biassed
open from a first position to a second position having a gap greater than or
equal
a diameter of the guidewire.
Thus, the present inventor has discovered a balloon catheter which
combines the advantages of the "over the wire" approach (i.e., optimum
steerability, smoother movement of the guidewire with respect to the catheter
(due to the coaxial relationship thereof), optimum torque control and the
ability
to exchange the guidewire while leaving the catheter in place) with the
principal
advantage ofthe "monorail" approach (i.e., rapid exchange of the balloon
catheter
with another balloon catheter while leaving the guidewire in place).
As used throughout this specification, the term "tubular member", when
used in the context of the present balloon dilation catheter is intended to
mean a
portion of the catheter generally tubular in construction and generally
representing the large majority of the overall length of the balloon dilation
catheter. Typically, the tubular member will be at least about 75%, more
preferably at least about 85%, most preferably at least about 95%, of the
overall
length of the balloon dilation catheter.
Thus, the present inventor has discovered a balloon dilation catheter
which have one or more of the number of novel features. For example, it has
been discovered that a particularly advantageous approach in facilitating
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-7-
guidewire removal from the catheter is to utilize materials for the tubular
member
which, when finely slitted, results in the longitudinal edges of the slit
portion
being abutting or touching engagement. This obviates or mitigates back
bleeding
through that portion of the catheter which is outside the patient during use.
Another independently novel feature ofthe present balloon dilation catheter is
the
adapter located at the proximal end of the catheter. Specifically, the present
inventor has discovered that the use of resilient valve in the adapter having
a slit
disposed therein (preferably aligned with the slit in the tubular member)
allows
for advantageous removal of the guidewire from balloon dilation catheter while
obviating or mitigating unintended or accidental removal of the guidewire or
damage thereto.
BRIEF DESCRIPTION OF THE DRAWINGS
Embodiments of the present invention will be described with reference to
the accompanying drawings wherein like numerals designate like parts and in
which:
Figure 1 illustrates a perspective view of an embodiment of the present
balloon dilation catheter;
Figure 2 is a sectional view along line II-II in Figure 1;
Figure 3 is a sectional view along line TII-11I in Figure 1;
Figure 4 illustrates an exploded view of modified proximal end of the
balloon dilation catheter illustrated in Figure 1;
Figures 5-11 illustrate steps in various catheterization techniques
employing the present balloon dilation catheter;
Figure 12 illustrates a modified balloon for use in the present balloon
dilation catheter;
Figure 13 illustrates a preferred embodiment of a modified tubular
member for use in the present balloon dilation catheter;
Figures 14a,14b and 14c illustrate preferred embodiments of a modified
tubular member for use in the present balloon dilation catheter;
Figure 15 illustrates an alternate embodiment of the balloon dilation
catheter illustrated in Figure 4;
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-8-
Figure 15a is an enlarged sectional view of region B of Figure 15; and
Figure 16 illustrates a preferred embodiment of an adapter at the proximal
end of the present balloon dilation catheter.
BEST MODE FOR CARRYING OUT THE INVENTION
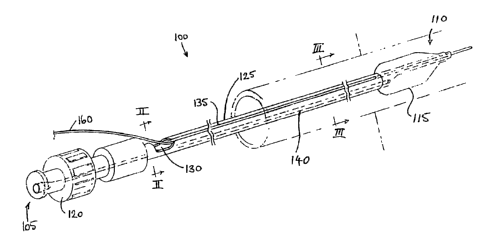
Thus, with reference to Figures 1-3, there is illustrated a balloon dilation
catheter 100. Balloon dilation catheter 100 comprises a proximal end 105 and a
distal end 1 10. Distal end 110 of balloon dilation catheter 100 comprises an
expandable balloon 115. Proximal end 105 of balloon dilation catheter 100
comprises an single lumen Luer-type adaptor 120. Disposed between adaptor 120
and balloon 115 is a tubular member 125.
As will be apparent from Figure 1, disposed in tubular member 125 is an
opening 130. Also disposed in tubular member 125 is a slit 135 which extends
from opening 130 to a point in tubular member 125 just proximal balloon 115.
With particular reference to Figures 2 and 3, tubular member 125
comprises a first lumen 140 and a second lumen 150. First lumen 140 is
designed
to be in communication with an interior of balloon 115. The design of the
interface between balloon 115 and first lumen 140 is conventional - see for
example Horzewski referred to hereinabove. The construction of tubular member
125 having opening 130, slit 135, first lumen 140 and second lumen 150 is
conventional - see Horzewski referred to hereinabove.
With further reference to Figures 1-3, it will be apparent that opening 130
is designed to receive a guidewire 160. Guidewire 160 passes through second
lumen 150 and out of a distal opening of tubular member 125 beyond balloon
115.
Preferably, slit 135 has a width which is less than the diameter of
guidewire 160, In a more preferred embodiment, the longitudinal edges of slit
135 are abutting or touching engagement thereby proving containment of
guidewire 160 therein. Slit 135 may be formed by an suitable means, including
the use of a very thin slicing member being run longitudinally along the
surface
oftubularmember 125 corresponding to second lumen 150. Other manufacturing
means will be apparent to those of skill in the art.
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-9-
In Figure 4, there is illustrated a modification of balloon dilation catheter
100 illustrated in Figures 1-3.
Specifically, in Figure 4, Luer-type adaptor 120a is modified to contain
a lumen 150a in communication with a slit 135a. As will be apparent to those
of
skill in the art, lumen 150a is in communication with second lumen 150 in
tubular
member 125. Further, slit 135a is in communication with slit 135 in tubular
member 125. The modification of balloon dilation catheter 100 illustrated in
Figure 4 eliminates the need for having opening 130 disposed in tubular member
125 illustrated in Figure 1.
With reference to Figures 5-9, the delivery ofballoon dilation catheter 100
will be described.
As is known in the art, catheterization is normally performed to alleviate
a lesion in an artery. This is shown schematically in Figures 6-9 wherein a
lesion
in the form of a blockage 15 obstructs an artery 20. In certain cases, it is
desirable to deploy a stent at the site of the lesion to maintain the patency
of
artery 20 at the site of blockage 15. As shown in Figure 5, catheterization is
performed through an incision in the groin area of the patient.
Thus, with reference to Figures 6 and 7, a guide catheter 25 is initially
delivered into artery 20 to a region proximal of blockage 15. The proximal end
of guide catheter 25 remains outside the patient.
Balloon dilation catheter 100 (Figure 1) has mounted on balloon 115
thereof a stent 30. Further, guidewire 160 in second lumen 150 such that it
emanates from opening 130 and from distal end 110 of balloon dilation catheter
100. Preferably, this is achieved in a conventional manner by feeding
guidewire
160 into second lumen 150 at distal end 110 ofballoon dilation catheter 100
until
the proximal end of guidewire 160 emanates from opening 130.
At this point, balloon dilation catheter 100 is inserted into guide catheter
25 and guidewire 160 is navigated through artery 20 to a point distally of
blockage 15 (Figure 7).
Alternatively, it is possible to advance guidewire 160 to a point distally
of blockage 15, after which the distal end of second lumen 150 of balloon
dilation
catheter 100 is passed onto the proximal end of guidewire 160. If it becomes
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-10-
difficult to advance guidewire 160 across blockage 15 using this technique, it
is
possible to advance balloon dilation catheter over the proximal end of
guidewire
160 until that end exits opening 130 and the system may used in the "over-the-
wire" approach described herein.
In Figure 8, there is illustrated removal of guidewire 160 while leaving
balloon dilation catheter 100 in position at point proximal to blockage 15.
This
is an advantageous feature of the present balloon dilation catheter which is
not
possible with the balloon dilation catheter taught in Horzewski. Thus,
guidewire
160 may simply be replaced with another guidewire by removing the original
guidewire from proximal end 105 of balloon dilation catheter 100 and simply
inserting a replacement guidewire (not shown) into the proximal end 105 of
balloon dilation catheter 100 and through tubular member 125. Thereafter, the
replacement guidewire may be navigated so that it emanates from distal end 110
of balloon dilation catheter 100. The replacement guidewire is navigated to a
point distal of blockage 15.
Balloon dilation catheter 100 is then navigated over the replacement
guidewire such that stent 30 is in proper position with respect to blockage 15
(Figure 8). Once the guidewire and balloon dilation catheter 100 are in the
correct position, fluid is injected into first lumen 150 thereby expanding
balloon
115 and stent 30 mounted thereon. Deployment of a stent in this manner is
conventional and within the purview of a person skilled in the art.
In Figures 10 and 11, there is illustrated rapid exchange of balloon
dilation catheter 100 while leaving guidewire 160 in place. In this case, for
clarity, stent 30 is not shown on balloon 115. One of the features of the
present
balloon dilation catheter which distinguishes it from that in Horzewski is
that
guidewire 160 emanates from a proximal portion of balloon dilation catheter
100
which is always outside the body of the patient. This provides the
practitioner
with the "over-the-wire" approach described above. Thus, either opening 130 is
located outside the body at all times during use of catheter 100 illustrated
in
Figure 1 or it is necessarily emanating from the proximal end of balloon
dilation
catheter 100 if the modified embodiment in Figure 4 is utilized.
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-11-
When it is desired to exchange balloon dilation catheter 100, the balloon
dilation catheter is withdrawn from artery 20 while leaving guidewire 160 in
place. As balloon dilation catheter 100 is withdrawn from the body ofthe
patient,
it may be readily separated from guidewire 160 via slit 135 along virtually
the
entire length of tubular member 125 - this is one of the principal advantages
of
the present balloon dilation catheter which, to the knowledge of the present
inventors, has not been achieved with a prior balloon dilation catheter. Once
distal end 110 of balloon dilation catheter 100 is withdrawn from the body,
balloon 115 may be exchanged from guidewire 160 in a conventional manner.
A replacement balloon dilation catheter may then be fed over guidewire
100 and navigated into artery 20 in the area of blockage 15.
With reference to Figure 12, there is illustrated yet a further altemate
embodiment to the present balloon dilation catheter. In this case, a slit 135b
is
provided in balloon 11 5b such that slit 135 is in communication with slit
135b on
balloon 115b. This modification of balloon catheter 100 is particularly
advantageous when the catheter is being used in an angioplasty application
(i.e.,
without a stent mounted on balloon 115) as a pre-dilation balloon catheter
allowing for enhanced rapid exchange features by facilitating withdrawal of
guidewire 160 in a rapid exchange manner along virtually the entire length of
tubular member 125 and balloon 115b via the combination of slits 135 and 135b.
This feature is generally advantageous since it facilitates withdrawal of the
balloon dilation catheter from the patient.
With reference to Figure 13, there is illustrate a preferred modification to
tubular member 125 of balloon catheter 100. Specifically, a third lumen 180 is
provided along substantially the entire length of tubular member 125. Disposed
within third lumen 180 is a stiffening member 185 which serves to improve the
"torqueability" of balloon dilation catheter. Unlike, the approach in
Horzewski
described above wherein a single lumen does double duty for receiving: (i) a
stiffening member along most of the length of the catheter and (ii) the
guidewire
along a minor portion of its length, the approach shown in Figure 13 is a
significant improvement over Horzewski since it maximizes both the distance
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-12-
over which rapid exchange may be effected and the distance over which
stiffening may be conferred to the tubular member.
With reference to Figures 14a, 14b and 14c, there is illustrated a
modification to the balloon dilation catheter illustrated in Figures 1-3 and
described hereinabove. In Figures 1-3 and 14a, 14b and 14c, like numerals
designate like elements. As will be evident tubular member 125 has been
modified to provide a weakened region A. Weaken region A comprises a thinned
wall 135a (Figure 14a), 135b (Figure 14b) and 135c (Figure 14c). When it is
desired to exchange balloon catheter 100, guidewire 160 is separated from
tubular
member 125. The separation force causes incision of thinned wall 135a (Figure
14a), 135b (Figure 14b) and 135c (Figure 14c). This causes in situ formation
of
a slit as guidewire 160 is separated from tubular member 125. The feature of
in
situ slit formation is a significant advantage over the Schiffer catheter
design
above since, in this embodiment of the present balloon dilation catheter, in
situ
slit formation and guidewire separation from the tubular member are achieved
simultaneously in a one-step operation. Additionally, provision of thinned
wall
135a (Figure 14a),135b (Figure 14b) and 135c (Figure 14c) obviates or
mitigates
reduction in the integrity of tubular member 125 since a slit is not formed
therein
until the guidewire is removed (i.e., until a point in time after the catheter
has
been navigated to the target location).
With reference to Figure 15, there is illustrated a modification of balloon
dilation catheter 100 illustrated in Figures 1-4.
Specifically, in Figure 15, there is illustrated a balloon dilation catheter
200 comprising a proximal end 205 and a distal end 210. Distal end 210 of
balloon dilation catheter100 comprises an expandable balloon 215. Proximal end
205 of balloon dilation catheter 200 comprises adapter 220a which is similar
to
adapter 120a in Figure 4. Disposed between adapter 220a and balloon 215 is a
tubular member 225. As will be apparent from Figure 15, disposed in tubular
member 225 is a slit 235. Slit 235 is similar to slit 135 in Figure 4 with the
exception that slit 235 ends at a spot more proximally than slit 135. Disposed
distally of slit 235 is a port or an opening 250 which is in communication
with
a guidewire receiving lumen 255 extending along the entire length of tubular
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-13-
member 225 and through the distal end of balloon 215. Disposed at the distal
region of opening 250 is a ramp 260 whose function will be described below.
Ramp 260 may be fonned by any suitable means. In one preferred
embodiment, ramp 260 is formed in situ by piercing the outer wall of tubular
member 225 on a surface corresponding to guidewire lumen 255 using a hot
instrument which results in formation of small mass on the inside surface of
guidewire lumen 255 in the form of ramp 260. Again, other suitable means for
manufacturing ramp 260 will be apparent to those of skill in the art.
Balloon dilation catheter 200 may be used in the following manner.
The initial steps of using balloon dilation catheter 200 are similar to those
described above in relation to the use ofballoon dilation catheter 100. Thus,
once
the balloon dilation catheter has been withdrawn it is to be replaced with
another
balloon dilation catheter, the replacement balloon dilation catheter may be a
conventional "monorail"-type balloon dilation catheter such as the one taught
in
Horzewski or balloon dilation catheter 200. Specifically, with reference to
Figures 15 and 15a, balloon dilation catheter 200 is fed over guidewire 160 in
the
direction of arrow C. Once the proximal end of guidewire 160 approaches the
area in guidewire lumen 255 corresponding to opening 250, it is biassed
upwardly
and outwardly from opening 250 by ramp 260, thereby rendering the replacement
catheter 200 a "monorail"-type catheter. As stated above, the replacement
catheter could be a conventional "monorail"-type catheter.
With reference to Figure 16, there is illustrated an adapter 300 which is
particularly preferred for use in the present balloon dilation catheter. Thus,
adapter 300 comprises an outer housing 305, typically constructed from plastic
or other durable material. Tubular member 125 as described above in relation
to
Figure 1-4 is disposed within housing 305 as illustrated and is sealed with
respect
to housing 305 by adhesive 310 or any other suitable adhesive/sealant
material.
As illustrated, first lumen 140 which is in communication with the interior of
the
balloon (not shown) of the catheter, emanates from tubular member 125 into a
first arm 305 of adapter 300. This proximal end of first lumen 140 is then
able
to receive injection fluid to inflate the balloon.
CA 02616856 2008-02-08
WO 01/58383 PCT/CA01/00154
-14-
Disposed at the proximal end of adapter 300 within housing 305 is a
resilient valve 315. Resilient valve 315 may be made out of a polymeric
material
based on silicone and the like. This resiliency is particularly beneficial
since the
formation of a slit in valve 315 allows the edges of the slit to be abutting
or
touching engagement until such time as the guidewire is to be removed - indeed
the view in Figure 16 is a sectional view taken along the slit (not shown as
such).
As the guidewire is removed, it biases open the slit in resilient valve 315.
After
removal of the guidewire since valve 315 is made from a resilient material,
the
slit "closes" that the edges thereof are abutting or touching engagement (or a
gap
less than the diameter of the guidewire separates the edges). The advantages
from this approach include: mitigating or obviating back bleeding through the
catheter, mitigating or obviating unintentional displacement of the guidewire
fonn adapter 300 and the like.
While this invention has been described with reference to illustrative
embodiments, this description is not intended to be construed in a limiting
sense.
For example, while the illustrated embodiments depict use of the present
balloon
dilation catheter in delivery of a stent, those of skill in the art will
innnediately
appreciate that the present balloon dilation catheter may be used in
percutaneous
transluminal coronary angioplasty techniques. Further, as will be apparent to
those of skill in the art, it is possible to combine, in a single catheter,
the slit
illustrated in Figures 1-3 with the weakened region illustrated in Figures
14a,14b
and/or 14c. Further, while preferred, it is not strictly necessary for the
weakened
region illustrated in Figures 14a,14b and/or 14c to extend along substantially
the
entire length of the tubular member. Still further, the specific nature of the
weakened region illustrated in Figures 14a, 14b and 14c is not particularly
restricted provided that it can be readily incised as the guidewire separated
from
the catheter - e.g., a perforated region or a region comprising a plurality of
small,
partial cuts is also useful. Various modifications ofthe illustrative
embodiments,
as well as other embodiments of the invention, will be apparent to persons
skilled
in the art upon reference to this description. It is therefore contemplated
that the
appended claims will cover any such modifications or embodiments.
CA 02616856 2008-02-08
WO 01/58383 PCT/CAOI/00154
-15-
All publications, patents and patent applications referred to herein are
incorporated by reference in their entirety to the same extent as if each
individual
publication, patent or patent application was specifically and individually
indicated to be incorporated by reference in its entirety.