Note: Descriptions are shown in the official language in which they were submitted.
CA 02617370 2008-02-01
WO 00/69470 PCTIUSOOn3260
IMPROVED CELLULAR UPTAKE OF BIOACTIVE AGENTS
Background of the invention
Absorption of biomolecules, such as amino acids and proteins, is critical
to cellular function. About 75 percent of the solids in the mammalian body are
proteins, including enzymes, polypeptides such as cytokines, nucleoproteins,
transport proteins, and structural proteins. The principal functional
constituents
of these proteins, amino acids, polypeptides and isolated amino acids, are
also
important for cellular metabolic functions. The amino acid glutamine, for
example, serves important functions in metabolism, including transport of
carbon and nitrogen between tissues. It is a precursor for hepatic and renal
gluconeogenesis, as well as urea synthesis in the liver and ammonia production
in the kidney. A number of cell types, particularly the cells of the
intestinal
mucosa, also utilize large amounts of glutamine as their major source of
respiratory fuel.
The effectiveness of amino acid supplementation for treatment of a
variety of physiologic disorders has been demonstrated. D-serine
supplementation, for example, augments the beneficial effects of
antipsychotics
for the treatment of schizophrenia. (Tsai, G., et al., Biol. Psychiatry (1998)
44(11): 1081-1089.) L-tryptophan or 5-hydroxytryptophan supplementation has
been shown to improve symptoms of depression, anxiety, insomnia and pain in
patients with fibromyalgia. (Juhl, J.H., Altern. Med. Rev. (1998) 3(5): 367-
375.)
Dietary supplementation with 8 essential and 9 nonessential amino acids
provided improved health, tone, and mood in dialysis patients, in whom protein
malnutrition is a common problem. (Mastroiacovo, P., et al., Clin. Ther.
(1993)
15(4): 698-704.) Nutritional supplementation with aspartic acid has been
suggested for the treatment of Canavan disease, a rare recessive autosomal
genetic disorder generally resulting in death within several years of onset.
(Baslow, M.H., et al., J. Mol. Neurosci. (1997) 9(2): 109-125.) L-lysine has
also been demonstrated to have therapeutic use for lesions associated with
herpes
simplex virus type 1 (HSV-1). (Ayala, E. And D. Krokorian, J. Med. Virol.
(1989) 28(1): 16-20.)
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
2
Glutamine supplementation has been shown to provide numerous
benefits, including stimulation of certain cells of the immune system and
general
promotion of cellular growth. Depletion of glutamine results in atrophy of
epithelial tissue, with associated bacterial translocation. Clinical
supplementation of glutamine reduces epithelial atrophy and accelerates
recovery.
Dietary glutamine supplementation has been proposed for the treatment
of patients recovering from surgery or suffering from sepsis, inflammation,
burns, or trauma. Topical administration, usually in the form of a "swish and
swallow" solution for oral use to repair the damaged epithelial tissue of
mouth or
esophageal sores, can be effective in many patients who have undergone bone
marrow transplantation or chemotherapy. (Skubitz, et al., J. Lab. Clin. Med.
(1996) 127(2): 223-8; Anderson, et al., Bone Marrow Transplant (1998) 22(4):
339-44.)
The effectiveness of amino acid supplementation has been limited in
some individuals due to aging or disease. Effective supplementation with
certain
amino acids is further limited to varying degrees by the low aqueous
solubility
and limited cellular uptake of some amino acids. Glutamine, for example,
exhibits a low solubility in water (48 g/l at 30 C, 26 g/l at 18 C, 18 g/1 at
0 C;
The Merck Index, 12th Edition) and a low chemical stability in aqueous
solution
(11 days at 22-24 C). (Cardona, P., Nutr. Hosp. (1998) 13(1): 8-20.).
Transport of small molecules into various cell types is controlled by
alternate transport systems, making it more difficult to devise methods for
increasing cellular uptake into particular cell types. Despite the need for
methods to enhance the uptake of amino acids and other small molecules,
methods for increasing initial direct absorption of amino acids, peptides and
other compounds into cells such as epithelial cells, the type of cells
initially
responsible for initial uptake of many bioactive compounds, has not been
described.
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
3
Therefore, a continuing need exists for methods to increase cellular
uptake of bioactive compounds into mammalian cells.
Summary of the Invention
The invention provides a composition and a method for increasing
cellular uptake of bioactive agents, particularly those compounds termed
"small
molecules" into the cells of mammalian tissue, such as the epithelial cells of
the
mucosa. The composition is a solution dispersion or suspension comprising an
aqueous vehicle and an effective amount of a bioactive compound, in
combination with an amount of carbohydrate effective to reduce the absolute
solubility of the bioactive agent in the aqueous vehicle, so as to achieve
increased transport (absorption) of the bioactive agent into the target cells.
The
transport (absorption) is increased over the amount that would enter the cells
under physiological conditions, i.e., under homeostatic conditions, when the
cells are contacted with the agent dissolved or suspended in water or in a
physiological salt solution. Preferably, the transport (absorption) is
increased by
a factor of at least about 100-2000 times that is obtainable by a saturated
aqueous
solution of the active agent. It is believed that the carbohydrate(s) act by
reducing the amount of free/available water in the composition, which induces
increased transport into mammalian cells, in vitro or in vivo.
The carbohydrate carrier can comprise a monosaccharide, such as
glucose, a disaccharide, such as sucrose, or a combination of monosaccharides
and disaccharides. The carbohydrate carrier can also comprise a sugar alcohol
such as mannitol, sorbitol or xylitol. The carbohydrate carrier can also
comprise
a polysaccharide such as high fructose corn syrup or corn syrup solids,
wherein
the corn syrup or corn syrup solids, hydrous or anhydrous, constitute a
solution
phase for the active agent(s). The carrier can be combined with water, or with
a
mixture of water with pharmaceutically acceptable alkanols, alkylene glycols
or
polyols such as glycerol, to form a solution. Preferably the organic solvents
constitute a minor proportion of the aqueous phase, preferably < 5-10 vol-%.
The solution can be a true solution or a flowable "solid solution." It can
be administered by a variety of means for the administration of liquids,
including
toothpaste, chewing gum, hard or soft gelatin capsules, suppositories, or
other
CA 02617370 2008-04-24
WO 00/69470 PCTIUSOO/13260
4
liquid dosage forms such as topically applied lotions, drinks, such as a
shake, an
enema, or mouthwash.
Administration of the composition of the invention can provide treatment
for a variety of physiologic disorders ameliorated by enhancement of
absorption
of bioactive agents into damaged or intact tissues, especially disorders
affecting
the endothelial cells and fibroblasts of epithelial tissue. Such physiologic
disorders involving damaged tissue, include, for example, lesions of the oral
and
esophageal mucosa following radiation or chemotherapy in patients treated for
cancer or in whom bone marrow transplant is performed, gastric and peptic
ulcers, bums, major and minor trauma wounds, viral lesions, inflammatory
bowel disorder, Crohn's disease, Sjoren's syndrome, xerostoma, and
cryptosporidiosis.
A pharmaceutical dosage composition is also provided, consisting of
either bulk-packaged or individually-packaged pre-mixed dry or liquid
formulations of a therapeutically effective dose of amino acid in admixture
with
an amount of carbohydrate carrier effective to achieve increased absorption of
the amino acid into epithelial cells. Kits can also be provided comprising,
separately packaged in one container, dry formulation(s) and pre-measured
aqueous vehicle(s).
For example, the present invention relates to a dry composition
comprising:
(a) 57.9 wt-% L-glutamine;
(b) 34.8 wt-%o sucrose;
(c) 3.2 wt-% sorbitol; and
(d) 2.9 wt-% glycerin.
The present invention also relates to a dry composition comprising
L-glutamine, sucrose, sorbitol and glycerin in amounts so that, when mixed
with water, the dry composition yields a suspension having 50% w/v
L-glutamine, 30% w/v sucrose, 2.8 w/v % sorbitol and 2.5 w/v % glycerin.
CA 02617370 2008-04-24
WO 00/69470 PCT/US00/13260
4a
Brief Description of the Drawings
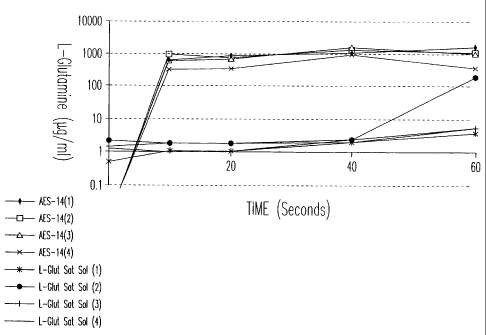
Fig. 1 and Fig. 2 are graphs illustrating the increased amino acid uptake
achieved using a composition and method of the invention. The amino acid
glutamine was administered to CaCo cells in combination with an effective
amount of carbohydrate carrier (7:1 ratio carbohydrate carrier to amino
TM
acid)(Aesgen-14), with amino acid administered as a saturated solution without
additional components (L-Glut Sat Sol) as a control. As indicated by the
figure
legend and the graph, intracellular glutamine concentration was increased
significantly in cells treated with a combination of amino acid and
carbohydrate
carrier, as compared to that achieved by glutamine administration alone.
Incubation time in seconds is indicated on the X axis, with cellular glutamine
uptake on the Y axis.
Fig. 3 depicts the relative effect of vehicle on L-glutamine cellular
uptake.
20
30
CA 02617370 2008-02-01
WO 00/69470 PCT/USOO/13260
Fig. 4 depicts the relative effect of vehicle on glycylsarcosine cellular
uptake.
Fig. 5 depicts the relative effect of vehicle on L-asparagine cellular
uptake.
5 Fig. 6 depicts the relative effect of vehicle on acyclovir cellular uptake.
Fig. 7 depicts the relative effect of vehicle on L-glutamine cellular uptake
(from half saturation).
Fig. 8 depicts the CaCo-2 permeability of L-glutamine.
Fig. 9 depicts the CaCo-2 permeability of glycylsarcosine.
Fig. 10 depicts the CaCo-2 permeability of L-asparagine.
Fig. 11 depicts the CaCo-2 permeability of acyclovir.
Fig. 12 depicts the CaCo-2 permeability of L-glutamine (from half
saturation).
Fig. 13 depicts the effect of Aesgen-14 on L-glutamine uptake into
human fibroblasts (right boxes) vs. saturated L-glutamine (left boxes).
Fig. 14 depicts the effect of Aesgen-14 on L-glutamine uptake into
human umbilical and endothelial cells.
Detailed Description of the Invention
The inventors have discovered a new composition that increases the
cellular uptake of bioactive agents into mammalian cells in vitro or in vivo.
Using the composition and method of the invention, increased gastrointestinal
epithelial cell uptake of the amino acid glutamine by a factor of over 150X
within ten seconds after administration has been demonstrated. The present
invention also provides a method for treating patients suffering from a number
of
pathophysiological conditions, using the composition to increase cellular
uptake
of bioactive agents in therapeutic amounts.
As used herein, the term "bioactive agent" refers to a molecule that exerts
a therapeutic or nutritive effect on a mammal following absorption of an
effective amount of the molecule by the target cells.
As used herein, the term "effective amount" refers to an amount that
causes a detectable biological change in a target cell population, and
preferably
an amount that accomplishes a therapeutic effect, i.e., reduces at least one
symptom of a pathology or disease afflicting said mammal.
CA 02617370 2008-02-01
6
As used herein, "amino acid" includes, for example, a1anine, arginine,
i
aspartic acid, asparagine, cysteine, glutamic acid, glutamine,=glyeine,
histidine,
isoleucine, leucine, lysine, methionine, phenylalanine, prolinc, serine,
threonine,
tryptophan, tyrosine, valine, citrulline, g-aminobutyric acid,
lFydroxyproline, and
ornithine, as well as dipeptides such as glutamyl glutamate and tripeptides
such
as glutathione. (See Remington's Pharmaceutical Sciences (1!9th ed.) at pages
389-392_) The composition and method are particularly useful, however, for
increasing absorption of those amino acids which exhibit limi,,ted aqueous
solubility and/or poor cellular uptake, such as glutamie. Liztrted aqueous
solubility, as used herein, is defined as a solubility of less than about 5
grams
amino acid in 100 ml water at 22-25 C.
The present solutions can also enhance the in vitro or in vivo cellular
absorption of a wide variety of bioactive agents, preferably in;therapeutic
amounts, particularly of the entities generally referred to as "small
molecules."
As used herein, the term "small molecule" includes single molecular
entities such as amino acids, steroids, cytokines, hormones, hormonal
regulators,
enzymes, vitamins and the like that generally have a molecular weight of less
than 30 kD, preferably less than 25 kD, most preferably less than 10 kD, i.e.,
a
molecular weight of s 5000 daltons_
,,
As used herein, the term "oligopeptide" is a peptide ccanprised of 2 to 20
amino acids.
Enhanced absorption of bioactive agents into the skin or intact mucosal
tissue of the gut can also be used to administer bioactive ager9s having an
effect
on organs or tissues remote from the site of administration. Such agents can
include small molecules such as enzymes (enzyme deficiencies), short chain
fatty acids (1BD), pamidronate (osteoporosis), pyruvate (kidney failure),
interferons (immunoregulation), TGF-P (atherosclerosis), hormones (prostate,
breast and other cancers), steroids (testosterone), and chemotli' rapeutic
agents
TM
(taxol, TMX and the like).
Other small molecules that may be potentiated using the present method
include antiviral drugs and antibiotics such as those agents that ligate to
binding
sites or receptors on the exterior surface of the cell membrane* These
antiviral
agents may include analogs of the viral binding amino acid sequences or
analogs
CA 02617370 2008-02-01
WO 00/69470 PCTIUS00I13260
7
of receptor groups, which inactivate the binding sequence of the virus, or
toxins
that are attached to receptor ligands which are used as a lethal agent to kill
the
infected cell, or agents that slow viral replication by inhibiting reverse
transcriptase. (See Remington's Pharmaceutical Sciences (19th ed.) at pages
1237-1241.)
Analogs of nucleosides, nucleotides or nucreosides may be used as
antiviral agents as well. Additional antiviral agents include macrophages
activated by muramyl tripeptides or other ligands on liposomes; antiseptics;
astringents; and B-propiolactone.
Specific antiviral agents include acyclovir, acyclovir sodium, amantadine
hydrochloride, cytarabine, idoxuridine, ribavirin, rifampin, suramin,
trifluridine,
vidarabine, zidovudine (AZT or ZDY), HPA-23, abacavir (Ziagen ), and any of
the interferons and any combination thereof Additional antiviral agents
include
rCD4-ricin A chain complex; AL-721 which is a combination of tumor necrosis
factor and gamma-interferon; ampligen, which is poly-IC 12U; ansamycin
(Rifabutin); (E)-5-(2-bromovinyl-2'-doxyuridine)(BVDU); butylated
hydroxytoluene; castanospermine; dextran sulfate; dideoxycitidine (DDC);
dideoxyadenosine; dideoxyinosine (DDI); foscamet;
dihydromethylpyridinylcarbonyloxyazidodide-oxythymidine; 2'-fluoro-2'-deoxy-
5-iodo-ara C (F1AC) and its uridine analog (FIAU); ganciclovir (9-[2-hydroxy-l-
(hydroxymethyl) ethoxymethyl] guanine (DPHG); Peptide-T; phosphonoformate
(foscarnet sodium); amantadine hydrochloride; and any combination thereof.
Other bioactive proteins that may be potentiated using the present method
include the group of proteins that are generally referred to as nerve growth
factors. These include nerve growth factor itself (NGF), Brain-Derived
neurotrophic factor (BDNF), neutrotrophin-3 (NT-3) and ciliary neurotropic
factor (CNTF). NGF (total dose infused i.v. = I ug) has been reported to
ameliorate cholinergic neuron atrophy and spatial memory impairment in aged
rats by W. Fischer et al., Nature, 324, 65 (1987). Recombinant human beta NGF
has been produced which has potent in vitro and in vivo neurotropic activity.
See J. Barrett et al., Exp. Neurol., uQ, 11 (1990). Therefore, exogenous
administration of neuronal growth factors may be helpful to treat pathological
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
8
disorders involving degenerative processes, including Alzheimer's disease or
diabetic associated polyneuropathy.
The present method can also be used to deliver insulin. Since it has been
demonstrated that there is a widespread distribution of insulin receptors in
brain,
insulin is likely to also have important functions in the central nervous
system.
It is suggested that insulin may function as a neurotrophic factor and
neuromodulator by D. G. Baskin et al., Trends Neurol.,11, 107 (1988) and D. G.
Baskin et al., Ann. Rev. Physiol., 42, 335 (1987).
Another class of proteins are the neuroreceptors or soluble peptides
isolated therefrom. These include receptors for neurotransmitters
(epinephrine,
norepinephrine, dopamine, serotonin, GABA, glycine, glutamate, and the like);
neuropeptides (P-endorphin, enkephalins, somatostatin, neurotensin,
angiotensin
vasoactive intestinal peptide, and the like); and neurohormones (luteinizing
hormone releasing hormone, thyrotrophin-releasing hormone, substance P, and
the like).
High molecular weight bioactive agents can also be employed in the
present method and compositions, including nucleic acids such as DNA and
RNA, i.e., linearized or plasmid DNA. The DNA can encode "sense" or
antisense RNA to block an undesirable cellular function. The DNA can encode
polypeptides such as hormones, and cytokines in amounts effective to
accomplish "gene therapy," i.e., the correction of metabolic diseases and
defects.
"Carbohydrate," as used herein, includes those sugars known as
monosaccharides and disaccharides, polyols, hydroxy analogs or sugar alcohols,
such as, for example, xylitol, sorbitol, and mannitol, and their polymers,
such as
dextrins, high fructose corn syrup, and corn syrup solids. It is well known in
the
art that certain mono- and disaccharides form sugar alcohols, or hydroxy
analogs. Certain of these hydroxy analogs, particularly sorbitol and xylitol,
have
proven to provide the benefit of a sugar taste without the cariogenic
properties of
the mono- and disaccharides from which they are derived.
It is believed that the carbohydrate, or mixture thereof, as used in the
present invention, acts at least in part by reducing the free water available
to
solubilize the bioactive agent(s), thereby promoting absorption of the amino
acids into the cytosol of the target cells. Preferably, there will be a major
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
9
proportion by weight of carbohydrate in the final composition, e.g., greater
than
80-90 weight percent. In some cases the composition can be essentially free of
added water, i.e., can be a "solid solution," the carbohydrate acting as a
"solvent" for the active ingredient. Such "solid solutions" can be flowable,
semisolid or even solid. The ratio of carbohydrate to active agent can be
approximately 1.5 : I w/w to 20: 1 w/w in a dry preparation, and preferably 4
: 1
w/v to 15 : 1 w/v in final aqueous solution, most preferably greater than 7: 1
w/v,
achieved either by constitution of the preparation with aqueous solvent or by
delivery into the aqueous environment of the extracellular fluids surrounding
the
target tissue.
"Cell," as used herein, includes any cell that can be contacted by the
present composition in accord with the present method, such as epithelial
cells,
endothelial cells, skin cells, fibroblasts or neuronal cells. More
specifically, cells
in which the composition and method of the present invention have been
demonstrated to increase absorption of the amino acid glutamine are
gastrointestinal epithelial cells., including cells of the mouth, throat,
esophagus,
stomach, intestines, colon and rectum, endothelial cells and fibroblasts.
"Constitution with aqueous solvent," as used herein, includes constitution
with water, physiological salt solutions or buffers, fruit juice or other
liquid
which contains a high percentage of water, or with extracellular fluids
surrounding the tissue to which the composition is applied, such as saliva,
mucous, gastric fluids, spinal fluid, and the like.
Formulation of a Composition for Increasing Solubility and Absorption of
an Amino Acid
In accord with the present invention, at least one bioactive agent is
combined with a carbohydrate in the presence of water, so as to form an
aqueous
solution. The carbohydrate can be a monosaccharide, including, for example,
allose, altrose, arabinose, dihydroxyacetone, erythrose, erythrulose,
fructose,
galactose, glucose, glyceraldehyde, gulose, lyxose, idose, mannose, psicose,
ribose, ribulose, sorbitol, tagatose, threose, xylose, xylulose, and their
respective
hydroxy analogs, such as sorbitol from sorbose, mannitol from mannose, and
xylitol from xylose. Alternatively, the carbohydrate can be a disaccharide,
such
as maltose or sucrose, or both, or their polymers, such as dextrins,
maltodextrins,
CA 02617370 2008-02-01
US001326C
1
Lt
and high fructose corn syrup products; The carbohydrate carrier can also be
composed of any combination of monosaccharides, disacchandes, or both. For
many applications, the hydroxy analog of the sugar is preferable, particularly
where a noncariogenic sugar is needed- Examples of hydroxy analogs include
5 the sugar alcohols, xylitol, sorbitol, and mannitol.
Carbohydrate concentration, measured as weight/weight, in the solid
composition is preferably 20% to 99%. At a certain concentration, the
carbohydrate will complex and reduce the amount of free watt available as a
solute for the active agent, so that the transport of the active ix gent into
the target
10 cell is significantly increased.
A preferred embodiment of the composition provides;a mixture of solids
including about 5-50% w/w glutarnine (most preferably L-glutamine), about 15-
50%'w/w carbohydrate carriers, including a disaccharide (moot preferably
sucrose), a sugar alcohol or polyol (most preferably sorbitol),land glycerin,
an
effective amount of buffer, or buffering compound (most preferably anhydrous
monobasic sodium phosphate), about 1-5% w/w modified cellulose (most
preferably Avieelo Cellulose Gel), with the remainder optionally comprising
,
stabilizers and emulsifying agents (xanthan gum, carrageenaii), preservatives
(methylparaben, potassium sorbate), a defoamant (simethiconp), and flavoring.
A more preferred embodiment provides approximately, 5-15% w/w
glutamine, 30-50% w/w carbohydrate carriers, including a disaccharide (most
preferably sucrose), a sugar alcohol or polyol (most preferable sorbitol), and
glycerin, with the remainder of dry solids comprising an effective amount of a
buffer, or buffering compound (most preferably anhydrous monobasic sodium
phosphate), modified cellulose (most preferably Avicel' Cellulose Gel), and
optionally comprising stabilizers and emulsifiers (xanthan gum, carrageenan),
preservatives (methylparaben, potassium sorbate), defoamants (simethicone), it
and flavoring.
A preferred liquid composition provides 5-25% w/v L.=glutamine, 20-
40% w/v carbohydrate carrier, including a disaccharide, a sugar alcohol, and
glycerin, 5-10%w/v citric acid, and an effective amount of buffer (preferably
0.4-0.8% sodium phosphate), with optional stabilizers, preservatives,
emulsifiers
and flavorings.
AMENDED SHEET .
Lmnfnnec701t 17..1811 71:41
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
11
Use of a carbohydrate carrier in the composition can increase the cellular
absorption of the amino acid by at least ten times over direct administration
of
the amino acid in water. For example, a preferred aqueous composition of 38%
w/v L-glutamine, 30% w/v sucrose, and 2.8% w/v sorbitol produced a 360-fold
increase in glutamine uptake by CaCo cells (an epithelial mucosa cell line)
over
that obtained by use of an aqueous glutamine solution alone.
Excipients can also be added to the composition, provided that the
necessary concentration of carbohydrate carrier is maintained. These can
include
a sweetener/solvent, such as glycerin; emulsifying and stabilizing agents,
such
as cellulose gel ( for example, Avicel Microcrystalline Cellulose Gel (FMC
Corp., Philadelphia, Pennsylvania)), xanthan gum or carrageenan; preservatives
and stabilizers, such as citric acid, and methylparaben; a defoamant/base
ingredient, such as simethicone; flavoring, or other ingredients which improve
the stability and administration of the composition.
Delivery of an Increased Concentration of an Active Agent
The invention provides a method of delivery of increased concentrations
of active agent to target cells in vivo or in vitro by a number of alternate
routes.
For example, the active agent can be mixed with a carbohydrate and water, and
optionally gelling or thickening agents. The mixture can be administered as a
solution, gel, or suspension. Where desired, undissolved materials can be
removed by allowing the mixture to stand to allow undissolved particles to
settle
out, or can be centrifuged to isolate the supernatant. The supernatant
solution
can then be parenterally, or orally applied to target tissue, as by
intravenous
injection of infusion.
Application of the preparation can include, but is not limited to, topical
administration by swabbing directly on a wound resulting from, for example,
burn, trauma, or viral infection, e.g., in ointment, gel or liquid form,
including
administration by transdermal patches. The preparation can be applied to oral,
nasal, and esophageal lesions by oral rinse, a gel, or an ingestible drink.
For
either oral rinse or ingestible drink, the carbohydrate carrier can be chosen
from
among a number of monosaccharides, disaccharides, or a combination of both, or
from their polymers, such as dextrins, maltodextrins, and high fructose corn
syrup products. Preferred carbohydrate carriers include sucrose, sorbitol and
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
12
high fructose corn syrup products. Either a suspension or a drink can be
provided as a dry mixture of carbohydrate carrier and an effective amount of
amino acid, for reconstitution with water, juice, or other liquid. Bulk
packaging
of the dry mixture or packets containing single applications can be provided
to a
patient, health care provider, or any individual for whom the delivery of an
increased concentration of active agent is desired. Premixed liquid bulk or
unit
dosage forms can also be employed.
Application of the composition having a relatively low concentration of
free water can also be accomplished by providing a lozenge or a form of candy
or other medicated confection, such as a common lollipop, which utilizes a
suitable carbohydrate carrier, such as sucrose or sorbitol, and a gelling or
thickening agent, as needed. Chewing gum can also be used to deliver the
carbohydrate carrier, such as sucrose, xylitol, sorbitol, or corn syrup
solids, and
amino acid. In a preferred form, the chewing gum can incorporate a central
pocket of flavored syrup, composed of the appropriate mixture of carbohydrate
carrier, such as xylose, sorbitol, or sucrose, and an effective amount of the
amino
acid. Formulations for preparation of chewing gum with a soft core portion are
described in U.S. Patent No. 4,352,823 (Cherukuri, et al., Oct. 5, 1982) and
U.S.
Patent No. 4,352,825 (Cherukuri, et al., Oct. 5, 1982). Alternatively, a solid
solution of a biologically active agent can be used in the preparation of
chewing
gum, lozenges, or a candy form such as a lollipop. Such solid solutions can be
formed from comelts, coprecipitates, or by mechanical activation of the
carbohydrate carrier and the biologically active agent.
A toothpaste can also be formed to incorporate a carbohydrate carrier and
active agent. Microencapsulation of ingredients in toothpaste compositions has
been described in U.S. Patent No. 4,348,378 (Kosti, September 7, 1982), U.S.
Patent No. 4.071,614 (Grimm, January 31, 1978), and U.S. Patent No. 3,
957,964 (Grimm, May 18, 1976), which describe the addition of encapsulated
flavorings and anti- plaque ingredients to standard toothpaste preparations.
The composition of the present invention can also be delivered by
suppository to epithelial tissues of the colon and rectum. Methods of
preparation
of suppository formulations are known in the art. One such method has been
described in U.S. Patent No. 4,439, 194 (Harwood, et al., March 27, 1984),
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
13
which describes a water and drug delivery system for suppository use. An
enema preparation can also be formed of a carbohydrate carrier and an amino
acid, incorporating a sufficient amount of water to form an aqueous solution.
A
solid solution of the biologically active agent in the carbohydrate carrier
can also
be administered in a suppository or enema, drawing the aqueous component
from the colon or rectum.
When delivery to the stomach is preferred, a filled capsule can be used.
One such method has been described in U.S. Patent 5,569,466 (Tanner, et al.,
October 29, 1996), which describes the preparation of fill compositions for
soft
elastic gelatin capsules. Enteric coated capsules or tablets, or enteric
coated
microparticles can be employed to deliver the compositions to the upper or
lower
intestines.
The composition can be delivered in ice cream formulations, as well as
frozen confections such as the common popsicle. Frozen formulations can be
especially effective for the treatment of oral and esophageal ulcers, since
they
can combine, for example, both the beneficial effects of glutamine, as well as
the
soothing effects of the cold mixture.
The composition of the present invention has been shown to improve
solubility and cellular absorption of a dietary amino acid, glutamine, into
human
gastrointestinal epithelial cells, as illustrated in the following example.
Example 1.
Evaluation of Cellular Uptake of Glutamine in Combination With Sucrose
and Sorbitol
1. Materials and Methods
Distilled, deionized water (107 ml) was added to 207 grams of a mixture of
sucrose, sorbitol, and glutamine with excipients (Aesgen-14) as listed in
Table 1.
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
14
Table 1. Aesgen-14 (AES-14)
L-glutamine 240.0 Kg 57.94 w%* 50.00%
w/v**
Sucrose 144.0 Kg 34.77 w% 30.00% w/v
Crystalline Sorbitol 13.44 Kg 3.24 w% 2.80% w/v
Glycerin 14.0 Kg 2.92 w% 2.52% w/v
Sodium Phosphate Monobasic (Anhydrous) 2.6 Kg 0.63 w% 0.54% w/v
Avicel Cellulose Gel Type CL-611 874.0 g 0.18 w% 0.17% w/v
Citric Acid (Anhydrous) 280.0 g 0.07 w% 0.06% w/v
Xanthan Gum 230.0 g 0.05 w% 0.04 %o w/v
Carrageenan 230.0 g 0.05 w% 0.04% w/v
Artificial Flavor 230.0 g 0.05 w% 0.04% w/v
Methylparaben 207.0 g 0.04 w%
0.04% w/v
Potassium Sorbate Powder 180.0 g 0.04 w% 0.04% w/v
30% Simethicone Emulsion 115.0 g 0.02 w% 0.02% w/v
* Weight percents are expressed as percent of total weight of dry
ingredients for reconstitution with water in a 240 ml bottle.
** Weight/volume percents are expressed as percent of total volume in
aqueous mixture.
As a control, 200 milliliters of distilled, deionized water was added to 50
grams of L-glutamine (Ajinomoto, Raleigh NC) and mixed by agitation. Both
samples were allowed to stand for 1 day at room temperature. The supernatant
was decanted from the residue and used for the cellular uptake determination.
On Day 1, cells from a human gastrointestinal epithelial cell line (CaCo)
were plated at a density of 0.5 x 106 cells per well in a 6-well tissue
culture dish.
On Day 2, culture media was replaced with either normal growth medium or
medium deficient in L-glutamine.
On Day 3, cells cultured in both normal growth medium ("normal") and
L-glutamine deficient growth medium ("starved") were evaluated for
comparison of glutamine uptake using the Aesgen-14 solution in parallel with
the L-glutamine solution, according to the following protocol: Two milliliters
of
test material (either Aesgen- 14 or L-glutamine solution) was added to the
appropriate wells, then incubated at 37 C. At time points 0, 10, 20, 40, and
60
seconds the test material was aspirated and the cells washed three times (3x)
with
chilled (4 C) phosphate buffered saline (PBS), followed by the addition of 1.0
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
ml of perchloric acid. Cells were harvested by scraping, then aspiration by
pipet
into a 1.7 ml tube.
The harvested cells were sonicated for 10 seconds, and 500 gl of
sonicated cells were transferred into a 1.7 ml tube. The perchloric acid was
5 neutralized by the addition of 130 gl of 2M KHCO3, and the resulting mixture
was frozen overnight at -80 C.
Upon thawing, the sample was centrifuged for 10 minutes at 14,000 rpm
and the supernatants were transferred to new 1.7 ml tubes and frozen at -80 C.
The resulting clarified samples were thawed and diluted 1:3 with deionized
10 water. Fifty microliters were withdrawn, added to 10 microliters complete o-
phthaldialdehyde (Sigma P-0532), and mixed by agitation. After incubation for
two minutes at room temperature, a 20 g1 sample was injected on a Hypersil
C18 Elite 5 gm HPLC column using 70:30 acetonitrile:water as the mobile
phase. Glutamine levels, measured as gg/ml, were detected at 340 nm.
2. Results
Results are shown in Table 2 as gg/ml mean cellular glutamine uptake:
Table 2
Incubation Time (Seconds) 0 10 20 40
Normal cells + Aesgen 14 1.00 1568.55 900.60 1185.88
1765.13
Normal cells + L-glutamine 3.53 10.30 2.48 3.23
25 4.85
Starved cells +Aesgen 14 0.00 613.10 672.93 1213.40
1053.85
Starved cells + L-glutamine 1.33 1.43 1.49 2.23
49.96
As summarized above, glutamine uptake is significantly increased in both
normal cells (363x) and in starved cells (21x) in cells treated with Aesgen-14
as
compared to cells treated with aqueous L-glutamine alone.
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
16
Example 2.
Effect of AES-14 on Drug Uptake and Permeability
The cellular uptake and permeability enhancing effect of a
pharmaceutical vehicle on four model drugs (L-glutamine, L-asparagine,
glycylsarcosine, acyclovir, along with half saturation L-glutamine) across
Caco-
2 cell monolayers were measured in this experiment. Uptake and permeability
of each compound was measured in the apical-to-basolateral direction, with and
without vehicle.
Methods
Materials. Two amino acids (L-glutamine, L-asparagine), a dipeptide
(glycylsarcosine), and a therapeutic agent (and acyclovir) with low
permeability
were studied. Each compound was tritiated. 14C-mannitol was used as an
evaluation of monolayer/cell integrity (i.e. as a low uptake/permeability
marker).
Uptake and Permeability Assessments. Compound cellular uptake into
and permeability across Caco-2 monolayers was measured. Caco-2 monolayers
were grown using a recently developed, rapid culture system, that requires 4
days rather than 21 days. Lentz et al., (2000), Int. J. Pharm., 200(1): 41-51.
Uptake and permeability studies were conducted in duplicate at 37 C and
50 oscillations per min across Caco-2 monolayers in either (a) blank AES-14
(i.e., AES-14 without L-glutamine) or (b) Hank's balanced salt solution (HBSS)
containing 10 mM HEPES buffer (solution pH=6.8). HBSS was used when no
pharmaceutical vehicle was present for each of the four compounds. Blank
AES-1 14 was the matrix for L-asparagine, glycylsarcosine, acyclovir, and
"half-
saturation" L-glutamine studies when a vehicle effect is considered. AES-14,
which contains L-glutamine, was studied for L-glutamine. Monolayer integrity
was monitored using 14C-mannitol permeability. Mannitol uptake was also
studied.
Uptake and permeability studies were conducted using Transwell
inserts in the apical to basolateral direction, at intervals of 10 sec., 60
sec., and 5
min. Donor solution included a nine saturated systems (except half strength L-
glutamine) were the source solutions for the uptake/permeability studies.
Saturated solutions were obtained by utilizing 5.4g L-glutamine/100ml, 1 g L-
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
17
asparagine/lOml, 2g glycylsarcosine/lOml, and 16mg acyclovir/lOml system
concentrations (Kristol, (1999), J. Pharm. Sci., 88: 109-110), wherein excess
solid solute was present to assure saturation:
Saturated solution of L-glutamine in HBSS (5.4g/10Oml)
Saturated solution of L-asparagine in HBSS (lg/lOml)
Saturated solution of glycylsarcosine in HBSS (2g/lOml)
Saturated solution of acyclovir (16mg/1Oml) in HBSS AES-14
Saturated solution of L-asparagine in blank AES- 14(1 g/ I Oml)
Saturated solution of glycylsarcosine in blank AES-14 (16mg/lOml)
2.3g/100ml L-glutamine in blank AES-14 (i.e. half-saturated L-
glutamine)
14C-mannitol and 3H-drugs were quantified by liquid scintillation
counting. For uptake studies, at designated time points (10 sec, 60 sec, and 5
min), the donor solution was aspirated off. The cell monolayer was washed
twice with ice cold HBSS to remove any residual binding and then dissolved in
1
ml of the cell solubilizing agent, Solvable . The cell lysate (0.5ml) was
added
to 5 ml scintillation cocktail (Econosafe ) and counted on liquid
scintillation
counter (Beckman LS5801, Columbia, MD). For permeability studies, 0.5ml of
received solution was added to 5 ml scintillation cocktail (Econosafe ) and
counted on liquid scintillation counter.
Since saturated solutions of unknown concentration of drugs were used,
absolute uptake could not be calculated. Hence, the vehicle effect on uptake
is
considered below (Fig.3-7) in terms of the relative drug uptake into cell
monolayer from vehicle vs non-vehicle (i.e., ratio of uptake, after normalized
for
slight differences in radiolabel tracer).
Permeability (3) in each experiment was calculated (Fig.8-12) using eq 1:
dM
P= /t
A id
where P is permeability, dM/dt is rate of drug mass accumulation (i.e.,
radioactivity) in receiver compartment, A is area, and Cd is donor drug
concentration (i.e., radioactivity). Polli et al., (1998), Pharm. Res., 15: 47-
52.
CA 02617370 2008-02-01
1 i-U/-zuui US0013260
18
Permeability is an absolute measure (units of cm/Sec or velocity) and can be
determined even though the absolute drug concentrations were not known.
Results
Uptake. In Fig_ 3-7, the relative effect of vehicle on -glutamine,
glycylsarcosine, L-asparagine, acyclovir, and L-glutamine (half-strength)
uptake
into cells is shown. If uptake (normalized for slight differences in donor
radiolabel) were identical from each vehicle and HESS, the relative uptake
would be 1Ø For all four drugs and half-strength L-glutamine, the relative
uptake exceeded 1Ø In Fig. 3-6, for L-glutamine, L-asparagine,
glycylsarcosine, and acyclovir, vehicle enhanced cellular drug uptake about
four-
fold- To perhaps a lesser extent, vehicle enhanced half-strength L-glutamine
(Fig. 7).
In Table 2 below, vehicle had no effect on mannitol relative uptake-
These mannitol studies, which were performed simultaneously with those in Fig-
3-7, indicated the vehicle effect differentiates man itol from the other
compounds, in terms of uptake enhancement. Thus, the uptake of the
saccharides per se is apparently not increased, and the term "biologically
active
agent" can be read to exclude the saccharides present in the solution,
dispersion,
or gel.
Table 2. Relative Effect of vehicle on MannitoJ Cellular Uptake
r
Time (sec) L-glutamine Glycyl- L-argine AcybIovir L-glutamine
study sarcosine study study (half-
study strength)
study
5 0.52 0.83 1.65 1.24; 1.20
60 0.80 1.51 0.77 0-851 0.57
300 0.63 1.06 0.43 0.43; 0.30
Permeability. In Fig. 8-12, the relative effect of Aesgen-14 vehicle on L-
glutamine, glycylsarcosine, L-asp aragine, acyclovir, and L-glutamine (half-
strength) permeability is shown. Unlike the uptake data presented above, which
shows the relative vehicle effect on uptake (i_e., the ratio of intake with
vehicle
AMENDED SHEET
Pmnf9nZQ701t I i..lnI i 71:41
CA 02617370 2008-02-01
WO 00/69470 PCTIUSOO/13260
19
vs without vehicle), permeability is an absolute measurement, and is
calculated
for each formulation (no vehicle and with vehicle). Since two-fold variation
in
permeability is within typical experimental variation, these results indicate
that
vehicle had no effect on permeability. Similarly, vehicle had no effect on
mannitol permeability (Table 3).
In Fig. 14 the effect of Aesgen-14 vehicle on L-glutamine uptake into
human fibroblasts (right boxes) vs. uptake of saturated L-glutamine (left
boxes).
Figure 14 depicts the effect of vehicle on L-glutamine absorption into human
endothelial cells. On the chart, the effect of saturated L-glutamine alone was
not
visible.
It should be noted that 5 min. represents a very brief time frame for
traditional Caco-2 permeability studies. It is unlikely that steady-state is
achieved after 5 min., reducing the probability of observing any possible
vehicle
effect.
Summary
L-glutamine, L-asparagine, glycylsarcosine, and acyclovir represent two
amino acids, a peptide, and an anti-viral agent, each with poor passive
membrane
penetration properties under normal physiological conditions. Hence,
enhancement of their cellular uptake and membrane permeability is
advantageous, from a drug delivery perspective. For saturated solutions of L-
glutamine, L-asparagine, glycylsarcosine, and acyclovir, vehicle AES-14
enhanced their cellular drug uptake about four-fold. This enhancement of drug
uptake into cells occurred immediately (i.e., <<1 min), and was sustained over
the time period studies (5 min.). To perhaps a lesser extent, vehicle enhanced
half-saturated L-glutamine. Vehicle had no effect to mannitol uptake.
Regarding permeability over a very brief 5 min. period, vehicle had no effect
for
any compound.
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
Table 3. Caco-2 Permeability of Mannitol
Study Mannitol Permeability Mannitol Permeability
without Vehicle (cm/sec) with Vehicle (cm/sec.)
L-glutamine 3.80x 10"9 9.16x107
5 Glycylsarcosine Below LOQ 1.48x106
L-asparagine 3.80x 10"6 9.48x 10-7
Acyclovir 1.14x 10.6 1 .46x 10.6
L-glutamine (half- 1.49x10"6 Below LOQ
strength)
Method for Treating Mammalian Subjects by Enhancing Amino Acid
Absorption
The composition of the present invention, and its various methods of
delivery, can be used in a method for treating a variety of mammalian,
especially
human, physiologic disorders. The method is most effective for treatment of
disorders involving epithelial tissue, particularly gastrointestinal
epithelium
(including oropharynx, esophagus, stomach, intestines and colon).
The method provides the previously described composition, a
combination of therapeutically effective dosage of a selected amino acid, or a
combination of amino acids, with an effective amount of carbohydrate
carrier(s)
which increase(s) aqueous solubility and cellular absorption of the amino acid
or
amino acids for adn,"i nz Lration to the epithelial tissue of the patient.
The invention is particularly useful for delivery of therapeutic levels of
amino acids which exhibit limited aqueous solubility, such as the dietary
amino
acids tryptophan, tyrosine, glutamine, aspartic acid, asparagine, glutamic
acid,
histidine, isoleucine, leucine, methionine, and phenylalanine. Both D- and L-
amino acids, as well as amino acids such as citrulline, g-aminobutyric acid,
hydroxyproline, and ornithine, for example, can be delivered by the method to
increase cellular absorption.
Carbohydrate carriers useful for the composition administered in the
method of the invention can be chosen from among the sugars, either
monosaccharide or disaccharide, including, for example, D-allose, D-altrose, D-
arabinose, D-erythrose, D-erythrulose, D-fructose, D-galactose, D-glucose, D-
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
21
glyceraldehyde, D-gulose, D-lyxose, D-idose, D-mannose, D-psicose, D-ribose,
D-ribulose, D-sorbose, D-tagatose, D-talose, D-threose, D-xylose, D-xylulose,
maltose, lactose, and sucrose. In some patients or physiological conditions,
as,
for example, when it is important to choose a carbohydrate carrier which will
not
promote tooth decay or cause a sudden increase in blood glucose levels, it may
be preferable to choose a polyol, or sugar alcohol, such as, for example,
sorbitol,
erythritol, maltitol, mannitol, or xylitol.
For children, particularly, a sugar alcohol may be a preferable carrier, and
can produce added benefit beyond the desired therapeutic effect on the target
tissue. For example, xylitol reduces the growth of Streptococcus pneumoniae
and has been shown to have a preventive effect against acute otitis media when
incorporated into chewing gum for children. (Uhari, M., et al., Brit. Med. J.
(1996) 313(7066): 1180-1184.) Use of xylitol as a carbohydrate carrier for
glutamine in a chewing gum formulation used to treat damaged oral or
esophageal epithelial tissue after chemotherapy or bone marrow transplant can,
therefore, also provide a protective benefit against a pathogenic organism.
The method comprises identification of physiologic disorders for which
amino acid supplementation is indicated. More particularly, it provides a
method for delivering increased intracellular amino acid supplementation to
patients who exhibit symptoms of a physiologic disorder for which amino acid
supplementation may be of therapeutic value. Numerous physiologic disorders,
or diseases, have been linked, for example, to defective amino acid metabolism
or defective absorption. In many situations, it is desirable to deliver large
intracellular concentrations of an amino acid. In most situations, it is also
preferable to do so by administering a limited dose of the selected amino acid
or
amino acids. This has not previously been possible, however, since many amino
acids exhibit limited aqueous solubility and intracellular absorption--and
must
therefore be administered in large doses to achieve a desired effect.
Physiological conditions for which amino acids supplementation has been
indicated, and for which the method of the present invention is therefore
beneficial for increasing intracellular delivery of amino acid supplements,
are
described below. These examples are not intended to limit the use of the
method
described herein, but are presented as examples of the wide variety of
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
22
physiologic disorders for which the method of the present invention will be
useful.
Enhancing Amino Acid Absorption for the Treatment of Children and
Adults with Short Bowel Syndrome
Short bowel syndrome is associated with surgical resection of the large
intestine,- and results in decreased surface area for absorption. The tissue
of the
bowel is often irritated, with accompanying symptoms such as cramping and
diarrhea. An amino-acid based complete infant formula has been demonstrated
to be effective in improving feeding tolerance, eliminating the need for
parenteral nutrition, and improving intestinal function in children with
severe
short bowel syndrome. (Bines, J., et al., J. Pediatr. Gastroenterol. Nutr.
(1998)
26(2): 123-128.) The present invention provides a method for increasing
absorption of amino acids, particularly those amino acids which exhibit
limited
aqueous solubility and cellular uptake (e.g., tryptophan, tyrosine, glutamine,
aspartic acid, asparagine, glutamic acid, histidine, isoleucine, leucine,
methionine, and phenylalanine), in both children and adults with short bowel
syndrome. When used for the treatment of patients with short bowel syndrome,
the combination of therapeutically effective concentrations of amino acids and
an effective amount of carbohydrate carrier provide increased levels of
cellular
uptake of amino acids into the intestinal epithelium, thereby providing a
greater
benefit to the patient and decreasing the amounts of amino acids that must be
administered in order to achieve satisfactory therapeutic levels.
The combination of amino acids and carbohydrate carrier can be
administered by a variety of pharmaceutically acceptable routes, including
tablets, caplets, or capsules coated for delivery to the intestines or colon,
as well
as enema solutions or suspensions. Therapeutic dosages can be determined by
the patient's physician, taking into consideration the age, size, and
nutritional
status of the patient.
Enhancing Amino Acid Absorption in Dialysis Patients
Dialysis patients commonly exhibit malnutrition. However,
supplementation with a mixture of 8 essential and 9 nonessential amino acids
has
been shown to improve both health and mood of dialysis patients.
(Mastroiacovo, P., et al., Clin. Ther. (1993) 15(4): 698-704.) In the method
of
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
23
the present invention, a combination of amino acids, in therapeutically
effective
amounts, is combined with an effective amount of a carbohydrate carrier to
enhance solubility and cellular uptake of the amino acids, thereby increasing
the
therapeutic effect of amino acid supplementation and decreasing the dosage of
amino acid required to achieve therapeutic effect.
A preferred mode of administration for dialysis patients is an enteric
coated capsule, caplet, tablet, or coated bead containing a therapeutically
effective amount of each of a variety of amino acids in combination with an
effective amount of a carbohydrate carrier, such as sucrose or a polyol such
as
xylitol or sorbitol. For administration to diabetic patients, the preferred
carbohydrate carrier is a polyol.
Enhanced Absorption of Glutamine for the Treatment of Wounds
Glutamine is precursor for the synthesis of nucleotides. It is both an
activator of protein synthesis, and an inhibitor of protein degradation. It is
an
activator of glycogen synthesis, as well as a metabolic substrate for rapidly
dividing cells. It is also an energy source for epithelial cells. Treatment of
wounds, whether superficial or non-superficial, with the composition described
for enhancing amino acid absorption, increases the absorption of glutamine
into
epithelial tissues, promoting more rapid wound healing. In addition to
promoting wound healing by increasing glutamine absorption, however, the
method provides a treatment which protects the wound from infection with
pathogenic organisms. Filling infected wounds with sugar has been a practice
for centuries. Honey has long been known to have antibacterial properties,
due,
in part, to the hypertonic sugar concentration. (Basson, N. et al., J. Dent.
Assoc.
S. Afr. (1994) 49(7): 339-341; Jeddar, A., et al., S. Afr_ Med. J. (1985)
67(7):
257-258; Willix, D., et al., J. Appl. Bacteriol. (1992) 73(5): 388-394.)
A combination of sugar and povidone-iodine has been effective in
promoting rapid healing, reducing bacterial contamination, and filling of
defects
with granulation tissue when used to treat patients for wounds, bums, and
ulcers.
(Knutson, R., et al., South Med. J. (1981) 74(11: 1329-1335.) However, while
adding to the antibacterial properties of the hypertonic sugar environment,
povidone-iodine kills white blood cells.
CA 02617370 2008-02-01
WO 00/69470 PC1'/US00/13260
24
Combining glutamine with a carbohydrate carrier, therefore, provides a
dual benefit for wound care: the increased glutamine absorbed by the
epithelial
cells provides an energy source for the epithelial cells, promoting cell
division
and healing, while also providing an energy source for the white blood cells
needed to protect the underlying tissues from bacterial invasion, and the
carbohydrate carrier protects the surface of the wound from bacterial
contamination by providing an environment in which the high osmotic pressure
and low water availability prevents microbial growth.
For wound care, the combination of a therapeutically effective amount of
glutamine and a carbohydrate carrier, preferably sucrose or honey, is applied
topically as a semi-solid formulation of a high concentration of sugar mixed
with
water and glutamine. Alternately, the combination is provided as a thick syrup
for topical application to the affected area. Another alternative method of
application is to provide the formulation as a solid to be applied to the
wound
area, drawing its aqueous fraction from the wound environment. Such a
preparation, if provided in powdered or crystalline form, can be easily placed
in
a first-aid kit or other emergency care kit for wound treatment.
The combination can be especially effective for the treatment of burns,
where the primary goals of treatment are protection of the tissue from
infection
and rapid regeneration of new tissue.
Enhancing Glutamine Absorption for the Treatment of Mucositis and
Stomatitis
Mucositis is an inflammatory reaction, characterized by burn-like lesions
or ulcerative lesions of the epithelial tissue of the gastrointestinal tract
from
mouth to anus. It may result from exposure to either ionizing radiation or
chemotherapeutic agents. Stomatitis is any inflammatory reaction affecting the
oral mucosa, with or without accompanying ulceration. Mucositis, particularly,
is often further complicated by infection of the ulcerative tissue.
Studies have previously shown that oral application of glutamine
solutions can improve the symptoms accompanying mucositis in some bone
ma row transplant patients and chemotherapy patients. (Skubitz, K., and P.
Anderson, J. Lab. Clin. Med. (1996) 127(2): 223-228; Anderson, P., et al.,
Bone
Marrow Transplant (1998) 22(4): 339-344; Anderson, P., et at., Cancer (1998)
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
83(7): 1433-1439; U.S. Patent No. 5,545,668 (Skubitz, et al., August 13,
1996);
and U.S. Patent No. 5,438,075 (Skubitz, et al., August 1, 1995.) Using the
composition and method described herein, increased and effective intracellular
glutamine concentrations can be delivered to epithelial tissues of the
5 gastrointestinal system for the treatment of mucositis or stomatitis without
increasing the absolute glutamine dosage.
In the method of the invention, the composition can be provided, for
example, as a mouthwash, swish and swallow preparation, lozenge, or hard
candy for treatment of oral ulcerations. For esophageal ulcers, a drink,
including
10 a sugared drink, a milkshake, or a frozen slurry can be used. Biodegradable
inserts can also be used to treat the mouth and throat. Children, as well as
adults,
with mucositis or stomatitis can be treated using any of these preparations,
but
may prefer a preparation of carbohydrate, glutamine, and flavorings delivered
as
a popsicle or in combination with sherbet, an ice, or ice cream. These methods
15 of delivery provide the added benefit of soothing cold on the ulcerative
tissue. A
chewing gum preparation, preferably a chewing gum with a semi-solid or liquid
center, can also be used for the treatment of oral and esophageal ulcers.
For gastric ulcer therapy, tablets, capsules, capsules, or coated beads
containing the carbohydrate/glutamine composition can be administered. For
20 intestinal ulcerations, coated tablets, caplets, capsules, or coated beads
can be
administered for either enteric or colonic delivery. Methods for providing
enteric coatings or coatings for colonic delivery are known in the art and
have
been described previously herein.
Enhancement of Glutamine Absorption for the Treatment of
25 Cryptosporidiosis
Cryptosporidium parvum is a leading cause of persistent diarrhea in
developing countries. Due to its resistance to chlorine, it has also become a
threat in some United States water supplies. Cryptosporidiosis is particularly
problematic in AIDS patients, the elderly, and the very young, in whom it
causes
a severe, life-threatening diarrhea. Cryptosporidium parvum infects the
intestinal tissue, but does not infect beyond the most superficial surface of
the
intestinal epithelium. In a piglet model, approximately two-thirds of the
intestinal villus surface area was damaged during Cryptosporidia infection. In
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
26
the remaining epithelial tissue, increased glutamine metabolism is associated
with a sodium-hydrogen exchange coupled to a chloride transport mechanism.
Because of its direct association with the chloride transport mechanism,
glutamine can be particularly therapeutic for repair of tissue damaged by
Cryptosporidium infection. (Guerrant, R., Emerging Infectious Diseases (1997)
3(1): 51-57.) Infected tissue has lost much of the absorptive surface area,
however, and the method of the present invention, by treating the patient with
the composition of carbohydrate carrier and a therapeutic dose of glutamine,
enhances glutamine uptake in the remaining cells to compensate for the
decreased absorptive surface area.
The composition can be administered using a coated capsule, tablet, or
caplet for intestinal delivery. Alternately, the composition can be infused or
administered as an enema solution to coat the intestinal lining with the
glutamine/carbohydrate carrier and enhance glutamine absorption into the
remaining intestinal epithelial cells.
The method can also be useful as a factor in disease prevention, since
glutamine is known to provide a primary energy source for white blood cells,
which migrate among the cells of the intestinal lining and are responsible for
destruction of pathogenic organisms such as C. parvum. Enhancement of
glutamine absorption into the epithelial and white blood cells by the method
of
the present invention therefore provides a method for improving the immune
response while maintaining the structural integrity of the epithelial lining
of the
intestine. For patients at risk for Cryptosporidium infection, enteric-coated
capsules can be administered to maintain epithelial cell integrity and improve
the
immune response.
Enhancement of Glutamine Absorption to Improve Post-Surgical Wound
Healing in the Gastrointestinal Tract
Following surgical resection within the oral cavity, the intestine, or
bowel, epithelial tissue damage can be treated by the method of the present
invention to increase tissue integrity and promote wound healing. Following
oral surgery, a swish and swallow preparation, mouthwash, lozenge, candy, or
chewing gum preparation containing the composition of the present invention
can be provided to the patient to allow easy administration of a
therapeutically
CA 02617370 2008-02-01
WO 00/69470 PCT/USOO/13260
27
effective dose of glutamine in combination with a carbohydrate carrier.
Particularly in patients who have undergone oral surgery, non-cariogenic
carbohydrate carriers are preferred. Such sugar carriers include, for example,
maltitol, lactitol, sorbitol, and xylitol. The most preferable polyol
carbohydrate
carrier for incorporation into the composition is xylitol.
Following intestinal surgery, the composition can be administered in the
form of a coated tablet, caplet, capsule, or coated bead. The tablet, caplet,
capsule, or coated bead can be coated with an organic solvent, such as, for
example, cellulose acetate phthalate, cellulose acetate trimellitate,
cellulose
acetate succinate, hydroxypropyl methyl cellulose phthalate, hydroxypropyl
methyl cellulose acetate succinate, and carboxy methyl ethyl cellulose, for
enteric delivery. A tablet, caplet, or capsule can be coated with an acrylic-
based
resin to dissolve at higher pH (pH 7) to provide delivery to the distal ileum
and
colon. Alternatively, delivery of the glutamine/carbohydrate carrier
composition
can be provided in the form of a suppository, using a base such as cocoa
butter or
other glyceride, or as a rectal tablet without a conventional suppository
base.
Such compositions for suppository use have been described by Mizuno, et al.,
in
U.S. Patent No. 4,462, 984, and Harwood, et al., in U.S. Patent No. 4,439,194.
For treatment of diabetic patients, xylitol is the preferred carbohydrate
carrier, as sorbitol is not absorbed in the intestine and could cause added
intestinal discomfort.
Enhancement of Glutamine Absorption for Treatment of Low Birth Weight
Infants
Neu, et al., have reported that very-low-birth-weight neonates who
receive enteral glutamine supplementation have an increased survival rate. (J.
Pediatrics, (1997) 131(5): 691-699.) The method of the present invention
provides increased therapeutic intracellular glutamine dosages with decreased
actual glutamine administration. In low-birth-weight neonates, particularly,
achievement of the desired effect with smaller doses of nutrient can be
essential.
For delivery of the composition, an enteral feeding tube is preferred.
Any one of a number of carbohydrate carriers can be chosen, although sucrose
and high fructose com syrup are preferred. The therapeutic dosage of glutamine
can be determined by the individual physician, using standard means of dosage
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
28
calculation, bearing in mind that glutamine absorption is enhanced by
combination with the carbohydrate carrier to levels of at least ten times
higher
than that achieved by administration of glutamine alone. Excipients can be
added to the feeding formula, including flavorings and stabilizers. Added
nutrients can also be included, including vitamins, amino acids, and
recommended nutrients such as lactoferrin.
Enhancement of Glutamine Absorption to Treat Dermatological Lesions of
Viral and Bacterial Origin
A number of viral illnesses can be recognized by epithelial lesions.
Among these are, for example, herpetic lesions around the mouth, the lesions
associated with impetigo, and the painful lesions known as shingles,
characteristic of varicella-zoster virus. The method of the present invention
can
be used to treat such lesions by topically applying the glutamine/carbohydrate
carrier composition to the affected area. The glutamine component of the
composition aids in healing by providing energy to the epithelial cells, while
the
sugar provides antibacterial properties to protect the damaged or infected
tissue
from further infection.
For topical application, a lotion or cream is preferred, incorporating
glutamine, a carbohydrate carrier, and excipients such as stabilizing agents,
gelling agents, or thickening agents.
Enhancement of Glutamine Absorption to Treat Patients Infected with
Human Immunodeficiency Virus
Gastrointestinal lymphoid tissue harbors more than 90% of the total
lymphocytes in the body. Studies have shown that the gastrointestinal
epithelium contains a large population of CD34+ CD4-progenitors. (Mattapallil,
J., et al., J. Virol. (1999) 73(5): 4518-4523.) The gastrointestinal tract has
also
been demonstrated to be a major site of CD4+ T cell depletion and viral
replication in simian immunodeficiency virus infection. Other studies have
shown that glutamine enhances production of T lymphocyte-derived cytokines.
(Yaqoob, P. and P. Calder, Cytokine (1998) 10(10): 790-794.) Enhancing
glutamine absorption into the intestinal mucosa by the method of the present
invention therefore can provide a therapeutic benefit to HN-infected patients,
particularly those patients who are in the early stages of infection.
Enhancement
CA 02617370 2008-02-01
WO 00/69470 PCTIUSOO/13260
29
of the cytokine response to the viral infection can contribute to viral
destruction
by the immune system at the site of significant viral replication.
The glutamine/carbohydrate carrier composition can be administered in
the form of an enteric-coated tablet, caplet, capsule, or coated bead.
Suitable
sugar carriers will preferably include, for example, sucrose, glucose, high
fructose corn syrup, and xylitol.
Daily administration of recommended dietary levels of glutamine is
preferred, since administration of this quantity of glutamine by the method of
the
present invention can result in an increased delivery of glutamine to the
intestinal
epithelium by a factor of, for example, 10-30x. Therefore, administration of
more moderate amounts can produce an even greater intracellular concentration
of glutamine than has been previously been achieved by administration of
higher
dosages of glutamine alone.
Enhancement of Glutamine Absorption for Cancer Therapy
Glutamine supplementation can be beneficial for cancer therapy for both
its direct and indirect results. Glutamine supplementation has been shown to
increase glutathione release from the gut in Fisher-344 rats. (Cao, Y., et
al., Z
Parenter. Enteral Nutr. (1998) 22(4): 224-227.) When given in conjunction with
either radiation or chemotherapy, glutamine has been demonstrated to increase
selectivity of either therapy for tumor cells. (Klimberg, V. and J. McClellan,
Amy. (1996) 172(5): 418-424.) In one study, tumor growth in rats
receiving glutamine, either by gavage or as a food additive, decreased by 40%
within three weeks. (Fahr, M., et al., J. Parenter. Enteral Nutt. (1994)
18(6):
471-476.) In a separate study, tumor volume loss in rats receiving
methotrexate
was nearly doubled when glutamine was added to the diet. (Klimberg, V., et
al.,
J. Parenter. Enteral Nutr. (1992) 16 (6 Suppl): 83S-87S.) Decreased tumor
growth in glutamine-supplemented rats has been correlated with greater natural
killer cell activity, presumably due to glutathione-mediated suppression of
prostaglandin E2 (PGE2) synthesis. (Klimberg, V., et al., J. Surg. Res. (1996)
63(1): 293-297.)
By providing normal cells with an energy source and a means to
accomplish cellular repair, glutamine supplementation has also been indirectly
associated with increased tolerance to chemotherapeutic agents.
CA 02617370 2008-02-01
WO 00/69470 PCT/US00/13260
The composition and method of the present invention provide increased
glutamine absorption into gastrointestinal epithelial cells. Once absorbed
into
these cells, more glutamine is made available to circulate to other tissues of
the
body. Enhancement of absorption of glutamine also provides a means to
5 increase glutathione production in the intestine. Cancer therapy can
therefore
consist of, or be enhanced by, daily administration of glutamine in admixture
with an amount of carbohydrate carrier, such as, for example, sucrose,
glucose,
xylose, xylitol, high fructose corn syrup or corn syrup solids effective to
increase
glutamine absorption into the gastrointestinal epithelium. The composition and
10 method can be used for both human and veterinary cancer therapy.
Daily doses of glutamine will be determined by the individual patient's
physician, taking into consideration factors which are known by those of skill
in
the art to affect dosage calculation, such as, for example, body size and age.
Recommended daily doses of glutamine for cancer therapy are preferably at
least
15 at the maximum dietary intake of 3-4 grams per day, although lower doses
can
be administered, since the composition and method of the present invention
increase glutamine absorption by at least a factor of ten, and more
preferably,
100.
Other Uses for a Method for Increased Amino Acid Absorption
20 Although the method for treating physiological disorders in patients has
been described primarily in terms of administration of glutamine, the
invention
is not intended to be limited to a method of administering enhanced levels of
glutamine alone. For example, D-serine has been demonstrated to be therapeutic
for the treatment of schizophrenia when administered in conjunction with
25 antipsychotic medications. (Tsai, G., et al., Biol. Psychiatry (1998)
44(11):
1081-1089.) Enhanced absorption of D-serine into the intestinal epithelia
after
oral administration, can, therefore, provide a method for increasing available
D-
serine for systemic circulation. Canavan disease, an autosomal genetic
disorder,
is proposed to benefit from supplementation of dietary aspartic acid. (Baslow,
30 M. And T. Resnik, J. Mol. Ne uroc i. (1997) 9(2): 109-125.) Early detection
of
the disease, therefore, can be accompanied by aspartic acid supplementation by
the method of the present invention to enhance uptake of aspartic acid, an
amino
CA 02617370 2008-02-01
WO 00/69470 PCTIUSOO/13260
31
acid with an aqueous solubility of only 0.778 g/1 00g at 25 C, to protect
against
the progressive degeneration of the brain which is characteristic of the
disease.
These are only two examples of a number of physiologic conditions
which can be therapeutically treated using enhanced amino acid absorption
provided by the method of the present invention. As amino acids are identified
as having therapeutic value, dietary supplementation can be further enhanced
by,
providing the amino acid supplement in combination with a carbohydrate carrier
as described by the method of the invention.
Veterinary Use for Enhanced Amino Acid Absorption into Epithelial Cells
The early-weaned pig develops intestinal atrophy, and glutamine
supplementation has been proposed to prevent intestinal epithelial damage and
provide a benefit in swine production. (Wu, et al., J. Nutr. (1996) 126 (10):
2578-84.) The composition and method of the present invention can be used to
enhance amino acid absorption into those epithelial tissue cells, thereby
decreasing costs associated with amino acid supplementation. The composition
and method are also useful for veterinary treatment of dogs and other mammals
in whom chemotherapy has been initiated. For example, doxorubicin, associated
with gastrointestinal ulcers in human chemotherapy patients, is the
recommended treatment for a number of other mammalian cancers, including
canine hemangiosarcoma. The composition and method of the present invention
provide enhanced amino acid absorption into the damaged epithelium of the
mammalian subject, as well as increasing systemically available amino acid by
increasing absorption into the gastrointestinal epithelium.
Stable Glutamine Preparations for Administration to a Patient
The present invention also describes a composition for providing
glutamine to a patient in a form which has improved aqueous solubility and
stability. In one form, the composition can be provided as a granulated or
powdered drink mix, contained in bulk packaging or packaged as individual
doses. Before administration, the preparation can be constituted with water,
juice, or other liquid to provide for easy administration and increase the
absorption of glutamine into the epithelial tissue. Glutamine can also be
provided in stable form with the sugar carrier as a solid solution in the form
of a
candy or lozenge. The patient can administer the glutamine/carbohydrate
carrier
CA 02617370 2008-02-01
WO 00/69470 PCTIUSOO/13260
32
composition by simply placing the candy or lozenge into his mouth and allowing
it to remain there while the surrounding fluids dissolve it. In this aqueous
environment, the carbohydrate can provide the carrier to facilitate absorption
of
the glutamine into the epithelial cells of the oral cavity, the esophagus, and
the
stomach.
Either the granulated/powdered formulation or the solid solution can also
be administered to the environment of the small intestine or the large
intestine by
adding an enteric coating or an acrylic-based resin as previously described
for
delivery to the distal ileum or colon.
In any of these preparations, glutamine has a stable shelf-life and can be
provided to the patient well in advance of the time of administration. The
preparations can be stored in the clinic or the patient's home for
administration
as needed.
The invention is described with reference to various specific and
preferred embodiments and techniques. However, it should be understood that
many variations and modifications may be made while remaining within its
scope.