Note: Descriptions are shown in the official language in which they were submitted.
CA 02618608 2008-01-15
INTEGRATED HYDROMETALLURGICAL AND PYROMETALLURGICAL
PROCESSING OF BASE-METAL SULPHIDES
Field of the Invention
The present invention relates to the recovery of base metals, in particular
but not
exclusively copper, via integrated hydrometallurgical and pyrometallurgical
processing of
base-metal sulphides, in particular but not exclusively iron-containing base-
metal
sulphides.
The present invention relates more particularly but not exclusively to the
recovery of
copper from iron-containing copper sulphide concentrates in which the copper
is present
as chalcopyrite and/or bornite, by first processing the initial iron-bearing
copper sulphide
concentrate feedstock into three separate fractions by means of froth
flotation or other
beneficiation processes, the fractions being a high-grade concentrate
fraction, a low-grade
concentrate fraction, and tailings fraction. Typically the high- and low-grade
concentrates
will have copper contents greater than about 25%, and less that about 25%,
respectively.
The three separate fractions thereby recovered are individually and
collectively treated in
an integrated hydrometallurgical and pyrometallurgical flowsheet to recover
copper in the
metallic state or another suitable form in such a manner that the overall
copper recovery is
greater than that which would be obtained by direct smelting or roasting of
the single iron-
bearing copper sulphide concentrate.
The present invention relates more particularly but not exclusively to the
recovery of
copper from iron-containing copper sulphide concentrates whereby the high-
grade copper
concentrate recovered by froth flotation or beneficiation of the initial iron-
bearing copper
sulphide concentrate feedstock is further upgraded to yield a product
containing about
50% copper or higher by reaction with a copper sulphate solution.
The present invention relates more particularly but not exclusively to the
recovery of
copper from iron-containing copper sulphide concentrates whereby the copper
sulphate
solution used to further upgrade the high-grade concentrate recovered by froth
flotation or
beneficiation is produced by leaching the low-grade copper concentrate
recovered from
the froth flotation circuit.
CA 02618608 2008-01-15
2
The present invention also relates more particularly but not exclusively to
the combined
recovery of copper from iron-containing copper sulphide concentrates and from
pyrometallurgical smelter slags, dusts and/or fumes by means of an integrated
hydrometallurgical and pyrometallurgical process.
The present invention also relates more particularly but not exclusively to
the separation of
any uranium or other potential byproducts such as cobalt, nickel, and rare
earths present
in the iron-containing copper sulphide concentrate feedstock or in the
flotation tailings.
The present invention also relates more particularly but not exclusively to
the recovery of
precious metals, in particular gold and silver, from iron-containing copper
sulphide
concentrates by means of an integrated hydrometallurgical and
pyrometallurgical process.
The present invention also relates more particularly but not exclusively to
the rejection of
environmentally stable arsenic-containing compounds from iron-containing
copper
sulphide concentrates by means of an integrated hydrometallurgical and
pyrometallurgical
process.
The present invention also relates more particularly but not exclusively to
additional
upgrade of high grade concentrate after reaction with copper sulphate solution
by means of
froth flotation, beneficiation or further treatment of a portion of the
upgraded concentrate
to make copper sulphate solution.
Back2round of the Invention
In this specification, where a document, act or item of knowledge is referred
to or
discussed, this reference or discussion is not an admission that the document,
act or item
of knowledge or any combination thereof was, at the priority date:
(i) part of common general knowledge; or
(ii) known to be relevant to attempt to solve any problem with which this
specification is concerned.
Copper can be recovered from a range of copper-containing ores and
concentrates by both
hydrometallurgical and pyrometallurgical routes. Where practical the copper-
containing
run-of-mine ore is concentrated by means of various physical beneficiation
techniques,
CA 02618608 2008-01-15
3
/ especially froth flotation. Some copper ore treatment plants sacrifice
overall recovery in
order to maximise the copper grade of the flotation concentrate.
The dominant copper-containing mineral in most copper sulphide deposits is
chalcopyrite.
Minor copper-containing minerals include chalcocite, bornite, covellite and
enargite.
Pyrite and to a lesser extent pyrrhotite are typically present as gangue
sulphide minerals.
High-grade copper sulphide-containing concentrates (typically greater than
about 25% Cu)
are commonly treated by pyrometallurgical routes, whereas hydrometallurgical
routes are
generally applied to lower-grade copper concentrates. The economically and
technically
most favourable processing route is also influenced by the presence and
concentration of
minor metals such as zinc and lead, valuable metals such as silver, gold and
platinum, as
well as deleterious metals such as arsenic, present in the feed material.
Hydrometallurgical
processing routes are generally characterised as being complex with many unit
steps and
large circulating loads of copper.
The three dominant pyrometallurgical routes for high-grade copper sulphide
concentrates
are smelting to a matte followed by converting to blister copper, direct to
blister smelting,
and roasting. The efficiency of the smelting technology is determined by,
amongst other
things, the Cu/S ratio and the concentration of slag forming components,
especially iron,
magnesium and silica. Conventional smelting processes are generally not
applicable to
lower grade copper concentrates. Not all of the copper content of the original
feed is
recovered as blister copper, the remaining copper reporting to the slag and to
the smelter
dusts or fumes recovered from the smelter off-gases.
Roasting involves conversion of the copper content to a water-soluble form,
which is
recovered from the roaster calcine by leaching, solvent extraction and
electrowinning.
Roasting is often inefficient because copper-containing insoluble ferrite
phases can form
during the roasting stage.
Many hydrometallurgical processes have been proposed for treating copper-
containing
concentrates, especially chalcopyrite-containing concentrates. This is a
reflection of the
so-called refractory nature of chalcopyrite. The more significant of these
processes
include:
CA 02618608 2008-01-15
4
(a) Oxidative leaching under acidic conditions at elevated temperatures and
pressures;
(b) Oxidative leaching using bacteria;
(c) Oxidative leaching with ferric sulphate at ambient pressure;
(d) Oxidative leaching under alkaline (ammoniacal) conditions at elevated
temperatures and pressures; and
(e) Chloride or chlorine leaching.
Few of the proposed processes have attained full-scale commercial development
for a
number of reasons, including the need for ultrafme grinding, extended
retention times,
problems with the generation and neutralisation/precipitation of high ferric
iron liquors,
difficulties in recovery of any precious metals in the original feedstock, and
the generation
of relatively dilute copper solutions. All of these factors increase both the
capital and
operating costs, especially when applied to relatively low-grade copper
(chalcopyrite)
concentrates. The overall copper recoveries from many of the proposed
processes is often
less than economically acceptable because of incomplete reaction and/or losses
to leach
residues via precipitation and/or absorption processes.
The recovery of copper from chalcopyrite-containing copper concentrate that
also contains
an appreciable uranium content is substantially more complex as it is
necessary to effect
an efficient copper-uranium separation ahead of the recovery of
electrowon/electrorefined
copper. Under most hydrometallurgical processing conditions it is difficult to
achieve
selective leaching of copper over uranium or uranium over copper, so that most
hydrometallurgical circuits yield a pregnant copper-uranium solution.
Separation of the
soluble copper from the uranium requires the installation and operation of a
complex
solvent extraction circuit that results in increased capital and operating
costs. Processing of
the copper-uranium ore produced at the Olympic Dam project is a typical
example of such
a complex flowsheet.
As an alternative to direct hydrometallurgical treatment of a copper
concentrate, various
means of chemically upgrading the copper content of the feed have been
proposed. The
upgraded copper-containing intermediate product may be then further processed
by known
CA 02618608 2008-01-15
pyrometallurgical and hydrometallurgical technologies. One such approach
involves the
so-called metathesis process in which the chalcopyrite component of the
concentrate is
reacted with a copper sulphate solution to produce low-iron copper sulphide
(e.g. digenite)
and an acidic ferrous sulphate solution.
5 3CuFeS2 + 6CuSO4 + 4H20 -* 5Cu1.8S + 3FeSO4 + 4H2SO4 (1)
A similar reaction also occurs for any bomite present in the copper
concentrate.
3Cu5FeS4 + 6CuSO4 + 4H20 ' 5CuI.8S + 6Cu2S + 3FeSO4 + 4H2S04 (2)
Similar reactions occur representing the recovery of arsenic and iron from
enargite, as well
as cobalt and nickel from a variety of minerals that may coexist with the
predominant
copper minerals, for example cobaltite and carrolite, etc.
One or both of these reactions (1) and (2) constitute one aspect of US Patent
Nos 2 568
963, 2 662 009, 2 744 172 and 4 024 218, Canadian Patent No 1 258 181, and
WIPO
Patent Publication No WO 2004/106561. All of these patents propose to forward
the
upgraded copper sulphide concentrate, which typically contains about 50% Cu,
to either a
smelter or further processed by other means. The claimed flowsheets are
deficient in a
number of aspects in that, for example, they do not disclose how the copper
sulphate
leachant is generated, involve additional flotation steps, require the
addition of a reductant
to facilitate the process metallurgy, or achieve incomplete conversion
(metathesis) to
ensure that the resultant discarded acidic ferrous sulphate solution has an
acceptably low
copper content.
The present invention overcomes these deficiencies and is capable of
maximising overall
copper recovery more particularly but not exclusively from all flotation
concentrates and
tailings, or smelter dusts, fumes or slags, and together where appropriate an
efficient
copper-uranium separation from the concentrate, while at the same time
minimising the
capital and operating cost components of the total processing flowsheet.
The objects and advantages of the invention described in the present
specification are to be
read disjunctively with the object of at least providing the public with a
useful alternative.
Summary of the Invention
CA 02618608 2008-01-15
6
In one aspect, the present invention provides a method for the recovery from a
metal value
bearing concentrate of one or more metal values including at least a primary
metal value,
the method using an integrated hydrometallurgical and pyrometallurgical
process and
including the steps of:
i. perfonning a separation grading step by subjecting the metal-bearing
concentrate to one or more beneficiation steps to enable the formation of at
least
three separate metal-bearing fractions including a high-grade metal
concentrate
fraction, a low-grade metal concentrate fraction, and a tailing fraction;
ii. subjecting the low-grade metal concentrate fraction to an acidic leaching
process to enable formation of an acidic upgrading solution containing a
solubilised form of the primary metal value;
iii. subjecting the high-grade metal concentrate fraction to a metal value
upgrading step via reaction with at least a portion of the acidic upgrading
solution
formed in step (ii) to produce an upgraded metal concentrate fraction having a
higher w/w concentration of the primary metal value; and
iv. subjecting the upgraded metal concentrate fraction derived from step (iii)
to
a smelting process to obtain at least the primary metal value.
The primary metal value present in the metal value-bearing concentrate may
include
various metals including copper, zinc or nickel. Preferably, the primary metal
value
present in the metal value-bearing concentrate is copper. Preferably, the
acidic upgrading
solution in step (ii) is an acidic copper sulphate solution.
The one or more beneficiation steps of step (i) can include milling, gravity,
magnetic
and/or flotation processes.
Preferably, the tailing fraction obtained in step (i) can be subjected to an
acidic leaching
process to form an acidic solution for use in the processes of step (ii) or
step (iii), and/or to
further hydrometallurgical treatment to recover any additional soluble metal
values that
may be present in the tailing fraction.
In a preferred embodiment, the smelter flue dust or slag derived from the
smelting process
of step (iv) is further subjected to hydrometallurgical treatment with an acid
solution to
CA 02618608 2008-01-15
7
form an underflow stream containing insoluble components and an overflow
stream
containing soluble forms of the primary metal value, wherein the underflow
stream is
recycled for use in step (iii) or step (iv) and/or the overflow stream is
recycled for
processing with the low-grade metal concentrate fraction or tailing fraction
for facilitating
recovery of any contained metal value. Preferably, the further step of
treating the smelter
flue dust or slag is a one or two stage process, whereby in the second stage
the underflow
product formed is further treated, prior to use in step (iii) or step (iv), by
reaction with a
stronger acid solution to facilitate further removal of any ferric iron or
other leachable
impurities that may be present. Preferably, the underflow stream is also
treated through
regrinding and/or flotation processes to provide a concentrate feed for use in
step (iii) or
step (iv) for combination with the high-grade metal concentrate fraction.
Preferably, the acidic leaching process of step (ii) includes a pressure
oxidation circuit
where an oxidative leach reaction is continued until substantially all of the
primary metal
value present in the low-grade metal concentrate fraction is dissolved in the
acidic
upgrading solution and the ferric ion concentration of the acidic upgrading
solution is
below about 10 g/L. More preferably, any leach residues formed in the pressure
oxidation
circuit are further processed or recycled with the tailing fraction.
The metal value-bearing concentrate can also include a precious metal value,
typically
gold and silver, wherein any leach residues formed in any of the
hydrometallurgical and
pyrometallurgical steps are further processed for recovering the precious
metal value.
More preferably, the further process for recovering the precious metal value
is via cyanide
leaching.
Preferably, the metal value-bearing concentrate is an ore sluny containing at
least one of
the minerals selected from the group consisting of covellite, chalcocite,
chalcopyrite,
bornite and enargite.
In one preferred embodiment, the high-grade metal concentrate fraction
includes greater
than 20-35% w/w of the primary metal value and the low-grade metal concentrate
fraction
includes less than 20-35% w/w of the primary metal value.
CA 02618608 2008-01-15
8
The separated high-grade metal concentrate fraction and/or low-grade metal
concentrate
fraction from step (i) can also be subjected to further processing including
grinding,
flotation, filtering, dilution, thickening, washing and/or other cleaning
processes.
Preferably, prior to step (iii) or step (iv), the difference between the
concentration of the
primary metal value in the high-grade metal concentrate fraction compared to
that in the
low-grade metal concentrate fraction is increased through further processing
to facilitate
the recovery of the primary metal value.
In one embodiment of the invention, the hydrometallurgical upgrading step
(iii) includes
the use of a single or multi-comparlrnented autoclave or digestor to produce
an upgraded
metal concentrate fraction having an increased primary metal value
concentration.
A source of sulphur dioxide can also be obtained from off-gases of the
smelting process of
step (iv) and used in the hydrometallurgical upgrading step (iii) to
facilitate reduction of
any ferric ions that may be present.
In another preferred embodiment, the primary metal value concentration in the
upgraded
metal concentrate fraction is increased to a concentration of greater than
above about 45-
50% w/w.
Prior to the smelting process step (iv), the upgraded metal concentrate
fraction obtained in
step (iii) can be further processed by at least one of washing, flotation,
dewatering, or
drying and mixing with fluxes, and/or subjected to further separation grading
to form a
further upgraded feed for the smelting process step (iv) and a secondary
recycle feed for
step (iii).
In another embodiment of the invention, secondary or precious metal values
such as
uranium, cobalt, nickel, gold and silver, are recovered, typically during the
smelting
process, when present in the metal value bearing concentrate or any one of the
tailings
fraction, low grade metal concentrate fraction or high grade metal concentrate
fraction.
Furthermore, any arsenic present in the metal value bearing concentrate can be
transformed into an environmentally stable form acceptable for further
storage.
For convenience, the following summary of the invention as well as the
detailed
description of the invention is specifically directed to the recovery of
copper from
CA 02618608 2008-01-15
9
chalcopyrite, bomite and/or enargite containing concentrates through the
application of the
integrated hydrometallurgical and pyrometallurgical process. Those skilled in
the art will
appreciate that the same processing principles can be applied to other base-
and precious-
metal containing feedstocks and as such the inventors include within the scope
of the
present invention the application of such feedstocks.
Those skilled in the art will also appreciate that efficient operation of the
integrated
hydrometallurgical and pyrometallurgical flowsheet depends on strict control
of water,
mass and heat balances. As such, where process streams are split ahead of
forwarding to
both downstream and upstream unit stages, the proportions of such splits or
fractions is
determined by meeting these balance requirements and will be determined, in
part, by the
chemical, physical and mineralogical properties of each process stream.
Furthermore, in one particular aspect the present invention provides a novel
flowsheet first
involving treatment of ore sluny, such as typical run-of-mine ore slurry,
after any
necessary and appropriate comminuting stage or stages, to a conventional froth
flotation
step to form three streams or fractions: a high-grade copper concentrate
fraction (typically
greater than about 25% Cu), a low-grade copper concentrate fra.ction
(typically less than
about 25% Cu), and a tailings fraction. Each of these streams or fractions is
then
subjected to further processing by means of the integrated hydrometallurgical
and
pyrometallurgical flowsheet.
If appropriate, the separate high- and low-grade copper concentrates produced
from the
original copper-containing feedstock can be reground and refloated, filtered,
washed or
cleaned by other known methods prior to treatment by the integrated
hydrometallurgical
and pyrometallurgical processing flowsheet if the mineral separation is
improved by a
finer size distribution or deleterious water-soluble chemical species, eg.,
halides, can be
conveniently separated from the concentrate.
The mass and copper split between the high- and low-grade copper concentrate
fractions
can be adjusted to optimise recovery of copper for upgrading and smelting, and
restrict the
deportment of iron minerals, particularly pyrite and pyrrhotite, to the high-
grade stream.
In one embodiment, the high-grade concentrate fraction can be reacted with a
copper rich,
low ferric ion solution in a single or multi-compartmented agitated autoclave
or unagitated
CA 02618608 2008-01-15
digestor to produce an upgraded copper concentrate, typically containing above
about 45-
50% Cu, according to reactions (1) and (2). The upgraded copper concentrate
can be
washed, dewatered, dried and mixed with fluxes before smelting by known
methods, to
produce blister copper and copper-containing smelter slag and smelter dust by-
products.
5 The copper rich, low ferric ion solution can be obtained by oxidative
leaching of, for
example, the low-grade concentrate, or optionally supplemented with overflow
from the
smelter dust leach circuit.
The low-grade concentrate slurry can be leached in a single or multi-
comparhnented
agitated autoclave in the presence of sparged oxygen or air, ferric ions and
sulphuric acid
10 or optionally supplemented with overflow from the smelter dust leach
circuit. The
reaction conditions can be adjusted for temperature, copper and ferric ions in
solution.
The sluny pulp density can be adjusted to suit the reactivity and size
distribution of the
low-grade concentrate, and to control the thermal and water balance and/or
free acid
concentration across the leaching processes. Preferably, the oxidative leach
reaction is
continued in one or more compartments with or without interstage thickening
until
essentially all of the copper minerals contained in the low-grade concentrate
are dissolved
into the pregnant leach solution while the ferric ion concentration is
controlled to below
about 10 g/L. The product from the low-grade concentrate leach circuit can
then be
treated by suitable means, such as a conventional or pressure thickener, for
solid/liquid
separation purposes. The thickener underflow or overflow can be flashed cooled
to
release heat from the circuit. The copper-rich liquor overflow can then be
forwarded as
the soluble copper source for the high-grade concentrate leach or transferred
under
pressure to preserve the heat content for downstream processing. In this
preferred
embodiment, the copper-depleted sluny (underflow) is then forwarded to the
tailings
circuit.
The flotation tailings can be thickened to manage the water balance in the
milling,
flotation and tailings leach circuits, with the underflow forwarded optionally
to a leach
circuit where it is reacted with a ferric rich solution derived from the low-
grade
concentrate leach circuit supplemented with additional sulphuric acid and
optionally, with
the overflow from a separate smelter dust leach circuit, in order to promote
the leaching of
the residual copper and any impurity metals contained in the flotation
tailings. The
CA 02618608 2008-01-15
11
tailings leach temperature and retention time can be adjusted to suit the
reactivity of the
copper and impurity metals contained in the flotation tailings. In this
regard, flash steam
from the oxidative leach can optionally be employed to provide additional heat
to the
tailings leach. The slurry exiting the flotation tailings leach circuit can
then be subjected to
solid/liquid separation by suitable means, such as a conventional CCD
thickener. The
leach residue, as the CCD underflow, can be disposed of in a suitable residue
storage
facility. The pregnant leach solution, as the CCD overflow, is processed by
known
methods for copper recovery or further purified by separation and recovery of
uranium or
other metals as by-products.
The optional leaching of the smelter dusts can be operated using flexible
operating
conditions in order to maximise impurity removal while at the same time
assisting with the
overall water balance across the high-grade and low-grade concentrate leach
circuits. The
smelter dust leach circuit can be carried out in a single stage, or
alternatively in two
separate stages. In the latter configuration, the first stage involves
reaction with a
relatively low acid leachant to dissolve the easily soluble copper component.
This is
recovered by means of a suitable solid/separation stage, such as a
conventional CCD
thickener. The pregnant solution, as the CCD overflow can then be forwarded to
the low-
grade concentrate leach circuit. The first stage leach residue, as the CCD
underflow, is
forwarded to the second leach stage where it is reacted with a stronger acid
solution to
increase ferric iron removal from the smelter dust residue as well as dissolve
a greater
proportion of the impurities in the original smelter dust. In this preferred
embodiment,
after solid/liquid separation by suitable means, such as a conventional CCD
thickener, the
CCD overflow is forwarded to the flotation tailings leach circuit, with the
residue, as the
CCD underflow, which contains any insoluble (refractory) copper, is
transferred to the
high-grade concentrate upgrading step.
The optional treatment of the smelter slag can also involve a combination of
regrinding
and flotation stages to recover a concentrate rich in copper that can be
combined with the
high-grade concentrate fraction or upgrading feed. This additional copper-
containing feed
assists with the reduction of any ferric iron present in the high-grade
concentrate
upgrading circuit. Alternatively, a side stream of sulphur dioxide from the
smelter off-
CA 02618608 2008-01-15
12
gases can be injected into the high-grade concentrate upgrading circuit to
promote the
ferric ion reduction reactions.
An advantage of the present invention is that the overall recovery of copper
from the
original copper-containing feedstock is maximised. An additional advantage is
that
variable ore blends, concentrate mixtures, or diverse sources of copper ore
may be
economically treated without the need to dispose of any untreated component of
the
original feedstock.
Another advantage of the present integrated hydrometallurgical and
pyrometallurgical
process flowsheet is the ready transfer of solution products and energy from
circuit to
circuit so that the chemical efficiency and hence economics of the whole
integrated
process is improved.
The integrated hydrometallurgical and pyrometallurgical process flowsheet also
facilitates
the optimum recovery of by-products including uranium, cobalt, nickel and
precious
metals, particularly gold and/or silver, contained in the original feedstock.
The integrated hydrometallurgical and pyrometallurgical process flowsheet also
facilitates
the transformation of any arsenic contained in the original feedstock into a
form that is
environmentally benign and can be safely discharged for disposal in a
conventional
tailings/residue storage facility.
Brief Description of the Drawin2s
The invention is now described by way of example, with reference to the
accompanying
drawings, in which:
Figure 1 shows an overall flowsheet of one embodiment of the present invention
covering
an integrated hydrometallurgical and pyrometallurgical process for the
recovery of a
primary metal value from a base metal concentrate; and
Figure 2 shows an overall flowsheet of another embodiment of the present
invention
covering an integrated hydrometallurgical and pyrometallurgical process for
the recovery
of a primary metal value from a base metal concentrate.
Detailed Description of the Invention
CA 02618608 2008-01-15
13
The following detailed description of a preferred embodiment of the present
invention
refers to the overall process flowsheet outlined in Figure 1. This particular
preferred
embodiment incorporates the optional treatment of the smelter dusts as well as
the smelter
slag in order to maximise the total recovery of the copper content of the
original run-of-
mine ore.
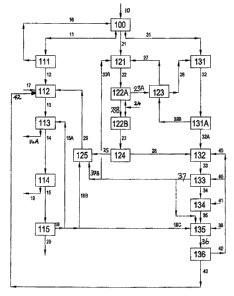
Slurry from a run-of-mine copper ore grinding circuit [10] is forwarded to a
froth flotation
circuit [100] where it is separated into three streams by known flotation
methods: A high-
grade concentrate [31 ], a low-grade concentrate [21 ] and tailings [ 11 ].
Typically the high-
grade and low-grade concentrates will have copper contents of greater than
about 25% and
less than about 25%, respectively. The high-grade and low-grade concentrates
can be
reground and further treated by known flotation methods to enhance the copper
split
between the concentrate streams and/or influence the extent of reaction in the
downstream
processes. The concentrate streams may also be washed and dewatered to remove
soluble
impurities, such as halides, before further treatment. All of the copper from
the run-of-
mine slurry exiting the grinding circuit [10] reports to one of the three
product streams
derived from the flotation circuit [100] and will report for treatment in the
overall
integrated hydrometallurgical and pyrometallurgical flowsheet. By this means,
the
recovery of copper from the initial run-of-mine ore is enhanced.
Typically the high-grade concentrate [31] will contain the bulk of the simple
copper
sulphide minerals (covellite and chalcocite) and well as the bulk of the iron-
containing
copper sulphide minerals (chalcopyrite and bomite). Typically the low-grade
concentrate
[21] will contain most of the remaining copper sulphide and iron-copper
sulphide minerals
with a minor portion of the iron sulphide minerals (pyrite and pyrrhotite).
Typically the
tailings [11] will contain the remaining iron sulphide minerals, gangue
minerals (ground
rock) and oxide minerals of impurity metals. Other copper and value metal
minerals may
be present in concentrates [21 ] and [31 ].
The high-grade concentrate [31] is dewatered and forwarded to an appropriate
repulp tank
[ 131 ] using copper sulphate solution [26] after which any uranium leached
can be
recovered via thickener/filter [ 131 A] where it is partially dewatered and/or
washed in
order to maintain the water balance around the high-grade circuit. The copper
sulphate
solution [26] overflows from the thickener treating the slurry exiting the
primary oxidative
CA 02618608 2008-01-15
14
leach reactor (autoclave) first compartment [ 122A] flash cooling thickening
circuit [ 123]
to the repulp tank [131]. The repulped high grade concentrate [32A] is
typically at the
ambient pressure boiling point and is forwarded to a medium temperature
reactor [132],
typically operating at 110-190 C and more typically at 140-180 C, to which is
added the
overflow liquor [28] from the thickener [ 124] treating the sluny exiting the
primary
oxidative leach reactor (autoclave) last compartment [ 122B] and a portion of
the smelter
dust leach residue [40] exiting the smelter dust leach thickener [136]. The
overflow liquor
temperature from the thickener is typically 200-210 C or about 100 C from
atmospheric
thickening.
The so-called metathesis reactions (1) and (2) proceed within the upgrading
reactor
(autoclave or digestor) [ 132]. The reaction rate will vary with the size
distribution,
temperature and mineralogy of the high-grade concentrate [31 ] and the reactor
process
conditions. Copper in solution replaces iron in the iron-containing copper
sulphide
minerals by displacement to raise the copper content of the upgraded
concentrate [33],
typically to above about 50%.
The upgraded concentrate [33] and the remaining portion of the smelter dust
leach residue
[40] are subjected to washing, dewatering and drying steps [133] using known
processes,
or may be further treated as shown in Figure 2 by flotation or other known
separation
methods to split the upgraded concentrate into streams of different grades for
oxidative
leaching [33A] and smelting [33B]. The hot upgraded concentrate dewatering
liquor
[37A,37B] is recycled to the low-grade concentrate and tailings circuits. The
dried
upgraded concentrate [34] is combined with suitable fluxes [41] and fed to a
conventional
copper smelter [134]. The smelter output (blister copper) is forwarded to a
conventional
copper anode casting/electrorefining circuit. Any precious metals (gold and
silver)
initially present in the high-grade concentrate report with the upgraded
concentrate and
can be recovered from the anode slimes produced in the copper electrorefining
circuit. The
smelter off-gases are captured, cleaned and converted to sulphuric acid in a
contact acid
plant. Electrolyte bleed from the refinery can alternatively supplement the
smelter dust
leach solution, repulp low-grade or high-grade concentrate, or other circuit
balance
purposes depending on the water, copper or acid flows.
CA 02618608 2008-01-15
The dust [35] recovered from the smelter off-gases is slurried with a portion
of the acidic
raffinate [ 18C] exiting the solvent extraction circuit [ 115] used to recover
soluble copper
from the tailings [ 11 ] treatment circuit, or another suitable barren liquor
or bleed
electrolyte, together with a suitable amount of sulphuric acid [38] derived
from the acid
5 contact plant or from some other source. The dust leach [ 135] can be
carried out in one
stage, or in two counter current stages to conserve acid consumption and
control
undesirable impurity dissolution.
The leached smelter dust [36] is partially dewatered in a conventional
thickener [136].
Dust leach thickener overflow [42] carries soluble impurities to the low grade
leach or
10 tailings leach process [ 112] depending on the copper balance. Dust leach
thickener
underflow [40] is returned upstream to the upgrading reactor (autoclave or
digestor) [132]
and/or the upgraded concentrate washing/filtering/drying circuit [ 133] and
forms part of
the feed [34] for the flash smelting circuit [134]. By this means the
refractory copper
content of the smelter dust will ultimately be recovered during the
smelting/refining steps.
15 The low-grade concentrate [21 ] produced in the run-of-mine ore
beneficiation circuit [ 100]
is dewatered in a conventional thickener or other suitable means to maintain
the water
balance through the low-grade leach circuit. It is then repulped [ 121 ] with
primary
oxidation reactor (autoclave) first compartment flash cooled thickener
underflow slurry
[27] and/or upgraded concentrated dewatering liquor [37A] and/or downgraded
concentrate [33A] to form the primary oxidation feed [22]. The primary
oxidation feed
slurry [22] is oxidized in a compartmented agitated autoclave [122] into which
oxygen
and/or oxygen-enriched air [24] is injected. The first compartment of the
primary
oxidation autoclave [ 122A] is sized to accommodate about 80-95% of the
oxidation
reactions and is typically operated at about 200-220 C with and oxygen partial
pressure of
about 600 kPa. The operating temperature of the first compartment of the
primary
oxidation autoclave [ 122A] is maintained by means of a flash
cooling/thickener recycle
cooling circuit [ 123]. The primary oxidation first compartment partly
discharges [23A] to
the flash cooling thickener step [123] and permits thermal stability while
operating the
oxidative autoclave at high concentrate sluny densities. The remaining partial
oxidized
slurry from the first compartment [23B] flows to the downstream compartments
of the
primary oxidation autoclave [122B]. The ferrous iron in [37A] is oxidised in
the autoclave
CA 02618608 2008-01-15
16
ultimately to basic ferric sulphate but not without participating in the
oxidation of the low-
grade concentrate. The return of [37A] to [121] ensures that any un-
precipitated copper
from the liquors in [ 132] is lock-cycled within the combined oxidation and
upgrade
autoclave circuits. Only small quantities of soluble copper exit the
concentrate oxidation
and upgrade circuits in liquor streams [25] and [37B].
The primary oxidation discharge sluny [23] exiting the last comparhnent of the
primary
oxidation autoclave [122B] is separated by suitable means such as a pressure
thickener or
flashed to atmospheric pressure prior to conventional thickening[ 124]. The
overflow
stream [28] consists of an acidic copper sulphate solution and is forwarded to
the
upgrading autoclave [ 132] as the copper-rich solution or leachant (reactant)
for the
metathesis reactions that take place in the upgrading autoclave [132].
The primary oxidation product thickener underflow [25] is forwarded to the
ferric leach
circuit [ 125] for further dissolution of iron. The ferric iron content of the
thickener
underflow [25] is leached with the required portion [37B] exiting the
upgrading thickener
[ 133] overflow and the required portion [ 18B] of the acidic raffinate
exiting the solvent
extraction circuit [ 115] used to recover soluble copper from the last stage
of the tailings
retreatment circuit. The acidic femc solution [29] generated in the ferric
leach circuit
[125] is forwarded to the tailings leach stage [112] where it is used to
dissolve the
secondary copper sulphide-containing phases present in the flotation tailings.
The flotation tailings [ 11 ] derived from the run-of-mine ore beneficiation
circuit [ 100] are
thickened [ 111 ] in order to maintain the water balance in the milling and
flotation circuits
[100]. The thickener overflow [16] returns to the upstream circuits [100] or
to storage
(not shown) before re-use. The thickener underflow [ 12] is leached at
atmospheric
pressure in a suitable agitated reactor [ 112] with sulphuric acid [ 17],
derived from the
contact acid plant used to treat the smelter off-gases, and supplemented by
the ferric rich
liquor [29] derived from the ferric leach circuit [ 125] that treats the
primary oxidation
thickener underflow [25]. Any copper-containing phases together with uranium
and other
soluble impurity minerals present in the tailings underflow will be dissolved
by the acidic
ferric leachant. Direct steam or flash steam from the autoclave can be
injected into the
tailings leach tanks to raise the slurry temperature to increase the rate of
reaction and
maximise the extent of leaching the copper and uranium contents.
CA 02618608 2008-01-15
17
The leached slurry [13] is subjected to CCD and washing stages [113] using
recycled
raffinate [ 1 8A], with the CCD underflow [ 14A] being discharged as fmal
residue to a
suitable tailings impoundment. The pregnant CCD overflow [ 14] is clarified by
known
means (not shown) before the soluble copper is recovered by known solvent
extraction and
electrowinning stages [ 114]. The barren liquor [ 15] is further processed [
115] to recover
uranium, impurity and/or by-product metals by solvent extraction,
precipitation or other
known methods. The barren raffinate solution [ 18] is distributed to wash and
leach
applications [ 18A,18B,18C] in the three process circuits, or may be stored
for future use.
Example 1
A copper-iron sulphide concentrate containing about 34.5% copper and 1.9% iron
and
consisting predominantly of chalcopyrite and having a size range of about 80%
passing 75
micron was subjected to pressure oxidation at 225 C using a pulp density of
20% solids
(w/w). More than 99% of the contained copper was leached after 120 minutes.
A second portion of the same concentrate was ground to 80% passing 23 micron
and was
reacted at a pulp density of 40% solids (w/w) at 180 C with a copper sulphate
solution
containing 90 g/L of copper derived from the above pressure oxidation test. No
reagents
or chemicals were added to the upgrading step. Copper in solution dropped to
less than 1
g/L after 15 minutes, and the copper content of the concentrate upgraded to
above 45%
w/w while the iron content was lowered to about 14% w/w.
Example 2
The same copper-iron sulphide concentrate described in Example 1 was subjected
to
pressure oxidation at 210 C and a pulp density of 25% solids (w/w) for a
period of 180
minutes. Copper extraction was in excess of 97%.
Another portion of the same concentrate was reacted at a pulp density of 21.5
% solids
(w/w) at 180 C with a copper sulphate solution containing 82 g/L of copper
derived from
the above pressure oxidation step. No reagents or chemical were added to the
upgrading
stage. Copper in solution dropped to less than 6 g/L after 180 minutes, and
the copper
content of the concentrate upgraded to above 55% w/w while the iron content
was lowered
to below 9% w/w.
CA 02618608 2008-01-15
18
In the preceding description of the invention and in the claims which follow,
except where
the context requires otherwise due to express language or necessary
implications, the
words "comprise" or variations such as "comprises" or "comprising" are used in
an
inclusive sense, ie., specify the presence of the stated features, but not to
preclude the
presence or addition of further features in various embodiments of the
invention.
It is to be understood that this invention and the preferred embodiments are
not limited to
the particular materials described, as these may vary. It is also to be
understood that the
terminology used herein is for the purpose of describing particular
embodiments only, and
is not intended to limit the scope of the present invention in any way.
It is also to be noted that, as used herein, the singular forms of "a", "an"
and "the" include
the plural unless the contact clearly requires otherwise. Unless defined
otherwise, all
technical and scientific terms herein have the same meanings as commonly
understood by
one of ordinary skill in the art to which the invention belongs.