Note: Descriptions are shown in the official language in which they were submitted.
~ CA 02618888 2008-02-06
Printed: 23-11-2007 ~ ~P~7 Oti 1Z'Pt ~
Europaisches P~ DESCPAMD PJ~ PCT/EP 2006/007 949
n~qn~tstelle Berlm
0 4. MAI 1001 deaa, C.a
pjl
Ani..
Acyltryptophanols for Fertility Control
The present patent application relates to novel acyltryptophanols, process for
their
preparation, pharmaceutical compositions comprising the compounds according to
the
invention, and the use thereof for fertility control in men or in women.
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are together
responsible for the control of male and female fertility and of the production
of sex
steroids.
In the female mammal, FSH controls the early ripening of ovarian primary
follicles and
the biosynthesis of sex steroids. In the advanced stage of differentiation
(preantral
follicles), the influence of LH becomes increasingly important for further
development of
the follicles until ovulation occurs.
In male mammals, FSH is primarily responsible for the differentiation and
stimulation of -
Sertoli cells. Their function consists of assisting spermatogenesis on many
levels. LH is
primarily responsible for stimulating the Leydig cells and thus androgen
production.
FSH, LH and TSH (thyrotropic hormone) together form the group of glycoprotein
hormones which are formed in the pituitary and are secreted from there.
Whereas the
alpha subunit is common to the three hormones, their specificity of action is
determined
by the beta chain which is unique in each case. The molecular weight of FSH
including
the sugar portion is about 30 kD.
FSH and the other glycoprotein hormones act specifically via their selectively
expressed
G protein-coupled receptor (GPCR). FSH stimulates, through binding to its
receptor, the
association thereof with a stimulating G protein (Gg) which is thereby
stimulated to
hydrolyse guanosine triphosphate (GTP) and to activate the membrane-associated
adenylate cyclase. Cyclic adenosine monophosphate (cAMP) is accordingly an
important and readily quantifiable secondary messenger substance of FSH (G.
Vassart,
L. Pardo, S. Costagliola, Trends Biochem. Sci. 2004, 29, 119-126).
The importance of FSH for male fertility is the subject of intensive research.
It has been
possible to show that FSH influences several processes of spermatogenesis such
as
the proliferation of spermatogonia, the antiapoptotic effect on spermatogonia
and
spermatocytes and the stimulation of sperm maturation including motility
thereof.
The following arguments are also in favour of the FSH receptor as target for
male
fertility control:
1/4 AMENDED SHEET 04-05-20~67
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
2
1. The FSH receptor is exclusively expressed on Sertoli cells (high
specificity).
2. Contraceptive vaccination against FSH beta chain or the FSH receptor
induces
infertility in male primates (N. R. Mougdal, M. Jeyakumar, H. N.
Krishnamurthy, S.
Sridhar, H. Krishnamurthy, F. Martin, Human Reproduction Update 1997, 3, 335-
346).
3. Naturally occurring mutations in the FSH receptor or the FSH beta chain may
lead
to sub- or infertility in men (I. Huhtaniemi, Journal of Reproduction and
Fertility
2000, 119, 173-186; L. C. Layman, P. G. McDonough, Molecular and Cellular
Endocrinology 2000, 161, 9-17).
4. Neutralizing FSH antiserum has no effect on testis weight and testosterone
production (V. Sriraman, A. J. Rao, Molecular and Cellular Endocrionology
2004,
224, 73-82). Adverse effects of FSH blockade on androgen production therefore
appear unlikely.
In accordance with the stated arguments, it is to be expected that effective
FSH
antagonists are suitable for spermatogenesis inhibition (prevention) in men.
However, a
suitable FSH antagonist also leads to infertility in women, because it will
suppress
follicle ripening and thus also ovulation.
On the other hand, the skilled person expects advantages from non-peptidergic
FSH
agonists when used to promote fertility in women (stimulation of follicle
ripening). There
are no reports of experience on the use of FSH or FSH agonists in male
infertility, but
specific indications are also conceivable in this connection.
New findings demonstrate that there is also a direct effect of FSH on cells of
bone
metabolism. Two fundamentally different cell types need to be distinguished:
osteoclasts play a central role in bone resorption (breakdown of bone).
Osteoblasts
simulate bone density (anabolic effect).
FSH receptors have been detected in osteoclasts but not osteoblasts. In vitro,
FSH
stimulates bone resorption by mouse osteoclasts ( Li Sun et al. 2006. FSH
directly regu-
lates bone mass. Cell 2006; 125: 247-60). A clinical correlation between the
height of
the serum FSH levels and low bone density has been observed in postmenopausal
women (Devleta et al, 2004; Hypergonadotropic amenorrhea and bone density: new
approach to an old problem. J. Bone Miner. Metab. 22: 360-4).
This and other findings suggest that FSH stimulates loss of bone mass, and
accordingly
FSH antagonists will display an antiresorptive effect on bone and are
therefore suitable
CA 02618888 2008-02-06
~.. --
Prjnted: 23-11-2007~~ DESCPAMD PCT/EP 2006/007 949
3
for the therapy and/or prevention of peri- and postmenopausal loss of bone
mass and
osteoporosis.
In recent years, some low molecular weight FSH receptor modulators, FSH
receptor
antagonists and FSH receptor agonists from various classes of substances have
been
published.
FSH receptor modulators are disclosed in WO 2004/056779, WO 2004/056780; J.
Med.
Chem. 2005, 48, 1697 [Tetrahydroquinolines]; WO 02/70493, Bioorg. Med. Chem.
Lett.
2004, 14, 1713 and 1717 [Diketopiperazines]; and WO 01/47875 [Sulphonamides].
FSH receptor agonists are disclosed in WO 02/09706; J. Comb. Chem. 2004, 6,
196
[Thiazolidinones]; WO 2003/020726 and WO 03/20727, Chem. Biochem. 2002, 10,
1023 (Thieno[2,3-d] pyrimidines); WO 01/87287 [Pyrazoles]; WO 00/08015
[Carbazoles].
Examples of FSH receptor antagonists are disclosed in WO 03/004028
[fetrahydroquinolines], WO 02/09705 [Thiazolidinones], WO 00/58277, Bioorg.
Med.
Chem. 2002, 10, 639 [Sulphonic acids]; WO 00/58276, WO 00/58277 [Aryl sulfonic
acids], Endocr. 2002, 143, 3822; Synth. Comm. 2002, 32, 2695 [Azo compounds].
WO 2004/081011 describes 2-aryl substituted 3-oxo-1,3-dihydropyrazolo[4,3-
c]cmnolm
derivatives with immunomodulatory activity and discloses 4-(8-fluoro-3-oxo-1,3-
dihydro-
pyrazolo[4,3-c]cinnolin-2-yl-N-[3-hydroxy-2-(1-H-indol-3-yl)-propyl]benzamide
explicitly,
which incidentally falls within the scope of the present invention.
The object of the present invention was therefore to provide alternative
compounds
having an FSH receptor antagonistic effect.
The object has been achieved according to the present invention by the
compounds of
the formula I
2J4 , AMENDED SHEET 04-05-2007''
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
4
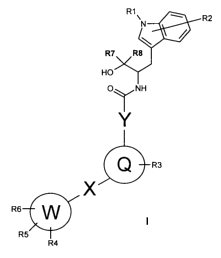
in which
R1 may be hydrogen, C,-C6-alkyl, C3-C6-alkenyl, C3-C6-alkynyl, C3-C7-
cycloalkyl, C,-C6-alkyloxy-C,-C6-alkylene, C3-C7-cycloalkyloxy-C,-Cs-
alkylene, C,-C6-alkylamino-C,-Cs-alkylene, di(C,-C6-alkyl)amino-C,-Cs-
alkylene, phenyloxy-C,-C6-alkylene;
where the hydrocarbon chains therein may optionally be substituted one
or more times by fluorine, cyano, hydroxy, amino or the groups:
0
-N/~ . I -N 0 -N \S -N S~.
\J \---/ 'o
-N /-\ N-Ca Cs -Alkyl -N~~//~Co Cs Alkyl \N
~N-Co Cs Alkyl
R2 may be hydrogen, halogen, cyano, -SO2Me, C,-C6-alkyl, C2-C6-alkenyl,
C2-C6-alkynyl, C,-C6-alkyloxy or benzyloxy,
where the hydrocarbon chains therein may optionally be substituted one
or more times by fluorine;
R3 may be hydrogen, hydroxy, halogen, nitro, amino, cyano, C,-C6-alkyl,
C2-C6-alkenyl or C2-C6-alkynyl, C3-C,-cycloalkyl, hydroxy-C,-C6-
alkylene, hydroxy-C3-C6-alkenylene, hydroxy-C3-C6-alkynylene, C1-C6-
alkyloxy, C,-Cs-alkyloxy-C,-C6-alkylene, C3-C7-cycloalkyloxy, C3-C,-
cycloalkyl-C,-C6-alkylenoxy, C3-C,-cycloalkyloxy-C,-C6-alkylene, C,-C6-
alkyloxy-C3-C6-alkenylene, C,-C6-alkyloxy-C3-C6-alkynylene, C,-C6-
alkyloxyphenyl-C,-C6-alkylene, C,-C6-alkylamino-C,-C6-alkylene, di(C,-
C6-alkyl)amino-C,-C6-alkylene, phenyloxy-C,-C6-alkylene;
where the hydrocarbon chains therein may optionally be substituted one
or more times by fluorine, cyano, hydroxy, amino or the groups
/~ 0
-N, I -N O -N \S -N \S,
~J \-/ , o
-N /--\
N-Co Cs AIkyI -Nv~Ca Cs Alkyl ~N
N-Co Cs Alkyl
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
R4, R5, R6 may be independently of one another hydrogen, hydroxy, halogen,
nitro,
amino, cyano, phenyl, C,-C6-alkyl, C2-C6-alkenyl or C2-C6-alkynyl, C3-
5 C7-cycloalkyl, C3-C7-cycloalkyl-C,-C6-alkylene, C3-C7-heterocycloalkyl,
where the hydrocarbon chains therein may optionally be substituted one
or more times by fluorine, cyano or the radicals:
/~ 0
- N, I -N O - N \S - N \S .
~J o
~ \N~
-N ~N-Co Cs Alkyl -NaCo Cs Alkyl
~N-Co C fi Alkyl
or
independently of one another hydroxy-C,-C6-alkylene, hydroxy-C3-C6-
alkenylene, hydroxy-C3-Cs-alkynylene, C,-C6-alkyloxy, C3-C7-
cycloalkyloxy, C3-C7-cycloalkyl-C,-C6-alkylenoxy, C,-C6-alkyloxy-C,-C6-
alkylene, C3-C,-cycloalkyloxy-C,-C6-alkylene, C1-C6-alkyloxy-C3-C6-
alkenylene, C,-C6-alkyloxy-C3-C6-alkynylene, C,-C6-alkyloxyphenyl-C,-
C6-alkylene, phenyloxy-C,-C6-alkylene,
C,-C6-alkylamino, di(C,-C6-alkyl)amino, C,-C6-alkylamino-C,-Cs-
alkylene, di(C,-C6)-alkylamino-C,-C6-alkylene, C3-C7-cycloalkyl-(Co-C6-
alkyl)amino,
C,-C6-acyl-(Co-Cs-alkyl)amido, C,-Cs-alkylaminocarbonyl, di(C,-Cs-
alkyl)aminocarbonyl, (C3-C,-cycloalkyl)aminocarbonyl, di(C3-C7-
cycloalkyl)aminocarbonyl, C3-C,-cycloalkyl-C,-C6-alkyleneamino-
carbonyl,
C,-C6-alkylcarbonyl, C3-C,-cycloalkylcarbonyl,
carboxy, carboxamido [-C(O)NH2], C,-C6-alkyloxycarbonyl,
C,-C3-alkylsulphanyl, C,-C6-alkysulphonyl, C3-C,-cycloalkylsulphonyl,
C3-C,-cycloalkyl-C,-C6-alkylenesulphonyl,
C,-C6-alkylaminosulphonyl, di(C,-C6-alkyl)aminosulphonyl, (C3-C7-
cycloalkyl)aminosulphonyl, di(C3-C,-cycloalkyl)aminosulphonyl, C3-C7-
cycloalkyl-C,-C6-alkyleneaminosulphonyl, C,-C6-alkylsulphonylamido,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
6
-N(Co-C6-alkyl)-C(O)-C,-C6-alkyl, -N(Co-C6-alkyl)-C(O)-C3-C7-cycloalkyl,
-N(Co-C6-alkyl)-C(O)-N-di(Co-C6-alkyl), -N(Co-C6-alkyl)-C(O)-O-(Co-
C6)alkyl, -N(Co-C6-alkyl)-C(O)-NH-C3-C,-cycloalkyl,
-N(Co-C6-alkyl)-SO2-C,-Cs-alkyl, -N(Co-C6-alkyl)-SO2-C3-C7-cycloalkyl,
-N(Co-C6-alkyl)-SO2-N-di(Co-C6-alkyl), -N(Co-C6-alkyl)-S02-NH-(C3-C,)-
cycloalkyl,
-C(O)-N(H)-C2-C6-alkylene-(C,-C6-alkyl)amine, -C(O)-N(H)-C2-C6-
alkylene-[di(C,-C6-alkyl)]amine, -C(O)-N(H)-C2-C6-alkylene-(C3-C7-
cycloalkyl)amine, -C(O)-N(H)-C2-C6-alkylene-(C3-C7-cycloalkyl-C,-C6-
alkyl)amine,
-S(02)-N(H)-C2-C6-alkylene-(C,-C6-alkyl)amine, -S(O2)-N(H)-CZ-C6-
alkylene-[di(C,-C6-alkyl)]amine, -S(02)-N(H)-C2-C6-alkylene-(C3-C7-
cycloalkyl)amine, -S(02)-N(H)-C2-C6-alkylene-(C3-C7-cycloalkyl-C,-C6-
alkylene)amine,
-O-C2-C6-alkylene-(C,-C6-alkyl)amine, -O-C2-Cs-alkylene-[di(C,-C6-
alkylene)]amine,
or the radicals:
N (N) (N) U (N)
O S
o N~o
(N)
N N
N N
Co Cs alkyl Co Cs alkyl Co Cs alkyl
CN) (N) (N) N
C~
O S
.5..
0 0
N N
(N)
N N
C C -alk I
Co Cs alkyl o s y Co Cs alkyl
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
7
0=S=0 0=S=0 0=S=0 0=S=0
O (N) C:) 5
O O
0=s=0 0=s=0 0=s=0
N p C
C> >
N N
Co Cs alkyl Co-Cs-alkYI Co Cs alkyl
Co Cs alkyl Co Cs alkyl Co Cs alkyl Co Cs alkyl
Oy NI-I OvN~ Oy N~ Oy N~
O C:) () g ,51.
O O
Co Cs alkyl Co Cs alkyl Co Cs alkyl
O\ /N Oy N~ O\ /N~
NC;) >
N
Co Cs alkyl Co-Cs-alkYI Co Cs alkyl
NCo Cs alkyl N'Co Cs alkyi N'Co Cs alkyl N.Co Cs alkyl
I I I I
0=S=0 0=S=0 0=S=0 0=S=0
U (N) C:) N
C~
,,S%.
O O
N'Co Cs alkyl N~Co Cs alkyl N'Co Cs alkyl
I I I
0=S=0 0=S=0 0=S=0
N C
C;) ~
Co Cs alkyl Co Cs alkYl Co Cs alkyl
R7, R8 may be independently of one another hydrogen, methyl, ethyl,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
8
where the methyl and ethyl radicals may be fluorinated one or more
times;
where
R2 may substitute one or more positions of the aryl or heteroaryl ring in
the indole residue;
R3 may substitute one or more positions of the aryl or heteroaryl ring in
the radical Q;
R5 and R6 may together form heterocycloalkyl, cycloalkyl;
Q and W may be independently of one another aryl, heteroaryl;
X may be a bond, C,-C4-alkylene, C2-C4-alkenylene, C2-C4-alkynylene,
C,-C3-alkyleneoxy, C,-C3-alkyleneoxy-C,-C3-alkylene,
Y may be a bond, C,-C4-alkylene.
The object has likewise been achieved according to the present invention by
the com-
pounds of the formula I in which R7 and R8 are a hydrogen, that is to say by
the com-
pounds of the formula Ia
R1
~N ~ R2
\ /
HO
OyNH
Y
Q R3
X
R6
V V la
R5
R4
where
CA 02618888 2008-02-06
Printed: 23-11-2007 DESCPAMD PCT/EP 2006/007 949'4
9
Q and W may be independently of one another aryl, heteroaryl;
X may be a bond, C,-C4-alkylene, C2-C4-alkenylene, C2-C4-alkynylene,
C,-C3-alkyleneoxy, C,-C3-alkyleneoxy-C,-C3-alkylene,
Y may be a bond, C,-C4-alkylene;
with the provision that the formula I does not include 4-(8-fluoro-3-oxo-1,3-
dihydro-
pyrazolo[4,3-c]cinnolin-2-yl-N-[3-hydroxy-2-(1-H-indol-3-yl)-propyl]benzamide.
The object has likewise been achieved according to the present invention by
the com-
pounds of the formula I in which R7 and R8 are a hydrogen, that is to say by
the com-
pounds of the formula Ia
R1\N ~ R2
~ ~
HO
Oy NH
Y
Q R3
X
R6
YY la
R5
R4
where
R1 may be hydrogen, C,-C6-alkyl, C3-Ce-alkenyl or C3-Cs-alkynyl, where
the hydrocarbon radicals therein may optionally be substituted one or
more times by fluorine;
R2 may be hydrogen,.halogen, C,-C6-alkyl, CZ-CB-alkenyl or CZ-Ce-alkynyl,
C,-C4-alkyloxy, where the hydrocarbon chain therein may optionally be
substituted one or more times by fluorine; or benzyloxy;
3/4 AMENDED SHEET 04-05-2007
CA 02618888 2008-02-06
Printed: 23-11-2007' DESCPAMD" PCT/EP 2006/007 949=
R3 may be hydrogen, halogen, nitro, amino, cyano, C,-CB-alkyl, C2-Ce-
alkenyl or Cz-Ce-alkynyl, C,-Ca-alkykoxy, where the hydrocarbon chain
therein may optionally be substituted one or more times by fluorine;
R4, R5, R6 may be independently of one another hydrogen, halogen, C,-Ce-alkyl,
5 C2-Ce-alkenyl, CZ-CB-alkynyl, C,-C4-alkyloxy, where the hydrocarbon
chain therein may optionally be substituted one or more times by fluorine,
C,-C3-alkylsulphanyl, acetamido, C,--Cg-alkylaminocarbonyl; hydroxy,
cyano, hydroxy-C,-C4-alkyl;
where
10 R2 and R3 may substitute one or more positions of the aryl or heteroaryl
ring in
each case in the radical Q and in the indole residue;
R5 and R6 may together form heterocycloalkyl, cycloalkyl;
Q and W may be independently of one another aryl, heteroaryl;
X may be a bond, CI-C4-alkylene, Ci-C4-alkenylene, C,-C4-afkynylene,
C,-C3-alkyleneoxy, C,-C3-alkyleneoxy-C,-C3-alkylene,
Y may be a bond, C,-C4-aikylene;
with the provision that the formula la does not include 4-(8-fluoro-3-oxo-1,3-
dihydro-
pyrazolo[4, 3-c]cinnolin-2-yl-N-[3-hydroxy-2-( 9 -H-indol-3-yl)-propyl]benzam
ide.
The present invention relates to both possible enantiomeric forms at the
stereocentre of
the tryptophanol residue.
The unbranched C,-CB-alkyl groups for the radicals R1 to R6 may be for example
a
methyl, ethyl, propyl, butyl, pentyl or a hexyl group; and the branched C3-Ce-
alkyl
groups for the radicals Rl to R6 may be an isopropyl, isobutyl, sec-butyl,
tert-butyl,
isopentyl, 2-methylbutyl, 1-methylbutyl, 1-ethylpropyl, neopentyl, 1, 1 -
dimethylpropyl, 4-
methylpentyl, 3-methylpentyl, 2-methylpentyl, 1-methylpentyl, 2-ethylbutyl, 1-
ethylbutyl,
3,3-dimethylbutyl, 2,2-dimethylbutyl, 1,1-dimethylbutyl, 2,3-dimethylbutyl,
1,3-
dimethylbutyl or a 1,2-dimethylbutyl group.
The branched or unbranched C3-CS-aikenyl groups for the radical R1 may be for
example an allyi, (E)-2-methylvinyl, (Z)-2-methylvinyl, homoallyl, (E)-but-2-
enyl, (Z)-but-
2-enyl, (E)-but-l-enyl, (Z)-but-l-enyt, pent-4-enyl, (E)-pent-.3-enyl, (Z)-
pent-3-enyl, (E)-
pent-2-enyl, (Z)-pent-2-enyl, (E)-pent-1-enyl, (Z)-pent-l-enyl, hex-5-enyl,
(E)-hex-4-
enyl, (Z)-hex-4-enyl, (E)-hex-3-enyl, (Z)-hex-3-enyl, (E)-hex-2-enyl, (Z)-hex-
2-enyl, (E)-
hex-l-enyl, (Z)-hex-l-enyl, isopropenyl, 2-methylprop-2-enyl, 1-methylprop-2-
enyl, 2-
4/4 AMENDED SHEET 04-06-200f
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
11
The C2-C6-alkenyl groups for the radicals R2 to R6 may, in addition to the C3-
C6-
alkenyl groups mentioned for the radical R1, be for example a vinyl group.
The C2-C6-alkynyl groups for the radicals R2 to R6 may, in addition to the C3-
C6-
alkynyl groups mentioned for the radical R1, be for example an ethynyl group.
The C,-Cs-alkyloxy groups for the radicals R2 to R6 may be for example a
methyloxy,
ethyloxy, propyloxy, isopropyloxy, butyloxy, isobutyloxy, sec-butyloxy, tert-
butyloxy,
pentyloxy, isopentyloxy, (2-methylbutyl)oxy, (1-methylbutyl)oxy, (1 -ethyl
propyl)oxy,
neopentyloxy, (1,1-dimethylpropyl)oxy, hexyloxy, (4-methylpentyl)oxy, (3-
methylpentyl)-
oxy, (2-methylpentyl)oxy, (1-methylpentyl)oxy, (1 -ethyl butyl)oxy, (2-
ethylbutyl)oxy, (3,3-
dimethylbutyl)oxy, (2,2-dimethylbutyl)oxy, (1,1-dimethylbutyl)oxy, (2,3-
dimethyl-
butyl)oxy, (1,3-dimethylbutyl)oxy or a (1,2-dimethylbutyl)oxy group.
The halogens for the radicals R2 to R6 are fluorine, chlorine, bromine or
iodine.
The C,-C3-alkylsulphanyl groups for the radicals R4 to R6 may be for example a
methylsulphanyl (CH3S-), ethylsulphanyl (CH3CH2S-), propylsulphanyl,
isopropylsulphanyl group.
The C,-C6-alkylaminocarbonyl groups for the radicals R4 to R6 may be for
example a
methylaminocarbonyl-, ethylaminocarbonyl-, propylaminocarbonyl-,
isopropylaminocarbonyl-, butylaminocarbonyl-, isobutylaminocarbonyl-, sec-
butylaminocarbonyl-, tert-butylaminocarbonyl-, pentylaminocarbonyl-,
isopentylaminocarbonyl-, (2-methylbutyl)aminocarbonyl-, (1-
methylbutyl)aminocarbonyl-
, (1-ethylpropyl)aminocarbonyl-, neopentylaminocarbonyl-, (1,1-
dimethylpropyl)aminocarbonyl-, hexylaminocarbonyl-, (4-
methylpentyl)aminocarbonyl-,
(3-methylpentyl)aminocarbonyl-, (2-methylpentyl)aminocarbonyl-, (1-
methylpentyl)aminocarbonyl-, (1-ethylbutyl)aminocarbonyl-, (2-
ethylbutyl)aminocarbonyl-, (3,3-dimethylbutyl)aminocarbonyl-, (2,2-
dimethylbutyl)aminocarbonyl-, (1,1-dimethylbutyl)aminocarbonyl-, (2,3-
dimethylbutyl)aminocarbonyl-, (1,3-dimethylbutyl)aminocarbonyl- or a (1,2-
dimethylbutyl)aminocarbonyl group.
The hydroxy-C,-Cs-alkylene groups for the radicals R3 to R6 may be a
hydroxymethyl
(HOCH2-), 2-hydroxyethyl (HOCH2CH2-), 1-hydroxyethyl [CH3CH(OH)-], 3-
hydroxypropyl (HOCH2CH2CH2-), 2-hydroxypropyl [CH3CH(OH)CH2-], 1-hydroxypropyl
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
12
[CH3CH2CH(OH)-], 2-hydroxy-l-methylethyl [HOCH2CH(CH3)-], 1-hydroxy-l-
methylethyl [(CH3)2C(OH)-], 4-hydroxybutyl (HOCH2CH2CH2CH2-), 3-hydroxybutyl
[CH3CH(OH)CH2CH2-], 2-hydroxybutyl [CH3CH2CH(OH)CH2-], 1 -hydroxybutyl
[CH3CH2CH2CH(OH)-], 3-hydroxy-l-methylpropyl [HOCH2CH2CH(CH3)-], 2-hydroxy-l-
methylpropyl [CH3CH(OH)CH(CH3)-], 1-hydroxy-l-methylpropyl [CH3CH2C(CH3)(OH)-
],
1-(hydroxymethyl)propyl [CH3CH(CH2OH)-], 3-hydroxy-2-methylpropyl
[HOCH2CH(CH3)CH2-], 2-hydroxy-2-methylpropyl [(CH3)2C(OH)CH2-], 1-hydroxy-2-
methylpropyl [CH3CH(CH3)CH(OH)-] or a 2-hydroxy-1,1-dimethylethyl group
[HOCH2C(CH3)2-].
The heterocycloalkyl groups which may form the radicals R5 and R6 together may
be
for example the following groups:
O-_ O HN 0\1S HN
\O~ CO N~ X
H H O
0
C O, C C
HN S~ / HN~ /
O O O H C O H 0 S
0 0
The cycloalkyl groups which may form the radicals R5 and R6 together may be
for
example the following groups:
cOG
The C3-C7-cycloalkyl groups for the radicals R1 to R6 may be for example a
cyclopro-
pyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl group.
The C3-C,-heterocycloalkyl groups for the radicals R1 to R6 may be for example
a
cyclopropyl, cyclobutyl, cycopentyl, cyclohexyl, cycloheptyl group in which
one or two
carbon atoms of the ring are replaced independently of one another by an
oxygen, ni-
trogen or sulphur atom.
The aryl groups for the radicals Q and W may be for example a phenyl, naphthyl
group
which is linked via substitutable positions.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
13
The heteroaryl groups for the radicals Q and W may be for example a pyridinyl,
pyrimidinyl, quinolinyl, isoquinolinyl, quinazolinyl, quinoxalinyl,
phthalazinyl, 1.5-
naphthyridinyl, 1,6-naphthyridinyl, 1,7-naphthyridinyl, 1,8-naphthyridinyl,
benzofuranyl,
benzothienyl, 1,3-benzodioxolyl, 2,1,3-benzothiadiazolyl, indolyl, furanyl,
thienyl,
oxazolyl, isoxazolyl, thiazolyl, pyrrolyl, pyrazolyl or an imidazolyl group
which is linked
via substitutable positions.
The C,-C4-alkylene groups for the radicals X and Y may be for example a
methylene (-
CH2-), ethylidene [-CH(CH3)-], ethylene (-CH2CH2-), prop-1,3-ylene (-CH2CH2CH2-
),
prop-1,2-ylene [-CH2CH(CH3)-], but-1,4-ylene (-CH2CH2CH2CH2-), but-1,3-ylene [-
CH2CH2CH(CH3)-], but-1,2-ylene [-CH2CH(CH2CH3)-], but-2,3-yiene [-CHCH(CH3)-],
2-
methylprop-l,2-yiene [-CH2C(CH3)2-] or a 2-methylprop-1,3-ylene group [-
CH2CH(CH3)CH2-].
The C2-C4-alkenylene groups for the radical X may be for example an ethen-1,2-
ylidene
(-CH=CH-), prop-2-en-1,3-ylidene (-CH2-CH=CH-), prop-l-en-l,3-ylidene (-CH=CH-
CH2-), but-1-en-1,4-ylidene (-CH=CH-CH2-CH2-), but-2-en-1,4-ylidene (-CH2-
CH=CH-
CH2-) or a but-3-en-1,4-ylidene group (-CH2-CH2-CH=CH-).
The C2-C4-alkynylene groups for the radical X may be for example an ethyn-1,2-
ylidene
(-C=C-), prop-2-yn-1,3-ylidene (-CHZ-C=C-), prop-l-yn-l,3-ylidene (-C=C-CH2-),
but-1-
yn-1,4-ylidene (-C=C-CH2-CH2-), but-2-yn-1,4-ylidene (-CH2-C=C-CH2-) or a but-
3-yn-
1,4-ylidene group (-CH2-CH2-C=C-).
The C,-C3-alkyleneoxy groups for the radical X may be for example an
oxymethylene (-
O-CHZ-), methyleneoxy (-CH2-O-), ethane-l,2-diyloxy (-CH2-CH2-O-), oxyethane-
1,2-
diyl (-O-CH2-CH2-), propane-1,3-diyloxy (-CH2-CH2-CH2-O-) or an oxypropane-1,3-
diyl (-
O-CH2-CH2-CH2-) group.
The C,-C3-alkyleneoxy-C,-C3-aIkyl groups for the radical X may be for example
an
oxybis(methylene) (-CH2-O-CH2-), methyleneoxyethane-2,1-diyl [-CH2-O-(CH2)2-],
ethane-l,2-diyloxymethylene [-(CH2)2-O-CH2-], methyleneoxypropane-3,1-diyl [-
CH2-O-
(CH2)3-], propane-l,3-diyloxymethylene [-(CH2)3-O-CH2-], oxybis(ethane-2,1-
diyl) [-
(CH2)2-0-(CH2)2-], propane-l,3-diyloxyethane-2,1-diyl [-(CH2)3-0-(CH2)2-],
ethane-1,2-
diyloxypropane-3,1-diyl [-(CH2)2-0-(CH2)3-] or an oxybis(propane-3,1-diyl)
group [-
(CH2)3-0-(CH2)3-].
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
14
The C3-C,-cycloalkyloxy groups for the radicals R1 to R6 may be for example a
cyclo-
propyloxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, cycloheptyloxy group.
The C,-C6-alkylamino groups for the radicals R1 to R6 may be for example
methyl-
amino, ethylamino, propylamino, isopropylamino, butylamino, isobutylamino, sec-
butyl-
amino, tert-butylamino, pentylamino, isopentylamino, (2-methylbutyl)amino,
(1-methylbutyl)amino, (1-ethylpropyl)amino, neopentylamino, (1,1-
dimethylpropyl)-
amino, hexylamino, (4-methylpentyl)amino, (3-methylpentyl)amino,
(2-methylpentyl)amino, (1-methylpentyl)amino, (1-ethylbutyl)amino, (2-
ethylbutyl)amino,
(3,3-dimethylbutyl)amino, (2,2-dimethylbutyl)amino, (1,1-dimethylbutyl)amino,
(2,3-dimethylbutyl)amino, (1,3-dimethylbutyl)amino or a (1,2-
dimethylbutyl)amino group.
In the di(C,-C6-alkyl)amino groups for the radicals R1 to R6, each of the two
radicals on
the nitrogen atom of the dialkylamino group may be chosen independently of one
an-
other from the following radicals: possible examples are a methyl, ethyl,
propyl, isopro-
pyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, (2-
methylbutyl),
(1-methylbutyl), (1-ethylpropyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-
methylpentyl),
(3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-
ethylbutyl), (3,3-
dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl),
(1,3-
dimethylbutyl) or a (1,2-dimethylbutyl) group.
In the C3-C,-cycloalkyl-C,-C6-alkyleneoxy groups for the radicals R1 to R6 it
is possible
to combine each of the C3-C7-cycloalkyl groups of the C3-C,-cycloalkyl-C,-C6-
alkyleneoxy group, for example of a cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl or
cycloheptyl group, independently of one another with each C0-C6-alkyleneoxy
group,
for example with a methyleneoxy, ethyleneoxy, propyleneoxy, butyleneoxy,
pentylene-
oxy, hexyleneoxy group.
In the hydroxy-C3-C6-alkenylene groups for the radicals R1 to R6 it is
possible for the
hydroxy group to be located on any desired position of the C3-Cs-alkenyl
group, for ex-
ample of an allyl, (E)-2-methylvinyl, (Z)-2-methylvinyl, homoallyl, (E)-but-2-
enyl, (Z)-but-
2-enyl, (E)-but-1-enyl, (Z)-but-l-enyl, pent-4-enyl, (E)-pent-3-enyl, (Z)-pent-
3-enyl, (E)-
Pent-2-enyl-, (Z)-Pent-2-enyl-, (E)-Pent-l-enyl-, (Z)-Pent-l-enyl-, hex-5-enyl-
, (E)-hex-
4-enyl, (Z)-hex-4-enyl, (E)-hex-3-enyl, (Z)-hex-3-enyl, (E)-hex-2-enyl, (Z)-
hex-2-enyl,
(E)-hex-l-enyl, (Z)-hex-l-enyl, isopropenyl, 2-methylprop-2-enyl, 1-methylprop-
2-enyl,
2-methyl prop- 1 -enyl, (E)- 1 -methyl prop- 1 -enyl, (Z)- 1 -methyl prop- 1 -
enyl, 3-methylbut-3-
enyl, 2-methylbut-3-enyl, 1-methylbut-3-enyl, 3-methylbut-2-enyl, (E)-2-
methylbut-2-
enyl, (Z)-2-methylbut-2-enyl, (E)-1-methylbut-2-enyl, (Z)-1-methylbut-2-enyl,
(E)-3-
methylbut-l-enyl, (Z)-3-methylbut-1-enyl, (E)-2-methylbut-1-enyl, (Z)-2-
methylbut-1-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
enyl, (E)-1-methylbut-l-enyl, (Z)-1-methylbut-l-enyl, 1,1-dimethylprop-2-enyl,
1-
ethyl prop- 1 -enyl, 1-propylvinyl, 1-isopropylvinyl, 4-methylpent-4-enyl, 3-
methylpent-4-
enyl, 2-methylpent-4-enyl, 1-methylpent-4-enyl, 4-methylpent-3-enyl, (E)-3-
methylpent-
3-enyl, (Z)-3-methylpent-3-enyl, (E)-2-methylpent-3-enyl, (Z)-2-methylpent-3-
enyl, (E)-1-
5 methylpent-3-enyl, (Z)-1-methylpent-3-enyl, (E)-4-methylpent-2-enyl, (Z)-4-
methylpent-
2-enyl, (E)-3-methylpent-2-enyl, (Z)-3-methylpent-2-enyl, (E)-2-methylpent-2-
enyl, (Z)-2-
methylpent-2-enyl, (E)-1-methylpent-2-enyl, (Z)-1-methylpent-2-enyl, (E)-4-
methylpent-
1-enyl, (Z)-4-methylpent-l-enyl, (E)-3-methyl pent- 1 -enyl, (Z)-3-methylpent-
1-enyl, (E)-2-
methylpent-1-enyl, (Z)-2-methylpent-1-enyl, (E)- 1 -methyl pent- 1 -enyl, (Z)-
1-methylpent-
10 1-enyl, 3-ethylbut-3-enyl, 2-ethylbut-3-enyl, 1-ethylbut-3-enyl, (E)-3-
ethylbut-2-enyl, (Z)-
3-ethylbut-2-enyl, (E)-2-ethylbut-2-enyl, (Z)-2-ethylbut-2-enyl, (E)- 1 -ethyl
but-2-enyl, (Z)-
1 -ethyl but-2-enyl, (E)-3-ethyl but- 1 -enyl, (Z)-3-ethyl but- 1 -enyl, 2-
ethylbut-l-enyl, (E)-1-
ethylbut-1-enyl, (Z)-1-ethylbut-1-enyl, 2-propylprop-2-enyl, 1-propylprop-2-
enyl, 2-
isopropylprop-2-enyl, 1-isopropylprop-2-enyl, (E)-2-propyl prop- 1 -enyl, (Z)-
2-propylprop-
15 1-enyl, (E)-1-propylprop-l-enyl, (Z)-1-propylprop-l-enyl, (E)-2-isopropyl
prop- 1 -enyl, (Z)-
2-isopropylprop-l-enyl, (E)-1-isopropylprop-l-enyl, (Z)- 1 -isopropyl prop- 1 -
enyl, (E)-3,3-
dimethylprop-1 -enyl, (Z)-3,3-dimethylprop-1-enyl or a 1 -(1, 1 -
dimethylethyl)ethenyl
group, and to be combined independently of one
another.
In the hydroxy-C3-C6-alkynyl groups for the radicals R1 to R6 it is possible
for the hy-
droxy group to be located at any desired position of the C3-C6-alkynyl group,
for exam-
ple of a prop-l-ynyl, prop-2-ynyl, but-1-ynyl, but-2-ynyl, but-3-ynyl, pent-l-
ynyl, pent-2-
ynyl, pent-3-ynyl, pent-4-ynyl, hex-1-ynyl, hex-2-ynyl, hex-3-ynyl, hex-4-
ynyl, hex-5-ynyl,
1-methylprop-2-ynyl, 2-methylbut-3-ynyl, 1-methylbut-3-ynyl, 1 -m ethyl but-2-
ynyl, 3-
methylbut-l-ynyl, 1 -ethyl prop-2-ynyl, 3-methylpent-4-ynyl, 2-methylpent-4-
ynyl, 1-
methylpent-4-ynyl, 2-methylpent-3-ynyl, 1-methylpent-3-ynyl, 4-methylpent-2-
ynyl,
1-methylpent-2-ynyl, 4-methylpent-1 -ynyl, 3-methylpent-1-ynyl, 2-ethylbut-3-
ynyl,
1 -ethyl but-3-ynyl, 1-ethylbut-2-ynyl, 1-propylprop-2-ynyl, 1-isopropylprop-2-
ynyl, 2,2-di-
methylbut-3-ynyl, 1, 1 -dimethylbut-3-ynyl, 1,1-dimethylbut-2-ynyl or a 3,3-
dimethylbut-1 -
ynyl group.
In the C,-C6-alkyloxy-C3-C6-alkenylene groups for the radicals R1 to R6 it is
possible for
the C,-C6-alkyloxy group, for example a methyloxy, ethyloxy, propyloxy,
isopropyloxy,
butyloxy, isobutyloxy, sec-butyloxy, tert-butyloxy, pentyloxy, isopentyloxy,
(2-methylbutyl)oxy, (1-methylbutyl)oxy, (1-ethylpropyl)oxy, neopentyloxy, (1,1-
dimethyl-
propyl)oxy, hexyloxy, (4-methylpentyl)oxy, (3-methylpentyl)oxy, (2-
methylpentyl)oxy,
(1-methylpentyl)oxy, (1-ethylbutyl)oxy, (2-ethylbutyl)oxy, (3,3-
dimethylbutyl)oxy, (2,2-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
16
dimethylbutyl)oxy, (1,1-dimethylbutyl)oxy, (2,3-dimethylbutyl)oxy, (1,3-
dimethylbutyl)oxy
or a (1,2-dimethylbutyl)oxy group, to be located on any desired position of
the C3-C6-
alkenyl group, for example of an allyl, (E)-2-methylvinyl, (Z)-2-methylvinyl,
homoallyl,
(E)-but-2-enyl, (Z)-but-2-enyl, (E)-but-l-enyl, (Z)-but-l-enyl, pent-4-enyl,
(E)-pent-3-
enyl, (Z)-pent-3-enyl, (E)-pent-2-enyl, (Z)-pent-2-enyl, (E)-pent-l-enyl, (Z)-
pent-l-enyl,
hex-5-enyl, (E)-hex-4-enyl, (Z)-hex-4-enyl, (E)-hex-3-enyl, (Z)-hex-3-enyl,
(E)-hex-2-
enyl, (Z)-hex-2-enyl, (E)-hex-l-enyl, (Z)-hex-l-enyl, isopropenyl, 2-
methylprop-2-enyl,
1-methylprop-2-enyl, 2-methylprop-l-enyl, (E)-1-methylprop-l-enyl, (Z)-1-
methylprop-l-
enyl, 3-methylbut-3-enyl, 2-methylbut-3-enyl, 1-methylbut-3-enyl, 3-methylbut-
2-enyl,
(E)-2-methylbut-2-enyl, (Z)-2-methylbut-2-enyl, (E)- 1 -m ethyl but-2-enyl,
(Z)-1-methylbut-
2-enyl, (E)-3-methylbut-l-enyl, (Z)-3-methylbut-l-enyl, (E)-2-methylbut-1-
enyl, (Z)-2-
methylbut-l-enyl, (E)-1-methylbut-l-enyl, (Z)-1-methylbut-l-enyl, 1,1-
dimethylprop-2-
enyl, 1 -ethyl prop- 1 -enyl, 1-propylvinyl, 1-isopropylvinyl, 4-methylpent-4-
enyl,
3-methylpent-4-enyl, 2-methylpent-4-enyl, 1-methylpent-4-enyl, 4-methylpent-3-
enyl,
(E)-3-methylpent-3-enyl, (Z)-3-methylpent-3-enyl, (E)-2-methylpent-3-enyl, (Z)-
2-
methylpent-3-enyl, (E)-1-methylpent-3-enyl, (Z)-1-methylpent-3-enyl, (E)-4-
methylpent-
2-enyl, (Z)-4-methylpent-2-enyl, (E)-3-methylpent-2-enyl, (Z)-3-methylpent-2-
enyl, (E)-2-
methylpent-2-enyl, (Z)-2-methylpent-2-enyl, (E)-1-methylpent-2-enyl, (Z)-1-
methylpent-
2-enyl, (E)-4-methyl pent- 1 -enyl, (Z)-4-methylpent-l-enyl, (E)-3-methyl pent-
1 -enyl, (Z)-3-
methylpent-1-enyl, (E)-2-methylpent-l-enyl, (Z)-2-methyl pent- 1 -enyl, (E)- 1
-methyl pent-
1-enyl, (Z)-1-methylpent-l-enyl, 3-ethylbut-3-enyl, 2-ethylbut-3-enyl, 1 -
ethyl but-3-enyl,
(E)-3-ethylbut-2-enyl, (Z)-3-ethylbut-2-enyl, (E)-2-ethylbut-2-enyl, (Z)-2-
ethylbut-2-enyl,
(E)-1-ethylbut-2-enyl, (Z)-1-ethylbut-2-enyl, (E)-3-ethyl but- 1 -enyl, (Z)-3-
ethyl but- 1 -enyl,
2-ethylbut-l-enyl, (E)- 1 -ethyl but- 1 -enyl, (Z)-1-ethylbut-l-enyl, 2-
propylprop-2-enyl,
1-propylprop-2-enyl, 2-isopropylprop-2-enyl, 1-isopropylprop-2-enyl, (E)-2-
propylprop-1-
enyl, (Z)-2-propyl prop- 1 -enyl, (E)-1-propylprop-l-enyl, (Z)-1-propylprop-l-
enyl, (E)-2-
isopropylprop-1-enyl, (Z)-2-isopropylprop-l-enyl, (E)-1-isopropylprop-l-enyl,
(Z)-1-
isopropylprop-1-enyl, (E)-3,3-dimethylprop-l-enyl, (Z)-3,3-dimethylprop-l-enyl
or a
1-(1,1-dimethylethyl)ethenyl group and to be combined independently of one
another.
In the C,-C6-alkyloxy-C3-C6-alkynylene groups for the radicals R1 to R6 it is
possible for
the C,-Cs-alkyloxy group, for example a methyloxy, ethyloxy, propyloxy,
isopropyloxy,
butyloxy, isobutyloxy, sec-butyloxy, tert-butyloxy, pentyloxy, isopentyloxy,
(2-methylbutyl)oxy, (1-methylbutyl)oxy, (1 -ethyl propyl)oxy, neopentyloxy,
(1,1-dimethyl-
propyl)oxy, hexyloxy, (4-methylpentyl)oxy, (3-methylpentyl)oxy, (2-
methylpentyl)oxy, (1-
methylpentyl)oxy, (1-ethylbutyl)oxy, (2-ethylbutyl)oxy, (3,3-
dimethylbutyl)oxy, (2,2-
dimethylbutyl)oxy, (1,1-dimethylbutyl)oxy, (2,3-dimethylbutyl)oxy, (1,3-
dimethylbutyl)oxy
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
17
or a (1,2-dimethylbutyl)oxy group, to be located at any desired position of
the C3-C6-
alkynyl group, for example of a prop-1-ynyl, prop-2-ynyl, but-1-ynyl, but-2-
ynyl, but-3-
ynyl, pent-1-ynyl, pent-2-ynyl, pent-3-ynyl, pent-4-ynyl, hex-1-ynyl, hex-2-
ynyl, hex-3-
ynyl, hex-4-ynyl, hex-5-ynyl, 1-methylprop-2-ynyl, 2-methylbut-3-ynyl, 1-
methylbut-3-
ynyl, 1-methylbut-2-ynyl, 3-methylbut-1-ynyl, 1 -ethyl prop-2-ynyl, 3-
methylpent-4-ynyl,
2-methylpent-4-ynyl, 1-methylpent-4-ynyl, 2-methylpent-3-ynyl, 1-methylpent-3-
ynyl,
4-methylpent-2-ynyl, 1-methylpent-2-ynyl, 4-methylpent-1-ynyl, 3-methylpent-1-
ynyl,
2-ethylbut-3-ynyl, 1 -ethyl but-3-ynyl, 1 -ethyl but-2-ynyl, 1-propylprop-2-
ynyl,
1-isopropylprop-2-ynyl, 2,2-dimethylbut-3-ynyl, 1,1-dimethylbut-3-ynyl, 1,1-
dimethylbut-
2-ynyl or a 3,3-dimethylbut-1-ynyl group, and to be combined independently of
one an-
other.
In the C,-Cs-alkyloxyphenyl-C,-Cs-alkylene groups for the radical R1 to R6 it
is possible
for the C,-Cs-alkyloxy group to be selected independently of one another from
methy-
loxy, ethyloxy, propyloxy, isopropyloxy, butyloxy, isobutyloxy, sec-butyloxy,
tert-
butyloxy, pentyloxy, isopentyloxy, (2-methylbutyl)oxy, (1-methylbutyl)oxy, (1-
ethylpro-
pyl)oxy, neopentyloxy, (1,1-dimethylpropyl)oxy, hexyloxy, (4-methylpentyl)oxy,
(3-
methylpentyl)oxy, (2-methylpentyl)oxy, (1-methylpentyl)oxy, (1-ethylbutyl)oxy,
(2-ethyl-
butyl)oxy, (3,3-dimethylbutyl)oxy, (2,2-dimethylbutyl)oxy, (1,1-
dimethylbutyl)oxy, (2,3-
dimethylbutyl)oxy, (1,3-dimethylbutyl)oxy or a (1,2-dimethylbutyl)oxy, and to
be com-
bined independently of one another with C,-Cs-alkylene groups such as, for
example,
methylene, ethylene, propylene, butylene, pentylene, hexylene.
In the C3-C,-cycloalkyl-(Co-C6)-alkyleneamino groups of the radicals R3 to R6
it is pos-
sible for each of the C3-C,-cycloalkyl groups of the C3-C,-cycloalkyl-(Co-C6)-
alkyleneamino group, for example of a cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl
or cycloheptyl group, to be combined independently of one another with each C0-
C6-
alkylene group, for example with a bond, a methylene, ethylene, propylene,
butylene,
pentylene, hexylene group.
In the C,-C6-alkyloxy-C,-C6-alkylene groups for the radical R1 to R6, it is
possible for
the C,-C6-alkyloxy group to be selected independently for example from
methyloxy,
ethyloxy, propyloxy, isopropyloxy, butyloxy, isobutyloxy, sec-butyloxy, tert-
butyloxy,
pentyloxy, isopentyloxy, (2-methylbutyl)oxy, (1-methylbutyl)oxy, (1 -ethyl
propyl)oxy,
neopentyloxy, (1,1-dimethylpropyl)oxy, hexyloxy, (4-methylpentyl)oxy, (3-
methylpentyl)-
oxy, (2-methylpentyl)oxy, (1-methylpentyl)oxy, (1 -ethyl butyl)oxy, (2-
ethylbutyl)oxy, (3,3-
dimethylbutyl)oxy, (2,2-dimethylbutyl)oxy, (1,1-dimethylbutyl)oxy, (2,3-
dimethyl-
butyl)oxy, (1,3-dimethylbutyl)oxy or a(1,2-dimethylbutyl)oxy and to be
combined inde-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
18
pendently of one another with C,-C6-alkylene groups such as, for example,
methylene,
ethylene, propylene, butylene, pentylene, hexylene.
In the di(C,-C6-alkyl)amino-C,-Cs-alkylene group for the radical R1 it is
possible for
each of the two radicals on the nitrogen atom of the amino group to be
selected inde-
pendently for example from methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
sec-butyl,
tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1-
ethylpropyl), neopentyl,
(1,1-dimethylpropyl), hexyl, (4-methylpentyl), (3-methylpentyl),
(2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl),
(2,2-dimethylbutyl), (1, 1 -dimethylbutyl), (2,3-dimethylbutyl), (1,3-
dimethylbutyl) or a (1,2-
dimethylbutyl) group, and to be combined with C,-C6-alkylene groups such as,
for ex-
ample, methylene, ethylene, propylene, butylene, pentylene, hexylene.
The C3-C,-cycloalkyl-C,-C6-alkylene groups for the radicals R1 to R6 may be
for ex-
ample a cyclopropyloxymethylene, cyclopropyloxyethylene,
cyclopropyloxypropylene,
cyclopropyloxybutylene, cyclopropyloxypentylene, cyclopropyloxyhexylene,
cyclobuty-
loxymethylene, cyclobutyloxyethylene, cyclobutyloxypropylene,
cyclobutyloxybutylene,
cyclobutyloxypentylene, cyclobutyloxyhexylene, cyclopentyloxymethylene,
cyclopenty-
loxyethylene, cyclopentyloxypropylene, cyclopentyloxybutylene,
cyclopentyloxypenty-
lene, cyclopentyloxyhexylene, cyclohexyloxymethylene, cyclohexyloxyethylene,
cyclo-
hexyloxypropylene, cyclohexyloxybutylene, cyclohexyloxypentylene,
cyclohexyloxy-
hexylene, cycloheptyloxymethylene, cycloheptyloxyethylene,
cycloheptyloxypropylene,
cycloheptyloxybutylene, cycloheptyloxypentylene, cycloheptyloxyhexylen group.
In the C,-C6-alkylamino-C,-C6-alkylene groups for the radicals R1 to R6 it is
possible
for the C,-Cs-alkylamino group to be selected independently for example from
methyl-
amino, ethylamino, propylamino, isopropylamino, butylamino, isobutylamino, sec-
butyl-
amino, tert-butylamino, pentylamino, isopentylamino, (2-methylbutyl)amino,
(1-methylbutyl)amino, (1-ethylpropyl)amino, neopentylamino, (1,1-
dimethylpropyl)-
amino, hexylamino, (4-methylpentyl)amino, (3-methylpentyl)amino, (2-
methylpentyl)-
amino, (1-methylpentyl)amino, (1-ethylbutyl)amino, (2-ethylbutyl)amino, (3,3-
dimethyl-
butyl)amino, (2,2-dimethylbutyl)amino, (1,1-dimethylbutyl)amino, (2,3-dimethyl-
butyl)amino, (1,3-dimethylbutyl)amino or a (1,2-dimethylbutyl)amino and to be
com-
bined with C,-Cs-alkylene groups such as, for example, methylene, ethylene,
propylene,
butylene, pentylene, hexylene.
The phenyloxy-C,-C6-alkylene groups for the radicals R1 to R6 may be for
example a
phenyloxymethyl, phenyloxyethyl, phenyloxypropyl, phenyloxybutyl,
phenyloxypentyl,
phenyloxyhexyl group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
19
In the C,-C6-acyl-(Co-C6-alkyl)amido groups for the radicals R4 to R6, it is
possible for
each of the C,-C6-acyl groups, for example a formyl, acetyl, propionyl, 2-
methylpro-
pionyl, 2,2-dimethylpropionyl, butyryl, 2-methylbutyryl, 3-methylbutyryl, 2,2-
dimethyl-
butyryl, 2-ethylbutyryl, pentanoyl, 2-methylpentanoyl, 3-methylpentanoyl,
4-methylpentanoyl or a hexanoyl group, to be combined independently of one
another
with each (Co-C6-alkyl)amido group, for example a hydrogen atom, a
methylamido,
ethylamido, propylamido, isopropylamido, butylamido, isobutylamido, sec-
butylamido,
tert-butylamido, pentylamido, isopentylamido, (2-methylbutyl)amido, (1-methyl-
butyl)amido, (1-ethylpropyl)amido, neopentylamido, (1,1-dimethylpropyl)amido,
hexyla-
mido, (4-methylpentyl)amido, (3-methylpentyl)amido, (2-methylpentyl)amido,
(1-methylpentyt)amido, (1-ethylbutyl)amido, (2-ethylbutyl)amido, (3,3-dimethyl-
butyl)amido, (2,2-dimethylbutyl)amido, (1,1-dimethylbutyl)amido, (2,3-dimethyl-
butyl)amido, (1,3-dimethylbutyl)amido or a (1,2-dimethylbutyl)amido group.
The C,-C6-alkylaminocarbonyl groups for the radicals R4 to R6 may be for
example a
methylaminocarbonyl, ethylaminocarbonyl, propylaminocarbonyl, isopropylamino-
carbonyl, butylaminocarbonyl, isobutylaminocarbonyl, sec-butylaminocarbonyl,
tert-
butylaminocarbonyl, pentylaminocarbonyl, isopentylaminocarbonyl, (2-
methylbutyl)-
aminocarbonyl, (1-methylbutyl)aminocarbonyl, (1-ethylpropyl)aminocarbonyl,
neopen-
tylaminocarbonyl, (1,1-dimethylpropyl)aminocarbonyl, hexylaminocarbonyl,
(4-methylpentyl)aminocarbonyl, (3-methylpentyl)aminocarbonyl, (2-methylpentyl)-
aminocarbonyl, (1-methylpentyl)aminocarbonyl, (1-ethylbutyl)aminocarbonyl,
(2-ethylbutyl)aminocarbonyl, (3,3-dimethylbutyl)aminocarbonyl, (2,2-
dimethylbutyl)-
aminocarbonyl, (1,1-dimethylbutyl)aminocarbonyl, (2,3-
dimethylbutyl)aminocarbonyl,
(1,3-dimethylbutyl)aminocarbonyl or a (1,2-dimethylbutyl)aminocarbonyl group.
In the di(C,-C6-alkyl)aminocarbonyl groups for the radicals R4 to R6, each of
the two
C,-C6-alkyl radicals on the nitrogen atom of the di(C,-C6-alkyl)aminocarbonyl
group may
be independently of one another for example a methyl, ethyl, propyl,
isopropyl, butyl,
isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-
methylbutyl),
(1 -ethyl propyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl),
(3-methyl-
pentyl), (2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl),
(3,3-
dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl),
(1,3-
dimethylbutyl) or a (1,2-dimethylbutyl) group.
The (C3-C,-cycloalkyl)aminocarbonyl groups for the radicals R4 to R6 may be
for ex-
ample a cyclopropylaminocarbonyl, cyclobutylaminocarbonyl, cyclopentylaminocar-
bonyl, cyclohexylaminocarbonyl or cycloheptylaminocarbonyl group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
In the di(C3-C7-cycloalkyl)aminocarbonyl groups for the radicals R4 to R6,
each of the
two C3-C7-cycloalkyl radicals on the nitrogen atom of the di(C3-C,-cyclo-
alkyl)aminocarbonyl group may be independently of one another for example a
cyclo-
propyl, cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl group.
5 In the C3-C,-cycloalkyl-C,-C6-alkyleneaminocarbonyl groups of the radicals
R4 to R6 it
is possible for each of the C3-C,-cycloalkyl groups of the C3-C7-cycloalkyl-C,-
C6-
alkyleneaminocarbonyl groups, for example of a cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl or cycloheptyl group, to be combined independently of one another
with
each CI-Cs-alkyleneaminocarbonyl group, for example with a
methyleneaminocarbonyl,
10 ethyleneaminocarbonyl, propyleneaminocarbonyl, butyleneaminocarbonyl, penty-
leneaminocarbonyl, hexyleneaminocarbonyl group.
The C,-C6-alkylcarbonyl groups for the radicals R4 to R6 may be for example a
methyl-
carbonyl, ethylcarbonyl, propylcarbonyl, isopropylcarbonyl, butylcarbonyl,
isobutylcar-
bonyl, sec-butylcarbonyl, tert-butylcarbonyl, pentylcarbonyl,
isopentylcarbonyl, (2-
15 methylbutyl)carbonyl, (1-methylbutyl)carbonyl, (1-ethylpropyl)carbonyl,
neopentylcar-
bonyl, (1,1-dimethylpropyl)carbonyl, hexylcarbonyl, (4-methylpentyl)carbonyl,
(3-
methylpentyl)carbonyl, (2-methylpentyl)carbonyl, (1-methylpentyl)carbonyl, (1-
ethylbutyl)carbonyl, (2-ethylbutyl)carbonyl, (3,3-dimethylbutyl)carbonyl, (2,2-
dimethylbutyl)carbonyl, (1,1-dimethylbutyl)carbonyl, (2,3-
dimethylbutyl)carbonyl, (1,3-
20 dimethylbutyl)carbonyl or a (1,2-dimethylbutyl)carbonyl group.
The C3-C,-cycloalkylcarbonyl groups for the radicals R4 to R6 may be for
example a
cyclopropylcarbonyl, cyclobutylcarbonyl, cyclopentylcarbonyl,
cyclohexylcarbonyl or
cycloheptylcarbonyl group.
The C,-C6-alkyloxycarbonyl groups for the radicals R4 to R6 may be for example
a
methyloxycarbonyl, ethyloxycarbonyl, propyloxycarbonyl, isopropyloxycarbonyl,
buty-
loxycarbonyl, isobutyloxycarbonyl, sec-butyloxycarbonyl, tert-
butyloxycarbonyl, penty-
loxycarbonyl, isopentyloxycarbonyl, (2-methylbutyl)oxycarbonyl, (1-methyl-
butyl)oxycarbonyl, (1 -ethyl propyl)oxycarbonyl, neopentyloxycarbonyl, (1,1-
dimethylpropyl)oxycarbonyl, hexyloxycarbonyl, (4-methylpentyl)oxycarbonyl, (3-
methylpentyl)oxycarbonyl, (2-methylpentyl)oxycarbonyl, (1-
methylpentyl)oxycarbonyl,
(1 -ethyl butyl)oxycarbonyl, (2-ethylbutyl)oxycarbonyl, (3,3-
dimethylbutyl)oxycarbonyl,
(2,2-dimethylbutyl)oxycarbonyl, (1,1-dimethylbutyl)oxycarbonyl, (2,3-dimethyl-
butyl)oxycarbonyl, (1,3-dimethylbutyl)oxycarbonyl or a (1,2-
dimethylbutyl)oxycarbonyl
group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
21
The C,-C6-alkylsulphonyl groups for the radicals R4 to R6 may be for example a
me-
thylsulphonyl, ethylsulphonyl, propylsulphonyl, isopropylsulphonyl,
butylsulphonyl, iso-
butylsulphonyl, sec-butylsulphonyl, tert-butylsulphonyl, pentylsulphonyl,
isopentyl-
sulphonyl, (2-methylbutyl)sulphonyl, (1-methylbutyl)sulphonyl, (1-
ethylpropyl)sulphonyl,
neopentylsulphonyl, (1,1-dimethylpropyl)sulphonyl, hexylsulphonyl, (4-methyl-
pentyl)sulphonyl, (3-methylpentyl)sulphonyl, (2-methylpentyl)sulphonyl, (1-
methylpentyl)sulphonyl, (1-ethylbutyl)sulphonyl, (2-ethylbutyl)sulphonyl, (3,3-
dimethylbutyl)sulphonyl, (2,2-dimethylbutyl)sulphonyl, (1,1-
dimethylbutyl)sulphonyl,
(2,3-dimethylbutyl)sulphonyl, (1,3-dimethylbutyl)sulphonyl or a (1,2-
dimethylbutyl)sulphonyl group.
The C3-C7-cycloalkylsulphonyl groups for the radicals R4 to R6 may be for
example a
cyclopropylsulphonyl, cyclobutylsulphonyl, cyclopentylsulphonyl,
cyclohexylsulphonyl or
cycloheptylsulphonyl group.
In the C3-C,-cycloalkyl-C,-Cs-alkylenesulphonyl groups of the radicals R4 to
R6 it is
possible for each of the C3-C,-cycloalkyl groups of the C3-C,-cycloalkyl-C,-C6-
alkylenesulphonyl groups, for example of a cyclopropyl, cyclobutyl,
cyclopentyl, cyclo-
hexyl or cycloheptyl group, to be combined independently of one another with
each C,-
C6-alkylenesulphonyl group, for example with a methylenesulphonyl,
ethylenesulphonyl,
propylenesulphonyl, butylenesulphonyl, pentylenesulphonyl, hexylenesulphonyl
group.
The C,-C6-alkylaminosulphonyl groups for the radicals R4 to R6 may be for
example a
methylaminosulphonyl, ethylaminosulphonyl, propylaminosulphonyl,
isopropylaminosul-
phonyl, butylaminosulphonyl, isobutylaminosulphonyl, sec-butylaminosulphonyl,
tert-
butylaminosulphonyl, pentylaminosulphonyl, isopentylaminosulphonyl, (2-methyl-
butyl)aminosulphonyl, (1-methylbutyl)aminosulphonyl, (1-
ethylpropyl)aminosulphonyl,
neopentylaminosulphonyl, (1,1-dimethylpropyl)aminosulphonyl,
hexylaminosulphonyl,
(4-methylpentyl)aminosulphonyl, (3-methylpentyl)aminosulphonyl, (2-
methylpentyl)-
aminosulphonyl, (1-methylpentyl)aminosulphonyl, (1-ethylbutyl)aminosulphonyl,
(2-
ethylbutyl)aminosulphonyl, (3,3-dimethylbutyl)aminosulphonyl, (2,2-
dimethylbutyl)-
aminosulphonyl, (1,1-dimethylbutyl)aminosulphonyl, (2,3-
dimethylbutyl)aminosulphonyl,
(1,3-dimethylbutyl)aminosulphonyl or a (1,2-dimethylbutyl)aminosulphonyl
group.
In the di(C,-C6-alkyl)aminosulphonyl groups for the radicals R4 to R6, each of
the two
C,-C6-alkyl radicals on the nitrogen atom of the di(C,-C6-alkyl)aminosulphonyl
group
may be independently of one another for example a methyl, ethyl, propyl,
isopropyl,
butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-
methylbutyl), (1-
ethylpropyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl), (3-
methylpentyl),
(2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl),
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
22
(2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-
dimethylbutyl) or a (1,2-
dimethylbutyl) group.
The (C3-C7-cycloalkyl)aminosulphonyl groups for the radicals R4 to R6 may be
for ex-
ample a cyclopropylaminosulphonyl, cyclobutylaminosulphonyl,
cyclopentylaminosul-
phonyl, cyclohexylaminosulphonyl or cycloheptylaminosulphonyl group.
In the di(C3-C7-cycloalkyl)aminosulphonyl groups for the radicals R4 to R6,
each of the
two C3-C7-cycloalkyl radicals on the nitrogen atom of the di(C3-C,-cycloalkyl)-
aminosulphonyl group may be independently of one another for example a
cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl group.
In the C3-C,-cycloalkyl-C,-Cs-alkyleneaminosulphonyl groups of the radicals R4
to R6,
each of the C3-C,-cycloalkyl groups of the C3-C,-cycloalkyl-C,-C6-
alkyleneaminosulphonyl groups, for example of a cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl or cycloheptyl group, can be combined independently of one another
with
each C,-C6-alkyleneaminosulphonyl group, for example with a methyleneaminosul-
phonyl, ethyleneaminosulphonyl, propyleneaminosulphonyl,
butyleneaminosulphonyl,
pentyleneaminosulphonyl, hexyleneaminosulphonyl group.
The C,-C6-alkylsulphonylamido groups for the radicals R4 to R6 may be for
example a
methylsulphonylamido, ethylsulphonylamido, propyisulphonylamido,
isopropylsulphon-
ylamido, butylsulphonylamido, isobutylsulphonylamido, sec-butylsulphonylamido,
tert-
butylsulphonylamido, pentylsulphonylamido, isopentylsulphonylamido, (2-
methylbutyl)-
sulphonylamido, (1-methylbutyl)sulphonylamido, (1-ethylpropyl)sulphonylamido,
neopentylsulphonylamido, (1,1-dimethylpropyl)sulphonylamido,
hexylsulphonylamido,
(4-methylpentyl)sulphonylamido, (3-methylpentyl)sulphonylamido, (2-
methylpentyl)-
sulphonylamido, (1-methylpentyl)sulphonylamido, (1-ethylbutyl)sulphonylamido,
(2-
ethylbutyl)sulphonylamido, (3,3-dimethylbutyl)sulphonylamido, (2,2-
dimethylbutyl)-
sulphonylamido, (1,1-dimethylbutyl)sulphonylamido, (2,3-
dimethylbutyl)sulphonylamido,
(1,3-dimethylbutyl)sulphonylamido or a (1,2-dimethylbutyl)sulphonylamido
group.
In the -N(Co-C6-alkyl)-C(O)-C,-C6-aIkyl groups of the radicals R4 to R6, each
of the
(Co-C6-alkyl) groups on the nitrogen atom of the -N(Co-Cs-alkyl)-C(O)-C,-C6-
aIkyl
groups, for example a hydrogen, a methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-
butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1 -
ethyl propyl),
neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl), (3-methylpentyl),
(2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl),
(2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-
dimethylbutyl) or a
(1,2-dimethylbutyl) group, may be combined independently of one another with
each
C,-C6-alkyl group on the carbonyl group of the amide, for example with a
methyl, ethyl,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
23
propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl,
(2-methylbutyl),
(1-methylbutyl), (1 -ethyl propyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-
methylpentyl),
(3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-
ethylbutyl), (3,3-
dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl),
(1,3-
dimethylbutyl) or a (1,2-dimethylbutyl) group.
In the -N-(Co-C6-alkyl)-C(O)-C3-C7-cycloalkyl groups of the radicals R4 to R6,
each of
the (Co-Cs-alkyl) groups on the nitrogen atom of the -N(Co-C6-alkyl)-C(O)-C,-
C6-alkyl
groups, for example a hydrogen, a methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-
butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1-
ethylpropyl),
neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl), (3-methylpentyl), (2-
methyl-
pentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl),
(2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-
dimethylbutyl) or a (1,2-
dimethylbutyl) group, may be combined independently of one another with each
C3-C7-
cycloalkyl group on the carbonyl group of the amide, for example with a
cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl group.
In the -N(Co-C6-alkyl)-C(O)-N-di(Co-C6-alkyl) groups of the radicals R4 to R6,
all three
(Co-C6-alkyl) groups may be independently of one another a hydrogen, a methyl,
ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl,
(2-methylbutyl),
(1-methylbutyl), (1-ethylpropyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-
methylpentyl),
(3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-
ethylbutyl), (3,3-
dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl),
(1,3-
dimethylbutyl) or a (1,2-dimethylbutyl) group.
In the -N(Co-Cs-alkyl)-C(O)-O-(Co-C6-alkyl) groups of the radicals R4 to R6,
both
(Co-C6-alkyl) groups may be independently of one another a hydrogen, a methyl,
ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl,
(2-methylbutyl),
(1-methylbutyl), (1-ethylpropyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-
methylpentyl),
(3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-
ethylbutyl), (3,3-
dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl),
(1,3-
dimethylbutyl) or a (1,2-dimethylbutyl) group.
In the -N(Co-C6-alkyl)-C(O)-NH-(C3-C,-cycloalkyl) groups of the radicals R4 to
R6, each
of the (Co-C6-alkyl) groups on the nitrogen atom of the -N(Co-C6-aIkyl)-C(O)-
NH-(C3-C,-
cycloalkyl) groups, for example a hydrogen, a methyl, ethyl, propyl,
isopropyl, butyl,
isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-
methylbutyl),
(1-ethylpropyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl),
(3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-
ethylbutyl), (3,3-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
24
dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl),
(1,3-
dimethylbutyl) or a (1,2-dimethylbutyl) group, may independently of one
another be
combined with each C3-C7-cycloalkyl group on the terminal nitrogen atom of the
urea,
for example with a cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or
cycloheptyl
group.
In the -N(Co-C6-alkyl)-SO2-(C,-C6-alkyl) groups of the radicals R4 to R6, each
of the
(Co-Cs-alkyl) groups on the nitrogen atom of the -N(Co-Cs-alkyl)-SO2-(C,-Cs-
alkyl)
group, for example a hydrogen, a methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-
butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1-
ethylpropyl),
neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl), (3-methylpentyl),
(2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl),
(2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-
dimethylbutyl) or a (1,2-
dimethylbutyl) group, may independently of one another be combined with each
C,-C6-
alkyl group on the sulphonyl group of the sulphonamide, for example with a
methyl,
ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl,
isopentyl, (2-
methylbutyl), (1-methylbutyl), (1-ethylpropyl), neopentyl, (1,1-
dimethylpropyl), hexyl, (4-
methylpentyl), (3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-
ethylbutyl), (2-
ethylbutyl), (3,3-dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl),
(2,3-
dimethylbutyl), (1,3-dimethylbutyl) or a (1,2-dimethylbutyl) group.
In the -N(Co-C6-alkyl)-SO2-C3-C,-cycloalkyl groups of the radicals R4 to R6,
each of the
(Co-C6-alkyl) groups on the nitrogen atom of the -N(Co-Cs-alkyl)-SO2-C3-C,-
cycloalkyl
group, for example a hydrogen, a methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-
butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1 -
ethyl propyl),
neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl), (3-methylpentyl),
(2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl),
(2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-
dimethylbutyl) or a (1,2-
dimethylbutyl) group, may be combined independently of one another with each
C3-C,-
cycloalkyl group on the sulphonyl group, for example with a cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl or cycloheptyl group.
In the -N(Co-Cs-alkyl)-SO2-N-di(Co-C6-alkyl) groups of the radicals R4 to R6,
all three
(Co-C6-alkyl) groups may be independently of one another a hydrogen, a methyl,
ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl,
(2-methylbutyl),
(1-methylbutyl), (1-ethylpropyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-
methylpentyl),
(3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-
ethylbutyl), (3,3-
dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl),
(1,3-
dimethylbutyl) or a (1,2-dimethylbutyl) group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
In the -N(Co-C6-alkyl)-SO2-NH-(C3-C,)-cycloalkyl groups of the radicals R4 to
R6, the
Co-Cs-alkyl group of the -N(Co-C6-alkyl)-SO2-NH-(C3-C7)-cycloalkyl group, for
example
a hydrogen, a methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl,
tert-butyl, pen-
tyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1 -ethyl propyl),
neopentyl, (1,1-
5 dimethylpropyl), hexyl, (4-methylpentyl), (3-methylpentyl), (2-
methylpentyl),
(1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-dimethylbutyl), (2,2-
dimethylbutyl),
(1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-dimethylbutyl) or a (1,2-
dimethylbutyl)
group, may be combined independently of one another with each C3-C,-cycloalkyl
group, for example with a cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or
cycloheptyl
10 group.
In the -C(O)-N(H)-C2-C6-alkylene-(C,-C6-alkyl)amine groups of the radicals R4
to R6,
each of the C2-C6-alkylene groups on the nitrogen atom of the -C(O)-N(H)-C2-C6-
alkylene-(C,-C6-alkyl)amine group, for example an ethylene, propylene,
butylene, penty-
lene or hexylene group, may be combined independently of one another with each
C,-
15 C6-alkyl group on the amino group, for example with a methyl, ethyl,
propyl, isopropyl,
butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-
methylbutyl), (1-
ethylpropyl), neopentyl, (1,1-dimethylpropyl), hexyl, (4-methylpentyl), (3-
methylpentyl),
(2-methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl),
(2,2-dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-
dimethylbutyl) or a (1,2-
20 dimethylbutyl) group.
In the -C(O)-N(H)-C2-C6-alkylene-[di(C,-Cs-alkyl)]amine groups of the radicals
R4 to
R6, each of the C2-C6-alkylene groups on the nitrogen atom of the -C(O)-N(H)-
Cz-C6-
alkylene-[di(C,-C6-aIkyl)]amine group, for example an ethylene, propylene,
butylene,
pentylene or hexylene group, may be combined independently of one another with
each
25 of the two identically or different C,-C6-alkyl groups on the amino group,
for example
with a methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-
butyl, pentyl,
isopentyl, (2-methylbutyl), (1-methylbutyl), (1-ethylpropyl), neopentyl, (1,1-
dimethyl-
propyl), hexyl-, (4-methylpentyl), (3-methylpentyl), (2-methylpentyl), (1-
methylpentyl),
(1-ethylbutyl), (2-ethylbutyl), (3,3-dimethylbutyl), (2,2-dimethylbutyl), (1,1-
dimethylbutyl),
(2,3-dimethylbutyl), (1,3-dimethylbutyl) or a (1,2-dimethylbutyl) group.
In the -C(O)-N(H)-CZ-C6-alkylene-(C3-C7-cycloalkyl)amine groups of the
radicals R4 to
R6, each of the (C2-C6-alkylene) groups of the -C(O)-N(H)-C2-C6-alkylene-(C3-
C,-cyclo-
alkyl)amine group, for example an ethylene, propylene, butylene, pentylene or
hexylene
group, may be combined independently of one another with each C3-C7-cycloalkyl
group on the amine, for example with a cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl
or cycloheptyl group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
26
In the -C(O)-N(H)-C2-Cs-alkylene-(C3-C,-cycloalkyl-C,-C6-alkylene)amine groups
of the
radicals R4 to R6, each of the (C2-Cs-alkylene) groups of the -C(O)-N(H)-C2-C6-
alkylene-(C3-C6-cycloalkyl-C1-C6-alkylene)amine group, for example an
ethylene, pro-
pylene, butylene, pentylene or hexylene group, may be combined independently
of one
another with each C3-C7-cycloalkyl-C,-C6-alkyiene group on the amine, for
example with
a cyclopropylmethylene, cyclopropylethylene, cyclopropylpropylene,
cyclopropylbuty-
lene, cyclopropylpentylene, cyclopropylhexylene, cyclobutylmethylene,
cyclobutylethyl-
ene, cyclobutylpropylene, cyclobutylbutylene, cyclobutylpentylene,
cyclobutylhexylene,
cyclopentylmethylene, cyclopentylethylene, cyclopentylpropylene,
cyclopentylhexylene,
cyclohexylmethylene, cyclohexylethylene, cyclohexylpropylene,
cyclohexylbutylene,
cyclohexylpentylene, cyclohexylhexylene, cycloheptylmethylene,
cycloheptylethylene,
cycloheptylpropylene, cycloheptylbutylene, cycloheptylpentylene or
cycloheptylhexylene
group.
In the -S(OZ)-N(H)-C2-C6-alkylene-(C,-Cs-alkyl)amine groups of the radicals R4
to R6,
the (C2-C6-alkylene) groups of the -S(02)-N(H)-C2-C6-alkylene-(C,-Cs-
alkyl)amine
group, for example an ethylene, propylene, butylene, pentylene or hexylene
group, may
be combined independently of one another with each C,-Cs-alkyl group on the
amino
group, for example with a methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
sec-butyl, tert-
butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1-ethylpropyl),
neopentyl, (1,1-
dimethylpropyl), hexyl, (4-methylpentyl), (3-methylpentyl), (2-methylpentyl),
(1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-dimethylbutyl), (2,2-
dimethylbutyl),
(1, 1 -dimethylbutyl), (2,3-dimethylbutyl), (1,3-dimethylbutyl) or a (1,2-
dimethylbutyl)
group.
In the -S(O2)-N(H)-CZ-C6-alkylene-[di(C,-C6-alkyl)]amine groups of the
radicals R4 to
R6, the C2-C6-alkylene group of the -S(02)-N(H)-C2-C6-alkylene-[di(C,-C6-
alkyl)]amine
group, for example an ethylene, propylene, butylene, pentylene or hexylene
group, may
be combined independently of one another with each of the two C,-C6-alkyl
groups on
the amino group, for example with a methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-
butyl, tert-butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1-
ethylpropyl),
neopentyl, (1,1-dimethylpropyl), hexyl-, (4-methylpentyl), (3-methylpentyl),
(2-
methylpentyl), (1-methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-
dimethylbutyl), (2,2-
dimethylbutyl), (1,1-dimethylbutyl), (2,3-dimethylbutyl), (1,3-dimethylbutyl)
or a (1,2-
dimethylbutyl) group.
In the -S(OZ)-N(H)-C2-C6-alkylene-(C3-C,-cycloalkyl)amine groups of the
radicals R4 to
R6, the Cz-C6-alkylene group of the -S(OZ)-N(H)-CZ-C6-alkylene-(C3-C7-
cycloalkyl)-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
27
amine group, for example an ethylene, propylene, butylene, pentylene or
hexylene
group, may be combined independently of one another with each C3-C7-cycloalkyl
group on the amino group, for example with a cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl or cycloheptyl group.
In the -S(02)-N(H)-C2-C6-alkylene-(C3-C,-cycloalkyl-C,-C6-alkylene)amine
groups
of the radicals R4 to R6, each C2-C6-alkylene group of the -S(O2)-N(H)-CZ-Cs-
alkylene-
(C3-C7-cycloalkyl-C,-C6-alkylene)amine group, for example an ethylene,
propylene, bu-
tylene, pentylene or hexylene group, may be combined independently of one
another
with each C3-C7-cycloalkyl-C,-C6-alkylene group on the amine, for example with
a
cyclopropylmethylene, cyclopropylethylene, cyclopropylpropylene,
cyclopropylbutylene,
cyclopropylpentylene, cyclopropylhexylene, cyclobutylmethylene,
cyclobutylethylene,
cyclobutylpropylene, cyclobutylbutylene, cyclobutylpentylene,
cyclobutylhexylene,
cyclopentylmethylene, cyclopentylethylene, cyclopentylpropylene,
cyclopentylhexylene,
cyclohexylmethylene, cyclohexylethylene, cyclohexylpropylene,
cyclohexylbutylene,
cyclohexylpentylene, cyclohexylhexylene, cycloheptylmethylene,
cycloheptylethylene,
cycloheptylpropylene, cycloheptylbutylene, cycloheptylpentylen or
cycloheptylhexylene
group.
In the -O-C2-Cs-alkylene-(C,-C6-alkyl)amine groups of the radicals R4 to R6,
the C2-C6-
alkylene group of the -O-C2-Cs-alkylene-(C,-C6-alkyl)amine group, for example
an eth-
ylene, propylene, butylene, pentylene or hexylene group, may be combined
independ-
ently of one another with each C,-C6-alkyl group on the amino group, for
example a
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl,
pentyl, isopentyl, (2-
methylbutyl), (1-methylbutyl), (1-ethylpropyl), neopentyl, (1,1-
dimethylpropyl), hexyl, (4-
methylpentyl), (3-methylpentyl), (2-methylpentyl), (1-methylpentyl), (1-
ethylbutyl), (2-
ethylbutyl), (3,3-dimethylbutyl), (2,2-dimethylbutyl), (1,1-dimethylbutyl),
(2,3-
dimethylbutyl), (1,3-dimethylbutyl) or a (1,2-dimethylbutyl) group.
In the -O-C2-C6-alkylene-[di(C,-C6-alkyl)]amine groups of the radicals R4 to
R6, the C2-
C6-alkylene group of the -O-C2-C6-alkylene-[di(C,-C6-alkyl)]amine group, for
example an
ethylene, propylene, butylene, pentylene or hexylene group, may be combined
inde-
pendently of one another with two freely selectable C,-C6-alkyl groups on the
amino
group, for example with a methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
sec-butyl, tert-
butyl, pentyl, isopentyl, (2-methylbutyl), (1-methylbutyl), (1-ethylpropyl),
neopentyl, (1,1-
dimethylpropyl), hexyl-, (4-methylpentyl), (3-methylpentyl), (2-methylpentyl),
(1-
methylpentyl), (1-ethylbutyl), (2-ethylbutyl), (3,3-dimethylbutyl), (2,2-
dimethylbutyl), (1,1-
dimethylbutyl), (2,3-dimethylbutyl), (1,3-dimethylbutyl) or a (1,2-
dimethylbutyl) group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
28
Compounds preferred according to the present invention are those of the
formula II and
III
R1\ R2
N
R7 R8
HO
O NH
R6
T TZ
~ R3
R5 ~\T,T1 II
R4
R1\ R2
N
R7 R8
HO
O NH
T 4~T
4R3
X~N T~T2
i
R6 \/~/
VV III
R5
R4
in which the radicals R1 to R8 and W have the same meaning as in formula I and
X is a bond, C,-C4-alkylene, C2-C4-alkenylene, C2-C4-alkynylene;
T is a nitrogen atom or a CH group;
T1, T2, T3, T4 are each independently of one another a nitrogen atom or an R3-
C
group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
29
Compounds likewise preferred according to the present invention are those of
formula
Ila and Illa
R1\ ~ R2
N
\ /
HO
0 NH
T 4-, T
I I 4R3
_ N T~Tz
X/ ,
R6 \/~/
VV Illa
R5
R4
in which the radicals R1 to R6 and W have the same meaning as in formula Ia
and
X is a bond, C,-C4-alkylene, C2-C4-alkenylene, C2-C4-alkynylene;
T is a nitrogen atom or a CH group;
T1, T2, T3, T4are each independently of one another a nitrogen atom or an R3-C
group.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
Compounds particularly preferred according to the present invention are those
of the
formula IV and V
R1\ R2
N
R7 R8 H
HO
O NH
H
R3
R6 \/~/
~V H
R5 IV
5 R4
R1\ R2
N
R7 R8 H
HO
O NH
H
R3
R6 ' A' N
VV V
R5
R4
10 in which the radicals R1 to R8 and W have the same meaning as in formula I.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
31
Compounds likewise particularly preferred according to the present invention
are those
of the formula IVa and Va
R1
\ ~ R2
N
~ /
H
HO
O NH
H
R3
R6 \/~/
VV
R5 IVa
R4
R1\ ~ R2
N
~ /
H
HO
O NH
H
R3
R6 \/~/
VV Va
R5
R4
in which the radicals R1 to R6 and W have the same meaning as in formula Ia.
The following compounds are very particularly preferred:
I N-[(R,S)-2-(5-Bromo-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
2 N-[(R,S)-1-(Hydroxymethyl)-2-(5-methyl-1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
3 N-[(R,S)-1-(Hydroxymethyl)-2-(4-methyl-1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
4 N-[(R,S)-1-(Hydroxymethyl)-2-(6-methyl-1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
5 N-[(R)-1-(Hydroxymethyl)-2-(1-methyl-lH-indol-3-yl)ethyl]-2-(3,4,5-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
32
trimethoxyphenyl)quinoline-4-carboxamide;
6 N-[(R,S)-2-(5-Fluoro-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
7 N-[(R,S)-1-(Hydroxymethyl)-2-(5-methoxy-1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
8 N-[(R, S)-2-(6-Fluoro-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-2-(3,4, 5-
trimethoxyphenyl)quinoline-4-carboxamide;
9 N-[(R,S)-1-(Hydroxymethyl)-2-[5-(phenylmethoxy)-1 H-indol-3-yl]ethyl]-2-
(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
N-[(R,S)-1-(Hydroxymethyl)-2-(7-methyl-1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
11 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
12 N-[(S)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
13 2-(4-Chloro-3-methylphenyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yI)ethyl]quinoline-4-carboxamide;
14 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(3,4, 5-
trimethoxyphenyl)quinoline-4-carboxamide;
6-Bromo-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
16 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(2,3,4-
trimethoxyphenyl)quinoline-4-carboxamide;
17 6-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
18 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-iodo-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
19 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-nitro-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
6-Amino-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
21 N-[(R,S)-1-(Hydroxymethyl)-2-(5-fluoro-1 H-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
22 N-[(R)-1-(Hydroxymethyl)-2-(1-methyl-1 H-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
23 N-[(R,S)-2-(6-Fluoro-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-6-methoxy-2-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
33
(3,4,5-trimethoxyphenyl)quinoline-4-carboxamide;
24 2-(3,4-Dimethoxyphenyl)-N-[(S)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]quinoline-4-carboxamide;
25 2-(3,4-Dimethoxyphenyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]quinoline-4-carboxamide;
26 2-(3,4-Dimethylphenyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yI)ethyl]quinoline-4-carboxamide;
27 2-(2,3-Dihydro-1,4-benzodioxin-6-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-
3-
yi)ethyl]quinoline-4-carboxamide;
28 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyt]-2-[4-
(trifluoromethoxy)phenyl]quinoline-4-carboxamide;
29 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-[4-
(methylsulphanyl)phenyl]quinoline-4-carboxamide;
30 2-(3,5-Dimethoxyphenyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]quinoline-4-carboxamide;
31 2-[3-(Acetylamino)phenyl]-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yI)ethyl]quinoline-4-carboxamide;
32 2-(4-Chlorophenyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]quinoline-4-
carboxamide;
33 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(4-
methoxyphenyl)quinoline-
4-carboxamide;
34 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3-
methoxyphenyl)quinoline-
4-carboxamide;
35 N-[(R)-2-(1-Ethyl-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-6-methoxy-2-
(3,4,5-tri-
methoxyphenyl)quinoline-4-carboxamide;
36 2-(2,3-Dihydrobenzofuran-5-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yI)ethyl]quinoline-4-carboxamide;
37 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(7-
methoxybenzofuran-2-yl)quinoline-4-carboxamide;
38 2-[(Z)-2-(3,4-Dimethoxyphenyl)ethenyl]-N-[(R)-1-(hydroxymethyl)-2-(1 H-
indol-
3-yl)ethyl]-6-methoxyquinoline-4-carboxamide;
39 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-4'-methoxy[1,1'-biphenyl]-
2-
carboxamide;
40 N-[(S)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3',4'-dimethoxy[1,1'-
biphenyl]-
3-carboxamide;
41 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4'-dimethoxy[1,1'-
biphenyl]-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
34
3-carboxamide;
42 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-2',3',4'-trimethoxy[1,1'-
biphenyl]-3-carboxamide;
43 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4',5'-trimethoxy[1,1'-
biphenyl]-3-carboxamide;
44 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4',5'-trimethoxy[1,1'-
biphenyl]-4-carboxamide;
45 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4',5'-trimethoxy-2-
methyl[1,1'-biphenyl]-4-carboxamide;
46 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-2',3',4'-trimethoxy[1,1'-
biphenyl]-2-carboxamide;
47 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4',5'-trimethoxy[1,1'-
biphenyl]-2-carboxamide;
48 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',3',4'-trimethoxy-6-
methyl[1,1'-biphenyl]-3-carboxamide;
49 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4',5'-trimethoxy-6-
methyl[1,1'-biphenyl]-3-carboxamide;
50 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',3',4'-trimethoxy[1,1'-
biphenyl]-4-carboxamide;
51 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',3',4'-trimethoxy-2-
methyl[1,1'-biphenyl]-4-carboxamide;
52 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',3',4,4'-
tetramethoxy[1,1'-
biphenyl]-2-carboxamide;
53 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3',4,4',5'-
tetramethoxy[1,1'-
biphenyl]-2-carboxamide;
54 4'-(Hydroxymethyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-
methyl[1,1'-biphenyl]-3-carboxamide;
55 4'-(Hydroxymethyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-
methyl[1,1'-biphenyl]-4-carboxamide;
56 4'-(Hydroxymethyl)-N-[(R)-1-(hydroxymethyl)-2-(1H-indol-3-yl)ethyl][1,1'-
biphenyl]-2-carboxamide;
57 4'-(Hydroxymethyl)-N-[(R)-1-(hydroxymethyl)-2-(1H-indol-3-yl)ethyl][1,1'-
biphenyl]-3-carboxamide;
58 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4-methoxy-3'-(1-
methylethyl)[1,1'-biphenyl]-2-carboxamide;
59 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3'-(1-methylethyl)[1,1'-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
biphenyl]-3-carboxamide;
60 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methyl-3'-(1-
methylethyl)[1,1'-biphenyl]-3-carboxamide;
61 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3'-(1-methylethyl)[1,1'-
biphenyl]-4-carboxamide;
62 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-2-methyl-3'-(1-
methylethyl)[1,1'-biphenyl]-4-carboxamide;
63 4'-(Hydroxymethyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4-
methoxy[1,1'-biphenyl]-2-carboxamide;
64 3',4',5'-Trifluoro-N-[(R)-1-(hydroxymethyl)-2-(1H-indol-3-yl)ethyl][1,1'-
biphenyl]-
2-carboxamide;
65 3',4',5'-Trifluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl][1,1'-
biphenyl]-
3-carboxamide;
66 3',4',5'-Trifluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-
methyl[1,1'-
biphenyl]-3-carboxamide;
67 3',4', 5'-Trifluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl][ 1,1'-
biphenyl]-
4-carboxamide;
68 3',4',5'-Trifluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yi)ethyl]-2-
methyl[1,1'-
biphenyl]-4-carboxamide;
69 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',5'-dimethoxy[1,1'-
biphenyl]-
2-carboxamide;
70 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',4,5'-trimethoxy[1,1'-
biphenyl]-2-carboxamide;
71 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',5'-dimethoxy-6-
methyl[1,1'-
biphenyl]-3-carboxamide;
72 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-2',5'-dimethoxy[1,1'-
biphenyl]-
4-carboxamide;
73 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-2',5'-dimethoxy-2-
methyl[1,1'-
biphenyl]-4-carboxamide;
74 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3',4'-dimethoxy[1,1'-
biphenyl]-
2-carboxamide;
75 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4,4'-trimethoxy[1,1'-
biphenyl]-2-carboxamide;
76 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4'-dimethoxy-6-
methyl[1,1'-
biphenyl]-3-carboxamide;
77 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4'-dimethoxy[1,1'-
biphenyl]-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
36
4-carboxamide;
78 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3',4'-dimethoxy-2-
methyl[1,1'-
biphenyl]-4-carboxamide;
79 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4'-methoxy[1,1'-
biphenyl]-2-carboxamide;
80 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4,4'-
dimethoxy[1,1'-
biphenyl]-2-carboxamide;
81 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4'-methoxy[1,1'-
biphenyl]-3-carboxamide;
82 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4'-methoxy-6-
methyl[1,1'-biphenyl]-3-carboxamide;
83 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4'-methoxy[1,1'-
biphenyl]-4-carboxamide;
84 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4'-methoxy-2-
methyl[1,1'-biphenyl]-4-carboxamide;
85 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3',4-dimethoxy[1,1'-
biphenyl]-
2-carboxamide;
86 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3'-(1-methylethyl)[1,1'-
biphenyl]-2-carboxamide;
87 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2',5'-dimethoxy[1,1'-
biphenyl]-
3-carboxamide;
88 3',4',5'-Trifluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4-
methoxy[1,1'-biphenyl]-2-carboxamide;
89 3-(Benzofuran-2-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yI)ethyl]benzamide;
90 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3-(5-methoxybenzofuran-2-
yl)-
benzamide;
91 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-[(3,4,5-
trimethoxyphenyl)methoxy]phenylpropanamide
92 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4-[[(3,4,5-
trimethoxyphenyl)methoxy]methyl]benzamide;
93 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3-[(3,4,5-
trimethoxyphenyl)methoxy]thiophene-2-carboxamide;
94 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4-[(3,4,5-
trimethoxyphenyl)methoxy]phenylacetamide;
95 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3-[(3,4,5-
trimethoxyphenyl)-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
37
methoxy]phenylpropanamide;
96 2-[2-(3,4-Dimethoxyphenyl)ethyl]-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yi)ethyl]-6-methoxyquinoline-4-carboxamide;
487 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yi)ethyl]-2-(3,4, 5-
trimethoxyphenyl)-
1,6-naphthyridine-4-carboxamide;
488 6-Bromo-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)-1,8-naphthyridine-4-carboxamide;
97 2-(6-Methoxynaphthalen-2-yl)quinoline-4-carboxylic acid [(R)-1-
hydroxymethyl-
2-(1 H-indol-3-yl)ethyl]amide;
98 6-Methoxy-2-(3-methoxyphenyl)quinoline-4-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yi)ethyl]amide;
99 2-(4-Fluoro-3-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
100 2-(3-Iodo-4-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
101 2-(3-Hydroxyphenyl)-6-methoxyquinoline-4-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yi)ethyl]amide;
102 2-(4-Hydroxy-3,5-dimethoxyphenyl)-6-methoxyquinoline-4-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
103 2-(3,5-Difluoro-4-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid [(R)-
2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
104 2-(3-Ethylphenyl)-6-methoxyquinoline-4-carboxylic acid [(R)-1-
hydroxymethyl-
2-(1 H-indol-3-yl)ethyl]amide;
105 2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
106 2-(3-Fluoro-4-methoxyphenyl)-6-methylquinoline-4-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
107 6-Methyl-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
108 6-Bromo-2-(2,4-dimethylthiazol-5-yl)quinoline-4-carboxylic acid [(R)-2-
hydroxy-
1-(1 H-indol-3-ylmethyl)ethyl]amide;
109 2-(7-Methoxybenzofuran-2-yl)-6-trifluoromethoxyquinoline-4-carboxylic acid
[(R)-2-hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
110 2-(3-Fluoro-4-methoxyphenyl)-6-iodoquinoline-4-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
111 2-(3-Fluoro-4-methoxyphenyl)-6-trifluoromethoxyquinoline-4-carboxylic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
38
[(R)-2-hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
112 2-(3-Fluoro-4-methoxyphenyl)-6,8-dimethylquinoline-4-carboxylic acid [(R)-
2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
113 2-(3,4-Dimethoxyphenyl)-6-methoxy-3-methylquinoline-4-carboxylic acid [(R)-
1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
114 6-Amino-2-(3-fluoro-4-methoxyphenyl)quinoline-4-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
115 2-(4,6-Dimethoxybenzofuran-2-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yI)ethyl]-6-methoxyquinoline-4-carboxamide;
116 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(5-
methoxybenzofuran-2-yl)quinoline-4-carboxamide;
117 2-(7-Ethoxybenzofuran-2-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]-6-
methoxyquinoline-4-carboxamide;
118 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(6-
methoxybenzofuran-2-yl)quinoline-4-carboxamide;
119 2-(7-Fluorbenzofuran-2-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]-6-
methoxyquinoline-4-carboxamide;
120 2-(4-Fluorbenzofuran-2-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]-6-
methoxyquinoline-4-carboxamide;
121 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(5-
methylbenzofuran-2-yl)quinoline-4-carboxamide;
122 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(7-
methylbenzofuran-2-yl)quinoline-4-carboxamide;
123 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-(4-
methoxybenzofuran-2-yl)quinoline-4-carboxamide;
124 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-6-methoxy-2-[5-
(trifluoromethoxy)benzofuran-2-yl]quinoline-4-carboxamide;
125 4-Ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-indol-3-
yl)ethyl]amide;
126 4-Ethoxy-3'-fluoro-4'-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1H-
indol-3-ylmethyl)ethyl]amide;
127 2-Ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(6-
methoxypyridin-3-
yl)-benzamide;
128 4-Ethoxy-2'-fluoro-3'-methoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1H-
indol-3-ylmethyl)ethyl]amide;
129 4'-Acetylamino-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1H-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
39
indol-3-yl)ethyl]amide;
130 2-Ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(2-
methoxypyrimidin-5-yl)-benzamide;
131 4-Ethoxy-5'-fluoro-3'-methoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
132 4-Ethoxy-3',4'-difluoro-5'-methoxybiphenyl-3-carboxylic acid [(R)-2-
hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
133 4-Ethoxy-4'-fluoro-3'-methoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
134 3',5'-Dimethoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
135 4-Ethoxy-3'-hydroxymethylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
136 4-Ethoxy-3'-methylsulphanylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1
H-
indol-3-ylmethyl)ethyl]amide;
137 3'-Cyano-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-
indol-
3-yI)ethyl]amide;
138 2-Ethoxy-5-(6-fluoro-5-methylpyridin-3-yl)-N-[(R)-2-hydroxy-1-(1 H-indol-3-
ylmethyl)ethyl]benzamide;
139 4-Ethoxy-4'-trifiuoromethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
140 5-Benzo[b]thiophene-3-yl-2-ethoxy-N-[(R)-2-hydroxy-1-(1 H-indol-3-
ylmethyl)ethyl]benzamide;
141 4-Ethoxy-2'-trifluoromethylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1
H-
indol-3-ylmethyl)ethyl]amide;
142 4-Ethoxy-2'-trifluoromethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
143 4-Ethoxy-3'-trifluoromethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1H-
indol-3-yimethyl)ethyl]amide;
144 4-Ethoxy-3'-fluorobiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-indol-
3-
ylmethyl)ethyl]amide;
145 4'-Chloro-4-ethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-indol-
3-
ylmethyl)ethyl]amide;
146 4-Ethoxy-4'-methylsulphanylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1
H-
indol-3-ylmethyl)ethyl]amide;
147 4-Ethoxy-3'-trifluoromethylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1
H-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
indol-3-ylmethyl)ethyl]amide;
148 3'-Chloro-4-ethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-indol-
3-
ylmethyl)ethyl]amide;
149 4-Ethoxy-3'-methylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-
indol-
3-yl)ethyl]amide;
150 5-Benzofuran-2-yl-2-ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-
yI)ethyl]benzamide;
151 4-Ethoxy-2'-methylsulphanylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1
H-
indol-3-ylmethyl)ethyl]amide;
152 2-Ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(1 H-indol-4-
yl)benzamide;
153 2-Ethoxy-N-[(R)-2-hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]-5-(4-
methylthiophen-2-
yI)-benzamide;
154 3'-Acetylamino-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-
indol-3-yl)ethyl]amide;
155 4-Ethoxy-2'-methylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-
3-yI)ethyl]amide;
156 2-Ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(5-methylfuran-
2-yl)-
benzamide;
157 3'-Chloro-4-ethoxy-4'-methylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1H-
indol-3-ylmethyl)ethyl]amide;
158 5-(2-Chloro-6-methylpyridin-3-yl)-2-ethoxy-N-[(R)-2-hydroxy-1-(1 H-indol-3-
ylmethyl)ethyl]benzamide;
159 4-Ethoxy-4'-fluorobiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1H-indol-3-
ylmethyl)ethyl]amide;
160 2-Ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-naphthalen-1-yl-
benzamide;
161 5-Benzo[b]thiophene-2-yl-2-ethoxy-N-[(R)-2-hydroxy-1-(1 H-indol-3-
ylmethyl)ethyl]benzamide;
162 4-Ethoxy-4'-methylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-
3-yI)ethyl]amide;
163 2-Ethoxy-N-[(R)-2-hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]-5-thiophen-3-yl-
benzamide;
164 4-Ethoxy-4'-methoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-yl)ethyl]amide;
165 2',4'-Dichloro-4-ethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-
indol-3-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
41
ylmethyl)ethyl]amide;
166 4'-Methoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-yl)ethyl]amide;
167 4-Propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-indol-3-
yl)ethyl]amide;
168 5-Benzofuran-2-yI-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-2-
propoxybenzamide;
169 3'-Chloro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-indol-
3-
ylmethyl)ethyl]amide;
170 5-Benzo[b]thiophene-2-yI-N-[(R)-2-hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]-2-
propoxybenzamide;
171 3'-Fluoro-4'-methyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
172 4-Propoxy-3'-trifluoromethylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
173 2'-Fluoro-5'-methoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
174 4-Propoxy-3',5'-bis-trifluoromethylbiphenyl-3-carboxylic acid [(R)-2-
hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
175 4'-Chloro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-indol-
3-
ylmethyl)ethyl]amide;
176 5-Benzo[b]thiophene-3-yl-N-[(R)-2-hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]-2-
propoxybenzamide;
177 4-Propoxy-4'-trifluoromethylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
178 3'-Hydroxy-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-yl)ethyl]amide;
179 N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-2-propoxy-5-quinolin-6-yl-
benzamide;
180 5-(6-Fluoro-5-methylpyridin-3-yl)-N-[(R)-2-hydroxy-1-(1 H-indol-3-
ylmethyl)ethyl]-2-propoxybenzamide;
181 N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(6-methoxypyridin-3-yl)-
2-
propoxybenzamide;
182 3'-Chloro-4'-methyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
183 N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-2-propoxy-5-pyridin-4-yl-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
42
benzamide;
184 3'-Chloro-4'-fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
185 3'-Acetylamino-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
186 3',4'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1H-
indol-3-
ylmethyl)ethyl]amide;
187 3',5'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-
indol-3-
ylmethyl)ethyl]amide;
188 3'-Cyano-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-yl)ethyl]amide;
189 5-(2,4-Dimethoxypyrimidin-5-yl)-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-
yl)ethyl]-
2-propoxybenzamide;
190 2',3'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1H-
indol-3-
ylmethyl)ethyl]amide;
191 2',5'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-
indol-3-
ylmethyl)ethyl]amide;
192 5-[(E)-2-(4-Fluorophenyl)-vinyl]-N-[(R)-2-hydroxy-1-(1 H-indol-3-
ylmethyl)ethyl]-
2-propoxybenzamide;
193 5-(5-Cyanothiophene-2-yl)-N-[(R)-2-hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]-
2-
propoxybenzamide;
194 2'-Fluoro-3'-methoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
195 N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(2-methoxypyrimidin-5-
yl)-2-
propoxybenzamide;
196 4'-Chloro-2',6'-difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-
hydroxy-l-
(1 H-indol-3-ylmethyl)ethyljamide;
197 3',5'-Dimethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1H-
indol-3-yl)ethyl]amide;
198 N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-2-propoxy-5-quinolin-3-yl-
benzamide;
199 4'-Acetylamino-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
200 4-Propoxy-3'-(2,2,2-trifluoroethoxy)biphenyl-3-carboxylic acid [(R)-2-
hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
201 3'-Ethoxy-5'-fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1 H-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
43
indol-3-ylmethyl)ethyl]amide;
202 5'-Ethoxy-2'-fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1H-
indol-3-ylmethyl)ethyl]amide;
203 3'-Ethoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-yl)ethyl]amide;
204 4-Propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1-methyl-1 H-
indol-3-yl)ethyl]amide;
205 5-Benzofuran-2-yl-N-[(R)-1-hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]-
2-
propoxybenzamide;
206 5-Benzo[b]thiophen-2-yl-N-[(R)-2-hydroxy-1-(1-methyl-1 H-indol-3-
ylmethyl)ethyl]-2-propoxybenzamide;
207 2'-Fluoro-4'-methyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
208 4'-Fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1-methyl-
1 H-
indol-3-ylmethyl)ethyl]amide;
209 2'-Fluoro-5'-methoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
210 3'-Fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1-methyl-
1H-
indol-3-ylmethyl)ethyl]amide;
211 N-[(R)-1-Hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]-2-propoxy-5-
pyridin-3-
yl-benzamide;
212 5-Benzo[b]thiophen-3-yI-N-[(R)-2-hydroxy-1 -(1-methyl-1 H-indol-3-
ylmethyl)ethyl]-2-propoxybenzamide;
213 3'-Cyano-4'-fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
214 N-[(R)-1-Hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]-5-(6-
methoxypyridin-3-
yl)-2-propoxybenzamide;
215 3'-Acetylamino-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1-
methyl-1 H-indol-3-yl)ethyl]amide;
216 3',4'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1-
methyl-
1 H-indol-3-ylmethyl)ethyl]amide;
217 3',5'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1-
methyl-
1 H-indol-3-ylmethyl)ethyl]amide;
218 5-(2,4-Dimethoxypyrimidin-5-yl)-N-[(R)-1-hydroxymethyl-2-(1-methyl-1 H-
indol-
3-yl)ethyl]-2-propoxybenzamide;
219 2',5'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1-
methyl-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
44
1 H-indol-3-ylmethyl)ethyl]amide;
220 5-[(E)-2-(4-Fluorophenyl)-vinyl]-N-[(R)-2-hydroxy-1 -(1 -methyl-1 H-indol-
3-
ylmethyl)ethyl]-2-propoxybenzamide;
221 5-(5-Cyanothiophen-2-yl)-N-[(R)-2-hydroxy-1-(1-methyt-1 H-indol-3-
ylmethyl)ethyl]-2-propoxybenzamide;
222 N-[(R)-1-Hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]-5-(2-
methoxypyrimidin-5-yl)-2-propoxybenzamide;
223 N-[(R)-1-Hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]-2-propoxy-5-
quinoline-
3-yl-benzamide;
224 5'-Fluoro-3'-methoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
225 4-Propoxy-3'-(2,2,2-trifluoroethoxy)biphenyl-3-carboxylic acid [(R)-2-
hydroxy-1-
(1-methyl-1 H-indol-3-ylmethyl)ethyl]amide;
226 5'-Ethoxy-2'-fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
227 4'-Methoxy-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-3-
yl)-1-
hydroxymethylethyl]amide;
228 5-Benzofuran-2-yl-N-[2-(5-fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-2-
propoxybenzamide;
229 3'-Methyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-3-yl)-
1-
hydroxymethylethyl]amide;
230 5-Benzo[b]thiophen-2-yl-N-[2-(5-fluoro-1 H-indol-3-yl)-1-
hydroxymethylethyl]-2-
propoxybenzamide;
231 2'-Fluoro-5'-methoxy-4-propoxybiphenyl-3-carboxylic acid [1 -(5-fluoro-1 H-
indol-
3-ylmethyl)-2-hydroxyethyl]amide;
232 4-Propoxy-3',5'-bis-trifluoromethylbiphenyl-3-carboxylic acid [1-(5-fluoro-
1 H-
indol-3-ylmethyl)-2-hydroxyethyl]amide;
233 N-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-2-propoxy-5-pyridin-3-
yl-
benzamide;
234 5-Benzo[b]thiophen-3-yl-N-[2-(5-fluoro-1 H-indol-3-yl)-1-
hydroxymethylethyl]-2-
propoxybenzamide;
235 3'-Cyano-4'-fluoro-4-propoxybiphenyl-3-carboxylic acid [1-(5-fluoro-1 H-
indol-3-
ylmethyl)-2-hydroxyethyl]amide;
236 N-[1 -(5-Fluoro-1 H-indol-3-ylmethyl)-2-hydroxyethyl]-5-(6-fluoro-5-
methylpyridin-
3-yl)-2-propoxybenzamide;
237 N-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-5-(6-methoxypyridin-3-
yl)-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
2-propoxybenzamide;
238 3'-Chloro-4'-fluoro-4-propoxybiphenyl-3-carboxylic acid [1 -(5-fluoro-1 H-
indol-3-
ylmethyl)-2-hydroxyethyl]amide;
239 3'-Acetylamino-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-
3-yl)-
1-hydroxymethylethyl]amide;
240 3',4'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [1 -(5-fluoro-1 H-indol-
3-
ylmethyl)-2-hydroxyethyl]amide;
241 3',5'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-
3-yl)-1-
hydroxymethylethyl]amide;
242 2',5'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [1 -(5-fluoro-1 H-indol-
3-
ylmethyl)-2-hydroxyethyl]amide;
243 N-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-5-[(E)-2-(4-
fluorophenyl)-
vinyl]-2-propoxybenzamide;
244 5-(5-Cyanothiophen-2-yl)-N-[2-(5-fluoro-1 H-indol-3-yl)-1-
hydroxymethylethyl]-2-
propoxybenzamide;
245 2'-Fluoro-3'-methoxy-4-propoxybiphenyl-3-carboxylic acid [1 -(5-fluoro-1 H-
indol-
3-ylmethyl)-2-hydroxyethyl]amide;
246 N-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-5-(2-methoxypyrimidin-
5-
yl)-2-propoxybenzamide;
247 N-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-2-propoxy-5-quinolin-
3-yl-
benzamide;
248 4-Propoxy-3'-(2,2,2-trifluoroethoxy)biphenyl-3-carboxylic acid [1-(5-
fluoro-1 H-
indol-3-ylmethyl)-2-hydroxyethyl]amide;
249 5'-Ethoxy-2'-fluoro-4-propoxybiphenyl-3-carboxylic acid [1-(5-fluoro-1 H-
indol-3-
ylmethyl)-2-hydroxyethyl]amide;
250 3'-Methoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1-
methyl-1 H-indol-3-yl)ethyl]amide;
251 3'-Chloro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -(1 -methyl-
1 H-
indol-3-ylmethyl)ethyl]amide;
252 4-Propoxy-3',5'-bis-trifluoromethylbiphenyl-3-carboxylic acid [(R)-2-
hydroxy-1-
(1-methyl-1 H-indol-3-ylmethyl)ethyl]amide;
253 3',4',5'-Trifluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
254 4-Propoxy-4'-trifluoromethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
255 4-Propoxy-4'-trifluoromethylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
46
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
256 5-(6-Fluoro-5-methylpyridin-3-yl)-N-[(R)-2-hydroxy-1-(1-methyl-1 H-indol-3-
ylmethyl)ethyl]-2-propoxybenzamide;
257 5-(3,5-Dimethylisoxazol-4-yl)-N-[(R)-1-hydroxymethyl-2-(1-methyl-1 H-indol-
3-
yl)ethyl]-2-propoxybenzamide;
258 3'-Chloro-4'-fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
259 3'-Cyano-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1-
methyl-1 H-indol-3-yl)ethyl]amide;
260 2',3'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1-
methyl-
1 H-indol-3-ylmethyl)ethyl]amide;
261 3',5'-Dimethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1-
methyl-1 H-indol-3-yl)ethyl]amide;
262 3'-Ethoxy-5'-fluoro-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
263 5'-Fluoro-3'-hydroxy-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1-
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
264 4,3'-Dipropoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1-methyl-
1H-
indol-3-yl)ethyl]amide;
265 3'-Chloro-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-3-yl)-
1-
hydroxymethylethyl]amide;
266 3'-Fluoro-4'-methyl-4-propoxybiphenyl-3-carboxylic acid [1-(5-fluoro-1 H-
indol-3-
ylmethyl)-2-hydroxyethyl]amide;
267 2'-Fluoro-4'-methyl-4-propoxybiphenyl-3-carboxylic acid [1-(5-fluoro-1H-
indol-3-
ylmethyl)-2-hydroxyethyl]amide;
268 4-Propoxy-3'-trifluoromethylbiphenyl-3-carboxylic acid [1-(5-fluoro-1 H-
indol-3-
ylmethyl)-2-hydroxyethyl]amide;
269 3'-Isopropyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-3-
yl)-1-
hydroxymethylethyl]amide;
270 3'-Methylsulphanyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-3-
yl)-1-hydroxymethylethyl]amide;
271 4-Propoxy-4'-trifluoromethoxybiphenyl-3-carboxylic acid [1 -(5-fluoro-1 H-
indol-3-
ylmethyl)-2-hydroxyethyl]amide;
272 N-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-2-propoxy-5-quinolin-
6-yl-
benzamide;
273 3'-Chloro-4'-methyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
47
3-yl)-1-hydroxymethylethyl]amide;
274 5-(3,5-Dimethylisoxazol-4-yl)-N-[2-(5-fluoro-1 H-indol-3-yl)-1-
hydroxymethylethyl]-2-propoxybenzamide;
275 2',3'-Difluoro-4-propoxybiphenyl-3-carboxylic acid [1 -(5-fluoro-1 H-indol-
3-
ylmethyl)-2-hydroxyethyl]amide;
276 3',5'-Dimethyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1H-indol-3-
yl)-1-
hydroxymethylethyl]amide;
277 5'-Ethoxy-3'-fluoro-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-3-
yl)-1-hydroxymethylethyl]amide;
278 3'-Fluoro-5'-hydroxy-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-
3-yl)-1-hydroxymethylethyl]amide;
279 4,3'-Dipropoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1H-indol-3-yl)-1-
hydroxymethylethyl]amide;
280 3'-Ethoxy-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1H-indol-3-yl)-
1-
hydroxymethylethyl]amide;
281 4'-Hydroxymethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide;
282 3'-Hydroxymethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide;
283 4-Propoxybiphenyl-3,4'-dicarboxylic acid 3-{[(R)-1-hydroxymethyl-2-(1H-
indol-
3-yl)ethyl]amide} 4'-methylamide;
284 N-[(R)-2-Hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]-5-(5-
hydroxymethylthiophen-2-
yl)-2-propoxybenzamide;
285 5'-Fluoro-4-propoxybiphenyl-3,3'-dicarboxylic acid 3-{[(R)-2-hydroxy-1-(1H-
indol-3-ylmethyl)ethyl]amide} 3'-methylamide;
286 3'-Chloro-4-propoxybiphenyl-3,4'-dicarboxylic acid 3-{[(R)-2-hydroxy-1-(1H-
indol-3-ylmethyl)ethyl]amide} 4'-methylamide;
287 3'-Hydroxymethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1-methyl-1 H-indol-3-yl)ethyl]amide;
288 3'-Hydroxymethyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-3-
yl)-1 -hydroxymethylethyl]amide;
289 3'-Chloro-4-propoxybiphenyl-3,4'-dicarboxylic acid 3-{[2-(5-fluoro-1 H-
indol-3-
yl)-1-hydroxymethylethyl]amide} 4'-methylamide;
290 N-[(R)-2-Hydroxy-1 -(1 -methyl-1 H-indol-3-ylmethyl)ethyl]-5-(5-
hydroxymethylthiophen-2-yl)-2-propoxybenzamide;
291 3'-Chloro-4-propoxybiphenyl-3,4'-dicarboxylic acid 3-{[(R)-2-hydroxy-1-(1-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
48
methyl-1 H-indol-3-ylmethyl)ethyl]amide} 4'-methylamide;
292 4'-Hydroxymethyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-3-
yI)-1-hydroxymethylethyl]amide;
293 4-Propoxybiphenyl-3,4'-dicarboxylic acid 3-{[2-(5-fluoro-1 H-indol-3-yl)-1-
hydroxymethylethyl]amide} 4'-methylamide;
294 5'-Fluoro-4-propoxybiphenyl-3,3'-dicarboxylic acid 3-{[2-(5-fluoro-1 H-
indol-3-yl)-
1-hydroxymethylethyl]amide} 3'-methylamide;
295 4-Ethoxy-3'-fluoro-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
296 4-Ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3'-methoxy[1,1'-
biphenyl]-3-carboxamide;
297 4-Ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-N-methyl[1,1'-
biphenyl]-3,3'-dicarboxamide;
298 4-Ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4',5'-
trimethoxy[1,1'-biphenyl]-3-carboxamide;
299 4-Ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3',4'-
dimethoxy[1,1'-
biphenyl]-3-carboxamide;
300 4-Ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3'-(1-
methylethyl)[1,1'-biphenyl]-3-carboxamide;
301 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4',5'-trimethoxy-4-
propoxy[1,1'-biphenyl]-3-carboxamide;
302 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-3',4'-dimethoxy-4-
propoxy[1,1'-biphenyl]-3-carboxamide;
303 N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-3'-methoxy-4-propoxy[1,1'-
biphenyl]-3-carboxamide;
304 N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-M-methyl-4-propoxy[1,1'-
biphenyl]-3,3'-dicarboxamide;
305 4,3',4',5'-Tetramethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1H-
indol-3-yl)ethyl]amide;
306 4,3',4'-Trimethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-
3-yI)ethyl]amide;
307 3'-Fluoro-4,4'-dimethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1H-
indol-
3-ylmethyl)ethyl]amide;
308 4,3'-Dimethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-indol-
3-
yI)ethyl]amide;
309 5-Benzo[1,3]dioxol-5-yl-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-2-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
49
methoxybenzamide;
310 3',4'-Difluoro-4,5'-dimethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1 -
(1 H-
indol-3-ylmethyl)ethyl]amide;
311 4-Isopropoxy-3'-methoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
312 5-Benzo[1,3]dioxol-5-yI-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-2-
isopropoxybenzamide;
313 4-Isopropoxy-3',4',5'-trimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
314 3'-Fluoro-4-isopropoxy-4'-methoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-
l-
(1 H-indol-3-ylmethyl)ethyl]amide;
315 4-Isopropoxy-3',4'-dimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-
2-(1 H-indol-3-yl)ethyl]amide;
316 4-Isopropoxy-3'-methylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1H-
indol-3-yl)ethyl]amide;
317 4'-Fluoro-4-isopropoxy-3'-methylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-
l-
(1 H-indol-3-ylmethyl)ethyl]amide;
318 3',4'-Difluoro-4-isopropoxy-5'-methoxybiphenyl-3-carboxylic acid [(R)-2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide;
319 4,3',4',5'-Tetramethoxy-5-methylbiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
320 4,3',4'-Trimethoxy-5-methylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide;
321 3'-Fluoro-4,4'-dimethoxy-5-methylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-
l-
(1 H-indol-3-ylmethyl)ethyl]amide;
322 5-Benzo[1,3]dioxol-5-yI-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-2-
methoxy-3-methylbenzamide;
323 4,3'-Dimethoxy-5-methylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
324 4-Methoxy-5,3'-dimethylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-
indol-3-yl)ethyl]amide;
325 4'-Fluoro-4-methoxy-5,3'-dimethylbiphenyl-3-carboxylic acid [(R)-2-hydroxy-
l-
(1 H-indol-3-ylmethyl)ethyl]amide;
326 3',4'-Difluoro-4,5'-dimethoxy-5-methylbiphenyl-3-carboxylic acid [(R)-2-
hydroxy-
1 -(1 H-indol-3-ylmethyl)ethyl]amide;
327 3'-Hydroxy-4-isopropoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
(1 H-indol-3-yl)ethyl]amide;
328 3',4',5'-Trimethoxy-4-(3-methyl-but-2-enyloxy)biphenyl-3-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
329 3'-Butoxy-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-
3-yI)ethyl]amide;
330 4-Ethoxy-3'-isopropoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1H-
indol-3-yl)ethyl]amide;
331 N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(7-methoxybenzofuran-2-
yl)-
2-propoxybenzamide;
332 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-2-(6-
chlor-1 H-indol-3-yl)-1-hydroxymethylethyl]amide;
333 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-1-
hydroxymethyl-2-(2-methyl-1 H-indol-3-yl)ethyl]amide;
334 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [1-
hydroxymethyl-2-(6-methyl-1 H-indol-3-yl)ethyl]amide;
335 N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-6-methoxy-2-(3-
methoxyphenyl)quinoline-4-carboxamide;
336 N-[ (R)-1-(Hydroxymethyl)-2-(1-propyl-1 H-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
337 N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-2-(3,5-
difluoro-4-
methoxyphenyl)-6-methoxyquinoline-4-carboxamide;
338 N-[ (R)-1-(Hydroxymethyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-6-methoxy-2
(3-
methoxyphenyl)-quinoline-4-carboxamide;
339 N-[ (R)-1-(Hydroxymethyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-2-(3,5-
difluoro-4-
methoxyphenyl)-6-methoxyquinoline-4-carboxamide;
340 N-[ (R)-1-(Hydroxymethyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-trimethoxyphenyl)quinoline-4-carboxamide;
341 N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-2-(3-fluoro-4-
methoxyphenyl)-6-trifluoromethoxyquinoline-4-carboxamide;
342 N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-2-(7-
methoxybenzofuran-2-yl)-6-trifluoromethoxyquinoline-4-carboxamide;
343 N-[ (R)-1-(Hydroxymethyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-2-(3-fluoro-
4-
methoxyphenyl)-6-trifluoromethoxyquinoline-4-carboxamide;
344 N-[ (R)-1-(Hydroxymethyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-2-(7-
methoxybenzofuran-2-yl)-6-trifluoromethoxyquinoline-4-carboxamide;
345 N-[ (R)-1-(Hydroxymethyl)-2-(1-n hexyl-lH-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
51
trimethoxyphenyl)quinoline-4-carboxamide;
346 N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-4-ethoxy-3'-
methoxybiphenyl-3-carboxamide;
333 N-[ (R)-1-(Hydroxymethyl)-2-(1-isopropyl)-1 H-indol-3-yl)ethyl]-4-ethoxy-
3'-
methoxybiphenyl)-3-carboxamide;
347 N-[(R)-2-(1-Ethyl-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-3'-fluoro-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
348 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1-propyl-1 H-indol-3-yl)ethyl]-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
349 N-[(R)-2-(1-Butyl-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-3'-fluoro-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
350 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-[1-(3-methylbutyl)-1 H-indol-3-
yi]ethyl]-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
351 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-(1-pentyl-1 H-indol-3-yl)ethyl]-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
352 3'-Fluoro-N-[(R)-2-(1-hexyl-1H-indol-3-yl)-1-(hydroxymethyl)ethyl]-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
353 4-Ethoxy-3'-methoxybiphenyl-3-carboxylic acid [2-(5,6-difluoro-1H-indol-3-
yl)-1-
hydroxymethylethyl]amide;
354 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [2-(5,6-
difluoro-1 H-indol-3-yi)-1-hydroxymethylethyl]amide;
355 N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl-5-fluoro-1H-indol-3-yl) ethyl]-6-
methoxy-2-
(3,4,5-trimethoxyphenyl)quinoline-4-carboxamide;
356 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [2-(1-
ethyl-5-
fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]amide;
357 6-(3,4,5-Trimethoxyphenyl)quinoline-8-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide;
358 3-(3,4,5-Trimethoxyphenyl)naphthalene-1-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yi)ethyl]amide;
359 4-Methoxy-5-(3,4,5-trimethoxyphenyl)thiophene-3-carboxylic acid [(R)-2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide;
360 6-(3,4,5-Trimethoxyphenyl)-1 H-benzoimidazol-4-carboxylic acid [(R)-2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide;
361 2-(3,4,5-Trimethoxyphenyl)thiazol-4-carboxylic acid [(R)-2-hydroxy-1-(1 H-
indol-
3-ylmethyl)ethyl]amide;
362 5-(3,4,5-Trimethoxyphenyl)thiophene-2-carboxylic acid [(R)-2-hydroxy-1 -(1
H-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
52
indol-3-ylmethyl)ethyl]amide;
363 5-(3,4,5-Trimethoxyphenyl)benzo[b]thiophene-2-carboxylic acid [(R)-2-
hydroxy-
1 -(1 H-indol-3-ylmethyl)ethyl]amide;
364 2-(3-Fluoro-4-methoxyphenyl)-N-[(R)-2-hydroxy-1 -(1 H-indol-3-
ylmethyl)ethyl]-
6-methylisonicotinamide;
365 2-(3-Fluoro-4-methoxyphenyl)-6-methylpyrimidine-4-carboxylic acid [(R)-2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide;
366 6-(4-Methoxyphenyl)pyrimidine-4-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-yl)ethyl]amide;
367 2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinazoline-4-carboxylic acid [(R)-2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide;
368 2-(3-Fluoro-4-methoxyphenyl)-6-iodoquinazoline-4-carboxylic acid [(R)-2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide;
369 2-(4-Methoxyphenyl)quinazoline-4-carboxylic acid [(R)-1-hydroxymethyl-2-(1
H-
indol-3-yl)ethyl]amide;
370 2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinazoline-4-carboxylic acid [(R)-1-
(1-ethyl-1 H-indol-3-ylmethyl)-2-hydroxyethyl]amide;
371 2-(3,4,5-Trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-2-hydroxy-1 -(1
H-
indol-3-ylmethyl)-2-methylpropyl]amide;
372 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-2-
hydroxy-1 -(1 H-indol-3-ylmethyl)-2-methylpropyl]amide;
373 6-(4-Hydroxybut-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic
acid
[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
374 6-(5-Hydroxypent-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic
acid
[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
375 6-(3-Hydroxyprop-l-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic
acid
[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
376 6-(3-Methoxyprop-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic
acid
[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
377 5-(4-Hydroxybut-1-ynyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid [(R)-
1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
378 5-(3-Hydroxyprop-1 -ynyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
379 5-(5-Hydroxypent-1-ynyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yI)ethyl]amide;
380 3',4',5'-Trimethoxy-5-(3-methoxyprop-1-ynyl)biphenyl-3-carboxylic acid
[(R)-1-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
53
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
381 3',4'-Dimethoxy-5-(3-methoxyprop-1-ynyl)biphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
382 5-(3-Hydroxyprop-1-ynyl)-3',4'-dimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
383 3',4',5'-Trimethoxy-5-(4-methoxyphenylethynyl)biphenyl-3-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
384 3',4',5'-Trimethoxy-5-((Z)-3-methoxypropenyl)biphenyl-3-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
385 5-((Z)-4-Hydroxybut-l-enyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yi)ethyl]amide;
386 5-((Z)-3-Hydroxypropenyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
387 5-((Z)-5-Hydroxypent-l-enyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
388 6-(5-Hydroxypentyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
389 6-(4-Hydroxybutyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
390 6-(3-Hydroxypropyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
391 6-(3-Methoxypropyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yi)ethyl]amide;
392 3',4',5'-Trimethoxy-4-(3-methoxypropyl)biphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
393 3',4',5'-Trimethoxy-5-(3-methoxypropyl)biphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
394 3',4'-Dimethoxy-5-(3-methoxypropyl)biphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yi)ethyl]amide;
395 5-(3-Hydroxypropyl)-3',4'-dimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxy-
methyl-2-(1 H-indol-3-yl)ethyl]amide;
396 5-(5-Hydroxypentyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
397 5-(3-Hydroxypropyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
398 5-(4-Hydroxybutyl)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxy-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
54
methyl-2-(1 H-indol-3-yl)ethyl]amide;
399 3',4',5'-Trimethoxy-5-[2-(4-methoxyphenyl)ethyl]-biphenyl-3-carboxylic
acid
[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
400 N-[(R)-2-[1-(2-Cyanoethyl)-1 H-indol-3-yl]-1-(hydroxymethyl)ethyl]-3'-
fluoro-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
401 3'-Fluoro-N-[(R)-2-(1-heptyl-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
402 N-[(R)-2-[1-(4-Cyanobutyl)-1 H-indol-3-yl]-1-(hydroxymethyl)ethyl]-3'-
fluoro-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
403 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-[1-(3-phenoxypropyl)-1 H-indol-3-
yl]ethyl]-
4'-methoxy[1,1'-biphenyl]-3-carboxamide;
404 3'-Fluoro-N-[(R)-1-(hydroxymethyl)-2-[1-(2-methoxyethyl)-1 H-indol-3-
yl]ethyl]-
4'-methoxy[1,1'-biphenyl]-3-carboxamide;
405 N-[(R)-2-[1-(3-Cyanopropyl)-1 H-indol-3-yl]-1-(hydroxymethyl)ethyl]-3'-
fluoro-4'-
methoxy[1,1'-biphenyl]-3-carboxamide;
406 4-Ethoxy-3'-methoxybiphenyl-3-carboxylic acid [(R)-2-(1-cyanomethyl-1 H-
indol-
3-yI)-1-hydroxymethylethyl]amide;
407 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-2-(1-
cyanomethyl-1 H-indol-3-yl)-1-hydroxymethylethyl]amide;
408 6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid {(R)-2-[1-
(4-
cyano-butyl)-1 H-indol-3-yl]-1-hydroxymethylethyl}amide;
409 4-Hydroxy-3',4' ,5'-trimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-
(1 H-indol-3-yI)ethyl]amide;
410 4-(3-Cyanopropoxy)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
411 4-Cyclopentyloxy-3'-fluoro-4'-methoxybiphenyl-3-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
412 4-Cyclopentyloxy-3'-methylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide;
413 3'-(1-Butyl-3-methylureido)-4-cyclopentyloxybiphenyl-3-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
414 4-Cyclopentyloxy-4'-fluoro-3'-methylbiphenyl-3-carboxylic acid [(R)-2-
hydroxy-
1 -(1 H-indol-3-ylmethyl)ethyl]amide;
415 4-Cyclopentyloxy-3'-methoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-(1 H-indol-3-yl)ethyl]amide;
416 4-Cyclopentyloxy-3',4'-dimethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxy-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
methyl-2-(1 H-indol-3-yl)ethyl]amide;
417 5-Benzo[1,3]dioxol-5-yl-2-cyclopentyloxy-N-[(R)-1-hydroxymethyl-2-(1 H-
indol-
3-yl)ethyl]benzamide;
418 4-Cyclopentyloxy-3',4',5'-trimethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxy-
methyl-2-(1 H-indol-3-yl)ethyl]amide;
419 4-Cyclopentyloxy-3',4'-difluoro-5'-methoxybiphenyl-3-carboxylic acid [(R)-
2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide;
420 3'-[Butyl[(1,1-dimethylethoxy)carbonyl]amino]-4-ethoxy-N-[(R)-1-
(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl][1,1'-biphenyl]-3-carboxamide;
421 3'-(Butylamino)-4-ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl][1,1'-
biphenyl]-3-carboxamide;
422 3'-[Butyl[(methylamino)carbonyl]amino]-4-ethoxy-N-[(R)-1-(hydroxymethyl)-2-
(1 H-indol-3-yl)ethyl][1,1'-biphenyl]-3-carboxamide;
423 3'-[Butyl[(1,1-dimethylethoxy)carbonyl]amino]-N-[(R)-1-(hydroxymethyl)-2-
(1H-
indol-3-yl)ethyl]-4-propoxy[1,1'-biphenyl]-3-carboxamide;
424 3'-(1-Butyl-3-methylureido)-4-methoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
425 3'-(1-Butyl-3-methylureido)-4-methoxy-5-methylbiphenyl-3-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
426 3'-(1-Butyl-3-methylureido)-4-isopropoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
427 3'-(2-Dimethylaminoethoxy)-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
428 4'-Ethoxy-3'-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethylcarbamoyl]-
biphenyl-3-
carboxylic acid methyl ester;
429 4-Ethoxy-[1,1';3',1"]terphenyl-3-carboxylic acid [1-hydroxymethyl-2-(1H-
indol-3-
yl)ethyl]amide;
430 3'-Acetyl-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-
indol-
3-yl)ethyl]amide;
431 4-Ethoxy-3'-pyrrolidin-l-ylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide;
432 4'-Cyanomethyl-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
433 4'-Dimethylamino-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide;
434 4'-Hydroxymethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-
2-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
56
(1 H-indol-3-yl)ethyl]amide;
435 4-Propoxybiphenyl-3,4'-dicarboxylic acid 4'-diethylamide 3-{[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide};
436 3'-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethylcarbamoyl]-4'-
propoxybiphenyl-4-
carboxylic acid;
437 4'-Acetyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-yi)ethyl]amide;
438 4'-Ethanesulphonyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-
(1H-
indol-3-ylmethyl)ethyl]amide;
439 3'-Cyanomethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
440 3'-Methanesulphonylamino-4-propoxybiphenyl-3-carboxylic acid [(R)-2-
hydroxy-1-(1 H-indol-3-ylmethyl)ethyl]amide;
441 3'-Cyclopropylmethoxy-4-propoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yi)ethyl]amide;
442 3'-Methanesulphonyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
443 4-Propoxybiphenyl-3,3'-dicarboxylic acid 3'-[(2-dimethylaminoethyl)-amide]
3-
{[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide} ;
444 3'-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethylcarbamoyl]-3-methoxy-4'-
propoxybiphenyl-4-carboxylic acid methyl ester;
445 3'-Chloro-4-propoxybiphenyl-3,4'-dicarboxylic acid 4'-amide 3-{[(R)-2-
hydroxy-
1-(1 H-indol-3-ylmethyl)ethyl]amide};
446 3'-Dimethylsulphamoyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1
-
(1 H-indol-3-ylmethyl)ethyl]amide;
447 4'-(Propane-2-sulphonyl)-4-propoxybiphenyl-3-carboxylic acid [(R)-2-
hydroxy-
1-(1 H-indol-3-ylmethyl)ethyl]amide;
448 4'-Methylsulphamoyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
449 4'-Dimethylsulphamoyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
450 4-Propoxybiphenyl-3,4'-dicarboxylic acid 4'-amide 3-{[(R)-1-hydroxymethyl-
2-
(1 H-indol-3-yl)ethyl]amide};
451 3'-Methylsulphamoyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1 H-indol-3-ylmethyl)ethyl]amide;
452 3'-Methanesulphonyl-4-propoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-l-
(1-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
57
methyl-1 H-indol-3-ylmethyl)ethyl]amide;
453 3'-[(R)-1-Hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethylcarbamoyl]-3-
methoxy-
4'-propoxybiphenyl-4-carboxylic acid methyl ester;
454 4-Propoxybiphenyl-3,4'-dicarboxylic acid 4'-[(2-dimethylaminoethyl)amide]
3-
{[(R)-1-hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]amide};
455 3'-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethylcarbamoyl]-4'-
propoxybiphenyl-4-carboxylic acid;
456 3'-Methanesulphonylamino-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-
1 H-indol-3-yl)-1-hydroxymethylethyl]amide;
457 3'-Methanesulphonyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-
3-yl)-1-hydroxymethylethyl]amide;
458 4-Propoxybiphenyl-3,3'-dicarboxylic acid 3'-[(2-dimethylaminoethyl)amide]
3-
{[2-(5-fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]amide};
459 3'-Chloro-4-propoxybiphenyl-3,4'-dicarboxylic acid 4'-amide 3-{[2-(5-
fluoro-1 H-
indol-3-yl)-1-hydroxymethylethyl]amide};
460 3'-Dimethylsulphamoyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-3-yl)-1-hydroxymethylethyl]amide;
461 4'-(Propane-2-sulphonyl)-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-
1 H-
indol-3-yl)-1-hydroxymethylethyl]amide;
462 4'-Dimethylsulphamoyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-3-yl)-1-hydroxymethylethyl]amide;
463 4-Propoxybiphenyl-3,4'-dicarboxylic acid 4'-diethylamide 3-{[2-(5-fluoro-1
H-
indol-3-yl)-1-hydroxymethylethyl]amide} ;
464 3'-Methylsulphamoyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-
3-yl)-1-hydroxymethylethyl]amide;
465 3'-Acetyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1-
methyl-1 H-indol-3-yl)ethyl]amide;
466 4-Propoxy-[1,1';3',1"]terphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1-
methyl-1 H-indol-3-yl)ethyl]amide;
467 3'-Cyanomethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1-methyl-1 H-indol-3-yl)ethyl]amide;
468 3'-Methanesulphonylamino-4-propoxybiphenyl-3-carboxylic acid [(R)-2-
hydroxy-1 -(1-methyl-1 H-indol-3-ylmethyl)ethyl]amide;
469 4'-Cyanomethyl-4-propoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1-methyl-1 H-indol-3-yl)ethyl]amide;
470 4-Propoxybiphenyl-3,3'-dicarboxylic acid 3'-[(2-dimethylaminoethyl)amide]
3-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
58
{[(R)-1-hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]amide};
471 4-Fluoro-3'-[(R)-2-hydroxy-1 -(1 -methyl-1 H-indol-3-
ylmethyl)ethylcarbamoyl]-4'-
propoxybiphenyl-3-carboxylic acid;
472 3'-Chloro-4-propoxybiphenyl-3,4'-dicarboxylic acid 4'-amide 3-{[(R)-2-
hydroxy-
1 -(1 -methyl-1 H-indol-3-ylmethyl)ethyl]amide};
473 4-Propoxybiphenyl-3,4'-dicarboxylic acid 4'-diethylamide 3-{[(R)-1-
hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]amide};
474 4'-Dimethylamino-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-
indol-3-
yl)-1-hydroxymethylethyl]amide;
475 4'-Acetyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-3-yl)-
1-
hydroxymethylethyl]amide;
476 3'-Acetyl-4-propoxybiphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-3-yl)-
1-
hydroxymethylethyl]amide;
477 4-Propoxy-[1,1';3',1"]terphenyl-3-carboxylic acid [2-(5-fluoro-1 H-indol-3-
yl)-1-
hydroxymethylethyl]amide;
478 3'-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethylcarbamoyl]-3-methoxy-
4'-
propoxybiphenyl-4-carboxylic acid methyl ester;
480 4-Propoxybiphenyl-3,4'-dicarboxylic acid 4'-amide 3-{[2-(5-fluoro-1 H-
indol-3-yl)-
1 -hydroxymethylethyl]amide};
481 4-Ethoxy-4'-methoxymethylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
482 4-Ethoxybiphenyl-3,3'-dicarboxylic acid 3'-amide 3-{[(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide};
483 4'-Ethanesulphonyl-4-ethoxybiphenyl-3-carboxylic acid [(R)-2-hydroxy-1-(1H-
indol-3-ylmethyl)ethyl]amide;
484 4-Ethoxy-4'-(4-methylpiperazine-l-carbonyl)biphenyl-3-carboxylic acid [(R)-
1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
485 3'-Cyclopropylmethoxy-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
486 3'-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethylcarbamoyl]biphenyl-2-
carboxylic
acid methyl ester.
The present invention also relates to a process for preparing the compounds
according
to the invention. Compounds of the general formula I or Ia can be prepared as
shown in
Scheme 1 by an amide-formation reaction between the tryptophanol derivative VI
or VIa
and the carboxylic acid VII. Reagents suitable for this purpose are all
suitable peptide-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
59
coupling reagents which are known to the skilled person and which convert the
carboxylic acid, where appropriate in the presence of a base, into an
intermediate active
ester, for example PyBOP ([(1H-benzotriazol-1-yl)oxy]tris(pyrrolidin-1-
yl)phosphonium
hexafluorophosphate), HATU (2-(7-aza-lH-benzotriazol-1-yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate), HBTU (2-(1H-benzotriazol-1-yl)-
1,1,3,3-
tetramethyluronium hexafluorophosphate), EDC (N-[3-(dimethylamino)propyl]-N'-
ethylcarbodiimide hydrochloride) / HOBt (1 -hydroxy-1 H-benzotriazole). It is
possible as
alternative for the carboxylic acid to be converted, where appropriate in the
presence of
a base, into the carbonyl chloride and reacted with the tryptophanol VI or VIa
to give the
product of the general formula I or Ia.
Scheme I
R1
N R2
R7 R8
OTOH HO R2 O NH
R1eR8
Y R7 Y
+ Q R3
HO
NH2 R6 X R3
R6 X
R5~
VI R4 VII
Via: R7=R8=H R5/W Ia: R7=R8=H
R4
Compounds of general formulae II, Ila, III and Illa can be prepared as shown
in Scheme
2 by an amide-formation reaction between the tryptophanol derivative VI or Vla
and the
appropriate carboxylic acid VIII or IX. Reagents suitable for this purpose are
all known
peptide-coupling reagents which convert the carboxylic acid, where appropriate
in the
presence of a base, into an intermediate active ester, for example PyBOP ([(1
H-
benzotriazol-1-yl)oxy]tris(pyrrolidin-1-yl)phosphonium hexafluorophosphate),
HATU (2-
(7-aza-1 H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate),
HBTU
(2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate), EDC
(N-[3-
(dimethylamino)propyl]-N'-ethylcarbodiimide hydrochloride) / HOBt (1 -hydroxy-
1 H-
benzotriazole). It is possible as alternative for the carboxylic acid to be
converted,
where appropriate in the presence of a base, into the carbonyl chloride and
reacted with
the tryptophanol VI or Vla to give the product of the general formula 11, Ila,
III or Illa.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
Scheme 2
R1\ ~ R2
N
R1\ R2
N O OH R7 R8
R7 R8 + R6 LR3 HO
HO RS X\T/O NH
NHz R4 R6
T3/ Tz
VI VIII X~ ~-R3
R5 T4~ ,T
T
Vla: R7=R8=H R4
11
Ila: R7=R8=H
R1\ ~ R2
N
0 OH
R1\ R2 T R7 R8
N T 4~T
~ \ R3 -- HO
R7 R8 + X N T~ 0 NH
R6
HO
T
NHz R5 R4 --R3
T~ tT ',
X \N ~ Tz
vl IX R6
Via: R7=R8=H
R5
R4 III
Illa: R7=R8=H
Compounds of general formulae IV, IVa, V and Va can be prepared as shown in
5 Scheme 3 by an amide-formation reaction between the tryptophanol derivative
VI or Vla
and the appropriate carboxylic acid X or XI. Reagents suitable for this
purpose are all
suitable peptide-coupling reagents which are known to the skilled person and
which
convert the carboxylic acid, where appropriate in the presence of a base, into
an
intermediate active ester, for example PyBOP ([(1 H-benzotriazol-1 -
yl)oxy]tris(pyrrolidin-
10 1-yl)phosphonium hexafluorophosphate), HATU (2-(7-aza-lH-benzotriazol-1-yl)-
1,1,3,3-
tetramethyluronium hexafluorophosphate), HBTU (2-(1H-benzotriazol-1-yi)-
1,1,3,3-
tetramethyluronium hexafluorophosphate), EDC (N-[3-(dimethylamino)propyl]-N'-
ethylcarbodiimide hydrochloride) / HOBt (1-hydroxy-1H-benzotriazole). It is
possible as
alternative for the carboxylic acid to be converted, where appropriate in the
presence of
15 a base, into the carbonyl chloride and reacted with the tryptophanol VI or
Via to give the
product of the general formula IV, IVa, V or Va.
Scheme 3
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
61
R1\ R2
N
R7 R8
Ri ~ R2 0 OH H
N \ HO
H 0 NH
R7 R8
H + R3
H
HO R6 W
R3
H
NH2
R5 R6 W
R4 H
R5
VI x R4 IV
Via: R7=R8=H IVa: R7=R8=H
Ri ~ R2
O OH N
R1\ R2 H R7 R8
N
R3
R7 R8 + \ \ ~ HO
H R6 ~/ N O\ NH
HO
R5
NH2 R4
H
R3
R6 W N
VI XI R5 V
Via: R7=R8=H R4 Va: R7=R8=H
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
62
The present invention further relates to the carboxylic acids of the formulae
VII, VIII, IX,
X and XI as intermediates of the process according to the invention for
preparing the
compounds according to the invention, namely:
2-(4-Chloro-3-methylphenyl)quinoline-4-carboxylic acid;
6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid;
6-Methoxy-2-(2,3,4-trimethoxyphenyl)quinoline-4-carboxylic acid;
6-Fluoro-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid;
6-Iodo-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid;
6-Nitro-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid;
2-[4-(Trifluoromethoxy)phenyl]quinoline-4-carboxylic acid;
2-(3,5-dimethoxyphenyl)quinoline-4-carboxylic acid;
2-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-6-methoxyquinoline-4-carboxylic acid;
2',3',4'-Trimethoxy[1,1'-biphenyl]-3-carboxylic acid;
3',4',5'-Trimethoxy[1,1'-biphenyl]-4-carboxylic acid;
3',4',5'-Trimethoxy-2-methyl[1,1'-biphenyl]-4-carboxylic acid;
2',3',4'-Trimethoxy-6-methyl[1,1'-biphenyl]-3-carboxylic acid;
2',3',4'-Trimethoxy[1,1'-biphenyl]-4-carboxylic acid;
2',3',4'-Trimethoxy-2-methyl[1,1'-biphenyl]-4-carboxylic acid;
3',4,4',5'-Tetramethoxy[1,1'-biphenyl]-4-carboxylic acid;
4'-(Hydroxymethyl)-6-methyl[1,1'-biphenyl]-3-carboxylic acid;
4'-(Hydroxymethyl)-2-methyl[1,1'-biphenyl]-4-carboxylic acid;
4-methoxy-3'-(1-methylethyl)[1,1'-biphenyl]-2-carboxylic acid;
3'-(1-methylethyl)[1,1'-biphenyl]-3-carboxylic acid;
6-methyl-3'-(1-methylethyl)[1,1'-biphenyl]-3-carboxylic acid;
3'-(1-methylethyl)[1,1'-biphenyl]-4-carboxylic acid;
2-methyl-3'-(1-methylethyl)[1,1'-biphenyl]-4-carboxylic acid;
4'-(Hydroxymethyl)-4-methoxy[1,1'-biphenyl]-2-carboxylic acid;
3',4',5'-Trifluoro[1,1'-biphenyl]-2-carboxylic acid;
3',4',5'-Trifluoro[1,1'-biphenyl]-3-carboxylic acid;
3',4',5'-Trifluoro-6-methyl[1,1'-biphenyl]-3-carboxylic acid;
3',4',5'-Trifluoro[1,1'-biphenyl]-4-carboxylic acid;
3',4',5'-Trifluoro-2-methyl[1,1'-biphenyl]-4-carboxylic acid;
2',4,5'-Trimethoxy[1,1'-biphenyl]-2-carboxylic acid;
2',4,5'-Trimethoxy[1,1'-biphenyl]-2-carboxylic acid;
2',5'-dimethoxy[1,1'-biphenyl]-4-carboxylic acid;
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
63
2',5'-dimethoxy-2-methyl[1,1'-biphenyl]-4-carboxylic acid;
3',4,4'-Trimethoxy[1,1'-biphenyl]-2-carboxylic acid;
3',4'-dimethoxy-6-methyl[1,1'-biphenyl]-2-carboxylic acid;
3',4'-dimethoxy-2-methyl[1,1'-biphenyl]-4-carboxylic acid;
3'-Fluoro-4'-methoxy[1,1'-biphenyl]-2-carboxylic acid;
3'-Fluoro-4,4'-dimethoxy[1,1'-biphenyl]-2-carboxylic acid;
3'-Fluoro-4'-methoxy[1,1'-biphenyl]-3-carboxylic acid;
3'-Fluoro-4'-methoxy-6-methyl[1,1'-biphenyl]-3-carboxylic acid;
3'-Fluoro-4'-methoxy-2-methyl[1,1'-biphenyl]-4-carboxylic acid;
3',4'-dimethoxy[1,1'-biphenyl]-2-carboxylic acid;
3'-(1-methylethyl)[1,1'-biphenyl]-2-carboxylic acid;
2',5'-dimethoxy[1,1'-biphenyl]-3-carboxylic acid;
3',4',5'-Trifluoro-4-methoxy[1,1'-biphenyl]-2-carboxylic acid;
3-(Benzofuran-2-yl)benzoic acid;
3-(5-methoxybenzofuran-2-yl)benzoic acid;
2-[(3,4,5-Trimethoxyphenyl)methoxy]phenylpropanoic acid;
4-[[(3,4,5-Trimethoxyphenyl)methoxy]methyl]benzoic acid;
3-[(3,4,5-Trimethoxyphenyl)methoxy]thiophene-2-carboxylic acid;
4-[(3,4,5-Trimethoxyphenyl)methoxy]phenylacetic acid;
3-[3-((3,4,5-Trimethoxyphenyl)methoxy)phenyl]propionic acid;
2-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-6-methoxyquinoline-4-carboxylic acid;
2-(4-Fluoro-3-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid;
2-(3-Iodo-4-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid;
2-(3-Hydroxyphenyl)-6-methoxyquinoline-4-carboxylic acid;
2-(4-Hydroxy-3,5-dimethoxyphenyl)-6-methoxyquinoline-4-carboxylic acid;
2-(3,5-Difluoro-4-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid;
2-(3-Ethylphenyl)-6-methoxyquinoline-4-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinoline-4-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-methylquinoline-4-carboxylic acid;
6-Methyl-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid;
6-Bromo-2-(2,4-dimethylthiazol-5-yl)quinoline-4-carboxylic acid;
2-(7-Methoxybenzofuran-2-yl)-6-trifluoromethoxyquinoline-4-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-iodoquinoline-4-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-trifluoromethoxyquinoline-4-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6,8-dimethylquinoline-4-carboxylic acid;
2-(3,4-Dimethoxyphenyl)-6-methoxy-3-methylquinoline-4-carboxylic acid;
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
64
2-(4,6-Dimethoxybenzofuran-2-yl)-6-methoxyquinoline-4-carboxylic acid;
6-Methoxy-2-(5-methoxybenzofuran-2-yl)quinoline-4-carboxylic acid;
2-(7-Ethoxybenzofuran-2-yl)-6-methoxyquinoline-4-carboxylic acid;
6-Methoxy-2-(6-methoxybenzofuran-2-yl)quinoline-4-carboxylic acid;
2-(7-Fluorobenzofuran-2-yl)-6-methoxyquinoline-4-carboxylic acid;
2-(4-Fluorobenzofuran-2-yl)-6-methoxyquinoline-4-carboxylic acid;
6-Methoxy-2-(5-methylbenzofuran-2-yl)quinoline-4-carboxylic acid;
6-Methoxy-2-(7-methylbenzofuran-2-yl)quinoline-4-carboxylic acid;
6-Methoxy-2-(4-methoxybenzofuran-2-yl)quinoline-4-carboxylic acid;
6-Methoxy-2-[5-(trifluoromethoxy)benzofuran-2-yl]quinoline-4-carboxylic acid;
4-Ethoxy-3'-fluoro-4'-methoxy[1,1'-biphenyl]-3-carboxylic acid;
4-Ethoxy-3'-methoxy[1,1'-biphenyl]-3-carboxylic acid;
4-Ethoxy-3'-[(methylamino)carbonyl][1,1'-biphenyl]-3-carboxylic acid;
4-Ethoxy-3',4',5'-trimethoxy[1,1'-biphenyl]-3-carboxylic acid;
4-Ethoxy-3',4'-dimethoxy[1,1'-biphenyl]-3-carboxylic acid;
4-Ethoxy-3'-(1-methylethyl)[1,1'-biphenyl]-3-carboxylic acid;
3',4',5'-Trimethoxy-4-propoxy[1,1'-biphenyl]-3-carboxylic acid;
3',4'-Dimethoxy-4-propoxy[1,1'-biphenyl]-3-carboxylic acid;
3'-Methoxy-4-propoxy[1,1'-biphenyl]-3-carboxylic acid;
3'-[(Methylamino)carbonyl]-4-propoxy[1,1'-biphenyl]-3-carboxylic acid;
4,3',4',5'-Tetramethoxybiphenyl-3-carboxylic acid;
4,3',4'-Trimethoxybiphenyl-3-carboxylic acid;
3'-Fluoro-4,4'-dimethoxybiphenyl-3-carboxylic acid;
4,3'-Dimethoxybiphenyl-3-carboxylic acid;
5-Benzo[1,3]dioxol-5-yl-2-methoxybenzoic acid;
3',4'-Difluoro-4,5'-dimethoxybiphenyl-3-carboxylic acid;
4-Isopropoxy-3'-methoxybiphenyl-3-carboxylic acid;
5-Benzo[ 1, 3]dioxol-5-yl-2-isopropoxybenzoic acid;
4-Isopropoxy-3',4',5'-trimethoxybiphenyl-3-carboxylic acid;
3'-Fluoro-4-isopropoxy-4'-methoxybiphenyl-3-carboxylic acid;
4-isopropoxy-3',4'-dimethoxybiphenyl-3-carboxylic acid;
4-Isopropoxy-3'-methylbiphenyl-3-carboxylic acid;
4'-Fluoro-4-isopropoxy-3'-methylbiphenyl-3-carboxylic acid;
3',4'-Difluoro-4-isopropoxy-5'-methoxybiphenyl-3-carboxylic acid;
4,3',4',5'-Tetramethoxy-5-methylbiphenyl-3-carboxylic acid;
4,3',4'-Trimethoxy-5-methylbiphenyl-3-carboxylic acid;
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
3'-Fluoro-4,4'-dimethoxy-5-methylbiphenyl-3-carboxylic acid;
5-Benzo[1,3]dioxol-5-yl-2-methoxy-3-methyl-benzoic acid;
4,3'-Dimethoxy-5-methylbiphenyl-3-carboxylic acid;
4-Methoxy-5,3'-dimethylbiphenyl-3-carboxylic acid;
4'-Fluoro-4-methoxy-5,3'-dimethylbiphenyl-3-carboxylic acid;
3',4'-Difluoro-4,5'-dimethoxy-5-methylbiphenyl-3-carboxylic acid;
3'-Hydroxy-4-isopropoxybiphenyl-3-carboxylic acid;
3',4',5'-Trimethoxy-4-(3-methyl-but-2-enyloxy)biphenyl-3-carboxylic acid;
5-(7-Methoxybenzofuran-2-yl)-2-propoxybenzoic acid;
6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid;
2-(3,4,5-Trimethoxyphenyl)thiazol-4-carboxylic acid;
5-(3,4,5-Trimethoxyphenyl)thiophene-2-carboxylic acid;
5-(3,4,5-Trimethoxyphenyl)-benzo[b]thiophene-2-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-methylisonicotinic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-methylpyrimidine-4-carboxylic acid;
6-(4-Methoxyphenyl)-pyrimidine-4-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinazoline-4-carboxylic acid;
2-(3-Fluoro-4-methoxyphenyl)-6-iodoquinazoline-4-carboxylic acid;
2-(4-methoxyphenyl)-quinazoline-4-carboxylic acid;
4-Hydroxy-3',4',5'-trimethoxybiphenyl-3-carboxylic acid;
4-(3-Cyano-propoxy)-3',4',5'-trimethoxybiphenyl-3-carboxylic acid;
4-Cyclopentyloxy-3'-fluoro-4'-methoxybiphenyl-3-carboxylic acid;
4-Cyclopentyloxy-3'-methylbiphenyl-3-carboxylic acid;
3'-(1-Butyl-3-methylureido)-4-cyclopentyloxybiphenyl-3-carboxylic acid;
4-Cyclopentyloxy-4'-fluoro-3'-methylbiphenyl-3-carboxylic acid;
4-Cyclopentyloxy-3'-methoxybiphenyl-3-carboxylic acid;
4-Cyclopentyloxy-3',4'-dimethoxybiphenyl-3-carboxylic acid;
5-Benzo[1,3]dioxol-5-yl-2-cyclopentyloxybenzoic acid;
4-Cyclopentyloxy-3',4',5'-trimethoxybiphenyl-3-carboxylic acid;
4-Cyclopentyloxy-3',4'-difluoro-5'-methoxybiphenyl-3-carboxylic acid;
3'-[Butyl[(1,1-dimethylethoxy)carbonyl]amino]-4-propoxy[1,1 '-biphenyl]-3-
carboxylic acid;
3'-(1-Butyl-3-methylureido)-4-methoxybiphenyl-3-carboxylic acid;
3'-(1-Butyl-3-methylureido)-4-methoxy-5-methylbiphenyl-3-carboxylic acid;
3'-(1-Butyl-3-methylureido)-4-isopropoxybiphenyl-3-carboxylic acid
N-[(R)-1-(Methoxycarbonyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-6-methoxy-2-(3-
methoxyphenyl)quinoline-4-carboxamide;
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
66
N-[(R)-1-(Methoxycarbonyl)-2-(1-propyl-1 H-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-2-(3, 5-difluoro-
4-
methoxyphenyl)-6-methoxyquinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-6-methoxy-2 (3-
methoxyphenyl)- quinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-2-(3,5-
difluoro-4-
methoxyphenyl)-6-methoxyquinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-2-(3-fluoro-4-
methoxy-
phenyl)-6-trifluoromethoxyquinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-2-(7-
methoxybenzofuran-2-
yI)-6-trifluoromethoxyquinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-2-(3-fluoro-4-
methoxy-
phenyl)-6-trifluoromethoxyquinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-isopropyl-1 H-indol-3-yl)ethyl]-2-(7-
methoxybenzo-
furan-2-yl)-6-trifluoromethoxyquinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-n-hexyl-1 H-indol-3-yl)ethyl]-6-methoxy-2-
(3,4,5-tri-
methoxyphenyl)quinoline-4-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-ethyl)-1 H-indol-3-yl)ethyl]-4-ethoxy-3'-
methoxy-
biphenyl-3-carboxamide;
N-[(R)-1-(Methoxycarbonyl)-2-(1-isopropyl)-1 H-indol-3-yl)ethyl]-4-ethoxy-3'-
methoxy-
biphenyl)-3-carboxamide;
6-Bromoquinoline-8-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-indol-3-
yl)ethyl]amide;
3-Bromonaphthalene-1 -carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-indol-3-
yl)ethyl]amide;
5-Bromo-4-methoxythiophene-3-carboxylic acid [(R)-2-hydroxy-1 -(1 H-indol-3-
ylmethyl)ethyl]amide;
6-Bromo-1 H-benzimidazole-4-carboxylic acid [(R)-2-hydroxy-1 -(1 H-indol-3-
ylmethyl)ethyl]amide;
5-Bromo-2-ethoxy-N-[(R)-2-hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]benzamide;
N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-iodo-2-propoxybenzamide;
N-[(R)-1-Hydroxymethyl-2-(1-methyl-1 H-indol-3-yl)ethyl]-5-iodo-2-
propoxybenzamide;
N-[2-(5-Fluoro-1 H-indol-3-yl)-1-hydroxymethylethyl]-5-iodo-2-propoxybenzamide
and the methyl, ethyl, propyl and butyl esters thereof.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
67
Pharmacological investigations
HTRF assay for measuring cAMP in cells
The method is based on a competitive immunoassay between native cAMP, which
has
been produced by the cells, and cAMP which is labelled with XL665. The tracer
binding
was visualized by a monoclonal antibody, anti-cAMP labelled with cryptate
[HTRF =
homogeneous time-resolved fluorescence].
The specific signal is inversely proportional to the cAMP concentration of the
samples
employed.
The 665nm/ 620nm fluorescence ratio was evaluated.
The following material was used: 96-well plates for the tissue culture, 96-
well plates with
black edge and black base (e.g. Fluotrac 600 from Greiner), 96-well plates for
the
substance dilutions of polypropylene and cAMP Femtomolar (4000wells Kit, CIS
Bio
International # 62AMIPEC).
The following reagents were used: BSA (bovine serum albumin) Fraction V
protease-
free, IBMX (3-isobutyl-l-methylxanthine), hFSH (human follicle stimulating
hormone),
Triton X-100 analytical grade, potassium fluoride analytical grade, G 418
(Geneticin)
and Accutase.
Buffer 1 (washing and testing buffer) contained PBS, 1 mM CaCI2, 1 mM MgCl2,
0.2%
glucose; 0.1% BSA, 1 mM IBMX.
Buffer 2 (2x lysis buffer) contained 1% Triton X-100 in PBS (without CaCl2 and
MgCI2).
Buffer 3 (assay buffer) contained 50 mM potassium phosphate buffer (pH 7.0);
800 mM
potassium fluoride; 0.2% BSA (always added fresh).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
68
Procedure:
On day 1, the cells were seeded in 96-well plates (3x104 cells per well hFSHR
clone 16
cells (CHO cells stably transfected with the human FSH receptor in 150 NI of
medium).
The next day, test substance dilutions were made up. For this purpose, all the
substances were diluted in ice-cold buffer 1 (with or without hFSH), and the
substance
dilutions were placed on ice until applied to the cells.
The cell supernatant was then aspirated off, and the cells were washed 2x with
200 NI
of buffer 1. The cells were treated with 60 NI of the appropriate substance
concentrations at 37 C for 2h. The cells were then lysed with 60 NI of buffer
2 (put onto
the supernatant) (on a plate shaker at RT for 30 min).
The test conjugates (XL-665 and anti-cAMP cryptate) were diluted in buffer 3
in
accordance with the manufacturers' information. The actual mixture for
measurement
was pipetted into a black 96-well plate (in each case 15 NI of the cell lysate
diluted with
35 NI of buffer 1; firstly 25 NI of XL-665 conjugate were pipetted and, after
10 min, 25 NI
of the anti-cAMP cryptate were added). This is followed by incubation at RT
for 90
minutes. The measurement was carried out in a PheraStar (BMG).
Tissue culture conditions
1) hFSHr clone 16 Ham's F12
PSG
10% FCS
700 pg/mI G 418 (Geneticin) from PAA.
Dose-effect curve (hFSH) for the human receptor: 1 e-8, 3e-9, 1 e-9, 3e-10, 1
e-10, 3e-
11, 1 e-11, 3e-12 mol/l.
The test substances were employed in suitable dilutions in the absence (test
for
agonism) and in the presence of le-9 mol/I hFSH.
Evaluation
The values of the well ratio were averaged and then entered directly in
SigmaPlot
versus the concentrations. The maximum and minimum values were determined for
each plate, and half the difference is to be regarded as ICso=
The test results (Table 1) show that the compounds according to the invention
have an
FSH-antagonistic effect.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
69
Table 1. FSH-antagonistic effect of selected compounds in the HTRF assay
Compound [Ex. #] IC50
1 7 NM
8 1 NM
9 200 nM
1 NM
16 4 NM
17 400 nM
19 6 pM
22 300 nM
26 9 NM
36 6 NM
38 900 nM
59 6 NM
86 8pM
87 10 NM
88 10 pM
91 6 NM
96 7 NM
97 4NM
108 3 NM
120 1.5 NM
130 4 NM
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
Table 2. (continuation) FSH-antagonistic effect of selected compounds in the
HTRF assay
Compound [Ex. #] IC50
162 1.5 NM
307 450 nM
333 450 nM
337 3.5 pM
345 1 NM
361 4 NM
368 2.5 pM
373 8 NM
379 4.5 pM
388 400 nM
392 1.5 NM
396 3,5 pM
403 100 nM
418 300 nM
430 400 nM
483 1 NM
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
71
Dosage
Satisfactory results are generally to be expected if the daily doses comprise
a range
from 5 pg to 50 mg of the compound according to the invention per kg of body
weight. A
recommended daily dose for larger mammals, for example humans, is in the range
from
pg to 30 mg per kg of body weight. Suitable dosages for the compounds
according
to the invention are from 0.005 to 50 mg per day per kg of body weight,
depending on
the age and constitution of the patient, it being possible to administer the
necessary
daily dose by single or multiple delivery.
10 Pharmaceutical products based on the novel compounds are formulated in a
manner
known per se by processing the active ingredient with the carrier substances,
fillers,
substances which influence disintegration, binders, humectants, lubricants,
absorbents,
diluents, test modifiers, colorants etc. which are used in pharmaceutical
technology, and
converting into the desired administration form. Reference should be made in
this
connection to Remington's Pharmaceutical Science, 15th ed. Mack Publishing
Company, East Pennsylvania (1980).
Suitable for oral administration are in particular tablets, coated tablets,
capsules, pills,
powders, granules, pastilles, suspensions, emulsions or solutions.
Preparations for
injection and infusion are possible for parenteral administration.
Appropriately prepared
crystal suspensions can be used for intraarticular injection. Aqueous and oily
solutions
for injection or suspensions and corresponding depot preparations can be used
for
intramuscular injection. The novel compounds can be used for rectal
administration in
the form of suppositories, capsules, solutions (e.g. in the form of enemas)
and ointments
both for systemic and for local therapy. Formulations possible for topical
application are
gels, ointments, greasy ointments, creams, pastes, dusting powders, milk and
tinctures.
The dosage of the compounds of the general formula I in these preparations
should be
0.01 % - 20% in order to achieve an adequate pharmacological effect. Topical
use can
also take place by means of a transdermal system, for example a patch.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
72
The invention likewise encompasses the compounds according to the invention of
the
general formula I as therapeutic active ingredient. The invention further
includes the
compounds according to the invention of the general formula I as therapeutic
active
ingredients together with pharmaceutically suitable and acceptable excipients
and
carriers. The invention likewise encompasses a pharmaceutical composition
which
comprises one of the pharmaceutically active compounds according to the
invention or
mixture thereof and a pharmaceutically suitable salt or pharmaceutically
suitable
excipients and carriers.
The present invention therefore also relates to pharmaceutical compositions
which
comprise at least one compound of the general formula I, where appropriate
together
with pharmaceutically suitable excipients and/or carriers.
Suitable for forming pharmaceutically suitable salts of the compounds
according to the
invention of the general formula I are, by methods known to the skilled
person, as
inorganic acids inter alia hydrochloric acid, hydrobromic acid, sulphuric acid
and
phosphoric acid, nitric acid, as carboxylic acids inter alia acetic acid,
propionic acid,
hexanoic acid, octanoic acid, decanoic acid, oleic acid, stearic acid, maleic
acid, fumaric
acid, succinic acid, benzoic acid, ascorbic acid, oxalic acid, salicylic acid,
tartaric acid,
citric acid, lactic acid, glycolic acid, malic acid, mandelic acid, cinnamic
acid, glutamic
acid, aspartic acid, and as sulphonic acids inter alia methanesulphonic acid,
ethanesulphonic acid, toluenesulphonic acid, benzenesulphonic acid and
naphthalenesulphonic acid.
These pharmaceutical compositions and medicaments may be intended for oral,
rectal,
subcutaneous, transdermal, percutaneous, intravenous or intramuscular
administration.
They comprise besides conventional carriers and/or diluents at least one
compound of
the general formula I.
The medicaments of the invention are produced using the customary solid or
liquid
carriers or diluents and the excipients customarily used in pharmaceutical
technology, in
accordance with the desired mode of administration with a suitable dosage in a
known
manner. The preferred preparations consist of a dosage form which is suitable
for oral
administration. Examples of such dosage forms are tablets, film-coated
tablets, sugar-
coated tablets, capsules, pills, powders, solutions or suspensions or else
depot forms.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
73
The pharmaceutical compositions which comprise at least one of the compounds
according to the invention are preferably administered orally.
Parenteral preparations such as solutions for injection are also suitable.
Preparations
which may also be mentioned for example are suppositories.
Appropriate tablets can be obtained for example by mixing the active
ingredient with
known excipients, for example inert diluents such as dextrose, sugar,
sorbitol, mannitol,
polyvinylpyrrolidone, disintegrants such as maize starch or alginic acid,
binders such as
starch or gelatine, lubricants such as magnesium stearate or talc and/ or
agents to
achieve a depot effect such as carboxylpolymethylene, carboxylmethylcellulose,
cellulose acetate phthalate or polyvinyl acetate. The tablets may also consist
of a
plurality of layers.
Correspondingly, coated tablets can be produced by coating cores which have
been
produced in analogy to the tablets with agents normally used in tablet
coatings, for
example polyvinylpyrrolidone or shellac, gum Arabic, talc, titanium oxide or
sugar. The
tablet coating may also consist of a plurality of layers, it being possible to
use the
excipients mentioned above for tablets.
Solutions or suspensions with the compounds according to the invention of the
general
formula I may additionally comprise taste-improving agents such as saccharin,
cyclamate or sugar and, for example, flavourings such as vanillin or orange
extract.
They may additionally comprise suspending aids such as sodium
carboxymethylcellulose or preservatives such as p-hydroxybenzoates.
Capsules comprising the compounds of the general formula I can be produced for
example by the compound(s) of the general formula I being mixed with an inert
carrier
such as lactose or sorbitol and encapsulated in gelatine capsules.
Suitable suppositories can be produced for example by mixing with carriers
intended for
this purpose, such as neutral fats or polyethylene glycol or derivatives
thereof.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
74
The compounds according to the invention of the general formula I can be
prepared as
described below.
Abbreviations used:
ACN Acetonitrile
DIBAC Diisobutylaluminium hydride
DMF N,N-Dimethylformamide
EDC N-Ethyl-N'-(3-dimethylaminopropyl)carbodiimide
EtOH Ethanol
HATU O-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophosphate
FMOC (9H-Fluoren-9-ylmethoxy)carbonyl
HOBt 1 -Hydroxy-1 H-benzotriazole
MeCN Acetonitrile
MeOH Methanol
MTBE Methyl tert-butyl ether
NMM 4-methylmorpholine
NMP N-Methylpyrrolidinone
Rf Reflux
RT Room temperature
TBAF Tetrabutylammonium fluoride
TFA Trifluoroacetic acid
THF Tetrahydrofuran
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
Compounds of the general formula I or Ia can in principle be prepared as shown
in
Scheme 4 by an amide-formation reaction between a tryptophanol derivative VI
or Vla
and a carboxylic acid VII. The reagents typically used for the coupling are
EDC and
5 HOBt.
Scheme 4
R1
\ ~
N R2
~ /
R7 R8
R1eR8 OyOH HO
R2
NH
Y Oy
R7 + Q R3 Y
HO
R6 X Q R3
NHZ
R6 X
R5/ W I
VI R4 VII
Via: R7=R8=H R5 la: R7=R8=H
R4
The tryptophanol derivatives of the formula VI can be prepared as shown in
Scheme 5
from the corresponding amino acids which can be purchased or are known from
the
literature.
Scheme 5
R1\ R2 Rl~ R2 Rl~ R2 Ri
R2
N N N N
0 a~ O\ ~ b) ~~
HO HO HO HO
NHZ HN, FMOC HN, FMOC NH2
vl
Reagents: a) FMOC-CI, dioxane, 10% Na2CO3 solution in water, 0 C-RT; b)
i)EtOC(O)CI, THF,
NMM, -10 C; ii) NaBH4, MeOH, 0 C; c) piperidine, NaOH, RT.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
76
The carboxylic acids of the general formula VII can be prepared as shown in
Scheme 6
by a Suzuki reaction between a boronic acid XII or XVI and a halogen compound
XIII or
XV (Hal = I, Br, CI).
Scheme 6
OH
( O~OEt
R6 X-"\OH y a)
W +
R5 R4 ~ R3
Hal O'rOEt
xii xiii
Y
XHal O~OEt Q R3 b
R6 Y a) R6 X ~- Vil
W ~
R5 R4 HO" Q R3 RS W
g R4
I
OH
xV XVi XiV
Reagents: a) TBAF, Pd(PPh3)4, THF, Rf; b) KOH, MeOH;.
Carboxylic acids of the formula XIX can be prepared as shown in Scheme 7 in a
so-
called Pfitzinger reaction from a methyl ketone and an isatin derivative
XVIII.
Scheme 7
0 OH
O
X CH3 T4\ T'~~T
R6 + O \T~3 a) Tf~3
N TZ X N T
R5 H T R6
R4
R5
XVii XViii R4 XiX
Reagents: a) KOH, EtOH.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
77
Carboxylic acids of the general formulae XXI and XXII can likewise be prepared
by a
Pfitzinger reaction as shown in Scheme 8.
Scheme 8
0
0
T
R6- W CH3 + '', T3 a)
O I -~- R3 --
R5 N T!TZ
R4
xx xviii
O OH O OH
T
4-:~f R3 b) \--r-R3
TZ ,~ z
T
R6- W N T~ R6 W N T/
R5 R5
R4
XXi R4 XXii
Reagents: a) KOH; b) H2, Pd/C.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
78
Carboxylic acids of the general formula XXVIII can be prepared in an ether
synthesis as
shown in Scheme 9.
Scheme 9
Oy OMe Oy OMe
R6 ~ Hal Y R6 ~ OH Y
W + W +
R5/ Q R3 R5/ Q R3
R4 HO R4 Hal
m m
XXII I XXIV XXV XXVI
n1,2,3 n0,1,2,3
m=0,1,2,3 m=1,2,3
a) a)
OyOMe
Y
R6 Q R3
O
m
R5Z n XXVII
R4
b)
Oy OH
Y
R6 Q R3
O
m
R5Z n XXVI11
R4
Reagents: a) Cs2CO3, MeCN, Rf; b) KOH, MeOH.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
79
Synthesis of the compounds according to the invention
Example 1
N-[(R,S)-2-(5-Bromo-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-2-(3,4,5-
'
/ Br
HO
O NH
N
-O
trimethoxyphenyl)quinoline-4-carboxamide -
1 a) (R, S)-5-Bromo-a-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-1 H-indole-3-
propanoic acid
A solution of 0.36 mmol (92 mg) of 9-fluorenylmethyl chloroformate in 1.11 ml
of
dioxane was slowly added, while stirring and cooling to 0 C in an ice bath, to
a solution
of 0.35 mmol (100 mg) of 5-bromo-DL-tryptophan in 0.55 ml of dioxane and 1.11
ml of
10% strength aqueous sodium carbonate solution. After the addition was
complete, the
mixture was stirred at 0 C for one hour and at room temperature for a further
three
hours, cooled again to 0 C and 24 ml of water were added dropwise. Then 1.0 ml
of
concentrated hydrochloric acid is used to acidify, whereupon the protected
amino acid
precipitated. After the precipitate had been stored in a refrigerator and
filtered, 163 mg
of white amorphous solid product were obtained.
'H-NMR (400 MHz, DMSO-d6): S[ppm] = 12.73 s(1H, COOH); 11.07 s(1H, NH); 7.87 d
(J=7.5 Hz, 2H, aryl); 7.75 s(1 H, aryl); 7.70 d (J = 8.1 Hz, 1 H, aryl); 7.63
m (2H, aryl);
7.39 m (2H, aryl); 7.27 m (4H, aryl); 7.17 d (J = 6.9 Hz, 1 H, aryl); 4.18 m
(2H, CH); 3.56
s (2H, OCH2); 3.18 dd (J = 14.5 Hz / 4.7 Hz, 1 H, CH); 3.14 dd (J = 14.5 Hz /
4.4 Hz, 1 H,
CH).
MS (ESI, +): 505 (M+1).
1 b,c) (9H-Fluoren-9-ylmethyl) [(R,S)-2-(5-bromo-1 H-indol-3-yl)-1-
(hydroxymethyl)ethyl]carbamate
0.32 mmol (35 NI) of N-methylmorpholine was added to a stirred solution of
0.32 mmol
(163 mg) of the protected amino acid prepared as in 1 a) in 1.7 ml of THF at -
10 C,
followed by 0.32 mmol (31 NI) of ethyl chloroformate. The mixture was then
stirred for a
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
further hour at the stated temperature. Subsequently, 0.96 mmol (36 mg) of
sodium
borohydride was added in one portion.
When the reaction mixture had reached the temperature of 0 C, 3.2 ml of
methanol
were added dropwise. The solution was stirred for a further 10 minutes and
then
5 neutralized with 0.4 ml of 1 M hydrochloric acid. The organic solvents were
removed in
vacuo. The residue was taken up in water and extracted with methyl tertiary
butyl ether.
The resulting organic phase was dried over magnesium sulphate, filtered and
concentrated in vacuo. 157 mg of the target compound were obtained as a
colourless
foam.
10 'H-NMR (400 MHz, DMSO-ds): 6[ppm] = 11.00 s(1 H, NH); 7.86 d (J = 7.5 Hz,
2H,
aryl); 7.76 s(1 H, aryl); 7.64 m (2H, aryl); 7.39 m (2H, aryl); 7.29 m (3H,
aryl); 7.16 m
(3H, aryl); 4.74 t(J = 5.6 Hz, 1 H, OH); 4.17 m (4H, CH, OCH2); 3.74 m (2H,
OCH2);
2.91 dd (J = 14.3 Hz / 5.8 Hz, 1 H, CH); 2.70 dd (J = 14.4 Hz / 8.4 Hz, 1 H,
CH).
MS (ESI,+): 491 (M+1).
1 d) (R, S)-(3-Amino-5-bromo-1 H-indole-3-propanol
0.30 mmol (150 mg) of the protected amino alcohol prepared as in 1 b,c) was
stirred in
4 ml of piperidine at room temperature for one hour. After the solution had
been cooled
to 0 C, 2 ml of water were added dropwise. The resulting precipitate was
filtered off,
and a total of 1.5 g of potassium hydroxide powder was added in portions to
the filtrate
while stirring. The piperidine phase was separated off and concentrated in
vacuo with
addition of toluene. 110 mg of the amino alcohol still contaminated with
piperidine were
obtained.
MS(ESI,+): 269(M+1).
1 e) N-[(R, S)-2-(5-Bromo-1 H-indol-3-yl)-1-(hydroxymethyl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide
0.38 mmol (130 mg) of 2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid
were
dissolved in 3 ml of DMF and, at room temperature, 0.38 mmol (59 mg) of 1-
hydroxy-
1H-benzotriazole hydrate and 0.38 mmol (73 mg) of N-[3-(dimethylamino)propyl]-
N'-
ethylcarbodiimide hydrochloride were added. The mixture was stirred at the
stated
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
81
temperature for 30 minutes and then about 0.35 mmol (100 mg) of the amino
alcohol
obtained as in 1 d) was added.
After a further hour, the reaction mixture was added to saturated aqueous
sodium
hydrogen carbonate solution, and the precipitate was filtered and washed with
water.
Purification by chromatography on silica gel with the eluent cyclohexane /
ethyl acetate
affords 50 mg of the amide as yellowish solid.
'H-NMR (400 MHz, DMSO-d6): 8[ppm] = 11.09 s (1 H, NH); 8.64 d (J = 8.3 Hz, 1
H,
aryl); 8.07 d (J = 8.4 Hz, 1 H, aryl); 7.98 s (1 H, aryl); 7.82 s (1 H, aryl);
7.74 m (2H, aryl);
7.53 s (2H, aryl); 7.46 t (J = 7.5 Hz, 1 H, aryl); 7.34 d (J = 8.5 Hz, 1 H,
aryl); 7.26 s(1 H,
aryl); 7.16 d (J = 7.3 Hz, 1 H, aryl); 4.92 t (J = 5.0 Hz, 1 H, OH); 4.36 m(1
H, CH); 3.93 s
(6H, OCH3); 3.76 s (3H, OCH3); 3.59 m (2H, OCH2); 3.06 dd (J = 14.6 Hz / 5.4
Hz, 1 H,
CH); 2.89 dd (J = 14.6 Hz / 8.5 Hz, 1 H, CH).
MS (APCI, -): 588 (M-1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
82
The following compounds were obtained in analogy to the preparation methods
described in detail:
Ex. Product; a aio9 ~S 'H-NMR (400 MHz) S Structure
reagents to Ippm]
2 N-[(R,S)-1-(Hydroxymethyl)- I (DMSO-d6): 10.72 s(1 H,
2-(5-methyl-1 H-indol-3- NH); 8.63 d (J = 8.3 Hz, Ho
1 H, NH); 8.07 d (J o NH
yl)ethyl]-2-(3,4,5-trimethoxy-
phenyl)quinoline-4- 8.4Hz, 1 H, aryl); 7.97s -
-O
carboxamide; (1 H, aryl); 7.81 d (J = 8.4 o
Hz, 1 H, aryl); 7.76 t(J =
(R, S)-p-Amino-5-methyl-1 H- 7.6 Hz, 1 H, aryl); 7.52 s
indole-3-propanol (2H, aryl); 7.49 t (J = 7.6
and Hz, 1 H, aryl); 7.41 s(1 H,
aryl); 7.24 d (J = 8.2 Hz,
2-(3, 4, 5- 1 H, aryl); 7.14 s(1 H, aryl);
Trimethoxyphenyl)quinoline- 6.88 d (J = 8.2 Hz, 1 H, a-
4-carboxylic acid ryl); 4.89 t (J = 5.7 Hz, 1 H,
OH); 4.38 m(1 H, CH);
3.93 s (6H, OCH3); 3.76 s
(3H, OCH3); 3.60 m (2H,
OCH2); 3.06 dd (J =14.4
Hz / 5.5 Hz,1 H, CH); 2.89
dd (J = 14.4 Hz / 8.3 Hz,
1 H, CH); 2.30 s (3H, CH3).
MS(ESI; +): 526.
3 N-[(R,S)-1-(Hydroxymethyl)- 1 (DMSO-d6): 10.82 s(1H, q
2-(4-methyl-1 H-indol-3- NH); 8.66 d (J = 8.6
HO
yl)ethyl]-2-(3,4,5-trimethoxy- Hz,1 H, NH); 8.07 d (J = o NH
phenyl)quinoline-4- 8.4 Hz, 1 H, aryl); 8.01 s -o
carboxamide; (1 H, aryl); 7.78 m (2H, - ,o
aryl); 7.54 s (2H, aryl);
(R, S)-AAmino-4-methyl-1 H- 7.50 t (J = 7.6 Hz, 1 H,
indole-3-propanol and aryl); 4.97 t (J = 5.7 Hz,
2-(3, 4, 5- 1 H, OH); 4.39 m(1 H, CH);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
83
Product;
ea 'H-NMR (400 MHz) S Structure
a alog ~S
Ex.
9ents to IPP ]
Trimethoxyphenyl)quinoline- 3.93 s (6H, OCH3); 3.76 s
4-carboxylic acid (3H, OCH3); 3.67 m(1 H,
OCH); 3.59 m(1 H, OCH);
3.31 m(1 H, CH); 3.00 m
(1 H, CH); 2.70 s (3H,
CH3).
MS (ESI; +): 526 (M+1).
4 N-[(R,S)-1-(Hydroxymethyl)- I (DMSO-d6): 10.68 s(1H, H
2-(6-methyl-1 H-indol-3- NH); 8.62 d (J = 8,4
HO
yl)ethyl]-2-(3,4,5- Hz,1 H, NH); 8.07 d (J = O NH
trimethoxyphenyl)quinoline- 8.4 Hz, 1 H, aryl); 7.99 s -O i~~
4-carboxamide; (1 H, aryl); 7.82 d (J = 8.3 - ,o
Hz, 1 H, aryl); 7.76 t (J =
(R, S)-P-Amino-6-methyl-1 H- 7.6 Hz, 1 H, aryl); 7.53 s
indole-3-propanol (2H, aryl); 7.49 m (J = 7,7
and Hz, 2H, aryl); 7.12 s(1 H,
aryl); 7.10 s(1 H, aryl);
2-(3, 4, 5- 6.78 d(J = 8.1 Hz, 1 H, a-
Trimethoxyphenyl)quinoline- ryl), 4.88 t (J = 5,5 Hz, 1 H,
4-carboxylic acid OH); 4.38 m(1 H, CH);
3.92 s (6H, OCH3); 3.76 s
(3H, OCH3); 3.59 m (2H,
OCHZ); 3.05 dd (J = 14,5
Hz / 5.7 Hz, 1 H, CH); 2.90
dd (J = 14.4 Hz / 8.3 Hz,
1 H, CH); 2.37 s (3H, CH3).
MS (APCI; -): 524 (M-1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
84
Ex. Product; a aiogo S 'H-NMR (400 MHz) S Structure
reagents to [ppm]
N-[(R)-1-(Hydroxymethyl)-2- 1 (DMSO-d6): 8.65 d (J = 8.4 \
(1-methyl-1 H-indol-3- Hz, 1 H, NH); 8.08 d (J =
HO
yI)ethyl]-2-(3,4,5- 8.4 Hz, 1 H, aryl); 8.03 s o H
trimethoxyphenyl)quinoline- (1 H, aryl); 7.81 d (J = 8.4 1 N
4-carboxamide Hz, 1 H, aryl); 7.77 t (J = 7.6 -o
.0
Hz, 1 H, aryl); 7.68 d (J =
(R)-fl-Amino-1-methyl-1 H-
.9 Hz, 1 H, aryl); 7.55 s
indole-3-propanol (2H, aryl); 7.48 t (J = 7.5
2-(3, 4, 5- Hz, 1 H, aryl); 7.41 d (J =
Trimethoxyphenyl)quinoline- 8=2 Hz, 1 H, aryl); 7.16 s
4-carboxylic acid (1 H, aryl); 7.13 t(J = 7.8
Hz, 1 H, aryl); 7.00 t (J = 7.4
Hz, 1 H, aryl); 4.90 t (J = 5.7
Hz, 1 H, OH); 4.38 m(1 H,
CH); 3.93 s (6H, OCH3);
3.76 s (3H, OCH3); 3.74 s
(3H, NCH3); 3.60 m (2H,
OCHZ); 3.07 dd (J = 14.5
Hz / 5.8 Hz, 1 H, CH); 2.91
dd (J = 14.5 Hz / 8.1 Hz,
1 H, CH).
MS (ESI;+): 526 (M+1).
6 N-[(R,S)-2-(5-Fluoro-1 H- 1 (DMSO-d6): 10.96 s(1 H, H
F
indol-3-yl)-1- NH); 8.65 d (J = 8.4 Hz,
HO
(hydroxymethyl)ethyl]-2- 1 H, NH); 8.07 d (J = 8.6 0 NH
(3,4,5-trimethoxyphenyl)- Hz, 1 H, aryl); 8.00 s (1 H, ;
quinoline-4-carboxamide; aryl); 7.76 m (2H, aryl); _o ~~ N
7.54 s (2H, aryl); 7.47 t (J ~
(R,S)-fl-Amino-5-fluoro-1H- = 7.5 Hz, 1H, aryl); 7.40 d
indole-3-propanol (J - 10,0 Hz, 1 H, aryl);
2-(3, 4, 5- 7.35 m(1 H, aryl); 7.28 s
(1 H, aryl); 6.89 t(J = 10.2
Trimethoxyphenyl)quinoline-
Hz, 1 H, aryl); 4.91 t (J =
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analogous
reagents to IPPm]
4-carboxylic acid 5.4 Hz, 1 H, OH); 4.37 m
(1 H, CH); 3.93 s (6H,
OCH3); 3.76 s (3H, OCH3);
3.60 m (2H, OCH2); 3.05
dd (J = 14.4 Hz / 5.6 Hz,
1 H, CH); 2.89 dd (J = 14,4
Hz / 8,1 Hz, 1 H, CH).
19F-NMR (400 MHz,
DMSO-d6): -124.84 m
(1 F).
MS (APCI; -): 528 (M-1).
7 N-[(R,S)-1-(Hydroxymethyl)- 1 (DMSO-d6): 10.69 s(1H, \ 0
2-(5-methoxy-1 H-indol-3- NH); 8.65 d (J = 8.4 Hz,
HO""~
yI)ethyl]-2-(3,4,5- 1 H, NH); 8.07 d (J = 8,3 a NH
trimethoxyphenyl)quinoline- Hz, 1 H, aryl); 7.99 s(1 H, ~~
I
4-carboxamide; aryl); 7.82 d (J = 8.3 Hz, I~ "~
1 H, aryl); 7.76 t(J = 7,0 "0
(R, S)-,QAmino-5-methoxy- Hz, 1 H, aryl); 7.53 s (2H,
1H-indole-3-propanol aryl); 7.48 t (J - 7.5 Hz,
and 1 H, aryl); 7.24 d (J = 8,7
Hz, 1 H, aryl); 7.16 s (2H,
2-(3, 4, 5- aryl); 6.71 d (J = 8.7 Hz,
Trimethoxyphenyl)quinoline- 1 H, aryl); 4.89 t (J = 5,7
4-carboxylic acid Hz, 1 H, OH); 4.38 m(1 H,
CH); 3.93 s (6H, OCH3);
3.76 s (3H, OCH3); 3,68 s
(3H, OCH3); 3.60 m (2H,
OCH2); 3.04 dd (J = 14,5
Hz / 5.6 Hz, 1 H, CH); 2.90
dd (J = 14,5 Hz / 8.3 Hz,
1 H, CH).
MS(ESI;+): 542 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
86
Product;
ea 'H-NMR (400 MHz) S Structure
a alog ~S
Ex.
9ents to Ipp ]
8 N-[(R,S)-2-(6-Fluoro-1 H- I (DMSO-d6): 10.93 s(1 H, H_ F
indol-3-yl)-1- NH); 8.64 d (J = 8.3 Hz,
(hydroxymethyl)ethyl]-2- 1 H, NH); 8.07 d (J = 8.4 Ho
O NH
(3,4,5-trimethoxyphenyl)- Hz, 1 H, aryl); 8.00 s(1 H,
I
quinoline-4-carboxamide; aryl); 7.78 m (2H, aryl); o N
7.64 m(1 H, aryl); 7.54 s 'o
(R, S)-~3-Amino-6-fluoro-1 H- 'o
(2H, aryl); 7.48 t (J = 7.5
indole-3-propanol Hz, 1 H, aryl); 7.20 s(1 H,
and aryl); 7,19 d (J = 10,2 Hz,
1 H, aryl); 6.82 t (J = 8.0
2-(3, 4, 5- Hz, 1 H, aryl); 4.91 t (J =
Trimethoxyphenyl)quinoline- 5.7 Hz, 1 H, OH); 4.38 m
4-carboxylic acid (1 H, CH); 3.93 s (6H,
OCH3); 3.76 s (3H, OCH3);
3.59 m (2H, OCH2); 3.06
dd (J = 14,5 Hz / 5,6 Hz,
1 H, CH); 2.91 dd (J = 14.5
Hz / 8.3 Hz, 1 H, CH).
19F-NMR (400 MHz,
DMSO-d6): -121.73 m
(1 F).
MS(ESI;+): 530 (M+1).
9 N-[(R,S)-1-(Hydroxymethyl)- 1 (DMSO-d6): 10.71 s(1H, \
2-[5-(phenylmethoxy)-1H- NH); 8.66 d (J = 8.4 Hz,
Ho
indol-3-yI]ethyl]-2-(3,4,5- 1 H, NH); 8.07 d (J = 8.4 NH
~ . ~
trimethoxyphenyl)quinoline- Hz, 1 H, aryl); 7.96 s (1 H,
4-carboxamide; aryl); 7.81 d (J = 8.4 Hz, _ ,
1 H, aryl); 7.76 t (J = 7,5
(R, S)-P-Amino-5-methoxy- Hz, 1 H, aryl); 7.51 s (1 H,
1 H-indole-3-propanol aryl); 7.48 t (J = 7.5 Hz,
1 H, aryl); 7.32 m (8H, a-
and
ryl); 7.18 s(1 H, aryl); 6.77
2-(3, 4, 5- d (J = 8.7 Hz, 1 H, aryl);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
87
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. analogous
reagents to IpPm]
Trimethoxyphenyl)quinoline- 4.98 d (J = 11.7 Hz, 1 H,
4-carboxylic acid OCH); 4.38 m(1 H, CH);
3.91 s (6H, OCH3); 3.75 s
(3H, OCH3); 3.61 m (2H,
OCHZ); 3.04 dd (J = 14.4
Hz / 5.4 Hz, 1 H, CH); 2.89
dd (J = 14.5 Hz / 8.4 Hz,
1H, CH).
MS (ESI;+): 618 (M+1).
N-[(R,S)-1-(Hydroxymethyl)- 1 (DMSO-d6): 10.82 s(1H,
H
2-(7-methyl-1 H-indol-3- NH); 8.65 d (J = 8.3 N ~ i
yl)ethyl]-2-(3,4,5- Hz,1 H, NH); 8.07 d (J = Ho
trimethoxyphenyl)quinoline- 8.4 Hz, 1 H, aryl); 8.02 s 0 NH
4-carboxamide; (1 H, aryl); 7.83 d (J = 8.3 1;
I~ N
Hz, 1 H, aryl); 7.76 t(J =
(R, S)-,&Amino-7-methy1-1 H- o
7.5 Hz, 1 H, aryl); 7.54 s
indole-3-propanol (2H, aryl); 7.48 m (2H, a-
and ryl); 7.18 s(1 H, aryl); 6.86
m (2H, aryl); 4.90 t (J = 5.6
2-(3,4,5- Hz, 1H, OH); 4.39 m(1H,
Trimethoxyphenyl)quinoline- OH); 3.93 s (6H, OCH3);
4-carboxylic acid 3.76 s (3H, OCH3); 3.58 m
(2H, OCH2); 3.08 dd (J =
14.4 Hz / 5.6 Hz, 1 H, OH);
2.93 dd (J = 14.4 Hz / 8.2
Hz, 1 H, CH); 2.46 s (3H,
CH3).
MS (ESI; +): 526 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
88
Ex. Product; a aioyo S 'H-NMR (400 MHz) S Structure
reagents to Ippm]
11 N-[(R)-1-(Hydroxymethyl)-2- le (CDCI3): 8.27 s(1H, NH); N
Ho~
(1 H-indol-3-yI)ethyl]-2-(3,4,5- 8.13 d (J = 8.4 Hz, 1 H, a-
rimethoxyphenyl)quinoline-4- ryl); 7.85 d (J = 8.0 Hz, 1 H, o HN o
carboxamide; aryl); 7.72 d (J = 8.0 Hz, o
1 H, aryl); 7.70 s(1 H, aryl);
D-Tryptophanol 7.69 dd (J = 8.4 Hz / 8.0
and Hz, 1 H, aryl); 7.38 m (2H,
aryl); 7.29 s (2H, aryl);
2-(3, 4, 5-Trimethoxyphenyl)- 7.19 dd (J = 8.0 Hz / 8.0
quinoline-4-carboxylic acid Hz, 1 H, aryl); 7.11 d (J =
8.0 Hz, 1 H, aryl); 7.08 d (J
= 2.6 Hz, 1 H, aryl); 6.51 d
(J = 8.0 Hz, 1 H, NH); 4.66
m(1 H, CH); 3.94 s(6H,
OCH3); 3.90 s (3H, OCH3);
3.94 m(1 H, CH2OH); 3.81
dd (J = 11.0 Hz / 5.1 Hz,
1H, CHzOH); 3.19 d (J
=
7.2 Hz, 2H, CH2).
12 N-[(S)-1-(Hydroxymethyi)-2- le (CDCI3): 8.21 s(1H, NH); OH
(1 H-indol-3-yI)ethyl]-2-(3,4,5- 8.14 d (J = 8.4 Hz, 1 H, a- o NH N
H
rimethoxyphenyl)quinoline-4- ryl); 7.86 d (J = 8.0 Hz, 1 H, i
N
carboxamide; aryl); 7.72 d (J = 8.0 Hz, . \ I
1H, aryl); 7.71 s (1H, aryl); '
L-Tryptophanol 7.69 dd (J = 8.4 Hz / 8.0
and Hz, 1 H, aryl); 7.38 m (2H,
aryl); 7.29 s (2H, aryl);
2-(3, 4, 5-Trimethoxyphenyl)- 7.20 dd (J = 8.0 Hz / 8.0
quinoline-4-carboxylic acid Hz, 1 H, aryl); 7.12 d (J =
8.0 Hz, 1 H, aryl); 7.09 d (J
= 2.6 Hz, 1 H, aryl); 6.47 d
(J = 7.6 Hz, 1 H, NH); 4.67
m(1 H, CH); 3.95 s (6H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
89
Ex. Product; a alog ~S 'H-NMR (400 MHz) S Structure
reagents to [ppm]
OCH3); 3.91 s (3H, OCH3);
3.93 m(1 H, CH2OH); 3.83
dd (J = 11.0 Hz / 5.1 Hz,
1 H, CH2OH); 3.20 d (J
=
7.2 Hz, 2H, CH2).
MS (ESI;+): 512 (M+1).
[a]p = -16.5 (c = 0.475,
MeOH / CH2CI2 1:1).
Example 13
2-(4-Chloro-3-methylphenyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]quinoline-4-carboxamide
OH
0 H N
H
N
cl
13a) 2-(4-Chloro-3-methylphenyl)quinoline-4-carboxylic acid
2.05 mmol (135 mg) of potassium hydroxide were slowly added to a stirred
solution of
0.68 mmol (100 mg) of isatin in 7 ml of ethanol and 0.82 mmol (138 mg) of 4-
chloro-3-
methylacetophenone. After the addition was complete, the mixture was stirred
at 80 C
for six hours. The solution was cooled and then the ethanol was removed in
vacuo. The
residue was taken up in water and acidified with 2 ml of 1 M aqueous
hydrochloric acid.
The aqueous phase was extracted with ethyl acetate. The resulting organic
phase was
dried over magnesium sulphate, filtered and concentrated in vacuo. Flash
chromatography resulted in 145 mg of the target compound.
'H-NMR (400 MHz, pyridine-d5): 8[ppm] = 9.33 d (J = 8 Hz, 1 H, aryl); 8.81 s
(1 H, aryl);
8.41 d (J = 8 Hz, 1 H, aryl); 8.28 s(1 H, aryl); 8.15 dbr (J = 8 Hz, 1 H,
aryl); 7.75 dd (J = 8
Hz / 7 Hz, 1 H, aryl); 7.56 m(1 H, aryl); 7.51 m(1 H, aryl); 2.34 s (3H, Me).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
13b) 2-(4-Chloro-3-methylphenyl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]quinoline-4-carboxamide
In analogy to Example le), 67 mg of the title compound were obtained from 0.31
mmol
5 (93 mg) of 2-(4-chloro-3-methylphenyl)quinoline-4-carboxylic acid and 0.26
mmol
(50 mg) of D-tryptophanol.
' H-NMR (400 MHz, pyridine-d5): 6[ppm] = 11.98 s(1 H, NH); 9.65 d (J = 8.4 Hz,
1 H,
NH); 8.54 d (J = 7.6 Hz, 1 H, aryl); 8.31 d (J = 8.4 Hz, 1 H, aryl); 8.20 d (J
= 7.6 Hz, 1 H,
aryl); 8.16 s (1 H, aryl); 8.06 s (1 H, aryl); 7.93 dd (J = 8.4 Hz / 2.1 Hz, 1
H, aryl); 7.68 dd
10 (J = 8.0 Hz / 7.6 Hz, 1 H, aryl); 7.65 d (J = 8.4 Hz, 1 H, aryl); 7.56 s(1
H, aryl); 7.48 d (J =
8.4 Hz, 1 H, aryl); 7.45 dd (J = 8.4 Hz / 8.0 Hz, 1 H, aryl); 7.33 dd (J = 8.0
Hz / 7.6 Hz,
1 H, aryl); 7.25 dd (J = 8.4 Hz / 8.0 Hz, 1 H, aryl); 5.38 m (1 H, CH); 4.38
dd (J = 10.5 Hz /
4.6 Hz, 1 H, CH2OH); 4.33 dd (J = 10.5 Hz / 5.5 Hz, 1 H, CH2OH); 3.73 dd (J =
14.3 Hz/
6.7 Hz, 1 H, CH2); 3.68 dd (J = 14.3 Hz/ 6.7 Hz, 1 H, CH2); 2.31 s(3H, CH3).
15 The following compounds were obtained in analogy to the preparation methods
described in detail:
Product; Method Structure
Ex. eagents anai~oous'H-NMR (400 MHz) S[ppm]
14 N-[(R)-1-(Hydroxymethyl)-2- 13 (CDCI3): 8.19 s(1H); 8.04 d H
N ~
1 H-indol-3- I eth I 6
( y) y]- - (J = 8.4 Hz, 1 H); 7.71 d (J
HO
methoxy-2-(3,4,5-trimethoxy- = 8.0 Hz, 1 H); 7.68 s(1 H); o H
phenyl)quinoline-4- 7.38 m(3H); 7.20 m(1 H); 0,
carboxamide;
7.11 m(2H); 6.52 d (J = 8.0 N Hz, 1 H); 4.71 m(1 H); 3.99 0 D-Tryptophanol
s(6H); 3.91 s(3H); 3.91 m
and (1 H); 3.82 m(1 H); 3.72 s
(3H); 3.22 m (2H); 2.62 t (J
6-Methoxy-2-(3, 4, 5- = 5.7 Hz, 1 H).
trimethoxyphenyl)quinoline- MS (ESI; +): 542 (M+1).
4-carboxylic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
91
Product; Method Structure
Ex. reagents a"ai=o "S'H-NMR (400 MHz) S[ppm]
15 6-Bromo-N-[(R)-1-(hydroxy- 13 (DMSO-d6): 10.86 s(1H); \
methyl)-2-(1 H-indol-3- 8.79 d (J = 8.4 Hz, 1 H);
HO
I)ethyl]-2-(3,4,5- 8.25 d (J = 2.1 Hz, 1 H); 0 H
rimethoxyphenyl)quinoline-4- 8.07 m (2H); 7.92 dd (J B'
O N
carboxamide; 2.1 Hz / 8.9 Hz, 1 H); 7.69 d
(J = 8.0 Hz, 1 H); 7.58 s
D-Tryptophanol (2H); 7.35 d (J = 8.0 Hz,
und 1 H); 7.25 d (J = 2.1 Hz,
1 H); 7.06 m(1 H); 6.96 m
6-Bromo-2-(3,4,5- (1 H); 4.92 m(1H); 4.39 m
trimethoxyphenyl)quinoline- (1 H); 3.92 s (6H); 3.79 s
4-carboxylic acid (3H); 3.68 m(2H); 3.10 dd
(J = 6.3 Hz / 14.8 Hz, 1 H);
2.98 dd (J = 7.6 Hz / 14.3
Hz, 1 H).
MS (ESI; +): 591 (M+1)
16 N-[(R)-1-(Hydroxymethyl)-2- 13 (CDCI3): 8.23 s(1H); 8.03 H
(1 H-indol-3-yl)ethyl]-6- d (J = 9.1 Hz, 1 H); 7.81 s
methoxy-2-(2,3,4-trimethoxy- (1 H); 7.70 d (J = 7.8 Hz, HO
H
phenyl)quinoline-4- 1 H); 7.58 m(2H); 7.37 m o~,
carboxamide; (2H); 7.12 m(3H); 6.80 d (J (~ -N
= 8.8 Hz, 1 H); 6.51 d (J = ~o ~ o
~o
D-Tryptophanol and 7.8 Hz, 1 H); 4.61 m(1 H);
6-Methoxy-2-(2,3,4- 3.98 s (3H); 3.95 s (3H);
trimethoxyphenyl)quinoline- 3.84 s (3H); 3.69 s (3H);
4-carboxylic acid 3.20 d (J = 7.1 Hz, 2H);
2.68 s (1 H).
MS (ESI; +): 542 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
92
Product; Method Structure
Ex. reagents anai~oous'H-NMR (400 MHz) S[ppm]
"
17 6-Fluoro-N-[(R)-1-(hydroxy- 13 (CDCI,): 8.22 s(1H); 8.15 S
N methyl)-2-(1 H-indol-3- m(1 H); 7.71 m (2H); 7.63
I)ethyl]-2-(3,4,5- dd (J = 2.8 Hz / 9.9 Hz, "o
O "
rimethoxyphenyl)quinoline-4- 1 H); 7.48 m(1 H); 7.39 d (J I F
= 8.1 Hz, 1 H); 7.20 m(1 H); -N
carboxamide;
7.11 m (2H); 6.50 d (J = 7.6
D-Tryptophanol and 'o
Hz, 1 H); 4.69 m(1 H); 3.99
6-Fluoro-2-(3,4,5-trimethoxy- s (6H); 3.91 s (3H); 3.85 m
phenyl)quinoline-4-carboxylic (1 H); 3.21 d (J = 7.1 Hz,
acid 2H); 2.60 s(1 H).
MS (ESI; +): 530 (M+1).
18 N-[(R)-1-(Hydroxymethyl)-2- 13 (DMSO-d6): 10.82 s(1H); H
(1 H-indol-3-yI)ethyl]-6-iod-2- 8.73 d (J = 8.3 Hz, 1 H);
(3,4,5- 8.45 s (1H); 8.02 m (2H); "o "
rimethoxyphenyl)quinoline-4- 7.88 d (J = 8.8 Hz, 1 H);
carboxamide; 7.64 d (J = 7.8 Hz, 1 H); o -N
7.52 s (2H); 7.31 d (J = 8.3 'o
D-Tryptophanol and .
Hz, 1 H); 7.03 m(1 H); 6.92
6-lodo-2-(3, 4, 5- m(1 H); 5.72 s(1 H); 4.88 m
trimethoxyphenyl)quinoline- (1 H); 4.32 m(1 H); 3.90 s
4-carboxylic acid (6H); 3.71 s (3H); 3.58 m
(2H); 3.04 m (1 H); 2.93 m
(1 H).
MS (ESI; +): 638 (M+1).
19 N-[(R)-1-(Hydroxymethyl)-2- 13 (DMSO-d6): 10.80 s(1H); N
(1 H-indol-3-yI)ethyl]-6-nitro-2- 8.98 d (J = 2.6 Hz, 1 H);
(3,4,5- 8.84 d(J = 8.5 Hz, 1 H); HO
H 0
"'o
-
rimethoxyphenyl)quinoline-4- 8.46 dd (J = 2.6 Hz / 9,4 7-NC
carboxamide; Hz, 1 H); 8.28 m(2H); 7.64 0s (1 H); 7.61 s(2H); 7.29 d o
D-Tryptophanol
(J=7.9Hz, 1H);7.21 d (J=
2.1 Hz, 1 H); 6.99 m(1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
93
Product' Method Structure
Ex. reagents anai~oous'H-NMR (400 MHz) S[ppm]
and 6.88 m(1 H); 4.90 m(1 H);
4.35 m(1 H); 3.92 s (6H);
6-Nitro-2-(3,4,5-trimethoxy- 3.73 s (3H); 3.60 s (2H);
phenyl)quinoline-4-carboxylic 3.09 dd (J = 6.2 Hz / 14.7
acid Hz, 1 H); 3.95 dd (J = 7.7
Hz / 14.5 Hz, 1 H).
MS (ESI; +): 557 (M+1).
Example 20
6-Amino-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-(3,4,5-
trimethoxyphenyl)quinoline-4-carboxamide
I oll
0 OHO
NH
0 ~ N
N H
NH2
5.21 mmol (2.9 g) of the compound prepared in Example 19), and the catalyst
palladium
on carbon (10%, 500 mg) were suspended in methanol (40 ml) and hydrogenated
with
hydrogen under atmospheric pressure and at room temperature. After hydrogen
uptake
was complete, the catalyst was filtered off and the solvent was distilled off
in a rotary
evaporator. Oil-pump drying resulted in 2.15 g (78% yield) of the crystalline
title
compound.
'H-NMR (400 MHz, DMSO-d6): 6[ppm] = 10.81 s(1 H); 8.48 d (J = 8.1 Hz, 1 H);
7.74 m
(2H); 7.68 d (J = 7.8 Hz, 1 H); 7.40 s (2H); 7.31 d (J = 8.1 Hz, 1 H); 7.21 d
(J = 2.3 Hz,
1 H); 7.13 dd (J = 2.5 Hz / 9.1 Hz, 1 H); 7.03 m (2H); 6.98 m(1 H); 5.70 s
(2H); 4.82 m
(1 H); 4.29 m(1 H); 3.88 s (6H); 3.70 s (3H); 3.58 m(1 H); 3.51 m(1 H); 3.06
dd (J = 6.6
Hz / 14.7 Hz, 1 H); 2.93 dd (J = 7.6 Hz / 14.7 Hz, 1 H).
MS (ESI; +): 527 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
94
The following compounds were obtained in analogy to the preparation methods
described in detail:
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gousto LPPm]
21 N-[(R,S)-1-(Hydroxymethyl)- I (DMSO-d6): 10.94 s(1 H, N
F
2-(5-fluoro-1 H-indol-3- NH); 8.71 d (J = 8.4 Hz,
yl)ethyl]-6-methoxy-2-(3,4,5- 13 1 H, NH); 8.00 d (J = 8.6 Ho NH
trimethoxyphenyl)quinoline-4- Hz, 1 H, aryl); 7.97 s(1 H, 0.
I
carboxamide;
aryl); 7.50 s (2H, aryl); N
-o ~
7.41 m (3H, aryl); 7.32 m 'o
(R, S)-fi-Amino-5-fluoro-1 H- (1 H, aryl); 7.29 m(1 H,
indole-3-propanol aryl); 6.88 t (J = 8.9 Hz,
and 1 H, aryl); 4.96 s(1 H,
OH); 4.37 m(1 H, CH);
6-Methoxy-2-(3, 4, 5- 3.93 s(6H, OCH3); 3.75
trimethoxyphenyl)quinoline-4- s (6H, OCH3); 3.60 m
carboxylic acid (2H, OCH2); 3.01 dd (J =
14.4 Hz / 5.6 Hz, 1 H,
CH); 2.91 dd (J = 14.4
Hz / 7.8 Hz, 1 H, CH).
19F-NMR (400 MHz,
DMSO-d6): -124.81 m
(1 F).
MS (ESI; +): 560 (M+1).
22 N-[(R)-1-(Hydroxymethyl)-2- 1 (DMSO-d6): 8.67 d (J = \ N
(1-methyl-1 H-indol-3-yl)ethyl]- 8.3 Hz, 1 H, NH); 8.00 d
6-methoxy-2-(3,4,5- 13 (J = 7.8 Hz, 1 H, aryl); HO
O H
trimethoxyphenyl)quinoline-4- 7.99 s(1H, aryl); 7.68 d o
carboxamide (J = 7.9 Hz, 1 H, aryl); .10 N
7.51 s(2H, aryl); 7.41 m-o I~
(R)-fi-Amino-l-methyl-lH- (3H, aryl); 7.18 s (1H, -O
indole-3-propanol aryl); 7.12 t (J = 7.5 Hz,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gousto Ippm]
and 1 H, aryl); 6.99 t (J = 7.4
Hz, 1 H, aryl); 4.91 t(J =
6-Methoxy-2-(3, 4, 5- 5.3 Hz, 1 H, OH); 4.38 m
trimethoxyphenyl)quinoline-4- (1 H, CH); 3.93 s(6H,
carboxylic acid OCH3); 3.75 s (6H,
OCH3); 3.72 s (3H,
NCH3); 3.60 t (J = 5.2
Hz, 2H, OCHZ); 3.03 dd
(J = 14.4 Hz / 6.1 Hz,
1 H, CH); 2.96 dd (J =
14.4 Hz / 7.5 Hz, 1 H,
CH).
MS (APCI; +): 556
(M+1).
23 N-[(R,S)-2-(6-Fluoro-1 H- I (DMSO-d6): 10.90 s(1 H, H F
N
indol-3-yl)-1- NH); 8.67 d (J = 8.4 Hz,
(hydroxymethyl)ethyl]-6- 13 1 H, NH); 8.00 dd (J = 8.6 HO'-"~
methoxy-2-(3,4,5-trimethoxy- Hz, 1 H, aryl); 7.97 s(1 H, 0 NH
phenyl)quinoline-4- aryl); 7.63 m(1 H, aryl);
carboxamide; 7.50 s(2H, aryl); 7.42 m,
(2H, aryl); 7.22 s (1 H, ~O
(R, S)-p-Amino-6-fluoro-1 H-
aryl); 7.10 d (J = 10,2
indole-3-propanol Hz, 1 H, aryl); 6.81 t (J
=
and 8.1 Hz, 1 H, aryl); 4.92 t
(J = 5.4 Hz, 1 H, OH);
6-Methoxy-2-(3, 4, 5- 4.39 m (1 H, CH); 3.60 t
trimethoxyphenyl)quinoline-4- (J = 5.3 Hz, 2H, OCH2);
carboxylic acid 3.04 dd (J = 14.5 Hz /
5.8 Hz, 1 H, CH); 2.93 dd
(J = 14.6Hz / 7.9 Hz, 1 H,
CH).
19F-NMR (400 MHz,
DMSO-d6): -121.70 m
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
96
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gousto Ippm]
(1 F).
MS (APCI; +): 560
(M+1).
24 2-(3,4-Dimethoxyphenyl)-N- 13 (Pyridin-d5): 11.97 s oH
[(S)-1-(hydroxymethyl)-2-(1H- (1H, NH); 9.63 d (J = 8.4 o NH N
H
indol-3-yl)ethyl]quinoline-4- Hz, 1 H, NH); 8.53 d (J = i
O N I
carboxamide; 7.6 Hz, 1 H, aryl); 8.32 d\\
(J = 8.4 Hz, 1 H, aryl);
L-Tryptophanol
8.28 s(1 H, aryl); 8.20 d
and (J = 7.6 Hz, 1 H, aryl);
8.13 d(J = 2.1 Hz, 1 H,
2-(3,4-Dimethoxyphenyl)- aryl); 7.70 dd (J = 8.4 Hz
quinoline-4-carboxylic acid / 2.1 Hz, 1 H, aryl); 7.65
dd (J = 8.0 Hz / 7.6 Hz,
1 H, aryl); 7.64 d (J = 8.0
Hz, 1 H, aryl); 7.58 d (J =
2.1 Hz, 1 H, aryl); 7.41 dd
(J = 8.4 Hz / 8.0 Hz, 1 H,
aryl); 7.33 dd (J = 7.6 Hz
/ 7.0 Hz, 1 H, aryl); 7.26
dd (J = 8.0 Hz / 7.0 Hz,
1 H, aryl); 7.00 d (J = 8.4
Hz, 1 H, aryl); 5.38 m
(1 H, CH); 4.35 m (2H,
CH2OH); 3.78 s (3H,
OCH3); 3.76 s (3H,
OCH3); 3.70 m (2H,
CH2). [a]p = -11.0 (c =
0.330, MeOH / CH2CI2
1:1)
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
97
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gousto [ppm]
25 2-(3,4-Dimethoxyphenyl)-N- 13 (pyridine-d5): 11.97 s OH
[(R)-1-(hydroxymethyl)-2-(1H- (1 H, NH); 9.63 d (J = 8.4 o H IN
indol-3-yl)ethyl]quinoline-4- Hz, 1 H, NH); 8.53 d (J =
i H
7.6 Hz, 1 H, aryl); 8.32 d 0 N
carboxamide;
(J = 8.4 Hz, 1 H, aryl); 'o
D-Tryptophanol 8.28 s(1 H, aryl); 8.20 d
(J = 7.6 Hz, 1 H, aryl);
and 8.13 d(J = 2.1 Hz, 1 H,
aryl); 7.70 dd (J = 8.4 Hz
2-(3,4-Dimethoxyphenyl)- / 2.1 Hz, 1 H, aryl); 7.65
dd (J = 8.0 Hz / 7.6 Hz,
quinoline-4-carboxylic acid
1 H, aryl); 7.64 d (J = 8.4
Hz, 1 H, aryl); 7.58 d(J =
2.1 Hz, 1 H, aryl); 7.41 dd
(J = 8.4 Hz / 8.0 Hz, 1 H,
aryl); 7.33 dd (J = 8.4 Hz
/ 8.0 Hz, 1 H, aryl); 7.26
dd (J = 8.0 Hz / 7.6 Hz,
1 H, aryl); 7.00 d (J = 8.4
Hz, 1 H, aryl); 5.38 m
(1 H, CH); 4.35 m (2H,
CH2OH); 3.78 s (3H,
OCH3); 3.76 s (3H,
OCH3); 3.70 m (2H,
CHZ).
[a]p = +12.1 (c = 0.500,
MeOH / CH2CI2 1:1)
26 2-(3,4-Dimethylphenyl)-N-[(R)- 13 (CDCI3): 8.15 s(1 H, o"
1-(hydroxymethyl)-2-(1 H- NH); 8.13 d (J = 8.0 Hz, o H I
N
indol-3-yl)ethyl]quinoline-4- 1 H, aryl); 7.93 d (J = 8.4 "
carboxamide; Hz, 1 H, aryl); 7.89 s(1 H, N I
aryl); 7.74 d (J = 8.0 Hz, I
D-Tryptophanol 1 H, aryl); 7.69 dd (J =
8.0 Hz / 8.0 Hz, 1 H, aryl);
and 7.68 d(J = 8.0 Hz, 1 H,
aryl); 7.65 s (1 H, aryl);
2-(3,4- 7.39 dd (J = 8.0 Hz / 8.0
Dimethylphenyl)quinoline-4- Hz, 2H, aryl); 7.25 d (J =
8.4 Hz, 1 H, aryl); 7.23 dd
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
98
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gousto Ippm]
carboxylic acid (J = 8.0 Hz / 8.0 Hz, 1 H,
aryl); 7.15 d (J = 8.0 Hz,
1 H, aryl); 7.12 s(1 H, a-
ryl); 6.43 d (J = 7.6 Hz,
1 H, NH); 4.66 m(1 H,
CH); 3.93 d (J = 11.0 Hz,
1H, CH2OH); 3.85 dd (J
= 11.0 Hz / 5.0 Hz, 1 H,
CH2OH); 3.22 d (J = 7.2
Hz, 2H, CHZ); 2.38 s (3H,
CH3); 2.35 s (3H, CH3).
27 -(2,3-Dihydro-1,4- 13 (CDCI3): 8.22 s(1H, OH NH); 8.06 d (J = 8.4 Hz, /
benzodioxin-6-yl)-N-[(R)-1- o ~IIH N
(hydroxymethyl)-2-(1H-indol- 1 H, aryl); 7.92 d (J = 8.0 - "
3-yl)ethyl]quinoline-4- Hz, 1 H, aryl); 7.72 d(J = ro \ I -N
8.0 Hz, 1 H, aryl); 7.65 dd 'o
carboxamide;
(J = 8.4 Hz / 8.0 Hz, 1 H,
D-Tryptophanol aryl); 7.58 d (J = 2.1 Hz,
1 H, aryl); 7.46 dd (J =
and 8.4 Hz / 2.1 Hz, 1 H, aryl);
2-(2,3-Dihydro-1,4- 7.44 s (1 H, aryl); 7.38 dd
benzodioxin-6-yl)quinoline-4- (J = 8.0 Hz/ 8.0 Hz, 1 H,
carboxylic acid aryl); 7.36 dd (J = 8.0 Hz
/ 8.0 Hz, 1 H, aryl); 7.22
dd (J = 8.0 Hz / 8.0 Hz,
1 H, aryl); 7.15 d(J = 8.0
Hz, 1 H, aryl); 7.11 d (J =
2.5 Hz, 1 H, aryl); 6.96 d
(J = 8.4 Hz, 1 H, aryl);
6.43 d (J = 7.6 Hz, 1 H,
NH); 4.63 m (1H, CH);
4.32 s (4H, CH2O); 3.93
dd (J = 11.0 Hz / 3.8 Hz,
1H, CH2OH); 3.83 dd (J
= 11.0 Hz / 5,5 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
99
Ex. Product; aMetho nalotl 'H-NMR (400 MHz) S Structure
reagents gousto IPPm]
CH2OH); 3.22 dd (J =
15.0 Hz / 8.0 Hz, 1 H,
CH2); 3.17 dd (J = 15.0
Hz / 6.7 Hz, 1 H, CH2).
28 N-[(R)-1-(Hydroxymethyl)-2- 13 (Pyridin-ds): 11.99 s OH (1 H-indol-3-
yl)ethyl]-2-[4- (1 H, NH); 9.60 d(J = 8.0 0 H N
(trifluoromethoxy)phenyl]- Hz, 1 H, NH); 8.52 d (J "
7.6 Hz, 1 H, aryl); 8.28 d F N
uinoline-4-carboxamide F'=o =
(J = 8.4 Hz, 1 H, aryl); F
D-Tryptophanol 8.18 d (J = 7.6 Hz, 1 H,
aryl); 8.15 d (J = 8.9 Hz,
2-(4-
1 H, aryl); 8.10 s(1 H, a-
(Trifluoromethoxy) phen yl]-
ryl); 7.67 dd (J = 8.0 Hz/
quinoline-4-carboxylic acid
7.6 Hz, 1 H, aryl); 7.65 d
(J = 8.4 Hz, 1 H, aryl);
7.57 d (J = 2.5 Hz, 1H,
aryl); 7.44 dd (J = 8.4 Hz
/ 8.0 Hz, 1 H, aryl); 7.36 d
(J = 8.9 Hz, 1 H, aryl);
7.33 dd (J = 8.0 Hz / 7.6
Hz, 1 H, aryl); 7.24 dd (J
=8.4Hz/8.OHz, 1H,
aryl); 5.38 m(1 H, CH);
4.38 dd (J = 10.5 Hz /
5.1 Hz, 1H, CH2OH);
4.32 dd (J = 10.5 Hz /
5.5 Hz, 1 H, CH2OH);
3.72 dd (J = 16.4 Hz /
6,7 Hz, 1H, CHz); 3.68
dd (J = 16.4 Hz / 6.7 Hz,
1H, CH2).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
100
Ex. Product; aMetho nalotl 'H-NMR (400 MHz) S Structure
reagents gousto IPpm]
29 N-[(R)-1-(Hydroxymethyl)-2- 13 (CDCI,): 8.16 s(1H, OH NH); 8.11 d (J = 8.4
Hz,
(1H-indol-3-yl)ethyl]-2-[4- o ~-IIH N
(methylsuipha- 1 H, aryl); 7.93 d (J = 8.6 "
nyl)phenyl]quinoline-4- Hz, 1 H, aryl); 7.93 d (J = ~ I N ~ I
8.0 Hz, 1 H, aryl); 7.73 d I'S ~
carboxamide;
(J = 8.0 Hz, 1 H, aryl);
D-Tryptophanol 7.56 s(1 H, aryl); 7.69 dd
(J = 8.4 Hz / 8.0 Hz, 1 H,
and aryl); 7.41 d (J = 8.0 Hz,
2-[4- 1 H, aryl); 7.40 dd (J =
(Methyl- 8.0 Hz / 8.0 Hz, 1 H, aryl);
sulphanyl)phenyl]quinoline-4- 7.35 d (J = 8.6 Hz, 1 H,
carboxylic acid aryl); 7.24 dd (J = 8.0 Hz
/ 8.0 Hz, 1 H, aryl); 7.14
dd (J = 8.0 Hz / 8.0 Hz,
1 H, aryl); 7.12 s(1 H, a-
ryl); 6.40 d (J = 7.6 Hz,
1 H, NH); 4.66 m(1 H,
CH); 3.94 dd (J = 11.4
Hz / 3.8 Hz, 1 H,
CHzOH); 3.85 dd (J =
11.4 Hz / 5.5 Hz, 1 H,
CH2OH); 3.23 dd (J =
15.6 Hz / 7.2 Hz, 1 H,
CHZ); 3.20 dd (J = 15.6
Hz / 7.2 Hz, 1 H, CHZ);
2.56 s (3H, SCH3).
30 2-(3,5-Dimethoxyphenyl)-N- 13 (PYridine-ds): 11.96 s oH
[(R)-1-(hydroxymethyl)-2-(1 H- (1 H, NH); 9.66 d (J = 8.0 o H
indol-3-YI)ethYI]quinoline-4- Hz, 1 H, NH); 8.52 d (J = H
~ ~
7.6 Hz, 1 H, aryl); 8.32 d o ~~ I
carboxamide N
(J = 8.4 Hz, 1 H, aryl);
D-Tryptophanol 8.32 s(1 H, aryl); 8.20 d .10
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
101
Ex. Product; aMetho neloa 'H-NMR (400 MHz) S Structure
reagents gousto IPpm]
2-(3,5- (J = 7.6 Hz, 1 H, aryl);
Dimethoxyphenyl)quinoline-4- 7.66 dd (J = 8.0 Hz/ 7.6
carboxylic acid Hz, 1 H, aryl); 7.63 d (J =
8.4 Hz, 1 H, aryl); 7.58 d
(J = 2.3 Hz, 2H, aryl);
7.57 s(1 H, aryl); 7.43 dd
(J = 8.4 Hz / 8.0 Hz, 1H,
aryl); 7.32 dd (J = 8.4 Hz
/ 8.0 Hz, 1 H, aryl); 7.26
dd (J = 8.0 Hz / 7.6 Hz,
1 H, aryl); 6.78 t (J = 2.3
Hz, 1 H, aryl); 5.37 m
(1 H, CH); 4.34 m (2H,
CH2OH); 3.71 s (6H,
OCH3); 3.69 m (2H,
CHZ).
31 -[3-(Acetylamino)phenyl]-N- 13 (Pyridine-d5): 11.91 s o"
[(R)-1-(hydroxymethyl)-2-(1H- (1H, NH); 10.91 s(1H, o H N
indol-3-yl)ethyl]quinoline-4- NH); 9.57 d (J = 8.4 Hz, ~o - H
carboxamide; 1 H, NH); 8.90 s(1 H, a- HN -N
ryl); 8.51 d (J = 7.6 Hz,
D-Tryptophanol 1 H, ary l); 8.21 d (J = 8.4
Hz, 1 H, aryl); 8.20 s(1 H,
and aryl); 8.19 d (J = 7.6 Hz,
1 H, aryl); 8.15 d (J = 8.0
2-(4-(Acetylamino)phenyl]- Hz, 1 H, aryl); 7.82 d (J =
quinoline-4-carboxylic acid 8.0 Hz, 1 H, aryl); 7.65 d
(J = 8.4 Hz, 1 H, aryl);
7.64 dd (J = 8.0 Hz/ 7.6
Hz, 1 H, aryl); 7.63 s(1 H,
aryl); 7.43 dd (J = 8.4 Hz
/ 8.0 Hz, 1 H, aryl); 7.41
dd (J = 8.0 Hz / 8.0 Hz,
1 H, aryl); 7.33 dd (J =
8.4 Hz / 8.0 Hz, 1 H, aryl);
7.26 dd (J = 8.0 Hz / 7.6
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
102
Ex. Product; eMetho na otl 'H-NMR (400 MHz) S Structure
reagents gousto Ippm]
Hz, 1 H, aryl); 5.36 m
(1 H, CH); 4.36 m (2H,
CH2OH); 3.71 s (6H,
CHzOH); 3.73 dd (J =
14.6 Hz / 6.7 Hz, 1H,
CHZ); 3.68 dd (J = 14.6
Hz / 7.2 Hz, 1 H, CHz);
2.24 s (3H, CH3).
32 2-(4-Chlorophenyl)-N-[(R)-1- 13 (CDCI3): 8.14 s(1H, o"
(hydroxymethyl)-2-(1H-indol- NH); 8.13 d (J = 8.4 Hz, o H I
N
3-yI)ethyl]quinoline-4- 1 H, aryl); 7.98 d (J = 8.0 H
carboxamide; -- Hz, 1 H, aryl); 7.95 d (J = N
8.6 Hz, 2H, aryl); 7.74 d Cil I
o-Tryptophanol (J = 8.0 Hz, 1 H, aryl);
7.72 dd (J = 8.4 Hz / 8.0
and Hz, 1 H, aryl); 7.56 s(1 H,
aryl); 7.47 d (J = 8.6 Hz,
2-(4-Chlorophenyl)quinoline-4- 2H, aryl); 7.45 dd (J =
8.0 Hz/ 8.0 Hz, 1 H, aryl);
carboxylic acid
7.44 d (J = 8.0 Hz, 1 H,
aryl); 7.25 dd (J = 8.0 Hz
/ 8.0 Hz, 1 H, aryl); 7.14
dd (J = 8.0 Hz / 8.0 Hz,
1 H, aryl); 7.14 s(1 H, a-
ryl); 6.37 d (J = 7.6 Hz,
1 H, NH); 4.69 m(1 H,
CH); 3.96 d (J = 11.4 Hz,
1 H, CH2OH); 3.86 d (J
=
11.4 Hz, 1 H, CH2OH);
3.26 dd (J = 14.8 Hz /
6.3 Hz, 1 H, CHZ); 3.21
dd (J = 14.8 Hz / 7.6 Hz,
1H, CHZ).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
103
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gousto [ppm]
33 -[(R)-1-(Hydroxymethyl)-2- 13 (CDCI3): 8.16 s(1H, o"
(1 H-indol-3-yl)ethyl]-2-(4- NH); 8.09 d (J - 8.4 Hz, 0 H H
methoxyphenyl)quinoline-4- 1 H, aryl); 7.94 d (J = 8.9
carboxamide;
Hz, 2H, aryl); 7.91 d(J =\ i N
8.0 Hz, 1 H, aryl); 7.73 d
D-Tryptophanol (J = 8.0 Hz, 1 H, aryl);
and 7.67 dd (J = 8.4 Hz / 8.0
Hz, 1 H, aryl); 7.53 s(1 H,
2-(4-Methoxyphenyl)quinoline- aryl); 7.40 d (J = 8.0 Hz,
4-carboxylic acid 1 H, aryl); 7.37 dd (J =
8.0 Hz/ 8.0 Hz, 1 H, aryl);
7.24 dd (J = 8.0 Hz / 8.0
Hz, 1 H, aryl); 7.14 dd (J
=8.OHz/8.OHz, 1H,
aryl); 7.10 d (J = 2.5 Hz,
1 H, aryl); 7.00 d(J = 8.9
Hz, 2H, aryl); 6.40 d (J =
8.0 Hz, 1 H, NH); 4.65 m
(1 H, CH); 3.92 d (J =
11.4 Hz, 1H, CH2OH);
3.89 s (3H, CH3); 3.84 d
(J = 11.4 Hz, 1 H,
CH2OH); 3.21 m (2H,
CH2).
34 N-[(R)-1-(Hydroxymethyl)-2- 13 (Pyridine-d5): 11.97 s oH
(1 H-indol-3-yl)ethyl]-2-(3- (1 H, NH); 9.63 d (J = 8.0 o H
H
methoxyphenyl)quinoline-4- Hz, 1 H, NH); 8.52 d (J = 11
I
carboxamide 7.6 Hz, 1 H, aryl); 8.31 d 0 N (J = 8.4 Hz, 1 H, aryl);
o-Tryptophanol 8.23 s (1 H, aryl); 8.20 d
(J = 7.6 Hz, 1 H, aryl);
2-(3-Meth ox yph en yl) q uinolin e-
4-carboxylic acid 8.03 dd (J = 2.1 Hz / 1,7
Hz, 1 H, aryl); 7.67 dd (J
=8.OHz/7.6Hz, 1H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
104
Ex. Product; aMetho nalotl 'H-NMR (400 MHz) 8 Structure
reagents gousto IPPm]
aryl); 7.64 dd (J = 8.0 Hz
/ 1.7 Hz, 1 H, aryl); 7.64 d
(J = 8.4 Hz, 1 H, aryl);
7.56 s (1 H, aryl); 7.43 dd
(J = 8.4 Hz / 8.0 Hz, 1 H,
aryl); 7.39 dd (J = 8.0 Hz
/ 7.6 Hz, 1 H, aryl); 7.34
dd (J = 8.0 Hz / 7.6 Hz,
1 H, aryl); 7.26 dd (J =
8.4 Hz / 8.0 Hz, 1 H, aryl);
7.08 dd (J = 7.6 Hz / 2.1
Hz Hz, 1 H, aryl); 5.38 m
(1 H, CH); 4.36 m (2H,
CH2OH); 3.71 s (3H,
OCH3); 3.71 m (2H,
CH2).
35 N-[(R)-2-(1-Ethyl-1 H-indol-3- 13 (DMSO-d6): 8.69 d (J
yI)-1-(hydroxymethyl)ethyl]-6- 8.3Hz, 1H, NH), 8.00 d N~
methoxy-2-(3,4,5-trimethoxy- J = 8.3Hz, 1 H, aryl); 7.99 HO
phenyl)quinoline-4- s (1 H, aryl); 7.67 d (J = 0 H
o"
carboxamide; 7.84, 1 H, aryl); 7.50 s -0 N
(2H, aryl); 7.44 m (3H, -o
(R)-2-Amino-3-(1-ethyl-1 H- ~o
aryl); 7.24 s(1 H, aryl);
indol-3-yl)-propan-l-ol 7.10 t(J = 7.7 Hz, 1 H,
and aryl); 6.97 t (J = 7.3 Hz,
1 H, aryl); 4.93 t (J = 5.7
6-Methoxy-2-(2,3,4-tri- Hz, 1 H, OH); 4.38 m(1 H,
methoxyphenyl)quinoline-4- CH); 4.13 q (J = 7.17 Hz,
carboxylic acid 2H, NC2H5); 3.92 s (6H,
OCH3); 3.75 s (6H,
OCH3); 3.61 m (2H,
OCH2); 3.03 dd (J = 14.4
Hz / 5.8 Hz, 1 H,CH);
2.95 dd (J = 14.4 Hz /
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
105
Ex. Product; aMetho nalotl 'H-NMR (400 MHz) S Structure
reagents gousto [ppm]
7.6 Hz, 1 H, CH); 1.27 t (J
= 7.17 Hz, 3H, NC2H5).
MS (APCI;+): 570(M+1)
36 2-(2,3-Dihydrobenzofuran-5- (CDC13): 8.19 s (1 H, OH
I)-N-[(R)-1-(hydroxymethyl)- NH); 8.06 d(J= 8.3 Hz, o H- N
H
2-(1 H-indol-3- 1 H, aryl); 7.93 s(1 H,
I)ethyl]quinoline-4- aryl); 7.89 d (J = 8.3 Hz, N
0
carboxamide; 1 H, aryl); 7.72 d (J = 7.8
Hz, 1 H, aryl); 7.65 dd (J
D-Tryptophanol = 8.3 Hz / 8.3 Hz, 1 H,
and aryl); 7.64 d (J = 8.3 Hz,
1 H, aryl); 7.49 s(1 H,
2-(2, 3-Dihydrobenzofuran-5- aryl); 7.39 d (J = 8.3 Hz,
I)quinoline-4-carboxylic acid 1 H, aryl); 7.35 dd (J =
8.3 Hzl 8.3 Hz, 1 H, aryl);
7.22 dd (J = 8.3 Hz / 7.0
Hz, 1 H, aryl); 7.13 dd (J
= 7.8 Hz / 7.0 Hz, 1H,
aryl); 7.08 d (J = 2.3 Hz,
1 H, aryl); 6.85 d (J = 8.3
Hz, 1 H, aryl); 6.44 d (J =
7.8 Hz, 1 H, NH); 4.63 m
(1 H, CH); 4.64 t (J = 8.7
Hz, 2H, CH2O); 3.90 dd
(J = 11.1 Hz / 3.8 Hz,
1 H, CH2OH); 3.82 dd (J
= 11.1 Hz / 5.1 Hz, 1 H,
CH2OH); 3.27 t (J = 8.7
Hz, 2H, CH2); 3.21 dd (J
= 15.7 Hz/6.8 Hz, 1H,
CHZ); 3.17 dd (J = 15.7
Hz / 6.8 Hz, 1 H, CHZ).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
106
Metho
Ex. Product; analoa 'H-NMR (400 MHz) S Structure
reagents gousto [ppm]
37 N-[(R)-1-(Hydroxymethyl)-2- (Pyridine-d5): 11.85 s OH
(1 H-indol-3-yl)ethyl]-6- (1 H, NH); 9.64 d (J = 8.3 0 H N
H
methoxy-2-(7- Hz, 1 H, NH); 8.45 s(1 H, o~
methoxybenzofuran-2-yl)- aryl); 8.20 d (J = 9.2 Hz,
uinoline-4-carboxamide; 2H, aryl); 8.06 d (J = 2.8
Hz, 1 H, aryl); 7.68 s(1 H,
D-Tryptophanol aryl); 7.62 s (1 H, aryl);
and 7.59 d(J = 8.0 Hz, 1 H,
aryl); 7.44 dd (J = 9.2 Hz
6-Methoxy-2-(7-methoxy- / 2.8 Hz, 1 H, aryl); 7.33 d
benzofuran-2-yl)quinoline-4- (J = 7.8 Hz, 1 H, aryl);
carboxylic acid 7.31 dd (J = 8.0 Hz / 8.0
Hz, 1 H, aryl); 7.29 dd (J
=9.2Hz/8.OHz, 1H,
aryl); 7.24 dd (J = 7.8 Hz
/ 7.8 Hz, 1 H, aryl); 6.93 d
(J = 7.8 Hz, 1 H, aryl);
5.37 m (1 H, CH); 4.38 dd
(J = 10.9 Hz / 4.5 Hz,
2H, CH2OH); 4.30 dd (J
= 10.9 Hz / 6.0 Hz, 2H,
CH2OH); 3.92 s (3H,
OCH3); 3.70 s (3H,
OCH3); 3.68 m (2H,
CHZ).
38 2-[(E)-2-(3,4- 13 (DMSO-d6): 10.80 s H-
N &
Dimethoxyphenyl)ethenyl]-N- (1 H); 8.55 d (J = 8.5 Hz,
HO \ ~
[(R)-1-(hydroxymethyl)-2-(1 H- 1 H); 7.85 d (J = 9.6 Hz, o H
indol-3-yl)ethyl]-6- 1 H); 7.60 m (3H); 7.33 m -~
~ \ \ N
methoxyquinoline-4- (5H); 7.19 m (2H); 6.98 o
carboxamide; m (3H); 4.85 t (J = 5.7
Hz, 1 H); 4.38 m(1 H);
D-Tryptophanol
3.84 s (3H); 3.79 s (3H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
107
Ex. Product; aMet nahoa 'H-NMR (400 MHz) S Structure
reagents gousto IPpm]
=
and 3.68 s (3H); 3.57 t (J
5.7 Hz, 1 H); 3.02 dd (J =
2-[(E)-2-(3,4- 14.9 Hz / 6,2 Hz, 1 H);
Dimethoxyphenyl)ethenyl]-6- 2.91 m (1 H).
ethoxyquinoline-4-carboxylic MS (ESI; +): 538 (M+1).
acid
Example 39
N-[(R)-1-(Hydroxymethyl)-2-(1H-indol-3-yl)ethyl]-4'-methoxy[1,1'-biphenyl]-2-
carboxamide
OH
~
O H I N
H
I
39a) ethyl 4'-methoxy[1,1'-biphenyl]-2-carboxylate
0.66 mmol (100 mg) of 4-methoxyphenylboronic acid, 0.88 mmol (0.88 ml) of a 1
molar
solution of tetrabutylammonium fluoride in tetrahydrofuran and 0.044 mmol (51
mg) of
tetrakis(triphenylphosphine)palladium(0) were added to a solution of 0.44 mmol
(69 pl)
of ethyl 2-bromobenzoate in 4.4 ml of toluene and 2.2 ml of ethanol. The
mixture was
heated to boiling for four hours. After cooling, the reaction mixture was
diluted with
saturated aqueous sodium hydrogen carbonate solution and extracted with ethyl
acetate. The combined organic phases were washed with saturated aqueous sodium
chloride solution, dried over sodium sulphate, filtered and concentrated in
vacuo. Flash
chromatography resulted in 107 mg of the target compound.
'H-NMR (400 MHz, CDC13): S[ppm] = 7.79 d (J = 7.8 Hz, 1 H, aryl); 7.50 dd (J =
7.6 Hz /
7.3 Hz, 1 H, aryl); 7.37 dd (J = 7.8 Hz / 7.3 Hz, 1 H, aryl); 7.36 d (J = 7.6
Hz, 1 H, aryl);
7.26 d (J = 8.6 Hz, 1 H, aryl); 6.93 d (J = 8.6 Hz, 1 H, aryl); 4.12 q (J =
7.1 Hz, 2H,
OCHz); 3.85 s (3H, OCH3); 1.06 t (J = 7.1 Hz, 3H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
108
39b) 4'-methoxy[1,1'-biphenyl]-2-carboxylic acid
0.39 mmol (100 mg) of the compound prepared as in 39a) were stirred in 4 ml of
methanol with 2.39 ml of a 2 molar aqueous sodium hydroxide solution at room
temperature for 16 hours. The reaction mixture was concentrated in vacuo,
acidified to
pH 4 with 1 M aqueous hydrochloric acid and stirred for a further hour. 82 mg
of the
target compound were obtained by filtering off the precipitate with suction.
' H-NMR (400 MHz, CDCI3): S[ppm] = 7.92 d (J = 7.8 Hz, 1 H, aryl); 7.54 dd (J
= 7.6 Hz /
7.6 Hz, 1 H, aryl); 7.39 dd (J = 7.8 Hz / 7.6 Hz, 1 H, aryl); 7.36 d (J = 7.6
Hz, 1 H, aryl);
7.27 d (J = 8.7 Hz, 1 H, aryl); 6.93 d (J = 8.6 Hz, 1 H, aryl); 3.85 s(3H,
OCH3).
39c) N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-4'-methoxy[1,1'-
biphenyl]-2-
carboxamide
In analogy to Example 1e), 89 mg of the title compound were obtained from 0.33
mmol
(75 mg) of the compound prepared as in 39b) and 0.26 mmol (50 mg) of D-
tryptophanol.
'H-NMR (400 MHz, CDC13): S[ppm] = 8.05 s (1 H, NH); 7.63 d (J = 7.8 Hz, 1 H,
aryl);
7.54 d (J = 7.8 Hz, 1 H, aryl); 7.44 dd (J = 7.8 Hz / 7.5 Hz, 1 H, aryl); 7.34
d (J = 8.0 Hz,
1 H, aryl); 7.33 dd (J = 7.8 Hz / 7.5 Hz, 1 H, aryl); 7.31 d (J = 7.8 Hz, 1 H,
aryl); 7.29 d (J
= 8.8 Hz, 2H, aryl); 7.19 dd (J = 7.8 Hz / 7.1 Hz, 1 H, aryl); 7.09 dd (J =
8.0 Hz / 7.1 Hz,
1 H, aryl); 6.89 d (J = 8.8 Hz, 2H, aryl); 6.84 d (J = 2.3 Hz, 1 H, aryl);
5.61 d (J = 7.6 Hz,
1 H, NH); 4.26 m (1 H, CH); 3.78 s(3H, OCH3); 3.48 m(2H, CH2OH); 2.77 d (J =
6.6 Hz,
2H, CH2).
The following compounds were obtained in analogy to the preparation methods
described in detail:
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
109
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gous to IPpm]
40 N-[(S)-1-(Hydroxymethyl)-2- 39 (CDCI,): 8.15 s(1H, OH
(1 H-indol-3-yI)ethyl]-3',4'- NH); 7.87 s(1 H, aryl); o NH I H
imethoxy[1,1'-biphenyl]-3- 7.73 d (J = 8.0 Hz, 1 H,
I
carboxamide; aryl); 7.65 d (J = 7.6 Hz, o
1 H, aryl); 7.51 d (J = 7.6
L-Tryptophanol Hz, 1 H, aryl); 7.40 dd (J
=8.OHz/8.OHz, 1H,
and aryl); 7.40 dd (J = 7.6 Hz
/ 7.6 Hz, 1 H, aryl); 7.22
3;4'-Dimethoxy[1,1'-biphenyl]-
dd (J = 8.0 Hz / 8.0 Hz,
3-carboxylic acid 1 H, aryl); 7.15 d (J = 8.0
Hz, 1 H, aryl); 7.12 d(J =
2.1 Hz, 1 H, aryl); 7.09 dd
(J = 8.0 Hz / 2.1 Hz, 1 H,
aryl); 7.07 s(1 H, aryl);
6.94 d (J = 8.0 Hz, 1 H,
aryl); 6.55 d (J = 7.6 Hz,
1H, NH); 4.51 m (1H,
CH); 3.94 s (6H, OCH3);
3.85 dd (J = 11.0 Hz /
4.2 Hz, 1 H, CH2OH);
3.80 dd (J = 11.0 Hz /
5.4 Hz, 1 H, CH2OH);
3.18 d (J = 6.7 Hz, 2H,
CH2).
Ho = -45.7 (c = 0.980,
MeOH / CH2CIZ 1:1)
41 N-[(R)-1-(Hydroxymethyi)-2- 39 OH
(CDC13): 8.14 s (1 H,
(1H-indol-3-yl)ethyl]-3',4'- NH); 7.87 s(1H, aryl); H H
dimethoxy[1,1'-biphenyl]-3- 7.73 d (J = 8.0 Hz, 1 H,
o
carboxamide; aryl); 7.65 d (J = 7.6 Hz, ,
1 H, aryl); 7.51 d (J = 7.6
D-Tryptophanol Hz, 1 H, aryl); 7.40 dd (J
= 8.0 Hz / 8.0 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
110
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gous to [PPm]
and aryl); 7.40 dd (J = 7.6 Hz
/ 7.6 Hz, 1 H, aryl); 7.22
3', 4'-Dimethoxy(1,1 '-biphenyl]- dd (J = 8.0 Hz / 8.0 Hz,
3-carboxylic acid 1 H, aryl); 7.15 d(J = 8.0
Hz, 1 H, aryl); 7.13 d (J =
2.1 Hz, 1 H, aryl); 7.09 dd
(J = 8.0 Hz / 2.1 Hz, 1 H,
aryl); 7.07 s(1 H, aryl);
6.94 d(J = 8.0 Hz, 1 H,
aryl); 6.55 d (J = 7.6 Hz,
1 H, NH); 4.51 m(1 H,
CH); 3.94 s (6H, OCH3);
3.85 dd (J = 11.0 Hz /
4.2 Hz, 1 H, CH2OH);
3.80 dd (J = 11.0 Hz /
5.4 Hz, 1 H, CH2OH);
3.18 d (J = 6.7 Hz, 2H,
CHZ).
[a]p = +51.5 (c = 0,690,
MeOH / CH2CI2 1:1)
42 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8,20 s(1H, OH
(1H-indol-3-yI)ethyl]-2',3',4'- NH); 7.77 s H a Ioi
(1 ry ), H
rimethoxy[1,1'-biphenyl]-3- 7.71 d (J = 7,8 Hz, 1 H, I
carboxamide; aryl); 7.62 d (J = 7.5 Hz,
0
1 H, aryl); 7.60 d (J = 8.0
D-Tryptophanol Hz, 1 H, aryl); 7.39 dd (J
= 8.0 Hz / 7.5 Hz, 1H,
and
aryl); 7.36 d (J = 8.3 Hz,
2',3;4'-Trimethoxy(1,1 '- 1 H, aryl); 7.19 dd (J =
biphenyl]-3-carboxylic acid 7,8 Hz / 7.0 Hz, 1 H, aryl);
7.11 dd (J = 8.3 Hz / 7.0
Hz, 1 H, aryl); 7.11 d (J =
2.5 Hz, 1 H, aryl); 6.97 d
(J = 8.6 Hz, 1 H, aryl);
6.74 d(J = 8.6 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
111
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents yous to [ppm]
aryl); 6.55 d (J = 7.3 Hz,
1 H, NH); 4.49 m(1 H,
CH); 3.94 s (3H, OCH3);
3.91 s (3H, OCH3); 3.80
m (2H, CH2OH); 3.62 s
(3H, OCH3); 3,16 d (J
6.8 Hz, 2H, CH2).
43 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.16 s(1H, H
(1H-indol-3-yi)ethyl]-3',4',5'-tri- NH); 7.90 s(1H, aryl); H N
methoxy[1,1'-biphenyl]-3- 7.72 d (J = 8.0 Hz, 1 H, ~ H
I
aryl); 7.65 d (J = 7.8 Hz, .
carboxamide;
1 H, aryl); 7.53 d (J = 7.8 o
Hz, 1 H, aryl); 7.41 dd (J ,
D-Tryptophanol
= 7.8 Hz / 7,8 Hz, 1 H,
and aryl); 7.38 d (J = 8.0 Hz,
1 H, aryl); 7.21 dd (J =
3',4',5'-Trimethoxy(1,1 '-bi- 8.0 Hz / 7.0 Hz, 1 H, aryl);
henyl]-3-carboxylic acid 7.12 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 7.12 d(J =
2.3 Hz, 1 H, aryl); 6.74 s
(2H, aryl); 6.56 d (J = 6.5
Hz, 1 H, NH); 4.51 m(1 H,
CH); 3.91 s (6H, OCH3);
3.90 s (3H, OCH3); 3.82
m (2H, CH2OH); 3.18 d
(J = 6.8 Hz, 2H, CHZ).
44 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.18 s(1H, oH
(1 H-indol-3-yl)ethyl]-3',4',5'-tri- NH); 7.73 d (J = 7,8 Hz,
methoxy[1,1'-biphenyl]-4- 1 H, aryl); 7.70 d (J = 8.5 H
carboxamide; Hz, 2H, aryl); 7.54 d (J =
8.5 Hz, 2H, aryl); 7.39 d
D-Tryptophanol (J = 8.0 Hz, 1 H, aryl);
~
7.23 dd (J = 7.8 Hz / 7.0
o
and Hz, 1 H, aryl); 7.17 dd (J o
= 8.0 Hz / 7.0 Hz, 1 H,
3; 4; 5'-Trimethoxy(1,1 '-bi- aryl); 7.12 d (J = 2.3 Hz,
1 H, aryl); 6.76 s (2H, a-
henyl]-4-carboxylic acid
ryl); 6.54 d (J = 7.5 Hz,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
112
Product; Method 'H-NMR (400 MHz) S Structure
Ex. rea ents analo-
9 gous to [ppm]
1 H, NH); 4.51 m(1 H,
CH); 3.92 s (6H, OCH3);
3.89 s (3H, OCH3); 3.82
m (2H, CH2OH); 3.18 d
(J = 6,8 Hz, 2H, CH2).
45 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.24 s(1H, oH
(1 H-indol-3-yl)ethyl]-3',4',5'-tri- NH); 7.74 d (J = 8.0 Hz,
o H
methoxy-2-methyl[1,1'- 1 H, aryl); 7.52 m (1 H, H
biphenyl]-4-carboxamide; aryl); 7.48 d (J = 8.0 Hz,
1 H, aryl); 7.39 d (J = 8.0
D-Tryptophanol and Hz, 1 H, aryl); 7.23 d(J =
8.0 Hz, 1 H, aryl); 7.23 dd
q o
3 ; 4 ; 5'-Trimethoxy-2- (J = 8.0 Hz / 7.0 Hz, 1 H, o
ethy111, 1 ,-biphenylJ-4- aryl); 7.16 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.12 d
carboxylic acid (J = 2.3 Hz, 1 H, aryl);
6.46 s (2H, aryl); 6.53 d
(J = 7.3 Hz, 1 H, NH);
4.50 m(1 H, CH); 3.90 s
(3H, OCH3); 3.85 s (6H,
OCH3); 3.82 m (2H,
CH2OH); 3.18 d (J = 6.8
Hz, 2H, CH2).
46 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.09 s(1H, OH
(1 H-indol-3-yl)ethyl]-2',3',4'-tri- NH); 7.66 d (J = 7.5 Hz, ,a 0 H N
methoxy[1,1'-biphenyl]-2- 1 H, aryl); 7.56 d (J = 8.0 0~~ ~ H
carboxamide; Hz, 1 H, aryl); 7.44 dd (J o\
=7.5Hz/7.5Hz, 1H,
D-Tryptophanol
aryl); 7.38 dd (J = 7.5 Hz
and / 7.5 Hz, 1 H, aryl); 7.33 d
(J = 8.0 Hz, 1 H, aryl);
2',3;4'-Trimethoxyjl,1 '- 7.24 d(J = 7.5 Hz, 1 H,
biphenylJ-2-carboxylic acid aryl); 7.18 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.08
dd (J = 8.0 Hz / 7.0 Hz,
1 H, aryl); 6.92 d (J = 1.5
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
113
Ex. Product; aMetho nalotl 'H-NMR (400 MHz) S Structure
reagents 9oõS to [ppm]
Hz, 1 H, aryl); 6.86 d (J
=
8.5 Hz, 1 H, aryl); 6.65 d
(J = 8.5 Hz, 1 H, aryl);
6.04 d (J = 8.0 Hz, 1 H,
NH); 4.26 m(1 H, CH);
3.90 s (3H, OCH3); 3.85
s (3H, OCH3); 3.59 m
(1 H, CHZOH); 3.57 s
(3H, OCH3); 3.46 m (1 H,
CHzOH); 2.84 m (2H,
CH2).
47 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.09 s(1 H, OH
(1 H-indol-3-yl)ethyl]-3',4',5'-tri- NH); 7.65 d (J = 7.5 Hz, "o 0 H N
methoxy[1,1'-biphenyl]-2- 1 H, aryl); 7.54 d (J = 8.0 o~~
carboxamide; Hz, 1 H, aryl); 7.46 dd (J
= 7.5 Hz / 7.5 Hz, 1H,
D-Tryptophanol
aryl); 7.40 dd (J = 7.5 Hz
and / 7.5 Hz, 1 H, aryl); 7.34 d
(J = 7.5 Hz, 1 H, aryl);
3',4 ; 5'-Trimethoxy(1,1'-bi- 7.33 d(J = 8.0 Hz, 1 H,
henyl)-2-carboxylic acid aryl); 7.17 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.07
dd (J = 8.0 Hz / 7.0 Hz,
1 H, aryl); 6.75 d (J = 2.5
Hz, 1 H, aryl); 6.59 s (2H,
aryl); 5.68 d (J = 7.3 Hz,
1 H, NH); 4.25 m(1 H,
CH); 3.87 s (3H, OCH3);
3.83 s (6H, OCH3); 3.50
m (2H, CHZOH); 2.78 d
(J = 7.0 Hz, 2H, CHZ).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
114
Ex. Product; aMetho nalo~ 'H-NMR (400 MHz) S Structure
reagents 9ous to [ppm]
48 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.16 s(1H, OH
(1 H-i ndol-3-yl)ethyl]-2', 3',4'-tri- NH); 7.69 d(J= 8.0 Hz, o H N
H
methoxy-6-methyl[1,1'- 1 H, aryl); 7.55 dd (J = 'o
biphenyl]-3-carboxamide; 8.0 Hz / 2.0 Hz, 1 H, aryl); 'o \ I \
0
7.45 d(J = 2.0 Hz, 1 H, ~
D-Tryptophanol aryl); 7.34 d (J = 8.0 Hz,
and 1 H, aryl); 7.25 d (J = 8.0
Hz, 1 H, aryl); 7.17 dd (J
2;3;4'-Trimethoxy-6- = 8.0 Hz / 7.0 Hz, 1 H,
ethyl11,1'-biphenyl]-3- aryl); 7.08 dd (J = 8.0 Hz
carboxylic acid / 7.0 Hz, 1 H, aryl); 7.09 d
(J = 2.3 Hz, 1 H, aryl);
6.78 d(J = 8.5 Hz, 1 H,
aryl); 6.72 d (J = 8.5 Hz,
1 H, aryl); 6.48 d (J = 7.0
Hz, 1 H, NH); 4.45 m(1 H,
CH); 3.93 s (3H, OCH3);
3.92 s (3H, OCH3); 3.79
m (2H, CH2OH); 3.52 s
(3H, OCH3); 3.14 m (2H,
CH2); 2.19 s (3H, CH3).
49 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.15 s(1H, OH
(1H-indol-3-yl)ethyl]-3',4',5'-tri- NH); 7.69 d (J = 7,8 Hz, o H N
H
methoxy-6-methyl[1,1'- 1 H, aryl); 7.53 dd (J = ~
~o I
biphenyl]-3-carboxamide; 8.0 Hz / 2.0 Hz, 1 H, aryl); I
0
7.50 d(J = 2.0 Hz, 1 H, 1 o,
D-Tryptophanol aryl); 7.35 d (J = 8.0 Hz,
and 1 H, aryl); 7.26 d (J = 8.0
Hz, 1 H, aryl); 7.18 dd (J
3', 4', 5'-Trimethoxy-6- = 8.0 Hz / 7.0 Hz, 1 H,
ethyl11,1 '-biphenyl]-3- aryl); 7.07 dd (J = 8.0 Hz
carboxylic acid / 7.0 Hz, 1 H, aryl); 7.10 d
(J = 2,3 Hz, 1 H, aryl);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
115
Product; Method 'H-NMR (400 MHz) S Structure
Ex. rea ents analo-
9 yous to Ippml
6,44 s (2H, aryl); 6.49 d
(J = 6.8 Hz, 1 H, NH);
4.48 m(1 H, CH); 3.92 s
(3H, OCH3); 3.85 s (6H,
OCH3); 3.79 m (2H,
CH2OH); 3,15 m (2H,
CH2); 2,29 s (3H, CH3).
50 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.21 s(1H, OH -
(1H-indol-3-yl)ethyl]-2',3',4'-tri- NH); 7.73 d (J = 8.0 Hz,
O H
methoxy[1,1'-biphenyl]-4- 1 H, aryl); 7.68 d (J = 8.5 H
carboxamide; Hz, 2H, aryl); 7.51 d (J = I
8.5 Hz, 2H, aryl); 7.38 d ~o
D-Tryptophanol ~
(J = 8.0 Hz, 1 H, aryl); o~
und 7.22 dd (J = 7,8 Hz / 7.0 1 o.
Hz, 1 H, aryl); 7.15 dd (J
2; 3; 4'-Trimethoxy(1,1 '-bi- = 8.0 Hz / 7.0 Hz, 1 H,
henylj-4-carboxylic acid aryl); 7.11 d (J = 2,3 Hz,
1 H, aryl); 7.01 d (J = 8.6
Hz, 1 H, aryl); 6.74 d (J =
8.6 Hz, 1 H, aryl); 6.55 d
(J = 7.2 Hz, 1 H, NH);
4.51 m(1 H, CH); 3.93 s
(3H, OCH3); 3.90 s (3H,
OCH3); 3.81 m (2H,
CHZOH); 3,64 s (3H,
OCH3); 3.17 d (J = 6.8
Hz, 2H, CH2).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
116
Ex. Product; aMet nehoa 'H-NMR (400 MHz) S Structure
reagents gous to [PPmI
-
51 V-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.19 s(1H, OH
(1H-indol-3-yI)ethyl]-2',3',4'- NH); 7.74 d (J = 8.0 Hz, o H
rimethoxy-2-methyl[1,1'- 1 H, aryl); 7.50 s(1 H, a- H
biphenyl]-4-carboxamide; ryl); 7.47 d (J = 8.0 Hz,
~
1 H, aryl); 7.39 d (J = 8.0 o
D-Tryptophanol
Hz, 1 H, aryl); 7.22 dd (J o
and = 8.0 Hz / 7.0 Hz, 1 H, oNI
aryl); 7.19 d (J = 8.0 Hz,
2; 3; 4'-Trimethoxy-2-methyl- 1 H, aryl); 7.16 dd (J =
1,1 '-biphenylJ-4-carboxylic 8.0 Hz / 7.0 Hz, 1 H, aryl);
acid 7.12 d(J = 2.3 Hz, 1 H,
aryl); 6.78 d (J = 8.6 Hz,
1 H, aryl); 6.71 d (J = 8.6
Hz, 1 H, aryl); 6.51 d (J =
7.0 Hz, 1H, NH); 4.50 m
(1 H, CH); 3.92 s (3H,
OCH3); 3.90 s (3H,
OCH3); 3.82 m (2H,
CHZOH); 3.54 s (3H,
OCH3); 3.18 m (2H,
CH2); 2.14 s (3H, CH3).
52 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.07 s(1 H, OH
(1 H-indol-3-yI)ethyl]-2',3',4,4'- NH); 7.56 d (J = 8.0 Hz, o o H N
etramethoxy[1,1'-biphenyl]-2- 1 H, aryl); 7.33 d (J = 8.0 o~~ ~ H
carboxamide; Hz, 1 H, aryl); 7.22 d (J = o.
2.8 Hz, 1 H, aryl); 7.17 dd
D-Tryptophanol (J = 7,8 Hz / 7.0 Hz, 1 H,
and aryl); 7.14 d (J = 8.5 Hz,
1 H, aryl); 7.08 dd (J =
2;3',4,4'-Tetramethoxy(1,1'- 8.0 Hz / 7.0 Hz, 1 H, aryl);
biphenylJ-4-carboxylic acid 6.98 dd (J = 8.5 Hz / 2.8
Hz, 1 H, aryl); 6.98 d (J =
2.3 Hz, 1 H, aryl); 6.83 d
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
117
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents yous to Ippml
(J = 8,7 Hz, 1 H, aryl);
6.63 d(J = 8.7 Hz, 1 H,
aryl); 6.05 d (J = 7.9 Hz,
1 H, NH); 4.25 m(1 H,
CH); 3.90 s (3H, OCH3);
3.83 s (6H, OCH3); 3.58
s (3H, OCH3); 3.58 m
(1 H, CH2OH); 3.44 m
(1 H, CH2OH); 2.81 m
(2H, CH2).
53 N-[(R)-1-(Hydroxymethyl)-2- 39 (DMSO-d6): 10.79 s(1H, OH
/
o
(1H-indol-3-yl)ethyl]-3',4,4',5'- NH); 7.81 d (J = 8.1 Hz, o o ~-IH N
etramethoxy[1,1'-biphenyl]-2- 1 H, NH); 7.58 d (J = 7.9 o~
carboxamide; Hz, 1 H, aryl); 7.33 d (J = I ~ I o,
8.5 Hz, 1 H, aryl); 7.32 d
D-Tryptophanol (J = 7.6 Hz, 1 H, aryl);
and 7.09 d(J = 2.8 Hz, 1 H,
aryl); 7.05 dd (J = 7.6 Hz
3; 4, 4; 5'-Tetramethoxy(1,1 '- / 7.0 Hz, 1 H, aryl); 7.02
biphenyl]-4-carboxylic acid dd (J = 8.5 Hz / 2.8 Hz,
1 H, aryl); 6.96 dd (J =
7.9 Hz / 7.0 Hz, 1 H, aryl);
6,80 d(J = 2,6 Hz, 1 H,
aryl); 6,62 s (2H, aryl);
4,01 m (1H, CH); 3.76 s
(3H, OCH3); 3.73 s (6H,
OCH3); 3.64 s (3H,
OCH3); 3.35 m (1 H,
CH2OH); 3.25 m (1 H,
CH2OH); 2.83 dd (J =
14,3 Hz / 7.0 Hz, 1 H,
CH2); 2.71 dd (J = 14,3
Hz / 7.0 Hz, 1 H, CH2).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
118
Ex. Product; eMetho natoa 'H-NMR (400 MHz) S Structure
reagents gous to IPpm]
54 '-(Hydroxymethyl)-N-[(R)-1- 39 (CD3OD): 7.66 d (J = 7,8 0"
~ /
(hydroxymethyl)-2-(1 H-indol- Hz, 1 H, aryl); 7.64 dd (J o H 1 N
3-yl)ethyl]-6-methyl[1,1'- = 7.5 Hz / 2.0 Hz, 1 H, H
biphenyl]-3-carboxamide; aryl); 7.58 d (J = 2.0 Hz,
1 H, aryl); 7.43 d (J = 8.5
D -Tryptophanol o"
Hz, 2H, aryl); 7.32 d (J =
and 8.0 Hz, 1 H, aryl); 7.31 d
(J = 7.5 Hz, 1 H, aryl);
4' (Hydroxymethyl)-6-methyl- 7.29 d (J = 8.5 Hz, 2H,
1, 1 '-biphenyl]-3-carboxylic aryl); 7.10 s (1 H, aryl);
acid 7.06 dd (J = 7,8 Hz / 7.0
Hz, 1 H, aryl); 6.96 dd (J
= 8.0 Hz / 7.0 Hz, 1H,
aryl); 4.67 s (2H,
CH2OH); 4.44 m (1 H,
CH); 3.68 m (2H,
CH2OH); 3.13 dd (J =
14.6 Hz / 6.8 Hz, 1H,
CH2); 3.05 dd (J = 14.6
Hz / 8.0 Hz, 1 H, CH2);
2.27 s (3H, CH3).
55 '-(Hydroxymethyl)-N-[(R)-1- 39 (CD3OD): 7.69 d (J = 7,8 OH (hydroxymethyl)-
2-(1 H-indol- Hz, 1 H, aryl); 7.63 s(1 H,
3-yI)ethyl]-2-methyl[1,1'- aryl); 7.61 d (J = 7.8 Hz, 0 H H
biphenyl]-4-carboxamide; 1 H, aryl); 7.43 d (J = 8.0 i
Hz, 2H, aryl); 7.32 d (J =
o-Tryptophanol 8.3 Hz, 1 H, aryl); 7.28 d i
and (J = 8.0 Hz, 2H, aryl);
7.23 d(J = 7.8 Hz, 1 H, OH
4'-(Hydroxymethyl)-2- aryl); 7.12 s(1 H, aryl);
ethyl(1,1 '-biphenyl]-4- 7.08 dd (J = 7.8 Hz / 7.0
carboxylic acid Hz, 1 H, aryl); 7.01 dd (J
= 8.3 Hz / 7.0 Hz, 1H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
119
Ex. Product; aMet nahoa 'H-NMR (400 MHz) S Structure
reagents yous to LPpm]
aryl); 4.66 s (2H,
CH2OH); 4.46 m (1 H,
CH); 3.71 m (2H,
CH2OH); 3.16 dd (J =
14.6 Hz / 6.8 Hz, 1 H,
CHZ); 3.07 dd (J = 14.6
Hz / 7.0 Hz, 1 H, CH2);
2.26 s (3H, CH3).
56 '-(Hydroxymethyl)-N-[(R)-1- 39 (CD3OD): 7.57 d (J = 7.8 0"
(hydroxymethyl)-2-(1 H-indol- Hz, 1 H, aryl); 7.46 dd (J OH
o H N
H
3-yl)ethyl][1,1'-biphenyl]-2- = 7.3 Hz / 7.3 Hz, 1 H,
carboxamide; aryl); 7.38 dd (J = 7.3 Hz
/ 7.3 Hz, 1 H, aryl); 7.37 d
D-Tryptophanol
(J = 7.8 Hz, 1 H, aryl);
and 7.35 d (J = 7.3 Hz, 2H,
aryl); 7.29 d (J = 8.0 Hz,
4' (Hydroxymethyl)(1,1 '-bi- 2H, aryl); 7.24 d (J = 8.0
henyl]-2-carboxylic acid Hz, 2H, aryl); 7.10 dd (J
=7.8Hz/7.0Hz, 1H,
aryl); 6.99 dd (J = 7,8 Hz
/ 7.0 Hz, 1 H, aryl); 6.97 s
(1 H, aryl); 4.56 s (2H,
CH2OH); 4.22 m (1H,
CH); 3,49 dd (J = 11.0
Hz / 5.2 Hz, 1 H,
CH2OH); 3.41 dd (J =
11.0 Hz / 5.7 Hz, 1 H,
CH2OH); 2.93 dd (J =
14.6 Hz / 6.5 Hz, 1H,
CHZ); 2.81 dd (J = 14.6
Hz / 7.3 Hz, 1 H, CH2).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
120
Ex. Product; aMetho naloa 'H-NMR (400 MHz) 8 Structure
reagents 9ous co [ppm]
57 '-(Hydroxymethyl)-N-[(R)-1- 39 (CD30D): 7.95 d (J = 1.8 OH (hydroxymethyl)-
2-(1 H-indol- Hz / 1.5 Hz, 1 H, aryI); o H N
3-yl)ethyl][1,1'-biphenyl]-3- 7.75 d (J = 7.8 Hz, 1 H, H
carboxamide; aryl); 7.71 d (J = 7.8 Hz,
1 H, aryI); 7.68 d (J = 7.8
~-Tryptophanol o"
Hz, 1 H, aryl); 7.61 d(J =
and 8.0 Hz, 2H, aryl); 7.48 dd
(J = 7.8 Hz / 7.8 Hz, 1 H,
4'-(Hydroxymethyl)(1,1 '-bi- aryl); 7.45 d (J = 8.0 Hz,
henyl]-3-carboxylic acid 2H, aryl); 7.32 d (J = 8.0
Hz, 1 H, aryl); 7.13 s(1 H,
aryl); 7.08 dd (J = 7.8 Hz
/ 7.0 Hz, 1 H, aryl); 6.99
dd (J = 8.0 Hz / 7.0 Hz,
1 H, aryl); 4.66 s (2H,
CH2OH); 4.47 m (1 H,
CH); 3.72 m (2H,
CH2OH); 3.17 dd (J =
14,8 Hz / 6.8 Hz, 1 H,
CH2); 3.08 dd (J = 14.8
Hz / 7.9 Hz, 1 H, CHZ).
58 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDC13): 7.97 s(1H, OH
(1 H-indol-3-yl)ethyl]-4- NH); 7.54 d (J = 8.0 Hz, o H H
methoxy-3'-(1- 1 H, aryl); 7.33 d(J = 8.0
methylethyl)[1,1'-biphenyl]-2- Hz, 1 H, aryl); 7.32 dd (J o-
carboxamide = 8.2 Hz / 7.6 Hz, 1 H,
aryl); 7.26 d (J = 8.5 Hz,
o-Tryptophanol 1 H, aryl); 7.25 d (J = 8,2
1 H, aryl); 7.22 s(1 H,
4-Methoxy-3'-(1-methylethyl)- Hz, 1,1 '-biphenyl]-2-carboxylic aryl); 7.18 dd
(J = 8.0 Hz
acid / 7.0 Hz, 1 H, aryl); 7.16 d
(J = 7.6 Hz, 1 H, aryl);
7.08 dd (J = 8.0 Hz / 7.0
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
121
Ex. Product; aMetho naloa 'H-NMR (400 MHz) 8 Structure
reagents 9ous to Ippml
Hz, 1H, aryl); 7.01 dd (J
= 8.5 Hz / 2.8 Hz, 1H,
aryl); 6.98 d (J = 2.3 Hz,
1 H, aryl); 6.82 d (J = 2.8
Hz, 1 H, aryl); 5.54 d (J =
7.5 Hz, 1 H, NH); 4.20 m
(1 H, CH); 3.85 s (3H,
OCH3); 3,38 m (2H,
CH2OH); 2.93 sept (J =
7.0 Hz, 1 H, CH); 2.70 m
(2H, CHZ); 1.26 d (J =
7.0 Hz, 6H, CH3).
59 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.12 s(1H, oH
(1H-indol-3-yl)ethyl]-3'-(1- NH); 7.88 dd (J- 1.8 Hz 0 H H
methylethyl)[1,1'-biphenyl]-3- / 1,5 Hz, 1 H, aryl); 7.73 d
carboxamide; (J = 8.0 Hz, 1 H, aryl); ~ I \
7.69 d (J = 7.5 Hz, 1 H,
D-Tryptophanol aryl); 7.57 d (J = 7.3 Hz,
and 1 H, aryl); 7.42 dd (J =
7.5 Hz / 7.3 Hz, 1 H, aryl);
3'-(1-Methylethyl)(1,1'- 7.41 s(1H, aryl); 7.38 d
biphenylJ-3-carboxylic acid (J = 7.3 Hz, 1 H, aryl);
7.37 dd (J = 7.3 Hz / 7.3
Hz, 1 H, aryl); 7.33 d (J =
7.3 Hz, 1 H, aryl); 7.25 d
(J = 8.0 Hz, 1 H, aryl);
7.22 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 7.14 dd (J
= 8.0 Hz / 7.0 Hz, 1H,
aryl); 7.12 d (J = 1.8 Hz,
1 H, aryl); 6.54 d (J = 7.0
Hz, 1 H, NH); 4.51 m(1 H,
CH); 3.82 m (2H,
CH2OH); 2.98 sept (J =
6,8 Hz, 1 H, CH); 3.18 m
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
122
Ex. Product; aMet nahoa 'H-NMR (400 MHz) S Structure
reagents yous to [ppm]
(2H, CH2); 1.30 d (J =
6.8 Hz, 6H, CH3).
60 N-[(R)-1-(Hydroxymethyl)-2- 39 0"
(CDC13): 8.12 s (1 H,
(1 H-indol-3-yl)ethyl]-6-methyl- NH); 7.70 d (J = 8.0 Hz, 0 H I H
3'-(1-methylethyl)[1,1'- 1 H, aryl); 7.55 dd (J = I
biphenyl]-3-carboxamide; 7.7 Hz / 2.0 Hz, 1 H, aryl); \ I ~
7.50 d(J = 2.0 Hz, 1 H,
D-Ttyptophanol aryl); 7.34 dd (J = 8.0 Hz
and / 7,8 Hz, 1 H, aryl); 7.34 d
(J = 8.0 Hz, 1 H, aryl);
6-Methyl-3' (1- 7.27 d(J = 8.0 Hz, 1 H,
ethylethyl)(1,1 '-biphenyl]-3- aryl); 7.23 d (J = 7.8 Hz,
carboxylic acid 1 H, aryl); 7.17 dd (J =
8.0 Hz / 7.0 Hz, 1 H, aryl);
7.12 s(1 H, aryl); 7.09 dd
(J = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 7.09 d (J = 1.8 Hz,
1 H, aryl); 7.06 d (J = 7.7
Hz, 1 H, aryl); 6,48 d (J =
7.0 Hz, 1 H, NH); 4,47 m
(1 H, CH); 3.82 dd (J =
11.0 Hz / 3.5 Hz, 1H,
CH2OH); 3.76 dd (J =
11.0 Hz / 5.3 Hz, 1H,
CH2OH); 3.15 m (2H,
CH2); 2.95 sept (J = 7.0
Hz, 1 H, CH); 2.27 s (3H,
CH3); 1.29 d (J = 7.0 Hz,
6H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
123
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gous to [ppm]
61 N-[(R)-1-(Hydroxymethyl)-2- 39 oH
(CDC13): 8.14 s(1 H, I\ ~
(1 H-indol-3-yl)ethyl]-3'-(1 - NH); 7.74 d (J = 8.0 Hz, o H
N
methylethyl)[1,1'-biphenyl]-4- 1 H, aryI); 7.71 d (J = 8.5 H
carboxamide; Hz, 2H, aryl); 7.59 d (J =
8.5 Hz, 2H, aryl); 7.43 s
D-Tryptophanol (1 H, aryl); 7.40 d (J = 8.0
and Hz, 1 H, aryl); 7.39 d (J =
8.0 Hz, 1 H, aryl); 7.37 dd
3' (1-Methylethyl)(1,1 '- (J = 8.0 Hz / 8.0 Hz, 1 H,
iphenyl]-4-carboxylic acid aryl); 7.26 m(1 H, aryl);
7.23 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 7.16 dd (J
= 8.0 Hz / 7.0 Hz, 1H,
aryl); 7.13 d (J = 2.5 Hz,
1 H, aryl); 6.53 d (J = 7.0
Hz, 1 H, NH); 4.51 m(1 H,
CH); 3.83 m (2H,
CH2OH); 3.19 m (2H,
CHZ); 2.98 sept (J = 7.0
Hz, 1 H, CH); 1.30 d(J =
7.0 Hz, 6H, CH3).
62 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.16 s(1 H, OH (1 H-indol-3-
yl)ethyl]-2-methyl- NH); 7.74 d (J = 8.0 Hz,
O H
3'-(1-methylethyl)[1,1'- 1H, aryl); 7.47 d(J= 8.0 H
biphenyl]-4-carboxamide; Hz, 1 H, aryl); 7.09 d (J =
8.0 Hz, 1 H, aryl); 7.13 s
D-Tryptophanol (1 H, aryl); 7.39 d (J = 8.0
and Hz, 1 H, aryl); 7.23 m
(2H, aryl); 7.34 dd (J =
2-Methyl-3' (1- 8.0 Hz / 8.0 Hz, 1H, aryl);
methylethyl)[1,1 '-biphenyl]-4- 7.52 s(1 H, aryl); 7.23 dd
carboxylic acid (J = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 7.17 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.13 d
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
124
Ex. Product; aMetho neloa 'H-NMR (400 MHz) 8 Structure
reagents 9ous to [pp-n]
(J = 1,5 Hz, 1 H, aryl);
6.51 d(J = 6.8 Hz, 1 H,
NH); 4.50 m(1 H, CH);
3.83 m (2H, CH2OH);
3.18 m (2H, CH2); 2.94
sept (J = 7.0 Hz, 1 H,
CH); 2,24 s (3H, CH3);
1.28 d (J = 7.0 Hz, 6H,
CH3).
63 i'-(Hydroxymethyl)-N-[(R)-1- 39 (CD3OD): 7.58 d (J = 8.0 0"
/
(hydroxymethyl)-2-(1 H-indol- Hz, 1 H, aryl); 7.34 d (J = H o H I N
3-yI)ethyl]-4-methoxy[1,1'-bi- 8.0 Hz, 1 H, aryl); 7.27 d ~ I H
phenyl]-2-carboxamide; (J = 8.3 Hz, 1 H, aryl); o.~
7.25 d (J = 8.3 Hz, 2H,
D-Tryptophanol
aryl); 7.21 d (J = 8.3 Hz,
and 2H, aryl); 7.09 dd (J =
7.8 Hz / 7.0 Hz, 1 H, aryl);
4'-(Hydroxymethyl)-4- 7.02 d (J = 8.3 Hz, 1 H,
ethoxy[l,1 '-biphenyl]-2- aryl); 7.00 dd (J = 7.8 Hz
carboxylic acid / 7.0 Hz, 1 H, aryl); 6.98 s
(1 H, aryl); 6.89 d (J = 2.8
Hz, 1 H, aryl); 4.55 s (2H,
CH2OH); 4.22 m (1 H,
CH); 3.79 s (3H, OCH3);
3.50 dd (J = 10.8 Hz /
5,1 Hz, 1 H, CHZOH);
3.43 dd (J = 10.8 Hz /
5,6 Hz, 1H, CH2OH);
2.94 dd (J = 14.7 Hz /
6,3 Hz, 1 H, CH2); 2.82
dd (J = 14.7 Hz / 7.6 Hz,
1H, CHZ).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
125
Ex. Product; aMetho na oa 'H-NMR (400 MHz) S Structure
reagents yous to [ppm]
64 3',4',5'-Trifluoro-N-[(R)-1- 39 (CD3OD): 7.59 d (J = 8.0 OH
-
/
(hydroxymethyl)-2-(1 H-indol- Hz, 1 H, aryl); 7.49 dd (J F 0 HI N
3-yI)ethyl][1,1'-biphenyl]-2- = 7.3 Hz / 7.3 Hz, 1 H, F I H
carboxamide; aryl); 7.39 dd (J = 7.3 Hz
/ 7.3 Hz, 1 H, aryl); 7.34 d
D-Tryptophanol
(J = 8.0 Hz, 1 H, aryl);
and 7.34 d(J = 7.3 Hz, 1 H,
aryl); 7.32 d (J = 7.3 Hz,
3; 4; 5'-Trifluoro(1,1 '-biphenyl]- 1H, aryl); 7.11 m (2H,
2-carboxylic acid aryl); 7.11 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.06 d
(J = 1.8 Hz, 1 H, aryl);
6.99 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 4.27 m
(1H, CH); 3.53 m (2H,
CHzOH); 3.00 dd (J =
15.1 Hz / 6.8 Hz, 1 H,
CHZ); 2.87 dd (J = 15.1
Hz / 6.8 Hz, 1 H, CHz).
65 3',4',5'-Trifluoro-N-[(R)-1- 39 (CD30D): 7.88 s(1H, OH
-
(hydroxymethyl)-2-(1 H-indol- aryl); 7.78 d (J = 7.7 Hz, 0 H N
3-yI)ethyl][1,1'-biphenyl]-3- 1 H, aryl); 7.75 d (J = 7.7 H
carboxamide; Hz, 1 H, aryl); 7.66 d (J = F I
8.0 Hz, 1 H, aryl); 7.51 dd F
D-Tryptophanol F
(J = 7.7 Hz / 7.7 Hz, 1 H,
and aryl); 7.42 m (2H, aryl);
7.32 d(J = 8. 0 Hz, 1 H,
3;4;5'-Trifluoro[l,1'-biphenyl]- aryl); 7.12 d(J= 1.8 Hz,
3-carboxylic acid 1 H, aryl); 7.06 dd (J =
8.0 Hz / 7.0 Hz, 1 H, aryl);
6.97 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 4,48 m
(1 H, CH); 3.72 m (2H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
126
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents yous to LPpm]
CH2OH); 3.16 dd (J =
14.5 Hz / 6.4 Hz, 1 H,
CHz); 3.07 dd (J = 14.5
Hz / 7.2 Hz, 1 H, CH2).
66 3',4',5'-Trifluoro-N-[(R)-1- 39 (CD,OD): 7.68 dd (J = o"
I
(hydroxymethyl)-2-(1 H-indol- 7.9 Hz / 1.9 Hz, 1 H, aryI); o N
3-yI)ethyl]-6-methyl[1,1'- 7.64 d (J = 8.0 Hz, 1 H, H
biphenyl]-3-carboxamide; aryl); 7.50 d (J = 1.9 Hz, F 1 H, aryI); 7.35 d (J =
7.9 F
D-Tryptophanol Hz, 1 H, aryl); 7.30 d (J = F
8.0 Hz, 1 H, aryl); 7.10 m
and (2H, aryl); 7.10 d (J = 1.8
Hz, 1 H, aryl); 7.04 dd (J
3;4;5'-Trifluoro-6-methyl(1,1 ' = 8.0 Hz / 7.0 Hz, 1 H,
5iphenyl]-3-carboxylic acid aryl); 6.94 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 4,44
m(1 H, CH); 3.69 m(2H,
CH2OH); 3.13 dd (J =
14.5 Hz / 6.7 Hz, 1 H,
CH2); 3.04 dd (J = 14.5
Hz / 7.2 Hz, 1 H, CH2);
2.29 s (3H, CH3).
67 3',4',5'-Trifluoro-N-[(R)-1- 39 (CD3OD): 7,84 d(J - 8,7 oH
(hydroxymethyl)-2-(1 H-indol- Hz, 2H, aryl); 7.67 d (J =
O NH I N
3-yI)ethyl][1,1'-biphenyl]-4- 8,7 Hz, 2H, aryl); 7.67 d H
carboxamide; (J = 8.0 Hz, 1 H, aryl);
7.46 m(2H, aryl); 7.32 d
D-Tryptophanol (J = 8.0 Hz, 1 H, aryl);
7.11 d (J = 1.8 Hz, 1 H,
and
aryl); 7.08 dd (J = 8.0 Hz F
F F
/ 7.0 Hz, 1 H, aryl); 7.00
3; 4; 5'-Trifluoro[l,1 '-biphenyl]- dd (J = 8.0 Hz / 7.0 Hz,
4-carboxylic acid 1 H, aryl); 4.47 m(1 H,
CH); 3.71 m (2H,
CH2OH); 3.16 dd (J =
15,1 Hz / 6.8 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
127
Ex. Product; aMetho nalo~ 'H-NMR (400 MHz) S Structure
reagents 9oõS to [ppm]
CH2); 3.06 dd (J = 15.1
Hz / 7.3 Hz, 1 H, CH2).
68 3',4',5'-Trifluoro-N-[(R)-1- 39 (CD3OD): 7.68 d (J - 7.7 OH (hYdroxYmethYI)-
2-(1 H-indol- Hz, 1 H, arYI); 7.64 s (1 H,
3-yI)ethyl]-2-methyl[1,1'- aryl); 7.63 d (J = 8.0 Hz, H
biphenyl]-4-carboxamide; 1 H, aryl); 7.32 d (J = 8.0
Hz, 1 H, aryl); 7.25 d(J =
D-Tryptophanol 7.7 Hz, 1 H, aryl); 7.11 m ~
(2H, aryl); 7.08 dd (J = F~ F
and 8.0 Hz / 7.0 Hz, 1 H, aryl); F
7.08 d(J = 1.8 Hz, 1 H,
3; 4; 5' Trifluoro-2-methyl(1,1 '- aryl); 7.00 dd (J = 8.0 Hz
biphenyl]-4-carboxylic acid / 7.0 Hz, 1 H, aryl); 4.46
m(1 H, CH); 3.71 m(2H,
CH2OH); 3,16 dd (J =
14.8 Hz / 7.2 Hz, 1 H,
CHZ); 3.07 dd (J = 14.8
Hz / 7.3 Hz, 1 H, CH2);
2.28 s (3H, CH3).
69 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD30D): 7.57 d (J = 8.0 OH (1 H-indol-3-
yl)ethyl]-2',5'- Hz, 1 H, aryl); 7.51 d (J = o o H'
N
dimethoxy[1,1'-biphenyl]-2- 7.5 Hz, 1 H, aryl); 7.47 dd ~ I H
L~
carboxamide; (J = 7.5 Hz / 7.5 Hz, 1 H, o~
aryl); 7.38 dd (J = 7.5 Hz
o-Tryptophanol / 7.5 Hz, 1 H, aryl); 7.31 d
und (J = 8.0 Hz, 1 H, aryl);
7.26 d(J = 7.5 Hz, 1 H,
2;5'-Dimethoxy[1,1'-biphenyl]- aryl); 7.07 dd (J = 8.0 Hz
2-carboxylic acid / 7.0 Hz, 1 H, aryl); 6.98 d
(J = 1.8 Hz, 1 H, aryl);
6.97 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 6.86 m
(2H, aryl); 6.77 s (1 H,
aryl); 4.16 m(1 H, CH);
3.73 s (3H, OCH3); 3.60
s (3H, OCH3); 3.39 dd (J
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
128
Ex. Product; aMet nahoa 'H-NMR (400 MHz) S Structure
reagents gous to [ppm]
= 10,9 Hz / 4.5 Hz, 1H,
CH2OH); 3.30 dd (J =
10.9 Hz / 5.3 Hz, 1 H,
CH2OH); 2.80 dd (J =
14.7 Hz / 7.6 Hz, 1 H,
CHz); 2.71 dd (J = 14.7
Hz / 5.8 Hz, 1 H, CH2).
70 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.57 d (J = 8.0 0"
. /
(1 H-indol-3-yl)ethyl]-2',4.5'-tri- Hz, 1 H, aryl); 7.31 d (J = o O H ~ N
methoxy[1,1'-biphenyl]-2- 8.0 Hz, 1 H, aryl); 7.17 d H
carboxamide; (J = 8.8 Hz, 1 H, aryl); 01~ o.
7.07 dd (J = 8.0 Hz / 7.0
D-Tryptophanol Hz, 1 H, aryl); 7.03 s(1 H,
and aryl); 7.02 dd (J = 8,8 Hz
/ 2.8 Hz, 1 H, aryl); 6.98 d
2;4.5' Trimethoxy(1,1'- (J = 1.8 Hz, 1 H, aryl);
iphenyl]-2-carboxylic acid 6.97 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 6.85 d(J =
8.6 Hz, 1 H, aryl); 6.83 dd
(J = 8.6 Hz / 2.5 Hz, 1 H,
aryl); 6.75 d (J = 2.5 Hz,
1 H, aryl); 4.15 m(1 H,
CH); 3.81 s (3H, OCH3);
3.73 s (3H, OCH3); 3.60
s (3H, OCH3); 3.40 dd (J
= 10.9 Hz / 4.5 Hz, 1 H,
CH2OH); 3.31 m (1 H,
CH2OH); 2.80 dd (J =
14.4 Hz / 7.6 Hz, 1 H,
CH2); 2.72 dd (J = 14.4
Hz / 6.3 Hz, 1 H, CH2).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
129
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents yous to IPpm]
71 V-[(R)-1-(Hydroxymethyl)-2- 39 (DMSO-ds): 10.74 s(1H, o"
(1 H-indol-3-yl)ethyl]-2',5'- NH); 8.10 d (J = 8.1 Hz, o " N
dimethoxy-6-methyl[1,1'- 1 H, NH); 7.73 dd (J = 7.8
o H
biphenyl]-3-carboxamide; Hz / 1.8 Hz, 1 H, aryl);
7.63 d(J = 8. 0 Hz, 1 H,
D-Tryptophanol aryl); 7.62 s (1 H, aryl); .
7.30 d(J = 8.0 Hz, 1 H,
and
aryl); 7.30 d (J = 7,8 Hz,
2;4.5' Trimethoxy(1,1 '- 1 H, aryl); 7.10 d (J = 1.8
iphenyl]-2-carboxylic acid Hz, 1 H, aryl); 7.03 dd (J
= 8.0 Hz / 7.0 Hz, 1H,
aryl); 7.03 d (J = 8.8 Hz,
1 H, aryl); 6.95 dd (J =
8.8 Hz / 3.0 Hz, 1 H, aryl);
6.94 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 6.69 d (J =
3.0 Hz, 1 H, aryl); 4.23 m
(1 H, CH); 3.74 s (3H,
OCH3); 3.64 s (3H,
OCH3); 3.50 m (1 H,
CH2OH); 3.44 m (1 H,
CHZOH); 2.99 dd (J =
14.7 Hz / 6,1 Hz, 1 H,
CHZ); 2.89 dd (J = 14.7
Hz / 7.8 Hz, 1 H, CHZ).
72 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.78 d(J - 8.6 0"
(1 H-i ndol-3-yl)ethyl]-2', 5'- Hz, 2H, aryl); 7.69 d (J = o H N
dimethoxy[1,1'-biphenyl]-4- 8.0 Hz, 1 H, aryl); 7.55 d H
I
carboxamide; (J = 8.6 Hz, 2H, aryl);
7.32 d(J = 8.0 Hz, 1 H,
D-Tryptophanol
aryl); 7.12 d(J = 1.8 Hz, o.
and 1 H, aryl); 7.08 dd (J =
8.0 Hz / 7.0 Hz, 1 H, aryl);
2;5'-Dimethoxy[1,1 '-biphenyl]- 7.01 d (J = 8.6 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
130
Ex. Product; Mnalotl 'H-NMR (400 MHz) S Structure
reagents 9ous to Ippm]
4-carboxylic acid aryl); 7.00 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 6.91
dd (J = 8.6 Hz / 3.0 Hz,
1 H, aryl); 6.87 d (J = 3.0
Hz, 1 H, aryl); 4.46 m
(1 H, CH); 3.78 s (3H,
OCH3); 3.72 s (3H,
OCH3); 3.71 m (2H,
CH2OH); 3.16 dd (J =
14.4 Hz / 6,8 Hz, 1 H,
CH2); 3.07 dd (J = 14.4
Hz / 7.1 Hz, 1 H, CH2).
73 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.69 d (J = 8.0 oH
(1 H-indol-3-yl)ethyl]-2',5'- Hz, 1 H, aryl); 7.59 s(1 H,
O H I
H
dimethoxy-2-methyl[1,1'- aryl); 7.58 d (J = 7,8 Hz,
biphenyl]-4-carboxamide; 1 H, aryl); 7.33 d (J = 8.0
Hz, 1 H, aryl); 7.15 d(J =
~-Tryptophanol "
7.8 Hz, 1 H, aryl); 7.13 d ~
0
and (J = 1.8 Hz, 1 H, aryl);
7.09 dd (J = 8.0 Hz / 7.0
2; 5'-Dimethoxy-2-methyl(1,1 '- Hz, 1H, aryl); 7.01 dd (J
iphenylj-4-carboxylic acid = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 6.98 d (J = 9.1 Hz,
1 H, aryl); 6.92 dd (J =
9.1 Hz / 3.0 Hz, 1 H, aryl);
6.65 d (J = 3.0 Hz, 1 H,
aryl); 4,45 m(1 H, CH);
3.76 s (3H, OCH3); 3.70
m (2H, CH2OH); 3.66 s
(3H, OCH3); 3.16 dd (J =
14.4 Hz / 6.3 Hz, 1 H,
CHZ); 3.07 dd (J = 14.4
Hz / 6.6 Hz, 1 H, CH2);
2.12 s (3H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
131
Method '
Ex. Product; anaio- H-NMR (400 MHz) S Structure
reagents gous to [ppm]
74 V-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.58 d (J = 8.0 OH
(1 H-indol-3-yl)ethyl]-3',4'- Hz, 1 H, aryl); 7.49 - 7.31 0 0 H
H
imethoxy[1,1'-biphenyl]-2- m (4H, aryl); 7.35 d (J = 'o I I
carboxamide; 8.0 Hz, 1 H, aryl); 7.09 dd
(J = 8.0 Hz / 7.0 Hz, 1H,
D-Tryptophanol
aryl); 7.00 d (J = 1.8 Hz,
and 1 H, aryl); 6.99 dd (J =
8.0 Hz / 7.0 Hz, 1 H, aryl);
3; 4 '-Dimethoxy[l, 1 ' biphenyl]- 6.96 d (J = 2.1 Hz, 1 H,
2-carboxylic acid aryl); 6.77 dd (J = 8.3 Hz
/ 2.1 Hz, 1 H, aryl); 6.71 d
(J = 8.3 Hz, 1 H, aryl);
4.21 m(1 H, CH); 3.78 s
(3H, OCH3); 3.77 s (3H,
OCH3); 3.47 dd (J = 11,1
Hz / 5.3 Hz, 1 H,
CH2OH); 3.40 dd (J =
11.1 Hz / 5.7 Hz, 1 H,
CH2OH); 2.91 dd (J =
14.5 Hz / 7.0 Hz, 1 H,
CHZ); 2,78 dd (J = 14.5
Hz / 7.0 Hz, 1 H, CH2).
75 N-[(R)-1-(Hydroxymethyl)-2- 39 0"
(CD30D): 7.58 d (J = 8.0
(1 H-indol-3-yl)ethyl]-3',4,4'-tri- Hz, 1 H, aryl); 7.34 d (J = o 1 o H H
methoxy[1,1'-biphenyl]-2- 8.0 Hz, 1 H, aryl); 7.27 d '-o
carboxamide; (J = 8.7 Hz, 1 H, aryl);
7.09dd(J=8.OHz/7.0
D-Tryptophanol
Hz, 1 H, aryl); 7.01 d (J =
and 2.6 Hz, 1 H, aryl); 7.00 dd
(J = 8.7 Hz / 2.6 Hz, 1 H,
3;4,4' Trimethoxy(1,1 '- aryl); 6.99 dd (J = 8.0 Hz
biphenyl]-2-carboxylic acid / 7.0 Hz, 1 H, aryl); 6.92 d
(J = 1.8 Hz, 1 H, aryl);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
132
Ex. Product; aMet nahoa 'H-NMR (400 MHz) S Structure
reagents 9ous to [ppm]
6.90 d(J = 2.6 Hz, 1 H,
aryl); 6.74 dd (J = 8.3 Hz
/ 1.8 Hz, 1 H, aryl); 6.70 d
(J = 8.3 Hz, 1 H, aryl);
4.21 m(1 H, CH); 3.79 s
(3H, OCH3); 3.78 s (3H,
OCH3); 3.76 s (3H,
OCH3); 3.47 dd (J = 11.1
Hz / 5.3 Hz, 1 H,
CH2OH); 3.41 dd (J =
11.1 Hz / 5,7 Hz, 1H,
CH2OH); 2.91 dd (J =
14.5 Hz / 6.2 Hz, 1H,
CH2); 2.78 dd (J = 14.5
Hz / 7.0 Hz, 1 H, CH2).
76 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.65 d (J - 8.0 oH
(1 H-indol-3-yl)ethyl]-3',4'- Hz, 1 H, aryl); 7.62 dd (J 0 H H
dimethoxy-6-methyl[1,1'- = 7.9 Hz / 1.9 Hz, 1 H, i ~
biphenyl]-3-carboxamide; aryl); 7.59 d (J = 1.9 Hz, 0'
I
'0
1 H, aryl); 7.31 d (J = 7.9
D-Tryptophanol
Hz, 1 H, aryl); 7.30 d (J =
and 8.0 Hz, 1 H, aryl); 7.10 d
(J = 1.8 Hz, 1 H, aryl);
3; 4'-Dimethoxy-6-methyl(1,1 '- 7.05 dd (J = 8.0 Hz / 7.0
iphenylJ-2-carboxylic acid Hz, 1 H, aryl); 7.02 d (J =
8.1 Hz, 1 H, aryl); 6.95 dd
(J = 8.0 Hz / 7.0 Hz, 1H,
aryl); 6.89 d (J = 2.1 Hz,
1 H, aryl); 6.85 dd (J =
8.1 Hz / 2.1 Hz, 1 H, aryl);
4.44 m(1 H, CH); 3.88 s
(3H, OCH3); 3.84 s (3H,
OCH3); 3.68 m (2H,
CH2OH); 3.13 dd (J =
14.3 Hz / 6.2 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
133
Ex. Product; aMetho neloa 'H-NMR (400 MHz) S Structure
reagents gous to [ppm]
CH2); 3.05 dd (J = 14.3
Hz / 7.2 Hz, 1 H, CH2).;
2.29 s (3H, CH3)
77 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.81 d (J = 8.7 OH (1 H-indol-3-
yI)ethyl]-3',4'- Hz, 2H, aryl); 7.68 d (J =
O H
dimethoxy[1,1'-biphenyl]-4- 8.0 Hz, 1 H, aryl); 7.65 d H
carboxamide;
(J = 8.7 Hz, 2H, aryl);
7.32 d(J = 8. 0 Hz, 1 H,
o-Tryptophanol aryl); 7.23 d (J = 1.7 Hz,
and 1 H, aryl); 7.22 dd (J = eo
9.0 Hz / 1.7 Hz, 1 H, aryl);
3', 4'-Dimethoxy[1,1 '-biphenyl]- 7.12 d (J = 1.8 Hz, 1 H,
4-carboxylic acid aryl); 7.08 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.04 d
(J = 9,0 Hz, 1 H, aryl);
7.00 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 4.47 m
(1 H, CH); 3.90 s (3H,
OCH3); 3.87 s (3H,
OCH3); 3.71 m (2H,
CH2OH); 3.16 dd (J =
14.7 Hz / 6.6 Hz, 1 H,
CH2); 3.08 dd (J = 14,7
Hz / 7.3 Hz, 1 H, CHZ).
78 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.69 d(J= 8.0 oH -
(1 H-indol-3-yI)ethyl]-3',4'- Hz, 1 H, aryl); 7.62 s(1 H, o H
dimethoxy-2-methyl[1,1'- aryl); 7.60 d (J = 7.9 Hz, H
biphenyl]-4-carboxamide; 1 H, aryl); 7.33 d (J = 8.0
Hz, 1 H, aryl); 7.24 d (J =
D-Tryptophanol
7.9 Hz, 1 H, aryl); 7.13 d o
and (J = 1.8 Hz, 1 H, aryl); ~O
7.08dd(J=8.OHz/7.0
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
134
Ex. Product; aMet nahoa 'H-NMR (400 MHz) 8 Structure
reagents gousto Ippm]
3; 4'-Dimethoxy-2-methyl(1,1'- Hz, 1 H, aryl); 7.02 d (J =
iphenylJ-4-carboxylic acid 8= 1 Hz, 1 H, aryl); 7.01 dd
(J = 8.0 Hz / 7.0 Hz, 1H,
aryl); 6,86 dd (J = 8.1 Hz
/ 2.1 Hz, 1 H, aryl); 6,80 d
(J = 2.1 Hz, 1 H, aryl);
4.46 m(1 H, CH); 3.87 s
(3H, OCH3); 3.84 s (3H,
OCH3); 3.71 m (2H,
CH2OH); 3,16 dd (J =
14.7 Hz / 7.0 Hz, 1 H,
CH2); 3.07 dd (J = 14,7
Hz / 6.8 Hz, 1 H, CHZ);
2.29 s (3H, CH3).
79 3'-Fluoro-N-[(R)-l-(hydroxy- 39 (CD3OD): 7.60 d (J = 8.0 OH methyl)-2-(1 H-
indol-3-yl)ethyl]- Hz, 1 H, aryl); 7.45 m o~ 0 H N
'-methoxy[1,1'-biphenyl]-2- (1 H, aryl); 7.35 m (3H, F H
carboxamide; aryl); 7.33 d (J = 8.0 Hz,
1 H, aryl); 7.12 dd (J =
o-Tryptophanol 12.4 Hz / 2.2 Hz, 1 H,
and aryl); 7.10 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.06 d
3'-Fluoro-4'-methoxy[1,1'- (J = 1.8 Hz, 1 H, aryl);
biphenyl]-2-carboxylic acid 7.00 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 6.89 ddd (J
= 8.5 Hz / 2.2 Hz / 1.1
Hz, 1 H, aryl); 6.73 dd (J
= 8.7 Hz / 8.5 Hz, 1H,
aryl); 4.25 m(1 H, CH);
3.79 s (3H, OCH3); 3.54
dd (J = 11.0 Hz / 5.3 Hz,
1H, CH2OH); 3.47 dd (J
= 11.0 Hz / 5.8 Hz, 1 H,
CH2OH); 2.98 dd (J =
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
135
Ex. Product; aMet nahoa 'H-NMR (400 MHz) S Structure
reagents gous to [ppm]
14.7 Hz / 6.4 Hz, 1 H,
CH2); 2.83 dd (J = 14.7
Hz / 7.5 Hz, 1 H, CH2).
80 3'-Fluoro-N-[(R)-1-(hydroxy- 39 (CD3OD): 7.60 d(J - 8.0 oH --
/
methyl)-2-(1 H-indol-3-yI)ethyl]- Hz, 1 H, aryl); 7.36 d (J = o o H~ N
I,4'-dimethox 1,1'-bi hen I - ~ I H
Y[ P Y] 8.0 Hz, 1 H, aryl); 7.24 d F
2-carboxamide; (J = 8.5 Hz, 1 H, aryl);
7.09 dd (J = 8.0 Hz / 7.0
D-Tryptophanol Hz, 1 H, aryl); 7.08 d (J =
and 11.3 Hz, 1 H, aryI); 7.06 d
(J = 1.8 Hz, 1 H, aryl);
3 '-Fluoro-4,4 '-dimethoxy(1,1 '- 7.00 dd (J = 8.0 Hz / 7.0
iphenyl]-2-carboxylic acid Hz, 1 H, aryl); 7.00 dd (J
= 8.5 Hz / 2,6 Hz, 1 H,
aryl); 6.86 d (J = 2.6 Hz,
1 H, aryl); 6.85 ddd (J =
8.7 Hz / 2.1 Hz / 1,1 Hz,
1 H, aryl); 6.72 dd (J =
8.7 Hz / 8.7 Hz, 1 H, aryl);
4.25 m(1 H, CH); 3.79 s
(3H, OCH3); 3.78 s (3H,
OCH3); 3.54 dd (J = 11.0
Hz / 5.3 Hz, 1 H,
CHZOH); 3.48 dd (J =
11.0 Hz / 5.8 Hz, 1 H,
CHZOH); 2.98 dd (J =
14.7 Hz / 6.3 Hz, 1 H,
CH2); 2.84 dd (J = 14.7
Hz / 7.5 Hz, 1 H, CHZ).
81 3'-Fluoro-N-[(R)-1-(hydroxy- 39 (CD3OD): 7.90 dd (J = OH
methyl)-2-(1 H-indol-3-yI)ethyl]- 1,7 Hz / 1.5 Hz, 1 H, aryl); 0 H N
H
'-methoxy[1,1'-biphenyl]-3- 7.71 d (J = 7.9 Hz, 1 H,
carboxamide; aryl); 7.69 d (J = 7.7 Hz, F~ I \
"0
1 H, aryl); 7.68 d (J = 8.0
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
136
Method '
Ex. Product; anaio- H-NMR (400 MHz) S Structure
reagents gous to [ppm]
D-Tryptophanol Hz, 1 H, aryl); 7.46 dd (J
= 7.9 Hz / 7.7 Hz, 1H,
and aryl); 7.42 d (J = 9.0 Hz,
1 H, aryl); 7.39 d(J =
3' Fluoro-4' methoxy(1,1 ' bi- 10.2 Hz, 1 H, aryl); 7.32 d
henyl]-3-carboxylic acid
(J = 8.0 Hz, 1 H, aryl);
7.16 dd (J = 9.0 Hz / 8.5
Hz, 1 H, aryl); 7.12 d (J =
1.8 Hz, 1 H, aryl); 7.08 dd
(J = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 6.98 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 4.47
m(1 H, CH); 3.92 s (3H,
OCH3); 3.72 m (2H,
CH2OH); 3.16 dd (J =
15,1 Hz / 6.8 Hz, 1 H,
CHZ); 3.07 dd (J = 15.1
Hz / 7.5 Hz, 1 H, CH2).
82 3'-Fluoro-N-[(R)-1-(hydroxy- 39 (CD3OD): 7.65 d (J = 8.0 OH
methyl)-2-(1 H-indol-3-yI)ethyl]- Hz, 1 H, aryl); 7.63 dd (J 0 H N
H
1'-methoxy-6-methyl[1,1'- = 7.9 Hz / 2.1 Hz, 1 H, ~ I
biphenyl]-3-carboxamide; aryl); 7.55 d(J = 2.1 Hz,
.1o
1 H, aryl); 7.31 d (J = 8.0
o-Tryptophanol
Hz, 1 H, aryl); 7.31 d (J =
and 7.9 Hz, 1 H, aryl); 7.15 dd
(J = 9.0 Hz / 8.3 Hz, 1 H,
3'-Fluoro-4'-methoxy-6- aryl); 7.10 d (J = 1.8 Hz,
ethyl(1,1'-biphenyl]-3- 1 H, aryl); 7.06 dd (J =
carboxylic acid 8.0 Hz / 7.0 Hz, 1 H, aryl);
7.06 m (2H, aryl); 6.96
dd (J = 8.0 Hz / 7.0 Hz,
1 H, aryl); 4,43 m(1 H,
CH); 3.92 s (3H, OCH3);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
137
Ex. Product; aMetho naloa 'H-NMR (400 MHz) 8 Structure
reagents gous to [ppm]
3.72 m (2H, CH2OH);
3.13dd(J=14.9Hz/
7.3 Hz, 1 H, CH2); 3.05
dd (J = 14.9 Hz / 6.6 Hz,
1H, CH2); 2.28 s (3H,
CH3).
83 3'-Fluoro-N-[(R)-1-(hydroxy- 39 (CD3OD): 7.81 d (J = 8.7 OH methyl)-2-(1 H-
indol-3-yl)ethyl]- Hz, 2H, aryl); 7.68 d(J =
'-methoxy[1,1'-biphenyl]-4- 8.0 Hz, 1 H, aryl); 7.63 d 0 H H
carboxamide; (J = 8.7 Hz, 2H, aryl);
7.42 m (2H, aryl); 7.32 d
D-Tryptophanol (J = 8.0 Hz, 1 H, aryl);
7.16 dd (J = 8,7 Hz / 8.5 F
and
Hz, 1 H, aryl); 7.12 d (J =
3'-Fluoro-4'-methoxy(1,1'- 1.8 Hz, 1 H, aryl); 7.08 dd
biphenyl]-4-carboxylic acid (J = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 7.00 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 4.47
m(1 H, CH); 3.91 s(3H,
OCH3); 3.71 m (2H,
CH2OH); 3.16 dd (J =
15.1 Hz / 6,8 Hz, 1 H,
CH2); 3.07 dd (J = 15.1
Hz / 7.7 Hz, 1 H, CHZ).
84 3'-Fluoro-N-[(R)-1-(hydroxy- 39 (CD30D): 7.68 d (J = 8.0 OH methyl)-2-(1 H-
indol-3-yl)ethyl]- Hz, 1 H, aryl); 7.62 s(1 H,
O H I N
'-methoxy-2-methyl[1,1'- aryl); 7.60 dd (J = 9.2 Hz H
biphenyl]-4-carboxamide; / 1.3 Hz, 1 H, aryl); 7.32 d
(J = 8.0 Hz, 1 H, aryl);
D-Tryptophanol 7.22 d (J = 9,2 Hz, 1 H,
and aryl); 7.15 dd (J = 8,7 Hz F
/ 8.5 Hz, 1 H, aryl); 7.12 d
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
138
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents 9ous to [ppm]
3' Fluoro-4' methoxy-2- (J = 1.8 Hz, 1 H, aryl);
ethyl[1,1 '-biphenyl]-4- 7.08 dd (J = 8.0 Hz / 7.0
carboxylic acid Hz, 1 H, aryl); 7.06 m
(2H, aryl); 7.01 dd (J =
8.0 Hz / 7.0 Hz, 1 H, aryl);
4.46 m(1 H, CH); 3.91 s
(3H, OCH3); 3.71 m (2H,
CH2OH); 3.15 dd (J =
14,7 Hz / 7.0 Hz, 1 H,
CH2); 3.05 dd (J = 14,7
Hz / 7.0 Hz, 1 H, CH2);
2.27 s (3H, CH3).
85 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.57 d (J = 8.0 0" ~
(1 H-indol-3-yl)ethyl]-3',4- Hz, 1 H, aryl); 7.33 d (J J = o H
~ H
dimethoxy[1,1'-biphenyl]-2- 8.0 Hz, 1 H, aryl); 7.29 d o~ I ~
carboxamide; (J = 8.5 Hz, 1 H, aryl);
7.16 dd (J = 7.9 Hz / 7.9
D-Tryptophanol Hz, 1 H, aryl); 7.08 dd (J
=8.OHz/7.OHz, 1H,
and
aryl); 7.02 dd (J = 8.5 Hz
3',4'-Dimethoxy(1,1'-biphenyl]- / 2.8 Hz, 1 H, aryl); 6.99 d
2-carboxylic acid (J = 1.8 Hz, 1 H, aryl);
6.98 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 6.91 m
(1 H, aryl); 6.89 d (J = 2.8
Hz, 1 H, aryl); 6.86 d (J =
7.9 Hz, 1 H, aryl); 6.82 d
(J = 7,9 Hz, 1 H, aryl);
4.20 m(1 H, CH); 3.78 s
(3H, OCH3); 3.75 s (3H,
OCH3); 3.45 dd (J = 11,1
Hz / 5.3 Hz, 1 H,
CH2OH); 3,39 dd (J =
11,1 Hz/5,7Hz, 1H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
139
Ex. Product; aMetho nalotl 'H-NMR (400 MHz) S Structure
reagents yous to Ippm]
CH2OH); 2.88 dd (J =
14,7 Hz / 7.2 Hz, 1 H,
CHZ); 2.76 dd (J = 14.7
Hz / 7.3 Hz, 1 H, CH2).
86 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.53 d (J = 8.0 OH
(1 H-indol-3-yl)ethyl]-3'-(1 - Hz, 1H, aryl); 7.43 dd (J 0 H H
methylethyl)[1,1'-biphenyl]-2- = 7.2 Hz / 7.2 Hz, 1 H,
carboxamide aryl); 7.34 m (2H, aryl);
7.32 d (J = 8.0 Hz, 1 H,
D-Tryptophanol
aryl); 7.30 dd (J = 8,2 Hz
3'-(1-Methylethylff1,1 '- / 7.6 Hz, 1 H, aryl); 7.22 s
(1 H, aryl); 7.16 m (2H,
iphenylJ-2-carboxylic acid
aryl); 7.11 m(1 H, aryl);
7.04 dd (J = 8.0 Hz / 7.0
Hz, 1 H, aryl); 6.93 dd (J
= 8.0 Hz / 7.0 Hz, 1 H,
aryl); 6.92 d (J = 1.8 Hz,
1 H, aryl); 4.13 m(1 H,
CH); 3.37 dd (J = 11.1
Hz / 5.3 Hz, 1 H,
CH2OH); 3.29 m (1 H,
CH2OH); 2.82 m (1 H,
CH); 2.80 dd (J = 15.1
Hz / 7.0 Hz, 1 H, CHZ);
2.70 dd (J = 15.1 Hz /
7.2 Hz, 1 H, CH2); 1.19 d
(J = 7.0 Hz, 6H, CH3).
87 N-[(R)-1-(Hydroxymethyl)-2- 39 (CD3OD): 7.85 dd (J = o"
(1 H-indol-3-yl)ethyl]-2',5'- 1.7 Hz / 1.3 Hz, 1 H, aryl); o H N
dimethoxy[1,1'-biphenyl]-3- 7.68 d (J = 8.0 Hz, 1 H,
~ H
=
carboxamide; aryl); 7.68 d (J = 7.7 Hz, ~ 1 H, aryl); 7.63 d (J = 7.7
o-Tryptophanol ~.
Hz, 1 H, aryl); 7.42 dd (J
= 7.7 Hz / 7.7 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
140
Ex. Product; aMetho nalotl 'H-NMR (400 MHz) S Structure
reagents yo-,S to IPpml
and aryl); 7.31 d (J = 8.0 Hz,
1 H, aryl); 7.12 d(J = 1.8
2;5'-Dimethoxy(1,1'-biphenyl]- Hz, 1 H, aryl); 7.06 dd (J
3-carboxylic acid = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 7.00 d (J = 9.6 Hz,
1 H, aryl); 6.97 dd (J =
8.0 Hz / 7.0 Hz, 1 H, aryl);
6.90 dd (J = 9.6 Hz / 3.0
Hz, 1 H, aryl); 6.80 d (J =
3.0 Hz, 1 H, aryl); 4.46 m
(1 H, CH); 3.78 s (3H,
OCH3); 3.70 s (3H,
OCH3); 3.70 m (2H,
CH2OH); 3,15 dd (J =
14.5 Hz / 6,2 Hz, 1 H,
CH2); 3.07 dd (J = 14.5
Hz / 7.2 Hz, 1 H, CH2).
88 3',4',5'-Trifluoro-N-[(R)-1- 39 (CD3OD): 7.59 d (J = 8.0 0" F
(hydroxymethyl)-2-(1 H-indol- Hz, 1 H, aryl); 7.34 d(J = F o H N
3-yI)ethylJ-4-methoxy[1,1'- 8.0 Hz, 1 H, aryl); 7.27 d F biphenyl]-2-
carboxamide; (J = 8.7 Hz, 1 H, aryl);
7.09 dd (J = 8.0 Hz / 7.0
D-Tryptophanol Hz, 1 H, aryl); 7.07 d (J =
and 1.6 Hz, 1 H, aryl); 7.07 m
(2H, aryl); 7.01 dd (J =
3;4;5'-Trifluoro-4- 8,7 Hz / 2,7 Hz, 1H, aryl);
ethoxy[1,1 '-biphenyl]-2- 7.00 dd (J = 8.0 Hz / 7.0
carboxylic acid Hz, 1 H, aryl); 6.79 d (J =
2.7 Hz, 1 H, aryl); 4.28 m
(1H, CH); 3.75 s (3H,
OCH3); 3.55 m (2H,
CH2OH); 3.01 dd (J =
14.5 Hz / 5,8 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
141
Ex. Product; eMetho nalo~ 'H-NMR (400 MHz) S Structure
reagents yous to [ppm]
CH2); 2.87 dd (J = 14.5
Hz / 7.7 Hz, 1 H, CHZ).
89 3-(Benzofuran-2-yl)-N-[(R)-1- 39 0"
(CD30D): 8.24 s (1 H,
(hydroxymethyl)-2-(1 H-indol- aryl); 8.01 d (J = 7,9 Hz, 0 " H
3-yI)ethyl]benzamide; 1 H, aryl); 7.73 d (J = 7.7
D-Tryptophanol Hz, 1 H, aryl); 7.69 d (J = _ o
8.0 Hz, 1 H, aryl); 7.61 d
and (J = 7.4 Hz, 1 H, aryl);
7.53 d (J = 7.4 Hz, 1 H,
3-(Benzofuran-2-y1)benzoic aryl); 7.51 dd (J = 7.9 Hz
acid / 7.7 Hz, 1 H, aryl); 7.32 d
(J = 8.0 Hz, 1 H, aryl);
7.30 dd (J = 8.3 Hz / 7.4
Hz, 1 H, aryl); 7.23 dd (J
= 8.3 Hz / 7.4 Hz, 1 H,
aryl); 7.20 s(1 H, aryl);
7.14 d (J = 1.8 Hz, 1 H,
aryl); 7.08 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H, aryl); 7.00
dd (J = 8.0 Hz / 7.0 Hz,
1 H, aryl); 4.49 m(1 H,
CH); 3.73 m (2H,
CHZOH); 3.17 dd (J =
14.7 Hz / 7.0 Hz, 1 H,
CH2); 3.09 dd (J = 14.7
Hz / 7.3 Hz, 1 H, CH2).
90 N-[(R)-1-(Hydroxymethyl)-2- 39 (DMSO-d6): 10.77 s(1H, OH (1 H-indol-3-
yl)ethyl]-3-(5- NH); 8.37 d J = 8.3 Hz,
( - H
methoxybenzofuran-2- 1 H, NH); 8.33 s(1 H, a-
I)benzamide; ryl); 8.02 d (J = 7.8 Hz, o o
1 H, aryl); 7.84 d (J = 7.8
D-Tryptophanol Hz, 1 H, aryl); 7.68 d (J =
and 8.0 Hz, 1 H, aryl); 7.57 dd
(J = 7.8 Hz / 7,8 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
142
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents yous to LPPmI
3-(5-Methoxybenzofuran-2-y1)- aryl); 7.56 d (J = 9.1 Hz,
5enzoic acid 1 H, aryl); 7.43 s (1 H, a-
ryl); 7.32 d (J = 8.0 Hz,
1 H, aryl); 7.16 s(1 H, a-
ryl); 7.05 dd (J = 8.0 Hz /
7.0 Hz, 1 H, aryl); 6.98 dd
(J = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 6.93 dd (J = 9.1 Hz
/ 2.5 Hz, 1 H, aryl); 4.28
m(1 H, CH); 3.81 s(3H,
OCH3); 3.57 m (1 H,
CH2OH); 3.53 m (1 H,
CHZOH); 3.05 dd (J =
14.4 Hz / 5.8 Hz, 1H,
CH2); 2.96 dd (J = 14.4
Hz / 8.1 Hz, 1 H, CH2).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
143
Example 91
N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-[(3,4,5-
trimethoxyphenyl)methoxy]phenylpropanamide
OYH- I \ /
~O O N
H
.~O /
\ I O
~ I
91 a) Methyl 2-[(3,4,5-trimethoxyphenyl)methoxy]phenylpropanoate
0.38 mmol (68 mg) of methyl 2-hydroxyphenylpropanoate and 0.384 mmol (125 mg)
of
caesium carbonate were added to a solution of 0.38 mmol (100 mg) of 1-
(bromomethyl)-3,4,5-trimethoxybenzene in 4 ml of acetonitrile. The mixture was
heated
to boiling for four hours. After cooling, the reaction mixture was diluted
with saturated
aqueous sodium hydrogen carbonate solution and extracted with ethyl acetate.
The
combined organic phases were washed with saturated aqueous sodium chloride
solution, dried over sodium sulphate, filtered and concentrated in vacuo.
Flash
chromatography resulted in 122 mg of the target compound.
'H-NMR (400 MHz, CDC13): S[ppm] = 7.18 d (J = 7.3 Hz, 1 H, aryl); 7.19 dd (J =
8.3 Hz /
7.6 Hz, 1 H, aryl); 6.91 dd (J = 8.3 Hz / 7.3 Hz, 1 H, aryl); 6.90 d (J = 7.6
Hz, 1 H, aryl);
6.67 s (2H, aryl); 5.02 s (2H, OCH2); 3.88 s (6H, OCH3); 3.86 s(3H, OCH3);
3.64 s (3H,
OCH3); 3.02 t (J = 7.9 Hz, 2H, CHZ); 2.67 t (J = 7.9 Hz, 2H, CH2).
91 b) 2-[(3,4,5-Trimethoxyphenyl)methoxy]phenylpropanoic acid
In analogy to Example 39b), 112 mg of the title compound were obtained from
0.32 mmol (115 mg) of the compound prepared as in 91a) in 6.4 ml of methanol
with
1.6 ml of a 2 molar aqueous sodium hydroxide solution.
'H-NMR (400 MHz, CD3OD): S[ppm] = 7.16 m(21 H, aryl); 6.86 dd (J = 7.5 Hz /
7.3 Hz,
1 H, aryl); 6.98 d (J = 8.5 Hz, 1 H, aryl); 6.78 s (2H, aryl); 5.05 s (2H,
OCH2); 3.83 s (6H,
OCH3); 3.75 s (3H, OCH3); 2.96 t (J = 7.9 Hz, 2H, CHZ); 2.60 t (J = 7.9 Hz,
2H, CHz).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
144
91 c) N-[(R)-1-(Hydroxymethyl)-2-(1 H-indol-3-yl)ethyl]-2-[(3,4,5-
trimethoxyphenyl)methoxy]phenylpropanamide
In analogy to Example le), 87 mg of the title compound were obtained from 0.27
mmol
(95 mg) of the compound prepared as in 91 b) and 0.26 mmol (50 mg) of D-
tryptophanol.
'H-NMR (400 MHz, DMSO-d6): 6 [ppm] = 10.74 s(1 H, NH); 7.63 d (J = 8.3 Hz, 1
H, NH);
7.60 d (J = 8.0 Hz, 1 H, aryl); 7.31 d (J = 8.0 Hz, 1 H, aryl); 7.16 dd (J =
7.9 Hz / 7.7 Hz,
1 H, aryl); 7.12 d (J = 8.1 Hz, 1 H, aryl); 7.05 s(1 H, aryl); 7.04 dd (J =
8.1 Hz / 7.7 Hz,
1 H, aryl); 7.04 d (J = 7.9 Hz, 1 H, aryl); 6.95 dd (J = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 6.84 dd (J
= 8.0 Hz / 7.0 Hz, 1 H, aryl); 6.79 s (2H, aryl); 5.04 s (2H, OCH2); 3.97 m (1
H, CH); 3.76
s(6H, OCH3); 3.65 s(3H, OCH3); 3.33 m(2H, CH2OH); 2.87 dd (J = 14.5 Hz / 7.9
Hz,
1 H, CHZ); 2.83 m(2H, CHZ); 2.71 dd (J = 14.5 Hz / 7.0 Hz, 1 H, CH2); 2.38 m
(2H, CH2).
The following compounds were obtained in analogy to the preparation methods
described in detail:
Ex. Product; aMetho na oa 'H-NMR (400 MHz) S Structure
reagents gous to [ppm]
92 N-[(R)-1-(Hydroxymethyl)-2- 91 (DMSO-ds): 10.74 broad oH
(1 H-indol-3-yl)ethyl]-4- s (1H, indole-NH); 8.13 d "~ H
[[(3,4,5- (J = 8.3 Hz, 1 H, amide);
trimethoxyphenyl)methoxy]- 7.83 d (J = 8.4 Hz, 2H, o
methyl]benzamide; aryl); 7.64 d (J = 8.0 Hz, - ~ I
1 H, aryl); 7.42 d (J - 8.4 ~
D-Tryptophanol Hz, 2H, aryl); 7.31 d (J = o\
and 8.0 Hz, 1 H, aryl); 7.12 d
(J = 2.1 Hz, 1 H, aryl);
4-(((3, 4, 5- 7.04 dd (J = 8.0 Hz / 7.0
Trimethoxy- Hz, 1 H, aryl); 6.96 dd (J
phenyl)methoxy]methyl]benzo = 8.0 Hz / 7.0 Hz, 1 H,
ic acid aryl); 6.66 s (2H, aryl);
4.77 dd (J = 5,7 Hz / 5.7
Hz, OH); 4.57 s (2H,
CH2O); 4.47 s (2H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
145
Ex. Product; aMetho nelotl 'H-NMR (400 MHz) S Structure
reagents yous to [ppm]
CHZO); 4.23 m (1 H,
CHNH); 3.76 s (6H, 0-
Me); 3.64 s (3H, OMe);
3.52 m (1 H, CH2OH);
3.48 m (1 H, CH2OH);
3.02 dd (J = 15.1 Hz/
5.8 Hz; 1 H, CH2-indole);
2.91 dd (J = 15.1 Hz /
7.7 Hz; 1 H, CH2-indole).
93 N-[(R)-1-(Hydroxymethyl)-2- 91 (DMSO-d6): 10.80 s(1H, H P
-
(1H-indol-3-yl)ethyl]-3-[(3,4,5- indole-NH); 7.68 d (J O ~' " H
~~ , ~/=i s
trimethoxyphenyl)methoxy]- 5.5 Hz, 1 H, aryl); 7.57 d ~ ~/
thiophene-2-carboxamide; (J = 8.0 Hz, 1 H, aryl);
7.55 d(J = 8.1 Hz, 1 H,
D-Tryptophanol amide); 7.31 d (J = 8.0
=
and Hz, 1 H, aryl); 7.22 d (J
5.5 Hz; 1 H, aryl); 7.05 dd
3-((3, 4, 5-Trimethoxyphenyl)- (J = 8.0 Hz / 7.0 Hz, 1 H,
methoxyJthiophene-2- aryl); 6.98 d (J = 2.3 Hz,
carboxylic acid 1 H, indole); 6.95 dd (J =
8.0 / 7.0 Hz, 1 H, aryl);
6.76 s (2H, aryl); 5.21 d
(J =11.7 Hz, 1 H, CH2O);
5.14 m(1 H, CHNH); 5.13
d(J = 11.7 Hz, 1H,
CH2O); 4.95 dd (J = 5.3
Hz / 5.3 Hz; 1 H, OH);
3.93 dd (J = 14.7 Hz /
7,0 Hz, 1H, CHZ); 3.81
dd ( J = 14.7 Hz / 6.4 Hz,
1H, CHz); 3.73 s (6H,
OMe); 3.63 s (3H, OMe);
3.44 m(1 H, CH2); 3.40
m (1H, CHZ).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
146
Ex. Product; aMet nehoa 'H-NMR (400 MHz) S Structure
reagents yous to [ppm]
94 N-[(R)-1-(Hydroxymethyl)-2- 91 (DMSO-d6): 10.76 s(1H, O"
(1H-indol-3-yl)ethyl]-4-[(3,4,5- indole-NH); 7.82 d (J= 0 " 1 H
trimethoxyphenyl)methoxy]- 8.1 Hz, 1H, amide); 7.58
phenylacetamide; d (J = 8.0 Hz, 1 H, aryl); 'o
7.32 d(J = 8. 0 Hz, 1 H, ,
D-Tryptophanol aryl); 7.08 d (J = 8.7 Hz,
and 2H, aryl); 7.07 s(1 H, a-
ryl); 7.05 dd (J = 8.0 Hz /
4-((3, 4, 5-Trimethoxyphenyl)- 7.0 Hz, 1 H, aryl); 6.95 dd
methoxy]benzenessigsaure (J = 8.0 Hz / 7.0 Hz, 1 H,
aryl); 6.89 d (J = 8.7 Hz;
2H; aryl); 6.76 s (2H, a-
ryl); 4.97 s (2H, CH2O);
4.73 dd (J = 5.5 Hz / 5.5
Hz, 1 H, OH); 3.95 m(1 H,
CHNH); 3.77 s (6H, 0-
Me); 3.65 s (3H, OMe);
3.36 m (2H, CH2OH);
3.33 s (2H, CHz); 2.90 dd
(J=14.1 Hz/6.2Hz,
1H, CH2-indole); 2.74 dd
(J = 14.1 Hz / 7.5 Hz,
1 H, CH2-indole).
95 N-[(R)-1-(Hydroxymethyl)-2- 91 (DMSO-d6): 10.75 s(1H, o"
~/
(1H-indol-3-yl)ethyl]-3-[(3,4,5- indole-NH); 7.68 d (J = o "~ N
H
rimethoxyphenyl)methoxy]- 8.3 Hz, 1 H, amide); 7.60
~~
phenylpropanamide; d (J = 8.0 Hz, 1 H, aryl); o'
"o
7.31 d(J = 8.O Hz, 1 H, ~
D-Tryptophanol
aryl); 7.17 dd (J = 7.9 Hz o,
and / 7.7 Hz, 1 H, aryl); 7.07 d
(J = 2.1 Hz, 1 H, indole);
3-(3-((3, 4, 5-trimethoxyphenyl)- 7.05 dd (J = 8.0 Hz / 7.0
methoxy)phenyl]-propionsaure Hz, 1 H, aryl); 6.87 s(1 H,
aryl); 6.85 dd (J = 8.0 Hz
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
147
Ex. Product; aMet nahoa 'H-NMR (400 MHz) S Structure
reagents gous to Ippml
/ 7.0 Hz, 1 H, aryl); 6.82
dd (J = 7.9 Hz / 2.5 Hz,
1 H, aryl); 6.76 dd (J =
7.7 Hz / 2.5 Hz, 1 H, aryl);
6.75 s (2H, aryl); 4.96 s
(2H, CH2O); 4.70 dd (J =
5.7 Hz / 5.5 Hz, 1 H, OH);
3.76 s (6H, OMe); 3.98
m (1H, CHNH); 3.65 s
(3H, OMe); 3.33 m (2H,
CH2OH); 2.89 dd (J =
14,1 Hz / 6.6 Hz, 1 H,
CH2); 2.74 m (2H, CH2);
2.72 dd (J = 14.1 Hz /
7.0 Hz, 1H, CHZ); 2.35 m
(2H, CH2).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
148
Example 96
2-[2-(3,4-dimethoxyphenyl)ethyl]-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]-
HO I
O NH N
H
O,
0 N
6-methoxyquinoline-4-carboxamide "0
96a) 2-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-6-methoxyquinoline-4-carboxylic
acid
4-methoxyisatin (4 g, 22.5 mmol) and (E)-3,4-dimethoxybenzylideneacetone (4.6
g,
22.5 mmol) were suspended in 30% strength aqueous KOH (20 ml) and heated under
reflux for 8 hours. The reaction mixture was cooled and diluted with water,
and the solid
was filtered off. The residue on the filter was boiled three times with sodium
hydroxide
solution (1 N, 100 ml), and the combined mother liquors were acidified by
adding acetic
acid. A solid precipitates out of the solution after it has stood in a
refrigerator overnight.
The precipitate was filtered off, washed with water (100 ml) and dried in
vacuo. 1.67 g
(20% yield) of the title compound were obtained and could be employed in the
next
stage without further purification.
'H-NMR (400 MHz, DMSO-d6): 6[ppm] = 8.18 s(1 H); 8.08 d (J = 2.6 Hz, 1 H);
7.94 d (J
= 9.2 Hz, 1 H); 7.71 d (J = 16.2 Hz, 1 H); 7.44 m (2H); 7.40 d (J = 16.3 Hz, 1
H); 7.22 d (J
= 8.1 Hz, 1 H); 6.96 d (J = 8.5 Hz, 1 H); 3.86 s (3H); 3.81 s (3H); 3.76 s
(3H).
96b) 2-[2-(3,4-dimethoxyphenyl)ethyl]-6-methoxyquinoline-4-carboxylic acid
2-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-6-methoxyquinoline-4-carboxylic acid
(500 mg)
was dissolved in methanol (10 ml) and aqueous sodium hydroxide solution (1 N,
5 ml)
and concentrated to dryness in vacuo. The residue is dissolved in methanol (5
ml), a
spatula tip of Pd/C is added, and hydrogenation is carried out under low
pressure and at
room temperature until no further uptake of hydrogen is to be observed. The
catalyst
was filtered off and the filtrate was concentrated in a rotary evaporator.
Acidification with
aqueous hydrochloric acid (1 N), removal of the precipitate by filtration and
drying in
vacuo resulted in 277 mg of the title compound which could be employed in the
next
stage without further purification.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
149
'H-NMR (400 MHz, DMSO-d6): S[ppm] = 13.70 s broad (1 H, acid); 8.07 d (J =2.8
Hz,
1 H, aryl); 7.93 d (J = 9.3 Hz, 1 H, aryl); 7.83 s (1 H, aryl); 7.42 dd (J =
9.1 Hz / 2.8 Hz;
1 H, aryl); 6.85 d (J =1.8 Hz, 1 H, aryl); 6.79 d (J = 8.1 Hz, 1 H, aryl);
6.72 dd (J = 8.1 Hz /
1.5 Hz, 1 H, aryl); 3.85 s (3H, OMe); 3.66 s (6H, OMe); 3.18 m (2H, CHZ); 2.97
m (2H,
CH2).
96c) 2-[2-(3,4-dimethoxyphenyl)ethyl]-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]-6-methoxyquinoline-4-carboxamide
In analogy to method 1e), D-tryptophanol (103 mg, 0.54 mmol) and the
quinolinecarboxylic acid from method 98b) (100 mg, 0.27 mmol) were reacted to
give
the title compound and purified by recrystallization from ethanol. 122 mg of
the title
compound were obtained.
'H-NMR (400 MHz, DMSO-d6): S[ppm] = 10.80 s(1 H, indole-NH); 8.47 d (J = 8.7
Hz,
1 H, amide); 7.86 d (J = 9.9 Hz, 1 H, aryl); 7.62 d (J = 7.7 Hz, 1 H, aryl);
7.30 m (4H, aryl);
7.17 d (J = 1.9 Hz, 1 H, aryl); 7.03 dd (J = 7.0 Hz / 7.0 Hz, 1 H, aryl); 6.94
dd (J = 7.0 Hz
/ 7.0 Hz, 1 H, aryl); 6.87 d (J = 1.5 Hz, 1 H, aryl); 6.80 m (2H, aryl); 4.84
dd (J = 5.5 Hz /
5.5 Hz, 1 H, OH); 4.35 m(1 H, CHz); 3.68 s (3H, OMe); 3.67 s (3H, OMe); 3.66 s
(3H,
OMe); 3.54 dd (J =5.6 Hz / 5.6 Hz, 2H, CH2); 3.10 m (2H, CH2); 2.92 m (4H,
CH2).
Ex. Product; aMet nehotl 'H-NMR (400 MHz) S Structure
reagents gous to Lppm]
97 2-(6-Methoxy-naphthalen-2- 13 (DMSO-ds): 10.85 s(1 H); - H
/ N
yl)quinoline-4-carboxylic acid 8.67 s (1 H); 8.64 d (J
=
[(R)-1-hydroxymethyl-2-(1 H- 8.3 Hz, 1 H); 8.33 dd (J = HO
O NH
indol-3-yl)ethyl]amide; 2.0 Hz / 8.8 Hz, 1 H); 8.09 ~ I
- 7.96 m(4H); 7.84 d(J = N
(~)-Tryptophanol I ~
8.3 Hz, 1 H); 7.76 m(1 H); o
and 7.66 d (J = 7.8 Hz, 1 H);
7.48 m(1 H); 7.37 m (2H);
2-(6-Methoxy-naphthalen-2- 7.24 dd (J = 2.5 Hz / 9.1
yl)quinoline-4-carboxylic acid Hz, 1 H); 7.18 m (1 H);
7.06 m(1 H); 6.95 m(1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
150
Product; Method 'H-NMR (400 MHz) S Structure
Ex. rea ents analo-
9 gous to [PPm]
4.39 m (1H); 3.89 s (3H);
3.55 m (2H); 3.08 dd (J =
5.8 Hz / 14.7 Hz, 1 H);
2.92 dd (J = 8.3 Hz / 14.4
Hz, 1 H).
98 6-Methoxy-2-(3- 13 (DMSO-d6): 10.81 s (1H); H
/ N
methoxyphenyl)quinoline-4- 8.63 d (J = 8.6 Hz, 1 H);
carboxylic acid [(R)-1- 7.98 d (J = 9.1 Hz, 1 H); Ho HNH
hydroxymethyl-2-(1 H-indol-3- 7.89 s(1 H); 7.75 s(1 H); o
~,
yI)ethyl]amide; 7.71 d (J = 7.8 Hz, 1 H); ~0 N 7.64 d(J = 7.8 Hz, 1 H);
(D)-Tryptophanol 7.45 m (3H); 7.31 d (J =
and 8.1 Hz, 1 H); 7.18 s(1 H);
7.05 m (1 H); 6.93 m (1 H);
6-Methoxy-2-(3- 4.89 m(1 H); 4.38 m(1 H);
methoxyphenyl)quinoline-4- 3.84 s (3H); 3.71 s (3H);
carboxylic acid 3.57 m (2H); 3.03 dd (J =
5.6 Hz / 14.7 Hz, 1 H); dd
(J = 8.1 Hz / 14.7 Hz,
1 H).
99 2-(4-Fluoro-3- 13 (DMSO-d6): 10.80 s(1 H); - H
N
methoxyphenyl)-6- 8.62 d (J = 8.7 Hz, 1 H);
methoxyquinoline-4- 7.96 m (2H); 7.90 s(1 H); Ho H
O NH
carboxylic acid [(R)-2- 7.69 m(1 H); 7.61 d (J =
hydroxy-1-(1 H-indol-3- 7.9 Hz, 1 H); 7.42 m(3H); ~~
O ~ I N /
ylmethyl)ethyl]amide; 7.19 s(1 H); 7.03 m(1 H); F
6.93 m(1 H); 4.87 m(1 H);
(o)-Tryptophanol 4.37 m(1 H); 3.96 s (3H);
and 3.71 m (3H); 3.57 m (2H);
2-(4-Fluoro-3- 3.01 m(1 H); 2.91 m(1 H).
methoxyphenyl)-6-
methoxyquinoline-4-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
151
Ex. Product; aMetho naloa 'H-NMR (400 MHz) 8 Structure
reagents gous to IPPm]
carboxylic acid
100 2-(3-Iodo-4-methoxyphenyl)- 13 (DMSO-d6): 10.79 s(1 H); N
6-methoxyquinoline-4- 8.62 m(1 H); 8.15 dd (J =
carboxylic acid [(R)-2- 2.1 Hz / 8.5 Hz, 1 H); 7.95 Ho H
O NH
hydroxy-1-(1 H-indol-3- d (J = 9.6 Hz, 1 H); 7.84 s
oll
ylmethyl)ethyl]amide; (1 H); 7.63 d (J = 7.9 Hz, , I N
1 H); 7.40 s(1 H); 7.30 m o I~
(D)-Tryptophanol I
(2H); 7.18 m (2H); 7.04 m
and (1 H); 6.94 m(1 H); 4.87 m
(1 H); 4.36 m(1 H); 3.90 s
2-(3-1odo-4-methoxyphenyl)- (3H); 3.70 s (3H); 3.57 m
6-methoxyquinoline-4- (2H); 3.01 m(1 H); 2.90 m
carboxylic acid (1 H).
101 2-(3-Hydroxyphenyl)-6- 13 (DMSO-d6): 10.79 s(1 H); N
methoxyquinoline-4- 9,60 s(1 H); 8.62 d (J = ?
carboxylic acid [(R)-1- 8.7 Hz, 1 H); 7.94 d (J = Ho
O NH
hydroxymethyl-2-(1 H-indol-3- 9.6 Hz, 1 H); 7.82 s(1 H); o~,
yI)ethyl]amide; 7.64 m (2H); 7.51 d(J = HO N
8.1 Hz, 1 H); 7.41 m (2H); (D)-Tryptophanol 7.32 m (2H); 7.18 s(1 H);
and 7.03 m(1 H); 6.93 m(1 H);
6.87 m (1 H); 4.88 m (1 H);
2-(3-Hydroxyphenyl)-6- 4.38 m(1 H); 3.71 s(3H);
methoxyquinoline-4- 3.56 m(2H); 3.01 m(1 H);
carboxylic acid 2.93 m (1 H).
102 2-(4-Hydroxy-3,5- 13 (DMSO-d6): 10.79 s(1 H); H
N
dimethoxyphenyl)-6- 8.78 s (1 H); 8.61 d (J =
methoxyquinoline-4- 8.8 Hz, 1 H); 7.90 m(2H); Ho NH
carboxylic acid [(R)-1- 7.63 d (J = 7.8 Hz, 1 H); o,~
hydroxymethyl-2-(1 H-indol-3- 7.47 s (2H); 7.39 m (3H); -o ~ N 7.30 d J= 8.1
Hz, 1 H; HO yl)ethyl]amide; ( ) o
7.19 s(1 H); 7.02 m(1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
152
Ex. Product; aMetho naloa 'H-NMR (400 MHz) 8 Structure
reagents gous to Ippm]
(o)-Tryptophanol 6.93 m(1 H); 4.86 m(1 H);
4.37 m(1 H); 3.87 s(6H);
and 3.69 s (3H); 3.57 m (2H);
3.02dd(J=5.8Hz/15.1
2-(4-Hydroxy-3,5- Hz, 1 H); 2.91 dd (J = 7.6
dimethoxyphenyl)-6- Hz / 14.4 Hz, 1 H).
methoxyquinoline-4-
carboxylic acid
103 2-(3,5-Difluoro-4- 13 (DMSO-d6): 10.80 s(1 H); - N
/
methoxyphenyl)-6- 8.59 d (J = 8.7 Hz, 1 H);
methoxyquinoline-4- 7.93 m (4H); 7.63 d (J = Ho
O NH
carboxylic acid [(R)-2- 7.9 Hz, 1 H); 7.41 m(2H); o
1~
hydroxy-1-(1 H-indol-3- 7.31 d (J = 7.9 Hz, 1 H); F N
~
ylmethyl)ethyl]amide; 7.18 s(1 H); 7.02 m(1 H); o
F
6.92 m(1 H); 4.87 m(1 H);
(D)-Tryptophanol 4.37 m(1 H); 3.99 s(3H);
and 3.70 s (3H); 3.57 m (2H);
3.01 dd(J=6.4Hz/14.7
2-(3, 5-Difluoro-4- Hz, 1 H); 2.89 dd (J = 8.1
methoxyphenyl)-6- Hz / 14.3 Hz, 1 H).
methoxyquinoline-4-
carboxylic acid
104 2-(3-Ethylphenyl)-6- 13 (DMSO-d6): 10.80 s(1 H); H
~ N
methoxyquinoline-4- 8.61 d (J = 8.6 Hz, 1 H);
carboxylic acid [(R)-1- 7.96 m (4H); 7.86 s(1H); Ho
O NH
hydroxymethyl-2-(1 H-indol-3- 7.63 d (J = 7.8 Hz, 1 H); o,
yl)ethyl]amide; 7.41 m (4H); 7.32 d (J = I~ N 8.1 Hz, 1 H); 7.18 s(1 H);
(D)-Tryptophanol 7.03 m(1 H); 6.93 m(1 H);
and 4.87m(1H);4.37m(1H);
3.71 s (3H); 3.57 m (2H);
2-(3-Ethylphenyl)-6- 2.95 dd (J = 5.6 Hz / 14.7
methoxyquinoline-4- Hz, 1 H); 2.91 dd (J = 8.1
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
153
Ex. Product; aMetho ne otl 'H-NMR (400 MHz) S Structure
reagents gous to Ippm]
carboxylic acid Hz / 14.7 Hz, 1 H); 2.71 m
(2H); 1.21 m (3H).
105 2-(3-Fluoro-4- 13 (DMSO-d6): 10.80 s(1 H);
methoxyphenyl)-6- 8.59 d (J = 8.7 Hz, 1 H); Ho NH ~\/
N
H
methoxyquinoline-4- 8.00 m (3H); 7.85 s(1 H); ~,
carboxylic acid [(R)-2- 7.63 d (J = 7.7 Hz, 1 H); N
hydroxy-1-(1 H-indol-3- 7.35 m (4H); 7.18 d (J = F
ylmethyi)ethyl]amide; 2.1 Hz, 1 H); 7.04 m(1 H);
6.93 m(1 H); 4.86 m(1 H);
(o)-Tryptophanol 4.37 m(1 H); 3.91 s(3H);
and 3.70 s (3H); 3.57 m (2H);
2-(3-Fluoro-4- 3.02 m(1 H); 2.93 m(1 H).
methoxyphenyl)-6-
methoxyquinoline-4-
carboxylic acid
106 2-(3-Fluoro-4- 13 (DMSO-d6): 10.83 s(1 H); - H
N
methoxyphenyl)-6- 8.56 d (J = 8.7 Hz, 1 H);
methylquinoline-4-carboxylic 8.06 dd (J = 2.1 Hz / 13.0 HO
acid [(R)-2-hydroxy-1-(1 H- Hz, 1 H); 7.98 d (J = 9.8 0 NH
indol-3-ylmethyl)ethyl]amide; Hz, 1 H); 7.91 d (J = 8.5
Hz, 1 H); 7.84 s(1 H); 7.62 N
o)-Tryptophanol
( m (3H); 7.32 m (2H); 7.18 ol
and s(1 H); 7.05 m(1 H); 6.94
m(1 H); 4.88 m(1 H); 4.38
2-(3-Fluoro-4- m(1 H); 3.91 s(3H); 3.57
methoxyphenyl)-6- m(2H); 3.03 m(1H); 2.90
methylquinoline-4-carboxylic m (1 H); 2.38 s (3H).
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
154
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. rea ents analo-
9 gous to EPPm]
107 6-Methyl-2-(3,4,5- 13 (DMSO-d6): 10.81 s (1H);
Ho
trimethoxyphenyl)quinoline-4- 8.58 d (J = 8.3 Hz, 1 H); 0 NH N
carboxylic acid [(R)-1- 7.93 m (2H); 7.62 m (2H); hydroxymethyl-2-(1 H-indol-3-
7.58 dd (J = 1.8 Hz / 8.6 0 N
0
yl)ethyl]amide; Hz, 1 H); 7.48 s (2H); 7.32 ~,
d (J = 8.1 Hz, 1 H); 7.18 s
(D)-Tryptophanol
(1 H); 6.95 m(1 H); 6.93 m
and (1 H); 4.86 m(1 H); 4.36 m
(1 H); 3.89 s (6H); 3.72 s
6-Methyl-2-(3,4,5- (3H); 3.57 m (2H); 3.04 m
trimethoxyphenyl)quinoline-4- (1 H); 2.91 m(1 H); 2.65 s
carboxylic acid (3H).
108 6-Bromo-2-(2,4-dimethyl- 13 (DMSO-d6): 10.81 s(1 H); Ho
~ NH ~
thiazol-5-yl)quinoline-4- 8.72 d (J = 8.7 Hz, 1 H); N
Br
U N~ ~
carboxylic acid [(R)-2- 8.15 s(1 H); 7.88 s (2H); / ~\ \
N
hydroxy-1-(1 H-indol-3- 7.63 s(1 H); 7.58 d (J =
ylmethyl)ethyl]amide; 7.9 Hz, 1 H); 7.32 d (J =
8.1 Hz, 1 H); 7.17 d(J =
(D)-Tryptophanol 2.1 Hz, 1 H); 7.02 m(1 H);
and 6.92 m(1 H); 4.88 m(1 H);
4.33 m(1 H); 3.55 m (2H);
6-Bromo-2-(2,4-dimethyl- 3.02 dd (J = 5.3 Hz / 14.7
thiazol-5-yl)quinoline-4- Hz, 1 H); 2.90 dd (J = 8.3
carboxylic acid Hz / 14.7 Hz, 1 H); 2.64 s
(3H); 2.62 s (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
155
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents gous to [ppm]
109 2-(7-Methoxybenzofuran-2- 13 (DMSO-d6): 10.79 s(1 H); N
yI)-6- 8.81 d (J = 8.9 Hz, 1 H); ~
HO
trifluoromethoxyquinoline-4- 8.23 d (J = 9.2 Hz, 1 H); o NH
carboxylic acid [(R)-2- 8.07 s(1 H); 7.93 s(1 H); o~,F
F
N /
hydroxy-1-(1 H-indol-3- 7.80 m (2H); 7.62 d (J o
ylmethyl)ethyl]amide; 7.7 Hz, 1 H); 7.33 m (3H); 0
~
(D)-Tryptophanol 7.18 s(1 H); 7.03 m (3H);
and 4.90 m(1 H); 4.36 m(1 H);
2-(7-Methoxybenzofuran-2- 3.99 s (3H); 3.56 m (2H);
yl)-6- 3.02 m(1 H); 2.92 m(1 H).
trifluoromethoxyquinoline-4-
carboxylic acid
110 2-(3-Fluoro-4- 13 (DMSO-d6): 10.80 s(1 H); - H
N
methoxyphenyl)-6- 8.68 d (J = 8.5 Hz, 1 H);
iodoquinoline-4-carboxylic 8.44 s(1H); 8.00 m(3H); Ho
0 NH
acid [(R)-2-hydroxy-1-(1 H- 7.91 s(1 H); 7.80 d (J =
indol-3-ylmethyl)ethyl]amide; 8.9 Hz, 1 H); 7.65 d (J = F
7,9 Hz, 1 H); 7.33 m (2H); ~a
(o)-Tryptophanol
7.18 s(1 H); 7.04 m(1 H);
and 6.94 m(1 H); 4.34 m(1 H);
3.92 s (3H); 3.57 m (2H);
2-(3-Fluoro-4- 3.03 m(1 H); 2.92 m(1 H).
methoxyphenyl)-6-
iodoquinoline-4-carboxylic
acid
111 2-(3-Fluoro-4- 13 (DMSO-d6): 10.84 s(1 H); - N
methoxyphenyl)-6- 8.76 d (J = 8.5 Hz); 8.76 ~
trifluoromethoxyquinoline-4- d (J = 8.5 Hz, 1 H); 8.21 d Ho NH
carboxylic acid [(R)-2- (J = 9.23 Hz, 1 H); 8.16 - oFF
hydroxy-1-(1 H-indol-3- 8.04 m (4H); 7.81 dd (J = F I I N F
ylmethyl)ethyl]amide; 2.8 Hz / 8.5 Hz, 1 H); 7.68
d (J = 7.7 Hz, 1 H); 7.37 m
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
156
Ex. Product; aMetho na otl 'H-NMR (400 MHz) S Structure
reagents yous to [ppm]
(o)-TryPtophanol (2H); 7.21 d (J = 2.1 Hz,
1 H); 7.07 m (1 H); 6.97 m
and (1 H); 4.91 m(1 H); 4.38 m
(1 H); 3.97 s (3H); 3.61 m
2-(3-Fluoro-4- (2H); 3.09 dd (J = 6.2 Hz
methoxyphenyl)-6-
/ 14.7 Hz, 1 H); 2.94 dd (J
trifluoromethoxyquinoline-4-
7.5 Hz / 14.5 Hz, 1 H).
carboxylic acid
112 2-(3-Fluoro-4- 13 (DMSO-d6): 10.86 s(1 H); N
methoxyphenyl)-6,8- 8.55 d (J = 8.5 Hz, 1 H); i
dimethylquinoline-4- 8.14 dd (J = 2.1 Hz / 15.3 HO
carboxylic acid [(R)-2- Hz, 1 H); 8.04 d (J = 8.7 0 NH
hydroxy-1-(1 H-indol-3- Hz, 1 H); 7.87 s(1 H); 7.69
ylmethyl)ethyl]amide; d (J = 7.9 Hz, 1 H); 7.49 d N
(J = 2.3 Hz, 1 H); 7.37 d (J 0
(D)-Tryptophanol = 8.7 Hz, 2H); 7.22 s
and (1 H); 7.10 m(1 H); 6.99 m
(1H); 4.90 m (1H); 4.40 m
2-(3-Fluoro-4- (1 H); 3.96 s (3H); 3.60 m
methoxyphenyl)-6, 8- (2H); 3.08 m(1 H); 2.95 m
dimethylquinoline-4- (1 H); 2.76 s (3H); 2.38 s
carboxylic acid (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
157
Example 113
2-(3,4-Dimethoxyphenyl)-6-methoxy-3-methylquinoline-4-carboxylic acid [(R)-1-
H
N
HO
O NH
O,
O N
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide; 'Ox
113a) 2-(3,4-Dimethoxyphenyl)-6-methoxy-3-methylquinoline-4-carboxylic acid
3',4'-Dimethoxy-l-phenylpropiophenone (1.5g) and 5-methoxyisatin (1.4 g) were
heated
together in aqueous 30% strength potassium hydroxide solution (20 ml) under
reflux
overnight. The reaction mixture was added to water and the remaining residue
was fil-
tered off with suction. The filtrate was acidified with glacial acetic acid
and placed in a
refrigerator overnight. The precipitated reaction product was filtered off,
dried in vacuo
and employed without further purification in the next stage (yield 34%).
(DMSO-d6): 7,90 d (J = 9.2 Hz); 7.39 dd (J = 9.2 Hz / 2.8 Hz, 1 H); 7.04 m
(4H); 3.85 s
(3H); 3.79 s (3H); 3.76 s (3H); 2.35 s(3H).
113b) 2-(3,4-Dimethoxyphenyl)-6-methoxy-3-methylquinoline-4-carboxylic acid
[(R)-
1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide
The quinolinecarboxylic acid from the previous stage (200 mg) was stirred
together with
(D)-tryptophanol (108 mg), HOBt (87 mg), EDC (109 mg) and
diisopropylethylamine
(0.099 ml) in DMF (10 ml) at room temperature overnight. The mixture was added
to
water and stirred for 10 minutes, and the precipitate was filtered off. The
crude product
was purified by column chromatography using Flashmasters and crystallized from
diisopropyl ether. The title compound is obtained in 30% yield (90 mg).
=
(DMSO-d6): 10.76 s (1 H); 8.56 d (J = 8.9 Hz, 1 H); 7.83 d (J = 9.2 Hz, 1 H);
7.59 d (J
7.7 Hz, 1 H); 7.27 m (3H); 7.02 m (5H); 4.90 m(1 H); 4.47 m(1 H); 3.79 s (6H);
3.56 m
(2H); 2.96 m(1 H); 2.69 m(1 H); 2.05 s(3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
158
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Ex. Product; aMetho naloa 'H-NMR (400 MHz) S Structure
reagents 9ous to [ppm]
114 6-Amino-2-(3-fluoro-4- 20 (DMSO-d6): 10.82 s (1 H); H
N
methoxyphenyl)quinoline-4- 8.43 d (J = 8.3 Hz, 1 H);
carboxylic acid [(R)-2- 7.94 d (J = 2.3 Hz / 13.1 Ho
= o NH
hydroxy-1-(1H-indol-3- Hz, 1 H); 7.84 d (J 9.4 NHZ
ylmethyl)ethyl]amide; Hz, 1 H); 7.70 m(2H); 7.19 F I N ~
s(1 H); 7.14 m(1 H); 7.03 . I ~
0
2-(3-Fluoro-4- m(1 H); 6.96 m(1 H); 5.69
methoxyphenyl)- 6- (2H); 4.82 (m, 1 H); 4.28
nitroquinoline-4-carboxylic m(1 H); 3.89 s(3H); 3.55
acid ((R)-2-hydroxy-1-(1H- m(2H); 3.03 m(1H); 2.96
indol-3-y1methyl)ethy1]amide m (1 H).
Example 115
2-(4,6-Dimethoxybenzofuran-2-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl]-
OH
I \ /
O NH N
H
0,
O N
O ~ ~
0
6-methoxyquinoline-4-carboxamide; '
115a) 1-(4,6-Dimethoxybenzofuran-2-yl)ethanone
4,6-Dimethoxysalicylaldehyde (500 mg), 1-chloro-2-propanone (241 NI),
potassium car-
bonate (379 mg) were stirred in 2-butanone (20 ml) under a nitrogen atmosphere
at
90 C for 8 hours. The reaction mixture was diluted with water and extracted
with ethyl
acetate, and the combined organic phases were washed with saturated aqueous
NaCI
solution. The solvent was distilled out in vacuo, and the crude product was
purified by
Flashmaster chromatography. The title compound was obtained in 29% yield (174
mg).
(CDCI3): 7.53 s(1 H); 6.64 m(1 H); 6.32 s(1 H); 3.91 s(3H); 3.86 s(3H); 2.53
s(3H).
115b) 2-(4,6-Dimethoxybenzofu'ran-2-yl)-6-methoxyquinoline-4-carboxylic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
159
1-(4,6-Dimethoxybenzofuran-2-yl)-ethanone (169 mg), 5-methoxyisatin (136 mg)
were
stirred together with potassium hydroxide solution (30% strength in water, 2.7
ml) under
a nitrogen atmosphere at 80 C for 8 hours. The reaction mixture was added to
150 ml
of water and, while cooling in ice, acidified with 70% strength acetic acid
until a pH of 5-
6 was reached. After 30 minutes, stirring with n-butanol/ethyl acetate (1:1,
20 ml) and
back-extraction with ethyl acetate were carried out. The combined organic
phases were
washed with saturated aqueous NaCI solution. The solvent was distilled out in
vacuo.
Crystallization from dichloromethane/methanol results in the title compound in
78%
yield (227 mg).
(DMSO-d6): 8.39 s (1 H); 8.10 s (1 H); 8.00 m (J = 9.3 Hz, 1 H); 7.64 s(1 H);
7.48 m(1 H);
6.95 s(1 H); 6.44 s(1 H); 3.88 s (6H); 3.81 s (3H).
11 5c) 2-(4,6-Dimethoxybenzofuran-2-yl)-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-
3-
yl)ethylj-6-methoxyquinoline-4-carboxamide
The quinolinecarboxylic acid (120 mg) was converted into the title compound in
64%
yield (93 mg) in analogy to general method 113b.
(DMSO-d6): 10.85 s ( 1 H); 8.68 d (J = 8.8 Hz, 1 H); 7.97 d (J = 9.1 Hz, 1 H);
7.92 s(1 H);
7.66 d (J = 8.0 Hz, 1 H); 7.63 d (J = 0.8 Hz, 1 H); 7.44 dd (J = 9,1 Hz / 2.8
Hz, 1 H); 7.39
d(J = 2.8 Hz, 1 H); 7.36 d(J = 8.0 Hz, 1 H); 7.22 d(J = 2.0 Hz, 1 H); 7.08 dd
(J = 8.0 Hz /
7.0 Hz, 1 H); 6.99 dd (J = 8.0 Hz / 7.0 Hz, 1 H); 6.98 s (1 H); 6.49 s (1 H);
4.93 m (1 H);
4.42 m(1 H); 3.94 s (3H); 3.86 s (3H); 3.73 s (3H); 3.61 m (2H); 3.05 dd (J =
14.9 Hz /
6.3 Hz, 1 H); 2.93 dd (J = 14.9 Hz / 7.8 Hz, 1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
160
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) S Structure
Ex. rea ents analo-
9 gous to IPPm]
116 N-[(R)-1-(Hydroxymethyl)-2- 115 (DMSO-d6): 10.85 s o"
(1 H-indol-3-yl)ethyl]-6- (1 H); 8.71 d(J = 8.6 Hz, o NH N
H
methoxy-2-(5- 1 H); 8.02 d (J = 9.1 Hz, o,
O N
methoxybenzofuran-2- 1 H); 7.94 s(1 H); 7.67 d
yl)quinoline-4-carboxamide; (J = 8.0 Hz, 1 H); 7.66 d -o
(J = 8.6 Hz, 1 H); 7.65 s
D-Tryptophanol and
(1 H); 7.46 dd (J = 9.1 Hz
6-Methoxy-2-(5- / 2.8 Hz, 1 H); 7.41 d (J =
methoxybenzofuran-2- 2.8 Hz, 1 H); 7.36 d (J =
yl)quinoline-4-carboxylic acid 8=0 Hz, 1 H); 7.27 d (J =
2.8 Hz, 1 H); 7.23 d (J =
2.0 Hz, 1 H); 7.09 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 7.02
dd (J = 8.6 Hz / 2.8 Hz,
1 H); 6.99 dd (J = 8.0 Hz /
7.0 Hz, 1 H); 4.93 m(1 H);
4.43 m(1 H); 3.84 s (3H);
3.74 s (3H); 3.61 m (2H);
3.06 dd (J = 14.7 Hz / 5.6
Hz, 1 H); 2.94 dd (J =
14.7 Hz / 8.1 Hz, 1 H).
117 2-(7-Ethoxybenzofuran-2-yl)- 115 (DMSO-d6): 10.84 s o"
NH ~ H
N-[(R)-1-(hydroxymethyl)-2- (1 H); 8.74 d (J = 8.6 Hz, 0
(1 H-indol-3-yl)ethyl]-6- 1 H); 8.04 d (J = 9.1 Hz, \_o o; o,
methoxyquinoline-4- 1 H); 7.96 s(1 H); 7.70 s N
carboxamide; (1 H); 7.68 d (J = 8.0 Hz,
1 H); 7.47 dd (J = 9.1 Hz /
D-Tryptophanol and 2.8 Hz, 1 H); 7.42 d (J =
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
161
Product; Method 1
Ex. analo- H-NMR (400 MHz) S Structure
reagents 9oUs to IPPm]
2-(7-Ethoxybenzofuran-2-y1)- 2.8 Hz, 1 H); 7.36 d (J =
6-methoxyquinoline-4- 8.0 Hz, 1 H); 7.32 d (J =
carboxylic acid 7.8 Hz, 1 H); 7.24 d (J =
2.3 Hz, 1 H); 7.23 dd (J =
7.8 Hz / 7.8 Hz, 1 H); 7.08
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 7.03 d (J = 7.8 Hz,
1H);7.01dd(J=8.0Hz/
7.0 Hz, 1 H); 4.94 m(1 H);
4.45 m (1 H); 4.30 q (J =
6.9 Hz, 2H); 3.73 s (3H);
3.62 m (2H); 3.06 dd (J =
14.7 Hz / 5.6 Hz, 1 H);
2.95 dd (J = 14.7 Hz / 8.3
Hz, 1 H); 1.48 t(J = 6.9
Hz, 3H).
118 N-[(R)-1-(Hydroxymethyl)-2- 115 (DMSO-d6): 10.85 s OH
(1 H-indol-3-yl)ethyl]-6- (1 H); 8.70 d(J = 8.6 Hz, 0 "H ~ N
methoxy-2-(6- 1 H); 7.99 d (J = 9.1 Hz, o,
a,
"
methoxybenzofuran-2- 1 H); 7.90 s(1 H); 7.67 d o r~~
yi)quinoline-4-carboxamide; (J = 8.0 Hz, 1 H); 7.65 d
(J = 8.6 Hz, 1 H); 7.64 s
D-Ttyptophanol and (1 H); 7.45 dd (J = 9.1 Hz
6-Methoxy-2-(6- / 2.8 Hz, 1 H); 7.41 d (J =
methoxybenzofuran-2- 2.8 Hz, 1 H); 7.36 d (J =
yl)quinoline-4-carboxylic acid 8.0 Hz, 1 H); 7.27 d (J =
2.1 Hz, 1 H); 7.23 d (J =
2.3 Hz, 1 H); 7.09 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 6.99
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.97 dd (J = 8.6 Hz /
2.3 Hz, 1 H); 4.92 m(1 H);
4.44 m(1 H); 3.87 s (3H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
162
Product; Method 'H-NMR (400 MHz) S Structure
Ex. rea ents analo-
g gous to (ppm]
3.73 s (3H); 3.61 m (2H);
3.06 dd (J = 14.4 Hz / 5.6
Hz, 1 H); 2.98 dd (J =
14.4 Hz / 8.1 Hz, 1 H).
119 2-(7-Fluorobenzofuran-2-yl)- 115 (DMSO-d6): 10.85 s H
N-[(R)-1-(hydroxymethyl)-2- (1 H); 8.75 s(1 H); 8.05 d o HI N
H
(1 H-indol-3-yi)ethyl]-6- (J = 9.1 Hz, 1 H); 7.99 s o"
F O IN /
methoxyquinoline-4- (1 H); 7.83 d (J = 3.0 Hz,
carboxamide; 1 H); 7.67 d (J = 8.0 Hz,
1 H); 7.62 m (1 H); 7.49 dd
D-Ttyptophanol and (J = 9.1 Hz / 2.8 Hz, 1 H);
2-(7-Fluorobenzofuran-2-y1)- 7.43 d(J = 2.8 Hz, 1 H);
6-methoxyquinoline-4- 7.36 d (J = 8.0 Hz, 1 H);
carboxylic acid 7.34 m (2H); 7.23 d (J =
2.0 Hz, 1 H); 7.08 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 6.99
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 4.9 5 m (1 H); 4.44 m
(1 H); 3.74 s (3H); 3.62 m
(2H); 3.05 dd (J = 14.7
Hz / 5.6 Hz, 1 H); 2.94 dd
(J = 14.7 Hz / 8.1 Hz,
1 H).
120 2-(4-Fluorobenzofuran-2-yl)- 115 (DMSO-d6): 10.88 s OH
N-[(R)-1-(hydroxymethyl)-2- (1 H); 8.75 d (J = 8.6 Hz, o H N
H
(1 H-indol-3-yl)ethyl]-6- 1 H); 8.04 d (J = 9.1 Hz, o,~
o IN /
methoxyquinoline-4- 1 H); 8.02 s(1 H); 7.83 d
carboxamide; (J = 1.0 Hz, 1 H); 7.66 d F
(J = 8.3 Hz, 1 H); 7.66 d
D-Tryptophanol and (J = 8.0 Hz, 1 H); 7.48 dd
2-(4-Fluorobenzofuran-2-y1)- (J = 9.1 Hz / 2.8 Hz, 1 H);
6-methoxyquinoline-4- 7.45 ddd (J = 8.3 Hz / 8.3
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
163
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents gousanelo-to [ppm]
carboxylic acid Hz / 5.8 Hz, 1 H); 7.42 d
(J = 2.8 Hz, 1 H); 7.36 d
(J = 8.0 Hz, 1 H); 7.23 d
(J = 2.0 Hz, 1 H); 7.19 dd
(J = 9.6 Hz / 8.3 Hz, 1 H);
7.08dd(J=8.0Hz/7.0
Hz, 1 H); 6.98 dd (J = 8.0
Hz / 7.0 Hz, 1 H); 4.97 m
(1 H); 4.43 m(1 H); 3.74 s
(3H); 3.62 m (2H); 3.06
dd (J = 14.7 Hz / 5.8 Hz,
1 H); 2.94 dd (J = 14.7 Hz
/ 8.1 Hz, 1 H).
121 N-[(R)-1-(Hydroxymethyl)-2- 115 (DMSO-d6): 10.86 s OH
(1 H-indol-3-yl)ethyl]-6- (1 H); 8.72 d (J = 8.6 Hz, 0 NH I N
H
methoxy-2-(5- 1 H); 8.01 d (J = 9.1 Hz, ,~
O ~N I /
methylbenzofuran-2- 1 H); 7.94 s (1 H); 7.67 d
yI)quinoline-4-carboxamide; (J = 8.0 Hz, 1 H); 7.64 s
(1 H); 7.63 d (J = 8.6 Hz,
D-Tryptophanol and 1 H); 7.56 d (J = 1.5 Hz,
6-Methoxy-2-(5- 1 H); 7.46 dd (J = 9.1 Hz /
methylbenzofuran-2- 2.8 Hz, 1 H); 7.41 d (J =
yl)quinoline-4-carboxylic acid 2.8 Hz, 1 H); 7.36 d (J =
8.0 Hz, 1 H); 7.24 dd (J =
8.6 Hz / 1.5 Hz, 1 H); 7.23
d (J = 2.0 Hz, 1 H); 7.09
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.99 dd (J = 8.0 Hz /
7.0 Hz, 1 H); 4.94 m(1 H);
4.43 m(1 H); 3.74 s (3H);
3.61 m (2H); 3.06 dd (J =
14.7 Hz / 5.8 Hz, 1 H);
2.94 dd (J = 14.7 Hz / 8.1
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
164
Product; Method 'H-NMR (400 MHz) S Structure
Ex. rea ents analo-
9 gous to IPPm]
Hz, 1 H); 2.44 s (3H).
122 N-[(R)-1-(Hydroxymethyl)-2- 115 (DMSO-d6): 10.86 s OH
(1 H-indol-3-yl)ethyl]-6- (1 H); 8.75 d (J = 8.6 Hz, o NH N
H
methoxy-2-(7- 1 H); 8.03 d (J = 9.1 Hz, o,
methylbenzofuran-2- 1 H); 7.98 s(1 H); 7.70 s
yI)quinoline-4-carboxamide; (1 H); 7.67 d (J = 8.0 Hz,
1 H); 7.60 m(1 H); 7.47 dd
D-Tryptophanol and (J = 9.1 Hz / 2.8 Hz, 1 H);
6-Methoxy-2-(7- 7.43 d (J = 2.8 Hz, 1 H);
methylbenzofuran-2- 7.36 d(J = 8.0 Hz, 1 H);
yl)quinoline-4-carboxylic acid 7.24 m (3H); 7.08 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 6.99
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 4.95 m(1 H); 4.43 m
(1 H); 3.74 s (3H); 3.62 m
(2H); 3.06 dd (J = 14.7
Hz / 5.6 Hz, 1 H); 2.95 dd
(J = 14.7 Hz / 8.3 Hz,
1 H); 2.61 s (3H).
123 N-[(R)-1-(Hydroxymethyl)-2- 115 (DMSO-d6): 10.85 s OH
(1 H-indol-3-yl)ethyl]-6- (1 H); 8.70 d (J = 8.6 Hz, o NH
H
methoxy-2-(4- 1 H); 8.01 d (J = 9.1 Hz, o,
methoxybenzofuran-2- 1 H); 7.99 s(1 H); 7.73 s
yI)quinoline-4-carboxamide; (1 H); 7.67 d (J = 8.0 Hz, 0
~
1 H); 7.46 dd (J = 9.1 Hz /
D-Tryptophanol and 2.8 Hz, 1 H); 7.41 d (J =
6-Methoxy-2-(4- 2.8 Hz, 1 H); 7.37 m(2H);
methoxybenzofuran-2- 7.36 d (J = 8.0 Hz, 1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
165
Product; Method 'H-NMR (400 MHz) S Structure
Ex. rea ents analo-
3 gous to IPPm]
yl)quinoline-4-carboxylic acid 7.22 s (1 H); 7.08 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 6.99
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.88 dd (J = 7.1 Hz/
1.8 Hz, 1 H); 4.94 m(1 H);
4.43 m(1 H); 3.98 s (3H);
3.73 s (3H); 3.61 m (2H);
3.05 dd (J = 14.7 Hz / 5.6
Hz, 1 H); 2.93 dd (J =
14.7 Hz / 8.1 Hz, 1 H).
124 N-[(R)-1-(Hydroxymethyl)-2- 115 (DMSO-d6): 10.86 s OH
(1 H-indol-3-yl)ethyl]-6- (1 H); 8.75 d (J = 8.6 Hz, " H
0,
methoxy-2-[5- 1 H); 8.04 d (J = 9.1 Hz, o
N
(trifluoromethoxy)benzofuran- 1 H); 7.98 s (1 H); 7.90 d
F
F~--O
2-yi]quinoline-4-carboxamide; (J = 8.8 Hz, 1 H); 7.83 d F
(J = 1.5 Hz, 1 H); 7.78 d
D-Tryptophanol and
(J = 0.8 Hz, 1 H); 7.66 d
6-Methoxy-2-[5- (J = 8.0 Hz, 1 H); 7.49 dd
(trifluoromethoxy)benzofuran- (J = 9.1 Hz / 2.8 Hz, 1 H);
2-yl]quinoline-4-carboxylic 7.43 dd (J = 8.8 Hz / 1.5
acid Hz, 1 H); 7.42 d (J = 2.8
Hz, 1 H); 7.36 d (J = 8.0
Hz, 1 H); 7.23 d (J = 2.0
Hz, 1 H); 7.08 dd (J = 8.0
Hz / 7.0 Hz, 1 H); 6.99 dd
(J = 8.0 Hz / 7.0 Hz, 1 H);
4.95 m (1 H); 4.43 m
(1 H); 3.74 s(3H); 3.61 m
(2H); 3.06 dd (J = 14.7
Hz / 5.6 Hz, 1 H); 2.94 dd
(J = 14.7 Hz / 8.1 Hz,
1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
166
Example 125
4-Ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-indol-3-
H
N
HO
O NH
~
yl)ethyl]amide;
125a) Ethyl 5-bromo-2-ethoxybenzoate
5-Bromo-2-hydroxybenzoic acid (5g) and potassium carbonate (6.37 g) in acetone
(230 ml) were stirred under reflux under a nitrogen atmosphere and, at the
boiling point,
iodoethane (5 x 5 ml) was slowlly added at intervals of 1 hour. Stirring under
reflux was
continued for 4 hours. The solvent was distilled out in a rotary evaporator,
the residue
was taken up in ethyl acetate and extracted with water and saturated aqueous
NaCl
solution, and the combined organic phases were freed of solvent. Flash
chromatogra-
phy resulted in 4.1 g (65% yield) of the title compound. MS (ESI, +): 274
(M+1).
125b) 5-Bromo-2-ethoxybenzoic acid
Ethyl 5-bromo-2-ethoxybenzoate (5g) were stirred under reflux in potassium
hydroxide
(10% strength in ethanol, 50 ml) for twelve hours. The cooled reaction mixture
was
mixed with water, and the remaining ethanol was distilled out in a rotary
evaporator.
The remaining aqueous phase was washed with diethyl ether and acidified by
adding
2N HCI. The precipitated reaction product was filtered off and washed with
water. Dry-
ing in vacuo resulted in 4.25 g (95% yield) of the title compound, which was
employed
without further purification in the next stage.
MS (ESI, +): 246 (M+1)
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
167
125c) Methyl (R)-2-(5-bromo-2-ethoxybenzoylamino)-3-(1 H-indol-3-yl)-
propionate
5-Bromo-2-ethoxybenzoic acid (500 mg), (D)-tryptophan methyl ester
hydrochloride
(520 mg), EDC (390 mg), HOBt (310 mg) and diisopropylethylamine (0.36 ml) in
DMF
(10 ml) were stirred together at room temperature overnight. The reaction
mixture was
concentrated, taken up in ethyl acetate and extracted several times with
water. The
combined organic phases were freed of solvent, and the reaction mixture was
purified
by flash chromatography. 660 mg of the title compound (73% yield) were
obtained. MS
(ESI, +): 446 (M+1)
125d) 5-Bromo-2-ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-
yl)ethyl]benzamide
A solution of methyl (R)-2-(5-bromo-2-ethoxybenzoylamino)-3-(1 H-indol-3-yl)-
propionate (500 mg) in THF (10 ml) was cooled to -10 C, and a solution of
lithium
borohydride in THF (0.84 ml, 2 mmol/ml) was slowly added dropwise. The mixture
was
stirred overnight and then 1 N HCI was cautiously added. The solvent was
distilled out in
a rotary evaporator, and the remaining aqueous phase was extracted with ethyl
acetate.
The combined organic phases were freed of solvent and dried in vacuo. 435 mg
of the
title compound (93% yield) were obtained after crystallization from ethanol.
MS (ESI, +):
418 (M+1)
125e) 4-Ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-indol-3-
yI)ethyl]amide
5-Bromo-2-ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]benzamide (200
mg),
phenylboronic acid (64 mg), sodium carbonate (2 M solution in water, 1 ml) and
Pd(PPh3)4 (6 mg) were heated to reflux together in toluene (6 ml) and ethanol
(0.4 ml)
overnight. The reaction mixture was filtered and the filtrate was
concentrated. The resi-
due was taken up in ethyl acetate and extracted with water. The organic phases
were
dried and the solvent was distilled off in a rotary evaporator. Flash
chromatography re-
sulted in 45 mg of the title compound (21 % yield).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
168
(DMSO-ds): 10.78 s (1 H); 8.37 d (J = 8 Hz, 1 H); 8.13 s (1 H); 7.76 -7.50
broad m (5H);
7.42 m (2H); 7.29 m (2H); 7.03 m (1H); 6.91 m (1H); 4.30 m (1H); 4.11 m (2H);
3.42 m,
(2H): 2.95 m (2H); 1.28 m (3H).
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents g ~ o [ppm]
126 4-Ethoxy-3'-fluoro-4'- 125 (DMSO-d6): 10.78 s(1 H); - N
propoxybiphenyl-3-carboxylic 8.36 d (J = 8.1 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H-indol- 8.08 s(1 H); 7.70 m (2H); HO
0 H
3-ylmethyl)ethyl]amide; 7.47 d (J = 12.9 Hz, 1 H);
7.37 m(1 H); 7.35 m(1 H): F
5-Bromo-2-ethoxy-N-((R)-2-
7.15 m(4H); 7.03 m(1 H); o
hydroxy-1-(1 H-indol-3-
6.93 m(1 H); 4.90 m(1 H);
ylmethyl)ethyl]benzamide 4.22 m(1 H); 4.12 m (2H);
and 3.46 m(1 H); 3.40 m(1 H);
3-Fluoro-4-propoxyphenyl- 2.95 m (2H); 1.73 m (2H);
boronic acid 1.27 m(3H); 0.98 m (3H).
127 2-Ethoxy-N-[(R)-1- 125 (DMSO-d6): 10.79 s(1H); N
hydroxymethyl-2-(1 H-indol-3- 8.40 d (J = 2.5 Hz, 1 H); i
yI)ethyl]-5-(6-methoxypyridin-3- 8.36 d (J = 8.1 Hz, 1 H); Ho
yI)benzamide; 8.07 s (1 H); 7.94 dd (J = 0 NH
2.8 / 8.6 Hz, 1 H); 7.68 m
5-Bromo-2-ethoxy-N-[(R)-2-
(2H); 7.30 d (J = 8.1 Hz, N
hydroxy-1-(1H-indol-3- 11
1 H); 7.18 d (J = 8.6 Hz, 0
ylmethyl)ethyl]benzamide
1 H); 7.13 s (1 H); 7.03 m
and (1 H); 6.93 m(1 H); 6.88 m
(1 H); 4.22 m(1 H); 4.12 m
2-Methoxy-5-pyridineboronic (2H); 3.86 s (3H); 3.47 m
acid (2H); 2.95 m (2H); 1.28 m
(3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
169
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. reagents gous ~ o [ppm]
128 4-Ethoxy-2'-fluoro-3'- 125 (DMSO-d6): 10.79 s(1 H); H
methoxybiphenyl-3-carboxylic 8.36 d (J = 8.1 Hz, 1 H);
acid [(R)-2-hydroxy-l-(1 H-indol- 8.02 s(1 H); 7.65 d(J = HO
O H
3-ylmethyl)ethyl]amide; 8.1 Hz, 1 H); 7.60 m(1 H);
5-Bromo-2-ethoxy-N-((R)-2- 7.29 d (J = 8.1 Hz, 1 H); 'o I
7.18 m (4H); 7.00 m (2H); I
hydroxy-1-(1 H-indol-3-
ylmethyl)ethylJbenzamide 6.93 m(1 H); 4.90 m(1 H);
4.23 m(1 H); 4.13 m (2H);
and 3.84 s (3H); 3.46 m(1 H);
2-Fluoro-3-methoxyphenyl- 3.38 m(1 H); 2.95 m (2H);
boronic acid
1.28 m (3H).
129 4'-Acetylamino-4- 125 (DMSO-d6): 10.82 s(1 H); S
H ethoxybiphenyl-3-carboxylic
10.04 s(1 H); 8.43 d (J =
acid [(R)-1-hydroxymethyl-2- 8.1 Hz, 1 H); 8.19 d (J Ho
O NH
(1 H-indol-3-yl)ethyl]amide; 2.5 Hz, 1 H); 7.89 s (1 H);
5-Bromo-2-ethoxy-N-[(R)-2- 7.73 m(2H); 7.58 m (1 H); o
hydroxy-1-(1H-indol-3- A N
7.33 m (3H); 7.26 d (J = H
ylmethyl)ethylJbenzamide 8.9 Hz, 1 H); 7.18 s(1 H);
and 7.07 m(1 H); 6.96 m(1 H);
4-Acetamidophenylboronic acid 4 29 m(1 H); 4.17 m(2H);
3.48 m (2H); 2.99 m (2H);
2.08 s (3H); 1.33 m (3H).
130 2-Ethoxy-N-[(R)-1- 125 (CDCI3): 8.88 s (2H); 8.49 - N
hydroxymethyl-2-(1 H-indol-3- d (J - 7.6 Hz, 1 H); 8.41 s \
yI)ethyl]-5-(2-methoxypyrimidin- (1 H); 8.17 s(1 H); 7.70 d Ho
5-yl)benzamide; o NH
(J = 7.6 Hz, 1 H); 7.56 d(J
5-Bromo-2-ethoxy-N-[(R)-2-
= 8.3 Hz, 1 H); 7.37 d (J =
hydroxy-1-(1H-indol-3- N~
8.1 Hz, 1 H); 7.19 m(1 H); =o) 'N
ylmethyl)ethylJbenzamide 7.11 m (2H); 7.02 d (J -
and 8.3 Hz, 1 H); 4.59 m (2H);
2-Methoxypyrimidine-5-boronic 4.10 m(5H); 3.84 m(2H);
acid 3.16 m (2H); 1.28 m (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
170
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents 9 ~ o [ppm]
131 4-Ethoxy-5'-fluoro-3'- 125 (DMSO-d6): 10.78 s(1 H); H
methoxybiphenyl-3-carboxylic 8.35 d (J = 7.8 Hz, 1 H);
acid [(R)-2-hydroxy-l-(1 H-indol- 8.10 s (1H); 7.77 dd (J = HO
O H
3-ylmethyl)ethyl]amide; 2.5 Hz / 8.8 Hz, 1 H); 7.66
(J = 7.6 Hz, 1 H); 7.61 -
5-Bromo-2-ethoxy-N-[(R)-2- d
7.49 m (3H); 7.30 d (J =
hydroxy-1-(1 H-indol-3- 8.1 Hz, 1 H); 7.18 d (J = F
ylmethyl)ethylJbenzamide 7.8 Hz, 1 H); 7.13 s(1 H);
and 7.04 - 6.91 m (3H); 6.77
m(1 H); 4.90 m(1 H); 4.21
3-Fluoro-5-methoxyphenyl- m(1 H); 4.12 m(2H); 3.81
boronic acid s (1 H); 3.42 m (2H); 2.95
m (2H); 1.30 m (3H).
132 4-Ethoxy-3',4'-difluoro-5'- 125 (DMSO-d6): 10.78 s(1 H); H
methoxybiphenyl-3-carboxylic 8.34 d (J 8.1= Hz, 1 H);
acid [(R)-2-hydroxy-l-(1 H-indol- 8.11 s(1 H); 7.78 dd (J = Ho
O H
3-ylmethyl)ethyl]amide; 2.5 Hz / 8.6 Hz, 1 H); 7.67 \ o~
d (J = 7.8 Hz, 1 H); 7.30 do
5-Bromo-2-ethoxy-N-((R)-2-
(J = 8.1 Hz, 1 H); 7.25 - F
hydroxy-l-(1H-indol-3- 7.17 m(3H); 7.13 s(1H); F
ylmethyl)ethylJbenzamide 7.02 m (1 H); 6.93 m (1 H);
and 4.22 m(1 H); 4.14 m (2H);
3.94 s (3H); 3.47 m(1 H);
3,4-Difluoro-5-methoxyphenyl- 3.39 m(1H); 2.95 m (2H);
boronic acid 1.29 m (3H).
133 4-Ethoxy-4'-fluoro-3'- 125 (DMSO-d6): 10.78 s(1 H); H
N
methoxybiphenyl-3-carboxylic 8.36 d (J = 8.1 Hz, 1 H);
acid [(R)-2-hydroxy-l-(1H-indol- 8.10 s(1H); 7.72 dd (J- Ho
3-ylmethyl)ethyl]amide; 0 H
2.6 Hz / 8.7 Hz, 1 H); 7.65
5-Bromo-2-ethoxy-N-[(R)-2-
d (J = 8.8 Hz, 1 H); 7.19 m o I~
hydroxy-l-(1H-indol-3- (6H); 7.02 m(1H); 6.93 mF
ylmethyl)ethylJbenzamide (1 H); 4.25 m(1 H); 4.14
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
171
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents 9 ~ o [ppm]
and (m 2H); 3.90 s (3H); 3.45
4-Fluoro-3-methoxyphenyl- m (2H); 2.95 m (2H); 1.28
boronic acid m (3H).
134 3',5'-Dimethoxy-4- 125 (DMSO-d6): 10.78 s(1 H); - H
N
propoxybiphenyl-3-carboxylic 8.31 d (J =8.2 Hz, 1 H); ~
acid [(R)-1-hydroxymethyl-2- 8.11 s(1 H); 7.71 dd (J = Ho
0 NH
(1 H-indol-3-yl)ethyl]amide; 2.6 Hz / 8.7 Hz, 1 H); 7.65
d (J = 7.9 Hz, 1 H); 7.30 d o I~
N-((R)-1-Hydroxymethyl-2-(1 H-
(J = 8.1 Hz, 1 H); 7.15 d (J indo1-3-y1)ethylJ-5-iod-2- = 8.9 Hz, 1 H); 7.12 s
propoxybenzamide
(1 H); 7.02 m(1 H); 6.93 m
and (1 H); 6.70 s (2H); 6.45 s
(1 H); 4.89 m(1 H); 4.24 m
3,5-Dimethoxyphenylboronic (1 H); 4.03 m (2H); 3.77 s
acid pinacol ester (6H); 3.46 m (2H); 2.95 m
(2H); 1.65 m (2H); 0.91 m
(3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
172
Example 135
4-Ethoxy-3'-hydroxymethylbiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1
H-
H
N
HO .
0 NH
I \ 0'_.,
indol-3-yi)ethyl]amide; HO
5-Bromo-2-ethoxy-N-[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]benzamide (0.2
M so-
lution in THF, 500 NI, prepared by general method 125a-d), triethylamine (0.6
M solu-
tion in THF, 200 NI), palladium(II) acetate (0.0375 M in THF, 250 NI),
triotolylphosphine
(0.05 M solution in THF, 400 NI), 3-hydroxymethyl-phenylboronic acid (0.4 M
solution in
THF, 200 NI) and water (200 NI) were pipetted into a glass reactor of a
microwave and
provided with a stirring bar. The mixture was stirred in the microwave at 1200
W, and at
120 C under pressure for 30 minutes.
The THF was stripped off in a centrifuge, and the residue was then dissolved
in 2 ml of
DMSO and purified by HPLC.
HPLC-MS: Column Purospher Star RP C18 4.6x125 5pm; detection wavelength
214 nm; flow rate 1 mi/min; eluents A: 0.1% TFA in H20, B 0.1 % TFA in ACN;
gradient
in each case based on B: 5% to 95% (10') to 95% (2') to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1): 445.5
Retention time: 8.3 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
173
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method HPLC-MS conditions / Structure
Ex. analo- I
reagents gousto H-NMR (400 MHz) S [ppm]
136 4-Ethoxy-3'- 135 Column Purospher Star RP N
methylsulphanylbiphenyl-3- C18 4.6x125 5pm; detec-
tion
wavelength 214 nm; Ho
carboxylic acid [(R)-2-hydroxy- o HNH
1- 1H-indol-3- flow rate 1 ml/min; eluents
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B I'S
0.1 % TFA in ACN; gradient
5-Bromo-2-ethoxy-N-[(R)-2- in each case based on B:
hydroxy-l-(1H-indol-3- 5% to 95% (10') to 95% (2')
ylmethyl)ethyl]benzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
461.6
3-(Methylthio)phenylboronic Retention time: 10.11 min.
acid
137 3'-Cyano-4-ethoxybiphenyl-3- 135 Column Purospher Star RP S
Ncarboxylic acid [(R)-1-hydroxy- C18 4.6x125 5Nm; detec-
methyl-2-(1 H-indol-3- tion wavelength 214 nm;
HO H
flow rate 1 mI/min; eluents p NH
yl)ethyl]amide;
A: 0.1% TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-l-(1H-indol-3- in each case based on B:
ylmethyl)ethyl]benzamide 5% to 95% (10') to 95% (2') I I
to 5% (0.5') to 5% (2.5') N
and
Molecular peak (ESI, M+1):
3-Cyanophenylboronic acid 440.5
Retention time: 9.28 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
174
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents sousto H-NMR (400 MHz) 5[ppm]
138 2-Ethoxy-5-(6-fluoro-5- 135 Column Purospher Star RP N
methylpyridin-3-yl)-N-[(R)-2- C18 4.6x125 5pm; detec-
hydroxy-1-(1 H-indol-3- tion wavelength 214 nm; Ho H
ylmethyl)ethyl]benzamide; flow rate 1 ml/min; eluents o NH
A: 0.1% TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-1-(IH-indol-3- in each case based on B: F"
ylmethyl)ethylJbenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2-Fluoro-3-methylpyridine-5- 448.5
boronic acid Retention time: 9.1 min.
139 4-Ethoxy-4'- 135 Column Purospher Star RP N
trifluoromethoxybiphenyl-3- C18 4.6x125 5Nm; detec-
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; "o "NH
1-(1H-indol-3- flow rate 1 mI/min; eluents I\
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B F F ~ '
x .~
0.1 % TFA in ACN; gradient F o
5-Bromo-2-ethoxy-N-[(R)-2- in each case based on B:
hydroxy-l-(1H-indol-3- 5% to 95% (10') to 95% (2')
ylmethyl)ethylJbenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
499.5
4-(Trifluoromethoxy)phenyl- Retention time: 10.55 min.
boronic acid
140 5-Benzo[b]thiophene-3-y1-2- 135 Column Purospher Star RP _ H
ethoxy-N-[(R)-2-hydroxy- 1 -(1 H- C18 4.6x125 5pm; detec-
indol-3- tion wavelength 214 nm; Ho H
flow rate 1 ml/min; eluents o""
ylmethyl)ethyl]benzamide;
A: 0.1 % TFA in H20, B - I~
\ -
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient s ~ I
hydroxy-l-(1H-indol-3- in each case based on B:
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
175
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
ylmethyl)ethyl]benzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
1-Benzothiophen-3-ylboronic 471.6
acid Retention time: 10.68 min.
141 4-Ethoxy-2'- 135 Column Purospher Star RP N
trifluoromethylbiphenyl-3- C18 4.6x125 5pm; detec- ~/
tion wavelength 214 nm;
carboxylic acid [(R)-2-hydroxy-
1-(1 H-indol-3- flow rate 1 mI/min; eluents HO H NH
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B
0.1% TFA in ACN; gradient
5-Bromo-2-ethoxy-N-[(R)-2- in each case based on B: F
hydroxy-1-(1H-indol-3- 5% to 95% (10') to 95% (2') F F
ylmethyl)ethyl]benzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and 483.5
2-(Trifluoromethyl)phenyl- Retention time: 10.05 min.
boronic acid
142 4-Ethoxy-2'- 135 Column Purospher Star RP =: N
trifluoromethoxybiphenyl-3- C18 4.6x125 5pm; detec-
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm;
flow rate 1 ml/min; eluents HO H%
1-(1 H-indol-3- o NH
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B \ o~
0.1 % TFA in ACN; gradient
~
5-Bromo-2-ethoxy-N-[(R)-2- in each case based on B: F F
~ I x
hydroxy-l-(1H-indol-3- 5% to 95% (10') to 95% (2') O F
ylmethyl)ethyl]benzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
499.5
2-(Trifluoromethoxy)phenyl- Retention time: 10.25 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
176
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
143 4-Ethoxy-3'- 135 Column Purospher Star RP N
trifluoromethoxybiphenyl-3- C18 4.6x125 5pm; detec- S
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; "o "NH
flow rate 1 mI/min; eluents
1-(1 H-indol-3- o"",
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B FXo
F F \
0.1 % TFA in ACN; gradient
5-Bromo-2-ethoxy-N-[(R)-2- in each case based on B:
hydroxy-1-(1H-indol-3- 5% to 95% (10') to 95% (2')
ylmethyl)ethylJbenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
499.5
3-(Trifluoromethoxy)phenyl- Retention time: 10.52 min.
boronic acid
144 4-Ethoxy-3'-fluoro-biphenyl-3- 135 Column Purospher Star RP
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5pm; detec- N
1-(1H-indol-3- tion wavelength 214 nm;
HO H
ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents o NH
A: 0.1 % TFA in H20, B \ o
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-1-(1H-indol-3- in each case based on B: ~ i
ylmethyl)ethylJbenzamide 5% to 95% (10') to 95% (2') F
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Fluorophenylboronic acid 433.5
Retention time: 9.8 min.
145 4'-Chloro-4-ethoxybiphenyl-3- 135 Column Purospher Star RP H
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5pm; detec-
tion wavelength 214 nm; o
1-(1 H-indol-3- H H
ylmethyl)ethyl]amide; flow rate 1 mi/min; eluents o N"
\ o~
A: 0.1% TFA in H20, B i
5-Bromo-2-ethoxy-N-[(R)-2- 0.1% TFA in ACN; gradient I
ci
hydroxy-1-(1H-indol-3- in each case based on B:
5% to 95% (10') to 95% (2')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
177
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents so~s to
ylmethyl)ethyl]benzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
449.9
4-Chlorophenylboronic acid Retention time: 10.32 min.
146 4-Ethoxy-4'- 135 Column Purospher Star RP N
methylsulphanylbiphenyl-3- C18 4.6x125 5pm; detec-
tion wavelength 214 nm;
carboxylic acid [(R)-2-hydroxy- Ho .
flow rate I mI/min; eluents o"H
1-(1 H-indol-3-
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B I~
0.1 % TFA in ACN; gradient I
5-Bromo-2-ethoxy-N-[(R)-2- in each case based on B:
hydroxy-1-(1H-indol-3- 5% to 95% (10') to 95% (2)
ylmethyl)ethyl]benzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
461.6
4-(Methylthio)pheny1boronic Retention time: 10.17 min.
acid
147 4-Ethoxy-3'- 135 Column Purospher Star RP N
trifluoromethylbiphenyl-3- C18 4.6x125 5pm; detec-
tion wavelength
carboxylic acid [(R)-2-hydroxy- 214 nm; Ho
1-(1H-indol-3- flow rate 1 mI/min; eluents O HNH
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B
0.1 % TFA in ACN; gradient
5-Bromo-2-ethoxy-N-((R)-2- in each case based on B:
hydroxy-1-(1H-indol-3- 5% to 95% (10') to 95% (2') F
F F
ylmethyl)ethyl]benzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
483.5
3-(Trifluoromethyl)-phenyl- Retention time: 10.35 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
178
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) 8 [ppm]
148 3'-Chloro-4-ethoxybiphenyl-3- 135 Column Purospher Star RP H
N
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5Nm; detec-
1-(1H-indol-3- tion wavelength 214 nm; Ho H
ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents 0 NH
A: 0.1 % TFA in H20, B
ci
5-Bromo-2-ethoxy-N-((R)-2- 0.1 % TFA in ACN; gradient I
hydroxy-1-(1H-indol-3- in each case based on B:
ylmethyl)ethylJbenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Chlorophenylboronic acid 449.9
Retention time: 10.3 min.
149 4-Ethoxy-3'-methylbiphenyl-3- 135 Column Purospher Star RP N
carboxylic acid [(R)-1-hydroxy- C18 4.6x125 5pm; detec-
methyl-2-(1 H-indol-3- tion wavelength 214 nm; HO H
flow rate 1 mI/min; eluents 0 NH
yl)ethyl]amide;
A: 0.1 % TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-1-(1H-indol-3- in each case based on B:
ylmethyl)ethylJbenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Methylphenylboronic acid 429,5
Retention time: 10,17 min.
N
150 5-Benzofuran-2-yl-2-ethoxy-N- 135 Column Purospher Star RP _H
[(R)-1-hydroxymethyl-2-(1 H- C18 4.6x125 5pm; detec- ~/
indol-3-yl)ethyl]benzamide; tion wavelength 214 nm; Ho o NH
flow rate 1 mI/min; eluents
5-Bromo-2-ethoxy-N-[(R)-2- A: 0.1% TFA in H20, B hydroxy-1-(1H-indol-3- 0.1%
TFA in ACN; gradient o
ylmethyl)ethyl)benzamide in each case based on B:
5% to 95% (10') to 95% (2)
and
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
179
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
Benzo(bJfuran-2-boronic acid Molecular peak (ESI, M+1):
455.5
Retention time: 10.61 min.
151 4-Ethoxy-2'- 135 Column Purospher Star RP N
methylsulphanylbiphenyl-3- C18 4.6x125 5pm; detec-
tion wavelength
carboxylic acid [(R)-2-hydroxy- 214 nm; Ho
1-(1H-indol-3- flow rate 1 mI/min; eluents o HNH
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B o~
0.1 % TFA in ACN; gradient ~
5-Bromo-2-ethoxy-N-((R)-2- in each case based on B: s-
hydroxy-l-(1H-indol-3- 5% to 95% (10') to 95% (2')
ylmethyl)ethylJbenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
461.6
2-(Methylthio)phenylboronic Retention time: 10.17 min.
acid
152 2-Ethoxy-N-[(R)-1- 135 Column Purospher Star RP N
hydroxymethyl-2-(1 H-indol-3- C18 4.6x125 5pm; detec-
yl)ethyl]-5-(1 H-indol-4- tion wavelength 214 nm; HO
flow rate 1 mI/min; eluents o H NH
yl)benzamide;
A: 0.1% TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-l-(1H-indol-3- in each case based on B:
ylmethyl)ethyl)benzamide 5% to 95% (10') to 95% (2') H~
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
1 H-Indole-4-boronic acid 454.5
Retention time: 9.11 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
180
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
153 2-Ethoxy-N-[(R)-2-hydroxy-1- 135 Column Purospher Star RP N
(1 H-indol-3-ylmethyl)ethyl]-5- C18 4.6x125 5pm; detec-
(4-methylthiophene-2- tion wavelength 214 nm; Ho H
flow rate 1 mI/min; eluents o NH
yl)benzamide;
A: 0.1% TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-1-(1H-indol-3- in each case based on B: S
ylmethyl)ethyl]benzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Methylthiophene-2-boronic 435.6
acid Retention time: 10.07 min.
154 3'-Acetylamino-4- 135 Column Purospher Star RP N
ethoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- \A AO
tion wavelength
acid [(R)-1-hydroxymethyl-2- 214 nm; Ho
(1 H-indol-3-yl)ethyl]amide; flow rate 1 mI/min; eluents 0 H NH
A: 0.1 % TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy- 1-(1 H-indol-3- in each case based on B:
ylmethyl)ethyl]benzamide 5% to 95% (10') to 95%(2,) HNr
to 5% (0.5') to 5% (2.5') 0
and
Molecular peak (ESI, M+1):
3-Acetamidophenylboronic acid 472.6
Retention time: 8.3 min.
155 4-Ethoxy-2'-methylbiphenyl-3- 135 Column Purospher Star RP
H
C18 4.6x125 5pm; detec- ~/ N
carboxylic acid [(R)-1-hydroxy- AO
methyl-2-(1 H-indol-3- tion wavelength 214 nm;
HO H
flow rate 1 mi/min; eluents O NH
yl)ethyl]amide;
A: 0.1 % TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1% TFA in ACN; gradient
hydroxy-1-(1H-indol-3- in each case based on B:
ylmethyl)ethyl]benzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
181
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to 'H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
429.5
2-Methylphenylboronic acid Retention time: 10.35 min.
156 2-Ethoxy-N-[(R)-1- 135 Column Purospher Star RP N
hydroxymethyl-2-(1 H-indol-3- C18 4.6x125 5Nm; detec- /
tion wavelength 214 nm;
yl)ethyl]-5-(5-methyl-furan-2-
flow rate 1 mI/min; eluents HO H%
yl)benzamide; o NH
A: 0.1 % TFA in H20, B
5-Bromo-2-ethoxy-N-((R)-2- 0.1 % TFA in ACN; gradient
hydroxy-l-(1H-indol-3- in each case based on B: o
ylmethyl)ethylJbenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
5-Methylfuran-2-boronic acid 419.5
Retention time: 9.08 min.
157 3'-Chloro-4-ethoxy-4'- 135 Column Purospher Star RP N
methylbiphenyl-3-carboxylic C18 4.6x125 5pm; detec-
tion wavelength 214 nm;
acid [(R)-2-hydroxy- 1 -(1 H- HID =
indol-3-ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents 0 NH
A: 0.1% TFA in H20, B
ci
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient I
hydroxy-l-(1H-indol-3- in each case based on B:
ylmethyl)ethylJbenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Chloro-4-methyl- 464.0
phenylboronic acid Retention time: 10.83 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
182
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gousto H-NMR (400 MHz) S[ppm]
158 5-(2-Chloro-6-methylpyridin-3- 135 Column Purospher Star RP N
yl)-2-ethoxy-N-[(R)-2-hydroxy- C18 4.6x125 5pm; detec- ?
1-(1H-indol-3- tion wavelength 214 nm;
HO .
ylmethyl)ethyl]benzamide; flow rate 1 mI/min; eluents 0 NH
5-Bromo-2-ethoxy-N-[(R)-2- A: 0.1 % TFA in H20, B "',
hydroxy-l-(1H-indol-3- 0.1% TFA in ACN; gradient
ylmethyl)ethylJbenzamide in each case based on B: " cl
and 5% to 95% (10') to 95% (2)
2-Chloro-6-methylpyridine-3- to 5% (0.5') to 5% (2.5')
boronic acid Molecular peak (ESI, M+1):
465.0
Retention time: 9.33 min.
159 4-Ethoxy-4'-fluoro-biphenyl-3- 135 Column Purospher Star RP _ N
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5pm; detec- ~
tion wavelength 214 nm;
1-(1 H-indol-3- HO H
ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents 0 NH
A: 0.1 % TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-l-(1H-indol-3- in each case based on B: F
ylmethyl)ethyl)benzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Fluorophenylboronic acid 433.5
Retention time: 9.73 min.
160 2-Ethoxy-N-[(R)-1- 135 Column Purospher Star RP N
hydroxymethyl-2-(1 H-indol-3- C18 4.6x125 5pm; detec-
yl)ethyl]-5-naphthalen-1 - tion wavelength 214 nm; HO H
flow rate 1 ml/min; eluents NH
ylbenzamide;
A: 0.1 % TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1% TFA in ACN; gradient
hydroxy-1-(1H-indol-3- in each case based on B:
ylmethyl)ethylJbenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
183
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents sousto 'H-NMR (400 MHz) S [ppm]
and Molecular peak (ESI, M+1):
465.6
1-Naphthaleneboronic acid Retention time: 10.55 min.
161 5-Benzo[b]thiophene-2-yl-2- 135 Column Purospher Star RP N
ethoxy-N-[(R)-2-hydroxy-1-(1H- C18 4.6x125 5pm; detec-
tion wavelength 214 nm;
indol-3- Ho H'
ylmethyl)ethyl]benzamide; flow rate 1 mI/min; eluents o""
A: 0.1% TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient s
hydroxy-l-(1H-indol-3- in each case based on B:
ylmethyl)ethyl]benzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
Benzo[b]thiophene-2-boronic 471.6
acid Retention time: 10.92 min.
162 4-Ethoxy-4'-methylbiphenyl-3- 135 Column Purospher Star RP N
carboxylic acid [(R)-1-hydroxy- C18 4.6x125 5pm; detec-
methyl-2-(1 H-indol-3- tion wavelength 214 nm; Ho H
flow rate 1 ml/min; eluents o NH
yl)ethyl]amide;
A: 0.1% TFA in H20, B
5-Bromo-2-ethoxy-N-[(R)-2- 0.1 % TFA in ACN; gradient
hydroxy-l-(1H-indol-3- in each case based on B:
ylmethyl)ethyl]benzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Methylphenylboronic acid 429.5
Retention time: 10.14 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
184
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gousto 'H-NMR (400 MHz) S[ppm]
163 2-Ethoxy-N-[(R)-2-hydroxy-1- 135 Column Purospher Star RP H
C18 4.6x125 5Nm; detec- N
(1 H-indol-3-ylmethyl)ethyl]-5- i
tion wavelength 214 nm;
thiophene-3-ylbenzamide; HO H
flow rate 1 ml/min; eluents 0 NH
5-Bromo-2-ethoxy-N-[(R)-2- A: 0.1 % TFA in H20, B
hydroxy-l-(1H-indol-3- 0.1% TFA in ACN; gradient
ylmethyl)ethyl]benzamide in each case based on B: S ~
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
Thiophene-3-boronic acid Molecular peak (ESI, M+1):
421.5
Retention time: 9.72 min.
164 4-Ethoxy-4'-methoxybiphenyl-3- 135 HPLC-MS: _ H
carboxylic acid [(R)-1-hydroxy- Column Purospher Star RP
methyl-2-(1 H-indol-3- C18 4.6x125 5pm; detec- HO H
tion wavelength 214 nm; 0 NH
yl)ethyl]amide;
flow rate 1 mI/min; eluents
5-Bromo-2-ethoxy-N-[(R)-2- A: 0.1 % TFA in H20, B
o
hydroxy-1-(1 H-indol-3- 0.1 % TFA in ACN; gradient
ylmethyl)ethyl]benzamide in each case based on B:
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
4-Methoxyphenylboronic acid Molecular peak (ESI, M+1):
445.5
Retention time: 9.61 min.
165 2',4'-Dichloro-4-ethoxybiphenyl- 135 HPLC-MS: N
3-carboxylic acid [(R)-2- Column Purospher Star RP
C18 4.6x125 5pm; detec- Ho
hydroxy-1 -(1 H-indol-3- %
ylmethyl)ethyl]amide; tion wavelength 214 nm; o NH
flow rate 1 mI/min; eluents c' ~
5-Bromo-2-ethoxy-N-[(R)-2- A: 0.1 % TFA in H20, B I ci
hydroxy-l-(1 H-indol-3- 0.1 % TFA in ACN; gradient
ylmethyl)ethyl]benzamide in each case based on B:
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
185
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm)
reagents so~s to
and 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
2,4-Dichlorophenylboronic acid Molecular peak (ESI, M+1):
484.4
Retention time: 10.78 min.
166 4'-Methoxy-4-propoxybiphenyl- 135 Column Purospher Star RP _
C18 4.6x125 5Nm; detec- ~ HO
\/
3-carboxylic acid [(R)-1- o HN H
~ ,"~
hydroxymethyl-2-(1 H-indol-3- tion wavelength 214 nm; o I ~
yl)ethyl]amide; flow rate 1 mI/min; eluents
A: 0.1% TFA in H20, B I
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient o
indol-3-y1)ethyl]-5-iod-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Methoxyphenylboronic acid 459.6
Retention time: 9.67 min
167 4-Propoxybiphenyl-3-carboxylic 135 Column Purospher Star RP N H
acid [(R)-1-hydroxymethyl-2- C18 4.6x125 5pm; detec-
(1 H-indol-3-yl)ethyl]amide; tion wavelength 214 nm; H o' \\'
flow rate 1 ml/min; eluents 0H o
N-((R)-1-Hydroxymethyl-2-(1H- A: 0.1% TFA in H20, B
indol-3-y1)ethyl]-5-iodo-2- 0.1% TFA in ACN; gradient
propoxybenzamide in each case based on B:
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
Phenylboronic acid Molecular peak (ESI, M+1):
429.5
Retention time: 9.75 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
186
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
168 5-Benzofuran-2-yl-N-[(R)-1- 135 Column Purospher Star RP
hydroxymethyl-2-(1 H-indol-3- C18 4.6x125 5pm; detec-
tion wavelength 214 nm;
yl)ethyl]-2-propoxybenzamide;
flow rate 1 ml/min; eluents H o
N-[(R)-1-Hydroxymethyl-2-(1 H- A: 0.1 % TFA in H20, B N I H=. NH 0
indol-3-y1)ethyl]-5-iodo-2- 0.1 % TFA in ACN; gradient oH
propoxybenzamide in each case based on B:
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
Benzo(b]furan-2-boronic acid Molecular peak (ESI, M+1):
469.6
Retention time: 10.52 min.
169 3'-Chloro-4-propoxybiphenyl-3- 135 Column Purospher Star RP c,
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5Nm; detec-
1-(1H-indol-3- tion wavelength 214 nm; " H0
NH
ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents OH
l
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
indol-3-y1)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Chlorophenylboronic acid 464
Retention time: 10.38 min.
170 5-Benzo[b]thiophen-2-yl-N-[(R)- 135 Column Purospher Star RP H
N
2-hydroxy-1 -(1 H-indol-3- C18 4.6x125 5pm; detec-
ylmethyl)ethyl]-2- tion wavelength 214 nm; HO
H
propoxybenzamide; flow rate 1 ml/min; eluents H o
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient -
indol-3-y1)ethyl]-5-iodo-2- in each case based on B: S~
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
187
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[pprn]
and Molecular peak (ESI, M+1):
485.6
Benzo[b]thiophene-2-boronic Retention time: 10.84 min.
acid
171 3'-Fluoro-4'-methyl-4- 135 Column Purospher Star RP "
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N
acid [(R)-2-hydroxy-1 -(1 H- tion wavelength 214 nm; HO
H
indol-3-ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents " 0
A: 0.1% TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient F
indol-3-y1)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Fluoro-4-methyl- 461.6
phenylboronic acid Retention time: 10.37 min.
172 4-Propoxy-3'- 135 Column Purospher Star RP "
trifluoromethylbiphenyl-3- C1 8 4.6x1 25 5pm; detec- - "
"
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; HO
1-(1H-indol-3- flow rate 1 ml/min; eluents ~o" 0
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B F
0.1 % TFA in ACN; gradient F
N-[(R)-1-Hydroxymethyl-2-(1H- in each case based on B:
indol-3-y1)ethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
497.5
3-(Trifluoromethyl)- Retention time: 10.41 min.
phenylboronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
188
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to 'H-NMR (400 MHz) S [ppm]
173 2'-Fluoro-5'-methoxy-4- 135 Column Purospher Star RP H
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- \hN
acid [(R)-2-hydroxy-1 -(1 H- tion wavelength 214 nm; HO
H
indol-3-ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents o H 0
A: 0.1 % TFA in H20, B
\ / F
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
indol-3-y1)ethyl]-5-iod-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2') -O
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2-Fluoro-5-methoxyphenyl- 477.6
boronic acid Retention time: 9.79 min.
174 4-Propoxy-3',5'-bis- 135 Column Purospher Star RP H
C18 4.6x125 5pm; detec- h
trifluoromethylbiphenyl-3-
H
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; HO
1-(1 H-indol-3- flow rate 1 ml/min; eluents ~,o H 0
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B
0.1 % TFA in ACN; gradient cF
N-((R)-1-Hydroxymethyl-2-(1H- in each case based on B:
indol-3-y1)ethyl]-5-iod-2- 5% to 95% (10') to 95% (2') CF3
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
565.5
3,5-Bis-(Trifluoromethyl)- Retention time: 11.01 min.
phenylboronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
189
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S [ppm]
175 4'-Chloro-4-propoxybiphenyl-3- 135 Column Purospher Star RP H
C18 4.6x125 5pm; detec- "
carboxylic acid [(R)-2-hydroxy-
1-(1H-indol-3- tion wavelength 214 nm; Ho
H
ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents --,\H o
,o
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1 H- 0.1 % TFA in ACN; gradient
indol-3-y1)ethyl]-5-iod-2- in each case based on B: ci
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Chlorophenylboronic acid 464
Retention time: 10.44 min.
176 5-Benzo[b]thiophene-3-yl-N- 135 Column Purospher Star RP TNH
[(R)-2-hydroxy-1-(1 H-indol-3- C18 4.6x125 5Nm; detec- ylmethyl)ethyl]-2- tion
wavelength 214 nm;
flow rate 1 ml/min; eluents ,OH
propoxybenzamide; I ~ H
A: 0.1 % TFA in H20, B ~ o
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient S/
indol-3-yl)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
1-Benzothiophen-3-yl-boronic
Molecular peak (ESI, M+1):
acid
485.6
Retention time: 10.56 min.
177 4-Propoxy-4'- 135 Column Purospher Star RP H
C18 4.6x125 5pm; detec- N
trifluoromethylbiphenyl-3-
H
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; HO
1 -(1 H-indol-3- flow rate 1 mI/min; eluents o
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1H- in each case based on B: ~ F
F F
indo1-3-y1)ethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
190
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents gous co
and Molecular peak (ESI, M+1):
497.5
4-(Trifluoromethyl)- Retention time: 10.42 min.
phenylboronic acid
178 3'-Hydroxy-4-propoxybiphenyl- 135 Column Purospher Star RP _ H
3-carboxylic acid [(R)-1- C18 4.6x125 5pm; detec- ~~ N
hydroxymethyl-2-(1 H-indol-3- tion wavelength 214 nm; Ho H
yl)ethyl]amide; flow rate 1 mI/min; eluents HN o
A: 0.1% TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient o
indol-3-y1)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Hydroxyphenylboronic acid 445.5
Retention time: 8.56 min.
179 N-[(R)-1-Hydroxymethyl-2-(1H- 135 Column Purospher Star RP H
indol-3-yl)ethyl]-2-propoxy-5- C18 4.6x125 5Nm; detec-
OH
quinoline-6-ylbenzamide; tion wavelength 214 nm; H NH
flow rate 1 mI/min; eluents o 01-
N-((R)-1-Hydroxymethyl-2-(1H- A: 0.1% TFA in H20, B
indol-3-y1)ethyl]-5-iodo-2- 0.1 % TFA in ACN; gradient
propoxybenzamide in each case based on B: N
~
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
Quinoline-6-boronic acid Molecular peak (ESI, M+1):
480.6
Retention time: 6.78 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
191
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents gous to
180 5-(6-Fluoro-5-methylpyridin-3- 135 Column Purospher Star RP _ H
C18 4.6x125 5pm; detec- N
yl)-N-[(R)-2-hydroxy-1 -(1 H- -
indol-3-ylmethyl)ethyl]-2- tion wavelength 214 nm; HO
flow rate 1 mI/min; eluents o H"
propoxybenzamide; o
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
indol-3-y1)ethyl]-5-iodo-2- in each case based on B: N
F
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2-Fluoro-3-methylpyridine-5- 462.5
boronic acid Retention time: 9.2 min.
181 N-[(R)-1-Hydroxymethyl-2-(1H- 135 Column Purospher Star RP _ H
indol-3-yl)ethyl]-5-(6- C1 8 4.6x1 25 5pm; detec- N
~
H
methoxypyridine-3-yl)-2- tion wavelength 214 nm; HO
propoxybenzamide; flow rate 1 ml/min; eluents HN o
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient N
indol-3-yl)ethyl]-5-iodo-2- in each case based on B: o-
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2-Methoxypyridine-5-boronic 460.6
acid Retention time: 8.57 min.
182 3'-Chloro-4'-methyl-4- 135 Column Purospher Star RP H
C18 4.6x125 5pm; detec- N
propoxybiphenyl-3-carboxylic
acid [(R)-2-hydroxy-1 -(1 H- tion wavelength 214 nm; HO
H H
60\r indol-3-ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents
A: 0.1 % TFA in H20, B N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
ci
indol-3-y1)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
192
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
and to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
3-Chloro-4-methylboronic acid 478
Retention time: 10.94 min.
183 N-[(R)-1-Hydroxymethyl-2-(1 H- 135 Column Purospher Star RP _
H
indol-3-yl)ethyl]-2-propoxy-5- C18 4.6x125 5Nm; detec- N
pyridine-4-ylbenzamide; tion wavelength 214 nm; HO
flow rate 1 mI/min; eluents HN H o
N-((R)-1-Hydroxymethyl-2-(1H- A: 0.1% TFA in H20, B --\,-O
indol-3-yl)ethylj-5-iodo-2- 0.1 % TFA in ACN; gradient
propoxybenzamide in each case based on B: N
5% to 95% (10') to 95% (2)
and
to 5% (0.5') to 5% (2.5')
Pyridine-4-boronic acid Molecular peak (ESI, M+1):
430.5
Retention time: 6.14 min.
184 3'-Chloro-4'-fluoro-4- 135 Column Purospher Star RP
N
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- - acid [(R)-2-hydroxy-1 -
(1 H- tion wavelength 214 nm; HO
H H
indol-3-ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents o
A: 0.1% TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1 H- 0.1 % TFA in ACN; gradient ci
indol-3-y1)ethylj-5-iodo-2- in each case based on B: F
propoxybenzamide 5% to 95% (10') to 95% (2')
and to 5% (0.5') to 5% (2.5')
3-Chloro-4-fluorophenylboronic Molecular peak (ESI, M+1):
acid 482
Retention time: 10.41 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
193
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
185 3'-Acetylamino-4- 135 Column Purospher Star RP \~ N
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- ,
acid [(R)-1-hydroxymethyl-2- tion wavelength 214 nm; H0 HN H
(1 H-indol-3-yl)ethyl]amide; flow rate 1 mI/min; eluents '\-O _
A: 0.1% TFA in H20, B H
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient o
indol-3-y1)ethylJ-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Acetamidophenylboronic acid 486.6
Retention time: 8.32 min.
186 3',4'-Difluoro-4- 135 Column Purospher Star RP _
H
propoxybiphenyl-3-carboxylic C18 4.6x125 5Nm; detec- - N
"
acid [(R)-2-hydroxy-1-(1H- tion wavelength 214 nm; HO
indol-3-ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents o
A: 0.1% TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient F
indol-3-y1)ethylJ-5-iodo-2- in each case based on B: F
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular weight, calc.
3, 4-Difluorophenylboronic acid 464.5
Molecular peak (ESI, M+1):
465.5
Retention time: 9.97 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
194
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) 8[ppm]
187 3',5'-Difluoro-4- 135 Column Purospher Star RP _
C18 4.6x125 5Nm; detec- N
propoxybiphenyl-3-carboxylic
"
acid [(R)-2-hydroxy-1 -(1 H- tion wavelength 214 nm; HO
indol-3-ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents " 6Qr A: 0.1% TFA in
H20, B N-((
R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient F
indol-3-yl)ethyl]-5-iodo-2- in each case based on B:
F
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3, 5-Difluorophenylboronic acid 465.5
Retention time: 10.07 min.
188 3'-Cyano-4-propoxybiphenyl-3- 135 Column Purospher Star RP N
carboxylic acid [(R)-1- C18 4.6x125 5pm; detec-
hydroxymethyl-2-(1 H-indol-3- tion wavelength 214 nm; HO "
flow rate 1 mI/min; eluents HN
yl)ethyl]amide;
A: 0.1% TFA in H20, B
N-[(R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
indol-3-yl)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Cyanophenylboronic acid 454.6
Retention time: 9.42 min.
189 5-(2,4-Dimethoxypyrimidin-5- 135 Column Purospher Star RP _ H
I N- R 1-h drox meth I 2- C18 4.6x125 5pm; detec- ~~ N
Y )- [( )- Y Y Y -
(1 H-indol-3-yl)ethyl]-2- tion wavelength 214 nm; Ho
propoxybenzamide; flow rate 1 mi/min; eluents HN H 0
A: 0.1% TFA in H20, B
o-
N-[(R)-1-Hydroxymethyl-2-(1 H- 0.1 % TFA in ACN; gradient
N
indol-3-y1)ethyl]-5-iodo-2- in each case based on B: ' NA 0
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
195
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents 9ous to H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
491.6
2,4-Dimethoxypyrimidine-5- Retention time: 7.16 min.
boronic acid
190 2',3'-Difluoro-4- 135 Column Purospher Star RP _
"
propoxybiphenyl-3-carboxylic C1 8 4.6x1 25 5Nm; detec-
H
acid [(R)-2-hydroxy-1-(1 H- tion wavelength 214 nm; HO
indol-3-ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents " o
A: 0.1% TFA in H20, B F
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient F
indo1-3-yl)ethylJ-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2, 3-Difluorophenylboronic acid 465.5
Retention time: 9.88 min.
191 2',5'-Difluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- ~
acid [(R)-2-hydroxy- 1 -(1 H- tion wavelength 214 nm; HO
indol-3-ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents H H o
A: 0.1% TFA in H20, B --'-,-o
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient F
~
indol-3-y1)ethylJ-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2') F
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2, 5-Difluorophenylboronic acid 465.5
Retention time: 9.82 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
196
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
192 5-[(E)-2-(4-Fluorophenyl)-vinyl]- 135 Column Purospher Star RP "
C18 4.6x125 5pm; detec- "
N-[(R)-2-hydroxy-1 -(1 H-indol-3-
ylmethyl)ethyl]-2- tion wavelength 214 nm; HO "
propoxybenzamide; flow rate 1 ml/min; eluents --\,O"
A: 0.1% TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1 H- 0.1 % TFA in ACN; gradient
indol-3-y1)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2') F
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
(E)-2-(4-Fluorphenyl)viny1- 473.6
boronic acid Retention time: 10.31 min.
193 5-(5-Cyanothiophen-2-yl)-N- 135 Column Purospher Star RP _
[(R)-2-hydroxy-1 -(1 H-indol-3- C18 4.6x125 5pm; detec- \/ N
ylmethyl)ethyl]-2- tion wavelength 214 nm; HO flow rate 1 ml/min; eluents H H
propoxybenzamide; o
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
indol-3-y1)ethyl]-5-iodo-2- in each case based on B:
/
propoxybenzamide 5% to 95% (10') to 95% (2') s
to 5% (0.5') to 5% (2.5') N
and
Molecular peak (ESI, M+1):
5-Cyanothiophene-2-boronic 460.6
acid Retention time: 9.48 min.
194 2'-Fluoro-3'-methoxy-4- 135 Column Purospher Star RP "
C18 4.6x125 5pm; detec- "
propoxybiphenyl-3-carboxylic
acid [(R)-2-hydroxy-1 -(1H- tion wavelength 214 nm; HO " "
indol-3-ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents
A: 0.1 % TFA in H20, B F
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient .
indol-3-yl)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
197
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents sousco H-NMR (400 MHz) S[ppm]
and to 5% (0.5') to 5% (2.5')
2-Fluoro-3-methoxyphenyl- Molecular peak (ESI, M+1):
boronic acid 477.6
Retention time: 9.59 min.
195 N-[(R)-1-Hydroxymethyl-2-(1H- 135 Column Purospher Star RP _ H
indol-3-yl)ethyl]-5-(2- C18 4.6x125 5pm; detec- ~/ "
H
methoxypyrimidine-5-yl)-2- tion wavelength 214 nm; HO
propoxybenzamide; flow rate 1 mI/min; eluents H" o
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1 H- 0.1 % TFA in ACN; gradient N
indol-3-y1)ethyl]-5-iodo-2- in each case based on B: N-A o
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
2-Methoxypyrimidine-5-boronic 461.5
acid Retention time: 8.09 min.
196 4'-Chloro-2',6'-difluoro-4- 135 Column Purospher Star RP _
H
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- ~
acid [(R)-2-hydroxy-1 -(1 H- tion wavelength 214 nm; Ho
indol-3-ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents -\-o H H 0
A: 0.1% TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient 1 F
~
indol-3-yl)ethyl]-5-iodo-2- in each case based on B: F~~
propoxybenzamide 5% to 95% (10') to 95% (2') ci
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Chloro-2, 6-difluoro- 500
phenylboronic acid Retention time: 10.43 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
198
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
197 3',5'-Dimethyl-4- 135 Column Purospher Star RP _ H
propoxybiphenyl-3-carboxylic C18 4.6x1 25 5Nm; detec- N
H
acid [(R)-1-hydroxymethyl-2- tion wavelength 214 nm; HO
(1 H-indol-3-yl)ethyl]amide; flow rate 1 ml/min; eluents HN o
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
indol-3-y1)ethylJ-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3, 5-Dimethylphenylboronic acid 457.6
Retention time: 10.71 min.
198 N-[(R)-1-Hydroxymethyl-2-(1H- 135 Column Purospher Star RP _
N
indol-3-yl)ethyl]-2-propoxy-5- C1 8 4.6x1 25 5pm; detec- tion wavelength 214
nm; Ho
quinoline-3-ylbenzamide; HN H
flow rate 1 mi/min; eluents
N-((R)-1-Hydroxymethyl-2-(1H- A: 0.1% TFA in H20, B
indol-3-yl)ethylJ-5-iodo-2- 0.1% TFA in ACN; gradient
N~
propoxybenzamide in each case based on B:
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
3-Quinolineboronic acid Molecular peak (ESI, M+1):
480.6
Retention time: 7.07 min.
H
199 4'-Acetylamino-4- 135 Column Purospher Star RP 31H
opoxybiphenyl-3-carboxylic C18 4.6x1 25 5Nm; detec- - N
pr
acid [(R)-1-hydroxymethyl-2- tion wavelength 214 nm; Ho (1 H-indol-3-
yl)ethyl]amide; flow rate 1 ml/min; eluents HN o
A: 0.1 % TFA in H20, B
N-[(R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient
indol-3-y1)ethylJ-5-iodo-2- in each case based on B: NH
propoxybenzamide 5% to 95% (10') to 95% (2') '~
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
199
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gousto H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
486.6
4-Acetamidophenylboronic acid Retention time: 8.12 min.
200 4-Propoxy-3'-(2,2,2- 135 Column Purospher Star RP
C18 4.6x125 5pm; detec- OH
/
trifluoroethoxy)biphenyl-3- NH
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; 0 HN H
1 -(1 H-indol-3- flow rate 1 mI/min; eluents 0
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B
0.1 % TFA in ACN; gradient ~ I
0
N-[(R)-1-Hydroxymethyl-2-(1H- in each case based on B: cF
3
indol-3-y1)ethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
527.6
3-(2,2,2-Trifluorethoxy)phenyl- Retention time: 10.15 min.
boronic acid
201 3'-Ethoxy-5'-fluoro-4- 135 Column Purospher Star RP H
C18 4.6x125 5pm; detec- N
propoxybiphenyl-3-carboxylic ~
H
acid [(R)-2-hydroxy-1-(1 H- tion wavelength 214 nm; HO
indol-3-ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents H 0
A: 0.1% TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1 H- 0.1 % TFA in ACN; gradient F
indol-3-yl)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2) ro
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Ethoxy-5-fluorophenylboronic 491.6
acid Retention time: 10.42 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
200
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gousto H-NMR (400 MHz) S[ppm]
202 5'-Ethoxy-2'-fluoro-4- 135 Column Purospher Star RP
H
C18 4.6x125 5pm; detec-
propoxybiphenyl-3-carboxylic
N
tion wavelength 214 nm; HO
acid [(R)-2-hydroxy-1 -(1 H-
indol-3-ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents H"
0
A: 0.1 % TFA in H20, B
N-[(R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient F
indol-3-y1)ethylJ-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2') r o
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
5-Ethoxy-2-fluorophenylboronic 491.6
acid Retention time: 10.23 min.
203 3'-Ethoxy-4-propoxybiphenyl-3- 135 Column Purospher Star RP /
carboxylic acid [(R)-1-hydroxy- C18 4.6x125 5pm; detec- ~ o" NH
methyl-2-(1 H-indol-3- tion wavelength 214 nm; o HN
yl)ethyl]amide; flow rate 1 ml/min; eluents i
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1H- 0.1% TFA in ACN; gradient I
indol-3-y1)ethylJ-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Ethoxyphenylboronic acid 473.6
Retention time: 10.13 min.
204 4-Propoxybiphenyl-3-carboxylic 135 Column Purospher Star RP
acid [(R)-1-hydroxymethyl-2-(1- C18 4.6x125 5pm; detec- N,
methyl-1 H-indol-3- tion wavelength 214 nm;
OH
yl)ethyl]amide; flow rate 1 ml/min; eluents HN H
A: 0.1 % TFA in H20, B o
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient O
methyl-lH-indol-3-y1)ethylJ-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
201
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents sousto H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
443.6
Phenylboronic acid Retention time: 10.76 min.
205 5-Benzofuran-2-yl-N-[(R)-1- 135 Column Purospher Star RP
C18 4.6x125 5pm; detec- I N,
hydroxymethyl-2-(1-methyl-1 H-
indol-3-yl)ethyl]-2- tion wavelength 214 nm;
H oH
propoxybenzamide; flow rate 1 mi/min; eluents HN o
A: 0.1% TFA in H20, B %1 o
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-1 H-indol-3-y1)ethyl]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
Benzo(b]furan-2-boronic acid 483.6
Retention time: 11.22 min.
206 5-Benzo[b]thiophen-2-yl-N-[(R)- 135 Column Purospher Star RP
2-hyd roxy- 1 -(1 -methyl- 1 H- C1 8 4.6x1 25 5pm; detec- N-
indol-3-ylmethyl)ethyl]-2- tion wavelength 214 nm; H oH
flow rate 1 mi/min; eluents HN
propoxybenzamide; o
A: 0.1 % TFA in H20, B o
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient ~
methyl-lH-indol-3-yl)ethyl]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
Benzo[b]thiophene-2-boronic 499.6
acid Retention time: 11.52 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
202
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
207 2'-Fluoro-4'-methyl-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N,
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; OH
methyl-1 H-indol-3- flow rate 1 mI/min; eluents F HN' H
A: 0.1 % TFA in H20, B c
o ylmethyl)ethyl]amide; 0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-y1)ethyl]-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
475.6
2-Fluoro-4- Retention time: 11.07 min.
methylphenylboronic acid
208 4'-Fluoro-4-propoxybiphenyl-3- 135 Column Purospher Star RP
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5pm; detec- N,
1-(1-methyl-lH-indol-3- tion wavelength 214 nm; oH
ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents HN"H
A: 0.1 % TFA in H20, B
F o
\ ~ \
N-((R)-1-Hydroxymethy1-2-(1- 0.1% TFA in ACN; gradient
methyl-1H-indol-3-y1)ethy1]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Fluorophenylboronic acid 461.5
Retention time: 10.61 min.
209 2'-Fluoro-5'-methoxy-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N,
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm;
OH
methyl-1 H-indol-3- flow rate 1 ml/min; eluents HN.=H
ylmethyl)ethyl]amide; s A: 0.1 % TFA in H20, B ~ o
0.1 % TFA in ACN; gradient o
o
N-((R)-1-Hydroxymethy1-2-(1- in each case based on B:
methyl-1H-indol-3-yl)ethyl]-5- 5% to 95% (10') to 95% (2')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
203
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents so~s co
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
2-Fluoro-5- 491.6
methoxyphenylboronic acid Retention time: 10.42 min.
210 3'-Fluoro-4-propoxybiphenyl-3- 135 Column Purospher Star RP
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5Nm; detec- N,
1 -(1 -methyl-1 H-indol-3- tion wavelength 214 nm;
OH
ylmethyl)ethyl]amide; flow rate 1 ml/min; eluents HNH
A: 0.1 % TFA in H20, B F 0
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient o
methyl-1H-indol-3-y1)ethyl]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Fluorophenylboronic acid 461.5
Retention time: 10.63 min.
211 N-[(R)-1-Hydroxymethyl-2-(1- 135 Column Purospher Star RP
methyl-1 H-indol-3-yl)ethyl]-2- C18 4.6x125 5pm; detec- N,
propoxy-5-pyridine-3-yl- tion wavelength 214 nm;
OH
benzamide; flow rate 1 mi/min; eluents HNH
A: 0.1 % TFA in H20, B N O
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient O
methy1-1H-indol-3-y1)ethy1]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
Pyridine-5-boronic acid 444.5
Retention time: 6.73 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
204
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gousto H-NMR (400 MHz) 5 [ppm]
212 5-Benzo[b]thiophene-3-yl-N- 135 Column Purospher Star RP
[(R)-2-hydroxy-1 -(1-methyl-1 H- C18 4.6x125 5pm; detec- N,
indol-3-ylmethyl)ethyl]-2- tion wavelength 214 nm;
OH
H,
propoxybenzamide; flow rate 1 ml/min; eluents HN
A: 0.1 % TFA in H20, B o
N-((R)-1-Hydroxymethyl-2-(1- 0.1 % TFA in ACN; gradient s O
methyl-1H-indol-3-yl)ethylJ-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
Benzo[b]thiophene-3-boronic 499.6
acid Retention time: 11.37 min.
213 3'-Cyano-4'-fluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N,
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; oH
methyl-1 H-indol-3- flow rate 1 mI/min; eluents N HN'~H
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B F o
0.1 % TFA in ACN; gradient - - o
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-yl)ethylJ-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
486.6
4-Fluoro-3-cyanophenylboronic Retention time: 10.16 min.
acid
214 N-[(R)-1-Hydroxymethyl-2-(1- 135 Column Purospher Star RP
methyl-1 H-indol-3-yl)ethyl]-5- C18 4.6x125 5pm; detec- N-
(6-methoxypyridine-3-yl)-2- tion wavelength 214 nm; oH
propoxybenzamide; flow rate 1 mI/min; eluents N HN o
._ _
A: 0.1 % TFA in H20, B ~~ o
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-1 H-indol-3-yl)ethylJ-5- in each case based on B:
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
205
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents sousto 'H-NMR (400 MHz) 8 [ppm]
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
2-Methoxypyridine-5-boronic 474.6
acid Retention time: 9.62 min.
215 3'-Acetylamino-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C1 8 4.6x1 25 5pm; detec- N-
acid [(R)-1-hydroxymethyl-2-(1- tion wavelength 214 nm; o"
flow rate 1 mI/min; eluents 0 H
"" "
methyl-1 H-indol-3- ~ o
A: 0.1 % TFA in H20, B ~\ i\ o
yl)ethyl]amide;
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-y1)ethyl]-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
500.6
3-Acetamidophenylboronic acid Retention time: 9.06 min.
216 3',4'-Difluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C1 8 4.6x1 25 5pm; detec- N,
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; oH
methyl-1 H-indol-3- flow rate 1 mI/min; eluents F HN' o
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B F o
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-yl)ethyl]-5- 5% to 95% (10') to 95% (2)
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
479.5
3, 4-Difluorophenylboronic acid Retention time: 10.65 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
206
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
217 3',5'-Difluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N,
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm;
OH
methyl-1 H-indol-3- flow rate 1 ml/min; eluents F HN. H
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B o
0.1 % TFA in ACN; gradient o
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-y1)ethyl]-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
479.5
3,5-Difluorophenylboronic acid Retention time: 10.74 min.
218 5-(2,4-Dimethoxypyrimidin-5- 135 Column Purospher Star RP
yl)-N-[(R)-1-hydroxymethyl-2- C1 8 4.6x1 25 5pm; detec- N-
tion wavelength
(1-methyl-1 H-indol-3-yl)ethyl]- 214 nm; oH
flow rate 1 ml/min; eluents % 0 HN o
2-propoxybenzamide;
A: 0.1% TFA in H20, B 0~N-\
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-1 H-indol-3-yl)ethyl]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
and to 5% (0.5') to 5% (2.5')
2,4-Dimethoxypyrimidine-5- Molecular peak (ESI, M+1):
boronic acid 505.6
Retention time: 7.76 min.
219 2',5'-Difluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5Nm; detec- N,
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; oH
methyl-1 H-indol-3- flow rate 1 mi/min; eluents HN H
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B F o
0.1% TFA in ACN; gradient - ~~ o
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-y1)ethy1]-5- 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
207
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents yous to H-NMR (400 MHz) S[ppm]
iodo-2-propoxybenzamide Molecular peak (ESI, M+1):
479.5
and
Retention time: 10.51 min.
2, 5-Difluorophenylboronic acid
220 5-[(E)-2-(4-Fluorophenyl)vinyl]- 135 Column Purospher Star RP
N
C18 4.6x125 5pm; detec- i H
N-[(R)-2-hydroxy-1 -(1-methyl-
1 H-indol-3-ylmethyl)ethyl]-2- tion wavelength 214 nm; H propoxybenzamide;
flow rate 1 mI/min; eluents
A: 0.1% TFA in H20, B ~
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient ~ i
methyl-1 H-indol-3-yl)ethyl]-5- in each case based on B: F
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
and to 5% (0.5') to 5% (2.5')
(E)-2-(4-Fluorophenyl)-vinyl- Molecular peak (ESI, M+1):
boronic acid 487.6
Retention time: 11.07 min.
221 5-(5-Cyano-thiophen-2-yl)-N- 135 Column Purospher Star RP
i N
[(R)-2-hydroxy-1-(1-methyl-1 H- C18 4.6x125 5pm; detec- Ho
indol-3-ylmethyl)ethyl]-2- tion wavelength 214 nm; HN
propoxybenzamide; flow rate 1 mI/min; eluents
A: 0.1% TFA in H20, B s
N=
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-1H-indol-3-y1)ethyl]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
5-Cyanothiophene-2-boronic 474.6
acid Retention time: 10.33 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
208
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents gous to
222 N-[(R)-1-Hydroxymethyl-2-(1- 135 Column Purospher Star RP
methyl-1 H-indol-3-yl)ethyl]-5- C18 4.6x125 5pm; detec- N-
(2-methoxypyrimidine-5-yl)-2-
propoxybenzamide; tion wavelength 214 nm; oH
flow rate 1 mI/min; eluents N HNH
_
A: 0.1 % TFA in H20, B N io
N-((R)-1-Hydroxymethyl-2-(1-
0.1 % TFA in ACN; gradient
methyl-1 H-indol-3-yl)ethyl]-5-
in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2)
and
to 5% (0.5') to 5% (2.5')
2-Methoxypyrimidine-5-boronic Molecular weight, calc.
acid 474.6
Retention time: 8.83 min.
223 N-[(R)-1-Hydroxymethyl-2-(1- 135 Column Purospher Star RP
methyl-1 H-indol-3-yl)ethyl]-2- C1 8 4.6x1 25 5pm; detec- N,
propoxy-5-quinoline-3-yl- tion wavelength 214 nm; H oH
flow rate 1 mI/min; eluents HN
benzamide; QN o
A: 0.1% TFA in H20, B o
N-[(R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-1H-indol-3-y1)ethyl]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
Quinoline-3-boronic acid 494.6
Retention time: 7.64 min.
224 5'-Fluoro-3'-methoxy-4- 135 Column Purospher Star RP
C18 4.6x125 5pm; detec- N,
propoxybiphenyl-3-carboxylic
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; oH
methyl-1H-indol-3- flow rate 1 mI/min; eluents F HN~H
0
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B
o
0.1% TFA in ACN; gradient o
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1 H-indol-3-yl)ethyl]-5- 5% to 95% (10') to 95% (2)
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
209
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
491.6
3-Fluoro-5- Retention time: 10.6 min.
methoxyphenylboronic acid
225 4-Propoxy-3'-(2,2,2- 135 Column Purospher Star RP
trifluoroethoxy)biphenyl-3- C1 8 4.6x1 25 5pm; detec- "-
tion wavelength 214 nm; F F oH
carboxylic acid [(R)-2-hydroxy- F~ H
1-(1-methyl-1H-indol-3- flow rate 1 ml/min; eluents o "" o
ylmethyl)ethyl]amide; amide~ A: 0.1% TFA in H20, B
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methy1-lH-indol-3-yl)ethylJ-5- 5% to 95% (10') to 95% (2)
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-(2, 2, 2-Trifluorethoxy)phenyl- 541.6
boronic acid Retention time: 10.89 min.
226 5'-Ethoxy-2'-fluoro-4- 135 Column Purospher Star RP \
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N_
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; oH
methyl-1 H-indol-3- flow rate 1 mI/min; eluents HN H 0
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B ~SO
0.1 % TFA in ACN; gradient o
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1 H-indol-3-yl)ethylJ-5-
5%to95%(10')to95%(2)
iodo-2-propoxybenzamide o 0
to5/o(0.5)to5/o(2.5)
and Molecular peak (ESI, M+1):
2-Fluoro-5-
505.6
ethoxyphenylboronic acid Retention time:.10.9 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
210
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
Column Purospher Star RP F
227 4'-Methoxy-4-propoxybiphenyl- 135
3-carboxylic acid [2-(5-fluoro- C18 4.6x125 5pm; detec- NH
1H-indol-3-yl)-1- tion wavelength 214 nm; H oH
hydroxymethylethyl]amide; flow rate 1 ml/min; eluents HN
0
A: 0.1 % TFA in H20, B ~~ o
N-[2-(5-Fluoro- 1 H-in dol- 3-yl) - 1 - 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-Methoxyphenylboronic acid 477.5
Retention time: 9.78 min.
228 5-Benzofuran-2-yl-N-[2-(5- 135 Column Purospher Star RP F I
C18 4.6x125 5pm; detec- NH
fluoro-1 H-indol-3-yl)-1-
tion wavelength 214 nm;
hydroxymethylethyl]-2- H oH
propoxybenzamide; flow rate 1 ml/min; eluents HN o
A: 0.1 % TFA in H20, B
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
Benzo(b]furan-2-boronic acid 487.5
Retention time: 10.58 min.
229 3'-Methyl-4-propoxybiphenyl-3- 135 Column Purospher Star RP F \
carboxylic acid [2-(5-fluoro-1 H- C18 4.6x125 5pm; detec- NH
indol-3-yl)-1- tion wavelength 214 nm;
OH
hydroxymethylethyl]amide; flow rate 1 ml/min; eluents HN H
A: 0.1 % TFA in H20, B o
N-[2-(5-Fluoro-lH-indol-3-yl)-1- 0.1% TFA in ACN; gradient o
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
211
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gousto 'H-NMR (400 MHz) 8 [ppm]
and Molecular peak (ESI, M+1):
461.5
3-Methylphenylboronic acid Retention time: 10.36 min.
Column Purospher Star RP F
230 5-Benzo[b]thiophene-2-yl-N-[2- 135
(5-fluoro-1 H-indol-3-yl)-1- C18 4.6x125 5pm; detec- NH
hydroxymethylethyl]-2- tion wavelength 214 nm; H OH
propoxybenzamide; flow rate 1 ml/min; eluents HN o
A: 0.1 % TFA in H20, B
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
Benzo[b]thiophene-2-boronic 503.6
acid Retention time: 10.92 min.
231 2'-Fluoro-5'-methoxy-4- 135 Column Purospher Star RP F \
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- I NH
acid [1-(5-fluoro-1 H-indol-3- tion wavelength 214 nm; OH
ylmethyl)-2- flow rate 1 mI/min; eluents HN H
F o
hydroxyethyl]amide; A: 0.1% TFA in H20, B Q 0.1 % TFA in ACN; gradient o o
N-[2-(5-Fluoro-lH-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
495.5
2-Fluoro-5-methoxyphenyl- Retention time: 9.86 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
212
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents gous to
232 4-Propoxy-3',5'-bis- 135 Column Purospher Star RP F
trifluoromethylbiphenyl-3- C18 4.6x125 5pm; detec- NH
carboxylic acid [1-(5-fluoro-1H- tion wavelength 214 nm; oH
indol-3- Imeth I-2- flow rate 1 mI/min; eluents F F F HN H
y y) o
hydroxyethyl]amide; A: 0.1% TFA in H20, B o
0.1 % TFA in ACN; gradient F ~
N-[2-(5-Fluoro-1H-indol-3-yl)-1- F F
in each case based on B:
hydroxymeth ylethyl]-5-iodo-2-
5% to 95% (10') to 95% (2)
propoxybenzamide 5% 0
to5/o (0.5)to5/o (2.5)
and Molecular peak (ESI, M+1):
3, 5-Bistrifluoromethylphenyl- 583.5
boronic acid
Retention time: 10.97 min.
233 N-[2-(5-Fluoro-1H-indol-3-yl)-1- 135 Column Purospher Star RP F
hydroxymethylethyl]-2-propoxy- C18 4.6x125 5pm; detec- NH
5-pyridin-3-yl-benzamide; tion wavelength 214 nm; oH
flow rate 1 mi/min; eluents
HN H
N-[2-(5-Fluoro-1H-indol-3-yl)-1- A: 0.1% TFA in H20, B N o
hydroxymethylethyl]-5-iodo-2- 0.1 % TFA in ACN; gradient o
propoxybenzamide in each case based on B:
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
Pyridine-3-boronic acid Molecular peak (ESI, M+1):
448.5
Retention time: 6.26 min.
234 5-Benzo[b]thiophene-3-yl-N-[2- 135 Column Purospher Star RP F
(5-fluoro-1H-indol-3-yl)-1- C18 4.6x125 5pm; detec- I NH
hydroxymethylethyl]-2- tion wavelength 214 nm; oH
H
propoxybenzamide; flow rate 1 ml/min; eluents HN
A: 0.1 % TFA in H20, B
N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 0.1 % TFA in ACN; gradient S
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
213
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
503.6
Benzo[b]thiophene-3-boronic Retention time: 10.63 min.
acid
235 3'-Cyano-4'-fluoro-4- 135 Column Purospher Star RP F I
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [1-(5-fluoro-1 H-indol-3- tion wavelength 214 nm; oH
ylmethyl)-2- flow rate 1 mI/min; eluents N HN H
0
hydroxyethyl]amide; A: 0.1% TFA in H20, B F o
0.1 % TFA in ACN; gradient
N-[2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
490.5
3-Cyano-4-fluorophenylboronic Retention time: 9.52 min.
acid
236 N-[1-(5-Fluoro-1H-indol-3- 135 Column Purospher Star RP F 1
ylmethyl)-2-hydroxyethyl]-5-(6- C1 8 4.6x1 25 5pm; detec- H
tion wavelength 214 nm; OH
fluoro-5-methylpyridine-3-yl)-2-
H
propoxybenzamide; flow rate 1 ml/min; eluents N_ HN o
A: 0.1% TFA in H20, B F\ o
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
2-Fluoro-3-methylpyridine-5- 480.5
boronic acid Retention time: 9.26 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
214
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents yous to H-NMR (400 MHz) S[ppm]
Column Purospher Star RP F
237 N-[2-(5-Fluoro-1H-indol-3-yl)-1- 135
I
hydroxymethylethyl]-5-(6- C18 4.6x125 5pm; detec- NH
tion wavelength
methoxypyridine-3-yl)-2- 214 nm; oH
flow rate 1 ml/min; eluents HN
propoxybenzamide; N
o
A: 0.1 % TFA in H20, B ~_~ 0
N-[2-(5-Fluoro-lH-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2-Methoxypyridine-5-boronic 478.5
acid Retention time: 8.77 min.
238 3'-Chloro-4'-fluoro-4- 135 Column Purospher Star RP F I~
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- H
tion wavelength 214 nm; OH
acid [1-(5-fluoro-1H-indol-3-
ylmethyl)-2- flow rate 1 mI/min; eluents ~~ HN o
A: 0.1% TFA in H20, B F~~ ~~
hydroxyethyl]amide; - _ 0
0.1 % TFA in ACN; gradient
N-[2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-Chloro-4-fluorophenylboronic 500
acid Retention time: 10.54 min.
239 3'-Acetylamino-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x1 25 5pm; detec- NH
acid [2-(5-fluoro-1 H-indol-3-yl)- tion wavelength 214 nm; oH
flow rate 1 ml/min; eluents "IN HN H
1-hydroxymethylethyl]amide; o
A: 0.1% TFA in H20, B b_c~o
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
215
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S [PPm]
and to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
3-Acetamidophenylboronic acid 504.6
Retention time: 8.37 min.
240 3',4'-Difluoro-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
tion wavelength 214 nm; oH
acid [1 -(5-fluoro-1 H-indol-3-
ylmethyl)-2- flow rate 1 mi/min; eluents F HN H
hydroxyethyl]amide; A: 0. 1 % TFA in H20, B F o
0.1 % TFA in ACN; gradient
N-(2-(5-Fluoro-lH-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2)
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
483.5
3,4-Difluorophenylboronic acid Retention time: 10.08 min.
241 3',5'-Difluoro-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [2-(5-fluoro-1 H-indol-3-yl)- tion wavelength 214 nm;
OH
1 -hydroxymethylethyl]amide; flow rate 1 ml/min; eluents F HN H
A: 0.1 % TFA in H20, B 0-- o
/
N-(2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient _
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3, 5-Difluorophenylboronic acid 483.5
Retention time: 10.07 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
216
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
242 2',5'-Difluoro-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [1 -(5-fluoro-1 H-indol-3- tion wavelength 214 nm; oH
ylmethyl)-2- flow rate 1 mI/min; eluents F HN H
hydroxyethyl]amide; A: 0. 1 % TFA in H20, B o
0.1 % TFA in ACN; gradient 0
N-[2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
483.5
2,5-Difluorophenylboronic acid Retention time: 9.95 min.
Column Purospher Star RP F
243 N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 135
hydroxymethylethyl]-5-[(E)-2- C18 4.6x125 5pm; detec- NH
(4-fluorophenyl)-vinyl]-2- tion wavelength 214 nm; OH
flow rate 1 mI/min; eluents HN o H propoxybenzamide; o
A: 0.1 % TFA in H20, B F~~
N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 0.1 % TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
and to 5% (0.5') to 5% (2.5')
(E)-2-(4-Fluorphenyl)vinyl- Molecular peak (ESI, M+1):
boronic acid 491.5
Retention time: 10.46 min.
244 5-(5-Cyano-thiophene-2-yl)-N- 135 Column Purospher Star RP F
[2-(5-fluoro-1H-indol-3-yl)-1- C18 4.6x125 5pm; detec- I NH
hydroxymethylethyl]-2- tion wavelength 214 nm; oH
propoxybenzamide; flow rate 1 ml/min; eluents N HN H
0
A: 0.1 % TFA in H20, B
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
217
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents yous to H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
478.6
5-Cyanothiophene-2-boronic Retention time: 9.5 min.
acid
245 2'-Fluoro-3'-methoxy-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- I NH
acid [1-(5-fluoro-1 H-indol-3- tion wavelength 214 nm; OH
ylmethyl)-2- flow rate 1 mI/min; eluents _o HN H
0
hydroxyethyl]amide; A: 0. 1 % TFA in H20, B
\ i o
0.1% TFA in ACN; gradient
N-[2-(5-Fluoro-1 H-indol-3-yl)-1-
in each case based on B:
h yd rox ym e th yl e th yl]- 5-i o d o- 2-
5%to95%(10')to95%(2')
propoxybenzamide o o
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
2-Fluoro-3-methoxyphenyl- 495.5
boronic acid
Retention time: 9.57 min.
Column Purospher Star RP F
246 N-[2-(5-Fluoro-1H-indol-3-yl)-1- 135
hydroxymethylethyl]-5-(2- C18 4.6x125 5pm; detec- NH
tion wavelength
methoxypyrimidin-5-yl)-2- 214 nm; OH
flow rate 1 mI/min; eluents HN H.
propoxybenzamide;
A: 0.1% TFA in H20, B 0~N o
N-(2-(5-Fluoro-lH-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
2-Methoxypyrimidine-5-boronic 479.5
acid Retention time: 8.27 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
218
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) 8 [ppm]
Column Purospher Star RP F
247 N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 135
hydroxymethylethyl]-2-propoxy- C18 4.6x125 5pm; detec- NH
tion wavelength 214 nm; oH
5-quinoline-3-yl-benzamide; H
flow rate 1 mi/min; eluents "N
N-[2-(5-Fluoro-1H-indol-3-yl)-1- A: 0.1% TFA in H20, B ;NN r !\ o
hydroxymethylethyl]-5-iodo-2- 0.1 % TFA in ACN; gradient
propoxybenzamide in each case based on B:
5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
Quinoline-3-boronic acid Molecular peak (ESI, M+1):
498.6
Retention time: 7.05 min.
Column Purospher Star RP F
248 4-Propoxy-3'-(2,2,2- 135
trifluoroethoxy)biphenyl-3- C18 4.6x125 5pm; detec- NH
tion wavelength 214 nm;
carboxylic acid [1-(5-fluoro-1 H- F~ H. OH
indol-3-ylmethyl)-2- flow rate 1 ml/min; eluents O HN
hydroxyethyl]amide; A: 0.1 % TFA in H20, B
0.1 % TFA in ACN; gradient
N-(2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-(2, 2, 2-Trifluoroethoxy)- 545.5
phenylboronic acid Retention time: 10.27 min.
249 5'-Ethoxy-2'-fluoro-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- I NH
acid [1-(5-fluoro-1 H-indol-3- tion wavelength 214 nm; OH
ylmethyl)-2- flow rate 1 mI/min; eluents F HN H.
/\ \
hydroxyethyl]amide; A: 0. 1 % TFA in H20, B
_
0.1 % TFA in ACN; gradient j r
N-[2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
219
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
509.6
2-Fluoro-5- Retention time: 10.33 min.
ethoxyphenylboronic acid
250 3'-Methoxy-4-propoxybiphenyl- 135 Column Purospher Star RP
3-carboxylic acid [(R)-1- C18 4.6x125 5pm; detec- N,
hydroxymethyl-2-(1-methyl-1 H- tion wavelength 214 nm; oH
indol-3-yl)ethyl]amide; flow rate 1 mI/min; eluents -o HN o
A: 0.1 % TFA in H20, B
- _ o
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-lH-indol-3-y1)ethylJ-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Methoxyphenylboronic acid 473.6
Retention time: 10.31 min.
251 3'-Chloro-4-propoxybiphenyl-3- 135 Column Purospher Star RP
carboxylic acid [(R)-2-hydroxy- C18 4.6x125 5pm; detec- N,
1-(1-methyl-1H-indol-3- tion wavelength 214 nm; oH
ylmethyl)ethyl]amide; flow rate 1 mI/min; eluents ci HN' o
A: 0.1% TFA in H20, B \ ~ ~_\ o
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-1H-indol-3-y1)ethylJ-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Chlorophenylboronic acid 478
Retention time: 11.14 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
220
Product; Method HPLC-MS conditions I Structure
Ex. analo- I
MHz) reagents gous to H-NMR (400 S [ppm]
252 4-Propoxy-3',5'-bis- 135 Column Purospher Star RP
trifluoromethylbiphenyl-3- C18 4.6x125 5pm; detec- N-
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; OH
flow rate 1 mI/min; eluents FF F HN,H
1-(1-methyl-1 H-indol-3- o
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B ~~ o
0.1 % TFA in ACN; gradient FF F 11_~
N-[(R)-1-Hydroxymethyl-2-(1-
in each case based on B:
methyl-1 H-indol-3-yl)ethyl]-5-
5%to95%(10')to95%(2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3, 5-Bistrifluoromethylphenyl- 579.6
boronic acid
Retention time: 11.68 min.
253 3',4',5'-Trifluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic Cl 8 4.6x1 25 5pm; detec- N,
tion wavelength 214 nm; oH
acid [(R)-2-hydroxy-1-(1-
methyl-1 H-indol-3- flow rate 1 mI/min; eluents F HN o
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B F~ o
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-yl)ethyl]-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and 497.5
3,4,5-Trifluorophenylboronic Retention time: 11.01 min.
acid
254 4-Propoxy-4'- 135 Column Purospher Star RP
trifluoromethoxybiphenyl-3- C18 4.6x125 5pm; detec- N-
tion wavelength
carboxylic acid [(R)-2-hydroxy- 214 nm; H OH
1-(1-methyl-1H-indol-3- flow rate 1 ml/min; eluents HN o
\,
ylmethyl)ethyl]amide; A: 0.1% TFA in H20, B FF F 0
0.1 % TFA in ACN; gradient
N-[(R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-yl)ethyl]-5- 5% to 95% (10') to 95% (2)
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
221
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
527.6
4-Trifluoromethoxyphenyl- Retention time: 11.2 min.
boronic acid
255 4-Propoxy-4'- 135 Column Purospher Star RP
trifluoromethylbiphenyl-3- C18 4.6x125 5pm; detec- N-
carboxylic acid [(R)-2-hydroxy- tion wavelength 214 nm; oH
1-(1-methyl-1H-indol-3- flow rate 1 ml/min; eluents F HN' o
o
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B FF \
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-y1)ethyl]-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
511.6
4-Trifluoromethylphenylboronic
Retention time: 11.00 min.
acid
256 5-(6-Fluoro-5-methylpyridine-3- 135 Column Purospher Star RP
yl)-N-[(R)-2-hydroxy-1-(1- C18 4.6x125 5pm; detec- N-
methyl-1 H-indol-3- tion wavelength 214 nm; oH
ylmethyl)ethyl]-2- flow rate 1 ml/min; eluents N_ HN' o
roPoxYbenzamide, A: 0.1% TFA in H20, B F\ propoxybenzamide; o
~
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-y1)ethyl]-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
476.6
2-Fluoro-3-methylpyridine-5- Retention time: 10.02 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
222
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
257 5-(3,5-Dimethyl-isoxazol-4-yl)- 135 Column Purospher Star RP
N-[(R)-1-hydroxymethyl-2-(1- C18 4.6x125 5pm; detec- N,
methyl-1 H-indol-3-yl)ethyl]-2- tion wavelength 214 nm; oH
flow rate 1 mI/min; eluents HN H
propoxybenzamide;
A: 0.1% TFA in H20, B N o
'
N-((R)-1-Hydroxymethyl-2-(1- 0.1 % TFA in ACN; gradient o~ 0
methyl-1H-indol-3-yl)ethylJ-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3, 5-Dimethylisoxazole-4- 462.6
boronic acid Retention time: 9.32 min.
258 3'-Chloro-4'-fluoro-4- 135 Column Purospher Star RP
C18 4.6x125 5pm; detec- I N-
propoxybiphenyl-3-carboxylic
tion wavelength 214 nm; oH
acid [(R)-2-hydroxy-1-(1-
methyl-1 H-indol-3- flow rate 1 ml/min; eluents oi HNH
A:
ylmethyl)ethyl]amide; 0.1 % TFA in H20, B F!~
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-yl)ethylJ-5- 5% to 95% (10') to 95% (2)
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and 496
3-Chloro-4-fluorophenylboronic Retention time: 11.22 min.
acid
259 3'-Cyano-4-propoxybiphenyl-3- 135 Column Purospher Star RP
carboxylic acid [(R)-1- C18 4.6x125 5pm; detec- N,
hydroxymethyl-2-(1-methyl-1H- tion wavelength 214 nm; oH
indol-3-yl)ethyl]amide; flow rate 1 mI/min; eluents N HN'~H
A: 0.1% TFA in H20, B o
N-((R)-1-Hydroxymethyl-2-(1- 0.1 % TFA in ACN; gradient 0
methyl-lH-indol-3-yl)ethylJ-5- in each case based on B:
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
223
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents yo~s to
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-Cyanophenylboronic acid 468.6
Retention time: 10.1 min.
260 2',3'-Difluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- ~ i N,
tion wavelength 214 nm;
acid [(R)-2-hydroxy-1-(1-
OH
methyl-1 H-indol-3- flow rate 1 ml/min; eluents F HN'~H
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B o
0.1 % TFA in ACN; gradient o
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-yl)ethyl)-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
479.5
2,3-Difluorophenylboronic acid Retention time: 10.54 min.
261 3',5'-Dimethyl-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N,
acid [(R)-1-hydroxymethyl-2-(1- tion wavelength 214 nm; OH
methyl-1 H-indol-3- flow rate 1 mI/min; eluents HN'H
0
yl)ethyl]amide; A: 0.1 % TFA in H20, B o
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-yl)ethylJ-5- 5% to 95% (10') to 95% (2)
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
471.6
3,5-Dimethylphenylboronic acid Retention time: 10.18 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
224
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents 9ous to H-NMR (400 MHz) S[ppm]
262 3'-Ethoxy-5'-fluoro-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- N,
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; oH
methyl-1 H-indol-3- flow rate 1 mI/min; eluents F HN H
A: 0.1 % TFA in H20, B \ ~ o
ylmethyl)ethyl]amide; \
0
0.1 % TFA in ACN; gradient j -
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-1H-indol-3-yl)ethylJ-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
505.6
5-Ethoxy-3-fluorophenylboronic Retention time: 10.98 min.
acid
263 5'-Fluoro-3'-hydroxy-4- 135 Column Purospher Star RP
C18 4.6x125 5pm; detec- ~ N,
propoxybiphenyl-3-carboxylic
acid [(R)-2-hydroxy-1-(1- tion wavelength 214 nm; oH
methyl-1 H-indol-3- flow rate 1 mI/min; eluents F HN' o
ylmethyl)ethyl]amide; A: 0.1 % TFA in H20, B O_C~
o 0.1 % TFA in ACN; gradient Ho
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-y1)ethylJ-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-Fluoro-5- 477.5
hydroxyphenylboronic acid Retention time: 9.5 min.
264 4,3'-Dipropoxybiphenyl-3- 135 Column Purospher Star RP
carboxylic acid [(R)-1- C18 4.6x125 5pm; detec- N-
tion wavelength
hydroxymethyl-2-(1-methyl-1H- 214 nm; H oH
indol-3-yl)ethyl]amide; flow rate 1 ml/min; eluents HN 0
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-lH-indol-3-yl)ethylJ-5- in each case based on B:
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
225
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents 9ousto H-NMR (400 MHz) S[ppm]
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-Propoxyphenylboronic acid 501.6
Retention time: 11.21 min.
265 3'-Chloro-4-propoxybiphenyl-3- 135 Column Purospher Star RP F
carboxylic acid [2-(5-fluoro-1 H- C18 4.6x125 5pm; detec- NH
indol-3-yl)-1- tion wavelength 214 nm;
OH
flow
hydroxymethylethyl]amide; rate 1 ml/min; eluents cb-C HN H
A: 0.1 % TFA in H20, B o
N-[ 2-(5-Fluoro-lH-indol-3-yl)-1- 0.1% TFA in ACN; gradient o
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Chlorophenylboronic acid 482
Retention time: 10.42 min.
266 3'-Fluoro-4'-methyl-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- I NH
acid [1 -(5-fluoro-1 H-indol-3- tion wavelength 214 nm; OH
ylmethyl)-2- flow rate 1 ml/min; eluents F HN H
0
hydroxyethyl]amide; A: 0.1% TFA in H20, B
0.1 % TFA in ACN; gradient
N-[2-(5-Fluoro-lH-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
479.5
3-Fluoro-4-methylphenyl- Retention time: 10.08 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
226
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
267 2'-Fluoro-4'-methyl-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [1-(5-fluoro-1H-indol-3- tion wavelength 214 nm; oH
ylmethyl)-2- flow rate 1 mI/min; eluents F HN H
0
hydroxyethyl]amide; A: 0.1 % TFA in H20, B i\ ~\ o
0.1 % TFA in ACN; gradient
N-[2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2)
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
479.5
2-Fluoro-4-methylphenyl- Retention time: 10.27 min.
boronic acid
268 4-Propoxy-3'- 135 Column Purospher Star RP F
trifluoromethylbiphenyl-3- C18 4.6x125 5pm; detec- NH
carboxylic acid [1-(5-fluoro-1 H- tion wavelength 214 nm; oH
flow rate 1 mI/min; eluents HN
indol-3-ylmethyl)-2- FF F o
hydroxyethyl]amide; A: 0.1 % TFA in H20, B \- \
0.1 % TFA in ACN; gradient
N-(2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and 515,5
3-Trifluoromethylphenylboronic Retention time: 10,41 min.
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
227
Product; Method HPLC-MS conditions / Structure
Ex. reagents goanalo- us to 'H-NMR (400 MHz) S[ppm]
269 3'-Isopropyl-4-propoxybiphenyl- 135 Column Purospher Star RP F
3-carboxylic acid [2-(5-fluoro- C18 4.6x125 5pm; detec- NH
1H-indol-3-yl)-1- tion wavelength 214 nm; OH
hydroxymethylethyl]amide; flow rate 1 mI/min; eluents HN H
0
A: 0.1 % TFA in H20, B
- _ o
N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 0.1 % TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Isopropylphenylboronic acid 489.6
Retention time: 10.96 min.
Column Purospher Star RP F
270 3'-Methylsulphanyl-4- 135
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
tion wavelength 214 nm; OH
acid [2-(5-fluoro-1 H-indol-3-yl)-
1-hydroxymethylethyl]amide; flow rate 1 mI/min; eluents _S HN H
0
A: 0.1 % TFA in H20, B
- _ o
N-(2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3- 493.6
Methylsulphanylphenylboronic Retention time: 10.31 min.
acid
Column Purospher Star RP F
271 4-Propoxy-4'- 135
trifluoromethoxybiphenyl-3- C18 4.6x125 5pm; detec- NH
tion wavelength
carboxylic acid [1-(5-fluoro-1 H- 214 nm; oH
HN H
o
indol-3-ylmethyl)-2- flow rate 1 ml/min; eluents 0
hydroxyethyl]amide; A: 0.1 % TFA in H20, B FF ~~ 0
0.1% TFA in ACN; gradient
N-[2-(5-Fluoro-lH-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
228
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
531.5
4-Trifluoromethoxyphenyl- Retention time: 10.69 min.
boronic acid
Column Purospher Star RP F
272 N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 135
hydroxymethylethyl]-2-propoxy- C18 4.6x125 5pm; detec- NH
tion wavelength 214 nm; OH
5-quinoline-6-yl-benzamide; H
flow rate 1 ml/min; eluents HN o
N-[2-(5-Fluoro-lH-indol-3-yl)-1- A: 0.1% TFA in H20, B .N p<~ o
hydroxymethylethyl]-5-iodo-2- 0.1 % TFA in ACN; gradient
propoxybenzamide in each case based on B:
5% to 95% (10') to 95% (2)
and
to 5% (0.5') to 5% (2.5')
Quinoline-6-boronic acid Molecular peak (ESI, M+1):
498.6
Retention time: 6.9 min.
\
273 3'-Chloro-4'-methyl-4- 135 Column Purospher Star RP F I
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [2-(5-fluoro-1 H-indol-3-yl)- tion wavelength 214 nm; oH
1 -hydroxymethylethyl]amide; flow rate 1 mUmin; eluents ci HN H
A: 0.1% TFA in H20, B
0
N-(2-(5-Fluoro-1H-indol-3-y1)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
and to 5% (0.5') to 5% (2.5')
3-Chloro-4- Molecular peak (ESI, M+1):
methylphenylboronic acid 496
Retention time: 11.03 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
229
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
274 5-(3,5-Dimethyl-isoxazol-4-yl)- 135 Column Purospher Star RP F
N-[2-(5-fluoro-1H-indol-3-yl)-1- C18 4.6x125 5pm; detec- NH
hydroxymethylethyl]-2- tion wavelength 214 nm; OH
propoxybenzamide; flow rate 1 ml/min; eluents HN H
A: 0.1% TFA in H20, B N o
N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 0.1 % TFA in ACN; gradient ~ o
hydroxymethylethyl]-5-iodo-2-
in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
and
to 5% (0.5') to 5% (2.5')
3, 5-Dimethylisoxazole-4-
Molecular peak (ESI, M+1):
boronic acid
466.5
Retention time: 8.77 min.
275 2',3'-Difluoro-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- I~ NH
acid [1-(5-fluoro-1H-indol-3- tion wavelength 214 nm; OH
ylmethyl)-2- flow rate 1 ml/min; eluents 6-C HN H
hydroxyethyl]amide; A: 0.1 % TFA in H20, B o
0.1 % TFA in ACN; gradient
N-[2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and 483.5
2,3-Difluorophenylboronic acid Retention time: 10.05 min.
276 3',5'-Dimethyl-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [2-(5-fluoro-1H-indol-3-yl)- tion wavelength 214 nm; OH
1-hydroxymethylethyl]amide; flow rate 1 ml/min; eluents HN H
A: 0.1% TFA in H20, B o
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient \~ ~\ o
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
230
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents 9ous to H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
475.6
3,5-Dimethylphenylboronic acid Retention time: 10.71 min.
277 5'-Ethoxy-3'-fluoro-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [2-(5-fluoro-1 H-indol-3-yl)- tion wavelength 214 nm; oH
1-hydroxymethylethyl]amide; flow rate 1 mI/min; eluents F HN H
0
A: 0.1 % TFA in H20, B 0-
hydroxymethylethyl]-5-iodo-2- I N-[2-(5-Fluoro-1 H-indol-3-yl)-1- 0.1 % TFA in
ACN; gradient o in each case based on B: ~
propoxybenzamide 5% to 95% (10') to 95% (2')
and to 5% (0.5') to 5% (2.5')
5-Ethoxy-3-fluorphenylboronic Molecular peak (ESI, M+1):
acid
509.6
Retention time: 10.41 min.
278 3'-Fluoro-5'-hydroxy-4- 135 Column Purospher Star RP F \
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [2-(5-fluoro-1 H-indol-3-yl)- tion wavelength 214 nm; oH
1-hydroxymethylethyl]amide; flow rate 1 ml/min; eluents F HN H
0
A: 0.1 % TFA in H20, B /~ ~ o
N-(2-(5-Fluoro-lH-indol-3-yl)-1- 0.1% TFA in ACN; gradient Ho -
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
and to 5% (0.5') to 5% (2.5')
3-Fluoro-5- Molecular peak (ESI, M+1):
hydroxyphenylboronic acid 481.5
Retention time: 9.03 min.
Column Purospher Star RP F
279 4,3'-Dipropoxybiphenyl-3- 135
carboxylic acid [2-(5-fluoro-1 H- C18 4.6x125 5pm; detec- NH
indol-3-yl)-1- tion wavelength 214 nm; OH
flow rate 1 mI/min; eluents HN H
hydroxymethylethyl]amide;
A: 0.1 % TFA in H20, B
N-(2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
231
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents gous to
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-n-Propoxyphenylboronic acid 505.6
Retention time: 10.67 min.
280 3'-Ethoxy-4-propoxybiphenyl-3- 135 Column Purospher Star RP F Q,NH
carboxylic acid [2-(5-fluoro-1 HC18 4.6x125 5Nm; detec- tion wavelength 214
nm;
indol-3-yl)-1- H
hydroxymethylethyl]amide; flow rate 1 mi/min; eluents \_ HN H
0
A: 0.1 % TFA in H20, B
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Ethoxyphenylboronic acid 491.6
Retention time: 10.29 min.
281 4'-Hydroxymethyl-4- 135 Column Purospher Star RP H
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- - N
H
acid [(R)-1-hydroxymethyl-2- tion wavelength 214 nm; HO HN
flow rate 1 ml/min; eluents
(1 H-indol-3-yl)ethyl]amide; _O
A: 0.1 % TFA in H20, B
N-((R)-1-Hydroxymethyl-2-(1 H- 0.1 % TFA in ACN; gradient
OH
indol-3-y1)ethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2)
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-(Hydroxymethyl)-phenyl- 459.5
boronic acid Retention time: 8.16 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
232
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents gous to
282 3'-Hydroxymethyl-4- 135 Column Purospher Star RP _ H
N
propoxybiphenyl-3-carboxylic C1 8 4.6x1 25 5pm; detec- ~~
HO
acid[(R)-1-hydroxymethyl-2- tion wavelength 214 nm; HNH
flow rate 1 ml/min; eluents
(1 H-indol-3-yl)ethyl]amide; -O
A: 0.1 % TFA in H20, B H
N-((R)-2-Hydroxy-l-(1 H-indol-3- 0.1% TFA in ACN; gradient
ylmethyl)ethylJ-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-(Hydroxymethyl)- 459.5
phenylboronic acid Retention time: 8.28 min.
283 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP H
dicarboxylic acid 3-{[(R)-1- C18 4.6x125 5pm; detec- N
hydroxymethyl-2-(1 H-indol-3- tion wavelength 214 nm; Ho ! H
yl)ethyl]amide} 4'-methylamide; flow rate 1 ml/min; eluents HN
A: 0.1 % TFA in H20, B
N-[(R)-2-Hydroxy-l-(1H-indol-3- 0.1% TFA in ACN; gradient
ylmethyl)ethylJ-5-iodo-2- in each case based on B: o
propoxybenzamide 5% to 95% (10') to 95% (2) HN
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
4-(N-Methylaminocarbonyl)- 486.5
phenylboronic acid Retention time: 7.84 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
233
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents sousto H-NMR (400 MHz) S[ppm]
284 N-[(R)-2-Hydroxy-1-(1 H-indol-3- 135 Column Purospher Star RP qj1
C18 4.6x125 5Nm; detec- ylmethyl)ethyl]-5-(5- ylmethyl)ethyl]-5-(5-
hydroxymethylwavelength 214 nm; HO
flow rate 1 ml/min; eluents H" 0
propoxybenzamide;
A: 0.1% TFA in H20, B ~~O
N-((R)-2-Hydroxy-1-(1H-indol-3- 0.1% TFA in ACN; gradient
ylmethyl)ethyl]-5-iod-2- in each case based on B: s ~
propoxybenzamide 5% to 95% (10') to 95% (2')
HO
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
5-(Hydroxymethyl)thiophene-2- 465.5
boronic acid Retention time: 8.21 min.
285 5'-Fluoro-4-propoxybiphenyl- 135 Column Purospher Star RP H
C18 4.6x125 5pm; detec- "
3,3'-dicarboxylic acid 3-{[(R)-2-
hydroxy-1-(1H-indol-3- tion wavelength 214 nm; HO H
ylmethyl)ethyl]amide} 3'-methyl- flow rate 1 mI/min; eluents --\,o" o
amide; A: 0.1% TFA in H20, B
0.1 % TFA in ACN; gradient F
N-((R)-2-Hydroxy-l-(1H-indol-3- in each case based on B:
ImethY)1 ethYI1-5-~ ~odo-2- 5% to 95% 10' to 95% (2')
"~ o
Y ( ) propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
504.5
3-Fluoro-5-(methylcarbamoyl)- Retention time: 8.46 min.
phenylboronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
234
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
286 3'-Chloro-4-propoxybiphenyl- 135 Column Purospher Star RP H
C18 4.6x125 5Nm; detec- N
3,4'-dicarboxylic acid 3-{[(R)-2-
hydroxy-1-(1 H-indol-3- tion wavelength 214 nm; "O H
ylmethyl)ethyl]amide} 4'-methyl- flow rate 1 mI/min; eluents H ~o 0
A: 0.1 % TFA in H20, B
amide;
0.1% TFA in ACN; gradient / c'
N-((R)-2-Hydroxy-1-(1H-indol-3- in each case based on B:
HN~
ylmethyl)ethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and 521
3-Chloro-4-(N- Retention time: 8.11 min.
methylcarbamoyl)-
phenylboronic acid
287 3'-Hydroxymethyl-4- 135 Column Purospher Star RP
propoxybiphenyl-3-carboxylic C1 8 4.6x1 25 5pm; detec- N-
acid [(R)-1-hydroxymethyl-2-(1- tion wavelength 214 nm; OH
methyl-1 H-indol-3- flow rate 1 mi/min; eluents HO "N o
yl)ethyl]amide; A: 0.1 % TFA in H20, B
0.1% TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1- in each case based on B:
methyl-lH-indol-3-yl)ethyl]-5- 5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
473.6
3-Hydroxymethylphenylboronic Retention time: 8.92 min.
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
235
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
288 3'-Hydroxymethyl-4- 135 Column Purospher Star RP F
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [2-(5-fluoro-1 H-indol-3-yl)- tion wavelength 214 nm; oH
1-hydroxymethylethyl]amide; flow rate 1 mI/min; eluents HO HN H
o
A: 0.1 % TFA in H20, B ~\ r\
- _ o
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Hydroxymethylphenylboronic 477.5
acid Retention time: 8.45 min.
Column Purospher Star RP F
289 3'-Chloro-4-propoxybiphenyl- 135
3,4'-dicarboxylic acid 3-{[2-(5- C18 4.6x125 5pm; detec- NH
fluoro-1 H-indol-3-yl)-1- tion wavelength 214 nm; OH
HN H
hydroxymethylethyl]amide} 4'- flow rate 1 mI/min; eluents o cl o 11
methylamide;
A: 0.1 % TFA in H20, B -H ~
0.1 % TFA in ACN; gradient
N-[2-(5-Fluoro-1 H-indol-3-yl)-1-
in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-Chloro-4-(methylamino- 539
carbonyl)phenylboronic acid
Retention time: 8.2 min.
290 N-[(R)-2-Hydroxy-1-(1-methyl- 135 Column Purospher Star RP
1 H-indol-3-ylmethyl)ethyl]-5-(5- C18 4.6x125 5pm; detec- N-
hydroxymethylthiophen-2-yl)-2- tion wavelength 214 nm; OH
propoxybenzamide; flow rate 1 mI/min; eluents Ho HN' o
A: 0.1% TFA in H20, B ~o
N-((R)-1-Hydroxymethyl-2-(1- 0.1% TFA in ACN; gradient
methyl-1H-indol-3-yl)ethyl]-5- in each case based on B:
iodo-2-propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
236
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
and Molecular peak (ESI, M+1):
479.6
5-(Hydroxymethyl)thiophene-2- Retention time: 8.87 min.
boronic acid
291 3'-Chloro-4-propoxybiphenyl- 135 Column Purospher Star RP
3,4'-dicarboxylic acid 3-([(R)-2- C18 4.6x125 5pm; detec- N-
tion wavelength
hydroxy-1 -(1 -methyl-1 H-indol- 214 nm; H oH
flow rate 1 mI/min; eluents cl HN
3-yimethyl)ethyl]amide} 4'- 0 / \ / \ o
A: 0.1% TFA in H20, B -H ~
methylamide;
0.1 % TFA in ACN; gradient
N-((R)-1-Hydroxymethyl-2-(1-
in each case based on B:
methyl-1 H-indol-3-yl)ethyl]-5-
5% to 95% (10') to 95% (2')
iodo-2-propoxybenzamide to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
3-Chloro-5-(methylcarbamoyl)- 535
phenylboronic acid
Retention time: 8.82 min.
Column Purospher Star RP F
292 4'-Hydroxymethyl-4- 135
propoxybiphenyl-3-carboxylic C18 4.6x125 5pm; detec- NH
acid [2-(5-fluoro-1 H-indol-3-yl)- tion wavelength 214 nm; oH
1-hydroxymethylethyl]amide; flow rate 1 mI/min; eluents HN o
_
A: 0.1% TFA in H20, B Ho ~/ /~ O
N-[2-(5-Fluoro-1H-indol-3-yl)-1- 0.1% TFA in ACN; gradient
hydroxymethylethyl]-5-iodo-2- in each case based on B:
propoxybenzamide 5% to 95% (10') to 95% (2')
to 5% (0.5') to 5% (2.5')
and Molecular peak (ESI, M+1):
4-Hydroxymethylphenylboronic 477.5
acid Retention time: 8.24 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
237
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents 9ous to H-NMR (400 MHz) S[ppm]
293 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP F
dicarboxylic acid 3-{[2-(5-fluoro- C18 4.6x125 5pm; detec- NH
1 H-indol-3-yl)-1- tion wavelength 214 nm; oH
HN H
hydroxymethylethyl]amide} 4'- flow rate 1 ml/min; eluents 0
A: 0.1% TFA in H20, B H ~ r - ~
methylamide;
0.1 % TFA in ACN; gradient
N-(2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B:
hydroxymethylethyl]-5-iodo-2- 5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1):
and
504.6
4-(Methylaminocarbonyl)- Retention time: 7.97 min.
phenylboronic acid
Column Purospher Star RP F
294 5'-Fluoro-4-propoxybiphenyl- 135
3,3'-dicarboxylic acid 3-{[2-(5- C18 4.6x125 5pm; detec- ~ NH
fluoro-1 H-indol-3-yl)-1- tion wavelength 214 nm; oH
hydroxymethylethyl]amide} 3'- flow rate 1 ml/min; eluents F HN H
methylamide; A: 0.1 % TFA in H20, B o
0.1 % TFA in ACN; gradient O NH
N-(2-(5-Fluoro-1H-indol-3-yl)-1- in each case based on B: hydroxymethylethylJ-
5-iodo-2-
5% to 95% (10') to 95% (2')
propoxybenzamide to 5% (0.5') to 5% (2.5')
and
Molecular peak (ESI, M+1):
3-Fluoro-5-(methylcarbamoyl)- 522.6
phenylboronic acid
Retention time: 8.62 min.
295 4-Ethoxy-3'-fluoro-N-[(R)-1- 39 (DMSO-d6): 10.82 s(1H); OH (hydroxymethyl)-
2-(1 H-indol-3- 8.39 d (J = 8.1 Hz, 1 H); H N
H
yl)ethyl]-4'-methoxy[1,1'- 8.12 d (J = 2.5 Hz, 1 H); F\
biphenyl]-3-carboxamide; 7.75 dd (J = 8.7 Hz / 2.5 'o
Hz, 1 H); 7.70 d (J = 8.0 Hz,
D-Tryptophanol and 1 H); 7.51 dd (J = 12.9 Hz /
4-Ethoxy-3' fluoro-4'- 2.2 Hz, 1 H); 7.43 d (J = 8.8
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
238
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents 9ous to
methoxy[l,1'-biphenyl]-3- Hz, 1 H); 7.34 d (J = 8.0 Hz,
carboxylic acid 1 H); 7.24 dd (J = 8.8 Hz /
8.8 Hz, 1 H); 7.19 d (J = 8.7
Hz, 1 H); 7.16 d (J = 2.3 Hz,
1 H); 7.06 dd (J = 8.0 Hz /
7.0 Hz, 1 H); 6.97 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 4.93
m(1 H); 4.26 m(1 H); 4.15
m (2H); 3.88 s (3H); 3.50 m
(1 H); 3.45 m(1 H); 3.00 dd
(J = 14.4 Hz / 7.6 Hz, 1 H);
2.97 dd (J = 14.4 Hz / 5.8
Hz, 1 H); 1.31 t (J = 7.0 Hz,
3H).
296 4-Ethoxy-N-[(R)-1- 39 (DMSO-d6): 10.82 s(1H); OH
(hydroxymethyl)-2-(1 H-indol-3- 8.40 d (J = 8.1 Hz, 1 H); o NH N
H
yI)ethyl]-3'-methoxy[1,1'- 8.16 d (J = 2.5 Hz, 1 H); 0 biphenyl]-3-
carboxamide; 7.77 dd (J = 8.6 Hz / 2.5
Hz, 1 H); 7.70 d (J = 8.0 Hz,
D-Tryptophanol and 1 H); 7.38 dd (J = 8.3 Hz /
4-Ethoxy-3' methoxy(1,1 '- 7.8 Hz, 1 H); 7.34 d (J = 8.0
biphenylJ-3-carboxylic acid Hz, 1 H); 7.21 d (J = 8.6 Hz,
1 H); 7.19 d (J = 7.8 Hz,
1 H); 7.17 d (J = 2.3 Hz,
1 H); 7.14 dd (J = 2.5 Hz /
1.8 Hz, 1 H); 7.06 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 6.97
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.92 dd (J = 8.3 Hz /
2.5 Hz, 1 H); 4.93 m(1 H);
4.27 m(1 H); 4.16 m (2H);
3.83 s(3H); 3.50 m(1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
239
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents gous to
3.46 m(1 H); 3.01 dd (J =
14.4 Hz / 7.3 Hz, 1 H); 2.96
dd (J = 14.4 Hz / 6.3 Hz,
1 H); 1.32 t (J = 7.0 Hz, 3H).
297 4-Ethoxy-N-[(R)-1- 39 (DMSO-d6): 10.82 s(1H); OHT -
(hydroxymethyl)-2-(1 H-indol-3- 8.61 q (J = 4.6 Hz, 1 H); 0 NH ~ N
yI)ethyl]-M-methyl[1,1'- 8.41 d (J = 8.1 Hz, 1 H); 1 ol H
biphenyl]-3,3'-dicarboxamide; 8.25 d (J = 2.5 Hz, 1 H);
8.10 s(1 H); 7.84 dd (J =
D-Tryptophanol and N'
8.6 Hz / 2.5 Hz, 1 H); 7.80 d
4-Ethoxy-3' (J = 7.8 Hz, 1 H); 7.78 d (J =
[(methylamino)carbonyl](1,1'- 7.6 Hz, 1 H); 7.71 d (J = 8.0
biphenyl]-3-carboxylic acid Hz, 1 H); 7.54 dd (J = 7.8
Hz/7.6 Hz, 1H); 7.33 d(J
= 8.0 Hz, 1 H); 7.25 d (J =
8.6 Hz, 1 H); 7.17 d (J = 2.3
Hz, 1 H); 7.06 dd (J = 8.0
Hz / 7.0 Hz, 1 H); 6.97 dd (J
= 8.0 Hz / 7.0 Hz, 1 H); 4.94
m(1 H); 4.28 m(1 H); 4.17
m (2H); 3.51 m(1 H); 3.46
m(1 H); 3.01 dd (J = 14.4
Hz / 7.3 Hz, 1 H); 2.98 dd (J
= 14.4 Hz / 6.1 Hz, 1 H);
2.82 d (J = 4.6 Hz, 3H);
1.33 t (J = 7.0 Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
240
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
298 4-Ethoxy-N-[(R)-1- 39 (DMSO-d6): 10.82 s(1H);
(hydroxymethyl)-2-(1 H-indol-3- 8.41 d (J = 7.9 Hz, 1 H);
yl)ethyl]-3',4',5'-trimethoxy[1,1'- 8.14 d (J = 2.5 Hz, 1 H); NH
biphenyl]-3-carboxamide; 7.78 dd (J = 8.7 Hz / 2.5 HO NH
Hz, 1 H); 7.70 d (J = 8.0 Hz,
D-Tryptophanol and
1 H); 7.33 d (J = 8.0 Hz,
4-Efhoxy-3;4;5 1 H); 7.20 d (J = 8.7 Hz,
' I ~
trimethoxy(1,1 '-biphenylJ-3- 1 H); 7.17 s (1 H); 7.06 dd (J
carboxylic acid = 8.0 Hz / 7.0 Hz, 1 H); 6.97
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.86 s(2H); 4.93 m
(1 H); 4.26 m(1 H); 4.16 m
(2H); 3.86 s (6H); 3.69 s
(3H); 3.50 m(1 H); 3.45 m
(1 H); 3.01 dd (J = 14.2 Hz /
7.0 Hz, 1 H); 2.97 dd (J =
14.2 Hz / 6.2 Hz, 1 H); 1.32
t (J = 7.0 Hz, 3H).
299 4-Ethoxy-N-[(R)-1- 39 (DMSO-d6): 10.82 s(1H);
(hydroxymethyl)-2-(1 H-indol-3- 8.41 d (J = 8.1 Hz, 1 H);
NH
yI)ethyl]-3',4'-dimethoxy[1,1'- 8.13 d (J = 2.5 Hz, 1 H);
biphenyl]-3-carboxamide; 7.74 dd (J = 8.6 Hz / 2.5 Ho NH o
Hz, 1 H); 7.70 d (J = 8.0 Hz, o
D-Tryptophanol and 1 H); 7.33 d (J = 8.0 Hz,
4-Ethoxy-3 ; 4' dimethoxy(1,1 '- 1 H); 7.18 d (J = 8.6 Hz,
biphenyl]-3-carboxylic acid 1 H); 7.16 s (2H); 7.15 dd (J o ~
= 8.1 Hz / 2.3 Hz, 1 H); 7.06
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 7.03 d (J = 8.1 Hz,
1H);6.97dd(J=8.0Hz/
7.0 Hz, 1 H); 4.93 m(1 H);
4.27 m(1 H); 4.15 m (2H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
241
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents 9o~s to
3.85 s (3H); 3.79 s (3H);
3.50 m(1 H); 3.45 m(1 H);
3.01 dd (J = 14.2 Hz / 7.3
Hz, 1 H); 2.97 dd (J = 14.2
Hz / 5.8 Hz, 1 H); 1.32 t(J =
7.0 Hz, 3H).
300 4-Ethoxy-N-[(R)-1- 39 (CDCI3): 8.14 s(1H); 8.52 OH
(hydroxymethyl)-2-(1 H-indol-3- m(1 H); 8.51 d (J = 2.5 Hz, 0 NH
N
H
yl)ethyl]-3'-(1-methylethyl)[1,1'- 1 H); 7.71 d (J = 8.0 Hz, 0
biphenyl]-3-carboxamide; 1 H); 7.66 dd (J = 8.6 Hz
2.5 Hz, 1 H); 7.48 s(1 H);
D-Tryptophanol and 7.42 d (J = 7.8 Hz, 1 H);
4-Ethoxy-3'-(1- 7.36 d(J = 8.0 Hz, 1 H);
methylethyl)[1,1 '-biphenyl]-3- 7.35 dd (J = 7.8 Hz / 7.6
carboxylic acid Hz, 1 H); 7.20 d (J = 7.6 Hz,
1 H); 7.19 dd (J = 8.0 Hz /
7.0 Hz, 1 H); 7.12 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 7.11 d
=
(J = 2.3 Hz, 1 H); 6.97 d (J
8.6 Hz, 1 H); 4.58 m(1 H);
4.06 m (2H); 3.84 d (J =
10.9 Hz, 1 H); 3.77 dd (J =
10.9 Hz / 5.1 Hz, 1 H); 3.17
dd (J = 15.2 Hz / 6.8 Hz,
1 H); 3.14 dd (J = 15.2 Hz /
6.8 Hz, 1 H); 2.97 sept (J =
6.8 Hz, 1 H); 1.30 d(J = 6.8
Hz, 6H); 1.26 t (J = 7.0 Hz,
3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
242
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) 8[ppm]
reagents gous to
301 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.49 d (J = 7.5 Hz, OH (1 H-indol-
3-yl)ethyl]-3',4',5'- 1 H); 8.47 d(J = 2.6 Hz, 0 NH 'H
trimethoxy-4-propoxy[1,1'- 1 H); 8.12 s(1 H); 7.71 d (J o 1 o,-,-
biphenyl]-3-carboxamide; = 8.0 Hz, H); 7.61 dd (J = ~o
8.7 Hz / 2.6 Hz, 1 H); 7.36 d "o
D-Tryptophanol and
(J = 8.0 Hz, 1 H); 7.20 dd (J
3;4;5'-Trimethoxy-4- = 8.0 Hz / 7.0 Hz, 1H); 7.11
propoxy[l,1 '-biphenylJ-3- dd (J = 8.0 Hz / 7.0 Hz,
carboxylic acid 1 H); 7.11 s(1 H); 6.98 d(J
= 8.7 Hz, 1 H); 6.79 s (2H);
4.60 m(1 H); 3.97 m (2H);
3.92 s (6H); 3.89 s (3H);
3.81 m (2H); 3.15 m (2H);
1.66 m (2H); 0.95 t (J = 7.4
Hz, 3H).
302 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDC13): 8.49 d (J = 7.4 Hz, OH (1 H-indol-
3-yI)ethyl]-3',4'- 1 H); 8.47 d(J = 2.5 Hz, o NH 'N
H
dimethoxy-4-propoxy[1,1'- 1 H); 8.11 s(1 H); 7.71 d (J biphenyl]-3-
carboxamide; = 8.0 Hz, 1 H); 7.62 dd (J
8.7 Hz / 2.5 Hz, 1 H); 7.36 d
D-Tryptophanol and (J = 8.0 Hz, 1 H); 7.19 dd (J
3;4'-Dimethoxy-4-propoxy[l,1 '- = 8.0 Hz / 7.0 Hz, 1 H); 7.12
biphenylJ-3-carboxylic acid m (4H); 6.97 d (J = 8.7 Hz,
1 H); 6.93 d (J = 8.3 Hz,
1 H); 4.59 m (1 H); 3.97 m
(2H); 3.95 s (3H); 3.92 s
(3H); 3.81 m (2H); 3.18 dd
(J = 15.1 Hz / 6.8 Hz, 1 H);
3.13 dd (J = 15.1 Hz/7.9
Hz, 1 H); 1.65 m (2H); 0.94 t
(J = 7.4 Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
243
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents 9o~s to
303 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI3): 8.51 d (J = 2.6 Hz, OH
\~
(1 H-indol-3-yl)ethyl]-3'- 1 H); 8.46 d (J = 7.5 Hz, o N'H ~N
H
o \ ~ i
methoxy-4-propoxy[1,1'- 1 H); 8.09 s(1 H); 7.71 d (J
biphenyl]-3-carboxamide; = 8.0 Hz, H); 7.65 dd (J = ~~
8.7 Hz / 2.6 Hz, 1 H); 7.36 d
D-Tryptophanol and
(J = 8.0 Hz, 1 H); 7.34 dd (J
3'-Methoxy-4-propoxy(1,1'- = 7.9 Hz / 7.9 Hz, 1 H); 7.20
biphenyl]-3-carboxylic acid d (J = 7.9 Hz, H); 7.19 dd (J
= 8.0 Hz / 7.0 Hz, 1H);7.14
s(1H); 7.11 dd(J=8.0Hz/
7.0 Hz, 1 H); 7.11 s(1 H);
6.98 d (J = 8.7 Hz, 1 H);
6.88 dd (J = 7.9 Hz / 2.5
Hz, 1 H); 4.59 m(1 H); 3.96
m (2H); 3.86 s (3H); 3.80 m
(2H); 3.16 m (2H); 1.65 m
(2H); 0.93 t (J = 7.4 Hz,
3H).
304 N-[(R)-1-(Hydroxymethyl)-2- 39 (CDCI,): 8.47 d(J= 2.5 Hz, OH
(1 H-indol-3-yl)ethyl]-N-methyl- 1 H); 8.42 d (J = 7.4 Hz, 0 NH N
H
4-propoxy[1,1'-biphenyl]-3,3'- 1 H); 8.21 s(1 H); 7.97 dd (J I~ o.~
dicarboxamide; = 1.7 Hz / 1.7 Hz, 1 H); 7.72
m (3H); 7.66 dd (J = 8.7 Hz
D-Tryptophanol and H~
/ 2.5 Hz, 1 H); 7.47 dd (J =
3'-[(Methylamino)carbonyl]-4- 7.7 Hz / 7.5 Hz, 1 H); 7.36 d
propoxy[1,1 '-biphenyl]-3- (J = 8.0 Hz, 1 H); 7.19 dd (J
carboxylic acid = 8.0 Hz / 7.0 Hz, 1 H); 7.11
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.97 d (J = 8.7 Hz,
1 H); 6.37 m (1 H); 4.58 m
(1 H); 3.95 m (2H); 3.80 m
(2H); 3.16 dd (J = 15.1 Hz /
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
244
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents 9ous to H-NMR (400 MHz) S[ppm]
6.8 Hz, 1 H); 3.13 dd (J =
15.1 Hz / 7.9 Hz, 1 H); 3.04
d (J = 4.9 Hz, 3H); 1.65 m
(2H); 0.93 t (J = 7.4 Hz,
3H).
305 4,3',4',5'- 39 (DMSO-d6): 10.85 s(1 H); - N
~
Tetramethoxybiphenyl-3- 8.19 d (J = 7.8 Hz, 1 H); i
carboxylic acid [(R)-1- 8.05 d (J = 2.3 Hz, 1 H); Ho\
hydroxymethyl-2-(1 H-indol-3- 7.76 dd (J = 8.6 Hz, J = 2.3 0 NH
yi)ethyl]amide; Hz, 1 H); 7.69 d (J = 7.8 Hz, I o1~
1 H); 7.33 d (J = 7.8 Hz,
0 (D)-Tryptophanol
1 H); 7.18-7.20 m (2H); 7.06 0
t(J = 7.2 Hz, 1 H); 6.97 t (J oll
and
= 7.4 Hz, 1 H); 6.83 s (2H);
4-Methoxy-3 ; 4; 5' 4.93 t (J = 5.2 Hz, 1 H);
trimethoxybiphenyl-3-carboxylic 4.20-4.23 m (1 H); 3.85 s
acid (6H); 3.84 s (3H); 3.68 s
(3H); 3.40-3.56 m (2H);
2.95-3.05 m (2H).
306 4,3',4'-Trimethoxybiphenyl-3- 39 (DMSO-d6): 10.85 s(1 H); - N
carboxylic acid [(R)-1-hydroxy- 8.20 d (J = 7.8 Hz, 1 H); \~
methyl-2-(1 H-indol-3- 8.04 d (J = 2.3 Hz, 1 H); Ho
yI)ethyl]amide; 7.73 dd (J = 8.6 Hz, J= 2.3 0 NH
Hz, 1 H); 7.69 d (J = 7.8 Hz,
(D)-Tryptophanol 1 H); 7.34 d (J = 8.2 Hz, 0 and
1 H); 7.12-7.20 m (4H); 7.06 o
t(J = 7.6 Hz, 1 H); 7.02 d (J
= 8.2 Hz, 1 H); 6.98 t (J =
.4Hz, 1H);4.94t(J=5.1
4, 3 ; 4' Trimethoxybiphenyl-3- 7Hz, 1 H); 4.21-4.29 m(1 H);
carboxylic acid
3.84 s (3H); 3.83 s (3H);
3.78 s (3H); 3.40-3.56 m
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
245
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents yous to H-NMR (400 MHz) S[ppm]
(2H); 2.96-3.05 m (2H).
307 3'-Fluoro-4,4'- 39 (DMSO-d6): 10.85 s(1 H); ~ H
dimethoxybiphenyl-3-carboxylic 8.18 d (J = 7.8 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H- 8.01 d (J = 2.3 Hz, 1 H); HO
indol-3-ylmethyl)ethyl]amide; 7.73 dd (J = 8.7 Hz, J = 2.3 o H
Hz, 1 H); 7.69 d(J = 7.8 Hz, ~
(D)-Tryptophanol 1 H); 7.49 dd (J = 12.9 Hz, j F
= 1.9 Hz, 1 H); 7.40 d (J = q
and I
8.6 Hz, 1 H); 7.34 d (J = 8.2
3' Fluoro-4, 4'- Hz, 1 H); 7.23 t(J = 8.8 Hz,
dimethoxybiphenyl-3-carboxylic 1 H); 7.17-7.19 m (2H); 7.06
acid t (J = 7.4 Hz, 1 H); 6.98 t (J
= 7.4 Hz, 1 H); 4.93 t (J =
5.2 Hz, 1 H); 4.20-4.28 m
(1 H); 3.87 s (3H); 3.83 s
(3H); 3.40-3.56 m (2H);
2.95-3.05 m (2H). !
308 4,3'-Dimethoxybiphenyl-3- 39 (DMSO-d6): 10.85 s(1 H); - N
carboxylic acid [(R)-1-hydroxy- 8.19 d (J = 8.2 Hz, 1 H); ~~
methyl-2-(1 H-indol-3- 8.06 d (J = 2.7 Hz, 1 H); Ho
yl)ethyl]amide; 7.76 dd (J = 8.8 Hz, J = 2.5 0 NH
Hz, 1 H); 7.69 d (J = 7.8 Hz, o~
(D)-Tryptophanol 1 H); 7.36 t(J = 8.0 Hz, 1 H); 0'~ I~
7.34 d (J = 8.2 Hz, 1 H);
and
7.16-7.21 m (3H); 7.12
4,3' Dimethoxybiphenyl-3- (1 H); 7.06 t (J = 7.4 Hz,
carboxylic acid 1 H); 6.98 t (J = 7.4 Hz, 1 H);
6.91 dd (J = 8.2 Hz, J = 2.3
Hz, 1 H); 4.93 t (J = 5.2 Hz,
1 H); 4.21-4.29 m(1 H); 3.83
s (3H); 3.82 s (3H); 3.41-
3.56 m (2H); 2.95-3.06 m
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
246
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents 9ous to H-NMR (400 MHz) 8[ppm]
(2H).
309 5-Benzo[1,3]dioxol-5-yI-N-[(R)- 39 (DMSO-d6): 10.84 s(1H); - N
~
1-hydroxymethyl-2-(1 H-indol-3- 8.17 d (J = 7.8 Hz, 1 H);
yI)ethyl]-2-methoxybenzamide; 8.04 d (J = 2.3 Hz, 1 H); Ho
7.97 d (J = 2.3 Hz, 1 H); O NH
(D)-Tryptophanol ON
7.66-7.70 m(1 H); 7.34 d (J o
and = 7.8 Hz, 1 H); 7.15-7.19 m <
0
(3H); 7.04-7.08 m (2H);
5-Benzo[1,3]dioxol-5-y1-2- 6.96-6.99 m(2H); 6.05 s
methoxybenzoic acid (2H); 4.93 t (J = 5.2 Hz,
1 H); 4.20-4.28 m(1 H); 3.82
s (3H); 3.40-3.56 m (2H);
2.96-3.05 m (2H).
310 3',4'-Difluoro-4,5'- 39 (DMSO-d6): 10.84 s(1H); N
dimethoxybiphenyl-3-carboxylic 8.17 d (J = 7.8 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H- 8.04 d (J = 1.9 Hz, 1 H); HO
o H
indol-3-yimethyl)ethyl]amide; 7.80 dd (J = 8.8 Hz, J = 2.3 I 0~
Hz, 1 H); 7.69 d(J = 7.8 Hz, ,0 ~ I
(D)-Tryptophanol ~
1 H); 7.33 d (J = 7.8 Hz, F
F
and 1 H); 7.19-7.27 m(4H); 7.06
t(J = 7.4 Hz, 1 H); 6.97 t (J
3; 4' Difluoro-4, 5'- = 7.4 Hz, 1 H); 4.93 t (J =
dimethoxybiphenyl-3-carboxylic 4.8 Hz, 1 H); 4.20-4.28 m
acid (1 H); 3.97 s(3H); 3.84 s
(3H); 3.40-3.56 m (2H);
2.95-3.05 m (2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
247
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents 9ous to H-NMR (400 MHz) S[ppm]
311 4-Isopropoxy-3'- 39 (DMSO-d6): 10.82 s(1H); -/ N
~ ~
methoxybiphenyl-3-carboxylic 8.49 d (J = 8.20 Hz, 1 H);
acid [(R)-1-hydroxymethyl-2- 8.19 d (J = 2.3 Hz, 1 H); o NH
(1 H-indol-3-yl)ethyl]amide; 7.75 dd (J = 8.6 Hz, J = 2.7 \ I% oT,~,
(D)-Tryptophanol Hz, 1 H); 7.71 d(J = 7.8 Hz, /
1 H); 7.37 t (J = 8 Hz, 1 H);
and 7.33 d (J = 8.2 Hz, 1 H);
7.24 d (J = 8.6 Hz, 1 H);
4-Isopropoxy-3' 7.19 d(J = 7.8 Hz, 1 H);
methoxybiphenyl-3-carboxylic 7.14-7.16 m (2H); 7.06 t (J
acid = 7.6 Hz, 1 H); 6.97 t (J =
7.2 Hz, 1 H); 6.92 dd (J =
8.2 Hz, J = 2 Hz, 1H);4.97t
(J = 5.1 Hz, 1 H); 4.78-4.84
m(1 H); 4.23-4.30 m(1 H);
3.82 s (3H); 3.42-3.54 m
(2H); 2.95-3.04 m (2H);
1.28 d (J = 5.6 Hz, 3H);
1.27 d (J = 5.6 Hz, 3H).
312 5-Benzo[1,3]dioxol-5-yi-N-[(R)- 39 (DMSO-d6): 10.82 s(1H); - N
~
1-hydroxymethyl-2-(1 H-indol-3- 8.48 d (J = 8.20 Hz, 1 H);
HO
yi)ethyl]-2- 8.10 d (J = 2.3 Hz, 1 H); 0 NH
isopropoxybenzamide; 7.71 d (J = 7.8 Hz, 1 H); I oIT"
7.67 dd (J = 8.6 Hz, J = 1.9
(D)-Tryptophanol ~o I
Hz, 1 H); 7.33 d (J = 8.2 Hz,
and 1 H); 7.19-7.21 m (2H); 7.15
(1 H); 7.04-7.10 m (2H);
5-Benzo[1,3]dioxol-5-y1-2- 6.95-6.99 m(2H); 6.05 s
isopropoxybenzoic acid (2H); 4.97 t (J = 5.1 Hz,
1 H); 4.75-4.81 m(1 H);
4.22-4.29 m (1H); 3.42-3.54
m (2H); 2.94-3.04 m (2H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
248
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents yous to H-NMR (400 MHz) S [ppm]
1.28 d(J = 5.6 Hz, 3H);
1.27 d (J = 5.6 Hz, 3H).
313 4-Isopropoxy-3',4',5'- 39 (DMSO-d6): 10.82 s(1 H); -/ N
trimethoxybiphenyl-3-carboxylic 8.49 d (J = 8.20 Hz, 1 H);
acid [(R)-1-hydroxymethyl-2- 8.16 d (J = 2.3 Hz, 1 H); HO
O NH
(1 H-indol-3-yI)ethyl]amide; 7.75 dd (J = 8.6 Hz, J = 2.3 0~
Hz, 1 H); 7.71 d (J = 7.8 Hz, o I
(D)-Tryptophanol
1 H); 7.33 d (J = 7.8 Hz, o
and 1 H); 7.24 d (J = 8.6 Hz, I o1~
1 H); 7.15 (1 H); 7.06 t(J =
4-Isopropoxy-3;4;5' 7.6 Hz, 1 H); 6.97 t (J = 7.4
trimethoxybiphenyl-3-carboxylic Hz, 1 H); 6.85 s (2H); 4.97 t
acid (J = 4.7 Hz, 1 H); 4.78-4.84
m(1 H); 4.23-4.30 m(1 H);
3.86 s (3H); 3.69 s (3H);
3.42-3.54 m (2H); 2.95-3.04
m (2H); 1.28 d (J = 5.6 Hz,
3H); 1.27 d (J = 5.6 Hz,
3H).
314 3'-Fluoro-4-isopropoxy-4'- 39 (DMSO-d6): 10.83 s(1 H); - N
methoxybiphenyl-3-carboxylic 8.48 d (J = 8.20 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H- 8.15 d (J = 2.3 Hz, 1 H); HO
O H
indoi-3-ylmethyl)ethyl]amide; 7.71 d (J = 7.4 Hz, 1 H);
7.50 dd (J = 12 Hz, J = 1.9 F \ I
(D)-Tryptophanol
Hz, 1 H); 7.42 d (J = 8.6 Hz, i
and 1 H); 7.33 d (J = 8.2 Hz,
1 H); 7.24 t(J = 8.2 Hz, 1 H);
3' Fluoro-4-isopropoxy-4' 7.22 d (J = 8.8 Hz, 1 H);
methoxybiphenyl-3-carboxylic 7.16 (1 H); 7.06 t (J = 7.4
acid Hz, 1 H); 6.97 t (J = 7.4 Hz,
1 H); 4.98 t (J = 5.1 Hz, 1 H);
4.77-4.83 m (1 H); 4.23-4.30
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
249
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents yous to H-NMR (400 MHz) 8[ppm]
m(1 H); 3.87 s (3H); 3.42-
3.55 m (2H); 2.95-3.05 m
(2H); 1.28 d (J = 5.6 Hz,
3H); 1.27 d (J = 5.6 Hz,
3H).
315 4-Isopropoxy-3',4'- 39 (DMSO-d6): 10.82 s(1 H) - N
dimethoxybiphenyl-3-carboxylic 8.50 d (J = 7.8 Hz, 1 H);
acid [(R)-1-hydroxymethyl-2- 8.16 d (J = 2.3 Hz, 1 H); Ho~
O NH
(1 H-indol-3-yl)ethyl]amide; 7.71 dd (J = 8.6 Hz, J = 2.7 0-r
Hz, 1 H); 7.34 d (J = 8.2 Hz, o
(D)-Tryptophanol
1 H); 7.22 d (J = 8.9 Hz, o ~
1 H); 7.12-7.16 m (3H); 7.06
and
t(J = 7.4 Hz, 1 H); 7.02 d(J
4-Isopropoxy-3;4' = 8.6 Hz, 1 H); 6.97 t(J =
dimethoxybiphenyl-3-carboxylic 7.4 Hz, 1 H); 4.97 t (J = 5.1
acid Hz, 1 H); 4.76-4.82 m(1 H);
4.23-4.30 m (1 H); 3.84 s
(3H); 3.79 s (3H); 3.42-3.54
m (2H); 2.94-3.04 m (2H);
1.28 d (J = 5.6 Hz, 3H);
1.27 d (J = 5.6 Hz, 3H).
316 4-Isopropoxy-3'- 39 (DMSO-d6): 10.82 s(1 H); N
methylbiphenyl-3-carboxylic 8.50 d (J = 8.2 Hz, 1 H);
acid [(R)-1-hydroxymethyl-2- 8.20 d (J = 2.3 Hz, 1 H); Ho
O NH
(1 H-indol-3-yl)ethyl]amide; 7.72 dd (J = 8.6 Hz, J = 2.7
Hz, 1 H); 7.45 (1 H); 7.41 d
(D)-Tryptophanol
(J = 7.8 Hz, 1 H); 7.31-7.35
and m(2H); 7.24 d (J = 8.6 Hz,
1 H); 7.14-7.16 m (2H); 7.06
4-1sopropoxy-3' t (J = 7.4 Hz, 1 H); 6.97 t (J
methylbiphenyl-3-carboxylic = 7 Hz, 1 H); 4.97 t (J = 5.1
acid Hz, 1 H); 4.77-4.83 m(1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
250
Product; Method HPLC-MS conditions I Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents sous to
4.24-4.31 m (1 H); 3.43-3.55
m (2H); 2.95-3.05 m (2H);
2.38 s (3H); 1.28 d (J = 5.6
Hz, 3H); 1.27 d (J = 5.6 Hz,
3H).
317 4'-Fluoro-4-isopropoxy-3'- 39 (DMSO-d6): 10.82 s(1 H); N
methylbiphenyl-3-carboxylic 8.50 d (J = 8.2 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H- 8.17 d (J = 2.3 Hz, 1 H); HO
H
indol-3-ylmethyl)ethyl]amide; 7.74-7.70 m(2H); 7.56 d (J I o~
= 7 Hz, 1 H); 7.45-7.48 (1 H);
(D)-Tryptophanol
7.34 d (J = 8.2 Hz, 1 H); F
and 7.20-7.25 m (2H); 7.16
(1 H); 7.06 t (J = 7.4 Hz,
4'-Fluoro-4-isopropoxy-3' 1 H); 6.97 t (J = 7.4 Hz, 1 H);
methylbiphenyl-3-carboxylic 4.97 t (J = 5.1 Hz, 1 H);
acid 4.77-4.83 m(1 H); 4.23-4.31
m (1 H); 3.43-3.55 m (2H);
2.95-3.05 m (2H); 2.31 s
(3H); 1.28 d (J = 5.6 Hz,
3H); 1.27 d(J = 5.6 Hz,
3H).
318 3',4'-Difluoro-4-isopropoxy-5- 39 (DMSO-d6): 10.82 s(1 H); -/ N
methoxybiphenyl-3-carboxylic 8.48 d (J = 7.8 Hz, 1 H);
HO
acid [(R)-2-hydroxy- 1 -(1 H- 8.18 d (J = 1.6 Hz, 1 H); o H
indol-3-ylmethyl)ethyl]amide; 7.78 dd (J = 8.74 Hz, J= 1.8 /o 1 o~
Hz, 1 H); 7.72 d (J = 7.8 Hz, I
(D)-Tryptophanol 1 H); 7.33 d (J = 8.2 Hz, F F
and 1 H); 7.28-7.23 m(2H); 7.20
d (J = 6.62 Hz, 1 H); 7.16
3;4'-Difluoro-4-isopropoxy-5'- (1 H); 7.06 t (J = 7.4 Hz,
methoxybiphenyl-3-carboxylic 1 H); 6.97 t (J = 7.4 Hz, 1 H);
acid 4.97 t (J = 5.1 Hz, 1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
251
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
4.80-4.86 m (1 H); 4.23-4.30
m(1 H); 3.98 s (3H); 3.42-
3.55 m (2H); 2.95-3.04 m
(2H); 1.28 d (J = 5.6 Hz,
3H); 1.27 d (J = 5.6 Hz,
3H).
319 4,3',4',5'-Tetramethoxy-5- 39 (DMSO-ds): 10.81 s(1 H); - H
~ N
methylbiphenyl-3-carboxylic 8.26 d (J = 7.8 Hz, 1 H); \
acid [(R)-1-hydroxymethyl-2- 7.69 d (J = 7.8 Hz, 1 H); HO
(1 H-indol-3-yl)ethyl]amide; 7.63-7.65 m (2H); 7.32 d (J 0 NH
(D)-Tryptophanol = 7.8 Hz, 1 H); 7.18 (1 H); 0~
o
7.05 t (J = 7.2 Hz, 1 H); 6.97 I
and t (J = 7.4 Hz, 1 H); 6.84 s o
(2H); 4.90 t (J = 5.2 Hz, 01~1
4, 3; 4; 5' Tetramethoxy-5- 1 H); 4.22-4.30 m(1 H); 3.86
methylbiphenyl-3-carboxylic s (6H); 3.69 s(3H); 3.62 s
acid (3H); 3.44-3.58 m (2H);
3.03. 2.96 AB (J, = 14.4
Hz, J2 = 6.9 Hz, 2H); 2.31 s
(3H).
320 4,3',4'-Trimethoxy-5- 39 (DMSO-d6): 10.81 s(1 H); - H
N
methylbiphenyl-3-carboxylic 8.25 d (J = 8.2 Hz, 1 H);
acid [(R)-1-hydroxymethyl-2- 7.69 d (J = 7.8 Hz, 1 H); Ho\
O NH
(1 H-indol-3-yl)ethyl]amide; 7.59-7.61 m (2H); 7.32 d (J
i ~ o1~
= 7.8 Hz, 1 H); 7.18 (1 H); o \ I~
(D)-Tryptophanol 7.12-7.15 m (2H); 7.01-7.07 I
O
m (2H); 6.97 t (J = 7.4 Hz, I
and
1 H); 4.89 t (J = 5.2 Hz, 1 H);
4, 3; 4'-Trimethoxy-5- 4.23-4.30 m(1 H); 3.84 s
methylbiphenyl-3-carboxylic (6H); 3.70 s (3H); 3.61 s
acid (3H); 3.44-3.58 m (2H);
3.03. 2.95 AB (J, = 14.3
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
252
Product; Method HPLC-MS conditions I Structure
Ex. analo- MHz) reagents 9ous to H-NMR (400 S [PPm]
Hz, J2 = 7 Hz, 2H); 2.31 s
(3H).
321 3'-Fluoro-4,4'-dimethoxy-5- 39 (DMSO-d6): 10.81 s(1 H); - H
methylbiphenyl-3-carboxylic 8.23 d (J = 8.2 Hz, 1 H);
acid [(R)-2-hydroxy-l-(1 H- 7.69 d (J = 7.8 Hz, 1 H); Ho
indol-3-ylmethyl)ethyl]amide; 7.58-7.59 m (2H); 7.49 dd 0 H
(J = 13.1 Hz, J= 1.7 Hz, i 0~
(D)-Tryptophanol F
1 H); 7.40 d (J = 8.2 Hz,
and 1 H); 7.33 d (J = 7.8 Hz,
1 H); 7.23 t (J = 9 Hz, 1 H);
3'-Fluoro-4, 4'-dimethoxy-5- 7.17 d (J = 1.6 Hz, 1 H);
methylbiphenyl-3-carboxylic 7.06 t (J = 7.4 Hz, 1 H); 6.97
acid t (J = 7.4 Hz, 1 H); 4.89 t (J
= 5.4 Hz, 1 H); 4.23-4.30 m
(1 H); 3.88 s(3H); 3.60 s
(3H); 3.44-3.58 m (2H);
3.03, 2.95 AB (J, = 14.2
Hz, J2 = 7 Hz, 2H); 2.29 s
(3H).
322 5-Benzo[1,3]dioxol-5-yl-N-[(R)- 39 (DMSO-d6): 10.80 s(1H); - N
~
1-hydroxymethyl-2-(1 H-indol-3- 8.21 d (J = 8.2 Hz, 1 H);
I eth I 2-methox 3-meth I- 7.68 d (J 7.8 Hz, 1 H; "o
y) y]- y- y ( ) O NH
benzamide; 7.54 s(2H); 7.30 d (J = 8.2
Hz, 1 H); 7.17-7.18 m (2H); o I~
(D)-Tryptophanol ~ I ~
7.04-7.08 m(1 H); 6.95-6.99 0
and m (2H); 6.06 s (2H); 4.88 t
(J = 5.1 Hz, 1 H); 4.22-4.30
5-Benzo[1,3]dioxol-5-y1-2- m(1H); 3.60 s (3H); 3.44-
methoxy-3-methyl-benzoic acid 3.57 m (2H); 3.02, 2.94 AB
(J, = 14.3 Hz, J2 = 6.6 Hz,
2H); 2.28 s (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
253
Product; Method HPLC-MS conditions I Structure
Ex. analo-
reagents sous to H-NMR (400 MHz) S[ppm]
323 4,3'-Dimethoxy-5- 39 (DMSO-d6): 10.81 s (1H); - H
~
methylbiphenyl-3-carboxylic 8.25 d (J = 8.2 Hz, 1 H);
acid [(R)-1-hydroxymethyl-2- 7.69 d (J = 7.8 Hz, 1 H); Ho
(1H-indol-3-yI)ethyl]amide; 7.61-7.63 m (2H); 7.36 t (J 0 NH
= 8.2 Hz, 1 H); 7.33 d (J = I o~
(D)-Tryptophanol o I
8.2 Hz, 1 H); 7.14-7.18 m
and (3H); 7.06 t (J = 7.4 Hz,
1 H); 6.97 t (J = 7.4 Hz, 1 H);
4, 3' Dimethoxy-5- 6.92 dd (J = 8.2 Hz, J= 1.9
methylbiphenyl-3-carboxylic Hz, 1 H); 4.89 t (J = 5.1 Hz,
acid 1 H); 4.23-4.31 m(1 H); 3.82
s (3H); 3.62 s (3H); 3.45-
3.58 m (2H); 3.03, 2.95 AB
(J, = 14.5 Hz, J2 = 6.6 Hz,
2H); 2.31 s (3H).
324 4-Methoxy-5,3'- 39 (DMSO-d6): 10.80 s(1 H); , H
N
dimethylbiphenyl-3-carboxylic 8.24 d (J = 8.2 Hz, 1 H); ~~ i
acid [(R)-1-hydroxymethyl-2- 7.69 d (J = 7.8 Hz, 1 H); HO
(1 H-indol-3-yI)ethyl]amide; 7.60, 7.63 AB (J, = 14.8 0 NH
Hz, J2 = 1.9 Hz, 2H); 7.43
(D)-Tryptophanol (1 H); 7.39 d (J = 7.8 Hz,
and 1 H); 7.32-7.35 m (2H);
7.15-7.17 m (2H); 7.06 t (J
4-Methoxy-5, 3'- = 7.4 Hz, 1 H); 6.97 t (J =
dimethylbiphenyl-3-carboxylic 7.4 Hz, 1 H); 4.88 t (J = 5.1
acid Hz, 1 H); 4.23-4.31 m(1 H);
3.61 s (3H); 3.45-3.58 m
(2H); 3.03, 2.95 AB (J, =
14.3 Hz, J2 = 6.6 Hz, 2H);
2.38 s (3H); 2.31 s (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
254
Product; Method HPLC-MS conditions / Structure
Ex. analo-
reagents gous to H-NMR (400 MHz) S[ppm]
325 4'-Fluoro-4-methoxy-5,3'- 39 (DMSO-d6): 10.81 s(1 H); - H
~ N
dimethylbiphenyl-3-carboxylic 8.24 d (J = 7.8 Hz, 1 H);
acid [(R)-2-hydroxy- 1 -(1 H- 7.69 d (J = 7.8 Hz, 1 H); HO
indol-3-ylmethyl)ethyl]amide; 7.59-7.60 m (2H); 7.54 d (J H
= 7.4 Hz, 1 H); 7.42-7.45 m
(D)-Tryptophanol
(1 H); 7.33 d (J = 8.2 Hz,
1 H); 7.18-7.22 m (2H); 7.06 F
and
t (J = 7.4 Hz, 1 H); 6.97 t (J
4'-Fluoro-4-methoxy-5, 3' = 7.4 Hz, 1 H); 4.89 t (J =
dimethylbiphenyl-3-carboxylic 5.1 Hz, 1 H); 4.23-4.31 m
acid (1 H); 3.61 s (3H); 3.45-3.58
m (2H); 3.03, 2.95 AB (J, _
14.5 Hz, J2 = 7.1 Hz, 2H);
2.30 s (6H).
326 3',4'-Difluoro-4,5'-dimethoxy-5- 39 (DMSO-d6): 10.80 s(1 H); N
methylbiphenyl-3-carboxylic 8.23 d (J = 8.2 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H- 7.69 d (J = 7.8 Hz, 1 H); Ho
O H
indol-3-ylmethyl)ethyl]amide; 7.66, 7.63 AB (J, = 14.8 oll
Hz, J2 = 1.9 Hz, 2H); 7.32 d~o
(D)-Tryptophanol
(J = 8.2 Hz, 1 H); 7.18-7.26 F
F
and m (3H); 7.05 t (J = 7.4 Hz,
1 H); 6.96 t (J = 7.4 Hz, 1 H);
3; 4'-Difluoro-4, 5'-dimethoxy-5- 4.88 t (J = 5.1 Hz, 1 H);
methylbiphenyl-3-carboxylic 4.22-4.30 m (1 H); 3.97 s
acid (3H); 3.62 s (3H); 3.45-3.58
m (2H); 3.03, 2.95 AB (Jl _
14.5 Hz, J2 = 6.8 Hz, 2H);
2.31 s (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
255
Product; Method HPLC-MS conditions / Structure
Ex. analo- H-NMR (400 MHz) S[ppm]
reagents 9o~s to
327 3'-Hydroxy-4- 39 (DMSO-d6): 10.82 s(1 H); \ N
isopropoxybiphenyl-3- 9.53 s (1 H); 8.50 d (J = 7.8
carboxylic acid [(R)-1- Hz, 1 H); 8.17 s(1 H); 7.71 0 HNH
hydroxymethyl-2-(1 H-indol-3- m (2H); 7.34 d (J = 7.8 Hz, o~
yI)ethyl]amide; 1 H); 7.24 m (2H); 7.16 s HO
(1 H); 7.10 m(4H); 6.75 d (J
(D)-Tryptophanol
= 7.8 Hz, 1 H); 4.98 m(1 H);
and 4.79 m(1 H); 4.27 m(1 H);
3.51 m (2H); 3.00 m (2H);
3'-Hydroxy-4- 1.27 m (6H).
isopropoxybiphenyl-3-
carboxylic acid
328 3',4',5'-Trimethoxy-4-(3-methyl- 39 (DMSO-d6): 10.78 s(1 H); \~ N
but-2-enyloxy)biphenyl-3- 8.31 d (J = 8.1 Hz, 1 H);
HO
carboxylic acid [(R)-1-hydroxy- 8.09 d (J = 2.6 Hz, 1 H); o NH
methyl-2-(1 H-indol-3- 7.73 dd (J = 2.5 Hz / 8.7 o i~ o"'y
yI)ethyl]amide; Hz, 1 H); 7.63 d (J = 7.9 Hz, o
1 H); 7.59 m(2H); 7.31 d (J (D)-Tryptophanol
= 7.9 Hz, 1 H); 7.21 d (J =
and 8.9 Hz, 1 H); 7.10 s(1 H);
7.02 m (1 H); 6.93 m (1 H);
3;4;5'-Trimethoxy-4-(3-methyl- 6.81 s(2H); 5.35 m(1H);
but-2-enyloxy)biphenyl-3- 4.87 m(1 H); 4.62 m (2H);
carboxylic acid 4.20 m (1 H); 3.82 s (6H);
3.65 s (3H); 3.42 m (2H);
2.94 m (2H); 1.66 m (6H).
Example 329
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
256
3'-Butoxy-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1 H-indol-
3-
H
/
HO\ N
O NH
yl)ethyl]amide;
329a) 3-n-Butoxyphenylboronic acid pinacol ester
3-Hydroxyphenylboronic acid pinacol ester (1 g), potassium carbonate (1.57 g)
and
n-butyl iodide (2.6 ml) were dissolved in DMF (20 ml) and stirred at a bath
temperature
of 100 C overnight. The cooled reaction mixture was filtered and the filtrate
was freed of
solvent. The remaining residue was triturated with diisopropyl ether and the
residue was
filtered off in vacuo and discarded. The mother liquor was concentrated and
the crude
product was purified by flash chromatography. 710 mg of the title compound
were ob-
tained. MS (ESI,+): 277 (M+1).
329b) 3'-Butoxy-4-ethoxybiphenyl-3-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-
indol-3-
yl)ethyl]amide
3-n-Butoxyphenylboronic acid pinacol ester (200 mg), 5-bromo-2-ethoxy-N-[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]benzamide (201 mg), dihydrogen
dichlorobis(di-
tert-butylphosphinito-kappa)dipalladate (12 mg) and potassium carbonate (200
mg) in
DMF (5 ml) were stirred at 100 C overnight. The mixture was diluted with water
and
extracted several times with ethyl acetate. The combined organic phases were
dried
over magnesium sulphate and freed of solvent. Purification by HPLC resulted in
the title
compound in 32% yield (114 mg).
(DMSO-d6): 10.83 s(1 H); 8.41 d (J = 8.1 Hz, 1 H); 8.16 (J = 2.6 Hz, 1 H);
7.79 dd (J =
2.5 Hz / 8.5 Hz, 1 H); 7.72 d (J = 7.7 Hz, 1 H); 7.36 m (2H); 7.20 m (3H);
7.14 m(1 H);
7.07 m(1 H); 6.98 m(1 H); 6.92 dd (J = 1.9 Hz / 7.9 Hz, 1 H); 4.20 m(1 H);
4.15 m (2H);
4.05 m (2H); 3.50 m (2H); 3.00 m (2H); 1.73 m (2H); 1.45 m (2H); 1.33 m (3H);
9.96 m
(3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
257
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method '
reagents (400 MHz) S Structure
Ex. analogous
9ents to [Pp ]
330 4-Ethoxy-3'- 329 (DMSO-ds): 10.83 s(1 H); \/ N
isopropoxybiphenyl-3- 8.43 d (J = 7.9 Hz, 1 H); ~
HO
carboxylic acid [(R)-1- 8.15 d (J = 2.6 Hz, 1 H); 0 NH
hydroxymethyl-2-(1 H-indol-3- 7.75 dd (J = 2.5 Hz / 8.5
yl)ethyl]amide; Hz, 1 H); 7.71 d (J = 7.7 Hz,
1 H); 7.36 m (2H); 7.18 m
5-Bromo-2-ethoxy-N-((R)-1-
(2H);7.11 m(1H);6.98m
hydroxymethyl-2-(1 H-indol-3-
(1 H); 6.92 m (1 H); 4.72 m
yl)ethylJbenzamide
(1 H); 4.20 m(1 H); 4.16 m
and (2H); 3.51 m (2H); 3.00 m
(2H); 1.31 m (9H).
3-Isopropylox yphen ylboronic
acid pinacol ester
Example 331
N-[(R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(7-methoxybenzofuran-2-yl)-2-
H
N
NH O~~
OHO
~ O
propoxybenzamide; O\
331 a) Methyl 5-(7-methoxybenzofuran-2-yl)-2-propoxybenzoate
A solution of 7-methoxybenzofuran (500 mg) in THF (3 ml) was cooled to 0 C,
and a
solution of n-BuLi in hexane (1.6 M, 2.11 ml) was slowly added, whereupon the
tem-
perature rose to 15 C. The mixture was then stirred at 5 C for 1 hour, zinc
chloride (1 M
solution in THF, 3.71 ml), Pd(PPh3)4 (39 mg) and a solution of methyl 5-bromo-
2-
propoxybenzoate (1.08 g) in THF (3 ml) were added, and the mixture was then
stirred
under reflux overnight. The mixture was diluted with ethyl acetate and
extracted with
aqueous ammonium chloride solution. The combined organic phases were dried
over
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
258
sodium sulphate, and the solvent was distilled off in a rotary evaporator. The
title com-
pound was obtained after purification by flash chromatography in 11% yield
(127 mg).
MS (ESI,+): 341 (M+1).
331 b) 5-(7-Methoxybenzofuran-2-yl)-2-propoxybenzoic acid
A solution of methyl 5-(7-methoxybenzofuran-2-yl)-2-propoxybenzoate (120 mg)
in
methanol (5 ml) was mixed with potassium hydroxide solution (10% strength in
metha-
nol, 2 ml) and stirred at 50 C for 5 hours. The mixture was concentrated and
extracted
with MTBE. The aqueous phase was acidified with 1 N HCI and again extracted
with
MTBE, and the combined organic phases were freed of solvent. The title
compound
was employed without further purification in the next stage (yield 97%, 112
mg). MS
(ESI,+): 327 (M+1).
331 c) N-((R)-1-Hydroxymethyl-2-(1 H-indol-3-yl)ethyl]-5-(7-methoxybenzofuran-
2-yl)-2-
propoxybenzamide
5-(7-Methoxybenzofuran-2-yl)-2-propoxybenzoic acid (85 mg) were reacted with
(D)-
tryptophanol (59 mg) in analogy to general method 113b. The title compound was
ob-
tained in 32% yield (41 mg).
(DMSO-d6): 10.79 s(1 H); 8.37 d (J = 2.5 Hz, 1 H); 8.32 d (J = 8.3 Hz, 1 H);
7.96 dd (J =
2.5 Hz / 8.6 Hz, 1 H); 7.68 d (J = 7.8 Hz, 1 H); 7.30 m (2H); 7.24 d (J = 8.8
Hz, 1 H); 7.15
m (3H); 7.03 m(1 H); 6.95 m(1 H); 6.90 m(1 H); 4.91 m(1 H); 4.26 m(1 H); 4.06
m (2H);
3.95 s (3H); 3.45 m (2H); 2.96 m (2H); 1.66 m (2H); 0.91 m(3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
259
Example 332
6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-2-(6-
chloro-
I o'
o
O N
I
O
HN O
OH
N
H
1 H-indol-3-yl)-1-hydroxymethylethyl]amide; cil
Preswell 0.2 mmol of unloaded Wang resin in 1.5 ml of DMF for 15 min. Then 6
eq of
Fmoc-amino acid (R)-3-(6-chloro-1 H-indol-3-yl)-2-(9H-fluoren-9-
ylmethoxycarbonyl-
amino)propionic acid (0.3M in NMP); 10 eq of pyridine (dried) and 6 eq of 2,4-
dichlorobenzoyl chloride (dried) are added and coupled to the resin by shaking
for 20 h.
After washing 5x with 2 ml of DMF, capping is carried out with 1.5 ml of
acetic ahydride
10% in DMF for 5 minutes, followed by washing 5 x with 2 ml of DMF.
Deprotection with
2 ml of 20% PIP in DMF (1 x 5 minutes, 1 x 15 minutes) is followed by washing
a further
5 x with 2 ml of NMP.
6-Methoxy-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid is coupled on
by add-
ing 2 eq of 0.3M acid, 6 eq of N-methylmorpholine 3M in NMP+ 2.5% DMAP and 3
eq
of HATU 0.3M in NMP (double coupling 2 x 4h). This is followed by washing 3 x
with 2
ml of NMP and 5 x with 2 ml of THF. For the reductive elimination, 2 ml of
DIBAL 1 M in
THF are added at 0 C under N2 and stirred for 12 h. Warming to room
temperature is
followed by filtration and washing with 4 x 1.5 ml of THF.
HPLC-MS: Column Purospher Star RP C18 4.6x125 5pm; detection wavelength
214 nm; flow rate 1 ml/min; eluents A: 0.1 % TFA in H20, B 0.1 % TFA in ACN;
gradient
in each case based on B: 5% to 95% (10') to 95% (2') to 5% (0.5') to 5% (2.5')
Molecular peak (ESI, M+1): 577
Retention time: 7.96 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
260
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method HPLC-MS conditions I Structure
Ex. eagents yousto 'H-NMR (400 MHz) S[ppm]
333 6-Methoxy-2-(3,4,5- 318 Column Purospher Star o
trimethoxyphenyl)quinoline-4- RP C18 4.6x125 5Nm; o
carboxylic acid [(R)-1- detection wavelength o ~ N Nzt
hydroxymethyl-2-(2-methyl- 214 nm; flow rate 1 o
ml/min; eluents A: 0.1 % HN 0
1 H-indol-3-yl)ethyl]amide;
TFA in H20, B 0.1 % TFA H
(R)-2-(9H-Fluoren-9- oH
in ACN; gradient in each
ylmethoxycarbonylamino)-3- ~ N
case based on B: 5% to - H
(2-methyl-1H-indol-3-yl)- 95% (10') to 95% (2) to
propionic acid o o
5/0(0.5')to5/o(2.5)
and Molecular peak (ESI,
6-Methoxy-2-(3, 4, 5- M+1): 557.6
trimethoxyphenyl) quinoline-4-
Retention time: 7.61 min.
carboxylic acid
334 6-Methoxy-2-(3,4,5- I (DMSO-d6): 10.65 s H
trimethoxyphenyl)quinoline-4- (1 H); 8.64 d (J = 8.6 Hz, ~~~
carboxylic acid [1- 1 H); 7.99 d (J = 9.0 Hz, Ho
O NH
hydroxymethyl-2-(6-methyl- 1 H); 7.96 s(1 H); 7.51 d o
,
1 H-indol-3-yl)ethyl]amide; (J = 8.5 Hz, 1 H); 7.49 s - N
(2H); 7.43 s(1 H); 7.40 s -
(2-RS)-Amino-3-(6-methyl- (1 H); 7.10 d (J - 4.3 Hz, 0
1H-indol-3-yl)-propan-1-ol 2H); 6.76 d (J - 7.8 Hz,
and 1 H); 4.90 t (J = 5.4 Hz,
1 H); 4.38 m(1 H); 3.92 s
6-Methoxy-2-(3,4,5- (6H); 3.73 s(3H); 3.72 s
trimethoxyphenyl)quinoline-4- (3H); 3.59 t (J = 5.2 Hz,
carboxylic acid 2H); 2.99 dd (J = 14.3 Hz
/ 8.1 Hz, 1 H); 2.92 dd (J
= 14.3 Hz / 5.4 Hz, 1 H);
2.35 s (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
261
Example 335
N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl) -1H-indol-3-yl) ethyl]-6-methoxy-2-(3-
I
N
HO
H
O NH
O~
.0*0 N
methoxyphenyl)quinoline-4-carboxamide;
335a) Methyl (R)-2-tert-butoxycarbonylamino-3-(1 H-indol-3yl)-propionate
15.72 mmol (2.18 ml) of triethylamine were added dropwise to a solution of
3.93 mmol
(1g) of D-tryptophan methyl ester hydrochloride in 35 ml of dichloromethane
with stirring
and then 7.85 mmol (1.71 g) of di-tert-butyl dicarbonate, dissolved in 5 ml of
dichloromethane, were added, followed by 0.39 mmol (48 mg) of
dimethylaminopyridine. The mixture was stirred at room temperature for about
1.5 h.
Then 25 ml of 10% strength sodium bisulphite solution were added to the
reaction mix-
ture and stirred for 15 minutes. After phase separation, the aqueous phase was
ex-
tracted with dichloromethane. The resulting organic phase was dried over
magnesium
sulphate, filtered and concentrated in vacuo. Purification by chromatography
on silica
gel with the eluent cyclohexane/ethyl acetate affords 800 mg of the compound
as a
white solid.
'H-NMR (400 MHz,DMSO-d6):6 [ppm] = 10.84 s(1 H, NH); 7.45 d (J = 7.8 Hz, 1 H,
aryl)
7.32 d (1 H, aryl); 7.14 s (1 H, aryl); 7.05 t (J = 7.4 Hz, 1 H, aryl); 6.97 t
(J = 7.9 Hz, 1 H,
aryl); 4.20 m(1 H, CH); 3.59 s (3H, OCH3); 3.08 dd (J = 14.4 Hz / 5.8 Hz, 1 H,
CH); 3.00
dd (J = 14.4 Hz / 8.2 Hz, 1 H, CH); 1.33 s (9H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
262
335b) Methyl (R)-2-tert-butoxycarbonylamino-3-(1-ethyl-1 H-indol-3-
yl)propionate
3.27 mmol (183 mg) of potassium hydroxid powder were added in portions to a
stirred
solution of 2.51 mmol (800 mg) of the protected amino acid prepared in a), in
8 ml of
DMSO, slightly cooling in water. This mixture was stirred for 5 minutes and
then 3.27
mmol (0.26 ml) of ethyl iodide, dissolved in 2 ml of DMSO, were added
dropwise. Stir-
ring was continued at room temperature for 2 hours, and the reaction mixture
was then
added to saturated aqueous ammonium chloride solution and extracted with ethyl
ace-
tate. The resulting organic phase was dried over magnesium sulphate, filtered
and con-
centrated in vacuo with the addition of toluene. 871 mg of the target compound
are
obtained.
'H-NMR (400 MHz,DMSO-ds):b [ppm] = 7.48 d (J = 7.8 Hz, 1 H, aryl); 7.41 d (J =
7.8
Hz, 1 H, aryl); 7.23 d(J = 7.4 Hz, 1 H, aryl); 7.11 t(J = 7.6 Hz, 1 H, aryl);
7.00 t(J = 7.8
Hz, 1 H, aryl); 4.20 m(1 H, CH); 4.19 q (J = 7.0 Hz, 2H, CH2); 3.07 dd (14.3
Hz / 5.3 Hz,
1 H, CH); 3.01 dd (J = 14.3 Hz / 8.2 Hz, 1 H, CH); 1.33 s (9H, CH3); 1.3 t (J
= 7.0 Hz, 3H,
CH3).
335c) Methyl (R)-2-amino-3-(1-ethyl-1 H-indol-3-yl)propionate
2.48 mmol (860 mg) of the compound prepared in b) were dissolved in 10 ml of
di-
chloromethane and then 24.8 mmol (1.91 ml) of trifluoroacetic acid were added
drop-
wise at room temperature. After 1 hour, 20 ml of saturated sodium bicarbonate
solution
were cautiously added dropwise to the mixture until the neutral point was
reached. After
phase separation, the aqueous phase was extracted with dichloromethane. The
result-
ing organic phase was dried over magnesium sulphate, filtered and concentrated
in
vacuo. 588 mg of the product are obtained.
1H-NMR (400 MHz,DMSO-d6): b[ppm] = 7.47 d (J = 7.8 Hz, 1 H, aryl); 7.39 d (J =
7.8
Hz, 1 H, aryl); 7.15 s (1 H, aryl); 7.09 t (J = 7.5 Hz, 1 H, aryl); 6.98 t (J
= 7.8 Hz, 1 H, aryl);
4.14 q (J = 7.0 Hz, 2H, CH2); 3.57 m(1 H, CH); 3.54 s (3H, OCH3); 2.98 dd (J =
14.2 Hz
/ 5.4 Hz, 1 H, CH); 2.93 dd (J = 14.2 Hz / 8.4 Hz, 1 H, CH); 1.79 s(2H, NH2);
1.31 t (J
=
7.0 Hz, 3H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
263
N-[ (R)-1-(Methoxycarbonyl)-2-(1-ethyl) -1H-indol-3-yl) ethyl]-6-methoxy-2-(3-
methoxyphenyl)quinoline-4-carboxamide
The title compound was prepared in analogy to general method le.
'H-NMR (400 MHz, DMSO-ds): b[ppm] = 9.42 d (7.2 Hz, 1 H, NH); 8.22 d (J = 9.0
Hz,
1 H, aryl); 8.05 m(3H, aryl); 7.97 s (1 H, aryl); 7.80 d (J = 8.6 Hz, 1 H,
aryl); 7.60 d (J
=
7.8 Hz, 1 H, aryl); 7.45 d (J = 8.2 Hz, 1 H, aryl); 7.37 t (J = 8.6 Hz, 1 H,
aryl); 7.32 s (1 H,
aryl); 7.13 t(J = 7.8 Hz, 1 H, aryl); 7.01 t(J = 7.5 Hz, 1 H, aryl); 4.80 m(1
H, CH); 4.14 q
(J = 7.0 Hz, 2H, CH2); 3.96 s (3H, OCH3); 3.72 s (3H, OCH3); 3.30 dd (J = 14.2
Hz / 5.1
Hz, 1 H, CH); 3.24 dd (J = 14.2 Hz / 8.2 Hz, 1 H, CH); 1.29 t(J = 7.0 Hz, 3H,
CH3).
335d) N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl)-1H-indol-3-yl) ethyl]-6-methoxy-2-
(3-
methoxyphenyl)quinoline-4-carboxamide
0.19 mmol (94 NI) of 2M lithium borohydride solution was added dropwise to a
solution
of 0.19 mmol (115mg) of the carboxamide (prepared in analogy to 1e) in 3 ml of
THF at
0 C. This mixture is then stirred at room temperature for 4-6 hours. It was
then neutral-
ized at 0 C with 1 N hydrochloric acid and, after addition of water, extracted
with ethyl
acetate. The organic phase was dried over magnesium sulphate, filtered and
concen-
trated in vacuo. Purification by chromatography on silica gel with the eluent
cyclohex-
ane/acetone affords 36.9 mg of pale yellow foam.
'H-NMR (400 MHz,DMSO-ds): b[ppm] = 8.76 d (J = 7.7 Hz, 1 H), 8.20 d (J = 9.0
Hz,
1 H, aryl) 8.13 m(2H, aryl); 8.06 s(1 H, aryl); 8.01 s(1 H, aryl); 7.76 d (J =
7.4 Hz, 1 H,
aryl); 7.65 d (J = 7.8 Hz, 1 H, aryl); 7.40 t (J = 7.4 Hz, 2H, aryl); 7.24 s
(1 H, aryl); 7.10 t
(J = 7.8 Hz, 1 H, aryl); 6.97 t (J = 7.4 Hz, 1 H, aryl); 4.92 t (J = 5.4 Hz, 1
H, OH); 4.36 m
(1 H, CH); 4.14 q (J = 7.0 Hz, 2H, CH2); 3.96 s(3H, OCH3); 3.60 m(2H, OCHZ);
3.05 dd
(J = 14.3 Hz / 5.6 Hz, 1 H, CH); 2.97 dd (J = 14.3 Hz / 8.2 Hz, 1 H, CH); 1.29
t (J = 7.0
Hz, 3H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
264
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method Structure
Ex. reagents gousio 'H-NMR (400 MHz) S[ppm]
336 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.66 d (J = 8.5 _
propyl-1 H-indol-3-yl) ethyl]-6- Hz, 1 H, NH); 8.00 d (J = 8.6 (N ~ i
~
methoxy-2-(3,4,5- Hz, 1 H, aryl); 7.98 s(1 H,
HO
trimethoxyphenyl)quinoline-4- aryl); 7.66 d (J = 8.4Hz, 1 H, o NH
carboxamide; aryl); 7.50s (2s, aryl); 7.43 m o
q
N (3H, aryl); 7.22 s(1 H, aryl); -o N-[ (R)- 1 -(Methoxycarbonyl)-2- 7.09 t (J
= 7.5 Hz, 1 H, aryl); ~O
(1-propyl-1 H-indol-3-yl) ethyl]- 6.97 t (J = 7.4 Hz, 1 H, aryl);
6-methoxy-2-(3, 4, 5- 4.91 t (J = 5.3 Hz, 1 H, OH);
trimethoxyphenyl)quinoline-4- 4.38 m (1 H, CH) 4.05 t (J =
carboxamide
7.0 Hz, 2H, CH2); 3.92 s (6H,
OCH3); 3.75 (6H, OCH3);
3.60 m (2H, OCH2); 3.03 dd
(J = 14.3 Hz / 5.7 Hz, 1 H,
CH); 2.97 dd (J = 14.3 Hz /
8.2 Hz, 1H, CH); 1.68 m(2H,
CHZ); 0.75 t (J = 7.0 Hz, 3H,
CH3).
337 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.63 d (J = 8.6
ethyl)-1 H-indol-3-yl) ethyl]-2- Hz, 1 H, NH); 7.99 m(3H, N
(3,5-difluoro-4-methoxyphenyl)- aryl), 7.95 s(1 H, aryl); 7.67 d HO H
0 H
6-methoxyquinoline-4- (J = 7.8 Hz, 1 H, aryl), 7.43 m
carboxamide; (3H, aryl); 7.25 s(1 H, aryl), F N
I~
7.11 t (J = 7.4 Hz, 1 H, aryl);
N-[ (R)-1-(Methoxycarbony!)-2- F
6.98 t (J = 7.4 Hz, 1 H, aryl);
(1-ethyl)-1 H-indol-3-yl) ethyl]-2- 4.91 t (J = 5.5 Hz, 1 H, OH);
(3,5-difluoro-4-methoxyphenyl)- 4,37 m(1 H, CH); 4.14 q(J -
6-methoxyquinoline-4- 7.0 Hz, 2H, CH2); 4.03 s (3H,
carboxamide OCH3); 3.60 m (2H, OCHZ);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
265
Product; Method Structure
Ex. reagents 9o~sto 'H-NMR (400 MHz) S[ppm]
3.03 dd (J = 14.2 Hz / 5.3
Hz, 1 H, CH); 2.96 dd (J =
14.2 Hz / 8.3 Hz, 1 H, CH);
1.29 t (J = 7.0 Hz, 3H, CH3).
338 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.66 d (J = 8.3
isopropyl-1 H-indol-3-yl) ethyl]- Hz, 1 H, NH); 8.01 d (J = 7.7
6-methoxy-2 (3- Hz, 1 H, aryl); 7.90 s(1 H, Ho NH
methoxyphenyl)-quinoline-4- aryl); 7.77 s (1 H, aryl); 7.74 d / 0,
carboxamide; (J = 7.9 Hz, 1 H, aryl): 7.66 d
(J = 7.8 Hz, 1 H, aryl); 7.46 m
N-[ (R)- 1 -(Methoxycarbonyl)-2- (4H, aryl), 7.33 s (1 H,aryl);
(1-isopropyl -1H-indol-3-yl) 7.08 m (2H, aryl); 6.99 t (J
-
ethyl]-6-methoxy-2 (3-
7.0 Hz, 1 H, aryl); 4.92 t (J
=
methoxyphenyl)-quinoline-4- 5.5 Hz, 1 H, OH); 4.68 m(1 H,
carboxamide
CH), 4.38 m(1 H, CH), 3.87 s
(3H, OCH3); 3.75 s (3H,
OCH3); 3.60 m (2H, OCH2);
3.04 dd (J = 14.3 Hz / 5.5
Hz, 1 H, CH); 2.96 dd (J =
14.3 Hz / 8.3 Hz, 1 H, CH);
1.37 (J = 7.0 Hz, 6H, CH3).
339 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.64 dd (J = 8.5
isopropyl-1 H-indol-3-yl) ethyl]- Hz, 1 H, NH); 8.01 d (J = 7.7 N~
2-(3,5-difluoro-4- Hz, 1 H, aryl); 8.00 s (1 H, Ho
methoxyphenyl)-6- aryl); 7.96 d (J = 6.0 Hz, 2H, O H
0,
methoxyquinoline-4- aryl); 7.66 d (J = 7.8 Hz, 1 H,
F ~ I N /
carboxamide; aryl); 7.49 s(1 H, aryl); 7.45 o I~
m(2H, aryl); 7.34 s(1 H, aryl) F
N-[ (R)-1-(Methoxycarbonyl)-2- 7.10 t(J - 7.4 Hz, 1 H, aryl);
(1-isopropyl -1 H-indol-3-yl) e- 7.00 t (J = 7.9 Hz, 1 H, aryl);
thyl]-2-(3, 5-difluoro-4- 4.92 t (J = 5.4 Hz, 1 H, OH);
methoxyphenyl)-6- 4.69 m(1 H, CH); 4.39 m
methoxyquinoline-4-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
266
Product; Method ~ Structure
Ex. reagents 9o~sto H-NMR (400 MHz) S[ppm]
carboxamide (1 H, CH); 4.03 s (3H, OCH3);
3.74 s (3H, OCH3); 3.60 m
(2H, OCH2); 3.04 dd (J =
14.4 Hz / 5.6 Hz, 1 H, CH);
2.96 dd (J = 14.4 Hz / 8.4
Hz, 1 H, CH); 1.37 t (J = 7.0
Hz, 6H, CH3).
340 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.66 d(J= 8.7
isopropyl-1 H-indol-3-yl) ethyl]- Hz, 1 H, NH); 8.01 d (J = 7.6 N
6-methoxy-2-(3,4,5- Hz, 1 H, aryl); 7.99 s (1 H, Ho
0 NH
trimethoxyphenyl)quinoline-4- aryl); 7.66 d (J = 8.2 Hz, 1 H, o,
carboxamide; aryl); 7.50 s (2H, aryl); 7.46 'o N
m (3H, aryl); 7.33 s(1 H, a- 'o ;
N-[ (R)-1-(Methoxycarbonyl)-2-
ryl); 7.10 t (J = 7.7 Hz, 1 H,
(1-isopropyl-1 H-indol-3-yl) aryl); 6.98 t (J = 7.6 Hz); 1 H,
ethylJ-6-methoxy-2-(3, 4, 5-
aryl); 4.91 t (J = 5.4 Hz, 1 H,
trimethox yph en yl) quinoline-4-
OH); 4.68 m(1 H, CH); 4.41
carboxamide m(1 H, CH); 3.91 s(6H,
OCH3); 3.75 s (3H, OCH3);
3.73 s (3H, OCH3); 3.61 m
(2H, OCHZ); 3.03 dd (J =
14.4 Hz / 5.7 Hz, 1 H, CH);
2.97 dd (J = 14.4 Hz / 8.2
Hz, 1 H, CH); 1.36 t(J = 6.3
Hz, 6H, CH3).
341 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.76 d (J = 7.7
ethyl)-1 H-indol-3-yl) ethyl]-2-(3- Hz, 1 H, NH). 8.20 d (J = 9.0
fluoro-4-methoxyphenyl)-6- Hz, 1 H, aryl); 8.13 m (2H, HO
0 H
trifluoromethoxyquinoline-4- aryl), 8.06 s (1H,aryl); 8.01 s o F
X
carboxamide (1 H, aryl), 7.76 d (J = 7.4 Hz, F N F F
1 H, aryl); 7.65 d (J = 7.8 Hz,
N-[ (R)-1-(Methoxycarbonyl)-2-
1 H, aryl); 7.40 t (J = 7.4 Hz,
(1-ethyl)-1 H-indol-3-yl) ethyl]-2-
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
267
Product; Method ~ Structure
Ex. ' analo- H-NMR (400 MHz) S[ppm]
reagents gousto
(3-fluoro-4-methoxyphenyl)-6- 2H, aryl); 7.24 s (1 H, aryl);
trifluoromethoxyquinoline-4- 7.10 t (J = 7.8 Hz, 1 H, aryl);
carboxamide 6.97 t (J = 7.4 Hz, 1 H, aryl);
4.92 t (J = 5.4 Hz, 1 H, OH);
4.36m(1H,CH);4.14q(J=
7.0 Hz, 2H, CHZ); 3.96 s (3H,
OCH3); 3.60 m (2H, OCH2);
3.05 dd (J = 14.3 Hz / 5.6
Hz, 1 H,CH); 2.97 dd (J =
14.3 Hz / 8.2 Hz, 1 H, CH);
1.29 t (J = 7.0 Hz, 3H, CH3).
342 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.86 d (J = 8.2
ethyl)-1 H-indol-3-yl) ethyl]-2-(7- Hz, 1 H, NH); 8.25 d (J = 9.4
methoxybenzofuran-2-yl)-6- Hz, 1 H, aryl); 8.11 s(1 H, "o H
trifluoromethoxyquinoline-4- aryl); 7.96 s(1 H, aryl); 7.83 F
\ . ~ / F F
carboxamide; m (2H, aryl); 7.66 d (J = 7.8 o N
Hz, 1 H, aryl); 7.42 d (J = 8.2 p
N-[ (R)-1-(Methoxycarbonyl)-2-
Hz, 1 H, aryl); 7.37 d (J = 7.8
(1-ethyl)-1H-indol-3-yl) ethylJ-2-
Hz, 1 H, aryl); 7.30 t (J = 7.7
(7-methoxybenzofuran-2-yl)-6- Hz, 2H, aryl); 7.25 s(1H,
trifluoromethoxyquinoline-4-
aryl); 7.09 m (2H, aryl); 7.00
carboxamide
t (J = 7.1 Hz, 1 H, aryl); 4.95 t
(J = 5.4 Hz, 1 H, OH); 4.38 m
(1 H, CH); 4.16 q (J = 7.1 Hz,
2H, CHZ); 4.02 s (3H, OCH3);
3.61 m (2H, OCHZ); 3.05 dd
(J = 14.2 Hz / 5.3 Hz,
1 H,CH); 2.94 dd (J = 14.2 Hz
/ 8.1 Hz, 1 H, CH); 1.29 t(J =
7.1 Hz, 3H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
268
Product; Method Structure
Ex. reagents 9o~sto 'H-NMR (400 MHz) S[ppm]
343 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.76 d (J = 8.6
isopropyl -1 H-indol-3-yl) ethyl]- Hz, 1 H, NH); 8.20 d (J = 9.4 N
2-(3-fluoro-4-methoxyphenyl)-6- Hz, 1 H, aryl); 8.06 m (4H, Ho
0 H
trifluoromethoxyquinoline-4- aryl); 7.77 d (J = 9.0 Hz, 1 H, 0 F
carboxamide; aryl); 7.65 d (J = 8.2 Hz, 1 H, F N
0
aryl); 7.40 d (J = 8.2 Hz, 1 H,
N-[ (R)-1-(Methoxycarbonyl)-2-
aryl); 7.37d (J = 9.0 Hz, 1 H,
(1-isopropyl -1 H-indol-3-yl) e-
aryl); 7.33 s(1 H, aryl), 7.09 t
thyl]-2-(3-fluoro-4-
(J = 7.4 Hz, 1 H, aryl), 6.97 t
methoxyphenyl)-6-
(J = 7.4 Hz, 1 H, aryl); 4.93 t
trifluoromethoxyquinoline-4- (J = 5.5 Hz, 1 H, OH); 4.68 m
carboxamide (1 H,CH); 4.37 m(1 H, CH);
3.96 s (3H, OCH3); 3.60 m
(2H, OCH2); 3.06 dd (J =
14.4 Hz / 5.6 Hz, 1 H, CH);
2.94 dd (J = 14.4 Hz / 8.1
Hz, 1 H, CH); 1.37 t(J =
7.0Hz, 6H, CH3).
344 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.86 d (J = 8.4
isopropyl-1 H-indol-3-yl) ethyl]- Hz, 1 H, NH); 8.25 d (J = 9.4
2-(7-methoxybenzofuran-2-yl)- Hz, 1 H, aryl); 8.11 s(1 H, HO H H
6-trifluoromethoxyquinoline-4- aryl); 7.98 s(1 H, aryl); 7.82 i >CF
N
carboxamide; m (2H, aryl); 7.65 d (J = 7.8 o
Hz, 1 H, aryl); 7.44 d (J = 8.2
N-( (R)-1-(Methoxycarbonyl)-2-
Hz, 1 H, aryl); 7.33 m (3H,
(1-isopropyl-1 H-indol-3-yl) e-
aryl); 7.29 t (J = 8.3 Hz, 1 H,
thyl]-2-(7-methoxybenzofuran-
aryl); 7.09 m (2H, aryl); 7.00
2-yl)-6- t (J = 7.4 Hz, 1 H, aryl); 4.95 t
trifluoromethoxyquinoline-4- (J - 5.5 Hz, 1 H, OH); 4.70 m
carboxamide (1 H, CH); 4.41 m(1 H, CH);
4.01 s (3H, OCH3); 3.61 m
(2H, OCH2); 3.05 dd (J =
14.3Hz / 5.5 Hz, 1 H, CH);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
269
Product; Method Structure
Ex. reagents gousto 'H-NMR (400 MHz) S[ppm]
2.94 dd (J = 14.3 Hz / 8.2
Hz, 1 H, CH); 1.40 d(J = 6.3
Hz, 3H, CH3); 1.37 d (J = 6.7
Hz, 3H, CH3).
345 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.65 d(J= 8.6
n hexyl-1 H-indol-3-yl) ethyl]-6- Hz, 1 H, NH); 8.00 d (J = 7.5
methoxy-2-(3,4,5- Hz, 1 H, aryl); 7.99 s(1 H, N~ i
trimethoxyphenyl)quinoline-4- aryl); 7.65 d (J = 8.3 Hz, 1 H, Ho
carboxamide; aryl); 7.50 s (2H, aryl), 7.45 O NH
o,
m(3H, aryl); 7.22 s(1 H, a- -o N
N-((R)-1-(Methoxycarbonyl)-2- ,o ~o
(1-n hexyl-1 H-indol-3-yl) ethylJ- ryl); aryl); 7.09 t (J = 7.6 Hz, 1 H, 6.99
t (J = 7.5 Hz, 1 H,
6-methoxy-2-(3,4,5- aryl); 4.91 t (J = 5.3 Hz, 1 H,
trimethoxyphenyl) quinoline-4-
OH); 4.39 m (1 H, CH); 4.06 t
carboxamide
(J = 7.1 Hz, 2H, CH2); 3.91 s
(6H, OCH3); 3.75 s (3H,
OCH3); 3.73 s (3H, OCH3);
3.61 t (J = 5.5 Hz, 2H,
OCH2); 3.02 dd (J = 14.3 Hz
/ 5.5 Hz, 1 H, CH); 2.98 dd (J
= 14.3 Hz / 8,1 Hz, 1 H, CH),
1.62 m (2H, CH2); 1.10 m
(6H, CHZ); 0.73 m (3H, CH3).
346 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.42 d (J = 7.8
ethyl)-1 H-indol-3-yl) ethyl]-4- Hz, 1 H, NH); 8.13 s(1 H, a-
ethoxy-3'-methoxybiphenyl-3- ryl); 7.70 d (J = 8.5 Hz, 1 H, Ho H
carboxamide; aryl); 7.69 d (J = 7.8 Hz, 1 H, O NH
CH); 7.40 m (2H, aryl); 7.29 1~
N-[ (R)-1-(Methoxycarbonyl)-2- m (3H, aryl); 7.12 m (2H, (1-ethyl) -1H-indol-3-
yl) ethylJ- aryl), 6.99 t (J - 7.4 Hz, 1 H,
4-ethoxy-3'-methoxybiphenyl-3-
aryl); 6.92 d (J = 6.3 Hz, 1 H,
carboxamide
aryl); 4.94 t (J = 5.5 Hz, 1 H,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
270
Product; Method Structure
Ex. reagents go~sto 'H-NMR (400 MHz) S[ppm]
OH); 4.24 m(1 H, CH); 4.14
q (J = 7.0 Hz, 2H, CH2); 3.82
s (3H, OCH3); 3.50m (2H,
OCH2); 2.98 m (2H, CH2);
1.30 m (6H, CH3);
347 N-[ (R)-1-(Hydroxymethyl)-2-(1- 335 (DMSO-d6): 8.40 d (J = 8.2
isopropyl) -1 H-indol-3-yl) ethyl]- Hz, 1 H, NH); 8.13 s(1 H, a- N~
4-ethoxy-3'-methoxybiphenyl)- ryl); 7.76 d (J = 8.5 Hz, 1 H, HO
3-carboxamide; aryl); 7.70 d (J = 7.8 Hz, 1 H, 0 NH
aryl); 7.43 d (J = 8.2 Hz, 1 H, o
N-[ (R)-1-(Methoxycarbonyl)-2- aryl); 7.36 t (J = 7.8 Hz, 1 H,
(1-isopropyl) -1 H-indol-3-yl)
aryl); 7.29 s(1 H, aryl); 7.19 t
ethylJ-4-ethoxy-3'
(J = 8.2 Hz, 2H, aryl); 7.13 s
methoxybiphenyl)-3-
(1 H, aryl); 7.10 t (J = 7.8 Hz,
carboxamide
1 H, aryl); 6.98 t (J = 7.4 Hz,
1 H, aryl); 6.93 d (J = 7.4 Hz,
1 H, aryl); 4.94 s (J = 5.4 Hz,
1 H, OH); 4.76 m(1 H, CH)
4.24 m(1 H, CH); 4.14 q (J
=
7.0 Hz, 2H, CH2); 3.82 s (3H,
OCH3); 3.47 m (2H, OCHZ);
2.98 m (2H, CH2); 1.40 t (J
=
6.6 Hz, 6H, CH3); 1.31 t (J
=
7.0 Hz; 3H, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
271
Product; Method Structure
Ex. reagents 9oUSto 'H-NMR (400 MHz) S[ppm]
348 N-[(R)-2-(1-Ethyl-1H-indol-3-yl)- 39 (CDCI3): 7.73 s(1H); 7.70 d ~~
1-(hydroxymethyl)ethyl]-3'- (J = 8.0 Hz, 1 H); 7.61 d (J =
fluoro-4'-methoxy[1,1'- 7.6 Hz, 1 H); 7.56 d (J = 7.8
J= 7.8 Hz HO NH
biphenyl]-3-carboxamide; Hz, 1 H); 7.41 dd (
/ 7.6 Hz, 1 H); 7.35 d (J = 8.0
1-Ethyl-L-tryptophanol and
Hz, 1 H); 7.28-7.20 m (3H);
7
3'-Fluoro-4' methoxy[1,1' 7.12 dd (J = 8.0 Hz / 7.0 Hz, F~ I
biphenyl]-3-carboxylic acid 1 H); 7.04 s(1 H); 7.02 dd (J
.
= 8.0 Hz / 7.0 Hz, 1 H); 6.51
d (J = 6.6 Hz, 1 H); 4.48 m
(1 H); 4.14 q(J = 7.3 Hz, 2H);
3.94 s (3H); 3.85 m(1 H);
3.80 m(1 H); 3.18 dd (J =
14.7 Hz / 7.1 Hz, 1 H); 3.15
dd (J = 14.7 Hz / 6.6 Hz,
1 H); 1.42 t(J = 7.3 Hz, 3H).
349 3'-Fluoro-N-[(R)-1- 39 (DMSO-d6): 8.28 d(J= 8.3 /~
(hydroxymethyl)-2-(1-propyl- Hz, 1 H); 8.03 s(1 H); 7.78 d
1 H-indol-3-yl)ethyl]-4'- (J = 7.8 Hz, 1 H); 7.76 d (J =
HO H
methoxy[1,1'-biphenyl]-3- 7.6 Hz, 1 H); 7.68 d (J = 8.0 N
carboxamide; Hz, 1 H); 7.65 dd (J = 13.1
Hz / 2.3 Hz, 1 H); 7.53 d(J =
1-Propyl-L-tryptophanol and
8.8 Hz, 1 H); 7.49 dd (J = 7.8 F
O
3'-Fluoro-4' methoxy[l,1' Hz / 7.6 Hz, 1 H); 7.28 d (J =
biphenyl]-3-carboxylic acid 8.0 Hz, 1 H); 7.28 dd (J = 8.8
Hz / 8.8 Hz, 1 H); 7.17 s(1 H);
7.09 dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.98 dd (J = 8.0 Hz / 7.0
Hz, 1 H); 4.82 m(1 H); 4.25 m
(1 H); 4.03 t (J = 6.8 Hz, 2H);
3.90 s (3H); 3.55 m (1H);
3.51 m (1 H); 3.04 dd (J =
14.2 Hz / 5.8 Hz, 1 H); 2.93
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
272
Product; Method Structure
Ex. reagents 9o~sto 'H-NMR (400 MHz) S[ppm]
dd (J = 14.2 Hz / 7.6 Hz,
1 H); 1.66 m (2H); 0.71 t (J
=
7.4 Hz, 3H).
350 N-[(R)-2-(1-Butyl-1 H-indol-3-yl)- 39 (DMSO-d6): 8.27 d (J = 8.3 ~~
1-(hydroxymethyl)ethyl]-3'- Hz, 1 H); 8.04 s(1 H); 7.78 d ~
~ N
fluoro-4'-methoxy[1,1'- (J = 7.8 Hz, 1 H); 7.76 d(J =
HO N
biphenyl]-3-carboxamide; 7.8 Hz, 1 H); 7.67 d (J = 8.0
Hz, 1 H); 7.65 dd (J = 12.9 0 ~ I
1-Butyl-L-tryptophanol and
Hz / 2.3 Hz, 1 H); 7.53 d (J
= ~ I
3'-Fluoro-4' methoxy(1,1' 8.8 Hz, 1 H); 7.49 dd (J = 7.8 F
biphenyl]-3-carboxylic acid Hz / 7.8 Hz, 1 H); 7.37 d(J =
8.0 Hz, 1 H); 7.28 dd (J = 8.8
Hz / 8.8 Hz, 1 H); 7.16 s(1 H);
7.09 dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.99 dd (J = 8.0 Hz / 7.0
Hz, 1 H); 4.82 m(1 H); 4.25 m
(1 H); 4.06 t (J = 6.9 Hz, 2H);
3.90 s (3H); 3.56 m(1 H);
3.51 m (1 H); 3.04 dd (J =
14.4 Hz / 5.8 Hz, 1 H); 2.93
dd (J = 14.4 Hz / 7.8 Hz,
1 H); 1.61 m(2H); 1.11 m
(2H); 0.72 t (J = 7.3 Hz, 3H).
351 3'-Fluoro-N-[(R)-l- 39 (DMSO-d6): 8.27 d (J = 8.3 ~~
(hydroxymethyl)-2-[1-(3- Hz, 1 H); 8.04 s(1 H); 7.78 d N
methylbutyl)-1 H-indol-3- (J = 7.8 Hz, 1 H); 7.76 d(J = Ho \
NH
yI]ethyl]-4'-methoxy[1,1'- 7.6 Hz, 1 H); 7.66 d (J = 8.0 0
biphenyl]-3-carboxamide; Hz, 1 H); 7.64 dd (J = 12.9
~
Hz / 2.3 Hz, 1 H); 7.53 d(J = ~ I
1-(3-Methylbutyl)-L-tryptophanol F
8.8 Hz, 1 H); 7.49 dd (J = 7.8 ~o
and Hz / 7.6 Hz, 1 H); 7.36 d(J =
3'-Fluoro-4' methoxy(1,1' 8.0 Hz, 1 H); 7.28 dd (J = 8.8
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
273
Product; Method Structure
Ex. eagents analo- 'H-NMR (400 MHz) S[ppm]
gousto
biphenyl]-3-carboxylic acid Hz / 8.8 Hz, 1 H); 7.16 s(1 H);
7.09 dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.99 dd (J = 8.0 Hz / 7.0
Hz, 1 H); 4.82 m(1 H); 4.25 m
(1 H); 4.07 t (J = 6.8 Hz, 2H);
3.89 s (3H); 3.56 m(1 H);
3.51 m (1 H); 3.04 dd (J =
14.4 Hz / 5.8 Hz, 1 H); 2.93
dd (J = 14.4 Hz / 8.1 Hz,
1 H); 1.51 td (J = 7.2 Hz / 7.0
Hz, 1 H); 1.36 m (2H); 0.77 d
(J = 7.4 Hz, 6H).
352 3'-Fluoro-N-[(R)-l- 39 (DMSO-d6): 8.27 d (J = 8.1 ~~
(hydroxymethyl)-2-(1-pentyl- Hz, 1 H); 8.03 s(1 H); 7.78 d ~ N
1 H-indol-3-yl)ethyl]-4'- (J = 7.8 Hz, 1 H); 7.76 d(J = Ho ~
NH
methoxy[1,1'-biphenyl]-3- 7.6 Hz, 1 H); 7.66 d (J = 8.0 0
carboxamide; Hz, 1 H); 7.64 dd (J = 12.9
Hz / 2.3 Hz, 1 H); 7.53 d(J = F 1-Pentyl-L-tryptophanol and ~o
8.8 Hz, 1 H); 7.49 dd (J = 7.8
3' Fluoro-4' methoxy(1,1' Hz / 7.6 Hz, 1 H); 7.37 d (J =
biphenyl]-3-carboxylic acid 8.0 Hz, 1 H); 7.28 dd (J = 8.8
Hz / 8.8 Hz, 1 H); 7.17 s(1 H);
7.09 dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.98 dd (J = 8.0 Hz / 7.0
Hz, 1 H); 4.82 m(1 H); 4.25 m
(1 H); 4.05 t (J = 6.9 Hz, 2H);
3.89 s (3H); 3.56 m(1 H);
3.51 m (1 H); 3.04 dd (J =
14.7 Hz / 5.8 Hz, 1 H); 2.93
dd (J = 14.7 Hz / 8.1 Hz,
1 H); 1.63 m (2H); 1.13 m
(2H); 1.10 m (2H); 0.70 t (J =
7.1 Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
274
Product; Method Structure
Ex. reagents analo- 'H-NMR (400 MHz) S[ppm]
gousto
353 3'-Fluoro-N-[(R)-2-(1-hexyl-1 H- 39 (DMSO-d6): 8.27 d (J = 8.1 /~
indol-3-yl)-1- Hz, 1 H); 8.04 s(1 H); 7.78 d ~ N
(hydroxymethyl)ethyl]-4'- (J = 7.8 Hz, 1 H); 7.76 d(J = HO NH
methoxy[1,1'-biphenyl]-3- 7.6 Hz, 1 H); 7.66 d (J = 8.0 ~
carboxamide; Hz, 1 H); 7.65 dd (J = 12.9
Hz / 2.3 Hz, 1 H); 7.53 d(J = F
1-Hexyl-L-tryptophanol and "
8.8 Hz, 1 H); 7.49 dd (J = 7.8
3' Fluoro-4'-methoxy(1,1' Hz / 7.6 Hz, 1 H); 7.37 d (J =
biphenyl]-3-carboxylic acid 8.0 Hz, 1 H); 7.28 dd (J = 8.8
Hz / 8.8 Hz, 1 H); 7.17 s(1 H);
7.09 dd (J = 8.0 Hz / 7.0 Hz,
1 H); 6.98 dd (J = 8.0 Hz / 7.0
Hz, 1 H); 4.82 m(1 H); 4.25 m
(1 H); 4.05 t (J = 6.9 Hz, 2H);
3.89 s (3H); 3.56 m(1 H);
3.51 m (1 H); 3.04 dd (J =
14.4 Hz / 5.8 Hz, 1 H); 2.93
dd (J = 14.4 Hz / 8.1 Hz,
1 H); 1.61 m(2H); 1.10 m
(6H); 0.73 t (J = 7.1 Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
275
Example 354
4-Ethoxy-3'-methoxybiphenyl-3-carboxylic acid [2-(5,6-difluoro-1 H-indol-3-yl)-
1-
F
N ~ \
~ ~ F
HO
O NH
hydroxymethylethyl]amide;
354a) (5,6-Difluoro-1 H-indol-3-yl)-acetaldehyde
At 0 C, phosphoryl chloride (22.03 g) was slowly added dropwise to DMF (19.1
g), and
the mixture was stirred at 0-5 C for half an hour and then at room temperature
for one
hour. The mixture was cooled again to 0 C, and a solution of 5,6-difluoro-1 H-
indole
(20 g) in DMF (20 g) was slowly added dropwise. The mixture was stirred at 0 C
for
30 minutes and then at room temperature for a further 15 hours. The reaction
mixture
was poured onto ice (200 g) and basified to pH 10 with NaOH. The crystalline
title com-
pound was filtered off, washed with water and dried in vacuo (yield 22.7 g,
96%). MS
(ESI,+): 196 (M+1).
354b) [2-(5,6-Difluoro-1 H-indol-3-yl)ethyl]diethylamine
Sodium triacetoxyborohydride (26.3 g) was added in portions to a solution of
(5,6-
difluoro-1 H-indol-3-yl)acetaldehyde (15 g) and diethylamine (6.66 g) in
absolute di-
chloromethane (300 ml) with 2 drops of trifluoroacetic acid, and the mixture
was stirred
at room temperature for 24 hours. The solvent was distilled off in a rotary
evaporator,
and the residue was mixed with 10% strength aqueous sodium bicarbonate
solution and
extracted with ethyl acetate. The combined organic phases were dried over
sodium
sulphate and concentrated in a rotary evaporator. The crude product was
purified by
flash chromatography, and the title compound was obtained in 68% yield (13.5
g). MS
(ESI,+): 253 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
276
354c) Ethyl 3-(5,6-difluoro-1 H-indol-3-yl)-2-nitropropionate
A mixture of gramine (8 g) and ethyl 2-nitroacetate (8.9 g) was stirred in
absolute tolu-
ene at 90-100 C for 4 hours. The reaction mixture was concentrated in a rotary
evapo-
rator, and the crude product was purified by flash chromatography (chloroform
: metha-
nol 19 :1), after which the title compound was obtained in a yield of 11.7 g
as a 1:2 mix-
ture with ethyl 2-nitroacetate. MS (ESI,+): 299 (M+1).
354d) Ethyl 2-amino-3-(5,6-difluoro-1 H-indol-3-yl)propionate
The mixture from the above stage was stirred with ammonium formate (9.9 g) and
Pd
(4.1 g. 10% on activated carbon) in 300 ml of ethanol under reflux for 15
hours. The
reaction mixture was concentrated in a rotary evaporator, diluted with water
(100 ml)
and extracted with ethyl acetate. The combined organic phases were dried over
sodium
sulphate and concentrated in a rotary evaporator. The residue was purified by
flash
chromatography (silica, chloroform : methanol 19 :1) and recrystallized as HCI
salt from
ethanol. The title compound was obtained in a yield of 2.7 g. MS (ESI,+): 269
(M+1).
354e) 4-Ethoxy-3'-methoxybiphenyl-3-carboxylic acid [2-(5,6-difluoro-1H-indol-
3-yl)-1-
hydroxymethylethyl]amide
0.39 mmol (143 mg) of the acid was dissolved in 5 ml of dimethylformamide and,
at
room temperature, 0.39 mmol (59 mg) of 1-hydroxy-1 H-benzotriazole hydrate and
0.39 mmol (74 mg) of N-[3-(dimethylamino)propyl]-N'-ethylcarbodiimide
hydrochloride
were added. The mixture was stirred at the stated temperature for 60 minutes,
and then
0.3 mmol (80 mg) of the difluorotryptophan ethyl ester was added. After a
further hour,
the reaction mixture was added to saturated sodium bicarbonate solution, and
the pre-
cipitate was filtered and washed with water. Purification by chromatography on
silica gel
with the eluent cyclohexane/ethyl acetate affords 64 mg of the compound as
yellow
foam.
0.15 mmol (76 NI) of 2M lithium borohydride solution was added dropwise to a
solution
of 0.1 mmol (63mg) of the carboxamide in 2ml of THF at 0 C. This mixture is
then stirred
at room temperature for 4-6 hours. It was subsequently neutralized with 1 N
hydrochloric
acid at 0 C and, after addition of water, extracted with ethyl acetate. The
organic phase
was dried over magnesium sulphate, filtered and concentrated in vacuo.
Purification by
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
277
chromatography on silica gel with the eluent cyclohexane/ethyl acetate affords
37 mg of
pale yellow powder.
(DMSO-d6): 11.00 s(1 H) 8.40 d (J = 7.8 Hz, 1 H); 8.12 s(1 H); 7.77 d (J = 8.6
Hz, 1 H);
7.65 m (1 H); 7.36 m (3H); 7.20 m (3H); 7.13 s (1 H); 6.91 d (J = 9.7 Hz, 1
H); 4.98 t (J =
5.4 Hz, 1 H); 4.16 m (3H); 3.82 s (3H); 3.45 m(2H); 2.95 m (2H); 1.33 t (J =
7.0 Hz, 3H).
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents analo-
gousto [Ppm]
355 6-Methoxy-2-(3,4,5- 354 (DMSO-6): 11.00 s(1 H);
N F
trimethoxyphenyl)quinoline-4- 7 d (J = 8.6 Hz, 1 H); 8.00 F
carboxylic acid [2-(5,6-difluoro- d (J = 9.0 Hz, 1 H); 7.96 s HO
0 NH
1 H-indol-3-yl)-1- (1 H); 7.59 m(1 H); 7.49 s ~ 0"
H N I
hydroxymethylethyl]amide; (2); 7.42 d (6.6 Hz, 1 H);
7.31 m(1 H); 7.29 s(1 H);
2-Amino-3-(5, 6-difluoro-1 H- 4.93 t (J = 5.3 Hz, 1 H); /
indol-3-yl)-propan-1-ol 4.35 m(1 H); 3.92 s (6H);
and 3.75 s (6H); 3.60 t (J =
5.5 Hz, 2H); 3.00 dd (J =
6-Methoxy-2-(3, 4, 5- 14.3 Hz / 5.6 Hz, 1 H);
trimethoxyphenyl)quinoline-4- 2.90 dd (J = 14.4 Hz / 8.0
carboxylic acid Hz, 1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
278
Example 356
N-[ (R)-1-(Hydroxymethyl)-2-(1-ethyl-5-fluoro-1 H-indol-3-yl) ethyl]-6-methoxy-
2-
C -
N F
HO
O NH
O"
_O N
,O
(3,4,5-trimethoxyphenyl)quinoline-4-carboxamide; '
0.2 mmol (12 mg) of potassium hydroxide powder was added in portions, cooling
slightly with water, to a stirred solution of 0.09 mmol (50 mg) of 6-methoxy-2-
(3,4,5-
trimethoxyphenyl)quinoline-4-carboxylic acid [R-1-(5-fluoro-1 H-indol-3-
ylmethyl)-2-
hydroxyethyl]amide in 1 ml of DMSO. This mixture was stirred for 5 minutes and
then
0.2 mmol (17.2 NI) of ethyl iodide, dissolved in 0.3 ml of DMSO, was added
dropwise.
Stirring was then continued at room temperature for 2 hours, and the reaction
mixture
was subsequently added to saturated aqueous ammonium chloride solution and ex-
tracted with ethyl acetate. The resulting organic phase was dried over
magnesium sul-
phate, filtered and concentrated in vacuo with addition of toluene.
Purification by chro-
matography on silica gel with the eluent cyclohexane/acetone affords 41.9 mg
of the
compound as pale yellow foam.
(DMSO-d6): 8.67 d (J = 8.6 Hz, 1 H, NH); 8.01 d (J = 8.5 Hz, 1 H, aryl); 7.98
s (1 H, aryl);
7.50 s (2H, aryl); 7.42 m (4H, aryl); 7.32 s (1 H, aryl); 6.94 t (J = 7.3 Hz,
1 H, aryl), 6.94 t
(J = 7.3 Hz, 1 H, aryl); 4.91 t (J = 5.4 Hz, 1 H, OH); 4.36 m(1 H, CH); 4.13 q
(J = 7.0 Hz,
CHZ); 3.92 s (6H, OCH3); 3.60 t (J = 5.5 Hz, 2H, OCH2); 2.98 dd (J = 14.4 Hz /
5.7 Hz,
1 H, CH); 2.92 dd (J = 14.4 Hz / 8.1 Hz, 1 H, CH); 1.27 t (J = 7.0 Hz, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
279
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents 9~ o IPpm]
357 6-Methoxy-2-(3,4,5- 356 (DMSO-d6): 8.67 d (J trimethoxyphenyl)quinoline-4-
8.6 Hz, 1 H, NH); 8.01 d (J carboxylic acid [(R)-2-(1-ethyl- = 8.5 Hz, 1H,
aryl); 7.98 s Ho
0 NH
5-fluoro-1 H-indol-3-yl)-1- (1 H, aryl); 7.50 s (2H, a- oN
hydroxymethylethyl]amide; ryl); 7.42 m (4H, aryl); '0 N
7.32 s(1 H, aryl); 6.94 t (J - ;
6-Methoxy-2-(3, 4, 5- = 7.3 Hz, 1 H, aryl), 6.94 t
trimethoxyphenyi)quinoiine-4-
(J = 7.3 Hz, 1 H, aryl); 4.91
carboxylic acid((R)-1-(5-fluoro- t (J = 5.4 Hz, 1 H, OH);
1 H-indol-3-ylmethyl)-2- 4.36 m(1 H, CH); 4.13 q (J
hydroxyethylJamide
= 7.0 Hz, CH2); 3.92 s
(6H, OCH3); 3.60 t (J = 5.5
Hz, 2H, OCH2); 2.98 dd (J
= 14.4 Hz / 5.7 Hz, 1 H,
CH); 2.92 dd (J = 14.4 Hz
/ 8.1 Hz, 1 H, CH); 1.27 t(J
= 7.0 Hz, CH3).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
280
Example 358
6-(3,4,5-Trimethoxyphenyl)quinoline-8-carboxylic acid [(R)-1-hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide;
H
~ N
\
HO
O NH
N
o
358a) 6-Bromoquinoline-8-carboxylic acid
Concentrated sulphuric acid (20.5 mi) was added to a soluton of 2-amino-5-
bromobenzoic acid (25 g), glycerol (35 ml) and nitrobenzene (7.3 ml) (highly
exother-
mic) and the reaction was stirred at 150 C for 5 hours. The cooled reaction
mixture was
poured into ice-water (750 ml), and KOH (22.4 g) was added. The precipitate
was fil-
tered off, and the residue on the filter was dissolved in KOH (5 g) in water
(350 ml). Ac-
tivated carbon was added, and the mixture was stirred at 50 C for half an
hour. The
mixture was filtered through a short layer of silica gel, and the filtrate was
acidified with
acetic acid. The resulting precipitate was filtered off, washed with water and
dried in air.
Recrystallization from acetonitrile yielded 7.5 g (26%) of the title compound.
MS
(ESI,+): 253 (M+1).
358b) 6-Bromoquinoline-8-carboxylic acid [(R)-1-hydroxymethyl-2-(1H-indol-3-
yl)ethyl]amide
The quinolinecarboxylic acid was reacted with (D)-tryptophanol to give the
title com-
pound in analogy to general method 113b.
358c) 6-(3,4,5-Trimethoxyphenyl)quinoline-8-carboxylic acid [(R)-1-
hydroxymethyl-2-
(1 H-indol-3-yl)ethyl]amide
The aryl bromide was arylated under the Suzuki conditions to give the title
compound in
analogy to general method 125e.
(DMSO-d6): 11.17 d (J = 7.8 Hz, 1 H); 10.83 s(1 H); 8.87 s (2H); 8.52 dd (J =
1.5 Hz /
8.3 Hz, 1 H); 8.44 s (1 H); 7.73 d (J = 7.8 Hz, 1 H); 7.60 m (2H); 7.33 d (J =
7.8 Hz, 1 H);
7.26 s(1 H); 7.05 m (3H); 6.94 m(1 H); 5.03 m(1 H); 4.42 m(1 H); 3.88 s (6H);
3.71 s
(3H); 3.67 m(1 H); 3.54 m(1 H); 3.12 m (2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
281
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents y~ o [ppm]
359 3-(3,4,5-Trimethoxyphenyl)- 113b (DMSO-ds): 10.79 s(1 H); H
N
naphthalene-l-carboxylic acid 8.40 d (J = 8.3 Hz, 1 H);
[(R)-1-hydroxymethyl-2-(1 H- 125e 8.24 s(1 H); 7.99 m(1 H);
HO
indol-3-yl)ethyl]amide; 7.75 d (J = 1.7 Hz, 1 H); O NH
7.65 d (J = 7.7 Hz, 1 H);
3-Bromo-naphthalene-1- 7.52 m(1 H); 7.44 m(1 H);
carboxylic acid ((R)-1- 0
7.30 d(J = 7.9 Hz, 1 H);
hydroxymethyl-2-(1 H-indol-3-
7.18 s(1 H); 7.03 m (4H);
O
yl)ethylJamide ~
6.92 m(1 H); 4.83 m(1 H);
and 4.33 m(1 H); 3.87 s (6H);
3.70 s (3H); 3.58 m(1 H);
3,4,5-Trimethoxyphenylboronic 3.51 m (1 H); 3.04 m(1H);
acid 2.92 m (1 H).
360 4-Methoxy-5-(3,4,5- 113b (CDCI3): 10.81 s(1 H); N
trimethoxyphenyl)thiophene-3- 7.95 s(1 H); 7.88 d (J =
carboxylic acid [(R)-2-hydroxy- 125e 8.3 Hz, 1 H); 7.68 d (J =
1-(1 H-indol-3- 7.8 Hz, 1 H); 7.33 (J = 7.8 HO
O H
ylmethyl)ethyl]amide; Hz, 1 H); 7.17 s(1 H); 7.06
5-Bromo-4-methoxy-thiophene- m(1 H); 6.98 m(1 H); 6.90 O S
3-carboxylic acid ((R)-2- s(2H); 4.94 m(1 H); 4.25
hydroxy-1-(1H-indol-3- m(1H); 3.82 s (6H); 3.70 %
ylmethyl)ethylJamide s (3H); 3.54 s (3H); 3.51 -'0
and m(1 H); 3.46 m(1 H); 2.97
3, 4, 5-Trimethoxyphenylboronic m (2H).
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
282
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents g ~ o [ppm]
361 6-(3,4,5-Trimethoxyphenyl)-1 H- 113b (DMSO-d6): 12.94 s(1 H); N
benzoimidazole-4-carboxylic 10.73 s(1 H); 10.02 d (J
acid [(R)-2-hydroxy-1-(1 H- 125e
7.7 Hz, 1 H); 8.47 s(1 H); Ho
indol-3-ylmethyl)ethyl]amide; 8.10 s(1 H); 7.93 s(1 H); o H
6-Bromo-1 H-benzimidazole-4- 7.70 d (J = 7.7 Hz, 1 H);
carboxylic acid [(R)-2-hydroxy- 7.28 d (J = 7.9 Hz, 1 H); o \>
N
1-(1H-indol-3- 7.14 s(1H); 6.91 m(4H); H
ylmethyl)ethylJamide
4.92 m(1 H); 4.33 m(1 H);
and 3.85 s (6H); 3.68 s (3H);
3,4,5-Trimethoxyphenylboronic 3.56 m ~~ m ~~N), . 2.98 m {2,u N,
acid
362 2-(3,4,5- 125 (DMSO-d6): 10.77 s(1 H); S
NTrimethoxyphenyl)thiazole-4- 8.23 s(1 H); 8.04 d(J =
carboxylic acid [(R)-2-hydroxy- 8.6 Hz, 1 H); 7.66 d (J =
HO
1-(1 H-indol-3- 7.8 Hz, 1 H); 7.28 d(J = o H
ylmethyl)ethyl]amide; 8.1 Hz, 1 H); 7.23 s (2H);
7.15 s(1 H); 7.01 m(1 H); N~ s
(D)-Tryptophanol 6.92 m(1 H); 4.91 m(1 H);
o
and 4.19 (1 H); 3.87 s (6H); 3.70 s (3H); 3.56 m(1 H); 0
~
2-(3,4,5- 3.49 m(1H); 2.99 m (2H).
Trimethoxyphenyl)thiazole-4-
carboxylic acid
363 5-(3,4,5- 125 (DMSO-d6): 10.73 s(1 H); H
Trimethoxyphenyl)thiophene-2- 8.17 d (J = 8.3 Hz, 1 H);
carboxylic acid [(R)-2-hydroxy- 7.91 s (1 H); 7.74 d(J =
HO
1-(1 H-indol-3- 4.0 Hz, 1 H); 7.60 d (J = o H
ylmethyl)ethyl]amide; 7.8 Hz, 1 H); 7.48 d (J =
s
3.8 Hz, 1 H); 7.28 d (J =
(D)-Tryptophanol 8.1 Hz, 1 H); 7.10 s(1 H); o
and 7.01 m(1H); 6.90 m(1H); _o
0
4.80 m(1 H); 4.15 m(1 H); ~
5-(3,4,5- 3.81 s (6H); 3.64 s (3H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
283
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents g ~ o [ppm]
Trimethoxyphenyl)thiophene-2- 3.48 m (2H); 2.97 m (1 H);
carboxylic acid 2.85 m(1 H).
364 5-(3,4,5-Trimethoxyphenyl)- 125 (DMSO-d6): 10.73 s(1 H); N
benzo[b]thiophene-2-carboxylic 8.52 d (J = 8.1 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H-indol- 8.16 d (J = 1.5 Hz, 1 H);
HO
3-ylmethyl)ethyl]amide; 8.14 s(1 H); 8.02 d (J = 0 H
8.3 Hz, 1 H); 7.73 dd (J =
(D)-Tryptophanol s
2.0 Hz / 8.6 Hz, 1 H); 7.61
and d (J = 8.1 Hz, 1 H); 7.28 d
(J = 8.1 Hz, 1 H); 7.12 s ~\
5-(3, 4, 5-Trimethoxyphenyl)- ~ _
(1H);7.01 m(1H);6.95s
benzo[b]thiophene-2-carboxylic (2H); 6.92 m(1 H); 4.82 m 0 \ / 0
acid (1 H); 4.18 m(1 H); 3.85 s
(6H); 3.67 s (3H); 3.50 m
(2H); 3.01 m(1 H); 2.95 m
(1 H).
365 2-(3-Fluoro-4-methoxyphenyl)- 125 (DMSO-d6): 10.74 s(1 H); - H
N-[(R)-2-hydroxy-1-(1 H-indol-3- 8.46 d(J - 8.3 Hz, 1 H); i
ylmethyl)ethyl]-6-methyl- 7.91 m (3H); 7.62 d (J =
HO
isonicotinamide; 7.7 Hz, 1 H); 7.47 s(1 H); 0 NH
(D)-Tryptophanol 7.26 m(2H); 7.11 s(1 H);
I
and 7.01 m(1 H); 6.93 m(1 H); F ~ N
2-(3-Fluoro-4-methoxyphenyl)- 4.81 m(1 H) 2.53 s(3H). ~o I~
6-methyl-isonicotinic acid
366 2-(3-Fluoro-4-methoxyphenyl)- 125 (CDC13): 8.39 d (J = Hz, - N
6-methylpyrimidine-4-carboxylic 1 H); 8.31 s(1 H); 8.23 d (J
~ l i
acid [(R)-2-hydroxy-1-(1 H-indol- = 8.4 Hz, 1 H); 8.00 dd (J = Ho
3-ylmethyl)ethyl]amide; 12.9 Hz / 2.3 Hz, 1 H); O NH
7.81 s(1 H); 7.72 d (J = N' I
(D)-Tryptophanol 7.8 Hz, 1 H); 7.42 d (J = F Nz~ N
and 8.1 Hz, 1 H); 7.22 m (2H); "o
7.12 m(1 H); 7.01 m(1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
284
Product; Method +H-NMR (400 MHz) 8 Structure
Ex. reagents 9~ o Ippm]
2-(3-Fluoro-4-methoxyphenyl)- 4.49 m(1 H); 3.97 s(3H);
6-methylpyrimidine-4-carboxylic 3=89 m (2H); 3.20 m (2H);
acid 2.71 s (3H).
367 6-(4-Methoxyphenyl)pyrimidine- 125 (DMSO-d6): 10.79 s(1H);
4-carboxylic acid [(R)-1- 9.28 s(1 H); 8.69 d (J
hydroxymethyl-2-(1 H-indol-3- 8.9 Hz, 1 H); 8.42 s(1 H); NH
yI)ethyl]amide; 8.27 d (J = 9.0 Hz, 1 H);
(D)-Tryptophanol 7.70 d (J = 7.7 Hz, 1 H); OH o N\
and 7.33 d (J = 7.9 Hz, 1 H); 1
7.14 m (3H); 7.06 m(1 H); N
6-(4-Methoxyphenyl)pyrimidine-
6.98 m(1 H); 4.28 m(1 H);
4-carboxylic acid
3.87 s (3H); 3.52 m (2H); 3.03 m (2H). 0
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
285
Example 368
2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinazoline-4-carboxylic acid [(R)-2-
H
N
HO
O NH
N~ O1~
O Zt,
hydroxy-l-(1H-indol-3-ylmethyl)ethyl]amide; F
368a) 5-Methoxyisatin sodium salt
A solution of 1 N KOH (100 ml) was slowly added in portions to a suspension of
5-methoxyisatin (17.7 g) in water (100 ml), and the mixture was heated to
about 40 C. It
was stirred until almost all the isatin had dissolved. The undissolved residue
was filtered
off, and the filtrate was evaporated to dryness in a rotary evaporator.
Absolute ethanol
(200 ml) was added to the residue, and the solid was stirred at room
temperature, and
the sodium salt of 5-methoxyisatin was filtered off and dried in vacuo at room
tempera-
ture. Yield 20.6 g (96%).
368b) [2-(3-Fluoro-4-methoxybenzoylamino)-5-methoxyphenyl]oxoacetic acid
Dimethylaminopyridine (3.5 g), and then triethylamine (75 ml) and subsequently
a solu-
tion of 3-fluoro-4-methoxybenzoyl chloride (37.7 g) in THF (200 ml) were added
drop-
wise to a solution of the sodium salt of 5-methoxyisatin (21.5 g) in THF (300
ml), and
the reaction mixture was stirred at room temperature for 20 hours. Water (30
ml) was
added to the reaction mixture and stirred for a 4 hours. The insoluble residue
was fil-
tered off and the filtrate was evaporated to dryness. The residue was again
dissolved in
water (900 ml) and acidfied to pH 1 with 1 N HCI. The precipitate which
separated out
was filtered off, washed with water and dried in air. Recrystallization from
benzene
yielded 11.1 g (32%) of the title compound. MS (ESI,+): 348 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
286
368c) 2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinazoline-4-carboxylic acid
Anhydrous ammonia (5 g) was added to a solution of [2-(3-fluoro-4-
methoxybenzoylamino)-5-methoxyphenyl]oxoacetic acid (3.47 g) in ethanol (50
ml). The
reaction mixture was heated in a sealed tube at 120 C under autogenous
conditions for
6 hours. Solvent and ammonia were distilled out in a rotary evaporator, and
the dry
residue was suspended in water (100 ml) and acidified to pH 3-4 with acetic
acid. The
resulting precipitate was filtered off, washed with water and recrystallized
from ethanol
in an autoclave at 150 C. Yield 2.1 g (65%). MS (ESI,+): 329 (M+1).
368d) 2-(3-Fluoro-4-methoxyphenyl)-6-methoxyquinazoline-4-carboxylic acid [(R)-
2-
hydroxy-1 -(1 H-indol-3-ylmethyl)ethyl]amide
The title compound was obtained by reaction with (D)-tryptophanol in analogy
to gen-
eral method 113b.
(DMSO-d6): 10.81 s (1 H); 8.90 d (J = 8.6 Hz, 1 H); 8.31 m (2H); 8.12 s (1 H);
7.97 m
(1 H); 7.34 m (2H); 7.21 s(1 H); 7.00 m(1 H); 6.90 m(1 H); 4.95 m(1 H); 4.35
m(1 H);
3.93 s (3H); 3.82 m (3H); 3.58 m (2H); 3.08 m(1 H); 3.03 m(1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
287
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analto~
reagents IPpm]
369 2-(3-Fluoro-4-methoxyphenyl)- 368 (DMSO-d6): 10.81 s(1 H); - N
6-iodoquinazoline-4-carboxylic 9.11 s (1 H); 8.95 d (J = A i
acid [(R)-2-hydroxy-1-(1H-indol- Hz, 1 H); 8.35 m (2H); 8.23 H(D
3-ylmethyl)ethyl]amide; m (1 H); 7.81 d (J = 8.8 Hz, H 0
1 H); 7.66 d(J = 7.8 Hz,
N (D)-Tryptophanol
1 H); 7.29 m(2H); 7.21 s ~
o
and (1H); 7.00 m(1H); 6.90 m
(1 H); 4.93 m(1 H); 4.32 m
2-(3-Fluoro-4-methoxyphenyl)- (1 H); 3.94 s (3H); 3.58 m
6-iodoquinazoline-4-carboxylic (2H); 3.05 m (2H).
acid
370 2-(4-Methoxyphenyl)- 368 (DMSO-d6): 10.84 s(1 H); H
~ N
quinazoline-4-carboxylic acid 8.43 d (J = 8.6 Hz, 1 H);
[(R)-1-hydroxymethyl-2-(1 H- 8.48 m (2H); 8.39 d(J =
HO
indol-3-yl)ethyl]amide; 8.3 Hz, 1 H); 7.98 m (2H); 0 NH
7.66 m(1 H); 7.59 m(1 H);
(D)-Tryptophanol N
7.33 d (J = 7.3 Hz, 1 H); "N
and 7.21 d (J = 2.0 Hz, 1 H);
7.12 m (2H); 7.03 m (1H);
2-(4-methoxyphenyl)- 6.94 m(1 H); 4.93 m(1 H);
quinazoline-4-carboxylic acid 4.36 m(1 H); 3.85 s (3H);
3.56 m (2H); 3.08 m (1H);
3.01 m (1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
288
Product; Method 'H-NMR (400 MHz) S Structure
Ex. reagents analogous
O1S [ppm]
371 2-(3-Fluoro-4-methoxyphenyl)- 368 (DMSO-d6): 8.94 d (J 6-methoxy-
quinazoline-4- 8.6 Hz, 1 H); 8.34 m (2H);
N~
carboxylic acid [(R)-1-(1-ethyl- 8.17 s(1 H); 8.00 d (J = 9.4
HO
1 H-indol-3-ylmethyl)-2- Hz, 1 H); 7.69 d (J = 7.5 0 H
hydroxyethyl]amide; Hz, 2H) 7.38 t (J = 10.9 N~ \ 0
Hz, 2H); 7.28 s(1 H); 7.08 t . ~ ~
(R)-2-Amino-3-(1-ethyl-1H- N
(J = 7.5 Hz, 1 H; 6.96 t (J indol-3-yl)-propan-l-ol 7.1 Hz, 1 H); 4.98 t (J =
5.4 F
and Hz, 1 H); 4.37 m(1 H); 4.12
q(J = 7.0 Hz, 2H); 3.64 m
2-(3-Fluoro-4-methoxyphenyl)- (2H); 3.10 dd (J = 14.3 Hz
6-methoxy-quinazoline-4- / 5.6 Hz, 1 H); 3.03 dd (J =
carboxylic acid 14.3 Hz / 8.2 Hz, 1 H); 1.26
t (J = 7.0 Hz, 3H).
Example 372
2-(3,4,5-Trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-2-hydroxy-l-(1 H-
indol-
3-ylmethyl)-2-methylpropyl]amide;
H
X
N
HO
O NH
-O N I
-O
"o
3.33 mmol (1.11 ml) of 3M methylmagnesium bromide solution were added dropwise
to
a solution of 0.22 mmol (120 mg) of methyl (R)-3-(1 H-indol-3-yl)-2-{[2-(3,4,5-
trimethoxyphenyl)quinoline-4-carbonyl]amino}propionate in 4.5 ml of THF at 0
C. This
mixture is then stirred at room temperature for about 30 minutes and then
added to
saturated aqueous ammonium chloride solution and extracted with ethyl acetate.
The
organic phase was dried over magnesium sulphate, filtered and concentrated in
vacuo.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
289
Purification by chromatography on silica gel with the eluents
cyclohexane/ethyl acetate
affords 107 mg of pale red foam.
(DMSO-d6):10.81 s(1 H); 8.50 d (J = 9.7 Hz, 1 H); 8.04 d (J = 8.7 Hz, 1 H);
7.74 s(1 H);
7.72 d (J = 7.4 Hz, 1 H); 7.62 d (J = 7.9 Hz, 1 H); 7.49 d (J = 8.3 Hz, 1 H);
7.46 s (2H);
7.41 d(J=7.8Hz, 1 H); 7.36 d (J = 8.2 Hz, 1 H); 7.18 s (1 H); 7.06 t (J = 7.8
Hz, 1H);
6.96 t (J = 7.4 Hz, 1 H); 4.68 s (1 H); 4.40 t (J = 9.8 Hz, 1 H); 3.92 s (6H);
3.74 s(3H);
3.26 d (J = 14.1 Hz, 1 H); 2.83 t (J = 14.4 Hz, 1 H); 1.38 s (3H); 1.27 s
(3H).
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) g Structure
X. reagents g ~ o [ppm]
373 6-Methoxy-2-(3,4,5- 372 (DMSO-d6): 10.77 s(1 H); N -
trimethoxyphenyl)quinoline-4- 8.53 d (J = 9.4 Hz, 1 H);
carboxylic acid [(R)-2- 7.97 d (J = 9.0 Hz, 1 H); "o
O NH
hydroxy-1-(1 H-indol-3- 7.68 s (1 H); 7.60 d (J = o,
ylmethyl)-2- 7.8 Hz, 1 H); 7.41 s (3H); -o ~ N methylpropyl]amide; 7.31 d (J =
8.2 Hz, 1 H); -o
"o
7.21 d (J = 9.8 Hz, 2H);
Methyl (R)-3-(1 H-indol-3-yl)-2- 7.04 t (J = 7.7 Hz, 1 H);
{(6-methoxy-2-(3, 4, 5-
6.94 t (J = 7.8 Hz, 1 H);
trimethox yphen yl) quinoline-4-
4.69 s(1 H); 4.38 t (J =
carbonyl]amino}propionate
10.1 Hz, 1 H); 3.92 s (6H);
3.75 s (3H); 3.63 s (3H);
3.24 d (J = 14.0 Hz, 1 H);
2.82 t (J = 14.7 Hz, 1 H);
1.37 s (3H); 1.27 s (3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
290
Example 374
6-(4-Hydroxybut-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid
[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
H ~
N ~
HO
O NH OH
I / \
N
o
374a1 6-lodo-2-(3-4.5-trimethoxvnhenvl)auinoline-4-carboxvlic acid
--, - ---- - .-. ,- _.....-- - õ- , , , .
The title compound was obtained in analogy to general methods 13a. MS (ESI,+):
466
(M+1).
374b) Methyl 6-iodo-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylate
The carboxylic acid (1 g) was dissolved in methanol (100 ml) and acidified
with a few
drops of conc. sulphuric acid. The mixture was stirred at room temperature
overnight,
and the title compound was precipitated by addition of water, filtered off and
washed
with water, and the residue was dried in vacuo. Yield 910 mg. MS (ESI,+): 480
(M+1).
374c) Methyl 6-(4-hydroxybut-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-
carboxyate
Methyl 6-iodo-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylate (1 g), but-3-
yn-l-ol
(0.32 ml), Cul (397 mg), Pd(PPh3)4 (241 mg) and triethylamine (2.89 ml) were
sus-
pended in THF (20 ml) and stirred together at room temperature overnight. The
mixture
was added to water and extracted with ethyl acetate. The combined organic
phases
were dried over sodium sulphate and freed of solvent in a rotary evaporator.
The title
compound was obtained after flash chromatography in 41 % yield (360 mg). MS
(ESI,+):
422 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
291
374d) 6-(4-Hydroxybut-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic
acid
The title compound was obtained in analogy to general method 39b.
374e) 6-(4-Hydroxybut-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic
acid
[(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide
The title compound was obtained by reaction with (D)-tryptophanol in analogy
to gen-
eral method 113b.
(DMSO-d6): 10.79 s(1 H); 8.70 d(J = 8.3 Hz, 1 H); 8.01 m (3H); 7.65 m(2H);
7.50 s
(2H); 7.32 d (J = 7.8 Hz, 1 H); 7.20 s (1 H); 7.02 m (1 H); 6.93 m (1 H); 4.31
m (1 H); 3.89 s
(6H); 3.73 s (3H); 3.60 m (4H); 3.03 m(1 H); 2.95 m(1 H); 2.60 m (2H).
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; eeethod 'H-NMR (400 MHz) S Structure
Ex. 9~ o [ppm]
reagents
375 6-(5-Hydroxypent-1-ynyl)-2- 374 (DMSO-ds): 10.79 s(1H); OH
(3,4,5- 8.70 d (J = 8.3 Hz, 1 H);
trimethoxyphenyl)quinoline-4- 8.07 s(1 H); 8.00 m(2H); HN ~ ~ ~
carboxylic acid [(R)-1- 7.69 m (2H); 7.50 s (2H);
NH I
OHO
hydroxymethyl-2-(1 H-indol-3- 7.31 d (J = 8.1 Hz, 1 H);
i ~N
yl)ethyl]amide; 7.19 s(1H); 7.02 m(1H);
6.93 m(1H); 4.33 m(1H); I
6-lodo-2-(3,4,5- 3.90 s (6H); 3.73 s (3H); \c o~
.110
trimethoxyphenyl)quinoline-4-
3.51 m(4H); 3.03 m (1 H);
carboxylic acid ((R)-1-
2.96 m(1 H); 2.50 m (2H);
hydroxymethyl-2-(1 H-indol-3-
1.70 m (2H).
yl)ethylJamide
and
Pent-4-yn-l-ol
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
292
Product; Method 1H-NMR (400 MHz) S Structure
Ex. analo-
reagents sousto [ppm]
376 6-(3-Hydroxyprop-1-ynyl)-2- 374 (DMSO-d6): 10.78 s(1H);
OH
(3,4,5- 8.72 d (J = 8.3 Hz, 1 H);
HN ~ II
trimethoxyphenyl)quinoline-4- 8.11 s(1 H); 8.02 m(2H);
o NH
carboxylic acid [(R)-1- 7.72 d (J = 2.0 Hz / 8.8
%
hydroxymethyl-2-(1 H-indol-3- Hz, 1 H); 7.64 d (J = 7.8
yI)ethyl]amide; Hz, 1 H); 7.51 s(2H); 7.31
6-lodo-2-(3, 4, 5- d (J = 8.1 Hz, 1 H); 7.20 s .o ~
0
trimethoxyphenyl)quinoline-4- (1 H); 7.02 m (1 H); 6.93 m ~o
carboxylic acid ((R)-1- (1 H); 4.35 m (3H); 3.90 s
hydroxymethyl-2-(1H-indol-3- (6H); 3.73 s (3H); 3.55 m
yl)ethylJamide (2H); 3.04 m(1 H); 2.94 m
and (1 H).
Prop-2-yn-l-ol
377 6-(3-Methoxyprop-1-ynyl)-2- 374 (DMSO-d6): 10.78 s(1H);
H O
(3,4,5- 8.72 d(J = 8.3 Hz, 1 H); N
trimethoxyphenyl)quinoline-4- 8.13 s(1 H); 8.03 m (2H); carboxylic acid [(R)-1-
7.76 d (J = 1.8 Hz, 1 H); NH
hydroxymethyl-2-(1 H-indol-3- 7.73 d (J = 1.8 Hz, 1 H); oHO
-N
yl)ethyl]amide; 7.52 s (2H); 7.30 d (J =
6-lodo-2-(3, 4, 5- 8.1 Hz, 1 H); 7.19 d (J = ~o o
trimethoxyphenyl)quinoline-4- 2.3 Hz, 1 H); 7.02 m (1 H); ~o
carboxylic acid ((R)-1- 6.92 m(1 H); 4.37 s(2H);
hydroxymethyl-2-(1H-indol-3- 4.33 m(1H); 3.90 s (6H);
yl)ethyl]amide 3.73 s (3H); 3.57 s (3H);
and 3.59 m (2H); 3.02 m(1 H);
3-Methoxypropyne 2.95 m (1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
293
Product; nnechod 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents s m to [ppm]
378 5-(4-Hydroxybut-1-ynyl)- 39a-b/ (DMSO-d6): 10.75 s(1H); _ H
3',4', 5'-trimethoxybiphenyl- 8.35 d (J = 7.8 Hz, 1 H);
374c- 7.97 s(1 H); 7.82 s(1 H); HO
3-carboxylic acid [(R)-1- e 7.80 s(1 H); 7.65 d (J 0 NH
hydroxymethyl-2-(1 H-indol-
7.8 Hz,1 H); 7.30 d(J = o
3-yl)ethyl]amide;
7.8 Hz,1 H); 7.13 s(1 H); o OH
7.03 t(J = 7.4 Hz, 1 H);
6.94 s (2H); 4.95 t (J =
(D)-Tryptophanol 5.5 Hz,1 H); 4.80 t (J = 5.5
Hz, 1 H); 4.24 m(1 H);
and
3.88 s (6H); 3.70 s (3H);
5-(3-Hydroxybut-1-ynyl)- 3.62 m(2H); 3.54 m(1H);
3',4',5'-trimethoxybiphenyl-3- 3.49 m(1 H); 2.97 m(2H);
carboxylic acid 2.80 t (J = 6.6 Hz, 2H).
379 5-(3-Hydroxyprop-1-ynyl)- 39a-b/ (DMSO-d6): 10.76 s(1H); - H
N
~
3',4',5'-trimethoxybiphenyl-3- 8.38 d (J = 8.2 Hz, 1 H);
carboxylic acid [(R)-1- 374c- 8.02 s(1 H); 7.86 s(1 H); Ho
0 NH
hydroxymethyl-2-(1 H-indol-3- e 7.84 s(1 H); 7.65 d (J
=
yl)ethyl]amide; 7.8 Hz, 1 H); 7.30 d (J = 0 8.2 Hz, 1 H); 7.14 s(1 H); 0 oH
(D)-Tryptophanol 7.04 t(J = 7 Hz, 1 H); I
and 6.94-6.97 m (3H); 5.40 t
(J = 5.9 Hz, 1 H); 4.81 t (J
5-(3-Hydroxyprop-1-ynyl)- = 5.5 Hz, 1 H); 4.35 d (J =
3;4;5'trimethoxybiphenyl-3- 5.5 Hz, 2H); 4.20-4.28 m
carboxylic acid (1 H); 3.88 s (6H); 3.70 s
(3H); 3.46-3.59 m (2H);
3.03 dd (J = 14.4 Hz, J =
7.8 Hz, 1 H); 2.96 dd (J =
14.4 Hz; J= 7.8 Hz, 1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
294
Product; Method +H-NMR (400 MHz) S Structure
Ex. analo-
reagents sousto [ppm]
380 5-(5-Hydroxypent-1-ynyl)- 39a-b/ (DMSO-d6): 10.76 s(1H); ~/ N
~ ~
3',','-trimethoxybiphenyl-3- 8.36 d (J = 8.2 Hz, 1 H);
carboxylic acid [(R)-1- 37ec 7.97 s(1 H); 7.80 d (J = Ho NH
hydroxymethyl-2-(1 H-indol-3- 4,3 Hz, 1 H); 7.66 d (J I
yI)ethyl]amide; 7.8 Hz, 1 H); 7.30 d (J
= o P OH
7.8 Hz, 1 H); 7.14 s(1 H); ~ 0,
(D)-Tryptophanol 7.04 t (J = 7.4 Hz, 1 H);
and 6.94-6.97 m (3H); 4.81 t
(1 H); 4.58 t (J = 3.9 Hz,
5-(5-Hydroxypent-1-ynyl)- 1 H); 4.20-4.28 m (1 H);
3;4;5'-trimethoxybiphenyl-3- 3.88 s (6H); 3.70 s (3H);
carboxylic acid 3.46-3.58 m (4 H); 3.02
dd (J = 14.4 Hz, J = 7.8
Hz, 1 H); 2.93 dd (J = 14.4
Hz, J = 7.8 Hz, 1 H); 2.49-
2.53 m (2H); 1.72 q (J =
6,6 Hz, 2H).
381 3',4',5'-Trimethoxy-5-(3- 39a-b/ (DMSO-d6): 10.76 s(1 H); - H
~ N
methoxyprop-1-ynyl)biphenyl- 8.36 d (J = 8.2 Hz, 1 H);
o
3-carboxylic acid [(R)-1- 374ce- 8.04 s(1H); 7.89 (s, 2H); H o NH
hydroxymethyl-2-(1 H-indol-3- 7.66 d (J = 7.8 Hz, 1 H);
I eth I amide; 7.30 d (J 8.2 Hz ; 7.14 0
Y) Yl ( ) I I
o
s (1 H); 7.04 t (J = 7.4 Hz, o ~
(D)-Tryptophanol o~
1 H); 6.94-6.96 m (3H);
and 4.81 t (J = 5.9 Hz, 1 H);
4.36 s(2H); 4.52 m(1 H);
3;4;5'-Trimethoxy-5-(3- 3.88 s (6H); 3.70 s (3H);
methoxyprop-1-ynyl)biphenyl- 3.47-3.59 m (2H); 3.37 s
3-carboxylic acid (3H); 3.03 dd (J = 14.4
Hz, J = 5.8 Hz, 1 H); 2.94
dd (J = 14.4 Hz, J = 7.8
Hz, 1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
295
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. analo-
reagents yousto [ppm]
_
382 3','-Dimethoxy-5-(3- 39a-b/ (DMSO-d6): 10.76 s (1 H); H
N
methoxyprop-1-ynyl)biphenyl- 8.39 d (J = 8.2 Hz, 1 H); ~/
374c- 8.04 s(1 H); 7.85 s (2H); Ho
3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3- e 7.66 d (J = 7.8 Hz, 1 H); o NH
7.26-7.31 m (3H); 7.14
yl)ethyl]amide; o
(1 H); 7.02-7.07 m (2H); o,
o
(D)-Tryptophanol 6.96 t (J = 7.42; 1 H); 4.82
t (J = 5.8 Hz, 1 H); 4.37 s
and
(2H); 4.21-4.29 m (1H);
3;4'-Dimethoxy-5-(3- 3.87 s(3H); 3.80 s (3H);
methoxyprop-1-ynyl)bipheny1- 3.46-3.58 m (2H); 3.37 s
3-carboxylic acid (3H); 3.03 dd (J = 14.4
Hz; J = 5.8 Hz, 1 H); 2.93
dd (J = 14.4 Hz, J = 7.8
Hz, 1 H).
; H
383 5-(3-Hydroxyprop-1 -ynyl)- 39a-b/ (DMSO-d6): 10.76 s (1 H) N
3',4'-dimethoxybiphenyl-3- 8.38 d (J = 8.2 Hz, 1 H);
carboxylic acid [(R)-1- 374c- 8.02 s(1 H); 7.83 s(1 H); HO
hydroxymethyl-2-(1 H-indol-3- e 7.80 s (1 H); 7.65 d (J = 0 NH
yI)ethyl]amide; 7.8 Hz, 1 H); 7.30 d (J = o \\ I
8.2 Hz, 1 H); 7.25-7.27 m oH
0
(D)-Tryptophanol (2H); 7.13 (1 H); 7.02-7.07 ~
m (2H); 6.96 t (J = 7.42
and Hz, 1 H); 5.40 m(1 H);
5-(3-Hydroxyprop-1-ynyl)- 4.81 t (J = 5.8 Hz, 1 H);
3;4'-dimethoxybiphenyl-3- 4.35 m (2H); 4.21-4.29 m
carboxylic acid (1 H); 3.86 s (3H); 3.80 s
(3H); 3.46-3.58 m (2H);
3.03 dd (J = 14.5 Hz, J
6 Hz, 1 H); 2.93 dd (J =
14,5 Hz, J = 7.8 Hz, 1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
296
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. analo-
reagents gousto [ppm]
384 3',4',5'-Trimethoxy-5-(4- 39a-b/ (DMSO-d6): 10.77 s(1 H); H
methoxyphenylethy- 8.40 d (J = 7.8 Hz, 1 H);
374c- 8.03 s(1 H); 7.95 s (2H); Ho
nyl)biphenyl-3-carboxylic acid e o NH
7.67 d (J = 7.8 Hz, 1 H);
[(R)-1-hydroxymethyl-2-(1 H- I
~~ I \
indol-3-yl)ethyl]amide; 7.55 s (1 H); 7.53 s (1 H); 0
7.31 (J = 8.2 Hz, 1 H); 0
i
o" o
(D)-Tryptophanol 7.15 (1 H); 6.94-7.06 m
(6H); 4.83 t (J = 5.5 Hz,
and 1 H); 4.22-4.30 m(1 H);
3',4',5'-Trimethoxy-5-(4- 3.89 s(6H); 3.80 s (3H);
methoxy- 3.71 s (3H); 3.48-3.60 m
phenylethynyl)biphenyl-3- (2H); 3.03 dd (J = 14.8
carboxylic acid Hz, J = 6 Hz, 1 H); 2.95
dd (J = 14.4 Hz, J = 7.4
Hz, 1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
297
Example 385
3',4',5'-Trimethoxy-5-((Z)-3-methoxypropenyl)biphenyl-3-carboxylic acid [(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyi]amide;
H
~ N
~
HO
O NH
ON.
O / \ I /
O
620 mg of zinc dust are suspended in 3.6 ml of water. Argon is passed through
the vig-
orously stirred suspension for 15 min. Then 62 mg of copper(II) acetate are
added, and
the mixture is stirred for 15 min. Subsequently 62 mg of sliver nitrate are
added and
stirring is continued for 30 min. The metal is filtered off with suction under
argon. It is
washed with 2 x 1.8 ml of water, 2 x 1.8 ml of methanol, 2 x 3.6 ml of acetone
and 2 x
3.6 ml of diethyl ether.
The activated zinc obtained in this way is transferred while still moist with
ether into a
solution of 50 mg of 5-(3-hydroxyprop-1-ynyl)-3',4',5'-trimethoxybiphenyl-3-
carboxylic
acid [(R)-1-hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide (Example # 381) in 1.3
ml of
methanol and 0.5 ml of water. The reaction mixture is stirred until the
reaction is com-
plete. The metal is filtered off with suction (caution: the remaining metal is
pyrophoric),
and washed with methanol, and the solvent is evaporated. The title compound is
ob-
tained as a colourless foam (45 mg, 89% of theory).
'H-NMR (400 MHz) S[ppm] (DMSO-d6): 10.77 s(1 H); 8.30 d (J = 8.2 Hz, 1 H);
7.93 s
(1 H); 7.66 d (J = 7.8 Hz, 1 H); 7.62 s (2H); 7.30 d (J = 8.2 Hz, 1 H); 7.15
s(1 H); 7.03 t (J
= 7.4 Hz, 1 H); 6.93-6.97 m(3H); 6.68 d (J = 12.1 Hz, 1 H); 5.88-5.94 m(1 H);
4.82 t (J =
5.6 Hz, 1 H); 4.20-4.26 m(3H); 3.88 s(6H); 3.71 s (3H); 3.47-3.60 m(2H); 3.27
s(3H);
3.04 dd (J = 14.5 Hz, J= 5.8 Hz, 1 H); 2.95 dd (J = 14.4 Hz, J= 7.8 Hz, 1 H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
298
,TTie following compounds were obtained in analogy to the preparation methods
de-
scribed in detail:
Product; Method Structure
Ex. analo- 'H-NMR (400 MHz) S [ppm]
reagents sousto
386 5-((Z)-4-Hydroxybut-1-enyl)- 385 (DMSO-d6): 10.76 s(1H); N
3',4',5'-trimethoxybiphenyl-3- 8.27 d (J = 8.2 Hz, 1 H); 7.90
carboxylic acid [(R)-1- s(1 H); 7.74 s(1 H); 7.74 d (J HO
NH
hydroxymethyl-2-(1 H-indol-3- = 7.8 Hz, 1 H); 7.70 s(1 H); oH
0
yl)ethyl]amide; 7.66 (J = 7.8 Hz, 1 H); 7.30 d
0
(J = 8.2 Hz, 1 H); 7.16 s(1 H); I
5-(4-Hydr oxybut-l-ynyl)-
7.03 t (J = 7.4 Hz, 1 H); 6.95-
3', 4, 5'-trimethoxybiphenyl-3-
6.97 m(3H); 6.58 d (J = 11.7
carboxylic acid ((R)-1-
Hz,1 H) 5.78-5.84 m(1 H);
hydroxymethyl-2-(1 H-indol-3-
4.81 t (J = 5,8 Hz, 1 H); 4.69 t
yl)ethyl]amide (J = 4.7 Hz, 1 H); 4.20-4.28 m
(1 H); 3.88 s(6H); 3.71 s
(3H); 3.47-3.60 m (4H); 3.04
dd (J = 14.8 Hz, J = 6 Hz,
1 H); 2.95 dd (J = 14.4 Hz, J
7.8 Hz, 1 H); 2.47-2.52 m
(2H).
387 5-((Z)-3-Hydroxypropenyl)- 385 (DMSO-d6): 10.77 s(1 H); - H
N
3',','-trimethoxybiphenyl-3- 8.29 d (J = 7.8 Hz, 1 H); 7.91
carboxylic acid [(R)-1- s(1 H); 7.67 d (J = 7.8 Hz, Ho\
O NH
hydroxymethyl-2-(1 H-indol-3- 1 H); 7.64 s(1 H); 7.61 s(1 H); OH
yl)ethyl]amide; 7.30 (J=8.2Hz, 1H);7.16
(1 H); 7.03 t (J = 7.4 Hz, 1 H);
5-(3-Hydroxyprop- 1 -ynyl)-
6.94-6.97 m (3H); 6.58 d (J =
3', 4, 5'-trimethoxybiphenyl-3-
11.7 Hz, 1 H); 5.88-5.94 m
carboxylic acid ((R)-1- (1 H); 4.96 t(J = 5,1 Hz, 1 H);
hydroxymethyl-2-(1 H-indol-3-
4.82 t (J = 4.8 Hz, 1 H); 4.21-
yl)ethyl]amide 4.30 m (3H); 3.88 s(6H);
3.71 s (3H); 3.47-3.60 m
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
299
Product; Method Structure
Ex. analo- 'H-NMR (400 MHz) S [ppm]
reagents gousto
(2H); 3.27 s (3H); 3.04 dd (J
= 14.5 Hz, J = 5.8 Hz, 1 H);
2.95 dd (J = 14.4 Hz, J = 7.8
Hz, 1 H).
388 5-((Z)-5-Hydroxypent-l-enyl)- 385 (DMSO-ds): 10.76 s(1H); N
3',4',5'-trimethoxybiphenyl-3- 8.26 d (J = 8.2 Hz, 1 H); 7.89
HO
carboxylic acid [(R)-1- s(1 H); 7.69 s (2H); 7.66 s O NH
0 H
hydroxymethyl-2-(1 H-indol-3- (1 H); 7.30 (J = 8.2 Hz, 1 H); ol~
yI)ethyl]amide; 7.16 s (1 H); 7.04 t (J = 7.4 o,
Hz, 1 H); 6.94-6.97 m (3H);
5-(5-Hydroxypent-1-ynyl)- 6.52 d(J = 11,3 Hz, 1 H);
3 ; 4, 5'-trimethoxybiphenyl-3-
5.75-5.81 m (1 H); 4.81 t (J
=
carboxylic acid ((R)-1-
5.6 Hz, 1 H); 4.51 t (J = 5 Hz,
hydroxymethyl-2-(1 H-indol-3-
1H); 4.21-4.28 m(1H); 3.88 s
yl)ethyl]amide
(6H); 3.71 s (3H); 3.43-3.60
m (4H); 3.05 dd (J = 14.5 Hz,
J= 5.8 Hz, 1 H); 2.95 dd (J =
14.4 Hz, J= 7.8 Hz, 1 H);
2.39 q (J = 7.4 Hz, 2H); 1.58-
1.65 m (2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
300
Example 389
6-(5-Hydroxypentyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid [(R)-
1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide;
H ~
N ~
HO
O NH
\ OH
O N I /
O
"lo
6-(5-Hydroxypent-1-ynyl)-2-(3,4,5-trimethoxyphenyl)quinoline-4-carboxylic acid
[(R)-1-
hydroxymethyl-2-(1 H-indol-3-yl)ethyl]amide (100 mg) and palladium on carbon
(10%,
50 mg) were suspended in methanol (10 ml) and hydrogenated under hydrogen at
atmospheric pressure at room temperature. After hydrogen uptake ceased, the
catalyst
was filtered off and the mother liquor was stripped off in a rotary
evaporator. Drying in
vacuo resulted in the title compound in 42% yield (42 mg).
(DMSO-d6): 10.79 s(1 H); 8.60 d (J = 8.3 Hz, 1 H); 7.93 m(2H); 7.70 s(1 H);
7.62 m
(2H); 7.49 s (2H); 7.32 d (J = 8.1 Hz, 1 H); 7.19 s(1 H); 7.03 m(1 H); 6.93
m(1 H); 4.85 m
(1 H); 4.32 m(1 H); 3.89 s(6H); 3.72 s(3H); 3.56 m(2H); 3.36 m(2H); 3.05 m(1
H); 2.93
m (1H); 2.63 m(2H); 1.55 m(2H); 1.39 m(2H); 1.30 m(2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
301
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. analo-
reagents yousto [ppm]
390 6-(4-Hydroxybutyl)-2-(3,4,5- 389 (DMSO-d6): 10.79 s(1 H); OH
trimethoxyphenyl)quinoline-4- 8.59 d (J = 8.5 Hz, 1 H); HN
carboxylic acid [(R)-1- 7.97 d (J = 8.5 Hz, 1 H);
o,~
hydroxymethyl-2-(1 H-indol-3- 7.94 s(1 H); 7.71 s(1 H); NH
yl)ethyl]amide; 7.62 m(2H); 7.49 s (2H); N
7.31 d (J = 8.1 Hz, 1 H);
6-(4-Hydroxybut-1-ynyi)-2- 7.19 s(1H); 7.04 m(1H); 'o I~ o
o
(3,4,5- 6.93 m (1H); 4.85 m (1H); .1
trimethoxyphenyl)quinoline-4- 4.40 m(2H); 3.89 s (6H);
carboxylic acid ((R)-1-
3.72 s (3H); 3.56 m (2H);
hydroxymethyl-2-(1 H-indol-3-
3.38 m (2H); 3.03 m(1 H);
yl)ethyl]amide
2.94 m(1 H); 2.64 m(2H);
1.59 m (2H); 1.43 m (2H).
391 6-(3-Hydroxypropyl)-2-(3,4,5- 389 (DMSO-d6): 10.83 s(1 H);
trimethoxyphenyl)quinoline-4- 8.64 d (J = 8.5 Hz, 1 H); HN OH
carboxylic acid [(R)-1- 8.00 d (J = 8.7 Hz, 1 H);
NH
hydroxymethyl-2-(1 H-indol-3- 7.97 s (1 H); 7.76 d (J = OH yl)ethyl]amide; 1.5
Hz, 1H); 7.66 m(2H);
7.53 s (2H); 7.35 d (J = NNI
6-(3-Hydroxyprop-1-yny1)-2- o o
7.9 Hz, 1 H); 7.23 s(1 H);
(3,4,5- 7.06 m (1H); 6.97 m (1H); "o
trimethoxyphenyl)quinoline-4-
4.91 m(1 H); 4.59 m(1 H);
carboxylic acid [(R)-1- 4.40 m(1 H); 3.93 s(6H);
hydroxymethyl-2-(1 H-indol-3- 3.76 s(3H); 3.59 m (2H);
yl)ethyl]amide 3.45 m(2H); 3.06 dd (J =
5.8 Hz / 14.9 Hz, 1H);
2.96 dd (J = 8.3 Hz / 14.7
Hz, 1 H); 2.73 m (2H); 1.76
m (2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
302
Product; Method ,H-NMR (400 MHz) S Structure
Ex. analo-
reagents gous to [ppm]
392 6-(3-Methoxypropyl)-2-(3,4,5- 389 (DMSO-d6): 10.78 s(1H);
trimethoxyphenyl)quinoline-4- 8.61 d (J = 8.3 Hz, 1 H); o
HN
carboxylic acid [(R)-1- 7.97 d (J = 8.7 Hz, 1 H);
NH /
hydroxymethyl-2-(1 H-indol-3- 7.94 s(1 H); 7.72 s(1 H); o~ ~ I
yI)ethyl]amide; 7.62 m (2H); 7.49 s (2H);
I ~N
7.31 d (J = 8.1 Hz, 1 H);
6-(3-Methoxyprop-1-ynyl)-2- i
7.19 s(1 H); 7.03 m(1 H); o o
(3,4,5- 6.93 m (1H); 4.85 m (1H); ~,o
trimethoxyphen yl)quinoline-4-
4.36 m(1 H); 3.89 s (6H);
carboxyiic acid 1'(R)-1-
3.72 s (3H); 3.56 m (2H);
hydroxymethy1-2-(1 H-indol-3-
3.31 m (2H); 3.21 s (3H);
yl)ethylJamide 3.03 m(1 H); 2.92 m(1 H);
2.68 m (2H); 1.77 m (2H).
393 3',4',5'-Trimethoxy-4-(3- 389 (DMSO-ds): 10.79 s(1 H); H
methoxypropyl)biphenyl-3- 8.17 d (J = 8.2 Hz, 1 H); /~~
carboxylic acid [(R)-1- 7.63 d (J = 7.8 Hz, 1 H); NH
OH
hydroxymethyl-2-(1H-indol-3- 7.58-7.61 m(1H); 7.43 s o
yl)ethyl]amide; (1 H); 7.31 d (J = 7.8 Hz,
1 H); 7.26 d (J = 7.8 Hz, ~
3 ; 4', 5'-Trimethoxy-4-(3- o 0
methoxyprop-1-ynyl)biphenyl- 1 =7 H); .4 7.1Hz,7 1 s (1 H);6 H); 7.94t.03 (J t
= (J o\
3-carboxylic acid ((R)-1- 7.4 Hz, 1 H); 6.87 s (2H);
hydroxymethyl-2-(1 H-indol-3- 4.80 t (J = 5.6 Hz, 1 H);
yl)ethylJamide 4.18-4.26 m(1 H); 3.85 s
(6H); 3.70 s (3H); 3.51-
3.58 m(1 H); 3.42-3.48 m
(1 H); 3.19-3.22 m (5H);
3.03 dd (J = 14.5 Hz, J=
5.8 Hz, 1 H); 2.86 dd (J =
14.4 Hz, J= 8.6 Hz, 1 H);
2.62-2.70 m (2H); 1.66-
1.73 m (2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
303
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents 9 ut [ppm]
394 3',4',5'-Trimethoxy-5-(3- 389 (DMSO-d6): 10.76 s(1 H); N H
o'
methoxypropyl)biphenyl-3- 8.21 d (J = 8.2 Hz, 1 H); O)NH
carboxylic acid [(R)-1- 7.84 s (1 H); 7.67 d (J = o r~
hydroxymethyl-2-(1 H-indol-3- 7.8 Hz, 1 H); 7.62 s (2 H); I
yl)ethyl]amide; 7.30 d (J = 7.8 Hz, 1 H); I
. 0 Oi
3', 4; 5' Trimethoxy-5-(3- 7.Hz1,5 1 s H); (1 6 H); .92 7.03 t .97 (J m = 7.4
(3H); \
methoxyprop-1-ynyl)biphenyl- 4.81 t (J = 5.6 Hz, 1 H);
3-carboxylic acid ((R)-1-
4.20-4.28 m(1 H); 3.88 s
hydroxyrnethyl-2-(1 H-indoi-3-
(6H); 3.70 s (3H); 3.46-
yl)ethyl]amide
3.59 m (2H); 3.36 t (J =
6.2 Hz, 2H); 3.25 s (3H);
3.04 dd (J = 14.5 Hz, J =
5.8 Hz, 1 H); 2.94 dd (J =
14.4 Hz, J= 8.3 Hz, 1 H);
2.72 t (J = 7.6 Hz, 2H);
1.83-1.91 m (2H).
395 3',4'-Dimethoxy-5-(3- 389 (DMSO-d6): 10.76 s(1 H); - N
~
methoxypropyl)biphenyl-3- 8.21 d (J = 8.2 Hz, 1 H);
carboxylic acid [(R)-1- 7.84 s(1 H); 7.67 d (J = HO
0 NH
hydroxymethyl-2-(1 H-indol-3- 7.8 Hz, 1 H); 7.59 s (2 H);
yI)ethyl]amide; 7.30 d (J = 7.8 Hz, 1 H); 0 o~,
7.22-7.24 m (2H); 7.14 0 ~
3;4'-Dimethoxy-5-(3- (1H); 7.02-7.06 m (2H);
methoxyprop-1-ynyl)biphenyl- 6.96 t(J - 7.4 Hz, 1 H);
3-carboxylic acid ((R)-1- 4.81 t(J - 5.6 Hz, 1 H);
hydroxymethyl-2-(1 H-indol-3- 4.21-4.29 m(1 H); 3.86 s
yl)ethyl]amide
(3H); 3.80 s (3H); 3.46-
3.59 m (2H); 3.35 t (J =
6.2 Hz, 2H); 3.25 s (3H);
3.04 dd (J = 14.5 Hz, J =
5.8 Hz, 1 H); 2.94 dd (J =
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
304
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. analo-
reagents gO1S to [Ppm]
14.4 Hz, J= 7.8 Hz, 1 H);
2.71 t (J = 7.8 Hz, 2H);
1.83-1.90 m (2H).
396 5-(3-Hydroxypropyl)-3',4'- 389 (DMSO-d6): 10.75 s(1 H); - / N
~
dimethoxybiphenyl-3- 8.21 d (J = 8.2 Hz, 1 H);
carboxylic acid [(R)-1- 7.83 s(1 H); 7.67 d (J = Ho
0 NH
hydroxymethyl-2-(1 H-indol-3- 7.4 Hz, 1 H); 7.59 s (2 H);
yl)ethyl]amide; 7.30 d (J = 7.8 Hz, 1 H); 0 oH
7,22-7.24 m (2H); 7.14 0
5-(3-Hydroxypropyl)-3;4' (1H); 7.02-7.06 m (2H);
dimethoxybiphenyl-3- 6.96 t (J = 7.4 Hz, 1 H);
carboxylic acid ((R)-1- 4.80 t (J = 5.6 Hz, 1 H);
hydroxymethyl-2-(1 H-indol-3- 4.52 (J - 5.1 Hz; 1 H);
yl)ethyl]amide 4.21-4.29 m(1H); 3.86 s
(3H); 3.80 s (3H); 3.42-
3.59 m (4H); 3.04 dd (J =
14.5 Hz, J= 5.8 Hz, 1 H);
2.94 dd (J = 14.4 Hz, J =
7.8 Hz, 1 H); 2.71 t (J = 7.6
Hz, 2H); 1.75-1.82 m (2H).
397 5-(5-Hydroxypentyl)-3',4',5'- 389 (DMSO-d6): 10.76 s(1H); H
trimethoxybiphenyl-3- 8.20 d (J = 7.8 Hz, 1 H);
carboxylic acid [(R)-1- 7.83 s (1 H); 7.67 d (J = HO NH 0H
0
hydroxymethyl-2-(1 H-indol-3- 7.8 Hz, 1 H); 7.60 s (2 H);
yI)ethyl]amide; 7.30 d (J = 7.8 Hz, 1 H);
7.15 s (1 H); 7.03 t (J = 7.4 0
5-(5-Hydroxypent- 1-ynyl)- Hz, 1 H); 6.95 t (J - 7.4 0 0-
3', 4; 5'-trimethoxybiphenyl-3- Hz, 1 H); 6.92 s (2H); 4.81
carboxylic acid ((R)-1- t (J = 4.7 Hz, 1 H); 4.37 t (J
hydroxymethyl-2-(1 H-indol-3- = 4.5 Hz, 1 H); 4.20-4.29 m
yl)ethyl]amide
(1 H); 3.88 s (6H); 3.70 s
(3H); 3.47-3.59 m (2H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
305
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents gous to [ppm]
3.37-3.41 m (2H); 3.04 dd
(J = 14.5 Hz, J 5.8 Hz,
1 H); 2.94 dd (J = 14.4 Hz,
J = 7.8 Hz, 1 H); 2.68 t (J
=
7.6 Hz, 2H); 1.60-1.67 m
(2H); 1.44-1.51 m (2H);
1.31-1.38 m (2H).
398 5-(3-Hydroxypropyl)-3',4',5'- 389 (DMSO-d6): 10.76 s(1H); Q N
trimethoxvbiphenyl-3- 8.21 d(J = 8.2 Hz, 1 H); ~
carboxylic acid [(R)-1- 7.83 s(1 H); 7.67 d (J = Ho T
O NH
hydroxymethyl-2-(1 H-indol-3- 7.8 Hz, 1 H); 7.62 (2 H);
I
yI)ethyl]amide; 7.30 d (J = 7.8 Hz, 1 H); 0 oH
7.14 s (1 H); 7.03 t (J = 7.4 0
5-(3-Hydroxypropyl)-3', 4, 5'- o~
Hz, 1 H); 6.95 t(J = 7.4 Hz,
trimethoxybiphenyl-3- 1 H); 6.92 s (2H); 4.81 t (J
carboxylic acid ( ( R ) - 1 -
= 5.4 Hz, 1 H); 4.53 t(J = 5
hydroxymethyl-2-(1 H-indol-3- Hz, 1 H); 4.20-4.28 m(1 H);
yl)ethylJamide 3.88 s (6H); 3.70 s (3H);
3.43-3.59 m (4H); 3.03 dd
(J = 14.5 Hz, J= 5.8 Hz,
1 H); 2.94 dd (J = 14.4 Hz,
J = 7.8 Hz, 1 H); 2.72 t (J
=
7.6 Hz, 2H); 1.76-1.83 m
(2H).
399 5-(4-Hydroxybutyl)-3',4',5'- 389 (DMSO-d6): 10.76 s(1 H); - H
N
\
trimethoxybiphenyl-3- 8.20 d (J = 8.2 Hz, 1 H);
carboxylic acid [(R)-1- 7.84 s (1 H); 7.67 d (J = Ho NH
hydroxymethyl-2-(1 H-indol-3- 7.7 Hz, 1 H); 7.61 s (2 H);
yI)ethyl]amide; 7.30 d (J = 7.8 Hz, 1 H); 0 oH
7.15s(1H); 7.03t(J=7.4
5-(4-Hydroxybut-1-yny1)- Hz, 1 H); 6.95 t(J = 7.4 Hz,
3', 4; 5'-trimethoxybiphenyl-3- 1 H); 6.92 s(2H); 4.81 t(J
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
306
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents 9 us t [pPm]
carboxylic acid [(R)-1- = 5.5 Hz, 1 H); 4.41 t (J
=
hydroxymethyl-2-(1 H-indol-3- 5.1 Hz, 1 H); 4.20-4.28 m
yl)ethyl]amide (1 H); 3.88 s (6H); 3.70 s
(3H); 3.46-3.60 m (2H);
3.41-3.46 m (2H); 3.04 dd
(J = 14.5 Hz, J 5.8 Hz,
1 H); 2.94 dd (J = 14.4 Hz,
J = 7.8 Hz, 1 H); 2.68 t (J
7.6 Hz, 2H); 1.63-1.70 m
(2H); 1.44-1.51 m (2H).
400 3',4',5'-Trimethoxy-5-[2-(4- 389 (DMSO-d6): 10.78 s(1 H); N
methoxyphenyl)ethyl]- 8.22 d (J = 8.2 Hz, 1 H);
HO
biphenyl-3-carboxylic acid 7.84 s (1 H); 7.70-7.67 m 0 NH
[(R)-1-hydroxymethyl-2-(1H- (2H); 7.53 s (1 H); 7.31 d i
indol-3-yl)ethyl]amide; (J = 7.8 Hz, 1 H); 7.15-7.17 1~ o
m (3H); 7.04 t (J = 7.4 Hz, 0. 1
3', 4 ; 5' Trimethoxy-5-(4- 1 H); 6.96 t (J = 7.4 Hz,
methoxyphenylethy- 1 H); 6.83-6.86 m (4H);
nyl)biphenyl-3-carboxylic acid 4.83 t (J = 5.5 Hz, 1 H);
((R)-1-hydroxymethyl-2-(1H- 4.21-4.29 m (1H); 3.87 s
indol-3-y1) ethyl]amide
(3H); 3.70 s (6H); 3.48-
3.60 m (2H); 3.86-3.07 m
(6H).
401 N-[(R)-2-[1-(2-Cyanoethyl)- 39 (DMSO-d6): 8.31 d (J 1 H-indol-3-yl]-1- 8.1
Hz, 1 H); 8.05 s(1 H); _~N
N
(hydroxymethyl)ethyl]-3'- 7.78 d (J = 7.8 Hz, 1 H);
HO
fluoro-4'-methoxy[1,1'- 7.78 d (J = 7.6 Hz, 1 H); NH
biphenyl]-3-carboxamide; 7.73 d (J = 8.0 Hz, 1 H); o
7.65 dd (J = 12.9 Hz / 2.3
1-(2-Cyanoethyl)-L- Hz, 1 H); 7.53 d (J = 8.8
tryptophanol and F
Hz, 1 H); 7.51 d (J = 8.0 ~O
Hz, 1 H); 7.50 dd (J = 7.8
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
307
Product; Method +H-NMR (400 MHz) S Structure
Ex. analo-
reagents sousto [ppm]
3'-Fluoro-4' methoxy(1,1'- Hz / 7.6 Hz, 1 H); 7.29 dd
(J = 9.1 Hz / 8.8 Hz, 1 H);
biphenyl]-3-carboxylic acid
7.26 s (1 H); 7.13 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 7.03
dd (J = 8.0 Hz / 7.0 Hz,
1 H); 4.81 m(1 H); 4.42 t (J
= 6.6 Hz, 2H); 4.25 m
(1 H); 3.90 s (3H); 3.54 m
(1 H); 3.50 m(1 H); 3.03 dd
(J = 14.4 Hz / 6.6 Hz, 1 H);
2.95 t (J = 6.6 Hz, 2H);
2.94 m (1 H).
402 3'-Fluoro-N-[(R)-2-(1-heptyl- 39 (DMSO-d6): 8.27 d (J = OH
1 H-indol-3-yl)-1- 8.1 Hz, 1 H); 8.03 s(1 H); o HNH N
(hydroxymethyl)ethyl]-4'- 7.78 d (J = 7.8 Hz, 1 H);
methoxy[1,1'-biphenyl]-3- 7.76 d (J = 7.8 Hz, 1 H); F I~ I~
carboxamide; 7.66 d (J = 8.0 Hz, 1 H);
7.64 dd (J = 12.9 Hz / 2.3
1-Heptyl-L-tryptophanol and
Hz, 1 H); 7.53 d (J = 8.8
3'-Fluoro-4' methoxy(1,1'- Hz, 1 H); 7.49 dd (J = 7.8
biphenyl]-3-carboxylic acid Hz / 7.8 Hz, 1 H); 7.36 d (J
= 8.0 Hz, 1 H); 7.28 dd (J =
8.8 Hz / 8.8 Hz, 1 H); 7.17
s (1 H); 7.08 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H); 6.98 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 4.82
m(1 H); 4.25 m(1 H); 4.05
t(J = 6.9 Hz, 2H); 3.89 s
(3H); 3.56 m(1 H); 3.51 m
(1 H); 3.04 dd (J = 14.4 Hz
/ 5.8 Hz, 1 H); 2.93 dd (J =
14.4 Hz / 8.1 Hz, 1 H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
308
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents gousto [ppm]
1.61 m (2H); 1.09 m (8H);
0.76 t(J = 7.1 Hz, 3H).
N
403 N-[(R)-2-[1-(4-Cyanobutyl)- 39 (DMSO-d6): 8.27 d (J = ?,.-
-indol-3-yl]-1- 8.1 Hz, 1 H); 8.04 s(1 H); N
1 H
(hydroxymethyl)ethyl]-3'- 7.78 d(J = 7.7 Hz, 1 H); Ho H
fluoro-4'-methoxy[1,1'- 7.77 d (J = 7.5 Hz, 1 H); o
biphenyl]-3-carboxamide; 7.68 d (J = 8.0 Hz, 1 H);
7.65 dd (J = 13.0 Hz / 2.3 F~
1-(4-Cyanobutyl)-~- Hz 1 I-I); 7.54 d (J = 8.7 .10
tryptophanol and
Hz, 1 H); 7.50 dd (J = 7.7
3'-Fluoro-4' methoxy(1,1' Hz / 7.5 Hz, 1 H); 7.41 d (J
biphenyl]-3-carboxylic acid = 8.0 Hz, 1 H); 7.28 dd (J =
9.0 Hz / 8.7 Hz, 1 H); 7.18
s (1 H); 7. 10 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H); 7.00 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 4.81
m (1H); 4.13t(J=6.8 Hz,
2H); 4.25 m(1 H); 3.90 s
(3H); 3.55 m(1 H); 3.52 m
(1 H); 3.03 dd (J = 14.6 Hz
/ 6,2 Hz, 1 H); 2.96 t(J =
7.1 Hz, 2H); 2.93 dd (J =
14.6 Hz / 7.4 Hz, 1 H);
1.74 m (2H); 1.40 m (2H).
404 3'-Fluoro-N-[(R)-l- 39 (DMSO-d6): 8.27 d(J= o"
(hydroxymethyl)-2-[1-(3- 8.1 Hz, 1H); 8.03 s(1H);
phenoxypropyl)-1 H-indol-3- 7.78 d (J = 7.7 Hz, 1 H);
F o
yI]ethyl]-4'-methoxy[1,1'- 7.77 d (J = 7.5 Hz, 1 H);
biphenyl]-3-carboxamide; 7.68 d (J = 8.0 Hz, 1 H);
7.64 dd (J = 13.0 Hz / 2.3
1-(3-Phenoxypropyl)-L- Hz, 1 H); 7.52 d (J = 8.9
tryptophanol and
Hz, 1 H); 7.49 dd (J = 7.7
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
309
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents gO1stO [ppm]
3' Fluoro-4' methoxy(1,1' Hz / 7.5 Hz, 1 H); 7.38 d(J
biphenyl]-3-carboxylic acid = 8=0 Hz, 1 H); 7.27 dd (J =
8.9 Hz / 8.9 Hz, 1 H); 7.24
dd (J = 7.6 Hz / 7.6 Hz,
2H); 7.19 s(1 H); 7.06 dd
(J = 8.0 Hz / 7.0 Hz, 1 H);
6.98dd(J=8.0Hz/7.0
Hz, 1 H); 6.90 dd (J = 7.6
Hz / 7.6 Hz, 1 H); 6.84 d (J
= 7.6 Hz, 2H); 4.79 m
(1 H); 4.26 t (J = 6.7 Hz,
2H); 4.25 m(1 H); 3.88 s
(3H); 3.54 m(1 H); 3.51 m
(1 H); 3.02 dd (J = 14.1 Hz
/ 6.4 Hz, 1 H); 3.80 t(J =
6.0 Hz, 2H); 2.93 dd (J =
14.1 Hz / 7.7 Hz, 1 H);
2.10 m (2H).
405 3'-Fluoro-N-[(R)-l- 39 (DMSO-d6): 8.30 d (J = H
~/
(hydroxymethyl)-2-[1-(2- 8.1 Hz, 1 H); 8.05 s(1 H); o"" ~N
methoxyethyl)-1 H-indol-3- 7.78 d (J = 7.8 Hz, 1 H); F -
~
yl]ethyl]-4'-methoxy[1,1'- 7.78 d (J = 7.6 Hz, 1 H); l ~~
biphenyl]-3-carboxamide; 7.68 d (J = 8.0 Hz, 1 H);
7.65 dd (J = 13.1 Hz / 2.3
1-(2-Methoxyethyl)-L- Hz, 1 H); 7.53 d(J = 8.8
tryptophanol and
Hz, 1 H); 7.50 dd (J = 7.8
3'-Fluoro-4' methoxy(1,1' Hz / 7.6 Hz, 1 H); 7.40 d (J
biphenyl]-3-carboxylic acid = 8=0 Hz, 1 H); 7.28 dd (J =
8.8 Hz / 8.8 Hz, 1 H); 7.18
s (1 H); 7.09 dd (J = 8.0 Hz
/ 7.0 Hz, 1 H); 6.99 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 4.82
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
310
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents gousto [ppm]
m(1 H); 4.25 m(1 H); 4.23
t (J = 5.3 Hz, 2H); 3.90 s
(3H); 3.56 t (J = 5.3 Hz,
2H); 3.55 m(1 H); 3.51 m
(1 H); 3.10 s (3H); 3.04 dd
(J = 14.4 Hz / 6.0 Hz, 1 H);
2.93 dd (J = 14.4 Hz / 7.8
Hz, 1 H).
406 N-[(R)-2-[1-(3-Cyanopropyl)- 39 (CDCI3): 7.72 d (J = 8.0 /N
1 H-indol-3-yi]-1- Hz, 1 H); 7.72 s(1 H); 7.61 /~
~
(hydroxymethyl)ethyl]-3'- d (J = 7.9 Hz, 1 H); 7.59 d N
~
fluoro-4'-methoxy[1,1'- (J = 7.5 Hz, 1 H); 7.41 dd
HO H
biphenyl]-3-carboxamide; (J = 7.9 Hz / 7.5 Hz, 1 H); N
7.34 d(J = 8.0 Hz, 1 H); 0 1-(3-Cyanopropyl)-L- ~
7.03 s(1 H); 7.29-7.19 m
tryptophanol and
(3H); 7.13 dd (J = 8.0 Hz
3'-Fluoro-4'-methoxy(1,1'- 7.0 Hz, 1 H); 7.01 dd (J = F
biphenyl]-3-carboxylic acid 8.0 Hz / 7.0 Hz, 1 H); 6.58
d (J = 7.4 Hz, 1 H); 4.49 m
(1 H); 4.26 t (J = 6.0 Hz,
2H); 3.94 s (3H); 3.83 m
(1 H); 3.79 m(1 H); 3.17 m
(2H); 2.18 m (2H); 2.16 m
(2H).
407 4-Ethoxy-3'-methoxybiphenyl- 335 (DMSO-d6): 8.42 d (J = N
3-carboxylic acid [(R)-2-(1- 7.8 Hz, 1 H); 8.15 s(1 H); N
cyanomethyl-1 H-indol-3-yl)-1- 7.53 d (J = 8.2 Hz, 1 H);
hydroxymethylethyl]amide; 7.36 t (J = 8.2 Hz, 1 H); Ho
0 NH
7.25 s(1 H); 7.20 m(3H); o~
Methyl (R)-3-(1-cyanomethyl-
7.13 m (3H); 6.91 d (9.3 ~o
1 H-indol-3-yl)-2-((4-ethoxy-3'-
Hz, 1 H); 5.49 s(2H); 4.99
methoxybiphen yl-3-carbon yl)-
m(1 H); 4.25 m(1 H); 4.15
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
311
Product; Method 'H-NMR (400 MHz) 5 Structure
Ex. analo-
reagents sousto IPpm]
amino]propionate q (J = 6.3 Hz, 2H); 3.82 s
(3H); 3.50 m(1 H); 3.46 m
(1H); 3.00 m (2H); 1.31 t
(J = 6.6 Hz, 3H).
408 6-Methoxy-2-(3,4,5- 335 (DMSO-ds): 8.71 d (J = ;
trimethoxyphenyl)quinoline-4- 8.2 Hz, 1 H); 8.00 t (J = 4.3 N
carboxylic acid [(R)-2-(1- Hz, 2H); 7.73 d (J = 7.9
cyanomethyl-1 H-indol-3-yl)-1- Hz, 1 H); 7.54 d (J = 7.8 Ho
O NH
hydroxymethylethyl]amide; Hz, 1 H); 7.50 s(2H); 7.46
s(1 H); 7.41 m(1 H); 7.30 N
Methyl (R)-3-(1-cyanomethyl- s(1 H); 7.21 t (J - 7.6 Hz, o ~
1 H-indol-3-yl)-2-{(6-methoxy- 1 H); 7.09 t(J - 7.5 Hz, ~o
2-(3,4,5- 1 H); 5.50 s (2H); 4.95 t (J
trimethoxyphenyl) quinoline-4-
= 5.5 Hz, 1 H); 4.40 m
carbon yl]amino} propionate
(1 H); 3.92 s (6H); 3.76 s
(3H); 3.74 s (3H); 3.74 m
(2H); 3.03 dd (J = 14.4 Hz
/ 5.5 Hz, 1 H); 2.95 dd (J =
14.4 Hz / 8.1 Hz, 1 H).
409 6-Methoxy-2-(3,4,5- 335 (DMSO-d6): 8.67 d (J = N
trimethoxyphenyl)quinoline-4- 8.6 Hz, 1 H); 8.01 d (J =
carboxylic acid {(R)-2-[1-(4- 7.8 Hz, 1 H); 8.00 s(1 H); N\~
cyano-butyl)-1 H-indol-3-yl]-1- 7.68 d (J = 7.8 Hz, 1 H);
hydroxymethylethyl}amide; 7.51 s (2H); 7.48 s (1 H); Ho o NH
7.43 m(2H); 7.24 s(1 H); 0,
Methyl (R)-3-(1-(4- 7.11 t(J - 7.8 Hz, 1 H); 'o N
-
cyanobutyl)-1 H-indol-3-yl]-2- 6.98 t(J - 7.4 Hz, 1 H); \o 0
{[6-methoxy-2-(3, 4, 5-
4.90 m (1H); 4.38 m (1H);
trimethoxyphenyl)quinoline-4- 4.14 m (2H); 3.92 s (6H);
carbonyl]amino]propionate 3.75 s (6H); 3.60 m (2H);
2.99 m (2H), 2.40 t (J =
7.0, Hz, 2H);1.76 t (J = 7.5
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
312
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents yous to [ppm]
Hz, 2H); 1.45 t (J = 7.4
Hz, 2H).
410 4-Hydroxy-3',4',5'- 39 (DMSO-d6): 12.49 s(1 H); - N
~
trimethoxybiphenyl-3- 10.76 s(1 H); 8.67 d (J =
carboxylic acid [(R)-1- 8.1 Hz, 1 H); 8.10 d (J = HO
hydroxymethyl-2-(1 H-indol-3- 2,1 Hz, 1 H); 7.65 m(1 H); 0 NH
OH
yI)ethyl]amide; 7,28 d (J = 7.9 Hz, 1 H);
~1o
7.12 s(1H); 6.93 m (3H); \o
(D)-Tryptophanol
6.84 s(2H); 4,88 m(1 H);
o
and 4.26 m(1 H); 3.84 s (6H);
3.65 s (3H); 3.50 m (2H);
4-Hydroxy-3;4;5' 2.97 m (2H).
trimethoxybiphenyl-3-
carboxylic acid
411 4-(3-Cyanopropoxy)-3',4',5'- 39 (DMSO-d6): 10.78 s(1 H); \ N
trimethoxybiphenyl-3- 8.21 d (J = 8.1 Hz, 1 H);
HO
carboxylic acid [(R)-1- 8.02 d (J = 2.6 Hz, 1 H); O NH
N
hydroxymethyl-2-(1 H-indol-3- 7.71 dd (J = 2.5 Hz / 8.5 a~
yI)ethyl]amide; Hz, 1 H); 7.68 d (J = 7.7
'0
Hz, 1 H); 7.30 d (J = 7.9
4-(3-Cyanopropoxy)-3', 4, 5 ' -
H z , 1 H); 7.19 d(J = 8.7
trimethoxybiphenyl-3- Hz, 1 H); 7.14 s(1 H); 7.03
carboxylic acid
m (1 H); 6.94 m (1 H); 6.82
and s(2H); 4.94 m(1 H); 4.16
m(1 H); 4.13 m (2H); 3.83
(D)-Tryptophanol s (6H); 3.66 s (3H); 3.48
m (2H); 2.94 m (2H); 2.60
m (2H); 1.99 m (2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
313
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents sousto [ppm]
412 4-Cyclopentyloxy-3'-fluoro-4'- 39 (DMSO-d6): 10.81 s(1 H); -,"~
methoxybiphenyl-3-carboxylic 8.36 d (J = 8.2 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H- 8.16 (1 H); 7.70-7.74 m o H
indol-3-ylmethyl)ethyl]amide; (2H); 7.51 d (J = 12.9 Hz, 0"0
1 H); 7.42 d (J = 8.6 Hz, F
(D)-Tryptophanol
1 H); 7.33 d (J = 7.8 Hz, o
and 1 H); 7.23 t (J = 8.8 Hz,
1H);7.18d(J8.9Hz,
4-Cyclopentyloxy-3'-fluoro-4'- 1 H); 7.15 s(1 H); 7.06 t (J
methoxybiphenyl-3-carboxylic = 7.4 Hz, 1 H); 7.15 s(1 H);
acid 6.97 t (J = 7.4 Hz, 1 H);
5.01 m (1 H); 4.97 t (J =
4.9 Hz, 1 H); 4.24-4.31 m
(1 H); 3.87 s (3H); 3.42-
3.54 m (2H); 2.93-3.04 m
(2H); 1.78-1.96 m (3H);
1.52-1.70 m (5H).
413 4-Cyclopentyloxy-3'- 39 (DMSO-d6): 10.81 s(1 H); N
methylbiphenyl-3-carboxylic 8.36 d (J = 8.2 Hz, 1 H); /
acid [(R)-1-hydroxymethyl-2- 8.21 (1H); 7.70-7.75 m NH o10
OH (1 H-indol-3-yl)ethyl]amide; (2H); 7.40-7.45 m (2H);
7.31-7.35 m (2H); 7.20 d
(D)-Tryptophanol
(J = 8.6 Hz, 1 H); 7.15 m
and (2H); 7.06 t (J = 7.3 Hz,
1 H); 6.97 t (J = 7.4 Hz,
4-Cyclopentyloxy-3'- 1 H); 5.02 m (1 H); 4.97 t (J
methylbiphenyl-3-carboxylic = 4.9 Hz, 1 H); 4.24-4.31 m
acid (1 H); 3.42-3.54 m (2H);
2.94-3.04 m (2H); 2.38 s
(3H); 1.79-1.94 m (3H);
1.64-1.73 m (3H); 1.51-
1.61 m (2H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
314
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents sous to [ppm]
414 3'-(1-Butyl-3-methylureido)-4- 39 (DMSO-ds): 10.81 s(1H); N H
cyclopentyloxybiphenyl-3- 8.37 d (J = 8.2 Hz, 1 H); E5Pa
carboxylic acid [(R)-l- 8.22 d (J = 2.3 Hz, 1 H); "H o~
hydroxymethyl-2-(1 H-indol-3- 7.76 dd (J = 8.6 Hz, J= ~
yl)ethyl]amide; 2.3 Hz, 1 H); 7.70 d (J =
7.8 Hz, 1 H); 7.53-7.50 m
(D)-Tryptophanol
(1 H); 7.48 t (J = 7.8 Hz, ~
Hi O
=
and 1 H); 7.43 (1 H); 7.31 d (J
8.2 Hz, 1 H); 7.22 d (J =
3'-(1-Butyl-3-methylureido)-4- 8.6 Hz, 1 H); 7.14-7.18 m
cyclopentyloxybiphenyl-3- (2H); 7.05 t (J = 7.4 Hz,
carboxylic acid 1 H); 6.96 t (J = 7.4 Hz,
1 H); 5.66 q (J = 4.3 Hz,
1 H); 5.03 m (1 H); 4.97 t (J
= 5.1 Hz, 1 H); 4.24-4.32 m
(1 H); 3.61 t (J = 7.4 Hz,
1 H); 3.42-3.54 m (2H);
2.94-3.04 m (2H); 2.54 d
(J = 4.3 Hz, 3H); 1.79-1.95
m (3H); 1.52-1.73 m (5H);
1.36-1.43 m (2H); 1.21-
1.29 m (2H) 0.84 t (J = 7.4
Hz, 3H).
415 4-Cyclopentyloxy-4'-fluoro-3'- 39 (DMSO-d6): 10.81 s(1 H); H
methylbiphenyl-3-carboxylic 8.37 d (J = 8.2 Hz, 1 H);
acid [(R)-2-hydroxy-1-(1 H- 8.18 (1 H); 7.72 s(1 H); OH "" o10
indol-3-ylmethyl)ethyl]amide; 7.70 s (1 H); 7.55 d (J = 1
7.4 Hz, 1 H); 7.44-7.48 m
(D)-Tryptophanol
(1 H); 7.33 d (J = 8.2
and Hz,1 H); 7.20 t (J = 7 Hz, F
1 H); 7.15 s (1 H); 7.06 t (J
4-Cyclopentyloxy-4'-fluoro-3'- = 7.4 Hz, 1 H); 6.97 t (J =
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
315
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents s usto [ppm]
methylbiphenyl-3-carboxylic 7.4 Hz, 1 H); 5.02 m(1 H);
acid 4.97 t (J = 5.2 Hz, 1 H);
4.25-4.32 m (1 H); 3.42-
3.54 m (2H); 2.94-3.04 m
(2H); 2.31 s (3H); 1.79-
1.96 m (3H); 1.51-1.73 m
(5H).
416 4-Cyclopentyloxy-3'- 39 (DMSO-d6): 10.80 s(1H); ,"~
methoxybipheny!-3-carboxy!ic 8.37 d(J = 8.2 Hz, 1 H);
acid [(R)-1-hydroxymethyl-2- 8.19 d (J = 2.3 Hz, 1 H); OH NH 0 (1 H-indol-3-
yl)ethyl]amide; 7.76 dd (J = 8.6 Hz, J =
2.3 Hz, 1 H); 7.70 d (J =
(D)-Tryptophanol 7.8 Hz, 1 H); 7.36 t (J = 8
0
and Hz, 1 H); 7.33 d (J = 7.8
Hz, 1 H); 7.18-7.21 m (2H);
4-Cyclopentyloxy-3' 7.14 s (2H); 7.05 t (J = 7.4
methoxybiphenyl-3-carboxylic Hz, 1 H); 6.96 t (J = 7.2
acid Hz, 1 H); 6.92 dd (J = 8
Hz, J = 2 Hz 1 H); 5.03 m
(1 H); 4.96 t(J = 5.1 Hz,
1 H); 4.24-4.31 m(1 H);
3.82 s (3H); 3.42-3.53 m
(2H); 2.93-3.04 m (2H);
1.79-1.95 m (3H); 1.52-
1.72 m (5H).
417 4-Cyclopentyloxy-3',4'- 39 (DMSO-d6): 10.81 s(1 H); N
dimethoxybiphenyl-3- 8.37 d (J = 7.8 Hz, 1 H); ~
HO
carboxylic acid [(R)-1- 8.16 d (J = 2.3 Hz, 1 H); 0 NH
hydroxymethyl-2-(1H-indol-3- 7.69-7.73 m (2H); 7.36 d 0,10
y!)ethyl]amide; (J = 8.2 Hz, 1 H); 7.33 d (J ~o
= 7.8 Hz, 1 H); 7.14-7.19 m
(D)-Tryptophanol (4H); 7.03 t (J = 8.2 Hz,
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
316
Product; Method 'H-NMR (400 MHz) 8 Structure
Ex. analo-
reagents 9 usto [ppm]
and 1 H); 6.96 t (J = 7.42 Hz,
1 H); 5.02 m (1 H); 4.96 t (J
4-Cyclopentyloxy-3 ; 4'- = 5 Hz, 1 H); 4.24-4.31 m
dimethoxybiphenyl-3- (1 H); 3.84 s(3H); 3.79 s
carboxylic acid (3H); 3.41-3.53 m (2H);
2.93-3.04 m (2H); 1.79-
1.93 m (3H); 1.52-1.72 m
(5H).
418 5-8enzo[ 1,3]dioxoi-5-y1-2- 39 (DMSO-ds): 10.80 s(1H); - N
~
cyclopentyloxy-N-[(R)-1- 8.35 d (J = 7.8 Hz, 1 H);
HO
hydroxymethyl-2-(1 H-indol-3- 8.11 d (J = 1.9 Hz, 1 H); 0 NH
yl)ethyl]benzamide; 7.66-7.71 m (2H); 7.34 d 0'0
(J = 8.2 Hz, 1 H); 7.14-7.19 <o I~
(D)-Tryptophanol m (3H); 7.04-7.10 m (2H);
and 6.94-6.99 m (2H); 6.05 s
(2H); 5.0 m (1 H); 4.95 t (J
5-Benzo[1, 3]dioxo1-5-y1-2- = 5 Hz, 1 H); 4.24-4.31 m
cyclopentyloxybenzoic acid (1 H); 3.42-3.53 m (2H);
2.93-3.04 m (2H); 1.77-
1.93 m (3H); 1.54-1.69 m
(5H).
419 4-Cyclopentyloxy-3',4',5'- 39 (DMSO-d6): 10.81 s(1 H); \ ~ N
trimethoxybiphenyl-3- 8.37 d (J = 7.8 Hz, 1 H); ~
HO
carboxylic acid [(R)-1- 8.16 d (J = 2.3 Hz, 1 H); 0 NH
hydroxymethyl-2-(1 H-indol-3- 7.76 dd (J = 9 Hz, J = 2.7 0"0
I
yI)ethyl]amide; Hz, 1 H); 7.70 d (J = 7.8
0
Hz, 1 H); 7.33 d (J = 8.2 o'~
(D)-Tryptophanol Hz, 1 H); 7.19 d (J = 8.9
and Hz, 1 H); 7.14 d(J = 1.9
Hz, 1 H); 7.05 t(J = 7.4
4-Cyclopentyloxy-3 ; 4; 5' Hz, 1 H); 6.96 t (J = 7.4
trimethoxybiphenyl-3- Hz, 1 H); 6.85 s (2 H); 5.04
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
317
Product; Method 'H-NMR (400 MHz) S Structure
Ex. analo-
reagents sousto [ppm]
carboxylic acid m(1 H); 4.97 t (J = 5 Hz,
1 H); 4.24-4.31 m(1 H);
3.86 s (6H); 3.69 s (3H);
3.41-3.53 m (2H); 2.93-
3.04 m (2H); 1.79-1.95 m
(3H); 1.51-1.73 m (5H).
420 4-Cyclopentyloxy-3',4'- 39 (DMSO-d6): 10.81 s(1 H); N
difluoro-5'-methoxybiphenyl-3- 8.37 d (J = 8.2 Hz, 1 H);
HO
r.arhnxy Iir aniri r(R)-2- 8,19 d(.l = 2,3 H7 1 H); n IH
hydroxy-1-(1 H-indol-3- 7.78 dd (J = 8.7 Hz, J= ~
I
ylmethyl)ethyl]amide; 2.6 Hz, 1 H); 7.71 d (J = '~
7.8 Hz, 1 H); 7.33 d (J = F F
(D)-Tryptophanol
7.8 Hz, 1 H); 7.20-7.26 m
and (3H); 7.15 (1 H); 7.06 t(J =
7.4 Hz, 1 H); 6.96 t (J = 7.4
4-Cyclopentyloxy-3; 4'- Hz, 1 H); 5.04 m(1 H); 4.97
difluoro-5' methoxybiphenyl-3- t (J = 5.1 Hz, 1 H); 4.24-
carboxylic acid 4.32 m(1 H); 3.98 s (3H);
3.42-3.55 m (2H); 2.94-
3.04 m (2H); 1.79-1.95 m
(3H); 1.52-1.74 m (5H).
Example 421
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
318
3'-[Butyl[(1,1-dimethylethoxy)carbonyl]amino]-4-ethoxy-N-[(R)-1-
(hydroxymethyl)-
2-(1 H-i ndol-3-yl)ethyl] [1,1'-bi phenyl]-3-carboxam ide;
OH ~
I \ ~
O NH N
H
Oy N
-~O
421 a) tert-Butyl (3-bromophenyl)-n-butylcarba mate
tert-Butyl (3-bromophenyl)carbamate (56 g) were dissolved in DMF (250 ml), and
NaH
(60%, 10g) was added in portions. The mixture was stirred until gas evolution
was no
longer observable and then 1-bromobutane (35 g) was slowly added dropwise. The
mixture was stirred at 80 C for two hours, cooled and poured into water (1000
ml). It
was extracted with ethyl acetate (150 ml), and the organic phases were washed
with
water (3 x 100 ml), concentrated in a rotary evaporator and dried by
azeotropic distilla-
tion with toluene. The title compound was obtained in quantitative yield
(68g). MS
(ESI,+): 329 (M+1).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
319
421 b) 3-(tert-Butoxycarbonylbutylamino)phenylboronic acid
Butyllithium (1.6 M in hexane, 70 ml) was added dropwise to a solution of tert-
butyl
(3-bromophenyl)-n-butylcarbamate (31.4 g) in THF (400 ml) at -80 C and, after
stirring
for 30 minutes, trimethyl borate (21.5 ml) was added dropwise. The reaction
was
thawed to room temperature, diluted with water (300 ml) and extracted with
ethyl ace-
tate, and the organic phases were dried over sodium sulphate. The residue was
di-
gested with hexane (200 ml) and water (20 ml) and stored in a refrigerator
overnight.
The product was filtered off and washed with cold hexane. Yield of the title
compound
54% (16g). MS (ESI,+): 294 (M+1).
407c) 3'-[Butyl[(1,1-dimethylethoxy)carbonyl]amino]-4-ethoxy-N-[(R)-1-
(hydroxymethyl)-
2-(1 H-indol-3-yl)ethyl][1,1'-biphenyl]-3-carboxamide
The title compound was obtained in a Suzuki reaction in analogy to general
method
125e.
(CD30D): 8.27 d (J = 2.5 Hz, 1 H); 7.72 dd (J = 8.7 Hz / 2.5 Hz, 1 H); 7.66 d
(J = 8.0 Hz,
1 H); 7.50 d (J = 7.9 Hz, 1 H); 7.45 m (1 H); 7.33 d (J = 8.0 Hz, 1 H); 7.44
dd (J = 7.9 Hz /
7.7 Hz, 1 H); 7.17 d (J = 7.7 Hz, 1 H); 7.15 d (J = 8.7 Hz, 1 H); 7.14 s(1 H);
7.07 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 6.95 dd (J = 8.0 Hz / 7.0 Hz, 1 H); 4.49 m(1 H); 4.11
m(1 H); 4.03
m(1 H); 3.69 m (2H); 3.67 m (2H); 3.14 m (2H); 1.53 m (2H); 1.45 s (9H); 1.35
m (2H);
1.24 t (J = 7.2 Hz, 3H); 0.92 t (J = 7.4 Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
320
Example 422
3'-(Butylamino)-4-ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl][1,1'-
biphenyl]-3-carboxamide;
OH
I \ /
O NH N
H
\I
HN
422a ) 3-Butylaminophenylboronic acid
Ethereal HCI (saturated, 6 ml) was added to a solution of tert-butyl (3-
bromophenyl)-n-
butylcarbamate (500 mg) in dichloromethane (5 ml) and stirred at room
temperature for
six hours. The precipitate was filtered off, washed with diethyl ether, taken
up in water
(5 ml) and mixed with aqueous sodium bicarbonate solution. The precipitate was
filtered
off and washed with water. The title compound was obtained in 90% (350 mg)
yield.
422b) 3'-(Butylamino)-4-ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1 H-indol-3-
yl)ethyl][1,1'-
biphenyl]-3-carboxamide
The title compound was obtained in a Suzuki reaction in analogy to general
method
125e.
(CDCI3): 8.50 d (J = 7.3 Hz, 1 H); 8.48 d (J = 2.5 Hz, 1 H); 8.13 s(1 H); 7.71
d (J = 8.0
Hz, 1 H); 7.63 dd (J = 8.6 Hz / 2.5 Hz, 1 H); 7.36 d (J = 8.0 Hz, 1 H); 7.22
dd (J = 7.8 Hz /
7.8 Hz, 1 H); 7.19 dd (J = 8.0 Hz / 7.0 Hz, 1 H); 7.11 dd (J = 8.0 Hz / 7.0
Hz, 1 H); 7.10 s
(1 H); 6.95 d (J = 8.6 Hz, 1 H); 6.93 d (J = 7.8 Hz, 1 H); 6.86 m (1 H); 6.60
d (J = 7.8 Hz,
1 H); 4.58 m(1 H); 4.05 m (2H); 3.84 dd (J = 10.9 Hz / 3.5 Hz, 1 H); 3.77 dd
(J = 10.9 Hz /
5.3 Hz, 1 H); 3.17 m (2H); 3.15 m(2H); 1.63 m(2H); 1.46 m(2H); 1.25 t(J = 6.9
Hz,
3H); 0.97 t (J = 7.3 Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
321
Example 423
3'-[Butyl[(methylamino)carbonyl]amino]-4-ethoxy-N-[(R)-1-(hydroxymethyl)-2-(1
H-
indol-3-yl)ethyl][1,1'-biphenyl]-3-carboxamide;
OH
I \ ~
O NH N
H
/ I
\
Oy N
~ INH
423a) 3-(1-Butyl-3-methylureido)phenylboronic acid
A solution of 3-butylaminophenylboronic acid (350 mg) and methyl isocyanate
(103 mg)
in THF (5 ml) was stirred at room temperature for one hour, a further 0.05 ml
of methyl
isocyanate was added, and the mixture was stirred at room temperature for a
further
three hours. The solvent was distilled off in a rotary evaporator, and the
residue was
recrystallized from ethanol. The title compound was obtained in 33% yield (150
mg).
423b) 3'-[Butyl[(methylamino)carbonyl]amino]-4-ethoxy-N-[(R)-1-(hydroxymethyl)-
2-(1 H-
indol-3-yl)ethyl][1,1'-biphenyl]-3-carboxamide
The title compound was obtained in a Suzuki reaction in analogy to general
method
125e.
(CD3OD): 8.28 d (J = 2.5 Hz, 1 H); 7.75 dd (J = 8.7 Hz / 2.5 Hz, 1 H); 7.65 d
(J = 8.0 Hz,
1 H); 7.61 d(J = 7.9 Hz, 1 H); 7.52 dd (J = 7.9 Hz / 7.7 Hz, 1 H); 7.49 m(1
H); 7.33 d(J =
8.0 Hz, 1 H); 7.20 d (J = 7.7 Hz, 1 H); 7.16 d (J = 8.7 Hz, 1 H); 7.14 s(1 H);
7.07 dd (J =
8.0 Hz / 7.0 Hz, 1 H); 6.95 dd (J = 8.0 Hz / 7.0 Hz, 1 H); 4.49 m(1 H); 4.11
m(1 H); 4.02
m(1 H); 3.69 m(2H); 3.67 m(2H); 3.15 m(2H); 2.67 m(3H); 1.51 m(2H); 1.34 m
(2H);
1.25 t (J = 7.0 Hz, 3H); 0.91 t (J = 7.4 Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
322
The following compounds were obtained in analogy to the preparation methods de-
scribed in detail:
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
424 3'-[Butyl[(1,1-dimethyleth- 421 (CDC13): 8.48 d(J= 2.5 Hz,
oxy)carbonyl]amino]-N- 1 H); 8.44 d (J = 7.1 Hz, 1 H);
[(R)-1-(hydroxymethyl)-2- 7.71 d (J = 8.0 Hz, 1 H); 7.63 Ho -vH
(1H-indol-3-yl)ethyl]-4- dd (J = 8.7 Hz / 2.5 Hz, 1H); o NH _f
propoxy[1,1'-biphenyl]-3- 7.44 d (J = 8.1 Hz, 1 H); 7.43
carboxamide;
m(1 H); 7.37 dd (J = 8.1 Hz
8.1 Hz, 1 H); 7.36 d (J = 8.0 Q D
-Tryptophanol and Hz, 1 H); 7.19 dd (J = 8.0 Hz / N
3' (Butyl((1,1- 7.0 Hz, 1 H); 7.14 m(1 H); o~ ~
0
dime- 7.11 dd (J = 8.0 Hz / 7.0 Hz, thylethoxy)carbonyl]amino 1 H); 7.10 s (1
H); 6.99 d (J
= /\
]-4-propoxy[1,1'-biphenyl]- 8.7 Hz, 1 H); 4.59 m(1 H);
3-carboxylic acid 3.96 m (2H); 3.88 m(1 H);
3.78 m(1 H); 3.66 m (2H);
3.15 m (2H); 1.64 m (2H);
1.54 m (2H); 1.45 s (9H); 1.32
m(2H); 0.94 t (J = 7.4 Hz,
3H); 0.90 t (J = 7.4 Hz, 3H).
425 3'-(1-Butyl-3- 423 (DMSO-d6): 10.85 s(1 H); N
methylureido)-4- 8.19 d (J = 7.8 Hz, 1 H); 8.19
methoxybiphenyl-3- (1 H); 7.78 d (J = 8.6 Hz, 1 H); NH o~
H
carboxylic acid [(R)-1- 7.69 d (J = 7.8 Hz, 1 H); 7.53 o
hydroxymethyl-2-(1 H- d (J = 7.8 Hz, 1 H); 7.47 t (J =
indol-3-yl)ethyl]amide; 7.8 Hz, 1 H); 7.41 (1 H); 7.33 d
(J = 8.2 Hz, 1 H); 7.22 -7.15 m NN
(D)-Tryptophanol H
(3H); 7.06 t (J = 7.4 Hz, 1 H);
and 6.98 t (J = 7.4 Hz, 1 H); 5.65 q
(J = 4.3 Hz, 1 H); 4.94 (1 H);
3'-(1-Butyl-3- 4.21-4.29 m(1H); 3.84 s (3H);
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
323
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
methylureido)-4- 3.60 t (J = 7.2 Hz, 2H); 3.41-
methoxybiphenyl-3- 3.57 m (2H); 2.95-3.06 m
carboxylic acid (2H); 2.53 d (J = 4.3 Hz, 3H);
1.35-1.42 m (2H); 1.21-1.26
m (2H); 0.83 t (J = 7.4 Hz,
3H).
426 3'-(1-Butyl-3- 423 (DMSO-d6): 10.80 s(1H); S
H N
/
methylureido)-4-methoxy- 8.25 d (J = 8.2 Hz, 1 H); 7.68
5-mPthvlhinhPnvl-3- d(J = 7.8 Hz_ 1 H)' 763 m HO
O NH
carboxylic acid [(R)-1- (2H); 7.46-7.52 m (2H); 7.43 s
I o~
hydroxymethyl-2-(1 H- (1 H); 7.32 d (J = 8.2 Hz, 1 H);
indol-3-yI)ethyi]amide; 7.18 m (2H); 7.06 t (J = 7.4
Hz, 1 H); 6.97 t(J = 7.4 Hz, Ny O
(D)-Tryptophanol
1 H); 5.65 q (J = 4.3 Hz, 1 H); HN"
and 4.89 t (J = 5.5 Hz, 1 H); 4.23-
4.31 m(1 H); 3.62 s(3H);
3'-(1-Butyl-3- 3.53-3.59 m (3H); 3.45-3.50
methylureido)-4-methoxy- m(1 H); 3.04 dd (J = 14.4 Hz,
5-methylbiphenyl-3- J= 6.6 Hz, 1 H); 2.94 dd (J =
carboxylic acid 14.4 Hz, J = 6.6 Hz, 1 H); 2.54
d (J = 4.3 Hz, 3H); 2.31 s
(3H); 1.35-1.42 m (2H); 1.21-
1.28 m (2H); 0.83 t (J = 7.4
Hz, 3H).
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
324
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
427 3'-(1-Butyl-3- 423 (DMSO-d6): 10.82 s(1H); H
methylureido)-4- 8.48 d (J = 8.2 Hz, 1 H); 8.20 ONH isopropoxybiphenyl-3- d(J
= 2.3 Hz, 1 H); 7.75 dd (J o~
carboxylic acid [(R)-1- = 8.2 Hz, J = 2.1 Hz, 1H); 1
hydroxymethyl-2-(1 H- 7.71 d (J = 7.8 Hz, 1 H); 7.54
indol-3-yl)ethyl]amide; d (J = 7.8 Hz, 1 H); 7.48 t (J = ~ 1
7.8 Hz, 1 H); 7.42 (1 H); 7.33 d o N
(D)-Tryptophanol ~
(J = 8.2 Hz, 1 H); 7.26 d (J =
anri 8.9 Hz, 1 H); 7.16-7.18 m
(2H); 7.05 t (J = 7.4 Hz, 1 H);
3'-(1-Butyl-3- 6.98 t (J = 7.2 Hz, 1 H); 5.65 q
methylureido)-4- (J = 4.3 Hz, 1 H);4.97 t (J
=
isopropoxybiphenyl-3- 4.8 Hz, 1 H); 4.79-4.85 m
carboxylic acid (1 H); 4.23-4.30 m (1 H); 3.61 t
(J = 7.4 Hz, 2H); 3.421-3.54
m (2H); 2.94-3.04 m (2H);
2.53 d (J = 4.3 Hz, 3H); 1.36-
1.43 m (2H); 1.21-1.29 m
(8H); 0.84 t (J = 7.4 Hz, 3H).
428 3'-(2-Dimethylminothoxy)- 329 (CDCI3): 8.78 s(1 H); 8.50 d H
N
4-ethoxybiphenyl-3- (J = 7.3 Hz, 1 H); 8.46 d (J
=
carboxylic acid [(R)-1- 2.5 Hz, 1 H); 7.71 d (J = 7.8 HO
0 NH
hydroxymethyl-2-(1 H- Hz, 1 H); 7.56 dd (J = 2.5 Hz
indol-3-yl)ethyl]amide; 8.6 Hz, 1 H); 7.32 m (2H); 5-Bromo-2-ethoxy-N-((R)-
7.13 m (3H); 7.06 m (2H); o
1-hydroxymethyl-2-(1H- 6.89 m(2H); 4.55 m(1H); N f
4.11 m (2H); 3.97 m (2H); i
indol-3-y1)eth yl]benzamide
and 3.74 m(2H); 3.12 m (2H);
3-(2-Dimethylamino- 2.78 m (2H); 2.37 s(6H); 1.21
ethoxY)-phenYlboronic m (3H).
acid pinacol ester
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
325
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
429 4'-Ethoxy-3'-[(R)-1- 135 Column Purospher Star RP ~ H
hydroxymethyl-2-(1 H- C18 4.6x125 5pm; detection
indol-3-yl)ethylcarbamoyl]- wavelength 214 nm; flow rate
HO
biphenyl-3-carboxylic acid 1 mI/min; eluents A: 0.1 % o NH
methyl ester; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
5-Bromo-2-ethoxy-N-((R)-
based on B: 5% to 95% (10') XMe
1-hydroxymethyl-2-(1H- to 95% (2') to 5% (0.5') to 5%
indol-3-y1)ethylJbenzamide (2.5')
and Molecular peak (ESI, M+1):
3- 473.5
Methoxycarbonylphenyl- Retention time: 9.95 min.
boronic acid
430 4-Ethoxy- 135 Column Purospher Star RP ~ H
[1,1';3',1 "]terphenyl-3- C18 4.6x125 5pm; detection ~~
carboxylic acid [1- wavelength 214 nm; flow rate Ho
hydroxymethyl-2-(1H- 1 mI/min; eluents A: 0.1% o tvH
indol-3-yl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
5-Bromo-2-ethoxy-N-((R)- I ~
based on B: 5% to 95% (10')
1-hydroxymethyl-2-(1H- to 95% (2') to 5% (0.5') to 5%
indol-3-y1)ethylJbenzamide (2.5') and
Molecular peak (ESI, M+1):
Biphenyl-3-boronic acid 491.6
Retention time: 11.1 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
326
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
431 3'-Acetyl-4- 135 Column Purospher Star RP H
ethoxybiphenyl-3- C18 4.6x125 5pm; detection
carboxylic acid [(R)-1- wavelength 214 nm; flow rate
HO .
hydroxymethyl-2-(1 H- 1 ml/min; eluents A: 0.1 % o NH
indol-3-yl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
5-Bromo-2-ethoxy-N-((R)- based on B: 5% to 95% (10')
1-hydroxymethyl-2-(1H- to 95% (2') to 5% (0.5') to 5% 0
indol-3-y1)ethyl]benzamide (2.5')
and Molecular peak (ESI, M+1):
3-Acetylphenylboronic 457.5
acid Retention time: 9.1 min.
432 4-Ethoxy-3'-pyrrolidin-1-yl- 135 Column Purospher Star RP H
biphenyl-3-carboxylic acid C18 4.6x125 5pm; detection
[(R)-1-hydroxymethyl-2- wavelength 214 nm; flow rate H NH oJ
(1 H-indol-3-yl)ethyl]amide; 1 ml/min; eluents A: 0.1 % OH
~
TFA in H20, B 0.1% TFA in
5-Bromo-2-ethoxy-N-((R)- ACN; gradient in each case
1-hydroxymethyl-2-(1 H-
based on B: 5% to 95% (10')
indol-3-y1)ethy1]benzamide to 95% (2') to 5% (0.5') to 5%
and (2.5')
(3-Pyrolidine-l-
ylphenyl)boronic acid Molecular peak (ESI, M+1):
484.6
Retention time: 8.75 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
327
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
433 4'-Cyanomethyl-4- 135 Column Purospher Star RP H
ethoxybiphenyl-3- C18 4.6x125 5pm; detection
carboxylic acid [(R)-1- wavelength 214 nm; flow rate
H NH O
hydroxymethyl-2-(1H- 1 mI/min; eluents A: 0.1% OH
~
indol-3-yl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
5-Bromo-2-ethoxy-N-[(R)- based on B: 5% to 95% (10')
1 -hydroxymethyl-2-(1 H-
to95%(2')to5%(0.5')to5%
indol-3-yl)ethylJbenzamide (25)
and
Molecular peak (ESI, M+1):
(4- 454.5
Cyanomethylphe-
Retention time: 9.03 min.
nyl)boronic acid
434 4'-Dimethylamino-4- 135 Column Purospher Star RP ~~
' oH
propoxy-biphenyl-3- C18 4.6x125 5pm; detection HN ~
carboxylic acid [(R)-1- wavelength 214 nm; flow rate H NH o
hydroxymethyl-2-(1 H- 1 mI/min; eluents A: 0.1 % o
indol-3-yl)ethyl]amide; TFA in H20, B 0.1% TFA in
N-((R)-1-Hydroxymethyl-2- ACN; gradient in each case
(1H-indol-3-y1)ethy1J-5- based on B: 5% to 95% (10') .N~
iodo-2-propoxy- to 95% (2') to 5% (0.5') to 5%
benzamide (25)
and Molecular peak (ESI, M+1):
4-(Dimethylamino)phenyl- 472.5
boronic acid
Retention time: 6.95 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
328
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
435 4-Propoxybiphenyl-3,3'- 135 Column Purospher Star RP
dicarboxylic acid 3'- C18 4.6x125 5pm; detection OH NH
diethylamide 3-{[(R)-1- wavelength 214 nm; flow rate HN
"
~ 0
hydroxymethyl-2-(1 H- 1 mI/min; eluents A: 0.1 %
indol-3-yl)ethyl]amide}; TFA in H20, B 0.1% TFA in
ACN; gradient in each case I~ 1
N-((R)-1-Hydroxymethyl-2- 5% o "
based on B: 5/o to 95% (10')
(1 H-indol-3-yl)ethylJ-5-
to95%(2')to5%(0.5')to5%
iodo-2-propoxy- (2.5')
benzamide
Molecular peak (ESI, M+1):
and
528.5
3-(N, N- Retention time: 8.95 min.
Diethylaminocarbonyl)-
phenylboronic acid
436 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP
dicarboxylic acid 4'- C18 4.6x125 5pm; detection H \ NH
diethylamide 3-{[(R)-1- wavelength 214 nm; flow rate 0 HN H
hydroxymethyl-2-(1 H- 1 mI/min; eluents A: 0.1 % I~
indol-3-yl)ethyl]amide}; TFA in H20, B 0.1% TFA in
ACN; gradient in each case I
N-((R)-1-Hydroxymethyl-2- based on B: 5% to 95% (10') o N
(1 H-indol-3-yl)ethylJ-5- to 95% (2') to 5% (0.5') to 5% J
iodo-2-propoxy- (2.5')
benzamide
Molecular peak (ESI, M+1):
and
528.5
4-(N,N- Retention time: 8.88 min.
Diethylaminocarbonyl)-
phenylboronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
329
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
437 3'-[(R)-1-Hydroxymethyl-2- 135 Column Purospher Star RP H
N
(1 H-indol-3- C18 4.6x125 5pm; detection -
yl)ethylcarbamoyl]-4'- wavelength 214 nm; flow rate Ho H
HN
propoxybiphenyl-4- 1 mI/min; eluents A: 0.1 % 0
carboxylic acid; TFA in H20, B 0.1 % TFA in
ACN; gradient in each case
N-((R)-2-Hydroxy-l-(1H- based on B: 5% to 95% (10') o
indol-3-ylmethyl)ethyl]-5- HO
to95%(2')to5%(0.5')to5%
iodo-2-propoxy- (2 5,)
benzamide
Molecular peak (ESI, M+1):
and
473.5
4-Carboxyphenylboronic Retention time: 8.35 min.
acid
438 4'-Acetyl-4- 135 Column Purospher Star RP H
N
propoxybiphenyl-3- C18 4.6x125 5pm; detection
carboxylic acid [(R)-1- wavelength 214 nm; flow rate Ho HN 0
H
hydroxymethyl-2-(1 H- 1 mI/min; eluents A: 0.1 % ~o
indol-3-yl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
N-((R)-2-Hydroxy-1-(1 H- o
based on B: 5% to 95% (10')
indol-3-ylmethyl)ethyl]-5- to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (2 5,)
benzamide
Molecular peak (ESI, M+1):
and
471.5
4-Acetylphenylboronic Retention time: 9.15 min.
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
330
Product; Method Structure
Ex. analogous 'H-N M R(400 MHz) S[ppm]
reagents to
439 4'-Ethanesulphonyl-4- 135 Column Purospher Star RP
propoxy-biphenyl-3- C18 4.6x125 5pm; detection oH
NH
carboxylic acid [(R)-2- wavelength 214 nm; flow rate 0 HN
hydroxy-1 -(1 H-indol-3- 1 mI/min; eluentsA: 0.1%
ylmethyl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
N-((R)-2-Hydroxy-1-(1 H- -
based on B: 5% to 95% (10') S1
indol-3-ylmeth yl) eth yl]-5-
to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (2.5')
benzamide
Molecular peak (ESI, M+1):
and
521.5
4-(Ethylsulphonyl)-
Retention time: 8.73 min.
phenylboronic acid
440 3'-Cyanomethyl-4- 135 Column Purospher Star RP
propoxy-biphenyl-3- C18 4.6x125 5pm; detection oH
NH
carboxylic acid [(R)-1- wavelength 214 nm; flow rate o HN H
~ 0
hydroxymethyl-2-(1 H- 1 ml/min; eluents A: 0.1 %
indol-3-yl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case "
N-((R)-2-Hydroxy-1-(1 H-
based on B: 5% to 95% (10')
indol-3-ylmethyl)ethyl]-5- to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (2 5,)
benzamide
Molecular peak (ESI, M+1):
and
468.5
3-(Cyanomethyl)phenyl- Retention time: 9.13 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
331
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
441 3'-Methanesulphonyl- 135 Column Purospher Star RP
amino-4-propoxy- C18 4.6x125 5pm; detection OH ~
NH
biphenyl-3-carboxylic acid wavelength 214 nm; flow rate O HN FI
[(R)-2-hyd roxy- 1 -(1 H- 1 mI/min; eluents A: 0.1% I~ 0
indol-3-ylmethyl)ethyl]- TFA in H20, B 0.1% TFA in
i
amide; ACN; gradient in each case ~ I NH
o: ~
based on B: 5% to 95% (10') o_slI
N-[(R)-2-Hydroxy-1-(1H- to 95% (2') to 5% (0.5') to 5%
indol-3-ylmeth yl) eth ylJ-5-
(2.5')
iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
522.5
and
Retention time: 8.56 min.
3-(Methylsulphonylamino)-
phenylboronic acid
442 3'-Cyclopropylmethoxy-4- 135 Column Purospher Star RP
propoxy-biphenyl-3- C18 4.6x125 5pm; detection H
NH
carboxylic acid [(R)-1- wavelength 214 nm; flow rate 0 HN H
hydroxymethyl-2-(1 H- 1 ml/min; eluents A: 0.1 % 0
indol-3-yl)ethyl]amide; TFA in H20, B 0.1% TFA in
N-((R)-2-Hydroxy-l-(1 H- ACN; gradient in each case o
based on B: 5% to 95% (10') LIL
indol-3-ylmethyl)ethy1J-5- to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and 499.5
3-(Cyclopropylmethoxy)- Retention time: 10.5 min.
phenylboronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
332
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) 8 [ppm]
reagents to
443 3'-Methanesulphonyl-4- 135 Column Purospher Star RP H
N
propoxy-biphenyl-3- C18 4.6x125 5pm; detection
carboxylic acid [(R)-2- wavelength 214 nm; flow rate HO H
hydroxy-1-(1H-indol-3- 1 mI/min; eluentsA: 0.1% ---o Q
ylmethyl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case 60
N-[(R)-2-Hydroxy-1-(IH- based on B: 5% to 95% (10')
indol-3-ylmethyl)ethyl]-5- to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
507.5
3-(Methylsuofonyl)phen yl-
Retention time: 8.45 min.
boronic acid
444 4-Propoxybiphenyl-3,3'- 135 Column Purospher Star RP
/
dicarboxylic acid 3'-[(2- oH -
C18 4.6x125 5pm; detection NH
dimethyl- wavelength 214 nm; flow rate O HN H
aminoethyl)amide] 3-{[(R)- I
1 ml/min; eluents A: 0.1 % ~
1-hydroxymethyl-2-(1 H- TFA in H20, B 0.1% TFA in
indol-3-yl)ethyl]amide}; ACN; gradient in each case I o
N-[(R)-2-Hydroxy-1-(IH- HN~
based on B: 5% to 95% (10')
indol-3-ylmethyl)ethyl]-5- to 95% (2') to 5% (0.5') to 5% i
iodo-2-propoxy- (2.5')
benzamide
and Molecular peak (ESI, M+1):
3-(2-N, N-Dimeth ylamino- 543.5
eth ylaminocarbonyl)-
phenylboronic acid Retention time: 6.67 min.
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
333
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
445 3'-[(R)-1-Hydroxymethyl-2- 135 Column Purospher Star RP
(1 H-indol-3-yl)ethylcarba- C18 4.6x125 5pm; detection oH ~ NH
moyl]-3-methoxy-4'- wavelength 214 nm; flow rate o HN H
~ 0
propoxybiphenyl-4-car- 1 mI/min; eluents A: 0.1 % I~
boxylic acid methyl ester; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
N-((R)-2-Hydroxy-1-(1 H-
based on B: 5% to 95% (10') o 0
indol-3-ylmethyl)ethyl]-5- to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
517.5
3-Methoxy-4-(methoxy-
Retention time: 9.13 min.
carbonyl)phenylboronic
acid
446 3'-Chloro-4- 135 Column Purospher Star RP
oH ~
propoxybiphenyl-3,4'- C18 4.6x125 5pm; detection NH
dicarboxylic acid 4'-amide wavelength 214 nm; flow rate 0 HN H
0
3-{[(R)-2-hydroxy-1-(1H- 1 ml/min; eluentsA: 0.1% I~
indol-3- TFA in H20, B 0.1% TFA in
ylmethyl)ethyl]amide}; ACN; gradient in each case c,
based on B: 5% to 95% (10') HZN 0
N-((R)-2-Hydroxy-l-(1H- to 95% (2') to 5% (0.5') to 5%
indo1-3-ylmeth yl)ethyl]-5-
(2.5')
iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
507
and
Retention time: 7.85 min.
4-(Aminocarbonyl)-3-
chlorophenylboronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
334
Product; Method Structure
Ex. analogous 'H-N M R(400 MHz) S[ppm]
reagents to
447 3'-Dimethylsulphamoyl-4- 135 Column Purospher Star RP
propoxy-biphenyl-3- C18 4.6x125 5pm; detection oH NH
carboxylic acid [(R)-2- wavelength 214 nm; flow rate "N
o
hydroxy-1-(1 H-indol-3- 1 mI/min; eluents A: 0.1%
ylmethyl)ethyl]amide; TFA in H20, B 0.1% TFA in
N-((R)-2-Hydroxy-1-(1 H- ACN; gradient in each case o:o
indol-3-ylmethyl)ethyl]-5- based on B: 5% to 95% (10')
iodo-2-propoxy- to 95% (2') to 5% (0.5') to 5%
benzamide (2.5')
and Molecular peak (ESI, M+1):
3-(N, N- 536.5
Dimethylsulphonamido-
phenyl)boronic acid Retention time: 9.13 min.
448 4'-(Propane-2-sulphonyl)- 135 Column Purospher Star RP QY\"
4-propoxy-biphenyl-3- C18 4.6x125 5Nm; detection o" NH
carboxylic acid [(R)-2- wavelength 214 nm; flow rate o HN Fi
hydroxy-1-(1H-indol-3- 1 mI/min; eluentsA: 0.1%
ylmethyl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case -
oõ
N-((R)-2-Hydroxy-1-(1H- based on B: 5% to 95% (10') o=sy
indo1-3-ylmethyl)ethyl]-5- to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
535.5
4-
Retention time: 9.03 min.
(Isopropylsulphonylphe-
nyl)boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
335
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
449 4'-Methylsulphamoyl-4- 135 Column Purospher Star RP
~ OH
propoxy-biphenyl-3- C18 4.6x125 5pm; detection
NH
carboxylic acid [(R)-2- wavelength 214 nm; flow rate o HN H
hydroxy-1 -(1 H-indol-3- 1 ml/min; eluentsA: 0.1% I~
ylmethyl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
N-((R)-2-Hydroxy-1-(1 H- s,
based on B: 5% to 95% (10') O p NH
indol-3-ylmethyl)ethyl]-5-
to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
522.5
(4-Methylaminosulphonyl-
Retention time: 8.49 min.
phenyl)boronic acid
450 4'-Dimethylsulphamoyl-4- 135 Column Purospher Star RP
propoxy-biphenyl-3- C18 4.6x125 5pm; detection o"
NH
carboxylic acid [(R)-2- wavelength 214 nm; flow rate O HN
hydroxy-1-(1H-indol-3- 1 ml/min; eluents A: 0.1% 1 o
ylmethyl)ethyl]amide; TFA in H20, B 0.1% TFA in ~
ACN; gradient in each case ~ i
N-((R)-2-Hydroxy-1-(1 H-
based on B: 5% to 95% (10') o o'N~
indol-3-ylmethyl)ethyl]-5-
to95%(2')to5%(0.5')to5%
iodo-2-propoxy-
(2.5')
benzamide
Molecular peak (ESI, M+1):
and
536.5
(4-Dimethylamino- Retention time: 9.1 min.
sulphonylphenyl)boronic
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
336
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
451 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP N
dicarboxylic acid 4'-amide C18 4.6x125 5pm; detection '
HO
3-{[(R)-1-hydroxymethyl-2- wavelength 214 nm; flow rate HNH
(1 H-indol-3-yl)ethyl]- 1 mi/min; eluents A: 0.1 % tio
amide); TFA in H20, B 0.1% TFA in
ACN; gradient in each case
N-((R)-2-Hydroxy-l-(1 H- "~"
based on B: 5% to 95% (10')
indol-3-ylmethyl)ethyl]-5- to 95% (2') to 5% (0.5') to 5%
iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
472.5
4-Aminocarbonyl-
Retention time: 7.59 min.
phenylboronic acid
452 3'-Methylsulphamoyl-4- 135 Column Purospher Star RP Q
propoxy-biphenyl-3- C18 4.6x125 5Nm; detection o" ~ NH
carboxylic acid [(R)-2- wavelength 214 nm; flow rate "N "
I ~ o
hydroxy-1-(1H-indol-3- 1 mI/min; eluentsA: 0.1%
ylmethyl)ethyl]amide; TFA in H20, B 0.1% TFA in
ACN; gradient in each case N
N-((R)-2-Hydroxy-1-(1 H- 'so \
based on B: 5% to 95% (10')
indol-3-ylmethyl)ethyl]-5-
to95%(2')to5%(0.5')to5%
iodo-2-propoxy- (2 5,)
benzamide
Molecular peak (ESI, M+1):
and
522.5
(3-Methylaminosulphonyl- Retention time: 8.57 min.
phenyl)boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
337
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
453 3'-Methanesulphonyl-4- 135 Column Purospher Star RP
propoxy-biphenyl-3- C18 4.6x125 5pm; detection N-
carboxylic acid [(R)-2- wavelength 214 nm; flow rate OH
hydroxy-1 -(1-methyl-1 H- 1 mi/min; eluents A: 0.1 % o S HN'"
0
indol-3-ylmethyl)ethyl]- TFA in H20, B 0.1% TFA in - ~~
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-((R)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5%
(1-methyl-1 H-indol-3-
(2.5')
y,)c~t hyI]-5-lvdir2-propoxy-
benzamide Molecular peak (ESI, M+1):
521.6
and
Retention time: 9.22 min.
3-Methylsulphonyl-
phenylboronic acid
454 3'-[(R)-1-Hydroxymethyl-2- 135 Column Purospher Star RP
(1-methyl-1 H-indol-3- C18 4.6x125 5pm; detection ~ "-
yl)ethylcarbamoyl]-3- wavelength 214 nm; flow rate OH
O HN' H
methoxy-4'- 1 ml/min; eluents A: 0.1 % a~~
propoxybiphenyl-4- TFA in H20, B 0.1% TFA in - - - 0
carboxylic acidmethyl es- ACN; gradient in each case
ter; based on B: 5% to 95% (10')
to 95% (2') to 5% (0.5') to 5%
N-((R)-1-Hydroxymethyl-2- (25)
(1-methyl-1 H-indol-3-
yl)ethylJ-5-iodo-2- Molecular peak (ESI, M+1):
propoxybenzamide 531.6
and Retention time: 9.92 min.
3-Methoxy-4-(methoxy-
carbonyl)phenylboronic
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
338
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
455 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP N
4
dicarboxylic acid 4'-[(2- C18 4.6x125 5pm; detection 0 i HO
dimethylaminoethyl)- wavelength 214 nm; flow rate N o o-/
amide] 3-{[(R)-1-hydroxy- 1 mI/min; eluents A: 0.1%
methyl-2-(1 -methyl- 1 H- TFA in H20, B 0.1% TFA in
indol-3-yl)ethyl]amide}; ACN; gradient in each case o NH
based on B: 5% to 95% (10')
N-((R)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5% -N
(1-methyl-1 H-indol-3-yl)-
(2.5')
zthylJ-S-iodo-2-pr opoxy-
benzamide Molecular peak (ESI, M+1):
557.7
and
Retention time: 6.91 min.
3-(2-N, N-Dimethylamino-
ethylaminocarbonyl)-
phenylboronic acid
456 3'-[2-(5-Fluoro-1 H-indol-3- 135 Column Purospher Star RP F
yl)-1-hydroxymethyl- C18 4.6x125 5pm; detection NH
ethylcarbamoyl]-4'- wavelength 214 nm; flow rate OH
HN H.
propoxybiphenyl-4- 1 mI/min; eluents A: 0.1 % Ho _ o
\ / ~ \ -
carboxylic acid; TFA in H20, B 0.1% TFA in o o
ACN; gradient in each case
N-(2-(5-Fluoro-1H-indol-3- based on B: 5% to 95% (10')
yl)-1-hydroxymethyl-ethylJ- to 95% (2') to 5% (0.5') to 5%
5-iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and 491.5
4-Carboxyphenylboronic
Retention time: 8.37 min.
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
339
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
457 3'-Methanesulphonyl- 135 Column Purospher Star RP F
amino-4-propoxy-bi- C18 4.6x125 5pm; detection ~ NH
phenyl-3-carboxylic acid wavelength 214 nm; flow rate o OH
-$-N HN H.
[2-(5-fluoro-1 H-indol-3-yl)- 1 ml/min; eluents A: 0.1 % o
1-hydroxymethylethyl]- TFA in H20, B 0.1% TFA in - - ~
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]- (25)
5-iodo-2-pr ;,poxy-
benzamide Molecular peak (ESI, M+1):
540.6
and
Retention time: 8.57 min.
3-
(Meth ylsul ph on ylami-
no)phenylboronic acid
458 3'-Methanesulphonyl-4- 135 Column Purospher Star RP F I
propoxy-biphenyl-3- C18 4.6x125 5pm; detection ~ NH
carboxylic acid [2-(5- wavelength 214 nm; flow rate OH
O HN H
fluoro-1 H-indol-3-yl)-1- 1 ml/min; eluents A: 0.1 % 's o
hydroxymethy- TFA in H20, B 0.1% TFA in ~~ c ~~ 0
lethyl]amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1 H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]- (2.5')
5-iodo-2-
propoxybenzamide Molecular peak (ESI, M+1):
525.6
and
Retention time: 8.61 min.
3-Methylsulphonylphenyl-
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
340
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
459 4-Propoxybiphenyl-3,3'- 135 Column Purospher Star RP f
dicarboxylic acid 3'-[(2- C18 4.6x125 5pm; detection /\ HO 0 o
dimethylaminoethyl)- wavelength 214 nm; flow rate
amide] 3-{[2-(5-fluoro-1 H- 1 mI/min; eluents A: 0.1 % F ~
I
indol-3-yl)-1-hydroxy- TFA in H20, B 0.1% TFA in o
methylethyl]amide}; g ~NH
ACN; radient in each case
based on B: 5% to 95% (10') N
N-[2-(5-Fluoro-1 H-indol-3-
yl)-1-hydroxymethylethyl]- to 95% (2') to 5% (0.5') to 5%
5-iodo-2-propoxybenz- (25)
amide Molecular peak (ESI, M+1):
and 561.7
3-(2-N, N-Dimethylamino-
ethylaminocarbonyl)- Retention time: 6.85 min.
phenylboronic acid
460 3'-Chloro-4-propoxy- 135 Column Purospher Star RP - N
biphenyl-3,4'-dicarboxylic C18 4.6x125 5pm; detection oH
acid 4'-amide 3-{[2-(5- wavelength 214 nm; flow rate HNH 0
fluoro-1H-indol-3-yl)-1- 1 ml/min; eluentsA: 0.1%
hydroxymethylethyl]- TFA in H20, B 0.1% TFA in -
HzN
amide}; ACN; gradient in each case o
based on B: 5% to 95% (10')
N-[2-(5-Fluoro-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]- (25)
5-iodo-2-propoxybenz-
amide Molecular peak (ESI, M+1):
525
and
Retention time: 7.99 min.
4-(Aminocarbonyl)-3-
chlorophenylboronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
341
Product; Method Structure
Ex. analogous IH-NMR (400 MHz) S [ppm]
reagents to
461 3'-Dimethylsulphamoyl-4- 135 Column Purospher Star RP F
propoxy-biphenyl-3- C18 4.6x125 5pm; detection ~ NH
carboxylic acid [2-(5- wavelength 214 nm; flow rate OH
O N- HN H
fluoro-1 H-indol-3-yl)-1- 1 mI/min; eluents A: 0.1 % 'S o
hydroxymethylethyl]- TFA in H20, B 0.1% TFA in 6-- c \ o
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-lH-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]- (2 5,)
5-iodo-2-pr opoxybenz-
amide Molecular peak (ESI, M+1):
554.7
and
Retention time: 9.17 min.
3-(N, N-Dimethylsulphon-
amidophenyl)boronic acid
462 4'-(Propane-2-sulphonyl)- 135 Column Purospher Star RP H
N HO ~
4-propoxy-biphenyl-3- C18 4.6x125 5pm; detection N
H
carboxylic acid [2-(5- wavelength 214 nm; flow rate F
fluoro-1 H-indol-3-yl)-1- 1 mI/min; eluents A: 0.1 %
hydroxymethylethyl]- TFA in H20, B 0.1% TFA in ~'o
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-lH-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]- (2.5')
5-iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
553.7
and
Retention time: 9.18 min.
4-(Isopropylsulphonyl-
phenyl)boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
342
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
463 4'-Dimethylsulphamoyl-4- 135 Column Purospher Star RP F
propoxy-biphenyl-3- C18 4.6x125 5pm; detection NH
OH
carboxylic acid [2-(5- wavelength 214 nm; flow rate HN H
fluoro-1 H-indol-3-yl)-1- 1 mI/min; eluents A: 0.1 % os a
hydroxymethylethyl]- TFA in H20, B 0.1% TFA in
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]-
(2.5')
~ - -- ~ - -- - - -
a--oao----piopoxy-
benzamide Molecular peak (ESI, M+1):
and 554.7
4-(N,N-Dimethylsulphon- Retention time: 9.12 min.
amidophenyl)boronic acid
464 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP F
dicarboxylic acid 4'- C18 4.6x125 5pm; detection NH
OH
diethylamide 3-{[2-(5- wavelength 214 nm; flow rate HN H.
fluoro-1H-indol-3-yl)-1- 1 mI/min; eluents A: 0.1%
r--N ~ r r_~ O
hydroxymethy- TFA in H20, B 0.1% TFA in
lethyl]amide}; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]- (2.5')
5-iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
546.7
and
Retention time: 9.07 min.
4-(N, N-Dimethylamino-
carbon yl)-phenylboronic
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
343
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
465 3'-Methylsulphamoyl-4- 135 Column Purospher Star RP F
propoxy-biphenyl-3- C18 4.6x125 5pm; detection ~ NH
carboxylic acid [2-(5- wavelength 214 nm; flow rate \ OH
O NH H
fluoro-1 H-indol-3-yl)-1- I ml/min; eluents A: 0.1 % o=s HN
0
hydroxymethylethyl]- TFA in H20, B 0.1% TFA in
- _ o
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]-
(2.5')
5-:--do-2-propoxy-
benzamide Molecular peak (ESI, M+1):
540.6
and
Retention time: 8.72 min.
(3-Methylaminosulphonyl-
phenyl)-boronic acid
466 3'-Acetyl-4-propoxy- 135 Column Purospher Star RP
biphenyl-3-carboxylic acid C18 4.6x125 5pm; detection ~"-
[(R)-1-hydroxymethyl-2-(1- wavelength 214 nm; flow rate OH
H
HN'
0
methyl-1 H-indol-3-yl)- 1 mi/min; eluents A: 0.1 % 0
ethyl]amide; TFA in H20, B 0.1% TFA in ~
ACN; gradient in each case
N-((R)-1-Hydroxymethyl-2- based on B: 5% to 95% (10')
(1-methyl-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)ethyl]-5-iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
485.6
3-Acetylphenylboronic Retention time: 9.8 min.
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
344
P rod uct; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
467 4-Propoxy- 135 Column Purospher Star RP
[1.1';3'.1"]terphenyl-3- C18 4.6x125 5pm; detection ~ "-
carboxylic acid [(R)-1- wavelength 214 nm; flow rate HN H H
hydroxymethyl-2-(1- 1 mI/min; eluentsA: 0.1%
methyl-1 H-indol-3-yl)- TFA in H20, B 0.1% TFA in 0 *-~
ethyl]amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-((R)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5%
(1-methyl-1 H-indol-3-
(2.5')
yl)ethy,"
.,-5-iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
519.7
and
Retention time: 11.62 min.
Biphenyl-3-boronic acid
468 3'-Cyanomethyl-4- 135 Column Purospher Star RP
I ,
propoxy-biphenyl-3- C18 4.6x125 5pm; detection "
carboxylic acid [(R)-1- wavelength 214 nm; flow rate OH
h drox meth I-2- 1- 1 mI/min; eluents A: 0.1% N HNH
y y y ( O
methyl-1 H-indol-3-yl)- TFA in H20, B 0.1% TFA in
ethyl]amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-((R)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5%
(1-methyl-1 H-indol-3-
(2.5')
yl) eth yl]-5-iodo-2-propox y-
benzamide Molecular peak (ESI, M+1):
482.6
and
Retention time: 9.81 min.
3-Cyanomethylphenyl-
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
345
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
469 3'-Methansulphonylamino- 135 Column Purospher Star RP
4-propoxy-biphenyl-3- C18 4.6x125 5pm; detection ~ N-
carboxylic acid [(R)-2- wavelength 214 nm; flow rate o OH
-N HN H
hydroxy-1 -(1 -methyl-1 H- 1 ml/min; eluents A: 0.1% 0
indol-3-ylmethyl)ethyl]- TFA in H20, B 0.1% TFA in
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-((R)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5%
(1-methyl-1 H-indol-3-
(2.5')
yl)ethyi;-5-iodo-2-pr opoxy-
benzamide Molecular peak (ESI, M+1):
436.7
and
Retention time: 9.1 min.
3-(Methylsulphonamido)-
phenylboronic acid
470 4'-Cyanomethyl-4- 135 Column Purospher Star RP
propoxy-biphenyl-3- C18 4.6x125 5pm; detection ~ N-
carboxylic acid [(R)-1- wavelength 214 nm; flow rate OH
HN, H
hydroxymethyl-2-(1- 1 mi/min; eluents A: 0.1% 0
methyl-1 H-indol-3-yl)- TFA in H20, B 0.1% TFA in 0 *-~
ethyl]amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-ftR)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5%
(1-methyl- 1 H-indol-3- (25)
yl) e th ylJ-5-iodo-2-propox y-
benzamide Molecular peak (ESI, M+1):
482.6
and
Retention time: 9.67 min.
4-Cyanomethylphenyl-
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
346
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
471 4-Propoxy-biphenyl-3.3'- 135 Column Purospher Star RP
dicarboxylic acid 3'-[(2- N HO
C18 4.6x125 5pm; detection I H
dimethylamino-ethyl)- wavelength 214 nm; flow rate "~
amide] 3-{[(R)-1-hydroxy- 1 mI/min; eluents A: 0.1%
methyl-2-(1-methyl-1 H- TFA in H20, B 0.1 % TFA in r NH
indol-3-yl)ethyl]amide}; ACN; gradient in each case NJ
N-((R)-1-Hydroxymethyl-2- based on B: 5% to 95% (10')
(1-methyl-IH-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)ethyl]-5-iodo-2-propoxy- (25)
benzarr-ide
and Molecular peak (ESI, M+1):
3-(2-N, N-Dimethylamino- 557.7
e th yl amin oca rb on yl) -
phenylboronic acid Retention time: 7.19 min.
472 4-Fluoro-3'-[(R)-2- 135 Column Purospher Star RP
hydroxy-1-(1-methyl-1H- C18 4.6x125 5pm; detection ~ N-
indol-3-ylmethyl)ethyl- wavelength 214 nm; flow rate HO HNH o"
carbamoyl]-4'-propoxy- 1 mI/min; eluents A: 0.1 % F~~
biphenyl-3-carboxylic acid; TFA in H20, B 0.1% TFA in
ACN; gradient in each case
N-((R)-1-Hydroxymethyl-2- based on B: 5% to 95% (10')
(1-methyl-lH-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)ethyl]-5-iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
505.6
3-Carboxy-4-fluorphenyl- Retention time: 8.94 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
347
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
473 3'-Chloro-4-propoxy- 135 Column Purospher Star RP
biphenyl-3,4'-dicarboxylic C18 4.6x125 5pm; detection ~ N-
acid 4'-amide 3-{[(R)-2- wavelength 214 nm; flow rate OH
HN H
hydroxy-1-(1-methyl-1 H- 1 mI/min; eluents A: 0.1 % HZN ci
- ~ ~
indol-3-ylmethyl)ethyl]- TFA in H20, B 0.1% TFA in 0 o
amide}; ACN; gradient in each case
based on B: 5% to 95% (10')
N-((R)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5%
(1-methyl-1 H-indol-3-
(2.5')
y!) ?t.hy.;1-5-iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
521
and
Retention time: 8.44 min.
3-Chloro-5-(carbamoyl)-
phenylboronic acid
474 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP
dicarboxylic acid 4'- C18 4.6x125 5pm; detection ~ N-
OH
diethylamide 3-{[(R)-1- wavelength 214 nm; flow rate HN H
hydroxymethyl-2-(1- 1 ml/min; eluents A: 0.1% ~~
/--N ~ ~ _ 0
methyl-1 H-indol-3-yl)- TFA in H20, B 0.1% TFA in
ethyl]amide}; ACN; gradient in each case
based on B: 5% to 95% (10')
N-((R)-1-Hydroxymethyl-2- to 95% (2') to 5% (0.5') to 5%
(1-methyl-1H-indol-3- (2.5')
yl)ethyl]-5-iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
542.7
and
Retention time: 9.7 min.
4-(N, N-Diethylamino-
carbonyl)phenylboronic
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
348
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
475 4'-Dimethylamino-4- 135 Column Purospher Star RP F I
propoxy-biphenyl-3- C18 4.6x125 5pm; detection ~ NH
carboxylic acid [2-(5- wavelength 214 nm; flow rate OH
fluoro-1 H-indol-3-yl)-1- 1 mI/min; eluents A: 0.1 % HN o
hydroxymethylethyl]- TFA in H20, B 0.1% TFA in o
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1 H-indol-3-
to95%(2')to5%(0.5')to5%
yl)-1-hydroxymethylethylJ- (25)
-2-propox-benz-
amide Molecular peak (ESI, M+1):
490.6
and
Retention time: 6.91 min.
4-Dimethylaminophenyl-
boronic acid
476 4'-Acetyl-4-propoxy- 135 Column Purospher Star RP F I
biphenyl-3-carboxylic acid C18 4.6x125 5pm; detection ~ NH
[2-(5-fluoro-1 H-indol-3-yl)- wavelength 214 nm; flow rate OH
1-hydroxymethylethyl]- 1 mI/min; eluentsA: 0.1% HN o
amide; TFA in H20, B 0.1% TFA in oO~o
ACN; gradient in each case
N-(2-(5-Fluoro-1 H-indol-3-
based on B: 5% to 95% (10')
yl)-1-hydroxymethylethylJ- to 95% (2') to 5% (0.5') to 5%
5-iodo-2-propoxy- (25)
benzamide
Molecular peak (ESI, M+1):
and
489.6
4-Acetylphenylboronic Retention time: 9.28 min.
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
349
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
477 3'-Acetyl-4-propoxy- 135 Column Purospher Star RP F
biphenyl-3-carboxylic acid C18 4.6x125 5Nm; detection ~ NH
[2-(5-fluoro-1 H-indol-3-yl)- wavelength 214 nm; flow rate OH
H
1-hydroxymethylethyl]- 1 mI/min; eluents A: 0.1 % 0 HN o
amide; TFA in H20, B 0.1% TFA in o
ACN; gradient in each case
N-[2-(5-Fluoro- I H-indol-3- based on B: 5% to 95% (10')
yl)-1-hydroxymethylethyl]- to 95% (2') to 5% (0.5') to 5%
5-iodo-2-propoxy- (2.5')
benzamide
Molecular peak (ESI, M+1):
and
489.6
3-Acetylphenylboronic
Retention time: 9.33 min.
acid
478 4-Propoxy- 135 Column Purospher Star RP F I
[1.1';3'.1"]terphenyl-3- C18 4.6x125 5pm; detection ~ NH
carboxylic acid [2-(5- wavelength 214 nm; flow rate OH
fluoro-1H-indol-3-yl)-1- 1 mi/min; eluentsA: 0.1% HN Ho
hydroxymethylethyl]- TFA in H20, B 0.1% TFA in o
amide; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]-
(2.5')
5-iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
523.6
and
Retention time: 10.86 min.
Biphenyl-3-boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
350
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
479 3'-[2-(5-Fluoro-1 H-indol-3- 135 Column Purospher Star RP F
yl)-1-hydroxymethyl- C18 4.6x125 5pm; detection NH
ethylcarbamoyl]-3- wavelength 214 nm; flow rate OH
O HN H
methoxy-4'-propoxy- 1 ml/min; eluents A: 0.1 % o~\ o
biphenyl-4-carboxylic acid TFA in H20, B 0.1% TFA in -~ - 0
methylester; ACN; gradient in each case
based on B: 5% to 95% (10')
N-(2-(5-Fluoro-1H-indol-3- to 95% (2') to 5% (0.5') to 5%
yl)-1-hydroxymethylethyl]- (2 5,)
5-iodo-2-propoxy-
benzamide Molecular peak (ESI, M+1):
535.6
and
Retention time: 9.16 min.
3-Methoxy-4-(methoxy-
carbonyl)phenylboronic
acid
480 4-Propoxybiphenyl-3,4'- 135 Column Purospher Star RP F
dicarboxylic acid 4'-amide C18 4.6x125 5pm; detection NH
HN H OH
3-{[2-(5-fluoro-1 H-indol-3- wavelength 214 nm; flow rate
yl)-1-hydroxymethylethyl]- 1 ml/min; eluents A: 0.1% H2N - O
\ / ~ \ -
amide}; TFA in H20, B 0.1 % TFA in o o
ACN; gradient in each case
N-(2-(5-Fluoro-1 H-indol-3- based on B: 5% to 95% (10')
yl)-1-hydroxymethylethyl]- to 95% (2') to 5% (0.5') to 5%
5-iodo-2-propoxy- (2.5')
benzamide
Molecular peak (ESI, M+1):
and
490.5
4-Aminocarbonylphenyl-
Retention time: 7.64 min.
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
351
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
481 4-Ethoxy-4'-methoxy- 125 (DMSO-d6): 10.79 s(1 H); H
N
methyl-biphenyl-3- 8.36 d (J = 8.1 Hz, 1 H); 8.14
carboxylic acid [(R)-1- d (J = 2.5 Hz, 1 H); 7.73 dd (J NH oJ
hydroxymethyl-2-(1 H- = 2.8 Hz / 8.6 Hz, 1 H); 7.68 d o
indol-3-yI)ethyl]amide; (J = 7.8 Hz, 2H); 7.59 d (J =
8.3 Hz, 2H); 7.36 d(J = 8.3
5-Bromo-2-ethoxy-N-((R)- Hz, 2H); 7.29 d (J = 8.1 Hz,
1-hydroxymethyl-2-(1 H- 1 H); 7.19 d (J = 8.8 Hz, 1 H);
indol-3-yl)ethylJbenzamide
7.13 s(1 H); 7.03 m(1 H); 6.94
and m(1 H); 4.90 m(1 H); 4.41 s
(2H); 4.23 m(1 H); 4.12 m
4-Methoxymethylphenyl- (2H); 3.47 m(1H); 3.41 m
boronic acid (1 H); 2.95 m (2H); 1.28 m
(3H).
482 4-Ethoxybiphenyl-3,3'- 125 (DMSO-d6): 10.78 s(1 H); H
dicarboxylic acid 3'-amide 8.37 d (J = 8.1 Hz, 1 H); 8.21
3-{[(R)-1-hydroxymethyl-2- d (J = 2.6 Hz, 1 H); 8.11 s ~ 'H
NH O
(1 H-indol-3-yI)ethyl]- (2H); 7.79 m (2H); 7.69 d (J = o H
amide}; 7.9 Hz, 1 H); 7.50 m(1 H);
7.40 s(1 H); 7.31 d (J = 8.1
5-Bromo-2-ethoxy-N-((R)-
Hz; 1 H); 7.23 d (J = 8.7 Hz,
1-h ydroxymeth yl-2-(1 H- NH2
1 H); 7.14 s(1 H); 7.02 m(1 H);
indol-3-yl)ethylJbenzamide 6.94 m(1 H); 4.90 m(1 H);
and 4.18 m(1 H); 4.14 m(2H);
3.47 m (2H); 2.96 m (2H);
3-Aminocarbonylphenyl- 1.29 m (3H).
boronic acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
352
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
483 4'-Ethanesulphonyl-4- 125 (DMSO-d6): 10.78 s(1 H); H
N
ethoxybiphenyl-3- 8.35 d (J = 8.1 Hz, 1 H); 8.20
carboxylic acid [(R)-2- d (J = 2.5 Hz, 1 H); 7.91 s NH oJ
hydroxy-1-(1 H-indol-3- (4H); 7.85 dd (J = 2.5 Hz / 8.6 O H
ylmethyl)ethyl]amide; Hz, 1 H); 7.67 d (J = 7.8 Hz,
1 H); 7.30 d (J = 8.1 Hz, 1 H);
5-Bromo-2-ethoxy-N-((R)- 7.24 d (J = 8.8 Hz, 1 H); 7.14 ~ I
1-hydroxymethyl-2-(1 H- ~ =o
s(1 H); 7.03 m(1 H); 6.93 m
indol-3-y1)ethyl]benzamide (1 H); 4.90 m (1 H); 4.23 m
and (2H); 4.15 m (2H); 4.00 m
(1 H); 3.47 m(1 H); 3.41 m
4-(Ethylsulphonyl)phenyl- (1 H); 2.96 m (2H); 1.29 m
boronic acid (3H); 1.10 m (3H).
484 4-Ethoxy-4'-(4- 125 (DMSO-d6): 10.79 s(1 H); H
methylpiperazin-l- 8.37 d (J = 8.3 Hz, 1 H); 8.18
carbonyl)biphenyl-3- d (J = 2.5 Hz, 1 H); 7.79 dd (J NH oJ
H
carboxylic acid [(R)-1- = 2.6 Hz / 7.8 Hz, 1 H); 7.72 d o
hydroxymethyl-2-(1 H- (J = 8.5 Hz, 2H); 7.67 d (J =
indol-3-yI)ethyl]amide; 7.7 Hz, 1 H); 7.53 d (J = 8.3
Hz, 1 H); 7.29 d (J = 8.1 Hz,
5-Bromo-2-ethoxy-N-[(R)- 1 H); 7.22 d (J = 8.2 Hz, 1 H); N
1-h ydroxymeth yl-2-(1 H-
7.13 s(1 H); 7.03 m(1 H); 6.93
indol-3-y1)ethyl]benzamide m(1 H); 4.22 m(1 H); 4.13 m
and (2H); 3.57 m (6 H, broad),
3.43 m (2H); 3.09 m (2H);
4-(4-Methylpiperazin-l- 2.95 m (2H); 2.80 s (3H); 1.29
carbonyl)-phenylboronic m (3H).
acid
CA 02618888 2008-02-06
WO 2007/017289 PCT/EP2006/007949
353
Product; Method Structure
Ex. analogous 'H-NMR (400 MHz) S [ppm]
reagents to
485 3'-Cyclopropylmethoxy-4- 125 (MeOD): 8.30 d (J = 2.5 Hz, H
ethoxy-biphenyl-3- 1 H); 7.75 dd (J = 2.5 Hz / 8.7
carboxylic acid [(R)-1- Hz, 1 H); 7.69 d (J = 7.9 Hz, ~ NH oJ
hydroxymethyl-2-(1 H- 1 H); 7.35 m (2H); 7.17 m Ho ~
indol-3-yl)ethyl]amide; (4H); 7. 11 m (1 H); 6.99 m
(1 H); 6.93 m(1 H); 4.53 m
5-Bromo-2-ethoxy-N-[(R)- (1 H); 4.16 m(1 H); 4.05 m o
1-hydroxymethyl-2-(1 H- (1 H); 3.92 d (J = 7.0 Hz, 1 H);
indol-3-y1)ethyl]benzamide
3.70 d(J = 4.9 Hz, 1 H); 3.17
and d(J = 6.4 Hz, 1 H); 1.27 m
(4H); 0.67 m (2H); 0.41 m
3-(Cyclopropylmethoxy)- (2H),
phenylboronic acid
486 3'-[(R)-1-Hydroxymethyl-2- 125 (DMSO-d6): 10.72 s(1H); H
(1 H-indol-3-yI)ethylcarba- 8.21 d (J = 8.1 Hz, 1 H); 7.77 N
moyl]biphenyl-2-carboxylic m (3H); 7.63 m (2H); 7.44 m
acid methyl ester; (3H); 7.36 m (1 H); 7.26 d (J = HO
3-Bromo-N-[(R)-1- 8.1 Hz, 1H); 7.09 s(1H); 7.01 O NH
hydroxymethyl-2-(1 H- m(1 H); 6.92 m(1 H); 4.76 m O O
indol-3-y1)ethyl]benzamide (1 H); 4.23 m(1 H); 3.52 s
and (3H); 3.49 m (2H); 2.97 m
(1 H); 2.88 m (1 H).
(2-Methoxycarbonyl-
phenyl)boronic acid