Note: Descriptions are shown in the official language in which they were submitted.
CA 02618970 2014-03-06
LISOFYLLINE ANALOGS AND METHODS FOR USE
Government Funding
[001] The invention described herein was made with government support under
Grant Numbers DK 63521 and R21DK 063521 awarded by the National Institute of
Health. The United States Government has certain rights in the invention.
[002]
Background of the Invention
[003] Type 1 diabetes is an autoimmune disorder which results from the immune-
mediated inflammatory destruction of insulin-producing I3-cells in pancreatic
islets.
Although the specific pathogenic mechanisms in Type 1 diabetes are not known,
it
is believed that activated T cells and macrophages are required for the
initiation.
Once activated, macrophages secrete several inflammatory cytokines, such as
interleukin 113(IL-113), interleukin 12 (IL-12) and tumor necrosis factor a
(TNF-a),
and trigger interferon-7 (IFN-7) production from activated T cells (see Z. D.
Yang,
M. Chen, R. Wu, M. McDuffle, J. L. Nadler, Diabetologia, 2002, 45, 1307-1314).
These cytokines are reported to be cytotoxic to 0 cells and enhance Thl-
mediated
inflammatory responses, which are believed to be responsible for the 13 cell
destruction (see M. Chen, Z. D. Yang, R. Wu, J. L. Nadler, Endocrinology,
2002,
143(6), 2341-2348).
[004] The anti-inflammatory compound Lisofylline (LSF; 1-(5-R-hydroxyhexyl)-
3,7-dimethylxanthine)) has been shown to be able to protect I3-cells from
multiple
inflammatory cytokine-mediated injuries by its ability to maintain insulin
secretory
capability and cell viability.
[005] Agents such as Lisofylline may have clinical utility in preventing 13-
cell
damage during the development of Type 1 diabetes. This hypothesis is supported
by
the studies that showed Lisofylline could significantly reduce
I
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
spontaneous Type 1 diabetes development in the non-obese diabetic (NOD)
mouse (see Yang). However, the disadvantages of Lisofylline may limit its
clinical development because it is not orally bioavailable and has relatively
weak
potency. The structure of LSF is Formula I.
[006] Currently, there is a need for novel, potent, and selective agents
based
on the Lisofylline backbone which have enhanced potency, selectivity, and oral
bioavailability. The present invention satisfies this need.
Summary of the Invention
[007] The present invention provides analogs of a Lysofylline (LSF), and
synthetic methods for the preparation of such analogs. The analogs can have
greater potency and oral bioavailability than LSF. The analogs have the active
side chain moiety (5-R-hydroxyhexyl) of LSF. The invention also includes
derivatives of LSF. LSF has formula I:
OH 0 CH3
ONN
CH3
I.
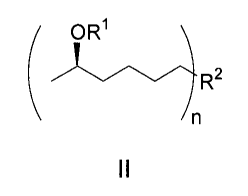
[008] The analogs of the invention can be substituted with a variety of
nitrogen-contained heterocyclic compounds or on the hydroxyl group of the side
chain. Accordingly, the invention provides 1-substituted 5-hexanol compounds
having additional substitution on the hydroxyl group. The compounds have the
formula
( OR1
R2
In
II
where R1 is hydrogen or a group having the formula ¨C(=0)R3, wherein R3 is
lower alkyl. R2 is selected from the group consisting of
2
CA 02618970 2014-03-06
. ,
H H
Br * H 0
\ N o9 ,, N
* I NH
N N
HI
C 0 N Y , * 0
0 0 0
0
HN )-N---c 5 11H HN 5 N ?NH
0 N N - A\J
00 'N 0
I, OH, , H ,
,C) 0 0 H
0
N *
NO 401 S 1NH (10 11H la
H N--N 0
, , , ,
F 0 H
HN H
N
0 :: * S* SI N le . 44,
0
OH
0 H H H
fi N ilo
N-H N eek 4) N ill
ii
NN
OH , , ,and .
,
and n is 1 or 2, or a pharmaceutically acceptable salt thereof
In another embodiment, the present invention provides a compound having
formula II:
/ OR1 \
)'\/µR2
\ /n
II
3
CA 02618970 2014-03-06
wherein RI is hydrogen or a group having the formula ¨C(=0)R3, wherein R3 is
hydrogen or C1-C6 alkyl, and R2 is selected from the group consisting of
0 0
0
N N
NH HNN401 10 ri\IN H
0 N N N I 0 , OH ,
F 0
COS NH 40
OH
wherein the H3C-C(ORI)-C4H8- moiety replaces the H atom of one or more NH or
one or more OH or one or more NH and OH group of R2; and n is 1 or 2; or a
pharmaceutically acceptable salt thereof.
[009] In another embodiment, the present invention provides analogs having
formula III:
0
NR4
* N
N R4
III
where each R4 is independently hydrogen or ¨(CH2)m-ORI, where RI is hydrogen
or a group having the formula ¨C(=0)R3, wherein R3 is lower alkyl and m is an
integer
from 2 to about 22, provided that at least one R4 group is not hydrogen.
3a
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[010] In another embodiment, the present invention provides analogs
having formula W:
0
* NR5
N
NR5
Iv
where each R5 is independently hydrogen or -(CH2)1-(CH0R1,)-(CH2)i-CH3,
where R1 is hydrogen or a group having the formula -C(=0)R3, wherein R3 is
lower alkyl i is an integer from 1 to about 20, and j is an integer from 0 to
about
20, provided that the sum of i and j in each R5 group is from 2 to about 22
and at
least one R5 group is not hydrogen.
[011] In another embodiment, the present invention provides analogs of
LSF having the ability to protect cell viability, particularly the ability to
protect
pancreatic (3-cells. Thus, the analogs of the invention can allow the pancreas
to
maintain its insulin secretory capability.
[012] In another embodiment, the present invention provides analogs of
LSF which are effective in treating Type 1 diabetes. In another embodiment,
the
present invention provides analogs of LSF which can inhibit the development of
Type 1 diabetes.
[013] In another embodiment, the present invention provides analogs of
LSF which are effective in treating Type 1 diabetes. In another embodiment,
the
present invention provides analogs of LSF which can lead to reversal of type I
diabetes by allowing the body to regenerate beta cells.
[014] In another aspect, the present invention also provides:
a pharmaceutical composition comprising a compound of formula II, formula
DI, formula IV, or pharmaceutically acceptable salts thereof, and a
pharmaceutically acceptable carrier or excipient (the composition preferably
comprises an effective amount of the compound or salt);
4
CA 02618970 2014-03-06
. ,
a method of treating or preventing Type 1 diabetes, comprising administering
to
a mammal (e.g., a human) in need of such treatment, a compound of formula II,
formula III, formula IV, or pharmaceutically acceptable salts thereof;
a method for protecting cell viability, particularly the ability to protect
pancreatic
13-cells comprising contacting (in vitro or in vivo) the cells with an
effective
protective amount of a compound of formula II, formula III, formula IV, or a
pharmaceutically acceptable salt thereof;
a compound of formula II, formula III, formula IV, or a pharmaceutically
acceptable salt thereof for use in medical treatment (e.g., the treatment of
Type 1
diabetes); and
the use of a compound of formula II, formula III, formula IV, or a
pharmaceutically acceptable salt thereof to prepare a medicament for treating
Type 1
diabetes in a mammal (e.g., a human).
[015] The analogs of the invention can be useful in the treatment of
inflammatory
and autoimmune diseases. Non-limiting examples of such diseases include
atherosclerosis, type 2 diabetes, disorders associated with visceral obesity
such as
non-alcoholic steatohepatitis (NASH), multiple sclerosis, inflammatory bowel
disease, psoriasis, rheumatoid arthritis, Alzheimer's disease and the like.
5
CA 02618970 2014-03-06
In another embodiment, the present invention provides a use of an effective
amount of a compound according to the present disclosure, or a
pharmaceutically
acceptable salt thereof, for preventing or treating a pathological condition
or symptom in
a mammal wherein the protection of I3-cells from Thl cytokine-induced
dysfunction or
the onset of Type 1 diabetes is reduced.
In another embodiment, the present invention provides a use of an effective
amount of a compound according to the present disclosure, or a
pharmaceutically
acceptable salt thereof, in the preparation of a medicament for preventing or
treating a
pathological condition or symptom in a mammal wherein the protection of 13-
cells from
Thl cytokine-induced dysfunction or the onset of Type 1 diabetes is reduced.
In another embodiment, the present invention provides a compound according to
the present disclosure, or a pharmaceutically acceptable salt thereof, for use
in preventing
or treating a pathological condition or symptom in a mammal wherein the
protection of P-
eens from Thl cytokine-induced dysfunction or the onset of Type 1 diabetes is
reduced.
In another embodiment, the present invention provides a use of an effective
protective amount of a compound according to the present disclosure, or a
pharmaceutically acceptable salt thereof, for protecting pancreatic 13-cells
to prevent or
treat a pathological condition or symptom in a mammal.
In another embodiment, the present invention provides a use of an effective
protective amount of a compound according to the present disclosure, or a
pharmaceutically acceptable salt thereof, in the preparation of a medicament
for
protecting pancreatic 13-cells to prevent or treat a pathological condition or
symptom in a
mammal.
In another embodiment, the present invention provides a compound according to
the present disclosure, or a pharmaceutically acceptable salt thereof, for use
in protecting
pancreatic I3-cells to prevent or treat a pathological condition or symptom in
a mammal.
In another embodiment, the present invention provides a use of an effective
amount of a compound according to the present disclosure, or a
pharmaceutically
acceptable salt thereof, for treating an inflammatory and autoimmune condition
to
prevent or treat a pathological condition or symptom in a mammal.
5a
CA 02618970 2014-03-06
. .
In another embodiment, the present invention provides a use of an effective
amount of a compound according to the present disclosure, or a
pharmaceutically
acceptable salt thereof, in the preparation of a medicament for treating an
inflammatory
and autoimmune condition to prevent or treat a pathological condition or
symptom in a
mammal.
In another embodiment, the present invention provides a compound according to
the present disclosure, or a pharmaceutically acceptable salt thereof, for use
in treating an
inflammatory and autoimmune condition to prevent or treat a pathological
condition or
symptom in a mammal.
In another embodiment, the present invention provides a compound according to
the present disclosure, for use in medical therapy, wherein the medical
therapy is
prevention or treatment of Type 1 diabetes.
In another embodiment, the present invention provides a use of a compound
according to the present disclosure for medical therapy, wherein the medical
therapy is
prevention or treatment of Type 1 diabetes.
In another embodiment, the present invention provides a use of a compound
according to the present disclosure, to prepare a medicament for prevention or
treatment
of a pathological condition or symptom in a mammal, wherein the pathological
condition
or symptom is an inflammatory or autoimmune condition.
[016] The invention also provides methods for testing the activity of the
compounds of the invention. Methods not disclosed are known to those of
ordinary skill
in the art. One of ordinary skill will appreciate that many techniques are
available to
determine whether compounds of the invention produce the desired result.
[017] The invention also provides a kit for administering compounds of the
invention.
[018] The invention also provides novel intermediates and processes disclosed
herein that are useful for preparing analogs of formula II, formula III, or
formula IV,
including the generic and specific intermediates as well as the synthetic
processes
described in the Charts and Examples herein.
5b
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
Brief Description of the Drawings
[019] Figure 1 depicts specific examples of compounds of the invention.
[020] Figure 2 illustrates a scheme for preparing compound CPW11.
[021] Figures 3A-3D illustrate schemes for preparing analogs having an R2
group based on 2,3-dihydro-phthalazine-1,4-dione.
[022] Figure 4 illustrates schemes for preparing 2,3-dihydro-pyridazino[4,5-
d]pyridazine-1,4-dione and a related analog.
[023] Figures 5A-5P illustrate the 13-cell protective effect of LSF and
additional analogs of the invention for mouse-origin I3-TC6 cells and mouse
islet
1() cells.
[024] Figure 6 illustrates the release of insulin from mouse islet cells.
[025] Figures 7A-7D illustrate the release of insulin from mouse islet
cells.
Figure 7A illustrates the basal-stimulated Insulin Release. Figure 7B
illustrates
the glucose-stimulated Insulin Release. Figure 7C illustrates the ATP
concentrations and Figure 7D illustrates the 13-cell viability.
[026] Figures 8a-8c illustrate the ability of the analogs of the invention
to
stimulate insulin release from f3-cells.
[027] Figures 9A-9P illustrate the ability of LSF and additional analogs to
effect insulin release in mouse-origin 13-TC6 cells.
[028] Figure 10 illustrates the ability of compounds (analogs) of the
invention to reduce STAT4 phosphorylation in murine splenocytes (Immuno-
dot-blot).
Detailed Description
Abbreviations
[029] Unless defined otherwise, all technical and scientific terms used
herein have the same meaning as commonly understood by one of ordinary skill
in the art to which this invention belongs. Although any methods and materials
similar or equivalent to those described herein can be used in the practice or
testing of the present invention, the preferred methods and materials are
described herein.
6
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[030] In describing and claiming the invention, the following terminology
will be used in accordance with the definitions set forth below.
[031] For purposes of the description of this invention, the articles "a"
and
"an" are used herein to refer to one or to more than one (i.e., to at least
one) of
the grammatical object of the article. By way of example, "an element" means
one element or more than one element.
[032] A disease or disorder is "alleviated" if the severity of a symptom of
the disease, condition, or disorder, or the frequency with which such a
symptom
is experienced by a subject, or both, are reduced.
[033] As used herein, an "analog" of a chemical compound is a compound
that, by way of example, resembles another in structure but is not necessarily
an
isomer (e.g., 5-fluorouracil is an analog of thymine).
[034] A "control" cell, tissue, sample, or subject is a cell, tissue,
sample, or
subject of the same type as a test cell, tissue, sample, or subject. The
control
may, for example, be examined at precisely or nearly the same time the test
cell,
tissue, sample, or subject is examined. The control may also, for example, be
examined at a time distant from the time at which the test cell, tissue,
sample, or
subject is examined, and the results of the examination of the control may be
recorded so that the recorded results may be compared with results obtained by
examination of a test cell, tissue, sample, or subject. The control may also
be
obtained from another source or similar source other than the test group or a
test
subject, where the test sample is obtained from a subject suspected of having
a
disease or disorder for which the test is being performed.
[035] A "test" cell, tissue, sample, or subject is one being examined or
treated.
[036] A "pathoindicative" cell, tissue, or sample is one which, when
present, is an indication that the animal in which the cell, tissue, or sample
is
located (or from which the tissue was obtained) is afflicted with a disease or
disorder.
[037] A tissue "normally comprises" a cell if one or more of the cell are
present in the tissue in an animal not afflicted with a disease or disorder.
7
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[038] The terms "compound or analog," are used interchangeably. As used
herein, these terms refer to any type of substance or agent that is commonly
considered a drug, or a candidate for use as a drug, combinations, and
mixtures
of the above, as well as polypeptides and antibodies of the invention.
[039] As used herein, a "derivative" of a compound refers to a chemical
compound that may be produced from another compound of similar structure in
one or more steps, as in replacement of a hydrogen atom by an alkyl, acyl, or
amino group.
[040] As used herein, an "effective amount" means an amount sufficient to
produce a selected effect.
[041] The use of the word "detect" and its grammatical variants is intended
to refer to measurement of the species without quantification, whereas use of
the
word "determine" or "measure" with their grammatical variants are meant to
refer to measurement of the species with quantification. The terms "detect"
and
"identify" are used interchangeably herein.
[042] As used herein a "disease" is a state of health of an animal wherein
the animal cannot maintain homeostasis, and wherein if the disease is not
ameliorated then the animal's health continues to deteriorate.
[043] In contrast, as used herein a "disorder" in an animal is a state of
health in which the animal is able to maintain homeostasis, but in which the
animal's state of health is less favorable than it would be in the absence of
the
disorder. Left untreated, a disorder does not necessarily cause a further
decrease
in the animal's state of health.
[044] As used herein, a "functional" analog or molecule is a molecule in a
form in which it exhibits a property or activity by which it is characterized.
[045] As used herein, the term "inhibit," refers to the ability of a
compound
of the invention to reduce or impede a described function. Preferably,
inhibition
is by at least 10%, more preferably by at least 25%, even more preferably by
at
least 50%, and most preferably, the function is inhibited by at least 75%.
[046] As used herein, "instructional material(s)" includes a publication, a
recording, a diagram, or any other medium of expression which can be used to
communicate the usefulness of the analog of the invention in the kit for
effecting
8
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
alleviation of the various diseases or disorders recited herein. Optionally,
or
alternately, the instructional material may describe one or more methods of
alleviating the diseases or disorders in a cell or a tissue of a mammal. The
instructional material of the kit of the invention may, for example, be
affixed to a
container which contains the identified compound invention or be shipped
together with a container which contains the identified compound.
Alternatively,
the instructional material may be shipped separately from the container with
the
intention that the instructional material and the compound be used
cooperatively
by the recipient.
[047] As used herein, the term "purified" and similar terms relate to an
enrichment of a molecule or compound relative to other components normally
associated with the molecule or compound in a native environment. The term
"purified" does not necessarily indicate that complete purity of the
particular
molecule has been achieved during the process. A "highly purified" compound
as used herein refers to a compound that is greater than 90% pure.
[048] As used herein, the term "pharmaceutically acceptable carrier"
includes any of the standard pharmaceutical carriers, such as a phosphate
buffered saline solution, water, emulsions such as an oil/water or water/oil
emulsion, and various types of wetting agents. The term also encompasses any
of the agents approved by a regulatory agency of the US Federal government or
listed in the US Pharmacopeia for use in animals, including humans. Examples
of these and other pharmaceutically acceptable carriers are described in
Remington's Pharmaceutical Sciences (1991, Mack Publication Co., New
Jersey).
[049] A "sample," as used herein, refers preferably to a biological sample
from a subject, including, but not limited to, normal tissue samples, diseased
tissue samples, biopsies, blood, saliva, feces, semen, tears, and urine. A
sample
can also be any other source of material obtained from a subject which
contains
cells, tissues, or fluid of interest. A sample can also be obtained from cell
or
tissue culture.
[050] The term "standard," as used herein, refers to something used for
comparison. For example, a standard can be a known standard agent or
9
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
compound which is administered or added to a control sample and used for
comparing results when measuring said compound in a test sample. Standard
can also refer to an "internal standard," such as an agent or compound which
is
added at known amounts to a sample and is useful in determining such things as
purification or recovery rates when a sample is processed or subjected to
purification or extraction procedures before a marker of interest is measured.
[051] A "subject" of analysis, diagnosis, or treatment is an animal. Such
animals include mammals, preferably a human.
[052] As used herein, the terms "treating or treatment" includes
prophylaxis
of the specific disorder or condition, or alleviation of the symptoms
associated
with a specific disorder or condition and/or preventing or eliminating said
symptoms.
[053] As used herein, a "prophylactic" treatment is a treatment
administered
to a subject who does not exhibit signs of a disease or exhibits only early
signs of
the disease for the purpose of decreasing the risk of developing pathology
associated with the disease.
[054] As used herein, a "therapeutic" treatment is a treatment administered
to a subject who exhibits signs of pathology for the purpose of diminishing or
eliminating those signs.
[055] As used herein, a "therapeutically effective amount" of a compound
or analog is an amount of compound which is sufficient to provide a beneficial
effect to the subject to which the compound is administered.
Chemical Definitions
[056] As used herein, the term "halogen" or "halo" includes bromo, chloro,
fluoro, and iodo. The term "haloalkyl" as used herein refers to an alkyl
radical
bearing at least one halogen substituent, for example, chloromethyl,
fluoroethyl
or trifluoromethyl and the like.
[057] The term "lower alkyl" refers to alkyl groups having from one (1) to
(6) carbon atoms. Typically, C1-C6 alkyl groups include, but are not limited
to,
methyl, ethyl, n-propyl, iso-propyl, butyl, iso-butyl, sec-butyl, tert-butyl,
pentyl,
hexyl, and the like.
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
..
[058] It will be appreciated by those skilled in the art that compounds of
the
invention having a chiral center may exist in and be isolated in optically
active
and racemic forms. Some compounds may exhibit polymorphism. It is to be
understood that the present invention encompasses any racemic, optically-
active,
polymorphic, or stereoisomeric form, or mixtures thereof, of a compound of the
invention, which possess the useful properties described herein, it being well
known in the art how to prepare optically active forms (for example, by
resolution of the racemic form by recrystallization techniques, by synthesis
from
optically-active starting materials, by chiral synthesis, or by
chromatographic
separation using a chiral stationary phase) and how to detenaine cADPR agonist
or antagonist activity using the standard tests described herein, or using
other
similar tests which are well known in the art.
[059] The compounds of the present invention may exist in tautomeric
forms and the invention includes both mixtures and separate individual
tautomers. For example the following structure:
N."NH
\-/ is understood to represent a mixture of the structures:
N,NNH HN^N
\-/ and \--/
=
[060] The term "pharmaceutically-acceptable salt" refers to salts which
retain the biological effectiveness and properties of the compounds of the
present
invention and which are not biologically or otherwise undesirable. In many
cases, the compounds of the present invention are capable of forming acid
and/or
base salts by virtue of the presence of amino and/or carboxyl groups or groups
similar thereto.
[061] Pharmaceutically-acceptable base addition salts can be prepared from
inorganic and organic bases. Salts derived from inorganic bases, include by
way
of example only, sodium, potassium, lithium, ammonium, calcium and
magnesium salts. Salts derived from organic bases include, but are not limited
to,
salts of primary, secondary and tertiary amines, such as alkyl amines, dialkyl
amines, trialkyl amines, substituted alkyl amines, di(substituted alkyl)
amines,
11
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
tri(substituted alkyl) amines, alkenyl amines, dialkenyl amines, trialkenyl
amines, substituted alkenyl amines, di(substituted alkenyl) amines,
tri(substituted
alkenyl) amines, cycloalkyl amines, di(cycloalkyl) amines, tri(cycloalkyl)
amines, substituted cycloalkyl amines, disubstituted cycloalkyl amine,
trisubstituted cycloalkyl amines, cycloalkenyl amines, di(cycloalkenyl)
amines,
tri(cycloalkenyl) amines, substituted cycloalkenyl amines, disubstituted
cycloalkenyl amine, trisubstituted cycloalkenyl amines, aryl amines, diaryl
amines, triaryl amines, heteroaryl amines, diheteroaryl amines, triheteroaryl
amines, heterocyclic amines, diheterocyclic amines, triheterocyclic amines,
mixed di- and tri-amines where at least two of the substituents on the amine
are
different and are selected from the group consisting of alkyl, substituted
alkyl,
alkenyl, substituted alkenyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl,
substituted cycloalkenyl, aryl, heteroaryl, heterocyclic, and the like. Also
included are amines where the two or three substituents, together with the
amino
nitrogen, form a heterocyclic or heteroaryl group. Examples of suitable amines
include, by way of example only, isopropylamine, trimethyl amine, diethyl
amine, tri(iso-propyl) amine, tri(n-propyl) amine, ethanolamine, 2-
dimethylaminoethanol, tromethamine, lysine, arginine, histidine, caffeine,
procaine, hydrabamine, choline, betaine, ethylenediamine, glucosamine, N-
alkylglucamines, theobromine, purines, piperazine, piperidine, morpholine, N-
ethylpiperidine, and the like. It should also be understood that other
carboxylic
acid derivatives would be useful in the practice of this invention, for
example,
carboxylic acid amides, including carboxamides, lower alkyl carboxamides,
dialkyl carboxamides, and the like.
[062] Pharmaceutically acceptable acid addition salts may be prepared from
inorganic and organic acids. Salts derived from inorganic acids include
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric
acid,
and the like. Salts derived from organic acids include acetic acid, propionic
acid,
glycolic acid, pyruvic acid, oxalic acid, malic acid, malonic acid, succinic
acid,
maleic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, cinnamic
acid,
mandelic acid, methanesulfonic acid, ethanesulfonic acid, p-toluene-sulfonic
acid, salicylic acid, and the like.
12
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[063] Pharmaceutically acceptable salts may be obtained using standard
procedures well known in the art, for example by reacting a sufficiently basic
compound such as an amine with a suitable acid affording a physiologically
acceptable anion. Alkali metal (for example, sodium, potassium or lithium) or
alkaline earth metal (for example calcium) salts of carboxylic acids can also
be
made.
[064] In another embodiment, the present invention provides kits for use in
administering or using compounds of the invention.
[065] In cases where compounds are sufficiently basic or acidic to form
acid or base salts, use of the compounds as salts may be appropriate. Examples
of acceptable salts are organic acid addition salts formed with acids which
form a
physiological acceptable anion, for example, tosylate, methanesulfonate,
acetate,
citrate, malonate, tartarate, succinate, benzoate, ascorbate, a-ketoglutarate,
and a-
glycerophosphate. Suitable inorganic salts may also be formed, including
= hydrochloride, sulfate, nitrate, bicarbonate, and carbonate salts.
[066] Specific and preferred values listed below for radicals,
substituents,
and ranges, are for illustration only; they do not exclude other defined
values or
other values within defined ranges for the radicals and substituents.
[067] Specifically, (Ci-C6)alkyl can be methyl, ethyl, propyl, isopropyl,
butyl, iso-butyl, sec-butyl, pentyl, 3-pentyl, hexyl and the like.
[068] The compounds of the present invention are generally named
according to the IUPAC or CAS nomenclature system. Abbreviations which are
well known to one of ordinary skill in the art may be used (e.g. "Ph" for
phenyl,
"Me" for methyl, "Et" for ethyl, "h" for hour or hours, "rt" for room
temperature,
and "rac" for racemic mixture).
[069] The invention also provides compounds of formula 11, formula III, or
formula IV, for use in medical therapy.
[070] A specific value for R3 is hydrogen, methyl, ethyl or propyl.
[071]3 i
A more specific value for R s methyl, or ethyl.
[072]3 i
A more specific value for R s methyl.
[073] A more specific value for R3 is hydrogen.
13
CA 02618970 2008-02-08
WO 2007/027719 PCT/US2006/033777
[074] A specific value for R2 is
0 0
H
H 0
0 NO --N-NH HNA Ni 0 riFi
o' 0 NI
ON7L---N1 N
I or OH ..
[075] In one aspect, specific analogs of the invention can have having the
formulas:
AcS,
, _______________________________________________________ 1
/
Br *
N N
Br /
\ 10 \ ,C)
\ \ 0 N
\ _______________________________________________________ \
Ac0) ,
Ac0 HO
Acg, Hq,
? 1 Ac0õ,
ix-
NC) NO
ON-
0 N -
\ \ lio NO
\ \
HO) __ 3
HO
Na0, HOõ
jr.
H OAc
0 N NO ,,.0 * N-d
101 8
o o. ,
0 H OH OAc 0 OH 0
,--Ns
ON)N dNN)---N
410 II/) I I
, , ,
14
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
0 0 0 OAc
0
NH 0 NH
7N 7 N 7 N
0-y-
OAc , OH, OAc,
0 OH 0 OAc
_
LW 7 N 7 N
0,.....õõ,,_,Thr 0.-..-y
OAc, OH ,
O OH OAc 0 OH 0
40 N-: N 40 TWN
1
1161
7 N
0 0 0 0
0.õõ7 ,-,....õ....õ7-y
OH
O 0 0 OH
.
*11H 40 NH40 NCF3
7 N 7 N 7 N
O,(CF3 OCF3 OrCF3
OH, OAc, OAc
,
0 OH 0 OH 0 OH
_
isN-CF340 N 7.2---CF3 Is N/='--CF3
7 N 7 N 7 N
0..,...õ,..--,,,õ,..-yCF3 CyCF3 0CF3
OAc, OAc, OAc,
CA 02618970 2008-02-08
WO 2007/027719 PCT/US2006/033777
F 0 F 0 F 0 OAc
*NH 5 NH
N 5
_. ,-- N ---N
0-...i.- 0õ,,.........,,,,....õ,-y 0..õ.........,-
....,....õ...-y
OAc, OH, OAc,
F 0 OH F 0 OAc F 0 OH
-
0 ri =,,,./\% 0 0
0...,.......õ.Thr- 0........,......---.......-y
0..õ...........õ...-..y..--
OAc , OH, OH,
0 OAc 0 OH 0 OAc
_
NN-- NN-7' N-)Thl
II I II I II I
N./,.N N .-.,..N N.-,N
0....---y- 0......,....- 0,..............õ....-
y-
OAc, OAc, OH,
0 OH 0 0
N---L'NH N---NH
ii I m 1 11 1
N ..,-yN N.,.y-N NN
0,.........- 0...õ.....õ---r- 0........õ.õ--
..,..............¨......y
OH, OAc, OH,
0 0 0
riLl N pAc rA. N ¨ \__ C\M_irc
N ,--- NI
OAc ' N 0 OAc N' N0 OH ' N 0
9 9
0
0 (:)
1.I 0 OAc
OH N 'N 0 N--'0 OH N
/T\/\.)
, 9 9
16
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
0 OAc
0 0 -
N=\/\.-='
lel s'N-----()H 0 s'N----/----/-1)Ac lel N IV
9
0 OH
is 1,,,.....___.....,,,õ..,..õ,. NN OH, N 0 ma- lei N &
-"
9 0 9 0 IW ,
OH N
W 0 Aco 0 N 0 Aco N
S S 0 10
, , 9
HO
OH, N 0 N 10
Aco
0 /10/N 40/
0
9 9
0
Z
Z
OH, N 0 Ac0 is N 0 Ac0 410 N .
9 9 9
Z ...../.
oHO N . Ac0 41 N 1104 0-HO N 1104
9 9 9
and pharmaceutically acceptable salts thereof.
[076] More specific analogs of the invention can have having the formulas:
17
CA 02618970 2008-02-08
WO 2007/027719 PCT/US2006/033777
HQ,.
0 OH
401 N 0
9
40 NH
OH 0
N
)
ONN
1 ,and OH .
[077] Processes for preparing analogs of formula IL formula III, or
formula
IV, are provided as further embodiments of the invention and are illustrated
by
the following procedures in which the meanings of the generic radicals are as
given above unless otherwise qualified. A general scheme for preparing the
analogs of the invention having formula IL is provided in Scheme 1:
Scheme 1
OAc
OAc
+ _________________________________________ =
CI
OH
________________ =
[078] Scheme 2 (Figure 2) provides a synthetic scheme route to prepare
analog 11. The preparation of additional analogs of the invention having
formula ill, or formula IV, are illustrated in Figures 3A-3D and Figure 4.
[079] The compounds of formula I can be formulated as pharmaceutical
compositions and administered to a mammalian host, such as a human patient in
a variety of forms adapted to the chosen route of administration, i.e., orally
or
parenterally, by intravenous, intramuscular, topical or subcutaneous routes.
18
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
1080] Thus, the present compounds may be systemically administered,
e.g.,
orally, in combination with a pharmaceutically acceptable vehicle such as an
inert diluent or an assimilable edible carrier. They may be enclosed in hard
or
soft shell gelatin capsules, may be compressed into tablets, or may be
incorporated directly with the food of the patient's diet. For oral
therapeutic
administration, the active compound may be combined with one or more
excipients and used in the form of ingestible tablets, buccal tablets,
troches,
capsules, elixirs, suspensions, syrups, wafers, and the like. Such
compositions
and preparations should contain at least 0.1% of active compound. The
percentage of the compositions and preparations may, of course, be varied and
may conveniently be between about 2 to about 60% of the weight of a given unit
dosage form. The amount of active compound in such therapeutically useful
compositions is such that an effective dosage level will be obtained.
[081] The tablets, troches, pills, capsules, and the like may also contain
the
following: binders such as gum tragacanth, acacia, corn starch or gelatin;
excipients such as dicalcium phosphate; a disintegrating agent such as corn
starch, potato starch, alginic acid and the like; a lubricant such as
magnesium
stearate; and a sweetening agent such as sucrose, fructose, lactose or
aspartame
or a flavoring agent such as peppermint, oil of wintergreen, or cherry
flavoring
may be added. When the unit dosage form is a capsule, it may contain, in
addition to materials of the above type, a liquid carrier, such as a vegetable
oil or
a polyethylene glycol. Various other materials may be present as coatings or
to
otherwise modify the physical form of the solid unit dosage form. For
instance,
tablets, pills, or capsules may be coated with gelatin, wax, shellac or sugar
and
the like. A syrup or elixir may contain the active compound, sucrose or
fructose
as a sweetening agent, methyl and propylparabens as preservatives, a dye and
flavoring such as cherry or orange flavor. Of course, any material used in
preparing any unit dosage form should be pharmaceutically acceptable and
substantially non-toxic in the amounts employed. In addition, the active
compound may be incorporated into sustained-release preparations and devices.
[082] The active compound may also be administered intravenously or
intraperitoneally by infusion or injection. Solutions of the active compound
or
its salts can be prepared in water, optionally mixed with a nontoxic
surfactant.
19
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
Dispersions can also be prepared in glycerol, liquid polyethylene glycols,
triacetin, and mixtures thereof and in oils. Under ordinary conditions of
storage
and use, these preparations contain a preservative to prevent the growth of
microorganisms.
[083] The pharmaceutical dosage forms suitable for injection or infusion
can include sterile aqueous solutions or dispersions (suspensions) or sterile
powders comprising the active ingredient which are adapted for the
extemporaneous preparation of sterile injectable or infusible solutions or
dispersions, optionally encapsulated in liposomes. In all cases, the ultimate
dosage form should be sterile, fluid and stable under the conditions of
manufacture and storage. This dispersion or solution may be formulated
according to the known art, and may comprise, in addition to the active
ingredient, additional ingredients such as the dispersing agents, wetting
agents,
or suspending agents. The liquid carrier or vehicle can be a solvent or liquid
dispersion medium comprising, for example, water, ethanol, a polyol (for
example, glycerol, propylene glycol, liquid polyethylene glycols, and the
like),
vegetable oils, nontoxic glyceryl esters, and suitable mixtures thereof. Other
acceptable diluents and solvents include, but are not limited to, Ringer's
solution, isotonic sodium chloride solution, and fixed oils such as synthetic
mono- or di-glycerides. The proper fluidity can be maintained, for example, by
the formation of liposomes, by the maintenance of the required particle size
in
the case of dispersions or by the use of surfactants. The prevention of the
action
of microorganisms can be brought about by various antibacterial and antifungal
agents, for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal,
and the like. In many cases, it will be preferable to include isotonic agents,
for
example, sugars, buffers or sodium chloride. Prolonged absorption of the
injectable compositions can be brought about by the use in the compositions of
agents delaying absorption, for example, aluminum monostearate and gelatin.
[084] Sterile solutions are prepared by incorporating the active
compound
in the required amount in the appropriate solvent with various of the other
ingredients enumerated above, as required, followed by filter sterilization.
In the
case of sterile powders for the preparation of sterile solutions, the
preferred
methods of preparation are vacuum drying and the freeze drying techniques,
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
which yield a powder of the active ingredient plus any additional desired
ingredient present in the previously sterile-filtered solutions. The sterile
solutions or powders can be combined with suitable carriers and administered
via injection. In addition, the sterile solutions or powders can be combined
with
suitable carriers and/or propellants and administered via inhalation.
[085] For topical administration, the present compounds may be applied
in
pure form, i.e., when they are liquids. However, it will generally be
desirable to
administer them to the skin as compositions or formulations, in combination
with a dermatologically acceptable carrier, which may be a solid or a liquid.
[086] Useful solid carriers include finely divided solids such as talc,
clay,
microcrystalline cellulose, silica, alumina and the like. Useful liquid
carriers
include water, alcohols or glycols or water-alcohol/glycol blends, in which
the
present compounds can be dissolved or dispersed at effective levels,
optionally
with the aid of non-toxic surfactants. Adjuvants such as fragrances and
additional antimicrobial agents can be added to optimize the properties for a
given use. The resultant liquid compositions can be applied from absorbent
pads, used to impregnate bandages and other dressings, or sprayed onto the
affected area using pump-type or aerosol sprayers.
[087] Thickeners such as synthetic polymers, fatty acids, fatty acid salts
and
esters, fatty alcohols, modified celluloses or modified mineral materials can
also
be employed with liquid carriers to form spreadable pastes, gels, ointments,
soaps, and the like, for application directly to the skin of the user.
[088] Useful dosages of the compounds of formula I can be determined by
comparing their in vitro activity, and in vivo activity in animal models.
Methods
for the extrapolation of effective dosages in mice, and other animals, to
humans
are known to the art; for example, see U.S. Pat. No. 4,938,949.
[089] Generally, the concentration of the compound(s) of formula I in a
liquid composition, such as a solution or suspension, will be from about 0.1-
25
wt %, preferably from about 0.5-10 wt %. The concentration in a semi-solid or
solid composition such as a gel or a powder will be about 0.1-5 wt %,
preferably
about 0.5-2.5 wt %.
21
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[090] The amount of the compound, or an active salt or derivative thereof,
required for use in treatment will vary not only with the particular salt
selected
but also with the route of administration, the nature of the condition being
treated
and the age and condition of the patient and will be ultimately at the
discretion of
the attendant physician or clinician.
[091] In general, however, a suitable dose will be in the range of from
about
0.5 to about 20 mg/kg, e.g., from about 1 to about 18 mg/kg of body weight per
day, such as 3 to about 16 mg per kilogram body weight of the recipient per
day,
preferably in the range of 6 to 14 mg/kg/day, most preferably in the range of
9 to
11 mg/kg/day.
[092] The compound is conveniently administered in unit dosage form; for
example, containing 5 to 1000 mg, conveniently 10 to 750 mg, most
conveniently, 50 to 500 mg of active ingredient per unit dosage form.
[093] Ideally, the active ingredient should be administered to achieve peak
plasma concentrations of the active compound of from about 0.5 to about 75 M,
preferably, about 1 to 50 M, most preferably, about 2 to about 30 M. This
may be achieved, for example, by the intravenous injection of a 0.05 to 5%
solution of the active ingredient, optionally in saline, or orally
administered as a
bolus containing about 1-100 mg of the active ingredient. Desirable blood
levels
may be maintained by continuous infusion to provide about 0.01-5.0 mg/kg/hr or
by intermittent infusions containing about 0.4-15 mg/kg of the active
ingredient(s).
[094] The desired dose may conveniently be presented in a single dose or as
divided doses administered at appropriate intervals, for example, as two,
three,
four or more sub-doses per day. The sub-dose itself may be further divided,
e.g.,
into a number of discrete loosely spaced administrations; such as multiple
inhalations from an insufflator or by administration of a plurality of
tablets.
[095] Compounds which are identified using any of the methods described
herein may be formulated and administered to a subject for treatment of any of
the diseases and disorders described herein. However, the use of compounds of
the invention should not be construed to include only the diseases and
disorder
described herein. Preferably, the subject is a human.
22
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[096] The formulations of the pharmaceutical compositions described
herein may be prepared by any method known or hereafter developed in the art
of pharmacology. In general, such preparatory methods include the step of
bringing the active ingredient into association with a carrier or one or more
other
accessory ingredients, and then, if necessary or desirable, shaping or
packaging
the product into a desired single- or multi-dose unit.
[097] Although the descriptions of pharmaceutical compositions provided
herein are principally directed to pharmaceutical compositions which are
suitable
for ethical administration to humans, it will be understood by the skilled
artisan
that such compositions are generally suitable for administration to animals of
all
sorts.
[098] Modification of pharmaceutical compositions suitable for
administration to humans in order to render the compositions suitable for
administration to various animals is well understood, and the ordinarily
skilled
veterinary pharmacologist can design and perform such modification with merely
ordinary, if any, experimentation. Subjects to which administration of the
pharmaceutical compositions of the invention is contemplated include, but are
not limited to, humans and other primates, and mammals, including
commercially relevant mammals such as cattle, pigs, horses, sheep, cats, and
dogs.
[099] A pharmaceutical composition of the invention may be prepared,
packaged, or sold in bulk, as a single unit dose, or as a plurality of single
unit
doses. As used herein, a "unit dose" is a discrete amount of the
pharmaceutical
composition comprising a predetermined amount of the active ingredient. The
amount of the active ingredient is generally equal to the dosage of the active
ingredient which would be administered to a subject or a convenient fraction
of
such a dosage such as, for example, one-half or one-third of such a dosage.
23
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[0100] The relative amounts of the active ingredient, the
pharmaceutically
acceptable carrier, and any additional ingredients in a pharmaceutical
composition of the invention will vary, depending upon the identity, size, and
condition of the subject treated and further depending upon the route by which
the composition is to be administered. By way of example, the composition may
comprise between 0.1% and 100% (w/w) active ingredient.
[0101] In addition to the active ingredient, a pharmaceutical
composition of
the invention may further comprise one or more additional pharmaceutically
active agents. Particularly contemplated additional agents include anti-
emetics
and scavengers such as cyanide and cyanate scavengers.
[0102] Controlled- or sustained-release formulations of a pharmaceutical
composition of the invention may be made using conventional technology.
[0103] In some cases, the dosage forms to be used can be provided as
slow
or controlled-release of one or more active ingredients therein using, for
example, hydropropylmethyl cellulose, other polymer matrices, gels, permeable
membranes, osmotic systems, multilayer coatings, microparticles, liposomes, or
microspheres or a combination thereof to provide the desired release profile
in
varying proportions. Suitable controlled-release formulations known to those
of
ordinary skill in the art, including those described herein can be readily
selected
for use with the pharmaceutical compositions of the invention. Thus, single
unit
dosage forms suitable for oral administration, such as tablets, capsules,
gelcaps,
and caplets that are adapted for controlled-release are encompassed by the
present invention.
[0104] Most controlled-release formulations are designed to initially
release
an amount of drug that promptly produces the desired therapeutic effect, and
gradually and continually release of other amounts of drug to maintain this
level
of therapeutic effect over an extended period of time. In order to maintain
this
constant level of drug in the body, the drug must be released from the dosage
form at a rate that will replace the amount of drug being metabolized and
excreted from the body.
24
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[0105] Controlled-release of an active ingredient can be stimulated by
various inducers, for example pH, temperature, enzymes, water, or other
physiological conditions or compounds.
[0106] Powdered and granular formulations of a pharmaceutical
preparation
of the invention may be prepared using known methods. Such formulations may
be administered directly to a subject, used, for example, to form tablets, to
fill
capsules, or to prepare an aqueous or oily suspension or solution by addition
of
an aqueous or oily vehicle thereto. Each of these formulations may further
comprise one or more of dispersing or wetting agent, a suspending agent, and a
preservative. Additional excipients, such as fillers and sweetening,
flavoring, or
coloring agents, may also be included in these formulations.
[0107] A formulation of a pharmaceutical composition of the invention
suitable for oral administration may be prepared, packaged, or sold in the
form of
a discrete solid dose unit including, but not limited to, a tablet, a hard or
soft
capsule, a cachet, a troche, or a lozenge, each containing a predetermined
amount
of the active ingredient. Other formulations suitable for oral administration
include, but are not limited to, a powdered or granular formulation, an
aqueous
or oily suspension, an aqueous or oily solution, a paste, a gel, a toothpaste,
a
mouthwash, a coating, an oral rinse, or an emulsion. The terms oral rinse and
mouthwash are used interchangeably herein.
[0108] A tablet comprising the active ingredient may, for example, be
made
by compressing or molding the active ingredient, optionally with one or more
additional ingredients. Compressed tablets may be prepared by compressing, in
a suitable device, the active ingredient in a free flowing form such as a
powder or
granular preparation, optionally mixed with one or more of a binder, a
lubricant,
an excipient, a surface-active agent, and a dispersing agent. Molded tablets
may
be made by molding, in a suitable device, a mixture of the active ingredient,
a
pharmaceutically acceptable carrier, and at least sufficient liquid to moisten
the
mixture. Pharmaceutically acceptable excipients used in the manufacture of
tablets include, but are not limited to, inert diluents, granulating and
disintegrating agents, binding agents, and lubricating agents. Known
dispersing
agents include, but are not limited to, potato starch and sodium starch
glycollate.
Known surface-active agents include, but are not limited to, sodium lauryl
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
sulphate. Known diluents include, but are not limited to, calcium carbonate,
sodium carbonate, lactose, microcrystalline cellulose, calcium phosphate,
calcium hydrogen phosphate, and sodium phosphate. Known granulating and
disintegrating agents include, but are not limited to, corn starch and alginic
acid.
Known binding agents include, but are not limited to, gelatin, acacia, pre-
gelatinized maize starch, polyvinylpyrrolidone, and hydroxypropyl
methylcellulose. Known lubricating agents include, but are not limited to,
magnesium stearate, stearic acid, silica, and talc.
[0109] Tablets may be non-coated or they may be coated using known
methods to achieve delayed disintegration in the gastrointestinal tract of a
subject, thereby providing sustained release and absorption of the active
ingredient. By way of example, a material such as glyceryl monostearate or
glyceryl distearate may be used to coat tablets. Further by way of example,
tablets may be coated using methods described in U.S. Patent Nos. 4,256,108;
4,160,452; and 4,265,874 to form osmotically-controlled release tablets.
Tablets
may further comprise a sweetening agent, a flavoring agent, a coloring agent,
a
preservative, or some combination of these in order to provide for
pharmaceutically elegant and palatable preparation.
[0110] Hard capsules comprising the active ingredient may be made using
a
physiologically degradable composition, such as gelatin. Such hard capsules
comprise the active ingredient, and may further comprise additional
ingredients
including, for example, an inert solid diluent such as calcium carbonate,
calcium
phosphate, or kaolin.
[0111] Soft gelatin capsules comprising the active ingredient may be
made
using a physiologically degradable composition, such as gelatin. Such soft
capsules comprise the active ingredient, which may be mixed with water or an
oil medium such as peanut oil, liquid paraffin, or olive oil.
[0112] Liquid formulations of a pharmaceutical composition of the
invention
which are suitable for oral administration may be prepared, packaged, and sold
either in liquid form or in the form of a dry product intended for
reconstitution
with water or another suitable vehicle prior to use.
26
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[0113] Injectable formulations may be prepared, packaged, or sold in
unit
dosage form, such as in ampules or in multi dose containers containing a
preservative. Formulations for parenteral administration include, but are not
limited to, suspensions, solutions, emulsions in oily or aqueous vehicles,
pastes,
and implantable sustained-release or biodegradable formulations. Such
formulations may further comprise one or more additional ingredients
including,
but not limited to, suspending, stabilizing, or dispersing agents. In one
embodiment of a formulation for parenteral administration, the active
ingredient
is provided in dry (i.e., powder or granular) form for reconstitution with a
suitable vehicle (e.g., sterile pyrogen free water) prior to parenteral
administration of the reconstituted composition.
[0114] A pharmaceutical composition of the invention may be prepared,
packaged, or sold in a formulation suitable for buccal administration. Such
formulations may, for example, be in the form of tablets or lozenges made
using
conventional methods, and may, for example, 0.1 to 20 % (w/w) active
ingredient, the balance comprising an orally dissolvable or degradable
composition and, optionally, one or more of the additional ingredients
described
herein. Alternately, formulations suitable for buccal administration may
comprise a powder or an aerosolized or atomized solution or suspension
comprising the active ingredient. Such powdered, aerosolized, or aerosolized
formulations, when dispersed, preferably have an average particle or droplet
size
in the range from about 0.1 to about 200 nanometers, and may further comprise
one or more of the additional ingredients described herein.
[0115] As used herein, "additional ingredients" include, but are not
limited
to, one or more of the following: excipients; surface active agents;
dispersing
agents; inert diluents; granulating and disintegrating agents; binding agents;
lubricating agents; sweetening agents; flavoring agents; coloring agents;
preservatives; physiologically degradable compositions such as gelatin;
aqueous
vehicles and solvents; oily vehicles and solvents; suspending agents;
dispersing
or wetting agents; emulsifying agents, demulcents; buffers; salts; thickening
agents; fillers; emulsifying agents; antioxidants; antibiotics; antifungal
agents;
stabilizing agents; and pharmaceutically acceptable polymeric or hydrophobic
27
CA 02618970 2014-03-06
materials. See Genaro, ed., 1985, Remington's Pharmaceutical Sciences, Mack
Publishing Co., Easton, PA,
[0116] The compound can be administered to a subject as frequently as
several times daily, or it may be administered less frequently, such as once a
day,
once a week, once every two weeks, once a month, or even less frequently, such
as once every several months or even once a year or less. The frequency of the
dose will be readily apparent to the skilled artisan and will depend upon any
number of factors, such as, but not limited to, the type and severity of the
disease
being treated, the type, and age of the subject, etc.
[0117] The invention also provides a pharmaceutical pack or kit comprising
one or more containers filled with one or more of the ingredients of the
pharmaceutical compositions of the invention. In accordance with one
embodiment, a kit is provided for treating a subject in need of immuno-
modulation. Preferably, the subject is a human. In one embodiment, the kit
comprises one or more of the S113 analogs of the present invention and may
also
include one or more known immuno-suppressants. These pharmaceuticals can
be packaged in a variety of containers, e.g., vials, tubes, microtiter well
plates,
bottles, and the like. Other reagents can be included in separate containers
and
provided with the kit; e.g., positive control samples, negative control
samples,
buffers, cell culture media, etc. Preferably, the kits will also include
instructions
for use.
[0118] Although any methods and materials similar or equivalent to those
described herein can be used in the practice or testing of the present
invention,
the preferred methods and materials are described herein.
[0119] In accordance with the present invention, as described above or as
discussed in the Examples below, there can be employed conventional clinical,
chemical, cellular, histochemical, biochemical, molecular biology,
microbiology,
and recombinant DNA techniques which are known to those of ordinary skill in
the art. Such techniques are fully explained in the literature.
Examples
[0120] The invention is now described with reference to the following
Examples and Embodiments. Without further description, it is believed that one
28
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
of ordinary skill in the art can, using the preceding description and the
following
illustrative examples, make and utilize the present invention and practice the
claimed methods. The following working examples therefore, are provided for
the purpose of illustration only and specifically point out some embodiments
of
the present invention, and are not to be construed as limiting in any way the
remainder of the disclosure. Therefore, the examples should be construed to
encompass any and all variations which become evident as a result of the
teaching provided herein.
Example 1: LSF Analogs
[0121] The side chain moiety (5-R-hydroxyhexyl) was kept constant and a
variety of nitrogen-contained heterocyclic compounds, including xanthine-like
(5-aza-7-deazaxanthine-LSF) and non-xanthine-like skeletons, were prepared for
substitution with the hydroxyhexyl group. Most of the heterocyclic compounds
were commercially available. 5-aza-7-deazaxanthine (in compounds 10 and 11),
which was synthesized from a 1,3,5-triazine derivative, cyanuric chloride. See
J.
D. Hepworth. Org. Synth. 5, 27-29; R. Calabretta, C. Giordano, C. Gallina, V.
Morea, V. Consalvi and R. Scandurra. Eur. J. Med. Chem. 1995, 30, 931-941; S.
Horrobin. J. Chem. Soc. 1963, 4130-4145; and S. H. Kim, D. G. Bartholomew,
and L. B. Allen. J. Med. Chem. 1978, 21(9), 883-888. The general scheme for
synthesis of the LSF analogs is provided in scheme 1. Specific examples of
compounds of the invention are depicted in Figure 1. The synthesis of
compound 11 (CPW11) is illustrated in Scheme 2, Figure 2.
Example 2: Biological Evaluations of LSF Analogs
[0122] Analogs of the invention (CPW1-15) were evaluated in pancreatic 0
cell lines for apoptosis protection after treatment with inflammatory
cytokines
(reflected by reduced 0D450) and insulin release (see Figures 5 and 6). For
the
protective effect of 0-cells, some analogs showed comparative potency with LSF
and additional analogs were able to protect cells at low concentrations (nm
and
lower; see Figure 5). For the insulin release assay, some compounds
demonstrated effects similar to those of LSF in response to glucose (CPW1-15;
Figure 6).
29
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[0123] Further evaluations were performed for some of the LSF analogs of
the invention in MDS assays, solubility, oral bioavailability, and stability
(see
Table 1).
Table 1- rCYP450 Inhibition and Metabolic Stabilities
rCYP % Inhibition
Metabolic
Compound Formula
Stability
No. Weight
1A2 2C9 2C19 2D6 3A4 Percent
Remaining
CPW1 338.24 58% 86% 99% 80% 57% 0%
CPW4 296.2 56% 38% 94% 50% 32% 0%
CPW6 342.47 13% 3% 14% 16% 14% 77%
CPW7 271.29 12% 5% 12% 8% 12% na
CPW8 319.36 7% 5% 24% 10% 12% 45%
CPW9 277.32 5% -12% 1% 2% -4% 106%
CPW11 280.32 13% 12% 21% 3% 0% 82%
CPW12 262.3 18% 30% 30% 12% 4% 75%
CPW20 283.36 95% 86% 98% 73% 50% 19%
CPW13 362.46 25% 92% 89% -28% -159% 38%
[0124] Analogs having greater than 50% inhibitory activity were
considered
to have high activity; analogs having from 20-49% inhibitory activity were
considered to have medium activity; and analogs having less than 20 inhibitory
activity were considered to have low activity
Example 3: Biological Evaluations of LSF Analogs in Human islets
[0125] Four compounds (CPW7, CPW8, CPW11, and CPW12) were also
evaluated on human islets for the induction of insulin secretion,
intracellular
ATP concentrations and the effect of reducing cell death exposed to cytokines
(combination of human IL-113,1FN-7,and TNF-a). The results are illustrated in
Figures 7A-7D.
[0126] The results of the testing indicate that CPW12 indicates that
there
were positive effects in n-cell protection and insulin release on mouse islet
cell
line, the analog shows low cytochrome p450 inhibition (it was a safe compound
with low potential for drug interactions). The Caco-2 permeability test
results
shows CPW12 could be orally absorbed from the GI track, which is consistent
with good oral bioavailability. The CPW12 analog shows acceptable stability
and solubility.
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
[0127] In the evaluation on human islet, CPW12 shows positive effects to
improve insulin secretion in the presence of inflammatory cytokines and has a
beneficial effect in maintaining mitochondrial function and cell viability in
human 13-cells.
Example 4: Effects of LSF and Analogs on 13-cells
[0128] The effects of LSF analogs on 13-cells in the mouse insulin-
secreting
INS-1 cell line were investigated. Cells were maintained in RPMI 1640 medium
(Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% heat-
inactivated FBS, 10 mm HEPES, 200 lam L-glutamine, 1 mm sodium pyruvate, 5
nm 2-mercaptoethanol, 50 U/m1 penicillin, and 50 g/m1 streptomycin at pH 7.4.
The cells were cultured at 37 C in a humidified incubator supplied with 5%
carbon dioxide. Fresh medium was replaced every 2 days. The cells were plated
at a density of 105/cm2. Culture vessels were coated with poly-D-lysine and
gelatin (Sigma, St. Louis, MO) to retain detached and dead cells so that
seeding
cell numbers reflect the actual cell numbers after all treatment conditions.
INS-1
cells were treated with the combination of recombinant mouse IL-1f3 (5 ng/ml),
IFNy (100 ng/ml), and TNFoc (10 ng/ml; R&D Systems, Inc., Minneapolis, MN)
suspended in complete RPMI medium. LSF (provided by Cell Therapeutics, Inc.,
Seattle, WA) and analogs were added simultaneously with the cytokines in
complete RPMI medium. All treatments were performed for 18 hours.
[0129] The results (illustrated in Figures 8a-8c) showed that the LSF
analogs
could protect 13-cells from cytokine-induced cell death. INS-1 cells were
treated
with a combination of recombinant IL-113, INFy, and TNFoc with 10-5-103 M
LSF and its analogs. At a concentration of 10-2 M, LSF showed a maximum
protective effect (Fig. 8a). There was no further increase in 13-cell
protection
when the LSF concentration was increased from 10-2 to 102 M. The LSF
analogs showed some protective effects on I3-cells, especially compounds
CPW11 (Fig. 8b) and CPW12 (Fig. 8c), which proved effective at low
concentrations. Both of these compounds showed comparative activity with LSF
at concentration of 10-2 M. No further increases in 13-cell protection were
observed when analog concentrations were increased.
31
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
Example 5: Effects of LSF and Analogs on Insulin Secretion
[0130] The effects of LSF analogs on insulin secretion in INS-1 cells
with
and without inflammatory cytokines. 13-Cells were treated with apoptosis
detecting dye for 2-3 hours at room temperature. Apoptotic cells were
recognized with purple-red color under a microscope. After washing to
eliminate free dye and adding dye release reagent, color density was
quantified
by reading at OD 450 nM. At the end of treatment, cells were washed with
Krebs-Ringer-bicarbonate-HEPES buffer (KRB) containing 134 mm NaCl, 4.7
mm KC1, 1.2 mm KH2PO4, 1.2 mm MgSO4, 1.0 mm CaC12, 10 mm HEPES, and
0.1% BSA at 37 C, pH 7.4. The cells were preincubated in the same buffer for
30 min, followed by 60-min incubation in KRB supplemented with 15 mM D-
glucose (J. T. Baker, Phillipsburg, NJ). The supernatant was harvested and
subjected to centrifugation to eliminate residue cells. Insulin secreted into
the
supernatant was measured by RIA with mouse insulin as a standard. The cells
are maintained in basal (3 mM) and glucose-stimulated (28 mM). Insulin release
was observed by 20 IIM of LSF and its analogs. Insulin secretion in INS-1
cells:
(a) LSF, (b) compound CPW11, and (c) compound CPW12. =
w/compound, w/o cytokines, 3 mM glucose; -C-G3 = w/o compound, w/o
cytokines, 3 mM glucose; +C-G28 = w/compound, w/o cytokine, 28 mM
glucose; -C-G28 = w/compound, w/o cytokine, 28 mM glucose; +C+G3 = w/
compound, w/cytokines, 3 mM glucose; -C+G3 = w/o compound, w/cytokines, 3
mM glucose;+C+G28 = w/compound, w/cytokines, 28 mM glucose; -C+G28 =
w/o compound, w/cytokines, 28 mM glucose). The results are illustrated in
Figures 9a-9c.
Example 6: Development of LSF analogs based on CPW12.
[0131] Schemes are provided for additional analogs of CPW12, (see
Figures
3A-3D, Schemes 3A-3D and Figure 4, Scheme 4). In Figures 3A-3D, Schemes
3A-3D modifications of the side chain moiety are illustrated. (See V. Cere, C.
Mazzini, C. Paolucci, S. Pollicino and A. Fava J. Org. Chem., 1993, 58, 4567-
4571; C. Paolucci, S. Pollicino and A. Fava J. Org. Chem., 1995, 60, 169-175;
F. Sato, Y. Tomuro, H. Ishikawa and M. Sato. Chem. Lett. 1980, 99-102; and G.
32
CA 02618970 2008-02-08
WO 2007/027719
PCT/US2006/033777
Cerichelli, C. Grande, L. Luchetti and G. Mancini .1. Org. Chem., 1991, 56,
3025-3030.)
[0132] In Figure 4, Scheme 4 a modification of the nuclear moiety (R2)
is
illustrated. (See N. Haider, E. Mavrokordatou and A. Steinwender. Syn. Comm.
1999, 29(9), 1577-1584.)
Example 7: Mouse-insulin secreting cell line j3-TC6
Cell preparation
[0133] The 13-TC6 cell line is maintained in RPMI 1640 medium (Life
Technologies, Rockville, MD) supplemented with 10% heat inactivated fetal
bovine serum, 10 mM HEPES, 200 pM L-glutamine, 50 units/ml penicillin and
50 i.tg/m1 streptomycin at pH 7.4. The cells are cultured in a 37 C,
humidified
incubator supplied with 5% carbon dioxide. Fresh media is replaced every two
days. Unless otherwise stated, the cells are plated at a density of 105/cm2.
Culture
vessels (dishes and chamber slides) used for experiments were coated with poly-
D-lysine and gelatin (Sigma, St. Louis, MO) to retain detached and dead cells
so
that seeding cell numbers reflect the actual cell numbers after all treatment
conditions.
Cytokine and LSF analog treatment of B-TC6 cells.
[0134] 13-TC6 cells are treated with vehicle alone or with the
combination of
recombinant mouse IL-113 (5 ng/ml), IFNI, (100 ng/ml) and TNF-a (10 ng/ml)
(R&D Systems, Minneapolis, MN) suspended in complete RPMI medium. LSF
(Cell Therapeutics, Inc. Seattle, WA) or the analogs are added simultaneously
with the cytokines in complete RPMI medium in the concentrations ranging 1
nM-20 pM. All treatments are conducted for 18 hours. The results are
illustrated
in Figures 8A-8P.
Example 8: Static insulin secretion.
[0135] At the end of treatment, cells are washed with Krebs-Ringer-
bicarbonate-HEPES buffer (KR13) containing in mM: 134 NaCl, 4.7 KC1, 1.2
KH2PO4, 1.2 MgSO4., 1.0 CaCl2, 10 HEPES, and 0.1 % bovine serum albumin at
37 C, at a pH of 7.4. The cells are pre-incubated in the same buffer for 30
min
33
CA 02618970 2014-03-06
. .
followed by 60-min incubation in KRB supplemented with 15 mM D-glucose (J.T.
Baker, Phillisburg, NJ). The supernatant is harvested and subjected to
centrifugation to
eliminate residue cells. Insulin secreted into the supernatant is measured
using EIA with
mouse insulin as a standard. The results are illustrated in Figures 9A-9P.
Example 9: STAT4 Phosphorylation
[0136] Murine splenocytes are equally plated in 96-well plates and are treated
with or
without LPS (1.0 ng/ml) supplemented with LSF or a series of analogs for 18
hours. The
protein lysates are transferred onto the Hybond-P membranes, and are
subsequently
probed with a polyclonal antibody against phosphorylated STAT4. The hybridized
membranes are subjected to ECL and autoradiography. Samples are repeated in
triplicate.
The results are illustrated in Figure 10.
[0137] Other methods which were used but not described herein are well known
and
within the competence of one of ordinary skill in the art of clinical,
chemical, cellular,
histochemical, biochemical, molecular biology, microbiology and recombinant
DNA
techniques.
[0138] One skilled in the art will readily appreciate that the present
invention is well
adapted to carry out the objects and obtain the ends and advantages mentioned,
as well as
those inherent therein.
[0139] The abbreviations used herein have their conventional meaning within
the
chemical and biological arts. In the case of any inconsistencies, the present
disclosure,
including any definitions therein will prevail. The invention has been
described with
reference to various specific and preferred embodiments and techniques. The
scope of
the claims should not be limited by the preferred embodiments set forth in the
examples,
but should be given the broadest interpretation consistent with the
description as a whole.
34