Note: Descriptions are shown in the official language in which they were submitted.
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
APPARATUS FOR ASSAY, SYNTHESIS AND STORAGE, AND METHODS OF
MANUFACTURE, USE, AND MANIPULATION THEREOF
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
60/707,501, which was filed August 11, 2005; and is a continuation-in-part of
U.S. Patent
Application No. 10/315,832, which was filed on December 10, 2002, which is a
divisional application of U.S. Patent Application No. 09/975,496, which was
filed on
October 10, 2001, and is now issued as U.S. Patent No. 6,716,629, and which
claims the
benefit of U.S. Provisional Application No. 60/239,538, filed October 10,
2000, U.S.
Provisional Application No. 60/268,894, filed February 14, 2001, and U.S.
Provisional
Application No. 60/284,710, filed April 18, 2001; each of the foregoing
applications are
hereby incorporated by reference in their entirety.
FIELD OF THE INVENTION
This invention relates to devices for molecular synthesis, storage and
screening,
and other chemical, biochemical, biological, and physical experiments, and to
methods of
making, using, and manipulating the same.
BACKGROUND OF THE INVENTION
High throughput methods for creating and analyzing chemical and biochemical
diversity play a vital role in technologies including drug discovery and
development.
Specific applications of high throughput methods include drug discovery,
optimization of
reaction conditions (e.g., conditions suitable for protein crystallization),
genomics,
proteomics, genotyping, polymorphism analysis, examination of RNA expression
profiles
in cells or tissues, sequencing by hybridization, and recombinant enzyme
discovery.
Rapid, high throughput methods for synthesizing (e.g., using combinatorial
chemistry methods) and screening large numbers of these compounds for
biological and
physicochemical properties are desired, for example, to increase the speed of
discovery
and optimization of drug leads.
Similarly, due in part to the large amount of sequence data from the human
genome project, efforts are underway to rapidly obtain x-ray crystallography
data for the
protein products of many newly discovered genes. One of the rate limiting
steps in this
process is the search for appropriate solution conditions (e.g., pH, salt
concentration) to
cause protein crystallization. There is also a need to determine the function
of each of;the
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
newly discovered genes (i.e., "functional genomics") and to map protein-
protein
interactions (i.e., "proteomics"). Given the large number of human genes,
protein
modifications, and protein binding partners, higher throughput methods are
desired.
Another advance in biotechnology is the creation of surfaces with high-density
arrays of biopolymers such as oligonucleotides or peptides. High-density
oligonucleotide
arrays are used, for example, in genotyping, polymorphism analysis,
examination of RNA
expression profiles in cells or tissues, and hybridization-based sequencing
methods as
described, for example, in U.S. Patent Nos. 5,492,806, 5,525,464, and
5,667,972 to
Hyseq, Inc. Arrays containing a greater number of probes than currently
provided are
desirable.
The process of discovering and improving recombinant enzymes for industrial or
consumer use has emerged as an important economic activity in recent years. A
desire to
discover very rare, activity-improving mutations has further stimulated the
search for
higher throughput screening methods. Such methods often require screening
100,000 to
1,000,000 members of a genetic library in parallel, and then rapidly detecting
and
isolating promising members for further analysis and optimization.
One of the challenges in the development of high throughput methods is that
conventional liquid handling techniques such as pipetting, piezoelectric
droplet
dispensing, split pin dispensing, and microspritzing are generally unsuitable
for rapidly
loading or transferring liquids to or from plates of high density (e.g.,
plates having more
than about 384 wells). For example, these techniques can cause substantial
splashing,
resulting, for example, in contamination of neighboring wells and loss of
sample volume.
Also, as the number of wells increases, the time necessary to reformat
compounds from
the previous generation of plates to the higher density plates generally
increases, thus
limiting the utility of higher density plates. Evaporation can also be
problematic with
times greater than a few seconds. Moreover, entrapped air bubbles can result
in
inconsistencies in the loading of small fluid volumes (e.g., less than about
one microliter).
Significant bottlenecks in high throughput screening efforts include library
storage, handling, and shipping. As the number of compounds in a library
increases, the
number of 96- or 384-well plates, and the total volume needed to store the
libraries, also
increases. For compounds that are stored in frozen solvent such as DMSO or
water,
thawing, dispensing, and refreezing pose the hazard of crystallization,
precipitation, or
degradation of some compounds, making it difficult to dispense accurate
quantities in the
future. Having samples stored in low-density plates requires a time consuming
step of
2
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
reformatting the samples into high-density plates before the high-density
technology can
be utilized.
SUMMARY OF THE INVENTION
The invention features methods of making devices, or "platens", having a high-
density array of through-holes, as well as methods of cleaning and
refurbishing the
surfaces of the platens. The invention further features methods of making high-
density
arrays of chemical, biochemical, and biological compounds, having many
advantages
over conventional, lower-density arrays. The invention includes methods by
which many
physical, chemical or biological transformations can be implemented in serial
or in
parallel within each addressable through-hole of the devices. Additionally,
the invention
includes methods of analyzing the contents of the array, including assaying of
physical
properties of the samples.
In various embodiments, the reagents can be contained within the through-holes
by capillary action, attached to the walls of the through-holes, or attached
to or contained
within a porous material inside the through-hole. The porous material can be,
for
example, a gel, a bead, sintered glass, or particulate matter, or can be the
inner wall of a
through-hole that has been chemically etched. In particular embodiments, the
arrays can
include individual molecules, complexes of molecules, viruses, cells, groups
of cells,
pieces of tissue, or small particles or beads. The members of the arrays can
also, for
example, function as transducers that report the presence of an analyte (e.g.,
by providing
an easily detected signal), or they can function as selective binding agents
for the
retention of analytes of interest. Using these methods, arrays corresponding
to a large
plurality of human genes (e.g., using nucleic acid probes) can also be
prepared.
On embodiment of the invention features a method of making a platen of a
desired
thickness having a plurality of through-holes. The method includes the steps
of (a)
providing a plurality (e.g., 2, 3, 5, 8, 10, 100, 1000 or more) of plates
having upper and
lower surfaces, wherein one or both of the upper and lower surfaces of at
least some of
said plurality of plates has continuous, substantially parallel grooves
running the length of
said surfaces; (b) bonding the upper surfaces of all but one of said plurality
of plates to
the lower surfaces of the other plates (i.e., the upper surface of the first
plate is bonding to
the lower surface of the second plate; the upper surface of the second plate
is bonded to
the lower surface of the third plate; and so on; the upper surface of the last
plate is not
bonded to anything else); and (c) if necessary to achieve the desired
thickness, slicing the
3
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
platen substantially perpendicularly to the through-holes, thereby creating a
platen of a
desired thickness having a plurality of through-holes. Step c) can optionally
be repeated
make a plurality of platens. . By "a plurality of through-holes" is meant at
least 2 (e.g.,
2, 5, 10, 20, 25, 50, 100, 200, 250, 500, 25,000, 50,000 or more). For
example, a platen
the size of a conventional microscope slide may have about 3,072 holes, while
a platen
the size of a microtiter plate may have about 24,576. The number of through-
holes on a
microtiter plate can be 50,000, 100,000, 200,000 or more. Of course, the
number of
through-holes will vary depending on the diameter of the hole and the size of
the platen.
For example, where the through-hole has a diameter of less than about 400
micrometers,
the through-hole density is at least 1.6 through-holes per square millimeter.
The plates can be made from any material that can be bonded (e.g., plastic,
metal,
glass, or ceramic), and each can have a thickness from, e.g., about 0.01 mm to
2.0 mm,
preferably 0.1 mm to 1 mm; the grooves have a depth from, e.g., 0.005 mm to
2.0 mm
(i.e., less than the thickness of the plates); and the grooves can have a
width from, e.g.,
0.1 mm to 1.0 mm.
The plates can be bonded in a configuration in which the grooves of one plate
are
substantially parallel to the grooves of each of the other plates, or can be
bonded so that
the grooves of certain plates are perpendicular to, or at acute angles to, the
grooves of
certain other plates.
In another embodiment, the invention features a device for the immobilization
of
probes, cells, or solvent. The device includes a platen (optionally having
hydrophobic
upper and lower surfaces) having a plurality of through-holes (e.g., from the
upper
surface to the lower surface), where at least some of the through-holes
contain a porous
material such as a gel (e.g., polyacrylamide), silica, sintered glass, or
polymers for the
immobilization of probes, cells, or solvent.
In still another embodiment, the invention features a method of making a
platen
having opposing hydrophobic surfaces and a plurality of hydrophilic through-
holes. The
method includes the steps of: (a) coating a plate with a material (e.g., gold,
silver, copper,
gallium arsenide. metal oxides, or alumina) that reacts with amphiphilic
molecules (e.g.,
alkane thiols, alkanephosphates, alkane carboxylates); (b) forming through-
holes in the
plate (e.g., by micromachining methods such as drilling, electrospark
discharge
machining (EDM), punching, stamping, or etching; and (c) treating (e.g.,
dipping or
spraying) the plate with a solution or vapor of an amphiphilic molecule to
provide a
platen having hydrophobic coating on surfaces of the platen but not on the
walls of the
4
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
through-holes. The invention also includes the platens made by this method, as
well as a
method of regenerating the hydrophobic coating on the platen after use. This
method
includes the steps of: (a) removing residual hydrophobic coating, if any
(e.g., by washing
the platen with oxidant, reductant, acid, base, or detergent, or by heating,
electropolishing, irradiating, or burning); and (b) treating the platen with a
solution or
vapor of an amphiphilic molecule to regenerate the hydrophobic coating.
In yet another embodiment, the invention features a method of selectively
making
a coating on the surfaces of a platen having a plurality of through-holes. The
method
includes the steps of: (a) selectively coating the surfaces of the platen with
a material that
reacts with amphiphilic molecules; and (b) treating the platen with a solution
or vapor of
an amphiphilic molecule to regenerate the hydrophobic coating.
Still another embodiment of the invention features a platen having two
opposing
surfaces and a plurality of through-holes extending between the surfaces. The
surfaces
have different chemical properties relative to the walls of the through-holes,
such that the
walls and surfaces can be independently functionalized. For example, the walls
can be
coated with gold (e.g., by coating the entire platen, including both the walls
and the
opposing surfaces with gold, and then electropolishing the surfaces to remove
the gold
therefrom), allowing the walls to be rendered hydrophobic upon treatment with
alkane
thiols. Conversely, the surfaces (but not the walls) could be coated with
metal oxides so
that alkanephosphates can be bound thereto.
In another embodiment, the invention features a method of making a plastic
platen
of a desired thickness, having through-holes. The method features the steps
of: a) potting
a plurality of capillaries (e.g., glass or plastic capillaries) in the through-
holes of a stack
of platens comprising at least two platens having through holes; b) separating
adjacent
platens by a distance equal to the desired thickness; c) injecting a plastic-
forming material
into the space between the separated platens; d) forming (e.g., heat-setting
or curing) the
plastic; and e) slicing at the interface between the platens and the plastic
to form the
chips. The plastic-forming material can be, for example, a photo-, thermo-, or
chemical-
curable material such as a UV-curable material, e.g., polymethylmethacrylate
(PMMA),
polystyrene, or epoxy, and the forming step can entail exposing the material
to ultraviolet
light; or the plastic-forming material can be a molten thermoplastic material
and the
forming step can involve cooling the material.
In still another embodiment, the invention features a method of making a
plastic
chip of a desired thickness, having through-holes. The method features the
steps of:
5
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
a) potting a plurality of fibers or wires in the through-holes of a stack of
platens
comprising at least two platens having through holes; b) separating adjacent
platens by a
distance equal to the desired thickness; c) injecting a plastic-forming
material into the
space between the separated platens; d) forming the plastic; e) withdrawing
the fibers or
wires from the plastic to form through-holes; and f) slicing at the interface
between the
platens and the plastic to form the chips.
Still another embodiment of the invention is a method of creating a chemical
array. The method includes the steps of: a) providing a platen having a
plurality of
through-holes and two opposing surfaces; b) applying a mask to one or both
surfaces of
the platen to block at least some of the through-holes, while leaving other
through-holes
open; c) exposing a surface of the platen to a reagent (e.g., e.g., a liquid,
a gas, a solid, a
powder, a gel, a solution, a suspension such as a slurry, a cell culture, a
virus preparation,
or electromagnetic radiation; e.g., by spraying.the platen with a solution or
suspension of
the reagent, or by condensing, pouring, depositing, or dipping the reagent
onto the platen)
so that the reagent enters at least one of the open through-holes; and d)
repeating steps b)
and c) (e.g., at least once, generally at least three times; for creation of
nucleic acid
arrays, the steps can be repeated four times, the length of the desired
nucleic acid chains;
for creation of protein arrays, the steps can be repeated twenty times the
length of the
desired peptide chains) with at least one different mask and at least one
different reagent
to create a chemical array. The masks can be reusable or disposable, and can
be applied
mechanically (e.g., robotically) or manually. The mask can, in some cases,
initially
include the reagent (e.g., absorbed onto or contained within it). The mask can
be flexible
or rigid, for example, and can be made of a polymer, an elastomer, paper,
glass, or a
semiconductor material. The mask can, for example, include mechanical valves,
pin
arrays (e.g., posts, pistons, tubes, plugs, or pins), or gas jets. In some
cases, the
"applying" step forms a hermetic seal between the mask and the platen. The
mask can
also be translated (e.g., moved between the repetitions of the method) to
expose different
through-holes. In some cases, the mask has co-registration pins and holes such
that
alignment of pins and holes in the mask register with the through-holes in the
platen. In
these cases, multiple masks can be made part of a flexible tape, and the
multiple masks
are registered with the through-holes of the platen by advancing the tape
(e.g., the masks
can be on a spool, ribbon, or roll, and can be advanced in a manner analogous
to the
advancing of film in a camera). Arrays created by any of these methods are
also
considered to be an aspect of the invention.
6
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
In yet another embodiment, the invention features a method of creating a
chemical
array. The method includes the steps of: a) providing a platen having a
plurality of
through-holes and two opposing surfaces; b) applying a mask that has one or
more
reagents on its surface to one or both surfaces of the platen to transfer the
reagent from
the mask to at least some of the through-holes; and c) repeating step b) with
at least one
different mask and at least one different reagent to create a chemical array.
The invention also features a method for separating samples within a chemical
array in a platen. The method includes the steps of a) providing a platen
having a
plurality of through-holes and two opposing surfaces; b) electrophoretically
transporting a
charged reagent into at least some of the through-holes by placing the platen
into an
electrophoresis apparatus containing the reagent and applying an electric
field parallel to
the through-holes; and c) repeating step b) with at least one different
reagent to create a
chemical array.
In still another embodiment, the invention features a method of creating a
spatially
addressable array. The method includes the following steps: a) providing a
platen having
a spatially addressable plurality of discrete through-holes each having an
inner wall,
wherein said platen has opposing hydrophobic surfaces; and b) covalently or
non-
covalently immobilizing at least one reagent or probe on the inner walls of at
least some
of the through-holes or on a bead contained within at least one of the through-
holes to
form a spatially addressable array. In this method, the through-holes can be
either non-
communicating (i.e., the contents of adjacent through-holes do not mix with
each other)
or selectively communicating (i.e., the walls of at least some of the through-
holes act as
semi-permeable membranes) through-holes. In some cases, the method can also
include
the step of: c) flowing reagents (e.g., monomers, wash solutions, catalysts,
terminators,
denaturants, activators, polymers, cells, buffer solutions, luminescent and
chromatogenic
substrate solutions, beads, heated or cooled liquids or gases, labelled
compounds, or
reactive organic molecules) into or through a predetermined subset of the
through holes.
Yet another embodiment of the invention is a method of creating a stochastic
array. The method includes a) providing a platen having a plurality of through-
holes; and
b) applying each of a plurality of reagents to the through-holes in a random
or semi-
random manner (e.g., spatially random or random with respect to distribution
of reagents)
to create a stochastic array. The "applying" step can include, for example,
providing a
plurality of dispensing devices addressing at least some of the through-holes,
dispensing
different combinations of reagent solutions (e.g., as solutions, neat, or in
suspension) into
7
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
each through-hole, and repositioning the dispensing devices at least once to
address a
different set of through-holes. In this case, the method can also involve
dispensing a fluid
that is immiscible with the reagent solutions into at least one through-hole.
In another embodiment, the invention features a method of identifying
combinations of reagents having a biological, chemical or physical property of
interest.
The method involves, for example, the use of radiolabelled probes, or the
measurement of
chemiluininescence. The method features the steps of: a) creating a stochastic
array using
the above method; b) assaying the stochastic array for combinations having a
property of
interest; and c) identifying the reagents that have the property of interest.
Non-limiting
examples of properties of interest include catalysis (see, e.g., Weinberg et
al., Current
Opinion in Solid State & Materials Science, 3:104-110 (1998)); binding
affinity for a
particular molecule (see, e.g., Brandts et al., Ainerican Laboratory 22:3041
(1990); or
Weber et al., J. Am. Chena. Soc. 16:2717-2724 (1994)); ability to inhibit
particular
chenlical and biochemical reactions; thermal stability (see, e.g., Pantaliano
et al., U.S.
Patent Nos. 6,036,920 and 6,020,141); luminescence (see, e.g., Danielson et
al., Nature
389:944-948 (1997)); crystal structure (see, e.g., Hindeleh et al., Journal
afMaterials
Science 26:5127-5133 (1991)); crystal growth rate; diastereoselectivity (see,
e.g., Burgess
et al., Angew. Chem. 180:192-194 (1996)); crystal quality or polymorphism;
surface
tension; (see, e.g., Erbil, J. Phys. Chena. B., 102:9234-9238 (1998)); surface
energy (see,
e.g., Leslot et al., Plays. Rev. Lett. 65:599-602 (1990)); electromagnetic
properties (see,
e.g., Briceno et al., Science 270:273-275 (1995); or Xiang et al., Science
268:1738-1740
(1995)); electrocliemical properties (see, e.g., Mallouk et al., Extended
Abstracts; Fuel
Cell seminar: Orlando, Florida, 686-689 (1996)); and optical properties (see,
e.g., Levy et
al., Advanced Materials 7:120-129 (1995)); toxicity, antibiotic activity,
binding, and other
biological properties; fluorescence and other optical properties; and pH,
mass, binding
affinity, and other chemical and physical properties.
In another embodiment yet, the invention features a method of loading a platen
having a plurality of through-holes, where the platen has opposing surfaces
(e.g., the
surfaces are hydrophobic and the through-holes have hydrophilic walls). The
method
includes the steps of: a) dipping the platen into a liquid sample (e.g., a
neat liquid, a
solution, a suspensions, or a cell culture) that includes a sample to be
loaded into the
through-holes, thereby loading at least some of the through-holes with the
sample; and b)
passing the platen through a liquid that has an affinity for the surfaces of
the platen but
that is immiscible with the liquid sample, thereby cleaning the surface of the
platen of
8
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
excess sample mixture (e.g., by adding, on top of the sample mixture, the
immiscible
liquid, where the liquid has a lower density than the sample mixture (e.g.,
mineral oil);
and removing the platen from the sample mixture through the liquid; device
comprising a
barrier between the sample and the liquid).
The invention also features another method of loading a platen having a
plurality
of through-holes, where the platen has opposing surfaces. The method includes:
a) dipping the platen into a liquid sample coinprising a sample to be loaded
into the
through-holes, thereby loading at least some of the through-holes with the
sample; and
b) contacting the platen with a liquid that has an affinity for the surfaces
of the platen but
is immiscible with the liquid sample, thereby cleaning the surface of the
platen of excess
sample mixture.
The invention also features a method of maintaining the viability of an
aerobic
organism in a platen having a plurality of through-holes. The method includes
the steps
of: a) loading the aerobic organism (e.g., a cell or an embryo) into at least
some of the
through-holes of the platen, and b) submerging the platen into a gas permeable
liquid.
The organism can be, for example, in a fluid such as a growtli medium, in
which case the
gas permeable liquid should be immiscible with the fluid. The method can also
include
assaying one or more physical properties of the aerobic organism.
The gas permeable liquid can be, for example, a fluorocarbon such as
perfluorodecalin, a silicone polymer, or a monolayer (e.g., a monolayer of a
lipid or high
molecular weight alcohol.
In another embodiment still, the invention features a method of mixing
volatile
samples with other samples (whether volatile or non-volatile). The method
include the
steps of: a) providing a platen having a plurality of through-holes; b)
optionally loading
some or all of the through-holes with one or more non-volatile samples (if
any);
c) loading at least some of the through-holes of the platen with one or more
volatile
samples to allow the samples in each through-hole to mix with other samples in
the same
through-hole; and d) submerging the platen in a liquid immiscible with the
volatile
samples, where steps b), c) and d) can be performed in any order. In preferred
embodiments, step d) is performed prior to introduction of volatile samples.
The samples
to be mixed can be initially provided in two separate platens that are
contacted while
submerged in said immiscible liquid to allow mixing. The immiscible liquid can
be, for
example, a fluorocarbon, a silicone polymer, mineral oil, or an alkane.
9
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The invention also features a method of mixing an array of samples. The method
entails: a) providing a platen having a plurality of through-holes, wherein at
least some of
the through holes are loaded with a first sample or set of samples; b)
providing a
substantially flat surface comprising an array of a second sample or set of
samples,
wherein the second sample or set of samples on the flat surface can be
registered (e.g.,
the second sample or set of samples can be arranged in a spatial pattern that
allows it to
line up with at least some of the through-holes of the platen) with the sample
in the
platen; c) registering the platen with the array of the second sample or set
of samples on
the flat surface; and d) contacting the platen with the flat surface, wherein
the sample in
the platen is aligned with the sample on the flat surface. This method can be
used, for
example, to avoid cross-contamination; also, registering and contacting can be
done
simultaneously. In some cases, either the first or second sample or set of
samples can
include one or more probes. The method can also include the further step of
analyzing a
physical property (such as fluorescence or other optical properties, pH, mass,
binding
affinity; e.g., using radiolabelled probes and film, chemiluminescence) of a
sample
contained in the platen. In some cases, the flat surface can also include a
hydrophobic
pattern matching the pattern of the platen array (e.g., to prevent cross-
contamination).
In another embodiment, the invention features a method for transferring a
reagent
or probe to a receptacle (e.g., into a bottle, a tube, another platen, a
microtiter plate, or a
can) from a specific through-hole of a platen comprising a plurality of
through-holes.
The method includes the steps of: a) placing the platen over the receptacle;
and
b) applying a burst of gas, liquid, solid, or a pin (e.g., a piston, a tube, a
post, a plug) to
the specific through-hole to transfer the reagent or probe into the
receptacle. The burst of
gas, liquid, or solid can be generated, for example, with a syringe, or by
depositing a
photodynamic or photothermal material (carbon black, plastic explosives, water
droplets)
in or above the through-hole, and then exposing the photodynamic or
photothermal
material to a laser beam of frequency and intensity suitable to activate the
photodynamic
or photothermal material.
In another embodiment, the invention features a device for filling or draining
through-holes in a platen having a plurality of through-holes. The device
includes: a) a
holder adapted to accept the platen; b) a nozzle having an aperture of a
suitable size to
inject a sample into a single through-hole in said platen; and c) a valve that
controls a
flow of a sample through said nozzle, wherein the holder and nozzle can move
with
respect to each other. The nozzle can be, for example, positioned so as to
contact the
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
platen (or not). The device can optionally include a microplate (e.g., a
microtiter plate)
positioned to receive samples from the platen, as well as a computer that can
control the
valve and control the positions of the holder and nozzle (and, optionally, the
microplate)
relative to one other. The optional microplate, the holder, and the nozzle
can, in some
cases, be moved independently of each other in at least two dimensions.
Alternatively,
the nozzle can be held in a single position while the holder and nozzle can be
moved
independently of each other in at least two dimensions.
In another embodiment, the invention features a method of analyzing the
kinetics
of one or more reactions occurring in at least one of the through holes of a
platen. The
method includes: a) providing a first platen having a plurality of through-
holes, wherein
the through-holes are loaded with a first sample or set of samples; b)
introducing the
platen into a detection device; c) introducing a second platen having a
plurality of
through-holes into the detection device, wherein the through holes are loaded
with sample
or reagent; d) registering and contacting the platens such that contents of
the through-
holes of said first platen can mix with contents of corresponding through-
holes of said
second platen; and e) detecting a change in a physical property of the
contents of at least
some of the through-holes over time.
In another embodiment, the invention features a method of analyzing a physical
property of a sample in an array. The method includes the steps of a)
providing a platen
having a plurality of through-holes, where the through-holes are loaded with a
sample;
b) placing the platen between two partially transmitting mirrors; c)
illuminating the
samples through one of the mirrors (e.g., with a laser, atomic lamp, or other
light source,
including white light sources); and d) detecting optical output from the
sample.
Optionally, mirrors that reflect at only one wavelength and transmit at all
others can be
used, and non-linear optical effects can also be observed. The "imaging" step
can
involve, for example, measuring light emanating from the array or measuring
light
emitted from the mirror opposite from the illumination source. The platen can
also be
placed within a laser cavity, and an optical gain medium can be positioned
between the
two mirrors.
The invention also features a method of ineasuring sample output from an
array.
The method includes the steps of: a) providing a platen having a plurality of
through-
holes, wherein the through-holes are loaded with sample; b) introducing the
sample into
an array of capillaries; c) eluting the samples through the capillaries using
pulse pressure,
creating a non-continuous flow; d) spotting the eluting samples onto a surface
that is
11
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
moving relative to the capillaries (e.g., a web, a tape, a belt, or a film),
wherein the spots
are discrete and no mixing of the samples occurs; and e) analyzing a physical
property of
the spots.
The invention also features a method of storing a plurality of saniples in an
assay-
ready, high-density format. The method includes the steps of a) providing a
platen having
a plurality of through-holes; b) loading the through-holes with the samples
(e.g., small
molecules) dissolved in a mixture comprising two solvents, a first solvent
having a low
vapor pressure (e.g., dimethyl sulfoxide (DMSO)) and a second solvent having a
higher
vapor pressure relative to the first solvent (e.g., ethanol; preferably, both
solvents are inert
and are able to dissolve the sample); and c) evaporating the second solvent to
result in a
plurality of samples in first solvent (preferably as films on the walls of the
through-holes).
The volume of the first solvent in each solution can be, for example, less
than about 25 nl
(e.g., less than 10 nl, 1 nl, 250 p1, 100 p1, or even less than about 25 pl;
e.g., a
"microdroplet"). In some embodiments, the sample dissolved in the first
solvent forms a
film on the wall of a through-hole.
The invention features a method of forming a high throughput assay. The method
includes: a) providing a platen having a plurality of through-holes, wherein
at least some
of the through-holes contain a sample dissolved in a solvent having a low
vapor pressure
(such as a array of samples prepared for storage according to the above
method);
b) cooling the platen to a temperature sufficient to freeze the dissolved
sample, c) dipping
the platen into a solution comprising a reagent, wherein the temperature of
the solution is
less than the freezing point of the sample, but greater than the freezing
point of the
reagent solution, d) removing the platen from the reagent solution, and e)
warming the
platen to a temperature greater than the freezing point of the sample. The
reagent solution
can be, for example, an aqueous solution.
Yet another embodiment of the invention features a filtration device, having
first
and second platens, each having a plurality of through-holes, and a semi-
permeable
membrane. The platens are aligned such that the through-holes of the first
platen are
substantially aligned with the through-holes of the second platen and the
membrane is
sandwiched in between the two platens. Optionally, the platens can have
hydrophobic
surfaces. The semi-permeable membrane can be, for example, a nitrocellulose
membrane, or can include a layer of cells.
In yet another aspect, the invention provides a cell chip containing first and
second platens, each having a plurality of through-holes, and a porous
membrane,
12
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
wherein the platens are aligned such that the through-holes of the first
platen are
substantially aligned with the through-holes of the second platen and the
membrane is
sandwiched in between the two platens. By "cell chip" is meant at least a
platen
containing a plurality of through-holes and containing in at least one through-
hole a cell
and culture media.
In another aspect, the invention provides a cell chip containing in order from
top
to bottom: (a) a coverslip in contact with a spacer; (b) a spacer that
separates the
covership from a first platen; (c) a first platen having a plurality of
through-holes; (d) a
gasket containing a plurality of through-holes that provides a seal between
the first platent
and the membrane; (e) a porous membrane containing aluminum oxide and having
pores
between 0.1 and 1 m sandwiched between the gasket and the second platen; (f)
a second
platent having a plurality of through holes; and (g) a solid support in
contact with the
second platen.
In yet another aspect, the invention provides a method of culturing a cell on
a cell
chip, the metllod involving providing a cell chip of any previous aspect
containing cell
culture medium; contacting the porous membrane with a cell; and incubating the
cell
under conditions suitable for cell survival. In one embodiment, the conditions
include
contacting the cell chip a gas permeable liquid (e.g., perfluorodecalin). In
yet another
aspect, the cell chip further comprises a hydrophobic fluid (e.g.,
perfluorodecalin, silicone
oil or mineral oil) in contact with the cell culture medium.
In yet another aspect, the invention provides a method of constructing a cell
chip
of any previous aspect, the method involving filling a first platen having a
plurality of
through-holes with cell culture medium; contacting the first platen with a
porous
membrane; and contacting the membrane with a second platen having a plurality
of
through-holes, such that the through-holes are substantially aligned, thereby
constructing
a cell chip. In one embodiment, the cell chip further contains a solid support
in contact
with the first platen. In another embodiment, the cell chip further comprises
a spacer in
contact with the second platen, wherein the spacer is in contact with a cover
slip. In yet
another embodiment, the cell chip further comprises a gasket sandwiched
between the
first platen. In still another embodiment, the gasket comprises a flexible
material (e.g., a
biocompatible elastomer, such as teflon, silicone, or rubber.
In yet another aspect, the invention provides a method for identifying an
agent
having a desired biological activity, the method including bontacting a cell
chip of any
previous aspect containing a cell with a platen containing an agent;
contacting the cell
13
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
with the agent; and detecting an alteration in the cell, thereby identifying
an agent having
a desired biological activity. In one embodiment, the agent is present in cell
growtli
medium, or is contacted with the cell using a slotted pin or syringe, or by
adding the cell
to a well containing the agent. In another embodiment, the agent is a
polypeptide, nucleic
acid molecule (e.g., siRNA, microRNA, or an aptamer), or small compound. In
another
embodiment, the alteration is an alteration in gene expression, polypeptide
expression,
cell growth, proliferation or survival, in the intracellular localization of a
cellular
component, morphological change, or change in motility. In yet another
embodiment, the
alteration is detected in an immunoassay, an enzymatic assay, highthroughput
gene
expression profiling, reverse transcriptase polymerase chain reaction (RT-
PCR),
quantitative PCR, real time PCR, methylation, or high content screening (HCS)
using
quantitative fluorescence microscopy and automated image acquisition. In yet
another
embodiment, the high content screening detects alterations in protein
translocation. In yet
another embodiment, the cell is lysed and the proteins or nucleic acid
molecules are
bound on a binding surface. In yet another embodiment, the binding surface is
a weak
catio'nic exchange medium. In another embodiment, the bound proteins or
nucleic acid
molecules are analysed for a characteristic selected from the group consisting
of
sequence, molecular weight, binding characteristic, and expression level. In
another
embodiment, the binding characteristic is detected in an immunoassay or by
polypeptide
binding.
In various embodiments of any of the above aspects, the membrane comprises
pores that are no more than half the through-hole diameter (e.g., pores of
between about
0.2 - 250 m) In various embodiments pores are about 0.2 m, 0.5 m, or 1.0 m
in
diameter. In other embodiments of any previous aspect, the membrane comprises
aluminum oxide or polycarbonate. In still other embodiments, the polycarbonate
comprises 1 m pores. In still other embodiments of any previous aspect, the
membrane
is coated with fibronectin, laminin, collagen, or another substrate that
supports cell
adhesion. In still other embodiments of any previous aspect, the platens
comprise metal
(e.g., gold, Tungsten or stainless steel), polystyrene, or a flexible material
(e.g., silicone,
rubber, teflon). In still other embodiments of any previous aspect, the chip
further
comprises a gasket that seals off individual wells, such as a removable
gasket. In still
other embodiments, the gasket contains a plurality of through-holes aligned
with those of
the platens. In still other embodiments of any previous aspect, the chip
further comprise a
hydrophobic compound that prevents lateral diffusion, such as a hydrophobic
compound
14
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
that provides a watertight seal between the membrane and the platen. In still
other
embodiments of any previous aspect, the platen comprises a flexible
biocompatible
material and the other platen is a rigid platen that supports the flexible
platen. In various
embodiments, the flexible platen comprises silicone, polypropylene, or rubber.
In still
other embodiments of any previous aspect, the two platens are attached by
raised surfaces
on one platen that fit into a recessed surface on the other platen. In still
other
embodiments of any previous aspect, the total thickness of the cell chip is
less than about
mm, 5 mm, or 1 mm. In still other embodiments of any previous aspect, a solid
support (e.g., a microscope slide) in contact with the first platen. In
another embodiment,
10 the chip further containing a coverslip in contact with the second platen.
In still other
embodiments, the coverslip, microscope slide, and cell chip are secured
together. In still
other embodiments of any previous aspect, the filling of the chip with media
is
accomplished by placing the first platen on a solid support and centrifuging
the platen and
solid support. In one example, the first platen is contacted with a gasket and
cell medium
is then overlayed on the platen.
A "spatially addressable through-hole" has a position and dimensions that are
known to a high degree of certainty (e.g., relative to a reference position on
the device).
The degree of certainty is sufficient that the through-holes of two platens
placed one on
top of the other can align, allowing reagents to transfer in a parallel
fashion. The degree
of certainty is also sufficient such that a sample in any given through-hole
can be
retrieved by a robotic device that knows only the position in which that hole
should be
found relative to a reference point on the device. The term "planar array of
through-
holes" refers to an array of through-holes on a platen such as that described
in PCT
application W099/34920.
A "reagent" is a chemical compound, a gas, a liquid, a solid, a powder, a
solution,
a gel, a bead, or electromagnetic radiation.
The term "probe" or "chemical probe" refers to a chemical, biological,
mechanical, or electronic structure that detects a specific analyte by a
specific binding or
catalytic event. The binding or catalytic event can be transduced into a
signal readable by
an operator. One type of chemical probe is an affinity probe (e.g., a specific
nucleic acid
that binds to another nucleic acid). Examples of mechanical probes include a
cantilever
that has a ligand immobilized on its surface and a material whose properties
(e.g., strain,
inertia, surface tension) change in response to a chemical or biological
event.
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The term "chemical detection event" refers to a chemical reaction between
molecule(s) of interest and probe molecule(s) that in turn produces a signal
that can be
observed by an operator. For example, the hydrolysis of fluorescein di-(3-
galactoside by
the enzyme 0-galactosidase, to produce the fluorescent molecule fluorescein,
is a
chemical detection event. In some cases, the chemical detection event can
involve a
series of chemical reactions triggered by an initial interaction of analyte
and probe (e.g.,
activation of a signal transduction pathway in a probe cell by the binding of
a ligand to a
surface receptor).
The term "linker molecule" means a molecule that has a high affinity for or
covalently links to the surface of a platen or bead. The linker molecule can
have a spacer
segment such as a carbon chain, and can also have a functional group at its
end to enable
attachment of probe molecules covalently or with high affinity.
The term' "immobilized" means substantially attached at the molecular level
(i.e.,
through a covalent or non-covalent bond or interaction).
The term "photocleavable compound" refers to a compound that contains a moiety
that, when exposed to light, dissociates into multiple independent molecules.
The term "small molecule" refers to a molecule having a mass less than about
3000 daltons.
The term "hybridization" refers to complementary, specific binding of two or
more molecules (e.g., nucleic acids) to one another.
"Solid phase synthesis" refers to a chemical synthesis process in which at
least
one of the starting materials in the synthesis reaction is attached to a solid
material such
as a polymer bead, a gelatinous resin, a porous solid, or a planar surface.
The term "blotter" refers to a material capable of capturing excess liquids by
absorption.
The term "bead" means a small particle, generally less than about 1 mm (e.g.,
less
than about 100 m) in any dimension, with the ability to have reagents
attached to its
surface or stored in its interior. A bead can be made from one or more of a
variety of
materials, including organic polymers, glass, and metals. The reagent is
typically
attached to the bead by chemical reaction with a reactive functional group
such as a
carboxyl, silanol, or amino group on its surface. Reagents can, for example,
be confined
to the bead by covalent chemical attachment or by physical adsorption to the
bead
surface. The bead shape can be nearly spherical, irregularly shaped, or of an
intermediate
shape.
16
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The term "stringency" refers to the degree to which non-specific molecular
interactions are disrupted during a washing step.
The term "electrophoretic washing" refers to the removal of non-specifically
bound, ionic molecules from a probe by applying an electric field.
"Specific interactions" are interactions between two molecules resulting from
a
unique three-dimensional structure of at least one of the molecules involved.
For
example, enzymes have specific interactions with transition state analogues
due to their
evolution toward stabilizing reaction intermediates.
The term "micro-plate" refers to a collection plate used to transfer the
contents of
the through-holes of an array, where no cross contamination of the through-
holes occurs
in the transfer.
The term "micro-droplet" means a drop of liquid having a volume of 50 nl or
less
(e.g., less than about 50 nl, 25 nl, 10 nl, 5 nl, 1 nl, 500 pl, 250 pl, 100
pl, 50 pl, or less).
The term "physical properties" means any measurable property of an object or
system, including electrical, magnetic, optical, thermal, mechanical,
biological, nuclear,
and chemical properties.
The new methods have numerous advantages. For example, the new methods
allow optimization of processes in a parallel manner. For instance, synthesis
of a
particular molecular species often requires tedious quantitative investigation
of different
synthetic methods with a view towards optimizing product yield. Using the new
methods, process parameters can be varied on a through-hole-by-through-hole
basis in the
array, and the product analyzed to determine the protocol best suited for high
yield
synthesis.
Another advantage of the invention over conventional arrays of chemical probes
on a planar substrate is that each chemical detection event takes place in a
physically
isolated container (i.e., the through-hole), allowing amplification of the
signal by catalysis
(e.g., releasing detectable molecules into the solution contained in each
through-hole).
Such detectable molecules include, for example, fluorescent products of a
fluorogenic
enzyme substrate, and chromogenic products of a chromogenic substrate.
Physical
isolation of samples retained in the array also prevents cross-contamination
by
eliminating lateral communication between the through-holes.
Another advantage of the invention is that each through-hole can have a
precise
and known spatial location in the array. Each through-hole is then spatially
addressable,
thereby facilitating the insertion and removal of liquids from each through-
hole, the
17
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
analysis of the contents of each through-hole, and the alignment of multiple
arrays for
highly parallel transfer of reagents.
Another advantage of the invention is that the relative volumes of the members
of
two arrays can be easily adjusted by changing the depth of one array with
respect to the
other.
Still another advantage of the invention is that substances that bind to
chemical
probes contained in the through-hole array can easily be recovered as distinct
samples for
further analysis. For example, the bound contents of the well can be eluted
onto a planar
substrate for analysis by matrix-assisted laser desorption and ionization
(MALDI) or
surface-enhanced laser desorption and ionization (SELDI) mass spectrometry, or
nuclear
magnetic resonance (NMR) spectroscopy. Alternatively, the contents of the
through-hole
can be electrosprayed directly from the through-hole into a mass spectrometer.
The
contents of the through-hole can also be crystallized and analyzed with x-ray
or electron
diffraction techniques (e.g., to determine crystal structure). This aspect of
the invention
allows for sensitive detection of unlabelled analytes.
Yet another advantage of the invention is that the samples can be introduced
or
removed from the platen by electrophoresis, as the through-holes can allow for
conduction of an electric field.
Another advantage of the invention is that samples are accessible from both
sides
of the platens. This means, for example, that samples can be removed from the
platens by
applying pressure, an air or gas stream, or an explosive charge to a through-
hole of
interest and then collecting the material from the opposing face of the
platen. Alternately,
samples can be sucked out of the platen without creating a vacuum. Thus, the
volume of
the samples in not limited by the current state-of-the-art microfluidics
techniques, and a
minimum quantity of fluid is lost upon the collection of the sample. A
pressure can be
applied, for example, in the form of a solid pin (acting, e.g., as a piston),
or in the form of
a burst of inert gas. Another implication of this advantage is that it is
relatively easy to
perform electrospray ionization mass spectrometry directly from the platen.
Simultaneous measurement of luminescence from two spectrally distinct
luminescent
probes located in the microchannel array can be performed in either a trans-
or epi-
illuinination optical configuration, including, for example, a light source,
an optical filter,
and a CCD camera. Optical signals can be collected from both sides of the
platen
simultaneously.
18
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The numerous samples contained in the platen can be rapidly transferred to a
flat
surface or membrane, facilitating processes such as SELDI mass spectrometric
analysis
and growth of bacterial cells (e.g., cells contained in the through-holes), to
form
individual colonies for storage and further analysis. Transfer from a planar
material to
the array can also be accomplished, as in electroblotting from a
polyacrylamide 2-D
protein gel into the array.
Advantageously, the surface area of the liquid exposed to the environment is
minimized by the high aspect ratio geometry, thus limiting evaporation.
Still another advantage of the new methods is that the sample contained in a
given
through-hole constitutes a small thermal mass and can, therefore, reach
thermal
equilibrium quickly and uniformly. The fact is relevant, for example, to
synthetic
methods that involve heating and/or cooling steps (e.g., replication of
nucleic acids using
the polymerase chain reaction, PCR).
Unless otherwise defined, all technical and scientific terms used herein have
the
sanie meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. Although methods and materials similar or equivalent to
those
described herein can be used in the practice or testing of the present
invention, suitable
methods and materials are described below. All publications, patent
applications, patents,
and other references mentioned herein are incorporated by reference in their
entirety. In
case of conflict, the present specification, including definitions, will
control. In addition,
the materials, methods, and examples are illustrative only and not intended to
be limiting.
Other features and advantages of the invention will be apparent from the
following detailed description, and from the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is an exploded top corner view of a platen with top and bottom masks.
FIG. 2 is a cross-sectional view of an interlocking array system.
FIG. 3 is an illustration of a method for transferring small volume drops into
specific through-holes in an array with a pin array.
FIG. 4 is an illustration of a method for producing a mask by use of UV
curable
epoxy.
FIG. 5 is an illustration of a method for producing a mask by use of an array
of
pins having a precision fit into a matching array of through-holes.
19
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
FIG. 6 is an illustration of a method for storing liquid from individual wells
from a
microtiter plate in a bundle of capillary tubing for transference into high-
density through-
hole arrays.
FIG. 7 is an illustration of a method for transferring fluid from wells in a
microtiter plate to through-holes in an array using a flexible member.
FIG. 8 is a drawing of an array in which the through-hole cross-sections are
shaped to hold only one microsphere per through-hole, and the longitudinal
cross-section
is tapered such that the microsphere sits in the hole either at or below the
array surface.
FIG. 9 is an illustration of a method for transfer with a single sampling
device
with a fast sequential positioning of a mechanical plunger over the through-
holes to be
sampled and pushing the plunger through the hole to transfer the hole's
contents to the
well of a microtiter plate located at a small distance below the through-hole
array.
FIG. 10 is an illustration of a method for transferring materials from a
through-
hole in a through-hole array into a well of a microtiter plate using a gas jet
generated by
spatially localized heating with a focused laser beam.
FIG. 11 is an illustration of the transfer of material from a through-hole in
the
array to a well in a microtiter plate with a gas jet caused by localized
ignition of an
explosive charge randomly distributed in a thin sheet overlaid on one surface
of the
through-hole array.
FIG. 12 is an illustration of a sheet with an explosive charge pattern
matching the
through-hole positions in the array.
FIG. 13 is an illustration of a method to interface massively parallel HPLC
separation with inherently serial analytical methods such as mass
spectrometry.
FIG. 14 is an illustration of a chromatographic device.
FIG. 15 is an illustration of a linear MALDI-TOF mass spectrometer.
FIG. 16 is an illustration of an array of chamfered through-holes machined in
a
block of material, a syringe bank with the same center-to-center spacing as
the through-
hole array, wherein the syringe needles pass through a metal block that is
attached to the
syringe bank holder by pneumatically-actuated, spring-loaded pins.
FIG. 17 is an illustration of the array of FIG. 16, wherein the capillary
channels
are pressurized by the syringes.
FIG. 18 is an illustration of an array similar to that of FIG. 17, with the
exception
that the syringe bank is bolted to the capillary tube array.
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
FIG. 19 is an illustration of an array similar to that of FIG. 18, with the
exception
that the syringe bank is bolted to the capillary tube array.
FIG. 20 is an illustration of a method of manufacturing a platen having a
plurality
of through-holes and two opposing surfaces, by bonding together multiple
grooved
surfaces.
FIG. 21 depicts an array positioned inside an optical resonator featuring a
source
of illumination and two partially reflective surfaces.
FIG. 22 depicts a device for removal of the contents of a through-hole array
and
transfer of those contents. The device has a nozzle, a stage for holding the
through-hole
array and a stage for holding a capture chamber. Movement in two dimensions of
the
nozzle or the through-hole array can be achieved.
FIG. 23 depicts a method for wiping excess fluids from the surface of the
platen.
The device has enables a through-hole array to be loaded with sample and
removed
through a wiping fluid in an efficient manner.
FIG. 24 depicts a device for aligning platens having a plurality of through-
holes
inside a detection device, wherein the platens are held in place through
device coinprising
two pins attached to a flat base.
FIG. 25 shows a flow chart of a cell-chip microarray. A platen of rigid
material
such as metal or polystyrene with pores of 50 - 200 m in diameter is attached
to a
porous membrane forming an array of microwells with a porous bottom. Cells are
added
and allowed to adhere to the membrane before test compound is added with a
microspotting pin. After incubation, the membrane may be processed for
analysis of
protein or mRNA expression, or any assay or assay component of interest.
FIG. 26 shows polycarbonate membranes, having 1.0 m pore size, soaked in 100
ug/mLl fibronectin and air dried for 2 hours. The membrane was attached to the
bottom
of a Petri dish by the addition of 10% agarose at the edges. PKC(3-GFP
transfected 293
cells were added at a concentration of 5 x 105 /mL and incubated 37 C, 5%
COa. Images
were acquired by confocal microscopy.
FIG. 27 shows polycarbonate membranes, 1.0 ,um pore size, soaked in 100 ug/mL
fibronectin and air dried for 2 hours. The membrane was attached to the bottom
of a Petri
dish by the addition of 10% agarose at the edges. PKC(3-GFP transfected 293
cells were
added at a concentration of 5 x 105 /mL and incubated 37 C, 5% CO2. Then, 1
uM
phorbol-l2-myristate-13-acetate (PMA) was added. After 1 hour at 37 C, 5%
CO2,
images were acquired by confocal microscopy.
21
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
FIG. 28 shows attachment of cells to the membrane in the cell-chip device
comprised of gold platen and porous membrane assembled as demonstrated in
Figure 1.
FIG. 29 shows microspotting on gold platen. A 300 mesh gold platen (50 uma
holes) was wetted with medium. Fluorescein in 10% DMSO/PBS was spotted on
platen
using a FP9 floating pin (VP-scientific.) Spots were examined under a confocal
microscope at 5x magnification.
FIG. 30 shows cell uptake of Hoechst stain. PKC(3-GFP cells were incubated
overnight in 300 mesh gold platen (50 um2 holes) and washed with medium.
Hoechst
stain was then spotted on the platen that was dried by blotting.
FIG. 31 shows microspotting on a gold platen. A 300 mesh gold platen (50 um2
holes) was wetted with medium or briefly dried by blotting. A drop of mineral
oil was
added to the platen before adding Fluorescein in 10% DMSO/PBS using a FP9
floating
pin. Spots were examined under a confocal microscope at 5x magnification.
FIG. 32 shows cell uptake of Hoechst stain by PKC(3-GFP cells that were
incubated overnight in a stainless steel cell-chip and then washed with
medium. Hoechst
was spotted on a first platen that was dried by blotting. The stainless steel
platen
(National Jet Company, LaVale, M.D.) tested was a 1-inch square and 400- m
thick with
pores 150 m in diameter. A second platen was attached using adhesive sealing
film with
the center cut out. The chip was placed in a cytospin sample chamber with the
funnel
removed with a small gasket between the platen and the top of the chamber.
Fig. 33 shows an anopore membrane sandwiched between two stainless steel
platens (as shown in Figure 8). PKC(3-GFP cells were added and incubated
overnight.
Note unequal distribution of cells in the well, possibly due to leaks of media
between
platen and membrane
Fig. 34 shows a cell microarray prototype based on a 200-,um thick Tungsten
platen. The platens (National Jet Company, LaVale, M.D.) had pores of 300 m
in
diameter and were attached with 4 screws.
Fig. 35 shows cell culture on an anopore membrane sandwiched between two
tungsten platens (as shown in Figure 34). PKC(3-GFP cells were added and
incubated 48
hrs. Note more random cell distribution with more secure attachment; however,
the
platen is not yet optimized for cell attachment.
Fig. 36 shows an open array-based cell chip and delivery of C12-resazurin to a
single well on the array using a floating pin.
22
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Fig. 37 shows an open array-based cell chip with PKC(3-GFP transfected cells,
added at 5 x 105 /mL and incubated 37 C, 5% COZ. Images were acquired by
confocal
microscopy.
Fig. 38 shows essentially that which is depicted in Fig 12, except here the
platen is
not shown.
Fig. 39 shows one particular embodiment of the invention - specifically, an
apparatus for in vitro and ex-vivo analysis. A platen of rigid material such
as metal or
polystyrene with pores of 10 - 300 m in diameter is attached to a porous
membrane or
modified glass surface forming an array of microwells with a porous bottom.
Cells are
added and allowed to adhere to the membrane overnight before test compound is
added
with a micro spotting pin. After incubation, high content image analysis is
performed.
Membranes are processed for analysis of protein or mRNA expression, or other
assay or
assay component of interest.
Fig. 40 is essentially that which is depicted in Figure 15, but here shows the
cross-
section of an individual well.
Fig. 41 shows structural enhancements to increase pressure on the member
and/or
gaskets to seal off individual wells.
DETAILED DESCRIPTION OF THE INVENTION
The invention provides methods of creating, storing, and screening diverse
chemical and biological compositions, each contained in a through-hole that
traverses a
platen, as well as methods for making and using platens, particularly platens
containing
cells. In certain embodiments, the methods include transmitting reagents to a
selected
group of holes in a dense array of through-holes. Additional rounds of reagent
transmission are provided as needed. The invention also provides for placing a
series of
masks over a planar array of through-holes and flowing reagents through the
masks to
build a defined pattern of probes or reagents such that the contents of each
through-hole
can be known. In an alternate embodiment, the invention provides distributing
probe-
holding particles, such as beads or cells, into the array of through-holes.
Such probes
include, but are not limited to, nucleic acids, peptides, small molecules, and
chemical
sensing cells. Uses of the arrays include screening of genetic libraries,
producing and
screening compound libraries for discovery of pharmaceutical leads,
optimization of
reaction conditions, gene expression analysis, clinical diagnostics, genomics,
functional
genomics, pharmacogenomics, structural genomics, proteomics, production and
23
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
optimization of industrial catalysts, chemical genetics, identification of
suitable
conditions for reactions (e.g., conditions suitable for protein
crystallization), genotyping,
polymorphism analysis, examination of RNA expression profiles in cells or
tissues,
sequencing by hybridization, and recombinant enzyme discovery.
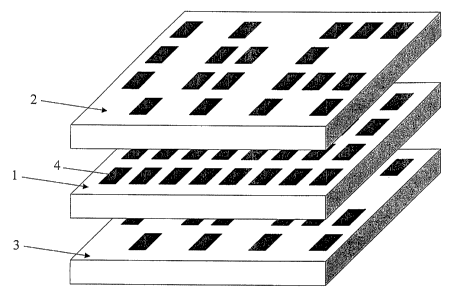
A platen having a high-density array of through-holes in accordance with one
embodiment of the present invention is illustrated in FIGS. 1(top view) and 2
(cross-
sectional side view). The platen can be made of silicon or other rigid
materials, such as
metal, glass, or plastic. The platen material can be chemically inert, or can
be rendered so
by appropriate surface treatments.
Referring to FIG. 1, each through-hole has a square cross-section, although
circular or rectangular cross-sections can alternatively be used. The diameter
of each
through-hole is less than 1 mm (e.g., less than about 600 m, 300 m, 100 m,
10 m, 1
m, or 100 nm), typically 200 - 250 m, and the depth of the platen can be 10-
2000 ,um
or more, generally about 250-1000 .m.
Greater depths can be achieved using a bundle of glass capillaries.
Alternatively,
platens having greater depths can achieved by bonding together multiple
surfaces having
parallel grooves, creating a long three dimensional object having through-
holes running
throughout the length of the object. This object can be subsequently sliced
horizontally,
allowing flexibility in the depth of the through-holes. The result is the
ability to use
arrays with compatible positions of through-holes, wherein the depths of the
through-
holes can vary from array to array.
For spatial addressability, center-to-center spacing of through-holes should
be
fairly precise. Hole-to-hole spacing depends on the dimension of the through-
holes
within the platen. The though-holes can be arranged in regular rows and
columns,
hexagonal arrays, or other configurations (e.g., groupings of through-holes
into smaller
sub-arrays). Multiple platens can be fabricated with the same arrangement of
through-
holes so that the pattern is reproducible, and each through-hole can be
identified by its
own address within the array.
When three platens having through-holes are stacked, the total volume of a
single
channel (i.e., three through-holes stacked) is typically - 100 nl. Using this
volume as an
example, if the entire channel were filled from a dense yeast cell culture (-
107/ml), each
channel would thus contain approximately 103 yeast cells. Based on a yeast
cell volume
of 70 m3, the maximum number of cells per 100 nl channel is on the order of
106. A
minimum of 100 cells per microchannel can be adequate to compensate for cell-
to-cell
24
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
variability of yeast cell response to the bioassays. However, this volume can
vary
depending not only on the diameter of the through-holes, but on the depth of
the through-
holes. This ability to vary the volume of samples allows flexibility, enabling
the use of a
wider variety of materials, concentrations, and reaction conditions.
Optional features such as binary identification codes, or holes and grooves
for
indexing and alignment, can also be incorporated into each platen.
1. Methods of making devices having arrays of through-holes.
Fabrication of an array of through-holes by casting in resin.
Conventional technologies for manufacturing high-density through-hole arrays
include micro-machining, electrospark discharge machining (EDM), or chemical
etching.
Alternatively, the arrays can be cast in a polymer or resin. A casting mold
can be
designed such that the inner diameter of the mold will be equal to or larger
than the final
outer diameter of the array device. The depth of the casting mold can be as
little as 0.5
mm for a single array, or 1 meter or longer. In the case where a long block of
resin is
cast, the resin can be cross-sectioned into slices of desired thickness and
the surfaces can
be polished or smoothed. The through-holes can be defined in the cast by
several
methods. Solid wire, fiber, or an array of pins of the desired geometry and
diameter can
be arranged within the casting mold. If necessary, the wire, fiber or pins can
be
immobilized in place with the use of one or more positioning jigs within the
casting mold.
The chemistries of the wire, fiber, or pins must be chosen such that they will
not form a
permanent bond with the resin or polymer as it solidifies, so that they can be
pulled out to
produce the through-holes. For example, the fibers may be
ethyleneterephthalate and the
resin is polymethylmethacrylate (PMMA). Alternatively, the surfaces of the
wire, fiber,
or pins can be coated with a release agent such as an oil, a fluoropolymer,
water, or a,
polymerization inhibitor that will facilitate the removal of the wire, fiber,
or pins from the
cast resin or polymer once the final curing, setting, or polymerization is
complete.
In an alternate system, the through-holes can be defined by positioning an
array of
hollow tubing or capillaries within the casing mold. The hollow tubes can be
immobilized within the casting mold in a positioning jig. Use of tubing of
different
internal diameter results in an array with through-holes of different
diameters. The
chemistry of the hollow tubes and polymer will ideally be chosen such that a
permanent
bond will form between the outside hollow tube and the resin or polymer that
is cast. The
inner surface of the hollow tubes will then make up the through-holes of the
array. The
hollow tubes can be made of glass or fused silica, a polymer, or a metal.
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The chemistry of the resin or polymer that is cast can be selected such that
the
surface of the array device is of a desired hydrophobic or hydrophilic
character. The
chemistry of casting resins, such as acrylate or polystyrene, can be modified
with
hydrophobic groups to result in an array with the desired surface chemistry.
Alternatively, the surface chemistry of the array device can be modified with
standard
techniques after slicing and polishing. In addition, the chemical or physical
properties of
the polymer can be modified by the addition of other materials. For example,
to control
the electrical conductivity of the device, particles of a conductive metal can
be mixed into
the resin or polymer prior to casting the mold to confer conductivity to the
device.
Generally, the more metal particles that are mixed with the resin or polymer
prior to
casting, the greater the conductivity of the devices will be.
Additives to the resin or polymer can be used to improve the sensitivity of
optical
imaging of the array. For example, metal particles can be added to make the
material
between the through-holes. The metal particles enable light to scatter,
causing a
fluorescent signal generated by a probe in a through-hole to reflect toward
the detector
and to prevent cross-talk of signals. Alternatively, carbon black may be added
to make
the material, preventing cross-talk and minimizing signal from light scattered
off the
surface of the array. More preferably, a combination of a light scattering
agent such as
titanium dioxide and a light absorbing agent, such as carbon black are added
to the resin
or polymer to achieve maximum optical density between the holes.
Using hollow tubes in the casting mold to form through-holes allows the
chemical
properties of the tubes to be varied according to the needs of the
application. Tubes
manufactured from a biologically inert polymer (e.g., polyetheretherketone
(PEEK) or
poly(tetrafluoroethylene) (PTFE)) are desirable for some applications.
Alternatively,
fused silica tubing can be used to form the through-holes. The interior
surface of the
fused silica can be derivatized prior to casting, allowing for virtually any
level of desired
hydrophilicity or hydrophobicity. A metal or alloy tubing can also be used to
form the
through-holes of the array. Metal tubing can be coated to make it
biocompatible. For
example, metals can be coated with thin layers of gold and the gold surfaces
can be
readily coated with a variety of reagents possessing thiol moieties.
The inner surfaces of the tubes or capillaries can, for example, be coated
with
materials that facilitate the use of the resulting slices as a probe array.
For instance, each
tube or capillary can be coated with a different nucleic acid probe, so that
when the block
26
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
of resin is sliced, the resulting platens can be used as genosensors.
Alternatively, the
probes can be immobilized on a porous material contained in the capillaries.
Once a block is cast from the desired resin or polymer, it can be sliced to an
appropriate thiclcness to form a platen having an array of through-holes. In
certain
embodiments, the thickness of the platen ranges from 0.2 to 25.0 mm. However,
using
this technique, arrays of through-holes can be manufactured having the same
length as the
casting mold that is used. If desired, casts that are many meters in length
can be prepared.
Standard techniques for producing silicon wafers by slicing and polishing
silicon ingots in
the semiconductor industry can be directly applied to the manufacture of an
array of
through-holes. Flowing a coolant through the through-holes in the cast array
while
slicing can prevent heat buildup that could otherwise melt the polymer or
degrade
coatings or probes inside the holes. Examples of coolants include cold water,
cold
aqueous ethylene glycol, and cold isopropanol.
If solid wires are arranged in the cast and the resulting block is then
sliced, the
wires can be eroded by electrodeposition onto a plate in an electrochemical
cell or by
chemical degradation such as by placing the slice in concentrated nitric acid
to give an
array of through-holes. The slices can be bonded to a metal sheet, the polymer
eroded,
and the slices used as an electrode for production of through-hole arrays by
sink-EDM.
The polymer can be eroded by chemical means, melted, or burned off.
Method of making an array by stacking and bonding multiple grooved surfaces.
One method of manufacturing an array of through-holes is to stack and bond
together grooved plates having upper and lower surfaces, creating a three-
dimensional
array. Any number of materials can be used to manufacture the array of through-
holes
including, but not limited to, silicon, glass, plastics, resins, or metals.
Depending on the
material used, grooves can be machined into the individual surfaces using a
variety of
techniques (for example, micro-machining, chemical etching, embossing, or
stamping).
The depth and width of each groove determines the dimensions of the through-
holes in
the completed platens and can be machined according to the desired
specifications.
A precise layering or stacking of the grooved plates into a three-dimensional
array
can be accomplished with the use of an external or internal jig into which
each surface is
precisely placed. Alternatively, registration devices, (for example notches,
posts,
tongues, etc.) can be precisely integrated into each surface to facilitate
accurate stacking.
After stacking the individual grooved surfaces, they are bonded together in a
permanent
manner. The use of traditional adhesives to bond the plates together is a
disfavored
27
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
approach because excess adhesive can migrate into the grooves and result in
the blockage
of some of the through-holes. A preferred method for the bonding process is
the use of a
combination of elevated temperature and pressure, resulting in a fusion of the
chosen
materials. In some cases, one or both surfaces of the grooved platens are
coated with a
material (for example, gold) that, upon the application of an appropriate
amount of
temperature and pressure, diffuses into the surfaces and results in a
permanent bond. The
grooved plates can be made from materials that include a thermoplastic, a
ceramic, a glass
or a metal such as silicon.
In a preferred embodiment, the width of each of the grooved plates is
equivalent
to the width of the final platen of through-holes, but the length of each
grooved plate is
much larger than the desired thickness of the final platen of through-holes.
This results in
a three-dimensional array of through-holes that is much thicker than required.
Individual
platens of through-holes can then be precisely cut from this thick block to
the desired
specification. The platens can further require polishing after they have been
cut in order
to yield an optically flat surface. The required surface cheniistries are then
be applied to
the platens. An advantage of this method of manufacturing is the creation of
platens with
straight-walled through-holes that are niuch deeper than those made using
traditional
micro-machining technology.
Minor misalignments in the stacking of the individual grooved plates can
result in
small imperfections in the registration of the through-holes in the final
platens. This can
interfere with operations using the platens of through-holes (for example,
stacking of two
or more platens to initiate massively parallel reaction). However, even if
minor errors in
positional registration exist, adjacent slices cut from each thick array of
through-holes
will be a near-perfect match and the required stacking operations can be
accomplished.
Formation of a Silicon Oxide Layer.
In one embodiment, a dense array of through-holes is produced in a silicon
wafer.
A silicon oxide layer is created uniformly on all surfaces of the array by
heating the
silicon array in a furnace to a temperature high enough to cause oxidation.
Oxygen
and/or humidity levels in the furnace can be raised above the ambient to speed
oxidation
process (see, e.g., Atalla et al., The Bell Systena Technical.Iournal, pp. 749-
783, May
1959). The silicon oxide layer is advantageous because it enables the
application of
various chemical surface treatments to the array surfaces. Examples of surface
treatments
are found in Irnmobilized Affinity Ligand Techniques (Hermanson et al,
Academic Press,
San Diego, 1992) and technical literature available from United Chemical
Technologies,
28
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Inc., Bristol, PA. Use of silicon oxide provides and additional advantage,
allowing the
optical reflectivity of the surfaces to be controlled by adjusting the
thiclcness of the silicon
oxide film (see, e.g., Priraciples of Optics, M. Born & E. Wolf, Pergamon
Press, 1980,
pages 59-66).
II. Methods of modifying the surfaces of array devices and walls of through-
holes.
The surfaces of the through-hole arrays can be modified in various ways (e.g.,
to
change their physical and chemical properties). Types of surface modification
can
include, but are not limited to, the application of polymer coatings,
deposition of metals,
chemical derivitization, and mechanical polishing.
Selectively Modifying the Surface Chemistry of Array Faces: In order to
prevent
aqueous solutions from adhering to array surfaces and cross-communication
between the
various through-holes during loading and other manipulations, it is desirable
to coat the
surfaces of the platen with a hydrophobic coating. It is also desirable to
coat the inner
surfaces of the through-holes with a hydrophilic coating so that they retain
fluids. The
inner coating can further be blocked, preventing non-specific binding, or
derivatized with
affinity ligands. This combination of hydrophobic surfaces and hydrophilic
through-
holes prevents aqueous solutions from adhering to the surfaces of the array
while
allowing instantaneous loading of the through-holes.
Generally, a platen having a dense array of through-holes is produced in
silicon
and coated in silicon oxide by oxidation. The platen is then cleaned, removing
organic
materials, by soaking in a mixture of hydrogen peroxide and sulfuric acid, or
other caustic
solvent cleaning solution. This treatment results in clean silicon oxide with
a high surface
energy. Hydrophobic coatings produced using this method are stable under high
humidity and they can be used repeatedly.
The top and bottom faces of the arrays are made hydrophobic through exposure
to
vapor from a solution containing an appropriate silanizing agent (such as
polydimethylsiloxane sold as Glassclad 216 by United Chemical Technologies,
Inc.) in a
volatile solvent. The silanizing agent reacts with the hydroxyl and silanol
groups on the
array surface and creates covalent bonds to hydrophobic alkyl groups.
Selective
modification of the surface chemistry can be achieved by selection of
alternative
silanizing agents.
29
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
In one surface modification method, a positive pressure of inert gas is
applied to
the surface of the platen opposite the surface being treated. The positive
pressure within
the through-holes prevents the silanizing vapor from reaching the interior
through-holes.
Silanizing agents suitable for rendering glassy surfaces hydrophobic include
alkyltrichlorosilanes and alkyltrimethoxysilanes, many of which are
commercially
available.
In another method, a first platen is made from silicon, and then oxidized. A
second silicon platen is produced with identical size and alignment holes, but
without
through-holes. The tlirough-hole array platen is placed on top of the solid
platen on the
stacking jig such that each through-hole becomes a well. The jig and plates
are then
treated with a surface-modifying chemical reagent. The air trapped at the
bottom of the
wells prevents the fluid from entering the wells. After sufficient time for
the reaction to
occur, the plate is removed from solution, dried, and heat-treated if
appropriate. The
backing is removed, the array is flipped over, and the process repeated,
coating the
second surface.
Yet another method for creating hydrophobic exterior surfaces is to place the
array
on a matching array of pins such that each single pin narrowly passes through
its
corresponding through-hole. This array of pins can be the electrode used to
create the
array of through-holes using sink EDM. Next a coating of a hydrophobic polymer
such
as polypropylene or Teflon is deposited on the exposed surfaces using gas
phase
deposition method such as evaporative vapor deposition. The array of through-
holes is
removed from the matching pin array, inverted, and the coating process is
repeated to
cover the opposite surface.
Another method for producing hydrophobic coatings on the platen surfaces
involves coating platen surfaces with a metal such as gold (then exposing the
arrays to a
chemical that selectively reacts with the metal, but not with the uncoated
through-hole
surfaces. For example electron beam vapor deposition can be used to coat the
outer
surfaces of a platen containing a plurality of through-holes with gold, other
metal or semi-
conductor. Electron beam vapor deposition will preferentially deposit the gold
on surfaces
normal to the beam direction. The gold-coated surface can then react with
alkane thiols to
attach a hydrophobic alkyl groups (Z. Hou et al., Langmuir, 14:3287-3297,
1998). The
inner surfaces of the through-holes that are not coated with gold will remain
hydrophilic.
Alkane thiols are also reactive towards other materials including silver,
copper and
gallium arsenide (Y Xia and G.M. Whitesides, Annu. Rev. Mater. Sci., 28: 153-
84, 1998).
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Amphiphiles besides alkane thiols can be chemically reacted to other inorganic
solid
materials to produce hydrophobic coatings. Examples of such coatings include,
but are
not limited to, allcanephosphates on metal oxides (D. Brovelli et al.,
Langmuir, 15:4324-
4327, 1999 and R. Hofer et al., Langmuir, 17: 4014-4020, 2001), and alkane
carboxylates on alumina (P.E. Laibinis et al., Science, 245: 845, 1989).
Selectively Modifing the Surface Chemistry of Through-Hole Surfaces.
In one method, multiple, identical through-hole arrays are prepared, aligned,
and
stacked. A chemical reagent is passed through the continuous channels formed
by the
stacked arrays. The reagent can be a solution, a suspension, a liquid, a
vapor, or fine
powder. The stack of arrays is then washed, dried, and heated, as appropriate
for the
particular coating. The stack of chips is then physically separated from one
another and
the arrays on the top and bottom of the stack ("sacrificial arrays") are
discarded.
Another method for selectively coating through-hole surfaces involves using a
robot to position a fine needle or an array of fine needles proximal to the
entrance of each
through-hole. Chemical surface-modifying reagents can then be delivered
through this
needle/capillary directly into individual holes
In another method, all surfaces of a silicon through-hole array are chemically
modified. The array faces are then mechanically polished to restore the
original surface
character. The array faces can then be coated again.
In still another method, the array faces are coated with a material that is
inert
towards the desired surface-modifying chemicals, and then the entire array is
exposed to
it. For example, a gold coating can be applied to both faces of an oxidized
silicon
through-hole array by electron beam deposition. The array can then be
submerged in a
solution of chemical reagent such as a silanizing reagent that reacts
selectively with the
siliceous through-hole surfaces.
In another method, the inner and outer surfaces of a through-hole array are
coated
by filling the holes with a removable, impermeable material. For example, the
interior
surfaces of the through-holes in an array can be protected by filling them
with a solid that
can later be removed. Examples of such a solid include a wax, a plastic, a
frozen oil, ice,
dry ice, or a polymer such as poly(ethylene-glycol). In one example, the
through-holes
are filled, excess material removed from the surface of the platen, and the
surface coated,
leaving the interiors uncoated. Methods for removing excess materials include
scraping,
sanding, polishing, dissolving with a solvent, melting or burning. The solid
material in
31
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
the interior of the through-holes can be removed under similar conditions. The
interiors
of the through-holes can then be selectively coated or modified.
Derivatizing Through-Hole Surfaces.
In many cases it is desirable to immobilize probes on the inner walls of all,
some,
or one of the through-holes. There are many techniques for covalently
attaching probes
to glass or plastic surfaces (see, e.g., Inntnobilized AffZnity Ligand
Techniques, Hermanson
et al., Academic Press, 1992). Those methods useful for glass can also be used
for the
oxidized surfaces of a silicon substrate. For example, the inner walls of the
holes in an
oxidized silicon through-hole array can be reacted with g-glycidoxypropyl
trimethoxysilane in the presence of acid and heated to provide a glycerol
coating. This
glycerol coating can then be covalently linked to peptide or nucleic acid
probes.
Rendering the Inner Walls Porous: The inner wall of a through-hole can be made
porous by chemical etching following the procedure as outlined by Wei et al.
(Nature,
399:243-246, 1999). The larger area of the porous region increases surface
area and thus
the amount of chemical reagent attached to the through-hole inner wall. When
used for
synthetic transformation, the increased reagent loading of porous through-
holes increases
yield. When used for detection, the increased reagent loading increases
sensitivity.
Furthermore, the material between adjacent through-holes can be made porous
allowing
for communication between through-holes by liquids or gases. This method can
be useful
for the controlled delivery of reagents stored in adjacent through-holes,
allowing mixing
of reagents and reactions to occur. All or part (e.g., just the middle
portion) of the
through-hole can be made porous.
Polymer Scaffolding for Protein and Cell Immobilization in a Platen
The interior walls of the through-holes of the platen can be derivatized to
allow
covalent or non-covalent attachment of proteins or cells. Any signal arising
from the
protein or cell thus attached is then confined to the perimeter of the well,
unless the signal
is enzymatically amplified, as in an ELISA assay. Even if amplified in this
way,
however, the signal may be weakened, for example, by low analyte
concentration. In the
case of cell attachment, because the wells are bottomless, cells can attach
only to the
interior walls and can grow upwards in two dimensions. An alternative to
passive protein
adsorption and covalent attachment to the walls of the array through-holes is
the
introduction of a three-dimensional hydrophilic scaffold such as a hydrophilic
linear, gel
or foam polymer filling activated for protein coupling or capable of protein
or cell
entrapment within the well.
32
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Covalent attachment of proteins to polymer-filled through-holes of the platen
Because the interior surface of the through-holes is hydrophilic, a
hydrophilic or
water soluble pre-polymer can easily be loaded into the wells of the platen
and the
polymerization reaction can be initiated by a change in temperature or pH or
by the
addition of initiator. Protein coupling can be carried out during
polymerization, provided
that the polymerization conditions do not affect protein structure and
function.
Alternatively, protein coupling can be carried out after polymerization.
Proteins can be
coupled using any of a variety of reactions, including reactions of free
amines, free
carboxylic acid groups, and free sulfide groups. Reactions that form isourea
linkages,
diazo linkages, or peptide bonds are among those typically used to couple
proteins to
surfaces, but any aqueous based polymer reaction that is easily controlled can
be used.
Examples of polymers scaffolds include dextran and polyamides.
Dextran is a polysaccharide polymer that is very hydrophilic. The sugar
residues
of dextran contain hydroxyl groups, which can be chemically activated for
covalent bond
formation. Hydroxyl groups also form hydrogen bonds with water molecules, and
thereby create an aqueous environment in the support. When activated with an
aldehyde,
dextran can be easily coupled with proteins via amine groups (e.g., using
sodium
cyanoborohydride). When activated with hydrazide, dextran can be coupled with
proteins
via aldehyde or carboxyl groups using 1-ethyl-3-(3-dimethylaminopropyl
carbodiimide
hydrochloride) (EDC). However, use of dextran for certain applications is
limited by its
susceptibility to microbial attack.
An alternative method for covalent immobilization of proteins or enzymes is
through various derivatized polyamides, such as Nylon . Polyamides are best
suited for
the immobilization of high molecular weight substrates. Chemically, many
polyamides
are thermoplastic polymers with high mechanical strength, superficial
hardness, and
resistance to abrasive conditions caused by the intermolecular hydrogen bond
interactions
established between the amide groups of parallel chains. These characteristics
make
polyamides useful for immobilized enzymes, because they provide a favorable
hydrophilic microenvironment to support both catalytic activity and enzyme
structure.
Proteins covalently attached to a polymer scaffold are amenable to
conventional
biochemical assays such as ELISAs, binding assays, and activity assays.
Encapsulation of proteins (or cells).
Both proteins and cells can be immobilized by encapsulation within a web,
matrix,
or pores of semipermeable membranes, gels, or foams. Encapsulation of cells
requires
33
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
special consideration of the following factors: diffusion of materials within
and througli
the support; non-toxicity of the starting materials and of the polymer to
cells; and physical
properties such as optical clarity, temperature stability, flexibility, and
resistance to
chemical and microbial attack. The support is preferably resilient and
flexible, capable of
hydrogen bonding, and resistant to proteolysis and hydrolysis.
Given an appropriate scaffold, mammalian cells can be cultured in three
dimensions in a platen through-hole, enabling both the performance of many
types of
cell-based assays and the potential for imaging of cells within the through-
hole using
confocal techniques. Examples of types of assays that can be carried out
include reporter
gene assays, cell growth assays, apoptosis assays, and assays involving events
occurring
at the cell surface or within the cytoplasmic region (e.g., measurements of
calcium efflux
from the endoplasmic reticulum). Such assays are useful for functional studies
of
chemical and biological libraries. Examples of materials amenable to
encapsulation
include Poly-(2-hydroxyethyl methacrylate) (poly-HEMA) gel, Poly(carbamoyl
sulfonate) (PCS) hydrogels, and Phosphorylated polyvinyl alcohol (PVA) gel.
Poly-(2-hydroxyethyl methacrylate), "poly-HEMA," gel has superior mechanical
properties, temperature tolerance, and resistance to microbial attack than
many natural
polymers (e.g., gelatin, agarose, carrageenan, cellulose, and albumin). Poly-
HEMA gels
can be used for entrapment of both proteins and cells. The process of
biocatalyst
immobilization in poly-HEMA hydrogels generally allows for high retention of
immobilized enzyme activity, well-controlled porosity (e.g., to ensure
sufficient mass
transfer of reactants and products), and good chemical resistance. Poly-HEMA
hydrogels
also protect entrapped proteins and cells from bacterial degradation, by
resisting bacteria
entry into the gel support. Additionally, Poly-HEMA hydrogels can retain a
large
quantity of water, providing a microenvironment that approximates ira vivo
conditions.
Poly(carbamoyl sulfonate), "(PCS) hydrogels," afford adjustable gelation time,
high mechanical stability, and resistance to microbial attack. PCS hydrogels
also have a
high degree of flexibility, which can be exploited for filtering or expelling
samples.
Phosphorylated polyvinyl alcohol, " phosphorylated PVA" gels can be used to
immobilize cells of bacteria or yeast. These gels are economical, nontoxic,
durable and
support a high cell viability. Applications using phophorylated PVA gels are
limited by
their poor gas permeability.
Growth of cells on membranes
34
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The invention further methods for growing and analyzing eulcaryotic cells in a
format where a porous membrane is sandwiched between platens whose holes are
aligned
on both sides of the membrane. Cells are grown in the wells formed by one of
the platens
holes and the membrane. A removable gasket may be placed on top of the device
to
allow cells to be added. Test compounds may be introduced to the wells on
either side of
the membrane with micro-spotting pins or by aligning a solid surface pre-
spotted with test
compounds over the wells. The device may be covered on both sides with cover
slips
separated by spacers to reduce evaporation. A solid substrate, such as a glass
microscope
slide may be used to support the device. Iinage analysis can be performed
macroscopically with conventional scanning devices or microscopically using
light or
fluorescence detection. Biochemical analysis may be done on supematants or
cell lysates
using standard centrifugal or vacuum filtration devices.
In particular embodiments, platens used to create micro-wells are sufficiently
rigid
to make an even contact on the membrane. The material is preferably
biocompatible and
may be metal, plastic or ceraniic. The holes formed by the platen may be less
than 0.5
mm in diameter spaced less than 0.1 mm apart, or any other configuration and
dimension
that allows growth of adherent eukaryotic cells. To prevent lateral diffusion,
a watertight
seal may be formed between the membrane and the platen. This may be
accomplished by
coating the platen where it contacts the membrane with a hydrophobic substance
or by
using a secondary platen made of a flexible biocompatible material such as
silicon,
rubber, or other suitable material supported by a rigid platen on top. The two
platens may
be attached by raised surfaces on one platen fitting into recessed surfaces on
the opposing
platen. The total platen thickness on either side of the membrane whether a
single rigid
platen or a rigid platen on a flexible platen is typically less than 1 mm.
In related embodiments, adherent cell growth is maintained on commercially
available membranes or any biocompatible porous membrane that will allow cell
attachment. Optimal cell growth for adherent cells is maintained on membranes
exposed
to nutrient containing medium on either side.
The construction of the chip with medium and cells begins by filling the
through-
holes in a first platen with medium. This may be done by placing the first
platen on a
solid support. A gasket may be placed on the platen and cell mediuin is then
overlayed
on the platen. After centrifugation, the air in the platen holes is displaced
with medium.
Next, a pre-wetted porous membrane is place on top of the first platen
followed by
placement of a second platen(s). Guide holes in all of the platens will allow
holes to be
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
aligned visually. Alternatively, pins attached to the bottom support spaced in
the same
configuration as the guide holes on the platens may be used to line up the
platens. After
platen assembly, a gasket may be placed on top of the platen and medium
containing cells
id added. After centrifugation to allow cells to enter through-holes, excess
medium may
be removed. Spacers (-< 0.5 mm thiclc) may be placed on the edges of the top
platen
followed by a cover slip. The device is then clamped together and placed in a
humidified
chamber compatible with cell growth.
Pre-spotted test compounds such as proteins, small molecular weight compounds,
RNAi or other nucleic acids may be applied to the cells using a solid
substrate with
compounds arranged in the same configuration as the platen through-holes. In
such
embodiments, the compounds are spotted on a solid substrate in a medium with
low
volatility such as glycerol to reduce evaporation. The compounds are then
applied to the
solid substrate to have spatial orientation as the platen through-holes. The
solid substrate
to which the compounds are applied may also have a,guide pin that fits into
the cell-chip
so that each hole is aligned with a compound. In addition, a layer of mineral
oil may be
applied to either or both platens prior to compound contact to prevent lateral
diffusion and
cross contamination of compounds. Contact of the compound into the through-
holes of
the platen then allows diffusion into the through-holes and dispersal to cells
attached to
the membrane. Cells may then be incubated until analysis.
In other embodiments, analysis after addition of test compound may include
image and/or biochemical analysis. Image analysis may be done with existing
technologies such as visible light, laser scanners, visible or fluorescence
microscopy to
detect morphological changes in the cell or biochemical changes by visible or
fluorescent
dyes. Biochemical components of the cell lysates or supernatants such as RNA
or protein
may be analyzed by existing technologies such as centrifugal or vacuum
filtration to
separate components onto a membrane or module for analysis by qPCR, or SELDI
MS.
In other embodiments, compounds and other test substances are delivered using
a
standard microarray-spotting pin. The micro format allows high throughput
screening
using an array spotter. Cell viability or morphological changes can be
measured directly
on the membrane. Protein or mRNA expression can be determined after membrane
processing. Applications of this technology include lead generation screening
for drug
discovery or gene function studies using RNAi, cDNA and RNA.
36
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Fiberglass Chin
A through-hole array having holes filled with fibers can be created in a
simple and
inexpensive manner. Examples of useful fibers include fiberglass filters, mesh
glass fiber
filters, and polymer filters such as Nylon and polyethersulfone. These
materials can be
surface modified for specific interactions prior to fusing.
In one method, a sheet of loose fiberglass mesh is fused between through-holes
of
an array. The fusion can be achieved by pressing the fiberglass against a
through-hole
array created from silicon or other suitable metal that has been heated to a
temperature
sufficient to fuse the fiberglass, and then removing the array. Non-reactive
lubricants
such as graphite or molybdenum grease can be used to aid in the separation of
the heated
array and fiberglass arrays. Alternately, the fiberglass sheet can be pressed
between two,
aligned, heated through-hole arrays.
The resulting fiberglass array can be treated to make the regions between the
porous holes hydrophobic. Such treatment can be accomplished by blowing gas
through
the array while exposing the opposing face to a silanizing vapor. The porous
areas in the
resulting fiberglass array can be used for immobilizing probes such as nucleic
acids or
peptides with the advantage of very high surface areas for attachment, ease of
flow
through the array and the optical transparency of glass.
An alternate method for producing the fiberglass arrays is to inject or cause
a
polymerization of a thermoplastic material in the space between the desired
holes, for
example, by using a printing technique to deposit a polymer, epoxy, monomer,
or
polymerization initiator, or by photo-initiating polymerization or curing in
the desired
areas using one or more photomasks.
Growing porous glass in the through-holes.
Another method for producing an array of through-holes containing a porous
material is to machine an array by an appropriate method such as EDM and then
to grow
material in the array. Porous glass can be introduced into the array by using
pressure to
force a mixture of potassium silicate mixed with formamide into the array and
then
baking for several hours. An array of capillaries that is fused or embedded in
a binding
agent can be filled with porous glass by this method and then sectioned with a
cooled
sectioning saw to create multiple, thin platens of porous-glass filled through-
hole arrays.
By including particles such as porous silica or polymer beads in the potassium
silicate
mix, the affinity properties and porosity of the material can be adjusted as
desired.
37
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
III. Methods of loading array devices with samples.
Synthesis of an array by masking.
Certain methods feature applying at least one mask to a platen, or an aligned
stack
of such platens, such that, when a reagent is applied to the mask, the reagent
communicates with only the through-holes selected by the mask. In particular
embodiments, the reagent can be selected from an aqueous solution, an organic
solution, a
dry powder, a gel, a gas, or an electromagnetic radiation (e.g., heat, light,
X-rays,
ultraviolet radiation, a magnetic field). Methods for introducing reagents
through the
mask and into the array include applying a mechanical or optical pressure to
the reagent
reservoir, diffusion, and electrophoresis. Measures to prevent cross-
contamination of
neighboring wells include placing a second, identical mask on the face of the
platen
opposite the face to which the reagent is applied, and using a blotter to
absorb excess
liquid flowing through the through-holes. The masks and arrays can be held
together by
electrostatic, magnetic, or gravitational forces, or by applied pressure
(e.g., by applying a
clamp to the periphery of the stacked platens). Masks and arrays can be
separated when
liquid fills the through-holes by application of electrostatic, magnetic, or
gravitational
forces, or by application of a negative pressure to the stacked platens.
Stacks of platens
can optionally be dried to facilitate separation.
The liquid samples in the through-holes can be subdivided by placing an empty
array beneath a filled array or array stack, and then applying positive or
negative pressure
to force a portion of the liquid into the empty array. A small (typically less
than 100 m)
air gap is maintained between the filled array and the empty array to
facilitate the
physical separation of the two arrays after the fluid transfer is complete.
Through repetitive cycles of adding masks, introducing reagents, and
optionally
washing the array, a defined pattern of chemicals can be created in the
through-holes of
the platen by using solution phase chemistry.
By first derivatizing the inner surfaces of the through-holes with a linker
molecule
that contains a free functional group, the inner surface of each hole can be
coated with a
member of a library of molecules. The patterned through-hole array is then
used to
analyze chemical information. As described above for solution phase systems,
repeatedly
adding masks, introducing reagents, and washing the array, can result in a
defined pattern
of chemicals attached to the linker molecules in the through-holes. FIG. 1 is
an
illustration of this process. A platen (1) having through-holes with
derivatized inner
38
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
surfaces (4) is brought into contact with a mask (2) configured such that only
select
through-holes in the platen communicate with the reagents to which the masked
platen is
exposed. In this embodiment, two identical masks (2,3) are placed in contact
with the top
and bottom surfaces of the platen so as to prevent fluid from entering the
covered
through-holes. Preferably, the masks and platen are flat and polished (e.g.,
to an optical
finish) so that they create an airtight seal when contacted. Alternatively,
the mask or
platen, or both, can be coated with a soft polymer or gasket-forming material
to facilitate
sealing, or the mask and platen surfaces can be manufactured with contoured
surfaces
such that one fits into the other to form a large contacting surface area. The
contoured
surfaces can also provide alignment features that can aid in co-alignment of
through-holes
in the masks and platens. One example of an interlocking array design is shown
in cross-
section in FIG. 2, where the through-hole arrays are contoured to have
opposing and
matching geometrical features. The requisite geometrical features can be
obtained, for
example, by patterned chemical etching or micromilling.
Similar approaches can be applied to the manufacture of a system having two
masks with a platen sandwiched in between. The mask-platen sandwich can then
be
loaded with a reagent, such that the reagent enters into the open (i.e., non-
masked)
through-holes. After the reagent has reacted with the linker molecules located
inside the
through-holes, the excess reagent can be washed from the sandwich, and the
process can
be repeated with a new reagent. The mask can then be removed and a new mask
applied,
or the synthesized material can be removed from each through-hole.
Another embodiment uses only one mask to block one end of the through-hole
with an airtight seal. The air trapped in the through-hole prevents liquid
from entering
into the through-hole.
In another embodiment, a porous material derivatized with a linker molecule
having a free functional group is in each through-hole and members of a
library of
chemical probes are attached to the linker molecules. Alternatively, the inner
surfaces of
the through-holes are made porous (e.g., by etching with hydrofluoric acid),
and the
library of probes is attached. Containment of probes within the porous polymer
or
surface (i.e., as opposed to immobilization of library members on the inner
surface of the
through-hole) increases the density of library molecules in each through-hole.
For chemical synthesis, a porous polymer derivatized with a linker molecule
having a free functional group can be inserted into the through-hole. By
repeatedly
adding masks, introducing reagents, and, optionally, washing the array, a
defined pattern
39
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
of chemicals attached to the linker molecules can be created in the through-
holes.
Containment of linker molecules within the porous polymer increases the
density of
synthesized molecules in each through-hole.
A similar synthetic procedure can be used with a molecular library immobilized
inside a porous through-hole surface.
Mask production methods.
An embodiment of the invention provides for producing a mask by use of a
through-hole array substantially identical to the array used for synthesis or
analysis. In
one embodiment, a mask can be fabricated from metal, dielectric (glass,
polymer) or
semiconductor (e.g., silicon, germanium, gallium arsenide). Inertial drilling
is a suitable
manufacturing process for fabrication of masks in polymers, glass or metals.
Patterned
chemical etching processes, such as deep reactive ion etching (DRIE), provide
another
suitable manufacturing process for fabrication of masks in semiconductors and
dielectrics
such as glass. Electrospark discharge machining (EDM) is anotlier suitable
manufacturing process for fabrication of masks in conductive materials (e.g.
conductive
semiconductors and metals).
Another embodiment of the invention, shown in FIG. 4, provides for producing a
mask by use of a through-hole array substantially identical to the array used
for synthesis
or analysis. A solution (1) is added to each hole of a through-hole array (2)
such that the
solution contains a molecule or mixture of molecules that polymerize upon
irradiation
(Step 1). The solution could be for example, an aqueous solution of polymer
and a photo-
reactive molecule that produces free-radical initiators of polymerization when
irradiated
with ultraviolet light (3) (Step 2). An example of a UV curable polymer
suitable for mask
fabrication can be found in the class of UV-curable polyurethane epoxies. By
shining
ultraviolet light onto each hole that the artisan wishes to be blocked in a
given step of
adding reagent to a through-hole array, a mask (4) is built (Step 3). The
resulting polymer
is impervious to the fluids to which the mask is exposed. The through-holes to
be
blocked can be illuminated through an optically opaque mask or illuminated
sequentially
with focused light.
Another embodiment, shown in FIG. 5, uses an array of pins or posts to make a
mask. The external dimensions of the static pin array are selected such that
they have a
precision fit into a matching through-hole. Viewed in cross-section, a pre-
fabricated pin
array (1) selectively blocks those through-holes from communicating with a
reagent as
part of a synthesis sequence. A through-hole array (2) is prepared with the
inside surface
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
of the through-hole derivatized with a linker molecule having a free
functional group
(Step 1). A pin array is inserted into the through-hole array (Step 2) and by
the process of
introducing reagent and optionally washing the array, a defined pattern of
cliemicals in
the open through-holes is created attached to the linker molecules (Step 3).
The pin array
maslc is removed and the process is repeated with a different mask and
reagents resulting
in a defined pattern of chemicals created in the through-hole array (3)
attached to the
linker molecules (Step 4).
Pins in the array are fabricated to precisely fit into each matching through-
hole
forming a hermetic seal. Polymer coatings (e.g., Teflon) can be applied to
each pin to
facilitate sealing. An advantage of this approach is that the post arrays are
reusable and
need only to be made once. The large contact area between the pin and through-
hole
interior surface ensures a viable hermetic seal. As opposed to the plate
masks, only the
pins contact the array plate, thereby facilitating decoupling of the mask from
the array
plate after completion of a synthesis cycle. A further advantage is gained by
application
of only one pin array to seal through-holes in an array. This is achieved by
physical
blockage of the hole by the inserted pin or by the pressure of air entrapped
in the through-
hole. The pin array can be manufactured by a variety of fabrication
techniques. One
example is to electro-spark discharge machine (EDM) a regular array of pins
having the
precision cross sections needed to hermetically seal a through-hole. With a
die-sinking
EDM, selected posts could be machined away with a die to form the spatial
pattern of
pins matching a particular mask configuration. A second example is to start
with a plate
having holes in the spatial pattern matching a mask configuration. The pins
are fitted into
each through-hole and simultaneously soldered in place.
Another embodiment uses an actuated pin array forming a mask to hermetically
seal selected through-holes in an array. The actuated pin array is similar in
design to the
static pin array except that each pin can be extended or retracted such as to
reconfigure
the pin array to make a different mask. This is different from the static pin
array in that
each different inask requires a different pin array. Extended pins are
inserted into
through-holes whilst retracted pins are not. Individual pins in the array are
electronically
addressable to be actuated by one of several types of methods including:
piezoelectric,
electromagnetic (solenoid), magnetostrictive, shape memory alloy or conducting
polymer.
One advantage of this approach is a single pin array can be reconfigured to
produce n!/(n-
2)! number of different masks where n is the number of pins in the array. A
second
41
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
advantage is generation of different masks in an automated manner. This is
important
when the processes requiring masking of the through-hole array are also
automated.
In still another embodiment, the mask has holes that allow reagents to flow to
selected positions in the through-hole plate. In other positions, the mask
includes raised
features (e.g., pins or bumps) that fit into the holes to be blocked. This
approach aids in
alignment of the mask and platen, allows a single mask to be used, and ensures
a good
seal between the mask and the platen.
The through-hole array can also be fabricated with valves on one side of the
through-hole array. Each through-hole thus has a valve that either blocks or
unblocks one
end of the through-hole. Pressure of air entrapped in the through-hole
prevents liquid
from entering the open end of the blocked through-hole. The valve can be
formed as a
bilayer actuated by shape memory alloy, electrostrictive, electroporous,
piezoelectric,
magnetostrictive, or conducting polymer materials. Microsolenoid activated
valves can
also be used to perform a similar function.
Another method for producing a flow-mask is to laminate a platen on at least
one
side with a non-permeable membrane such as an adhesive tape. The mask is then
created
by selectively perforating the laminate material. Methods by which the
laminate can be
perforated are: by an actuated pin array, by laser machining, by contacting
with a platen
that allow heat to be applied in a localized manner, thus melting or burning a
hole in the
laminate. Serial dilutions can be performed during loading.
This operation can be performed to fill a series of through-holes with
different
concentrations of the same solute. In a typical example, a microsyringe, or
other fluid
transfer device, is positioned over the first,through-hole and used to fill
the first and
second through-holes with a 16X solution of the solute. The outer surface of
the syringe
tip and the faces of the array must be nonwetting toward the solution being
dispensed.
For each hole, a sufficient volume of solution, referred to below as "Y nl,"
is dispensed to
overfill the hole enough to create positive menisci. The microsyringe tip is
then rinsed
three times and filled with solvent. The syringe tip is positioned above the
second
through-hole and Y nl of solvent is expelled such that it forms a droplet at
the end of the
syringe tip. The syringe tip is lowered until the solvent droplet contacts the
solution
surface, causing the two liquids to mix and produce an 8X solution. The
syringe plunger
is then withdrawn to suck up Y nl of 8X solution and dispense it into the next
through-
hole. Another droplet of solvent is then formed and the process can be
repeated to
dispense 4X, 2X, 1X, etc. into individual array through-holes.
42
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Serial ArraYof Masks on a Flexible Sheet
Since syntliesis of an array of probes in through-holes can involve the use of
many
masks, a rapid and automated method for interchanging masks is desirable. One
method
involves creating the multiple maslcs in a single, flexible tape, such as a
metal or plastic
tape with a width greater than that of the array to be synthesized. The first
mask can be
aligned with the array and reagents can be transferred to the array. The tape
can then be
advanced to reveal the next mask prior to addition of the second reagent. This
process
can be repeated until the solid-phase synthesis is complete. For steps that
require
washing the entire array, a single large hole, or holes corresponding to each
position in
the array, can be produced on the tape. For additional through-put and
customization of
the synthesis, the tape can be produced concurrently with the synthesis, for
example, by
having an array of punches or a micro-positioned laser drilling system to
create holes as
the tape advances. A second tape or blotter can be used on the opposite side
of the array
(e.g., to prevent cross-talk). Various methods for aligning the masks with the
arrays can
be used, including placing precise alignment notches in the tape, and using
optical or
amperometric detection to determine mask position relative to the array.
Synthesis of arrays Using Masks and a Membrane.
The mask-synthesis methods described here can also be used with non-
addressable porous membranes (e.g., a filter), instead of with the rigid
platen.
CapillarYtube array
Viewed in cross-section, a capillary tube array (FIG. 6) is constructed from
capillary tubing (1) with an external diameter that fits precisely into the
through-holes of
a second array. The tubing array (3) is designed such that tubing at one end
has a center-
to-center spacing equal to the spacing between holes in a through-hole array
and tubing at
the opposite end has a center-to-center spacing equal to the center-to-center
spacing of
wells in a microtiter plate (2). Plates with through-holes having these
separations serve as
jigs (4) to hold the tubing in a regular array. Additional through-hole plates
placed
between the two ends are spacer jigs providing additional support for the
tubing array as
the center-to-center spacing is changed over the tubing length.
The internal volume of each tube in the array is slightly greater than the
total
volume of a column of aligned holes in the array stack. For example, if the
through-hole
dimensions in the array are 250 m x 250 m x 1000 m giving a volume per
through-
hole equal to 62.5 nl, then the volume of one set of holes in a stack of 100
arrays is 6.25
l (100 x 62.5 nl). Capillary tubing with an internal diameter of 200 m and an
external
43
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
diameter of 245 m is readily available; thus a minimum tube length of 200 mm
stores
the volume of fluid needed to fill this set of through-holes.
One end of the tubing array is inserted into the wells of a microtiter plate
where
each tube is inserted into a matching well. A negative pressure is applied
across the
length of tubing, drawing liquid from each well into its corresponding tube.
Negative
pressure can be applied to each tube individually or as shown in FIG. 6, the
ends of the
tube array can terminate in a chamber that can be partially evacuated. After
filling each
tube of the array, the microtiter plate is removed. The liquid can be stored
in the tubing
array for an indefinite period of time, either frozen or in a humidified
environment.
Multiple tubing arrays can be filled from the same microtiter plate (assuming
there is
sufficient volume of liquid per well) or different tubing arrays can be filled
from different
microtiter plates.
Transfer from a Microtiter Plate with an Array of Flexible Members
As illustrated in FIG. 7, fluid can be transferred from individual wells of a
microtiter plate (3) with an array of flexible members (2) (e.g., shape memory
alloy
fibers). The fiber diameter is equal to or less than the inside dimension of
the through-
holes in the array (1) into which fluid will be transferred. The number of
fibers in the
bundle can, for example, be equal to the number of wells in the microtiter
plate. The ends
of the fibers at one end of the bundle can have a center-to-center spacing
equal to the
spacing of the holes in the through-hole array, while the ends of the fibers
at the opposite
end can have a center-to-center spacing equal to the spacing of wells in the
microtiter
plate. The fibers can be held in place with a series of through-hole jigs
designed to
increase the spacing between fibers from one end of the bundle to another.
Once fixed in
place, shape memory alloy fibers can be heated above their critical transition
temperature
to make the imposed fiber curvature permanent. After they are cooled to room
temperature, the fibers can be removed from the holding jig, with the change
in fiber
center-to-center spacing intact. The close packed end of the fiber bundle can
then be
inserted into the through-hole array into which fluid from each well in the
plate is to be
placed. The opposite end can be arranged such that each fiber is positioned
above a well
in the microtiter plate, and the ends of the fibers can be immersed in the
fluid contained in
each well. On retraction, a small volume drop (4) can remain attached (e.g.,
by surface
tension) to the end of each fiber. A force can be applied to the opposite end
of the fiber
bundle to pull the bundle through the holes of the through-hole array, such
that the fluid is
brought into contact with the corresponding through-holes. As the fibers are
pulled
44
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
through the hole, surface tension can act to hold the liquid in the through-
hole as the fiber
is removed.
Pressure loadin~
The array can also be loaded by applying a pressure across the platen, thereby
causing a dilute solution of reagent and/or sample to flow through the array
of through-
holes. This method can be advantageous if the tlirough-holes are already
loaded with
reagents, and a reaction with a second set of reagents is desired.
Bead loading
Bead loading can be used to load an entire combinatorial library immobilized
on
microscopic polymer spheres of uniform size. In this method, the through-holes
in an
array can be shaped so as to hold only one microsphere per through-hole. The
through-
holes can additionally have a tapered cross-section, such that the
microspheres sits in the
holes either at or below the array surface (FIG. 8).
Transferring Contents from a Second Array
Replicating a platen containing through-holes reproduce an array wherein each
channel contains a colony of cells having a unique genetic profile. The master
plate is
prepared from a suspension of cells of diverse genetic characters. The
suspension can be
diluted such that when an array is loaded from the dilute solution, an average
of one cell
is transferred into each channel. The array is then incubated in an enclosed
humidity
chamber with the appropriate temperature and agitation for the cell type until
the cells
have reached mid log phase. The cell number density can be estimated by
observing
select channels under an optical microscope, measuring the amount of
scattering when
light is incident on the arrays, or if the cells also contain a gene for green
fluorescence
protein production, by measuring the intensity of fluorescence from each
channel.
Various methods can be used to transfer a portion of the cells from each
channel into
corresponding channels in a second array.
One method of transfer involves freeze-drying the contents of the through-
holes.
A master through-hole array is prepared from a suspension of cells of diverse
genetic
characters as described above. A second identical through-hole array is filled
with
growth media. The two through-hole arrays are aligned and stacked to mix
contents of
corresponding through-holes. The stacked through-hole arrays are freeze-dried
and
separated. The colonies are reconstituted by filling the array with media. In
the case of
robust cells such as bacteria and yeast cells, dehydration by evaporation can
be sufficient
to remove the liquid without significantly compromising cell viability. This
method is
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
also useful for storing compound libraries such as small molecule libraries in
a dry form.
For example, the crystalline compounds can adhere to the walls of the
channels.
Compounds can then be stored for long periods of time and reconstituted by the
addition
of solvent. Compound libraries can also be stored in a powdered or crystalline
form
while frozen in an inert matrix. A suspension of crystalline compounds can be
made in a
low molecular weight perfluorinated hydrocarbon and stored frozen. An exainple
of a
perfluorinated hydrocarbon that can be used for this purpose is
perfluorohexane, which
has a melting point of -4 C and a boiling point of about 59 C. Upon retrieval
of the
sample, the hydrocarbon can readily evaporate at atmospheric pressure or under
an
applied vacuum; the samples can then be reconstituted with DMSO, water, or
other
solvent.
Transfering/Mixing Samples in a Through-Hole Array with Samples on a Flat
Surface
Liquid samples contained in a through-hole array can be transferred in part or
in
whole to a flat surface having a pattern of hydrophilic and hydrophobic
regions. The
hydrophilic regions must be spatially isolated from one another and must match
the
spacing of the through-holes such that when the array is contacted with the
surface, the
contents of each through-hole contacts at most one hydrophilic region. The
hydrophobic
regions on the surface also serve to isolate the transferred fluids from one
another.
The surface may support an array of samples that can be registered with an
array
of probes contained in a through-hole array. The samples must be spatially
isolated from
one another and must match the spacing of the through-holes such that when the
array is
contacted with this surface, the contents of each through-hole contacts at
most one
sample. The surface can also contain a hydrophobic pattern matching the
pattern of the
through-hole array to prevent cross-contamination after the surface and array
are
contacted. If a hydrophobic pattern is not provided, the platen and surface
can be pressed
tightly against one another to form a hermetic seal and thus prevent mixing
between
adjacent samples.
Alternatively the flat surface can support an array of probes, such as
fluorescently
labeled oligonucleotides, chemical substrates, or cells, matched to a through-
hole array
containing samples. The probes can be attached to the surface in a variety of
methods:
they can be chemically or physically absorbed on the surface, trapped in a
porous matrix,
attached with an adhesion layer, or contained in a drop of liquid. The probes
can be used
to generate a change in a detectable physical property of the sample (such as
46
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
fluorescence, optical absorption or mass) in response to a chemical or
biological
characteristic of the sample as binding activity or enzyme activity.
Plunger sterilization
Plunger sterilization can be an important aspect of a serial sampling scheme.
One
approach is to have at least two plungers -while one plunger is sampling, the
other is
being sterilized. The two plungers can be located in a common mechanical
housing, for
example, mounted to rotate about an axis parallel to the plunger axis. Plunger
sterilization can be accomplished by heat or exposure to sterilizing agent
(e.g., 70%
ethanol). A wire (e.g., platinum) loop inside a ceramic sheath is an example
of a suitable
plunger design. The ceramic sheath imparts mechanical rigidity and is an
electrical
insulator whereas the wire loop permits heating with an electrical current. As
an
example, assume that a platinum wire loop is used, having a specific heat of 4
J/kg- C.
To electrically heat a 10-4 kg wire to 1000 C in 0.2 s requires a maximum
current of 4.5
A, which is easily achieved with medium power thyristors. Rapid cooling can be
achieved from the spray of a volatile sterilizing agent (e.g., ethanol), the
high latent heat
of vaporization of which can aid in cooling the heated wire. Alternatively,
the wire can
be rapidly cooled by a spray of gas or liquefied gas such as liquid nitrogen.
Sequential sampling with a single sampling device can be extended to a linear
array of sampling devices as, for example, a linear array of mechanical
plungers. There
can be a substantial time saving (e.g., 1/M for an M x M array of through-
holes), since
motion along one orthogonal direction can be avoided. Similar time saving
considerations are applied to two-dimensional sampling techniques. In an
alternate
approach, pressure generated by spatially localized jets of liquid, solid, or
gas can be used
in place of the plungers.
Loading with High Protein/Surfactant Media
Loading the platen by submerging the platen into a reagent of interest when
the
reagent or media is high in protein or surfactant and therefore low in surface
tension, can
be a challenge. Droplets of the low surface tension fluid can remain on the
surface of the
platen after removal from the fluid to be loaded. If droplets or a surface
coating of
protein rich media remains on the surface, it can result in contamination of
the assay or
crosstalk between through-holes. This problem is significantly lessened by
pulling the
platen up through a layer of a hydrophobic fluid that is immiscible with the
fluid to be
loaded. This provides a wiping or "liquid squeegee" effect, removing the
proteins or
47
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
surfactants adhering to the surface of the platen. The wiping fluid setup can
be generated
one of at least three methods.
Submerging the platen in the fluid to be loaded, ensuring the through-holes
are
filled. The platen is withdrawn from the fluid to be loaded, and submerged in
a
hydrophobic fluid that has a greater affinity for the proteins or surfactants
than the protein
or surfactant has for the surface of the platen. Such fluids can include but
are not limited
to perfluorodecalin, silicone oil and mineral oil.
Alternatively, the platen is first submerged into the fluid to be loaded. Then
a
small amount of a less dense, hydrophobic fluid such as but not limited to
mineral oil and
silicone oil is gently layered on the surface so that the surface of the
loading fluid is
completely covered with this wiping fluid. Then the platen is slowly removed
from the
loading fluid, up through the wiping fluid.
Additionally, a container can be used as in Fig 23. The container has a baffle
that
extends from one side if the container to another, but does not extend to the
bottom of the
container. The container is filled to a level midway up the baffle with the
fluid to be
loaded. This creates two open surfaces of loading fluid connected by a channel
underneath. Wiping fluid is gently layered on one of the surfaces, and is kept
from the
other surface by the baffle. The platen is submerged into the fluid to be
loaded,
underneath the baffle, and removed through the wiping fluid. This method for
employing
the wiping fluid is well suited for high throughput and automation methods.
Synthesis of arrays by Selective LoadingLof Fluids into throu h-g holes.
An array of pins (1) can be fabricated with pins arranged to be co-registered
and
co-aligned with a second regular array of through-holes (2) (FIG. 3). Each
through-hole
can be prepared such that linker molecules suitable for chemical synthesis are
immobilized on the interior surface of each through-hole. The ends or tips of
the pins can
be made hydrophilic over a pre-determined surface area whilst the remainder of
the array
surface area is made hydrophobic. In one embodiment, the tips of the pins in
the pin
array are brought into contact with the fluid to be loaded into the through-
holes of the
through-hole array (3). When retracted, a small volume liquid drop adheres to
the
hydrophilic region of each pin (e.g., by virtue of surface tension). The
volume of liquid
adhering to the pin is determined by the relative surface energies between the
liquid and
solid surface and the depth of immersion of the pin into the liquid relative
to the
hydrophobic surface area. The pin array with the adherent liquid drops can be
arranged
relative to the through-hole array such that the through-holes into which
liquid is to be
48
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
placed are aligned relative to each pin with an adherent liquid drop. The two
arrays can
be brouglit into contact such that the drops enter into the corresponding
through-holes
(e.g., by capillary pressure). Removal of the pin array leaves behind the
fluid placed into
the through-holes of the array (4). A chemical reaction can thus be initiated
between the
linker molecules immobilized on the interior surface of the through-hole and
the liquid
placed in the through-hole. The chemical reaction rate can be increased by
raising
temperature and/or changing the partial pressure and/or composition of gas in
the
atmosphere surround the through-hole array. After the reaction is complete,
the array can
be washed to remove unreacted components, and dried to remove excess solvent.
A
second pin array with either the same or different pin configuration can be
loaded with
fluid and the synthesis process can be repeated. Because the array loading
process can
rely on simple mechanical motions, the array loading can be quite rapid. The
rate-
limiting step, therefore, would be the synthesis step itself.
An alternative embodiment features the use of a pin array sparsely populated
with
pins aligned with respect to the through-holes of a regular array. The pins
can be
fabricated such that their length is at least twice the thickness of the
through-hole array
platen. Each through-hole can be prepared such that linker molecules suitable
for
chemical synthesis can be immobilized onto the interior surface of each
through-hole.
The end of each pin can, for example, by made hydrophilic and the remainder of
the array
can be made hydrophobic. The lateral dimension of the pins can be set such
that the pins
can be inserted into the matching through-holes in a regular array. The pin
array can then
be inserted through the second through-hole array such that the pins extend
through to the
opposite side of the platen. This assembly can be arranged relative to the
surface of a
fluid that is to be placed into the through-holes through which pins have been
inserted.
The tips of the pins can be brought into contact with the fluid surface, and,
on retraction,
small volume drops can adhere to the end of each pin. As the pin array is
retracted
relative to the through-hole array, the liquid drops can come into contact
with the
through-hole into which the pin has been inserted. As the pin leaves the
through-hole,
surface tension can keep the fluid volume inside the througli-hole.
An advantage of both embodiments is that they provide a rapid, simple, and
precise method by which fluid can be loaded into through-holes of an array.
Fluids
containing surfactants can, for example, be easily transferred into the array
with minimal
contamination between adjacent through-holes because of the long path along
the array
surface separating the ends of adjacent pins.
49
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Synthesis of a stochastic array.
A stochastic array can be created using a nozzle moving randomly to different
through-holes on the array. Loading the through-holes in a stochastic method,
wherein
one variable of the sample is varied in a random manner has many applications.
For
example, the stochastic loading can vary with respect to the concentration of
a particular
reagent in the sample loaded. The difference in concentration of the reagent
allows
simultaneous sampling, in a controlled manner, of many different reaction
conditions.
For example, this sort of application of samples can be used to optimize
reaction
conditions in chemical synthesis or can optimize parameters of a
crystallization
experiment.
IV. Reactions/experiments in the platens.
Synthesis of Combinatorial Libraries.
The present invention provides new methods for producing a combinatorial
library
in a platen. The types of combinatorial libraries that can be produced using
the new
methods include, but are not limited to, nucleic acid arrays, peptide arrays,
protein arrays,
polymer arrays, and arrays of small molecules.
Certain of the new methods include immobilizing a linker molecule on the inner
walls of the platen's through-holes, or in a porous material located inside
the through-
holes, and sequentially flowing reagents through masks to build a pattern of
chemicals.
For example, to create a nucleic acid array, phosphoramidite monomers can be
sequentially placed in the through-holes in defined patterns with activation,
reaction,
washing, and deprotection steps in between each addition of monomer. To create
an
array of small molecules using solid or liquid phase synthetic chemistry,
linker molecules
with, for example, protected amide groups can be sequentially placed in the
through-holes
in defined patterns with washing and deprotection steps between each synthetic
reagent
addition step. Chemical synthesis with solid phase chemistry can be carried
out on core
molecules linked to the interior surface of a through-hole, inside a porous
material placed
in a through-hole, or on a polymer bead placed in the through-hole. Core
molecules with
chemically active side groups can also be prepared in the through-holes using
solution
phase chemistry.
Chemical and Physical Process Optimization
Optimization of a chemical or physical process requires searching a
multivariate
space of experimental conditions for a subset of those parameters producing
the desired
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
outcome. The search strategy can be either systematic or stochastic; either or
both
strategies can be implemented as embodiments of the present invention.
Systematic
optimization is aided by producing an array of chemical or physical conditions
from one
through-hole to the next in a known and regular way.
Stochastic variation of reagent concentrations from one through-hole to the
next
can be accomplished, for example, by first uniformly loading an array with a
first reagent.
A container holding a second reagent can then be positioned above the array,
for example,
on a motorized two-axis mount, and the second reagent can be dispensed through
a nozzle
with an electronically controlled valve. The nozzle can be moved to different
randomly
selected array positions (or to positions determined by an algorithm), and the
amount of
liquid dispensed through the nozzle can be determined by a randomly selected
(or
algorithm-selected) time duration less than a pre-selected maximum. In this
manner,
different amounts of the second reagent are dispensed into through-holes
containing the
first reagent. This process can be repeated with additional reagents as
needed. The
reactions with optimal outcomes can be identified by analyzing the contents of
the
through-holes. If the second reagent is distributed randomly, rather than
according to an
algorithm, lack of specific knowledge regarding the starting conditions for
these reactions
can make duplication difficult. However, if one is interested in the reaction
products
alone, then a stochastic approach can provide a facile method for rapidly
searching a large
experimental parameter space for a desired reaction outcome. Moreover, the
reaction
conditions can sometimes be inferred, for example, by examining the contents
of other
through-holes in the array where little or no reaction occurred, and then
combining the
results in a multivariate plot. Optimal reaction conditions can be inferred
from domains
containing little or no data. Another approach to assess initial reaction
conditions is to
produce a replica plate using the same dispensing protocol but into an array
uniformly
loaded with solvent without any of the first reagent. Comparison of through-
holes
showing the desired chemical activity with the contents of the corresponding
through-
hole in the replica plate would provide information as to the most likely
starting
conditions of the observed reaction.
If large numbers of conditions need to be tested, the multiple reagents can be
randomly sprayed onto the array until all of the through-holes have been
filled. The
surface can then be wiped with a rubber spatula to remove excess fluid. A
reaction can be
initiated by stacking the array with a second array, and the result can be
probed optically.
Because the contents of each address in the array will be unknown, one can
either chose
51
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
promisingaddresses, and then analyze the contents to determine what was in the
hole, or
else replicate the plate prior to initiating the reaction, and then use the
replicate plate to
determine the optimal conditions.
These examples also allow for physical parameters to be either systematically
or
randomly varied from one through-hole to the next. For example, a temperature
gradient
can be imposed across one or two-dimensions of an array fabricated from
thermally
conductive material, for example, by holding the edges at different
temperatures. If the
temperature of a heating/cooling source is changed with time, then the
temperature
distribution across the array can be varied. The rate of temperature change is
generally
proportional to temperature; thus, both the temperature and the rate of
temperature change
can vary from one through-hole to the next. Alternatively, a focused laser
beam can be
directed to heat each through-hole independently, thus enabling control of the
liquid in
each through-hole with time as described, for example, in U.S. Patent No.
5,998,768,
incorporated by reference in its entirety.
Protein Crystallization
X-ray diffraction from crystallized proteins is an important analytical tool
for
determination of protein structure and function. Proteins can be difficult to
crystallize
because they generally include a multiplicity of hydrophobic and hydrophilic
molecular
groups. As a consequence, proteins often crystallize only under a specific set
of solvent,
pH, salt concentration, and temperature conditions. A high throughput method
for protein
crystallization is enabled by the present invention.
Screening Methods
The parameters that determine whether or not a biochemically efficacious
compound is suitable for further development as a pharmaceutical compound
include
absorption, distribution, metabolism, excretion, and toxicology ("ADMET"). As
the
number of potential drug leads increases due to advances in primary high
throughput
screening, ADMET testing can become increasingly rate limiting in the drug
discovery
process.
Adsorption.
Oral administration is the preferred route of administration for small
molecule
drugs. For an orally administered drug to have biological efficacy it must be
bio-
available (i.e. it must have the ability to pass through the gut and into the
bloodstream).
The ability to assess the ability of a drug candidate to pass through the
lining of the gut in
an in vitro assay is highly desirable. Typically, such absorption assays
utilize a
52
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
monolayer of cells grown on a semipermeable membrane that separates two liquid-
filled
chambers. The drug candidate is added to one of the chambers and after
sufficient time
for diffusion or transport, the concentration of the molecule in the other
chamber is
quantitatively analyzed.
One such absorption assay commonly used in the bio-pharmaceutical industry is
the CaCo-2 absorption assay. The CaCo-2 assay interrogates the ability of a
molecule to
pass through a single layer of a colon cell line, known as CaCo-2. Typically,
the rate of
both apical to basal and basal to apical diffusion across the cell layer is
determined. The
CaCo2 assay is usually performed in an apparatus that provides two chambers of
fluid
separated by a porous membrane. The merpbrane is permeable to cell growth
products,
but acts as a support and impermeable barrier for the cells. A monolayer of
cells is grown
across the surface of the membrane, and the active or passive transport of
molecules from
one chamber to the other across this cell barrier is assayed.
The platens containing arrays of through-holes can be configured several ways
to
provide an array of absorption assays (e.g. using CaCo-2 or other cells), thus
increasing
throughput and minimizing reagent volumes. In one embodiment, a isotropically
porous
membrane (such as, but not limited, to a PTFE filter) treated to provide a
biologically
compatible surface for growing adherent cells. The membrane has dimensions at
least the
dimensions of the array of through-holes, and is placed on one platen so that
it covers the
through-holes of the platen. A second platen with matching through-holes is
placed on
the membrane so that the membrane is sandwiched between the two platens.
Pressure is
applied to the platens so that the membrane is collapsed between adjacent
through-holes
and no chemical crosstalk may occur between non-opposing through-holes, but
allowing
chemical communication between opposing through-holes for the assay.
In a second embodiment, the membrane is anisotropically porous, consisting of
parallel pores through the membrane. Examples of this type of membrane are
Isopore
and Nucleopore filters sold by Millipore Corporation. As in the previous
embodiment,
the membrane is sandwiched between two platens, and pressure is applied. In
this
embodiment, pressure is not required to collapse the membrane between adjacent
through-holes, but only to seal between adjacent through-holes. The parallel
pores of the
membrane allow chemical communication between opposing through-holes but not
adjacent or non-opposing through-holes.
In a third embodiment, the membrane is patterned with regions of porosity
spaced
and sized like the pattern of through-holes in the platen. The membrane is
sandwiched
53
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
between two platens so that the areas of porosity match up with the through-
holes,
allowing chemical communication between opposing through-holes but not
adjacent or
non-opposing through-holes.
In a fourth embodiment, the membrane is made from a platen of through-holes,
in
which the through-holes contain a porous material, such as but not limited to
porous
silica. In this embodiment, the membrane platen is sandwiched between two
platens of
though holes, allowing chemical communication between opposing through-holes
but not
adjacent or non-opposing through-holes.
Metabolism.
For metabolism studies, compounds can be tested for their propensity to be
degraded by various cytochrome P-450 (CYP-450) enzymes or by liver microsome
preparations. Propensity for causing drug-drug interactions can be estimated
by assaying
inhibition of various CYP450 enzymes by each drug or drug candidate.
An embodiment of this invention provides for measurement of cellular
metabolism of compounds from a library. As described above in connection with
adsorption assays, the compounds can be small organic molecules, peptides,
oligonucleotides, or oligosaccharides. The cells can either be suspended in
liquid, or can
be grown as a monolayer of cells inside the through-holes or on a membrane
having high
longitudinal permeability and low lateral permeability. The membrane can
include, for
example, polymerized monomers in the through-holes of a plate, or
hydrophilic/hydrophobic domains in a flexible membrane with domain size and
center-to-
center spacing equal to that of the through-hole array. Volumes of known
concentrations
can be loaded from the compound library into one through-hole array. The cell
array or
layer can be placed in contact with the library array and incubated, and the
array
composition can be analyzed to determine the change in compound composition or
amount with cellular metabolism.
Toxici .
An embodiment of this invention provides for measurement of cellular toxicity
of
compounds from a library. As described above in connection with adsorption and
metabolism assays, the compounds can be small organic molecules, peptides,
oligonucleotides or oligosaccharides, and can either be suspended in liquid or
grown as a
monolayer of cells inside the through-holes or on a membrane having high
longitudinal
permeability and low lateral permeability. The membrane can include, for
example,
polymerized monomers in the through-holes of a plate or
hydrophilic/hydrophobic
54
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
domains in a flexible membrane with domain size and center-to-center spacing
equal to
that of a through-hole array. Volumes of known concentrations are loaded from
the
compound library into one through-hole array. The cell layer can be placed in
contact
with the library array and incubated, and the cells in each through-hole can
be analyzed
for viability.
Ligand Screening by Affinity.
It can be desirable to measure or rank the affinity of various members of a
compound library toward a particular target macromolecule, or to measure the
affinity of
an analyte toward various members of a probe array. Such screening can be
carried out
using the new methods described herein. For example, affinity experiments can
be
carried out by immobilizing a target in many holes of the through-hole array
and probing
with a library of potential ligands, or by immobilizing a ligand library in an
array and
probing with a target.
Thermal Denaturation Rankin~.
As new drug targets are rapidly being discovered, methods are needed to find
molecules with affinity to these targets in the absence of a functional assay.
Fulfillment
of this goal can be accomplished by immobilizing the target biomolecule on the
inner
surfaces of an array, incubating the holes of the array with a library of
compounds, and
detecting those members of the array that retain a compound. Bound compounds
also
stabilize target molecules to thermal denaturation to a degree that can
correlates with the
degree of affinity. By detecting unfolding of protein as a function of
temperature or
denaturing solvent condition, affinities can be ranked, as described, for
example, in U.S.
Patent No. 6,020,141 to Pantoliano et al.
Gene Probes.
An embodiment of the invention provides for the production of a through-hole
array containing numerous, known nucleic acid sequences, adding a nucleic acid
solution
that has at least some unknown sequences to each of the holes in the array,
providing
sufficient time, temperature, and solution conditions for the unknown nucleic
acid to bind
specifically to complementary nucleic acids in the through-holes, and
analyzing the
degree of hybridization between the nucleic acids of known and unknown
sequence in
each through-hole. After binding, the array can be washed with a solution of
the desired
stringency. Often, the unknown nucleic acid has a fluorescent probe attached.
An
advantage of the invention is that amplification of the signal can be achieved
by using an
enzyme reaction that is associated with the hybridized nucleic acids. For
example, the
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
unknown nucleic acid can be labeled with horseradish peroxidase and incubated
with a
substrate that produces a luminescent, fluorescent or chromogenic signal upon
reaction
with the enzyme following the binding and washing steps. Such amplification
techniques
can be incompatible with conventional nucleic acid arrays on planar surfaces,
since the
activated substrate in solution generally cannot be assigned to a particular
point on the
array due to diffusion. PCR or other thermal-cycling reactions may be
performed in the
array by submerging the array in a water-immiscible liquid such as an oil,
alkane, or
perfluorinated solvent. The array and water-immiscible liquid may be contained
in a
thermally conducting container such as a metal box, and then inserted into a
thermal
cycler adapted to receive the box.
Long-Term or Hi ng Temperature Culture of Cells
In order to minimize evaporation, it is known in the art to layer a small
amount of
a low volatility, immiscible liquid on top of a small volume of aqueous
reaction media.
For example, a small amount of mineral oil can be layered on a reaction vial
containing a
PCR (Polymerase Chain Reaction) reaction, in order to minimize evaporation
during
heating cycles. It is also known that fluids with high oxygen solubility
contents can be
used to enable oxygen transport to systems that require oxygen. It is a novel
aspect of
this invention that an immiscible fluid with a high oxygen solubility content
can be used
to eliminate evaporation from the array of sub-microliter samples, while
facilitating
oxygen transport to maintain cell viability.
In order to culture non-adherent cells in a nano-volume format for long
periods
(e.g., greater than 12 hours), it can be desirable to reduce evaporation by
containing the
cells in a hydrophobic, low volatility fluid. To allow for aerobic respiration
or other gas
exchange process to occur, an oxygenated emulsion of perfluorinated compounds
can be
used. These compounds have been the subject of clinical testing as artificial
blood
substitutes. Examples include perfluorodecalin, which is sold as Fluosol-DATM
by Green
Cross Corp. of Japan; OxycyteTM, which is being developed by Synthetic Blood
International; and OxygentTM-brand perfluorooctylbromide, which is being
tested by
Alliance Pharmaceuticals. In a typical application of these methods and
materials using
the through-hole array, cells are grown in a platen that is submerged in
perfluorodecalin,
while oxygen or air is bubbled through the medium in a manner that does not
disturb the
cells.
56
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Culture of cells that adhere to the walls of the through-holes or to porous
substances immobilized in the through-holes is comparatively simpler, as
oxygenated
aqueous media can be perfused through or around the platen as required.
Culturing thermophilic organisms under aerobic conditions at low volumes and
high temperature can be particularly problematic, since evaporation tends to
act quickly at
temperatures such as 90 C. By submerging the array of through-holes in an
appropriate
fluorinated solvent, these temperatures can be used without significant
evaporation, while
maintaining a supply of oxygen to the cells.
Often the act of assay detection or sample "picking" may require that a platen
be
exposed to non-humidified environments or bright lights for a period of time.
These are
conditions under which evaporation from the through-holes can be problematic.
In a
typical experiment, sample evaporation is minimized by performing operations
under a
layer of immiscible fluid such as but not limited to perfluorodecalin, and
samples may be
added to or removed from the through-holes with a microsyringe while submerged
under
such a fluid. Other operations, such as imaging and platen manipulation such
as platen
stacking may be performed under the immiscible fluid as well.
V. Methods of analyzing and manipulating output from array devices.
Methods of transferring samples from throu h-gholes.
Still another method features transfer into microtiter plates. In order to
recover
samples giving a positive response to a test, there is often a need to
transfer fluid from
selected through-holes in a high-density array plate to a microtiter plate
having a lower
density of wells. Often, this transfer process must be performed with sterile
technique.
This will allow for sampling of materials held in through-holes with selected
properties
from a larger collection of samples. There are three general methods for
transferring
fluids from the high density array plate to the wells of a microtiter plate:
transfer with a
single sampling device, transfer with a linear array of sampling devices and
transfer with
a two-dimensional array of sampling devices. Samples from proscribed through-
holes
can be removed by spatially localized mechanical action. One general
embodiment is to
insert a member through the hole to mechanically displace the material out the
opposite
side and into a receptacle positioned beneath the hole. A second general
embodiment is
to apply a localized gas or liquid jet to cause material in the hole to be
displaced out the
opposite end and into a,receptacle positioned beneath the hole. A third, but
slower,
method is to transfer liquid from the hole to a waiting receptacle by
transferring liquid
57
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
onto a pin or into a syringe, moving the pin or syringe to the receptacle and
dispensing the
liquid.
In order to maximize throughput of the transfer system, the number of times
that
the low-density plate is moved should be minimized. If the high-density array
is imaged
and the imaged stored on a computer, the coordinates of the desired through-
holes is
available for input into the transfer apparatus. By aligning the high-density
array above
the low-density plate, and calculating which desired samples sit above an
empty well in
the low-density plate, a maximum number of samples can be transferred without
re-
positioning the low-density plate. The low-density plate can then be moved to
a position
that allows the greatest possible number of samples to be transferred in the
next step. In
order to minimize the cost of the transfer apparatus, the high-density through-
hole array
should remain stationary to avoid use of a high-precision alignment system.
Some high-
throughput systems that can be used in this way are described below.
Another method features transfer with a single sampling device. This method
can
be accomplished, for example, by fast sequential positioning of a mechanical
plunger
over the through-holes to be sampled and pushing the plunger through the hole
to transfer
the hole's contents to the well of a microtiter plate located at a small
distance below the
through-hole array (6) (FIG. 9). The mechanical plunger (1) can be actuated
by, for
example, a linear electromagnetic motor (2). This process is repeated until
each well (3)
of the microtiter plate (4) contains the contents of a different through-hole.
Once
complete, the filled plate is replaced with an empty plate and the process is
repeated. A
servo-controlled, linear motor-actuated two axis stages (5) with magnetic or
air bearings
can position a mechanical plunger whose diameter is slightly less than a
through-hole
diameter to within <1/100 of a hole diameter (-l m) with velocities up to 1
m/s. Thus if
1% of a 100,000 hole array is to be transferred to microtiter plates and the
time to sample
each hole is on average 0.2 s, then 1% of the array can be sampled in about
200 s. This
time excludes, of course, the time required to change microtiter plates, the
time needed to
sterilize the plunger between samples and, if needed,athe time to position a
well below the
sampled through-hole.
Spatially localized gas, liquid, or solid jets
With reference to FIG. 10, a beam from a laser (1) passes through a shutter
(2) and
can be directed by a beam scanning system (3) to be focused onto a specified
through-
hole (4) in a high-density array of through-holes (5). The laser wavelength
can be chosen
to coincide with an absorption band of a fluid in a targeted through-hole. The
58
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
corresponding absorption coefficient can be such that a large percentage of
the incident
laser radiation is absorbed in a thin layer at the top of the liquid column.
The shutter can
control the length of time the liquid in the through-hole is exposed to laser
radiation.
When the shutter opens, laser light illuminates the liquid, and sufficient
energy is
absorbed during the exposure time to rapidly heat a thin liquid layer to
vaporization,
causing the rapid build-up of pressure at one end of the through-hole. The
resulting force
from the expanding vapor causes ejection of liquid from the opposite end of
the hole (6)
into a well of a microtiter plate located below the through-hole array (7). A
further
increase in force is possible if the volume above the heated surface is
hermetically sealed
thus increasing the pressure applied to the liquid. Rapid vaporization and
expulsion of
liquid from the column requires the laser energy to be deposited in a time
less than the
thermalization time. Rapid expulsion is needed to increase throughput and to
prevent
substantial degradation of the cells or reagents contained in the liquid.
The case of a water-filled through-hole provides an illustrative example.
Indeed,
in many cases, the analytical substance is in water. The absorption
coefficient of water at
10.6 m is -1000 cm 1 indicating 99% of the incident radiation will be
absorbed within
46 m of the surface -a small fraction of the water column's length assuming a
length of
0.5 mm or greater. The thermalization time, z, is the time required for the
water column
to reach thermal equilibrium and is given by z=l 2 14tx where l is the
distance from the
source of thermal energy and a is thermal diffusivity (= iclcp ) in which the
thermal
conductivity is K, c is the specific heat and p is the density. Inputting
appropriate values
for water, the thermalization time for a column of water 1 mm in length is
1.75 s while for
a column 0.5 mm long, z is 0.44 s. Adiabatic heating with the focused laser
beam will
take place if the laser pulse length At is less than z
The peak pressure generated by the instantaneous vaporization of a volume of
water 46 m thick by 200 m in extent can be estimated assuming the water
vapor is an
ideal gas. The pressure, P, in this volume, V (=1.4 x 10-12 m3) , when the
liquid is
vaporized is P= nRTIV where n is the moles of water (= 88 nanomoles), T is the
gas
temperature (= 373 K) and R is the ideal gas constant (= 8.2 m3-Pa/mole- K).
Inputting
these values gives Pma,, = 186 x 106 Pa equal to a force of 5.8 N on the water
column;
sufficient to expel the liquid from the through-hole.
The laser power to vaporize a 46 prn thick layer of water in a 200 m diameter
through-hole can be found by computing the energy, Q, to vaporize this volume
of water:
59
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The thermal energy is found for Q = m (cAT +OHõap) where in is the mass of the
water
in this volume (1.6 x 10"9 lcg), c is the specific heat of water (= 4184 J/kg/
K), dT is the
temperature change (353 K) and AH,,,p is water's latent heat of vaporization
(=2.3
MJ/kg). Inputting these values gives Q equal to 6 mJ. Assuming 99% absorption
of the
incident laser energy, 6 mJ is deposited in the sample by a 10 W laser
illuminating the
liquid surface for 0.6 ms. For random-access scaiining, typical settling times
for
galvanometer-steered mirrors is 10 ms and for 1000 through-holes in an array
to be
individually addressed, it will take approximately 10.6 seconds.
In alternative embodiments, solids (e.g., powders) or liquids can be used to
displace or force out (e.g., under pressure) the contents of specific through-
holes or the
contents of the through-holes in general. (See FIG. 22) Examples of liquids
that can be
used include liquids that are miscible with the contents of the through-holes
(e.g., water
when the contents of the through-holes are aqueous) or liquids that are
immiscible with
the contents of the through-holes (such as oils or organic solutions when the
contents of
the through-holes are aqueous). Application of pressurized liquid to an array
can be used
to wash contents of each hole into, for example, a common container.
Explosive charge.
An extension of the previous embodiment is the expulsion of the liquid from a
through-hole by a pressure wave generated by rapidly expanding gas on ignition
of an
explosive charge located in proximity to one end of the through-hole. With
reference to
FIG. 10, an array of discrete slow-burning explosive charges (1) is co-
registered with
respect to the through-hole array such that one charge is located above one
through-hole.
Each charge is placed in a chamber having a thin membrane as a common wall
with the
through-hole. The charge array is bonded (or tightly attached to) the through-
hole array.
Examples of explosive material for this application include plastic explosive
sheets such
as "C4", or trinitrotoluene (TNT) embedded in plastic. The charge array is
addressable,
for example, electrically or optically. An individual charge can be ignited by
passing an
electrical current through a resistive element (2) located in the chamber or
by the thermal
energy deposited in the chamber by a focused laser beam. Once ignited, the
expanding
gas from the explosion generates sufficient pressure to burst the separating
membrane and
drive liquid (3) from the through-hole (4) into the well (5) of a microtiter
plate (6) located
below the through-hole array (7).
Alternatively, as shown in FIG. 11, the explosive charge can be embedded as a
uniform stochastic distribution in a thin plastic sheet (1). Conversely, the
explosive
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
chemicals could be printed onto the sheet in the same pattern as the through-
hole array, as
shown in FIG. 12. Printed onto the sheet are resistive elements (2) at
discrete spatial
locations with the same pattern as the through-hole array. Alternatively,
spatial locations
on the sheet are addressable by a focused laser beam providing the energy
required to
ignite the explosive chemicals. The sheet is bonded to the through-hole array
(3) and the
charges ignited above the through-holes whose contents (4) are to be
transferred into a
well (5) of a microtiter plate (6) located below the through-hole array.
To increase the inertia imparted to the liquid column from the expanding gas
charge, metal or ceramic microspheres are mixed with the explosive charge and
are
accelerated by the explosion. Alternatively, a plug of material between the
explosive
charge and liquid column accelerated by the explosion will act as a mechanical
plunger to
expel liquid from the through-hole.
Forming the exit of the chamber containing the explosive charge into a nozzle
will
increase the spatial localization and inertia of the exiting gas before
impacting the liquid
column. The nozzle exit either is fluid with the chamber surface or protrudes
slightly to
insert into the opposing through-hole.
61
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Sample aspiration.
Liquid samples in a through-hole or group of through-holes can be transferred
out
of the through-holes by aspiration into a tube or channel. The tip of a piece
of flexible or
rigid tubing, generally having an outer diameter narrower than the inner
diameter of the
through-hole, can be aligned within the through-hole. Application of negative
pressure to
the distal end of the tubing can then be used to aspirate fluid from the
through-hole into
the tubing. The amount of fluid to be aspirated can be accurately controlled
by
manipulating several variables. For example, the length and internal diameter
of the tube
can be determinative of the pressure drop across that piece of tubing, which
can in turn
affect the rate of flow through that piece of tubing for a given amount of
applied negative
pressure. A metered amount of fluid can be aspirated from the through-holes
into a valve
assembly, from which the fluid can be moved by positive or negative pressure
to any type
of fluidic circuit that is required by a given application. In one embodiment,
fluid is
aspirated from a through-hole into a fluidic valve. Actuation of that valve
introduces the
fluid via positive pressure to a mass spectrometer for analysis of that fluid.
Alternatively,
further sample preparation or characterization (e.g., chromatograpliy,
spectroscopy) can
be performed on the fluid once it has been aspirated from the through-hole.
Electrophoresis and Electroblotting: The invention also provides methods for
introducing an ionic sample into a through-hole array containing chemical
probes, for
modulating the stringency of the binding between the probes and certain ions
in the
sample, and for removing an ionic sample from the through-hole array. The
method
includes placing the through-hole array containing chemical probes localized
in the holes
into a buffer in an electrophoresis apparatus. A sample containing ions is
introduced into
the electrophoresis apparatus on one side of the planar through-hole array,
such that when
an electric field is applied, the ions of the appropriate charge will migrate
in the direction
of the through-hole array. If a particular ion does not bind to the array and
the electric
field is applied for sufficient time, that ion will migrate through the hole
to the opposite
side of the through-hole array. By periodically changing the direction of the
electric field,
approach to equilibrium in the binding between the charged species and the
chemical
probes in the through-holes can be accelerated. Partial purification of the
sample to be
analyzed can optionally be achieved by electrophoresis of the sample through a
gel prior
to its migration to the through-hole array. Once the analytes of interest in
the sample
have associated with the chemical probes, the field can be applied for
sufficient time and
with sufficient strength to dissociate non-specifically bound ions from the
chemical
62
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
probes. The through-hole array can then be taken from the electrophoresis
apparatus, and
the pattern of binding can be analyzed. The bound analytes can also be removed
for
further analysis, for example, by electroblotting onto a membrane, and then
removing the
membrane for further analysis.
Chromatic analysis of samples in an array of throu h-g holes.
For many applications, sainples must be isolated, purified, or concentrated by
a
chromatographic step. Many different types of chromatography can be performed
on
liquid samples. Examples of well-known chromatographic methods include, but
are not
limited to, ion exchange, reversed phase, size exclusion, bio-affinity, and
gel permeation
chromatography. The chromatography matrix can be in the form of an insoluble
bead,
gel, resin, polymer, or slurry. The matrix can alternatively be a micro-
machined
structure. One embodiment of such a micromachined structure is a grouping of
square
through-holes or channels with sides of dimension on the order of 0.01 to 10
m. The
walls of these through-holes can be coated with a surface having a desired
affinity such
that a separation is achieved as sample is flowed through the group of through-
holes. The
analyte mixture of interest is then introduced to this matrix and selective
binding of
components of the analyte mixture to the matrix takes place. Analytes of
interest or
contaminants can then be selectively eluted from the matrix by changing the
physical or
chemical environment of the matrix.
Liquid chromatography is typically performed in a column in which the
chromatography matrix is immobilized and the analyte is flowed through the
column,
allowing chemical and/or physical interaction between the sample and the
chromatography medium to take place. The length and internal diameter of the
array of
capillaries generally determines the amount of chromatography matrix that can
be loaded
into each column and, therefore, is directly related to the loading capacity
of each
column.
Immobilizing a chromatography matrix inside an array of through-holes can
create
an array of miniature liquid chromatography columns. A suitable length-to-
diameter ratio
of the array of through-holes can be selected. Typically, a minimum length-to-
diameter
ratio of at least about 10 is required to form an effective chromatography
column. In
certain embodiments, the internal diameter of the columns formed in the
through-holes is
less than a millimeter, allowing for precise and accurate manipulation of very
small
amounts of sample. The chromatography matrix can be immobilized within the
chromatography columns by positioning a porous frit at the exit end of the
column or by
63
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
chemically binding a porous polymeric ceramic or glass substrate to the inside
of the
column. The porous ceramic or glass substrate can either act as a frit to
immobilize a
bead or resin chromatography media or as a method to increase the total
surface area
within a column. In surface-effect driven chromatographic methods (e.g., ion
exchange,
affinity, or reversed phase chromatography), chemical derivatization of the
interior
surface of the columns can provide the necessary separation.
In one embodiment, samples are loaded into the array of columns with syringes.
A submicroliter volume of sample can be drawn into the needle of the syringe
or a bank
of syringes with a spacing co-registered to the spacing of the array of
columns, and then
transferred to the array of columns. The barrels of the syringes can contain a
larger
quantity of liquid for performing wash and/or elution steps in the bundle of
capillaries.
The sample in the needle of the syringes and the liquid in the barrel of the
syringes can be
isolated from one another by drawing up a small amount of air into the
syringes. The
needles of syringes can be docked into the array of capillaries with a liquid-
tight
compression fitting. As the content of the syringes are ejected into the array
of columns
the samples in the needles of the syringes initially elute onto the column
bed. The
chromatography media used and buffer drawn into the barrel of the syringe will
dictate
the chromatographic separation. Once the samples have been loaded onto the
columns,
the syringes can be removed from their docking ports and a second aliquot of a
similar or
different buffer can be drawn into the syringes. As many wash and/or elution
steps as
necessary can be performed by re-docking the syringes to the array of
capillaries by
tightening the compression fitting and ejecting the liquid from the syringes.
A first array
of columns can also be mated to a second array of columns for further
separation.
It is often desirable to analyze the eluate from a chromatography column in
real
time using a variety of spectroscopic methods. Spectroscopic devices for
interrogating
the eluate from a chromatography column are well known. If required by the
specific
application, the eluate from each column in a bundle of columns can be
analyzed on-line
in real time, spectroscopically (e.g., by absorption, fluorescence, or Raman
spectroscopy),
electrochemically, or otherwise. The light from the spectroscopic light source
can be
delivered to and recovered from the eluate of each column in a bundle of
columns as it
passes through an observation window machined into the exit capillary of that
column
with a fiber-optic cable.
64
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Mating an array of through-holes to an array of liquid chromatography channels
A device can be manufactured to include an array of chromatography columns
with a chromatography matrix immobilized within the array of columns, for
example,
with spacing such that it can be co-registered with the through-holes in an
array of
through-holes that do not contain a chromatography matrix. The physical size
of the
array of through-holes can determine the size of the array of columns.
Preferably, the
internal diameter of each column in an array of columns will be similar to the
internal
diameter of the corresponding co-registered through-hole in an array of
through-holes.
The array of columns should be mated to the array of through-holes in a manner
that allows the application of a positive or negative pressure across the
device and does
not result in cross talk between the individual samples. The number of columns
need not
be in a one-to-one ration with the through holes. Radial diffusion of samples
between the
layers of a stack of arrays of through-holes in response to an applied
external pressure
may result in cross talk and must be avoided. Inter-sample cross talk can be
eliminated
by forming a liquid-tight seal hermetic seal between the through-holes to
eliminate radial
diffusion. An elastomer sheet with holes co-registered with the through-holes
in the array
can be compressed between the layers of a stack to form such a seal.
Alternatively, a
thin, inert, porous polymer sheet can be placed between the two arrays such
that, when
the two columns are pressed together, liquid can flow through the pores in the
sheet, but
cannot flow laterally. Another approach entails manufacturing the array of
through-holes
such that one side of the immediate area around each through hole is raised
relative to the
rest of the array. An o-ring can then be placed around this raised area. When
two or
more arrays of through-holes are compressed together, the o-rings will form a
hermetic
seal, thereby eliminating radial diffusion and sample cross talk. The cross-
section of any
pair of mating arrays can also be fabricated to be interlocking with an
elastomer gasket or
coating between the mating surfaces, to provide a leak-tight fluidic seal.
One or more arrays of through-holes can be mated to an array of columns in a
similar manner. A top plate can then be affixed to the assembly that allows
for the
necessary chromatographic wash and/or elution buffers to be applied to the
each through-
hole. The liquid can be forced through the device in such a manner that the
sample will
be pushed through the array of through-holes into the array of columns upon
which the
chromatography will take place. If required by a specific application, the
wash and/or
elution buffers from the chromatography columns can be transferred via
capillaries to
another chromatography device, chromatographic fraction collector, or another
array of
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
through-holes. An illustration of such a device is shown in FIG. 14. The
fluidic
connections that lead to and from the device can be machined for easy coupling
to
standard fluidic connections using standard fluidic components such as ferules
and
compression fittings.
Device for fraction collection
Chromatography generally entails separation of a mixture of compounds on the
basis of differential chemical and/or physical interactions of the individual
components of
that mixture with a chromatographic matrix. A sudden or gradual change in the
physical
and/or chemical environment can affect the interactions between the components
of a
mixture and the chromatographic matrix. Typically, each component of a mixture
elutes
individually from the chromatographic matrix as the physical and/or chemical
conditions
are varied. It can be desirable to isolate a given component of a mixture of
compounds
for furtlier analysis or chromatography. In some cases, a component of
interest can be
identified by online spectroscopic analysis.
Devices for fractionating the eluant of a chromatographic column and storing
individual fractions are well known. One embodiment of the present invention
features a
system for collecting chromatographic fractions from an array of columns. The
column
eluate can be spotted dropwise onto another array of through-holes. By
controlling the
speed at which the array of through-holes is moved with respect to the array
of columns,
the volume of each fraction can be controlled. A fluid bridge can be formed
between the
exit capillary from the array of columns and a through-hole in a through-holes
array if the
interior surface of the through-hole is coated with a material with the
appropriate affinity
for the eluant. The maximum number of fractions that can be collected from a
given
column will depend on the size of each fraction, on the speed at which the
collection
array of through-holes is moved, and on the density of the array of columns.
If a large
number of fractions must be collected from each column, a linear array of
columns can be
used, its output being collected in a two-dimensional array of through-holes.
If the
fraction collection array of through-holes is then moved perpendicularly to
the linear
array of columns, the number of fractions that can be collected is limited
only by the
physical size of the collection array.
For some applications, a single chromatographic separation can take several
minutes or longer to complete. If long periods are required, it is possible
that evaporation
of liquid from the array of through-holes in the fraction collection device
will occur. To
avoid evaporative loss from the through-holes in the fraction collection
device, the entire
66
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
array of through-holes used to collect fractions can be placed within an
environmentally
controlled enclosure. If a high-humidity environment is maintained within the
enclosure,
evaporative losses can be minimized. Additionally, it can be desirable to
maintain a
certain teinperature within the enclosure (e.g., 4 C) to maintain compound
stability. The
elution capillaries from the array of columns can enter the enclosure through
a series of
precision-machined holes to maintain the integrity of the enclosure while
allowing for
introduction of the eluant from the array of columns. Elastomer gaskets may be
used to
ensure a good seal around the enclosure.
An array of through-holes containing the fractions collected from an array of
columns can be stacked with another array of through-holes to initiate a
second mixing
operation to initiate a chemical reaction. A second chromatography application
can be
initiated by stacking the array of through-holes into which the fractions were
collected
with a second array of columns. Alternatively, further spectroscopic or
spectrometric
analyses can be performed on the collected fractions at this time.
Spectrometric AnLlysis of Compounds in an Array of Through-Holes
Atmospheric pressure ionization mass spectrometa (API-MS)
Samples in an array of through-holes can be analyzed by a spectrometric
technique such as atmospheric pressure ionization mass spectrometry (API-MS).
The
spectrometric analyses are typically performed serially. Therefore, the chips
should be
environmentally isolated in a controlled temperature and humidity environment
to avoid
loss of sample due to evaporation. In API-MS, one simple method for
introducing the
sample to the mass spectrometer features aspirating a selected sample directly
from a
particular through-hole into a valve using a length of capillary tubing (e.g.,
as described
herein). A metered volume of sample can then be introduced into a mass
spectrometer
using standard API-MS protocols.
Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry
(MALDI TOF-MS)
In MALDI TOF-MS analysis, a sample of interest is generally mixed with one or
more matrix-forming compounds. Typically, a saturated solution of an organic
matrix
material (e.g., derivatives of hydroxycinnamic acid) is mixed with an equal
volume of
sample. In some applications of MALDI TOF-MS, the organic matrix compound is
replaced by inorganic nanoparticles (e.g., colloidal gold, quantum dots, or
porous silica).
The mixture is then spotted in the form of a regular and addressable array on
a flat plate
67
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
and allowed to evaporate completely. The sample plate is then positioned in
the mass
spectrometer, and the samples are ionized by irradiation from a pulsed laser.
Samples in an array of through-holes are well suited for analysis using MALDI
TOF-MS and related applications, since the necessary sample preparations steps
can
easily be accoinplished in a parallel fashion. For example, a second array of
through-
holes can be loaded witll a saturated solution of an organic matrix or a
slurry of an
inorganic matrix compound. The array of through-holes can either be dip-loaded
uniforrnly, or, if desired, any number of different matrix compounds can be
loaded into
individual through-holes in an addressable fashion. The sample and matrix
arrays of
through-holes can be mixed together by bringing the chips together (e.g., as
described
herein). After allowing the solvent to completely evaporate, the array of
through-holes
can be placed in a slightly modified receptacle in most commercially available
MALDI
TOF mass spectrometers. The conventional flat metal MALDI plate can be
machined
down to compensate for the thickness of the array of through-holes. The array
of
through-holes can be affixed with a temporary adhesive within the recessed
area of the
standard sample holder.
The laser used for sample ionization in the MALDI TOF mass spectrometer can
be focused within the through-hole to provide the necessary irradiance for
sample
ionization. Internal reflection of the laser beam within the through-hole can
possibly
increase the amount of laser energy absorbed by the matrix and transferred to
the sample,
thereby increasirig the amount of sample ionization. Additionally, an array of
through-
holes can allow for a very high density of samples to be spatially located in
a small
footprint without inter-sample contamination.
Typical MALDI-MS sample plates are solid surfaces onto which samples are
spotted. The laser used to ionize the samples must be on the same side of the
plate as the
inlet of the flight tube of the mass spectrometer, since the sample plate is
opaque to the
laser energy. The use of an array of through-holes as the sample plate allows
for the
source of laser irradiation and the inlet to the TOF mass spectrometer to be
located on
opposite faces of the sample plate. A scheme of this linear MALDI TOF mass
spectrometer is shown in FIG. 15. Translocation of the sample plate in front
of the inlet
of the flight tube allows for the laser ionization of a selected sample.
Alternatively, an array of posts or pins, precision-machined to fit into an
array of
through-holes, can be coated with the MALDI matrix material by dipping the
array into a
bulk matrix solution. After the solvent has evaporated, the pin array can be
inserted into
68
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
the through-hole array. Fluid contained in each through-hole is transferred to
the
corresponding pin surface. After the solvent has evaporated, the pin array can
be placed
at the input to a TOF mass spectrometer and the pins can be illuminated
sequentially with
a focused laser beam. In such a pin array, a portion of the sample from each
through-hole
can be held isolated from its neighbor by the air gap between each pin.
Through-hole array/Surface method.
When placed into an electric field or driven by pressure, the through-hole
array
can be used as a parallel capillary electrophoresis, electrokinetic
chromatography or
chromatography device. An array of samples in one through-hole array can be
introduced
into a second, typically longer, through-hole array. The second through-hole
array can be
filled with a gel (e.g. silica), a polymer (e.g., polyacrylamide) or a resin
and can have a
coating on its walls to prevent or enhance electro-osmosis or protein binding.
However,
if electrophoresis or chromatography is performed in such an array, it will be
difficult to
analyze the output of each column as molecules emerge from it. One way to
alleviate
this problem is to pass the output through a moving surface (e.g,
nitrocellulose sheet) with
an affinity for the' analyte molecules and then move the web to an imaging
detector. For
example, fluorescently labeled DNA or protein could be eluted onto a moving
nitrocellulose membrane and passed to a fluorescent imager to analyze. A
continuously
moving surface, moving in the manner of a tape, would cause smearing of the
samples,
therefore it is advantageous to reduce or reverse the polarity of the electric
field or
pressure during those periods of time when the surface is moving. An increase
in
sensitivity of the detection system is can be achieved by further delaying the
movement of
the surface and the voltage or pressure while the detector is acquiring an
image.
Alternately, the imager could be a line-scanner such as a fluorescence laser
line-scanner.
The surface could be fed from a long spool if desired (e.g., in a tape like
manner). The
surface can further be taken up on a second spool.
Readout methods.
A method of using wetting properties of the array to detect chemical binding
to the walls
of the through-holes.
The binding between two proteins, such as between an antibody and an antigen
is
detected via its affect on the surface energy of the channel interiors.
69
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
In a preferred embodiment, a library (e.g., a library of antibodies,
receptors,
macromolecules, or molecular probes) is bound to the interior walls of a
through-hole
array having hydrophilic channel walls and hydrophobic faces. The array is
rinsed with a
blocking agent, which binds to non-specific protein absorption sites on the
channel walls
but does not change the hydrophilic character of the wall surface. Such
blocking agents
include bovine serum albumin (BSA), powdered milk, and gelatin. The array is
then
immersed in a solution that contains antigen and is incubated for sufficient
time to allow
binding of antigen to complementary antibody. The arrays are then removed from
the
antigen solution and washed with buffer. The array is dried and dipped into an
aqueous
solution containing a chromophore or fluorophore. The presence of the antigen
on the
surfaces lowers the surface energy sufficiently such that the liquid is
prevented from
entering the through-holes. The empty through-holes can be identified by
imaging the
array. The empty through-holes then correspond to the antibodies in the
library that
effectively bind the ligand.
In another embodiment, the interior walls of a set of small array devices,
each
having roughly 1- 100 through-holes, are coated such that each array has a
different
antibody. The arrays are placed together in a solution of antigen and
incubated for
sufficient time to allow binding of antigen to complementary antibody. The
arrays are
then removed from the antigen solution and washed with buffer. All arrays are
then
placed together in a separation bath that contains a liquid that is non-
destructive to the
proteins and can be adjusted in density in some manner (e.g., by addition of a
higher
density liquid, or by adding a thickening agent).
When the holes remain empty, the density of the array in the bath is reduced
and,
under appropriate conditions, the array containing bound antigen can float to
the surface.
The presence of the antigen on the surfaces lowers the surface energy
sufficiently such
that the liquid is prevented from entering the through-holes. These floating
arrays are
removed and the identity of the antibody bound to each array is determined by
mass
spectroscopy, or from a code on each array, or by some other means such as a
bar code or
radio transponder built into the arrays. For this method the array can be a
porous structure
such as an aerogel or a porous bead.
A device for the analysis of an array of through-holes by mass spectrometry.
Samples or aliquots of samples can be removed from an array of through-holes
for
analysis by mass spectrometry by one of several different methods. One such
method
features drawing the sample or an aliquot thereof into a tube with the
application of
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
negative pressure. In one example of this approach, the tip of a syringe is
inserted into a
selected through-hole in an array and a metered amount of sample is drawn into
the
syringe. Alternatively a vacuum could be used to aspirate the samples into a
length of
tubing, a valve, or a container for storage.
For certain applications, it can be desirable to assay each sample in an array
of
through-holes by a serial process such as mass spectrometry. Application of a
serial
process to a large number of samples in an array of through-holes, even if
done very
rapidly, can still require a significant amount of time. If humidity
conditions and
temperature are not strictly regulated during this time, evaporation of
samples from the
through-holes can occur and artificially bias assay results.
One approach to controlling evaporation and facilitating the aspirating of
individual samples from an array of through-holes is to design an additional
array of
through-holes in which each of the through-holes is coregistered with a sample
through-
hole in the assay. This additional array of through-holes can be placed on top
of the
arrays of through-holes used in the assay to create a top plate. The through-
holes in this
top plate can be designed sucli that the diaineter of each through-hole can be
made to be
much larger at the outer surface than the diameter in the surface that
contacts the arrays of
through-holes used in the assay. The conical shape formed by such a through-
hole will
act as a guide for a syringe needle into a selected through-hole and
facilitate efficient
sampling. The outer surface of this top plate can also be coated with a thin
film of
polymer similar to that used in lamination. The sealed surface will act to
retard
evaporation. The syringe needle used for aspirating the sample out of the
through-holes
can easily perforate this thin film and will not hinder efficient sampling.
Once the sample is aspirated into a syringe, it can be delivered into a mass
spectrometer for analysis by any one of many techniques known by those skilled
in the
art. These can include atmospheric pressure ionization techniques such as
electrospray
ionization (ESI) or atmospheric pressure chemical ionization (APCI).
In another embodiment of the invention, a metal plate can be used as a bottom
plate for the array of through-holes. The solvent used in the assay can be
allowed to
evaporate and a solid sample can form in a footprint on the bottom plate that
corresponds
to the internal diameter of the through-hole. If desired, a matrix can be
added to the
samples before complete evaporation. Alternatively, a matrix can be added to
the surface
of the metal bottom plate before it is stacked with the arrays of through-
holes used in the
assay. Once the samples have completely evaporated the metal bottom plate can
be
71
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
removed from the arrays of through-holes and each sample can be analyzed by
matrix
assisted laser-desorption ionization (MALDI) or a similar surface based
ionization mass
spectrometry technique generally practiced by those skilled in the art.
In another embodiment of the invention, an array of pins coregistered with the
array of through-holes can be dipped into the array of through-holes and
removed.
Sample that is residually removed with the array of through-holes can be
allowed to
evaporate on the tips of the array of pins. As in the previous embodiment,
this evaporated
sample can be used for a surface based mass spectrometry method.
Time-gated fluorescence imaging of a through-hole array
Many biological assays are configured to give a fluorescent readout that can
be
acquired from an array of through-holes by fluorescence imaging. Typically,
light from
an excitation lamp or laser is passed through an excitation filter, through
the array,
through an emission filter and then to a CCD camera. In many cases, the
sensitivity of
the signal is limited by background light due to imperfect performance of the
filters, and
by inelastic and elastic scattering of light by the sample and optical
components.
Whereas the fluorophores of interest have fluorescence lifetimes of about 1 ns
to 1 ms,
scattering occurs at much shorter timescales. Thus removal of background light
can be
accomplished by the technique of time-gating. Time-gating the process of
illuminating
the sample while preventing the camera from acquiring data, quickly removing
the
excitation light, then waiting for a delay time before acquiring the
fluorescence emission
image. By not collecting photons emitted during the first 1 to 100 ps of after
excitation,
background noise is significantly reduced and signal to noise is improved. A
similar
apparatus can be used to repeat the data acquisition with varying delay times,
thus
yielding fluorescence lifetime information for each of the through-holes in
the array.
Various strategies can be used to construct a time-gated fluorescence imaging
system. A pulsed excitation source is needed and can be either a flash lamp or
laser such
as a passive or active mode-locked or Q-switched laser. If a laser is used, a
beam
expander and diffuser plate will give uniform irradiation of the platen. A
continuous
excitation source can also be used with a means for rapidly blocking and un-
blocking the
light such as an electro-optical, an acousto-optical cell or a rapidly
rotating disk with slits.
A pulse generator can be used to trigger the illumination source and detector
at a given
delay. The CCD camera can be electronically shuttered or physically shuttered
as with a
rotating disk with slits that is out of phase with the excitation pulsing.
72
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Optical Readout Based on Insertion of a Through-Hole Array into an Optical
Resonator
or Interferometer
A through-hole array can be inserted into either an optical resonator or an
optical
interferometer for simultaneous and parallel interaction of an optical field
with material
contained in each through-hole. (See FIG. 21) Such a method can be
advantageous when
the through-hole array (2) is placed in an optical resonator (Irradiation (1)
is shined and
amplified between mirrors (2) and (3).) so that the optical path length is
increased over
the length of the through-hole array, to increase absorption (5). The optical
path length is
increased as a multiple of the through-length, to increase optical absorption.
For
example, for simultaneous initiation of chemical reactions by the enhanced
optical field
characteristic of an optical resonator. It can also be advantageous as a means
for
simultaneous analysis of materials and interactions between materials
contained within
the through-holes, for example, by recording changes in incident optical field
intensity,
phase, polarization, or frequency, or by recording of these parameters, of
light emitted
from as a result of interaction between an incident optical field (e.g.
fluorescence,
phosphorescence), the materials contained in a through-hole, or a change in
the material
itself (e.g. luminescence).
One advantage of measuring these parameters with the chip as part of an
optical
resonator is that the resonator's resonance condition will change on
interaction of the
optical field contained within the resonator structure with the materials
contained within
each through-hole. These changes (e.g., phase, intensity, polarization,
frequency) are
intimately related to the composition and physical state of the material
contained in the
through-hole. The change in optical field parameters changes the resonant
condition of
the cavity, which, in turn, changes the intensity of light passed through or
reflected from
the resonator.
One can also take advantage of the increased optical field strength
characteristic
of optical resonant structures, for example, to initiate photochemical
reactions or non-
linear optical effects (multi photon absorption, harmoriic conversion, etc.)
as a probe to
measure properties of the materials contained in the through-holes. The
optical field
incident on the resonator can be either continuous in time or it can vary with
time as in an
optical pulse.
Examples of optical resonator structures that can be used in this embodiment
include Fabry-Perot-style interferometers with two planar mirrors and confocal
Fabry-
73
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Perot-style interferometers having two curved mirrors or one planar and one
curved
mirror.
The through-hole array can either be part of or be inserted into a two-beam
interferometer. Examples of such interferometers are many, and include
Michelson,
Twyman-Green, Sagnac, and Mach-Zhender-type interferometers. In the embodiment
where the array is inserted into one of the optical paths of a two-beam
interferometer, the
phase of the light is delayed according to the complex refractive index
(refractive index
and absorption) as a function of wavelength. The interferometer can be
illuminated with
a beam of white light of sufficient width to also illuminate the through-hole
array. For
each optical path length difference between the two arms of the
interferometer, a camera
at the interferometer output can record the pattern of light exiting the
interferometer and
corresponding to light that has passed through each hole in the array. A
series of images
can thus be acquired for each optical path length difference, and taking the
Fourier
transform of each pixel of the image as a function of optical path length can
generate an
optical absorption or emission spectrum for the materials in each through-hole
of the
array.
Another embodiment uses a two-beam interferometer to analyze light passed
through or emitted from a through-hole array. A camera records the light
pattern from the
interferometer for each optical path length difference imposed between the two
plane
mirrors that make up the interferometer. After recording a sequence of images
corresponding to each path length difference, individual interferograms can be
generated
from each set of pixels co-registered across the image sequence. Application
of the
Fourier transform to each interferogram can generate an absorption or emission
spectrum
at each spatial position in the image. In this way, the spectral content of
light interacting
with material contained in each through-hole of the array can be determined.
Application of the approach described in U.S. Patent No. 6,088,100,
incorporated
herein by reference in its entirety, adapted for full-field imaging, can also
allow for
capture of absorption spectroscopic information from each through-hole in a
stack of
through-hole arrays.
Image Center of Array as a Function of Thermal Perturbation.
The invention is compatible with many systems for detecting the output of the
arrays of chemical probes. Commercially available fluorescence scanners can be
used if
desired. Because each position in the array has two apertures (i.e., on the
top and bottom
faces of the platens), the array can be exposed to electromagnetic radiation
on one face of
74
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
the platen, and the optical properties of the samples in the array can be
measured via
detection at the opposite face of the platen. The positions of the array can
be imaged in
parallel or by serial scanning techniques. Both static and kinetic analysis of
reactions can
be utilized.
One method of detecting binding between an analyte and probes immobilized in
the walls of the through-holes includes observing the distribution of analyte
within each
through-hole as a function of a perturbation. For example, a ligand of
interest can be
covalently attached to the inner walls of each of 10,000 through-holes in a 2
sq cm.
platen. The chip can then be stacked with another chip containing, for
example, a peptide
library such that each member of the library occupies its own through-hole and
has a
fluorescent tag. After allowing sufficient time for non-covalent binding
reactions to reach
equilibrium, the chip can be rinsed with buffer to remove unbound material. By
observing the fluorescence distribution in each hole as a function of a
perturbation such as
increasing temperature or increasing formamide concentration, the members of
the library
can be ranked as to binding energy. Binding kinetics can be determined by
following the
fluorescence distribution as a function of time following a rapid perturbation
such as a
temperature jump. The sensitivity of the assay can be improved by using a mask
that
includes of an array of through-holes complementary to the chip, but of
smaller diameter,
that spatially filters the light such that only the interior of each through-
hole is observed.
Another method of increasing signal-to-noise ratio includes applying a
periodic or
stochastic perturbation such as temperature, and then observing only signal
correlated
with the perturbation.
An increasingly common technique in drug discovery is millisecond time-scale
fluorescence analysis of cell populations. Existing commercially available
devices utilize
a bank of syringes that add reagents from one 384-well microplate containing
drug
candidates to a second 384-well microplate containing cells loaded with a
fluorescent
indicator of calcium such as Fura-2, followed by laser scanning of the
underside of the
second plate to generate millisecond kinetic measurements of calcium release
from the
endoplasmic reticulum of the cells. It would be advantageous to use a white-
light
excitation source and a digital caniera to acquire such kinetic data due to
lower cost and
greater choice of excitation wavelengths. This was previously difficult
because the
syringe bank would obstruct the light path in such a system and would haved to
be moved
on a millisecond time scale, which is not typically possible. Use of a white-
light source is
possible by initiating mixing of samples stored in an array of through-holes
with cells
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
growing in a second array, both arrays being in the camera system. An
additional benefit
of this method is that the throughput of samples collected is greatly
increased by the use
of through-hole arrays containing as many as 20,000 or more samples.
Typically, the
system is automated and will collect data that begins at the moment of mixing
or
otherwise indexes the times associated with collected data points to the
moment of
stacking. The types of assays possible with this method are not limited to
cell or
fluorescence assays, any assay with an optical read-out that occurs on a time
scale of less
than minutes can benefit from the invention.
A preferred embodiment of the invention includes at least two stacked and co-
aligned platens containing through-hole arrays consists of (i) a detection
device; (ii) a
means for introducing platens into the detection device (iii) a means to
register the platens
to cause fluid to communicate between at least some of the co-registered
through-holes
and (iv) a means of contacting the platens to initiate mixing of reagents
simultaneously in
at least some of the through-holes. The detection device (Figure 24) can be an
imaging
device, such as a CCD camera, with optical filters and a light source for
ilhtmination.
Light from an optical source (1) illuminates parabolic collimation mirrors (3)
after
passage through a flexible, bifurcated fiber bundle (2). The light then
illuminates the
stack of arrays at an oblique angle such that light rays transmitted through
the array
through-holes does not enter into the camera lens (5). This optical
arrangement is
desirable as a simple means to decrease optical background for increased
optical
sensitivity.
Separation Methods.
A method of separating a stack of two or more arrays filled with liquid.
It can be desirable to separate arrays once the contents of individual through-
holes
have been combined by stacking. For example, in order to perform a dilution by
two an
array filled with a chemical library is stacked onto an array containing
solvent buffer.
After sufficient time for mixing of the two sets of liquids (approximately 15
seconds for
100 nl), the plates are separated to produce two identical arrays of libraries
members at
half the initial concentration. This process can be repeated to produce a
dilution series.
When two through-hole arrays are stacked such that their contents mix, the two
plates are not readily separated with out cross-contamination between
neighboring
76
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
through-holes. Pulling one plate against the additive surface tension created
by many
microscopic columns of fluid invariably introduces shear forces that mixes the
contents of
individual through-holes together as the chips are separated.
A method is proposed to separate each fluid column into two shorter columns
separated by a small vapor phase. Small electrodes at the interface between
the two
stacked arrays produce a small volume of gas in each through-hole. As the
bubble grows
it recreates the liquid vapor interfaces between the two arrays. After each
column is
cleaved in two the arrays are separated mechanically.
Alternatively an inert, humid gas is introduced into the atmosphere above
and/or
below the stacked arrays and nucleated at the interface between the two
stacked arrays.
Alternatively the gas can be pumped into the center of each through a matching
array of very fine hollow tubes.
Centrifuging (performing gravimetric separation in) an array of through-holes.
Many biological or chemical assays require centrifugation and/or filtration of
samples. In many it can be desirable to filter or centrifuge samples in a
biological or
chemical assay that is performed in array of tlirough-holes. The following
invention
pertains to a device for the centrifugation and or filtration of an array of
through-holes.
A metal jig with two flat surfaces larger than the array of through-holes can
be
built. A single or a stack of arrays of through-holes are placed between the
two flat
surfaces and evenly compressed together with the application of force on the
metal plates.
The metal jig will be machined such that the amount of compression can be
adjusted as
desired, preferably by a simple tightening of several screws holding the jig
together.
In some applications the filtrate or pellet will need to be recovered from the
centrifuged sample. In other cases the pellet formed after centrifugation will
need to be
removed. A simple solution for this is to machine a plate that contains an
array of wells
or dimples of a metered volume that are coregistered to the array of through-
holes. The
array(s) of through-holes can be stacked atop this bottom plate with
coregistered wells. If
desired, a filter can be placed between the bottom plate and the array(s) of
through-holes.
After the centrifugation is complete, the bottom plate can be removed and the
filtrate or
contaminating precipitate can be removed. Alternatively, if the supematant is
the desired
fraction, it can be aspirated directly from the array of through-holes without
the need for
removal of the bottom plate.
In certain applications a large centrifugal force can need to be applied to
the
array(s) of through-holes. Even with a bottom plate and/or a filter
application of large
77
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
amounts of centrifugal force a stack of arrays of through-holes can result in
a lateral
displacement of samples that are forced into the spaces between the individual
layers of
the arrays of through-holes. Coating of the contacting surfaces of the array
of through-
holes with a hydrophobic material will inhibit lateral diffusion between
layers at
relatively low centrifugal forces. When high levels of centrifugal forces are
required a
material can be staclced within each individual array of through-holes such
that when the
array of through-holes are compressed together by the metal jig a leak-tight
seal will be
formed between the two arrays of through-holes. If the material used to form
the seal is
porous it will not impede the flow of liquid between the layers of through-
holes, yet it
will form a tight seal against lateral flow.
VI. Miscellaneous uses.
To fully realize the potential of reagent volume savings and increased
throughput
provided by nanoliter volume fluid handling, means and methods of storing
chemical and
biological samples at high density in low volumes are necessary. These storage
systems
must be easily access by microfluidic screening instrumentation.
One embodiment of the invention provides the a method to store chemical or
biochemical samples at high density and in low volumes. Additionally, samples
stored
using the devices and methods of the invention can be assayed with a minimum
of liquid
handling steps or other manipulations. The method includes placing a small
volume of
compounds dissolved in a solvent in an array of through-holes and adding a
second
solvent to the array of through-holes without causing substantial migration of
the
compounds out of the through-holes. The result is an array of compounds
dissolved in a
liquid that is primarily comprised of the second solvent.
The invention further provides a method of storing compounds in a manner
wherein the samples can be readily introduced into aqueous medium for
performance of
an assay. The step include dispensing a volume of compound in a solvent that
is much
less than the volume of the container that it is dispensed in, storing for
some time, and
adding an aqueous medium to fill the remaining volume of the container in
preparation
for an assay.
In a preferred embodiment, integration of low-volume compound library storage
and screening includes the following steps: (1) Dispensing volumes of compound
dissolved in a non-aqueous solvent that are smaller than the total volume of
the containers
to be used for screening. Usually, the volume dispensed will be less than half
and could
78
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
be 1/10th of ,1/40th of or less than the total capacity of the container.
Usually, the
containers will be part of an array of containers. The container is preferably
a hydrophilic
area surrounded by hydrophobic areas, such as a channel of a through-hole
array, or a
spot on a glass slide with spots of hydrophobic areas in a hydrophilic
background. (2)
Storing the compounds for some period of time. Storage conditions may consist
of a low
temperature such as 4 C, -20 C, -80 C, submerged in liquid nitrogen, or a
lower
temperature. Desiccation is usually desirable. (3) Removing the compounds from
storage, and elevating them to above the freezing point of the aqueous media
to be loaded
into the containers, but above the freezing. (4) Adding aqueous medium to the
containers. Preferably, the aqueous medium is chilled to above its freezing
point and
below the freezing point of the non-aqueous medium. The addition could be done
by
dispensers, but is more rapidly and inexpensively done by dipping the
containers into a
bath of the aqueous medium. It is advantageous to have the non-aqueous solvent
be in
the solid form so as to minimize loss of the compounds into the aqueous medium
and to
prevent communication of compounds in adjacent or nearby channels. (5) Waiting
for a
time sufficient to allow mixing of the compound with the aqueous medium. And
(6)
Performing an assay or measurement upon the samples.
Methods for dispensing small volumes into large holes.
It is advantageous to screen samples such as drug candidates in very small
volumes in order to conserve reagents and increase through-put. A typical
through-hole
array format will have channels that hold 60 nl of fluid and a typical assay
will be done
with two stacked chips, holding a total of 120- nl. If the maximum
concentration of
DMSO acceptable in the assay is 2%, then a total of 2.4 nl must be dispensed
into one of
the channels and this is extremely difficult to do with conventional liquid
handling
systems. One way to solve this problem is to dissolve the compounds in a
volatile solvent
such as ethanol, DMSO, water, dispense the compounds and allow the solvent to
evaporate, leaving a spot of dried compound in the container. This has some
major
disadvantages in that the compound may crystallize into a form that does not
easily re-
solubalize, and is not sufficiently immobilized. Another approach would be to
dispense
the compound in a volatile solvent such as DMSO and allow the DMSO to
evaporate to
leave the desired compound in a lower volume of DMSO. In this case, it may be
difficult
to evenly evaporate the solvent, which may interfere with the assay to be
performed on
the samples.
79
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
A more robust approach is to dissolve the compound in a first volatile solvent
such as DMSO and a second, more volatile solvent, such as ethanol or methanol,
dispense
the mixture into the containers and allow the second solvent to evaporate,
leaving
coinpound dissolved in to first solvent. Ethanol and methanol are good choices
since they
will dissolve most drug-like compounds and are hydrophilic enough to remain
contained
in a hydrophilic container surrounded by hydrophobic barriers, although other
solvents
could be used. DMSO is a good solvent for use as the first solvent since it
dissolves most
compounds, will stay in place due to its hydrophilicity and is not very
volatile,
evaporating more slowly than water.
The invention also features a method for storing compounds dry in an array of
sub-microliter containers separated by hydrophobic barrier. Compounds are
dissolved in
a volatile solvent or combination of solvents, introduced into the array of
containers and
the solvent is allowed to dry, leaving a thin film of solid compound. If
necessary, a
biocompatible adhesive is added to keep the film attached to the walls of the
container.
A solvent mixture containing a first volatile solvent and a second less-
volatile solvent is
added to substantially all of the containers in the array. In a preferred
embodiment, the
solvent mixture comprises an alcohol such as ethanol and DMSO. The more
volatile
solvent is allowed to evaporate, leaving a residue of the less-volatile
solvent that dissolves
at least most of the compounds in the array. Typically, the less-volatile
solvent will
comprise the minority of the solvent mixture, is usually less than 20%, and is
often less
than 5% of the solvent mixture. The array is then prepared for assay by
introducing an
aqueous media that is compatible with the assay such as water or a buffer. The
aqueous
media may be dispensed by the methods described above.
EXAMPLES
The invention is further described in the following examples, which do not
limit
the scope of the invention described in the claims.
Example 1 -- DNA Probe Array.
A 50,000-channel through-hole array is fabricated from silicon. Using the gene
database, series of 80 spatial filter top masks with another 80 identical
bottom masks are
fabricated. Arrays are aligned and derivatized with 3-
glycidoxypropyltrimethoxysilane in
order to provide a free functional group coating on the interior surfaces of
the through-
holes.
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
A stack of ten coated platens is aligned. Alignment is verified by observing
the
optical transmission of the various channels. Mask #1 is aligned with the top
of the stack
and an identical mask aligned in the same orientation and the bottom of the
stack. Mask
#1 is constructed such that the positions in the mask that corresponded to a
gene in the
database with an adenosine in the first position are open and allow flow
through the
through-holes in those addressable positions. The stack is rinsed with dry
acetonitrile. A
phosphoramidite monomer at a concentration of 0.1 M acetonitrile and tetrazole
is added
by a pressurized flow against the mask. The coupling reaction is allowed to
proceed for 3
minutes. An oxidizing solution of iodine/lutidine/acetonitrile/water is
introduced into the
chip stack and incubated for 2 minutes, followed by rinses with acetonitrile
and
dichloromethane. The chip is dried by vacuum.
The masks are removed and replaced with a top and bottom Mask #2
corresponding to positions to which a T monomer is to be added. A deprotection
agent is
added, and the T coupling reaction is commenced.
This process is repeated with Mask #3 for G in the first position, and Mask #4
for
C in the first position. Mask #5 corresponds to A in the second position, and
so on. After
the synthesis is complete the chip is stored in 30% ammonia for 12 hours.
To test the array, a fluorescein end-labeled 20-mer corresponding to one of
the
intended probes in the array is synthesized by standard methods, dissolved in
a
hybridizing solution of 6X SSC/0.5% SDS, and introduced into all through-holes
of the
array simultaneously. The array is washed with 0.5X SSC and the chip is imaged
fluorescently. Only the position in the array corresponding to the probe
complementary
to the test oligonucleotide shows a significant increase in fluorescent
intensity over the
background level determined by averaging the signal from the remaining
positions in the
array.
Example 2 -- Catalyst Screening.
A recombinant enzyme library is screened in a dense array of through-holes
against a fluorogenic substrate. Genetically diverse E. coli containing the
gene for the
protease subtilisin with a poly histidine tag is created by mutagenesis. A
dilute solution
of the bacteria is added to a nickel-coated array such that there is an
average of 1 to 2
bacteria per through-hole. The bacteria are allowed to grow to the log phase
and replicate
plated (as described above). The bacteria in the array are lysed by heating,
allowing the
tagged subtilisin to attach to the nickel coated walls of the array. Another
array
81
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
containing the fluorogenic substrate and reaction buffer (Boehringer) in each
well is
staclced with the first array. The stack is immediately placed in a CCD camera-
based
fluorescent imaging system, and the rate of increase in fluorescence intensity
is measured
for each througli-hole. The enzyme with the fastest rate is selected and the
corresponding
bacteria in the replica plate are grown for further studies.
Example 3 -- High Throughput Screening with Beads.
A 100,000 member combinatorial library immobilized via a photocleavable linker
on 10 micron diameter beads is purchased from Affymax. A platen is prepared
with
100,000 through-holes of a diameter such that only one bead fits in each hole.
The beads
are washed in PBS, suspended in a solution containing a fluorogenic substrate
for the
enzyme to be screened, and spread over the platen with a rubber spatula. An
ultraviolet
lamp is used to decouple the members of the library from the beads. A second
platen
filled with a solution of the enzyme in a reaction buffer is aligned and
stacked on top of
the first chip. The stack is immediately imaged by epi-fluorescence to
determine the rate
of increase in fluorescence intensity as a function of channel position. Those
holes that
exhibit decreased enzyme rates are selected for further analysis as drug
leads.
Example 4-- Absorption Assay.
A single-cell layer of CaCo-2 cells is grown on two identical anisotropic
membranes coated with collagen slightly larger than the array of through-
holes. The cell
culture conditions, media, and membrane coatings used in the in vitro growth
and
maintenance of CaCo-2 cells are well known by those skilled in the art. Once a
uniform
layer of cells is established, each membrane is sandwiched between two
identical arrays
of through holes dip-loaded with culture maintenance media. The array and
membrane
assemblies are cultured an additional day to allow for the CaCo-2 cells to
equilibrate and
form an intact layer within the through-holes. Two additional arrays with
through-holes
co-registered with the CaCo-2 arrays are loaded with a chemical diversity
library of small
molecules. A compound known not to pass through CaCo-2 cells (e.g. mannitol)
is used
as a negative control to assess the integrity of the CaCo-2 cell monolayer,
while a
compound known to easily diffuse through CaCo-2 cell monolayers is used as a
positive
control. One of the arrays containing the chemical library is stacked on the
apical side of
one of the CaCo-2 cell monolayer arrays to assess apical to basal absorption
while the
82
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
second identical chemical library array is placed on the basal side of the
other CaCo-2
cell monolayer array to assess basal to apical absorption. The completed
arrays are
incubated for 1 hour, allowing time for transport or penneation of the library
compounds.
The oral bio-availability of the chemical library is assessed by quantifying
the amount of
library compound diffused through the CaCo-2 cell monolayer (e.g. by mass
spectrometry).
Example 5 -- Ligand Fishing by Blotting from a 2-D Gel.
Cellular proteins exhibiting an affinity for a ligand are identified using a 2-
D gel.
A platen having 500,000 through-holes is derivatized in order to covalently
link a
segment of the human epidermal growth factor receptor to the inside of each
through-
hole. Each hole is filled with a buffer solution. A cellular extract of a
human cell line is
then separated on a 2-D gel of a size similar to that of the platen, and then
aligned with
the platen. The proteins in the 2-D gel are then blotted onto the chip by
applying a buffer
above the chip and drawing fluid through the chip by placing the chip on a
blotter. The
proteins are thus transferred through the chip and those that have an affinity
for the
epidermal growth factor receptor are retained in the chip. A denaturing
solution
composed of 1M fonnamide in 100mM Tris buffer at pH 8 is used to blot the
contents of
the chip onto a platen for mass spectrometric analysis. The location of those
holes that
contain a protein with affinity for the receptor and the mass of the proteins
contained in
those holes are used to determine the identity of the binding proteins. These
proteins can
be targets for drugs that block the EGF signaling pathway as a treatment for
certain
cancers.
Example 6 -- Screening for Antibiotics.
A 500,000 member combinatorial peptide library is prepared in a platen that
includes 500,000 through-holes such that the peptides are dissolved in a
sterile cell
culture medium within the holes. The library is prepared such that the
identity of the
peptide present in each through-hole is known. A dilute culture of
Enterococcus faecium
bacteria is prepared such that each through-hole will receive on average 10
bacteria in cell
culture medium. The bacteria platen is stacked with the peptide library platen
to achieve
mixing then incubated at 30 C for 5 hours. The stacked platens are then imaged
by light
scattering measurement to determine the degree of bacterial growth in each
through-hole.
83
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The holes showing a greater than 99% reduction in growth are identified, and
larger quantities of the corresponding peptides are synthesized for further
analysis.
Exam-ple 7 -- Finding the Peptide Target of a Kinase by Radiolabeling.
A protein suspected of being a protein kinase is isolated. The protein is
incubated
with radiolabeled ATP substrate in the presence of 100,000 different proteins,
all
occupying unique positions in a platen having through-holes. After incubation
for a
sufficient time (e.g., about 20 minutes), the platen containing through-holes
is washed
with water and the presence of radiolabeled proteins is detected by a phosphor-
imaging
system. The protein target for the kinase is thus identified.
Example 8 -- Cytochrome P450 Inhibition Assays by Fluorescence.
The purpose of this experiment is to examine the potential of a library of
compounds to inhibit a specific CYP450 enzyme. The protocol is adapted from
Crespi et
al., Anal. Biochem. 248:188-190, 1997. The fluorometric substrate is 3-cyano-7-
ethoxycoumarin for CYP 1 A2, CYP2C9, CYP2C 19 and CYP2D6, and resoufin benzyl
ether (BzRes) for CYP3A4. These reagents are obtained from Pharmazyme. A
compound library is loaded into one platen array device for each of the CYP450
enzymes
to be tested at a concentration equivalent to the Km of each enzyme. The
appropriate
substrate containing reaction mixture is added to a second platen array device
and the
enzyme is added to a third platen array device. The chips are stacked to
initiate the
reaction, and the increase in fluorescent signal is monitored continuously by
fluorescent
imaging. The relative rates of P450 inhibition are used to select a drug lead
from the
candidate compound library.
Example 9 -- High Throughput Protein Crystallization.
A protein in a solvent is uniformly loaded into a through-hole array. The
array
through-holes are randomly filled with solutions containing different salts,
randomly
changing the concentration and relative abundance of the salts. Acidic and
basic
solutions are subsequently loaded into the through-holes, varying the pH. A
temperature
gradient is applied to the array device (or the array can alternatively be
held at a constant
temperature), causing the solvent to evaporate at a given rate. Additionally,
the partial
pressure of solvent can also be changed in the container in which the array is
placed to
change the evaporation rate. Those through-holes in which protein
crystallization is
84
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
observed are exposed to a beam of X-rays or electrons and the diffraction
pattern
recorded for analysis. An important benefit is the rapid and efficient
discovery of
experimental conditions leading to crystallization. Furthermore, protein
crystals can be
analyzed directly in the through-hole array.
Sixteen different crystallant solutions, and eleven different buffers, are
obtained
from Emerald Biostructures (Bainbridge Island, WA), and randomly loaded into
the
platen through-holes together with lysozyme. A temperature gradient is applied
across
the platen, and the excess liquid is removed with a rubber spatula. The system
is sealed
in a container with 20 ml of precipitant solution. Optimal solution conditions
are
determined for crystallization using LC-MS (liquid chromatography-mass
spectrometry).
Example 10 -- Ultra High Throughput Mixture Separation and Screening in Dense
Arrays of Through-Holes.
In many situations such as screening of natural products for pharmacologically
active molecules, complex mixtures need to be rapidly and efficiently
separated and
screened against protein targets. The purpose of this experiment is to
separate and screen
in a dense array of through-holes a complex mixture of natural products
against a
fluorogenic substrate. The natural product sample is first prepared in the
normal manner
for high pressure liquid chromatography (HPLC). As liquid elutes from the
chromatographic column, equi-volume samples are acquired and stored
sequentially in
the array through-holes. A replicate plate can be generated simultaneously.
Fluorogenic
substrate is then loaded uniformly into a second through-hole array. After
completion of
chromatographic separation, each sample in the array is exposed to the
substrate by
stacking the sample plate onto the substrate plate. Optical monitoring the
fluorescence
signal from the assayed fractions selects samples for further evaluation
either from the
assayed plate or the replicate plate.
An extension of this method to applications where multiple mixtures are stored
in
a dense array of through-holes is also described (e.g., separation and
identification of the
active component in a mixture). In one embodiment, a capillary tube array
having the
same center-to-center spacing as through-holes in the platen is brought into
contact with
the sample array. Each tube is located to spatially address one hole in the
array. The
opposite end of the tubing array is inserted into a stack of arrays where each
capillary
tube addresses a single column of through-holes on spatially co-registered
arrays. The
number of stacked arrays equals the number of elution samples to be captured
and
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
analyzed. Each tube is pre-filled with a porous gel suitable for
chromatographic
separation of the mixtures. An array plate filled with buffer is stacked onto
the sample
plate and a pressure is applied to drive the buffer through each through-hole
and into the
corresponding capillary tube. As liquid exits from each tube, the through-hole
in which
the tube resides is filled. As the tube array is slowly withdrawn from the
array stack the
liquid sample is retained in the through-hole. One advantage of this scheme is
that
fractions eluted from the array of capillary tubes are simultaneously
collected. Once an
array is filled, it can be removed from the stack and assayed, (e.g.,
contacting with a
platen array containing a fluorogenic substrate). Active compounds revealed by
fluorescence emission are then removed from the plate for analysis.
Alternatively, the
flow rate of liquid from the tubing array and withdrawal velocity can be
chosen such that
two plates are filled with essentially the same fraction eluted from the
column. The two
plates are removed; one is assayed while the other serves as a replicate
plate.
A further extension of this method to interface parallel HPLC separation with
inherently serial analytical methods such as mass spectrometry is now
described. FIG. 13
depicts, an array of capillary tubing (1) is interfaced with a sample filled
through-hole
array (2). However the opposite end of the tubing array is splayed in such a
manner so as
to increase the distance between consecutive rows (or columns) of the array
whilst
keeping the others intact. A series of spacers (3) through which the tubing is
inserted to
form the array provides structural integrity. The sample array is brought into
contact and
co-registered with the capillary tubing array. Each capillary tube in the
array is pre-filled
with porous gel suitable for chromatographic separation of the mixtures
contained in the
array through-holes. Pressurized carrier solvent is forced through the holes
in the array
and carries one sample into one capillary tube. Lengths of the tubing in the
array are
chosen so as to give the desired separation efficiency for the components in
the mixtures
analyzed. A fiber or thin tape runs just below and parallel to a row or column
of the
capillary tubing array. Lateral motion of the tubing array relative to the
fiber brings the
tubing ends in one column/row in the array into contact with the fiber (or
tape) (4). Fluid
from each tube is transferred to discrete spatial locations along the fiber.
After the fluid is
transferred, the fiber is advanced through a vacuum interface (5) and the
fluid drops are
sequentially presented to the mass spectrometer (6) for analysis. After one
set of drops is
deposited, the surface (e.g., nitrocellulose fiber) is advanced a distance
sufficient for next
set of drops to be deposited. Note there are only two displacements required,
the surface
in one direction and the array in the orthogonal direction.
86
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
With a combined time to make a mass spectral measurement and move the fiber of
about 300 ms, a row of 100 drops is transported and analyzed in 30 seconds.
The entire
10,000-tube array is analyzed in 3000 seconds (50 minutes). As shown in U.S.
Patent No.
6,005,664, stochastic sampling can substantially reduce the number of data
points (by up
to a factor of 10) needed to reconstruct a signal compared with equi-spaced
sampling.
Implementation of a stochastic sampling protocol could,greatly reduce the
analysis time.
Example 11 -- Optimization of chemical synthesis conditions.
Optimization of a chemical synthesis by changing one or more process
parameters
and recording the amount of material synthesized for a set of reaction
conditions.
Modified process parameters include types of reagents, reagent concentration,
sequence
of addition/mixing, temperature, and time.
A series of hydantoin compounds, pharmaceutical drugs useful for treatment of
epilepsy, are prepared using the new methods in solid-phase synthesis. Each
step in the
synthesis process consists of reaction with a solvated reagent under a given
set of time
and temperature conditions and then a wash to remove the excess (unreacted)
reagents.
Microspheres made from resins having different functional groups (e.g.,
hydrogen,
phenyl, methyl, benzyl, and s-butyl) and a protected amide group are either
purchased or
synthesized. An array is manufactured with tapered holes such that as the
beads are
spread across the surface, only one resin bead fits into each through-hole.
Microspheres
made from different resins are mixed together and spread across the array in a
dilute
solution such that all the holes are filled, each with a microsphere made from
a different
resin. Next, a mask is placed over the regular array of through-holes and the
amine
groups of the exposed resin beads are deprotected by exposure to a strong acid
(e.g.
trifluoroacetic acid) or base. After a wash step to remove the acid or base,
the same
resins are exposed to one member from an isocyanate group library consisting
of different
chemical and structural moieties. Reaction between the isocyanates and amide
groups
from a urea with a variable moiety. Such chemical units include hydrogen,
butyl, allyl, 2-
trifluorotoyl and 4-methoxyphenyl. The reaction proceeds at an elevated
temperature for
a certain time after which the exposed holes are washed to remove unreacted
reagents.
This process is repeated with a new mask exposing some of the original holes
as well as
new ones to expose these microspheres to a new isocyanate library group. Upon
completion, the hydantoins are screened against targets to find those potent
compounds.
Varying the reagents, their concentrations, the reagent sequences, the
reaction
87
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
temperature and reaction time provides a rapid and efficient method to
optimize the
synthetic rout.
Example 12 -- Selection of Phage Antibody Libraries (Multi-Chip Method)
A library of phage antibodies against a target antigen in a dense array of
through-
holes is screened. The protocol was adapted from (Winter,
http://axiintl.iint.uni-
marburg.de/-rek/AEPStart.html).
Through application of recombinant DNA technology a large (109 to 1010) and
diverse monoclonal antibody library is produced and stored as DNA plasmids in
bacterial
(E. coli) colonies. A single round of conventional affinity selection is
performed in an
immunotube coated with the target antigen. After incubating, blocking, and
washing, the
specifically bound phage are eluted and used to reinfect additional E. coli.
Two 10,000-through-hole silicon arrays are fabricated. Both array devices are
loaded with a solution of purified target antigen. The array devices are then
incubated at
4 C for approximately 12 hours, washed and filled with a blocking solution
consisting of
4% dry milk powder in phosphate buffered saline (PBS). After one hour the
blocking
solution is removed, the array devices are rinsed and refilled with a buffered
solution of
IPTG to induce phage expression in the bacteria.
A sufficiently dilute solution of the phage-infected bacteria is added to a
third
10,000 through-hole array such that there is an average of 1-10 bacteria per
through-hole.
The resulting through-hole array, henceforth referred to as the Library
Expression Chip, is
incubated until the bacteria reach log phase. The Library Expression Chip is
stacked
between the two antigen coated through-hole arrays. The stack is then
incubated for two
hours in a high humidity chamber to allow binding of the phage antibodies to
the antigen.
The antigen through-hole arrays are washed to remove bacteria and unbound
phage and
separated from the Library Expression Chip.
The bound phage from one of the two antigen through-hole arrays, henceforth
referred to as the Phage Inoculation Chip, is eluted by filling the through-
holes with a
solution of 100 mM triethylamine, incubated for approximately 10 minutes, then
neutralized by stacking onto another 10,000 through-hole array containing 2X
Tris-HCl
buffer. The eluted phage is used to inoculate uninfected bacteria contained in
a fourth
88
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
10,000 channel array. This array, henceforth referred to as the Positive
Expression Chip,
is grown to log phase and stored.
Meanwhile, the second antigen-phage chip, the Antibody Selection Chip, is
analyzed for bound phage antibody via an indirect ELISA assay. The protocol is
adapted
from Current Protocols in Inanaunology, Supplement 15, pages 11.2.2-11.2.5.
This
protocol is an indirect ELISA to detect specific antibodies. Other forms of
ELISA assays,
such as direct competitive ELISA assays can be implemented in the through-hole
arrays
with minor modifications to the procedure described in this example.
The Antibody Selection Chip is filled with a solution of the developing
reagent,
an-M13 conjugated to horseradish peroxidase in blocking solution. After
incubating for
thirty minutes at room temperature, the array is washed, blocked, and washed
again. The
array is then filled with a solution of fluorescent substrate, and incubated
for 30 minutes
at room temperature. A fluorescent image is then collected every 5 minutes for
one hour.
All positive through-holes are identified by an increase in fluorescent
intensity with time.
Each corresponding bacterial culture is then extracted from the Positive
Expression Chip
and dispersed onto agar (containing ampicilin) in a separate cell culture
well. These
selected bacterial cultures constitute a source of additional phage antibody
for subsequent
analysis.
In an alternative method, the Library Expression plate, rather than the chip
is
replica plated. Only a single antigen through-hole array is exposed to the
antibody
library. After identifying positive interactions in the Antigen Selection
chip, the bacteria
from the corresponding through-holes of the replicated Library Expression
plate are
diluted and dispersed across another 10,000 through-hole array. The assay is
repeated
until to ensure that all selected bacterial colonies are monocultures.
Example 13 -- Selection of Phage Antibodies (Single Plate Method).
A library of phage display antibodies is screened against a target antigen
using a
single 10,000 channel array of through-holes. By performing the selection in a
single
array simplifies the screening process, the phage must be reconstructed from
their DNA,
ecause the ELISA assay renders selected phage incapable of reinfecting
bacteria.
The target antigen is immobilized on the walls of the through-holes by filling
an
array with antigen solution and incubating in the environmental chamber. Non-
specific
binding sites are blocked by washing the array with blocking solution.
89
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
A solution of the phage-infected bacteria is added to all through-holes in the
array
such that there is an average of 1-10 bacteria per through-hole. The assay
device is then
incubated in a humidified chamber until the bacteria reach log phase. The
assay device is
submerged in a solution of IPTG for a period of time sufficient for diffusion
of IPTG into
through-holes, but insufficient to permit bacteria to diffuse out of the
array. The assay
device is incubated to allow expression of phage antibody, and antibody-
antigen binding.
The expression of phage antibody in the presence of the target antibody is
counter to
current methods in the art, and can reduce the number of antibodies selected
in the screen.
Next, the array is washed to remove bacteria, supernatant, and unbound phage
from through-holes. Bound phage antibody is then detected by an indirect ELISA
assay.
After collecting a fluorescent image, the plate is washed and filled with
elution reagent to
elute bound phage. Solutions in through-holes that are identified
corresponding to all
positive through-holes by cherry picking to individual wells of a 96-well or
higher density
microtiter plate. Phage DNA is then amplified by PCR and sequenced. The DNA
sequences are used to identify the structure of selected antibodies and to
reproduce more
phage antibody for further experimentation.
Exa mple 14 -- Protein Chip.
One hundred thousand protein-binding probes are produced using phage display
technology according to the methods described in Sheets et al., Proc. Natl.
Acad. Sci.
USA, 95:6157-6162, 1998, incorporated by reference in its entirety, such that
each probe
selectively binds a particular human protein with high affinity and
specificity. The probes
are transferred into a platen, such that different protein-binding probe in
each of its
through-holes. The platen has a TEFLON (R) surface to prevent wetting and
protein
absorption.
Tissue samples are homogenized, and the protein portions are extracted and
equilibrated with the platen. The platen is washed to remove non-specifically
bound
molecules. The contents of each through-holes are analyzed by heating the
platen from
ambient temperature to 100 C over a two minute period while using Raman
imaging to
detect protein desorption from the walls of the through-holes into the centers
of the
through-holes.
Example 15 -- Construction of an Array of Micro-HPLC Columns for Rapid
Parallel
Sample Separation and Purification
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
An array of twelve through-holes in a linear arrangement is machined in a
block
of material (e.g., metal, ceramic, or plastic). The through-holes have a
diameter of
250 ,um, a total length of 20 mm, and a center-to-center spacing of 9
millimeters. The
internal diameter of the distal end of the array of through-holes is increased
and threaded
to accept standard 1/16" outer diameter HPLC tubing using a standard nut-and-
ferule
compression fitting. A 2 cm long piece of 50 m internal diameter, 1/16" outer
diameter
tubing is mated to each of the through-holes in the array through machined
compression
fittings. A 1/16" outer diameter stainless steel frit is placed inside the
through-hole and is
held in place with the compression fitting. Chromatography media is then
packed into the
each through-hole in the array in the form of a slurry. The chromatography
media is
immobilized within the through-hole due to the stainless steel frit at the
distal end, and
creates a micro-HPLC column. When pressurized, sample, wash, and elution
buffers will
flow through the chromatography media and the frit, and elute from the through-
holes
through the mated 50 m internal diameter tubing at the distal end.
Samples are loaded onto the array of micro-HPLC columns by mating a bank of
syringes to the micro-HPLC array as detailed in examples 2 and 3. Wash and
elution
buffers are flowed through the micro-column array using the same syringe bank.
A flow
rate of 1 to 20 l of liquid per minute is used to wash and elute the samples
from the
micro-columns. The use of narrow bore tubing at the distal end of micro-HPLC
columns
results in the liquid eluting from the column to form small droplets. The
column eluate is
collected in an array of wells (e.g., a microtiter plate). The eluate is
fractionated by
collecting individual droplets as they elute from the micro-HPLC columns in
different
wells of the microtiter plate. Chemical and physical analysis (e.g.,
spectroscopy or
spectrometry) of the samples can be performed on the samples within the wells.
Example 16 -- Fluidic Seals for Parallel HPLC in Microcapillary Channels.
With reference to FIG. 16, an array of through-holes is machined in a block of
material (e.g., -metal, ceramic, or plastic). The through-holes have a
diameter of less than
1 mm and an aspect ratio greater than 10. The through-holes are chamfered to
accept an
o-ring gasket. A syringe bank is fabricated with the same center-to-center
spacing as the
through-hole array. The syringe needles pass through a metal block that is
attached to the
syringe bank holder by pneumatically actuated, spring-loaded pins. 0-ring
gaskets are
placed onto the syringe needles protruding through the block. The syringes are
loaded
with fluid and inserted into the capillary tubes. The pins are pneumatically
actuated to
91
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
bring the metal block in contact with the top surface of the capillary tube
block and press
the o-ring gaskets into the hole chamfer and around the syringe needle. This
makes a leak
tight seal between the needle and capillary channel thus allowing the
capillary channels to
be pressurized by the syringes (FIG. 117). FIGS. 18 and 19 illustrate a
similar approach,
except that the syringe bank in those figures is shown bolted to the capillary
tube array to
result in a rigid structure for easy handling.
Example 17 -- Identiing a Ligand to a Biomolecular Target
The purpose of this experiment is to use low volume chromatograpliy using
through-hole arrays to identify ligands in a chemical, biochemical or
biological mixture
that bind to a biomolecular target, in this case a protein. Two linear through-
hole arrays
are constructed such that each through-hole holds 50 nl of liquid when filled.
The
exterior surfaces of the arrays are treated to be hydrophobic and the
interiors are treated to
be hydrophilic. A center to center spacing of 9 mm between each of the through-
holes is
used and registration holes are includes to ensure alignment and co-
registration of the
through-holes upon stacking of the arrays using pins on a precision jig. A
bank of
syringes is used to dispense 50 nl of target protein solution into the first
array and 50 nl 'of
different compound libraries into each through-hole of a second array. The
arrays are
stacked using a precision jig and the fluids are allowed to mix in each of the
co-registered
through-hole positions. A bank of syringes with compression fittings is filled
with 1 ml
of eluant, followed by a small air bubble and the used to draw up the 100 nl
of the
protein-library reaction mixture. The reaction mixture is held in the tip of
the syringe and
kept separate from the eluant reservoir by the air gap. The syringe tips are
the placed into
the orifice of an array of size exclusion columns and the compression fittings
are
tightened to ensure a seal. The samples are dispensed into the column,
followed by the
eluant. By monitoring the UV absorbance of the sample exiting at least one
column, one
may determine when protein bound to ligand is exiting the column. This protein
is then
recovered onto an array of reverse phase columns by coupling the two column
arrays.
The ligands are removed from the protein by reverse phase chromatography,
recovered,
and analyzed to determine their identity.
Example 18 -- Protection of Through-holes by Coating with Wax.
Paraffin wax with a melting point of 54 C is heated above its melting point. A
through-hole array is submerged in a thin layer of molten poly(ethylene
glycol) wax with
92
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
an average molecular weight of 1500. The through-hole array and wax are cooled
to
cause the wax to harden. Excess wax is removed from the surface by scraping
with a
sharp blade and then polishing. The wax-filled array is then exposed for 10
seconds to
vapor from a solution of (tridecafluoro- 1, 1,2,2-tetrahydrooctyl)- 1 -
trichlorosilane diluted
1:20 in xylenes. The coating is then cured by baking the platen at 110 C for
30 minutes
while allowing the wax to melt and drip out of the platen. Wax residues can be
removed
from the channel interiors by washing with water. The platen is then rinsed
for 10
seconds in sulfuric acid/ hydrogen peroxide (2:1), and then with water, to
remove
residues and ensure oxidation of the channel interiors. The resulting through-
hole array
has a hydrophobic exterior and a hydrophilic interior.
Example 19: Photo-initiation method of fiberglass through-hole array
production.
A sheet of fiberglass filter such as that available from Millipore (Bedford,
MA) is
soaked in a polymerizable solution (e.g., a solution containing methyl
methacylate
monomer and a photoinitiator such as benzoin methyl ether) and placed between
two
quartz plates, each having an array of dots that serve to mask the areas that
will remain
open and porous Polymerization of the monomer solution is effected by
illuminating the
photomasks with ultraviolet light.
Example 20: Application of an assay to an array of through-holes.
A common assay used in drug discovery efforts is the cytochrome P450
inhibition
assay. Compounds that inhibit the activity of P450 enzymes are generally
undesirable as
pharmaceuticals as they have the potential to cause serious side-effects and
have the
potential for negative reactions if taken in conjunction with other
pharmaceuticals. A
common assay that is used to screen compounds that are potential candidates
for
becoming pharmaceuticals is to incubate the compounds in a cellular, extra-
cellular, or
recombinant system than expresses P450 enzyrnes and to assay the activity of
the
enzymes in the presence of the candidate compound. Such an assay could be
modified
for analysis in a massively parallel manner in an array of through-holes.
Four co-registered arrays of through-holes in which the through-holes are
used.
The enzyme or cellular compartrnent (e.g., microsomal compartment of a liver
cell) that
expresses P450 enzymes can be loaded into one array of through-holes such that
each
through-hole contains an equivalent volume and concentration of P450s. A known
substrate for a chosen P450 enzyme along with a source of NADPH (a source of
energy)
93
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
can be loaded onto a second array of through-holes such that each through-hole
contains
an equivalent volume and concentration of substrate and NADPH. A third plate
can be
loaded with known concentrations (e.g., serial dilutions) of the compounds
that are to be
assayed along with positive and negative controls. An invention for the rapid
loading of
multiple arrays of through-holes has been described elsewhere in this
document. The
assay is started when these 3 arrays of through-holes are brought in to
contact with one
another. As has been described elsewhere in this document, by controlling the
size,
density, and surface cheinistry of the through-holes and the arrays themselves
the
contents of the 3 arrays can be made to rapidly mix with one another while
inter-through-
hole contamination between two or more adjacent through-holes can be avoided.
The stacked array of through-holes is then incubated at 37 C in a controlled
humidity environment to allow the reaction to proceed. By maintaining a high
humidity
environment during this incubation evaporative loss from the through-holes can
be
minimized. After 30 minutes a stop solution (e.g., organic solvent, urea, high
salt
solution etc.) is added to the each through-hole by stacking a fourth array
filled with the
stop solution with the assay arrays. The stop solution works to stop the assay
by causing
the P450 enzymes to denature and/or precipitate out of solution. This
precipitate can be
pelleted by centrifugation using an invention described elsewhere in this
document. The
top array of through-holes can then be removed from the others and used for
analysis. In
many assays, when the substrate is metabolized by the P450 enzymes it is
transformed
from a non-fluorescent compound into a fluorescent one. The amount of
fluorescence
will be directly proportional to the amount of P450 activity. If the compound
being
assayed inhibits the P450 enzyme, the less the metabolism that will occur and
less
fluorescent material will be generated. The concentration at which the P450
enzyme is
inhibited by 50% (IC50) can be determined if multiple concentrations of
inhibitors were
used in the assay. Fluorometric and/or mass spectrometric analysis of the
samples can be
performed using inventions described elsewhere in this document.
Example 21 -- Washing protein from a surface of a through-hole array.
A through-hole array is prepared from silicon with a fluoro-chloro-alkane
coating
on its surface and is treated with an oxidizing solution that renders the
interiors of the
holes hydrophilic. The array is dipped in water and frozen to -80 C, then
quickly dipped
94
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
into a solution of the fluoropolymer FluoroPel (Cytonix Corp., Beltsville,
MD). The
array is baked at 200 C for 20 minutes, and then dipped into cell media
containing 10%
fetal calf serum. Dipping the through-hole array into the medium fills the
holes in the
array and wets the surface. By then dipping the through-hole array into
perfluorooctane
and withdrawing slowly, the surface is cleaned of all aqueous media.
EXAMPLE 22 -- DNA sequencing.
Fluorescently labeled DNA fragments are prepared by Sanger sequencing and
arrayed in an array of 2500 through-holes in a platen of 0.5 mm thickness.
This platen is
then stacked on a second platen of 80 mm thickness containing a gel and
inserted into an
actively cooled electrophoresis tank. As fluorescent oligos emerge from the
column array
they bind to a nitrocellulose menlbrane unwound from a spool. A computer
controls both
the movement of the membrane and turns off the electric field while the
nitrocellulose
membrane (in the forrn of a tape) is moving. The membrane is then moved to
cause
exposure to a light source and CCD camera, analyzing the images and creating
elution
profiles for each of the through-holes. This achieves reconstruction of the
DNA
sequence.
EXAMPLE 23 -- Method of manufacturing a platen using a plurality of grooved
plates.
Platens having through-holes are to be manufactured from silicon plates having
parallel grooves. See FIG 20. The resulting array has 5000 through-holes,
wherein the
through-holes are approximately square with each side 0.25 mm in length and
the platens
are about lmm deep.
Nine inch silicon wafers (0.5 millimeter thickness) are used. The circular
wafers
are precisely cut to yield rectangular pieces (60 in total) that are 55 x 160
millimeters (a
total of three surfaces per wafer are obtained). Grooves of 250 microns width
and 250
micron depth are then etched lengthwise into each piece of silicon. A total of
100
grooves are etched into 50 of the silicon pieces and the distance between each
groove is
adjusted to about 250 microns. The remaining 10 pieces of silicon are not
etched.
Chemical etching of silicon is a process known to those skilled in the art,
and involves the
masking the appropriate areas of silicon and treating with acid. The unmasked
areas are
then etched away in a controlled manner. A total of 25 millimeters from each
side is not
etched, providing a solid border to the finished platens.
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Once the chemical etching of the surfaces is complete, the mask used in the
etching process is removed and the non-grooved surface of each piece of
silicon is
sputter-coated with a thin layer of gold. The pieces of silicon are then
stacked together in
a jig having a flat surface with a right-angle bracket. Five pieces of silicon
without
grooves are first stacked, followed by the 50 grooved surfaces, aiid another 5
pieces
without grooves. The entire jig containing the pieces of silicon is moved to a
press and
pressure is applied to the stacked pieces of silicon. The assembly is heated
and allowed
to remain under elevated pressure and temperature for about 16 hours. This
treatment
results in permanent bonding of the platens into a single large piece of
silicon with 55 x
30 x 160 mm dimensions having 5000 through-holes (in a 100 x 50 array) and a
25 mm
solid border of silicon around the through-holes. A wire saw is used to cut
the individual
platens to a thickness of 0.5 millimeter. The platens are cut by slicing in
the plane
orthogonal to the through-holes. See Figure 20. The wire used in the saw has a
thickness of 150 microns and as a result this amount of material is lost
during the sawing
process. Including the removal of uneven platens created at either end, a
total of 230
platens fitting the required specifications are cut from this large piece of
silicon.
Both surfaces of the platens are polished in a lapping machine to ensure a
flat
surface, resulting in an insignificant amount of silicon being removed.
Finally, surface
chemistry is applied to the platens to provide the finished product, having
the desired
physical characteristics.
Example 24 - Storing and screening a nonoliter volume compound library.
Compounds are reformatted from 96 well plates into a through-hole array as
follows: Compounds in 96 well plates are dissolved in DMSO to 100 times the
concentration that they willed be assayed at - for example, 100 uM for a luM
final
concentration in the assay. The total volume of DMSO sample solution in each
well of
the 96 well plate is 10 ul. An additional 90 ul of ethanol is mixed into each
well and the
samples are immediately drawn into a syringe bank for dispensing. The
automated
syringe bank the dispensed 60 nl volume into each stack of a through-hole
array having
the same footprint as a 96 well plate. This process is repeated with new 96-
well plates
until the through-hole arrays are substantially full. The alcohol is then
allowed to
evaporate, leaving a residue of approximately 600 pl of DMSO dissolved
compound in
each channel of each through-hole array. After storage of the compounds for
the desired
time under desiccation at -80 C, a through-hole array containing the arrayed
compounds
96
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
is removed, brought to 10 C so that the DMSO remains frozen and dipped into a
bealcer
containing aqueous assay solution that has been chilled to 10 C. Removal of
the
through-hole array from the beaker under a humidified environment results in
the
through-holes of the chip being filled with the aqueous medium. The through-
hole array
is warmed to ambient temperature to allow mixing of the solvents. A second
through-
hole array is uniformly filled with a freshly prepared, chilled assay buffer
containing an
enzyme and a fluorogenic substrate; in this case a Matrix Metalloprotease
assay.
Stacking of the two chips, warming to ambient temperature and fluorescent
imaging at
several time points over 30 minutes gives a primary screen for enzyme
inhibition.
Example 24 - Selecting membranes for cell culture
The invention provides methods for the growth of various cell types, including
eukaryotic cells, including but not limited to adult stem cells, chondrocytes,
embryonic
stem cells, endothelial cells, epithelial cells, fibroblasts, hematopoietic
cells, muscle cells,
including cardiac muscle cells of the heart, neurons, osteocytes). A schematic
of this
method is shown in Fig. 25. To facilitate the growth of these cells, porous
membranes
were assayed for their ability to support the attachment, survival, growth, or
proliferation
of an exemplary cell type, HEK 293 cells transfected with a PKCb - GFP
expression
vector (Fig. 26). The cells were plated into wells of a 24-well plate (BD-
Biocoat 24 well)
that contained inserts to be assayed. 3 m pore inserts that were uncoated or
that were
coated with fibronectin, laminin, and collagen were tested, as were membranes
fabricated
from aluminum oxide having a uniform capillary pore structure, ANOPORE tissue
culture inserts, of 0.2- m pore size (Nunc Inc.). The membranes were incubated
overnight with 200,000 cells. Following this incubation, the ANOPORE membranes
were completely confluent (see Fig. 25). The laminin coated membrane was about
20%
confluent. The other membranes were less than 10% confluent. Cells seeded at
75,000
per well on an Anopore insert continued to grow until confluency at day 6 (see
Fig. 27).
Example 25 - Cell chip construction methods
For cell chip construction, anodisc membranes, which are the membranes in the
ANOPORE inserts, were used. First, membranes were placed on a glass slide and
a 400
m thick stainless steel platen with 150 m diameter pores was placed over the
membrane. The device was placed in a the chamber of a Cytospin slide
centrifuge and
cells were added to the top of the platen. After a twenty-four hour incubation
the platen
97
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
was washed three times with cell culture medium. Although there were cells
adhering to
the membrane, the cells were rounded and there were no firm attachments or
spreading as
seen in previous experiments using the anopore insert. With the inserts, the
membrane
was not placed on a solid support, but was suspended within the platen, which
allow the
membrane to be in contact with medium on both surfaces.
A double platen was constructed with a anodisc membrane in between the platens
as shown in Fig. 28. The bottom platen was pre-wetted by placing a drop of
medium over
the platen and moving a glass cover slip perpendicular to the platen over the
surface to
push the liquid into the pores. This was repeated on the top platen using a
drop of cell
suspension at 1 x 10G cells/mL. A pre-wetted membrane was then placed between
the
platens, and the platens were adjusted under a microscope so that the pores of
both
platens were brought into register or alignment. The device was clamped and
incubated
overnight. Examination of the membrane after washing showed that the cells had
a
characteristic adherent morphology similar to that seen with the inserts (Fig
28). Cells
were examined every 24 hours and proliferated until confluent (Fig. 28).
Example 26 - ArrU spotting
To assess the potential of the cell-chip to be used with an array spotter for
compound testing, single pin spotting was characterized (FIG. 29). Fig. 30
shows
microspotting on gold platen using Hoechst dye. Figure 36 shows microspotting
of C12
resazurin to determine cell viability. C12 resazurin is a detection agent used
to study
cellular metabolism. The reduction product of C12-resazurin is C12-resorufin,
which
exhibits enhanced cellular retention and detection relative to the reduction
product of
resazurin. Metabolically active cells reduce C12 resazurin to C12 resorufin
which
fluoresces red. Hoechst stain was included as a counter stain. After briefly
spotting on
the platen, the cell-chip was incubated for 15 minutes at 37 C, 5% CO2. The
chip was
examined using fluorescence microscopy. Several wells in the area of the spot
showed
Hoechst staining and contained metabolically active cells. This area was about
300 uM in
diameter, about 1.5 times the diameter of the pin (see Fig. 29). Fig. 36 shows
an open
array-based cell chip and delivery of C12-resazurin to a single well on the
array using a
floating pin.
The technology for microarray spotting allows the generation of high density
microarrays by spotting cDNAs and/or oligonucleotides on a solid chip surface.
In this
report, the chip surface is modified providing for improved performance of an
ultra-high
98
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
throughput cell-based screening assay. This novel chip technology includes an
ANODISC membrane selected for its ability to support cell attachment and
viability and a
microwell stainless steel platen.
Membranes with either 3 um or 0.2 m pore diameter were tested for their
ability
to support PKCb-GFP cell attachment and growth. An ANODISC 0.2 m membranes
was covered with a confluent layer of cells after an overnight incubation. In
contrast, less
than 10% confluency was present when a polycarbonate membrane having a 3 m
pore
size and treated with laminin, collagen, fibronectin or untreated. These
experiments
suggest that membranes having a smaller pore size have an enhanced ability to
support
cell attachment, differences in membrane material cannot be excluded.
Alternatively,
difference in the fabrication material can not be excluded.
When cells were cultured with a cell-chip consisting of a membrane on a glass
slide covered by a stainless steel platen, the cells appeared round and did
not attach well.
In contrast, cells cultured on membranes in framed inserts (i.e., on a
membrane between
two platens) that provided contact with culture medium on both sides solved
this problem.
This configuration could can be adapted for use in a variety of analystical or
culture
systems. In one embodinient, a chip including cells on a membrane is placed
over
another chip having a membrane designed for protein or RNA attachment. Cells
are
lysed and the lysed contents is spotted onto the second membrane by vacuum
filtration for
dot blot analysis.
These experiments described herein further demonstrated that a compound could
be spotted directly onto the cell-chip and processed by metabolically active
cells with
minimal diffusion on the platen. In order to decrease diffusion further and
achieve high
density spotting, the platen surface may be treated with a hydrophobic agent.
Mineral oil
and silicone coating dramatically limited diffusion and created a small spot
in studies
carried out using membranes having 50 m pore in a gold platen (Fig. 31).
Because of the small well size, this novel technology provides for the culture
and
analysis of rare cell types and further provides ultra high throughput single
cell screening
(uHTS). Single cell uHTS may have a transformational effect on antibody
engineering as
activated B cells may be tested without fusion (variable regions amplified
from positive
cells).
The present invention overcomes limitations present in the prior art. In
particular,
using the cell-chip configuration described herein, living cells can be
analyzed using
fluorescence markers for cell function. Further more, cultured cells may be
lysed and the
99
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
contents transferred through a membrane for further biochemical analysis, such
as protein
analysis or gene expression.
Example 27-Hoechst staining of cells
Cells were incubated overnight on a stainless steel cell-chip and then washed
with
medium. Hoechst was spotted on a first platen that was dried by blotting. The
stainless
steel platen (National Jet Company, LaVale, M.D.) tested was a 1-inch square,
400- m
thick with pores 150 m in diameter. A second platen was attached to the first
platen
using adhesive sealing film with the center cut out. The cliip was placed in a
cytospin
sample chamber with a small gasket between the platen and the top of the
chamber. No
funnel was used. The results of this experiment are shown in Figure 32. Fig.
33 shows
an anopore membrane sandwiched between two stainless steel platens. PKC(3-GFP
cells
were added and incubated overnight.
Example 28-Tun sg ten platens
A cell microarray prototype was constructed on a 200- m thick Tungsten platen.
The platens (National Jet Company, LaVale, M.D.) had pores of 300 m in
diameter and
were attached with 4 screws. The prototype is shown in Fig. 34. An Anopore
membrane
of aluminum oxide was sandwiched between two tungsten platens (as shown in
Figure
34). PKC(3-GFP cells were added and incubated for 48 hours (Fig. 35). In this
experiment, random cell distribution was observed and the cells were more
securely
attached to the substrate. Metal platens having biocompatibility may be used
in such
methods. Metals that are not biocompatible may be coated with a biocompatible
polymer
(PEG) or metal.
Example 29-Confocal images of cultured cells
Fig. 37 shows an open array-based cell chip with PKC(3-GFP transfected cells,
added at 5 x 105 /mL and incubated 37 C, 5% COz. Images were acquired by
confocal
microscopy. Fig. 38 shows essentially that which is depicted in Fig 37, except
here the
platen is not shown.
Example 30-Rigid materials may be attached to the porous membrane
A platen of rigid material, such as metal or polystyrene having pores between
10 -
300 m in diameter is attached to a porous membrane or modified glass surface
to form
100
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
an array of microwells having a porous bottom (Fig. 39). Cells are added and
allowed to
adhere to the membrane overnight. Subsequently, one or more test compounds is
added
using a micro spotting pin. After incubation, high content image analysis is
performed.
Membranes are processed to analyse protein, mRNA expression, or to detect
changes in a
biological fu.netion of interest. Fig. 40 shows a cross-section of an
individual well
depicted in Figure 39. The present invention can be adapted as shown in Fig.
41. Figure
41 depicts structural enhancements to increase pressure on the member and/or
gaskets to
seal off individual wells.
Cell chip assays were carried out using the following methods and materials.
Cell Culture Media
DMEM supplemented with 10% FCS and 0.22 ug/mL hygromycin was used to
maintain PKCb - GFP cells. In some experiments, DMEM without phenol red
supplemented with 10% FCS and 100 IU/mL penicillin, 100 ug/mL streptomycin was
used.
Cells
Human PKCI3II cDNA (GenBank Accession No.:X07109) was isolated by PCR
from reverse transcribed human spleen marathon-ready cDNA (Clontech) and
subcloned
into the pCDNA3 vector containing a hygromycin resistant gene (Invitrogen).
The gene
was inserted downstream of sequences encoding a green Xuorescent protein
(ZsGFP)
(Clontech). A stable cell line expressing GFP-PKCBII was obtained by
transfecting
pcDNA3/GFP-PKCI3II vector into human embryonic kidney (HEK) 293 cells (QBI,
Montreal, Quebec, Canada) followed by selection with 600 g/ml hygromycin B.
Drug
resistant colonies were picked and screened for PKCI3II protein expression by
immunoblotting using an anti-PKCI3II antibody (Santa Cruz Biotechnology, Santa
Cruz,
CA). The expression of GFP was tested by Xuorescence microscopy. Positive
clones
were maintained in the growth medium containing 300 g/ml of hygromycin B.
HEK293
cells transfected with GFP-tagged PKC-Bll protein were maintained in DMEM
containing
10% FBS, glutamine, antibiotics, and hygromycin B at 37 C. See, Ilyin et al.,
Methods
37: 280-288, 2005, which is hereby incorporated by reference.
Cell culture on membranes
101
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
Cell culture inserts from BD-Biocoat membranes were obtained in 3 uM pores
size (BD Biosciences, San Jose, CA). The inserts were pre-coated with lamalin,
collagen
or fibronectin. Anopore inserts having a 0.2 uM pore size (Nunc) membranes
were also
tested. Inserts were placed in a 24-well culture plate. Cell culture medium
was added
until the level reached the membrane. Cells were added to the inserts in 200
uL of
medium. Plates were incubated overnight at 37 C, 5% CO2, In subsequent
experiments
using membranes without framed inserts, anodisc membranes having 0.2 or 0.1 m
(Whatman, Clifton, N.J.) pores were used.
Platens
Stainless steel shims 400 m thick were obtained and sent to National Jet
Company (LaVale, M.D.) for manufacture of a 10 x10 platen of 150 uM diameter
holes
spaced 50 m apart using micro electro-discharge machining. Each shim was cut
to about
1 inch square with the platen in the center.
Spotting pins
An FP9 0.229 mm diameter floating tube pin with a volume delivery range of 5-
1 5 nL (V&P Scientific , Inc., San Diego, CA) was used in all experiments.
Spotting
solutions were made in an eppendorf tube. The spotting pin was dipped in
solution and
then spotted on the platen by briefly touching perpendicular to the platen.
Cell viability assay
C 12- Resazurin (molecular probes) was diluted in DMEM 10% FCS w/o phenol
red. Hoechst 33258 (molecular probes) was included to control for spotting
efficiency.
Solution was spotted onto platen on top of cells as described above. The cell
chamber
was incubated at 37 C, 5% CO2 for 15 minutes. After incubation, the chamber
was
examined by fluorescence microscopy for Hoechst DNA staining. This area was
then
analyzed using confocal microscopy for conversion of C12- Resazurin to red-
fluorescent
resorufin by viable cells ( Abs/Em 563/587).
OTHER EMBODIMENTS
From the foregoing description, it will be apparent that variations and
modifications may be made to the invention described herein to adopt it to
various usages
and conditions. Such embodiments are also within the scope of the following
claims.
102
CA 02619250 2008-02-11
WO 2007/022026 PCT/US2006/031534
The recitation of a listing of elements in any definition of a variable herein
includes definitions of that variable as any single element or combination (or
subcombination) of listed elements. The recitation of an embodiment herein
includes that
embodiment as any single embodiment or in combination with any other
embodiments or
portions thereof.
All patents and publications mentioned in this specification are herein
incorporated by
reference to the same extent as if each independent patent and publication was
specifically and individually indicated to be incorporated by reference.
103