Note: Descriptions are shown in the official language in which they were submitted.
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
HYDROGEN HAiVDLING OR DISPENSING SYSTEM
Field of Invention
This invention generally relates to systems and methods for handling and
dispensing
diatomic hydrogen.
Background of the Invention
Hydrogen has been used unbonded fi-om other elements for various purposes, in
both
a liquid and gaseous form For instance, liquid hydrogen has been used in
aerospace
applications including manned space flight. In fact, liquid hydrogen was used
as the fuel for
the Saturn V rockets that propelled astronauts on their journey to the moon.
Hydrogen gas has also been used, though on a relatively small scale, for
various
purposes including in dirigibles and as a fuel for vehicles, such as
automobiles and boats.
Use of hydrogen as a fuel has been proposed on a larger scale because hydrogen
typically
produces much less pollution than alternative fuels and methods of energy
storage. In fact,
President George W. Bush advocated research into the use of hydrogen in
vehicles in his
2003 State of the Union Address. It has been proposed that hydrogen may be
used, for
example, as a fuel for internal combustion engines, or in fuel cells. Hydrogen
also produces
more power per weight than other fuels, providing advantages in aerospace
applications and
other uses where weight is criticaL
The product of combustion when hydrogen is burned is water vapor, so when pure
hydrogen is burned in internal combustion engines, the traditional polluta&s
associated with
fossil fuels, hydrocarbons, carbon monoxide, and air toxics, are not produced
at all. In
addition, the green house gas unavoidably produced by the combustion of fossil
fuels,
carbon dioxide, is also not produced at all in the combustion of hydrogen.
Using electrolysis, hydrogen has been produced by separating hydrogen and
oaygen
that form water. When used in its gaseous form, hydrogen has been stored at
various
pressures to reduce the amount of space that is required for storage.
Electrolysis has
typically been performed at atmospheric pressure, so compressors have been
used to
compress the hydrogen gas for storage. Such equipment and systems for the
production of
hydrogen have typically been powered by electricity, a substantial amount of
which is used
to drive the compressor. If hydrogen is to be used on a large scale, the
amount of electrical
power that will be used for this purpose will most likely be significant.
CA 02619895 2008-02-19
In addition, if hydrogen is to be used on a larger scale, the need exists for
systems and equipment
for handling, distributing, and dispensing hydrogen. For instance, if hydrogen
is to be used on a
larger scale in vehicles, a need exists for hydrogen dispensing or refueling
stations. Such
hydrogen refueling stations have been contemplated, for example, in U. S.
Patent No. 6,432, 283.
A need exists for suitable hydrogen refueling stations that may be located,
for example, in urban
areas where pollution levels are high, and hydrogen usage is likely to be
particularly beneficial.
As mentioned above, at one time, hydrogen gas was used in dirigibles, and the
famous
Hindenburg disaster dramatized that hydrogen is quite flammable and can be
dangerous if not
handled in a safe manner. Thus, fire codes and other standards have required a
high level of
safety precautions for systems that handle or dispense hydrogen.
For instance, systems for dispensing hydrogen have been very spread-out by
requiring
large "set-back"distances between hydrogen and buildings or other fuels,
electric power lines,
and areas accessible to the public, so that leaks of the flammable substance,
and any resulting
fires or explosions, are not likely to damage other equipment or endanger
users or the public.
Storage tanks or pressure vessels were typically mounted next to the ground in
a
horizontal position, which fiitther increased the amount of land required for
a hydrogen refueling
station. Pressure vessels were also typically penetrated on both ends
providing multiple potential
leakage points. Such hydrogen handling and dispensing facilities have
typically been enclosed
with high industrial fencing, for example, chain-link fencing, usually with
barbed wire at its top
to create a barrier for the public from the hazards of the gas. But such
configurations are not
suitable for applications in urban areas, for example, where land is limited
and/or the value of
land is high. If hydrogen is to become a fuel for motor vehicles, possibly
including marine, fuel
cell, or hybrid vehicles, then a need exists for hydrogen handling and
dispensing systems (which
may include hydrogen production and/or storage) to be compatible with existing
motor vehicle
refueling facilities located in light commercial areas generally accessible to
the public.
In addition, hydrogen handling and dispensing systems have typically required
elaborate
active fire detection and suppression systems in the event of a leak which is
ignited. In addition,
such systems were typically custom designed and fabrica.ted and tested on the
site. These
systems are expensive to construct, test, and maintain, and the fact that
these activities have been
performed at the site has increased their cost.
2
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
on the site. These systems are ea-pensive to construct, test, and maintain,
and the fact that
these activities have been performed at the site has increased their cost.
Thus, a need exists for hydrogen handling and dispensing systems that are safe
and
yet occupy a relatively small amount of land. A need exists for such systems
to require a
m.inimum amount of active fire detection and suppression systems, and that
they be
= relatively simple, inexpensive to manufacture, easy to erect in the field,
test, and maintain.
_
In addition, since large-scale hydrogen production and handling consumes a
considerable amount of energy, a need exists to minimize the amount of energy
that is
required to produce and/or compress hydrogen, and a need exists to be able to
conduct these
activities (and consume the associated electrical power) during time periods
when electricity
is plentiful and inexpensive, for example, when other demands for electricity
are relatively
low. Since the production of electricity is much more economical when the
production is at
a steady and predictable rate, a need exists for systems that facilitate at
least some control by
the electrical power companies of when hydrogen is produced. On the other
hand, hydrogen
. users need to be able to obtain hydrogen at times that are convenient to
them. Thus, a need
exists for hydrogen handling and dispensing systems to include pressure
vessels configured
to store hydrogen, from approximately the time that it is produced or
compressed until it is
distributed or dispensed. A need also exists that these pressure vessels
occupy a relatively
small amount of land, require a minimum amount of active fire detection and
suppression
systems, and be relatively inexpensive to manufacture, erect in the field,
test, cerEify, and
maintain.
Other needs also exist that may be apparent from this document to a person of
skill
in the relevant fields of this invention.
Summarv of the Invention
Various embodiments of the present invention include systems and methods for
handling or dispensing diatomic hydrogen, which may include distributing,
compressing,
and/or storing hydrogen. Exemplary embodiments include hydrogen refueling
stations that
are suitable to be located in urban areas, for instance, that are relatively
safe and yet
occupying a relatively small amount of land. Some embodiments are relatively
inexpensive
to manufacture, erect in the field, test, and maintain, for instance,
requiring a minimunz
amount of active fire detection and suppression systems. Some embodiments
minimize the
amount of energy that is required to produce and/or compress hydrogen, and/or
conduct
3
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
these activities during time periods when electricity is plentiful and
inexpensive, for
instance, when other demands for electricity are relatively low.
Various embodiments of the present invention provide systems for hydrogen
handling and dispensing that include hydrogen storage pressure vessels, so
that hydrogen
may be stored in these pressure vessels from the time that it is produced
until it is used or
dispensed. These pressure vessels may occupy a relatively small amount of
land, require a
minimum amount of active fire detection and suppression systems, and be
relatively
inexpensive to manufacture, erect in the field, test, and maintain. Some
embodiments
provide complete or partial enclosures, for instance, for the purpose of
improving safety.
Other embodiments and advantages thereof also exist that may be apparent from
this
document to a person of skill in the art. These include a number of aspects of
the present
invention which are applicable to systems and methods for handling or
dispensing other
flammable fluids, especially gasses that are lighter than air.
In a particular exemplary embodiment, the present invention provides a system
for
dispensing hydrogen gas, which may have a hydrogen source configured to
provide diatomic
hydrogen gas, a pressurizing apparatus configured to obtain the result of the
hydrogen gas
being pressurized, at least one pressure vessel configured to store hydrogen
gas, and/or
piping configured to convey the hydrogen gas at least from the hydrogen source
and to the
pressure vesseL The pressure vessel may be configured in the shape of a
cylinder oriented
substantially vertically with a top end and a bottom end. The system may be
assembled,
tested, and/or certified, in the shop.
In some embodiments, the system may be configured to refuel vehicles that
consume
substantially pure compressed hydrogen gas, for example, in an internal-
combustion engine
or a fuel cell. In some embodiments, the system may be configured to refuel
interna.l-
combustion engine powered vehicles that consume a mixture of hydrogen gas and
at least
one other flammable gas. The other flammable gas may be natural gas, for
example, and the
system may be configured to dispense at least a plurality of substantially
different mixture
ratios of hydrogen gas and natural gas.
Some embodiments may have a plurality of pressure vessels, and each pressure
30 vessel may be a cylinder oriented with a substantially vertical axis. There
may be at least
one vent pipe configured to vent the hydrogen gas to the atmosphere near or
above the top
end of the pressure vessel, and the vent pipe may be oriented with a
substantially vertical
axis, and may be located near the pressure vessel. Venting of hydrogen through
the vent
4
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
pipe may occur, for example, through a pressure relief valve, in the event of
an over-
pressurization condition. In some embodiments, the piping may have at least
one supply
pipe configured carry the hydrogen gas to the pressure vessel, and the supply
pipe may be
connected to the pressure vessel at or substantially near the top end of the
pressure vessel.
The supply pipe may be at least partially located inside the vent pipe. In
some
embodiments, all penetrations into the pressure vessel may be on top, for
example, at least -
within the top quarter of the pressure vessel.
In some embodiments, the bottom end of the pressure vessel may be below grade,
and may have secondary containment forming an interstitial space at least
between the
bottom end of the pressure vessel and the secondaiy containment. The
interstitial space may
be ducted to the vent pipe. In some embodiments, the hydrogen source may be a
hydrogen
generator configured to generate hydrogen gas. For instance, the hydrogen
generator may
include at least one electrolysis unit configured to generate the hydrogen gas
by electrolysis
of water, and the pressurizing apparatus may include at least one pump
configured
pressurize the feed water. As an example, the pump may be configured to pump
the water to
a pressure of at least 150 psi. The pressurizing apparatus alternatively, or
in addition, may
include at least one compressor configured to compress the hydrogen gas.
In some embodiments, the hydrogen generator may include at least one reformer
configured to generate the hydrogen gas, for example, froiin fossil fuels such
as natural gas,
.20 or from bio-mass. The pressurizing apparatus for the reformed hydrogen may
be a
compressor. There may also be a compressor configured to pressurize the
natural gas, and
the system may be configured to dispense at least one mu.'ture of hydrogen gas
and
compressed natural gas. For some embodiments, the pressurizing apparatus may
provide at
least half of the pressurization before the hydrogen is generated.
In another exemplary embodiment, the present invention also provides a system
for
handling hydrogen that includes cea=tain hydrogen handling equipment,
typically including
piping, that is at least partially contained within a structure having walls,
a floor, and an
open top. At least one of the walls of the structure may be configured to lean
away from the
equipment so that the open top has a larger area than the area of the floor.
In some
embodiments, at least two of the walls may be configured to lean away from the
equipment.
The hydrogen handling equipment within the structure may include, for example,
a
hydrogen generator, a compressor, a hydrogen gas storage pressure vessel, or
some
combination of these.
5
CA 02619895 2008-02-19
WO 200i/019721 PCT/US2004/020983
In an even fiuther exemplary embodiment, the present invention provides a
system
for handling hydrogen that includes certain hydrogen handling equipment,
generally
including at least piping and valves, which are contained within at least one
substantially
sealed enclosure. The system may be a stationary facility configured to
refu.el vehicles that
consume hydrogen gas. In addition to or in lieu of refueling vehicles, the
system may be
configured to dispense hydrogen gas into a stationary system, for example, a
natural gas -
delivery or distribution system. The enclosure may be vented to atmosphere
through a vent
pipe terminating at a location safely overhead and away from people and other
equipment,
and the enclosure and the vent pipe may be configured to withstand the
detonation of a
mixture of hydrogen and air in the enclosure and vent pipe. Some embodiments
have more
than one pressure vessel, and there niay be a separate enclosure for the
control valves for
each pressure vessel. The enclosures may be cylindrical.
There may be at least one dispenser configured to dispense the hydrogen gas to
vehicles, and the system may have at least one dispensing pipe configured to
carry the
hydrogen gas to the dispenser. In some embodiments, there may be a fire
suppression
system, which may, for example, be configured to introduce a substantially
inert gas into the
enclosure. In some embodiments, the enclosure may normally be filled with an
inert gas to
prevent combustion of any hydrogen gas within.
Brief Descrintion of the Drawings
The figures in this document illustrate various exemplary embodiments of the
present invention. Embodiments of the present invention may include part or
all of the
features shown in one of these drawings, or may include features from two or
more figures.
Embodiments of the present invention may also include features descrnbed in
the
specification, or limitations to features described in the specification.
Furthermore,
embodiments of the present invention may include features that would be
familiar to a
person of ordinary skill in the art having studied this document.
FIG. 1 is a block diagram illustrating various components of one embodiment of
a
system for handling or dispensing hydrogen in accordance with the present
invention;
FIG. 2 is a fi=ont view of a system for handling or dispensing hydrogen in a
structure in
accordance with the present invention; and
FIG. 3 is a side view illustrating one embodiment of a control valve enclosure
and
connections to a hydrogen storage pressure vessel.
6
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
Detailed Descrintion of Exemplarv Embodiments
In general, various embodiments of the present invention include systems for
handling and dispensing hydrogen. Embodiments include stationaty and mobile
refueling
stations for vehicles that use diatomic hydrogen as a fuel, either alone or
mixed with one or
= more other fuels. The hydrogen may be in a gaseous form and may be
compressed, or may
be in liquid form. Various embodiments of the present invention may include
hydrogen
handling equipment, that may include, for example, a hydrogen source or
hydrogen
generator, a pressurizing apparatus or compressor, one or more pressure
vessels, various
interconnecting piping, valves, one or more vent pipes, or some combination of
these items.
Various embodiments of the present invention also include enclosures or
enclosing walls or
stracture.
In some embodinients, one or more pressure vessels configured to store
hydrogen
gas are oriented substantially vertically (i. e., with the major axis
substantially vertical), in
pait to reduce the amount of land or floor space that is required for the
system. In some
embodiments, hydrogen handling equipment is at least partially contained
within or
surrounded by a structure that has walls, at least some of which may be
configured to lean
away from the equipment. These structures may have an open top to allow gasses
and
pressure to escape safely, and the way the walls lean away in some embodiments
may
facilitate the open top being large relative to, for example, the floor area
where the
equipment may be located. As a result, in the event of a hydrogen leak that
forms an
explosive mixture within the stracture, the structure may be able to withstand
a resulting
explosion or detonation and direct the heat and forces upwards, partially or
fiilly protecting
human life and property outside the structure.
In some embodiments, hydrogen handling equipment may be contained within a
substantially sealed enclosure that may be vented to atmosphere through a vent
pipe that
may terminate overhead or higher than the hydrogen handling equipment. The
enclosure
may be configured to withstand the pressure generated by an explosion and/or
fire within the
enclosure resulting from a hydrogen leak within. Any hydrogen leak within
the'enclosure
that is ignited may quickly be smothered by lack of oxygen. Some embodiments
incorporate
hydrogen leak and/or fire detection and alarm systems and some have active
fire suppression
systems. (As used herein, the term "and/or" means one or more of the items
listed.)
7
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
Embodiments of the present invention include hydrogen refueling stations for
refueling vehicles, such as automobiles, that consume hydrogen. The vehicles
may consume
pure or substantially pure hydrogen, for instance, in internal combustion
engines, or fuel
cells, or may consume a mixture of hydrogen and one or more other flammable
gasses. As
used herein, hydrogen is considered to be substantially pure if it comprises
more than 50
percent hydrogen by weight. = ,
As an example of a mixture of hydrogen and another flammable gas, a mixture of
compressed hydrogen and compressed natural gas (CNG) may be dispensed. As used
herein, a gas is considered to be compressed if it is at more than 150 pounds
per square inch
(psi) of pressure (gauge). Hydrogen may also be used for other purposes,
including being
added to natural gas lines or storage tanks to reduce the emissions resulting
from the
combustion of the natural gas.
A hydrogen source may include hydrogen production equipment or at least one
hydrogen generator, which may be, for example, an electrolysis unit or a
reformer.
Electrolysis equipment may generate hydrogen gas by performing electrolysis of
water, and
may be configured to operate during off-peak periods when electricity is
readily available
and inexpensive. Reformers may reform hydrocarbons such as fossil fuels, for
example, like
natural gas.
The hydrogen gas used in the present invention may be pressurized for storage,
and
the pressurizing apparatus may be a compressor. In embodiments where
electrolysis is used,
the electrolysis may be performed at high pressure to reduce or eliminate the
need to
compress the hydrogen gas, and the pressurizing apparatus may be a pump
configured to
pressui7ze the water entering the electrolysis unit. In some embodiments, high
pressure
membrane electrolysis may be used. In embodiments where reforming is used, the
pressurizing apparatus may pressurize before or after reforming.
Referring now to FIG. 1, which illustrates various exemplary embodiments of
the
present invention, system 100 is configured to handle hydrogen and dispense it
to one or
more vehicles 180 at a time. In the exemplary embodiment illustrated, system
100 includes
various hydrogen handling equipment, including hydrogen generator 120,
pressure vessel
110, and various piping and valves. Piping of system 100 includes pipe 114,
122, 124, 132,
134, 144, 172, 174, 182, 192, 194, 197, 198, 199, and vent pipe 140, and
performs various
functions described herein. As used herein, the term "piping" and "pipe"
includes any
suitable device configured to convey fluids under pressure such as, for
example, pipe,
8
CA 02619895 2008-02-19
WO 2005/019721 PCT/US200~t/020983
piping, tabing, hose, and like conduit. System 100 may also include one or
more dispensers
170, which may be configured to dispense hydrogen and/or a mixture of hydrogen
and one
or more other flammable substances. In addition, system 100 may include
equipment for
handling the other flamma.ble substance, such as natural gas system 190, also
illustrated in
FIG. 1.
System 100 may stand alone or may be incorporated into another system, such
as, for
er.ample, a conventional vehicle refueling station, a gas station, or a CNG
refueling station
(e.g:, system 190). System 100 may be located in an urban area, such as an
area that is
zoned Cl or for gasoline motor vehicle refueling facilities. Thus, system 100
may be
integrated with equipment and systems, which may be known in the art, for
delivering fuels
to vehicles and performing other ancillaiy services such as providing vehicle
maintenance
and repair, towing disabled vehicles, distributing or selling food, beverages,
and various
convenience items, and/or charging for electric vehicles. System 100 may also
be integrated
with equipment and systems that provide financial transactions, Internet
access, advertising,
communication, and/or notifications (e.g., of high pollution days). The
present invention
may also be incorporated into other systems such as electric power plants,
natural gas
distribution systems, refmeries, farms, foresthy facilities, mines, and/or the
like. Various
aspects of the present invention make it safe and compatible with such other
systems,
businesses, and facilities. Accordingly, various embodiments of the present
invention
occupy minimal land or floor space, are relatively safe for people to be in
close proximity to,
and are capable of being operated and maintained by personnel of ordinary
skill in such
industries.
The various piping in accordance with the present invention, for example,
supply
pipe 144, vent pipe, 140 and pipes 132, 134, 172, and 174, may be fabricated
from steel or
stainless steel, for instance, type 316 stainless steel, of the schedule or
wall thiclcness
suitable for the pressure at that location. Piping may be sized to provide the
required flow,
but yet to minimize the rate or quantity of leakage should a pipe fail. As an
example, pipes
134, 144, and 172 may be 3/8-inch nomin.al diameter type 316 stainless steel.
The pipe wall
thickness or schedule may be selected to have a minimum factor of safety of 4,
for example,
against hoop stress from internal pressure. Pipe joints may be welded, and
some or all may
be non-destructively tested, for example, x-ray (radiographically) inspected.
For instance,
15 percent of welds may be radiographed. In some embodiments, piping may be
hydrostatically tested or pressure tested, for example, in accordance with the
ASMB code,
9
CA 02619895 2008-02-19
WO 2005/019721 PCT/1JS2004/020983
for instance, using water, nitrogen, or helium as a test medium. Some or all
piping may be
coaxial, having two walls and an interstitial space in between. The
interstitial space may be
vented, for example, to vent pipe 140, or may contain an inert gas.
Valves may include shut-off valves, control valves, safety or pressure relief
valves
(prv's), check valves, pressure regulators, flow control valves, water release
valves. float-
operated valves, and the like. Control valves may be servo valves, solenoid
valves, spool
valves, three-way valves, and/or the like, and may be electrically operated,
pneumatic/electrically operated and/or pilot operated. Valves may be suitably
configured
for the pressure involved, and may have a minimum 25 percent safety margin
above
maximum operating pressure. System 100 may have one or more in-line filters to
remove
debrig and/or moisture, for example, located in pipe 132 downstream of
hydrogen generator
120, in pipe 134 downstream of compressor 130, and/or in pipe or hose 182
downstreani of
dispenser 170.
As used herein, the "generation" of hydrogen (or production, or other similar
terms),
includes separating the hydrogen from other elements to which the hydrogen was
bonded, to
form diatomic hydrogen unbonded from other elements. Thus, as the terms are
used herein,
hydrogen generator 120 generates hydrogen by separating hydrogen from other
elements the
hydrogen was bonded to, and forms diatomic hydrogen, which in some embodiments
is in
the form of hydrogen gas.
System 100 may include a hydrogen source, for example, hydrogen generator 120.
Hydrogen generator 120 illustrated in FIG. 1 may divide water into hydrogen
and oxygen
through electrolysis. In such embodiments, hydrogen generator 120 may receive
water from
water main 127 or from another source such as a water-storage tank or
reservoir. The water
may be filtered or otherwise treated before entering hydrogen generator 120,
for example, in
pipe 122 or 124. In some embodiments of the present invention, hydrogen
generator 120
may produce hydrogen at a rate between 300 standard cubic feet per hour
(scfhr) and 4500
scfhr.
The oxygen that is produced by an electrolysis-type hydrogen generator 120 may
also be used, and although not shown, a compressor, pressure vessel, piping,
or a
combination thereof may be provided for that purpose. In some embodiments,
oxygen may
be bottled and used or sold for medical applications, diving, or metal cutting
purposes, for
example.
{
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
In some embodiments, hydrogen generator 120 may produce hydrogen by refining
another substance. For example, hydrogen generator 120 may reform natural gas,
gasoli.ne,
coal, biomass, or a substance produced from biomass. Sources of hydrogen from
biomass
may include microorganisms, including algae, plant matter, including forestry,
yard, and
farm waste products, and sewage. Hydrogen may be produced directly, for
example, with
the aid of genetically engineered microorganisms, or hydrocarbons may be
produced from
the biomass, which may then be reformed to produce hydrogen. In the reforrning
process,
the hydrogen atoms are stripped from the hydrocarbon chains, producing
diatomic hydrogen
and a byproduct that is rich in carbon. Production of hydrogen from biomass
may have
other benefits including making other products such as fertilizer, building
soil fertility, and
removing carbon and other greenhouse gasses from the atmosphere.
In the enibodinZent illustrated in FIG. 1, the substance for reforming may be
supplied
through pipe 194. Natural gas may be reformed through steam methane reforming,
for
instance. In the example of reforming natural gas, some of the natural gas may
be refined
into hydrogen in hydrogen generator 120, while other natural gas may be mixed
with the
hydrogen that is produced. This mixing may occur, for example, at dispenser
170.
Some embodiments of system 100 may have a hydrogen source that is not a
hydrogen generator 120, and for example, does not separate hydrogen fiorn
other elements
to form diatomic hydrogen. Other examples of hydrogen sources include hydrogen
distnbution pipelines, storage tanks, and the like. Further examples of
hydrogen sources
include liquid hydrogen storage (e.g., through an evaporator), commercial
hydrogen gas tube
trailers, and the like. In some embodiments, hydrogen sources may supply
mixtin=es of
diatomic hydrogen and other gasses or hydrogen rich gasses rather than, for
example,
substantially pure diatomic hydrogen.
System 100 may comprise a pressurizing apparatus, which may include, for
example,
at least one of a compressor 130 and a pump 133. The pressurizing apparatus
may obtain
the result of the hydrogen being pressurized, for instance, by pressurizing or
compressing
the hydrogen directly, for example, with compressor 130, by pressurizing the
substance from
which the hydrogen is generated, for example, pumping the water for
electrolysis to the
desired pressure with pump 133, or a combination thereof.
In embodiments having a compressor 130, compressor 130 may be a diaphragm-type
compressor, and may be hydraulically operated. In other embodiments,
compressor 130
may be a piston type, or a screw type compressor. Compressor 130 may have
multiple
11
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
stages, and the hydrogen gas being compressed may be cooled between stages.
Compressor
130 may be driven by an electrical motor or an internal combustion engine, for
example. In
the exemplary embodiment illustrated, hydrogen may flow from hydrogen
generator 120 to
compressor 130 through pipe 132, which may contain valve 139.
In various embodiments, compressor 130 may have a ma.ximum output pressure
within the range of about 5000 to 12,000 psi. For instance, compressor 130 may
have a
ma~imum output pressure within the range of about 5500 to 7000 or 8000 psi.
Compressor
130 may have a suction pressure, for example, of atmospheric pressure, 100
psi, 150 psi, or
higher. Compressor 130 may have a capacity at maximum output pressure of about
5 to 100
standard cubic feet per minute (sefin). Compressor 130 may also have at least
one safety
valve or pressm=e relief valve (prv) 138 that may be piped or vented to vent
pipe 140,
described in more detail below. In some embodiments, system 100 may include
more than
one compressor 130, and there may be a low or intermediate pressure storage
tank or
pressure vessel between the compressors, for example, at approximately 100 to
200 psi, or a
higher intermediate pressure.
In some embodiments, compressor 130 may be used to compress the same gas more
than once. For example, hydrogen produced by hydrogen generator 120 may be
compressed
by compressor 130 to an intermediate pressure and stored in one or more
pressure vessels
110. Then this intermediate pressure hydrogen may be directed to the inlet of
at least one
stage of compressor 130, where it may be compressed again to a higher
pressure, and stored
in another pressure vessel 110. The different pressure vessels 110 may be
configured or
dedicated for different pressures, or may be interchangeable. In some
embodiments, fewer
than all of the stages of compressor 130 may be used at the higher pressure.
The hydrogen
may be cooled by a separate chiller or cooler, or allowed to cool in pressure
vessel 110 at the
intermediate pressure before being compressed to the higher pressure.
In embodiments wherein hydrogen generator 120 produces hydrogen by refining
another substance, the pressurizing apparatus may include a compressor or pump
located in
pipe 194, for example. This compressor or pump may be in addition to or in
lieu of
compressor 130, compressor 193 (described below), or both. In other words, in
some
embodiments, compressor 130 may be located in pipe 194 rather than where shown
in
FIG. I or their may be a compressor at each location. In such embodiments, the
refining
process taking place in hydrogen generator 120 may occur at a higher or
intermediate
pressure. Thus, the pressurizing apparatus may provide at least part of the
pressurization
12
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
before the hydrogen is generated. For instance, in some embodiments, the
pressurizing
apparatus may provide at least half of the pressurization before the hydrogen
is generatecL
In fact, in some embodiments, the pressurizing apparatus may provide all of
the
pressurization before the hydrogen is generated.
Embodiments that provide at least part of the pressurization before the
hydrogen is
generated may use less energy to provide the pressurization. In embodiments
where the
substance being refined is a liquid, providing at least part of the
pressurization before the
hydrogen is generated may be done with a pump located in pipe 194. Such
embodiments
may have similar advantages and characteristics described below for pump 133.
But even
where the substance being refined is a gas, providing at least part of the
pressurization
before the hydrogen is generated may require less energy provided the
substance being
refined has less volume or fewer molecules than the gasses that would need to
be
pressurized after refining at a lower pressure.
In various embodiments, including those wherein hydrogen generator 120 is an
electrolysis unit, system 100 may include pump 133 which may increase the
pressure of the
water, for example from water main 127. Pump 133 may provide at least part of
the
pressurization (performed by the pressurization apparatus) before the hydrogen
is generated.
Thus, pump 133 may serve as a pressurizing apparatus, either alone or in
combination with
other equipment, such as compressor 130. For instance, in some embodiments,
pump 133
may provide at least one quarter or one half of the pressurization before the
hydrogen is
generated. In some embodiments, pump 133 may provide all of the
pressurization. In other
embodiments, pump 133 may provide a smaller fraction of the pressurization.
For example,
pump 133 may provide an output pressure of at least 150 psi.
Although a centrifugal pump 133 is shown, pump 133 may be a positive
displacement pump such as a diaphragm type pump or gear pump. Thus, pump 133
may
have an output pressure, for example, above 150 psi, or as high as 12,000 psi,
for example.
In the embodiment illustrated, water may flow from water main 127, through
pipe 122 to
pump 133, and from pump 133 through pipe 124 to hydrogen generator 120. As
mentioned
above, system 100 may include various valves, for example valve 1291ocated in
water pipe
122. Valve 129 may be used, for example, for shutting off the water from water
main 127
when system 100 is not in service. Some valves of the present invention may be
automatically or electrically operated, while other valves may be manually
operated.
13
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
In embodiments of system 100 having pump 133, electrolysis may be performed at
a
pressure substantially above atmospheric pressure, for example, at 5000 psi
absolute. This
may result in the hydrogen and oxygen being produced at substantially the same
pressure
and may reduce or eliminate the equipment and/or energy required for
compressor '130.
Thus, in embodiments wherein hydrogen generator 120 is an electrolysis unit,
the overall
energy consumption of system 100 may be reduced by pump 133, for example, in -
comparison to systems that do not have pump 133 and rely on compressor 130
and/or other
compressors as the pressurizing apparatus. The size of system 100 may also be
reduced, for
example, by reducing the size of or eliminating compressor 130. The initial
cost and/or
maintenance and repair cost may be reduced as well. In other embodiments,
however, pump
133 may be omitted, and electrolysis may be performed at lower pressure. In
some
embodiments, electrolysis may be performed at atmospheric pressure, for
example.
System 100 may consume electrical energy, which may be supplied from
electrical
power utility grid 160. For instance, in embodiments wherein hydrogen
generator 120 is an
electrolysis unit, electricity may be used for electrolysis. Pump 133,
compressor 130,, and
other components of system 100 may also consume electricity. Power from grid
160 may be
provided, for example, through wires or electrical circuits 162, 163, and 164
to hydrogen
generator 120, compressor 130, and pump 133 respectively. Circuits 162, 163,
and 164 may
contain disconnect switches, on/off switches, circuit breakers, fuses, meters,
and/or the like,
and may be controlled by various automatic control equipment, for example, as
described
herein.
System 100 and the equipment forming system 100 may be controlled, in whole or
in
part, by automatic control equipment, which may include, for example, one or
more
processors, pressure switches, timers, relays, manually-operated switches or
buttons, load
25. controllers, or the like. This control equipment, may, for example, start,
adjust, or terminate
hydrogen generator 120, compressor 130, and/or pump 133. The control equipment
may
also control, for instance, valves 119 and/or 219 thereby determining which
pressure vessel
110 is being filled or emptied at a particular time. Control equipment may
also control
dispenser 170. Dispenser 170 may contain control equipment for activation,
which may
accept payment, for example, via credit card, and may allow the user to select
the type of
fuel or mixture ratio dispensed. Control equipment may include or interface
with leak and
fire detection, alarm, and suppression systems and/or equipment.
14
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
Electricity from grid 160 may be consumed, in whole or in part, during off-
peak
periods, when the demand for electricity from grid 160 is generally low, and
electricity is
readily available. This may be accomplished, for example, by controlling the
time of day
that system 100 produces and stores hydrogen (e.g., hydrogen generator 120 and
compressor
130 operate), or one or more electric utility company may remotely control
when system
100 operates. Such control may take into consideration, for example, the
pressure within -
pressure vessel or vessels 110, anticipated demand for hydrogen, and/or the
fike. In some
embodiments, the electricity for electrolysis, compressor 130, pump 133, or
some
combination thereof may come fi=om an off-line source, such as an engine-
generator set. In
still other embodiments, - one or more components of system 100 may be driven
by
something other than an electric motor, such as an internal combustion engine,
a turbine, a
water wheel, a wind turbine, or the like. For instance, in embodiments that
involve natural
gas, some of the natural gas may be burned in an internal combustion engine or
turbine that
may drive compressor 130. Such an internal combustion engine may perform other
functions as well, such as, for example, driving a generator to provide
electricity for use at
the site, providing heat for space heating, deicing, heating water, or other
purposes,
providing exhaust gas to be used as a substantially inert gas for fire
suppression, and/or the
like.
- System 100 may include one or more pressure vessels 110. Pressure vessel 110
may
be suitably configured to store hydrogen gas, for example at a pressure of
5000 to 12,000
psi. Pressure vessel 110 may have a pressure relief valve (prv) 118 that may
be vented to
vent pipe 140, which is described in more detail below. Pressure vessel 110
may be made
from a steel cylinder or section of pipe, and may have domed or dished ends
which may be
welded to the cylinder. In other embodiunents, pressure vessel 110 may be made
of a
composite material. The axis of pressure vessel 110 may, for example, be the
axis of the
cylinder. Pressure vessel 110 may be designed, fabricated, tested, and/or
certified, in
accordance with the American Society of Mechanical Engineers (ASME) Boiler and
Pressure Vessel Code, which is hereby incorporated by reference, and may be
stainped or
marked accordingly. Pressure vessel 110 may be designed, fabricated, tested,
certified,
and/or marked specifically for hydrogen service. Pressure vessel 110 may be
coated and/or
lined, for example, with a suitable paint.
In some embodiments of the present invention, pressure vessel 110 may be
oriented
substantially vertically, and may have a top end 111 and a bottom end 113. In
embodiments
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
having a plurality of pressure vessels 110, they may each be oriented
substantially vertically,
and may be parallel, for example as shown in FIG. 2. As used herein,
"substantially
vertically" means that the axis is closer to vertical than to horizontal. The
axis may be, for
example, the axis of the cylinder that forms pressure vessel 110. Being
oriented
substantially vertically may offer several advantages in particular
embodiments, including
that pressure vessel 110 may require less square footage of floor space or
land area. Thus,
system 100 may have a smaller footprint than the prior art.
In addition, the vertical orientation of pressure vessel 110 may facilitate
the safe
escape and dissipation of any hydrogen that leaks fiom pressure vessel 110,
the connecting
piping (e.g., supply pipe 144), various valves (e.g., valve 119 shown in FIG.
1) or the
various connections thereto. Thus, the veitical orientation of pressure vessel
110 may
enhance the safety of the hydrogen storage assembly. Top end I 11 of pressure
vessel 110
and any exposed portion of supply pipe 144 shown in FIG. 1 may be located, for
example,
about 30-feet above the ground. From this location any leaking hydrogen would,
in most
applications, rise harmlessly. In some embodiments, all penetrations through
pressure
vessel 110 may be in the top half, third, or quarter of pressure vessel 110.
In some
embodiments, some or all penetrations into pressure vessel 110 may be at,
near, or
substantially near top end 111. Thus, in the event hydrogen leaks and ignites,
the distance
from the fire to any personnel or vulnerable equipment may be great enough to
prevent harm
thereto.
Pressure vessel 110 may be connected to hydrogen generator 120 or compressor
130,
for example via pipe 134, supply pipe 144, and/or the like, and valves, for
example, valves
149, 119, or both. In the exemplary embodiment illustrated, pipes 132, 134,
and 144 are
configured to convey hydrogen from hydrogen generator 120, and to pressure
vessel 110.
Hydrogen flow into or out of pressure vessel 110 may be at the top end 111 of
pressure
vessel 110. Pressure vessel 110 may be equipped with a safety or pressure
relieve valve
(prv) 118 which may be located at or connected to top end 111. Pressure vessel
110 may
have a drain valve at (or piped to) bottom end 113, the discharge from which
may be piped
to vent pipe 140. For instance, pressure vesse1110 may have a drain that may
include a pipe
that penetrates pressure vessel 110 at top end 111, extends down through the
interior of
pressure vessel 110, and terminates at or near bottom end 113 inside pressure
vessel 110.
Thus, a drain may be provided in pressure vessel 110 even in embodiments where
all
penetrations through pressure vessel 110 are at or near top end 111. Vent pipe
140 may
16
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
contain a drain valve at its low point as well, for example, for removing any
water or other
liquid from within.
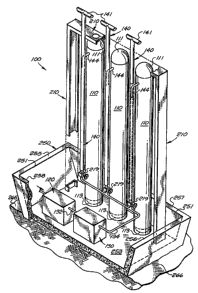
Pressure vessel or vessels 110 may be supported by a support structure 210
(shown
in FIG. 2), which may also support vent pipes 140, other hydrogen handling
equipment, or a
combination thereof. Support structure 210 may be fabricated from structural
steel, and may
be painted to prevent corrosion. In some embodiments, support structure 210
may be coated -
with a fire-resistant coating. In other embodiments, pressure vessel or
vessels 110 may be
self suppoiting and vent pipes 140 may be supported in whole or in patt by
pressure vessel
or vessels 110. Other hydrogen handling equipment may also be mounted on or
supported
by pressure vessel or vessels 110 or a common support structure 210. Support
structure 210,
pressure vessels 110, andlor vent pipes 140 may have lightening rods mounted
thereon.
Pressure vessel or vessels 110, support structure 210, enclosure 150, and the
various
hydrogen handling equipment, including dispenser 170, may be grounded with a
suitable
grounding grid.
In an exemplary embodiment of system 100, three pressure vessels 110 may
provide
a storage capacity of about 25,000 standard cubic feet (sco of hydrogen. In
another
exemplary embodiment, six pressure vessels 110 may provide a storage capacity
of about
50,000 sef.
In some embodiments of the present invention, hydrogen may be generated at
another location or site and delivered to system 100 by pipeline, ship,
railroad, or truck, for
example. In such embodiments, the hydrogen source at the site may be the
pipeline, ship,
railroad, or truck, for example. For instance, hydrogen may be generated
through
electrolysis of water at a dam site, geothermal site, wind farm, solar power
plant, and/or the
ldce, that has generating capability but does not necessarily have access to
power lines or a
power grid of sufficient size to utilize all of the electrical power that is
generated, at least at
some times. In such embodinlents, system 100 may lack a hydrogen generator
120, and may
also lack a pressurizing apparatus or compressor 130, or may have a compressor
130, which
may be, for example, configured to start compressing at a higher pressure.
Such
embodiments of system 100 may have one or more pressure vessels 110.
In some embodiments of system 100, pressure vessel or vessels 110 may be
smaller
in size or eliminated entirely, and system 100 may deliver hydrogen directly.
For example,
system 100 may directly fill pressure vessel 185 on vehicle 180, which are
described in
more detail below. In such embodiments, hydrogen generator 120, the
pressurizing
17
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
apparatus, for example, compressor 130, or both, may be larger. For instance,
hydrogen
generator 120 and/or compressor 130 may be configured to deliver hydrogen at a
sufficient
rate to fi11 pressure vessel 185 within an acceptable amount of time while the
user or driver
of vehicle 180 waits. Greater expense of hydrogen generator 120 and/or
compressor 130
may be partially or completely offset by savings on pressure vessel or vessels
110. If a
smaller pressure vessel 110 is used at all in such an embodiment of system
100, the smaller
pressure vessel 110's function may be to avoid excessive cycling on and off of
hydrogen
generator 120 and/or compressor 130, for example. Hydrogen generator 120
and/or
compressor 130, may be turned on and off by a pressure switch on pressure
vessel 110, for
example.
Such embodiments may have the disadvantage that they consume energy when
hydrogen is demanded, rather than being able to shift some or most energy
consumption to
off-peak periods. In addition, the number of vehicles 180 that can be refueled
in a short time
may be limited by the capacity of the hydrogen source or hydrogen generator
120 and/or
compressor 130, for example. In addition, additional cooling equipment for the
pressurized
hydrogen may be required, rather than allowing it to cool in pressure vessels
110. But these
embodiments may have the advantage of being able to produce more hydrogen, for
example,
around the clock, when needed, relative to the investment in equipment. In
addition, less
energy may be wasted by transferring compressed hydrogen gas at a higher
pressure in
pressure vesse1110 to a lower or initially lower pressure in pressure
vesse1185.
Some embodiments of system 100 may have a large hydrogen generator 120, a
large
pressurizing apparatus, and relatively large pressure vessel 110 capacity, and
may be
configured to fill pressure vessel 185 directly from hydrogen generator 120
and the
pressurizing apparatus, or from pressure vessels 110. For instance, the user
may determine
which fill scheme is used, and this decision may depend on how long the user
is willing to
wait. In other embodiments, system 100 may automatically employ a direct fill
scheme, for
example, if there is only one user, but may fill from pressure vessels 110 if
there are
multiple users or multiple vehicles 180, for example, at multiple dispensers
170. In such
embodiments, a cascade scheme (described in more detail below) may be used
when
hydrogen is taken from pressure vessels 110.
System 100 may include one or more vent stacks or vent pipes 140, which may be
substantially parallel to pressure vessel or vessels 110, may be substantially
vertical, may be
located near pressure vessel 110, and may terminate at end 141 to the
atmosphere overhead
18
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
and/or near or above the top end 111 of pressure vessel 110. As used herein,
the term "near"
when referring to proximity to pressure vessel 110 or to top end 111, unless
indicated
otherwise, means within two diameters of pressure vessel 110. Also as used
herein, the
phrase "substantially near" when referring to proximity to pressure vessel 110
or to top end
111, unless indicated otherwise, means within one diameter of pressure vessel
110. Vent
pipe 140 may be configured to vent leaking or escaping hydrogen to a location
where it can -
be released to the atmosphere relatively safely. Since hydrogen gas is lighter
than air, this
location may be overhead, as shown.
System 100 may be configured so that no hydrogen can be released, for
exa.rnple,
through a valve, except through vent pipe or pipes 140. Vent pipe or pipes 140
may be sized
so that all safety valves can release simultaneously, and the pressure in vent
stack 140
remains below a maximum pressure, for example, of 1000 psi. There may be a
temperature
monitor or sensor within vent stack 140, for example, a theimocouple, to
detect a fire within
vent pipe 140 or exhausting into vent pipe 140. Vent pipe 140 may be made, for
example,
from afenous material suitable for the temperature, pressures, and exposure to
hydrogen
that vent pipe 140 may encounter, for example, type 316 stainless steel.
In some embodiments of the present invention, vent pipe 140 may be designed to
withstand the stoichiometric detonation of hydrogen and air within vent stack
140. This
may be, for example, 300 to 900 psi for 0.5 to 2 milliseconds. In some
embodiments, vent
pipe 140 may be designed to withstand stoichiometric flame temperatures of
hydrogen and
air within the vent pipe 140 for short periods of time of, for example, 2045
degrees C.
Enclosure 150 (described below) may be designed to withstand similar pressures
and
temperatures.
End 141 of vent pipe 140 may include a tee (as shown), a 135 to 180 degree
bend, a
plurality of bends, or other shape or feature to exclude precipitation from
vent pipe 140, or
may be a plain open end. End 141 may be configured to withstand deflagration
or
detonation of hydrogen or another fuel. End 141 may be configured so that
leaking
hydrogen can burn at end 141 continuously without damaging end 141 or other
nearby
equipment or structures. End 141 may direct any pressure pulsation or flame
away from
people, equipment, or stiuctures.
FIG. 1 illustrates an exemplary embodiment having one vent pipe 140, and FIG.
2
illustrates an exemplary embodiment having three vent pipes 140 (and three
pressure vessels
110). In the embodiment illustrated in FIG. 2, each vent pipe 140 encloses
part of a supply
19
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
pipe 144, which is each connected to a pressure vessel 110 near top end 111.
In the
embodiment illustrated, each supply pipe 144 contains a valve 219, which in
FIG. 2 are
shown located within structure 250. Various exemplary embodiments of the
present
invention may include a fill valve, a block valve, a dispensing valve, and/or
a safety valve
118, for example, for each pressure vessel 110. The block valve may be located
in pipe 144
and may isolate all flow into and out of pressure vessel 110, except usually
for flows out of -
pressure relief valve 118. Some or all of these valves may be housed within
one enclosure
150 (described in more detail below), for exa.mple, for each pressure
vesse1110.
As mentioned, system 100 may include supply pipe 144, which may connect to
pressure vessel 110, for example, as shown. Supply pipe 144 may catry hydrogen
from
hydrogen generator 120, compressor 130, or other piping (e.g., pipe 134) to
pressure vessel
110. Supply pipe 144 may also carry hydrogen from pressure vessel 110 to
dispenser 170,
for example, via pipes 172 and 174, and/or to vehicle 180, for example, via
hose or pipe
182. Supply pipe 144 and/or other piping may be located at least partially
within vent pipe
140 so that any leakage fiom supply pipe 144 and/or the other piping may be
safely vented
through vent pipe 140. Supply pipe 144 may connect to pressure vessel 110
overhead or
near top end 111 so that any leakage from the connection is likely to
dissipate innocuously
upward. As used herein, "overhead" means at least above where users or members
of the
public are likely to be located. In some embodiments, all high-pressure
hydrogen carrying
piping may be located inside enclosures, or other piping that is vented
overhead, for
example through one or more vent pipes 140. Supply pipe 144 being located
within vent
pipe 140 may also eliminate potential flame impingement on adjacent surfaces
caused by
failure of supply pipe 144 and the ignition of the hydrogen leaking therefrom.
For instance,
vent pipe 140 may eliminate potential flame impingement on pressure vessel
110, adjacent
pressure vessels 110, and/or adjacent supply pipes 144. As would be apparent
to a person of
sl:.ill in the art who has studied this document, coaxial piping may be used
in other parts of
system 100 and may provide similar benefits.
Pressure vessel 110 may be completely or partially located below grade (below
the
ground surface), for example, in a hole, or in an underground vault. Thus,
bottom end 113
of pressure vessel 110 may be located below the ground surface. This may
reduce the height
of pressure vessel 110 above the ground surface, allow pressure vessel 110 to
be longer
(taller), provide support for pressure vessel 110, or a combination of these.
Reducing the
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
height of pressure vessel 110 may reduce wind loading, reduce the risk of
lightening stri7ces,
reduce visual impact, reduce solar heating, and/or the like.
In such a below-grade embodiment of pressure vessel 110, at least the portion
of
pressure vessel 110 that is below grade may have another enclosure or
secondary
containment 115 forming interstitial space 116 between pressure vessel 110 and
secondary
containment 115. In some embodiments, secondary containment 115 may continue
above
grade, and may completely enclose pressure vessel 110. In addition,
embodiments of the
present invention wherein pressure vessel or vessels 110 are entirely above
grade may have
secondary containment 115. Secondaty containment 115 may allow pressure
vessels 110 to
be located more closely to other buildings, for example.
Secondary containment 115 or pressure vessel 110 may be buried directly or may
be
embedded in concrete, for example. Interstitial space 116 may be ducted or
vented (directly =
or indirectly) to vent pipe 140. Interstitial space 116 or vent pipe 140 may
be monitored for
leakage of hydrogen thereto, or for the presence of water or other foreign
substances therein.
Pressure vessel 110 and/or secondary containment 115 may be coated, for
example to
prevent corrosion, and a pump, drain, or access may be provided to remove
water or other
liquids fiom interstitial space 116. Access may be provided to inspect
pressure vessel 110
within secondary containment 115.
As illustrated in FIG. 2, there may be more than one pressure vessel 110, and
bottom
end 113 of pressure vessel (or vessels) 110 may be located at or above floor
or ground level.
In the exemplary embodiment illustrated, there are three pressure vessels 110.
In
embodiments of system 100 having a plurality of pressure vessels 110, hydrogen
gas may be
stored at different pressures in the different pressure vessels 110.
Deliveries of hydrogen,
for example, via dispenser 170 to vehicle 180, may be made in a cascading
manner, first
fiom the pressure vessel 110 having the lowest pressure, and last from the
pressure vessel
110 having the highest pressure. Using such a cascade fill scheme, less energy
may be
wasted by releasing fully pressurized hydrogen to a location having little or
no pressure.
The more pressure vessels 110, each having a different pressure and each
cascaded in turn,
the closer to isentropic the process of filling pressure vesse1185 becomes.
Multiple pressure
vessels 110 also provide more storage capacity, assuming the size of the
pressure vessels are
the same. In addition, multiple pressure vessels 110 may facilitate expansion
of system 100
in the event demand for hydrogen increases over time, and may allow
maintenance or repair
of one pressure vessel 110 while the others remain in service.
21
CA 02619895 2008-02-19
WO 2005/019721 . PCT/US2004/020983
As mentioned, system 100 may supply hydrogen to vehicle 180 through dispenser
170. Although vehicle 180 is shown in FIG. I as being a car or automobile,
vehicle 180
may be a boat, ship, submarine, train, truck, golf cart, industrial cart,
personnel carrier such
as used in airports or at resorts, an industrial or commercial vehicle or
piece of equipment, a
construction vehicle or piece of equipment, a piece of lawn, gardening, or
farming
equipment or vehicle, a vehicle or equipment for mining, a military vehicle,
an all-terrain
vehicle, a race car, a wheel chair, a personal transportation device, a
motorcycle, a scooter, a
SEGWAY, a human transporter, an aircraft, a space vehicle, or the like.
Vehicle 180 may
have pressure vessel 185 located on board vehicle 180, which may be used to
store hydrogen
or a mixture containing hydrogen. Vehicle 180 may have engine 181 which may be
an
internal combustion engine, a steam or Rankin cycle engine, or a gas turbine,
for example,
and may burn hydrogen as a fuel, either alone or mixed with one or more other
fuels.
Engine 181 may drive the wheels of vehicle 180, for example, through a
transmission and
drive line, may drive a generator, or both. In lieu of or in addition to
engine 181, vehicle
180 may have a fael ce11 183, which may convert hydrogen to electricity.
Vehicle 180 may
by a hybrid electric vehicle, and may have battery, batteries, or battery pack
187, and/or an
electric motor or motor/generator.
Vehicle 180 may consume substantially pure compressed hydrogen gas, for
example,
via engine 181, fuel cell 183, or both. Such vehicles 180 may produce little
or no pollution,
and may be particularly well suited for uses where not polluting is critical.
For example,
vehicle 180 may be suitable for use indoors or in confined spaces, such as
tunnels and the
like, and may reduce or eliminate the need for ventilation in such areas, as
compared with
alternative fuels. These pure hydrogen consuming vehicles 180 may be suitable
for use
where the only other practical alternative is to use electric vehicles.
In other applications, vehicle 180 may consume a mixture of hydrogen gas and
at
least one other flamma.ble gas, which may be a fossil fueL Such a vehicle 180
may burn this
mixture in an internal combustion engine. The other flammable gas may be
natural gas, for
example, which may be compressed, i.e., compressed natural gas (CNG).
Consumption of
this mixture of hydrogen and CNG may produce less pollution than burning CNG
alone.
Dispenser 170 may be configured to dispense a plurality of substantially
different mixture
ratios of hydrogen and the other gas or gasses. For example, dispenser 170 may
dispense a
mixture of hydrogen and CNG in the ratios of 0, 5, 10, 15, 20, 30, 50 or 100
percent
hydrogen (by volume) for vehicles 180 of differing configurations or being
used for
22
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
different purposes. In some embodiments, the ratio of hydrogen to the other
fuel dispensed
by dispenser 170 may be continuously variable over a range, and the range may
be as great
as from 0 to 100 percent hydrogen. The mi~,~ture may be stored in pressure
vessel 185 on
vehicle 180, for example.
System 100 or dispenser 170 may produce the desired mixture, for example, by
metering the flow of each substance, which may be computer monitored and
controlled. -
The metering may be implemented, for example, with Coriolis meters or thermal
mass flow
meters. In one exemplary embodiment, dispenser 170 niay introduce a small
quantity of at
least one substance, and then may use the perfect gas equation to calculate
the volume of
pressure vessel 185 on vehicle 180, using the change in pressure resulting
from adding the
small quantity of at least one substance. System 100 or dispenser 170 may then
calculate the
amount of each substance to deliver to obtain the desired mixture ratio, and
may then
dispense metered quantities of each substance, for example, in turn or at
least partially
simultaneously.
In other embodiments, hydrogen and one or more other fuels may be stored
separately on vehicle 180, for example, in separate pressure vessels 185 or in
appropriate
storage tanks. Thus, vehicle 180 may be a duel-fuel or multi-fuel vehicle.
Vehicle 180 may
be configured to operate on different fuels, for example, in different
situations. As an
example, vehicle 180 may have engine 181 which may be configured to burn
hydrogen
when pollution-free exhaust is required, but may buin CNG, propane, gasoline,
another fuel,
or a mixture of hydrogen and one or more of these fuels when exhaust emissions
are not as
critical. For instance, vehicle 180 may burn hydrogen when operating in a
confined space,
but may burn other fuels or a mixture when outdoors or in a well-ventilated
space.
In some embodiments, vehicle 180 may consume a mixture of hydrogen and another
fuel during operations when emissions are more difficult to control, but may
reduce or
eliminate the amount of hydrogen that is burned, in comparison to the other
fuel, when
emissions are easier to control. For instance, vehicle 180 may consume
hydrogen or a
mixture of hydrogen and another fuel during start-up conditions, but may
switch to
exclusively the other fuel or reduce the proportion of hydrogen that is burned
when normal
operating temperature has been reached. In some embodiments, vehicle 180 may
use
hydrogen for one purpose, for example in a fuel cell 183, and may use another
fuel, or a
mixture of hydrogen and another fuel, for another purpose, for example, in
engine 181.
23
CA 02619895 2008-02-19
WO 20051019721 PCT/US2004/020983
Embodiments of system 100 that are configured to dispense mixtures of hydrogen
gas and another gas or gasses may include equipment for handling the other gas
or gasses.
Although system 190 in the exemplary embodinient handles natural gas, system
190 may
alternatively, or in addition, handle propane, methane, butane, or other
fossil fuels or
flammable gasses. In some embodiments, system 190 may handle gasoline,
alcohol, diesel
fuel, or other liquid fuels, although differences may exist due to such other
flaxnmable -
substances being liquids.
The exemplary embodiment of system 100 illustrated in FIG. 1 includes natural
gas
system 190 that receives natural gas from gas main 197 via pipe 192,
compresses the natural
gas with compressor 193 forming CNG, and stores the CNG in one or more
pressure vessels
191. In the exemplary embodinient illustrated, the CNG travels fiom compressor
193 to
pressure vessel 191 through pipe 198, and from pressure vessel 191 to
dispenser 170 through
pipe 199. Pressure vessel 191 is shown in FIG. 1 as being horizontal, but
pressure vessel
191 could be oriented substantially vertically, which would have many of the
benefits
described herein for such orientation of pressure vessel 110. In addition,
pipe 199 could be
connected to pipe 198 rather than to the opposite end of pressure vessel 191.
Pressure vessel
191 may otherwise be similar in configuration and operation to pressure vessel
110
described above, as may be apparent to a person skilled in the art.
In some embodiments of system 100, various equipment may be shared between the
hydrogen and at least one other flammable gas. For instance, hydrogen and
natural gas may
both be compressed by the same compressor (e.g., 130) and/or stored in the
same pressure
vessel or vessels 110. In such embodiments, the mixture ratio may be
controlled as the
hydrogen is produced (e.g., by hydrogen generator 120), and different mixture
ratios may be
produced at different times, compressed, and stored in different pressure
vessels 110.
Dispenser 170 may include various components including, for example, control
switches andlor control buttons, a display, a card reader, and/or the hqce. In
embodiments of
the present invention where hydrogen and another flammable substance or fuel
are stored
separately, dispenser 170 may be configured to allow the user to select the
mixture ratio of
hydrogen and one or more other fuels, and pay for the fuel purchased, for
example with a
credit card. Dispenser 170 may display or otherwise provide various
infoimation such as the
mixture or type of fuel being dispensed, the amount of fuel thus far dispensed
(e.g., in
volume, such as standard cubic feet, or by mass), the price of fuel delivered
so far, the
24
CA 02619895 2008-02-19
WO 2005/019721 PCT/US200-1/020983
pressure, and/or the like. Dispenser 170 may have a manually operated shut-off
switch for
emergency use.
In addition to or in lieu of dispensing hydrogen from dispenser 170 to vehicle
180,
the hydrogen produced by system 100 may be used for other purposes. For
example, the
hydrogen may be used for industrial purposes as a chemical (rather than as a
fuel), or added
to other fuels to reduce the pollution produced by the consumption of those
other fuels. In _
the exemplary embodiment illustrated in FIG. 1, system 100 may provide
hydrogen to
stationary natural gas system or natural gas main 197 via pipe 114. Thus, the
hydrogen may
be mixed with the natural gas to produce a mixture, that when bmmed, produces
less
pollution. This mixture may be delivered, for example, to urban areas where
pollution is a
particular problem, and/or at times when pollution is a particular problem.
In such applications, the pressurizing apparatus (e.g., compressor 130, pump
133, or
both) may not need to provide nearly as much pressure, and pressure vessel 110
may not be
needed or may be configured for lower pressure storage. In one embodiment of
such a
system 100, hydrogen generator 120 may be a natural gas reformer, the
pressurizing
apparatus may provide just enough pressure to obtain the desired flow of
natural gas from
gas main 197, through pipe 194 and natural gas reformer or hydrogen generator
120, and
back to natural gas main 197, for example, through pipe 114. Thus, in this
application,
system 100 may treat the natural gas to make it a cleaner burning fuel. In
other applications,
hydrogen generator 120 may be an electrolysis unit, and hydrogen may be
produced and
added to natural gas main 197 to increase the quantity of natural gas, for
example, in
applications or at times when electricity is plentiful and natural gas is in
short supply or
particularly valuable.
In the embodiments described so far, the hydrogen may be in gaseous form, and
may
be compressed for storage, for exarn ple, in pressure vessel 110. In other
embodiments of the
present invention, the hydrogen may be cooled, condensed, and stored in liquid
fornn. In
such embodiments, system 100 may include a chiller, for example, in lieu of
compressor 130
between pipes 132 and 134. Thus, hydrogen from a hydrogen source or produced
by
hydrogen generator 120 may be condensed to liquid form. Such embodiments of
system
100 may include a tank for storage of liquid hydrogen, for example, in lieu of
pressure
vessel 110. Such a tank may be vented to atmosphere, for example via vent pipe
140, and
may be insulated. Such a tank may also be refrigerated, or may be combined
with the
chiller. In other enlbodiments, hydrogen may be reformed in liquid form. In
embodiments
CA 02619895 2008-02-19
WO 2005/019721 ' PCT/US200 t/020983
where the hydrogen is liquid, the liquid hydrogen may be delivered in liquid
form to vehicle
180, for example via dispenser 170, and may be stored in liquid form at
approximately
atmospheric pressure in an insulated tank on vehicle 180.
Liquid hydrogen may be used, for example, in applications where vehicle 180 is
an
aircraft, a space craft, a race car, or the like. Use of hydrogen for such
applications may
reduce the weight of the fuel that is required for vehicle 180, and the use of
liquid hydrogen
rather than hydrogen gas may eliminate or reduce the weight of pressure
vesse1185 and may
reduce the space requi.red for fuel storage. Liquid hydrogen may be used, for
example, to
increase the load capacity, range, speed, altitude, or a combination thereof,
of commercial or
military aircraft. Hydrogen may also be used to increase the power, reduce the
weight,
and/or increase the safety of race cars. Using hydrogen with race cars, as
well as in other
applications, would also have the public relations benefit of substantially
reducing or
eliminating harmful exhaust emissions.
Even where hydrogen gas is used rather than liquid hydrogen, it may be
advantageous to cool the hydrogen gas, for example, after it leaves hydrogen
generator 120
or compressor 130, between stages of compressor 130, or while the hydrogen gas
is stored in
pressure vessel 110. Cooling the hydrogen gas may reduce the amount of energy
that is
required by compressor 130, increase the rate at which compressor 130 can
compress
hydi-ogen gas, increase the amount of hydrogen gas that can be stored in
pressure vessel 110
or 185, or a combination of these benefits. Ways to cool the hydrogen gas may
include air-
cooled heat exchangers before compressor 130 or between stages, painting
pressure vessel
110 white or a light color or shading it, cooling compressor 130, the heat
exchangers, or
pressure vessel 110 with evaporative cooling, or refrigerating pressure vessel
110.
One significant issue with flammable materials, including hydrogen, is safety.
As is
well known, if flammable materials leak out, they may ignite causing a fire or
even an
explosion, which may kill or injure people nearby, and may damage property.
Hydrogen is
lighter than air, so it tends to rise if it leaks. This distinguishes hydrogen
from most other
flammable materials. Various aspects of the present invention serve to improve
the safety of
hydrogen handling or distributing systems, particularly with respect to fires
or explosions
caused by hydrogen leaks.
Hydrogen may react with air to produce rapid combustion which can fall into
the
categories of deflagration (subsonic pressure wave) and detonation (sonic
pressure wave).
Deflagration can typically occur when mixture of air and hydrogen fall below
30% hydrogen
26
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004,/020983
stoichiom.etrically. Deflagration pressure wave may vary from 0.5 to 5 psi.
The detonation
of hydrogen in air typically occurs in air in a stoichiometric miz.=ture of 18
to 59% hydrogen
by volume. Pressure waves in the order of 600 to 900 psi during time inteivals
of 0.5 to 2.0
milliseconds may occur in hydrogen detonation. Hydrogen flame temperatures may
reach
2045 degrees C in hydrogen fires.
As mentioned above, pressure vessels 110 being oriented substantially
vertically, and
vent pipe or pipes 140, may facilitate the safe escape and dissipation of
hydrogen leakage
overhead from various potential leakage locations. Enclosure 150 and 195, and
fire
suppression system 155, all illustrated in FIG. 1, enclosure 350 shown in FIG.
3, walls 255,
256, 257, and 258 illustrated in FIG. 2, and other features of various
embodiments of the
present invention may also improve safety, as will be described next.
Referring again to FIG. 1, control panel or enclosure 150 may be substantially
sealed, and may contain various hydrogen handling equipment including that
described
herein. For instance, enclosure 150 may contain compressor 130, pipe 132,
valve 139, part
or all of pipe 134, valve 149, and the like, for example, as shown in FIG. 1.
In an exemplary
embodiment, enclosure 150 may just contain valves and piping, for example, for
one
pressure vessel 110. Although hydrogen generator 120 and pressure vessel 110
are located
outside enclosure 150 in the exemplary embodiment illustrated in FIG. 1, in
other
embodiments, hydrogen generator 120 and/or pressure vessel 110 may be located
within
enclosure 150. Enclosure 150 may include secondary containment 115.
Enclosure 150 may be fabricated fiom steel plate or sheet metal, or pipe,
which may
be galvanized, painted, or botb, and may have one or more doors or removable
panels, for
example, to access the hydrogen handli.ng equipment within, or all or part of
enclosure 150
may be removable for maintenance of the equipment within. The doors or panels
may have
gaskets or seals. Enclosure 150 may be, for example, rectangular, cylindrical,
spherical or
the like, or some combination thereof. Enclosure 150 may have various
penetrations and/or
connections for electrical conduit, piping, and the like, and penetrations may
be sealed in
whole or in paLt.
Enclosure 150 may be vented to atmosphere, for example, via vent pipe 140.
Enclosure 150 may be ducted to vent pipe 140, for example, via duct or pipe
152, which
may enclose various piping, such as pipe 134 shown in FIG. 1. Unless clearly
otherwise,
enclosure 150, as described herein, may include pipe 152 and/or vent pipe 140.
In
anticipation of a hydrogen leak within enclosure 150, which forms an explosive
mixture of
27
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
hydrogen and air and is then ignited, enclosure 150 may be configured to
withstand the
pressure generated by the detonation of such a mixture. This pressure may
spike and then be
relieved quickly through vent pipe 140. Thus, enclosure 150 may form a vented
coaxial
detonation compartment.
Enclosure 150 may be designed for the worst case scenario of such an explosion
or
detonation, which may be assumed to be the existence of a stoichiometric
mi.titure of ~
hydrogen and air throughout enclosure 150, for example, where the pressure
therein is at
atmospheric before detonation. It may be assumed that the stoichiometric
mixture exists in
pipe 152 and vent pipe 140 as well. But, as will be explained, in most
situations, a perfect
stoichiometric ratio will only exist (at most) for an instant.
Assuming that a small hydrogen leak forms within enclosure 150 and continues
to
leak at the same rate, the percentage of hydrogen within enclosure 150 will
gradually
increase. Before the stoichiometric ratio is established, the mixture will be
leaner than
stoichiometric, and after the stoichiometric ratio has been established, the
mixture will
become richer than stoichiometric. In fact, after a leak has existed for a
while, or quickly if
the leak is large, the hydrogen may displace the air substantially reducing or
eliminating the
potential of an explosion. In addition, the mix-ture may not be the same
throughout the
enclosure, so the mixture may be richer than stoichiometric near the leak, and
leaner than
stoichiometric away from it. Thus, in some embodiments, it may be acceptable
to assume a
maxinlum pressure that is less than that produced by a perfect stoichiometric
ratio. For
instance, enclosure 150 may be designed and/or configured to withstand a
pressure that is
half of that produced by a stoichiometric mixture throughout enclosure 150.
The enclosure 150 may be allowed to withstand some damage in an explosion,
provided it remains substantially sealed. As used herein "substantially
sealed" means sealed
sufficiently that air would not leak into enclosure 150 in sufficient quantity
to support a
significant fire therein, and hydrogen would not leak out of enclosure 150
through a leak in
enclosure 150 (but not including through end 141 of vent pipe 140) in
sufficient quantity to
support a significant fire at the location of the leak from enclosure 150. As
used herein,
enclosure 150 and/or vent pipe 140 are said to be able to withstand an
exTlosion or fire or
the pressure and/or heat of an explosion or fire, if they remain substantia]ly
sealed in the
event of such an explosion or fire within.
Enclosure 150 may be pressure or leakage tested to verify that it is
substantially
sealed. End 141 of vent pipe 140 may be configured to be plugged or attach a
pressurizing
28
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
device to pressure test vent pipe 140 and/or enclosure 150. In some
embodiments, a
particular amount of leakage from enclosure 150 may be tolerable.
The less unoccupied volume or free air volume within enclosure 150, the less
space
that is available for the accumulation of an explosive mixture. Thus, it may
be advantageous
to select or construct enclosure 150 so that it is as small as possible while
still containing the
= hydrogen handling equipment and providing access thereto for any required
maintenance or -
repair. Extra space or fiee air volume within enclosure 150 may be occupied or
eliminated
with filler material 157, which may be configured to collapse and absorb
pressure and
energy in the event of an explosion. For example, closed cell foam or empty
sealed
containers may be used for filler material 157, which may collapse, at least
to some extent,
in the event of the sudden pressure rise from an explosion. Some embodiments
of enclosure
150 may have pressure relief panels that may open in the event of substantial
internal
pressure, relieving the pressure, but may close upon dissipation of the
pressure to prevent
oxygen-containing air from traveling back into enclosure 150. Such pressure
relief panels
may be located where their sudden opening and release of potentially hot
gasses would not
pose a particularly high risk to people.
In some embodinlents, there may be separate enclosures 150, for example, for
separate pieces of hydrogen handling equipment (e.g., hydrogen generator 120
and
compressor 130), which may be separately ducted to vent pipe 140, or
partitions may exist
between different pieces of hydrogen handling equipment, forming distinct
zones or
compartments that may be separately ducted to vent pipe 140. There may be
check valves
or barometric dampers on the ducts to vent pipe 140 to prevent hydrogen that
leaks into one
enclosure or compartment fiom traveling into other enclosures or compartments,
or separate
vent pipes 140 may be used. Hydrogen handling equipment associated with each
pressure
vessel 110 may be segregated into its own enclosure 150, vent pipe 140, or
both, forming
separate pressure zones to prevent the cascading failure of adjacent zones.
These separate
enclosures 150 or partitions forming compartments may reduce the amount of
volume
within which an explosive mixture can form from a single hydrogen leak.
Fuither, dispenser
170 may have its own enclosure, and may have its own vent pipe.
In addition to containing explosions and smothering fires, enclosure 150,
pipes
located within other pipes (e.g., supply pipe 144 within vent pipe 140), and
secondary
containment (e.g., secondary containment 115 of pressure vessel 110) may
reduce or
eliminate flame impingement concerns on equipment should a leak occur and the
leaking
29
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
hydrogen ignite. For instance, in the exemplary embodiment illustrated in FIG.
1, if a leak
develops in supply 144, and the leaking hydrogen ignites at the location of
the leak, the wall
of pipe 140 may block the flames and prevent them from damaging or rupturing,
for
exaanple, pressure vessel 110. In this example, the wall of vent pipe 140 may
only have to
block the flame long enough for the oxygen within vent pipe 140 to be depleted
and the
flame to go out. -
Natural gas system 190 may contain enclosure 195, which may be similar to
enclosure 150, except that the flammable gas is natural gas rather than
hydrogen. Enclosure
195 may contain various pieces of natural gas handling equipment, including
compressor
193, piping 198, and/or pressure vessel 191. Enclosure 195 may also be ducted
to vent pipe
140, for example, via pipe 142, or to another relatively safe location. The
safest Iocation to
vent natural gas may be different than that for hydrogen, because of the
difference in density
of the two gasses. In some embodiments of system 100, the hydrogen handling
equipment
may share a common enclosure with other equipment, such as some or all of the
equipment
of natural gas system 190. In some enibodiments, system 100 may be configured
to handle
another fuel or flammable substance in addition to, or in lieu of, natural
gas, and such
embodiments may have various components similar to those of natural gas system
190,
including, for example, an enclosure similar to enclosure 150 and/or enclosure
195.
In the embodiment of enclosure 150 described above, enclosure 150 may also
safely
suppress a fire that starts within enclosure 150 by depriving the fire of
oxygen. For instance,
if a hydrogen leak begins from hydrogen handling equipment within enclosure
150, and the
leak is immediately ignited, then a fire may bum within enclosure 150, for
example, at the
location of the leak. But the fire will quickly consume the available oxygen
within
enclosure 150, suffocating the fire. Unburned hydrogen will further displace
the air within
enclosure 150, preventing combustion. In such an embodiment, enclosure 150 and
the
hydrogen handling equipment may be configured so that they are capable of
withstanding
the heat produced by a hydrogen fire within enclosure 150, at least for the
time required
until all of the oxygen within enclosure 150 is consumed. This may prevent the
fire from
causing a breach in enclosure 150 that would allow more air in, or the
creation of a more
severe hydrogen leak.
The above described embodiments of enclosure 150 are generally passive fire
suppression systems. Essentially no action may need to be taken by either
personnel or
automatic systems to prevent explosions or suppress fires. However, there are
advantages to
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
active or partially active fire suppression systems, and such advantages may
win out in
patticular applications. Embodiments of system 100 that have active fire
suppression
system may, for example, have fire suppression system 155 shown in FIG. 1 that
may be
configured to introduce air, a substantially inert gas, another fire
suppression substance, or a
combination of these, into enclosure 150, enclosure 195, or both.
In embodiments of system 100 having either active and passive fire suppression
-
systems, one or more locations may be monitored for the presence of hydrogen,
combustible
gasses, heat, products of combustion, the displacement of oxygen, infrared
radiation,
ultraviolet light, pressure spikes, noise generated by explosions or leaks,
and the like, which
may trigger an alarm, shut off equipment, operate valves, and/or the like. In
embodiments
of system 100 having enclosure 150, 195, or both, one or more of the locations
that is
monitored may be within the enclosure or enclosures, for example, at detectors
or sensors
156 and/or 196. In embodiments having one or more vent pipes 140, detectors or
sensors
156 and/or 196 or similar devices may be located within vent pipes 140. In
embodiments
having active fire suppression systems, for example, fire suppression system
155, the
monitoring (e.g., via sensor 156, 196, or both) may also trigger fire
suppression system 155
to take action.
In an exemplary embodiment, fire suppression system 155 may include a
ventilation
system, which may include a fan, and may blow air into or out of enclosure
150, enclosure
195, or both. For example, an exhaust fan may be installed in vent pipe 140.
Such an
exhaust fan may create a low pressure area or partial vacuum within enclosure
150 and may
prevent gasses within enclosure 150 from leaking out through any openings in
enclosure
150. A ventilation system having flow-through ventilation may prevent the
accumulation of
the flammable substance (e.g., hydrogen or natural gas) within the enclosure
(e.g., 150 or
195). Fire suppression system 155 may be configured to tum off the ventilation
system or
fan when a fire is detected, for example, by sensor or detector 156 or 196, or
one or more
sensors in vent pipe 140. In some embodiments, a damper may also close to
prevent air
from entering through the ventilation system. Thus, the fire may be smothered
inside
enclosure 150 or 195 for lack of oxygen. Such ventilated enlbodiments may
reduce or
eliminate the risk of an explosive mixture of the flammable substance (e.g.,
hydrogen or
natural gas) accumulating within enclosure 150 or 195, and yet may provide for
fire
suppression through consumption of oxygen. In such embodiments, it may not be
necessary
for enclosure 150 or 195 to be able to withstand as much pressure, as would be
required for
31
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
a purely passive fire suppression system that was designed to contain an
explosion within
the enclosure.
In some embodiments of the present invention, fire suppression system 155 may
be
configured to turn off the hydrogen, for example by closing valves 119, 132,
149, and/or
shutting off hydrogen generator 120 and/or compressor 130, when a hydrogen
leak or fire is
detected. In some embodiments, fire suppression system 155 may be configured
to release ~
some or all hydrogen through vent stack 140, for example, by opening valve
159, when a
hydrogen leak or fire is detected. Just hydrogen contained within piping may
be released or,
in some embodiments, hydrogen within pressure vessel 110 may also be released.
Such a
leak or fire may be detected, -for example, by sensor 156 or 196, or one or
more sensors in
vent pipe 140. Thus, a leak or fire may be stopped by eliminating the fuel
(hydrogen).
Similar schemes may be used for other flammable substances or fuels, for
example, for natural gas system 190. But release of other flammable substances
to the atmosphere'may
be more problematic with respect to safely since they are typically not
lighter than air, and
may tend to linger near the ground. Release of other flammable substances may
also have
more significant impacts to the environrnent, such as air pollution, ozone
depletion, and/or
contributing to global warming.
In some embodiments, fire suppression system 155 may be configured to
introduce a
substantially inert gas or fire suppression substance into enclosures 150,
195, vent pipe 140,
or a combination thei : r For example, fire suppression system may be
configured to
introduce helium, nitr argon, carbon dioxide, water, HALON, or a mixture
thereof,
such as exhaust gas fruternal combustion engine, into enclosures 150 or 195.
Such a
substance may be re.:: for instance, when a hydrogen leak or fire is detected,
for
example, by sensor 156 ~ ;6, or one or more sensors in vent pipe 140. The
substantially
in.ert gas (or water) m~. " isplace air, hydrogen, or both, substantially
eliminating the
opportunity for fire. The .splaced air, hydrogen, and the products of any
combustion may
be pushed out through vent pipe 140 to the atmosphere, where an.y unburned
hydrogen may
dissipate harmlessly upwar=,:,. Vent pipe 140 may be located and configured so
that any fire
at end 141 is relatively ha'. :.:ttss.
In some embodiments, there may be a lid, rapture disk, or low pressure
pressure-
relief valve, for example, on end 141 of vent pipe 140, that may normally be
closed sealing
enclosure 150, 195, or both. A substantially inert gas may be introduced
initially,
periodicaIly, or continuously into enclosures 150, 195, or both, to displace
oxygen and
32
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
create an environment within the enclosure (or enclosures) that is
incompatible with
explosions, fires, or both. In embodiments wherein the substantially inert gas
is introduced
into the enclosure continuously, a lid or a low pressure relief valve may not
be required. For
instance, in embodiments where compressor 130 is driven by an internal
combustion engine,
e.xhaust from the engine may be cooled, dehumidified, and introduced into
enclosure 150,
exiting through vent pipe 140. Thus, any hydrogen that leaks into enclosure
150, for
example, would be released to the atmosphere at the end 141 of vent pipe 140,
where an
explosive mixture is not at all likely to accumulate, and a fu=e would not
cause a significant
safety concein to either people or property. Such a system may be passive,
with the
exception of introduction of the substantially inert gas. Or such a system may
be active, for
example, introducing more substantially inert gas when the oxygen
concentration within the
enclosure (e.g., 150 or 195) reaches a particular level, or when.a leak or fue
is detected (e.g.,
as detected by sensors 156, or 196).
In embodiments of the present invention having enclosures 150 or 195, it may
be
advisable to take special precautions when the enclosures are opened, for
example, to
perform maintenance on hydrogen handling equipment or other equipment located
inside.
These precautions may include, for example, shutting off the hydrogen or other
flammable
substance, providing ventilation, disabling fire suppression system 155,
shutting off
electrical power, tagging or locking out electrical power and/or isolation
valves, checking
for the accumulation of hydrogen or another flamma.ble substance, and/or the
like.
Referring now to FIG. 2, hydrogen handling equipment and other equipment in
accordance with the present invention may be enclosed within a structure 250
with walls
255, 256, 257, and 258 to provide enhanced safety to people and property
located outside
the walls. An example of such an embodiment is illustrated in FIG. 2. The
structure may
have a floor 253, which may, for example, be a concrete floor (shown), a dirt
or gravel floor,
or metal. A concrete floor 253 may be reinforced, and may have embedments for
anchoring
hydrogen handling equipment, and/or other equipment, drains, and the h-ke.
Walls 255, 256, 257, and 258 may, for example, be concrete, and may be
reinforced,
or may be metal, for example, steel, and may be painted. In some embodiments,
walls, for
example, 255 and 256, may be earthen. Although four walls are shown, structure
250 may
have more or fewer walls. For example, structure 250 may have one circular,
spiral, or oval
wall that goes all of the way around structure 250. One or more of the walls
255 through
258 may have a door, and walls 255 through 258 and/or floor 253 may have
embedded or
33
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
buried piping, conduit, and/or penetrations therefore. Such penetrations may
be sealed
completely or partially, for example, with grout or foam In some embodiments,
the walls
may not continue a11 the way around structure 250, for example, where
protection is needed
only in particular directions.
Various hydrogen handling equipment may be located within sf.rachue 250, for
example, within the perimeter of walls 255 through 258, for instance, one or
more of =_
hydrogen generator 120, compressor 130, pressure vessel (or vessels) 110,
valves 219, and
at least part of piping 132, 134, and 144. Thus, structure 250 and walls 255
through 258
may at least partially contain a hydrogen leak and/or resulting fire or
explosion from such
hydrogen handling equipment. Various equipment for handling other flammable
substances,
for example, CNG, may also be located within structure 250. Leaks from such
other
flamnable substances may have consequences similar to hydrogen leaks, except
where
differences in density and other properties dictate different behavior. Walls
255 through 258
may be fire rated, for example, with a two-hour fire rating.
Structure 250 may have an open top 251. Thus, any hydrogen leak and/or
resulting
fire or explosion from the hydrogen handling equipment within structure 250
may be
directed upwards away from people and propeity outside of walls 255 through
258. Open
top 251 may allow leaking hydrogen to partially or fully dissipate upward to
the atmosphere,
preventing the accumulation of much if any explosive mixture of hydrogen and
air. In other
embodiments, structure 250 may have a complete or partial top that may be free
to lift up
from walis 255 through 258, for example. Such a top may protect the hydrogen
handling
equipment from the elements, but may lift up or off relieving the pressure
from any
explosion therein. Passive or forced ventilation or fans may reduce the
likelihood of the
accumulation of an explosive mixture within stracture 250. Ventilation fans
may turn on
and/or dampers may open when hydrogen is detected by sensors within structure
250, and/or
alamis may be initiated.
In some embodiments, of the present invention, some or all of the walls, for
e?.ample, 255 and 256, may tilt or lean away from the hydrogen handling
equipment (e.g.,
hydrogen generator 120 and compressor 130) and the top (e.g., 251) may have a
larger area,
for example, than the area of floor 253. This configuration may reduce the
loading on walls
255 through 258 in the event of an explosion within stracture 250. Structure
250 may have
some walls that are vertical (e.g., walls 257 and 258) and some walls that
lean away (eg,
walls 255 and 256). Wa11s 255 and/or 256 may lean away, for example, at an
angle within
34
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
the range of about 30 to 75 degrees from horizontal, for instance, at an angle
of
approximately 45 degrees or 60 degrees from horizontal. In other embodiments,
the angle
may be more or less. Although walLs 255 and 256 are shown flat, they may be
angled or
curved, for example concave upward or concave downward. The space 265 and 266
below
walls 255 and 256 may be unoccupied as shown, or may contain equipment,
supports, wall
material(e.g., concrete), .backfill, or the h7ce. Floor 253 may be at grade,
below grade, or -
above grade, depending on the application.
Structure 250 may have various fire detection and/or fire suppression systems
and
may have leak detection capabilities. Some embodiments may have fire
suppression
systems, which may include water sprinklers, HALON, or carbon dioxide, for
example. Fire
detection systems may detect hydrogen, heat, ionization, infrared radiation,
ultraviolet light,
and the like, and may trigger alarms, closure of valves, opening or closure of
da.nipers,
activation or deactivation of ventilation fans, activation of fire suppression
systeins,
releasing of hydrogen to vent stack or stacks 140, and/or the hke.
Various embodiments of the present invention may include grounding, among
other
things, to minimize the risk of a static electric discharge that may ignite
any leaking
hydrogen. For instance, each pressure vessel 110 may be grounded with a #4/0
standard,
bare copper ground cable. Each piping system may have a #14 ground wire
attached. Each
panel and enclosure 150, for example, may have a #4/0 ground cable, as may
structural steel
support structure 210, hydrogen generator 120, compressor 130, dispenser 170,
and such
hydrogen handling equipment. Electrical panels may be grounded in accordance
with the
National Electric Code (NEC).
Ventilation may be provided in the area of dispenser 170 and vehicle 180, for
example, to help dissipate any hydrogen or other fuel that leaks during
refaeling. Open
architecture may facilitate natural ventilation. Fans may provide forced
ventilation.
Evaporative coolers may be used for this purpose, and to improve comfort in
hot dry
climates.
Various hydrogen handling equipment forming system 100 may be shop assembled
and certified as opposed to being assembled and certified in the field. This
may include, for
example, non-destructive examination of welds, hydrostatic testing of pressure
vessels 110,
and/or certification, for example, in accordance with the ASME code. Hydrogen
handling
equipment may be inspected and/or functionally tested in the shop. Wiring may
be installed
and tested as may control equipment and/or fire detection, alarm, and
suppression systems.
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
Performing these functions in the shop may reduce the cost of assembly and
certification,
and may facilitate correction of any problems that arise.
Various aspects of the present invention facilitate shop assembly, testing,
and
certification, including the vertical orientation of cylinders 110, the close
proximity and/or
parallel arrangement of vent pipes 140 to pressure vessels 110, the existence
of enclosure
150, and the like. For example, various hydrogen handling equipment and vent
pipes 140 --
may be mounted off of pressure vessel or vessels 110, or fiom a common support
structure
210. In one embodiment, enclosure 150 is mounted off of and supported by
pressure vessels
110. Shop assembly and testing also assures consistent quality and facilitates
employment
of mass-production techniques reducing the cost and improving the efficiency
of fa.brication.
Parts may be interchangeable in whole or in part, an inventory of parts may be
available, an
assembly line may be used with particular workers specializing in particular
steps, and the
like.
All or part of some embodiments of system 100 may be preassembled in the shop,
and field installation may be limited to placing concrete with embedded anchor
bolts, for
example, of structure 250, and bolting system 100 (in some embodiments
excluding
structure 250) to the anchor bolts. System 100 may be designed and anchored,
for example,
to withstand an earthquake or a 150 mile/hour wind. In other embodiments, all
or part of
structure 250 may be included in the shop assembly, for example, where
structure 250 is
metal.
FIG. 3 illustrates another exemplary embodiment of the present invention
having a
particular embodiment of enclosure 150, enclosure 350. Enclosure 350 may be
cylindrical,
and may be made with a section of pipe 355, domed or dished end or head 354,
and flanges
357 and 358. These components of enclosure 350 may be steel, and section of
pipe 355 may
be welded to dished head 354 and flauge 357. Flange 357 may be bolted to
flange 358, and
there may be a gasket or o-ring between the two flanges 357 and 358. Flange
358 may be a
blind flange, and may have holes drilled through for vent pipe 140 and pipes
134 and 172
shown. Flange 358 may also have penetrations for electrical conduit, for
example, for
power and control wiring. Flange 358 may be welded to vent pipe 140, or
attached with a
screwed (e.g., NPT) or flanged joint. Pipes 134 and 172 may have a close ft
where they
pass through flange 358, or may be welded thereto, caulked, or otherwise
sealed Pipes 134,
172, or both may be enclosed within a coaxial pipe such as pipe 372
surrounding pipe 172.
36
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
Valves 219 and 319 may be located within enclosure 350 as shown. In the
exemplary embodiment illustrated, valves 219 and 319 are control valves. For
instance,
valve 219 may open to fill pressure vessel 110, and valve 319 may open to
dispense
hydrogen, for example, to vehicle 180 iIlustrated in FIG. 1. Other valves,
flow meters, and
the like, may also be located within enclosure 350. In embodiments having more
than one
pressure vessel 110, there may be a separate enclosure 350 for each pressure
vessel 110. In
embodiments having a compressor 130, there may be a separate or additional
enclosure 150
for compressor 130 (shown in FIG. 1).
Flange 358 may be supported, for example, by vent pipe 140 or by suppoit
structure
210, and the remainder of enclosure 150 may hang fiom and be supported by
flange 358.
Thus, it may be possible to remove flange 357, pipe 355 and dished head 354 as
an assembly
to access valves 219 and 319 by unbolting flange 357 from flange 358 and
lowering the
assembly.
Also as illustrated in FIG. 3, the connection to pressure vessel 110 may be at
the very
top of top end 111 of pressure vessel 110, for example, via pipe 344. In some
embodiments,
pipe 344 may be the only penetration into pressure vessel 110. As illustrated,
pipe 344 may
pass through structural steel suppoit structure 210 to reach pressure vessel
110. Th.us, in this
exemplaty embodiment, if pipe 344 were to break off and the leaking hydrogen
ignite,
pressure vessel 110 may be protected from the flame by support structure 210.
In
embodiments having more than one pressure vessel 110, the other pressure
vessels 110 may
be similarly protected. In addition, the flame may be directed upward from
this high
location, away from people and equipment.
FIG. 3 also illustrates that in some embodiments of the present invention,
pressure
relief or safety valve 118 may be vented directly to atmosphere rather than
being ducted to
vent pipe 140. In the exemplary embodiment illustrated, safety valve 118 is
positioned and
directed to release any hydrogen upward and away from pressure vessel 110,
other
equipment, or people.
The present invention has been described above for applications involving
diatomic
hydrogen. However, many aspects of the present invention may be applicable to
other
substances, for example, other flammable gasses and/or flanimable liquids. For
instance,
piping, valves, various equipment and/or the like containing flammable
substances may be
enclosed within larger piping or enclosures. I some embodiments, the larger
piping or
enclosures may be configured to withstand the pressure generated by an
explosion of the
37
CA 02619895 2008-02-19
WO 2005/019721 PCT/US2004/020983
flammable substance and air within the enclosure. In some embodiments the
enclosure may
also be configured to withstand the heat that may be generated by the
combustion of the
flammable substance within the enclosure, at least until the oxygen within the
enclosure is
depleted. The enclosure may be vented to atmosphere at a relatively safe
location. Other
aspects of the present invention described herein may also apply to other
substances,
although differences in density, as compared with hydrogen gas, may dictate
certain -
differences in function and/or structure.
Benefits, other advantages, and solutions to problems have been described
above
with regard to specific embodiments. However, the benefits, advantages,
solutions to
problems, and any element(s) that may cause any benefit, advantage, or
solution to occur or
become more pronounced are not to be construed as critical, required, or
essential features
or elements of any or all the claims. As used in this document, the terms
"comprises",
"comprising", or any other variation thereof, are intended to cover a non-
exclusive
inclusion, such that a process, method, article, or apparatus that comprises a
list of elements
does not include only those elements but may include other elements not
expressly listed or
inherent to such process, method, article, or apparatus. Further, no element
described in this
document is required for the practice of the invention imless expressly
described as
"essential" or "critical".
In addition, modifications may be made to the disclosed embodiments without
departing from the scope of the invention. The scope of the invention is
therefore not
limited to the disclosed embodiments but is defined by the appended claims.
Other
variations and modifications of the present invention will be apparent to
those of ordinary
skill in the art, and it is the intent of the appended claims that such
variations and
modifications be covered. The particular values and configurations discussed
above can be
varied, are cited to illustrate particular embodiments of the present
invention, and are not
intended to limit the scope of the invention. It is contemplated that the use
of the present
invention can involve components having different characteristics as long as
the elements of
at least one of the claims below, or the equivalents thereof, are included.
38