Note: Descriptions are shown in the official language in which they were submitted.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
1
MOISTENED DISPOSABLE WIPE FOR CONTROLLING ALLERGENS
Field
The present invention relates to moistened disposable wipes for household and
automotive use for the control of indoor allergens. The present invention also
relates to a
method for cleaning and reducing allergens from fabric-based materials around
the home
and automobile utilizing the moistened wipes of the present invention.
Background
House dust is comprised of many components. While its content can vary
considerably, a speck of dust can contain fabric fibers, human skin particles,
animal
dander, house dust mites, parts of cockroaches, mold spores, bacteria, food
particles, and
other debris. For numerous individuals, many of these dust components are
allergenic,
including the most common culprits, dust mites, pet dander, and mold spores.
One dust
particle may contain an agglomeration of these various components. These
components
are often associated with the onset of a runny or stuffy nose,
itchy/watery/red eyes, and
sneezing for allergy sufferers. Unfortunately, house dust is present even in
"clean"
homes. Some of the common reservoirs of indoor allergens include the fabrics
of
bedding, upholstery, drapery, and carpets.
Laundering is one approach to combat dust allergens. Yet, for many home
fabrics, such as upholstery and draperies, washing or dry-cleaning are not
easy or readily
available options. As a result, consumers will often report that they clean
these articles
routinely by vacuuming. Yet, in actuality, "routinely" translates into
vacuuming the
upholstery and draperies once or twice yearly, or cleaning the fabric surfaces
with a dust
cloth or old T-shirt a couple of times monthly. Unarguably, vacuuming and
dusting may
reduce the level of dust in home fabrics; however, it is believed that there
are many dust
components, including allergens, that are very difficult to remove by these
measures
(American College of Allergy, Asthma, & Immunology On-Line, Public Education:
Indoor Allergy Survival Tips, 2005). In addition, there is a growing concern
that these
practices may be contributing to the allergen problem. That is, both vacuuming
and
dusting have been implicated in rendering allergens airborne during the
cleaning process,
which can make things considerably worse for the allergy sufferer.
Consequently, these
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
2
normal housekeeping practices are neither sufficient nor conducted with the
necessary
frequency to reduce the level of indoor allergens on home fabrics.
This present invention embodies a moistened disposable wipe uniquely designed
to remove allergens from fabric surfaces. The moistened, disposable wipe
quickly, easily,
and gently removes dirt and contamination from the surfaces of fabrics without
fraying
and without leaving lint behind. The moistened disposable wipe also removes
allergens
(typically very small in size, generally ranging from about 0.1 micron to 100
microns)
from fabric surfaces so that they can be placed in the garbage and not in the
air or home,
while at the same time depositing allergen control agents on the fabric
surface contacted
by the wipe.
This and other features, aspects, advantages, and variations of the present
invention will become evident to those skilled in the art from a reading of
the present
disclosure with the appended claims and are covered within the scope of the
claims.
Summary
The present invention relates to a moistened disposable wipe for cleaning
household fabric-based materials. The wipe comprises a substrate and a
composition.
The composition comprises:
i) from about 0.01 % to about 25% by weight of an allergen control agent;
ii) from about 0.05% to about 15% by weight of an organic solvent; and
iii) balance water and optional components.
The present invention also relates to a method for cleaning household
surfaces.
The method comprises the steps of.
a) providing a disposable wipe wherein the disposable wipe comprises:
a substrate which is comprised of at least one ply which includes a
composition
applied to the substrate wherein the composition is applied to the substrate
in the amount
of from about 0.5 grams of the composition/gram of the substrate by weight to
about 8
grams of the composition/gram of the substrate by weight. The composition
comprises:
i) from about 0.01% to about 25% by weight of an allergen control agent;
ii) from about 0.05% to about 15% by weight of an organic solvent; and
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
3
iii) balance water and other optional components;
b) contacting the surface to be cleaned with the disposable wipe;
c) applying the composition to the surface; and
d) transferring dirt and contaminants from the surface to the disposable wipe.
The present invention additionally relates to a kit for cleaning household
surfaces.
The kit comprises:
a) a moistened disposable wipe comprising a substrate which is comprised of at
least- one ply which includes a composition applied to the substrate wherein
the
composition is applied to the substrate in the amount of from about 0.5 grams
of the
composition/gram of the substrate by weight to about 8 grams of the
composition/gram of
the substrate by weight. The composition comprises:
i) from about 0.01% to about 25% by weight of an allergen control agent;
ii) from about 0Ø01% to about 15% by weight of an organic solvent;
iii) balance water; and
b) an implement to which the moistened disposable wipe is attached for
facilitating contact of the moistened disposable wipe to the household
surface.
Brief Description of the Drawings
FIG. 1 is a perspective view of one embodiment of a moistened wipe substrate
made in accordance with the present invention.
FIG. 2 is a cross-sectional view of a portion of the wipe substrate of FIG. 1.
FIG. 3 is a magnified detail view of one bond site of a laminate substrate
made in
accordance with the present invention.
FIG. 4 is a top plan view of another embodiment of a substrate made in
accordance with the present invention.
FIG. 5 is a cross-sectional view of a portion of the substrate shown in FIG.
4.
Detailed Description
Reference will now be made in detail to various embodiments of the present
invention, examples of which are illustrated in the accompanying drawings
wherein like
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
4
numerals indicate the same elements throughout the views. All percentages,
ratios and
proportions herein are on a weight basis unless otherwise indicated.
Except as otherwise noted, all amounts including quantities, percentages,
portions, and proportions, are understood to be modified by the word "about",
and
amounts are not intended to indicate significant digits.
Except as otherwise noted, the articles "a", "an", and "the" mean "one or
more".
As used herein, the terms "cleaning sheet", "moistened disposable wipe", and
"moistened wipe", "disposable wipe" and "wipe" may be used interchangeably
herein.
As used herein, "comprising" means that other steps and other ingredients
which
do not affect the end result can be added. This term encompasses the terms
"consisting
of and "consisting essentially of'. The compositions and methods/processes of
the
present invention can comprise, consist of, and consist essentially of the
essential
elements and limitations of the invention described herein, as well as any of
the
additional or optional ingredients, components, steps, or limitations
described herein.
As used herein the term "fabric" encompasses articles of fabric including but
not
limited to: clothing, upholstery, linen, draperies, clothing accessories,
leather, floor
coverings, and the like. The term also encompasses other items made in whole
or in part
of fabric, including but not limited to tote bags, furniture covers, leather
upholstery and
other leather products, automobile interiors, tarpaulins, shoes, window
screens, stuffed
animals, pillows, mattresses, and the like.
As used herein, the term "moistened" refers to the addition of a liquid to the
substrate either prior to or at the time of use. The term "liquid" includes
any material
having a liquid phase, including but not limited to emulsions having a liquid
phase. The
substrate may be moistened with liquid during manufacture or it may be
moistened with
liquid after manufacture (e.g.; by the user at point of use).
As used herein, the term "disposable" is used to describe articles which are
not
intended to be laundered or otherwise restored but rather are intended to be
discarded
after use.
As used herein, the term "nonwoven" refers to a substrate that has a structure
of
individual fibers or threads which are interlaid, but not in any regular or
repeating
manner. Nonwoven substrates may be formed by a variety of processes including
but not
limited to meltblowing processes, spunbonding processes, and bonded carded
processes.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
As used herein, the term "microfibers", refers to small diameter fibers having
an average
diameter not greater than about 100 microns.
As used herein, the term "meltblown fibers", refers to fibers formed by
extruding a
molten thermoplastic material through a plurality of fine, usually circular,
die capillaries
as molten threads or filaments into a high velocity gas (e.g., air) stream
which attenuates
the filaments of molten thermoplastic material to reduce their diameter, which
may be to
a microfiber diameter. Thereafter, the meltblown fibers are carried by the
high velocity
gas stream and are deposited on a collecting surface to form a web of randomly
dispersed
meltblown fibers.
As used herein, the term "spunbonded fibers", refers to small diameter fibers
which are formed by extruding a molten thermoplastic material as filaments
from a
plurality of fine, usually circular, capillaries of a spinneret with the
diameter of the
extruded filaments then being rapidly reduced by drawing.
As used herein, "laminate" and "composite" are used interchangeably to
describe
substrates which may be used with the present invention. Both refer to a
substrate formed
from at least two webs joined in a face to face relationship to form a unitary
web
comprised of more than one ply or layer.
As used herein, the term "polymer" generally includes, but is not limited to,
homopolymers, copolymers, such as, for example, block, graft, random and
alternating
copolymers, terpolymers, etc., and blends and modifications thereof.
Furthermore, unless
otherwise specifically limited, the term "polymer" shall include all possible
geometrical
configurations of the material. These configurations include, but are not
limited to,
isotactic, syndiaotactic and random symmetries.
It should be understood that every maximum numerical limitation given
throughout this specification includes every lower numerical limitation, as if
such lower
numerical limitations were expressly written herein. Every minimum numerical
limitation given throughout this specification will include every higher
numerical
limitation, as if such higher numerical limitations were expressly written
herein. Every
numerical range given throughout this specification will include every
narrower
numerical range that falls within such broader numerical range, as if such
narrower
numerical ranges were all expressly written herein.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
6
Moistened Disposable Wipe
The moistened disposable wipe of the present invention comprises a substrate
and
a composition which is applied thereto wherein the composition is applied to
the
substrate in the amount of from about 0.5 grams of the composition/gram of the
substrate
by weight to about 8 grams of the composition/gram of the substrate by weight.
A. Substrate
The substrate of the present invention may be any suitable wipe substrate
including but not limited to baby wipes, cleaning wipes, towelettes, and the
like. The
substrate of the present invention may be made in accordance with (but not
limited to):
PCT Publication No. WO 2004/080265 published in the name of Hofte et al. on
September 23, 2004; U.S. Publication No. 2006/0052269 published in the name of
Panandiker et al. on March 9, 2006; U.S. Patent No. 6,716,805 issued to Sherry
et al. on
April 6, 2004, U.S. Patent No. 6,561,354 issued to Fereshtehkhou et al. on May
13, 2003;
U.S. Publication No. 2003/0028165 published in the name of Curro et al. on
February 6,
2003 and U.S. Publication No. 2002/0034912 published in the name of Curro et
al. on
March 21, 2002. The substrate of the present invention may be comprised of one
or more
plies. The substrate may comprise woven and/or nonwoven, unmodified and/or
modifed
natural fibers (one non-limiting example of which is a cellulosic-based fiber
such as
wood pulp fiber), synthetic fibers, or mixtures thereof.
Other natural fibers which may be used include but are not limited to cotton,
Esparto grass, bagasse, hemp, flax, silk, wool, wood pulp, chemically modified
wood
pulp, jute, ethyl cellulose, and/or cellulose acetate. Suitable synthetic
fibers include but
are not limited to polyvinyl chloride, polyvinyl fluoride,
polytetrafluoroethylene,
polyvinylidene chloride, polyacrylics such as ORLON , polyvinyl acetate,
rayon,
polyethylvinyl acetate, non-soluble or soluble polyvinyl alcohol, polyolefins
such as
polyethylene (e.g., PULPEX) and polypropylene, polyamides such as nylon,
polyesters
such as DACRON or KODEL , polyurethanes, polystyrenes, and the like,
including
fibers comprising polymers containing more than one monomer.
The fibers useful herein can be hydrophilic, hydrophobic, or can be a
combination
of both hydrophilic and hydrophobic fibers. As indicated above, the particular
selection
of hydrophilic or hydrophobic fibers depends upon the other materials included
in the
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
7
absorbent (and to some degree) the scrubbing layer described hereinafter. '
Suitable
hydrophilic fibers for use in the present invention include cellulosic fibers,
modified
cellulosic fibers, rayon, cotton, polyester fibers such as hydrophilic nylon
(HYDROFIL ). Suitable hydrophilic fibers can also be obtained by
hydrophilizing
hydrophobic fibers, such as surfactant-treated or silica-treated thermoplastic
fibers
derived from, for example, polyolefins such as polyethylene, polypropylene,
polyacrylics,
polyamides, polystyrenes, polyurethanes and the like.
Suitable wood pulp fibers include those obtained from well-known chemical
pulping processes such as the kraft and sulfite processes. It may be desirable
to derive
these wood pulp fibers from southern softwoods due to their premium absorbency
characteristics. These wood pulp fibers can also be obtained from mechanical
pulping
processes, such as stone groundwood, refiner mechanical, thermomechanical,
chemimechanical, and chemi-thermomechanical pulping processes. Recycled or
secondary wood pulp fibers, as well as bleached and unbleached wood pulp
fibers, can
also be used.
Another type of hydrophilic fiber which may be used in the present invention
is
chemically stiffened cellulosic fiber. As used herein, the term "chemically
stiffened
cellulosic fiber" means cellulosic fibers that have been stiffened by chemical
means to
increase the stiffness of the fibers under both dry and aqueous conditions.
Such means
can include the addition of a chemical stiffening agent that, for example,
coats and/or
impregnates the fibers. Such means can also include the stiffening of the
fibers by
altering the chemical structure, e.g., by crosslinking the polymer chains.
If fibers are used as the absorbent layer (or a constituent component
thereof), the
fibers can optionally be combined with a thermoplastic material. Upon melting,
at least a
portion of this thermoplastic material migrates to the intersections of the
fibers, typically
due to interfiber capillary gradients. These intersections become bond sites
for the
thermoplastic material. When cooled, the thermoplastic materials at these
intersections
solidify to form the bond sites that hold the matrix or substrate of fibers
together in each
of the respective layers. This can be beneficial in providing additional
overall integrity to
the cleaning wipe.
Various methods can be used to form a suitable substrate for use in the
present
invention. Suitable methods include but are not limited to spunbonding,
meltblowing,
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
S
carding, wet laying, and airlaying. Suitable techniques for binding the fibers
of the
substrate together include but are not limited to hydroentangling, needle
punching,
thermal bonding, ultrasonic bonding, chemical bonding, surface treating, and
laminating.
When utilizing a substrate comprised of more than one ply, wherein one of the
plies is an absorbent layer, the fibers can optionally be combined with a
thermoplastic
material. Upon melting, at least a portion of this thermoplastic material
migrates to the
intersections of the fibers, typically due to interfiber capillary gradients.
These
intersections become bond sites for the thermoplastic material. When cooled,
the
thermoplastic materials at these intersections solidify to form the bond sites
that hold the
matrix or substrate of fibers together in each of the respective plies. This
may be
beneficial in providing additional overall integrity to the wipe.
Thermoplastic materials useful in the present invention can be in any of a
variety
of forms including particulates, fibers, or combinations thereof. Suitable
thermoplastic
materials can be made from any thermoplastic polymer that can be melted at
temperatures that will not extensively damage the fibers that comprise the
primary
substrate or matrix of each ply. Typically, the melting point of this
thermoplastic
material will be less than about 190 C, and generally between about 50 C and
about
175 C.
Suitable thermoplastic fibers can be made from a single polymer
(monocomponent fibers), or can be made from more than one polymer (e.g.,
bicomponent
fibers). Sheath/core bicomponent fibers refers to thermoplastic fibers that
comprise a
core fiber made from one polymer that is encased within a thermoplastic sheath
made
from a different polymer. The polymer comprising the sheath often melts at a
different,
typically lower, temperature than the polymer comprising the core. As a
result, these
bicomponent fibers provide thermal bonding due to melting of the sheath
polymer, while
retaining the desirable strength characteristics of the core polymer.
Suitable bicomponent fibers for use in the present invention can include but
are not
limited to sheath/core fibers having the following polymer combinations:
polyethylene/
polypropylene, polyethylvinyl acetate/polypropylene, polyethylene/polyester,
polypropylene/polyester, copolyester/polyester, and the like. Particularly
suitable
bicomponent thermoplastic fibers for use herein are those having a
polypropylene or
polyester core, and a lower melting copolyester, polyethylvinyl acetate or
polyethylene
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
9
sheath (e.g., those available from Danaklon a/s, Chisso Corp., and CELBOND ,
available from Hercules). These sheath/core bicomponent fibers can be
concentric or
eccentric. As used herein, the terms "concentric" and "eccentric" refer to
whether the
sheath has a thickness that is even, or uneven, through the cross-sectional
area of the
bicomponent fiber: Eccentric bicomponent fibers may be desirable in providing
more
compressive strength at lower fiber thicknesses.
Suitable methods for preparing thermally bonded fibrous materials are
described in
U.S. Patent No. 5,607,414 issued to Richards et al. on March 4, 1997 and U.S.
Patent No.
5,549,589 issued to Horney et al. on August 27, 1996.
Another method of bonding the fibers is chemical bonding. Common chemical
bonding agents include but are not limited to solvent based and resin based
adhesives
(e.g.; latex, etc.).
The wipe may also be comprised of a HIPE-derived hydrophilic, polymeric foam.
Such foams and methods for their preparation are described in U.S. Patent No.
5,550,167
issued to DesMarais on August 27, 1996.
The substrate of the present invention typically has a basis weight of about
40
g/m2 to about 250 g/m2 or from about 50 g/m2 to about 120 g/m2 as measured in
accordance with ASTM D3776-96 and a caliper of from about 0.3 mm to about 2
mm.
The substrate of the present invention typically has a liquid holding capacity
of about 1
gram of liquid/gram of substrate to about 10 grams of liquid/gram of
substrate, or about 2
grams of liquid/gram of substrate to about 8 grams of liquid/gram of
substrate, or about 3
grams of liquid to about 5 grams of liquid/gram of substrate. The substrate
also typically
has a fuzz level of less than about 0.8 mg/cm2, or less than about 0.5 mg/cm2,
or less than
about 0.3 mg/cm2. The substrate typically has a dry cross direction ("CD")
stiffness
value of from about 0.01 g-cm to about 2 g-cm and a wet cross direction
stiffness value
of from about 0.005 g-cm to about 2 g-cm, or from about 0.1 g-cm to about 1.5
g-cm.
In one non-limiting embodiment, the substrate can be an airlaid nonwoven
fibrous
substrate comprising a combination of natural fibers, staple length synthetic
fibers and a
latex adhesive binder. The dry fibrous substrate can be about 20% to 80% by
weight
wood pulp fibers, about 10% to 60% by weight staple length polyester fibers,
and about
10% to 25% by weight binder.
CA 02620678 2010-06-18
In another non-limiting embodiment, the dry fibrous substrate can comprise at
least
about 50=%o by weight wood pulp fibers, and more preferably at least about 70%
by weight
wood pulp fibers. One particular airlaid nonwoven fibrous substrate which is
suitable for
use in the present invention comprises about 75% by weight Southern softwood
Kraft
wood pulp fibers having an average fiber length of about 2.6 mm; about 12% by
weight
polyester fibers having a denier of about 1.35 grams per 9000 meters of fiber
length and a
staple length of about 0.85 inch; and about 13% by weight of a binder
composition
comprising a styrene butadiene copolymer. The styrene butadiene copolymer may
have a
styrene to butadiene ratio of about 45 parts styrene to 55 parts butadiene. A
latex
TM
adhesive suitable for making the binder composition is ROVENE 5550 (containing
about
50 weight percent solids of styrene butadiene copolymer) available from
Mallard Creek
Polymers of Charlotte, North Carolina.
In a further non-limiting embodiment the substrate of the present invention is
formed by air laying a blend of natural and synthetic fibers to form a fibrous
web,
spraying water on the web, and then embossing the web. A latex adhesive binder
is then
applied to the web, followed by drying and curing of the latex adhesive binder
in an oven.
The nonwoven web may then be moistened with a liquid.
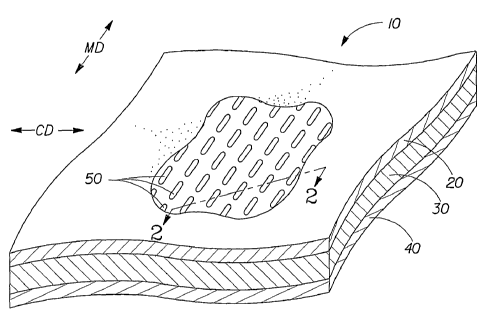
In yet another non-limiting embodiment the substrate is a laminate substrate
formed
from a laminate web 10 comprising at least three layers or plies, disposed in
a layered,
face-to-face relationship, as shown in FIG. 1. The layers should be
sufficiently thin to be
processible such as described in U.S. Publication No. 2003/0028165, but no
actual
thickness (i.e., caliper) is considered limiting. A first outer layer 20, is
typically
thermally bondable, and may be a nonwoven web comprising a sufficient quantity
of
thermoplastic material, the web having a predetermined extensibility and
elongation to
break. By "sufficient quantity" is meant a quantity of thermoplastic material
adequate to
enable enough thermal bonding upon application of heat and/or pressure to
produce a
unitary web. A second outer layer, 40, is typically the same material as first
outer layer
20, but may be a different material. Second outer layer, 40 is also generally
thermally
bondable and has a predetermined extensibility and elongation to break. At
least one
third central layer 30 may be disposed between the two outer layers.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
11
The laminate web 10 is processed by joining means, such as by ultrasonic
welding, or
thermal calendaring as described in U.S. Publication No. 2003/0025165 to
provide a
plurality of melt bond sites 50 that serve to couple the outer layers 20 and
40, and, in
some embodiments, portions of central layer 30, thereby forming the
constituent layers
into a unitary web. When joined together, the two outer layers form an
interior region
between them. The interior region is the space between the outer layers
surrounding the
bond sites 50. In one non-limiting embodiment, the third central layer 30
substantially
fills the interior region, the third central layer 30 being apertured
coincident the bond sites
50.
While the laminate web 10 is disclosed primarily in the context of nonwoven
webs
and composites, in principle the laminate web 10 can be made out of any web
materials
that meet the requirements, (e.g., melt properties, extensibility) as
disclosed herein. For
example, the outer layers 20 and 40 can be thermoplastic films, micro-porous
films,
apertured films, and the like. Central layer 30 can be paper, including tissue
paper; metal,
including metal foil; other non-thermoplastic web material, woven fabric, and
the like. In
general, it is required that outer layer materials be flexible enough to be
processed as
described herein. However, central layer can be a brittle, relatively stiff
material, as long
at it also can be processed as described herein, albeit possibly becoming
fractured,
broken, or otherwise broken up in the process.
The laminate substrate may be apertured, non-apertured, or a combination
thereof.
The laminate substrate may have an average aperture size of from about 0.01
mm2 to
about 4 mm2 or from about 0.5 mm2 to about 2.5 mm2. The percentage of the
laminate
which is comprised of apertures may be express as the percent open area. The
percent
open area of the laminate of the present invention may be from about 2 % to
about 25%
or from about 5% to about 20%. In one non-limiting embodiment as shown in
cross-
section in FIG. 2, central layer 30 can be apertured, without aperturing the
two outer
layers to provide a three-layer laminate characterized by the laminate web 10
(as a whole)
being unapertured, while the central layer 30 is apertured. The laminate
substrate web
can be made without requiring registration of the layers to ensure bonding of
the outer
layers through the apertures of the central layer(s). One way of describing
one
embodiment of a web 10 as described above, is that the unitary web 10, when
viewed
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
12
orthogonally by the un-aided human eye from a distance of approximately 50 cm,
exhibits
no apertures or perforations through the entire laminate, but bond sites 50
are nevertheless
visible.
The laminate web 10 is further characterized in that the joining of the three
plies
into a unitary web can be achieved in the absence of adhesive. Thus in some
embodiments no adhesive is required to bond the plies together. Joining is
achieved by
the input of energy into the constituent layers, such as by thermal melt
bonding of the two
outer layers together at the melt bond sites 50. In other embodiments, the
energy input
can be via ultrasonic bonding. Accordingly, a laminate web, that is a unitary
web, can be
formed without the use of adhesives. Not only does this simplify processing
and lower
the cost of the laminate web, when certain materials such as nonwoven webs are
used, it
results in a more flexible, softer web.
As shown in FIG. 2, central layer 30 is chosen such that when the constituent
web
layers of laminate web 10 are processed as aforementioned, portions of central
layer 30 in
the region of the melt bond sites 50 separate to permit the first outer layer
20 to melt bond
directly to the second outer layer 40 at the interface of the two materials 52
at melt bond
sites 50. Thus, apertures in the central layer 30 are formed in the lamination
step by
displacement, just prior to the bonding of the outer layers as detailed by the
method of the
present invention below. In this manner, central layer 30 can be provided as
an
unapertured web, avoiding complex registration steps to align apertures in
registry with
bond sites when laminated. Further, central layer 30 need not be thermally
compatible
with outer layers 20 and 40. Central layer need not be a thermoplastic
material, and need
not even have a melting point. It simply needs to be displaceable by the
forces exerted by
the processing equipment. Therefore, one way of describing the laminate web is
to
distinguish the central layer as being a material differentiated from the
materials of the
first or second layers by at least one material property selected from thermal
properties,
elongation properties, elastic properties, or conductive properties. By
"thermal
properties" it is meant primarily thermal melt properties, such that the
central layer has no
melting point, or if it has a melting point, it is typically at least about 10
degrees
Centigrade higher, or about 20 degrees Centigrade higher than either outer
layer, or about
100 degrees Centigrade higher than either outer layer. By "elongation
properties" it is
meant that in tension, the material of the central layer exhibits an
elongation to break that
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
13
is at least about 10% less than either outer layer, or about 50% less than
either outer layer,
or can be more than about 100% less than either outer layer. Thus, the central
layer can
be extensible, while either outer layer can be highly extensible. By "elastic
properties" it
is meant that the central layer can be, for example, elastic, while either
outer layer can be
highly elastic, as defined herein. Or the central layer can be non-elastic,
and the outer
layers elastic or highly elastic. By "conductive properties" as used herein it
is meant
electrically conductivity, such that the central layer can have an electrical
conductivity
that is about 10 times as great as the outer layers or about 100 or more times
as great as
the outer layers. Conductive properties may be facilitated by the central
layer being a
metallic foil, or by being a conductive polymer, including a conductive
nonwoven web.
Without being bound by theory, it is believed that to accomplish the
displacement of
central layer 30 to form apertures therein and to bond the outer layers, the
thermal point
calendaring as described in U.S. Publication No. 2003/0028165 should form
thermal bond
sites having a narrow width W dimension and a high aspect ratio. For example,
FIG. 3
shows the melt area of a single melt bond site 50 having a narrow width
dimension W
and a high aspect ratio, i. e., the length, L, is much greater than the width,
W. The length
L should be selected to permit adequate bond area while width W is
sufficiently narrow
such that the protuberance used to form the bond site (as described below) can
cut, shear,
displace, or otherwise pierce the central layer 30 at the region of the bond
sites by the
method described below. Width W can be between about 0.003 inches (.008 cm)
and
about 0.020 inches (.050 cm) or between about 0.005 inches (.012 cm) and about
0.010
inches (.025 cm), and may be adjusted depending on the properties of central
layer 30.
It is believed that the aspect ratio of melt bond site 50 can be as low as
about 3 (i.e.,
ratio of L/W equals 3/1). It can also be between about 4 and 20. It is
believed that the
aspect ratio of the melt bond sites 50 is limited only by the corresponding
aspect ratio of
the point bonding protuberances of the calendaring roller(s), as disclosed in
U.S.
Publication No. 2003/0028165.
In one embodiment, the longitudinal axis of each bond site, 1, which
corresponds
directionally to the length dimension of bond site 50, is disposed in a
regular, repeating
pattern oriented generally parallel to the machine direction, MD as shown in
FIG. 1. But
the longitudinal axis of each bond site may be disposed in a regular,
repeating pattern
oriented in the cross machine direction, or randomly oriented in a mixture of
cross and
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
14
machine directions. In one non-limiting example, the bond sites 50 can be
disposed in a
"herringbone" pattern.
FIG. 4 shows a partially cut-away representation of an apertured laminate. As
shown, the partial cut-away permits each layer or ply to be viewed in a plan
view. The
laminate web 10 shown in FIG. 4 is produced after the thermally bonded
laminate is
stretched in a direction orthogonal to the longitudinal axis of the melt bond
sites, in this
case, in the cross-machine direction, CD with sufficient elongation in the
direction of
extension to cause apertures to form. As shown, where formerly there were melt
bond
sites 50, apertures 60 are produced as the relatively weak bond sites fail in
tension. Also
as shown, central layer 30 can remain generally uniformly distributed within
laminate 10,
depending on the material properties of central layer 30. For example, if
central layer 30
is more extensible than outer layers 20 or 40, then it simply extends, either
elastically or
by plastic deformation, but remains generally uniformly distributed in the
unapertured
regions of web 10. For example, if a thermoplastic film is utilized as the
central layer 30,
it extends, either extensibly or elastically (depending on the type of film),
but can remain
generally uniform, for example, in density or basis weight.
One beneficial property of such a laminate web is that once apertured, fluid
communication with the central layer is facilitated. Thus, an absorbent
central layer 30
can be used between two relatively non-absorbent outer layers, and the
laminate 10 could
be an absorptive wiper with a relatively dry to the touch outer surface.
To the extent that central layer 30 is involved, or participates, in any
bonding
between outer layers 20 and 40, it also participates in the remnant of bonded
portions 62,
as shown in FIG. 4. The involvement may be due to some degree of actual melt
bonding
about the perimeter of bond site 50 (e.g., for thermoplastic central layers
30), or it may be
due to mechanical interaction, such as by entanglement (e.g., for cellulosic
fibrous central
layer 30 between fibrous nonwoven layers).
FIG. 5 is a schematic representation of the cross-section denoted in FIG. 4.
As
shown, apertures 60 form when the laminate web is elongated in the direction
T.
An example of one embodiment of a unitary web having a central layer having an
elongation to break less than either of the two outer layers, and less than
the actual
magnitude of extension, is shown partially cut-away in FIG. 5. The partial cut-
away
permits each layer or ply to be viewed in a plan view. As shown, after
extension, central
CA 02620678 2010-06-18
layer 30 becomes fragmented, forming discontinuous regions of the central
layer material.
These discontinuous regions may be relatively uniformly distributed, such as
in rows as
shown in FIG. 5, or may be relatively randomly distributed, depending on the
pattern of
melt bond sites 50, the physical properties of central layer 30, and the
method of
extension employed.
One example of a web 10 having a structure similar to that shown in FIG. 5 is
a web
having outer layers of relatively extensible nonwovens, with a central layer
of relatively
low extensibility tissue paper. Such a laminate would be an apertured laminate
web
having an absorbent central core, wherein the absorbent core material is in
fluid
communication with regions exterior to the laminate web. If a relatively
hydrophobic
nonwoven web is used for the outer layers, such a wipe could exhibit dry-to-
the-touch
properties along with high absorbency.
One example of a web 10 having a structure similar to that shown in FIG. 5 is
a
web having outer layers of relatively extensible nonwovens, with a central
layer of
relatively low extensibility tissue paper. One particularly interesting
structure
incorporates a highly hydrophobic outer layer combined with a highly absorbent
central
layer. A suitable hydrophobic material is described in U.S. Patent 3,354,022
Dettre et al.
Such a material has a water repellent surface having an intrinsic advancing
water contact
angle of more than 90 degrees and an intrinsic receding water contact angle of
at least 75
degrees. Such a material exhibits highly hydrophobic properties, similar to
the effect
known to exist on leaves from the Lotus plant. When such a material is
combined with
an absorbent central layer, one non-limiting example of which is a BOUNTY'
paper
towel tissue layer, the resulting composite can be highly absorbent while
retaining a very
clean and dry outer surface. The basis weight and porosity of the outer layer
can be
varied to achieve different degrees of absorbent performance.
Other webs and methods of making webs suitable for use in the present
invention
include but are not limited to those described in the following patents:
U.S. Patent No. 3,862,472 issued Jan. 28,
1975 to Norton et al.; U.S. Patent No. 3,905,863, issued September 16, 1975 to
Ayers;
U. S. Patent No. 3,974,025 issued August 10, 1976 to Ayers; U.S. Patent No.
3,918,126
issued Nov. 11, 1975 to Wood; U.S. Patent No. 3,982,302 issued Sept. 28, 1976
to
Vaalburg; U.S. Patent No. 4,004,323 issued Jan. 25, 1977 to Gotchel et al.;
U.S. Patent
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
16
No. 4,014,635 issued March 29, 1977 to Kroyer; U.S. Patent No. 4,057,669
issued
November 8, 1977 to McConnell; U.S. Patent No. 4,064,600 issued Dec. 27, 1977
to
Gotchel et al.; U.S. Patent No. 4,074,393 issued Feb. 21, 1978 to Hicklin et
al.; U.S.
Patent No. 4,097,965 issued July 4, 1978 to Gotchel et al.; U.S. Patent No.
4,130,915
issued Dec. 26, 1978 to Gotchel et al.; U.S. Patent No. 4,144,619 issued March
20, 1979
to White et al.; U.S. Patent No. 4,176,426 issued Dec. 4, 1979; U.S. Patent
No. 4,176,427
issued December 4, 1979 to Neuenschwander; U.S. Patent No. 4,1919,609 issued
March
4, 1980 to Trokhan; U.S. Patent No. 4,207,367 issued June 10, 1980 to Baker,
Jr.; U.S.
Patent No. 4,296,161 issued October 20, 1981 to Kaiser et al., U.S. Patent No.
4,309,469
issued January 5, 1982 to Varona; PCT Publication No. WO 00/08998 published in
the
name of Hanser et al. on February 24, 2000.
B. Composition
The substrate of the present invention includes a composition comprised of
from
about 0.01% to about 25% by weight based on the composition, or from about
0.05% to
about 15% by weight based on the composition, or from about 0.1% to about 5%
by
weight based on the composition of an allergen control agent. Suitable
allergen control
agents include but are not limited to substituted benzoic acids including but
not limited to
3,4,5-trihydroxybenzoic acid (also known as gallic acid), and preferably 3,4,5-
trimethoxybenzoic acid. Other non-limiting examples of suitable allergen
control agents
include benzoic acid esters, such as benzyl benzoate.
Other components of the composition which may be added if desired include
flocculating polymer, organic solvent, surfactant, soil suspending polymer,
perfume, and
combinations thereof. When used, these other components may be included in the
composition in the following amounts: from about 0.001% to about 0.5% by
weight of
flocculating polymer, from about 0. 0.05% to about 15% by weight of organic
solvent,
from about 0.001% to about 10% or from about 0.01% to about 2% by weight of
surfactant, from about 0.001% to about 0.5% by weight of soil suspending
polymer, and
from about 0.001% to about 1% by weight of perfume. Up to about 2% by weight
of
other optional ingredients may also be included as part of the composition.
Non-limiting
examples of these other optional ingredients include detersive builders,
enzymes, enzyme
stabilizers (non-limiting examples of which include propylene glycol, boric
acid and/or
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
17
borax), foam control agents, soil suspending agents, soil release agents, pH
adjusting
agents, chelating agents, phase stabilizers, solubilizers, brighteners,
preservatives,
antimicrobial agents, coloring agents, and mixtures thereof.
If desired, the composition may also optionally include microencapsulated
actives. One or more active may be contained within a single micro
encapsulate.
Different active-containing microencapsulates may also be used. The
composition may
be comprised of from about 0.01% to about 10% by weight of the
microencapsulated
active (i.e.; based on the microcapsule and the active contained therein), or
from about
0.025% to about 5% by weight of the microencapsulated active, or from about
0.05% to
about 1% by weight of the microencapsulated active. Non-limiting examples of
micro encapsulated actives include perfume, surfactant, silicone,
antimicrobial agents,
allergy control agents, emolients, softening agents, conditioning agents,
preservatives,
and the like. Micro encapsulated actives suitable for use in the present
invention include
but are not limited to those disclosed in U.S. Application Serial No.
60/685,815 filed on
May 31, 2005.
The ratio of the mass of the composition to the mass of the substrate is
typically in
the range of from about 10:1 to about 1:1 or from about 6:1 to about 3:1.
Without being bound by theory, it is believed that when used the flocculating
polymer is irreversibly adsorbed on the cellulosic component of the wipe so as
to
flocculate the dirt away from the surface being cleaned thereby holding the
dirt and
contaminants in the interior of the substrate. As the cellulosic layer forms
the central ply
of the web it is not in direct contact with the fabric being cleaned. This
prevents the dirt
from being smeared on the fabric. Further, the nonvolatile solvent solubilizes
the surface
dirt and makes it easier to be removed. Being nonvolatile, the solvent allows
sufficient
working time before the fabric dries out. This allows the user sufficient time
to clean the
fabric before the composition dries thereby alleviating excessive
reapplication of the
composition. This allows for more efficient cleaning with a single wipe.
While not wishing to bound by theory it is believed that it is undesirable to
overload the substrate with flocculating polymer as the polymer may have a
tendency to
be released onto the surface being cleaned thereby allowing for the dirt and
contaminants
to be flocculated/remain on the fabric being cleaned.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
18
Allergen Control Agent
The composition of the present invention includes an allergen control agent.
The
allergen control agent comprises from about 0.01 % to about 25% by weight of
the
composition, or from about 0.05% to about 15% by weight of the composition, or
from
about 0.1% to about 5% by weight of the composition. While not wishing to be
limited
by theory, it is believed that in addition to the surfactant component of the
composition,
the allergen control agent exhibits enzymatic activity on allergenic proteins,
rendering
them less immunogenic.
0
0 0,H
H
Benzoic Acid 3,4,5-trimethoxybenzoic acid
Allergen control agents may include benzyl derivatives or chemicals that
contain
benzyl moieties, non-limiting examples of which are shown in Table A below.
TABLE A
NON-LIMITING EXAMPLES OF BENZYL DERIVATIVES
SUBCATEGORY EXAMPLES
Benzyl Alcohols Benzyl Alcohol
o-Benzyl-p-Chlorophenol
Benzaldehydes Benzaldehyde
p-Methoxybenzaldehyde
m-Methoxy-p-Hydroxybenzaldehyde
Benzyl Esters Benzyl Acetate
Benzyl Benzoate
Benzyl Salicylate
Benzyl Cinnamate
Benzyl Glutamate
Benzyl Chlorocarbonate
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
19
Benzyl Propionate
Benzyl Butyrate
Benzyl Acids Benzoic Acid
Para-Aminobenzoic Acid
Benzoate Esters Methyl Benzoate
Methyl-p-Methylbenzoate
2-Hydroxybenzoate Esters Methyl 2-Hydroxybenzoate
Pentyl 2-Hydroxybenzoate
Benzyl 2-Hydroxybenzoate
Benzyl Halides Benzyl Chloride
Benzyl Bromide
Benzyl Iodide
N-Alkyl Dimethyl Benzyl Ammonium Chloride
Benzyl Ammonium Chloride
Benzyl Amines N-Benzyl-p-Phenylenediamine
Benzylamine
Di-Benzylamine
Benzyl Ethers Benzyl Ether
Other Benzyl Derivatives Benzyl Isoeugenyl
Benzyl Phthalate
Alkyl Benzyl Ketone
One suitable allergen control agent is benzoic acid. A preferred benzoic acid
useful for allergen control is 3,4,5-trimethoxybenzoic acid. This agent is
also known by
the following names: trimethylgallic acid, eudesmic acid, tri-o-methylgallic
acid, gallic
acid trimethyl ether, and 5-methoxy-veratric acid.
The benzyl moiety can be delivered in a number of ways non-limiting examples
of
which include: as a chemical agent, as a natural extract rich in benzyl
derivatives
(examples of which include but are not limited to lavender oil, geranium oil,
horseradish
extracts, eucalyptus extracts, cotton blossom extract, or juniper essences),
as a botanical
extract obtained through benzyl-mediated extractions or processes (examples of
which
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
include but are not limited to benzyl alcohol extraction or acid fractionation
with benzoic
acid), or combinations thereof.
Some allergen control agents may be crystalline in nature. When using an
allergen control agent which is crystalline, a miscibility agent such as an
alcohol may be
used to render the allergen control agent miscible with the composition. A
suitable
alcohol for this purpose includes but is not limited to alcohol. A typical
ratio of allergen
control agent to miscibility agent is from about 1:1, or from about 1:1.5, or
from about
1:2.5.
Organic acids and/or their esters may also be optionally included with the
allergen
control agent of the present invention. Suitable organic acids and/or their
esters include
but are not limited to: carboxylic acids and their esters, phenolic acids and
their esters,
polyphenolic acids and their esters, and mixtures thereof. While not wishing
to be limited
by theory, it is believed that the addition of an organic acid may help
enhance the
enzymatic activity of the allergen control agents, thereby enhancing the
ability of the
allergen control agent to denature the proteins which typically comprise the
allergen.
When used, the optional organic acid is typically added in a range from about
0.1 % to
about 5% by weight of the composition.
Flocculating Polymer
Compositions and systems of the present invention may comprise from about
0.001%, to about 0.5%, or from about 0.01 to about 0.1% of a flocculating
polymer,
wherein the polymer comprises at least one cationically charged unit, inter
alia,
quaternary ammonium moiety or unit which can form a cationic charge in situ,
inter alia,
an amine moiety. Stated in another way, the oligomer, polymer, or co-polymer
resulting
from the herein below described monomer units have one net cationic charge at
a pH = 7.
The charge can be distributed among any of the herein described units.
The flocculating polymer adsorbs irreversibly on the non-woven substrate and
helps flocculate or trap the dirt on it. This prevents the dirt from being
smeared around
on the surface that is being cleaned.
Cationic polymers in general and their method of manufacture are known in the
literature. For example, a detailed description of cationic polymers can be
found in an
article by M. Fred Hoover that was published in the Journal of Macromolecular
Science-
Chemistry, A4(6), pp 1327-1417, October, 1970. The entire disclosure of the
Hoover
CA 02620678 2010-06-18
21
article. Other suitable cationic polymers are those
used as retention aids in the manufacture of paper. They are described in
"Pulp and
Paper, Chemistry and Chemical Technology Volume III edited by James Casey
(1981).
The Molecular weight of these polymers is in the range of 2,000 - 5,000,000
daltons.
The flocculating polymers of this invention will be better understood when
read in
light of the Hoover article and the Casey book, the present disclosure and the
Examples
herein.
Suitable flocculating polymers include but are not limited to:
1. polyethyleneimine and its derivatives. These are commercially available
under
the trade name Lupasol ex. BASF AG of Ludwigschaefen, Germany.
2. Polyamidoamine-epichlorohydrin (PAE) Resins which are condensation
products of polyalkylenepolyamine with polycarboxylic acid. The most common
PAE
resins are the condensation products of diethylenetriamine with adipic acid
followed by a
subsequent reaction with epichlorohydrin. They are available from Hercules
Inc. of
Wilmington DE under the trade name Kymene or from BASF A.G. under the trade
name
Luresin.
These polymers are described in Wet Strength resins and their applications
edited
by L. L. Chan, TAPPI Press(1994).
Linear Polymer Units
3. Synthetic addition polymers of the general structure
R1 R2
I i
R1 Z
wherein R', R2, and Z are defined herein below. The linear polymer units are
typically
formed from linearly polymerizing monomers. Linearly polymerizing monomers are
defined herein as monomers which under standard polymerizing conditions result
in a
linear polymer chain or alternatively which linearly propagate polymerization.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
22
The linearly polymerizing monomers of the present invention have the formula:
R1 R2
C=C\ R' Z.
however, those of skill in the art recognize that many useful linear monomer
units are
introduced indirectly, inter alia, vinyl amine units, vinyl alcohol units, and
not by way of
linearly polymerizing monomers. For example, vinyl acetate monomers once
incorporated into the backbone are hydrolyzed to form vinyl alcohol units. For
the
purposes of the present invention, linear polymer units may be directly
introduced, i.e. via
linearly polymerizing units, or indirectly, i.e. via a precursor as in the
case of vinyl
alcohol cited herein above.
Each R1 is independently hydrogen, C1-C4 alkyl, substituted or unsubstituted
phenyl, substituted or unsubstituted benzyl, carbocyclic, heterocyclic, and
mixtures
thereof. Preferably R' is hydrogen, C1-C4 alkyl, phenyl, and mixtures thereof,
more
preferably hydrogen and methyl.
Each R2 is independently hydrogen, halogen, C1-C4 alkyl, C1-C4 alkoxy,
substituted or unsubstituted phenyl, substituted or unsubstituted benzyl,
carbocyclic,
heterocyclic, and mixtures thereof. Preferred R2 is hydrogen, C1-C4 alkyl, and
mixtures
thereof.
Each Z is independently hydrogen; hydroxyl; halogen; -(CH2)mR, wherein R is
hydrogen,
hydroxyl, halogen, nitrilo, -OR3, -O(CH2),,N(R3)2, -O(CH2)nN+(R3)3X -, -
000(CH2)nN(R3)2, -OCO(CH2)nN+(R3)3X -, -C(O)NH-(CH2)nN(R3)2, -
C(O)NH(CH2)nN+(R3)3X ", -(CH2)nN(R3)2, -(CH2),,N+(R3)3X a non-aromatic
nitrogen
heterocycle comprising a quaternary ammonium ion, a non-aromatic nitrogen
heterocycle
comprising an N-oxide moiety, an aromatic nitrogen containing heterocyclic
wherein one
or more or the nitrogen atoms is quaternized; an aromatic nitrogen containing
heterocycle
wherein at least one nitrogen is an N-oxide; -NHCHO (formamide), or mixtures
thereof;
wherein each R3 is independently hydrogen, C1-C8 alkyl, C2-C8 hydroxyalkyl,
and
mixtures thereof; X is a water soluble anion; the index n is from 1 to 6;
carbocyclic,
heterocyclic, or mixtures thereof; -(CH2)mCOR' wherein R' is -OR3, -
O(CH2),,N(R3)2, -
O(CH2)nN+(R3)3X -, -NR3(CH2)nN(R3)2, -NR3(CH2)nN+(R3)3X -, -(CH2)nN(R'3)2, -
(CH2)nN+(R3)3X -, or mixtures thereof, wherein R3, X, and n are the same as
defined
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
23
herein above. A preferred Z is -O(CH2)nN+(R3)3X wherein the index n is 2 to 4.
The
index m is from 0 to 6, preferably 0 to 2, more preferably 0.
Non-limiting examples of addition polymerizing monomers comprising a
heterocyclic Z unit includes 1-vinyl-2-pyrrolidinone, 1-vinylimidazole, 2-
vinyl-l,3-
dioxolane, 4-vinyl-l-cyclohexenel,2-epoxide, and 2-vinylpyridine.
The polymers and co-polymers of the present invention comprise Z units which
have a cationic charge or which result in a unit which forms a cationic charge
in situ.
When the co-polymers of the present invention comprise more than one Z unit,
for
example, Z1, Z2.... Zn units, at least about 1% of the monomers which comprise
the co-
polymers will comprise a cationic unit. Preferred cationic units include -
O(CH2)nN+(R3)3X - and -(CH2)nN+(R)3X -. When the co-polymers of the present
invention are formed from two monomers, Zl and Z2, the ratio of Z1 to Z2 is
preferably
from about 9:1 to about 1:9.
A non-limiting example of a Z unit which can be made to form a cationic charge
in situ is the -NHCHO unit, formamide. The formulator can prepare a polymer or
co-
polymer comprising formamide units some of which are subsequently hydrolyzed
to form
vinyl amine equivalents. For example the formulator may prepare a co-polymer
having
the general formula:
HYNH Z
O
X y
which comprises a formamide unit and then subsequently treat the co-polymer
such that
some of the formamide units are hydrolyzed to form a co-polymer comprising
vinyl
amine units, said polymer having the formula:
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
24
HY NH NH2 Z
O
x X y
wherein Z may be a cationic unit comprising or non-cationic unit comprising
moiety and
X'+X"=X.
Another class of preferred linearly polymerizable monomers comprise
cationically
charged heteroaromatic Z units having the formula:
R1 R2
~C=C
R
+
R6
an non-limiting example of which is 4-vinyl (N-alkyl)pyridine wherein R1 and
R2 are
each hydrogen and R6 is methyl.
Another class of preferred linearly polymerizable monomers which comprises a
heterocyclic ring includes Z units comprising an N-oxide, for example, the N-
oxide
having the formula:
R1 R2
/C=C~
R
\A O
a non-limiting example of which is 4-vinyl pyridine N-oxide.
N-alkyl vinylpyridine monomers and N-oxide vinylpyridine monomers can be
suitably combined with other non aromatic monomers, inter alia, vinyl amine.
However,
preferred polymers of the present invention include co-polymers derived from a
combination of quaternized, N-oxide, and nitrogen containing heteroaromatic
monomers,
non-limiting examples of which includes a copolymer of N-methyl vinyl pyridine
and
vinyl pyridine in a ratio of 4:1; a copolymer of N-methyl vinyl pyridine and
vinyl
pyridine in a ratio of 4:6; a co-polymer of poly(N-methyl vinyl pyridine) and
vinyl
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
pyridine N-oxide in a ratio of polymer to monomer of 4:1; poly(N-methyl vinyl
pyridine)
and vinyl pyridine N-oxide in a ratio of polymer to monomer of 4:6; and
mixtures thereof.
As described herein above, some preferred polymer residues may be formed by
treatment of the resulting polymer. For example, vinyl amine residues are
preferably
introduced via formamide monomers which are subsequently hydrolyzed to the
free
amino unit. Also vinyl alcohol units are obtained by hydrolysis of residues
formed form
vinyl acetate monomers. Likewise, acrylic acid residues may be esterified
after
polymerization, for example, units having the formula:
CH3
X
O D~/ ~ CH3
CH3
may be more conveniently formed after the backbone has been formed by
polymerization
with acrylic acid or acrylic acid precursor monomers.
II) Cyclic Units Derived from Cyclically Polymerizing Monomers
The polymers or co-polymers of the present invention can comprise one or more
cyclic polymer units which are derived from cyclically polymerizing monomers.
Cyclically polymerizing monomers are defined herein as monomers which under
standard
polymerizing conditions result in a cyclic polymer residue as well as serving
to linearly
propagate polymerization. Preferred cyclically polymerizing monomers of the
present
invention have the formula:
R4 -
x
R4-N R5
Rs
wherein each R4 is independently an olefin comprising unit which is capable of
propagating polymerization in addition to forming a cyclic residue with an
adjacent R4
unit; R5 is C1-C12 linear or branched alkyl, benzyl, substituted benzyl, and
mixtures
thereof; X is a water soluble anion.
Non-limiting examples of R4 units include allyl and alkyl substituted allyl
units.
Preferably the resulting cyclic residue is a six-member ring comprising a
quaternary
nitrogen atom.
R5 is preferably C1-C4 alkyl, preferably methyl.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
26
An example of a cyclically polymerizing monomer is dimethyl diallyl ammonium
having the formula:
N
H3C CH3
which results in a polymer or co-polymer having units with the formula:
N+ X"
H3C CH3
Z
wherein preferably the index z is from about 10 to about 50,000.
III) Mixtures thereof.
The polymers or co-polymers of the present invention retain a net cationic
charge,
whether the charged is developed in situ, or whether the polymer or co-polymer
itself has
a formal positive charge. Preferably the polymer or co-polymer has at least
10%, more
preferably at least about 25%, more preferably at least about 35%, most
preferably at least
about 50% of the residues comprise a cationic charge.
The polymers or co-polymers of the present invention can comprise mixtures of
linearly and cyclically polymerizing monomers, for example the
poly(dimethyldiallyl-
ammonium chloride/acrylamide) co-polymer having the formula:
Z1 Z2
X y
N+X-
H3C CH3
Z
wherein Z1, Z2, x, y, and z are the same as defined herein above and X is a
chloride ion.
One embodiment of this invention is the composition comprising a polymer based
on dimethyldiallylammonium chloride and a copolymer which is based upon
acrylamide
with a co-monomer selected from the group consisting of N, N
CA 02620678 2010-06-18
27
dialkylaminoalkyl(meth)acrylate, N, N dialkylaminoalkylacrylate, N,N
dialkylaminoalkylacrylamide, N,N dialkylaminoalkyl(meth)acrylamide, their
quaternized
derivatives and mixtures thereof.
Non-limiting examples of polymers suitable for use with the present invention
include flocculating copolymers comprising:
i) a first monomer selected from the group consisting of N, N
dialkylaminoalkyl(meth)acrylate, N, N dialkylaminoalkylacrylate, N,N
dialkylaminoalkylacrylamide, N,N dialkylaminoalkyl(meth)acrylamide,
their quaternized derivatives, vinylamine or its derivatives, allylamine or
its derivatives and mixtures thereof; and
ii) a second monomer selected from the group consisting of acrylic acid,
methacrylic acid, CI-C6 alkylmethacrylate, CI-C6 alkyl acrylate, C1-Cs
hydroxyalkylacrylate, CI-Cs hydroxyalkylmethacrylate, acrylamide, Cj-
C16 alkyl acrylamide, CI-CI6 diallrylacrylamide, 2-acrylamido-2-
methylpropane sulfonic acid or its alkali salt, methacrylamide, C1-C16
aklmethacrylamide, C1-C16 dialkyhnethacrylamide, vinyl formamide,
vinylacetamide, vinyl alcohol, C1-C5 vinylalkylether, vinyl pyridine,
itaconic acid, vinyl acetate, vinyl propionate, vinyl butyrate and mixtures
thereof;
4. Cationic polysaccharides preferably cationic hydroxyethyl cellulose,
cationic guar gum
TM
and cationic starches. Examples of cationic hydroxyehtyl cellulose is Ucare
Polymer JR
25M, Polymer JR 400, Polymer LK 400 and Polymer LR 400 all available from Dow
TM TM
Chemicals Co and Celquat H200 and Celquat L-200 available from National Starch
and
Chemical Company or Bridgewater, NJ.
TM
Examples of cationic guar gums are Jaguar Cl3 and Jaguar Excel available from
Rhodia
Examples of cationic starches are described by D. B. Solarek in Modified
Starches,
Properties and Uses published by CRC Press (1986). Cationic starches are
commercially
available from National Starch and Chemical Company under the Trade Name Cato.
CA 02620678 2010-06-18
28
Surfactant
If desired, the compositions herein may comprise from about 0.001 % to about
2%
by weight of a surfactant or from about 0.01% to about 0.5% by weight of
surfactant.
Detersive surfactants are preferably, zwitterionic or amphoteric or nonionic
type or can
comprise compatible mixtures of these types. Detergent surfactants useful
herein are
described in U.S. Patent 3,664,961, Norris, issued May 23, 1972, U.S. Patent
3,919,678,
Laughlin et al., issued December 30, 1975, U.S. Patent 4,222,905, Cockrell,
issued
September 16, 1980, and in U.S. Patent 4,239,659, Murphy, issued December 16,
1980.
Non-limiting examples of nonionic surfactants include:
a) C12-CIS alkyl ethoxylates, such as, NEODOL nonionic surfactants from
Shell;
b) C6-C12 alkyl phenol alkoxylates wherein the alkoxylate units are a mixture
of
ethyleneoxy and propyleneoxy units;
c) Cu-CIS alcohol and C6-CI2 alkyl phenol condensates with ethylene
oxide/propylene oxide block polymers such as Pluronic from BASF;
d) C14-C22 mid-chain branched alcohols, BA, as disclosed in U.S. Patent No.
6,150,322;
e) C14-C22 mid-chain branched alkyl alkoxylates, BAEX, wherein x 1-30, as
disclosed
in U.S. Patent Nos.: 6,153,577, 6,020,303, and 6,093,856;
f) alkyl polysaccharides as disclosed in U.S. Patent No. 4,565,647 issued to
Llenado on
January 26, 1986; specifically alkylpolyglycosides as disclosed in U.S. Patent
Nos.: 4,483,780 and 4,483,779;
g) Polyhydroxy fatty acid amides as disclosed in U.S. Patent No. 5,332,528,
WO 92/06162, WO 93/19146, WO 93/19038, and WO 94/09099;
h) ether capped poly(oxyalkylated) alcohol surfactants as disclosed in U.S.
Patent No. 6,482,994 and WO 01/42408.
Preferred surfactants for use herein are the alkylpolysaccharides that are
disclosed
in U.S. Patent Nos.: 5,776,872, entitled "Cleansing compositions", issued July
7, 1998, to
Giret et al.; 5,883,059, entitled '"Three in one ultra mild lathering
antibacterial liquid
personal cleansing composition" issued March 16, 1999, to Furman et al.;
5,883,062,
entitled "Manual dishwashing compositions", issued March 16, 1999, to Addison
et al.;
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
29
and 5,906,973, entitled "Process for cleaning vertical or inclined hard
surfaces" issued
May 25, 1999, to Ouzounis et al.
. Suitable alkylpolysaccharides for use herein are disclosed in U.S. Patent
No.
4,565,647, issued to Llenado on Jan. 21, 1986, having a hydrophobic group
containing
from about 6 to about 30 carbon atoms, preferably from about 10 to about 16
carbon
atoms and a polysaccharide, e.g., a polyglycoside, hydrophilic group. For
acidic or
alkaline cleaning compositions/solutions suitable for use in no-rinse methods,
the
preferred alkyl polysaccharide preferably comprises a broad distribution of
chain lengths,
as these provide the best combination of wetting, cleaning, and low residue
upon drying.
This "broad distribution" is defined by at least about 50% of the chainlength
mixture
comprising from about 10 carbon atoms to about 16 carbon atoms. Preferably,
the alkyl
group of the alkyl polysaccharide consists of a mixtures of chainlength,
preferably from
about 6 to about 18 carbon atoms, more preferably from about 8 to about 16
carbon
atoms, and hydrophilic group containing from about one to about 1.5
saccharide,
preferably glucoside, groups per molecule. This "broad chainlength
distribution" is
defined by at least about 50% of the chainlength mixture comprising from about
10
carbon atoms to about 16 carbon atoms. A broad mixture of chain lengths,
particularly
C8-C16, is highly desirable relative to narrower range chain length mixtures,
and
particularly versus lower (i.e., C8-C10 or C$-C12) chainlength alkyl
polyglucoside
mixtures. It is also found that the preferred C8_16 alkyl polyglucoside
provides much
improved perfume solubility versus lower and narrower chainlength alkyl
polyglucosides,
as well as other preferred surfactants, including the C8-C14 alkyl
ethoxylates. Any
reducing saccharide containing 5 or 6 carbon atoms can be used, e.g., glucose,
galactose
and galactosyl moieties can be substituted for the glucosyl moieties.
(optionally the
hydrophobic group is attached at the 2-, 3-, 4-, etc. positions thus giving a
glucose or
galactose as opposed to a glucoside or galactoside.) The intersaccharide bonds
can be,
e.g., between the one position of the additional saccharide units and the 2-,
3-, 4-, and/or
6- positions on the preceding saccharide units. 'The glycosyl is preferably
derived from
glucose.
Optionally, and less desirably, there can be a polyalkyleneoxide chain joining
the
hydrophobic moiety and the polysaccharide moiety. The preferred alkyleneoxide
is
ethylene oxide. Typical hydrophobic groups include alkyl groups, either
saturated or
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
unsaturated, branched or unbranched containing from 8 to 18, preferably from
10 to 16,
carbon atoms. Preferably, the alkyl group is a straight-chain saturated alkyl
group. The
alkyl group can contain up to about 3 hydroxyl groups and/or the
polyalkyleneoxide chain
can contain up to about 10, preferably less than 5, alkyleneoxide moieties.
Suitable alkyl
polysaccharides are octyl, nonyldecyl, undecyldodecyl, tridecyl, tetradecyl,
pentadecyl,
hexadecyl, heptadecyl, and octadecyl, di-, tri-, tetra-, penta-, and
hexaglucosides and/ or
galatoses. Suitable mixtures include coconut alkyl, di-, tri-, tetra-, and
pentaglucosides
and tallow alkyl tetra-, penta- and hexaglucosides.
To prepare these compounds, the alcohol or alkylpolyethoxy alcohol is formed
first and then reacted with glucose, or a source of glucose, to form the
glucoside
(attachment at the 1-position). The additional glycosyl units can then be
attached
between their 1-position and the preceding glycosyl units 2-,3-, 4- and/or 6-
position,
preferably predominantly the 2-position.
In the alkyl polyglycosides, the alkyl moieties can be derived from the usual
sources like fats, oils or chemically produced alcohols while their sugar
moieties are
created from hydrolyzed polysaccharides. Alkyl polyglycosides are the
condensation
product of fatty alcohol and sugars like glucose with the number of glucose
units defining
the relative hydrophilicity. As discussed above, the sugar units can
additionally be
alkoxylated either before or after reaction with the fatty alcohols. Such
alkyl
polyglycosides are described in detail in WO 86/05199 for example. Technical
alkyl
polyglycosides are generally not molecularly uniform products, but represent
mixtures of
alkyl groups and mixtures of monosaccharides and different oligosaccharides.
Alkyl
polyglycosides (also sometimes referred to as "APGs") are preferred for the
purposes of
the invention since they provide additional improvement in surface appearance
of the
surface being cleaned relative to other surfactants. The glycoside moieties
are preferably
glucose moieties. The alkyl substituent is preferably a saturated or
unsaturated alkyl
moiety containing from about 8 to about 18 carbon atoms, preferably from about
8 to
about 10 carbon atoms or a mixture of such alkyl moieties. C8-C16 alkyl
polyglucosides
are commercially available (e.g., Simusol surfactants from Seppic
Corporation, 75 Quai
d'Orsay, 75321 Paris, Cedex 7, France, and Glucopon 425 available from
Henkel). In
the present invention, the preferred alkyl polyglucosides are those which have
been
purified enough for use in personal cleansing. Most preferred are "cosmetic
grade" alkyl
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
31
polyglucosides, particularly C8 to C16 alkyl polyglucosides, such as Plantaren
2000 ,
Plantaren 2000 NO, and Plantaren 2000 N UP , available from Henkel Corporation
(Postfach 101100, D 40191 Dusseldorf, Germany).
Additional suitable nonionic surfactants include polyhydroxy fatty acid amides
of the
formula:
II I 1
R-C-N-Z
wherein R is a C9-17 alkyl or alkenyl, R1 is a methyl group and, Z is glucityl
derived from a reduced sugar or alkoxylated derivative thereof. Examples are N-
methyl
N-1-deoxyglucityl cocoamide and N-methyl N-1-deoxyglucityl oleamide. Processes
for
making polyhydroxy fatty acid amides are known and can be found in U.S. Patent
No.
2,965,576 issued to Wilson and U.S. Patent No. 2,703,798 issued to Schwartz.
Zwitterionic Surfactants
Non-limiting examples of zwitterionic surfactants include: derivatives of
secondary and tertiary amines, derivatives of heterocyclic secondary and
tertiary amines,
or derivatives of quaternary ammonium, quaternary phosphonium or tertiary
sulfonium
compounds. See U.S. Patent No. 3,929,678 issued to Laughlin et al., granted on
December 30, 1975 at column 19, line 38 through column 22, line 48, for
examples of
zwitterionic surfactants; betaine, including alkyl dimethyl betaine and
cocodimethyl
amidopropyl betaine, C8 to C18 (preferably C12 to C18) amine oxides and sulfo
and
hydroxy betaines, such as N-alkyl-N,N-dimethylammino-1-propane sulfonate where
the
alkyl group can be C8 to C18, preferably C10 to C14.
Ampholytic Surfactants
Non-limiting examples of ampholytic surfactants include: aliphatic derivatives
of
secondary or tertiary amines, or aliphatic derivatives of heterocyclic
secondary and
tertiary amines in which the aliphatic radical can be straight- or branched-
chain. One of
the aliphatic substituents contains at least about 8 carbon atoms, typically
from about 8 to
about 18 carbon atoms, and at least one contains an anionic water-solubilizing
group, e.g.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
32
carboxy, sulfonate, sulfate. See U.S. Patent No. 3,929,678 to Laughlin et al.,
issued
December 30, 1975 at column 19, lines 18-35, for examples of ampholytic
surfactants.
Anionic Surfactants
Nonlimiting examples of anionic surfactants useful herein include:
a) C11-C18 alkyl benzene sulfonates (LAS);
b) C10-C20 primary, branched-chain and random alkyl sulfates (AS);
c) C10-C18 secondary (2,3) alkyl sulfates having formulae (1) and (II):
OSO3- M+ OSO3- M+
CH3(CH2)X(CH)CH3 or CH3(CH2)y(CH)CH2CH3
(I) (II)
M in formulae (I) and (II) is hydrogen or a cation which provides charge
neutrality. For the purposes of the present invention, all M units, whether
associated with a surfactant or adjunct ingredient, can either be a hydrogen
atom
or a cation depending upon the form isolated by the artisan or the relative pH
of
the system wherein the compound is used. Non-limiting examples of preferred
cations include sodium, potassium, ammonium, and mixtures thereof. Wherein x
in formulae (I) and (II) is an integer of at least about 7, preferably at
least about 9;
y in formulae (I) and (II) is an integer of at least 8, preferably at least
about 9;
d) C10-C18 alkyl alkoxy sulfates (AEXS) wherein preferably x is from 1-30;
e) C10-C18 alkyl alkoxy carboxylates preferably comprising 1-5 ethoxy units;
f) mid-chain branched alkyl sulfates as disclosed in U.S. Patent Nos.
6,020,303 and 6,060,443;
g) mid-chain branched alkyl alkoxy sulfates as disclosed in U.S. Patent Nos.
6,008,181 and 6,020,303;
h) modified alkylbenzene sulfonate (MLAS) as disclosed in: WO 99/05243, WO
99/05242, WO 99/05244, WO 99/05082, WO 99/05084, WO 99/05241, WO
99/07656, WO 00/23549, and WO 00/23548.;
i) methyl ester sulfonate (MES); and
j) alpha-olefin sulfonate (AOS)
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
33
Cationic Surfactants
Non-limiting examples of anionic surfactants include: the quaternary ammonium
surfactants, which can have up to 26 carbon atoms.
a) alkoxylate quaternary ammonium (AQA) surfactants as disclosed in U.S.
Patent
No. 6,136,769;
b) dimethyl hydroxyethyl quaternary ammonium as disclosed in U.S. Patent No.
6,004,922;
c) polyamine cationic surfactants as dislosed in WO 98/35002, WO 98/35003, WO
98/35004, WO 98/35005, and WO 98/35006;
d) cationic ester surfactants as disclosed in U.S. Patent Nos. 4,228,042,
4,239,660
4,260,529 and 6,022,844; and
e) amino surfactants as disclosed in U.S. Patent No. 6,221,825 and WO
00/47708,
specifically amido propyldimethyl amine.
Semi-Polar Nonionic Surfactants
Non-limiting examples of semi-polar nonionic surfactants include: water-
soluble
amine oxides containing one alkyl moiety of from about 10 to about 18 carbon
atoms and
2 moieties selected from the group consisting of alkyl groups and hydroxyalkyl
groups
containing from about 1 to about 3 carbon atoms; water-soluble phosphine
oxides
containing one alkyl moiety of from about 10 to about 18 carbon atoms and 2
moieties
selected from the group consisting of alkyl groups and hydroxyalkyl groups
containing
from about 1 to about 3 carbon atoms; and water-soluble sulfoxides containing
one alkyl
moiety of from about 10 to about 18 carbon atoms and a moiety selected from
the group
consisting of alkyl and hydroxyalkyl moieties of from about 1 to about 3
carbon atoms.
See WO 01/32816, U.S. 4,681,704, and U.S. 4,133,779.
Organic Solvent
The compositions can also include one or more organic solvents. Suitable
organic
solvents include but are not limited to alcohols, glycols, glycol ethers,
ketones, aldehydes,
ethers, alkyl pyrrolidone, and terpenes. The organic solvent may include one
or more
nonvolatile organic solvents at effective levels, typically from about 0.05%
by weight of
the composition to about 15% by weight of the composition, or from about 0.1 %
by
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
34
weight of the composition to about 10% by weight of the composition, or from
about 1 %
by weight of the composition to about 5%, by weight of the composition.
One non-limiting class of organic solvents which may be used are glycol ethers
represented as:
R2 \ n
RI-O-CH-CH2-j--X-R3
wherein:
R = CI to C8 alkyl, or C6 to C8 alkly aryl moiety,
R2 = H or C 1 to C4 alkyl
R3= H or C1 to C6 alkyl, or C6 to C8 alkly aryl moiety
X = -O- or C(O)O- group
Examples of glycol ethers are ethyleneglycol methyl ether, ethyleneglycol
monoethyl ether, ethyleneglycol monopropyl ether, ethyleneglycol monobutyl,
ether,
ethyleneglycol monohexyl ether, diethylene glycol methyl ether, diethylene
glycol
monoethyl ether, diethylene glycol monopropyl ether, diethylene glycol
monobutyl ether,
diethylene glycol monohexyl ether, triethyleneglycol monomethylether,
triethyleneglycol
monoethyl ether, triethyleneglycol monobutylether, ethylene glycol
phenylether,
diethylene glycol phenylether, tri ethylene glycol phenylether, diethylene
glycol n-butyl
ether acetate, diethylene glycol methyl ether acetate, ethylene glycol methyl
ether acetate,
ethylene glycol butyl ether acetate, propylene glycol monomethyl ether,
diproppylene
glycol monomethyl ether, tripropylene glycol monomethyl ether, propylene
glycol
monoethyl ether, diproppylene glycol monoethyl ether, tripropylene glycol
monoethyl
ether, propylene glycol monopropyl ether, dipropylene glycol monopropyl ether,
tripropylene glycol monopropyl ether, propylene glycol monobutyl ether,
diproppylene
glycol monobutyl ether, tripropylene glycol monobutyl ether, propylene glycol
monohexyl ether, diproppylene glycol monohexyl ether, tripropylene glycol
monohexyl
ether,, propylene glycol phenyl ether, diproppylene glycol phenyl ether,
propylene glycol
methyl ether acetate, dipropylene glycol methyl ether acetate, ethylene glycol
dimethyl
ether, diethylene glycol dimethyl ether, triethylene glycol dimethyl ether,
tetraethylene
glycol dimethyl ether, propylene glycol dimethyl ether, dipropylene glycol
dimethyl
ether, tripropylene glycol dimethyl ether, polyethylene glycol dimethyl ether,
Ethylene
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
glycol dibutyl ether, diethylene glycol dibutyl ether, and polyethylene glycol
dibutyl
ether.
Nonlimiting examples of glycols are ethylene glycol, propylene glycol,
butylene
glycol, hexylene glycol, diethylene glycol, dipropylene glycol, polyethylene
glycol of
molecular weight less than 400, polypropylene glycol of molecular weight less
than 400
Nonlimiting examples of alcohols are methanol, ethanol, propyl alcohol, butyl
alcohol,
pentyl alcohol and hexyl alcohol, benzyl alcohol, cyclohexanol and their
derivatives.
Nonlimiting examples of esters are ethyl acetate, propyl acetate, butyl
acetate, pentyl
acetate, hexyl acetate, methyl propionate, ethyl propionate, butyl propionate,
pentyl
propionate, ethyl 3-ethoxy propionate (U-CAR ESTER EEP available from Dow
Chemicals of Midland, Michigan), glyceryl mono, di and triacetate, glyceryl
mono, di and
tripropionate, mixed esters of glycerine, methyl, propyl and butyl esters of
glycols,
preferably ethylene glycol methyl ester, propylene glycol methyl ester.
Nonlimiting examples of terpenes include hydrocarbons and terpene alcohols.
These may include limonene, ^ and ^ pinene, camphene, fenchene, myrcene, cis-
pinane,
p-8 menthene, 3-carene, cyrnene, terpinene, terpinolene, cineole, pinane,
cineole,
fenchone, linalool, fenchol, citronellal, terpinenol, neomenthol, bomeol,
isoborneol,
menthol, citronellol, neral, and geraniol. Additional examples of terpenes are
shown in
"Kirk-Othmer Encyclopedia of Chemical Technology Fourth Edition", Vol. 23,
pages
832-882 published by John Wiley and Son of New York City, New York.
Other suitable solvents are, pyrrolidone and N- alkyl pyrrolidone such as n-
octyl
pyrrolidone and n-dodecyl pyrrolidone sold under the trade name SURFADONE LP-
100
and SURFADONE LP-300 (available from International Specialty Products of
Wayne,
New Jersey). Preferably, the solvent is soluble in the composition at the
level used in the
composition. If an insoluble solvent is used, it is solubilized using an
appropriate co-
solvent or emulsified using an appropriate emulsifier. Preferably, the solvent
is non-
volatile. The nonvolatile organic solvent has a vapor pressure of less than
about 0.1 mm
of mercury at about 20 C or has a boiling point of at least about 2300C. Due
to their low
volatility, these solvents do not evaporate rapidly and allow sufficient
"working" time for
the wipe before it dries out. Preferred solvents are esters, alcohols, and
glycol ethers.
Such solvents typically have a terminal C3 - C6 hydrocarbon attached to from
about two to about three alkylene glycol moieties to provide the appropriate
degree of
CA 02620678 2010-06-18
36
hydrophobicity, high boiling point (or low vapor pressure) and, preferably,
surface
activity. Examples of commercially available hydrophobic cleaning solvents
based on
alkylene glycol chemistry include Triethyleneglycol monomethyl ether
(Methoxytriglycol
ether from Dow Chemical of Midland, Michigan), Diethylene glycol monoethyl
ether
(carbitol solvent from Dow Chemical), Triethyleneglylcol monoethyl ether
(Ethoxytriglycol from Dow Chemical), diethyleneglycol butylether (Butyl
Carbitol),
Triethyleneglycol monobutyl ether (Butoxytriglycol ether), Diethyleneglycol
monohexyl
TM
ether (Hexyl Carbitol), ethylene glycol phenyl ether (DOWANOL EPH),
TM
Dipropyleneglycol methyl ether (DOWANOL DPM), Tripropylene glycol methyl ether
TM
(DOWANOrTPM), Dipropylene glycol methylether acetate (DOWANOL DPMA),
Dipropylene glycol n-propyl ether (DOWANOLTTMDPnP), Tripropyleneglycol n
propyl
TM TM
ether (DOWANOL TPnP), dipropyleneglycol n-butyl ether (DOWANOL DPnB),
TM
Tripropylene glycol n-butyl ether (DOWANOL TPnB), Propyleneglycol phenyl ether
TM
(DOWANOL PPb). These solvents are commercially available from Dow Chemical of
Midland, Michigan.
Additional solvents of this class are available from Clariant GmbH of Work
Gendorf, Germany, examples of which include Methyl tetraglycol and buyl
polyglycol.
Soil Suspending Polymers
Compositions and systems of the present invention if desired may comprise from
about 0.001 % to about 0.5% or from about 0.01 % to about 0.15% of a soil
suspension
polymer. The polymer is a water soluble ethoxylated amine having soil removal
properties. These compounds are selected from ethoxylated monoamines,
ethoxylated
diamines, ethoxylated polyamines, ethoxylated amine polymers and mixtures
thereof.
One preferred soil suspension polymer is polyethyleneimine ("PEI") 600
ethoxylated (20
mole per nitrogen) available having a minimum molecular weight of about 9,000
daltons.
The soil suspending polymer enhances the cleaning efficacy of the composition
by
suspending particulate soils such that they can be more easily removed by the
substrate.
CA 02620678 2010-06-18
37
Method of Treating Household Fabric-Based Materials- With the Moistened
Disposable Wive Article of the Present Invention
The present invention also includes a method for treating the household fabric-
based materials with the premoistended disposable wipe of the present
invention. This
includes contacting the wipe to the surface to be cleaned, applying the
composition to the
surface to be cleaned, and transferring the dirt and contaminants from the
surface to be
cleaned to the wipe. In one non-limiting embodiment, a user sprays the wipe
with the
composition prior to contacting the surface to be cleaned with the wipe. In
yet another
non-limiting embodiment, a user sprays the surface to be cleaned with the
composition
prior to contact by the wipe. In another non-limiting embodiment, the
composition is
applied to the wipe during manufacture and provided to the user in a
premoistened form.
In a further non-limiting embodiment, the composition is applied to the wipe
during
manufacture. The user then moistens the wipe at the time of use.
Kit
The disposable wipe of the present invention may be provided alone or it may
also
optionally be provided in conjunction with an implement as a kit for cleaning
household
surfaces such as fabric-based materials around the house. In use, a user will
typically
attach the moistened disposable wipe to the implement to facilitate cleaning.
Non-
limiting implements which may be used in conjunction with the present
invention include
but are not limited to those disclosed in U.S. Publication No. 2006-0277706
filed on April 11, 2006; U.S. Publication No. 2005/0060827 published March 25,
2005;
U.S. Publication No. 2006/0048318 published March 9, 2006; U.S. Patent No.
6,484,346
issued to Kingry et al. on November 26, 2002, U.S. Patent No. 6,305,046 issued
to
Kingry et al. on October 23, 2001; U.S. Patent No. 6,669,391 issued to
Policicchio et al.
on December 20,2003; and U.S. Publication No. 2002/005001 published in the
name of
Willman et al. on May 2, 2002. Other implements which may be used in
conjunction
TM
with the moistened wipe of the present invention include but are not limited
to CLOROX
TM TM
READY MOP, SCOTCH BRITE TUB AND TILE SCRUBBER, and SCOTCH BRITE
BATHROOM FLOOR CLEANER.
The disposable wipe of the present invention may be overwrapped. Non-limiting
examples of suitable overwraps include shrink wrap, foil, or the like (not
shown). The
wipes may be provided in a package (not shown) such as a box, pouch, or
carton. The
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
38
box, pouch, or carton may optionally include a sample of the wipe so as to
allow the user
to touch, view, and/or smell the wipe, prior to purchase. The overwrap,
box/carton/pouch, or a combination thereof may include an opening or window so
as to
allow the user to view and/or touch at least some portion of the wipe and/or
optionally the
implement if included.
Use Identifiers
Use identifiers (not shown), may be used if desired in order to identify what
the
disposable wipe and/or optional cleaning implement may be used for. In
addition to or
alternatively, one or more use identifiers can also be utilized for example to
indicate the
types and/or forms of surfaces the disposable wipe and/or optional cleaning
implement
may be used on. The use identifier may be utilized to quickly and easily
communicate to
a user what type of surfaces the disposable wipe and/or optional cleaning
implement may
be used on. Use identifiers could be included if desired on one or more of the
following:
on the packaging for the kit, the cleaning implement, the disposable wipe, or
a
combination thereof; on the cleaning implement itself; on the disposable wipe
itself; on
the disposable wipe(s) and/or cleaning implement overwrap; on a label attached
for
instance to some part of the kit including but not limited to: the package,
the cleaning
implement, the disposable wipe, the disposable wipe overwrap, or combinations
thereof;
on the use instructions; on separate print advertising; on in-store displays
or the like; or
combinations thereof. Non-limiting examples of the form of the use identifier
could be in
the form of written words, pictorials, graphics, symbols/icons, and the like,
as well as
combinations thereof. Non-limiting examples would be a use identifier which
combines
an icon and one or more words to indicate for example that the disposable wipe
and/or
optional cleaning implement could be used on fabric. Additional non-limiting
examples
include combining an icon and one or more words to indicate that the
disposable wipe
and/or optional cleaning implement could be used on: upholstery; draperies;
pillows;
comforters; bedding including but not limited to bed linens and mattress
covers; car
fabrics; baby/infant fabric items including but not limited to strollers and
car seats, or the
like; clothing; fabric clothing accessories including but not limited to
purses, wallets, and
shoes; or combinations thereof. For instance, one non-limiting use identifier
could
comprise the combination of an icon of a car with the words "car fabrics".
Another non-
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
39
limiting use identifier could comprise the combination of an icon of a sofa
with the word
"upholstery". Another non-limiting use identifier could comprise an icon of a
stroller
with the words "baby items". Yet another non-limiting use identifier could
comprise an
icon of a bed with the words "bedding".
Self-Instructing Article of Commerce
The present invention also encompasses an article of commerce comprising the
disposable wipe described above. The article of commerce may also comprise a
kit
which includes the disposable wipe in conjunction with the cleaning implement
described
above. A set of instructions may be included in association with the article
of commerce
which directs the user to follow the method of cleaning surfaces around the
house with
the disposable wipe or the disposable wipe and cleaning implement. For
instance, in one
non-limiting embodiment, such instructions may direct the user to attach a
disposable
wipe to the implement and contact the area(s)/surface to be cleaned with the
cleaning
article. In another non-limiting embodiment, such instructions may direct the
user to
contact a surface to be cleaned using the disposable wipe without the
implement. In yet
another non-limiting embodiment, the user may be directed to remove a
disposable wipe
from whatever the wipe is packaged in such as an overwrap, box, carton, pouch,
or the
like, attach the wipe to the implement, and to contact the area(s)/surfaces to
be cleaned
with the wipe.
Herein, "in association with", when referring to such instructions, means the
instructions are either directly printed on the implement; directly printed on
the
packaging for the implement and/or the cleaning sheet; printed on a label
attached to the
packaging for the implement and/or the cleaning sheet; or presented in a
different manner
including, but not limited to, a brochure, print advertisement, electronic
advertisement,
broadcast or internet advertisements; and/or other media, so as to communicate
the set of
instructions to a consumer of the implement and/or the cleaning sheet.
Methods
Method for Determining Capacity
Capacity may be measured using the following technique which is adapted from
EDANA 10.1. A 2 inch x 6 inch (5 cm x 15 cm) sample of the substrate is cut,
weighed
and immersed in distilled water for 3 minutes. The sample is then removed and
allowed to
drip for 10 seconds and reweighed. The absorption capacity of the substrate
reported in
CA 02620678 2010-06-18
grams of liquid absorbed in the substrate per gram of substrate is calculated
by the
following equation:
(wet weight of substrate - dry weight of substrate) / dry weight of substrate
Method for Determining Fuzz Level
This method can be used as a quantitative prediction of the level of fuzz
associated with nonwoven or laminate materials. The fuzz level may be
determined in
accordance with the Fuzz Level Test disclosed in U.S. Publication No.
2002/0119720.
Method for Determining Caliper
Caliper is measured in accordance with EDANA (European Disposables and
Nonwovens Association) Method 30.5-99 using a caliper foot pressure of 0.5
kPa. An
instrument suitable for this purpose is the ProGage thickness tester available
from
Thwing-Albert Instrument Company of Philadelphia, PA.
Method for Determining Stiffness
Stiffness of a dry substrate is measured in accordance with ASTM D5650-97
entitled "Standard Test Method for Resistance to Bending of Paper of Low
Bending
Stiffness (Taber- Type Tester in 0 - 10 Taber Stiffness Unit Configuration)".
A suitable
instrument for measuring stiffness per this method is a V-5 Teledyne Taber
Stiffness
TM
Tester (model 150-B) available from Teledyne Taber Instruments of North
Tonawanda,
New York. If it is desired to determine the stiffness of a wet substrate, ASTM
D5650-97
is modified by immersing each sample of substrate to be tested in distilled
water for 3
minutes. The sample is then removed and allowed to drip for 10 seconds.
Stiffness is
then measured in accordance with ASTM D5650-97.
Method for Determining ft Average Agatm Size and the % Ones Area of thee
Substrate
The following method can be used for determining the average size i.e., area
of
the aperture in a substrate and the % open area of the substrate.
Apparatus:
HP Scanje t TMA 3970 scanner (or equivalent scanner with >_ 200 dpi
resolution)
available from Hewlett-Packard Company Palo Alto, CA 94304 (650) 857-1501
CA 02620678 2010-06-18
41
Certified millimeter ruler (0.1 mm divisions)
Black cardboard paper
TM
Image Pro Plus Software 4.0 or better avaiable from Media Cybernetics, Inc.
Silver Spring, MD 20910 (301) -495-3305
Computer
Printer
Sample Preparation:
Cut cardboard frames made 4.75 inches x 4.75 inches (12 cm x 12 cm) on the
outside
with inside of the frames cut out, leaving a 1 inch (2.54 cm) cardboard
perimeter. Cover
the frame with double-sided tape (1 inch wide) (2.54 cm) and place the frame
on a wound
roll, sticky side down, centering it over the area to be measured. With the
material
attached, out around the frame to remove it from the roll.
Data Collection - collecting the image:
Lay the ruler on the scanner with the millimeter side face down, then lay the
framed
sample over the ruler on the scanner and finally lay the black cardboard paper
on top of
the sample. Scan the image into the scanner per the scanner instruction making
sure the
resolution is set to >- 200 dpi and then use the zoom to adjust the area of
interest. Save the
image as a high resolution bitmap or other uncompressed image form.
Data Analysis Using Image Pro Software
Open and import the image into the Image Pro software per the instructions
with the
program. Calibrate the spacing of the picture using the image and ruler to set
a pixel /
mm value. Select the area of interest in the image and convert it to a
greyscale.
Adjust the grey scale ranges on the count sizes to highlight all the apertures
after
setting the software to aperture size and % open area. Note for aperture size-
do not
include partial apertures that are on the edge of the sample or picture area
that do not
represent full apertures, while for the % open area these apertures should be
included.
The software will then calculate the average aperture size area and % open
area.
Examples
Examples of Wipe Substrates
Non-limiting examples of substrates which may be used for the moistened wipe
of the
present invention are disclosed below.
In one non-limiting embodiment of a substrate made in accordance with the
present invention (shown as Test Substrate 1 in Table I below), a four layer
apertured
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
42
laminate composite is formed from nonwoven webs. The four layer composite is
comprised of 2 outer layers which are polypropylene ("PP") carded nonwoven
each of
which has a basis weight of approximately 31 g/m2 (commercially available from
BBA
Nonwovens of Simpsonville, SC under code number FPN336) and two inner layers
which are wetlaid cellulose each of which has a basis weight of approximately
23 g/m2
(commercially available from Cellu Tissue Corporation of East Hartford, CT
under code
number 7020 HWS). This four layer composite after aperturing has a basis
weight of
approximately 91 g/m2 and is commercially available from Precision Fabrics
Group
("PFG") of Greensboro, NC under style No. 36385000110000.
Another non-limiting embodiment of a laminate substrate made in accordance
with the present invention (shown as Test Substrate 2 in Table I below), is a
four layer
apertured laminate composite formed from nonwoven webs. The four layer
composite is
comprised of 2 outer layers, with each being a blend of 40% 2 denier
polypropylene
fiber, 40% 6 denier polypropylene fiber, and 20% rayon fiber provided as a
homogonously carded nonwoven. Each outer layer has a basis weight of
approximately
50 g/m2 (commercially available from BBA Nonwovens of Simpsonville, SC under
grade
number BD0216). The two inner layers are each comprised of wetlaid cellulose
each of
which has a basis weight of approximately 23 g/m2 (commercially available from
Cellu
Tissue Corporation of East Hartford, CT under code number 7020 HWS). This four
layer
composite, after aperturing, has a basis weight of approximately 127 g/m2.
Table I
Approx. Approx. Approx. CD* CD* Avg. Ope
Material Capacit Fuzz Caliper Dry Wet Aper- n
(g/g) y (mg/cm2 (mm) Stiffnes Stiffnes ture Area
(mg/cm2 ) s s Area (%)
(g-cm) (g-cm) (mm2
Test Substrate 1: 4.4 0.09 1.11 1.38 0.47 0.76 6.9
PFG 97 g/m2 PP/
cellulose
composite (Style
3638 50001
10000)
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
43
Test Substrate 2: 6.5 0.28 1.18 2.80 2.40 0.46 6.9
Laminate 37-2
*CD refers to the cross direction of the substrate sample.
In another non-limiting alternate embodiment of a four layer apertured
laminate
substrate (not shown in Table 1), the substrate is comprised of 2 outer layers
which are
polypropylene ("PP") carded nonwoven (commercially available from BBA
Nonwovens
of Simpsonville, South Carolina under code number FPN336) and two inner layers
of
cellulose each of which is comprised of BOUNTY towel (commercially available
from
the instant assignee).
Non-Limiting Examples of Liquid Cleaning Solutions Useful for the Wipes of the
Present Invention
Example Example Example Example
1 2 3 4
Wt% Wt% Wt% Wt%
C10 Alkylpolyglucoside'
0.05 0.05 0.05 0.05
(SURFACTANT)
Polyethyleneimine 2 0.02 0.02 0.02 0.02
Triethylene glycol monomethyl 1.0
ether3
Diethylene glycol monoethyl ether 3 -- 1.0 -- --
Octyl pyrrolidone4 -- -- 1.0
Butyl polyglycol5 -- -- -- 1.0
Polyethyleneimine 600 ethoxylated 0.15 0.15 0.15 0.15
(20 mol) per nitrogen'
Ethoxylated castor oil' 1 0.01 0.01 0.01 0.01
Diethylenetriamine pentaacetic acid12 0.4 0.4 0.4 0.4
3,4,5-trimethoxybenzoic acidl3 0.2 0.2 0.2 0.2
Benzyl alcohol 14 0.5 0.5 0.5 0.5
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
44
Perfume 0.0075 0.0075 0.0075 0.0075
Water To 100% To 100% To 100% To 100%
Example Example Example Example
6 7 8
Wt% Wt% Wt% Wt%
C10 Alkylpolyglucosidel 0.05 0.05 0.05 0.05
Polyvinyl formamide co-vinylamine6 0.02 -- -- --
Polydimethyldiallylammoniumchloride7 -- 0.02 --
Cationic guar gum8 -- -- 0.02 --
Setleze 30009 -- -- -- 0.02
Triethylene glycol monomethyl ether3 1.0 1.0 1.0 1.0
Polyethyleneimine 600 ethoxylated (20
0.15 0.15 0.15 0.15
mol) per nitrogen10
Ethoxylated castor oil11 0.01 0.01 0.01 0.01
Diethylenetriamine pentaacetic acid12 0.4 0.4 0.4 0.4
3,4,5-trimethoxybenzoic acid13 0.2 0.2 0.2 0.2
Benzyl alcohol 14 0.5 0.5 0.5 0.5
Perfume 0.0075 0.0075 0.0075 0.0075
Suds suppressors, preservative and
0.1 0.1 0.1
other minor ingredients 0.1
Water To 100% To 100% To 100% To 100%
1 Surfactant available from Cognis Corp of Cincinnati, OH under the trade name
Plantaren.
2 Cationic flocculating polymer available from BASF AG, under the trade name
Lupasol SK having a molecular weight of 2,000,000 daltons.
3. Non-volatile solvent available from Dow Chemicals, Midland MI
4: Non-volatile solvent available from International Specialty Products,
Wayne,
NJ
5 Non-volatile solvent available from Clariant GmbH of Gendorf, Germany
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
6: Cationic flocculating polymer available from BASF AG having a molecular
weight of 25,000 daltons.
7: Cationic flocculating polymers available from Calgon Corporation under the
trade name Merquat 100 having a molecular weight of 10,000 daltons.
8: Cationic flocculating polymer available from Aqualon Company of
Wilmington, Delaware under the tradename N-Hance 3000 having a molecular
weight of
1,000,000 daltons.
9: Copolymer of vinyl pyrrolidone and dimethyaminoethylmethacrylamide
available from International Specialty Products of Wayne, New Jersey.
10: Soil Suspension Polymer having a minimum molecular weight of 9,000
daltons, available from Nippon Shokubai Co., Ltd., of Osaka, Japan.
11. Solubilizer available from LCW of South Plainsfield, New Jersey.
12: Chelating agent available from BASF of Mount Olive, New Jersey.
13: Allergen Control Agent: TMBA (3,4,5 trimethoxybenzoic acid), available
from Spectrum Chemicals, New Brunswick, New Jersey.
14: Solvent for allergen control agent: Benzyl alcohol, supplied by
Mallinckrodt
Baker, Phillipsburg, New Jersey.
The liquid composition may be prepared by premixing the benzoic acid with
benzyl alcohol. The remainder of the ingredients, excluding water and perfume
(if used),
are mixed together to homogeneity. The allergen control premix is added to
this mixture
and the pH of this mixture is adjusted to approximately 6.5 to 7.5. The soil
suspending
agent is then added to this mixture. The benzoic acid/alcohol premix is added
to this
mixture. If used, the perfume is then added to the mixture. The pH is then
adjusted to
approximately 5. The liquid composition may then be applied to the laminate
substrate at
approximately a loading level of between about 3 grams of liquid
composition/gram of
dry substrate to about 4 grams of liquid composition/gram of dry substrate.
Example 9
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 1. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
46
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
Example 10
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 2. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
Example 11
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 3. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
Example 12
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 4. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
Example 13
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 5. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
CA 02620678 2008-02-28
WO 2007/027904 PCT/US2006/034047
47
Example 14
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 6. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
Example 15
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 7. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
Example 16
An apertured laminate substrate PFG-97 gsm PP/cellulose composite (Style 3638
50001
010000) is moistened with the liquid cleaning solution from Example 8. The
liquid is
thoroughly distributed to achieve a loading level of approximately 3 grams of
liquid
composition/gram of dry substrate to about 4 grams of liquid composition/gram
of dry
substrate.
Example 17
An apertured laminate substrate referred to as laminate 37-2 is moistened with
the liquid
cleaning solution from Example 1. The liquid is thoroughly distributed to
achieve a
loading level of approximately 3 grams of liquid composition/gram of dry
substrate to
about 4 grams of liquid composition/gram of dry substrate.
Example 18
A spunlaced substrate (Nubtex, 64 grams/sqm comprised of 70% rayon 30%
polyester
available from BBA Nonwovens, Simpsonville SC) maybe moistened with the liquid
cleaning solution from any of Examples 1-8. The liquid should be thoroughly
distributed
to achieve the loading of 3.0 g of liquid per gram of dry substrate.
CA 02620678 2010-06-18
48
Example 19
TM
An air-laid substrate Visorb X622 (basis weight I OOg/sgm, 84% NSK Pulp, 14%
bicomponent, Buckeye Technologies, Memphis TN) may be moistened with the
liquid
cleaning solution from any of Examples 1-8. The liquid should be thoroughly
distributed
to achieve the loading of 3.0 g of liquid per gram of dry substrate.
The liquid cleaning solution may be applied to the substrate via spraying,
roll
coating, extrusion, dipping, brushing, and any other ways that would be known
to those
of ordinary skill in the art. If desired, the wipe may be folded prior to
packaging via
folding boards or other mechanical manipulation that would be known to those
of
ordinary skill in the art. Non-limiting examples of equipment systems for
applying
cleaning solutions to wipes and folding the wipes are available from Paper
Converting
Machine Company of Green Bay, Wisconsin (soon to be Barry-Wehmiller Companies,
Inc.) which are commercially available under the Viper, Mermaid, Neptune or
Calypso
trade name.
The citation of any document is not to be
construed as an admission that it is prior art with respect to the present
invention.