Note: Descriptions are shown in the official language in which they were submitted.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
STABILIZED PROTEASE COMPOSITION
Field of the invention
The present invention relates to an enzyme
composition in which the enzyme is stabilized by certain
additives in an inventive combination. More particularly,
the invention concerns a serine protease composition
comprising a reversible inhibitor to the serine protease
and an additional stabilizing agent M as defined below.
Background
Serine proteases are a group of proteolytic enzymes
characterized by having a serine and a histidine residue
in their active site. Many well known enzymes belong to
this group, for example trypsin, kallikrein, thrombin and
plasmin. Several of them have found practical use.
Trypsin is used in the leather industry. Thrombin is used
as a haemostatic agent to stop bleeding from wounds.
Urokinase and tissue plasminogen activator, two other
serine proteases, are used clinically as thrombolytic
agents in the treatment of acute myocardial infarction. A
number of these enzymes have been used extensively as
research tools, for instance in protein structure
determination. Furthermore, the enzymes are used in
various diagnostic kits.
Common to most of the serine proteases are their
limited stability in solution. This is mainly caused by
autodegradation when left in solution, caused by their
property as proteases. This limited stability is a
problem when the material has to be stored in solution.
Commercial serine protease preparations available today
are essentially always in the form of frozen solutions
orlyophilized powders, with obvious drawbacks. The extra
time needed for dissolution of the powder or thawing of
the frozen solution to the correct temperature is the
most important issue. There are, however, other problems
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
2
with these preparations. For frozen solutions, there is a
need for controlled temperatures (-20 C) in all steps
from manufacture and transportation to storage. For
lyophilized powders, there is a need for a reconstitution
solution with an acceptable grade of purity and
stability. Also, the material frequently needs to be
prepared aseptically (by mixing of the two parts) in an
environment which may be non-controlled (such as
inclement weather or lack of a clean water supply), and
there is a need to verify that the powders have been
properly mixed. These are all major drawbacks of the
products available today, adding to their complexity of
use as well as their cost.
For thrombin, which preferably has to be immediately
available for use in arresting bleeding, the stability
problems have forced manufacturers to use lyophilized
thrombin or deep frozen solutions. These then require a
certain amount of time to prepare for use. The two
thrombolytic agents urokinase and tissue plasminogen
activator are sold in the form of lyophilized
preparations that have to be dissolved before use. Since
thrombolytic treatment of acute myocardial infarction has
to be started as early as possible after onset of the
infarction, any time delay caused by such preparation is
a problem.
Many efforts have been made to find ways to
stabilize the various serine proteases. For trypsin,
which degrades itself fairly rapidly, a simple and
efficient stabilizing agent is the calcium ion (Sipos T
and Merkel J, Biochemistry 9:2766 (1970)). Decreasing the
pH to below 4 is also a method that works with some of
the enzymes, like trypsin and plasmin, but is not
feasible with thrombin, since it is irreversibly
inactivated by a pH below 5. Reversible protease
inhibitors can be used, but are less popular, since they
interfere in a detrimental fashion with the action of the
enzyme when they are used by themselves (see below).
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
3
For stabilization of tissue plasminogen activator
(tPA), addition of the amino acid arginine is
conventionally used. The tPA material in clinical use
today contains arginine as stabilizer.
Also, a lot of effort has been devoted to find ways
to stabilize thrombin solutions. As examples of
stabilizing additives, the following proposals may be
mentioned: carboxylic acids in high concentrations, EDTA,
various amino acids, albumin, polymers such as
polyethylene glycol, polyvinyl pyrrolidone and polyvinyl
alcohol, glycerol, various inorganic salts,
carbohydrates, gelatin, collagen.
Japanese patent application JP2004191367 describes a
stabilized thrombin containing test reagent for testing
blood coagulation ability. The test reagent contains
thrombin and a thrombin inhibitor, and may also comprise
one or more thrombin stabilizing compounds selected from
calcium ion, an organic acid, a surfactant and a protein.
WO 02/100830, WO 02/22575, WO 00/20394, WO 99/11658,
WO 02/37937 and US 5,409,927 all describe different
serine protease inhibiting compounds and their use in
pharmaceutical compositions for treatment of various
disease conditions, such as thrombosis, wherein
inhibition of the corresponding serine proteases is
indicated.
Nakamura et al. (J. Chrom. A, 1009, (2003), 133-139)
describe the use of an immobilized protease inhibitor for
affinity chromatography of trypsin-like proteases.
Turner at al. (Biochemistry, 25, (1986), 4929-4935)
describe three p-amidinophenyl esters that irreversibly
inhibit human factor IXa.
Tsung Fu Yang at al. (Biomacromolecules, 25, (2004),
1926-1932) describe the synthesis of a cationic polymer,
N,N-diethylethylenediamin polyurethane, for use in gene
delivery.
US patent application 2001/0033837 (corresponding to
EP 1 136 084 Al) describes a thrombin preparation
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
4
containing a non-covalently bound inhibitor as
stabilizer. Furthermore, the inhibitor is combined with
other stabilizing additives, like sugars or carboxylic
acids, which have been previously described in patents or
other publications.
JP 2000300250 describes the stabilization of
thrombin solutions by addition of polyvinyl alcohol,
gelatin or polyvinyl pyrrolidone in different buffer
solutions.
In GB 1354761, proteases and amylases are stabilized
to various extents by a number of substances, such as
aliphatic alcohols, carboxylic acids, heterocyclic
compounds containing hydroxyl groups, and aliphatic or
alicyclic amines.
Thus, stabilization of a serine protease using
inhibitors has been described (for example US
2001/0033837 and JP 2004191367, supra). The problem with
this approach is that the inhibitor strongly diminishes
the effect of the enzyme, if it is not removed prior to
use of the preparation. If a potent inhibitor is used,
most of the enzymatic activity is lost. A better approach
is to use a reversible inhibitor of intermediate
strength. However, even in this case, a considerable part
of the initial enzymatic activity will be lost as
concentration of the inhibitor is increased in order to
get a good stabilization effect.
Disclosure of the invention
It is therefore an object of the present invention
to accomplish a serine protease composition, which is
stable in solution and retains a degree of enzymatic
activity which is sufficient for practical use of the
composition.
It is another object of the present invention to
provide a serine protease composition, which is amenable
to direct use without prior steps of preparation from
deep frozen or lyophilized material.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
It is a further object of the present invention to
enable practical use of reversible inhibitors of serine
proteases for stabilization purposes, through the
provision of an additional stabilizing component.
5 These, and other objects apparent from the present
text, are attained by the different aspects of the
present invention as claimed.
Thus, one aspect of the invention provides a
stabilized serine protease composition comprising a) a
serine protease; b) a reversible inhibitor of said serine
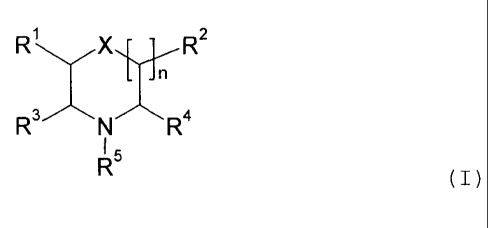
protease; and c) a stabilizing agent M having the formula
R2
N
(I)
wherein
15 n is 0, 1 or 2;
X is 0, N or CH2;
R'-R4 are the same or different, and selected from H,
-CI-12-R6, -0H2-0-R6, -CH2-S-R6, -CH2-NH-R6, -00-0-R6,
-CO-NH-R6, -0H2-NH-CO-R6, -CH2-0-CO-R6, -CH2-NH-00-NHR6,
-CH2-NH-00-0R6, -CH2-NH-CS-NHR6 and -CH2-0-CO-NHR6;
R5 is as R1-R4 or P-Q;
P is selected from -(CH2).- and -(0H2)m-Y-(CH2)m-,
wherein m is 1-6 and Y is 0, NH or S;
Q is selected from H, -SO3, -COOH, -NH2, -OH and
-CONH2;
each R6 individually being selected from H,
substituted or non-substituted lower alkyl, substituted
or non-substituted cycloalkyl, substituted or non-
substituted benzyl, substituted or non-substituted aryl
or mono-, bi-, or tricyclic unsubstituted or substituted
heteroaromatic ring(s) with one or more heteroatoms and
non-aromatic heterocycles, the substituents of the
substituted groups being selected from lower alkyl,
CA 02621059 2012-09-20
22819-623
6
halogens, substituted or non-substituted aryl, substituted or
non-substituted hetero-aromatic compounds, non-aromatic
heterocycles, alkyloxy, alkylamino; or a pharmaceutically
acceptable salt thereof. In one aspect of the invention, the
stabilizing agent is present in a concentration of 6.1 - 6.3 M.
The present invention derives from initial results
from a study on the stability of thrombin, in which it was
surprisingly found that the inventive combination of a
reversible inhibitor of the enzyme and a stabilizing agent M as
defined above had a strong stabilizing effect on the enzyme in
solution. Both the thrombin inhibitor and the stabilizing
agent M alone had stabilization effects on thrombin, but the
combination was several fold better than any of them (see
Example 1). Thus, when a low concentration of enzyme inhibitor
was combined with morpholine, MOPS or related compounds, a very
strong stabilizing effect on the enzyme was obtained. Some
tested compositions were stable, as indicated by less than 30%
decrease in activity, for more than 2 months at 37 C. This
would, according to data in prior publications and confirmed by
the present inventors, correspond to 6 months at room
temperature or 2.5 years at refrigerator temperature. The
results from the initial study were expanded to include
experiments on other serine proteases, and in these experiments
the surprising stabilizing effect was also observed.
As exemplified below, the composition according to
the invention exhibits a substantially improved stability as
compared to enzyme compositions without the inventive
combination of ingredients b) and c). With the inventive
approach, a low concentration of serine protease inhibitor may
CA 02621059 2012-09-20
22819-623
6a
be used, and a satisfactory degree of stabilization still
obtained. For example, the concentration of the inhibitor may
be lower than what has been suggested previously, e g in
US 2001/0033837. With such a low concentration of inhibitor,
much more of the enzymatic activity is retained in the
stabilized enzyme solution.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
7
It should be noted that the increase in
stabilization due to the combination of the reversible
serine protease inhibitor and stabilizing agent M is not
regarded as an additional inhibitory effect provided by
M. In fact M, as described in Illustrative Example A, may
lack any serine protease inhibiting capacity. Without
wishing to be bound by theory, the present inventors
believe that the surprisingly increased stabilizing
effect observed is achieved through a beneficial synergy
between reversible serine protease inhibitors and
stabilizing agents M of the inventive composition. The
present invention provides such a combination of a
reversible serine protease inhibitor and stabilizing
agent M in a stabilized serine protease composition and
use of such a combination for stabilizing a serine
protease composition.
In an embodiment of the invention, the serine
protease in the composition is selected from the group
consisting of trypsin, kallikrein, thrombin, plasmin,
urokinase, tissue plasminogen activator, active form of
factor IX, active form of factor X and active form of
factor XI. In a more specific embodiment, the serine
protease is thrombin. In another specific embodiment, the
serine protease is plasmin. In yet another specific
embodiment, the serine protease is trypsin.
Reversible inhibitors to serine proteases are known
to persons of skill in the art, and which one is the
optimal to use will vary depending on what specific
serine protease is used. In general, it is of importance
for the intended effect that the inhibitor is not of
great strength. In other words, the inhibitory effect has
to be moderate enough that the enzymatic activity remains
usefully high. As a guideline, it has been found that
inhibitors having a Ki of between 0.01 mM and 2 mM are
suitable for use in the composition according to the
invention, with from 0.04 mM to 0.5 mM as a preferred
range.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
8
In one embodiment, in which the serine protease is
thrombin, the reversible inhibitor may be selected from
N-(2'-phenoxy)-4-aminopyridin and derivatives thereof,
benzamidine, N,N-diethylethylenediamine,
aminobenzamidine, amidinopyridin and tert-butylamidin. In
another embodiment, in which the serine protease is
thrombin, the reversible inhibitor is selected from N-
(2'-phenoxy)-4-aminopyridin and derivatives thereof, N,N-
diethylethylenediamine, amidinopyridin and tert-
butylamidin. In a more specific embodiment, in which the
serine protease is thrombin, the reversible inhibitor is
N-(2'-phenoxy)-4-aminopyridin or a derivative thereof. In
another embodiment, in which the serine protease is
plasmin, the reversible inhibitor is selected from N,N-
diethylethylenediamine, aminobenzamidine and benzamidine.
In another embodiment, in which the serine protease is
trypsin, the reversible inhibitor is selected from
aminobenzamidine and benzamidine. These combinations of
enzymes and inhibitors are illustrative examples, and are
not to be interpreted as limiting.
In one embodiment of the invention, the value of n
in formula I is 1 or 2. In a more specific embodiment, n
in formula I is 1.
The composition according to the invention comprises
a stabilizing agent M with the general formula I given
above. In embodiments of the invention, stabilizing agent
M is a compound of formula II:
1
ROR2
I 5
(II)
wherein
R'-R4 are the same or different, and selected from H,
-CH2-R6;
R5 is as R1-R4 or P-Q;
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
9
P is selected from -(CH2)m- and -(CH2)m-Y-(CH2)m-,
wherein m is 1-6 and Y is 0, NH or S;
Q is selected from H, -SO3, -COOH, -NH2, -OH and
-00NH2;
each R6 individually being selected from H,
substituted or non-substituted lower alkyl, substituted
or non-substituted cycloalkyl, substituted or non-
substituted benzyl, substituted or non-substituted aryl
or mono-, bi-, or tricyclic unsubstituted or substituted
heteroaromatic ring(s) with one or more heteroatoms and
non-aromatic heterocycles, the substituents of the
substituted groups being selected from lower alkyl,
halogens, substituted or non-substituted aryl,
substituted or non-substituted hetero-aromatic compounds,
non-aromatic heterocycles, alkyloxy, alkylamino;
or a pharmaceutically acceptable salt thereof.
Accordingly, in some embodiments, stabilizing agent
M is a compound of formula III:
N
R5 (III)
wherein
R6 is -CH2-R6 or P-Q;
P is selected from -(CH2)m- or -(CH2)m-Y-(CH2)m-,
wherein m is 1-6 and Y is 0, NH or S;
Q is selected from Hr -S03, -COOHr -NH2r -OH and
-CONH2.
each R6 individually being selected from substituted
or non-substituted lower alkyl, substituted or non-
substituted cycloalkyl, substituted or non-substituted
benzyl, substituted or non-substituted aryl, the
substituents of the substituted groups being selected
from lower alkyl, halogens, substituted or non-
substituted aryl, substituted or non-substituted hetero-
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
aromatic compounds, non-aromatic heterocycles, alkyloxy,
alkylamino;
or a pharmaceutically acceptable salt thereof.
In some embodiments of the invention, stabilizing
5 agent M is selected from the group consisting of
morpholine, 3-(N-morpholino)propanesulfonic acid (MOPS),
morpholino butyl sulphonic acid, morpholino propyl
carboxylic acid, morpholino ethyl alcohol and morpholino
ethyl sulphonic acid. Thus, examples of compounds M for
10 use in the compositions of this aspect of the invention
are morpholine and 3-(N-morpholino)propanesulfonic acid
(MOPS). In a specific embodiment of the invention,
stabilizing agent M is morpholine.
A composition according to the invention which shows
the stabilization effect is one in which the serine
protease is thrombin, the reversible inhibitor is N-(2'-
phenoxy)-4-aminopyridin, and stabilizing agent M is
morpholine.
Another composition according to the invention which
shows the stabilization effect is one in which the serine
protease is thrombin, the reversible inhibitor is N-(2'-
phenoxy)-4-aminopyridin, and stabilizing agent M is 3-(N-
morpholino)propanesulfonic acid (MOPS).
Another composition according to the invention which
shows the stabilization effect is one in which the serine
protease is thrombin, the reversible inhibitor is
aminobenzamidine, and stabilizing agent M is morpholine.
Another composition according to the invention which
shows the stabilization effect is one in which the serine
protease is plasmin, the reversible inhibitor is N,N-di-
ethylethylenediamine and stabilizing agent M is
morpholine.
Another composition according to the invention which
shows the stabilization effect is one in which the serine
protease is plasmin, the reversible inhibitor is
aminobenzamidine and stabilizing agent M is morpholine.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
11
In serine protease compositions for topical
administration, e g to a wound site, it has been a
problem that the composition can easily flow or be rinsed
from the site where it is applied. In order to solve this
problem, it is possible to add to the enzyme composition
an adhesive polymer, which then serves the purpose of
making the composition more viscous and adherent to skin
or wound sites. As an embodiment of the present
invention, such an addition of an adhesive polymer to the
inventive composition may have an additional unexpected
and beneficial effect on its stability. The addition of a
polymer then serves the double purpose of increasing the
viscosity and adhesiveness of the composition, at the
same time as it helps the stabilization of the enzyme
even further.
In some embodiments of the invention, the
composition further comprises a viscous and adhesive
polymer selected from polysaccharides and gelatin. Thus,
the polymer may for example be a polysaccharide, such as
selected from starch, its derivatives, cellulose, its
derivatives, and mixtures thereof. Specific, non-limiting
examples of starches useful as additives to the
composition according to the invention include corn
starch and potato starch and mixtures thereof, whereas
non-limiting examples of useful cellulose derivatives are
carboxymethyl cellulose and ethyl hydroxyethyl cellulose
and mixtures thereof. In a specific embodiment the
polysaccharide is carboxymethyl chitosan. In further
embodiments of the invention, said polysaccharide is
present in a concentration of 0.1-5 %. However, it is
also envisaged that the polymer is gelatin, such as
gelatin from a cold water fish. In some embodiments of
the invention, said gelatin is present in a concentration
of 0.5-20 %.
In one embodiment of the invention, said serine
protease is present in a concentration of 0.001-2 mg/ml.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
12
In a more specific embodiment, said serine protease is
present in a concentration of 0.01-1 mg/ml.
In one embodiment of the invention, in which the
serine protease is thrombin, the concentration of
thrombin is between 5-3500 activity units/ml.
In one embodiment of the invention, in which the
serine protease is thrombin, the concentration of
thrombin is between 200-1000 activity units/ml. In one
embodiment of the invention, in which the serine protease
is thrombin, the concentration of thrombin is between 5-
activity units/ml.
In one embodiment of the invention, said reversible
inhibitor of said serine protease is present in a
concentration of 0.1-10 mM. In a more specific
15 embodiment, said reversible inhibitor of said serine
protease is present in a concentration of 0.5-2 mM.
In one embodiment of the invention, said stabilizing
agent M is present in a concentration of 0.02-0.5 M. In a
more specific embodiment, said stabilizing agent M is
20 present in a concentration of 0.1-0.3 M.
According to another aspect thereof, the present
invention provides use of a composition as described
above as a medicament. ,
Another aspect of the invention concerns use of said
composition, in which the serine protease is thrombin,
for the preparation of a medicament for establishing
haemostasis in a subject suffering from a bleeding. A
related aspect of the invention provides a method for
establishing haemostasis in a subject suffering from a
bleeding, comprising applying a composition according to
the invention, in which composition the serine protease
is thrombin, to the site of bleeding in an amount
sufficient to diminish or stop said bleeding.
In connection with such a use or method employing a
thrombin composition according to the invention as a
medicament, the stability of the inventive composition
offers benefits in the circumstances in which it is used.
CA 02621059 2008-03-03
WO 2007/035143
PCT/SE2005/001391
13
Often, thrombin compositions are used in the context of
emergency situations, wherein it is crucial to stop
subjects from bleeding. In these same situations, the use
of conventional, haemostatic thrombin preparations is
difficult, since they often require cumbersome and time-
consuming steps of thawing (if frozen) and/or dissolution
(if lyophilized). The present invention enables the
production of e g such haemostatic agents in the form of
solutions, whose stability is such that they can readily
be stored during extended periods of time, for example in
an ambulance or an emergency helicopter, until needed at
the site of an accident or the like. At this time, they
may be used as is, without any delay due to preparation.
The conventional preparations used to stop bleeding
contain fairly high concentrations of thrombin, between
200-1000 activity units/ml. In connection with plastic
surgery applications, this is seen as a risk for
increased scar formation. Low thrombin concentration
solutions are presently prepared in the clinic by
dilution of concentrated thrombin solutions. No ready to
use preparation is available. Therefore, in a further
aspect thereof, the present invention provides a
stabilized thrombin composition with a considerably lower
concentration of thrombin, between 5-20 activity
units/ml, and use thereof in plastic surgery.
Another aspect of the invention exploits the known
properties of plasmin, urokinase or tPA as thrombolytic
agents. Thus, the invention provides use of a composition
as described above, in which the serine protease is
selected from plasmin, urokinase and tissue plasminogen
activator, for the preparation of a medicament for
thrombolytic treatment. A related aspect provides a
method for thrombolytic treatment in a subject in need
thereof, comprising administering a composition as
described above, in which composition the serine protease
is selected from plasmin, urokinase and tissue
plasminogen activator, to the subject in an amount
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
14
sufficient for said treatment. In these two related
aspects, the thrombolytic treatment in question may, as
non-limiting examples, be performed in order to treat
myocardial infarction or in order to treat stroke.
As mentioned in the context of the composition
aspect of the invention, the increase in stabilization
due to the combination of the reversible serine protease
inhibitor and stabilizing agent M is not regarded as an
additional inhibitory effect provided by M. In fact M, as
described in Illustrative Example A, may lack any serine
protease inhibiting capacity. Without wishing to be bound
by theory, the present inventors believe that the
surprisingly increased stabilizing effect observed is
achieved through a beneficial synergy between reversible
serine protease inhibitors and stabilizing agents M of
the inventive composition.
Therefore, in another aspect thereof, the invention
provides the use of a combination of
a) a reversible serine protease inhibitor and
b) a stabilizing agent M of formula I:
RçXç R2
R5 (I)
wherein
n is 0, 1 or 2;
X is 0, N or CH2;
R1-R4 are the same or different, and selected from H,
-CH2-R6, -CH2-0-R6, -0H2-S-R6, -CH2-NH-R6, -00-0-R6,
-CO-NH-R6, -CH2-NH-CO-R6, -0H2-0-CO-R6, -0H2-NH-CO-NHR6,
-CH2-NH-00-0R6, -CH2-NH-CS-NHR6 and -CH2-0-00-NHR6;
R5 is as R'-R4 or P-Q;
P is selected from -(CH2).- and -(CH2)m-Y-(CH2)m-,
wherein m is 1-6 and Y is 0, NH or S;
Q is selected from H, -E03, -COOH, -NH2, -OH and
-CONH2;
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
each R6 individually being selected from H,
substituted or non-substituted lower alkyl, substituted
or non-substituted cycloalkyl, substituted or non-
substituted benzyl, substituted or non-substituted aryl
5 or mono-, bi-, or tricyclic unsubstituted or substituted
heteroaromatic ring(s) with one or more heteroatoms and
non-aromatic heterocycles, the substituents of the
substituted groups being selected from lower alkyl,
halogens, substituted or non-substituted aryl,
10 substituted or non-substituted hetero-aromatic compounds,
non-aromatic heterocycles, alkyloxy, alkylamino; or a
pharmaceutically acceptable salt thereof;
for stabilizing a serine protease composition,
wherein the reversible serine protease inhibitor and the
15 stabilizing agent M act in synergy to provide a serine
protease stabilizing effect.
In this inventive use of a combination of a
reversible serine protease inhibitor and a stabilizing
agent for stabilization of a serine protease composition,
the choices of particular components that may be used and
substituents for compounds M are as discussed above in
relation to the composition aspect of the invention.
In yet another aspect, the invention provides a
method for the stabilization of a serine protease, which
comprises admixing the serine protease with a) a
reversible inhibitor of said serine protease; and b) a
stabilizing agent M of formula I:
RXf R2
Fin
I 5
(I)
wherein
n is 0, 1 or 2;
X is 0, N or CH2;
R1-R4 are the same or different, and selected from H,
-CH2-R6, -CH2-0-R6, -CH2-S-R6, -CH2-NH-R6,
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
16
-CO-NH-R6, -CH2-NH-CO-R6, -CH2-0-CO-R6, -CH2-NH-CO-NNR6,
-CH2-NH-CO-0R6, -CH2-NH-CS-NHR6 and -CH2-0-CO-NHR6;
R5 is as R1-R4 or P-Q;
P is selected from -(CH2)m- and -(CH2)m-Y-(CH2)m-,
wherein m is 1-6 and Y is 0, NH or S;
Q is selected from H, -SO3, -COOH, -NH2, -OH and
-CONH2;
each R6 individually being selected from H,
substituted or non-substituted lower alkyl, substituted
or non-substituted cycloalkyl, substituted or non-
substituted benzyl, substituted or non-substituted aryl
or mono-, bi-, or tricyclic unsubstituted or substituted
heteroaromatic ring(s) with one or more heteroatoms and
non-aromatic heterocycles, the substituents of the
substituted groups being selected from lower alkyl,
halogens, substituted or non-substituted aryl,
substituted or non-substituted hetero-aromatic compounds,
non-aromatic heterocycles, alkyloxy, alkylamino;
or a pharmaceutically acceptable salt thereof.
In this inventive method for stabilization of a
serine protease composition, the choices of particular
components that may be used and substituents for
compounds M are as discussed above in relation to the
composition aspect of the invention.
A further aspect of the invention concerns the use
of the composition as described above for adsorption onto
a solid object, in order that this solid object may
provide the enzymatic activity in question. In
particular, it is of interest in many surgical
applications to enter and, in particular, exit arteries
while inflicting as little damage from bleeding as
possible. In order to stop bleeding from an artery, it
has previously been suggested to use a form of "arterial
plug" (such objects are also known as vascular sealing
devices, femoral access closure devices (when the femoral
artery is used for entry, e g in angiography), vascular
hemostasis devices and puncture closure devices), for
CA 02621059 2008-03-03
WO 2007/035143
PCT/SE2005/001391
17
example made from collagen or another biodegradable
material. According to the present aspect of the
invention, such a plug may advantageously be coated with
a composition according to the invention, in which the
serine protease is thrombin. Such a plug achieves faster
sealing of the opening of the artery, in that the
thrombin of the composition aids in blood clotting around
the plug. Thus, the invention provides, in this aspect, a
vascular haemostasis device having an amount of the
composition according to the invention, in which the
serine protease is thrombin, adsorbed on it. The vascular
haemostasis device is preferably made from a
biodegradable solid or semi-solid material, such as
collagen, chitosan or other biological polymer.
Another aspect of the invention concerns the novel
identification of N,N-diethylethylenediamine as a serine
protease inhibitor. Thus, in this aspect, the invention
provides use of N,N-diethylethylenediamine as an
inhibitor of a serine protease, as well as a method of
inhibiting a serine protease, comprising admixing
therewith an inhibitory amount of N,N-diethylethylene-
diamine. In some embodiments of this aspect of the
invention, the serine protease is plasmin. In other
embodiments of this aspect of the invention, the serine
protease is thrombin.
It is generally preferred, for the realization of
all the advantages of the invention's different aspects,
that the composition according to the invention is in a
form selected from a solution and a gel. In this regard,
aqueous solutions and aqueous gels are more preferred.
Definitions
As used herein, the term "lower alkyl" means an
unbranched or branched, cyclic, saturated or unsaturated
(alkenyl or alkynyl) hydrocarbyl radical which may be
substituted or unsubstituted. Where cyclic, the alkyl
group is preferably C3-C12, more preferably 05-C10, most
CA 02621059 2008-03-03
WO 2007/035143
PCT/SE2005/001391
18
preferably 05-07. Where acyclic, the alkyl group is
preferably 01-010, more preferably C1-C6, more preferably
methyl, ethyl, propyl (n-propyl, isopropyl), butyl
(branched or unbranched) or pentyl, most preferably
methyl.
As used herein, the term "aryl" means an aromatic
group, such as phenyl or naphthyl, or a mono-, bi-, or
tricyclic heteroaromatic group containing one or ore
heteroatom(s) preferably selected from N, 0 and S, such
as pyridyl, pyrrolyl, quinolinyl, furanyl, thienyl,
oxadiazolyl, thiadiazolyl, thiazolyl, oxazolyl,
pyrazolyl, triazolyl, tetrazolyl, isoxazolyl,
isothiazolyl, imidazolyl, pyrimidinyl, indolyl,
pyrazinyl, indazolyl, pyrimidinyl, thiophenetyl, pyranyl,
carbazolyl, acridinyl, quinolinyl, benzoimidazolyl,
benzthiazolyl, purinyl, cinnolinyl, pterdinyl.
As used herein, the term "functional group" means,
in the case of unprotected: hydroxy-, thiolo-,
aminofunction, carboxylic acid, and in the case of
protected: lower alkoxy, N-, 0-, S- acetyl, carboxylic
acid ester.
As used herein, the term "heteroaryl" means an
aromatic group containing one or more heteroatom(s)
preferably selected from N, 0 and S, such as pyridyl,
pyrrolyl, quinolinyl, furanyl, thienyl, oxadiazolyl,
thiadiazolyl, thiazolyl, oxazolyl, pyrazolyl, triazolyl,
imidazolyl, pyrimidinyl, indolyl, pyrazinyl or indazolyl.
As used herein, the term "non-aromatic heterocycle"
means a non-aromatic cyclic group containing one or more
heteroatom(s) preferably selected from N, 0 and S, such
as a cyclic amino group such as pyrrolidinyl, piperidyl,
piperazinyl, morpholinyl or a cyclic ether such as
tetrahydrofuranyl, monosaccharide.
As used herein, the term "halogen" means fluorine,
chlorine, bromine or iodine.
As used herein, the term "substituted" means that
the groups concerned are substituted with a functional
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
19
group such as hydroxyl, amine, sulf...le, silyl, carboxylic
acid, halogen, aryl, etc.
Examples of pharmaceutically acceptable addition
salts for use in the compositions of the present
invention include those derived from mineral acids, such
as hydrochlorid, hydrobromic, phosphoric, metaphosphoric,
nitric and sulphuric acids, and organic acids, such as
tartaric, acetic, citric, malic, lactic, fumaric,
benzoic, glycolic, gluconic, succinic, and arylsulphonic
acids. Pharmaceutically acceptable excipients described
herein, for example, vehicles, adjuvants, carriers or
diluents, are well known to those who are skilled in the
art and are readily available to the public. A
pharmaceutically acceptable carrier may be one which is
chemically inert to the active compounds and which have
no detrimental side effects or toxicity under the
conditions of use. Pharmaceutical formulations may be
found e g in Remington: The Science and Practice of
Pharmacy, 19th edition, Mack Printing Company, Easton,
Pennsylvania (1995).
As detailed in the description of the invention, a
possible choice of inhibitor for use in the composition
and methods according to the invention is "N-(2'-
phenoxy)-4-aminopyridin and derivatives thereof". By this
is meant a compound having the formula IV:
R4
X R3
R2 (IV)
wherein
Rl is selected from H, C1-C6-alkyl, C3-C7-cyclo
alkyl, phenyl, benzyl acetyl and benzoyl;
X is selected from oxygen, nitrogen and sulfur;
R2 and R2 is each individually selected from H,
halogen, hydroxyl, C1-C6-alkyl, C3-C7-cyclo alkyl, C1-C6-
alkyloxy; and
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
R4 is selected from H, C1-C6-alkyl, arylalkyl and
acyl.
Preferred such inhibitors have the formula V:
R3
)cN
R2 (V)
5 wherein
Rl is selected from C1-C6-alkyl, C3-07-cyclo alkyl,
phenyl and benzyl;
R2 and R3 is each individually selected from H,
halogen, hydroxyl, 01-06-alkyl, 03-C7-cyclo alkyl and 01-
10 06-alkyloxy.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
21
Examples
The following examples illustrate the invention, and
are not to be interpreted as limiting.
In the following description of experiments
conducted in accordance with the present invention, the
time it takes to reach 70% of the initial activity is
used as a numerical value for the stability of an enzyme
solution. This value, denoted "T 70%", is chosen since it
corresponds to what could be accepted as a maximum
permitted loss in activity during a life span of a
commercial product.
In the experimental studies, a high temperature
(37 C) has been used, as well as a high concentration of
enzyme. This has been made in order to obtain stability
data in reasonably short times, and not have to wait for
months or years. The stability's dependency on
temperature has been studied, and the results given in
Example 1. This study showed that the inactivation
process is about 3 times slower at room temperature, and
around 20 times slower at refrigerator temperature, than
the process actually measured (at 37 C).
Furthermore, the inactivation process is
concentration dependent, and is more rapid at higher
concentrations of the enzyme. The concentration of
thrombin used in Example 1 was 1 mg/ml (or 3300
units/ml), i e higher than the 0.1-0.3 mg/ml used in
present commercially available preparations and/or
devices. Studies of the concentration dependency has
shown that the inactivation process is 3-4 times slower
at those concentrations as compared to the concentration
used in Example 1.
Taking this together would give a factor of between
10-12, with which to multiply the T 70% value in order to
arrive at what corresponds to room temperature storage
conditions for a commercial product containing a serine
protease, such as a haemostatic preparation containing
thrombin. In Table 1, the composition with N-(2'-
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
22
phenoxy)-4-aminopyridine and MOPS has a T 70% of 120
days. That would correspond to a value, for a 0.1-0.3
mg/ml product, of more than 1200 days in room temperature
conditions, i e more than three years. This clearly
exceeds anything previously accomplished, regarding the
stabilization of thrombin in solution.
Example 1 - Stabilization of human thrombin
To determine the coagulant activity of thrombin
solutions, the time to clotting of a fibrinogen solution
(1.3 mg/ml) after additions of various dilutions of a
solution of a particular human thrombin (derived from
plasma, 3300 units/mg, Biovitrum AB, Sweden) was
measured. The clotting times were measured using an
Amelungen Kc 1 coagulometer (Amelungen, Germany). To
study the stabilities of thrombin solutions with
different additives, the samples were incubated in a
thermostat chamber kept at 37 C. Aliquots were taken out
at various time intervals, and the remaining thrombin
activity in these aliquots was measured. From the values
obtained, activity decay curves could be constructed.
Activity decay curves of 1 mg/ml thrombin solutions
in 10 mM HEPES, 0.13 M NaC1 buffer, pH 7.4, showed T 70%
values around 1.6 days at 37 C. Corresponding
experiments at room temperature (around 21 00) showed a T
70% value of 5.4 days, whereas after refrigerator storage
(around 5 00), the T 70% value was 36 days. Thus, as
expected, there is a strong temperature dependency.
Solutions containing 1 mg/ml of thrombin in 10 mM
HEPES and 0.13 M NaC1 at pH 7.4 with the indicated
stabilizing additive(s) were put in the thermostat
chamber, and their activity decay curves were determined.
The results obtained are shown in Table 1. Data on the
corresponding 1 mg/ml thrombin solution without additives
is included for comparison.
CA 02621059 2008-03-03
WO 2007/035143
PCT/SE2005/001391
23
Table 1
Stabilization of thrombin
Stabilizing additive(s) T 70%
(days)
None 1.6
0.20 M MOPS , 7.5
0.20 M morpholine 8.5
0.20 M morpholino butyl sulphonic acid 4.0
0.20 M morpholino propyl carboxylic acid 4.1
0.20 M morpholino ethyl alcohol 3.8
0.20 M morpholino ethyl sulphonic acid 3.2
0.5 mM aminobenzamidine 20
0.20 M MOPS +
68
0.5 mM aminobenzamidine
3.1 mM N-(2'-phenoxy)-4-aminopyridin 74
1.9 mM N-(2'-phenoxy)-4-aminopyridin 35
1.9 mM N-(2'-phenoxy)-4-aminopyridin +
68
0.5 mM aminobenzamidine
0.20 M MOPS +
120
1.9 mM N-(2'-phenoxy)-4-aminopyridin
0.20 M N,N-diethylethylenediamine 10
0.20 M MOPS +
22
0.20 M N,N-diethylethylenediamine
It is evident that the tested compounds all have a
stabilizing effect. However, there is a synergistic
effect of combinations of inhibitor and morpholine-
containing compound in accordance with the invention, as
evidenced by the superior results obtained with such com-
binations. As the table above illustrates, the addition
of 0.20 M MOPS alone gives an increase in stabilization
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
24
by a factor of 4.7, and the addition of 0.5 mM of the
reversible thrombin inhibitor aminobenzamidine gives a
stabilization increase by a factor of 12.5. The inventive
combination, however, stabilizes the thrombin composition
much better, by a factor of 42.5. The inventive
combination of MOPS and N,N-diethylethylenediamine is
also better at stabilizing the enzyme than the individual
components. Likewise, the combination of 0.20 M MOPS and
1.9 mM N-(2'-phenoxy)-4-aminopyridin is seen to confer a
very high stabilization increase, a factor of 75, whereas
the individual components increase stability by a factor
of 4.7 and 22, respectively.
The thrombin used in the study is human thrombin
derived from plasma. Recombinant human thrombin has also
been studied and has essentially shown the same behavior.
Example 2 - Stabilization of bovine thrombin
Stabilization of bovine thrombin was studied. The
experimental setup was the same as in Example 1, but the
concentration of bovine thrombin (Baxter) used was 0.4
mg/ml. Upon storage at 37 C, the thrombin solution in
HEPES buffer showed a T 70% value of 1.3 days. The
thrombin solution in HEPES buffer plus 3.0 mM N-(2'-
phenoxy)-4-aminopyridin and 0.20 M MOPS showed a T 70%
value of 54 days.
The results obtained show that bovine thrombin is
somewhat more labile than the preparations of human
thrombin studied, but that a very good stabilizing effect
is nevertheless obtained by the compositions of the
invention.
Example 3 - Low concentrations of thrombin
Stabilization of thrombin in compositions containing
low concentrations of thrombin was studied. The
stabilizing effect of the compositions according to the
invention was demonstrated to work also for comparatively
low concentrations of thrombin.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
A 15.0 activity units/ml solution of human thrombin
(derived from plasma, 3300 units/mg, Biovitrum AB,
Sweden)in HEPES buffer, pH 7.4, showed a T 70% value of
23 days. The corresponding solution in HEPES buffer, pH
5 7.4, plus 2.0 mM N-(2'-phenoxy)-4-aminopyridin and 0.20 M
MOPS exhibited a T 70% value of 92 days.
Example 4 - Stabilization of plasmin
The stabilization of solutions of plasmin in
10 accordance with the invention was tested. The activity of
plasmin was determined using chromogenic peptide
substrate Chromozym TH (Pentapharm, Switzerland) and
measurement of absorbance change at 405 nm in a
spectrophotometer. Solutions containing 100 pg/ml of
15 plasmin (specific activity 3.2 units/mg, Sigma-Aldrich)
in 10 mM HEPES and 0.13 M NaC1, pH 7.4, as well as
stabilizers as indicated in Table 2 below were incubated
at 37 C, and samples were taken out at various time
intervals for activity determination. The results
20 obtained are shown in Table 2.
Table 2
Stabilization of plasmin
Stabilizing additive(s) T 70%
(hours)
None 3
0.20 M morpholine 12
0.13 M N,N-diethylethylenediamine 8
1 mM benzamidine 16
1.3 mM aminobenzamidine 52
0.20 M morpholine +
22
0.13 M N,N-diethylethylenediamine
0.20 M morpholine +
216
1.3 mM aminobenzamidine
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
26
From these results, it is evident that a very strong
stabilization is obtained using the combination in
accordance with the invention. 0.20 M morpholine
increases the stability of the plasmin composition by a
factor of 4, 0.13 M N,N-diethylethylenediamine by a
factor of 2.7 and 1.3 mM aminobenzamidine by a factor of
17. However, the combination of morpholine and N,N-
diethylethylenediamine increases the stability of the
plasmin composition by a factor of 7.3, and the
combination of morpholine and aminobenzamidine increases
the stability by a factor of 72.
Example 5 - Stabilization of trypsin
The stabilization of solutions of trypsin in
accordance with the invention was tested. The activity of
trypsin was determined using tosyl arginine methyl ester
(TAME) as substrate and measuring the absorbance change
at 247 nm in a spectrophotometer. Solutions containing
100 pg/m1 of trypsin (TPCK-treated, Sigma-Aldrich) in 10
mM HEPES and 0.13 M NaCl, pH 7.4, as well as stabilizers
as indicated in Table 3 below were incubated at 37 C,
and samples were taken out at various time intervals for
activity determination. The results obtained are shown in
Table 3.
Table 3
Stabilization of trypsin
Stabilizing additive(s) T 70%
(hours)
None 0.6
0.5 M morpholine 8
1 mM benzamidine 43
0.5 M morpholine +
88
1 mM benzamidine
CA 02621059 2008-03-03
WO 2007/035143
PCT/SE2005/001391
27
Again, the stabilizing effect is the greatest in the
composition according to the invention. Thus, 0.5 M
morpholine alone gives an increase in stabilization by a
factor of 13, and 1 mM benzamidine alone gives an
increase in stabilization by a factor of 72. The
inventive combination, on the other hand, gives an
increase in stabilization by a factor of 147.
Example 6 - Stabilization of thrombin with CMC
Tests of thrombin solutions containing between 1.0
and 2.0% carboxymethyl cellulose (CMC) for their
adhesiveness to human skin showed that the addition of
CMC increased both viscosity and adhesiveness strongly.
Surprisingly, however, it was also found that the
stability of these thrombin solutions was further
increased. A 1 mg/ml human thrombin (derived from plasma,
3300 units/mg, Biovitrum AB, Sweden)solution in 0.5 mM
aminobenzamidine, 0.20 M MOPS, 10 mM HEPES, 0.13 M NaCl
being 2.0% with respect to CMC was incubated at 37 C,
and the activity decay curve was determined. The T 70%
value obtained was 175 days.
Example 7 - Stabilization with other adhesive polymers
Four other polymers were also studied: ethyl
25, hydroxyethyl cellulose (EHEC), potato starch, corn starch
and cold water fish gelatin. All four of these polymers
increased the adhesiveness and viscosity of thrombin
solutions. The compatibility and stability of thrombin
solutions with the polymers were further studied by
incubation at 37 C of 1 mg/ml human thrombin (derived
from plasma, 3300 units/mg, Biovitrum AB, Sweden)
solutions in 0.20 M MOPS, 0.5 mM aminobenzamidine, 10 mM
HEPES, 0.13 M NaC1, pH 7.4, containing the various
polymers. The concentrations of the polymers used were:
EHEC, 0.6%; the two different starches, 4.0%; and
gelatin, 12.8% . EHEC was fully compatible with thrombin,
and the same T 70% value, i.e. around 65 days, was
CA 02621059 2008-03-03
WO 2007/035143
PCT/SE2005/001391
28
obtained as with the corresponding solution without EHEC.
The starch containing solutions had T 70% values of 22
and 26 days. The stability of thrombin was very good in
gelatin with a T 70% value of more than 90 days, which
demonstrates additional stabilizing effect of cold water
fish gelatin.
Example 8 - Bleeding experiments
The ability of inventive compositions to stop
bleeding was tested in a series of experiments on
rabbits. The model chosen was incisions in the liver
which is a frequently used model. The abdomen of the
rabbit was opened and the liver exposed. Standardized
cuts of 3 mm length were made in the liver surface and an
0.10 ml amount of test solution was applied to the wound
using a syringe. The time to haemostasis was measured.
10-12 experiments were performed with each solution. An
average value of bleeding time was calculated after
removal of the highest and the lowest value in each
series of experiments. For comparison, the commonly used
haemostatic agent Tisseel (Baxter), a fibrin glue, was
also included in the study. Tisseel was used essentially
according to the manufacturer's recommendations. 0.2 ml
of solution was applied to each wound using a double
syringe with a mixing chamber. The results obtained are
given in Table 4 below.
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
29
Table 4
Bleeding experiments
Composition
Average bleeding time (s)
mM HEPES, 0.13 M NaCl, pH 7.4 106
10 mM HEPES, 0.13 M NaCl, pH 7.4 +
1.5% CMC
10 mM HEPES, 0.13 M NaC1, pH 7.4 +
1.5% CMC + 0.20 M MOPS + 1000 31
units/ml thrombin
10 mM HEPES, 0.13 M NaC1, pH 7.4 +
2 mM aminobenzamidine + 0.20 M 26
MOPS + 1000 units/ml thrombin
Tisseel (Baxter) 0.2 ml solution 31
As is evident from these results, the thrombin
solution stabilized according to the invention is the
5 most effective in quickly establishing haemostasis in a
bleeding subject, comparable to or better than a commonly
used agent.
Example 9 - Compatibility with porous materials
10 A solution containing 0.4 mg/ml human thrombin
(derived from plasma, 3300 units/mg, Biovitrum AB,
Sweden) in 10 mM HEPES, 0.14 M NaCl, 0.5 mM
aminobenzamidine, 0.20 M MOPS of pH 7.4 was adsorbed into
a piece of polyurethane plaster (marketed as Ligasone by
15 Hartmann Scandicare AB, Anderstorp, Sweden). An amount of
solution sufficient to saturate the polyurethane piece
was used. The piece was transferred to a tube, which was
then closed to prevent evaporation. The tube was kept at
37 C, and samples of solution were taken out at
20 intervals by a slight pressure on the polyurethane piece.
The activity decay curve showed a T 70% value of 74 days,
corresponding to a stability increase by a factor of 46.
CA 02621059 2008-03-03
WO 2007/035143
PCT/SE2005/001391
Example 10 - Adsorption of enzyme onto a solid phase
Adsorption of thrombin in stabilizing solutions to
surfaces was tested. Solid flakes of chitosan (at least
85 % deacetylated, Sigma-Aldrich), around 3 x 3 mm, were
5 incubated for 10 minutes in solutions of 400 units/ml
human thrombin (derived from plasma, 3300 units/mg,
Biovitrum AB, Sweden) in 10 mM HEPES, 0.13 M NaC1, pH
7.4. The solutions had the following additions: 1) none,
2) 0.10 M morpholine, 2 mM N-(2'-phenoxy)-4-amino-
10 pyridine, 3) 0.10 M morpholine, 2 mM N-(2'-phenoxy)-4-
aminopyridine, 0.5 % carboxymethyl cellulose. The flakes
were then taken up and dried on filter paper. To get a
measure of thrombin clotting activity, a flake was put in
a test tube and 0.4 ml of 1.3 mg/ml fibrinogen solution
15 was added. To improve clot detection, the tube also
contained a small steel ball. The clotting times obtained
initially on flakes from the various incubation mixtures
varied between 1 to 4 minutes. After incubation in
Eppendorf tubes at 37 C for 7 days, the clotting times
20 for flakes incubated in solution 1) were strongly
prolonged. The values were between 24 and 27 minutes. In
contrast, the clotting times for flakes incubated in
solutions 2) and 3) were in the range of from 1 to 2.5
minutes, i e the same as the starting values. Evidently,
25 a strong stabilization of thrombin activity is obtained
by using solutions 2) and 3). To test the in vivo
haemostatic activity chitosan flakes incubated in
solution 3) were applied to wounds in rabbit liver
according to the animal model described in Example 8. The
30 average time to haemostasis was 27 seconds (based on six
experiments).
Illustrative Example A - M'opholine is not a thrombin
inhibitor
The possibility that morpholine is an inhibitor of
thrombin was evaluated. The fibrinogen clotting activity
of thrombin is usually measured by clotting tests,
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
31
wherein the time to coagulation of a fibrinogen solution
is detected by mechanical or optical devices. The
clotting tests in this experimental setup were performed
in 0.01 M HEPES , 0.13 M NaC1 buffer of pH 7.4, which is
according to standard procedure (EU Pharmacopeia). A
human thrombin (derived from plasma, 3300 units/mg,
Biovitrum AB, Sweden) solution containing 89 units/ml was
used and dilutions of 1/5, 1/10 and 1/16 were tested.
Solutions of various concentrations of morpholine were
prepared in the HEPES buffer by adding a concentrated
morpholine solution adjusted to pH 7.4. When morpholine
is dissolved in water, the pH goes up to 9-10, so HC1 was
added to get a pH of 7.4. That also increased the ionic
strength of the solution. Table I shows the results
obtained. Observed clotting times were converted to
concentrations of thrombin using a standard curve.
Table 5
Inhibitory effect of morpholine
Morpholine NaC1 added Apparent thrombin
Apparent
concentration concentration
inhibition
(M) (M) (U/ml) (%)
0 0 89 0
0.05 0 87 4
0.10 0 71 20
0.15 0 49 45
0.20 0 51 43
0 0.05 74 17
0 0.10 53 40
0 0.15 58 34
0 0.20 55 37
As evident from these results, there was a prolongation
of clotting times with increasing concentration of
morpholine up to a certain level. However, the same thing
was observed when the ionic strength was increased with
CA 02621059 2008-03-03
WO 2007/035143 PCT/SE2005/001391
32
NaCl, and a similar profile was exhibited. Thus, the
prolongation effect was, in all likelihood, caused by the
increase in ionic strength. Further, it is known that an
increase of ionic strength from 0.15 M to 0.22 M causes a
change in the polymerization of fibrin (B. Blomback,
Thrombosis Research, vol. 83, (1996), p.1-75, especially
p.18). This actually corresponds to the range studied in
this experimental series, in which the initial
concentration of NaC1 was 0.13 M and then increased to
0.18 M and onwards up to 0.33 M. This also corresponds to
the plateau level observed. In conclusion, morpholine
itself is not an inhibitor of thrombin.