Note: Descriptions are shown in the official language in which they were submitted.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
1
OPHTHALMIC SYRINGE
RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application Serial Number
60/717,865 filed September 16, 2005, Attorney Docket No. EYE-036P, which is
hereby
incorporated in its entirety by reference.
FIELD OF THE INVENTION
The present invention relates to metliods of adininistering ophthalmic
medicines and
devices related thereto. In particular, the invention relates to intravitreous
injection using an
ophthahnic syringe and needle.
BACKGROUND OF THE INVENTION
Intravitreous (IVT) injection has been used in the treatment of huinan ocular
disease
for nearly a century beginning in 1911 as means to introduce air for retinal
tamponade and
repair of detachment (J. Ohm, Albrecht von Graefes Arch Ophthalmol 1911;
79:442-450).
Over the past two decades, the use of intravitreous injection has gained
increasing acceptance
in the therapeutic management of many intraocular diseases, particularly
disorders affecting
the posterior segment of the eye (Jager et al., Retina 24:676-698, 2004). IVT
injection is
increasingly being incorporated into management of ocular diseases and the
number of
approved products for IVT injection is anticipated to grow on the basis of
promising results
from ongoing clinical studies. Currently formivirsen sodium (Vitravene ,
Novartis AG,
Basel, Switzerland), ranibizumab injection (LucentisTM, Genentech, Inc., South
San Francisco,
CA) and pegaptanib sodium (Macugeng, (OSI) Eyetech, Inc. NY, NY) are three
medicines
approved by the Food and Drug Administration as IVT injections.
Advantages of IVT injection of medicines and diagnostics include the
achievement of
maximum vitreous concentrations while minimizing toxicity attributed to
systemic
administration. While these advantages are becoming widely appreciated, the
ophthalmology
community turns its focus to various complications potentially associated with
IVT injection.
Risks of IVT injection, some vision threatening, include endophthalmitis,
retinal detachinent,
iritis/uveitis, inflammation, intraocular hemorrhage, ocular hypertension,
hypotony,
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
2
pneumatic retinopexy, and cataract (R.D. Jager et al., Retina 24:676-698, 2004
and C.N. Ta,
Retina, 24:699-705, 2004).
Endophthalmitis is a condition in which the tissues inside the eyeball become
inflamed and is generally caused by bacterial infection. The most common
sources of
bacteria causing postoperative endophthalmitis are believed to be the
patient's conjunctiva or
eyelids. Unless treated effectively, endophthalmitis can rapidly lead to
severe vision loss or
blindness. The relative risks of developing postoperative endophthalmitis
depend on a
number of factors, including the presence of eyelid or conjunctival diseases,
the patient's
general health, the use of inununosuppressant medications, the type of
intraocular surgery,
and intraoperative complications. Of these factors, intraoperative
complications, particularly
breaks in the posterior capsule witli vitreous loss, carry the greatest risk
for the development
of endophthalmitis.
Although intravitreous injection is a simple procedure with a small wound, it
has been
demonstrated that bacteria potentially introduced by the procedure are
sufficient to induce
endophthalmitis, which is likely due to the inability of the vitreous to clear
the infectious
microorganisms. Other equally plausible explanations for the apparent high
risk of
endophthalmitis after intravitreous injections may be the very limited sample
size as well as
publication bias. It is important, nevertlieless, to minimize the risk of
developing
endoplithalmitis by reducing or eliminating bacteria from the ocular surface
at the time of the
injection and to strictly adhere to aseptic technique. The use of topical
antibiotics has been
shown to reduce conjunctival and eyelid bacterial flora, which may in turn
also decrease the
risk of endophthalmitis.
Because transient increases in intra-ocular pressure (IOP) may cause mild
discomfort
and can be associated in rare instances with irreversible damage to retinal
ganglion cells
and/or retinal vascular occlusion, many investigators reported using
prophylactic and/or
therapeutic measures to prevent increases in IOP after IVT injection. These
have included
the use of aqueous paracentesis, preoperative treatment with pressure-lowering
agents and
digital massage or the use of a Honan IOP reducer.
Particulate contaminants present in a drug, in a syringe, or in or on
materials used at
the time of injection also may have the potential to induce detrimental
effects when injected
into the vitreous. This has been demonstrated in the case of glove lubricants,
wllich are
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
3
highly inflammatory when injected into the posterior ocular chamber (H.S.
Park, Korean J.
Ophthalinol. 1997; 11:51-59).
Other serious complications rarely occurred after IVT injection, making it
difficult, in
most instances, to determine whether these were truly injection-related or
simply sporadic,
unrelated comorbidities.
Serious adverse events are for the most part transient and/or treatable, and
the risks of
serious adverse events reported after IVT injection is low. Even so, there is
a need for
improved devices and methods for IVT injection. The risks and benefits of IVT
injection will
likely carry increased weight in patient and clinician treatment as more
treatment options
become available.
Guidelines for IVT injection are continuing to evolve (L.P. Aiello et al.,
Retina,
24:S3-S19, 2004). For example, povidone iodine and an antibiotic are
adnzinistered prior to
IVT injection. Also, IVT injections are generally performed with a sterile
surgical drape and
lid speculum in place and a 27 or 30 gauge needle is typically used with an
injection site
3.5-4.0 mm posterior to the limbus.
As new treatment modalities for macular diseases become available, the number
of
intravitreous injections administered is expected to increase dramatically.
For example,
intravitreous injection of the vascular endothelial growth factor (VEGF)
inhibitor, Macugen ,
has become available for the treatment of age-related macular degeneration.
Also,
intravitreous injections of triamcinolone acetonide are now coinmonly used for
the treatment
of macular edema.
The prevalence of endophthalmitis after intravitreous injection of anti-VEGF
agents is
unknown. Due to the very limited data regarding the rate of endophthahnitis
after
intravitreous injections, it is difficult to speculate about the true
prevalence of
endophthalmitis after these types of procedures. The increased use of
intravitreous injections
for the delivery of these agents to the retina will provide data regarding the
prevalence and
risk factors for post-injection endophthalmitis and in the future define a
more accurate rate of
endophthalmitis.
Drug delivery into the eye is challenging because the anatomy, physiology and
biochemistry of the eye includes several defensive barriers that render ocular
tissues
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
4
impervious to foreign substances. Techniques used for administering active
agents into the
eye include systemic routes, intraocular injections, injections around the
eye, intraocular
implants, and topical applications. Patient acceptance and safety are key
issues that play a
key role as to which treatments are used.
Ocular bioavailability of drugs applied topically in formulations such as eye
drops is
very poor. The absorption of drugs in the eye is severely limited by some
protective
mechanisms that ensure the proper functioning of the eye, and by other
concomitant factors,
for exainple: drainage of the instilled solutions; lacrhyrnation, tear
evaporation; non-
productive absorption/adsoiption such as conjunctival absorption, poor corneal
permeability,
binding by the lachrymal proteins, and metabolism.
Alternative approaches to delivery include in situ activated gel-forming
systems,
mucoadhesive formulations, ocular penetration enhancers and ophthalmic
inserts. In situ
activated gel-for-ming systems are liquid veliicles that undergo a viscosity
increase upon
instillation in the eye, thus favoring pre-corneal retention. Such a change in
viscosity can be
triggered by a change in temperature, pH or electrolyte composition.
Mucoadhesive
formulations are vehicles containing polymers that adhere via non-covalent
bonds to
conjunctival inucin, thus ensuring contact of the medication with the pre-
comeal tissues until
mucin turnover causes elimination of the polymer. Ocular penetration enhancers
are mainly
surface active agents that are applied to the cornea to enhance the
permeability of superficial
cells by destroying the cell membranes and causing cell lysis in a dose-
dependent manner.
Ophtllalmic inserts are solid devices intended to be placed in the
conjunctival sac and to
deliver the drug at a comparatively slow rate. One such device is Ocusert , by
Alza
Corporation, which is a diffusion unit consisting of a drug reservoir enclosed
by two release-
controlling membranes made of a copolymer. M.F. Saettone provides a review of
continued
endeavors devoted to ocular delivery. ("Progress and Problems in Ophthalmic
Drug
Delivery", Business Briefing: Pharmatech, Future Drug Delivery, 2002, 167-
171).
Many types of ophthalmic surgeries such as cataract surgery require use of
various
fluids which are both delivered and removed from the eye over the course of
the surgery.
The simultaneous delivery of two or more therapeutics typically requires
multiple separate
needle penetrations. In areas where bacterial infection and/or structural
damage are a concern,
the risks associated with multiple injections may become unacceptable.
Multiple injections
may be circumvented by using a multi-compartment syringe or a double-barrel
syringe.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
Administration of multiple viscoelastic solutions witli a multi-compartment
syringe is
described in US Patent Application Publication No. 2004/0167480. A double-
barrel syringe
for ophthalmic surgeries is described in US Patent Application Publication No.
2004/0064102.
Such invasive intraocular administrations may not be favorable because they
cause
5 patient discomfort and sometimes fear, while risking permanent tissue
damage. A device
which allows the simultaneous or sequential delivery of a therapeutic while
requiring a single
needle penetration would significantly reduce any needle associated
complications.
SUMMARY OF THE INVENTION
The present invention provides a device for use in ophthalmology. In
particular, the
present invention provides a device for use in intravitreous administration of
ocular agents.
The present invention also provides methods of delivering one or more drugs to
a human eye.
In one aspect, the invention relates to ophthalmic drug delivery devices and
features a
device for delivery of a therapeutic agent to the eye of a mammal.
The invention features a drug delivery device for delivering a therapeutic
compound
to the eye and drug delivery methods related thereto. The invention also
features a syringe
for intravitreal delivery and metliods of using the syringe to treat an
ophthalmic disease,
disorder, or condition.
Other features and advantages of the invention will be apparent from the
following description, the drawings, and the claims.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
6
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic representation of a needle assembly comprising a luer
hub, a
cannula and a needle tip having a standard bevel.
Figure 2 is a schematic representation of a needle assembly comprising a luer
hub, a
cannula and a needle tip shield.
Figure 3 is a schematic representation of a syringe and needle assembly
comprising a
low dead space hub assembly.
Figure 4 shows drawings of a first embodiment of a double barrel syringe.
Figure 5 shows drawings of a first embodiment of a double barrel syringe.
Figure 6 is a schematic representation of a fluid exchange device.
Figure 7 is a schematic representation of a tandem syringe.
Figure 8 is a graph showing penetration force required by various needles.
DETAILED DESCRIPTION OF THE INVENTION
One aspect provides a syringe useful in ophthalmic applications for delivery
of a
material into the eye.
Needle
Any suitable needle may be used. Suitable needles provide facile penetration
of the
sclera with minimal injury. A needle typically includes an elongated tube with
an outside
surface, a proximal end, a distal end and an open bore therethrough. As seen
in Figure 1, the
needle assembly 20 may have a hub 23 attached to the proximal end of the
needle 22 that is
used to attach the needle to a syringe. In one embodiment the hub is a Luer
hub.
The needle may be attached to the syringe permanently (e.g., staked) or may be
attached to the syringe by a Luer fitting. The Luer fitting may be a standard
Luer fitting,
Luer slip fitting or a Luer lock fitting. The Luer fitting has either a tip
(male) or hub (female)
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
7
component, and provides the ability to insure leak-proof and mechanically
secure connections
to any other device having a mating Luer fitting. Luer connectors can comprise
round and
tapered male and matching female mating surfaces. Luer connectors can fonn a
locking
configuration by adding a threaded locking collar to the male luer connector,
which mates
with ears on the female luer connector, thereby providing a positive "locked"
connection.
Luer fittings have several advantages. Luer fittings provide compatibility
among various
medical devices, offering the clinician the benefits of choosing a preferred
needle. In
addition, Luer-lock connections insure against possibility of needle coming
off of the syringe
during the injection procedure. Standards for Luer fittings are described in
American
National Standard ANSI/HIMA MD 70.1-1983 and the International Standard ISO-
594-1 and
ISO-7886-1.
A non-standard Luer fitting may be used. Examples of non-standard Luer
fittings
include, but are not limited to, the Tru-LokTM fluid transfer adaptor by
Becton Dickinson.
Other non-standard fittings include Tyco Health Care, Kendall Monoject low
dead space
(LDS) needles featuring tri bevel, anti-coring, stainless steel needles.
Examples of low
waste space fittings are found in US Patent Nos. 6,840,291, 5,902,277
5,902,271, 5,902,270
5,902,269 5,782,803, the contents of each are hereby incorporated by reference
in its entirety.
The needle may also be attached to the syringe via a ceramic coated tip (CCT)
interface, i.e. 'press fit'.
In one embodiment, the needle is beveled and coated with a suitable silicone.
In one
embodiment, the needle is a PrecisionGlideg needle available from Becton-
Dickenson.
Suitable PrecisionGlide needles include but are not limited to a 1/2 inch 30
gauge needle
and a 1/z inch 27 gauge needle. In one embodiment, the needle is a
PrecisionGlide shown in
Figure 3. Referring to the figure, the needle comprises a polypropylene Luer
hub 33 and a
stainless steel cannula 34, lubricated with silicone, having a three-bevel
point, attached to the
hub via an epoxy joint.
The needle tip may have a standard bevel. In one embodiment, the needle may
have
more than one bevel. In one embodiment, the needle has three bevels. In one
einbodiment,
the needle has five bevels. Examples of a five-bevel needle are described in
US Patent No.
6,629,963, and 6,009,933, US patent Application publication Nos. 2044/0111066,
2004/0030303 and PCT Application No. 2005/016420
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
8
In one embodiment, the needle is a coated needle. In one embodiment, the
needle is a
lubricated needle. The needle optionally includes a lubricious coating applied
to and
adherent to the outside surface of the tube, as described in US Patent No.
5,911,711.
In one embodiment, the coating is a silicone coating. Any suitable silicone
coating
may be used. Examples of suitable coatings include, but are not limited to,
those available
from SurModics, Eden Prairie, MN (see US Patent Nos. 6,706,408, 6,669,994,
6,254,634
and 6,121,027).
In one embodiment the coating is a medicated coating.
Preferably the needle is a 27 gauge needle or smaller. In one embodiment the
needle
is a 30 gauge needle.
In one embodiment, the needle has a length of less than 1 inch. In another
embodiment, the needle has a length of about 0.5 inches.
Needle tip shield
As seen in Figure 2, the needle assembly 20 may comprises a needle tip shield
21
enclosing needle 22. Needle 22 is attached to luer hub 23 via epoxy joint 24.
In one
embodiment, the tip shield 21 is rigid. Examples of suitable rigid shields
include but are not
limited to those disclosed in US Patent No. 4,986,818. As depicted in Figure
2, the tip shield
is not in contact with the needle tip. Needle tip shields in contact with the
needle potentially
dull the needle and wipe away any lubrication on the needle. In another
einbodiment, the tip
shield comprises one or more apertures or is permeable to sterilizing gases.
The apertures
may facilitate sterilization by allowing sterilizing gasses or steam to access
the interior of the
needle shield. In a particular embodiment, the tip shield is synthetic
isoprene, ethylene
oxide (EtO) or hydrogen peroxide (H202) penneable. In another embodiment, the
syringe
barrels, stoppers and plunger rod components and assemblies can also be gamma
irradiated.
In one embodiment, the needle tip shield comprises a polypropylene. In another
embodiment,
the needle tip shield comprises a styrene block thermoplastic elastomer.
Penetration Force
The needles of the present invention are used for penetration of the scleral
tissue for
administration of the syringe contents into the vitreous. Preferably the
needles require a low
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
9
penetration force. Preferably the needles require a low penetration force with
low variability.
In one embodiment, the needles require a penetration force of less than 500
grains (g). In
another embodiment, the needles require a penetration force of less than 100
grams (g). In
another embodiment, the needles require a penetration force of less than 50
grams (g).
In one embodiment, the needles require a penetration force with a variability
range of
+/- 20 %. In one embodiment, the needles require a penetration force witli a
variability range
of +/- 50 g. In anotller embodiment, the needles require a penetration force
with a variability
range of +/- 20 g
In one embodiment, the penetration force is reduced by reducing the needles
coefficient of friction. In one einbodiment the penetration force is reduced
by using a
lubricious coating on the needle.
Syringe
The syringe barrel is typically made of glass or a thermoplastic material. In
one
embodiment the syringe is a 1 mL Type I glass barrel syringe sealed with a
bromobutyl
rubber stopper. Exainples of pre-filled syringes are found in US Patent No.
4,252,11 S. In
one embodiment the syringe is a BD Hypak SCF syringe. In a particular
einbodiment, the
syringe is a single dose, pre-filled syringe. In one embodiment, the syringe
barrel has a
volume of 1 mL or less. In a particular embodiment, the syringe barrel has a
microliter
volume. The syringe barrels of the present invention may further be provided
with
graduations to assist in precision filling of the barrel.
In one einbodiment, the syringe is a plastic syringe. In another einbodiment,
the
syringe comprises a cyclic olefin copolymer (COC). In another embodiment the
cyclic
olefin copolymer is TopPac (Schott).
In another embodiment, the final Luer formation is made using a platinum wire.
In a
particular embodiment, the syringe is substantially free of tungsten. Staked
needle
production requires a small hole and seat for gluing in the needle. The small
hole requires a
high temperature tungsten pin. Some of the tungsten pin material may shed into
the glass
during processing. Luer lock syringes are alternatively formed using a
platinum pin material.
The platinum may not leave a significant residue in the glass as compared to
tungsten.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
Optimal particulate matter concentrations may be achieved primarily through
strict control of
the environment and material cleanliness.
Volume
The ophthalmic injection solutions of the present invention are useful as
inicroliter
5 ( L)-volume injections. Microliter ( L)-volume injections may also be
referred to as "ultra-
low volume injections". In one embodiment, the ophthalmic injection solution
to be
delivered has a volunie of about 1.0 mL (1000 [tL) or less. In another
embodiment the
ophthalmic injection solution to be delivered has a volume of about 200 L or
less. In
another embodiment the ophthalmic injection solution to be delivered has a
volume of about
10 100 L or less. In another embodiment the ophthalmic injection solution to
be delivered has
a volume of about 90 L. In another embodiment the ophthalmic injection
solution to be
delivered has a volume of about 50 L.
,Sub-Visible Particulate Matter
The ophthalmic injection solutions of the present invention, including
solutions
constituted from sterile solids intended for parenteral use, as used herein
are substantially free
from particles that can be observed on visual inspection. There are also
strict controls on
sub-visible particulate matter for ophthalmic injections. The ophthalmic
injection solutions of
the present invention can be tested by a light obscuration procedure or may be
tested by a
microscopic procedure as described in USP Chapter <788>. United States
Pharmacopoeia
(USP) Chapters <788> Particulate Matter in Injections and <789> Particulate
Matter in
Ophthalmic Solutions describe physical tests for the purpose of enumerating
extraneous
particles within specific size ranges. The United States Pharmacopoeia, 28 th
revision and the
National Formulary, 23'd edition (USP28-NF23), The United States Pharmacopeial
Convention, Inc (2005), is hereby incorporated by reference in its entirety.
In one embodiinent, the ophthahnic solution contained within the syringe of
the
present invention has a l0 m-size or larger sub-visible particulate count of
less than or equal
to about 60 particles per mL, a 25 m-size or larger sub-visible particulate
count of less than
or equal to about 10 particles per mL, or a 50gm-size or larger sub-visible
particulate count
of less than or equal to about 5 particles per mL. In one particular
embodiment, the
concentration of sub-visible particulate matter is less than or equal to about
150 ppb.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
11
In one embodiment the ophthalmic solution contained within the syringe of the
present invention is subject to the particulate matter limits set forth in USP
<789> wherein
the average number of particles present in the units tested does not exceed
the values listed in
Table 1.
Table 1.
Diameter
>10 m >25 m >50 m
Number of particles 50 per mL 5 per mL 2 per mL
In one embodiment, the ophthalmic solution contained within the syringe of the
present invention has a 10 m-size or larger sub-visible particulate count of
less than or equal
to about 20 particles per mL, a 25 m-size or larger sub-visible particulate
count of less than
or equal to about 5 particles per mL, or a 50 m-size or larger sub-visible
particulate count of
less than or equal to about 2 particles per mL. In one particular embodiment,
the
concentration of sub-visible particulate matter contained within the syringe
of the present
invention is less than or equal to about 150 ppb.
Waste volume
As represented in Figure 3, the syringe assembly has a low waste space, which
is
defined as the volume located in the syringe tip 31 of syringe barre132,
needle hub 33 and
needle cannula 34. The International Standard ISO-7886-1 identifies the
maximum waste
space for a 3ml syringe tip to be 0.07 mL.
In a particular embodiment, the needle/syringe combination of the present
invention
has a low waste space. Examples of low waste space fittings are found in US
Patent
Nos. 6,840,291, 5,902,277 5,902,271, 5,902,270 5,902,269 5,782,803, the
contents of each is
hereby incorporated by reference in its entirety. An example of a
needle/syringe combination
having a low waste space includes Tru-lokTM fluid transfer adaptors by Becton
Dickinson
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
12
(US Patent No. 6,840,291) and Monoject0 low dead space (LDS) needles Tyco
Health Care,
Kendall (catalog Nos. 1188005058 and 1188001112) featuring tri bevel, anti-
coring, stainless
steel needles.
The needle/syringe combination of the present invention has a waste space of
less
than 0.1 mL. In one embodiment, the waste space is less than 0.05 mL. In
another
embodiment, the waste space is approx. 50-60 L. In one einbodiment, the waste
space for
the 1 mL Hypak Luer tip syringe is from about 0.040 to about 0.050 mL. In
another
embodiment, the waste space is less than 0.001 mL.
Syringe tip cap
The syringe assembly may comprise a syringe tip cap. The syringe tip cap is
used to
seal the barrel of a prefilled syringe. In one embodiment, the syringe tip cap
is a plastic rigid
tip cap. Examples of suitable syringe tip caps include, but are not limited
to, those found in
US Patent Nos. 6,190,364; 6,196,998; 6,520,935 and 5,833,653; US patent
Application
Publication No. 2004/0215148 and US design patent Nos. 457954S1 and 493526S1.
In one
embodiment, the rigid tip cap is an elastomeric formulation comprising an
elastomer,
reinforcement and a curing system. In another embodiment, the elastomer is a
synthetic
isoprene blend, the reinforcement is an inert material, and the curing system
is a resin. In
another embodiment, the syringe tip cap comprises a chloro/bromobutyl rubber
stopper.
Multiple barrel syringe
Another aspect of the invention provides a syringe comprising more than one
barrel.
The multiple barrel syringe may permit simultaneous, selective or sequential
delivery of one
or more different materials.
In one embodiment the syringe comprises a first and second barrel positioned
in side-
by-side relationship including a first and second plunger for telescoping
movement within
their respective chambers (see US Patent Application Publication No.
2004/0064102, which
is herby incorporated by reference in its entirety). The plungers are
optionally connected to a
common handle allowing for the dispensing of the inaterials from the two
chambers
simultaneously at the saine rate, as disclosed, for example, in US Patent No.
5,792,103. In
another embodiment, the plungers are detachably connected to the plunger
stopper.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
13
Figures 4 and 5 show examples of dual barrel syringes for the simultaneous or
sequential delivery of two or more therapeutic agents. The syringe includes a
first barrel, a
second barrel, and one or more needles. Each barrel contains a therapeutic
agent dissolved or
suspended in a liquid formulation. Referring now to the drawings, there is
seen in Figures 4
and 5 first and second embodiments of the double barrel syringe which differ
in the respect
that the first embodiment (Figure 4) is configured for direct filling of the
first and second
materials inside their respective barrels wllile the second embodiinent
(Figures 5) is
configured for insertion of pre-filled first and second carpules into the
first and second barrels,
respectively.
Referring to the first embodiment shown in Figure 4, the syringe coinprises
first and
second barrels 41, 42 each having an internal chamber 43, 44 for holding a
quantity of first
and second, liquefied materials therein, respectively. The first and second
barrels 41, 42 are
arranged in side-by-side relationship with each other. First and second
plungers 45, 46 are
positioned for sliding within the first and second barrels 41, 42 for
telescoping movement
therein, respectively. The syringe tip 47 is located adjacent the distal ends
of the first and
second barrels 41, 42 and is in common, fluid cominunication therewith via
exit orifices 48
and 49. The exit orifices 48 and 49 provide the pathway for the first and
second materials,
respectively, to tip 47 and thereby allowing passage of the first and second
therethrough. Tip
47 may be in the form of a needle directly attached to the syringe body, or
may be in the form
of a cannula which is attached to the syringe body via a Luer lock.
Referring to the second einbodiment shown in Figure 5, the syringe coinprises
first
and second carpules 51, 52 removably insertable within said first and second
barrels 53, 54,
respectively. First and second syringe needles 55, 561ocated in the first and
second barrels 53,
54 adjacent the distal ends thereof. As such, upon fully inserting the first
and second carpules
51, 52 in the first and second barrels 53, 54, the first and second syringe
needles 55, 56 pierce
the carpule plug provided at the respective carpule distal end. The first and
second needles 55,
56 extend into the tip 57.
A single needle with two or more hollow bores may perform both injections or
multiple needles may be used. When one needle is employed, two cannulas, one
affixed to
one of each barrel, may lead to the one needle. Alternatively, when one needle
is employed,
the hollow bores may be arranged in a concentric pattern. In such a concentric
pattern, one
bore is for introduction of a first fluid into the vitreous, and one bore is
for introduction of
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
14
second fluid into the vitreous. When two needles are employed, a second needle
may be
attached to the exterior of a first needle. Needles may be manufactured from
standard
materials, e.g., stainless steel, by methods known in the art.
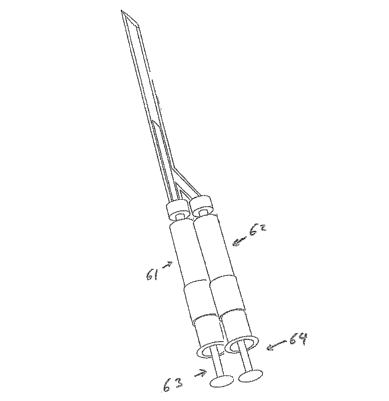
PCT Publication No. WO 2004/073765 describes, in part, a Fluid Exchange System
(FES) which is designed to remove a specific volume from a closed system and
sequentially
deliver a measured voluine. By replacing the vacuum chamber with a housing
that can accept
a pre-filled syringe and removing the air vents (manifolds) an addition
tllerapeutic may be
delivered utilizing the original needle penetration. Additional syringe
housings could also be
added to allow for multiple sequential adininistrations. Figure 6 illustrates
an apparatus
comprising a first and second housing 61, 62 capable of accepting a first and
second prefilled
syringe 63, 64.
In one aspect, multiple medicaments can be administered using a tandem
syringe. A
tandein syringe typically comprises two or more compartments within one
external barrel.
Examples of tandem syringes include but are not limited to those found in US
Patent Nos.
4,313,440; 4,715,854; 5,102,388; 5,298,024; 6,132,400; and US Patent
Application
Publication No. 2004/0167480, each of which is herby incorporated by reference
in its
entirety.
In one embodiment, the tandem syringe comprises an outer first coinpartment
including a first sealing member and an inner second compartment in which the
first sealing
member functions as the plunger for the outer first compartment (see Figure
7). Referring to
Figure 7, the outer first compartment 71 is filled with a first injection
solution. The inner
second compartment 72 is filled with a second injection solution. The inner
second
compartment comprises a first sealing member 73 and functions as the piston
for the first
compartment. When the first injection solution in completely administered, the
first sealing
member 73 is pierced using a piercing device 74 at the distal end of the outer
first chamber.
Once the first sealing member 73 is pierced, the second injection solution is
administered by
pushing stopper 75 with plunger 77 thereby forcing the second injection
solution past stopper
73 and out through the needle 76.
Each coinpartment may be pre-filled with its injection solution separately
providing
for storage of the injection solutions without mixing or contact with each
other.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
In one embodiment compartment 71 (inner compartment) contains the final
solution
to be injected. Coinpartment 71 is first loaded separately, then assembled
with the main
housing forming compartment 72. Compartment 72 in then loaded with the first
solution to
be injected.
5 Advantages
The syringes of the present invention have several advantages. Advantages
include
the benefits of ease of use, flexibility, cost effectiveness, patient comfort
and safety. An
advantage of using a non-fixed needle/syringe combination, such as one using a
Luer fitting,
as described herein is the allowance for a choice of application needle. For
example, a
10 practitioner may select either a 27 or 30 gauge disposable needle. A non-
fixed needle is
typically sharper than a fixed needle because the non-fixed needle will not be
susceptible to
dulling as a result of contact with the sheath needed for a fixed needle pre-
filled syringe.
Sharper needles reduce patient discomfort and reduce the risk of infection.
Definitions
15 The grammatically correct and preferred terin "intravitreous" is used
herein and in the
art. The term "intravitreal" is used colloquially as an alternative to the
term "intravitreous"
for injections into the eye's vitreous humor between the lens and the retina.
"Particulate matter" includes mobile, randomly sourced, extraneous substances,
other
than gas bubbles, that cannot be quantitated by chemical analysis because of
the small
amount of material they represent or because of their heterogeneous
composition.
The portion of the device that is toward the practitioner is tenned "proximal"
and the
portion of the device that is toward the patient is termed "distal."
"Penetration force" is the measure of force applied to the needle prior to the
needle
cutting the tissue. Penetration force is typically measured throughout the art
in grams (g).
"Drag force" is a measure of force applied to the needle required to continue
the
penetration into the tissue.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
16
An "Injection" is a preparation intended for parenteral administration.
Injections
include, but are not limited to, liquid preparations that are drug substances
or
solutions/suspensions thereof.
By "substantially constant pressure" is meant pressure that is constant with
minor,
teinporary variations due to filling, emptying, or a change in osmotic
pressure of the
surrounding liquid.
"Parenteral" articles are preparations intended for injection through the skin
or other
external boundary tissue, rather than through the alimentary canal, so that
the active
substances they contain are administered, using gravity or force, directly
into a blood vessel,
organ, tissue, or lesion. Parenteral articles are prepared by metllods
designed to ensure that
they meet Pharmacopeial requirements for sterility, pyrogens, particulate
matter, and other
contaminants (USP Chapter 1).
The designation "Small-Volume Injection" applies to an injection that is
packaged in
containers labeled as containing 100 mL or less.
The designation "Microliter-volume Injection" or "Ultra-Low-Volume Injection"
applies to an Injection that is packaged in containers labeled as containing
1.0 mL (1000 L)
or less.
As used herein, the term "dead space" or "Waste space" is the volume of
injection
solution within the syringe/needle asseinbly containing any residual injection
solution present
following an injection that does not get evacuated from the syringe during the
injection.
By "therapeutic agent" is meant any compound or mixture of compounds that
provide
a therapeutic effect for one or more diseases, disorders, or conditions. Such
compounds
include, without limitation, small organic or inorganic molecules, proteins
(e.g., antibodies),
peptides, lipids (e.g., steroids) and nucleic acids (e.g., aptamers).
Therapeutic agents are, for
example, antibiotics, analgesics, anti-inflammatory compounds, or any other
compound for
the treatment of a disease, disorder, or condition.
By "treating" is meant the medical management of a patient with the intent
that a cure,
amelioration, or prevention of a disease, pathological condition, or disorder
will result. This
tenn includes active treatment, that is, treatinent directed specifically
toward improvement of a
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
17
disease, pathological condition, or disorder, and also includes causal
treatment, that is,
treatment directed toward removal of the cause of the disease, pathological
condition, or
disorder. In addition, this tenn includes palliative treatment, that is,
treatment designed for the
relief of symptoms rather than the curing of the disease, pathological
condition, or disorder;
preventive treatment, that is, treatment directed to prevention of the
disease, patliological
condition, or disorder; and supportive treatment, that is, treatment employed
to supplement
another specific therapy directed toward the improvement of the disease,
pathological
condition, or disorder. The term "treating" also includes symptomatic
treatment, that is,
treatment directed toward constitutional syiuptoms of the disease,
pathological condition, or
disorder.
Ophthalmic solutions are sterile solutions, essentially free from foreign
particles,
suitably compounded and packaged for instillation or injection into the eye.
Preparation of
an ophthalmic solution requires careful consideration of such factors as the
inherent toxicity
of the drug itself, isotonicity value, the need for buffering agents, the need
for a preservative
(and, if needed, its selection), sterilization, and proper packaging.
While specific reference has been made to the use of the devices of the
present
invention to administer therapeutic agents to the eye, it is to be understood
that the present
invention can be used to deliver a therapeutic agent to any desired site,
including, but not
limited to, intraorbital, intraocular, intraaural, intratympanic, intrathecal,
intracavitary,
peritumoral, intratumoral, intraspinal, epidural, intracranial, and
intracardial.
A device of the invention may be used in the treatment of any eye disease. A
device
of the invention may also be used to direct a therapeutic agent to a
particular eye tissue, e.g.,
the retina or the choroid. The therapeutic agent or combination of agents will
be chosen
based on the disease, disorder, or condition being treated. In addition to a
therapeutic agent
for a particular condition, other compounds may be included for secondary
effects, for
example, an antibiotic to prevent microbial growth. The amount and frequency
of the dosage
will depend on the disease, disorder, or condition being treated and the
therapeutic agent
employed. One skilled in the art can make this determination.
Therapeutic agents that may be employed in the device of the invention
include,
without limitation, small molecules, honnones, proteins, peptides, aptamers,
antibodies, lipids,
glycolipids, DNA, RNA, PNA, enzymes, sugars, saccharides, glycoproteins,
polymers,
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
18
metalloproteases, transition metals, or chelators. In addition, nucleic acid
vectors can also be
delivered wherein the nucleic acid may be expressed to produce a protein that
may have a
variety of pharmacological, physiological or immunological activities.
Macromolecules with
a molecular weight of about 5 kDa to about 500 kDa may also be used in
accordance with the
invention.
For oplithahnic drug delivery applications, exeinplary disease states include
macular
degeneration, diabetic retinopathy, glaucoma, optic disc neovascularization,
iris
neovascularization, retinal neovascularization, choroidal neovascularization,
pannus,
pterygiuin, macular edema, vascular retinopathy, retinal vein occlusion,
histoplasmosis,
ischemic retinal disease, retinal degeneration, uveitis, inflammatory diseases
of the retina,
keratitis, cytomegalovirus retinitis, an infection, conjunctivitis, cystoid
macular edema,
cancer, and proliferative vitreoretinopathy.
Classes of therapeutic agents include anti-infectives including, without
limitation,
antibiotics, antivirals, and antifungals; analgesics; antiallergenic agents;
mast cell stabilizers;
steroidal and non-steroidal anti-inflammatory agents; decongestants; anti-
glaucoma agents
including, without limitation, adrenergics, beta-adrenergic blocking agents,
alpha-adrenergic
blocking agonists, parasyinpathomimetic agents, cholinesterase inhibitors,
carbonic
anhydrase inlubitors, and protaglandins; antioxidants; nutritional
supplements; angiogenesis
inhibitors; antimetabolites; fibrinolytics; wound modulating agents;
neuroprotective drugs;
angiostatic steroids; mydriatics; cyclopegic mydriatics; miotics;
vasoconstrictors;
vasodilators; anticlotting agents; anticancer agents; immunomodulatory agents;
VEGF
antagonists; immunosuppresant agents; and combinations and prodrugs thereof.
Specific therapeutic agents include MACUGEN (pegaptanib sodium injection) as
described in U.S. Patent No. 6,051,698, herein incorporated in its entirety by
reference.
Pegaptanib sodium is also referred to as EYE001 or NX183S.
Pegaptanib sodiuin is a covalent conjugate of an oligonucleotide of twenty-
eight
nucleotides in length that terminates in a pentylamino linker, to which two 20-
kilodalton
(kDa) monomethoxypolyethylene glycol (PEG) units are covalently attached via
the two
amino groups on a lysine residue. The molecular formula for pegaptanib sodium
is
C294H342F13N107Na28O188P28(CZH4O)õ (where n is approximately 900) and the
molecular
weight is approximately 50 kDa.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
19
The chemical name for pegaptanib sodium is as follows: RNA, ((2'-deoxy-2'-
fluoro)C-G,,; G,,; AA-(2'-deoxy-2'-fluoro)U-(2'-deoxy-2'-fluoro)C-A,,; G,n (2'-
deoxy-2' -
fluoro)U-G,n Ap A,,; (2'-deoxy-2'-fluoro)U-G,n (2'-deoxy-2'-fluoro)C-(2'-deoxy-
2'-fluoro)U-
(2'-deoxy-2'-fluoro)U-A,ri (2'-deoxy-2'-fluoro)U-A,n (2'-deoxy-2'-fluoro)C-A,n
(2'-deoxy-2'-
fluoro)U-(2'-deoxy-2'-fluoro)C-(2'-deoxy-2'-fluoro)C-G,n (3'-->3')-dT), 5'-
ester with a, a'-
[4,12-dioxo-6- [[ [5-(phosphoonoxy)p entyl] amino] carbonyl]-3,13-dioxa-5,11-
diaza-1,15-
pentadecanediyl]bis[co-methoxypoly(oxy-1,2-ethanediyl)], sodium salt.
MACUGEN (pegaptanib sodium injection) is a sterile, aqueous solution
containing
pegaptanib sodium for intravitreous injection. Macugen is supplied in a single-
dose, pre-
filled syringe and is formulated as a 3.47 mg/mL solution, measured as the
free acid form of
the oligonucleotide. The active ingredient is 0.3 mg of the free acid form of
the
oligonucleotide without polyethylene glycol, in a nominal volume of 90 L.
This dose is
equivalent to 1.6 mg of pegaptanib sodium (PEGylated oligonucleotide) or 0.32
mg when
expressed as the sodium salt form of the oligonucleotide moiety. The product
is a sterile,
clear, preservative-free solution containing sodium chloride, monobasic sodium
phosphate
monohydrate, dibasic sodiuin phosphate heptahydrate, hydrochloric acid, and/or
sodium
hydroxide to adjust the pH and water for injection. Macugen is formulated to
have an
osmolality of 280-360 mOsn1/Kg, and a pH of 6-7.
Dosage levels of pegaptanib sodium on the order of about 1 gg/kg to 100 mg/kg
of
body weight per administration are useful in the treatment of neovascular
disorders.
Examples of formulations are found in WO 03/039404, which is hereby
incorporated by
reference in its entirety. In some embodiments, pegaptanib sodium is
administered at a
dosage of about 0.1 mg to about 1.0 mg locally into the eye, wherein the
treatment is
effective to treat occult, minimally classic, and predominantly classic forms
of wet macular
degeneration. When administered directly to the eye, the dosage range is about
0.3 mg to
about 3 mg per eye, in some embodiments the dosage range is about 0.1 mg to
about 1.0 mg
per eye. In one embodiment, pegaptanib sodium is administered in a
therapeutically effective
amount of about 0.003 - 3.0 mg, 0.1 - 1.0 mg, or about 0.3 mg. In one
embodiment,
pegaptanib sodium is present in an ophthalmic injection solution fonnulation
at a
concentration ranging from 0.003 to 3.0 mg/mL. According to one embodiment,
the carrier
comprises sodium phosphate and sodium chloride. According to one specific
embodiment
the carrier comprises 10 mM sodium phosphate and 0.9% sodium chloride.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
According to one embodiment, the dose is effective to achieve a vitreous
concentration of the anti-VEGF aptamer of about 10-30 ng/mL. According to
another
embodiinent, the dose is effective to maintain a vitreous concentration of the
anti-VEGF
aptamer of about 10-30 nghnL throughout a 6 week dosing interval.
5 In alternative embodiments, the anti-VEGF agent is an anti-VEGF aptamer and
is
administered at a dosage of less than 0.3 mg to about 0.003 mg locally into
the eye. In some
embodiments, the anti-VEGF aptamer is administered at a dosage less than about
0.30 mg.
Examples of such formulations are found in US Patent Application Serial No.
60/692,727;
which is hereby incorporated by reference in its entirety.
10 Specific therapeutic agents also include the anti-PDGF aptamer ARC-127
(Archemix
Corp., Cainbridge, MA), a PEGylated, anti-PDGF aptamer having the sequence
CAGGCUACGN CGTAGAGCAU CANTGATCCU GT (SEQ ID NO: 10 from U.S. Patent
No. 6,582,918, incorporated herein by reference in its entirety) having 2'-
fluoro-2'-
deoxyuridine at positions 6, 20 and 30, 2'-fluoro-2'-deoxycytidine at
positions 8, 21, 28, and
15 29, 2'-O-Methyl-2'-deoxyguanosine at positions 9, 15, 17, and 31, 2'-O-
Methyl-2'-
deoxyadenosine at position 22, hexaethylene-glycol phosphoramidite at "N" in
positions 10
and 23, and an inverted orientation T (i.e., 3'-3'-linked) at position 32.
A combination therapy for the treatment of ocular neovascular disorders using
a
VEGF antagonist and a PDGF antagonist is described in PCT Application
20 No. WO 2005/020972, whicll is incorporated herein by reference in its
entirety. An example
of such a therapy comprises the administration of a coinbination of Macugen
and ARC127.
According to another embodiment, the present invention features a method for
treating a patient suffering from an ocular disease, which method includes the
following
steps: (a) administering to the patient an effective amount of an anti- VEGF
aptamer; and (b)
providing the patient with phototherapy, such as photodynainic tllerapy or
thermal laser
photocoagulation as further described in PCT WO 03/039404, incorporated in its
entirety by
reference.
In one embodiment of the invention, the photodynamic therapy (PDT) includes
the
steps of: (i) delivering a photosensitizer to the eye tissue of a patient; and
(ii) exposing the
photosensitizer to light having a wavelength absorbed by the photosensitizer
for a time and at
an intensity sufficient to inhibit neovascularization in the patient's eye
tissue. A variety of
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
21
pllotosensitizers may be used, including but not limited to, benzoporphyrin
derivatives (BPD),
monoaspartyl chlorine, zinc phthalocyanine, tin etiopurpurin, tetrahydroxy
tetraphenylporphyrin, and porfimer sodium (PHOTOFRIN), and green porphyrins.
Other therapeutic agents include 4,9(11)-pregnadien-17a,21-diol-3,20-dione,
4,9(I 1)-
pregnadien-17a,21-dio1-3,20-dione-21-acetate, coinbretastatin, timolol,
betaxolol, atenolol,
brimonidine, acetazolamide, methazolamide, dichlorphenamide, diamox,
nimodipine,
eliprodil, colchicine, vincristine, cytochalasin B, tetracycline,
chlortetracycline, bacitracin,
neomycin, polymyxin, gramicidin, oxytetracycline, chlorainphenicol,
gentamycin,
erythromycin, sulfonamides, sulfacetainide, sulfamethizole, sulfisoxazole,
fluconazole,
nitrofurazone, amphotericin B, ketoconazole, trifluorothymidine, acyclovir,
ganciclovir,
didanosine, AZT, foscainet, vidarabine, idoxuridine, ribavirin, protease
inhibitors, anti-
cytomegalovirus agents, methapyriline; chlorpheniramine, pyrilamine
pheniramine,
hydrocortisone, dexamethasone, fluocinolone, prednisone, prednisolone,
methylprednisolone,
fluorometholone, betamethasone, triaincinolone, phenylephrine, naphazoline,
tetrallydrozoline, pilocarpine, carbachol, diisopropylfluorophosphate,
echothiophate iodide,
demecarium bromide, atropine sulfate, cyclopentolate, homatropine,
scopolamine,
tropicamide, eucatropine, epinephrine, heparin, antifibrinogen, fibrinolysin,
anti clotting
activase, acetohexamide, chlorpropamide, glipizide, glyburide, tolazamide,
tolbutamide,
insulin, aldose reductase inhibitors, thalidomide, folic acid, 5-fluorouracil,
adriamycin,
asparaginase, azacytidine, azathioprine, bleomycin, busulfan, carboplatin,
cannustine,
chlorambucil, cisplatin, cyclophosphamide, cyclosporine, cytarabine,
dacarbazine,
dactinomycin, daunorubicin, estramustine, etoposide, etretinate, filgrastim,
floxuridine,
fludarabine, fluoxymesterone, flutamide, goserelin, hydroxyurea, ifosfamide,
leuprolide,
levamisole, lomustine, nitrogen mustard, melphalan, mercaptopurine,
methotrexate,
,25 mitomycin, mitotane, pentostatin, pipobroman, plicamycin, procarbazine,
sargramostim,
streptozocin, tamoxifen, taxol, teniposide, thioguanine, uracil mustard,
vinblastine, vindesine,
pituitary hormones, , insulin-related growth factor, thyroid hormones, growth
horinones, heat
shock proteins, iinmunological response modifiers such as muramyl dipeptide,
interferons
(including a, 0, and y interferons), interleukin-2, cytokines; FK506, tumor
necrosis factor,
thymopentin, transforming factor beta2, erythropoietin; antineogenesis
proteins, monoclonal
antibodies, brain neive growth factor (BNGF), celiary nerve growth factor
(CNGF), vascular
endothelial growth factor (VEGF), monoclonal antibodies or aptamers directed
against
growth factors, and combinations and prodrugs thereof:
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
22
A therapeutic agent may be present in any suitable formulation for delivery to
the eye.
Methods well known in the art for making formulations are found, for example,
in
Renaington: Th.e Science and Practice of Pharmacy (20th ed., A.R. Gennaro ed.,
Lippincott:
Philadelphia, 2000). Therapeutic agents may be administered to humans,
domestic pets,
livestock, or other animals with a pharmaceutically acceptable diluent,
carrier, or excipient.
Therapeutic formulations may be liquid solutions, suspensions, or other
formulations
deliverable via a needle. Formulations may, for exainple, contain excipients,
sterile water,
saline, polyalkylene glycols such as polyethylene glycol, oils of vegetable
origin, or
hydrogenated napthalenes.
The therapeutic agent may be admixed witll a pharmaceutically acceptable
carrier
adapted to provide sustained release of the therapeutic agent. Sustained
release carriers
include emulsions, suspensions, polymeric matrices, microspheres,
microcapsules,
microparticles, liposomes, multivesicular liposomes, lipospheres, hydrogels,
salts, and
polymers with the therapeutic agent reversibly bound electrostatically,
chemically or by
entrapment. Suitable sustained release forinulations which may be used are
known in the art
and are disclosed in, for example, U.S. Patent Nos. 4,865,846, 4,115,544,
5,185,152,
4,078,052, 4,241,046, 4,853,224, 4,865,846, 6,309,669, 5,326,761, 6,071,534,
6,132,766 and
6,277,413 and PCTs WO 01/74400, WO 03/24420, WO 03/028765, WO 02/15888, WO
03/092665 and WO 03/070219, all of which are hereby incorporated in their
entirety by
reference.
Formulations of the drug may also include a transscleral diffusion promoting
agent,
such as dimethylsulfoxide, ethanol, dimethylformamide, propylene glycol, N-
methylpyrolidone, oleic acid, isopropyl myristate, polar aprotic solvents,
polar protic solvents,
steroids, sugars, polymers, small molecules, charged small molecules, lipids,
peptides,
proteins, and surfactants.
A therapeutic agent may be optionally administered as a pharmaceutically
acceptable
salt, such as a non-toxic acid addition salts or metal complexes that are
commonly used in the
pharmaceutical industry. Examples of acid addition salts include organic acids
such as acetic,
lactic, pamoic, maleic, citric, malic, ascorbic, succinic, benzoic, palmitic,
suberic, salicylic,
tartaric, methanesulfonic, toluenesulfonic, or trifluoroacetic acids or the
like; polymeric acids
such as tannic acid, carboxymethyl cellulose, or the like; and inorganic acids
such as
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
23
hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, or the
like. Metal
complexes include cations, such as divalent cations including calcium and
magnesium, zinc,
iron, and the like. In addition, a therapeutic agent may be optionally
administered as a
phannaceutically acceptable prodrug, e.g., an ester or amide.
The chemical compounds for use in such therapies may be produced and
isolated as described herein or by any standard technique known to those in
the field
of medicinal chemistry. Conventional pharmaceutical practice may be employed
to
provide suitable formulations or compositions to administer the identified
compound
to patients suffering from a disease, disorder, or condition of the eye.
Administration
may begin before, during, or after the patient is symptomatic.
Although the above process was described using the syringe of the invention,
alternative methods of injection can be employed. Other variations on these
configurations
will be apparent to one skilled in the art.
EXAMPLES
The following examples serve to illustrate certain usefal embodiments and
aspects of
the present invention and are not to be construed as limiting the scope
thereof. Alternative
materials and methods can be utilized to obtain similar results.
Example 1
Macugen Formulation
Macugen ((OSI) Eyetech, Inc., NY, NY) is formulated at 0.3mg/90 L having a
tungsten particulate count of less than 150 ppb. The solution is presented in
USP Type I
glass barrel syringes fitted with a Luer lock hub and sealed with a bromobutyl
rubber plunger
stopper. The syringe is fitted with a Luer lock 27-gauge, multi-beveled,
silicone coated
needle with a rigid plastic outer shield. The needle requires a penetration
force of less than
100 g.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
24
Example 2
Macugen0 Formulation
Macugen (Eyetech Pharmaceuticals, NY, NY) is fonnulated at 0.3mg/90 L,
0.031ng/90 L or 0.003mg/90 L and presented in USP Type I glass barrel syringes
sealed
with a bromobutyl rubber plunger stopper. The syringe is fitted with a Luer
lock 27-gauge
needle with a rigid plastic outer shield. The stoppered syringe is packaged in
a foil pouch. A
plastic plunger rod and flange adapter are also supplied for administration
purposes. These
components are provided in a separate foil pouch. Use of the flange is
optional and is not
required to administer the injection. The drug product is preservative-free
and intended for
single use by intravitreous injection only. The product should not be used if
cloudy or if
particles are present.
Active Ingredient: Pegaptanib Sodium Injection formulated as:
= 0.0347mg/mL solution to deliver a dose of 0.003mg
pegaptanib sodium injection
= 0.347mg/mL solution to deliver a dose of 0.03mg
pegaptanib sodium injection
= 3.47mg/mL solution to deliver a dose of 0.3mg pegaptanib
sodium injection
Excipients: Sodium Chloride, USP
Sodium Phosphate Monobasic, Monohydrate, USP
Sodium Phosphate Dibasic, Heptahydrate, USP
Sodium Hydroxide, USP (as needed)
Hydrochloric acid, USP (as needed)
Water for injection, USP
Preparation
The drug product pegaptanib sodium is a ready-to-use sterile solution provided
in a
single-use glass syringe. Administration of the syringe contents involves
attaching the
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
threaded plastic plunger rod to the rubber stopper inside the barrel of the
syringe. The rubber
end cap is then reinoved to allow administration of the product. An optional
flange is
provided for adininistrative purposes.
5 Example 3
Intravitreous Injection
1% Mydriacyl and 2.5% Phenylephrine are applied topically to the study eye to
achieve adequate pupillary dilation. Two to three drops of 50% saline diluted
10% povidone-
iodine (betadine) solution are instilled into the eye. In the event of allergy
to iodine, a drop of
10 topical antibiotic is placed on the conjunctiva in place of iodine. A
subconjunctival injection
of 0.5 m12% xylocaine without epinephrine is administered in the
inferotemporal quadrant in
all patients - 3.0 to 3.5 inm from the limbus in aphakic/pseudophakic
patients, and 3.5 to 4.0
mm in phakic patients. Investigators are instructed to select one of two pre-
injection
procedures (Options A and B, below). For patients witll iodine allergy,
investigators are
15 required follow Option A, instilling one additional drop of antibiotic
instead of povidone-
iodine.
A. Adininister topical ofloxacin, levofloxacin, or an antibiotic drop with
comparable
antimicrobial coverage for three days prior to the treatment followed by three
consecutive drops of antibiotic and several drops of 5% povidone-iodine
iminediately
20 before the treatment
B. Administer three consecutive drops of antibiotic and a 5% povidone-iodine
flush of
the fornices and caruncle with at least 10 cc of solution just prior to
treatinent.
Prior to treatment, topical antibiotic drops are administered 3 times
separated by at
least 5 minutes within one hour prior to treatment.
25 For patients who are prepared under Option A, following the last dose of
antibiotic,
the investigator instills two or three drops of 5% povidone-iodine into the
eye. Using sterile
gloves and cotton-tip applicators soaked in 5% povidone iodine, the
investigator scrubs the
eyelids, the upper and lower eyelid margins, and the caruncle 3 times. In the
event of allergy
to iodine, one additional drop of antibiotic is instilled instead of povidone-
iodine.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
26
For patients who are prepared under Option B, the investigator waits at least
5
minutes after the last dose of antibiotic to perform a 5% povidone-iodine
flush, irrigating the
fornices and the caruncle with at least 10 cc of 5% povidone-iodine using a
forced stream
from a syringe connected to an angio-catheter to effect mechanical
debridement.
After changing gloves, the investigator isolates the ocular field with a
drape, pinning
the eyelashes to the eyelids, and places one or two drops of 5% povidone-
iodine on the ocular
surface at the intended treatment site. An eyelid speculum is used for all
injections.
Example 4
Needle Penetration of Porcine Sclera
Data was recorded on a Universal Material Testing Machine (Instron
Corporation,
Norwood, MA) to mimic insertion of the needle into the eye 6 inm below the
sclera. The
data was then transferred to a MINITAB statistical software (Minitab, Inc,
State College,
PA) for analysis.
The sainples (n=15) were tested as follows:
Control: Becton Dickenson (BD) 1 cc TB syringe paired with a 27Ga 1/2 inch
PrecisionGlideTM needle.
Group 1: HYPAK 1 mL long syringe 27Ga five-bevel %2 inch needle
Group 2: HYPAK 1 mL long syringe 29Ga five-bevel %2 inch needle
Group 3: BD 1mL TB syringe paired with a 30Ga %Z inch precision glide needle
A porcine eye is fixed in test stand and pressurized to standard conditions
for blade
tests to simulate live conditions. The syringe is placed in the test position
on the Instron
device. The crosshead speed is set to 150 millimeters per minute. The needle
is penetrated
about %2 the way into the sclera. The penetration location is about 6 inm
below the center of
the eye pointing toward the center axis of the eye. A new eye is used for each
test (60 eyes
total). The penetration force resulting from various needles are shown in
Table 2 and Figure
8.
CA 02622573 2008-03-13
WO 2007/035621 PCT/US2006/036260
27
Table 2.
Needle Mean penetration Mean Std Dev. Range(grams)
force (normalized to Force
PrecisionGlide 27G) (grams)
PrecisionGlide 27G 1 68.01 19.67 32.93 - 104.10
PrecisionGlide 30G 0.88 59.96 22.92 23.26 - 119.48
HYPAK 27G 2.80 190.3 53.5 113.4 - 318.1
HYPAK 29G Ph siolis 3.24 220.25 61.9 138.8 - 323.3
Incorporation by Reference
The patent and scientific literature referred to herein establishes knowledge
that is
available to those of skill in the art. All patents, patent applications, and
published references
cited herein are hereby incorporated by reference in their entirety.
Equivalents
Those skilled in the art will recognize, or be able to ascertain, using no
more than
routine experimentation, numerous equivalents to the specific embodiments
described
specifically herein. Such equivalents are intended to be encoinpassed in the
scope of the
following claims.