Note: Descriptions are shown in the official language in which they were submitted.
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
ORAL DOSAGE COMBINATION PHARMACEUTICAL PACKAGING
The present invention relates to the packaging of pharmaceuticals and drugs
for
medical uses. The invention has particular utility in the packaging of
combinations of
two or more pharmaceuticals and drugs for the same or co-morbid therapy, and
will be
described in connection with such utility, although other utilities are
contemplated.
The convenience of co-administered two or more active pharmaceutical
ingredients in a unit dosage form, as opposed to the administration of a
number of
separate doses of two or more pharmaceuticals at regular intervals, has been
recognized
in the pharmaceutical arts and is described in our prior U.S. Patent Nos.
6,428,809 and
6,702,683, and co-pending application Nos. 10/756,124 and 10/479,438 and
Provisional
Application No. 60/727,029. Advantages to the patient and clinician include
(1)
minimization or elimination of local and/or systemic side effects; (2) more
effective
treatment of co-morbid conditions; (3) improved polypharmacy; and (4) better
patient
compliance with overall disease management, which in turn may lead to reduced
costs
due to fewer trips to the physician, reduced hospitalization, and improved
patient well-
being.
In our aforesaid U.S. Patent Nos. 6,428,809 and 6,702,683 we have described
packaging two or more active pharmaceuticals or drugs, segregated from one
another, in
a readily ingestible pharmaceutical delivery package which may take the form
of, for
example, a tablet or capsule. Various drug combinations are described and
claimed in
our aforesaid patents.
The present invention provides improveinents over the pharmaceutical delivery
packages described in our aforesaid patents. An embodiment of the present
invention
provides a fixed dose combination medication delivery package which is simple
to
manufacture. More particularly, the embodiments of the present invention
provide a
pharmaceutical delivery package comprising fixed unit dose quantities of two
or more
different active pharmaceutical ingredients (a) combined in a single delivery
package,
and (b) segregated from one another within said package wherein said package
comprises
a core containing a first active pharmaceutical ingredient surrounded at least
in part by a
capsule containing a second active pharmaceutical ingredient. The active
pharmaceutical
1
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
ingredient is defined here as either single pharmaceutical ingredient,
optionally combined
with appropriate excipients, or more than one pharmaceutical ingredient,
optionally
combined with appropriate excipients. The present invention provides certain
unique and
advantageous combinations of drugs that address or overcome one of several
issues
relating to combinational drug therapy, including more efficient treatment of
co-morbid
conditions, polypharmacy, reduction of adverse side effects, adjuctive therapy
and known
drug interactions. In one embodiment of the invention, the delivery package is
designed
to provide for essentially simultaneous release of the two or more
pharmaceutical
ingredients. In another embodiment, the pharmaceutical delivery package
provides for
different release rates of the two or more pharmaceutical ingredients, or
differential
release of the two or inore pharmaceutical ingredients. By way of example, the
invention
provides a combination medication delivery package that includes
pharmaceutical
ingredients providing combinational therapy or polypharmacy for treatment of
diabetes
such as diabetes and hyperlipidemia, and diabetes and hypertension. And yet
another
exeinplary embodiment, the invention provides combinational pharmacology for
treating
hyperlipidemia and liypertension. In a particular embodiment, the present
invention
provides a package in which the active ingredients are segregated from one
another by a
physical barrier suclz that the package may be broken or split into two
halves, with the
active medications divided essentially equally between the halves.
As used herein the term "fixed dose combination medication delivery package"
is
one in which two or more drug components are packaged together, isolated from
one
another, in a single dosage form. The drug components may each comprise an
active
pharmaceutical ingredient or one of the drug components may comprise an active
pharmaceutical ingredient while the other coinprises a substance that effects
the other
ingredient, such as, through an acid base reaction, or a substance that
potentiates or
suppresses the other in a known and predictable manner, or a substance that
suppresses or
increases absorption time or uptake of the other ingredient, or a substance
that suppresses
or increases metabolism through enzymatic activity and effect absorption of
the other
ingredient. Also, in yet another embodiment, the pharmaceutical delivery
package
includes two or more pharmaceutical ingredients packaged in a manner whereby
one or
more of the ingredients will be released at different sites within the
alimentary canal.
2
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
Further features and advantages of the present invention will become clear
from
the following detailed description taken in conjunction with the accompanying
drawings,
wherein like numerals depict like parts, and wherein:
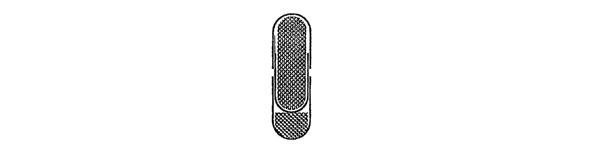
Figs. lA - 1H diagrammatically illustrate the formation of a combination
medication delivery system in accordance with one embodiment of the present
invention;
Figs. 2A - 2E diagrammatically illustrate the formation of a combination
medication delivery system in accordance with a second embodiment of the
present
invention;
Figs. 3A - 3B diagrammatically illustrate how a combination medication
delivery
system of Fig. 2 may be divided or split into two half doses.
Figs. 4A - 4C diagrammatically illustrate embodiments of the combination
medication delivery system according to the present invention enabling
differential
release of the active pharmaceutical ingredients.
Figs. 5A - 51 diagrammatically illustrate embodiments of the combination
medication deli'very system.
Referring first to Figs. 1A - 1B, there is diagrammatically illustrated the
formation
of a combination medication delivery system in accordance with one embodiment
of the
invention. Referring first to Fig. lA, a core 10 comprising a controlled
amount of a first
pharmaceutical ingredient may be formed in a conventional manner as a tablet,
capsule or
caplet by coinbining the active pharmaceutical ingredient with a filler and
binders, and
shaping and forming the tablet, capsule or caplet in known manner. The core
tablet,
capsule or caplet 10 is then coated with a barrier material 12 such as a
gelatin, a starch or
a cellulose such as hydroxypropylmethylcellulose shown in phantom at 12.
Alternatively, core 10 may be sealed within a two-piece capsule 14, 16 formed
of, for
example, gelatin such as press fit gel caps available from Capsugel, Inc. of
Morris Plains,
New Jersey.
Referring to Figs. 1C - 1D, a controlled amount of a second active
pharmaceutical
ingredient 18 is loaded into the bottom of a capsule half shell 20. The
capsule half shell
20 which is sized to fit over the core 10 is assembled to the core 10 and
fixed in place by
shrink or press fitting to form a medication delivery system comprising two
pharmaceuticals, indicated generally at 22.
3
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
The active pharmaceutical ingredients 10 and 18 can be in the form of a
powder,
including fine powder, coarse powder, or powder comprising several different
fractions,
as well as in the form of pellets or beads or a tablet. Additionally the
active
pharmaceutical ingredients 10 and 18 can be in a liquid or semi-liquid form,
which can
facilitate precision dosing. The liquid can be formed as a mixture of the drug
and a
solvent, or as a solution of the drug in a solvent, with the solvent
preferably quickly
evaporating or the liquid formulation quickly solidifying after dosing into
the capsule or
half-capsule. The liquid formulation of the drug can be additionally used to
create an
attachment force between the components of the combination medication delivery
system
in accordance with an embodiment of the present invention. Upon complete
evaporation
of solvent, a strong bond can be formed between the components of the
combination
medication delivery system such as the capsule half shell 20 and capsule part
14.
In yet another embodiment of the present invention, a powder form of the
active
pharmaceutical ingredient 18 is loaded into the capsule half shell 20, and a
small quantity
of a suitable solvent, such as water, is further dosed into the capsule half
shell 20 thus
creating a viscous mixture of the active pharmaceutical ingredient 18 and the
solvent.
Upon complete evaporation of solvent, a strong bond can be fonned between the
components of the combination medication delivery system such as the capsule
half shell
and capsule part 14.
20 Referring now to another embodiment of the present invention shown in Figs.
lE
and 1F, a liquid formulation of the active pharmaceutical ingredient can be
coated on the
outside of the capsule part 14 by using dip coating, as shown in Fig 1E and
then covered
with the capsule half she1120, as shown in Fig. 1F. Upon complete evaporation
of
solvent, a strong bond can be formed between the components of the combination
medication delivery system such as the capsule half she1120 and capsule part
14.
Referring now to Fig. 1 G, another embodiment of the present invention is
illustrated,
wlierein larger quantity of semi-solid formulation of the active
pharmaceutical ingredient
is placed into the capsule half shel120.
Referring now to Fig. 1H, another embodiment of the present invention is
illustrated, wherein more than 2 different active pharmaceutical ingredients
are
4
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
incorporated into the combination medication delivery system according to the
present
invention.
Alternatively, as illustrated in Figs. 2A-2E, the second active pharmaceutical
ingredient 18 may be split and loaded into two capsule half shells 20, 24
which are
assembled to the core 10 and press or shrink fitted to one another. If
desired, each
capsule half shell 20, 24 may contain controlled amounts of different
medications.
Referring now to Figs. 3A - 3B, it is illustrated how a combination medication
delivery system of Fig. 2 may be divided or split into two half doses.
In another embodiment of the present invention, combinations of active
pharmaceutical ingredients are incorporated into a combination medication
delivery
system enabling simultaneous release, differential release, and/or extended
release of
ingredients in the patient's alimentary canal. For simultaneous release, two
or more active
pharmaceutical ingredients incorporated into a combination medication delivery
system
are released practically simultaneously as the capsules or capsule components
dissolve in
the patient's alimentary canal. For differential release applications,
specific capsule
assemblies are enabled wherein one of the components is released before or
after another
component or components, using design and wall thickness and/or wall
composition,
including solubility in acidic and/or alkaline media. Specifically, by varying
capsule wall
composition, porosity capsule material curing, and wall thickness,
differential release of
the active pharmaceutical ingredients is achieved.
Referring now to Fig 4A, active pharmaceutical ingredients inside compartments
50 and 52 are released first, as the capsule walls of compartments 50 and 52
are
dissolving faster due to protection of the compartment 51 by the walls of the
compartments 50 and 52.
Referring now to Figs 4B and 4C, some of the compartments ofthe combination
medication delivery system are shown as having, for illustration purposes, a
tliicker wall
or walls. The thicker wall as shown in these figures indicates slower
dissolution rate of
the wall due to higher thickness, different wall material, or both. Different
material
composition of the wall of the compartments shown in Figures 4B and 4C also
can make
the corresponding compartment resistant to immediate dissolution, thus
delaying the
release of the active pharmaceutical ingredient from the corresponding
compartment.
5
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
This can result in desirable late release or release in a different location
along the
alimentary canal. Furthermore, a compartment wall not soluble in acidic
environment of
the stomach can be made soluble in more neutral to alkaline environment of the
small
intestine, thus enabling release of the active pharmaceutical ingredient
incorporated in
said compartment in the small intestine. Thus one or more of several active
pharmaceutical ingredients contained in the combination medication delivery
system
according to the present invention can be delivered to the stomach, while
another active
pharmaceutical ingredient or ingredients can be delivered to small intestine.
The above embodiments permit simultaneous or differential time release as well
as differential spatial release of active pharmaceutical ingredients. In
addition to delivery
to different parts of gastro-intestinal tract, combinations of active
pharmaceutical
ingredient with a fast action delayed action is possible, such as pain
medication. Another
application of the present invention is for delivery combinations wherein for
example,
first active pharmaceutical ingredients should be taken by the patient before
food intake,
while second active pharmaceutical ingredient should be taken after food
intake. The
combination medication delivery system taken before food intake, with delayed
release of
the second active pharmaceutical ingredient. Another embodiment of the present
invention comprises combination medication delivery system wherein active
pharmaceutical ingredients are released differentially because they can
interact if released
simultaneously due to chemical interactions between ingredients, changes in
the pH or
other parameters in the vicinity of the dissolving ingredient, or ingredients
which can
have a detrimental effect on the action or absorption of another ingredient.
Referring now to Figs. 5A - 51, the combination medication delivery system
assembly can be further reinforced or components of the assembly joined by
utilizing a
tight components fit. In an embodiment, a locking ring or locking ring-groove
combination, as shown in Figs. 5A; 513, 5C, and 5D, or a polymer band, as
shown in Figs.
5E, 5F, and 5G. In addition, a mechanism comprising a locking tight fit groove
as
illustrated by Figs. 5H and 51 enables secure assembly of the combination
medication
delivery system of the present invention. Other methods, including forming a
bond
between components as was described above and depicted in Figures 1 E and 1 F
above
are possible. Still other methods of securing combination medication delivery
system
6
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
assembly are possible, including a hydroalcoholic or other liquid seal, using
shrink wrap-
like securing mechanism and the like. The mechanisms of securing the
combination
medication delivery system assembly are not limited to these described above
and other
mechanisms are also possible.
- As discussed in our aforesaid parent patents and patent applications, there
are
many combinations of drugs that advantageously may be employed for treatment
of co-
morbid diseases, polypharmacy and/or reduce side effects of treatment. By way
of
example, eighty plus percent of diabetics reportedly are also hypertensive.
Hyperlipidemia also is frequently concurrent with diabetes. Thus, an anti-
diabetic agent
conventionally used for treating diabetes such as a sulfonylurea, a
meglitinide, a
biguanide, an insulin sensitizer such as thiazolidinedione, or an alpha-
glucosidase
inhibitor may be combined with a drug useful for treating hypertension or
hyperlipidemia. For example, a dose of sulfonylurea (e.g., Glipizide) can be
combined in
a single delivery system with a dose of a statin (e.g., Atorvastatin), a
fibrate, a bile acid
sequestrant (e.g., Cholestipol), a cholesterol absorption inhibitor or niacin.
Likewise, a
sulfonylurea can be combined with a bile acid sequestrant. Similarly, a drug
for treating
diabetes may be combined with an ACE inhibitor, an angiotension II antagonist,
a
calcium blocker, a beta-blocker, or a diuretic. An example is a combination of
a
biguanide (e.g., Metformin) coadministered with a calcium channel blocker
(e.g.,
Amlodipine). Another example would be the combination of a meglitinide (e.g.,
Repaglinide) and an angiotension II antagonist (e.g., Losartan). Also, drug
combinations
may be selected based on the following criteria:
= The possibility of a pharmacodynamic interaction. Drug combinations may be
selected which exhibit affinity for the same receptors or may produce similar
effects on physiologic function, related or not to their mechanism of action.
= The possibility of a pharmacokinetic interaction. A pharmacokinetic
interaction
can manifest in several ways, some of which can be monitored in vivo and some
of which cannot. One drug product may be selected based on its ability to
alter
the absorption or excretion of another product, change its distribution into
one or
more tissues, or change its pattern or rate of metabolism. Drugs may compete
for
7
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
serum protein binding, resulting in an increase in circulating free levels and
tissue
uptake of one drug.
= The possibility of a toxicologic interaction (e.g., where the target organs
for
toxicity are similar for each drug). A possible lowering of a previously
determined no-effect dose for one or both drug products and/or more severe
toxicities in the affected organs should be considered, wllere applicable.
= The margin of safety for each drug product. If one or more of the drugs has
a
narrow margin of safety (i.e., causes serious toxicity at exposures close to
the
predicted clinical exposure), then the possibility of drug interaction needs
to be
considered.
= The possibility that the drugs compete for or alter the activity or
endogenous
levels of the same enzymes or other intracellular molecules should be
considered
(e.g., co-administration of two prooxidants could deplete endogenous levels of
glutathione).
= The possibility of a chemical interaction. One drug may chemically modify
another drug (e.g., one drug may oxidize, methylate, or ethylate the other
drug).
This could result in new molecular entities with new toxicities. However, this
effect can largely be avoided by providing for delayed release of one of the
drugs.
= The possibility that one drug may conipromise the effectiveness of another
drug.
Various embodiments of the invention will now be further described with
reference to the following non-limiting examples:
(1) Combination #1: Enalapril maleatel and analogs and isomers tliereof are
ACE inhibitors used for the treatment of hypertension. This drug may be used
with the
following and analogs and isomers of beta adrenergic-blocking agents,
methyldopa,
nitrate, calcium blocking agents, Hydralazine6, Prazosin7 and Digoxin8 without
clinically
significant side effects. One or more of these agents may be packaged as above
described
with a drug for treatment of diabetes such as a sulfonylurea, a meglitimide, a
biguanide,
an insulin sensitizer or an alpha-glucosidase inhibitor.
8
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
(2) Combination #2: A hypoglycermic agent such as Metformin HC12 and
analogs and isomers thereof may be packaged as above described with an
angiotensin
converting enzyme inhibitor (ACE inhibitor).
(3) Combination #3: A diabetes drug as above described in Combination #1
or #2 may be packaged as above described with an angiotensin II receptor
antagonist
such as Losartan potassium3 aiid/or Valsartan4.
(4) Combination #4: A diabetes drug as above described may be packaged as
above described with a Beta Adrenergic Blocking Agent such as Bioprolol
fumarate 5 or
Metoprolol succinate6.
(5) Combination #5: A diabetes drug as above described may be packaged as
described in Combinations # 1 or #2 may be packaged with a Calcium Channel
Blocking
Agent sucli as Amlodipine7 or Nifedipine8.
(6) Combination #6: A diabetes drug as above described may be packaged
with a Periferal Adrenergic Blocking Agent such as Prazosin hydrochloride9.
(7) Combination #7: A diabetes drug as above described may be packaged
with an Adrenergic central stimulant such as Methyldopa10 or Clonidinel l
(8) Combination #8: A biguanide such as Metformin14 may be packaged as
above described with a sulfonylurea such as Glipizidels
(9) Combination #9: A biguanide such as Metformin14 may be packaged as
above described with a thiazolidinedione such as rosiglitazone maleate16
(10) Combination #10: A biguanide such as Metformin14 may be packaged as
above described with an alpha glucosidase inhibitor such as Cerivastatin17.
(11) Combination #11: A sliort acting oral insulin may be packaged as above
described with sustained release oral insulin.
The drug delivery system of the present invention also allows three drug
combinations such as diabetes drugs and ACE Inhibitors combined with Beta
Blockers,
methyldopa nitrates, calcium channel blockers, Hydralazine12, Prazosin13,
Digoxin14 as
well as multiple combinations of drugs.
(12) Combination #12: A diabetes drug may be packaged with an ACE
Inhibitor and a Beta Blocker.
9
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
(13) Combination #13: A diabetes drug such as described in Combinations #1
,
or #2 may be paclcaged with a HMG-CoA reductase inllibitor such as
Simvastatin35
Atorvastatin36, or Pravastatin37, and with a bile acid sequestrant such as
Colestipol
hydrohloride38.
(14) Combination #14: A diabetes drug such as described in Combinations #1
or #2 may be packaged with a HMG-CoA reductase inhibitor and with a niacin
compound.
(15) Combination #15: A diabetes drug such as described in Combinations #1
or #2 may be packaged with a HMG-CoA reductase inhibitor or Combination #14,
and
with a hypolipidemia agent such as Gemf brozi139
While the above embodiments of the invention has been described with
particular
drug combinations segregated from one another, it will be understood that some
of the
above-listed drug conibinations also may be blended and packaged in a single
tablet,
capsule or caplet when chemical interaction is not a problem.
Other embodiments of the present invention are directed towards combinations
of
at least one active phannaceutical ingredient and at least one substance which
can be an
active pharmaceutical ingredient or non- pharmaceutical ingredient and which
is
mitigating the negative effects of said first active pharmaceutical
ingredient, or
promoting/enhancing action of said first active pharmaceutical ingredient, or
is
promoting general health and well-being of the patient taking said first
active
pharmaceutical ingredient. The following non-limiting examples are
illustrating this
aspect of the embodiments of the present invention:
Example 16: A combination of first active pharmaceutical ingredient which may
cause a side effect with a second active pharmaceutical ingredient medication
mitigating
side effect of the first active pharmaceutical ingredient are combined in a
single delivery
package. Examples include first active pharmaceutical ingredient with side
effect
causing, e.g., constipation, nausea, gas/bloating, heartburn, pain or cramps;
and a second
active pharmaceutical ingredient, mitigating the above side effect of the
first ingredient,
e.g. correspondingly laxative medication, nausea treatment medication, anti-
gas and anti-
bloating medication, anti-acid medication, pain reliever & muscle relaxant
medication.
More specific example may include pain medication causing constipation and
nausea,
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
e.g. oral narcotic with the second ingredient containing stool softener and
anti-nausea
components.
Example 17. In another embodiment of the present invention, a first active
pharmaceutical ingredient is combined with a second active pharmaceutical
ingredient
which controls and stops the action of the first ingredient after the time
necessary for the
action of the first ingredient. As an example, a combination of anti-cancer
drug such as
Methetrexate with immediate release, and the "quencher" substance, such as L-
leukovorin, with delayed release, can be advantageously delivered within the
combination medication delivery system.
Example 18: In another embodiment of the present invention, a first active
phairnaceutical ingredient is combined with a second active pharmaceutical
ingredient or
a substance which optimizes the pH in the immediate vicinity of the first
active
pharmaceutical i.ngredient for facilitating dissolution, and/or absorption of
the first active
pharmaceutical ingredient. Additionally, control and/or neutralization of the
stomach acid
to slow down first active pharmaceutical ingredient breakdown can be affected
thus
improving the bioavailability of the first active pharmaceutical ingredient.
Non-limiting
examples of pH controlling substances include pH buffering compounds known in
the
art.
Example 19: In another embodiment of the present invention, a first active
pharmaceutical ingredient which is fat soluble is combined with a second
active
pharmaceutical ingredient or a substance containing oil for better drug
solubility and
absorption.
Example 20: In another embodiment of the present invention, a first active
pharmaceutical ingredient is combined with an enzyme wherein said enzyme
facilitates
active pharmaceutical ingredient absorption and/or bio-availability or
mitigates side
effects.
Example 21: In another embodiment of the present invention, a first active
pharmaceutical ingredient is combined with a nutraceutical or a vitamin. Non-
limiting
examples include combination of (i) Nexium (esomeprazole) which changes the pH
in the
stomach and thus prevents absorption of B 12 vitamin which can only happen at
low pH,
11
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
with B-group vitamins and (ii) Anti-viral active pharmaceutical ingredients
with vitamin
C or multivitamin supplements.
Example 22: In another embodiment of the present invention, a first active
pharmaceutical ingredient is combined with a surfactant which facilitates
absorption or
vice versa, inhibits absorption in the certain part of the alimentary canal.
Example 23: In another embodiment of the present invention, a first active
pharmaceutical ingredient is combined with a sleeping aid.
Another embodiment of the present invention is directed towards combinations
of
at least two active pharmaceutical ingredients within the same class of
pharmaceuticals
treating or preventing the same symptoms or same disease (polypharmacy), such
as
infectious disease, metabolic disorders, cardiovascular disease, pain, cancer,
transplant-
related treatment, gastrointestinal disorders, respiratory diseases,
autoimmune diseases,
vaccines, etc. The following non-limiting examples are illustrating this
embodiment of
the present invention:
Example 24: Combination of anti-infective active pharmaceutical ingredients,
with examples including at least two antibiotics combined, resulting in a
broad spectrum
anti-bacterial action. Another example includes a combination of anti-viral
and anti-
bacterial pharmaceutical ingredients resulting in a treatment of an infection
with
unknown pathogen as well as treatment of bacterial infections often following
viral
infections. Yet another example 'includes a combination of at least two active
phannaceutical ingredients which are treating cancer or managing the symptoms
of
cancer, for example topoisomerase inhibitor drug and anti-cancer monoclonal
antibody
drug. Another example includes a combination of antibiotic with antibiotic
potentiators.
Potentiators confer increased activity to pha.rmaceutical agents, such as, for
instance,
antibiotics. Although potentiators may lack themselves any antibacterial
activity, in
combination with antibiotics, such as for example, erythromycin,
chloramphenicol,
tetracycline, linezolid, clindamycin or rifampin, potentiators promote and
significantly
increase the activity of the pharmaceutical agent, in this example,
antibiotic.
Example 25: In another embodiment of the present invention, the same active
pharmaceutical ingredient is combined in at least two formulations, including
a fast
release or fast action and a slow release or long term action formulation. The
slow release
12
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
or long term action can be achieved by differential release capsule components
design, as
discussed above, or by formulation of the drug, excipients and tablet forming
means, and
other means available to these skilled in the art, with beneficial effects
including better
treatment or relief of symptoms and potential for the decrease of the overall
medication
intake. Specific non-limiting examples include: nitroglycerin, with fast
acting/fast
dissolving formulation providing for a fast action for acute treatment with a
slow release
formulation for maintenance; antibiotic with fast action / fast dissolution
formulation for
immediate increase of the concentration in blood plus slow release; pain
medication, with
a fast acting formulation for immediate pain relief help combined with a slow
release
pain maintenance medication; sleeping aid with a fast dissolving or fast
acting
formulation for immediate effect combined with a delayed release for
maintenance
throughout the night, with specific non-limiting example including Ambien.
Example 26: In another embodiment of the present invention, at least two anti-
cholesterol pharmaceutical ingredients such as statins of different types are
combined in
the combination medication delivery system. Since effects of statins are
highly
individual, a combination medication is advantageous.
Example 27: In another embodiment of the present invention, a broad spectrum
anti-hypertensive combination comprises two or more hypertension-reducing
drugs in the
combination medication delivery system, including medications of the same
type, such as
beta-blockers or diuretics, or medications of different types or classes, such
as beta-
blocker and diuretic.
Various other changes may be made without departing from the spirit and scope
of the invention. For example, the above-described capsules may be used with
various
drug combinations as described in our earlier U.S. Patent Nos. 6,428,809 and
6,702,783,
and the drug combinations described in our co-pending application Nos.
10/756,124 and
10/479,438. Still other drug combinations, which term may also include
vitamins, dietary
supplements, minerals and nutraceuticals, which may be used with the above-
described
capsules or with the combination capsules, tablets or caplets described in our
earlier
patents and pending applications, include combination drug therapies for
treating
infectious disease, e.g., AIDS, TB and malaria, and for pain management, e.g.,
13
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
nonsteroidal anti-inflammatory drugs/proton pump inhibitors (NSAIDS/PPI).
These
include, by way of example, and not limitation:
Example 28. In another embodiment of the present invention, at least two anti-
malaria drugs are combined in the combination medication delivery system.
Specific
Examples of potential drug combinations include, Artesunate and Mefloquine;
Artemether and Lumefantrine; Chloroquine and Paracetamol. More generally, a
combination of at least two of the following representative anti-malaria drugs
in the
combination medication delivery system are exemplified: Artemether;
Lumefantrine;
Artensunate; Amodiaquine HCI; Atovaquone-proguanil; Quinine Sulfate;
Chloroquine
Sulfate; Hydroxychloroquine Sulfate; Doxycycline; Mefloquine; Primaquine;
Sulfadoxine; Pyrimethainine; Paracetamol.
Example 29. In another embodiment of the present invention, at least two HIV
treatment medications are combined in the combination medication delivery
system.
Specific Examples of potential drug combinations include, at least two of the
nucleoside
reverse transcriptase inhibitor (NRTI) medications, including e.g. Abacavir;
lamivudine;
Didanosine; Emtricitabine; Stavudine; Tenofovir. Another example includes
combining a
non-nucleoside reverse transcriptase inhibitor (NNRTI) and a nucleoside
reverse
transcriptase inhibitor (NRTI) e.g. Nevirapine (NNRTI) and didanozine (NRTI);
Efavirenz (NNRTI) and abacavir sulfate (NRTI). Yet another example includes
combining two NRTI's and one NNRTI e.g. Abacavir and lamivudine and efavirenz
or
Abacavir and lainivudine and nevirapine. Still another Example includes
combining at
least two 2 NRTI's and a PPI: Abacavir and lamivudine and lopinavir/ritonavir.
Still
another example includes a combination of at least two of the anti-HIV drugs
selected
from the group comprising: abacavir sulfate; didanozine; stavudine; tenofovir;
disoproxil;
fumarate; zidovudine; lamivudine; emtricitabine; lopinavir/ritonavir;
nevirapine;
efavirenz; nelfinavir. Still other combinations include combination of AZT
and3TC;
combination of abacavir and AZT and 3TC; a combination of lopinavir and
ritonavir;
combinations of ABC and 3TC; and combination of emtricitabine and tenofovir.
Example 30. In another embodiment of the present invention, at least two of
Tuberculosis treatment medications are combined in the combination medication
delivery
system. Specific Examples of potential combinations include at least two of
the following
14
CA 02625776 2008-04-11
WO 2007/047371 PCT/US2006/039894
medications: Isoniazid; Rifampicin; Pyrazinamide; Ethambutol HCI;
Streptomycin;
Capreomycin; Cycloserine; Protionamide; Macrolides; Fluoroquinolones; p-
Salicylic
acid.
Example 31. In another embodiment of the present invention, at least two of
the
pain treatment medications are combined in the combination medication delivery
system.
Specific Examples of potential combinations include at least two of the
following
medications: Aspirin; Carbex; Codeine; Luvox; Marplan; Nardil; Neurotin;
OxyContin;
Pamate; Topamax; Tylenol/Acetaminophen; Vicodin; Xyrem; Zarontin; Zoloft;
Zomig.
Example 32. Anotlier embodiment of the present invention is a combination of
aspirin or acetylsalicylic acid combined in the combination medication
delivery system
with a active ingredient mitigating side effects of aspirin, such as effects
related to the
acidity of aspirin. Specific Examples of potential combinations include
buffering
compounds and anti-acid compounds in combination with aspirin.
Example 33. Another embodiment of the present invention is a combination
therapy for treatment of lupus nephritis. Specific example includes
combination of
methylprednisolone and cyclophosphainide.
Still other changes are permissible. For example, a pre-formed tablet, capsule
or
caplet containing one pharmaceutical ingredient may be obtained from the
manufacturer.
Then, a compounding pharmacist may encase that pre-formed tablet within an
outer
capsule in which a second pharmaceutical ingredient is loaded. This permits a
compounding pharmacist to produce custom drug combination packages. Also, if
desired, the pharmaceutical delivery system may be scored adjacent its mid-
point 26 so
that the delivery system may be broken into two equal halves 28A, 28B, so that
the user
may create half dose tablets each half containing equal amounts of both
medications.
(See Figs. 3A - 3B).
Various other changes may be possible without departing from the spirit and
scope of the invention. For example, the core may comprise a capsule
containing a liquid
or gel. Still other changes are possible.