Note: Descriptions are shown in the official language in which they were submitted.
CA 02627196 2008-04-24
SELF-EXPANDING MEDICAL OCCLUSION DEVICE
BACKGROUND OF THE INVENTION
[000111. Field of the Invention
[0002] The present invention relates to a self-expanding medical occlusion
device for treating
heart defects in patients, in particular closing abnormal openings in tissue,
whereby the occlusion
device is introduced into the body of a patient in minimally invasive fashion
using a catheter
system and consists of a braiding of thin threads, whereby the braiding
exhibits a first
preliminarily definable shape as the occlusion device is being inserted into
the patient's body and
a second preliminarily definable shape in the implanted state of the occlusion
device, whereby
the braiding of said occlusion device in the first profile form is in a
collapsed state and the
braiding in the second profile form is in expanded state.
[0003] 2. Description of the Related Art
[0004] The principle behind this type of occlusion device is known to at least
some extent in
medical technology. For example, an occlusion device for treating septum
defects is known from
DE 10 338 702 of Aug. 22, 2003, consisting of a braiding of thin wires or
threads and given a
suitable profile in a molding and heat treatment process. The known occlusion
device has a
proximal retention area which is particularly distinctly flat, a distal
retention area, and a
cylindrical crosspiece between said proximal and distal retention areas. The
ends of the wires
forming the braiding converge into a holder in the distal retention area. This
is hence designed as
such so that the two retention areas of the known occlusion device will
position on the two sides
of a shunt to be occluded in a septum, usually by means of an intravascular
surgical procedure,
while the crosspiece will transverse the shunt.
[0005] Medical technology has long endeavored to be able to occlude septal
defects, for instance
atrioseptal defects, by means of non-surgical transvenous catheter procedures,
in other words,
without having to perform an operation in the literal sense. Various different
occlusion systems
have been proposed, each with their own pros and cons, without any one
specific occlusion
system having yet become widely accepted.
[0006] In making reference to these different systems, the following will use
the terms "occluder"
or "occlusion device." In all interventional occlusion systems, a self-
expanding umbrella system
is introduced transvenously into a defect to be occluded in a septum. This
type of system might
comprise two umbrellas: one, for example, positioned at the distal side of the
septum (i.e. the
side furthest from the median plane of the body/heart) and one at the proximal
side of the septum
(i.e. the side closer to the median plane of the body), whereby the two
uinbrella prostheses are
subsequently secured to a double umbrella in the septal defect. Thus, in the
assembled state, the
occlusion system usually consists of two clamped umbrellas connected to one
another by means
1
CA 02627196 2008-04-24
of a short bolt transversing the defect.
[0007] However, a disadvantage to such prior art occlusion devices turns out
to be the relatively
complicated, difficult and complex implantation procedure. Apart from the
complicated
implantation of the occlusion system in the septal defect to be occluded, the
umbrellas utilized
are susceptible to material fatigue along with fragment fracture. Furthermore,
thromboembolic
complications are frequently to be anticipated.
[0008] In order to enable the inventive occlusion device to be introduced by
means of a surgical
insertion instrument andlor guidewire, a holder is provided at the end of the
distal retention area
which can engage with the insertion instrument andlor guidewire. It is thereby
intended that this
engagement can be readily disengaged after positioning the occlusion device in
the defect. For
example, it is possible to devise the braiding at the end of the distal
retention area of the
occlusion device in such a manner so as to create an internal threading in the
holder to engage
with the insertion instrument. Of course, other embodiments are naturally also
conceivable.
[0009] With another type of occlusion device, the so-called Lock-Clamshell
umbrella system,
two stainless steel preferably Dacron-covered umbrellas are provided, each
stabilized by four
arms. This type of occluder is implanted into the patient through a vein.
However, seen as
problematic with the Lock-Clamshell occluder is the fact that the insertion
instruments necessary
to implant the device need to be of relatively large size. A further
disadvantage seen with other
systems, for example the Amplatz occluder, is that many different occluder
sizes are needed in
order to cope with the respective dimensions of the septal defects to be
occluded. It thus turns out
that the umbrellas do not flatten out completely in the inserted state if the
length or the diameter
of the crosspiece inserted into the defect is not of an optimum match. This
results in incomplete
endothelialization. It has furthermore been shown that many of the systems
implanted into
patients' bodies exhibit material fatigue and fractures in the metallic
structures due to the
substantial mechanical stresses over a longer period. This is especially the
case given permanent
stress between an implant and the septum.
[0010] In order to overcome these disadvantages, self-centering occlusion
devices have been
developed which are inserted into the body of the patient and introduced into
the septal defect to
be occluded by way of a minimally invasive procedure, for example using a
catheter and
guidewires. Their design is based on the principle that the occlusion device
can be tapered to the
dimensions of the insertion instrument and/or catheter used for the
intravascular procedure. Such
a tapered occlusion device is then introduced by catheter into the septal
defect to be occluded,
respectively into the shunt of the septum defect to be occluded. The occluder
is then discharged
from the catheter, upon which the self-expanding umbrellas, retention plates
respectively,
subsequently unfold against the two sides of the septum. The umbrellas in turn
comprise fabric
inserts made from or covered by, for example, Dacron, with which the
defect/shunt is occluded.
The implants remaining in the body are more or less completely ingrown by the
body's own
tissue after a few weeks or months.
2
CA 02627196 2008-04-24
[0011 ] An example of a self-centering occlusion device of the type specified
is known from WO
99/12478 Al, which is a further development of the occlusion device known as
the "Amplatz
occluder" in accordance with US printed U.S. Pat. No. 5,725,552. Same consists
of a braiding of
a plurality of fine, intertwined nitinol wire strands in the shape of a yo-yo.
Each braiding is
produced in its original form as a rounded braiding having loose wire ends
both at its leading end
(its proximal side, respectively) as well as at its trailing end (its distal
side, respectively). During
the subsequent processing of the rounded braiding, each of these loose ends
must then be
gathered into a sleeve and welded together. After the appropriate processing,
both the proximal
side as well as the distal side of the finished occluder exhibit a protruding
collar. Dacron patches
are sewn into the distal and proximal retention umbrellas and the interposed
crosspiece. Because
of the memory effect exhibited by the nitinol material used, the two retention
umbrellas unfold
by themselves upon exiting the catheter, initially in a balloon-like
intermediate stage, whereby
the retention umbrellas ultimately positioned on the two sides of the septum
eventually assume a
more or less flattened form. The crosspiece centers itself automatically into
the shunt to be
occluded during the positioning of the umbrellas.
[0012] The shape memory nitinol material known from prior art occlusion
devices and that as
previously described has, however, proven to have certain disadvantages with
respect to
occlusion devices. Nitinol, which is an atomistic alloy of nickel and
titanium, is only
conditionally suitable as a shape memory material for medical occlusion
devices because the
maximum deformation for nitinol between the first preliminarily definable
shape given as the
occlusion device is being inserted into the body of the patient and the second
preliminarily
definable shape given when the occlusion device is in implanted state only
amounts to about 8%.
In other words, this means that the shape memory nitinol material is only
conditionally suitable
for collapsing an occlusion device as small as possible for the implantation
procedure. Hence, the
implantation procedure when using a medical occlusion device which has
braiding made from
nitinol is not a particularly gentle one on the patient. Moreover, being an
alloy of nickel and
titanium, nitinol constitutes a permanent foreign body such that once in the
implanted state,
relevant defense system reactions can be expected from the body.
[0013] On the basis of the problematic task as set forth, which is in
particular coupled with the
use of nitinol as a shape memory material for medical occlusion devices, the
task on which the
present invention is based is that of improving upon a self-expanding medical
occlusion device
of the type specified at the outset to afford the patient a gentler
implantation of the device.
SUMMARY OF THE INVENTION
[0014] This task is solved in accordance with a self-expanding medical
occlusion device of the
type specified at the outset which inventively has the threads of the braiding
including a shape
memory polymer composite so that the braiding is deformed from a temporary
shape to a
permanent shape by means of an external stimulus, whereby the temporary shape
is given in a
first profile form and the permanent shape is given in a second profile form.
3
CA 02627196 2008-04-24
[0015] The inventive solution has a number of significant advantages over the
known and above-
described medical occlusion devices of the prior art. Especially because a
shape memory polymer
composite exhibits considerably better memory properties than nitinol, a far
gentler implantation
is afforded when implanting said medical occlusion device. Compared to known
shape memory
materials, for example the nitinol shape memory alloy; i.e., an atomistic
alloy of nickel and
titanium, shape memory polymers are far superior in terms of their memory
properties. Only little
effort is required in the (heating/cooling) process to program the temporary
shape or,
respectively, to restore the permanent shape. Moreover, in the case of
nitinol, for example, the
maximum deformation between permanent and temporary shape amounts to just 8%.
In contrast,
shape memory polymers exhibit substantially higher deformability capabilities
of up to 1100%.
[0016] The inventive polymer composite also exhibits advantages over the prior
art with respect
to the manufacturing process since conventional processing methods can be
used. For example,
the polymer could conceivably be initially given its permanent shape using
conventional
processing methods such as injection molding or extrusion. The synthetic can
then be
subsequently deformed and fixed in its desired temporary shape, which is a
process known as
"programming." This procedure can ensue with polymers such that the specimen
is heated,
deformed and then cooled. Or the polymer/synthetic can also be deformed at
lower temperature,
a process known as "cold drawing." The permanent form thus becomes a memory
shape which is
remembered while still in temporary form. Once an external stimulus acts on
the molded polymer
body, this leads to the shape memory effect being triggered and thus to a
restoring of the
permanent memory shape. Cooling the specimen effects an irreversible
degeneration of the
temporary shape, which is why this is referred to as a so-called one-way shape
memory effect.
The original temporary form--as well as other forms--can be reprogrammed upon
new
mechanical deformation being effected.
[0017] Shape-memory polymers are included in a group which is known as smart
polymers in
English and are polymers which exhibit a shape memory effect; i.e., which are
able to change
their outer form in response to external stimuli such as, for example, a
change in temperature.
The above-described process of programming and shape restoration is depicted
schematically in
FIG. 1.
[0018] A particularly preferred implementation provides for the external
stimulus being a
definable switching temperature. It is thus conceivable that in order to
trigger the shape memory
effect and thus the restoring of the permanent memory shape, the braiding of
the molded polymer
body must be heated to a higher temperature than the switching temperature.
Appropriately
selecting the chemical composition to a polymer composite allows the initial
specifying of such a
specific transition temperature.
[0019] It is thus particularly preferred to set the switching temperature
within a range of between
room temperature and the patient's body temperature. This is of particular
advantage as regards
the application of the occlusion device as an implant in the body of a
patient. As such, all that
must be ensured when implanting the occlusion device is that the device is not
warmed up to the
4
CA 02627196 2008-04-24
patient's body temperature (36° C.), which would trigger the polymer's
shape memory
effect, until in the implanted state.
[0020] One possible implementation of the inventive occlusion device in which
the external
stimulus is a definable switching temperature provides for the polymer
composite to comprise
polymer switching elements, whereby the temporary shape of the braiding is
stabilized below the
definable switching temperature based on the characteristic phase transitions
of the polymer
switching elements.
[0021] It is thus conceivable, for example, that should the polymer composite
exhibit a
crystalline or semi-crystalline polymer network having crystalline switching
segments,
[0022] the temporary shape to the braiding is fixed and stabilized by freezing
the crystalline
switching segments at crystallization transition, whereby the switching
temperature is a function
of the crystallization temperature, the switching temperature of the
crystalline switching
segments respectively.
[0023] On the other hand, in the case of a polymer composite such as an
amorphous polymer
network having amorphous switching segments, it is feasible to fix and
stabilize the temporary
shape of the braiding at glass transition by freezing of the amorphous
switching segments,
whereby the switching temperature is a function of the glass transition
temperature of the
amorphous switching segments.
[0024] In accordance with these preferred embodiments, characteristic phase
transitions can thus
be used to stabilize the temporary shape of the shape memory polymers; i.e.,
crystallization in the
case of crystalline or semi-crystalline polymers and glass transition in the
case of amorphous
polymers. Accordingly, in the case of elastic polymers composed of covalent
bonded polymer
networks, the mechanism of shape memory transition is based on the one hand on
the stabilizing
of the permanent shape by chemical bonding of the polymer chains and, on the
other, by the
fixing of the temporary form by crystallization of segments (semi-crystalline
polymer networks)
or by freezing the switching segments in the case of glass transition
(amorphous polymer
networks). The Ttrans switching temperature, the exceeding of which
triggers the shape
memory effect, is accordingly contingent upon the synthetic's Tm melting
temperature, the
Tg glass transition temperature respectively, in the corresponding
temperature range.
[0025] FIG. 2 schematically depicts the molecular mechanism of a thermally-
induced shape-
memory transition for a semi-crystalline polymer network. When the ambient
temperature is
higher than Ttrans (Tm) of the crystalline switching segments, these
segments are then
flexible and can be elastically deformed, for example stretched.
[0026] The temporary shape which is formed is fixed by cooling below
Ttrans (Tm);
i.e., by the crystalline areas forming upon cooling, acting quasi as physical
crosslinks. When the
polymer is heated above Ttrans (Tm), the permanent shape is once
again restored. The
5
CA 02627196 2008-04-24
thermodynamic force driving the resumption of the permanent shape is the
entropic gain thereby
realized. Amorphous polymer networks having shape memory effect function
similar to the semi-
crystalline polymer networks having shape memory effect, whereby the switching
temperature
represents the glass transition temperature and the temporary shape is fixed
by freezing the
mobility of the amorphous switching segments.
[0027] Another advantageous implementation or development of the previously-
cited
embodiments of the inventive occlusion device provides for the polymer
composite to comprise a
linear, phase-segregated multiblock copolymer network which can exhibit at
least two different
phases, whereby the first phase is a hard segment-forming phase in which a
plurality of hard
segment-forming blocks are formed in the polymer which serve the physical
crosslinking of the
polymer structure and define and stabilize the permanent shape to the
braiding, and whereby the
second phase is a switching segment-forming phase, in which a plurality of
switching segment-
forming blocks are formed in the polymer which serve to fix the temporary
shape of the braiding,
whereby the transition temperature from the switching segment-forming phase to
the hard
segment-forming phase is the switching temperature, and whereby conventional
methods such as
injection molding or extrusion processes can be used to set the profile form
to the braiding above
the transition temperature of the hard segment-forming phase.
[0028] With respect to the chemical composition of the polymer composite of
which the braiding
of the inventive medical occlusion device is comprised, a preferred
implementation of the
inventive medical occlusion device having a braiding consisting of a shape
memory polymer
composite can provide for the polymer composite to have thermoplastic
polyurethane elastomers
of a multiblock structure, whereby the hard segment-forming phase is formed by
conversion of
diisocyanates, in particular methylene-bis(4-phenylisocyanate) or
hexamethylene diisocyanate,
with diols, in particular 1,4-butanediol, and whereby the switching segment-
forming phase yields
from oligomeric polyether/poly-esterdiols, in particular based on OH-
terminated
poly(tetrahydrofuran), poly(.epsilon.-caprolactone), poly(ethylene adipate),
poly(ethylene
glyocol) or poly(propylenglycol).
[0029] In an alternative yet advantageous implementation, it is conceivable
for the phase-
segregated diblock copolymers of the polymer composite to exhibit an amorphous
A-block and a
semi-crystallized B-block, whereby the glass transition of the amorphous A-
block constitutes the
hard segment-forming phase, and whereby the melting temperature of the semi-
crystalline B-
block serves as the switching temperature for the thermal shape memory effect.
[0030] It is advantageously provided in the latter preferred implementation
with respect to the
polymer composite for this compound to have polystyrol as the amorphous A-
block and
poly(1,4-butadiene) as the semi-crystalline B-block.
[0031 ] In consequence thereof, the linear phase-segregated multiblock
copolymers constitute an
important group of shape memory polymers. These polymers have two separate
phases, whereby
the one phase with the higher transition temperature serves the physical
crosslinking and for
6
CA 02627196 2008-04-24
defining the permanent shape. Conventional processes for profile shaping such
as injection
molding or extrusion can be used above this melting temperature. As indicated
above, the second
phase is then molecular switching and serves to fix the temporary shape,
whereby the transition
temperature of the switching phase (Ttrans) can be a melting or a glass
transition
temperature.
[0032] Included among the shape memory polymers which function in accordance
with this
operating principle based on linear block copolymers are thermoplastic
polyurethane elastomers
having a multiblock structure.
[0033] As shown in FIGS. 3 and 4, the hard segment-forming phase is usually a
process of
converting commercial diisocyanates such as, for example, methylene-bis(4-
phenyliso-cyanate)
(MDI) or hexamethylene diisocyanate (HMDI) with commercial diols such as, for
example, 1,4-
butanediol. The switching segment-forming phase then yields from the
commercially-available
oligomeric polyether/polyesterdiols used such as, for example, based on OH-
terminated
poly(tetrahydro furan), poly(. epsilon. -caprolactone), poly (ethylene
adipate), poly(ethylene
glyocol) or poly(propylenglycol).
[0034] An example yielding from MDI/1,4-butanediol as the hard segment-forming
phase and
poly(.epsilon.-caprolactone) is semi-crystalline linear block copolymers
having shape memory
effect and a switching temperature of Tm=44-55° C. at a molecular
weight of from
1600 to 8000 g/mol. In contrast thereto, linear shape-memory block copolymers
having an
amorphous phase and a switching temperature of Tg=-5 to 48° C.
from MDI/1,4-
butanediol as the hard segment-forming phase and flexible poly(ethylene
adipate) can have a
molecular weight of from 300 to 2000 g/mol.
[0035] Alternatively to the embodiment in which the polymer composite, of
which the braiding
of inventive medical occlusion device is composed, exhibits a phase-segregated
diblock
copolymer, it is provided for the polymer composite to exhibit a phase-
segregated triblock
copolymer having a semi-crystalline central B-block and two amorphous terminal
A-blocks
whereby the A-blocks constitute the hard segment and the B-block establishes
the switching
temperature.
[0036] It would be conceivable here for the polymer composite to have semi-
crystalline poly-
(tetrahydrofuran) as the central B-block and amorphous poly(2-methyloxazolin)
as the terminal
A-blocks.
[0037] Pursuant thereto, other shape memory polymers based on linear block
copolymers and
which function according to the above-described operating principle are the
claimed phase-
segregated diblock or triblock copolymers, which would include, for example,
AB-block
copolymers of 34 wt. % polystyrol (PS) as the amorphous A-block and 66 wt. %
poly(1,4-
butadiene) (PB) as the semi-crystallized B-block.
7
CA 02627196 2008-04-24
[0038] FIG. 5 shows the structure of such diblock or triblock copolymers
having shape memory
effect. The glass transition of PS is known to be 90° C. and
constitutes the hard segment-
forming phase. The melting temperature of the PB crystallite serves as the
switching temperature
for the thermal shape memory effect and is between 45 and 65° C.
[0039] Another example likewise shown in FIG. 5 depicts ABA triblock
copolymers of semi-
crystalline poly(tetrahydrofuran) (PTHF) as the central B-block and amorphous
poly(2-
methyloxazolin) (POX) as the terminal A-blocks. The A-blocks having an average
molecular
weight of 1500 g/mol exhibit a glass transition temperature of 80° C.
and constitute the
hard segment. The B-block having a molecular weight of between 4100 and 18800
g/mol is
semi-crystallized and melts between 20 and 40° C. depending upon the
molecular weight.
The switching temperature can thus vary within this range.
[0040] Polymer compounds having polynorbomene, polyethylene/nylon-6-graft
copolymers
and/or crosslinked poly(ethylene-co-vinyl acetate) copolymers have been
determined to be
advantageous with respect to the chemical composition of the polymer composite
used in the
inventive medical occlusion device.
[0041 ] Likewise proven to be advantageous is for the polymer composite to
exhibit a covalent
crosslinked polymer network formed by polymerization, polycondensation and/or
polyaddition of
difunctional monomers or macromers with additive of tri or higher funetional
crosslinking,
whereby given an appropriate selection of the monomers, their functionality
and ratio of
crosslinkers, the chemical, thermal and mechanical properties of the polymer
network as formed
can be specifically and selectively set. This thus enables the precise and
advance establishing of
the properties for the occlusion device at the transition from the first
preliminary definable
profile shape to the second preliminary definable profile shape, and in
particular, the precise and
advance establishing of the course of events upon expansion of the occlusion
device.
[0042] A particularly preferred implementation of the latter embodiment
provides for the
polymer composite to be a covalent polymer network which constitutes a
crosslinker by
crosslinking copolymerization of stearylacrylate and methacrylic acid with
N,N'-
methylenebisacrylamide, whereby the shape memory effect of the polymer
composite is based on
crystallizing stearyl-side chains.
[0043] It is likewise feasible for the polymer composite to exhibit a covalent
crosslinked polymer
network which is formed by subsequent crosslinking of linear or branched
polymers.
[0044] Additionally conceivable here would be, for example, activating the
crosslinking by
ionizing radiation or by thermal fission of radical-forming groups.
[0045] Hence, a large group of shape-memory polymers constitute the covalent
crosslinked
polymer networks as previously indicated at the outset. Based on their
structure, two different
strategies for synthesis are advantageously followed:
8
CA 02627196 2008-04-24
[0046] Polymerization, polycondensation or polyaddition of difunctional
monomers or
macromers with additive of tri or higher functional crosslinking. Given the
appropriate selection
of the monomers, their functionality and the ratio of crosslinkers, the
chemical, thermal and
mechanical properties of the polymer network as formed can be specifically and
selectively set.
[0047] A second synthesis variant for covalent shape-memory polymer networks
is given by the
subsequent crosslinking of linear or branched polymers. Cross-linking density
is hereby heavily
dependent on the reaction conditions selected. Here, the crosslinking is
usually activated by
ionizing radiation or by thermal fission of radical-forming groups. For
example, polyethylene
films receive heat-shrinking properties from irradiating polyethylene with
.gamma.-steel or cross-
linked polyethylene-polyvinylacetate copolymers obtain shape memory effect by
homogenous
addition of the dicumylperoxide radical initiator.
[0048] FIG. 6 shows feasible monomers for covalent shape memory polymer
networks. Here, for
example, covalent polymer networks have shape memory effect obtained by
crosslinked
copolymerization of stearylacrylate STA and methacrylic acid MAA with N,N'-
methylene-
bisacrylamide MBA as the crosslinker, the shape memory effect of which is
based on the
crystallizing stearyl-side chains. Based on the relative stearylacrylate
ratio, a melting or switching
temperature of between 35 and 50° C. results.
[0049] It can be established in summary that both basic types of shape memory
polymers; i.e.,
thermoplastic elastomers and covalent polymer networks, differ in their
properties, their
processing methods and their programming procedures. The thermoplastic
elastomers need a
minimum part by weight of hard segment-forming polymer chains to ensure the
physical
crosslinks. In the case of covalent networks, the ratio of hard segment-
forming polymer chains
can be higher. It is, of course, conceivable for the described shape memory
polymers to find
potential application across a wide range of technologies, for example with
respect to self-
repairing auto bodies, switching elements, sensors and right on up to smart
packaging.
[0050] Of particular interest with respect to the use of medical occlusion
devices are implant
materials which are synthetically biodegradable. Degradable materials,
respectively polymers,
have bonds which are fissionable under physiological conditions. Degradable-
ness is the term
used if a material decomposes from loss of mechanical properties due to or
within a biological
system. An implant's external form and dimensions may in fact remain intact
during the
decomposition. What is meant with respect to degradation time, provided no
additional
quantifying data is given, is the time it takes for the complete loss of
mechanical properties.
Biostable materials refer to materials which remain stable within biological
systems and which
degrade at least only partially over the long term.
[0051 ] The present invention provides for medical occlusion devices of the
type specified at the
outset and in accordance with the previously-cited preferred embodiments to
consist of a braiding
which is synthesized from a polymer composite comprising at least one bio-
degradable material.
9
CA 02627196 2008-04-24
[0052] A particularly preferred implementation of the latter embodiment
provides for the
polymer composite to exhibit a hydrolytically degradable polymer, in
particular poly(hydroxy
carboxylic acids) or the corresponding copolymers. Hydrolytic degradation has
the advantage that
the rate at which degradation occurs is independent of the site of
implantation since water is
present throughout the system.
[0053] However, making use of enzymatically degradable polymers is also
conceivable in
another embodiment. Feasible in particular is that the polymer composite
exhibit a biodegradable
thermoplastic amorphous polyurethane-copolyester polymer network.
[0054] Likewise requisite for the chemical composition to the polymer
composite for the
inventive medical occlusion device is that the polymer composite exhibit a
biodegradable elastic
polymer network, obtained from crosslinking of oligomer diols with
diisocyanate.
[0055] Having polymer composites be formed as covalent networks based on
oligo(epsilon.-
caprolactone)dimethacrylate and butylacrylate is a conceivable alternative
thereto.
[0056] For the braiding from which the inventive occlusion device is
configured, the invention
claims both hydrolytically as well as enzymatically degradable polymer
composites for the
degradable polymers. As stated above, hydrolytic degradation has the advantage
that the rate at
which degradation occurs is independent of implant location. In contrast,
local enzyme
concentrations vary greatly. Given biodegradable polymers or materials,
degradation can thus
occur through pure hydrolysis, enzymatically-induced reactions or through a
combination
thereof.
[0057] Typical hydrolyzable chemical bonds for the polymer composites of the
occlusion device
are amide, ester or acetal bonds. Two mechanisms can be noted with respect to
the actual
degradation. With surface degradation, the hydrolysis of chemical bonds
transpires exclusively at
the surface. Because of the hydrophobic character, polymer degradation is
faster than the water
diffusion within the material. This mechanism is seen especially with
poly(anhydrides) and
poly(orthoesters).
[0058] As relates to the poly(hydroxy carboxylic acids) particularly
significant especially to the
present invention such as poly(lactic acid) or poly(glycol acid), the
corresponding copolymers
respectively, polymer degradation transpires throughout the entire volume. The
step which
determines the rate here is the hydrolytic fission of the bonds since water
diffusion in the
somewhat hydrophilic polymer matrix occurs at a relatively fast rate.
[0059] Decisive for the use of biodegradable polymers is that, on the one
hand, they degrade at a
controlled or variable speed and, on the other, that the products of
decomposition are non-toxic.
[0060] The concept of polymer material resorption refers to the substance or
mass degrading
CA 02627196 2008-04-24
through to the complete removal of a material from the body by way of the
natural metabolism.
In the case of homogenous implants (occlusion devices) of only one degradable
polymer,
resorption begins as of that point in time of the complete loss of the
mechanical properties.
Specification of the resorption time covers the period starting from
implantation and running
through to the complete elimination of the implant.
[00611 Among the most important biodegradable synthetic classes of polymers
from which the
braiding of the inventive occlusion device is advantageously synthesized are:
[0062] polyesters
such as poly(lactic acid) PLA, poly(glycol acid) PGA, poly(3-hydroxybutyric
acid) PBA, poly(4-
hydroxyvalerate acid) PVA or poly(.epsilon.-caprolactone) PCL or the
respective copolymers;
[0063] polyanhydrides synthesized from dicarboxylic acids such as, for
example, glutar PAG,
amber PAB or sebacic acid PAS; [0064] poly(amino acids) or polyamides such as,
for example,
poly(serine ester) PSE or poly(aspartic acid) PAA (FIG. 9).
[0065] FIG. 7 shows examples of biodegradable polyesters while FIG. 8 shows
examples of
biodegradable polyanhydrides, poly(amino acids) and polyamides.
[0066] In summary, it can be stated that shape memory properties play a
significant role with
respect to implants, particularly in terms of minimally invasive medicine.
Degradable implants
having shape memory properties are particularly effective in this regard.
[0067] For example, this type of degradable implant can be introduced into the
body in
compressed (temporary) form through a small incision and once in place, then
assume the
memory shape relevant to its application after being warmed by the body
temperature. The
implant will then degrade after a given interval of time, thereby doing away
with the need for a
second operation to remove it.
[0068] Based on the known biodegradable polymers, structural elements can be
derived for the
synthesizing of biodegradable shape memory polymers. In so doing, suitable
crosslinks, which
fix the permanent form, and network chains, which serve as switching elements,
must be selected
such that, on the on hand, the switching temperature can be realized through
the physiological
conditions, and on the other, toxicological problems with respect to any
products of
decomposition are excluded. Thus, suitable switching segments for
biodegradable shape memory
polymers can be selected based on the thermal properties of known degradable
implant material.
Of particular interest in this regard is a thermal transition of the switching
elements in the
temperature range of between room temperature and body temperature. For this
transition
temperature range, biodegradable polymer segments can be selectively
synthesized by varying
the stochiometric relationship of the known starting monomers and the
molecular weight of the
formed polymers in the range of from approx. 500 to 10000 g/mol.
[0069] Suitable polymer segments are e.g. poly(.epsilon.-caprolactone)diols
with melting
temperatures between 46 and 64° C. or amorphous copolyesters based on
lactic and glycol
acid with glass transition temperatures between 35 and 50° C. The phase
transition
11
CA 02627196 2008-04-24
temperatures hereby; i.e., the melting or glass transition temperature of the
polymer switching
segments, can be further diminished by their chain length or by degradation of
specific end
groups. The polymer switching elements thus customized can then be integrated
into physical or
covalent crosslinked polymer networks, yielding the selectively composed
biodegradable shape-
memory polymer material.
[0070] In one possible embodiment, biodegradable thermoplastic amorphous
polyurethane
copolyester polymer networks having shape memory properties are used as the
material for the
inventive occlusion device. First, suitable biodegradable star-shaped
copolyester polyols are
synthesized here based on commercially available dilactide DL (cyclic lactic
acid dimer),
diglyocolide DG (cyclic glycol acid dimer) and trimethylolpropane TP
(functionality F=3) or
pentaerythrit PE (F=4) with glass transition temperatures between 36 and
59° C., which
are then crosslinked with commercial trimethylhexa-methylene diisocyanate TMDI
in forming a
biodegradable polyurethane network.
[0071] FIG. 9 shows an example of monomer components for amorphous
polyurethane
copolyester polymer networks having shape memory properties.
[0072] The amorphous polyurethane copolyester polymer networks having shape
memory
properties as formed have a glass transition temperature Tg between 48
and 66° C.
and exhibit a modulus of elasticity in extension of between 330 and 600 MPa, a
tensile strength
respectively of between 18.3 and 34.7 MPa. Heating these networks to
approximately 20°
C. above this switching temperature yields elastic materials which can be
deformed 50-265%
into a temporary shape. Cooling down to room temperature occasions the forming
of deformed
shape memory polymer networks which have a clearly higher modulus of
elasticity in extension
of from 770 to 5890 MPa. Upon subsequent reheating to 70° C., the
examples of
deformed specimens thereby produced retransform back into the permanent
corkscrew-like shape
after approx. 300 s. What was ultimately shown was that polyurethane
copolyester polymer
networks in an aqueous phosphate buffer decomposed fully at 37° C. over
a period of
between approximately 80 and 150 days.
[0073] By optimizing the composition of the biodegradable switching segments,
degradable
polyurethane copolyester polymer networks having shape memory properties can
be produced
substantially faster, e.g. within 14 days.
[0074] Similar biodegradable elastic shape memory polymer networks can be
yielded from
crosslinking of oligomer diols with diisocyanate TMDI which have melting
temperatures
between 38 and 85° C. and which are likewise suitable for the inventive
occlusion device.
Using these materials, a fiber was synthesized and stretched 200% into a
temporarily longer fiber
and a loose knot formed therefrom. After fixing the two ends of the knot and
heating the knot to
40° C.; i.e., higher than the switching temperature, the knot tightened
itself again after
approx. 20 s into the semi-permanent length through the transition of the
thread. Degradableness
was also ultimately assessed, whereby for these polymers in an aqueous
phosphate buffer at
12
CA 02627196 2008-04-24
37° C., a 50% loss of mass was seen after approximately 250 days.
[0075] In one possible realization of the inventive occlusion device, the
braiding is formed from
a biodegradable shape memory polymer on covalent networks based on
oligo(.epsilon.-capro-
lactone)dimethacrylate and butylacrylate. It has been seen that subsequent
subcutaneous
implantation, this polymer composite has no negative impacts on the wound
healing process. The
synthesis of such biodegradable shape memory polymers can follow from n-
butylacrylate which,
because of the low glass transition temperature of -55° C. for pure
poly(n-butylacrylate),
can be used as the soft segment-forming component.
[0076] FIG. 10 shows monomer components for covalent biodegradable networks.
The bio-
degradable segments are introduced here via the oligo(.epsilon.-
caprolactone)dimethacrylate
crosslinker. Network synthesis ensues through photopolymerization. Based on
the molar mass of
the macromolecular oligo(.epsilon.-caprolactone)dimethacrylate and the content
of comonomer
n-butylacrylate, the switching temperature and the mechanical properties of
the covalent network
can be controlled. Thus in an implementation of the inventive solution, the
molar mass of the
oligo(.epsilon.-caprolactone)dimethacrylate varies between 2000 and 10000
g/mol and the n-
butylacrylate content between 11 and 90 mass %. In the case of a polymer
network based on a
mixture of the low molecular oligo(.epsilon.-caprolactone)-dimethacrylate at
11 mass % of n-
butylacrylate, a melting point of 25° C. was realized.
[0077] The biodegradable covalent and physical polymer networks having shape
memory effect
as described above can also be used as a matrix for a controlled active
substance release. Yet also
conceivable would be biodegradable polyurethane multiblock copolymers having
shape memory
effect based on poly(p-dioxanone) PDO as the hard segment and TMDI as the
diisocyanate.
[0078] FIG. 11 shows polymer segments in biodegradable poly(p-dioxanone)-
polyurethane
multi-block copolymers. The combination with the poly(lactid-co-glycolid) PDLG
or
poly(.epsilon.-caprolactone) PCL switching segments yields multiblock
copolymers having a
switching temperature of 37 or 42° C. respectively. The hydrolytic
degrading of the
polymers shows that the polymers based on PCL degrading at a lesser rate. In a
trial on the PCL
polymers, 50 to 90% of the initial mass was still present after 266 days of
hydrolysis while in the
case of the PDLG polymers, 14 to 26% was detectable after only just 210 days.
[0079] It can be maintained that biodegradable shape memory polymer networks
can be
synthesized from a combination of physical or covalent shape memory polymer
networks having
biodegradable polymer segments. Selectively choosing the components allows
setting optimal
parameters for each respective application such as the mechanical properties,
the deformability,
the phase transition temperatures and, above all, the switching temperature,
as well as the rate of
polymer decomposition.
[0080] With respect to the profile form to the inventive medical occlusion
device, it is
advantageously provided for the second preliminarily definable shape of the
occlusion device to
13
CA 02627196 2008-04-24
be configured to close an abnormal tissue opening in a patient's heart,
whereby in its expanded
state, the occlusion device exhibits a proximal retention area, a distal
retention area and a center
section between the two, and in which the occlusion device exhibits a smaller
diameter at the
center segment than at the proximal and/or distal retention areas. The
advantage to this
embodiment is in particular seen in that an intravascular occlusion device is
provided which is
particularly applicable to treating septal defects, patent foramen ovale
defects and persistent
ductus arteriosus defects and in which the occlusion device can be introduced
to the defect to be
occluded by means of a catheter system.
[0081] Septal defects refer to atrial septal defects (ASD); i.e., a hole in
the heart's interatrial
partition, and ventricular septal defects (VSD); i.e., a hole in the
interventricular partition.
[0082] A patent foramen ovale defect (PFO) is an oval opening (slit) in the
interatrial partition of
the heart which is normally closed after birth by adhesion of the flap-like
edges, although
imperfect adhesion (persistence) occurs in approximately 25% of all births,
leaving an open
foramen oval.
[0083] The term "persistent ductus arteriosus defect" (PDA) refers to an open
passageway
between the aorta and the pulmonary artery, one which normally closes after
birth.
[0084] The main objective of the present invention is to provide a reliable,
simple occluding
device to be used in the heart which is configured so as to be able to treat
patent foramen oval
defects (PFO), atrial septal defects (ASD), ventricular septal defects (VSD)
and patent ductus
arteriosus (PDA) and to do so in a form in which the braiding--as already
described above--is
replaced by that of a shape memory polymer or biologically degradable shape
memory polymer.
[0085] In configuring the second preliminarily definable shape to the medical
occlusion device
from the braiding composed of a polymer, there is a plurality of flexible
strands or threads,
whereby the threads are braided in such a way so as to produce an elastic
material. This braided
fabric is then deformed so that it will conform to the outer surface of a
molding element. The
braided fabric is positioned on the surface of the molding element and subject
to thermal
treatment at increased temperature. The duration and temperature for the
thermal treatment is
selected so as to retain the deformation to the braided fabric. Subsequent the
thermal treatment,
the braided fabric is removed from the molding element, retaining its
deformation. A braided
fabric treated in this way corresponds to the second preliminarily defined
(expanded) shape to the
medical occlusion device which can be introduced into a channel in the patient
in collapsed state
by means of a catheter system.
[0086] Types of application for the present invention include special shapes
for medical devices,
which can then be made in accordance with the present invention in order to be
used in specific
medical cases. The devices, having a flat expanded shape and which can be
disposed with
collapsed clamps, can be attached to an end of the insertion device or
guidewire in order to
retract the device after positioning. In use, a catheter is introduced into
the body of the patient to
14
CA 02627196 2008-04-24
the point where the distal end of the catheter positions exactly at the
location which is in need of
physiological treatment. A medical device previously selected in accordance
with the present
invention is then collapsed into a preliminarily defined second shape and
inserted into the
catheter opening. The device is pushed through the catheter and exits again at
its distal end
where, due to its memory properties, springs back into its original shape next
to the site to be
treated. The guidewire or inserting catheter then releases from the clamp and
is retracted.
[0087] In its second preliminarily defined shape, the occlusion device
preferentially exhibits an
oblong shape with a tube as its center section and an expanded diameter
segment at each end of
said center section. The thickness to the center section corresponds roughly
to the wall thickness
of the organ to be occluded, for example the thickness of the septum.
[0088] The center of at least one expanded diameter segment (proximal or
distal retention area),
can be offset relative the center of the center section. A membranous
ventricular septal defect can
thus be closed with simultaneous application of a support device large enough
to reliably close
the abnormal opening of the septum. Each braided end of the device is held by
a clamp. These
clamps are retracted in the expanded diameter parts of the device, whereby the
overall length of
the device is reduced and a more flush closure mechanism is yielded.
[0089] In another type of application, the device takes on the appearance of a
bell having an
oblong body with a tapered and a larger endpiece. The larger end has a fabric
plate which upon
unfolding, positions generally perpendicular the axis of the channel in which
the device unfolds.
The clamps holding the braided ends together retract into the center of the
"bell" and thus yield a
flush device having lower overall height.
[0090] Since the proximal retention area of the braiding exhibits a flaring
toward the proximal
end of the occlusion device in a particularly preferred embodiment, this
allows for the occlusion
device to adjust automatically to the septal defect in particularly
advantageous manner--
independent of the relative diameter of the defect to be occluded and
independent of the
thickness of the septal wall--and to do so with no part of the occlusion
device projecting beyond
the plane of the septal wall with the defect at the proximal side of the
defect. There is thus no
occurrence of the usual complications which normally arise in such cases. To
emphasize, this
means that the occlusion device used is ingrown by the body's own tissue
substantially faster than
is the case with the occluding systems known in the prior art. Using a
braiding composed of thin
threads as the starting material for the inventive occlusion device yields the
further advantage of
long-term mechanical stability. This thus largely prevents structural
fractures in the inserted
implant. In addition, the braiding is afforded sufficient rigidity. The
flaring to the proximal end of
the braiding's proximal retention area additionally allows the proximal
retention area of the
device to flatten completely against the lateral edge of the defect in the
inserted state and to do so
virtually independently of the diameter to the defect or the thickness of the
septal wall. As a
result, the occlusion device can be used for a wide range of differently sized
septal defects.
Because there is then no need for a holder for the bundled or merging braiding
at the proximal
retention area, neither do any components of the occlusion device protrude
past the septum wall,
CA 02627196 2008-04-24
which prevents components of the implant from being in constant contact with
the blood. This
yields the advantage of there being no threat that the body will mount defense
mechanism
reactions or of there being thrombembolic complications.
[0091] A particular preferred embodiment provides for the center of the
proximal/distal retention
area to be offset relative the center of the center segment. By so doing, a
membranous ventricular
septal defect can be occluded and, at the same time, a support device can be
used which is large
enough to close the abnormal opening in the septum.
[0092] Thereto, it can be provided that each braided end of the occlusion
device be held by a
clamp. These clamps are withdrawn from the occlusion device's expanded
diameter parts
(proximal and distal retention areas), yielding a reduced overall length to
the occlusion device
and a more flush closure mechanism.
[0093] One advantageous embodiment provides for the interior of the proximal
and/or distal
retention area to exhibit a concave profile form in the second preliminarily
definable shape of the
occlusion device in the expanded state. This allows the expanded occlusion
device to attain an
especially good positioning in the defect to be occluded. It is particularly
preferred for the
braiding from which the occlusion device is produced to be tapered in a first
preliminarily
definable shape to the diameter of the catheter system used in the
intravascular procedure. This
thus enables the occlusion device for occluding a defect to be inserted with a
catheter introduced,
for example, through a vein, eliminating the need for an operation in the
actual sense. When the
braiding includes a shape-memory polymer material, as described above, the
occlusion device
tapered to the diameter of the catheter is known as a "self-expanding device"
which unfolds
automatically upon exiting the catheter such that the two retention areas can
position accordingly
at the proximal/distal sides of the defect. The design to the contiguous
braiding of the inventive
occlusion device moreover occasions an occlusion device which is a self-
expanding and self-
positioning occluding system which prevents permanent mechanical stress from
occurring
between the inserted occlusion device and the septum wall. Provided as a
conceivable
implementation is that the proximal retention area of the braiding exhibit a
bell-shaped flaring
toward the proximal end.
[0094] It would furthermore be conceivable for the proximal retention area to
exhibit a bell-
shaped flaring to the proximal end. This would thus allow the occlusion device
to be used in the
treatment of various different defects, in particular ventricular septal
defects (VSD), atrioseptal
defects (ASD) as well as persistent ductus arteriosus Botalli (PDA), whereby
an optimized
contouring to the proximal retention area can in principle be selected for a
plurality of defects of
differing sizes and types. Of course, other profiles are also conceivable in
this regard such as, for
example, a barbell-like shape. In order to enable a particularly good
positioning of the expanded
occlusion device at the retention area, it is advantageously provided for the
length of the center
section to be dimensioned such that the peripheral edge of the distal or
proximal retention area
overlaps the peripheral edge of the other retention area.
16
CA 02627196 2008-04-24
[0095] A particularly preferred embodiment of the inventive occlusion device
provides for the
proximal and/or distal retention area to exhibit a recess in which the holder
for bundling the ends
of the braiding is disposed. By arranging the holder in the recess provided at
the proximal or
distal end of the occlusion device, no components of the occlusion device will
protrude beyond
the septum wall, preventing the components of the implant from coming into
constant contact
with the blood. This has the advantage of there being no threat that the body
will mount defense
mechanism reactions or of there being thrombembolic complications. Especially
because the
expanded occlusion device positions and fixes itself in the defect with the
distal and proximal
retention areas being radially stressed, the occlusion device can be used for
a wide range of
defects of various hole sizes.
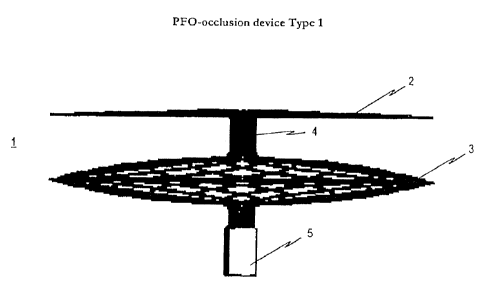
[0096] A particularly preferred embodiment of the inventive occlusion device
in which the distal
retention area exhibits a recess further provides for the distal end of the
occlusion device to be
further disposed with a connective element in the recess, whereby said
connective element can
engage with a catheter. This connective element, which is arranged on the
occlusion device so as
not to protrude beyond the septum wall such that no components of the implant
come into
constant contact with the blood, provides the inventive occlusion device with
the added
functionality of retrievability. Moreover, a connective element which can
engage with a catheter
facilitates implantation and positioning of the occlusion device (collapsed
during the actual
implanting) in the defect to be occluded. Various devices are conceivable as
connective elements.
For example, latching members would be feasible, as would even be
hooks/eyelets which force-
fit with the correspondingly configured complementary connective elements of a
catheter.
[0097] Another advantageous embodiment provides for the occlusion device to be
configured so
as to be reversibly collapsible inward and outward so that the device can be
collapsed in its
expanded state, for example with the help of an explantation catheter. In
conjunction hereto, it is
conceivable for a catheter in the explantation procedure to, for example,
engage with connective
elements configured at the distal end of the occlusion device and occasion the
collapsing of the
occlusion device in response to external manipulation of the catheter. The
occlusion device is
thereby fully reversibly retractable in the catheter, enabling the complete
removal of the device.
[0098] Last but not least, it is particularly preferred for the occlusion
device to have at least one
fabric insert disposed in or on the distal retention area or in the center
section of the occlusion
device to occasion complete closure of a defect. This fabric insert serves to
close the gaps which
remain in the center section and in the expanding diameters of the occlusion
device following
insertion and expansion of the device in the defect to be occluded. The fabric
insert is, for
example, affixed to the braiding of the occlusion device at the distal
retention area such that it
can be stretched over the distal retention area like a cloth. The advantage to
this design lies in the
fact that the lateral edge of the distal retention area is flush with the
septum and less foreign
material is introduced into the body of the patient. The fabric inserts can be
made of, for
example, Dacron. Other materials and other positionings to the fabric insert
in or on the
occlusion device are of course also conceivable here.
17
CA 02627196 2008-04-24
[0099] There has thus been outlined, rather broadly, some features consistent
with the present
invention in order that the detailed description thereof that follows may be
better understood, and
in order that the present contribution to the art may be better appreciated.
There are, of course,
additional features consistent with the present invention that will be
described below and which
will form the subject matter of the claims appended hereto.
[0100] In this respect, before explaining at least one embodiment consistent
with the present
invention in detail, it is to be understood that the invention is not limited
in its application to the
details of construction and to the arrangements of the components set forth in
the following
description or illustrated in the drawings. Methods and apparatuses consistent
with the present
invention are capable of other embodiments and of being practiced and carried
out in various
ways. Also, it is to be understood that the phraseology and terminology
employed herein, as well
as the abstract included below, are for the purpose of description and should
not be regarded as
limiting.
[0101] As such, those skilled in the art will appreciate that the conception
upon which this
disclosure is based may readily be utilized as a basis for the designing of
other structures,
methods and systems for carrying out the several purposes of the present
invention. It is
important, therefore, that the claims be regarded as including such equivalent
constructions
insofar as they do not depart from the spirit and scope of the methods and
apparatuses consistent
with the present invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0102] The following will make reference to the drawings in providing a more
precise detailing
of preferred embodiments of the inventive occlusion device.
[0103] FIG. 1 is a schematic representation of the shape memory effect;
[0104] FIG. 2 is a schematic representation of the molecular mechanisms which
occur upon
shape-memory transition of a semi-crystalline polymer network;
[0105] FIG. 3 depicts a synthesis diagram of thermoplastic polyurethane
multiblock copolymers;
[0106] FIG. 4 depicts the chemical structure to monomer components of
thermoplastic
polyurethane multiblock copolymers;
[0107] FIG. 5 depicts the structure to diblock or triblock copolymers having
shape-memory
effect;
[0108] FIG. 6 is a representation of monomers for covalent shape memory
polymer networks;
[0109] FIG. 7 depicts examples of biodegradable polyesters;
18
CA 02627196 2008-04-24
[0110] FIG. 8 depicts examples of biodegradable polyanhydrides, poly(amino
acids) and
polyamides;
[0111 ] FIG. 9 depicts monomer components for amorphous polyurethane
copolyester polymer
networks having shape memory properties;
[0112] FIG. 10 depicts monomer components for covalent biodegradable networks;
[0113] FIG. 11 depicts polymer segments in biodegradable poly(p-dioxanone)-
polyurethane
multiblock copolymers;
[0114] FIGS. 12a, b depict a side and stereoscopic view of a Type 1 PFO
occlusion device;
FIGS. 13a, b are a stereoscopic representation of a Type 1 PFO occlusion
device;
[0115] FIG. 14 depicts a side view of a Type 2 PFO occlusion device;
[0116] FIG. 15 depicts a side view of a Type 3 PFO occlusion device;
[0117] FIG. 16 depicts a top plan view of a Type 3 PFO occlusion device;
[0118] FIGS. 17a, b depict a conventionalized side view and sectional
representation of a Type 4
PFO occlusion device;
[0119] FIG. 18a depicts an enlarged detail view of a section as seen through a
Type 1 occlusion
device (Type 1 ASD occlusion device) for occluding an atrial septal
defect(ASD); the occlusion
device is elongated and extends partially out of the opening of an insetting
catheter;
[0120] FIG. 18b depicts an enlarged detail view of a section as seen through a
Type 2 occlusion
device in a conventional embodiment;
[0121 ] FIG. 18c depicts an enlarged detail view of a section as seen through
a Type 3 ASD
occlusion device with polymer threads thermally bundled in the left atrial
curve;
[0122] FIG. 18d depicts an enlarged detail view of a section as seen through a
Type 4 ASD
occlusion device having a type of braiding comparable to that of Type 1(FIG.
19-2 1);
[0123] FIG. 19a depicts a frontal view of the Type 1 ASD occlusion device
pursuant FIG. 18a in
its pre-formed shape;
[0124] FIG. 19b depicts the ASD occlusion device pursuant FIG. 19a in slightly
elongated form;
[0125] FIG. 19c depicts a side view of the ASD occlusion device pursuant FIG.
19a in further
19
CA 02627196 2008-04-24
elongated form;
[0126] FIG. 20a depicts a frontal view of a Type 2 ASD occlusion device
pursuant FIG. 18b in
its pre-formed shape;
[0127] FIG. 20b depicts the ASD occlusion device pursuant FIG. 20a in slightly
elongated form;
[0128] FIG. 20c depicts the ASD occlusion device pursuant FIG. 20a in further
elongated form;
[0129] FIG. 21 a depicts a frontal view of a Type 3 ASD occlusion device
pursuant FIG. 18c in its
pre-formed shape;
[0130] FIG. 21b depicts a side view of the ASD occlusion device pursuant FIG.
21 a in slightly
elongated form;
[0131 ] FIG. 21 c depicts a side view of the ASD occlusion device pursuant
FIG. 21 a in further
elongated form;
[0132] FIG. 22a depicts a frontal view of the Type 4 ASD occlusion device
pursuant FIG. 18d in
its pre-formed shape;
[0133] FIG. 22b depicts a side view of the ASD occlusion device pursuant FIG.
22a in slightly
elongated form;
[0134] FIG. 22c depicts a side view of the ASD occlusion device pursuant FIG.
22a in slightly
further elongated form;
[0135] FIG. 23 depicts a detailed view of a section as seen through the side
of an ASD occlusion
device pursuant FIG. 21 in the ASD of a heart;
[0136] FIG. 24 depicts an enlarged frontal view of an occlusion device for
occluding a VSD in
its pre-formed shape;
[0137] FIG. 25 depicts a side view of the VSD occlusion device pursuant FIG.
24;
[0138] FIG. 26 depicts a detail view of a section as seen through the front of
the VSD occlusion
device pursuant FIG. 24;
[0139] FIG. 27 depicts a surface depiction from above of the VSD occlusion
device pursuant
FIG. 24;
[0140] FIG. 28 depicts a surface depiction from below of the VSD occlusion
device pursuant
FIG. 24;
CA 02627196 2008-04-24
[0141] FIG. 29 depicts an enlarged frontal view of another VSD occlusion
device in its pre-
formed shape;
[0142] FIG. 30 depicts a detail view of a section as seen through the side of
the VSD occlusion
device pursuant FIG. 29;
[0143] FIG. 31 depicts an enlarged frontal view of another VSD occlusion
device in its pre-
formed shape;
[0144] FIG. 32 depicts a detail view of a section as seen through the side of
the VSD occlusion
device pursuant FIG. 31;
[0145] FIG. 33 depicts an enlarged frontal view of another VSD or PDA
occlusion device in its
pre-formed shape;
[0146] FIG. 34 depicts a detailed view of a section as seen through the side
of the VSD or PDA
occlusion device pursuant FIG. 33;
[0147] FIG. 35 depicts a perspective view of a medical occlusion device in
accordance with the
present invention;
[0148] FIG. 36 depicts a side view of the occlusion device pursuant FIG. 35;
[0149] FIG. 37 depicts a top plan view of the occlusion device pursuant FIG.
35;
[0150] FIG. 38 depicts a partial sectional view through a molding element used
for shaping the
occlusion device pursuant FIG. 35;
[0151 ] FIG. 39 depicts a perspective view of a medical occlusion device in
accordance with the
present invention without the associated sleeve in the proximal area as in the
occlusion device
pursuant FIG. 35;
[0152] FIG. 40 depicts a perspective detail view of a section through the
heart with the occlusion
device pursuant FIG. 35 unfolded in a central shunt of the patient's blood
vessel;
[0153] FIG. 41 depicts an enlarged frontal view of an occlusion device used in
the occluding of a
PDA;
[0154] FIG. 42 depicts a detail view of a section through the PDA occlusion
device pursuant
FIG. 41;
[0155] FIG. 43 depicts a topplan view of the PDA occlusion device pursuant
FIG. 41;
= 21
CA 02627196 2008-04-24
[0156] FIG. 44 depicts a plan view from below of the PDA occlusion device
pursuant FIG. 41;
and
[0157] FIG. 45 depicts a PDA occlusion device within an insertion catheter.
DESCRIPTION OF THE INVENTION
[0158] The present invention relates to a percutaneous catheter-guided
occlusion device which
serves to close abnormal openings such as, for example, atrial septal defects
(ASD, PFO),
ventricular septal defects (VSD), patent ductus arteriosus (PDA) and the like.
The present
invention furthermore provides for a method of forming a medical device from a
flat or tubular
synthetic or polymer fabric. Both the flat as well as the tubular fabric is
comprised of a plurality
of wire strands having a predefined relative arrangement to one another. The
tubular fabric has
synthetic strands distinguishing two sets of essentially parallel spiral
strands, whereby the strands
of one set have a rotation direction counter to that of the other strands.
This fabric is also known
in the industry as a tubular braid.
[0159] The braided form is used primarily in Type 2 (FIG. 14) and Type 3 (FIG.
15) PFO
devices, whereby the wires/threads of the proximal curves are thermally
bundled at proximal end
2 and specifically in an element which is designated as a"thermal holder."
Thermal energy acts
here to fuse the wires together.
[0160] The tubular fabric 10 is used in comparable manner in the Type 2 ASD
(FIG. 18b, and
FIG. 20a-20c) and Type 3 devices (FIG. 18c, FIG. 21a-21c) in addition to the
VSD types of
devices (FIG. 24, FIG. 25, FIG. 26), the type of device in accordance with
FIGS. 35 and 39, and
last but not least, the PDA device in accordance with FIG. 41.
[0161 ] Using the braiding method as developed by JEN.meditec GmbH in
accordance with the
Aug. 22, 2003 German patent application No. 10 338 702 as cited at the outset
yields additional
preferable forms of the device which are particularly economical in terms of
material and the
method used to produce such braided material enables the PFO and ASD devices
to have flatter
final forms. The medical devices produced with this braiding method comprise
the PFO, Type 1
(FIG. 12a, b and FIG. 13a, b) and Type 4 (FIG. 17a, b) types of devices and
the ASD Type 1
(FIG. 18a, FIG. 19a-19c) and Type 4 (FIG. 18d, FIG. 22a-22c) types of devices.
[0162] The pitch to the synthetic strands and the pick (i.e., the number of
turns per unit length) as
well as other factors such as the number of wires used in the tubular braiding
are essential in
defining a number of important properties for the device. The tighter the pick
and the pitch of
fabric 10, meaning the closer the synthetic strands are woven to one another,
the more rigid the
device. A greater wire density means a larger wire surface, thus increasing
the device's occluding
ability. Such thrombogenicity can either be increased, e.g. by coating with a
thrombolytic agent,
or decreased, e.g. by means of a lubricious anti-thrombogenic coating.
22
CA 02627196 2008-04-24
[0163] In the forrning of device 1 in accordance with the present invention, a
tubular or flat
synthetic fabric 10 of corresponding size is inserted into a mold in which the
fabric 10 conforms
to the cavities of the mold. These cavities are configured such that the
synthetic fabric 10
assumes the shape of the desired device. The ends of the synthetic strands of
the tubular or flat
synthetic fabric 10 should be secured in order to prevent fraying. A clamp can
be used to this end
(e.g. Type 2 PFO and ASD devices as described above) or the ends of the
synthetic strands can
be thermally treated, for example welded (e.g. Type 3 PFO and ASD devices).
[0164] In the case of a tubular braiding, a molding element can be inserted
into the tube of the
braiding prior to the braiding being inserted into the mold. This occasions an
even more precise
defining of the molded surface. When the ends of the tubular synthetic fabric
have been clamped
or welded, the molding element can be introduced into the tube manually by
bending apart the
synthetic strands of fabric 10. This type of molding element serves to provide
a very precise
control over the final size and shape of the device by ensuring that the
fabric conforms to the
cavities of the mold.
[0165] A material can be selected for the molding element which can be broken
into smaller
pieces or removed from the inside of the synthetic fabric. The molding element
can thus, for
example, be made from a brittle or friable material. After thermally treating
the material with the
molding element in the mold cavity, the molding element is broken into small
pieces easily
removed from the synthetic fabric.
[0166] Usually, however, molding tools (molding elements) can be used for all
the medical
devices described here which precisely define the shape of the medical devices
based on an outer
sleeve (fractionable into different individual pieces). Since the medical
devices are made from
synthetic material having a melting point below 350° C., the molding
elements of the
molding tool can be made of aluminum, tool steel, non-ferrous metal or even
titanium or titanium
alloys.
[0167] It is, however, to be pointed out that the specific form of a
particular molding element
will yield a specific shape and that other molding elements having other
configurations can also
be used as desired. If a complex shape is desired, molding elements and molds
can have
additional components, including cammed connections. For simpler shapes, the
mold can also
have fewer components. The number of components in a given mold and their
shape depend
almost exclusively on the shape of the desired device to which the synthetic
fabric will conform.
In its relaxed state, the synthetic strands of the tubular braiding assume a
previously-defined
orientation relative one another. When the tubular braiding is compressed
along its axis, the
fabric pitches away from the axis in expanding according to the shape of the
mold. In deformed
fabric, the relative orientation to the wire strands of the synthetic fabric
changes. Compressing
the mold occasions the synthetic fabric to conform to the surface of the
cavity. The device has a
pre-determined expanded configuration and collapsed configuration so that it
can be introduced
by means of a catheter or such similar inserting device. The expanded
configuration is a function
23
CA 02627196 2008-04-24
of the shape of the fabric after having been formed to the surface of the
mold.
[0168] Once the tubular or flat synthetic fabric has been inserted into the
selected mold, whereby
the fabric is flush against the surface of the mold's cavity, thermal
treatment then follows with
fabric 10 thereby remaining in the mold. The wire strands of the synthetic
fabric are re-aligned
and re-formed relative one another by the thermal treatment, whereby the
fabric conforms to the
mold. The fabric is then removed from the mold and retains the given shape of
the surface of the
mold's cavity, now constituting the desired device. The thermal treatment
depends to a large
extent on the specific material from which the wire strands of the synthetic
fabric are made, yet
duration and temperature for the thermal treatment should be selected such
that the fabric is fixed
in its new shape; i.e., the wire strands assume their relative re-orientation
subsequent the fabric
conforming to the surface of the mold.
[0169] After being thermally treated, the fabric is removed from the molding
element and retains
its new form. In those cases where a molding element has been used, same is
now removed again
as described above. The duration of and temperature for the thermal treatment
depends heavily
on the material composition to the wire strands and has already been described
in detail above.
[0170] After device 1 has been brought into the previously specified form, it
can be used for
treating a patient. A device is selected based on its being suitable for
treating the respective
medical problem. Such a device is to be consistent with one of the above-
described types of
application. Once the corresponding device is selected, a catheter or other
inserting device is
introduced into the patient and positioned such that the distal end of the
inserting device
positions next to the site to be treated, e.g. thus directly adjacent to (or
at the same height of) a
shunt of an abnormal opening in an organ.
[0171] Insertion devices can be of various shapes but should, however,
preferably comprise a
pliable metal shaft with threading at its distal end. The insertion device
hereby serves in pushing
the medical device through the tube of the catheter and positioning it in the
patient. When the
device is pushed out the distal end of the catheter, it is thus still being
held. Not until the device
is positioned within the shunt of the abnormal opening is the shaft of the
catheter rotated about
its axis in order to unscrew the device from the catheter.
[0172] As long as the device is still connected to the catheter, the surgeon
can move the device
forward and backward relative the abnormal opening until that point at which
it is exactly
positioned as desired within the shunt. Using a threaded clamp, as attached to
the device, the
surgeon can control the movement of the device out the distal end of the
catheter. Once device 1
has been pushed out of the catheter, it will spring back into the expanded
form it assumes in
consequence of the fabric having been thermally treated. At that moment at
which it springs back
into its original form, it may happen that it impacts the distal end of the
catheter and is thereby
urged forward. This can result in an incorrect seating of the device,
especially critical if same is
to be positioned in a shunt between two blood vessels. The surgeon can keep
hold of the device
during its positioning by means of the threaded clamp; the device will not
spring out
24
CA 02627196 2008-04-24
uncontrollably and can be positioned accurately.
[0173] The device is collapsed and inserted into the opening of the catheter.
The collapsed form
of the device should be such that it can be easily inserted into the tube of
the catheter and can
withdraw correctly at the distal end of same. Thus an ASD occluding device
can, for example,
have a relatively oblong collapsed form, whereby the individual components are
disposed along
the axis (see FIGS. 18a-18d)). This can be attained in that one pulls the
device in opposite
directions along its axis by e.g. manually holding the clamps and pulling
apart so that the
expanded diameter segments fold inward toward the axis.
[0174] The PDA occluding device also functions in similar fashion. It can also
be collapsed to
allow insertion into a catheter by stretching it along its axis (see FIG. 45),
as it folds into itself
when pulled in opposite directions.
[0175] If the device is to serve to permanently close a channel in a patient,
the catheter is simply
pulled out. The device remains in the patient's vascular system to close the
blood vessel or the
respective channel. In some cases, the device can be affixed to an inserting
system such that the
device is fixedly connected to the end of the insertion device. Before the
catheter can be removed
from such a system, it may be necessary to unhook the medical device from the
insertion device
prior to withdrawing the catheter and insertion device.
[0176] Although the device springs back into its original expanded shape
(i.e., the form which it
held before it was collapsed so as to enable its insertion into the catheter),
it must be made clear
here that it does not always assume its original shape in full measure. It can
thus be desirable, for
example, for the device to have a maximum outer diameter in its expanded shape
which is at
least as large and preferably larger than the inner diameter of the lumen of
the abnormal opening
at which it is to be affixed. When such a device is fit to a blood vessel or
an abnormal opening
having a small lumen, it expands until it fills out the lumen. When doing so,
it can thereby
happen that the device will not have the need to expand fully into its
original expanded shape. It
is nevertheless properly affixed because it shuts off the inner wall of lumen
and remains fixed
there.
[0177] When the device is deployed in a patient, thrombi form on the surface
of the wires. In the
case of greater wire density, the total surface area of the wires is increased
such that the
thrombotic activity at the device also increases and the blood vessel in which
it is affixed closes
at a relatively fast rate. Should it be desired to accelerate the occluding
time, a number of
thrombotic means can be disposed on the device.
[0178] The devices (occlusion devices 1) in accordance with FIGS. 12-17 are
introduced in order
to close defects such as the so-called patent foramen ovale (PFO). With the
Type 1 to 4 PFO
variants depicted here (exclusively synthetic fibers), cases of critical
defects can also be treated at
the locality. A detailed description of a Type 1 PFO occluder configured from
nitinol material
can be found in the previously-cited Aug. 22, 2003 JEN.meditec patent
application Ser. No.
CA 02627196 2008-04-24
10/338,702.
[0179] FIGS. 18-22 show a further form of application for the present devices
(occlusion devices
1), with which atrial septal defects (ASD) can be corrected. The devices
(occlusion devices 1)
shown in FIGS. 19-22 are a depiction of frames of the Type 1-4 ASD devices in
their relaxed,
unexpanded state through to partially expanded state.
[0180] ASD is a congenital anomaly of the atrial septal resulting from a
structural weakness of
the interatrial septum. There can be a shunt in the interatrial septum through
which the blood
flows from the right into the left atrium. When there is a large defect with
significant shunts from
left to right through the defect, the right atrium and the right ventricle
overflow and the excess
empties into a pulmonary vessel of low resistance.
[0181] Pulmonary vessel closure and pulmonary atrial hypertension develop in
adults. Patients
suffering secondary ASD with a considerable shunt (the ratio of the pulmonary
blood flow to the
blood flow of the system being greater than 1.5) are preferably operated on at
the age of 5 or as
soon as the diagnosis is made in later life. With the advent of two-
dimensional echocardiography
and Doppler color flow mapping, the exact anatomy of the defect can be
visualized. The
appropriate ASD device is selected based on the size of the defect.
[0182] The size of the ASD occluder valve is proportional to the size of the
shunt to be occluded.
In its relaxed state, the synthetic fabric is shaped such that two plate-like
members, retention
areas 2 and 3 (FIG. 19a) respectively, are in axial alignment and connected to
a short cylindrical
segment, or center section 4, respectively. The length of cylindrical segment
4 is to correspond to
the thickness of the interatrial partition; i.e., 2 to 20 mm thick. Proximal
plate 2 and distal plate 3
have an outer diameter which is much larger than the shunt so as to exclude
any slippage of
device 1. Proximal plate 2 is relatively flat while distal plate 3 is curved
toward the proximal end
such that it overlaps proximal plate 2 to some degree. Given the above, the
springing open of
device 1 presses the peripheral edge of distal plate 3 flush with the side
wall of the septum. The
outer edge of proximal plate 2 is pressed against the septum's opposite side
wall in like manner.
[0183] The ends of device 1, made of metal tubular braiding fabric 10, are
welded or clamped to
holder 5, similar to the clamps as described above, to prevent fraying. Holder
5, which holds the
wire strands together at an end, also serves in connecting the device to the
inserting system (see
FIG. 18). In the application as shown, the generally cylindrical holder 5 has
a recess for the ends
of the metal fabric so that the wires of the braided fabric 10 cannot shift
relative one another. A
threading is disposed in the recess of holder 5, configured such that it can
receive and hold the
distal end of an insertion system.
[0184] ASD occluder device 1 can be advantageously produced as a form of
application for the
present invention using the method specified above.
[0185] FIG. 23 depicts a detail sectional view through the side of the ASD
occluder of FIG. 21 in
26
CA 02627196 2008-04-24
the ASD of a heart.
[0186] FIGS. 24-28 show different variants of an occluder device, preferably
used in cases of
membranous VSD. In their preset form, these devices 1 have two expanded
diameter sections
(retention areas) 2 and 3 with a smaller diameter segment (center section) 4
disposed between
said two expanded diameter sections 2 and 3. Each expanded diameter section 2
and 3 is
disposed with a recess projecting inwardly from the outer surface of expanded
diameter sections
2 and 3. A clamp 7 is provided in the recesses at each end of the tubular
synthetic fabric 10.
[0187] The smaller diameter segment (center section) 4 has a length which
corresponds to the
thickness of the abnormal opening in the septum wall. The VSD device can be
deformed in its
expanded preset form, thereby reducing its cross-section, so that it can be
introduced through a
channel in the body as described above. The inner surfaces of the expanded
diameter sections can
be concave or curved so that the outer periphery will come into contact with
each diameter
section given in the septum.
[0188] At least one diameter section 2 or 3 can also be arranged to be offset
relative the smaller
diameter section 4. In the case of abnormal openings adjacent the aorta, this
thus prevents the
offset support device or the expanded diameter sections 2 and 3 from closing
off the aorta after
insertion.
[0189] FIGS. 29 and 30 show a VSD device 1 in which the center of both
expanded diameter
sections 2 and 3 and the smaller diameter section 4 are along one line. Clamps
(not explicitly
shown) are affixed to the ends of synthetic fabric 10 and pulled inwardly in
order to yield a flat
occluding device. The clamps can have an inner or outer threading for the
fastening of an
inserting device or guidewire. This type of VSD device 1 is preferably used to
close muscular
ventricular septum defects. The VSD device is inserted as described above.
[0190] FIGS. 31 and 32 show another form of application for device 1 in the
closing of a VSD.
The device pursuant FIG. 32, while similar to the VSD device of FIGS. 29 and
30, does have a
few differences: the length of the smaller diameter section 4 has been reduced
and both expanded
diameter sections 2 and 3 have been compressed in order to reduce the
thickness of each
diameter section.
[0191] FIGS. 33 and 34 show another form of application for a device 1 which
is similar to that
as depicted in FIGS. 31 and 32. The device pursuant FIGS. 33 and 34 can
occlude a patent ductus
arteriosus (PDA) in which the patient is suffering from pulmonary
hypertension. Both expanded
diameter sections 2 and 3 are formed with a thin cross-section so as not to
hinder the flow of
fluid through the pulmonary vein or the aorta. In addition, the smaller
diameter section 4 tapers to
a point in order to increase the fabric contact area around the defect.
[0192] PDA is essentially the condition in which two blood vessels, usually
the aorta and the
pulmonary artery near the heart, present with a shunt between their two lumen.
In this condition,
27
CA 02627196 2008-04-24
blood will flow from one blood vessel to the other directly through the shunt,
obstructing the
patient's normal bloodstream flow. The PDA device in accordance with FIG. 35
and FIGS. 36-37
has a bell-shaped body 3 and a forward section 2 projecting outwardly. The
bell-shaped body 3 is
adapted for affixing to the shunt between the blood vessels while the forward
section 2 is adapted
for positioning in the aorta in order to hold the body of the device in the
shunt. The size of body
3 and end 2 can be matched to the respective size of the shunt as desired.
Body 3 can thus have,
e.g., in its generally thin center section, a diameter of approximately 10 mm
with a length to its
axis of approximately 25 mm.
[0193] The base of the PDA device body is to extend radially to the outer
diameter of forward
section 2, which has a diameter on an order of magnitude of approximately 20
mm.
[0194] Base 4 should have a distinct flaring in order to form the shoulder
piece which tapers out
radially from the center of body 3. When the PDA device is inserted into the
blood vessel, this
shoulder piece then abuts the edge of the lumen to be treated at high
pressure. Forward section 2
is held in the blood vessel and presses against the lower end of body 3 so
that the shoulder piece
nestles against the vascular wall. This thus prevents the device from
dislodging from within the
shunt.
[0195] The PDA occluder device as a form of application of the present
invention can be readily
produced in accordance with the above-described method by deforming a tubular
metal fabric
such that it will conform to the surface of a mold; the fabric is then subject
to thermal treatment
in order to fix its new form.
[0196] The PDA device pursuant FIG. 39 realizes a simplification in that the
use of synthetic
material allows the sleeve in the proximal area since the synthetic wires are
welded flush together
at this location.
[0197] FIG. 40 is a drawing of a PDA device in the heart of a patient for the
purpose of PDA
occlusion. The drawing shows the device in a shunt extending from the "A"
aorta to the "P"
pulmonary artery. The device is guided through the PDA in collapsed state by a
catheter.
Subsequent thereto, the shoulder piece is allowed to spring back into its
"remembered shape" as
occasioned by its prior thermal treatment upon pushing the device out through
the catheter's
distal end. The shoulder piece should be larger than the shunt lumen of the
PDA.
[0198] One then pulls somewhat on the device so that the shoulder piece
affixes to the wall of
the "P" pulmonary artery. If pulling continues on the catheter, the device
will affix to the wall of
the PDA, thereby pulling its body section 3 out of the catheter. Body section
3 can now expand.
Body section 3 should be dimensioned such that it engages in the lumen of the
PDA shunt by
means of friction. The device is held in its place on the one hand by friction
between body
section 3 and the lumen of the shunt and on the other hand by the aorta's
blood pressure against
the shoulder piece of the device. Thrombi develop in and on the device within
a short time and
occlude the PDA. Occluding of the device as shown here can be even further
accelerated by
28
CA 02627196 2008-04-24
coating same with a thrombolytic agent, filling it with polyester fibers or a
nylon material, or
braiding a larger amount of wire strands together.
[0199] FIGS. 41 to 44 show another variant of the PDA device. This device has
a cylindrical
body 3, 4 which tapers to a point and a shoulder piece 2 extending out
radially from an end of the
body. The ends of the braided fabric are pressed inward in the cavity of body
section 3. Clamps
are thereby disposed at each end of the device's tubular fabric, by means of
which the entire
length of the PDA device is shortened and its manipulation is simplified.
[0200] It is emphasized that the realization of the invention is not limited
to the embodiments
associated with the figures, but rather can be realized in a plurality of
variants without departure
from the scope of the invention herein involved. It is intended that all
matter contained in the
above description, as shown in the accompanying drawings, the specification,
and the claims
shall be interpreted in an illustrative, and not limiting sense.
29