Note: Descriptions are shown in the official language in which they were submitted.
CA 02627408 2008-04-25
1
OCCLUSION INSTRUMENT FOR CLOSING A CARDIAC AURICLE
AND A METHOD FOR PRODUCING SUCH AN OCCLUSION INSTRUMENT
Description
The present invention relates to a self-expanding
occlusion device for occluding an atrial auricula
consisting of a braiding of thin wires or threads given a
suitable form by means of a molding and heat treatment
procedure, whereby the occlusion device has a rear proximal
retention area and a front distal retention area and
whereby the ends of the wires or threads converge in a
holder in the distal retention area. The occlusion device
moreover has a center section between said proximal and
said distal retention area.
The occlusion device is configured in such a manner
that it can be introduced into the body of a patient in
collapsed state and positioned in the atrial auricula of
the patient in a minimally invasive procedure using a
catheter. The invention furthermore relates to a method for
the production of such an occlusion device.
The principle behind this type of occlusion device is
known to at least some extent in medical technology. For
example, an occlusion device for treating septum defects is
known from DE 10 338 702 of August 22, 2003, consisting of
a braiding of thin wires or threads and given a suitable
profile in a molding and heat treatment process. The known
occlusion device has a proximal retention area which is
particularly distinctly flat, a distal retention area, and
a cylindrical crosspiece between the proximal and distal
retention areas. The ends of the wires forming the braiding
CA 02627408 2008-04-25
2
converge into a holder in the distal retention area. This
structural design thus allows the two retention areas of
the known occlusion device to position on the two sides of
a shunt to be occluded in a septum, usually by means of an
intravascular surgical procedure, while the crosspiece
transverses the shunt.
Medical technology has long endeavored to be able to
occlude septal defects, for instance atrioseptal defects,
by means of non-surgical transvenous catheter procedures,
in other words, without having to perform an operation in
the literal sense. Various different occlusion systems have
been proposed to this end, each with their own pros and
cons, without any one specific occlusion system having yet
become widely accepted. In making reference to these
different systems, the following will use the terms
"occluder" or "occlusion device." In all interventional
occlusion systems, a self-expanding umbrella system is
introduced transvenously into a defect to be occluded in a
septum. This type of system might comprise two umbrellas;
one, for example, positioned at the distal side of the
septum (i.e. the side furthest from the median plane of the
body/heart) and one at the proximal side of the septum
(i.e. the side closer to the median plane of the body),
whereby the two umbrella prostheses are subsequently
secured to a double umbrella in the septal defect. Thus, in
the assembled state, the occlusion system usually consists
of two clamped umbrellas connected to one another by means
of a short bolt transversing the defect.
However, a disadvantage to such prior art occlusion
devices turns out to be the relatively complicated,
difficult and complex implantation procedure. Apart from
the complicated implantation of the occlusion system in the
septal defect to be occluded, the umbrellas utilized are
susceptible to material fatigue along with fragment
CA 02627408 2008-04-25
3
fracture. Furthermore, thromboembolic complications are
frequently to be anticipated.
In order to enable the inventive occlusion device to
be introduced by means of a surgical insertion instrument
and/or guidewire, a holder is provided at the end of the
distal retention area for engaging with the insertion
instrument and/or guidewire. It is thereby intended that
this engagement can be readily disengaged after positioning
the occlusion device in the defect. For example, it is
possible to devise the braiding at the end of the distal
retention area of the occlusion device in such a manner so
as to create an internal threading in the holder to engage
with the insertion instrument. Of course, other embodiments
are naturally also conceivable.
With another type of occlusion device, the so-called
Lock-Clamshell umbrella system, two stainless steel
preferably Dacron-covered umbrellas are provided, each
stabilized by four arms. This type of occluder is implanted
into the patient through a vein. However, seen as
problematic with the Lock-Clamshell occluder is the fact
that the insertion instruments necessary to implant the
device need to be of relatively large size. A further
disadvantage seen with other systems, for example the
Amplatz occluder, is that many different occluder sizes are
needed in order to cope with the respective dimensions of
the septal defects to be occluded. It thus turns out that
the umbrellas do not flatten out completely in the inserted
state if the length or the diameter of the crosspiece
inserted into the defect is not of an optimum match. This
results in incomplete endothelialization. It has
furthermore been shown that many of the systems implanted
into patients' bodies exhibit material fatigue and
fractures in the metallic structures due to the substantial
mechanical stresses over a longer period. This is
CA 02627408 2008-04-25
4
especially the case given permanent stress between an
implant and the septum.
In order to overcome these disadvantages, self-
centering occlusion devices have been developed which are
inserted into the body of the patient and introduced into
the septal defect to be occluded by way of a minimally
invasive procedure, for example using a catheter and
guidewires. Their design is based on the principle that the
occlusion device can be tapered to the dimensions of the
insertion instrument/catheter used for the intravascular
procedure. Such a tapered occlusion device is then
introduced by catheter into the septal defect to be
occluded, respectively into the shunt of the septum defect
to be occluded. The occluder is then discharged from the
catheter, upon which the self-expanding umbrellas,
retention plates respectively, subsequently unfold against
the two sides of the septum. The umbrellas in turn comprise
fabric inserts made from or covered by, for example,
Dacron, with which the defect/shunt.is occluded. The
implants remaining in the body are more or less completely
ingrown by the body's own tissue after a few weeks or
months.
An example of a self-centering occlusion device of
the type specified at the outset and in accordance with the
pre-characterizing part of claim 1 is known from WO
99/12478 Al, which is a further development of the
occlusion device known as the "Amplatz occluder" in
accordance with US printed patent No. 5,725,552. Same
consists of a braiding of a plurality of fine, intertwined
nitinol wire strands in the shape of a yo-yo. Each braiding
is produced in its original form as a rounded braiding
having loose wire ends both at its leading end (its
proximal side, respectively) as well as at its trailing end
(its distal side, respectively). During the subsequent
processing of the rounded braiding, each of these loose
CA 02627408 2008-04-25
ends must then be bundled into a sleeve and welded
together. After the appropriate processing, both the
proximal as well as the distal side of the finished
occluder exhibit a protruding collar. Dacron patches are
5 sewn into the distal and proximal retention umbrellas and
the interposed crosspiece. Because of the memory effect
exhibited by the nitinol material used, the two retention
umbrellas unfold by themselves upon exiting the catheter,
initially in a balloon-like intermediate stage, whereby the
retention umbrellas ultimately positioned on the two sides
of the septum eventually assume a more or less flattened
form. The crosspiece centers itself automatically into the
shunt to be occluded as the umbrellas unfold.
Yet embolic-related problems can arise with an
inserted implant due to the protruding collar at the
proximal retention area of the occluder, consecutive
embolization in particular. Such embolic-related problems
arise in particular in cases of patients suffering from so-
called atrial fibrillation. Atrial fibrillation is a
condition in which the atria of the heart experiences
frequent electrical discharge, leading to the atria not
contracting. One consequence of this lack of contraction to
the atria of the heart is that there is no effective
delivery or mixing of the blood and thrombi can form in the
atrium. A considerable risk of thrombi developing in an
atrium in consequence of atrial fibrillation is that such
thrombi can be carried along in the bloodstream and enter
the arterial circulation. Strokes are an especially
frequent consequence of such embolization, occurring at a
rate of roughly 5% per year in patients with atrial
fibrillation when not treated chronically with so-called
dicumerol to inhibit blood clots. However, effecting the
inhibition of blood clots with so-called dicumerol is
likewise not without risk. Since the side effects of
dicumerol treatment include increased bleeding,
contraindications for this treatment arise for
CA 02627408 2008-04-25
6
approximately 20% of all patients with atrial fibrillation
and patients also have to come to terms with the risk of
stroke when weighing the hemorrhage/stroke risks.
In the great majority of cases, thrombi forming in
the atrium of the heart develop in the so-called atrial
auricula. The atrial auricula are appendages found in the
atrium of the human heart. The right atrial auricula is
situated near the aorta ascendens, the left near the large
pulmonary artery. Blood clots which could potentially lead
to strokes develop most frequently in the left atrial
auricula in patients with atrial fibrillation.
Because of the risks and problems cited in connection
with the above-described formation of thrombi in the atrial
auricula, the task facing the present invention is that of
providing an occlusion device which can be used to occlude
the atrial auricula of the left atrium in order to
significantly reduce the formation of thrombi coupled with
the risk of stroke. To be provided in particular is an
occlusion device with which the risk of stroke is also
reduced for those patients for whom inhibiting blood clots
with dicumerol (so-called anticoagulation) is
contraindicated due to bleeding tendencies.
This task is solved with a self-expanding occlusion
device for occluding an atrial auricula, whereby said
occlusion device consists of a braiding of thin wires or
threads given a suitable form in a molding and heat
treatment procedure. It is provided for the occlusion
device to have a rear proximal retention area and a front
distal retention area as well as a center section arranged
between said proximal and said di'stal retention areas,
whereby the ends of the wires or threads converge into a
holder in the distal retention area and whereby the
occlusion device can be introduced in collapsed state into
the body of a patient and positioned in the atrial auricula
CA 02627408 2008-04-25
7
of the patient in a minimally invasive procedure using a
catheter. It is thereby provided in accordance with
invention for the proximal retention area to have a flanged
area which positions at the inner walls of the atrial
auricula when the occlusion device is in expanded state in
the atrial auricula to be occluded, forming a force-fit
connection with the inner walls of the atrial auricula in
order to hold the implanted and expanded occlusion device
in the atrial auricula by the distal retention area of the
occlusion device closing the opening in the atrial
auricula.
The inventive solution has a number of substantial
advantages over the occlusion devices known from the prior
art as described above. Firstly, the inventive occluder is
a self-expanding device which is especially easy to
implant, for example with the appropriate insertion
catheter. A procedure indicated as an example would be
puncturing a vein in the patient's groin area and guiding
the insertion catheter system through to the septum of the
right atrium. The left atrium of the heart can be reached
by puncturing the septum of the atrium, for example by a
known transseptal puncture, so that the insertion catheter
system can subsequently be introduced from the groin vein
into the left atrial auricula. The self-expanding occlusion
device for occluding the atrial auricula can then be
introduced by the insertion catheter system.
The occlusion device, which remains in collapsed
state during the implantation, preferably has a diameter of
from 6 to 10 French so that the surgical procedure for
occluding the atrial auricula is minimally invasive.
After the collapsed occlusion device has been
positioned in the atrial auricula to be occluded using, for
example, an insertion catheter, the occlusion device is
released from the catheter, upon which it unfolds in
CA 02627408 2008-04-25
8
response to its self-expanding nature and assumes its
distinctive shape induced by the molding and heat treatment
procedure used during its manufacture. In this expanded
state, the rear proximal retention area with the flanged
area configured thereupon is unfolded completely and
positions against the inner walls of the atrial auricula to
be occluded. In so doing, the proximal retention area with
the flanged area configured thereupon serves in the fixing
and positioning of the expanded occlusion device in the
atrial auricula. The center section extending toward the
opening in the atrial auricula from the proximal retention
area as well as the distal retention area provided at the
distal end of the center section thereby fill the open area
of the atrial auricula virtually in complete fashion so
that the entire expanded occlusion device in inserted state
serves as a plug in occluding the atrial auricula. The
formation of thrombi coupled with the risk of stroke can
thus be considerably reduced in an especially simple and
minimally invasive way.
Especially because the positioning and fixing of the
occlusion device makes use of the flanged area which
positions at the inner walls of the atrial auricula, the
occlusion device can dispense with the fastening hooks or
other anchoring means as normally used with such occlusion
devices for fixing and positioning the device in the
tissue. Of particular consideration in this respect is that
because the tissue walls near the atrial auricula are
extremely thin, conventionally-used fastening hooks cannot
provide a permanent fixing and positioning for the
occlusion device. The inventive solution and especially the
flanged area disposed on the proximal retention area can
circumvent the problems associated with fixing the
occlusion device to the extremely thin-walled and easily-
lacerated tissue of the atrial auricula when using hooks.
CA 02627408 2008-04-25
9
Preferred embodiments of the occlusion device are
given in the subclaims.
It is thus preferably provided for the occlusion
device to have its proximal retention area with the flanged
area be configured such that this area distends outward
upon expanding of the occlusion device so as to thus
position against the inner walls of the atrial auricula in
the inserted state. This preferred embodiment enables an
insertion catheter system to be able to urge the inventive
self-expanding occlusion device particularly deep into the
atrial auricula to be occluded. The distal retention area,
which is configured in particularly advantageous manner as
a distal umbrella, unfolds and positions thereafter; i.e.
after the catheter system has inserted the occlusion device
into the atrial auricula to be occluded, whereby the
umbrella abuts against the edge of the atrial auricula
opening at the entrance to the atrial auricula. At the same
time, the proximal area of the occlusion device; i.e., the
proximal umbrella, expands and in the process of expanding,
the proximal umbrella of the occlusion device's proximal
retention area is pulled farther into the atrial auricula
and a tractive force is thus exerted on the distal umbrella
across the center section. As a direct consequence thereof,
the distal umbrella, the distal retention area
respectively, is held by imposed permanent stress at the
opening to the atrial auricula. In other words, the
advantageous embodiment of the self-expanding occlusion
device provides a self-positioning and self-retaining
occlusion device, whereby the position held by the distal
umbrella is preferably flush with the opening of the atrial
auricula by means of the proximal umbrella distending
outwardly by itself.
A preferred embodiment furthermore provides for the
proximal retention area of the occlusion device to exhibit
a shape which flares toward the proximal end. What this
CA 02627408 2008-04-25
thereby realizes in particularly advantageous manner is
that the occlusion device automatically fits to the inner
wall of the atrial auricula - independent of the relative
diameter of the atrial auricula to be occluded and
5 independent of the thickness to the atrial auricula wall -
which, in particular, ensures a secure hold of the expanded
occlusion device in the atrial auricula. Configuring the
occlusion device as a fabric and due to the proximal
retention area's tapered shape to the proximal end further
10 enables the implanted occlusion device to independently
partake in certain independent movements of the atrial
auricula; this plays a considerable role, above all as
regards material fatigue and the long-lasting and reliable
functioning of the occlusion device - in particular,
precluding the usual complications which normally arise in
such cases.
The flexible and yet at the same time force-fit
positioning of the flanged area at the inner wall of the
atrial auricula moreover enables the inserted occlusion
device to be fully ingrown by the body's own tissue
considerably faster than is the case with the known prior
art occluding systems.
Further advantages can yield from using a braiding
made of thin wires or threads as the starting material for
the inventive occlusion device in that same exhibits long-
term mechanical stability. As already indicated, structural
fractures or other types of material fatigue can be largely
prevented in the inserted implant. Such a braiding moreover
has sufficient rigidity.
The flanged area configured at the proximal end
together with the tapered profile to the proximal retention
area of the braiding additionally allows the proximal
retention area of the occlusion device to unfold completely
at the inner walls of the atrial auricula in the inserted
CA 02627408 2008-04-25
11
and expanded state, and to do so virtually independent of
the diameter to the hole in the atrial auricula or the
thickness of the atrial auricula's inner walls. Because a
holder on the proximal retention area for bundling or
gathering the braiding together can be dispensed with, no
components of the occlusion device protrude any farther
into the atrial auricula so that neither is there any
threat of the body mounting defense mechanism reactions or
of there being any other conceivable complications.
A particularly advantageous embodiment of the
inventive occlusion device provides for the flanged area
configured at the proximal retention to be flared toward
the distal end by folding back of the proximal retention
area. This is an especially easy realized and thereby
effective way to configure the flanged area for the
occlusion device. In particular, it is thus possible to
form the entire occlusion device from one integral braiding
such that, on the one hand, no mechanic connective element
will be needed between the flanged area and the proximal
end and, on the other, the dimensions to the occlusion
device in its collapsed state can be even further reduced.
Other embodiments for configuring the flanged area at the
proximal retention area are, of course, also conceivable.
In order to have the distal retention area of the
occlusion device flatten fully in the implanted and
expanded state at the lateral edge of the atrial auricula
hole, and to do so virtually independently of the diameter
to said atrial auricula hole, a particularly advantageous
development of the above-mentioned embodiment of the
occlusion device provides for the distal retention area to
be provided with a recess in which the holder is disposed.
By arranging the holder in the recess provided at the
distal end of the occlusion device, no components of the
occlusion device protrude beyond the atrial auricula wall,
thus preventing components of the implant from being in
CA 02627408 2008-04-25
12
constant contact with the blood. This yields the advantage
of there being no threat that the body will mount defense
mechanism reactions or of there being thrombembolic
complications. Especially because the expanded occlusion
device expands, positions and fixes itself in the opening
of the atrial auricula, whereby the distal and proximal
retention areas are radially pre-stressed, the occlusion
device can be used for a wide range of atrial auricula
openings of various different sizes.
A particularly preferred development of the latter
embodiment of the inventive occlusion device in which the
distal retention area exhibits a recess further provides
for the distal end of the occlusion device to further
exhibit a connective element in the recess, whereby said
connective element can engage with a catheter. This
connective element, which is arranged on the occlusion
device such that it does not protrude beyond the atrial
auricula wall, thereby preventing components of the implant
from coming into constant contact with the blood, further
provides the inventive occlusion device with the
functionality of retrievability. In addition, a connective
element which can engage with a catheter facilitates
implantation and positioning of the occlusion device
(collapsed during the implanting procedure) in the atrial
auricula to be occluded. Various devices are conceivable as
connective elements. For example, latching members or even
hooks/ eyelets which force-lock with the correspondingly
configured complementary connective elements of a catheter
would be feasible.
Another advantageous embodiment provides for the
occlusion device to be configured so as to be reversibly
collapsible inward and outward so that the device can be
collapsed in its expanded state, for example with the help
of an explantation catheter in the disengaging of the
force-fit connection between the flanged area configured on
CA 02627408 2008-04-25
13
the proximal retention area and the inner wall of the
atrial auricula. In conjunction hereto, it is conceivable
for a catheter in the explantation procedure to, for
example, engage with connective elements configured at the
distal end of the occlusion device and to have the
collapsing of the occlusion device be occasioned in
response to external manipulation of the catheter. The
occlusion device is thereby fully reversibly retractable in
the catheter, enabling the complete removal of the device.
In order to ensure that the braiding of the occlusion
device will maintain the shape it was given by means of its
molding and heat treatment procedure, a particularly
preferable embodiment provides for the braiding to be made
from a shape memory material, in particular nitinol or a
polymer synthetic. Using nitinol for occluders is known.
Shape-memory polymers are included in the group known as
smart polymers and are polymers which exhibit a shape
memory effect; i.e., which are able to change their
external form in response to external stimuli such as, for
example, a change in temperature.
To this end, the polymer is first given its permanent
shape using conventional processing methods such as
injection molding or extrusion. The synthetic is then
subsequently deformed and fixed in its desired temporary
shape, a process known as "programming." In the case of
polymers, this process can ensue by heating, deforming and
then cooling a specimen. Or the polymer/synthetic can also
be deformed at lower temperature, a process known as "cold
drawing." The permanent shape thus becomes a remembered
memory shape while still in its current temporary shape.
Should the molded polymer body now be heated to a
temperature which is higher than the switching temperature,
this leads to a triggering of the shape memory effect and
thus to a restoring of the permanent memory shape. Cooling
the specimen effects an irreversible degeneration of the
CA 02627408 2008-04-25
14
temporary shape, which is why this is referred to as a so-
called one-way shape memory effect.
Shape memory polymers are far superior in terms of
memory properties than the known shape memory materials
such as, for example, the nitinol shape memory alloy, an
atomistic alloy of nickel and titanium. Only little effort
is required from the (heating/cooling) process to program
the temporary shape or, respectively, to restore the
permanent shape. Moreover, in the case of nitinol, for
example, the maximum deformation between permanent and
temporary form amounts to just 8%. In contrast, shape
memory polymers exhibit substantially higher deformability
capabilities of up to 1100%. In accordance with the present
invention, all afore-mentioned shape memory polymers are
claimed for biomedical application of the occlusion device
as specified at the outset.
An advantageous development of the latter above-
described embodiment of the inventive occlusion device in
which the braiding is made from a shape memory material
provides for the material to be a biologically degradable
shape memory polymer material. Synthetic, biodegradable
implant materials are particularly well-suited hereto. Such
types of degradable materials or polymers have bonds which
are fissionable under physiological conditions.
"Degradableness" is the term used if a material decomposes
from loss of mechanical properties due to or within a
biological system. An implant's external form and
dimensions may in fact remain intact during the
decomposition. What is meant with respect to degradation
time, provided no additional quantifying data is given, is
the time it takes for the complete loss of mechanical
properties. Biostable materials refer to materials which
remain stable within biological systems and which degrade
at least only partially over the long term.
CA 02627408 2008-04-25
In terms of degradable polymers, a distinction is
made between hydrolytically and enzymatically degradable
polymers. Hydrolytic degradation has the advantage that the
rate at which degradation occurs is independent of the
5 implant site since water is present throughout the system.
Given biodegradable polymers or materials, degradation can
thus occur through pure hydrolysis, enzymatically-induced
reactions or through a combination thereof. Typical
hydrolyzable chemical bonds are amide, ester or acetal
10 bonds. Two mechanisms can be noted with respect to the
actual degradation. With surface degradation, the
hydrolysis of chemical bonds transpires exclusively at the
surface. Because of the hydrophobic character, polymer
degradation is faster than the water diffusion within the
15 material. This mechanism is seen especially with
poly(anhydrides) and poly(orthoesters). As relates to the
poly(hydroxy carboxylic acids) particularly significant
especially to the shape memory effect such as poly(lactic
acid) or poly(glycol acid), the corresponding copolymers
respectively, polymer degradation transpires throughout the
entire volume. The step which determines the rate here is
the hydrolytic fission of the bonds since water diffusion
in the somewhat hydrophilic polymer matrix occurs at a
relatively fast rate. Decisive for the use of biodegradable
polymers is that, on the one hand, they degrade at a
controlled or variable speed and, on the other, that the
products of decomposition are non-toxic.
All cited biologically-degradable shape memory
polymers are claimed in accordance with the present
invention.
In terms of the shape to the inventive occlusion
device, it is particular preferred for the occlusion
device to exhibit a bell-shaped profile, whereby the
tapered end of this bell-shaped contouring forms the distal
retention area. Alternatively, the occlusion device can
CA 02627408 2008-04-25
16
also exhibit a mushroom-shaped profile, whereby the cap of
this mushroom shape forms the proximal or distal retention
area. It is additionally conceivable for the occlusion
device to exhibit a barbell-shaped profile, whereby the
center segment of this barbell shape forms the center
section between the proximal and the distal retention area
of the occlusion device. Of course, other contourings, as
chosen on the basis of the intended application, would be
just as conceivable.
It is particularly preferred for the braiding of the
inventive occlusion device to be tapered to the diameter of
a catheter to be used in a minimally invasive surgical
procedure. The advantage to this embodiment can be seen in
that it allows the catheter system used in the implantation
and explantation to have a considerably smaller internal
diameter, which substantially increases the maneuverability
of the occlusion device to be implanted and thus improves
the positioning accuracy of the device in the atrial
auricula. In the case of an occluder made from nitinol, the
internal diameter of a catheter used for its implantation
or explantation will measure between 8 and 10 French
whereas when using occlusion devices made from a synthetic
polymer, the internal diameter need only be between 6 and 8
French.
Last but not least, it is particularly preferred for
the occlusion device to have at least one fabric insert
arranged in or on the distal retention area or in the
center section of the occlusion device so as to completely
occlude the atrial auricula. This fabric insert serves in
closing the gaps remaining in the center area and in the
expanding diameters of the occlusion device following
insertion and expanding of the device in the atrial
auricula. For example, the fabric insert is affixed to the
braiding at the distal retention area in such a manner that
it can be stretched over the distal retention area like a
CA 02627408 2008-04-25
17
cloth. The advantage to this design lies in the fact that
the lateral edge of the distal retention area is flush with
the opening in the atrial auricula and less foreign
material is introduced into the body of the patient. The
fabric inserts can be made of Dacron, for example. Of
course, other materials and other.positionings to the
fabric insert in or on the occlusion device are also
feasible here.
The inventive procedure affords the prospect of
realizing a particularly simple manufacturing of the
occlusion device described above. First, a funnel-shaped
hollow braiding is formed, for example using a round
braiding machine. The technology used here can be one in
which the configured braiding is bundled at the end of the
length of the braiding; i.e., at what will later be the
distal end of the occlusion device, while the start of the
length of the braiding; i.e., what will later be the
proximal end of the occlusion device, remains open. It is
thereby possible to produce a funnel-shaped hollow
braiding, the bundled end of which corresponds to the
distal end of the finished occlusion device and the
opposite open end to the proximal end of the finished
occlusion device. Because a known braiding procedure can be
used to produce the occlusion device, the occlusion device
produced exhibits mechanical properties in terms of, for
example, expansion, stability, strength, etc., which can be
individually adapted to the later use of the occlusion
device. In advantageous manner, metallic wires or even
organic threads can be worked into the braiding.
With respect to the method, it is preferably provided
for the process step of forming the retention areas and the
center section to include a procedural step of molding
and/or heat treatment. This is of particular advantage when
the configured, funnel-shaped hollow braiding is made of
nitinol or of another material, especially polymer, which
CA 02627408 2008-04-25
18
has shape memory properties or effect. Preferably provided
for the inventive occlusion device is forming the braiding
from a shape memory polymer which is based, for example, on
a polyanhydride matrix or on polyhydroxycarboxylic acids.
These are synthetic, biodegradable materials which have a
thermally-induced shape memory effect. Yet also conceivable
would be other shape memory polymers such as, for example,
block copolymers as described e.g. in the special edition
of Angewandte Chemie 2002 114 by A. Lendlein and S. Kelch,
pages 2138 to 2162. It is a simple matter to bring such
materials into the applicable final form using a
combination of molding and heat treatment procedural steps.
A final formed occluder can then be tapered to the
dimensions of a catheter, for example. After exiting the
catheter, the occlusion device then unfolds by itself and
again assumes that shape to the funnel-shaped hollow
braiding to which the occlusion device was molded during
the manufacturing process by means of the molding and heat
treatment step.
It is preferred for the funnel-shaped hollow braiding
to be manufactured in such a manner that the thin wires or
threads constituting the finished braiding intertwine at
the proximal end of said braiding when forming the funnel-
shaped hollow braiding. This represents a conceivable and
readily realizable manner of producing an occlusion device
in accordance with the present invention, the proximal
retention area of which exhibits a form flared to the
proximal end. Of course, other manufacturing methods are
naturally also conceivable.
The following will make reference to the drawings in
providing a more precise detailing of preferred embodiments
of the inventive occlusion device.
Shown are:
CA 02627408 2008-04-25
19
Fig. 1 a side view of a preferred
embodiment of an occlusion device according to the present
invention;
Fig. 2 a top plan view of the proximal
end of the occlusion device shown in Fig. 1;
Fig. 3 a perspective view of the
occlusion device of in Fig. 1;
Fig. 4 an occlusion device pursuant
Fig. 1 implanted into the atrial auricula of a patient's
left atrium;
Fig. 5 a representative device for
producing a wire braiding of which the inventive occlusion
device is made;
Fig. 6 the wire braiding prior to heat
treatment (annealing); and
Fig. 7 a sectional view of a multi-
part device for producing the final form of an inventive
occlusion device.
Fig. 1 shows a side view of a preferred embodiment of
the inventive self-expanding occlusion device. Figures 2
and 3 show a top plan view of the proximal end and a
stereoscopic representation of the embodiment pursuant Fig.
1.
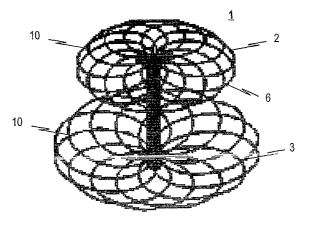
The inventive occlusion device 1 of the embodiment as
depicted consists of a braiding 10 of thin wires or threads
given a suitable form by means of a molding and heat
treatment procedure. The shape to occlusion device 1
depicted in Figs. 1-3 is that of a barbell-like profile
consisting of a front distal retention area 3, a center
CA 02627408 2008-04-25
section 5 and a rear proximal retention area 2. The ends of
the wires or threads of braiding 10 converge into a holder
4 in distal retention area 3. In contrast, proximal
retention area 2 exhibits a form tapering toward the
5 proximal end.
Proximal retention area 2 is moreover depicted to
have a flanged area 6, which is formed by the at least
partly folding over of proximal retention area 2 to the
10 distal end.
Braiding 10 is formed from wires or threads which are
preferably made from nitinol or other material having shape
memory properties or effect. It would also be conceivable
15 here to make use of a polymer synthetic which has shape
memory properties, as would be the use of a biologically
degradable shape memory material. What is essential is that
braiding 10 exhibit enough flexibility so that occlusion
device 1 can be tapered to the diameter of a catheter
20 (explicitly not shown) which would be used in a minimally
invasive, in particular intravascular, surgical procedure.
Because of the material's memory effect, an occlusion
device 1 tapered in such fashion exhibits a shape memory
function such that subsequent exiting the catheter, the
device 1 will expand automatically and re-assume the pre-
determined shape corresponding to its application. This
usually occurs after the occlusion device 1, initially
arranged in the catheter, has been positioned at the site
to be treated, in particular in the atrial auricula of a
patient's heart.
The pre-determined shape to the occlusion device can
also be one not unlike a bell, with the tapering end of the
bell-shaped form constituting distal retention area 3. It
would also be conceivable for occlusion device 1 to exhibit
a mushroom-shaped profile, whereby the cap of the mushroom
profile would form the proximal or distal retention area 2,
CA 02627408 2008-04-25
21
3. In contrast, the embodiment of the inventive occlusion
device depicted in Figs. 1-3 exhibit - as stated above - a
barbell-like shape, whereby the crosspiece of said barbell
shape forms the center section 5 between proximal and
distal retention areas 2, 3 of occlusion device 1.
Of course, other profile shapes to the occlusion
device, however constituted and application-specific, are
also conceivable. The barbell-like profile depicted here
serves only in the describing of a preferred embodiment of
the occlusion device and is in particular not to limit the
invention's scope of protection in any way.
In Figs. 1-3, the inventive occlusion device 1 is
shown in its expanded state. As indicated above, occlusion
device 1 exhibits a proximal retention area 2, a distal
retention area 3 as well as a concave, cylindrical center
section S. Proximal retention area 2 with its configured
flanged area 6 primarily serves in the affixing and holding
of the implanted and expanded occlusion device 1 in the
patient's atrial auricula. To this end, it is provided that
flanged area 6 partially positions on the inner walls of
the atrial auricula in the atrial auricula to be occluded,
realizing a force-fit connection with the inner walls of
the atrial auricula; the implanted and expanded occlusion
device 1 in thus held in the atrial auricula. It would also
be conceivable, for example, for proximal retention area 2
and/or flanged area 6 to be radially pre-stressed so as to
ensure a secure hold for the expanded occlusion device 1
given a relatively wide variation of atrial auricula
openings.
In implanted and expanded state, distal retention
area 3 serves to occlude the atrial auricula as optimally
as possible. How the individual retention areas function
will be described in greater detail in the following with
reference being made to Fig. 4.
CA 02627408 2008-04-25
22
The design of inventive occlusion device 1 is based
on the principle that occlusion device 1 can be tapered to
the size of a catheter. After it exits the catheter,
retention areas 2, 3 then unfold by themselves and position
against the inner walls of the atrial auricula. Hence, to a
certain degree, the inventive design thus entails a self-
positioning and self-centering system. Center section 5
thereby has a fixed length pre-defined for the application
in order to ensure closing of the atrial auricula opening.
Distal retention area 3 further exhibits a recess 7
in which holder 4 is arranged, into which the ends of the
wires or threads of braiding 10 converge. This ensures that
in implanted state, no material of the implanted occlusion
device 1 can protrude beyond the plane of the atrial
auricula into the patient's bloodstream. The provision of
such a recess 7 in distal retention area 3 can additionally
allow for disposing a connective element 8 at distal
retention area 3 without the patient's body mounting any
defense mechanism reactions since connective element 8
disposed within recess 7 is effectively prevented from
coming into contact with the blood. Connective element 8
can be configured so as to engage with a catheter.
The flexible property to inventive occlusion device 1
afforded by the material used and by braiding 10 allows
device 1 to be of a configuration which is reversibly
collapsible inward and outward so that an occlusion device
1 already expanded can be re-collapsed, for example by
using an explantation catheter, whereby the force-fit
joining of flanged area 6 and the inner walls of the atrial
auricula can then be disengaged.
Fig. 4 shows the preferred embodiment of inventive
occlusion device 1 in implanted state. Specifically, the
occlusion device is inserted into the left atrial auricula
CA 02627408 2008-04-25
23
of the patient's heart and serves to occlude the atrial
auricula. In detail, flanged area 6 of proximal retention
area 2 abuts the inner walls of the atrial auricula and
serves to position and fix the implanted occlusion device
1. In implanted state, distal retention area 3 closes the
opening of the atrial auricula, whereby the periphery of
the retention area positions against the wall of the atrial
auricula opening while center section 5 extends through the
opening. The inventive occlusion device 1 thus represents
an occluding system which is introduced into the body of a
patient and positioned at a specific location for the
purpose by means of a minimally invasive procedure; i.e.,
using a catheter and guidewires, for example. Hereto,
distal retention area 3 of device 1 in particular is
configured such that no material of implanted occlusion
device 1 can extend beyond the atrial auricula wall into
the patient's bloodstream. The edge of distal retention
area 3 is thereby flush with the atrial auricula wall. This
occurs over a relatively wide area independent of the
diameter of the atrial auricula or the thickness of the
atrial auricula wall at the atrial auricula opening. This
thus allows for a complete endothelialization to be
realized relatively quickly subsequent implantation of
occlusion device 1 and the patient's body will not mount
any defense mechanism reactions since the blood is
effectively prevented from coming into contact with the
material of implant 1.
Although not explicitly depicted in the figures,
inventive occlusion device 1 can further-more comprise a
fabric insert, same consisting of Dacron material, for
example. It would be conceivable here to work fabric
inserts into the interior of center section 5 or at the
distal end of retention area 3 for the purpose of
completely occluding the atrial auricula opening. The
inclusion of fabric inserts can ensue, e.g. by bracing same
within occlusion device 1. The implant inserted into the
CA 02627408 2008-04-25
24
body is then completely ingrown by the body's own tissue
after just a few weeks or months.
Fig. 5 depicts a representative device for producing
a wire braiding. This round braiding machine 11 is
configured such that it forms a funnel-shaped hollow
braiding 10 when in operation, whereby the hollow braiding
is gathered at a first distal end and remains opens at
an opposite second proximal end.
Fig. 6 shows a separate representation of the funnel-
shaped hollow braiding 10 made with round braiding machine
11. According to this design to the funnel-shaped hollow
braiding, proximal retention area 2 at the open second end
of braiding 10, distal retention area 3 at the gathered
first end of braiding 10, and a center section arranged
between said proximal and distal retention areas 2, 3 are
formed by means of a heat treatment. Subsequently, a holder
4 is provided at the bundled distal end of funnel-shaped
hollow braiding 10. The structuring of the inventive
occlusion device's braiding 10 is described in detail in
the DE 103 38 702 patent application cited at the outset.
Fig. 7 shows a sectional view of a multi-part device
for producing the final form of an inventive occlusion
device.
It is emphasized that the realization of the
invention is not limited to the embodiments associated with
the figures, but rather can be realized in a plurality of
variants.
CA 02627408 2008-04-25
List of Reference Numerals
1 occlusion device
5 2 proximal retention area
3 distal retention area
4 holder
5 center section
6 flanged area
10 7 recess
8 connective element
10 braiding
11 braiding machine