Note: Descriptions are shown in the official language in which they were submitted.
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
MICRONEEDLE ARRAYS AND METHODS OF PREPARING SAME
Cross-Reference to Related Applications
This application claims priority to International Patent Application Serial
No.
US2005/041858, filed on November 18, 2005, and to U.S. Provisional Application
Serial
No. 60/747618, filed on May 18, 2006, both of which are incorporated herein by
reference.
Field
The present invention relates to microneedle arrays and methods of preparing
the
sanle, and in particular to microneedle arrays where a drug, such as a
vaccine, is coated on
the microneedles.
Background
Devices including arrays of many small piercing structures, sometimes referred
to
as microneedles, microblades, or micro-pins, have been disclosed for use in
connection
with the delivery of drugs and other substances through the skin and other
surfaces. The
devices are typically pressed or driven against the skin in an effort to
pierce the stratum
comeum such that the drugs and other substances can pass through that layer
and into the
tissues below.
Some microneedle devices have a fluid reservoir and conduits through which a
therapeutic substance may be delivered into or through the skin. Others have a
coating on
the surface of the microneedles that releases into the target tissue after
penetration.
Summary of the Invention
It has been found, however, that the ability to provide a consistent coating
in one or
more desired locations on the microneedle array is an important feature for a
microneedle
device having an active substance-containing matrix coating disposed on the
surface of the
microneedle array. Altliough there are numerous well known niethods for
providing dried
coatings on generally flat surfaces, coating of a microneedle array provides a
challenge
due to the high surface irregularity inherent in the array design.
1
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
In a first aspect, the present invention provides a microneedle array
comprising a
substrate and a plurality of microneedles extending out from the substrate
with a multi-
phase matrix coating on at least a portion of the microneedle surface. The
multi-phase
matrix coating comprises an active substance and has a first solid phase and a
liquid phase.
The first solid phase comprises a water-soluble polymer.
In a second aspect, the present invention provides a method of providing an
active
substance-containing matrix coating on a microneedle array. A microneedle
array is
provided having a substrate and a plurality of microneedles extending out from
the
substrate. A water-soluble polymer is applied to the microneedle surface to
form a dried
coating of water-soluble polymer on at least a portion of the microneedles. A
coating
solution comprising an active substance, a liquid capable of phase separating
from the
water-soluble polymer, and a carrier fluid is prepared and applied to the
dried coating of
water-soluble polymer. At least a portion of the carrier fluid is removed from
the array to
provide the active substance-containing matrix coating on a microneedle array.
In certain embodiments, the present invention may provide one or more of the
following benefits: protection of active substance(s) from contact, and thus
possible
irreversible adhesion, with a microneedle array; protection of active
substance(s) from
accidental removal from a microneedle array; relative ease of removal of
active
substance(s) from a microneedle array under ordinary conditions of usage; and
efficient
placement of active substance(s) at or near the tips of the microneedles.
The invention will be further understood by those skilled in the art upon
consideration of the remainder of the disclosure, including the Detailed
Description and
the appended claims.
As used herein, certain terms will be understood to have the meaning set forth
below:
"Array" refers to the medical devices described herein that include one or
more
structures capable of piercing the stratum corneum to facilitate the
transdermal delivery of
therapeutic agents or the sampling of fluids through or to the skin.
"Microstructure," "microneedle" or "microarray" refers to the specific
microscopic
structures associated with the array that are capable of piercing the stratum
corneum to
facilitate the transdermal delivery of therapeutic agents or the sampling of
fluids througli
2
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
the skin. By way of example, microstructures can include needle or needle-like
structures
as well as other structures capable of piercing the stratum corneum.
The features and advantages of the present invention will be understood upon
consideration of the detailed description of the prefeiTed embodiment as well
as the
appended claims. These and other features and advantages of the invention may
be
described below in connection with various illustrative embodiments of the
invention.
The above summary of the present invention is not intended to describe each
disclosed
embodiment or every implementation of the present invention. The Figures and
the
detailed description that follow more particularly exemplify illustrative
embodiments.
Brief Description of the Drawings
Preferred embodiments of the invention will now be described in greater detail
below with reference to the attached drawings, wherein:
FIG. 1A is a microphotograph of a multi-phase matrix coating.
FIG. 1 B is a microphotograph of a porous solid coating remaining after the
liquid
was removed from the multi-phase matrix coating in FIG. 1A.
FIGS. 2A-5A are microphotographs of other multi-phase matrix coatings.
FIGS. 2B-5B are microphotographs of porous solid coatings remaining after the
liquid was removed from the multi-phase matrix coatings of FIGS. 2A-5A,
respectively.
Detailed Description
One embodiment of the present invention comprises a microneedle array having a
substrate and a plurality of microneedles extending out from the substrate
with a multi-
phase matrix coating on at least a portion of the microneedle surface of the
microneedle
array. The multi-phase matrix coating comprises an active substance and has a
first solid
phase and a liquid phase. The first solid phase comprises a water-soluble
polymer.
Microneedle arrays useful in the various embodiments of the invention may
comprise any of a variety of configurations, such as those described in the
following
patents and patent applications, the disclosures of which are herein
incorporated by
reference. One embodiment for the microneedle arrays comprises the structures
disclosed
in United States Patent Application Publication No. 2003/0045837. The
disclosed
microstructures in the aforementioned patent application are in the form of
microneedles
3
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
having tapered structures that include at least one channel formed in the
outside surface of
each microneedle. The microneedles may have bases that are elongated in one
direction.
The channels in microneedles with elongated bases may extend from one of the
ends of
the elongated bases towards the tips of the microneedles. The channels formed
along the
sides of the microneedles may optionally be terminated short of the tips of
the
microneedles. The microneedle arrays may also include conduit structures
forined on the
surface of the substrate on which the microneedle array is located. The
channels in the
microneedles may be in fluid communication with the conduit structures.
Another
embodiment for the microneedle arrays comprises the structures disclosed U. S.
Patent
Application Publication No. 2005/026 1 63 1, which describes microneedles
having a
truncated tapered shape and a controlled aspect ratio. Still another
embodiment for the
microneedle arrays comprises the structures disclosed in United States Patent
No.
6,091,975 (Daddona, et al.) which describes blade-like microprotrusions for
piercing the
skin. Still another embodiment for the microneedle arrays comprises the
structures
disclosed in United States Patent No. 6,313,612 (Sherman, et al.) which
describes tapered
structures having a hollow central channel. Still another embodiment for the
microneedle
arrays comprises the structures disclosed in U. S. Patent No. 6,379,324
(Gartstein, et al.)
which describes hollow microneedles having at least one longitudinal blade at
the top
surface of the tip of the microneedle.
The microneedles are typically less than 1000 microns in height, often less
than
500 microns in height, and sometimes less than 250 microns in height. The
microneedles
are typically more than 5 microns in height, often more than 25 microns in
height, and
sometimes more than 100 microns in height. The microneedles may be
characterized by
an aspect ratio. As used herein, the term "aspect ratio" is the ratio of the
height of the
microneedle (above the surface surrounding the base of the microneedle) to the
maximum
base dimension, that is, the longest straight-line dimension that the base
occupies (on the
surface occupied by the base of the microneedle). In the case of a pyramidal
microneedle
with a rectangular base, the maximum base dimension would be the diagonal line
connecting opposed corners across the base. Microneedles typically have an
aspect ratio
of between about 2:1 to about 5:1 and sometimes between about 2.5:1 to about
4:1.
The microneedles may be made of any suitable material, such as polymers,
metals,
or ceramics. Among polymeric materials, it may be preferred that the
microneedles be
4
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
manufactured of thermoplastic polymeric materials. Suitable polymeric
materials for the
microneedles of the present invention may include, but are not limited to:
polyacrylonitrile, polyacrylonitrile-butadiene, polybutadiene-styrenes,
polyphenyl
sulfides, polycarbonates, polypropylenes, acetals, acrylics, polyetherimides,
polybutylene
terephthalates, and polyethylene terephthalates. Polymeric microneedles may be
manufactured of a single polymer or a mixture/blend of two or more polymers.
In one
embodiment, the polymeric material is water-insoluble.
The microneedle arrays are generally characterized as having a substrate from
which a plurality of microneedles protrudes. The area of the substrate from
which the
microneedles protrude may have any suitable size, but will typically have a
planar area of
between about 0.5 cm2 and about 10 cm2.
A multi-phase matrix coating is present on at least a portion of the
microneedle
surface of the microneedle array. The multi-phase matrix coating may be
substantially
evenly distributed on the microneedle surface of the microneedle array. In
another
embodiment, the multi-phase matrix coating is preferentially present on the
substrate. In
another embodiment, the multi-phase matrix coating is preferentially present
on the
microneedles. By preferentially present it is meant that the amount of multi-
phase matrix
coating per unit surface area will be greater in one area than in another. In
one
embodiment, the multi-phase matrix coating is preferentially present on or
near the tips of
the microneedles. In some cases more than 20%, sometimes more than 50%, and
occasionally more than 75% of the multi-phase matrix coating by weight is
present on the
microneedles. In some cases the multi-phase matrix coating preferentially
resides on the
upper half of the microneedles, that is, the portion of the microneedles away
from the
substrate. In one embodiment, substantially no multi-phase matrix coating is
present on
the substrate, that is, substantially all of the multi-phase matrix coating is
present on the
microneedles. In one embodiment, substantially all of the multi-phase matrix
coating is
present on the upper half of the microneedles. By substantially all, it should
be understood
that insignificant amounts of multi-phase matrix coating, for example less
than about 5%
by weight, preferably less than about 1% by weight of the multi-phase matrix
coating are
present on the lower half of the microneedles and the substrate. The thickness
of the
multi-phase matrix coating may vary depending on the location on the
microneedle array
and the intended application use for the coated microneedle array. Typical
thicknesses of
5
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
the multi-phase matrix coating are less than 50 microns, often less than 20
microns and
sometimes less than 10 microns. It may be desirable for the coating thickness
to be
smaller near the tip of the microneedle so as not to interfere with the
ability of the
microneedle to effectively pierce into the skin.
The multi-phase matrix coating comprises a first solid phase comprising a
water-
soluble polymer. Examples of suitable polymers include polyvinylpyrrolidone,
polyvinyl
alcohol, polyethylene glycol, poly(ethyl oxazoline), polyacrylamide, poly(N-
vinyl
formamide), poly(N-vinyl acetamide), poly(N,N-dimethyl acrylamide), polyvinyl
oxazolidone, poly(ethylene oxide), poly(acrylic acid) and its partial or
complete salts,
cellulose derivatives, such as ethyl cellulose, hydroxyethyl cellulose,
hydroxypropyl
cellulose, and hydroxypropyl methyl cellulose, guar gum, xanthan gum, agarose,
and
copolyiners and/or mixtures tlzereof. In one embodiment the water soluble
polymer is
polyvinyl alcohol. In one embodiment the water soluble polymer is
polyvinylpyrrolidone.
For purposes of the present invention it is sufficient that some portion of
the polymer be
water-soluble under readily achievable conditions. So, for example, it may be
necessary
to heat certain polymers to allow them to dissolve in water. Likewise it
should be
appreciated that these polymers may contain high-molecular weight fractions or
be
partially cross-linked so that some portion of the polymer may not be able to
dissolve in
water.
The multi-phase matrix coating further comprises a liquid phase. In its most
general sense a liquid may be considered a material whose molecules are free
to move past
one another (i.e., not solid) while remaining in sliding contact with one
another (i.e., not
gaseous). Such a definition, however, may potentially apply to any amorphous
material.
For example, amorphous high-molecular weight polymers may flow over very long
time
scales (e.g., months, years, etc.) if held at temperatures above their glass
transition
temperature but below their molten temperature, but will not generally be
considered to be
liquid. For purposes of the present invention, a liquid is considered to be a
material where
the molecules can readily undergo flow during a time scale relevant to
formation of the
multi-phase matrix coating, which is typically on the order of less than one
minute to 30
minutes, and under conditions relevant to formation and use of the multi-phase
matrix
coatings, such as room temperature and pressure. Determination of flow may be
perforined by any suitable test method, such as ASTM D 4359-90(2000)el,
"Standard
6
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
Test Method for Determining Whether a Material is a Liquid or a Solid".
Typical liquid
phases will have a viscosity of between about 10-4 and 100 Pa-s, often between
about 10"3
and 10 Pa-s, and sometimes between about 0.1 and 5 Pa=s. It should be
appreciated,
however, that the liquid phase may be held in place in the multi-phase matrix
and/or on the
microneedle surface by capillary or surface tension forces, since these forces
may be
sufficient to prevent bulk flow of microscopic layers or channels of liquid.
Even in the
absence of bulk flow, however, the molecules within the microscopic layers or
channels of
liquid will still be able to flow with respect to each other.
Suitable liquids include those capable of phase separating from the water-
soluble
polymer. In one embodiment the liquid phase comprises a surfactant. Examples
of
suitable surfactants include polyoxyethylene sorbitan esters, such as
polyoxyethylene (20)
sorbitan monooleate (available as TWEEN 80), polyoxyethylene (20) sorbitan
monostearate (available as TWEEN 60), polyoxyethylene (5) sorbitan monooleate
(available as TWEEN 81), and polyoxyethylene (20) sorbitan monolaurate
(available as
TWEEN 20); sorbitan esters, such as sorbitan monolaurate (available as SPAN
20),
sorbitan monooleate (available as SPAN 80), and sorbitan trioleate (available
as SPAN
85); mono and diglycerides, such as glycerol monooleate; and mixtures thereof.
Other
suitable liquids include glycerol and propylene glycol. In one embodiment, the
liquid
phase comprises an oily liquid having a viscosity greater than that of water.
In one embodiment, the liquid phase is a continuous phase and the first solid
phase
is present as discrete particles suspended in the liquid phase and/or
contacting the
microneedle array surface. Such discrete solid particles may take any shape,
such as
spherical, needle-shaped, plate-like, and the like. The solid particles may
have relatively
smooth surfaces, irregular or rough surfaces, or some combination thereof and
may be
either solid or porous.
In another embodiment, the multi-phase matrix coating is bicontinuous, that
is, the
liquid phase is a continuous phase and the first solid phase is present as an
interlocking
continuous phase within the liquid phase. In still another embodiment, the
liquid phase is
present as discrete domains. For example, the liquid phase may be present as
droplets
suspended within a continuous, first solid phase, or as droplets contained
within
depressions within the first solid phase. Presence of a continuous solid phase
may be
7
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
beneficial in that the liquid phase will be generally contained within the
solid phase and
thus potentially protected from accidental removal from the microneedle array.
The weight ratio of first solid phase to liquid phase is typically greater
than about
5:95, often greater than about 10:90, and sometimes greater than about 20:80.
The weight
ratio of first solid phase to liquid phase is typically less than about 90:10,
often less than
about 80:20, and sometimes less than about 50:50.
The matrix coating on the microneedle array comprises one or more of a
biologically active material, a pharmaceutically effective substance, and a
therapeutically
active substance, which are collectively referred to throughout as active
substances. In
one embodiment, microneedle devices suitable for use in the present invention
may be
used to deliver drugs (including any pharmacological agent or agents) through
the skin in
a variation on transdermal delivery, or to the skin for intradermal or topical
treatment,
such as vaccination. In one aspect, drugs that are of a large molecular weight
may be
delivered transdermally. Increasing molecular weight of a drug typically
causes a
decrease in unassisted transdermal delivery. Microneedle arrays suitable for
use in the
present invention have utility for the delivery of large molecules that are
ordinarily
difficult to deliver by passive transdermal delivery. Examples of such large
molecules
include proteins, peptides, nucleotide sequences, monoclonal antibodies, DNA
vaccines,
polysaccharides, such as heparin, and antibiotics, such as ceftriaxone.
In another aspect, microneedle arrays suitable for use in the present
invention may
have utility for enhancing or allowing transdermal delivery of small molecules
that are
otherwise difficult or impossible to deliver by passive transdermal delivery.
Examples of
such molecules include salt forms; ionic molecules, such as bisphosphonates,
including
sodium alendronate or pamedronate; and molecules with physicochemical
properties that
are not conducive to passive transdermal delivery.
In another aspect, microneedle arrays suitable for use in the present
invention may
have utility for enhancing delivery of molecules to the skin, such as in
dermatological
treatments, vaccine delivery, or in enhancing immune response of vaccine
adjuvants.
Examples of suitable vaccines include flu vaccine, Lyme disease vaccine,
rabies vaccine,
measles vaccine, mumps vaccine, chicken pox vaccine, small pox vaccine,
hepatitis
vaccine, pertussis vaccine, rubella vaccine, diphtheria vaccine, encephalitis
vaccine,
yellow fever vaccine, recombinant protein vaccine, DNA vaccine, polio vaccine,
8
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
therapeutic cancer vaccine, herpes vaccine, pneumococcal vaccine, meningitis
vaccine,
whooping cough vaccine, tetanus vaccine, typhoid fever vaccine, cholera
vaccine,
tuberculosis vaccine, and combinations thereof. The term "vaccine" thus
includes,
without limitation, antigens in the forms of proteins, polysaccarides,
oligosaccarides, or
weakened or killed viruses. Additional examples of suitable vaccines and
vaccine
adjuvants are described in United States Patent Application Publication No.
2004/0049150, the disclosure of which is hereby incorporated by reference.
In one embodiment, the present invention is a method of applying an active
substance-containing matrix coating to the surface of a microneedle array. A
microneedle
array having a microneedle surface comprising a substrate and a plurality of
microneedles
is provided. A water-soluble polymer is applied to the microneedle surface to
form a dried
coating of water-soluble polymer on at least a portion of the microneedles
surface. In one
embodiment, the water-soluble polymer coating may be sterilized, for example,
by
exposure to gamma radiation or ethylene oxide gas.
For example, a thin coating of water-soluble polymer may be applied to the
entire
surface of the array prior to application of the coating solution, described
in more detail
below. Such a coating of water-soluble polymer may alter the hydrophilicity or
hydrophobicity of the array and tliereby affect the ability of the coating
solution to wet the
array. Such a coating of water-soluble polymer may also be partially or
totally miscible
with the coating solution, so that the water-soluble polymer is at least
partially taken up
into the coating solution before the carrier solvent completely evaporates,
thereby leaving
a mixture of the water-soluble polymer and the coating material subsequently
applied.
Examples of suitable water-soluble polymers are described above. Such a
polymer
coating may be prepared on the array by applying a polymer solution (e.g., an
aqueous or
ethanolic solution) to the array and allowing the solvent to evaporate,
thereby leaving a
dried polymer coating behind. Alternatively, the coating of water-soluble
polymer may be
directly applied as a solid material, such as through use of heat or plasma
deposition. In
certain embodiments, it may be desirable to add a surfactant to the polymer
solution to aid
in spreading the polymer across the entire surface of the microneedle array.
The surfactant
used to aid spreading of the polymer solution may be the same or different
from the
surfactant used in the coating solution that is subsequently applied to the
polymer coating.
In some instances, the surfactant may also aid in spreading of the coating
solution that is
9
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
subsequently applied to the dried coating of water-soluble polymer. Other
additives or
excipients may also be included in the coating of water-soluble polymer.
A coating solution comprising a carrier fluid and one or more active
substances is
subsequently prepared and applied to the dried coating of water-soluble
polymer. In one
embodiment, the coating solution is allowed to remain in contact with the
dried coating of
water-soluble polymer for a length of time sufficient to allow at least a
portion of the dried
coating of water-soluble polymer to be dissolved, at which time the carrier
fluid is
removed thus leaving behind any non-volatile components, such as active
substances and
other inactive pharmaceutical excipients.
The coating solution comprises an active substance, a liquid, and a carrier
fluid or
solvent. The carrier fluid or solvent should be selected so that it may
dissolve or disperse
the active substance. In one embodiment, the carrier fluid is selected so that
it may
dissolve at least a portion of the initially formed dried coating of water-
soluble polymer.
Dispersed material may be in the form of a suspension, that is, as particles
dispersed or
suspended in a carrier fluid or solvent. Examples of suitable carrier fluids
or solvents
include water, ethanol, methanol, isopropanol, ethyl acetate, hexane, and
heptane. Water
is particularly preferred as a carrier fluid. The coating solution may contain
additional
excipients such as viscosity modifiers, stabilizers, pH modifiers, and other
additives.
Examples of suitable additional excipients include sugars, such as sucrose,
trehalose,
raffinose, and lactose; proteins, such as ovalbumin; and salts, such as
monosodium citrate,
disodium citrate, trisodium citrate, monopotassium citrate, dipotassium
citrate, and
tripotassium citrate. In one embodiment, the coating solution may contain a
water-soluble
polymer, which may or may not be the same as the water-soluble polymer used to
form the
dried coating of water-soluble polymer. Additional solid excipients may mix
with the
water-soluble polymer to form the first solid phase. Alternatively, additional
solid
excipients may phase separate from the first solid phase comprising water-
soluble polymer
to form a second, or additional, solid phase(s).
In one embodiment, the coating solution will preferably spread relatively
uniformly across the array. Such spreading will desirably lead to a relatively
uniform
application of coated material to the microneedle array. That is, the amount
of coated
material at and near the edges of the array will be similar to the amount of
coated material
at or near the center of the array. Alternatively, the surface properties of
the coating
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
solution may be adjusted to control the amount of spreading of the coating
solution,
thereby allowing for application of controlled amounts of coating material at
specified
locations on the microneedle array. Removal of the carrier fluid is typically
accomplished
by evaporation, which may be allowed to take place at ambient conditions or
may be
adjusted by altering the temperature or pressure of the atmosphere surrounding
the
microneedle array. Evaporation conditions are desirably selected so as to
avoid
degradation of the coating material. In certain embodiments, the active agent
may
preferentially adsorb onto the dried polymer coating prior to complete removal
of the
carrier fluid, as described in United States Patent Application Serial No.
60/754786,
"Methods for Coating Microneedles", filed on Dec. 29, 2005, the disclosure of
which is
herein incorporated by reference.
In some embodiments removal of all or substantially all of the carrier fluid
is
desired. It should be understood, however, that relatively small amounts of
carrier fluid
may remain in the resultant matrix coating. For example, where the carrier
fluid
comprises water, the resultant matrix coating may typically contain between
about 0.1 to
30% by weight of water, often between about 1% to 20% by weight of water, and
sometimes between about 1% and 10% by weight of water.
Although not wishing to be bound by theory, it is believed that in certain
embodiments the following mechanism allows for formation of the aforementioned
multi-
phase matrix coating having a continuous, first solid phase. The dried coating
of water-
soluble polymer allows for a hydrophilic coating solution to wet out most or
all of the
surface of the microneedle array. The coating solution then dissolves a
portion of the
water-soluble polymer, while simultaneously evaporating. A thin layer of water-
soluble
polymer in intimate contact with the microneedle array is not dissolved, and
thus prevents
or minimizes interaction of active substance with the microneedle array
surface. The
water-soluble polymer that dissolves into the coating solution subsequently
phase
separates when the carrier fluid is removed (e.g., by evaporation) from the
coating
solution. Once the carrier fluid is removed, the water-soluble polymer forms a
porous
structure that is filled with non-volatile components of the coating solution.
The non-
volatile components of the coating solution may include materials that form a
liquid phase,
as well as materials that may form a second solid phase within the pores of
the first solid
phase formed by the water-soluble polymer. Thus several benefits are achieved.
The non-
11
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
volatile components of the coating solution (e.g., active substance, etc.) are
protected from
contact, and thus possible irreversible adhesion, with the microneedle
surface. The non-
volatile components of the coating solution are retained on the surface of the
microneedle
array by the porous polymer structure, thus minimizing the potential that
active substance
can be removed accidentally from the surface. The non-volatile components of
the
coating solution, and in particular the active substance(s), however, are not
intimately
mixed with the water-soluble polymer. As such, they do not need to slowly
diffuse from
the polymer-rich phase of the matrix and are thus relatively easily removed
from the
matrix when the matrix is brought into contact with a hydrophilic medium, such
as the
interstitial fluid in a skin layer. It should be understood, however, that
some of the non-
volatile components may mix with the water-soluble polymer and that some or
all of the
water-soluble polymer may be delivered from the array in other embodiments.
In another embodiment, a masking fluid may be applied to the microneedle array
prior to application of the coating solution as described in International
Patent Application
Publication No. WO 06/055799, the disclosure of which is herein incorporated
by
reference. For example, a masking fluid, such as a hydrofluoroether, may be
applied to
partially cover the microneedle array and then the coating solution may be
applied to the
surface of the masking fluid. In some embodiments, use of a masking fluid may
help to
control the location of matrix coating ultimately left on the microneedle
array.
One or more surfaces of the microneedle arrays may be altered with a surface
pre-
treatment prior to application of the water-soluble polymer. Typical surface
pre-
treatinents include a variety of plasma treatments capable of altering surface
functionality.
For example, polycarbonate may be plasma treated with a nitrogen plasma to
cause amide
functionalization or with an oxygen plasma to cause carboxylate
functionalization. A
combination of nitrogen and oxygen plasma treatment may be used to give a
mixed
surface functionality.
In one embodiment, different portions of the coating material may be
preferentially
deposited in different locations on the microneedle array. For example, where
the coating
material comprises a pharmaceutically effective substance (such as an
antigen), it may be
desirable to preferentially deposit the pharmaceutically effective substance
on or near the
tips of the microneedles. In some cases more than 30%, sometimes more than
50%, and
occasionally more than 75% of the pharmaceutically effective substance by
weight is
12
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
deposited on the microneedles. In some cases the pharmaceutically effective
substance
preferentially resides on the upper half of the microneedles, that is, the
portion of the
microneedles away from the substrate. In one embodiment substantially no
pharmaceutically effective substance is deposited on the substrate, that is,
substantially all
of the pharmaceutically effective substance is deposited on the microneedles.
In one
embodiment, substantially all of the pharmaceutically effective substance is
deposited on
the upper half of the microneedles. By substantially all, it should be
understood that
insignificant amounts of pharmaceutically effective substance, for example
less than about
5% by weight, preferably less than about 1% by weight of the pharmaceutically
effective
substance is not deposited on the upper half of the microneedles. The total
amount of
material deposited on the microneedle array (i.e., water-soluble polymer,
surfactant, other
excipients, etc.) may be distributed differently from the distribution of
active substance.
For example, more than 50% by weight of the total amount of material deposited
may be
deposited on the substrate of the array.
In any of the foregoing embodiments, any number of conventional coating
methods may be used to apply the coating solution to the microneedle array
including
dipping, brushing, drop coating, precision volumetric dispensing, gravure
coating, and
spray coating. In one embodiment, the coating solution may be applied as a
metered
amount of one or more droplets that are allowed to spread across the array
substrate.
In one embodiment, a microneedle array may be applied to a skin surface in the
form of a patch, such as described in International Patent Application
Publication WO
05/123173, the disclosure of which is herein incorporated by reference. A
microneedle
device may comprise a patch in the form of a combination of an array, a
pressure sensitive
adhesive and backing. Microneedles may protrude from all or a portion of the
microneedle array substrate surface. The microneedles may be arranged in any
desired
pattern, such as uniformly spaced rows, or distributed over the microneedle
substrate
surface randomly. In one embodiment, arrays of the present invention have a
distal-facing
surface area of more than about 0.1 cm2 and less than about 20 cm2, preferably
more than
about 0.5 cm2 and less than about 5 cm2. In one embodiment, a portion of the
substrate
surface of the patch is non-patterned. In one embodiment the non-patterned
surface has an
area of more than about 1 percent and less than about 75 percent of the total
area of the
device surface that faces a skin surface of a patient. In one embodiment the
non-patterned
13
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
surface has an area of more than about 0,10 square inch (0.65 cm2) to less
than about 1
square inch (6.5 cm2). In another embodiment, the microneedles are disposed
over
substantially the entire surface area of the array.
Microneedle devices may be used for immediate delivery, that is where they are
applied and immediately removed from the application site, or they may be left
in place
for an extended time, which may range from a few minutes to as long as 1 week.
In one
aspect, an extended time of delivery may be from 1 to 30 minutes to allow for
more
complete delivery of a drug than can be obtained upon application and
immediate
removal. In another aspect, an extended time of delivery may be from 4 hours
to 1 week
to provide for a sustained release of drug.
Examples
The following examples are presented merely to further illustrate features,
advantages, and other details of the invention. It is to be expressly
understood, however,
that while the examples serve this purpose, the particular materials and
amounts used as
well as other conditions and details are not to be construed in a matter that
would unduly
limit the scope of this invention.
Tetanus toxoid total-array content by high performance liquid chromatography
(HPLC)
A sample extraction solvent was prepared containing 50 mM potassium
perchlorate, 50
mM potassium citrate, 20 mM sodium phosphate, 376 mM sodium chloride, and 100
g/mL bovine serum albumin. An HPLC sample solution was prepared by placing an
array into a polypropylene cup, adding 1.0 mL of the sample extraction solvent
to the cup,
snapping a cap onto the sample cup, and sonicating for 30 minutes. Gradient
elution
HPLC (Mobile phase A): 0.2% (v/v) perchloric acid; Mobile phase B: 10% water,
88%
acetonitrile, 2% isopropanol, 0.2% perchloric acid (70%); Solvent Program:
0.00 min,
22% B, 1.0 mL/min; 6.00 min, 58% B, 1.0 mL/min; 6.01 min, 100% B, 1.0 mL/min;
6.50
min, 100% B, 0.5 mL/min; 10.0 min, 0% B, 0.5 mL/min; Injection Volume: 100 L;
Column: Zorbax 300SB-C8 50 x 4.6mm, 3.5 micron) was used to quantify tetanus
toxoid
in the HPLC sample solution. Non-adjuvanted tetanus toxoid (TT) vaccine
(Aventis) was
calibrated against a lyophilized TT primary standard (List Biologics) and used
as a
working standard. The working standard was used to obtain a calibration curve
from
14
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
approximately 1 g-TT/mL to 28 g-TT/mL. The correlation coefficient for the
linear
regression of the calibration curve was typically greater than 0.999. Tetanus
toxoid
content results are the average of between 6 and 10 replicates.
Tetanus toxoid tip-content by high performance liquid chromatography (HPLC)
Tetanus toxoid content on the tips of the microneedles was measured by fixing
the
toxoid in place on the substrate and lower portions of the microneedles so
that it could not
be extracted into the HPLC sample solution. A microneedle array was placed on
a flat
surface with the needles pointing upward and 10 L of an oil-based
polyurethane coating
solution (Minwax Fast-Drying Polyurethane) was applied to the array and
allowed to coat
the substrate of the array. The polyurethane was allowed to cure for at least
3 hours at
ambient conditions. The array was subsequently extracted and analyzed as
described in
the total content method.
Microneedle arrays
Microneedle arrays were prepared as follows. A circular disk (area 2 cm2,
thickness 1.02 mm) that was partially patterned with an array of microneedles
(37 x 37) in
a square shape (1 cm2) centered on one side of the disk was prepared. The
needles were
regularly spaced with a distance of 275 microns between the tips of adjacent
needles in a
square-shaped pattern. Individual needles were pyramidal in shape with a
height of 250
microns and a square base having a side-length of 83.3 microns. The tips were
truncated
with a flat, square-shaped top having a side-length of 5 microns. Arrays were
injection
molded according to the general description provided in International Patent
Application
Publication No. WO 05/82596 and made from polycarbonate (LEXAN HPS1R-1125, GE
Plastics, Pittsfield, MA). The center of the disk was then die cut to provide
a microneedle
array (area = 1 cm2) having microneedles on approximately 90% of the surface
of the
patterned side of the disk. The microneedle array had approximately 1200
microneedles,
Example 1
A polyvinylpyrrolidone (PVP) stock solution was prepared by adding 825 mg PVP
(Plasdone K-29/32, Povidone USP, ISP Technologies, Wayne, NJ) to 25 mL water
and
mixing until the PVP was dissolved. A stock solution was prepared by adding 50
mg
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
polysorbate 80 (TWEEN 80, Sigma Chemical Co., St. Louis, MO) to 25 mL ethanol.
A
diluted stock solution was prepared by adding 2 mL of the polysorbate stock
solution to 18
mL ethanol. A PVP coating solution was prepared by adding 1 mL of the PVP
stock
solution to 9 mL of the diluted polysorbate stock solution. A microneedle
array was
placed on a flat surface with the needles pointing upward and an aliquot of 30
L of the
PVP coating solution was applied to the center of the array using a pipette
and allowed to
spread across the array. The PVP coating solution was allowed to dry at
ambient
conditions.
TWEEN -80 (90 mg) was added to water (30 mL) to prepare a TWEEN -80 stock
solution with a concentration of 3 mg/mL. PVP (1.8 g) was added to water (20
mL) to
prepare a PVP stock solution with a concentration of 90 mg/mL. Sucrose (1.8 g)
was
added to water (20 mL) to prepare a sucrose stock solution with a
concentration of 90
mg/mL. Potassium citrate (1.8 g) was added to water (20 mL) to prepare a
potassium
citrate stock solution with a concentration of 90 mg/mL. An antigen coating
formulation
was prepared by mixing tetanus toxoid (Statens Serum Institute Lot 92-1, 888
Lf/mL) with
aliquots of the TWEEN -80, PVP, sucrose and potassium citrate stock solutions.
An aliquot (15 L) of masking fluid (FC-43 FLUORINERT Electronic Liquid)
was applied to the center of the array using a pipette and allowed to spread
across the
array. A 10 L aliquot of the antigen coating formulation was applied to the
center of the
masking fluid on the array using a pipette. The nominal amount of tetanus
toxoid in the
applied antigen coating formulation was 10 g. The nominal amount of TWEEN -80
in
the applied antigen coating formulation was 6 g. The nominal amounts of PVP,
sucrose,
and potassium citrate were 100 g. The volatile components of the antigen
coating
formulation and the masking fluid were allowed to evaporate at ambient
conditions for
approximately 30 minutes to provide an antigen-containing coating on the
array. Tetanus
toxoid total-array content as measured by reversed phase HPLC was 11.9 g (st.
dev.=0.5
g). Tetanus toxoid tip-content was measured as 5.0 g (st. dev.=1.2 g).
Exainples 2-5
Coated arrays were prepared according to the procedure described in Example 1
with the exception that the nominal amounts of PVP, sucrose and potassium
citrate were
varied, as shown in Table 1. Tetanus toxoid content of the coated array as
measured by
16
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
reversed phase HPLC and tetanus toxoid content on the tips of the microneedles
was
measured. The results are shown in Table 1.
Table 1
Tetanus toxoid content
Ex. PVP Sucrose Potassium citrate Total-array, Tip-content,
No. [ g] [ g] [ g] Mean (st. dev) Mean (st. dev) [ g]
[ g]
1 100 100 100 11.9 (0.5) 5.0 (1.2)
2 100 100 10 13.1 (0.4) 8.5 (0.5)
3 10 10 100 11.6 (0.8) 9.3 (1.3)
4 10 100 10 11.5 (0.3) 9.1(1,4)
100 10 10 12.3 (0.3) 6.9 (0.7)
5 In vivo tetanus toxoid deposition
Microneedle devices were prepared by adhering antigen coated arrays as
described
in Examples 1 to 5 to an adhesive baclcing. The arrays were applied to
hairless guinea
pigs using an applicator as generally described in U. S. Patent Application
Serial No.
60/578,65 1, the disclosure of which is hereby incorporated by reference. The
applicator
piston mass was 5.08 g and the devices were applied at a velocity of 8.07
meters/second.
The devices were applied to sites on the soft tissue of the abdomen and muscle
on the
lower back below the ribs and just above the pelvis. The application sites
were cleaned
with 70% isopropyl alcohol and allowed to air dry for at least 30 seconds
prior to device
application. Devices (N = 5) were removed at specified time points and the
tetanus toxoid
content remaining on the arrays was measured by HPLC. The results are
summarized in
Table 2.
Table 2
Tetanus toxoid content [ g]
Array Example T= 0 min T= 1 min T= 5 min T= 10 min T= 20 min
No.
1 11.9 9.8 10.3 8.6 8.1
17
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
2 13.1 10.9 10.1 8.2 8.1
3 11.6 9.1 8.1 7.6 6.3
4 11.5 9.9 8.6 8.2 7.0
12.3 11.1 9.8 10.0 8.5
Example 6
A polyvinyl alcohol coating solution was prepared as follows. An amount (250
mg) of polyvinyl alcohol (80% hydrolyzed, typical MW = 9,000 - 10,000, CAS
9002-89-
5 5, Aldrich, St. Louis, MO) was added to water (25 mL) to prepare a polyvinyl
alcohol
stock solution. An aliquot of polyvinyl alcohol stock solution (2 inL) was
added to
ethanol (18 mL) to prepare a polyvinyl alcohol coating solution. A microneedle
array was
placed on a flat surface with the needles pointing upward and an aliquot of 30
L of the
polyvinyl alcohol coating solution was applied to the center of the array
using a pipette
and allowed to spread across the array. The polyvinyl alcohol coating solution
was
allowed to dry at ambient conditions. An aliquot (15 L) of masking fluid (FC-
43
FLUORINERT Electronic Liquid) was then applied to the center of the array
using a
pipette and allowed to spread across the array. A 10 L aliquot of the antigen
coating
formulation was applied to the center of the masking fluid on the array using
a pipette.
Antigen coating formulations were prepared according to the general procedure
described
in Example 1. The nominal amount of tetanus toxoid in the applied antigen
coating
formulation was 10 g. The nominal amount of TWEEN-80 in the applied antigen
coating
formulation was 6 g. The nominal amounts of PVP, sucrose, and potassium
citrate were
100 g. The volatile components of the antigen coating forinulation and the
masking fluid
were allowed to evaporate at ambient conditions for approximately 30 minutes
to provide
an antigen-containing coating on the array. Tetanus toxoid total-array content
as measured
by reversed phase HPLC was 10.4 g (st. dev.=0.7 g). Tetanus toxoid tip-
content was
measured as 9.3 g (st. dev.=0.4 g).
Examples 7-14
Coated arrays were prepared according to the procedure described in Example 6
with the exception that the nominal amounts of PVP, sucrose and potassium
citrate were
varied, as shown in Table 3. Tetanus toxoid content of the coated array as
measured by
18
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
reversed phase HPLC and tetanus toxoid content on the tips of the microneedles
was
measured. The results are shown in Table 3.
Table 3
Tetanus toxoid content
Ex. PVP Sucrose Potassium citrate Total-array, Tip-content,
No. [ g] [ g] [ g] Mean (st. dev) Mean (st. dev)
[ g] [ g]
6 100 100 100 10.4 (0.7) 9.3 (0.4)
7 100 100 10 10.4 (0.3) 8.2 (0.8)
8 100 10 100 10.2 (0.6) 9.1 (0.3)
9 10 100 100 9.5 (0.9) 6.7 (1.0)
10 10 100 9.3 (0.4) 5.8 (1.1)
11 10 100 10 9.5 (0.5) 6.9 (0.9)
12 100 10 10 10.2 (0.4) 5.6 (1.4)
13 10 10 10 7.9 (0.2) 4.5 (0.7)
14 55 55 55 10.6 (0.4) 8.4 (0.5)
Exam lp e 15
5 A coated array was prepared according to the procedure described in Example
7.
Tetanus toxoid total-array content as measured by reversed phase HPLC was 10.7
g (st.
dev.= 0.9 g). Tetanus toxoid tip-content was measured as 8.7 g (st. dev.=
0.6 gg).
Arrays were applied to hairless guinea pigs as described above in the section
"in vivo
tetanus toxoid deposition". The amount of tetanus toxoid remaining on the
array after
10 removal from the hairless guinea pig was measured by HPLC. The results are
summarized
in Table 4.
Example 16
A coated array was prepared according to the procedure described in Example 8.
Tetanus toxoid total-array content as measured by reversed phase HPLC was 11.4
g (st.
dev.= 0.3 g). Tetanus toxoid tip-content was measured as 8.6 g (st. dev.=
0.5 g).
Arrays were applied to hairless guinea pigs as described above in the section
"in vivo
tetanus toxoid deposition". The amount of tetanus toxoid remaining on the
array after
19
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
removal from the hairless guinea pig was measured by HPLC. The results are
summarized
in Table 4.
Exam in e 17
A coated array was prepared according to the procedure described in Example 9.
Tetanus toxoid total-array content as measured by reversed phase HPLC was 10.8
g (st,
dev.= 0.3 Tetanus toxoid ti -content was measured as 6.8 st. dev.= 0.9
g)= p g ( g)=
Arrays were applied to hairless guinea pigs as described above in the section
"in vivo
tetanus toxoid deposition". The amount of tetanus toxoid remaining on the
array after
removal from the hairless guinea pig was measured by HPLC. The results are
suinmarized
in Table 4.
Example 18
A coated array was prepared according to the procedure described in Example
13.
Tetanus toxoid total-array content as measured by reversed phase HPLC was 11.7
g (st.
dev.= 0.3 g). Tetanus toxoid tip-content was measured as 5.3 g (st. dev.=
1.0 g).
Arrays were applied to hairless guinea pigs as described above in the section
"in vivo
tetanus toxoid deposition". The amount of tetanus toxoid remaining on the
array after
removal from the hairless guinea pig was measured by HPLC. The results are
summarized
in Table 4.
Table 4
tetanus toxoid content [ g]
ArrayExample T= 0 min T= 1 min T= 5 min T= 10 min T= 20 min
No.
15 10.7 10.6 8.8 7.8 6.3
16 11.4 10.2 8.4 8.1 7.3
17 10.8 9.3 9.2 8.4 7.5
18 11.7 9.7 9.7 8.3 7.9
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
Example 19
A glass substrate (50 mm x 75 mm microscope slide) was treated with a Basic
Plasma Cleaner (available from Harrick Scientific, Ithaca, New Yorlc) for 2
minutes. A
0.3 wt-% solution of poly(vinyl alcohol) in water was prepared using 85-89%
hydrolyzed
poly(vinyl alcohol) having a 4% solution viscosity of 100 cP (available from
Spectrum
Chemical Manufacturing Co., Gardena, California as catalog no. P1180). An
aliquot of
200 L of the poly(vinyl alcohol) solution was spread over a 20 cm2 area of
the glass
substrate and allowed to dry overnight at ambient conditions. A placebo
coating solution
was prepared having a concentration of 2.3 wt-% polyoxyethylene (20) sorbitan
monooleate (available from Uniqema, New Castle, Delaware as TWEEN-80) in
water. An
aliquot of 200 L of the coating solution was applied to the 20 cm2 area of
the glass
substrate covered by the dried poly(vinyl alcohol). The glass substrate was
then placed in
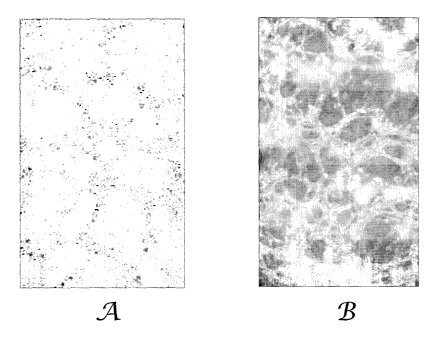
an oven for 30 minutes at 45 C to allow the water to evaporate. A photograph
of the
multi-phase matrix coating (using a Nikon Eclipse ME600 microscope with a 40x
objective and a Nikon DXM 1200F digital camera with a l Ox ocular) is shown in
Figure
lA. The multi-phase matrix coating was subsequently washed with an ethyl
acetate/heptane mixture (1:1 by weight) to remove the polyoxyethylene (20)
sorbitan
monooleate. The washed sample was then dried to remove the ethyl
acetate/heptane
mixture. A photograph of the remaining porous solid coating, using the same
conditions
as above, is shown in Figure 1B.
Exam lp e 20
A sample was prepared as described in Example 19, with the exception that the
coating solution had a concentration of 1.84 wt-% polyoxyethylene (20)
sorbitan
monooleate and 0.46% sucrose in water. A photograph of the multi-phase matrix
coating
is shown in Figure 2A and the remaining porous solid coating (after washing)
is shown in
Figure 2B.
Example 21
A sample was prepared as described in Example 19, with the exception that the
coating solution had a concentration of 2.07 wt-% polyoxyethylene (20)
sorbitan
monooleate and 0.23% sucrose in water. A photograph of the multi-phase matrix
coating
21
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
is shown in Figure 3A and the remaining porous solid coating (after washing)
is shown in
Figure 3B.
Example 22
A sample was prepared as described in Example 20, with the exception that the
coating solution contained trehalose instead of sucrose. A photograph of the
multi-phase
matrix coating is shown in Figure 4A and the remaining porous solid coating
(after
washing) is shown in Figure 4B.
Example 23
A sample was prepared as described in Example 21, with the exception that the
coating solution contained trehalose instead of sucrose. A photograph of the
multi-phase
matrix coating is shown in Figure 5A and the remaining porous solid coating
(after
washing) is shown in Figure 5B.
Exam lp e 24
A poly (vinyl alcohol) - (PVA) stock solution was prepared by adding 72 mg of
PVA (MW 9,000-10,000, 80% hydrolyzed, Aldrich, Milwaukee, WI) to 5 mL of
diluent
(12 mg/mL sucrose in phosphate buffered saline) and mixed until dissolved. A
diluted
PVA solution was prepared by adding 1 mL of the PVA stock solution to 9 mL of
methanol. A microneedle array was placed on a flat surface with the needles
pointing
upward and an aliquot of 30 L of the diluted PVA stock solution was applied
to the
center of the array using a pipette and allowed to spread across the array.
The PVA
solution was allowed to dry at ambient conditions. Antigen coating
formulations were
prepared by mixing tetanus toxoid (Statens Serum Institute Lot 92-1, 888
Lf/mL) with
equal volumes of either PBS or PBS containing sucrose (12 mg/mL).
An aliquot (15 L) of masking fluid (FC-43 FLUORINERT Electronic Liquid)
was applied to the center of the array using a pipette and allowed to spread
across the
array. A 10 L aliquot of the antigen coating formulations were applied to the
center of
the masking fluid on an array using a pipette. The nominal amount of tetanus
toxoid in the
applied antigen coating formulations was 18 g. The nominal amount of sucrose
applied
in the sucrose containing antigen coating formulation was 60 g. The volatile
components
22
CA 02627542 2008-04-28
WO 2007/059289 PCT/US2006/044551
of the antigen coating formulation and masking fluid were allowed to evaporate
at ambient
conditions for approximately 30 minutes to provide an antigen-containing
coating on the
arrays. Tetanus toxoid total-array content as measured by reversed phase HPLC
for the
PBS and PBS containing sucrose were 17.14 incg (st.dev. = 0.39 g) and 17,24
g (st.dev.
= 0.54 g), respectively. Tetanus toxoid tip-content for the PBS and PBS
containing
sucrose were measured as 9.17 g (st.dev. = 1.61 g) and 9.57 g (st.dev. =
2.99 g),
respectively.
23