Note: Descriptions are shown in the official language in which they were submitted.
CA 02628145 2008-05-01
IMTM GmbH 1
Novel dual-use peptidase inhibitors as prodrugs
for a therapy of inflammatory and other diseases
The invention relates to novel substances and compounds which are capable of
concertedly inhibiting the enzymes dipeptidyl peptidase IV (DPIV) as well as
peptidases having an analogous enzymatic effect and alanyl aminopeptidase N
(APN) as well as peptidases having an analogous enzymatic effect ("dual inhibi-
tors"). Furthermore, the invention relates to processes to prepare the novel
dual
inhibitors of DPIV and APN. The invention also relates to the afore-mentioned
novel compounds for a use in the medical field. Moreover, the invention
relates
to a use of the afore-mentioned dual inhibitors for a prophylaxis and a
therapy
of diseases showing an excessive immune response and having an inflamma-
tory genesis, of neuronal diseases and of cerebral damage, of tumor diseases,
of skin diseases, of diabetes of the type II and of SARS.
The enzyme dipeptidyl peptidase IV (DPIV, CD26, EC 3.4.14.5) is a serine pro-
tease existing ubiquituously and catalyzing the hydrolysis of peptides specifi-
cally after proline and - to a lesser extent - after alanine or - with
restrictions -
after further amino acids like serine, threonine, valine and glycine at the
second
position of the N-terminus. Enzymes belonging to the gene family of enzymes
having DPIV-analogous enzymatic effect are - inter alia - DP 8, DP 9 and
FAP/seprase [T. Chen et al.: Adv. Exp. Med. Biol. 524, 79, 2003]. A substrate
specifity analogous to DPIV was also found for attractin (mahagony protein)
[J.
S. Duke-Cohan et al.: J. lmmunol. 156, 1714, 1996]. Said enzyme is also inhib-
ited by DPIV inhibitors.
Belonging to the group of alanyl aminopeptidases (also existing ubiquituously)
are the aminopeptidase N (APN, CD13, EC 3.4.11.2) predominantly appearing
as a membrane protein of the type II, as well as the cytosolic soluble alanyl
aminopeptidase (EC 3.4.11.14, puromycine-sensitive aminopeptidase, amino-
peptidase PS, encephaline-degrading aminopeptidase). Alanyl aminopepti-
CA 02628145 2008-05-01
IMTM GmbH 2
dases (including the afore-mentioned aminopeptidases) act in dependency of a
metal, for example in dependency of zinc, and catalyze the hydrolysis of
peptide
bonds after the N-terminal amino acids of oligopeptides, in the case of APN
with
a preference of alanine at the N-terminus [A. J. Barrett et al.: Handbook of
Pro-
teo-lytic Enzymes, Academic Press 1998]. All inhibitors of aminopeptidase N
also inhibit the cytosolic alanyl aminopeptidase, while specific inhibitors of
the
cytosolic aminopeptidase exist [M. Komodo et al.: Bioorg. and Med. Chem. 9,
121, 2001].
For both groups of enzymes, important biologic functions were proved in differ-
ent cell systems. This is true - inter alia - for the immune system [U.
Lendeckel
et al.: Intern. J. Mol. Med. 4, 17, 1999; T. Kahne et al.: Intern. J. Mol.
Med. 4, 3,
1999; I. De Meester et al.: Advanc. Exp. Med. Biol. 524, 3, 2002;
International
Patent Application No. WO 01/89,569; International Patent Application No. WO
02/053,170; International Patent Application No. PCT/EP 03/07,199]; the neu-
ronal system [International Patent Application No. WO 02/053,169 and German
Patent Application No. 103 37 074.9]; the fibroblasts [German Patent Applica-
tion No. 103 30 842.3]; the keratinocytes [International Patent Application
No.
WO 02/053,170]; the sebaceous gland cells/sebatocytes [International Patent
Application No. PCT/EP 03/02,356]; tumors as well as for virus-caused infec-
tions as, for example coronavirusses [D. P. Kontoyiannis et al.: Lancet 361,
1558, 2003].
The capability, of DPIV, of specifically inactivating the incretory hormones
GIP
and GLP led to the development of a new therapeutic concept for treating glu-
cose metabolic disorders [D. M. Evans: Drugs 5, 577, 2002].
For both groups of enzymes, there are known different inhibitors [reviews are
found in: D. M. Evans: Drugs 5, 577, 2002; and: M.-C. Fournie-Zaluski and B.
P.
Roques: in J. Langner and S. Ansorge: Ectopeptidases, Kluwer Aca-
demic/Plenum Publishers, p. 51, 2002].
CA 02628145 2008-05-01
IMTM GmbH 3
The isolated inhibition of the alanyl aminopeptidases and of the dipeptidyl
pepti-
dase IV as well as the inhibition of enzymes having an analogous substrate
specificity, in particular the combined inhibition of enzymes of both groups
of
enzymes, results into a strong inhibition of the DNA synthesis of immune cells
and, hence, into a strong inhibition of the cell propagation as well as into a
change of the cytokine production, particularly into an induction of the
(immune-
regulatory effective) TGF-[31 [International Patent Application No. WO
01/89,569; International Patent Application No. WO 02/053,170] as well as into
an inhibition of the generation and release of inflammatory cytokines of the
type
TH1 and TH2, e. g. interleukine-4 (IL-4) [International Patent Application No.
WO 02/053,170 and German Patent Application No. 101 02 392.8]. Inhibitors of
alanyl aminopeptidase effect a strong induction of TGF-R1 at regulatory T-
cells
[International Patent Application No. PCT/EP 03/07,199]. In the neuronal sys-
tem, a decrease or retardation of acute and chronic cerebral damage processes
was proved by an inhibition of both enzyme systems [International Patent Appli-
cation No. WO 02/053,169 and German Patent Application No. 103 37 074.9].
Moreover, it was proved for fibroblasts [German Patent Application No. 103 30
842.3], keratinocytes [International Patent Application No. WO 02/053,170) and
sebatocytes [International Patent Application No. PCT/EP 03/02,356] that the
combined inhibition of alanyl aminopeptidase N and DPIV effects an inhibition
of
the growth and a change of the cytokine production.
This results into the surprising fact that the alanyl aminopeptidases and the
dipeptidyl peptidase IV as well as enzymes having an analogous effect perform
fundamental central biologic functions in different organs and cell systems,
and
that a combined inhibition of both groups of enzymes represents a new
effective
therapeutic principle for the treatment of various - im most cases chronic -
dis-
eases.
CA 02628145 2008-05-01
IMTM GmbH 4
In accepted animal models, the applicants could show in the meantime that, in
particular, the combined administration of inhibitors on both groups of said
pep-
tidases results into an inhibition of the growth of different cell systems and
into a
suppression of an excessive immune response, of chronic inflammatory proc-
esses and of cerebral damages, also in vivo [International Patent Application
No. WO 01/89,569]. The isolated administration of single known inhibitors re-
sults into a diminished effect.
The results reported up to now were obtained predominantly by means of
known inhibitors of alanyl aminopeptidase N and dipeptidyl peptidase IV alone,
being described in the literature and being - in part - commercially available
but
in particular by combinations of inhibitors of enzymes of both groups.
Now, surprisingly, novel, predominantly non-peptidic low molecular weight sub-
stances were found, which may be employed as prodrugs and which may react
under physiological and pathological conditions to effective agents or to a
mix-
ture of effective agents and which inhibit alanyl aminopeptidase N and enzymes
having an analogous substrate specificity, and dipeptidyl peptidase IV and en-
zymes having an analogous substrate specificity as well, in a dual manner. The
conversion of the prodrugs is conducted by a reduction of -S-S- or -Se-Se-
bridges, preferably by cellular thiols (compounds bearing -SH- groups).
Hence, prodrugs of the type disclosed here preferably act at cells and
tissues.
Moreover, by the use of said prodrugs, a reduction of the inhibitory capacity
of
the inhibitors by binding to free peptidases in the blood plasma can be pre-
vented from occurring.
Hence, the invention relates to novel substances which are capable of specifi-
cally inhibiting peptidases cleaving Ala-p-nitroanilide as well as peptidases
cleaving Gly-Pro-p-nitroanilide and, hence, combine the capability of a con-
certed inhibition of both groups of peptidases in one substance, only.
CA 02628145 2008-05-01
IMTM GmbH 5
Moreover, the invention relates to novel substances which may be used as
such, but also may be used as starting materials for other substances, for a
prophylaxis and a therapy of diseases having an excessive immune response
(autoimmune diseases, allergies and transplant rejections, sepsis), of other
chronic-inflammatory diseases, including arteriosclerosis , neuronal diseases,
and cerebral damage, skin diseases (inter alia acne and psoriasis), of tumor
diseases and specific virus infections (inter alia SARS) as well as type II
diabe-
tes.
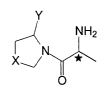
The invention relates to compounds of the general formulae (1) and (2)
A-B-D-B'-A' (1) and
A - B - D - E (2), in which
- A and A' may be identical or different and are the residue
Y
NH2
r_~ X\/N Y*
0
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH, and Y is H or CN, and *
designates a chiral carbon atom;
- B and B' may be identical or different and are an 0, N or S containing or
non-containing, unsubstituted or substituted, unbranched or branched al-
kylene residue, cycloalkylene residue, aralkylene residue, heterocycloal-
kylene residue, heteroarylalkylene residue, arylamidoalkylene residue, het-
eroarylamidoalkylene residue, unsubstituted or mono- or poly-substituted
CA 02628145 2008-05-01
IMTM GmbH 6
arylene residue or heteroarylene residue having one or more five-, six- or
seven-membered ring(s);
- D is -S-S- or -Se-Se-; and
- E is the group -CH2-*CH(NHZ)-R9 in which R9 is an 0, N or S containing or
non-containing, unsubstituted or substituted, unbranched or branched alkyl
residue, cycloalkyl residue, aralkyl residue, heterocycloalkyl residue, het-
eroarylalkyl residue, arylamidoalkyl residue, heteroarylamidoalkyl residue,
unsubstituted or mono- or poly-substituted aryl residue or heteroaryl residue
having one or more five-, six- or seven-membered ring(s) and * designates a
chiral carbon atom;
- or the acid addition salts thereof with organic and/or inorganic acids.
Preferred embodiments of the compounds of the general formulae (1) and (2)
result from subclaims 2 to 10.
The invention also relates to a process to prepare compounds of the general
formulae (1) and (2) according to the general scheme of synthesis given on the
following page in which A, A', B, B', D and E have the meanings mentioned
above in detail, in which according to the following scheme of synthesis 1
CA 02628145 2008-05-01
IMTM GmbH 7
Scheme of synthesis 1 for compounds A - B - D - B' - A' and A - B - D -
E
NH-(SG)
HOTI-I> HO NH-(SG)
S-S-Z
yl-~
Y 0
Y
X-_/NH r-~NH
Y X-_/
~ NH-(SG) Y
N~ ~ NH-(SG)
X~ S-S-Z
0 X-_/N y -~ S-S-Z
0
1. HS-CHZ-CH-R9
2. - (SG) NH-(SG)
Y NH-(SG) HS-CH2-CH-R9 Y
NH-(SG) ~ NH2
N~ 2. (SG)
LX~ S- X~/ N S-S-CHZ-CH-R9
0 2 0 yl~2- 1
NH2
Y
-B-D-E
NH A
\(SG)
Y
NH-(SG) I NH2 0
[HO_] X\/N S-S NX
O 2 0 Y
NH2 /I
Y
A-B-D-B'-A'
CA 02628145 2008-05-01
IMTM GmbH 8
- compounds of the general formula
HO - C(=O) - CH[-NH (SG)] -D
in which (SG) is a protecting group and -D is a structure element of B
are transformed with a heterocyclic compound of the general formula
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the ob-
tained condensation product of the formula
Y
N - C(=O) - CH[-NH (SG)] _>
X_/
is transformed with a compound of the general formula
S-S
in which is a structure element of B; and the resulting reaction product
is transformed being cleaved from the protecting groups (SG) into a com-
pound of the general formula A - B - D - B' - A' (1) in which A and A' may
be identical or different and B and B' may be identical or different and in
which A, A', B, B' and D may have the afore-mentioned meanings; or
- compounds of the general formula
HO - C(=O) - CH[-NH (SG)] ~
in which (SG) is a protecting group and -> is a structure element of B
are transformed with a heterocyclic compound of the general formula
CA 02628145 2008-05-01
IMTM GmbH 9
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the re-
sulting condensation product of the formula
Y
N - C(=0) - CH[-NH (SG)] _>
X-/
is transformed with a compound of the general formula
S-S
in which I <is a structure element of B; and the resulting reaction product is
transformed with a compound of the general formula
HS-CH2-CH [-NH-(SG)]-(R9)
and is transformed being cleaved from the protecting groups (SG) into a
compound of the general formula A - B - D - E (2), in which A, B, D and E
may have the afore-mentioned meanings; or
- a compound of the general formula
HO - C(=O) - CH[-NH (SG)] ~I - S-S -Z
in which (SG) is a protecting group, Ul is a structure element of B and
Z is a residue, which activates an -S-S-group for a thiol exchange, is
transformed with a heterocyclic compound of the general formula
CA 02628145 2008-05-01
IMTM GmbH 10
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the ob-
tained condensation product of the formula
Y
N-C(=O)CH[-NH(SG) - S - S - Z
X-/
is transformed with a compound of the general formula
HS - CHZ - CH [- NH - (SG)] - (R9)
being cleaved from the protecting groups (SG) into a compound of the general
formula A - B - D - E (2), in which A, B, D and E may have the afore-
mentioned meanings; or
- a compound of the general formula
[ HO - C(=O) - CH [-NH (SG)] E:I- S]2
in which SG is a protecting group and F] is a structure element
of B is transformed with a heterocyclic compound of the general formula
CA 02628145 2008-05-01
IMTM GmbH 11
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; and the
obtained reaction product is transformed with a compound of the general
formula
HS-CHz-CH [-NH-(SG)]-R9
into a compound of the general formula A - B - D - E (2) in which A, B, D
and E may have the afore-mentioned meanings; or
- a compound of the general formula
[ HO - C(=0) - CH [-NH (SG)] ~I- S]Z
in which SG is a protecting group and is a structure element of B, is
transformed with a heterocyclic compound of the general formula
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; and the
obtained reaction product is transformed being cleaved from the protecting
groups into a compound of the general formula A - B - D - B' - A' (1) in
which A and A' may be identical or different and B and B' may be identical or
CA 02628145 2008-05-01
IMTM GmbH 12
different and in which A, A', B, B' and D may have the afore-mentioned
meanings or
- compounds of the general formula
HO - C(=O) - CH[-NH (SG)] -D
in which (SG) is a protecting group and -D is a structure element of B
are transformed with a heterocyclic compound of the general formula
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the ob-
tained condensation product of the formula
Y
N - C(=O) - CH[-NH (SG)]
X-/
is transformed with a compound of the general formula
I1-S-S-z
in which 11 \ is a structure element of B and Z is a residue which activates
an -S-S-group for a thiol exchange and (SG) is a protecting group; and the
obtained reaction product of the formula
Y
N-C(=O)CH[-NH(SG)M- S - S - Z
X-/
CA 02628145 2008-05-01
IMTM GmbH 13
is transformed with a compound of the general formula
HS-CH2-CH [-NH-(SG)]-(R9)
being cleaved from the protecting group (SG) into a compound of the gen-
eral formula A - B - D - E (2) in which A, B, D and E may have the afore-
mentioned meanings.
The invention also relates to the afore-mentioned and in the following in
detail
described compounds of the general formulae (1) and (2) to be used in medi-
cine.
Furthermore the invention relates to the afore-mentioned and in the following
in
detail described compounds of the general formulae (1) and (2) being inhibitor
precursors or inhibitor prodrugs.
Preferred embodiments result from subclaims 14 to 16.
Furthermore, the invention relates to the use of at least one compound of the
afore-mentioned and in the following in more detail described general formulae
(1) and (2) for the prophylaxis and therapy of diseases with exceeding immune
response and inflammatory genesis including arteriosclerosis , neuronal dis-
eases, cerebral damages, skin diseases, tumour diseases and virus-caused
diseases, as well as type II diabetes.
The invention also relates to the use of at least one of the compounds of the
afore-mentioned and in the following in detail described general formulae (1)
and (2) for the preparation of a medicament for the prophylaxis and therapy of
diseases with exceeding immune response and inflammatory genesis including
arteriosclerosis , neuronal disease, cerebral damages, skin diseases, tumour
diseases and virus-caused diseases, as well as type II diabetes.
CA 02628145 2008-05-01
IMTM GmbH 14
Preferred embodiments of the use are claimed in claims 19 to 20.
Furthermore, the invention relates to a process to generate at least one
inhibitor
of dipeptidyl peptidase IV (DPIV) and of peptidases with analogous enzymatic
effect, as well as of analyl aminopeptidase N (APN) and of peptidases with
analogous enzymatic effect from at least one of the compounds of general for-
mulae (1) and (2) according to the following detailed description, wherein at
least one compound of the general formulae (1) and (2) is according to the fol-
lowing detailed description exposed to reducing conditions as they are present
in cells and tissues.
Preferred embodiments of the process result from claim 22.
The invention relates also to pharmaceutical or cosmetic preparations compris-
ing at least one of the compounds of at least one of the general formulae (1)
and (2) according to one of the claims 1 to 10 and according to the following
detailed description, optionally in combination with one or more
pharmaceutical
or cosmetic acceptable carrier(s), auxiliary compound(s) and/or adjuvant(s).
According to the invention the new compounds have either the general formula
(1):
A-B-D-B'-A' (1)
or the general formula (2):
A-B-D-E (2),
It was surprisingly found that the compounds of said formulae themselves have
inhibitory effects with regard to the enzymes mentioned below and moreover
CA 02628145 2008-05-01
IMTM GmbH 15
react to compounds under defined conditions which are inhibitors of dipeptidyl
peptidase IV (DPIV) and of peptidases with analogous enzymatic effect, the
analyl aminopeptidase N (APN) and of peptidases with analogous enzymatic
effect.
Using the term "dipeptidyl peptidase IV" (DPIV, CD26, EC 3.4.14.5) in the fol-
lowing description and in the claims, the serine protease is recognized which
catalyzes the hydrolysis of peptide bonds specifically after proline and to a
lesser degree alanine and - with restrictions - after other amino acids like
ser-
ine, threonine, valine, and glycine respectively at the second position of the
N-
terminus of peptides.
Using the term "peptidases with dipeptidyl peptidase IV analogous enzymatic
effect" peptidases are recognized in the present description and in the claims
which catalyze the hydrolysis of peptides specifically after proline or
alanine at
the second position of the N-terminus. Examples for peptidases with dipeptidyl
peptidase IV analogous enzymatic effect are, without restricting the invention
to
those, DP 8, DP 9 and FAP/seprase [T. Chen et al., a. a. 0.] and attractin (ma-
hagony protein) [J. S. Duke-Cohan et al., a. a. 0.].
Using the term "alanyl aminopeptidase N" (APN, CD13, EC 3.4.11.2) in the pre-
sent description and in the claims the protease is recognized which operates
metal- (zinc-) dependent and catalyzes the hydrolysis of peptide bonds specifi-
cally after N-terminal amino acids of peptides and preferably alanine at the N-
terminus.
Using the term "peptidases with alanyl aminopeptidase N analogous enzymatic
effect" peptidases are recognized in the present description and in the claims
which - like APN - operate metal-dependent and catalyze the hydrolysis of
peptide bonds specifically after N-terminal amino acids of peptides and
prefera-
bly after alanine at the N-terminus. An example of a peptidase with alanyl
amin-
CA 02628145 2008-05-01
IMTM GmbH 16
opeptidase N analogous enzymatic effect is, without restricting the invention
thereunto, the cytosolic soluble alanyl aminopeptidase (EC 3.4.11.14, puromy-
cine-sensitive aminopeptidase, aminopeptidase PS, encephaline-degrading
aminopeptidase) [A. J. Barret et al., a. a. 0.].
Using the term "inhibitor" in the present description and in the claims such
com-
pounds of natural origin, synthetic origin or natural origin with synthetic
modifi-
cation are recognized having a regulatory, particularly inhibitory effect on
an
enzyme or a group of enzymes. The regulatory effect can be based on most
different effects without limiting the afore-mentioned wide definition of the
term
"inhibitor". Preferred inhibitors according to the invention are inhibitors
with an
inhibitory effect on enzymes, more preferred on groups of enzymes for example
inhibitors with inhibitory effect on dipeptidyl peptidase IV (DPIV) and on
pepti-
dases with dipeptidyl peptidase IV analogous enzymatic effect or inhibitors
with
inhibitory effect on alanyl aminopeptidase N (APN), respectively, and on pepti-
dases with alanyl aminopeptidase N analogous enzymatic effect as defined
above.
Using the term "precursor" in the present description and in the claims
naturally
occurring or synthetic or naturally occurring but synthetically modified com-
pounds are recognized from which other compounds can be derived chemically
under defined conditions. Hence inhibitor precursors are recognized to be com-
pounds of natural or synthetic origin or natural but synthetically modified
com-
pounds which can react to inhibitors systematically.
Using the term "prodrug" in the present description and in the claims
naturally
occurring or synthetic or naturally occurring but synthetically modified com-
pounds are recognized from which other compounds can be derived chemically
under defined conditions preferably under physiological or pathological condi-
tions whereat these other compounds develop a pharmacological effect which
differs qualitatively and/or quantitatively of the starting substance. Hence
inhibi-
CA 02628145 2008-05-01
IMTM GmbH 17
tor prodrugs are recognized to be compounds of natural or synthetic origin or
natural but synthetically modified compounds which can react systematically
preferably under physiological or pathological conditions, more preferred
under
physiological or pathological conditions in a mammal for example a human to
new substances with inhibitory effect. This does not exclude that prodrugs per
se are able, before being transformed into drugs with a defined
pharmacological
(for example inhibitory) effect, to develop a pharmacological effect (for
example
to inhibit one of the two afore-mentioned enzymes). Conditions of the transfor-
mation of prodrugs into drugs in a mammal or a human respectively can be of
that ilk like they are regularly present in the physiological surroundings of
a
mammal for example a human or in the body of a mammal for example a hu-
man. Alternatively, such physiological conditions might only be present under
defined conditions in a mammal as for example in a human like for example a
defined physiological condition as present in for example a disease pattern or
they can be induced or be invoked by external influences for example (without
restriction) by medical influences to the organism of a mammal like for
example
the organism of a human being.
In the compounds of the afore-mentioned general formulae (1) and (2) A and A'
which might be identical or different are a residue
Y
NH2
X-_/
0
in which X is S, 0, CH2, CH2CH2, CH2O, or CH2NH and Y is H or CN and * is a
chiral carbon atom. Particularly preferred are according to the invention com-
pounds or the general formula (1) in which A and A' are identical as well as
CA 02628145 2008-05-01
IMTM GmbH 18
compounds of the general formulae (1) and (2) in which the afore-mentioned A-
representing residue X is S, CH2 or CH2CH2 and/or Y is H or CN.
In further preferred embodiments of the invention such compounds of the gen-
eral formulae (1) and (2) are prodrugs for particularly effective inhibitors
in
which the chiral carbon atom designated by * has either an S- or L-
configuration.
In the compounds of the afore-mentioned general formulae (1) and (2) B and B'
can be identical or different and are a residue containing an 0, N or S
contain-
ing or non-containing unsubstituted or substituted unbranched or branched al-
kylene-residue, cycloalkylene-residue aralalkylene-residue, heterocycloal-
kylene-residue, heteroarylalkylene-residue, arylamidoalkylene-residue, het-
eroarylamidoalkylene-residue, unsubstituted or mono- or polysubsituted ary-
lene-residue or heteroaryl-residue having one or more five-, six-, or seven-
membered ring(s).
Using the term "alkyl-residue" In the present description and in the claims a
monovalent straight-chained ("unbranched") or branched residue made of car-
bon atoms linked by single bonds to each other with hydrogen atoms bound to
the carbon atoms is recognized. Hence alkyl-residues are according to the pre-
sent invention saturated monovalent hydrocarboned residues. Preferably the
alkyl-residues in the compounds of the general formulae (1) and (2) comprise 1
to 18 carbon atoms and are thus selected from the residues methyl, ethyl, n-
propyl, i-propyl and the numerous different straight-chained and branched iso-
mers of the residues butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl,
undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl and
octadecyl.
Particularly preferred are straight-chained and branched alkyl-residues having
1
to 12 carbon atoms; straight-chained and branched alkyl-residues having 1 to 6
carbon atoms are even more preferred. Most preferred are residues methyl,
ethyl, n-propyl, i-propyl, n-butyl, i-butyl, sec-butyl and tert-butyl.
CA 02628145 2008-05-01
IMTM GmbH 19
Accordingly in the present description and in the claims the terms "alkenyl-
residue" and "alkinyl-residue" monovalent straight-chained ("unbranched") or
branched residues of carbon atoms linked to each other by single bonds and at
least one double bond or triple bond, respectively at an arbitrary but defined
position in the molecule with hydrogen atoms bound to the remaining bonds of
the carbon atoms are recognized having at least 2 carbon atoms and up to 18
carbon atoms. Such residues are for example preferably vinyl-residues or allyl-
residues; however, carbon-carbon multiple bond-containing residues are not
restricted to said residues.
In the present descriptions and in the claims the term "alkylene-residue" is
rec-
ognized to be a divalent straight-chained ("unbranched") or branched residue
of
carbon atoms linked to each other by single bonds with hydrogen atoms bound
to the carbon atoms. Hence alkylene-residues are according to the present in-
vention saturated divalent hydrocarbon-residues. Preferably alkylene-residues
in the compounds of the general formulae (1) and (2) comprise 1 to 18 carbon
atoms and are therefore selected from the residues methylene, ethylene, n-
propylene, 2,2-propylene, 1,2-propylene and numerous different straight-
chained and branched isomers of the residues butylene, pentylene, hexylene,
heptylene, octylene, nonylene, decylene, undecylene, dodecylene, tridecylene,
tetradecylene, pentadecylene, hexadecylene, heptadecylene and octadecylene.
Particularly preferred are straight-chained and branched alkylene-residues hav-
ing 1 to 12 carbon atoms and straight-chained and branched alkylene-residues
having 1 to 6 carbon atoms are more preferred. Most preferred are the residues
methylene, ethylene, n-propylene, 2,2-propylene, 1,2-propylene and the numer-
ous different butylene-position isomers).
In the alkyl-residues and/or the alkylene-residues which can be according to
the
invention part of the compounds of the general formulae (1) and (2) the chains
of carbon atoms might be interrupted by 0-atoms, N-atoms or S-atoms; Hence
CA 02628145 2008-05-01
IMTM GmbH 20
in the course of the chain there might exist instead of one or more -CH2-
group(s) one or more group(s) of the group -0-, -NH- and -S-, whereas usually
not two of the groups -0-, -NH- and/or -S- follow each other in the chain.
Said
one or more group(s) -0-, -NH- or -S- can be inserted at arbitrary positions
in
the molecule. Preferably if a hetero group of that ilk is present, a group of
that
ilk is present in the molecule.
Straight-chained as well as branched alkyl- or alkylene-residues might be sub-
stituted according to the invention in the compounds of the general formulae
(1)
and (2) in a further embodiment with one or more substituents preferably with
one substituent. The substituent(s) can be located at arbitrary positions of
the
backbone, formed by the carbon atoms and can preferably, without restricting
the invention hereunto, be selected from the group consisting of halogen atoms
like fluorine, chlorine, bromine and iodine, particularly preferred chlorine
and
bromine, alkyl-groups having 1 to 6 C-atoms each, particularly preferred alkyl-
groups having 1 to 4 C-atoms, alkoxy-groups having 1 to 6 C-atoms in the alkyl-
residue preferably having 1 to 3 C-atoms in the alkyl-residue, unsubstituted
or
with one or two alkyl-residue(s) containing 1 to 6 C-atoms independently from
each other, preferably 1 to 3 C-atoms, substituted amino-groups, carbonyl-
groups and carboxyl-groups. The latter can also be present in form of salts or
esters with alcohols having 1 to 6 carbon atoms in the alkyl-residue; hence
the
term "carboxyl-groups" includes groups of the general structure -COO- M+ (with
M = monovalent metal atom such as an alkali metal-atom or an accordant
equivalent of a polyvalent metal atom such as half an equivalent of a divalent
metal atom like an earth alkali metal atom) or of the general structure-COORX
(with R. = alkyl-groups having 1 to 6 carbon atoms). The substituting alkyl-
groups are selected from alkyl-groups mentioned above in detail and are par-
ticularly preferred methyl-groups, ethyl-groups, n-propyl-groups, i-propyl-
groups, n-butyl-groups, i-butyl-groups, sec-butyl-groups or tert-butyl-groups.
Alkoxygroups are alkyl-groups in the above-defined sense which are bound via
an 0-atom to the backbone formed by the carbon atoms. They are preferably
CA 02628145 2008-05-01
IMTM GmbH 21
selected from the group consisting of the residues methoxy, ethoxy, n-propoxy,
i-propoxy; n-butoxy, i-butoxy, sec-butoxy and tert-butoxy. Amino-groups are
groups of the general structure -NRX Ry in which the residues RX and Ry might
independently from each other designate, hydrogen or alkyl-groups (according
to the afore-mentioned definition) having 1 to 6 carbon atoms particularly pre-
ferred having 1 to 3 C-atoms in which the residues Rx and Ry might be
identical
or different from each other. Such amino-groups being particularly preferred
as
substituents are -NH2, -NH(CH3), -N(CH3)2, -NH(C2H5) and -N(C2H5)2. The term
"amino-groups" contains also groups of the above-defined structure which are
present as quaternated ammonium ions, either because of salt formation with
organic acids or inorganic acids (e.g. residues of the structure RX RY RZ N+ Q-
, in
which RX, Ry and RZ might be identical or different preferably identical and
RX
and Ry might have the above-defined meanings and at least one of the residues
is hydrogen from the quaternation with the organic or inorganic acid and Q is
an
acid-residue from an acid of the organic or inorganic acid) or because of salt
formation with suitable quaternation reagents which are known to a person
skilled in the field such as (without restriction hereunto) with alkyl
halogenids.
In the present description and in the claims the term "cycloalkyl" is used for
un-
substituted or substituted monovalent residues of -CH2-groups linked to each
other in form of closed rings. According to the invention said rings might
contain
preferably 3 to 8 atoms forming the ring and might either contain exclusively
carbon atoms or contain one or more hetero atom(s) which is/are selected from
-0-, -S- and -NRX in which Rx is hydrogen or a alkyl-residue (as defined
above) having 1 to 6 carbon atoms. In case hetero atoms are inserted in the
rings said hetero atoms can be - in case of more than one hetero atoms - iden-
tical or different. Preferably in case hetero atoms are present one hetero
atom is
inserted into the ring. Particularly preferred among purely carbocyclic rings
are
the residues cyclopentyl, cyclopentenyl, cyclopentadienyl, cyclohexyl,
cyclohex-
enyl, cyclohexadienyl, cycloheptyl, cycloheptenyl, cyclohepadienyl and cyclo-
heptatrienyl. Examples for hetero atoms containing cycloalkyl-residues which
CA 02628145 2008-05-01
IMTM GmbH 22
are often referred to as heterocycloalkyl-residues in further embodiments of
the
invention the residues tetrahydrofuranyl, pyrrolidinyl, pyrazolidinyl,
imidazolid-
inyl, piperidinyl, piperazinyl and morpholinyl.
Possible substituents at the carbocyclic or heterocyclic cyloalkyl-residues
might
be preferably, without restricting the invention hereunto, selected from the
afore-mentioned group of substituents for linear alkyl-groups. Particularly
pre-
ferred substituents for cycloalkyl-groups are the substituents -Cl, -Br, -
methyl,
ethyl, n-propyl, i-propyl, n-butyl, i-butyl, sec-butyl or tert-butyl, methoxy,
ethoxy,
n-propoxy, i-propoxy; n-butoxy, i-butoxy, sec-butoxy and tert-butoxy, -NH2, -
NH(CH3), -N(CH3)2, -NH(C2H5) and -N(C2H5)2, carbonyl and carboxyl.
In the present description and in the claims the term "cycloalkylene" is used
for
unsubstituted or substituted divalent residues of -CH2-groups linked to closed
rings. According to the invention these can preferably contain three to eight
at-
oms in the ring and can consist either exclusively of carbon atoms or contain
one or more hetero atom(s) which is/are selected from -0-, -S- and -NRx , in
which RX is hydrogen or a alkyl-residue (as defined above) having 1 to 6
carbon
atoms. Particularly preferred among the purely carbocyclic rings are the resi-
dues cyclopentylen, cyclopentenylen, cyclopentadienylen, cyclohexylen, cyclo-
hexenylen, cyclohexadienylen, cycloheptylen, cycloheptenylen, cycloheptadi-
enylen and cycloheptatrienylen. Also, the heterocyclic groups defined above
with regard to the cycloalkyl-residues can appear in compounds of the general
formulae (1) and (2) as groups "B" in form of divalent residues and
particularly
preferred are such cyclic divalent residues in which one group -0- or -NRX is
inserted into the ring. In those cases both valences are localized at
arbitrary C-
atoms in the ring. Preferably one hetero atom or two hetero atom(s) is/are in-
serted into the ring and in particularly preferred embodiments of such groups
the divalent residues are derived from tetrahydrofuran, pyrrolidin,
pyrazolidin,
imidazolidin, piperidin, piperazin and morpholin.
CA 02628145 2008-05-01
IMTM GmbH 23
Possible substituents at these carbocyclic or heterocyclic cycloalkylene-
residues can be preferably, without restricting the invention hereunto,
selected
from the afore-mentioned group of substituents for linear alkyl-groups.
Particu-
larly preferred substituents for cycloalkylene-groups are the substituents -
Cl, -
Br, methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, sec-butyl or tert-
butyl, meth-
oxy, ethoxy, n-propoxy, i-propoxy; n-butoxy, i-butoxy, sec-butoxy and tert-
butoxy, -NH2, -NH(CH3), -N(CH3)2, -NH(C2H5) and -N(C2H5)2, carbonyl and
carboxyl.
Using the term "aryl-residue" in the present description and in the claims a
monovalent hydrocarbon-residue is recognized which is derived from a cyclic
molecule with aromatic character (4n + 2 in ring orbitals delocalized rr-
electrons) which might be unsubstituted or substituted. The ring structure of
such an aryl-residue can be a five-, six- or seven-membered ring structure
with
one ring or a structure formed by two or more ("annelated") rings bound to
each
other whereat the annelated rings have identical or different numbers of ring
members, particularly of C-atoms. In case of systems consisting of more than
one rings condensated to each other, benzocondensated rings are particularly
preferred, i.e. a ring system in which at least one of the rings is an
aromatic,
exclusively C-atoms containing six-membered ring (phenyl ring). Typical but
not
limiting examples of aryl rings are cyclopentadienyl-residues (C5H5 )(being a
five-membered ring), phenyl-residues (being a six-membered ring), cyclohepta-
trienyl-residues (C7H7+) (being an seven-membered ring) naphthyl-residues (be-
ing a ring system comprising two annelated six-membered rings) as well as
monovalent residues being derived from anthracen and phenanthren (being
three annelated six-membered rings). According to the invention most preferred
aryl-residues are phenyl- and naphthyl-residues.
Possible substituents of carbocyclic aryl-residues can be selected preferably
from the groups of substituents mentioned above for linear alkyl-groups,
without
restricting the invention to theses substituents. Particularly preferred
substitu-
CA 02628145 2008-05-01
IMTM GmbH 24
ents for aryl-groups are substituents -Cl, -Br, -methyl, ethyl, n-propyl, i-
propyl,
n-butyl, i-butyl, sec-butyl or tert-butyl, methoxy, ethoxy, n-propoxy, i-
propoxy; n-
butoxy, i-butoxy, sec-butoxy and tert-butoxy, -NH2, -NH(CH3), -N(CH3)2, -
NH(C2H5) and -N(C2H5)2, carbonyl and carboxyl. One or more substituent(s) of
this ilk which might be identical or different from each other can be bound to
one
aryl-residue according to the present invention. The substituted position(s)
at
the aryl-ring (-sytem) can be chosen arbitrarily.
A comparable definition as in case of the aryl-residues applies to the present
description and the claims with regard to the definition of the term "arylene-
residue": in this regard a divalent residue is recognized which elemenrary com-
position the selection thereof and the substituent(s) thereof are comparable
with
the afore-mentioned definitions of the aryl-residues with the exception that
it is a
divalent residue which insertion can be carried out at two arbitrary carbon at-
oms.
In the present description and in the claims the term "heteroaryl-residue" an
aryl-residue is recognized (in accordance with the afore-mentioned definition)
which ring structure contains one or more hetero atom(s) preferably from the
group 0, N or S without losing the aromatic character of the molecule. Het-
eroaryl-residues can be unsubstituted or substituted according to the
invention.
The ring structure of such a heteroaryl-residue can either be a five-membered,
a six-membered or a seven-membered ring structure with one ring or be a
structure formed by two or more ("annelated") rings bound to each other
wherein the annelated rings might have an identical or a different number of
ring
members. The hetero atom(s) can occur in one ring alone or in more than one
ring of the ring system. The heteroaryl-residues preferably consist of one or
two
rings. In case of systems consisting of more than one ring condensated to each
other, benzocondensated rings are especially preferred, i.e. ring systems in
which at least one of the rings is an aromatic carbocyclic (i.e. only C-atoms
con-
taining) six-membered ring. Particularly preferred heteroaryl-residues are se-
CA 02628145 2008-05-01
IMTM GmbH 25
lected from furanyl, thiophenyl, pyridyl, indolyl, cumaronyl, thionaphthenyl,
chi-
nolinyl (benzopyridyl), chinazolinyl (bezopyrimidinyl) and chinoxylinyl (ben-
zopyrazinyl).
Possible substituents at these heteroaryl-residues can be preferably selected
from the afore-mentioned group of substituents for linear alkyl-groups without
restricting the invention to these substituents. Particularly preferred
substituents
for heteroaryl-groups are the substituents -Cl, -Br, methyl, ethyl, n-propyl,
i-
propyl, n-butyl, i-butyl, sec-butyl or tert-butyl, methoxy, ethoxy, n-propoxy,
i-
propoxy; n-butoxy, i-butoxy, sec-butoxy, tert-butoxy, -NH2, -NH(CH3), -
N(CH3)2,
-NH(C2H5) and -N(C2H5)2, carbonyl and carboxyl. One or more substituents of
that ilk which might be identical or different from each other might be bound
to
one heteroaryl-residue according to the present invention. The substituted
posi-
tion(s) at the heteroaryl-ring (-system) can be selected arbitrarily.
A comparable definition as in the case of the heteroaryl-residues applies to
the
present description and the claims with regard to the definition of the term
"het-
eroarylene-residue": in this regard a divalent residue is recognized which gen-
eral composition and which selection and which substituents are comparable to
the afore-mentioned definition of "heteroaryl-residues" with the exception
that it
is a divalent residue which insetion can be carried out at two arbitrary
carbon
atoms of the ring or the ring system respectively or at a nitrogen atom as
well.
In the context of the present description and in the claims the terms "aralkyl-
residue", "heteroarylalkyl-residue", "heterocycloalkyl-residue",
"arylamidoalkyl-
residue" and heteroarylamidoalkyl-residue", mean alkyl-residues ( - or more
specifically - alkylene-residues) according to the afore-mentioned general and
specific definition which are substituted at one of their bonds with an aryl-
residue (according to the afore-mentioned general and specific definition),
hetroaryl-residue (according to the afore-mentioned general and specific
defini-
tion), heterocyclyl-residue (according to the afore-mentioned general and spe-
CA 02628145 2008-05-01
IMTM GmbH 26
cific definition of the cycloalkyl-residues substituted with hetero atoms)
aryla-
mido-residues (according to the following general and specific definition) or
het-
eroarylamido-residue (according to the following general and specific
definition).
These residues can be unsubstituted or substituted.
In preferred embodiments of the invention aralkyl-residues are residues of
that
ilk, in which the aryl-residue is a phenyl-residue, substituted phenyl-
residue,
naphthyl-residue or substituted naphthyl-residue and the alkyl(ene)-group is
straight-chained or branched having 1 to 6 carbon atoms. In a very particular
and advantageous way the residues benzyl, phenethyl, naphthylmethyl and
naphthylethyl can be used as aralkyl-residues of which benzyl-residues are par-
ticularly preferred.
Possible substituents at the aryl-groups of the aralkyl-residues can be
prefera-
bly selected from the afore-mentioned group of substituents for linear alkyl-
groups without restricting the invention to those substituents. Particularly
pre-
ferred substituents for aryl-groups of the aralkyl-residues are the
substituents -
Cl, -Br, methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, sec-butyl or
tert-butyl,
methoxy, ethoxy, n-propoxy, i-propoxy; n-butoxy, i-butoxy, sec-butoxy, tert-
butoxy, -NH2, -NH(CH3), -N(CH3)2, -NH(C2H5) and -N(C2H5)2, carbonyl and
carboxyl. One ore more substituents of that ilk which might be identical or
dif-
ferent from each other can be bound to one aryl-group of an aralkyl-residue ac-
cording to the present invention. The substituted position(s) at the aryl ring
(-
system) can be chosen arbitrarily.
In preferred embodiments of the invention the heteroalkyl-residues are such
residues in which the heteroaryl-residue of the heteroarylalkyl-residue
according
to the invention is substituted and the alkylene-group is straight-chained or
branched having 1 to 6 carbon atoms. The ring structure of such a heteroaryl-
residue can be a ring structure with one ring or a structure formed by two or
more than two ("annelated") rings bound to each other wherein the annelated
CA 02628145 2008-05-01
IMTM GmbH 27
rings might have an identical or different number of ring members. The hetero
atom(s) can occur in one or more ring(s) of the ring system. The heteroaryl-
residues of the heteroarylalkyl-residue consist preferably of one or two
rings. In
case of heteroarylalkyl systems composed of more than one rings condensated
to each other, benzocondensated rings are especially preferred, i.e. ring sys-
tems in which at least one of the rings is an aromatic carbocyclic six-
membered
ring. Particularly preferred heteroaralalkyl-residues are selected from
furanyl-
methyl and -ethyl, thiophenylmethyl and -ethyl, pyridylmethyl and -ethyl, indo-
lylmethyl and -ethyl, cumaronylmethyl and -ethyl, thionaphthenylmethyl and -
ethyl, chinolinyl-(bezopyridyl-)methyl and -ethyl, chinazolinyl-
(benzopyrimidinyl-
) and chinoxylinyl-(benzopyrazinyl-)methyl and -ethyl.
Possible substituents at these heteroaryl-groups of heteroarylalkyl-residues
can
be preferably selected from the afore-mentioned group of substituents for
linear
alkyl-groups without restricting the invention to thereunto. Particularly
preferred
substituents for heteroaryl-groups are the substituents -CI, -Br, methyl,
ethyl, n-
propyl, i-propyl, n-butyl, i-butyl, sec-butyl or tert-butyl, methoxy, ethoxy,
n-
propoxy, i-propoxy; n-butoxy, i-butoxy, sec-butoxy, tert-butoxy, -NH2, -
NH(CH3),
-N(CH3)2, -NH(C2H5) and -N(C2H5)2, carbonyl and carboxyl. One or more sub-
stituent(s) of that ilk which can be identical or different from each other
can be
bound to a heteroarylalkyl-residue according to the present invention. The sub-
stituted position(s) at the heteroaryl ring (-system) can be chosen
arbitrarily.
In preferred embodiments of the invention heterocycloalkyl-residues are
cycloal-
kyl-residues according to the afore-mentioned general and specific definition
which contain one or more hetero atom(s) which is/are selected from -0-, -S-
and -NRx-, in which RX is hydrogen or an alkyl-residue having 1 to 6 carbon at-
oms (as defined above) and the alkyl(ene)-groups of the heterocycloalkyl-
residues are straight-chained or branched having 1 to 6 carbon atoms. In case
of more than one hetero atoms inserted into the ring(s) these can be identical
or
different. Preferably one hetero atom is incorporated in the ring. Preferred
ex-
amples for hetero atoms containing cycloalkyl-residues which are also referred
CA 02628145 2008-05-01
IMTM GmbH 28
to as heterocycloalkyl-residues are in further embodiments of the invention
the
residues tetrahydrofuranyl, pyrrolinidyl, pyrazolidinyl, imidazolidinyl,
piperidinyl,
piperazinyl and morpholinyl.
Possible substituents at these heterocycloalkyl-residues can preferably be se-
lected from the afore-mentioned group of substituents for linear alkyl-groups,
without restricting the invention to those substituents. Particularly
preferred sub-
stituents for heteroaryl-groups are the substituents -Cl, -Br, methyl, ethyl,
n-
propyl, i-propyl, n-butyl, i-butyl, sec-butyl or tert-butyl, methoxy, ethoxy,
n-
propoxy, i-propoxy; n-butoxy, i-butoxy, sec-butoxy, tert-butoxy, -NH2, -
NH(CH3),
-N(CH3)2, -NH(C2H5) and -N(C2H5)2, carbonyl and carboxyl. One or more sub-
stituent(s) of that ilk which might be identical or different from each other
can be
bound to one heterocycloalkyl-residue according to the present invention. The
substituted position(s) at the heterocacloalkyl-ring (-system) can be chosen
ar-
bitrarily.
Using the terms "arylamidoalkyl-residue" and "heteroarylamidoalkyl-residue" in
the present description and in the claims alkyl-residues ( - more precisely -
alkylene-residues) according to the afore-mentioned general and specific
defini-
tion are recognized which are substituted at one of their bonds by an
arylamido-
residue or heteroarylamido-residue of the general formula Ar-NRx-C(=O)- or the
general formula Ar-C(=O)-NRX in which RX is hydrogen or an alkyl having 1 to 6
carbon atoms and Ar is an arbitrary aryl-residue or heteroaryl-residue
according
to the afore-mentioned general or specific definition. These aryl- or
heteroaryl-
residues can be unsubstituted or substituted. Preferred examples for an aryla-
midoalkyl-residue - without restricting the invention - are 2-, 3- or 4-benzoe-
acid-amino-n-butyl-residues or 2-nitro-3-, -4-, -5- or -6-benzoe-acid-amido-n-
butyl-residues; preferred but not limiting examples for heteroarylamidoalkyl-
residues are 2-, 4-, 5- or 6-pyridin-3-carbonacid-amido-n-butyl-residues.
CA 02628145 2008-05-01
IMTM GmbH 29
Possible substituents at these arylamidoalkyl-residues and heteroarylamidoal-
kyl-residues can preferably be selected from the afore-mentioned group of sub-
stituents for linear alkyl-groups, without restricting the invention to those
sub-
stituents. Particularly preferred substituents for aryl-groups or heteroaryl-
groups
of the arylamidoalkyl-residues and heteroarylamidoalkyl-residues are the sub-
stituents -Cl, -Br, methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, sec-
butyl or
tert-butyl, methoxy, ethoxy, n-propoxy, i-propoxy; n-butoxy, i-butoxy, sec-
butoxy, tert-butoxy, -NH2, -NH(CH3), -N(CH3)2, -NH(C2H5) and -N(C2H5)2, car-
bonyl and carboxyl. One or more substituent(s) of that ilk which can be
identical
or different from each other can be bound to an aryl- or heteroaryl-group of
the
arylamidoalkyl-residues or heteroarylamidoalkyl-residues according to the pre-
sent invention. The substituted position(s) at the aromatic ring (-system) can
be
chosen arbitrarily.
A comparable definition as for the aralkyl-residues, heteroarylalkyl-residues,
heterocycloalkyl-residues, arylamidoalkyl-residues and heteroarylamidoalkyl-
residues applies in the context of the present description and the claims with
regard to the definition of the terms "aralkylene-residue",
"heteroarylalkylene-
residue", "heterocycloalkylene-residue", "arylamidoalkylene-residue" and "het-
eroarylamidoalkylene-residue": These are understood to be divalent residues
which general composition and the selection thereof and the substituent(s)
thereof are comparable to the afore-mentioned definition of "aralkyl-residue",
"heteroarylalkyl-residue", "heterocycloalkyl-residue", "arylamidoalkyl-
residue"
and "heteroarylamidoalky-residue" with the exception that it is in either case
a
divalent residue which insertion can be carried out at two arbitrary carbon
atoms
of the ring or the ring system of the alkylene-group respectively, or also at
a ni-
trogen atom of the heteroaryl or heterocyclyl ring system.
In the general formulae (1) and (2) the residue D is -S - S- or -Se - Se-.
These
two S- or Se-atoms respectively, form a bridge between two parts of the mole-
cules of the compounds of the general formulae (1) and (2) which can be
CA 02628145 2008-05-01
IMTM GmbH 30
cleaved under natural, particularly under reducing conditions. Thereby two
parts
of the molecule are released which develop an inhibitory effect on dipeptidyl
peptidase IV (DPIV) and on peptidases with analogous enzymatic effect as well
as on alanyl aminopeptidase N (APN) and on peptidases with analogous enzy-
matic effect.
In the afore-mentioned general formula (2) E is the group -CH2 - C*H (NH2) -R9
in which R9 is an 0, N or S containing or non-containing unsubstituted or
substi-
tuted unbranched or branched alkyl-residue, cycloalkyl-residue, aralkyl-
residue,
heterocycloalkyl-residue, heteroarylalkyl-residue, arylamidoalkyl-residue, het-
eroarylamidoalkyl-residue, unsubstituted or mono-substituted or polysubstitued
aryl-residue or heteroaryl-residue with one or more five-, six- or seven-
membered ring(s). With regard to the inventory-conform usable or preferred
examples of the alkyl-residues, cycloalkyl-residues, arylalkyl-residues,
hetero-
cycloalkyl-residues, heteroarylalkyl-residues, arylamidoalkyl-residues, het-
eroarylamidoalkyl-residues, unsubstituted or mono- or poly-substituted aryl-
residues or heteroaryl-residues with one or more five-, six-, or seven-
membered
ring(s) as well as with regard to conceivable and preferred substituents for
these residues it can be referred to the afore-mentioned definitions of the ac-
cordant residues and their preferred embodiments; these definitions are identi-
cally applicable for the residues of general formula (2) designated by E.
In said formula for E, the carbon atom, which is substituted with the amino-
group is a chiral carbon atom, symbolized by *. In a further preferred embodi-
ment of the invention such compounds of general formula (2) are prodrugs for
particularly effective inhibitors in which the chiral carbon atom designated
by *
has either an S- or an L-configuration.
According to the invention E preferably designates 2-aminoalkylene-residues,
for example a 2-amino-3-phenylpropyl-residue or an unsubstituted or by hetero
atoms such as -S-, -S(=0)-, -N- or -0-substituted 2-aminoalkylene-residues,
CA 02628145 2008-05-01
IMTM GmbH 31
for example a 2-amino-4-methylpentyl-residue, a 2-amino-4-methylthiobutyl-
residue or a 2-amino-4-methyl-sulfoxybutyl-residue.
In further preferred embodiments of the invention the residues B and/or B' are
in
the general formulae (1) and (2) a residue R' which is a straight-chained or
branched alkylene-residue having 1 to 6 carbon atoms. Particularly preferred
compounds of the general formulae (1) and (2) comprise residues B and/or B' in
form of one or more of the group(s) selected from -CH2-(methylene), -CH2-
CH2(ethylene) or (H3C)Z-C<(2,2-propylene).
In alternative also further preferred embodiments B and/or B' is a residue -
(CH2)n - R2 - R3 - R4-, in which n is an integer from 1 to 5; R2 is -NH- or -
NH-
C(=NH)-NH-, if R3 is O= C< or -SOZ- or in which R2 is O= C< if R3 is -NH-; R4
is 0, N or S containing or non-containing unsubstituted or substituted un-
branched or branched alkylene-residue, cycloalkylene-residue, arylalkylene-
residue, heterocycloalkylene-residue, heteroarylalkylene-residue,
unsubstituted
or mono-substituted or poly-substituted arylene-residue or heteroarylene-
residue with one or more five-, six- or seven-membered ring(s). Further pre-
ferred, n is an integer from 1 to 5, so that preferred examples of the afore-
mentioned residues contain a methylene-group, ethylene-group, propylene-
group, butylene-group and pentylene-group; R2 and R3 in combination prefera-
bly form an amido-group - C(=O)-NH- or -NH-C(=0)-. Further preferred are
such compounds of the general formulae (1) and (2) with residues B and/or B'
in which B is the afore-mentioned formula and R4 is an amino-substituted al-
kylene-residue, for example an aminoethylene-residue or an unsubstituted or
(for example by a nitro-group) substituted phenylene-residue or an unsubsti-
tuted or substituted pyridyl-2,5-ene-residue.
In alternative also further preferred embodiments B and/or B' is a residue of
the
formula - R' - R8 -, in which R' is a mono- or poly-substituted benzylene
residue
and R 8 is a single bond or an 0, N or S containing or non-containing unsub-
CA 02628145 2008-05-01
IMTM GmbH 32
stitued or substituted unbranchend or branched alkylene residue, cycloalkylene
residue, aralalkylene residue, heterocycloalkylene residue or
heteroarylalkylene
residue, which might contain as functional groups preferably one or more amino
groups, carbonyl groups or carboxyl groups or an unsubstituted or mono- or
poly-substituted arylene residue or heteroaryiene residue with one or more
five-,
six-, or seven-membered ring(s). With regard to the definition of the afore-
mentioned residues and their conceivable inventory substituents it can be re-
ferred to the afore-mentioned general or specific definition of the particular
resi-
dues or substituents.
Further preferred are according to the invention compounds of the general for-
mulae (1) or (2) in which B and B' might be identical or different and which
des-
ignate a residue -(CH2)n - R2 - R3 - R4 in which R2 is -NH- or -NH-C(=NH) -
NH- if R3 is O= C < or -SOZ- or in which R 2 is O=C< if R3 is - NH - and in
which R4 is
--CH(COOH)-R1-, in which R' has the afore-mentioned meaning if R2 is
O=C< and R3 is -NH-; or
C(=O)-NH-CH(COOH)-R'- (substitution at position 2, 3 or 4)
in which R' has the afore-mentioned meaning if R2 is O= C <and R3 is
-NH-; or
--CH (NHRS) -R1- if R 2 is -NH- or -NH-C(=NH) -NH- and R3 is O= C< in
which R5 is H or an acyl residue prefarably a benzyloxycarbonyl residue,
a fluorene-9-yimethoxycarbonyl residue, a tert-butyloxycarbonyl residue
or a benzoyl residue; or
CA 02628145 2008-05-01
IMTM GmbH 33
-(substitution at position 2, 3 or 4), in which R4 is phenylene
and R2 is -NH- or -NH-C(=NH)-NH- if R3 is O= C< or -
SO2- or in which R2 is O= C < if R3 is -NH-; or
NH-C(=O)-CH (NHR5)-R'- (substitution at position 2, 3 or 4)
in which R5 is H or an acyl residue preferably a benzyloxycarbonyl resi-
due, a fluoren-9-ylmethoxycarbonyl residue or a benzoyl residue and R2
is -NH- or -NH-C(=NH) -NH- if R3 is O= C< or -SO2- or in which R2 is 0
= C < if R3 is -NH-; or
NH-C (=0)-alkylene- (substitution at position 2, 3 or 4),
__(O in which alkylene is an unbranched or branched alkylene residue having
1 to 6 carbon atoms and R2 is -NH- or -NH-C(=NH)-NH- if R3 is O= C<
or -SO2- or in which R2 is 0 C < if R3 is -NH-; or
- C(=0) - NH - alkylene- (substitution at position 2, 3 or 4),
in which alkylene is an unbranched or branched alkylene residue having
1 to 6 carbon atoms and R2 is -NH- or -NH-C(=NH)-NH- if R3 is O= C<
or -SO2- or in which R2 is 0 = C < if R3 is -NH-; or
CA 02628145 2008-05-01
IMTM GmbH 34
0
~
in which and R2 are -NH- or -NH-C(=NH) -NH- if R3 is O= C< or -SO2-
or in which R2 is O= C < if R3 is -NH- ; or
R6 (substitution. at position 2, 3 or 4)
(substitution at the ring depending on the position of
R6) in which R6 is H, NO2, CN, halogen or an acyl residue and R2 is -NH-
or -NH-C(=NH) -NH- if R3 is O= C< or -SO2- or in which R2 is O= C < if
R3 is-NH-; or
R6 (substitution at position 3, 4 or 6), in which R6 is H,
NO2, CN, halogen or an acyl residue and R2 is -NH- or -NH-C(=NH) -
NH- if R3 is O= C< or -SO2- or in which R2 is O= C < if R3 is -NH- ; or
R6 (substitution at position 4, 5 or 6), in which R6 is H,
NO2, CN, halogen or an acyl residue and R2 is -NH- or -NH-C(=NH) -
NH- if R3 is 0 = C< or -SO2- or in which R2 is 0 = C < if R3 is -NH; or
CA 02628145 2008-05-01
IMTM GmbH 35
- Alkyl
-Alkylene -
in which and R2 is -NH- or -NH-C(=NH) -NH- if R3 is O= C< or -SO2- or
in which R2 is O= C < if R3 is -NH-.
Alternatively thereto according to the invention compounds of the general for-
mulae (1) and (2) are further preferred, in which B and B' may be identical or
different and designate a residue - R' - R 8 - in which R' and R8 in
combination
are a residue
R 8
- CH2
R6
(in which R7 is the residue above without R8 and in which the position of R6
is
dependent of the position R 8) in which R 8 and R6 have the afore-mentioned
meanings, i. e. in which R6 is H, NO2, CN, halogen or an acyl residue and in
which R 8 is a single bond or an O, N or S containing or non-containing unsub-
stituted or substituted unbranched or branched alkylene residue, cycloalkylene
residue, aralkylene residue, heterocycloalkylene residue or heteroarylalkylene
residue which preferably contains as functional groups one or more amino-
group(s), carbonyl group(s) or carboxyl group(s) or which is unsubstituted or
mono- or poly-substituted arylene residue or heteroarylene residue with one or
more five-, six- or seven-membered ring(s).
Further preferred compounds of the general formulae (1) and (2) are such in
which B and B' are identical or different and designate independently from
each
CA 02628145 2008-05-01
IMTM GmbH 36
other a residue - R' - R 8 - in which R' is a mono- or poly-substituted
benzylene
residue of the afore-mentioned formula (without R$) and R8 is
- NH - or - Cl- to C6-alkylene - NH - in combination with
- C(=0) - Cl- to C6-alkylene - or
- - C (=0) - arylene - or
- SOz - Cl- to C6-alkylene - or
- - SO2 - arylene - or
C(=0) - NH - Cl- to C6-alkylene -
- -S02
(substitution at position 2, 3 or 4) or
- C(=0) - CH (NHR5) - R' in which R' and R5 have the afore-mentioned
meanings; or
- O= C< in combination with
-- NH - Cl- to C6-alkylene - or
- - NH - aryiene - or
- NH - CH (COOH) - R' - in which R' has the afore-mentioned mean-
ings; or
- O- C,- to C6-alkylene - or
- - O - arylene - or
- O- Cl- to C6-alkylene - NH - C(=0) - CH (NH2) - R' - in which R'
has the afore-mentioned meanings, or
- O- Cl- to C6-alkylene - C(=0) - NH - CH (COOH) - R' -, in which R'
has the afore-mentioned meanings.
CA 02628145 2008-05-01
IMTM GmbH 37
According to the invention the compounds of the general formulae (1) and/or
(2)
are present in form of neutral molecules and are according to the invention
used
as neutral molecules. Alternatively to that the compounds of the general formu-
lae (1) and/or (2) can also be present in form of their acid addition salts
with
inorganic and/or organic acids. Because of the presence of alkaline centers
(mostly of alkaline nitrogen atoms) in the molecule such acid addition salts
are
formed by the addition of one or more molecules of H-acid compounds
(Bronstedt acids) preferably one molecule of an H-acid compound and provide
an improved solubility of the molecules on polar media like for example in
water.
The latter characteristic is of particular impact for such compounds which de-
velop pharmacological effect.
In preferred embodiments of the invention acid addition salts are salts of
phar-
maceutically acceptable acids and are advantageously chosen (but without lim-
iting the present invention) from the group consisting of hydrochlorides,
trifluoroacetates, tartrates, succinates, formiates and/or citrates of the com-
pounds of the general formulae (1) or (2).
Particularly preferred and advantageously usable compounds of the general
formula (1) are characterized by the general formula (1a)
Y
N NH2 p Y_1~
B~S~S"B N~X
G NH2 p
Y (1a)
in which X, Y and B have the afore-mentioned meanings. With particular advan-
tage usable and thus subject of the invention are acid addition salts of the
com-
pound of the general formulae (1a) preferably the acid addition salts with
phar-
CA 02628145 2008-05-01
IMTM GmbH 38
maceutical acceptable inorganic and/or organic acids thereof particularly with
acids of the afore-mentioned group.
Exceptionally advantageous compounds of the general formula (1a) result from
the following table 1 without restricting the invention to those compounds:
Table 1
Examples of compounds of general formula A - B - D- B' - A' (1)
No. B x Y Empirical formula
I -CH2- -CH2- H C14H26N402S2
II - CH2- S H C12H22N402S4
III - CH2- -CH2- CN C16H24N602S2
IV -(CH2)4-NH- I - i H-CH2- S H C24H46N804S4
O NH2
V / \ S H C32H42N808S4
-(CH2)~NH- II
O
NO2
N
VI -(CH2)4-NH-C ~ S H C30H42N804S4
I I /_
O
and the acid addition salts thereof preferably the acid addition salts thereof
having pharmacologically acceptable inorganic and/or organic acids pref-
erably from the afore-mentioned groups of pharmacologically acceptable ac-
ids.
CA 02628145 2008-05-01
IMTM GmbH 39
Particularly preferred and advantageously usable compounds of the general
formula (2) are characterized by the general formula (2a).
Y
NH2
r_~
X-,/ N Yl~ BSS R -,-,-y
O NH2
(2a)
in which X, Y, R9 and B have the afore-mentioned meanings and the acid addi-
tion salts thereof preferably the acid addition salts thereof with
pharmaceutically
acceptable inorganic and/or organic acids preferably from the afore-mentioned
group of pharmaceutically acceptable acids.
Exceptionally preferred compounds of the general formula (2a) result from fol-
lowing table 2 without restricting the invention to theses compounds:
CA 02628145 2008-05-01
IMTM GmbH 40
Table 2
Examples of compounds of general formula A - B - D - E (2)
No. B R9 X Y Empirical
formula
VII - CH2 -CHZ OS H C15H23N3OS3
CH3
VI I I _ ~_ -CH2 ~ ~ S H C H27N3OS3
I -
CH3
IX ~ ~
(CH2)~NH-C -CH2 - S H C25H33N504S3
11
O
N02
N
X _(CH2)4-NH-C CH2 / \ S H C24H33N502S3
O
XI -(CH~~-NH-C \ / -CH2 / \ S H
- C29H42N603S3
CH2-NH- li -CH-CH~
O NH2
XII -(CH~,,~-NH-C \ / NH- Ii -CH-CH~ -CHZ / \ S H
il C28H40N603S3
O O NH2 -
X(II - CH2 \ / -NH-iI -CH-CH2 -CH2 ~ ~ S H C24H33N502S3
O NH2
and the acid addition salts thereof preferably the acid addition salts thereof
with
pharmaceutically acceptable inorganic and/or organic acids.
CA 02628145 2008-05-01
IMTM GmbH 41
The invention also relates to a process to prepare compounds of the general
formulae
A-B-D-B'-A' (1) and
A-B-D-E (2).
In said formulae (1) and (2) A, A', B, B', D and E have the meanings mentioned
above in detail. With regard to the process of the preparation of the new com-
pounds (1) and/or (2) according to the afore-mentioned scheme of synthesis 1
either
- compounds of the general formula
HO - C(=O) - CH[-NH (SG)] -D
in which (SG) is a protecting group and > is a structure element of B are
transformed with a heterocyclic compound of the general formula
Y
N-H
X
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the ob-
tained condensation product of the formula
Y
N - C(=O) - CH[-NH (SG)] _>
X-/
is transformed with a compound of the general formula
71- S-S E
CA 02628145 2008-05-01
IMTM GmbH 42
in which < is a structure element of B; and the resulting reaction product
is transformed being cleaved from the protecting group (SG) into a com-
pound of the general formula A - B - D - B' - A' (1) in which A and A' may
be identical or different and B and B' may be identical or different and in
which A, A', B, B' and D may have the afore-mentioned meanings; or
- compounds of the general formula
HO - C(=O) - CH[-NH (SG)] ~
in which (SG) is a protecting group and > is a structure element of B are
transformed with a heterocyclic compound of the general formula
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the re-
sulting condensation product of the formula
Y
N - C(=O) - CH[-NH (SG)] _>
X-/
is transformed with a compound of the general formula
>I1-s-sL
in whichl </ is a structure element of B; and the resulting reaction product
is
transformed with a compound of the general formula
HS - CH2 - CH [- NH - (SG)] - (R)
CA 02628145 2008-05-01
IMTM GmbH 43
and is transformed being cleaved from the protecting groups (SG) into a
compound of the general formula A - B - D - E (2), in which A, B, D and E
may have the afore-mentioned meanings; or
- a compound of the general formula
HO - C(=O) - CH[-NH (SG)] ~I- S-S -Z
in which (SG) is a protecting group, El is a structure element of B and
Z is a residue, which activates an -S-S-group for a thiol exchange, is
transformed with a heterocyclic compound of the general formula
Y
N-H
X--/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the ob-
tained condensation product of the formula
Y
N-C(=0)CH[-NH(SG)12 - S - S - Z
X-/
is transformed with a compound of the general formula
HS - CH2 - CH [- NH - (SG)] - (R9)
and is transformed being cleaved from the protecting groups (SG) into a com-
pound of the general formula A - B - D - E (2), in which A, B, D and E may
have the afore-mentioned meanings; or
CA 02628145 2008-05-01
IMTM GmbH 44
- a compound of the general formula
[ HO - C(=O) - CH [-NH (SG)] F-I- S]2
in which SG is a protecting group and E is a structure element
of B is transformed with a heterocyclic compound of the general formula
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; and the
obtained reaction product is transformed with a compound of the general
formula
HS-CH2-CH [-NH-(SG)]-R9
into a compound of the general formula A - B - D - E (2) in which A, B, D
and E may have the afore-mentioned meanings; or
- a compound of the general formula
[ HO - C(=O) - CH [-NH (SG)] U-I- S]2
in which SG is a protecting group and El is a structure element of B, is
transformed with a heterocyclic compound of the general formula
Y
N-H
X-/
CA 02628145 2008-05-01
IMTM GmbH 45
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; and the
obtained reaction product is cleaved from the protecting groups into a com-
pound of the general formula A - B - D - B' - A' (1) in which A and A' may
be identical or different and B and B' may be identical or different and in
which A, A', B, B' and D may have the afore-mentioned meanings or
- compounds of the general formula
HO - C(=O) - CH[-NH (SG)] -D
in which (SG) is a protecting group and f> is a structure element of B are
transformed with a heterocyclic compound of the general formula
Y
N-H
X
in which X is S, 0, CH2, CH2CH2, CHZO or CH2NH and Y is H or CN; the ob-
tained condensation product of the formula
Y
N - C(=O) - CH[-NH (SG)] _>
X-/
is transformed with a compound of the general formula
>I1-s-s-z
in which ~ is a structure element of B and Z is a residue which activates
an -S-S-group for a thiol exchange and (SG) is a protecting group; and the
obtained reaction product of the formula
CA 02628145 2008-05-01
IMTM GmbH 46
Y
H-C(=O)CH[-NH(SG)- S - S - Z
X-/
is transformed with a compound of the general formula
HS - CH2 - CH [- NH - (SG)] - (R9)
and is transformed being cleaved from the protecting group (SG) into a com-
pound of the general formula A - B - D - E (2) in which A, B, D and E may
have the afore-mentioned meanings.
Protecting groups which have been inserted in the context of the afore-
mentioned process steps into the intermediate of the synthesis of compounds of
the general formulae (1) and (2) ("SG") can be arbitrary protecting groups
which
are known to a person in the field of organic synthesis from his practical
experi-
ence and they are according to the invention not restricted. Preferred
protecting
groups are protecting groups for protecting amino acid side-chains such as ure-
thane protecting groups for example benzyloxycarbonyl-residues, fluorene-9-
ylmethoxycarbonyl-residues, tert-butyloxycarbonyl-residues ect..
The groups Z which have been inserted in the context of the afore-mentioned
process steps into intermediates of the synthesis of compounds of the general
formulae (1) and (2) are residues which activate a molecule containing an S-S-
group for a thiol-exchange, hence an exchange of a group bound to the S-atom
for an S-atom (optionally attached to a substituent) of another molecule. Such
groups are sufficiently known to a person skilled in the field of organic
synthesis
and the invention is not restricted to certain groups which activate for a
thiol-
exchange. Preferred but not restricting examples are the groups 3-nitro-2-
pyridyl, 5-nitro-2-pyridyl, 2-, 3- or 4-pyridyl, 2-nitrophenyl,
methoxycarbonyl or
N-methyl-N-phenylcarbamoyl.
CA 02628145 2008-05-01
IMTM GmbH 47
The symbols
-D , E and
used in the general reaction scheme for the synthesis leading to compounds of
the general formulae (1) and (2) and also used in the afore-going and in the
fol-
lowing descriptions of process steps mean structure elements of the residues B
or B', respectively, in the compounds of the general formulae (1) and (2).
Using
the term "structure elements" it is understood in this context that the
residues B
or B', respectively are composed of parts of the molecule having a reactive
group each which is added in the context of an organic synthesis forming a co-
valent bond between the two reactive groups which become thereby adjacent
bond partners in the newly formed molecule. Particular but not restricting
exam-
ples for such structure elements are a carboxyl-group in one and a hydroxy-
group in the second molecule which add to one another forming an ester-group
and releasing water or a carboxyl-group in one and an amino-group in the sec-
ond molecule which add to one another forming an amido-group and releasing
water. This is symbolized by the combination of the two symbols shown above
on the left to give the symbol shown above on the right.
With particular advantage the compounds (1) and/or (2) can be prepared in
processes which are presented in the following schemes of synthesis 2 to 17:
A compound of the general formulae
HO - C(=O) - CH [-NH (SG)l] - (CH2)n - R2 - (SG)2
in which (SG)' is a protecting group for an amino-group, (SG)2 is a protecting
group at the substituent R2 and R2 and n have the afore-mentioned general or
CA 02628145 2008-05-01
IMTM GmbH 48
specific meanings, is transformed with a heterocyclic compound of the general
formula
Y
N-H
X-/
in which X is S, 0, CH2, CH2CH2, CH2O or CH2NH and Y is H or CN; the
resulting condensation product of the formula
N-C(=O)-CH[-NH (SG)'] -(CH2)n- R2 - (SG)2
X
is transformed after cleavage of the protecting group (SG)2 with a compound of
the general formula
[F' -R3-R4-S-]2
in which R3 and R4 might have the afore-mentioned general and specific mean-
ings and Fl is one part of the functional group which can react with one part
of
the other functional group; and the resulting reaction product is transformed
re-
leasing the protecting group (SG)l into a compound of a general formula A - B
- D - B' - A' (1) in which A and A' might be identical or different and B and
B'
might be identical or different and in which A, A', B, B' and D might have the
afore-mentioned meanings; in case of the following reaction scheme 2 A and A'
are identical and are said X and Y containing heterocyclic compound with a car-
bonyl amino-group bound to a nitrogen atom; B and B' are also identical and
CA 02628145 2008-05-01
IMTM GmbH 49
are the group of the formula -(CH2),, - R2 - R3 - R4 - with the afore-
mentioned
general or specific meanings for n, R2 - R3 and R4 and D is -S-S-.
Scheme 2
Synthesis of symmetric compounds A-B-D-B-A (1)
with B = -(CH2)n-R2 -R3-R4-)
Y
(SG)l NH r_~ (SG)' NH
HO X NH ~N (
(CH2)- i 2 X~ CHz)Rz ~ s. scheme 4
n
(SG)2 O (SG)z
1. - (SG)2
2. EF1-R3-R4-S -1 2
Y
~(SG)l NH
N 30 s. scheme 3
X-_/ (CH2)n R2-R3-R4-S-
O 2
- (SG)'
Y
1N
r_\
X-_/ ( NH2CH2)n R2-R3-R4-S-
O 2
The protecting groups (SG)' and (SG)2 are in general protecting groups as they
are known by a person skilled in the field of organic synthesis for the
intermedi-
ate protection of certain groups in organic molecules and are preferably ure-
thane protecting groups, which are used for example as amino acid protecting
CA 02628145 2008-05-01
IMTM GmbH 50
groups. Not restricting examples are a benzyloxyl carbonyl-residue, a fluorene-
9-ylmethoxylcarbonyl-residue or a tert-butyloxycarbonyl residue.
Using the first reaction steps of the preceding reaction scheme 2 - starting
from
the dimer which results from the transformation with the compound of the for-
mula [F'- R3 - R4 - S-]2 - an unsymmetric compound of the formula A - B - D -
E (2) can be obtained releasing the protecting group (SG)3 while being trans-
formed with a compound of the general formula HS - CH2 - CH (R) -NH (SG)3
in which R9 has the afore-mentioned general and specific meaning and (SG)3 is
a protecting group for the amino-group. This is shown in the following
reaction
scheme 3
Scheme 3
Synthesis of unsymmetric compounds A-B-D-E with
B = -(CH2)n-R2 -R3-R4-)
Y
~(SG)~ NH
N
X-_/ (CH2)n R2-R3-R4-S-
O 2
1. HS-CH2-CH-R9
NH-(SG)3
2. - (SG)', - (SG)3
Y
1
~\N NH2
X-_/ (CH2)n-R2-R3-R4-S-S-CH2-CH-R9
0 NH2
In the unsymmetric compound obtained having the general formula A - B - D -
E (2) A is the afore-mentioned X and Y containing heterocyclic compound with
an a-aminocarbonyl-residue bound to the nitrogen; B is the group of the
formula
CA 02628145 2008-05-01
IMTM GmbH 51
-(CH2)n - R2 - R3 - R4 - with the afore-mentioned general or specific meanings
for n, R2, R3 and R4, D is -S -S- and E is the group - CH2 - CH (NH2) - R9 in
which R9 has the afore-mentioned general and specific meanings.
In a second path of synthesis leading to unsymmetric compounds the residue B
- starting from the first intermediate of the reaction scheme 2 shown above -
is
composed in subsequent reaction steps finally obtaining the same product like
in the reaction of reaction scheme 3, as shown in the following reaction
scheme
4:
The afore-mentioned first intermediate (see reaction scheme 2) is transformed
being cleaved form the protecting group (SG)2 into a compound with a suitable
leaving group F2 for the subsequent reaction with the compound of the general
formulae F3 - R3 - R4a -(SG)4; in which F2 and F3 mean parts of functional
(leaving-) groups. If R3 has the afore-mentioned general and specific meaning
R4a is a part of the group R4 and (SG)4 is a protecting group. The latter is
trans-
formed being cleaved into a suitable leaving group F4. The transformation with
a
compound of the general formula F5 - R4b - S- S -Z in which F5 is a suitable
functional leaving group, R4b is a second part of R4 and Z is a residue which
activates the molecule for a thiol exchange and the subsequent transformation
with the thiol of the general formula HS - CH2 - CH (R) - NH (SG)3 after cleav-
ing the two protecting groups (SG)' and (SG)3 lead to unsymmetric compounds
of the general formula A - B - D - E (2) in which A is the afore-mentioned X
and Y containing heterocyclic compound with an a-aminocarbonyl-residue
bound to a nitrogen; B is the group of the formula -(CH2)n - R2 - R3 - R4 -
with
the afore-mentioned general or specific meanings for n, R2, R3 and R4, D is -S-
S- and E is the group - CH2 - CH (NH2) - R9 in which R9 has the afore-
mentioned general and specific meanings.
CA 02628145 2008-05-01
IMTM GmbH 52
Scheme 4
Synthesis of unsymmetric compounds A-B-D-E
with B = -(CH2)n-R2-R3-R4
Y
/N (SG) NH Y
I~ ~(SG)-NH
2
x~ O (CH2)n ( SG)2 x~N (CH2)n R2-F2
(SG)2 ( 0
1. F3-R3-Raa_(SG)4
2. - (SG)4
Y
(SG)I-NH
~X-_/N II lCH2)n R2-R3-R4a-F4
0
F5-R4b-S-S-Z
Y
r_~(SG)I-NH
x__1N __~(CH2)n R2-R3-R4a-R4b S-S-Z
0
1. HS-CH2-CH-R9
NH-(SG)3
2. - (SG)l, - (SG)a
Y
1 NH2
r_\
N
X-_/ (CH2)n R2-R3-R4a-R4b S-S-CH2-CH-R9
0 NH2
In the preceding reaction scheme 4(SG)1, (SG)2, (SG)3 and (SG)4 are in gen-
eral protecting groups as they are known to a person skilled in the field of
or-
ganic chemistry for the intermediate protection of certain groups in organic
molecules and are preferably urethane protecting groups which are usually
used for example as amino acid protecting groups. Not restricting examples are
CA 02628145 2008-05-01
IMTM GmbH 53
a benzyloxyicarbonyl-residue, a fluorene-9-ylmethyloxycarbobyl-residue or a
tert-butyloxycarbonyl-residue.
The residues FZ, F3, F4 and F5 are parts of functional groups and designate
leaving groups such as for example -H, -OH, -CI etc. which are particularly
suited to be cleaved from the molecule. Z are residues which activate a mole-
cule containing an -S-S-group for a thiol exchange thus an exchange of a group
for an S-atom (optionally attached to a substituent) of another molecule. Such
groups are sufficiently known to a person skilled in the field of organic
synthesis
and the invention is not restricted to certain groups which activate for a
thiol ex-
change. Preferred but not restricting examples are the groups 3-nitro-2-
pyridyl,
5-nitro-2-pyridyl, 2-, 3- or 4-pyridyl, 2-nitrophenyl, methoxycarbonyl or N-
methyl-
N-phenylcarbamoyl. In the preceding scheme the residues R4a an R4b mean in
combination the residue R4 with the afore-mentioned general and specific
meaning.
The starting compound of the following reaction scheme 5 in which R' has the
meaning mentioned above in general and in detail can be transformed directly
with the afore-mentioned heterocyclic compound of the general formula
Y
N-H
X-/
releasing the protecting group (SG)l into the symmetric compound of the gen-
eral formula A - B - D - B' - A' (1) with B = R' in which X, Y and (SG), have
the
afore-mentioned general and specific meanings:
CA 02628145 2008-05-01
IMTM GmbH 54
Scheme 5
Synthesis of symmetric compounds A-B-D-B-A (1)
with B = R)
(SG)'-NH O
HO R1i- S',S,~R' OH
O NH-(SG)l
Y
HN\-2X
2. - (SG)l
Y
F~ NHz O
N
Y_I~ R~iS~S~R1 N
O NH2 P
Y
Using the preceding reaction path for example a final product is obtainable in
which Y is CN by carrying out the afore-mentioned reaction with the
heterocyclic
compound
Y
N-H
X_/
in which Y is -C (=0) -NH2 and the symmetric reaction product in which Y is
still -C (=0) -NH2 is exposed to conditions under which the carbonyl amino
group is transformed to a cyano-residue releasing water. This reaction results
from the following reaction scheme 5a:
CA 02628145 2008-05-01
IMTM GmbH 55
Scheme 5a
Synthesis of symmetric compounds A - B - D - B - A
with Y = CN
(SG)~-NH O -)A HO 1iS"'S'-R1 OH
NH-(SG)l
HN
C
O NH2
H2N % (SG)l
C I
~ NH O
X~N _~A R~~S"S~R~ N
O NH ~
(SG)~ pC\
NH2
1. - H20
2. - (SG)l
CN
NH2 O
X~N RliS-S- ~
N/--X
O NH2
CN
Unsymmetric compounds of the general formula A - B - D - E, in which B is R'
and R' has the afore-mentioned general and specific meanings, are obtainable
from the N-protected reaction product of reaction scheme 5 (see above) by
transformation with a thiol compound of the general formula HS - CH2 - CH [-
NH (SG)3] -R9 and cleavage of the protecting groups. This is shown in a reac-
tion scheme 6:
CA 02628145 2008-05-01
IMTM GmbH 56
Scheme 6
Synthesis of unsymmetric compounds A-B-D-E (2)
with B = R'
Y
HN-(SG)' O
r_~ X___ZN S"IS"R N/-X
0 (SG)'-NH p
Y
1. HS-CH2-CH-R9
NH-(SG)3
2. - (SG)', - (SG)3
Y
~
NH2
N
X-,/ W-S-S-CH2-CH-R9
O NHz
The same unsymmetrical compounds A - B - D - E (2) with B R' as in reac-
tion scheme 6 are also obtainable following the path of synthesis as shown in
reaction scheme 7 in which firstly a starting compound is transformed with the
heterocyclic compound
Y
N-H
X-/
The subsequent transformation with a thiol compound of the general formula
HS - CH2 - CH [-NH (SG)3] -R9 and the cleavage of protecting groups (SG)'
and (SG)3 also leads to unsymmetrical compounds A - B - D - E (2) with B
R' in which R' may have the afore-mentioned general and specific meanings.
CA 02628145 2008-05-01
IMTM GmbH 57
Scheme 7
Synthesis of unsymmetric compounds A-B-D-E (2)
with B = R'
(SG)~-NH
HO R~iS~S~ Z
O
Y
~
1. HN\-- X
Y
~ NH-(SG)l
X~N RiiS-S"Z
O
1. HS-CH2-CH-R9
NH-(SG)3
2. - (SG)', - (SG)3
Y
~ NH2
N
X---/ R1-S-S-CH2-CH-R9
O NH2
The following schemes of synthesis 8 and 9 show paths leading to the synthesis
of symmetric compounds A - B - D - B - A (1) with B = R7 - R$- (scheme 8)
and - starting from the N-protected product of the reaction of the scheme 8 -
to
the synthesis of unsymmetric compounds A - B - D - E - (2) using paths of
synthesis which are similar to the ones described above:
CA 02628145 2008-05-01
IMTM GmbH 58
Scheme 8
Synthesis of symmetric compounds A-B-D-B-A (1)
with B = -R -R$-)
Y
1 NH-(SG)'
X~ \~ N R7-R$a-Fs
O
CF7~-Rsb-S ] 2
Y
1 NH-(SG)'
X~ \N R7-R$a-R$b-S-
-_/
O
2
- (SG)1
Y
1 NH2
[XN R~-R$a-RBb-S-
O
2
CA 02628145 2008-05-01
IMTM GmbH 59
Scheme 9
Synthesis of unsymmetric compounds A-B-D-E (2)
with B = -R7 -R8-
Y
1 NH-(SG)' X~~/\N R7-R$a-R$b-S-
O
2
1. HS-CH2-CH-R9
NH-(SG)3
2. - (SG)', - (SG)3
Y
1 NH2
r-\
N
X-_/ RT-Rsa-Rsb- S-S-CH2-CH-R9
O NH2
In the reaction schemes 8 and 9 the residues Rsa and Rsb are these residues
which compose R 8 within two steps of the synthesis along said path.
Two further parts of synthesis which lead as well as the path of synthesis
shown
in reaction scheme 9 to unsymmetrical compounds A - B - D - E (2) with B=-
R' - R8 and which are comparable to the reactions of synthesis as already
shown above are presented in the following schemes of synthesis 10 and 11:
CA 02628145 2008-05-01
IMTM GmbH 60
Scheme 10
Synthesis of unsymmetric com~ounds A-B-D-E (2)
with B = -R7 -R -
Y
SG)~ NH
N R7-R8-SH
O
CI-S-Z
Y
~(SG)~ NH
N ""-~ R'-R$-S-S-Z
X~/
O
1. HS-CH2-CH-R9
NH-(SG)3
2. - (SG)l, - (SG)3
Y
NH2
r_~ N
X-_/ R7- RB-S-S-CH2-CH-R9
O NH2
In all mentioned reaction schemes the residues F2, F3, F4, F5 and F6 are parts
of
functional groups and designate leaving groups such as for example -H, -OH, -
Cl etc. which are particularly suited to be cleaved from the molecule. Z are
resi-
dues which activate the molecule containing an - S-S-group for a thiol ex-
change, hence an exchange of a group bound to the S-atom for an S-atom (op-
tionally attached to a substituent) of another molecule. Such groups are suffi-
ciently known to a person skilled in the field of organic synthesis and the
inven-
tion is not restricted to certain groups which activate for a thiol exchange.
Pre-
ferred but not restricting examples are the groups 3-nitro-2-pyridyl, 5-nitro-
2-
pyridyl, 2-, 3- or 4-pyridyl, 2-nitrophenyl, methoxycarbonyl or N-methyl-N-
CA 02628145 2008-05-01
IMTM GmbH 61
phenylcarbamoyl. In the preceding scheme the residues R4a and R4b mean in
combination the residue R4 and the residues R$a and R 8b mean in combination
the residue R8 both having the afore-mentioned general and specific meanings.
Scheme 11
Synthesis of unsymmetric compounds A-B-D-E (2)
with B = -R'-R$-
Y
~(SG)l NH
X__/N R~ R8a-Fe
O
F8 Rsb S-S-Z
Y
~(SG)~ NH
N 7 8a_ 8b
X~ R -R R S-S-Z
O
1. HS-CH2-CH-R9
NH-(SG)3
2. - (SG)l, - (SG)3
Y
r__~N NH2
X-_/ R7 -R$a-Rab S-S-CH2-CH-R9
O NH2
The afore-mentioned and in the reaction schemes depicted reactions leading
according to the invention to compounds of the general formulae (1) and (2)
are
carried out under conditions which are sufficiently known to a person skilled
on
the field of organic synthesis and do not require in this regard any further
elabo-
ration. Typical solvents used are polar organic solvents or mixtures of
solvents.
Particularly ethers are conceivable such as THF, esters such as acetic acid
ethyl ester or DMF without restricting the invention hereunto. The temperature
CA 02628145 2008-05-01
IMTM GmbH 62
of reaction conforms with the transformation to be carried out and with the
sol-
vent used but lies usually in the range of -20 C to +50 C preferably in the
range of 10 C to 40 C.
According to the invention the compounds of the general formulae (1) and (2)
accrue during their synthesis in form of acid addition salts according to one
of
the afore-mentioned processes particularly when their precise characterization
and subsequent use in an aqueous milieu is to be carried out. Preferably acid
addition salts with certain physiological (i.e. pharmacologically and/or
cosmeti-
cally) acceptable inorganic or organic acids are prepared for the characteriza-
tion and for the subsequent use of the compounds of the general formulae (1)
and (2). In preferred embodiments of the invention salts of pharmaceutically
acceptable acids from the group consisting of hydrochlorides,
trifluoroacetates,
tartrates, succinates, formiates and/or citrates of the compounds of the
general
formulae (1) or (2) are prepared as acid addition salts.
The compounds mentioned above in general and in detail (referring to formulae
(1) and (2)) which can be prepared for example using one of the previously
mentioned processes without restricting the preparation of the compounds to
one of the said processes can be used for numerous purposes. Surprisingly it
was found by the invention that the compounds can be used in the field of
medicine. Particularly it was found that the new compounds according to the
present invention themselves are inhibitors of the dipeptidyl peptidase IV or
of
enzymes with analogous enzymatic effect and of alanyl aminopeptidase N or of
enzymes with analogous enzymatic effect.
In preferred embodiments of the invention the compounds can be successfully
used as inhibitor precursors or inhibitor prodrugs. With regard to the
definitions
the terms "inhibitor", "precursor" and "prodrug" it can be referred to the
afore-
mentioned definitions.
CA 02628145 2008-05-01
IMTM GmbH 63
Further preferred compounds according to the invention serve for the use as
prodrugs for inhibitors of dipeptidyl peptidase IV (DPIV) and peptidases with
analogous enzymatic effect as well as of alanyl aminopeptidases N (APN) and
peptidases with analogous enzymatic effect. Even more preferred are com-
pounds belonging to said compounds which serve for the use as prodrugs for
inhibitors of dipeptidyl peptidase IV (DPIV) and alanyl aminopeptidase N
(APN).
Namely it was surprisingly found that compounds according to the invention can
react under physiological or pathological conditions particularly under
reducing
conditions - as they are present in cells and tissues - to such compounds
which
are highly effective inhibitors of dipeptidyl peptidase IV (DPIV) and
peptidases
with analogous enzymatic effect, as well as for alanyl aminopeptidase N (APN)
and peptidases with analogous enzymatic effect. According to the invention one
of the new compounds according to formulae (1) or (2) mentioned above can be
used according to the general or special description or more than one of the
compounds mentioned above can be used in combination. Said combinations of
more than one compounds can compirse either more than one compounds of
the general formulae (1) or more than one compounds of the general formulae
(2) or more than one compounds which are arbitrarily selected of the group of
compounds of the general formulae (1) and (2) in combination. It is preferred
to
apply one compound of the general formulae (1) or (2) exclusively.
The invention also relates to the use of one or more compound(s) at least one
compound particularly preferred exactly one compound of the general formulae
(1) and/or (2) according to the afore-mentioned general and detailed
description
for the prophylaxis and therapy of diseases with exceeding immune response
and inflammatory genesis, including arteriosclerosis, neuronal disease,
cerebral
damages, skin diseases, tumour diseases and virus-caused diseases as well as
type II diabetes.
Furthermore the invention relates to the use of one or more compound(s), at
least of one compound, particularly preferred exactly one compound of the gen-
CA 02628145 2008-05-01
IMTM GmbH 64
eral formulae (1) and/or (2) according to the afore-mentioned general and de-
tailed description for the preparation of a medicament or a cosmetic
preparation
for the prophylaxis and therapy of diseases with exceeding immune response
and inflammatory genesis including arteriosclerosis, neuronal diseases, cere-
bral damages, skin diseases, tumour diseases and virus-caused diseases as
well as type II diabetes.
In preferred embodiments of the invention compounds of the general formulae
(1) and/or (2) are used in general and preferably compounds according to the
two afore-mentioned tables solely or the use in combination or in form of phar-
maceutical or cosmetic preparations which comprise one or more of said com-
pounds for the prophylaxis and therapy of diseases such as for example multi-
ple sclerosis, Morbus Crohn, colitis ulcerosa, and other autoimmune diseases
as well as inflammatory diseases, asthma bronchiale and other allergic dis-
eases, skin- and mucosa-diseases, for example psoriasis, acne as well as de-
rmatological diseases with hyper proliferation and modified conditions of
differ-
entiation of fibroblasts, benign fibrosing and sclerosing skin diseases and ma-
ligne fibroblastic conditions of hyper proliferation, acute neuronal diseases
such
as for example ischemia-caused cerebral damages after an ischemia - or
haemorrhagic apoplexia, cranio-cerebral injury, cardiac arrest, heart attack
or
as a consequence of cardio surgical intervention, of chronic neuronal diseases
for example of Morbus Alzheimer, of the Pick-disease, a progressive supra-
nuclear palsy, the corticobasal degeneration, the frontotemporal dementia, of
Morbus Parkinson, especially parkinsonism coupled to chromosome number
17, of Morbus Huntington, of prion-caused conditions of diseases and amyotro-
phic lateral sclerosis, of arteriosclerosis, arterial inflammation, stent-
restenosis
of chronic obstructive pulmonary disease (COPD), of tumours, metastases, of
prostate carcinoma, of severe acute respiratory syndrome (SARS) and of sep-
sis and sepsis-like conditions as well as diabetes II.
CA 02628145 2008-05-01
IMTM GmbH 65
In a further preferred embodiment of the invention the compounds of the gen-
eral formulae (1) and/or (2) are used in general and preferably compounds ac-
cording to the two afore-mentioned tables solely or the use in combination or
in
form of pharmaceutical or cosmetic preparations which comprise one or more of
said compounds are used for the prophylaxis and therapy of the rejection of
transplanted tissues and cells. As an example of such a use the use of one or
more of the afore-mentioned compounds or of a pharmaceutical preparation
containing one or more of the afore-mentioned compounds can be mentioned
with regard to allogene or xenogene transplanted organs, tissues and cells
such
as kidney-, heart-, liver- pancreas-, skin- or stem cell transplantation as
well as
graft versus host diseases.
In a further preferred embodiment of the invention the compounds of the gen-
eral formulae (1) and/or (2) in general and preferably the compounds according
to the afore-mentioned tables are used solely or in combination or pharmaceuti-
cal or cosmetic preparations containing one or more of said compounds are
used for the prophylaxis and therapy of reactions concerning rejection or in-
flammation at - or caused by - medical devices implanted into an organism.
These can be for examples stents, joint implants (knee joint implants, hip
joint
implants), bone implants, cardio pace maker or other implants. In a further
pre-
ferred embodiment of the invention the compounds of the general formulae (1)
and/or (2) are used in general and preferably the compounds according to the
afore-mentioned tables are used solely or in combination or pharmaceutical or
cosmetic preparations are used containing one or more of said compound(s) in
that way that the compound(s) or preparation(s) are applied in form of a
coating
or a wetting onto the item(s) or at least one of the compounds or preparations
is
materially admixed to the item(s). Also in this case it is certainly possible
to ap-
ply one of the compounds or preparations - if applicable subsequently or paral-
lelly - locally or systemically.
CA 02628145 2008-05-01
IMTM GmbH 66
In the same way as mentioned above - and for comparable purposes or for
prophylaxis and therapy of the above exemplary but not completely mentioned
diseases and conditions - the compounds of the general formulae (1) and (2) in
general and the compounds according to the two afore-mentioned tables in pre-
ferred embodiments as well as the following pharmaceutical and cosmetic
preparations containing said compounds can be used solely or in combination
of more than one of them to prepare medicaments for the treatment of the
afore-mentioned diseases and conditions. These can comprise the afore-
mentioned compounds in amounts mentioned in the following, optionally in
combination with per se known carrier substances auxiliary substances and/or
additives.
In preferred embodiments the use of the compounds of the afore-mentioned
general formulae (1) and/or (2) comprises such cases in which the used com-
pound(s) generate(s) at least one inhibitor of dipeptidyl peptidase IV (DPIV)
and
peptidases with analogous enzymatic effect as well as alanyl aminopeptidase N
(APN) and peptidases with analogous enzymatic effect. The generation of at
least one of such inhibitors preferably two or more than two of such
inhibitors
particularly preferred one or two inhibitors from a compound leads to a
surpris-
ing specificity of the use according to the invention in the medical fields
particu-
larly as inhibitors of one or more enzymes of the afore-mentioned groups.
In a further preferred embodiment the use of one or more compounds of the
afore-mentioned formulae (1) and/or (2) or of cosmetic or pharmaceutical
preparations comprising at least one of the compounds of the general formulae
(1) and/or (2) leads to at least one inhibitor preferably to one inhibitor or
two
inhibitors of enzymes of the group mentioned above if reducing conditions are
present particularly if reducing conditions are present at a position at which
the
resulting compound(s) which is/are formed develop(s) its/their pharmacological
effect.
CA 02628145 2008-05-01
IMTM GmbH 67
Using the term "reducing conditions" in this context conditions of a chemical
reaction are recognized in which the compound(s) of the general formula (1) or
the compound(s) of the general formula (2) or one or more compound(s) of the
general formulae (1) and (2) in combination are exposed to reaction conditions
at the site of an effect in which an electron-donor supplies electrons for the
ac-
ceptance by the compound(s) of the general formulae (1) and/or (2). As it was
found according to the invention compounds of the general formulae (1) and/or
(2) are transformed under reducing conditions cleaving an - S-S- or -Se-Se-
bridge designated by D in the general formulae (1) and (2) into two compounds
with an -SH-terminal group or an -SeH-terminal group, respectively.
It is for the invention without any crucial importance by which means the
reduc-
ing physiological reaction conditions are achieved as long as they achieve a
reliable generation of the accordant molecules with an -SH- or an - Se-
terminating group. The compounds according to the invention regularly meet
the conditions which lead to the in- vivo generation of suitable compounds
being
inhibitors of the afore-mentioned enzymes.
The amounts of at least one of the compounds of the general formulae (1)
and/or (2) in general or of the afore-mentioned compounds accordant to the two
preceding tables are in the scope of the inventive use in the range of 0.01 to
1000 mg with regard to at least one of the compounds of the general formulae
(1) and/or (2) per application unit preferably in the range of 0.1 to 100 mg
per
application unit.
In the context of the inventive use an application of at least one of said com-
pounds of general formulae (1) and/or (2) can be achieved on any pathway per
se known which a person normally skilled in this technical field knows. The ap-
plication of compounds of the general formulae (1) and/or (2) in general and
further preferred the compound according to the afore-mentioned tables or
pharmaceutical or cosmetic preparations, respectively, which comprise one or
CA 02628145 2008-05-01
IMTM GmbH 68
more of the afore-mentioned compounds in combination with per se known
usual carrier substances, auxiliary substances and/or additives is either
carried
out as topic application in form of for example cremes, salves, pastes, gels,
so-
lutions, sprays, liposomes and nanosomes, shake mixtures, "pegylated" formu-
lations degradable (e.g. under physiological conditions degradable) depot-
matrices, hydrocolloid-bandages, plasters, micro-sponges, prepolymers and
similar new carrier substrates, jet-injection or other dermatological princi-
ples/vehicles including instillative application and on the other hand as
systemic
application for oral, transdermal, intravenous, subcutane, intracutane,
intramus-
cular, intrathecal application in suitable formulations or suitable galenic,
thus in
form of tablets, dragees, lozenges, capsules, aerosols, sprays, solutions,
emul-
sions and suspensions.
The invention also relates to a process for the inhibition of the alanyl
aminopep-
tidase N activity or of the activity of peptidases with analogous enzymatic
effect
as well as the dipeptidyl peptidase IV activity or of the activity of
peptidases with
analogous enzymatic effect either solely or in combination with other
inhibitors
of alanyl aminopeptidase N or inhibitors of peptidases with analogous enzy-
matic effect and/or other inhibitors of DPIV or inhibitors of peptidases with
analogous enzymatic effect by the application of at least one compound of the
general formulae (1) and/or (2) or a pharmaceutical or cosmetic preparation
which comprises at least one of the compounds of the general formulae (1)
and/or (2) according to the afore-mentioned detailed description in an amount
necessary for the inhibition for the enzyme activity. The amounts of the com-
pounds of the general formulae (1) and/or (2) in general or the compounds ac-
cording to the two afore-mentioned tables, respectively, are in the range - as
mentioned above - of 0.01 to 1000 mg of at least one compound per application
unit, preferably in the range of 0.1 to 100 mg per application unit.
Further the invention relates to a process for the topic influence of the
alanyl
aminopeptidase N activity or of the activity of peptidases with analogous enzy-
CA 02628145 2008-05-01
IMTM GmbH 69
matic effect as well as of the activity dipeptidyl peptidase IV or of the
activity of
peptidases with analogous enzymatic effect either solely or in combination
with
other alanyl aminopeptidase N inhibitors or inhibitors of peptidases with
analo-
gous enzymatic effect and for other DPIV inhibitors or inhibitors of
peptidases
with analogous enzymatic effect by the application of at least one compound of
the general formulae (1) and/or (2) or of pharmaceutical or cosmetic prepara-
tions according to the following detailed description in an amount necessary
for
the manipulation of the enzyme activity. Also in these cases the amounts of
the
compound(s) of the general formulae (1) and/or (2) are in the range mentioned
above.
Furthermore the invention relates to a process for to generate at least one in-
hibitor of dipeptidyl peptidase IV (DPIV) and peptidases with analogous enzy-
matic effect as well as alanyl aminopeptidase N (APN) and peptidases with
analogous enzymatic effect from at least one of the compounds of the general
formulae (1) and (2). The process according to the invention comprises the
step
that at least one of the compounds of the general formulae (1) and (2) is ex-
posed to reducing conditions according to the afore-mentioned description. As
previously described a skilled person is not restricted with regard to
reducing
conditions for the transformation of at least one compound of the general
formu-
lae (1) and/or (2) which can insofar as well be regarded as intermediate in
the
synthesis of inhibitors according to the invention of dipeptidyl peptidase IV
(DPIV) and inhibitors of peptidases with analogous enzymatic effect as well as
inhibitors of the alanyl aminopeptidase N (APN) and inhibitors of peptidases
with analogous enzymatic effect into the actual inhibitors of a dipeptidyl
pepti-
dase IV (DPIV) and inhibitors of peptidases with analogous enzymatic effect as
well as inhibitors of alanyl aminopeptidase N (APN) and inhibitors of
peptidases
with analogous enzymatic effect.
Furthermore the invention relates to a process for the prophylaxis and therapy
of numerous diseases, for example diseases with exceeding immune response
CA 02628145 2008-05-01
IMTM GmbH 70
(autoimmune diseases, allergies and transplant rejections) of other chronic in-
flammatory diseases, neuronal diseases and cerebral damages, skin diseases
(inter alia acne and psoriasis), tumour diseases and particular virus
infections
(inter alia SARS) as well as type II diabetes and particularly the diseases
men-
tioned above in detail. This includes: processes for the prophylaxis and
therapy
of diseases such as for example multiple sclerosis, Morbus Crohn, colitis ul-
cerosa, and other autoimmune diseases as well as inflammatory diseases,
asthma bronchiale and other allergic diseases, skin- and mucosa diseases for
example psoriasis, acne as well as dermatological diseases with hyperprolifera-
tion and modified conditions of differentiation of fibroblasts, benigne
fibrosing
and scierosing skin diseases and malign fibroblastic hyper proliferation condi-
tions, acute neuronal diseases, such as for example ischemia-caused cerebral
damages after an ischemia- or haemorrhagic apoplexia, cranio-cerebral injury,
cardiac arrest, heart attack or as a consequence of cardio-surgical interven-
tions, of chronic neuronal diseases such as for example Morbus Alzheimer, the
Pick-disease, the progressive supra-nuclear palsy, the corticobasal denegera-
tion, the frontotemporal dementia, of Morbus Parkinson, particularly parkinson-
ism coupled to chromosome 17, of Morbus Huntington, or disease conditions
caused by prions and of amyotrophic lateral sclerosis, of arteriosclerosis,
arte-
rial inflammations, stent-restenosis, of chronic obstructive pulmonary
diseases
(COPD), of tumours, metastases, of prostate carcinoma, of severe acute respi-
ratory syndrome (SARS) and of sepsis and sepsis-like conditions. The process
comprises an application of at least one compound or pharmaceutical prepara-
tion according to the following detailed description in an amount necessary
for
the prophylaxis or therapy of the accordant disease. Also in these cases the
amounts of compound(s) is/are in the afore-mentioned range of 0.01 to 1000
mg of a compound per application unit preferably in a range of 0.1 to 100 mg
per application unit.
The invention also relates to pharmaceutical preparations which comprise at
least one compound of at least one of the general formulae (1) and (2) of the
CA 02628145 2008-05-01
IMTM GmbH 71
afore-mentioned general and detailed description optionally in combination
with
one or more pharmaceutically acceptable carrier substance(s), auxiliary sub-
stance(s) and/or adjuvant(s).
Furthermore the invention also relates to cosmetic preparations which comprise
at least one of the compounds of at least one of the general formulae (1) and
(2) of the afore-mentioned general and detailed description if applicable in
com-
bination with one or more cosmetically acceptable carrier substance(s),
auxiliary
substance(s) and/or adjuvant(s).
With regard to these preparations the pharmaceutically or cosmetically accept-
able carrier substances, auxiliary substances and/or adjuvants are
sufficiently
known to a person skilled in the pharmaceutical or cosmetic field and do not
require any further detailed mentioning.
The mentioned pharmaceutical or cosmetic preparations, might contain at least
one compound of the general formulae (1) or (2), preferably one or two com-
pounds of the general formulae (1) and/or (2) in such amount(s) which is/are
necessary for the desired effect in the pharmaceutical or cosmetic field. The
amount(s) is/are not particularly restricted and is/are dependent on a number
of
parameters such as for example the application pathway, the specific disease
pattern or the cosmetic status, of the constitution of the addressee who can
be
a mammal such as for example a human, of the bio-availablility of the used
compound(s) etc.. In particularly preferred embodiments a pharmaceutical ap-
plication unit or a cosmetic application unit, respectively, contains an
amount of
at least one compound of the general formula (1) and/or (2) which is in the
range of 0.01 to 1000 mg of a compound per application unit, preferably in the
range of 0.1 to 100 mg per application unit. Usually the application units can
be
of that ilk (and contain such concentrations of at least one compound of the
general formulae (1) and/or (2)) that the application of one or less further
pre-
ferred two or three application units per day is sufficient to apply an amount
CA 02628145 2008-05-01
IMTM GmbH 72
necessary for a systematic pharmaceutical or cosmetic treatment with regard to
at least one of the compounds (1) and/or (2) to the patient such as for
example
a mammal, particularly a human.
The invention is explained in the following by examples of particularly
preferred
embodiments. The following examples of embodiments are not to restrict the
invention but only to give an exemplary illustration.
Examples
Example 1
Preparation of compounds of the general formula (1)
Compounds of the general formula (1) were prepared using the following pro-
cess:
(a) Compound II (Scheme 12)
440 mg (1 mmol) (Boc-Cys-OH)2 1 were dissolved in 5 ml dry THF. Under ar-
gon 158 pl (2 mmol) thiazolidine were added to the solution and after 25 min
at
0 C gradually 460 mg (2,4 mmol) N'-(3-dimethylaminopropyl)-N-
ethylcarbodiimid (EDC) were added. The transformation was controlled at RT
by means of thin layer chromatography (TLC). After 6 h a further activation
was
carried out using 460 mg (2.4 mmol) EDC and 79 pl (1 mmol) thiazolidine at 0
C. After 20 h the THF was removed by distillation and the solid residue was
dissolved in ethylacetate. The ehtylacetate phase was rinsed 3 times with 5 /o
KHSO4-solution, once with NaCI-solution, 3 times with saturated NaHCO3-
solution and 3 times with NaCI-solution. It was dried above Na2SO4, filtrated
and
concentrated.
CA 02628145 2008-05-01
IMTM GmbH 73
The obtained raw product was purified by crystallisation from a mixture of
ethy-
lacetate/petrolether.
468 mg (80 %) of the accordant Boc-protected compound were obtained. In
order to cleave the protecting groups 468 mg (0.8 mmol) of this compound were
dissolved in 1.2 ml (16 mmol) trifluoroacetic acid and 12 ml
methylenechloride.
The reaction was controlled by means of TLC and dry ether was added to the
reaction batch after the transformation was completed. The precipitating solid
was filtered and rinsed several times with ether.
Yield: 446 mg (91 %) II.
Scheme 12
HNS
0
II ~
Boc-NH-CH-COOH H2N-CH-C-N S
~~
SH2 2) TFA/CH2C12 SHZ
S S CH2 CHz 0
II
Boc-NH-CH-COOH HzN-CH-C-N~S
1 II
(b) Compound VI (scheme 13)
(i) Boc-Lys-thiazolidide 3
1.4 g (3 mmol) Boc-Lys(Fmoc)-OH 2 and 382 pl (3 mmol) 4-ethylmorpholin
were dissolved in 7 ml dry THF. The reaction batch was cooled to -15 C and at
this temperature 390 lal (3 mmol) chloroformic acid isobutyl ester were added.
CA 02628145 2008-05-01
IMTM GmbH 74
After a reaction time of 15 minutes at -15 C 284 pl (3.6 mmol) thiazolidine
were
added, the batch was agitated for another hour at -15 C and subsequently over
night at RT.
To complete the transformation half of the afore-mentioned amounts were again
activated cooling the batch to -15 C after the addition of 4-ethlymorpholine
and
chloroformic acid isobutyl ester and thiazolidine were added as described.
After
another 4 hours of reaction time at RT it was concentrated until completely
dried
and the residue was dissolved in ethylacetate.
The ethylacetate phase was rinsed 3 times with 5 % KHSO4-solution, once with
NaCI-solution, 3 times with saturated NaHCO3-solution and subsequently with
NaCI-solution until neutral.
After drying above Na2SO4 the ethylacetate phase was concentrated and the
residue was dissolved in 30 ml morpholine to cleave the Fmoc-protecting group.
After 1.5 h of reaction time at RT the morpholine was removed by distillation.
6
ml ice-cold methanol were added to the residue. The solution was filtered from
hardly soluble 4-fluoroene-9-ylmethylmorpholine and the solution was concen-
trated.
Yield: 875 mg (92 %) 3
(ii) Synthesis of VI
To the solution of 181 mg (0.5 mmol) 4 in 3 ml DMF gradually 240 mg (1.25
mmol) EDC were added at 0 C and subsequently 317 mg (1 mmol) 3 were
added.
CA 02628145 2008-05-01
IMTM GmbH 75
The reaction batch was agitated for 1 h at 0 C and subsequently 6 h at RT.
After the addition of another 120 mg (0.63 mmol) EDC and 6 hours of incuba-
tion time the DMF was removed by distillation and the residue was dissolved in
ethylacetate. The ethylacetate phase was washed 3 times with 5 % KHSO4-
solution, once with NaCI-solution, 3 times with saturated NaHCO3-solution and
subsequently with NaCI-solution until neutral.
After drying above Na2SO4 and removal of the ethylacetate by distillation 440
mg of the raw product remained.
Using chromatographic purification on silica gel with the eluent chloro-
form/methanol 92/8 the yield obtained was 250 mg (55 %) 8.
In order to the protecting groups 20 mg (0.022 mmol) 8 were dissolved in 220
pl
1 M HCI in pure acetic acid. After standing for 6 hours at RT dry ether was
added to the reaction batch and the precipitating product was filtered and
rinsed
with ether.
Yield: 15 mg (88 %) VI
CA 02628145 2008-05-01
IMTM GmbH 76
Scheme 13
Boc-NH-CH-COOH COOH
I
(CH2)4 ~
NH N HZN-CH-CO-N~s
Fmoc CO-NH-(CHz)4
2 S
S N
1) HN~S
~ I N
S
2) Morpholin
COOH HCI/AcOH
Boc-NH-CH-CO-NS 4 8 N~
(CH2)a
NH2 CO-NH-(CHZ)4
3 H2N-CH-CO-N~
VI
The compounds obtained were characterised by ESI-MS. The following table 3
presents 6 examples of compounds of the general formula (1) according to the
invention
Table 3
Examples for compounds of the general formulae A - B - D -B' - A' (1)
Y
NH2 p
X~ B~S~S~B N//--X
0 NH2 P
Y
CA 02628145 2008-05-01
IMTM GmbH 77
No. B x Y Empirical for- Molar ESI-MS
mula weight m/e
I -CH2- -CH2- H C14H26N402S2 346,5 347,2
II -CH2- S H C12H22N402S4 382,6 383,0
III -CH2- -CH2- CN C16H24N602S2 396,5 397,1
IV -(CH2)4-NH-iI - i H-CHZ- S H C24H46N804S4 638,9 639,2
O NH2
V-(CH2)4-NH-!! / \ S H C32H42N808S4 795,0 795,2
O
NOz
N
VI _(CH2)4-NH-C ~ ~ S H C30H42N804S4 707,0 707,2
O -
Example 2
Preparation of compounds of the general formula (2)
Compounds of the general formula (2) were prepared using the following proc-
ess:
(a) Compound VI! (scheme 14)
(i) Synthesis of 6
188 mg (0.5 mmol) Boc-Cys(Npys)-OH 5 were dissolved in 2.5 ml dry THF. Un-
der argon 40 pl (0.5 mmol) thiazolidine and gradually 115 mg (0.6 mmol) EDC
were added at 0 C. The reaction was tracked at RT by means of TLC and ter-
minated after 4 h. For this purpose the reaction batch was concentrated, the
CA 02628145 2008-05-01
IMTM GmbH 78
solid residue was dissolved in ethylacetate and the organic phase was rinsed
subsequently 3 times with 5 % KHSO4-solution, once with NaCI-solution, 3
times with saturated NaHCO3-solution and 3 times with NaCI-solution. After dry-
ing above Na2SO4 it was filtrated and the ethylacecate phase was concentrated.
Yield: 191 mg (86 %) 6
(ii) Synthesis of Vll
29 mg (0.065 mmol) 6 were dissolved in 5 mi ACN/phosphate buffer (1 : 1), pH
8Ø To this solution a solution of 18 mg (0.065 mmol) (3-aminothiol 7 (synthe-
sized in three steps starting from (S)-phenylalaninol according the "Fournier-
Zaluski, M.C.; Coric, P.; Turcaud, S.; Bruetschy, L., Lucas, E.; Noble, F.;
Roques, B.P. J. Med. Chem. 1992, 35, 1259-1266"; "Fournier-Zaluski, M.C.;
Coric, P.; Turcaud, S.; Lucas, E.; Noble, F.; Maldonado R.; Roques, B.P. J.
Med. Chem. 1992, 35, 2473-2481") in 5 ml ACN/phosphate buffer was added
under argon at RT. After a reaction time of 3 h (HPLC-control) the reaction
was
terminated by concentrating the reaction batch, dissolving the solid residue
in
ethylacetate and by rinsing this phase 3 times with NaCI-solution. It was
dried
above Na2SO4, filtrated and concentrated. The obtained raw product was chro-
matographically purified on silica gel with ethylacetate/petrolether. The
yield
was 20 mg (55 %) of the accordant Boc-protected compound. In order to cleave
the protecting groups 10 mg (0.018 mmol) of this compound was dissolved in
28 pl (0.36 mmol) trifluoroacetic acid and 280 pl methylenechloride. The reac-
tion was tracked by means of TLC and terminated by adding dry ether to the
reaction batch. The precipitating product was rinsed several times with ether.
Yield: 8 mg (76 %) VII.
CA 02628145 2008-05-01
IMTM GmbH 79
Scheme 14
HN~S
O
\
Boc-NH-CH-COOH Boc-NH-CH-C-N S
CH2 CHZ
S
OZN 02N
N N
6
1) HS-CH2-CH-CH2
NH
I
Boc 7
2) TFA/CH2CI2
O
II ~
H2N-CH-C- ~~
CH2
CHz
HZN-CH-CH2 0
VII
(b) Compound X (Scheme 15)
73 mg (0.08 mmol) 8 and 20 mg (0.075 mmol) P-aminothiol 7 were agitated in 3
ml methanol for 24 hours at RT. After the removal of the methanol by
distillation
the raw product was purified on silica gel with the eluent
ehylacetate/petrolether
95/5. The yield was 34 mg Boc-protected disulfide (63 %) which was dissolved
in order to cleave the protecting group in 750 pl methylenechloride and 75 pl
trifluoroacetic acid were added. After standing for 6 hours at RT dry ether
was
CA 02628145 2008-05-01
IMTM GmbH 80
added and the precipitating product was filtered and rinsed with ether. Yield:
29
mg (83 %) X.
Scheme 15
Boc-NH-CH-CO-N~S
I U
CO-NH-(CH2)4
N H2N-CH-CO-NS
~ I \-j
CO-NH-(CH2)4
S ~) 7 N
~ - I
2) TFA/CHZCI2
S
I
N-- S
CHZ-CH-CHz 0
CO-NH-(CH2)4 NH2
I
Z~
Boc-NH-CH-CO-NS
x
8
(c) Compound X! (Scheme 16)
(i) Synthesis of 10
190 mg (0.5 mmol) 3-(Fmoc-aminomethyl)-benzoe acid 9 and 64 NI (0.05 mmol)
4-ethylmorpholine were dissolved in 1 ml DMF and cooled under argon to -15
C. To this solution 65 pl (0.5 mmol) chloroformic acid isobutyl ester was
added,
the solution was agitated for 20 min at -15 C and subsequently a solution of
159 mg (0.5 mmol) Boc-Lys-thiazolidide 3 in 1.25 ml DMF was added and the
solution was agitated for 1 h at -15 C.
CA 02628145 2008-05-01
IMTM GmbH 81
After 20 h at RT 32 pl (0.25 mmol) 4-ethyl-morpholine and 33 NI (0.25 mmol)
chloroformic acid isobutyl ester were again added at -15 C for another activa-
tion.
The DMF was distilled after 40 h (TLC-control) and dissolved in ethylacetate.
The ethylacetate phase was rinsed 3 times with 5 % KHSO4-solution, once with
NaCI-solution, 3 times with saturated NaHCO3-solution, once with NaCI-
solution, 3 times with saturated NaHCO3-solution and 3 times with NaCI-
solution. It was dried above Na2SO4, filtered and subsequently concentrated.
The obtained raw product was purified chromatographically on silica gel with
ethylacetate.
The yield was 174 mg (52 %) of the according Fmoc-protected compound. In
order to cleave the protecting groups 150 mg (0.22 mmol) of this compound
was dissolved in 2.2 ml morpholine and after 2 h the excessive morpholine was
removed by distillation. The residue was dissolved in 1 ml ice-cold methanol,
filtrated from the insoluble 4-fluorene-9-ylmethylmorpholine and concentrated.
Yield: 92 mg (93 %) 10
(ii) Synthesis of 11
78 mg (0.21 mmol) Boc-Cys(Npys)-OH 5 and 27 NI (0.21 mmol) 4-ethyl-
morpholine were dissolved in 0.4 ml THF and cooled under argon to -15 C. To
this solution 28 pl (0.21 mmol) chloroformic acid isobutyl ester were added,
it
was agitated for 20 min at -15 C at subsequently a solution of 95 mg (0.21
mmol) 10 in 0.75 ml THF were added. After 1 h at -15 C and 20 h at RT 13 pl
4-ethylmorpholine (0.10 mmol) and 13 pl (0.10 mmol) chloroformic acid isobutyl
ester were added at -15 C for another activation step.
CA 02628145 2008-05-01
IMTM GmbH 82
The THF was removed after 24 h (TLC-control) by distillation and the reaction
batch was processed as in case of compound 10.
The obtained raw product was subsequently purified chromatographically on
silica gel with methanol/chloroform. Yield: 100 mg (60 %) 11.
(iii) Synthesis of XI
67 mg (0.08 mmol) 11 were dissolved in 8 mg acetonitrile/phosphate buffer (1 :
1), pH 8Ø A solution of 21 mg (0.08 mmol) 7 in 8 ml acetonitrile/phosphate
buffer was added under argon at RT. The reaction was tracked by means of
HPLC and the reaction batch was concentrated after terminated reaction. The
solid residue was dissolved in ethylacetate and rinsed 3 times with NaCI-
solution, dried above Na2SO4, filtered and concentrated. The raw product was
subsequently purified chromatographically on silica gel with ethylace-
tate/petrolether. The yield was 29 mg (40 %) of the according Boc-protected
compound. In order to cleave the protecting group 22 mg (0.024 mmol) of this
compound were dissolved in 55 pl trifluoroacetic acid and 550 pl methyle-
nechloride. The reaction was checked by means of TLC and ether was added to
the reaction batch after the termination of the cleavage. The precipitated
prod-
uct was filtered and rinsed several times with ether. Yield: 16 mg (70%) XI.
CA 02628145 2008-05-01
IMTM GmbH 83
Scheme 16
CH2-NH-Fmoc
1) HOOC O
~ ~ 9 ~ n
Boc-NH-CH-C-N S Boc-NH-CH-C-N S
(CH~4 \~ 2) Morpholin (CH~4 ~~ CH2-NH2
NHZ NHCO
3 10
Boc-NH-CH-COOH
I
CHz
S
O2N
1) 7
~ ~ 2) TFA/CH2CI2 0
~
HzN- i H-C-N~~ Boc-NH- i H-C- ~
(CH2)a (CHz)a
NH NH
I I
CO CO
\ \ ~
CH2 CH2
NH NH
CO CO
CH-NH2 CH-NH-Boc
CH2-S-S-CHZ-CH-CH2 0 CHZ
NH2
XI 02N N
11
(d) Compound XIII (Scheme 17)
CA 02628145 2008-05-01
IMTM GmbH 84
(i) Synthesis of 13
2.5 g (5 mmol) Boc-p-Amino-Phe(Fmoc)-OH 12 were dissolved in 25 ml dry
THF. Under argon 394 lal (5 mmol) thiazolidine were added at 0 C and gradu-
ally 1.15 g (6 mmol) EDC. The reaction was checked by means of TLC at RT.
After 6 h 197 NI (2.5 mmol) thiazolidine and 954 mg (5 mmol) EDC were added
at 0 C for another activation step. The reaction was terminated after another
2
h at RT by concentrating the reaction batch dissolving the solid residue in
ethy-
lacetate and by rinsing the organic phase 3 times with 5 % KHSO4-solution,
once with NaCI-solution, 3 times with saturated NaHCO3-solution, 3 times with
NaCI-solution. Subsequently it was dried above Na2SO4, filtered and concen-
trated.
The yield was 2.58 g (90 %) of the corresponding Fmoc-protected compound
which was dissolved in 45 ml morpholine in order to cleave the protecting
group. After 2 h at RT the excessive morpholine was removed by distillation
and
the residue was dissolved in 6 ml ice-cold methanol. The insoluble 4-fluorene-
9-
ylmethyl-morpholine was filtered off and the solution was concentrated.
Yield: 1.23 g (78%) 13
(ii) Synthesis of 14
375 mg (1 mmol) 5 and 127 NI (1 mmol) 4-ethylmorpholine were dissolved in 3
ml dry THF and under argon cooled to -15 C. To this solution 131 pl (1 mmol)
chloroformic acid disobutyl ester were added and after 20 min at -15 C a solu-
tion of 351 mg (1 mmol) 13 in 0.5 ml THF was added. After 1 h at -15 C and 20
h at RT (TLC-control) 64 pl (0.5 mmol) 4-ethylmorpholine and 66 pl (0.5 mmol)
chloroformic acid isobutyl ester were added at -15 C for another activation
step. The reaction was terminated after another 2 h by concentrating the reac-
CA 02628145 2008-05-01
IMTM GmbH 85
tion batch, dissolving the solid residue in ethylacetate and by processing an
ethylacetate phase as described in the context of compound 13.
The obtained raw product was purified chromatographically on silica gel with
ethylacetate. Yield: 350 mg (50%) 14.
(iii) Synthesis of XIII
65 mg (0.09 mmol) 14 were dissolved in 9 ml acetonitrile/phosphate buffer (1 :
1), pH 8Ø Under argon a solution of 25 mg (0.09 mmol) 7 in 9 ml acetoni-
trile/phosphate buffer were added at RT. The reaction was tracked by means of
HPLC and the reaction batch was processed after approx. 3 h after the concen-
tration the solid residue was dissolved in ethylester and the organic phase
was
subsequently rinsed 3 times with NaCI-solution. It was dried above Na2SO4,
filtrated and concentrated. The raw product was purified chromatographically
on
silica gel with ethylacetate/petrolether (60 : 40). The yield was 35 mg (47 %)
of
the corresponding Boc-protected compound. In order to cleave the protecting
groups 30 mg of this compound were dissolved in 84 NI (1.1 mmol) trifluoroace-
tic acid and 840 NI methylenechloride. The reaction was controlled by means of
TLC. After completion dry ether was added to the product and the precipitating
solid was rinsed with ether several times. Yield: 22.5 mg (70 %) XIII.
The following table 4 presents 7 examples of the compounds of the general for-
mula (2) according the invention.
CA 02628145 2008-05-01
IMTM GmbH 86
Scheme 17
1) HN~S 0
~~ I I
v
Boc-NH-CH-COOH Boc-NH-CH-C-N S
CH2 2) Morpholin CH2
\ \
NH NH2
Fmoc
12 13
1) 7
0 2) TFA/CH2CI2 0
II ~ E Boc-NH-CH-CN~S
H2N-CH-C-N S
CHZ Li CHz Li
\ \
NH NH
I
CO CO
I
CH-NH2 CH-NH-Boc
CHZ-S-S-CHZ-CH-CH2 CH2
NH2 - S
Xlii
02N
N
14
CA 02628145 2008-05-01
IMTM GmbH 87
Table 4
Examples for compounds of the general formulae A - B - D - E (2)
Y
NH2
r-~N Yl~ B/SS R 9
--,-y
0 NH2
s Empirical Molar ESI-
formula B R X Y formula weight MS
[mle]
VII -CH2 -CH2 / \ S H C15H23N30S3 357,6 358,0
CH3
VI I I -CHZ / \ S H C17H27N3OS3 385,6 386,0
-
CH3
IX _ CH NH-C / \ -CH2 / \ S H C25H33N5O4Sg 563,8 564,1
( 2)4- II _
O
N02
N
X-(CH2)4-NH-C -CH2 0 S H C24H33N502S3 519,8 520,1
O
XI _(CH~q-NH-C
~\ / -CH2 / \ S H C29H42N603S3 618,9 619,2
CH2-NH- II -CH-CH~ -
O NH2
XII _(CH~,4-NH-II ~ ~ NH- li -CH-CH~ 2 ~ ~
-CH S H C28H4pN603S3 604,8 605,2
O O NH2 -
XII I- CH2 \ / NH C CH CH2 _CH2 / \ S H C24H33N502S3 519,8 520,2
11 1
O NH2 -
CA 02628145 2008-05-01
IMTM GmbH 88
Dual inhibition of APN and DPIV after release of the inhibitors of prodrugs
of the general formulae (1) and (2)
Eight compounds were selected from the compounds prepared in the afore-
mentioned examples 1 and 2 (compounds I and II of the general formulae (1)
and (2) and compounds VII, VIII, X, XI, XII and XIII of the general formulae
(1)
and (2). The mentioned compounds were treated reductively with dithiothreitol
(DTT) using the following process to release the inhibitors from the prodrugs.
Process of the reduction with DTT:
Same volumes of the 7- 10"2 M solution of the inhibitor in water or DMF and a
0.35 M aqueous DTT-solution (5 equivalents) were agitated 4 hours at RT.
Subsequently to the reductive treatment of the prodrugs the IC50-values were
estimated using the following process:
Determination of the IC50-values after reduction:
Dipeptidyl peptidase IV from pig kidney (EC 3.4.14.5.), Iyophilisated powder
(Sigma).
Substrate: Ala-Pro-AMC, [S] = 3- 10"5 M (2 Km) in the reaction batch.
Buffer: 40 mM Tris/HCI-buffer, pH 7.6, I= 0.125 in the reaction batch.
Leucin aminopeptidase, microsomal, from pig kidney (EC 3.4.11.2).
Suspension in 3.5 M (NH4)2SO4-solution (Sigma).
Substrate: Leu-AMC, [S] = 1.5 - 10-4 M (2 Km) in the reaction batch.
Buffer: 40 mM Tris/HCI-buffer, pH 7.2 in the reaction batch.
CA 02628145 2008-05-01
IMTM GmbH 89
Procedure:
By dilution with water 10 to 12 solutions with defined inhibitor concentration
were prepared from the reduction batch. These were selected from a relevant
concentration range, i.e. between complete inhibition and non-influenced en-
zyme activity.
60 pl of these inhibitor solutions each or water (standard), were incubated
with
80 NI of the according diluted enzymes in buffer for 15 minutes at 30 C and
subsequently 60 pl of the according AMC-substrate in water (total volume 200
pl) were added. The release of the 7-Amino-4-methyl-cumarin was traced con-
tinuously over a period of 20 minutes at 30 C using a fluorence-pladt-reader
NOVOstar (BMG Labtechnologies). The enzyme concentration was chosen to
give a linear increase of the fluorescence during the measuring period.
The excitation- and emission-wavelengths, respectively, were 390 and 460 nm.
The measurements were performed in duplicates. The turn-over rates (increase
of fluorescence/minutes) were plotted against the concentration of the
inhibitor
and the IC50-values were calculated using the computer program GraFit.
For comparison the IC50-values of established inhibitor against DPIV (H-
Lys[Z(N02)]-thiazolidide) and APN (actinonine) were estimated.
The results can be derived from table 5.
CA 02628145 2008-05-01
IMTM GmbH 90
Table 5
Dual inhibition of APN and DP IV after the release (reduction) of the inhibi-
tors from the prodrugs
Compound APN (IC 50 [M]) DP IV (IC 50 [M])
1,84 - 10-4 2,25. 10"5
II 1,08. 10"4 8,07. 10-6
VI I 1,66 = 10"' 1,18 10"5
V(II 1,08. 10-' 4,51 = 10-6
X 1,33 = 10"' 1,25 = 10"'
X1 1, 90 = 10"' 2, 80 = 10-'
XII 2,07 = 10-' 2,56 = 10"'
XI I I 2,40 = 10-' 7,19 = 10"6
Lys[Z(N02)]- 4,34 = 10"'
thiazolidide
Actinonine 3,02 = 10-'
Example 3
Inhibition of phytohaemaglutinine-induced proliferation of mononuclear
cells (MNZ) of healthy donors by compounds V to XIII (tables 3 and 4)
MNZ were isolated from the peripheral blood of healthy donors by density gra-
dient centrifugation and stimulated in serum-free medium (AIMV) with 1 pg/mi
phytohaemaglutinine (PHA). 5 x 104 MNZ each were incubated in the presence
CA 02628145 2008-05-01
iMTM GmbH 91
of different inhibitor concentrations (triple measurements) at 37 C and 5%
CO2
in micro test plates for 48 hours. As controls unstimulated cells as well as
PHA-
stimulated MNZ without inhibitor were cultivated under identical conditions.
The
proliferation rate of the cells was determined by the incorporation of bro-
modesoxyuridine in newly synthesized DNA (Biotrak-Assay, GE Healthcare).
The curves shown in figure 1 each represent accumulated data (relative values
based on PHA-stimulated cells in absence of inhibitor) of at least three
different
donors.
Example 4
Therapeutic effect of compound VII (table 4) in the mouse model of dex-
tranesulfate-induced colitis
The therapeutic potential of the substance(s) was/were tested in an
established
chemical induced model of colitis. In this model an inflammatory reaction is
in-
duced in the colon of the animals by dextranesulfate-sodium (DSS) which histo-
logical pattern is similar to the human form of chronic inflammatory colon dis-
ease (acute push) called colitis ulcerosa. The extent of the inflammatory reac-
tion is dependent on the DSS-concentration. The severity of the illness is
meas-
ured using an established scoring system in which changes of the stool consis-
tence the detection of blood in faeces as well as the extent of the loss of
body
weight are being taken into consideration and are valued by scores.
By the application of the inflammatory DSS during the complete duration of the
experiments the illness permanently progressed. From a score of 10 a poten-
tially deadly condition of the illness had to be assumed. The maximum reach-
able therapeutic success was to stop the progression of the inflammatory reac-
tion.
CA 02628145 2008-05-01
IMTM GmbH 92
Female Balb/c-mice (8 weeks old) having an average weight of 20 g were fed 3
%(w/v) dextranesulfate-sodium with drinking water (ad libitum) during the com-
plete duration of the experiment. After 2 to 3 days colitis-like symptoms
(haem-
orrhagic diarrhoea and loss of body weight) were detectable with all animals.
12
animals each received with day 3 of the experiment daily each 100 pg of sub-
stance VII dissolved in physiological salt solution (PBS) or the according
amount of solvent (PBS) = placebo-control) by intraperitoneal injection.
In the graph of figure 2 the average severity (score) of those mice treated
with
substance VII is presented compared to placebo treated animals. The average
scores of those mice treated with substance VII was significantly lower on
days
to 7 of the experiment as those of the placebo treated animals. At the end of
the experiment those animals treated with substance VII reached on average
approximately half the score of the control animals.
Example 5
Therapeutic success of several compounds of the general formulae (1)
and (2) given in table 3 and 4 in the mouse model of DSS-induced colitis
In figure 3 the therapeutic potential of various exemplary substances on day 7
of the experiment (end of experiment) is shown for the mouse model of DSS-
induced colitis. The colitis activity score was determined according to the
method described in example 4.