Note: Descriptions are shown in the official language in which they were submitted.
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
METHOD OF OPERATING A FUEL CELL STACK
AT LOW PRESSURE AND LOW POWER CONDITIONS
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a method of operating fuel cell stacks, in
particular, operating solid polymer fuel cell stacks under low pressure and
low power
operating conditions.
Description of the Related Art
Electrochemical fuel cells convert fuel and oxidant into electricity. Solid
polymer electrochemical fuel cells generally employ a membrane electrode
assembly
that includes an ion exchange membrane or solid polymer electrolyte disposed
between
two electrodes typically comprising a layer of porous, electrically conductive
sheet
material, such as carbon fiber paper or carbon cloth. The membrane electrode
assembly
comprises a layer of catalyst, typically in the form of finely comminuted
platinum, at
each membrane electrode interface to induce the desired electrochemical
reaction. In
operation, the electrodes are electrically coupled for conducting electrons
between the
electrodes through an external circuit. Typically, a number of membrane
electrode
assemblies are electrically coupled in series to form a fuel cell stack having
a desired
power output.
The membrane electrode assembly is typically interposed between two
electrically conductive bipolar flow field plates or separator plates wherein
the bipolar
flow field plates may comprise polymeric, carbonaceous, graphitic, or metallic
materials. These bipolar flow field plates act as current collectors, provide
support for
the electrodes, and provide passages for the reactants and products. Such
bipolar flow
field plates may comprise flow fields to direct the flow of the fuel and
oxidant reactant
fluids to the anode and cathode electrodes of the MEA, respectively, and to
remove
excess reactant gases and reaction products, such as water formed during fuel
cell
operation.
1
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
A certain amount of pressure is typically needed to deliver reactant
fluids to the fuel cell and to run other fuel cell system components, all of
which governs
the operating pressure of the fuel cell. Thus, during fuel cell stack
operation, the
reactant streams are typically pressurized to an operating pressure by means
of a
compressor, pump, blower, fan, or the like. In most cases, a lower operating
pressure is
desirable in order to lower the amount of parasitic power that is needed to
pressurize the
anode and cathode reactant streams. Specifically, a high efficiency blower or
fan is
desirable to pressurize the reactant streams because it consumes a lower
amount of
parasitic power than compressors and pumps. However, most commercially
available
blowers and fans pressurize the reactants to significantly lower pressures
than
conventional fuel cells, for example, up to 0.21 barg, thereby undesirably
limiting the.
highest operating pressure.
During regular fuel cell operation, water is produced at the cathode,
which may condense into water droplets within the catalyst layer, within the
gas
diffusion layer, in the reactant flow fields, or surfaces thereof. An excess
of water
droplets is undesirable because the water droplets contribute to unstable
performance
(for example, water "flooding" in the anode and/or cathode), and may cause non-
uniform reactant fluid flow and reactant starvation.
The most common approaches to solving this problem are to increase the
pressure drop of the flow fields, supply a greater amount of reactant fluid
than
stoichiometrically required, operate at a higher operating pressure, and/or
increase the
operating temperature.
For example, the anode and cathode flow field geometry may be
designed to have a high pressure drop to passively enhance the flow of
reactant fluids
through the fuel cell and the removal of reactant product fluids, for example,
water, out
of the fuel cell. However, this results in an increase in the operating
pressure to
compensate for the pressure drop of the flow fields, thus increasing parasitic
power
consumption and decreasing fuel efficiency. Thus, pressure drop in the flow
fields is
usually minimized, for example, to as low as 150 mbar, particularly for low
pressure
fuel cells. However, when operating low pressure fuel cells at low power,
unstable fuel
cell performance is often observed because a low amount of reactants.are
delivered to
2
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
the fuel cells, thereby -resulting in a reactant flow velocity that is
inadequate to clear
excessive liquid water in the flow fields of the low pressure fuel cell,
particularly in the
anode flow fields wherein the stoichiometry is typically minimized to maximize
fuel
efficiency.
Alternatively, water may be removed by supplying an excess of reactants
to the anode and cathode flow fields, increasing the reactant pressure, or
increasing the
operating temperature. The former methods remove water droplets by inducing a
shearing force to get rid of excess liquid water in the cathode while the
latter method
removes water droplets by evaporating the liquid water in the fuel cell.
However, all of
these methods are also undesirable because they increase parasitic power
consumption
and decrease fuel efficiency.
One method of clearing excess water in the flow fields is described in
Published U.S. Pat. Appl. No. 2004/0137293, wherein a fuel cell system and its
control
method is capable of removing condensed water only from a place where flooding
is
generated. Heating means is arranged on a separator and its switch is turned
on when
the moisture for hydration of an electrolyte membrane is condensed, so that
current is
supplied to the heating means from a power source so as to evaporate the
*condensed
water. Heating means is provided on at least one of the separators, and
actuation and
de-actuation of the heating means is controlled according to the state of the
fuel cell.
However, power consumption is increased and fuel efficiency is decreased in
order to
run other system components to evaporate or remove excess liquid water.
Another method is described in Published PCT No. WO 2004/107839
wherein the temperatures of cathodes of fuel cells are maintained sufficiently
above the
temperatures of corresponding anodes either before, during, or after cold
starts and after
freeze-thaw cycles to cause migration of water from cathodes to anodes,
thereby
imposing a temperature differential between the electrodes by warming the
cathode side
or cooling the anode side, so as to influence the flow of water, causing it to
be more
toward the anode, to preserve or restore performance. Temperatures may be
controlled
by heaters or by heating process air, by flowing air through just the anode to
be cooled
by vaporization, by flowing air through both anodes and the cathodes, the
cathode's air
being warmer, or by bleeding H2 into cathodes momentarily. Again, these
methods
3
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
consume extra parasitic power in order to prevent water from excessively
flooding the
cathode or to evaporate excess water, thereby preventing/recovering fuel cell
performance loss due to cathode flooding.
During low power operation (for example, at a current density of less
than 0.5A/cm2), water produced at the cathode may migrate to the anode due to
a water
vapor pressure differential between the fuel stream and the oxidant stream in
the anode
and cathode flow fields, respectively, thus resulting in anode flooding. Anode
flooding
is difficult to mitigate because fuel is usually supplied at a stoichiometry
that is as low
as possible in order to maximize fuel efficiency while sustaining the required
power
generation. Furthermore, when running at low pressure (for example, in cases
when
blowers and/or fans are used to deliver the reactants to the fuel cell stack),
the reactant
flow velocity is insufficient to remove the condensed water vapor in the anode
flow
fields, thereby increasing performance instability.
Accordingly, there remains a need in the art to minimize unstable
performance of a fuel cell stack operating at low pressure and low power
conditions.
The present invention fulfills this need and provides further advantages.
BRIEF SUMMARY OF THE INVENTION
In brief, a method is provided for operating a low pressure drop fuel cell
stack with improved performance stability at low pressure and low power
operating
conditions, wherein each fuel cell of the fuel cell stack comprises an anode
flow field
plate, a cathode flow field plate and a membrane electrode assembly, such that
during
low pressure and low power operation, the cathode flow field plate of each
fuel cell has
a higher heat transfer rate than the anode flow field plate of the same fuel
cell.
In the practice of operating a low pressure drop fuel cell stack
comprising a plurality of fuel cells wherein during low pressure and low power
operation, a heat transfer rate of a cathode flow field plate of each fuel
cell is greater
than the heat transfer rate of an anode flow field plate of the same fuel
cell. During fuel
cell stack operation, the reactants are delivered to the anode flow field
plate and the
cathode flow field plate of each fuel cell by means of a blower or fan.
4
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
To create a higher heat transfer rate in the cathode flow field plate than
the heat transfer rate in the anode flow field plate, in one embodiment, the
cathode flow
field plate material has a higher thermal conductivity than the anode flow
field plate
material, for example, by using different materials for the anode and cathode
flow field
plates. Thus, during low pressure and power operation of the low pressure drop
fuel
cell stack, heat rejection from the reactant in the cathode flow fields of
each fuel cell is
higher than heat rejection from the reactant in the anode flow fields of the
same fuel
cell, thereby keeping the cathode of each fuel cell warmer than the anode of
the same
fuel cell.
- In another embodiment, wherein at least one of the anode and cathode
flow field plates comprises coolant flow fields, the web thickness of the
anode flow
field plate is made greater than the web thickness of the cathode flow field
plate to
ensure a greater heat transfer rate in the cathode flow field plate than in
the anode flow
field plate. In other words, the distance of the bottom of the anode flow
fields to the
parallel edge of the coolant flow fields is greater than the distance of the
bottom of the
cathode flow fields to the opposite edge of the coolant flow fields of the
bipolar flow
field plate.
These and other aspects of the invention will be evident in view of the
attached figures and following detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
In the figures, identical reference numbers identify similar elements or
acts. The sizes and relative positions of elements in the figures are not
necessarily
drawn to scale. For example, the shapes of various elements and angles are not
drawn
to scale, and some of these elements are arbitrarily enlarged and positioned
to improve
figure legibility. Further, the particular shapes of the elements, as drawn,
are not
intended to convey any information regarding the actual shape of the
particular
elements, and have been solely selected for ease of recognition in the
figures.
Figure 1 is a cross-sectional diagram of a bipolar flow field plate with
low pressure drop anode and cathode flow fields.
5
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
Figure 2 is a cross-sectional diagram of a low pressure drop fuel cell and
fuel cell stack.
Figure 3 is a cell voltage vs. cell position diagram showing the average
performance of each cell in a first 10-cell stack using a first set of anode
flow field
plates.
Figure 4 is a cell voltage vs. cell position diagram showing the average
performance of each cell in a second 10-cell stack using a second set of anode
flow
field plates.
DETAILED DESCRIPTION OF THE INVENTION
In the following description, certain specific details are set forth in order
to provide a thorough understanding of various embodiments of the invention.
However, one skilled in the art will understand that the invention may be
practiced
without these details. In other instances, well-known structures associated
with fuel
cells, fuel cell stacks, and/or fuel cell systems have not been shown or
described in
detail to avoid unnecessarily obscuring descriptions of the embodiments of the
invention.
Unless the context requires otherwise, throughout the specification and
claims which follow, the word "comprise" and variations thereof, such as
"comprises"
and "comprising" are to be construed in an open, inclusive sense, that is as
"including
but not limited to".
Reference throughout this specification to "one embodiment" or "an
embodiment" means that a particular feature, structure or characteristic
described in
connection with the embodiment is included in at least one embodiment of the
present
invention. Thus, the appearances of the phrases "in one embodiment" or "in an
embodiment" in various places throughout this specification are not
necessarily all
referring to the same embodiment. Furthermore, the particular features,
structures, or
characteristics may be combined in any suitable manner in one or more
embodiments.
The invention is characterized by a method of operating a fuel cell stack
at a low pressure (for example, less than 0.21 barg) and low power (for
example, less
than 0.5 A/cmz), wherein the performance of each fuel cell in the fuel cell
stack is
6
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
stabilized by establishing different heat transfer rates for the anode and
cathode flow
field plates of each fuel cell, such that the heat transfer rate of the
cathode flow field
plate of each fuel cell is greater than the heat transfer rate of the anode
flow field plate
of the same fuel cell.
Figure 1 shows the cross-section of an exemplary bipolar flow field plate
2 comprising anode flow field plate 4 with anode flow fields 8 on a first
surface 10 and
cathode flow field plate 6 with cathode flow fields 12 on a first surface 14.
To facilitate
the low pressure operation, anode flow fields 8 and cathode flow fields 12
should have
a low pressure drop, preferably less than 150 mbar. Anode flow field plate 4
and
cathode flow field plate 6 may be adhesively joined together around a
peripheral edge
thereof (not shown) such that a second surface 18 of anode flow field plate 4
faces and
contacts second surface 20 of cathode flow field plate 6. Alternatively, anode
flow field
plate 4 and cathode flow field plate 6 are not adhesively joined together.
Furthermore,
at least one of the second surfaces of the anode flow field plate and the
cathode flow
field plate may further comprise at least one coolant flow field. At least one
coolant
flow field for circulating a coolant fluid is typically employed for fuel
cells to remove
heat from the reactants in the anode and cathode flow fields, thereby keeping
the fuel
cell at an optimum operating temperature. In addition, the coolant fluid helps
distribute
heat evenly throughout the fuel cell to prevent hot spots from forming
therein, which
may damage components of the membrane electrode assembly (hereinafter referred
to
as "MEA"). For example, in Figure 1, cathode flow field plate 6 further
comprises
coolant flow fields 22 on a second surface 20 of cathode flow field plate 6.
In another
alternative, the second surfaces of both the anode and cathode flow field
plates may
comprise coolant channels (not shown).
The heat transfer rate of cathode flow field plate 6 is preferably greater
than the heat transfer rate of anode flow field plate 4 during operation. For
example,
during low pressure and low power operation, oxidant is delivered to cathode
flow
fields 12 by means of a blower or fan (not shown), fuel is delivered to anode
flow fields
8 by means of a blower or fan (not shown) (which may optionally go through a
reformer prior to entering the anode flow fields 8), and a coolant fluid is
flowing in
coolant flow fields 22. The amount of heat conducted or removed from the
oxidant in
7
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
cathode flow fields 12 to the coolant fluid in coolant flow fields 22 is
greater than the
amount of heat conducted or removed from the fuel in anode flow fields 8 to
the coolant
fluid in coolant flow fields 22. Thus, if the reactants are supplied at the
same
temperature to the anode and cathode flow fields, the fuel will be at a higher
temperature than the oxidant.
As mentioned before, creating different heat transfer rates for the anode
and cathode flow field plate of each fuel cell is of particular importance for
low
pressure and low power operation, for example, less than about 0.21 barg and
less than
about 0.5 A/cm2, respectively, in order to reduce parasitic power loss and to
increase
fuel efficiency. Preferably, only the stoichiometrically required amount of
fuel is
supplied to the anode flow fields because this reduces the amount of power
needed to
run the blowers and/or fans that deliver the fuel and improves fuel
utilization, thereby
improving fuel efficiency. However, this approach minimizes the amount of fuel
being
delivered to the anode flow fields, particularly if the fuel is 100% hydrogen
and the
temperature of the fuel cell is not particularly high (because voltage is high
at low
power), which may cause an inadequate flow velocity in the anode flow fields
and too
low of a fuel cell temperature to sufficiently remove water that has migrated
from the
cathode to the anode. At low pressure and low power operating conditions, it
is
preferable to keep water at the cathode because a relatively higher amount of
oxidant is
supplied to the cathode when using air as the oxidant (because air only
comprises 21%
oxygen), which means that the amount of oxidant being delivered to the cathode
flow
fields is significantly higher than the amount of fuel being delivered to the
anode flow
fields, and is also supplied at a significantly higher reactant flow velocity
than the fuel.
One way of achieving different heat transfer rates for the anode flow
field plate and the cathode flow field plate of each fuel cell during low
pressure and low
power operation is to use materials of different thermal conductivities for
each plate,
such that the thermal conductivity of cathode flow field plate 6 is greater
than the
thermal conductivity of anode flow field plate 4. For example, various blends
of
materials, such as carbon, graphite, metal and/or polymer, may be used to
obtain the
desired thermal conductivity of the anode and cathode flow field plates. In
one
example, different resins may be used for each of the flow field plates to
vary its
8
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
thermal conductivity. Alternatively, layered plate structures with different
materials for
each layer of the plate may also be used to control the thermal conductivity
and/or heat
transfer rate of each flow field plate during operation, such as incorporating
a metallic
layer to increase thermal conductivity or incorporating a relatively thermally
insulating
layer to decrease thermal conductivity, or using metallic plates with
different coatings
for the anode and cathode flow field plates. In another alternative, the
amount of resin
on one surface of the plate may be higher than an opposite surface of the flow
field
plate. In a further alternative, materials with anisotropic thermal properties
may also be
used as part of the layered. structure to obtain and/or control the desired
heat transfer
rate of each flow field plate. One example of an anisotropic material is
expanded
graphite; its in-plane thermal conductivity is orders of magnitude greater
than its
through-plane thermal conductivity. One of ordinary skill in the art will
recognize that
although different materials and blends thereof may be used for the anode and
cathode
flow field plates, the coefficient of thermal expansion for each of the plate
materials
should not be so different as to create large thermal stresses in the bipolar
flow field
plate and the fuel cells.
Another way of achieving different heat transfer rates for the anode and
cathode flow field plate of each fuel cell during low pressure and low power
operation
is to control their web thicknesses. The web thickness is defined as the cross-
sectional
distance of the bottom of a reactant flow field on the first surface of the
flow field plate
to the opposing second surface of the same flow field plate. In the case where
the
opposing second surface further comprises coolant flow fields, the web
thickness is the
distance from the bottom of the reactant fluid flow field to a bottom of the
coolant flow
field.
For example, in Figure 1, anode plate web thickness 34 of anode flow
field plate 4 is the distance from surface 36 of anode flow field 8 to second
surface 18
of anode flow field plate 4. Similarly, for cathode flow field plate 6,
cathode plate web
thickness 35 is the distance from surface 38 of cathode flow field 12 to
surface 40 of
coolant flow field 22. A bipolar flow field plate 2 is formed by contacting
second
surface 18 of anode flow field plate 4 with second surface 20 of cathode flow
field plate
8.
9
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
An embodiment of the present method is discussed in reference to Figure
2. Figure 2 shows fuel cell stack 42 comprising two fuel cells 30 and 30-1.
Fuel cell 30
comprises anode flow field plate 4, cathode flow field plate 6 and MEA 32,
wherein
MEA 32 comprises anode electrode 24, cathode electrode 26, and membrane 28,
and
further-comprising anode flow fields 8, cathode flow fields 12, and coolant
flow fields
22. Adjacent fuel cell 30-1 similarly comprises anode flow field plate 4-1,
cathode flow
field plate 6-1 and MEA 32-1 having anode electrode 24-1, cathode electrode 26-
1, and
membrane 28-1, and further comprising anode flow fields 8-1, cathode flow
fields 12-1,
and coolant flow fields 22-1.
During low pressure and low power operation of this fuel cell stack, a
coolant fluid is circulated in coolant flow fields 22 on second surface 20 of
cathode
flow field plate 6, the coolant fluid being in contact with the second surface
18-1 of
anode flow field plate 4-1 of fuel cell 30-1 to evenly remove and/or
distribute heat
within fuel cells 30 and 30-1. The temperature of the reactant fluid in anode
flow fields
8-1 in contact with anode electrode 24-1 is different than the temperature of
the reactant
fluid in cathode flow fields 12 in contact with cathode electrode 26 due to
the difference
in relative plate web thicknesses (e.g., anode web thickness 34 is great than
cathode
web thickness 35). Preferably, the reactant fluid in the anode flow fields is
maintained
at a higher temperature than the reactant fluid in the cathode flow fields in
order to
reject more heat from the reactant in the cathode flow field than from the
reactant to the
anode flow field. This encourages water vapor condensation in cathode flow
fields and
minimizes water back-diffusion from the cathode of each fuel cell to the anode
of the
same fuel cell during low pressure and low power operation, thereby reducing
anode
flooding. The heat transfer rate of the cathode flow field plate is greater
than the heat
transfer rate of the adjacent anode flow field plate by the means previously
described
and/or other methods known in the art for passively inducing different heat
transfer
rates for the anode and the cathode flow field plates.
Referring to Figure 2, fuel cell stack 42 may be formed by stacking fuel
cell 30 next to an adjacent fuel cell 30-1 such that second surface 20 of
cathode flow
field plate 6 of fuel cell 30 is in contact with second surface 18-1 of anode
flow field
plate 4-1 of adjacent fuel cell 30-1. In fuel cell stack 42, coolant flow
fields 22 and 22-
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
1 are formed on the second surface of cathode flow field plates 6 and 6-1,
respectively.
For example, cathode flow field plate 6 of fuel cell 30 comprises coolant flow
fields 22
so that a coolant fluid may flow between cathode flow field plate 6 of fuel
cell 30 and
anode flow field plate 4-1 of adjacent fuel cell 30-1. Again, during low
pressure and
low power operation, the heat transfer rate of the cathode flow field plate of
each fuel
cell is greater than the heat transfer rate of the adjacent anode flow field
plate of an
adjacent fuel cell, as discussed above.
In fuel cell stack 42, one or both of the second surfaces of anode flow
field plates 4 and 4-1 and cathode flow field plates 6 and 6-1 may comprise
coolant
flow fields 22 and 22-1, respectively. Alternatively, no coolant flow fields
may be
present on the second surface of either anode flow field plate 4 or cathode
flow field
plate 6. Instead, bipolar flow field plate 2 further comprises an additional
coolant plate
disposed between second surface 18-1 of anode flow field plate 4-1 and second
surface
of cathode flow field plate 6, and coolant flow fields are formed on the
coolant plate
15 (not shown). In one embodiment, the coolant flow field plate may be such
that during
low pressure and low power operation, it produces a higher heat transfer rate
in the
cathode flow field plate than the anode flow field plate (e.g., the amount of
heat
removed from the cathode flow field plate is greater than the amount of heat
removed
from the anode flow field plate) by using different materials with different
thermal
20 conductivities and/or by orienting the coolant flow fields such that they
are closer to the
cathode flow fields than the anode flow fields and/or other methods known in
the art for
passively inducing different heat transfer rates for the anode and the cathode
flow field
plates.
The following example is provided to illustrate certain aspects and
embodiments of the invention but should not be construed as limiting in any
way.
EXAMPLE
Two 10-cell fuel cell stacks were tested under the following conditions:
diluted fuel (74% hydrogen, 20% carbon dioxide, 6% nitrogen) was supplied to
the
anode at a pressure of 17.2 kPag, a humidification temperature of 57 C and a
11
CA 02629087 2008-05-08
WO 2007/058657 PCT/US2005/041863
stoichiometry of 1.25, while air was supplied to the cathode at a pressure of
10.7 kPag,
a humidification temperature of 57 C and a stoichiometry of 2Ø The anode
flow fields
of both stacks had a pressure drop of 120 mbar while the cathode flow fields
of both
stacks had a pressure drop of 100 mbar. The anode flow field plate web
thickness of
the anode flow field plates in the first stack was 1.88-millimeters, while the
anode flow
field plate web thickness of the anode flow field plates in the second stack
was 3.6-
millimeters. Both stacks were operated at 0.285A/cm2 for about 15 minutes.
Figure 3 shows the average performance of each fuel cell in the first 10-
cell stack comprising 1.88-millimeter web thickness anode flow field plates.
The
average performance was unstable and had a large cell-to-cell voltage
variability,
greater than 50 mV difference. between the best performing cell and the worst
performing cell. The average performance at 0.285A/cm2 was 544mV.
Figure 4 shows the average performance of each fuel cell in the second
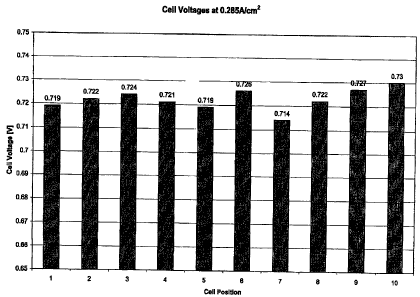
10-cell stack comprising 3.6-millimeter web thickness anode flow field plates.
Performance was stable and had a much lower cell-to-cell voltage variability,
less than
17 mV difference between the best performing cell and the worst performing
cell. The
average performance at 0.285A/cm2 was 722mV, which was significantly better
than
the first 10-cell stack at only 544mV.
A reduction in water back-diffusion from the cathode to the anode was
verified by collecting water that was condensed from a water knockout at the
anode
outlet of the fuel cell. The same two 10-cell fuel cell stacks were operated
for 8 hours
at 0.221 A/cm2. A total of 15.4 grams/hour of water was collected from the
first 10-cell
fuel cell stack while a total of only 1.2 grams/hour of water was collected
from the
second 10-cell fuel cell stack, thus illustrating the significant influence of
anode flow
field plate web thickness on water back-diffusion from the cathode to the
anode and the
reduction in anode flooding with low pressure drop anode flow fields.
While particular elements, embodiments, and applications of the present
invention have been shown and described, it will be understood that the
invention is not
limited thereto since modifications may be made by those skilled in the art
without
departing from the spirit and scope of the present disclosure, particularly in
light of the
foregoing teachings.
12