Note: Descriptions are shown in the official language in which they were submitted.
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
ANTIMICROBIAL CELLULOSIC SHEET
Claim for Priority
This non-provisional application is based upon U.S. Provisional Patent
Application
Serial No. 60/748,499, of the same title, filed December 8, 2005. The priority
of U.S.
Provisional Patcnt Application Scrial No. 60/748,499 is hcrcby claimed and the
disclosurc
thereof is incorporated into this application by reference.
Technical Field
The present invention relates to paper towels used as hand towels. An anti-
microbial
lotion on the towel increases water absorbency times (WAR) to further promote
lotion
transfer to the skin and increase transfer effectiveness. Most preferably,
lotion is applied as a
micro-emulsion.
Background
Frequent hand washing is a simple and effective means to ensure proper hygiene
and
prevent contamination of food and the spread of disease. Complex systems have
been
proposed to encourage food service and health care workers to adequately
cleanse their hands
frequently, in view of the rclativcly high potential for undcsirablc
contamination associated
with their activities.
Washing of the skin, especially the hands, with antimicrobial soap
formulations can
remove many viruses and bacteria from the washed surfaces. Rcmoval of thc
viruses and
bacteria is due to the surfactancy of the soap and the mechanical action of
the wash procedure.
Therefore, it is known and recommended that the people wash frequently to
reduce the spread
of viruses and bacteria. Recent surveys, however, have revealed that while
nearly 95% of
people claim to have washed their hands after use of public restrooms, actual
observations
reveal that this figure does not exceed about 66%. Notwithstanding increased
awareness,
there is a tendency to rush the hand washing process which leads to inadequate
hygiene. A
number systems and devices to encourage longer and more thorough handwashing
have
accordingly been developed.
1
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
Collopy in United States Patent No. 6,832,916 discloses a hand-washing device
containing a display panel that encourages the user to wash their hands for
about 15 seconds
to remove germs. Gorra, United States Patent No. 5,945,910 discloses method
and apparatus
for monitoring and reporting hand washing, which includes a sensor for
signaling the
dispcnsation of a cleaning agcnt from a dispenscr, and a reporting and
monitoring modulc.
Allen et al., United States Patent No. 5,781,942 discloses wash stations and
method of
operation, which monitors hand washing and assists in hand washing. These
systems are
relatively expensive and difficult to implement; oftentimes involving training
and monitoring
personnel. Even when such steps have been taken, there is little certainty
that all personnel
have followed proper washing procedures.
Frequent hand washing has the drawback that harsh soaps and cleansing agents
can
irritate the skin and damage the acid mantle of the skin.
Cellulosic substrates coated with lotions are well known in the art. For
example,
United States Patent No. 5,665,426 to Krzysik et al., is directed towards a
lotion formula that
can be applied to a tissue, which transfers the lotion to the user's skin in
order to reduce
irritation and redness. United States Patent No. 5,871,763 to Luu et al., as
well is directed
towards a lotion formula that is applied to a substrate for skin care
treatment. The lotion
composition of '763 is melted by the heat produced by the hands of a user of
the cellulosic
substrate to enable the lotion's transfer to the user's skin. Another lotion-
treated substrate is
dcscribcd in United States Patent No. 5,525,345 to Warner et al. The lotion
composition of
'345 comprises a plastic or fluid emollient that is solid or semi solid at
room temperature and
an immobilizing agent with a melting point above room temperature, which
stabilizes the
lotion composition on the surface of the substrate. See also United States
Application No.
10/483,633 (Publication No. US 2005/0031847), where two separate and distinct
phases, lipid
and aqueous, are applied to a substrate to facilitate cleansing of skin.
Further, there is
described in United States Patent No. 4,987,632 to Rowe et al., a cleaning
wipe treated with a
composition containing detergent, which is leached out upon contact with
water.
There are also known lotions containing anti-microbial and pH balancing agents
to
protect the skin. For example, United States Patent No. 6,238,682 to Klofta et
al. is directed
2
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
towards a tissue treated with anhydrous skin lotion containing antimicrobial
components in
addition to hydrophilic solvents and surfactants. See also United States
Patent No. 6,352,700
to Luu et al., which is directed towards a substrate treated with a lotion
that contains a skin pH
balancing compound for maintaining a proper skin acid mantle. Other lotions
containing
antimicrobial agents include United States Patent Application No. 10/608,661
(Publication
No. US 2004/0039353), which is dircctcd towards wet wipcs containing a Yucca
species
extract as an antimicrobial agent; United States Patent Application No.
09/851,273
(Publication No. US 2002/0031486), which is directed towards an antimicrobial
cleansing
composition, containing little or no volatile alcohol, that may be used alone
or in combination
with lotions and a like; United States Patent No. 6,436,885 to Biedermann et
al., which is
directed towards an antimicrobial cleansing compositions that has a pH of from
about 2 to
about 5.5.; United States Patent No. 6,383,505 to Kaiser et al. which is
directed towards an
antimicrobial lotion for topical use in a form of oil-in-water emulsion;
additionally, similar
subject matter is disclosed in United States Patent No. 6,482,423 to Beerse et
al.; United
States Patent No. 6,488,943 to Beerse et al.; United States Patent No.
6,284,259 to Beerse et
al.; United States Patent No. 6,258,368 to Beerse et al.; United States Patent
No. 6,183,763 to
Beerse et al.; and United States Patent No. 6,210,695 to Beerse et al., as
well.
Despite plentiful art, there exists a need for simple and effective means for
promoting
hygiene and skin care concurrently in connection with hand washing.
Summary of the Invention
A salicnt aspcct of the invention involves application of a suitable anti-
microbial
lotion to a substrate in amounts that will actually increase WAR times of the
cellulosic sheet.
This feature, while usually undesirable in a towel product, promotes anti-
microbial lotion
transfer to the skin, since a user will rub the towel longer when drying his
or her hands.
Lotion transfer is extremely important for both skin care and anti-microbial
effectiveness as
will be appreciated. by one of the skill in the art.
There is provided in one aspect of the invention an anti-microbial cellulosic
sheet for
paper towel including: a) a cellulosic web; b) a transferable lotion
composition comprising an
emollient and anti-microbial agent, the lotion composition being immobilized
on the
cellolosic web in a solid or semi-solid form, wherein the transferable lotion
composition is
3
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
selected from lotion compositions which are transferable upon contact with
water or lotion
compositions which are transferable upon application of heat; and c) the
transferable lotion
composition disposed on the web is selected and applied in amounts such that
it imparts a
water absorption rate delay of at least about 25% to the cellulosic web. In
preferred
embodiments, the transferable lotion composition disposed on the web is
selected and applied
in amounts such that it imparts a water absorption ratc delay to the
ccllulosic wcb of at least
about 50%, 75%, 100%, or more. So also, the unlotioned cellulosic web
preferably has
substantially the same SAT value as the lotioned cellulosic web. The
cellulosic sheet may
have SAT values of at least about 3 g/g, at least about 3.5 g/g, at least
about 4 g/g or at least
about 4.5 g/g. Typically, the cellulosic sheet has a SAT value of from about 3
g/g to about 5
9/g=
The lotioned cellulosic sheet typically has a WAR value of at least about 40
or 50
seconds, with WAR values of from about 55 seconds to about 75 seconds being
generally
suitable.
The transferable lotion is suitably applied to the cellulosic web in an amount
of from
about 3 weight percent to about 20 weight percent such as in an amount of from
about 5
percent by weight to about 15 percent by weight or
in an amount of from about 8 percent by weight to about 10 percent by weight
(based on the
combined weight of towel and lotion). The unlotioned cellulosic web may have a
basis
weight of from about 15 g/mZ to about 65 g/m2 such as from about 25 g/m2 to
about 50 g/m2.
A basis weight of from about 30 g/m2 to about 40 g/m2 is typical and the
ccllulosic web
consists predominantly of softwood fiber. The web may include at least about
65 or 70
percent by weight softwood fiber and typically from about 70 percent by weight
softwood
fiber to about 90 percent by weight softwood fiber. A preferred softwood fiber
is Douglas fir
fiber especially for electronic (motion sensored) dispensers. The sheet
suitably has an eight
sheet caliper of from about 35 to about 90 mils, consists predominantly of
softwood fiber and
is in the form of a single ply towel.
Typically, the lotion composition comprises from about 0.01 % to about 10% by
weight anti-microbial agent; preferably from about 0.05% to about 5% by weight
anti-
microbial agent. The anti-microbial agent may be selected from: 2,4,4'-
trichloro-2'-
4
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
hydroxydiphenyl ether; 3,4,4'-trichlorocarbanilide; 3,4,4'-trifluoromethyl-
4,4'-d-
ichlorocarbanilide; 5-chloro-2-methyl-4-isothiazolin-3-one;
iodopropynlbutylcarbamate;
8-hydroxyquinoline; 8-hydroxyquinoline citrate; 8-hydroxyquinoline sulfate; 4-
chloro-3,5-
xylenol; 2-bromo-2-nitropropane-1,3-diol; diazolidinyl urea; butoconazole;
nystatin;
terconazole; nitrofurantoin; phenazopyridine; acyclovir; clortrimazole;
chloroxylenol;
chloncexidine; chloncexidine gluconatc; miconazole; tcrconazolc; butylparaben;
cthylparabcn;
methylparaben; methylchloroisothiazoline; methylisothiazoline; a mixture of
1,3-
bis(hydroxymethyl)-5,5-dimethylhydantoin and 3-iodo-2-propynyl butyl
carbamate;
oxyquinoline; EDTA; tetrasodium EDTA; p-hydroxyl benzoic acid ester; alkyl
pyridinum
compounds; coco phosphatidyl PG-dimonium chloride; chlorhexidene digluconate;
chlorhexidene acetate; chlorhexidene isethionate; chlorhexidene hydrochloride;
benzalkonium chloride; benzethonium chloride; polyhexamethylene biguanide,
zinc salts and
mixtures thereof.
The web optionally includes a wet strength agent selected from aldehyde-
containing
polyols, aldehyde-containing cationic starch, glyoxal, glutaraldehyde,
dialdehydes, boric acid
carbonate, zirconium ammonium carbonate, glyoxalated polyacrylamide, polyamide-
epichlorohydrin, polyamine-epichlorohydrin, urea-formaldehyde, melamine-
formaldehyde,
polyethyleneimine, and latex emulsions.
One preferred embodiment is an anti-microbial cellulosic sheet for paper towel
including: a) a cellulosic towel web; b) a lotion emulsion including an anti-
microbial agent
disposed on the web, the lotion emulsion including a polar emollient and a non-
polar
emollient as well as a surfactant composition comprising a nonionic
surfactant, wherein the
lotion emulsion is substantially liquid at room temperature, the emollients
and surfactant
composition are selected such that the lotion emulsion is immobilized on the
web in a semi-
solid or solid state and wherein further the lotion emulsion is capable of
forming an aqueous
gel upon contact with water; and c) the lotion emulsion disposed. on the web
is selected. and
applied in amounts such that it imparts a water absorption rate delay of at
least 25% to the
cellulosic web. In connection with this class of lotioned sheet, the lotion
emulsion typically
comprises polar emollient in an amount of from about 2% to about 40% by weight
of the
lotion emulsion and the lotion emulsion may include a polar polyhydroxy
emollient selected
from propylene glycol, glycol, glycerol, diethylene glycol, methylene glycol,
polypropylene
5
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
glycol, polyethylene glycol and sorbitol. The lotion emulsion also preferably
includes a non-
polar emollient in the amount of from about 10% to about 40% by weight of the
lotion
emulsion, which non-polar emollient may be selected from aromatic or linear
esters, Guerbet
ester, mineral oil, squalane, liquid paraffin, and mixtures thereof. Suitable
non-polar
emollients thus include: isopropyl myristate; C12-CI5 alkyl benzoate esters;
tri-octyldodecyl-
citrate; mixtures of C12-C15 alkyl bcnzoatc cstcrs; and carnation oil. The
surfactant
composition may include non-ionic surfactant including a fatty alcohol in the
amount of from
about 40% to about 70% by weight of the lotion emulsion. Suitable non-ionic
surfactants
may be selected from PEG-20 methyl glucose sesquistearate, PPG-20 methyl
glucose ether,
PPG-20 methyl glucose ether distearate, PEG-20 methyl glucose distearate, PEG-
120 methyl
glucose dioleate, ethoxylated methyl glucose having from about 10 to about 20
repeating
ethoxy units, a mixture thereof and the like.. The surfactant composition most
preferably
includes a co-surfactant in an amount of from about 0.1% to about 20% by
weight of the
lotion emulsion. The co-surfactant is suitably selected from C12 -C18 fatty
alcohols, behenyl
alcohol, cetyl alcohol, stearyl alcohol, iso-cetyl alcohol, and iso-stearyl
alcohol, myristyl
alcohol, and mixtures of cetyl alcohol (C16) and stearyl alcohol (C18).
Typically, the lotion
emulsion is substantially waterless.
Another preferred embodiment is an anti-microbial cellulosic sheet comprising:
a) a
cellulosic web; b) a waterless micro-emulsion which is substantially liquid at
room
temperature immobilized on the web in a semi-solid or solid state; wherein the
waterless
micro-emulsion comprises an anti-microbial agent, a polar emollient, a non-
polar emollient
and a surfactant composition including a nonionic surfactant; and whcrcin
further the
waterless micro-emulsion is capable of forming an aqueous micro-emulsion upon
contact with
water; and c) the waterless micro-emulsion disposed on the web is selected and
applied in
amounts such that it imparts a water absorption rate delay of at least 25% to
the cellulosic
web.
Still another aspect of the invention is an anti-microbial cellulosic sheet
for paper
towel comprising: a) a cellulosic web; b) a transferable lotion composition
disposed on the
web comprising an emollient, an anti-microbial agent, and a retention/release
agent such that
the lotion has a AH above about 37 C of more than about 10 calories/gram, a
total heat of
melting of above about 25 calories/gram, and an onset of melting temperature
of at least about
6
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
30 C; and c) the transferable lotion composition disposed on the web is
selected and applied
in amounts such that it imparts a water absorption rate delay of at least
about 25% to the
cellulosic web. The lotion composition on this sheet optionally further
comprises a surfactant
composition in the amount of from about 10% to about 15% by weight of the
lotion
composition. The surfactants may be selected from methyl glucoside
sesquistearate,
cthoxylated mcthyl glucoside scsquistcaratc containing 20 molcs of oxycthylene
units,
mixtures of PEG-20 methyl glucose sesquistearate and methyl glu.cose
sesquistearate, or
combinations of the foregoing. Suitably the lotion composition comprises an
emollient in the
amount of from about 5% to about 75% by weight of the lotion composition. The
emollient
may be an aromatic ester emollient, a fatty alcohol ester of a non-fatty
organic acid emollient,
or mixtures thereof. Typical aromatic ester emollients may be benzoate ester
emollients
selected from C12 - C15 alkyl benzoate, stearyl benzoate, octyl dodecyl
benzoate, isostearyl
benzoate, methyl gluceth-20 benzoate, stearyl ether benzoate, poloxamer 182
dibenzoate,
poloxamer 105 benzoate, or mixtures thereof. A suitable fatty alcohol ester of
a non-fatty
organic acid emollient comprises C12 - C15 octanoate.
The heat-sensitive lotion composition typically includes a retention/release
agent in
the amount of from about 25% to about 95% by weight of the lotion composition,
wherein the
retention/release agent may be a C12 - C1g fatty alcohol. Suitable fatty
alcohols are selected
from dodecanol, tridecanol, tetradecanol, pentadecanol, hexadecanol,
heptadecanol,
octadecanol, mixtures of cetyl alcohol and stearyl alcohol and combinations of
the foregoing.
Further aspects of the invention will bccomc apparent from the discussion
which
follows.
Brief Description of the Drawings:
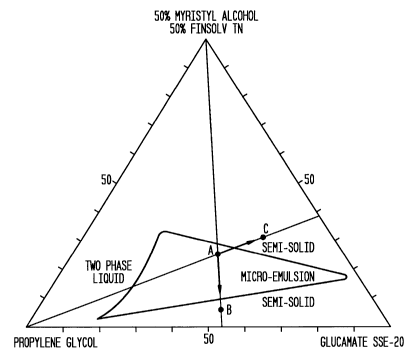
Figure 1 is a partial phase diagram of the composition of Example 1 showing
the
phase characteristics of a waterless micro-emulsion; and
Figure 2 is a partial phase diagram of the composition of Example 1 with water
showing the phase behavior of a mixture of the composition of Example 1 with
water.
7
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
Detailed Description
The invention is described in detail below for purposes of illustration only.
Modifications within the spirit and scope of the invention, set forth in the
appended claims,
will be readily apparent to one of skill in the art. As used herein,
terminology and
abbreviations have their ordinary meaning; for example, "cps" refers to
centipoises; g refers
to grams, mg refcrs to milligrams, m2 rcfcrs to squarc mctcrs and so forth.
Absorbency of the inventive products is measured with a simple absorbency
tester.
The simple absorbency tester is a particularly useful apparatus for measuring
the
hydrophilicity and absorbency properties of a sample of tissue, napkins, or
towel. In this test a
sample of tissue, napkins, or towel 2.0 inches in diameter is mounted between
a top flat
plastic cover and a bottom grooved sample plate. The tissue, napkin, or towel
sample disc is
held in place by a 1/8 inch wide circumference flange area. The sample is not
compressed by
the holder. De-ionized water at 73 F is introduced to the sample at the center
of the bottom
sample plate through a 1 mm. diameter conduit. This water is at a hydrostatic
head of minus 5
mm. Flow is initiated by a pulse introduced at the start of the measurement by
the instrument
mechanism. Water is thus imbibed by the tissue, napkin, or towel sample from
this central
entrance point radially outward by capillary action. When the rate of water
imbibation
decreases below 0.005 gm water per 5 seconds, the test is terminated. The
amount of water
removed from the reservoir and absorbed by the sample is weighed and reported
as grams of
water per square meter of sample or grams of water per gram of sheet. In
practice, an M/K
Systems Inc. Gravimetric Absorbency Testing System is used. This is a
commercial system
obtainable from M/K Systems Inc., 12 Garden Strcct, Danvcrs, Mass., 01923. WAC
or water
absorbent capacity also referred to as SAT is actually determined by the
instrument itself.
WAC is defined as the point where the weight versus time graph has a "zero"
slope, i.e., the
sample has stopped absorbing. The termination criteria for a test are
expressed in maximum
change in water weight absorbed over a fixed time period. This is basically an
estimate of
zero slope on the weight versus time graph. The program uses a change of
0.005g over a 5
second time interval as termination criteria; unless "Slow SAT" is specified
in which case the
cut off criteria is 1 mg in 20 seconds.
Water absorbency rate or WAR, is measured in seconds and is the time it takes
for a
sample to absorb a 0.1 gram droplet of water disposed on its surface by way of
an automated
8
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
syringe. The test specimens are preferably conditioned at 23 Ct 1 C (73.4 f
1.8 F) at 50 %
relative humidity. For each sample, 4 3x3 inch test specimens are prepared.
Each specimen is
placed in a sample holder such that a high intensity lamp is directed toward
the specimen. 0.1
ml of water is deposited on the specimen surface and a stop watch is started.
When the water
is absorbed, as indicated by lack of further reflection of light from the
drop, the stopwatch is
stopped and the time recorded to the ncarest 0.1 seconds. The procedurc is
rcpcatcd for each
specimen and the results averaged for the sample. WAR is measured in
accordance with
TAPPI method T-432 om-99.
The water absorption rate delay in percent is calculated from the WAR values
of the
unlotioned cellulosic web and lotioned sheet product of the invention as
follows:
Absorption rate delay =
(WAR value of lotioned cellulosic sheet - WAR value of unlotioned cellulosic
web) =(WAR
value of unlotioned cellulosic web) X 100%
"Aqueous gel" refers to viscous lotion/water compositions typically having a
room
temperature viscosity of above about 500 cps at room temperature and typically
above about
1000 cps at room tcmpcraturc. Prcfcrred lotion compositions form gels of morc
than 1500
cps at room temperature as is seen in Table 2 below.
"Basis weight", BWT, bwt and so forth is expressed in grams per square meter
or
pounds per 3000 square foot rcam of product as is indicated.
The term "cellulosic", "cellulosic sheet" and the like is meant to include any
product
incorporating papermaking fiber having cellulose as a major constituent.
"Papermaking
fibers" include virgin pulps or recycle (secondary) cellulosic fibers or fiber
mixes comprising
cellulosic fibers. Fibers suitable for making the webs of this invention
include fibers such as
those obtained from deciduous and coniferous trees, including softwood fibers,
such as
northern and southern softwood kraft fibers; hardwood fibers, such as
eucalyptus, maple,
birch, aspen, or the like as well as nonwood cellulosic fibers. Papermaking
fibers can be
9
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
liberated from their source material by any one of a number of chemical
pulping processes
familiar to one experienced in the art including sulfate, sulfite,
polysulfide, soda pulping, etc.
The pulp can be bleached if desired by chemical means including the use of
chlorine, chlorine
dioxide, oxygen, alkaline peroxide and so forth. The products of the present
invention may
comprise a blend of conventional fibers (whether derived from virgin pulp or
recycle sources)
and high coarscncss lignin-rich tubular fibers, such as blcachcd chemical
thcrmomcchanical
pulp (BCTMP). "Furnishes" and. like terminology refers to aqueous compositions
including
papermaking fibers, optionally wet strength resins, debonders and the like for
making paper
products.
Preferably, the fiber in the towel products of the invention consists
predominantly
(more than 50% by weight of fiber based on fiber content) of softwood (SW)
fiber such as
Douglas fir. Southern Softwood Kraft (SSWK) is also a preferred fiber.
Softwood fibers
provide strength to the product; Southern softwoods are generally preferred
for towel of the
invention; however thin and flexible Northern softwood may be used in some
fiber mixtures.
Percent means weight percent unless otherwise indicated and refers to weight
percent
without water unless the inclusion of the water weight is expressly indicated.
Weight percent
softwood fiber and like terminology or expressions refer to the weight percent
of softwood
fiber based on fiber content of a product or composition only, exclusive of
other ingredients_
Room temperature is refers to a temperature of from about 20 C to about 25 C.
Dry tensile strengths (MD and CD), stretch, ratios thereof, modulus, break
modulus,
stress and strain are measured with a standard Instron test device or other
suitable elongation
tensile tester which may be configured in various ways, typically using 3 or 1
inch wide strips
of tissue or towel, conditioned in an atmosphere of 23 t 1 C (73.4 f 1 F) at
50% relative
humidity for 2 hours. The tensile test is run at a crosshead speed of 2
in/min. Break modulus
is expressed in grams/3 inches/ %strain. % strain is dimensionless and need
not be specified.
Tensile ratios are simply ratios of the values determined by way of the
foregoing
methods. Unless otherwise specified, a tensile property is a dry sheet
property.
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
The wet tensile of the tissue of the present invention is measured using a
three-inch
wide strip of tissue that is folded into a loop, clamped in a special fixture
termed a Finch Cup,
then immersed in a water. The Finch Cup, which is available from the Thwing-
Albert
Instrument Company of Philadelphia, Pa., is mounted onto a tensile tester
equipped with a 2.0
pound load cell with the flange of the Finch Cup clamped by the tester's lower
jaw and the
ends of tissuc loop clamped into the uppcr jaw of the tensilc tcstcr. The
sample is immcrsed in
water that has been adjusted to a pH of 7.0+- 0.1 and the tensile is tested
after a 5 second
immersion time.
"Waterless", "substantially waterless" and like terminology refers to
compositions
which include generally less than about 10% by weight water. In cases where
water is present
at all, water is preferably not added as such, but is contained in other
ingredients.
In some preferred embodiments of the present invention, the lotion composition
is a
"cold" lotion such as the lotions described in United States Application
Serial No. 10/141,442
(United States Publication No. 2003/0211124) filed on May 7, 2002 and
incorporated herein
by reference in its entirety. "Cold" lotions refer to lotions that are
substantially liquid at room
temperature and can be applied as such to substrates. Due to the liquid state
of the "cold"
lotions at room temperature, they do not require heating or melting equipment
and can be
applied to the substrates by several available technologies such as spraying,
printing, coating,
extrusion or other techniques.
The cold lotion used in the present invention contains a micro-emulsion
composition
containing predominantly an emollient composition and a surfactant
composition. The small
particle size of the micro-emulsion increases the surface area of its
constituents so it
contributes to the utility of the present composition in increasing the
interaction between the
emollient and the skin surface; a desirable property for restoring the oil
layer of the skin.
Preferably, the micro-emulsion composition contains an external continuous non-
polar or
polar emollient, an internal discontinuous polar or non-polar emollient, a
surfactant and a
mixture of fatty alcohol co-surfactants. The lotion composition may also
contain optional
ingredients, including typical cosmetic additives, preservatives, plant
extracts, fragrances, and
medicinal agents. Any suitable combination or proportion of ingredients which
produces a
micro-emulsion can be used.
11
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
An important aspect of the cold lotion employed is when the liquid lotion
contacts the
fibers or non-woven substrate, it undergoes an in-situ phase change from
liquid to
immobilized semi-solid or solid form. This phase change of the lotion results
when the
substrate web surface fibers absorb the continuous outer phase of the micro-
emulsion, which
may bc a non-polar or polar-emollient. Subscqucntly, the pcrccnt of the outer
phase of the
micro-emulsion within the composition is reduced, resulting in increase in the
percent of the
internal phase of the micro-emulsion and shuft of the original lotion
composition from point
A (liquid micro-emulsion) to points B or C (semi-solid state), which are
located outside of
the micro emulsion region (see Figure 1). The immobilized antimicrobial lotion
is restorable
to transferable forrn upon contact with water and is capable of forming an
aqueous gel. The
compositions .ofthe present invention are preferably chosen to lie within the
micro-emulsion
regionof a given formulation. All percentages, ratios, and proportions of the
ingredients
within the compositions of the present invention are determined by the micro-
emulsion region
of a ternary phase diagram of the polar emollient/non-polar emollient/co-
surfactant/non-ionic
surfactant formulations (PE/NPE/COS/NIS). Outside of the micro-emulsion region
on the
low percent side of the polar or non-polar emollients, a semi-solid or solid
region is preferably
present. A micro-emulsion is thermodynamically stable and is essentially
transparent in the
visible region of the spectrum, which typically indicates that particle size
diameter is
preferably less than about 0.1 micron, or so. When the particle size diameter
is greater than
about 3,200 A (about 0.32 micron), the liquid is no longer considered a micro-
emulsion but is
an emulsion which can often appear turbid and be thermodynamically unstable.
The micelle
structure of a micro-emulsion is cithcr a"dircct" type (hcad out/tail in) or
an "invcrsc" type
(head in/tail out). The liquid micro-emulsion increases the surface area of
the lipophilic
constituent so it contributes significantly to the utility of the present
composition in neat form.
Fluidity on the skin surface, small particle size, high surface area and high
hydrophilic
character, are highly desirable properties for cleansing purposes either when
the substrate is
used by itself or when lotioned products are rewet with water. Any combination
or proportion
of these ingredients which produces a micro-emulsion can be used.
A hot lotion composition used in connection with the present invention is
chosen such
that its AH of above about 37 C is above about 10 calories/gram, AH of below
about 37 C is
above about 15 calories/gram, AH total (total energy to melt) of above about
37 C is above
12
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
about 25 calories/gram. Further, the retention/release agent is preferably
selected to have a
melting point substantially higher than about room temperature but lower than
about 65 C,
such that the lotion onset of inelting temperature is within the range of from
about 30 C to
about 45 C. This enables the lotion composition to maintain a substantially
solid state at
about room temperature and partially melted state at human skin temperature.
It should be noted that for purposes of this description, the temperature of
human skin
is between about 30 C to about 37 C and room temperature is between about 20 C
to about
25 C.
An important aspect of a hot lotion used is that it is partially melted by
body heat to
enable transfer to the skin of partially liquefied and partially solid
emollient(s), particles of
retention/release agent and other ingredients. The partial melting of the
lotion is important,
because when the lotion is completely melted to liquid by body heat it is
perceived as too
greasy, and when a lotion is not sufficiently melted by body heat, it would
not spread easily
on the skin. At least a portion of the partially melted lotion resolidifies on
the skin to form a
smooth and moisturizing layer. Further details as to suitable hot lotion
compositions are
found in United States Patent No. 5,871,763, the disclosure of which is
incorporated herein in
its entirety.
Optionally included in the anti-microbial lotions are suitable anti-viral
agents
including those effective against, or at least retardant toward Corona virus,
Picoma virus,
Rhino virus, Hcrpcs simplex, Hcrpcs genitalis, Hcrpes labialis, Respiratory
Syncytial Virus
(RSV), Para influenza, Cytomegalovirus, Adenovirus, Condyloma and certain
synergistic
disease states that can involve a virus and a protozoa or a virus and any
unfriendly enzymes,
e.g., protease, lipase and amylase, that cause a compromised skin as a
precursor state for a
viral infection to occur. Specific anti-viral agents suitable for use in the
lotions include
bioflavonoids such as hesperitin, naringin, catechin and. certain selected
amino acids of
leguminous origin such as L-canavanine and an analog of L-arginine;
dicarboxylic acids such
as malonic, glutaric, citric, succinic, and diglycolic acids; alpha hydroxy
carboxylic acid such
as D-galacturonic acid from Sterculia urens; neem seed oil (Azadirachta
indica) in its un-
denatured form; sandalwood oil (Santalum album L.) in its un-denatured form.
Optionally,
13
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
the anti-viral agent could be admixed with at most about 50% by weight of the
anti-viral agent
of a protease inhibitor such as zinc oxide or other suitable zinc salt.
The cold or hot lotion composition can include other optional components
typically
present in lotions of this type. These optional components include a botanical
extract, such as
aloc cxtract, avocado oil, basil cxtract, sesamc oil, olive oil, jojoba oil,
chamomile extract,
eucalyptus extract, peppermint extract, as well as animal oils such as emu
oil, cod liver oil,
orange roughy oil, mink oil, and the like.
The lotion of the present invention can also optionally include a humectant.
Humectants are hygroscopic materials with a two-fold moisturizing action
including water
retention and water absorption. Humectants prevent the loss of moisture from
skin and help
to attract moisture from the environment. Preferred humectants include
glycerol, hydrolyzed
silk, ammonium lactate, hydroxypropyltrimonium hydrolyzed silk, hydroxypropyl
chitosan,
hydroxypropyltrimonium hydrolyzed wheat protein, lactamidopropyltrimonium
chloride, and
ethyl ester of hydrolyzed silk. The botanical extract, animal oil or humectant
is preferably
present in an amount of less than about 3% when used in the base formulation
of the lotion.
Further optional components include a skin refreshing agent such as
encapsulated water in oil,
eucalyptus oil, and menthol oil. All of these optional materials are well
known in the art as
additives for such formulations and can be employed in appropriate amounts in
the lotion
compositions of the present invention by those skilled in the art.
Thc lotion can optionally include a fragrance. The fragrance can be present in
an
amount of from 0.01 % to about 2%. Suitable fragrance includes volatile
aromatic esters, non-
aromatic esters, aromatic aldehydes, non-aromatic aldehydes, aromatic
alcohols, non-aromatic
alcohols, heterocyclic aroma chemicals, and natural floral fragrances, such as
blossom,
carnation, gardenia, geranium, iris, hawthorne, hyacinth and jasmine.
The lotion can also optionally include natural or synthetic powder like talc,
mica,
boron nitride, silicone, or mixtures thereof.
The towel web of the present invention can be any suitable cellulosic
substrate web,
optionally wet-strengthened, and optionally including synthetic fibrous
material such as melt-
14
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
blown polyethylene, polypropylene, copolymers of polyethylene. The substrate
also may be
embossed.
Wet strength agents which may be added include temporary as well as permanent
wet
strength agents. Suitable wet strength agents include glyoxal; glutaraldehyde;
uncharged
chemical moicties selectcd from a group consisting of dialdchydcs, aldchyde-
containing
polyols, uncharged. aldehyde-containing polymers, and cyclic ureas and
mixtures thereof, and
aldehyde-containing cationic starch; mixtures of polyvinyl alcohol and salts
of multivalent
anions, such as boric acid or zirconium ammonium carbonates; glyoxalated
polyacrylamide;
polyamide-epichlorohydrin; polyamine-epichlorohydrin; urea-formaldehyde;
melamine-
formaldehyde; polyethyleneimine; and latex emulsions.
The present invention includes a web of cellulosic fibers treated on at least
one side
thereof, preferably in an amount of from about 0.1% to about 25%, more
preferably from
about 0.5% to about 20%, by weight of the dried fiber web with an anti-
microbial lotion.
The cellulosic substrate can be prepared according to conventional processes
(including TAD, CWP and variants thereof) known to those skilled in the art. A
preferred
towel web is a fabric-creped towel web as is used in Example 18. Lotion can be
applied to
the substrate according to conventional application methods known to those
skilled in the art.
Examples 1-7
Formulations of thc watcrlcss lotion were prepared in which, the components,
their
ratios and the conditions selected to provide micro-emulsion subject to in-
situ phase change
upon contact with a cellulosic substrate were varied as shown in the following
Examples.
In preparing each formulation the following, a general procedure was used. The
polar
phase propylene glycol was mixed with surfactant and. co-surfactant in a
heated container at
about 60 C to about 70 C until the chemicals were completely melted. The non-
polar oil
phase was added to the mixture with moderate agitation for about 10 minutes,
then cooled to
room temperature. At this point the lotion was in clear liquid form and ready
to apply to the
substrate. The micro-emulsion formed spontaneously without the need for a high
shear
mechanical device and is stable indefinitely.
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
Examples 1 to 7 were prepared in accordance with United States Patent
Application
No. 10/141,442, the disclosure of which is incorporated herein by reference.
These lotion
formulas were liquid at room temperature, transparent, very stable and
accordingly the lotion
ingredient ratios were inside the micro-emulsion region of phase diagrams such
as Figure 1
which is a partial phase diagram of the composition of Example 1.
Surprisingly, the lotion of
the prescnt invention is characterized as having a good hand-fccl perception
and non-greasy
hand-feel, which is thought to be due to the particle size of the micro-
emulsion being too
small to be detected in the oil phase by the fingertips.
Tablel
Ingredients Ex.l Ex. 2 Ex. 3 Ex. 4 Ex. 5 Ex. 6 Ex. 7
% (%) (%) % % % %
Propylene 1 col 35 35 5 15 15 30 35
Finsolv TN 12.5 0 16 0 30 15 0
Carnation oil 0 0 0 0 0 0 12.5
Iso ro 1 myristate 0 15 0 30 0 0 0
Lambert CE 2000 0 0 4 0 0 0 0
M s l alcohol C14 12.5 15 0 0 0 0 12.5
Kalcol1618 0 0 7.5 0 5.5 5.5 0
Glucam P-20 0 0 67.5 0 49.5 49.5 0
Distearate(5) Glucamate SSE-20 40 35 0 55 0 0 40
Finsolv TN: C12-C15 alkyl benzoate ester from Finetex Inc.
(2) Carnation: Mincral oil from Witco Corp.
(') Lambert CT 2000 - tri-octyldodecyl-citrate (Guerbet ester) from Lambert
Technologies.
(4) Kalcol 1618: Mixture 50/50 of cetyl alcohol (C 16) and stearyl alcohol (C
18) from Kao Corp.
(5),Giucan P-20 Distearate: PEG-20 methyl glucose distearate from Amer-chol.
(6) Glucamate SSE-20: PEG-20 methyl glucose sesquistearate from Amer-chol.
Example 8
The lotion prepared in Example 1 was applied to a tissue basesheet at a 5% add-
on
level, then converted to a two ply tissue product. The product was tested for
the amount of
lotion transferred. to the skin. The results were compared with commercially
available
lotioned tissues by comparing the light reflection of cold lotion residual on
glass relative to
that from two other products. The scattering of light caused by lotion smeared
onto the glass
microscope slide was measured by using the UV/visible spectrophotometer in the
wavelength
region from 700 nm to 400 nm. Lotion was transferred to the slide by holding
it between two
layers of lotioned tissue for 30 seconds and then rubbing the tissue over the
slide 20 times in
15 seconds. The lotion smeared glass slide was placed in the sample beam of a
double beam
16
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
UV/Visible spectrometer to measure the light scattering. The results show that
scattering of
light caused by lotion smeared onto the slide rubbed with the tissue treated
with the lotion in
Example 1, looked identical to the control (untreated tissue). However, the
two commercially
available lotioned facial tissue products tested produced a significant amount
of light
scattering compared to the lotioned tissue of the present invention. In fact,
the containers for
thcsc commercial products specifically state "not rccommcndcd for cleaning
cycglasscs." In
addition, from the lab test result, the amount of lotion transferred. by the
lotioned substrate of
the present invention to the skin was measured to be about 4.2 mg/cmZ.
The lotioned substrate product of the present invention was able to transfer
lotion to
the skin for enhancing skin care benefits, while also being able to "wipe
eyeglasses and still
maintain clear vision." These properties of the present invention represent
significant
advantages over the lotioned facial tissues of the prior art.
The waterless emulsion compositions of the present invention have numerous
attributes which make them particularly suitable for paper towels. For one,
the waterless
micro-emulsions form low viscosity aqueous micro-emulsions with relatively
small amounts
of water such that an immobilized lotion on the substrate is restorable to
readily transferable
form when wetted or mixed with water. Thus, when contacted with wet hands of a
paper
towel user, for example, the lotion is readily transferred from the towel to
the skin of a user.
Another unique characteristic of the invention is that the lotion emulsions
are capable
of forming viscous gels with watcr as the amount of water mixed with the
lotion is incrcascd.
Gels are generally more glutinous than liquids, thus being more desirable as
hand lotions.
Details as to these characteristics appear in Examples 9-16 below.
Examples 9-16
The composition of Example 1 was mixed with water and tested for viscosity
using a
Brookfield Digital Viscometer at 73 F. Examples 9, 10, 11 and 16 were tested
with a No. 2
spindle, while Examples, 12, 13, 14 and 15 were tested with a No. 5 spindle.
Details as to
composition and test conditions appear in Table 2 below.
17
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
Table 2 - Aqueous Phasing Properties
Example # Speed Viscosity Appearance and
Description S indlc # (RPM) c s Pro crtics
9/ 100% Lotion
Example #1 2 50 182 Clear Liquid
/ 95% Lotion
Example #1 + 5%
Water 2 50 218 Clear Liquid
11 / 90% Lotion
Example #1 + 10%
Water 2 50 348 Clear Liquid
12 / 85% Lotion
Example #1 + 15%
Water 5 10 4,600 Viscous gel
13 / 80% Lotion
Example #1 + 20%
Water 5 10 22,000(2) Elastic get
14 / 70% Lotion
Example #1 + 30%
Water 5 10 13,000 (2&3) Crystalline gel
15/ 50 /a Lotion
Example #1 + 50% Viscous turbid
Water 5 10 3,500 el
16/ 20% Lotion
Example #1 + 80% Turbid
Water 2 50 140 emulsion
It is seen in Table 2 that the water/emulsion mixtures remained a micro-
emulsion up to
5 a water concentration of between 10% and 15% by weight of the composition
(Examples 9-
12). At 15% water, the lotion emulsion turned into a viscous gel, which became
even more
viscous as additional water was added. At 20% water, the composition was an
elastic gel
having a viscosity of 22,000 cps, making viscosity measurement difficult. At
30% watcr
(Example 14), the gel exhibited some opacity and appeared to have some
crystalline structure
10 appearing almost brittle. Due to the difficulty of viscosity measurement as
well as the elastic
and adhesive properties of the elastic gel of Example 13, the actual
difference in viscosity
between Examples 13, 14 may be less than indicated in Table 2.
At 50% by weight water, viscosity fell off dramatically and the composition
appeared
to be a viscous, turbid gel which was somewhat translucent. While the
viscosities of
18
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
Examples 12 and 15 were similar, the composition of Example 14 exhibited
considerably
more turbidity. At 80% water, viscosity was low again; however, the
composition was no
longer clear and appeared to be an emulsion which was somewhat turbid.
The phase behaviors of the mixtures of Table 2 are illustrated in the partial
phase
diagram of Figure 2, whcrc it is seen that Examplcs 9, 10 and 11 arc within
the micro-
emulsion region of the phase diagram. Examples 12, 13, 14 and 15 are in "semi
solid" form,
while Example 16 is a two-phase liquid.
Example 17 and Comparative Example A
Still further features of the invention which are highly desirable include WAR
delay
which promotes lotion transfer to the skin and anti-microbial action of paper
towel. These
features are appreciated form the discussion which follows.
Towel basesheet was prepared using 100% Douglas Fir Kraft fiber by way of a
fabric
crepe/Yankee dry process of the class disclosed in co-pending United States
Patent
Application Serial No. 11/451,111, entitled "Fabric-Creped Sheet for
Dispensers", filed June
12, 2006 (Attorney Docket No. 20079; GP-05-10), the relevant disclosure of
which is
incorporated herein by reference in its entirety. To the basesheet, lotion was
applied in 1"
bands along the machine direction (alternating with 1" bands of unlotioned
towel) using a
DynatecTM applicator of the class seen in United States Patent Nos.:
5,904,298; 5,902,540;
and 5,882,573, the disclosures of which are incorporated herein by reference.
The lotion
formulation of Example 1 was uscd, containing additionally 2% by weight lotion
triclosan
anti-microbial compound, 2, 4,4'-trichloro-2'-hydroxy diphenyl ether. Further
details appear
in Table 3 below.
The towel was treated for anti-microbial properties by placing a wetted
specimen disk
of towel in a Petri dish on inoculated agar. The anti-microbial properties are
termed
"negative" if microbe contamination is observed on or at the towel after
incubation and
"positive" if a "ring" around the test specimen is observed, indicating that
microbe growth
was inhibited by the towel.
19
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
Results of anti-microbial testing also appear in Table 3.
Table 3 - Anti-microbial and Towel Properties
Example A Exam lc 17
Properties No Lotion Lotioned
Anti-microbial Properties:
Sta h lococcus aureus Negative Positive
E. coli Negative Positive
Salmonella sps Negative Positive
Physical Properties:
Add on rate (% of product wei ht 0% 8 to 10%
Basis Weight lbs/rm 22.2 23.5
Caliper (mils/8 sheets) 46.0 46.1
Dry MD Tensile (g/3") 6531.2 5528.9
Dry CD Tensile 3" 3912.0 3435.1
MD Stretch (%) 7.4 7.7
CD Stretch (%) 3.3 3.7
Wet MD Cured Tensile (g/3")
(Finch) 1976.1 2040.1
Wet CD Cured Tensile (g/3")
(Finch) 1041.0 1122.1
WAR (seconds) TAPPI 34.3 67.6
MacBeth 3100 Brightness (%)
UV Excluded 77.5 75.5
O aci (%) 60.2 56.6
SAT Ca aci rn~2 125.1 123.0
SAT Time (seconds) 643.7 823.6
GM Break Modulus 1025.2 829.0
It is seen in Table 3 that the anti-microbial lotion was effective against
staphylococcus
aureous, E. coli and salmonella sps.
It is also sccn that, with the absorbent capacity (SAT) of the control and the
lotioncd
towel remained. substantially the same, WAR times, or absorption rates were
considerably
lengthened, perhaps due to gel blockage; consistent with the data in Table 2
above. Higher
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
WAR values are generally not desired; however, the glutinous gel feel and
initial "wetness"
experienced by a towel user is a positive consequence, offsetting lower
measured absorption
rates and encouraging more wiping action so the anti-microbial lotion is more
effective in
preventing or ameliorating contamination. The apparent gel blockage also
appeared to
increase CD wet tensile, a common source of towel failure.
Examples 18-22
The lotion compositions in the following examples comprise a base lotion with
and
without a pH balancing agent. Suitable pH balancing agents include glycolic
acid, alpha-
acetyl glycolic acid, lactic acid, tartaric acid, alpha-acetyl lactic acid,
alpha-hydroxy
isobutyric acid, salicylic acid, mandelic acid, ortho-acetyl mandelic acid,
benzilic acid, ortho-
acetyl benzilic acid, malic acid, citric acid, gluconic acid, pyruvic acid,
sorbic acid and
combinations thereof. Examples 18 and 19 are comparative and contain no pH
balancing
agent, and Examples 20-22 relate to lotions compositions combined with a pH
balancing
agent. Further detail is seen in United States Patent No. 6,352,700, the
disclosure of which is
incorporated herein in its entirety.
The lotions in Examples 20-22 were prepared according to the following
procedure:
the base lotion ingredients, i.e., emollient(s), release and retention agent
and surfactants were
mixed together and heated to 75 C until the mixture was completely melted.
Note lotion
composition components in Table 4. The lotion composition mixture was
maintained at 75 C
for about 15 minutes with moderate agitation. The pH balancing compound was
then added,
using high agitation, until the compound was complctcly mclted and blendcd.
The pH valuc
for each lotion was determined by emulsifying 0.276 g of solid lotion
(equivalent to the lotion
amount contained in 5 sheets of 15% lotionized tissue) in 20 ml tap water
(pH=8.65) at 23 C.
The emulsion was shaken for 5 minutes before measuring pH using a standard
calibrated pH
meter.
21
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
N
N
'n M N M
cd
N
~
o O 1.O O c~
V'1
co
O N
..~
M O O N O
cd
cd O~
tn
O O O O~
I -~~+\
cn
N
.--i W
cd
03
00
~
Cd
K
a)
cn
O
~ U
-- cd
co
kn
c'n
cc ~ ~,
U , N~ ~ UU N ~" N cn_ '
cd m +cd w
~ o V N'b w? U~ U
y_
= W
V r3.~"OD VUC7 C7a onC7 1
22
CA 02630112 2008-05-15
WO 2007/097818 PCT/US2006/060718
While the invention has been described in connection with numerous examples,
modifications to those examples within the spirit and scope of the invention
will be readily
apparent to those of skill in the art. In view of the foregoing discussion,
relevant knowledge
in the art and references including co-pending applications discussed above,
the relevant
disclosures of which are all incorporated herein by reference, further
description is deemed
unneccssary.
23