Note: Descriptions are shown in the official language in which they were submitted.
CA 02630438 2008-05-20
1
Prodrugs of ER[i-Selective Substances, Process for their Production, and
Pharmaceutical Compositions that Contain These Compounds
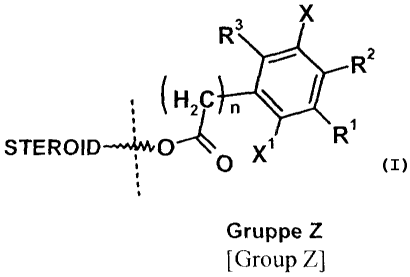
The invention relates to prodrugs of ER13-selective substances of general
formula (I),
R3 x
R2
(HZC n
STEROtD-~~~~O Xi R I
O
Gruppe Z
(I)
[Group Z]
a process for their production, pharmaceutical compositions that contain these
compounds,
and their use for the production of pharmaceutical agents.
Estrogens play an important role in the organism in both sexes. In the
maturing
organism, estrogens are involved in the imprinting of sex characteristics. In
both sexes,
estrogens control the changes in the organism during sexual maturation, such
as growth
spurts and then the completion of bone growth. In all phases of life,
estrogens play a central
role (1, 4) in bone metabolism in both sexes. Their loss results in the
degradation of bone
substance and involves the risk of an elevated brittleness of the bone.
In women, the estrogens that are secreted by the ovary predominate in the
organism.
In pregnancy, the placenta forms large amounts of estrogen. In men, estrogens
are produced
primarily "peripherally" by the aromatization of testosterone or the adrenal
androgens in
various effector organs, such as the central nervous system (CNS), the bones
or the intestinal
epithelium. This adaptation makes possible physiological estrogen effects in
men at very low
estradiol levels in the blood. In men and women with a genetic defect of the
aromatase or the
estrogen receptor, the bones are severely disrupted relative to growth and
development (2).
While for natural estrogens, the oral administration (10) is problematic owing
to its
low oral bioavailability, conventional chemically modified estrogens with
improved
bioavailability (for example ethinyl estradiol) often have the drawback of
producing a
considerably increased estrogenic action in the liver (3, 9, 10). This hepatic
estrogeneity
CA 02630438 2008-05-20
2
relates to a number of functions, such as transport proteins, lipometabolism,
blood pressure
regulation and clotting factors (5, 7, 11, 12, 14). Also, the especially
important secretion of
IGF-I (8) for the preservation of muscles and bones is negatively affected by
hepatic
estrogenic actions (12, 13, 6).
In WO 01/77139, new 8(3-substituted estratrienes are described, whereby the
8(3-
substituent can be a straight-chain or branched-chain, optionally partially or
completely
halogenated alkyl or alkenyl radical with up to 5 carbon atoms, an ethinyl or
a prop- l -inyl
radical, which as pharmaceutical active ingredients show a higher in vitro
affinity to estrogen
receptor preparations of rat prostates than to estrogen receptor preparations
of rat uteri and
have in vivo a preferential action on bone in comparison to the uterus and/or
a pronounced
action with respect to the stimulation of the expression of 5HT2a-receptors
and -transporters.
These compounds can preferably be used for treating diseases that are caused
by an estrogen
deficiency.
Drawbacks of these 8p-substituted estratrienes are their deficient oral
bioavailability
as well as the metabolic instability.
From WO 01/91797, steroidal compounds are known that are bonded via a group
-SO2NRtR2 to erythrocytes and accumulate there. The concentration ratio of the
compounds
between erythrocytes and plasma is 10-1000:1, preferably 30-1000:1, such that
a depot
formation in the erythrocytes can be mentioned. By the strong bond of the
compounds to the
erythrocytes, the metabolization is avoided during the liver passage.
Disadvantageously,
despite a reduced metabolization with the indicated dosages, no therapy-
relevant active
ingredient levels are given.
It is therefore the object of this invention to provide prodrugs of ER(3-
selective
compounds, which make the ER(3-selective compounds orally bioavailable.
This object is achieved by sulfamoyl compounds of 80-substituted estratrienes
of
CA 02630438 2008-05-20
3
general formula (I), in which group Z is bonded to the steroid that is to be
released
R3 x
R2
(H2C n
R
STEROID-~~~~O ~ X,
Gruppe Z
[Group ZI
in which n can mean a number 0- 4,
R1 means a radical -SO2NH2 or -NHSO2NHZ,
whereby R2, R3 and X, Xl, independently of one another, stand for a hydrogen
atom, a halogen atom, a nitrile group, a nitro group, a C1 _S-alkyl group, a
CpFZp+l group with p=1-3, a group OC(O)-R20, COOR20, OR20, C(O)NHRZO or
OC(O)NH-R2',
whereby R20 and RZ1 are a Ci_5-alkyl group, a C3_g-cycloalkyl group, an aryl
group, a C14-alkylene aryl group, a C]4-alkylene-C3_8-cycloalkyl group or a
C3_8-cycloalkylene-CI-4-alkyl group, and
R20 in addition can mean a hydrogen atom, or
R2 can mean a radical -SOZNHZ or -NHSO2NH2,
whereby R~, R3 and X, Xl, independently of one another, stand for a hydrogen
atom, a halogen atom, a nitrile group, a nitro group, a C1_5-alkyl group, a
CFF2p+l group with p=l -3, a group OC(O)-R20, COOR20, ORZO, C(O)NHRZO or
OC(O)NH-R21,
whereby R20 and Rz1 are a C1_5-alkyl group, a C3_g-cycloalkyl group, an aryl
group, a C]4-alkylene aryl group, a C14-alkylene-C3_8-cycloalkyl group or C3_
8-cycloalkylene-CI_4-alkyl group, and
R20 in addition can mean a hydrogen, or
R3 can mean a radical -SO2NH2 or -NHSO2NH2,
CA 02630438 2008-05-20
4
whereby R1, R2 and X, Xl, independently of one another, stand for a hydrogen
atom, a halogen atom, a nitrile group, a nitro group, a C1_5-alkyl group, a
CpF2p+l group with p=1-3, a group OC(O)-R20, COOR20, OR20, C(O)NHRZO or
OC(O)NH-RZ',
whereby R20 and R21 are a C1_5-alkyl group, a C3_g-cycloalkyl group, an aryl
group, a C1_4=alkylene aryl group, a C1_4-alkylene-C3_g-cycloalkyl group or
C3_
g-cycloalkylene-C1_4-alkyl group, and
R20 in addition can mean a hydrogen, and
STEROID stands for a steroidal ABCD-ring system of formula (A):
R"
11)_R16
( \
R3 ~ (A)
whereby the radicals R3, R8, R16 and R17 have the following meaning:
R3 can be Z and
R17 can be an OH group, a tri(Cj-C4-alkyl)silyloxy group or a group OC(O)-
R20or
R3 can be OH, OMe, a tri(C1-C4-alkyl)silyloxy group, or a group OC(O)-R20 and
R17 can be Z
and
R 8 can be a branched or straight-chain, optionally partially or completely
halogenated alkyl, alkenyl or alkinyl radical with up to 3 carbon atoms,
R16 can be a hydrogen atom, a halogen atom, or a methyl group,
whereby the substituents R16 and R17 in each case can be both in a- and in j3-
position,
and their pharmaceutically acceptable salts.
In addition, this invention comprises the new compounds as pharmaceutical
active
ingredients, their production, their therapeutic application and
pharmaceutical dispensing
forms that contain the new substances.
The invention relates to estrogen derivatives that themselves cannot bind to
the
estrogen receptor and from which the contained mother estrogen is released in
the body,
CA 02630438 2008-05-20
process for their production, and pharmaceutical compositions that contain
these compounds.
The compounds according to the invention are prodrugs that release an ER(3-
selective
estrogen (mother estrogen) after saponification of the ester group Z.
By absolute and relatively greatly weakened action via the ER a, undesirable
estrogen
effects of any standard estrogen therapy on the uterus, the mammary glands and
the liver are
avoided, as they are typical for non-dissociated estrogens. The compounds
according to the
invention have therapeutically advantageous estrogenic activities if they are
mediated by the
ER (3, in particular in the central nervous system, in the circulatory system
and in the bones.
The substances according to the invention are preferably used for oral
therapy.
Compared to their mother estrogens, the compounds according to the invention
have a clearly
increased oral bioavailability, an increased systemic, but generally reduced
hepatic
estrogeneity. By this dissociation of desirable and undesirable hormonal
effects,
simultaneously more therapeutically effective and, in comparison to the prior
art, more
compatible pharmaceutical agents are made possible.
The substances according to the invention are cleaved enzymatically or
hydrolytically
in the body, whereby no steroid sulfatases (STS), such as, for example, for
cleavage of
estradiol-3-sulfamate, are required. Thus, the inhibition of the steroid
sulfatase that is typical
for estrogen-3-sulfamates and disadvantageous for achieving strong estrogenic
effects can
also be avoided, which is typical for estrogen sulfamates in humans. In the
case of oral
therapy with natural estrogens (estradiol, estradiol valerate, estrone
sulfate, conjugated
estrogens), but also in that with estradiol sulfamate, high levels of estrone
dominate in the
blood (10). Unlike in the cycle, the concentrations of estradiol in the blood
are lower than
that of estrone. This is therefore disadvantageous, since estrone is a less
effective estrogen
than estradiol.
An advantage of the substances according to the invention in comparison to
those in
the prior art is the preferable release of the respective mother estrogen,
thus, for example, 80-
ethylestradiol, 8(3-methylestradiol, 8B-vinylestradiol and 8B-
difluorovinylestradiol instead of
the inactive estrone derivatives.
The compounds of general formula (I) according to the invention or their
pharmaceutically accceptable salts can be used as individual components in
pharmaceutical
preparations or in combination in particular with antiestrogens or gestagens.
The
combination with ERa-selective antiestrogens or with antiestrogens that are
peripherally-
CA 02630438 2008-05-20
6
selectively active, i.e., that do not pass through the blood-brain barrier, is
especially
preferred.
The substances and the pharmaceutical agents containing them are especially
suitable
for the treatment of perimenopausal and postmenopausal symptoms, in particular
hot flashes,
sleep disorders, irritability, mood swings, incontinence, vaginal atrophy, and
hormone-
deficiency-induced mental disorders. The substances are also suitable for
hormone
substitution and for the treatment of hormone-deficiency-induced symptoms in
ovarian
dysfunction that is caused by surgery, medication, etc. Prevention of bone
mass loss in
postmenopausal women and andropausal men, in women who have undergone
hysterectomies or in women who were treated with LHRH antagonists or agonists
is also part
of this.
The prodrugs of ERB-selective agoriists according to the invention can be used
alone
or in combination with antiestrogens, aromatase inhibitors or selective
estrogen receptor
modulators (SERM) for the treatment of prostate hyperplasia to avoid estrogen
deprivation or
to reduce the effects thereof.
As antiestrogen, preferably 7alpha-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]-
nonyl]estra-1,3,5(10)-triene-3,17I3-diol (fulvestrant) is used.
As the aromatase inhibitors that are to be used, the following are considered:
anastrozole, atamestane, fadrozole, formestane, and letrozole.
As SERM, compounds that are selected from the following group are considered:
raloxifene, tamoxifen, and 5-(4-{5-[(RS)-(4,4,5,5,5-
pentafluoropentyl)sulfinyl]-
pentyl } phenyl)-6-phenyl-8,9-dihydro-7H-benzocyclohepten-2-ol (WO 00/03979).
The compounds are also suitable for alleviating symptoms of andropause and
menopause, i.e., for male and female hormone replacement therapy (HRT),
naniely both for
prophylaxis and for treatment, in addition for treating symptoms accompanied
by a
dysmenorrhea as well as for treating acne.
In addition, the substances can be used for prophylaxis against hormone-
deficiency-
induced bone mass loss and osteoporosis, for preventing cardiovascular
diseases, in particular
vascular diseases such as arteriosclerosis, for inhibiting the proliferation
of arterial smooth
muscle cells, and for treating primary pulmonary high blood pressure.
CA 02630438 2008-05-20
7
In addition, the substances can be used for treating inflammatory diseases and
diseases of the immune system, in particular autoimmune diseases, such as,
e.g., rheumatoid
arthritis, multiple sclerosis, Crohn's disease as well as endometriosis.
The compounds can be used in particular according to therapies that result in
estrogen
deprivation, for example after treatment with aromatase inhibitors or GnRH
antagonists or
agonists, for treatment of arthritic symptoms.
In addition, the compounds can be used for treatment of male fertility
disorders and
prostatic diseases. The compounds according to the invention are suitable for
estrogen
treatment of prostate cancer.
The compounds can also be used in combination with the natural vitamin D3 or
with
calcitriol analogs for bone building or as supporting therapies to therapies
that cause a bone
mass loss (for example, a therapy with glucocorticoids, aromatase inhibitors,
GnRH agonists
or antagonists (chemotherapy)).
Finally, the compounds of general formula (1) in connection with progesterone
receptor modulators, for example mesoprogestins, such as asoprisnil, can be
used,
specifically especially for use in hormone replacement therapy and for
treatment of
gynecological disorders.
In addition, the compounds of general formula (I) according to the invention
can be
used for the treatment of alopecia, caused by, for example, chemotherapy.
A therapeutic product that contains an estrogen and a pure antiestrogen for
simultaneous, sequential or separate use for the selective estrogen therapy of
perimenopausal
or postmenopausal conditions is already described in EP-A 0 346 014.
In terms of this invention, "Cl_5-alkyl group" is defined as a branched or
straight-
chain alkyl radical with up to 5 carbon atoms, which can be substituted by,
for example,
halogens, OH, or CN. As examples, methyl, ethyl, n-propyl, i-propyl, n-butyl,
i-butyl, tert-
butyl or n-pentyl can be mentioned.
The above-mentioned "C3_g-cycloalkyl group" according to the invention means a
monocyclic or bicyclic group, which can be substituted by, for example,
halogens such as
fluorine, chlorine or bromine, OH or CN, such as, for example, a cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl or a hydroxycyclohexyl group.
In terms of this application, the term "aryl group" is defined as a
substituted or
unsubstituted aryl radical with 6 to 15 carbon atoms, for example a phenyl
group, a
CA 02630438 2008-05-20
8
substituted phenyl group, such as a halophenyl group, or a nitrophenyl group,
or a naphthyl
group.
In terms of this application, the term "Cl-4-alkylene aryl group" is defined
as a di-
substituted alkyl radical, which is substituted with at least one aryl
radical. Both radicals
together have 7 to 15 carbon atoms, whereby the aryl radical can carry
additional
substituents, such as, for example, a halogen atom. Examples are a benzyl
group or a
halobenzyl group.
In terms of this application, the term "C1_4-alkylene-C3_8-cycloalkyl group"
is defined
as a di-substituted alkyl radical that is substituted with a C3_g-cycloalkyl
radical. Both
radicals together have 4 to 12 carbon atoms, whereby the cycloalkyl radical
can carry
additional substituents, such as, for example, a halogen atom. Examples are a
cyclopentylethyl, cyclohexylmethyl or cyclohexylethyl group.
In terms of this application, the term "C3_8-cycloalkylene-Ct_4-alkyl group"
is defined
as a di-substituted C3_8-cycloalkylene radical that is substituted with a C1_4-
alkyl radical.
Both radicals together have 4 to 12 carbon atoms, whereby the group can carry
additional
substituents, such as, for example, a halogen atom. Examples are a
propylcyclohexyl or
butylcyclohexyl group.
A trialkylsilyloxy group is, for example, a trimethylsilyloxy group or a tert-
butyldimethylsilyloxy group.
Within the scope of this invention, the term "halogen atom" is defined as a
fluorine,
chlorine, bromine or iodine atom. Fluorine, chlorine and bromine are
preferred.
The number "n" is preferably 0, 1 or 2.
Ri preferably means a group -SOzNH2, whereby R2, R3, X' and X, independently
of
one another, are preferably an H, F, or Cl atom, or an OH or a methoxy group.
RZ preferably means a group -SO2NH2, whereby R~, R3, X~ and X, independently
of
one another, are preferably an H, F, or Cl atom, or an OH or a methoxy group.
R3 preferably means a group -SO2NH2, whereby R~, R2, X' and X, independently
of
one another, preferably are an H, F, or Cl atom, or an OH or a methoxy group.
Xj is preferably an H atom.
R8 is preferably methyl, ethyl, vinyl, difluorovinyl, ethinyl or prop-l-inyl.
Methyl, ethyl, vinyl or difluorovinyl are especially preferred for R8.
CA 02630438 2008-05-20
9
Y is preferably OH, OMe, a trimethylsilyloxy, a tert-butyldimethylsilyloxy, a
benzoate, a sulfamoyl benzoate, acetate, propionate, valerate, butcyclate or
cyclopentylpropionate radical.
R17 preferably means an OH, a trimethylsilyloxy, an acetate, propionate,
valerate, a
benzoate, or an optionally halogenated sulfamoyl benzoate radical.
In terms of this invention, especially preferred compounds are cited below:
1) (3'-Hydroxy-8'(3-methyl-estra-I',3',5'(10')-trien-17'(3-yl) 3-sulfanioyl
benzoate
2) (3'-Hydroxy-8'(3-ethyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl
benzoate,
3) (3'-Hydroxy-8'p -vinyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl
benzoate,
4) (3'-Hydroxy-8'(3-methyl-estra-1',3',5'(I0')-trien-I7'(3-yl) 4-sulfamoyl
benzoate,
5) (3'-Hydroxy-8'p -ethyl-estra-1',3',5'(10')-trien-I7'(3-yl) 4-sulfamoyl
benzoate,
6) (3'-Hydroxy-8'(3-vinyl-estra-1',3',5'(10')-tri en-17'(3-yl) 4-sulfamoyl
benzoate,
7) (3'-Acetoxy-8'p-methyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfarnoyl
benzoate,
8) (3'-Acetoxy-8'(3-ethyl-estra-1',3',5'(10')-tri en-17'(3-yl) 3-sulfamoyl
benzoate,
9) (3'-Acetoxy-8'0-vinyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl
benzoate,
10) (3'-Acetoxy-8'(3-methyl-estra-1',3',5'(10')-trien-17'p-yl) 4-sulfainoyl
benzoate,
11) (3'-Acetoxy-8'(3-ethyl-estra-1',3',5'(10')-trien-17'(3-y1) 4-sulfamoyl
benzoate,
12) (3'-Acetoxy-8'(3-vinyl-estra-1',3',5'(10')-trien-17'(3-yl) 4-sulfamoyl
benzoate,
13) (3'-Benzoyloxy-8'(3-methyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl
benzoate,
14) (3'-Benzoyloxy-8'(3-methyl-estra-1',3',5'(10')-trien-17'p-yl) 4-sulfamoyl
benzoate,
15) (3'-Benzoyloxy-8'(3-ethyl-estra-1',3',5'(10')-trien-I7'(3-yl) 3-sulfamoyl
benzoate,
16) (3'-Benzoyloxy-8'(3-ethyl-estra-1',3',5'(10')-tri en-17'(3-yI) 4-sulfamoyl
benzoate,
CA 02630438 2008-05-20
17) (3'-Benzoyloxy-8'(3-vinyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl
benzoate,
18) (3'-Benzoyloxy-8'(3-vinyl-estra-1',3',5'(10')-trien-17'(3-yl) 4-sulfamoyl
benzoate,
19) (3'-Hydroxy-8' (3-vinyl-estra-1 ',3',5'(10' )-trien-17' (3-yl) 2-chloro-5-
sulfamoyl
benzoate,
20) (3'-Hydroxy-8'(3-vinyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl-4-
chloro-
benzoate,
21) (3'-Hydroxy-8'(3-methyl-estra-1',3',5'(10')-trien-17'(3-yl) 2-chloro-5-
sulfamoyl benzoate,
22) (3'-Hydroxy-8'(3-methyl-estra-1',3',5'(10')-trien-17' j3-yl) 3-sulfamoyl-4-
chlorobenzoate,
23) (3'-Hydroxy-8'(3-ethyl-estra-1',3',5'(10')-trien-l 7'(3-yl) 2-chloro-5-
sulfamoyl
benzoate,
24) (3'-Hydroxy-8'(3-ethyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl-4-
chloro-
benzoate,
25) (17'(3-(n-Pentanoyloxy)-8'(3-vinyl-estra-1',3',5'(10')-trien-3'-yl) 3-
sulfamoyl
benzoate,
26) (17'(3-(n-Pentanoyloxy)-8'(3-methyl-estra-1',3',5'(10')-trien-3'-yl) 3-
sulfamoyl benzoate,
27) (17' (3-(n-Pentanoyloxy)-8' (3-ethyl-estra-1',3',5'(10')-trien-3'-yl) 3-
sulfamoyl
benzoate,
28) (17'(3-Benzoyloxy-8'(3-vinyl-estra-I',3',5'(10')-trien-3'-yl) 3-sulfamoyl
benzoate,
29) (17' j3-Benzoyloxy-8'(3-methyl-estra-1',3',5'(10')-trien-3'-yl) 3-
sulfamoyl
benzoate,
30) (17'0-Benzoyloxy-8'0-ethyl-estra-1',3',5'(10')-trien-3'-yl) 3-sulfamoyl
benzoate,
31) (17'(3-(n-Pentanoyloxy)-8'(3-vinyl-estra-1,3',5'(10')-trien-3'-yl) 4-
sulfamoyl
benzoate,
CA 02630438 2008-05-20
11
32) (17'(3-(n-Pentanoyloxy)-8'(3-methyl-estra-1',3',5'(10')-trien-3'-yl) 4-
sulfamoyl benzoate,
33) (17'(3-(n-Pentanoyloxy)-8'(3-ethyl-estra-1',3',5'(10')-trien-3'-yl) 4-
sulfamoyl
benzoate,
34) (17'0-Benzoyloxy-8'0-vinyl-estra-1',3',5'(10')-trien-3'-yl) 4-sulfamoyl
benzoate,
35) (17'0-Benzoyloxy-8'p-methyl-estra-1',3',5'(10')-trien-3'-yl) 4-sulfamoyl
benzoate,
36) (17'(3-Benzoyloxy-8'(3-ethyl-estra-1',3',5'(10')-trien-3'-yl) 4-sulfamoyl
benzoate,
37) (17'P-Acetoxy-8'(3-vinyl-estra-1',3',5'(10')-trien-3'-yl) 3-
sulfamoylbenzoate,
38) (17'(3-Acetoxy-8'(3-ethyl-estra-1',3',5'(10')-tri en-3'-yl) 3-sulfamoyl
benzoate,
39) (17'(3-Acetoxy-8'(3-methyl-estra-1',3',5'(10')-trien-3'-yl) 3-sulfamoyl
benzoate,
40) (17'(3-Acetoxy-8'(3-vinyl-estra-1',3',5'(10')-trien-3'-yl) 4-sulfamoyl
benzoate,
41) (17'(3-Acetoxy-8'(3-ethyl-estra-1',3',5'(10')-trien-3'-yl) 4-sulfamoyl
benzoate,
42) (17'(3-Acetoxy-8'(3-methyl-estra-I',3',5'(10')-trien-3'-yl) 4-sulfamoyl
benzoate,
43) (3'-Methoxy-8'(3-vinyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl
benzoate,
44) (3'-Methoxy-8'(3-ethyl-estra-1',3',5'(10')-tri en-17'p-yl) 3-sulfamoyl
benzoate,
45) (3'-Methoxy-8'(3-methyl-estra-1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl
benzoate,
46) (3'-Methoxy-8' J3-vinyl-estra-1',3',5'(10')-trien-17'J3-yl) 4-sulfamoyl
benzoate,
47) (3'-Methoxy-8'(3-ethyl-estra-1',3',5'(10')-trien-17'(3-yl) 4-sulfamoyl
benzoate,
48) (3'-Methoxy-8'(3-methyl-estra-l',3',5'(10')-trien-17'p-yl) 4-sulfamoyl
benzoate.
CA 02630438 2008-05-20
12
In-Vivo Tests
Principle of the Test and Test Description:
Adult Wistar rats were ovariectomized 14 days after this operation for the
study of the
substances according to the invention. A treatment extended over 3 days (days
1-3), and on
day 4, the animals were sacrificed. Then, the recovery of plasma for hormone-
analytical and
clinical-chemical determinations and the determination of uterus weights were
carried out. In
satellite tests, correspondingly conditioned animals were sacrificed and
samples were taken
of their blood after one-time treatment and at other times (see Figures 1 and
2).
Figure 1 Pharmacokinetics of 8-Vinyl-E2 vs. (3'-Hydroxy-8(3'-vinyl-estra-
1',3',5'(10')-trien-17'(3-y1) 3-Sulfamoyl Benzoate (Single p.o. Administration
of 1000 g)
each point: n= 3 rats
7000
E 6000
5000
J
E = ..~,.=,
=.......
4000
W 3000
~
~ 2000
00
1000
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
hours
~-vehicle/p.o. =d= (3'-Hydroxy-8'b-vinyl-estra-1',3' 5'(10')-trien-17'b-yl) 3-
sulfamoylbenzoateF ~8-Vin I-E2/ .o.
P.O.
Increase of the 8(3-vinylestra-1,3,5(10)-triene-3,17(3-diol level (8-vinyl-E2)
in the plasma of
rats after I x oral administration of 1 mg/animal. Considerably greater
increase of the 8-
vinyl-E2 level after administration of I mg/animal of (3'-hydroxy-8(3'-vinyl-
estra-
1',3',5'(10')-trien-17'(3-yl) 3-sulfamoyl benzoate than after oral
administration of 8-vinyl-E2.
CA 02630438 2008-05-20
13
Figure 2 Kinetics of 8-Vinyl-E2 vs. (3'-Hydroxy-8'(3-vinylestra-1',3',5'(10')-
trien-
17'(3-yl) 4-Sulfamoyl Benzoate After p.o. Administration of 1 mg of 8-Vinyl-E2
or
Equimolar (3'-Hydroxy-8'p-vinylestra-1',3',5'(10')-trien-17'(3-yl) 4-Sulfamoyl
Benzoate
4000
3750 ....... 3500 3250
3000
2750
2500
2250
E 2000
rn 1750
Q' 1500
W 1250
= 1000
750
500
00 250 O
0
0 2 4 6 8 10 12 14 16 18 20 22 24
Time After Application
(hl
tVehiclepo y8-Vinyl-E2Jp.o. =Q=(3'-Hydroxy-8'b-vinylesira-1',3',5'(10')-trien-
17'4-yl)4-sulfamoylbenzoate/p.o.
Increase of the 8p-vinylestra-1,3,5(10)-triene-3,17p-diol level (8-vinyl-E2)
in the
plasma of rats after a one-time oral administration of I mg/animal. A greater
increase of the
8-vinyl-E2 level can be observed clearly after the administration of 1
mg/animal of (3'-
hydroxy-8'(3-vinylestra-1',3',5'(10')-trien-17'(3-yl) 4-sulfamoyl benzoate
than after oral
administration of 8-vinyl-E2.
In in vivo experiments in rats, it was found that after oral administration of
the
compounds according to the invention, an unexpectedly high increase of the
mother estrogen
can be noted. This is not the case, for example, in the 17-benzoates and 17-
acetates of 8(3-
vinyl-estradiol.
Unlike conventional estrogens, the substances according to the invention do
not have
any action on the uterus, the ovary and the liver.
CA 02630438 2008-05-20
14
In-Vitro Tests
a) Blood Plasma Concentration Ratio - Test Principle and Test Description:
The SO2-NH2 group of the substances according to the invention can lead to a
concentration in erythrocytes by binding to carbonic anhydrases. The
displacement of
estradiol-3-sulfamate from the erythrocyte bond is measured by test
substances.
Test preparation: Human blood is mixed with a mixture that consists of 14C-
labeled
and unlabeled estradiol sulfamate. At the selected working point, the
erythrocytes are
saturated, and the distribution in plasma/erythrocytes is 40:60. A second
blood sample is
mixed with a mixture that consists of 14C-labeled etradiol sulfamate and
unlabeled test
substance. The relative binding affinity is calculated from the portion of J4C-
labeled estradiol
sulfamate in the plasma: high proportion = strong displacement of'4C-estradiol
sulfamate
from the erythrocytes by the test substance = high binding affinity.
In contrast to the results published in WO 01/91797, the concentration ratios
of the
compounds according to the invention between erythrocytes and plasma do not
lie in a range
of 10-1000:1, but rather in a range <10:1. In the case of (3'-hydroxy-8(3'-
vinyl-estra-
1',3',5'(10')-trien-17'P-yl) 3-sulfamoyl benzoate, the ratio is, for example,
approximately
1.4:1.
b) Carbonic Anhydrase Inhibition - Test Principle and Test Description:
Carbonic anhydrases catalyze the CO2 hydration.
Test preparation: A constant CO2 stream is directed through a buffer that was
mixed
with carbonic anhydrase I or carbonic anhydrase II. The measuring parameter is
the time that
is required to drop the pH within defined limits. This parameter reflects the
formation of
H2CO3 in the medium. IC50-Inhibiting values are determined by test substances
being
pipetted into the test preparation. In the concentration areas that are
examined, the test
substances cause no to complete inhibition of the above-mentioned enzymes.
CA 02630438 2008-05-20
Table 1: IC50-Inhibiting Values of Human Carbonic Anhydrases I and tt
CAI CAII
Inhibitor
IC50 (nM) ICso (nM) IC50 (nM) IC50 (nM)
Literature Literature
Estradiol-3-sulfamate 157 10.6 - 21.6 1.5 -
(3'-H yd roxy-8' beta-vi nyl-
estra-1',3',5'(10')-trien- 3900 - 570 -
17'beta-yI) 3-
sulfamoylbenzoat
(3'-Hyd roxy-8'beta-vi nyl-
estra-1',3',5'(10')-trien- >10000 - >10000 -
17'beta- I -benzoat
Acetazolamid 1200 1900 60 90
(bekannter CA-Hemmer)
iLiterature: C. Landolfi, M. Marchetti, G. Ciocci, and C. Milanese, Journal of
Pharmacological and Toxicological Methods 38, 169-172 (1997).
[Key to Table 1:
-benzoat = -benzoate
Acetazolamid (bekannter CA-Hemmer) = Acetazolamide (of known CA inhibitors)
Despite the low blood-plasma concentration ratios, a binding (inhibition) to
the two
isoenzymes carbonic anhydrases CA I and CA II in the erythrocytes could be
shown in all
cases. The binding to erythrocytes induced by affinity to the carbonic
anhydrases is
important for the properties as estrogen. This binding is essential for a
reduced extraction of
the orally administered substance in the first liver passage. High or low
affinity to the
erythrocytic carbonic anhydrases, faster or delayed release from this depot
and subsequent
hydrolysis determine the therapeutic applicability of the substances according
to the
invention. The compounds according to the invention thus open up the
possibility of
achieving higher, shorter-term or uniformly low and longer-lasting hormone
levels with an
equimolar amount of substance administered. As a result, active strength and
duration of
action are varied and make possible a therapy adapted to the organism.
These test results open up many possible applications in the compounds of
general
formula (I) according to the invention for hormone replacement therapy (HRT)
and in
hormonally-induced diseases in men and women.
CA 02630438 2008-05-20
16
Subjects of this invention are therefore also pharmaceutical compositions that
contain
at least one compound of general formula (1), optionally together with
pharmaceutically
compatible adjuvants and vehicles.
Compared to their mother estrogens, the substances according to the invention
have
pharmacologically and pharmacokinetically improved properties that are based
on a reduced
hepatic extraction and more uniform and longer-lasting blood levels of the
released estrogen.
Dosage
The Erp-selective compounds of general formula (I) are administered orally for
use
according to the invention.
Suitable dosages of the compounds according to the invention in human$ for the
treatment of perimenopausal and postmenopausal symptoms, hormone-deficiency-
induced
symptoms, gynecological disorders such as ovarian dysfunction and
endometriosis, male and
female fertility disorders, hormone-induced tumor diseases as well as for the
use in male and
female hormone replacement therapy are, depending on indication, 5 g to 2000
mg per day,
depending on age and constitution of the patient, whereby the necessary daily
dose can be
administered one or more times.
For gynecological disorders such as ovarian dysfunction and endometriosis, in
this
case dosages of between 0.5 and 100 mg are considered; for the treatment of
male and female
fertility disorders, dosages of 5 g to 50 mg are considered; for hormone-
induced tumor
diseases, dosages of 5 to 500 mg are considered, and for male or female
hormone
replacement therapy, dosages of 5 g to 100 mg are considered.
In addition to commonly used vehicles and/or diluents, the pharmaceutical
compositions contain at least one compound of general formula I. The
substances according
to the invention can also be used therapeutically in combination with a
gestagen, antigestagen
or mesoprogestin. The substances according to the invention are preferably
used individually
as active ingredients in pharmaceutical preparations.
The pharmaceutical agents of the invention are produced in a known way with
the
commonly used solid or liquid vehicles or diluents and the commonly used
pharmaceutical-
technical adjuvants corresponding to the desired type of administration with a
suitable
dosage. The preferred preparations exist in a form for dispensing that is
suitable for oral
CA 02630438 2008-05-20
17
administration. Such forms for dispensing are, for example, tablets, film
tablets, coated
tablets, capsules, pills, powders, solutions or suspensions or else depot
forms.
Corresponding tablets can be obtained by, for example, mixing active
ingredient with
known adjuvants, for example inert diluents such as dextrose, sugar, sorbitol,
mannitol,
polyvinylpyrrolidone, explosives such as corn starch or alginic acid, binders
such as starch or
gelatins, lubricants such as magnesium stearate or talc and/or agents for
achieving a depot
effect, such as carboxylpolymethylene, carboxyl methyl cellulose, cellulose
acetate phthalate
or polyvinyl acetate. The tablets can also consist of several layers.
Coating cores, which are produced analogously to the tablets, with agents that
are
commonly used in tablet coatings, for example polyvinyl pyrrolidone or
shellac, gum Arabic,
talc, titanium oxide or sugar, can accordingly produce coated tablets. In this
case, the shell of
the coated tablet can also consist of several layers, whereby the adjuvants
that are mentioned
above in the tablets can be used.
Solutions or suspensions with the compounds of general formula I according to
the
invention can contain additional taste-improving agents such as saccharine,
cyclamate or
sugar, as well as, e.g., flavoring substances such as vanilla or orange
extract. In addition,
they can contain suspending adjuvants such as sodium carboxy methyl cellulose
or
preservatives, such as p-hydroxybenzoates.
The capsules that contain compounds of general formula I can be produced, for
example, by the compound(s) of general formula I being mixed with an inert
vehicle such as
lactose or sorbitol and encapsulated in gelatin capsules.
Suitable suppositories can be produced by, for example, mixing with vehicles
that are
provided for this purpose, such as neutral fats or polyethylene glycol or
derivatives thereof.
CA 02630438 2008-05-20
18
The examples below explain this invention without limiting it.
Example 1
(3'-Hydroxy-8'-vinylestra-1 ',3',5'(10')-trien-17'p-y1)-3-sulfamoyl benzoate
3,17 j3-Bis-(tert-butyldimethylsilyloxy)-8-vinyl-estra-1,3,5(10)-triene
1.0 g of 8-vinyl-estra-1,3,5(l0)-tri ene-3,170-diol was added in 18 ml of DMF
and
mixed with 2.8 g of imidazole and 3.6 g of tert-butyldimethylchlorosilane. The
solution was
stirred for 2 hours at room temperature and then extracted with n-hexane. The
organic phase
was washed with saturated aqueous common salt solution and water, dried on
sodium sulfate,
and concentrated by evaporation in a vacuum. In this way, 2.0 g of crude
3,17(3-bis-(tert-
butyldimethylsilyloxy)-8-vinyl-estra-1,3,5(10)-triene is obtained.
I H-NMR (CDCl3): 0.01, 0.03 (s, 3H, SiMe,t-Bu), 0.17 (s, 6H, SiMe,t-Bu), 0.73
(s,
3H, H-18), 0.88 (s, 9H, SiMe2t-Bu), 0.96 (s, 9H, SiMezt-Bu), 3.54 (t, 1H, H-
17), 6.49 (d, 1H,
H-4), 6.58 (dd, 1 H, H-2), 7.08 (d, 1 H, H-1).
3-(tert-Butyldimethylsilyloxy)-8-vinylestra-1,3,5(10)-trien-17 j3-o1
Variant 1
3.79 g of crude 3,17(3-bis-(tert-butyldimethylsilyloxy)-8-vinyl-estra-
1,3,5(10)-triene
from the last stage was dissolved at room temperature in 245 ml of THF and 145
ml of
acetonitrile. Then, a solution that consists of 240 ml of acetonitrile, 0.4 ml
of water, and 1.2
ml of chlorotrimethylsilane was produced, and 140 ml from this solution was
added in drops
to the steroid solution. After 21 hours, it was mixed with methylene chloride,
washed with
water, dried on sodium suflate and concentrated by evaporation in a vacuum.
The thus
obtained 2.84 g of crude product was purified by column chromatography on
silica gel
(cyclohexane/ethyl acetate 8:2). In this way, 640 mg (22%) of 3-(tert-
butyldimethyl-
silyloxy)-8-vinylestra-1,3,5(10)-trien-17(3-ol was obtained.
'H-NMR (CDC13): 0.17 (s, 6H, SiMet-Bu), 0.78 (s, 3H, H-18), 0.96 (s, 9H,
SiMe2t-
Bu), 3.63 (t, 1 H, H-17), 6.49 (d, 1 H, H-4), 6.58 (dd, 1 H, H-2), 7.08 (d,
IH, H-1).
CA 02630438 2008-05-20
19
Variant 2
100 mg of 3,17p-bis-(tert-butyldimethylsilyloxy)-8-vinyl-estra-1,3,5(10)-
triene was
dissolved in 30 ml of acetone, mixed with 3.5 ml of 5% hydrochloric acid and
stirred for 2
hours at room temperature. Then, it was mixed with water and ethyl acetate,
the organic
phase was separated, washed with water, dried on sodium sulfate and
concentrated by
evaporation in a rotary evaporator. After chromatographic purification of the
crude product
on silica gel (cyclohexane/ethyl acetate 9:1), 31 mg (40%) of colorless 3-
(tert-
butyldimethylsilyloxy)-8-vinylestra-1,3,5(l0)-trien-170-ol was obtained.
(3 '-(tert-Butyldimethylsilyloxy)-8'-vinylestra-1 ',3 ',5 '(10')-trien-17'P-
yl)-3-
chlorosulfonylbenzoate
300 mg of 3 -(tert-butyl dimethyl silyloxy)- 8 -vinylestra- 1,3,5 (1 0)-trien-
17 0 -ol was
dissolved in 15 ml of tetrahydrofuran (THF) and mixed with 150 mg of sodium
hydride.
Then, a solution that consists of 0.3 ml of 3-(chlorosulfonyl)-benzoyl
chloride in 3 ml of THF
was added in drops and refluxed for 4 hours. The cooled reaction solution was
poured onto
ice water, extracted with methylene chloride, the organic phase was dried on
sodium sulfate
and concentrated by evaporation in a rotary evaporator. After column-
chromatographic
purification on silica gel (cyclohexane), 198 mg (44%) of (3'-(tert-
butyldimethylsilyloxy)-8'-
vinylestra-1',3',5'(10')-trien-17'0-yl)-3-chlorosulfonylbenzoate was obtained
in this way.
(3'-(tert-Butyldimethylsilyloxy)-8'-vinylestra-1 ',3',5'(10')-trien-17' p-yl)-
3-sulfamoyl
benzoate
198 mg of (3'-(tert-butyldimethylsilyloxy)-8'-vinylestra-1 ',3',5'(10')-trien-
17'p-yl)-
3-chlorosulfonylbenzoate was mixed with 20 ml of inethylene chloride and 20
rnl of 25%
aqueous ammonia solution and stirred at room temperature. After 2 hours, it
was mixed with
water and methylene chloride, the phases were separated, and the organic phase
was washed
neutral with water. Affter drying on sodium sulfate, it was concentrated by
evaporation in a
rotary evaporator. Thus, 144 mg (75%) of (3'-(tert-butyldimethylsilyloxy)-8'-
vinylestra-
1 ',3',5'(10')-trien-17'P-yl)-3-sulfamoyl benzoate was obtained.
'H-NMR (CDC13): 0.15 (s, 6H, SiMet-Bu), 0.93 (s, 9H, SiMe2t-Bu), 0.93 (s, 3H,
H-
18), 4.83 (t, 1 H, H-17), 6.47 (d, 1 H, H-4), 6.56 (dd, 1 H, H-2), 7.09 (d, 1
H, H-1).
CA 02630438 2008-05-20
(3'-Hydroxy-8'-vinylestra-1 ',3',5'(10')-trien-17'0-yl)-3-sulfamoyl benzoate
90 mg of tetrabutylammonium fluoride was added to 144 mg of (3'-(tert-
butyldimethylsilyloxy)-8'-vinylestra-1 ',3',5'(10')-trien-17'p-yl)-3-sulfamoyl
benzoate in 10
ml of tetrahydrofuran, stirred for 2 hours at room temperature, and then mixed
with water and
methylene chloride. The organic phase was washed neutral with water, dried on
sodium
sulfate, and concentrated by evaporation in a rotary evaporator. The foamy
crude product
was purified by column chromatography on silica gel (cyclohexane/ethyl acetate
8:2). Thus,
36 mg (31%) of (3'-hydroxy-8'-vinylestra-1',3',5'(10')-trien-17'(3-yl)-3-
sulfamoyl benzoate
was obtained.
'H-NMR (CDC13): 0.92 (s, 3H, H-18), 4.82 (t, 1H, H-17), 6.39 (d, 1H, H-4),
6.48
(dd, 1 H, H-2), 7.00 (d, 1 H, H-1).
Example 2
(3'-Hydroxy-8'-vinylestra-1 ',3',5'(10')-trien-17'(3-yl)-4-sulfamoyl benzoate
((3'-(tert-Butyldimethylsilyloxy)-8'-vinylestra-1 ',3',5' (10')-trien-17'(3-
yl)-4-sulfamoyl
benzoate
750 mg (2.6 mmol) of 4-sulfamido-benzoyl chloride and 28 mg of 4-
dimethylaminopyri dine were added to 300 mg of 3-(tert-butyldimethylsilyloxy)-
8-vinylestra-
1;3,5(10)-trien-17(3-ol in 4 ml of pyridine and stirred at room temperature
for 2 hours. The
reaction mixture was poured into ice water, the precipitate was filtered off,
and the thus
obtained crude product (913 mg) was used without additional purification in
the next stage.
(3'-Hydroxy-8'-vinylestra-1 ',3',5'(10')-trien-17'p-yl)-4-sulfamoyl benzoate
278 mg of tetrabutylammonium fluoride was added to 913 mg of crude ((3'-(tert-
butyldimethylsilyloxy)-8'-vinylestra-1 ',3',5'(10')-trien-17'P-yl)-4-sulfamoyl
benzoate in 30
ml of tetrahydrofuran, and it was stirred at room temperature for 2 hours.
Then, the reaction
solution was taken up in methylene chloride and water, the organic phase was
washed with
water, dried on sodium sulfate and concentrated by evaporation in a vacuum.
The crude
product was purified by column chromatography on silica gel (cyclohexane,
ethyl acetate
1:1) and recrystallized from methanol. In this way, 147 mg (42%) of (3'-
hydroxy-8'-
vinylestra-1 ',3',5'(10')-trien-17'(3-yl)-4-sulfamoyl benzoate was obtained.
CA 02630438 2008-05-20
21
'H-NMR (CDC13): 0.96 (s, 3H, H-18), 4.87 (t, 1H, H-17), 6.51 (d, 1H, H-4),
6.60
(dd, 1 H, H-2), 7.10 (d, 1 H, H-1).
Without further elaboration, it is believed that one skilled in the art can,
using the
preceding description, utilize the present invention to its fullest extent.
The following
preferred specific embodiments are, therefore, to be construed as merely
illustrative, and not
limitative of the remainder of the disclosure in any way whatsoever.
In the foregoing and in the following examples, all temperatures are set forth
uncorrected in degrees Celsius, and all parts and percentages are by weight,
unless otherwise
indicated.
The preceding examples can be repeated with similar success by substituting
the
generically or specifically described reactants and/or operating conditions of
this invention
for those used in the preceding examples.
From the foregoing description, one skilled in the art can easily ascertain
the essential
characteristics of this invention and, without departing from the spirit and
scope thereof, can
make various changes and modifications of the invention to adapt it to various
usages and
conditions.
References:
l. Cummings, S. R.; Browner, W. S.; Bauer, D.; Stone, K.; Ensrud, K.; Jamal,
S. and Ettinger, B. (1998), Endogenous Hormones and the Risk of Hip and
Vertebral Fractures Among Older Women. N. Engl. J. Med. 339, 733-38.
2. Frank, G. R. (1995), The Role of Estrogen in Pubertal Skeletal Physiology:
Epiphyseal Maturation and Mineralization of the Skeleton. Acta Paediatr.
84(6), 627-30.
3. Goldzieher, J. W. (1990), Selected Aspects of the Pharmacokinetics and
Metabolism of Ethinyl Estrogens and their Clinical Implications. Am. J.
Obstet. Gynecol. 163, 318-22.
4. Gustafsson, J. A. (2000), Novel Aspects of Estrogen Action. J. Soc.
Gynecol. Investig. 7, S8-S9.
CA 02630438 2008-05-20
22
5. Helmer, O. M., and Griffith, R.S. (1952), The Effect of the Administration
of Estrogens on the Renin-Substrate (Hypertensinogen) Content on Rat
Plasma. Endocrinology 51, 421-6.
6. Kelly, J. J.; Rajkovic, I.A.; O'Sullivan, A. J.; Semia, C. and Ho, K. K. Y.
(1993), Effects of Different Oral Estrogen Formulations on Insulin-like
Growth Factor-I, Growth Hormone and Growth Hormone Binding Protein
in Post-Menopausal Women. Clin. Endocrinol. 39, 561-67.
7. Krattenmacher, R.; Knauthe, R.; Parczyk, K.; Walker, A.; Hilgenfeldt, U.
and Fritzemeier, K.-H. (1994), Estrogen Action on Hepatic Synthesis of
Angiotensinogen and IGF-I: Direct and Indirect Estrogen Effects. J.
Steroid. Biochem. Mol. Biol. 48, 207-14.
8. Le Roith and Butler, A. A. (1999), Insulin-like Growth Factors in Pediatric
Health and Disease. J. Clin. Endocrinol. Metab. 84, 4355-61.
9. Mandel, F. P.; Geola, F. L.; Lu, J. K. H.; Eggena, P.; Sambhi, M. P.;
Hershman, J. M. and Judd, H. L. (1982), Biologic Effects of Various
Doses of Ethinyl Estradiol in Postmenopausal Women. Obstet. Gynecol.
59, 673-9.
10. Mashchak, C. A.; Lobo, R. A.; Dozono-Takano, R.; Eggena, P.; Nakamura,
R. M.; Brenner, P. F. and Mishell, D. R., Jr. (1982), Comparison of
Pharmacodynamic Properties of Various Estrogen Formulations. Am. J.
Obstet. Gynecol. 144, 511-18.
11. Oelkers, W. K. H. (1996), Effects of Estrogens and Progestagens on the
Renin-Aldosterone System and Blood Pressure. Steroids 61, 166-7 1.
12. O'Sullivan, A. J. and Ho, K. K. Y. (1995), A Comparison of the Effects of
Oral and Transdermal Estrogen Replacement on Insulin Sensitivity in
Postmenopausal Women. J. Clin. Endocrinol. Metab. 80, 1783-8.
13. Span, J. P.T.; Pieters, G. F. F. M.; Sweep, C. G. J.; Herrnus, A. R. M. M.
and Smals, A. G. H. (2000), Gender Difference in Insulin-like Growth
Factor I Response to Growth Hormone (GH) Treatment in GH-Deficient
Adults: Role of Sex Hormone Replacement. J. Clin. Endocrinol. Metab. 85,
1121-5.
CA 02630438 2008-05-20
23
14. von Schoultz, B.; Carlstrom, K.; Coliste, L.; Eriksson, A.; Henriksson,
P.;
Pousette, A. and Stege, R. (1989), Estrogen Therapy and Liver Function -
Metabolic Effects of Oral and Parenteral Administration. Prostate 14, 389-
95.