Note: Descriptions are shown in the official language in which they were submitted.
CA 02633231 2017-01-13
CARDIAC MECHANICAL ASSESSMENT USING ULTRASOUND
CROSS-REFERENCE TO RELATED APPLICATION
This application claims the benefit of U.S. Provisional
Patent Application 60/941,778.
FIELD OF THE INVENTION
The present invention relates generally to systems and
methods for medical diagnosis, and specifically to systems
and methods for assessing the function of a moving organ,
such as the heart.
BACKGROUND OF THE INVENTION
Methods for three-dimensional (3-D) mapping of the
endocardium (i.e., the inner surfaces of the heart) are known
in the art. For example, U.S. Patent 5,738,096, describes a
method for constructing a map of the heart. An invasive probe
is brought into contact with multiple locations on the wall
of the heart. The position of the invasive probe is
determined for each location, and the positions are combined
to form a structural map of at least a portion of the heart.
In some systems, such as the one described by U.S.
Patent 5,738,096 cited above, additional physiological
properties, as well as local electrical activity on the
surface of the heart, are also acquired by the catheter. A
1
CA 02633231 2017-01-13
_
-
corresponding map incorporates the acquired local
information.
Some systems use hybrid catheters that incorporate
ultrasound imaging and position sensing, as well as
electrical sensing. For example, U.S. Patent 6,690,963,
describes a locating system for determining the location and
orientation of an invasive medical instrument that may
include an ultrasound imaging head, as well as an electrode.
A catheter with acoustic transducers may be used for
non-contact imaging of the endocardium. For example, U.S.
Patents 6,716,166 and 6,773,402, describe a system for 3-D
mapping and geometrical reconstruction of body cavities,
particularly of the heart.
As another example, U.S. Patent 5,876,345, describes an
ultrasonic catheter for two-dimensional (2-D) imaging or 3-D
reconstruction. The ultrasonic catheter includes at least two
ultrasonic arrays having good near and far field resolutions.
The catheter provides an outline of a heart chamber, in order
to assist in interpreting images obtained by the catheter.
Several methods are known in the art for non-contact
reconstruction of the endocardial surface using intracardial
ultrasonic imaging. For example, PCT Patent Publication WO
00/19908, whose disclosure is incorporated herein by
reference, describes a steerable transducer array for
intracardial ultrasonic imaging. The array forms an
ultrasonic beam, which is steered in a desired direction by
an active aperture. U.S. Patent 6,004,269, describes an
2
CA 02633231 2017-01-13
acoustic imaging system based on an ultrasound device that is
incorporated into a catheter. The ultrasound device directs
ultrasonic signals toward an internal structure in the heart
to create an ultrasonic image.
PCT Patent Publication WO 99/55233, describes a method
for delineating a 3-D surface of a patient's heart. A 3-D
mesh model is developed using training data, to serve as an
archetypal shape for a population of patient hearts. Multiple
ultrasound images of the patient's heart are taken in
different image planes. Anatomical locations are manually
identified in each of the images. The mesh model is rigidly
aligned with the images, in respect to the predefined
anatomical locations.
Other methods of contour extraction and 3-D modeling
using ultrasonic images are described in European Patent
Application EP 0961135. As another example, PCT Patent
Publication WO 98/46139, describes a method for combining
Doppler and B-mode ultrasonic image signals into a single
image using a modulated nonlinear mapping function.
U.S. Patent Application Publication 2006/0241445,
describes a method for modeling of an anatomical structure.
A plurality of ultrasonic images of the anatomical structure
are acquired using an ultrasonic sensor, at a respective
plurality of spatial positions of the ultrasonic sensor.
Location and orientation coordinates of the ultrasonic sensor
are measured at each of the plurality of spatial positions.
Contours-of-interest that refer to features of the anatomical
structure are marked in one or more of the ultrasonic images.
3
CA 02633231 2017-01-13
A three-dimensional (3-D) model of the anatomical structure
is constructed, based on the contours-of-interest and on the
measured location and orientation coordinates.
Other patents and patent applications of relevance to
the present invention include U.S. Patent 6,139,500, U.S.
Patent Application Publication 2005/0283075, U.S. Patents
6,447,453 and 6,447,454, U.S. Patent Application Publication
2005/014377, U.S. Patent Application
Publication
2005/0137661, and U.S. Patent 6,556,695.
SUMMARY OF THE INVENTION
Embodiments of the present invention that are described
hereinbelow provide improved methods for modeling and
analyzing motion of organs in the body, and particularly of
the heart.
In some of these embodiments, an acoustic imaging probe, such
as an ultrasound catheter within the heart, captures a
sequence of 2-D images as the heart beats.
Contours of a
heart chamber are identified, either
4
CA 02633231 2008-06-02
automatically or manually, in one of the 2-D images. An
image processor then automatically identifies these
contours in the other images in the sequence. The image
processor may analyze changes in the contours during the
heart cycle in order to determine parameters of motion of
the heart wall, such as local velocity and strain.
Additionally or alternatively, the image processor
may use the contours in segmenting the images and
reconstructing a "4-D" image of the heart, i.e., a 3-D
anatomical image that changes over time, showing the
motion of the heart. The moving image may be enhanced,
by addition of pseudocolor, for example, to show changes
over time in other physiological parameters, such as
local electrical parameters measured by a catheter inside
the heart.
There is therefore provided, in accordance with an
embodiment of the present invention, a method for
diagnosis, including:
capturing a sequence of two-dimensional ultrasound
images of a moving organ within a body of a patient;
identifying at least one contour of the organ in a
succession of the images in the sequence; and
processing the at least one identified contour to
generate an output indicative of motion of the organ over
time.
Processing the at least one identified contour may
include computing a displacement of the contour over a
5
CA 02633231 2008-06-02
period of cyclical movement of the organ, a velocity
vector of one or more segments of the contour, or a
strain in the organ responsively to a change in length of
the contour.
In disclosed embodiments, the moving organ is a
heart of the patient, and processing the at least one
identified contour includes analyzing the motion of a
wall of at least one chamber of the heart over one or
more cycles of the heart. Typically,
capturing the
sequence of the two-dimensional ultrasound images
includes inserting a catheter, including an acoustic
transducer and a position sensor, into the heart, and
capturing the two-dimensional ultrasound images using the
transducer while tracking coordinates of the catheter
using the position sensor. In one embodiment, analyzing
the motion of the wall includes find a location of scar
tissue in the wall responsively to the motion. In
another embodiment, analyzing the motion of the wall
includes comparing the motion of two or more chambers of
the heart so as to detect improper synchronization of the
motion of the chambers.
There is also provided, in accordance with an
embodiment of the present invention, a method for
diagnosis, including:
capturing multiple ultrasound input images of a
moving organ within a body of a patient;
6
CA 02633231 2008-06-02
collecting data that are indicative of respective
local values of a physiological parameter at locations on
a surface of the moving organ; and
generating a sequence of three-dimensional images,
responsively to the input images and the collected data,
showing movement of the organ while superimposing an
indication of changes in the local values on the surface
in the three-dimensional images as the organ moves in the
three-dimensional images in the sequence.
In some embodiments, capturing the multiple
ultrasound input images includes capturing two-
dimensional ultrasound images from multiple different
positions of an acoustic transducer, and recording
is location and orientation coordinates of the acoustic
transducer in the multiple different positions, and
generating the sequence includes combining the two-
dimensional ultrasound images using the location and
orientation coordinates to reconstruct the three-
dimensional images. Typically,
capturing the two-
dimensional ultrasound images includes recording
respective times of capture of the two-dimensional
ultrasound images relative to an annotation point in a
cycle of motion of the organ, and combining the two-
dimensional ultrasound images includes grouping the two-
dimensional ultrasound images according to the respective
times of capture in order to generate the three-
dimensional images corresponding to the respective times
in the cycle. In a
disclosed embodiment, the moving
organ is a heart of the patient, and capturing the two-
dimensional ultrasound images includes inserting a
7
CA 02633231 2008-06-02
catheter, including the acoustic transducer and a
position sensor, into the heart, and capturing the two-
dimensional ultrasound images using the transducer while
tracking coordinates of the catheter using the position
sensor.
Typically, generating the sequence includes coloring
the surface of the moving organ in the three-dimensional
images responsively to the values of physiological
parameter. In a disclosed embodiment, the moving organ
is a heart of the patient, and collecting the data
includes collecting electrical data, and coloring the
surface includes displaying variations in electrical
activity of the heart over an area of a chamber of the
heart in the course of one or more heart cycles.
There is additionally provided, in accordance with
an embodiment of the present invention, diagnostic
apparatus, including:
an acoustic transducer, which is configured to
capture a sequence of two-dimensional ultrasound images
of a moving organ within a body of a patient; and
an image processor, which is configured to identify
at least one contour of the organ in a succession of the
images in the sequence, and to process the at least one
identified contour to generate an output indicative of
motion of the organ over time.
8
CA 02633231 2008-06-02
There is further provided, in accordance with an
embodiment of the present invention, diagnostic
apparatus, including:
an acoustic transducer, which is configured to
capture multiple ultrasound input images of a moving
organ within a body of a patient;
an invasive probe, which is configured to collect
data that are indicative of respective local values of a
physiological parameter at locations on a surface of the
moving organ; and
an image processor which is configured to generate,
responsively to the input images and the collected data,
a sequence of three-dimensional images showing movement
of the organ while superimposing an indication of changes
in the local values on the surface in the three-
dimensional images as the organ moves in the three-
dimensional images in the sequence.
The present invention will be more fully understood
from the following detailed description of the
embodiments thereof, taken together with the drawings in
which:
BRIEF DESCRIPTION OF THE DRAWINGS
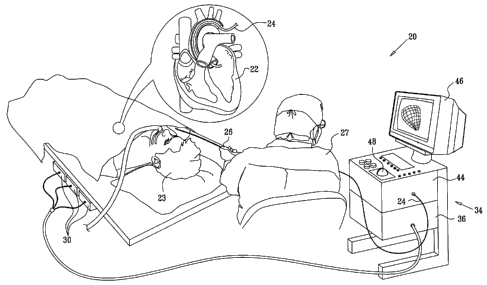
Fig. 1 is a schematic, pictorial illustration of a
system for cardiac mapping and imaging, in accordance
with an embodiment of the present invention;
Fig. 2 is a schematic side view of the distal end of
a catheter, in accordance with an embodiment of the
present invention;
9
CA 02633231 2008-06-02
Figs. 3 and 4 are schematic representation of
ultrasound images of a heart chamber at different,
respective points in the heart cycle, showing a moving
contour of the heart chamber in accordance with an
embodiment of the present invention;
Fig. 5 is a flow chart that schematically
illustrates a method for heart tissue characterization,
in accordance with an embodiment of the present
invention; and
Fig. 6 is a flow chart that schematically
illustrates a method for cardiac imaging, in accordance
with an embodiment of the present invention.
DETAILED DESCRIPTION OF EMBODIMENTS
SYSTEM DESCRIPTION
Reference is now made to Figs. 1 and 2, which
schematically illustrate a system 20 for imaging and
mapping a heart 22 of a patient 23, in accordance with an
embodiment of the present invention. The
system
comprises a catheter 24, which is inserted by a physician
27 into a chamber of the heart through a vein or artery.
Fig. 1 is a pictorial view of the system as a whole,
while Fig. 2 shows details of the distal end of the
catheter.
Catheter 24 is used, as described hereinbelow, to
acquire ultrasound images inside the heart and may, in
some embodiments, acquire other local physiological data,
as well, such as electophysiological data. Catheter 24
typically comprises a handle 26 for operation of the
CA 02633231 2008-06-02
catheter by the physician. Suitable controls (not shown)
on the handle enable the physician to steer, position and
orient the distal end of the catheter as desired.
Alternatively, the principles of the present invention
may be implemented using images captured by ultrasound
probes of other types, such as a transesophageal probe or
a non-invasive trans-thoracic probe.
System 20 comprises a positioning sub-system that
measures location and orientation coordinates of catheter
24. (Throughout this patent application and in the
claims, the term "location" refers to the spatial
coordinates of the catheter, and the term "orientation"
refers to its angular coordinates. The term "position"
refers to the full positional information of the
catheter, comprising both location and orientation
coordinates.)
In one embodiment, the positioning sub-system
comprises a magnetic position tracking system that
determines the location and orientation of catheter 24.
The positioning sub-system generates magnetic fields in a
predefined working volume its vicinity and senses these
fields at the catheter. For this purpose, the positioning
sub-system typically comprises a set of external
radiators, such as field generating coils 30, which are
located in fixed, known positions external to the patient
and generate electromagnetic fields in the vicinity of
heart 22. The generated fields are sensed by a position
sensor 32 inside catheter 24. In an alternative
embodiment, a radiator, such as a coil, in the catheter
11
CA 02633231 2017-01-13
.. -
generates electromagnetic fields, which are received by
sensors outside the patient's body.
Position sensor 32 transmits, in response to the sensed
fields, position-related electrical signals over cables 40
running through the catheter to a console 34. Alternatively,
the position sensor may transmit signals to the console over
a wireless link. The console comprises a positioning
processor 36, which controls coils 30 and calculates the
location and orientation of the distal end of catheter 24
based on the signals sent by position sensor 32. Positioning
processor 36 typically receives, amplifies, filters,
digitizes, and otherwise processes signals from catheter 24.
Some position tracking systems that may be used for this
purpose are described, for example, in U.S. Patents
6,690,963, 6,618,612 and 6,332,089, and U.S. Patent
Application Publications 2002/0065455 Al, 2004/0147920 Al and
2004/0068178 Al. Although the positioning sub-system shown in
Fig. 1 uses magnetic fields, the methods described below may
likewise be implemented using any other suitable positioning
sub-system, such as systems based on electrical impedance,
acoustic or ultrasonic measurements.
System 20 enables physician 27 to perform a variety of
mapping and imaging procedures, including display and
analysis of two-dimensional (2-D) ultrasound images, as well
as reconstruction of three-dimensional (3-D) images
12
CA 02633231 2008-06-02
of target structures, such as chambers of the heart,
based on the 2-D ultrasound images. The system can also
register, overlay and display a parametric map, such as
an electrophysiological information map or an electro-
anatomical map on the ultrasound images, as well as
registering the ultrasound images with a 3-D image
acquired from an external system, such as a computed
tomography (CT) or magnetic resonance imaging (MRI)
system. Some of these aspects of system 20 are described
in the above-mentioned US 2006/0241445, while other novel
aspects are described further hereinbelow.
As shown in Fig. 2, the distal end of catheter 24
comprises an ultrasonic imaging sensor 38, which
typically comprises an array of ultrasonic transducers
40, such as piezo-electric transducers.
Transducers 40
operate as a phased array, jointly transmitting an
acoustic beam. (Although the transducers are shown
arranged in a linear array configuration, other array
configurations can be used, such as circular or convex
configurations.) In one embodiment, the array transmits
a short burst of ultrasound energy and then switches to a
receiving mode for receiving the ultrasound signals
reflected from the surrounding tissue.
Typically, transducers 40 are driven individually in
a controlled manner in order to steer the ultrasound beam
in a desired direction. By
appropriate timing of the
transducers, the ultrasound beam can produced by sensor
38 be given a concentrically curved wave front, so as to
focus the beam at a given distance from the transducer
-.- --MSNP 13
CA 02633231 2008-06-02
array. Thus,
system 20 uses the transducer array as a
phased array and implements a transmit/receive scanning
mechanism that enables the steering and focusing of the
ultrasound beam, so as to produce 2-D ultrasound images.
After receiving the reflected ultrasound echoes,
transducers 30 send electric signals based on the
reflected echoes over cables 42 through catheter 24 to an
image processor 44 in console 34. The
image processor
transforms the signals into 2-D ultrasound images, which
are typically sector-shaped. Image
processor 44
typically computes or receives catheter position
information from positioning processor 36 and uses this
information in performing image reconstruction and
analysis functions, which are described in greater detail
below. In some embodiments, the image processor uses the
ultrasound images and the positional information to
produce a 3-D image or 4-D image sequence of a target
structure, which is presented to the physician as a 2-D
projection on a display 46. The physician may interact
with the displayed image and with console 34 generally by
means of a user interface device 48, such as a trackball
or other pointing device.
In some embodiments, the distal end of catheter 24
also comprises at least one electrode 49 for performing
diagnostic and/or therapeutic functions, such as electro-
physiological mapping and/or radio frequency (RF)
ablation. In one embodiment, electrode 49 is used for
sensing local electrical potentials. The electrical
potentials measured by electrode 49 may be used in
14
CA 02633231 2008-06-02
mapping the local electrical activity on the endocardial
surface. When electrode 49 is brought into contact or
proximity with a point on the inner surface of the heart,
it measures the local electrical potential at that point.
The measured potentials are converted into electrical
signals and sent through the catheter to the image
processor for processing and display. In other
embodiments, the local electrical potentials are obtained
from another probe, such as a second catheter (not shown
in the figures), comprising suitable electrodes and a
position sensor, all connected to console 34.
In alternative embodiments, catheter 24 may comprise
sensors in other configurations. For
example, although
electrode 49 is shown as being a single ring electrode,
the catheter may comprise any number of electrodes in any
form.
Additionally or alternatively, the catheter may
sense other physiological parameters, such as various
tissue characteristics, temperature and/or blood flow.
Position sensor 32 is typically located within the
distal end of catheter 24, adjacent to electrode 49 and
transducers 40. Typically, the mutual locational and
orientational offsets between the position sensor,
electrode, and transducers are constant. These
offsets
are used by positioning processor 36 to derive the
coordinates of ultrasonic sensor 38 and of electrode 49,
given the measured position of position sensor 32.
Further characteristics of the position sensor and its
use are described in the above-mentioned US 2006/0241445.
CA 02633231 2008-06-02
Typically, both the ultrasound images and the
position measurements are synchronized with the heart
cycle, by gating signal and image capture relative to a
body-surface electrocardiogram (ECG) signal or intra-
cardiac electrocardiogram. Since
features of the heart
change their shape and position during the heart's
periodic contraction and relaxation, console 34 records
the timing of each image captured by sensor 38 relative
to an annotation point (such as the QRS peak of the ECG)
in the heart cycle, along with the corresponding position
measurement. Thus, the images may be grouped according
to the different points in the heart cycle at which they
were captured. In some
embodiments, additional
measurements taken by the catheter, such as measurements
of electrical and other tissue characteristics, are also
synchronized to the ECG signal, as well as with the
corresponding position measurements. The
results of
these additional measurements may then be overlaid on the
reconstructed 3-D ultrasound image, as described further
hereinbelow.
Typically, positioning processor 36 and image
processor 44 comprise one or more general-purpose
computer processors, which are programmed in software to
carry out the functions described herein. The software
may be downloaded to the computer in electrical form,
over a network, for example, or it may, alternatively or
additionally, be stored on tangible media, such as
optical, magnetic or electronic memory media. The
positioning processor and image processor may be
implemented using separate computers or using a single
16
CA 02633231 2008-06-02
computer, or may be integrated with other computing
functions of system 20.
Additionally or alternatively,
at least some of the positioning and image processing
functions may be performed using dedicated hardware.
TRACKING AND ANALYSIS OF CONTOURS
Reference is now made to Figs. 3-5, which
schematically illustrate a method for heart tissue
characterization based on ultrasound images, in
accordance with an embodiment of the present invention.
Figs. 3 and 4 show 2-D ultrasound images 50 and 52,
respectively, of heart 22, which are used in the method,
while Fig. 5 is a flow chart that presents the steps of
the method itself. Images 50
and 52 are processed by
image processor 44 to identify contours 54 and to perform
other functions that are described hereinbelow on the
basis of these contours. As noted
earlier, images for
this sort of processing may be acquired not only using an
ultrasound catheter, but also using any other suitable
type of acoustic imaging system that is known in the art.
To acquire images 50 and 52, the user (such as
physician 27) moves catheter 24 inside the heart until
the desired point of view is achieved, such as the view
shown in Figs. 3 and 4. The user then operates system 20
to capture a "clip," i.e., a sequence of 2-D ultrasound
images at the desired position, at an image capture step
60. The images show a certain "slice" of a heart chamber
and the surrounding tissue at multiple points in time
17
CA 02633231 2008-06-02
over the course of one or more heart cycles.
(Typically
the clip is about 2.5 seconds long.)
The user freezes an ultrasound image in the sequence
and draws contour 54 on the 2-D image, at a contour
identification step 62.
Alternatively or additionally,
processor 44 may apply automatic edge detection to locate
the contour. The image is marked with the point in the
heart cycle at which it was captured.
Typically, as
noted earlier, the timing of the image is marked relative
to an annotation point in the electrocardiogram (ECG)
signal, which is captured using skin-surface electrodes
and a suitable monitor (not shown), but any other
suitable means for identifying the annotation point may
alternatively be used. Figs. 3 and 4 show a contour of
one chamber of the heart, but the methods described
herein may similarly be applied to multiple contours of
multiple chambers.
Contour 54 is initially drawn on one of the images
in the sequence, typically (although not necessarily) the
image captured at the annotation point itself. For the
sake of illustration, it will be assumed that image 50 is
the annotation image on which the contour is initially
drawn. After contour 54 has been drawn on image 50,
image processor 44 uses this contour to find the
corresponding contours in all the other images of the
image sequence between successive annotation points, at a
contour propagation step 64. Thus, based on contour 54
in image 50, the image processor finds the corresponding
contour in image 52. The
frame rate in the video
18
CA 02633231 2008-06-02
sequence is typically 30 frames per second, but rates up
to 100 frames per second may enable better estimation of
the tissue characteristics.
In addition to detecting the contours, image
processor 44 may calculate velocity vectors,
corresponding to the movement of a contour or contours
during the sequence, at a velocity calculation step 66.
To determine the local velocity of segments 56 of a
contour, for example, the image processor sweeps a
rectangular window over the selected contour in
successive image frames. Any suitable window size may be
used, for example, 5 x 10 pixels. The processor computes
a correlation function between windows from the
successive frames as a function of displacement between
the windows. The movement in the x and y directions that
maximizes the correlation function gives the local
displacement of the contour in the window in x and y
directions. Knowing
the time difference between
successive frames and the displacement, the local
velocity can be calculated as the quotient of the
displacement divided by the time difference. The
velocity vector is the combination of the velocity
components in the x and y directions.
Referring to Figs. 3 and 4, it can be seen that the
segments in the central part of contour 54 have velocity
components mainly in the upward direction.
The image processor may also perform strain
analysis, at a local strain calculation step 68. To
19
CA 02633231 2017-01-13
, ..
,
compute the strain along contour 54, the contour is segmented
into a number of segments 56 of known length.
In the
subsequent image frame, the same contour is identified and
segmented into the same number of segments. The difference
between the lengths of two corresponding segments from the
two frames divided by the length of the segment in the first
frame gives the strain on the segment.
Further information regarding strain computations of
this sort are presented by Stoylen in a thesis entitled,
"Strain Rate Imaging of the Left Ventricle by Ultrasound,"
Norwegian University of Science and Technology (2001), which
is available
at
http://folk.ntnu.no/stoylen/strainrate/thesis_AS.pdf.
Other calculations can also be done on the identified
moving contours.
For example, the displacement of contours
and segments of contours during the heart cycle may be
calculated.
Image processor 44 outputs the calculation results, at
an output step 70, typically by showing 2-D or 3-D images on
display 46.
The results can be displayed on the actual
ultrasound images in the video sequence, for example, showing
the identified contours and the calculated parameters
(velocity vectors, strain, etc.)
The magnitudes of a
parameter of interest over segments 56 may be shown by color-
coding the segments accordingly.
CA 02633231 2008-06-02
The parameters that are derived and output in this
manner may be used in characterizing the tissue, either
automatically by processor 44 or visually by a user of
system 20. Anomalies in the velocity and/or displacement
of certain contour segments can be used, for example, for
scar tissue identification (particularly in combination
with information provided by other imaging modalities,
such as MRI). As
another example, differences in the
instantaneous velocity between contours in different
parts of the heart (such as in different chambers) can be
used to assess the synchronization between the chamber
walls, as well as other diagnostic indicators of the
mechanical functioning of the heart. Some of
these
indicators may be combined with electrophysiological
diagnostic information, which may be provided by catheter
24 or by another mapping catheter within the heart. For
example, some of the methods for cardiac mechanical and
electromechanical diagnosis that are described in the
above-mentioned U.S. Patent 5,738,096 may also be
applied, mutatis mutandis, using the diagnostic
information provided by the moving contours that are
detected in ultrasound images as described above.
4-D IMAGE SEQUENCES BASED ON CONTOUR MAPPING
Fig. 6 is a flow chart that schematically
illustrates a method for cardiac imaging, in accordance
with an embodiment of the present invention. In this
method, the moving contours provided by sequences of
ultrasound images are combined with electro-anatomical
21
CA 02633231 2008-06-02
mapping data, such as the type data produced by the CARTO
mapping system (Biosense Inc., Diamond Bar, California).
A user, such as physician 27, aims catheter 24 in a
desired direction in heart 22, and captures a clip of 2-D
ultrasound images, at an image capture step 72. The user
operates the system as described above with reference to
Fig. 5 so as to identify contours in all the frames in
the clip. The user
then moves the catheter, captures
another clip of images, and identifies new contours if
necessary. Alternatively, the user may move the catheter
continually while acquiring the images. In any case, as
explained above, each of the ultrasound images is
associated with a certain point in time relative to an
annotation point in the heart cycle and the position of
the catheter at which the image was recorded. Each image
is thus marked with the time of acquisition, relative to
the annotation point, and with the catheter position
coordinates at the time of acquisition.
In addition, for each time slot in the heart cycle,
a corresponding CARTO map is generated, at a mapping step
74. For
example, at a frame rate of 30 frames per
second, there will be maps in time slots of 33 ms. For
this purpose, the user brings electrode 46 on catheter 24
(or an electrode or electrodes on a separate mapping
catheter) into contact with points on the inner surface
of one or more of the heart chambers. Although steps 72
and 74 are shown in Fig. 6 as occurring separately and
sequentially, the order of these steps may be reversed,
or the steps may be interleaved, without any particular
22
CA 02633231 2017-01-13
_
constraints on the order of acquisition of ultrasound images
relative to acquisition of electrical mapping data.
When the user has finished imaging, mapping and
identifying all the desired contours, image processor 44
produces a moving image of the heart overlaid with an
electro-anatomical CARTO map for every time slot, at an image
output step 76. The image processor uses the position data
provided by position sensor 32 in the catheter in order to
align the ultrasound images with the CARTO data in the same
3-D coordinate frame. Each contour in the ultrasound images
is thus associated with the CARTO map for the corresponding
time slot.
The geometrical shape of the CARTO map may be
updated according to the contours, as described, for example,
in the above-mentioned US 2006/0241445, as well as in U.S.
Patent Application Publication 2007/0106146.
To reconstruct 3-D and 4-D images, the 2-D fan images are
grouped by acquisition time (relative to the heart cycle).
Typically, the images are divided into between fifteen and
thirty time groups in this manner. The images in each group
are then combined, using the location and orientation
coordinates, into a 3-D volume matrix.
In other words, the
images are stored in 3-D matrices, with a corresponding
matrix for each time slot. System 20 may give the user an
indication of the amount of data acquired in each time slot
matrix so as to assist the user in knowing when to terminate
data acquisition.
23
CA 02633231 2008-06-02
To segment the 3-D images, processor 44 may select a seed
point inside the heart chamber that is to be segmented.
It then spreads the chamber volume outward from this seed
point in order to segment the chamber, using the contours
that were found at step 72. Alternatively, other methods
that are known in the art may be used to reconstruct the
surfaces of the heart chamber. At the conclusion of this
stage, for each time slot there is a segmented CT-like
image generated from the 3-D volume.
Following step 76, processor 44 is able to display
the moving volumes of the heart using 3-D volume-
rendering techniques, with numbers or other visual cues
to show the electrical activity on the inner heart
surface. These 3-D
images can be displayed as a clip,
showing the heart motion and electrical activity in a
"four-dimensional" (4-D - 3-D plus time) display. By
interpolation of the electrical activity in the CARTO
maps, the electrical parameters of interest may be
interpolated over the entire heart wall surface, and the
map of the heart can be colored according to the
parameters. The colors change and move over the course
of each heart cycle, thereby enabling the user to
visualize the interaction between the electrical and
mechanical activity of the heart. Other parameters, such
as temperature or chemical parameters, may be displayed
in 4-D in a similar manner.
Alternatively, upon the
user's command, system 20 may display only the moving
contours, and optionally the calculated mechanical
parameters, such as the velocity vector and strain, that
24
CA 02633231 2008-06-02
were described above. Volume
calculations can also be
performed on the 4-D images.
The user of system 20 views and analyzes the moving
images in order to identify characteristics of the heart
tissue, at a diagnosis step 78. For
example, the user
may identify areas of scar tissue based on their weak
electrical parameters and deviant mechanical behavior.
As another example, the user may use the moving images to
diagnose improper coordination between different chambers
of the heart, as expressed by abnormal timing of
mechanical and/or electrical changes over the course of a
heart cycle. Such
abnormalities typically occur, for
example, in congestive heart failure. The user may then
apply the visual information provided by system 20 in
deciding where to place pacing leads in the heart for
purposes of cardiac resynchronization therapy or to meet
other therapeutic goals.
It will be appreciated that the embodiments
described above are cited by way of example, and that the
present invention is not limited to what has been
particularly shown and described hereinabove. Rather,
the scope of the present invention includes both
combinations and subcombinations of the various features
described hereinabove, as well as variations and
modifications thereof which would occur to persons
skilled in the art upon reading the foregoing description
and which are not disclosed in the prior art.