Note: Descriptions are shown in the official language in which they were submitted.
CA 02633676 2008-06-17
WO 2007/111739
PCT/US2006/062042
THE USE OF MOFS IN PRESSURE SWING ADSORPTION
BACKGROUND OF THE INVENTION
[0001] The present invention relates to adsorption processes, and
more particularly to
pressure swing adsorption processes. The process employs metal-organic
framework
materials having a high porosity and high surface areas, and are useful in the
separation of
hydrocarbons from hydrogen streams.
[0002] It is often necessary to separate one or more components from a
gas mixture to
generate a purified gas. This can be done for removing an impurity from a gas
stream or for
concentrating a component or components within a gas stream.
[0003] One technique for separation of one component in a gas from a
mixture uses
adsorption of one or more components from the mixture onto an adsorbent. This
process is
further enhanced through pressure swing adsorption (PSA). Pressure swing
adsorption entails
passing a feedstream over an adsorbent where one, or more, components of the
feedstream are
selectively adsorbed onto the adsorbent, and where the process of adsorption
is performed at a
relatively high pressure. The adsorbent is regenerated by reducing the
pressure over the
adsorbent, and a process of desorption is performed at the relatively low
pressure. The
desorption process can also be accompanied by the passing of a purge gas
having a low
concentration of the adsorbate to enhance desorption.
[0004] The separation of gases from a gas mixture through adsorption in
a pressure swing
adsorption process is controlled by the pressures used in the process and the
capacity of the
adsorbent for one, or more, of the components in the gas mixture. The process
usually entails
a tradeoff between the range in pressure, and the load capacity of the
adsorbent for many of
the materials used. It is desirable to be able to use materials that can
overcome some of these
tradeoffs.
SUMMARY OF THE INVENTION
[0005] The invention is a pressure swing adsorption process for removing
hydrocarbons
from a hydrogen stream. The process passes the hydrogen stream over a metal
organic
framework material at a high adsorption pressure, generating an effluent
stream with a
reduced hydrocarbon content. The process then reduces the pressure over the
metal organic
CA 02633676 2013-07-30
=
framework material and releases the hydrocarbon from the material, and
generates a stream
having hydrocarbons. The process steps are then repeated. In one embodiment,
the process
uses multiple adsorption beds comprising the metal organic framework material
and cycles
the pressures sequentially through the beds to produce a continuous process.
[0005.1] According to one aspect of the present invention there is provided a
pressure
swing adsorption process for the removal of hydrocarbons from a hydrogen
feedstream
comprising (a) passing the feedstream comprising hydrogen and at least one
hydrocarbon
constituent over an adsorbent, wherein the adsorbent comprises a metal organic
framework
(MOF) material, in an adsorption zone at a temperature and adsorption pressure
sufficient to
adsorb at least a portion of the hydrocarbon constituent in the feedstream and
thereby
generating an effluent hydrogen stream having a reduced hydrocarbon content,
continuing to
pass the feedstream over the adsorbent for a time until the adsorbent has
substantially reached
its adsorption capacity; (b) reducing the pressure in the adsorption zone to a
desorption
pressure and for time sufficient to desorb at least a portion of the
hydrocarbon therefrom and
withdrawing a desorption effluent stream having an enriched hydrocarbon
content; and
repressurizing the adsorption zone to the adsorption pressure and repeating
the steps (a) and
(b).
[0006] Additional objects, embodiments and details of this invention can
be obtained
from the following detailed description of the invention.
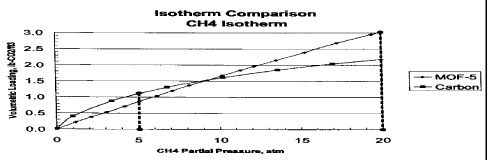
[0007] The Figure is the comparison of CH4 adsorption on carbon and MOF-5.
DETAILED DESCRIPTION OF THE INVENTION
[0008] The separation of gases from a gas mixture through adsorption in
a pressure swing
adsorption process is controlled by the difference between adsorption and
desorption
pressures and capacity of one of the components in the gas mixture. The
process usually
entails a tradeoff between the pressure differences and the capacity for many
of the materials
used. The capacity is the amount of material adsorbed by the adsorbent. It is
desirable to be
able to use materials that can overcome some of these tradeoffs.
[0009] In pressure swing adsorption, a gas made up of at least two
constituents, is
separated using the differences in selectivity of one of the constituents.
Usually, the gas is
- 2 -
CA 02633676 2013-07-30
=
purified by selectively removing an undesired constituent of the gas. The gas
is typically fed
into an adsorption unit at an elevated pressure, where one of the constituents
is preferentially
adsorbed onto an adsorbent. While one constituent is preferentially adsorbed,
other
constituents are also adsorbed, and it is desired to use adsorbents that have
significant
differences in the adsorption of the desired constituents.
100101 The adsorbent is regenerated through reversing the adsorption
process to desorb
the constituents. This is done by changing the conditions of the adsorbent
environment
through reducing the pressure. At a defined time or conditions, the gas feed
to the adsorption
unit is stopped, and the adsorption unit is depressurized. Preferably, the gas
feed is stopped
when the adsorption unit is near or at capacity for the adsorbent with the
desired constituent.
The adsorption unit is depressurized to a specified level where the adsorbed
constituents
desorb generating a desorbent stream that is relatively rich in the
constituent that is more
strongly adsorbed onto the adsorbent. The desorption process can use an inert
gas, or a non-
hydrocarbon gas to facilitate the desorption process. The desorption gas is
passed over the
=
- 2a -
CA 02633676 2008-06-17
WO 2007/111739
PCT/US2006/062042
adsorbent to remove the adsorbed constituents as they desorb from the
adsorbent. Preferably,
the desorption gas is passed over the adsorbent in a direction opposite the
direction of the
feed gas to regenerate the adsorbent.
[0011] An aspect of a pressure swing adsorption system is the isotherm
for adsorbing a
component in a gas dictates the operating pressures and loading onto the
adsorbent. Most
materials have an isotherm, wherein the saturation limit is rapidly
approached, and then there
is a small incremental improvement in adsorption for a relatively large
increase in pressure.
The working capacity of an adsorbent is defined as difference in the amount of
the adsorbed
components on the adsorbent between the adsorption pressure and the
desorption, or
regeneration, pressure. Lowering the regeneration pressure can increase the
capacity of the
adsorbent for selectively removing a component from a gas, but the effluent
stream from the
regeneration step may need to be recompressed. However, a lower regeneration
pressure
increases the recompression costs.
[0012] In pressure swing adsorption, there are many classes of
adsorbents that are
suitable. The selection is dependent upon the feed gas constituents and other
factors
generally known to those skilled in the art. In general, suitable adsorbents
include molecular
sieves, silica gels, activated carbons, activated aluminas, and other porous
metal oxides.
When purifying methane containing streams, the methane is often adsorbed along
with the
impurities that one wishes to remove. The choice of adsorbent presents
problems in selecting
an adsorbent that has the greatest differential in adsorption between hydrogen
and selected
impurities, especially light hydrocarbons such as methane and ethane.
[0013] To overcome the tradeoffs and improve PSA, the search is for a
high permeability
material that also has a high capacity for use in a pressure swing adsorber.
This means a
material with a very high surface area and a high porosity. It is desired to
increase the loading
of the adsorbent, while minimizing recompression requirements. This translates
to higher
desorption pressures.
[0014] One embodiment of the invention is a process using pressure
swing adsorption to
remove methane and other light hydrocarbon compounds, such as ethane, from a
hydrogen
feedstream. The process comprises passing a hydrogen feedstream having
hydrocarbons over
an adsorbent in an adsorption zone, and at a temperature and pressure
sufficient to adsorb a
portion of the hydrocarbons. The remaining gases in the feedstream becomes an
effluent
- 3 -
CA 02633676 2008-06-17
WO 2007/111739
PCT/US2006/062042
stream having a reduced hydrocarbon content. The adsorbent in the process is a
material
known as a metal organic framework (MOF), and has a high surface area and high
porosity.
The surface area of the material is greater than 1500 m2/gm. The pressure in
the adsorption
zone is then reduced to a pressure for desorbing the hydrocarbons, and
generates a desorption
effluent stream having an enriched hydrocarbon content. The effluent stream
will have an
increased methane content, as methane is the primary light hydrocarbon in the
hydrogen
feedstream. Other light hydrocarbons include ethane, propane, butanes, and
small amounts of
other hydrocarbons. The process during desorption can include passing a carbon
dioxide lean
purge gas over the adsorbent.
[0015] The process can be carried out by either passing the adsorbent bed
through a high
pressure adsorption zone, and then moving the adsorbent bed to a low pressure
desorption
zone, such as occurs with an adsorbent wheel in a rotating drum adsorber. The
process can
also be carried out by alternately pressurizing the adsorbent bed and passing
the feedstream
over the bed, and depressurizing the adsorbent bed and passing a purge gas
over the bed.
[0016] These processes are improved and made continuous by using a sequence
of at least
two adsorbent beds, wherein the beds are cycled through the adsorption and
desorption steps
in a sequential manner to provide a continuous operation. The process of
cycling the
adsorbent beds comprises pressurizing a first adsorbent bed to an adsorption
pressure and
flowing the feedstream over the first adsorbent bed, while depressurizing a
second adsorbent
bed to a desorption pressure and flowing a purge stream over the second
adsorbent bed.
Switching the feedstream and the purge streams to the second adsorbent bed and
first
adsorbent bed respectively, and pressurizing the second adsorbent bed to the
adsorption
pressure and flowing the feedstream over the second adsorbent bed, while
depressurizing the
first adsorbent bed to the desorption pressure and flowing the purge stream
over the first
adsorbent bed. The process can be further smoothed with respect to pressure
changes by
additional beds, wherein intermediate beds are pressurized or depressurized
before switching
flows.
[0017] In the process for reducing hydrocarbon content, and
particularly methane, in a
hydrogen feedstream, the feedstream is passed over the adsorbent, in a first
adsorbent zone, at
the highest pressure of the process, with the hydrocarbons adsorbed,
generating a hydrocarbon
depleted hydrogen stream. The hydrocarbon depleted hydrogen discharges from
the
- 4 -
CA 02633676 2008-06-17
WO 2007/111739
PCT/US2006/062042
adsorption zone so that hydrocarbon adsorption front is formed in the zone at
the hydrogen
feedstream inlet end and progressively moves toward the outlet. Preferably,
the adsorption
zone is sized to produce a hydrogen gas product with a hydrocarbon
concentration less than
1% by volume. The feedstream to the adsorbent unit is terminated when either
the
hydrocarbon adsorption front is at a predetermined point in the adsorption
unit, or when there
is an increase in the hydrocarbon in the hydrogen stream to above a
predeteimined value. The
feedstream is then terminated to the first adsorption zone, and directed to a
second adsorption
zone. The first adsorption zone is depressurized and a purge gas is passed
through the first
adsorption zone to regenerate the adsorbent in the first adsorption zone. The
purge gas
preferably flows in a counter current direction relative to the flow of the
feedstream in the
adsorption zones to remove the hydrocarbons in the reverse direction that they
were adsorbed.
[0018] When the first zone has been regenerated, it is repressurized to
the pressure level
for the feedstream, the feedstream is switched to the first zone, and the
second adsorption
zone is depressurized and regenerated with a purge gas at regeneration
conditions, and the
process cycle is repeated.
[0019] The operating conditions for the pressure swing adsorption
process include
adsorption pressures from 2 MPa (20 atms.) to 5 MPa (50 atms.). The desorption
pressure is
in a range from 1 kPa (1 atm) to 1.5 MPa (15 atms.), with a preferred range
from 500 kPa (5
atm) to 1 MPa (10 atms.). The desorption step is preferably operated at a
pressure sufficient
to minimize recompressing the desorption effluent stream. The adsorbent needs
to be
thermally stable for a range of temperatures, and operation is at temperatures
between 0 C to
400 C.
[0020] The process can further comprise passing a purge stream at
desorption conditions
over the adsorbent to facilitate the desorption of the hydrocarbons. The
desorbent effluent
stream can be recompressed and directed to a fuel system. It is preferred to
desorb the
adsorbate at moderate pressures to minimize repressurization of the desorbent
effluent stream.
A repressurized desorbed hydrocarbon stream can be used as a fuel gas.
[0021] New materials have been found to have good properties for
adsorption separation.
These materials are MOFs, or metal-organic framework materials. MOFs have very
high
surface areas per unit volumes, and have very high porosities. MOFs are a new
generation of
porous materials which have a crystalline structure comprising repeating units
having a metal
- 5 -
CA 02633676 2013-07-30
or metal oxide with a positive charge and organic units having a balancing
counter charge.
MOFs provide for pore sizes that can be controlled with the choice of organic
structural unit,
where larger organic structural units can provide for larger pore sizes. The
capacity and
adsorption characteristics for a given gas is dependent on the materials in
the MOF, as well as
the size of the pores created. Structures and building units for MOFs can be
found in
US 2005/0192175 published on September 1, 2005 and WO 2002/088148 published on
November 7, 2002.
[0022] The materials of use for this process include MOFs with a
plurality of metal, metal
oxide, metal cluster or metal oxide cluster building units, hereinafter
referred to as metal
building units, where the metal is selected from the transition metals in the
periodic table, and
beryllium. Preferred metals include zinc (Zn), cadmium (Cd), mercury (Hg), and
beryllium
(Be). The metal building units are linked by organic compounds to form a
porous structure,
where the organic compounds for linking the adjacent metal building units
include 1,3,5-
benzenetribenzoate (BTB); 1,4-benzenedicarboxylate (BDC); cyclobutyl 1,4-
benzenedicarboxylate (CB BDC); 2-amino 1,4 benzenedicarboxylate (I-12N BDC);
tetrahydropyrene 2,7-dicarboxylate (HPDC); terphenyl dicarboxylate (TPDC); 2,6
naphthalene dicarboxylate (2,6-NDC); pyrene 2,7-dicarboxylate (PDC); biphenyl
dicarboxylate (BDC); or any dicarboxylate having phenyl compounds.
[0023] Specific materials that show improvement in adsorption properties
have a three-
dimensional extended porous structure and include: MOF-177, a material having
a general
formula of Zn40(1,3,5-benzenetribenzoate)2; MOF-5, also known as IRMOF-1, a
material
having a general formula of Zn40(1,4-benzenedicarboxylate)3; IRMOF-6, a
material having a
general formula of Zn40(cyclobutyl 1,4-benzenedicarboxylate); IRMOF-3, a
material having
a general formula of Zn40(2-amino 1,4 benzenedicarboxylate)3; and IRMOF-11, a
material
having a general formula of Zn40(terphenyl dicarboxylate)3,or
Zn40(tetrahydropyrene 2,7-
dicarboxylate)3; and IRMOF-8, a material having a general formula of Zn40(2,6
naphthalene
dicarboxylate)3.
[0024] These materials have high capacities due to the high surface
areas, and have
favorable isotherms where the adsorbent releases a significant amount of the
adsorbate, at
moderate pressures of around 5 atm. (0.5 MPa).
- 6 -
CA 02633676 2008-06-17
WO 2007/111739
PCT/US2006/062042
EXAMPLE
[0025] The use of a metal organic framework improves the removal of
methane (CH4)
and other light hydrocarbons from a high pressure stream comprising hydrogen
(H2). In this
particular example, this is a high waste pressure application where the waste
gas stream is
directed to a fuel system. By regenerating the adsorbent at moderate
pressures, there is
significant savings from the reduced repressurization needed. The fuel systems
are typically
operated at pressures from 4 atm to 7 atm (400 kPa to 700 kPa). In this
example, the primary
impurity is methane, and the adsorbent activity of MOF-5 is compared with the
activity of
activated carbon in a PSA system. The isotherms for methane over the
adsorbents are shown
in the Figure using the basis of lbs of methane per cubic foot of adsorbent
bed. The feed
stream has a methane partial pressure of 20 atm which is then desorbed at a
pressure of 5 atm.
The loadings for the activated carbon and the MOF-5 are 1.05 and 2.15 lbs-
CH4/ft3,
respectively. The MOF-5 exhibits a loading capacity of more than double that
of carbon. To
increase the carbon loading, the desorption pressure can be reduced to 1 atm,
with a resulting
loading on the carbon of 1.8. The low pressure used for carbon must be
accompanied with a
significant increase in power usage to recompress the methane released during
the desorption
stage to return the methane effluent stream to a fuel system pressure.
[0026] One aspect of the invention is to have a material, or
combination of materials, that
changes the shape of the isotherm, so that the capacity-pressure curve does
not taper off as
pressure increases, but still retains significant capacity increases as the
pressure is increased
over the normal operating ranges for a pressure swing adsorber. MOFs provide
some of this
capability.
[0027] While the invention has been described with what are presently
considered the
preferred embodiments, it is to be understood that the invention is not
limited to the disclosed
embodiments, but it is intended to cover various modifications and equivalent
arrangements
included within the scope of the appended claims.
- 7 -