Note: Descriptions are shown in the official language in which they were submitted.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
1
METHOD AND APPARATUS FOR PRODUCING COMBUSTIBLE FLUID
INTRODUCTION AND BACKGROUND TO THE INVENTION
This invention relates to a method and apparatus for producing combustible
fluid. More particularly this invention relates to a method and apparatus for
producing hydrogen and oxygen through the electrolysis of an aqueous
electrolytic solution.
In this specification, the term "combustible fluid" includes within its scope
combustible gas containing predominantly hydrogen and oxygen.
US patent number 4,379,043 discloses an apparatus for decomposing water
and producing detonating gas by electrolysis. The apparatus includes a
plurality
of annular carbon electrodes which are arranged concentrically about a common
vertical axis. The annular electrodes are perforated and have upper and lower
ends, the lower ends being positioned adjacent to sealing and insulating
elements in order to form a plurality of concentrically-arranged cells for
containing an electrolyte, such as water. A solid cylindrical carbon electrode
is
positioned within the smallest concentric electrode and along the common axis.
A voltage of 12 V and current of 80 A are applied to the apparatus by a direct
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
2
current source in order to evolve the detonating gas from the electrolyte in
the
cells by electrolysis.
A disadvantage of the above described known apparatus is that the ratio
between power consumed and combustible fluid produced is unfavourable, so
that its efficiency is relative low, i.e. the energy produced is less than 65%
of the
energy consumed in the process.
OBJECT OF THE INVENTION
It is accordingly an object of the present invention to provide an alternative
method and apparatus for the production of combustible fluid overcoming the
above disadvantage by producing combustible fluid at relatively higher
efficiency
rates, i.e. the energy produced is substantially more than 65% of the energy
consumed in the process.
SUMMARY OF THE INVENTION
According to a first aspect of the invention there is provided a method for
the
production of combustible fluid from an aqueous electrolytic solution
including
the steps of:
- providing an aqueous electrolytic solution;
providing an electrolysing cell having at least two spaced apart
electrodes defining a passage between them; and
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
3
- passing the solution along the passage whilst applying a DC voltage
across the electrodes to electrolyse the solution, the voltage being in
the range of from 1 V to 6 V.
The two spaced apart electrodes may be a first outer electrode and a second
inner electrode, and the method may include the further step of providing a
plurality of intermediate electrodes disposed between the first and second
electrodes, the arrangement being such that a plurality of passages, each
having an inlet and an outlet, are defined between the electrodes, and the
step
of passing the solution along the passage may include the further step of
passing the solution along the passages whilst applying the voltage across the
electrodes.
The step of applying the DC voltage across the electrodes may include the step
of applying a DC voltage in the range of from 2 V to 4 V, preferably in the
range
of from 2.75 V to 3.25 V across the electrodes.
The step of applying the DC voltage across the electrodes may include the
further step of applying a pulsed DC voltage across the electrodes.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
4
The step of applying the pulsed DC voltage across the electrodes may include
the further step of applying a pulsed DC voltage having a duty cycle of from
10% to 90% and a frequency of from 5 kHz to 20 kHz.
The voltage may be pulsed at a duty cycle of from 30% to 70%, preferably from
40% to 60%.
The voltage may be pulsed at a frequency of from 10 kHz to 15 kHz, preferably
13 kHz.
The solution may be passed continuously along the passages from the inlets to
the outlets.
In passing the solution along the passages, the solution may be pumped from
the inlets to the outlets of the passages.
The combustible fluid may be produced on the surface of the electrodes and in
between the electrodes in the passages between the electrodes, in the form of
gas bubbles and the step of electrolysing the solution may include the further
step of physically removing the gas bubbles from the surfaces of the
electrodes
and from the passages, and moving the bubbles towards the outlets of the
passages by the stream of the solution flowing along the passages.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
The step of providing the aqueous electrolytic solution may include the
further
step of providing a sodium hydroxide solution in water of from 1% to 5% on a
mass per mass basis, preferably a 3% sodium hydroxide solution in water.
5
According to a second aspect of the invention there is provided apparatus for
the production of combustible fluid from an aqueous electrolytic solution
comprising:
- an electrolysing cell for electrolysing the aqueous electrolytic solution,
the
electrolysing cell having a first electrode and a second electrode spaced
from the first electrode and a passage defined between the electrodes,
the passage having an inlet and an outlet;
- a circulating means for circulating the solution from the inlet, along the
passage, to the outlet and back to the inlet via a separate passage; and
- a power supply for applying a DC voltage across the electrodes to
electrolyse the solution whilst passing along the passage, the voltage
being in the range of from 1 V to 6 V.
The DC voltage applied across the electrodes may preferably be in the range of
from 2 V to 4 V, more preferably in the range of from 2.75 V to 3.25 V, most
preferably, the voltage may be in the range of from 2.85 V to 2.95 V.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
6
The apparatus may include a pulsing means for applying a pulsed voltage
across the electrodes.
The pulsing means may be adapted to apply the pulsed DC voltage at a duty
cycle of from 10% to 90% and a frequency of from 5 kHz to 20 kHz.
More particularly, the pulsing means may be adapted to apply the pulsed DC
voltage at a duty cycle of from 30% to 70%, preferably from 40% to 60%.
Further more particularly, the pulsing means may be adapted to pulse the DC
voltage at a frequency of from 10 kHz to 15 kHz, preferably 13 kHz.
The electrolytic solution may be in the form of a sodium hydroxide solution in
water.
The electrolytic solution may be a sodium hydroxide solution in water of from
1 %
to 5% on a mass per mass basis, preferably a 3% sodium hydroxide solution in
water.
The electrodes may be tubular and elongate and may be arranged
concentrically with each other.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
7
The first electrode may be an outer electrode, with the second electrode being
an inner electrode disposed within the outer electrode.
A plurality of intermediate tubular concentrically arranged electrodes may be
disposed between the first and second electrodes, the arrangement being such
that a plurality of passages, each having an inlet and an outlet, and along
which
the solution may be circulated, are defined between adjacent electrodes.
The longitudinal axes of the electrodes may extend vertically so that the
passages also extend vertically and the inlets may be provided towards the
lower end of the electrolysing cell and the outlets may be provided towards
the
upper end of the electrolysing cell.
The inlet of each of the passages may be defined by the lower ends of the
electrodes and the outlet of each of the passages may be defined by the upper
ends of the electrodes.
The opposite ends of the electrodes may be interposed between isolators.
The electrodes may further be electrically connected to two conductors, the
arrangement being such that the electrodes are connected in a parallel
configuration in that every second electrode may be connected to an upper
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
8
conductor, which is electrically connected to one pole of the power supply and
the other electrodes may be connected to a lower conductor, which is
electrically
connected to an opposite pole of the power supply.
Alternatively, the electrodes may be connected in a series configuration with
the
plurality of intermediate tubular concentrically arranged electrodes being
floating
electrodes disposed between the first and second electrodes, with the first
electrode having an opposite polarity to the second electrode.
The spacing between the electrodes may be from 1 mm to 8 mm.
In the case where the electrodes are connected in the parallel configuration,
the
spacing between the electrodes may be the same between all adjacent
electrodes.
Alternatively, in the case where the electrodes are in the series
configuration,
the spacing between adjacent electrodes may increase radially outwardly.
The electrodes may be made from conductive material and may be elongate in
nature, the first outer electrode generally having an opposite polarity to the
second inner electrode.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
9
The electrodes may be made from conductive material, more specifically the
electrodes may be made of grade 316 stainless steel.
The electrolysing cell may be completely filled with the aqueous electrolytic
solution, such that the electrodes are submerged in the solution.
The circulating means may be in the form of a pump and may continuously
pump the solution in an upwardly direction from the lower inlet of the
passages
to the upper outlet thereof.
The circulating means may be connected to the electrolysing cell via the
separate passage, so that the solution is pumped from the inlet, along the
passages, to the outlet and back to the inlet via the separate passage.
According to a third aspect of the invention there is provided an internal
combustion engine used in conjunction with the apparatus.
According to a fourth aspect of the invention there is provided a fuel cell
used in
conjunction with the apparatus.
According to a fifth aspect of the invention there is provided a torch for
cutting or
welding used in conjunction with the apparatus.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will now be described further by way of a non-limiting example
with reference to the accompanying drawings wherein:
5
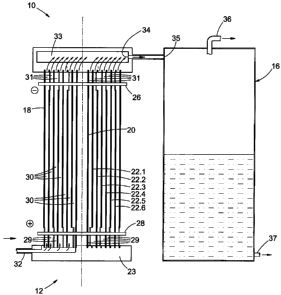
figure 1 is a schematic representation of an apparatus 10 according to a
preferred embodiment of the invention for the production of
combustible fluid from an aqueous electrolytic solution, the
apparatus including an electrolysing cell 12, a power supply 14
10 and a separator 16;
figure 2 is a longitudinal-sectional side view of the electrolysing cell 12
and
the separator 16 shown schematically in figure 1;
figure 3A is a perspective view from one end of electrodes 18, 20 and 22 of
the electrolysing cell 12;
figure 3B is a perspective view from another end of electrodes 18, 20 and 22
of the electrolysing cell 12
figure 4 is a view from below of an upper isolator 25;
figure 5 is a view from above of a lower isolator 23;
figure 6 is a graph showing current measurements and average current
calculated as drawn by the electrolysing cell 12 in operation;
figure 7 is a graph showing voltage measurements and average voltage as
used by the electrolysing cell 12;
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
11
figure 8 is a is a graph showing power and average power calculated and
as consumed by the electrolysing cell 12;
figure 9 is another graph showing voltage measurements for voltage drawn
by the cell 12; and
figure 10 is another graph showing current measurements for current drawn
by the cell 12.
DESCRIPTION OF A PREFERRED EMBODIMENT OF THE INVENTION
Referring to figure 1, an apparatus according to a preferred embodiment of the
invention for producing combustible fluid from an aqueous electrolytic
solution is
generally designated by reference numeral 10.
The apparatus 10 comprises an electrolysing cell 12 for electrolysing the
aqueous electrolytic solution; a power supply 14 for supplying a DC voltage;
and
a separator 16 wherein the combustible fluid is separated from the aqueous
electrolytic solution.
Referring further to figures 2 to 5, the electrolysing cell 12 includes a
first
electrode 18 and a second electrode 20 spaced from the first electrode 18. The
first electrode 18 is an outer electrode, with the second electrode 20 being
an
inner electrode disposed within the outer electrode 18. Intermediate
electrodes
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
12
22.1 to 22.6 (collectively referred to as 22) are disposed between the first
and
second electrodes 18 and 20 respectively.
The electrodes 18, 20 and 22 are tubular, elongate and made from grade 316
stainless steel and are arranged concentrically with each other with their
longitudinal axes extending vertically, as shown in detail in figure 3. The
opposite ends of the electrodes 18, 20 and 22 are interposed between a lower
inlet isolator 23 and an upper outlet isolator 25. The electrodes are further
electrically connected in a parallel fashion with to an upper conductor 26 and
a
lower conductor 28. The arrangement is such that every second electrode 22.1,
22.3. 22.5 and 18 is connected to the upper conductor 26 and the other
electrodes 20, 22.2, 22.4 and 22.6 are connected to the lower conductor 28.
The
upper conductor 26, and thus the electrodes 22.1, 22.3, 22.5 and 18, are
electrically connected to one pole of the power supply 14, in this case to the
negative pole, and the lower conductor 28, and thus the electrodes 20, 22.2,
22.4 and 22.6, are electrically connected to an opposite pole of the power
supply
14, in this case to the positive pole.
The electrodes 18, 20 and 22 could alternatively be connected in a series
configuration with the intermediate electrodes 22 being floating electrodes
disposed between the first and second electrodes 18 and 20. The first
electrode
18 would have an opposite polarity to the second electrode 20.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
13
The inlet and outlet isolators 23 and 25 are made from a non-conductive
material, such as Perspex. The isolators 23 and 25 each define a plurality of
grooves 23.1 and 25.1, shown in figures 4 and 5, wherein the electrodes 18, 20
and 22 are located. The upper isolator 25 further defines outlet passages 25.2
and the lower isolator defines an inlet passage 23.2.
The electrodes 18, 20 and 22 are located in the grooves 23.1 and 25.1 of the
isolators 23 and 25 so as to retain the electrodes 18, 20 and 22 in position
and
at a distance of from 4 mm to 8 mm apart. The length of the electrodes 18, 20
and 22 is 350 mm each. Electrode 20 has a diameter of 25.4 mm, electrode 22.1
has a diameter of 38.1 mm, electrode 22.2 has a diameter of 50.8 mm, electrode
22.3 has a diameter of 63.5 mm, electrode 22.4 has a diameter of 76.2 mm,
electrode 22.5 has a diameter of 88.9 mm, electrode 22.6 has a diameter of
101.6 mm, and electrode 18 has a diameter of 114.3 mm. Therefore, the
distance between electrodes 20 and 22.1 is 4.85 mm, between electrodes 22.1
and 22.2 is 4.85 mm, between electrodes 22.2 and 22.3 is 4.85 mm, between
electrodes 22.3 and 22.4 is 4.85 mm, between electrodes 22.4 and 22.5 is 4.85
mm, between electrodes 22.5 and 22.6 is 4.85 mm, and between electrodes
22.6 and 18 is 4.85 mm. Furthermore, the conductive areas of the electrodes
(without subtracting cut-outs, variance between inner and outer diameters of
the
1.5 mm thick electrodes, and holes defined in the electrodes) are as follows:
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
14
electrode 20 has a circumference of 79.83 mm and a single side area of 0.0279
m2; electrode 22.1 has a circumference of 119.74 mm and a double side area of
0.0838 m2; electrode 22.2 has a circumference of 159.66 mm and a double side
area of 0.1118 m2; electrode 22.3 has a circumference of 199.57 mm and a
double side area of 0.1397 m2; electrode 22.4 has a circumference of 239.49
mm and a double side area of 0.1676 m2; electrode 22.5 has a circumference of
279.40 mm and a double side area of 0.1956 m2, electrode 22.6 has a
circumference of 319.31 mm and a double side area of 0.2235 m2; and
electrode 18 has an inside circumference of 349.80 mm and a single side area
of 0.1224 m2. The conductive areas of the electrodes therefore amount to
1.0723 m2.
In the case where the electrodes 18, 20 and 22 are connected in the series
configuration, the spacing between adjacent electrodes 18, 20 and 22 increases
as they are located further from the second electrode 20 radially outwardly.
A plurality of passages 30 are defined by the electrodes 18, 20 and 22, the
arrangement being such that the electrolytic solution contained within the
electrolytic cell 12 can freely pass along said passages 30. The passages 30
each have a lower inlet 29 defined by the lower ends of the electrodes 18, 20
and 22 and an upper outlet 31 defined by the upper ends of the electrodes 18,
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
20 and 22, and the solution passes from the lower inlets 29 to the upper
outlets
31 along the passages 30.
The electrolysing cell 12 is provided with a first inlet 32, located towards a
lower
5 end of the electrolysing cell 12 for allowing electrolytic solution to pass
into the
electrolysing cell 12 via the inlet passage 23.2 of the lower isolator 23. The
electrolysing cell 12 is further provided with a first outlet 34, located
towards an
upper end of the electrolysing cell 12, for allowing solution containing
combustible fluid to flow from the passages 30 through the outlet passages
25.2
10 to a chamber 33. The combustible fluid produced by the apparatus 10, in
use,
thus flows from the electrolysing cell 12 via the first outlet 34 to the
separator 16,
having a second inlet 35 connected in fluid flow communication with the
electrolysing cell 12 via the first outlet 34.
15 In passing the solution along the passages form the first inlet 32 via the
inlet
passage 23.2 of the lower isolator 23, the solution moves in a swirling
motion.
This motion is initiated by the triangular shape of the inlet passage 23.2.
The
swirling motion assists with the even flow of the solution along the passages
and
over the surfaces of the electrodes 18, 20 and 22, thus avoiding cold spots,
where the concentration of combustible fluid will reduce conductivity and thus
current density, between the electrodes. The triangular shape of the inlet
passage 23.2 further assists in the flow rate of the solution being
proportional to
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
16
the diameters of the passages 30. Equal volumetric flow of the solution over
the
surfaces of the electrodes 18, 20 and 22 is thus achieved to maintain an equal
current density between all the electrodes.
In the separator 16, the solution is separated into combustible fluid and its
solution components, and the fluid flows from the separator 16 via a fluid
outlet
36, located towards the top of the separator 16. The separator 16 is further
provided with a second outlet 37, located towards the lower end thereof. The
second outlet 37 is connected to the first inlet 32 via a separate passage to
a
circulating means in the form of a pump (not shown), such that the solution is
continuously circulated through the apparatus 10 and along the passages 30 in
an upwardly direction, as indicated by the arrows in figure 2. The pump
circulates the solution through the apparatus 10 at a rate of approximately
100
litres per hour. The pump is a 12 V, 600 mA pump.
The power supply 14 includes a pulsing means and applies a pulsed DC voltage
of from 2 V to 4 V, specifically in the order of 2.85 V, at a frequency of
from 5
kHz to 20 kHz, specifically 13 kHz, and at a duty cycle of from 10% to 90%,
specifically 60%, across the electrodes 18, 20 and 22 of the electrolysing
cell 12.
During operation, the power supply 14 is connected to the upper and lower
conductors 26 and 28, such that the electrodes 22.1, 22.3, 22.5 and 18 are
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
17
connected to the negative pole of the power supply 14 and electrodes 20, 22.2,
22.4 and 22.6 to the positive pole of the power supply 14, or vice versa.
The electrolytic solution is prepared from 99% pure sodium hydroxide and is in
the form of a 3% sodium hydroxide solution in water on a mass per mass basis.
However, there are numerous other electrolytic solutions known in the art that
would also suffice.
In use, the electrolysing cell 12 and the passages 30 are filled completely
with
the electrolytic solution, such that the electrodes 18, 20 and 22 are
submerged
in the solution, and the separator 16 is filled approximately halfway with the
solution. The pump continuously circulates the solution to pass along the
passages 30 of the electrolysing cell 12 and to the separator 16. Only when
the
solution is circulated along the passages 30, is the power supply 14 switched
on
to apply the pulsed DC voltage across the electrodes. Electrolysis takes place
in the electrolysing cell 12. The solution containing the fluid, which is in
the form
of gas bubbles formed on the surfaces of the electrodes 18, 20 and 22 and
between the electrodes 18, 20 and 22, is physically removed from the surfaces
of the electrodes 18, 20 and 22 and from the passages 30 towards the upper
outlets 31 of the passages 30 by the stream of solution flowing along the
passages 30. The fluid then flows through the outlet passages 25.2 of the
isolator 25 and into the chamber 33. From the cell 12 the solution flows to
the
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
18
separator 16, via the first outlet 34 to the second inlet 35 of the separator,
where
the combustible fluid is separated from the solution. In the separator 16 the
fluid
flows out of the separator 16 via the fluid outlet 36 and the solution is
pumped
via the second outlet 37 to the first inlet 32.
From time to time the level of the solution in the separator 16 is topped up,
so
that the separator is filled to approximately half of its volume.
EXAMPLE 1
An experiment was conducted to measure the power dissipated and
combustible fluid produced by the apparatus 10 during electrolysis of the
electrolytic solution as described above.
RESULTS 1
A 6 V power supply was connected to the apparatus 10 to supply a pulsed
voltage at a frequency of 15 kHz at a duty cycle of 56.5%. The current and
average current drawn by the cell 12, the voltage and average voltage used by
the cell 12 and the power and average power dissipated by the cell, was
measured at the battery terminals. It was calculated that the average power
dissipated by the cell 12 was 218 W. Figures 6 to 8 show the graphs of
current,
voltage and power respectively, obtained from the measurements taken.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
19
EXAMPLE 2
In another experiment conducted to measure the time averaged power
dissipated by the cell and the pump during the electrolysis process. The
following results were obtained.
RESULTS 2
A 6 V power supply was connected to the apparatus 10 to supply a pulsed
voltage at a frequency of 15 kHz at a duty cycle of approximately 60%. The
current and average current drawn by the cell 12, the voltage and average
voltage used by the cell 12 and the power and time averaged power dissipated
by the cell, was measured at the battery terminals. It was calculated that the
average power dissipated by the cell 12 was 157.73 W and the average power
dissipated by pump was 6.74 W. The time taken to generate 250 ml combustible
fluid was 10 seconds and combustible fluid was thus produce at a rate of 1.51
litres per minute (I/min), which is 9.12 litres per kilowatt minute (VkWmin).
EXAMPLE 3
In another experiment conducted to measure the power consumed by the cell,
the following results were obtained.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
RESULTS 3
The power supply was connected to the apparatus 10 to supply a pulsed voltage
5 at a frequency of 15 kHz at a duty cycle of 44%. The average voltage drawn
by
the cell 12 was 2.88 V, as depicted in the graph of figure 9, and the average
current drawn by the cell 12 was 104 A, as depicted in figure 10. From these
measurements, it was calculated that the average power dissipated by the cell
12 was 299 W.
The applicant has found that the apparatus 10 performs far more superior than
the prior art since it utilises relatively low voltage and current and is
relatively
much more efficient in the production of combustible fluid than any of the
prior
art apparatus hitherto known to the public. The apparatus 10 is furthermore
compact and relatively easy to operate compared to the prior art.
The fluid may be used as a source of energy in any number of applications,
such as for generating heat or electricity, welding machines, rocket or jet
engines, or for running an internal combustion engine of a vehicle or a fuel
cell
vehicle.
CA 02636760 2008-07-10
WO 2007/080534 PCT/IB2007/050050
21
It will be appreciated that variations in detail are possible with a method
and
apparatus for producing combustible fluid according to the invention without
departing from the scope of the appended claims. For example, the electrodes
could also be arranged horizontally with an inlet towards one end of the cell
and
the outlet towards the other end of the cell.