Note: Descriptions are shown in the official language in which they were submitted.
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
TITLE
[0001] High Efficiency Absorption Heat Pump and Methods of Use
FIELD OF THE INVENTION
[0002] The invention is directed generally to heat pumps, and more
specifically to
a high pressure absorption heat pump using carbon dioxide and a low vapor
pressure
absorber as the circulating fluid.
BACKGROUND
[0003] Heat pumps are well known in the art. A heat pump is simply a device
for
delivering heat or cooling to a system, whereas a refrigerator is a device for
removing
heat from a system. Thus, a refrigerator may be considered a type of heat
pump.
Throughout the application, the invention will be referred to as a heat pump
with the
understanding that the designation of refrigerator, air conditioner, water
heater,
cogeneration system (also referred to as combined heat and power or CHP
system, which
is the use of a heat engine or a power station to simultaneously generate both
electricity
and useful heat), and trigeneration system (a cogeneration system that
additionally
produces cooling) could be substituted without changing the operation of the
device. The
inherent feature of a heat pump is to transport / move thermal energy from a
heat source
to a heat sink. The use of the term heat pump, thus is broadly applied as the
transport of
thermal energy from one enthalpy / entropy state to another. Thus, the
utilization of heat
pumps is not restricted to the generation of heating or cooling, but also for
the intrinsic
movement of thermal energy in virtually any thermodynamic cycle including
means to
convert such thermal energy into power generation (e.g., electrical or
mechanical energy).
[0004] In absorption heat pumps, an absorbent such as water absorbs the
refrigerant, typically ammonia, thus generating heat. When the combined
solution, also
referred to as binary solutions, is pressurized and heated f-u.rther, the
refrigerant is
expelled. When the refrigerant is pre-cooled and expanded to a low pressure,
it provides
cooling. The low pressure refrigerant is then combined with the low pressure
depleted
solution to complete the cycle.
[0005] Many current absorption heat pump/refrigerators make use of either a
water-ammonia couple, or a water-lithium bromide couple. These two absorption
couples
suffer from certain drawbacks. The water-arnrnonia couple raises security
problems in
view of the toxicity and flammability of ammonia, and LiBr is corrosive and
very failure
-1-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
prone due to low pressure operation, i.e., small leaks create contamination.
Moreover, the
tendency to crystallize can be a clogging problem. Operating at very low
pressures is
often impossible due to the freezing of water. Other absorption processes have
been
proposed, but generally involve working fluids that are toxic, flammable,
ozone-
depleting, or have high atmospheric green house effects.
[0006] United States Patent No. 6,374,630 for "Carbon dioxide absorption heat
pump" to Jones discloses a traditional absorption cycle utilizing
supercritical carbon
dioxide. The '630 patent does not anticipate an absorber having either a very
low vapor
pressure, a boiling point less than 50 C, or any means to achieve a
coefficient of
performance better than 0.70. The '630 patent further does not anticipate any
non-
thermal means to reduce desorption temperature, nor the extraction of
expansion energy.
It is understood that the term carbon dioxide and the abbreviations for carbon
dioxide
used are interchangeable that include CO2 and C02. Likewise, the term
water and
the abbreviations for water used are interchangeable that include H2 0
and H20.
[0007] United States Patent Application No. US 2003/0182946 for "Method and
apparatus for using magnetic fields for enhancing heat pump and refrigeration
equipment
performance" to Sami et al. utilizes a magnetic field that is operable to
disrupt
intermolecular forces and weaken intermolecular attraction to enhance
expansion of the
working fluid to the vapor phase. Magnetic field energy has been found to
alter the
polarity of refrigerant molecules and disrupt intermolecular Van der Waals
dispersion
forces between refrigerant molecules, though Sam.i et al. does not anticipate
the utilization
of a magnetic field to reduce desorption energy.
[0008] United States Patent No. 6,434,955 for "Electro-adsorption chiller: a
miniaturized cooling cycle with applications from microelectronics to
conventional air-
conditioning" to Ng et al. presents the combination of absorption and
thermoelectric
cooling devices. The governing physical processes are primarily surface rather
than bulk
effects, or involve electron rather than fluid flow. The '955 patent does not
anticipate a
continuous absorption process, but rather the transfer of thermal energy from
a batch
desorption process into the sequentially processed batch for subsequent
desorption.
[0009] United States Patent Application No. US 2003/0221438 for "Energy
efficient sorption processes and systems" to Rane, et al. devises adsorption
modules with
heat transfer passages in thermal contact with the adsorption module wall and
switchable
heat pipes. Adsorption module of this invention leads to lower cycle times as
low as 5
minutes that yields an efficient multi-stage regeneration processes for
regenerating liquid
-2-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
desiccant using rotating contacting disks. The '438 patent does not anticipate
either a
continuous process or an absorption process.
[0010] United States Patent Application No. US 2002/0078696 for "Hybrid heat
pump" and United States Patent No. 6,539,728 for "Hybrid heat pump ", both to
Korin,
disclose a hybrid heat pump system that includes (i) a membrane permeator
having a*
permselective membrane capable of selectively removing vapor from a vapor-
containing
gas to yield a dry gas, and (ii) a heat pump having (a) an internal side for
exchanging
thermal energy with a process fluid, (b) an external side for exchanging
thermal energy
with an external environment, and (c) a thermodynamic mechanism for pumping
thermal
energy between the internal side and the extemal side in either direction.
Korin uses
membranes to pre-condition air in conjunction with a refrigeration air
conditioning
system, and does not perform or anticipate any phase separation within the
refrigerant
itself. Furthermore, although membranes have been used in various separation
applications, their use for heat pump systems has been limited. U.S. Patent
Nos.
4,152,901 and 5,873,260 propose to improve an absorption heat pump by using of
a
semipermeable membrane and pervaporation membrane, respectively. U.S. Patent
No.
4,467,621 proposes to improve vacuum refrigeration by using sintered metal
porous
membrane, and U.S. Patent No. 5,946,931 describes a cooling evaporative
apparatus
using a microporous PTFE membrane. These patents do not anticipate the use of
membranes for phase separation within an absorption system, but rather within
adsorption
systems.
[00111 United States Patent No. 4,152,901 to Munters discloses a method and
apparatus for transferring energy in an absorption heating and cooling system
where the
absorbent is separated from the working medium by diffusing the mixture under
pressure
through a semi-permeable membrane defining a zone of relatively high pressure
and a
zone of relatively low pressure, higher than the arnbient pressure. The '901
patent does
not anticipate supercritical operation, as it explicitly states that the
"dilute solution of
working medium is passed to the evaporator upon being depressurized, while the
concentrated absorbent solution, upon being reduced to the ambient pressure,
is passed
into the sorption station".
[0012] United States Patent No. 5,873,260 for "Refrigeration apparatus and
method" to Linhardt, et al. utilizes the increased pressure of the
absorbent/refrigerant
solution that is then supplied to a pervaporation membrane separator to
provide as one
output stream a vapor-rich refrigerant, and as another output stream a
concentrated liquid
-3-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
absorbent. The '260 patent does not anticipate supercritical fluids as
explicitly stated "the
pressure of the substantially vaporized refrigerant input to the absorber is
less than 50
psia" and "the pressure of the absorbent/refrigerant solution entering the
membrane
separator is within the range of about 250 to 400 psia." The '260 patent
further notes that
"Osmotic-membrane-absorption refrigeration cycles are also capable of reaching
low
temperatures and may have a COP higher than conventional ammonia/water heat-
separation systems, but require very high pressures, of the order of 2,000
psia or more to
force the refrigerant through the pores of the osmotic membrane." It is to be
noted that a
pervaporation membrane operates in a totally different fashion from the prior
art
membrane separation processes used in refrigeration and heat pump systems.
Such prior
art membrane systems rely on osmotic pressure to force the refrigerant through
the
membrane thereby separating the refrigerant from other constituents. For the
ammonia-
water pair, this conventionally requires pressures of the order of magnitude
of 2,000 to
4,000 PSI and higher. Osmotic membranes are porous which allows the ammonia to
pass
through the membrane. Pervaporation membranes are not porous, but pass
constituents
through the membrane by dissolving the selected material into the membrane.
This
allows a much lower driving force, significantly less than 400 PSI, to act as
the driver. In
the case of an ammonia-water mixture, the pervaporation membrane, selectively
passes
ammonia and water vapor and rejects liquid water.
[0013] United States Patent No. 6,739,142 for "Membrane desiccation heat
pump" to Korin discloses a system that includes a membrane permeator for
removing
vapor from a process gas and for providing a vapor-depleted process. This
patent does
not disclose the use of any supercritical fluids.
[0014] United States Patent No. 6,918,254 for "Superheater capillary two-phase
thennodynamic power conversion cycle system" to Baker discloses a two-phase
thermodynamic power system including a capillary device, vapor accumulator,
superheater, an inline turbine, a condenser, a liquid pump and a liquid
preheater for
generating output power as a generator through the generation of a staggered
or pulsed
release of vapor flow. The capillary device, such as a loop heat pipe or a
capillary
pumped loop, is coupled to a vapor accumulator, superheater, the inline
turbine for
generating output power for power generation, liquid pump and liquid
preheater. The
capillary device receives input heat that is used to change phase of liquid
received from
the liquid preheater, liquid pump and condenser into vapor for extra heating
in the
superheater used to then drive the turbine. A superheater in combination with
a liquid
-4-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
pump and preheater are implemented for use with the evaporator for improved
thermal
efficiency while operating at maximum cycle temperatures well below other
available
power conversion cycles. The '254 patent requires a capillary device including
ioop heat
pipes and pumped loop in order to increase the single working fluid (i.e., to
achieve the
pressure differential resulting from the gain in thermal energy) pressure in
lieu of the
traditional utilization of a compressor to increase pressure within a
thermodynamic power
conversion cycle. Furthermore, the '254patent utilizes the superheater stage
to eliminate
any liquid drops in order to avoid liquid impingement within turbine blades.
The '254
patent is also a low pressure device having low pressure differentials between
the high
pressure and low pressure stage as specifically noted by its reference to
capillary wicks
with pores sizes of about one micron (commercially available) that can sustain
a pressure
differential of approximately ten psi_ In conclusion, '254the patent does not
enable the
utilization of working fluids including fluids characterized as supercritical,
binary
composition, and / or non-toxic fluids. The patent `254 is dependent on the
utilization of
a capillary device as a means to achieve a pressure differential.
[0015] United States Patent No. 5,899,067 for "Hydraulic engine powered by
introduction and removal of heat from a working fluid" to Hagernan discloses a
thermal
source as a means to increase a working fluid's pressure which in turn drives
a piston for
pumping, or alternatively refers to the piston being connected to a generator
to result in
electricity. The '067 patent is dependent in its operation of sequentially
heating and
cooling a fluid to enable the pressure on the piston to be increased by
heating and then
decreased by cooling to enable recovery from the fully expanded to fully
compressed
positions. The '067 patent is both a low pressure device, utilizes a single
working fluid,
and being comprised of a moving piston, has relatively very little surface,
area all
resulting in slow power conversion rates and large physical size.
[0016] "Poly(ionic liquid)s as New Materials for C02 Absorption" by Youqing
Shen et al., Department of Chemical and Petroleum Engineering, University of
Wyoming,
Laramie, Wyoming 82071, USA, received for publication on February 9, 2005,
identifies
that simply making ionic liquids into polymeric forms significantly increases
CO2
sorption capacity as compared with ionic liquids. Shen et al., fizrther notes
that especially
the polymers of tetraalkylamrnonium-based ionic liquids have CO2 sorption
capacities 6.0-7.6 times of those of room temperature ionic liquids. The
CO2
sorption and desorption of the polymer solids are very fast, and the
desorption is
-5-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
completely reversible. Shen et al., then specifically note the utilization of
said polymers
as being "very prospective as sorbent and membrane materials for CO2
separation".
[0017] Exemplary poly(ionic liquid)s, as noted by Shen et al., are comprised
of
ionic liquids PF6 anions having the highest CO2 -sorption capacity.
More
specifically, poly(ionic liquids) include 1-[2-(Methylacryloyloxy)ethyl]-3-
butyl-
imidazolium tetrafluoroborate ([MABI][BF_sub.4]) and 1-(p-vinylbenzyl)-3-butyl-
imidazolium tetrafluoroborate ([VBBI][BF4], poly[ 1-(4-vinylbenzyl)-3-
butylimidazolium tetrafluoroborate] (PVBIT), poly[(l-(4-vinylbenzyl)-3-
butylimidazolium hexafluorophosphate] (PVBIH), and poly[2-(1-butylimidazolium-
3-
yl)ethyl methacrylate tetrafluoroborate] (PBIMT). Specific results testing
particle size
yielded the conclusion that CO2 absorption capacity is mainly dependent
on
chemical structure of poly(ionic liquid)s, while the rate of CO2
absorption is
dependent on particle size.
[0018] Shen et al., clearly by the polymer being stationary as either a
sorbent or
membrane materials, does not anticipate the utilization of poly(ionic liquid)s
as being
heat transfer fluid or working fluid within a thermodynamic cycle.
[0019] The prior art lacks a lugh efficiency, a system with a coefficient of
performance greater than 0.7, environmentally friendly and efficient
absorption cycle that
uses a non-toxic, non-corrosive working fluid with a positive working
pressure.
SUlVIlYIARY
[0020] A safe, environmentally friendly absorptive cooling, heating, and
energy
generation process is provided. The process uses a carbon dioxide absorption
cycle that
utilizes a liquid, non-toxic absorbent such as ionic liquids, from which the
carbon dioxide
gas is absorbed. Only the carbon dioxide refrigerant is circulated to the
evaporator and
condenser heat exchangers, the components directly in contact with breathable
air, thus
avoiding a series of drawbacks associated with the absorber. The further
incorporation of
a thermodynamic hydraulic pump increases the energy efficiency, especially in
combustion power generation cycles, as it eliminates a substantial portion of
energy
utilized for compression prior to combustion.
[0021] One aspect of the invention is to integrate an absorption heat pump
with
integral power extraction capabilities to a standard vapor compression heat
pump as a
means of increasing total power conversion and cooling coefficient of
performance.
-6-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
[0022] The figures depicted within the specification of the invention provide
exemplary configurations of the most important components of the energy
conversion
system. A detailed description of the figures is provided in the following
paragraphs.
BRIEF DESCRIPTION OF DRAWINGS
[0023] Fig. 1 is a flowchart view of the absorption heat pump depicted with an
expansion turbine configuration as the mechanical energy extraction device.
[0024] Fig. 2 is a flowchart view of the absorption heat pump depicted with an
expansion turbine configuration as the mechanical energy extraction device
driving a
vapor compression pump (i.e., compressor).
[0025] Fig. 3 is a flowchart view of the absorption heat pump depicted with a
magnetic refrigeration heat pump configuration as non-thermal means of
increasing
strong solution temperature.
[0026] Fig. 4 is a flowchart view of the absorption heat pump depicted with a
sealed containment of an expansion turbine configuration
[0027] Fig. 5 is a flowchart view of the absorption heat pump depicted with a
multiple stage heat pump system's condenser pre-heating strong solution.
[0028] Fig. 6 is a three dimensional view of the absorption heat pump depicted
with a pre-heating of strong solution through the containment of combustor and
recuperator.
100291 Fig. 7 is a cross-sectional view of the absorption heat pump depicted
with
the strong solution desorption thermal energy obtained by an integral
microchannel heat
exchanger within solar collector.
[0030] Fig. 8 is a flowchart view of an absorption heat pump depicted in a
Goswami cycle.
[0031] Fig. 9A and Fig. 9B are flowchart views of a thermodynamic hydraulic
pUMp=
[0032] Fig. 10 is a flowchart view of non-thermal nanofiltration membrane to
desorb refrigerant from strong solution.
[0033] Fig. 11A, 11B, 11C, and 11D are flowchart views of multiple
configurations of two stage absorption heat pump systems.
[0034] Fig. 12 is a flowchart view of multiple use refrigerant desorbed from
an
absorption heat pump system.
-7-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
[0035] Fig. 13 is a flowchart view of multiple use weak solution and/or
refrigerant
to clean combustion byproducts.
[0036] Fig. 14 is a flowchart view of an absorption heat pump system as an
integral component of a biomass to biofuel conversion process.
[0037] Fig. 15 is a flowchart view of an integrated liquid desiccant and
combustion sytem.
[0038] Fig. 16 is a flowchart view of a membrane filtration system with
pressure
equilibrium across the membrane.
[0039] Fig. 17 is a flowchart view of an integrated combustion system having
independent control of a compressor and energy extraction device.
[0040] Fig. 18 is a flowchart view of a cavitation enhanced absorption heat
pump
and enhanced biomass to biofuel conversion process.
[00411 Fig. 19 is a flowchart view of a absorption heat pump utilizing bottom
cycle waste heat to power the compressor.
[0042] Fig. 20 is a flowchart view of a thermal bus switching circuit.
[0043] Fig. 21 is a flowchart view of a thermal bus and a range of thermal
sources.
[0044] Fig. 22 is a flowchart view of a thermal bus and.a range of thermal
sinks.
DETAILED DESCRIPTION OF THE PREFERRED EMBODMENTS
[0045] The inventive high efficiency absorption heat pump device, hereinafter
also referred to as "ScHPX", is now set forth as a device principally
comprised of a
supercritical absorption heat pump, low vapor pressure absorbers and a series
of integral
components to achieve desorption using non-thermal means.
[0046] The term "thermodynamic cycle" is defined as a process in which a
working fluid undergoes a series of state changes and finally returns to its
initial state.
[0047) The term "solar energy" is defined as energy derived from the sun,
which
most often refers to the direct conversion of radiated photons into electrons
or phonons
through a wide range of means. Solar energy is also indirectly converted into
additional
energy forms such as the heating of ground water (a.k.a. geothermal water).
[0048] The term "geothermal" is defined as relating to the internal heat of
the
earth, which is impacted by absorbed solar energy.
[00491 The term "ionic liquids" "ILs" is defined as liquids that are highly
solvating, non-coordinating medium in which a variety of organic and inorganic
solutes
-8-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
are able to dissolve. They are effective solvents for a variety of compounds,
and their
lack of a measurable vapour pressure.makes them a desirable substitute for
Volatile
Organic Compounds (VOCs). Ionic liquids are attractive solvents as they are
non-
volatile, non-flammable, have a high thermal stability, and are relatively
inexpensive to
manufacture. The key point about ionic liquids is that they are liquid salts,
which means
they consist of a salt that exists in the liquid phase and have to be
manufactured; they are
not simply salts dissolved in liquid. Usually one or both of the ions is
particularly large
and the cation has a low degree of symmetry. These factors result in ionic
liquids having
a reduced lattice energy and hence lower melting points.
[0050] The term "electride" is defined as being like alkalides except that the
anion
is presumed to be simply an electron which is localized to a region of the
crystal between
the complexed cations.
[0051] The term "alkalide" is defined as a class of ionic compounds where the
Anions are of the Type I group (Alkali) elements Na, K, Rb, Cs (no known
`Lithide'
exists). The cation is an alkali cation complexed by a large organic
complexant. The
resulting chemical form is A+ [Complexant] B-, where the complexant is either
a
Cryptand, Crown Ether, or Aza-Crown.
[0052] The term "nanofluid" is defined as a fluid that contains nanoscale
powders,
which are powders having a diameter of less than about 1 micron and preferably
less than
about 100 nanometers.
[0053] The term "supercritical" is defined as the point at which fluids have
been
exploited above their critical temperatures and pressures.
[0054] The term "heat pump" is defined as the transport of thermal energy
extracted from a heat source to a heat sink by means including vapor
compression,
absorption, and adsorption.
[0055] The term "cyclic compound" is one in which a series of carbon atoms are
connected together to form a loop or ring. Benzene is a well known example.
[0056] The term "polycyclic" is used when more than one ring is combined in a
single molecule, and the term "macrocycle" is used for a ring containing more
than a
dozen atoms.
[0057] The term "electron acceptor" is a compound that receives or accepts an
electron during cellular respiration. The process starts with the transfer of
an electron
from an electron donor. During this process (electron transport chain) the
electron
acceptor is reduced and the electron donor is oxidized. Examples of acceptors
include
-9-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
oxygen, nitrate, iron (III), manganese (IV), sulfate,.carbon dioxide, or in
some cases the
chlorinated solvents such as tetrachloroethene (PCE), trichloroethene (TCE),
dichloroethene (DCE), and vinyl chloride (VC).
[0058] The term "absorption" is widely accepted in the application of heat
pumps
for cooling. Absorption, in chemistry, is a physical or chemical phenomenon or
a process
in which atoms, molecules, or ions enter some bulk phase - gas, liquid or
solid material.
This is a different process from adsorption, since the molecules are taken up
by the
volume, not by surface. A more general term is sorption which covers
adsorption,
absorption, and ion exchange.
[0059] The term "stoichiometric combustion" is the ideal combustion process
during which a fuel is burned completely. A complete combustion is a process
which
burns all the carbon (C) to (CO2), all hydrogen (H) to (H2 0) and
all sulfur (S)
to (SO2). If there are unburned components in the exhaust gas such as C,
H2,
CO the combustion process is incomplete.
[0060] The term "excess gas" is defined as the amount of gas in excess of the
stoichiometric amount.
[006][] The term "process intensification reactor" is defined as the
miniaturization
of chambers in which chemical reactions take place. The utilization of
micromixing,
particularly with supercritical fluids, achieves high mass transfer and fast
reactiori times.
Supercritical fluids include gases such as carbon dioxide, methane, methanol,
anunonia,
ethanol, butanol, and hydrogen. The supercritical fluids can be prepared into
emulsions,
which preferably are nanoemulsions as a means of increasing surface area
significantly.
Devices include hydrodynamic cavitation devices, microchannel reactors,
spinning disk,
spinning tube in tube, oscillating flow reactors, and reactive distillation
reactors.
[0062] The ScHPX, an extension of the Champagne Heat Pump as developed by
Jones, establishes novel methods to decrease the desorption temperature and
total energy
requirements to achieve desorption. The refrigerant, which is the circulating
working
fluid, is comprised of any environmentally friendly fluid (a.k.a. greenhouse
friendly)
whereby the fluid expands into a gas within the evaporator. A wide range of
refrigerants,
specifically those known in the art for absorption heat pumps are compatible
with
ScHPX. The preferred refrigerant is selected from the group of ammonia and
carbon
dioxide. The more preferred refrigerant is carbon dioxide, which has reduced
toxicity and
perceived safety. The specifically preferred refrigerant operates within the
supercritical
or transcritical range, as determined by the specific refrigerant.
-10-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
[0063] The inventive heat pump also achieves superior desorption through a
modified spinning disc reactor "SDR". SDR's have extremely high heat and mass
transfer
coefficients. The strong solution is simultaneously pumped into the center of
the disc and
forms a thin film as the liquid moves outwards. The centrifugal force creates
intense
interfering waves, which generate high heat transfer between the strong
solution and the
spinning disc. The SDR can also be used through the intense local mixing to
accelerate
the absorption of the supercritical CO2. into the weak solution.
[0064] The ScHPX is further comprised of an absorber in which the refrigerant
is
absorbed as a method to either increase temperature lift (i.e., transform a
relatively low
temperature fluid to a higher temperature (a.k.a. higher quality) of a thermal
source, or
provide cooling. The energy requirements of an absorption system is limited to
traditionally a thermal source for desorption, and mechanical or electrical
energy to pump
/ pressurize the strong solution. The term "energy efficiency" is the energy
output
divided by the energy input required to produce the desired output. A high
efficiency
absorption system, which is characterized in terms of coefficient of
performance "COP",
requires methods to reduce principally the desorption energy requirements.
Desorption is
effectively the process in which the refrigerant separates from the absorber.
[0065] The inventive ScHPX utilizes a range of absorbers which includes at
least
one absorber selected from the group consisting of ionic liquids, ionic
solids, electride
solutions, and alkalide solutions. Ionic liquids and solids are recognized in
the art of
environmentally friendly solvents. Electride and alkalide solutions are
recognized in the
art of chemical reduction methods and oxidation methods respectively. ScHPX
uniquely
features ionic liquids "IL", which have very low if not negligible vapor
pressure,
preferably ionic liquids compatible with supercritical carbon dioxide "scCO2".
The
inventive combination of scCO2 and ILs have excellent carbon dioxide
solubility and
simple phase separation due to their classification as partially miscible
fluid
combinations. Partially miscible fluids are both miscible and immiscible as a
direct
function of both pressure and temperature. A partially miscible fluid in its
immiscible
state can be simply decanted for phase separation, which is inherently a low
energy
separation method. The phase behavior of CO2 with ionic liquids and how
the
solubility of the gas in the liquid is influenced by the choice and structure
of the cation
and the anion.
. [0066] The preferred embodiment of the working fluid is an ionic liquid and
poly(ionic liquid) "emulsion" having the combined benefits of fluid flow of
the ionic
-] ]-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
liquid monomers and the enhanced absorption/ desorption properties of the
poly(ionic
liquid) polymers, also referred to as ionic polymers. The standard
categorization of the
ionic liquid "emulsions" is the characterization as one phase of the emulsion
as being an
"ionic liquid monomer" or abbreviated as "ILM" phase and the other phase as
being an
"ionic liquid polymer" or abbreviated as "ILP" phase. The ILM and ILP phases
are
described also as an ionic liquid slurry, hereinafter referred to as "ILS". A
preferred ILS
is comprised of at least one ionic liquid monomer and at least one ionic
liquid polymer.
The preferred ILS is comprised of an ILP having particle size approximately
between
about 0.1 nanometers and about 500 microns. The particularly preferred ILS is
comprised of an ILP having particle size. approximately between about 10
nanometers and
about 5 microns. And the specifically preferred ILS is comprised of an ILP
having
particle size approximately between about 0.1 nanometers and about 500
nanometers.
Prior work utilizing nanoscale powders has identified 100 nanometers, without
being
bound by theory, as a significant size threshold having a quantum effect on
heat transfer.
Nanoscale powder size is a highly non-linear process in which particles of 50
nanometers
have superior results as compared to 100 nanometers. And likewise, 30, 20, and
10
nanometers are each superior to the respective larger size. Another
significant threshold
is 10 nanometers, again without being bound by theory, as a size threshold
wherein
powder sizes of less than 10 nan6meters have heat transfer performance
benefits that are
not realized for powder sizes above 10 nanometers. The mean free path of
phonons is
accepted as being less than 10 nanometers.
[0067] Most noted are the inclusion of binary working fluids having at least
one
fluid selected from at least one from the group of ionic liquid, poly(ionic
liquid) polymer,
electride, alkalide, and nanofluid solutions. The particularly preferred
working fluids
have at least one fluid selected from the group consisting of ionic liquids,
combination of
ionic liquids and poly(ionic liquid) polymers. The specifically preferred
working fluid is
comprised of a heat transfer fluid comprised of at least one ionic liquid and
at least one
poly(ionic liquid) polymer. The further inclusion of nanoscale powders
including
conductive powders, semiconductive powders, or combinations thereof increase
the
thermal conductivity of the working fluid.
[0068] The utilization of a poly(ionic liquid) polymer and at least one
additional
working fluid selected from the group consisting of ionic liquids, non-
polymeric solid
adsorbents, and combinations thereof maintains the ability of the working
fluid to be
-12-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
pumped and circulated through heat exchangers for increased heat transfer
while
demonstrating superior absorption and desorption of refrigerant rates.
[0069] A specifically preferred ionic liquid or ionic polymer is itself
magnetic
having distinct advantages including higher rates of absorption and desorption
when
subjected/removed from magnetic fields, and the ability to isolate said
materials from the
refrigerant more readily by non-thermal means.
[0070] The further addition of at least one non-ionic compound selected from
the
group consisting of cyclic, polycyclic, and macrocycle compounds, and
combinations
thereof including antioxidants, polyphenols, lignans, and vitamins, provides
the working
fluid with enhanced thermal stability and operating life, and without being
bound by
theory enhanced heat transfer and electron transfer.
[0071] Electron transfer mediators include polycationic protein, thialoto-
bridged
complexes, thiolated complexes, metalloproteins, protein complexes having an
iron-sulfur
cluster, trehalose complexes, iron-sulfur cluster, sodium-ammonia, sulfur-
ammonia, a
chitosan complex including chitosan lactate, chitosan alpha lipoic acid, or
thiolated
chitosan, or combinations thereof. Additional additives impacting electron
transfer
include iron salts, derivatives of iron salts, potassium salts, lactic acid
salts, derivatives of
potassium salts, derivatives of lactic acid salts, phytic acid, gallic acid
and combinations
thereof. '
[0072] Energy conversion including absorption heat pumps are particularly
preferred when fiirther comprised of at least one additive selected from the
group
consisting of electron transfer mediator, electron donor, electron acceptor,
ultraviolet
absorber, infrared absorber, quantum dot, nanoscale powder, and combinations
thereof.
The utilization of nanoscale powders enhances heat transfer and electrical
conductivity by
quantum means, without being bound by theory. The addition of additives,
preferably in
the nanoscale range, has an impact on the conversion of photons to phonons,
photons to
electrons, electrons to phonons, phonons to electrons, etc.
[0073] The particularly preferred application of the heat transfer fluid is
operable
within thermal energy conversion devices including devices selected from the
group
consisting of solar thermal flat panels, solar thermal concentrator receivers,
thermionics
emission cell, thermovoltaic cell, electricity generator, compressor, and heat
pump. And
the specifically preferred application is whereby the fluid and at least one
absorbed gas
(preferably CO2) operable with the transcritical or supercritical region
in solution
whereby the subsequently desorbed gas is utilized within a thermodynamic cycle
-13-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
including cycles selected from the group consisting of Goswarni, Uehara,
Kalina,
Rankine, Carnot, Joule-Brayton, Ericsson, and Stirling.
[0074] Additional combinations of refrigerants and absorbers are recognized in
the art as having partial miscibility. A further aspect of the inventions is
the achievement
of phase separation as a function of at least one function selected from the
group
consisting of temperature, pressure, and pH. The preferred solution further
includes the
utilization of small amounts of pH to vary solubility of the refrigerant
within the absorber.
The more preferred solution varies temperature and pressure, in combination
with pH
control using methods including electrodialysis. An additional method to
enable phase
separation is the application of electrostatic fields, as electrostatic fields
increase
solubility of ionic fluids.
[0075] The inventive ScHPX further leverages electride and alkalide solutions.
The preferred electride solution is comprised of ammonia. The principal
benefit of
electrides is centered around the transfer of free electrons (i.e., energy
state) between the
cathode and anode. An additional benefit, which is important to the later
incorporation of
nanoscale powders, is the electride's strong reducing characteristics. This is
important as
nanoscale powders, specifically metals, readily oxidize due in part to the
powder's high
surface area.
[00761 Yet another embodiment of the invention is the further inclusion of at
least
one nanoscale powder selected from of the group consisting of conductive, semi-
conductive, ferroelectric, and ferromagnetic powders. Nanoscale powders, as
recognized
in the art, maintain colloidal dispersions while enhancing or varying a range
of properties
including magnetism, thermophysical properties (e.g., therrnal conductivity),
electrical
conductivity, and absorption characteristics. The more preferred nanoscale
powders are
further comprised of nanoscale powders having nanoscale surface modifications,
including surface modifications selected from the group of monolayer, and
nanoscale
multi-layers (i.e., surface coatings of less than 100 nanometers). The
specifically
preferred nanoscale powders enhance more than one parameter selected from the
group
consisting of thermophysical properties, electrical conductivity, and solar
light spectrum
absorption.
[0077] A yet further feature of the inventive ScHPX is the integration of
mechanical energy extraction devices. The mechanical energy extraction devices
enhance
efficiency (i.e., COP) by extracting energy during the expansion stage of the
refrigerant
following the desorption step. Referring to Fig. 1, the mechanical energy can
be
-14-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
transforrned by utilizing the refrigerant desorbed from the desorber 50
through a valve or
flow regulator 20 into a wide range of useful forms of energy as known in the
art,
including an expansion turbine 65. The ScHPX, depending on the operating
conditions,
has further cooling capacity through a heat exchanger 25 prior to refrigerant
being
absorbed in the absorber 30. These forms include transforming mechanical
energy to
electrical energy (e.g., alternating or direct current electricity
generation), or driving
pumps, compressors, or motors. These include energy extraction devices
selected from
the group consisting of gerotor, Quasiturbine, piston, spherical engine,
expansion turbine,
expansion pump, Stirling cycle engine, Ericsson cycle engine, and ramjet
turbine. The
preferred mechanical extraction device leverages the refrigerants
supercritical state,
which features relatively high mass flow "density" and operations within the
supersonic
range. Referring to Fig. 2, the more preferred mechanical extraction device is
an integral
supersonic device selected from the group consisting of compressor 15 and
turbine 65.
The specifically preferred device operates on either the ramjet or pulsejet
principle. The
result is a relatively compact high efficiency compressor or turbine for
respectively
inputting mechanical energy by pressurizing the strong solution or extracting
mechanical
energy by reducing the pressure during the expansion of the refrigerant.
[0078] Referring to Fig. 2, the ScHPX has the ability to be in fluid
communication with a traditional vapor compression system, such as compressor
15. The
refrigerant desorbed from the desorber 50 is further compressed with the vapor
compressor 15 that elevates both the temperature and pressure as a method to
increase the
coefficient of performance when cooling is desired, as compressor energy is
required only
to incrementally increase the pressure gain beyond the desorber 50 pressure,
which is
significantly less electrically/mechanically energy intensive. The refrigerant
is in fluid
communication with a heat exchanger 25 that acts effectively as a condenser,
which
thermal energy can be transferred for many purposes including a second stage
absorption
heat pump desorber, preheating combustion air, preheating combustion fuel,
heating a
secondary heat transfer fluid, or combinations thereof.
[00791 As noted earlier, the most critical aspect to the efficiency in an
absorption
heat pump is the desorption energy. The ScHPX achieves desorption by the
inventive
combination of both non-thermal methods and traditional thermal methods.
Traditional
thermal methods, as known in the art, are achieved by simple heat transfer
through air-to-
liquid or liquid-to-liquid heat exchangers whereby a relatively hotter fluid
transfers
thermal energy to the relatively cooler strong solution. The preferred non-
therrnal
-15-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
methods are selected from the group consisting of magnetic refrigeration,
vapor
compression heat pump, solar activated direct spectrum light absorption,
electrostatic
field, electrodialysis, membrane separation, electrodesorption, pervaporation,
gas
centrifuge, vortex tube CO2-liquid absorber, and decanting. Membranes
used for
CO2 removal do not operate as filters, where small molecules are
separated from
larger ones through a medium with pores. Instead, they operate on the
principle of
solution-diffusion through a nonporous membrane. The CO2 first dissolves
into the
membrane and then diffuses through it. Because the membrane does not have
pores, it
does not separate on the basis of molecular size. Rather, it separates based
on how well
different compounds dissolve into the membrane and then diffuse through it. An
array of
polyvinylchloride vinylacetate membranes, for example, allows for quicker
permeation of
CO2. Very small molecules and highly soluble molecules, small molecules
(e.g.,
CO2) permeate faster than large molecules.
[00801 Referring to Fig. 10, an additional non-thermal means of desorption
includes microwave and/or radio frequency energy. The preferred working fluid
containing ionic liquids and ionic polymers are unique in their ability to
absorb
microwave energy. A preferred embodiment is the utilization of a
nanofiltration device
400 that is void of materials that absorb microwave energy, absorb radio
frequency
energy, disrupt electrostatic field, disrupt magnetic field, or combinations
thereof. The
localized.exposure of the strong solution to the aforementioned fields yields
rapid and
energy efficient desorption.
[0081] Membrane separation includes traditional ultra-filtration and nano-
filtration as a method to separate components by means including molecular
weight and
particle size separation.
[0082] Referring to Fig. 3, the more preferred non-thermal method utilizes the
combination of ferroelectric / ferromagnetic nanoscale powders in combination
with
magnetic refrigeration 105 that utilizes the magnetocaloric effect to raise
the strong
solution 100 to higher than the desorption temperature, and the subsequent
removal of the
working fluid from the magnetic field enables the refrigerant 120 to desorb
yielding weak
solution 115 transforming the strong solution into weak solution by either
utilizing less
thermal energy from a heat exchanger 25 or even no thermal energy (i.e.,
without any
heat exchangers). The specifically preferred implementation continuously and
sequentially pulses the strong solution into at least two desorption zones.
Sequentially
-16-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
pulsing the strong solution into the desorption zone enables a reduction of
the pumping
energy required to pressurize the strong solution into the desorption zone.
[0083] Yet another aspect of the invention is the absence of a compressor in
the
standard absorption design. The only moving part is limited to a very small
pump where
small is in terms of energy consumed as compared to total system energy. The
utilization
of a free-piston pump offers the opportunity for high efficiency, quiet, low
cost and oil
free vapor compression. The absence of oil is important in achieving benefits
including
avoidance of oil solubilizing in the preferred supercritical carbon dioxide,
which presents
significant complexities, and eliminating the oil boundary layer created on
the heat
transfer surfaces, which presents a deterioration of heat transfer. An ultra
high COP
ScHPX does incorporate a vapor compression stage as a method to achieve COPs
comparable and beyond the highest vapor compression heat pumps. The preferred
compressors are also oil-free, which is achieved by incorporating many
techniques as
known in the art for reducing friction, including diamond coatings, diamond
like coatings,
ultrafine diamond coatings, air bearings, magnetically levitation and solid
lubricants.
[0084) Another aspect of the invention further avoids the complexities
associated
with leak-free pumps or compressors. Referring to Fig. 4, the ScHPX thus
further
includes a sealed container 35, whereby the sealed container captures
refrigerant leaked
by the pumping system that is periodically evacuated into the weak solution.
The sealed
container captures low pressure strong solution which is leaked into the
sealed container.
A controller monitors the pressure within the sealed container to determine
when a
control valve is switched whereby the pump 460 between the absorber 30 and
desorber
50, which normally pressurizes the strong solution into the desorber, now
pressurizes the
losses into the sealed container into the absorber.
[0085] The physical size and the rate of absorption are additional important
components of any absorption system. The inventive ScHPX further includes a
cavitation
device, whereby the cavitation device enhances the absorption rate by creating
micro-
bubbles with significantly greater surface area. The more preferred cavitation
device is
selected from the category of devices that create hydrodynamic cavitation.
[00861 Physical size of the ScHPX is further reduced by the utilization of
microchannel heat exchangers, whereby the supercritical fluids have reduced
surface
tension that counteract the fluid friction associated with high surface area
heat
exchangers.
-17-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
[0087] ScHPX System Configuration
[0088] The inventive ScHPX is unique not only due to specific components but
also in terms of operational configuration. A multistage absorption heat pump
system,
also known as a cascading system, whereby one distinct refrigerant A is used
in at least
one distinct stage and at least one other distinct refrigerant B is used in at
least one other
distinct stage. Each stage is in effect a distinct thermodynamic cycle, though
each stage
is coupled to the other as one's output is the other's input. The preferred
ScHPX
leverages the differences in desorption temperature of a refrigerant A and
absorption
temperature of refrigerant B. Referring to Fig. 5, in other words, the
condensing thermal
source (i.e. condenser 259) of one stage is the desorption thermal source of
the other stage
(i.e. condenser 258).
[0089] Yet another configuration is the ScHPX having direct infusion of a
parallel
energy generation system or combustor such that its exhaust is infused into
the absorber.
One key advantage is the capture of latent energy from the exhaust stream. A
more
preferred implementation utilizes techniques as known in the art to
selectively enable the
refrigerant to enter the absorber, thus the exhaust air is treated to remove
byproducts,
whereby byproducts include NOx and sulfur. This implementation achieves .
concurrent carbon dioxide sequestration. The cooling available from the ScHPX
is then
utilized to precool the combustion air to increase turbine capacity and
energy.efficiency.
[0090] Referring to Fig. 6, a further gain in efficiency is obtained by
capturing
thermal energy directly recovered from thermal conduction losses of a
combustion
chamber 230 and combustion recuperator 220. Recuperators are often utilized to
capture
waste heat, though thermal conduction through the external walls of the
recuperator limit
total energy recovered, especially for space constrained implementations such
as mobile
vehicle applications.
[0091] Thermal energy of the inventive ScHPX uniquely utilizes low quality
thermal sources. One such source is a non-concentrated solar collector. The
more
preferred solution has an integral heat exchanger within the solar collector.
Referring to
Fig. 7, a more preferred implementation is a solar collector 300 that achieves
at least one
benefit selected from the group consisting of concentrating solar energy 310
as a means
of reducing thermal losses and cooling photovoltaic cells 320. A specifically
preferred
implementation is an integral microchannel heat exchanger 340 to further
reduce thermal
losses and heat exchanger size. And the particularly preferred implementation
has a
-18-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
translucent film 330 separating the solar collector and heat exchanger,
whereby photons
from the solar spectrum enable photon stimulated desorption, thus reducing the
desorption temperature. Stimulated desorption is also achieved by external
electrical and
electromagnetic fields. The further inclusion of nanoscale powders, including
quantum
dots and ultraviolet absorbers, enhance efficiency whereby the colloidal
dispersion of
powders within the absorber enhances direct conversion of photons to
electrons, and
subsequent electron transmission between cathode and electrode. The optimal
solution
has at least one solar collector stage followed by at least one solar
concentrator stage
where each stage creates an independent pressure zone (i.e., a superheated
vapor state).
[0092] The utilization of the inventive ScHPX as noted earlier yields higher
power generating efficiency when the working fluid is further elevated to
higher vapor
states. The elevation of the working fluid to a first vapor state through the
utilization of a
relatively lower temperature heat source, such as waste heat or non-
concentrated solar
energy, is subsequently elevated to a higher vapor state through means
including
traditional vapor compressor, concentrated solar energy, a combustion source,
a relatively
higher temperature heat source, or combinations thereof. This, elevation from
one lower
vapor state to subsequent higher vapor states can be repeated. The optimal
energy
efficiency replaces the utilization of the traditional vapor compressor with
staggered
increases in vapor states as a means of elevating vapor state through a series
of
themaodynamic stages via a thermal-hydraulic compressor / pump. The
utilization of a
high surface area heat exchanger as an integral component of the thermal-
hydraulic
pressure increasing zone enables rapid increases in pressure. The ability to
rapidly
increase the pressure within each zone enables the expansion device to receive-
a working
fluid with a constant pressure.
[0093] Numerous methods and devices exist to isolate one zone from the other.
One such means is a valve-less hydraulic pump comprised of a rotating cylinder
having
microchannels on the exterior portion of the rotating cylinder. The internal
part of the
rotating cylinder is exposed to the thermal source'. The rotating cylinder is
within a
further extemal cylinder that seals each microchannel thus isolating each zone
within the
microchannel from the other zones. During the period of rotation, the working
fluid
within the microchannel increases in both temperature and thus pressure. The
fluid enters
an individual microchannel, preferentially from an inlet duct that is
perpendicular to the
microchannel along the entire length of the microchannel. Likewise, the exit
duct has the
-19-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
same orientation with respect to the microchannel, but offset rotationally
along the
cylinder.
[00941 Alternatively, the thermal-hydraulic compressor / pump incorporates a
high surface area "solid" / slurry heated up to a specified teinperature, that
is
subsequently placed into a "sealed container". The working fluid is then
infused into the
sealed container leading to a rapid increase in pressure. The further
incorporation of a
spring piston to create a counter-force, preferentially such that the spring
creates a
constant force at least equivalent to the desired entry pressure of the
expansion device.
The spring further enables all of the superheated vapor to be ejected from the
pressure
zone and to maintain a constant pressure. The further utilization of a
flexible bladder or
springs enhances the constant pressure output from one pressure zone into the
next or to
the expansion device. A further advantage is that each pressure zone is
essentially
emptied for full occupancy by the prior pressure zone.
[0095] Referring to Fig. 9B, independent pressure zones are alternatively
produced by the utilization of input diode 200, also referred to as input flow
control
devices. One such device utilized to regulate the output is an output diode
240, also
referred to as a pressure relief valve. The utilization of a series of
pressure relief valves,
such that the cracking pressure is set incrementally to increase from the
first pressure
relief valve to the last with incremental increases for each pressure relief
valve, is an
effective way to prevent backflow and to provide inherently controllable means
to
increase working fluid vapor state. The aggregate of the series of pressure
relief valves
within a heat exchanger, heater 250, or displacement pump heater 220 is
hereinafter
referred to as a "pressure train" heat exchanger. Thus the pressure relief
valve creates
effectively independent zones within the pressure train. There are numerous
methods
known in the art to achieve precise and / or relative pressure control.
[0096] It is anticipated that the optimal scenario is such the last
independent zone
enables output flow to occur at a precise pressure, whether the pressure be
controlled by
an electronic pressure control in conjunction with a pressure sensor or a
mechanical
pressure relief valve. Such a relief valve can also be activated at a
differential pressure
between the prior output zone and the subsequent input zone.
[0097] Multiple parallel pressure train heat exchangers, enable a constant
pressure
output to the power extraction device, such that an increase in either or both
the number
of pressure relief valves within the pressure train and / or the number of
multiple parallel
pressure trains leads to a most constant pressure output.
-20-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
[0098] Referring to Fig. 9B, additional devices that both create independent
pressure zones include a Quasiturbine, quasiturbine used as positive
displacement pump,
positive displacement pump comprised of an inlet duct 210, an internal heater
220, and an
exit duct 230, and hydraulic pump.
[0099] Referring to Fig. 8, the final implementation feature of the ScHPX
achieves higher efficiency by operating with the Goswami, Kalina, Baker, or
Uehara
cycle. Under the Goswami cycle, the ScHPX can be optimized to provide maximum
levels of heating, cooling or energy, in addition to optimal total energy
efficiency.
[001001 An absorption heat pump system is depicted having at least two
pressure stages wherein each sequential stage has increasing pressure with
first stage P 1
less than second stage P2. The utilization of at least one "compression" stage
comprised
of absorption utilizes significantly less mechanical/electrical energy as
compared to
traditional vapor compression compressors. An absorption heat pump uniquely
transforms thermal energy, which is often waste heat or readily available from
supporting
processes, into pressure due to lower energy requirements of compressing a
"incompressible" liquid versus a compressible vapor. The benefits are realized
under
numerous configurations including, referring to Fig. 11A where working fluid /
absorbent
450, such as ILs, is blended with strong solution from absorber 430, such as
solid
adsorbent (Al), into a second stage absorber 431. The blended strong solution
(A2) is
subsequently pumped 460 from a lower pressure (Pl) to an increased pressure
(P2) that is
in fluid communication with desorber 50. The desorbed refrigerant can
optionally be
compressed via a traditional vapor compressor 15 for numerous purposes
including
increasing condenser temperature and elevating pressure for a subsequent
extraction
process. Referring to Fig. 11B, refrigerant desorbed from a first stage
absorption heat
pump desorber 50 and then regulated with flow valve 20 into a second stage
absorption
heat pump absorber 30 to be elevated to an increased pressure by pumping 460
the strong
solution, which is incompressible. Referring to Fig. 11C, refrigerant desorbed
from a first
stage absorption heat pump desorber 50 and then regulated with flow valve 20
into a
second stage vapor compression compressor 15 to be elevated to an increased
pressure.
Referring to Fig. 11D, alternatively depicts the vapor compression compressor
15 as the
first stage, such as instances when the initial pressure PO is not sufficient
for the
refrigerant to be absorbed into the weak solution of the second stage
absorption heat
pump absorber 30, which is then subsequently elevated to a yet higher pressure
with high
mechanical/electrical energy efficiency by higli pressure pump 460. All of
these
-21-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
aforementioned configurations utilize less mechanical/electrical energy as
compared to a
single, or even multiple, stage vapor compression compressor.
[00101) Example 1
[001021 The absorption heat pump system wherein the operating mode to
increase pressure from the initial pressure P0 to the second stage pressure P2
is selected
from the group consisting of (1) having a first adsorption or absorption stage
having
pressure P 11 including solid or liquid adsorbents and a second
adsorption or
absorption stage having pressure P21 wherein first stage adsorbent
Al1 is
combined with a second stage liquid non-compressible adsorbent A2 1 and
wherein
P11 is less than P2 1, or (2) having a first stage non-absorption
compression
stage including compressors or turbochargers wherein first stage increases
pressure from
initial pressure PO2 to operating pressure P12 and a second stage
comprised of
an absorption stage including solid or liquid adsorbents wherein Pl2 is
less than
P22.
[00103} Example 2
[00104] Example 1 is further comprised of a third stage to further increase
the pressure wherein pressure increasing means includes a non-absorption
compression
stage (i.e., traditional compressors; turbochargers, etc.) or an absorption
pumping stage.
[001051 Referring to Fig. 12, the desorbed refrigerant, from desorber 50,
wherein the refrigerant is subsequently processed in at least one post
desorbtion process
stage selected from the group consisting of reaction chemistry (includes
enzymatic
chemistry, fermentation chemistry), component extraction, supercritical
combustion, and
combinations thereof, wherein the combined mechanical and electrical energy
(E I)
required to increase working fluid pressure to operating pressure (P1) is at
least ten
percent lower than the combined mechanical and electrical energy (E2)
required to
increase working fluid pressure to operating pressure (P1) by compressing the
compressible portion of working fluid. This configuration is an enabling
approach to
increase the utilization of benefits recognized in the art of supercritical
extraction,
supercritical combustion, and process intensification reactors "PIR". A wide
range of
specific devices as known in the art are recognized for PIR including
hydrodynamic
cavitation, microchannel, spinning disk, spinning tube in tube, oscillating
flow, and
reactive distillation reactors. The further incorporation of nanoscale
catalysts within the
PIR, and more specifically with the utilization of supercritical working
fluids increases
the reaction rates dramatically due to high mass transfer rates and lower
viscosi-ty. An
-22-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
post desorbtion process stage, most notably within biomass to biofuel
conversion
applications, is an enzymatic reaction that is further comprised of
immobilized enzymes.
[001061 Biomass to biofuel conversion, most notably cellulose to ethanol, is
widely known in the art to utilize enzymes. However, the failure to solubilize
cellulose
demands the use of "free" as compared to immobilized enzymes to obtain
acceptable
enzymatic conversion rates. The inventive utilization of ionic liquids, and
preferably
poly(ionic liquid) polymers of which a wide range are known in the art having
the ability
to solubilize cellulose, uniquely enables iminobilized enzymes to be utilized
in
combination. The further utilization of refrigerants, particularly
supercritical fluids
including carbon dioxide significantly lowers the viscosity of the solubilized
cellulose
within the ILs. Poly(ionic liquid) polymer "PILP" are superior to ILs due to
the relative
ease in recovering the immobilized enzymes, which are incorporated into the
PILP by
means known in the art of immobilizing enzymes into polymers, especially when
utilizing
membrane filtration as a means to isolate the enzymatic converted biomass from
the ILs
and PILP. The combination of ILs and PILP provides the benefit of both
immobilized
enzymes, while having the relatively easy circulation of ILs in comparison to
PILPs thus
achieving effective biomass transport to the immobilized enzymes. A preferred
embodiment of the inventive application has the unique ability for the
immobilized
enzymes to be reused to yield a dramatic economic and conversion rate, with
the fizrther
advantage of having the subsequent ability to remove the spent enzymes from
the IL and
PILP slurry, which are then subsequently replenished with active enzymes, and
again
further subsequently immobilized within the PILP.
[001071 The immobilized enzymes, which are specialty proteins that
catalyze chemical reactions are removed from ILs by the further addition of
different
enzymes that effectively transform the immobilized enzymes into byproducts
including
amino acids, protein hydrolysates, or combinations thereof. Short chain amino
acids and
protein hydrolysates have increased water solubility, thus can be washed from
the IL and
PILP slurry easily. Thus, the removal of immobilized enzymes takes advantage
of the
byproducts being insoluble or partially immiscible into the IL or PILP phase.
The
determination of when either/both the IP and PILP, and inunobilized enzymes
are "spent"
with the requirement to be removed/regenerated/replaced is by placement of
detectors to
monitor at least one condition selected from the group consisting of ionic
liquid
absorption rate, ionic liquid desorption rate, catalytic conversion rate,
enzymatic
conversion rate, and combinations thereof.
-23-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
[00108] Referring to Fig. 12, a series of sensors 70 are placed to monitor
the strong solution, the weak solution, and the refrigerant within the
absorption heat
pump, and both prior and post to the subsequent process 470 that utilizes
means to
accelerate the biomass to biofuel conversion rate including catalysts and
enzymes. An
alternative subsequent process to the absorption heat pump system is
supercritical
combustion. The prior noted benefits of achieving supercritical pressures by
utilizing
waste heat, including from said supercritical combustion process, enables the
parasitic
process loss reduction of-energy generated from the combustion process (i.e.,
energy
coupled with the_energy extraction device such as turbine) to be utilized to
generate
additional mechanical/electrical energy, while the low quality thermal energy
is recovered
to drive the compression of intake air.
[00109] Referring to Fig. 13, a yet further advantage of the present
embodiment of the absorption heat pump, particularly the low energy
availability of
supercritical fluids, enables at least one component of the combustion waste
byproducts
to be removed from within the working fluid (e.g., CO2). The weak
solution
desorbed from desorber 50, which contains iLs and/or PILP, and/or the desorbed
refrigerant into the combustion.process 480. It is widely recognized that
supercritical
carbon dioxide and ionic liquids, both individually and in combination, are
superior
solvents, thus operating the supercritical combustion process discontinuously
enables the
non-combustion portion of the discontinuous operation to clean away the
combustion
byproducts. The further utilization of a fuel containing excess gas greater
than the gas
required for stoichiometric combustion enables continuous removal of
byproducts,
specifically when the excess gas is supercritical C02. Regardless of whether
the weak
solution and/or refrigerant are utilized to clean the combustion chamber from
the
combustion process 480, the "cleaning" fluid must have combustion waste
byproducts
removed froin means known in the art for separation, including nanofiltration
400, prior
to being utilized again within the absorption heat pump cycle.
[00110] Referring to Fig. 14, is another preferred embodiment wherein the
absorption/adsorption/ion exchange of byproducts from biomass to biofuel
conversion
process, which include carbon dioxide, methane, methanol, or combinations
thereof, are
utilized to produce additional biofuels. Traditional corn-based starch to
ethanol
conversion is widely recognized as producing significant quantities of C02,
and
additionally both utilizes a significant quantity of thermal energy while
producing low
quality waste heat. This waste heat recovered from biomass process 490 through
heat
-24-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
exchanger 25 is in fluid communication with desorber 50 through heat exchanger
25.
Another benefit of the utilization of supercritical combustion is the ability
to add at least
one fuel additive 510 including chitosan, glycerine, cellulose, and lignan.
The preferred
embodiment is such that the chitosan, cellulose, and lignan are precipitated
out of IL and
PILP slurry by water injection, and specifically preferred within a
microchannel as a
means of creating particle size less than about 10 microns and more preferably
less than
about 1 micron and particularly preferred sizes less than about 100
nanometers. The high
surface area enables more complete combustion 520, which reduces the
production of
char, ash, and tar. An excellent carrier for the biomass precipitates include
at least one
fuel additive selected from the group consisting of biodiesel, natural gas,
butanol, ethanol,
gasoline, carbon dioxide, ammonia, hydrogen, and water. Yet additional
additives
include water, wet biomass, glycerine, glycerol, glycol including a glycol,
dimethyleglycol, trimethylene glycol, or combinations thereof. A fuel
containing
colloidal suspensions of biomass precipitates is ideally suited for
supercritical combustion
within a porous combustion chamber, as recognized in the art to produce
effectively zero
emissions. Alternatively, the desorbed refrigerant that is at supercritical
pressures enables
more effective process intensification reactions 530. The reactions include at
least one
further conversion process selected from the group consisting of catalytic
reactions,
combustion reactions, enzymatic reactions, and combinations thereof. A
particularly
preferred embodiment is the conversion of biomass byproducts into additional
biofuels
that are electrochemically converted 500 into a liquid or gaseous biofuel. The
specifically
preferred configuration transforms waste heat to produce electricity which, at
least in part,
powers the electrochemical conversion process. This configuration
significantly
increases the revenue yield of the biomass to biofuel conversion plant, as the
revenue per
unit of energy produced is much greater than the wholesale price of
electricity. The
ability to remain off-grid and produce methanol (e.g., from C02 + H20 in a
reverse fuel
cell) or other electrochemical reaction products has numerous benefits
including more
carbon dioxide neutrality, increased revenue, faster reaction rates due in
part to
supercritical pressures, higher electrical conductivity due in part to IL,
PILP, electron
transfer mediators, etc. and process intensification devices 530.
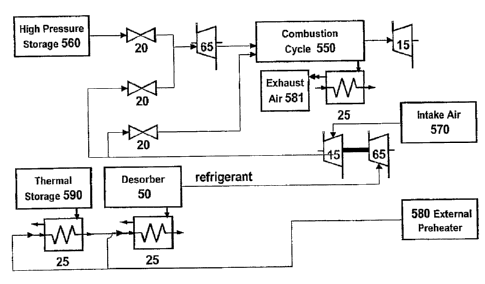
[00111] Referring to Fig. 15, another embodiment is an absorption heat
pump system in fluid communication with a liquid desiccant system. A preferred
embodiment includes the conversion of combustion processes to be supercritical
combustion 520 where exhaust waste heat is recovered. The combination uniquely
-25-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
enables the waste heat utilized from the liquid desiccant system 540 to be
recovered via
heat exchanger 25 to desorb 50 the strong solution, which can be subsequently
recovered
to preheat subsequent combustion cycle 550 including at least one selected
from the
group consisting of combustion cycle air intake, combustion cycle fuel, and
combinations
thereof Continuing the process of waste heat recovery enables the combustion
exhaust to
be recovered to produce increased cooling, power, or combinations thereof by
means
including desorbing working fluid, regenerating spent/wet liquid desiccant
system, or
combinations thereof. Another configuration is an energy conversion system
wherein the
spent liquid desiccant from the liquid desiccant system 540, either as dry
desiccant/wet
desiccant and with/without refrigerant desorbed from the absorption system
desorber 50
is further utilized as the fuel or one component of the fuel for the
combustion cycle 550.
The preferred liquid desiccant is comprised of glycerine, glycerol, or glycol
including a
glycol selected from the group consisting of dimethyleglycol and trimethylene
glycol, or
combinations thereof. This distinct capability yields fundamental advantages
for
integrating a variety of biofuel production into one plant, specifically the
integration of a
biodiesel plant having significant thermal energy and glycerine as byproducts,
both being
valuable inputs for ethanol production. A yet preferred embodiment recovers
latent
energy from said combustion cycle 550 exhaust becoming spent liquid absorbent
and
wherein said spent liquid absorbent is then utilized as the fuel or orie
component of the
fuel for the combustion cycle. The spent liquid desiccant can be further
comprised of at
least one fuel selected from the group consisting of biodiesel, natural gas,
butanol,
ethanol, gasoline, carbon dioxide, ammonia, and hydrogen. -
[001121 Referring to Fig. 16 is an enabling feature for the utilization of
membrane filtration, including micro- and nanofiltration, under conditions of
supercritical
pressures. A series of detectors/controllers are required to maintain the
pressure across
the desorption chamber membrane nanofiltration 400 wherein the pressure
differential
across the membrane is less than maximum membrane operating pressure. A
minimum
of two detectors/sensors 70 are required to monitor the pressure on each side
of the
membrane. Flow valves 20 are required to vary the flow of the strong solution
into the .
input side of the membrane while utilizing and controlling the flow of
refrigerant to
achieve precise pressure control. This occurs by simultaneously controlling
the flow
through flow valves 20 of refrigerant into the output side of the membrane to
maintain the
pressure differential into the acceptable operating levels in accordance to
the membrane
specifications. The isolated refrigerant can be optionally stored in a high
pressure storage
-26-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
tank 560, and can be fizrther pressurized using a traditional vapor
compression
compressor 15 in order to maintain adequate pressure particularly during
startup
conditions. Each flow valve 20 of the working fluids is individually
controlled for both
sides of the chamber membrane.
[00113] Referring to Fig. 17 is another feature realized by implementing a
preferred embodiment of the absorption heat pump system, which is an energy
conversion
system comprised of an individually controlled compressor and energy
extraction device,
and a fuel combustion chamber wherein the compression energy is dynamically
controlled or switched to maximize power generation. The compression energy is
provided from at least one source selected from the group consisting of (a)
thermal
storage system 590, (b) high pressure storage tank 560 including air, working
fluid, or
hydraulic oil, (c) external preheater 580 including thermal energy from said
fuel
combustion chamber, solar, and geothermal sources, and (d) absorption heat
pump
utilizing waste heat for desorber 50 from at least one source selected from
the group
consisting of said fuel combustion chamber, biomass to biofuel conversion
process, solar,
and geothermal sources where expansion energy extracted from turbine 65 drives
a
compressor 15 to compressor intake air 570.
j001141 Referring to Fig. 18 is yet another feature wherein the refrigerant
desorbed from desorber 50 is comprised of C02. The C02 is supercritical has
distinct
advantages in the preprocessing of biomass 600 wherein the working fluid
passes through
a separation process including nanofiltration 400 as a means of isolating
carbon dioxide
from other components within said working fluid including water, minerals,
mineral salts,
non-combustibles, combustion byproducts, or combinations thereof. The
additional
separation of cyclic, polycyclic, and macrocycle compounds including
polyphenols,
aromatic ring containing compounds from the biomass prior to biomass to
biofuel
conversion process 610 has benefits including increasing the conversion rate
to biofuels
and extracting high value add components to increase the revenue stream_ The
isolating
of C02 is an effective means of sequestering C02, particularly because the C02
is
already at supercritical pressure therefore avoiding the significant energy
penalty
associated with traditional C02 sequestration. Yet another preferred
embodiment is the
further inclusion of cavitation devices that enhance at least one rate
selected from the
group consisting of absorption 710, desorption 720, or combinations thereof.
The
relatively high viscosity of ILs and PILPs, especially with high solids
biomass gains
-27-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
significantly from the use of cavitation devices which provides intimate
mixing while
operating in the absorption mode, and stripping while operating in the
desorption mode.
[00115] Referring to Fig. 19, another embodiment is an absorption heat
pump system in fluid conzmunication with a combustion process. A preferred
embodiment includes the recovery of exhaust air 581 waste heat through
recuperator 863
from the combustion process 480 such that the bottom cycle low quality energy
is
transformed into useful energy by the absorption heat pump system high
pressure
desorbed from desorber 50 refrigerant through a pressure exchanger 861, such
as gerotor
or a combination compressor and expander (e.g., turbine) with a common shaft,
for
"compressing" the intake air 570 to a higher pressure (preferably
supercritical pressure).
Another preferred embodiment preheats the intake air 570 through a condenser
860 that
recovers the heat of absorption from absorber 30 (which has gained thermal
energy via
evaporator 862 within an air conditioning / refrigeration cycle). Another more
preferred
embodiment utilizes the exhaust air 581 in fluid communication downstream of
recuperator 863 to at least in part provide thermal energy to desorb
refrigerant and then to
provide thermal energy through a heat exchanger 25 for a wide range of
purposes-
including domestic hot water and preheating of process water. The resulting
thermal
energy from the aforementioned combustion process 480 can be utilized for a
wide range
of thermal energy conversion processes including steam cycle, process heat,
boiler, and
supercritical boiler.
[001161 Referring to Fig. 20, another embodiment is a dynamic thermal bus
for switching a series of thermal sources, depicted here as a general heat
exchanger 25
and heat pump 850 (i.e., in this scenario the thermal diode 93 reverses to be
in fluid
communication with condenser). The preferred embodiment has a switching array
94
comprised of at least an incoming switching circuit 95 and outgoing switches
92
(including normally open as shown with preferably one normally closed 91 as
shown), as
known in the art, having the capability to switch any thermal. source from a
series of
thermal sources to any thermal bus circuit of a series of thermal bus
circuits. A
representative exarnple of the varying thermal bus circuits is a series of
circuits having a
target temperature that deviates from the thermal bus temperature mean 834.
This
example utilizes three circuits having a temperature target differential of
ten degrees
Celsius below the mean respectively 833, 832, and 831, in addition to three
circuits
having a temperature target differential of ten degrees Celsius above the mean
respectively 835, 836, and 837. Each thermal bus circuit has at least one
sensor 70
-28-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
including temperature sensor to detect the actual circuit temperature.
Additional
detector/measurements include mass flow rate, thermal energy flow rate, and
pressure.
Measuring the pressure is critical, especially when the pressures are in the
supercritical
range in order to maintain the pressure below bursting pressure, to minimize
pressure
losses. A preferred embodiment of the dynamic thermal bus is to integrate a
series of
sensors 70 to detect/monitor critical parameters, particularly parameters
(hereinafter
referred to as "non-linear parameters") to identify the non-linear algorithm
for thermal
source energy efficiency, thermal sink energy efficiency, thermal =source end
product
coefficient of performance, and thermal sink end product coefficient of
performance.
(e.g., refrigeration, electrical energy produced, etc. divided by total energy
input) as a
function of at least one parameter selected from the group consisting of
thermal bus heat
exchanger inlet temperature, thermal bus heat exchanger outlet temperature,
thermal bus
mass flow rate, thermal source inlet temperature, thermal source outlet
temperature, and
thermal source mass flow rate. Numerous methods know in the art are
anticipated to
control fluid flow including valves, smart materials whose properties change
as a function
preferably though not limited to temperature, variable speed pumps, flow
switches and
thermal diodes.
[00117] Referring to Fig.2 1, an alternative embodiment is depicted showing
a series of heat sources wherein a thermal source has insufficient thermal
energy being
transported away to the thermal bus from the thermal source leading to the
switching
circuit routing the thermal source thermal energy into thermal
contact/communication
directly to a thermal sink including heat pump 850 for temperature lift. Yet
another
embodiment is where a lower temperature circuit, such as supporting a
refrigerator
evaporator 920 is then directed in fluid communication to a thermal source
including heat
pump 851 for subcooling. The multiple circuit thermal bus is represented by an
example
of three circuits 810, 820, and 830 which are in switchable fluid
communication by
methods known in the art for switching flows and/or thermal transport as
represented by
switched circuit 840. Yet another embodiment is a configuration where the
thermal
sources within any one circuit are in series of thermal sources by
sequentially increasing
thermal source inlet temperature as a method to maximize heat transfer of each
thermal
source. Alternatively where the thermal sinks within any one circuit are in
series of
thermal sinks by sequentially decreasing thermal sink inlet temperature as a
method to
maximize heat transfer of each thermal sink.
-29-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
[00118] A wide range of thermal sinks or sources are anticipated botll
within residential/commercial/industrial environments including refrigerator
condenser
910, refrigerator evaporator 920, dish washer waste heat recovery 930 (also
optionally
with water recovery), oven cooler 940, water faucet sink 950, shower 960,
electronics
cooler 970, lighting cooler 980 (including LEDs particularly a series of
LEDs), a heat
pump condenser 990, heat pump evaporator 991, one or more external heat
exchangers
992, and/or a window heat exchanger 993. The aforementioned window heat
exchanger
transforms non-visible light (i.e., ultraviolet and/or infrared spectrum) into
thermal energy
that is in thermal contact with a thermal bus circuit. The optimal
implementation of the
window heat exchanger is a visible light transparent composite, preferably
comprised of a
high thermal conductivity nanocomposite to transport the thermal energy into
the thermal
bus. The more preferred embodiment includes a visible light transparent
nanocomposite
film having high thermal conductivity, contained within the multi-pane cavity,
in thermal
communication with the window heat exchanger (preferably a supercritical
pressure fluid
heat exchanger, and particularly preferred a fluid having nanoscale additives
with low
visible light absorption and high infrared and/or high ultraviolet light
absorption). The
particularly preferred embodiment of the window heat exchanger is further
comprised of
a nanocomposite film having an exterior film reflecting-to the interior
infrared and/or
ultraviolet spectrum waves. The heat exchanger is further comprised of a
nanocomposite
film on an interior pane that reflects infrared and/or ultraviolet spectrum
waves from the
exterior facing to back to the thermally conductive film and reflects infrared
and/or
ultraviolet spectrum waves from the interior of the building (in which the
window heat
exchanger is built) back into the occupied building space to minimize thermal
losses. The
window heat exchanger and/or thermally conductive film is preferably further
comprised
of aerogels as one such method to minimize thermal losses. The thermally
conductive
film and window heat exchanger are further thermally isolated from the window
structure
as known in the art.
[00119] Referring to Fig. 22, the thermal bus fluid flow rate is controlled
within the preferred embodiment by a variable speed control of pump 460
utilizing a
series of sensors 70 that detect/monitor a range of parameters to determine
energy
efficiency including incoming and outgoing temperature, fluid flow rate,
energy
consumption kilowatt hour "kwh", power generation kwh, BTU (i.e., thermal
energy)
meter. Due to non-linearity of thermodynamics, achieving the optimal total
system
energy efficiency is not simply dependent on maximizing thermal waste heat
recovery
-30-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
from thermal sources but rather precise flow control as the preferred method
to impact the
temperature change (i.e., delta T) across thermal sources. The thermal bus is
controlled
to maximize the temperature gain of the highest thermal bus circuit within the
constraints
of thermal energy sinks maximum thermal energy demand including maximum flow
rate
and maximum temperature (i.e., the system will not increase the flow rate
beyond the
maximum useful level by aggregate of energy sinks or beyond the maximum useful
temperature of any energy sink while concurrently sacrificing the energy
efficiency of
other energy sinks). The determination to maximize temperature of a thermal
circuit has
many penalty conditions in terms of individual component energy efficiency
including (a)
the reduction of total amount of recovered waste heat, (b) reducing the
subcooling/precooling post condenser within a vapor compression system which
may lead
to lower energy efficiency to achieve air conditioning / refrigeration, (c)
biomass
preprocesses 600 and/or biomass fermentation processes have clear maximum
process
temperatures in which enzymatic reactions will deteriorate and enzymes could
even
become inactivated, (d) increasing temperature for removal of absorption
energy within
absorber 30 leads to lower absorption cooling, (e) increasing temperature
beyond critical
desorption temperature simply increases the amount of energy needing to be
removed in
the subcooling portion of the absorption cooling cycle, (f) increasing
temperature beyond
design limits of components such as turbine 65 blades may gain energy
efficiency but at
the cost of system lifetime where incremental revenue gain of power generation
may not
exceed incremental increase in maintenance expense, and (g) numerous thermal
sinks are
not operated in either steady state/equilibrium conditions including liquid
desiccant
cooling system 540 which in fact is discontinuously regenerated. Another
feature of the
preferred embodiment utilizes the aforementioned switcher circuit 840 to
determine the
output from a particular selected thermal bus circuit (e.g., highest
temperature circuit 837)
being routed in fluid communication to a particular selected thermal sink
including
devices such as refrigeration condenser 910, or intake air 570 for a
subsequent
combustion process.
[00120] The thermal bus fluid flow direction/pathway is controlled by a
series of algorithms based on non-linear parameters representing the thermal
sources and
thermal sinks in fluid communication (i.e., connected) to the thermal bus. The
thermal
source(s) and thermal sink(s) are connected to at least one thermal bus
circuit by a
thermal interface including thermal diode and/or thermal switch (including
thermal
diode/switch of the following types as known in the art: liquid metal
switches, phase
-31-
CA 02637488 2008-07-16
WO 2007/082103 PCT/US2007/001120
change materials, smart materials, switches comprised of movable thermal
contact
including high thermal conductivity nanocomposites such as a carbon nanotube
array
composite). The particular preferred thermal source/sink is connected via a
switch circuit
array having the means to vary thermal communication with at least two thermal
bus
circuits. The specifically preferred dynamic thermal bus switch circuit
is.controlled in
accordance to a thermal bus control system comprised of at least a series of
non-linear
parameters and at least one thermal diode/switch. The dynamic thermal bus is
further
comprised of thermal storage devices preferably further comprised of sensors
to provide
'real-time feedback of storage capacity level and temperature. The control
system modes
of operation include: (a) method to maximize total thermal energy to
mechanical/electrical energy conversion, (b) method to maximize mass flow rate
at
highest achievable temperature, (c) method to maximize mass'flow rate at
lowest
achievable temperature, (d) method to minimize energy consumption from fuel
sources
having green house gas emissions, (e) method to minimize total energy
consumption cost
from all sources where cost includes any green house gas emissions penalties,
(f)
aforementioned method "e" with further comprised of parametric operating
constraints
that ensure each thermal source and thermal sink (hereinafter also referred to
as
"equipment") meets minimum operating conditions, and (g) aforementioned method
"f'
further comprised of quantitative costs for failure to meet minimum operating
conditions."
The control system is further comprised of data including calendars, equipment
operating
schedules, predictive equipment operating schedules, predictive weather, and
building
occupancy schedules, and further comprised of non-linear algorithms including
equipment energy consumption algorithms and equipment energygeneration
algorithms.
-32-