Note: Descriptions are shown in the official language in which they were submitted.
CA 02637517 2008-07-14
COLD STORAGE OF MODIFIED PLATELETS
FIELD OF THE INVENTION
The present invention relates to method for storing and using platelets and an
associated platelet structure. The present invention also relates to the use
of the stored
platelets for treating subjects.
BACKGROUND OF THE INVENTION
Transfusion of platelets (a commonly transfused cellular component of blood)
is a cornerstone of modem medical care for a number of acute and chronic
conditions
characterized by either excessive bleeding or insufficiency of endogenous
platelet
production or function. Unlike red blood cells, which can be efficiently
stored at 1-
6 C (mean 4 C), platelets are irreversibly injured when temperatures,
repeatedly drop
below approximately 20 C for short periods of time or are kept at less than 20
C for
long periods of time. This injury is termed the "platelet cold storage
lesion".
Importantly, this platelet cold storage lesion begins to occur even after
brief exposure
to temperatures less than 20 C and is even seen in patients undergoing surgery
in
which the temperature of the whole body or of parts of the body is decreased
to
temperatures less than 20 C and leads to bleeding abnormalities.
FIG. 1 depicts effects on platelets of cooling platelets from 37 C to 4 C, in
accordance with the related art. Exposure of platelets to temperatures less
than 20 C
results in structural injury and functional activation of control (normal)
platelets. In
portion A of FIG. 1, significant morphological changes occur when platelets
are
cooled from 37 C to 4 C as shown by the appearance of filopodia using phase
contrast
microscopy. In portion B of FIG. 1, temperature dependent activation of
platelets is
1
CA 02637517 2008-07-14
further demonstrated by anti-phosphotyrosine Western Blot analysis of
platelets
incubated for 30 min at 37 C (lanes 1, 3) or 4 C (lanes 2, 4), in the absence
(in lanes
1, 2) or presence (in lanes 3, 4) of a membrane-active compound. The blot was
stripped and probed for actin as a loading control.
As shown in FIG. 1, key characteristics of this platelet cold storage lesion
are:
(1) reversible to irreversible morphological change from a discoid cell to
spiculated
spheres with protruding filopodia, depending on time at temperatures less than
20 C;
(2) irreversible immune-independent microaggregation of platelets (i.e.,
increased
cell:cell interaction); (3) membrane clustering of the glycoprotein GPIb on
the surface
of platelets resulting in the formation of a neoantigen; and (4) subsequent
recognition
and phagocytosis by macrophages of the microaggregates and/or neoantigen-
expressing platelets upon transfusion into a recipient. In addition, there is
a
significant reduction in circulation half-life of chilled platelets introduced
into a
recipient of the chilled platelets. As a consequence of this platelet cold
storage lesion,
platelets must be stored at 20-24 C (mean of 22 C) in order to maintain
acceptable
function and viability in the transfused patient (see American Association of
Blood
Banks (AABB) Technical Manual). Unfortunately, maintaining platelets at a mean
temperature of 22 C for prolonged periods of time greatly increases the risk
of
adverse medical events due to bacterial growth in the platelet product.
Current
estimates are that 1 in every 3000 platelet units are affected by microbial
contamination (see Kleinman SH et al., "Two-year experience with aerobic
culturing
of apheresis and whole blood-derived platelets", Transfusion 2006, 46:1787-
1794).
Risks are associated with transfusion of cellular blood components in Canada
(see
Transfusion Medicine Reviews, 17:120-163). Because of this microbial risk,
platelets
2
CA 02637517 2008-07-14
can only be stored at 20-24 C for a maximum of 5 days before they must be
destroyed.
Rosiello (International Publication No. WO 2006/044790 A2) discloses a
method for the cold storage (-80 C to 15 C) of platelets for periods of 3 days
to 28
days, by modifying the platelet membrane with a glycan-modifying agent, namely
a
sugar, a monosaccharide sugar, a nucleotide sugar, sialic acid, sialic acid
precursors,
CMP-sialic acid, UDP-galactose, and UDP-galactose precursors. Rosiello's
method
is not practical, however, because it is known that glycosylation (i.e.,
binding
saccharides to proteins and/or lipids) fails to restore, the functionality of
chilled
platelets in vivo.
For example, the inventors of the present invention were present at a seminar
at the Center for Blood Research at the University of British Columbia on
April 26,
2006 at which Dr. Karin Hoffmeister gave a public presentation entitled
"Platelet
Glycosylation and the "In and Outs" of Platelet Transfusion" during which Dr.
Hoffmeister talked about the problems that had been encountered with
glycosylation,
said problems including the fact that glycosylation does not protect platelets
in chilled
platelet concentrates. The results presented at the seminar were also
published in a
peer-review journal (Blood, 2008, 111: 3249-56).
In addition, Hans Wandall of Zymequest, Inc. gave a public presentation in
California at the annual meeting of the California Blood Bank Society on April
28,
2006 in which Hans Wandall substantiated that "glycosylation of platelets does
not
work, at least after extended storage in the cold and not for larger volumes,"
which
was confirmed by an attendee of said public presentation by Hans Wandall to an
inventor of the present invention via email correspondence on June 22, 2006.
3
CA 02637517 2008-07-14
At a meeting of the American Society of Hematology on December 11, 2006,
S. J. Schlichter et al. reported the result of studies relating to
galactosylated platelets
derived from humans and stored a 4 C and concluded: "The data show that,
following
two days of 4 C storage, the recoveries and survivals of the galactosylated
platelets
are no different than the non-galactosylated 4 C stored platelets from the
same
volunteer. Although the recoveries of the 4 C stored platelets with and
without
galactosylation are well-maintained compared to the 22 C stored platelets, the
survivals are markedly reduced as had been previously shown for 4 C stored
platelets
(Br J Haematol 1976;34:403)." (see S. J. Schlichter et al., Abstract
HEMO6L1_379:
Contract View, American Society of Hematology, December 9, 2006,
http=//127 0 0.1:9080/HEMO6/view.y?nu=HEMO6L1 379&terms=580).
Further, very few cryoprotectants are available to store platelets at
temperatures below 0 C. Known cryoprotectants are dimethylsulfoxide (DMSO),
hydroxyethyl starch (HES), polyethylene glycol (PEG) and glycerol. These
cryoprotectants are usually added to plasma containing platelets. Because they
either
interfere with the blood coagulation mechanism or are toxic, they are usually
removed
from the platelet suspension before the transfusion (Transfusion Medicine
Reviews,
2003, 17: 263-271).
It has been reported that DMSO is the best of these three options as it best
preserves platelet morphology and function (Rothwell et al. 2000 Transfusion,
40:988-993). A concentration of 5-6% (w/v) DMSO provides the best results for
long-term storage of platelets at -80 C. However, since DMSO is very toxic,
removal
of the cryoprotective agent present in the thawed cell suspension is a
necessary step
before transfusion of cryopreserved platelets. This process, currently
performed by
centrifugation, is labor intensive and negatively affects platelet viability.
Indeed, cells
4
CA 02637517 2008-07-14
washed by centrifugation, which results in a pellet, must be left undisturbed
to give
the cells time to recover from the washing before resuspension. It has been
shown that
the release of PF4 and the expression of CD62P are significantly higher with
centrifuged platelets, which are signs of platelet activation. In a nutshell,
DMSO could
be an appropriate cryoprotectant but since it has to be removed from the
platelet
suspension prior to transfusion, it increases the risk for bacterial
contamination and
adds a mechanical stress on the platelets.
With respect to the storage and freezing of platelets, it is known in the art
that
in vitro results are predictive of in vivo functionality (Rothwell et al. 2000
Transfusion
40: 988-993). It is also recognized in the art that it is not necessary for
frozen and
thawed platelet substitutes to retain 100 percent of in vitro function to have
satisfactory results in vivo (Rothwell et al. 2000 Transfusion 40: 988-993).
Thus, there is a need for a method for storing platelets at temperatures below
4 C such that the stored/thawed platelets have acceptable platelet
functionality and
viability. There is also a need for a non-toxic cryoprotectant.
SUMMARY OF THE INVENTION
This application relates to method for storing platelets and more specifically
to
methods for storing platelets at freezing temperatures.
According to a first aspect, the present application provides a method for
storing platelets. This method comprises forming at least one modified
platelet
comprising at least one platelet and at least one polymerated chemical. Each
polymerated chemical either comprises (i) a polymer covalently bonded directly
to the
platelet membrane of the platelet or (ii) a polymer and a linker molecule such
that the
linker molecule is covalently bonded to the platelet membrane of the platelet
and the
5
CA 02637517 2008-07-14
polymer is covalently attached to the linker molecule. The polymer of each
polymerated chemical of each modified platelet may be independently selected
from
the group consisting of polyethylene glycol (PEG) and a PEG derivative. The
method
also comprises storing the at least one modified platelet in at temperature of
about or
below 0 C or 4 C for a time period of at least one hour. In an embodiment, the
storage temperature is about or below -18 C, about or below -80 C or about or
below -
210 C. In another embodiment, the storage time exceeds 12 days. In a further
embodiment, the at least one modified platelet in a platelet is also stored in
an additive
solution. In yet another embodiment, the method further comprises, prior to
the
formation of the at least one modified platelet, that the at least one
platelet is provided
from whole blood-derived platelet rich plasma (PRP) platelets, whole blood-
derived
buffy coat platelets, and/or apheresis platelets. In yet a further embodiment,
the
polymer of the polymerated chemical consists of PEG and/or a PEG derivative.
In yet
another embodiment, the at least one modified platelet consists of a plurality
of
modified platelets, wherein a polymer of a polymerated chemical of a first
modified
platelet of the plurality of modified platelets consists of a first PEG
derivative, and
wherein a polymer of a polymerated chemical of either the first modified
platelet or a
second modified platelet of the plurality of modified platelets consists of a
second
PEG derivative that differs from the first PEG derivative. In still another
embodiment, wherein if after said storing is performed the at least one
modified
platelet were introduced into a subject in need thereof, then the at least one
modified
platelet introduced into the subject would restore at least one platelet
function in the
subject wherein a same number of non-modified platelets introduced into the
subject
after being stored in the temperature range for the time period would not
restore the at
least one platelet function. In still a further embodiment, the at least one
platelet
6
CA 02637517 2008-07-14
function is selected from the group consisting of platelet adhesion, platelet
activation,
platelet aggregation, clot fornlation, clot retraction, cytokine production
and
coagulation.
According to another aspect, the present application provides a platelet
structure comprising at least one modified platelet at a temperature of about
or below
0 C or 4 C. Each modified platelet comprises a platelet and at least one
polymerated
chemical. Each polymerated chemical either comprises (i) a polymer covalently
bonded directly to the platelet membrane of the platelet or (ii) a polymer and
a linker
molecule such that the linker molecule is covalently bonded to the platelet
membrane
of the platelet and the polymer is covalently attached to the linker molecule.
The
polymer of each polymerated chemical of each modified platelet is
independently
selected from the group consisting of polyethylene glycol (PEG) and a PEG
derivative. In an embodiment, wherein if the at least one modified platelet
was
subsequently introduced into a subject in need thereof, the at least one
modified
platelet would restore at least one platelet function in the subject, whereas
a same
number of non-modified platelets introduced into the subject after being
stored in the
temperature range for the time period would not restore the at least one
platelet
function. In a further embodiment, the at least one platelet function is
selected from
the group consisting of platelet adhesion, platelet activation, platelet
aggregation, clot
formation, clot retraction, cytokine production and coagulation. In yet
another
embodiment, the storage temperature of the platelet structure is about or
below -18 C,
about or below -80 C and/or about or below -210 C. In still another
embodiment, the
structure further comprises a platelet additive solution. In yet a further
embodiment,
the polymer of the polymerated chemical consists of PEG and/or a PEG
derivative.
In still a further embodiment, the at least one modified platelet consists of
a plurality
7
CA 02637517 2008-07-14
of modified platelets, wherein a polymer of a polymerated chemical of a first
modified platelet of the plurality of modified platelets consists of a first
PEG
derivative, and wherein a polymer of a polymerated chemical of either the
first
modified platelet or a second modified platelet of the plurality of modified
platelets
consists of a second PEG derivative that differs from the first PEG
derivative.
In yet another aspect, the present application provides a method of treating a
condition associated with a reduced platelet function in a subject in need
thereof. The
method comprising administering at least one modified platelet produced by the
method described herein or the platelet structure described herein to the
subject,
thereby treating the condition in the subject. In an embodiment, the subject
is a
mammal and, in yet another embodiment, the subject is human or a non-human
mammal. The condition can be, but is not limited to, thrombocytopenia,
idiopathic
thrombocytopenic purpura, thrombotic thrombocytopenic purpura, drug-induced
thrombocytopenia, Gaucher's disease, aplastic anemia, alloimmune disorder,
fetomaternal alloimmune thrombocytopenia, transfusion reaction, HELLP
syndrome,
hemolytic-uremic syndrome, chemotherapy-induced thrombocytopenia, dengue and
alpha-delta platelet storage pool deficiency. In yet another embodiment, the
subject
in need thereof has a low platelet count. In still a further embodiment, the
method
restores normal platelet count in the subject. In yet another embodiment, the
at least
one modified platelet or the platelet structure has been mixed with a plasma
protein
prior to its administration to the subject.
In still another aspect, the present application provides use of the modified
platelets as described herein for the treatment of a subject in need thereof
as described
herein.
8
CA 02637517 2008-07-14
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 depicts effects on platelets of cooling platelets from 37 C to 4 C, in
accordance with the related art.
FIG. 2 is a schematic representation of a modified platelet, in accordance
with
embodiments of the present invention.
FIG. 3 is a flow chart of a method of forming and using modified platelets, in
accordance with embodiments of the present invention.
FIG. 4 contrasts mPEG grafted platelets with normal platelets with respect to
the respective platelets being chilled, in accordance with embodiments of the
present
invention.
FIG. 5 depicts modification of platelets with 10mM BTC-PEG (5000kDa), in
accordance with embodiments of the present invention.
FIG. 6 depicts the effect on morphological changes and microaggregation of
cooling and subsequent rewarming of PEG-modified platelets, in accordance with
embodiments of the present invention.
FIG. 7 depicts PEGylation of 7 day old platelet concentrates, in accordance
with embodiments of the present invention.
FIG. 8 depicts the response of PEGylated platelets to platelet agonists, in
accordance with embodiments of the present invention.
FIGS. 9A and 9B depict thromboelastography (TEG) of PEGylated platelets,
in accordance with embodiments of the present invention.
FIG. 10 depicts the platelet count (number of cells recovered of PEGylated
and untreated platelets) after freezing, storing for up to 12 days at -80 C
and thawing
of platelet suspensions.
9
CA 02637517 2008-07-14
FIG. 11 depicts the morphology of (A) control fresh platelets, (B) PEGylated
fresh platelets and (C) PEGylated frozen/thawed platelets.
FIG. 12 depicts the morphology of (A and D) control untreated platelets, (B
and E) PEGylated platelets and (C and F) DMSO-treated platelets. Phase
contrast
micrographs of (A, B, C) fresh and (D, E, F) frozen/thawed cells are shown.
FIG. 13 depicts flow cytometry results of (A) untreated fresh platelets, (B)
fresh PEGylated platelets, (D, F) frozen/thawed PEGylated platelets, (C) fresh
DMSO-treated platelets, (E, G) frozen/thawed DMSO-treated platelets. Scatter
plots
are shown in A, B, C, D and E while CD9 and CD62-P expression are shown in F
and
G.
FIG. 14 depicts automated optical platelet counts measured with the Advia
120. Optical platelet counts of (A) control fresh untreated platelets, (B)
fresh
PEGylated platelets and (C) control frozen/thawed untreated platelets and (D)
frozen/thawed PEGylated platelets.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a method, system, and structure for safely
storing modified platelets at temperatures of less than 0 C subsequent to
formation of
the modified platelets. The modified platelets are formed by covalent
modification of
the platelet membrane of the platelets with polyethylene glycol ("PEG") or
derivatives
of poly(ethylene glycol) such as methoxypolyethylene glycol ("mPEG"). The
covalent modification of the platelets with PEG or a PEG-derivative blocks the
adverse effects of the platelet cold storage lesion while maintaining
acceptable platelet
function and viability (e.g., normal platelet function and viability). As
indicated
above, PEG has been previously used as an additive in platelet suspensions as
a
CA 02637517 2008-07-14
cryoprotectant (Transfusion Medecine Reviews, 2003, 17: 263-271). In the prior
art,
contrary to what is described herein, PEG has not been covalently linked to
the
surface of the platelet and interfered with the blood coagulation properties
of the
thawed platelet suspension. Surprisingly, when PEG or mPEG is covalently
attached
to the surface of the platelet, as described herein, it does not alter the
blood
coagulation properties of the modified platelets and act as an excellent
cryoprotectant.
As indicate herein, in the art, there are very few techniques for storing
platelet
solutions at freezing temperatures. As used herein, the term "freezing
temperature"
refers to a temperature below 4 C or below 0 C. Cell (e.g. platelet) solutions
and
suspensions can be stored in freezers having an optimal temperature of -18 C.
But
generally, in the art, cells (e.g. platelets) suspensions and solutions are
stored in
industrial freezers having an optimal temperature of -80 C or in liquid
nitrogen
having an optimal temperature of -210 C.
Normal in vitro platelet functionality (or platelet function) is defined as
full
aggregation of platelets in plasma in response to 2 IU/mL thrombin (75 - 100%
increase in light transmission measured by platelet aggregometry test, as
illustrated in
portion 72 of FIG. 8, described infra) and the potential to recover from mild
stress,
i.e., recover resting morphology after mild temperature or osmotic stress.
Normal in
vivo functionality is defined as 67 percent mean post-transfusion recovery
(range 50-
80%) of stored platelets compared to fresh platelets and 50 percent mean post-
transfusion survival (range 30-70%) of stored platelets compared to fresh
platelets
measured. 1 hour or 24 hours after transfusion with both fresh and stored
platelets
being obtained from the same human being or mammal (Slichter S J et a12006
"Viability and function of 8-day-stored apheresis platelets", Transfusion. 46
1763-9;
11
CA 02637517 2008-07-14
and Murphy S. 2006 "The case for a new approach for documenting platelet
viability", Transfusion. 46 Suppl. 49S-51 S).
As it is known in the art, normal platelet function includes various aspects
leading to or facilitating clot formation. Normal platelet functions include,
but are not
limited to, platelet adhesion, platelet activation, platelet aggregation, clot
retraction,
pro-coagulation and cytokine signaling. Platelets are activated by contacting
an
activating agent (such as collagen, thrombin, a negatively charged surface,
etc). Once
activated, platelets release a number of different coagulation factors and
platelet
activating factors. They also aggregate and adhere to the inner surface of a
damaged
vessel wall. Because platelets are rapidly deployed to sites of injury or
infection, they
also modulate the inflammatory process by interacting with leukocytes and by
secreting cytokines, chemokines and other inflammatory mediators.
With the present invention, modified platelets can be stored for prolonged
periods of time (e.g., more than 12 days) at temperatures less than 0 C (e.g.,
-18 C,
80 C and/or -210 C). This temperature range significantly inhibits bacterial
growth
during the cold storage of the platelet suspensions or solutions. This
advantage is
applicable in the traditional blood banking environment as well as in specific
medical
interventions involving the transient cooling of the whole or partial body to
a
temperature of less than 22 C. Thus, the results provided herein satisfy a
long-felt,
previously unsatisfied need in transfusion medicine for storing platelets
under cooling
temperature conditions that inhibit microbial growth while maintaining
acceptable
platelet function and viability.
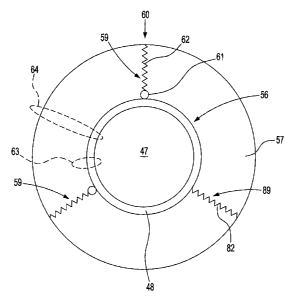
FIG. 2 is a schematic representation of a modified platelet 60, in accordance
with embodiments of the present invention. The modified platelet 60 comprises
a
platelet 56 and at least one polymerated chemical 59. In one embodiment, the
at least
12
CA 02637517 2008-07-14
one polymerated chemical 59 consists of a plurality of polymerated chemicals
59.
The platelet 56 includes a platelet core 47 and a platelet membrane 48 that
surrounds
the platelet core 47. Each polymerated chemical 59 is covalently bonded to the
platelet membrane 48 of the platelet 56. More specifically in one embodiment,
each
polymerated chemical 59 comprises a linker molecule 61 and a polymer 62,
wherein
the polymer 62 is covalently attached to the linker molecule 61 and the linker
molecule 61 is covalently bonded to the platelet membrane 48 at a bonding site
(e.g.,
at a protein or at a carbohydrate) of the platelet membrane 48. The linker
molecule
serves to activate the covalent linkage of the polymer 62 to the platelet 56
at the
platelet membrane 48.
In an alternative embodiment, a polymerated chemical 89 comprises a polymer
82 covalently bonded directly to the platelet membrane 48 at a bonding site
(e.g., at a
protein or at a carbohydrate) of the platelet membrane 48. The polymerated
chemical
89 is analogous to the polymerated chemical 59, except that the polymerated
chemical
89 does not comprise a linker molecule 61, and the polymer 82 is analogous to
the
polymer 62. Although the discussion infra describes the present invention for
the
embodiment of the polymerated chemical 59 that comprises the linker molecule
61
and the polymer 62, it should be understood that unless otherwise indicated or
otherwise inapplicable, said discussion infra applies likewise to the
alternative
embodiment of the polymerated chemical 89 that comprises the polymer 82,
wherein
the polymer 82 is covalently bonded directly to the platelet membrane 48.
The space defined by the at least one polymerated chemical 59 is an envelope
57 that envelopes the platelet 56 due to a "long chain length" of each polymer
62 (i.e.,
a chain length that has sufficient magnitude to fill the space around itself).
The
envelope 57 provides a immunocamouflage functionality. A small membrane
protein
13
CA 02637517 2008-07-14
63 (such as CD9 = p24) is covered by the envelope 57 and cannot bind its
respective
antibody. A large, extended membrane protein 64 (such as CD42b = GPIb) is
partially covered by the envelope 57 and reaches through the envelope 57, and
can
still be recognized and bound by the respective antibody as well as other
proteins
important for the hemostatic function of platelets. The envelope 57 prevents
the
formation and/or immunologic recognition of GPIb-clusters and
microaggregation.
The polymer 62 in each polymerated chemical 59 is independently selected
from the group consisting of polyethylene glycol (PEG) and a PEG derivative.
Polyethylene glycol has the formula H(OCH2CH2)r,OH, wherein n is greater than
or
equal to 4, with a molecular weight of up to about 20,000 Daltons. Various
derivatives of polyethylene glycol may substitute for the H or OH end groups,
forming, for example, polyethylene glycol ethers (e.g., PEG-O-R; PEG-O-CH3 ;
CH3-PEG-OH); 2,4-dinitrophenyl ethers of PEG), polyethylene glycol esters
(e.g.,
PEG-OZC(CH2)14CH3 ; PEG-O2CCH2CH2CO2-atropine), polyethylene glycol amides
(e.g., PEG-OZC(CHZ)7CONHR; mPEG-O2CCH2CH2CONH(CH3)CHCH2C6H5;
PEG-OZCCHzCHZCONHCHZCHZ-NAD+), polyethylene glycol amines (e.g.,
PEG-NH2; PEG-NH(CH2)6NH2; PEG-OCH2CH2NH2; mPEG-NH2), polyethylene
glycol acids (e.g., PEG-OZC(CHZ)ZCO2H; PEG-O-CH2CO2H;
PEG-OZC-(CH2)7-CO2H), polyethylene glycol aldehydes (e.g.,
PEG-O-CHZ-CHO), and electrophilic derivatives (e.g., PEG-Br; PEG-OSO2CH3;
PEG-O). Various phenyl moieties can also be substituted for the H or OH of
PEG,
such as the 2,4-dinitrophenyl ether of PEG mentioned above. The particular
polyethylene glycol derivatives listed above are exemplary only, and the
invention is
not intended to be limited to those particular examples.
The linker molecule 61 may comprise, inter alia, cyanuric chloride, imidazolyl
14
CA 02637517 2008-07-14
formate, succinimidyl succinate, succinimidyl carbonate, succinimidyl
glutarate, N-
hydroxysuccinimide, 4-nitrophenol, and 2,4,5-trichlorophenol. The linker
molecules
listed above are exemplary only, and the invention is not intended to be
limited to
those particular examples. Any linker molecule capable of covalently attaching
to the
polymer 62 and mediating the linkage of the polymer to the platelet membrane
48
may be similarly used.
FIG. 3 is a flow chart of a method of forming and using modified platelets, in
accordance with embodiments of the present invention. The flow chart of FIG. 3
comprises steps 31-34.
Step 31 prepares at least one platelet (e.g., a plurality of platelets), using
any
known platelet preparation method such as, inter alia, whole blood-derived
platelet
rich plasma (PRP) platelets, whole blood-derived buffy coat platelets, or
apheresis
platelets.
Step 32 forms at least one modified platelet from the at least one platelet
prepared in step 31. Each modified platelet conforms to the modified platelet
60 of
FIG. 2 and comprises a platelet and at least one polymerated chemical. Each
polymerated chemical either comprises a polymer covalently bonded directly to
the
platelet membrane of the platelet or comprises the polymer and a linker
molecule such
that the linker molecule is covalently bonded to the platelet membrane of the
platelet
and the polymer is covalently attached to the linker molecule. The polymer of
each
polymerated chemical of each modified platelet is independently selected from
the
group consisting of polyethylene glycol (PEG) and a PEG derivative. Step 32
does
not comprise modifying the platelet membrane of the platelets with a glycan-
modifying agent, because it is known that glycosylation (i.e., binding
saccharides to
proteins and/or lipids) fails to preserve the functionality of chilled
platelets in vivo as
CA 02637517 2008-07-14
indicated supra. Indeed, it is totally outside of the scope of the present
invention to
modify the platelet membrane of the platelets with a glycan-modifying agent.
In one embodiment, a polymer of a polymerated chemical of a modified
platelet of the at least one modified platelets consists of PEG. For example,
the
modified platelet 60 of FIG. 2 comprises at least one polymerated chemical,
and the
polymer of one polymerated chemical of the at least one polymerated chemical
may
consist of PEG.
In one embodiment, a polymer of a polymerated chemical of a modified
platelet of the at least one modified platelet consists of a PEG derivative.
For
example, the modified platelet 60 of FIG. 2 comprises at least one polymerated
chemical, and the polymer of one polymerated chemical of the at least one
polymerated chemical may consist of a PEG derivative.
In one embodiment, a polymer of a polymerated chemical of a first modified
platelet of the at least one modified platelet consists of a first PEG
derivative, and a
polymer of a polymerated chemical of either the first modified platelet or a
second
modified platelet of the at least one modified platelet consists of a second
PEG
derivative that differs from the first PEG derivative. The preceding
embodiment is
describing cases in which two different PEG derivatives (e.g., PEG-O-CH3 and
CH3-PEG-OH) are present in a plurality of modified platelets, wherein the
plurality
of modified platelets comprise a first modified platelet and a second modified
platelet.
These two different PEG derivatives are denoted as a first PEG derivative and
a
second PEG derivative. In one case, both the first PEG derivative and the
second
PEG derivative are in the first modified platelet. In another case, the first
PEG
derivatives is in the first modified platelet and the second PEG derivative is
in the
second modified platelet.
16
CA 02637517 2008-07-14
Step 33 stores the modified platelets formed in step 32 in a temperature range
below 4 C or below 0 C for a time period of at least one hour. In one
embodiment,
the modified platelets are stored in a platelet additive solution. In one
embodiment,
the temperature range below 4 C is a single temperature characterized by an
approximately constant value of temperature (e.g., 0 C, 4 C, 10 C, etc.). In
one
embodiment, the temperature range below 4 C (or below 0 C) is, inter alia:
from -210
C to below 4 C, from -80 C to below 4 C, from -18 C to below 4 C, etc. The
time
period of at least one hour may, inter alia: be in a range from 1 day to five
days,
exceed 5 days, be in a range from more than 5 days to 30 days, be in a range
from 30
days to 3 months, exceed 3 months, be in a range from 3 months to 1 year, etc.
The storage of the modified platelets in the temperature range below 4 C for
the time period of at least one hour in step 33 prevents and/or retards
microbial
growth on the stored platelets during the time period.
In one embodiment, the platelets prepared in step 31 were obtained from a
subject such as an animal (i.e., a mammal) and after the storing step 33 has
been
performed, the modified platelets have a post-transfusion recovery in the
animal of
50% to 80%, relative to fresh platelets from the subject, at a post-
transfusion time in a
range of 1 hour to 24 hours measured from a time of transfusion of the
modified
platelets and the fresh platelets into the subject. This means that if the
post-stored
platelets were transfused into the subject, then the percentage of the
transfused post-
stored platelets that would be recovered in the recipient's circulation is 50%
to 80% of
the percentage of fresh platelets that would recover in the recipient's
circulation at a
post-transfusion time in a range of 1 hour to 24 hours measured from a time of
the
transfusion of the post-stored platelets and the fresh platelets into the
subject. In this
embodiment, the subject may be the same animal or mammal into which the
modified
17
CA 02637517 2008-07-14
platelets are introduced in step 34 (described infra) or the animal may be
another
mammal. The modified platelets consist of at least N modified platelets, N
being a
minimum number of modified platelets necessary for a determination of the post-
transfusion recovery to have a statistical error not exceeding a specified
threshold
percent. The specified threshold percent may be in a range of 1% to 20% or any
subset thereof (e.g., 5%, 10%, 5 to15%, 10% to 20%, 20%, etc.). In this
embodiment,
the post-transfusion recovery is an acceptable post-transfusion recovery.
In one embodiment, the platelets prepared in step 31 were obtained from a
subject such as an an animal (i.e., a mammal) and after the storing step 33
has been
performed, the modified platelets have a post-transfusion survival in the
animal of
30% to 70%, relative to fresh platelets from the subject, at a post-
transfusion time in a
range of 1 hour to 24 hours measured from a time of transfusion of the
modified
platelets and the fresh platelets into the subject. This means that if the
post-stored
platelets were transfused into the subject, then the percentage of the
transfused post-
stored platelets that would survive is 30% to 70% of the percentage of fresh
platelets
that would survive, at a post-transfusion time in a range of 1 hour to 24
hours
measured from a time of the transfusion of the post-stored platelets and the
fresh
platelets into the subject. In this embodiment, the animal may be the same
mammal
into which the modified platelets are introduced in step 34 (described infra)
or the
animal may be another mammal. The modified platelets consist of at least N
modified
platelets, N being a minimum number of modified platelets necessary for a
determination of the post-transfusion survival to have a statistical error not
exceeding
a specified threshold percent. The specified threshold percent may be in a
range of
1% to 20% or any subset thereof (e.g., 5%, 10%, 5 tol5%, 10% to 20%, 20%,
etc.). In
this embodiment, the post-transfusion survival is an acceptable post-
transfusion
18
CA 02637517 2008-07-14
survival.
Step 34 introduces the modified platelets into a mammal after having been
stored at a temperature below 4 C for the time period in step 33. In one
embodiment,
the subject is a mammal such as a human being. In one embodiment, the subject
is a
non-human mammal (e.g., dog, cat, horse, rat, etc.).
The modified platelets introduced into the subject in step 34 have a longer
circulation half-life in the subject than would a same number of non-modified
platelets introduced into the subject after being stored in the temperature
range below
4 C or 0 C for the time period. The non-modified platelets would be processed
in
accordance with the flow chart of FIG. 3 except that step 32 is not performed.
Thus,
the non-modified platelets are prepared as in step 31, stored at temperature
below 4 C
or 0 C for the time period of at least one hour as in step 33, and introduced
into the
animal as in step 34.
FIG. 4 contrasts mPEG grafted platelets with normal platelets with respect to
the respective platelets being cooled, in accordance with embodiments of the
present
invention.
In the upper portion 5 of FIG. 4, normal platelets 10 comprise glycoprotein
(GP) lb 12 and other membrane proteins 14 inherent to the platelet membrane
16.
The normal platelets 10, upon being cooled from 37 C to 4 C, aggregate with
significant shape change wherein the GP lb 12 form GP lb clusters 13 at the
platelet
membrane 16 outer surface in the transformation of the normal platelets 10 to
the
cooled platelets 20. After introduction of the cooled platelets 20 into a
subject, the
GPIb clusters 13 are recognized by CR3 receptors of liver macrophages, which
leads
to the phagocytosis of the previously cooled platelets 20.
19
CA 02637517 2008-07-14
In the lower portion 6 of FIG. 4, the polymerated chemical 59 of FIG. 2
surrounds the platelet 56 to form the modified platelet 60, which is cooled
from 37 C
to 4 C, wherein the envelope 57 provides a immunocamouflage functionality that
prevents microaggregation of the platelets and reduces platelet shape change
upon
said cooling. Furthermore, the formation and/or immunologic recognition of
GPIb-
clusters 13 and other membrane proteins is attenuated due to the envelope 57.
FIG. 5 depicts modification of platelets with 10mM BTC-PEG (5000kDa), in
accordance with embodiments of the present invention. FIG. 5 comprises normal
platelets acting as a control in panels 41-43 and PEG-modified in panels 44-
46.
Panels. 41 and 44 depict the normal and modified platelets, respectively, as
fresh
platelets or platelets following 24 fours of storage at or above 20 C. Panels
42 and 45
depict the normal and modified platelets, respectively, at 20 C. Panels 43 and
46
depict the normal and modified platelets, respectively, at 4 C.
As seen in panels 41-42 and 44-45, the platelet modification of the modified
platelets does not change platelet morphology of fresh platelets or following
24 hours
storage at or above 20 C. Furthermore, PEGylation of platelets prevents
platelet
activation and microaggregation at 4 C, as shown for the modified platelets in
panel
46 in comparison with the control platelets in panel 43.
FIG. 6 depicts the effect on morphological changes and microaggregation of
cooling and subsequent rewarming of PEG-modified platelets, in accordance with
embodiments of the present invention. FIG. 6 comprises panels 67A, 67B, 68,
and
69. Panel 67A depicts normal control platelets from platelet concentrates or
platelet
rich plasma (PRP) fixed at 4 C. Panel 67B depicts microaggregation of normal
control platelets from platelet concentrates or platelet rich plasma (PRP)
fixed at 4 C.
Panel 68 depicts PEGylated platelets in plasma fixed at 4 C. Panel 69 depicts
CA 02637517 2008-07-14
PEGylated platelets rewarmed and fixed at 37 C after exposure to 4 C. FIG. 6
shows
that PEGylation of platelets prevents both significant morphological changes
and
microaggregation of platelets at or after 30 minutes at 4 C. Furthermore,
PEGylated
platelets regain normal morphology upon rewarming to 37 C.
As seen in panel 67, the normal control platelets from platelet concentrates
or
PRP undergo severe morphological changes and form small aggregates when
exposed
to low temperature (4 C). Phase contrast microscopy shows long pseudopods and
platelet-platelet interactions. As seen in panel 68, PEGylation inhibits
severe
morphological changes as well as platelet interactions at 4 C. As seen in
panel 69, a
smooth, resting morphology was restored by incubation at 37 C, which indicates
that
upon rewarming from 4 C to 37 C, PEGylated platelets are viable and minor
morphological changes caused by chilling are reversible.
FIG. 7 depicts PEGylation of 7 day old platelet concentrates, in accordance
with embodiments of the present invention. PEGylation of 7 day old platelet
concentrates prevents recognition of platelet surface (e.g., CD9) and
activation (e.g.,
CD62) markers. Shown is anti-CD9 binding to 7 day old platelets (washed before
and
after reaction with 0 or 10 mM BTC-PEG5000). CD9 antigens were effectively
masked on washed PEGylated platelets, which was shown as complete inhibition
of
FITC-labeled anti-CD9 binding to these platelets. In contrast, control
platelets
demonstrated -100% anti-CD9 binding and therefore -100% of platelets have
fluorescently (FITC) labeled antibody bound to them. In FIG. 7 the extent of
FITC
labeling is shown as % Fluorescence.
FIG. 8 depicts the response of PEGylated platelets to platelet agonists, in
accordance with embodiments of the present invention. FIG. 8 shows that
PEGylated
platelets are fully functional and aggregate in vitro in response to platelet
agonists
21
CA 02637517 2008-07-14
(e.g., thrombin). In portion 71 of FIG. 8, phase contrast microscopy of
control plate-
lets in plasma and PEGylated platelets in plasma shows that PEGylated
platelets
maintain a smooth, resting morphology. In portion 72 of FIG. 8, in response to
2
IU/mL thrombin, control platelets and PEGylated platelets fully aggregate at
37 C
with 1000 rpm stir speed in the aggregometer (ChronoLog). In portion 73 of
FIG. 8,
control and PEGylated platelets form microscopically very similar thrombin-
induced
clots demonstrating normal biological function. Aggregates depicted in portion
73 of
FIG. 8 came from samples fixed at the end of the experiment shown in portion
72.
FIGS. 9A and 9B depicts thromboelastography (TEG) of PEGylated platelets,
in accordance with embodiments of the present invention. The TEG in FIGS. 9A
and
9B demonstrates normal platelet function for the PEGylated platelets.
In FIG. 9A, platelet mapping with TEG determines total platelet function. The
two symmetric arms show the same results. The parameter definitions are: R:
time
required for initial fibrin formation); Kc: time to reach a certain level of
clot strength
(clot kinetics); Angle: speed of fibrin build-up and cross-link (clot
strengthening);
MA: maximum amplitude: dynamic properties of fibrin and platelet bonding
through
GPIIb-Ilfa.
In FIG. 9B, representative findings obtained with acid citrate dextrose (ACD)
anticoagulated control platelets and PEGylated platelets are overlaid on the
generic
tracing expected for normal whole blood. Both control and PEGylated platelet
products fall within the expected ranges; i.e., the speed of fibrin formation
and build-
up is equivalent and the dynamic properties of fibrin as well as platelet
bonding
through GPIlb-IIIa / fibrinogen are the same for control and PEGylated
platelets.
In another aspect, the present invention provide a method of storing platelet
at
freezing temperatures wherein the method limits the loss in normal platelet
function
22
CA 02637517 2008-07-14
of the thawed platelet. The platelets are produced by the method described
above and
illustrated in FIG. 3, except that, in steps 33 and 34, the platelets are
stored at a
freezing temperature (e.g. less than 0 C, -18 C, -80 C or -210 C). Because
modified
platelets described herein are non-toxic (e.g. do no exhibit cytotoxicity),
unlike
DMSO-treated platelets, once the modified platelets are thawed, there is no
need to
wash them prior to their administration to a subject.
In another embodiment, the invention also provides a method of treating a
condition associated with a reduced platelet function in a subject in need
thereof. The
method comprises the step of administering at least one modified platelet
produced by
the method described herein or the platelet structure described herein. In an
embodiment, the modified platelet or platelet structure has been thawed prior
to its
administration to the subject. In another embodiment, the thawed modified
platelet or
platelet structure does not need to be washed prior to its administration to
the subject.
As used herein, a subject in need thereof is defined as a subject having a low
platelet count or dysfunctional platelet activity. Such subjects have a
tendency to
abnormally bleed. The severity of the dysfunction correlates directly with the
severity
of the bleeding problem. In the art, it is recognized that normal platelet
count in a
healthy subject is between 150,000 and 400,000 per mm3 of blood (150-400 x
109/L).
The vast majority of healthy subjects (around 95%) will have platelet counts
in this
range. As used herein, a healthy subject is either a subject having a normal
platelet
count or a subject not afflicted by a condition related to decrease platelet
function. On
the other hand, a subject in need thereof, as used herein, refers either to a
subject
having a low platelet count (e.g. less than 100 x 109/L, 80 x 109/L, 50 x
109/L or even
5 x 109/L) or having platelets that do not perform at least one normal
platelet function
23
CA 02637517 2008-07-14
as indicated above. In a further embodiment, the subject in need thereof or
the
healthy subject is a mammal, a human or a non-human mammal.
The method provided herewith is advantageous for the treatment of subjects
afflicted with at least one of thrombocytopenia, idiopathic thrombocytopenic
purpura,
thrombotic thrombocytopenic purpura, drug-induced thrombocytopenia, Gaucher's
disease, aplastic anemia, alloimmune disorder, fetomatemal alloimmune
thrombocytopenia, transfusion reaction, HELLP syndrome, hemolytic-uremic
syndrome, chemotherapy-induced thrombocytopenia, dengue and/or alpha-delta
platelet storage pool deficiency. In some of the conditions listed above, the
modified
platelets are administered therapeutically to restore a platelet function and
ideally,
restore all platelet functions. In other conditions (such as chemotherapy-
induced
thrombocytopenia of subjects suffering from hematologic malignancies), the
modified
platelets are administered prophylactically because the subjects in need
thereof
become severely thrombocytopenic but do not (yet) bleed.
The present invention will be more readily understood by referring to the
following examples which are given to illustrate the invention rather than to
limit its
scope.
EXAMPLE I - Determination of cold storage on PEGylated platelets
Platelet modification with PEG or PEG derivatives is done by mixing a
concentration of platelets with chemically activated PEG or PEG derivatives.
The
concentration of platelets can range from very low counts to very high counts
as
required by the application; for clinical purposes, a single unit of platelet
rich plasma
(PRP) should contain at least 5.5 x 1010 platelets (see AABB Technical Manual,
12'h
edition, 1996 American Association of Blood Banks, page 144). Activation of
PEG
24
CA 02637517 2008-07-14
or PEG derivatives is accomplished by chemically modifying one or both
terminal
reactive groups of PEG or PEG derivatives with a chemical reactive linker
group of
an associated linker molecule.
Multiple mixing methods can be used to achieve the desired platelet-PEG
ratio. In one embodiment, whole blood is collected in ACD (acid citrate
dextrose)
anticoagulant. Platelet rich plasma (PRP) is prepared from the whole blood by
centrifugation (150 x g for 12 minutes). Platelet numbers are determined using
an
automated cell counter. The PRP is mixed with the desired concentration of
activated
PEG or PEG-derivative using an automated mixing instrument so as to achieve a
uniform platelet-PEG ratio. The platelet-PEG mixture is collected and allowed
to
react for 30 minutes at room temperature. Both the reaction time and
temperature can
be varied. For example, the reaction time could range from 1 minute to greater
than
60 minutes. The reaction time is governed in part by the reactivity of the
linker
molecule as well as the desired efficiency of the reaction. The temperature
should be
greater than 20 C to avoid cold induced injury prior to the protection
afforded by the
grafted PEG or PEG-derivative.
Following derivatization, the modified platelets can be used as is, or can
undergo gentle washing and centrifugation in physiologic solutions (e.g.,
isotonic
saline, ACD, or platelet additive solution that does not contain any plasma
proteins).
In one embodiment of washing, modified platelets are washed using an excess of
a
washing buffer consisting of a 1:1 ratio of phosphate buffered saline and ACD
at
physiologic pH (pH 7-7.8). The platelet-wash solution is mixed gently (e.g.,
inverting
the tube of platelet-wash solution several times) followed by centrifugation
at 600 g
for 3 minutes. Following washing, the wash supernatant is removed. Platelet
counts
are determined via automated cell counters and the platelets are resuspended
to the
CA 02637517 2008-07-14
desired modified platelet count per unit volume using physiologic solutions
(e.g.,
plasma, saline, platelet additive solutions). At this point, the platelets are
suitable for
storage at < 0 C and/or experimental or clinical usage. In other embodiments,
the
washing step is automated using clinical cell washers.
Once the frozen modified platelets are thawed, they can either be used as is,
or
they can be mixed with plasma (e.g. normal plasma) prior to their transfusion
into a
subject in need thereof.
In other preparation embodiments, the source of platelets can be whole blood,
leukoreduced whole blood, whole blood derived buffy coat platelets or
apheresis
platelets. Alternatively for non-clinical or veterinary use, a wide range of
other
platelet preparations (e.g., purified platelets obtained using magnetic bead
technology,
cell culture and expansion, or via cell sorter technology) can be similarly
derivatized.
Platelet concentration can also be significantly varied relative to the PEG or
PEG-
derivative concentration and/or physiologic media.
Depending on the PEG/PEG derivative and the linker group used in the
preceding methodology for forming modified platelets, either: (1) the
associated
linker molecule may remain part of the final structure of the polymerated
chemical (as
in the polymerated chemical 59 of FIG. 2; e.g., cyanuric chloride activated
mPEG); or
(2) the linker group may mediate the chemical reaction between PEG/PEG
derivative
and a protein of the platelet membrane but nonetheless function as a leaving
group so
that the associated linker molecule is not part of the final structure of the
polymerated
chemical (as in the polymerated chemical 89 of FIG. 2; e.g., benzotriazole
carbonate
activated mPEG).
As described supra, the present invention fulfills a long-felt, unsatisfed
need
in transfusion medicine to store platelets under cooling temperature
conditions by
26
CA 02637517 2008-07-14
inhibiting microbial growth while maintaining acceptable platelet function and
viability. The current invention addresses this long-felt, unsatisfied need,
by
covalently modifying the platelet membrane with PEG or a PEG derivative (FIG.
4).
As a consequence of this covalent modification of the platelet membrane, the
detrimental effects of cold storage/exposure are inhibited/prevented as
evidenced by:
maintenance of / return to normal platelet morphology upon transition from 4
to
>15 C (FIGS. 5 and 6); prevention of platelet microaggregation (FIGS. 5 and
6);
attenuation of cold storage activation of platelets during storage (FIG. 7);
maintenance
of normal platelet activation and clot formation upon stimulation (FIGS. 8 and
9); and
inhibition and/or attenuation of GPlb clustering and immunologic recognition
of
platelet surface proteins (FIGS. 4 and 7).
EXAMPLE II- Effects of a freeze/thaw cycle on PEGylated platelets
Platelet enrichment and storage
Whole blood was collected in acid citrate dextrose (ACD) and centrifuged at
150 x g for 12 min at 26 C to obtain platelet rich plasma (PRP). Platelet
counts were
measured with the ADVIA 120 automated hematology analyzer (Beckman Coulter).
The plasma was removed by centrifugation at 500 x g for 5 min at 25 C
followed by aspiration of the supernatant and the platelet pellet was
resuspended in a
platelet storage solution SSP+ (Macopharma, 5mL total volume). The resultant
suspension was split into three aliquots of 1.5mL each (Control, PEGylated and
DMSO-treated samples). An equal volume of SC-mPEG5000 solution in aqueous
buffer was mixed with the platelet suspension for a final concentration of
10mM SC-
mPEG5000. For DMSO-treatment the platelet suspension was mixed with 10%
DMSO at a 1:1 ratio for a final concentration of 5%. All samples were manually
mixed and subsequently kept gently rocked on a shaker for 30 min at room
27
CA 02637517 2008-07-14
temperature to complete the mPEGylation reaction. Samples were flash frozen in
liquid nitrogen and kept at -80 C for at least 12 hours and up to 6 days
before they
were thawed. Samples were thawed at room temperature for 30 min with gentle
agitation.
Platelet cell counts, morphology, flow cytometry and thrombin activity
Fresh and thawed samples were analyzed on the ADVIA 120. Aliquots were
fixed with paraformaldehyde (2% final concentration) for 1 hour at room
temperature
for inspection by phase contrast microscopy to determine the platelet
morphology. For
flow cytometry platelets were washed and resuspended in phosphate buffered
saline
(PBS). Antibodies to the small surface protein CD9 (anti-CD9-FITC obtained
from
BD Biosciences), and the activation marker CD62P (anti-CD62P-PE obtained from
Immunotech) were added prior to incubation at room temperature for 45 min. The
antibody-labeled platelet preparations were then fixed with 0.2% formalsaline.
For light transmission aggregometry, control and PEGylated platelets were
centrifuged at 900 x g for 10 min at 25 C, and the pellet resuspended in
autologous
plasma. Platelet suspensions were kept at room temperature and were incubated
at
37 C for 5 min. before the test was started. 450 uL of each sample was stirred
at 1000
rpm in the ChronoLog lumi-aggregometer and activated with a final
concentration of
2 IU/mL thrombin.
Results
After a freeze/thaw cycle, and as shown on Figure 10, 90% or more PEGylated
platelet cells remained intact and did not lyse, whereas less than 20% of
control
untreated platelets were recovered. Further, frozen/thawed PEGylated platelets
showed a 50% response to 2 IU/mL thrombin compared to fresh control platelets.
On
the other hand, frozen/thawed untreated control platelets did not show a
detectable
28
CA 02637517 2008-07-14
response to thrombin. During this experiment, all pH values stayed in the
physiological range (7.1 - 7.3).
The PEGylation of platelets also modulated cell morphology of frozen/thawed
platelets. As shown on Figure 11, control fresh platelets (A), PEGylated fresh
platelets (B) and frozen/thawed PEGylated platelets (C) display normal
platelet
morphology whereas as control untreated cells did not withstand the
freeze/thaw cycle
(data not shown). Fresh PEGylated platelets have normal morphology and do not
form microaggregates.
The protective effects of PEGylation on the morphology of the frozen/thawed
cells are similar to those of DMSO. As shown on Figure 12, untreated control
frozen/thawed platelets form microaggregates, lyse and form small cell
fragments
(Figure 12D), whereas PEGylated (12E) or DMSO-treated (12F) platelets show
typical platelet morphological characteristics.
The high recovery of platelets following freeze/thaw was also measured with
flow cytometry (Figure 13). Before freezing, the platelet populations (count
and size)
are very comparable as indicated by very similar scatter plots (side scatter
SSC vs.
forward scatter FSC, FIG. 13A, B and C). After a freeze/thaw cycle, control
untreated
platelets did not survive. Thawed populations for PEGylated and DMSO-treated
platelets are similar but shifted down indicating a reduction in platelet size
(FIG. 13D
and E). As shown in FIG. 13F and G, the number of events in the quadrants
marked in
grey indicated that platelet activation, measured as CD62-P expression, was
lower in
PEGylated platelets when compared to DMSO-treated platelets. Further,
PEGylation
also prevented, before and after the freeze/thaw cycle, CD9 recognition.
The flow cytometry results were confirmed by the optical automated
hematology analyzers results generated with the Advia 120 (Figure 14). The
29
CA 02637517 2008-07-14
freeze/thaw cycle did not substantially affect the morphology of the PEGylated
platelets.
EXAMPLE III - Platelet Transfusion in Rabbits comparing control, PEGylated and
DMSO-treated platelets
New Zealand white rabbits first receive gamma irradiation (Cobalt 550cG rads
for 10-11 min.). Six days later, rabbits are injected with ethyl palmitate
(34% solution,
Fluka) and sheep anti-rabbit platelet serum to induce thrombocytopenia (e.g.a
platelet
count of approximately 20 x 109 cells/L).
Concentrated platelet suspensions are prepared from platelet concentrates
drained into 50 mL conical tubes and centrifuged at 1000 x g for 5 min. at 19-
20 C.
With a syringe, 48 mL of the supematant is removed and the pellet is
resuspended in
the remaining supematant. Eight concentrates are combined to obtain a
transfusion
dose per rabbit of 20 x 1010 platelets in 10 mL. Samples for platelet counts,
microscopy and flow cytometry are prepared as described in Example I.
The rabbits are secured and anesthetized. Platelets are transfused through the
ear vein. To measure the ear bleeding time, an indicator of platelet function,
the other
rabbit ear is placed in a 0.9% sodium chloride solution at 37 C with slow
stirring. One
hour after platelet transfusion, the ear is cut with a scalpel and the
bleeding time is
measured until the bleeding stops or to a maximum of 15 min. The bleeding time
is
determined in duplicates from two different sites. The rabbit is allowed to
rest until
the next time point for measuring the bleeding time at 3 and 5 hours post
transfusion.
While particular embodiments of the present invention have been described
herein for purposes of illustration, many modifications and changes will
become
apparent to those skilled in the art. Accordingly, the appended claims are
intended to
CA 02637517 2008-07-14
encompass all such modifications and changes as fall within the true spirit
and scope
of this invention.
31