Note: Descriptions are shown in the official language in which they were submitted.
CA 02638146 2008-08-01
- 1 - 200700553
E v o n i k G o 1 d s c h m i d t GmbH, Essen
Use of ionic liquids for the lubrication of components .in
wind power plants
The invention relates to the use of ionic liquids for the
lubrication of movable parts in wind power plants, in
particular for gearbox lubrication.
Gearboxes are movable combinations of parts for
transmitting and converting force or for conducting parts
on a track. Within the gearbox, friction occurs between
the surfaces of the individual components of the gearbox,
which can comprise, for example, drive and offtake shafts
and toothed wheels. The friction has to be reduced by use
of suitable lubricants since otherwise damage (wear) and
malfunctions, in particular of the toothed wheels, can
occur. The lubricants can significantly prolong the life
of components. In addition, the lubricant can contribute
to reducing the force which has to be applied to produce
movement and the energy consumption associated therewith,
so as to achieve a higher efficiency.
Wind energy is the kinetic energy of the moving air
masses of the atmosphere. Wind energy is kinetic energy
of the air particles which move at the velocity v. The
power of the wind which, for instance, a wind generator
can utilize as electric power is considerably lower
because the velocity cannot be brought down to "0" in a
wind turbine. This fact is allowed for by the Betz
factor.
This Betz factor is not an efficiency but a "utilization
factor" since the wind energy which is not utilized is
largely retained, firstly in the abovementioned residual
CA 02638146 2008-08-01
- 2 - 200700553
motion energy of the wind passing through the wind
turbine and secondly because the wind evades the wind
turbine and flows around it without a reduction in its
speed.
A further important parameter is the tip speed ratio k
(lambda). It is the ratio of the circumferential velocity
of the rotor (blade tip speed) to the wind speed. Three-
blade rotors, as are now standard in large units, reach
the greatest efficiency at a tip speed ratio of from 7 to
8. This corresponds to blade tip speeds in the order of
about 250-300 km/h regardless of the rotor diameter. The
operating point having the highest power coefficient and
the design tip speed ratio also gives the design wind
velocity.
A wind energy plant (WEP) converts the kinetic energy of
the wind into electric energy and feeds this into the
power grid. This is achieved by the kinetic energy of the
wind flow acting on the rotor blades and thus setting the
rotor into rotational motion. The rotor transmits the
rotational energy to a generator which converts it into
electric power.
In everyday speech, and partly also in the technical
literature, the term wind power plant (WPP) has likewise
become established, and wind power station or wind energy
converter (WEC) is sometimes also used.
Wind energy plants can be used in all climatic zones, at
sea and in all land forms (coast, inland, mountains) to
generate electric power.
A wind energy plant consists essentially of a rotor with
hub and rotor blades, a machine pod which accommodates
CA 02638146 2008-08-01
- 3 - 200700553
the generator and frequently a gearbox (except in the
case of some wind energy plant manufacturers such as
Enercon, Scanwind and Vensys). It is mounted in a
rotatable fashion on a tower whose foundation gives the
necessary stability. In addition, there are the
monitoring and control systems and the power grid
connections in the machine pod and in the base of the
tower or outside it.
Wind energy plants having a horizontal axis of rotation
have now become established for power generation. Wind
energy plants having a horizontal rotor axis have to be
pointed in the direction of the wind. The pod is mounted
on the tower so as to be able to be rotated in a
horizontal plane by means of an azimuthal bearing. The
wind direction is in the case of large units determined
via the wind direction indicator. The rotor is then
pointed into the wind by means of servomotors.
Asynchronous or synchronous AC generators are used for
electromechanical energy conversion. The rotational speed
of the generator can be constant, have two settings (low
and high wind velocity) or be able to be adjusted
steplessly. Different variants of asynchronous generators
and directly coupled, multipolar synchronous generators
have become established in industry.
The generator and any gearbox are optimized in terms of
life, weight, size, maintenance requirement and costs. A
further parameter is the number of pairs of poles of the
generator, which fixes the transmission ratio of any
gearbox.
The power of the rotational motion of the rotor of a wind
power plant is transmitted to the generator via the drive
CA 02638146 2008-08-01
- 4 - 200700553
shaft, the gearbox and the offtake shaft.
The most practical solution for transmitting the rotor
frequency to an AC generator is the installation of a
gearbox, which is customary in precisely the opposite
direction in many industrial machines and in conjunction
with automobile engines. With the aid of a gearbox, the
power at a low rotational speed and a high torque, as is
obtained from the rotor of a wind power plant, is
converted into power at a high rotational speed and a low
torque, as is required for the generator.
There are multistage gearboxes and wind power plants
without gearboxes; most gearboxes are three-stage. The
first stage is optimum for low wind velocities (the rotor
can turn more easily in the first stage). The
intermediate and high-speed stages (second and third
stages) are particularly well suited for strong wind
(similar to the case of bicycle gears).
Since the noise level from wind energy plants represents
a problem, especially in more densely populated inland
regions, the noise level can in some plants be
individually matched to the specific site requirements by
programming of the plant control.
The gearbox is usually mounted on the pod by means of
rubber elements.
Movable components in a wind power plant which have to be
lubricated comprise, in particular, the following seven
important lubrication points:
- the main or tracking gearbox, which achieves gearing
up of the rotational speed of the rotor to the
CA 02638146 2008-08-01
- 5 - 200700553
rotational speed of the generator,
- the azimuthal gearbox, which is responsible for
tracking the wind direction,
- the pitch gearbox, which performs the adjustment of
the rotor blades,
- the main bearing, which essentially ensures the
mounting of the rotor,
- the pitch bearing, which acts as mounting for the
rotor blades,
- the azimuthal bearing, which provides the rotatable
connection of the wind power plant between tower and
pod and
- the generator bearing, which acts as mounting for
the generator shaft.
During each movement, there is relative motion between
components and therefore friction between the surfaces.
It is therefore necessary to apply a lubricant between
the moving components. Mineral oils, poly-alpha-olefins
(PAOs), natural oils (e.g. rapeseed oils), synthetic
ester oils or low-viscosity polyglycols are usually used
for lubrication.
When choosing the lubricants for wind power plants, it is
generally necessary to ensure that the material has very
low coefficients of friction, a satisfactory cold flow
capability (down to -60 C), good thermal stability (at
least to 200 C), good compatibility with surface coatings
and seals, low toxicity and ecotoxicity, high shear
stability, high viscosity indices, low corrosivity, good
compatibility with additives and very good wear
protection. The gearbox oil is subjected to particularly
demanding requirements since it plays a critical role in
determining the life of the gearbox. Grey specks and
consequent pitting and also roller bearing damage are
CA 02638146 2008-08-01
- 6 - 200700553
frequently occurring problems which increase the
frequency of damage. In the offshore area in particular,
maintenance and repair work is associated with very high
costs. Gearbox oils have to be particularly clean and
therefore have to be continually cleaned by means of fine
filtration.
In the gearboxes in particular, the very high pressure
stresses at low relative speeds result in boundary
friction or extreme mixed friction which places very
specific demands on the lubricant.
The lubricants known from the prior art have various
disadvantages. Natural oils have considerable
deficiencies in terms of low-temperature behaviour,
ageing behaviour and in the heat resistance and water
resistance. Mineral oils display low viscosity indices,
high vaporization losses, limited suitability at low
temperatures and moderate thermal stability. Polyglycols
display low thermal stability, a high vaporization loss,
low viscosities and poor compatibility with seals. Ester
oils are not stable to hydrolysis and display low shear
stability.
It was therefore an object of the present invention to
provide an alternative lubricant for the lubrication of
movable parts in wind power plants, in particular for
gearbox lubrication, which does not have one or more of
the abovementioned disadvantages of the lubricants known
from the prior art. The alternative lubricant should
preferably allow simple handling and display an improved
property profile, so that the life of the movable parts
can be increased, the intervals between maintenance and
repairs can be lengthened and/or the efficiency of the
wind power plant can be increased.
CA 02638146 2008-08-01
- 7 - 200700553
It has surprisingly been found that this object can be
achieved by the use of at least one ionic liquid or a
mixture of ionic liquids according to Claim 1.
The present invention therefore provides for the use of a
lubricant in components of a wind power plant or wind
energy plant, which is characterized in that the
lubricant comprises, in particular as lubricating
material, at least one ionic liquid.
The present invention likewise provides wind power plants
comprising at least one component having movable elements
and a lubricant, characterized in that the lubricant
contains an ionic liquid.
The use according to the invention of at least one ionic
liquid as lubricating material provides a novel lubricant
for wind power plants, in particular for their gearboxes,
which has an excellent property profile.
The use of ionic liquids in or as lubricants also has the
advantage that the property profile can be modified over
a wide range by selection of appropriate ionic liquids.
An appropriate choice of the ionic liquid(s) used enables
the property profile to be modified in terms of
viscosity, density, thermal stability, anticorrosion
properties, oxidation resistance, materials
compatibility, wear resistance, low-temperature
suitability, V-T behaviour, miscibilities, hydrolysis
stabilities, toxicity and ecotoxicity. In this way, a
tailored lubricant which fully meets requirements in
respect of the abovementioned properties can be provided
for each location/climatic zone. Thus, use of a lubricant
comprising an ionic liquid or a mixture of ionic liquids
CA 02638146 2008-08-01
- 8 - 200700553
in wind power stations, in particular in their gearboxes,
enables an increase in the life of the components and a
lengthening of the maintenance intervals to be achieved.
The lubricants according to the invention have, in
particular, the advantage that they do not lose their
lubrication properties at very low temperatures. Thus,
the lubricants according to the invention can, in
particular, be used advantageously in climatic zones in
which temperatures below 0 C, in particular below -40 C,
occur.
For the purposes of the present invention, ionic liquids
are salts which melt at low temperatures (_ 100 C) and
represent a novel class of liquids which are made up
exclusively of ions. In contrast to classical salt melts,
which are high-melting, highly viscous and very corrosive
media, ionic liquids are liquid even at low temperatures
(< 100 C) (K.R. Seddon J. Chem. Technol. Biotechnol.
1997, 68, 351-356).
The use according to the invention is described below by
way of example, without the invention being restricted to
these illustrative embodiments. When ranges, general
formulae or classes of compounds are indicated below,
these are intended to encompass not only the specific
ranges or groups of compounds which are explicitly
mentioned, but also all subranges and subgroups of
compounds which can be obtained by leaving out individual
values (ranges) or compounds. If documents are cited in
the present description, their contents are fully
incorporated by reference into the disclosure content of
the present invention.
The use according to the invention of a lubricant in
CA 02638146 2008-08-01
- 9 - 200700553
components of a wind power station is characterized in
that the lubricant comprises at least one ionic liquid.
The lubricant can comprise not only one ionic liquid but
also a plurality of ionic liquids. Suitable choice of
ionic liquids enables the properties of the lubricant to
be set.
According to the invention, the lubricant is preferably
used in components such as bearings or gearboxes of wind
power plants. When used in these components in
pa'rticular, the lubricant comprising ionic liquids can
achieve a great improvement and simplification. The
component of the wind power plant in which the lubricant
according to the invention is used can preferably be, for
example, a main or tracking gearbox for gearing up the
rotational speed of the rotor to the rotational speed of
the generator, an azimuthal gearbox for tracking the wind
direction, a pitch gearbox for adjusting the rotor
blades, a generator bearing for mounting the generator
shaft, a pitch bearing for mounting the rotor blades, a
main bearing for mounting the rotor or an azimuthal
bearing for providing the rotatable connection between
tower and pod of the wind power plant.
It can be advantageous for the lubricant used to contain
exclusively one or more ionic liquids as lubricating
material.
However, it is likewise possible for the lubricant used
to contain one or more further materials in addition to
the ionic liquid(s). Such materials can be, for example,
extreme pressure additives (EP additives; e.g. tricresyl
phosphate, zinc dialkyldithiophosphate - Additin RC 3048
from Rheinchemie) to optimize the friction- and wear-
producing properties or corrosion inhibitors, e.g. fatty
CA 02638146 2008-08-01
- 10 - 200700553
acid diethanolamide - REWOCOROS AC 28, fatty acid
monoethanolamide - REWOCOROS AC 101 (both products of
Evonik Goldschmidt GmbH). In addition to the ionic
liquids and any further materials present, the lubricant
used according to the invention can contain one or more
further lubricating materials. Such lubricating materials
can be, for example, mineral oils, poly-a-olefins (PAO),
synthetic esters or polyglycols, with the abovementioned
groups representing a selection and not a restriction.
The addition of further materials and/or lubricating
materials enables the property profiles of the lubricants
to be adjusted very finely to match them to requirements.
The lubricant used according to the invention preferably
has a pour point of from 0 C to -80 C, more preferably
from -25 C to -80 C and particularly preferably from
-40 C to -75 C.
The proportion of ionic liquids in the lubricant used
according to the invention is preferably from 0.1 to
99.98% by weight, more preferably from 75 to 99.95% by
weight and particularly preferably from 85 to 99.9% by
weight.
The lubricant can contain all known ionic liquids as
ionic liquids. The lubricant preferably contains ionic
liquids which give the lubricant a viscosity at operating
temperature of from 10 to 5000 mPas, preferably from 50
to 1000 mPas and particularly preferably from 100 to
500 mPas. Overviews of ionic liquids, their preparation
and their properties may be found, for example, in "Ionic
Liquids in Synthesis", P. Wasserscheid, T. Welton (eds.),
Wiley, in "Green Industrial Applications of Ionic Li-
quids", NATO Science Series. Li. Mathematics, Physics and
Chemistry, 92, or in "Ionic Liquids: Industrial
CA 02638146 2008-08-01
- 11 - 200700553
Applications for Green Chemistry", Robin D. Rogers (ed.),
Acs. Symposium Series, 818.
Preference is given to at least one salt of the formula
(I)
[A]n+ [Y]n (I)
where
n is 1, 2, 3 or 4,
[A]+ is a quaternary ammonium cation, an oxonium cation,
a sulphonium cation or a phosphonium cation (where
these cations can in each case be substituted or
unsubstituted) and
[Y]n' is a monovalent, divalent, trivalent or tetravalent
anion, or a
mixed salt having one of the general formulae (IIa) to
(IIc)
[Al]+[A2]+ [y]2- (IIa),
[Ai]+[A2]+[A3]+ [Y]s- (IIb) or
[Al] + [A2] + [A3] + [A9] + [Y] 4- ( IIc) ,
where
[Al]+, [A2]+ [A3]+ and [A4]+ are selected independently
from the groups mentioned for [A]+ and
[Y]2- to [Y]4- have the meanings given for [Y]n- in formula
(I), or
a mixed salt having one of the general formulae (IIIa) to
(IIIj)
[Al]+[A2]+[A3]+[M1]+ [Y] 4- (IIIa),
[Al]+[A2]+[Ml]+[M2]+ [Y]9- (IIIb),
CA 02638146 2008-08-01
- 12 - 200700553
[Al]+[M']+[M2]+[M3]+ [Y]9 (IIIc),
[Ai]+[A2]+[Mi]+ [y]3- (IIId),
[Ail+[Mi]+[M2]+ [Y]3- (IIIe),
[Al]+[Ml]+ [Y]2- (IIIf) ,
[A1]+[A2]+[M4]2+ [Y]4- (IIIg)
[A1]+[M1]+[Ma]2+ [y]9- (IIIh),
[A1]+[M5]3+ [Y]9- (IIIi) or
[Ai]+[M9]2+ [Y]3- (IIIj),
where
[Al] +, [A2] + and [A3] + are selected independently from the
groups mentioned for [A]+,
[y]2- to [y]4- have the meanings given for [Y] n- in formula
I, and
[Ml ] +, [M2 ] +, [M3 ] + are monovalent metal cations,
[M4] 2+ is a divalent metal cation and
[M513+ is a trivalent metal cation,
or a mixture of a plurality of salts of the formulae I to
IIIj
being present as ionic liquid in the lubricants according
to the invention.
Preferred ionic liquids have substituted or
unsubstituted, preferably substituted, ammonium,
phosphonium, pyridinium or imidazolium cations as
cations.
CA 02638146 2008-08-01
- 13 - 200700553
The ionic liquids which are preferably used according to
the invention preferably comprise at least one cation of
the general formulae:
R1RZR3R9N+ (IV)
R1R2N+=CR3R9 (V)
R1R2R3R4P+ (VI)
R1R2P+=CR3R4 (VII)
R1R2R3S+ ( VI I I )
where
Rl, R2, R3, R4 are identical or different and are each
hydrogen, a linear or branched aliphatic hydrocarbon
radical which has from 1 to 30 carbon atoms and may
contain a double bond, a cycloaliphatic hydrocarbon
radical which has from 5 to 40 carbon atoms and may
contain a double bond, an aromatic hydrocarbon
radical having from 6 to 40 carbon atoms, an
alkylaryl radical having from 7 to 40 carbon atoms, a
linear or branched aliphatic hydrocarbon radical
which has from 2 to 30 carbon atoms and is
interrupted by one or more heteroatoms (oxygen, NH,
NR' where R' is a C1-C30-alkyl radical which may
contain double bonds, in particular -CH3) and may
contain double bonds, a linear or branched aliphatic
hydrocarbon radical which has from 2 to 30 carbon
atoms and is interrupted by one or more functions
selected from the group consisting of -0-C(O)-,
- (0) C-O-, -NH-C (0) -, - (0) C-NH, - (CH3) N-C (O) -,
- (O) C-N (CH3) -, -S (02) -0-, -O-S (02) -, -S (02) -NH-, -NH-
S (02) -, -S (02) -N (CH3) -, -N (CH3) -S (02) -, and may
contain double bonds, a terminally by OH, OR', NH2,
N (H) R' , N(R')2r
where
R' is a C1-C30-alkyl radical which may contain double
CA 02638146 2008-08-01
- 14 - 200700553
bonds, a functionalized linear or branched
aliphatic or cycloaliphatic hydrocarbon radical
which has from 1 to 30 carbon atoms and may
contain double bonds or a polyether which may
have a block or random structure and has the
formula - (RS-0) õ-R6,
where
R5 is a linear or branched hydrocarbon radical
containing from 2 to 4 carbon atoms,
n is from 1 to 100, preferably from 2 to 60, and
R6 is hydrogen, a linear or -branched aliphatic
hydrocarbon radical which has from 1 to 30 carbon
atoms that may contain double bonds, a
cycloaliphatic hydrocarbon radical which has from
5 to 40 carbon atoms and may contain double
bonds, an aromatic hydrocarbon radical having
from 6 to 40 carbon atoms, an alkylaryl radical
or arylalkyl radical having from 7 to 40 carbon
atoms or a-C(0)-R7 radical where
R7 is a linear or branched aliphatic hydrocarbon
radical which has from 1 to 30 carbon atoms and
may contain double bonds, a cycloaliphatic
hydrocarbon radical which has from 5 to 40 carbon
atoms and may contain double bonds, an aromatic
hydrocarbon radical having from 6 to 40 carbon
atoms, an alkylaryl radical or arylalkyl radical
having from 7 to 40 carbon atoms.
As cations, the ionic liquid can likewise contain cations
derived from saturated or unsaturated cyclic compounds or
aromatic compounds having in each case at least one
trivalent nitrogen atom in a 4- to 10-membered,
preferably 5- or 6-membered, heterocyclic ring which may
be substituted. Such cations can be described in
simplified form (i.e. without indication of precise
CA 02638146 2008-08-01
- 15 - 200700553
position and number of the double bonds in the molecule)
by the general formulae (IX), (X) and (XI) below, where
the heterocyclic rings may also contain a plurality of
heteroatoms.
RI 2 Rl R
~N R R~~ R \N=C
N=C X
R (IX) R (X) R (XI)
Here, R1 and R2 are as defined above,
R may be a hydrogen atom, a linear or branched
aliphatic hydrocarbon radical which has from 1 to
30 carbon atoms and may contain double bonds, a
cycloaliphatic hydrocarbon radical which has from 5
to 40 carbon atoms and may contain double bonds, an
aromatic hydrocarbon radical having from 6 to
40 carbon atoms or an alkylaryl radical or arylalkyl
radical having from 7 to 40 carbon atoms.
X may be an oxygen atom, a sulphur atom or a
substituted nitrogen atom (X = 0, S, NRl) .
Examples of cyclic nitrogen compounds of the above-
mentioned type are pyrrolidine, dihydropyrrole, pyrrole,
imidazoline, oxazoline, oxazole, thiazoline, thiazole,
isoxazole, isothiazole, indole, carbazole, piperidine,
pyridine, the isomeric picolines and lutidines, quinoline
and isoquinoline. The cyclic nitrogen compounds of the
general formulae (IX), (X) and (XI) can be unsubstituted
(R = H) or monosubstituted or polysubstituted by the
radical R, and in the case of multiple substitution by R,
the individual radicals R can be different.
As cations, the ionic liquid can also contain ions
derived from saturated acyclic, saturated or unsaturated
CA 02638146 2008-08-01
- 16 - 200700553
cyclic compounds or from aromatic compounds having in
each case more than one trivalent nitrogen atom in a 4-
to 10-membered, preferably 5- or 6-membered, heterocyclic
ring. These compounds can be substituted both on the
carbon atoms and on the nitrogen atoms. They can also be
fused with unsubstituted or substituted benzene rings
and/or cyclohexane rings to form polycyclic structures.
Examples of such compounds are pyrazole,
3,5-dimethylpyrazole, imidazole, benzimidazole, N-methyl-
imidazole, dihydropyrazole, pyrazolidine, pyridazine,
pyrimidine, pyrazine, 2,3-, 2,5- and 2,6-dimethyl-
pyrazine, cinnoline, phthalazine, quinazoline, phenazine
and piperazine. Cations derived from imidazole and its
alkyl and phenyl derivatives have been found to be
particularly useful as constituents of ionic liquids.
As cations, the ionic liquid can likewise contain cations
which contain two nitrogen atoms and have the general
formula (XII)
R9
~
Ra-N O N-Rlo
RI i ~
(XII)
where
R8, R9, Rlo, R11, R12 are identical or different and are
each hydrogen, a linear or branched aliphatic
hydrocarbon radical which has from 1 to 30 carbon
atoms and may contain double bonds, a cycloaliphatic
hydrocarbon radical which has from 5 to 40 carbon
atoms and may contain double bonds, an aromatic
hydrocarbon radical having from 6 to 40 carbon atoms,
an alkylaryl radical or arylalkyl radical having from
CA 02638146 2008-08-01
- 17 - 200700553
7 to 40 carbon atoms, a linear or branched aliphatic
hydrocarbon radical which has from 1 to 30 carbon
atoms and is interrupted by one or more heteroatoms
(oxygen, NH, NR' where R' is a C1-C30-alkyl radical
which may contain double bonds) and may contain
double bonds, a linear or branched aliphatic
hydrocarbon radical which has from 1 to 30 carbon
atoms and is interrupted by one or more functions
selected from the group consisting of -0-C(O)-,
- (0) C-O-, -NH-C (0) -, - (0) C-NH, - (CH3) N-C (O) -,
-(0)C-N(CH3)-, -S(02)-0-, -O-S(02)-, -S(02)-NH-,
-NH-S (02) -, -S (02) -N (CH3) -, -N (CH3) -S (OZ) -, and may
contain double bonds, a linear or branched aliphatic
or cycloaliphatic hydrocarbon radical which has from
1 to 30 carbon atoms and is functionalized terminally
by OH, OR' , NH2, N(H) R' , N(R' ) 2, where R' is a C1-C3o-
alkyl radical which may contain double bonds, and may
contain double bonds or a polyether which may have a
block or random structure and is made up of
- (R5-0) n-R6i
where
R5 is a hydrocarbon radical containing from 2 to
4 carbon atoms,
n is from 1 to 100 and
R6 is hydrogen, a linear or branched aliphatic
hydrocarbon radical which has from 1 to
carbon atoms and may contain double bonds, a
cycloaliphatic hydrocarbon radical which has
from 5 to 40 carbon atoms and may contain double
30 bonds, an aromatic hydrocarbon radical having
from 6 to 40 carbon atoms, an alkylaryl radical
having from 7 to 40 carbon atoms or a -C(O) -R'
radical where
R7 is a linear or branched aliphatic hydrocarbon
radical which has from 1 to 30 carbon atoms and
CA 02638146 2008-08-01
- 18 - 200700553
may contain double bonds, a cycloaliphatic
hydrocarbon radical which has from 5 to
40 carbon atoms and may contain double bonds, an
aromatic hydrocarbon radical having from 6 to
40 carbon atoms, an alkylaryl radical having
from 7 to 40 carbon atoms.
As cations of the formula (XII), the ionic liquids
particularly preferably contain imidazolium ions selected
from among 1-methylimidazolium, 1-ethylimidazolium,
1-(1-butyl)imidazolium, 1-(1-octyl)imidazolium,
1-(1-dodecyl)imidazolium, 1-(1-tetradecyl)imidazolium,
1-(1-hexadecyl)imidazolium, 1,3-dimethylimidazolium,
1-ethyl-3-methylimidazolium, 1-(1-butyl)-3-methyl-
imidazolium, 1-(1-butyl)-3-ethylimidazolium, 1-(1-hexyl)-
3-methylimidazolium, 1-(1-hexyl)-3-ethylimidazolium,
1-(1-hexyl)-3-butylimidazolium, 1-(1-octyl)-3-methyl-
imidazolium, 1-(1-octyl)-3-ethylimidazolium, 1-(1-octyl)-
3-butylimidazolium, 1-(1-dodecyl)-3-methylimidazolium,
1-(l-dodecyl)-3-ethylimidazolium, 1-(1-dodecyl)-3-butyl-
imidazolium, 1-(1-dodecyl)-3-octylimidazolium, 1-(1-tetra-
decyl)-3-methylimidazolium, 1-(1-tetradecyl)-3-ethyl-
imidazolium, 1-(1-tetradecyl)-3-butylimidazolium,
1-(1-tetradecyl)-3-octylimidazolium, 1-(1-hexadecyl)-3-
methylimidazolium, 1-(1-hexadecyl)-3-ethylimidazolium,
1-(1-hexadecyl)-3-butylimidazolium, 1-(1-hexadecyl)-
3-octylimidazolium, 1,2-dimethylimidazolium,
1,2,3-trimethylimidazolium, 1-ethyl-2,3-dimethyl-
imidazolium, 1-(1-butyl)-2,3-dimethylimidazolium,
1-(1-hexyl)-2,3-dimethylimidazolium, 1-(1-octyl)-2,3-
dimethylimidazolium, 1,4-dimethylimidazolium,
1,3,4-trimethylimidazolium, 1,4-dimethyl-3-ethyl-
imidazolium, 3-butylimidazolium, 1,4-dimethyl-3-octyl-
imidazolium, 1,4,5-trimethylimidazolium, 1,3,4,5-tetra-
methylimidazolium, 1,4,5-trimethyl-3-ethylimidazolium,
CA 02638146 2008-08-01
- 19 - 200700553
1,4,5-trimethyl-3-butylimidazolium and 1,4,5-trimethyl-3-
octylimidazolium.
As cations, the ionic liquid can likewise contain ions
which, in particular, are made up of the abovementioned
cations as a result of dimerization, trimerization or
polymerization to form dications, trications or
polycations. These include, in particular, dications,
trications and polycations which have a polymeric
backbone, for example one based on siloxanes, polyethers,
polyesters, polyamides or polyacrylates, in particular
branched and hyperbranched polymers.
In a preferred embodiment of the present invention, the
lubricant contains ionic liquids in which the cation [A]+
is a pyridinium ion (XIIIa)
R3
R4 R2
R5 i R,
R (XIIIa)
where
R is as defined above for formula IX, one of the
radicals
R1 to R5 is methyl, ethyl or chlorine and the remaining
radicals R1 to R5 are hydrogen;
R3 is dimethylamino and the remaining radicals Rl, R2, R4
and R5 are hydrogen;
all radicals R1 to R5 are hydrogen;
R2 is carboxy or carboxamide and the remaining radicals
Rl, R2, R4 and R5 are hydrogen; or
Rl and R2 or R2 and R3 are 1, 4-buta-1, 3-dienylene and the
remaining radicals R1r R2, R4 and R5 are hydrogen. The
CA 02638146 2008-08-01
- 20 - 200700553
cation [A]+ is preferably a pyridinium ion (XIIIa) in
which
R1 to R5 are hydrogen; or one of the radicals
R1 to R5 is methyl or ethyl and the remaining radicals R1
to R5 are hydrogen.
The ionic liquid very particularly preferably has a
pyridinium ion (XIIIa) selected from among
1-methylpyridinium, 1-ethylpyridinium,
1-(1-butyl)pyridinium, 1-(1-hexyl)pyridinium,
1-(1-octyl)pyridinium, 1-(l-hexyl)pyridinium,
1-(1-octyl)pyridinium, 1-(1-dodecyl)pyridinium,
1-(1-tetradecyl)pyridinium, 1-(l-hexadecyl)pyridinium,
1,2-dimethylpyridinium, 1-ethyl-2-methylpyridinium,
1-(l-butyl)-2-methylpyridinium, 1-(1-hexyl)-2-methyl-
pyridinium, 1-(l-octyl)-2-methylpyridinium, 1-(1-dodecyl)-
2-methylpyridinium, 1-(1-tetradecyl)-2-methylpyridinium,
1-(1-hexadecyl)-2-methylpyridinium, 1-methyl-2-ethyl-
pyridinium, 1,2-diethylpyridinium, 1-(1-butyl)-2-ethyl-
pyridinium, 1-(1-hexyl)-2-ethylpyridinium, 1-(1-octyl)-2-
ethylpyridinium, 1-(l-dodecyl)-2-ethylpyridinium,
1-(1-tetradecyl)-2-ethylpyridinium, 1-(l-hexadecyl)-
2-ethylpyridinium, 1,2-dimethyl-5-ethylpyridinium,
1,5-diethyl-2-methylpyridinium, 1-(1-butyl)-2-methyl-
3-ethylpyridinium, 1-(1-hexyl)-2-methyl-3-ethylpyridinium
and 1-(1-octyl)-2-methyl-3-ethylpyridinium,
1-(1-dodecyl)-2-methyl-3-ethylpyridinium, 1-(1-tetra-
decyl)-2-methyl-3-ethylpyridinium and 1-(1-hexadecyl)-2-
methyl-3-ethylpyridinium as cation.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a pyridazinium ion (XIIIb)
CA 02638146 2008-08-01
- 21 - 200700553
R2
R3 R,
O
/ N
R4 I
R (XIIIb)
where
R is as defined above for formula IX,
R1 to R4 are each hydrogen, or one of the radicals R1 to
R4 is methyl or ethyl and the remaining radicals R1
to R4 are hydrogen.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a pyrimidinium ion (XIIIc)
R2
R3 R
N
R4 N R, (XIIIc)
where
R is as defined above for formula IX,
R1 is hydrogen, methyl or ethyl and
R2 to R4 are each, independently of one another, hydrogen
or methyl or
R1 is hydrogen, methyl or ethyl, R2 and R4 are each
methyl and R3 is hydrogen.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
CA 02638146 2008-08-01
- 22 - 200700553
the cation [A]+ is a pyrazinium ion (XIIId)
R
I
R3 N R2
R4 N R, (XIIId)
where
R is as defined above for formula IX,
R1 is hydrogen, methyl or ethyl and
R2 to R4 are each, independently of one another, hydrogen
or methyl,
R1 is hydrogen, methyl or ethyl, R2 and R4 are each
methyl and R3 is hydrogen,
Rl to R4 are each methyl or
R1 to R4 are each methyl or hydrogen.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a pyrazolium ion (XIIIf), (XIIIg) or
(XIIIg')
R
Ri I
I
R2 N /R R4 +O~ R4 N N \ R, R,
Ra R4 R3 R2 R3 R2
(XIIIf) (XIIIg) (XIIIg')
where
R is as defined above for formula IX,
R1 is hydrogen, methyl or ethyl and
CA 02638146 2008-08-01
- 23 - 200700553
R2 to R4 are each, independently of one another, hydrogen
or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a pyrazolium ion (XIIIh)
R
I
R2 /0\
N
R3
R, R4 (XIIIh)
where
R is as defined above for formula IX
R1 to R4 are each, independently of one another, hydrogen
or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a 1-pyrazolinium ion (XIIIi)
R
I
Rs NQ+~
N
R5
R R,
4
R3 R2 (XIIIi)
where
R is as defined above for formula IX,
R1 to R6 are each, independently of one another, hydrogen
or methyl.
. CA 02638146 2008-08-01
- 24 - 200700553
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a 2-pyrazolinium ion (XIIIj)
R~
I '., R
R6
N
R5
R4
R3 R2 (XIIIj )
where
R is as defined above for formula IX,
R1 is hydrogen, methyl, ethyl or phenyl and
R2 to R6 are each, independently one another, hydrogen or
methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a 3-pyrazolinium ion (XIIIk) or
(XIIIk' )
R, R,
1~R
R6 QN ~ R2 R6 N\Q R2
R
\ \ `
R3 R3
R5 R4 R5 R4
(XIIIk) (XIIIk' )
where
R is as defined above for formula IX,
R1 and R2 are each, independently of one another,
hydrogen, methyl, ethyl or phenyl and
. CA 02638146 2008-08-01
- 25 - 200700553
R3 to R6 are each, independently of one another, hydrogen
or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is an imidazolinium ion (XIIIl)
R6 R5
'*"~ H- -**' R
N o~
R1 R2
R4 a (XIIIl)
where
R is as defined above for formula IX,
R1 and R2 are each, independently of one another,
hydrogen, methyl, ethyl, 1-butyl or phenyl, R3 and R4
are each, independently of one another, hydrogen,
methyl or ethyl and
R5 and R6 are each, independently of one another,
hydrogen or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is an imidazolinium ion (XIIIm) or
(XIIIm')
R5 R4 R5 R4
Rs Rs R6 Rs
R, /N / O\R Ri ~E)/ N
R2 R2
(XIIIm) (XIIIm')
CA 02638146 2008-08-01
- 26 - 200700553
where
R is as defined above for formula IX,
R1 and R2 are each, independently of one another,
hydrogen, methyl or ethyl and
R3 to R6 are each, independently of one another, hydrogen
or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is an imidazolinium ion (XIIIn) or
(XIIIn')
R5 R4 ,R R4
~ 6 H
~N NQCH3 O N
R~ R~
H3C CH3 R2 R3
(XIIIn) (XIIIn')
where
R is as defined above for formula IX,
R1 to R3 are each, independently of one another,
hydrogen, methyl or ethyl and
R4 to R6 are each, independently of one another, hydrogen
or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a thiazolium ion (XIIIo) or (XIIIo')
or an oxazolium ion (XIIIp)
CA 02638146 2008-08-01
- 27 - 200700553
R2 /R R2
N N R2 R
R3 S R, R3 S R, OO
I Rs 0 R,
(XIIIo) (XIIIo') (XIIIp)
where
R is as defined above for formula IX,
R1 is hydrogen, methyl, ethyl or phenyl and
R2 and R3 are each independently of one another, hydrogen
or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a 1,2,4-triazolium ion (XIIIq),
(XIIIq') or (XIIIq " )
R R3
R3 R R ;
N N
N N N N
O @ Ri R2 R, N Rz R~ N O R2
I
R
(XIIIq) (XIIIq' ) (XIIIq" )
where
R is as defined above for formula IX,
R1 and R2 are each, independently of one another,
hydrogen, methyl, ethyl or phenyl and
R3 is hydrogen, methyl or phenyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
CA 02638146 2008-08-01
- 28 - 200700553
the cation [A]+ is a 1,2,3-triazolium ion (XIIIr),
(XIIIr' ) or (XIIIr")
R1~ /
N N N N /
N
T N
N N R3 R
R3 p R3
R2 R2 R2
(XIIIr) (XIIIr') (XIIIr " )
where
R is as defined above for formula IX,
R1 is hydrogen, methyl or ethyl and
R2 and R3 are each, independently of one another,
hydrogen or methyl or R2 and R3 together are 1,4-
butyl-1,3-dienylene.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a pyrrolidinium ion (XIIIs)
Rt R5
RRa
Rg Rs
R / \ R2
9
R, R (XIIIs)
where
R is as defined above for formula IX,
R1 is hydrogen, methyl, ethyl or phenyl and
R2 to R9 are each, independently of one another, hydrogen
or methyl.
CA 02638146 2008-08-01
- 29 - 200700553
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is an imidazolidinium ion (XIIIt)
R5
R6 N~~
R7 R3
O
R8 / \ R2
R, R (XIIIt)
where
R is as defined above for formula IX,
R1 and R4 are each, independently of one another,
hydrogen, methyl, ethyl or phenyl and
R2 and R3 and also R5 to R8 are each, independently of one
another, hydrogen or methyl.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is an arnmonium ion (IV)
R1
OI
R (N R2
R3 (IV)
where
R is as defined above for formula IX,
Rl to R3 are each, independently of one another, C1-C18-
alkyl or
R1 to R3 are each, independently of one another, hydrogen
or C1-C18-alkyl and
R4 is 2-hydroxyethyl, or
R1 and R2 together are 1,5-pentylene or 3-oxa-1,5-
CA 02638146 2008-08-01
- 30 - 200700553
pentylene and
R3 is C1-C18-alkyl, 2-hydroxyethyl or 2-cyanoethyl. As
particularly preferred ammonium ions (IV), mention
may be made, in particular, of methyltri-
(1-butyl)ammonium, 2-hydroxyethylammonium, bis-
(2-hydroxyethyl)dimethylammonium, N,N-dimethyl-
piperidinium and N,N-dimethylmorpholinium.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+"is a guanidinium ion (IVv),
RNR
,.
. ,
R2\ R5
N N~
I I
R3 R4 (IVv)
where
R is as defined above for formula IX,
R1 to R5 are each methyl,
Rl to R5 are each, independently of one another, C1-C1S-
alkyl or
R1 to R5 are each, independently of one another,
hydrogen or C1-C1B-alkyl or 2-hydroxyethyl.
A very particularly preferred guanidinium ion (IVv) is
N,N,N',N',N",N"-hexamethylguanidinium.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a derivative of an ethanolamine, e.g.
a cholinium ion (XIIIw) or of a diethanolamine (XIIIw')
or of a triethanolamine (XIIIw " )
CA 02638146 2008-08-01
- 31 - 200700553
Ri OR4 OR4
OI 0) O C~~
R- i OR3 R-N OR3 R-N OR4
R2 I Z OR5
(XIIIw) (XIIIw') (XIIIw " )
where
R is as defined above for formula IX,
R1 and R2 are each, independently of one another,
methyl, ethyl, 1-butyl or 1-octyl and
R3 is hydrogen, methyl, ethyl, acetyl, -S020H or
-PO (OH) 2, or
R1 is methyl, ethyl, 1-butyl or 1-octyl, R2 is a
-CH2-CH2-OR9 group and
R3 and R4 are each, independently of one another,
hydrogen, methyl, ethyl, acetyl, -S020H or -PO(OH)2,
or
Rl is a -CH2-CH2-OR4 group,
R2 is a-CH2-CH2-OR5 group and
R3 to R5 are each, independently of one another,
hydrogen, methyl, ethyl, acetyl, -S020H or -PO(OH)2,
or
R1 is methyl, ethyl, 1-butyl, 1-octyl, acetyl, -SO2OH,
or -PO(OH)2 and
R3 to R5 are each, independently of one another,
hydrogen, methyl, ethyl, acetyl, -S020H, -PO(OH)2, or
-(CõH2nO) mRl where n = 1 to 5 and m = 1 to 100.
In a further preferred embodiment of the present
invention, the lubricant contains ionic liquids in which
the cation [A]+ is a phosphonium ion (VI) in which
Rl to R3 are each, independently of one another, C1-C18-
alkyl, in particular butyl, isobutyl, 1-hexyl or 1-octyl.
CA 02638146 2008-08-01
- 32 - 200700553
Among the abovementioned cations, the pyridinium ions
(XIIIa), imidazolium ions (XII) and ammonium ions (IV)
are particularly preferred as cations. The lubricants
according to the invention very particularly preferably
contain ionic liquids which have one or more cations
selected from among 1-methylpyridinium, 1-ethyl-
pyridinium, 1-(1-butyl)pyridinium, 1-(l-hexyl)pyridinium,
1-(1-octyl)pyridinium, 1-(1-hexyl)pyridinium, 1-(1-
octyl)pyridinium, 1-(1-dodecyl)pyridinium, 1-(1-
tetradecyl)pyridinium, 1-(l-hexadecyl)pyridinium, 1,2-
dimethylpyridinium, 1-ethyl=2-methylpyridinium, 1-(1-
butyl)-2-methylpyridinium, 1-(1-hexyl)-2-methyl-
pyridinium, 1-(1-octyl)-2-methylpyridinium, 1-(1-
dodecyl)-2-methylpyridinium, 1-(1-tetradecyl)-2-
methylpyridinium, 1-(1-hexadecyl)-2-methylpyridinium,
1-methyl-2-ethylpyridinium, 1,2-diethylpyridinium, 1-(1-
butyl)-2-ethylpyridinium, 1-(1-hexyl)-2-ethylpyridinium,
1-(1-octyl)-2-ethylpyridinium, 1-(1-dodecyl)-2-ethyl-
pyridinium, 1-(l-tetradecyl)-2-ethylpyridinium, 1-(1-
hexadecyl)-2-ethylpyridinium, 1,2-dimethyl-5-ethyl-
pyridinium, 1, 5-diethyl-2-methylpyridinium, 1- (1-butyl) -
2-methyl-3-ethylpyridinium, 1-(1-hexyl)-2-methyl-3-ethyl-
pyridinium, 1-(1-octyl)-2-methyl-3-ethylpyridinium, 1-(1-
dodecyl)-2-methyl-3-ethylpyridinium, 1-(1-tetradecyl)-2-
methyl-3-ethylpyridinium, 1-(1-hexadecyl)-2-methyl-3-
ethylpyridinium, 1-methylimidazolium, 1-ethylimidazolium,
1-(1-butyl)imidazolium, 1-(1-octyl)imidazolium, 1-(1-
dodecyl)imidazolium, 1-(1-tetradecyl)imidazolium, 1-(1-
hexadecyl) imidazolium, 1, 3-dimethylimidazolium, 1-ethyl-
3-methylimidazolium, 1-(1-butyl)-3-methylimidazolium,
1-(1-hexyl)-3-methylimidazolium, 1-(1-octyl)-3-methyl-
imidazolium, 1-(1-dodecyl)-3-methylimidazolium, 1-(1-
tetradecyl)-3-methylimidazolium, 1-(1-hexadecyl)-3-
methylimidazolium, 1,2-dimethylimidazolium, 1,2,3-
trimethylimidazolium, 1-ethyl-2,3-dimethylimidazolium,
CA 02638146 2008-08-01
.
- 33 - 200700553
1-(1-butyl)-2,3-dimethylimidazolium, 1-(l-hexyl)-2,3-
dimethylimidazolium, and 1-(1-octyl)-2,3-dimethyl-
imidazolium, 1,4-dimethylimidazolium, 1,3,4-trimethyl-
imidazolium, 1,4-dimethyl-3-ethylimidazolium,
3-butylimidazolium, 1,4-dimethyl-3-octylimidazolium,
1,4,5-trimethylimidazolium, 1,3,4,5-tetramethyl-
imidazolium, 1,4,5-trimethyl-3-ethylimidazolium, 1,4,5-
trimethyl-3-butylimidazolium, 1,4,5-trimethyl-3-octyl-
imidazolium and 2-hydroxyethylammonium as cations.
The metal cations [M1]+, [M2]+~ [M3*][M4]2+ and [M5] 3+
mentioned in the formulae (IIIa) to (IIIj) are preferably
metal cations of Groups 1, 2, 6, 7, 8, 9, 10, 11, 12 and
13 of the Periodic Table. Particularly preferred metal
cations are, for example, Li+, Na+, K+, Cs+, Mg2+, Ca2+,
Ba2+, Cr3+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Ag+, ZnZ+ and A13+.
The ionic liquids which are preferably used according to
the invention comprise at least one of the abovementioned
cations in combination with at least one anion in each
case. As anions, it is in principle possible to use all
anions which in combination with the cation lead to an
ionic liquid.
The anion [Y]n- of the ionic liquid can, for example, be
selected from:
the group of halides and halogen-containing compounds of
the formulae: F, Cl-, Br-, I-, BF4-, PF6-, AlCl4-, A12C17-,
A13Cllo_, AlBr4-, FeCl4 , BC14-, SbF6-, AsF6-, ZnC13-, SnCl3-,
CuC12-, CF3SO3-, (CF3SO3)2N-, CF3C02-, CC13CO2-, CN-, SCN-,
OCN-, N02 , N03-, N (CN) ;
the group of sulphates, sulphites and sulphonates of the
general formulae: S092 , HSO4-, S032", HS03 , RaOS03 , RaS03-;
the group of phosphates of the general formulae: P043-,
CA 02638146 2008-08-01
- 34 - 200700553
HP042-, H2P04`, RaP042-, HRaP04-, RaRbP04 ;
the group of the phosphonates and phosphinates of the
general formulae: RaHP03', RaRbP02 , RaRbP03-;
the group of phosphites of the general formulae: P033-,
HP032-, H2PO3-, RaP032-, RaHP03-, RaRbP03-;
the group of phosphonites and phosphinites of the general
f ormulae : RaRbP02 , RaHP02 , RaRbPO , RaHPO ;
the group of carboxylates of the general formula: RaCO0-;
the group of borates of the general formulae: B033-,
HB032-, H2B03 , RaRbBO3-, RaHB03-, RaB032-,
B (ORa) (ORt') (ORc) (ORd) -, B (HSOq) -, B (RaSO4) -;
the group of boronates of the general formulae RaB022-,
RaRbBO-;
the group of carbonates and carbonic esters of the
general formulae: HC03-, C032-, RaC03-;
the group of silicates and silicic esters of the general
formulae: Si04 4-, HSiO93-, H2Si092-, H3SiO4-, RaSi043-,
RaRbSi092-, RaRbRCSiO4 , HRaSi04 2 a a b
, H2R Si09 , HR R Si04-
the group of alkylsilane or arylsilane salts of the
general formulae: RaSi033 , RaRbSi022 , RaRbRcSiO-,
RaRbRcSi 03 , RaRbRcSi.02-, RaRbSi032-;
the group of carboximides, bis(sulphonyl)imides and
sulphonylimides of the general formulae:
O 11/o ,\ ~0
Ra 4 Ra S~ Re S/ Rb Rb - S Rb
O 11
0 O
the group of methides of the general formula:
CA 02638146 2008-08-01
- 35 - 200700553
1O2Ra
RaO2S/eS02R
the group of alkoxides and aryloxides of the general
formula: Ra0-;
the group of the halometalates of the general formula
[MrHalt) s-, where M is a metal and Hal is fluorine,
chlorine, bromine or iodine, r and t are positive
integers and indicate the stoichiometry of the complex
and s is a positive integer and indicates the charge on
the complex;
the group of sulphides, hydrogensulphides, polysulphides,
hydrogenpolysulphides and thiolates of the general
formulae: SZ-, HS-, [S,] 2-, [HSV] [RaS] -, where v is a
positive integer from 2 to 10;
the group of complex metal ions such as Fe(CN)63-,
Fe ( CN ) 64-, Mn04, Fe ( CO ) 4
where Ra, Rb, R and Rd can each be, independently of one
another:
hydrogen;
C1-C30-alkyl or an aryl-, heteroaryl-, cycloalkyl-,
halogen-, hydroxy-, amino-, carboxy-, formyl-, -0-, -CO-,
-CO-O- or -CO-N-substituted derivative thereof, for
example methyl, ethyl, 1-propyl, 2-propyl, 1-butyl,
2-butyl, 2-methyl-l-propyl (isobutyl), 2-methyl-2-propyl
(tert-butyl), 1-pentyl, 2-pentyl, 3-pentyl, 2-methyl-
1-butyl, 3-methyl-l-butyl, 2-methyl-2-butyl, 3-methyl-
2-butyl, 2,2-dimethyl-l-propyl, 1-hexyl, 2-hexyl,
3-hexyl, 2-methyl-l-pentyl, 3-methyl-l-pentyl, 4-methyl-
1-pentyl, 2-methyl-2-pentyl, 3-methyl-2-pentyl, 4-methyl-
2-pentyl, 2-methyl-3-pentyl, 3-methyl-3-pentyl, 2,2-
dimethyl-l-butyl, 2,3-dimethyl-l-butyl, 3,3-dimethyl-l-
butyl, 2-ethyl-l-butyl, 2,3-dimethyl-2-butyl, 3,3,-di-
CA 02638146 2008-08-01
- 36 - 200700553
methyl-2-butyl, heptyl, octyl, nonyl, decyl, undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl,
heptadecyl, octadecyl, nonadecyl, icosyl, henicosyl,
docosyl, tricosyl, tetracosyl, pentacosyl, hexacosyl,
heptacosyl, octacosyl, nonacosyl, triacontyl,
phenylmethyl (benzyl), diphenylmethyl, triphenylmethyl,
2-phenylethyl, 3-phenylpropyl, cyclopentylmethyl,
2-cyclopentylethyl, 3-cyclopentylpropyl, cyclohexyl-
methyl, 2-cyclohexylethyl, 3-cyclohexylpropyl, methoxy,
ethoxy, formyl, acetyl or CqF2(q-a)+(1-b)H2a+b where q < 30, 0
S a:~ q and b = 0 or 1( for example CF3r C2F5, CH2CH2-C (q-
2)F2(q-2)+1. C6F13, C8F17. C1oF21, C12F25) ;
C3-C12-cycloalkyl or an aryl-, heteroaryl-, cycloalkyl-,
halogen-, hydroxy-, amino-, carboxy-, formyl-, -0-, -CO-
or -CO-O-substituted derivative thereof, for example
cyclopentyl, 2-methyl-l-cyclopentyl, 3-methyl-
1-cyclopentyl, cyclohexyl, 2-methyl-l-cyclohexyl,
3-methyl-l-cyclohexyl, 4-methyl-l-cyclohexyl or CqF2(q-a)-
(1-b)H2a-b where q< 30, 0< a- q and b = 0 or 1;
C2-C30-alkenyl or an aryl-, heteroaryl-, cycloalkyl-,
halogen-, hydroxy-, amino-, carboxy-, formyl-, -0-, -CO-
or -CO-0-substituted derivative thereof, for example
2-propenyl, 3-butenyl, cis-2-butenyl, trans-2-butenyl or
CqF2 (q-a) -( i-b) H2a-b where q< 30, 0< a <- q and b = 0 or 1;
C3-C12-cycloalkenyl or an aryl-, heteroaryl-, cycloalkyl-,
halogen-, hydroxy-, amino-, carboxy-, formyl-, -0-, -CO-
or -CO-0-substituted derivative thereof, for example
3-cyclopentenyl, 3-cyclohexenyl, 3-cyclohexenyl,
2, 5-cyclohexadienyl or CqF2(q-a)-3(1-b)H2a-3b where q!~ 30, 0
a< q and b= 0 or 1;
aryl or heteroaryl having from 2 to 30 carbon atoms or an
alkyl-, aryl-, heteroaryl-, cycloalkyl-, halogen-,
hydroxy-, amino-, carboxy-, formyl-, -0-, -CO- or
-CO-O-substituted derivative thereof, for example phenyl,
2-methylphenyl (2-tolyl), 3-methylphenyl (3-tolyl),
. CA 02638146 2008-08-01
- 37 - 200700553
4-methylphenyl, 2-ethyiphenyl, 3-ethyiphenyl, 4-ethyl-
phenyl, 2,3-dimethylphenyl, 2,4-dimethylphenyl, 2,5-
dimethylphenyl, 2,6-dimethylphenyl, 3,4-dimethylphenyl,
3,5-dimethylphenyl, 4-phenylphenyl, 1-naphthyl,
2-naphthyl, 1-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, 2-
pyridinyl, 3-pyridinyl, 4-pyridinyl or C6F(5-a)Ha where 0
< a < 5; or
two radicals form an unsaturated, saturated or aromatic
ring which may be substituted by functional groups, aryl,
alkyl, aryloxy, alkyloxy, halogen, heteroatoms and/or
heterocycles and may be interrupted by one or more oxygen
and/or sulphur atoms and/or one or more substituted or
unsubstituted imino groups.
The ionic liquids used according to the invention
preferably have anions selected from among halides,
carboxylates, phosphates, thiocyanates, isothiocyanates,
dicyanamides, sulphates, alkylsulphates, sulphonates,
alkylsulphonates, tetrafluoroborate, hexafluorophosphate
and bis(trifluoromethylsulphonyl)imide.
Preferred anions are chloride, bromide, iodide,
thiocyanate, hexafluorophosphate, trifluoromethane-
sulphonate, methanesulphonate, formate, acetate,
glycolate, lactate, mandelate, nitrate, nitrite,
trifluoroacetate, sulphate, hydrogensulphate, methyl
sulphate, ethyl sulphate, 1-propyl sulphate, 1-butyl
sulphate, 1-hexyl sulphate, 1-octyl sulphate, phosphate,
dihydrogenphosphate, hydrogenphosphate, C1-C9-dialkyl-
phosphates, propionate, tetrachloroaluminate, A1ZC1,-,
chlorozincate, chloroferrate, bis(trifluoromethyl-
sulphonyl)imide, bis(pentafluoroethylsulphonyl)imide,
bis(methylsulphonyl)imide, bis(p-toluenesulphonyl)imide,
tris(trifluoromethylsulphonyl)methide, bis(pentafluoro-
ethylsulphonyl)methide, p-toluenesulphonate, tetra-
. CA 02638146 2008-08-01
- 38 - 200700553
carbonylcobaltate, dimethylene glycol monomethyl ether
sulphate, oleate, stearate, acrylate, methacrylate,
maleate, hydrogencitrate, vinylphosphonate, bis(penta-
fluoroethyl)phosphinate, borates such as bis-
[salicylato(2-)]borate, bis[oxalato(2-)]borate, bis[1,2-
benzoldiolato(2-)-0,O']borate, tetracyanoborate, tetra-
fluoroborate, dicyanamide, tris(pentafluoroethyl)-
trifluorophosphate, tris(heptafluoropropyl)trifluoro-
phosphate, cyclic arylphosphates such as catechol-
phosphate (C6H,02) P(O) 0- or chlorocobaltate.
Particularly preferred anions are anions from the group
consisting of halides, bis(perfluoroalkylsulphonyl)amides
and bis(perfluoroalkylsulphonyl)imides such as bis(tri-
fluoromethylylsulphonyl)imide, alkyltosylates and aryl-
tosylates, perfluoroalkyltosylates, nitrate, sulphate,
hydrogensulphate, alkylsulphates and arylsulphates,
polyether sulphates and suiphonates, perfluoro-
alkylsulphates, sulphonate, alkylsulphonates and
arylsulphonates, perfluorinated alkylsulphonates and
arylsulphonates, alkylcarboxylates and arylcarboxylates,
perfluoroalkylcarboxylates, perchlorate, tetrachloro-
aluminate, saccharinate, dicyanamide, thiocyanate,
isothiocyanate, tetraphenylborate,
tetrakis(pentafluorophenyl)borate, tetrafluoroborate,
hexafluorophosphate, polyether phosphates,
dialkylphosphates and phosphates.
Very particularly preferred anions are chloride, bromide,
hydrogensulphate, tetrachloroaluminate, thiocyanate,
methylsulphate, ethylsulphate, methanesulphonate,
forrnate, acetate, glycolate, lactate, dimethylphosphate,
diethylphosphate, p-toluenesulphonate, tetrafluoroborate
and hexafluorophosphate.
. CA 02638146 2008-08-01
- 39 - 200700553
The lubricant very particularly preferably contains ionic
liquids or mixtures thereof which contain a combination
of a 1,3-dialkylimidazolium, 1,2,3-trialkylimidazolium,
1,3-dialkylimidazolinium or 1,2,3-trialkylimidazolinium
cation with an anion selected from the group consisting
of halides, bis(trifluoromethylylsulphonyl)imide, per-
fluoroalkyltosylates, alkylsulphates and alkyl-
sulphonates, perfluorinated alkylsulphonates and
alkylsulphates, perfluoroalkylcarboxylates, perchiorate,
dicyanamide, thiocyanate, isothiocyanate,
tetraphenylborate, tetrakis(pentafluorophenyl)borate,
tetrafluoroborate, hexafluorophosphate, dimethylphosphate
and diethyiphosphate.
It is also possible to use commercially available,
acyclic quaternary ammonium salts such as TEGOO IL T16ES
[quaternary fatty amine ethoxylate], TEGOO IL K5MS
[coconut alkylpentaethoxymethylammonium ethosulphate],
TEGOO IL DS [distearyldimethylammonium chloride] or TEGO
IL 2MS [dimethyldiethanolammonium methylsulphonate] (all
products of Evonik Goldschmidt GmbH) and also cyclic
quaternary nitrogen compounds selected from the groups of
imidazolium salts, pyridinium salts, pyrrolidinium salts,
etc., e.g. TEGOO IL IM ES [1-ethyl-3-methylimidazolium
ethylsulphate] (product of Evonik Goldschmidt GmbH) as
ionic liquids in the lubricant according to the
invention.
Owing to the fact that some ionic liquids can be selected
according to their property profile so that they are
stable at high temperatures, noncombustible, corrosion-
inhibiting and in terms of viscosity, density, oxidation
stability, materials compatibility, wear protection,
suitability for use at low temperatures, V-T behaviour,
miscibility and hydrolysis resistance can be matched
- CA 02638146 2008-08-01
- 40 - 200700553
precisely to the respective specifications, these ionic
liquids can be used particularly advantageously as
lubricants in wind power plants, in particular in their
gearboxes.
It can be advantageous to use ionic liquids which are
biodegradable and/or nontoxic, e.g. imidazolium salts and
pyridinium salts, in particular 1-butyl-3-
methylimidazolium tetrafluoroborate, 1-ethyl-3-
methylimidazolium ethylsulphate. Apart from the
abovementioned advantages, it is precisely these two
additional properties which are important criteria in the
selection of lubricants for wide use in the industrial
environment, in particular use of the lubricants in wind
power plants which are operated in ecologically sensitive
places, e.g. in drinking water catchment areas, bodies of
water (e.g. offshore plants) and permafrost regions.
The lubricant used according to the invention preferably
contains ionic liquids which either on their own or when
mixed with others have a melting point of < 100 C,
preferably < 80 C, particularly preferably < 50 C and
very particularly preferably S room temperature.
The lubricant used according to the invention more
preferably contains ionic liquids which either on their
own or when mixed with others are liquid at a temperature
of from -85 C to 400 C, preferably from -70 C to 250 C,
particularly preferably from -60 C to 150 C and very
particularly preferably from -55 C to 100 C.
Ionic liquids which are liquid at low temperatures are,
for example, imidazolium salts or pyridinium sulphates,
in particular 1-ethyl-3-methylimidazolium ethylsulphate,
1-methyl-3-octylimidazolium tetrafluoroborate or 1-ethyl-
CA 02638146 2008-08-01
- 41 - 200700553
3-methylpyridinium ethylsulphate.
The lubricant used according to the invention preferably
contains ionic liquids which either on their own or when
mixed with others have a decomposition temperature of
> 150 C, preferably > 250 C, more preferably 200 C and
particularly preferably > 300 C.
The choice of the ionic liquid or the mixture of ionic
liquids depends on the property profile of the machinery
selected and the respective climatic and environmental
conditions (locations).
The present invention is illustrated by figures 1 to 3,
without the invention being restricted to the embodiment
presented there.
Fig. 1 shows the typical curve of the coefficient of
friction as a function of the rubbing speed, divided into
the regions of solids contact, boundary friction, mixed
friction and hydrodynamic friction. This depiction is
referred to as the Stribeck curve.
An ideal lubricant should display a very low level of
friction in all the regions mentioned, in particular in
the region of boundary friction. The Stribeck curve
ideally runs parallel to the x axis.
Fig. 2 shows the results of the comparative experiments
of the example comparing a commercially available
completely additivated poly-alpha-olefin, a commercially
available completely additivated mineral oil of group 3
(Castrol SLX 0W-30 from Castrol), TEGO IL IMES, TEGO IL
IMES containing an EP additive from Evonik Goldschmidt
GmbH (lubricant 4) and TEGO IL IMES containing a
= CA 02638146 2008-08-01
- 42 - 200700553
commercially available EP additive (lubricant 5). The
measured values for lubricant 1 (PAO) are shown as dots,
those for lubricant 2 (mineral oil) as diamonds, those
for lubricant 3 (ionic liquid 1-ethyl-3-methylimidazolium
ethylsulphate) as squares, those for lubricant 4 as
crosses and those for lubricant 5 as plus signs.
In the examples described below, the present invention is
described by way of example without the invention, whose
scope is determined by the total description and the
claims, being restricted to the embodiments mentioned in
the examples.
Examples:
The choice of ionic liquids generally depends on the
expected lubrication requirements, the load uptake (load
uptake capability) and the temperature prevailing in the
lubrication region. To reduce the energy consumption in
apparatuses/machines/gearboxes and thus increase the
efficiency, it is useful to work at low viscosities which
nevertheless do not run the risk of increasing the mixed
friction. If there is a risk of solids contact friction,
for instance at high loads and/or low speeds of the
moving surfaces, it may be necessary to achieve boundary
lubrication by means of friction-reducing additives (EP
additives). Apart from a high viscosity index (VI), which
describes the stability of the viscosity at relatively
high temperatures, it is therefore also necessary to
ensure boundary lubrication. A high VI is a guarantee
that a constant lubricating action is achieved with
increasing speed of the moving surfaces and an associated
temperature increase. This region of lubrication is
referred to as hydrodynamic lubrication. The film which
is then formed between the surfaces to be lubricated
CA 02638146 2008-08-01
- 43 - 200700553
displays the lowest friction which is then dependent
essentially only on the viscosity of the liquids.
The viscosity index is a dimensionless parameter and is
used to characterize the viscosity-temperature behaviour
(VT) of a liquid (mainly lubricating oils). A high
viscosity index means good VT behaviour, i.e. the
viscosity changes only slightly with temperature. Good
oils should have a VI of > 150. This can be set by means
of additives. In general, a very high VI at a relatively
low viscosity of the oil is sought. The lower the
viscosity of the oil, the lower the energy consumption,
since the oil has a lower frictional resistance. A good
mineral oil of group 3 has a VI of about 130, so that it
has to be increased by means of additives.
The following lubricants were examined in the examples:
lubricant 1: poly-alpha-olefin (PAO 8), lubricant 2: a
commercially available completely additivated mineral oil
having a KV 40 of 31 cSt (Castrol SLX OW-30 from
Castrol), lubricant 3: TEGO IL IMES [ionic liquid from
Evonik Goldschmidt GmbH having a KV 40 (kinematic
viscosity at 40 C) of 39 cSt], lubricant 4: TEGO IL IMES
containing 0.3% by mass of REWOCOROS EAK 8190 (Evonik
Goldschmidt GmbH), lubricant 5: TEGO IL IMES containing
0.3% by mass of the commercially available EP additive
tricresyl phosphate.
Example 1:
Determination of the viscosity index (VI)
The viscosity indices shown in Table 1 were calculated in
accordance with DIN ISO 2909 from the kinematic
viscosities at 40 C and 100 C.
= CA 02638146 2008-08-01
= - 44 - 200700553
Table l:
Viscosity indices (in accordance with DIN ISO 2909)
Lubricant Viscosity index
Lubricant 1 ca. 170
Lubricant 2 ca. 160
Lubricant 3 ca. 172
Lubricant 4 ca. 172
Lubricant 5 ca. 172
Example 2:
Determination of the frictional force as a function of
the frictional speed
The dependence of the frictional force (the coefficient
of friction) on the frictional speed was measured using a
"mini traction machine" (MTM2 from PCS Instruments) at
80 C and 30 N. Fig. 1 shows an idealized Stribeck curve.
In Fig. 2, the measurement points obtained in the studies
on the various lubricant compositions are shown in the
form of a Stribeck curve.
The Stribeck curves at 80 C show that the mixed friction
part, i.e. increasing coefficient of friction, commences
at speeds of < 250 mm/s in the case of lubricants 1 and 2
and lubricants 3 to 5. The coefficients of friction of
the lubricants 3 to 5 are significantly below those of
the conventional lubricants 1 and 2. Only in the boundary
region at speeds of < 20 mm/s does the coefficient of
friction of the ionic liquid without additives (lubricant
3) rise above that of the commercial lubricants 1 and 2.
Addition of cresyl phosphate as EP additive (lubricant 5)
or REWOCOROS EAK 8190 (lubricant 4) enables the boundary
friction to be reduced below the values for the
commercial lubricants.
= CA 02638146 2008-08-01
- 45 - 200700553
Over virtually the entire speed range, the lubricants
containing ionic liquids (lubricants 3 to 5) display a
significantly lower coefficient of friction than
commercially available lubricants. When additives are
added to the ionic liquids, the ionic liquids display
significantly lower coefficients of friction than
commercially available lubricants over the entire speed
range.
Example 3:
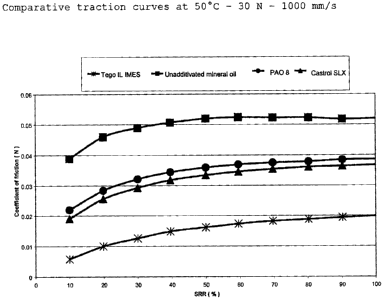
Determination of the traction curves
Traction curves can also be measured by means of the
MTM2. Here, the slide-to-roll ratio varies continuously
at a constant temperature and constant pressure. The
slide-to-roll ratio is the ratio of the sliding speed to
the rolling speed. At a slide-to-roll ratio of 0, the
plate and the ball move at the same surface speed (pure
rolling). At a slide-to-roll ratio of 2, one of the two
surfaces remains still (pure sliding). Since various
proportions of sliding can occur in the case of different
components, the traction curve makes it possible to show
the lubricant behaviour under these different conditions.
Conditions with a high proportion of sliding at the
toothed wheel contacts prevail in gearboxes in
particular. The measurements show that ionic liquids
display significantly lower coefficients of friction than
mineral oils, fully additivated mineral oils and poly-
alpha-olefins (PAOs) at comparable viscosities over the
entire range of slide-to-roll ratios (SRRs) . The results
for Tego IL IMES (ionic liquid from Evonik Goldschmidt
GmbH), Castrol SLX 0W-30 (fully additivated mineral oil
from Castrol), PAO 8 (poly-alpha-olefin) and a
nonadditivated mineral oil (CAS No. 8042-47-5) are shown
CA 02638146 2008-08-01
- 46 - 200700553
in Fig. 3.