Note: Descriptions are shown in the official language in which they were submitted.
CA 02638270 2008-07-24
NMS-053
THERAPEUTIC COMBINATION COMPRISING AN AURORA KINASE
INHIBITOR AND IMATINIB
FIELD OF THE INVENTION
The present invention relates in general to the field of cancer treatment and,
more
particularly, provides an anti-tumor composition comprising an Aurora kinase
inhibitor
and a BCR/ABL kinase inhibitor having a synergistic or additive antineoplastic
effect
BACKGROUND OF THE INVENTION
Survival rates in Chronic Myelogenous Leukemia patients have improved
dramatically since the introduction of Imatinib (Glivec, Gleevec) in 2001, a
tyrosine kinase
inhibitor, that is highly effective against most cases of CML in chronic
phase, but remains
poorly active in patients in the blast phase. Imatinib targets BCR-ABL, which
is the major
cause of CML and a subset of ALL patients bearing the Philadelphia chromosome.
For
review see: Deininger M, Buchdunger E, Druker BJ. The development of Imatinib
as a
therapeutic agent for chronic myeloid leukemia. Blood 2005;105:2640-53.
In particular patients in advanced phases of CML are resistant a priori or
frequently
develop resistance to Imatinib therapy, which is often due to the emergence of
mutant
forms of Bcr-Abl bearing point mutations in the kinase domain. These mutations
interfere
either directly with binding of the drug or prevent the adoption of the
inactive
conformation required for binding. Since Dasatinib and Nilotinib have been
launched most
of the mutations have a treatment option, with the exception of one of the
most common
identified mutations, which is located in the gatekeeper residue Threonine 315
of Abl and
which is mutated towards an Isoleucine (T3151). Against this mutation the most
advanced
1
CA 02638270 2008-07-24
second generation BCR-ABL inhibitors such as Dasatinib, Nilotinib, Bosutinib
or Inno-
406 are inactive.
Compound 1 has been identified based on a biochemical screen for inhibitors of
Aurora kinases and shows cross-reactivity with Abl kinase (see P. Carpinelli
et al., Mol
Cancer Ther 6: 3158-3168.)
The Aurora kinase inhibitor Compound 1 was also tested preclinically for its
activity to inhibit proliferation of cell lines expressing wildtype or
Imatinib resistant BCR-
ABL mutants including the T315I mutant and its crystal structure in complex
with T315I
Abl mutant has been solved (see Modugno et al., Crystal structure of the T3151
Abl mutant
in complex with the aurora kinases inhibitor Compound 1. Cancer Res. 2007 Sep
1;67(17):7987-90). In these cells both Abl and Aurora kinase activity were
inhibited and
Compound 1 showed pharmacological synergy with Imatinib in cell lines with a
partial
resistance to Imatinib. Strong antiproliferative activity is also seen in
CD34+ cells from
CML patients in chronic phase or blast crisis, including those bearing the
T3151 mutation
(Gontarewicz, A. et al. Simultaneous targeting of Aurora kinases and Bcr-Abl
kinase by
the small molecule inhibitor Compound I is effective against Imatinib-
resistant BCR-ABL
mutations including T3151. - Blood (2008) vol. 111, p. 4355-4364).
There is a continuous need of combination of known anticancer drugs in order
to
optimise the therapeutic treatment.
Some pyrrolopyrazoles have been demonstrated to be potent inhibitors of Aurora
kinase enzymes. One of these compounds is currently in development as an anti-
cancer
agent. Aurora kinase inhibitors are understood to trigger an aberrant mitosis,
dependent on
2
CA 02638270 2008-07-24
the genetic background of cells leading to a G2/M block, endoreduplication
and/or
apoptosis.
The present invention provides new combinations of a kinase inhibitor,
targeting
Aurora kinases as well as wild-type and mutant ABL kinase, with known
pharmaceutical
agents that are particularly suitable for the treatment of proliferative
disorders, especially
CML. More specifically, the combinations of the present invention are very
useful in
therapy as antitumor agents and lack, in terms of both toxicity and side
effects, the
drawbacks associated with currently available antitumor drugs.
SUMMARY OF THE INVENTION
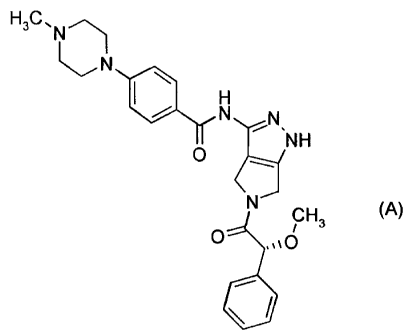
The present invention provides a therapeutic combination comprising (a)
Compound 1 of formula (A):
H3C,N
N
I~ H
N N
NH
O
N CH3 (A)
O
and (b) a BCR-ABL kinase inhibitor, wherein the active ingredients are present
in each
case in free form or in the form of a pharmaceutically acceptable salt or any
hydrate
thereof.
The present invention also provides a method of treating or delaying the
progression of a proliferative disorder, wherein said method comprises the
simultaneous,
3
CA 02638270 2008-07-24
sequential or separate administration to a patient in need thereof of the
above-mentioned
therapeutic combination.
The present invention further provides a pharmaceutical composition comprising
the above-identified therapeutic combination admixed with a pharmaceutically
acceptable
carrier, diluent or excipient.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides, in a first embodiment, a therapeutic
combination
comprising (a) Compound 1 of formula (A):
H3C' N--)
N
H
N N
O NH
N CH3 (A)
O ,,%0
and (b) a BCR-ABL kinase inhibitor, wherein the active ingredients are present
in each
case in free form or in the form of a pharmaceutically acceptable salt or any
hydrate
thereof.
A further embodiment of the combination according to the invention is a
combined
preparation for simultaneous, separate or sequential use.
A still further embodiment relates to the combination according to the
invention in
a method of treating or delaying the progression of a proliferative disorder,
wherein the
4
CA 02638270 2008-07-24
method comprises the simultaneous, sequential or separate administration to a
patient in
need thereof of the therapeutic combination.
In a still further embodiment the invention provides a pharmaceutical
composition
comprising a combination according to the invention admixed with a
pharmaceutically
acceptable carrier, diluent or excipient.
Another embodiment relates to the use of a compound 1 of formula (A) as
defined
above in the preparation of a medicament for the treatment of a proliferative
disorder,
wherein said treatment comprises simultaneously, sequentially or separately
administering
a compound of formula (A) as defined above and a BCR-ABL kinase inhibitor
selected
from the group consisting of Imatinib, Dasatinib, Nilotinib, Bosutinib and
Inno-406, to a
patient in need thereof.
Still another embodiment relates to the use of a compound of formula (A) as
defined above and a BCR-ABL kinase inhibitor, in the preparation of a
medicament for
treating a proliferative disorder.
The compound 1 of formula (A) has the chemical name N-[5-(2-Methoxy-2-
phenyl-acetyl)-1,4,5,6-tetrahydro-pyrrolo [3,4-c]pyrazol-3-yl]-4-(4-methyl-
piperazin- l yl)-
benzamide. This compound was described and claimed in the international patent
application W02005/005427, published on December 20, 2005, which also
disclosed the
process for its preparation (incorporated herein by reference). The compound 1
of formula
(A) is endowed with protein kinase inhibitory activity and is thus useful in
therapy as an
antitumor agent.
Pharmaceutically acceptable salts of the compound 1 of formula (A) include the
acid addition salts with inorganic or organic acids, e.g., nitric,
hydrochloric, hydrobromic,
CA 02638270 2008-07-24
sulphuric, perchloric, phosphoric, acetic, trifluoroacetic, propionic,
glycolic, lactic, oxalic,
malonic, malic, maleic, mesylate, tartaric, citric, benzoic, cinnamic,
mandelic,
methanesulphonic, isethionic and salicylic acid and the like.
According to a preferred embodiment of the invention, the BCR-ABL inhibitors
are
selected from the group consisting of Imatinib, Dasatinib, Nilotinib,
Bosutinib and Inno-
406. In a more preferred embodiment of the invention, the BCR-ABL inhibitor is
Imatinib.
Imatinib can be administered, e.g., in the form as it is marketed, e.g. under
the
trademark Glivec or Gleevec . Dasatinib can be administered, e.g., in the
form as it is
marketed, e.g. under the trademark Sprycel . Nilotinib can be administered,
e.g., in the
form as it is marketed, e.g. under the trademark Tasigna .
In the present invention, each of the active ingredients of the combination is
in an
amount effective to produce a synergistic or additive antineoplastic effect.
The present invention also provides a method for lowering the side effects
caused
by antineoplastic therapy with an antineoplastic agent in mammals, including
humans, in
need thereof, the method comprises administering to said mammal a combined
preparation
comprising the compound 1 of formula (A) as defined above and a BCR-ABL
inhibitor
selected from the group consisting of Imatinib, Dasatinib, Nilotinib,
Bosutinib and Inno-
406, in amounts effective to produce a synergistic or additive antineoplastic
effect.
By the term "a synergistic antineoplastic effect" as used herein is meant the
inhibition of the growth of the tumor, preferably the complete regression of
the tumor, by
administering an effective amount of the combination of a the compound of
formula (A) as
defined above and a BCR-ABL inhibitor selected from the group consisting of
Imatinib,
Dasatinib, Nilotinib, Bosutinib and Inno-406 to mammals, including humans.
6
CA 02638270 2008-07-24
The term "combined preparation" as used herein defines especially a "kit of
parts"
in the sense that the combination of components (a) and (b) as defined above
can be dosed
independently or by use of different fixed combinations with distinguished
amounts of the
combination components (a) and (b), i.e. simultaneously or at different time
points. The
elements of the kit of parts can then, e.g., be administered simultaneously or
chronologically staggered, that is at different time points and with equal or
different time
intervals for any part of the kit of parts. More preferably, the time
intervals are chosen such
that the effect on the treated disease in the combined use of the parts is
greater than the
effect which would be obtained by use of only any one of the combination
components (a)
and (b). The ratio of the total amounts of the combination component (a) to
the
combination component (b) to be administered in the combined preparation can
be varied,
e.g. in order to cope with the needs of a patient sub-population to be treated
or the needs of
the single patient which different needs can be due to the particular disease,
age, sex, body
weight, etc. of the patients. Preferably, there is at least one beneficial
effect, e.g., a mutual
enhancing of the effect of the combination components (a) and (b), in
particular a
synergism, e.g. a more than additive effect, additional advantageous effects,
less side
effects, less toxicity, and more preferably a strong synergism of the
combination
components (a) and (b). In addition, a beneficial effect is a combined
therapeutic effect in a
dosage where component (a) and/or component (b) has no therapeutic effect
alone under
such dosage.
By the term "administered" or "administering" as used herein is meant
parenteral
and /or oral administration. By "parenteral" is meant intravenous,
subcutaneous and
intramuscular administration.
7
CA 02638270 2008-07-24
In the method of the subject invention, for the administration of the compound
1 of
formula (A), the course of therapy generally employed is in the range from 100
mg/m2/day
to 1500 mg/m2/day of body surface area for up to 21 consecutive days. More
preferably,
the course therapy employed is from about 150 mg/m2/day to about 350 mg/m2/day
of
body surface area for up to 21 consecutive days. In a particularly preferred
regimen, the
compound of formula (A) is administered in a dose of 250, 330, or 400
mg/m2/day of body
surface area for six hours infusion on days 1, 8 and 15 of a four weeks cycle.
Other
possible therapeutic schedules are disclosed, for example, in WO 2008/052931
published
May 8, 2008 (incorporated herein by reference).
The compound 1 of formula (A) can be administered in a variety of dosage
forms,
e.g., orally, in the form of tablets, capsules, sugar or film coated tablets,
liquid solutions or
suspensions; rectally in the form of suppositories; parenterally, e.g.,
intramuscularly, or
through intravenous and/or intrathecal and/or intraspinal injection or
infusion.
For the administration of a BCR-ABL inhibitor the course of therapy generally
employed for Imatinib is from 150 mg/m2/day to 700 mg/m2/day, more preferably,
from
about 200 mg/m2/day to 350 mg/m2/day. For Dasatinib, the dose regimen is about
70 mg
per os bid.
The antineoplastic therapy of the present invention is in particular suitable
for
treating gastro-intestinal tumour (GIST) or hematopoietic malignant tumours
such as
leukaemias and lymphoma (i.e. Acute Lymphoblastic Leukaemia (ALL), Chronic
Lymphocytic Leukaemia (CLL), Multiple Myeloma (MM), Chronic Myeloid Leukaemia
(CML), Acute Myeloid Leukaemia (AML)).
8
CA 02638270 2008-07-24
As stated above, the effect of the combination of the invention is
significantly
increased without a parallel increased toxicity. In other words, the combined
therapy of the
present invention enhances the antitumoral effects of the component (a) and/or
of
component (b) of the combination of the invention and thus yields the most
effective and
less toxic treatment for tumors.
Pharmaceutical compositions according to the invention are useful in
anticancer
therapy.
The present invention further provides a commercial package comprising, in a
suitable container means, (a) a compound 1 of formula (A) as defined above,
and (b) a
BCR-ABL inhibitor, wherein the active ingredients are present in each case in
free form or
in the form of a pharmaceutically acceptable salt or any hydrate thereof,
together with
instructions for simultaneous, separate or sequential use thereof.
In a package according to the invention each of components (a) and (b) are
present
within a single container means or within distinct container means.
Another embodiment of the present invention is a commercial package comprising
a pharmaceutical composition or product as described above.
Due to the key role of the Aurora kinases in the regulation of cellular
proliferation,
the combinations of the present invention are also useful in the treatment of
a variety of
cell proliferative disorders such as, for example, benign prostate
hyperplasia, familial
adenomatosis, polyposis, neurofibromatosis, psoriasis, vascular smooth cell
proliferation
associated with atherosclerosis, pulmonary fibrosis, arthritis,
glomerulonephritis and post-
surgical stenosis and restenosis.
9
CA 02638270 2008-07-24
The activities of the combination of the present invention are shown for
instance by
the following in vitro and in vivo tests, which are intended to illustrate but
not to limit the
present invention.
The synergistic antineoplastic effect of the combined preparations of the
present
invention is shown, for instance, by the following in vitro test, which is
intended to
illustrate the present invention without posing any limitation to it.
Example 1: In vitro anti-proliferative effect of Compound 1 in combination
with
Imatinib.
Table 1 reports the results obtained testing in vitro the cytotoxic effect of
Compound 1 in combination with Imatinib.
Materials and Methods: Exponentially growing human myelogenous leukemia K-
562 cell line was seeded and incubated at 37 C in a humidified 5% CO2
atmosphere. Drugs
were added to the experimental culture, and incubations were carried out at 37
C for 72
hours in the dark. Scalar doses of Compound 1 and Imatinib were added to the
medium 24
hours after seeding.
Three treatment schedules were tested: A) simultaneous administration (both
drugs
administered to cells for 72 hours); B) sequential administration (Compound 1
administered 24 hours before Imatinib). C) sequential administration (Imatinib
administered 24 hours before Compound 1).
Drug solutions were prepared immediately before use. At the end of treatment,
cell
proliferation was determined by counting the cell number using a Coulter
Counter.
Inhibitory activity was evaluated comparing treated versus control data using
Assay
Explorer (MDL) program. The dose inhibiting 50% of cell growth was calculated
using
CA 02638270 2008-07-24
sigmoidal interpolation curve. Combination indices (C.I.) were calculated
using a
computer program for multiple drug effect analysis based on the equation of
Chou-Talalay
(Adv Enzyme Regul 1984; 22:27-55) for mutually nonexclusive drugs, where a
C.I. <1
indicates a more than additive effect (C.I.>3 indicates strong antagonism;
1.3<C.I.<3,
antagonism; 0.8<C.I.<1.2, additivity; 0.3<C.I.<0.8, synergism; C.I.<0.3,
strong
synergism).
Results. The administration to human myelogenous leukemia K-562 cell lines of
Compound 1 in combination with Imatinib resulted in a synergistic antitumor
effect.
Table 1
Cell Line Schedule Drug C.I. at 70% of Effect of
RATIO fraction affected Combination
K-562 A 1:0.5 0.24 strong synergism
1:1 0.21 strong synergism
1:2 0.51 synergism
1:4 0.49 synergism
B 1:0.05 0.06 strong synergism
1:0.1 0.01 strong synergism
1:0.2 0.19 strong synergism
1:0.4 0.09 strong synergism
C 1:0.05 0.22 strong synergism
1:0.1 0.30 synergism
1:0.2 0.74 synergism
1:0.4 0.55 synergism
11
CA 02638270 2008-07-24
Example 2: In vivo antitumor efficacy in combination with Imatinib
SCID female mice, from Harlan (Italy), were maintained in cages with paper
filter
cover, food and bedding sterilized and water acidified. Human myeloid leukemia
K-562
cell line was maintained in vitro at 37 C in a humidified 5% CO2 atmosphere.
For in vivo experiments 107 K562 cells were implanted subcutaneously in SCID
mice. K562 cell line was selected as it is a BCR-ABL positive model carrying
the
chromosomal translocation known as Philadelphia chromosome and because it was
previously demonstrated that it is sensitive to Imatinib.
On day 7, when tumors reached an estimated weight of 100 to 150 mg, animals
were assigned to 4 experimental groups by random selection and received the
following
treatments: group 1, control, vehicle solution; group 2, Compound 1 twice a
day
intraperitoneally at a dose of 15 mg/kg for 9 consecutive days (days 7, 8, 9,
10, 11, 12, 13,
14, 15); group 3, Imatinib twice a dayper os at 100 mg/kg for 9 consecutive
days (days 7,
8, 9, 10, 11, 12, 13, 14, 15); and group 4 Compound 1 twice a day
intraperitoneally at a
dose of 15 mg/kg (days 7, 8, 9, 10, 11, 12, 13, 14, 15) and Imatinib twice a
dayper os at
100 mg/kg (days 7, 8, 9, 10, 11, 12, 13, 14, 15).
Tumor growth and body weight were measured every 3 days. Tumor growth was
assessed by caliper. The two diameters were recorded and the tumor weight was
calculated
according the following formula: length (mm) x width2/2 The effect of the
antitumor
treatment was evaluated as the delay in the onset of an exponential growth of
the tumor
(see for references, Anticancer drugs 7:437-60,1996). This delay (T-C value)
was defined
as the difference of time (in days) required for the treatment group (T) and
the control
group(C) tumors to reach a predetermined size (1 g).
12
CA 02638270 2008-07-24
Toxicity was evaluated on the basis of body weight reduction. The results are
reported in Table 2 below. Compound 1 combined with Imatinib produced a clear
therapeutic advantage: the T-C observed when Compound 1 was combined with
Imatinib
was clearly superior to the one obtained with Imatinib or Compound 1 as single
agent. No
toxicity was observed in any of the treatment group.
Table 2
Treatment Time to reach T-C (days) Toxicity
1 g (days)
Compound 1 27.3 11.5 0/7
15 mg/kg*
Imatinib 25.1 9.3 0/7
100 mg/kg**
Imatinib 35.9 20 0/7
100 mg/kg +
Compound 1
15 mg/kg***
*Treatments made intraperitoneally twice a day on days 7,8,9,10,11,12,13,14,15
**Treatments made per os at days 7,8,9,10,11,12,13,14,15
*** Days 7,8,9,10,11,12,13,14,15 Imatinib treatments; days 7,8,9,11,12,13,15
Compound 1 treatments.
13