Note: Descriptions are shown in the official language in which they were submitted.
CA 02638310 2008-07-28
SWIRL COATING APPLICATOR
BACKGROUND
1. Technical Field
The present disclosure relates generally to filament coating systems and
methods,
more specifically to systems and methods for coating sutures.
2. Background of Related Art
Surgical sutures are primarily used during surgery to stitch together sections
of tissue
to aid in post-surgical healing. Sutures are often coated with various
substances to improve
their knot tie-down characteristics. In addition, a coating may increase a
suture's surface
lubricity which reduces the friction associated with passing of the suture
through tissue,
thereby reducing tissue trauma. Conventionally, suture coatings have been
applied by
brushing, wiping, spraying or dipping. Dip coating involves submergence of a
suture line
into a coating composition contained in a vessel. The coating composition may
be injected
into the vessel through one or more injection ports.
1
CA 02638310 2008-07-28
The application of coatings has also been accomplished using filling heads.
This
method may involve passing a suture line through a V-shaped notch to obtain a
more even
coating. Coating composition injected into the notch contacts and coats the
suture line.
Although the coating system using filling heads may provide more consistent
coating for the
suture line, the contact time for the coating solution to penetrate into the
suture may be less
(e.g., less than about 0.1 seconds) than that provided by conventional dip
coating
mechanisms.
Improved coating systems and methods for coating medical devices, including
sutures, remain desirable.
SUMMARY
According to one aspect of the present disclosure, an applicator for coating a
suture
line is disclosed. The applicator includes a coating cavity having an inlet
port for entry of the
suture line into the coating cavity and an outlet port for exit of the suture
line out of the
coating cavity. The applicator also includes one or more injection ports
configured to supply
a coating composition into the coating chamber in a direction substantially
tangential to the
coating cavity.
According to another aspect of the present disclosure, an applicator for
coating a
suture line is disclosed. The applicator includes a coating cavity having an
inlet port for entry
of the suture line into the coating cavity and an outlet port for exit of the
suture line out of the
coating cavity. The applicator also includes one or more injection ports
cOnfgured to inject a
coating composition into the coating chamber in a direction substantially
tangential to the
2
CA 02638310 2008-07-28
coating cavity thereby generating rotational circulation therein and thereby
further promoting
the uniformity of the coating composition and flow distribution inside the
coating cavity for
the passing suture line.
According to a further aspect of the present disclosure, an applicator for
coating a
suture line is disclosed. The applicator includes a coating cavity having an
inlet port for entry
of the suture line into the coating cavity and an outlet port for exit of the
suture line out of the
coating cavity. Each of the inlet port and the outlet port includes a seal
having an eyelet sized
to allow the at least one suture line to pass therethrough with minimal
clearance thereby
minimizing loss of the coating composition. The applicator also includes two
or more
injection ports configured to inject a coating composition into the coating
chamber in a
direction substantially tangential to the coating cavity thereby generating
rotational
circulation therein and thereby further promoting the uniformity of the
coating composition
and flow distribution inside the coating cavity for the passing suture line.
BRIEF DESCRIPTION OF THE DRAWINGS
The above and other aspects, features, and advantages of the present
disclosure will
become more apparent in light of the following detailed description when taken
in
conjunction with the accompanying drawings in which:
Fig. 1 is a schematic diagram of a suture coating system according to one
embodiment
of the present disclosure;
3
CA 02638310 2008-07-28
Fig. 2 is a top cross-sectional view of a coating applicator according to the
present
disclosure; and
Fig. 3 is a side cross-sectional view of the coating applicator of Fig. 2
according to the
present disclosure.
DETAILED DESCRIPTION
Particular embodiments of the present disclosure will be described herein
below with
reference to the accompanying drawings. In the following description, well-
known functions
or constructions are not described in detail to avoid obscuring the present
disclosure in
unnecessary detail.
The present disclosure provides for a swirl coating system. The system
includes one
or more input winders inputting a suture line into a coating applicator. The
coating applicator
includes a coating cavity and one or more injection ports for injecting a
coating composition
in the cavity. The injection ports may be disposed tangentially to the cavity
and may be
configured to generate rotational circulation therein, thereby swirling the
coating
composition. Upon coating, the suture line may be dried and thereafter the
line guided to
winding rolls.
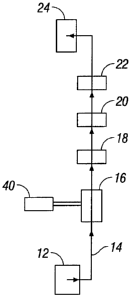
Fig. 1 shows a coating system 10 according to the present disclosure for
coating a
suture line and/or other filaments (e.g., wire). The coating system 10
includes at least one
input winder 12 for passing a suture line 14 through a coating applicator 16,
an optional air
wiper 18, a dryer 20, an optional air cooler 22 and a take-up winder 24. As
the line 14 passes
4
CA 02638310 2008-07-28
through the coating applicator 16 it is submerged in a coating composition in
order to apply
the coating composition thereto. The coating is supplied by pump 40. The
optional air wiper
18 is disposed between the coating applicator 16 and the dryer 20 and is
configured to blow
gas (e.g., air, nitrogen, etc.) on the passing line 14 to blow off excess
coating composition.
The optional air cooler 22 is disposed between the dryer 20 and the take-up
winder 24 and
may be configured to blow cool air on the line 14 to provide cooling for the
dried line. After
passing through the coating applicator 16, the line 14 is wound by at least
one take-up winder
24.
The input winder(s) 12 disperses the line 14 which may be a monofilament or a
multifilament braided suture. Prior to dispersing, the line 14 may be prepared
for coating, in
embodiments by calendaring the line 14 to facilitate penetration of the
coating composition
into the interstices of a multifilament braided suture. This may be especially
useful where the
present system is used to apply a second or third coating composition to the
suture line. An
example of a suitable calendaring apparatus and method of use thereof is
disclosed in
commonly owned U.S. Patent No. 5,312,642 entitled "Method and Apparatus for
Calendering and Coating/Filling Sutures":
Figs. 2 and 3 show in more detail the coating applicator 16 in accordance with
an
embodiment of the present disclosure. The coating applicator 16 includes a
housing 30
which may have a tubular or block structure having a coating cavity 32 defined
therein. The
coating cavity 32 may be fonrned within the housing using traditional milling,
casting, and/or
drilling techniques and may have a diameter from about 2 mm to about 20 mm, in
5
CA 02638310 2008-07-28
embodiments from about 3 mm to about 10 mm, and a height from about 5 mm to
about 100
mm, in embodiments from about 10 mm to about 50 nun. The housing 30 may be
formed
from metals, such as stainless steel, titanium, high-alloy cast steel, and the
like, ceramics, or
plastics, such as polytetrafluroethylene (PTFE), perfluoroalkoxy fluorocarbon
(PFA),
polypropylene, polyethylene, polycarbonate, polystyrene, and the like,
depending upon
material compatibility and corrosion and/or erosion considerations. If metal
is used, it may
be desirable to passivate the tube to reduce its reactivity. Passivation
methods and materials
are within the purview of those skilled in the art. Those skilled in the art
will also appreciate
that the cylindrical shape is merely only one embodiment of the coating cavity
32 and that the
cavity may have a variety of shapes (e.g., tubular, rectangular, triangular,
pentagonal or
hexagonal cross-sectional shapes, etc.).
The housing 30 also includes one or more injection ports 34 which are disposed
tangentially with respect to the coating cavity 32. A coating composition 38
is supplied
through the injection ports 34 to fill the cavity 32. The coating composition
38 is supplied by
a pump 40 (Fig. 1) which is connected to the injection ports 34 via tubing.
The pump 40 may
be any pump, such as centrifugal, rotary, diaphragm, gear, reciprocating, and
the like. Those
skilled in the art will appreciate that the tubing used to interconnect the
pump 40 and the
coating applicator 16 may be manufactured from any materials rigid or flexible
as well as
chemically inert to a variety of solvents. In one embodiment, the tubing may
be made from
PTFE or PFA.
The coating composition 38 is pumped into the cavity 32 through the injection
ports
34 until the cavity 32 is substantially filled with the coating composition
38. The coating
6
CA 02638310 2008-07-28
cavity 32 includes an inlet port 33 through which the line 14 enters the
cavity 32 and an
output port 35 through which the line 14 exits the cavity 32. The inlet and
outlet ports 33 and
35 may include an eyelet 36 configured to guide the line 14 therethrough. Each
of the eyelets
36 includes a passageway 41 drilled and/or formed therethrough. The passageway
41 has a
diameter sized to allow the line 14 to pass therethrough with minimal
clearance to eliminate
or minimize the loss of the coating composition 38 through the bottom eyelet
36. The
diameter of the passageway 41, sometimes referred to herein as the inner
diameter of the
eyelets 36, may be from about 0.9 mm to about 5 mm, in embodiments from about
1 mm to
about 3 mm, depending on the thickness of the line 14. The eyelets 36 may be
attached to the
housing 30 using one or more bolts 39. Each of the eyelets 36 may also include
seal 37 (e.g.,
an 0-ring). The seals 37 may be made from suitable materials, including
fluoroelastomers
such as those commercially available as VITON fluroelastomers (from DuPont),
PTFE,
fluoroelastomer encapsulated materials, including TEFLON encapsulated
silicone,
TEFLON encapsulated VITON , TEFLON encapsulated ethylene propylene diene
monomer (EPDM), and other suitable materials.
As shown in Fig. 3, once the cavity 32 is partially filled with the coating
composition
38, the line 14 is passed vertically through the coating applicator 16 along
the central axis "y"
thereof so that the line 14 is in direct contact with the coating composition
38. As stated
above, the injection ports 34 are disposed tangentially with respect to the
cavity 32 such that
injection streams of the coating composition 38 are directed tangentially
around the center of
thP r.avitv '12 inlilrP in nnnvPntinnal r.natina apnlinatnrc, where the
iniantinn nnrt ic dicnnePrl
> > c applicators,
perpendicular to the suture line so that the injection stream is directly
hitting the line. One
potential problem with the conventional injection port arrangements is the
opposite side of
7
CA 02638310 2008-07-28
the suture line may be subjected to a different flow distribution which
results in roughness of
the coating surface and/or dry spots.
The injection streams directed by the injection ports 34 generate rotational
circulation
as represented by the arrows 42 in Fig. 2. This eliminates uneven flow which
is a side effect
of pulsation generated by the pumping of the positive displacement pump 40
and/or non-
uniform distribution of flow inside the cavity 32 due to perpendicular
orientation of the
injection port 34. The swirling resulting from the configuration of the
present disclosure also
results in more uniform coating due to more uniform flow pattern of the
coating composition
38 as the coating composition 38 is swirled around the line 14.
The injection ports 34 may have a funnel shape as depicted in Fig. 3 narrowing
toward the cavity 32. This configuration is useful for connection to supply
tubes but may be
useful for increasing flow velocity of the coating composition 38 which, in
turn, may provide
for increased circulation of the coating composition 38. In embodiments,
multiple injection
ports 34 may be used depending on the flow rate, solution density and
viscosity of the coating
composition 38 and whether it is a homogenous solution or dispersion. As seen
in Fig. 2, the
injection ports 34 may be disposed such that the injection streams 40 are
injected in the same
direction (e.g., clockwise or counterclockwise) to the circulation of the
composition in the
cavity and, thus, not cancel each other out.
In Fig. 2 and 3, the injection ports 34 are disposed on the same horizorital
plane with
the streams 40 being injected in the clockwise direction. In embodiments,
multiple injection
ports 34 may be disposed on multiple horizontal planes to provide for
circulation along the
entire height of the cavity 32.
8
CA 02638310 2008-07-28
Any coating composition known to be useful for coating medical devices may be
applied to a medical device using the present methods and apparatus. The
coating
composition can be a solution, dispersion, emulsions or combinations thereof.
Suitable
coatings may contain, for example, one or more polymeric materials and/or one
or more
bioactive agents.
In some embodiments, the coating composition includes a polymer, or a
combination
of polymers. The polymer is most suitably biocompatible, including polymers
that are non-
toxic, non-inflammatory, chemically inert, and substantially non-immunogenic
in the applied
amounts. The polymer may be either bioabsorbable or biostable. Bioabsorbable
polymers
may be gradually absorbed or eliminated by the body by hydrolysis, metabolic
process, bulk,
or surface erosion. Examples of suitable bioabsorbable materials include, but
are not limited
to, polyesters, polyorthoesters, polyphosphoesters, poly (amino acids),
cyanoacrylates,
copoly(ether-esters), polyalkylene oxalates, polyphosphazenes,
polyiminocarbonates,
aliphatic polycarbonates, combinations thereof, and the like. Specific
examples of suitable
bioabsorbable materials include, but are not limited to, polycaprolactone
(PCL), poly-D, L-
Iactic acid (DL-PLA), poly-L-lactic acid (L-PLA), lactide, glycolide,
poly(lactide-co-
glycolide), poly(hydroxybutyrate), poly(hydroxybutyrate-co-valerate),
polydioxanone,
polyanhydride, poly(glycolic acid), poly(glycolic acid-cotrimethylene
carbonate),
polyphosphoester urethane, poly(trimethylene carbonate), poly(iminocarbonate),
and
combinations thereof. Biomolecules such as heparin, fibrin, fibrinogen,
cellulose, starch, and
collagen may also be suitable fnr noatings.
9
CA 02638310 2008-07-28
A biostable polymer does not break down in the body, and thus a biostable
polymer is
present in the body for a substantial amount of time after implantation.
Examples of biostable
polymers include para-xylylene, also known as parylene, and its derivatives
including poly-
para-xylylene (parylene N), poly-monochloro-para-xylylene (parylene C), poly-
dichloro-para-
xylylene (parylene D), and fluorinated parylenes (parylene HT) (all of which
are
commercially available from SPECIALTY COATING SYSTEMSTM), polyurethanes (for
example, segmented polyurethanes such as BIOSPANTM), polyethylene,
polypropylene,
polyethlyene teraphthalate, ethylene vinyl acetate, silicone, polyethylene
oxide, and
polytetrafluoroethylene (PTFE).
In some embodiments, the coating compositions of the present disclosure may
also
include a fatty acid component that contains a fatty acid or a fatty acid salt
or a salt of a fatty
acid ester. Suitable fatty acids may be saturated or unsaturated, and include
higher fatty acids
having more than about 12 carbon atoms. Suitable saturated fatty acids
include, for example,
stearic acid, palmitic acid, myristic acid and lauric acid. Suitable
unsaturated fatty acids
include oleic acid, linoleic acid, and linolenic acid. In addition, an ester
of fatty acids, such
as sorbitan tristearate or hydrogenated castor oil, may be used.
Suitable fatty acid salts include the polyvalent metal ion salts of C6 and
higher fatty
acids, particularly those having from about 12 to about 22 carbon atoms, and
mixtures
thereo Fatty acid salts including the calcium, magnesium, barium, aluminum,
and zinc salts
of stearic, palmitic and oleic acids may be useful in some embodiments of the
present
disclosure. Particularly useful salts include commercial "food grade" calcium
stearate which
CA 02638310 2008-07-28
consists of a mixture of about one-third C 16 and two-thirds C 18 fatty acids,
with small
amounts of the C 14 and C22 fatty acids.
Suitable salts of fatty acid esters which may be included in the coating
compositions
applied in accordance with the present disclosure include calcium, magnesium,
aluminum,
barium, or zinc stearoyl lactylate; calcium, magnesium, aluminum, barium, or
zinc palmityl
lactylate; calcium, magnesium, aluminum, barium, or zinc olelyl lactylate;
with calcium
stearoyl-2-lactylate (such as the calcium stearoyl-2-lactylate commercially
available under the
tradename VERV from American Ingredients Co., Kansas City, Mo.) being useful
in some
embodiments. Other fatty acid ester salts which may be utilized include
lithium stearoyl
lactylate, potassium stearoyl lactylate, rubidium stearoyl lactylate, cesium
stearoyl lactylate,
francium stearoyl lactylate, sodium palmityl lactylate, lithium palmityl
lactylate, potassium
palmityl lactylate, rubidium palmityl lactylate, cesium palmityl lactylate,
francium palmityl
lactylate, sodium olelyl lactylate, lithium olelyl lactylate, potassium olelyl
lactylate, rubidium
olelyl lactylate, cesium olelyl lactylate, and francium olelyl lactylate.
Where utilized, the amount of fatty acid component can be in an amount from
about 5
percent to about 50 percent by weight of the total coating composition, in
embodiments from
about 10 percent to about 20 percent by weight of the total coating
compositions.
In some embodiments, the coating composition contains one or more bioactive
agents. The term "bioactive agent", as used herein, is used in its broadest
sense and includes
any substance or mixture of substances that have clinical use. Consequently,
bioactive agents
may or may not have pharmacological activity per se, e.g., a dye.
Alternatively a bioactive
agent could be any agent which provides a therapeutic or prophylactic effect,
a compound
11
CA 02638310 2008-07-28
that affects or participates in tissue growth, cell growth, cell
differentiation, a compound that
may be able to invoke a biological action such as an immune response, or could
play any
other role in one or more biological processes.
Examples of classes of bioactive agents which may be utilized in coatings
applied in
accordance with the present disclosure include antimicrobials, analgesics,
antipyretics,
anesthetics, antiepileptics, antihistamines, anti-inflammatories,
cardiovascular drugs,
diagnostic agents, sympathomimetics, cholinomimetics, antimuscarinics,
antispasmodics,
hormones, growth factors, muscle relaxants, adrenergic neuron blockers,
antineoplastics,
immunogenic agents, immunosuppressants, gastrointestinal drugs, diuretics,
steroids, lipids,
lipopolysaccharides, polysaccharides, and enzymes. It is also intended that
combinations of
bioactive agents may be used.
Suitable antimicrobial agents which may be included as a bioactive agent in
the
coating applied in accordance with the present disclosure include triclosan,
also known as
2,4,4'-trichloro-2'-hydroxydiphenyl ether, chlorhexidine and its salts,
including chlorhexidine
acetate, chlorhexidine gluconate, chlorhexidine hydrochloride, and
chlorhexidine sulfate,
silver and its salts, including silver acetate, silver benzoate, silver
carbonate, silver citrate,
silver iodate, silver iodide, silver lactate, silver laurate, silver nitrate,
silver oxide, silver
palmitate, silver protein, and silver sulfadiazine, polymyxin, tetracycline,
aminoglycosides,
such as tobramycin and gentamicin, rifampicin, bacitracin, neomycin,
chloramphenicol,
miconazole, quinolones such as oxolinic acid, norfloxacin, nalidixic acid,
pefloxacin,
enoxacin and ciprofloxacin, penicillins such as oxacillin and pipracil,
nonoxynol 9, fusidic
acid, cephalosporins, and combinations thereof. In addition, antimicrobial
proteins and
12
CA 02638310 2008-07-28
peptides such as bovine lactoferrin and lactoferricin B may be included as a
bioactive agent
in the coatings.
Other bioactive agents which may be included as a bioactive agent in the
coating
composition applied in accordance with the present disclosure include: local
anesthetics;
non-steroidal antifertility agents; parasympathomimetic agents;
psychotherapeutic agents;
tranquilizers; decongestants; sedative hypnotics; steroids; sulfonamides;
sympathomimetic
agents; vaccines; vitamins; antimalarials; anti-migraine agents; anti-
parkinson agents such as
L-dopa; anti-spasmodics; anticholinergic agents (e.g. oxybutynin);
antitussives;
bronchodilators; cardiovascular agents such as coronary vasodilators and
nitroglycerin;
alkaloids; analgesics; narcotics such as codeine, dihydrocodeinone,
meperidine, morphine
and the like; non-narcotics such as salicylates, aspirin, acetaminophen, d-
propoxyphene and
the like; opioid receptor antagonists, such as naltrexone and naloxone; anti-
cancer agents;
anti-convulsants; anti-emetics; antihistamines; anti-inflammatory agents such
as hormonal
agents, hydrocortisone, prednisolone, prednisone, non-hormonal agents,
allopurinol,
indomethacin, phenylbutazone and the like; prostaglandins and cytotoxic drugs;
estrogens;
antibacterials; antibiotics; anti-fungals; anti-virals; anticoagulants;
anticonvulsants;
antidepressants; antihistamines; and immunological agents.
Other examples of suitable bioactive agents which may be included in the
coating
composition include viruses and cells, peptides, polypeptides and proteins,
analogs, muteins,
and active fragments thereof, such as immunoglobulins, antibodies, cytokines
(e.g.
lymphokines, monokines, chemokines), blood clotting factors, hemopoietic
factors,
interleukins (IL-2, IL-3, IL-4, IL-6), interferons ((3-IFN, (a-IFN and y-IFN),
erythropoietin,
13
CA 02638310 2008-07-28
nucleases, tumor necrosis factor, colony stimulating factors (e.g., GCSF, GM-
CSF, MCSF),
insulin, anti-tumor agents and tumor suppressors, blood proteins,
gonadotropins (e.g., FSH,
LH, CG, etc.), hormones and hormone analogs (e.g., growth hormone), vaccines
(e.g.,
tumoral, bacterial and viral antigens); somatostatin; antigens; blood
coagulation factors;
growth factors (e.g., nerve growth factor, insulin-like growth factor);
protein inhibitors,
protein antagonists, and protein agonists; nucleic acids, such as antisense
molecules, DNA
and RNA; oligonucleotides; and ribozymes.
A single bioactive agent may be utilized to form the coating composition or,
in
alternate embodiments, any combination of bioactive agents may be utilized to
form the
coating composition applied in accordance with the present disclosure.
The amounts of coating composition to be applied to a suture may vary
depending
upon the specific construction of the suture, the size and the material of
this construction. In
general, the coating composition applied to an unfilled suture may account for
from about 0.5
percent by weight to about 4 percent by weight of thb coated suture, in
embodiments from -
about 1 percent to about 3 percent by weight of the coated suture. For a
filled (i.e.,
containing a storage stabilizing agent) braided suture, amounts of coating
composition may
generally vary from about 0.2% to about 3%, in embodiments from about 0.5% to
about 2%.
As a practical matter and for reasons of economy and general performance, it
may be
desirable to apply the minimum amount of coating composition consistent with
good surface
lubricity and/or knot tie-down characteristics, which amount may be readily
determined
experimentally for any particular suture.
14
CA 02638310 2008-07-28
The described embodiments of the present disclosure are intended to be
illustrative
rather than restrictive, and are not intended to represent every embodiment of
the present
disclosure. Various modifications and variations can be made without departing
from the
spirit or scope of the disclosure as set forth in the following claims both
literally and in
equivalents recognized in law.