Note: Descriptions are shown in the official language in which they were submitted.
CA 02639126 2008-08-26
ENEMA DEVICE
BACKGROUND
An enema is a common medical procedure whereby fluid is injected into the
rectum of a patient in order to induce bowel movement. The need for such a
procedure
typically arises in patients suffering from certain physical ailments in which
voluntary
bowel control is impaired.
Medical equipment currently exists in the art for administering an enema to
patients in need of this medical procedure. At least one type of equipment
consists of an
enema squeeze bottle filled with the fluid intended to induce bowel movement,
which is
capped by a short applicator nozzle to be inserted into the patient's rectum.
The applicator
nozzle of this type of conventional enema application device often causes
discomfort and
irritation when being inserted. Therefore, it is desired to have an enema
device that
safely and effectively administers the enema to a patient without causing
discomfort.
Moreover, enemas are often administered to a patient at home when the need for
medical assistance does not necessitate a doctor or another health care
assistant.
However, it is often difficult for the patient to administer the enema to him
or herself
since the applicator nozzle must be inserted into such a small, sensitive
area. Moreover,
it is difficult for the patient to administer the fluid while steadily holding
the enema in the
required area. Often the patient is assisted by another individual; however,
assistance
may not always be available, if, for instance, the patient lives alone. Thus,
there is also a
need for an enema device that can be effectively self-administered.
SUMMARY
An enema device for use with a source of enema fluid is provided. The enema
device includes a hollow elongated body having first and second ends an
interior
IRFRO 181 IAP.DOC - 1 -
CA 02639126 2008-08-26
extending between the first and second ends, a feed portion formed at the
first end of the
hollow elongated body, and an applicator portion disposed at the second end of
the
elongated body. The feed portion is in fluid communication with the interior
of the
hollow elongated body and is adapted to be placed into fluid communication
with a
source of enema fluid. The applicator portion defines a discharge opening in
communication with the interior of the hollow elongated body.
This summary is provided to introduce a selection of concepts in a simplified
form that are further described below in the Detailed Description. This
summary is not
intended to identify key features of the claimed subject matter, nor is it
intended to be
used as an aid in determining the scope of the claimed subject matter.
DESCRIPTION OF THE DRAWINGS
The foregoing aspects and many of the attendant advantages of the present
disclosure will become more readily appreciated by reference to the following
detailed
description, when taken in conjunction with the accompanying drawings,
wherein:
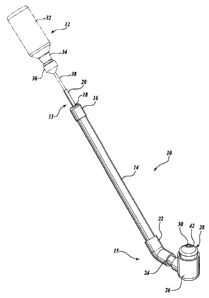
FIGURE 1 is an environmental view of an enema device constructed in
accordance with one embodiment of the present disclosure, wherein the enema
device is
shown in use with a pre-packaged enema bottle;
FIGURE 2 is an isometric view of the enema device of FIGURE 1;
FIGURE 3 is a cross-sectional isometric view of the enema device of FIGURE 2;
FIGURE 4 is a first alternate embodiment of a portion of the enema device of
FIGURE 2;
FIGURE 5 is a second alternate embodiment of a portion of the enema device of
FIGURE 2; and
FIGURE 6 is a partial cross-sectional view of an alternate embodiment of an
enema device, wherein the enema device is shown in use with a pre-packaged
enema
bottle.
DETAILED DESCRIPTION
An enema device 10 shown in accordance with one embodiment of the present
disclosure is best seen by referring to FIGURE 1, wherein the enema device is
being used
by a patient A sitting on a toilet B. The enema device 10 is shown in use with
a
prepackaged disposable enema 12 that contains a suitable medicated fluid used
for
IRFR\3181 I AP. DOC -2-
CA 02639126 2008-08-26
enemas. It should be appreciated that the enema device 10 may instead be used
with any
suitable enema, such as an enema bag or a bulb syringe. The enema device 10
may be
used by a patient A to self-administer the enema when sitting on the toilet B;
however,
the patient A may instead be standing or sitting in any suitable position such
that the
enema device 10 may effectively be used.
Referring to FIGURES 2 and 3, the enema device 10 includes a hollow, rigid
elongated body 14 that acts as a lever against the toilet B or another
suitable abutment
device to brace the lower enema device 10 against the patient's buttocks (see
FIGURE 1).
The elongated body 14 is preferably circular in cross section or any other
suitable cross
sectional shape. The elongated body 14 is substantially straight such that it
acts as a
suitable lever against the toilet B; however, it should be appreciated that
the elongated
body 14 may instead be suitably curved or contoured to position the enema
device 10
against the front interior portion C of a toilet B or similar device.
Moreover, an
adjustable brace (not shown) may also be received on the elongated body 14 to
help
position and maintain the elongated body 14 against the front interior portion
C of a
toilet B. The elongated body 14 may also include a telescoping feature (not
shown) that
would allow the patient to lengthen or shorten the enema device 10 as needed.
The
elongated body 14 is preferably made of any suitable rigid material, such as
polyvinyl
chloride (PVC).
The enema device 10 includes a feed portion 13 disposed at the upper end of
the
elongated body 14 that is adapted to engage the prepackaged enema 12 and
receive the
medicated fluid from the prepackaged enema 12. The feed portion 13 includes a
hollow
cap 16 that is tightly received on the upper end of the elongated body 14 to
enclose the
upper end of the elongated body 14. The cap 16 includes a relatively small
opening 17 in
substantially the center of the cap that is adapted to receive a transport
tube 18. The
transport tube 18 is preferably made of a flexible material, such as rubber,
and it extends
throughout the length of the elongated body 14 and upwardly and outwardly from
the
cap 16 through the opening 17. The protruding portion of the transport tube 18
defines a
feed tube portion 20 that is of a length and diameter to tightly receive, for
instance, a
nozzle 38 of the prepackaged enema 12 within the upper end of the feed tube
portion 20.
It should be appreciated that the transport tube 18 may instead receive a
separate
feed tube (not shown) thereon that is adapted to receive the nozzle 38 of the
prepackaged
IRFR\3181 I AP.DOC -3-
CA 02639126 2008-08-26
enema 12. The feed tube could be made of a flexible or semi-flexible material,
such as
rubber, and may be of a suitable length to act as an extension of the enema
device 10 and
facilitate easy insertion of the nozzle 38 within the feed tube at a desired
distance from
the enema device 10 or by another person.
In an alternative configuration, the transport tube 18 may not utilize a feed
tube
portion 20. Rather, a tapered opening may be formed in the cap 16 for
receiving the
nozzle 38 of the prepackaged enema 12. Such opening is tapered to receive
nozzles 38 of
various sizes. In this configuration, the tube 18 is in fluid flow
communication with the
tapered opening formed in the cap 16.
The enema device 10 further includes an applicator portion 15 disposed at the
lower end of the elongated body 14 that is adapted to position the enema
device 10
against the buttocks of the patient A for application of the enema. The
applicator
portion 15 includes a first elbow fitting 22 that is tightly received at one
end on the lower
end of the elongated body 14. The first elbow fitting 22 preferably includes
only a slight
bend such that an obtuse angle is formed between the elongated body 14 and the
first
elbow fitting 22. A sleeve fitting 24 is tightly received within the other end
of the first
elbow fitting 22, and a second elbow fitting 26 is tightly received on the
sleeve fitting 24.
The second elbow fitting 26 includes a substantially horizontal portion that
is secured to
the sleeve fitting 24 and a substantially vertical portion that defines a
circular opening
adapted to receive an end cap 28 therewithin.
The end cap 28 is circular in cross section and includes curved or contoured
edges
such that it is comfortably received against the patient's buttocks. The end
cap 28
includes an end face 44 having a discharge opening 30 formed in substantially
the center
of the end face 44 that is adapted to receive the lower end of the transport
tube 18 tightly
therewithin. The transport tube 18 is positioned within the opening 30 such
that the end
of the tube 18 is substantially flush with the end face 44 of the end cap 28.
Preferably, the end cap 28 is contoured to help align the opening 30 against
the
patient's rectum and provide comfort to the patient A during use. Any suitable
end cap
shape may be used. As a non-limiting example, the end cap 28 may include a
self-centering upward protrusion 42 formed around the opening 30, as shown in
FIGURES 1-3. The self-centering upward protrusion 42 may be any suitable
contoured
shape to engage the opening of the rectum. For instance, the end face 44 may
be slightly
IRF'R\3I S 1 I AP, DOC -4-
CA 02639126 2008-08-26
concave and extend upwardly toward the opening 30 to define the self-centering
protrusion 42 and provide a soft, curved engagement surface. The self-
centering
protrusion 42 may instead be a small dome (not shown) formed on a
substantially flat end
face 44.
In the alternative configuration of cap 16 discussed above, the nozzle 38 of
the
enema bottle cap 36 is conveniently engaged within the tapered opening formed
in cap 16
of the device feed portion 13. It will be appreciated that the cap 16 may be
formed with a
thicker or alternative configuration end portion than shown in FIGURE 3, so as
to
provide sufficient length for the tapered opening formed therein. Once the
nozzle 38 is
engaged within such tapered opening, the prepackaged enema 12 is placed in
secure fluid
communication with the enema device 10.
FIGURE 4 depicts a first alternate embodiment of an end cap 128, wherein the
end cap 128 includes a substantially flat end face 144 with an opening 130
formed
therein. The end cap 128 includes a curved perimeter edge to provide a
contoured
engagement surface.
FIGURE 5 depicts a second alternate embodiment of an end cap 228, wherein the
end cap 228 includes a convex, dome surface 244 having a central opening 230
formed
therein. It should be appreciated that any suitable contoured end cap shape
may instead
be used. Moreover, the end cap 28 may be any suitable cross-sectional shape,
such as
round, elliptical, etc.
Although the enema device 10 has been illustrated and described as having many
different parts, it should be appreciated that the rigid components of the
enema device 10
(i.e. the cap 16, elongated body 14, first elbow fitting 22, sleeve fitting
24, second elbow
fitting 26, and end cap 28) may instead be formed together as one hollow piece
formed by
injection molding or another suitable method. Moreover, the enema device 10
may be
any suitable shape such that it may effectively be used to administer an
enema, as
described below. Furthermore, the enema device 10 need not include a transport
tube 18.
Rather, the medicated fluid may instead be received and transported within the
rigid
components of the enema device 10.
Referring to FIGURE 2, the feed portion 13 of the enema device 10 is adapted
to
receive the medicated fluid from the prepackaged disposable enema 12 or any
other
suitable enema. The prepackaged disposable enema 12 preferably includes an
enema
IRFR\3181 1 AP.DOC -5-
CA 02639126 2008-08-26
bottle 32 having a threaded bottle neck 34 that receives a twist-on threaded
bottle cap 36.
The enema bottle 32 is preferably prefilled with a medicated fluid that is
well known in
the art. The upper end of the bottle cap 36 includes an opening that is in
communication
with a nozzle 38 secured to the bottle cap 36. The nozzle 38 is adapted to be
tightly
received within the feed tube portion 20 of the transport tube 18 to place the
prepackaged
enema 12 into fluid communication with the enema device 10.
As can best be seen be referring to FIGURE 6, the bottle cap 36 includes a
one-way anti-reflux valve 40 that is positioned within the bottle cap 36 such
that it
encloses the opening in the bottle neck 34. The one-way valve 40 is preferably
made
from a non-porous flexible material and can be circular in shape, and is of a
size which
permits accommodation into the bottle cap 36. The one-way valve 40 prevents
reflux of
the medicated fluid back up into the bottle 32, thereby holding back any
contaminants as
well as helping to sustain the collapsibility of the bottle 32.
Referring to FIGURE 1, the enema device 10 is most easily used when the
patient
is seated on a toilet B. To use the enema device 10, the patient A positions
the applicator
portion 15 beneath his or her buttocks such that a portion of the elongated
body 14 and
the feed portion 13 extend upwardly and outwardly from the toilet B in front
of the
patient A. The patient A then positions the end cap 28 properly against the
rectum such
that the self-centering protrusion 42 is received therein and the opening 30
is in fluid
communication therewith.
The patient A thereafter pushes the top portion of the elongated body 14
outwardly to engage the elongated body 14 on the front interior portion C of
the toilet B
and pivot the elongated body 14 on the front interior portion C. The top
portion of the
elongated body 14 is pushed outwardly until the end cap 28 is firmly engaged
against the
rectum. While holding the enema device 10 in this position, the patient
inserts the nozzle
38 of the prepackaged enema 12 into the feed tube 20. The patient A then
squeezes the
bottle 32 to force the medicated fluid out of the valve 40, through the nozzle
38, and into
the transport tube 18. The fluid travels forcibly down through the transport
tube 18 until
it reaches the opening 30 in the end cap 28. The medicated fluid exits the
opening 30 and
projects upwardly into the patient's rectum. In this manner, the patient is
able to
administer an enema without the help of another person, and without inserting
an
IRFR\3181IAP.DOC -6-
CA 02639126 2008-08-26
uncomfortable tip into the rectum. However, it should be appreciated that the
enema
device 10 may instead be used with the assistance of a second person.
Now referring to FIGURE 6, an alternate embodiment of an enema device 310
includes an end cap 328 that is adapted to be received onto the bottle neck 34
of a
disposable enema bottle 32. The enema device 310 includes a sleeve fitting 324
that is
adapted to be received over the lower portion of the bottle neck 34. An end
cap 328,
substantially similar in shape and size to the end cap 28 of the enema device
10 is tightly
received over at least a portion of the sleeve fitting 324.
The end cap 328 and sleeve fitting 324 are adapted to receive a cap 336 and
nozzle 338 substantially similar to the cap 36 and nozzle 38 that come with
the
prepackaged enema 12 except that the nozzle 338 is shorter in length. The end
cap 328
includes an opening 330 that is adapted to receive at least a portion of the
tip 338 such
that the tip 338 does not protrude from the opening 330 of the end cap 328, or
is
otherwise flush with the exterior surface of the end cap 328.
The cap 336 and tip 338 are secured within the sleeve fitting 324 and the end
cap 328 by first filling the upper portion of the cap 328 with an epoxy or
thermosetting
material 342 and thereafter press fitting the cap 336 and tip 338 therewithin.
The tip 338
is received within the opening 330 and the cap 336 is secured within the upper
end of the
interior of the end cap 328. The sleeve 324, cap 336, tip 338, and end cap 328
cooperatively define the enema device 310. The enema device 310 replaces the
standard
cap 36 and nozzle 38 that comes attached to the bottle 32 of the prepackaged
enema 12.
It should be appreciated that the enema device 310 may be instead made in any
other
suitable manner.
To use the enema device 310, the patient first removes the cap 36 that is
attached
to the bottle 32 of the prepackaged enema 12. The patient then secures the
enema
device 310 to the bottle 32 by inserting the threaded portion of the bottle
neck 34 into the
cap 336 and thereafter twisting the end cap 328. The patient then positions
the enema
device 310 such that the end cap 322 abuts the buttocks and the opening 330 is
aligned
with the rectum. While holding the bottle 32 and enema device 310 in this
position, the
patient then squeezes the bottle 32 to force the medicated liquid out of the
one-way
valve 40, through the tip 338, and outwardly through the opening 330 of the
enema
device 310 into the patient's rectum. Although the enema device 310 can be
used to
IRFRU I 81 I AP. DOC -7-
CA 02639126 2008-08-26
self-administer the enema, it is preferred that another person positions the
enema
device 310 and administers the enema to the patient.
While the preferred embodiment has been illustrated and described, it will be
appreciated that various changes can be made therein without departing from
the spirit
and scope of the present disclosure.
IRFR\3181 I AP. DOC - O-